1. Introduction
More than 100 years ago, vitamin A was discovered as a minor but vital lipid growth factor in ordinary foodstuff (McCollum, 1913; Osborne, 1913). Soon after, it was realized that certain yellow plant pigments, named carotenes, possess the same activity (Steenbock, 1919). In 1930, Moore showed that purified carotene was converted to an active growth promoting substance in animal physiology (Moore, 1930). Determination of the chemical structures of β-carotene and retinaldehyde by Paul Karrer and colleagues verified the kinship between the provitamin and its vitamin metabolite (Karrer P, 1930).
A connection between diet and vision was known since ancient times because malnutrition was strongly associated with night blindness (Wolf, 2001). The association between night blindness and vitamin A was made in 1925 by Frederica and Holm (Frederica, Holm, 1925), who recognized that vitamin A-deprived rats formed visual pigments at a slower rate than their normal-fed counterparts. Yudkin showed that vitamin A existed in the pig retina (Yudkin, 1931). However, it was Wald who, starting in 1935 and continuing into subsequent years, found that the visual pigments of the retina, called rhodopsin, consists of a protein, opsin, combined with “retinene” (later shown to be retinaldehyde). In the following years, Wald and colleagues elegantly described the basics of the visual cycle that regenerates light sensitivity of rhodopsin after a bleach (Wald, 1968). Research in the biochemical and molecular basis of the visual action of vitamin A led to the discovery of visual G protein-coupled receptors and culminated in the determination of their atomic structures (Palczewski et al., 2000).
In the late 1920s, additional cellular processes were identified to be vitamin A-dependent (Wolbach, 1925). Researchers showed that this action is mainly connected with the acidic form of the vitamin, all-trans-retinoic acid (RA). Further research on RA has shown that the acidic form of the vitamin functions in diverse processes including cell differentiation and survival, immunity, reproduction, and embryonic development (Hall et al., 2011; Rhinn, Dolle, 2012; von Lintig et al., 2010). In the 1980s, it became clear that RA exerts these functions by modulating gene expression in various cell types of the body (Chytil, 1986; Lotan et al., 1980). In 1987, Chambon (Petkovich et al., 1987) and Evans (Giguere et al., 1987), and their respective co-workers, discovered the retinoic acid receptors (RARs). RARs are transcription factors of the nuclear hormone receptor gene family, which in conjunction with retinoid X receptors (RXRs), control gene transcription by binding to conserved DNA motifs (retinoic acid response elements) in promoter regions of about 500 target genes in the human genome (Balmer, Blomhoff, 2002). This type of action established RA as a hormone that is synthesized from dietary precursor molecules.
To support these functions, vitamin A precursors must be rendered available from the diet, transported, and metabolically converted into chromophore and RA hormone. Work by Olson (Olson, Hayaishi, 1965) and others delineated major steps in this metabolism. This was followed by identification of the importance of the serum transport protein for vitamin A by Goodman (Kanai et al., 1968) as well as intracellular retinoid-binding proteins by Chytil and Ong (Chytil, Ong, 1987). Norum and Blomhoff (Blomhoff et al., 1990) and many other researchers then established the basic facts of absorption and liver storage mechanisms for vitamin A. Vitamin A is the only vitamin that is stored in significant amounts in the body of vertebrates, thereby providing the key pro-survival advantage to endure periods with little to no dietary supplies of dietary precursor molecules.
Our laboratory entered this research field by studying insect vision and analyzing chromophore-deficient Drosophila mutants. Setting the stage for future research across diverse animal groups, we identified and characterized proteins for carotenoid absorption and bioconversion to chromophore (Kiefer et al., 2002; Oberhauser et al., 2008; von Lintig et al., 2001; von Lintig, Vogt, 2000). Subsequently, we showed that homologous proteins have similar functions in vertebrates, including humans (Amengual et al., 2011b; Amengual et al., 2013; Hessel et al., 2007; Kiefer et al., 2001; Voolstra et al., 2006). Our research also revealed that carotenoid absorption and bioconversion to vitamin A is regulated by demand and availability of dietary precursor molecules (Lobo et al., 2013; Lobo et al., 2010b). Comparative research in different animal classes provided insights as to how carotenoids and retinoids are transported and distributed throughout the body (Amengual et al., 2014a; Babino et al., 2015a; Isken et al., 2008; Widjaja-Adhi et al., 2015). This review summarizes and balances the advancements that have been made in the past two decades in our and others’ laboratories with a particular focus on the role of carotenoids as chromophore precursor. We will follow the carotenoids’ metabolic fate in the body from the gut to the eyes and consider the different biochemical strategies that animal classes have evolved to supply photoreceptors with chromophore.
2. Carotenoids
Carotenoids are a familiar sight as colorful pigments of fruits, flowers and vegetables. This class of isoprenoids comprises more than 1000 related compounds which contain up to 15 conjugated double bonds. The best known and eponymous representative of carotenoids is β-carotene (Figure 1). Other trivial names for carotenoids such as lycopene, lutein, zeaxanthin, and astaxanthin were in use long before the exact chemical structures of these compounds were determined. In hindsight, the monikers of carotenoids may seem undue but developed under functional analysis as opposed to stringent chemical elucidation. In 1974, The Nomenclature of Carotenoids was issued by the IUPAC with hundreds having been identified and even more looming. An online database searchable across several factors including chemical structures is maintained and updated with new discoveries (Yabuzaki, 2017).
Figure 1.
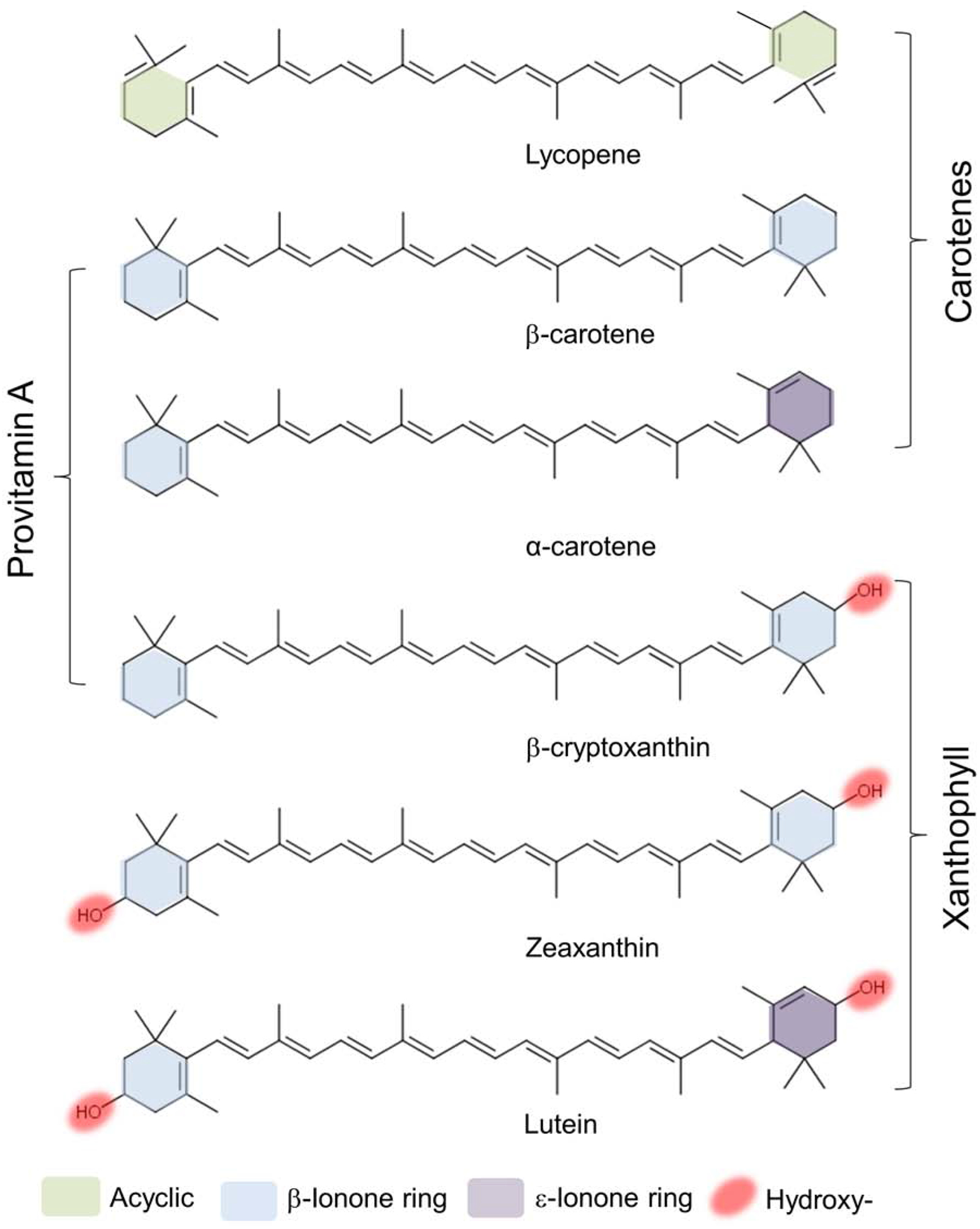
Structures of the six major carotenoids in human blood.
Photosynthetic plants, protists and bacteria, as well as some heterotrophic bacteria and fungi can synthesize carotenoids. The initial steps of this pathway follow a common scheme that is like sterol and isoprenoid biosynthesis in most species (1,2). The first step exclusively devoted to carotenoid synthesis is the condensation of two molecules of geranylgeranyl pyrophosphate (C20) to phytoene (C40) catalyzed by phytoene synthase (Schledz et al., 1996). Synthesis of phytoene is an important branching point in plant isoprenoid metabolism and regulated by phytochrome and light during leaf development (von Lintig et al., 1997; Welsch et al., 2000; Welsch et al., 2003). The conversion of the colorless phytoene to the red colored lycopene occurs through four desaturation steps. In none-photosynthetic bacteria as well as in fungi, these steps are carried out by a single enzyme (Giuliano et al., 1986). In plants, this pathway employs several proteins that catalyze a sequence of desaturation and isomerization steps (Isaacson et al., 2004). This multistep pathway in higher plants is evidenced by mutant variants such as tangerine tomatoes which accumulate its tetra-cis-lycopene intermediate (Isaacson et al., 2002). Downstream in the biosynthetic pathway, all-trans-lycopene is the substrate for the introduction of ionone ring structures at both ends of the linear carbon chain of the carotenoid (Hugueney et al., 1995). These cyclization reactions yield β-carotene and α-carotene. The addition of functional groups is another notable strategy (Bouvier et al., 1998). Insertion of hydroxyl groups into the ionone rings creates lutein, the bright yellow pigment of daffodils and marigolds. Addition of keto-groups results in asthaxanthin, the vermilion color of flamingoes, salmon and crustaceans. The enormous diversity of carotenoids is achieved by modulation in length of the polyene chromophore and shifts of the conjugated double bonds that result in changes of the absorption spectra (Moise et al., 2014). Additionally, carotenoids can be chemically transformed to apocarotenoid metabolites through the introduction of oxygen at specific double bonds of their polyene backbone. The geometric forms all-(E)-form (all-trans) and (Z)-geometric isomers (cis) contribute to the chemically diversity of this class of natural compounds. Finally, chiral centres in cyclic end-groups also provide further multiplicity of optical isomers. Lutein, for example, contains three chiral centres at the 3, 3’ and 6’-carbons with either R or S configurations. Zeaxanthin and meso-zeaxanthin, the other macular pigments of the human eyes, are limited to two at the 3 and 3’-carbons which exist in 3R,3R’ configuration in zeaxanthin and 3R.3S’ configuration in meso-zeaxanthin.
3. Carotenoid functions
Carotenoids are accessory pigments in the antennae of chloroplasts, where they augment the light-harvesting capacity by absorbing light in the blue-green range of the visible spectrum (450–550 nm) and transferring the energy to chlorophyll. They are also involved in photo-protection of the photosynthetic apparatus and other cellular structures (Demmig-Adams, Adams, 2002). The properties of carotenoids in photosynthetic systems continue to place a great deal of inquiry and inspire innovation in the field. For reviews based on this subject matter, carotenoid excited singlet states, we refer to (Hashimoto et al., 2018) and (Musser, Clark, 2019). Time-resolved spectroscopy of singlet fission in carotenoid aggregates even has revealed them as potential singlet fission sensitizers for solar panels (Billsten et al., 2005; Musser et al., 2015; Sineshchekov et al., 1972).
In daily life, carotenoids delight our senses as colors and expedite communication even over evolutionary borders. These colorful pigments attract pollinators such as birds and insects and advertise ripe fruits in exchange for seed dispersal. They maintain ranks in schools of fish and influence the mating choice of birds (Blount et al., 2003; Faivre et al., 2003). Feathers of some birds even contain crimson and burgundy colored carotenoids that are metabolically derived from dietary plant-produced carotenoid precursor molecules (Berg et al., 2013). Crustaceans such as lobster use carotenoids for blue coloration (Cianci et al., 2002). Once boiled the original reddish carotenoid color appears due to denaturing of the blueish protein-carotenoid complex.
Human blood and tissues also retain considerable amounts of carotenoids (Figure 2). In the eyes, the macular pigments have been chemically identified as the carotenoids lutein, zeaxanthin, and meso-zeaxanthin (Bernstein et al., 2016; Bone et al., 1985). Though distributed throughout the retina, these carotenoids are enriched in the fovea in primate retinas and confer its yellow colour. Hence, the fovea is traditionally known as the macula lutea, or ‘yellow spot’. The macular pigments may protect the retina against light damage (Barker et al., 2011; Widjaja-Adhi et al., 2018) and reduce the adverse impact of light scattering and chromatic aberration, thereby optimizing contrast sensitivity of the retina (Hammond et al., 2013). These properties have led to the hypothesis that macula pigments may protect against the development of age related macular degeneration (AMD) (Bernstein et al., 2016; Mares, 2016). Epidemiological studies support this role and revealed that individuals with lower concentrations of serum carotenoids and macular pigment optical density measurements are at a higher risk of developing AMD. Conversely, nutritional supplementation and diets rich in lutein and zeaxanthin readily impact MP concentrations and reduce the risk of progression to advanced AMD (Chew et al., 2014). The light filtering properties of carotenoids also can provide modest protection against ultraviolet (UV)-induced erythema in the skin (Kopcke, Krutmann, 2008).
Figure 2.
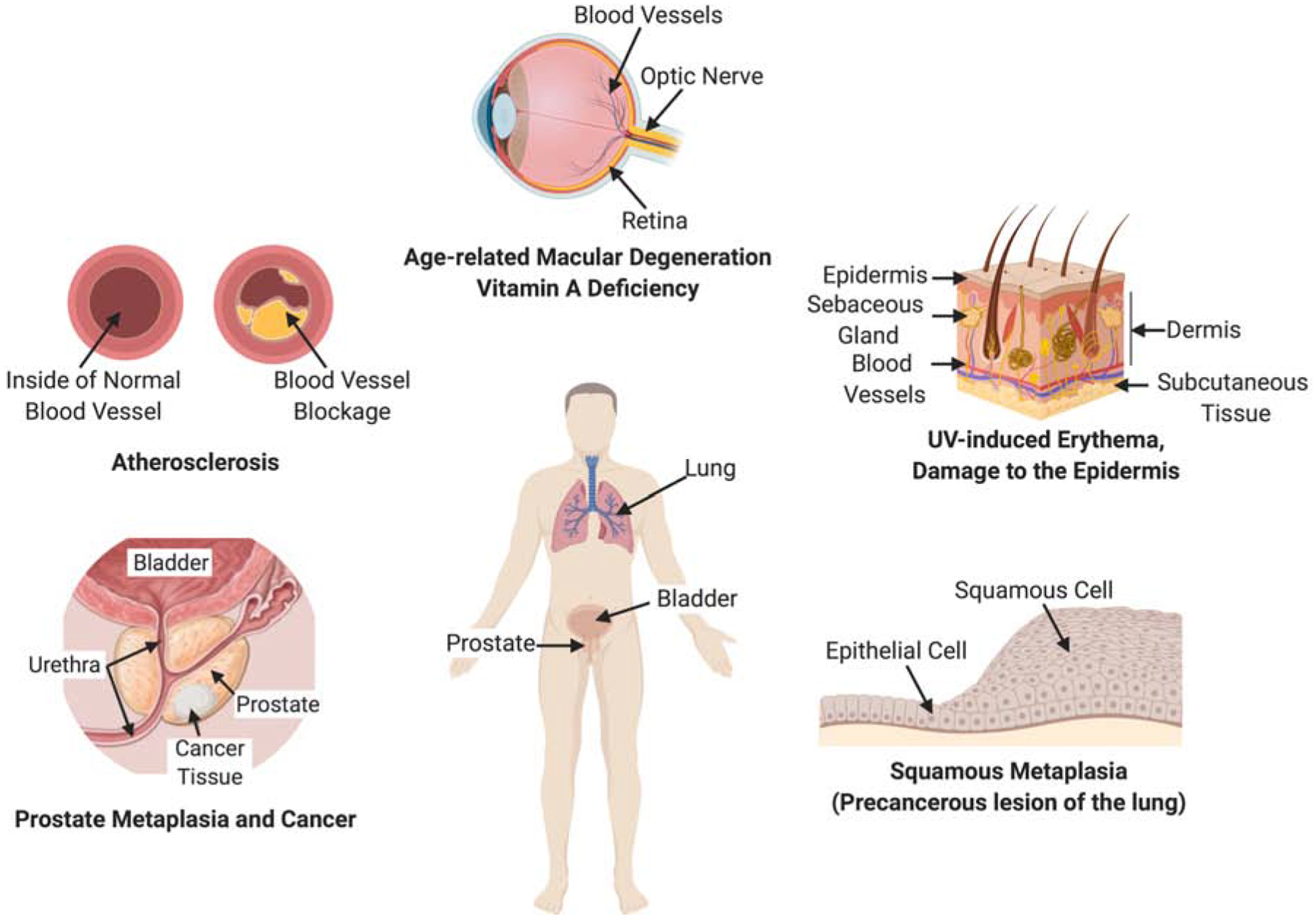
Health and disease states related to carotenoids.
Several epidemiological studies have reported that plasma carotenoid levels are inversely correlated with the incidence of cardiovascular diseases (Bohn et al., 2017). This association has been reported for total plasma carotenoids and individual carotenoids such as lycopene, β-carotene, and lutein (Leermakers et al., 2016). There are several potential mechanisms through which carotenoid consumption may affect the etiology of these diseases. The most commonly cited is through the antioxidant action of carotenoids. The antioxidant properties of carotenoids may decrease lipid peroxidation and eventually reduce oxidative stress and inflammation responses in human cells and tissues (Edge, Truscott, 2018). Additionally, local production of signaling molecules including RA from carotenoids may influence the activity of nuclear receptors (Alvarez et al., 2014).
Higher blood levels of carotenoids have been also associated with prevention of various forms of cancer, particularly prostate cancer. The effects may involve antioxidant properties, interactions with signaling molecules such as NFκB (Linnewiel-Hermoni et al., 2014), and interactions with the metabolism and transport of other lipids, particularly cholesterol (Palozza et al., 2012). Notably, also adverse effects of certain carotenoids, β-carotene, on cancer have been reported (Omenn et al., 1996; Wang, Russell, 1999). Studies revealed that levels, environmental factors, and genetics may play a role for this process. However, many molecular details of carotenoid action remain undefined and more research is required to analyze the distribution, metabolism, and function of carotenoids in relation to different forms of cancer.
Living organisms chemically transform carotenoids by oxidative cleavage at specific alkene bonds to generate a unique series of apocarotenoid metabolites (Figure 3). In all kingdoms of life, apocarotenoids serve as chromophore of photopigments. The occurrence of retinaldehyde in bacteriorhodopsin qualifies this lipid as evolutionarily among the oldest photochemical compounds (Jung, 2007). Similarly, most animals metabolize carotenoids to chromophore. The chromophore function of retinoids is clearly defined by the compounds’ chemical and physical properties. The interaction of delocalized π-electron system with electromagnetism underlies the light harvesting and light filtering properties of these compounds.
Figure 3.
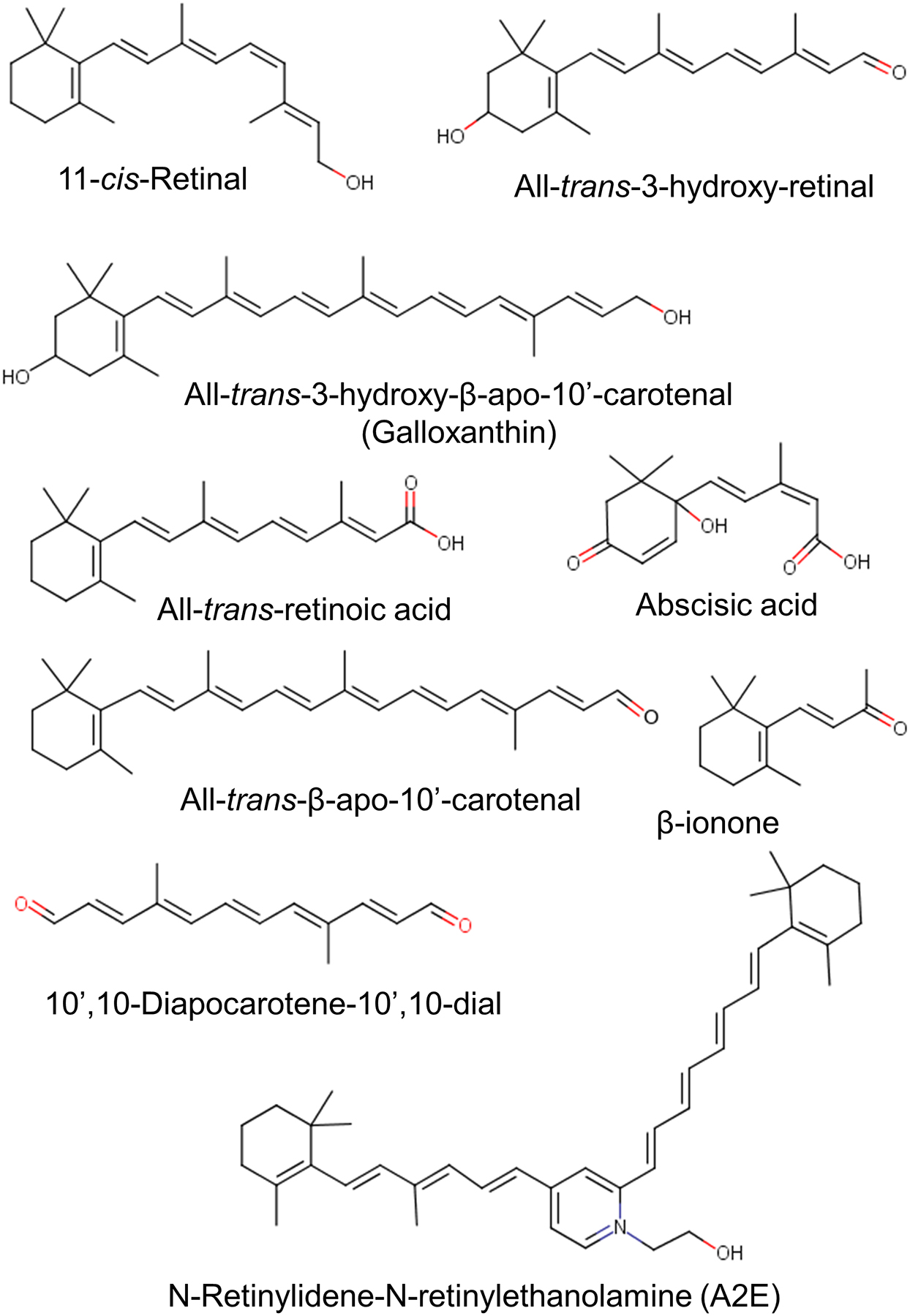
Structure of various natural apocarotenoids.
Cleavage of carotenoids also yields β-ionone, α-ionone, bixin (annatto), saffron, and norisoprenoids. These apocarotenoids are of significant economic value as food colorants, cosmetic (Bouvier et al., 2003a; Bouvier et al., 2003b), and aromatic compounds (Mendes-Pinto, 2009).
Apocarotenoids also are used as hormones which upon receptor activation regulate various physiological processes. In plants, abscisic acid and strigolactones influence processes, as diverse as seed dormancy, morphogenesis and environmental adaptation (Al-Babili, Bouwmeester, 2015; Schwartz et al., 1997). More recently, β-cyclo-citral and zaxinone have been identified as growth factors in plants (Dickinson et al., 2019; Wang et al., 2019). In vertebrates, RA regulates gene expression throughout the life cycle and is responsible for most, if not all, non-visual physiological effects of the vitamin. Interestingly, some cyanobacteria can also produce RA and its hydroxylated derivatives from carotenoids (Alder et al., 2009). Cyanobacteria blooms may result in aquatic concentrations of this hormone that can harm amphibians in eutrophic habitats (Wu et al., 2012). Animals also transform carotenoids into long chain apocarotenoids (>C20). Birds use 3-hydroxy-dehydro-β−10’-apocarotenol (galloxanthin) in oil droplets of the retina to modulate spectral sensitivity of visual pigments in adjacent cone photoreceptors (Toomey et al., 2016). In mammals, emerging evidence suggests these compounds are biologically active modulators of physiological processes (Aydemir et al., 2016; Harrison, Quadro, 2018; Lian et al., 2007; Narayanasamy et al., 2017). For instance, it has been reported that pharmacological doses of apo-10’-lycopenoic acid can protect against liver damage (Ip et al., 2013). Furthermore, β−13-apo-carotenone and certain lycopenoids act as RAR antagonists (Eroglu et al., 2012; Narayanasamy et al., 2017). A recent study indicate that apocarotenoids different than RA may act as signal molecules on their own right in physiological processes of mammals (Costabile et al., 2016).
4. The enzymatic conversion of carotenoids into apocarotenoids
4.1. The discovery of carotenoid cleavage dioxygenases
Oxidative cleavage of carotenoids at a specific position of the polyene chain had been proposed as the method for apocarotenoid production in all living organisms. However, the identity and molecular nature of the involved enzymes remained elusive for a long time. By analyzing the genetic basis of the abscisic acid-deficient corn mutant, vp14 (viviparous 14), the first gene encoding a carotenoid cleavage dioxygenase (CCD) was identified and the encoded enzyme biochemically characterized (Schwartz et al., 1997). Recombinant VP14 catalyzes oxidative cleavage at position C10, C11 of 9-cis-violaxanthin to yield xanthoxin.. Xanthoxin is then further metabolized to abscisic acid. The researchers proposed that related CCDs catalyze oxidative cleavage of carotenoids in other organisms. We succeeded in the identification of three distinct types of metazoan CCDs encoded by the ninaB, BCO1, and BCO2 genes (Kiefer et al., 2001; von Lintig, Vogt, 2000). Simultaneously, a classical protein purification and sequencing approach conducted by Adrian Wyss identified the BCO1 gene from chicken (Wyss et al., 2000). We used a cloning and expression strategy to biochemically characterize these enzymes which employed carotenoid producing E. coli strains (Figure 4). These E. coli strains lose their bright yellow or red colors and turn pale when a CCD is expressed that splits the pigments into apocarotenoids. The discoloration occurs because the blue-shifted spectrum and lower extinction coefficient of the apocarotenoid products. This robust and reliable test system was instrumental in overcoming initial difficulties with the heterologous expression and biochemical characterization of these enzymes. These enzymes catalyze a reaction at the lipid aqueous interface and can form insoluble misfolded aggregates when expressed in E. coli. The challenging biochemistry of CCDs had led to many conflicting results about this enzyme class. For instance, the retina pigment epithelium protein of 65 kDa (RPE65), the first molecularly identified metazoan member of the CCD family (Hamel et al., 1993), was proposed to be a retinoid-binding protein rather than an enzyme catalyst (Gollapalli et al., 2003; Mata et al., 2004). Moreover, the existence of a vitamin A forming enzyme was questioned by some researchers because of the failure to measure its activity in cell free extracts (Hansen, Maret, 1988). Over the years, advanced protocols for the heterologous expression and biochemical characterization of CCDs were established and enzymatic properties of CCDs were scrutinized. Amongst others, these analyses revealed an additional enzymatic property of this enzyme class that is critical for their visual function. Some CCDs possess intrinsic isomerase activity and catalyze geometric isomerization of double bonds within the polyene backbone of carotenoids. The CCD, encoded by the insect neither inactivation nor after potential gene B (ninaB) gene (von Lintig et al., 2001), catalyzes a combined oxidative cleavage at position C15,C15’ and isomerization at position 11’,12’ of its carotenoid substrates to directly yield visual chromophore (Babino et al., 2016; Oberhauser et al., 2008) (Figure 5). In vertebrates, oxidative cleavage of the carotenoid and geometric isomerization of the resulting retinoid are catalyzed by two distinct family members, respectively encoded by the BCO1 and RPE65 genes (Figure 6). BCO1 encodes a β-carotene-15,15’-dioxygenase that splits β-carotene into two molecules of retinaldehyde (Wyss et al., 2000). RPE65 acts as a retinoid isomerase and catalyst of the conversion of retinyl esters (RE) to 11-cis-retinol (Jin et al., 2005; Moiseyev et al., 2005; Redmond et al., 2005a). The third vertebrate CCD is encoded by the BCO2 gene and catalyzes cleavage at position C9,C10 and C9’,C10’ of carotenoids (Kiefer et al., 2001). Though originally characterized as β-carotene metabolizing enzyme, BCO2 is promiscuous and displays broad substrate specificity for various carotenoids and apocarotenoids, including lycopene, zeaxanthin, lutein, and canthaxanthin (Amengual et al., 2011b; Dela Sena et al., 2016; Hu et al., 2006; Kelly et al., 2018; Lobo et al., 2012; Mein et al., 2010). One study indicates that human BCO2 is enzymatically inactive and that this inactivity favors the accumulation of macula pigment in the retina (Li et al., 2014). However, other studies provided evidence that human BCO2 catalyzes carotenoid breakdown when expressed in E. coli cells (Palczewski et al., 2014; Poliakov et al., 2017). Additionally, BCO2 cloned from macaques, a primate with macula pigments, converts zeaxanthin into apocarotenoids (Babino et al., 2015a), indicating that ocular accumulation of carotenoids is not dependent on an inactive BCO2 enzyme.
Figure 4. Reactions catalyzed by the two mammalian carotenoid cleavage dioxygenases.
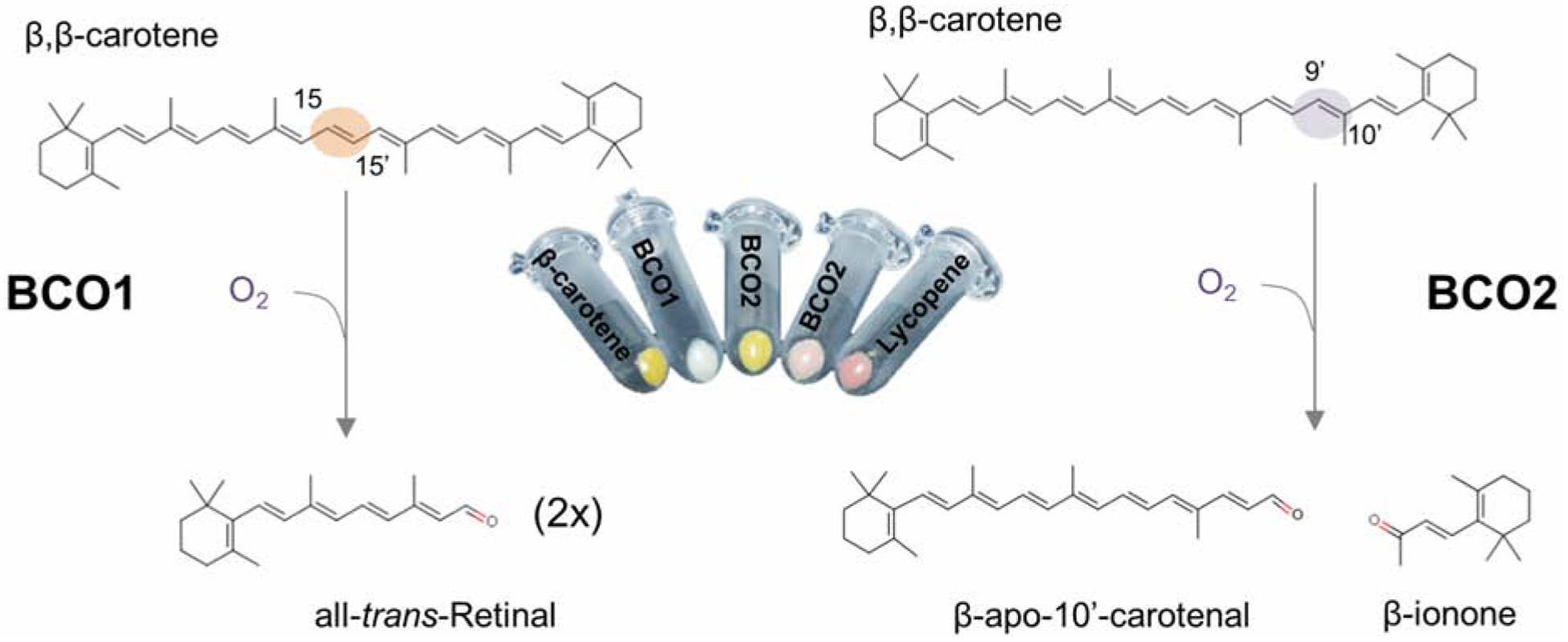
Carotenoid conversion by β-carotene-15,15’-dioxygenase (encoded by the BCO1 gene) (left) and β-carotene-9’,10’-dioxygenase (encoded by the BCO2 gene) (right). The cleavage sites within the carbon backbone of β-carotene are highlighted. In the middle, pellets of bacteria are shown that produce either β-carotene (left) or lycopene (right). Expression of recombinant mouse BCO1 or BCO2 leads to color shifts of the pellets caused by carotenoid conversion to apocarotenoids.
Figure 5. NinaB and chromophore production in arthropods.
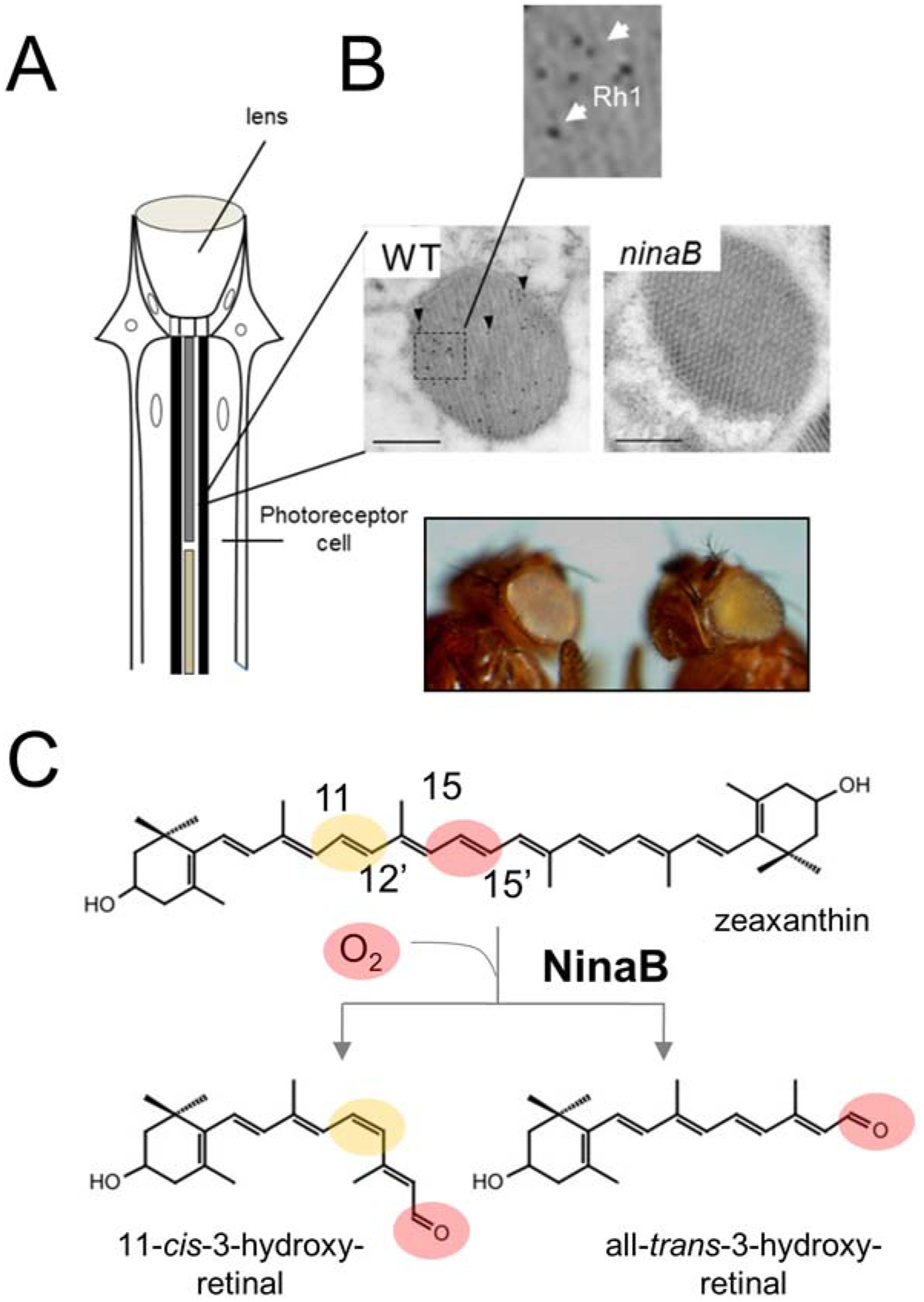
(A) Scheme of an omatidium of the insect compound eyes. (B) Electromicrographs of cross sections of rhabdomers immuno-gold stained for rhodopsin 1 (Rh1 encoded by the ninaE gene) of wild type and ninaB mutant flies. Note that ninaB360d flies lack Rh1. Photographs of wild type and ninaB360d mutants on a white eye genetic background. The eyes of ninaB360d flies appear yellow because of carotenoid accumulation. (C) Reaction catalyzed by NinaB protein with zeaxanthin. The sites for geometric isomerization and oxidative cleavage are respectively highlighted by yellow and red color.
Figure 6. Chromophore production in vertebrates.
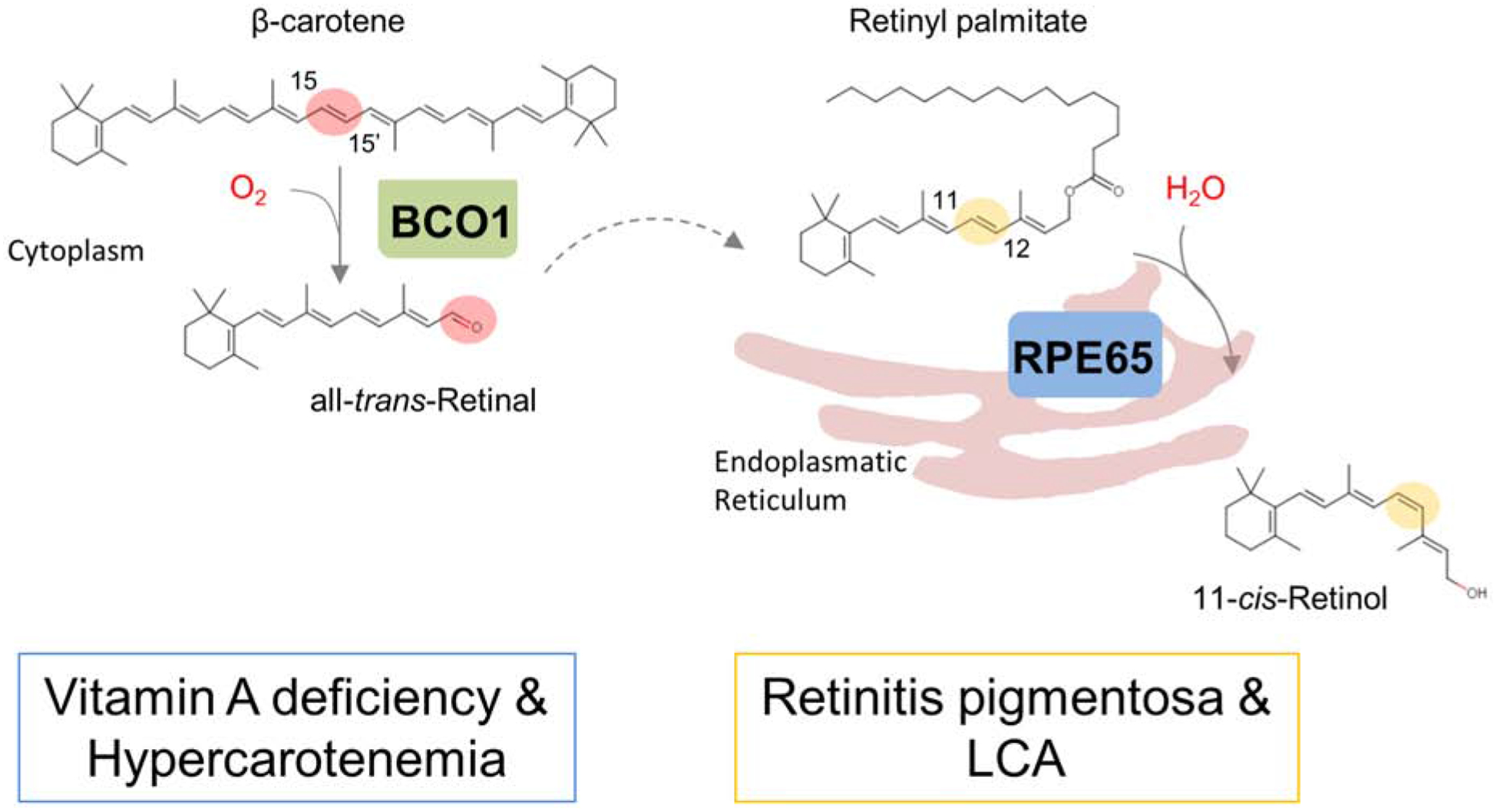
In vertebrates, two carotenoid cleavage oxygenases are involved in chromophore production. The β-carotene-15,15’-dioxygenase (encoded by the BCO1 gene) converts the β-carotene into all-trans-retinal. All-trans-retinal is further converted to retinyl palmitate. The retinoid isomerase (encoded by the retinal pigment epithelium protein of 65 kDa) splits all-trans-retinyl palmitate into 11-cis-retinol and palmitate.
4.2. Physiological roles of BCO1 and BCO2 in mammals
All three CCDs display distinct subcellular localizations. BCO1 exists in the cytoplasm, RPE65 at the endoplasmic reticulum, and BCO2 resides at the inner mitochondrial membrane (Golczak et al., 2010; Kowatz et al., 2013; Palczewski et al., 2014). On tissue and organ levels, BCO1 and BCO2 are expressed in many human tissues and cell types (Lindqvist, Andersson, 2002; Lindqvist et al., 2005), whereas RPE65 expression is restricted to the mammalian RPE that nourishes and maintains the adjacent photoreceptor in the retina with chromophore (Hamel et al., 1993). The expression pattern of BCO1 and BCO2 indicates that most cell types and tissues can metabolize carotenoids to apocarotenoids and can synthesize vitamin A. In enterocytes of the intestine, BCO1 expression is controlled by vitamin status via the transcription factor ISX (Lobo et al., 2013). In other tissues, BCO1 expression is controlled by peroxisome proliferator activator receptors (Boulanger et al., 2003; Lobo et al., 2010a). Knowledge about the transcriptional control of the BCO2 gene is limited. A recent study showed that BCO2 mRNA is highly expressed in the peripheral retina and at low level in the central (macula) retina of the human eye. This expression pattern may contribute to the specific carotenoid distribution in the human retina (Voigt et al., 2019). Differential expression of BCO2 in tissues has also been associated with skin coloration in birds (Eriksson et al., 2008) and more recently in lizards (Andrade et al., 2019). In human cell lines, BCO2 expression is induced by oxidative stress (Babino et al., 2015a). The latter regulation and the broad substrate specificity of BCO2 indicate that the enzyme acts as a carotenoid scavenger and may explain why carotenoid levels decrease in chronic disease states that are associated with inflammation and oxidative stress.
The generation of mouse models deficient for BCO1 and BCO2 set the stage for an in-depth analysis of the metabolism of dietary carotenoids (Amengual et al., 2011b; Hessel et al., 2007). These studies identified BCO1 as major β-carotene metabolizing enzyme and key enzyme for vitamin A production (Amengual et al., 2013). In its absence, only trace amounts of β-apo-10’-carotenols are produced from supplemented β-carotene by BCO2 (Amengual et al., 2011a; Amengual et al., 2013). Similarly, humans with inherited BCO1 deficiency show elevated β-carotene and reduced vitamin A levels (Lindqvist et al., 2007). Consistent with BCO2’s enzymatic properties and subcellular localization, xanthophylls accumulate in the mitochondria of BCO2-deficient mice (Amengual et al., 2011b). This accumulation of xanthophyll increases oxidative stress in tissues and reduces mitochondrial respiration rates (Amengual et al., 2011b; Lobo et al., 2012; Palczewski et al., 2016). Xanthophylls accumulate in oxidized form in mouse tissues (Amengual et al., 2011b; Palczewski et al., 2016). 4′,5′-Didehydro-retro-β-carotene-3,3′-dione (rhodoxanthin) and (6RS,6RS)-ε,ε-Carotene-3,3’-di-one were respectively identified as zeaxanthin oxidation products in white adipose tissues (Widjaja-Adhi et al., 2015) and in blood and liver (Amengual et al., 2011b; Palczewski et al., 2016). Lower levels of oxidized carotenoids were measured in lung and heart of CCD deficient mice (Amengual et al., 2011b; Amengual et al., 2013; Hessel et al., 2007). Xanthophyll also accumulate in the eyes of CCD knockout mice with higher levels in the RPE than in the neuronal retina (Li et al., 2014; Widjaja-Adhi et al., 2015). However, levels of carotenoids in the eyes were low when compared to liver, fat, and blood (Babino et al., 2015a). This observation clearly indicates that mice lack mechanisms for ocular carotenoid accumulation. BCO2-deficient mice also accumulate lycopene in different tissues, whereas levels of supplemented lycopene were indistinguishable between wild type control and BCO1-deficient mice (Ford et al., 2010).
Naturally occurring mutations in the BCO2 gene have been associated with the yellow skin phenotype in chicken (Eriksson et al., 2008) and the yellow fat phenotype in cattle and sheep (Berry et al., 2009; Tian et al., 2009; Vage, Boman, 2010). These findings demonstrate that turnover of carotenoids by CCDs plays an important role in the control of carotenoid homeostasis (Figure 7). Given the importance of carotenoids in vision, development, reproduction, and immunity, it has been speculated that BCO2 mutations in domestic animals help to adapt them to artificial diets low in carotenoids that are vastly different from those of their wild-living ancestors (Fallahshahroudi et al., 2019).
Figure 7. BCO2 mutations and the yellow fat phenotype.
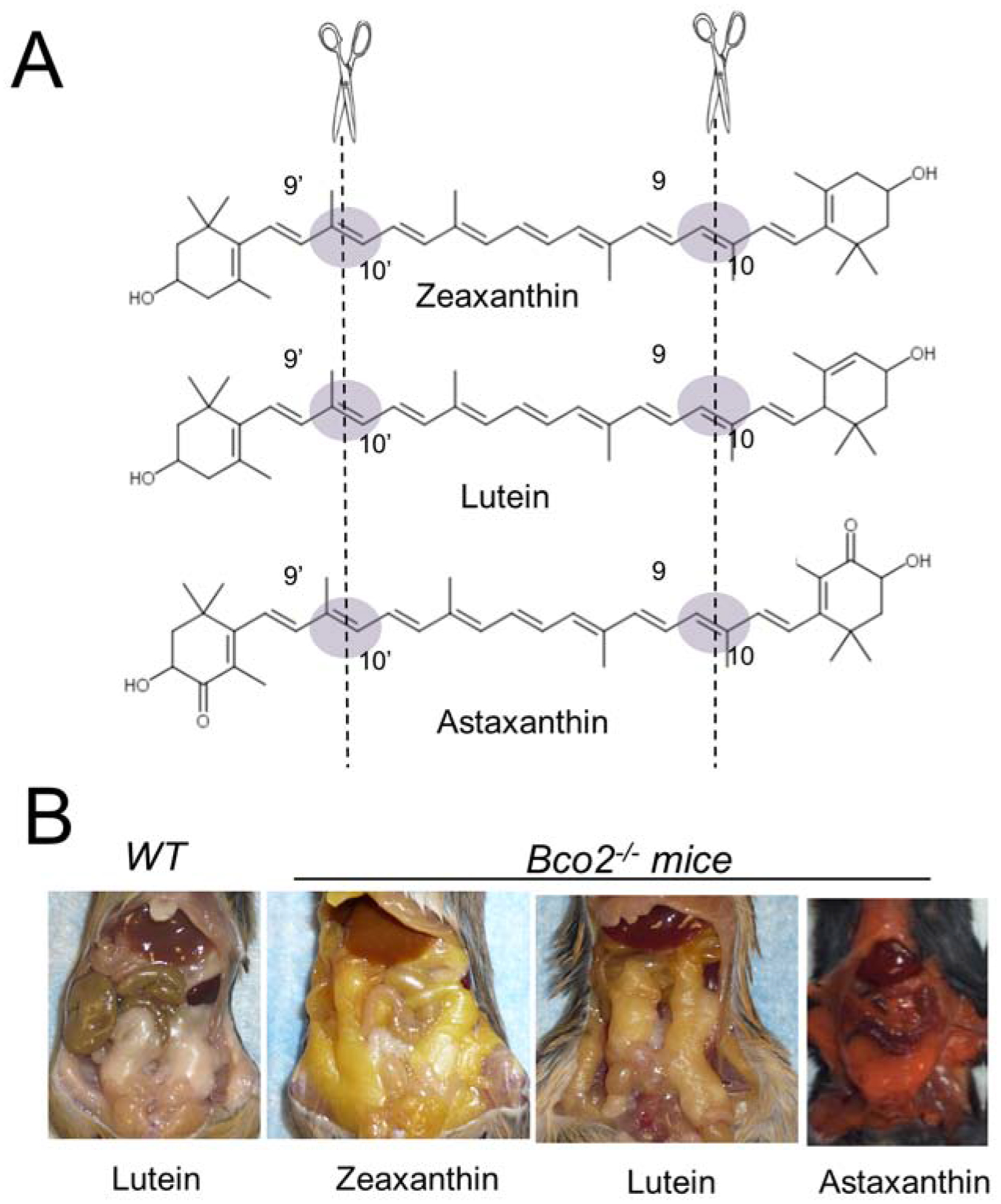
(A) Cleavage sites of β-carotene-9’,10’-dioxygenase in zeaxanthin, lutein, and astaxanthin substrates. (B) Yellow and red fat phenotype of Bco2−/− mice supplemented with zeaxanthin, lutein, and astaxanthin. Wild type mice do not display fat coloration when supplemented with carotenoids.
4.3. CCD structure and chromophore production
The structures of an apocarotenoid-oxygenase (ACO) (Kloer et al., 2005; Sui et al., 2014), the plant enzyme VP14 (Messing et al., 2010), and bovine RPE65 (Kiser et al., 2012; Kiser et al., 2009) have been resolved at atomic resolution. These analyses revealed that the overall architecture of CCDs is well conserved. The basic structural fold is a rigid seven-bladed β-propeller covered by a half-dome (Figure 8A). The Fe (II) of the active center is accessible through long non-polar tunnels. The ferrous iron center in CCDs is invariably coordinated by four His residues, with three Glu residues forming a second coordination sphere. Moderate resolution of the crystal structures of ACO and RPE65 show average Fe-Nε bond lengths of ~2.1–2.2 Å, a distance consistent with the 2.15 Å Fe-Nε bond length measured for RPE65 by X-ray absorption spectroscopy (XAS) (Khadka et al., 2019; Kiser et al., 2012). As indicated in the structure of iron-free ACO, this platform for iron-binding is rigid and does not change upon iron-binding (Kloer et al., 2005). Mutagenesis studies of the key metal-binding first and second sphere His and Glu residues indicate that iron is absolutely required for CCDs to perform their catalytic roles and that both first and second sphere ligands contribute to iron-binding and catalytic function (Poliakov et al., 2005; Redmond et al., 2005b; Sui et al., 2017; Sui et al., 2016; Takahashi et al., 2005). The requirement for a divalent iron in CCD catalysis is well documented by several studies (Kiefer et al., 2001; Lindqvist, Andersson, 2002; Moiseyev et al., 2006; Redmond et al., 2001; Schwartz et al., 1997). The putative role of Fe(II) in canonical double bond cleaving CCDs such as BCO1, ACO and VP14 is to activate oxygen for cleavage of carotenoid/apocarotenoid substrates (Borowski et al., 2008; Kloer, Schulz, 2006). It is now generally accepted that the incorporation of dioxygen follows a dioxygenase reaction mechanism (Babino et al., 2016; Dela Sena et al., 2014; Schmidt et al., 2006; Sui et al., 2015). In the RPE65 catalyzed isomerization reaction, Fe(II) acts as a Lewis acid that polarizes the ester group and is not involved in the activation of molecular oxygen (Kiser et al., 2015). For a detailed review of the structural arrangement of the active center and the mode of enzyme catalysis of CCDs we here refer to an excellent review article (Daruwalla, Kiser, 2019).
Figure 8. Structure and function of vertebrate CCDs.
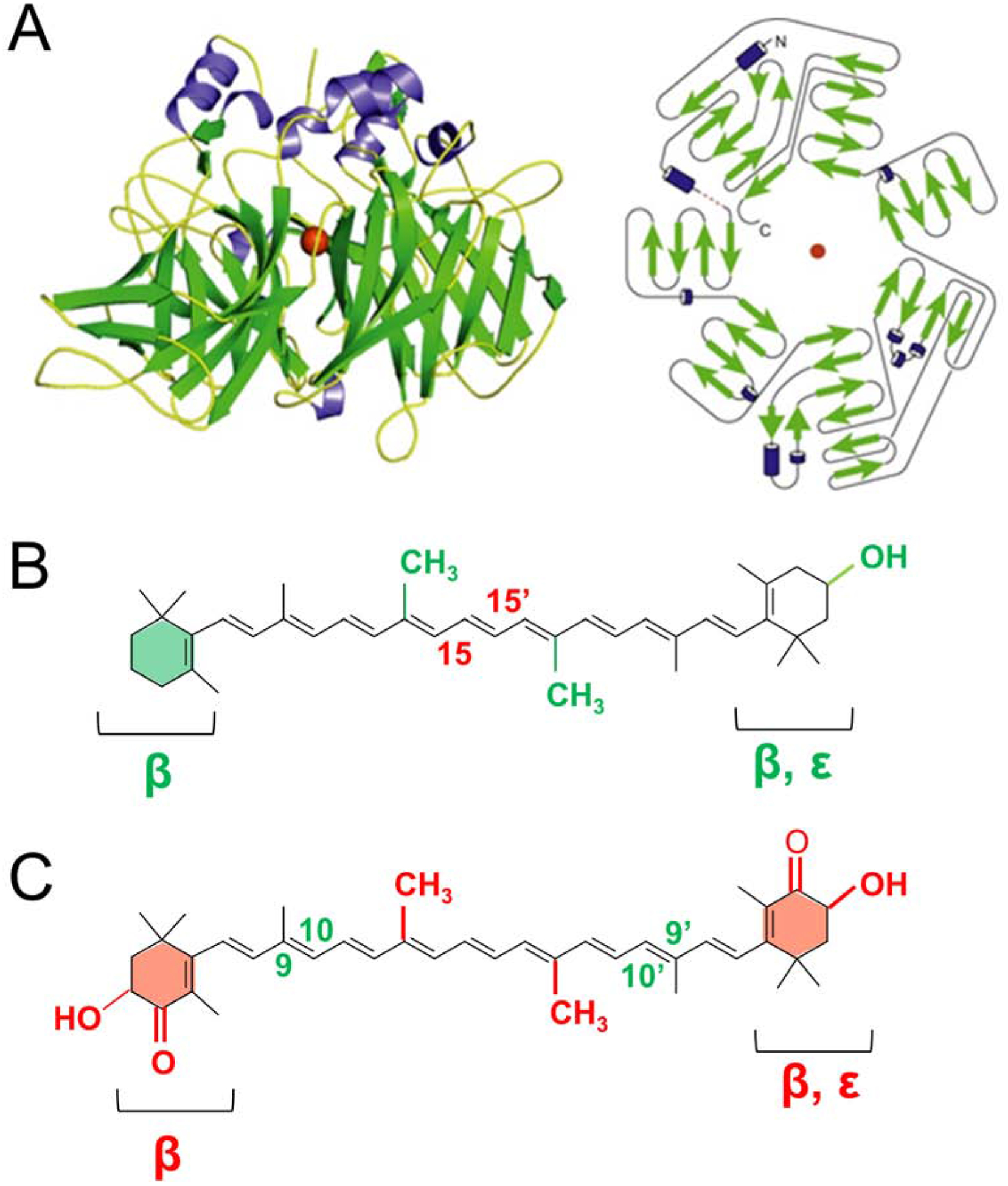
(A) Crystal structure and topology diagram of bovine RPE65. (PDB accession codes: 3FSN). The ferrous catalytic iron is colored in red. Secondary structural elements consisting of α-helices and β-sheets are colored in blue and in green, respectively. (B) Scheme of substrates of BCO1. The displayed structure highlights in green chemical allowed modifications of BCO1’s classical substrate β-carotene. These modifications include 3-hydroxy substitutions of the β-ionone ring, replacement of the β- by an ε-ionone, removal of methyl groups (nor). The central cleavage site at position C15, C15’ of BCO1 is indicated in red. Note that BCO1 substrates must contain at least one none-substituted β-ionone ring. (C) Scheme of substrates of recombinant mouse BCO2. The structure highlights in red the allowed chemical modifications of BCO2’s classical substrate BC. These modifications include 3-hydroxy substitutions and 4-oxo-substitutions of the β-ionone ring, replacement of the β- by an ε-ionone, or removal of methyl groups (nor). The eccentric cleavage sites at positions C9, C10 and C9, C10’ of BCO2 are indicated in green. Note that BCO2 cleaves substrates with assorted ionone ring site modifications.
Native bovine RPE65 has been crystallized in complex with the retinoid-mimetic drug ACU-4429 (emixustat) (Kiser et al., 2015). This study revealed that the active retinoid-binding cavity is located near the membrane-interacting surface as well as a Fe-bound palmitate ligand positioned in an adjacent pocket. The structural arrangement of the enzyme-substrate complex (Michaelis complex) of a canonical double bond cleaving CCD has not yet been determined. Our efforts to yield structures of recombinant BCO1 and BCO2 were met with a major difficulty. The greatest obstacles were co-purification of the bacterial chaperone GroEL and a lack of a native tissue source from which the enzymes can be purified in enough amounts. For recombinant ACO a crooked, rod-shaped electron density feature observed in the active site cavity after soaking ACO crystals with substrate was attributed to a bound apocarotenoid (Kloer et al., 2005). However, electron densities for both the β-ionone ring and alcohol tail of the apocarotenoid were invisible and only the central part of the substrate density was observed. These crystals were obtained in the presence of polyethylene glycol and the detergent octylpolyoxyethylene, molecules that also bear elongated carbon chains resembling an isoprene chain. The possibility that the observed electron density is a detergent was recently confirmed by a refined structural analysis of ACO (Sui et al., 2014). Improved protocols for the expression of CCDs as well as methods for the generation of metal substituted CCDs provides novel tools to analyze the interaction between CCDs and their carotenoid substrates (Sui et al., 2018).
The current knowledge allows us to hypothesize that the architecture of the substrate tunnels of CCDs significantly contributes to the selectivity of chromophore production. The overwhelming majority of animals use a single chromophore to avoid ambiguity in spectral sensitivity of visual pigments: Vertebrates use retinal (vitamin A1) and 3-dehydroretinal (vitamin A2), arthropods use retinal and 3-hydroxy-retinal (vitamin A3), and some crustaceans use 4-hydroxy-retinal (vitamin A4) (Goldsmith, 2013). For vitamin A1 production in mammals, BCO1 selectively interacts with the non-substituted β-ionone ring of provitamin A carotenoids (Kelly et al., 2018; Lindqvist, Andersson, 2002) (Figure 8B). Xanthophylls such as zeaxanthin and lutein with two hydroxylated β-ionone ring sites are not a substrate for the recombinant BCO1 enzyme (Kelly et al., 2018). Though the structure of BCO1 has not been solved, it can be assumed that the architecture of the enzyme hinders substituted ionone rings from entering the substrate tunnel. When comparing the predicted structures of BCO1 and BCO2, we reasoned that the diameter of the entrance of the substrate tunnel may be critical for substrate selectivity. The opening of the substrate tunnel should be wider in BCO2 than in BCO1 because BCO2 can accommodate bulky hydroxylated ionone rings in the substrate tunnel (Figure 8C). Comparison of the predicted structures of BCO1 and BCO2 identified two candidate amino acids (Trp270 and Leu168) that narrow the mouth of the substrate tunnel in BCO1. Exchange of these amino acids with the corresponding amino acids of BCO2 (Trp270Phe and Leu168Gly) by site-directed mutagenesis produced a mutant BCO1 enzyme that was able to cleave zeaxanthin with two hydroxylated ionone rings (Kelly et al., 2018). Thus, a small change in an overall conserved fold provides the structural basis of the ring site selectivity of BCO1 and BCO2.
A directional interaction of CCDs with ionone rings of the carotenoid substrates also plays a role in substrate recognition of insect NinaB (Oberhauser et al., 2008). Studies with recombinant NinaB from the wax moth Galleria showed that the enzyme only uses carotenoids with two ionone rings as substrates (Babino et al., 2016). In this respect, NinaB differs from mammalian CCDs which can convert apocarotenoids with one ionone ring site (Kelly et al., 2018) as well as the acyclic lycopene (dela Sena et al., 2013; Kiefer et al., 2001). Additionally, moth NinaB prefers 3-hydroxy-β-ionone ring sites over non-substituted ring sites to synthesize 3-hydroxy-retinal (Oberhauser et al., 2008), the unique vitamin A3 chromophore of higher flies and butterflies (Seki, 1998; Vogt, 1983). Mammalian BCO2 also preferentially interact with hydroxylated ionone rings of carotenoids (Kelly et al., 2018; Mein et al., 2010). This enzymatic property of BCO2 plays an important role for quality control of chromophore production in mammals (Fig. 8). BCO2 removes the non-canonical ring sites from asymmetric provitamin A carotenoids such as β-cryptoxanthin (Kelly et al., 2018). The resulting β−10’-apocarotenal is subsequently converted by BCO1 into vitamin A1-aldehyde. In the mouse intestine, all metabolites of this pathway were identified upon β-cryptoxanthin supplementation (Kelly et al., 2018).
Specific modification of the β-ionone ring by cytochrome P450 family member is another notable concept for chromophore synthesis. In lower vertebrates, the cytochrome P450 family member, CYP27c1, converts retinal (vitamin A1) into 3,4-didehydroretinal (vitamin A2). Genetic disruption of cyp27c1 abrogates the zebrafish’ ability to red-shift its photoreceptor spectral sensitivity (Enright et al., 2015). A cytochrome P450 enzyme also has been implicated in the conversion of retinaldehyde (vitamin A1) into 3-hydroxy-retinal (vitamin A3) in flies (Seki et al., 1998). Drosophila can also convert supplemented β-carotene to zeaxanthin which then is converted by NinaB into vitamin A3 (Voolstra et al., 2010). Though not directly related to chromophore production, the cytochrome P450 enzyme CYP2J19, acts as a carotenoid ketolase in astaxanthin synthesis in birds (Lopes et al., 2016). Thus, stereo- and region-selectivity of CCDs as well modifications of the β-ionone ring play critical roles for chromophore production in different animal species.
5. The Absorption of Carotenoids
5.1. Analysis of Drosophila ninaD mutant revealed protein-dependency of fat soluble vitamin absorption
In contrast to carotenogenic organisms, animals must acquire carotenoids from the diet. For many years it was assumed that the uptake of dietary lipids, including carotenoids, occurs via passive diffusion. However, studies in cell culture revealed that carotenoid but not retinoid absorption is saturable (During et al., 2002) and it is now well accepted that it depends on scavenger receptors (Reboul, Borel, 2011). To our knowledge, the protein-dependency of this process was firstly demonstrated by our analysis of the Drosophila mutant ninaD (Kiefer et al., 2002) (Figure 9). Initially, this mutant was identified in a large screen for visual pigment-deficient mutants and was shown to lack chromophore when raised on standard corn meal chow (Giovannucci, Stephenson, 1999; Stephenson et al., 1983). We searched the genomic region to which the ninaD gene was mapped and focused on a gene that encodes a class B scavenger receptor. Class B scavenger receptors were previously characterized to facilitate cholesterol transport between high density lipoproteins and cells in mammals (Acton et al., 1996; Kozarsky et al., 1997). We reasoned that NinaD may facilitate carotenoid uptake in the fly because of the similar chemical characteristics between carotenoids and cholesterol. Our candidate approach identified a nonsense mutation in the ninaD gene that led to a premature stop codon. We also showed that expression of a wild type ninaD transgene rescued the blind phenotype and restored visual pigment synthesis in the mutant (Kiefer et al., 2002). Later, it was shown that ninaD mutant flies also lack tocopherols (vitamin E) and that ninaD is expressed in the midgut of the larva (Voolstra et al., 2006; Wang et al., 2007). This expression pattern coincides with the time point when the larva acquires carotenoids for chromophore production during compound eye development in the pupal stage of this holometabolous insect. When expressed in Drosophila Schneider cells, NinaD localizes to the plasma membrane and facilitates carotenoid uptake from synthetically mixed micelles (Voolstra et al., 2006) (Figure. 9 B, C). A second NinaD homologous scavenger receptor encoded by the santa maria gene facilitates carotenoid uptake into neuronal and glial cells where carotenoids are converted to chromophore by NinaB (Wang et al., 2007). The transport of chromophore to photoreceptors employs a CRAL-TRIO domain protein encoded by the pinta gene (Wang, Montell, 2005). Eyes of the pinta mutant fly strain contain chromophore but display highly reduced levels of rhodopsin (Voolstra et al., 2010). This finding indicates that Pinta is required for the transport of chromophore from its place of production to photoreceptor cells. The dependency of retinoid transport from Pinta is demonstrated by another study. Ectopic expression of a ninaB transgene renders the fly eyes, carotenoid and chromophore deficient (Voolstra et al., 2010). Retinoids only can be used as chromophore precursors in the fly when supplied in very high doses with the chow (Wang et al., 2010) or when an alcoholic retinoid solution is directly applied on the eyes with a brush(Voolstra et al., 2010).
Figure 9. NinaD and carotenoid transport.
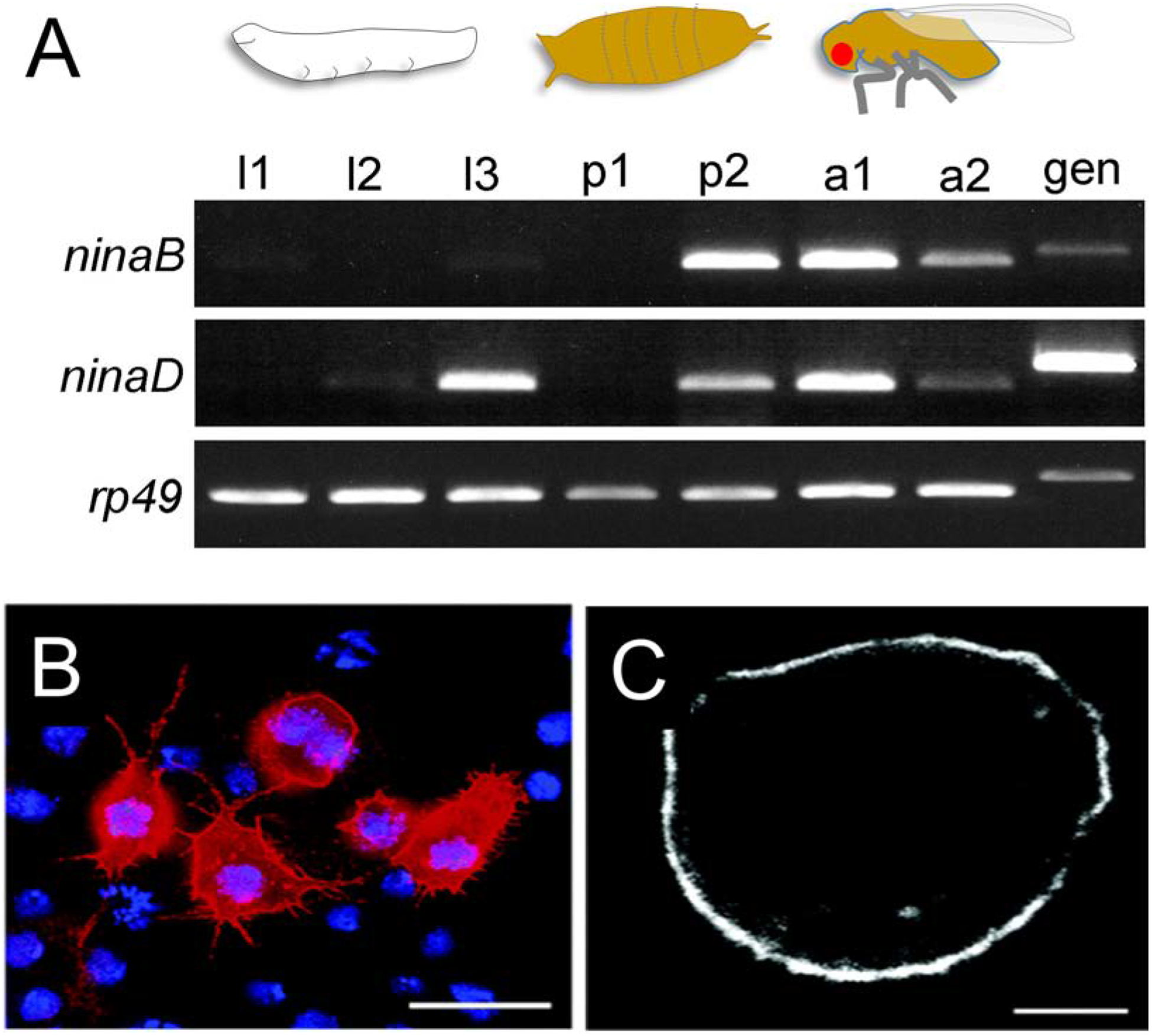
(A) Temporal expression patterns of NinaD and NinaB during the flies’ life cycle. NinaD is expressed at late larval stages and helps rendering carotenoids available from the chow. NinaB expression increases at late pupal stages timely coinciding with rhodopsin synthesis in the compound eyes. l1–3, first, second and third instar larval stages; p1, early pupa; p2, late pupa, a1, adult (< 3 days old); a2, adult (10 days old), gen, genomic DNA control. (B, C) Immunocytochemistry of transiently transfected S2 cells expressing ninaD-I. Panels B shows an epifluorescence image showing labeling of NinaD-I, which was detected with an antibody raised against the V5 epitope of the recombinant NinaD fusion protein and Cy3-conjugated secondary antibody (red). Cell nuclei are stained with DAPI (blue). Scale bar is 10 μm. Panels C shows a confocal image depicting the immunofluorescence labeling of NinaD-I in one optical section that is approximately 0.26 μm thick. Scale bars are 2.5 μm.
Research in other insect species confirmed the initial findings about the ninaD gene and carotenoid transport. Analysis in the silk worm Bombyx mori showed that carotenoid absorption is mediated by a NinaD-related protein encoded by the Yellow Cocoon gene (Sakudoh et al., 2010). Additionally, a carotenoid-binding protein (CBP) is encoded by the Yellow Blood gene. CBP is a member of the family of steroidogenic acute regulatory (StAR) proteins and required for carotenoid accumulation in the silk gland (Sakudoh et al., 2010).
5.2. Class B scavenger receptors in vertebrate fat-soluble vitamin absorption
In mammalian cell lines, the scavenger receptor class B type 1 (SR-B1) (During et al., 2005; During et al., 2008; Goncalves et al., 2014; Reboul et al., 2006; van Bennekum et al., 2005; Voolstra et al., 2006; Widjaja-Adhi et al., 2015) and cluster determinant 36 (CD36) facilitate carotenoid uptake from micelles (During et al., 2005; Reboul et al., 2005). The involvement of SR-B1 in the intestinal absorption of β-carotene and zeaxanthin was later confirmed in mice (van Bennekum et al., 2005; Widjaja-Adhi et al., 2015). Studies in mice also demonstrated that SR-B1 facilitates the intestinal absorption of fat soluble vitamins E and K (Goncalves et al., 2014; Reboul et al., 2006). Moreover, it was demonstrated that CD36 also contributes to the intestinal absorption of dietary isoprenoids in mice (Borel et al., 2013; Shyam et al., 2017b). The critical role of SR-B1 in this process is beautifully demonstrated by the white recessive mutation of canary birds. This phenotype is caused by a splice site mutation in the SR-B1 gene (Toomey et al., 2017). The mutant bird shows white feather coloration and very low levels of carotenoids in blood and tissues. These birds also suffer from severe vitamin A deficiency and depend on supplementation with preformed vitamin A via the diet (Preuss et al., 2007).
Scavenger receptors are glycosylated transmembrane proteins with a large extracellular domain. Structural prediction, based on the crystal structure of the CD36 family member lysosomal membrane protein 2 (LIMP II), (Neculai et al., 2013) indicate the presence of a large cavity traversing the entire length of the protein that serves as a tunnel for lipid transfer from extracellular to cellular compartments (Rodrigueza et al., 1999; Yu et al., 2012). Mammalian CD36 and SRB1 have been associated with a wide range of physiological processes. Their broad substrate specificity relies on their ability to recognize similar molecular patterns rather than specific epitopes. CD36 substrates include carotenoids, long chain fatty, native or modified lipoproteins, thrombospondin-1, collagen, apoptotic cells, amyloid B, and malaria-infected erythrocytes (Febbraio, Silverstein, 2007). CD36 is expressed in muscle, adipose tissue, intestine and the capillary endothelium, where it facilitates long chain fatty acid uptake into target cells in capillary beds of tissues (Abumrad et al., 1993; Greenwalt et al., 1992), (Goldberg et al., 2009). CD36-deficient mice display among other defects, significantly increased levels of circulating fatty acids (Cifarelli, Abumrad, 2018). SR-B1 binds high-density lipoproteins (HDLs) (Connelly et al., 1999) and facilitates selective cellular uptake of cholesterol in mammalian steroidogenic tissues (Acton et al., 1996). SR-B1 is expressed in the liver, intestine, macrophages, adrenal gland, and ovary (Acton et al., 1994; Cifarelli, Abumrad, 2018). SR-B1-deficient mice develop hypercholesterolemia and multiple pathologies, including male sterility (Acton et al., 1996).
5.3. Regulation of carotenoid absorption and vitamin A production in the intestine
Studies in human and experimental animals provided evidence that the vitamin A status of the subject affects carotenoid absorption (Borel et al., 2011; Meyers et al., 2014) and bioconversion to vitamin A in the intestine (Lala, Reddy, 1970; van Vliet et al., 1996; Villard, Bates, 1986). It is now generally acknowledged that vitamin A status affects β-carotene absorption and bioconversion efficiency via a negative feedback loop (Bachmann et al., 2002; Borel et al., 2015). The control of vitamin A production in intestinal enterocytes is a rare example of negative transcriptional feedback regulation. The Bco1 gene is expressed by default when there is a demand for vitamin A (Bachmann et al., 2002; Seino et al., 2008). However, if preformed vitamin A or β-carotene is present in the diet, enterocytes rapidly cease production of vitamin A (Bachmann et al., 2002; Lobo et al., 2010b; Seino et al., 2008). Similarly, when the body’s vitamin A stores are filled, the gene expression of Bco1 is repressed in brush border cells (Lobo et al., 2010b). This regulation avoids excess vitamin A production that can be detrimental (Lobo et al., 2013; Widjaja-Adhi et al., 2017).
The repressor protein of vitamin A production has been identified as the intestine specific homeobox transcription factor ISX (Choi et al., 2006; Seino et al., 2008). This transcription factor binds to conserved motifs in promoter region of the Bco1 gene (Lobo et al., 2013; Widjaja-Adhi et al., 2015) (Figure 10). The small molecule inducer that regulates the activity of the repressor protein is retinoic acid (Lobo et al., 2010b). The acidic form of the vitamin binds to retinoic acid receptors to induce Isx mRNA expression in the intestine (Lobo et al., 2010b). In turn, ISX binds to the promoters of the Bco1 and SR-B1 gene and represses their mRNA expression (Lobo et al., 2013; Widjaja-Adhi et al., 2015).Thus, in the presence of elevated levels of RA protein levels of SR-B1 and BCO1 decrease in the enterocytes and absorption and bioconversion of carotenoids are diminished. This indirect transcriptional regulation of the repressor protein is required because the Bco1 gene promoter is controlled by vitamin A-independent mechanisms in other cell types of the body (Boulanger et al., 2003; Lobo et al., 2010b). In the intestine of vitamin A sufficient mice, ISX is expressed at higher levels in distal rather than in proximal parts (Seino et al., 2008). The ISX target genes, encoding BCO1 and SRB1, reveal a reverse pattern of expression (Seino et al., 2008) which guarantees that provitamin A carotenoids are effectively acquired from the diet in the vitamin A deficient state. In a vitamin A sufficient scenario, the shutdown of vitamin A production prevents excessive accumulation of retinoids and imbalances in intestinal RA production (Widjaja-Adhi et al., 2017).
Figure 10. A diet-responsive regulatory network controls intestinal vitamin A production and carotenoid absorption.
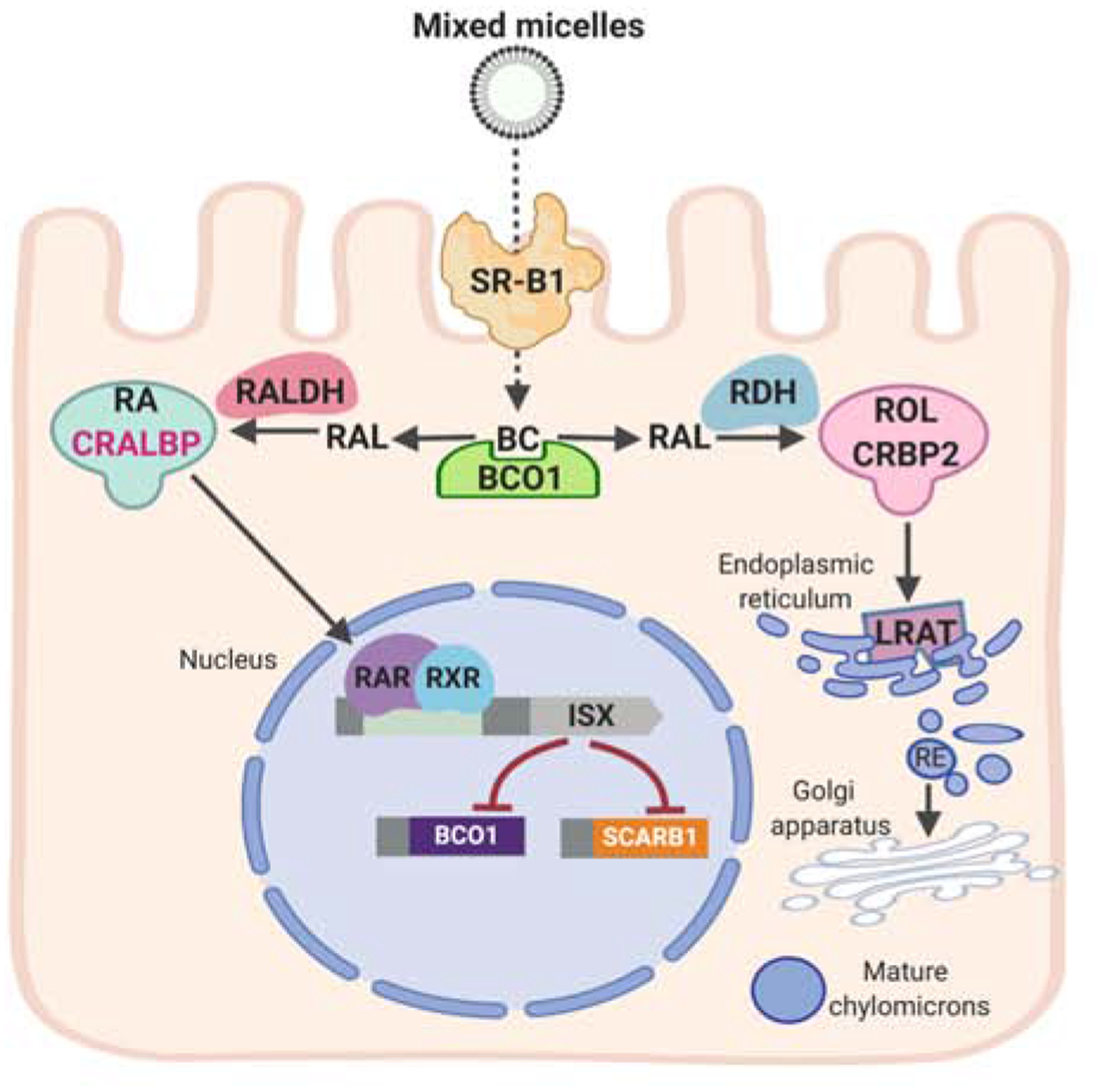
β-Carotene (BC) absorption is facilitated by scavenger receptor class B type 1 (SR-B1). The conversion of BC by β-carotene-15,15’-dioxygenase (BCO1) is an important branching point in retinoid metabolism. The primary product retinaldehyde (RAL) is reduced to retinol (ROL) by retinal dehydrogenases (RDH) in a process that involves cellular retinol-binding protein (CRBP2). ROL is the converted to retinyl esters (RE) by lecithin: retinol acyl transferase (LRAT) and packed in chylomicrons for body distribution. Some RAL is also converted to retinoic acid (RA) by retinal dehydrogenase (RALDH). RA binds to retinoic acid receptors (RARs) that in conjunction with retinoid X receptors (RXR) regulate the expression of intestine specific homeobox (ISX) transcription factor. ISX is a transcriptional repressor of the genes encoding SCRAB1 and BCO1.
Significant SNPs were found in the genes encoding BCO1, SR-B1, and the transcription factor ISX (Borel et al., 2011; Borel et al., 2015; Ferrucci et al., 2009; Leung et al., 2008). Interestingly, SNPs in BCO1 are associated with macular pigment density (Meyers et al., 2013). This finding is surprising at first glance since xanthophylls are not a substrate for the vitamin A forming enzymes. However, the vitamin A-dependent regulation of SR-B1 expression in the intestine explains the interaction between xanthophyll accumulation and β-carotene metabolism. It has long been known in chicken that absorption of xanthophyll is reduced in the presence of dietary preformed vitamin A (Thompson, 1975).
Genetic makeup also influences the conversion rate of provitamin A carotenoids (Grune et al., 2010; Leung et al., 2008; Lietz et al., 2012) and may constitute the basis for the low and high responder phenotypes described in the general European male population (Borel et al., 1998). Additionally, several SNPs in proteins that affect lipid and lipoprotein metabolism have been associated with carotenoid blood levels (Borel, Desmarchelier, 2018). The latter association indicates significant interactions between carotenoid and lipid metabolism. Such interactions have been demonstrated in mouse models (Palczewski et al., 2016). Most of the genetic data in humans stem from the analyses of β-carotene, lycopene, and lutein fasting blood levels, but it is assumed that these genetic variations also modulate the bioavailability of the other carotenoids found in human blood and tissue (Borel, Desmarchelier, 2018).
6. Enzyme Classes Involved in Retinoid Metabolism
The term retinoid was coined by Sporn and colleagues in the mid-1970s (Sporn et al., 1976). Retinoids comprise both natural and synthetic compounds with structural resemblance to all-trans-retinol, with or without the biological activity of vitamin A. Hence, vitamin A (by definition all-trans-retinol) is a natural retinoid. The metabolism of retinoids has been recently reviewed in several excellent articles (Blaner et al., 2016; Chelstowska et al., 2016; D’Ambrosio et al., 2011; Kiser et al., 2014). For completeness, we will introduce the enzyme classes of these metabolic processes because they will later become important for the discussion of ocular retinoid metabolism (Figure 11). Retinaldehyde, the product of β-carotene splitting, can be either oxidized to all-trans-RA or reduced to all-trans-retinol. All-trans-retinol is converted to retinyl esters (RE). The alcohol and ester form of vitamin A are the predominant retinoids in tissues, whereas the acidic form of the vitamin exists only in very low (nanomolar) concentrations in most tissues (Kane et al., 2010). The tissue concentrations levels of RA are tightly controlled, and even small amounts of this hormone-like compound are sufficient to elicit profound cellular responses through the activation of RA receptors (Giguere et al., 1987; Petkovich et al., 1987). RARs belong to the superfamily of nuclear receptors. Nuclear receptors function as ligand-activated transcription factors. They were first recognized as the mediators of steroid hormone signaling and provided an important link between transcriptional regulation and physiology. The human genome encodes 48 members of this transcription factor family, including classic endocrine receptors that mediate the actions of steroid hormones, thyroid hormones, and the fat-soluble vitamins A and D, but also nuclear receptors that acts as lipid sensors and in many other cellular processes (Chawla et al., 2001). Nuclear receptors contain two major domains: A amino-terminal DNA binding domain with two conserved zinc finger motifs that is connected with a flexible hinge region to the carboxy-terminal ligand binding and dimerization domain. Upon ligand binding, nuclear receptors undergo a conformational change that result in the dissociation of corepressors and in the recruitment of coactivator proteins to enable transcriptional activation. RARs form obligate dimers with retinoid X receptors (RXRs) and control transcription by binding to conserved DNA motifs (retinoic acid response elements) in promoter regions of about 500 target genes in the human genome (Balmer, Blomhoff, 2002). The amount of RA in tissues is tightly controlled throughout the mammalian life cycle by cytochrome P450-dependent hydroxylases CYP26A1, CYP26B1 and CYP26C1 that catalyze the production of 4-hydroxy and 4-oxo-RA (Abu-Abed et al., 2001; Abu-Abed et al., 1998; Niederreither et al., 2002; Zhong et al., 2019). The responsiveness of CYP26A1 expression to RA indicates that this enzyme is a major contributor to the catabolism of acidic retinoids. This metabolic regulation is found for many CYP enzymes which catabolize dietary lipids and prevent their excessive accumulation. Regulation activities of the other RA catabolizing enzymes, CYP26B1 and CYP26C1, are more complex and description of their physiological roles are subject of ongoing research.
Figure 11. Overview of mammalian vitamin A metabolism.
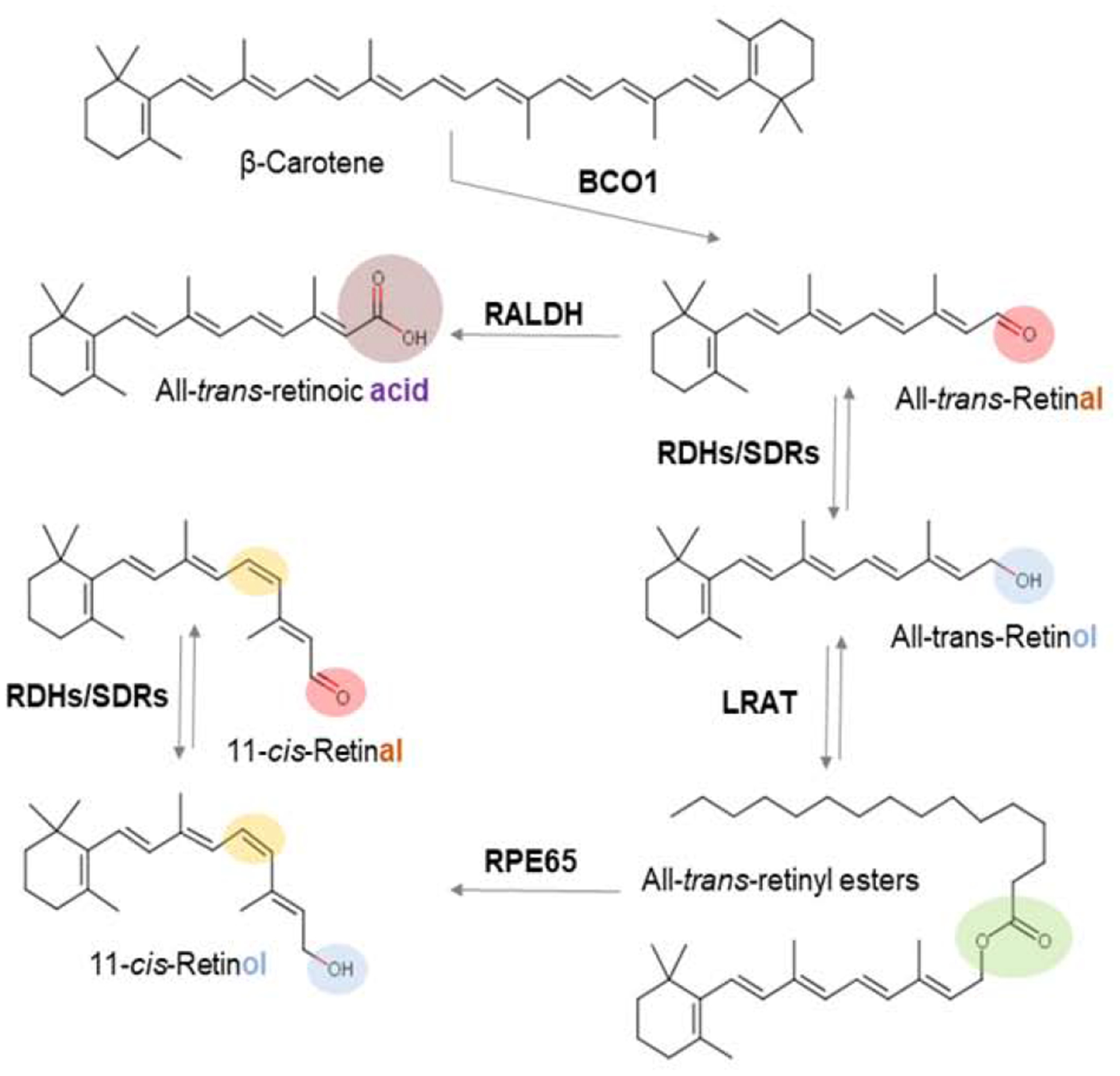
The different functional groups of retinoids are indicated by a color code (red, aldehyde; blue, alcohol; green, ester; purple, acid). The 11-cis-retinoid diastereoisomer is indicated by yellow color. Major retinoid metabolizing enzyme classes (see main text) catalyzing the chemical transformations of retinoids are highlighted.
The interconversion of retinol to retinal is catalyzed by cytosolic alcohol dehydrogenases (ADHs) and microsomal retinol dehydrogenases (RDH) (Kedishvili, 2013; Napoli, 2012). The latter belong to the short chain dehydrogenase/reductase protein family (SDR). Adenine dinucleotide cofactors NAD(H) and NADP(H) are the redox carriers of these reactions. The enzymes bind their cofactors by a conserved sequence motif, the Rossmann-fold, which consists of six to seven parallel β-strands flanked by three to four α-helices (Hofmann et al., 2016; Kiser et al., 2014). ADHs and RDHs use different catalytic mechanisms with either a zinc atom or a tyrosine in the active center, respectively. ADHs and RDHs catalyze an equilibrium reaction which depends on the oxidative state of their redox carriers. Under physiological conditions, the ratio of NAD/NADH is around 1000 in the cytoplasm, and the ratio of NADP/NADPH is 0.005. Thus, enzymes using NAD as redox carriers catalyze the oxidation while enzymes using NADP catalyze reduction of retinoids.
Though ADHs and RDHs can use retinol and retinal as substrates, most of these enzymes can metabolize other alcohols including sterols (Kedishvili, 2013). Therefore, knowledge about the physiological functions of these enzymes stem from loss-of-function studies in knockout mice. (Kedishvili, 2013; Kumar et al., 2012; Napoli, 2012). The observed consequences of the gene knockouts are discussed below. In extraocular retinoid metabolism, RDH10 plays a critical role in RA synthesis (Sandell et al., 2007). DHRS3 has been identified as critical retinal reductase in the embryo (Billings et al., 2013) and post-developmentally in adult tissues (Adams et al., 2014; Zolfaghari et al., 2012). RDH1 contributes to retinoid homeostasis in adult tissues as well (Napoli, 2012; Obrochta et al., 2015; Zhang et al., 2007). RDH1-deficient mice display increased hepatic retinyl ester stores and altered body fat mass (Zhang et al., 2007). RDH11 is required for the maintenance of all-trans-retinol steady-state levels in mouse liver and testis (Belyaeva et al., 2018).
The conversion of retinaldehyde to RA is catalyzed by specific members of the aldehyde dehydrogenases (ALDHs) family. The cytosolic Class I ALDHs were initially termed ALDH1 in human, Ahd2 in the mouse, and RALDH or RalDHI in rat (Napoli, 2012). Mammalian genomes encode three different family members which are now termed ALDH1A1, ALDH1A2 and ALDH1A3, respectively. Most knowledge about embryonic RA synthesis comes from genetic dissection in single and compound knockout mice for these enzymes (Duester, 2008; Rhinn, Dollé, 2012). These studies clearly identified Aldh1A2 as a major enzymatic system for the production of RA as indicated by early embryonic death of null mice (Niederreither et al., 1999). Aldh1A3 is expressed in specific compartments later during mouse development (Mic et al., 2000). Aldh1A3-null mice display eye defects and die at birth due to respiratory distress consistent with the enzyme’s role at later developmental stages (Vermot et al., 2003). In humans, Aldh1A3 gene mutations are associated with congenital microphthalmia (Yahyavi et al., 2013). Aldh1A1 knockout mice develop normally and are fertile (Fan et al., 2003b). These mice display several metabolic anomalies during adult life, e.g., they are resistant to diet-induced obesity (Ziouzenkova et al., 2007).
The conversion of retinol to RE is achieved by lecithin:retinol acyltransferase (LRAT) a vertebrate specific enzyme and has been molecularly cloned from several species. Studies in mice and zebrafish confirmed the enzyme’s critical role in retinoid metabolism and vitamin A homeostasis (Batten et al., 2004; Isken et al., 2007; Ruiz et al., 2001; Ruiz et al., 1999). Additionally, a diacylglycerol acyltransferase 1 (DGAT1) contributes to RE formation in some tissues (O’Byrne et al., 2005; Wongsiriroj et al., 2008). While DGAT1 uses coenzyme A as acyl donor for RE production. LRAT, a member of the ancestral NlpC/P60 thiol peptidase protein superfamily (Golczak et al., 2015; Pang et al., 2012), selectively transfers an acyl moiety from the sn-1 position of phosphatidylcholine (Golczak et al., 2015; MacDonald, Ong, 1988). The general structural motif of NlpC/P60 thiol peptidase is reminiscent of papain-like proteases and consists of a four-strand antiparallel β-sheet and three α-helices. The conserved catalytic residues Cys161, His60, and His72 define the active site. LRAT adopts an analogous catalytic strategy as thiol peptidases, whereby the deprotonated Cys161 serves as a nucleophile to attack the carbonyl carbon of an ester bond at the SN1 position of phosphatidylcholine, eventually leading to an acyl transfer and trans-esterification of retinol. LRAT-deficient mice lack liver and lung retinyl ester stores and are highly susceptible to vitamin A deficiency (Liu, Gudas, 2005; O’Byrne et al., 2005).
7. Carotenoid and Retinoid transport to the eyes
The hydrophobic nature of carotenoids and retinoids limits their solubility and diffusion in the aqueous environment of the body. Therefore, carotenoids and retinoids are transported in the main lipoprotein classes of the body together with other lipids such as fatty acids, cholesterol, and fat-soluble vitamins. Additionally, animals have evolved specific binding proteins for the pigments. In vertebrates, three classes of retinoid-binding proteins named for their selectivity (i.e. retinol-binding proteins (RBP1–4), cellular RA-binding protein (CRABP1 and 2), and cis-retinoid-specific cellular retinal-binding proteins (CRALBP) carry retinoids within cells. Additionally, the interstitial retinol-binding protein 3 (IRBP) transports vitamin A between RPE and photoreceptors of the eyes (Liou et al., 1982; Liou et al., 1989). Vertebrate RBPs and CRABPs are members of lipocalin family of lipid-binding proteins. CRALBP, like insect Pinta, belongs to the Sec14 protein family of CRAL-Trio proteins (Widjaja-Adhi, Golczak, 2019). An isoform of glutathione-S-transferase, GSTP1, and a steroidogenic acute regulatory domain protein, StARD3, were described as zeaxanthin and lutein-binding proteins in the human retina (Bhosale, Bernstein, 2007; Li et al., 2011). The StARD3 protein belongs to the same protein class as the CBP from silkworm (Bombyx mori) (Sakudoh et al., 2007).
7. 1. Vitamin A transport
For transport in lipoproteins, dietary vitamin A precursors are converted into RE and are incorporated into chylomicrons together with parent carotenoids and other dietary lipids (O’Byrne, Blaner, 2013; O’Byrne et al., 2005) (Figure 12). Chylomicron production occurs at the endoplasmic reticulum of enterocytes and requires surface proteins apolipoprotein B48 (apoB-48) and apolipoprotein A-IV. Microsomal triglyceride transport protein (MTP) catalyzes the lipidation of apoB-48 in the inner leaflet of the ER (Iqbal et al., 2008). The assembled pre-chylomicrons translocate to the Golgi apparatus where it acquires apolipoprotein A1. Mature chylomicrons are then secreted into the lymph and reach the circulation at the level of the subclavian vein. In the circulation, chylomicrons interact with lipoprotein lipase of peripheral tissues (He et al., 2018). The resulting chylomicron remnant is taken up by the liver for processing of its remaining lipid cargo. Retinyl esters are cleaved into fatty acid and retinol by a yet not molecularly identified retinyl ester hydrolase(s) and transported to stellate (Ito) cells. Here, they are re-esterified by LRAT and stored in lipid droplets (D’Ambrosio et al., 2011; O’Byrne et al., 2005).
Figure 12. Transport of vitamin A throughout the body.
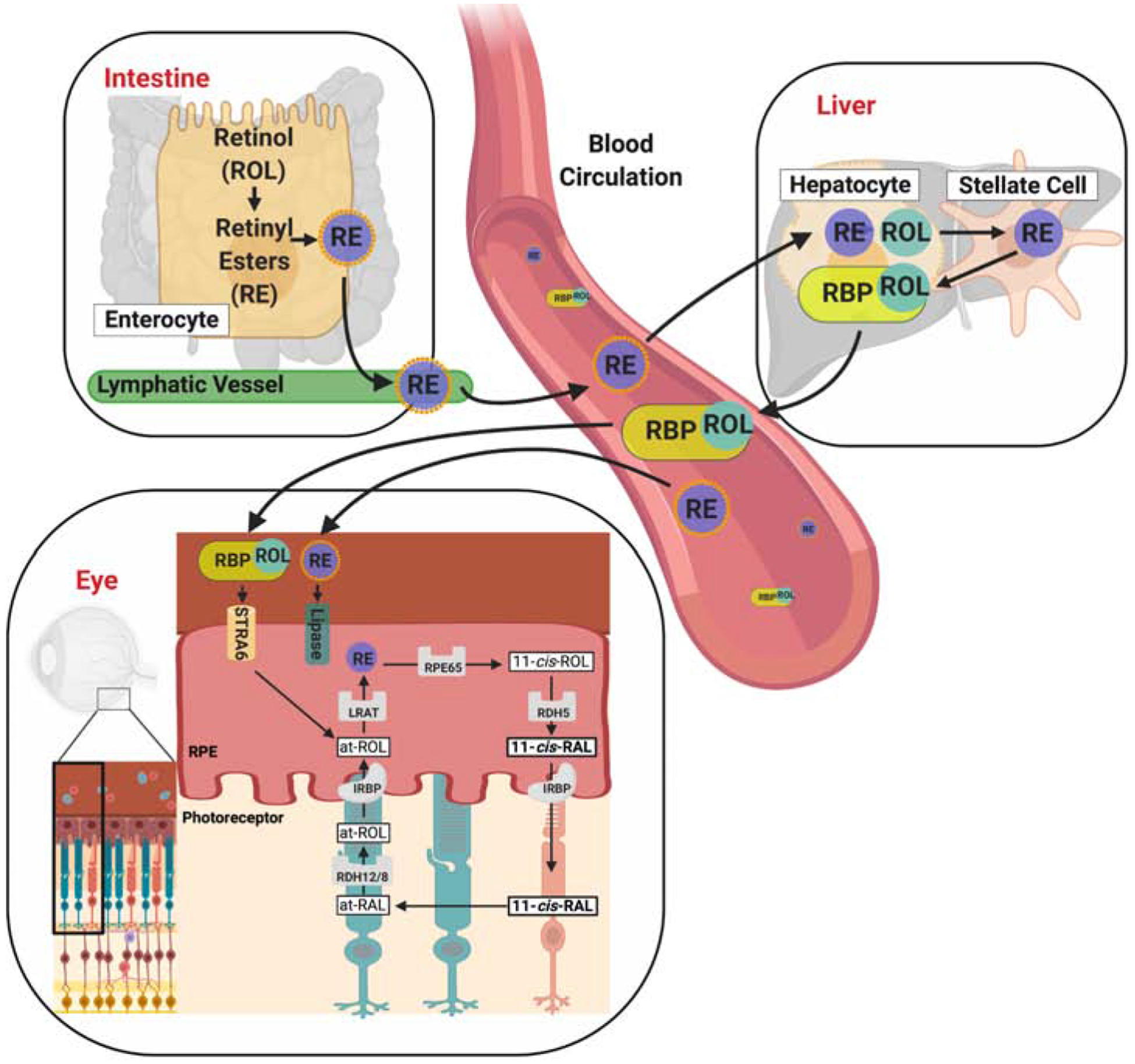
Carotenoids such as β-carotene are absorbed in brush border cells in the intestine, where they are converted into retinyl esters (RE), packaged into chylomicrons, and released into the circulation. The RE in chylomicrons can either be delivered to peripheral cells and taken up in a lipoprotein lipase-mediated process or be transported for receptor-mediated endocytosis and storage to the liver. In the liver, stored RE are converted back to retinol which binds to serum retinol-binding protein (RBP, encoded by the RBP4 gene). The retinol-RBP complex is released into the circulation. Vitamin A is taken up from holo-RBP in a STRA6-mediated transport process. In the retina pigment epithelium (RPE) of the eyes, vitamin A is esterified during uptake and converted to chromophore through the visual cycle. RAL, retinal, ROL, retinol, RDH, retinal dehydrogenase
All-trans-retinol bound to RBP4 (holo-RBP4) is the major form of vitamin A in the fasting circulation. RBP4 is mainly produced in the liver (Thompson et al., 2017) and its secretion depends on the presence of vitamin A (Quadro et al., 1999) (Figure 12). In the blood stream, holo-RBP4 forms a ternary complex with transthyretin (Episkopou et al., 1993). The levels of this complex are homeostatic and not affected by fluctuations in dietary vitamin A supply. For many years, it remained elusive how retinol, in its transport form can cross cell membranes and enter the cytoplasm of target cells. In the mid 1970’s, two groups independently reported evidence for the existence of a cellular receptor for RBP4. This molecule is present on the basolateral surface of bovine retinal pigment epithelium (RPE) cells (Heller, 1975; Heller, Bok, 1976) as well as on cells of the monkey’s small intestine (Rask, Peterson, 1976). Thirty years after the biochemical description of the RBP4 receptor, the molecular nature of this protein was elucidated. This breakthrough was achieved using an elegant strategy that stabilized the fragile interaction between recombinant tagged RBP4 and its receptor by protein cross-linking followed by high affinity purification of the complex (Kawaguchi et al., 2007). The RBP4 receptor protein is encoded by the stimulated by retinoic acid gene 6 (STRA6) that has been previously identified as a RA-inducible gene (Bouillet et al., 1997). Cell culture studies revealed that STRA6 gene product fulfills all three criteria expected for a bona fide RBP4 membrane receptor: 1) RBP4 binds to this membrane protein 2) STRA6 mediates cellular uptake of vitamin A, and 3) STRA6 is expressed in many tissues in which the native receptor has been previously described including RPE, the choroid plexus of the brain, and the Sertoli cells of the testis (Kawaguchi et al., 2007). We then showed that knockdown of STRA6 in zebrafish embryos rendered the larval eyes vitamin A deficient, thus providing physiological evidence for STRA6’s involvement in vitamin A transport (Isken et al., 2008).
Structural analysis revealed that STRA6 is assembled as an intricate dimer with 18 transmembrane helices (nine per protomer) and two long horizontal intramembrane helices interacting at the dimer core (Chen et al., 2016). The receptor complex displays a lipophilic cleft to which holo-RBP4 binds with high affinity (Chen et al., 2016; Kawaguchi et al., 2007). Studies in cell lines indicate that STRA6 facilitates the bidirectional flux of retinol between RBP4 and cells (Isken et al., 2008; Kawaguchi et al., 2011). Cellular accumulation of retinol is driven by esterification by LRAT (Amengual et al., 2012; Amengual et al., 2014b; Isken et al., 2008; Kawaguchi et al., 2011).
Mouse models for all major players of ocular vitamin A uptake from holo-RBP4 have been established. Early in life, Stra6−/− mice display very low ocular vitamin A levels and display highly reduced ERG responses (Amengual et al., 2014a; Ruiz et al., 2012). Blood and other tissues such as the lungs, fat, and liver display normal vitamin A levels when mice are bred and raised on vitamin A-rich diets (Amengual et al., 2014a; Berry et al., 2013). Ocular vitamin A levels increase with age when the mice are raised on diets rich in vitamin A (Amengual et al., 2014a; Kelly et al., 2016). A comparable phenotype has been reported for RBP4-deficient mice (Amengual et al., 2014a; Quadro et al., 1999). These findings clearly indicate that RE in chylomicrons can compensate at least in part for the RBP4/STRA6 system when dietary supply with the vitamin is abundant. Genetic disruption of the Lrat gene renders mice blind because of the inability to acquire vitamin A from circulating RBP4 (Amengual et al., 2012) and to produce REs for visual chromophore production (Batten et al., 2004). LRAT-deficient mice also lack major retinoid stores of the body and are highly susceptible to dietary vitamin A deficiency (Liu, Gudas, 2005; O’Byrne et al., 2005).
The STRA6, RBP4, and LRAT dependent storage and distribution system for vitamin A provides the advantage to endure prolonged periods with little to no supplies of dietary vitamin A precursors. Particularly, the eyes benefit from this system. Dowling and Wald already described how vitamin A deprivation in rats successively affects retinoid levels in liver, blood, and eyes (Dowling, Wald, 1958). We observed in mice that the eyes display normal levels of vitamin A even after 20 weeks of vitamin A deprivation, though vitamin A stores in the liver and lung were nearly exhausted under this condition (Amengual et al., 2012). Mice only show significant losses of ocular vitamin A when they are subjected to eight months of dietary deprivation (Hu et al., 2011). In contrast, the eyes of STRA6-deficient mice are highly susceptible to dietary vitamin A deprivation, demonstrating the importance of STRA6 for ocular vitamin A homeostasis under this condition (Kelly et al., 2016). The preferred delivery of stored vitamin A to the eyes is explained by the regulation STRA6 gene expression which depends on tissue type and vitamin A status. Stra6 is highly expressed in epithelia that constitute a blood tissue barrier, especially in the RPE, but also in Sertoli cells of the testis and ependymal cells of the choroid plexus (Amengual et al., 2014a; Berry et al., 2013; Kelly et al., 2016). In the eyes, which have the highest vitamin A requirement in the body, Stra6 gene expression is independent from retinoid status (Amengual et al., 2012; Kelly et al., 2016). This explains why ocular retinoid homeostasis is maintained for prolonged times under dietary vitamin A deficiency (Amengual et al., 2012). In testis and brain, Stra6 expression is higher than in most other tissues, but it is positively regulated by retinoids (Kelly et al., 2016). This regulation seemingly increases STRA6 expression in vitamin A sufficiency but reduces vitamin A consumption of these tissues when its dietary supply is limited, saving stored retinoids to be used in the eye. Other peripheral tissues such as lung and adipose tissues display very low expression of STRA6 and apparently depend on the delivery of postprandial dietary vitamin A in lipoproteins, which they can store. However, these tissues may play a role in quickly removing vitamin A from the circulation when it is present in excess through the sensitivity of Stra6 expression to RA (Amengual et al., 2012; Amengual et al., 2014a). Accordingly, STRA6 expression is induced in peripheral tissues and blood holo-RBP4 levels decline when mice are treated with pharmacological doses of RA (Amengual et al., 2012). The coupling of STRA6-dependent cellular vitamin A uptake with LRAT activity provides an additional layer of regulation and fine tuning in tissues with a high vitamin A demand (Amengual et al., 2012). From these findings one deduces that STRA6 acts like a ‘cellular faucet’ which allows for a higher cellular uptake of circulating vitamin A than is possible when the uptake is solely reliant on passive diffusion. The elaborated regulation of STRA6 expression in peripheral tissues ensures proper distribution of vitamin A depending on tissue demand and availability of the nutrient.
In humans, 24 missense and nonsense mutations in the STRA6 gene have been identified to cause a severe microphthalmic syndrome named Matthew-Wood syndrome (MWS) (Golzio et al., 2007; Pasutto et al., 2007). MWS is characterized by severe bilateral microphthalmia, often in combination with pulmonary dysplasia, cardiac defects, and diaphragmatic hernia, among other anomalies and malformations (Chassaing et al., 2009). The symptoms of MWS are consistent with the pivotal role of retinoids in mammalian embryonic development (Clagett-Dame, DeLuca, 2002), but are variable even within the same family ranging from isolated microphthalmia to fatal syndromes (Casey et al., 2011). Similarly, mutations in the RBP4 gene can cause congenital eye malformations, including microphthalmia (Chou et al., 2015; Khan et al., 2016; Seeliger et al., 1999). Consistently, even within the same family large variations in the phenotypic manifestation of the RBP4 mutation exist (Chou et al., 2015).
The severe ocular malformations in STRA6- and RBP4-deficient patients contrast the milder ocular phenotypes in mice. However, recent genetic analysis also revealed that mutations in the gene encoding the retinoic acid producing enzyme ALDH1a3 cause microphthalmia in humans (Yahyavi et al., 2013), but a milder ocular phenotype in mice (Matt et al., 2005; Molotkov et al., 2006). Importantly, variability in the extra-ocular phenotype associated with STRA6 mutations also may reflect maternal vitamin A status and delivery to the fetus. Studies in mice indicate that both dietary and stored vitamin A can be transported through the fetal-maternal-blood barrier (Quadro et al., 2005; Wassef, Quadro, 2011). Notably, dietary vitamin A restriction of Rbp4 knockout mice results in malformations that resemble the birth defects of MWS patients (Quadro et al., 2005). Biochemical evidence has been provided that an RBP4 receptor is expressed in the placenta (Redondo et al., 2008). In mice it is unclear whether this receptor is STRA6 or the recently identified RBP-receptor 2 since both RBP4 receptors are expressed in this tissue (Alapatt et al., 2012; Kawaguchi et al., 2007; Kim et al., 2008).
7.2. Carotenoid transport
Circulating carotenoid levels are influenced by many factors, including individual genetics, health status, dietary intake, and vitamin A status (Bohn et al., 2017). Generally, their levels are correlated with serum triglyceride and cholesterol levels. Body composition is another major factor that affects the circulating levels of carotenoids. A number of studies have reported an inverse association between body mass index (BMI) and plasma carotenoid concentrations (Bohn et al., 2017; Wang et al., 2008). Further dissection of this association suggests that carotenoid plasma concentrations are inversely related not only to fat mass, but also to lean body mass. This indicates that non-fat tissues, such as muscle, may also serve as a reservoir for carotenoids (Bohn et al., 2017). Notably, people with anorexia nervosa have very high levels of plasma carotenoids, most likely due to high levels of mobilization in this catabolic condition (Curran-Celentano et al., 1985).
In the blood, carotenoids are not equally distributed among lipoprotein classes. Zeaxanthin and lutein exist in higher concentrations in HDL than in low density and very low density lipoproteins (Palczewski et al., 2016; Thomas, Harrison, 2016). Carotenoid content and composition is variable between tissues. The accumulation of macula pigments in the retina indicate that specific transport mechanisms must exist for carotenoids. Studies in human retinal pigment epithelial cell lines indicate that scavenger receptor SR-B1 facilitates uptake of carotenoids from HDL (During et al., 2008; Thomas, Harrison, 2016). The retinal pigment epithelium cells acquire fat soluble vitamins and other nutrients to deliver them to the adjacent neuronal retina to support photoreceptor function. Carotenoid-binding proteins have been implicated in assisting macular pigment accumulation (Bernstein et al., 2016). However, given the very high levels of xanthophylls in the macula and the stoichiometry of their binding, it is unlikely that macular pigments predominantly exist in protein-bound form unless the carotenoid-binding proteins GSTP1 and StARD3 would be present in comparable amounts. β-Carotene in circulating LDL is absorbed by LDL receptor mediated endocytosis into the retinal pigment epithelium (Thomas, Harrison, 2016). Human RPE cells express relatively high levels of BCO1 (Yan et al., 2001), the vitamin A forming enzyme and local vitamin A synthesis may contribute to ocular retinoid homeostasis (Chichili et al., 2005).
8. Vitamin A metabolism in the eyes
Phototransduction, the process by which light energy is translated into a photoreceptor’s electrical response, has long been at the forefront of sensory transduction and cell signaling studies (Hardie, Raghu, 2001; Lamb, Pugh, 2004). Vertebrate and invertebrate photoreceptors sequester their transduction machinery in specialized subcellular compartments. These compartments are characterized by the need to maximize the amount of light-absorbing membranes and harbor the phototransduction machinery. Insects photoreceptors display tightly packed microvilli, which are organized in a cylindrical rhabdomere. Vertebrate rods achieve this with stacks of membranous discs that are internalized in the rod outer segment. The outer segment is separated from the rest of the cell by a short ciliary stalk. Vertebrate cones display invaginations of the plasma membrane (Hofmann, Palczewski, 2015).
The elucidation of the biochemical steps involved in phototransduction led to the discovery and characterization of visual G protein signaling and culminated in the determination of the structure of the heptahelical transmembrane receptor rhodopsin (Palczewski, 2006; Palczewski et al., 2000). These transmembrane proteins activate heterotrimeric G proteins and are involved in a broad range of physiological processes throughout the body, where they respond to a wide variety of chemical messengers including hormones, neurotransmitters, odorants, and food ingredients. Visual pigments comprise one class of G protein-coupled receptors and consist of an integral transmembrane protein (opsin) and a covalently bound retinylidene chromophore (von Lintig et al., 2010). Other chromophore-binding G protein coupled receptors, such as melanopsin, regulate circadian rhythms, suppress pineal melatonin, modify locomotor activity, and modulate pupil size in the mammalian eyes (Foster, Hankins, 2002).
The 11-cis-diastereomer of the chromophore binds by a Schiff-base linkage to a membrane-embedded Lys residue in the opsin molecule to form functional visual pigments. In vertebrates, absorption of light triggers a geometric isomerization of the chromophore that converts rhodopsin into an activated state termed Meta II. Meta II is catalytically active and binds transducin (Gt), a photoreceptor-specific G protein, thereby initiating a signal-amplifying cascade involving cGMP that results in plasma membrane hyperpolarization. In Drosophila, rhodopsin activation and binding of Gt initiates phosphoinositide signaling, culminating in the opening of transient receptor potential (TRP) channels and depolarization of the photoreceptor cell membrane.
To regenerate chromophore after photo-bleach, animals have evolved two strategies: In bistable visual pigments of invertebrates, the chromophore remains covalently bound to the opsin and absorption of a second photon isomerizes the all-trans- back to the 11-cis- configuration of chromophore (von Lintig, 2012; Wang, Montell, 2007). In ciliary photoreceptors of vertebrates, the visual pigments decay following photo-activation into opsin and free all-trans-retinal. To restore light sensitivity, the photoproduct must be regenerated by a multi-step enzymatic pathway, named the visual cycle (Kiser et al., 2014; Wald, 1968).
Research in our and other laboratories has challenged the strict classification into light-dependent and independent mechanisms for chromophore regeneration in the two main animal groups. In Drosophila, an enzyme-based pathway was discovered for the production and regeneration of chromophore (Oberhauser et al., 2008; Voolstra et al., 2010; Wang et al., 2010; Wang et al., 2012). In mice, it was observed that chromophore production is accelerated by light (Wenzel et al., 2005b). In keeping with this finding, a blue light-dependent photoreversal of rhodopsin has been described in the literature (Grimm et al., 2000; Grimm et al., 2001; Hubbard, Kropf, 1958; Williams, 1964). Additionally, evidence for blue light-dependent isomerization of phosphatidylamine-chromophore conjugates (Kaylor et al., 2017) and RPE-retinal G-protein–coupled receptor (RGR)-opsin-dependent mechanisms have been reported in the mammalian eyes (Chen et al., 2001; Diaz et al., 2017; Morshedian et al., 2019; Zhang et al., 2019).
8. 1. Chromophore production and recycling in insects
Retinoids are mainly required for vision in Drosophila (Harris et al., 1977). This characteristic is demonstrated through the significant reduction of the retinoid content in sine oculis fly mutants that lack compound eyes (Voolstra et al., 2010) (Figure 13 A,C). Transcriptomic analysis of this mutant also revealed an eye-enriched expression of major carotenoid and retinoid processing enzymes, including NinaB, Pinta, and the retinol dehydrogenases Pdh and RdhB (Xu et al., 2004) (Fig. 13 B). The latter three proteins are enriched in pigment cells, suggesting that this cell type displays an analogous function in chromophore metabolism to the RPE in vertebrate eyes (Wang, Montell, 2005; Wang et al., 2010; Wang et al., 2012).
Figure 13. The insect visual cycle.
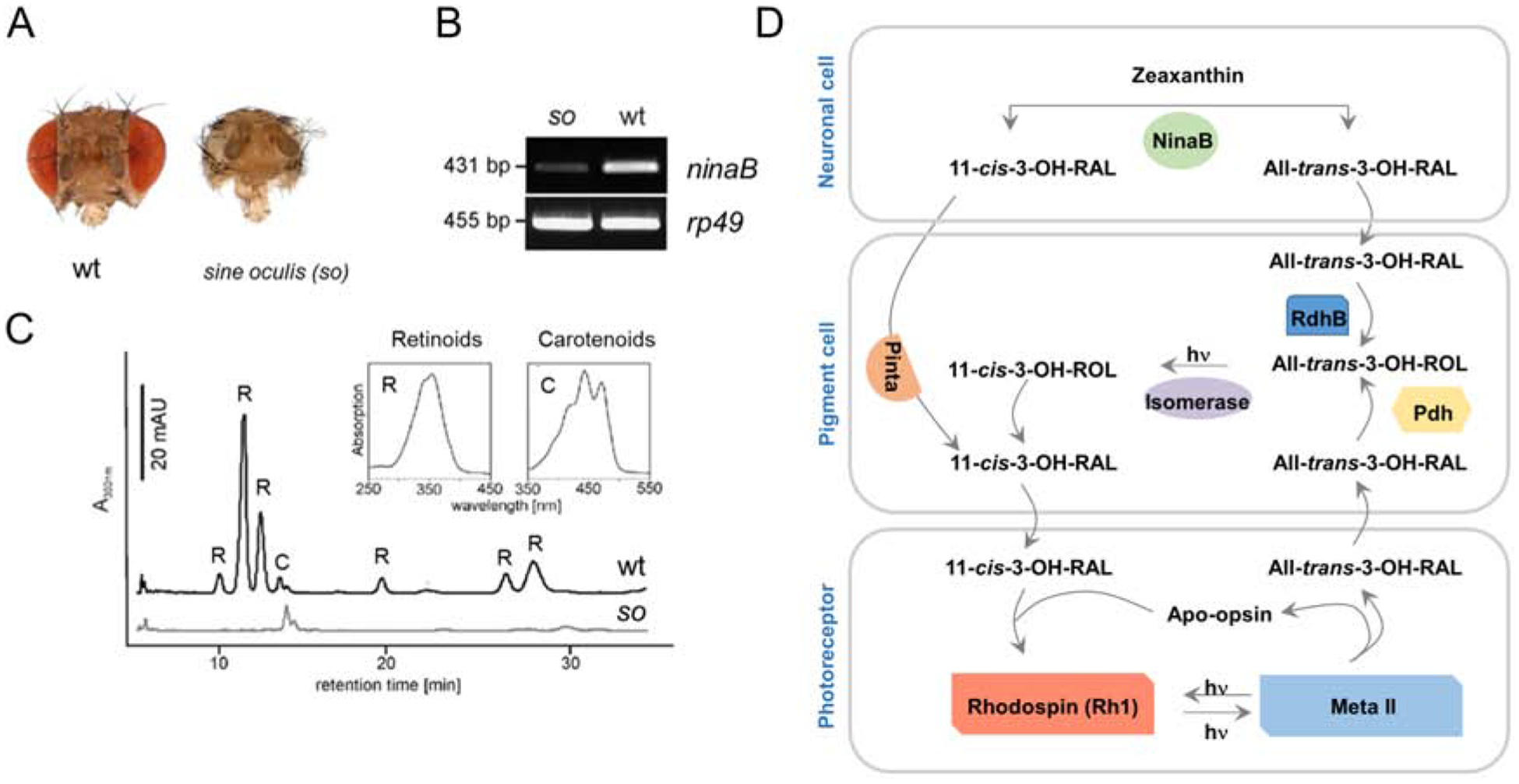
(A) Heads of wild type and sine oculis flies, (B) Semiquantitative RT-PCR analysis for ninaB expression with total RNA preparations from the heads of adult sine oculis and wild-type flies. (C) HPLC traces obtained from lipid extracts of 70 mg of wild-type (upper trace) and sine oculis (lower trace) fly heads. Different retinoids and carotenoids are indicated by numbers. Peaks: 1, 9/13-cis-3-hydroxy-retinal oxime (syn); 2, all-trans- (anti) and 11-cis-3-hydroxy-retinal oxime (syn); 3, all-trans-3-hydroxy-retinal oxime (syn); 4, zeaxanthin/lutein; 5, all-trans-3-hydroxy-retinol; 6, 9-cis anti-3-hydroxy-retinal oxime; 7, 13-cis-3-hydroxy-retinol; and 8, 11-cis-(anti)-3-hydroxy-retinal oxime. Insets show the spectra of peak 3, all-trans-retinal oxime (syn) and peak 4, zeaxanthin. (D) Scheme of the fly’s chromophore metabolism.
In fly photoreceptors, the opsin is synthesized at the endoplasmic reticulum, combined with chromophore, transported through the Golgi apparatus, and inserted into rhabdomeric membranes (Schopf, Huber, 2017). This synthesis requires the 11-cis-diastereomer of chromophore because the opsin is degraded in its absence (Huber et al., 1994; Oberhauser et al., 2008; Ozaki et al., 1993). Isono and colleagues observed that carotenoids but not retinoids promoted visual pigment synthesis in flies that were reared in darkness (Isono et al., 1988). The biochemical basis for this observation was provided by the identification of the ninaB gene and the characterization of the encoded enzyme. Missense and nonsense mutations in ninaB gene disrupt the de novo synthesis of chromophore and visual pigment (von Lintig et al., 2001). Supplementation of 11-cis-retinal but not all-trans-retinal restores visual pigment synthesis in the dark-reared ninaB mutant. Additionally, supplementation of carotenoids and/or 11-cis-retinal but not all-trans-retinal promoted visual pigment synthesis in wild type flies which were reared on vitamin A-deprived chow in the dark (Oberhauser et al., 2008). Analysis of the NinaB enzyme showed that it catalyzes an isomer-oxygenase reaction which converts a carotenoid into an 11-cis-retinal and all-trans-retinal product in 1:1 molar ratio (Figures 5 and 13 D) (Chai et al., 2019; Oberhauser et al., 2008). The unique reaction sequence follows a dioxygenase mechanism with a carbocation/radical intermediate (Babino et al., 2016). Pinta protein is required for transport of the 11-cis diastereomer of the chromophore to photoreceptor cells. The pinta mutant displays highly reduced visual pigment content in photoreceptors (Wang, Montell, 2005), though the mutant flies can produce the 11-cis-diastereomer of the chromophore (Voolstra et al., 2010).
The all-trans-retinal cleavage product of the NinaB-catalyzed conversion of a carotenoids is further metabolized by a light-dependent pathway. This pathway involves a (blue) light-dependent isomerization reaction that awaits molecular description (Ozaki et al., 1993; Schwemer et al., 1984; Smith, Goldsmith, 1991). The existence of this pathway explains why light-reared flies can synthesize visual pigments from supplemented all-trans-retinal as precursor.
In addition to NinaB and Pinta, a retinol dehydrogenase, encoded by the rdhB gene is required for visual pigment production in flies (Wang et al., 2012). An oxidoreductase, encoded by the ninaG gene, has been also implicated in this pathway. The ninaG mutant shows highly reduced content of Rh1, the major rhodopsin of the fly eyes (Ahmad et al., 2006; Sarfare et al., 2005). NinaG mutant flies accumulate 3-hydroxy-retinol and the NinaG protein has been implicated in the conversion of 3(R)-hydroxy-retinol into the 3(S)-enantiomer (Sarfare et al., 2005). A pathway for the conversion of all-trans-retinal into all-trans-3(S)-hydroxy-retinal has been described in flies, and evidence exists that Drosophila utilizes this enantiomer as chromophore (Seki et al., 1998).
Insect visual pigments are bistable. Once bound to the opsin, the chromophore remains covalently bound after a bleach. Absorption of a second photon isomerizes the all-trans-photoproduct back to 11-cis-diastereomer and restores light sensitivity to the visual pigment (von Lintig, 2012; Wang, Montell, 2007). Therefore, it was generally not assumed that flies exhibit a regeneration pathway for chromophore as commonly found in vertebrates. However, biochemical evidence from blow flies indicate the existence of a renewal pathway for rhodopsin (Schwemer, 1984). In Drosophila, it has been observed that photo-bleached visual pigments removed from rhabdomeric membranes under constant illumination conditions (Satoh, Ready, 2005; Wang et al., 2010). The analysis of the Drosophila pdh mutant provided genetic evidence that the chromophre of internalized visual pigments is regenerated through an enzymatic pathway (Wang et al., 2010) (Figure 13 D). The pdh gene encodes an enzyme which belongs to the short-chain dehydrogenase/reductase (SDR) family. The pdh mutant is characterized by a progressive light-dependent loss of visual pigments that is accompanied by degeneration of photoreceptors. The light-dependent degeneration of photoreceptors in Pdh-deficiency is likely explained by the toxicity of opsin-unbound chromophore. This assumption is based on experiments that showed that retinal degeneration of opsin-deficient fly mutants is prevented in chromophore-deficient photoreceptors. Additionally, it has been shown that free retinaldehyde but not retinol can induce oxidative stress and apoptosis in Drosophila S2 cells (Voolstra et al., 2010). Accordingly, dark-reared pdh mutant flies are protected against visual pigment loss and photoreceptor degeneration. Similarly, photoreceptor degeneration can be prevented in pdh mutants by the expression of human RDH12. This retinal dehydrogenase reduces the aldehyde form of the photoproduct into the corresponding alcohol (Haeseleer et al., 2002; Wang et al., 2010). Biochemical analysis confirmed that recombinant Pdh, similar to RDH12, catalyzes an interconversion of all-trans-3-hydroxy-retinal to all-trans-3-hydroxy-retinol (Hofmann et al., 2016). The all-trans-3-hydroxy-retinol can then be recycled to 11-cis-3-hydroxy-retinal by a pathway that depends on RdhB and light (Wang et al., 2012). The ability to recycle chromophore trough this pathway enables flies to maintain normal chromophore levels under conditions of prolonged dietary vitamin A deprivation (Wang et al., 2010).
8. 2. The visual cycle of vertebrates
In the disc membranes of rod outer segments (ROS) of the vertebrate eye, rhodopsin exists as an integral membrane protein and the chromophore is covalently bound via a Schiff base. Light induces a geometric isomerization of the protein-bound chromophore to initiate visual phototransduction (Arshavsky et al., 2002). Hydrolysis of the Schiff base linkage by bulk water entering from the cytoplasmic side liberates the all-trans-retinal photoproduct (Jastrzebska et al., 2011). The photoproduct must be converted back to chromophore to sustain vision. Individual steps in this cyclic biochemical pathway have been described by Wald and are now understood in biochemical and molecular detail (for an overview see Figure 14). Mutations in genes encoding these proteins are associated with inherited blinding diseases, including Leber congenital amaurosis and various forms of retinitis pigmentosa (Kiser, Palczewski, 2016; Thompson, Gal, 2003). Furthermore, the generation of knockout mouse models in these genes have confirmed the biochemical role of the encoded proteins and provided models to establish therapeutic intervention strategies (Garafalo et al., 2019; Sears et al., 2017).
Figure 14. The vertebrate visual cycle.
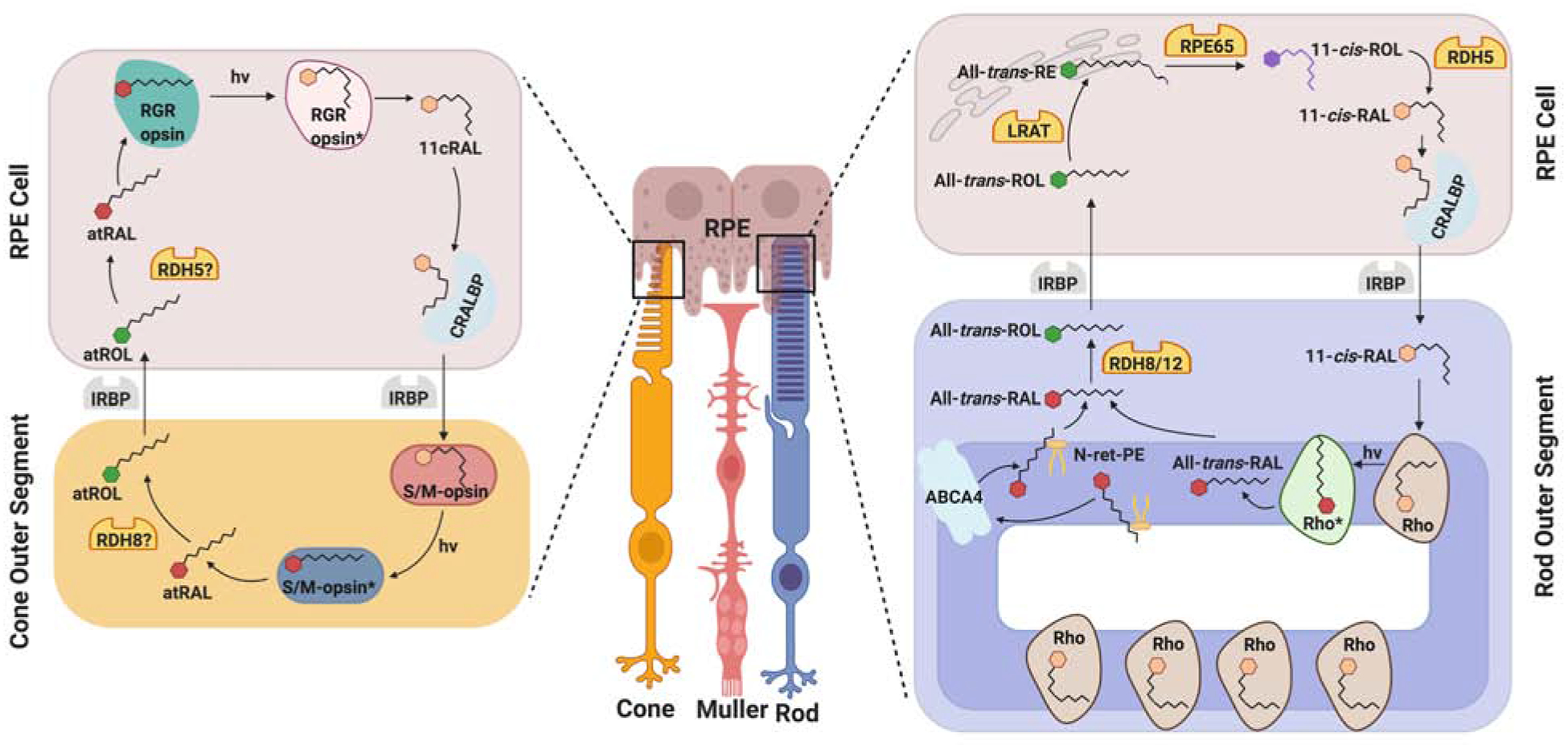
(central) Simplified schematic overview of the arrangement of retinal pigment epithelium cells, rod and cone photoreceptor cells, and Müller glial cells. (right) Biochemical key steps of the classical visual cycle. (left) Photic RGR-dependent visual cycle. N-ret-PE, N-retinylidene-phoshatidyl-ethanolamine, RAL, retinal, RE, retinyl ester, Rho, rhodopsin, ROL, retinol.
After its release from rhodopsin, all-trans-retinal enters the disk lumen and must be transferred to the cytosol by the ATP-binding cassette transporter 4 (ABCA4) (Molday et al., 2000; Molday et al., 2009; Tsybovsky et al., 2010). For this transport, all-trans-retinal forms conjugates with phosphatidylethanolamine, an abundant membrane lipid in disk membranes. ABCA4 catalyzes N-retinylidene-phosphatidylethanolamine import into the cytosol in an ATP-dependent fashion (Quazi et al., 2012; Tsybovsky et al., 2013). This transporter also plays an important role in the control of chromophore homeostasis in the dark-adapted photoreceptors (Quazi, Molday, 2014).
In the cytoplasm, the photoproduct is converted to all-trans-retinol in a reaction catalyzed by RDHs (Haeseleer et al., 1998; Rattner et al., 2000). Two enzymes, Rdh8 in photoreceptor outer segments and RDH12 in photoreceptor inner segments employ NADPH as a cofactor and are mainly responsible for catalyzing this reaction in mouse rod photoreceptors (Maeda et al., 2005). However, the redundancy of retinal reductase activity shown in mice suggests that photoreceptors may contain additional RDHs aside from RDH12 and RDH8 (Maeda et al., 2007a). This redundancy could be due to the need for a large enzymatic capacity to convert the chemically reactive aldehyde group of the photoproduct to the corresponding alcohol.
Impairment of all-trans-retinal clearance can cause severe retinal pathologies. The aldehyde group of the photoproduct can form adducts with primary amino groups such as phosphatidylethanolamine to form bisretinoids such as A2E. Ocular accumulation of A2E together with A2E-mediated redox reactions have been implicated in the pathology of eye diseases such as Stargardt disease and the age related macular degeneration (Sparrow, Boulton, 2005; Sparrow et al., 2020; Zhou et al., 2006). The importance for rapid clearance of the photoproduct is demonstrated by the pathological consequences of mutations in RDH12 and ABCA4 in humans (Allikmets et al., 1997; Janecke et al., 2004). Notably, ABCA4 knockout mice accumulate bisretinoids such as A2E (Mata et al., 2001; Radu et al., 2004). Retinal pathology is augmented when ABCA4 and RDH8 genes are genetically disrupted together in mice (Maeda et al., 2008). In addition of A2E formation, acute all-trans-retinal toxicity may contribute to the retinal pathology in the compound mutant (Maeda et al., 2009) by the formation of retinal-adducts with other cellular molecules, including proteins and ribonucleotides (Chen et al., 2012; Maeda et al., 2012).
All-trans-retinol formed in rod outer segments is transported to the RPE where it is esterified. This process is facilitated by IRBP that binds retinoids in the extracellular space. Missense mutations in the human IRBP gene have been identified and can cause autosomal recessive retinitis pigmentosa (den Hollander et al., 2009). CRBP1 is needed for the transport of all-trans-retinol within RPE cells (Edwards, Adler, 1994; Redmond et al., 1985). CRBP1 has recently identified as putative novel target for visual cycle modulation. Abnormal cannabidiol is a nonretinoid inhibitor of CRBP1, reduces the flux through the visual cycle, and can ameliorate consequences of bright light damage to the retina (Silvaroli et al., 2019).
The major ester synthase in RPE is LRAT (Batten et al., 2004; Saari, Bredberg, 1988). This membrane-anchored enzyme plays a central role in retinoid metabolism of the RPE. It is required for the STRA6-dependent uptake of all-trans-retinol from circulating holo-RBP4 (Amengual et al., 2012), helps to form oil droplet-like structures, named retinosomes (Imanishi et al., 2004), and produces RE as substrate for the retinoid isomerase RPE65. This latter enzyme catalyzes the transformation of all-trans-retinyl esters to 11-cis-retinol and palmitate (Jin et al., 2005; Moiseyev et al., 2005; Redmond et al., 2005a). The product of this isomerization reaction is subsequently oxidized in the final catalytic step of the visual cycle to 11-cis-retinal. Enzymatic activities of RDH5, RDH10 and RDH11 are mainly responsible for this reaction (Haeseleer et al., 2002; Sahu et al., 2015) but additional 11-cis-RDHs may participate within the RPE (Maeda et al., 2007b). Newly synthesized 11-cis-retinal is protected by binding to CRALBP which mediates its transport back to photoreceptor ROS where the chromophore covalently binds to opsin, thereby completing the visual cycle (Saari, Bredberg, 1987). Disrupting the enzymatic steps of chromophore regeneration in the RPE, especially those involving LRAT and RPE65, has severe pathological consequences on retinal health (Marlhens et al., 1997; Thompson, Gal, 2003). The resulting chromophore deficiency causes slow progressive death of rods that is attributed to continuous activation of visual phototransduction by unliganded opsin (Woodruff et al., 2003). Moreover, disordered processing and transport of cone visual pigments lacking bound-chromophore leads to very rapid cone degeneration in knockout mouse models (Zhang et al., 2008).
8.2.1. Modifications of the canonical visual cycle in diurnal animals
Mice are nocturnal animals and mainly rely on scotopic vision that is mediated by rods. Daylight vision is mediated by cones and is critical for visual acuity and color discrimination. Cone response kinetics differ significantly from rods. Following light flashes that generate similar membrane currents, cones recover sensitivity approximately 10-fold faster than rods (Perry, McNaughton, 1991). Moreover, the rod photo-response is saturated at photoisomerization rates above 500 per second (Baylor et al., 1984) but cones remain responsive to light at photoisomerization rates up to 1,000,000 per second (Schnapf et al., 1990).
In diurnal mammals, the different characteristics of cones and rods may necessitate specific mechanism(s) for chromophore regeneration to avoid competition for the unique chromophore, 11-cis-retinal. The competition between these two types of photoreceptors has been convincingly demonstrated in the retina of the R91W Rpe65 mutant mouse that can produce only minute amounts of chromophore. Due to the higher number of rods and the instability of cone opsin, cones suffer from chromophore deficiency and degenerate in the retina of this mouse mutant (Samardzija et al., 2009).
Evidence exists that modifications of the canonical visual cycle may help diurnal animals to cope with the increased demand for visual pigment regeneration under day light conditions. Moiseyev and colleagues showed that chicken RPE65 display significant increased isomerase efficiency when compared to its mouse counterpart (Moiseyev et al., 2008), thus allowing faster chromophore regeneration. Another hallmark of eyes with high resolution color vision, including the human eyes, is the existence of 11-cis-RE (Mustafi et al., 2016). How this vitamin A metabolite is synthesized and whether it can support cone vision was recently investigated in the zebrafish (Danio rerio) eyes (Babino et al., 2015b). The eyes of the zebrafish larva are amenable for genetic and pharmacological interventions and have been successfully used to study photoreceptor development and function (Fleisch, Neuhauss, 2010). These studies showed that the larval eyes express key components of the canonical visual cycle, including Stra6, Lrat, Rpe65, and Cralbp (Fleisch et al., 2008; Isken et al., 2008; Isken et al., 2007; Schonthaler et al., 2007). Similar to diurnal mammals, 11-cis-REs exist in the RPE of dark-adapted eyes of larval and adult zebrafish (Figure 15A, B). This was demonstrated by biochemical analyses as well as by two-photon microscopy of the larval eyes (Palczewska et al., 2010) (Figure 15C). The levels of 11-cis-REs are comparable to that of all-trans-REs, also known to be present in the RPE as storage pools for chromophore production. Wild type and cone-only mutant larvae were employed to test whether 11-cis-REs contribute to cone vision with a recovered sensitivity approximately 10-fold faster than rods (Baylor et al., 1979; Perry, McNaughton, 1991). The experiments revealed that after an initial drop in 11-cis-retinal, levels of the chromophore achieved a steady-state, while levels of 11-cis-REs continuously diminished to nearly undetectable levels after prolonged bleaching time. This finding indicated that steady state levels of the 11-cis-retinal were achieved at least in part though hydrolysis of 11-cis-REs. Interestingly, previous studies have reported that hydrolysis of 11-cis-RE also occurs in homogenates of human retinal epithelial cells (Blaner et al., 1987).
Figure 15. 11-cis-retinyl esters exist in the retinal pigment epithelium (RPE) in zebrafish larvae.
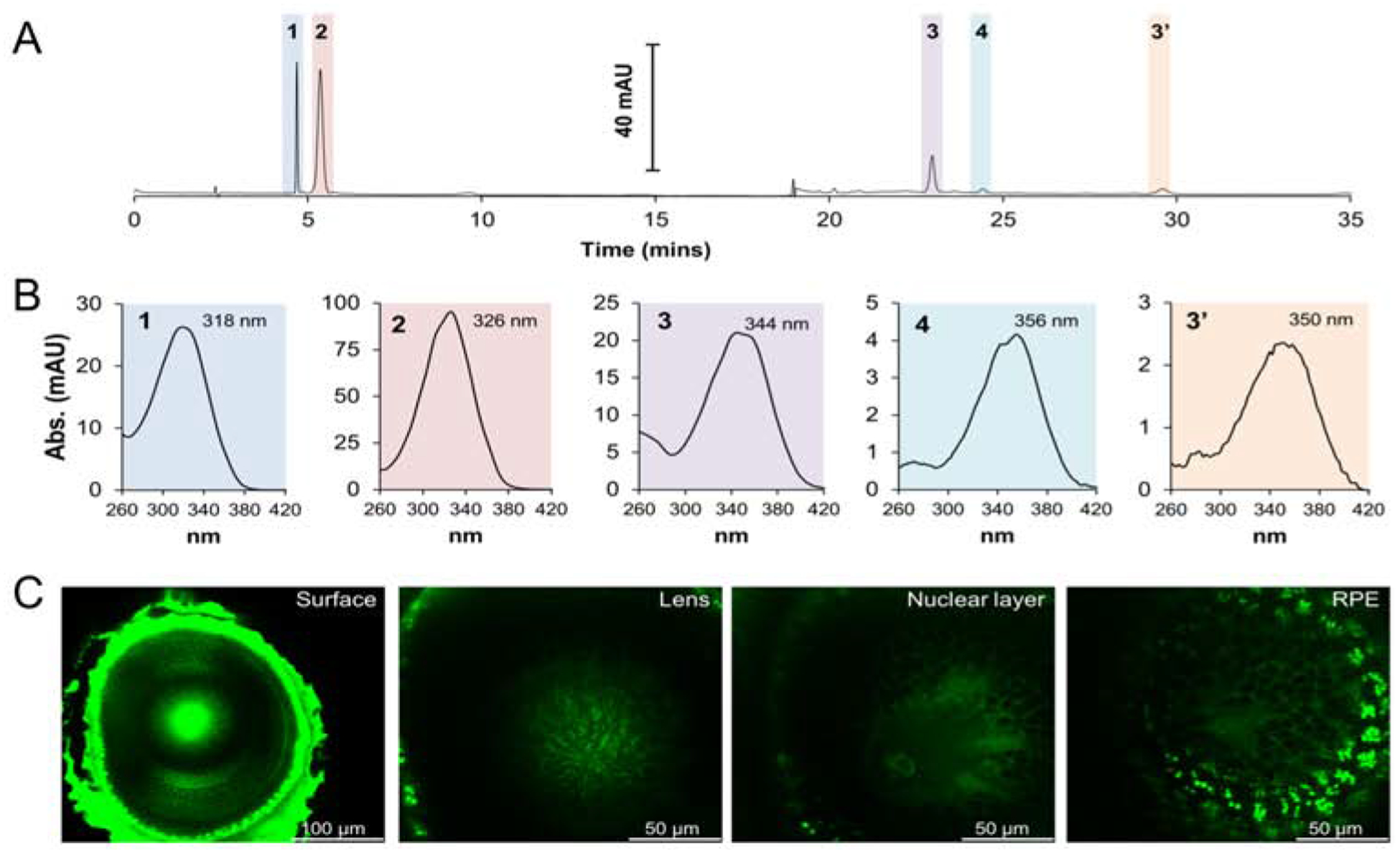
(A) Representative HPLC chromatogram at 325 nm of lipophilic extracts of 5-days post fertilization zebrafish larvae. (B) Spectral characteristics of extracted retinoids from zebrafish larvae. (C) In vivo two-photon microscopy images of different layers of the zebrafish larval eye. Retinosomes were exclusively detected in the RPE (far right image). 1, 11-cis-retinyl esters (11-RE); 2, all-trans-retinyl esters (at-RE); 3, syn 11-cis-retinal oxime (syn 11-RAL); 4, syn all-trans-retinal (all-RAL); 3’, anti 11-cis-retinal oxime (anti 11-RAL).
To elucidate the pathway by which 11-cis-REs is generated in the zebrafish RPE, retinyl amine, a potent inhibitor of the RPE65 isomerase of the visual cycle, was used (Schonthaler et al., 2007). This approach showed that the inhibitor prohibited the regeneration of 11-cis-REs under dark conditions in both wild-type and cone-only zebrafish larvae (Babino et al., 2015b).
These experiments indicate that 11-cis-REs are synthesized in the dark and provide a pool of 11-cis-retinol that can be utilized under bright light luminance (for an overview see Figure 16), when the relatively slow RPE65 catalyzed isomerization reaction becomes rate limiting (Lyubarsky et al., 2005; Wenzel et al., 2005b). Under this condition, 11-cis-REs are hydrolyzed to produce 11-cis-retinol and shuffled to cones, likely by binding to Cralbpa (in the RPE) and Cralbpb (in Müller glia) (Fleisch et al., 2008; Schonthaler et al., 2008). Here it would subsequently be oxidized by cis-stereoisomer specific retinal dehydrogenases into 11-cis-retinal. This ‘dark-generated’ pool of chromophore precursor elegantly and efficiently supplies cones of zebrafish larvae with chromophore under bright light illuminance. It remains to be clarified whether this pathway also exists in diurnal mammals.
Figure 16. Proposed 11-cis-retinyl ester cycle of the zebrafish eyes.
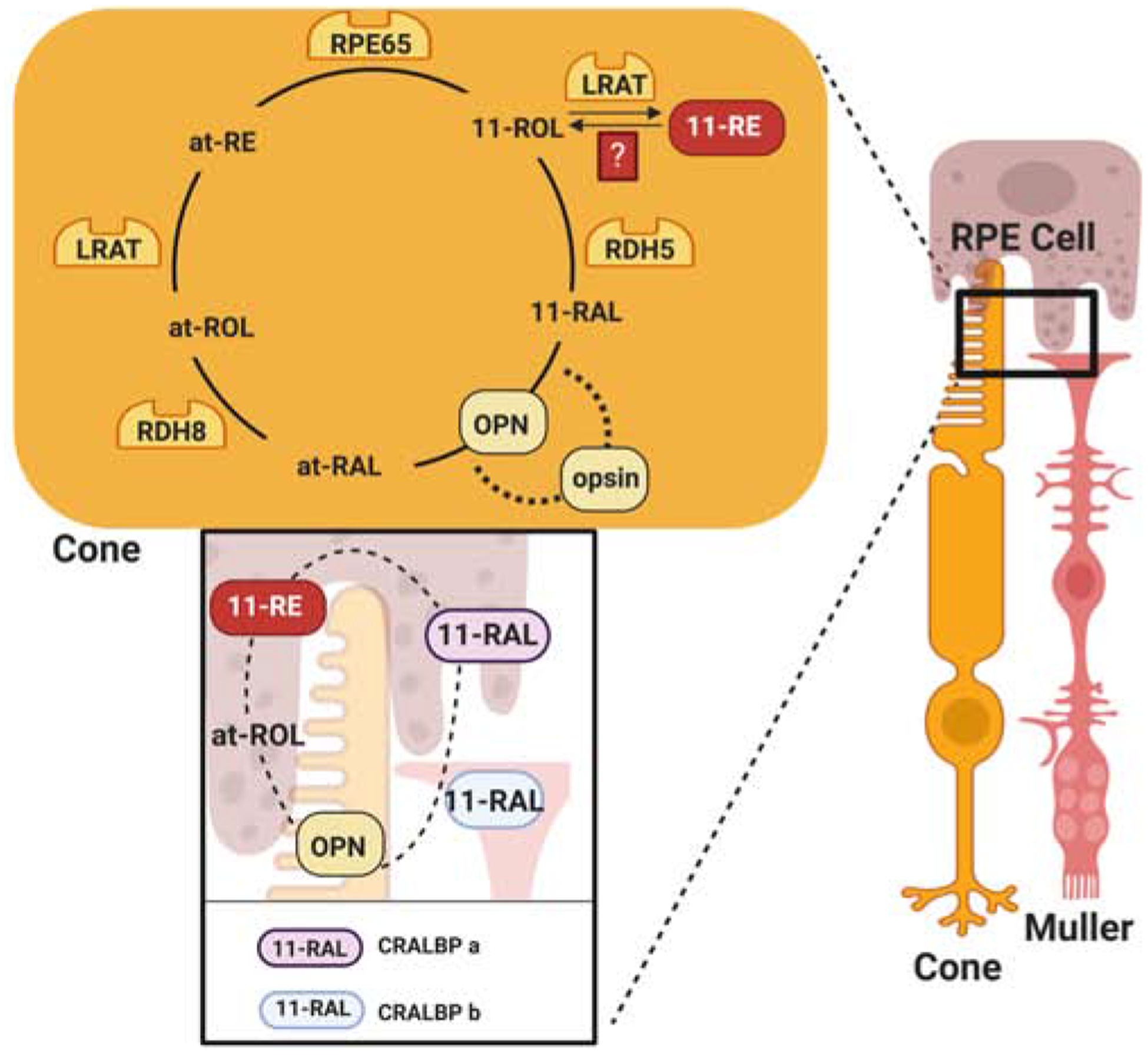
Within the RPE, under dark conditions, RPE65 forms 11-cis-retinol in a canonical fashion. Subsequently, this 11-cisretinol is esterified by LRAT for storage in retinosomes in the form of 11-cis-retinyl esters. An unidentified light-dependent hydrolase hydrolyzes 11-cis-retinol into 11-cis-retinol when demand for the chromophore would arise. The newly formed 11-cis-retinol would be oxidized to 11-cis-retinal and shuffled from the RPE to Müller glia cells via a CRALBPa and CRALBPb-dependent mechanism. In the cones, 11-cis-retinal binds to cone opsins to form the cone opsin pigment molecules for use in phototransduction. Recycling of the released chromophore, all-trans-retinal will proceed in a canonical fashion. 11-RAL, 11-cis-retinal; at-RAL, all-trans-retinal, 11-RE, 11-cis-retinyl ester; at-RTE, all-trans-retinyl ester. 11-ROL, 11-cis-retinol; at-ROL, all-trans-retinol.
8.3. Light-dependent regeneration pathways for chromophore in the vertebrate retina
Bleached rhodopsin may be regenerated, not only in the dark through enzymes, but also photochemically. In vitro, visible light of short wavelength (blue light) can regenerate rhodopsin by a process called photoreversal of bleaching and it has been demonstrated that it occurs in the living rat eye (Grimm et al., 2000; Grimm et al., 2001; Hubbard, Kropf, 1958; Williams, 1964). Recently, it was shown that blue light illumination of a bleached mouse retina results in a significant increase in 9-cis- and 11-cis-retinal, thus providing further evidence for light-dependent re-isomerization of chromophore in photoreceptors. Additionally, it was shown that the photoproduct all-trans-retinal can form conjugates with PE in photoreceptors that, when illuminated with blue light (450 nm), are converted to the corresponding 11-cis-N-ret-PE. The quantum efficiency of this enzyme-independent photoconversion is similar to rhodopsin and blue light (450 nm) induced visual pigment regeneration occurs in the living mouse retina (Kaylor et al., 2017).
Additionally, light dependent isomerases may exist that interconvert retinal diastereomers. In mollusks an opsin protein known as retinochrome and a retinoid-binding protein, which are phylogenetically related to RPE-retinal G-protein-coupled receptor (RGR)-opsin and to CRALBP are involved in this pathway (Albalat, 2012). RGR has been implicated as constituent of a photic visual cycle in vertebrates as well (Yang, Fong, 2002). Evidence has been provided that RGR contributes to chromophore regeneration in Rpe65−/− but not in Rpe65−/−Rgr−/− compound knockout mice (Van Hooser et al., 2002). However, the relatively low rate of 11-cis-retinal synthesis casted doubts on the physiological relevance of this finding (Hao, Fong, 1999). Recent biochemical studies revived the role of RGR in visual chromophore regeneration (Zhang et al., 2019). Using bovine RPE protein extracts, the Palczewski laboratory demonstrated high-level production of 11-cis-retinal in RPE membranes upon narrow band green light illumination (for an overview see Figure 14). The activity increased with protein concentration and displayed substrate saturation. The presence of CRALBP stimulated 11-cis-retinal production in these assays. Using specific inhibitors for RPE65, the researchers demonstrated that the activity was associated with RGR. Most importantly, recombinant RGR exhibited the same activity in cell-based and cell-free enzyme assays. Site directed mutagenesis and biochemical testing revealed that the isomerase reaction involves a Schiff’ base linkage of the all-trans-retinal substrate at Lys255 of RGR. Single-cell RNA-Seq analysis of the retina and RPE tissue showed that RGR is expressed in human and bovine RPE and Müller glia cells, whereas mouse RGR is expressed in RPE but at much lower levels in Müller glial cells (Zhang et al., 2019). The latter finding contrasts studies in mice which indicate that RGR in concert with a retinol dehydrogenase enzyme (Xue et al., 2017), contributes to cone visual pigment regeneration in Müller glia cells (Morshedian et al., 2019).
However, RGR also was associated with retinyl ester metabolism in the RPE and implicated as activator of RPE65 activity (Radu et al., 2008; Wenzel et al., 2005b). In the latter study, rhodopsin regeneration was found to be attenuated in Rgr−/− mice in both light and darkness. Additionally, it was shown that RGR regeneration was accelerated by light in an RGR-independent fashion (Wenzel et al., 2005b). Further research is needed to clarify the role of RGR in visual pigment regeneration in vertebrates.
8.4. An alternative retinoid cycle in the inner retina?
Studies in lower vertebrates showed that cone, but not rod, visual pigment regenerates independent of the RPE (reviewed in, (Fleisch, Neuhauss, 2010)), indicating that additional pathways for chromophore regeneration exist. Electrophysiological studies provided evidence that an intra-retinal pathway for cone visual pigment regeneration also exists in mice and humans (Wang, Kefalov, 2009). In this pathway, IRBP specifically delivers 11-cis-retinal to cone photoreceptors (Parker et al., 2011). Biochemical studies provided evidence that pharmacological inhibition of RPE65 impair but not completely abolish cone vision (Kiser et al., 2018). Furthermore, Müller glia is seemingly involved in this pathway because the genetic disruption of CRALBP expression in these cells impairs cone vision in the zebrafish (Fleisch et al., 2008) and mouse retina (Xue et al., 2015).
In the early 2000s, the Travis laboratory analyzed enzyme activities in the neuronal retina of chicken and ground squirrel and proposed an alternative visual cycle (Mata et al., 2002). In this alternative visual cycle, the photoproduct all-trans-retinal is reduced to all-trans-retinol by RDH8 in cone photoreceptors and IRBP-dependently transferred to Müller glial cells (Parker et al., 2011; Tang et al., 2013). All-trans retinol is isomerized to 11-cis-retinol by an isomerase in Müller glial cells (Mata et al., 2005). The endothermic isomerase reaction is driven by an AcylCoA-dependent esterification. An 11-cis-RE hydrolase in conjunction with a photoreceptor specific 11-cis-RDH synthesizes 11-cis-retinal and completes the cycle (Mata et al., 2002). Evidence for this pathway comes from zebrafish larvae in which gene knockdown and pharmacological inhibition of RPE65 does not significantly affect cone vision (Schonthaler et al., 2007; Ward et al., 2020). The putative alternative retinol isomerase later was identified as dihydroceramide desaturase-1 (DES1) (Kaylor et al., 2013). Recombinant DES1 displays retinoid isomerase activity in the presence of appropriate cofactors though the enzyme was originally characterized in sphingolipid metabolism. DES1 was shown to be expressed in Müller glial cells and to be associated with CRALBP (Kaylor et al., 2013). Because DES1 catalyzes an equilibrium reaction between various retinol diastereomers (Kaylor et al., 2013), a multifunctional O-acyltransferase (MFAT) is proposed to drive the isomerization reaction by sequestering 11-cis-retinol in the form of its fatty acid ester (Kaylor et al., 2014). MFAT is allosterically regulated by 11-cis-retinoids (Arne et al., 2017). In this positive-feedback regulation, esterification rates for 9-cis, 13-cis, or all-trans retinols decrease and enable preferential synthesis of 11-cis-RE (Arne et al., 2017).
Efforts to provide genetic evidence for the alternative isomerase in the cone visual cycle were largely unsuccessful. A Des1-deficient zebrafish larva displays normal cone responses (Ward et al., 2020). Moreover, a Müller cell specific Des1 knockout in mice revealed no changes in cone photoreceptor responses (Kiser et al., 2019). In line with these studies, gene knockout of RPE65, the retinoid isomerase of the canonical visual cycle, abolishes cone and rod photoreceptor response in mice (Redmond et al., 1998). Some residual light sensitivity of young RPE65 knockout mice could be attributed to rod photoreceptors (Seeliger et al., 2001) and are mediated by 9-cis-retinal and isorhodopsin (Fan et al., 2003a). Additionally, studies of Nrl−/− mice with a cone only retina demonstrated that Rpe65 is critical for cone vision in mice (Wenzel et al., 2007). Thus, the studies for an alternative light-independent visual cycle in the retina remain inconclusive.
8.5. Putative interaction between carotenoids and the visual cycle
Many vertebrates accumulate significant amounts of carotenoids in their retina. In birds, carotenoids and long-chain apocarotenoids act as filters, substantially modifying light detection by cone photoreceptors, thereby fine-tuning spectral sensitivity and improving color discrimination. Macular pigments in the primate eyes reduce the adverse impact of light scattering and chromatic aberration, thereby optimizing contrast sensitivity of the retina (Hammond et al., 2013). These macular pigments also absorb short-wavelength light and scavenge reactive oxygen species (Bernstein et al., 2016; Hammond et al., 2013), both hallmarks of light damage of the retina (Hunter et al., 2012; Maeda et al., 2012; Wenzel et al., 2005a). We recently observed in wild type mice that ultraviolet light (405 nm) illumination of bleached photoreceptors can trigger a geometric isomerization of the photoproduct that results in the accumulation of 9-cis- and 13-cis-retinal (Widjaja-Adhi et al., 2018). These aberrant cis-retinal diastereomers persist for prolonged time in the retina and their formation is associated with morphological damage of the tissue. Notably, A2E concentration is inversely related to the concentration of carotenoids in patients (Bhosale et al., 2009) and studies in quails indicate that carotenoids can reduce A2E levels in the eyes (Bhosale et al., 2009). Additionally, macular pigments prevent photo-oxidation of A2E in cell culture models (Kim et al., 2006). However, research about the pigments beneficial roles in vision is limited because mice, the most common and available ophthalmologic animal model, do not accumulate these compounds (Bernstein et al., 2016). To overcome this hurdle, we employed Bco2 knockout mice (Amengual et al., 2011b), which accumulate significant amounts of xanthophylls in their eyes (Amengual et al., 2011b; Li et al., 2014; Widjaja-Adhi et al., 2015). We observed that already relatively low amounts of carotenoids reduced the levels of aberrant cis-retinal diastereomers after blue light bleaching and alleviated the associated retinal pathology (Widjaja-Adhi et al., 2018). An in vitro test system revealed that zeaxanthin can efficiently prevent blue light-induced geometric isomerization of all-trans-retinal in the test tube.
The observed interaction between carotenoids and retinoids may also explain why nocturnal animals such as mice naturally don’t accumulate carotenoids in their retina. Their light absorbing properties would reduce sensitivity of visual pigments under dim light conditions by absorbing light in the range of 400 to 500 nm. Notably, owls have been reported to have pale oil droplets in the retina that lack carotenoids whereas diurnal birds accumulate these compounds (Bowmaker, Martin, 1978). The interaction between carotenoids and retinoids also may be considered in the recently proposed pathway for blue light-dependent chromophore regeneration (Kaylor et al., 2017; Morshedian et al., 2019) because the light absorbing properties of carotenoids would likely interfere with these processes. This interaction would be even more pronounced in the primate retina where macula carotenoids exist in very high concentrations.
Another notable interaction between carotenoids and the visual cycle is the putative involvement of RPE65 in macular pigment metabolism (Shyam et al., 2017a). Previously, it was shown that meso-zeaxanthin and RPE65 expression concomitantly appear in the RPE of the developing chicken embryos (Gorusupudi et al., 2016). Supply of lutein to human embryonic kidney (HEK293T) cells expressing recombinant RPE65 revealed turnover of lutein into meso-zeaxanthin. Furthermore, primary cultures of chicken RPE, also accumulated meso-zeaxanthin when incubated with lutein in a dose- and time-dependent manner. To test whether the reaction occurs in live animals, a small molecule inhibitor of RPE65 was injected into the yolk of chicken embryos and demonstrated to block meso-zeaxanthin synthesis. Thus, this studies show that RPE65 is involved in the synthesis of this unique ocular carotenoid. Further research will be required to understand the molecular basis of the unusual catalytic promiscuity of this visual cycle enzyme and its impact on retinoid metabolism of the eyes.
9. Conclusions and future perspectives
Much progress has been made in elucidating the metabolism of carotenoids related to vision. Comparative analyses in different animal classes has identified evolutionarily, well conserved key components in this metabolism. Mutations in their corresponding genes impair carotenoid metabolism and induce various visual pathologies in animal models. In humans, these genes are afflicted by mutations that can cause a wide spectrum of blinding diseases. The better understanding of the pathology of these diseases has paved the way for the development of therapeutic interventions. These include gene therapies and pharmacological interventions. Small molecules targeting visual cycle proteins have been developed and are in clinical tests. Just recently, small molecules targeting retinoid-binding protein CRBP1 have shown promising results in mice. An ancestral family of CCDs catalyzes the oxidative cleavage and geometric isomerization of carotenoid double bonds. The specific enzymatic properties of these enzymes are critical for chromophore production and its quality control. Studies in mouse models revealed that carotenoid metabolism is regulated on demand for vitamin A supply. This mechanism helps to prevent retinoid deficiency when the dietary supply is scant and prevents excess during times of food surplus. The emerging knowledge about host and environmental factors that affect carotenoid metabolism asks for careful reevaluation of existing intake recommendations for these nutrients and further studies of their eye protecting roles. The generation of mice that accumulate significant amounts of carotenoids in the retina will allow testing of the pigment’s ability to protect the retina and retinal pigment epithelium against damaging illumination and oxidative stress. These analyses should clarify putative interaction between carotenoids and the visual cycle since evidence exists that carotenoids can filter blue light that triggers geometric isomerization of the photoproduct and prevent the chemical modifications of visual cycle intermediates. A specific and selective transport system for stored vitamin A has been identified that helps the eyes maintain retinoid homeostasis even during times of prolonged dietary vitamin A deprivation. In STRA6-deficient mice the ocular retinoid content can be manipulated by dietary intervention thus allowing to study the consequences of conditions of mild to severe vitamin A deficiency that is widespread in children many parts of the world. With regards to the visual cycle, the question whether cones and rods rely on different regeneration pathways for chromophore is still a matter of debate. The role of photic regeneration pathways need to be studied in further details. Alternatively, modifications of the canonical visual cycle may well serve the higher demand for chromophore of cones. For this endeavor, researchers may strike out into new directions and take advantage of vertebrate models with cone-rich retinas. The zebrafish and chicken are promising models for such research and the newly established CRISPR-Cas technology offers opportunities to scrutinize their chromophore metabolism. Fostering our knowledge about the chemistry and biology of carotenoids in vision is and continues to be of substantial research interest. A better understanding of the involved molecular factors will aid in the development of nutritional intervention strategies to improve health in neonatal and adult life.
Highlights.
Carotenoid and apo-carotenoid functions.
Carotenoid absorption and its homeostatic regulation.
Carotenoid bioconversion to vitamin A.
Transport and body distribution of carotenoids and retinoids.
Chromophore metabolism in different animal classes.
Acknowledgments
We wish to thank many colleagues for fruitful discussions and collaborations. We are very thankful for the enthusiasm and research contributions of the past and present members of the von Lintig laboratory.
Funding
This work was supported by the National Institutes of Health Grants (EY02551, EY028121 and EY007157). This grant support was responsible for cited research that was carried out in the author’s laboratory and partially allowed for the writing of this review.
Abbreviations
- AMD
age related macular degeneration
- ADH
alcohol dehydrogenase
- ALDH
Aldehyde dehydrogenase
- BCO1
gene encoding β-carotene-15,15′-dioxygenase
- BCO2
gene encoding β-carotene-9,10’-dioxygenase
- CCD
Carotenoid Cleavage Dioxygenases
- CD36
Cluster of Differentiation 36
- CRALBP
Cellular retinal binding protein
- Cyp26a1
Cytochrome P450 Family 26 A1
- DES1
sphingolipid δ4 desaturase
- IRBP
interstitial retinol-binding protein
- ISX
Intestine-Specific Homeobox Transcription factor
- LCA
Leber congenital amaurosis
- LRAT
lecithin: retinol acyl transferase
- MFAT
multifunctional O-acyltransferase
- NinaD
neither inactivation nor afterpotential mutant D
- NinaB
neither inactivation nor afterpotential mutant B
- PDH
Pigment Cell enriched Dehydrogenase
- RPE65
Retina Pigment Epithelium-Specific Protein 65kDa
- Pinta
prolonged depolarization afterpotential is not apparent
- SR-B1
Scavenger Receptor Class B, Member 1
- STRA6
Stimulated by Retinoic Acid 6
- RALDH1–3
Retinal Dehydrogenase 1–3
- RAR
retinoic acid receptor
- RBP (1–4)
retinol-binding protein
- RDH
retinol dehydrogenase
- RGR
RPE-retinal G-protein-coupled receptor (RGR)-opsin
- RXR
retinoid X receptor
- SDR
short chain dehydrogenase/reductase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
The authors declare no conflict of interest or bias by other persons. Figure captions
References
- Abu-Abed S, et al. , 2001. The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev 15, 226–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Abed SS, et al. , 1998. Mouse P450RAI (CYP26) expression and retinoic acid-inducible retinoic acid metabolism in F9 cells are regulated by retinoic acid receptor gamma and retinoid X receptor alpha. J Biol Chem 273, 2409–2415. [DOI] [PubMed] [Google Scholar]
- Abumrad NA, et al. , 1993. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem 268, 17665–17668. [PubMed] [Google Scholar]
- Acton S, et al. , 1996. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 271, 518–520. [DOI] [PubMed] [Google Scholar]
- Acton SL, et al. , 1994. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J Biol Chem 269, 21003–21009. [PubMed] [Google Scholar]
- Adams MK, et al. , 2014. The retinaldehyde reductase activity of DHRS3 is reciprocally activated by retinol dehydrogenase 10 to control retinoid homeostasis. J Biol Chem 289, 14868–14880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad ST, et al. , 2006. The role of Drosophila ninaG oxidoreductase in visual pigment chromophore biogenesis. J Biol Chem 281, 9205–9209. [DOI] [PubMed] [Google Scholar]
- Al-Babili S, Bouwmeester HJ, 2015. Strigolactones, a novel carotenoid-derived plant hormone. Annual review of plant biology 66, 161–186. [DOI] [PubMed] [Google Scholar]
- Alapatt P, et al. , 2012. Liver retinol transporter and receptor for serum retinol binding protein (RBP4). J Biol Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albalat R, 2012. Evolution of the genetic machinery of the visual cycle: a novelty of the vertebrate eye? Mol Biol Evol 29, 1461–1469. [DOI] [PubMed] [Google Scholar]
- Alder A, et al. , 2009. In vitro characterization of Synechocystis CYP120A1 revealed the first nonanimal retinoic acid hydroxylase. FEBS J 276, 5416–5431. [DOI] [PubMed] [Google Scholar]
- Allikmets R, et al. , 1997. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science 277, 1805–1807. [DOI] [PubMed] [Google Scholar]
- Alvarez R, et al. , 2014. Functions, therapeutic applications, and synthesis of retinoids and carotenoids. Chemical reviews 114, 1–125. [DOI] [PubMed] [Google Scholar]
- Amengual J, et al. , 2012. Lecithin:Retinol Acyltransferase Is Critical for Cellular Uptake of Vitamin A from Serum Retinol-binding Protein. J Biol Chem 287, 24216–24227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amengual J, et al. , 2011a. Beta-Carotene Reduces Body Adiposity of Mice via BCMO1. PloS one 6, e20644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amengual J, et al. , 2011b. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J 25, 948–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amengual J, et al. , 2013. Two carotenoid oxygenases contribute to mammalian provitamin A metabolism. J Biol Chem 288, 34081–34096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amengual J, et al. , 2014a. STRA6 is critical for cellular vitamin A uptake and homeostasis. Human molecular genetics 23, 5402–5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amengual J, et al. , 2014b. STRA6 is critical for cellular vitamin A uptake and homeostasis. Human molecular genetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade P, et al. , 2019. Regulatory changes in pterin and carotenoid genes underlie balanced color polymorphisms in the wall lizard. Proc Natl Acad Sci U S A 116, 5633–5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arne JM, et al. , 2017. Allosteric modulation of the substrate specificity of acyl-CoA wax alcohol acyltransferase 2. J Lipid Res 58, 719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky VY, et al. , 2002. G proteins and phototransduction. Annu Rev Physiol 64, 153–187. [DOI] [PubMed] [Google Scholar]
- Aydemir G, et al. , 2016. Lycopene supplementation restores vitamin A deficiency in mice and possesses thereby partial pro-vitamin A activity transmitted via RAR signaling. Mol Nutr Food Res 60, 2413–2420. [DOI] [PubMed] [Google Scholar]
- Babino D, et al. , 2016. The Biochemical Basis of Vitamin A3 Production in Arthropod Vision. ACS Chem Biol 11, 1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babino D, et al. , 2015a. Characterization of the Role of beta-Carotene 9,10-Dioxygenase in Macular Pigment Metabolism. J Biol Chem 290, 24844–24857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babino D, et al. , 2015b. The role of 11-cis-retinyl esters in vertebrate cone vision. FASEB J 29, 216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann H, et al. , 2002. Feedback regulation of beta, beta-carotene 15,15′-monooxygenase by retinoic acid in rats and chickens. J Nutr 132, 3616–3622. [DOI] [PubMed] [Google Scholar]
- Balmer JE, Blomhoff R, 2002. Gene expression regulation by retinoic acid. J Lipid Res 43, 1773–1808. [DOI] [PubMed] [Google Scholar]
- Barker FM 2nd, et al. , 2011. Nutritional manipulation of primate retinas, V: effects of lutein, zeaxanthin, and n-3 fatty acids on retinal sensitivity to blue-light-induced damage. Invest Ophthalmol Vis Sci 52, 3934–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batten ML, et al. , 2004. Lecithin-retinol acyltransferase is essential for accumulation of alltrans-retinyl esters in the eye and in the liver. J Biol Chem 279, 10422–10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, et al. , 1979. The membrane current of single rod outer segments. The Journal of physiology 288, 589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, et al. , 1984. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. The Journal of physiology 357, 575–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyaeva OV, et al. , 2018. Retinol dehydrogenase 11 is essential for the maintenance of retinol homeostasis in liver and testis in mice. J Biol Chem 293, 6996–7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg CJ, et al. , 2013. Vibrational and electronic spectroscopy of the retro-carotenoid rhodoxanthin in avian plumage, solid-state films, and solution. Arch Biochem Biophys 539, 142–155. [DOI] [PubMed] [Google Scholar]
- Bernstein PS, et al. , 2016. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res 50, 34–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DC, et al. , 2013. The STRA6 Receptor Is Essential for Retinol-binding Protein-induced Insulin Resistance but Not for Maintaining Vitamin A Homeostasis in Tissues Other Than the Eye. J Biol Chem 288, 24528–24539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry SD, et al. , 2009. Mutation in bovine beta-carotene oxygenase 2 affects milk color. Genetics 182, 923–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhosale P, Bernstein PS, 2007. Vertebrate and invertebrate carotenoid-binding proteins. Arch Biochem Biophys 458, 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhosale P, et al. , 2009. Retinal carotenoids can attenuate formation of A2E in the retinal pigment epithelium. Arch Biochem Biophys 483, 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings SE, et al. , 2013. The retinaldehyde reductase DHRS3 is essential for preventing the formation of excess retinoic acid during embryonic development. FASEB J 27, 4877–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billsten HH, et al. , 2005. Self-assembled aggregates of the carotenoid zeaxanthin: Time-resolved study of excited states. J Phys Chem A 109, 1521–1529. [DOI] [PubMed] [Google Scholar]
- Blaner WS, et al. , 1987. Hydrolysis of 11-cis- and all-trans-retinyl palmitate by homogenates of human retinal epithelial cells. J Biol Chem 262, 53–58. [PubMed] [Google Scholar]
- Blaner WS, et al. , 2016. Vitamin A Absorption, Storage and Mobilization. Sub-cellular biochemistry 81, 95–125. [DOI] [PubMed] [Google Scholar]
- Blomhoff R, et al. , 1990. Transport and storage of vitamin A. Science 250, 399–404. [DOI] [PubMed] [Google Scholar]
- Blount JD, et al. , 2003. Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science 300, 125–127. [DOI] [PubMed] [Google Scholar]
- Bohn T, et al. , 2017. Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans. Mol Nutr Food Res 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone RA, et al. , 1985. Preliminary identification of the human macular pigment. Vision Res 25, 1531–1535. [DOI] [PubMed] [Google Scholar]
- Borel P, et al. , 2011. Genetic variants in BCMO1 and CD36 are associated with plasma lutein concentrations and macular pigment optical density in humans. Annals of medicine 43, 47–59. [DOI] [PubMed] [Google Scholar]
- Borel P, Desmarchelier C, 2018. Bioavailability of Fat-Soluble Vitamins and Phytochemicals in Humans: Effects of Genetic Variation. Annu Rev Nutr 38, 69–96. [DOI] [PubMed] [Google Scholar]
- Borel P, et al. , 2015. A Combination of Single-Nucleotide Polymorphisms Is Associated with Interindividual Variability in Dietary beta-Carotene Bioavailability in Healthy Men. J Nutr 145, 1740–1747. [DOI] [PubMed] [Google Scholar]
- Borel P, et al. , 1998. Low and high responders to pharmacological doses of beta-carotene: proportion in the population, mechanisms involved and consequences on beta-carotene metabolism. J Lipid Res 39, 2250–2260. [PubMed] [Google Scholar]
- Borel P, et al. , 2013. CD36 and SR-BI are involved in cellular uptake of provitamin A carotenoids by Caco-2 and HEK cells, and some of their genetic variants are associated with plasma concentrations of these micronutrients in humans. J Nutr 143, 448–456. [DOI] [PubMed] [Google Scholar]
- Borowski T, et al. , 2008. Reaction mechanism of apocarotenoid oxygenase (ACO): a DFT study. Chemistry 14, 2264–2276. [DOI] [PubMed] [Google Scholar]
- Bouillet P, et al. , 1997. Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mech Dev 63, 173–186. [DOI] [PubMed] [Google Scholar]
- Boulanger A, et al. , 2003. Identification of beta-carotene 15, 15′-monooxygenase as a peroxisome proliferator-activated receptor target gene. Faseb J 17, 1304–1306. [DOI] [PubMed] [Google Scholar]
- Bouvier F, et al. , 2003a. Biosynthesis of the food and cosmetic plant pigment bixin (annatto). Science 300, 2089–2091. [DOI] [PubMed] [Google Scholar]
- Bouvier F, et al. , 1998. Xanthophyll biosynthesis: molecular and functional characterization of carotenoid hydroxylases from pepper fruits (Capsicum annuum L.). Biochim Biophys Acta 1391, 320–328. [DOI] [PubMed] [Google Scholar]
- Bouvier F, et al. , 2003b. Oxidative remodeling of chromoplast carotenoids: identification of the carotenoid dioxygenase CsCCD and CsZCD genes involved in Crocus secondary metabolite biogenesis. Plant Cell 15, 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowmaker JK, Martin GR, 1978. Visual pigments and colour vision in a nocturnal bird, Strix aluco (tawny owl). Vision Res 18, 1125–1130. [DOI] [PubMed] [Google Scholar]
- Casey J, et al. , 2011. First implication of STRA6 mutations in isolated anophthalmia, microphthalmia, and coloboma: a new dimension to the STRA6 phenotype. Hum Mutat 32, 1417–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai C, et al. , 2019. Characterization of the novel role of NinaB orthologs from Bombyx mori and Tribolium castaneum. Insect Biochem Mol Biol 109, 106–115. [DOI] [PubMed] [Google Scholar]
- Chassaing N, et al. , 2009. Phenotypic spectrum of STRA6 mutations: from Matthew-Wood syndrome to non-lethal anophthalmia. Human mutation 30, E673–681. [DOI] [PubMed] [Google Scholar]
- Chawla A, et al. , 2001. Nuclear receptors and lipid physiology: opening the X-files. Science 294, 1866–1870. [DOI] [PubMed] [Google Scholar]
- Chelstowska S, et al. , 2016. Molecular Basis for Vitamin A Uptake and Storage in Vertebrates. Nutrients 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, et al. , 2001. A photic visual cycle of rhodopsin regeneration is dependent on Rgr. Nat Genet 28, 256–260. [DOI] [PubMed] [Google Scholar]
- Chen Y, et al. , 2016. Structure of the STRA6 receptor for retinol uptake. Science 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, et al. , 2012. Mechanism of all-trans-retinal toxicity with implications for stargardt disease and age-related macular degeneration. J Biol Chem 287, 5059–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew EY, et al. , 2014. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA ophthalmology 132, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichili GR, et al. , 2005. beta-Carotene conversion into vitamin A in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 46, 3562–3569. [DOI] [PubMed] [Google Scholar]
- Choi MY, et al. , 2006. A dynamic expression survey identifies transcription factors relevant in mouse digestive tract development. Development 133, 4119–4129. [DOI] [PubMed] [Google Scholar]
- Chou CM, et al. , 2015. Biochemical Basis for Dominant Inheritance, Variable Penetrance, and Maternal Effects in RBP4 Congenital Eye Disease. Cell 161, 634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chytil F, 1986. Retinoic acid: biochemistry and metabolism. J Am Acad Dermatol 15, 741–747. [DOI] [PubMed] [Google Scholar]
- Chytil F, Ong DE, 1987. Intracellular vitamin A--binding proteins. Annu Rev Nutr 7, 321–335. [DOI] [PubMed] [Google Scholar]
- Cianci M, et al. , 2002. The molecular basis of the coloration mechanism in lobster shell: beta-crustacyanin at 3.2-A resolution. Proc Natl Acad Sci U S A 99, 9795–9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifarelli V, Abumrad NA, 2018. Intestinal CD36 and Other Key Proteins of Lipid Utilization: Role in Absorption and Gut Homeostasis. Comprehensive Physiology 8, 493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clagett-Dame M, DeLuca HF, 2002. The role of vitamin A in mammalian reproduction and embryonic development. Annu Rev Nutr 22, 347–381. [DOI] [PubMed] [Google Scholar]
- Connelly MA, et al. , 1999. Comparison of class B scavenger receptors, CD36 and scavenger receptor BI (SR-BI), shows that both receptors mediate high density lipoprotein-cholesteryl ester selective uptake but SR-BI exhibits a unique enhancement of cholesteryl ester uptake. J Biol Chem 274, 41–47. [DOI] [PubMed] [Google Scholar]
- Costabile BK, et al. , 2016. beta-Apo-10′-carotenoids Modulate Placental Microsomal Triglyceride Transfer Protein Expression and Function to Optimize Transport of Intact beta-Carotene to the Embryo. J Biol Chem 291, 18525–18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran-Celentano J, et al. , 1985. Alterations in vitamin A and thyroid hormone status in anorexia nervosa and associated disorders. Am J Clin Nutr 42, 1183–1191. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio DN, et al. , 2011. Vitamin A Metabolism: An Update. Nutrients 3, 63–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daruwalla A, Kiser PD, 2019. Structural and mechanistic aspects of carotenoid cleavage dioxygenases (CCDs). Biochim Biophys Acta Mol Cell Biol Lipids, 158590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dela Sena C, et al. , 2013. Substrate specificity of purified recombinant human beta-carotene 15,15′-oxygenase (BCO1). J Biol Chem 288, 37094–37103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dela Sena C, et al. , 2014. The human enzyme that converts dietary provitamin a carotenoids to vitamin a is a dioxygenase. J Biol Chem 289, 13661–13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dela Sena C, et al. , 2016. Substrate Specificity of Purified Recombinant Chicken beta-Carotene 9′,10′-Oxygenase (BCO2). J Biol Chem 291, 14609–14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW 3rd, 2002. Antioxidants in photosynthesis and human nutrition. Science 298, 2149–2153. [DOI] [PubMed] [Google Scholar]
- den Hollander AI, et al. , 2009. A homozygous missense mutation in the IRBP gene (RBP3) associated with autosomal recessive retinitis pigmentosa. Invest Ophthalmol Vis Sci 50, 1864–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz NM, et al. , 2017. The Visual Cycle in the Inner Retina of Chicken and the Involvement of Retinal G-Protein-Coupled Receptor (RGR). Mol Neurobiol 54, 2507–2517. [DOI] [PubMed] [Google Scholar]
- Dickinson AJ, et al. , 2019. beta-Cyclocitral is a conserved root growth regulator. Proc Natl Acad Sci U S A 116, 10563–10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JE, Wald G, 1958. Vitamin a Deficiency and Night Blindness. Proc Natl Acad Sci U S A 44, 648–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duester G, 2008. Retinoic acid synthesis and signaling during early organogenesis. Cell 134, 921–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During A, et al. , 2005. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J Nutr 135, 2305–2312. [DOI] [PubMed] [Google Scholar]
- During A, et al. , 2008. Xanthophylls are preferentially taken up compared with beta-carotene by retinal cells via a SRBI-dependent mechanism. J Lipid Res 49, 1715–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During A, et al. , 2002. Carotenoid uptake and secretion by CaCo-2 cells: beta-carotene isomer selectivity and carotenoid interactions. J Lipid Res 43, 1086–1095. [DOI] [PubMed] [Google Scholar]
- Edge R, Truscott TG, 2018. Singlet Oxygen and Free Radical Reactions of Retinoids and Carotenoids-A Review. Antioxidants (Basel) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RB, Adler AJ, 1994. Exchange of retinol between IRBP and CRBP. Experimental eye research 59, 161–170. [DOI] [PubMed] [Google Scholar]
- Enright JM, et al. , 2015. Cyp27c1 Red-Shifts the Spectral Sensitivity of Photoreceptors by Converting Vitamin A1 into A2. Curr Biol 25, 3048–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Episkopou V, et al. , 1993. Disruption of the transthyretin gene results in mice with depressed levels of plasma retinol and thyroid hormone. Proc Natl Acad Sci 90, 2375–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson J, et al. , 2008. Identification of the yellow skin gene reveals a hybrid origin of the domestic chicken. PLoS Genet 4, e1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu A, et al. , 2012. Naturally occurring eccentric cleavage products of provitamin A beta-carotene function as antagonists of retinoic acid receptors. J Biol Chem 287, 15886–15895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre B, et al. , 2003. Immune activation rapidly mirrored in a secondary sexual trait. Science 300, 103. [DOI] [PubMed] [Google Scholar]
- Fallahshahroudi A, et al. , 2019. The Domestic BCO2 Allele Buffers Low-Carotenoid Diets in Chickens: Possible Fitness Increase Through Species Hybridization. Genetics 212, 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, et al. , 2003a. Isorhodopsin rather than rhodopsin mediates rod function in RPE65 knock-out mice. Proc Natl Acad Sci 100, 13662–13667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, et al. , 2003b. Targeted disruption of Aldh1a1 (Raldh1) provides evidence for a complex mechanism of retinoic acid synthesis in the developing retina. Mol Cell Biol 23, 4637–4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio M, Silverstein RL, 2007. CD36: implications in cardiovascular disease. Int J Biochem Cell Biol 39, 2012–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, et al. , 2009. Common variation in the beta-carotene 15,15′-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet 84, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch VC, Neuhauss SC, 2010. Parallel visual cycles in the zebrafish retina. Prog Retin Eye Res 29, 476–486. [DOI] [PubMed] [Google Scholar]
- Fleisch VC, et al. , 2008. Subfunctionalization of a retinoid-binding protein provides evidence for two parallel visual cycles in the cone-dominant zebrafish retina. J Neurosci 28, 8208–8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford NA, et al. , 2010. Loss of carotene-9′,10′-monooxygenase expression increases serum and tissue lycopene concentrations in lycopene-fed mice. J Nutr 140, 2134–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster RG, Hankins MW, 2002. Non-rod, non-cone photoreception in the vertebrates. Prog Retin Eye Res 21, 507–527. [DOI] [PubMed] [Google Scholar]
- Frederica LS, Holm E, 1925. Experimental contribution to the study of the relation between night blindness and malnutrition. Am J Physiol 73, 63–77. [Google Scholar]
- Garafalo AV, et al. , 2019. Progress in treating inherited retinal diseases: Early subretinal gene therapy clinical trials and candidates for future initiatives. Prog Retin Eye Res, 100827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere V, et al. , 1987. Identification of a receptor for the morphogen retinoic acid. Nature 330, 624–629. [DOI] [PubMed] [Google Scholar]
- Giovannucci DR, Stephenson RS, 1999. Identification and distribution of dietary precursors of the Drosophila visual pigment chromophore: analysis of carotenoids in wild type and ninaD mutants by HPLC. Vision Res 39, 219–229. [DOI] [PubMed] [Google Scholar]
- Giuliano G, et al. , 1986. The gene crtI mediates the conversion of phytoene into colored carotenoids in Rhodopseudomonas capsulata. J Biol Chem 261, 12925–12929. [PubMed] [Google Scholar]
- Golczak M, et al. , 2010. Importance of membrane structural integrity for RPE65 retinoid isomerization activity. J Biol Chem 285, 9667–9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golczak M, et al. , 2015. LRAT-specific domain facilitates vitamin A metabolism by domain swapping in HRASLS3. Nature chemical biology 11, 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg IJ, et al. , 2009. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. J Lipid Res 50 Suppl, S86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith TH, 2013. Evolutionary tinkering with visual photoreception. Vis Neurosci 30, 21–37. [DOI] [PubMed] [Google Scholar]
- Gollapalli DR, et al. , 2003. RPE65 operates in the vertebrate visual cycle by stereospecifically binding all-trans-retinyl esters. Biochemistry 42, 11824–11830. [DOI] [PubMed] [Google Scholar]
- Golzio C, et al. , 2007. Matthew-Wood syndrome is caused by truncating mutations in the retinol-binding protein receptor gene STRA6. Am J Hum Genet 80, 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves A, et al. , 2014. Intestinal scavenger receptors are involved in vitamin K1 absorption. J Biol Chem 289, 30743–30752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorusupudi A, et al. , 2016. Developmentally Regulated Production of meso-Zeaxanthin in Chicken Retinal Pigment Epithelium/Choroid and Retina. Invest Ophthalmol Vis Sci 57, 1853–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwalt DE, et al. , 1992. Membrane glycoprotein CD36: a review of its roles in adherence, signal transduction, and transfusion medicine. Blood 80, 1105–1115. [PubMed] [Google Scholar]
- Grimm C, et al. , 2000. Blue light’s effects on rhodopsin: photoreversal of bleaching in living rat eyes. Invest Ophthalmol Vis Sci 41, 3984–3990. [PubMed] [Google Scholar]
- Grimm C, et al. , 2001. Rhodopsin-mediated blue-light damage to the rat retina: effect of photoreversal of bleaching. Invest Ophthalmol Vis Sci 42, 497–505. [PubMed] [Google Scholar]
- Grune T, et al. , 2010. Beta-carotene is an important vitamin A source for humans. J Nutr 140, 2268S–2285S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeseleer F, et al. , 1998. Molecular characterization of a novel short-chain dehydrogenase/reductase that reduces all-trans-retinal. J Biol Chem 273, 21790–21799. [DOI] [PubMed] [Google Scholar]
- Haeseleer F, et al. , 2002. Dual-substrate specificity short chain retinol dehydrogenases from the vertebrate retina. J Biol Chem 277, 45537–45546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA, et al. , 2011. The role of retinoic acid in tolerance and immunity. Immunity 35, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel CP, et al. , 1993. Molecular cloning and expression of RPE65, a novel retinal pigment epithelium-specific microsomal protein that is post-transcriptionally regulated in vitro. J Biol Chem 268, 15751–15757. [PubMed] [Google Scholar]
- Hammond BR Jr., et al. , 2013. Glare disability, photostress recovery, and chromatic contrast: relation to macular pigment and serum lutein and zeaxanthin. Invest Ophthalmol Vis Sci 54, 476–481. [DOI] [PubMed] [Google Scholar]
- Hansen S, Maret W, 1988. Retinal is not formed in vitro by enzymatic central cleavage of beta-carotene. Biochemistry 27, 200–206. [DOI] [PubMed] [Google Scholar]
- Hao W, Fong HK, 1999. The endogenous chromophore of retinal G protein-coupled receptor opsin from the pigment epithelium. J Biol Chem 274, 6085–6090. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Raghu P, 2001. Visual transduction in Drosophila. Nature 413, 186–193. [DOI] [PubMed] [Google Scholar]
- Harris WA, et al. , 1977. Vitamin A deprivation and Drosophila photopigments. Nature 266, 648–650. [DOI] [PubMed] [Google Scholar]
- Harrison EH, Quadro L, 2018. Apocarotenoids: Emerging Roles in Mammals. Annu Rev Nutr 38, 153–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H, et al. , 2018. Understanding/unravelling carotenoid excited singlet states. J R Soc Interface 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He PP, et al. , 2018. Lipoprotein lipase: Biosynthesis, regulatory factors, and its role in atherosclerosis and other diseases. Clin Chim Acta 480, 126–137. [DOI] [PubMed] [Google Scholar]
- Heller J, 1975. Interactions of plasma retinol-binding protein with its receptor. Specific binding of bovine and human retinol-binding protein to pigment epithelium cells from bovine eyes. J Biol Chem 250, 3613–3619. [PubMed] [Google Scholar]
- Heller M, Bok D, 1976. A specific receptor for retinol binding protein as detected by the binding of human and bovine retinol binding protein to pigment epithelial cells. Am J Ophthalmol 81, 93–97. [DOI] [PubMed] [Google Scholar]
- Hessel S, et al. , 2007. CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J Biol Chem 282, 33553–33561. [DOI] [PubMed] [Google Scholar]
- Hofmann L, Palczewski K, 2015. Advances in understanding the molecular basis of the first steps in color vision. Prog Retin Eye Res 49, 46–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann L, et al. , 2016. Structural Insights into the Drosophila melanogaster Retinol Dehydrogenase, a Member of the Short-Chain Dehydrogenase/Reductase Family. Biochemistry 55, 6545–6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu KQ, et al. , 2006. The biochemical characterization of ferret carotene-9′,10′-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J Biol Chem 281, 19327–19338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, et al. , 2011. Comparison of ocular pathologies in vitamin A-deficient mice and RPE65 gene knockout mice. Invest Ophthalmol Vis Sci 52, 5507–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R, Kropf A, 1958. The Action of Light on Rhodopsin. Proc Natl Acad Sci U S A 44, 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber A, et al. , 1994. Opsin maturation and targeting to rhabdomeral photoreceptor membranes requires the retinal chromophore. Eur J Cell Biol 63, 219–229. [PubMed] [Google Scholar]
- Hugueney P, et al. , 1995. Metabolism of cyclic carotenoids: a model for the alteration of this biosynthetic pathway in Capsicum annuum chromoplasts. Plant J 8, 417–424. [DOI] [PubMed] [Google Scholar]
- Hunter JJ, et al. , 2012. The susceptibility of the retina to photochemical damage from visible light. Prog Retin Eye Res 31, 28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanishi Y, et al. , 2004. Retinosomes: new insights into intracellular managing of hydrophobic substances in lipid bodies. J Cell Biol 166, 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip BC, et al. , 2013. Lycopene metabolite, apo-10′-lycopenoic acid, inhibits diethylnitrosam-ineinitiated, high fat diet-promoted hepatic inflammation and tumorigenesis in mice. Cancer Prev Res (Phila) 6, 1304–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J, et al. , 2008. Microsomal triglyceride transfer protein enhances cellular cholesteryl esterification by relieving product inhibition. J Biol Chem 283, 19967–19980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson T, et al. , 2004. Analysis in Vitro of the Enzyme CRTISO Establishes a Poly-cis-Carotenoid Biosynthesis Pathway in Plants. Plant Physiol 136, 4246–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson T, et al. , 2002. Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of beta-carotene and xanthophylls in plants. Plant Cell 14, 333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isken A, et al. , 2008. RBP4 disrupts vitamin A uptake homeostasis in a STRA6-deficient animal model for Matthew-Wood syndrome. Cell Metab 7, 258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isken A, et al. , 2007. Sequestration of retinyl esters is essential for retinoid signaling in the zebrafish embryo. J Biol Chem 282, 1144–1151. [DOI] [PubMed] [Google Scholar]
- Isono K, et al. , 1988. Dependency on light and vitamin A derivatives of the biogenesis of 3-hydroxyretinal and visual pigment in the compound eyes of Drosophila melanogaster. J Gen Physiol 92, 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janecke AR, et al. , 2004. Mutations in RDH12 encoding a photoreceptor cell retinol dehydrogenase cause childhood-onset severe retinal dystrophy. Nat Genet 36, 850–854. [DOI] [PubMed] [Google Scholar]
- Jastrzebska B, et al. , 2011. Role of bulk water in hydrolysis of the rhodopsin chromophore. J Biol Chem 286, 18930–18937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, et al. , 2005. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell 122, 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KH, 2007. The distinct signaling mechanisms of microbial sensory rhodopsins in Archaea, Eubacteria and Eukarya. Photochem Photobiol 83, 63–69. [DOI] [PubMed] [Google Scholar]
- Kanai M, et al. , 1968. Retinol-binding protein: the transport protein for vitamin A in human plasma. J Clin Invest 47, 2025–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MA, et al. , 2010. Identification of 9-cis-retinoic acid as a pancreas-specific autacoid that attenuates glucose-stimulated insulin secretion. Proc Natl Acad Sci U S A 107, 21884–21889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer P H. A, Wehrli H, Wettstein A, 1930. Über die Konstitution des Lycopins und Cartoins. Hel. Chim. Acta 13, 1084. [Google Scholar]
- Kawaguchi R, et al. , 2007. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin a. Science 315, 820–825. [DOI] [PubMed] [Google Scholar]
- Kawaguchi R, et al. , 2011. Receptor-Mediated Cellular Uptake Mechanism That Couples to Intracellular Storage. ACS Chem Biol 6, 1041–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaylor JJ, et al. , 2014. Identification of the 11-cis-specific retinyl-ester synthase in retinal Muller cells as multifunctional O-acyltransferase (MFAT). Proc Natl Acad Sci U S A 111, 7302–7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaylor JJ, et al. , 2017. Blue light regenerates functional visual pigments in mammals through a retinyl-phospholipid intermediate. Nature communications 8, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaylor JJ, et al. , 2013. Identification of DES1 as a vitamin A isomerase in Muller glial cells of the retina. Nature chemical biology 9, 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedishvili NY, 2013. Enzymology of retinoic acid biosynthesis and degradation. J Lipid Res 54, 1744–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M, et al. , 2016. Transport of vitamin A across blood-tissue barriers is facilitated by STRA6. FASEB J 30, 2985–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly ME, et al. , 2018. The Biochemical Basis of Vitamin A Production from the Asymmetric Carotenoid beta-Cryptoxanthin. ACS Chem Biol 13, 2121–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachik F, et al. , 1997. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci 38, 1802–1811. [PubMed] [Google Scholar]
- Khadka N, et al. , 2019. Evidence for distinct rate-limiting steps in the cleavage of alkenes by carotenoid cleavage dioxygenases. J Biol Chem 294, 10596–10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan KN, et al. , 2016. Vitamin A deficiency due to bi-allelic mutation of RBP4: There’s more to it than meets the eye. Ophthalmic genetics, 1–2. [DOI] [PubMed] [Google Scholar]
- Kiefer C, et al. , 2001. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem 276, 14110–14116. [DOI] [PubMed] [Google Scholar]
- Kiefer C, et al. , 2002. A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila. Proc Natl Acad Sci 99, 10581–10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SR, et al. , 2006. Photooxidation of A2-PE, a photoreceptor outer segment fluorophore, and protection by lutein and zeaxanthin. Exp Eye Res 82, 828–839. [DOI] [PubMed] [Google Scholar]
- Kim YK, et al. , 2008. Retinyl ester formation by lecithin: retinol acyltransferase is a key regulator of retinoid homeostasis in mouse embryogenesis. J Biol Chem 283, 5611–5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser PD, et al. , 2012. Structure of RPE65 isomerase in a lipidic matrix reveals roles for phospholipids and iron in catalysis. Proc Natl Acad Sci U S A 109, E2747–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser PD, et al. , 2009. Crystal structure of native RPE65, the retinoid isomerase of the visual cycle. Proc Natl Acad Sci U S A 106, 17325–17330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser PD, et al. , 2014. Chemistry of the retinoid (visual) cycle. Chemical reviews 114, 194–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser PD, et al. , 2019. Conditional deletion of Des1 in the mouse retina does not impair the visual cycle in cones. FASEB J 33, 5782–5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser PD, Palczewski K, 2016. Retinoids and Retinal Diseases. Annual review of vision science 2, 197–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser PD, et al. , 2015. Catalytic mechanism of a retinoid isomerase essential for vertebrate vision. Nature chemical biology 11, 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser PD, et al. , 2018. Retinoid isomerase inhibitors impair but do not block mammalian cone photoreceptor function. J Gen Physiol 150, 571–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloer DP, et al. , 2005. The structure of a retinal-forming carotenoid oxygenase. Science 308, 267–269. [DOI] [PubMed] [Google Scholar]
- Kloer DP, Schulz GE, 2006. Structural and biological aspects of carotenoid cleavage. Cellular and molecular life sciences : CMLS 63, 2291–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopcke W, Krutmann J, 2008. Protection from sunburn with beta-Carotene--a meta-analysis. Photochem Photobiol 84, 284–288. [DOI] [PubMed] [Google Scholar]
- Kowatz T, et al. , 2013. Characterization of human beta,beta-carotene-15,15′-monooxygenase (BCMO1) as a soluble monomeric enzyme. Arch Biochem Biophys 539, 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarsky KF, et al. , 1997. Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature 387, 414–417. [DOI] [PubMed] [Google Scholar]
- Kumar S, et al. , 2012. Alcohol and aldehyde dehydrogenases: retinoid metabolic effects in mouse knockout models. Biochim Biophys Acta 1821, 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lala VR, Reddy V, 1970. Absorption of beta-carotene from green leafy vegetables in undernourished children. Am J Clin Nutr 23, 110–113. [DOI] [PubMed] [Google Scholar]
- Lamb TD, Pugh EN Jr., 2004. Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res 23, 307–380. [DOI] [PubMed] [Google Scholar]
- Leermakers ET, et al. , 2016. The effects of lutein on cardiometabolic health across the life course: a systematic review and meta-analysis. Am J Clin Nutr 103, 481–494. [DOI] [PubMed] [Google Scholar]
- Leung WC, et al. , 2008. Two common single nucleotide polymorphisms in the gene encoding {beta}-carotene 15,15′-monoxygenase alter {beta}-carotene metabolism in female volunteers. Faseb J. [DOI] [PubMed] [Google Scholar]
- Li B, et al. , 2011. Identification of StARD3 as a lutein-binding protein in the macula of the primate retina. Biochemistry 50, 2541–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, et al. , 2014. Inactivity of human beta,beta-carotene-9′,10′-dioxygenase (BCO2) underlies retinal accumulation of the human macular carotenoid pigment. Proc Natl Acad Sci U S A 111, 10173–10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian F, et al. , 2007. Apo-10′-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo. Carcinogenesis 28, 1567–1574. [DOI] [PubMed] [Google Scholar]
- Lietz G, et al. , 2012. Single nucleotide polymorphisms upstream from the beta-carotene 15,15′-monoxygenase gene influence provitamin A conversion efficiency in female volunteers. J Nutr 142, 161S–165S. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, Andersson S, 2002. Biochemical properties of purified recombinant human beta-carotene 15,15′-monooxygenase. J Biol Chem 277, 23942–23948. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, et al. , 2005. Cell type-specific expression of beta-carotene 9′,10′-monooxygenase in human tissues. J Histochem Cytochem 53, 1403–1412. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, et al. , 2007. Loss-of-function mutation in carotenoid 15,15′-monooxygenase identified in a patient with hypercarotenemia and hypovitaminosis A. J Nutr 137, 2346–2350. [DOI] [PubMed] [Google Scholar]
- Linnewiel-Hermoni K, et al. , 2014. Carotenoid derivatives inhibit nuclear factor kappa B activity in bone and cancer cells by targeting key thiol groups. Free Radic Biol Med 75, 105–120. [DOI] [PubMed] [Google Scholar]
- Liou GI, et al. , 1982. Vitamin A transport between retina and pigment epithelium--an interstitial protein carrying endogenous retinol (interstitial retinol-binding protein). Vision Res 22, 1457–1467. [DOI] [PubMed] [Google Scholar]
- Liou GI, et al. , 1989. Human interstitial retinoid-binding protein. Gene structure and primary structure. J Biol Chem 264, 8200–8206. [PubMed] [Google Scholar]
- Liu L, Gudas LJ, 2005. Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin A deficiency. J Biol Chem 280, 40226–40234. [DOI] [PubMed] [Google Scholar]
- Lobo GP, et al. , 2013. Genetics and diet regulate vitamin A production via the homeobox transcription factor ISX. J Biol Chem 288, 9017–9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo GP, et al. , 2010a. {beta},{beta}-Carotene Decreases Peroxisome Proliferator Receptor {gamma} Activity and Reduces Lipid Storage Capacity of Adipocytes in a {beta},{beta}-Carotene Oxygenase 1-dependent Manner. J Biol Chem 285, 27891–27899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo GP, et al. , 2010b. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin A production. FASEB J 24, 1656–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo GP, et al. , 2012. BCDO2 acts as a carotenoid scavenger and gatekeeper for the mitochondrial apoptotic pathway. Development 139, 2966–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes RJ, et al. , 2016. Genetic Basis for Red Coloration in Birds. Curr Biol 26, 1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan R, et al. , 1980. Comparison of the level of cellular retinoid-binding proteins and susceptibility to retinoid-induced growth inhibition of various neoplastic cell lines. J Natl Cancer Inst 64, 1259–1262. [PubMed] [Google Scholar]
- Lyubarsky AL, et al. , 2005. Mole quantity of RPE65 and its productivity in the generation of 11-cis-retinal from retinyl esters in the living mouse eye. Biochemistry 44, 9880–9888. [DOI] [PubMed] [Google Scholar]
- MacDonald PN, Ong DE, 1988. A lecithin:retinol acyltransferase activity in human and rat liver. Biochem Biophys Res Commun 156, 157–163. [DOI] [PubMed] [Google Scholar]
- Maeda A, et al. , 2009. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J Biol Chem 284, 15173–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A, et al. , 2008. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem 283, 26684–26693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A, et al. , 2005. Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo. J Biol Chem 280, 18822–18832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A, et al. , 2007a. Redundant and unique roles of retinol dehydrogenases in the mouse retina. Proc Natl Acad Sci 104, 19565–19570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A, et al. , 2007b. Redundant and unique roles of retinol dehydrogenases in the mouse retina. Proceedings of the National Academy of Sciences of the United States of America 104, 19565–19570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, et al. , 2012. Retinal photodamage mediated by all-trans-retinal. Photochem Photobiol 88, 1309–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mares J, 2016. Lutein and Zeaxanthin Isomers in Eye Health and Disease. Annu Rev Nutr 36, 571–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlhens F, et al. , 1997. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat Genet 17, 139–141. [DOI] [PubMed] [Google Scholar]
- Mata NL, et al. , 2004. Rpe65 is a retinyl ester binding protein that presents insoluble substrate to the isomerase in retinal pigment epithelial cells. J Biol Chem 279, 635–643. [DOI] [PubMed] [Google Scholar]
- Mata NL, et al. , 2002. Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron 36, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata NL, et al. , 2005. Chicken retinas contain a retinoid isomerase activity that catalyzes the direct conversion of all-trans-retinol to 11-cis-retinol. Biochemistry 44, 11715–11721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata NL, et al. , 2001. Delayed dark-adaptation and lipofuscin accumulation in abcr+/− mice: implications for involvement of ABCR in age-related macular degeneration. Invest Ophthalmol Vis Sci 42, 1685–1690. [PubMed] [Google Scholar]
- Matt N, et al. , 2005. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development 132, 4789–4800. [DOI] [PubMed] [Google Scholar]
- McCollum E, Davis M, 1913. The necessity of certain lipids in the diet during growth. J. Biol. Chem 15, 167–175. [Google Scholar]
- Mein JR, et al. , 2010. Enzymatic formation of apo-carotenoids from the xanthophyll carotenoids lutein, zeaxanthin and beta-cryptoxanthin by ferret carotene-9′,10′-monooxygenase. Arch Biochem Biophys 506, 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes-Pinto MM, 2009. Carotenoid breakdown products the-norisoprenoids-in wine aroma. Arch Biochem Biophys 483, 236–245. [DOI] [PubMed] [Google Scholar]
- Messing SA, et al. , 2010. Structural insights into maize viviparous14, a key enzyme in the biosynthesis of the phytohormone abscisic acid. Plant Cell 22, 2970–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers KJ, et al. , 2013. Genetic determinants of macular pigments in women of the Carotenoids in Age-Related Eye Disease Study. Invest Ophthalmol Vis Sci 54, 2333–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers KJ, et al. , 2014. Genetic evidence for role of carotenoids in age-related macular degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS). Invest Ophthalmol Vis Sci 55, 587–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mic FA, et al. , 2000. RALDH3, a retinaldehyde dehydrogenase that generates retinoic acid, is expressed in the ventral retina, otic vesicle and olfactory pit during mouse development. Mech Dev 97, 227–230. [DOI] [PubMed] [Google Scholar]
- Moise AR, et al. , 2014. Mechanistic aspects of carotenoid biosynthesis. Chemical reviews 114, 164–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseyev G, et al. , 2005. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proceedings of the National Academy of Sciences of the United States of America 102, 12413–12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseyev G, et al. , 2006. RPE65 is an iron(II)-dependent isomerohydrolase in the retinoid visual cycle. J Biol Chem 281, 2835–2840. [DOI] [PubMed] [Google Scholar]
- Moiseyev G, et al. , 2008. RPE65 from cone-dominant chicken is a more efficient isomerohydrolase compared with that from rod-dominant species. J Biol Chem 283, 8110–8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday LL, et al. , 2000. ABCR expression in foveal cone photoreceptors and its role in Stargardt macular dystrophy. Nat Genet 25, 257–258. [DOI] [PubMed] [Google Scholar]
- Molday RS, et al. , 2009. The role of the photoreceptor ABC transporter ABCA4 in lipid transport and Stargardt macular degeneration. Biochimica et biophysica acta 1791, 573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molotkov A, et al. , 2006. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development 133, 1901–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, 1930. Vitamin A and carotene VI. The conversion of carotene to vitamin A in vivo. Biochem. J 24, 692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshedian A, et al. , 2019. Light-Driven Regeneration of Cone Visual Pigments through a Mechanism Involving RGR Opsin in Muller Glial Cells. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser AJ, Clark J, 2019. Triplet-Pair States in Organic Semiconductors. Annu Rev Phys Chem 70, 323–351. [DOI] [PubMed] [Google Scholar]
- Musser AJ, et al. , 2015. The nature of singlet exciton fission in carotenoid aggregates. J Am Chem Soc 137, 5130–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafi D, et al. , 2016. Transcriptome analysis reveals rod/cone photoreceptor specific signatures across mammalian retinas. Human molecular genetics 25, 4376–4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli JL, 2012. Physiological insights into all-trans-retinoic acid biosynthesis. Biochim Biophys Acta 1821, 152–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanasamy S, et al. , 2017. Synthesis of apo-13- and apo-15-lycopenoids, cleavage products of lycopene that are retinoic acid antagonists. J Lipid Res 58, 1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neculai D, et al. , 2013. Structure of LIMP-2 provides functional insights with implications for SR-BI and CD36. Nature 504, 172–176. [DOI] [PubMed] [Google Scholar]
- Niederreither K, et al. , 2002. Genetic evidence that oxidative derivatives of retinoic acid are not involved in retinoid signaling during mouse development. Nat Genet 31, 84–88. [DOI] [PubMed] [Google Scholar]
- Niederreither K, et al. , 1999. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet 21, 444–448. [DOI] [PubMed] [Google Scholar]
- O’Byrne SM, Blaner WS, 2013. Retinol and retinyl esters: biochemistry and physiology. Journal of lipid research 54, 1731–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Byrne SM, et al. , 2005. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT). J Biol Chem 280, 35647–35657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhauser V, et al. , 2008. NinaB combines carotenoid oxygenase and retinoid isomerase activity in a single polypeptide. Proc Natl Acad Sci 105, 19000–19005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrochta KM, et al. , 2015. Insulin regulates retinol dehydrogenase expression and all-trans-retinoic acid biosynthesis through FoxO1. J Biol Chem 290, 7259–7268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JA, Hayaishi O, 1965. The enzymatic cleavage of beta-carotene into vitamin A by soluble enzymes of rat liver and intestine. Proc Natl Acad Sci U S A 54, 1364–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omenn GS, et al. , 1996. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 334, 1150–1155. [DOI] [PubMed] [Google Scholar]
- Osborne T, Mendel LB, 1913. The relation of growth to the chemical constituents of the diet. J. Biol. Chem 15, 311–326. [Google Scholar]
- Ozaki K, et al. , 1993. Maturation of major Drosophila rhodopsin, ninaE, requires chromophore 3-hydroxyretinal. Neuron 10, 1113–1119. [DOI] [PubMed] [Google Scholar]
- Palczewska G, et al. , 2010. Noninvasive multiphoton fluorescence microscopy resolves retinol and retinal condensation products in mouse eyes. Nat Med 16, 1444–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski G, et al. , 2014. Evidence for compartmentalization of mammalian carotenoid metabolism. FASEB J 28, 4457–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski G, et al. , 2016. Genetic dissection in a mouse model reveals interactions between carotenoids and lipid metabolism. J Lipid Res 57, 1684–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, 2006. G protein-coupled receptor rhodopsin. Annual review of biochemistry 75, 743–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, et al. , 2000. Crystal structure of rhodopsin: A G protein-coupled receptor. Science 289, 739–745. [DOI] [PubMed] [Google Scholar]
- Palozza P, et al. , 2012. Effect of lycopene and tomato products on cholesterol metabolism. Annals of nutrition & metabolism 61, 126–134. [DOI] [PubMed] [Google Scholar]
- Pang XY, et al. , 2012. Structure/function relationships of adipose phospholipase A2 containing a cys-his-his catalytic triad. J Biol Chem 287, 35260–35274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R, et al. , 2011. Interphotoreceptor retinoid-binding protein as the physiologically relevant carrier of 11-cis-retinol in the cone visual cycle. J Neurosci 31, 4714–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasutto F, et al. , 2007. Mutations in STRA6 Cause a Broad Spectrum of Malformations Including Anophthalmia, Congenital Heart Defects, Diaphragmatic Hernia, Alveolar Capillary Dysplasia, Lung Hypoplasia, and Mental Retardation. Am J Hum Genet 80, 550–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, McNaughton PA, 1991. Response properties of cones from the retina of the tiger salamander. The Journal of physiology 433, 561–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkovich M, et al. , 1987. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature 330, 444–450. [DOI] [PubMed] [Google Scholar]
- Poliakov E, et al. , 2005. Key role of conserved histidines in recombinant mouse beta-carotene 15,15′-monooxygenase-1 activity. J Biol Chem 280, 29217–29223. [DOI] [PubMed] [Google Scholar]
- Poliakov E, et al. , 2017. Phylogenetic analysis of the metazoan carotenoid oxygenase superfamily: a new ancestral gene assemblage of BCO-like (BCOL) proteins. Scientific reports 7, 13192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss SE, et al. , 2007. Vitamin A requirements of alipochromatic (‘recessive-white’) and coloured canaries (Serinus canaria) during the breeding season. The Veterinary record 160, 14–19. [DOI] [PubMed] [Google Scholar]
- Quadro L, et al. , 1999. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. Embo J 18, 4633–4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadro L, et al. , 2005. Pathways of vitamin A delivery to the embryo: insights from a new tunable model of embryonic vitamin A deficiency. Endocrinology 146, 4479–4490. [DOI] [PubMed] [Google Scholar]
- Quazi F, et al. , 2012. ABCA4 is an N-retinylidene-phosphatidylethanolamine and phosphatidylethanolamine importer. Nature communications 3, 925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quazi F, Molday RS, 2014. ATP-binding cassette transporter ABCA4 and chemical isomerization protect photoreceptor cells from the toxic accumulation of excess 11-cis-retinal. Proc Natl Acad Sci U S A 111, 5024–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu RA, et al. , 2008. Retinal pigment epithelium-retinal G protein receptor-opsin mediates light-dependent translocation of all-trans-retinyl esters for synthesis of visual chromophore in retinal pigment epithelial cells. J Biol Chem 283, 19730–19738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu RA, et al. , 2004. Light exposure stimulates formation of A2E oxiranes in a mouse model of Stargardt’s macular degeneration. Proc Natl Acad Sci U S A 101, 5928–5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask L, Peterson PA, 1976. In vitro uptake of vitamin A from the retinol-binding plasma protein to mucosal epithelial cells from the monkey’s small intestine. J Biol Chem 251, 6360–6366. [PubMed] [Google Scholar]
- Rattner A, et al. , 2000. Identification and characterization of all-trans-retinol dehydrogenase from photoreceptor outer segments, the visual cycle enzyme that reduces all-trans-retinal to all-trans-retinol. J Biol Chem 275, 11034–11043. [DOI] [PubMed] [Google Scholar]
- Reboul E, et al. , 2005. Lutein transport by Caco-2 TC-7 cells occurs partly by a facilitated process involving the scavenger receptor class B type I (SR-BI). Biochem J 387, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboul E, Borel P, 2011. Proteins involved in uptake, intracellular transport and basolateral secretion of fat-soluble vitamins and carotenoids by mammalian enterocytes. Prog Lipid Res 50, 388–402. [DOI] [PubMed] [Google Scholar]
- Reboul E, et al. , 2006. Scavenger receptor class B type I (SR-BI) is involved in vitamin E transport across the enterocyte. J Biol Chem 281, 4739–4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond TM, et al. , 2001. Identification, expression, and substrate specificity of a mammalian beta-carotene 15,15′-dioxygenase. J Biol Chem 276, 6560–6565. [DOI] [PubMed] [Google Scholar]
- Redmond TM, et al. , 2005a. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proceedings of the National Academy of Sciences of the United States of America 102, 13658–13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond TM, et al. , 2005b. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proceedings of the National Academy of Sciences of the United States of America 102, 13658–13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond TM, et al. , 1985. Isolation and characterization of monkey interphotoreceptor retinoid-binding protein, a unique extracellular matrix component of the retina. Biochemistry 24, 787–793. [DOI] [PubMed] [Google Scholar]
- Redmond TM, et al. , 1998. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet 20, 344–351. [DOI] [PubMed] [Google Scholar]
- Redondo C, et al. , 2008. Identification of the retinol-binding protein (RBP) interaction site and functional state of RBPs for the membrane receptor. FASEB J 22, 1043–1054. [DOI] [PubMed] [Google Scholar]
- Rhinn M, Dolle P, 2012. Retinoic acid signalling during development. Development 139, 843–858. [DOI] [PubMed] [Google Scholar]
- Rhinn M, Dollé P, 2012. Retinoic acid signalling during development. Development (Cambridge, England) 139, 843–858. [DOI] [PubMed] [Google Scholar]
- Rodrigueza WV, et al. , 1999. Mechanism of scavenger receptor class B type I-mediated selective uptake of cholesteryl esters from high density lipoprotein to adrenal cells. J Biol Chem 274, 20344–20350. [DOI] [PubMed] [Google Scholar]
- Ruiz A, et al. , 2001. Genomic organization and mutation analysis of the gene encoding lecithin retinol acyltransferase in human retinal pigment epithelium. Invest Ophthalmol Vis Sci 42, 31–37. [PubMed] [Google Scholar]
- Ruiz A, et al. , 2012. Retinoid content, visual responses, and ocular morphology are compromised in the retinas of mice lacking the retinol-binding protein receptor, STRA6. Invest Ophthalmol Vis Sci 53, 3027–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, et al. , 1999. Molecular and biochemical characterization of lecithin retinol acyltransferase. J Biol Chem 274, 3834–3841. [DOI] [PubMed] [Google Scholar]
- Saari JC, Bredberg DL, 1987. Photochemistry and stereoselectivity of cellular retinaldehyde-binding protein from bovine retina. J Biol Chem 262, 7618–7622. [PubMed] [Google Scholar]
- Saari JC, Bredberg DL, 1988. CoA- and non-CoA-dependent retinol esterification in retinal pigment epithelium. J Biol Chem 263, 8084–8090. [PubMed] [Google Scholar]
- Sahu B, et al. , 2015. Conditional Ablation of Retinol Dehydrogenase 10 in the Retinal Pigmented Epithelium Causes Delayed Dark Adaption in Mice. J Biol Chem 290, 27239–27247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakudoh T, et al. , 2010. A CD36-related transmembrane protein is coordinated with an intracellular lipid-binding protein in selective carotenoid transport for cocoon coloration. J Biol Chem 285, 7739–7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakudoh T, et al. , 2007. Carotenoid silk coloration is controlled by a carotenoid-binding protein, a product of the Yellow blood gene. Proc Natl Acad Sci U S A 104, 8941–8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samardzija M, et al. , 2009. In conditions of limited chromophore supply rods entrap 11-cis-retinal leading to loss of cone function and cell death. Human molecular genetics 18, 1266–1275. [DOI] [PubMed] [Google Scholar]
- Sandell LL, et al. , 2007. RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev 21, 1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfare S, et al. , 2005. The Drosophila ninaG oxidoreductase acts in visual pigment chromophore production. J Biol Chem 280, 11895–11901. [DOI] [PubMed] [Google Scholar]
- Satoh AK, Ready DF, 2005. Arrestin1 mediates light-dependent rhodopsin endocytosis and cell survival. Curr Biol 15, 1722–1733. [DOI] [PubMed] [Google Scholar]
- Schledz M, et al. , 1996. Phytoene synthase from Narcissus pseudonarcissus: functional expression, galactolipid requirement, topological distribution in chromoplasts and induction during flowering. Plant J 10, 781–792. [DOI] [PubMed] [Google Scholar]
- Schmidt H, et al. , 2006. The carotenase AtCCD1 from Arabidopsis thaliana is a dioxygenase. J Biol Chem 281, 9845–9851. [DOI] [PubMed] [Google Scholar]
- Schnapf JL, et al. , 1990. Visual transduction in cones of the monkey Macaca fascicularis. The Journal of physiology 427, 681–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonthaler HB, et al. , 2008. The zebrafish mutant lbk/vam6 resembles human multisystemic disorders caused by aberrant trafficking of endosomal vesicles. Development 135, 387–399. [DOI] [PubMed] [Google Scholar]
- Schonthaler HB, et al. , 2007. Evidence for RPE65-independent vision in the cone-dominated zebrafish retina. Eur J Neurosci 26, 1940–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopf K, Huber A, 2017. Membrane protein trafficking in Drosophila photoreceptor cells. Eur J Cell Biol 96, 391–401. [DOI] [PubMed] [Google Scholar]
- Schwartz SH, et al. , 1997. Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276, 1872–1874. [DOI] [PubMed] [Google Scholar]
- Schwemer J, 1984. Renewal of visual pigment in photoreceptors of the blowfly. Journal of Comparative Physiologie, 535–547. [Google Scholar]
- Schwemer J, et al. , 1984. Light-induced trans-cis isomerization of retinal by a protein from honey bee retina. Journal of Comparative Physiologie A 154, 549–554. [Google Scholar]
- Sears AE, et al. , 2017. Towards Treatment of Stargardt Disease: Workshop Organized and Sponsored by the Foundation Fighting Blindness. Translational vision science & technology 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeliger MW, et al. , 1999. Phenotype in retinol deficiency due to a hereditary defect in retinol binding protein synthesis. Invest Ophthalmol Vis Sci 40, 3–11. [PubMed] [Google Scholar]
- Seeliger MW, et al. , 2001. New views on RPE65 deficiency: the rod system is the source of vision in a mouse model of Leber congenital amaurosis. Nat Genet 29, 70–74. [DOI] [PubMed] [Google Scholar]
- Seino Y, et al. , 2008. Isx participates in the maintenance of vitamin A metabolism by regulation of beta-carotene 15,15′-monooxygenase (Bcmo1) expression. J Biol Chem 283, 4905–4911. [DOI] [PubMed] [Google Scholar]
- Seki T, et al. , 1998. The metabolic pathway of visual pigment chromophore formation in Drosophila melanogaster--all-trans (3S)-3-hydroxyretinal is formed from all-trans retinal via (3R)-3-hydroxyretinal in the dark. Eur J Biochem 257, 522–527. [DOI] [PubMed] [Google Scholar]
- Seki T, Vogt K, 1998. Evolutionary aspects of the diversity of visual pigment chromophores in the class Insecta. Comp Biochem Physiol 119B, 53264. [Google Scholar]
- Shyam R, et al. , 2017a. RPE65 has an additional function as the lutein to meso-zeaxanthin isomerase in the vertebrate eye. Proc Natl Acad Sci U S A 114, 10882–10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyam R, et al. , 2017b. All three human scavenger receptor class B proteins can bind and transport all three macular xanthophyll carotenoids. Arch Biochem Biophys 634, 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvaroli JA, et al. , 2019. Abnormal Cannabidiol Modulates Vitamin A Metabolism by Acting as a Competitive Inhibitor of CRBP1. ACS Chem Biol 14, 434–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sineshchekov VA, et al. , 1972. Chlorophyll and carotenoid aggregates and energy migration in monolayers and thin films. Photochem Photobiol 15, 187–197. [DOI] [PubMed] [Google Scholar]
- Smith WC, Goldsmith TH, 1991. The role of retinal photoisomerase in the visual cycle of the honeybee. J Gen Physiol 97, 143–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow JR, Boulton M, 2005. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res 80, 595–606. [DOI] [PubMed] [Google Scholar]
- Sparrow JR, et al. , 2020. Lessons learned from quantitative fundus autofluorescence. Prog Retin Eye Res 74, 100774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn MB, et al. , 1976. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids). Fed Proc 35, 1332–1338. [PubMed] [Google Scholar]
- Steenbock H, 1919. White Corn Vs. Yellow Corn and a Probable Relation between the Fat-Soluble Vitamine and Yellow Plant Pigments. Science 50, 352–353. [DOI] [PubMed] [Google Scholar]
- Stephenson RS, et al. , 1983. Drosophila mutants with reduced rhodopsin content. Symp Soc Exp Biol 36, 477–501. [PubMed] [Google Scholar]
- Sui X, et al. , 2018. Preparation and characterization of metal-substituted carotenoid cleavage oxygenases. Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry 23, 887–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui X, et al. , 2015. Utilization of Dioxygen by Carotenoid Cleavage Oxygenases. J Biol Chem 290, 30212–30223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui X, et al. , 2014. Analysis of Carotenoid Isomerase Activity in a Prototypical Carotenoid Cleavage Enzyme, Apocarotenoid Oxygenase (ACO). J Biol Chem 289, 12286–12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui X, et al. , 2017. Structure and Spectroscopy of Alkene-Cleaving Dioxygenases Containing an Atypically Coordinated Non-Heme Iron Center. Biochemistry 56, 2836–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui X, et al. , 2016. Key Residues for Catalytic Function and Metal Coordination in a Carotenoid Cleavage Dioxygenase. J Biol Chem 291, 19401–19412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, et al. , 2005. Identification of conserved histidines and glutamic acid as key residues for isomerohydrolase activity of RPE65, an enzyme of the visual cycle in the retinal pigment epithelium. FEBS Lett 579, 5414–5418. [DOI] [PubMed] [Google Scholar]
- Tang PH, et al. , 2013. New insights into retinoid metabolism and cycling within the retina. Prog Retin Eye Res 32, 48–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SE, Harrison EH, 2016. Mechanisms of selective delivery of xanthophylls to retinal pigment epithelial cells by human lipoproteins. J Lipid Res 57, 1865–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DA, Gal A, 2003. Vitamin A metabolism in the retinal pigment epithelium: genes, mutations, and diseases. Prog Retin Eye Res 22, 683–703. [DOI] [PubMed] [Google Scholar]
- Thompson SJ, et al. , 2017. Hepatocytes Are the Principal Source of Circulating RBP4 in Mice. Diabetes 66, 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SY, 1975. Role of carotene and vitamin A in animal feeding. World review of nutrition and dietetics 21, 224–280. [DOI] [PubMed] [Google Scholar]
- Tian R, et al. , 2009. Genetic variation in the beta, beta-carotene-9′, 10′-dioxygenase gene and association with fat colour in bovine adipose tissue and milk. Anim Genet 41, 253–259. [DOI] [PubMed] [Google Scholar]
- Toomey MB, et al. , 2016. Complementary shifts in photoreceptor spectral tuning unlock the full adaptive potential of ultraviolet vision in birds. eLife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toomey MB, et al. , 2017. High-density lipoprotein receptor SCARB1 is required for carotenoid coloration in birds. Proc Natl Acad Sci U S A 114, 5219–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsybovsky Y, et al. , 2010. The ATP-binding cassette transporter ABCA4: structural and functional properties and role in retinal disease. Adv Exp Med Biol 703, 105–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsybovsky Y, et al. , 2013. Molecular organization and ATP-induced conformational changes of ABCA4, the photoreceptor-specific ABC transporter. Structure 21, 854–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vage DI, Boman IA, 2010. A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries). BMC genetics 11, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bennekum A, et al. , 2005. Class B scavenger receptor-mediated intestinal absorption of dietary beta-carotene and cholesterol. Biochemistry 44, 4517–4525. [DOI] [PubMed] [Google Scholar]
- Van Hooser JP, et al. , 2002. Recovery of visual functions in a mouse model of Leber congenital amaurosis. J Biol Chem 277, 19173–19182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet T, et al. , 1996. beta-Carotene absorption and cleavage in rats is affected by the vitamin A concentration of the diet. J Nutr 126, 499–508. [DOI] [PubMed] [Google Scholar]
- Vermot J, et al. , 2003. Decreased embryonic retinoic acid synthesis results in a DiGeorge syndrome phenotype in newborn mice. Proc Natl Acad Sci U S A 100, 1763–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villard L, Bates CJ, 1986. Carotene dioxygenase (EC 1.13.11.21) activity in rat intestine: effects of vitamin A deficiency and of pregnancy. The British journal of nutrition 56, 115–122. [DOI] [PubMed] [Google Scholar]
- Vogt K, 1983. Is the Fly Visual Pigment a Rhodpsin? Zeitschrift für Naturforschung 38c, 329–333. [Google Scholar]
- Voigt AP, et al. , 2019. Molecular characterization of foveal versus peripheral human retina by single-cell RNA sequencing. Exp Eye Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lintig J, 2012. Metabolism of carotenoids and retinoids related to vision. J Biol Chem 287, 1627–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lintig J, et al. , 2001. Analysis of the blind Drosophila mutant ninaB identifies the gene encoding the key enzyme for vitamin A formation invivo. Proc Natl Acad Sci 98, 1130–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lintig J, et al. , 2010. The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends Biochem Sci 35, 400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lintig J, Vogt K, 2000. Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal. J Biol Chem 275, 11915–11920. [DOI] [PubMed] [Google Scholar]
- von Lintig J, et al. , 1997. Light-dependent regulation of carotenoid biosynthesis occurs at the level of phytoene synthase expression and is mediated by phytochrome in Sinapis alba and Arabidopsis thaliana seedlings. Plant J 12, 625–634. [DOI] [PubMed] [Google Scholar]
- Voolstra O, et al. , 2006. The Drosophila class B scavenger receptor NinaD-I is a cell surface receptor mediating carotenoid transport for visual chromophore synthesis. Biochemistry 45, 13429–13437. [DOI] [PubMed] [Google Scholar]
- Voolstra O, et al. , 2010. NinaB is essential for Drosophila vision but induces retinal degeneration in opsin-deficient photoreceptors. J Biol Chem 285, 2130–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald G, 1968. The molecular basis of visual excitation. Nature 219, 800–807. [DOI] [PubMed] [Google Scholar]
- Wang JS, Kefalov VJ, 2009. An alternative pathway mediates the mouse and human cone visual cycle. Curr Biol 19, 1665–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JY, et al. , 2019. The apocarotenoid metabolite zaxinone regulates growth and strigolactone biosynthesis in rice. Nature communications 10, 810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, et al. , 2008. Associations of plasma carotenoids with risk factors and biomarkers related to cardiovascular disease in middle-aged and older women. Am J Clin Nutr 88, 747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, et al. , 2007. Dissection of the pathway required for generation of vitamin A and for Drosophila phototransduction. J Cell Biol 177, 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Montell C, 2005. Rhodopsin formation in Drosophila is dependent on the PINTA retinoid-binding protein. J Neurosci 25, 5187–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Montell C, 2007. Phototransduction and retinal degeneration in Drosophila. Pflugers Arch 454, 821–847. [DOI] [PubMed] [Google Scholar]
- Wang X, et al. , 2010. Requirement for an enzymatic visual cycle in Drosophila. Curr Biol 20, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. , 2012. The Drosophila visual cycle and de novo chromophore synthesis depends on rdhB. J Neurosci 32, 3485–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XD, Russell RM, 1999. Procarcinogenic and anticarcinogenic effects of beta-carotene. Nutr Rev 57, 263–272. [DOI] [PubMed] [Google Scholar]
- Ward R, et al. , 2020. Non-photopic and photopic visual cycles differentially regulate immediate, early and late-phases of cone photoreceptor-mediated vision. J Biol Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassef L, Quadro L, 2011. Uptake of dietary retinoids at the maternal-fetal barrier: in vivo evidence for the role of lipoprotein lipase and alternative pathways. J Biol Chem 286, 32198–32207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch R, et al. , 2000. Regulation and activation of phytoene synthase, a key enzyme in carotenoid biosynthesis, during photomorphogenesis. Planta 211, 846–854. [DOI] [PubMed] [Google Scholar]
- Welsch R, et al. , 2003. Structural and functional characterization of the phytoene synthase promoter from Arabidopsis thaliana. Planta 216, 523–534. [DOI] [PubMed] [Google Scholar]
- Wenzel A, et al. , 2005a. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res 24, 275–306. [DOI] [PubMed] [Google Scholar]
- Wenzel A, et al. , 2005b. The retinal G protein-coupled receptor (RGR) enhances isomerohydrolase activity independent of light. J Biol Chem 280, 29874–29884. [DOI] [PubMed] [Google Scholar]
- Wenzel A, et al. , 2007. RPE65 is essential for the function of cone photoreceptors in NRL-deficient mice. Invest Ophthalmol Vis Sci 48, 534–542. [DOI] [PubMed] [Google Scholar]
- Widjaja-Adhi MA, et al. , 2015. A genetic dissection of intestinal fat-soluble vitamin and carotenoid absorption. Human molecular genetics 24, 3206–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widjaja-Adhi MAK, Golczak M, 2019. The molecular aspects of absorption and metabolism of carotenoids and retinoids in vertebrates. Biochim Biophys Acta Mol Cell Biol Lipids, 158571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widjaja-Adhi MAK, et al. , 2017. Transcription factor ISX mediates the cross talk between diet and immunity. Proc Natl Acad Sci U S A 114, 11530–11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widjaja-Adhi MAK, et al. , 2018. Protective role of carotenoids in the visual cycle. FASEB J, fj201800467R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TP, 1964. Photoreversal of Rhodopsin Bleaching. J Gen Physiol 47, 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbach S, Howe PR, 1925. Tissue changes following deprivation of fat-soluble A vitamin. J. Exp. Med 42, 753–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf G, 2001. The discovery of the visual function of vitamin A. J Nutr 131, 1647–1650. [DOI] [PubMed] [Google Scholar]
- Wongsiriroj N, et al. , 2008. The molecular basis of retinoid absorption: a genetic dissection. J Biol Chem 283, 13510–13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff ML, et al. , 2003. Spontaneous activity of opsin apoprotein is a cause of Leber congenital amaurosis. Nat Genet 35, 158–164. [DOI] [PubMed] [Google Scholar]
- Wu X, et al. , 2012. Cyanobacteria blooms produce teratogenic retinoic acids. Proc Natl Acad Sci U S A 109, 9477–9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss A, et al. , 2000. Cloning and expression of beta,beta-carotene 15,15′-dioxygenase. Biochem Biophys Res Commun 271, 334–336. [DOI] [PubMed] [Google Scholar]
- Xu H, et al. , 2004. A lysosomal tetraspanin associated with retinal degeneration identified via a genome-wide screen. Embo J 23, 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, et al. , 2017. The role of retinol dehydrogenase 10 in the cone visual cycle. Scientific reports 7, 2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, et al. , 2015. CRALBP supports the mammalian retinal visual cycle and cone vision. J Clin Invest 125, 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuzaki J, 2017. Carotenoids Database: structures, chemical fingerprints and distribution among organisms. Database : the journal of biological databases and curation 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahyavi M, et al. , 2013. ALDH1A3 loss of function causes bilateral anophthalmia/microphthalmia and hypoplasia of the optic nerve and optic chiasm. Human molecular genetics 22, 3250–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, et al. , 2001. Cloning and characterization of a human beta,beta-carotene-15,15′-dioxygenase that is highly expressed in the retinal pigment epithelium. Genomics 72, 193–202. [DOI] [PubMed] [Google Scholar]
- Yang M, Fong HK, 2002. Synthesis of the all-trans-retinal chromophore of retinal G protein-coupled receptor opsin in cultured pigment epithelial cells. J Biol Chem 277, 3318–3324. [DOI] [PubMed] [Google Scholar]
- Yu M, et al. , 2012. Contributions of a disulfide bond and a reduced cysteine side chain to the intrinsic activity of the high-density lipoprotein receptor SR-BI. Biochemistry 51, 10044–10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin AM, 1931. The Presence of Vitamin A in the Retina. Transactions of the American Ophthalmological Society 29, 263–272. [PMC free article] [PubMed] [Google Scholar]
- Zhang H, et al. , 2008. Trafficking of membrane-associated proteins to cone photoreceptor outer segments requires the chromophore 11-cis-retinal. J Neurosci 28, 4008–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, et al. , 2019. Photic generation of 11-cis-retinal in bovine retinal pigment epithelium. J Biol Chem 294, 19137–19154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, et al. , 2007. Altered vitamin A homeostasis and increased size and adiposity in the rdh1-null mouse. Faseb J 21, 2886–2896. [DOI] [PubMed] [Google Scholar]
- Zhong G, et al. , 2019. The retinoic acid hydroxylase Cyp26a1 has minor effects on postnatal vitamin A homeostasis, but is required for exogenous atRA clearance. J Biol Chem 294, 11166–11179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, et al. , 2006. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc Natl Acad Sci 103, 16182–16187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziouzenkova O, et al. , 2007. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat Med 13, 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolfaghari R, et al. , 2012. DHRS3, a retinal reductase, is differentially regulated by retinoic acid and lipopolysaccharide-induced inflammation in THP-1 cells and rat liver. Am J Physiol Gastrointest Liver Physiol 303, G578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]


