Abstract
Purpose
The prognostic implications of serum vitamin D status after a 5-year adjuvant endocrine therapy on the risk of late recurrence among hormone receptor (HR)-positive breast cancer patients remain unclear. Hence, we investigated this among Korean HR-positive breast cancer patients.
Methods
A total of 455 patients with HR-positive stage I–III invasive breast cancer who underwent curative surgery at St. Vincent's Hospital between February 2004 and April 2012 were included in this retrospective study. Patients were categorized based on their serum 25-hydroxyvitamin D (25(OH)D) levels after the 5-year adjuvant endocrine therapy. Initial recurrence sites were categorized. The primary clinical outcome was late recurrence-free survival (LRFS).
Results
Among the 455 patients, 242 and 213 were included in the 25(OH)D-deficient group and 25(OH)D-sufficient group, respectively. Forty-eight patients experienced late recurrence. Across all recurrence sites, the 25(OH)D-deficient group showed significantly worse LRFS rates than the 25(OH)D-sufficient group (hazard ratio [HR], 2.284; 95% confidence interval [CI], 1.155–4.515; p = 0.018). After patient subgrouping based on recurrence site, the 25(OH)D-deficient group also showed significantly worse LRFS rates in terms of regional lymph node (LN) (HR, 17.453; 95% CI, 2.46–128.83; p = 0.005), bone (HR, 2.394; 95% CI, 1.024–5.599; p = 0.044), and visceral (HR, 2.735; 95% CI, 1.182–6.328; p = 0.019) recurrence. However, there was no significant difference between the 2 groups in terms of local recurrence (p = 0.611).
Conclusions
We found that 25(OH)D deficiency after the 5-year adjuvant endocrine therapy was associated with worse LRFS among HR-positive breast cancer patients, particularly with respect to regional LN, bone, and visceral recurrence.
Keywords: Breast neoplasms, Hormone receptor, Recurrence, Vitamin D
INTRODUCTION
Hormone receptors (HRs), including estrogen receptors (ERs) and progesterone receptors (PRs), are important prognostic markers in breast cancer [1]. In general, HR-positive breast cancer has a better prognosis than HR-negative breast cancer subtypes, such as the human epidermal growth factor receptor 2 (HER2)-enriched or triple negative subtypes [2]. However, the recurrence rate of HR-negative breast cancer is high within the first 5 years of diagnosis but decreases thereafter, whereas that of HR-positive breast cancer is low within the first 5 years of diagnosis but does not considerably decrease after 5 years and is even sustained for up to 15 years [3]. Recurrence 5 years post-diagnosis is known as late recurrence and contributes to approximately 40% of all recurrent HR-positive breast cancer cases [4]. Although previous studies have reported several risk factors for late recurrence in HR-positive breast cancer, the mechanism of and predictive factors for late recurrence remain unclear.
Vitamin D is considered to have a favorable prognostic value in various cancers [5]. Several studies have reported the anticancer effects of vitamin D on breast cancer through the inhibition of cell growth, cell cycle arrest, or apoptosis [6,7,8]. Moreover, the serum vitamin D level at diagnosis is a significant prognostic factor among breast cancer patients [9,10,11,12,13,14]. Nevertheless, serum vitamin D status can be affected by adjuvant therapy [15]; hence, it should be measured after adjuvant therapy completion in HR-positive breast cancer patients.
One possible mechanism of late recurrence is the tumor dormancy theory [16,17]. In HR-positive breast cancer, latent tumor cells are dormant during adjuvant endocrine therapy. When the therapy is completed, the dormant tumor cells are exposed to estrogen and hence become activated tumor cells. Therefore, it is plausible that when adjuvant endocrine therapy is completed, serum vitamin D levels could subsequently be associated with the risk of late recurrence among HR-positive breast cancer patients. However, there are no previous studies that have investigated this hypothesis.
Therefore, we investigated the association between serum vitamin D status after a 5-year adjuvant endocrine therapy and the risk of late recurrence among Korean HR-positive breast cancer patients. Additionally, we performed a subgroup analysis of the recurrence site categories to investigate prognostic implications in the risk of late recurrence by site.
METHODS
Study population
We retrospectively reviewed the medical records of 588 female patients with HR-positive stage I–III invasive breast cancer who underwent curative surgery at the St. Vincent's Hospital between February 2004 and April 2012. All patient data were analyzed for various clinicopathologic factors, including age at diagnosis, body mass index at diagnosis, surgical methods administered, tumor size, histologic subtype, serum 25-hydroxyvitamin D [25(OH)D] level at completion of the 5-year adjuvant endocrine therapy, date of patient's blood sampling, adjuvant endocrine therapy medication, number of axillary lymph node (LN) metastases, number of retrieved axillary LNs, histologic grade, lymphatic invasion, vascular invasion, PR expression, HER2 expression, p53 expression, Ki-67 expression, and adjuvant treatment information (including use of systemic chemotherapy, radiotherapy, and trastuzumab therapy). All patients underwent either breast conserving surgery (BCS) plus axillary LN dissection or modified radical mastectomy plus axillary LN dissection. Subsequently, patients received adjuvant systemic chemotherapy, radiotherapy, endocrine therapy (tamoxifen, anastrozole, or letrozole), or trastuzumab therapy based on the clinician's discretion and according to institutional guidelines appropriate at the treatment time. We excluded patients who received neoadjuvant systemic chemotherapy, did not complete 5 years of adjuvant endocrine therapy, or had tumor recurrence during the 5 years of adjuvant endocrine therapy. Patients with bilateral breast cancer at initial diagnosis and patients without serum 25(OH)D level information upon completion of the 5-year adjuvant endocrine therapy were also excluded. Because the peak incidence of late recurrence among HR-positive breast cancer patients occurs approximately 7 years after diagnosis [18,19], patients with follow-up durations less than 7 years were excluded to remove possible bias. Using these criteria, 455 patients were included for analysis.
ER and PR expressions were considered positive if > 1% of cells stained positive on immunohistochemistry (IHC). HER2 expression was considered negative if the IHC score was 0 or 1+ and positive if the IHC score was 3+. If the HER2 IHC score was 2+, fluorescent in situ hybridization (FISH) was performed to assess HER2 amplification. Among the 38 patients with 2+ HER2 IHC scores, 36 patients had HER2 expression confirmed via FISH. The other 2 patient samples have not been assayed using FISH and were considered HER2-negative.
Patients were categorized after their 5-year adjuvant endocrine therapy based on serum 25(OH)D levels as sufficient (≥ 20 ng/mL) or deficient (< 20 ng/mL). Blood sampling to determine serum 25(OH)D levels was performed in an outpatient clinic at the time of adjuvant endocrine therapy completion. The date of sampling was categorized seasonally as follows: Spring (March–May), Summer (June–August), Autumn (September–November), and Winter (December–February). For all patients, the initial recurrence site was identified using biopsy or dynamic imaging examinations, including ultrasonography, computed tomography, or magnetic resonance imaging. The initial recurrence sites were categorized as follows: (1) local: recurrence in the skin adjacent to the chest wall, ipsilateral breast, or contralateral breast; (2) regional LN: recurrence in the axillary, internal mammary, supraclavicular, or infraclavicular LN; (3) bone: recurrence in the bone tissue; or (4) visceral: recurrence in the lung, liver, or brain tissue. The primary outcome examined in this study was late recurrence-free survival (LRFS), which was defined as the period from the breast cancer diagnosis date to the tumor recurrence or last follow-up date, until the end of data collection on April 2019.
All procedures involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (St. Vincent's Hospital [IRB No. VC19RISI0291]) and with the 2013 Declaration of Helsinki and its later amendments as well as other comparable ethical standards. All participants provided written informed consent for storage of medical information in the hospital database and use of this information for research purposes.
Statistical analyses
Differences between the clinicopathological characteristic subgroups were compared using independent t-tests or Pearson's χ2 test. Survival curves were generated using the Kaplan-Meier method, and significance was determined using log-rank tests. Multivariate analysis was conducted using Cox's proportional hazard regression model for survival, with the hazard ratio (HR) and 95% confidence interval (CI) estimated for each variable. All statistical tests were 2-sided, and the significance was set at p < 0.05. All statistical analyses were performed using SPSS Statistics for Windows, version 12.0 (SPSS Inc., Chicago, USA).
RESULTS
Patients and tumor characteristics
The mean patient age at diagnosis was 52.010 ± 10.265 years (range: 29–82 years). Among the 455 patients, 242 were included in the 25(OH)D-deficient group, whereas 213 were included in the 25(OH)D-sufficient group. The baseline patient characteristics are shown in Table 1.
Table 1. Baseline patient characteristics.
| Characteristics | Serum 25(OH)D status after 5-yr adjuvant endocrine therapy | |||
|---|---|---|---|---|
| Deficient (n = 242) | Sufficient (n = 213) | p-value | ||
| Age (yr) | 50.480 ± 10.408 | 53.740 ± 9.842 | 0.001 | |
| < 50 | 145 (59.9) | 74 (34.7) | < 0.001 | |
| ≥ 50 | 97 (40.1) | 139 (65.3) | ||
| BMI | 24.31 ± 3.57 | 24.50 ± 3.71 | 0.580 | |
| Serum 25(OH)D level after 5-yr adjuvant endocrine therapy (ng/mL) | 13.610 ± 3.792 | 29.350 ± 8.959 | < 0.001 | |
| Adjuvant endocrine therapy | < 0.001 | |||
| Tamoxifen | 165 (68.2) | 87 (40.8) | ||
| Anastrozole | 48 (19.8) | 69 (32.4) | ||
| Letrozole | 29 (12.0) | 57 (26.8) | ||
| Histologic subtype | 0.481 | |||
| IDC | 210 (86.8) | 180 (84.5) | ||
| ILC | 8 (3.3) | 12 (5.6) | ||
| Othera | 24 (9.9) | 21 (9.9) | ||
| Surgery | 0.001 | |||
| BCS | 142 (58.7) | 156 (73.2) | ||
| MRM | 100 (41.3) | 57 (26.8) | ||
| Tumor size (cm) | 2.129 ± 1.365 | 1.915 ± 1.224 | 0.081 | |
| ≤ 2 | 143 (59.1) | 146 (68.5) | 0.102 | |
| > 2, ≤ 5 | 92 (38.0) | 61 (28.6) | ||
| > 5 | 7 (2.9) | 6 (2.8) | ||
| No. of axillary LN metastases | 1.150 ± 2.883 | 1.270 ± 2.990 | 0.677 | |
| 0 | 170 (70.2) | 147 (69.0) | 0.493 | |
| 1–3 | 48 (19.8) | 39 (18.3) | ||
| 4–9 | 16 (6.6) | 22 (10.3) | ||
| ≥ 10 | 8 (3.3) | 5 (2.3) | ||
| No. of retrieved axillary LNs | 14.600 ± 6.086 | 14.370 ± 6.239 | 0.687 | |
| Histologic grade | 0.814 | |||
| 1 | 103 (42.6) | 97 (45.5) | ||
| 2 | 99 (40.9) | 83 (39.0) | ||
| 3 | 40 (16.5) | 33 (15.5) | ||
| Lymphatic invasion | 0.949 | |||
| No | 188 (77.7) | 166 (77.9) | ||
| Yes | 54 (22.3) | 47 (22.1) | ||
| Vascular invasion | 0.717 | |||
| No | 235 (97.1) | 208 (97.7) | ||
| Yes | 7 (2.9) | 5 (2.3) | ||
| Progesterone receptor | 0.251 | |||
| Negative | 43 (17.8) | 47 (22.1) | ||
| Positive | 199 (82.2) | 166 (77.9) | ||
| HER2 | 0.389 | |||
| Negative | 205 (84.7) | 174 (81.7) | ||
| Positive | 37 (15.3) | 39 (18.3) | ||
| p53 | 0.849 | |||
| Negative | 196 (81.0) | 174 (81.7) | ||
| Positive | 46 (19.0) | 39 (18.3) | ||
| Ki-67 (%) | 0.887 | |||
| < 14 | 130 (53.7) | 113 (53.1) | ||
| ≥ 14 | 112 (46.3) | 100 (46.9) | ||
| Adjuvant radiotherapy | 0.001 | |||
| No | 83 (34.3) | 44 (20.7) | ||
| Yes | 159 (65.7) | 169 (79.3) | ||
| Adjuvant chemotherapy | 0.444 | |||
| No | 138 (57.0) | 129 (60.6) | ||
| Yes | 104 (43.0) | 84 (39.4) | ||
| Adjuvant trastuzumab use | 0.354 | |||
| No | 228 (94.2) | 196 (92.0) | ||
| Yes | 14 (5.8) | 17 (8.0) | ||
| Season of patient's blood sampling | 0.631 | |||
| Spring | 59 (24.4) | 55 (25.8) | ||
| Summer | 62 (25.6) | 56 (26.3) | ||
| Autumn | 60 (24.8) | 59 (27.7) | ||
| Winter | 61 (25.2) | 43 (20.2) | ||
| Follow up duration (mo) | 109.580 ± 28.921 | 100.560 ± 23.672 | < 0.001 | |
| Follow up duration (mo) (from 5 years after diagnosis) | 49.540 ± 28.920 | 40.530 ± 23.698 | < 0.001 | |
Data are presented as mean ± standard deviation or number (%)
BMI = body mass index; 25(OH)D = 25-hydroxyvitamin D; IDC = invasive ductal carcinoma; ILC = invasive lobular carcinoma; BCS = breast conserving surgery; MRM = modified radical mastectomy; LN = lymph node; HER2 = human epidermal growth factor receptor 2.
aCribriform, medullary, micropapillary, mucinous, papillary, tubular subtype.
More patients were aged > 50 years in the 25(OH)D-sufficient group than in the 25(OH)D-deficient group (65.3% vs. 40.1%, p < 0.001). Additionally, patients in the 25(OH)D-sufficient group were more likely to have been treated with aromatase inhibitors (59.2% vs. 31.8%, p < 0 .001), BCS (73.2% vs. 58.7%, p = 0.01), and adjuvant radiotherapy (79.3% vs. 65.7%, p = 0.001) than those in the 25(OH)D-deficient group. The patient follow-up duration in the 25(OH)D-sufficient group was shorter than that in the 25(OH)D-deficient group (100.56 ± 23.672 vs. 109.58 ± 28.921 months, p < 0.001). Apart from these, there were no significant differences in patient characteristics between the 2 groups.
Late recurrence incidence by site and serum 25(OH)D status after the 5-year adjuvant endocrine therapy
The mean patient follow-up duration was 103.580 ± 27.143 months (range: 62–180 months). Among the 455 patients, 48 patients (10.5%) experienced late recurrence. Table 2 shows the number of patients who experienced late recurrence per recurrence site.
Table 2. Number of patients who experienced late recurrence (by site).
| Site of late recurrence | No. of patients |
|---|---|
| Ipsilateral breast | 1 |
| Skin | 1 |
| Regional LN | 4 |
| Bone | 8 |
| Lung | 3 |
| Liver | 1 |
| Bone + lung | 4 |
| Bone + brain | 2 |
| Lung + liver | 1 |
| Bone + lung + liver + brain | 3 |
| Skin + regional LN + bone + liver | 1 |
| Regional LN + lung | 1 |
| Regional LN + liver | 2 |
| Regional LN + bone + lung | 8 |
| Regional LN + bone + liver | 1 |
| Regional LN + lung + brain | 1 |
| Regional LN + liver + brain | 1 |
| Regional LN + bone + lung + liver | 3 |
| Regional LN + lung + liver + brain | 1 |
| Regional LN + bone + lung + liver + brain | 1 |
| Total | 48 |
LN = lymph node.
Next, we classified recurrence by site and patient serum 25(OH)D status after the 5-year adjuvant endocrine therapy. The total number of recurrence sites was 92, which consisted of the following: local (n = 3), regional LN (n = 24), bone (n = 32), and visceral (n = 33). After subgrouping according to patient serum 25(OH)D status post-adjuvant endocrine therapy, there were 76 recurrence sites in the deficient group (local [n = 2], regional LN [n = 23], bone [n = 25], and visceral [n = 26]) and 16 in the sufficient group (local [n = 1], regional LN [n = 1], bone [n = 7], and visceral [n = 7]; Table 3).
Table 3. Incidence of late recurrence by site, categorized by patient serum 25(OH)D status after 5-year adjuvant endocrine therapy.
| Site of late recurrence | Serum 25(OH)D status after 5-yr adjuvant endocrine therapy | ||
|---|---|---|---|
| Deficient | Sufficient | Total | |
| Local | 2 | 1 | 3 |
| Regional LN | 23 | 1 | 24 |
| Bone | 25 | 7 | 32 |
| Visceral | 26 | 7 | 33 |
| Total | 76 | 16 | 92 |
25(OH)D = 25-hydroxyvitamin D; LN = lymph node.
Association between serum 25(OH)D status after the 5-year adjuvant endocrine therapy and late recurrence risk
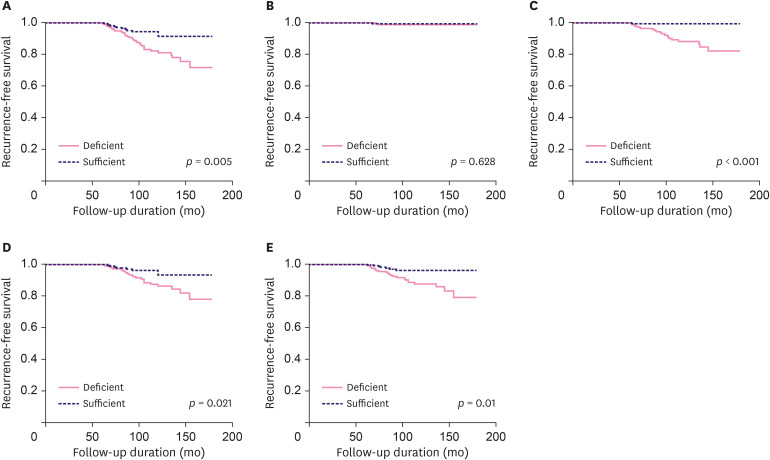
We also investigated the association between LRFS and patient serum 25(OH)D status after the 5-year adjuvant endocrine therapy (Figure 1). In all recurrence sites, the 25(OH)D-deficient group showed significantly worse LRFS rates than the 25(OH)D-sufficient group (84.7% vs. 94.8%, p = 0.005) (Figure 1A). After patient subgrouping per recurrence site, there was no significant difference in local recurrence (99.2% vs. 99.5%, p = 0.628) between both the groups (Figure 1B). However, the 25(OH)D-deficient group showed significantly worse LRFS rates in terms of regional LN (90.5% vs. 99.5%, p < 0.001) (Figure 1C), bone (89.7% vs. 96.7%, p = 0.021) (Figure 1D), and visceral recurrence (89.3% vs. 86.7%, p = 0.01) (Figure 1E).Subsequently, we performed multivariate analysis to investigate the clinical implications with respect to LRFS considering serum 25(OH)D status after the 5-year adjuvant endocrine therapy (Table 4). In all recurrence sites, the 25(OH)D-deficient group showed significantly worse LRFS than the 25(OH)D-sufficient group (HR, 2.284; 95% CI, 1.155–4.515; p = 0.018). With respect to recurrence site, the 25(OH)D-deficient group also showed significantly worse LRFS in regional LN (HR, 17.453; 95% CI, 2.46–128.83; p = 0.005), bone (HR, 2.394; 95% CI, 1.024–5.599; p = 0.044), and visceral (HR, 2.735; 95% CI, 1.182–6.328; p = 0.019) recurrence sites. However, there was no significant difference in local recurrence (HR, 2.195; 95% CI, 0.106–45.545; p = 0.611) between both the groups.
Figure 1. Association between late recurrence-free survival outcome by recurrence site and serum 25(OH)D status after 5-year adjuvant endocrine therapy: (A) all sites, (B) local recurrence, (C) regional lymph node recurrence, (D) bone recurrence, and (E) visceral recurrence.
25(OH)D = 25-hydroxyvitamin D.
Table 4. Multivariate analysis of late recurrence risk according to serum 25(OH)D status after 5-year adjuvant endocrine therapy.
| All sitesa | Locala | Regional LNa | Bonea | Viscerala | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Serum 25(OH)D status after 5-year adjuvant endocrine therapy | |||||||||||
| Sufficient | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | ||||||
| Deficient | 2.284 (1.155–4.515) | 0.018 | 2.195 (0.106–45.545) | 0.611 | 17.453 (2.460–128.83) | 0.005 | 2.394 (1.024–5.599) | 0.044 | 2.735 (1.182–6.328) | 0.019 | |
25(OH)D = 25-hydroxyvitamin D; LN = lymph node; HR = hazard ratio; CI = confidence interval.
aAdjusted for age, surgery, tumor size, number of axillary LN metastases, histologic grade, serum 25(OH)D status after 5-year adjuvant endocrine therapy, human epidermal growth factor receptor 2, Ki-67, lymphatic invasion, vascular invasion, and p53.
DISCUSSION
In the present study, we investigated the clinical implications of serum 25(OH)D status after the 5-year adjuvant endocrine therapy on the risk of late recurrence among HR-positive breast cancer patients. We found that insufficient serum 25(OH)D levels after the 5-year adjuvant endocrine therapy is a significant risk factor for late recurrence in HR-positive breast cancer, particularly in regional LN, bone, and visceral recurrence sites. To the best of our knowledge, this is first study to investigate the prognostic implications of serum 25(OH)D after the 5-year adjuvant endocrine therapy on late recurrence among HR-positive breast cancer patients.
The anticancer effects of vitamin D in breast cancer have been previously reported. Lundqvist et al. [6] reported that 1,25-dihydroxyvitamin D [1,25(OH)2D], the active form of 25(OH)D, inhibits proliferation of ER-positive breast cancer MCF7 cells. Moreover, Bohl et al. [20] reported the pro-apoptotic effects of 1,25(OH)2D in MCF7 cells. Further, Klopotowska et al. [21] reported that 1,25(OH)2D inhibits MCF7 cell growth by downregulating miR-125b and upregulating vitamin D receptor expression. In humans, serum 25(OH)D has a longer half-life and can be more easily measured than 1,25(OH)2D; thus, we considered serum 25(OH)D status as a standard indicator of patient vitamin D status [5]. Several previous retrospective clinical studies have reported the beneficial prognostic value of serum 25(OH)D sufficiency in the diagnosis of breast cancer patients; however, there were diverse definitions of 25(OH)D sufficiency in these studies [9,10,11,12,13,14]. Furthermore, patient serum vitamin D status after diagnosis can vary owing to several factors. As reported by Kim et al. [15], serum 25(OH)D levels in breast cancer patients are significantly reduced after adjuvant chemotherapy or endocrine therapy. Therefore, to retain the anticancer effects of vitamin D, it is important to maintain patient serum 25(OH)D levels within the normal range after the completion of adjuvant therapies.
On the other hand, some studies have reported on the risk factors for late recurrence among HR-positive breast cancer patients. Song et al. [3] reported that patients with more than 3 positive LNs (HR, 2.176; 95% CI, 1.071–4.421; p = 0.032) and histologic grade 3 disease (HR, 2.098; 95% CI, 1.300–3.385; p = 0.002) are significantly associated with a high late recurrence risk. Lee et al. [22] reported that tumor size (HR, 1.767; 95% CI, 1.033–3.023; p = 0.038) and axillary LN metastasis (HR, 4.274; 95% CI, 2.470–7.395; p < 0.001) are significant risk factors for distant late recurrence. Furthermore, several previous studies also reported that circulating tumor cell positivity or ER transcript levels in 21-gene recurrence score expression assays are associated with a late recurrence risk in HR-positive breast cancer [23,24]. However, these risk factors are generally unmodifiable factors. In contrast, our results indicate that insufficient serum 25(OH)D after the 5-year adjuvant endocrine therapy is a significant risk factor for late recurrence, which can be improved through medication and patient lifestyle modification [25]. We have thus identified a modifiable risk factor for late recurrence among HR-positive breast cancer patients.
Although the mechanism of late recurrence among HR-positive breast cancer patients remains under-evaluated, some previous reports have suggested that the tumor dormancy theory can explain it [16,17]. This theory states that remnant micro-metastatic breast cancer cells may persist in patients as circulating and disseminating tumor cells after primary tumor resection. In the disease-free period, the remnant tumor cells are balanced between apoptosis and proliferation, which is called the tumor cell dormant state. However, when the balance of tumor cell dormancy is disrupted, tumor cell proliferation overwhelms apoptosis and promotes tumor cell regrowth, which consequently presents as late recurrence. Although the mechanisms underlying the switch from clinical dormancy to tumor regrowth are poorly understood, it is possible that the cessation of endocrine therapy could trigger tumor regrowth. Considering this perspective, the improved LRFS among 25(OH)D-sufficient patients in our study suggest that sufficient serum vitamin D levels may contribute to maintain tumor dormancy after the 5-year adjuvant endocrine therapy, which could have resulted from the anti-proliferative and pro-apoptotic effect of vitamin D among HR-positive breast cancer patients.
After demonstrating the effectiveness of an extended endocrine therapy [26], it became crucial to determine the risk factors for late recurrence in HR-positive breast cancer to guide individualized extended endocrine therapy. Although the optimal predictive factor to select patients who will benefit from extended endocrine therapy remains unclear, it is commonly suggested that the prognostic benefit of extended endocrine therapy can be maximized among patients with a high disease relapse risk [27]. Based on our results, patients with 25(OH)D deficiency after the 5-year adjuvant endocrine therapy may be considered appropriate candidates for extended endocrine therapy.
Nevertheless, this study has several limitations. First, the design of this study was retrospective, which could potentially bias patient selection and clinicopathologic information collection. Although propensity score matching analysis is a powerful statistical method to adjust the difference between study groups, we were concerned of the reduction of the study cohort after propensity score matching because of the relatively small number of included patients and recurrence events in our study. Therefore, we did not perform this analysis in our study. Additionally, our study results should be interpreted with caution because we did not adjust several factors such as adjuvant endocrine therapy and radiotherapy in the multivariate analysis to avoid the risk of multicollinearity. Second, the total patient sample size and number of recurrence events were relatively small, which could decrease the statistical power of this study. In particular, the relatively smaller number of local recurrence events than events at other recurrence sites may affect the insignificant association between serum 25(OH)D status after the 5-year adjuvant endocrine therapy and the risk of late local recurrence. Third, the data on other confounding factors, such as patient's bone density before endocrine treatment, were not investigated. Finally, the data on environmental factors such as vitamin D supplement use, exercise, or outdoor activities were not collected, which could also affect the study results.
In conclusion, our study demonstrated that 25(OH)D deficiency after the 5-year adjuvant endocrine therapy is associated with decreased LRFS among HR-positive breast cancer patients, particularly in terms of regional LN, bone, and visceral recurrence. Therefore, we suggest that serum vitamin D status should be assessed before discontinuing adjuvant endocrine therapy and to maintain serum vitamin D levels within the normal range in HR-positive breast cancer patients after therapy. However, to investigate a more accurate association between patient's serum 25(OH)D, tumor dormancy, and late recurrence, a properly designed study with a larger cohort is warranted.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Lim ST, Jeon YW, Gwak H, Suh YJ.
- Data curation: Lim ST.
- Formal analysis: Lim ST, Suh YJ.
- Investigation: Lim ST.
- Methodology: Lim ST, Jeon YW.
- Supervision: Lim ST, Jeon YW, Gwak H, Suh YJ.
- Validation: Lim ST, Suh YJ.
- Visualization: Lim ST.
- Writing - original draft: Lim ST.
- Writing - review & editing: Suh YJ.
References
- 1.Chen X, Fan Y, Xu B. Distinct characteristics and metastatic behaviors of late recurrence in patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative breast cancer: a single institute experience of more than 10 years. Clin Breast Cancer. 2018;18:e1353–60. doi: 10.1016/j.clbc.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Rueda OM, Sammut SJ, Seoane JA, Chin SF, Caswell-Jin JL, Callari M, et al. Dynamics of breast-cancer relapse reveal late-recurring ER-positive genomic subgroups. Nature. 2019;567:399–404. doi: 10.1038/s41586-019-1007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song F, Zhang J, Li S, Wu J, Jin T, Qin J, et al. ER-positive breast cancer patients with more than three positive nodes or grade 3 tumors are at high risk of late recurrence after 5-year adjuvant endocrine therapy. Onco Targets Ther. 2017;10:4859–4867. doi: 10.2147/OTT.S142698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colleoni M, Sun Z, Price KN, Karlsson P, Forbes JF, Thürlimann B, et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the international breast cancer study group trials I to V. J Clin Oncol. 2016;34:927–935. doi: 10.1200/JCO.2015.62.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 6.Lundqvist J, Yde CW, Lykkesfeldt AE. 1α,25-dihydroxyvitamin D3 inhibits cell growth and NFκB signaling in tamoxifen-resistant breast cancer cells. Steroids. 2014;85:30–35. doi: 10.1016/j.steroids.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Segovia-Mendoza M, Díaz L, González-González ME, Martínez-Reza I, García-Quiroz J, Prado-Garcia H, et al. Calcitriol and its analogues enhance the antiproliferative activity of gefitinib in breast cancer cells. J Steroid Biochem Mol Biol. 2015;148:122–131. doi: 10.1016/j.jsbmb.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Marchionatti AM, Picotto G, Narvaez CJ, Welsh J, Tolosa de Talamoni NG. Antiproliferative action of menadione and 1,25(OH)2D3 on breast cancer cells. J Steroid Biochem Mol Biol. 2009;113:227–232. doi: 10.1016/j.jsbmb.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin PJ, Ennis M, Pritchard KI, Koo J, Hood N. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27:3757–3763. doi: 10.1200/JCO.2008.20.0725. [DOI] [PubMed] [Google Scholar]
- 10.Hatse S, Lambrechts D, Verstuyf A, Smeets A, Brouwers B, Vandorpe T, et al. Vitamin D status at breast cancer diagnosis: correlation with tumor characteristics, disease outcome, and genetic determinants of vitamin D insufficiency. Carcinogenesis. 2012;33:1319–1326. doi: 10.1093/carcin/bgs187. [DOI] [PubMed] [Google Scholar]
- 11.Vrieling A, Hein R, Abbas S, Schneeweiss A, Flesch-Janys D, Chang-Claude J. Serum 25-hydroxyvitamin D and postmenopausal breast cancer survival: a prospective patient cohort study. Breast Cancer Res. 2011;13:R74. doi: 10.1186/bcr2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tretli S, Schwartz GG, Torjesen PA, Robsahm TE. Serum levels of 25-hydroxyvitamin D and survival in Norwegian patients with cancer of breast, colon, lung, and lymphoma: a population-based study. Cancer Causes Control. 2012;23:363–370. doi: 10.1007/s10552-011-9885-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villaseñor A, Ballard-Barbash R, Ambs A, Bernstein L, Baumgartner K, Baumgartner R, et al. Associations of serum 25-hydroxyvitamin D with overall and breast cancer-specific mortality in a multiethnic cohort of breast cancer survivors. Cancer Causes Control. 2013;24:759–767. doi: 10.1007/s10552-013-0158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HJ, Lee YM, Ko BS, Lee JW, Yu JH, Son BH, et al. Vitamin D deficiency is correlated with poor outcomes in patients with luminal-type breast cancer. Ann Surg Oncol. 2011;18:1830–1836. doi: 10.1245/s10434-010-1465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HJ, Koh BS, Yu JH, Lee JW, Son BH, Kim SB, et al. Changes in serum hydroxyvitamin D levels of breast cancer patients during tamoxifen treatment or chemotherapy in premenopausal breast cancer patients. Eur J Cancer. 2014;50:1403–1411. doi: 10.1016/j.ejca.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Uhr JW, Pantel K. Controversies in clinical cancer dormancy. Proc Natl Acad Sci U S A. 2011;108:12396–12400. doi: 10.1073/pnas.1106613108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelao L, Criscitiello C, Fumagalli L, Locatelli M, Manunta S, Esposito A, et al. Tumour dormancy and clinical implications in breast cancer. Ecancermedicalscience. 2013;7:320. doi: 10.3332/ecancer.2013.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biganzoli E, Desmedt C, Fornili M, de Azambuja E, Cornez N, Ries F, et al. Recurrence dynamics of breast cancer according to baseline body mass index. Eur J Cancer. 2017;87:10–20. doi: 10.1016/j.ejca.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Demicheli R, Ardoino I, Boracchi P, Coradini D, Agresti R, Ferraris C, et al. Recurrence and mortality according to estrogen receptor status for breast cancer patients undergoing conservative surgery. Ipsilateral breast tumour recurrence dynamics provides clues for tumour biology within the residual breast. BMC Cancer. 2010;10:656. doi: 10.1186/1471-2407-10-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bohl LP, Liaudat AC, Picotto G, Marchionatti AM, Narvaez CJ, Welsh J, et al. Buthionine sulfoximine and 1,25-dihydroxyvitamin D induce apoptosis in breast cancer cells via induction of reactive oxygen species. Cancer Invest. 2012;30:560–570. doi: 10.3109/07357907.2012.700985. [DOI] [PubMed] [Google Scholar]
- 21.Klopotowska D, Matuszyk J, Wietrzyk J. Steroid hormone calcitriol and its analog tacalcitol inhibit miR-125b expression in a human breast cancer MCF-7 cell line. Steroids. 2019;141:70–75. doi: 10.1016/j.steroids.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Lee ES, Han W, Kim MK, Kim J, Yoo TK, Lee MH, et al. Factors associated with late recurrence after completion of 5-year adjuvant tamoxifen in estrogen receptor positive breast cancer. BMC Cancer. 2016;16:430. doi: 10.1186/s12885-016-2423-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sparano J, O'Neill A, Alpaugh K, Wolff AC, Northfelt DW, Dang CT, et al. Association of circulating tumor cells with late recurrence of estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4:1700–1706. doi: 10.1001/jamaoncol.2018.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dowsett M, Sestak I, Buus R, Lopez-Knowles E, Mallon E, Howell A, et al. Estrogen receptor expression in 21-gene recurrence score predicts increased late recurrence for estrogen-positive/HER2-negative breast cancer. Clin Cancer Res. 2015;21:2763–2770. doi: 10.1158/1078-0432.CCR-14-2842. [DOI] [PubMed] [Google Scholar]
- 25.Shin WK, Kim Z, Youn HJ, Cho J, Lee JE. Determinants of plasma 25-hydroxyvitamin D concentrations among breast cancer survivors in Korea. Nutrients. 2018;10:380. doi: 10.3390/nu10030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomized trial. Lancet. 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartlett JM, Sgroi DC, Treuner K, Zhang Y, Ahmed I, Piper T, et al. Breast cancer index and prediction of benefit from extended endocrine therapy in breast cancer patients treated in the Adjuvant Tamoxifen-To Offer More? (aTTom) trial. Ann Oncol. 2019;30:1776–1783. doi: 10.1093/annonc/mdz289. [DOI] [PMC free article] [PubMed] [Google Scholar]