Abstract
Introduction:
International travellers contribute to the rapid spread of Zika virus (ZIKV) and its sentinel identification globally. We describe ZIKV infections among international travellers seen at GeoSentinel sites with a focus on ZIKV acquired in the Americas and the Caribbean, describe countries of exposure and traveller characteristics, and assess ZIKV diagnostic testing by site.
Methods:
Records with an international travel-related diagnosis of confirmed or probable ZIKV from January 2012 through December 2019 reported to GeoSentinel with a recorded illness onset date were included to show reported cases over time. Records from March 2016 through December 2019 with an exposure region of the Americas or the Caribbean were included in the descriptive analysis. A survey was conducted to assess the availability, accessibility and utilization of ZIKV diagnostic tests at GeoSentinel sites.
Results:
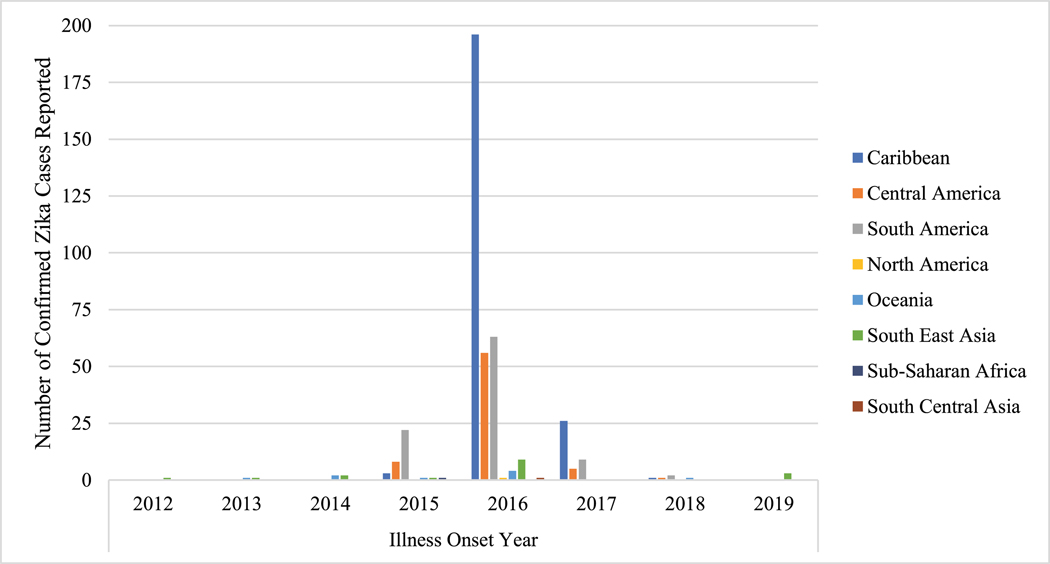
GeoSentinel sites reported 525 ZIKV cases from 2012 through 2019. Between 2012 and 2014, eight cases were reported, and all were acquired in Asia or Oceania. After 2014, most cases were acquired in the Americas or the Caribbean, a large decline in ZIKV cases occurred in 2018–19.
Between March 2016 and December 2019, 423 patients acquired ZIKV in the Americas or the Caribbean, peak reporting to these regions occurred in 2016 [330 cases (78%)]. The median age was 36 years (range: 3–92); 63% were female. The most frequent region of exposure was the Caribbean (60%). Thirteen travellers were pregnant during or after travel; one had a sexually acquired ZIKV infection. There was one case of fetal anomaly and two travellers with Guillain-Barré syndrome. GeoSentinel sites reported various challenges to diagnose ZIKV effectively.
Conclusion:
ZIKV should remain a consideration for travellers returning from areas with risk of ZIKV transmission. Travellers should discuss their travel plans with their healthcare providers to ensure ZIKV prevention measures are taken.
Keywords: Survey, Guillain-Barre syndrome, declining epidemic, sentinel surveillance, Zika diagnostics
Introduction
Zika virus (ZIKV) disease is caused by a flavivirus primarily transmitted by Aedes spp. mosquitoes. The World Health Organization (WHO) declared Zika a public health emergency of international concern in 2016,1 as a result of ZIKV outbreaks and their association with severe complications, such as congenital neurological abnormalities and Guillain-Barré syndrome (GBS). This was the first mosquito-borne viral disease to receive this declaration.
Less than 20 sporadic cases of human ZIKV infection were reported in Africa and Asia before 2007,2 likely due to a lack of disease awareness, poor access to diagnostics and underreporting. In 2007, the first recognized outbreak occurred in Yap, Federated States of Micronesia.3 Subsequently, an outbreak occurred in 2013–2014 in French Polynesia, followed by spread to Brazil. Although ZIKV disease in the continental Americas was first confirmed in May 2015 in Northeast Brazil, viral genome analyses combined with ecological and epidemiological data suggest that ZIKV was present in northeast Brazil by February 2014.4 Mathematical modelling concluded that introduction to Brazil may have occurred as early as July 2013.5
Travellers contributed substantially to the rapid ZIKV spread in and from the Americas,6 and helped to demonstrate ongoing transmission in Asia when few local cases were being reported.7–10 Countries with competent vectors receiving travellers from ZIKV-affected areas were at high-risk for introduction and further spread.6,11 In areas without competent vectors, sexual transmission has been documented.12,13 During the epidemic, travellers served as sentinels for ZIKV transmission in countries where outbreaks were not yet reported,14 were studied to quantify the incidence of birth defects,15 and pregnant travellers also helped define the ratio of symptomatic to asymptomatic maternal infections resulting in adverse fetal outcomes.16,17
This study’s aim was to describe ZIKV infections among international travellers seen at GeoSentinel sites cumulatively since 2012. We further focused on ZIKV acquired in the Americas and the Caribbean since 2016, describing countries of exposure and traveller characteristics. Finally, the availability, accessibility, and utilization of ZIKV diagnostic tests by GeoSentinel sites were assessed.
Methods
Data Source
GeoSentinel, a global sentinel surveillance network, is a collaboration between the US Centers for Disease Control and Prevention (CDC) and the International Society of Travel Medicine. The network, currently consisting of 68 specialized travel and tropical medicine sites in 29 countries, reports on travel-related illness among international travellers and migrants.18 Data collected includes demographic information, reason for most recent travel, travel duration, country and region of exposure, illness onset date, time between the symptom onset and presentation to the GeoSentinel site and hospitalization. GeoSentinel’s data collection protocol has been reviewed by a human subjects advisor at CDC’s National Center for Emerging and Zoonotic Infectious Diseases and is classified as public health surveillance and not human subjects research. Additional ethics clearances were obtained by sites as required by their respective institutions.
Inclusion Criteria
All records with an international travel-related diagnosis of confirmed or probable ZIKV disease from 1 January 2012 to 31 December 2019 with a recorded illness onset date were included to show reported cases over time. A confirmed case was defined as a positive culture or nucleic acid test from serum, urine, cerebrospinal fluid (CSF), tissue, or other body fluid; or ZIKV-specific IgM in serum or CSF and ZIKV antibody titers >4-fold higher than antibody titers for dengue/other flaviviruses; or a 4-fold rise in ZIKV IgG or plaque reduction neutralization test (PRNT) and ZIKV antibody titers >4-fold higher than antibody titers for dengue/other flaviviruses. A probable case was defined as ZIKV-specific IgM in serum or CSF but no PRNT done and dengue/other flaviviruses ruled out; or ZIKV-specific IgM in serum or CSF but ZIKV antibody titers <4-fold higher than antibody titers for dengue/other flaviviruses; or 4-fold rise in ZIKV IgG or PRNT but ZIKV antibody titers <4-fold higher than antibody titers for dengue/other flaviviruses.
Confirmed and probable international travel-related ZIKV disease records from 1 March 2016 to 31 December 2019 with an exposure region of North America, Central America, South America or the Caribbean were included in the descriptive analysis. Descriptive analyses of records with an exposure region in the Americas or Caribbean from 1 January 12013 to 29 February 2016,19 and records with an exposure region beyond the Americas from 1 January 2012 through 31 December 2016 have been published.14
Records were excluded from either analysis if there was a co-diagnosis of an additional flavivirus that may have interfered with Zika classification (i.e. confirmed dengue and probable Zika or probable dengue and probable Zika). Records were classified as ‘vector-acquired’ or ‘non-vector-acquired’ ZIKV disease based on clinical assessment.
Survey
Invitations to complete a multiple-choice survey was distributed by email to the 69 active GeoSentinel sites in June and July 2018. The survey evaluated molecular and serological test availability, including confirmatory testing by PRNT and types of body fluids that could be analyzed. Two reminders at roughly weekly intervals were sent. Each site was permitted one response although more than one response was allowed for Johannesburg due to the presence of satellite sites.
Statistical Analysis
Data were managed using Microsoft Access (Redmond, Washington, USA). Analyses were performed using SAS Version 9.4 (Cary, North Carolina, USA).
Results
Descriptive Analysis
Overall, 525 records with ZIKV were reported to GeoSentinel: 421 confirmed and 104 probable cases (Table 1). Confirmed cases with known illness onset date from 1 January 2012 through 31 December 2019 by region and year are shown in Figure 1. From 2012 to 2014, eight cases were reported; all were from Southeast Asia or Oceania (Table 1). In 2015, of 41 confirmed and probable cases, 38 (92.7%) acquired ZIKV in the Americas, 2 in Asia, and one in Africa. In 2016, 4% (15 of 394) of cases were acquired outside the Americas or Caribbean, and this increased in 2017 (7 of 65; 11%), 2018 (5 of 11; 45%) and 2019 (5 of 6; 83%). During this timeframe, the number of cases from the Americas and Caribbean continued to decrease. There was a large decline in ZIKV cases reported to GeoSentinel in 2018–2019 compared to 2015–2017.
Table 1.
Confirmed and probable cases of ZIKV infection among international travellers with known illness onset date seen at a GeoSentinel site, by year and region, 2012–2019 (n=525)
| Year of illness onset (total cases by region) | |||
|---|---|---|---|
| Confirmed | Probable | Annual total for region | |
| 2012 (n=1) | 1 | 0 | 1 |
| Southeast Asia | 1 | 0 | 1 |
| 2013 (n=2) | 2 | 0 | 2 |
| Oceania | 1 | 0 | 1 |
| Southeast Asia | 1 | 0 | 1 |
| 2014 (n=5) | 4 | 1 | 5 |
| Southeast Asia | 2 | 1 | 3 |
| Oceania | 2 | 0 | 2 |
| 2015 (n=41) | 36 | 5 | 41 |
| South America | 22 | 3 | 25 |
| Central America | 8 | 2 | 10 |
| Caribbean | 3 | 0 | 3 |
| Oceania | 1 | 0 | 1 |
| Southeast Asia | 1 | 0 | 1 |
| Sub-Saharan Africa | 1 | 0 | 1 |
| 2016 (n=394) | 330 | 64 | 364 |
| Caribbean | 196 | 23 | 219 |
| South America | 63 | 21 | 84 |
| Central America | 56 | 19 | 75 |
| Southeast Asia | 9 | 1 | 10 |
| Oceania | 4 | 0 | 4 |
| North America | 1 | 0 | 1 |
| South Central Asia | 1 | 0 | 1 |
| 2017 (n=65) | 40 | 25 | 65 |
| Caribbean | 26 | 9 | 35 |
| South America | 9 | 6 | 15 |
| Central America | 5 | 3 | 8 |
| Sub-Saharan Africa | 0 | 4 | 4 |
| Oceania | 0 | 1 | 1 |
| South Central Asia | 0 | 1 | 1 |
| Southeast Asia | 0 | 1 | 1 |
| 2018 (n=11) | 5 | 6 | 11 |
| South America | 2 | 1 | 3 |
| Central America | 1 | 1 | 2 |
| Caribbean | 1 | 0 | 1 |
| Oceania | 1 | 0 | 1 |
| Sub-Saharan Africa | 0 | 3 | 3 |
| South Central Asia | 0 | 1 | 1 |
| 2019 (n=6) | 3 | 3 | 6 |
| Southeast Asia | 3 | 1 | 4 |
| South America | 0 | 1 | 1 |
| Sub-Saharan Africa | 0 | 1 | 1 |
| Total | 421 | 104 | 525 |
Figure 1.
Confirmed cases of ZIKV infection among international travellers with known illness onset date seen at a GeoSentinel clinic, by year and region, 2012–2019 (n=421).
As data from 2012 to February 2016 have been published,14–18 our descriptive analysis focuses on 423 patients with ZIKV reported to GeoSentinel between 1 March 2016 and 31 December 2019 acquired in the Americas or Caribbean; 345 were confirmed and 78 were probable cases. Peak reporting was in 2016 with 330 cases (78%), followed by 80 in 2017 (19%), 12 in 2018 (3%) and 1 in 2019 (<1%). Sixty-three percent were female (Table 2). The median age was 36 years (range: 3–92) and 85% were aged 20–59 years; 195 (73%) were women of child-bearing age.The most frequent reasons for travel included tourism (56%), visiting friends and relatives (29%) and business (7%). The most frequent regions of ZIKV exposure were the Caribbean (60%), Central America (21%), South America (19%) and North America (<1%). The Dominican Republic (15%), Netherlands Antilles (9%), Martinique (8%), Mexico (8%) and Cuba (7%) were the most frequently reported countries of exposure. Seventy-five (26%) of 288 travellers with information available had a pretravel consultation with a healthcare provider. The median duration of travel in the country of exposure was 17 days [interquartile range (IQR): 12–32; n=378]. Among 335 travellers with ZIKV as their only diagnosis and with information available, the median time from symptom onset to GeoSentinel clinic presentation was 8 days (IQR: 4–21).
Table 2.
Demographics and travel characteristics of travellers with ZIKV infection acquired in the Americas and the Caribbean and evaluated at a GeoSentinel site, 1 March 2016–31 December 2019a (n=423)
| Characteristic | Travellers n (%) |
|---|---|
| Female | 267(63) |
| Agec | |
| <20 | 21(5) |
| 20–39 years | 228(54) |
| 40–59 years | 129(31) |
| ≥60 years | 43(10) |
| Reason for travel | |
| Tourism | 238(56) |
| Visiting friends and relatives | 121(29) |
| Business/corporate/conference | 29(7) |
| Missionary/volunteer/research/aid work | 16(4) |
| Education/student | 9(2) |
| Migrant worker | 7(2) |
| Migration | 2(1) |
| Military | 1(<1) |
| Region and country of exposure | |
| Caribbean | 252(60) |
| Dominican Republic | 59(23) |
| Netherlands Antilles | 36(14) |
| Martinique | 34(13) |
| Cuba | 30(12) |
| Guadeloupe | 28(11) |
| Trinidad and Tobago | 14(6) |
| Jamaica | 10(4) |
| Puerto Rico | 7(3) |
| Barbados | 6(2) |
| Saint Vincent and The Grenadines | 4(2) |
| Haiti | 3(1) |
| Saint Lucia | 3(1) |
| Dominica | 3(1) |
| Grenada | 2(1) |
| Saint Martin | 2(1) |
| Antigua and Barbuda | 1(<1) |
| Saint Barthelemy | 1(<1) |
| Virgin Islands, British | 1(<1) |
| Virgin Islands, USA | 1(<1) |
| Unascertainable | 7(3) |
| Central America | 89(21) |
| Mexicob | 34(38) |
| Nicaragua | 27(30) |
| Costa Rica | 14(16) |
| Panama | 5(6) |
| Guatemala | 3(3) |
| Belize | 1(1) |
| El Salvador | 1(1) |
| Honduras | 1(1) |
| Unascertainable | 3(3) |
| South America | 81(19) |
| Colombia | 16(20) |
| Brazil | 16(20) |
| Ecuador | 10(13) |
| Venezuela | 11(14) |
| Bolivia | 9(11) |
| Suriname | 8(10) |
| French Guiana | 3(4) |
| Peru | 2(3) |
| Argentina | 1(1) |
| Guyana | 1(1) |
| Unascertainable | 4(5) |
| North America | 1(<1) |
| USA | 1(100) |
Percentages may not sum to totals due to rounding.
Mexico is classified as Central America since GeoSentinel uses modified UN county classifications.
Missing for two patients.
Thirteen pregnant women were seen at a GeoSentinel site from 1 March 2016 through 31 December 2019 and diagnosed with ZIKV: nine were confirmed and four were probable cases. The median age of pregnant women was 29 years (range: 23–40). Twelve cases were vector acquired; one case was sexually transmitted. Among 11 pregnant women for whom information was available, 2 had an illness onset in 2015 after travel to South America, 8 had an illness onset in 2016 (7 after travel to the Caribbean and 1 after travel to Central America), and one had an illness onset in 2017 after travel to the Caribbean. Two (22%) of 9 pregnant women had a pretravel consultation with a healthcare provider. Seven pregnant women had timing of conception information available: three were pregnant before travel, two conceived during travel and two conceived after travel. Among five pregnant women whose trimester of ZIKV infection was known, all were infected in the first trimester. Among seven women in whom the pregnancy outcome was known; three carried their pregnancy to term with no congenital malformations reported, three elected to end their pregnancy (two after learning about their ZIKV diagnosis and one after a cerebral ultrasound at 14 weeks demonstrated asymmetric ventricles and unilateral ventriculomegaly), and one had a spontaneous abortion at 8 weeks.
Two cases of GBS were reported. The first was a 44-year-old male traveller exposed in Guyana in 2016, who presented to a GeoSentinel site in April 2016 with ascending lower extremity weakness and was hospitalized in an intensive care unit. The second was a 43-year-old woman who traveled to Colombia in 2015–2016, who presented to a GeoSentinel site in June 2016 with weakness and paresthesia.
Diagnostic Survey Results
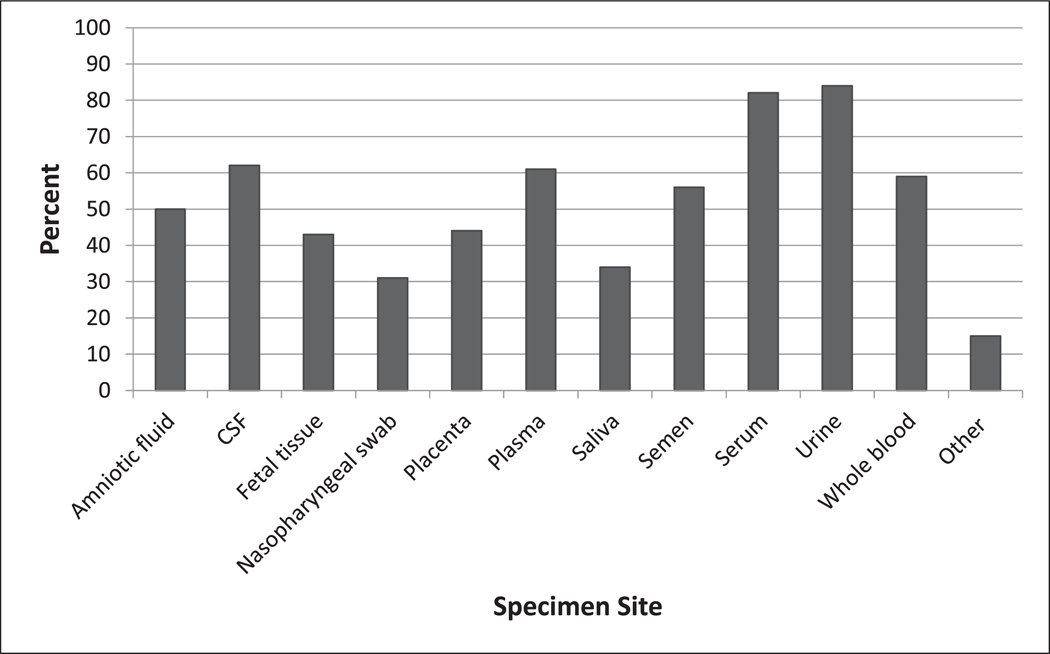
The survey response rate was 97% (70 of 72 sites); three responses were included from different clinics and hospitals at the Johannesburg, South Africa site. There were 103 responses in total (respondents could select more than one choice) for where ZIKV testing was sent: 58% of sites sent samples for ZIKV testing to a public laboratory, 30% to a hospital laboratory, 19% to a public reference laboratory, 16% to a private reference (commercial) lab and 11% to an academic research laboratory. The majority (88%) of sites (63 of 72) reported the capacity to conduct molecular testing, with an average turnaround time of 47 h (range: 3 h–15 days). Sixty (83%) sites reported performing serologic testing for ZIKV, and of those, 100% (60 of 60) performed anti-Zika IgM testing and 71% (51 of 60) anti-Zika IgG testing. Only 43% (29 of 68) were able to perform PRNT and, for those 29 sites, the average time from specimen submission to receipt of results for PRNT was 30 days (range: 1–100 days). Twenty-one percent (14 of 68) reported the capacity to culture ZIKV for diagnostic purposes. Sixty-eight sites responded to the question about their diagnostic capacity for ZIKV testing of the most common clinical specimens; these included urine (69%), serum (63%), and plasma (35%); fewer sites could test whole blood (21%) and other specimens (26%) (Figure 2).
Figure 2.
Percent of GeoSentinel sites above to test for ZIKV by specimen site, 2018 (n=103). CSF, cerebrospinal fluid; other: respondents noted that their site could run female and male genital tract swabs, urine and serum, or had no local capacity because all Zika testing was performed as send outs to commercial or public reference labs.
Major challenges identified regarding implementing diagnostics included the unavailability of serological tests (22%), lack of commercial molecular tests (19%), lack of funding for in-house tests (18%), cost of testing (18%), lack of funding to develop new tests (15%), insufficient capacity of personnel (13%) and unavailability of serology validation (10%), polymerase chain reaction validation (7%), positive controls for serology (3%) or positive controls for molecular testing (1%). Other challenges were written in by 17 of 68 (25%) sites, including difficulty obtaining insurance coverage, needing to send samples to regional and national labs due to lack of local infrastructure, limited capacity for testing, long turn-around time at referral centers and lack of testing standardization practices and assays.
Discussion
This analysis describes ZIKV infections among international travellers presenting to GeoSentinel sites and supports published findings, which demonstrated that early ZIKV cases were exposed in Southeast Asia and Oceania, and starting in 2015, cases were reported among travellers to the Americas and the Caribbean.4–6,8 Travellers reported to GeoSentinel during this period served as sentinels for this outbreak.14 Cases reported to GeoSentinel paralleled the explosive increase of cases in the Americas and the Caribbean in 201620 and the decline of cases in the Americas and Caribbean in 2017.In 2019,local transmission of ZIKV was documented in Europe21 and serves as a reminder that the absence of prior transmission does not indicate an absence of risk; sentinel surveillance remains critical.
The high number of infections among travellers (particularly tourists) to the Dominican Republic is likely due to its status as popular travel destinations. Over half of the ZIKV cases reported to GeoSentinel were among tourists, supporting the need to focus preventive efforts on these travellers who may not be considering vector-borne disease risks at their destination.
The ZIKV epidemic in the Americas and the Caribbean challenged vector-borne disease surveillance both before the outbreak (when Zika was likely misdiagnosed as dengue or chikungunya) and after the outbreak (when governments may have had other priorities such as preventing yellow fever in Brazil). GeoSentinel’s use as a sentinel surveillance system is well demonstrated: sentinel cases were identified before the outbreak peak from countries such as Kiribati14 and a large number (n=30) of travel-associated ZIKV infections were reported to GeoSentinel after travel to Cuba during the winter of 2016–2017 and summer of 2017, a year after peak transmission in neighbouring islands.22
A major concern with ZIKV infection is birth defects from maternal infection in pregnant travellers.23 Evidence suggests that many women of child-bearing age travel to Zika-endemic countries; three-quarters of travellers in one study who were planning to travel to a ZIKV-endemic country were of childbearing age.24 Few pregnant travellers with ZIKV were reported to GeoSentinel (despite many ZIKV infections among women of childbearing age), which may reflect pregnant travellers seeking care with their obstetricians rather than travel medicine providers, deciding not to travel, or having asymptomatic infection only detected by screening elsewhere. Given that less than one-quarter of pregnant travellers diagnosed with ZIKV had a pretravel consultation with a healthcare provider, targeting this population for pretravel care and advice by obstetricians, primary care physicians and travel medicine specialists depending on if the traveller is already pregnant or is planning pregnancy is paramount.
The decline of cases reported to GeoSentinel in 2018–2019 suggests that travellers may be acquiring ZIKV less frequently given the shifting epidemiology. This has implications for pretravel advice; current recommendations emphasize the need for a risk assessment by the healthcare provider,25 which depends on up-to-date information on ZIKV incidence. Unfortunately, there is no access to real-time data, as nationwide or regional data are often only made publicly available annually or surveillance systems may not capture all cases. Furthermore, transmission areas vary within a country, and ZIKV epidemiology at the local level may be lacking and result in difficulty in making an assessment for an individual traveller.
In endemic populations after an outbreak, although immunity among the local population may be high, ZIKV may be circulating at a low level and non-immune travellers may still be at risk. Box 1 summarizes WHO and CDC sources for updates on ZIKV transmission and its clinical sequelae in the post-epidemic era, to guide healthcare providers. Many areas now have very low transmission, rare sporadic cases, or unknown transmission, creating risk assessment challenges for healthcare providers when discussing potential for ZIKV exposure during a pretravel consultation.
Box 1:
WHO and CDC website sources on Zika virus and Zika epidemiology
Perceived low ZIKV risk by travellers or healthcare providers may result in travellers placing themselves at risk for both ZIKV infection and other Aedes-transmitted diseases such as dengue26 and chikungunya, which are reaching epidemic levels in some regions and also may be associated with negative maternal or fetal outcomes.23 Healthcare providers must provide specific pre and post-travel advice for women and men planning conception to mitigate the risk of maternal ZIKV (and other arboviral) infections,23 including mosquito precautions and condom use or abstinence.25,27
ZIKV diagnostics are complicated by issues with sensitivity and specificity of some serologic assays and a short-time window for molecular and serological testing.28 Low prevalence of Zika also impacts the positive predictive value of diagnostic tests (a positive test in a context of low prevalence has a high likelihood of being a false positive and can have important implications for pregnant woman). PRNT can help confirm recent infections and discriminate between cross-reacting antibodies, particularly in primary flaviviral infections, but access to PRNT among GeoSentinel sites was minimal. This is not surprising, as PRNT is time-consuming and requires expertise; therefore, it is usually confined to referral laboratories or research centers. Over a quarter of sites cited at least one challenge to successfully implementing ZIKV testing, which are necessary to overcome to ensure timely surveillance. Acquisition of appropriate diagnostics (including research for point-of-care assays), expertise, and personnel at the level of the healthcare provider is essential.
GeoSentinel data are subject to limitations. Since reporting is only by specialized sites and is not population-based, results may not be representative of all travellers with ZIKV, trends over time cannot be assessed, and rates and risks cannot be estimated. Outcome and risk factors are not routinely recorded nor is the information received at the pretravel consultation. Data are not routinely collected on reason for ZIKV testing; thus, it is unknown if travellers were asymptomatic and screened or presented with symptoms that prompted testing. GeoSentinel began collecting diagnostic testing information in October 2015, so confirmed or probable ZIKV reports before this date could not be validated.
In conclusion, the number and place of exposure of travellers with ZIKV seen at GeoSentinel sites reflects the evolution of the global epidemic and demonstrates a rapid decline in reported cases in 2018–2019. This decline suggests that travellers, including women of childbearing age, may be acquiring ZIKV less frequently given the shifting epidemiology, but since ZIKV is still circulating in many areas and given the severity of outcomes among pregnant women and their fetuses, these travellers should continue to discuss their travel plans with their healthcare providers to ensure precautionary measures are taken when appropriate. The expansion of ZIKV diagnostics to ensure timely sentinel case surveillance is essential to the further identification of ZIKV among international travellers.
Acknowledgements
The authors would like to thank Aisha Rizwan, M.P.H. and Kayce Maisel for their assistance with manuscript coordination.
Funding
GeoSentinel, the Global Surveillance Network of the International Society of Travel Medicine (ISTM), is supported by a Cooperative Agreement (U50CK00189) from the Centers for Disease Control and Prevention, as well as funding from the ISTM and the Public Health Agency of Canada. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. This analysis was partially funded by the European Union’s Horizon 2020 Research and Innovation program under Grant Agreement No. 734584 through the ZikaPLAN consortium.
Grants or funding
See text. A.W.S. is the Scientific Coordinator of ZikaPLAN.
§ Data from this analysis were presented at the International Society of Travel Medicine Conference, 7 June 2019, DC, USA.
Footnotes
Conflicts of interest
None declared.
References
- 1.Heymann DL, Hodgson A, Sall AA et al. Zika virus and microcephaly: why is this situation a PHEIC? The Lancet 2016; 387:719–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musso D, Gubler DJ. Zika virus. Clin Microbiol Rev 2016; 29:487–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffy MR, Chen TH, Hancock WT et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009; 360:2536–43. [DOI] [PubMed] [Google Scholar]
- 4.Faria NR, Quick J, Claro IM et al. Establishment and cryptic transmission of Zika virus in Brazil and the Americas. Nature 2017; 546:406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Hingrat Q, Perrier M, Charpentier C et al. Was Zika introduced to Brazil by participants at the 2013 Beach Soccer World Cup held in Tahiti: a phylogeographical analysis. Travel Med Infect Dis 2019; 32:101512. [DOI] [PubMed] [Google Scholar]
- 6.Quam MB, Wilder-Smith A. Estimated global exportations of Zika virus infections via travellers from Brazil from 2014 to 2015.J Travel Med 2016; 23. [DOI] [PubMed] [Google Scholar]
- 7.Duong V, Dussart P, Buchy P. Zika virus in Asia. Int J Infect Dis 2017; 54:121–8. [DOI] [PubMed] [Google Scholar]
- 8.Wilder-Smith A, Chang CR, Leong WY. Zika in travellers 1947–2017: a systematic review. J Travel Med 2018; 25. [DOI] [PubMed] [Google Scholar]
- 9.Kwong JC, Druce JD, Leder K. Zika virus infection acquired during brief travel to Indonesia. Am J Trop Med Hyg 2013; 89:516–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calleri G, Burdino E, Bonora S et al. Zika virus infection in two travelers returning from an epidemic area to Italy, 2016: algorithm for diagnosis and recommendations. Travel Med Infect Dis 2016; 14:506–8. [DOI] [PubMed] [Google Scholar]
- 11.Bogoch II, Brady OJ, Kraemer MU et al. Potential for Zika virus introduction and transmission in resource-limited countries in Africa and the Asia-Pacific region: a modelling study. Lancet Infect Dis 2016; 16:1237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Althaus CL, Low N. How relevant is sexual transmission of Zikavirus? PLoS Med 2016; 13:e1002157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grischott F, Puhan M, Hatz C, Schlagenhauf P. Non-vector-borne transmission of Zika virus: a systematic review. Travel Med Infect Dis 2016; 14:313–30. [DOI] [PubMed] [Google Scholar]
- 14.Leder K, Grobusch MP, Gautret P et al. Zika beyond the Americas: travelers as sentinels of Zika virus transmission. A GeoSentinel analysis, 2012 to 2016. PLoS One 2017; 12:e0185689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honein MA, Dawson AL, Petersen EE et al. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA 2017; 317:59–68. [DOI] [PubMed] [Google Scholar]
- 16.Paixao ES, Leong WY, Rodrigues LC, Wilder-Smith A. Asymptomatic prenatal Zika virus infection and congenital Zika syndrome. Open Forum Infect Dis 2018; 5:ofy073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crespillo-Andújar C, Díaz-Menéndez M, Elena T et al. Characteristics of Zika virus infection among international travelers: a prospective study from a Spanish referral unit. Travel Med Infect Dis 2019; 101543. [DOI] [PubMed] [Google Scholar]
- 18.Wilder-Smith A, Boggild AK. Sentinel surveillance in travel medicine: 20 years of GeoSentinel publications (1999–2018). J Travel Med 2018; 25. [DOI] [PubMed] [Google Scholar]
- 19.Hamer DH, Barbre KA, Chen LH et al. Travel-associated Zika virus disease acquired in the Americas through February 2016: a GeoSentinel analysis. Ann Intern Med 2017; 166:99–108. [DOI] [PubMed] [Google Scholar]
- 20.Huits R, Van Den Bossche D, Eggermont K et al. Incidence of Zika virus infection in a prospective cohort of Belgian travellers to the Americas in 2016. Int J Infect Dis 2019; 78:39–43. [DOI] [PubMed] [Google Scholar]
- 21.Giron S, Franke F, Decoppet A et al. Vector-borne transmission of Zika virus in Europe,southern France,Augsut 2019.Eurosurv 2019; 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grubaugh ND, Saraf S, Gangavarapu K et al. Travel surveillance and genomics uncover a hidden Zika outbreak during the waning epidemic. Cell 2019; 178:1057–71.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vouga M, Chiu YC, Pomar L et al. Dengue, Zika and Chikungunya during pregnancy: pre- and post-travel advice and clinical management. J Travel Med 2019; 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lammert S, Walker AT, Erskine S. et al. , Characteristics of US travelers to Zika virus–affected countries in the Americas, March 2015–October 2016. Emerg Infect Dis 2017; 23:324–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Caring for pregnant women. https://www.cdc.gov/pregnancy/zika/testing-follow-up/pregnant-woman.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fzika%2Fhc-providers%2Fpregnant-woman.html (2 December 2019, date last accessed).
- 26.Halstead S, Wilder-Smith A. Severe dengue in travellers: pathogenesis, risk, and clinical management. J Travel Med 2019; 26. [DOI] [PubMed] [Google Scholar]
- 27.Chen LH, Hamer DH. Zika virus and sexual transmission: updated preconception guidance. J Travel Med 2018; 25. [DOI] [PubMed] [Google Scholar]
- 28.Goncalves A, Peeling RW, Chu MC et al. Innovative and new approaches to laboratory diagnosis of Zika and dengue: a meeting report. J Infect Dis 2018; 217:1060–8. [DOI] [PMC free article] [PubMed] [Google Scholar]