Abstract
Organoids derived from mouse and human stem cells have recently emerged as a powerful tool to study organ development and disease. We here established a three‐dimensional (3D) murine bronchioalveolar lung organoid (BALO) model that allows clonal expansion and self‐organization of FACS‐sorted bronchioalveolar stem cells (BASCs) upon co‐culture with lung‐resident mesenchymal cells. BALOs yield a highly branched 3D structure within 21 days of culture, mimicking the cellular composition of the bronchioalveolar compartment as defined by single‐cell RNA sequencing and fluorescence as well as electron microscopic phenotyping. Additionally, BALOs support engraftment and maintenance of the cellular phenotype of injected tissue‐resident macrophages. We also demonstrate that BALOs recapitulate lung developmental defects after knockdown of a critical regulatory gene, and permit modeling of viral infection. We conclude that the BALO model enables reconstruction of the epithelial–mesenchymal‐myeloid unit of the distal lung, thereby opening numerous new avenues to study lung development, infection, and regenerative processes in vitro.
Keywords: BALO, BASC, lung organoids, stem cells
Subject Categories: Regenerative Medicine, Respiratory System
Resource work reports a new in vitro system to model the bronchioalveolar airways.
Introduction
In recent years, three‐dimensional (3D) organoid systems derived from mouse and human adult stem cells or induced pluripotent stem cells (iPSC) have emerged as a powerful technology for in vitro modeling of organ development and disease (Lancaster & Knoblich, 2014; Kretzschmar & Clevers, 2016). Adult somatic tissue‐resident stem/progenitor cells represent an excellent starting point to generate 3D organoid systems due to their ability to proliferate and differentiate into cell types found in the corresponding parental tissues. Murine lung organoids mimicking—to different extents—the cellular morphology and certain functional features of the native organ have been generated from tissue‐resident cells by growth factor supplementation or by co‐culture with feeder cells (Rock et al, 2009; Chen et al, 2012a; Barkauskas et al, 2013; Lee et al, 2014). For instance, single basal cells give rise to organoids termed “tracheospheres” consisting of basal and ciliated luminal cells (Rock et al, 2009). Type 2 alveolar epithelial cells (AEC II) co‐cultured with PDGFRα+ lung mesenchymal cells form alveolar‐like structures consisting of type 1 alveolar epithelial cells (AEC I) and AEC II (Chen et al, 2012a; Barkauskas et al, 2013). Furthermore, co‐culture of multipotent lung epithelial stem cells with endothelial cells gives rise to various organoid structures (Lee et al, 2014).
However, all 3D organoids described so far only recapitulate limited morphological and cellular features of the lung. A model that recapitulates the 3D complexity of the bronchioalveolar compartment of the lung is critically missing. Current lung organoid models mostly consist of only epithelial cells, and in rare cases, the mesenchymal compartment. Nonetheless, tissue‐resident cells of myeloid origin, which are important mediators of lung development, immune response, and tissue regeneration, remain absent. We have previously described an epithelial cell population of the distal lung that is characterized by the signature EpCAMhighCD24lowSca‐1+ and is enriched with epithelial stem/progenitor cells that exhibit high proliferative potential in response to influenza A virus (IAV)‐induced lung injury. We also showed that cells within this population proliferate and form organospheres in Matrigel cultures in the presence of fibroblast growth factor 10 and hepatocyte growth factor. Following orthotopic transplantation into IAV‐injured murine lungs, EpCAMhighCD24lowSca‐1+ cells regenerate the distal lung tissue and give rise to AEC I, thus demonstrating inherent stem cell properties (Quantius et al, 2016). Accordingly, bronchioalveolar stem cells (BASCs) represent an important stem/progenitor cell population that co‐expresses SCGB1A1 and SFTPC and can differentiate into club cells, AECII, and AECI in vitro (Giangreco et al, 2002; Lee et al, 2014). Our recently published data showed that BASCs contribute to regeneration of both bronchiolar epithelium and alveolar epithelium following lung injury including influenza virus‐induced damage in vivo (Salwig et al, 2019).
In the present study, we isolated an epithelial cell population that contains BASCs to develop a robust protocol for generation of bronchioalveolar lung organoids (BALOs) comprised of spatially organized bronchiolar‐like and alveolar‐like structures after co‐culture with defined subsets of lung‐resident mesenchymal cells (rMC). Within the organoids, cells in the alveolar‐like regions differentiated into mature AEC II and AEC I, whereas the airway‐like regions contained basal cells, secretory and ciliated cells. In addition, we provide evidence for successful engraftment of tissue‐resident, yolk sac‐derived alveolar macrophages (TR‐Mac) into the developing BALO. We also demonstrate the usefulness of our 3D model to study lung development by inhibition of microRNA 142‐3p (miR‐142-3p) gene expression. Finally, we show that BALOs can be infected with IAV, which opens new avenues to study and visualize processes of lung infection, injury, and repair.
Results
3D co‐culture of BASC and rMC results in the formation of BALO
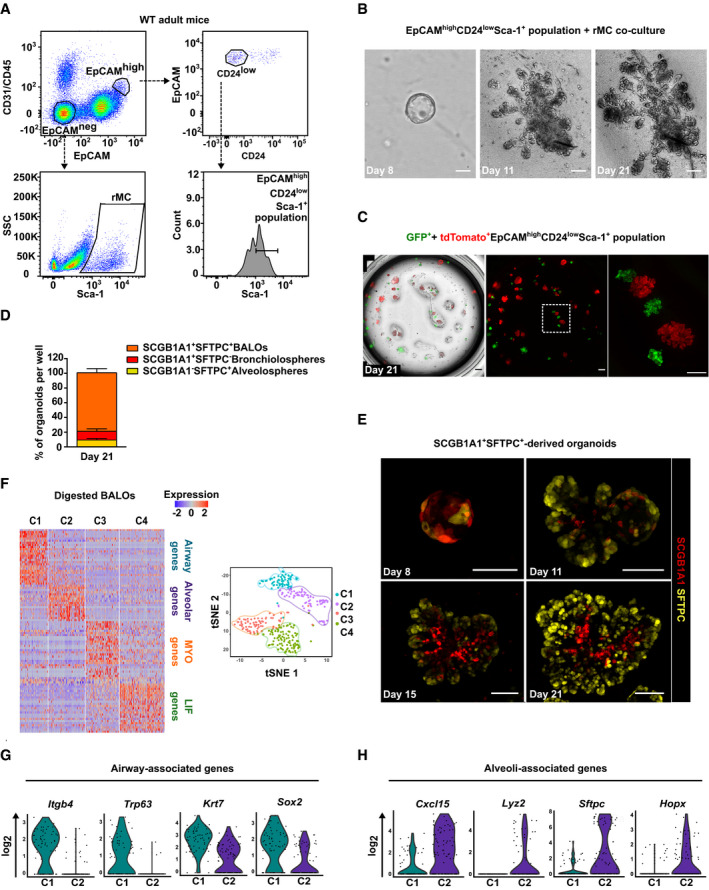
To establish lung organoids, rMC (defined as EpCAM−Sca‐1+) and epithelial stem/progenitor cells were isolated from leukocyte/endothelial cell‐depleted lung homogenates of wild‐type (WT) mice by FACS. The latter population was selected according to a previously defined signature enriching epithelial stem/progenitor cells, using the surface markers EpCAMhighCD24lowSca‐1+ (Quantius et al, 2016; Fig 1A). Flow‐sorted EpCAMhighCD24lowSca‐1+ cells in combination with rMC were seeded into Matrigel, and the development of organoid structures was monitored for at least 21 days. EpCAMhighCD24lowSca‐1+ cells expanded and formed colonies which developed into organospheres as illustrated in Fig 1B. After 10–11 days in culture, lung organoids started to develop buds that formed well‐defined central bronchiolar‐like structures and peripheral alveolar‐like structures by day 21 (Fig 1B and Movie EV1). Confocal microscopy showed that bronchiolar‐like structures within BALO were positive for the club cell marker secretoglobin family 1 A member 1 (SCGB1A1), whereas surrounding alveolar‐like regions stained positive for the AEC II marker surfactant protein C (SFTPC) (Appendix Fig S1A). In addition to BALOs, two other types of organoids formed, albeit at a far lower frequency. These structures resembled the previously described bronchiolar organoids composed of large tubes (bronchiolospheres) and alveolospheres characterized by a compact saccular arrangement (Appendix Fig S1B; Barkauskas et al, 2013; Lee et al, 2014). Our findings suggested that the EpCAMhighCD24lowSca‐1+ population used for organoid formation was not homogeneous but contained multilineage‐committed progenitor cells with bronchial epithelial cell and AEC differentiation potential. Quantitative analysis showed that about 80% of organoids formed per well were BALOs in an initial culture (passage 0; P0), whereas 6 and 14% revealed an alveolar and bronchiolar phenotype, respectively (Appendix Fig S1C). To verify that BALOs were generated by clonal expansion of a putative stem cell rather than by self‐assembly of several cell types contained within the EpCAMhighCD24lowSca‐1+ population, cells expressing membrane‐targeted tdTomato were cultured with a mixture of GFP‐expressing cells (Fig 1C). As would be expected for organoids derived from a single cell, only GFP+ or tdTomato+ organoids were observed but no mixed phenotypes. Taken together, these data highlight the potential of single EpCAMhighCD24lowSca‐1+ cells to proliferate and differentiate into a complete lung organoid in the presence of rMC.
Figure 1. 3D co‐culture of BASC and rMC results in the formation of BALO.
-
AGating strategy for sorting of EpCAMhighCD24lowSca‐1+ cells and rMC from lung homogenate of adult mice.
-
BTime course of epithelial stem/progenitor cell proliferation and differentiation within BALO at days 8, 11, and 21 of co‐culture with rMC.
-
CClonal expansion of EpCAMhighCD24lowSca‐1+ cells derived from either GFP‐expressing mice or tdTomato‐expressing mice at day 21 of co‐culture.
-
DPercentages of SCGB1A1+SFTPC+ (BALOs), SCGB1A1+SFTPC− (bronchiolospheres), and SCGB1A1−SFTPC+ (alveolospheres) organoids per well at day 21 of culture derived from EpCAMhighCD24lowSca‐1+ cells isolated from Scgb1a1 mCherry Sftpc YFP reporter mice (n = 4 biological replicates).
-
ERepresentative confocal images of days 8–21 of culture showing endogenous SCGB1A1 and SFTPC expression during BALO formation derived from EpCAMhighCD24lowSca‐1+SCGB1A1+SFTPC+ cells isolated from Scgb1a1 mCherry Sftpc YFP reporter mice.
-
F–HHeat map (left) and tSNE plot (right) (F) of digested day 21 BALO cultures depicting four distinct clusters (C1, airway, blue; C2, alveolar, purple; C3, MYO, orange; and C4, LIF, green). Violin plots of selected genes representing airway‐associated genes (G) (Itgb4, Trp63, Krt7, and Sox2) and alveoli‐associated genes (H) (Cxcl15, Lyz2, Sftpc, and Hopx). Each violin plot shows the frequency distribution of the mean transcript level (log2).
To further define the stem/progenitor cell phenotype within the EpCAMhighCD24lowSca‐1+ cell population, additional known epithelial stem cell markers were analyzed. BASCs are located at the bronchoalveolar duct junction and have been shown to be able to expand after injury, giving rise to differentiated alveolar as well as bronchiolar cells (Kim et al, 2005; Liu et al, 2019; Salwig et al, 2019). BASCs co‐express the club cell marker SCGB1A1 and the AEC II marker SFTPC. We used EpCAMhighCD24lowSca‐1+ cells from Scgb1a1 mCherry Sftpc YFP double reporter mice (SPC‐2A-YFP‐2A-tTA‐N, CCSP‐2A-mCherry‐2A-tTA‐C) to determine mCherry and YFP co‐expression within the EpCAMhighCD24lowSca‐1+ population (Salwig et al, 2019). About 5% of the cells were double‐positive for SCGB1A1 and SFTPC (Appendix Fig S1D). While most cells (95%) were SCGB1A1+SFTPC−, SCGB1A1−SFTPC+ cells were detected at a very low level in this fraction (< 0.5%). Although SCGB1A1+SFTPC+ cells were present at low frequency, mature (mCherry+YFP+) BALOs represented 80% of all organoids at day 21 (Fig 1D). Bronchiolospheres (composed solely of SCGB1A1+ cells) accounted for only 11%, whereas alveolospheres (composed solely of SFTPC+ cells) accounted for 9% of all organoids. To address the respective cell of origin of the different organoid phenotypes, EpCAMhighCD24lowSca‐1+ cells were flow‐sorted according to single or double expression of SCGB1A1 and SFTPC, and developing organoids were followed over time by confocal microscopy. Culture of SFTPC+ or SCGB1A1+ single‐positive EpCAMhighCD24lowSca‐1+ cells resulted in the formation of alveolospheres or bronchiolospheres, respectively, while the formation of BALOs was not detected in cultures with either of these two populations (Appendix Fig S1E and F). SCGB1A1+SFTPC+ double‐positive cells gave rise to BALOs at a colony‐forming frequency of 1:100, indicating their BASC phenotype (Fig 1E and Appendix Fig S1G). Given that 95% of EpCAMlowSFTPC+ AEC II (Quantius et al, 2016) are excluded by the EpCAMhighCD24lowSca‐1+ gating strategy, AEC II were isolated based solely on SFTPC single expression to evaluate their potential to generate organoids under our culture conditions. The resulting organoids formed at a colony‐forming frequency of 1:25 from SFTPC+ cells and were exclusively of alveolospheres phenotype, whereas isolation of only SCGB1A1+ cells without pre‐enrichment showed a colony‐forming frequency of 1:1,000 and generated bronchiolospheres (Appendix Fig S1G). We then used EpCAMhighCD24lowSca‐1+ cells from Scgb1a1 mCherry Sftpc YFP double reporter mice to determine mCherry and YFP expression during BALO formation. Within the early‐stage BALO, SCGB1A1+ and SFTPC+ cells were uniformly distributed with only a small fraction of double‐positive cells remaining at day 8 of culture. Day 21 BALOs were comprised of SCGB1A1+ central branches surrounded by SFTPC+ alveolar‐like structures (Figs 1E and EV1), confirming the cellular and structural composition of BALO at this later stage (Appendix Fig S1A). In addition, we used “BASC v‐race” (SPC‐2A-YFP‐2A-tTA‐N, CCSP‐2A-mCherry‐2A-tTA‐C, tetObiluc/Cre, Rosa26stopflox‐lacZ) mice in which BASCs and all their descendants are permanently labeled via β‐galactosidase activity to generate organoids. After 21 days of culture, completely LacZ+ BALOs were observed indicating that all cells in BALO originate from BASC (Appendix Fig S1H). Finally, to address whether BALOs still contained stem/progenitor cell pools, we digested only BALOs from the initial (P0) culture by manually removing all bronchiolospheres and alveolospheres and re‐cultured them in the presence of freshly sorted rMC (P1). As shown in Appendix Fig S1C, new BALOs formed in P1 cultures, although with a lower frequency as in the initial culture (19%), suggesting that within BALO there are progenitor cells capable of forming bronchiolospheres and alveolospheres, such as SCGB1A1+ bronchiolar and SFTPC+ alveolar progenitor cells. Nonetheless, BALOs were still formed after further passaging of these mixed organoids indicating the presence of BASCs within BALO (Appendix Fig S1C). Moreover, WT rMC were co‐cultured with BASCs that were isolated from the lungs of “BASC viewer” (SPC‐2A-YFP‐2A-tTA‐N, CCSP‐2A-mCherry‐2A-tTA‐C, tetObi lacZ/huGFP) mice, which harbor a split‐tTA construct at the endogenous gene loci to allow the identification of SCGB1A1+SFTPC+ double‐positive cells via β‐galactosidase labeling (Salwig et al, 2019). Notably, LacZ staining of the cultures revealed the presence of SCGB1A1+SFTPC+ BASCs within BALO, located at the distal regions even after 60 days of culture (Appendix Fig S1I).
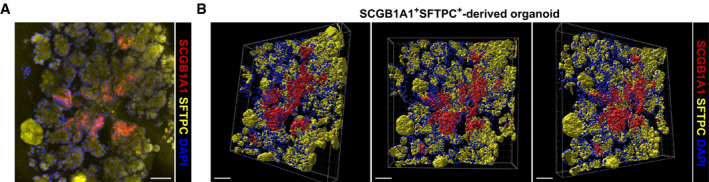
Figure EV1. 3D reconstruction of BALO depicts proximo‐distal cell specification.
-
A, BRepresentative confocal picture (A) and 3D reconstruction (B) of a day 21 culture showing endogenous SCGB1A1 and SFTPC expression within BALO derived from EpCAMhighCD24lowSca‐1+SCGB1A1+SFTPC+ cells isolated from Scgb1a1 mCherry Sftpc YFP reporter mice. Scale bars represent 50 μm.
To analyze the cellular composition of BALO at a mature stage, single‐cell RNA sequencing (scRNA‐Seq) was performed on digested day 21 BALOs. Data analysis revealed the presence of four distinct clusters including two epithelial (C1 and C2) and two mesenchymal subpopulations (C3, myofibroblasts (MYO); and C4, matrix fibroblasts/lipofibroblasts (LIFs)) (Fig 1F). Cells in the epithelial clusters expressed both airway‐ and alveoli‐associated genes (Fig 1G and H). Airway‐associated genes (C1) identified cellular markers for ciliated cells (Itgb4), basal cells (Trp63, Krt7), and respiratory epithelium (Sox2) (Fig 1G; Treutlein et al, 2014; Du et al, 2015, 2017). The alveolar cluster (C2) included genes described as lineage markers for AEC II such as Cxcl15, Lyz2, and Sftpc, and AEC I lineage markers such as Hopx (Fig 1H; Treutlein et al, 2014; Du et al, 2015, 2018).
The BALO model mimics the 3D morphology and cellular composition of the bronchioalveolar compartment with proximo‐distal patterning and pulmonary surfactant secretion
To further characterize and validate the cellular composition of BALO, day 0 and day 21 cultures were digested with dispase and analyzed by FACS (Appendix Fig S2A). Corresponding to the scRNA‐Seq data, BALOs were composed of an EpCAM+ epithelial and EpCAM− non‐epithelial fraction. The EpCAM+ BALO population consisted of cell populations that express typical markers of differentiated airway and alveolar epithelium. These subpopulations included a major fraction of AEC II (94.3 ± 0.2%), low‐frequent podoplanin (PDPN)+ AEC I (5.3 ± 0.6%), and EpCAMhighCD24high small airway (bronchial) epithelial cells (Appendix Fig S2A). Furthermore, we validated upregulation of lineage markers of differentiated cell types of the adult lung by qPCR. In day 21 BALO cultures, upregulated genes included Hopx for AEC I and Sftpc for AEC II, as well as Foxj1, Muc5ac, and p63 for ciliated, goblet cells, and basal cells, respectively (Fig 2A).
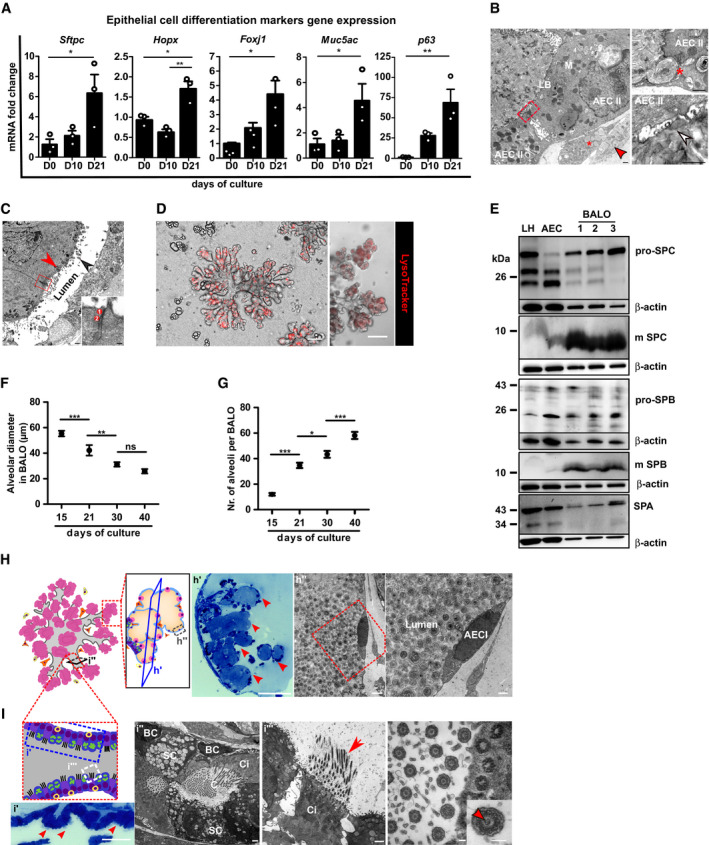
Figure 2. The BALO model mimics the 3D morphology and cellular composition of the bronchioalveolar compartment with proximo‐distal patterning and pulmonary surfactant secretion.
-
AmRNA expression analysis of epithelial cell differentiation markers Sftpc (AEC II), Hopx (AEC I), Foxj1 (ciliated cells), Muc5ac (secretory cells), and p63 (basal cells) in BALO at days 0, 10, and 21 of culture (n = 3 biological replicates with pooled cells from 4 cultures per replicate).
-
BElectron microscopy of BALO alveoli showing two cuboidal epithelial cells (AEC II) connected by tight junctions (red square) with numerous mitochondria (M) and lamellar bodies (LB). A LIF with numerous lipid droplets (*) and a MYO with cisterns of rough ER (red arrow) are located at the basal side (left). The alveolar lumen is filled with lamellar surfactant including tubular myelin (white arrow) (lower right). Exocytosis of a lamellar body (*) from an AEC II (upper right). Scale bars indicate 500 nm.
-
CElectron microscopy of bronchiolar‐like airway depicting columnar ciliated cells (red arrowhead) with basal bodies (black arrowhead) at the left side of the longitudinally sectioned lumen. The boxed area indicates an apical junctional complex between two ciliated cells: 1 = tight junction and 2 = adherens junction. Scale bar indicates 500 nm (in insert: 100 nm).
-
DStaining of lamellar bodies in BALO with RFP LysoTracker. Scale bars represent 100 μm.
-
EWestern blot analysis of the surfactant proteins: pro‐SPC, mature SPC, pro‐SPB, mature SPB, and SPA in lung homogenate (LH), AEC, and day 21 BALO (n = 3 biological replicates).
-
F, GAlveolar diameter (F) and number of alveoli (G) in tdTomato+ BALO at days 15, 21, 30, and 40 of culture were measured from n = 5 BALOs in n = 3 biological replicates BALOs.
-
H, IRepresentative scheme and images of day 40 BALO alveoli (H) and airway (I). BALO alveolar‐like structures (H) are shown (h′) (red arrowheads) in semi‐thin section (0.5 μm) stained with Toluidine blue. Scale bar indicates 100 μm (left). Electron microscopy showing AEC I (h″) ultrastructure within BALO. Scale bars indicate 2,500 nm (center) and 1,000 nm (right). An airway‐like structure (i′) is shown with secretory and ciliated cells (red arrowheads) in semi‐thin sections (0.5 μm) longitudinally cut and stained with Toluidine blue. Scale bar indicates 50 μm (far left). Electron microscopy of a bronchiolar‐like airway (I) depicting pseudostratified epithelium (i″) with a basal‐like cell (BC), not reaching the lumen in which cilia (C) are seen, located between a secretory (SC) and a ciliated cell (Ci). Scale bars indicate 1,000 nm (left and right). Mature cilia (i‴) in BALO at higher magnification depicting the 9 × 2 + 2 structure with central doubled microtubules (insert, red arrowhead), a characteristic for motile cilia. Scale bar indicates 100 nm (far right).
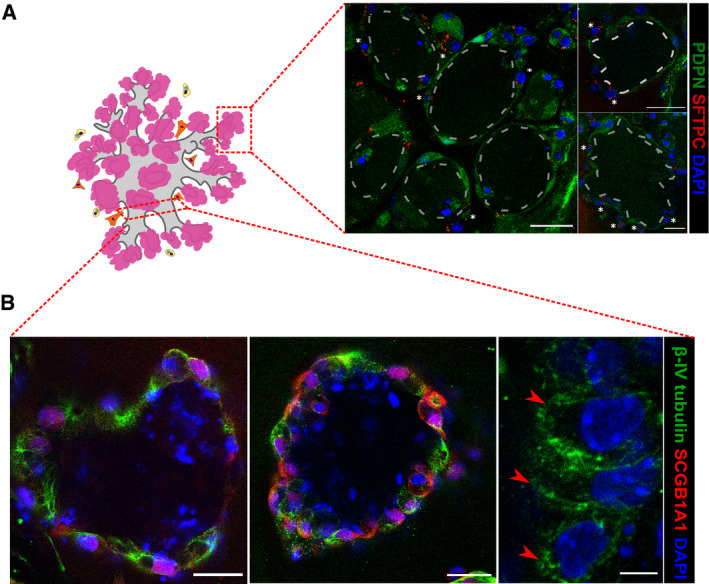
Moreover, electron microscopy analyses revealed that alveoli were lined with a single layer of epithelial cells with short microvillous protrusions. These cells were interconnected by tight junctions and contained abundant mitochondria and numerous lamellar bodies, which represent a characteristic feature of mature AEC II (Fig 2B). Notably, bronchoalveolar duct junction‐like regions containing bronchiolar and intermediate cells types that lead into alveolar‐like regions with flattened AEC I were identified within BALO (Appendix Fig S2B). Cells lining the airway‐like tubes were devoid of surfactant and showed different phenotypes from undifferentiated epithelial cells to ciliated airway cells found to be interconnected by typical junctional complexes at their apical surfaces (Fig 2C). To demonstrate the presence of surfactant production, BALOs were stained with LysoTracker, which has been shown to accumulate in lamellar bodies, and LipidTOX for phospholipids as a major component of pulmonary surfactant. As expected, alveolar‐like areas within BALO showed the presence of lamellar bodies and phospholipids (Fig 2D and Appendix Fig S2C). Furthermore, surfactant production in BALO was confirmed by Western blot analysis of pro‐SPC, mature SPC, pro‐SPB, mature SPB, and SPA (Fig 2E). Further proximo‐distal specification of BALO was demonstrated by identification of PDPN+ AEC I within the alveolar‐like regions in BALO generated from Pdpn GFP reporter mice and by staining of β‐IV tubulin+ ciliated cells located in the airway‐like sections (Fig EV2A and B).
Figure EV2. BALOs express markers of differentiated airways and alveoli.
-
A, BRepresentative scheme and confocal images of the BALO alveolar‐like (A) and airway‐like (B) structures. (A) Representative image of AEC II SFTPC staining (white asterisks) and AEC I endogenous PDPN expression in day 28 BALO isolated from the lungs of Pdpn GFP reporter mice. Scale bars represent 25 μm. Dotted lines indicate the alveoli. (B) Representative fluorescence images of BALO's airway‐like structures stained for β‐IV tubulin+ ciliated cells (red arrowheads) and SCGB1A1+ club cells. Scale bars represent 25 μm (left and middle) and 5 μm (right).
A recent stereological analysis has shown that the mean diameter of murine lung alveoli decreases during the first weeks after birth, while the number of alveoli increases before reaching a plateau throughout adulthood (Pozarska et al, 2017). In this regard, analysis of BALO distal structures at different stages revealed that the dynamic changes in terms of size and number followed a similar trend during BALO formation. The mean diameter of the distal alveolar‐like structures decreases by 23% between days 15 and 21, 13% between days 21 and 30, and remains unchanged until day 40. Correspondingly, BALOs showed a threefold increase in the mean number of alveolar‐like structures from day 15 to 30 (Fig 2F and G, and Appendix Fig S2D).
Notably, alveolar‐like structures within BALO showed a lumen filled with lamellar surfactant and lined by thin and elongated AEC I intercalated by cuboidal AEC II joined through tight junctions after 40 days of culture (Fig 2H and Appendix Fig S2E). The BALO airway‐like ultrastructure forms a pseudostratified epithelium comprised of basal‐like cells, differentiated secretory cells filled with secretory granules, and ciliated cells with mature cilia and basal bodies aligned underneath the apical cell surface. Of note, kinocilia with the classical central microtubule pair (9 × 2 + 2 configuration) could be identified in micrographs with higher magnification (Fig 2I).
Taken together, our in vitro model meets criteria that identify organoids based on the definition published by Lancaster and Knoblich, including (i) composition of multiple organ‐specific cell types, (ii) recapitulation of specific organ features such as lumen‐directed secretion of pulmonary surfactant by AEC II, and, importantly, (iii) spatially restricted cell lineage commitment with organization into defined airway‐ and alveolar‐like compartments with clear proximo‐distal patterning (Lancaster & Knoblich, 2014).
Distinct subsets of EpCAM−Sca‐1+ rMC‐derived fibroblasts are indispensable for BALO growth, cell differentiation, and branching morphogenesis, and model the mesenchymal niche of the lung
Having characterized the BALO epithelial compartment, we sought to phenotype the mesenchymal compartment during BALO development and after differentiation. EpCAM−Sca‐1+ of non‐leukocyte and non‐endothelial origin (Fig 1A, rMC) was previously found to support lung organoid formation (Quantius et al, 2016). In accordance with our scRNA‐Seq analysis and previously published data showing that rMC are a heterogeneous population including progenitors of MYO and LIF (Fig 1G; Perl & Gale, 2009; Al Alam et al, 2015), we found by microscopic analysis of BALO cultures that rMC gave rise to at least two distinct fibroblast cell types (Fig 3A and B, and Appendix Fig S3A). One population contained LipidTOX‐positive lipid bodies, characteristic of LIF, while the other population consisted of alpha‐smooth muscle actin (αSMA)‐positive, spindle‐shaped cells with long cellular extensions and ample cisterns of rough endoplasmic reticulum resembling MYO (Figs 2B and 3B, and Appendix Fig S3B).
Figure 3. Distinct subsets of EpCAM−Sca‐1+ rMC‐derived fibroblasts are indispensable for BALO growth, cell differentiation, and branching morphogenesis, and model the mesenchymal niche of the lung.
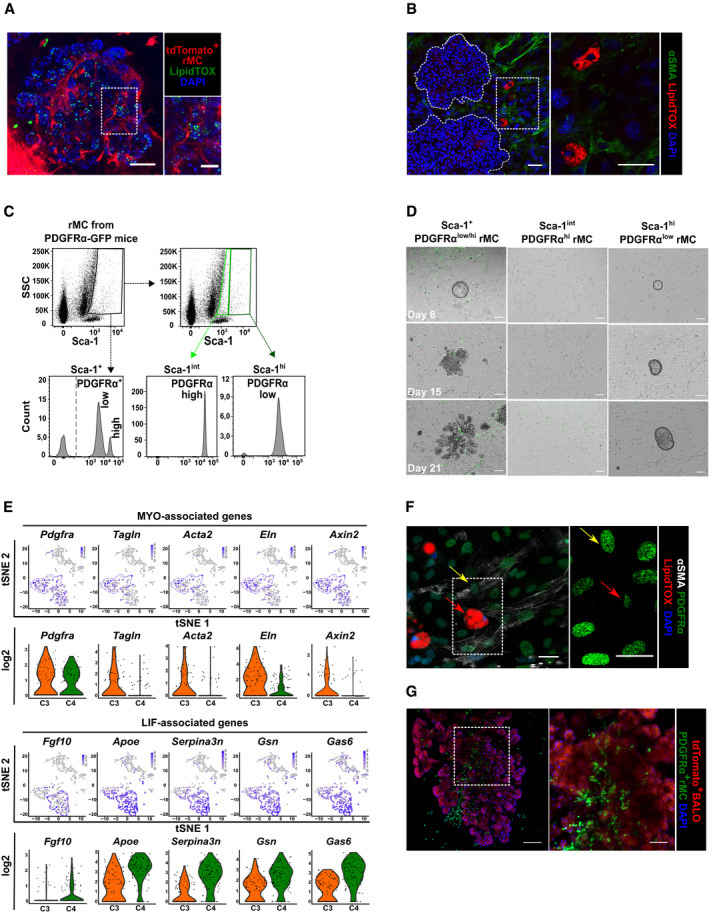
- Representative images of day 21 BALO and tdTomato+ rMC stained with LipidTOX (green). Scale bar represents 100 μm.
- Fluorescence images of αSMA (green) and neutral lipids (LipidTOX red) staining in WT‐derived rMC and BALO (dotted lines indicate single BALO or insert) at day 21 of culture. Scale bars represent 50 μm (left) and 25 μm (right).
- Representative flow cytometric dot plots and histograms of PDGFRα expression in rMC (EpCAM−CD45−CD31−Sca‐1+) isolated from the lung homogenate of Pdgfra GFP reporter mice.
- Representative images of BALO formation at day 8, 15, and 21 of co‐culture. WT BASCs were co‐cultivated with sorted rMC (total PDGFRα+ population) or rMC expressing either low or high levels of PDGFRα‐GFP. Scale bars represent 100 μm.
- tSNE plots and violin plots depicting selected genes representing MYO (Pdgfrα, Tagln, Acta2, Eln, and Axin2) (top panels) and LIF‐associated genes (Fgf10, Apoe, Serpina3n, Gsn, and Gas6) (bottom panels). Each violin plot shows the frequency distribution of the mean transcript level (log2). C3 (MYO, orange) and C4 (LIF, green) refer to the scRNA‐Seq experiment in Fig 1G.
- Fluorescence image of αSMA+PDGFRαhigh MYO (yellow arrows) and LipidTOX+PDGFRαlow LIF (red arrows) from sorted PDGFRα‐GFP rMC after 21 days of BALO culture. Scale bars represent 50 μm (left) and 25 μm (right).
- Fluorescence image containing PDGFRαhigh MYO from sorted PDGFRα‐GFP rMC within BALO derived from tdTomato+ mice at day 21 of culture. Scale bars represent 100 μm (left) and 50 μm (right).
It has been reported that MYO and LIF are defined by the differential expression of platelet‐derived growth factor receptor alpha (PDGFRα) and αSMA (Perl & Gale, 2009). Using a Pdgfra GFP knock‐in mouse line, two populations of rMC necessary for BALO formation were defined: a Sca‐1highPDGFRαlow and a Sca‐1intPDGFRαhigh fraction (Fig 3C and D). Intriguingly, although colony‐formation efficiency was the same as when both PDGFRα populations were present, Sca‐1highPDGFRαlow rMC allowed early organoid growth but were unable to support alveolar differentiation or sustain branching morphogenesis (Appendix Fig S3D and E). In contrast, co‐culture of BASCs with Sca‐1intPDGFRαhigh rMC did not support organoid outgrowth, suggesting that both populations were required for complete BALO formation (Fig 3D). To verify that Sca‐1intPDGFRαhigh rMC were necessary to drive branching morphogenesis, BALO cultures grown in the presence of Sca‐1highPDGFRαlow rMC fraction were complemented with Sca‐1intPDGFRαhigh rMC at day 7 of culture (shortly before first branches start to form in BALO). Branching morphogenesis could be rescued to a large extent; however, BALO formation was found to be delayed and did not reach BALO full size until day 28. These data indicate that Sca‐1intPDGFRαhigh rMC are essentially driving branching and that an early crosstalk between both rMC populations and BASCs might be essential for proper dynamics of BALO formation (Appendix Fig S3C).
Using our scRNA‐Seq data set (Fig 1G), we revealed two mesenchymal cell clusters. Among the genes expressed within these clusters are genes previously associated with MYO (C3) and LIF (C4) phenotypes including Fgf10, Pdgfrα, Tagln, Acta2, and Eln genes (Fig 3E and Table 1; Perl & Gale, 2009; McGowan & McCoy, 2014; Al Alam et al, 2015), thus confirming our morphological data. Of note, PDGFRα expression was higher in MYO than in LIF, in line with previous findings on these mesenchymal cell subsets in our BALO model (Perl & Gale, 2009). LIF‐associated genes previously defined by RNA‐Seq analysis of lung samples such as Apoe, Serpina3a, Gsn, and Gas6 were detected in the LIF cluster (Du et al, 2015, 2017). In addition, Axin2, a gene recently related to the MYO phenotype in the lung, was also expressed in the BALO MYO cluster (Zepp et al, 2017).
Table 1.
Adjusted P‐value of genes in LIF and MYO clusters
| MYO | P_val_adj | LIF | P_val_adj |
|---|---|---|---|
| Pdgfra | 3.78E‐04 | Apoe | 2.26E‐26 |
| Tagln | 4.37E‐07 | Serpina3n | 2.00E‐30 |
| Acta2 | 1.02E‐06 | Gsn | 3.88E‐19 |
| Eln | 3.44E‐15 | Gas6 | 8.32E‐21 |
| Axin2 | 2.95E‐04 | Fgf10 | 0.00217 |
Adjusted P‐value of genes associated with MYO (C3) and LIF (C4) phenotypes including Pdgfrα, Tagln, Acta2, Eln, Axin2 and Apoe, Serpina3n, Gsn Gas6, and Fgf10, respectively.
In the developing lung, lipid‐droplet‐containing LIFs are found in close proximity to the alveolar epithelium promoting epithelial growth and AEC II differentiation (El Agha et al, 2017). Moreover, LIFs allow primary murine AEC to form alveolospheres under co‐culture conditions by providing growth factors (Barkauskas et al, 2013). In accordance, we detected LipidTOX+ LIF frequently distributed around developing organospheres and around BALO. LipidTOX+ LIFs were found to be PDGFRαlow, whereas αSMA+ alveolar MYOs were confined to the PDGFRαhigh expressing fraction (Fig 3F). MYO had been demonstrated to deposit extracellular matrix in the neonatal lung, thereby providing the necessary scaffold for alveolarization (El Agha & Bellusci, 2014). Consistently, PDGFRαhigh MYO accumulated at branching sites within the center of the BALO (Fig 3G).
In summary, our data revealed the presence of distinct subsets of PDGFRα high‐ and low‐expressing MYO and LIF required for BALO formation. MYO and LIF are spatially organized within or in close proximity to alveolar‐like structures. Therefore, BALO might be useful for modeling the pulmonary mesenchymal niche and for studying of epithelial–mesenchymal crosstalk under different conditions.
TR‐Mac engraft into BALO alveolar‐like regions, maintain their identity, and drive epithelial cell differentiation
Although mature BALOs consist of epithelial and mesenchymal cell subsets, the model still lacked tissue‐resident immune cells that would be required to study processes dependent on these cells in lung development, homeostasis, and disease (Wynn & Vannella, 2016). Therefore, to complete the alveolar niche, TR‐Mac were introduced into BALO after isolation from bronchoalveolar lavage (BAL) fluid of adult tdTomato‐expressing reporter mice. Single‐cell TR‐Mac suspensions were prepared, and up to 50 TR‐Mac were microinjected at the rate of 6 cells/min under visual control into central regions of day 14 BALO (Appendix Fig S4A and B). Prior to microinjection, the TR‐Mac surface antigen signature (CD45+Ly6g−Gr1−CD11c+Siglec‐F+) was confirmed by FACS (Fig 4A). Notably, TR‐Mac efficiently engrafted at least until day 28 of culture (Fig 4B). Quantification of the numbers of TR‐Mac over time revealed that > 80% of initial TR‐Mac could be detected at 10 days post‐injection with ~ 87% viability (Appendix Fig S4C and D). Moreover, preservation of the TR‐Mac phenotype within the alveolar niche was proven by positive staining of the alveolar macrophage surface markers, CD206 and Siglec‐F, 14 days after microinjection (Fig 4C). To demonstrate direct TR‐Mac‐AEC interaction in the alveolar‐like niche of BALO, we performed electron microscopy analyses and revealed TR‐Mac filopodia in direct contact with AEC (Fig 4D). Of note, evidence of surfactant uptake and digestion by microinjected TR‐Mac was observed in the lumen of the BALO alveolar‐like structures (Appendix Fig S4E). A previous publication revealed that TR‐Mac express the tight junction molecule connexin 43 (Cx43) upon interaction with AEC in the lung (Westphalen et al, 2014). To confirm that TR‐Mac would similarly communicate with AEC in BALO after alveolar engraftment, and to visualize this intercellular communication, we stained TR‐Mac‐supplemented BALO cultures for Cx43 and revealed that TR‐Mac within alveolar‐like regions, but not TR‐Mac mono‐cultured in Matrigel without BALO, expressed Cx43 (Fig 4E). To identify whether TR‐Mac impacted epithelial growth and differentiation within BALO, the composition and differentiation stage of the BALO epithelium were evaluated by scRNA‐Seq 9 days post‐microinjection. Comparative analysis of digested day 23 BALO cultures with and without microinjected TR‐Mac showed the presence of 6 distinct clusters defined as club/secretory cells (C1; Scgb3a2, Muc5b, Bpifa1), basal cells (C2; Krt5, Krt14, Aqp3, Trp63), rMC (C3; Col1a2, Igfbp4, Apoe), AEC II (C4; Sftpc, Cxcl15, Sftpb), AEC I (C5; Hopx, Ager, Cldn18), and ciliated cells (C6; Foxj1, Tppp3, Lrrc23) (Fig 4F and Appendix Fig S4F). Data revealed that genes associated with cell proliferation such as Fos, Fosb, Areg, and Klf4 and with inflammatory processes and cellular stress such as Erg1 and Atf3 were downregulated in BALO with TR‐Mac, whereas genes related to cell differentiation, Neat1, and club cell maturation, Cyp2f2 and Ces1d, were upregulated in the cluster containing club/secretory cells (C1) (Fig 4G). The total percentage of terminally differentiated epithelial cells (AEC I and ciliated cells) was significantly higher in BALOs microinjected with TR‐Mac (Fig 4H), suggesting that the presence of TR‐Mac in BALO drives epithelial differentiation, while rather diminishing cell proliferation and stress signals, thereby accelerating maturation of BALO. In sum, BALOs can be complemented by TR‐Mac that engraft into the BALO and exert defined functions that are relevant for lung homeostasis.
Figure 4. TR‐Mac engraft into BALO alveolar‐like regions, maintain their identity, and drive epithelial cell differentiation.
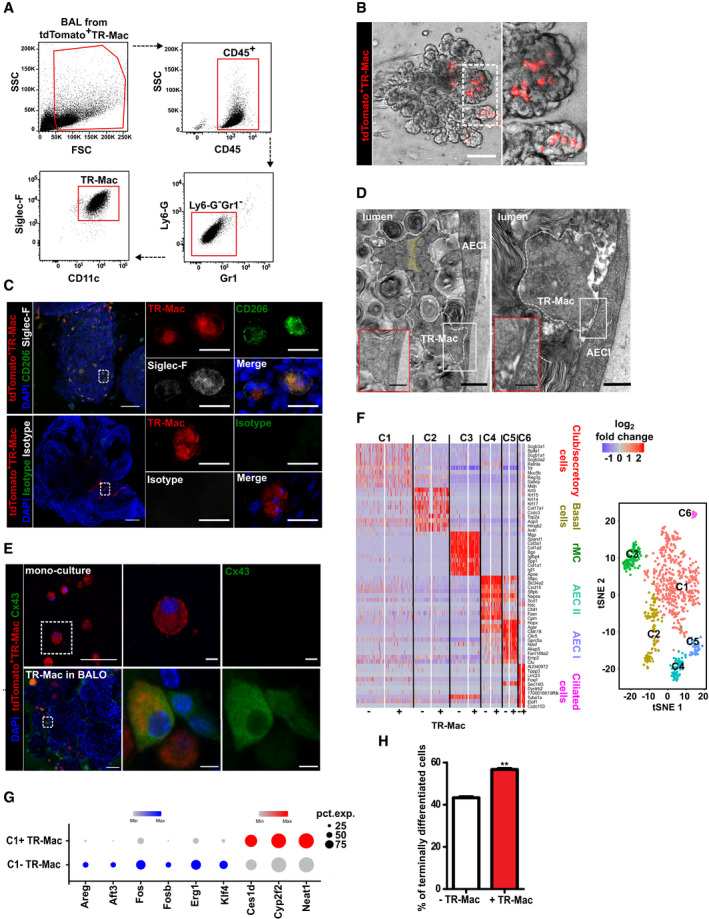
- Gating strategy to define TR‐Mac from BAL of adult tdTomato+ mice.
- Representative images of BALO after microinjection of tdTomato+ TR‐Mac at day 14. TR‐Mac are preferentially found in alveoli (right). Scale bars represent 100 μm (left) and 50 μm (right).
- Fluorescence confocal images of CD206, Siglec‐F, and isotype control staining 14 days after tdTomato+ TR‐Mac microinjection in a day 28 BALO. Scale bars represent 100 μm (left) and 25 μm (right).
- Electron microscopy depicting filopodium of TR‐Mac (white dashed lines) with a characteristic actin filament bundle (yellow background) (left panel) in contact with AEC I within BALO alveolar‐like structures. Uptake of surfactant by TR‐Mac is depicted by a (*). Scale bar indicates 1,000 nm (left, in insert: 500 nm) and 500 nm (right, in insert: 250 nm).
- Representative confocal images of Cx43 staining in tdTomato+ TR‐Mac monoculture in Matrigel and tdTomato+ TR‐Mac microinjected at day 14 and analyzed in a mature day 21 BALO. Scale bars represent 50 μm (overview) and 5 μm (close up).
- Heat map (left) and tSNE plot (right) of the comparative analysis of digested day 23 BALO cultures with (+) and without (−) microinjected TR‐Mac depicting six distinct clusters (C1, club/secretory cells, red; C2, basal cells, yellow; C3, rMC, green; C4, AEC II, blue; C5, AEC I, purple; and C6, ciliated cells, pink).
- Expression data dot plots of genes found differentially regulated in cluster C1 between day 23 BALO with (C1+) and without (C1−) microinjected TR‐Mac. The circle size illustrates the number of cells expressing a specific gene.
- Percentage of terminally differentiated cells (AEC I and ciliated cells) in day 23 BALO cultures with (+) and without (−) microinjected TR‐Mac.
Manipulation of WNT signaling pathway during BALO morphogenesis by knockdown of miR‐142‐3p gene expression recapitulates developmental defects observed in embryonic lung explants
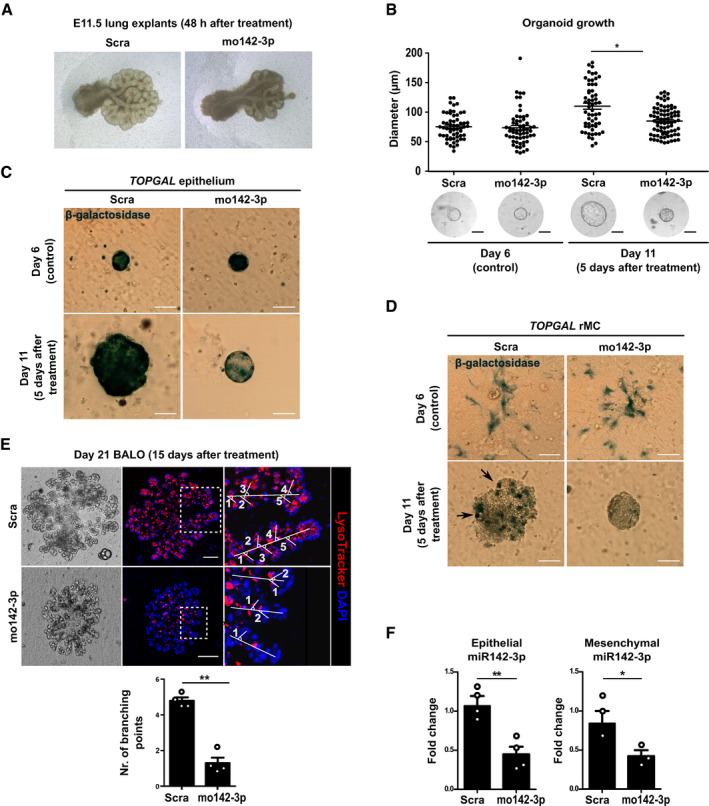
Following the detailed characterization of the structural and cellular composition of the BALO system, we next tested whether knockdown of important regulatory genes in BALO recapitulated developmental lung defects observed during in vivo loss‐of‐function experiments. Carraro et al (2014) have previously demonstrated that miRNA 142‐3p (miR‐142-3p) controls WNT‐dependent mesenchymal progenitor cell proliferation in the developing mouse lung. Inhibition of miR‐142-3p activity decreases proliferation of the lung mesenchyme and reduces lung growth and branching in embryonic lung explants (Fig 5A). To investigate whether, and if so, to what extent the effect of miR‐142-3p on the developing embryonic lung was recapitulated in the BALO model, miR‐142-3p‐specific morpholino antisense oligonucleotides (mo142‐3p) were diluted in media containing 0.2% FCS and applied to BALO cultures at day 6. Repetitive addition of mo142‐3p over a 5‐day period at an early organoid stage led to a significant decrease in organoid growth compared with addition of scrambled morpholino control (Scra) without affecting its colony‐forming efficiency (Fig 5B and Appendix Fig S5A). Of note, the treatment with mo142‐3p did not have adverse effects on cell viability when compared to untreated controls (Appendix Fig S5B). miR‐142-3p is known to regulate canonical WNT signaling by targeting adenomatosis polyposis coli (Apc), a gene involved in the β‐catenin destruction complex. Accordingly, gene expression of the miR‐142-3p target gene Apc was significantly upregulated in mo142‐3p‐treated cultures (Appendix Fig S5C). To identify the cellular compartment where downregulation of WNT signaling occurs within the BALO, TOPGAL mice allowing visualization of active canonical WNT signaling (by monitoring β‐galactosidase activity) were employed. For this purpose, BASCs from TOPGAL mice were co‐cultured with WT rMC or vice versa and treated with mo142‐3p or Scra starting at day 6 of culture. Similar levels of β‐galactosidase activity were detected in organospheres before addition of mo142‐3p or Scra at day 6 (Fig 5C). Interestingly, treatment with mo142‐3p led to a marked decrease in β‐galactosidase activity in the epithelial compartment of BALO and in reduced size compared with Scra controls. Visualization of WNT signaling in rMC revealed that mo142‐3p also targets the mesenchymal BALO compartment. Of note, β‐galactosidase+ mesenchymal cells were mainly detected inside the BALO by day 11 of culture (Fig 5D). Our data indicate that WNT signaling is activated in both epithelial and mesenchymal cells during organoid generation. mo142‐3p‐treated organoids were not only significantly smaller than Scra‐treated controls, but also exhibited impaired secondary branching (Fig 5E). Notably, numbers of AEC II and club cells were significantly reduced after treatment with mo142‐3p by FACS (Appendix Fig S5D). Knockdown of miR‐142-3p expression by mo142‐3p treatment in flow‐sorted EpCAM+ epithelial and EpCAM−Sca‐1+ mesenchymal cells from BALO was confirmed by qPCR (Fig 5F). The data demonstrate that (i) WNT signaling is involved in BALO morphogenesis and that (ii) BALO can serve as a platform for genetic manipulation of key developmental pathways to study their involvement in morphogenesis and response to injury.
Figure 5. Manipulation of WNT signaling pathway during BALO morphogenesis by knockdown of miR‐142‐3p gene expression recapitulates developmental defects observed in embryonic lung explants.
-
ARepresentative images of E11.5 lung explants after treatment for 48 h with 100 μM Scra or mo142‐3p reveal reduced size and branching morphogenesis after miR‐142-3p knockdown.
-
BOrganoid diameter (n = 30–50 per group) in μm before addition of 4 μM Scra or mo142‐3p at day 6 (control) and 5 days after treatment (at day 11 BALO culture) in n = 3 biological replicates.
-
C, DRepresentative images of β‐galactosidase staining in TOPGAL epithelium (C) and TOPGAL rMC (D) BALO cultures before (day 6, control) or 5 days after treatment with either 4 μM Scra or mo142‐3p (day 11 of co‐culture). BASCs and rMC were isolated from the lung homogenate of TOPGAL mice. β‐galactosidase+ rMC at day 11 of culture are indicated with arrows.
-
ERepresentative transmission and confocal images after LysoTracker staining indicating branching and number of branching points in n = 4 BALOs 15 days after treatment with 4 μM Scra or mo142‐3p (day 21 of co‐culture) in n = 3 biological replicates.
-
FmRNA levels of epithelial and mesenchymal miR‐142-3p expression in Scra and mo142‐3p-treated organoids 5 days after treatment (n = 3–4 biological replicates with pooled cells from 4 cultures per replicate).
BALOs support influenza virus infection and allow modeling of lung infection and injury
Lastly, the applicability of the BALO system for disease modeling was assessed in the context of influenza virus infection. In this regard, we next analyzed whether BALOs support influenza A virus infection using H7N7 and H1N1 IAV strains. To directly visualize infected epithelial cells, recently generated influenza reporter viruses expressing Cre recombinase or GFP, SC35MNS1_2A_Cre_2A_NEP and SC35MNS1_2A_GFP‐NEP (SC35M‐Cre and SC35M‐GFP, H7N7), were used (Reuther et al, 2015). To model proximal‐to‐distal epithelial infection as occurring in vivo, BALOs were infected with SC35M‐GFP virus by microinjection of the virus suspension into BALO central airway‐like structures (Fig 6A). Direct inoculation resulted in viral infection that spread efficiently from the proximal bronchiolar‐like regions toward the respective distal alveolar‐like regions within 12 h post‐infection (pi). Release of infectious influenza A virions from BALO was confirmed by plaque assay at 48 h pi (Fig 6B). To further demonstrate successful BALO infection, gene expression of H1N1 viral nucleoprotein (Np) in flow‐sorted infected EpCAM+/viral hemagglutinin (HA)+ versus non‐infected (EpCAM+HA−) cells was analyzed by qPCR. Np gene expression was detected in HA+ but not in HA− BALO epithelial cells (Fig 6C). Additionally, the proportion of infected EpCAM+ epithelial cells was determined by flow cytometry analysis of intracellular influenza virus NP expression. Viral infection was detected in approximately 8% of the cells (Fig 6D). Identification of IAV‐infected cells at a single‐cell resolution was achieved by using SC35M‐Cre in combination with BALO derived from reporter mice expressing tdTomato that switch from membrane‐targeted tdTomato to GFP after Cre‐mediated recombination. BALOs were monitored for 25 h pi to score the extent of infection, viral spread, and virus‐induced cell loss. We observed viral spread between 10 and 26 h pi, indicating that BALOs fully support viral replication (Fig 6E), which was also visualized over a time course of 26 h using live‐cell imaging of mTmG BALO where infection spreads to adjacent cells indicated by color switch from tdTomato to GFP (Movie EV2). Furthermore, 25 h pi we observed significant cell death in alveolar‐like areas that were previously infected at 14 h pi (Fig 6E; Herold et al, 2015). To define the host response to infection of BALO, interferon‐beta (Ifnb) expression in non‐ and IAV‐infected BALO cells was analyzed by qPCR (Fig 6F). A significant upregulation of Ifnb was observed in IAV‐infected EpCAM+HA+ BALO cells. To model macrophage–epithelial crosstalk under infection conditions, TR‐Mac were microinjected into BALO followed by infection with IAV or control inoculum (mock) for 48 h. Increased release of the pro‐inflammatory cytokines TNF‐α and IL‐6 was detected after BALO IAV infection. Remarkably, TNF‐α, IL‐6, and IL‐1β release was increased in IAV‐infected BALO with TR‐Mac compared to infected BALO without TR‐Mac (Fig 6G). These data indicate that the BALO epithelium not only supports IAV infection and spread but also mounts an antiviral response against IAV infection, which is amplified by TR‐Mac.
Figure 6. BALOs support influenza virus infection and allow modeling of lung infection and injury.
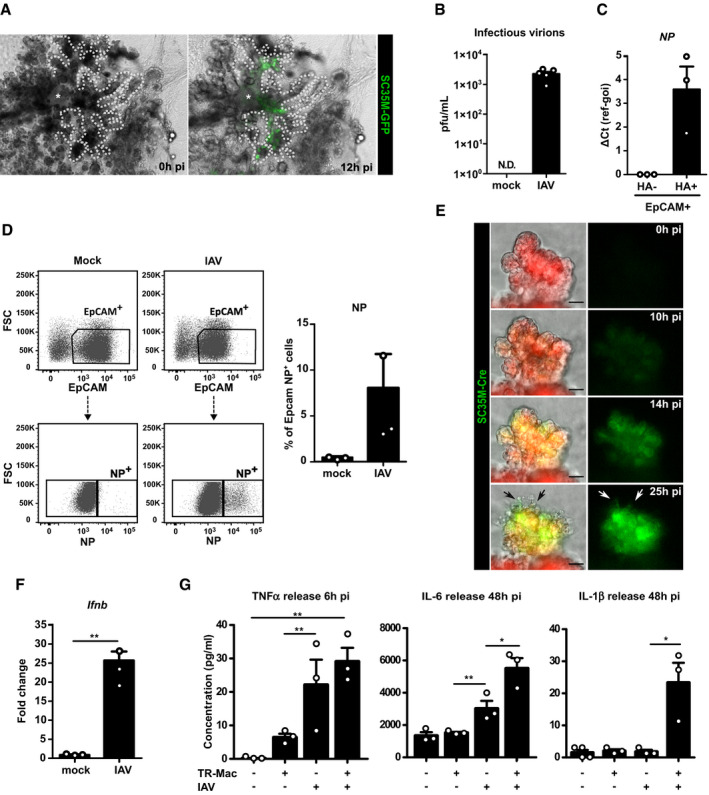
- Representative images of day 21 BALO after microinjection of SC35M‐GFP IAV (green) into the central airway‐like (*) after 0 and 12 h pi visualize IVA spread to the alveolar‐like regions. Dotted lines illustrate BALO borders. Scale bars represent 100 μm.
- Quantification of plaque‐forming units in supernatants from mock or SC35M IAV‐infected BALO 48 h after infection (n = 5 biological replicates). N.D.: not detectable.
- Relative NP expression in PR8 IAV‐infected (HA+) or non‐infected (HA−) epithelial cells isolated from BALOs 48 h pi, FACS‐sorted according to EpCAM and HA expression (n = 3 biological replicates with pooled cells from 4 cultures per replicate).
- Representative flow cytometric data showing the percentage of EpCAM+NP+ cells in mock‐ and SC35M IAV‐infected BALOs at 48 h pi in n = 3 biological replicates with pooled cells from 4 cultures per replicate.
- Representative fluorescence images of a day 21 distal region in BALO generated from mTmG reporter mouse and infected by SC35M‐Cre IAV after 0, 10, 14, and 25 h. Arrows indicate cell death. Scale bars represent 25 and 10 μm in the insert.
- mRNA expression of Ifnb in mock and PR8 IAV‐infected BALOs at 48 h pi (n = 3 biological replicates with pooled cells from 4 cultures per replicate).
- Release of TNF‐α (6 h pi), IL‐6 (48 h pi), and IL‐1β (48 h pi) detected by Bio‐Plex® Multiplex immunoassay in the supernatant of mock and IAV‐infected BALO cultures with and without TR‐Mac (n = 3 biological replicates).
Discussion
Several cellular and molecular processes cannot be easily studied in vivo, and traditional 2D in vitro cultures lack the conditions necessary to study distinct features involved in organ formation and regeneration. Consequently, 3D culture systems have recently emerged as a valuable platform for the better understanding of organogenesis and disease processes in vitro (Huch & Koo, 2015). BASCs have been shown to give rise to both airway and alveolar cell lineages upon different types of injury and although BASCs have not yet been identified in the human lungs and our understanding of the biology of these cells is limited, further genome profiling combined with lineage tracing may reveal specific cell markers for BASC identification in mice that could facilitate the discovery of a BASC equivalent in the human lung (Salwig et al, 2019). Therefore, in this work we describe the establishment of a robust protocol for isolation and 3D culture of murine progenitor cells with BASC signature and rMC giving rise to complete BALO that exhibits distinct bronchiolar‐like and alveolar‐like lung structures after 21 days of culture. Our data indicate that a high BALO purity of > 80% can be achieved when enriching these cells in the EpCAMhighCD24lowSca‐1+ fraction of the lung homogenate, thus preferentially excluding alveolosphere‐ and bronchiolosphere‐forming progenitor cells. As compared to previously described models, this system models much more closely the structural complexity and epithelial and mesenchymal cell composition of the murine lung with proximal structures containing airway epithelial cells (basal, ciliated, and club/secretory cells) and with distal alveolar‐like structures (AEC I and AEC II) including LIF and MYO that directly interact with the epithelium in a spatially defined manner (Barkauskas et al, 2013; Lee et al, 2014).
Most of the established human lung development and disease models are based on iPSC differentiated in 2D culture, therefore lacking a robust 3D structure including branched airways and distal alveoli (Rock et al, 2009; Kaisani et al, 2014; Dye et al, 2016; Nadkarni et al, 2016). Recently, iPSC‐derived human lung organoids were demonstrated to form airway‐like structures, but maintain a relatively high number of undifferentiated epithelial progenitor cells and low numbers of differentiated bronchial and alveolar cells, particularly AEC I (Dye et al, 2015). In addition, generation of lung‐like structures from human pluripotent stem cells is relatively time‐consuming and requires several months until the final differentiation stages are reached (Chen et al, 2017). Nonetheless, in a recent publication by Sachs et al (2019), human pseudostratified airway organoids were generated from adult tissue in conditions that allowed long‐term expansion of epithelial cells in vitro. Those airway organoids were comprised of basal cells, ciliated cells, and club/secretory cells, thus permitting effective modeling of airway diseases in vitro (Sachs et al, 2019).
Through investigation of the cellular composition of BALO by scRNA‐Seq, qPCR, immunofluorescence, electron microscopy, and FACS, we provide evidence that major cell types of the conducting airways such as basal, secretory, and ciliated cells, as well as alveoli, AEC I and AEC II, are present. Notably, differentiated AEC II within the distal BALO compartment were capable of surfactant secretion. Accordingly, scRNA‐Seq revealed expression of early AEC II markers such as Muc1, Slc34a2, and Scd1, which is indicative of AEC II progressive maturation during BALO formation (Treutlein et al, 2014). Furthermore, airway‐like regions in BALO at a later stage developed a pseudostratified epithelium and expressed well‐known basal cell markers Aqp3, Krt14, and Krt5 indicating the presence of basal‐like cells in BALO. To our knowledge, the BALO model is unique in terms of high cellular and structural complexity. Of note, BASC differentiation into basal cells has not been shown in vivo (Salwig et al, 2019), suggesting that BASC differentiation into basal cells in BALO may be for example driven by the complete absence of basal cells in the BALO airway‐like niche.
The phenotype and spatial organization of co‐cultured mesenchymal cells emerged as another important aspect of a successful lung organoid system. In this regard, besides providing a complex structural and cellular system, our in vitro model allows visualization of epithelial and mesenchymal interactions during BALO formation and differentiation, a feature that is not possible to achieve using current lung air–liquid interface cultures and most organoid systems (Rock et al, 2009; Schamberger et al, 2015; Rayner et al, 2019). During development, mesenchyme‐derived growth factors drive epithelial proliferation, differentiation, and branching morphogenesis (Herriges & Morrisey, 2014). Accordingly, CD45−CD31−EpCAM−Sca‐1+ rMC proved to be indispensable for proper BALO formation. Previous studies revealed also that LIFs are located at the base of the alveoli and express low levels of PDGFRα, whereas non‐lipid‐containing MYOs are PDGFRαhigh and reside at the alveolar entry ring (McGowan & Torday, 1997; Chen et al, 2012b). Using rMC isolated from PDGFRα reporter mice, we demonstrate that a PDGFRα‐positive fraction of sorted rMC likely contains progenitor cells for LIF and MYO phenotypes. A previous study in which AEC II were co‐cultured with PDGFRα+ cells revealed that these cells are necessary for alveolosphere formation (Chen et al, 2012b). Accordingly, αSMA+PDGFRαhigh MYOs were detected inside the organoid structures and seemed to provide a scaffold for tube formation (Barkauskas et al, 2013). Nonetheless, BASCs cultured with MYO precursors alone did not show any early organoid formation. In contrast, rMC‐derived LipidTOX+PDGFRαlow LIF supported BASC proliferation in the early organoid but did not support branching morphogenesis. These data indicate that both PDGFRαhigh and PDGFRαlow mesenchymal cell subsets are indispensable components of the stem cell niche and fulfill distinct tasks during BALO formation in the absence of endothelial cells (Lee et al, 2014). Of note, although MYO and LIF phenotype interconversion has been previously reported in vivo, it has only been observed under pathological conditions, suggesting that these processes may occur upon injury but not under homeostatic conditions (El Agha et al, 2017; Kheirollahi et al, 2019). Recently, Zepp et al (2017) described a subpopulation of mesenchymal cells in the alveolar niche co‐expressing Axin2 and Pdgfra capable of promoting AEC II self‐renewal and differentiation in vitro. In this regard, scRNA‐Seq demonstrated higher expression of Axin2 and Pdgfra in the MYO cluster, suggesting the presence of Axin2‐Pdgfra + cells in BALO cultures, whereas Lrg5 and Lrg6 expression was not detected at this time point of culture (Lee et al, 2017). Emergence of distinct MYO and LIF phenotypes from PDGFRαhigh and PDGFRαlow rMC within the developing BALO will enable researchers to study epithelial‐mesenchymal crosstalk mechanisms and the role of these subsets within the niche during organogenesis, injury, and repair (El Agha et al, 2017).
Another important component of the lung stem cell niche is TR‐Mac. Macrophage precursors seed the organ as early as embryonic day 10.5 in different waves and significantly contribute to lung development, branching morphogenesis, tissue regeneration, and remodeling (Chen et al, 2012a). We have established a microinjection protocol that enables us to successfully engraft these cells into BALO compartments to study and visualize such processes. It has been recently suggested that different subsets of macrophages might exert different roles during branching, alveolarization, and repair after injury (Lechner et al, 2017). Vice versa, it seems likely that the developing or repairing epithelium emanates signals important for programming of macrophages into distinct phenotypes. However, it has been difficult to address the molecular crosstalk between immune cells and lung epithelia and to visualize such processes. Interactions within BALO between TR‐Mac and AEC were detected by electron microscopy, suggesting that this model could facilitate future mechanistic studies. Additionally, scRNA‐Seq analysis revealed that addition of TR‐Mac induces changes in the transcriptional activity and differentiation of the developing epithelial cells. The AP‐1 transcription factor complex has been involved in several cellular functions including proliferation (Eferl & Wagner, 2003). Our data show that genes coding for components of the AP‐1 transcription factor complex, Fos and Fosb, as well as one of its target genes, Areg, were downregulated in the presence of TR‐Mac. Also, Erg1 and Atf3 genes, which regulate the expression of pro‐inflammatory genes such as Ccl4, Il6, and Il8, were downregulated (Park et al, 2013). Conversely, genes associated with epithelial cell maturation, Neat1, Cyp2f2, and Ces1d (Zemke et al, 2009; Standaert et al, 2014), were upregulated in BALO microinjected with TR‐Mac, and BALO with TR‐Mac contained a higher percentage of terminally differentiated epithelial cells. These data suggest that macrophages interact with the epithelium to drive cell differentiation, alongside with downregulating genes indicating inflammation‐related stress responses, a feature that has been previously ascribed to TR‐Mac, where these cells revealed an anti‐inflammatory phenotype during lung development (Furukawa et al, 2017). Notably, the presence of TR‐Mac amplified the pro‐inflammatory responses upon infection, suggesting that microinjected TR‐Mac may sense the signals from the injured epithelium. These data indicate that our system can be used to study macrophage–epithelium crosstalk in the context of viral infection.
Despite the advantages of the BALO model, we, however, need to mention the lack of an endothelial compartment in our model. Endothelial cells co‐develop with the lung epithelium, and it would be of great benefit to study endothelial/epithelial crosstalk in vitro or to model lung diseases confined to this compartment. Although CD31+ endothelial cells from adult lungs have been found to drive organoid development from BASC, a proper spatially organized endo‐epithelial network did not develop in such studies (Lee et al, 2014). The question remains whether better‐defined endothelial progenitor cells will be helpful to increase the cellular complexity of the BALO model.
To test whether BALOs are suitable for genetic loss‐of‐function approaches, we suppressed miR‐142-3p, a miRNA known to activate the canonical WNT β‐catenin pathway, by addition of morpholino oligos. This approach recapitulated developmental defects in BALOs including reduced growth and impaired branching morphogenesis previously observed in embryonic lung explants treated with mo142‐3p (Carraro et al, 2014). We reason that the knockdown of target genes at different stages of BALO development is a powerful tool to dissect the function of individual genes without damaging the organoid and without the need for organoid dissociation.
Furthermore, BALO can be used to model diseases that affect the bronchoalveolar compartment of the lung, such as viral infection. We demonstrate that the BALO epithelium infected with different strains of IAV supports viral replication and spread, and mounts an antiviral immune response. We were able to observe IAV infection of epithelial cells in real time using live‐cell imaging over a period of 25 h with a recombinant SC35M‐Cre reporter virus in combination with BALOs derived from a Cre reporter mouse strain. Microinjection of IAV into BALO airway‐like structures recapitulated proximal‐to‐distal spread of the infection and revealed substantial loss of AEC in the infected alveolar‐like areas, modeling this particular aspect of the in vivo situation of IAV pneumonia (Herold et al, 2015).
Altogether, the BALO model can be applied to (i) visualize lung developmental processes and, prospectively, tissue regeneration after injury induced by infection or other insults at high resolution and over time by microscopic analysis, and (ii) pinpoint the role of cells of mesenchymal, epithelial, and myeloid origin in these processes. A particular advantage of the BALO model is the presence of differentiated mesenchymal cell subsets that interact with the epithelium in a spatially defined manner with distinct roles in epithelial development, recapitulating features of myo‐ and lipofibroblasts that have been identified in vivo. The possibility to supplement the airway/alveolar space of BALO with different tissue‐resident or bone marrow‐derived leukocyte populations of interest adds a further level of complexity that has not been achieved in previously described models, and will allow visualizing the interactions with epithelial cells of different phenotypes during development, host defense, and repair. Furthermore, the employment of BASCs, rMC, and/or leukocytes carrying gain‐ or loss‐of‐function mutations, or from reporter mice, will enable characterization of cell‐specific functions of individual genes, a feature that is sometimes difficult to achieve in vivo due to the lack of appropriate cell subset‐specific transgenic mouse lines. In summary, the BALO model represents a valuable addition to the emerging repertoire of in vitro (and ex vivo) tools to study pulmonary development and disease.
Materials and Methods
Reagents and Tools table
| Reagent/Resource | Reference or Source | Identifier or Catalog Number |
|---|---|---|
| Experimental models | ||
| WT (Mus musculus) | Charles River Laboratory | C57BL/6JCrl |
| mTmG (M. musculus) | Jackson Laboratory | B6.129 (Cg)‐Gt (ROSA)26Sor tm4 (ACTB‐tdTomato‐EGFP)Luo /J |
| PDGFRαEGFP (M. musculus) | Jackson Laboratory | B6.129S4‐Pdgfra tm11(EGFP)Sor /J |
| PDPNEGFP (M. musculus) | Jackson Laboratory | B6;D2‐Tg (Pdpn,‐EGFP)16Dobb /J |
| UBI‐GFP (M. musculus) | Jackson Laboratory | (C57BL/6‐Tg(UBC‐GFP)30Scha/J |
| Scgb1a1 mCherry Sftpc YFP double transgenic mice (M. musculus) | Salwig et al (2019) | SPC‐2A-YFP‐2A-tTA‐N, CCSP‐2A-mCherry‐2A-tTA‐C |
| BASC viewer (M. musculus) | Salwig et al (2019) | SPC‐2A-YFP‐2A-tTA‐N, CCSP‐2A-mCherry‐2A-tTA‐C, tetObi lacZ/huGFP |
| BASC v‐race (M. musculus) | Salwig et al (2019) | SPC‐2A-YFP‐2A-tTA‐N, CCSP‐2A-mCherry‐2A-tTA‐C, tetObiluc/Cre, Rosa26stopflox‐lacZ |
| Antibodies | ||
| Anti‐goat Alexa Fluor® 488 | Thermo Scientific | Cat #A‐21467 |
| Anti‐goat Alexa Fluor® 647 | Thermo Scientific | Cat #A‐21469 |
| Anti‐mouse Alexa Fluor® 488 | Thermo Scientific | Cat #A‐21206 |
| Anti‐rabbit Alexa Fluor® 555 | Thermo Scientific | Cat #A‐31572 |
| Biotinylated rat anti‐mouse CD16/32 | BD Bioscience | Clone 2.4G2 |
| Biotinylated rat anti‐mouse CD31 | BD Bioscience | Clone MEC 13.3 |
| Biotinylated rat anti‐mouse CD45 | BD Bioscience | Clone 30‐F11 |
| Goat influenza A virus HA | Abcam | Cat# ab20841 |
| Goat SCGB1A1 | Santa Cruz Biotechnology | Clone T‐18 |
| Mouse Connexin 43 | Thermo Scientific | Clone CX‐1B1 |
| Mouse IgG1 Ctl | Abcam | Clone CT6 |
|
Mouse influenza A virus NP FITC |
Abcam | Cat #ab128193 |
| Mouse β‐IV tubulin | Abcam | Clone ONS.1A6 |
| Rabbit Mature SPB | Seven Hills | Cat #WRAB‐48604 |
| Rabbit pro‐SPB | Seven Hills | Cat #WRAB‐55522 |
| Rabbit purified pro‐SPC | Millipore | Cat #AB3786 |
| Rabbit SPA | Abcam | Cat #ab115791 |
| Rabbit β‐actin | Abcam | Cat #ab8227 |
| Rat CD11c FITC | BioLegend | Clone N4189 |
| Rat CD206 Alexa Fluor® 488 | BioLegend | Clone MMR |
| Rat CD24 PE‐Cy7 | BioLegend | Clone M1/69 |
| Rat CD31 Alexa Fluor® 488, PE or Pacific Blue | BioLegend | Clone 390 |
| Rat CD326 (EpCAM) APC‐Cy7 or APC‐eF780 | BioLegend | Clone G8.8 |
| Rat CD45 FITC, APC‐C7 or Horizon™ V450 | BD Biosciences | Clone 30‐F11 |
| Rat CD45 V500 | BioLegend | Clone 30‐F11 |
| Rat GR1 PE‐Cy7 | BioLegend | Clone RB6‐8C5 |
| Rat IgG2a Ctl | BioLegend | Clone RTK2758 |
| Rat Ly6A/E (SCA‐1) APC or Pacific Blue | BioLegend | Clone D7 |
| Rat Ly6G APC | BioLegend | Clone 1A8 |
| Rat Siglec‐F APC‐Cy7 | BD Biosciences | Clone E50‐2440 |
| Siglec‐F eFluor 660 and | Thermo Scientific | Clone 1RNM44N |
| Swine anti‐rabbit IgG | Agilent | Cat #F0054 |
| Syrian hamster IgG Ctl | BioLegend | Clone SHG‐1 |
| Syrian hamster PDPN APC | BioLegend | Clone 8.1.1 |
| Oligonucleotides and other sequence‐based reagents | ||
| 5S rRNA | This study | FP 5′‐TCTCGGAAGCTAAGCAGGGTC‐3′; RP 5′‐AGCCTACAGCACCCGGTATTC‐3′ |
| Apc | This study | FP 5′‐CATGGACCAGGACAAAAACC‐3′ RP5′‐GAACACACACAGCAGGACAGA‐3′ |
| Foxj1 | This study |
FP 5′‐GAGCCAGGCCTCACATTCG‐3′ RP 5′‐CGCTGGTAACCCAGACTCC‐3′ |
| Hopx | This study | FP 5′‐CAACAAGGTCAACAAGCACCC‐3′ RP 5′‐GCGCTGCTTAAACCATTTCTGC 3′ |
| Ifnb | This study | FP 5′‐GTTACACTGCCTTTGCCATCC‐3′ RP 5′‐GTGGAGTTCATCCAGGAGACG‐3′ |
| miR‐142-3p | This study | FP 5′‐ACTCCAGCTGGGTGTAGTGTTTC CTACTT‐3′; stem loop reverse 5′‐CTCAAC TGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCCATAAA‐3′ |
| miRNA | This study | Universal reverse 5′ TCAACTGGTG TCGTGGAGTCG‐3′ |
| Muc5ac | This study | FP 5′‐AGGACCACTGTATTGCTGGC‐3′ RP 5′‐TCCAGAACATGTGTTGGTGC‐3′ |
| NP | This study | FP 5′‐ACGAAGGTGGTCCCAAGAGG‐3′ RP 5′‐GATTTGGCCCGCAGATGCC‐3′ |
| Rps‐18 | This study |
FP 5′‐CCGCCATGTCTCTAGTGATCC‐3′ RP 5′‐TTGGTGAGGT CGATGTCTGC‐3′ |
| Sftpc | This study | FP 5′‐GGAGGAAGGGCATGATACTGG‐3′ RP 5′‐TTCTACCGACCCTGTGGATGC‐3′ |
| miR‐142-3p‐specific morpholino | Gene Tools, LLC | 5′TCCATAAAGTAG GAAACACTACACC‐3′ |
| Scramble morpholino | Gene Tools, LLC | 5′‐CCTCTTACCTCAGT TACAATTTATA‐3′ |
| Chemicals, enzymes, and other reagents | ||
| Biotin‐binder magnetic beads | Life Technologies | Cat #11947 |
| Insulin‐Transferrin‐Selenium | Biozym | Cat #41400‐045 |
| l‐Glutamine | Gibco | Cat #11539876 |
| Matrigel matrix | Corning | Cat #356231 |
| Paraformaldehyde | Merck | Cat #104005 |
| αMEM | Gibco | Cat #41061029 |
| DMEM | Gibco | Cat #12491023 |
| Dispase | Corning | Cat #354235 |
| DNase I | Serva | Cat #18535.01 |
| Heparin | Stem cell Technologies | Cat #07980 |
| Software | ||
| DIVA software (v8.02) | https://www.bdbiosciences.com | |
| FlowJo (v6.10.1) | https://www.flowjo.com | |
| GraphPad PRISM 5 (v5.01) | https://www.graphpad.com | |
| ImageJ (v1.53c) | https://imagej.nih.gov/ij | |
| R (v3.3.3) | https://cran.r-project.org/web/packages/Seurat/index.html | |
Methods and Protocols
Mice
Mice were between 8 and 10 weeks of age and were housed under specific pathogen‐free conditions with free access to food and water. All animal experiments were approved by the responsible animal ethics committee and by the local authorities.
Primary cell isolation
Primary murine lung cell isolation was modified from Herold et al (2006).
Mice were anesthetized with isoflurane inhalation and killed by cervical dislocation.
Lungs were perfused with 20 ml of sterile HBSS via the right ventricle until they were visually free of blood.
To obtain lung homogenate, 1.5 ml dispase was instilled through the trachea followed by lung harvest and 40‐min incubation at room temperature (RT).
Trachea was removed, and the lungs were homogenized in DMEM/2.5% HEPES with 0.01% DNase I using the gentleMACS® Dissociator (MACS Miltenyi Biotec).
Cell suspension was filtered through 70‐ and 40‐μm nylon filters and centrifuged for 10 min, 500 g at 4°C.
To remove red blood cells (RBCs), cells were incubated in 1 ml RBC lysis buffer (1.5 M NH4Cl, 100 mM NaHCO3, 10 mM EDTA, pH 7.4) for 90 s.
Lysis was stopped with addition of DMEM‐containing 10% FCS followed by and cell centrifugation for 10 min, 500 g at 4°C.
For leukocyte/endothelial cell depletion, cells were counted and the Dynabeads Magnetic Separation Technology protocol was followed (Thermo Fischer) to obtain the exact volume of antibody and beads needed.
Cells were incubated with an antibody mix containing biotinylated rat anti‐mouse CD45, CD16/32, and CD31 antibodies for 30 min at 37°C.
Cells were centrifuged for 10 min, 500 g at 4°C, and resuspended in DMEM‐containing streptavidin‐linked MagneSphere Paramagnetic Particles for 30 min with rotation at RT.
For the magnetic separation, cells were placed on the magnetic stand for 15 min at RT.
CD45/CD16/32/CD31-negative cells in the flow‐through were collected and washed once with DMEM to be used for further analysis.
Flow cytometry and cell sorting
Multicolor flow cytometry and cell sorting were performed on a BD LSRFortessa™ and a BD FACSAria™ III cell sorter using DIVA software (BD Bioscience).
Following primary cell isolation, cells were pelleted by centrifugation for 10 min, 500 g at 4°C, and resuspended in MACS buffer (PBS, 7.4% EDTA, 0.5% FCS, pH 7.2).
Cells were stained with the antibody cocktail‐containing gammaglobulins (sandoglobulin) (1:10), CD31 Alexa 488 (1:50), CD45 FITC (1:50), CD326 APC/Cy7 (1:50), CD24 PE/Cy7 (1:200), and Sca‐1 PB (1:50), and for 15 min at 4°C in MACS buffer.
Cells were washed once with MACS buffer and centrifuged for 5 min, 500 g at 4°C; resuspended in 300–500 μl of MACS buffer; and filtered with 40 μm mesh into FACS tubes for cell analysis.
Cell sorting was performed using an 85‐μm nozzle after doublet and dead cell exclusion.
BALO culture
Flow‐sorted EpCAMhighSca‐1+CD24low cells and EpCAM−Sca‐1+ rMC were co‐cultured in growth factor‐reduced Matrigel® (Corning) as follows:
Flow‐sorted cells were centrifuged for 5 min, 500 g at 4°C, and resuspended in medium containing α‐MEM, 10% FCS, 100 U/ml penicillin, 0.1 mg/ml streptomycin, 2 mM l‐glutamine, 1 × insulin/transferrin/selenium, and 0.0002% heparin.
For co‐cultivation, cell concentration was adjusted to 5 × 103 EpCAMhighCD24lowSca‐1+ cells and 3 × 104 rMC per 25 μl of medium per cell culture insert.
Mix of EpCAMhighCD24lowSca‐1+ cells and rMC (50 μl per insert) was diluted with cold growth factor‐reduced Matrigel® (Corning) at a 1:1 ratio as described before (Quantius et al, 2016).
For each insert, 90 μl of mixed EpCAMhighCD24lowSca‐1+ cells and rMC in Matrigel was added on top of a 12‐mm cell culture insert, placed in a 24‐well plate, and incubated for 5–10 min at 37°C for polymerization of Matrigel.
To obtain an air–liquid interface, 350 μl of medium was added per well and cultures were incubated at 37°C with 5% CO2 until further analysis.
Media were changed three times per week.
Organoid digestion and passaging
For passaging, FACS analyses, and single‐cell RNA‐Seq, BALOs were removed from cell culture inserts by addition of ice‐cold PBS and rigorous pipetting, collected in 1.5‐ml tubes, and centrifuged. The pellet was resuspended in 200 μl dispase and incubated for 30 min at 37°C with rotation. 1 ml DMEM was added, and tubes were centrifuged. Cells were washed twice with MACS buffer, stained with antibodies, and analyzed by flow cytometry, or resuspended in media and co‐cultured in Matrigel with freshly sorted rMC for passaging, alternatively 2,300 cells/μl in 1 × PBS/0.1% BSA for single‐cell RNA‐Seq.
Quantitative real‐time PCR
RNA was isolated from flow‐sorted cells and BALO cultures using RNeasy Micro Kit or miRNeasy Mini Kit (Qiagen). cDNA was synthesized using M‐MLV reverse transcriptase (Invitrogen) and random hexamer primer according to the manufacturer's instructions. For reverse transcription of miR‐142-3p, a stem loop reverse primer was used according to the protocol published by Tang et al (2006). Quantitative PCR was performed in a StepOnePlus™ Real‐Time PCR System (Thermo Scientific) using SYBR Green. The relative gene expression levels were normalized to ribosomal protein subunit S18 (RPS‐18) or 5S rRNA as indicated and presented as fold change in gene expression relative to day 0, scramble, or mock.
Single‐cell RNA sequencing
Single‐cell RNA‐Seq was performed as recommended by the manufacturer (Illumina protocol version: 1000000021452 v01). Briefly, cells were mixed with lysis buffer, barcoding bead suspension, and reverse transcription reagent. An emulsion‐containing droplets with single cells and a barcode bead were generated with the ddSEQ Single‐Cell Isolator (Bio‐Rad). Within the droplets, cells were lysed, allowing for binding of mRNA to the beads prior to reverse transcription. cDNA was purified and complemented by second‐strand cDNA synthesis. The resulting cDNA was fragmented with tagment enzyme and amplified. Finally, libraries were cleaned up, pooled, and subsequently sequenced on a NextSeq 500 System (Illumina). Upon sequencing, barcodes belonging to cells were identified using the Illumina BaseSpace platform. Reads were demultiplexed with a custom python script (available on request). Reads were mapped in the mouse genome using STAR (version 2.5.3a). Mapped reads were counted with the software featureCounts. Following, UMI‐tools was used to count the unique molecules per gene. Downstream analysis was performed in R (version 3.3.3) with the Seurat package (version 2.2, https://cran.r-project.org/web/packages/Seurat/index.html). Genes expressed in less than three cells were excluded, and cells with less than 200 detected genes were removed. UMI and number of genes ranged between 1,630 and 3,320 per cell. After filtering, the count values for each cell were normalized by using the NormalizeData method from Seurat and variable genes were detected with the FindVariableGenes method. To remove unwanted variation sources within the genes, data were regressed using the ScaleData method from Seurat combined with the number of UMIs per cell. Principal component analysis (PCA) was performed on the data, and results were visualized by using non‐linear dimensional reduction (tSNE). Finally, up‐ and downregulated genes were identified for each cluster and visualized as heat maps and violin plots. For the comparative analysis between BALO cells grown with and without macrophages (two conditions), a canonical correlation analysis (CCA) was performed using Seurat's RunCCA method. The top 1,000 genes with the highest variability for each condition were selected. Like the Seurat analysis of the data sets before, the cells were then clustered with the FindClusters function using a resolution of 0.4. UMI and number of genes ranged between 2,713 and 3,287 per cell. To identify possible detected cell types, marker genes (upregulated genes) conserved across the two conditions for each cluster were analyzed with the FindConservedMarkers function. The results were visualized as heat maps, violin plots, and dot plots using the DoHeatmap, VlnPlot, and SplitDotPlotGG methods.
Morpholino antisense oligo transfection
Scramble morpholino (Scra) and miR‐142-3p‐specific morpholino (mo142‐3p) were prepared in αMEM with 0.2% FCS at a concentration of 4 μM. At day 6 of culture, mo1423p or Scra was applied to BALOs overnight for three consecutive periods of 12 h, followed by application for 48 h for days 9–11. At day 11, treated organoids were removed from Matrigel and flow‐sorted based on EpCAM expression. Toxicity testing was performed using propidium iodide staining (1:100 dilution) followed by FACS analysis. For organoid formation experiments, BALOs were treated every 24 h with mo142‐3p or Scra until day 21 of culture.
Electron microscopy
Day 21 BALOs were fixed for 4 h in 1.5% glutaraldehyde (Merck, Darmstadt, Germany) and 2.5% PFA in 0.1 M phosphate buffer. Cultures were then washed in Tris–HCl and osmicated for 2 h at RT in 2% OsO4 (Sigma‐Aldrich, St. Louis, USA) diluted in distilled water. Samples were rinsed five times for 3 min in distilled water and stained overnight en bloc in half‐saturated uranyl acetate (Merck) in distilled water. Lastly, samples were rinsed again five times for 3 min in distilled water and were then routinely embedded in epon resin (Sigma‐Aldrich) through a graded series of ethanol and propylene oxide. Ultrathin sections (60–90 nm) were cut with a Reichert Ultracut E ultramicrotome (Leica, Bensheim, Germany) and viewed with a transmission electron microscope (EM 902; Zeiss, Wetzlar, Germany) equipped with a slow scan 2 K CCD camera (TRS; Tröndle, Moorenweis, Germany). Images for illustration were corrected for overall brightness, but not manipulated otherwise.
LysoTracker, phospholipid, neutral lipid, and viability staining
For staining of lamellar bodies, LysoTracker® Red DND‐99 (Thermo Scientific) stock solution was diluted in pre‐warmed α‐MEM according to the manufacturer's instructions. 50 nM LysoTracker was added to co‐cultures and incubated for 30 min at 37°C. Similarly, for staining of phospholipids, LipidTOX™ red phospholipidosis detection reagent (Thermo Scientific) was diluted 1:500 in α‐MEM and added to BALOs for 48 h. Microscopic pictures of co‐cultures were taken using the EVOS™ FL Auto Imaging System (Thermo Scientific). For staining of neutral lipids, co‐cultures were fixed with 4% PFA for 15 min, washed twice with PBS, and stained with 1× of PBS diluted LipidTOX™ neutral lipid staining solution (Thermo Scientific). After 6‐h incubation at RT, cultures were co‐stained with DAPI and mounted on glass slides for imaging. For the TR‐Mac viability staining, the cell viability imaging kit (Thermo Scientific) was used according to the manufacturer's instructions. A Leica SP8 microscope was employed for confocal imaging.
Immunofluorescence
BALO cultures were fixed with 4% PFA for 10 min at RT. Cultures were washed with buffer (0.2% BSA/PBS), permeabilized with 0.1% Triton X‐100/PBS for 5 min, and blocked in 5% horse serum, 2% BSA/PBS for 30 min at RT. For staining, cultures were incubated with primary antibodies over night at 4°C, followed by washing and addition of secondary antibodies for 2 h at RT. For the staining of PDGFRα‐GFP cultures, cultures were left unpermeabilized.
Western blot
Lysis buffer containing 50 mM Tris–HCl/pH 8.0, 5 mM EDTA, 150 mM NaCl, 1% (v/v) Triton X‐100, 0.5% (w/v) Na‐deoxycholate, and 1 mM PMSF was used for preparation of the protein extract. The probes were loaded to SDS–PAGE, followed by transfer onto PVDF membranes (Roth) in a wet blotting chamber according to the manufacturer's protocol (Bio‐Rad). Obtained immunoblots were blocked by incubating at RT for 1 h in blocking buffer containing either 5% (w/v) non‐fat milk for SPA or 3% (w/v) BSA for other antibodies. Next, immunostaining for the proteins of interest was performed overnight at 4°C. The primary antibodies used for Western blotting were mature SPB (1:1,000), pro‐SPB (1:1,000), mature SPC (1:1,000), pro‐SPC (Millipore, AB3786 1:1,000), and SPA (1:1,000). The blots were then incubated with horseradish peroxidase‐conjugated secondary swine anti‐rabbit IgG antibodies (1:10,000) for 1 h at RT. The Immobilon Western Chemiluminescent HRP substrate (Millipore) was used for the blots development, and emitted signal was detected with a chemiluminescence imager (Intas ChemoStar, Intas). Thereafter, blots were stripped for 20 min using Stripping Buffer (0.1 M glycine, pH 2.5), followed by re‐probing the blots with antibodies against the loading control β‐actin (1:5,000).
β‐galactosidase staining
BALOs were fixed with 4% PFA for 5 min at RT, washed once with PBS, and incubated with pre‐warmed LacZ buffer (5 mM [Fe(CN)6]3−, 5 mM [Fe(CN)6]4−, 2 mM MgCl2 in PBS) for 10 min at 37°C. LacZ buffer was replaced by staining solution (1 mg/ml X‐Gal in LacZ buffer) and incubated at 37°C. Stained cultures were rinsed once with PBS and kept in PBS at 4°C for further analysis.
Infection of BALO
BALOs were washed once in PBS and infected with 5 × 106 pfu (plaque‐forming units) of SC35M IAV (H7N7) or A/PR/8/34 (PR8) (H1N1, mouse‐adapted) in 600 μl of ice‐cold PBS for 2 h at RT and gentle agitation (200 rpm), followed by 30‐min incubation at 37°C with 5% CO2. The inoculum was removed, and inserts were washed twice with PBS. Inserts were placed in infection medium (α‐MEM, 0.2% BSA, 100 U/ml penicillin, 0.1 mg/ml streptomycin, 1 × insulin/transferrin/selenium, 2 mM l‐glutamine, 0.0002% heparin) and incubated for the indicated times. For direct inoculation approach, culture medium was removed and replaced by medium without additives, followed by microinjection of BALO airway‐like structures with SC35MNS1_2A_GFP‐NEP (SC35M‐GFP) IAV and SC35MNS1_2A_Cre_2A_NEP (SC35M‐Cre) IAV, diluted in PBS (Reuther et al, 2015). After an hour incubation, media were replaced by infection media and incubated for the indicated times. Images of infected BALOs were taken using EVOS™ FL Auto Imaging System (Thermo Scientific). The data shown are representative of three independent experiments.
Plaque assay
Infection of MDCK cells was performed in duplicate in 12‐well plates, using BALO supernatants at 48 h pi serially diluted in PBS for 1 h at 37°C. Pre‐warmed Avicel® overlay medium (MEM, 0.1% BSA, 100 U/ml penicillin, 0.1 mg/ml streptomycin, 0.1% HEPES, 2 μg/ml trypsin (TPCK‐treated), 1.25% Avicel) was added. Plates were incubated at 37°C, 5% CO2 without movement. After 48 h, the Avicel overlay was removed, and cells were fixed with 4% PFA in PBS for 15 min at RT. Cells were washed twice with PBS and permeabilized with 0.3% Triton X‐100 in PBS for 15 min at RT. Staining for viral protein was done using a polyclonal antibody against IAV NP (Abcam) in staining buffer (PBS, 10% horse serum, 0.1% Tween‐80) for 1 h at RT. Cells were washed three times with washing buffer (PBS, 0.05% Tween‐80) and incubated with horseradish peroxidase‐conjugated secondary antibody (Santa Cruz) followed by further washing steps. NP staining was visualized using TrueBlue™ peroxidase substrate (KPL), and stained plaques were counted.
Cytokine measurement
Release of the pro‐inflammatory cytokines IL‐6, TNF‐α, and IL‐1β was measured from the supernatant of mock‐ and IAV‐infected BALO cultures supplemented with and without TR‐Mac employing the Bio‐Plex® Multiplex Immunoassay System with Bio‐Plex Manager™ software (Bio‐Rad) according to the manufacturer's instructions.
Confocal microscopy, live‐cell imaging, and microinjection
For live‐cell imaging of BALO formation from day 15–20 of culture, a Leica DMI6000 B microscope was used. A Leica SP8 microscope was employed for confocal imaging. On day 14 of culture, microinjection of TR‐Mac isolated from the BALF of mTmG mice into the lung organoids was performed. For BALO infection imaging with SC35M‐Cre, the EVOS™ FL Auto Imaging System (Thermo Scientific) was used during the first 48 h. For SC35M‐GFP virus inoculation, viral infection was achieved by direct injection into the airway‐like regions within BALO. All microinjections were performed using a 10‐μm‐diameter capillary needle (Eppendorf). Cultures were kept in the incubator with 5% CO2 at 37°C until further analysis.
Culture of embryonic lung explants
Timed‐pregnant WT mice were sacrificed on post‐coitum day 11.5 (E11.5). Embryos were harvested, and lung explants were placed on 8 μm Nuclepore Track‐Etch membranes and cultured in DMEM:F12 medium (Gibco) with 0.5% FBS. Mo142‐3p and Scra (Gene Tools) were added at 100 μM to the lung explants for 48 h.
Statistics
All data are given as mean ± SEM. Statistical significance between two groups was estimated using the unpaired two‐sided Student's t‐test. For comparison of three groups, one‐way ANOVA and post hoc Tukey's test were performed. Calculations were done with GraphPad Prism 5. A P‐value lower than 0.05 was considered to be significant.
Author contributions
AIV‐A, MH, EEA, REM, IV, MS, SB, TB, WS, and SH were involved in study design and concept. AIV‐A, Mo.He, MCH, AS, GC, JPM, and WK acquired the data. AIV‐A, AH, JPM, TH, and AGo were involved in the ScRNA‐Seq experimental design and analysis. AIV‐A, MH, EEA, ISa, ISh, AGü, and SH conducted other data analysis, interpretation, and statistics.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Movie EV1
Movie EV2
Review Process File
Acknowledgements
This study was supported by the German Research Foundation (DFG; SFB 1021 C05 and Z02, SFB‐TR84 B9 and A6, KFO 309 P2/P5/P6/P7/P8/Z01; SFB 1160 to MS; Excellence Cluster Cardio Pulmonary System), the University Hospital Giessen and Marburg (FOKOOPV to SH and SB), and the German Center for Lung Research (DZL). SB was supported by DFG (BE4443/4‐1; BE4443/6‐1; and SFB1213). EE was also funded by SFB CRC1213‐project A04 and acknowledges the support of the Institute for Lung Health (ILH), DZL and the Cardio‐Pulmonary Institute (CPI, EXC 2026, Project ID: 390649896). We also acknowledge technical assistance by the Bioinformatics Core Facility/Professorship of Systems Biology at JLU Giessen and access to resources financially supported by the BMBF grant FKZ 031A533 to the BiGi center within the de.NBI network. We thank Dr. Katrin Ahlbrecht for kindly providing the PdpnGFP mice. This publication uses data collected within the frame work of the first author's PhD dissertation “Establishment of murine 3D bronchioalveolar lung organoids from adult somatic stem cells for organ development and disease modeling" published in 2018 at the Justus‐Liebig‐Universität Gießen, Germany. Open access funding enabled and organized by Projekt DEAL.
The EMBO Journal (2020) 39: e103476
See also: V Sontake & PR Tata (November 2020)
Data availability
The data sets and computer code produced in this study are available in the following database: Single‐cell RNA‐Seq data: SRA accession number PRJNA473688 (http://www.ebi.ac.uk/ena/data/view/PRJNA473688).
Additional data are available from authors upon reasonable request.
References
- Al Alam D, El Agha E, Sakurai R, Kheirollahi V, Moiseenko A, Danopoulos S, Shrestha A, Schmoldt C, Quantius J, Herold S et al (2015) Evidence for the involvement of fibroblast growth factor 10 in lipofibroblast formation during embryonic lung development. Development 142: 4139–4150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BLM (2013) Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 123: 3025–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraro G, Shrestha A, Rostkovius J, Contreras A, Chao C‐M, El Agha E, Mackenzie B, Dilai S, Guidolin D, Taketo MM et al (2014) miR‐142‐3p balances proliferation and differentiation of mesenchymal cells during lung development. Development 141: 1272–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Matsumoto K, Brockway BL, Rackley CR, Liang J, Lee J‐H, Jiang D, Noble PW, Randell SH, Kim CF et al (2012a) Airway epithelial progenitors are region specific and show differential responses to bleomycin‐induced lung injury. Stem Cells 30: 1948–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Acciani T, Le Cras T, Lutzko C, Perl A‐KT (2012b) Dynamic regulation of platelet‐derived growth factor receptor α expression in alveolar fibroblasts during realveolarization. Am J Respir Cell Mol Biol 47: 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y‐W, Huang SX, de Carvalho ALRT, Ho S‐H, Islam MN, Volpi S, Notarangelo LD, Ciancanelli M, Casanova J‐L, Bhattacharya J et al (2017) A three‐dimensional model of human lung development and disease from pluripotent stem cells. Nat Cell Biol 19: 542–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Guo M, Whitsett JA, Xu Y (2015) ‘LungGENS’: a web‐based tool for mapping single‐cell gene expression in the developing lung. Thorax 70: 1092–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Kitzmiller JA, Sridharan A, Perl AK, Bridges JP, Misra RS, Pryhuber GS, Mariani TJ, Bhattacharya S, Guo M et al (2017) Lung Gene Expression Analysis (LGEA): an integrative web portal for comprehensive gene expression data analysis in lung development. Thorax 72: 481–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye BR, Hill DR, Ferguson MAH, Tsai Y‐H, Nagy MS, Dyal R, Wells JM, Mayhew CN, Nattiv R, Klein OD et al (2015) In vitro generation of human pluripotent stem cell derived lung organoids. Elife 4: e05098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye BR, Dedhia PH, Miller AJ, Nagy MS, White ES, Shea LD, Spence JR (2016) A bioengineered niche promotes in vivo engraftment and maturation of pluripotent stem cell derived human lung organoids. Elife 5: 3025–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eferl R, Wagner EF (2003) AP‐1: a double‐edged sword in tumorigenesis. Nat Rev Cancer 3: 859–868 [DOI] [PubMed] [Google Scholar]
- El Agha E, Bellusci S (2014) Walking along the fibroblast growth factor 10 route: a key pathway to understand the control and regulation of epithelial and mesenchymal cell‐lineage formation during lung development and repair after injury. Scientifica (Cairo) 2014: 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Agha E, Moiseenko A, Kheirollahi V, De Langhe S, Crnkovic S, Kwapiszewska G, Kosanovic D, Schwind F, Schermuly RT, Henneke I et al (2017) Two‐way conversion between lipogenic and myogenic fibroblastic phenotypes marks the progression and resolution of lung fibrosis. Cell Stem Cell 20: 261–273.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa S, Moriyama M, Miyake K, Nakashima H, Tanaka A, Maehara T, Iizuka‐Koga M, Tsuboi H, Hayashida J‐N, Ishiguro N et al (2017) Interleukin‐33 produced by M2 macrophages and other immune cells contributes to Th2 immune reaction of IgG4‐related disease. Sci Rep 7: 42413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangreco A, Reynolds SD, Stripp BR (2002) Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol 161: 173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold S, von Wulffen W, Steinmueller M, Pleschka S, Kuziel WA, Mack M, Srivastava M, Seeger W, Maus UA, Lohmeyer J (2006) Alveolar epithelial cells direct monocyte transepithelial migration upon influenza virus infection: impact of chemokines and adhesion molecules. J Immunol 177: 1817–1824 [DOI] [PubMed] [Google Scholar]
- Herold S, Becker C, Ridge KM, Budinger GRS (2015) Influenza virus‐induced lung injury: pathogenesis and implications for treatment. Eur Respir J 45: 1463–1478 [DOI] [PubMed] [Google Scholar]
- Herriges M, Morrisey EE (2014) Lung development: orchestrating the generation and regeneration of a complex organ. Development 141: 502–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M, Koo BK (2015) Modeling mouse and human development using organoid cultures. Dev 142: 3113–3125 [DOI] [PubMed] [Google Scholar]
- Kaisani A, Delgado O, Fasciani G, Kim SB, Wright WE, Minna JD, Shay JW (2014) Branching morphogenesis of immortalized human bronchial epithelial cells in three‐dimensional culture. Differentiation 87: 119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirollahi V, Wasnick RM, Biasin V, Vazquez‐Armendariz AI, Chu X, Moiseenko A, Weiss A, Wilhelm J, Zhang JS, Kwapiszewska G et al (2019) Metformin induces lipogenic differentiation in myofibroblasts to reverse lung fibrosis. Nat Commun 10: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CFB, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T (2005) Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121: 823–835 [DOI] [PubMed] [Google Scholar]
- Kretzschmar K, Clevers H (2016) Organoids: modeling development and the stem cell niche in a dish. Dev Cell 38: 590–600 [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Knoblich JA (2014) Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345: 1247125 [DOI] [PubMed] [Google Scholar]
- Lechner AJ, Driver IH, Lee J, Conroy CM, Nagle A, Locksley RM, Rock JR (2017) Recruited monocytes and type 2 immunity promote lung regeneration following pneumonectomy. Cell Stem Cell 21: 120–134.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, Wagers AJ, Tseng YH, Ryeom S, Kim CF (2014) Lung stem cell differentiation in mice directed by endothelial cells via a BMP4‐NFATc1‐thrombospondin‐1 axis. Cell 156: 440–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J‐H, Tammela T, Hofree M, Choi J, Marjanovic ND, Han S, Canner D, Wu K, Paschini M, Bhang DH et al (2017) Anatomically and functionally distinct lung mesenchymal populations marked by Lgr5 and Lgr6. Cell 170: 1149–1163.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Liu K, Cui G, Huang X, Yao S, Guo W, Qin Z, Li Y, Yang R, Pu W et al (2019) Lung regeneration by multipotent stem cells residing at the bronchioalveolar‐duct junction. Nat Genet 51: 728–738 [DOI] [PubMed] [Google Scholar]
- McGowan SE, Torday JS (1997) The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu Rev Physiol 59: 43–62 [DOI] [PubMed] [Google Scholar]
- McGowan SE, McCoy DM (2014) Regulation of fibroblast lipid storage and myofibroblast phenotypes during alveolar septation in mice. Am J Physiol Lung Cell Mol Physiol 307: L618–L631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni RR, Abed S, Draper JS (2016) Organoids as a model system for studying human lung development and disease. Biochem Biophys Res Commun 473: 675–682 [DOI] [PubMed] [Google Scholar]
- Park S‐H, Do KH, Choi HJ, Kim J, Kim K‐H, Park J, Oh CG, Moon Y (2013) Novel regulatory action of ribosomal inactivation on epithelial Nod2‐linked proinflammatory signals in two convergent ATF3‐associated pathways. J Immunol 191: 5170–5181 [DOI] [PubMed] [Google Scholar]
- Perl A‐KT, Gale E (2009) FGF signaling is required for myofibroblast differentiation during alveolar regeneration. Am J Physiol Lung Cell Mol Physiol 297: L299–L308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozarska A, Rodríguez‐Castillo JA, Surate Solaligue DE, Ntokou A, Rath P, Mižíková I, Madurga A, Mayer K, Vadász I, Herold S et al (2017) Stereological monitoring of mouse lung alveolarization from the early postnatal period to adulthood. Am J Physiol Lung Cell Mol Physiol 312: L882–L895 [DOI] [PubMed] [Google Scholar]
- Quantius J, Schmoldt C, Vazquez‐Armendariz AI, Becker C, El Agha E, Wilhelm J, Morty RE, Vadász I, Mayer K, Gattenloehner S et al (2016) Influenza virus infects epithelial stem/progenitor cells of the distal lung: impact on Fgfr2b‐driven epithelial repair. PLoS Pathog 12: e1005544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner RE, Makena P, Prasad GL, Cormet‐Boyaka E (2019) Optimization of normal human bronchial epithelial (NHBE) cell 3D cultures for in vitro lung model studies. Sci Rep 9: 500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuther P, Gopfert K, Dudek AH, Heiner M, Herold S, Schwemmle M (2015) Generation of a variety of stable Influenza A reporter viruses by genetic engineering of the NS gene segment. Sci Rep 5: 11346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BLM (2009) Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA 106: 12771–12775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs N, Papaspyropoulos A, Zomer‐van Ommen DD, Heo I, Böttinger L, Klay D, Weeber F, Huelsz‐Prince G, Iakobachvili N, Amatngalim GD et al (2019) Long‐term expanding human airway organoids for disease modeling. EMBO J 38: e100300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salwig I, Spitznagel B, Vazquez‐Armendariz AI, Khalooghi K, Guenther S, Herold S, Szibor M, Braun T (2019) Bronchioalveolar stem cells are a main source for regeneration of distal lung epithelia in vivo . EMBO J 38: e102099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schamberger AC, Staab‐Weijnitz CA, Mise‐Racek N, Eickelberg O (2015) Cigarette smoke alters primary human bronchial epithelial cell differentiation at the air‐liquid interface. Sci Rep 5: 8163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert L, Adriaens C, Radaelli E, Van Keymeulen A, Blanpain C, Hirose T, Nakagawa S, Marine J‐C (2014) The long noncoding RNA Neat1 is required for mammary gland development and lactation. RNA 20: 1844–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Hajkova P, Barton SC, Lao K, Surani MA (2006) MicroRNA expression profiling of single whole embryonic stem cells. Nucleic Acids Res 34: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein B, Brownfield DG, Wu AR, Neff NF, Mantalas GL, Espinoza FH, Desai TJ, Krasnow MA, Quake SR (2014) Reconstructing lineage hierarchies of the distal lung epithelium using single‐cell RNA‐seq. Nature 509: 371–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS, Bhattacharya J (2014) Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature 506: 503–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Vannella KM (2016) Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44: 450–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemke AC, Snyder JC, Brockway BL, Drake JA, Reynolds SD, Kaminski N, Stripp BR (2009) Molecular staging of epithelial maturation using secretory cell‐specific genes as markers. Am J Respir Cell Mol Biol 40: 340–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepp JA, Zacharias WJ, Frank DB, Cavanaugh CA, Zhou S, Morley MP, Morrisey EE (2017) Distinct mesenchymal lineages and niches promote epithelial self‐renewal and myofibrogenesis in the lung. Cell 170: 1134–1148.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Movie EV1
Movie EV2
Review Process File
Data Availability Statement
The data sets and computer code produced in this study are available in the following database: Single‐cell RNA‐Seq data: SRA accession number PRJNA473688 (http://www.ebi.ac.uk/ena/data/view/PRJNA473688).
Additional data are available from authors upon reasonable request.


