Abstract
The human eosinophil, which typically comprises about 1–5% of all circulating leukocytes, has long been felt to favorably impact innate mucosal immunity but at times has also been incriminated in disease pathophysiology. Research into eosinophil biology, especially with the use of murine models, has uncovered a number of interesting contributions of eosinophils to health and disease. However, it appears that not all eosinophils from all species are created equal. It remains unclear, for example, exactly how having eosinophils benefits the human host when helminth infections in the developed world have become scarce. This review will focus on our current state of knowledge as it relates to human eosinophils from birth to death, from circulation to tissue accumulation, in sickness and in health. When information on aspects of human eosinophil biology are lacking, lessons learned from relevant mouse studies will be discussed, with the understanding that such information may or may not directly apply to human biology and disease. The use of recently approved biologics that selectively target eosinophils, (i.e., precision pharmacology) is now providing the medical community with an exciting opportunity to directly test hypotheses in people by defining the contribution of this cell in eosinophil-associated diseases such as asthma and others. While it is an exciting time to be an “eosinophilosopher”, there remain a number of important challenges and unmet needs in this field that provide opportunities for future studies and advancement as we explore the contributions of this enigmatic cell.
Keywords: Eosinophil, eosinophilia, hematopoiesis, cytokines, phenotype, adhesion, migration, function, eosinophil-related diseases, hypereosinophilic syndromes, biomarkers, biologics, treatments
INTRODUCTION
The eosinophil, one of several cells named by Paul Ehrlich in the late 1800’s, is one of the less common blood leukocytes. Its characteristically intense staining with the acidic dye eosin is due to the avidity of this stain for basically charged intracellular granules found uniquely within the cytoplasm, thus imparting this bilobed cell with distinct tinctorial properties. Normal numbers of circulating eosinophils range from 0–500 per μL in human blood, but in certain conditions can increase by 20-fold or more. Evolutionarily, the eosinophil or an eosinophil-like cell has been maintained in vertebrates, including reptiles and fish, over millions of years, strongly suggesting that this cell contributes important, favorable biology towards the well-being of these species (1, 2). In this regard, one prevailing theory is that the eosinophil participates in innate immunity to parasites, especially to helminths. With the availability of constitutive and conditionally eosinophil-deficient mouse strains and other tools, this traditional paradigm is being challenged (3, 4). Now that biologic agents that effectively and selectively deplete eosinophils in people with asthma and other eosinophil-related disorders can be prescribed, we are creating the equivalent of eosinophil-deficient humans with pharmacology (5). This places us at the beginning of a new era regarding our understanding of the role of the eosinophil in health and disease (6, 7). What follows is an overview of the role of the human eosinophil in this regard, highlighting gaps in our knowledge while also providing intriguing insights gained from mouse eosinophil biology that may or may not translate to its human counterpart. So, unless otherwise stated throughout the text, the term “eosinophil” will be equated with the term “human eosinophil”.
EOSINOPHIL HEMATOPOIESIS AND LINEAGE
Development during homeostasis
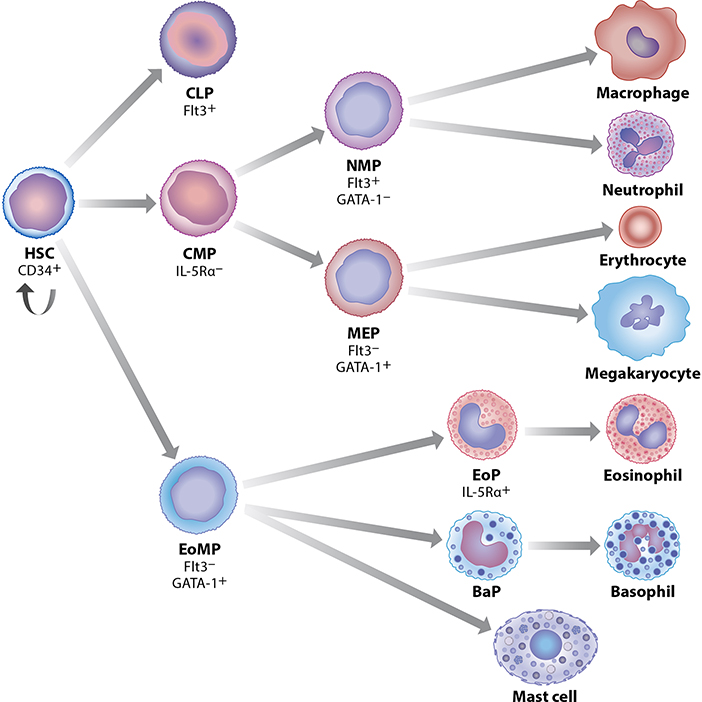
Eosinophils, along with the rest of the myeloid blood cell lineages, develop in the bone marrow microenvironment from multipotent hematopoietic stem cells, which give rise to a population of unique eosinophil-committed progenitors, termed EoPs, that are capable of terminally differentiating into mature eosinophils in the absence of any lineage-specific growth factors or cytokines, including IL-5 (8). The human EoP (hEoP) is defined by its surface expression of a number of receptors, the most important of which is the high-affinity alpha subunit of the IL-5 receptor (IL-5Rα). These IL-5Rα+ hEoPs differentiate exclusively into eosinophils, but not basophils or mast cells (Figure 1) (9). Under homeostatic conditions in healthy individuals, eosinophilopoiesis is regulated in part by a unique combinatorial program of transcription factors (10), that includes the requisite expression of GATA-1 (11), which occurs through utilization of an eosinophil-lineage specific enhancer in the GATA-1 gene itself (12). Notably, transgenic deletion of a unique high-affinity palindromic double GATA binding site in the HS-2 enhancer region of the GATA-1 gene produced an eosinophil-deficient mouse strain (ΔdoubleGATA) with essentially normal development of other GATA-requiring hematopoietic lineages, including the erythroid lineage (12). In addition to autoregulating eosinophil-specific GATA-1 expression in the mouse, these high affinity double GATA-1 binding sites are present and functionally important in many hallmark human eosinophil-affiliated genes whose expression defines the eosinophil lineage (13, 14), including those encoding eosinophil granule cationic proteins, e.g. Major Basic Protein-1 (MBP-1, via its eosinophil-specific P2 promoter), Eosinophil Peroxidase (EPX), the Charcot-Leyden Crystal protein (CLC)/Galectin-10, the eotaxin receptor CCR3 and the IL-5-binding alpha subunit of the IL-5 receptor (IL-5Rα) (14).
Figure 1.
Development of the eosinophil lineage in the context of normal human hematopoiesis. In the current paradigm, hematopoietic stem cells (HSC) give rise directly to eosinophil/mast cell progenitors (EoMP), from which IL-5R+ eosinophil progenitors (EoPs) develop and terminally differentiate into mature eosinophils. EoMPs also differentiate into basophil progenitors (BaP) and mast cells. Expression of GATA-1 versus Flt3 distinguishes between early multipotent progenitors that give rise to EoMP and megakaryocyte/erythroid progenitors (MEP) versus common lymphoid progenitors (CLP) and neutrophil/macrophage progenitors (NMP) (formerly GMP). Both NMP and MEP arise from a common myeloid progenitor (CMP) distinct from the EoMP population.
In addition to GATA-1, the eosinophil’s baseline combinatorial transcription factor program includes low levels of the ets factor PU.1, down-regulation of FOG-1, and temporally regulated expression of members of the C/EBP family (C/EBPα and C/EBPε), the latter of which is expressed during eosinophil development as a series of transcriptional activator and repressor isoforms (15) and is required for eosinophil terminal differentiation (16). Finally, baseline eosinophilopoiesis is regulated in part at the level of miRNAs and long non-coding RNAs (17–19), and epigenetically, by higher order regulatory mechanisms that are the topic of ongoing research, primarily in mouse models (20).
Changes during eosinophilia.
While basal eosinophilopoiesis requires the hierarchical expression, timing and levels of specific transcription factors, blood and tissue eosinophilia in allergic reactions, immunity to parasitic infections, and other eosinophil-associated responses is regulated principally by the lineage-specific cytokine IL-5, which amplifies proliferation and terminal differentiation of committed EoPs in the bone marrow. This IL-5 is produced by cells of both the innate and adaptive immune systems, including mast cells, ILC2s and activated Th2 lymphocytes. Of note, the number of hEoPs increases to represent ~10–20% of the common myeloid progenitor cell population in the bone marrow of patients with blood eosinophilia of various etiologies, indicating that the hEoP participates in expansion of eosinophilopoiesis in eosinophilic disorders. Thus, while the IL-5 knockout mouse is ostensibly eosinophil-deficient, it still develops small numbers of blood and tissue eosinophils through the baseline homeostatic transcriptional mechanisms noted above but cannot mount a blood or tissue eosinophilia in response to infection with helminths or sensitization and challenge with allergens because these responses are IL-5 dependent (21). In addition to IL-5, other cytokines and chemokines have been shown, at least in in vitro, to drive both murine and human EoPs to terminally differentiate. These include IL-3, GM-CSF and the eotaxin family of eosinophil-recruiting chemokines (CCL11, CCL24, CCL26).
EOSINOPHIL SURFACE PHENOTYPE
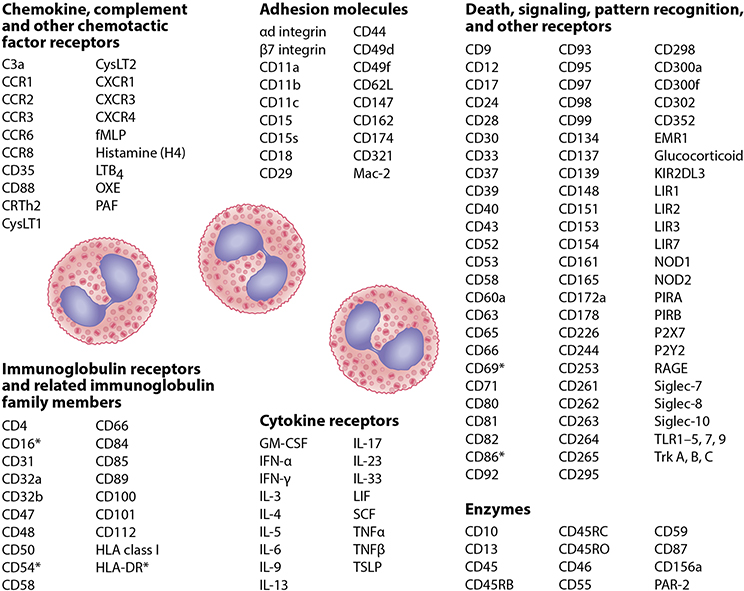
The eosinophil’s cell surface is richly adorned with cell surface receptors. While many are considered eosinophil-selective in their expression (e.g., CCR3, IL-5 receptor Siglec-8, and others), EMR-1 appears to be absolutely specific for eosinophils, although its function remains unknown (22, 23). Like all leukocytes, eosinophils express many cytokine and chemokine receptors and adhesion molecules (Figures 2 and 3) involved in their migration across the vascular endothelium and through the epithelium during recruitment into tissues. Eosinophils express receptors for the three key cytokines required for their differentiation, maturation, priming, activation and survival in the bone marrow and tissues, respectively, including the alpha subunits of the high affinity receptors for IL-3 (IL-3Rα/CD123), IL-5 (IL-5Rα/CD125) and GM-CSF (GMCSF-Rα/CD116) that heterodimerize with the shared β-common chain (CD131).
Figure 2.
Surface molecules expressed on human eosinophils. There is some overlap among categories for some of these proteins. Common names for chemokine (CC and CXC) receptors, toll-like receptors (TLRs), and others are used here instead of the CD names because of the greater use and familiarity of the former among most readers. The asterisk indicates molecules expressed on activated eosinophils. Abbreviations used: CRTh2, chemoattractant receptor-homologous molecule expressed on Th2 cells; CysLT, cysteinyl leukotriene; EMR1, EGF-like module-containing mucin-like hormone receptor-like 1; fMLP; N-Formyl-methionyl-leucyl-phenylalanine; IFN, interferon; IL, interleukin; KIR2DL3, Killer Cell Immunoglobulin Like Receptor, Two Ig Domains And Long Cytoplasmic Tail 3; LIF, Leukemia inhibitory factor; LIR, Leukocyte immunoglobulin-like receptor; Mac-2, epsilon binding protein; NOD, nucleotide-binding oligomerization domain; OXE, Oxoeicosanoid; P2X and P2Y, ATP-gated purinoreceptors; PAF, platelet activating factor; LTB, leukotriene B; PAR, Protease activated receptor; PIR, paired Ig-like receptor; RAGE, receptor for advanced glycation end products; SCF, stem cell factor; Siglec, sialic acid-binding, immunoglobulin-like lectin; TLR, toll-like receptor; TNF, tumor necrosis factor; Trk, Tropomyosin-receptor-kinase; TSLP, Thymic stromal lymphopoietin. Updated from (143) with permission.
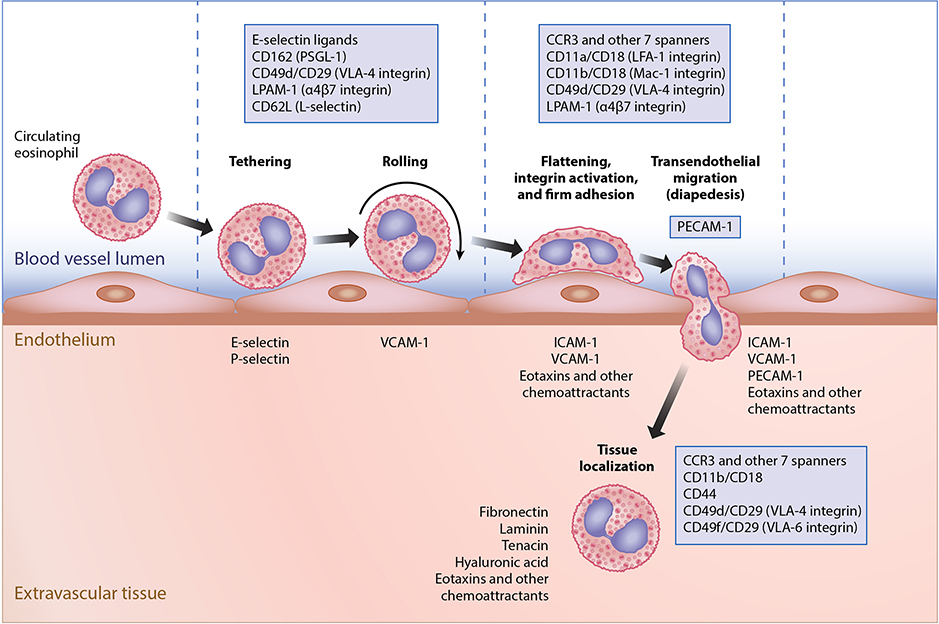
Figure 3.
Mechanisms involved in eosinophil extravasation during inflammation. Roles of adhesion molecules, chemoattractants and other molecules during the process of eosinophil migration from the circulation into tissues. Shown are the contributions of sets of leukocyte, endothelial, and tissue molecules during the steps of tethering, rolling, firm adhesion, transendothelial migration (diapedesis) and localization within tissues. Note that in addition to other adhesion molecules, PECAM-1 on both the leukocyte and the endothelium is uniquely involved in diapedesis.
Human (and mouse) eosinophils express high levels of the G-protein coupled receptor CCR3, a major receptor involved in eosinophil chemotaxis, migration, recruitment and degranulation in tissues. As for most chemokine receptors, CCR3 is promiscuous, binding multiple ligands besides eotaxin-1, −2 and −3 (CCL11, CCL24, CCL26). The eotaxins, along with IL-5, are the principal factors accounting for eosinophil maturation, recruitment and migration within tissues. Under physiologic conditions, CCL11 is the key CCR3 ligand for homeostatic recruitment of eosinophils into the gastrointestinal tract and other organs, a conclusion that is especially clear in mouse models (24). Eotaxin-3 (CCL26) pathologically recruits large numbers of human eosinophils into the esophagus in eosinophilic esophagitis (EoE) and is the most highly upregulated gene transcript in this immune-mediated, food allergy-associated remodeling disease of the esophagus (25). Eosinophils also express the prostaglandin D2 receptor 2 (PD2R2/chemoattractant receptor homologous molecule expressed on Th2 cells [CRTH2]) and can migrate in response to PGD2.
The eosinophil is endowed with multiple immunoglobulin receptors and related family members (Figure 2) involved in eosinophil-mediated functional activities, including antibody-mediated cellular cytotoxicity towards helminth parasites and other immune modulatory functions and pathologic activities in eosinophil-associated diseases (see below). For example, eosinophils express Fc-gamma RII-b (FcɣRIIb/CD32), a functional polymeric IgA, asialoglycoprotein receptors 1 and 2 (ASGPR 1, 2) and the IgA Fc receptor (CD89) for the IgA secretory component. CD89 is likely the major receptor for IgA-mediated eosinophil activation, e.g. in mucosal tissues of the gastrointestinal tract. Finally, although human eosinophils have been reported to express a number of the component chains of FcεRI, including the α and γ chains, this finding remains controversial. What is not controversial is that they lack the β chain of FcεRI expressed on basophils and mast cells.
One of the largest classes of membrane proteins expressed on the surface of eosinophils includes cell death, signal transduction, and pattern recognition receptors (Figure 2). Only a handful can be specifically mentioned here. Receptors that function in pattern recognition allow eosinophils to be stimulated directly during host innate immune responses by pathogen-associated molecular patterns (PAMPs) and/or damage-associated molecular patterns (DAMPs). These pattern recognition receptors are involved in eosinophil interactions with invading microorganisms (e.g. parasitic helminths, fungi and certain bacteria) and with its internal tissue microenvironment, where they help regulate eosinophil activation, tissue remodeling responses, survival and apoptotic cell death. Finally, these eosinophil-expressed sensors of innate immunity also include proteinase-activated receptors (PARs), PAR-1 and −2. PAR-2 may play a significant role in eosinophil activation in response to proteases released by aeroallergens such as dust mites, fungi, or pollens.
EVOLUTIONARY ORIGINS AND CONSIDERATIONS
The most recent (and comprehensive) consideration of this topic comes from a scholarly review by McGarry (1). Cells in a variety of invertebrates may represent evolutionary precursors of modern-day vertebrate eosinophils. Links based on biochemical or genetic similarities are limited, but include the expression of the myeloperoxidases, of which EPX is eosinophil-specific. Studies suggest that myeloperoxidase (expressed by neutrophils) and EPX diverged some 60–70 million years ago, but are not sufficiently robust to indicate when the earlier invertebrate to vertebrate evolution of the eosinophil lineage occurred.
Vertebrate eosinophils have been identified fairly extensively in representative species, from fish to mammals, at the light/histologic, electron microscopic and biochemical levels (26). Peroxidase-containing eosinophils have been definitively identified in embryonic and adult Zebra fish, which provide a potentially useful vertebrate model that can be genetically manipulated to study eosinophil development and functions (27). Observations in the frog support a role for the eosinophil in tissue remodeling events during metamorphosis (e.g., the shortening of the tadpole gut is accompanied by substantial infiltration of eosinophils) but the specific role of eosinophils in these complex metabolic, physiologic, and anatomical processes remains to be defined. Eosinophils are definitively present in most avian species. In the chicken, transcriptionally-regulated differentiation of eosinophil-committed myeloid progenitors (EoP) to mature eosinophils is remarkably similar to that of human eosinophils (10).
There are numerous published descriptions of mammalian eosinophils. Although these eosinophils are clearly well-equipped to kill and/or contain helminth parasites and their larval stages, their early appearance during evolution and accumulating studies of host immune responses to helminths and other parasites in eosinophil-deficient mouse strains (e.g. PHIL (28), ΔdblGATA (12), MBP-1−/−/EPX−/− double knock-out (29)) strongly argue against a significant selective advantage in host defense during the evolution of the eosinophil. The absence of significant, life-threatening developmental abnormalities or functional deficiencies in these eosinophil-deficient mouse strains, at least under the specific pathogen-free conditions present in most animal facilities, begs the question of why the eosinophil lineage continues to be ubiquitous in vertebrate species.
HUMAN EOSINOPHILS VERSUS OTHER SPECIES: SIMILARITIES AND DIFFERENCES
Human eosinophils differ to varying degrees from the eosinophils of other species, the mouse being of greatest interest. These differences are present at a number of levels including the origin of their hematopoietic progenitors, polymorphonuclear morphology, ultrastructure of their acidophilic specific granules, expression, types and amounts of their granule cationic and other major proteins, surface receptors, mechanisms of activation, secretion and degranulation, and other functionally relevant properties (30). That said, there continues to be some controversy, with ongoing revisions to the human and mouse hematopoietic trees based on improved reagents and approaches to identify these cells, their surface phenotypes, and most recently, transcriptomes at the single cell level.
Human and mouse eosinophils show significant differences in the cationic protein constituents of their specific granules. For example, although EPX, MBP-1 and −2 are well conserved, the human eosinophil contains only two cationic ribonucleases, EDN (RNase2) and ECP (RNase3) (31), whereas mouse eosinophil granules contain upwards of 7 members of an evolving family of eosinophil-associated ribonucleases (EARS) that are also expressed by other myeloid cells in the mouse and other rodents (32). Human eosinophils also express large amounts of the cytosolic auto-crystallizing CLC/Galectin-10, which represents ~7–10% of total cellular protein and is one of the earliest and most abundant mRNAs expressed during eosinophil development. In contrast, mouse eosinophils lack a gene encoding CLC/Galectin-10 (31). Although the function(s) of CLC/Galectin-10 in human eosinophil biology remains unclear, it may be required for effective granulogenesis during eosinophil development (29, 31).
Functional differences clearly exist between human eosinophils and those of mice and other species. There are numerous studies describing the role and specific functions of eosinophils in the development of allergic inflammatory “diseases” in mice, many of which have been ascribed to secretion of eosinophil-derived cytokines, chemokines and differences in eosinophil secretory potential between different species. Notably, the different pathways for eosinophil activation, degranulation and secretion of their cationic granule proteins and stored cytokine and chemokine inflammatory mediators, and particularly the mechanisms that regulate piecemeal degranulation (PMD), are based primarily on in vitro and in vivo studies of human eosinophils. These pathways of degranulation in the setting of allergic inflammatory reactions in tissues typically do not occur in most murine models of inflammation (33, 34). Finally, differences in cell surface protein and receptor phenotypes between eosinophils from humans and other species are considerable, but they are beyond the scope of this review. Ultimately, the significant differences between human eosinophils and those of other species, particularly in the context of genetically-modified mouse models used to assess eosinophil function in homeostasis and eosinophil-associated diseases, argue strongly for the need to confirm these aspects of eosinophil biology using human blood and tissue-derived eosinophils ex vivo and in vitro, and in humanized mouse models.
TISSUE EOSINOPHILIA AND EOSINOPHIL ACTIVATION WITHIN TISSUES
Once eosinophils mature within the bone marrow environment, they exit and circulate for about 1 day as estimated in normal adult humans using nuclear medicine tracer techniques (35). Using similar methods, the accumulation of eosinophils into the lung has been estimated and ranges from about 30 eosinophils per minute per mL of blood in healthy volunteers to rates 10–100 times higher or more in asthmatics and those with focal eosinophilic lung diseases (36). Under homeostatic conditions, the vast majority of eosinophils are headed for mucosal surfaces of the gastrointestinal tract, sparing the esophagus but including the stomach and small and large intestine. Once there, they are presumed to reside for days. Although their homeostatic lifespan within these organs is not known, it is almost certainly on the order of days rather than weeks. As is true for all circulating leukocytes, in order to leave the circulation and enter any extravascular compartment, a series of well-orchestrated steps involving leukocyte and endothelial adhesion molecules must occur (Figure 3). Initial events are mediated by selectin-sialoglycan interactions which, for eosinophils, are primarily mediated by carbohydrates displayed on P-selectin ligand (CD162) on the eosinophil and P-selectin on activated endothelium (37, 38). Eosinophils also express L-selectin and ligands for E-selectin on their surface, but their roles in eosinophil accumulation are less certain and do not appear to be as important as they are, for instance, in neutrophil or cutaneous T cell recruitment responses (39). Patients with leukocyte adhesion deficiency type 2 (CDG-IIc), whose leukocytes lack fucosylated selectin ligands, have elevated numbers of circulating neutrophils, but not eosinophils. The same phenomenon has been seen in clinical trials of a pan-selectin antagonist (GMI-1070, rivipansel), suggesting that selectin-mediated homeostatic recruitment is likely less important for eosinophils (40, 41).
Subsequent steps that are even more critical for recruitment for eosinophil extravasation beyond tethering and rolling involve integrins and their counter-ligands on activated endothelium. These molecules include the β1 integrin VLA-4 (α4β1 integrin, CD49d/CD29), which is not expressed by neutrophils but is found on other leukocytes and recognizes the endothelial ligand VCAM-1 (CD106) and β2 integrins, especially LFA-1 and Mac-1 (αLβ2 integrin, CD11a/CD18 and αMβ2 integrin CD11b/CD18, respectively), which are expressed by eosinophils, neutrophils and other cells and interact with endothelial ICAM-1 (CD54). Eosinophils, like neutrophils, use both ICAM-1 and PECAM-1 (CD31) during the process of transendothelial migration (39, 42). Whether eosinophils use CD99 and CD99L2 during this step, as has been described for neutrophils (43), is unknown. There may be an especially critical contribution via the selective interaction of α4β1 integrin with VCAM-1 on the endothelium and the selective induction of VCAM-1 expression caused by IL-4 and/or IL-13 (although IL-1 and TNFα can also influence VCAM-1 expression) (39). Further support for this concept comes from several clinical observations: 1) humans lacking β2 integrins have a markedly impaired ability to mobilize neutrophils, but not eosinophils or other leukocytes, into tissues during inflammation (44); 2) antibody blockade of α4β1 integrin with natalizumab (45) or of the common IL-4Rα chain (shared by both the IL-4R and IL-13R) with dupilumab, causes eosinophilia, not neutrophilia (46); and 3) blockade of α4β7 integrin (LPAM-1) with vedolizumab has no effect on circulating granulocyte numbers (47).
In addition to the role of adhesion molecules, eosinophils are equipped with a wide range of seven-spanner receptors for chemokines and other chemoattractants (Figure 2). Although the recent failure of an oral CCR3 antagonist to impact eosinophil numbers in the sputum of subjects with asthma or eosinophilic bronchitis challenges this paradigm (48), whether the contribution of CCR3 will be more substantial during eosinophil recruitment to other organs in other conditions or whether newer and potentially more effective CCR3 antagonists (49) will provide additional insight into this dilemma remains to be determined. Lastly, ongoing clinical trials with bertilimumab (anti-CCL11) in bullous pemphigoid, an eosinophil-rich skin disease, should tell us if this condition is driven by CCL11 (eotaxin-1).
There is abundant evidence that eosinophil integrins become activated in both the blood and in tissues in diseases such as asthma. Phenotypic changes occur with eosinophil activation and extravasation, including shedding of some surface proteins (e.g., L-selectin) and de novo expression of others (e.g., CD69), while others remain unchanged (e.g., Siglec-8). Another consequence of eosinophil activation is platelet adhesion. This not only complicates proteomic analyses of “purified eosinophils” but also results in platelet-dependent alteration of eosinophil function (50, 51).
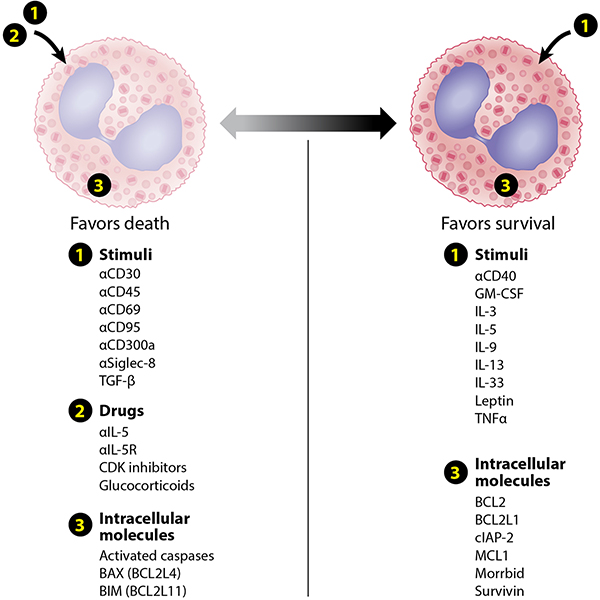
Because eosinophils are terminally differentiated cells that can no longer divide, eosinophilic inflammation must be the net result of combinations of enhanced recruitment and enhanced survival. Regarding the latter, numerous cytokines, such as IL-3, IL-5, GM-CSF and others, maintain eosinophil survival for weeks in vitro (Figure 4). Anti-IL-5 biologics reduce eosinophil numbers in tissues in asthma and eosinophilic esophagitis but not in the normal small intestine, suggesting that this cytokine is not exclusively responsible for prolonging eosinophil survival in vivo. While this may be due, in part, to a partial loss of IL-5 receptor expression on extravasated eosinophils (52), it must be the case that other mediators besides IL-5 are important in maintaining eosinophil longevity at sites of inflammation. Although GM-CSF seemed a likely candidate for this role, trials of anti-GM-CSF in asthma were disappointing (53).
Figure 4.
Examples of stimuli, drugs and intracellular molecules that enhance or reduce eosinophil survival. Abbreviations used: BAX, bcl-2-like protein 4; bcl-2, B-cell lymphoma 2; BCL2L, bcl-2-like protein; BIM, bcl-2-like protein 4; CDK, cyclin-dependent kinase; cIAP-2, cellular inhibitor of apoptosis 2; IFN, interferon; IL, interleukin; MCL1, myeloid cell leukemia 1; Siglec, sialic acid-binding, immunoglobulin-like lectin; TNF, tumor necrosis factor.
Eosinophil survival can be diminished by a number of pathways (Figure 4), as eosinophil death and removal can occur via many different mechanisms, including apoptosis, necrosis, autophagy, necroptosis, antibody-dependent cellular cytotoxicity (ADCC), and phagocytic cell recognition and clearance (efferocytosis) (54). Morrbid, a non-coding RNA found in leukocytes, downregulates transcription of the pro-apoptotic Bcl2l11 gene (previously called BIM) to promote survival in cells including eosinophils. Indeed, Morrbid as well as cellular inhibitor of apoptosis (cIAP-2) and survivin, all of which are anti-apoptotic, are abnormally over-expressed in eosinophils from subjects with hypereosinophilic syndrome (HES) compared to normal individuals (55). Therapeutically, anti-IL-5 antibodies such as mepolizumab and reslizumab reduce eosinophil hematopoiesis and induce eosinophil apoptosis, while afucosylated IgG1 monoclonal antibodies to the IL-5R (benralizumab) and Siglec-8 (AK002) actively deplete eosinophils via ADCC (56–58).
Separate from recruitment pathways and the competition between pro-survival and pro-death signals that eosinophils encounter in situ while in tissues, additional forms of activation result in secretion of a host of mediators ranging from preformed granule proteins to lipid mediators, cytokines, chemokines, enzymes, growth factors and other substances. Structures involved in secretion, such as vesicle-associated membrane proteins (VAMPs) including CD63, are found on the granule membranes themselves. During the process of degranulation, a key phenomenon by which eosinophils contribute to host defense and disease, preformed contents get released via at least three different pathways: 1) typical exocytosis, where granules fuse with the outer plasma membrane, 2) PMD involving intracellular vesicle formation associated with loss of granule integrity, followed by movement and fusion with the outer plasma membrane, and 3) ETosis, or cytolytic degranulation associated with plasma membrane rupture and release of free granules along with extracellular traps (4, 59, 60). Stimulated eosinophils can rapidly release other substances besides granule proteins, as has been observed when so-called traps containing mitochondrial DNA and granule proteins combine to form structures that then bind and kill bacteria (61).
While the exact mechanisms for various types of eosinophil degranulation in vivo in humans remain poorly characterized, in vitro studies have shown that engagement of FcαR by secretory IgA is particularly effective, while exposure to combinations of cytokines, chemokines, and other chemoattractants can also elicit secretion in an integrin/adhesion dependent manner. Finally, compared to other cells, eosinophils release relatively small amounts of cytokines, chemokines, and growth factors, and determining their relative contribution compared to other cells is difficult to do. In contrast, eosinophils make appreciable quantities of lipid mediators, especially leukotriene C4 (LTC4) via LTC4 synthetase located in the lipid bodies, and the 5-lipoxygenase product 5-hydroxyeicosatetraenoic acid (5-HETE), along with cyclooxygenase products such as thromboxane B2 and prostaglandins E1 and E2.
ROLES FOR EOSINOPHILS IN HEALTH
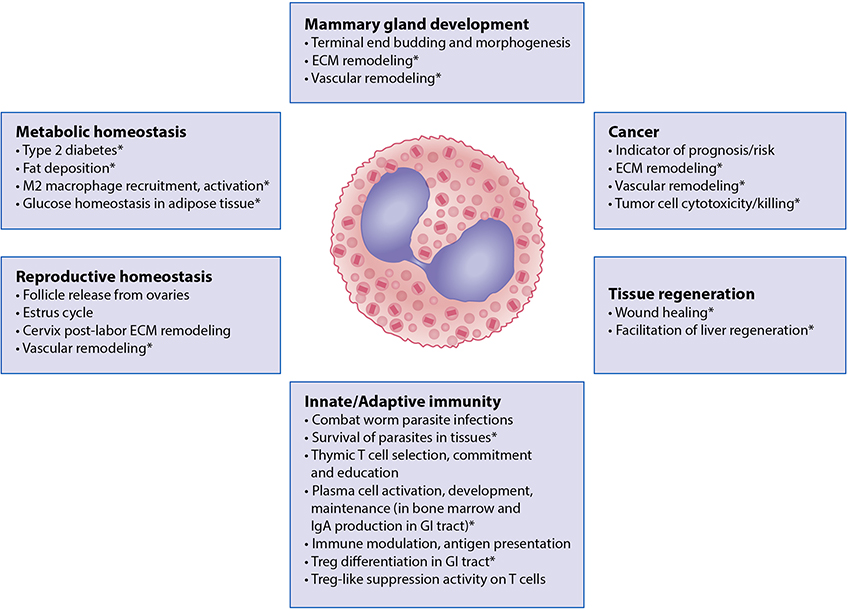
The association between eosinophilia and helminth infection was noted soon after the first description of eosinophils by Ehrlich. This association, coupled with studies demonstrating eosinophil killing of helminth larvae in vitro, led to the hypothesis that the primary role of eosinophils was in anti-pathogen responses, specifically those involving helminths. As helminth infection has become less common and eosinophils have persisted, the role of eosinophils in host defense against external pathogens has become less clear and other homeostatic functions of eosinophils have been described (Figure 5) (2).
Figure 5.
Roles of eosinophils in normal tissue and metabolic homeostasis in health. Major functions of the eosinophilic leukocyte include the maintenance of tissue microenvironments during normal organismal development, along with the establishment and regulation of host innate and adaptive immune responses. Findings from mouse models that have yet to be confirmed in humans are denoted with “*”. Abbreviations used: ECM, extracellular matrix protein; M2 macrophage, an alternatively activated macrophage that arises in response to exposure to Th2-type cytokines Treg, T regulatory cells.
Parasitic infections
Peripheral eosinophilia is commonly, but not always, associated with a wide variety of helminth infections, particularly those that involve migration of parasites through tissues, in ectoparasite infestations and rarely in the setting of protozoan infection (i.e., Sarcocystis myositis and Cystoisospora infection). Human and mouse eosinophils can adhere to and kill infective helminth larvae through ADCC and eosinophil granule protein deposition around dead and dying parasites has been demonstrated in tissue biopsies from infected patients (62). That said, the role that eosinophils play in protection remains uncertain. In murine models, eosinophils are sometimes protective (e.g., prevent secondary infection by Trichuris muris and Trichinella spiralis), sometimes of no consequence (e.g., do not affect granuloma formation in schistosomiasis) and sometimes required for parasite survival (e.g., maintenance of Trichinella larvae encysted in muscle through their effects on nurse cells) (63). Data supporting a role for human eosinophils in protection against helminth infection in vivo are scarce with the exception of schistosomiasis, where post-praziquantel eosinophil levels have been correlated with resistance to reinfection in many different epidemiologic settings (64).
Fungal infections
Many fungal infections are characterized by blood and/or tissue eosinophilia. Whereas data from most mouse models suggest that the presence of eosinophils is protective in fungal infection, eosinophilia appears to be associated with disseminated or more severe human fungal disease (65). The exception is allergic fungal disorders, including allergic bronchopulmonary aspergillosis and allergic fungal sinusitis, where eosinophilia can be dramatic and fungal elements are scarce. Eosinophil extracellular DNA traps appear to be involved in destroying fungal organisms (66).
Viral infections and others
Viral infections are typically associated with a decrease in circulating eosinophils in the blood. The most notable exception is human immunodeficiency virus (HIV) infection (67). Tissue eosinophilia in the absence of blood eosinophilia has been described in a variety of viral infections, including viral myocarditis and respiratory syncytial virus (RSV) pneumonia. Whether eosinophils play a role in antiviral defense, are responsible for tissue destruction, or simply recruited to sites of tissue damage is unknown. Although data from experimental murine infection with RSV and influenza A support the hypothesis that eosinophils play a predominantly protective role (68, 69), human studies have produced conflicting results with 1) comparable prevalence rates of respiratory viral infection but increased clinical severity in asthmatics with >3% sputum eosinophils (70), 2) similar airway inflammatory responses in response to experimental rhinovirus infection in asthmatics and healthy controls, despite increased eosinophils in the asthmatics, and finally 3) effects of mepolizumab (which substantially reduces blood and sputum eosinophils) on macrophage, B cell and neutrophil responses without effect on infection severity following rhinovirus challenge (71). Finally, whereas eosinopenia is the rule in acute bacterial infection, eosinophils may play a role in the host response to some chronic bacterial infections, including mycobacterial infection (72) and C. difficile colitis (73).
Anti-tumor responses
Our understanding of the role and contribution of eosinophils to human tumor biology and immunology is still evolving. Some of the more intriguing recent information on this topic comes from analyses of tumor biopsies and correlations between prognosis and numbers of eosinophils in the tumor microenvironment, detected histologically or based on the presence of eosinophil-specific gene signatures (74, 75). These approaches suggest that the presence of eosinophils within the tumor microenvironment can be good (e.g., breast cancer, melanoma), bad (e.g., lung cancer, Hodgkin’s lymphoma) or of unclear prognostic significance (e.g., brain cancer). The obvious disadvantage of this approach is that it provides no insight into the actual contribution of the eosinophil itself to tumor progression or remission, as tissue eosinophilia may simply be a biomarker of type 2 inflammation. It is worth pointing out that neither mice nor humans lacking eosinophils appear to be at increased risk of developing cancers. Ultimately, long-term safety data with biologics that selectively deplete eosinophils may be the best way to directly answer this question.
Eosinophil deficiency in humans
Despite multiple murine models demonstrating viability and reproductive capability of mice lacking eosinophils (12, 28), congenital eosinophil deficiency has not been described in humans to date. Although this may be due to underreporting in the absence of characteristic clinical features, an analysis of blood smears from 24,300 patients at University of Pittsburgh found no cases of unexplained eosinopenia (<1 eosinophil per 1,000 cells counted) (76). Rare cases of acquired eosinophil deficiency have been reported, most commonly in patients with thymoma and agammaglobulinemia (Good’s syndrome) (77), and do not appear to be associated with specific clinical features (78).
Hereditary abnormalities involving eosinophil granule proteins are also uncommon. Specific granule deficiency (SGD) is a rare primary immunodeficiency in which mutations in CEBPE, the gene encoding CCAAT/enhancer-binding protein-ε (C/EBPε), or SMARCD2, which encodes a factor that interacts with C/EBPε, lead to impaired transcription of granule components in neutrophils and eosinophils (79). Patients with SGD present with recurrent bacterial and fungal infections attributed to impaired neutrophil differentiation and function. Eosinophils from patients with SGD are deficient in three major components of eosinophil secondary granules (ECP, MBP and EDN) but do contain eosinophil peroxidase and respond to stimulation with GM-CSF (80). The clinical consequences of the eosinophil abnormalities in SGD are unknown. Abnormal eosinophil granule morphology without apparent clinical manifestations is also characteristic of the nearly 100 reported cases of hereditary eosinophil peroxidase deficiency (81).
FURTHER INSIGHTS ON EOSINOPHIL BIOLOGY FROM EXPERIMENTAL MODELS
Regulation of tissue remodeling and fibrosis
While it is clear that most eosinophil-associated disorders involve some sort of pathologic tissue remodeling and fibrosis, whether the eosinophil is directly contributory or is “guilty by association” is less clear. The most compelling data come from studies employing eosinophil-deficient and other genetically modified mouse strains, where roles in tissue remodeling associated with both normal physiologic processes and disease pathogenesis have been seen (Figures 5 and 6) (2). Eosinophils appear to be a major source of the profibrotic cytokine TGF-β in the allergic inflamed lung and in EoE (82–84). In addition, eosinophil granule cationic proteins have profibrogenic activities both in vitro (on fibroblasts and epithelial cells) and in vivo in mouse models (85). Eosinophil granule proteins induce production of IL-6 and related fibrogenic cytokines from fibroblasts, fibroblast proliferation and trans-differentiation to myofibroblasts, fibroblast-mediated collagen gel contraction, and expression of various matrix metalloproteinases involved in fibrogenesis (85, 86).
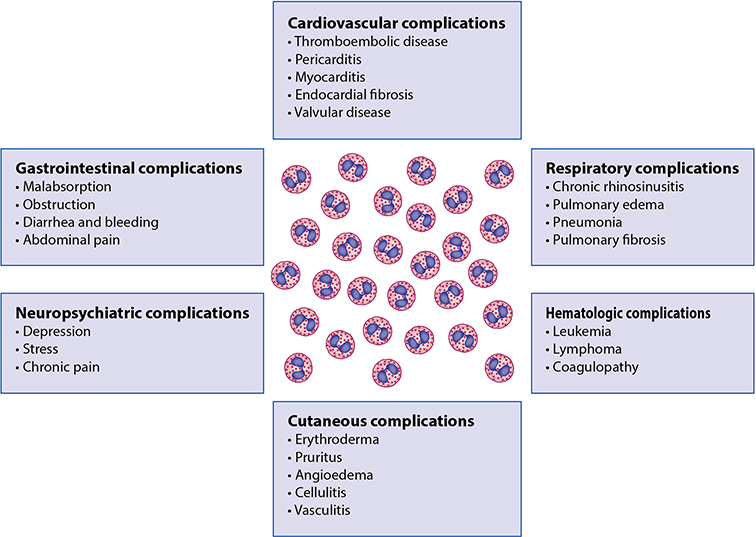
Figure 6.
Roles of eosinophils in disease pathogenesis. Contributions of eosinophils to complications of various diseases as separated by organ involvement that can occur independent of underlying disease pathogenesis.
Regulation of metabolism, adipose tissue and glucose homeostasis
A rather unexpected role for mouse eosinophils and their production of IL-4 emerged from a series of publications demonstrating an important role in adipose tissue and obesity by sustaining alternatively activated M2 macrophages, glucose homeostasis and the development of beige fat (87–90). Of note, this eosinophil/macrophage collaboration is regulated in part by ILC2s, which serve to sustain both the adipose eosinophils and the alternatively activated M2 macrophages (91). A recent study demonstrated that cross talk between the inhibitory receptor CD300f and IL-5 functionally modifies eosinophil regulation of metabolism (92). Whereas a convincing role has emerged for mouse eosinophils in regulating metabolic functions and adiposity, whether these findings will translate to human eosinophils requires further investigation.
Regulation of other immune responses
Eosinophils in the mouse bone marrow have been reported to secrete a number of survival factors including the plasma cell proliferation-inducing ligands APRIL and IL-6 that promote maintenance of the plasma cell niche (93). Subsequent studies showed that eosinophils promoted class switching toward secretory IgA, and were required for the development and maintenance of IgA-producing plasma cells (94, 95). Eosinophil deficiency was also associated with altered composition of gastrointestinal microbiota, altered development of Peyer’s patches, and decreased mucus production in the small intestine (96). However, two subsequent studies using ΔdblGATA eosinophil-deficient mice found that eosinophils were dispensable for the survival of plasma cells in the bone marrow and did not contribute to IgA antibody production or autoantibody-mediated disease (97, 98).
A potential role for eosinophil regulation of B cells has been proposed based on in vitro data showing that there is an IL-5-independent and cell-cell contact-independent but eosinophil-dependent enhancement of B cell proliferation and survival and a modest correlation between the number of circulating eosinophils and B cells in patients with HES (99). The demonstration of MHC class II expression on both mouse and human eosinophils and their ability to present antigen to T cells suggest that eosinophils may also play a role in antigen presentation (100–102). Despite these data, the relative contribution of human eosinophils to each of these processes has be difficult to define.
Roles for eosinophils in disease (Figure 6)
Definitions
Peripheral blood eosinophilia is generally defined as an absolute eosinophil count (AEC) ≥500/μL, although normal levels may vary depending on the patient population and method of quantification. An AEC ≥1,500/μL is considered marked peripheral eosinophilia (or hypereosinophilia). Tissue eosinophilia and hypereosinophilia are much more difficult to define as consensus guidelines have not been established for most tissues, and eosinophils themselves may be absent despite marked tissue deposition of eosinophil granule proteins consistent with eosinophilic inflammation. For the purposes of this chapter, HES will be defined according to a consensus definition developed by a multispecialty group of experts as 1) AEC ≥1,500/μL and clinical manifestations attributable to the eosinophilia or 2) tissue hypereosinophilia with blood eosinophilia (AEC above the upper limit of normal for the reference laboratory) (103). Of note, this definition does not distinguish between eosinophilia that is idiopathic or secondary to a known cause.
The definition of eosinophil-related diseases that do not meet the criteria for HES has evolved over the past decade (104, 105). These tend to be disorders where increased numbers of eosinophils in blood and/or tissues are felt to cause pathology, but often the eosinophilia or eosinophilic inflammation does not occur in isolation. Examples include common and uncommon human disorders, ranging from allergic conditions like asthma and atopic dermatitis, to eosinophilic gastrointestinal disorders (EGID), EGPA, bullous pemphigoid (Figure 7) and others. Due to the availability of eosinophil-targeted therapies, defining the role of eosinophils in eosinophil-related disorders may finally be feasible.
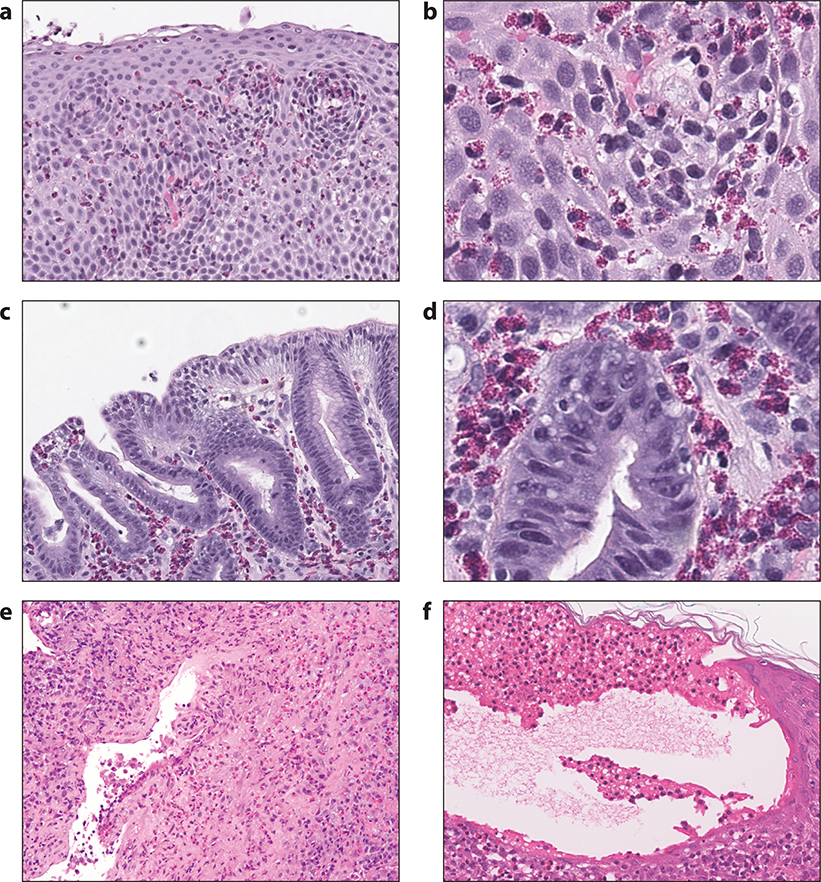
Figure 7.
Histologic findings in EGID, EGPA and bullous pemphigoid. Panels a and b: hematoxylin and eosin stained section of a biopsy from a patient with eosinophilic esophagitis, at both lower and higher power magnification, showing increased intraepithelial eosinophils, epithelial spongiosis and basal cell hyperplasia; Panels c and d: hematoxylin and eosin stained section of a biopsy from a patient with eosinophilic gastritis, at both lower and higher power magnification, showing increased eosinophils in the gastric lamina propria; Panel e: hematoxylin and eosin stained section of a lung biopsy from a patient with EGPA showing a dense interstitial infiltrate rich in eosinophils, lymphocytes and plasma cells involving a vessel wall with focal fibrinous changes; Panel f: hematoxylin and eosin stained section of a skin biopsy from a patient with bullous pemphigoid showing a sub-epidermal blister with numerous eosinophils aligned along the cutaneous basement membrane zone. Also present is significant epidermal edema (spongiosis) with a few intraepithelial eosinophils.
Clinical subtypes of HES
As defined above, HES comprises a diverse group of disorders related only by the presence of markedly increased numbers of blood and/or tissue eosinophils and evidence of eosinophil-mediated pathology. In an attempt to address this, a number of clinical subtypes have been described based on likely etiology and approach to management (106, 107).
1. Myeloid HES
Approximately 15–20% of patients who present with HES have definitive or presumptive evidence of a primary myeloid neoplasm. Of these, the vast majority (≥ 80% in most series) have an interstitial deletion in chromosome 4 giving rise to the fusion gene, FIP1L1-PDGFRA. Prior to the availability of imatinib, these patients had a very poor prognosis with a 30–50% 5-year mortality primarily due to the development of endomyocardial fibrosis and thromboembolic events. Imatinib response rates approach 100% and recent data suggests that a significant proportion of patients with this fusion gene may be cured after prolonged molecular remission (108). Other genetic abnormalities that can give rise to myeloid HES include PFGFRB and FGFR1 fusion genes as well as point mutations and translocations involving JAK2. The spectrum of myeloid HES also includes “chronic eosinophilic leukemia, not otherwise specified,” and patients without an identifiable mutation who have clinical and bone marrow characteristics suggestive of a myeloid neoplasm, including eosinophil dysplasia (Figure 8), involvement of other lineages, elevated serum vitamin B12 and/or tryptase levels, and splenomegaly (109). Myeloid HES involving abnormalities in PDGFR is almost exclusively seen in males, whereas other molecular phenotypes and idiopathic myeloid HES do not appear to have a gender preference.
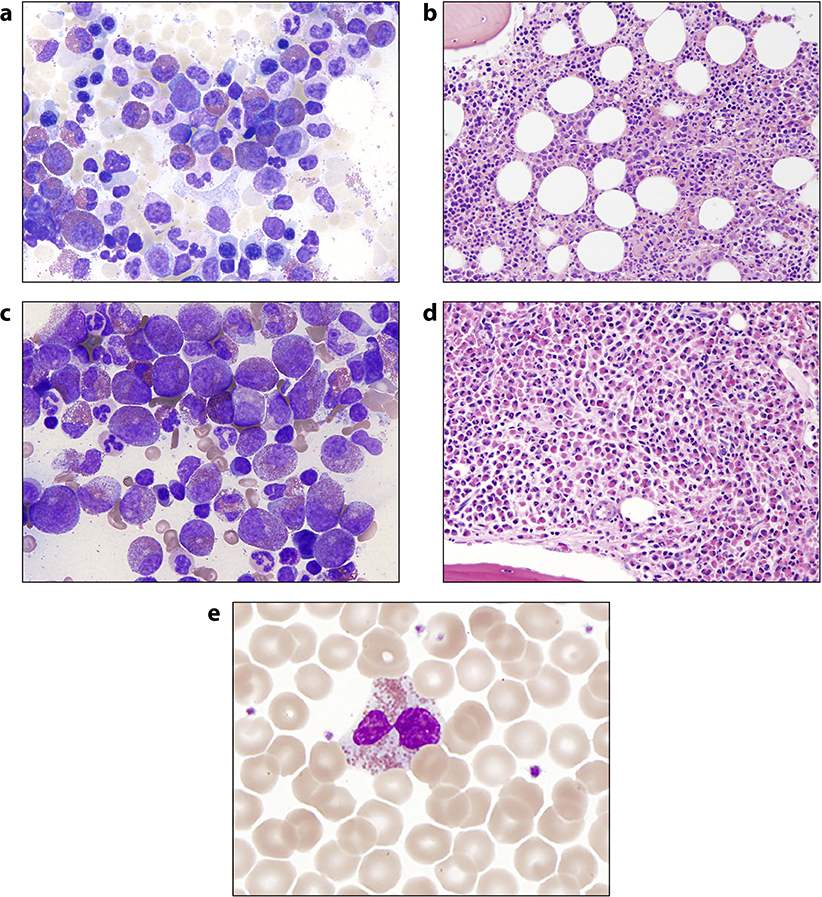
Figure 8.
Bone marrow and cytopathologic findings in HES. Giemsa-stained bone marrow aspirate and hematoxylin and eosin-stained bone marrow biopsy from a patient with idiopathic hypereosinophilic syndrome (Panels a and b, respectively) and FIP1L1-PDGFRA positive myeloid neoplasm (Panels c and d, respectively); Panel e: an example of a dysplastic eosinophil seen on a peripheral blood smear from a patient with HES that was accidentally mis-identified as a neutrophil in an electronic differential blood count.
2. Lymphocytic HES
Lymphocytic HES refers to patients with HES and the presence of a clonal and/or phenotypically aberrant T cell clone that secretes IL-5 or other cytokines that drive the eosinophilia (110). The most common abnormal T cell phenotype is CD3-CD4+. Similar to myeloid HES, this clinical subtype is a spectrum, ranging from an indolent lymphoproliferative syndrome to frank lymphoma. Skin manifestations appear to be most common, although any organ can be involved. Serum levels of IgE and the chemokine TARC (CCL17) are usually elevated. Up to 30% of patients with lymphocytic HES and no evidence of malignancy will ultimately develop lymphoma. This may be preceded by a change in the peripheral clonal population (increase or decrease) or a new cytogenetic abnormality.
Episodic angioedema with eosinophilia (Gleich’s syndrome) is an unusual HES variant characterized by the monthly occurrence of eosinophilia, neutrophilia, lymphocytosis, angioedema, urticaria and systemic symptoms that resolves spontaneously between episodes (111). Although CD3-CD4+ T cell clones are also detectable in the majority of patients with this syndrome and serum IL-5 levels (as well as a number of other soluble mediators) cycle, the role of the aberrant T cells in this multilineage disorder is unclear.
3. Overlap HES
The term “overlap HES” is used to denote single organ eosinophilic disorders, including EGID and eosinophilic fasciitis, and recognized multisystem eosinophilic syndromes with characteristic clinical features, such as EGPA. These disorders are distinguished from other forms of HES because of the collection of existing data, including specific approaches to treatment and prognostic factors. Conversely, they are included under the broad umbrella of HES because eosinophils are believed to play a primary role in disease pathogenesis. Moreover, the clinical presentation may be difficult to distinguish from that of other forms of HES.
4. Associated HES
Associated HES refers to HES in the setting of a defined cause for which treatment is not directed at the underlying eosinophilia, including parasitic infections, drug hypersensitivity, solid tumors, and primary immunodeficiency syndromes. Although it is beyond the scope of this review to discuss the broad list of secondary causes of HES, it should be noted that the clinical manifestations of hypereosinophilia can be identical irrespective of the cause. Secondary treatable causes of HES need to be considered and excluded in all patients presenting with AEC ≥1,500/μL.
5. Familial hypereosinophilia
Although familial clustering has been reported in EGID and EGPA (112, 113), clearly defined genetic transmission of hypereosinophilia has been described in only a handful of families. The best described is a large multigenerational cohort with autosomal dominant transmission mapped to a region on chromosome 5q31–33 that contains the IL-5 cytokine cluster. Despite hypereosinophilia from birth, most affected family members have remained completely asymptomatic. Although the genetic abnormality remains obscure, recent studies suggest that selective overexpression of IL-5 is responsible for driving the eosinophilia in this family (114).
6. Idiopathic HES
As diagnostic methods and our understanding of the mechanisms driving eosinophilia improve, the proportion of patients that cannot be classified into one of the above categories (i.e., with idiopathic HES) continues to decrease. That said, these patients represent a heterogenous mix with clinical manifestations ranging from relatively mild to life-threatening. Any organ can be affected, although skin, gastrointestinal tract, and pulmonary involvement are most common.
Therapeutic considerations in HES
Corticosteroids remain the first line therapy for most eosinophil-associated disorders, including HES, although long-term use is associated with significant toxicity and some patients do not respond (115). The exceptions are patients with myeloid HES who have targetable mutations or rearrangements, including FIP1L1-PDGFRA and translocations in PDGFRB. Conventional second-line therapies include hydroxyurea, interferon-α, imatinib (for patients with suspected myeloid HES) and methotrexate (116). The choice of second line agent typically depends on clinical subtype, concomitant medical issues, cost and patient and physician preference. Response rates to second line therapies vary and discontinuation of therapy is common due to lack of efficacy and side effects. Novel targeted agents with improved efficacy and toxicity profiles are desperately needed. Several such agents are currently FDA-approved and/or in clinical development for the treatment of eosinophilic disorders (Table 1).
Table 1.
Eosinophil-targeted therapies approved or in clinical development.
| Mepolizumab | Reslizumab | Benralizumab | AK002 | Dexpramipexole | |
|---|---|---|---|---|---|
| Target | IL-5 | IL-5 | IL-5R α | Siglec-8 | unknown |
| Antibody (parent) | Humanized IgG1κ (murine 2B6) | Humanized IgG4κ (rat 39D10) | Humanized afucosylated IgG1κ | Humanized non-fucosylated IgG1 | - |
| Max Dose in Clinical Trials | 10 mg/kg iv 300 mg sc |
3 mg/kg iv | 3 mg/kg iv 200 mg sc |
3 mg/kg iv | 300 mg orally/day |
| Approved Indications | Severe Eosinophilic Asthma (100 mg sc monthly) EGPA (300 mg sc monthly) |
Severe Eosinophilic Asthma (3 mg/kg iv monthly) | Severe Eosinophilic Asthma (30 mg sc monthly × 3 months and then every 2 months) | None | None |
| Pediatric Approval | >12 years of age | No | >12 years of age | No | No |
| Studies in multisystem HES | Phase 2 completed, phase 3 ongoing | Phase 2 completed | Phase 2 completed, Phase 3 planned | None | Phase 2 completed, Phase 3 planned |
| Studies in EGID | Phase 2 in EoE completed | Phase 2 in EoE completed | Phase 2 in eosinophilic gastritis ongoing | Phase 2 in eosinophilic gastritis and gastroenteritis ongoing | None |
| Studies in EGPA | Phase 3 completed | Phase 2 ongoing | Phase 2 ongoing | None | None |
| In vivo Effects on Target Cells | |||||
| Peripheral eosinophils | Profound reduction | Profound reduction | Complete depletion | Complete depletion (published abstract in JACI 2018) | Complete depletion |
| Tissue eosinophils | Partial depletion | Partial depletion | Complete depletion | NA | Complete depletion |
| Eosinophil precursors | Maturational arrest | NA | Complete depletion | NA | Maturational arrest |
| Basophils | NA | NA | Reduction | NA | Reduction |
| Mast cells | No effect | No effect | No effect | NA | No effect |
Note: therapies that target mutations associated with eosinophilic myeloid neoplasms, including the tyrosine kinase inhibitor imatinib, are not included in this table
NA = published data not available
The first randomized, placebo-controlled, double-blind clinical trial of a therapy for HES was conducted more than a decade ago with mepolizumab (750 mg iv monthly) and demonstrated that blocking IL-5 was well-tolerated and effective as an oral steroid-sparing agent in the treatment of steroid-responsive, PDGFRA-negative HES (117). Although this trial did not lead to FDA-approval of mepolizumab for HES, it provided a proof of principle. Subsequent trials confirmed the efficacy of mepolizumab in the treatment of eosinophilic asthma (at 100 mg sc monthly) and EGPA (at 300 mg sc monthly) and resulted in FDA-approval for these indications. Approval of reslizumab (3 mg/kg iv monthly) and benralizumab (30 mg sc monthly for 3 months followed by 30 mg sc every 2 months) for the treatment of eosinophilic asthma followed shortly thereafter.
As with other therapies, there appears to be considerable variability in the response to agents targeting IL-5 in patients with HES. For example, despite an 85% response rate in patients with systemic HES, mepolizumab has shown limited efficacy in the treatment of eosinophilic esophagitis. A similar lack of efficacy has been seen with reslizumab (118). Whether this is due to the lack of complete eosinophil depletion in tissue, involvement of other cells, such as mast cells, in the pathology, or issues with trial design (length of therapy, outcome measures) is unknown. Recent data examining high dose mepolizumab treatment of patients with life-threatening HES on a compassionate use protocol suggest that clinical subtype is an important factor in response to anti-IL-5 therapy (119). A phase 3 study of mepolizumab (300 mg sc monthly) is currently underway. Other biologics that target eosinophils currently in clinical development for the treatment of HES include benralizumab and AK002 in eosinophilic gastritis (a novel antibody targeting Siglec-8, a receptor on the surface of eosinophils and mast cells).
Whereas biologics account for the overwhelming majority of eosinophil-targeted agents in clinical development, safe, well-tolerated and effective oral agents for the treatment of HES would be highly desirable. Dexpramipexole, an oral agent developed for the treatment of amyotrophic lateral sclerosis and repurposed for the treatment of HES, shows promise in this regard. In a recent open-label phase 2 trial, 4/10 subjects with corticosteroid-responsive HES were able to taper their corticosteroid dose by ≥ 50% while on dexpramipexole (120). Dramatic reductions of both blood and tissue eosinophilia were observed in responders, concomitant with evidence of maturation arrest of eosinophil lineage development in the bone marrow. Only mild and transient treatment-related side effect were observed.
The availability of novel therapies that dramatically reduce blood and tissue eosinophilia has provided a unique opportunity to examine the side effects of acquired eosinopenia in humans. To date, there have been no reports of adverse consequences of eosinophil depletion in patients treated with therapies that specifically target eosinophils, including mepolizumab, reslizumab and benralizumab, despite the availability of some of these agents for almost two decades. Side effects have generally been mild; rare cases of anaphylaxis are reported. Although two cases of shingles occurred in patients receiving mepolizumab in pivotal clinical trials versus none in patients receiving placebo and herpes zoster vaccination is recommended in the package insert for this agent, the lack of an association between shingles and either reslizumab or benralizumab therapy suggests that eosinophil depletion is not the underlying mechanism.
Few human studies have directly examined homeostatic mechanisms affected by eosinophil depletion, unlike in murine models. These include a study of recall responses to immunization with tetravalent influenza vaccine in 103 patients enrolled in a placebo-controlled study of benralizumab (121) and assessment of B cell responses following rhinovirus challenge in 28 patients with eosinophilic asthma enrolled on a placebo-controlled trial of mepolizumab (71). In neither instance was eosinophil depletion detrimental. In fact, mepolizumab appeared to enhance B cell function and secretory IgA production in response to rhinovirus challenge (71).
UNMET NEEDS AND OPPORTUNITIES FOR EXPANDED UNDERSTANDING
Biomarkers for diagnosis and prognosis
As pointed out by expert panels, the need for biomarkers in the assessment of diagnosis, prognosis, choice of treatment, and disease severity and activity remains a hugely important unmet need in eosinophil-related diseases (104, 105). Fortunately, there are a few examples of highly useful diagnostic tests for the diagnosis of eosinophilic-related disorders, such as detecting the FIP1L1-PDGFRA fusion gene in blood or bone marrow cells in a subset of patients with HES; serum ANCA positivity in a minor subset of patients with EGPA; elevated serum levels of vitamin B12 and tryptase and dysplastic eosinophils seen in the myeloid variant of HES; and the finding, by flow cytometric immunophenotyping of whole blood, of aberrant T cell clones in the lymphocytic variant of HES (Figure 8) (106). With the exception of loss of detectable FIP1L1-PDGFRA during remission following treatment with imatinib (108, 122), what is urgently needed are tests to assess disease activity or that predict treatment responsiveness to a given agent. This deficiency is not due to lack of trying, as there are plenty of examples of failed efforts to find such biomarkers. For instance, measurements of eosinophil activation markers, both on the cell surface as well as levels in the serum of soluble proteins originating from the cell surface, such as the IL-5 receptor α subunit and Siglec-8, have so far not proven to be clinically useful (57, 123). Levels of chemokines, such as eotaxin-3 (CCL26) may be associated with mucosal inflammation in chronic eosinophilic rhinosinusitis (124) but in EGPA, their utility as a biomarker of disease remains controversial. So far, attempts to find serum biomarkers for eosinophilic esophagitis and gastritis, including measures of sizable panels of cytokines and chemokines, have been disappointing, even though the latter is much more frequently associated with peripheral blood eosinophilia. More promising is the use of a gene panel for analysis of biopsy material in eosinophilic esophagitis, where it has so far proven to be highly accurate in distinguishing disease from controls, and thus might be useful for following disease activity over time (125, 126). Regarding eosinophil-related disorders that especially affect the skin, CCL17 (TARC) is more commonly elevated in those with the lymphocytic variant of HES (127). In bullous pemphigoid (Figure 7), serum levels of anti-hemi-desmosomal protein antibodies, cytokines, chemokines, and other substances may be somewhat useful as biomarkers to assess disease severity or risk of relapse, but are far from optimal (128). Clearly, biomarkers beyond tracking the AEC are needed, including those that predict disease relapse and organ specificity of disease involvement and activity.
Less/minimally invasive biomarkers of disease activity and remission
Although one might expect that eosinophil-derived proteins, like the granule cationic proteins (EPX, MBP1, EDN, ECP) or CLC/Galectin-10, would serve as excellent peripheral biomarkers of eosinophil activation and secretion locally at tissue sites of allergic eosinophil-dominant inflammation, including host responses to helminth infestations. However, quantitative measurement of these proteins in blood has generally failed to be sufficiently sensitive and specific to be clinically useful for disease diagnosis or monitoring patient responses to treatment. This is likely because most of these granule cationic proteins bind strongly to negatively charged tissue elements with long half-live and thus fail to enter the peripheral circulation (129, 130). In fact, the peripheral blood AEC has been shown to correlate better with tissue eosinophilia in a number of biomarker studies assessing the utility of serum granule protein levels (131–133).
In asthma, although the eosinophilic phenotype can be identified through invasive bronchoalveolar lavage, quantitation of eosinophils in induced sputum currently serves this role, with ≥2% sputum eosinophils being considered diagnostic for eosinophilic asthma (134). However, performance of sputum eosinophil counts is both laborious and fraught with considerable lab-to-lab and patient-to-patient variability. Fortunately, efforts to develop rapid immunoassays, such as measurement of EPX or CLC/Galectin-10 in induced sputum extracts (135, 136), are showing considerable clinical promise and utility for identifying patients with eosinophilic asthma for targeted therapy with anti-eosinophilic agents.
To date, no single or panel of peripheral blood biomarkers has been identified that can reliably distinguish patients with active EoE from those with inactive (or successfully treated) EoE, patients with GERD from those with EoE, or even patients with EoE from healthy controls. Consequently, EoE patients are currently diagnosed and monitored with repeat endoscopy with biopsies. With the goal of developing a minimally invasive method for following mucosal eosinophilic inflammation in EoE, a novel capsule-based technology, the Esophageal String Test (EST)™, has been developed that captures a liquid biopsy containing esophageal luminal secretions, inflammatory and epithelial cells from the entire length of the esophagus, with quantitative measurement of eosinophil-associated protein biomarkers, CLC/Galectin-10 (137) and eotaxin-3 (138). The overnight EST showed considerable sensitivity and specificity comparable to histologic eosinophil counts in biopsies and the same biomarkers measured in biopsy extracts, and a clinically convenient 1-hr EST is currently being evaluated in a Phase-2 clinical validation study (138). Similar minimally invasive capsule-based devices, such as the Cytosponge™, have also shown promise in EoE, but may be restricted to use in adults due to the size of the capsule and swallowing difficulties for pediatric patients (139, 140).
Comparisons among available biologics
There are no data directly comparing the efficacy or safety of available biologics that target eosinophils in patients with eosinophil-related disorders. A meta-analysis of the clinical trial data from five studies of mepolizumab and reslizumab for the treatment of eosinophilic asthma found no differences in efficacy or safety by indirect comparison (141). A more recent indirect analysis of 11 published studies compared clinically significant impact on asthma exacerbations between mepolizumab, reslizumab and benralizumab and concluded that mepolizumab was more effective than either of the other two therapies (142). Theoretical differences between the three biologics include mode of administration (benralizumab and mepolizumab are approved as subcutaneous injections whereas reslizumab is administered intravenously), dosing (fixed dosing for benralizumab and mepolizumab versus weight-based dosing for reslizumab), and degree of depletion of tissue eosinophils (partial for mepolizumab and reslizumab versus more complete for benralizumab). Head-to-head comparisons of these three biologics in patients with the various eosinophil-related disorders are clearly needed to sort out these issues.
Long term safety of targeting eosinophils
Despite the lack of any worrisome safety signals to date, the effects of long-term depletion of eosinophils remain unknown. Whereas pharmacovigilance is clearly needed as these drugs are used in larger and more diverse populations (including populations in countries endemic for helminth infection), carefully designed clinical studies to assess the impact of eosinophil depletion on homeostatic mechanisms, including immune responses, tumor surveillance, metabolic pathways, and tissue remodeling, are needed.
CLOSING REMARKS
In the roughly 150 years since its discovery, the role of the eosinophil in health and disease has evolved tremendously. Just in the last decade or so, major developments in mouse models, especially those in which eosinophils are congenitally or conditionally absent, have shed light on both expected and unexpected roles for these cells in health. On the clinical side, the ability to selectively target eosinophils using the precision of approved biological therapies has helped to firmly cement our long-suspected role of the eosinophil in human asthma pathogenesis, especially asthma exacerbations, as well as EGPA. Ongoing clinical studies offer the potential to expand this list to include other eosinophil-associated skin diseases, including chronic rhinosinusitis with or without nasal polyps, EGID, HES and others. At the same time, there remains a need for novel eosinophil-targeted agents, including those that have the potential to be disease-modifying. Biomarkers other than AEC that will assist the physician in more confidently assessing the diagnosis, prognosis, choice of best treatment, disease severity, disease activity and risk of relapse would be a welcome addition to clinical practice. Also needed are head-to-head comparisons of anti-eosinophil therapies in patients suffering from various eosinophil-related disorders, with the goal of optimizing best care for each condition. Finally, continued monitoring for the emergence of any safety signals associated with long-term reductions of eosinophils remains important, and at the same time may advance our understanding of the unique contribution of the eosinophil to human health.
DISCLOSURE STATEMENT
Dr. Klion reports no conflicts of interest. Dr. Ackerman is a co-founder, chief scientific officer, board member, consultant, and holds equity in EnteroTrack, LLC, and is entitled to a share of royalties from the University of Illinois at Chicago/University of Colorado in conjunction with licensing of intellectual property to EnteroTrack, LLC. Dr. Bochner is a co-founder, scientific advisory board member and stockholder of Allakos, Inc. and is entitled to a share of royalties from Johns Hopkins University in conjunction with the licensing of intellectual property to Allakos, Inc. He also receives royalties for his role as an editor for UpToDate™ and Elsevier.
ACKNOWLEDGMENTS
The authors thank Jacqueline Schaffer for the numerous illustrations, and Drs. Guang-Yu Yang (Northwestern University Feinberg School of Medicine), Irina Maric (Department of Laboratory Medicine, Clinical Center, National Institutes of Health), Stefania Pittaluga (Laboratory of Pathology, National Cancer Institute, National Institutes of Health) Yi-Hua Chen (Northwestern University Feinberg School of Medicine) and Kyle Amber (University of Illinois College of Medicine) for providing photomicrographs.
This work was supported in part by funds from the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health (to ADK), grants from the Food and Drug Administration (R01FD004086) and the End Allergies Together Foundation (to SJA) and grants AI072265, AI105839, AI136443 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (to BSB).
LITERATURE CITED
- 1.McGarry MP. 2012. The evolutionary origins and presence of eosinophils in extant species. In Eosinophils in Health and Disease, ed. Lee JJ, Rosenberg HF, pp.13–8. Amsterdam: Elsevier [Google Scholar]
- 2.Abdala-Valencia H, Coden ME, Chiarella SE, Jacobsen EA, Bochner BS, et al. 2018. Shaping eosinophil identity in the tissue contexts of development, homeostasis, and disease. J. Leukoc. Biol. 104(1):95–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. 2010. Eosinophils in health and disease: the LIAR hypothesis. Clin. Exp. Allergy 40(4):563–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weller PF, Spencer LA. 2017. Functions of tissue-resident eosinophils. Nat. Rev. Immunol. 17(12):746–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klion A 2017. Recent advances in understanding eosinophil biology. F1000Res 61084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg HF, Dyer KD, Foster PS. 2013. Eosinophils: changing perspectives in health and disease. Nat. Rev. Immunol. 13(1):9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bochner BS. 2018. The Eosinophil: for better or worse, in sickness and in health. Ann. Allergy Asthma Immunol. 121150–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kopf M, Brombacher F, Hodgkin PD, Ramsay AJ, Milbourne EA, et al. 1996. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity 4(1):15–24 [DOI] [PubMed] [Google Scholar]
- 9.Mori Y, Iwasaki H, Kohno K, Yoshimoto G, Kikushige Y, et al. 2009. Identification of the human eosinophil lineage-committed progenitor: revision of phenotypic definition of the human common myeloid progenitor. J. Exp. Med. 206(1):183–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNagny K, Graf T. 2002. Making eosinophils through subtle shifts in transcription factor expression. J. Exp. Med. 195(11):F43–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirasawa R, Shimizu R, Takahashi S, Osawa M, Takayanagi S, et al. 2002. Essential and instructive roles of GATA factors in eosinophil development. J. Exp. Med. 195(11):1379–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu C, Cantor AB, Yang H, Browne C, Wells RA, et al. 2002. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J. Exp. Med. 195(11):1387–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du J, Stankiewicz MJ, Liu Y, Xi Q, Schmitz JE, et al. 2002. Novel combinatorial interactions of GATA-1, PU.1, and C/EBPepsilon isoforms regulate transcription of the gene encoding eosinophil granule major basic protein. J. Biol. Chem. 277(45):43481–94 [DOI] [PubMed] [Google Scholar]
- 14.Ackerman SJ, Du J. 2013. Transcriptional Regulation of Eosinophil Lineage Commitment and Differentiation In Eosinophils in Health and Disease, ed. Lee JJ, Rosenberg HF, pp.76–89. London: Elsevier [Google Scholar]
- 15.Bedi R, Du J, Sharma AK, Gomes I, Ackerman SJ. 2009. Human C/EBP-epsilon activator and repressor isoforms differentially reprogram myeloid lineage commitment and differentiation. Blood 113(2):317–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamanaka R, Lekstrom-Himes J, Barlow C, Wynshaw-Boris A, Xanthopoulos KG. 1998. CCAAT/enhancer binding proteins are critical components of the transcriptional regulation of hematopoiesis (Review). Int. J. Mol. Med. 1(1):213–21 [DOI] [PubMed] [Google Scholar]
- 17.Wagner LA, Christensen CJ, Dunn DM, Spangrude GJ, Georgelas A, et al. 2007. EGO, a novel, noncoding RNA gene, regulates eosinophil granule protein transcript expression. Blood 109(12):5191–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu TX, Lim EJ, Besse JA, Itskovich S, Plassard AJ, et al. 2013. MiR-223 deficiency increases eosinophil progenitor proliferation. J. Immunol. 190(4):1576–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu TX, Lim EJ, Itskovich S, Besse JA, Plassard AJ, et al. 2013. Targeted ablation of miR-21 decreases murine eosinophil progenitor cell growth. PLoS One 8(3):e59397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fulkerson PC. 2017. Transcription factors in eosinophil development and as therapeutic targets. Front. Med. (Lausanne) 4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. 1996. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J. Exp. Med. 183(1):195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamann J, Koning N, Pouwels W, Ulfman LH, van Eijk M, et al. 2007. EMR1, the human homolog of F4/80, is an eosinophil-specific receptor. Eur. J. Immunol. 37(10):2797–802 [DOI] [PubMed] [Google Scholar]
- 23.Legrand F, Tomasevic N, Simakova O, Lee CC, Wang Z, et al. 2014. The eosinophil surface receptor epidermal growth factor-like module containing mucin-like hormone receptor 1 (EMR1): a novel therapeutic target for eosinophilic disorders. J. Allergy Clin. Immunol. 133(5):1439–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews AN, Friend DS, Zimmerrmann N, Sarafi MN, Luster AD, et al. 1998. Eotaxin is required for the baseline level of tissue eosinophils. Proc. Natl. Acad. Sci. USA 95(11):6273–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, et al. 2006. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J. Clin. Invest. 116(2):536–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stacy NI, Raskin RE. 2015. Reptilian eosinophils: beauty and diversity by light microscopy. Vet. Clin. Pathol. 44(2):177–8 [DOI] [PubMed] [Google Scholar]
- 27.Balla KM, Lugo-Villarino G, Spitsbergen JM, Stachura DL, Hu Y, et al. 2010. Eosinophils in the zebrafish: prospective isolation, characterization, and eosinophilia induction by helminth determinants. Blood 116(19):3944–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, et al. 2004. Defining a link with asthma in mice congenitally deficient in eosinophils. Science 305(5691):1773–6 [DOI] [PubMed] [Google Scholar]
- 29.Doyle AD, Jacobsen EA, Ochkur SI, McGarry MP, Shim KG, et al. 2013. Expression of the secondary granule proteins major basic protein 1 (MBP-1) and eosinophil peroxidase (EPX) is required for eosinophilopoiesis in mice. Blood 122(5):781–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JJ, Jacobsen EA, Ochkur SI, McGarry MP, Condjella RM, et al. 2012. Human versus mouse eosinophils: “that which we call an eosinophil, by any other name would stain as red”. J. Allergy Clin. Immunol. 130(3):572–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Acharya KR, Ackerman SJ. 2014. Eosinophil granule proteins: form and function. J. Biol. Chem. 289(25):17406–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Dyer KD, Rosenberg HF. 2000. Evolution of the rodent eosinophil-associated RNase gene family by rapid gene sorting and positive selection. Proc. Natl. Acad. Sci. USA 97(9):4701–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malm-Erjefalt M, Persson CG, Erjefalt JS. 2001. Degranulation status of airway tissue eosinophils in mouse models of allergic airway inflammation. Am. J. Respir. Cell Mol. Biol. 24(3):352–9 [DOI] [PubMed] [Google Scholar]
- 34.Persson C, Uller L. 2014. Theirs but to die and do: primary lysis of eosinophils and free eosinophil granules in asthma. Am. J. Respir. Crit. Care Med. 189(6):628–33 [DOI] [PubMed] [Google Scholar]
- 35.Farahi N, Loutsios C, Simmonds RP, Porter L, Gillett D, et al. 2014. Measurement of eosinophil kinetics in healthy volunteers. Methods Mol. Biol. 1178165–76 [DOI] [PubMed] [Google Scholar]
- 36.Farahi N, Loutsios C, Tregay N, Wright AKA, Berair R, et al. 2018. In vivo imaging reveals increased eosinophil uptake in the lungs of obese asthmatic patients. J. Allergy Clin. Immunol. 142(5):1659–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Symon FA, Walsh GM, Watson SR, Wardlaw AJ. 1994. Eosinophil adhesion to nasal polyp endothelium is P-selectin-dependent. J. Exp. Med. 180(1):371–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woltmann G, McNulty CA, Dewson G, Symon FA, Wardlaw AJ. 2000. Interleukin-13 induces PSGL-1/P-selectin-dependent adhesion of eosinophils, but not neutrophils, to human umbilical vein endothelial cells under flow. Blood 95(10):3146–52 [PubMed] [Google Scholar]
- 39.Bochner BS. 2000. Road signs guiding leukocytes along the inflammation superhighway. J. Allergy Clin. Immunol. 106817–28 [DOI] [PubMed] [Google Scholar]
- 40.Sturla L, Puglielli L, Tonetti M, Berninsone P, Hirschberg CB, et al. 2001. Impairment of the Golgi GDP-L-fucose transport and unresponsiveness to fucose replacement therapy in LAD II patients. Pediatr. Res. 49(4):537–42 [DOI] [PubMed] [Google Scholar]
- 41.Wun T, Styles L, DeCastro L, Telen MJ, Kuypers F, et al. 2014. Phase 1 study of the E-selectin inhibitor GMI 1070 in patients with sickle cell anemia. PLoS One 9(7):e101301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenberg HF, Phipps S, Foster PS. 2007. Eosinophil trafficking in allergy and asthma. J. Allergy Clin. Immunol. 119(6):1303–10 [DOI] [PubMed] [Google Scholar]
- 43.Muller WA. 2016. Transendothelial migration: unifying principles from the endothelial perspective. Immunol. Rev. 273(1):61–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson DC, Springer TA. 1987. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu. Rev. Med. 38175–94 [DOI] [PubMed] [Google Scholar]
- 45.Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GP, et al. 2003. A controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 348(1):15–23 [DOI] [PubMed] [Google Scholar]
- 46.Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, et al. 2018. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N. Engl. J. Med. 378(26):2475–85 [DOI] [PubMed] [Google Scholar]
- 47.Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. 2009. The binding specificity and selective antagonism of vedolizumab, an anti-α4β7 integrin therapeutic antibody in development for inflammatory bowel diseases. J. Pharmacol. Exp. Ther. 330(3):864–75 [DOI] [PubMed] [Google Scholar]
- 48.Neighbour H, Boulet LP, Lemiere C, Sehmi R, Leigh R, et al. 2014. Safety and efficacy of an oral CCR3 antagonist in patients with asthma and eosinophilic bronchitis: a randomized, placebo-controlled clinical trial. Clin. Exp. Allergy 44(4):508–16 [DOI] [PubMed] [Google Scholar]
- 49.Grozdanovic M, Laffey KG, Abdelkarim H, Hitchinson B, Harijith A, et al. 2019. Novel peptide nanoparticle-biased antagonist of CCR3 blocks eosinophil recruitment and airway hyperresponsiveness. J. Allergy Clin. Immunol. 143(2):669–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilkerson EM, Johansson MW, Hebert AS, Westphall MS, Mathur SK, et al. 2016. The peripheral blood eosinophil proteome. J. Proteome Res. 15(5):1524–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laidlaw TM, Kidder MS, Bhattacharyya N, Xing W, Shen S, et al. 2012. Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood 119(16):3790–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, et al. 2002. Decreased expression of membrane IL-5 receptor alpha on human eosinophils: I. Loss of membrane IL-5 receptor alpha on airway eosinophils and increased soluble IL-5 receptor alpha in the airway after allergen challenge. J. Immunol. 169(11):6452–8 [DOI] [PubMed] [Google Scholar]
- 53.Molfino NA, Kuna P, Leff JA, Oh CK, Singh D, et al. 2016. Phase 2, randomised placebo-controlled trial to evaluate the efficacy and safety of an anti-GM-CSF antibody (KB003) in patients with inadequately controlled asthma. BMJ Open 6(1):e007709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robb CT, Regan KH, Dorward DA, Rossi AG. 2016. Key mechanisms governing resolution of lung inflammation. Semin. Immunopathol. 38(4):425–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kotzin JJ, Spencer SP, McCright SJ, Kumar DB, Collet MA, et al. 2016. The long non-coding RNA Morrbid regulates Bim and short-lived myeloid cell lifespan. Nature 537(7619):239–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Busse WW, Katial R, Gossage D, Sari S, Wang B, et al. 2010. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor alpha antibody, in a phase I study of subjects with mild asthma. J. Allergy Clin. Immunol. 125(6):1237–44 [DOI] [PubMed] [Google Scholar]
- 57.Legrand F, Cao Y, Wechsler J, Zhu X, Zimmermann N, et al. 2018. Siglec-8 in eosinophilic disorders: receptor expression and targeting using chimeric antibodies. J. Allergy Clin. Immunol. 10.1016/j.jaci.2018.10.066 [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rasmussen HS, Chang AT, Tomasevic N, Bebbington C. 2018. A randomized, double-blind, placebo-controlled, ascending dose phase 1 study of AK002, a novel Siglec-8 selective monoclonal antibody, in healthy subjects. J. Allergy Clin. Immunol. 141 AB403 [Google Scholar]
- 59.Ueki S, Tokunaga T, Melo RCN, Saito H, Honda K, et al. 2018. Charcot-Leyden crystal formation is closely associated with eosinophil extracellular trap cell death. Blood 132(20):2183–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Melo RCN, Weller PF. 2018. Contemporary understanding of the secretory granules in human eosinophils. J. Leukoc. Biol. 104(1):85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, et al. 2008. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 14(9):949–53 [DOI] [PubMed] [Google Scholar]
- 62.Kephart GM, Gleich GJ, Connor DH, Gibson DW, Ackerman SJ. 1984. Deposition of eosinophil granule major basic protein onto microfilariae of Onchocerca volvulus in the skin of patients treated with diethylcarbamazine. Lab. Invest. 50(1):51–61 [PubMed] [Google Scholar]
- 63.Huang L, Appleton JA. 2016. Eosinophils in helminth infection: defenders and dupes. Trends Parasitol. 32(10):798–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hagan P, Wilkins HA, Blumenthal UJ, Hayes RJ, Greenwood BM. 1985. Eosinophilia and resistance to Schistosoma haematobium in man. Parasite Immunol. 7(6):625–32 [DOI] [PubMed] [Google Scholar]
- 65.Harley WB, Blaser MJ. 1994. Disseminated coccidioidomycosis associated with extreme eosinophilia. Clin. Infect. Dis. 18(4):627–9 [DOI] [PubMed] [Google Scholar]
- 66.Ueki S, Konno Y, Takeda M, Moritoki Y, Hirokawa M, et al. 2016. Eosinophil extracellular trap cell death-derived DNA traps: Their presence in secretions and functional attributes. J. Allergy Clin. Immunol. 137(1):258–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cohen AJ, Steigbigel RT. 1996. Eosinophilia in patients infected with human immunodeficiency virus. J. Infect. Dis. 174(3):615–8 [DOI] [PubMed] [Google Scholar]
- 68.Percopo CM, Dyer KD, Ochkur SI, Luo JL, Fischer ER, et al. 2014. Activated mouse eosinophils protect against lethal respiratory virus infection. Blood 123(5):743–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Samarasinghe AE, Melo RC, Duan S, LeMessurier KS, Liedmann S, et al. 2017. Eosinophils promote antiviral immunity in mice infected with Influenza A virus. J. Immunol. 198(8):3214–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bjerregaard A, Laing IA, Backer V, Fally M, Khoo SK, et al. 2017. Clinical characteristics of eosinophilic asthma exacerbations. Respirology 22(2):295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sabogal Pineros YS, Bal SM, van de Pol MA, Dierdorp BS, Dekker T, et al. 2019. Anti-IL-5 in mild asthma alters rhinovirus-induced macrophage, B-cell, and neutrophil responses (MATERIAL). A placebo-controlled, double-blind study. Am. J. Respir. Crit. Care Med. 199(4):508–17 [DOI] [PubMed] [Google Scholar]
- 72.Driss V, Legrand F, Hermann E, Loiseau S, Guerardel Y, et al. 2009. TLR2-dependent eosinophil interactions with mycobacteria: role of alpha-defensins. Blood 113(14):3235–44 [DOI] [PubMed] [Google Scholar]
- 73.Buonomo EL, Cowardin CA, Wilson MG, Saleh MM, Pramoonjago P, Petri WA Jr. 2016. Microbiota-regulated IL-25 increases eosinophil number to provide protection during Clostridium difficile infection. Cell Rep. 16(2):432–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Varricchi G, Galdiero MR, Loffredo S, Lucarini V, Marone G, et al. 2018. Eosinophils: The unsung heroes in cancer? Oncoimmunology 7(2):e1393134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simon SCS, Utikal J, Umansky V. 2018. Opposing roles of eosinophils in cancer. Cancer Immunol. Immunother. 10.1007/s00262-018-2255-4 [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krause JR, Boggs DR. 1987. Search for eosinopenia in hospitalized patients with normal blood leukocyte concentration. Am. J. Hematol. 24(1):55–63 [DOI] [PubMed] [Google Scholar]
- 77.Kelesidis T, Yang O. 2010. Good’s syndrome remains a mystery after 55 years: A systematic review of the scientific evidence. Clin. Immunol. 135(3):347–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gleich GJ, Klion AD, Lee JJ, Weller PF. 2013. The consequences of not having eosinophils. Allergy 68(7):829–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Serwas NK, Huemer J, Dieckmann R, Mejstrikova E, Garncarz W, et al. 2018. CEBPE-mutant specific granule deficiency correlates with aberrant granule organization and substantial proteome alterations in neutrophils. Front. Immunol. 9588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosenberg HF, Gallin JI. 1993. Neutrophil-specific granule deficiency includes eosinophils. Blood 82(1):268–73 [PubMed] [Google Scholar]
- 81.Zabucchi G, Soranzo MR, Menegazzi R, Vecchio M, Knowles A, et al. 1992. Eosinophil peroxidase deficiency: morphological and immunocytochemical studies of the eosinophil-specific granules. Blood 80(11):2903–10 [PubMed] [Google Scholar]
- 82.Aceves SS, Broide DH. 2008. Airway fibrosis and angiogenesis due to eosinophil trafficking in chronic asthma. Curr. Mol. Med. 8(5):350–8 [DOI] [PubMed] [Google Scholar]
- 83.Rubinstein E, Cho JY, Rosenthal P, Chao J, Miller M, et al. 2011. Siglec-F inhibition reduces esophageal eosinophilia and angiogenesis in a mouse model of eosinophilic esophagitis. J. Pediatr. Gastroenterol. Nutr. 53(4):409–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cho JY, Doshi A, Rosenthal P, Beppu A, Miller M, et al. 2014. Smad3-deficient mice have reduced esophageal fibrosis and angiogenesis in a model of egg-induced eosinophilic esophagitis. J. Pediatr. Gastroenterol. Nutr. 59(1):10–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Letuve S, Pretolani M. 2013. Potential role of eosinophil granule proteins in tissue remodeling and fibrosis In Eosinophils in Health and Disease, ed. Rosenberg HF, Lee JJ, pp.393–8. London: Elsevier [Google Scholar]
- 86.Gomes I, Mathur SK, Espenshade BM, Mori Y, Varga J, Ackerman SJ. 2005. Eosinophil-fibroblast interactions induce fibroblast IL-6 secretion and extracellular matrix gene expression: implications in fibrogenesis. J. Allergy Clin. Immunol. 116(4):796–804 [DOI] [PubMed] [Google Scholar]
- 87.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, et al. 2011. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332(6026):243–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marichal T, Mesnil C, Bureau F. 2017. Homeostatic eosinophils: characteristics and functions. Front. Med. (Lausanne) 4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Y, Yang P, Cui R, Zhang M, Li H, et al. 2015. Eosinophils reduce chronic inflammation in adipose tissue by secreting Th2 cytokines and promoting M2 macrophages polarization. Int. J. Endocrinol. 2015565760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, et al. 2014. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 157(6):1292–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, et al. 2013. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med. 210(3):535–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rozenberg P, Reichman H, Zab-Bar I, Itan M, Pasmanik-Chor M, et al. 2017. CD300f:IL-5 cross-talk inhibits adipose tissue eosinophil homing and subsequent IL-4 production. Sci. Rep. 7(1):5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chu VT, Frohlich A, Steinhauser G, Scheel T, Roch T, et al. 2011. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat. Immunol. 12(2):151–9 [DOI] [PubMed] [Google Scholar]
- 94.Forman R, Bramhall M, Logunova L, Svensson-Frej M, Cruickshank SM, Else KJ. 2016. Eosinophils may play regionally disparate roles in influencing IgA(+) plasma cell numbers during large and small intestinal inflammation. BMC Immunol. 17(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jung Y, Wen T, Mingler MK, Caldwell JM, Wang YH, et al. 2015. IL-1beta in eosinophil-mediated small intestinal homeostasis and IgA production. Mucosal Immunol. 8(4):930–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chu VT, Beller A, Rausch S, Strandmark J, Zanker M, et al. 2014. Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity 40(4):582–93 [DOI] [PubMed] [Google Scholar]
- 97.Bortnick A, Chernova I, Spencer SP, Allman D. 2018. No strict requirement for eosinophils for bone marrow plasma cell survival. Eur. J. Immunol. 48(5):815–21 [DOI] [PubMed] [Google Scholar]
- 98.Haberland K, Ackermann JA, Ipseiz N, Culemann S, Pracht K, et al. 2018. Eosinophils are not essential for maintenance of murine plasma cells in the bone marrow. Eur. J. Immunol. 48(5):822–8 [DOI] [PubMed] [Google Scholar]
- 99.Wong TW, Doyle AD, Lee JJ, Jelinek DF. 2014. Eosinophils regulate peripheral B cell numbers in both mice and humans. J. Immunol. 192(8):3548–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang HB, Ghiran I, Matthaei K, Weller PF. 2007. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. J. Immunol. 179(11):7585–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weller PF, Rand TH, Barrett T, Elovic A, Wong DTW, Finberg RW. 1993. Accessory cell function of human eosinophils - HLA-DR-dependent, MHC-restricted antigen-presentation and IL-1a expression. J. Immunol. 150(6):2554–62 [PubMed] [Google Scholar]
- 102.Farhan RK, Vickers MA, Ghaemmaghami AM, Hall AM, Barker RN, Walsh GM. 2016. Effective antigen presentation to helper T cells by human eosinophils. Immunology 149(4):413–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Valent P, Klion AD, Horny HP, Roufosse F, Gotlib J, et al. 2012. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J. Allergy Clin. Immunol. 130(3):607–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bochner BS, Book W, Busse WW, Butterfield J, Furuta GT, et al. 2012. Workshop report from the National Institutes of Health Taskforce on the Research Needs of Eosinophil-Associated Diseases (TREAD). J. Allergy Clin. Immunol. 130(3):587–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Khoury P, Akuthota P, Ackerman SJ, Arron JR, Bochner BS, et al. 2018. Revisiting the NIH Taskforce on the Research needs of Eosinophil-Associated Diseases (RE-TREAD). J. Leukoc. Biol. 104(1):69–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Khoury P, Bochner BS. 2018. Consultation for elevated blood eosinophils: clinical presentations, high value diagnostic tests, and treatment options. J. Allergy Clin. Immunol. Pract. 6(5):1446–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Khoury P, Makiya M, Klion AD. 2017. Clinical and biological markers in hypereosinophilic syndromes. Front. Med. (Lausanne) 4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Khoury P, Desmond R, Pabon A, Holland-Thomas N, Ware JM, et al. 2016. Clinical features predict responsiveness to imatinib in platelet-derived growth factor receptor-alpha-negative hypereosinophilic syndrome. Allergy 71(6):803–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Klion AD, Noel P, Akin C, Law MA, Gilliland DG, et al. 2003. Elevated serum tryptase levels identify a subset of patients with a myeloproliferative variant of idiopathic hypereosinophilic syndrome associated with tissue fibrosis, poor prognosis, and imatinib responsiveness. Blood 101(12):4660–6 [DOI] [PubMed] [Google Scholar]
- 110.Roufosse F, Cogan E, Goldman M. 2007. Lymphocytic variant hypereosinophilic syndromes. Immunol. Allergy Clin. North Am. 27(3):389–413 [DOI] [PubMed] [Google Scholar]
- 111.Khoury P, Herold J, Alpaugh A, Dinerman E, Holland-Thomas N, et al. 2015. Episodic angioedema with eosinophilia (Gleich syndrome) is a multilineage cell cycling disorder. Haematologica 100(3):300–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alexander ES, Martin LJ, Collins MH, Kottyan LC, Sucharew H, et al. 2014. Twin and family studies reveal strong environmental and weaker genetic cues explaining heritability of eosinophilic esophagitis. J. Allergy Clin. Immunol. 134(5):1084–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bonatti F, Reina M, Neri TM, Martorana D. 2014. Genetic susceptibility to ANCA-associated vasculitis: State of the art. Front. Immunol. 5577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Prakash Babu S, Chen YK, Bonne-Annee S, Yang J, Maric I, et al. 2017. Dysregulation of interleukin 5 expression in familial eosinophilia. Allergy 72(9):1338–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Klion AD. 2015. How I treat hypereosinophilic syndromes. Blood 126(9):1069–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ogbogu PU, Bochner BS, Butterfield JH, Gleich GJ, Huss-Marp J, et al. 2009. Hypereosinophilic syndrome: A multicenter, retrospective analysis of clinical characteristics and response to therapy. J. Allergy Clin. Immunol. 124(6):1319–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rothenberg ME, Klion AD, Roufosse FE, Kahn JE, Weller PF, et al. 2008. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N. Engl. J. Med. 358(12):1215–28 [DOI] [PubMed] [Google Scholar]
- 118.Wechsler JB, Hirano I. 2018. Biological therapies for eosinophilic gastrointestinal diseases. J. Allergy Clin. Immunol. 142(1):24–31 e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kuang FL, Fay MP, Ware J, Wetzler L, Holland-Thomas N, et al. 2018. Long-term clinical outcomes of high-dose mepolizumab treatment for hypereosinophilic syndrome. J. Allergy Clin. Immunol. Pract 6(5):1518–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Panch SR, Bozik ME, Brown T, Makiya M, Prussin C, et al. 2018. Dexpramipexole as an oral steroid-sparing agent in hypereosinophilic syndromes. Blood 132(5):501–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zeitlin PL, Leong M, Cole J, Mallory RM, Shih VH, et al. 2018. Benralizumab does not impair antibody response to seasonal influenza vaccination in adolescent and young adult patients with moderate to severe asthma: results from the Phase IIIb ALIZE trial. J. Asthma Allergy 11181–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Klion AD, Robyn J, Akin C, Noel P, Brown M, et al. 2004. Molecular remission and reversal of myelofibrosis in response to imatinib mesylate treatment in patients with the myeloproliferative variant of hypereosinophilic syndrome. Blood 103(2):473–8 [DOI] [PubMed] [Google Scholar]
- 123.Wilson TM, Maric I, Shukla J, Brown M, Santos C, et al. 2011. IL-5 receptor α levels in patients with marked eosinophilia or mastocytosis. J. Allergy Clin. Immunol. 128(5):1086–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yamada T, Miyabe Y, Ueki S, Fujieda S, Tokunaga T, et al. 2019. Eotaxin-3 as a plasma biomarker for mucosal eosinophil infiltration in chronic rhinosinusitis. Front. Immunol. 1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wen T, Stucke EM, Grotjan TM, Kemme KA, Abonia JP, et al. 2013. Molecular diagnosis of eosinophilic esophagitis by gene expression profiling. Gastroenterology 145(6):1289–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dellon ES, Veerappan R, Selitsky SR, Parker JS, Higgins LL, et al. 2017. A gene expression panel is accurate for diagnosis and monitoring treatment of eosinophilic esophagitis in adults. Clin. Transl. Gastroenterol. 8(2):e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.de Lavareille A, Roufosse F, Schmid-Grendelmeier P, Roumier AS, Schandene L, et al. 2002. High serum thymus and activation-regulated chemokine levels in the lymphocytic variant of the hypereosinophilic syndrome. J. Allergy Clin. Immunol. 110(3):476–9 [DOI] [PubMed] [Google Scholar]
- 128.Giusti D, Le Jan S, Gatouillat G, Bernard P, Pham BN, Antonicelli F. 2017. Biomarkers related to bullous pemphigoid activity and outcome. Exp. Dermatol. 26(12):1240–7 [DOI] [PubMed] [Google Scholar]
- 129.Butterfield JH, Weiler D, Peterson EA, Gleich GJ, Leiferman KM. 1990. Sequestration of eosinophil major basic protein in human mast cells. Lab. Invest. 62(1):77–86 [PubMed] [Google Scholar]
- 130.Leiferman KM, Ackerman SJ, Sampson HA, Haugen HS, Venencie PY, Gleich GJ. 1985. Dermal deposition of eosinophil-granule major basic protein in atopic dermatitis. Comparison with onchocerciasis. N Engl J Med 313(5):282–5 [DOI] [PubMed] [Google Scholar]
- 131.Konikoff MR, Blanchard C, Kirby C, Buckmeier BK, Cohen MB, et al. 2006. Potential of blood eosinophils, eosinophil-derived neurotoxin, and eotaxin-3 as biomarkers of eosinophilic esophagitis. Clin Gastroenterol Hepatol 4(11):1328–36 [DOI] [PubMed] [Google Scholar]
- 132.Dellon ES, Rusin S, Gebhart JH, Covey S, Higgins LL, et al. 2015. Utility of a noninvasive serum biomarker panel for diagnosis and monitoring of eosinophilic esophagitis: a prospective study. Am. J. Gastroenterol. 110(6):821–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hines BT, Rank MA, Wright BL, Marks LA, Hagan JB, et al. 2018. Minimally invasive biomarker studies in eosinophilic esophagitis: A systematic review. Ann. Allergy Asthma Immunol. 121(2):218–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Szefler SJ, Wenzel S, Brown R, Erzurum SC, Fahy JV, et al. 2012. Asthma outcomes: biomarkers. J. Allergy Clin. Immunol. 129(3 Suppl):S9–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nair P, Ochkur SI, Protheroe C, Radford K, Efthimiadis A, et al. 2013. Eosinophil peroxidase in sputum represents a unique biomarker of airway eosinophilia. Allergy 68(9):1177–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wolfe MG, Mukherjee M, Radford K, Brennan JD, Nair P. 2018. Rapid quantification of sputum eosinophil peroxidase on a lateral flow test strip. Allergy (in press) [DOI] [PubMed] [Google Scholar]
- 137.Furuta GT, Kagalwalla AF, Lee JJ, Alumkal P, Maybruck BT, et al. 2013. The oesophageal string test: a novel, minimally invasive method measures mucosal inflammation in eosinophilic oesophagitis. Gut 62(10):1395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ackerman SJ, Kagalwalla AF, Hirano I, Gonsalves N, Menard-Katcher P, et al. 2019. The 1-hour esophageal string test: A non-endoscopic minimally invasive test to accurately detect disease activity in eosinophilic esophagitis. J. Allergy Clin. Immunol. 143(2):Ab309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Januszewicz W, Tan WK, Lehovsky K, Debiram-Beecham I, Nuckcheddy T, et al. 2019. Safety and acceptability of esophageal cytosponge cell collection device in a pooled analysis of data from individual patients. Clin. Gastroenterol. Hepatol. 17(4):647–56 e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Katzka DA, Geno DM, Ravi A, Smyrk TC, Lao-Sirieix P, et al. 2015. Accuracy, safety, and tolerability of tissue collection by Cytosponge vs endoscopy for evaluation of eosinophilic esophagitis. Clin Gastroenterol Hepatol 13(1):77–83 [DOI] [PubMed] [Google Scholar]
- 141.Henriksen DP, Bodtger U, Sidenius K, Maltbaek N, Pedersen L, et al. 2018. Efficacy, adverse events, and inter-drug comparison of mepolizumab and reslizumab anti-IL-5 treatments of severe asthma - a systematic review and meta-analysis. Eur. Clin. Respir. J. 5(1):1536097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Busse W, Chupp G, Nagase H, Albers FC, Doyle S, et al. 2019. Anti-IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: Indirect treatment comparison. J. Allergy Clin. Immunol. 143(1):190–200 [DOI] [PubMed] [Google Scholar]
- 143.Bochner BS. 2015. Novel therapies for eosinophilic disorders. Immunol. Allergy Clin. North Am. 35(3):577–98 [DOI] [PMC free article] [PubMed] [Google Scholar]