Abstract
The cytochrome P450 (CYP) enzyme family is the most important enzyme system catalyzing the phase 1 metabolism of pharmaceuticals and other xenobiotics such as herbal remedies and toxic compounds in the environment. The inhibition and induction of CYPs are major mechanisms causing pharmacokinetic drug–drug interactions. This review presents a comprehensive update on the inhibitors and inducers of the specific CYP enzymes in humans. The focus is on the more recent human in vitro and in vivo findings since the publication of our previous review on this topic in 2008. In addition to the general presentation of inhibitory drugs and inducers of human CYP enzymes by drugs, herbal remedies, and toxic compounds, an in-depth view on tyrosine-kinase inhibitors and antiretroviral HIV medications as victims and perpetrators of drug–drug interactions is provided as examples of the current trends in the field. Also, a concise overview of the mechanisms of CYP induction is presented to aid the understanding of the induction phenomena.
Keywords: Cytochrome P450, Inhibition, Induction, Drug–drug interaction, Herbal remedies, Environmental toxicants
Introduction
Inhibition and induction of cytochrome P450 (CYP) enzymes are central mechanisms, resulting in clinically significant drug–drug interactions (DDI). Today, characteristics and regulatory factors of various CYP enzymes have been elucidated to a considerable extent (Manikandan and Nagini 2018; Zanger and Schwab 2013). Detailed mechanisms of inhibition have been uncovered by studies on isolated or expressed enzymes and tissue fractions. Nuclear receptors as important xenobiotic-sensing transcription factors and as regulators of CYP induction have been elucidated (Wang et al. 2012).
Prediction on the basis of in vitro studies is now an integral part of early drug development (Lu and Di 2020) as well as of the medicines agency guidelines (EMA, FDA, and MHLW/PMDA). Computational models such as physiologically based pharmacokinetic models are now being used for quantitative prediction of in vivo interactions from in vitro experiments (Kato 2020; Min and Bae 2017), and these models are used extensively by drug developers before and during clinical trials. After preclinical studies, there is an ultimate need of human in vivo studies and observations on inhibition and induction. Obviously, such information is absolutely needed for clinical drug treatment to prevent possible adverse outcomes and ensure safety.
In addition to drugs, humans are exposed to a large number of other chemical substances through diet, use of cosmetics, in workplaces, by environmental pollutants, etc., and many of these chemicals are in vitro inhibitors or inducers of CYP enzymes but compared to pharmaceutics often poorly characterized. The risk posed by these chemicals is difficult or impossible to assess without reliable in vitro–in vivo extrapolation, which is only possible by having proven in vivo inhibitors or inducers (and non-effective substances) as reference items.
With these premises in mind, and pointing to the profound developments in drug research and regulation (see the guest editorial, Pelkonen et al., in this issue), we have collected and updated the information about human in vivo inhibitors and inducers, which would constitute a curated compilation for the use as a reference for other in-depth studies. The main focus is on data published after 2008, and in many instances, we point to our earlier review for references before 2008 (Pelkonen et al. 2008).
Progress since 2008
We previously reviewed CYP inhibition and induction 12 years ago (Pelkonen et al. 2008). In 2008, we stated that, because multiplicity and variability of CYP enzymes are an important complicating factor in pharmacological and toxicological research and regulation, and predictive and pre-empting measures are a top priority, and thus, the development of predictive in vitro approaches is necessary and should be based on the firm background of basic research on the phenomena of inhibition and induction and their underlying mechanisms. Consequently, we focused on covering both inhibition and induction of CYP enzymes, always keeping in mind the basic mechanisms on which to build predictive and preventive in vitro approaches to be validated by in vivo studies. These principles still apply today. Nevertheless, since 2008, further progress has been made in the research of CYP inhibition and induction and the application of the knowledge. Furthermore, very important development has happened in the characteristics of new drugs.
New pharmaceuticals since 2008
It is obvious that the spectrum of new drugs has changed since 2008 (see the guest editorial Pelkonen et al. in this issue and (de la Torre and Albericio 2020; Yu et al. 2019). Biological drugs, proteins, and peptides or oligonucleotides occupy nowadays a sizable share of new drugs (see Internet sites of major drug agencies: https://www.accessdata.fda.gov/scripts/cder/daf/; https://www.ema.europa.eu/en/medicines; https://www.pmda.go.jp/english/review-services/reviews/approved-information/drugs/0002.html) and their role in DDIs in general is supposed to be in the pharmacodynamics sphere; specifically, CYP-associated DDIs are not expected. Consequently, small-molecular new chemical entities represent a smaller contribution into the new drugs, and these are more thoroughly studied during the developmental phases with in vitro tools and during clinical trials with focus on specific enzymes and transporters depicted by the in vitro information. The efficiency of the in vitro and in vivo tools as formulated in guidance documents from major authorities (EMA 2012, FDA 2020, MHLW/PMDA 2018)1 is demonstrated by the fact that there have been no major surprises leading to drug withdrawals among novel drugs during the last 10–15 years. Advancements in the pharmacokinetic research include the recognition that many less-studied non-CYP enzymes and especially several transporters have emerged as interaction targets.
Shifts in approved drug classes have led to the situation that anticancer and antiviral (HIV) drugs are major molecules in CYP-associated DDIs. These shifts are probably behind the observation that CYP3A4 substrates form a majority of the drugs suspected or shown as causing CYP-associated interactions. The observation that there seem to be only a few inducers among newly approved drugs may be explained by the thrust in the development of small molecule drugs towards more potent and specific molecules. This has led to a relative decrease of clinical doses, which often are too small to cause a significant CYP induction.
Tyrosine (protein) kinase inhibitors as an example of CYP-mediated DDIs
Tyrosine kinase inhibitors (TKIs) form a relatively novel class of (mainly) anticancer agents, which has been expanding tremendously over the last 2 decades. Because of their “precision” targets, TKIs offer a more effective and safer option in many cancers compared to the cytostatic agents. Because their pharmacodynamic targets are a diverse, even if functionally related, set of enzymes, it is not surprising that their chemical structures as well as their metabolism and general pharmacokinetic characteristics are rather variable. However, TKIs actually are well represented in DDI sections of reference books and reviews, especially regarding their metabolic features and transporter involvements [see, e.g., (Gay et al. 2017; Hussaarts et al. 2019; Jackson et al. 2018)]. In this section, the TKI-associated CYP-DDIs are presented as an example of current concerns of clinically important CYP interactions.
Drugs selected
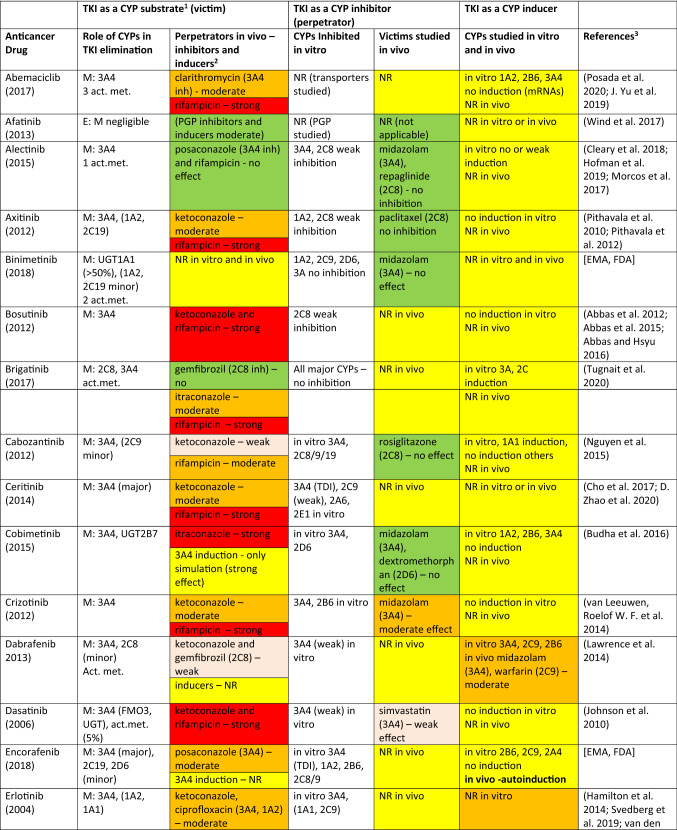
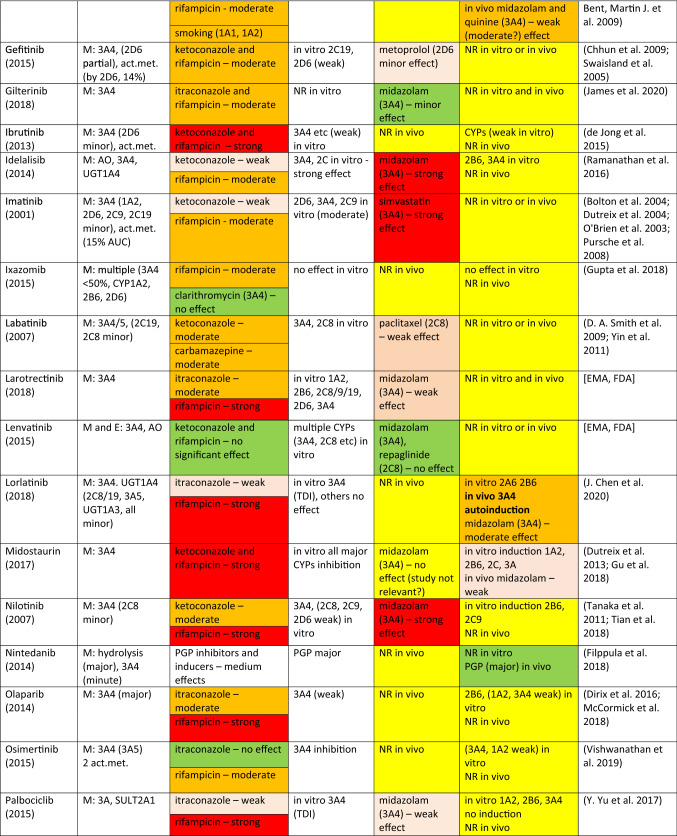
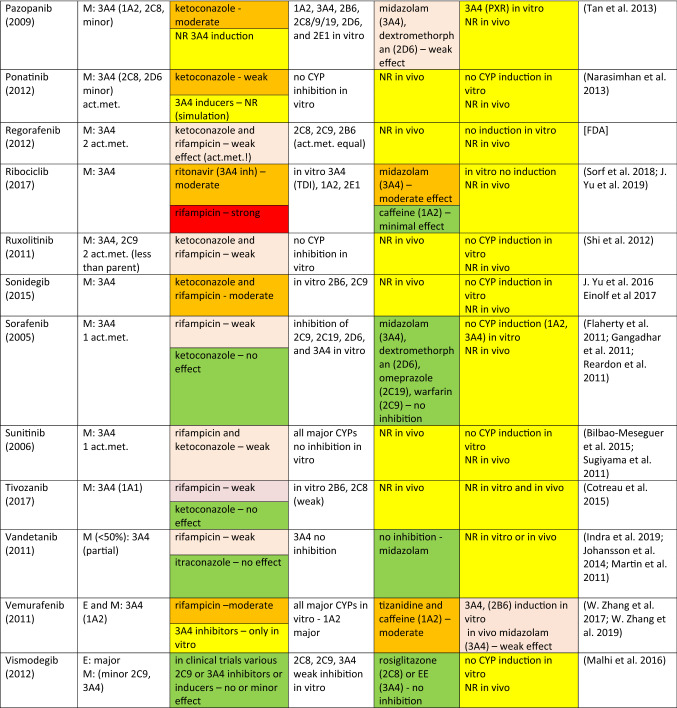
The drugs covered here include protein or tyrosine-kinase inhibitors (TKIs) approved by EMA and/or FDA until 2018. There are a number of TKIs that have been discarded in the last rounds of development, but this source of useful compounds remains largely untapped for the analysis of DDIs. However, a scan of literature and physician’s desk references demonstrate that many of the approved TKIs are predominantly CYP3A4 substrates and many of them display a potential to inhibit or induce CYP enzymes. Consequently, it is a good opportunity to look at various interaction characteristics of these TKIs for the purposes of this review. Some salient features are collected in Table 1.
Table 1.
Tyrosine (protein) kinase inhibitor anticancer drugs as CYP substrates, inhibitors, and inducers
Act.met. active metabolite(s) (if reported or published), PGP P-glycoprotein, NR no results or not reported, TDI time-dependent inhibition
1E: excretion of a drug as an unchanged parent. M: metabolism—the extent and contributions of CYP isoforms’ other xenobiotic-metabolizing enzymes if known
2Usually, strong inducers (rifampicin) and inhibitors (ketoconazole, itraconazole) of CYP3A4 were studied. Other perpetrators are assigned with appropriate CYP enzyme. Color code: red, strong effect; orange, moderate effect; light brown, weak/minor effect; green, no (significant) effect; yellow, information in need
3Major sources drug monographs from FDA, EMA, and FIMEA; the latest uploaded documents were retrieved. Publications in general literature were sought and used for additional evidence for conclusions
Key publications
An important element in research of TKIs is that the crucial development leading to authorization has occurred at the time when in vitro and in vivo studies for predicting and estimating CYP interactions have been refined to the extent that there has been a possibility for fact-based go/no-go decisions and that there are tools to estimate the contribution of particular CYP enzymes and their predictable interaction consequences. On the other hand, much of the available published material is of regulatory nature, i.e., drug monographs in national formularies, and thus detailed experimental and clinical results may not be available for open scrutiny. Thus, we have been mostly dependent on material that is not publicly peer-reviewed (naturally regulators have had access to original studies), but on the other hand, studies providing the basis for official drug monographs are expected to be of high quality. Furthermore, many of them have appeared in the public literature later on. Otherwise, publicly available studies are often rather sporadic regarding individual drugs, but, nevertheless, we have referred to them when they provide additional or confirmatory information.
TKI as a victim drug
As can be seen in Table 1, a large majority of TKIs, 41 out of 43 drugs, is metabolized by CYP3A4/5 at least to a certain extent. Other CYP enzymes, such as CYP1A2, CYP2B6, CYP2C, and CYP2D6, contribute to the metabolism of some TKIs, but only binimetinib is metabolized to a small extent by CYP1A2 and CYP2C9 and not at all by CYP3A4/5. It is perhaps appropriate to note that the exact contribution of any single CYP is often rather difficult to quantitate precisely, but usually it is possible to state, whether CYP3A4 is responsible for a major or minor share of the metabolism. In vitro studies with human liver preparations or human hepatocytes are often crucial in this respect. In any case, it is not often possible to find in regulatory filings important parameters to describe enzyme kinetics, although some information may be found in the public literature.
The extent and relative isoform contribution of CYP-associated metabolism of individual TKIs is one of the crucial factors leading to clinically significant DDI potential. As the anticancer effect is of paramount interest for the developer of the compound, the clinician, and ultimately the patient, some risks of off-target effects including DDIs are accepted that would not be deemed acceptable when developing drugs for other less serious indications.
In DDI clinical studies, it is customary to use inhibitors and inducers which are known to have a strong effect. In most cases, rifampicin is used as an inducer and ketoconazole or itraconazole as an inhibitor. However, the strength of effect of a perpetrator is dependent on the metabolic characteristics of a victim, i.e., affinity to the principal enzyme, relative contribution of a specific enzyme to overall metabolism or PK behavior of a drug, and alternative enzymatic and excretory clearance routes. Consequently, the interaction outcome of a “strong” perpetrator may be strong, moderate, or weak, dependent on a specific victim. The intensity of inhibition or induction is defined by the FDA on the basis of the AUC change (FDA 2020).2 Strong, moderate, and weak inhibitors give rise to an increase in AUC of a victim at least fivefold, between two and fivefold, and 1.25- to 2-fold, respectively. For induction, corresponding AUC classes are an AUC decrease by > 80%, between 50 and 80% and between 20 and 50%. As stated above, even a “strong” inhibitor or inducer could result in strong, moderate, or weak effect, dependent on characteristics of a victim. Obviously, this classification provides only a rough yardstick for assessing the likelihood or clinical significance of an interaction and many other factors such as concentration–effect relationships of a victim may be more significant.
Regarding 43 TKI drugs in Table 1, the metabolism of 30 of them is strongly or moderately and seven weakly inhibited and/or induced by “strong” CYP3A4 perpetrators and only five are classified as having no CYP3A4-associated DDIs as victims. Among these “negatives”, CYP3A4 plays either a minor or no role in elimination: afatinib is excreted mainly unchanged, binimetinib is metabolized by hydrolysis, lenvatinib is predominantly excreted unchanged and metabolized by aldehyde oxidase, nintedanib is eliminated by P-glycoprotein, and vismodegib is eliminated only to a minor extent by CYPs. It is fair to conclude that a majority of clinically used TKIs are CYP3A4 substrates, although the contribution of CYP3A4 to the overall elimination may be decreased by other metabolic or transporter routes [see, e.g., (Fenner et al. 2009; Yu et al. 2017a, b, 2019)].
TKIs as CYP inhibitors
Most TKIs in Table 1 have been screened for inhibitory potential using in vitro human liver microsomal assays consisting of major CYP activities from CYP1A2 to CYP3A4/5. In seven cases, no inhibition in vitro was detected, whereas for the rest of the drugs, the in vitro classifications ranged from “studied” to “some” or “weak inhibition”, and in a few cases even “moderate or strong inhibitory action”. However, on the basis of the published regulatory text, it is difficult to quantify “weak” or “strong” effect. Often, the regulatory text noted that inhibition was present or non-existent “at clinically relevant concentrations”. In certain cases, in vitro studies were followed by in vivo studies in which CYP-selective probe drugs were employed. For example, with respect to CYP3A4 substrates, inhibition was classified as strong for idelalisib–midazolam, imatinib–simvastatin and nilotinib–midazolam, moderate for crizotinib–midazolam, dasatinib–simvastatin, and ribociclib–midazolam, and weak for larotrectinib–midazolam, palbociclib–midazolam, and pazopanib–midazolam. Regarding CYP2D6 substrates, inhibition was classified as weak in two cases: gefitinib–metoprolol and pazopanib–dextromethorphan. Regarding CYP2C8, lapatinib inhibited weakly paclitaxel elimination, and with CYP1A2, vemurafenib inhibited moderately tizanidine and caffeine elimination. Altogether, it can be concluded that the cases CYP inhibition by TKIs, regarded worthy a warning in the regulatory desk reference, were rather few. However, occasionally, there were warnings that seemed to be based only on in vitro results and/or subsequent physiologically based pharmacokinetic (PBPK) simulations (Yu et al. 2019).
TKIs as CYP inducers
According to the guidelines of major regulatory agencies, potential CYP induction should be studied in human-cultured hepatocytes in vitro or in an analogous cellular system. In most cases, appropriate studies have been performed and the outcome registered in the drug monograph. In 14 cases, no information on in vitro induction studies could be found (in Table 1, these are marked by NR, no results or not reported). No induction of the major inducible CYPs has been found in 14 cases and a clear response emerged in 10 cases (brigatinib, dabrafenib, ibrutinib, idelalisib, midostaurin, nilotinib, olaparib, osimertanib, pazopanib, and vemurafenib). In vivo studies were performed with 4 TKIs which resulted in a moderate induction with erlotinib–quinine or midazolam, and dabrafenib–midazolam or warfarin, and a weak induction with midostaurin–midazolam and vemurafenib–midazolam. Encorafenib was suspected of exhibiting autoinduction. However, regulatory texts are not always reliable regarding negative findings and it may well be that additional in vitro and in vivo studies have been performed but not reported. Based on this analysis, it can be concluded that TKIs do not often display clinically significant induction potency in humans in vivo.
Active metabolites
At least 13 TKIs have at least one active metabolite. However, there may be several types of active metabolites regarding potential effects and outcomes. Several TKIs have pharmacodynamically active metabolites with a similar, although not necessarily equipotent, pharmacodynamic action as the parent. In some cases, a pharmacodynamically active metabolite may also have CYP-interaction potential. A special case is regorafenib, which has two CYP3A4-associated active metabolites with equal effect compared to the parent. This makes the assessment of interactions quite complex and uncertain. For example, although rifampicin exposure slightly decreased the AUC of the parent compound, it increased the AUC of one active metabolite by 2.6-fold. Thus, it is quite difficult to estimate the net pharmacodynamic effect.
Another mechanism is the so-called time-dependent inhibition (TDI), often due to the tight or irreversible binding of an active metabolite with the catalyzing enzyme leading to its inactivation (mechanism-based inhibition) or potentially due to formation of a more potent inhibitory metabolite. Both terms, TDI and mechanism-based inhibition, are used in this review. The evaluation of TDI would require appropriate in vitro studies, which were not usually available concerning TKIs. A recent review (Jackson et al. 2018) listed the following TKIs as potential candidates in this category: axitinib, bosutinib, dasatinib, imatinib, erlotinib, gefitinib, lapatinib, nilotinib, pazopanib, and sunitinib. However, company or authority data are not usually detailed enough in this respect, and more appropriate and detailed information is provided only rarely in published articles (Filppula et al. 2018; Kenny et al. 2012; Mao et al. 2016).
The generation of reactive metabolites has quite often been studied by drug companies developing the TKIs, since the reactive metabolites could potentially induce hepatotoxicity and form a threat for withdrawal during development or, worse, after the regulatory approval. Thus, at least in the following cases, reactive metabolites have been identified for clinically available tyrosine-kinase inhibitors: axitinib (Wang et al. 2020), dasatinib (Li et al. 2009), erlotinib (Li et al. 2009; Zhao et al. 2018), gefitinib (Li et al. 2009), imatinib (Li et al. 2014), lapatinib (Takakusa et al. 2011; Teng et al. 2010), ponatinib (Lin et al. 2017), and sunitinib (Amaya et al. 2018). It is, however, difficult to ascertain a specific reactive metabolite to cause a certain TDI, especially when the presence of a reactive metabolite has been deduced on the basis of trapping agents (Mao et al. 2016).
Antiretroviral HIV drugs
The antiretroviral human immunodeficiency virus (HIV) drugs (Table 2) are of considerable interest for DDIs in research and therapy for two main reasons. First, the group contains two drugs (ritonavir and cobicistat) that are mainly used as pharmacokinetic enhancers, “boosters”, due to their strong and mechanism-based inhibitory action towards CYP3A4, the predominant enzyme metabolizing anti-HIV-protease inhibitors (Tseng et al. 2017). These boosters are rather rare examples of intentional, beneficial utilization of CYP-DDIs. The second reason is due to the frequent use of combinations of various antiviral drugs; up to four drugs in fixed combinations, although pharmacodynamic benefits are the major reasons to use such combinations.
Table 2.
Antiretroviral HIV drugs as CYP substrates, inhibitors and inducers
| Antiretroviral drug | As a CYP substrate | As a CYP inhibitor | As a CYP inducer | Referencesb | ||
|---|---|---|---|---|---|---|
| As a victima | Perpetrators (effect assignments in parentheses) | Target enzymes | Victim drugs (effect assignments in parentheses) | |||
| Pharmacokinetic enhancers (boosters) | ||||||
| Cobicistat |
E: > 80% M: 3A4, 2D6 (minor) |
Strong 3A4 inducers (moderate) | 3A4 (mechanism-based), 2D6 (weak) | Atorvastatin, rosuvastatin, etc. | No significant in vitro | Cattaneo et al. (2019), Sherman et al. (2015), Tseng et al. (2017) |
| Ritonavir |
E: > 50% M: 3A4, 2D6 (minor) |
Strong 3A4 inhibitors ketoconazole (minor) Strong 3A4 inducers rifampicin (moderate) |
3A4 (mechanism-based), 2D6, 2C9 | 3A4-, 2D6- and 2C9-substrates variable effects | 1A2, 2B6, 2C8, 2C9, 2C19 in vitro; in vivo minor or moderate effects | Cattaneo et al. (2019), Cooper et al. (2003), Tseng et al. (2017) |
| Protease inhibitors | ||||||
| Atazanavir (+cobicistat) | M: 3A4 |
Strong 3A4 inducers rifampicin (strong) Efavirenz (moderate) |
3A4 (mechanism-based), 2C8 (weak) | 3A4 substrates (from weak to strong) | No effect in vitro or in vivo | Tseng et al. (2017) |
| Darunavir (+ritonavir) | M: 3A4, 2D6 | 3A4-inducers and inhibitors (variable observed or predicted effects) | 3A4, 2D6 | 3A4 substrates (from weak to moderate) | 2C9? warfarin | Tseng et al. (2017), Wagner et al. (2017) |
| Fosamprenavir (amprenavir) (+ritonavir) | M: 3A4 | 3A4-inducers and inhibitors (variable observed or predicted effects) | 3A4 | 3A4 substrates (from weak to moderate) | 3A4; in vivo effect minor or moderate | Justesen et al. (2003), Sale et al. (2002), Tran et al. (2002) |
| Lopinavir (+ritonavir) | M: 3A4 | 3A4-inducers and inhibitors (variable observed or predicted effects) | 3A4 | 3A4 substrates (from weak to moderate) | 3A4, in vivo effect minor at most | Wagner et al. (2017) |
| Nelfinavir | M: 3A4, 2C19 |
3A4-inducers and inhibitors (weak to moderate) 2C19-inhibitors (weak to moderate) |
3A4 | Midazolam (moderate) |
In vitro 1A2, 2B6, 2C19 In vivo 1A2 (moderate), 2B6 (weak) and 2C9 (weak) |
Kirby et al. (2011a, b) |
| Saquinavir (+ritonavir) | 3A4 | 3A4-inducers and inhibitors (variable observed or predicted effects) | 3A4 | Midazolam (strong) | 3A4, in vivo minor effect at most | Dickinson et al. (2008), Eagling et al. (2002) |
| Tipranavir (+ritonavir) | 3A4 | 2B6 and 3A4-inducers and inhibitors (variable observed or predicted effects) | 2D6 | NA | 3A4, 1A2, 2C19 combination in vivo moderate or strong effect | Tseng et al. (2017) |
| Integrase strand transfer inhibitors | ||||||
| Bictegravir | M: 3A4, UGT1A1 (about equal) |
3A4 inhibitors: voriconazole (weak), atanazavir (moderate) 3A4 inducers: rifabutin (moderate), rifampicin (strong) |
No significant effects in vitro/in vivo | NA | No significant effects in vitro/in vivo | Gallant et al. (2017), Sax et al. (2017), Zhang et al. (2017) |
| Dolutegravir |
E: ~ 50% M: UGT1A1; 3A4 (minor) |
Strong 3A4 inducers: ritonavir, efavirenz, rifampicin (no significant effect) | No effect in vivo | No effect in vivo | Kandel and Walmsley (2015) | |
| Elvitegravir |
E: 95% M: 3A4 (minor) |
Inducers; rifabutin, efavirenz, etc. (minor effect at most) | Minor effect in vitro at most | 2C9? | Lee et al. (2012), Tseng et al. (2017) | |
| Raltegravir |
E: major M: UGT1A, no CYPs |
No significant effects | No in vitro/in vivo | No in vitro/in vivo | Okeke and Hicks (2011) | |
| Non-nucleoside reverse transcriptase inhibitors | ||||||
| Doravirine | M: 3A4 |
Strong 3A4 inhibitors ritonavir, ketoconazole (moderate) Strong 3A4 inducers rifampicin (strong) |
No in vitro/in vivo | NA | In vivo 3A4 (weak) | Khalilieh et al. (2019) |
| Efavirenz | M: 2B6 (primary), 2A6, 3A4 | 2B6 and 3A4-inducers and inhibitors (variable observed or predicted effects) | 2C9, 2C19, 3A4 | In vivo variable effects |
3A4, 2B6 in vitro 2B6 autoinduction 2A6, 2B6, 2C19, 3A4 in vivo variable effects |
Best and Goicoechea (2008), Marzolini et al. (2017), McDonagh et al. (2015), Metzger et al. (2019) |
| Etravirine | M: 3A4, 2C9, 2C19 | Inhibitors and inducers variable effects | 2C9, 2C19 | In vitro variable effects | 3A4 | Havens et al. (2020) |
| Nevirapine | M: 3A4, 2B6 |
Rifampicin (moderate) Fluconazole (strong) |
3A4, 2B6 (both weak) | Weak or no effects in vitro or in vivo |
3A4, 2B6 In vivo autoinduction In vivo weak or moderate effect at most |
Ena et al. (2012) |
| Rilpivirine | M: 3A4 |
Rifampicin (moderate) Ketoconazole (moderate) |
3A4 | No/minor effects in vivo at most | No in vitro/in vivo | Crauwels et al. (2013) |
| C–C chemokine receptor type 5 | ||||||
| Maraviroc | M: 3A4 | Strong 3A4 inducers and inhibitors (strong) | 3A4 (weak) | No significant inhibition in vitro or in vivo | No induction in vitro or in vivo | Abel et al. (2009) |
aM, elimination by metabolism, E excretion as an unchanged drug
bPrincipal source for the information of this table is based on the AIDS Info: Panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Department of Health and Human Services. 2020 [cited 2020 March 20]. Available from: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf
The use of combinations makes it challenging to evaluate, especially in therapeutic situations, potential DDIs with other drug treatments of individual patients. The FDA or EMA-approved drug monographs contain extensive tabulated information about experimentally and/or clinically observed, or predicted DDIs, which often are difficult) to translate into clinically useful advice in actual patients. It is expected that in the future, DDI-predicting PBPK-models and artificial intelligence-based algorithms would aid clinical decisions [see, e.g., (Ryu et al. 2018; Varma et al. 2015)].
Cobicistat and ritonavir are especially employed in combination with HIV-protease inhibitors which are CYP3A4 substrates. CYP3A4-associated metabolism is very potently inhibited, because both boosters are mechanism-based inhibitors and block protease inhibitor metabolism and clearance almost completely thus extending drug exposure and the ensuing effect. They are also used in combination with other classes of HIV drugs, especially in fixed multidrug combinations containing protease inhibitors.
Pharmacokinetic interactions could also be based on processes involving transporters, e.g., P-glycoprotein. Many HIV drugs are ligands of various transporters and consequently interactions with other ligands may occur (Alam et al. 2016). This review will not cover transporter-mediated interactions as the focus is on CYP-DDIs.
Nucleoside reverse transcriptase inhibitors (abacavir, emtricitabine, lamivudine, tenofovir alafenamide, tenofovir disoproxil, and zidovudine) and the only fusion inhibitor (enfuvirtide) are devoid of CYP inhibition potential, because they are not metabolized by, or interacting with, CYP enzymes and most of them are renally eliminated. They are also not known to cause CYP induction.
Herbal/botanical natural products interacting with drugs
Herbal and/or botanical (medicinal) products are used in the treatment of various diseases, often as a ‘self-treatment’ by the patient and many times unbeknownst to the treating physician (Paine and Roe 2018). From the drug-interaction point of view, a challenge is that herbal products are usually complex mixtures of constituents that can vary substantially in both content and concentration depending on the preparation and, furthermore, when isolated they can behave very differently (Kellogg et al. 2019; Paine et al. 2018; Sevior and Ahokas 2017). These problems are exaggerated by inadequacies of product regulation and standardization, thus leaving a physician without essential information and thus being at the mercy of very variable and often blatantly poor-quality literature (Pelkonen et al. 2014). Especially, there is a dearth of quality scientific data on potential herb–drug interactions for even widely used herbal medicines. In this review, interactions resulting in induction of CYP enzymes are detailed in Table 14. Regarding inhibitory interactions, only a few well-characterized examples (resveratrol, quercetin) have been included as ‘clinically significant’ perpetrators (see Table 4). According to literature reviews on herbal-associated CYP interactions [see, e.g., (Hermann and von Richter 2012; Izzo and Ernst 2009)], a large number of herbal preparations are interacting with CYP enzymes at the level of in vitro incubations, but there are variable and uncertain evidence on interactions in vivo. Also, major agency guidances pay little attention to these natural products; only EMA has a rather general entry in the interaction guidance, while FDA is treating herbal products as food supplements. The WHO document on herbal–drug interactions is under preparation and is expected shortly; it is hoped to set the stage for further scientific research and regulatory guidance to assess the clinical significance of herb–drug interactions.
Table 14.
Nutritional exposures and herbal remedies as in vivo inducers of human cytochrome P450 enzymes. Some of the studies have been performed with purified compounds in high doses for drug development purposes. Food contaminants and compounds formed during food preparation are listed in Table 13
| Enzyme | Compound | Examples of sources | Receptor(s) implicated | Tissues | References |
|---|---|---|---|---|---|
| CYP1A2 | Indole-3-carbinol | Cruciferous vegetables | AHR | Liver (phenotyping) | Pantuck et al. (1979), Reed et al. (2005) |
| Resveratrol | Many plants including berries, grapes and peanuts, and red wine | AHR indirectly | Liver (phenotyping, studied only with a pharmacologic dose) | Chow et al. (2010) | |
| CYP2A6 | Genistein | Legumes such as soybeans | ER | Liver (phenotyping, studied only with a pharmacologic dose) | Chen et al. (2011) |
| Sulforaphane | Cruciferous vegetables | NRF2 | Liver (phenotyping) | Hakooz and Hamdan (2007) | |
| Quercetin | Tea, many vegetables, fruits, and berries | ER | Liver (phenotyping, studied only with a pharmacologic dose) | Chen et al. (2009) | |
| CYP2B6 | Baicalin | Baikal skullcap, an herbal remedy | CAR/PXR | Liver (phenotyping, studied only with a pharmacologic dose) | Fan et al. (2009) |
| Hyperforin | St. John’s wort, an herbal remedy | PXR | Liver (phenotyping) | Lei et al. (2010) | |
| Sodium ferulate | Several herbal remedies such as Angelica sinensis, Cimicifuga heracleifolia, and Lignsticum chuangxiong | PXR | Liver (phenotyping, studied only with a pharmacologic dose) | Gao et al. (2013, 2012) | |
| CYP2C9 | Hyperforin | St. John’s wort | PXR | Liver (phenotyping) | Jiang et al. (2004, 2006) |
| CYP2C19 | Baicalin | Yin Zi Huang, an herbal remedy with several herbs | CAR/PXR | Liver (phenotyping) | Fan et al. (2007) |
| Hyperforin | St. John’s wort | PXR | Liver (phenotyping) | Wang et al. (2004a, b) | |
| CYP2E1 | Ethanol | Alcoholic drinks | Stabilization | Liver (phenotyping and expression), blood lymphocytes, esophagus, placenta | Girre et al. (1994), Millonig et al. (2011), Oneta et al. (2002), Perrot et al. (1989), Rasheed et al. (1997), Raucy et al. (1997, 1999), Takahashi et al. (1993), Tsutsumi et al. (1989) |
| Unknown compound(s) in St. John’s wort | St. John’s wort | Unknown | Liver (phenotyping) | Gurley et al. (2002, 2005) | |
| CYP3A4 | Baicalin | Yin Zi Huang, an herbal remedy with several herbs | CAR/PXR | Liver (phenotyping) | Fan et al. (2007) |
| Unknown compounds in Echinacea purpurea | Echinacea purpurea, an herbal remedy | PXR | Liver (phenotyping) | Gorski et al. (2004), Penzak et al. (2010) | |
| Ethanol | Alcoholic drinks | Stabilization | Liver (phenotyping and expression), duodenum (phenotyping) | Liangpunsakul et al. (2005), Luceri et al. (2001), Niemela et al. (2000), Rahmioglu et al. (2011) | |
| Genistein | Legumes, soybeans, coffee | PXR | Liver (phenotyping, studied only with a pharmacologic dose) | Xiao et al. (2012) | |
| Ginkgolide A and B | Ginkgo biloba, an herbal remedy | PXR | Liver (phenotyping) | Markowitz et al. (2003), Robertson et al. (2008b) | |
| Hyperforin | St. John’s wort | PXR | Liver (phenotyping), duodenum | Durr et al. (2000); Piscitelli et al. (2000); Roby et al. (2000) | |
| Quercetin | Many vegetables, fruits, and berries (also one of the flavonoids in Ginkgo biloba) | PXR | Liver (phenotyping, studied only with a pharmacologic dose) | Duan et al. (2012) | |
| Tanshinone IIA and cryptotanshinone | Danshen (Salvia miltiorrhiza), an herbal remedy | CAR/PXR | Liver (phenotyping), duodenum (phenotyping) | Qiu et al. (2010), Qiu et al. (2013), Zhou et al. (2018) |
Table 4.
Substrates and inhibitors of CYP1A2 enzyme
| Reference substrates recommended by major regulatory agenciesa | ||||
|---|---|---|---|---|
| Drug | Reaction/assay measurement | Km (μM) in vitro (plasma conc)b | Specificity near Km | References |
| Phenacetin in vitro probe (withdrawn) | O-De-ethylation | 10–50 (na) | High | ☺ Zhou et al. (2009) |
| Ethoxyresorufin in vitro probe (non-drug) | O-De-ethylation | 0.11–0.23 (na) | Moderate (CYP1A1) | ☺ |
| Caffeine in vivo probe | N-Demethylation elimination rate (in vivo)c | 200–500 (20–50) | High | ☺ Thorn et al. (2012) |
| Theophylline in vivo probe | N-Demethylation elimination rate (in vivo) | 280–1230 (10–30) | High | ☺ Britz et al. (2019) |
| Tizanidine in vivo probe | Elimination rate (in vivo) | nk (0.6) | High | ☺ (Granfors et al. (2005), Karjalainen et al. (2008) |
| Substrates potentially affected by strong CYP1A2 inhibitorsc (Faber et al. 2005; Wang and Zhou 2009) | ||||
| Sensitive/moderate: agomelatine, alosteron, clozapine, duloxetine, flutamide, frovatriptan, guanabenz, leflunomide, lidocaine, melatonin, mexiletine, mirtazapine, olanzapine, pirfenidone, propranolol, ramelteon, ramosetron, riluzole, ropinirole, ropivacaine, tacrine, tasimelteon, thalidomide, triamterene, zolmitriptan, zolpidem, and zileuton | ||||
| Reference inhibitors recommended by major regulatory agenciesa | ||||
|---|---|---|---|---|
| Drug | Mode of inhibition | Ki/IC50 (μM) in vitro (plasma conc)b | CYP selectivity (other CYPs inhibited) | References |
| α-Naphthoflavone in vitro (non-drug) | Competitive | 0.01 (na) | Moderate (CYP1A1) | ☺ |
| Furafylline in vitro (withdrawn) | Mechanism-based | 0.6–0.7 (nk) | High | ☺ |
| Enoxacin in vivo | Competitive | 65–170 (3–12) | High | ☺ |
| Fluvoxamine in vivo | Competitive | 0.12–0.24 (0.2–0.7) | Moderate (minor 2B6, 2C9, 2C19, 2D6) | ☺ |
| Inhibitors of potential clinical significance | ||||
| Amiodarone (metabolites) | Mechanism-based | 0.46 (1.5–3) | Moderate (2D6, 3A4) | McDonald et al. (2015), Ohyama et al. (2000) |
| Ciprofloxacin | Competitive | 90–290 (7.5–12) | High | ☺ Granfors et al. (2004), Raaska and Neuvonen (2000) |
| Isoniazid | Competitive mechanism-based | 56 (36–73) | Low (2C19, 3A4, 2A6) | Wen et al. (2002) |
| Mexiletine | Competitive | 4.3–8.3 (3–11) | Moderate (1A1) | ☺ |
| Propafenone | Competitive | 21 (1–6) | Moderate (2D6, 3A4) | ☺ Dean (2012) |
| Thiabendazole | Mechanism-based | 1.4 (na) | nk | Bapiro et al. (2005), Coulet et al. (1998), Thelingwani et al. (2009) |
| Vemurafenib | Competitive | ~ 30 (100) | Moderate (2B6, 2C9, 3A4) | Zhang et al. (2017a, b) |
| Resveratrol (non-drug) | Competitive? | 500 (na) | poor (1A1, 3A4) | Chang et al. (2001), Chun et al. (1999) |
| Moderate/weak inhibitorsc: acyclovir, allopurinol, caffeine, cimetidine, daidzein, disulfiram, Echinacea, ethinylestradiol, famotidine, gestodene, norfloxacin, piperine, propafenone, propranolol, terbinafine, ticlopidine, verapamil, and zileuton | ||||
na not available, nk not known
☺For older references, see (Pelkonen et al. 2008). Newer inhibitors, since 2008, have been indicated in bold
aAppropriate guidance documents of EMA (2012), FDA (2020), and MHLW/PMDA (2018) recommending the listed reference compounds for in vitro and in vivo studies. The use of two structurally unrelated CYP3A4/5 substrates for evaluation of in vitro CYP3A4/5 inhibition is recommended
bKm or Ki/IC50 values were taken mostly from in vitro human microsomal incubations. Therapeutic (“control”) plasma concentrations were mainly taken from two compilations (Schulz et al. 2012, 2020) or the referenced publications listed
cThe list is compiled from various published reviews, databases, and guidelines and drug labels of major drug agencies (EMA, FDA, and MHLW/PMDA) as well as publicly available databases (Hoffmann et al. 2014; Preissner et al. 2010). Database address: http://bioinformatics.charite.de/transformer/
CYP substrates and inhibitors
General
Data on substrates and inhibitors of major xenobiotic-metabolizing CYP enzymes are collected in Tables 3, 4, 5, 6, 7, 8, 9, 10 and 11. It is obvious that due to the vast literature, this survey cannot include all the possible substrates and inhibitors for CYP enzymes, instead certain restrictions had to be applied. Obviously, ‘the clinical significance’ is one of the overriding criterium, although it is very difficult to define. In this review, ‘the clinical significance’ means that the first-hand assessment of the drug, mostly on the basis of information in the regulatory dossier, has resulted in the inclusion of the drug in the list (see above the section on tyrosine-kinase inhibitors). However, ‘the clinical significance’ is dependent on many determinants including in vitro studies, clinical trials with reference substrates and inhibitors (these studies may be available at the time of approval), published non-regulatory studies and clinical experiences, etc. In the end, we have to admit that a certain measure of personal experience has been applied in the current review. Predominantly, only currently used drugs are listed, but some well-established, although withdrawn drugs are provided as reference. Also a few well-studied examples of in vitro substances are included because of their use as reference substrates or inhibitors.
Table 3.
Substrates and inhibitors of CYP3A4/5 enzyme
| Reference substrates recommended by major regulatory agenciesa | ||||
|---|---|---|---|---|
| Drug | Reaction | Km (μM) in vitro (HLMs) (plasma conc)b | Specificity near Km | References |
| Midazolam in vitro, in vivo | 1′-Hydroxylation/elimination | 1–14 (0.8) | High | ☺ |
| Triazolam in vitro, in vivo | 4-Hydroxylation/elimination | 238–304 (0.06) | High | ☺ |
| Testosterone in vitro | 6β-Hydroxylation | 33–94 (na) | High | ☺ |
| Substrates potentially affected by strong CYP3A4 inhibitorsc | ||||
| Highly selective/sensitive: alfentanil, alprazolam, aprepitant, atorvastatin, avanafil, budesonide, buspirone, colchicine, conivaptan, cyclosporin A, darifenacin, darunavir, dasatinib, dihydroergotamine (and ergotamine), docetaxel, dronedarone, ebastine, eletriptan, eliglustat, eplerenone, everolimus, felodipine, fentanyl, flibanserin, guanfacine, ibrutinib, indinavir, lomitapide, lovastatin, lurasidone, maraviroc, midazolam, naloxegol, nifedipine, nisoldipine, pimozide, quetiapine, quinidine, rilpivirine, rivaroxaban, saquinavir, sildenafil, simeprevir, simvastatin, sirolimus, sonidegib, tacrolimus, tadalafil, ticagrelor, tipranavir, tolvaptan, triazolam, vardenafil, and vincristine | ||||
| Additional protein tyrosine-kinase inhibitors, see Table 1 for details | ||||
| Reference inhibitors recommended by major regulatory agenciesa | ||||
|---|---|---|---|---|
| Drug | Mode of inhibition | Ki/IC50 (μM) in in vitro (plasma conc)b | CYP selectivity and other CYPs inhibited | References |
| Ketoconazole in vitro, in vivo | Competitive | 0.0037–0.028 (2–6) | Moderate (2C, 1A2, 2D6) | ☺ |
| Itraconazole in vitro, in vivo | Competitive (metabolites) | 0.013–0.27 (0.6–2.8) | High | ☺ Yoshida et al. (2018) |
| Azamulin in vitro | Mechanism-based | 0.03–0.24 (na) | High | Parmentier et al. (2017), Stresser et al. (2004) |
| Fluconazole | Competitive | 5.4–13.1 (6–30) | Moderate (2C9, 2C19) | Niwa et al. (2005), Yoshida et al. (2018) |
| Troleandomycin in vitro | Mechanism-based | 0.26 | High | ☺ Yadav et al. (2018) |
| Verapamil | Mechanism-based | 2.3–2.9 (0.1–0.6) | High | ☺ |
| Ritonavir in vivo | Mechanism-based | 0.019–0.17 (7–15) | Moderate (2C9) | ☺ |
| Clarithromycin in vivo | Mechanism-based (comp) | 0.8 (5.5–10) (0.3–2.7) | High | ☺ |
| Erythromycin in vivo | Mechanism-based (comp) | 1.0 (16–19) (1–8) | High | Akiyoshi et al. (2013), Kanamitsu et al. (2000) |
| Inhibitors of potential clinical significance | ||||
| Voriconazole | Mechanism-based | 3.0 (4–17) | Poor (2B6, 2C9, 2C19) | Jeong et al. (2009a) |
| Posaconazole | Competitive | ? (< 0.1?) (1) | High | Groll et al. (2017), Krishna et al. (2009) |
| Indinavir | Competitive | 0.17–0.5 (> 0.16) | High | ☺ |
| Nelfinavir | Competitive | 1–4.8 (> 1.4) | Moderate (CYP2D6) | ☺ |
| Saquinavir | Mechanism-based | 0.65–2.99 (> 0.37) | High | ☺ |
| Diltiazem | Mechanism-based | 2.2–5.0 (0.1–0.6) | High | ☺ |
| Telithromycin | Mechanism-based (competitive) | 1.05 (3.65) (2.5) | High | Elsby et al. (2019) |
| Gestodene | Mechanism-based | 46 (0.02) | High | ☺ Palovaara et al. (2000) |
| Ceritinib | Mechanism-based | 0.16–0.2 (0.9–2.7) | Moderate (2C9) | Zhao et al. (2020) |
| Idelalisib | Mechanism-based (metabolite) | 5.1 (0.5–5) | High | Ramanathan et al. (2016) |
| Imatinib | Competitive? | 8 (1–4) | Moderate | O’Brien et al. (2003) |
| Lapatinib | Mechanism-based | 1.7 | High (3A5: 37.6 uM) | Chan et al. (2012), Teng et al. (2010) |
| Nilotinib | Competitive | 0.4–7 (2–3) | Moderate (2C8, 2C9, 2D6) | Tian et al. (2018) |
| Osimertinib | Mechanism-based competitive | 2.5–5.1 (1.5–3) | Moderate (2C8) | Pilla Reddy et al. (2018), Vishwanathan et al. (2019) |
| Stiripentol | Competitive | 80 (8–40) | Moderate (CYP1A2, 2D6) | Tran et al. (1997) |
| Dronedarone | Mechanism-based | 0.87 (0.15–0.3) | Moderate (2J2) | Hong et al. (2016) |
| Boceprevir | Mechanism-based | 6.1 (0.2–1.5) | High | Chu et al. (2013), Wilby et al. (2012) |
| Telaprevir | Mechanism-based | 0.19–0.36 (3–4.5) | High | Chapron et al. (2015) |
| Cobicistat | Mechanism-based | 0.032 (0.9) | Moderate | Hossain et al. (2017) |
| Netupitant | Competitive | 1.9–5.7 (0.3–1) | Moderate (2C9) | Giuliano et al. (2012) |
| Isavuconazole | Competitive | 0.62–1.93 (5.71) | Moderate (2C, 2D6) | Townsend et al. (2017), Yamazaki et al. (2017) |
| Grapefruit juice | Mechanism-based | Not applicable | Low? (multiple CYPs) | Bailey et al. (2013); Hanley et al. (2011) |
| Moderate inhibitorsc (regulatory documents): amprenavir, aprepitant, atazanavir, ciprofloxacin, crizotinib, darunavir/ritonavir, diltiazem, fosamprenavir, and gestodene | ||||
na not available, nk not known
☺For older references, see (Pelkonen et al. 2008). Newer inhibitors, since 2008, have been indicated in bold
aAppropriate guidance documents of EMA (2012), FDA (2020), and MHLW/PMDA (2018) recommending the listed reference compounds for in vitro and in vivo studies. The use of two structurally unrelated CYP3A4/5 substrates for evaluation of in vitro CYP3A4/5 inhibition is recommended
bKm or Ki/IC50 values were taken mostly from in vitro human microsomal incubations. Therapeutic (“control”) plasma concentrations were mainly taken from two compilations (Schulz et al. 2012, 2020) or the referenced publications listed
cThe list is compiled from various published reviews, databases, and guidelines and drug labels of major drug agencies (EMA, FDA, MHLW/PMDA) as well as publicly available databases (Hoffmann et al. 2014; Preissner et al. 2010). Database address: http://bioinformatics.charite.de/transformer/
Table 5.
Substrates and inhibitors of CYP2B6 enzyme
| Reference substrates recommended by major regulatory agenciesa | ||||
|---|---|---|---|---|
| Drug | Reaction/assay measurement | Km (μM) in vitro (plasma conc)b | Specificity near Km | References |
| Bupropion (in vitro, in vivo) | Hydroxylation | 89–130 (15–40) | High | ☺ |
| Efavirenz (in vitro, in vivo) | 8-Hydroxylation | 17–23 (3–10) | Moderate (CYP1A2, 3A4) | ☺ Manosuthi et al. (2013) |
| Substrates potentially affected by strong CYP2B6 inhibitorsc (Hedrich et al. 2016) | ||||
| Highly/moderately sensitive: artemether, artemisinin, cyclophosphamide, diazepam, Ifosfamide, ketamine, mephenytoin, mephobarbital, methadone, nicotine, pethidine (meperidine), propofol, piclamilast, selegiline, and temazepam | ||||
| Reference inhibitors recommended by major regulatory agenciesa | ||||
|---|---|---|---|---|
| Drug | Mode of inhibition | Ki/IC50 (μM) in vitro (plasma conc)b | CYP selectivity and other CYPs inhibited | References |
| Ticlopidine (in vitro, in vivo) | Mechanism-based | 0.2–0.8 (3–8) | Moderate (CYP1A2, 2C19, 2D6) | ☺ Palacharla et al. (2018) |
| ThioTEPA (in vitro) | Mechanism-based | 2.8–3.8 (3–20) | High | ☺ Bae et al. (2013) |
| Sertraline (in vivo) | Competitive | 3.2 (0.1–0.5) | Moderate | Hesse et al. (2000), Palacharla et al. (2018) |
| Phencyclidine (in vivo) | Mechanism-based | 2 (0.1–1) | Moderate | Jushchyshyn et al. (2006), Walsky and Obach (2007) |
| Inhibitors of potential clinical significance | ||||
| Canagliflozin | Competitive | 16 (0.6–3) | Poor (2E1, 3A4, 2C19, 2C9) | Yu et al. (2014) |
| Clopidogrel (pro-drug) | Mechanism-based | 1.1 (0.02) | Moderate (2C19, 2C9) | ☺ Backman et al. (2016), Wang et al. (2015) |
| 17-α-Ethynylestradiol | Mechanism-based | 0.8 (0.3 nM) | Moderate (1A2) | ☺ |
| Sonidegib | Competitive | 0.045 (0.3–1) | Moderate (CYP2C9) | Yu et al. (2017a, b) |
| Voriconazole | Competitive | 0.40 (5.7–11.5) | Poor (2C9, 2C19, 3A) | Jeong et al. (2009a, b) |
| Potential (moderate/weak) inhibitorsc | ||||
na not available, nk not known
☺For older references, see (Pelkonen et al. 2008). Newer inhibitors, since 2008, have been indicated in bold
aAppropriate guidance documents of EMA (2012), FDA (2020), and MHLW/PMDA (2018) recommending the listed reference compounds for in vitro and in vivo studies. The use of two structurally unrelated CYP3A4/5 substrates for evaluation of in vitro CYP3A4/5 inhibition is recommended
bKm or Ki/IC50 values were taken mostly from in vitro human microsomal incubations. Therapeutic (“control”) plasma concentrations were mainly taken from two compilations (Schulz et al. 2012, 2020) or the referenced publications listed
cThe list is compiled from various published reviews, databases, and guidelines and drug labels of major drug agencies (EMA, FDA, and MHLW/PMDA) as well as publicly available databases (Hoffmann et al. 2014; Preissner et al. 2010). Database address: http://bioinformatics.charite.de/transformer/
Table 6.
Substrates and inhibitors of CYP2C8 enzyme
| Reference substrates recommended by major regulatory agenciesa | ||||
|---|---|---|---|---|
| Drug | Reaction/assay measurement | Km (μM) in HLMs (plasma conc)b | Specificity near Km | References |
| Repaglinide (in vivo) | Oxidation | 24 (0.1–0.45) | Moderate (CYP3A4) | ☺ |
| Paclitaxel (in vitro) | 6α-Hydroxylation | 2.5–19 (0.3–0.8) | High | ☺ |
| Amodiaquine (in vitro) | N-De-ethylation | 1.9–3.4 (0.15) | High | ☺ Bohnert et al. (2016) |
| Substrates potentially affected by strong CYP2C8 inhibitors | ||||
| Highly selective: pioglitazone, rosiglitazone, and tazarotenic acid | ||||
| Moderately selective (other CYPs in parentheses): chloroquine (CYP3A4) and dasabuvir (3A4) | ||||
| Poorly selective (other CYPs in parentheses): amiodarone (CYP1A2, 2C19, 3A4) | ||||
| Reference inhibitors recommended by major regulatory agencies | ||||
|---|---|---|---|---|
| Drug | Mode of inhibition | Ki/IC50 (μM) in vitro (plasma conc)b | CYP selectivity and other CYPs inhibited | References |
| Montelukast in vivo | Competitive | 0.009–0.15 (0.05–0.5) | Moderate (CYP2C9, 3A4) | ☺ Bohnert et al. (2016) |
| Quercetin in vivo (non-drug) | Competitive | 1.1–1.6 (0.4) | Poor (CYP1A2, 2E1, 3A4) | ☺ |
| Phenelzine in vitro, in vivo | Mechanism-based | 1.2 (0.1–1.5) | Kahma et al. (2019) | |
| Clopidogrel in vitro, in vivo | Mechanism-based | na (0.02) | Moderate (CYP2C19, 2C9) | ☺ Backman et al. (2016), Kahma et al. (2019), Tornio et al. (2014) |
| Gemfibrozil (glucuronide) in vitro, in vivo | Mechanism-based | 52–75 (100) | High | ☺ Kahma et al. (2019) |
| Inhibitors of potential clinical significance | ||||
| Dabrafenib | Competitive | 8.2 | Poor (2C9, 2C19, 3A4) | Lawrence et al. (2014) |
| Deferasirox | na | na (50) | Moderate (1A2. 3A4) | Pakkir Maideen et al. (2018), Skerjanec et al. (2010), Tanaka (2014) |
| Trimethoprim | Competitive | 29–32 (4–9) | High | ☺ |
| Teriflunomide | na | na (100) | Moderate (1A2) | Cada et al. (2013) |
| Vorapaxar | Competitive? | 0.86 (0.15) | Moderate (2C9) | Yu et al. (2016a, b) |
| Belinostat | na | 100 (80) | Moderate (2C9) | Monograph |
| Idelalisib | Competitive? | 13 (4) | Moderate (3A4, 2C9) | Yu et al. (2016a, b) |
| Potential and/or putative inhibitors:c (Polasek et al. 2004) amiodarone, verapamil, nortriptyline, fluoxetine, and isoniazid. tasimelteon | ||||
na not available, nk not known
☺For older references, see (Pelkonen et al. 2008). Newer inhibitors, since 2008, have been indicated in bold
aAppropriate guidance documents of EMA (2012), FDA (2020), and MHLW/PMDA (2018) recommending the listed reference compounds for in vitro and in vivo studies. The use of two structurally unrelated CYP3A4/5 substrates for evaluation of in vitro CYP3A4/5 inhibition is recommended
bKm or Ki/IC50 values were taken mostly from in vitro human microsomal incubations. Therapeutic (“control”) plasma concentrations were mainly taken from two compilations (Schulz et al. 2012, Schulz et al. 2020) or the referenced publications listed
cThe list is compiled from various published reviews, databases, and guidelines and drug labels of major drug agencies (EMA, FDA, and MHLW/PMDA) as well as publicly available databases (Hoffmann et al. 2014; Preissner et al. 2010). Database address: http://bioinformatics.charite.de/transformer/
Table 7.
Substrates and inhibitors of CYP2C9 enzyme
| Reference substrates recommended by major regulatory agenciesa | ||||
|---|---|---|---|---|
| Drug | Reaction | Km (μM) in HLMs (plasma conc)b | Specificity near Km | References |
| S-warfarin in vitro, in vivo | 7-Hydroxylation | 3–4 (3–23) | High | ☺ |
| Diclofenac in vitro | 4-Hydroxylation | 2–22 (2–10) | High | ☺ |
| Tolbutamide in vivo | Hydroxylation | 60–580 (150–340) | High | ☺ |
| Substrates potentially affected by strong CYP2C9 inhibitors:c (Daly et al. 2017; Van Booven et al. 2010) bosentan, celecoxib, cyclophosphamide, flurbiprofen, fluvastatin, glibenclamide, glimepiride, glipizide, ibuprofen, indomethacin, irbesartan, lornoxicam, losartan, mefenamic acid, meloxicam, naproxen, nateglinide, phenytoin, tamoxiphen, and tenoxicam | ||||
| Reference inhibitors recommended by major regulatory agenciesa | ||||
|---|---|---|---|---|
| Drug | Mode of inhibition | Ki (μM) in HLMs (plasma conc)2 | CYP selectivity and other CYPs inhibited | References |
| Sulphaphenazole in vitro | Competitive | 0.3 (300–500) | High | ☺ |
| Tienilic acid in vitro (withdrawn) | Mechanism-based | 5 (150) | na | Hutzler et al. (2009) |
| Fluconazole in vivo | Mixed type | 7–8 (6–30) | Poor (2C19, 3A4) | Back et al. (1988), Kunze et al. (1996) |
| Inhibitors of potential clinical significance | ||||
| Amiodarone | Non-competitive | 95 (0.8–4) | Poor (2D6, 3A4) | Heimark et al. (1992), Ohyama et al. (2000) |
| Ceritinib | Mechanism-based | 0.24 (0.9–2.7) | Moderate (3A4) | Zhao et al. (2020) |
| Etravirine | Competitive? | na (0.7–5) | Moderate (2C19) | Havens et al. (2020) |
| Sonidegib | Competitive | 1.7 (0.3–1) | Moderate (3A4) | Yu et al. (2016a, b) |
| Stiripentol | Competitive | na (4–40) | Poor (1A2, 2D6, 3A4) | Tran et al. (1997) |
| Vemurafenib | Competitive | 5.9 (100) | Poor (1A2, 2B6, 3A4) | (RW.ERROR—unable to find reference:doc:5ef341eae4b0f33707a95cec) |
| Moderate/weak inhibitorsc: capecitabine, cotrimoxazole, fluvastatin, fluvoxamine, metronidazole, miconazole, oxandrolone, sulfinpyrazone, voriconazole, and zafirlukast (Wu et al. 2013) | ||||
na not available, nk not known
☺For older references, see (Pelkonen et al. 2008). Newer inhibitors, since 2008, have been indicated in bold
aAppropriate guidance documents of EMA (2012), FDA (2020), and MHLW/PMDA (2018) recommending the listed reference compounds for in vitro and in vivo studies. The use of two structurally unrelated CYP3A4/5 substrates for evaluation of in vitro CYP3A4/5 inhibition is recommended
bKm or Ki/IC50 values were taken mostly from in vitro human microsomal incubations. Therapeutic (“control”) plasma concentrations, either range or maximal, were mainly taken from two compilations (Schulz et al. 2012, 2020) or the referenced publications listed
cThe list is compiled from various published reviews, databases, and guidelines and drug labels of major drug agencies (EMA, FDA, and MHLW/PDMA) as well as publicly available databases (Hoffmann et al. 2014; Preissner et al. 2010). Database address: http://bioinformatics.charite.de/transformer/
Table 8.
Substrates and inhibitors of CYP2C19 enzyme
| Reference substrates recommended by major regulatory agenciesa | ||||
|---|---|---|---|---|
| Drug | Reaction | Km (μM) in HLMs (plasma conc)c | Specificity near Km | References |
| S-Mephenytoin (in vitro) | 4′-Hydroxylation | 23–169 (0.4–2) | High | ☺ |
| Omeprazole (in vivo) | 5-Hydroxylation elimination | 6–10 (0.2–10) | High | ☺ |
| Lanzoprazole (in vivo) | 5-Hydroxylation elimination | 15–17 (0.1–1) | Moderate (3A4) | ☺ |
| Substrates potentially affected by strong CYP2C19 inhibitorsc | ||||
| Citalopram (2D6, 3A4), clobazam, clomipramine, diazepam (3A4), lansoprazole (3A4), pantoprazole (3A4), phenytoin, proguanil (3A4), propranolol, and rabeprazole (CYP3A4) | ||||
| Reference inhibitors recommended by major regulatory agenciesa | ||||
|---|---|---|---|---|
| Drug | Mode of inhibition | Ki/IC50 (μM) in vitro (plasma conc)b | CYP selectivity and other CYPs inhibited | References |
| -(−)-N-3-Benzyl-phenobarbital in vitro (non-drug) | Competitive | 0.079–0.12 (na) | “Not specific” | Cai et al. (2004), Suzuki et al. (2002) |
| S-(+)-N-3-Benzyl-nirvanol in vitro (non-drug) | Competitive | 0.2 (na) | “Not specific” | Suzuki et al. (2002) |
| Nootkatone in vitro (non-drug) | nk | 0.5 (nk) | Poor (CYP2A6) | Tassaneeyakul et al. (2000) |
| Loratadine | Competitive | 0.76 (0.05) | Poor (2D6, 3°4, 2E1) | Barecki et al. (2001), Ramanathan et al. (2018) |
| Ticlopidine | Mechanism-based | 1.2 (3–8) | Poor (CYP2B6, 1°2, 2D6) | Ha-Duong et al. (2001), Ko et al. (2000), Turpeinen et al. (2006) |
| Inhibitors of potential clinical significance | ||||
| Omeprazole | Competitive | 2–3 (0.2–10) | Moderate (2C9, 3A4) | Chiba et al. (1993), Funck-Brentano et al. (1997) |
| Fluvoxamine | Competitive | 0.29 (0.13–0.53) | Moderate (1A2) | Iga (2016), Kong et al. (2014), Yasui-Furukori et al. (2004) |
| Modafinil | competitive | 39 (6–15) | High | Robertson et al. (2000), Rowland et al. (2018) |
|
Moderate/weak inhibitorsc: Wu et al. (2013) Carbamazepine, cimetidine, esomeprazole, etravirine, felbamate, fluconazole, fluoxetine, ketoconazole, moclobemide, and voriconazole | ||||
na not available, nk not known
☺For older references, see (Pelkonen et al. 2008). Newer inhibitors, since 2008, have been indicated in bold
aAppropriate guidance documents of EMA (2012), FDA (2020), and MHLW/PMDA (2018) recommending the listed reference compounds for in vitro and in vivo studies. The use of two structurally unrelated CYP3A4/5 substrates for evaluation of in vitro CYP3A4/5 inhibition is recommended
bKm or Ki/IC50 values were taken mostly from in vitro human microsomal incubations. Therapeutic (“control”) plasma concentrations, either range or maximal, were mainly taken from two compilations (Schulz et al. 2012, 2020) or the referenced publications listed
cThe list is compiled from various published reviews, databases, and guidelines and drug labels of major drug agencies (EMA, FDA, MHLW/PDMA) as well as publicly available databases(Hoffmann et al. 2014; Preissner et al. 2010). Database address: http://bioinformatics.charite.de/transformer/
Table 9.
Substrates and inhibitors of CYP2D6 enzyme
| Reference substrates recommended by major regulatory agenciesa | ||||
|---|---|---|---|---|
| Drug | Reaction | Km (μM) in vitro (plasma conc)b | Specificity near Km | References |
| Bufuralol (withdrawn) in vitro | 1′-Hydroxylation | 3–30 (2) | High | ☺ |
| Dextromethorphan in vitro, in vivo | O-Demethylation | 2.8–22 (0.5) | High | ☺ |
| Metoprolol in vivo | Elimination | 7.4 (1.85) | High | Dean (2011), Berger et al. (2018) |
| Desipramine in vivo | 2-Hydroxylation | 10–15 (2.0) | High | ☺ |
| Nebivolol in vivo | Elimination | 1.8 (0.05) | High | Hu et al. (2016), Lefebvre et al. (2007) |
| Substrates potentially affected by strong CYP2D6 inhibitorsc | ||||
| Highly sensitive: atomoxetine, codeine, nortriptyline, perphenazine, tolterodine, and R-venlafaxine | ||||
| Moderately sensitive (other CYPs in parentheses): eliglustat (CYP3A4), encainide, imipramine, propafenone (CYP3A4), propranolol, thioridazine (CYP2C19, CYP3A4), tramadol (CYP3A4), trimipramine, and S-venlafaxine | ||||
| Reference inhibitors recommended by major regulatory agenciesa | ||||
|---|---|---|---|---|
| Drug | Mode of inhibition | Ki (μM) in vitro (HLMs) (plasma conc)b | CYP selectivity and other CYPs inhibited | References |
| Quinidine in vitro, in vivo | Competitive | 0.018–0.06 (6–15) | High | ☺ |
| Paroxetine in vitro, in vivo | Competitive | 0.15 (0.01–0.2) | Moderate (2C9, 2C19) | ☺ |
| Fluoxetine in vivo | Competitive | 0.6 (0.5–1.6) | Moderate (2C9, 2C19) | ☺ |
| Mirabegron in vivo | Mechanism-based | 4.3 (0.01–0.2) | Moderate (CYP3A4) | Krauwinkel et al. (2014), Takusagawa et al. (2012) |
| Inhibitors of potential clinical significance | ||||
| Bupropion | Competitive | 21 (15–40) | High | Reese et al. (2008), Sager et al. (2017) |
| Sertraline | Competitive | 0.7 (0.02–0.5) | Moderate (2C9, 2C19) | ☺ |
| Terbinafine | Competitive | 0.028–0.044 (0.03–0.1) | High | ☺ |
| Stiripentol | Competitive | (4–40) | Poor | Tran et al. (1997) |
| Rolapitant | Competitive | >7 (1) | High | Wang et al. (2017), Wang et al. (2018) |
| Potential inhibitors (mostly weak and/or putative)c: aprepitant, alogliptin, cobicistat, crizotinib, eliglustat, and panobinostat | ||||
na not available, nk not known
☺For older references, see (Pelkonen et al. 2008). Newer inhibitors, since 2008, have been indicated in bold
aAppropriate guidance documents of EMA/EU (2012), FDA/USA (2020), and MHLW/PMDA (2018) recommending the listed reference compounds for in vitro and in vivo studies. The use of two structurally unrelated CYP3A4/5 substrates for evaluation of in vitro CYP3A4/5 inhibition is recommended
bKm or Ki/IC50 values were taken mostly from in vitro human microsomal incubations. Therapeutic (“control”) plasma concentrations, either range or maximal, were mainly taken from two compilations (Schulz et al. 2012, 2020) or the referenced publications listed
cThe list is compiled from various published reviews, databases, and guidelines and drug labels of major drug agencies (EMA, FDA, MHLW/PDMA) as well as publicly available databases (Hoffmann et al. 2014; Preissner et al. 2010). Database address: http://bioinformatics.charite.de/transformer/
Table 10.
Substrates and inhibitors of CYP2A6 enzyme
| Reference substrates (no recommendations by major regulatory agencies) | ||||
|---|---|---|---|---|
| Drug | Reaction/assay measurement | Km (μM) in in vitro HLMs (plasma conc)a | Specificity near Km | References |
| Nicotine in vitro (in vivo)c | N-1′-Oxidation (elimination) | 65–95 (0.03–0.2) | High | ☺ |
| Coumarin in vitro (in vivo)c | 7-Hydroxylation | 0.2–2.4 (max. 5) | High | ☺ |
|
Substrates potentially affected by strong CYP2A6 inhibitorsb (see (Tanner and Tyndale 2017) artemisinin, artesunate, caffeine, cotinine, letrozole, efavirenz, pilocarpine, tegafur, tyrosol, and valproic acid | ||||
| Reference inhibitors (no recommendations by major regulatory agencies) | ||||
|---|---|---|---|---|
| Drug | Mode of inhibition | Ki (μM) in HLMs (plasma conc)a | CYP selectivity and other CYPs inhibited | References |
| Tranylcypromine | Competitive | 0.08–0.2 (0.4) | Moderate (2E1) | ☺ |
| Methoxsalen | Mechanism-based | 0.2–0.8 (0.12–1) | Moderate (1A2) | ☺ |
| Inhibitors | ||||
| Letrozole | Competitive | 4.6 (0.5) | Moderate (2C19) | Jeong et al. (2009a, b) |
| Pilocarpine | Competitive | 1 (0.05) | High? | ☺ |
| Trans-cinnamic aldehyde (non-drug) | Mechanism-based | 6.1 (nk) | High | Chan et al. (2016) |
| Tryptamine (non-drug) | Competitive | 0.2 (nk) | Poor (CYP1A2) | ☺ |
na not available, nk not known
☺For older references, see (Pelkonen et al. 2008). Newer inhibitors, since 2008, have been indicated in bold
aKm or Ki/IC50 values were taken mostly from in vitro human microsomal incubations. Therapeutic (“control”) plasma concentrations, either range or maximal, were mainly taken from two compilations (Schulz et al. 2012, 2020) or the referenced publications listed
bThe list is compiled from various published reviews, databases, and guidelines and drug labels of major drug agencies (EMA, FDA, and MHLW/PMDA) as well as publicly available databases (Hoffmann et al. 2014; Preissner et al. 2010). Database address: http://bioinformatics.charite.de/transformer/
cNicotine and coumarin are used in various commodities, and could be used as probes also in vivo in small doses
Table 11.
Substrates and inhibitors of CYP2E1 enzyme
| Reference substrates (no recommendations by major regulatory agencies) | ||||
|---|---|---|---|---|
| Drug | Reaction | Km (μM) in vitro (HLMs) (plasma conc)a | Specificity near Km | References |
| Chlorzoxazonea,b | 6-Hydroxylation | 39–157 (170) | High | ☺ Ernstgård et al. (2004) |
| p-Nitrophenol (non-drug) | 3-Hydroxylation (nk) | 24–30 | High | ☺ Collom et al. (2008) |
| Aniline (non-drug) | 4-Hydroxylation | 6–24 | High | ☺ |
| Lauric acid (non-drug) | 11-Hydroxylation | 130 | Moderate (CYP4A) | ☺ |
| Substrates potentially affected by strong CYP2E1 inhibitorsb acetaminophen (paracetamol), theophylline, enflurane, and halothane | ||||
| Reference inhibitors (no recommendations by major regulatory agencies) | ||||
|---|---|---|---|---|
| Drug | Mode of inhibition | Ki/IC50 (μM) in vitro (plasma conc)b | CYP selectivity and other CYPs inhibited | References |
| 4-Methylpyrazole | Competitive | 2.0 (17–250) | High | Collom et al. (2008) |
| Diethyldithiocarbamate (DDC, non-drug) | Mechanism-based | 5.3–34 (na) | Poor (1A2, 2A6, 2B6, 2C8, 3A4) | ☺ Pratt-Hyatt et al. (2010) |
| Pyridine (non-drug) | Not known | 0.4, 11.8 (na) | High | ☺ Jones et al. (2011) |
| Disulfiram (in vivo) | Mechanism-based | Via DDC | Moderate (CYP2A6) | ☺ |
| Clomethiazole | Mechanism-based | 1.0 (10) | Moderate (2A6) | ☺ Stresser et al. (2016) |
| Diallyl sulfide (non-drug) | COMPETITIVE? | 6.3–17.3 (na) | High? | ☺ Rao et al. (2015) |
na not available, nk not known
☺For older references, see (Pelkonen et al. 2008)
a Km or Ki/IC50 values were taken mostly from in vitro human microsomal incubations. Therapeutic (“control”) plasma concentrations, either range or maximal, were mainly taken from two compilations (Schulz et al. 2012, 2020) or the referenced publications listed
bThe list is compiled from various published reviews, databases, and guidelines and drug labels of major drug agencies (EMA, FDA, MHLW/PMDA) as well as publicly available databases (Hoffmann et al. 2014; Preissner et al. 2010). Database address: http://bioinformatics.charite.de/transformer/
Reference substrates and inhibitors
Reference substrates and inhibitors recommended by major regulatory agencies, FDA, EMA, and MHLW/PMDA, have been collected in the upper part of Tables 3, 4, 5, 6, 7, 8, 9, 10 and 11. The basic requirement is that the compound is metabolized totally or preferably by a single CYP enzyme, and this has been demonstrated in vitro and in vivo. In in vitro assay, the formation of the CYP-associated metabolite is followed, but in in vivo studies, often, the elimination of the parent is measured due to, e.g., further metabolism of a CYP-associated metabolite. Naturally, in the human in vivo studies, approved drugs have to be used, but the lists contain also a few substances which are either withdrawn drugs or experimental substances (e.g., azamulin). These are used only in in vitro tests to investigate basic in vitro interactions in connection with early drug development or in mechanistic studies later on.
Sensitive substrates
In addition to reference substrates and inhibitors, appropriate lists of substrates and inhibitors of definitive clinical potential are compiled. Of potential substrates, only the so-called “strongly and/or moderately sensitive” substrates have been listed as extractions from reviews of individual CYP enzymes. Usually, sensitive substrates are metabolized almost completely or to a significant extent (> 25%) by the CYP enzyme concerned, so that the inhibition by a specific inhibitor will lead to a significant increase in the exposure to a substrate. However, there are a number of substrates which are actually metabolically activated by an enzyme and, consequently, the inhibition of metabolism leads to a pharmacodynamically reverse outcome and this is an important point to remember when assessing potential consequences of an interaction. However, perhaps, a more common situation is where pharmacologically active metabolites contribute to the action of the parent drug and the final outcome of the interaction may be more difficult to define.
Clinically significant inhibitors
Among inhibitors, the listed substances contain mostly “strong” or at least “moderate” inhibitors for a given CYP enzyme. This implies a relatively strong affinity to an enzyme at concentrations achieved in clinical situations. For this reason, an inhibition constant or a corresponding measure (IC50, Ki) and actual therapeutic concentration (if known) have been given in tables. Furthermore, mechanism of inhibition, most commonly competitive or mechanism-based inhibition, is of importance for the extent and length of inhibition.
The extent of inhibition is also heavily dependent on characteristics of a victim drug, its affinity to an enzyme, and a fraction of a victim metabolized by an enzyme. However, clinical situations could be much more complex. Consequently, quantitative measures of inhibitory potency are only guiding by nature, but may still suggest at least a significant possibility of inhibitory interaction in clinical drug use.
It should be kept in mind that the inhibition mechanisms may be very complex and may need extensive in-depth experiments to uncover the details of inhibition and the consequent in vitro and in vivo outcomes (Asaumi et al. 2018; Korzekwa et al. 2014; Lutz and Isoherranen 2012; Roberts et al. 2008; Varma et al. 2015). We have used a dichotomous expression of competitive vs mechanism-based inhibition, although the outcome of inhibition may be modified by more complex mechanisms.
It should also be stressed that the concentration of a drug interacting with the enzyme may be different from the plasma concentration, which is usually readily available from clinical trials and later monitoring activities. It has been suggested that the use of unbound cytosolic concentrations—as a proxy for total/unbound plasma concentrations—would improve the prediction of in vivo DDIs (Filppula et al. 2019). For practical reasons, we have listed the total plasma concentrations, not unbound concentrations, because there exists some uncertainty about which one is in better correlation with the drug concentration at the enzyme site. Also, it is not known whether there is a direct relation between unbound concentrations in plasma and cell cytosol. It has to be recognized that drugs bind to intracellular structures, mainly proteins and lipids, and the ensuing unbound concentration could be different from the unbound plasma concentration. A reliable method to measure the drug concentration at the effector site of an enzyme is needed.
Because the available literature on CYP inhibition is enormous, we have made use of our previous review (Pelkonen et al. 2008) as a collective reference to the older literature (Tables 3, 4, 5, 6, 7, 8, 9, 10, 11). In addition, we have referred to more recent papers if they have added significant new information. For many newer substances, publicly available regulatory dossiers have been a primary source of information, although they do not necessarily provide strictly quantitative information about DDIs.
Substrates and inhibitors of individual CYPs
CYP3A4/CYP3A5
Table 3 presents a collection of compounds participating as substrates and/or inhibitors in clinically relevant CYP3A4-associated DDIs, which is by far the most important area of CYP-based interactions. The table lists also > 10 inhibitors (in bold), which have come to the market since our previous review in 2008 (Pelkonen et al. 2008).
On the basis of analyses of Yu et al. (2014, 2016a, b, 2017a, b, 2018, 2019) on FDA-approved drugs (close to 150 between 2013 and 2017), roughly 65% were substrates, 30% inhibitors and about 5% inducers of CYP3A. This is not to say that a similar portion should cause DDI consequences of clinical significance, because the establishment of clinical significance would require at least some in vivo trials and/or observations. Currently, the use of reference perpetrators (e.g., ketoconazole and rifampicin) or substrates (e.g., midazolam) is practically mandatory to aid the assessment of clinical significance.
Usually, it is not possible to indicate what would be a contribution of CYP3A5 for the DDI effect. However, if need be there are in vitro tools to study the CYP3A5 contribution into the metabolism or the effect of a studied drug (Guo et al. 2020; Lolodi et al. 2017). The most comprehensive literature on the role of CYP3A5 is available for tacrolimus, see (Birdwell et al. 2015; Chen and Prasad 2018).
CYP1A2
The list of substrates potentially affected by CYP1A2 inhibitors (Table 4) contains at least 13 “new” drugs [compared with the previous review in 2008 (Pelkonen et al. 2008)], whereas only one inhibitor of potential clinical significance, vemurafenib (see also Table 1), has appeared since 2008. Resveratrol has been added to the table as an example of an ingredient in a large number of consumable products, including red wine. However, it seems to be a moderate CYP1A2 inhibitor at the best.
CYP2B6
There are only three “new” drugs added into the list of inhibitors, canagliflozin, sonidegib, and voriconazole, and the first two are probably only moderate-to-weak inhibitors. The list of substrates potentially affected by strong CYP2B6 inhibitors contains almost exclusively “old” drugs.
CYP2C8
In addition of recommended substrates and inhibitors, Table 6 lists 6 ‘new’ inhibitors of CYP2C8. However, in the immediate analysis, some recently registered drugs, which were shown to be CYP2C8 inhibitors in in vitro studies, were difficult to classify. For example, according to the regulatory dossier studies, tasimelteon was shown to be a weak in vitro inhibitor of CYP2C8 (IC50 > 100 µM), whereas vorapaxar was a relatively potent in vitro inhibitor (IC50 0.86 µM), but still both did not affect CYP2C8-associated rosiglitazone elimination in vivo [drug monographs, (Yu et al. 2016a, b)]. Consequently, tasimelteon is mentioned only in the group of putative inhibitors, waiting for additional in vivo investigations to classify more convincingly, whereas vorapaxar is listed in the category of inhibitors of potential clinical significance due to its low IC50 value as compared with the in vivo plasma concentration.
CYP2C9
The list of victim drugs of CYP2C9 (Table 7) is relatively long, altogether 20 substances. It reflects the importance of CYP2C9 in metabolizing clinically widely used drugs, practically all of which are “old” drugs and many of them used for 20–30 years. There are five “new” drugs as CYP2C9 inhibitors of potential clinical significance, three of them kinase inhibitors (ceritinib, sonidegib, and vemurafenib). The only “old” inhibitor is the widely used antiarrhythmic amiodarone, which is used in research projects as an example of a drug with a very long half-life, complex kinetics and multiple potential interactions (McDonald et al. 2015).
CYP2C19
Since the previous review (Pelkonen et al. 2008), only one “new” drug (modafinil) has been added in the list of inhibitors of potential clinical significance. Reference inhibitors recommended by major regulatory agencies are not specific for CYP2C19-mediated metabolism; however, they can be used together with other information such as data obtained from experiments done with recombinant enzyme systems.
CYP2D6
The classic polymorphic CYP enzyme was discovered decades ago, mainly based on debrisoquine hydroxylation studies. Debrisoquine, a classic probe drug [see (Pelkonen et al. 2008)], was withdrawn from clinical use a long time ago, and consequently from the lists of reference probe drugs. The current list of recommended reference inhibitors includes the only “new” drug, mirabegron (Table 9). In fact, there are not many “new” drugs listed in Table 9. One of the reasons may be the well-known problems related to CYP2D6 pharmacogenetics and drug–drug interactions, and likelihood of “killing” of molecules displaying CYP2D6 metabolism and/or inhibitory potency early in the drug development process.
CYP2A6
Since our review in 2008 (Pelkonen et al. 2008), only one drug (letrozole) has been added to the list of substrates or inhibitors (Table 10). Letrozole was added to the list of CYP2A6 inhibitors on the basis of an in vitro study (Jeong et al. 2009b); no clinical studies have been undertaken. Only 5 out of 102 FDA-approved drugs between 2013 and 2016 were at least partial substrates and/or inhibitors of CYP2A6 principally on the basis of in vitro experiments and none of them were considered as ‘clinically significant’ even potentially (Yu et al. 2018). Our own view over the years since 2007 (see the accompanying article, Pelkonen et al., this volume) is similar: although CYP2A6 was occasionally mentioned in drug labels as a target of in vitro inhibition (no quantitative information provided), no in vitro observations were translated into potentially clinical significance.
CYP2E1 is another enzyme that has been only rarely observed to associate with clinically significant interactions (Table 11). According to our own experiences (Pelkonen et al., this volume) and those of Yu et al. (2014, 2016a, b, 2017a, b, 2018, 2019), CYP2E1 has been mentioned only rarely in drug monographs and there have been no ‘clinically significant’ interactions since 2008. This is also reflected in a lack of officially recommended reference compounds to study metabolism or inhibition associated with CYP2E1. However, it is known that CYP2E1 is of importance in the metabolism of several small-molecular xenobiotics and its role in biochemical consequences of heavy alcohol consumption should be duly noted.
Mechanisms of CYP induction
Xenobiotic-sensing receptors as mediators of CYP induction
The induction of drug metabolism has been known since 1950s and it was early on understood to have important consequences for the action of drugs. However, the mechanistic basis behind induction remained enigmatic for decades. Discovery of the xenobiotic-sensing receptors, aryl hydrocarbon receptor (AHR) at 1970s and pregnane X receptor (PXR) and constitutive androstane receptor (CAR) at 1990s, as the molecular mediators of the CYP induction was a major step forward in understanding the mechanisms of induction (Baes et al. 1994; Honkakoski et al. 1998; Kliewer et al. 1998; Poland et al. 1976).
The xenobiotic-sensing receptors are ligand-activated transcription factors belonging structurally either to the nuclear receptors or the basic-helix–loop–helix Per-Arnt-Sim (bHLH-PAS) proteins. Today, activation of these receptors and subsequent CYP induction can be studied with a number of in silico, in vitro, and cell-based methods enabling relatively good prediction of in vivo induction (Bernasconi et al. 2019; Kato 2020; Pelkonen et al. 2008). However, not all the compounds found to be activators in cell or other in vitro assays are actual in vivo activators because of pharmacokinetic or other factors. It has also become clear that AHR, PXR, and CAR not only control the elimination of xenobiotics, but regulate also many other endogenous functions and signaling pathways and their activation may be involved in many chronic diseases such as metabolic diseases and cancer (Hakkola et al. 2018).
PXR and CAR, the xenobiotic-sensing nuclear receptors
PXR, systematic name NR1I2, and CAR, systematic name NR1I3, belong to the same subfamily of nuclear receptors. Their tissue expression profile is quite limited, and both are predominantly expressed in the liver, PXR also in the intestine (Wang et al. 2012). Low levels can be found in some other tissues. PXR and CAR ligand-binding sites have evolved to accommodate various foreign chemicals, and therefore, they play a major role in sensing of the chemical environment. The basis for their ligand promiscuity is large and flexible ligand-binding pockets that can accommodate a wide range of ligands with diverse structural and physicochemical properties (Buchman et al. 2018).
Especially, the PXR ligand-binding pocket is very large (1200–1600 Å3) and adaptable allowing a great number of compounds with different structures to bind and activate PXR, thus making PXR an ideal sensor for chemical environment (Buchman et al. 2018). The CAR ligand-binding pocket is smaller (~ 600 Å3) and less flexible than that of PXR and, therefore, apparently can accommodate a smaller number of chemicals (Buchman et al. 2018). However, also CAR can be activated with many different compounds. From the point of view of clinically important drug–drug interactions, PXR activation probably represents the most important induction mechanism. However, PXR and CAR also share many important pharmaceuticals as ligands.
While the DNA-binding domains of PXR and CAR are quite conserved across species, the ligand-binding domains differ significantly. Consequently, there are important species differences in the ligand preferences of these xenobiotic-sensing receptors hindering translation of in vivo results from the experimental animals to the humans (Blumberg et al. 1998; Lehmann et al. 1998). A classic example is rifampicin that induces efficiently the human PXR but poorly the mouse counterpart. Vice versa, PCN (pregnenolone-16α-carbonitrile) prefers the mouse PXR over the human PXR. Similarly, TCPOBOP (1,4-bis-[2-(3,5-dichloropyridyloxy)]benzene, 3,3′,5,5′-tetrachloro-1,4-bis(pyridyloxy)benzene) activates the mouse CAR, but not the human CAR, while CITCO (6-(4-Chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde-O-(3,4-dichlorobenzyl)oxime) is an agonist for the human CAR with little affinity to the mouse CAR (Chai et al. 2016). To overcome the problem of species differences in ligand preference, PXR and CAR-humanized mouse models have been developed (Scheer et al. 2008).
Aptly named as constitutive androstane receptor (or less frequently constitutively active receptor), CAR displays ligand-independent, constitutive transcriptional activity (Chai et al. 2016; Kobayashi et al. 2015). This has been especially evident in experiments utilizing exogenous expression of CAR in hepatic cell lines. In primary hepatocytes or in the liver in vivo, the constitutive activity may be limited by mainly cytoplasmic localization of the unliganded receptor as part of a multiprotein complex. Upon ligand binding, CAR dissociates from the chaperone proteins allowing translocation to nucleus. In addition to classical ligand binding, CAR may be activated indirectly. Phenobarbital is the prime example of an indirect CAR activator (Kobayashi et al. 2015). The mechanism of CAR activation by phenobarbital is complex and involves repression of epidermal growth factor (EGF) receptor (EGFR) signaling through the competitive inhibition of EGF–EGFR interaction. Subsequently, phosphorylation of receptor for activated C kinase 1 (RACK1) is reduced allowing RACK1 to interact with CAR and protein phosphatase 2A. This ternary interaction then enables CAR dephosphorylation and, consequently, translocation to nucleus (Kobayashi et al. 2015).
In response to ligand binding, both PXR and CAR transfer from the cytosol to the nucleus and form heterodimers with another nuclear receptor, retinoid X receptor (RXR). The heterodimer is then able to bind to the DNA elements including both direct and everted repeats of the sequence AGGTCA and its variants. The agonist-bound nuclear receptor activates transcription through coactivator recruitment modifying chromatin structure and engaging transcription initiation complex. In addition to this classical nuclear receptor function, PXR and CAR form also protein–protein interactions broadening the cellular functions under the control of these nuclear receptors (Oladimeji et al. 2016; Pavek 2016). This mode of action may be especially important for the gene repression by the receptors. Furthermore, the PXR and CAR function may be fine-tuned by phosphorylation status and other posttranslational modifications (Cui et al. 2016; Smutny et al. 2013; Staudinger et al. 2011).
PXR targets several CYP enzymes with major importance in drug metabolism including the most predominant drug-metabolizing CYP enzyme CYP3A4. Along with the CYP3A subfamily, PXR regulates many other important drug-metabolism CYPs. Chromatin immunoprecipitation sequencing (ChIP-Seq) analysis of PXR binding in HepG2 cells in response to rifampicin treatment detected rifampicin-induced regions close to CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP3A4, and CYP3A7 genes (Smith et al. 2014). In addition, several CYP genes with less-defined roles in drug metabolism and many phase 2 enzymes were found to interact with PXR (Smith et al. 2014).
CYP2B6 has been much studied as a classical CAR target gene, but the CAR target gene profile appears to be fairly overlapping with PXR (Kobayashi et al. 2015). No ChIP-Seq analysis revealing the CAR binding to human CYP genes has been published so far, although the human CAR interactome has been studied in a mouse model (Niu et al. 2018). Interestingly, this investigation showed that CAR targets several genes coding for other transcription factors including PXR and AHR introducing additional level of complexity to the induction mechanisms (Niu et al. 2018).
RXR functions as a binding partner for PXR and CAR as well as several other type 2 nuclear receptors. Although RXR is often regarded as a passive partner, RXR may also bind ligands such as 9-cis retinoic acid (de Almeida and Conda-Sheridan 2019) and it has been reported that RXR ligands may modulate function of the dimers formed by RXR and the xenobiotic-sensing receptors (Chen et al. 2010). It has also been reported that retinoids could induce CYP3A4 through RXR/VDR heterodimers and RXR homodimers (Wang et al. 2008).
AHR
Aryl hydrocarbon receptor (AHR) belongs to the bHLH-PAS family of transcription factors (Nebert 2017). AHR is activated especially by toxins and environmental contaminants including the classical activator 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and it has great toxicological significance (Kawajiri and Fujii-Kuriyama 2017). However, also some pharmaceutical ligands such as omeprazole activate AHR (Quattrochi and Tukey 1993). Many endogenous ligands have been identified for AHR including some originating from the microbiota (Bock 2019; Kawajiri and Fujii-Kuriyama 2017).
AHR is ubiquitously expressed in most tissues with high expression in placenta, lung, heart, pancreas, and liver (Dolwick et al. 1993). In absence of a ligand, AHR is sequestered to the cytosol in a complex with several proteins. Ligand binding-induced conformational change releases AHR from the chaperone proteins and allows translocation to the nucleus, where it heterodimerizes with another bHLH-PAS protein, aryl hydrocarbon receptor nuclear translocator (ARNT) (Kawajiri and Fujii-Kuriyama 2017; Nebert 2017). AHR/ARNT-dimer is then able to bind the so-called xenobiotic-response-elements (XRE) in the vicinity of the target genes to promote transcription. One of the target genes is aryl hydrocarbon receptor repressor (AHRR), which acts as a negative feedback mechanism (Bock 2019).
Among the CYPs, AHR mainly regulates the members of the CYP1 family, of which only CYP1A2 plays an important role in hepatic drug metabolism. In several extrahepatic tissues, AHR efficiently induces CYP1A1 and CYP1B1 (Bock 2019). In the other CYP families, AHR has been found to regulate some members in the CYP2 family including CYP2S1 (Saarikoski et al. 2005). In mouse, also Cyp2a5 is regulated by AHR, but no similar evidence exist for the human ortholog CYP2A6 (Arpiainen et al. 2005). AHR also regulates several phase 2 drug-metabolizing enzymes. In addition to drug metabolism, AHR plays important role in multiple physiological functions such as immunity, cell growth and differentiation, and prolonged activation may cause toxicity (Hakkola et al. 2018; Kawajiri and Fujii-Kuriyama 2017; Nebert 2017; Rothhammer and Quintana 2019).
Other transcriptional mechanisms mediating CYP induction
In addition to the xenobiotic-sensing receptors, some other transcription factors have been shown to mediate induction of CYP enzymes in response to chemical exposure. Some classical steroid receptors have been shown to regulate CYP genes. In contrast to the xenobiotic-sensing nuclear receptors, these nuclear receptors are more restricted in ligand preference and act as homodimer. Accordingly, estradiol induces CYP2A6 directly through estrogen receptor α (ERα) binding to the 5′-flanking region of the gene (Higashi et al. 2007).
Glucocorticoids regulate CYP expression; however, the mechanisms are diverse. Some glucocorticoids such as dexamethasone are PXR ligands explaining the observed CYP induction. However, others like methylprednisolone activate poorly the human PXR (Shukla et al. 2011). In fact, glucocorticoid receptor (GR) activation induces expression of PXR and CAR that may explain in many cases the CYP induction by glucocorticoids (Pascussi et al. 2001, 2003). However, also direct GR-mediated regulation of the CYP2C and CYP3A genes has been reported (Chen et al. 2003; Ferguson et al. 2005; Gerbal-Chaloin et al. 2002; Hukkanen et al. 2003; Matsunaga et al. 2004). For the CYP3A genes, this has been shown in the lung and fetal liver, i.e., in the absence of PXR and CAR expression (Hukkanen et al. 2003; Matsunaga et al. 2004).
Nuclear factor-erythroid 2-related factor 2 (NRF2) (the official name: Nuclear factor-erythroid-derived 2-like 2, NFE2L2) is a transcription factor belonging to the cap-n-collar subfamily of basic region–leucine zipper-type transcription factors (Suzuki and Yamamoto 2015). NRF2 expression is controlled at the level of protein stability and under unstressed conditions NRF2 is targeted to proteasomal degradation by its interaction partner Kelch-like ECH-associated protein 1 (KEAP1). KEAP1 functions as a redox sensor and contains several highly reactive cysteines that, upon modification by electrophilic molecules, prevent it from targeting NRF2 for proteasomal degradation. Therefore, in response to oxidative stress, NFR2 is stabilized, accumulates to the nucleus, and forms heterodimers with small musculoaponeurotic fibrosarcoma oncogene homologue (sMAF) proteins. The NRF2/sMAF-dimer binds to the antioxidant response element (ARE) in the regulatory regions of the target genes (Cuadrado et al. 2019).
NRF2 pathway is activated in response to oxidative stress produced by many toxic compounds such as heavy metals like cadmium and lead (Abu-Bakar et al. 2013). NRF2 regulates multiple cell functions, among them antioxidative response and xenobiotic biotransformation (Cuadrado et al. 2019). However, within the xenobiotic metabolism machinery, NRF2 mainly targets phase 2 enzymes, and among the CYP enzymes, only a limited number of CYP2 genes are regulated by NRF2 (K. C. Wu et al. 2012). The best-characterized CYP target is the mouse gene Cyp2a5 (Abu-Bakar et al. 2007; Lämsä et al. 2010). Also the closely related human gene CYP2A6 is regulated by NRF2 (Abu-Bakar et al. 2013; Yokota et al. 2011). Interestingly, the AHR and NRF2 pathways crosstalk at multiple levels (Köhle and Bock 2007).
Post-transcriptional regulation
Some CYPs are regulated at the post-transcriptional level. The most important example is CYP2E1. CYP2E1 protein has a short half-life and protein stabilization represents a major level of CYP2E1 regulation. The labile CYP2E1 protein is stabilized by xenobiotics such as ethanol, acetone, pyrazole, and isoniazid (Carroccio et al. 1994; Song et al. 1989). A few CYPs have been shown to be regulated by xenobiotics at the level of mRNA stability. mRNA stabilization has been shown convincingly for the mouse form Cyp2a5, which, in response to pyrazole treatment, is regulated by heterogeneous nuclear ribonucleoprotein A1 (hnRNP A1) binding to the 3′-untranslated region of the Cyp2a5 mRNA (Abu-Bakar et al. 2013). The human CYP2A6 appears to be regulated by a similar mechanism (Christian et al. 2004). During the recent years, many CYPs have been shown to be targeted by microRNAs that may also potentially mediate the post-transcriptional effects of chemical exposure (Yu et al. 2016a, b).
The in vivo induction of human CYP enzymes with drugs, herbal medicines, and environmental chemicals
In the following section, we will present the current status on the knowledge of the human in vivo induction. The following tables present the medications (Table 12), environmental contaminants (Table 13), and the herbal remedies and nutritional exposures (Table 14) known to induce human CYP enzymes. Only human in vivo inducers are listed based on the following criteria: the compound induces a specific CYP enzyme as assessed by (1) the pharmacokinetics of an established CYP-specific probe, (2) the established CYP-specific metabolic pathway of an endogenous metabolite (such as 6β-hydroxycortisol and 4β-hydroxycholesterol for CYP3A4), or (3) tissue-level expression of a CYP enzyme mRNA or protein. Also, supporting in vitro mechanistic evidence was required for compounds with only one published report of in vivo induction. However, the mechanistic evidence was not required if the inducer was a structural analog of a well-established inducer (this pertains especially to various barbiturates). Supporting evidence was not required if at least two studies report the induction. For medications, only those in current clinical use are listed. For withdrawn pharmaceuticals, reader is advised to consult previously published reviews (Hukkanen 2012; Zanger and Schwab 2013). Only CYP enzymes in families 1–3 are covered here.
Table 12.
Medications as in vivo inducers of human cytochrome P450 enzymes
| Enzyme | Class of inducers | Inducing medication | Receptor(s) implicated | Tissues | References |
|---|---|---|---|---|---|
| CYP1A1 | Proton pump inhibitors | Omeprazole | AHR | Duodenum | Buchthal et al. (1995), McDonnell et al. (1992) |
| CYP1A2 | Antibiotics | Rifampicin | PXR indirectly? | Liver (phenotyping) | Backman et al. (2006), Robson et al. (1984), Wietholtz et al. (1995) |
| Antiepileptics | Carbamazepine | CAR/PXR indirectly? | Liver (phenotyping and expression) | Lucas et al. (1998), Oscarson et al. (2006), Parker et al. (1998) | |
| Phenytoin | CAR/PXR indirectly? | Liver (phenotyping) | Miller et al. (1984), Wietholtz et al. (1989) | ||
| Antiretrovirals | Nelfinavir | PXR indirectly? | Liver (phenotyping) | Kirby et al. (2011) | |
| Ritonavir | PXR indirectly? | Liver (phenotyping) | Hsu et al. (1998), Kirby et al. (2011), Penzak et al. (2002) | ||
| Barbiturates | Pentobarbital | CAR and PXR indirectly? | Liver (phenotyping) | Dahlqvist et al. (1989) | |
| Phenobarbital | CAR and PXR indirectly? | Liver (phenotyping) | Landay et al. (1978), Saccar et al. (1985) | ||
| Secobarbital | CAR and PXR indirectly? | Liver (phenotyping) | Paladino et al. (1983) | ||
| Immunosuppressants | Teriflunomide | CAR? | Liver (phenotyping) | Aubagio summary of product characteristicsa | |
| Proton pump inhibitors | Omeprazole | AHR | Liver (phenotyping and expression) | Diaz et al. (1990), Rost et al. (1994), Rost and Roots (1994) | |
| CYP2A6 | Antiepileptics | Carbamazepine | CAR/PXR | Liver (phenotyping and expression) | Oscarson et al. (2006), Williams et al. (2010) |
| Antimalarials | Artemisinin | CAR/PXR | Liver (phenotyping) | Asimus et al. (2008) | |
| Antiretrovirals | Efavirenz | CAR/PXR | Liver (phenotyping) | Metzger et al. (2019) | |
| Barbiturates | Phenobarbital | CAR/PXR | Liver (expression) | Cashman et al. (1992), Kyerematen et al. (1990), Yamano et al. (1990) | |
| Estrogens | Ethinyl estradiol (of oral contraceptives) | ER | Liver (phenotyping) | Benowitz et al. (2006), Berlin et al. (2007), Sinues et al. (2008) | |
| CYP2B6 | Antibiotics | Rifampicin | PXR | Liver (phenotyping) | Chung et al. (2011), Loboz et al. (2006), Lopez-Cortes et al. (2002) |
| Antiepileptics | Carbamazepine | CAR/PXR | Liver (phenotyping and expression) | Ji et al. (2008), Ketter et al. (1995, Oscarson et al. (2006) | |
| Phenytoin | CAR/PXR | Liver (phenotyping) | Slattery et al. (1996), Williams et al. (1999) | ||
| Antimalarials | Arteether | CAR/PXR | Liver (phenotyping) | Elsherbiny et al. (2008) | |
| Artemether | CAR/PXR | Liver (phenotyping) | Elsherbiny et al. (2008) | ||
| Artemisinin | CAR/PXR | Liver (phenotyping) | Elsherbiny et al. (2008), Simonsson et al. (2003), Zang et al. (2014) | ||
| Artesunate | CAR/PXR | Liver (phenotyping) | Elsherbiny et al. (2008) | ||
| Dihydroartemisinin | CAR/PXR | Liver (phenotyping) | Elsherbiny et al. (2008) | ||
| Antipyretic analgesic | Metamizole | Unknown | Liver (phenotyping and expression) | Qin et al. (2012), Saussele et al. (2007) | |
| Antiretrovirals | Efavirenz | CAR/PXR | Liver (phenotyping), white blood cells | Kharasch et al. (2012), Meyer zu Schwabedissen et al. (2012), Ngaimisi et al. (2010), Robertson et al. (2008a, b) | |
| Nelfinavir | PXR | Liver (phenotyping) | Kirby et al. (2011a, b) | ||
| Ritonavir | PXR | Liver (phenotyping) | Kharasch et al. (2008), Kirby et al. (2011a, b) | ||
| Barbiturates | Phenobarbital | CAR/PXR | Liver (phenotyping) | Jao et al. (1972) | |
| CYP2C8 | Antibiotics | Rifampicin | PXR | Liver (phenotyping), small intestine enterocytes | Glaeser et al. (2005), Jaakkola et al. (2006), Niemi et al. (2000, 2004), Park et al. (2004) |
| Flucloxacillin | PXR | Liver (phenotyping) | Du et al. (2013) | ||
| Antiepileptics | Carbamazepine | CAR/PXR | Liver (expression) | Oscarson et al. (2006) | |
| CYP2C9 | Antiandrogens | Apalutamide | PXR? | Liver (phenotyping) | Duran et al. (2020) |
| Enzalutamide | PXR | Liver (phenotyping) | Gibbons et al. (2015) | ||
| Antibiotics | Dicloxacillin | PXR | Liver (phenotyping) | Stage et al. (2018) | |
| Nafcillin | PXR | Liver (phenotyping) | Kim et al. (2007), King et al. (2018) | ||
| Rifabutin | PXR | Liver (phenotyping) | Lutz et al. (2018) | ||
| Rifampicin | PXR | Liver (phenotyping), duodenum | Glaeser et al. (2005), O’Reilly (1974), Oscarson et al. (2007), Williamson et al. (1998), Zilly et al. (1975) | ||
| Antiemetics | Aprepitant | PXR | Liver (phenotyping) | Depre et al. (2005), Shadle et al. (2004) | |
| Antiepileptics | Carbamazepine | CAR/PXR | Liver (phenotyping and expression) | Herman et al. (2006), Lai et al. (1992), Oscarson et al. (2006) | |
| Phenytoin | CAR/PXR | Liver (phenotyping) | Chetty et al. (1998), Dickinson et al. (1985) | ||
| Antiretrovirals | Nelfinavir | PXR | Liver (phenotyping) | Kirby et al. (2011a, b) | |
| Ritonavir | PXR | Liver (phenotyping) | Kirby et al. (2011a, b), Lim et al. (2004), Yeh et al. (2006) | ||
| Barbiturates | Pentobarbital | CAR/PXR? | Liver (phenotyping) | Yoshida et al. (1993) | |
| Phenobarbital | CAR/PXR | Liver (phenotyping) | Goldberg et al. (1996), Orme and Breckenridge (1976) | ||
| Secobarbital | CAR/PXR? | Liver (phenotyping) | Breckenridge and Orme (1971), O’Reilly et al. (1980), Udall (1975) | ||
| Endothelin receptor antagonists | Bosentan | PXR | Liver (phenotyping) | van Giersbergen et al. (2002), Weber et al. (1999a) | |
| Kinase inhibitor | Dabrafenib | PXR | Liver (phenotyping) | Suttle et al. (2015) | |
| CYP2C19 | Antiandrogens | Apalutamide | PXR? | Liver (phenotyping) | Duran et al. (2020) |
| Enzalutamide | PXR | Liver (phenotyping) | Gibbons et al. (2015) | ||
| Antibiotics | Dicloxacillin | PXR | Liver (phenotyping) | Stage et al. (2018) | |
| Rifampicin | PXR | Liver (phenotyping), duodenum | Feng et al. (1998), Oscarson et al. (2007), Zhou et al. (1990), Zilly et al. (1975) | ||
| Antiepileptics | Carbamazepine | CAR/PXR | Liver (expression) | Oscarson et al. (2006) | |
| Phenytoin | CAR/PXR | Liver (phenotyping) | Richter et al. (1980) | ||
| Antimalarials | Arteether | CAR/PXR | Liver (phenotyping) | Asimus et al. (2007), Elsherbiny et al. (2008) | |
| Artemether | CAR/PXR | Liver (phenotyping) | Elsherbiny et al. (2008) | ||
| Artemisinin | CAR/PXR | Liver (phenotyping) | Asimus et al. (2007), Elsherbiny et al. (2008), Mihara et al. (1999), Svensson et al. (1998) | ||
| Antiretrovirals | Efavirenz | CAR/PXR | Liver (phenotyping) | Michaud et al. (2012) | |
| Ritonavir (with lopinavir or tipranivir) | PXR | Liver (phenotyping) | Dumond et al. (2010), Yeh et al. (2006) | ||
| Barbiturates | Pentobarbital | CAR/PXR? | Liver (phenotyping) | Heinemeyer et al. (1987) | |
| Phenobarbital | CAR/PXR | Liver (phenotyping and expression) | Lecamwasam et al. (1975), Richter et al. (1980) | ||
| CYP2E1 | Antibiotics | Isoniazid | Stabilization | Liver (phenotyping), blood lymphocytes | Chien et al. (1997), Mazze et al. (1982), O’Shea et al. (1997), Walubo et al. (2005), Zand et al. 1993) |
| Retinoid receptor modulators | All-trans-retinoic acid | RXR? | Liver (phenotyping) | Adedoyin et al. (1998) | |
| CYP2S1 | Retinoid receptor modulators | Topical all-trans retinoic acid | RXR? | Skin | Smith et al. (2003) |
| CYP3A4 | Antiandrogens | Apalutamide | PXR? | Liver (phenotyping) | Duran et al. (2020) |
| Enzalutamide | PXR | Liver (phenotyping) | Belderbos et al. (2018), Gibbons et al. (2015), Schwartzberg et al. (2017) | ||
| Antibiotics | Dicloxacillin | PXR | Liver (phenotyping) | Stage et al. (2018) | |
| Flucloxacillin | PXR | Liver (phenotyping) | Fan et al. (2019) | ||
| Nafcillin | PXR | Liver (phenotyping) | Lang et al. (2003) | ||
| Rifabutin | PXR | Liver (phenotyping) | Barditch-Crovo et al. (1999), Perucca et al. (1988) | ||
| Rifampicin | PXR | Liver (phenotyping and expression), duodenum | Greiner et al. (1999), Kolars et al. (1992), Marschall et al. (2005), McAllister et al. (1983), Ohnhaus and Park (1979), Perucca et al. (1988) | ||
| Rifapentine | PXR | Liver (phenotyping) | Birmingham et al. (1978), Vital Durand et al. (1986) | ||
| Antidiarrheals | Telotristat ethyl | PXR | Liver (phenotyping) | Yu et al. (2019) | |
| Antiemetics | Aprepitant | PXR | Liver (phenotyping) | Shadle et al. (2004) | |
| Antiepileptics | Carbamazepine | CAR/PXR | Liver (phenotyping, expression) | Crawford et al. (1990), Moreland et al. (1982), Oscarson et al. (2006) | |
| Phenytoin | CAR/PXR | Liver (phenotyping, expression) | Crawford et al. (1990), Thummel et al. (1994), Werk et al. (1964), Xu et al. (2006) | ||
| Oxcarbazepine | PXR | Liver (phenotyping) | Andreasen et al. (2007), Klosterskov Jensen et al. (1992, Zaccara et al. (1993) | ||
| Rufinamide | Unknown | Liver (phenotyping) | Perucca et al. (2008) | ||
| Topiramate | PXR | Liver (phenotyping) | Rosenfeld et al. (1997) | ||
| Antimalarials | Artemether | CAR/PXR | Liver (phenotyping) | Asimus et al. (2007) | |
| Artemisinin | CAR/PXR | Liver (phenotyping) | Asimus et al. (2007), Zang et al. (2014) | ||
| Dihydroartemisinin | CAR/PXR | Liver (phenotyping) | Asimus et al. (2007) | ||
| Antineoplastic agents | Vinblastine | CAR/PXR | Liver (phenotyping) | Smith et al. (2010) | |
| Antipyretic analgesic | Metamizole | Unknown | Liver (phenotyping and expression) | Caraco et al. (1999), Saussele et al. (2007) | |
| Antiretrovirals | Efavirenz | CAR/PXR | Liver (phenotyping) | Fellay et al. (2005), Mouly et al. (2002) | |
| Etravirine | PXR | Liver (phenotyping) | Kakuda et al. (2014), Scholler-Gyure et al. (2009) | ||
| Fosamprenavir (and metabolite amprenavir) | CAR/PXR | Liver (phenotyping) | Justesen et al. (2003), Kashuba et al. (2005), Tran et al. (2002) | ||
| Nevirapine | CAR/PXR | Liver (phenotyping) | Dailly et al. (2006), Mildvan et al. (2002), Solas et al. (2004) | ||
| Ritonavir | PXR | Liver (phenotyping) | Hsu et al. (1997), Ouellet et al. (1998) | ||
| Tipranavir | CAR/PXR | Liver (phenotyping) | Boehringer-Ingelheim (2005) | ||
| Barbiturates | Pentobarbital | CAR/PXR? | Liver (phenotyping) | Berman and Green (1971), Schellens et al. (1989) | |
| Phenobarbital | CAR/PXR | Liver (phenotyping) | Back et al. (1980), Burstein and Klaiber (1965) | ||
| Bile acid derivatives | Ursodeoxycholic acid | PXR | Liver (phenotyping) | Bodin et al. (2001), Marschall et al. (2005) | |
| Cystic fibrosis medications | Lumacaftor | PXR | Liver (phenotyping) | ORKAMBI summary of product characteristicsb | |
| Endothelin receptor antagonists | Bosentan | PXR | Liver (phenotyping) | Dingemanse et al. (2003), Weber et al. (1999b) | |
| Glucocorticoids | Dexamethasone | GR/PXR | Liver (phenotyping) | McCune et al. (2000), Roberts et al. (2008), Watkins et al. (1989) | |
| Methylprednisolone | GR | Liver (phenotyping) | Kuypers et al. (2004), Villikka et al. (2001) | ||
| Prednisolone | GR | Liver (phenotyping) | Press et al. (2010), van Duijnhoven et al. (2003) | ||
| Prednisone | GR | Liver (phenotyping) | Anglicheau et al. (2003) | ||
| Herpes virus medications | Amenamevir | Unknown | Liver (phenotyping) | Adeloye et al. (2018), Kusawake et al. (2017) | |
| Gout medications | Lesinurad | PXR | Liver (phenotyping) | Gillen et al. (2017) | |
| Retinoid receptor modulators | Alitretinoin (9-cis retinoic acid) | RXR | Liver (phenotyping) | Schmitt-Hoffmann et al. (2011) | |
| Bexarotene | RXR | Liver (phenotyping) | Padda et al. (2013), Wakelee et al. (2012) | ||
| Steroidogenesis inhibitors | Mitotane | PXR | Liver (phenotyping) | Bledsoe et al. (1964), van Erp et al. (2011) | |
| Stimulants | Modafinil (and its R-enantiomer armodafinil) | Unknown | Liver (phenotyping) | Darwish et al. (2008), Robertson et al. (2002) | |
| Kinase inhibitors | Dabrafenib | PXR | Liver (phenotyping) | Lawrence et al. (2014) | |
| Erlotinib | PXR | Liver (phenotyping | Svedberg et al. (2019) | ||
| Midostaurin | PXR | Liver (phenotyping) | Gu et al. (2018) | ||
| Vemurafenib | PXR | Liver (phenotyping) | Zhang et al. (2017) | ||
| CYP3A5 | Antibiotics | Rifampicin | PXR | Duodenum | Burk et al. (2004) |
| Glucocorticoids | Topical clobetasol 17-propionate | GR | Skin | Smith et al. (2006) | |
| CYP3A7 and CYP3A43 | Antibiotics | Rifampicin | PXR | Duodenum | Oscarson et al. (2007) |
| Antiepileptics | Carbamazepine | CAR/PXR | Liver (expression) | Oscarson et al. (2006) |
Only medications currently in clinical use are listed
ahttps://www.accessdata.fda.gov/drugsatfda_docs/label/2020/202992s010lbl.pdf
bhttps://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211358s000lbl.pdf
Table 13.
Chemical toxicants and radiation as in vivo inducers of human cytochrome P450 enzymes
| Enzyme | Class of inducers | Compound or exposure | Receptor(s) implicated | Tissues | References |
|---|---|---|---|---|---|
| CYP1A1 | Dioxins | Various environmental exposures, and a case of massive TCDD poisoning | AHR | Skin | Fabbrocini et al. (2015), Saurat et al. (2012) |
| PAHs | Charbroiled meat | AHR | Duodenum | Fontana et al. (1999) | |
| Smoking | AHR | Adipose tissue, lung, oral and pharyngeal mucosa, placenta, uroepithelium, fetal lung, fetal liver | Boyle et al. (2010), Chi et al. (2009), Dorrenhaus et al. (2007), Hukkanen et al. (2002), Huuskonen et al. (2008), McLemore et al. (1990), O’Shaughnessy et al. (2011), Pasanen et al. (1990), Tsai et al. (2018), Ullrich et al. (1997), Vyhlidal et al. (2013) | ||
| Topical coal tar | AHR | Skin, hair follicles | Merk et al. (1987), Smith et al. (2006) | ||
| Polychlorinated biphenyls | Consumption of contaminated rice oil | AHR | Placenta | Lucier et al. (1987) | |
| Radiation | Therapeutic ultraviolet-B radiation | AHR | Skin | Katiyar et al. (2000) | |
| CYP1A2 | Dioxins | Dioxins, mainly TCDD, from environmental and occupational exposures, an occupational accident, and a case of massive TCDD poisoning | AHR | Liver (phenotyping) | Abraham et al. (2002), Chernyak et al. (2016), Samer et al. (2020) |
| Heterocyclic aromatic amines | Pan-fried meat | AHR | Liver (phenotyping) | Sinha et al. (1994) | |
| PAHs | Charbroiled meat | AHR | Liver (phenotyping) | Fontana et al. (1999), Kappas et al. (1978), Pantuck et al. (1976) | |
| Coffee | AHR | Liver (phenotyping) | Djordjevic et al. (2008), Horn et al. (1995) | ||
| Smoking | AHR | Liver (phenotyping, expression in liver autopsy samples) | Baker et al. (2001), Hunt et al. (1976), Pantuck et al. (1972) | ||
| Topical coal tar | AHR | Skin | Smith et al. (2006) | ||
| Polybrominated and polychlorinated biphenyls | Consumption of contaminated fish and farm products | AHR | Liver (phenotyping) | Fitzgerald et al. (2005), Lambert et al. (1990) | |
| CYP1B1 | PAHs | Smoking | AHR | Adipose tissue, lung, oral mucosa, placenta, white blood cells, whole-blood cells, fetal lung | Boyle et al. (2010), Chi et al. (2009), Hukkanen et al. (2002), Huuskonen et al. (2008), Lampe et al. (2004), Tsai et al. (2018), van Leeuwen et al. (2007), Vyhlidal et al. (2013), Willey et al. (1997) |
| Topical coal tar | AHR | Skin | Smith et al. (2006) | ||
| Work in coke ovens and waste incinerators | AHR | White blood cells | Hanaoka et al. (2002), Hu et al. (2006) | ||
| Radiation | Therapeutic ultraviolet-B radiation | AHR | Skin | Katiyar et al. (2000) | |
| CYP2A6 | Heavy metals | Cadmium | NRF2 | Liver (phenotyping) | Satarug et al. (2004a, b) |
| CYP2E1 | Benzene derivatives | Smoking (cigarette smoke contains both styrene and toluene, see below) | Stabilization? | Liver (phenotyping), bronchial epithelium | Benowitz et al. (2003), Oyama et al. (2007) |
| Occupational exposure to styrene | Stabilization? | Blood lymphocytes, whole-blood cells | Prieto-Castello et al. (2010), Wongvijitsuk et al. (2011) | ||
| Toluene | Stabilization? | Blood lymphocytes | Mendoza-Cantu et al. (2006) | ||
| CYP2S1 | PAHs | Smoking | AHR | Bronchoalveolar macrophages | Thum et al. (2006) |
| Topical coal tar | AHR | Skin | Smith et al. (2003) | ||
| Radiation | Ultraviolet-B radiation | AHR | Skin | Smith et al. (2003) | |
| CYP3A4 | Organochlorine pesticides | Dichlorodiphenyltrichloroethane (DDT) | PXR | Liver (phenotyping) | Petersen et al. (2007), Poland et al. (1970) |
| Endrin | PXR | Liver (phenotyping) | Jager (1970) |
The search strategy included searching PubMed with the specific CYPs as keywords (e.g., CYP2B6 and [induction or inducer or induce]). Also searches with the specific probe compounds were performed (e.g., for CYP2B6 “bupropion and [induction or inducer or induce]”). The bibliographies of the publications were checked for additional articles. As the clinical and toxicological significance of the induction is often difficult to evaluate, the compounds are listed on the tables with no regard to the consequences or magnitudes of the induction. However, for CYP-inducing TKIs, Table 1 provides the estimates of potency. For the sake of brevity, the following paragraphs do not systematically repeat the data and the references given in Tables 12, 13 and 14.
The most important xenobiotic-activated receptor regulating the induction of enzymes in the CYP1 subfamily is AHR. Several environmental chemicals such as PAHs, dioxins, polychlorinated biphenyls, and heterocyclic aromatic amines induce CYP1A1, CYP1A2, and CYP1B1 enzymes via AHR (Tables 12, 13, 14). Human in vivo induction of CYP1A1 and CYP1B1 is difficult to study with phenotyping probes owing to their very low or non-existent hepatic expression and overlap with CYP1A2 substrates (Chang et al. 2003). However, their expression can be measured more easily as these enzymes are widely expressed in various extrahepatic tissues where tissue sampling is more convenient than with liver. Only one medication (omeprazole for CYP1A1 in duodenum and CYP1A2 in liver) (Buchthal et al. 1995; Diaz et al. 1990; McDonnell et al. 1992; Rost et al. 1994; Rost and Roots 1994) and one nutritional exposure (indole-3-carbinol present in cruciferous vegetables for hepatic CYP1A2) (Pantuck et al. 1979; Reed et al. 2005) are currently known to induce CYP1 enzymes via AHR-mediated pathways. PXR and CAR are not known to directly induce CYP1 enzymes but several CAR/PXR agonists do induce CYP1A2-related activities in vivo. It is quite likely that CAR/PXR agonists induce the expression of AHR and lead to the induction of CYP1 enzymes indirectly (Maglich et al. 2002; Oscarson et al. 2006). Recent evidence suggests that teriflunomide, an immunosuppressant, induces CYP1A2 activity as shown with caffeine phenotyping possibly via phenobarbital-like indirect CAR activation (Carazo et al. 2018).3
CYP2A6 is induced in humans in vivo by CAR, PXR, ERα, and NRF2 agonists (Tables 12, 13, 14). The regulation of CYP2A6 by ERα and NRF2 sets it apart as no other CYP enzyme is known to be regulated in vivo by these transcription factors. CYP2A6 is induced through ERα by phytoestrogens such as genistein (in legumes such as soybeans)(Y. Chen et al. 2011; Mazur 1998) and quercetin (in tea, vegetables, fruits, and berries) (Chen et al. 2009; Chun et al. 2012) as well as ethinyl estradiol of oral contraceptives (Benowitz et al. 2006; Berlin et al. 2007; Sinues et al. 2008). Exposure to cadmium measured as urine cadmium excretion is associated with CYP2A6 activity probed with coumarin 7-hydroxylation but only in non-smokers (Satarug et al. 2004a, b). In smokers, CYP2A6 activity is known to be reduced (inhibition) by an unknown mechanism (Hukkanen et al. 2005) and as smoking is also an important source of cadmium (induction), it is not surprising that smoking can confound the association between cadmium exposure and CYP2A6 activity. The effect of cadmium on CYP2A6 is most likely mediated by NRF2 as is the induction caused by sulforaphane present in cruciferous vegetables (Abu-Bakar et al. 2004; Yokota et al. 2011). All medications known to induce CYP2A6 are combined CAR/PXR activators and it is not known which nuclear receptor is more important for CYP2A6 induction in vivo as there is some evidence for the involvement of both (Itoh et al. 2006). Rifampicin treatment for 6 days had no effect on CYP2A6 activity measured as coumarin hydroxylation (Rautio et al. 1994) arguing against the role of PXR in the in vivo regulation.
Several medications with PXR and combined CAR/PXR-activating properties induce CYP2B6 (Table 12). The mechanism mediating the effect of metamizole, an antipyretic analgesic with spasmolytic properties, on the induction of CYP2B6 is currently unknown (Qin et al. 2012; Saussele et al. 2007). It is not acting as a direct ligand of PXR or CAR and an indirect phenobarbital-like mechanism has been suggested (Qin et al. 2012; Saussele et al. 2007). No environmental toxicant has been shown to induce CYP2B6 in vivo, but constituents of herbal remedies such as baicalin (CAR/PXR), hyperforin (PXR) of St. John’s wort, and sodium ferulate (PXR) induce CYP2B6 (Fan et al. 2009; Gao et al. 2012, 2013; Lei et al. 2010) (Table 14). The effects of baicalin and sodium ferulate on CYP2B6 were demonstrated only as purified compounds in high doses. Thus, it is not known if dosing as herbal preparations containing Angelica sinensis, Cimicifuga heracleifolia, or Lignsticum chuangxiong (sodium ferulate) or Baikal skullcap (Scutellaria baicalensis) (baicalin) induce CYP2B6.
The induction of CYP2C8 has been demonstrated only with a few CAR or PXR-activating pharmaceuticals (Table 12). No environmental chemicals or constituents of herbal remedies are known to induce CYP2C8 in vivo in humans. Similarly, CYP2C9 is not known to be induced by environmental toxicants and only one herbal preparation, St. John’s wort, induces CYP2C9-related activities in vivo (Jiang et al. 2004, 2006). However, a multitude of medications (PXR agonists and combined CAR/PXR activators) induce CYP2C9 (Table 12). CYP2C19 induction has been demonstrated with baicalin-containing Chinese multicomponent herbal preparation Yin Zhi Huang and hyperforin-containing St. John’s wort (Fan et al. 2007; Wang et al. 2004a, b) (Table 14), while no environmental chemical is known to induce CYP2C19. Several medications with PXR and CAR/PXR-activating properties induce CYP2C19 (Table 12).
The induction of CYP2E1 is regulated unlike any other CYP enzyme. The stabilization of mRNA and protein by inducing compounds, many of which are also CYP2E1 substrates, is the main mechanism of induction (Cederbaum 2006). Benzene derivatives such as styrene and toluene encountered by workers in print and plastic industries are known to induce CYP2E1 (Mendoza-Cantu et al. 2006; Prieto-Castello et al. 2010; Wongvijitsuk et al. 2011) and the same compounds may also be responsible for the CYP2E1 induction detected in tobacco smokers (Benowitz et al. 2003; Oyama et al. 2007) (Table 13). The most well-known toxicant inducing CYP2E1 is ethanol (Table 14). Two medications have been demonstrated to induce CYP2E1, namely isoniazid (stabilization) and oral all-trans retinoic acid with RXR agonism as the most likely mode of induction (Gyamfi et al. 2006) (Table 12). St John’s wort induces CYP2E1 in long-term administration (28 days), but the mechanism is unknown (Gurley et al. 2002, 2005). It is not known if the well-established PXR agonist hyperforin is involved or if some other St. John’s wort ingredient is responsible for the induction of CYP2E1.
CYP2S1 is induced in skin and bronchoalveolar macrophages with exposures containing AHR agonists such smoking and topical coal tar (G. Smith et al. 2003; Thum et al. 2006) (Table 13). Ultraviolet-B (UVB) radiation has been demonstrated to induce CYP2S1 in skin with AHR-mediated mechanism which is also involved in the induction of cutaneous CYP1A1 and CYP1B1 by UVB (Katiyar et al. 2000; Smith et al. 2003). UVB exposure leads to the formation of 6-formylindolo[3,2-b]carbazole, a tryptophan photoproduct and an endogenous AHR ligand (Fritsche et al. 2007). The only medication known to induce CYP2S1 expression is topical all-trans retinoic acid, possibly via RXR (McNeilly et al. 2012).
As CYP3A4 is involved in the metabolism of approximately 50% of all marketed medications (Zhou 2008), its induction is of special importance. There are also numerous pharmaceutical CYP3A4 inducers leading to increased risk of drug–drug interactions (Table 12). CAR, GR, and PXR are known to mediate the induction. The mechanism of induction is unknown for antiepileptic rufinamide, stimulants modafinil and its R-enantiomer armodafinil, antiherpetic medication amenamevir, and metamizole (Table 12). Also RXR agonists alitretinoin (9-cis retinoic acid) and bexarotene are known to induce CYP3A4-related activities in phenotyping studies (Padda et al. 2013; Schmitt-Hoffmann et al. 2011; Wakelee et al. 2012).
In addition to CYP3A4-inducing medications, quite many herbal remedies and food ingredients induce CYP3A4 (Table 14). Also the occupational and environmental exposure to organochlorine pesticides dichlorodiphenyltrichloroethane (DDT) and endrin is associated with the induction of CYP3A4 as measured with urinary 6β-hydroxycortisol (Petersen et al. 2007; Poland et al. 1970) (Table 13). One often neglected CYP3A4 inducer is ethanol. Chronic alcoholics had a higher ratio of urine 6β-hydroxycortisol/cortisol compared with healthy volunteers (Luceri et al. 2001). Also oral bioavailability of midazolam was significantly lower in subjects with moderate alcohol consumption in comparison with abstaining controls suggesting intestinal CYP3A4 induction (Liangpunsakul et al. 2005). In a twin study, alcohol consumption was significantly associated with greater St. John’s wort-induced CYP3A4 activity as assessed with quinine phenotyping (Rahmioglu et al. 2011). There are also indications that CYP3A4 protein could be induced in liver of the alcoholics with liver disease (Niemela et al. 2000).
The evaluation of induction phenomena of CYP3A enzymes is complicated by the closely related CYP3A5 enzyme. CYP3A4 and CYP3A5 have widely overlapping substrate specificities and their regulation shares certain features such as crucial role of PXR and CAR (Burk et al. 2004). A notable difference is the extensive influence of genetics on CYP3A5 expression. The CYP3A5*3 allele with severely decreased enzymatic activity is more common than the CYP3A5*1 allele (CYP3A5*3 allele frequency is ~ 90% in Caucasians and 50% in African–Americans) (Lamba et al. 2002). Thus, most Caucasians do not have a functional CYP3A5 enzyme. The phenotyping studies performed with probes metabolized by CYP3A4 and CYP3A5 are classified here as showing only CYP3A4 induction if there are no enzyme-specific data on CYP3A5 induction. It is conceivable that many of the CYP3A4 inducers are also CYP3A5 inducers in those patients carrying one or two functional CYP3A5*1 alleles. There are only a few known CYP3A5 in vivo inducers. Rifampicin induced duodenal CYP3A5 mRNA in the subjects carrying a CYP3A5*1 allele, while no induction was detected in CYP3A5*3/*3 subjects (Burk et al. 2004). Topical administration of the glucocorticoid clobetasol 17-propionate induced cutaneous CYP3A5 mRNA (Smith et al. 2006).
The induction of minor CYP3A forms has also been demonstrated. The use of carbamazepine is associated with the increased expression of hepatic CYP3A7 and CYP3A43 mRNA (Oscarson et al. 2006). Rifampicin induces intestinal CYP3A7 and CYP3A43 mRNA in healthy volunteers (Oscarson et al. 2007) (Table 12).
Consequences and relevance of CYP induction
The induction of CYP enzymes as a cause of DDIs, as distinct from the enzyme inhibition, is unique as the induction becomes apparent more slowly and it takes more time for the induction to abate. This is caused by the delay due to the synthesis of new enzymes when the inducer is introduced, and then for the additional enzymes to degrade after the inducer is withdrawn. These effects take usually days to even weeks to fully manifest when concerning rapidly metabolized compounds (Tran et al. 1999). The time-dependent effects are even slower when dealing with steady-state levels of compounds with long half-lives. Thus, the outcome of adding an inducer to the patient’s established drug regimen can be difficult to detect in clinical setting if the physician is unaware of the anticipated effect. The effect of the induction is even more difficult to discern when dealing with intermittent exposures as is common with environmental toxicants as both victims and perpetrators of induction. For drugs and toxicants active in their parent form, CYP induction increases the elimination of compounds and decreases therapeutic and toxic effects, respectively. For prodrugs and toxicants that have active metabolites formed by CYP enzymes, enhanced pharmacodynamic and toxic effects could result.
The consequences of CYP induction are even more difficult to evaluate when dealing with mixtures of chemical compounds comprised of all the pharmaceutical, herbal, and environmental chemical exposures encountered by individuals in their daily lives. This is due to newly emerging findings on the combinatorial effects of chemical mixtures as activators of xenobiotic-sensing receptors. This phenomenon has been best demonstrated with PXR. It has been shown that combinations of toxic compounds such as bisphenol A analogs (Sui et al. 2012), and drugs and toxicants such as the combination of pesticide trans-nonachlor and drug 17α-ethinylestradiol (Delfosse et al. 2015), potentiate the PXR activation even at the low concentrations incapable to activate PXR by themselves. The science of the combinations is still very much a work in progress.
Concluding remarks and lessons learnt
After intense investigation for several decades, the research field of CYP inhibition and induction has reached a rather matured stage. The basic mechanisms of both CYP inhibition and induction are now fairly well understood, although further details continue to be revealed.
The experimental tools to study CYP inhibition and induction in vitro have been well established and adopted in guidelines regulating drug development. The in vitro results can further guide the in vivo experiments. Indeed, we have moved from testing clinically commonly used individual drugs together to the rational design of studies using index drugs and reference inhibitors based on mechanistic understanding of drug–drug interactions (Tornio et al. 2019). Further development has been made in the computational tools, and the physiologically based pharmacokinetic modeling can be used to simulate in vivo conditions, extend the knowledge gained from the clinical studies, and even avoid unnecessary clinical studies (Shebley and Einolf 2019; Venkatakrishnan and Rostami-Hodjegan 2019). However, human in vivo DDI studies are still needed to definitively demonstrate the consequences of inhibition/induction, especially for the regulatory filings, and it is not likely that these studies would be deemed unnecessary in the near future.
As a result of the methodological developments, the CYP-mediated drug–drug interactions are identified early in the pharmaceutical development and no longer big surprises appear in the clinical use after approval. The early awareness of the potential CYP-mediated drug–drug interactions may also guide the drug development process to avoid strong inhibitors and inducers. Thus, especially the number of new inducers has been low among the recently approved drugs. However, there may still be unidentified inducers and inhibitors among the compounds present in our diet and various herbal remedies as well as in the environment as chemical toxicants.
The CYP-mediated interactions are now mastered rather well in the drug development process. The use of different databases and prescription aid tools has also improved application of the interaction data in the clinical practice. The widespread application of these information technology solutions is crucial as the amount of DDI data are too extensive for any individual physician to master. The progress in the pharmaceutical drug development during the recent years has resulted in design of small-molecular drugs with increasing metabolic stability. While this decreases the risk of CYP-mediated drug–drug interactions, this development may induce other types of interactions such as those mediated by various transporters (Venkatakrishnan and Rostami-Hodjegan 2019).
Although, in general, there is a good potential for prediction of the CYP inhibition and induction, unusual cases may still continue to provide surprises. For example, it was described that co-binding of two non-activating compounds to the active site of PXR may result in synergistic effect and receptor activation (Delfosse et al. 2015). This kind of cocktail effect may be possible among drugs, but perhaps more relevant in the toxicological exposure to complex mixtures. Naturally, also drugs and environmental compounds or natural substances could interact or act together. Thus, although much has been learned in the last decades regarding inhibition and induction of CYP enzymes, novel discoveries may still be made by inquiring minds.
Acknowledgements
The authors would like to pay tribute to the late Professor Pertti Neuvonen, a prominent scientist in the field of CYP-mediated drug–drug interaction, and extensively cited also in this review.
Author contributions
All authors participated in the literature search, data analysis, and writing of the manuscript. All authors have read and approved the manuscript.
Funding
Open access funding provided by University of Oulu including Oulu University Hospital. The original research by the authors is supported by the Academy of Finland (Grants 286743 and 323706) to JHa, Finnish Medical Foundation; the Finnish Foundation for Cardiovascular Research; the Northern Finland Health Care Support Foundation; and the Diabetes Research Foundation to JHu, the Northern Finland Health Care Support to MT.
Availability of data and material (data transparency)
All the data are available in the text and tables of the review.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Footnotes
EMA 2012, https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-drug-interactions-revision-1_en.pdf; FDA 2020, https://www.fda.gov/media/134582/download and https://www.fda.gov/media/134581/download; MHLW/PMDA 2018, https://www.pmda.go.jp/files/000228122.pdf.
Summary of Product Characteristics, https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/202992s010lbl.pdf.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbas R, Hsyu P. Clinical pharmacokinetics and pharmacodynamics of bosutinib. Clin Pharmacokinet. 2016;55:1191–1204. doi: 10.1007/s40262-016-0391-6. [DOI] [PubMed] [Google Scholar]
- Abbas R, Leister C, El Gaaloul M, Chalon S, Sonnichsen D. Ascending single-dose study of the safety profile, tolerability, and pharmacokinetics of bosutinib coadministered with ketoconazole to healthy adult subjects. Clin Ther. 2012;34:2011–2019.e1. doi: 10.1016/j.clinthera.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Abbas R, Boni J, Sonnichsen D. Effect of rifampin on the pharmacokinetics of bosutinib, a dual Src/Abl tyrosine kinase inhibitor, when administered concomitantly to healthy subjects. Drug Metab Pers Ther. 2015;30:57–63. doi: 10.1515/dmdi-2014-0026. [DOI] [PubMed] [Google Scholar]
- Abel S, Back DJ, Vourvahis M. Maraviroc: pharmacokinetics and drug interactions. Antivir Ther (Lond) 2009;14:607–618. [PubMed] [Google Scholar]
- Abraham K, Geusau A, Tosun Y, Helge H, Bauer S, Brockmoller J. Severe 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) intoxication: insights into the measurement of hepatic cytochrome P450 1A2 induction. Clin Pharmacol Ther. 2002;72:163–174. doi: 10.1067/mcp.2002.126408. [DOI] [PubMed] [Google Scholar]
- Abu-Bakar A, Satarug S, Marks GC, Lang MA, Moore MR. Acute cadmium chloride administration induces hepatic and renal CYP2A5 mRNA, protein and activity in the mouse: involvement of transcription factor NRF2. Toxicol Lett. 2004;148:199–210. doi: 10.1016/j.toxlet.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Abu-Bakar A, Lämsä V, Arpiainen S, Moore MR, Lang MA, Hakkola J. Regulation of CYP2A5 gene by the transcription factor nuclear factor (erythroid-derived 2)-like 2. Drug Metab Dispos. 2007;35:787–794. doi: 10.1124/dmd.106.014423. [DOI] [PubMed] [Google Scholar]
- Abu-Bakar A, Hakkola J, Juvonen R, Rahnasto-Rilla M, Raunio H, Lang MA. Function and regulation of the Cyp2a5/CYP2A6 genes in response to toxic insults in the liver. Curr Drug Metab. 2013;14:137–150. [PubMed] [Google Scholar]
- Adedoyin A, Stiff DD, Smith DC, Romkes M, Bahnson RC, Day R, Hofacker J, Branch RA, Trump DL. All-trans-retinoic acid modulation of drug-metabolizing enzyme activities: investigation with selective metabolic drug probes. Cancer Chemother Pharmacol. 1998;41:133–139. doi: 10.1007/s002800050719. [DOI] [PubMed] [Google Scholar]
- Adeloye T, Sahgal O, Puri A, Warrington S, Endo T, Dennison J, Johnston A. PMC6585933; amenamevir: studies of potential CYP3A-mediated pharmacokinetic interactions with midazolam, cyclosporine, and ritonavir in healthy volunteers. Clin Pharmacol Drug Dev. 2018;7:844–859. doi: 10.1002/cpdd.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyoshi T, Ito M, Murase S, Miyazaki M, Guengerich FP, Nakamura K, Yamamoto K, Ohtani H. Mechanism-based inhibition profiles of erythromycin and clarithromycin with cytochrome P450 3A4 genetic variants. Drug Metab Pharmacokinet. 2013;28:411–415. doi: 10.2133/dmpk.dmpk-12-rg-134. [DOI] [PubMed] [Google Scholar]
- Alam C, Whyte-Allman S, Omeragic A, Bendayan R. Role and modulation of drug transporters in HIV-1 therapy. Adv Drug Deliv Rev. 2016;103:121–143. doi: 10.1016/j.addr.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Amaya GM, Durandis R, Bourgeois DS, Perkins JA, Abouda AA, Wines KJ, Mohamud M, Starks SA, Daniels RN, Jackson KD. Cytochromes P450 1A2 and 3A4 catalyze the metabolic activation of sunitinib. Chem Res Toxicol. 2018;31:570–584. doi: 10.1021/acs.chemrestox.8b00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen AH, Brosen K, Damkier P. A comparative pharmacokinetic study in healthy volunteers of the effect of carbamazepine and oxcarbazepine on cyp3a4. Epilepsia. 2007;48:490–496. doi: 10.1111/j.1528-1167.2007.00924.x. [DOI] [PubMed] [Google Scholar]
- Anglicheau D, Flamant M, Schlageter MH, Martinez F, Cassinat B, Beaune P, Legendre C, Thervet E. Pharmacokinetic interaction between corticosteroids and tacrolimus after renal transplantation. Nephrol Dial Transplant. 2003;18:2409–2414. doi: 10.1093/ndt/gfg381. [DOI] [PubMed] [Google Scholar]
- Arpiainen S, Raffalli-Mathieu F, Lang MA, Pelkonen O, Hakkola J. Regulation of the Cyp2a5 gene involves an aryl hydrocarbon receptor-dependent pathway. Mol Pharmacol. 2005;67:1325–1333. doi: 10.1124/mol.104.008078. [DOI] [PubMed] [Google Scholar]
- Asaumi R, Toshimoto K, Tobe Y, Hashizume K, Nunoya K, Imawaka H, Lee W, Sugiyama Y. Comprehensive PBPK Model of rifampicin for quantitative prediction of complex drug–drug interactions: CYP3A/2C9 induction and OATP inhibition effects. CPT Pharmacomet Syst Pharmacol. 2018;7:186–196. doi: 10.1002/psp4.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asimus S, Elsherbiny D, Hai TN, Jansson B, Huong NV, Petzold MG, Simonsson US, Ashton M. Artemisinin antimalarials moderately affect cytochrome P450 enzyme activity in healthy subjects. Fundam Clin Pharmacol. 2007;21:307–316. doi: 10.1111/j.1472-8206.2007.00471.x. [DOI] [PubMed] [Google Scholar]
- Asimus S, Hai TN, Van Huong N, Ashton M. Artemisinin and CYP2A6 activity in healthy subjects. Eur J Clin Pharmacol. 2008;64:283–292. doi: 10.1007/s00228-007-0406-1. [DOI] [PubMed] [Google Scholar]
- Back DJ, Bates M, Bowden A, Breckenridge AM, Hall MJ, Jones H, MacIver M, Orme M, Perucca E, Richens A, Rowe PH, Smith E. The interaction of phenobarbital and other anticonvulsants with oral contraceptive steroid therapy. Contraception. 1980;22:495–503. doi: 10.1016/0010-7824(80)90102-x. [DOI] [PubMed] [Google Scholar]
- Back DJ, Tjia JF, Karbwang J, Colbert J. In vitro inhibition studies of tolbutamide hydroxylase activity of human liver microsomes by azoles, sulphonamides and quinolines. Br J Clin Pharmacol. 1988;26:23–29. doi: 10.1111/j.1365-2125.1988.tb03359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman JT, Granfors MT, Neuvonen PJ. Rifampicin is only a weak inducer of CYP1A2-mediated presystemic and systemic metabolism: studies with tizanidine and caffeine. Eur J Clin Pharmacol. 2006;62:451–461. doi: 10.1007/s00228-006-0127-x. [DOI] [PubMed] [Google Scholar]
- Backman JT, Filppula AM, Niemi M, Neuvonen PJ. Role of cytochrome P450 2C8 in drug metabolism and interactions. Pharmacol Rev. 2016;68:168–241. doi: 10.1124/pr.115.011411. [DOI] [PubMed] [Google Scholar]
- Bae SH, Kwon MJ, Choi EJ, Zheng YF, Yoon KD, Liu K, Bae SK. Potent inhibition of cytochrome P450 2B6 by sibutramine in human liver microsomes. Chem Biol Interact. 2013;205:11–19. doi: 10.1016/j.cbi.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Baes M, Gulick T, Choi HS, Martinoli MG, Simha D, Moore DD. A new orphan member of the nuclear hormone receptor superfamily that interacts with a subset of retinoic acid response elements. Mol Cell Biol. 1994;14:1544–1552. doi: 10.1128/mcb.14.3.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DG, Dresser G, Arnold JMO. Grapefruit-medication interactions: forbidden fruit or avoidable consequences? CMAJ. 2013;185:309–316. doi: 10.1503/cmaj.120951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JR, Satarug S, Reilly PE, Edwards RJ, Ariyoshi N, Kamataki T, Moore MR, Williams DJ. Relationships between non-occupational cadmium exposure and expression of nine cytochrome P450 forms in human liver and kidney cortex samples. Biochem Pharmacol. 2001;62:713–721. doi: 10.1016/s0006-2952(01)00716-x. [DOI] [PubMed] [Google Scholar]
- Bapiro TE, Sayi J, Hasler JA, Jande M, Rimoy G, Masselle A, Masimirembwa CM. Artemisinin and thiabendazole are potent inhibitors of cytochrome P450 1A2 (CYP1A2) activity in humans. Eur J Clin Pharmacol. 2005;61:755–761. doi: 10.1007/s00228-005-0037-3. [DOI] [PubMed] [Google Scholar]
- Barditch-Crovo P, Trapnell CB, Ette E, Zacur HA, Coresh J, Rocco LE, Hendrix CW, Flexner C. The effects of rifampin and rifabutin on the pharmacokinetics and pharmacodynamics of a combination oral contraceptive. Clin Pharmacol Ther. 1999;65:428–438. doi: 10.1016/S0009-9236(99)70138-4. [DOI] [PubMed] [Google Scholar]
- Barecki ME, Casciano CN, Johnson WW, Clement RP. In vitro characterization of the inhibition profile of loratadine, desloratadine, and 3-OH-desloratadine for five human cytochrome P-450 enzymes. Drug Metab Dispos. 2001;29:1173–1175. [PubMed] [Google Scholar]
- Belderbos BPS, Bins S, van Leeuwen RWF, Oomen-de Hoop E, van der Meer N, de Bruijn P, Hamberg P, Overkleeft ENM, van der Deure WM, Lolkema MP, de Wit R, Mathijssen RHJ. Influence of enzalutamide on cabazitaxel pharmacokinetics: a drug–drug interaction study in metastatic castration-resistant prostate cancer (mCRPC) Patients. Clin Cancer Res. 2018;24:541–546. doi: 10.1158/1078-0432.CCR-17-2336. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Peng M, Jacob P., III Effects of cigarette smoking and carbon monoxide on chlorzoxazone and caffeine metabolism. Clin Pharmacol Ther. 2003;74:468–474. doi: 10.1016/j.clpt.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Lessov-Schlaggar C, Swan GE, Jacob P., III Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79:480–488. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Berlin I, Gasior MJ, Moolchan ET. Sex-based and hormonal contraception effects on the metabolism of nicotine among adolescent tobacco-dependent smokers. Nicotine Tob Res. 2007;9:493–498. doi: 10.1080/14622200701243193. [DOI] [PubMed] [Google Scholar]
- Berman ML, Green OC. Acute stimulation of cortisol metabolism by pentobarbital in man. Anesthesiology. 1971;34:365–369. doi: 10.1097/00000542-197104000-00020. [DOI] [PubMed] [Google Scholar]
- Bernasconi C, Pelkonen O, Andersson TB, Strickland J, Wilk-Zasadna I, Asturiol D, Cole T, Liska R, Worth A, Müller-Vieira U, Richert L, Chesne C, Coecke S. Validation of in vitro methods for human cytochrome P450 enzyme induction: outcome of a multi-laboratory study. Toxicol In Vitro. 2019;60:212–228. doi: 10.1016/j.tiv.2019.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best BM, Goicoechea M. Efavirenz-still first-line king? Expert Opin Drug Metab Toxicol. 2008;4:965–972. doi: 10.1517/17425255.4.7.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbao-Meseguer I, Jose BS, Lopez-Gimenez LR, Gil MA, Serrano L, Castaño M, Sautua S, Basagoiti AD, Belaustegui A, Baza B, Baskaran Z, Bustinza A. Drug interactions with sunitinib. J Oncol Pharm Pract. 2015;21:52–66. doi: 10.1177/1078155213516158. [DOI] [PubMed] [Google Scholar]
- Birdwell KA, Decker B, Barbarino JM, Peterson JF, Stein CM, Sadee W, Wang D, Vinks AA, He Y, Swen JJ, Leeder JS, van Schaik R, Thummel KE, Klein TE, Caudle KE, IaM MacPhee. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for CYP3A5 genotype and tacrolimus dosing. Clin Pharmacol Ther. 2015;98:19–24. doi: 10.1002/cpt.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham AT, Coleman AJ, Orme ML, Park BK, Pearson NJ, Short AH, Southgate PJ. Antibacterial activity in serum and urine following oral administration in man of DL473 (a cyclopentyl derivative of rifampicin) [proceedings] Br J Clin Pharmacol. 1978;6:455P–456P. doi: 10.1111/j.1365-2125.1978.tb04626.x. [DOI] [PubMed] [Google Scholar]
- Bledsoe T, Island DP, Ney RL, Liddle GW. An Effect of O, P’-Ddd on the extra-adrenal metabolism of cortisol in man. J Clin Endocrinol Metab. 1964;24:1303–1311. doi: 10.1210/jcem-24-12-1303. [DOI] [PubMed] [Google Scholar]
- Blumberg B, Sabbagh W, Juguilon H, Bolado J, van Meter CM, Ong ES, Evans RM. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12:3195–3205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock KW. Aryl hydrocarbon receptor (AHR): from selected human target genes and crosstalk with transcription factors to multiple AHR functions. Biochem Pharmacol. 2019;168:65–70. doi: 10.1016/j.bcp.2019.06.015. [DOI] [PubMed] [Google Scholar]
- Bodin K, Bretillon L, Aden Y, Bertilsson L, Broome U, Einarsson C, Diczfalusy U. Antiepileptic drugs increase plasma levels of 4beta-hydroxycholesterol in humans: evidence for involvement of cytochrome p450 3A4. J Biol Chem. 2001;276:38685–38689. doi: 10.1074/jbc.M105127200. [DOI] [PubMed] [Google Scholar]
- Boehringer-Ingelheim (2005) Tipranavir: Antiviral Drugs Advisory Committee (AVDAC) Briefing Document. NDA 21-814
- Bohnert T, Patel A, Templeton I, Chen Y, Lu C, Lai G, Leung L, Tse S, Einolf HJ, Wang Y, Sinz M, Stearns R, Walsky R, Geng W, Sudsakorn S, Moore D, He L, Wahlstrom J, Keirns J, Narayanan R, Lang D, Yang X. Evaluation of a new molecular entity as a victim of metabolic drug–drug interactions-an industry perspective. Drug Metab Dispos. 2016;44:1399–1423. doi: 10.1124/dmd.115.069096. [DOI] [PubMed] [Google Scholar]
- Bolton AE, Peng B, Hubert M, Krebs-Brown A, Capdeville R, Keller U, Seiberling M. Effect of rifampicin on the pharmacokinetics of imatinib mesylate (Gleevec, STI571) in healthy subjects. Cancer Chemother Pharmacol. 2004;53:102–106. doi: 10.1007/s00280-003-0722-9. [DOI] [PubMed] [Google Scholar]
- Boyle JO, Gumus ZH, Kacker A, Choksi VL, Bocker JM, Zhou XK, Yantiss RK, Hughes DB, Du B, Judson BL, Subbaramaiah K, Dannenberg AJ. PMC2833216; effects of cigarette smoke on the human oral mucosal transcriptome. Cancer Prev Res (Phila) 2010;3:266–278. doi: 10.1158/1940-6207.CAPR-09-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge A, Orme M. Clinical implications of enzyme induction. Ann N Y Acad Sci. 1971;179:421–431. doi: 10.1111/j.1749-6632.1971.tb46919.x. [DOI] [PubMed] [Google Scholar]
- Britz H, Hanke N, Volz A, Spigset O, Schwab M, Eissing T, Wendl T, Frechen S, Lehr T. Physiologically-based pharmacokinetic models for CYP1A2 drug–drug interaction prediction: a modeling network of fluvoxamine, theophylline, caffeine, rifampicin, and midazolam. CPT Pharmacomet Syst Pharmacol. 2019;8:296–307. doi: 10.1002/psp4.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman CD, Chai SC, Chen T. A current structural perspective on PXR and CAR in drug metabolism. Expert Opin Drug Metab Toxicol. 2018;14:635–647. doi: 10.1080/17425255.2018.1476488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchthal J, Grund KE, Buchmann A, Schrenk D, Beaune P, Bock KW. Induction of cytochrome P4501A by smoking or omeprazole in comparison with UDP-glucuronosyltransferase in biopsies of human duodenal mucosa. Eur J Clin Pharmacol. 1995;47:431–435. doi: 10.1007/BF00196857. [DOI] [PubMed] [Google Scholar]
- Budha NR, Ji T, Musib L, Eppler S, Dresser M, Chen Y, Jin JY. Evaluation of cytochrome P450 3A4-mediated drug–drug interaction potential for cobimetinib using physiologically based pharmacokinetic modeling and simulation. Clin Pharmacokinet. 2016;55:1435–1445. doi: 10.1007/s40262-016-0412-5. [DOI] [PubMed] [Google Scholar]
- Burk O, Koch I, Raucy J, Hustert E, Eichelbaum M, Brockmoller J, Zanger UM, Wojnowski L. The induction of cytochrome P450 3A5 (CYP3A5) in the human liver and intestine is mediated by the xenobiotic sensors pregnane X receptor (PXR) and constitutively activated receptor (CAR) J Biol Chem. 2004;279:38379–38385. doi: 10.1074/jbc.M404949200. [DOI] [PubMed] [Google Scholar]
- Burstein S, Klaiber EL. Phenobarbital-induced increase in 6-beta-hydroxycortisol excretion: clue to its significance in human urine. J Clin Endocrinol Metab. 1965;25:293–296. doi: 10.1210/jcem-25-2-293. [DOI] [PubMed] [Google Scholar]
- Cada DJ, Demaris K, Levien TL, Baker DE. Teriflunomide. Hosp Pharm. 2013;48:231–240. doi: 10.1310/hpj4803-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Wang RW, Edom RW, Evans DC, Shou M, Rodrigues AD, Liu W, Dean DC, Baillie TA. Validation of (−)-N-3-benzyl-phenobarbital as a selective inhibitor of CYP2C19 in human liver microsomes. Drug Metab Dispos. 2004;32:584–586. doi: 10.1124/dmd.32.6.584. [DOI] [PubMed] [Google Scholar]
- Caraco Y, Zylber-Katz E, Fridlander M, Admon D, Levy M. The effect of short-term dipyrone administration on cyclosporin pharmacokinetics. Eur J Clin Pharmacol. 1999;55:475–478. doi: 10.1007/s002280050659. [DOI] [PubMed] [Google Scholar]
- Carazo A, Dusek J, Holas O, Skoda J, Hyrsova L, Smutny T, Soukup T, Dosedel M, Pávek P. Teriflunomide is an indirect human constitutive androstane receptor (CAR) activator interacting with epidermal growth factor (EGF) signaling. Front Pharmacol. 2018;9:993. doi: 10.3389/fphar.2018.00993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroccio A, Wu D, Cederbaum AI. Ethanol increases content and activity of human cytochrome P4502E1 in a transduced HepG2 cell line. Biochem Biophys Res Commun. 1994;203:727–733. doi: 10.1006/bbrc.1994.2242. [DOI] [PubMed] [Google Scholar]
- Cashman JR, Park SB, Yang ZC, Wrighton SA, Jacob P, III, Benowitz NL. Metabolism of nicotine by human liver microsomes: stereoselective formation of trans-nicotine N′-oxide. Chem Res Toxicol. 1992;5:639–646. doi: 10.1021/tx00029a008. [DOI] [PubMed] [Google Scholar]
- Cattaneo D, Cossu MV, Rizzardini G. Pharmacokinetic drug evaluation of ritonavir (versus cobicistat) as adjunctive therapy in the treatment of HIV. Expert Opin Drug Metab Toxicol. 2019;15:927–935. doi: 10.1080/17425255.2019.1685495. [DOI] [PubMed] [Google Scholar]
- Cederbaum AI. CYP2E1-biochemical and toxicological aspects and role in alcohol-induced liver injury. Mt Sinai J Med. 2006;73:657–672. [PubMed] [Google Scholar]
- Chai SC, Cherian MT, Wang Y, Chen T. Small-molecule modulators of PXR and CAR. Biochim Biophys Acta. 2016;1859:1141–1154. doi: 10.1016/j.bbagrm.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan ECY, New LS, Chua TB, Yap CW, Ho HK, Nelson SD. Interaction of lapatinib with cytochrome P450 3A5. Drug Metab Dispos. 2012;40:1414–1422. doi: 10.1124/dmd.112.044958. [DOI] [PubMed] [Google Scholar]
- Chan J, Oshiro T, Thomas S, Higa A, Black S, Todorovic A, Elbarbry F, Harrelson JP. Inactivation of CYP2A6 by the dietary phenylpropanoid trans-cinnamic aldehyde (Cinnamaldehyde) and estimation of interactions with nicotine and letrozole. Drug Metab Dispos. 2016;44:534–543. doi: 10.1124/dmd.115.067942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TK, Chen J, Lee WB. Differential inhibition and inactivation of human CYP1 enzymes by trans-resveratrol: evidence for mechanism-based inactivation of CYP1A2. J Pharmacol Exp Ther. 2001;299:874–882. [PubMed] [Google Scholar]
- Chang TK, Chen J, Pillay V, Ho JY, Bandiera SM. Real-time polymerase chain reaction analysis of CYP1B1 gene expression in human liver. Toxicol Sci. 2003;71:11–19. doi: 10.1093/toxsci/71.1.11. [DOI] [PubMed] [Google Scholar]
- Chapron B, Risler L, Phillips B, Collins C, Thummel K, Shen D. Reversible, time-dependent inhibition of CYP3A-mediated metabolism of midazolam and tacrolimus by telaprevir in human liver microsomes. J Pharm Pharm Sci. 2015;18:101–111. doi: 10.18433/j3288c. [DOI] [PubMed] [Google Scholar]
- Chen L, Prasad GVR. CYP3A5 polymorphisms in renal transplant recipients: influence on tacrolimus treatment. Pharmgenom Pers Med. 2018;11:23–33. doi: 10.2147/PGPM.S107710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Ferguson SS, Negishi M, Goldstein JA. Identification of constitutive androstane receptor and glucocorticoid receptor binding sites in the CYP2C19 promoter. Mol Pharmacol. 2003;64:316–324. doi: 10.1124/mol.64.2.316. [DOI] [PubMed] [Google Scholar]
- Chen Y, Xiao P, Ou-Yang D, Fan L, Guo D, Wang YN, Han Y, Tu JH, Zhou G, Huang YF, Zhou HH. Simultaneous action of the flavonoid quercetin on cytochrome P450 (CYP) 1A2, CYP2A6, N-acetyltransferase and xanthine oxidase activity in healthy volunteers. Clin Exp Pharmacol Physiol. 2009;36:828–833. doi: 10.1111/j.1440-1681.2009.05158.x. [DOI] [PubMed] [Google Scholar]
- Chen S, Wang K, Wan YJ. 2784018; Retinoids activate RXR/CAR-mediated pathway and induce CYP3A. Biochem Pharmacol. 2010;79:270–276. doi: 10.1016/j.bcp.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Xiao CQ, He YJ, Chen BL, Wang G, Zhou G, Zhang W, Tan ZR, Cao S, Wang LP, Zhou HH. Genistein alters caffeine exposure in healthy female volunteers. Eur J Clin Pharmacol. 2011;67:347–353. doi: 10.1007/s00228-010-0964-5. [DOI] [PubMed] [Google Scholar]
- Chen J, Xu H, Pawlak S, James LP, Peltz G, Lee K, Ginman K, Bergeron M, Pithavala YK. The effect of rifampin on the pharmacokinetics and safety of lorlatinib: results of a phase one, open-label, crossover study in healthy participants. Adv Ther. 2020;37:745–758. doi: 10.1007/s12325-019-01198-9. [DOI] [PubMed] [Google Scholar]
- Chernyak YI, Merinova AP, Shelepchikov AA, Kolesnikov SI, Grassman JA. Impact of dioxins on antipyrine metabolism in firefighters. Toxicol Lett. 2016;250–251:35–41. doi: 10.1016/j.toxlet.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Chetty M, Miller R, Seymour MA. Phenytoin auto-induction. Ther Drug Monit. 1998;20:60–62. doi: 10.1097/00007691-199802000-00011. [DOI] [PubMed] [Google Scholar]
- Chhun S, Verstuyft C, Rizzo-Padoin N, Simoneau G, Becquemont L, Peretti I, Swaisland A, Wortelboer R, Bergmann JF, Mouly S. Gefitinib-phenytoin interaction is not correlated with the C-erythromycin breath test in healthy male volunteers. Br J Clin Pharmacol. 2009;68:226–237. doi: 10.1111/j.1365-2125.2009.03438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi AC, Appleton K, Henriod JB, Krayer JW, Marlow NM, Bandyopadhyay D, Sigmon RC, Kurtz DT. 2764788; differential induction of CYP1A1 and CYP1B1 by benzo[a]pyrene in oral squamous cell carcinoma cell lines and by tobacco smoking in oral mucosa. Oral Oncol. 2009;45:980–985. doi: 10.1016/j.oraloncology.2009.05.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba K, Kobayashi K, Manabe K, Tani M, Kamataki T, Ishizaki T. Oxidative metabolism of omeprazole in human liver microsomes: cosegregation with S-mephenytoin 4′-hydroxylation. J Pharmacol Exp Ther. 1993;266:52–59. [PubMed] [Google Scholar]
- Chien JY, Peter RM, Nolan CM, Wartell C, Slattery JT, Nelson SD, Carithers RLJ, Thummel KE. Influence of polymorphic N-acetyltransferase phenotype on the inhibition and induction of acetaminophen bioactivation with long-term isoniazid. Clin Pharmacol Ther. 1997;61:24–34. doi: 10.1016/S0009-9236(97)90179-X. [DOI] [PubMed] [Google Scholar]
- Cho BC, Kim D, Bearz A, Laurie SA, McKeage M, Borra G, Park K, Kim S, Ghosn M, Ardizzoni A, Maiello E, Greystoke A, Yu R, Osborne K, Gu W, Scott JW, Passos VQ, Lau YY, Wrona A. ASCEND-8: a randomized phase 1 study of ceritinib, 450 mg or 600 mg, taken with a low-fat meal versus 750 mg in fasted state in patients with anaplastic lymphoma kinase (ALK)-rearranged metastatic non-small cell lung cancer (NSCLC) J Thorac Oncol. 2017;12:1357–1367. doi: 10.1016/j.jtho.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Chow HH, Garland LL, Hsu CH, Vining DR, Chew WM, Miller JA, Perloff M, Crowell JA, Alberts DS. PMC2933312; Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev Res (Phila) 2010;3:1168–1175. doi: 10.1158/1940-6207.CAPR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian K, Lang M, Maurel P, Raffalli-Mathieu F. Interaction of heterogeneous nuclear ribonucleoprotein A1 with cytochrome P450 2A6 mRNA: implications for post-transcriptional regulation of the CYP2A6 gene. Mol Pharmacol. 2004;65:1405–1414. doi: 10.1124/mol.65.6.1405. [DOI] [PubMed] [Google Scholar]
- Chu X, Cai X, Cui D, Tang C, Ghosal A, Chan G, Green MD, Kuo Y, Liang Y, Maciolek CM, Palamanda J, Evers R, Prueksaritanont T. In vitro assessment of drug–drug interaction potential of boceprevir associated with drug metabolizing enzymes and transporters. Drug Metab Dispos. 2013;41:668–681. doi: 10.1124/dmd.112.049668. [DOI] [PubMed] [Google Scholar]
- Chun YJ, Kim MY, Guengerich FP. Resveratrol is a selective human cytochrome P450 1A1 inhibitor. Biochem Biophys Res Commun. 1999;262:20–24. doi: 10.1006/bbrc.1999.1152. [DOI] [PubMed] [Google Scholar]
- Chun OK, Lee SG, Wang Y, Vance T, Song WO. Estimated flavonoid intake of the elderly in the United States and around the world. J Nutr Gerontol Geriatr. 2012;31:190–205. doi: 10.1080/21551197.2012.702530. [DOI] [PubMed] [Google Scholar]
- Chung JY, Cho JY, Lim HS, Kim JR, Yu KS, Lim KS, Shin SG, Jang IJ. Effects of pregnane X receptor (NR1I2) and CYP2B6 genetic polymorphisms on the induction of bupropion hydroxylation by rifampin. Drug Metab Dispos. 2011;39:92–97. doi: 10.1124/dmd.110.035246. [DOI] [PubMed] [Google Scholar]
- Cleary Y, Gertz M, Morcos PN, Yu L, Youdim K, Phipps A, Fowler S, Parrott N. Model-based assessments of CYP-mediated drug–drug interaction risk of alectinib: physiologically based pharmacokinetic modeling supported clinical development. Clin Pharmacol Ther. 2018;104:505–514. doi: 10.1002/cpt.956. [DOI] [PubMed] [Google Scholar]
- Collom SL, Laddusaw RM, Burch AM, Kuzmic P, Perry MD, Miller GP. CYP2E1 substrate inhibition. Mechanistic interpretation through an effector site for monocyclic compounds. J Biol Chem. 2008;283:3487–3496. doi: 10.1074/jbc.M707630200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CL, van Heeswijk RPG, Gallicano K, Cameron DW. A review of low-dose ritonavir in protease inhibitor combination therapy. Clin Infect Dis. 2003;36:1585–1592. doi: 10.1086/375233. [DOI] [PubMed] [Google Scholar]
- Cotreau MM, Siebers NM, Miller J, Strahs AL, Slichenmyer W. Effects of ketoconazole or rifampin on the pharmacokinetics of tivozanib hydrochloride, a vascular endothelial growth factor receptor tyrosine kinase inhibitor. Clin Pharmacol Drug Dev. 2015;4:137–142. doi: 10.1002/cpdd.145. [DOI] [PubMed] [Google Scholar]
- Coulet M, Dacasto M, Eeckhoutte C, Larrieu G, Sutra JF, Alvinerie M, Macé K, Pfeifer AM, Galtier P. Identification of human and rabbit cytochromes P450 1A2 as major isoforms involved in thiabendazole 5-hydroxylation. Fundam Clin Pharmacol. 1998;12:225–235. doi: 10.1111/j.1472-8206.1998.tb00946.x. [DOI] [PubMed] [Google Scholar]
- Crauwels H, van Heeswijk Rolf P G, Stevens M, Buelens A, Vanveggel S, Boven K, Hoetelmans R. Clinical perspective on drug–drug interactions with the non-nucleoside reverse transcriptase inhibitor rilpivirine. AIDS Rev. 2013;15:87–101. [PubMed] [Google Scholar]
- Crawford P, Chadwick DJ, Martin C, Tjia J, Back DJ, Orme M. 1368312; the interaction of phenytoin and carbamazepine with combined oral contraceptive steroids. Br J Clin Pharmacol. 1990;30:892–896. doi: 10.1111/j.1365-2125.1990.tb05457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado A, Rojo AI, Wells G, Hayes JD, Cousin SP, Rumsey WL, Attucks OC, Franklin S, Levonen A, Kensler TW, Dinkova-Kostova AT. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discov. 2019;18:295–317. doi: 10.1038/s41573-018-0008-x. [DOI] [PubMed] [Google Scholar]
- Cui W, Sun M, Zhang S, Shen X, Galeva N, Williams TD, Staudinger JL. A SUMO-acetyl switch in PXR biology. Biochim Biophys Acta. 2016;1859:1170–1182. doi: 10.1016/j.bbagrm.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlqvist R, Steiner E, Koike Y, von Bahr C, Lind M, Billing B. Induction of theophylline metabolism by pentobarbital. Ther Drug Monit. 1989;11:408–410. [PubMed] [Google Scholar]
- Dailly E, Tribut O, Tattevin P, Arvieux C, Perre P, Raffi F, Jolliet P. Influence of tenofovir, nevirapine and efavirenz on ritonavir-boosted atazanavir pharmacokinetics in HIV-infected patients. Eur J Clin Pharmacol. 2006;62:523–526. doi: 10.1007/s00228-006-0122-2. [DOI] [PubMed] [Google Scholar]
- Daly AK, Rettie AE, Fowler DM, Miners JO. Pharmacogenomics of CYP2C9: functional and clinical considerations. J Pers Med. 2017;8(1):1. doi: 10.3390/jpm8010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwish M, Kirby M, Robertson PJ, Hellriegel ET. Interaction profile of armodafinil with medications metabolized by cytochrome P450 enzymes 1A2, 3A4 and 2C19 in healthy subjects. Clin Pharmacokinet. 2008;47:61–74. doi: 10.2165/00003088-200847010-00006. [DOI] [PubMed] [Google Scholar]
- de Almeida NR, Conda-Sheridan M. A review of the molecular design and biological activities of RXR agonists. Med Res Rev. 2019;39:1372–1397. doi: 10.1002/med.21578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong J, Skee D, Murphy J, Sukbuntherng J, Hellemans P, Smit J, de Vries R, Jiao JJ, Snoeys J, Mannaert E. Effect of CYP3A perpetrators on ibrutinib exposure in healthy participants. Pharmacol Res Perspect. 2015;3:e00156. doi: 10.1002/prp2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre BG, Albericio F. The Pharmaceutical Industry in 2019. An analysis of FDA drug approvals from the perspective of molecules. Molecules. 2020;25(3):745. doi: 10.3390/molecules25030745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean L. Propafenone therapy and CYP2D6 genotype. In: Pratt VM, McLeod HL, Rubinstein WS, Scott SA, Dean LC, Kattman BL, Malheiro AJ, editors. Medical genetics summaries. Bethesda: National Center for Biotechnology Information (US); 2012. [PubMed] [Google Scholar]
- Delfosse V, Dendele B, Huet T, Grimaldi M, Boulahtouf A, Gerbal-Chaloin S, Beucher B, Roecklin D, Muller C, Rahmani R, Cavailles V, Daujat-Chavanieu M, Vivat V, Pascussi JM, Balaguer P, Bourguet W. PMC4569708; synergistic activation of human pregnane X receptor by binary cocktails of pharmaceutical and environmental compounds. Nat Commun. 2015;6:8089. doi: 10.1038/ncomms9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depre M, Van Hecken A, Oeyen M, De Lepeleire I, Laethem T, Rothenberg P, Petty KJ, Majumdar A, Crumley T, Panebianco D, Bergman A, de Hoon JN. Effect of aprepitant on the pharmacokinetics and pharmacodynamics of warfarin. Eur J Clin Pharmacol. 2005;61:341–346. doi: 10.1007/s00228-005-0907-8. [DOI] [PubMed] [Google Scholar]
- Diaz D, Fabre I, Daujat M, Saint Aubert B, Bories P, Michel H, Maurel P. Omeprazole is an aryl hydrocarbon-like inducer of human hepatic cytochrome P450. Gastroenterology. 1990;99:737–747. doi: 10.1016/0016-5085(90)90963-2. [DOI] [PubMed] [Google Scholar]
- Dickinson RG, Hooper WD, Patterson M, Eadie MJ, Maguire B. Extent of urinary excretion of p-hydroxyphenytoin in healthy subjects given phenytoin. Ther Drug Monit. 1985;7:283–289. doi: 10.1097/00007691-198507030-00008. [DOI] [PubMed] [Google Scholar]
- Dickinson L, Boffito M, Back DJ, Khoo SH, Pozniak AL, Mugyenyi P, Merry C, Autar RS, Burger DM, Aarons LJ. Population pharmacokinetics of ritonavir-boosted saquinavir regimens in HIV-infected individuals. J Antimicrob Chemother. 2008;62:1344–1355. doi: 10.1093/jac/dkn399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse J, Schaarschmidt D, van Giersbergen PL. Investigation of the mutual pharmacokinetic interactions between bosentan, a dual endothelin receptor antagonist, and simvastatin. Clin Pharmacokinet. 2003;42:293–301. doi: 10.2165/00003088-200342030-00004. [DOI] [PubMed] [Google Scholar]
- Dirix L, Swaisland H, Verheul HMW, Rottey S, Leunen K, Jerusalem G, Rolfo C, Nielsen D, Molife LR, Kristeleit R, Vos-Geelen Jd, Mau-Sørensen M, Soetekouw P, van Herpen C, Fielding A, So K, Bannister W, Plummer R. Effect of itraconazole and rifampin on the pharmacokinetics of olaparib in patients with advanced solid tumors: results of two phase I open-label studies. Clin Ther. 2016;38:2286–2299. doi: 10.1016/j.clinthera.2016.08.010. [DOI] [PubMed] [Google Scholar]
- Djordjevic N, Ghotbi R, Bertilsson L, Jankovic S, Aklillu E. Induction of CYP1A2 by heavy coffee consumption in Serbs and Swedes. Eur J Clin Pharmacol. 2008;64:381–385. doi: 10.1007/s00228-007-0438-6. [DOI] [PubMed] [Google Scholar]
- Dolwick KM, Schmidt JV, Carver LA, Swanson HI, Bradfield CA. Cloning and expression of a human Ah receptor cDNA. Mol Pharmacol. 1993;44:911–917. [PubMed] [Google Scholar]
- Dorrenhaus A, Muller T, Roos PH. Increased CYP1A1 expression in human exfoliated urothelial cells of cigarette smokers compared to non-smokers. Arch Toxicol. 2007;81:19–25. doi: 10.1007/s00204-006-0134-9. [DOI] [PubMed] [Google Scholar]
- Du QQ, Wang ZJ, He L, Jiang XH, Wang L. PXR polymorphisms and their impact on pharmacokinetics/pharmacodynamics of repaglinide in healthy Chinese volunteers. Eur J Clin Pharmacol. 2013;69(11):1917–1925. doi: 10.1007/s00228-013-1552-2. [DOI] [PubMed] [Google Scholar]
- Duan KM, Wang SY, Ouyang W, Mao YM, Yang LJ. Effect of quercetin on CYP3A activity in Chinese healthy participants. J Clin Pharmacol. 2012;52:940–946. doi: 10.1177/0091270011406278. [DOI] [PubMed] [Google Scholar]
- Dumond JB, Vourvahis M, Rezk NL, Patterson KB, Tien HC, White N, Jennings SH, Choi SO, Li J, Wagner MJ, La-Beck N, Drulak M, Sabo JP, Castles MA, Macgregor TR, Kashuba AD. 2882206; A phenotype-genotype approach to predicting CYP450 and P-glycoprotein drug interactions with the mixed inhibitor/inducer tipranavir/ritonavir. Clin Pharmacol Ther. 2010;87:735–742. doi: 10.1038/clpt.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran I, Carles J, Bulat I, Hellemans P, Mitselos A, Ward P, Jiao J, Armas D, Chien C. Pharmacokinetic drug-drug interaction of apalutamide, part 1: clinical studies in healthy men and patients with castration-resistant prostate cancer. Clin Pharmacokinet. 2020;59(9):1135–1148. doi: 10.1007/s40262-020-00882-2. [DOI] [PubMed] [Google Scholar]
- Durr D, Stieger B, Kullak-Ublick G, Rentsch KM, Steinert HC, Meier PJ, Fattinger K. St John’s Wort induces intestinal P-glycoprotein/MDR1 and intestinal and hepatic CYP3A4. Clin Pharmacol Ther. 2000;68:598–604. doi: 10.1067/mcp.2000.112240. [DOI] [PubMed] [Google Scholar]
- Dutreix C, Peng B, Mehring G, Hayes M, Capdeville R, Pokorny R, Seiberling M. Pharmacokinetic interaction between ketoconazole and imatinib mesylate (Glivec) in healthy subjects. Cancer Chemother Pharmacol. 2004;54:290–294. doi: 10.1007/s00280-004-0832-z. [DOI] [PubMed] [Google Scholar]
- Dutreix C, Munarini F, Lorenzo S, Roesel J, Wang Y. Investigation into CYP3A4-mediated drug–drug interactions on midostaurin in healthy volunteers. Cancer Chemother Pharmacol. 2013;72:1223–1234. doi: 10.1007/s00280-013-2287-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagling VA, Wiltshire H, Whitcombe IWA, Back DJ. CYP3A4-mediated hepatic metabolism of the HIV-1 protease inhibitor saquinavir in vitro. Xenobiotica. 2002;32:1–17. doi: 10.1080/00498250110085845. [DOI] [PubMed] [Google Scholar]
- Elsby R, Hare V, Neal H, Outteridge S, Pearson C, Plant K, Gill RU, Butler P, Riley RJ. Mechanistic in vitro studies indicate that the clinical drug–drug interaction between telithromycin and simvastatin acid is driven by time-dependent inhibition of CYP3A4 with minimal effect on OATP1B1. Drug Metab Dispos. 2019;47:1–8. doi: 10.1124/dmd.118.083832. [DOI] [PubMed] [Google Scholar]
- Elsherbiny DA, Asimus SA, Karlsson MO, Ashton M, Simonsson US. A model based assessment of the CYP2B6 and CYP2C19 inductive properties by artemisinin antimalarials: implications for combination regimens. J Pharmacokinet Pharmacodyn. 2008;35(2):203–217. doi: 10.1007/s10928-008-9084-6. [DOI] [PubMed] [Google Scholar]
- EMA (2012) Guideline on the investigation of drug interactions. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-drug-interactions-revision-1_en.pdf
- Ena J, Amador C, Benito C, Pasquau F. Pharmacological and clinical evidence of nevirapine immediate- and extended-release formulations. HIV AIDS (Auckl) 2012;4:169–179. doi: 10.2147/HIV.S35564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernstgård L, Warholm M, Johanson G. Robustness of chlorzoxazone as an in vivo measure of cytochrome P450 2E1 activity. Br J Clin Pharmacol. 2004;58:190–200. doi: 10.1111/j.1365-2125.2004.02132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrocini G, Kaya G, Caseiro Silverio P, De Vita V, Kaya A, Fontao F, Sorg O, Saurat JH. Aryl hydrocarbon receptor activation in acne vulgaris skin: a case series from the region of Naples, Italy. Dermatology. 2015;231:334–338. doi: 10.1159/000439402. [DOI] [PubMed] [Google Scholar]
- Faber MS, Jetter A, Fuhr U. Assessment of CYP1A2 activity in clinical practice: why, how, and when? Basic Clin Pharmacol Toxicol. 2005;97:125–134. doi: 10.1111/j.1742-7843.2005.pto_973160.x. [DOI] [PubMed] [Google Scholar]
- Fan L, Wang G, Wang LS, Chen Y, Zhang W, Huang YF, Huang RX, Hu DL, Wang D, Zhou HH. Herbal medicine yin zhi huang induces CYP3A4-mediated sulfoxidation and CYP2C19-dependent hydroxylation of omeprazole. Acta Pharmacol Sin. 2007;28:1685–1692. doi: 10.1111/j.1745-7254.2007.00617.x. [DOI] [PubMed] [Google Scholar]
- Fan L, Wang JC, Jiang F, Tan ZR, Chen Y, Li Q, Zhang W, Wang G, Lei HP, Hu DL, Wang D, Zhou HH. Induction of cytochrome P450 2B6 activity by the herbal medicine baicalin as measured by bupropion hydroxylation. Eur J Clin Pharmacol. 2009;65:403–409. doi: 10.1007/s00228-008-0594-3. [DOI] [PubMed] [Google Scholar]
- Fan Q, Liu W, Yang Y, Zhou J, Tang Y, Xiao M, Pan X, Zhou Y, Deng K, He F. A new similarity method for assessment of pharmacokinetic interaction between flucloxacillin and midazolam. Pharmazie. 2019;74:397–405. doi: 10.1691/ph.2019.9016. [DOI] [PubMed] [Google Scholar]
- FDA (2020) In vitro drug interaction studies—cytochrome P450 enzyme- and transporter-mediated drug interactions guidance for industry. https://www.fda.gov/media/134582/download
- Fellay J, Marzolini C, Decosterd L, Golay KP, Baumann P, Buclin T, Telenti A, Eap CB. Variations of CYP3A activity induced by antiretroviral treatment in HIV-1 infected patients. Eur J Clin Pharmacol. 2005;60:865–873. doi: 10.1007/s00228-004-0855-8. [DOI] [PubMed] [Google Scholar]
- Feng HJ, Huang SL, Wang W, Zhou HH. 1873992; the induction effect of rifampicin on activity of mephenytoin 4′-hydroxylase related to M1 mutation of CYP2C19 and gene dose. Br J Clin Pharmacol. 1998;45:27–29. doi: 10.1046/j.1365-2125.1998.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner KS, Troutman MD, Kempshall S, Cook JA, Ware JA, Smith DA, Lee CA. Drug–drug interactions mediated through P-glycoprotein: clinical relevance and in vitro–in vivo correlation using digoxin as a probe drug. Clin Pharmacol Ther. 2009;85:173–181. doi: 10.1038/clpt.2008.195. [DOI] [PubMed] [Google Scholar]
- Ferguson SS, Chen Y, LeCluyse EL, Negishi M, Goldstein JA. Human CYP2C8 is transcriptionally regulated by the nuclear receptors constitutive androstane receptor, pregnane X receptor, glucocorticoid receptor, and hepatic nuclear factor 4alpha. Mol Pharmacol. 2005;68:747–757. doi: 10.1124/mol.105.013169. [DOI] [PubMed] [Google Scholar]
- Filppula AM, Mustonen TM, Backman JT. In vitro screening of six protein kinase inhibitors for time-dependent inhibition of CYP2C8 and CYP3A4: possible implications with regard to drug–drug interactions. Basic Clin Pharmacol Toxicol. 2018;123:739–748. doi: 10.1111/bcpt.13088. [DOI] [PubMed] [Google Scholar]
- Filppula AM, Parvizi R, Mateus A, Baranczewski P, Artursson P. Improved predictions of time-dependent drug–drug interactions by determination of cytosolic drug concentrations. Sci Rep. 2019;9:5850. doi: 10.1038/s41598-019-42051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald EF, Hwang SA, Lambert G, Gomez M, Tarbell A. 1253751; PCB exposure and in vivo CYP1A2 activity among Native Americans. Environ Health Perspect. 2005;113:272–277. doi: 10.1289/ehp.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KT, Lathia C, Frye RF, Schuchter L, Redlinger M, Rosen M, O’Dwyer PJ. Interaction of sorafenib and cytochrome P450 isoenzymes in patients with advanced melanoma: a phase I/II pharmacokinetic interaction study. Cancer Chemother Pharmacol. 2011;68:1111–1118. doi: 10.1007/s00280-011-1585-0. [DOI] [PubMed] [Google Scholar]
- Fontana RJ, Lown KS, Paine MF, Fortlage L, Santella RM, Felton JS, Knize MG, Greenberg A, Watkins PB. Effects of a chargrilled meat diet on expression of CYP3A, CYP1A, and P-glycoprotein levels in healthy volunteers. Gastroenterology. 1999;117:89–98. doi: 10.1016/s0016-5085(99)70554-8. [DOI] [PubMed] [Google Scholar]
- Fritsche E, Schafer C, Calles C, Bernsmann T, Bernshausen T, Wurm M, Hubenthal U, Cline JE, Hajimiragha H, Schroeder P, Klotz LO, Rannug A, Furst P, Hanenberg H, Abel J, Krutmann J. 1885591; lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc Natl Acad Sci USA. 2007;104:8851–8856. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funck-Brentano C, Becquemont L, Lenevu A, Roux A, Jaillon P, Beaune P. Inhibition by omeprazole of proguanil metabolism: mechanism of the interaction in vitro and prediction of in vivo results from the in vitro experiments. J Pharmacol Exp Ther. 1997;280:730–738. [PubMed] [Google Scholar]
- Gallant JE, Thompson M, DeJesus E, Voskuhl GW, Wei X, Zhang H, White K, Cheng A, Quirk E, Martin H. Antiviral activity, safety, and pharmacokinetics of bictegravir as 10-day monotherapy in HIV-1-infected adults. J Acquir Immune Defic Syndr. 2017;75:61–66. doi: 10.1097/QAI.0000000000001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadhar TC, Cohen EEW, Wu K, Janisch L, Geary D, Kocherginsky M, House LK, Ramirez J, Undevia SD, Maitland ML, Fleming GF, Ratain MJ. Two drug interaction studies of sirolimus in combination with sorafenib or sunitinib in patients with advanced malignancies. Clin Cancer Res. 2011;17:1956–1963. doi: 10.1158/1078-0432.CCR-10-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao LC, Huang X, Tan ZR, Fan L, Zhou HH. The effects of sodium ferulate on the pharmacokinetics of bupropion and its active metabolite in healthy men. Eur Rev Med Pharmacol Sci. 2012;16:1192–1196. [PubMed] [Google Scholar]
- Gao L, He Y, Tang J, Yin J, Huang Z, Liu F, Ouyang D, Chen X, Zhang W, Liu Z, Zhou H. PMC3686783; genetic variants of pregnane X receptor (PXR) and CYP2B6 affect the induction of bupropion hydroxylation by sodium ferulate. PLoS One. 2013;8:e62489. doi: 10.1371/journal.pone.0062489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay C, Toulet D, Le Corre P. Pharmacokinetic drug–drug interactions of tyrosine kinase inhibitors: a focus on cytochrome P450, transporters, and acid suppression therapy. Hematol Oncol. 2017;35:259–280. doi: 10.1002/hon.2335. [DOI] [PubMed] [Google Scholar]
- Gerbal-Chaloin S, Daujat M, Pascussi JM, Pichard-Garcia L, Vilarem MJ, Maurel P. Transcriptional regulation of CYP2C9 gene. Role of glucocorticoid receptor and constitutive androstane receptor. J Biol Chem. 2002;277:209–217. doi: 10.1074/jbc.M107228200. [DOI] [PubMed] [Google Scholar]
- Gibbons JA, de Vries M, Krauwinkel W, Ohtsu Y, Noukens J, van der Walt JS, Mol R, Mordenti J, Ouatas T. PMC4580724; pharmacokinetic drug interaction studies with enzalutamide. Clin Pharmacokinet. 2015;54:1057–1069. doi: 10.1007/s40262-015-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillen M, Yang C, Wilson D, Valdez S, Lee C, Kerr B, Shen Z. Evaluation of pharmacokinetic interactions between lesinurad, a new selective urate reabsorption inhibitor, and CYP enzyme substrates sildenafil, amlodipine, tolbutamide, and repaglinide. Clin Pharmacol Drug Dev. 2017;6:363–376. doi: 10.1002/cpdd.324. [DOI] [PubMed] [Google Scholar]
- Girre C, Lucas D, Hispard E, Menez C, Dally S, Menez JF. Assessment of cytochrome P4502E1 induction in alcoholic patients by chlorzoxazone pharmacokinetics. Biochem Pharmacol. 1994;47:1503–1508. doi: 10.1016/0006-2952(94)90524-x. [DOI] [PubMed] [Google Scholar]
- Giuliano C, Lovati E, Funk C, Potthast M, Pietra C. In vitro drug–drug interaction studies with the antiemetic drug netupitant and its major metabolites M1 and M2, involving several human cytochrome P450 isoenzymes. Ann Oncol. 2012;23(suppl 9):ix520. [Google Scholar]
- Glaeser H, Drescher S, Eichelbaum M, Fromm MF. Influence of rifampicin on the expression and function of human intestinal cytochrome P450 enzymes. Br J Clin Pharmacol. 2005;59:199–206. doi: 10.1111/j.1365-2125.2004.02265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MR, Lo MW, Deutsch PJ, Wilson SE, McWilliams EJ, McCrea JB. Phenobarbital minimally alters plasma concentrations of losartan and its active metabolite E-3174. Clin Pharmacol Ther. 1996;59:268–274. doi: 10.1016/S0009-9236(96)80004-X. [DOI] [PubMed] [Google Scholar]
- Gorski JC, Huang SM, Pinto A, Hamman MA, Hilligoss JK, Zaheer NA, Desai M, Miller M, Hall SD. The effect of echinacea (Echinacea purpurea root) on cytochrome P450 activity in vivo. Clin Pharmacol Ther. 2004;75:89–100. doi: 10.1016/j.clpt.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Granfors MT, Backman JT, Neuvonen M, Neuvonen PJ. Ciprofloxacin greatly increases concentrations and hypotensive effect of tizanidine by inhibiting its cytochrome P450 1A2-mediated presystemic metabolism. Clin Pharmacol Ther. 2004;76:598–606. doi: 10.1016/j.clpt.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Granfors MT, Backman JT, Laitila J, Neuvonen PJ. Oral contraceptives containing ethinyl estradiol and gestodene markedly increase plasma concentrations and effects of tizanidine by inhibiting cytochrome P450 1A2. Clin Pharmacol Ther. 2005;78:400–411. doi: 10.1016/j.clpt.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Greiner B, Eichelbaum M, Fritz P, Kreichgauer HP, von Richter O, Zundler J, Kroemer HK. PMC408477; the role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. J Clin Investig. 1999;104:147–153. doi: 10.1172/JCI6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groll AH, Townsend R, Desai A, Azie N, Jones M, Engelhardt M, Schmitt-Hoffman AH, Brüggemann RJM (2017) Drug-drug interactions between triazole antifungal agents used to treat invasive aspergillosis and immunosuppressants metabolized by cytochrome P450 3A4. Transpl Infect Dis 19(5). 10.1111/tid.12751 [DOI] [PubMed]
- Gu H, Dutreix C, Rebello S, Ouatas T, Wang L, Chun DY, Einolf HJ, He H. Simultaneous physiologically based pharmacokinetic (PBPK) modeling of parent and active metabolites to investigate complex CYP3A4 drug–drug interaction potential: a case example of midostaurin. Drug Metab Dispos. 2018;46:109–121. doi: 10.1124/dmd.117.078006. [DOI] [PubMed] [Google Scholar]
- Guo Y, Lucksiri A, Dickinson GL, Vuppalanchi RK, Hilligoss JK, Hall SD. Quantitative prediction of CYP3A4- and CYP3A5-mediated drug interactions. Clin Pharmacol Ther. 2020;107:246–256. doi: 10.1002/cpt.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Hanley MJ, Venkatakrishnan K, Bessudo A, Rasco DW, Sharma S, O’Neil BH, Wang B, Liu G, Ke A, Patel C, Rowland Yeo K, Xia C, Zhang X, Esseltine D, Nemunaitis J. Effects of strong CYP3A inhibition and induction on the pharmacokinetics of ixazomib, an oral proteasome inhibitor: results of drug–drug interaction studies in patients with advanced solid tumors or lymphoma and a physiologically based pharmacokinetic analysis. J Clin Pharmacol. 2018;58:180–192. doi: 10.1002/jcph.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Cui Y, Ang CY. Cytochrome P450 phenotypic ratios for predicting herb–drug interactions in humans. Clin Pharmacol Ther. 2002;72:276–287. doi: 10.1067/mcp.2002.126913. [DOI] [PubMed] [Google Scholar]
- Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Cui Y, Ang CY. Clinical assessment of effects of botanical supplementation on cytochrome P450 phenotypes in the elderly: St John’s wort, garlic oil, Panax ginseng and Ginkgo biloba. Drugs Aging. 2005;22:525–539. doi: 10.2165/00002512-200522060-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyamfi MA, Kocsis MG, He L, Dai G, Mendy AJ, Wan YJ. The role of retinoid X receptor alpha in regulating alcohol metabolism. J Pharmacol Exp Ther. 2006;319:360–368. doi: 10.1124/jpet.106.108175. [DOI] [PubMed] [Google Scholar]
- Ha-Duong NT, Dijols S, Macherey AC, Goldstein JA, Dansette PM, Mansuy D. Ticlopidine as a selective mechanism-based inhibitor of human cytochrome P450 2C19. Biochemistry. 2001;40:12112–12122. doi: 10.1021/bi010254c. [DOI] [PubMed] [Google Scholar]
- Hakkola J, Bernasconi C, Coecke S, Richert L, Andersson TB, Pelkonen O. Cytochrome P450 induction and xeno-sensing receptors pregnane X receptor, constitutive androstane receptor, aryl hydrocarbon receptor and peroxisome proliferator-activated receptor α at the crossroads of toxicokinetics and toxicodynamics. Basic Clin Pharmacol Toxicol. 2018;123(Suppl 5):42–50. doi: 10.1111/bcpt.13004. [DOI] [PubMed] [Google Scholar]
- Hakooz N, Hamdan I. Effects of dietary broccoli on human in vivo caffeine metabolism: a pilot study on a group of Jordanian volunteers. Curr Drug Metab. 2007;8:9–15. doi: 10.2174/138920007779315080. [DOI] [PubMed] [Google Scholar]
- Hamilton M, Wolf JL, Drolet DW, Fettner SH, Rakhit AK, Witt K, Lum BL. The effect of rifampicin, a prototypical CYP3A4 inducer, on erlotinib pharmacokinetics in healthy subjects. Cancer Chemother Pharmacol. 2014;73:613–621. doi: 10.1007/s00280-014-2390-3. [DOI] [PubMed] [Google Scholar]
- Hanaoka T, Yamano Y, Pan G, Hara K, Ichiba M, Zhang J, Zhang S, Liu T, Li L, Takahashi K, Kagawa J, Tsugane S. Cytochrome P450 1B1 mRNA levels in peripheral blood cells and exposure to polycyclic aromatic hydrocarbons in Chinese coke oven workers. Sci Total Environ. 2002;296:27–33. doi: 10.1016/s0048-9697(02)00070-0. [DOI] [PubMed] [Google Scholar]
- Hanley MJ, Cancalon P, Widmer WW, Greenblatt DJ. The effect of grapefruit juice on drug disposition. Expert Opin Drug Metab Toxicol. 2011;7:267–286. doi: 10.1517/17425255.2011.553189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens JP, Podany AT, Scarsi KK, Fletcher CV. Clinical pharmacokinetics and pharmacodynamics of etravirine: an updated review. Clin Pharmacokinet. 2020;59:137–154. doi: 10.1007/s40262-019-00830-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich WD, Hassan HE, Wang H. Insights into CYP2B6-mediated drug–drug interactions. Acta Pharm Sin B. 2016;6:413–425. doi: 10.1016/j.apsb.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimark LD, Wienkers L, Kunze K, Gibaldi M, Eddy AC, Trager WF, O’Reilly RA, Goulart DA. The mechanism of the interaction between amiodarone and warfarin in humans. Clin Pharmacol Ther. 1992;51:398–407. doi: 10.1038/clpt.1992.39. [DOI] [PubMed] [Google Scholar]
- Heinemeyer G, Gramm HJ, Simgen W, Dennhardt R, Roots I. Kinetics of hexobarbital and dipyrone in critical care patients receiving high-dose pentobarbital. Eur J Clin Pharmacol. 1987;32:273–277. doi: 10.1007/BF00607575. [DOI] [PubMed] [Google Scholar]
- Herman D, Locatelli I, Grabnar I, Peternel P, Stegnar M, Lainscak M, Mrhar A, Breskvar K, Dolzan V. The influence of co-treatment with carbamazepine, amiodarone and statins on warfarin metabolism and maintenance dose. Eur J Clin Pharmacol. 2006;62:291–296. doi: 10.1007/s00228-006-0104-4. [DOI] [PubMed] [Google Scholar]
- Hermann R, von Richter O. Clinical evidence of herbal drugs as perpetrators of pharmacokinetic drug interactions. Planta Med. 2012;78:1458–1477. doi: 10.1055/s-0032-1315117. [DOI] [PubMed] [Google Scholar]
- Hesse LM, Venkatakrishnan K, Court MH, von Moltke LL, Duan SX, Shader RI, Greenblatt DJ. CYP2B6 mediates the in vitro hydroxylation of bupropion: potential drug interactions with other antidepressants. Drug Metab Dispos. 2000;28:1176–1183. [PubMed] [Google Scholar]
- Higashi E, Fukami T, Itoh M, Kyo S, Inoue M, Yokoi T, Nakajima M. Human CYP2A6 is induced by estrogen via estrogen receptor. Drug Metab Dispos. 2007;35:1935–1941. doi: 10.1124/dmd.107.016568. [DOI] [PubMed] [Google Scholar]
- Hoffmann MF, Preissner SC, Nickel J, Dunkel M, Preissner R, Preissner S. The transformer database: biotransformation of xenobiotics. Nucleic Acids Res. 2014;42:1113. doi: 10.1093/nar/gkt1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman J, Sorf A, Vagiannis D, Sucha S, Novotna E, Kammerer S, Küpper J, Ceckova M, Staud F. Interactions of alectinib with human ATP-binding cassette drug efflux transporters and cytochrome P450 biotransformation enzymes: effect on pharmacokinetic multidrug resistance. Drug Metab Dispos. 2019;47:699–709. doi: 10.1124/dmd.119.086975. [DOI] [PubMed] [Google Scholar]
- Hong Y, Chia YMF, Yeo RH, Venkatesan G, Koh SK, Chai CLL, Zhou L, Kojodjojo P, Chan ECY. Inactivation of human cytochrome P450 3A4 and 3A5 by dronedarone and N-desbutyl dronedarone. Mol Pharmacol. 2016;89:1–13. doi: 10.1124/mol.115.100891. [DOI] [PubMed] [Google Scholar]
- Honkakoski P, Zelko I, Sueyoshi T, Negishi M. The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol Cell Biol. 1998;18:5652–5658. doi: 10.1128/mcb.18.10.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn EP, Tucker MA, Lambert G, Silverman D, Zametkin D, Sinha R, Hartge T, Landi MT, Caporaso NE. A study of gender-based cytochrome P4501A2 variability: a possible mechanism for the male excess of bladder cancer. Cancer Epidemiol Biomark Prev. 1995;4:529–533. [PubMed] [Google Scholar]
- Hossain MA, Tran T, Chen T, Mikus G, Greenblatt DJ. Inhibition of human cytochromes P450 in vitro by ritonavir and cobicistat. J Pharm Pharmacol. 2017;69:1786–1793. doi: 10.1111/jphp.12820. [DOI] [PubMed] [Google Scholar]
- Hsu A, Granneman GR, Witt G, Locke C, Denissen J, Molla A, Valdes J, Smith J, Erdman K, Lyons N, Niu P, Decourt JP, Fourtillan JB, Girault J, Leonard JM. 163822; multiple-dose pharmacokinetics of ritonavir in human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 1997;41:898–905. doi: 10.1128/aac.41.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu A, Granneman GR, Bertz RJ. Ritonavir. Clinical pharmacokinetics and interactions with other anti-HIV agents. Clin Pharmacokinet. 1998;35:275–291. doi: 10.2165/00003088-199835040-00002. [DOI] [PubMed] [Google Scholar]
- Hu SW, Chen CC, Kuo CY, Lin WH, Lin P. Increased cytochrome P4501B1 gene expression in peripheral leukocytes of municipal waste incinerator workers. Toxicol Lett. 2006;160:112–120. doi: 10.1016/j.toxlet.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Hu X, Lan T, Dai D, Xu R, Yuan L, Zhou Q, Li Y, Cai J, Hu G. Evaluation of 24 CYP2D6 variants on the metabolism of nebivolol in vitro. Drug Metab Dispos. 2016;44:1828–1831. doi: 10.1124/dmd.116.071811. [DOI] [PubMed] [Google Scholar]
- Hukkanen J. Induction of CYP enzymes: a view on human in vivo findings. Expert Rev Clin Pharmacol. 2012;5:569–585. doi: 10.1586/ecp.12.39. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Pelkonen O, Hakkola J, Raunio H. Expression and regulation of xenobiotic-metabolizing cytochrome P450 (CYP) enzymes in human lung. Crit Rev Toxicol. 2002;32:391–411. doi: 10.1080/20024091064273. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Vaisanen T, Lassila A, Piipari R, Anttila S, Pelkonen O, Raunio H, Hakkola J. Regulation of CYP3A5 by glucocorticoids and cigarette smoke in human lung-derived cells. J Pharmacol Exp Ther. 2003;304:745–752. doi: 10.1124/jpet.102.038208. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- Hunt SN, Jusko WJ, Yurchak AM. Effect of smoking on theophylline disposition. Clin Pharmacol Ther. 1976;19:546–551. doi: 10.1002/cpt1976195part1546. [DOI] [PubMed] [Google Scholar]
- Hussaarts KGAM, Veerman GDM, Jansman FGA, van Gelder T, Mathijssen RHJ, van Leeuwen RWF. Clinically relevant drug interactions with multikinase inhibitors: a review. Ther Adv Med Oncol. 2019;11:1758835918818347. doi: 10.1177/1758835918818347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutzler JM, Balogh LM, Zientek M, Kumar V, Tracy TS. Mechanism-based inactivation of cytochrome P450 2C9 by tienilic acid and (+/−)-suprofen: a comparison of kinetics and probe substrate selection. Drug Metab Dispos. 2009;37:59–65. doi: 10.1124/dmd.108.023358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huuskonen P, Storvik M, Reinisalo M, Honkakoski P, Rysa J, Hakkola J, Pasanen M. Microarray analysis of the global alterations in the gene expression in the placentas from cigarette-smoking mothers. Clin Pharmacol Ther. 2008;83:542–550. doi: 10.1038/sj.clpt.6100376. [DOI] [PubMed] [Google Scholar]
- Iga K. Dynamic and static simulations of fluvoxamine-perpetrated drug–drug interactions using multiple cytochrome P450 inhibition modeling, and determination of perpetrator-specific CYP isoform inhibition constants and fractional CYP isoform contributions to victim clearance. J Pharm Sci. 2016;105:1307–1317. doi: 10.1016/j.xphs.2015.11.044. [DOI] [PubMed] [Google Scholar]
- Indra R, Pompach P, Martínek V, Takácsová P, Vavrová K, Heger Z, Adam V, Eckschlager T, Kopečková K, Arlt VM, Stiborová M. Identification of human enzymes oxidizing the anti-thyroid-cancer drug vandetanib and explanation of the high efficiency of cytochrome P450 3A4 in its oxidation. Int J Mol Sci. 2019;20(14):3392. doi: 10.3390/ijms20143392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Nakajima M, Higashi E, Yoshida R, Nagata K, Yamazoe Y, Yokoi T. Induction of human CYP2A6 is mediated by the pregnane X receptor with peroxisome proliferator-activated receptor-gamma coactivator 1alpha. J Pharmacol Exp Ther. 2006;319:693–702. doi: 10.1124/jpet.106.107573. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: an updated systematic review. Drugs. 2009;69:1777–1798. doi: 10.2165/11317010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Jaakkola T, Backman JT, Neuvonen M, Laitila J, Neuvonen PJ. Effect of rifampicin on the pharmacokinetics of pioglitazone. Br J Clin Pharmacol. 2006;61:70–78. doi: 10.1111/j.1365-2125.2005.02515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KD, Durandis R, Vergne MJ. Role of cytochrome P450 enzymes in the metabolic activation of tyrosine kinase inhibitors. Int J Mol Sci. 2018;19(8):2367. doi: 10.3390/ijms19082367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager KW. Aldrin, Dieldrin, Endrin and Telodrin: an epidemiological and toxicological study of long term occupational exposure. Amsterdam: Elsevier; 1970. [Google Scholar]
- James AJ, Smith CC, Litzow M, Perl AE, Altman JK, Shepard D, Kadokura T, Souda K, Patton M, Lu Z, Liu C, Moy S, Levis MJ, Bahceci E. Pharmacokinetic profile of gilteritinib: a novel FLT-3 tyrosine kinase inhibitor. Clin Pharmacokinet. 2020;59(10):1273–1290. doi: 10.1007/s40262-020-00888-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jao JY, Jusko WJ, Cohen JL. Phenobarbital effects on cyclophosphamide pharmacokinetics in man. Cancer Res. 1972;32:2761–2764. [PubMed] [Google Scholar]
- Jeong S, Nguyen PD, Desta Z. Comprehensive in vitro analysis of voriconazole inhibition of eight cytochrome P450 (CYP) enzymes: major effect on CYPs 2B6, 2C9, 2C19, and 3A. Antimicrob Agents Chemother. 2009;53:541–551. doi: 10.1128/AAC.01123-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S, Woo MM, Flockhart DA, Desta Z. Inhibition of drug metabolizing cytochrome P450s by the aromatase inhibitor drug letrozole and its major oxidative metabolite 4,4′-methanol-bisbenzonitrile in vitro. Cancer Chemother Pharmacol. 2009;64:867–875. doi: 10.1007/s00280-009-0935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji P, Damle B, Xie J, Unger SE, Grasela DM, Kaul S. Pharmacokinetic interaction between efavirenz and carbamazepine after multiple-dose administration in healthy subjects. J Clin Pharmacol. 2008;48:948–956. doi: 10.1177/0091270008319792. [DOI] [PubMed] [Google Scholar]
- Jiang X, Williams KM, Liauw WS, Ammit AJ, Roufogalis BD, Duke CC, Day RO, McLachlan AJ. Effect of St John’s wort and ginseng on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2004;57:592–599. doi: 10.1111/j.1365-2125.2003.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Blair EY, McLachlan AJ. Investigation of the effects of herbal medicines on warfarin response in healthy subjects: a population pharmacokinetic-pharmacodynamic modeling approach. J Clin Pharmacol. 2006;46:1370–1378. doi: 10.1177/0091270006292124. [DOI] [PubMed] [Google Scholar]
- Johansson S, Read J, Oliver S, Steinberg M, Li Y, Lisbon E, Mathews D, Leese PT, Martin P. Pharmacokinetic evaluations of the co-administrations of vandetanib and metformin, digoxin, midazolam, omeprazole or ranitidine. Clin Pharmacokinet. 2014;53:837–847. doi: 10.1007/s40262-014-0161-2. [DOI] [PubMed] [Google Scholar]
- Johnson FM, Agrawal S, Burris H, Rosen L, Dhillon N, Hong D, Blackwood-Chirchir A, Luo FR, Sy O, Kaul S, Chiappori AA. Phase 1 pharmacokinetic and drug-interaction study of dasatinib in patients with advanced solid tumors. Cancer. 2010;116:1582–1591. doi: 10.1002/cncr.24927. [DOI] [PubMed] [Google Scholar]
- Jones JP, Joswig-Jones CA, Hebner M, Chu Y, Koop DR. The effects of nitrogen-heme-iron coordination on substrate affinities for cytochrome P450 2E1. Chem Biol Interact. 2011;193:50–56. doi: 10.1016/j.cbi.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jushchyshyn MI, Wahlstrom JL, Hollenberg PF, Wienkers LC. Mechanism of inactivation of human cytochrome P450 2B6 by phencyclidine. Drug Metab Dispos. 2006;34:1523–1529. doi: 10.1124/dmd.106.010579. [DOI] [PubMed] [Google Scholar]
- Justesen US, Klitgaard NA, Brosen K, Pedersen C. Pharmacokinetic interaction between amprenavir and delavirdine after multiple-dose administration in healthy volunteers. Br J Clin Pharmacol. 2003;55:100–106. doi: 10.1046/j.1365-2125.2003.01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahma H, Filppula AM, Launiainen T, Viinamäki J, Neuvonen M, Evangelista EA, Totah RA, Backman JT. Critical differences between enzyme sources in sensitivity to detect time-dependent inactivation of CYP2C8. Drug Metab Dispos. 2019;47:436–443. doi: 10.1124/dmd.118.085498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuda TN, Van Solingen-Ristea RM, Onkelinx J, Stevens T, Aharchi F, De Smedt G, Peeters M, Leopold L, Hoetelmans RM. The effect of single- and multiple-dose etravirine on a drug cocktail of representative cytochrome P450 probes and digoxin in healthy subjects. J Clin Pharmacol. 2014;54:422–431. doi: 10.1002/jcph.214. [DOI] [PubMed] [Google Scholar]
- Kanamitsu S, Ito K, Green CE, Tyson CA, Shimada N, Sugiyama Y. Prediction of in vivo interaction between triazolam and erythromycin based on in vitro studies using human liver microsomes and recombinant human CYP3A4. Pharm Res. 2000;17:419–426. doi: 10.1023/a:1007572803027. [DOI] [PubMed] [Google Scholar]
- Kandel CE, Walmsley SL. Dolutegravir—a review of the pharmacology, efficacy, and safety in the treatment of HIV. Drug Des Dev Ther. 2015;9:3547–3555. doi: 10.2147/DDDT.S84850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappas A, Alvares AP, Anderson KE, Pantuck EJ, Pantuck CB, Chang R, Conney AH. Effect of charcoal-broiled beef on antipyrine and theophylline metabolism. Clin Pharmacol Ther. 1978;23:445–450. doi: 10.1002/cpt1978234445. [DOI] [PubMed] [Google Scholar]
- Karjalainen MJ, Neuvonen PJ, Backman JT. In vitro inhibition of CYP1A2 by model inhibitors, anti-inflammatory analgesics and female sex steroids: predictability of in vivo interactions. Basic Clin Pharmacol Toxicol. 2008;103:157–165. doi: 10.1111/j.1742-7843.2008.00252.x. [DOI] [PubMed] [Google Scholar]
- Kashuba AD, Tierney C, Downey GF, Acosta EP, Vergis EN, Klingman K, Mellors JW, Eshleman SH, Scott TR, Collier AC. Combining fosamprenavir with lopinavir/ritonavir substantially reduces amprenavir and lopinavir exposure: aCTG protocol A5143 results. AIDS. 2005;19:145–152. doi: 10.1097/00002030-200501280-00006. [DOI] [PubMed] [Google Scholar]
- Katiyar SK, Matsui MS, Mukhtar H. Ultraviolet-B exposure of human skin induces cytochromes P450 1A1 and 1B1. J Investig Dermatol. 2000;114:328–333. doi: 10.1046/j.1523-1747.2000.00876.x. [DOI] [PubMed] [Google Scholar]
- Kato H. Computational prediction of cytochrome P450 inhibition and induction. Drug Metab Pharmacokinet. 2020;35:30–44. doi: 10.1016/j.dmpk.2019.11.006. [DOI] [PubMed] [Google Scholar]
- Kawajiri K, Fujii-Kuriyama Y. The aryl hydrocarbon receptor: a multifunctional chemical sensor for host defense and homeostatic maintenance. Exp Anim. 2017;66:75–89. doi: 10.1538/expanim.16-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg JJ, Paine MF, McCune JS, Oberlies NH, Cech NB. Selection and characterization of botanical natural products for research studies: a NaPDI center recommended approach. Nat Prod Rep. 2019;36:1196–1221. doi: 10.1039/c8np00065d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny JR, Mukadam S, Zhang C, Tay S, Collins C, Galetin A, Khojasteh SC. Drug–drug interaction potential of marketed oncology drugs: in vitro assessment of time-dependent cytochrome P450 inhibition, reactive metabolite formation and drug–drug interaction prediction. Pharm Res. 2012;29:1960–1976. doi: 10.1007/s11095-012-0724-6. [DOI] [PubMed] [Google Scholar]
- Ketter TA, Jenkins JB, Schroeder DH, Pazzaglia PJ, Marangell LB, George MS, Callahan AM, Hinton ML, Chao J, Post RM. Carbamazepine but not valproate induces bupropion metabolism. J Clin Psychopharmacol. 1995;15:327–333. doi: 10.1097/00004714-199510000-00004. [DOI] [PubMed] [Google Scholar]
- Khalilieh SG, Yee KL, Sanchez RI, Fan L, Anderson MS, Sura M, Laethem T, Rasmussen S, van Bortel L, van Lancker G, Iwamoto M. Doravirine and the potential for cyp3a-mediated drug-drug interactions. Antimicrob Agents Chemother. 2019;63(5):e02016–e02018. doi: 10.1128/AAC.02016-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharasch ED, Mitchell D, Coles R, Blanco R (2008) Rapid clinical induction of hepatic cytochrome P4502B6 (CYP2B6) activity by ritonavir. Antimicrob Agents Chemother [DOI] [PMC free article] [PubMed]
- Kharasch ED, Whittington D, Ensign D, Hoffer C, Bedynek PS, Campbell S, Stubbert K, Crafford A, London A, Kim T. Mechanism of efavirenz influence on methadone pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2012;91(4):673–684. doi: 10.1038/clpt.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Frey RJ, Epplen K, Foruhari F. Interaction between warfarin and nafcillin: case report and review of the literature. Pharmacotherapy. 2007;27:1467–1470. doi: 10.1592/phco.27.10.1467. [DOI] [PubMed] [Google Scholar]
- King CA, Babcock KM, Godios RJ, King BS. PMC6243422; significant drug–drug interaction between warfarin and nafcillin. Ther Adv Drug Saf. 2018;9:667–671. doi: 10.1177/2042098618796186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BJ, Collier AC, Kharasch ED, Whittington D, Thummel KE, Unadkat JD. Complex drug interactions of HIV protease inhibitors 1: inactivation, induction, and inhibition of cytochrome P450 3A by ritonavir or nelfinavir. Drug Metab Dispos. 2011;39:1070–1078. doi: 10.1124/dmd.110.037523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BJ, Collier AC, Kharasch ED, Dixit V, Desai P, Whittington D, Thummel KE, Unadkat JD. Complex drug interactions of HIV protease inhibitors 2: in vivo induction and in vitro-to-in vivo correlation of induction of cytochrome P450 1A2, 2B6, and 2C9 by ritonavir or nelfinavir. Drug Metab Dispos. 2011;39:2329–2337. doi: 10.1124/dmd.111.038646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterström RH, Perlmann T, Lehmann JM. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- Klosterskov Jensen P, Saano V, Haring P, Svenstrup B, Menge GP. Possible interaction between oxcarbazepine and an oral contraceptive. Epilepsia. 1992;33:1149–1152. doi: 10.1111/j.1528-1157.1992.tb01773.x. [DOI] [PubMed] [Google Scholar]
- Ko JW, Desta Z, Soukhova NV, Tracy T, Flockhart DA. In vitro inhibition of the cytochrome P450 (CYP450) system by the antiplatelet drug ticlopidine: potent effect on CYP2C19 and CYP2D6. Br J Clin Pharmacol. 2000;49:343–351. doi: 10.1046/j.1365-2125.2000.00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Hashimoto M, Honkakoski P, Negishi M. Regulation of gene expression by CAR: an update. Arch Toxicol. 2015;89:1045–1055. doi: 10.1007/s00204-015-1522-9. [DOI] [PubMed] [Google Scholar]
- Köhle C, Bock KW. Coordinate regulation of Phase I and II xenobiotic metabolisms by the Ah receptor and Nrf2. Biochem Pharmacol. 2007;73:1853–1862. doi: 10.1016/j.bcp.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Kolars JC, Schmiedlin-Ren P, Schuetz JD, Fang C, Watkins PB. PMC443248; Identification of rifampin-inducible P450IIIA4 (CYP3A4) in human small bowel enterocytes. J Clin Investig. 1992;90:1871–1878. doi: 10.1172/JCI116064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong LM, Xu SY, Hu HH, Zhou H, Jiang HD, Yu LS, Zeng S. Identification of CYP2C19 inhibitors from phytochemicals using the recombinant human enzyme model. Pharmazie. 2014;69:362–366. [PubMed] [Google Scholar]
- Korzekwa K, Tweedie D, Argikar UA, Whitcher-Johnstone A, Bell L, Bickford S, Nagar S. A numerical method for analysis of in vitro time-dependent inhibition data. Part 2. Application to experimental data. Drug Metab Dispos. 2014;42:1587–1595. doi: 10.1124/dmd.114.058297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauwinkel W, Dickinson J, Schaddelee M, Meijer J, Tretter R, van de Wetering J, Strabach G, van Gelderen M. The effect of mirabegron, a potent and selective β3-adrenoceptor agonist, on the pharmacokinetics of CYP2D6 substrates desipramine and metoprolol. Eur J Drug Metab Pharmacokinet. 2014;39:43–52. doi: 10.1007/s13318-013-0133-1. [DOI] [PubMed] [Google Scholar]
- Krishna G, Moton A, Ma L, Savant I, Martinho M, Seiberling M, McLeod J. Effects of oral posaconazole on the pharmacokinetic properties of oral and intravenous midazolam: a phase I, randomized, open-label, crossover study in healthy volunteers. Clin Ther. 2009;31:286–298. doi: 10.1016/j.clinthera.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Kunze KL, Wienkers LC, Thummel KE, Trager WF. Warfarin-fluconazole. I. Inhibition of the human cytochrome P450-dependent metabolism of warfarin by fluconazole: in vitro studies. Drug Metab Dispos. 1996;24:414–421. [PubMed] [Google Scholar]
- Kusawake T, den Adel M, Groenendaal-van de Meent D, Garcia-Hernandez A, Takada A, Kato K, Ohtsu Y, Katashima M. PMC5702381; pharmacokinetic evaluation of the interactions of amenamevir (ASP2151) with ketoconazole, rifampicin, midazolam, and warfarin in healthy adults. Adv Ther. 2017;34:2466–2480. doi: 10.1007/s12325-017-0634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers DR, Claes K, Evenepoel P, Maes B, Coosemans W, Pirenne J, Vanrenterghem Y. Time-related clinical determinants of long-term tacrolimus pharmacokinetics in combination therapy with mycophenolic acid and corticosteroids: a prospective study in one hundred de novo renal transplant recipients. Clin Pharmacokinet. 2004;43:741–762. doi: 10.2165/00003088-200443110-00005. [DOI] [PubMed] [Google Scholar]
- Kyerematen GA, Morgan M, Warner G, Martin LF, Vesell ES. Metabolism of nicotine by hepatocytes. Biochem Pharmacol. 1990;40:1747–1756. doi: 10.1016/0006-2952(90)90351-k. [DOI] [PubMed] [Google Scholar]
- Lai ML, Lin TS, Huang JD. Effect of single- and multiple-dose carbamazepine on the pharmacokinetics of diphenylhydantoin. Eur J Clin Pharmacol. 1992;43:201–203. doi: 10.1007/BF01740672. [DOI] [PubMed] [Google Scholar]
- Lamba JK, Lin YS, Schuetz EG, Thummel KE. Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev. 2002;54:1271–1294. doi: 10.1016/s0169-409x(02)00066-2. [DOI] [PubMed] [Google Scholar]
- Lambert GH, Schoeller DA, Humphrey HE, Kotake AN, Lietz H, Campbell M, Kalow W, Spielberg SP, Budd M. 1567793; the caffeine breath test and caffeine urinary metabolite ratios in the Michigan cohort exposed to polybrominated biphenyls: a preliminary study. Environ Health Perspect. 1990;89:175–181. doi: 10.1289/ehp.9089175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe JW, Stepaniants SB, Mao M, Radich JP, Dai H, Linsley PS, Friend SH, Potter JD. Signatures of environmental exposures using peripheral leukocyte gene expression: tobacco smoke. Cancer Epidemiol Biomark Prev. 2004;13:445–453. [PubMed] [Google Scholar]
- Lämsä V, Levonen A, Leinonen H, Ylä-Herttuala S, Yamamoto M, Hakkola J. Cytochrome P450 2A5 constitutive expression and induction by heavy metals is dependent on redox-sensitive transcription factor Nrf2 in liver. Chem Res Toxicol. 2010;23:977–985. doi: 10.1021/tx100084c. [DOI] [PubMed] [Google Scholar]
- Landay RA, Gonzalez MA, Taylor JC. Effect of phenobarbital on theophylline disposition. J Allergy Clin Immunol. 1978;62:27–29. doi: 10.1016/0091-6749(78)90068-4. [DOI] [PubMed] [Google Scholar]
- Lang CC, Jamal SK, Mohamed Z, Mustafa MR, Mustafa AM, Lee TC. 1884262; evidence of an interaction between nifedipine and nafcillin in humans. Br J Clin Pharmacol. 2003;55:588–590. doi: 10.1046/j.1365-2125.2003.01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence SK, Nguyen D, Bowen C, Richards-Peterson L, Skordos KW. The metabolic drug–drug interaction profile of Dabrafenib: in vitro investigations and quantitative extrapolation of the P450-mediated DDI risk. Drug Metab Dispos. 2014;42:1180–1190. doi: 10.1124/dmd.114.057778. [DOI] [PubMed] [Google Scholar]
- Lecamwasam DS, Franklin C, Turner P. Effect of phenobarbitone on hepatic drug-metabolizing enzymes and urinary d-glucaric acid excretion in man. Br J Clin Pharmacol. 1975;2:257–262. doi: 10.1111/j.1365-2125.1975.tb01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JSF, Calmy A, Andrieux-Meyer I, Ford N. Review of the safety, efficacy, and pharmacokinetics of elvitegravir with an emphasis on resource-limited settings. HIV AIDS (Auckl) 2012;4:5–15. doi: 10.2147/HIV.S20993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre J, Poirier L, Poirier P, Turgeon J, Lacourciere Y. The influence of CYP2D6 phenotype on the clinical response of nebivolol in patients with essential hypertension. Br J Clin Pharmacol. 2007;63:575–582. doi: 10.1111/j.1365-2125.2006.02796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Investig. 1998;102:1016–1023. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei HP, Yu XY, Xie HT, Li HH, Fan L, Dai LL, Chen Y, Zhou HH. Effect of St. John’s wort supplementation on the pharmacokinetics of bupropion in healthy male Chinese volunteers. Xenobiotica. 2010;40:275–281. doi: 10.3109/00498250903509383. [DOI] [PubMed] [Google Scholar]
- Li X, He Y, Ruiz CH, Koenig M, Cameron MD, Vojkovsky T. Characterization of dasatinib and its structural analogs as CYP3A4 mechanism-based inactivators and the proposed bioactivation pathways. Drug Metab Dispos. 2009;37:1242–1250. doi: 10.1124/dmd.108.025932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AC, Yu E, Ring SC, Chovan JP. Structural identification of imatinib cyanide adducts by mass spectrometry and elucidation of bioactivation pathway. Rapid Commun Mass Spectrom. 2014;28:123–134. doi: 10.1002/rcm.6758. [DOI] [PubMed] [Google Scholar]
- Liangpunsakul S, Kolwankar D, Pinto A, Gorski JC, Hall SD, Chalasani N. Activity of CYP2E1 and CYP3A enzymes in adults with moderate alcohol consumption: a comparison with nonalcoholics. Hepatology. 2005;41:1144–1150. doi: 10.1002/hep.20673. [DOI] [PubMed] [Google Scholar]
- Lim ML, Min SS, Eron JJ, Bertz RJ, Robinson M, Gaedigk A, Kashuba AD. Coadministration of lopinavir/ritonavir and phenytoin results in two-way drug interaction through cytochrome P-450 induction. J Acquir Immune Defic Syndr. 2004;36:1034–1040. doi: 10.1097/00126334-200408150-00006. [DOI] [PubMed] [Google Scholar]
- Lin D, Kostov R, Huang JT-, Henderson CJ, Wolf CR. Novel pathways of ponatinib disposition catalyzed by CYP1A1 involving generation of potentially toxic metabolites. J Pharmacol Exp Ther. 2017;363:12–19. doi: 10.1124/jpet.117.243246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loboz KK, Gross AS, Williams KM, Liauw WS, Day RO, Blievernicht JK, Zanger UM, McLachlan AJ. Cytochrome P450 2B6 activity as measured by bupropion hydroxylation: effect of induction by rifampin and ethnicity. Clin Pharmacol Ther. 2006;80:75–84. doi: 10.1016/j.clpt.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Lolodi O, Wang Y, Wright WC, Chen T. Differential regulation of CYP3A4 and CYP3A5 and its implication in drug discovery. Curr Drug Metab. 2017;18:1095–1105. doi: 10.2174/1389200218666170531112038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Cortes L, Ruiz-Valderas R, Viciana P, Alarcon-Gonzalez A, Gomez-Mateos J, Leon-Jimenez E, Sarasanacenta M, Lopez-Pua Y, Pachon J. Pharmacokinetic interactions between efavirenz and rifampicin in HIV-infected patients with tuberculosis. Clin Pharmacokinet. 2002;41:681–690. doi: 10.2165/00003088-200241090-00004. [DOI] [PubMed] [Google Scholar]
- Lu C, Di L. In vitro and in vivo methods to assess pharmacokinetic drug–drug interactions in drug discovery and development. Biopharm Drug Dispos. 2020;41:3–31. doi: 10.1002/bdd.2212. [DOI] [PubMed] [Google Scholar]
- Lucas RA, Gilfillan DJ, Bergstrom RF. A pharmacokinetic interaction between carbamazepine and olanzapine: observations on possible mechanism. Eur J Clin Pharmacol. 1998;54:639–643. doi: 10.1007/s002280050527. [DOI] [PubMed] [Google Scholar]
- Luceri F, Fattori S, Luceri C, Zorn M, Mannaioni P, Messeri G. Gas chromatography-mass spectrometry measurement of 6beta-OH-cortisol/cortisol ratio in human urine: a specific marker of enzymatic induction. Clin Chem Lab Med. 2001;39:1234–1239. doi: 10.1515/CCLM.2001.198. [DOI] [PubMed] [Google Scholar]
- Lucier GW, Nelson KG, Everson RB, Wong TK, Philpot RM, Tiernan T, Taylor M, Sunahara GI. 1474460; placental markers of human exposure to polychlorinated biphenyls and polychlorinated dibenzofurans. Environ Health Perspect. 1987;76:79–87. doi: 10.1289/ehp.877679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz JD, Isoherranen N. In vitro-to-in vivo predictions of drug–drug interactions involving multiple reversible inhibitors. Expert Opin Drug Metab Toxicol. 2012;8:449–466. doi: 10.1517/17425255.2012.667801. [DOI] [PubMed] [Google Scholar]
- Lutz JD, Kirby BJ, Wang L, Song Q, Ling J, Massetto B, Worth A, Kearney BP, Mathias A. PMC6282692; cytochrome P450 3A induction predicts P-glycoprotein induction; part 2: prediction of decreased substrate exposure after rifabutin or carbamazepine. Clin Pharmacol Ther. 2018;104:1191–1198. doi: 10.1002/cpt.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglich JM, Stoltz CM, Goodwin B, Hawkins-Brown D, Moore JT, Kliewer SA. Nuclear pregnane X receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol. 2002;62:638–646. doi: 10.1124/mol.62.3.638. [DOI] [PubMed] [Google Scholar]
- Malhi V, Colburn D, Williams SJ, Hop CECA, Dresser MJ, Chandra P, Graham RA. A clinical drug–drug interaction study to evaluate the effect of a proton-pump inhibitor, a combined P-glycoprotein/cytochrome 450 enzyme (CYP)3A4 inhibitor, and a CYP2C9 inhibitor on the pharmacokinetics of vismodegib. Cancer Chemother Pharmacol. 2016;78:41–49. doi: 10.1007/s00280-016-3020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikandan P, Nagini S. Cytochrome P450 structure, function and clinical significance: a review. Curr Drug Targets. 2018;19:38–54. doi: 10.2174/1389450118666170125144557. [DOI] [PubMed] [Google Scholar]
- Manosuthi W, Sukasem C, Lueangniyomkul A, Mankatitham W, Thongyen S, Nilkamhang S, Manosuthi S, Sungkanuparph S. Impact of pharmacogenetic markers of CYP2B6, clinical factors, and drug–drug interaction on efavirenz concentrations in HIV/tuberculosis-coinfected patients. Antimicrob Agents Chemother. 2013;57:1019–1024. doi: 10.1128/AAC.02023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Tay S, Khojasteh CS, Chen Y, Hop CECA, Kenny JR. Evaluation of time dependent inhibition assays for marketed oncology drugs: comparison of human hepatocytes and liver microsomes in the presence and absence of human plasma. Pharm Res. 2016;33:1204–1219. doi: 10.1007/s11095-016-1865-9. [DOI] [PubMed] [Google Scholar]
- Markowitz JS, Donovan JL, Lindsay DeVane C, Sipkes L, Chavin KD. Multiple-dose administration of Ginkgo biloba did not affect cytochrome P-450 2D6 or 3A4 activity in normal volunteers. J Clin Psychopharmacol. 2003;23:576–581. doi: 10.1097/01.jcp.0000095340.32154.c6. [DOI] [PubMed] [Google Scholar]
- Marschall HU, Wagner M, Zollner G, Fickert P, Diczfalusy U, Gumhold J, Silbert D, Fuchsbichler A, Benthin L, Grundstrom R, Gustafsson U, Sahlin S, Einarsson C, Trauner M. Complementary stimulation of hepatobiliary transport and detoxification systems by rifampicin and ursodeoxycholic acid in humans. Gastroenterology. 2005;129:476–485. doi: 10.1016/j.gastro.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Martin P, Oliver S, Robertson J, Kennedy S, Read J, Duvauchelle T. Pharmacokinetic drug interactions with vandetanib during coadministration with rifampicin or itraconazole. Drugs R D. 2011;11:37–51. doi: 10.2165/11586980-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzolini C, Rajoli R, Battegay M, Elzi L, Back D, Siccardi M. Physiologically based pharmacokinetic modeling to predict drug–drug interactions with efavirenz involving simultaneous inducing and inhibitory effects on cytochromes. Clin Pharmacokinet. 2017;56:409–420. doi: 10.1007/s40262-016-0447-7. [DOI] [PubMed] [Google Scholar]
- Matsunaga T, Maruyama M, Harada E, Katsuyama Y, Sugihara N, Ise H, Negishi N, Ikeda U, Ohmori S. Expression and induction of CYP3As in human fetal hepatocytes. Biochem Biophys Res Commun. 2004;318:428–434. doi: 10.1016/j.bbrc.2004.04.041. [DOI] [PubMed] [Google Scholar]
- Mazur W. Phytoestrogen content in foods. Baillieres Clin Endocrinol Metab. 1998;12:729–742. doi: 10.1016/s0950-351x(98)80013-x. [DOI] [PubMed] [Google Scholar]
- Mazze RI, Woodruff RE, Heerdt ME. Isoniazid-induced enflurane defluorination in humans. Anesthesiology. 1982;57:5–8. doi: 10.1097/00000542-198207000-00002. [DOI] [PubMed] [Google Scholar]
- McAllister WA, Thompson PJ, Al-Habet S, Rogers HJ. 1547305; rifampicin reduces effectiveness and bioavailability of prednisolone. Br Med J (Clin Res Ed) 1983;286:923–925. doi: 10.1136/bmj.286.6369.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick A, Swaisland H, Reddy VP, Learoyd M, Scarfe G. In vitro evaluation of the inhibition and induction potential of olaparib, a potent poly(ADP-ribose) polymerase inhibitor, on cytochrome P450. Xenobiotica. 2018;48:555–564. doi: 10.1080/00498254.2017.1346332. [DOI] [PubMed] [Google Scholar]
- McCune JS, Hawke RL, LeCluyse EL, Gillenwater HH, Hamilton G, Ritchie J, Lindley C. In vivo and in vitro induction of human cytochrome P4503A4 by dexamethasone. Clin Pharmacol Ther. 2000;68:356–366. doi: 10.1067/mcp.2000.110215. [DOI] [PubMed] [Google Scholar]
- McDonagh EM, Lau JL, Alvarellos ML, Altman RB, Klein TE. PharmGKB summary: efavirenz pathway, pharmacokinetics. Pharmacogenet Genom. 2015;25:363–376. doi: 10.1097/FPC.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald MG, Au NT, Rettie AE. P450-based drug–drug interactions of amiodarone and its metabolites: diversity of inhibitory mechanisms. Drug Metab Dispos. 2015;43:1661–1669. doi: 10.1124/dmd.115.065623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell WM, Scheiman JM, Traber PG. Induction of cytochrome P450IA genes (CYP1A) by omeprazole in the human alimentary tract. Gastroenterology. 1992;103:1509–1516. doi: 10.1016/0016-5085(92)91171-y. [DOI] [PubMed] [Google Scholar]
- McLemore TL, Adelberg S, Liu MC, McMahon NA, Yu SJ, Hubbard WC, Czerwinski M, Wood TG, Storeng R, Lubet RA. Expression of CYP1A1 gene in patients with lung cancer: evidence for cigarette smoke-induced gene expression in normal lung tissue and for altered gene regulation in primary pulmonary carcinomas. J Natl Cancer Inst. 1990;82:1333–1339. doi: 10.1093/jnci/82.16.1333. [DOI] [PubMed] [Google Scholar]
- McNeilly AD, Woods JA, Ibbotson SH, Wolf CR, Smith G. Characterization of a human keratinocyte HaCaT cell line model to study the regulation of CYP2S1. Drug Metab Dispos. 2012;40:283–289. doi: 10.1124/dmd.111.042085. [DOI] [PubMed] [Google Scholar]
- Mendoza-Cantu A, Castorena-Torres F, Bermudez de Leon M, Cisneros B, Lopez-Carrillo L, Rojas-Garcia A, Aguilar-Salinas A, Manno M, Albores A. 1440770; occupational toluene exposure induces cytochrome P450 2E1 mRNA expression in peripheral lymphocytes. Environ Health Perspect. 2006;114:494–499. doi: 10.1289/ehp.8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merk HF, Mukhtar H, Kaufmann I, Das M, Bickers DR. Human hair follicle benzo[a]pyrene and benzo[a]pyrene 7,8-diol metabolism: effect of exposure to a coal tar-containing shampoo. J Investig Dermatol. 1987;88:71–76. doi: 10.1111/1523-1747.ep12465053. [DOI] [PubMed] [Google Scholar]
- Metzger IF, Dave N, Kreutz Y, Lu JBL, Galinsky RE, Desta Z. PMC6853154; CYP2B6 genotype-dependent inhibition of CYP1A2 and induction of CYP2A6 by the antiretroviral drug efavirenz in healthy volunteers. Clin Transl Sci. 2019;12:657–666. doi: 10.1111/cts.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer zu Schwabedissen HE, Oswald S, Bresser C, Nassif A, Modess C, Desta Z, Ogburn ET, Marinova M, Lutjohann D, Spielhagen C, Nauck M, Kroemer HK, Siegmund W. PMC3667667; compartment-specific gene regulation of the CAR inducer efavirenz in vivo. Clin Pharmacol Ther. 2012;92:103–111. doi: 10.1038/clpt.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud V, Ogburn E, Thong N, Aregbe AO, Quigg TC, Flockhart DA, Desta Z. Induction of CYP2C19 and CYP3A activity following repeated administration of efavirenz in healthy volunteers. Clin Pharmacol Ther. 2012;91(3):475–482. doi: 10.1038/clpt.2011.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara K, Svensson US, Tybring G, Hai TN, Bertilsson L, Ashton M. Stereospecific analysis of omeprazole supports artemisinin as a potent inducer of CYP2C19. Fundam Clin Pharmacol. 1999;13:671–675. doi: 10.1111/j.1472-8206.1999.tb00379.x. [DOI] [PubMed] [Google Scholar]
- Mildvan D, Yarrish R, Marshak A, Hutman HW, McDonough M, Lamson M, Robinson P. Pharmacokinetic interaction between nevirapine and ethinyl estradiol/norethindrone when administered concurrently to HIV-infected women. J Acquir Immune Defic Syndr. 2002;29:471–477. doi: 10.1097/00126334-200204150-00007. [DOI] [PubMed] [Google Scholar]
- Miller M, Cosgriff J, Kwong T, Morken DA. Influence of phenytoin on theophylline clearance. Clin Pharmacol Ther. 1984;35:666–669. doi: 10.1038/clpt.1984.92. [DOI] [PubMed] [Google Scholar]
- Millonig G, Wang Y, Homann N, Bernhardt F, Qin H, Mueller S, Bartsch H, Seitz HK. Ethanol-mediated carcinogenesis in the human esophagus implicates CYP2E1 induction and the generation of carcinogenic DNA-lesions. Int J Cancer. 2011;128:533–540. doi: 10.1002/ijc.25604. [DOI] [PubMed] [Google Scholar]
- Min JS, Bae SK. Prediction of drug–drug interaction potential using physiologically based pharmacokinetic modeling. Arch Pharm Res. 2017;40:1356–1379. doi: 10.1007/s12272-017-0976-0. [DOI] [PubMed] [Google Scholar]
- Morcos PN, Cleary Y, Guerini E, Dall G, Bogman K, De Petris L, Viteri S, Bordogna W, Yu L, Martin-Facklam M, Phipps A. Clinical drug–drug interactions through cytochrome P450 3A (CYP3A) for the selective ALK inhibitor alectinib. Clin Pharmacol Drug Dev. 2017;6:280–291. doi: 10.1002/cpdd.298. [DOI] [PubMed] [Google Scholar]
- Moreland TA, Park BK, Rylance GW. 1427548; microsomal enzyme induction in children: the influence of carbamazepine treatment on antipyrine kinetics, 6 beta-hydroxycortisol excretion and plasma gamma-glutamyltranspeptidase activity. Br J Clin Pharmacol. 1982;14:861–865. doi: 10.1111/j.1365-2125.1982.tb02050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouly S, Lown KS, Kornhauser D, Joseph JL, Fiske WD, Benedek IH, Watkins PB. Hepatic but not intestinal CYP3A4 displays dose-dependent induction by efavirenz in humans. Clin Pharmacol Ther. 2002;72:1–9. doi: 10.1067/mcp.2002.124519. [DOI] [PubMed] [Google Scholar]
- MHLW/PMDA (2018) Pharmaceuticals and medical devices safety information. https://www.pmda.go.jp/english/safety/info-services/drugs/medical-safety-information/0016.html. Accessed 20 June 2020
- Narasimhan NI, Dorer DJ, Niland K, Haluska F, Sonnichsen D. Effects of ketoconazole on the pharmacokinetics of ponatinib in healthy subjects. J Clin Pharmacol. 2013;53:974–981. doi: 10.1002/jcph.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW. Aryl hydrocarbon receptor (AHR): “pioneer member” of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of “sensors” of foreign and endogenous signals. Prog Lipid Res. 2017;67:38–57. doi: 10.1016/j.plipres.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngaimisi E, Mugusi S, Minzi OM, Sasi P, Riedel KD, Suda A, Ueda N, Janabi M, Mugusi F, Haefeli WE, Burhenne J, Aklillu E. Long-term efavirenz autoinduction and its effect on plasma exposure in HIV patients. Clin Pharmacol Ther. 2010;88:676–684. doi: 10.1038/clpt.2010.172. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Holland J, Miles D, Engel C, Benrimoh N, O’Reilly T, Lacy S. Pharmacokinetic (PK) drug interaction studies of cabozantinib: effect of CYP3A inducer rifampin and inhibitor ketoconazole on cabozantinib plasma PK and effect of cabozantinib on CYP2C8 probe substrate rosiglitazone plasma PK. J Clin Pharmacol. 2015;55:1012–1023. doi: 10.1002/jcph.510. [DOI] [PubMed] [Google Scholar]
- Niemela O, Parkkila S, Juvonen RO, Viitala K, Gelboin HV, Pasanen M. Cytochromes P450 2A6, 2E1, and 3A and production of protein-aldehyde adducts in the liver of patients with alcoholic and non-alcoholic liver diseases. J Hepatol. 2000;33:893–901. doi: 10.1016/s0168-8278(00)80120-8. [DOI] [PubMed] [Google Scholar]
- Niemi M, Backman JT, Neuvonen M, Neuvonen PJ, Kivisto KT. Rifampin decreases the plasma concentrations and effects of repaglinide. Clin Pharmacol Ther. 2000;68:495–500. doi: 10.1067/mcp.2000.111183. [DOI] [PubMed] [Google Scholar]
- Niemi M, Backman JT, Neuvonen PJ. Effects of trimethoprim and rifampin on the pharmacokinetics of the cytochrome P450 2C8 substrate rosiglitazone. Clin Pharmacol Ther. 2004;76:239–249. doi: 10.1016/j.clpt.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Niu B, Coslo DM, Bataille AR, Albert I, Pugh BF, Omiecinski CJ. In vivo genome-wide binding interactions of mouse and human constitutive androstane receptors reveal novel gene targets. Nucleic Acids Res. 2018;46:8385–8403. doi: 10.1093/nar/gky692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa T, Shiraga T, Takagi A. Effect of antifungal drugs on cytochrome P450 (CYP) 2C9, CYP2C19, and CYP3A4 activities in human liver microsomes. Biol Pharm Bull. 2005;28:1805–1808. doi: 10.1248/bpb.28.1805. [DOI] [PubMed] [Google Scholar]
- O’Brien SG, Meinhardt P, Bond E, Beck J, Peng B, Dutreix C, Mehring G, Milosavljev S, Huber C, Capdeville R, Fischer T. Effects of imatinib mesylate (STI571, Glivec) on the pharmacokinetics of simvastatin, a cytochrome p450 3A4 substrate, in patients with chronic myeloid leukaemia. Br J Cancer. 2003;89:1855–1859. doi: 10.1038/sj.bjc.6601152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnhaus EE, Park BK. Measurement of urinary 6-beta-hydroxycortisol excretion as an in vivo parameter in the clinical assessment of the microsomal enzyme-inducing capacity of antipyrine, phenobarbitone and rifampicin. Eur J Clin Pharmacol. 1979;15:139–145. doi: 10.1007/BF00609878. [DOI] [PubMed] [Google Scholar]
- Ohyama K, Nakajima M, Suzuki M, Shimada N, Yamazaki H, Yokoi T. Inhibitory effects of amiodarone and its N-deethylated metabolite on human cytochrome P450 activities: prediction of in vivo drug interactions. Br J Clin Pharmacol. 2000;49:244–253. doi: 10.1046/j.1365-2125.2000.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okeke NL, Hicks C. Role of raltegravir in the management of HIV-1 infection. HIV AIDS (Auckl) 2011;3:81–92. doi: 10.2147/HIV.S13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oladimeji P, Cui H, Zhang C, Chen T. PMC4992434; regulation of PXR and CAR by protein–protein interaction and signaling crosstalk. Expert Opin Drug Metab Toxicol. 2016;12:997–1010. doi: 10.1080/17425255.2016.1201069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oneta CM, Lieber CS, Li J, Ruttimann S, Schmid B, Lattmann J, Rosman AS, Seitz HK. Dynamics of cytochrome P4502E1 activity in man: induction by ethanol and disappearance during withdrawal phase. J Hepatol. 2002;36:47–52. doi: 10.1016/s0168-8278(01)00223-9. [DOI] [PubMed] [Google Scholar]
- O’Reilly RA. Interaction of sodium warfarin and rifampin. Studies in man. Ann Intern Med. 1974;81:337–340. doi: 10.7326/0003-4819-81-3-337. [DOI] [PubMed] [Google Scholar]
- O’Reilly RA, Trager WF, Motley CH, Howald W. Interaction of secobarbital with warfarin pseudoracemates. Clin Pharmacol Ther. 1980;28:187–195. doi: 10.1038/clpt.1980.149. [DOI] [PubMed] [Google Scholar]
- Orme M, Breckenridge A. Enantiomers of warfarin and phenobarbital. N Engl J Med. 1976;295:1482–1483. doi: 10.1056/nejm197612232952613. [DOI] [PubMed] [Google Scholar]
- Oscarson M, Zanger UM, Rifki OF, Klein K, Eichelbaum M, Meyer UA. Transcriptional profiling of genes induced in the livers of patients treated with carbamazepine. Clin Pharmacol Ther. 2006;80:440–456. doi: 10.1016/j.clpt.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Oscarson M, Burk O, Winter S, Schwab M, Wolbold R, Dippon J, Eichelbaum M, Meyer UA. Effects of rifampicin on global gene expression in human small intestine. Pharmacogenet Genom. 2007;17:907–918. doi: 10.1097/FPC.0b013e3280143dfc. [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy PJ, Monteiro A, Bhattacharya S, Fowler PA. Maternal smoking and fetal sex significantly affect metabolic enzyme expression in the human fetal liver. J Clin Endocrinol Metab. 2011;96:2851–2860. doi: 10.1210/jc.2011-1437. [DOI] [PubMed] [Google Scholar]
- O’Shea D, Kim RB, Wilkinson GR. Modulation of CYP2E1 activity by isoniazid in rapid and slow N-acetylators. Br J Clin Pharmacol. 1997;43:99–103. doi: 10.1111/j.1365-2125.1997.tb00039.x. [DOI] [PubMed] [Google Scholar]
- Ouellet D, Hsu A, Qian J, Locke CS, Eason CJ, Cavanaugh JH, Leonard JM, Granneman GR. Effect of ritonavir on the pharmacokinetics of ethinyl oestradiol in healthy female volunteers. Br J Clin Pharmacol. 1998;46:111–116. doi: 10.1046/j.1365-2125.1998.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama T, Sugio K, Uramoto H, Iwata T, Onitsuka T, Isse T, Nozoe T, Kagawa N, Yasumoto K, Kawamoto T. Increased cytochrome P450 and aryl hydrocarbon receptor in bronchial epithelium of heavy smokers with non-small cell lung carcinoma carries a poor prognosis. Front Biosci. 2007;12:4497–4503. doi: 10.2741/2404. [DOI] [PubMed] [Google Scholar]
- Padda SK, Chhatwani L, Zhou L, Jacobs CD, Lopez-Anaya A, Wakelee HA. Phase I and pharmacokinetic study of bexarotene in combination with gefitinib in the third-line treatment of non-small-cell lung cancer: brief report. Anticancer Drugs. 2013;24:731–735. doi: 10.1097/CAD.0b013e32836100d7. [DOI] [PubMed] [Google Scholar]
- Paine MF, Roe AL. “Green Medicine”: the past, present, and future of botanicals. Clin Pharmacol Ther. 2018;104:410–415. doi: 10.1002/cpt.1168. [DOI] [PubMed] [Google Scholar]
- Paine MF, Shen DD, McCune JS. Recommended approaches for pharmacokinetic natural product-drug interaction research: a NaPDI Center Commentary. Drug Metab Dispos. 2018;46:1041–1045. doi: 10.1124/dmd.117.079962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkir Maideen NM, Manavalan G, Balasubramanian K. Drug interactions of meglitinide antidiabetics involving CYP enzymes and OATP1B1 transporter. Ther Adv Endocrinol Metab. 2018;9:259–268. doi: 10.1177/2042018818767220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacharla RC, Nirogi R, Uthukam V, Manoharan A, Ponnamaneni RK, Kalaikadhiban I. Quantitative in vitro phenotyping and prediction of drug interaction potential of CYP2B6 substrates as victims. Xenobiotica. 2018;48:663–675. doi: 10.1080/00498254.2017.1354267. [DOI] [PubMed] [Google Scholar]
- Paladino JA, Blumer NA, Maddox RR. Effect of secobarbital on theophylline clearance. Ther Drug Monit. 1983;5:135–139. doi: 10.1097/00007691-198303000-00016. [DOI] [PubMed] [Google Scholar]
- Palovaara S, Kivistö KT, Tapanainen P, Manninen P, Neuvonen PJ, Laine K. Effect of an oral contraceptive preparation containing ethinylestradiol and gestodene on CYP3A4 activity as measured by midazolam 1'-hydroxylation. Br J Clin Pharmacol. 2000;50(4):333–337. doi: 10.1046/j.1365-2125.2000.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantuck EJ, Kuntzman R, Conney AH. Decreased concentration of phenacetin in plasma of cigarette smokers. Science. 1972;175:1248–1250. doi: 10.1126/science.175.4027.1248. [DOI] [PubMed] [Google Scholar]
- Pantuck EJ, Hsiao KC, Conney AH, Garland WA, Kappas A, Anderson KE, Alvares AP. Effect of charcoal-broiled beef on phenacetin metabolism in man. Science. 1976;194:1055–1057. doi: 10.1126/science.982059. [DOI] [PubMed] [Google Scholar]
- Pantuck EJ, Pantuck CB, Garland WA, Min BH, Wattenberg LW, Anderson KE, Kappas A, Conney AH. Stimulatory effect of brussels sprouts and cabbage on human drug metabolism. Clin Pharmacol Ther. 1979;25:88–95. doi: 10.1002/cpt197925188. [DOI] [PubMed] [Google Scholar]
- Park JY, Kim KA, Kang MH, Kim SL, Shin JG. Effect of rifampin on the pharmacokinetics of rosiglitazone in healthy subjects. Clin Pharmacol Ther. 2004;75:157–162. doi: 10.1016/j.clpt.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Parker AC, Pritchard P, Preston T, Choonara I. Induction of CYP1A2 activity by carbamazepine in children using the caffeine breath test. Br J Clin Pharmacol. 1998;45:176–178. doi: 10.1046/j.1365-2125.1998.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier Y, Pothier C, Delmas A, Caradec F, Trancart M, Guillet F, Bouaita B, Chesne C, Brian Houston J, Walther B. Direct and quantitative evaluation of the human CYP3A4 contribution (fm) to drug clearance using the in vitro SILENSOMES model. Xenobiotica. 2017;47:562–575. doi: 10.1080/00498254.2016.1208854. [DOI] [PubMed] [Google Scholar]
- Pasanen M, Haaparanta T, Sundin M, Sivonen P, Vakakangas K, Raunio H, Hines R, Gustafsson JA, Pelkonen O. Immunochemical and molecular biological studies on human placental cigarette smoke-inducible cytochrome P-450-dependent monooxygenase activities. Toxicology. 1990;62:175–187. doi: 10.1016/0300-483x(90)90108-s. [DOI] [PubMed] [Google Scholar]
- Pascussi JM, Drocourt L, Gerbal-Chaloin S, Fabre JM, Maurel P, Vilarem MJ. Dual effect of dexamethasone on CYP3A4 gene expression in human hepatocytes. Sequential role of glucocorticoid receptor and pregnane X receptor. Eur J Biochem. 2001;268:6346–6358. doi: 10.1046/j.0014-2956.2001.02540.x. [DOI] [PubMed] [Google Scholar]
- Pascussi JM, Busson-Le Coniat M, Maurel P, Vilarem M. Transcriptional analysis of the orphan nuclear receptor constitutive androstane receptor (NR1I3) gene promoter: identification of a distal glucocorticoid response element. Mol Endocrinol. 2003;17:42–55. doi: 10.1210/me.2002-0244. [DOI] [PubMed] [Google Scholar]
- Pavek P. Pregnane X receptor (PXR)-mediated gene repression and cross-talk of PXR with other nuclear receptors via coactivator interactions. Front Pharmacol. 2016;7:456. doi: 10.3389/fphar.2016.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkonen O, Turpeinen M, Hakkola J, Honkakoski P, Hukkanen J, Raunio H. Inhibition and induction of human cytochrome P450 enzymes: current status. Arch Toxicol. 2008;82:667–715. doi: 10.1007/s00204-008-0332-8. [DOI] [PubMed] [Google Scholar]
- Pelkonen O, Xu Q, Fan T. Why is research on herbal medicinal products important and how can we improve its quality? J Tradit Complement Med. 2014;4:1–7. doi: 10.4103/2225-4110.124323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzak SR, Hon YY, Lawhorn WD, Shirley KL, Spratlin V, Jann MW. Influence of ritonavir on olanzapine pharmacokinetics in healthy volunteers. J Clin Psychopharmacol. 2002;22:366–370. doi: 10.1097/00004714-200208000-00006. [DOI] [PubMed] [Google Scholar]
- Penzak SR, Robertson SM, Hunt JD, Chairez C, Malati CY, Alfaro RM, Stevenson JM, Kovacs JA. PMC3407958; echinacea purpurea significantly induces cytochrome P450 3A activity but does not alter lopinavir–ritonavir exposure in healthy subjects. Pharmacotherapy. 2010;30:797–805. doi: 10.1592/phco.30.8.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot N, Nalpas B, Yang CS, Beaune PH. Modulation of cytochrome P450 isozymes in human liver, by ethanol and drug intake. Eur J Clin Investig. 1989;19:549–555. doi: 10.1111/j.1365-2362.1989.tb00273.x. [DOI] [PubMed] [Google Scholar]
- Perucca E, Grimaldi R, Frigo GM, Sardi A, Monig H, Ohnhaus EE. Comparative effects of rifabutin and rifampicin on hepatic microsomal enzyme activity in normal subjects. Eur J Clin Pharmacol. 1988;34:595–599. doi: 10.1007/BF00615223. [DOI] [PubMed] [Google Scholar]
- Perucca E, Cloyd J, Critchley D, Fuseau E. Rufinamide: clinical pharmacokinetics and concentration-response relationships in patients with epilepsy. Epilepsia. 2008;49:1123–1141. doi: 10.1111/j.1528-1167.2008.01665.x. [DOI] [PubMed] [Google Scholar]
- Petersen MS, Halling J, Damkier P, Nielsen F, Grandjean P, Weihe P, Brosen K. Polychlorinated biphenyl (PCB) induction of CYP3A4 enzyme activity in healthy Faroese adults. Toxicol Appl Pharmacol. 2007;224:202–206. doi: 10.1016/j.taap.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Pilla Reddy V, Walker M, Sharma P, Ballard P, Vishwanathan K. Development, verification, and prediction of osimertinib drug–drug interactions using PBPK modeling approach to inform drug label. CPT Pharmacomet Syst Pharmacol. 2018;7:321–330. doi: 10.1002/psp4.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscitelli SC, Burstein AH, Chaitt D, Alfaro RM, Falloon J. Indinavir concentrations and St John’s wort. Lancet. 2000;355:547–548. doi: 10.1016/S0140-6736(99)05712-8. [DOI] [PubMed] [Google Scholar]
- Pithavala YK, Tortorici M, Toh M, Garrett M, Hee B, Kuruganti U, Ni G, Klamerus KJ. Effect of rifampin on the pharmacokinetics of Axitinib (AG-013736) in Japanese and Caucasian healthy volunteers. Cancer Chemother Pharmacol. 2010;65:563–570. doi: 10.1007/s00280-009-1065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pithavala YK, Tong W, Mount J, Rahavendran SV, Garrett M, Hee B, Selaru P, Sarapa N, Klamerus KJ. Effect of ketoconazole on the pharmacokinetics of axitinib in healthy volunteers. Investig New Drugs. 2012;30:273–281. doi: 10.1007/s10637-010-9511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland A, Smith D, Kuntzman R, Jacobson M, Conney AH. Effect of intensive occupational exposure to DDT on phenylbutazone and cortisol metabolism in human subjects. Clin Pharmacol Ther. 1970;11:724–732. doi: 10.1002/cpt1970115724. [DOI] [PubMed] [Google Scholar]
- Poland A, Glover E, Kende AS. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem. 1976;251:4936–4946. [PubMed] [Google Scholar]
- Polasek TM, Elliot DJ, Lewis BC, Miners JO. Mechanism-based inactivation of human cytochrome P4502C8 by drugs in vitro. J Pharmacol Exp Ther. 2004;311(3):996–1007. doi: 10.1124/jpet.104.071803. [DOI] [PubMed] [Google Scholar]
- Posada MM, Morse BL, Turner PK, Kulanthaivel P, Hall SD, Dickinson GL. Predicting clinical effects of CYP3A4 modulators on abemaciclib and active metabolites exposure using physiologically based pharmacokinetic modeling. J Clin Pharmacol. 2020;60:915–930. doi: 10.1002/jcph.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt-Hyatt M, Lin H, Hollenberg PF. Mechanism-based inactivation of human CYP2E1 by diethyldithocarbamate. Drug Metab Dispos. 2010;38:2286–2292. doi: 10.1124/dmd.110.034710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preissner S, Kroll K, Dunkel M, Senger C, Goldsobel G, Kuzman D, Guenther S, Winnenburg R, Schroeder M, Preissner R. SuperCYP: a comprehensive database on cytochrome P450 enzymes including a tool for analysis of CYP–drug interactions. Nucleic Acids Res. 2010;38:237. doi: 10.1093/nar/gkp970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press RR, Ploeger BA, den Hartigh J, van der Straaten T, van Pelt H, Danhof M, de Fijter H, Guchelaar HJ. 2868991; explaining variability in ciclosporin exposure in adult kidney transplant recipients. Eur J Clin Pharmacol. 2010;66:579–590. doi: 10.1007/s00228-010-0810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto-Castello M, Cardona A, Marhuenda D, Roel JM, Corno A. Use of the CYP2E1 genotype and phenotype for the biological monitoring of occupational exposure to styrene. Toxicol Lett. 2010;192:34–39. doi: 10.1016/j.toxlet.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Pursche S, Schleyer E, von Bonin M, Ehninger G, Said SM, Prondzinsky R, Illmer T, Wang Y, Hosius C, Nikolova Z, Bornhäuser M, Dresemann G. Influence of enzyme-inducing antiepileptic drugs on trough level of imatinib in glioblastoma patients. Curr Clin Pharmacol. 2008;3:198–203. doi: 10.2174/157488408785747656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin WJ, Zhang W, Liu ZQ, Chen XP, Tan ZR, Hu DL, Wang D, Fan L, Zhou HH. PMC3522813; Rapid clinical induction of bupropion hydroxylation by metamizole in healthy Chinese men. Br J Clin Pharmacol. 2012;74:999–1004. doi: 10.1111/j.1365-2125.2012.04304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F, Wang G, Zhang R, Sun J, Jiang J, Ma Y. PMC2883758; Effect of danshen extract on the activity of CYP3A4 in healthy volunteers. Br J Clin Pharmacol. 2010;69:656–662. doi: 10.1111/j.1365-2125.2010.03624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F, Jiang J, Ma Y, Wang G, Gao C, Zhang X, Zhang L, Liu S, He M, Zhu L, Ye Y, Li Q, Miao P. PMC3816049; opposite effects of single-dose and multidose administration of the ethanol extract of danshen on CYP3A in healthy volunteers. Evid Based Complement Altern Med. 2013;2013:730734. doi: 10.1155/2013/730734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrochi LC, Tukey RH. Nuclear uptake of the Ah (dioxin) receptor in response to omeprazole: transcriptional activation of the human CYP1A1 gene. Mol Pharmacol. 1993;43:504–508. [PubMed] [Google Scholar]
- Raaska K, Neuvonen PJ. Ciprofloxacin increases serum clozapine and N-desmethylclozapine: a study in patients with schizophrenia. Eur J Clin Pharmacol. 2000;56:585–589. doi: 10.1007/s002280000192. [DOI] [PubMed] [Google Scholar]
- Rahmioglu N, Heaton J, Clement G, Gill R, Surdulescu G, Zlobecka K, Hodgkiss D, Ma Y, Hider RC, Smith NW, Ahmadi KR. Genetic epidemiology of induced CYP3A4 activity. Pharmacogenet Genom. 2011;21:642–651. doi: 10.1097/FPC.0b013e3283498ecf. [DOI] [PubMed] [Google Scholar]
- Ramanathan S, Jin F, Sharma S, Kearney BP. Clinical Pharmacokinetic and pharmacodynamic profile of idelalisib. Clin Pharmacokinet. 2016;55:33–45. doi: 10.1007/s40262-015-0304-0. [DOI] [PubMed] [Google Scholar]
- Rao PSS, Midde NM, Miller DD, Chauhan S, Kumar A, Kumar S. Diallyl sulfide: potential use in novel therapeutic interventions in alcohol, drugs, and disease mediated cellular toxicity by targeting cytochrome P450 2E1. Curr Drug Metab. 2015;16:486–503. doi: 10.2174/1389200216666150812123554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed A, Hines RN, McCarver-May D. Variation in induction of human placental CYP2E1: possible role in susceptibility to fetal alcohol syndrome? Toxicol Appl Pharmacol. 1997;144:396–400. doi: 10.1006/taap.1997.8152. [DOI] [PubMed] [Google Scholar]
- Raucy JL, Schultz ED, Wester MR, Arora S, Johnston DE, Omdahl JL, Carpenter SP. Human lymphocyte cytochrome P450 2E1, a putative marker for alcohol-mediated changes in hepatic chlorzoxazone activity. Drug Metab Dispos. 1997;25:1429–1435. [PubMed] [Google Scholar]
- Raucy JL, Schultz ED, Kearins MC, Arora S, Johnston DE, Omdahl JL, Eckmann L, Carpenter SP. CYP2E1 expression in human lymphocytes from various ethnic populations. Alcohol Clin Exp Res. 1999;23:1868–1874. [PubMed] [Google Scholar]
- Rautio A, Salmela E, Arvela P, Pelkonen O, Sotaniemi EA. Assessment of CYP2A6 and CYP3A4 activities in vivo in different diseases in man. In: Lechner MC, editor. Cytochrome P450: biochemistry, biophysics and molecular biology. Paris: John Libbey Eurotext; 1994. pp. 519–521. [Google Scholar]
- Reardon DA, Vredenburgh JJ, Desjardins A, Peters K, Gururangan S, Sampson JH, Marcello J, Herndon JE, McLendon RE, Janney D, Friedman AH, Bigner DD, Friedman HS. Effect of CYP3A-inducing antiepileptics on sorafenib exposure: results of a phase II study of sorafenib plus daily temozolomide in adults with recurrent glioblastoma. J Neurooncol. 2011;101:57–66. doi: 10.1007/s11060-010-0217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed GA, Peterson KS, Smith HJ, Gray JC, Sullivan DK, Mayo MS, Crowell JA, Hurwitz A. A phase I study of indole-3-carbinol in women: tolerability and effects. Cancer Epidemiol Biomark Prev. 2005;14:1953–1960. doi: 10.1158/1055-9965.EPI-05-0121. [DOI] [PubMed] [Google Scholar]
- Reese MJ, Wurm RM, Muir KT, Generaux GT, St John-Williams L, McConn DJ. An in vitro mechanistic study to elucidate the desipramine/bupropion clinical drug–drug interaction. Drug Metab Dispos. 2008;36:1198–1201. doi: 10.1124/dmd.107.020198. [DOI] [PubMed] [Google Scholar]
- Richter E, Breimer DD, Zilly W. Disposition of hexobarbital in intra- and extrahepatic cholestasis in man and the influence of drug metabolism-inducing agents. Eur J Clin Pharmacol. 1980;17:197–202. doi: 10.1007/BF00561900. [DOI] [PubMed] [Google Scholar]
- Roberts PJ, Rollins KD, Kashuba AD, Paine MF, Nelsen AC, Williams EE, Moran C, Lamba JK, Schuetz EG, Hawke RL. 2770345; the influence of CYP3A5 genotype on dexamethasone induction of CYP3A activity in African Americans. Drug Metab Dispos. 2008;36:1465–1469. doi: 10.1124/dmd.107.020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson P, DeCory HH, Madan A, Parkinson A. In vitro inhibition and induction of human hepatic cytochrome P450 enzymes by modafinil. Drug Metab Dispos. 2000;28:664–671. [PubMed] [Google Scholar]
- Robertson PJ, Hellriegel ET, Arora S, Nelson M. Effect of modafinil on the pharmacokinetics of ethinyl estradiol and triazolam in healthy volunteers. Clin Pharmacol Ther. 2002;71:46–56. doi: 10.1067/mcp.2002.121217. [DOI] [PubMed] [Google Scholar]
- Robertson SM, Maldarelli F, Natarajan V, Formentini E, Alfaro RM, Penzak SR. Efavirenz induces CYP2B6-mediated hydroxylation of bupropion in healthy subjects. J Acquir Immune Defic Syndr. 2008;49:513–519. doi: 10.1097/QAI.0b013e318183a425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SM, Davey RT, Voell J, Formentini E, Alfaro RM, Penzak SR. Effect of Ginkgo biloba extract on lopinavir, midazolam and fexofenadine pharmacokinetics in healthy subjects. Curr Med Res Opin. 2008;24:591–599. doi: 10.1185/030079908x260871. [DOI] [PubMed] [Google Scholar]
- Robson RA, Miners JO, Wing LM, Birkett DJ. 1463637; theophylline-rifampicin interaction: non-selective induction of theophylline metabolic pathways. Br J Clin Pharmacol. 1984;18:445–448. doi: 10.1111/j.1365-2125.1984.tb02487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby CA, Anderson GD, Kantor E, Dryer DA, Burstein AH. St John’s Wort: effect on CYP3A4 activity. Clin Pharmacol Ther. 2000;67:451–457. doi: 10.1067/mcp.2000.106793. [DOI] [PubMed] [Google Scholar]
- Rosenfeld WE, Doose DR, Walker SA, Nayak RK. Effect of topiramate on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in patients with epilepsy. Epilepsia. 1997;38:317–323. doi: 10.1111/j.1528-1157.1997.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Rost KL, Roots I. Accelerated caffeine metabolism after omeprazole treatment is indicated by urinary metabolite ratios: coincidence with plasma clearance and breath test. Clin Pharmacol Ther. 1994;55:402–411. doi: 10.1038/clpt.1994.49. [DOI] [PubMed] [Google Scholar]
- Rost KL, Brosicke H, Heinemeyer G, Roots I. Specific and dose-dependent enzyme induction by omeprazole in human beings. Hepatology. 1994;20:1204–1212. [PubMed] [Google Scholar]
- Rothhammer V, Quintana FJ. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol. 2019;19:184–197. doi: 10.1038/s41577-019-0125-8. [DOI] [PubMed] [Google Scholar]
- Rowland A, van Dyk M, Warncken D, Mangoni AA, Sorich MJ, Rowland A. Evaluation of modafinil as a perpetrator of metabolic drug–drug interactions using a model informed cocktail reaction phenotyping trial protocol. Br J Clin Pharmacol. 2018;84:501–509. doi: 10.1111/bcp.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JY, Kim HU, Lee SY. Deep learning improves prediction of drug–drug and drug–food interactions. Proc Natl Acad Sci USA. 2018;115:E4304–E4311. doi: 10.1073/pnas.1803294115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarikoski ST, Rivera SP, Hankinson O, Husgafvel-Pursiainen K. CYP2S1: a short review. Toxicol Appl Pharmacol. 2005;207:62–69. doi: 10.1016/j.taap.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Saccar CL, Danish M, Ragni MC, Rocci MLJ, Greene J, Yaffe SJ, Mansmann HCJ. The effect of phenobarbital on theophylline disposition in children with asthma. J Allergy Clin Immunol. 1985;75:716–719. doi: 10.1016/0091-6749(85)90099-5. [DOI] [PubMed] [Google Scholar]
- Sager JE, Tripathy S, Price LSL, Nath A, Chang J, Stephenson-Famy A, Isoherranen N. In vitro to in vivo extrapolation of the complex drug–drug interaction of bupropion and its metabolites with CYP2D6; simultaneous reversible inhibition and CYP2D6 downregulation. Biochem Pharmacol. 2017;123:85–96. doi: 10.1016/j.bcp.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale M, Sadler BM, Stein DS. Pharmacokinetic modeling and simulations of interaction of amprenavir and ritonavir. Antimicrob Agents Chemother. 2002;46:746–754. doi: 10.1128/AAC.46.3.746-754.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samer CF, Gloor Y, Rollason V, Guessous I, Doffey-Lazeyras F, Saurat JH, Sorg O, Desmeules J, Daali Y. Cytochrome P450 1A2 activity and incidence of thyroid disease and cancer after chronic or acute exposure to dioxins. Basic Clin Pharmacol Toxicol. 2020;126(3):296–303. doi: 10.1111/bcpt.13339. [DOI] [PubMed] [Google Scholar]
- Satarug S, Ujjin P, Vanavanitkun Y, Nishijo M, Baker JR, Moore MR. Effects of cigarette smoking and exposure to cadmium and lead on phenotypic variability of hepatic CYP2A6 and renal function biomarkers in men. Toxicology. 2004;204:161–173. doi: 10.1016/j.tox.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Satarug S, Nishijo M, Ujjin P, Vanavanitkun Y, Baker JR, Moore MR. Effects of chronic exposure to low-level cadmium on renal tubular function and CYP2A6-mediated coumarin metabolism in healthy human subjects. Toxicol Lett. 2004;148:187–197. doi: 10.1016/j.toxlet.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Saurat JH, Kaya G, Saxer-Sekulic N, Pardo B, Becker M, Fontao L, Mottu F, Carraux P, Pham XC, Barde C, Fontao F, Zennegg M, Schmid P, Schaad O, Descombes P, Sorg O. The cutaneous lesions of dioxin exposure: lessons from the poisoning of Victor Yushchenko. Toxicol Sci. 2012;125:310–317. doi: 10.1093/toxsci/kfr223. [DOI] [PubMed] [Google Scholar]
- Saussele T, Burk O, Blievernicht JK, Klein K, Nussler A, Nussler N, Hengstler JG, Eichelbaum M, Schwab M, Zanger UM. Selective induction of human hepatic cytochromes P450 2B6 and 3A4 by metamizole. Clin Pharmacol Ther. 2007;82:265–274. doi: 10.1038/sj.clpt.6100138. [DOI] [PubMed] [Google Scholar]
- Sax PE, DeJesus E, Crofoot G, Ward D, Benson P, Dretler R, Mills A, Brinson C, Peloquin J, Wei X, White K, Cheng A, Martin H, Quirk E. Bictegravir versus dolutegravir, each with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection: a randomised, double-blind, phase 2 trial. Lancet HIV. 2017;4:e154–e160. doi: 10.1016/S2352-3018(17)30016-4. [DOI] [PubMed] [Google Scholar]
- Scheer N, Ross J, Rode A, Zevnik B, Niehaves S, Faust N, Wolf CR. A novel panel of mouse models to evaluate the role of human pregnane X receptor and constitutive androstane receptor in drug response. J Clin Investig. 2008;118:3228–3239. doi: 10.1172/JCI35483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellens JH, van der Wart JH, Brugman M, Breimer DD. Influence of enzyme induction and inhibition on the oxidation of nifedipine, sparteine, mephenytoin and antipyrine in humans as assessed by a “cocktail” study design. J Pharmacol Exp Ther. 1989;249:638–645. [PubMed] [Google Scholar]
- Schmitt-Hoffmann A, Roos B, Sauer J, Spickermann J, Maares J, Schoetzau A, Meyer I. Pharmacokinetic interactions between alitretinoin and ketoconazole or simvastatin or ciclosporin A. Clin Exp Dermatol. 2011;36(Suppl 2):24–28. doi: 10.1111/j.1365-2230.2011.04034.x. [DOI] [PubMed] [Google Scholar]
- Scholler-Gyure M, Kakuda TN, Raoof A, De Smedt G, Hoetelmans RM. Clinical pharmacokinetics and pharmacodynamics of etravirine. Clin Pharmacokinet. 2009;48:561–574. doi: 10.2165/10895940-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Schulz M, Iwersen-Bergmann S, Andresen H, Schmoldt A. Therapeutic and toxic blood concentrations of nearly 1000 drugs and other xenobiotics. Crit Care. 2012;16:R136. doi: 10.1186/cc11441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz M, Schmoldt A, Andresen-Streichert H, Iwersen-Bergmann S. Revisited: therapeutic and toxic blood concentrations of more than 1100 drugs and other xenobiotics. Crit Care. 2020;24:195. doi: 10.1186/s13054-020-02915-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzberg LS, Yardley DA, Elias AD, Patel M, LoRusso P, Burris HA, Gucalp A, Peterson AC, Blaney ME, Steinberg JL, Gibbons JA, Traina TA. A phase I/Ib study of enzalutamide alone and in combination with endocrine therapies in women with advanced breast cancer. Clin Cancer Res. 2017;23:4046–4054. doi: 10.1158/1078-0432.CCR-16-2339. [DOI] [PubMed] [Google Scholar]
- Sevior D, Ahokas JT. Chapter 5: interactions between conventional and herbal medicinal products. In: Pelkonen O, Duez P, Vuorela H, editors. Toxicology of herbal products. Switzerland: Springer International Publishing; 2017. pp. 81–99. [Google Scholar]
- Shadle CR, Lee Y, Majumdar AK, Petty KJ, Gargano C, Bradstreet TE, Evans JK, Blum RA. Evaluation of potential inductive effects of aprepitant on cytochrome P450 3A4 and 2C9 activity. J Clin Pharmacol. 2004;44:215–223. doi: 10.1177/0091270003262950. [DOI] [PubMed] [Google Scholar]
- Shebley M, Einolf HJ. Practical assessment of clinical drug–drug interactions in drug development using physiologically based pharmacokinetics modeling. Clin Pharmacol Ther. 2019;105:1326–1328. doi: 10.1002/cpt.1394. [DOI] [PubMed] [Google Scholar]
- Sherman EM, Worley MV, Unger NR, Gauthier TP, Schafer JJ. Cobicistat: review of a pharmacokinetic enhancer for HIV infection. Clin Ther. 2015;37:1876–1893. doi: 10.1016/j.clinthera.2015.07.022. [DOI] [PubMed] [Google Scholar]
- Shi JG, Chen X, Emm T, Scherle PA, McGee RF, Lo Y, Landman RR, McKeever EG, Punwani NG, Williams WV, Yeleswaram S. The effect of CYP3A4 inhibition or induction on the pharmacokinetics and pharmacodynamics of orally administered ruxolitinib (INCB018424 phosphate) in healthy volunteers. J Clin Pharmacol. 2012;52:809–818. doi: 10.1177/0091270011405663. [DOI] [PubMed] [Google Scholar]
- Shukla SJ, Sakamuru S, Huang R, Moeller TA, Shinn P, Vanleer D, Auld DS, Austin CP, Xia M. Identification of clinically used drugs that activate pregnane X receptors. Drug Metab Dispos. 2011;39:151–159. doi: 10.1124/dmd.110.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsson US, Jansson B, Hai TN, Huong DX, Tybring G, Ashton M. Artemisinin autoinduction is caused by involvement of cytochrome P450 2B6 but not 2C9. Clin Pharmacol Ther. 2003;74:32–43. doi: 10.1016/S0009-9236(03)00092-4. [DOI] [PubMed] [Google Scholar]
- Sinha R, Rothman N, Brown ED, Mark SD, Hoover RN, Caporaso NE, Levander OA, Knize MG, Lang NP, Kadlubar FF. Pan-fried meat containing high levels of heterocyclic aromatic amines but low levels of polycyclic aromatic hydrocarbons induces cytochrome P4501A2 activity in humans. Cancer Res. 1994;54:6154–6159. [PubMed] [Google Scholar]
- Sinues B, Fanlo A, Mayayo E, Carcas C, Vicente J, Arenaz I, Cebollada A. CYP2A6 activity in a healthy Spanish population: effect of age, sex, smoking, and oral contraceptives. Hum Exp Toxicol. 2008;27:367–372. doi: 10.1177/0960327107082224. [DOI] [PubMed] [Google Scholar]
- Skerjanec A, Wang J, Maren K, Rojkjaer L. Investigation of the pharmacokinetic interactions of deferasirox, a once-daily oral iron chelator, with midazolam, rifampin, and repaglinide in healthy volunteers. J Clin Pharmacol. 2010;50:205–213. doi: 10.1177/0091270009340418. [DOI] [PubMed] [Google Scholar]
- Slattery JT, Kalhorn TF, McDonald GB, Lambert K, Buckner CD, Bensinger WI, Anasetti C, Appelbaum FR. Conditioning regimen-dependent disposition of cyclophosphamide and hydroxycyclophosphamide in human marrow transplantation patients. J Clin Oncol. 1996;14:1484–1494. doi: 10.1200/JCO.1996.14.5.1484. [DOI] [PubMed] [Google Scholar]
- Smith G, Wolf CR, Deeni YY, Dawe RS, Evans AT, Comrie MM, Ferguson J, Ibbotson SH. Cutaneous expression of cytochrome P450 CYP2S1: individuality in regulation by therapeutic agents for psoriasis and other skin diseases. Lancet. 2003;361:1336–1343. doi: 10.1016/S0140-6736(03)13081-4. [DOI] [PubMed] [Google Scholar]
- Smith G, Ibbotson SH, Comrie MM, Dawe RS, Bryden A, Ferguson J, Wolf CR. Regulation of cutaneous drug-metabolizing enzymes and cytoprotective gene expression by topical drugs in human skin in vivo. Br J Dermatol. 2006;155:275–281. doi: 10.1111/j.1365-2133.2006.07317.x. [DOI] [PubMed] [Google Scholar]
- Smith DA, Koch KM, Arya N, Bowen CJ, Herendeen JM, Beelen A. Effects of ketoconazole and carbamazepine on lapatinib pharmacokinetics in healthy subjects. Br J Clin Pharmacol. 2009;67:421–426. doi: 10.1111/j.1365-2125.2009.03370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NF, Mani S, Schuetz EG, Yasuda K, Sissung TM, Bates SE, Figg WD, Sparreboom A. 3100585; Induction of CYP3A4 by vinblastine: role of the nuclear receptor NR1I2. Ann Pharmacother. 2010;44:1709–1717. doi: 10.1345/aph.1P354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RP, Eckalbar WL, Morrissey KM, Luizon MR, Hoffmann TJ, Sun X, Jones SL, Force Aldred S, Ramamoorthy A, Desta Z, Liu Y, Skaar TC, Trinklein ND, Giacomini KM, Ahituv N. Genome-wide discovery of drug-dependent human liver regulatory elements. PLoS Genet. 2014;10:e1004648. doi: 10.1371/journal.pgen.1004648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smutny T, Mani S, Pavek P. Post-translational and post-transcriptional modifications of pregnane X receptor (PXR) in regulation of the cytochrome P450 superfamily. Curr Drug Metab. 2013;14:1059–1069. doi: 10.2174/1389200214666131211153307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solas C, Poizot-Martin I, Drogoul MP, Ravaux I, Dhiver C, Lafeuillade A, Allegre T, Mokhtari M, Moreau J, Lepeu G, Petit N, Durand A, Lacarelle B. Therapeutic drug monitoring of lopinavir/ritonavir given alone or with a non-nucleoside reverse transcriptase inhibitor. Br J Clin Pharmacol. 2004;57:436–440. doi: 10.1046/j.1365-2125.2003.02020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BJ, Veech RL, Park SS, Gelboin HV, Gonzalez FJ. Induction of rat hepatic N-nitrosodimethylamine demethylase by acetone is due to protein stabilization. J Biol Chem. 1989;264:3568–3572. [PubMed] [Google Scholar]
- Sorf A, Hofman J, Kučera R, Staud F, Ceckova M. Ribociclib shows potential for pharmacokinetic drug–drug interactions being a substrate of ABCB1 and potent inhibitor of ABCB1, ABCG2 and CYP450 isoforms in vitro. Biochem Pharmacol. 2018;154:10–17. doi: 10.1016/j.bcp.2018.04.013. [DOI] [PubMed] [Google Scholar]
- Stage TB, Graff M, Wong S, Rasmussen LL, Nielsen F, Pottegard A, Brosen K, Kroetz DL, Khojasteh SC, Damkier P. PMC5809358; Dicloxacillin induces CYP2C19, CYP2C9 and CYP3A4 in vivo and in vitro. Br J Clin Pharmacol. 2018;84:510–519. doi: 10.1111/bcp.13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger JL, Xu C, Biswas A, Mani S. Post-translational modification of pregnane X receptor. Pharmacol Res. 2011;64:4–10. doi: 10.1016/j.phrs.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stresser DM, Broudy MI, Ho T, Cargill CE, Blanchard AP, Sharma R, Dandeneau AA, Goodwin JJ, Turner SD, Erve JCL, Patten CJ, Dehal SS, Crespi CL. Highly selective inhibition of human CYP3Aa in vitro by azamulin and evidence that inhibition is irreversible. Drug Metab Dispos. 2004;32:105–112. doi: 10.1124/dmd.32.1.105. [DOI] [PubMed] [Google Scholar]
- Stresser DM, Perloff ES, Mason AK, Blanchard AP, Dehal SS, Creegan TP, Singh R, Gangl ET. Selective time- and NADPH-dependent inhibition of human CYP2E1 by clomethiazole. Drug Metab Dispos. 2016;44:1424–1430. doi: 10.1124/dmd.116.070193. [DOI] [PubMed] [Google Scholar]
- Sugiyama M, Fujita K, Murayama N, Akiyama Y, Yamazaki H, Sasaki Y. Sorafenib and sunitinib, two anticancer drugs, inhibit CYP3A4-mediated and activate CY3A5-mediated midazolam 1′-hydroxylation. Drug Metab Dispos. 2011;39:757–762. doi: 10.1124/dmd.110.037853. [DOI] [PubMed] [Google Scholar]
- Sui Y, Ai N, Park SH, Rios-Pilier J, Perkins JT, Welsh WJ, Zhou C. 3295358; bisphenol A and its analogues activate human pregnane X receptor. Environ Health Perspect. 2012;120:399–405. doi: 10.1289/ehp.1104426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle AB, Grossmann KF, Ouellet D, Richards-Peterson L, Aktan G, Gordon MS, LoRusso PM, Infante JR, Sharma S, Kendra K, Patel M, Pant S, Arkenau HT, Middleton MR, Blackman SC, Botbyl J, Carson SW. Assessment of the drug interaction potential and single- and repeat-dose pharmacokinetics of the BRAF inhibitor dabrafenib. J Clin Pharmacol. 2015;55:392–400. doi: 10.1002/jcph.437. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Yamamoto M. Molecular basis of the Keap1-Nrf2 system. Free Radic Biol Med. 2015;88:93–100. doi: 10.1016/j.freeradbiomed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kneller MB, Haining RL, Trager WF, Rettie AE. (+)-N-3-benzyl-nirvanol and (−)-N-3-benzyl-phenobarbital: new potent and selective in vitro inhibitors of CYP2C19. Drug Metab Dispos. 2002;30:235–239. doi: 10.1124/dmd.30.3.235. [DOI] [PubMed] [Google Scholar]
- Svedberg A, Vikingsson S, Vikstrom A, Hornstra N, Kentson M, Branden E, Koyi H, Bergman B, Green H. Erlotinib treatment induces cytochrome P450 3A activity in non-small cell lung cancer patients. Br J Clin Pharmacol. 2019;85:1704–1709. doi: 10.1111/bcp.13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson US, Ashton M, Trinh NH, Bertilsson L, Dinh XH, Nguyen VH, Nguyen TN, Nguyen DS, Lykkesfeldt J, Le DC. Artemisinin induces omeprazole metabolism in human beings. Clin Pharmacol Ther. 1998;64:160–167. doi: 10.1016/S0009-9236(98)90149-7. [DOI] [PubMed] [Google Scholar]
- Swaisland HC, Ranson M, Smith RP, Leadbetter J, Laight A, McKillop D, Wild MJ. Pharmacokinetic drug interactions of gefitinib with rifampicin, itraconazole and metoprolol. Clin Pharmacokinet. 2005;44:1067–1081. doi: 10.2165/00003088-200544100-00005. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Lasker JM, Rosman AS, Lieber CS. Induction of cytochrome P-4502E1 in the human liver by ethanol is caused by a corresponding increase in encoding messenger RNA. Hepatology. 1993;17:236–245. [PubMed] [Google Scholar]
- Takakusa H, Wahlin MD, Zhao C, Hanson KL, New LS, Chan ECY, Nelson SD. Metabolic intermediate complex formation of human cytochrome P450 3A4 by lapatinib. Drug Metab Dispos. 2011;39:1022–1030. doi: 10.1124/dmd.110.037531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takusagawa S, Miyashita A, Iwatsubo T, Usui T. In vitro inhibition and induction of human cytochrome P450 enzymes by mirabegron, a potent and selective β3-adrenoceptor agonist. Xenobiotica. 2012;42:1187–1196. doi: 10.3109/00498254.2012.700140. [DOI] [PubMed] [Google Scholar]
- Tan AR, Gibbon DG, Stein MN, Lindquist D, Edenfield JW, Martin JC, Gregory C, Suttle AB, Tada H, Botbyl J, Stephenson JJ. Effects of ketoconazole and esomeprazole on the pharmacokinetics of pazopanib in patients with solid tumors. Cancer Chemother Pharmacol. 2013;71:1635–1643. doi: 10.1007/s00280-013-2164-3. [DOI] [PubMed] [Google Scholar]
- Tanaka C. Clinical pharmacology of deferasirox. Clin Pharmacokinet. 2014;53:679–694. doi: 10.1007/s40262-014-0151-4. [DOI] [PubMed] [Google Scholar]
- Tanaka C, Yin OQP, Smith T, Sethuraman V, Grouss K, Galitz L, Harrell R, Schran H. Effects of rifampin and ketoconazole on the pharmacokinetics of nilotinib in healthy participants. J Clin Pharmacol. 2011;51:75–83. doi: 10.1177/0091270010367428. [DOI] [PubMed] [Google Scholar]
- Tanner JA, Tyndale RF. Variation in CYP2A6 activity and personalized medicine. J Pers Med. 2017;7(4):18. doi: 10.3390/jpm7040018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassaneeyakul W, Guo LQ, Fukuda K, Ohta T, Yamazoe Y. Inhibition selectivity of grapefruit juice components on human cytochromes P450. Arch Biochem Biophys. 2000;378:356–363. doi: 10.1006/abbi.2000.1835. [DOI] [PubMed] [Google Scholar]
- Teng WC, Oh JW, New LS, Wahlin MD, Nelson SD, Ho HK, Chan ECY. Mechanism-based inactivation of cytochrome P450 3A4 by lapatinib. Mol Pharmacol. 2010;78:693–703. doi: 10.1124/mol.110.065839. [DOI] [PubMed] [Google Scholar]
- Thelingwani RS, Zvada SP, Dolgos H, Ungell AB, Masimirembwa CM. In vitro and in silico identification and characterization of thiabendazole as a mechanism-based inhibitor of CYP1A2 and simulation of possible pharmacokinetic drug–drug interactions. Drug Metab Dispos. 2009;37:1286–1294. doi: 10.1124/dmd.108.024604. [DOI] [PubMed] [Google Scholar]
- Thorn CF, Aklillu E, McDonagh EM, Klein TE, Altman RB. PharmGKB summary: caffeine pathway. Pharmacogenet Genom. 2012;22:389–395. doi: 10.1097/FPC.0b013e3283505d5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thum T, Erpenbeck VJ, Moeller J, Hohlfeld JM, Krug N, Borlak J. Expression of xenobiotic metabolizing enzymes in different lung compartments of smokers and nonsmokers. Environ Health Perspect. 2006;114:1655–1661. doi: 10.1289/ehp.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel KE, Shen DD, Podoll TD, Kunze KL, Trager WF, Hartwell PS, Raisys VA, Marsh CL, McVicar JP, Barr DM. Use of midazolam as a human cytochrome P450 3A probe: I. In vitro-in vivo correlations in liver transplant patients. J Pharmacol Exp Ther. 1994;271:549–556. [PubMed] [Google Scholar]
- Tian X, Zhang H, Heimbach T, He H, Buchbinder A, Aghoghovbia M, Hourcade-Potelleret F. Clinical pharmacokinetic and pharmacodynamic overview of nilotinib, a selective tyrosine kinase inhibitor. J Clin Pharmacol. 2018;58:1533–1540. doi: 10.1002/jcph.1312. [DOI] [PubMed] [Google Scholar]
- Tornio A, Filppula AM, Kailari O, Neuvonen M, Nyrönen TH, Tapaninen T, Neuvonen PJ, Niemi M, Backman JT. Glucuronidation converts clopidogrel to a strong time-dependent inhibitor of CYP2C8: a phase II metabolite as a perpetrator of drug–drug interactions. Clin Pharmacol Ther. 2014;96:498–507. doi: 10.1038/clpt.2014.141. [DOI] [PubMed] [Google Scholar]
- Tornio A, Filppula AM, Niemi M, Backman JT. Clinical studies on drug–drug interactions involving metabolism and transport: methodology, pitfalls, and interpretation. Clin Pharmacol Ther. 2019;105:1345–1361. doi: 10.1002/cpt.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend R, Dietz A, Hale C, Akhtar S, Kowalski D, Lademacher C, Lasseter K, Pearlman H, Rammelsberg D, Schmitt-Hoffmann A, Yamazaki T, Desai A. Pharmacokinetic evaluation of CYP3A4-mediated drug–drug interactions of isavuconazole with rifampin, ketoconazole, midazolam, and ethinyl estradiol/norethindrone in healthy adults. Clin Pharmacol Drug Dev. 2017;6:44–53. doi: 10.1002/cpdd.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran A, Rey E, Pons G, Rousseau M, d’Athis P, Olive G, Mather GG, Bishop FE, Wurden CJ, Labroo R, Trager WF, Kunze KL, Thummel KE, Vincent JC, Gillardin JM, Lepage F, Levy RH. Influence of stiripentol on cytochrome P450-mediated metabolic pathways in humans: in vitro and in vivo comparison and calculation of in vivo inhibition constants. Clin Pharmacol Ther. 1997;62:490–504. doi: 10.1016/S0009-9236(97)90044-8. [DOI] [PubMed] [Google Scholar]
- Tran JQ, Kovacs SJ, McIntosh TS, Davis HM, Martin DE. Morning spot and 24-hour urinary 6 beta-hydroxycortisol to cortisol ratios: intraindividual variability and correlation under basal conditions and conditions of CYP 3A4 induction. J Clin Pharmacol. 1999;39:487–494. [PubMed] [Google Scholar]
- Tran JQ, Petersen C, Garrett M, Hee B, Kerr BM. Pharmacokinetic interaction between amprenavir and delavirdine: evidence of induced clearance by amprenavir. Clin Pharmacol Ther. 2002;72:615–626. doi: 10.1067/mcp.2002.128868. [DOI] [PubMed] [Google Scholar]
- Tsai PC, Glastonbury CA, Eliot MN, Bollepalli S, Yet I, Castillo-Fernandez J, Carnero-Montoro E, Hardiman T, Martin TC, Vickers A, Mangino M, Ward K, Pietilainen KH, Deloukas P, Spector TD, Vinuela A, Loucks EB, Ollikainen M, Kelsey KT, Small KS, Bell JT. PMC6196025; Smoking induces coordinated DNA methylation and gene expression changes in adipose tissue with consequences for metabolic health. Clin Epigenet. 2018;10:126. doi: 10.1186/s13148-018-0558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng A, Hughes CA, Wu J, Seet J, Phillips EJ. Cobicistat versus ritonavir: similar pharmacokinetic enhancers but some important differences. Ann Pharmacother. 2017;51:1008–1022. doi: 10.1177/1060028017717018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi M, Lasker JM, Shimizu M, Rosman AS, Lieber CS. The intralobular distribution of ethanol-inducible P450IIE1 in rat and human liver. Hepatology. 1989;10:437–446. doi: 10.1002/hep.1840100407. [DOI] [PubMed] [Google Scholar]
- Tugnait M, Gupta N, Hanley MJ, Sonnichsen D, Kerstein D, Dorer DJ, Venkatakrishnan K, Narasimhan N. Effects of Strong CYP2C8 or CYP3A inhibition and CYP3A induction on the pharmacokinetics of brigatinib, an oral anaplastic lymphoma kinase inhibitor, in healthy volunteers. Clin Pharmacol Drug Dev. 2020;9:214–223. doi: 10.1002/cpdd.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpeinen M, Raunio H, Pelkonen O. The functional role of CYP2B6 in human drug metabolism: substrates and inhibitors in vitro, in vivo and in silico. Curr Drug Metab. 2006;7:705–714. doi: 10.2174/138920006778520633. [DOI] [PubMed] [Google Scholar]
- Udall JA. Clinical implications of warfarin interactions with five sedatives. Am J Cardiol. 1975;35:67–71. doi: 10.1016/0002-9149(75)90560-3. [DOI] [PubMed] [Google Scholar]
- Ullrich D, Munzel PA, Beck-Gschaidmeier S, Schroder M, Bock KW. Drug-metabolizing enzymes in pharyngeal mucosa and in oropharyngeal cancer tissue. Biochem Pharmacol. 1997;54:1159–1162. doi: 10.1016/s0006-2952(97)00347-x. [DOI] [PubMed] [Google Scholar]
- Van Booven D, Marsh S, McLeod H, Carrillo MW, Sangkuhl K, Klein TE, Altman RB. Cytochrome P450 2C9-CYP2C9. Pharmacogenet Genom. 2010;20:277–281. doi: 10.1097/FPC.0b013e3283349e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bent Martin J, Brandes AA, Rampling R, Kouwenhoven MCM, Kros JM, Carpentier AF, Clement PM, Frenay M, Campone M, Baurain J, Armand J, Taphoorn MJB, Tosoni A, Kletzl H, Klughammer B, Lacombe D, Gorlia T. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol. 2009;27:1268–1274. doi: 10.1200/JCO.2008.17.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijnhoven EM, Boots JM, Christiaans MH, Stolk LM, Undre NA, van Hooff JP. Increase in tacrolimus trough levels after steroid withdrawal. Transpl Int. 2003;16:721–725. doi: 10.1007/s00147-003-0615-1. [DOI] [PubMed] [Google Scholar]
- van Erp NP, Guchelaar HJ, Ploeger BA, Romijn JA, Hartigh J, Gelderblom H. Mitotane has a strong and a durable inducing effect on CYP3A4 activity. Eur J Endocrinol. 2011;164:621–626. doi: 10.1530/EJE-10-0956. [DOI] [PubMed] [Google Scholar]
- van Giersbergen PL, Treiber A, Clozel M, Bodin F, Dingemanse J. In vivo and in vitro studies exploring the pharmacokinetic interaction between bosentan, a dual endothelin receptor antagonist, and glyburide. Clin Pharmacol Ther. 2002;71:253–262. doi: 10.1067/mcp.2002.122473. [DOI] [PubMed] [Google Scholar]
- van Leeuwen DM, van Agen E, Gottschalk RW, Vlietinck R, Gielen M, van Herwijnen MH, Maas LM, Kleinjans JC, van Delft JH. Cigarette smoke-induced differential gene expression in blood cells from monozygotic twin pairs. Carcinogenesis. 2007;28:691–697. doi: 10.1093/carcin/bgl199. [DOI] [PubMed] [Google Scholar]
- van Leeuwen RWF, van Gelder T, Mathijssen RHJ, Jansman FGA. Drug–drug interactions with tyrosine-kinase inhibitors: a clinical perspective. Lancet Oncol. 2014;15:315. doi: 10.1016/S1470-2045(13)70579-5. [DOI] [PubMed] [Google Scholar]
- Varma MV, Pang KS, Isoherranen N, Zhao P. Dealing with the complex drug–drug interactions: towards mechanistic models. Biopharm Drug Dispos. 2015;36:71–92. doi: 10.1002/bdd.1934. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan K, Rostami-Hodjegan A. Come dance with me: transformative changes in the science and practice of drug–drug interactions. Clin Pharmacol Ther. 2019;105:1272–1278. doi: 10.1002/cpt.1433. [DOI] [PubMed] [Google Scholar]
- Villikka K, Varis T, Backman JT, Neuvonen PJ, Kivisto KT. Effect of methylprednisolone on CYP3A4-mediated drug metabolism in vivo. Eur J Clin Pharmacol. 2001;57:457–460. doi: 10.1007/s002280100340. [DOI] [PubMed] [Google Scholar]
- Vishwanathan K, So K, Thomas K, Bramley A, English S, Collier J. Absolute bioavailability of osimertinib in healthy adults. Clin Pharmacol Drug Dev. 2019;8:198–207. doi: 10.1002/cpdd.467. [DOI] [PubMed] [Google Scholar]
- Vital Durand D, Hampden C, Boobis AR, Park BK, Davies DS. 1400797; Induction of mixed function oxidase activity in man by rifapentine (MDL 473), a long-acting rifamycin derivative. Br J Clin Pharmacol. 1986;21:1–7. doi: 10.1111/j.1365-2125.1986.tb02816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyhlidal CA, Riffel AK, Haley KJ, Sharma S, Dai H, Tantisira KG, Weiss ST, Leeder JS. PMC3558855; Cotinine in human placenta predicts induction of gene expression in fetal tissues. Drug Metab Dispos. 2013;41:305–311. doi: 10.1124/dmd.112.049999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner C, Zhao P, Arya V, Mullick C, Struble K, Au S. Physiologically Based pharmacokinetic modeling for predicting the effect of intrinsic and extrinsic factors on darunavir or lopinavir exposure coadministered with ritonavir. J Clin Pharmacol. 2017;57:1295–1304. doi: 10.1002/jcph.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakelee HA, Takimoto CH, Lopez-Anaya A, Chu Q, Middleton G, Dunlop D, Ramlau R, Leighl N, Rowinsky EK, Hao D, Zatloukal P, Jacobs CD, Rodon J. The effect of bexarotene on atorvastatin pharmacokinetics: results from a phase I trial of bexarotene plus chemotherapy in patients with advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2012;69:563–571. doi: 10.1007/s00280-011-1772-z. [DOI] [PubMed] [Google Scholar]
- Walsky RL, Obach RS. A comparison of 2-phenyl-2-(1-piperidinyl)propane (ppp), 1,1′,1″-phosphinothioylidynetrisaziridine (thioTEPA), clopidogrel, and ticlopidine as selective inactivators of human cytochrome P450 2B6. Drug Metab Dispos. 2007;35:2053–2059. doi: 10.1124/dmd.107.015883. [DOI] [PubMed] [Google Scholar]
- Walubo A, Coetsee C, Arti D, Du Plessis JB. The effect of isoniazid containing regimen on CYP2E1 during antituberculosis therapy. Res Commun Mol Pathol Pharmacol. 2005;117–118:137–151. [PubMed] [Google Scholar]
- Wang B, Zhou S. Synthetic and natural compounds that interact with human cytochrome P450 1A2 and implications in drug development. Curr Med Chem. 2009;16:4066–4218. doi: 10.2174/092986709789378198. [DOI] [PubMed] [Google Scholar]
- Wang LS, Zhou G, Zhu B, Wu J, Wang JG, Abd El-Aty AM, Li T, Liu J, Yang TL, Wang D, Zhong XY, Zhou HH. St John’s wort induces both cytochrome P450 3A4-catalyzed sulfoxidation and 2C19-dependent hydroxylation of omeprazole. Clin Pharmacol Ther. 2004;75:191–197. doi: 10.1016/j.clpt.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Wang LS, Zhu B, Abd El-Aty AM, Zhou G, Li Z, Wu J, Chen GL, Liu J, Tang ZR, An W, Li Q, Wang D, Zhou HH. The influence of St John’s Wort on CYP2C19 activity with respect to genotype. J Clin Pharmacol. 2004;44:577–581. doi: 10.1177/0091270004265642. [DOI] [PubMed] [Google Scholar]
- Wang K, Chen S, Xie W, Wan YY. Retinoids induce cytochrome P450 3A4 through RXR/VDR-mediated pathway. Biochem Pharmacol. 2008;75:2204–2213. doi: 10.1016/j.bcp.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ong SS, Chai SC, Chen T. Role of CAR and PXR in xenobiotic sensing and metabolism. Expert Opin Drug Metab Toxicol. 2012;8:803–817. doi: 10.1517/17425255.2012.685237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Chen M, Zhu L, Yu L, Zeng S, Xiang M, Zhou Q. Pharmacokinetic drug interactions with clopidogrel: updated review and risk management in combination therapy. Ther Clin Risk Manag. 2015;11:449–467. doi: 10.2147/TCRM.S80437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang ZY, Powers D, Wang J, Lu S, Kansra V. Rolapitant absolute bioavailability and PET imaging studies in healthy adult volunteers. Clin Pharmacol Ther. 2017;102:332–339. doi: 10.1002/cpt.637. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang ZY, Arora S, Wang J, Lu S, Powers D, Kansra V. Effects of rolapitant administered intravenously on the pharmacokinetics of a modified cooperstown cocktail (midazolam, omeprazole, warfarin, caffeine, and dextromethorphan) in healthy subjects. J Clin Pharmacol. 2018 doi: 10.1002/jcph.1114. [DOI] [PubMed] [Google Scholar]
- Wang Y, Sparidans RW, Li W, Lebre MC, Beijnen JH, Schinkel AH. OATP1A/1B, CYP3A, ABCB1, and ABCG2 limit oral availability of the NTRK inhibitor larotrectinib, while ABCB1 and ABCG2 also restrict its brain accumulation. Br J Pharmacol. 2020;177:3060–3074. doi: 10.1111/bph.15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins PB, Murray SA, Winkelman LG, Heuman DM, Wrighton SA, Guzelian PS. Erythromycin breath test as an assay of glucocorticoid-inducible liver cytochromes P-450. Studies in rats and patients. J Clin Investig. 1989;83:688–697. doi: 10.1172/JCI113933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Banken L, Birnboeck H, Schulz R. Effect of the endothelin-receptor antagonist bosentan on the pharmacokinetics and pharmacodynamics of warfarin. J Clin Pharmacol. 1999;39:847–854. doi: 10.1177/00912709922008380. [DOI] [PubMed] [Google Scholar]
- Weber C, Schmitt R, Birnboeck H, Hopfgartner G, Eggers H, Meyer J, van Marle S, Viischer HW, Jonkman JH. Multiple-dose pharmacokinetics, safety, and tolerability of bosentan, an endothelin receptor antagonist, in healthy male volunteers. J Clin Pharmacol. 1999;39:703–714. doi: 10.1177/00912709922008344. [DOI] [PubMed] [Google Scholar]
- Wen X, Wang J, Neuvonen PJ, Backman JT. Isoniazid is a mechanism-based inhibitor of cytochrome P450 1A2, 2A6, 2C19 and 3A4 isoforms in human liver microsomes. Eur J Clin Pharmacol. 2002;57:799–804. doi: 10.1007/s00228-001-0396-3. [DOI] [PubMed] [Google Scholar]
- Werk EEJ, Macgee J, Sholiton LJ. 441983; effect of diphenylhydantoin on cortisol metabolism in man. J Clin Investig. 1964;43:1824–1835. doi: 10.1172/JCI105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietholtz H, Zysset T, Kreiten K, Kohl D, Buchsel R, Matern S. Effect of phenytoin, carbamazepine, and valproic acid on caffeine metabolism. Eur J Clin Pharmacol. 1989;36:401–406. doi: 10.1007/BF00558303. [DOI] [PubMed] [Google Scholar]
- Wietholtz H, Zysset T, Marschall HU, Generet K, Matern S. The influence of rifampin treatment on caffeine clearance in healthy man. J Hepatol. 1995;22:78–81. doi: 10.1016/0168-8278(95)80263-0. [DOI] [PubMed] [Google Scholar]
- Wilby KJ, Greanya ED, Ford JE, Yoshida EM, Partovi N. A review of drug interactions with boceprevir and telaprevir: implications for HIV and transplant patients. Ann Hepatol. 2012;11:179–185. [PubMed] [Google Scholar]
- Willey JC, Coy EL, Frampton MW, Torres A, Apostolakos MJ, Hoehn G, Schuermann WH, Thilly WG, Olson DE, Hammersley JR, Crespi CL, Utell MJ. Quantitative RT-PCR measurement of cytochromes p450 1A1, 1B1, and 2B7, microsomal epoxide hydrolase, and NADPH oxidoreductase expression in lung cells of smokers and nonsmokers. Am J Respir Cell Mol Biol. 1997;17:114–124. doi: 10.1165/ajrcmb.17.1.2783. [DOI] [PubMed] [Google Scholar]
- Williams ML, Wainer IW, Embree L, Barnett M, Granvil CL, Ducharme MP. Enantioselective induction of cyclophosphamide metabolism by phenytoin. Chirality. 1999;11:569–574. doi: 10.1002/(SICI)1520-636X(1999)11:7<569::AID-CHIR9>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Williams JM, Gandhi KK, Benowitz NL. 2952059; carbamazepine but not valproate induces CYP2A6 activity in smokers with mental illness. Cancer Epidemiol Biomark Prev. 2010;19:2582–2589. doi: 10.1158/1055-9965.EPI-10-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson KM, Patterson JH, McQueen RH, Adams KFJ, Pieper JA. Effects of erythromycin or rifampin on losartan pharmacokinetics in healthy volunteers. Clin Pharmacol Ther. 1998;63:316–323. doi: 10.1016/S0009-9236(98)90163-1. [DOI] [PubMed] [Google Scholar]
- Wind S, Schnell D, Ebner T, Freiwald M, Stopfer P. Clinical pharmacokinetics and pharmacodynamics of afatinib. Clin Pharmacokinet. 2017;56:235–250. doi: 10.1007/s40262-016-0440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongvijitsuk S, Navasumrit P, Vattanasit U, Parnlob V, Ruchirawat M. Low level occupational exposure to styrene: its effects on DNA damage and DNA repair. Int J Hyg Environ Health. 2011;214:127–137. doi: 10.1016/j.ijheh.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Wu KC, Cui JY, Klaassen CD. Effect of graded Nrf2 activation on phase-I and -II drug metabolizing enzymes and transporters in mouse liver. PLoS One. 2012;7:e39006. doi: 10.1371/journal.pone.0039006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Karnik S, Subhadarshini A, Wang Z, Philips S, Han X, Chiang C, Liu L, Boustani M, Rocha LM, Quinney SK, Flockhart D, Li L. An integrated pharmacokinetics ontology and corpus for text mining. BMC Bioinform. 2013;14:35. doi: 10.1186/1471-2105-14-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao CQ, Chen R, Lin J, Wang G, Chen Y, Tan ZR, Zhou HH. Effect of genistein on the activities of cytochrome P450 3A and P-glycoprotein in Chinese healthy participants. Xenobiotica. 2012;42:173–178. doi: 10.3109/00498254.2011.615954. [DOI] [PubMed] [Google Scholar]
- Xu Y, Hashizume T, Shuhart MC, Davis CL, Nelson WL, Sakaki T, Kalhorn TF, Watkins PB, Schuetz EG, Thummel KE. Intestinal and hepatic CYP3A4 catalyze hydroxylation of 1alpha,25-dihydroxyvitamin D(3): implications for drug-induced osteomalacia. Mol Pharmacol. 2006;69:56–65. doi: 10.1124/mol.105.017392. [DOI] [PubMed] [Google Scholar]
- Yadav J, Korzekwa K, Nagar S. Improved predictions of drug–drug interactions mediated by time-dependent inhibition of CYP3A. Mol Pharm. 2018;15:1979–1995. doi: 10.1021/acs.molpharmaceut.8b00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano S, Tatsuno J, Gonzalez FJ. The CYP2A3 gene product catalyzes coumarin 7-hydroxylation in human liver microsomes. Biochemistry. 1990;29:1322–1329. doi: 10.1021/bi00457a031. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Desai A, Goldwater R, Han D, Howieson C, Akhtar S, Kowalski D, Lademacher C, Pearlman H, Rammelsberg D, Townsend R. Pharmacokinetic effects of isavuconazole coadministration with the cytochrome P450 enzyme substrates bupropion, repaglinide, caffeine, dextromethorphan, and methadone in healthy subjects. Clin Pharmacol Drug Dev. 2017;6:54–65. doi: 10.1002/cpdd.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui-Furukori N, Takahata T, Nakagami T, Yoshiya G, Inoue Y, Kaneko S, Tateishi T. Different inhibitory effect of fluvoxamine on omeprazole metabolism between CYP2C19 genotypes. Br J Clin Pharmacol. 2004;57:487–494. doi: 10.1111/j.1365-2125.2004.02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh RF, Gaver VE, Patterson KB, Rezk NL, Baxter-Meheux F, Blake MJ, Eron JJJ, Klein CE, Rublein JC, Kashuba AD. Lopinavir/ritonavir induces the hepatic activity of cytochrome P450 enzymes CYP2C9, CYP2C19, and CYP1A2 but inhibits the hepatic and intestinal activity of CYP3A as measured by a phenotyping drug cocktail in healthy volunteers. J Acquir Immune Defic Syndr. 2006;42:52–60. doi: 10.1097/01.qai.0000219774.20174.64. [DOI] [PubMed] [Google Scholar]
- Yin OQP, Gallagher N, Fischer D, Zhao L, Zhou W, Leroy E, Golor G, Schran H. Effects of nilotinib on single-dose warfarin pharmacokinetics and pharmacodynamics: a randomized, single-blind, two-period crossover study in healthy subjects. Clin Drug Investig. 2011;31:169–179. doi: 10.2165/11538700-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Yokota S, Higashi E, Fukami T, Yokoi T, Nakajima M. Human CYP2A6 is regulated by nuclear factor-erythroid 2 related factor 2. Biochem Pharmacol. 2011;81:289–294. doi: 10.1016/j.bcp.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Oda Y, Nishi S, Abe J, Kaji A, Asada A, Fujimori M. Effect of barbiturate therapy on phenytoin pharmacokinetics. Crit Care Med. 1993;21:1514–1522. doi: 10.1097/00003246-199310000-00020. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Maeda K, Konagaya A, Kusuhara H. Accurate estimation of in vivo inhibition constants of inhibitors and fraction metabolized of substrates with physiologically based pharmacokinetic drug–drug interaction models incorporating parent drugs and metabolites of substrates with cluster newton method. Drug Metab Dispos. 2018;46:1805–1816. doi: 10.1124/dmd.118.081828. [DOI] [PubMed] [Google Scholar]
- Yu J, Ritchie TK, Mulgaonkar A, Ragueneau-Majlessi I. Drug disposition and drug–drug interaction data in 2013 FDA new drug applications: a systematic review. Drug Metab Dispos. 2014;42:1991–2001. doi: 10.1124/dmd.114.060392. [DOI] [PubMed] [Google Scholar]
- Yu A, Tian Y, Tu M, Ho PY, Jilek JL. MicroRNA pharmacoepigenetics: posttranscriptional regulation mechanisms behind variable drug disposition and strategy to develop more effective therapy. Drug Metab Dispos. 2016;44:308–319. doi: 10.1124/dmd.115.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Ritchie TK, Zhou Z, Ragueneau-Majlessi I. Key findings from preclinical and clinical drug interaction studies presented in new drug and biological license applications approved by the food and drug administration in 2014. Drug Metab Dispos. 2016;44:83–101. doi: 10.1124/dmd.115.066720. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhou Z, Owens KH, Ritchie TK, Ragueneau-Majlessi I. What can be learned from recent new drug applications? A systematic review of drug interaction data for drugs approved by the US FDA in 2015. Drug Metab Dispos. 2017;45:86–108. doi: 10.1124/dmd.116.073411. [DOI] [PubMed] [Google Scholar]
- Yu Y, Loi C, Hoffman J, Wang D. Physiologically Based Pharmacokinetic Modeling Of Palbociclib. J Clin Pharmacol. 2017;57:173–184. doi: 10.1002/jcph.792. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhou Z, Tay-Sontheimer J, Levy RH, Ragueneau-Majlessi I. Risk of clinically relevant pharmacokinetic-based drug–drug interactions with drugs approved by the U.S. Food and Drug Administration between 2013 and 2016. Drug Metab Dispos. 2018;46:835–845. doi: 10.1124/dmd.117.078691. [DOI] [PubMed] [Google Scholar]
- Yu J, Petrie ID, Levy RH, Ragueneau-Majlessi I. Mechanisms and clinical significance of pharmacokinetic-based drug–drug interactions with drugs approved by the U.S. Food and Drug Administration in 2017. Drug Metab Dispos. 2019;47:135–144. doi: 10.1124/dmd.118.084905. [DOI] [PubMed] [Google Scholar]
- Zaccara G, Gangemi PF, Bendoni L, Menge GP, Schwabe S, Monza GC. Influence of single and repeated doses of oxcarbazepine on the pharmacokinetic profile of felodipine. Ther Drug Monit. 1993;15:39–42. doi: 10.1097/00007691-199302000-00007. [DOI] [PubMed] [Google Scholar]
- Zand R, Nelson SD, Slattery JT, Thummel KE, Kalhorn TF, Adams SP, Wright JM. Inhibition and induction of cytochrome P4502E1-catalyzed oxidation by isoniazid in humans. Clin Pharmacol Ther. 1993;54:142–149. doi: 10.1038/clpt.1993.125. [DOI] [PubMed] [Google Scholar]
- Zang M, Zhu F, Li X, Yang A, Xing J. PMC4055232; auto-induction of phase I and phase II metabolism of artemisinin in healthy Chinese subjects after oral administration of a new artemisinin–piperaquine fixed combination. Malar J. 2014;13:214. doi: 10.1186/1475-2875-13-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138:103–141. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Zhang H, Custodio JM, Wei X, Wang H, Vu A, Ling J, Martin H, Quirk E, Kearney BP (2017) Clinical pharmacology of the HIV integrase strand transfer inhibitor bictegravir. In: Conference on retroviruses and opportunistic infections: abstract 40
- Zhang W, Heinzmann D, Grippo JF. Clinical pharmacokinetics of vemurafenib. Clin Pharmacokinet. 2017;56:1033–1043. doi: 10.1007/s40262-017-0523-7. [DOI] [PubMed] [Google Scholar]
- Zhang W, McIntyre C, Forbes H, Gaafar R, Kohail H, Beck JT, Plestina S, Bertran E, Riehl T. Effect of rifampicin on the pharmacokinetics of a single dose of vemurafenib in patients with BRAFV600 mutation-positive metastatic malignancy. Clin Pharmacol Drug Dev. 2019;8:837–843. doi: 10.1002/cpdd.643. [DOI] [PubMed] [Google Scholar]
- Zhao B, Zhang W, Yu D, Xu J, Wei Y. Erlotinib in combination with bevacizumab has potential benefit in non-small cell lung cancer: a systematic review and meta-analysis of randomized clinical trials. Lung Cancer. 2018;122:10–21. doi: 10.1016/j.lungcan.2018.05.011. [DOI] [PubMed] [Google Scholar]
- Zhao D, Chen J, Chu M, Long X, Wang J. Pharmacokinetic-based drug–drug interactions with anaplastic lymphoma kinase inhibitors: a review. Drug Des Devel Ther. 2020;14:1663–1681. doi: 10.2147/DDDT.S249098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SF. Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr Drug Metab. 2008;9:310–322. doi: 10.2174/138920008784220664. [DOI] [PubMed] [Google Scholar]
- Zhou HH, Anthony LB, Wood AJ, Wilkinson GR. 1368151; Induction of polymorphic 4′-hydroxylation of S-mephenytoin by rifampicin. Br J Clin Pharmacol. 1990;30:471–475. doi: 10.1111/j.1365-2125.1990.tb03799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SF, Yang LP, Zhou ZW, Liu YH, Chan E. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J. 2009;11(3):481–494. doi: 10.1208/s12248-009-9127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CH, Xu M, Yu HB, Zheng XT, Zhong ZF, Zhang LT. Effects of Danshen capsules on the pharmacokinetics and pharmacodynamics of clopidogrel in healthy volunteers. Food Chem Toxicol. 2018;119:302–308. doi: 10.1016/j.fct.2018.02.051. [DOI] [PubMed] [Google Scholar]
- Zilly W, Breimer DD, Richter E. Induction of drug metabolism in man after rifampicin treatment measured by increased hexobarbital and tolbutamide clearance. Eur J Clin Pharmacol. 1975;9:219–227. doi: 10.1007/BF00614021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data are available in the text and tables of the review.