Abstract
CMV (cucumber mosaic virus) is the most primitive virus infecting chilli (Capsicum annuum. L). The mosaic incidence with leaf filiformity, mosaic mottling and stunted growth was observed in major chilli growing regions of Tamil Nadu. CMV sap was inoculated on chilli, cowpea, bitter gourd, bottle gourd, ridge gourd, banana, cucumber, Nicotiana and Chenopodium plants. Host range studies revealed that CMV produced localized infection on Nicotiana and systemic symptoms on most of the test plants. The occurrence of CMV was confirmed through DAC-ELISA and RT-PCR analysis. Host plant samples tested with DAC-ELISA showed strong reaction with 1.7 optical density. For molecular characterization, total RNA isolated from infected plants used in RT-PCR with CMV specific primers. The specific amplicons were cloned and sequenced. The complete genome sequencing depicts CMV-RNA1 consist of 3339 nucleotides (nt), RNA2 and RNA3 with 3052nt and 2027nt respectively. Phylogenetic and nucleotide sequence analysis showed TN CMV isolates closely associated with subgroup IB rather than subgroup IA and II. Comparative sequence analysis indicates replicase protein to be more variable among five genes. CP sequence analysis showed 97–98 per cent identity with subgroup IB strains, 92–93 per cent identity with subgroup IA strains and 81–82 per cent identity with subgroup II strains. CMV-RNA3 was predicted to have recombination with Indian black pepper isolate (KU947031) between 165-505nt and Egyptian tomato isolate (KX014666) between 165-506nt positions.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02492-y) contains supplementary material, which is available to authorized users.
Keywords: CMV (cucumber mosaic virus), Chilli, RT-PCR, DAC-ELISA, Subgroup IB, Recombination and phylogenetic analysis
Introduction
Chilli (Capsicum annuum. L) is one of the most important commercial spice and vegetable crop in the world. India is the world's largest producer, consumer and exporter of chillies in the world (Iqbal et al. 2012). In India, chilli cultivated in parts of Andhra Pradesh, Telangana, Tamil Nadu, Karnataka and Madhya Pradesh (Anonymous 2019). Chilli is known to be infected by more than 30 economically important viruses (Zehra et al. 2017). Amongst, CMV (family: Bromoviridae; genus: Cucumovirus) is the most predominant and destructive virus pose a serious threat on chilli cultivation (Ramesh and Sreenivasulu 2018). It has a wider host range and infects more than 1287 plant species (Meena and Manivel 2019). CMV is transmitted by aphids in non-persistent manner and in some cases, through seeds (Palukaitis and Garcia-Arenal 2003). CMV first reported back on cucumber in 1916 (Doolittle 1916), since then the diseases were found simultaneously in chilli growing areas (Rahman et al. 2016). CMV is a sense, single-stranded RNA virus with isometric particles (28–30 nm diameter) and has tripartite genome (RNA1, RNA2 and RNA3) encodes five open reading frames (ORFs) (Palukaitis and Garcia-Arenal 2003). In general, monocistronic RNA1 encodes 1a protein (replicase) and RNA2 encodes 2a (replicase) and 2b protein (silencing suppressor). Alike, dicistronic RNA3 encodes 3a (movement protein) and 3b protein (coat protein) respectively (Revathy and Bhat 2017; Mochizuki and Ohki 2012). The coat protein (CP) is encoded by RNA3 but expressed from subgenomic RNA4 (Palukaitis and Garcia-Arenal 2003). CMV has been categorized into subgroup I and II based on serology, host range and peptide mapping. Further, subgroup I is divided into subgroup IA and IB based on 5′ non-coding region of RNA3 and sequence similarity (Roossinck 2001; Revathy and Bhat 2017; Nagendran et al. 2018). Subgroup IA and II have worldwide distribution whereas subgroup IB found in Asian countries and comparatively, subgroup I strains are more virulent than subgroup II strains. High genetic diversity of CMV provides an insight about role of genes in the virulence of virus (Mochizuki and Ohki 2012). CMV is difficult to manage due to its wider host range and lack of genetic resistance (Ntui et al. 2014). Understanding about genetic structure, diversity and evolutionary history of the viral population is an important aspect for developing management strategies. Moreover, CMV undergoes rapid genetic changes by genetic recombination and reassortment (Nouri et al. 2014; Pavithra et al. 2019). Hence, complete genome analysis is vital for the better conception about genetic structure and evolution of CMV. Here, we report on molecular properties, nucleotide diversity, phylogenetic and evolutionary relationship of CMV chilli isolate with Indian and other CMV isolates.
Materials and methods
Sample collection and inoculum maintenance
A survey was conducted during 2018 and 2019 in Western (Coimbatore, Dindigul and Theni), North Eastern (Thiruvannamalai), Southern (Thenkasi) and North Western (Namakkal, Salem and Krishnagiri) agro-climatic zones of Tamil Nadu, India. Infected chilli plants showing systemic mosaic, leaf malformation and leaf filiformity along with stunted growth were collected. Preliminary confirmation of CMV was done through RT-PCR using CMV coat protein gene-specific primer. Three TN CMV isolates (TN chilli 1, TN chilli 2 and TN chilli 3) were mechanically inoculated and maintained in cowpea and chilli plants as virus source for further analysis.
Symptomatological study
For host range study, CMV isolate was inoculated on Vigna unguiculata L. Walp, Capsicum annuum. L., Lagenaria siceraria L., Luffa acutangula (L) Roxb., Musa spp, Momordica charantia Linn., Nicotiana plumbaginifolia Viv., Solanum torvum, Cucumis sativus, Chenopodium amaranticolor and Nicotiana benthamiana at two-leaf stage under insect-proof greenhouse conditions. Infected chilli leaf samples (TN chilli 3 isolate) were grounded using phosphate buffer (pH-7), carborundum (600 mesh) and 0.02% mercaptoethanol. The sample extract inoculated on host plants by gentle abrasion on the leaf surface (Subramanian and Narayanasamy 1973). The inoculated plants were maintained at 30 ± 2ºC and observed for the symptom expression. Furthermore, virus transmission was confirmed through DAC-ELISA and RT-PCR analysis.
DAC-ELISA
Direct antigen coating ELISA was performed according to the methodology of Hobbs et al. (1987). Test plant samples with positive and negative control were ground using coating buffer (1:1, 1:20 and 1:50 dilutions) and centrifuged at 10,000 rpm for 10 min. 100 µl of crude extract was dispensed into wells of polystyrene ELISA plate and incubated at 37 °C for 3 hr. After incubation, the plate was washed thrice with PBS-Tween buffer at every step. Then, 100 µl of blocking buffer added and incubated at 37 °C for 3 hr. After that, 100 µl of polyclonal antiserum (obtained from ICAR-NRCB, Tiruchirappali, India) at 1:1000 dilutions was added into each wells and incubated. Subsequently, 100 µl of goat anti-rabbit enzyme-conjugated (Sigma) antiserum (1:5000) added and incubated. After repeated washings, 100 µl of substrate (p-nitro-phenyl-phosphate) added in dark and kept for 15–20 min. The absorbance was read at 405 nm using a microplate reader (BioTek).
RNA extraction and RT-PCR
Total RNA was extracted from the symptomatic leaf samples using TRIzol reagent (Chomczynski and Sacchi 2006). The reverse transcription step was performed to synthesize the first-strand complementary DNA (Thermo Fisher Scientific). The reaction mixture (9 µl–sterile water, 4 µl–5 × reaction buffer, 2 µl–dNTPs, 1 µl–random primer, 1 µl–reverse transcriptase, 1 µl–RNase inhibitor and 1 µg–total RNA) was prepared and incubated at 42 ºC for 60 min followed by 70 ºC for 5 min (Ramesh and Sreenivasulu 2018). To confirm the presence of CMV in test plants, replicase gene fragment was amplified through RT-PCR with the conditions of 94 ºC for 2 min of initial denaturation, 94 ºC for 30 s of denaturation, 48–56 ºC for 30 s of annealing, 72 ºC for 90 s of extension and 72 ºC for 10 min of final extension with 35 cycles.
Cloning of genome and phylogenetic analysis
The full-length genome of CMV was characterized by CMV specific primers covering RNA1 (3.3 kb), RNA2 (3 kb) and RNA3 (2.2 kb). RNA2 genome-specific primers were designed using Prime3 software and validated by multiple sequence alignment (CLUSTALW) of different CMV isolates retrieved from NCBI database (Supplementary Table 1). Besides, RNA1 and RNA3 specific primers were referred from Nagendran et al. (2018) (Supplementary Table 2). Amplification carried out with the cyclic condition of 94 ºC for 2 min of denaturation, annealing and extension specific to respective amplicons. The amplified products were cloned into pGEM-T Easy vector (Promega) and sequenced at both orientations with M/s Barcode Biosciences, Bangalore. The consensus sequences were assembled using BIOEDIT sequence alignment editor version 7.0. Database searches carried out with NCBI BLAST (https://blast.ncbi.nlm.nih.gov). Multiple alignments of nucleotide and amino acid sequences were carried out using CLUSTALW (www.ebi.ac.uk). The phylogenetic tree was constructed using MEGA 7.0 software (www.megasoftware.net) by Neighbor-joining tree method with 1000 bootstrap replication (Kumar et al. 2016). Recombination breakpoints were detected using RDP4 program with RDP, GENECONV, Bootscan, MacCN, MaxChi, SiScan, Phylopro and LARD methods. The analysis was carried out with default settings for different detection programs and Bonferroni-corrected p-value of 0.01. Furthermore, recombination events were reconfirmed using original SiScan method (Martin et al. 2015).
Results
CMV is the most bounteous virus infecting most of the vegetable crops throughout the globe. To evaluate the distribution of CMV, major chilli growing areas of Tamil Nadu were surveyed during 2018 and 2019 and the disease incidence was ranging from 50 to 60% in different agro-climatic zones of Tamil Nadu. Total 56 leaf samples were collected from chilli crops of different zones. Among them, 21 samples from the western zone, 7 samples from the north eastern zone, 9 samples from the southern zone and 19 samples from the north western zone were collected. The characteristic symptoms of CMV observed were systemic mosaic, leaf malformation, leaf filiformity (shoestring) and puckering along with stunted growth (Fig. 1). The preliminary screening to confirm the presence of CMV was done through RT-PCR using coat protein (CP) specific primer. Among 56 samples, 43 samples tested positive for CMV in RT-PCR. Based on the partial sequencing, Coimbatore (TN chilli 1), Theni (TN chilli 2) and Thenkasi (TN chilli 3) isolates, which showed more nucleotide variations, were used for further analysis.
Fig. 1.
Symptoms of CMV infected chilli plants: a, b Mosaic with puckering symptom, c Mosaic with leaf filiformity, d, e Mosaic mottling, f Stunted growth
Host range studies will help to understand the symptomology and transmission nature of CMV on different host plants. Since there was no difference in symptomatology between the CMV isolates (data not shown), a representative TN chilli 3 isolate maintained on chilli plant was sap inoculated on to different host plants. The inoculated host plants produced systemic mosaic and localized chlorotic and necrotic lesions after 5–7 days of post inoculation (dpi). The localized chlorotic and necrotic lesions on Nicotiana spp was observed at 4–5 dpi and Lagenaria siceraria produced chlorotic spots after 8 dpi. The systemic mosaic recorded in Momordica, Musa spp and Solanum torvum and severe mosaic with puckering symptom observed in Luffa acutangula after 7–10 dpi. The inoculated Vigna unguiculata and Chenopodium produced mosaic symptom at 3–6 dpi. The mosaic mottling observed in Cucumis sativus at 8–9 dpi and distinct mosaic mottling with filiformity was observed in Capsicum at 7–10dpi (Fig. 2). Symptomatological study indicated the wider host range and transmission nature of TN chilli 3 isolate to a number of test plants (Table 1). To confirm the symptomatological study, test plant samples were subjected to DAC-ELISA and RT-PCR analysis. DAC-ELISA depict that all of the test plant samples were positive at 1:10, 1:20 and 1:50 dilutions for CMV. The results indicated the minimal absorbance in Musa (0.8) and maximum absorbance in Capsicum annuum and Luffa acutangula followed by Momordica charantia, Cucumis sativus and Nicotiana spp. (1.5–1.7). Furthermore, test plants were confirmed through RT-PCR using replicase gene-specific primer (GKCMV Rep1F and GKCMV Rep1R) (Supplementary Fig. 1).
Fig. 2.
Symptomology of CMV upon sap inoculation: a Luffa acutangula, b Cucumis sativus, c Solanum torvum, d Lagenaria siceraria, e Capsicum annuum, f Leaf filiformity on chilli, g Vigna unguiculata, h Chenopodium amaranticolor, i Nicotiana plumbaginifolia (chlorotic lesion), j Momordica charantia, k Nicotiana plumbaginifolia (necrosis), l Nicotiana benthamiana, m Musa spp
Table 1.
Host response of sap inoculated CMV TN chilli isolate on different test plants
| Test plant(s) | Symptomology | Days taken for symptom expression | RT-PCR | DAC-ELISA |
|---|---|---|---|---|
| Luffa acutangula | Systemic mosaic; puckering | 7–10 days | + | + |
| Vigna unguiculata | Systemic mosaic | 3–4 days | + | + |
| Momordica charantia | Mosaic symptom | 6–7 days | + | + |
| Nicotiana benthamiana | Localized chlorotic lesions | 4–5 days | + | + |
| Nicotiana plumbaginifolia | Initially localized chlorotic lesions turns into necrotic lesion | 4–5 days | + | + |
| Solanum torvum | Yellow mosaic on inoculated leaves | 7–8 days | + | + |
| Capsicum annuum | Initially mosaic mottling, later filiformity of leaves | 7–10 days | + | + |
| Musa spp. | Mosaic symptoms on inoculated leaves | 10–15 days | + | + |
| Lagenaria siceraria | Chlorotic spots with yellow halo | 8–9 days | + | + |
| Cucumis sativus | Mosaic mottling | 8–9 days | + | + |
| Chenopodium amaranticolor | Mosaic symptom | 5–6 days | + | + |
The complete genome of TN Chilli 1, TN Chilli 2 and TN Chilli 3 isolates were amplified by RT- PCR using 10 sets of primers pairs and the aligned sequences submitted to NCBI Genbank (Supplementary Table 1). From the sequence analysis, the complete genome of CMV chilli isolates comprised of RNA1 (3339nt) with single ORF (1a), RNA2 (3052nt) with two ORFs (2a and 2b) and RNA3 (2207nt) with two ORFs (3a and 3b) (Supplementary Fig. 2). ORF1 encodes replicase protein (1a) contains 2982nt translated into 993 amino acids (aa). N-terminal region of replicase protein has putative amino acid methytransferase domain (1-146aa) and C-terminal region has helicase motif (646-993aa). Sequence analysis of TN chilli 1 and TN chilli 2 isolates showed 97.5–98% identity with Karnataka isolate followed by 97–97.50% identity with Kerala isolate of RNA1 segment. Likewise, TN chilli 3 isolate showed maximum identity (97.78%) with Kerala isolate followed by Karnataka isolate (97.20%) of RNA1 segment. The phylogenetic analysis of RNA1 showed two separate cluster formation of subgroup I and subgroup II. Indeed TN chilli 1 (MT410980), TN chilli 2 (MT410979) and TN chilli 3 (MT410981) isolates were grouped together with other isolates of subgroup I (Fig. 3). The comparative multiple sequence alignment revealed that lesser variation in the nucleotide sequences in the central region (1132-2760nt) than terminal regions. The amino acid 461 (Arg461 or Cys461) of 1a gene (replicase protein) is a determinant of necrosis induction. Indeed, TN CMV isolates possess Arg461 amino acid in 1a protein.
Fig. 3.
Phylogenetic analysis of TN chilli isolates with other CMV isolates reported worldwide based on RNA1 by MEGA 7.0. The evolutionary history was inferred from neighbor-joining method with 1000 bootstrap replication. Groundnut bud necrosis virus (MH591413) included as out group
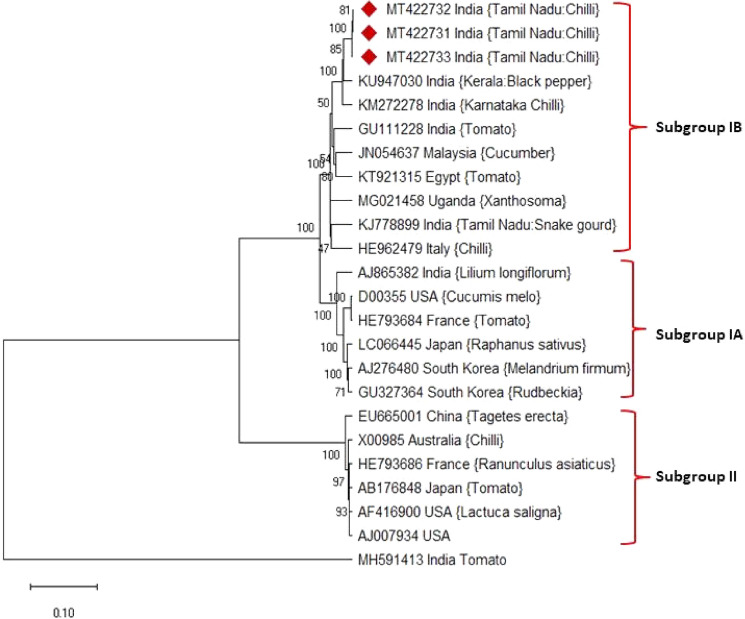
ORF 2a encodes larger replicase protein (2a) contains 2755nt translated to 859aa with GDD motif for RNA dependent RNA polymerase and ORF 2b encodes small silencing suppressor protein (2b) expressed from overlapping 3′ terminal of ORF 2a contains 336nt translated into 112aa. 2b gene is a viral suppressor of post-transcriptional gene silencing besides determinant of host range. RNA2 of TN chilli isolates showed 97–96.50% identity with Kerala isolate followed by 96–96.50% identity with Karnataka isolate of RNA2 segment. The phylogenetic analysis of RNA2 illustrates that TN chilli 1 (MT422732), TN chilli 2 (MT422731) and TN chilli 3 (MT422733) isolates were clustered with members of subgroup I isolates (Fig. 4). The comparative amino acid sequence analysis of 2a protein indicates highly conserved Gly-Asp-Asp amino acid sequence at 609–611 position essential for the replication of the virus. Furthermore, the amino acid substitution of Arg46 observed in the 2b protein of TN CMV isolates. ORF3a encodes movement protein (3a) contains 840nt (280aa) and a subgenomic RNA encodes the coat protein (3b) contain 657nt translated into 219aa. RNA3 of TN chilli 1 and TN chilli 2 isolates showed 96–97% identity with Karnataka isolate followed by 96% identity with Kerala isolate of RNA3 segment. Similarly, TN chilli 3 isolate had maximum identity (97.25%) with Kerala isolate followed by 96.78% identity with Karnataka isolate. The comparative amino acid sequence analysis of RNA3 revealed Ser14˗ Arg14 amino acid substitution in movement protein and Ser129 ˗ Pro129amino acid substitution in the coat protein of TN chilli 3 isolate.
Fig. 4.
Phylogenetic analysis of TN chilli isolates with other CMV isolates reported worldwide based on RNA2 by MEGA 7.0. The evolutionary history was inferred from neighbor-joining method with 1000 bootstrap replication. Groundnut bud necrosis virus (MH591413) included as out group
The phylogenetic analysis of CP sequence showed separate cluster formation of CMV subgroup I and II strains (Fig. 5). Precisely, TN chilli isolates clustered with subgroup IB clade which indicates TN chilli isolates belong to subgroup IB. The nucleotide sequences of CP showed 97–98% identity with subgroup IB, 92–93% identity with subgroup IA and 81–82% per cent identity with subgroup II. Comparably, amino acid sequences of CP indicated 98.33–98.93 per cent identity with members of subgroup IB, 92.15–92.85 per cent identity with subgroup IA and 75.19–75.98 per cent identity with subgroup II. The recombination analysis of RNA3 showed TN chilli 3 isolates to have recombination with Indian black pepper isolate (KU947031) between 166-505nt as a major parent and Egyptian tomato isolate (KX014666) between 166 and 506nt position as a minor parent (Supplementary Fig. 3). The recombination was predicted using RDP, GENECONV, Bootscan, MacCN, MaxChi, Phylopro and LARD methods with 4.98 × 10–11 of p-value and SiScan with 7.89 × 10−23of p-value (Supplementary Table. 3).
Fig. 5.
Phylogenetic analysis of TN chilli isolates with other CMV isolates reported worldwide based on RNA3 (CP) by MEGA 7.0. The evolutionary history was inferred from neighbor-joining method with 1000 bootstrap replication. Groundnut bud necrosis virus (MH591413) included as out group
Discussion
CMV is the most devastating virus with a wider host range and reported on most of the vegetable crops. Characterization of virus gives insight about genomic properties, genetic composition, variability (mutation or recombination), virulent determinant and evolutionary of virus (Dubey and Singh 2010). The better understanding on genetic structure, host range and evolution of the virus is crucial for disease diagnostic and management strategies (Revathy and Bhat 2017; García and Fraile 2013). This study evinces the prevalence of CMV over chilli growing areas of Tamil Nadu. Presence of CMV in collected samples was detected by RT-PCR as preliminary screening. Symptomatological study indicated the differential host range and sap transmissibility of TN chilli isolate of CMV. The sap inoculation of TN chilli 3 isolates on different host plants produced systemic and localized infection. Indeed, the systemic infection was observed in most of the host plants and Nicotiana spp. produced localized infection. The mechanical inoculation of snake gourd CMV isolate on Nicotiana glutinosa produced chlorotic rings, later turn into necrotic lesions (Nagendran et al. 2018). Furthermore, subgroup I strains of CMV certainly produce symptom and disease development on Nicotiana (Zhang et al. 1994). Similarly, sap transmission of CMV coleus isolates induced systemic mosaic mottling and leaf deformation on Capsicum annuum, Cucumis sativus and Cucurbita pepo plants (Pavithra et al. 2019). However, accumulating evidences suggest that, severe mosaic and stunting symptom developed by most of the subgroup I strains, in contrary most of subgroup II strains shows mild symptom or asymptomatic (Roossinck 2001; Mochizuki and Ohki 2012; Pavithra et al. 2019). In correlation with host plants response, TN CMV isolate alleged as subgroup I. All test plants showed a positive response in DAC-ELISA and maximum virus titer in the infected plants recorded as 1.5–1.7 OD. Comparably, higher absorbance reading of CMV in Catharanthus recorded as > 2.0, which suggest the occurrence of CMV at higher concentration in host plants (Mazidah et al. 2012). Besides, RT-PCR method is more sensitive than ELISA to detect the viruses in plant samples (Dubey and Singh 2010). Hence, CMV in test plants was also confirmed by RT-PCR using replicase gene-specific primer. The test plants showed a positive response in DAC-ELISA amplified for CMV in RT-PCR as well.
The complete genomes of CMV isolates of chilli were characterized by assembling the sequences. Based on sequence analysis, CMV genome (RNA1, RNA2 and RNA3) of three TN isolates shared maximum homology (96–98%) with chilli isolate of Karnataka and black pepper isolate of Kerala. RNA1 encodes 1a protein merely responsible for the synthesis of positive-strand RNAs (Mochizuki and Ohki 2012). Besides, amino acid 461 is a genetic determinant solely responsible for necrosis induction in the host (Salanki and Balazs 2004). The hydrophilic and hydrophobic α-helix domain observed betwixt of amino acid 443–472. Hence, amino acid 461 localized between the faces of these two α-helixes (Salanki and Balazs 2004). Mutation in amino acid 461 (C˗R, K, P or E) causes changes in amphiphilicity and integrity of helix structure led to alterations in interaction and membrane affinity of 1a protein with host factors (Mochizuki and Ohki 2012). Indeed, TN isolates induced necrotic lesions on Nicotiana in host range study may have a correlation with amino acid Arg461 of 1a protein for necrosis induction.
RNA2 encodes 2a protein is a component of the replicase complex and 2b gene is a viral suppressor of host gene silencing. 2a protein contains GDD motif (Gly-Asp-Asp) at 609–611 amino acid position conserved among all CMV isolates. It plays a vital role in the replication of virus by the synthesis of RdRp (Hu et al. 2012). 2b gene is a viral suppressor besides that involved in symptom induction in the host plant. Moreover, 2b gene attributed for the induction of mosaic and stunting symptoms in the host plant (Ding et al. 1995; Zhang et al.2006; Du et al. 2008). Indeed TN chilli isolates possess amino acid substitution of Arg46 in 2b protein. Lewsey et al. (2009) demonstrated the mutation of putative phosphorylation sites attenuated symptom expression without affecting siRNA binding property of 2b protein. In addition, a single amino acid (Arg46) of 2b gene associated with symptom severity of CMV in the host plant (Goto et al. 2007).
Movement protein (3a) encoded by RNA3 is vital for the systemic movement of the virus. Amino acid 14 is a putative phosphorylation site involved in polymerase interaction. Hwang et al. (2007) stated that amino acid 14 (Ser14) is critical for the interaction of movement protein with N-terminal amino acids and GDD motif of 2a protein. In contrary, TN chilli isolates showed amino acid substitution (serine to arginine) at 14th position of movement protein although, significant impacts of the amino acid substitution is not well understood. Coat protein of RNA3 is a fundamental determinant of encapsidation, symptom induction, aphid transmission, host range and movement of virus (Srivastava and Raj 2008; Takahashi et al. 2001; Mochizuki and Ohki 2012). Shoot apical meristem (SAM) tissues resist virus invasion by rapid RNAsilencing activity (Ratcliff et al. 1997; Valentine et al. 2002). Interestingly, RNA3 of CMV found to be required for the successful invasion of shoot apical meristem (SAM). In specific, amino acid 129 of coat protein is a preliminary determinant of SAM invasion (Mochizuki and Ohki 2005). Earlier studies demonstrated the point mutation of amino acid 129 (serine to proline) in the coat protein of CMV isolate (Y-CMV) detected in meristem tissues. Undeniably, coat protein gene sequence analysis indicates amino acid substitution of Ser129 ˗ Pro129 in TN chilli 3 isolate. The phylogenetic analysis of CP revealed TN CMV isolates closely associated with subgroup IB and clustered with Indian chilli isolates (HM348784 and KM272275) and black pepper isolate (KU947031) along with other members of subgroup IB. Nagendran et al. (2018) categorized TN snake gourd CMV isolates as subgroup IB based on CP gene sequence and these TN isolates clustered with a banana isolate of Kerala along with other Indian isolates under subgroup IB. Recombination analysis illustrates TN chilli 3 isolate have evolutionary ties with Indian black pepper isolate as a major parent and Egyptian tomato isolate as a minor parent. Apparently, the evolution of CMV presumably occurs due to the genetic exchange of recombination and reassortment (Pavithra et al. 2019; Bonnet et al. 2005; Roossink 2002). Comparatively, TN isolate 3 exhibited more variation in amino acids rather than other isolates. Among the tripartite genome, RNA2 showed more variable than RNA1 and RNA3 at amino acid and nucleotide levels. Among five genes, coat protein and movement protein genes observed to be with lesser variation in amino acids than replicase and suppressor genes. Previous studies also suggest that 2b gene is the most variable and coat protein is the most conserved among five genes of CMV at nucleotide and amino acid levels (Revathy and Bhat 2017; Koundal et al. 2011; Roossink 2002; Nouri et al. 2014).
Genome analysis of CMV gives insight into the genetic structure and evolutionary history of a virus. A single alteration in amino acids will imply on virion stability, vector transmission, symptom induction and movement of the virus. The evolutionary constrain imposed on the virus may inflict this apparent variation in amino acids and nucleotides. Hence, the variation in the genetic composition can occur due to recombination or reassortment led to the emergence of new phenotypes and interspecies transmission. In the present study, we characterized the complete genome of TN chilli isolates of CMV infecting chilli and depicted the genetic properties. Based on genome sequencing and phylogenetic relationships, TN chilli isolates found as subgroup IB. The evolution of TN isolates identified from Indian black pepper isolate by recombination between 165-505nt and Egyptian tomato isolate by recombination between 165-506nt positions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are thankful for Department of Biotechnology, NER- BPMC, New Delhi for the financial assistance and Professor and Head, Department of Plant Pathology, TNAU, Coimbatore for providing facilities during the course of study.
Compliance with ethical standards
Conflict of interest
Authors declare no conflict of interest.
Research involving in human and animal participants
This manuscript does not contain any experiments involving human or animals participants.
Contributor Information
J. Vinodhini, Email: vinojeeva194@gmail.com
L. Rajendran, Email: rajendran.l@tnau.ac.in
M. Raveendran, Email: raveendran.m@tnau.ac.in
V. Rajasree, Email: dr.rajashreeprabhu@gmail.com
G. Karthikeyan, Email: agrikarthi2003@gmail.com
References
- Anonymous . Chilli outlook. PJTSAU: Agricultural Market Intelligence Centre; 2019. [Google Scholar]
- Bonnet J, Fraile A, Sacristán S, Malpica JM, García-Arenal F. Role of recombination in the evolution of natural populations of cucumber mosaic virus, a tripartite RNA plant virus. Virology. 2005;332(1):359–368. doi: 10.1016/j.virol.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction: twenty-something years on. Nat Protoc. 2006;1(2):581–585. doi: 10.1038/nprot.2006.83. [DOI] [PubMed] [Google Scholar]
- Ding B, Li Q, Nguyen L, Palukaitis P, Lucas WJ. Cucumber mosaic virus 3a protein potentiates cell-to-cell trafficking of CMV RNA in tobacco plants. Virology. 1995;207(2):345–353. doi: 10.1006/viro.1995.1093. [DOI] [PubMed] [Google Scholar]
- Doolittle SP. A new infectious mosaic disease of cucumber. Phytopathology. 1916;6:145–147. [Google Scholar]
- Du Z, Chen F, Zhao Z, Liao Q, Palukaitis P, Chen J. The 2b protein and the C-terminus of the 2a protein of cucumber mosaic virus subgroup I strains both play a role in viral RNA accumulation and induction of symptoms. Virology. 2008;380(2):363–370. doi: 10.1016/j.virol.2008.07.036. [DOI] [PubMed] [Google Scholar]
- Dubey VK, Singh VP. Molecular characterization of cucumber mosaic virus infecting Gladiolus, revealing its phylogeny distinct from the Indian isolate and alike the Fny strain of CMV. Virus Genes. 2010;41(1):126–134. doi: 10.1007/s11262-010-0483-6. [DOI] [PubMed] [Google Scholar]
- García-Arenal F, Fraile A. Trade-offs in host range evolution of plant viruses. Plant Pathol. 2013;62:2–9. [Google Scholar]
- Goto K, Kobori T, Kosaka Y, Natsuaki T, Masuta C. Characterization of silencing suppressor 2b of cucumber mosaic virus based on examination of its small RNA-binding abilities. Plant Cell Physiol. 2007;48(7):1050–1060. doi: 10.1093/pcp/pcm074. [DOI] [PubMed] [Google Scholar]
- Hobbs HA, Reddy DVR, Rajeshwari R, Reddy AS. Use of direct antigen coating and protein a coating ELISA procedures. Plant Dis. 1987;71(8):747–749. [Google Scholar]
- Hu Z, Zhang T, Yao M, Feng Z, Miriam K, Wu J, Zhou X, Tao X. The 2a protein of cucumber mosaic virus induces a hypersensitive response in cowpea independently of its replicase activity. Virus Res. 2012;170(1–2):169–173. doi: 10.1016/j.virusres.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Hwang MS, Kim KN, Lee JH, Park YI. Identification of amino acid sequences determining interaction between the cucumber mosaic virus-encoded 2a polymerase and 3a movement proteins. J Gen Virol. 2007;88(12):3445–3451. doi: 10.1099/vir.0.83207-0. [DOI] [PubMed] [Google Scholar]
- Iqbal S, Ashfaq M, Shah H. Prevalence and distribution of cucumber mosaic virus (CMV) in major chilli growing areas of Pakistan. Pak J Bot. 2012;44(5):1749–1754. [Google Scholar]
- Koundal V, Haq QMR, Praveen S. Characterization, genetic diversity, and evolutionary link of cucumber mosaic virus strain New Delhi from India. Biochem Genet. 2011;49:25–38. doi: 10.1007/s10528-010-9382-8. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewsey M, Surette M, Robertson FC, Ziebell H, Choi SH, Ryu KH, Canto T, Palukaitis P, Payne T, Walsh JA, Carr JP. The role of the cucumber mosaic virus 2b protein in viral movement and symptom induction. Mol Plant Microbe Interact. 2009;22(6):642–654. doi: 10.1094/MPMI-22-6-0642. [DOI] [PubMed] [Google Scholar]
- Martin DP, Murrell B, Golden M, Khoosal A, Muhire B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus evolution. 2015;1(1):1–5. doi: 10.1093/ve/vev003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazidah M, Yusoff K, Habibuddin H, Tan YH, Lau WH. Characterization of cucumber mosaic virus (CMV) causing mosaic symptom on Catharanthus roseus (L.) G. Don in Malaysia. Pertanika J Trop Agric Sci. 2012;35(1):41–53. [Google Scholar]
- Meena RP, Manivel P. First report of cucumber mosaic virus infecting antamul vine (Tylophora indica) in India. Virus disease. 2019;30(2):319–320. doi: 10.1007/s13337-018-0501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T, Ohki ST. Amino acid 129 in the coat protein of cucumber mosaic virus primarily determines invasion of the shoot apical meristem of tobacco plants. J Gen Plant Pathol. 2005;71(4):326–332. [Google Scholar]
- Mochizuki T, Ohki ST. Cucumber mosaic virus: viral genes as virulence determinants. Mol plant pathol. 2012;13(3):217–225. doi: 10.1111/j.1364-3703.2011.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagendran K, Priyanka R, Aravintharaj R, Balaji CG, Prashant S, Basavaraj B, Mohankumar S, Karthikeyan G. Characterization of cucumber mosaic virus infecting snake gourd and bottle gourd in India. Physiol Mol Plant Pathol. 2018;103:102–106. [Google Scholar]
- Nouri S, Arevalo R, Falk BW, Groves RL. Genetic structure and molecular variability of cucumber mosaic virus isolates in the United States. PLoS ONE. 2014;9(5):e96582. doi: 10.1371/journal.pone.0096582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntui VO, Kong K, Azadi P, Khan RS, Chin DP, Igawa T, Mii M, Nakamura I. RNAi-mediated resistance to cucumber mosaic virus (CMV) in genetically engineered tomato. Am J Plant Sci. 2014;23:168–175. [Google Scholar]
- Palukaitis P, Garcia-Arenal F. Cucumoviruses. Adv Virus Res. 2003;62:241–323. doi: 10.1016/s0065-3527(03)62005-1. [DOI] [PubMed] [Google Scholar]
- Pavithra BS, Govin K, Renuka HM, Krishnareddy M, Jalali S, Samuel DK, Himabindu K. Characterization of cucumber mosaic virus infecting coleus (Plectranthus barbatus) in Karnataka. Virus Dis. 2019;30(3):403–412. doi: 10.1007/s13337-019-00536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MS, Akhter MS, Alam MM, Pervin N, Akanda AM. Prevalence of cucumber mosaic virus and its impact on growth and yield of different chili cultivar. Bull Inst Trop Agr, Kyushu Univ. 2016;39:65–74. [Google Scholar]
- Ramesh B, Sreenivasulu P. Detection of cucumber mosaic virus in brinjal and chilli by RT-PCR. Int J Pharm Biol Sci. 2018;8(3):128–131. [Google Scholar]
- Ratcliff F, Harrison BD, Baulcombe DC. A similarity between viral defense and gene silencing in plants. Science. 1997;276:1558–1560. doi: 10.1126/science.276.5318.1558. [DOI] [PubMed] [Google Scholar]
- Revathy KA, Bhat AI. Complete genome sequencing of cucumber mosaic virus from black pepper revealed rare deletion in the methyltransferase domain of 1a gene. Virusdisease. 2017;28(3):309–314. doi: 10.1007/s13337-017-0386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roossinck MJ. Cucumber mosaic virus, a model for RNA virus evolution. Mol Plant Pathol. 2001;2(2):59–63. doi: 10.1046/j.1364-3703.2001.00058.x. [DOI] [PubMed] [Google Scholar]
- Roossink MJ. Evolutionary history of cucumber mosaic virus deduced by phylogenetic analyses. J Virol. 2002;76:3382–3387. doi: 10.1128/JVI.76.7.3382-3387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanki K, Balazs E. The necrotic pathotype of the cucumber mosaic virus (CMV) Ns strain is solely determined by amino acid 461 of the 1a protein. Mol Plant Microbe Interact. 2004;17(8):837–845. doi: 10.1094/MPMI.2004.17.8.837. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Raj SK. Coat protein-mediated resistance against an Indian isolate of the cucumber mosaic virus subgroup IB in Nicotiana benthamiana. J Biosci. 2008;33(2):249–257. doi: 10.1007/s12038-008-0042-7. [DOI] [PubMed] [Google Scholar]
- Subramanian KS, Narayanasamy P. Mechanical transmission of whitefly borne yellow mosaic virus of Lablab niger Midikus. Curr Sci. 1973;47:92–93. [Google Scholar]
- Takahashi H, Suzuki M, Natsuaki K, Shigyo T, Hino K, Teraoka T, Hosokawa D, Ehara Y. Mapping the virus and host genes involved in the resistance response in cucumber mosaic virus-infected Arabidopsis thaliana. Plant Cell Physiol. 2001;42(3):340–347. doi: 10.1093/pcp/pce039. [DOI] [PubMed] [Google Scholar]
- Valentine TA, Roberts IM, Oparka KJ. Inhibition of tobacco mosaic virus replication in lateral roots is dependent on an activated meristem-derived signal. Protoplasma. 2002;219:184–196. doi: 10.1007/s007090200020. [DOI] [PubMed] [Google Scholar]
- Zehra SB, Ahmad A, Sharma A, Sofi S, Lateef A, Bashir Z, Husain M, Rathore JP. Chilli leaf curl virus an emerging threat to chilli in India. Int J Pure App Biosci. 2017;5(5):404–414. [Google Scholar]
- Zhang L, Hanada K, Palukaitis P. Mapping local and systemic symptom determinants of cucumber mosaic virus in tobacco. J Gen Virol. 1994;75:3185–3191. doi: 10.1099/0022-1317-75-11-3185. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yuan YR, Pei Y, Lin SS, Tuschl T, Patel DJ, Chua NH. Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Genes Dev. 2006;20(23):3255–3268. doi: 10.1101/gad.1495506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.