Abstract
Background
Efforts to minimize harms from opioid drug interactions may be hampered by limited evidence on which drugs, when taken concomitantly with opioids, result in adverse clinical outcomes.
Objective
To identify signals of opioid drug interactions by identifying concomitant medications (precipitant drugs) taken with individual opioids (object drugs) that are associated with unintentional traumatic injury
Design
We conducted pharmacoepidemiologic screening of Optum Clinformatics Data Mart, identifying drug interaction signals by performing confounder-adjusted self-controlled case series studies for opioid + precipitant pairs and injury.
Setting
Beneficiaries of a major United States-based commercial health insurer during 2000–2015
Patients
Persons aged 16–90 years co-dispensed an opioid and ≥1 precipitant drug(s), with an unintentional traumatic injury event during opioid therapy, as dictated by the case-only design
Measurements
Exposure: Precipitant-exposed (vs. precipitant-unexposed) person-days during opioid therapy. Outcome: Emergency department or inpatient International Classification of Diseases discharge diagnosis for unintentional traumatic injury. We used conditional Poisson regression to generate confounder adjusted rate ratios. We accounted for multiple estimation via semi-Bayes shrinkage.
Results
We identified 25,019, 12,650, and 10,826 new users of hydrocodone, tramadol, and oxycodone who experienced an unintentional traumatic injury. Among 464, 376, and 389 hydrocodone-, tramadol-, and oxycodone-precipitant pairs examined, 20, 17, and 16 (i.e., 53 pairs, 34 unique precipitants) were positively associated with unintentional traumatic injury and deemed potential drug interaction signals. Adjusted rate ratios ranged from 1.23 (95% confidence interval: 1.05–1.44) for hydrocodone + amoxicillin−clavulanate to 4.21 (1.88–9.42) for oxycodone + telmisartan. Twenty (37.7%) of 53 signals are currently reported in a major drug interaction knowledgebase.
Limitations
Potential for reverse causation, confounding by indication, and chance
Conclusions
We identified previously undescribed and/or unappreciated signals of opioid drug interactions associated with unintentional traumatic injury. Subsequent etiologic studies should confirm (or refute) and elucidate these potential drug interactions.
Registration
None
Keywords: Drug interactions, injury, opioid analgesics, pharmacoepidemiology, population health, self-controlled case series
Graphical Abstract
1. INTRODUCTION
Opioids, given their central nervous system (CNS)-depressant effects, have been consistently associated with numerous types of unintentional traumatic injury, a major cause of morbidity, disability, and death.[1,2] Unintentional traumatic injury is the leading cause of death in persons <45 years of age and the fourth leading cause of death among persons of all ages.[3] Among older adults, falls and motor vehicle crashes predominate,[4] leading to dramatic increases in mortality from injury beginning at 70 years of age.[3] Drug interactions are believed to be a major contributor to opioid-attributed unintentional traumatic injury via pharmacodynamic[5] and/or pharmacokinetic mechanisms.[6] Drug interactions are responsible for a substantial proportion of adverse drug events (ADEs), including those resulting in hospitalization and death[7,8] and are potentially preventable.[6] Therefore, it is unsurprising that opioids (and their drug interactions) are a high priority target for minimizing significant patient harms due to ADEs.[9] In fact, the National Academies of Sciences, Engineering, and Medicine and the United States (US) Department of Health and Human Services have endorsed the importance of studying opioid drug interactions to improve patient safety[10] and combat the opioid epidemic.[11]
Prior population-based studies of opioid drug interactions have been largely limited to overdose and/or injurious sequelae of concomitant use with alcohol,[12] benzodiazepines,[13] gabapentinoids,[14] and/or skeletal muscle relaxants.[15] While the initial focus on these CNS depressants is intuitive, this leaves a substantial knowledge gap in potential opioid ADEs when co-prescribed with hundreds of other commonly used medications. The lack of evidence is concerning since polypharmacy is common among persons treated with opioids,[16] with approximately one in four noncancer pain patients exposed to a potential opioid drug interaction.[17,18]
Given the critical need to identify potential opioid drug interactions, we conducted high-throughput pharmacoepidemiologic screening of administrative healthcare data to identify signals of potential clinically important interactions with opioids that increased rates of unintentional traumatic injuries. Our objective was to provide researchers with an evidence-based list of signals, such that limited available resources could be directed to confirm (or refute) and elucidate these potential interactions in future etiologic studies.
2. MATERIAL AND METHODS
2.1. Overview: Identifying opioid drug interaction signals via pharmacoepidemiologic screening
We conducted semi-automated, high-throughput pharmacoepidemiologic screening of US commercial health insurance data to identify signals of opioid drug interactions. We first identified candidate interacting precipitants, which we operationalized as orally administered drugs frequently co-dispensed with opioids. We subsequently identified drug interaction signals by performing hundreds of confounder-adjusted self-controlled case series studies to examine associations between individual opioids + candidate precipitants and unintentional traumatic injury (the primary outcome), typical hip fracture (a secondary outcome), and motor vehicle crash while the subject was driving (a secondary outcome).
For each opioid-precipitant pair, we conducted a bi-directional self-controlled case series study to examine the rate of each outcome (see §2.8) in an opioid-treated individual during time exposed vs. unexposed to the precipitant. Although the “case series” phrase within self-controlled case series may seem to imply the absence of a comparator, the approach is a rigorous controlled self-matched epidemiologic study design; it is the cohort analogue of the case-crossover design.[19] The self-controlled case series design is ideal for drug interaction screening because: a) the causal contrast is made within an individual and thus inherently controls for confounding by measured and unmeasured factors that remain constant within an individual over the observation period (e.g., sex, genetics, chronic diseases, frailty, socioeconomic status); b) the underlying statistical model can accommodate and control for time-varying factors;[20]; c) the approach is highly computationally-efficient,[21] since it includes only persons experiencing an outcome; and d) there is precedent for the use of high-throughput applications. Analogous pharmacoepidemiologic screening studies have identified drugs associated with hypoglycemia in users of insulin secretagogues,[22] rhabdomyolysis in users of statins,[23] and serious bleeding in users of clopidogrel[24] and anticoagulants[23,25,26] as examples.
2.2. Data source
We utilized Optum Clinformatics Data Mart (OptumInsight: Eden Prairie, MN, US)[27] administrative data from May 1, 2000 through September 30, 2015. Optum includes longitudinal enrollment and healthcare billing data from >71 million commercially insured and Medicare Advantage beneficiaries of the largest US-based private health insurer by market share.[28] See additional detail in Supplemental Methods.
2.3. Identifying candidate interacting precipitant drugs during opioid drug use
We used pharmacy claim dates and days’ supply values to identify prescription dispensings for the following opioids as object drugs of interest: codeine (including as an ingredient in antitussives); fentanyl; hydrocodone (including as an ingredient in antitussives); hydromorphone; levorphanol; meperidine; methadone; morphine; oxycodone; oxymorphone; tapentadol; and tramadol. We limited dispensings to the most commonly used route of administration per opioid (e.g., oral hydrocodone, transdermal fentanyl). During periods of apparent opioid use, we used pharmacy claim dates and days’ supply values to identify prescription dispensings for any orally administered concomitant medication (precipitant drugs of interest). We linked pharmacy claims to the Lexicon Plus Drug Database (Cerner Multum: Denver, CO, US) to categorize precipitants by medication class.
2.4. Creating study cohorts of new users of opioids
We constructed separate study cohorts for new users of each opioid object drug aged 16–90 years. We achieved this by requiring a baseline period (see §2.5) devoid of a dispensing for the given object opioid. We then utilized pharmacy claim dates and days’ supply values to build object drug exposure episodes consisting of ≥1 dispensing of the object opioid. We permitted a grace period—length calculated as days’ supply x 0.20, assuming 80% adherence—between contiguous opioid dispensings and at the end of the terminal dispensing.
2.5. Defining observation and baseline periods
For each new user meeting inclusion criteria, we began the observation period upon object drug initiation and censored it upon the earliest of: a) lapsed exposure to the object (permitting the grace period); b) a switch from the object to a pharmacologic alternative (i.e., non-object opioid or opioid agonist-antagonist); c) health plan disenrollment (permitting a maximum gap in enrollment of 45 days); or d) the end of the study dataset. Since the self-controlled case series design is a case-only approach, we required new users to experience an outcome (see §2.8) during their observation period. We did not censor upon outcome occurrence since this would violate an underlying assumption of the self-controlled case series design.[21,29]
We defined the baseline period as the 183 days immediately preceding yet excluding the observation period begin date. We required the baseline period to be devoid of an interruption in health plan coverage and a dispensing for the object drug under study. Regarding the latter, we did not exclude from study object episodes preceded by a baseline dispensing for a pharmacologic alternative; this permitted us to study second- and later-line opioid therapies. For example, we excluded from the hydrocodone cohort persons with a baseline hydrocodone dispensing, but not persons with a baseline tramadol dispensing. We further required the 30 days immediately preceding and including the observation period begin date to be event-free; this served to minimize reverse causation since opioids are used to treat injury-induced pain.
2.6. Categorizing observation period time based on precipitant drug exposure
We classified each observation period day as either precipitant-exposed or precipitant-unexposed. Precipitant-exposed days were defined by concomitant exposure to the candidate interacting precipitant drug. Precipitant-unexposed days were all other observation period days. To minimize exposure trend bias,[19] we included precipitant-unexposed days before and after precipitant-exposed days. Figure 1 provides a graphical representation of the design.
Figure 1. Example of object drug exposure episode eligible for inclusion.
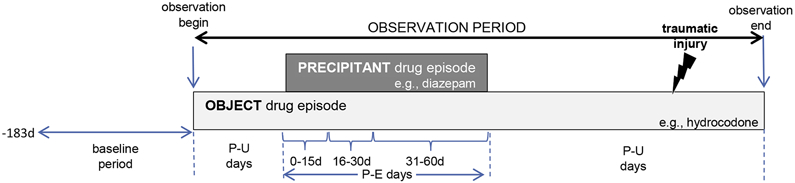
The presence of precipitant-unexposed person-days before and after precipitant-exposed person-days is indicative of a bi-directional implementation of the self-controlled case series design. P-E = precipitant-exposed; P-U = precipitant-unexposed.
Several studies have shown that the risk of an adverse event due to a drug interaction often peaks shortly after initiating concomitant therapy and declines thereafter.[30-32] We therefore examined a duration-response relationship for each object + precipitant pair by stratifying precipitant-exposed observation time into the following risk windows: 0–15, 16–30, 31–60, 61–120, and 121–180 days from initiation of concomitance.
2.7. Defining the exposure of interest and covariates
The exposure of interest was use of the candidate interacting precipitant drug. The self-controlled case series design implicitly controls for time-invariant (e.g., sex, genetic polymorphisms), but not time-varying, covariates.[29] We therefore included in each regression model the following time-varying covariates assessed during each day of observation time: a) opioid daily dose, in morphine milligram equivalents (see Supplemental Methods);[33] and b) prior traumatic injury of interest in any diagnostic position on any claim type (e.g., a prior secondary-position ambulatory care hip fracture diagnosis in an analysis of the hip fracture endpoint). The latter covariate is relevant because prior injury may predict subsequent injury,[34] and the self-controlled case series design does not censor observation time upon event occurrence.
2.8. Identifying outcomes
The primary outcome was unintentional traumatic injury, defined as an emergency department or inpatient hospitalization for fracture, dislocation, sprain/strain, intracranial injury, internal injury of thorax, abdomen, or pelvis, open wound, injury to blood vessels, crushing injury, injury to nerves or spinal cord, or certain traumatic complications and unspecified injuries. Consistent with the American College of Surgeons’ National Trauma Data Standard,[35] our definition excluded: a) late effects of injuries, poisonings, toxic effects, and other external causes; b) superficial injury; c) contusion with intact skin surface; and d) effects of a foreign body entering through orifice. Consistent with work by Sears et al,[36] our definition also excluded burns; such injuries are unlikely due to opioid use.
A secondary outcome was an inpatient hospitalization for typical hip fracture. We excluded: a) pathologic hip fractures, since these events are due to a localized process such as malignancy or infection;[37] and b) atypical hip fractures, since these events are infrequently traumatic and often attributed to bisphosphonate and/or glucocorticoid use.[38] Another secondary outcome was motor vehicle crash while the subject was driving, defined as an unintentional traumatic injury (see primary outcome above) plus an external cause of injury code for an unintentional traffic or nontraffic accident. We excluded crashes of a self-inflicted, assault, or undetermined manner, consistent with the Centers for Disease Control and Prevention’s injury mortality framework.[39] We provide operational outcome definitions, their performance characteristics, and other support for their use in Supplemental Table 1.
2.9. Statistical analysis
For each object-outcome pair, we constructed an analytic file in which the unit of observation was the person-day covered by an active prescription for an opioid. The binary dependent variable was whether the unintentional traumatic injury occurred on that day. Independent variables included a unique subject identifier, whether a given person-day was precipitant-exposed vs. precipitant-unexposed, and the time-varying covariates listed in §2.7. The parameter of interest was the outcome occurrence rate ratio during precipitant-exposed vs. precipitant-unexposed days, i.e., rateobject+precipitant / rateobject. In a secondary analysis, we separately examined rate ratios for the five mutually exclusive risk windows discussed in §2.6. We used conditional Poisson regression models (xtpoisson with fe option, Stata v.16: College Station, TX, US) to estimate rate ratios and 95% confidence intervals (CIs).[21,29,40] To avoid statistically unstable estimates, we did not estimate rate ratios when there were: a) <5 precipitant-exposed persons; or b) no events during precipitant-exposed time. Further, we do not report rate ratios from non-converged conditional Poisson regression models or if the variance of the beta estimate for the parameter of interest was >10.
To address multiple estimation inherent in calculating numerous rate ratios, we used a semi-Bayes shrinkage method. This approach increases the validity of effect estimates and preserves nominal type-1 error.[41,42] See additional detail in Supplemental Methods.
To contextualize findings, we compared drug interaction signals generated by our semi-automated approach to putative interactions documented in two drug interaction knowledgebases: Micromedex (IBM Watson Health: Cambridge, MA, US) and Clinical Drug Information (Wolters Kluwer: Alphen aan den Rijn, South Holland, Netherlands).
2.10. Institutional review board approval and role of funding source
The University of Pennsylvania’s institutional review board approved this research as protocol #831486. The US National Institutes of Health had no input on the conduct or interpretation of this research.
3. RESULTS
Table 1 summarizes characteristics of persons constituting object drug cohorts for analyses of unintentional traumatic injury. For the three most commonly used opioids, these cohorts consisted of 25019, 12650, and 10826 new users of hydrocodone, tramadol, and oxycodone respectively, all of whom by design experienced an outcome; the three most commonly occurring injuries were sprain/strain (39.9%), certain traumatic complications and unspecified injuries (27.1%), and fracture (25.7%). Median durations of observation for hydrocodone, tramadol, and oxycodone were 12, 13, and 19 days, respectively. The plurality of hydrocodone (40.6%), tramadol (47.3%), and oxycodone (40.3%) users were Caucasian adult females. Few hydrocodone (18.1%), tramadol (17.3%), and oxycodone (15.0%) users had multiple unintentional traumatic injuries during observation time. In analyses of secondary outcomes, cohorts consisted of 1142, 848, and 461, and 246, 98, and 113 users for typical hip fracture and motor vehicle crash, respectively. Supplemental Table 2 and Supplemental Table 3 summarize characteristics of persons constituting object drug cohorts for analyses of these outcomes.
Table 1.
Descriptors of object drug cohorts examining unintentional traumatic injury
| Object drug cohort | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Codeine | Fentanyl | Hydrocod. | Hydromor. | Levorph. | Meperidine | Methadone | Morphine | Oxycodone | Oxymorph. | Tapentadol | Tramadol | ||
| Persons | 6,065 | 1,435 | 25,019 | 828 | 2 | 248 | 439 | 1,612 | 10,826 | 224 | 163 | 12,650 | |
| Days of observation, median (Q1-Q3) per episode | 10.0(6.0-19.0) | 33.0(16.0-57.0) | 12.0(7.0-27.0) | 13.0(7.0-31.0) | 26.5(19.0-34.0) | 9.0(5.0-13.0) | 50.0(24.0-145.0 | 35.0(16.0-72.0) | 13.0(7.0-37.0) | 38.5(22.0-152.5) | 24.5(10.5-37.0) | 19.0(10.0-37.0) | |
| Days of observation, sum | 130,951 | 95,350 | 1,066,507 | 32,251 | 53 | 7,656 | 52,699 | 152,303 | 641,103 | 28,640 | 10,075 | 574,007 | |
| Unintentional traumatic injuries, sum | 7,790 | 1,894 | 33,567 | 1,023 | 2 | 293 | 644 | 2,082 | 14,071 | 299 | 284 | 17,385 | |
| Demographics | |||||||||||||
| Age in years, median (Q1-Q3) | 54.5(40.5-72.6) | 73.8(60.0-80.2) | 59.2(44.2-75.4) | 60.7(50.0-72.7) | 75.0(71.9-78.1) | 52.2(40.4-62.8) | 59.2(48.4-70.6) | 67.1(55.0-77.7) | 60.2(46.8-73.6) | 58.2(50.5-67.6) | 57.2(48.7-69.1) | 71.1(55.2-80.9) | |
| Sex, sum (%) female | 3,801(62.7) | 987(68.8) | 14,48(57.9) | 512(61.8) | 1(50.0) | 165(66.5) | 244(55.6) | 969(60.1) | 6,199(57.3) | 129(57.6) | 108(66.3) | 8,535(67.5) | |
| Race, sum (%) | Caucasian | 4,159(68.6) | 1,081(75.3) | 17,707(70.8) | 619(74.8) | 2(100.0) | 172(69.4) | 308(70.2) | 1,242(77.0) | 7,722(71.3) | 168(75.0) | 128(78.5) | 8,938(70.7) |
| African American | 471(7.8) | 110(7.7) | 2,206(8.8) | 69(8.3) | 0(0.0) | 21(8.5) | 43(9.8) | 122(7.6) | 1,047(9.7) | 27(12.1) | 12(7.4) | 1,439(11.4) | |
| Hispanic | 536(8.8) | 84(5.9) | 1,901(7.6) | 49(5.9) | 0(0.0) | 10(4.0) | 26(5.9) | 90(5.6) | 691(6.4) | 7(3.1) | 8(4.9) | 1,098(8.7) | |
| Asian | 172(2.8) | 16(1.1) | 381(1.5) | 13(1.6) | 0(0.0) | 1(0.4) | 8(1.8) | 21(1.3) | 145(1.3) | 1(0.4) | 3(1.8) | 182(1.4) | |
| Unknown | 727(12.0) | 144(10.0) | 2,824(11.3) | 78(9.4) | 0(0.0) | 44(17.7) | 54(12.3) | 13(8.5) | 1,221(11.3) | 21(9.4) | 12(7.4) | 993(7.8) | |
| Geographic division, sum (%) | East North Central | 320(5.3) | 80(5.6) | 820(3.3) | 48(5.8) | 0(0.0) | 1(0.4) | 19(4.3) | 85(5.3) | 533(4.9) | 8(3.6) | 2(1.2) | 568(4.5) |
| East South Central | 328(5.4) | 80(5.6) | 844(3.4) | 54(6.5) | 1(50.0) | 3(1.2) | 23(5.2) | 81(5.0) | 734(6.8) | 13(5.8) | 16(9.8) | 646(5.1) | |
| Middle Atlantic | 1,138(18.8) | 255(17.8) | 4,049(16.2) | 82(9.9) | 1(50.0) | 13(5.2) | 48(10.9) | 201(12.5) | 1,503(13.9) | 31(13.8) | 19(11.7) | 1,974(15.6) | |
| Mountain | 759(12.5) | 165(11.5) | 2,183(8.7) | 56(6.8) | 0(0.0) | 13(5.2) | 36(8.2) | 122(7.6) | 879(8.1) | 9(4.0) | 6(3.7) | 1,098(8.7) | |
| New England | 1,111(18.3) | 305(21.3) | 5,660(22.6) | 265(32.0) | 0(0.0) | 104(41.9) | 112(25.5) | 413(25.6) | 3,377(31.2) | 85(37.9) | 81(49.7) | 3,489(27.6) | |
| Pacific | 183(3.0) | 88(6.1) | 1,336(5.3) | 22(2.7) | 0(0.0) | 36(14.5) | 46(10.5) | 97(6.0) | 540(5.0) | 22(9.8) | 6(3.7) | 613(4.8) | |
| South Atlantic | 606(10.0) | 151(10.5) | 3,713(14.8) | 51(6.2) | 0(0.0) | 44(17.7) | 44(10.0) | 146(9.1) | 507(4.7) | 20(8.9) | 10(6.1) | 1,783(14.1) | |
| West North Central | 595(9.8) | 141(9.8) | 2,684(10.7) | 128(15.5) | 0(0.0) | 26(10.5) | 49(11.2) | 245(15.2) | 1,563(14.4) | 28(12.5) | 12(7.4) | 1,341(10.6) | |
| West South Central | 999(16.5) | 167(11.6) | 3,569(14.3) | 120(14.5) | 0(0.0) | 8(3.2) | 58(13.2) | 214(13.3) | 1,137(10.5) | 6(2.7) | 11(6.7) | 1,086(8.6) | |
| Unknown | 26(0.4) | 3(0.2) | 161(0.6) | 2(0.2) | 0(0.0) | 0(0.0) | 4(0.9) | 8(0.5) | 53(0.5) | 2(0.9) | 0(0.0) | 52(0.4) | |
| Time-varying covariates | |||||||||||||
| Object drug average daily dose (Q1-Q3), median, in MME | 9.0(4.5-18.0) | 60.0(60.0-120) | 22.5(15.0-32.1) | 64.0(48.0-96.0) | 65.2(44.0-65.2) | 20.0(10.0-30.0) | 240(80.0-600) | 60.0(43.5-120) | 60.0(30.0-90.0) | 120(60.0-180) | 80.0(60.0-120) | 15.0(10.0-20.0) | |
| Unintentional traumatic injury, ever prior,* person-days (%) | 93,889(71.7) | 75,649(79.3) | 774,045(72.6) | 26,384(81.8) | 53(100.0) | 3,759(49.1) | 41,364(78.5) | 119,191(78.3) | 485,679(75.8) | 23,681(82.7) | 8,957(88.9) | 425,268(74.1) | |
hydrocod. = hydrocodone; hydromor. = hydromorphone; levorph. = levorphanol; MME = morphine milligram equivalents; oxymorph. = oxymorphone; Q = quartile
diagnosis (any position, any claim type) ever prior to the day of observation
We identified 775, 656, and 731 candidate interacting precipitant drugs co-prescribed with hydrocodone, tramadol, and oxycodone respectively. After application of inclusion criteria, we examined 464 (59.9%), 376 (57.3%), and 389 (53.2%) precipitants in confounder-adjusted self-controlled case series studies of unintentional traumatic injury. Table 2 provides summary data on rate ratios for unintentional traumatic injury, before and after confounder adjustment; Supplemental Table 4 and Supplemental Table 5 provide summary data for typical hip fracture and motor vehicle crash, respectively. The heat map in Supplemental Figure 1 graphically depicts semi-Bayes shrunk confounder-adjusted rate ratios for all three outcomes; corresponding secondary analyses using an alternate variance parameter for semi-Bayes shrinkage yielded similar findings (Supplemental Figure 2). We present secondary findings that stratified precipitant-exposed observation time into risk windows in Supplemental Figure 3.
Table 2.
Summary data on semi-Bayes shrunk rate ratios for unintentional traumatic injury, by object drug cohort
| Object drug cohort | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Codeine | Fentanyl | Hydrocod. | Hydromor. | Levorph. | Meperidine | Methadone | Morphine | Oxycodone | Oxymorph. | Tapentadol | Tramadol | |||
| Unadjusted analyses | ||||||||||||||
| Candidate interacting precipitants examined, sum | 306 | 240 | 464 | 157 | Event count precluded examination | 61 | 142 | 244 | 389 | 75 | 41 | 376 | ||
| RR | DDI signals, sum (%) | 20(6.5) | 12(5.0) | 61(13.1) | 2(1.3) | 0(0.0) | 3(2.1) | 19(7.8) | 41(10.5) | 0(0.0) | 0(0.0) | 33(8.8) | ||
| Increased rate* | 8(2.6) | 1(0.4) | 17(3.7) | 1(0.6) | 0(0.0) | 0(0.0) | 11(4.5) | 17(4.4) | 0(0.0) | 0(0.0) | 15(4.0) | |||
| Decreased rate** | 12(3.9) | 11(4.6) | 44(9.5) | 1(0.6) | 0(0.0) | 3(2.1) | 8(3.3) | 24(6.2) | 0(0.0) | 0(0.0) | 18(4.8) | |||
| Range, min to max | 0.46 -2.66 | 0.42 -1.89 | 0.35 -1.73 | 0.43 -1.79 | 0.56 -1.30 | 0.40 -1.68 | 0.46 -2.24 | 0.37 -3.61 | 0.55 -2.45 | 0.83 -2.46 | 0.38 -2.60 | |||
| Confounder-adjusted analyses | ||||||||||||||
| Candidate interacting precipitants examined, sum | 305 | 240 | 464 | 157 | Event count precluded examination | 60 | 142 | 244 | 389 | 75 | 40 | 376 | ||
| RR | DDI signals, sum (%) | 25(8.2) | 16(6.7) | 67(14.4) | 2(1.3) | 0(0.0) | 3(2.1) | 25(10.2) | 41(10.5) | 0(0.0) | 0(0.0) | 37(9.8) | ||
| Increased rate* | 11(3.6) | 1(0.4) | 20(4.3) | 1(0.6) | 0(0.0) | 0(0.0) | 11(4.5) | 16(4.1) | 0(0.0) | 0(0.0) | 17(4.5) | |||
| Decreased rate** | 14(4.6) | 15(6.3) | 47(10.1) | 1(0.6) | 0(0.0) | 3(2.1) | 14(5.7) | 25(6.4) | 0(0.0) | 0(0.0) | 20(5.3) | |||
| Range, min to max | 0.49 -3.16 | 0.40 -1.95 | 0.31 -2.12 | 0.41 -1.87 | 0.52 -1.22 | 0.35 -1.78 | 0.44 -2.70 | 0.33 -4.21 | 0.50 -2.45 | 0.83 -2.59 | 0.38 -2.63 | |||
DDI = drug-drug interaction; max = maximum; min = minimum; RR = rate ratio
lower bound of the 95% confidence interval for the RR of interest excluded the null value
upper bound of the 95% confidence interval for the RR of interest excluded the null value
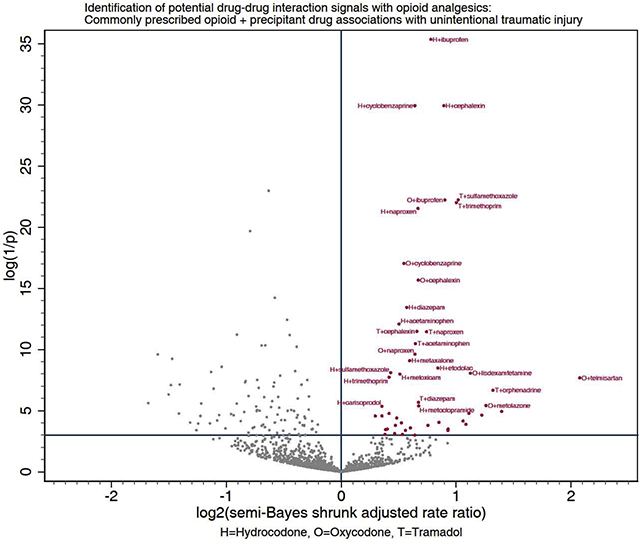
Among included precipitants co-prescribed with hydrocodone, tramadol, and oxycodone, 20 (4.3%), 17 (4.5%), and 16 (4.1%) respectively had statistically significantly elevated adjusted rate ratios for unintentional traumatic injury after semi-Bayes shrinkage. We therefore deemed these 53 opioid + precipitant pairs (34 unique precipitants) as potential drug interaction signals (Table 3 and Figure 2). Putative interacting precipitants were CNS agents (N = 21, including six nonsteroidal anti-inflammatory drugs and five skeletal muscle relaxants), anti-infective agents (N = 6), cardiovascular agents (N = 3), nutritional agents (N = 2), a gastrointestinal agent (N = 1), and a renal/genitourinary agent (N = 1). Rate ratios ranged from 1.23 (95% CI: 1.05–1.44) for hydrocodone + amoxicillin-clavulanate to 4.21 (1.88–9.42) for oxycodone + telmisartan. Twenty (37.7%) of the 53 potential drug interaction signals are currently reported in Micromedex, three of which are also reported in the Clinical Drug Information knowledgebase.
Table 3.
Opioid drug interaction signals of potential clinical concern given statistically significantly increased rates of unintentional traumatic injury, by commonly used object drug, by therapeutic category of precipitant drug
| Object | Rate ratio, semi-Bayes shrunk and adjusted |
95% confidence interval |
|---|---|---|
| HYDROCODONE | ||
| Anti-infective precipitant | ||
| amoxicillin-clavulanate | 1.23 | 1.05-1.44 |
| cephalexin | 1.86 | 1.64-2.10 |
| sulfamethoxazole | 1.35 | 1.15-1.58 |
| trimethoprim | 1.34 | 1.14-1.57 |
| Central nervous system precipitant | ||
| acetaminophen | 1.42 | 1.22-1.65 |
| bupropion | 1.32 | 1.03-1.70 |
| carisoprodol* | 1.28 | 1.08-1.52 |
| cyclobenzaprine* | 1.56 | 1.42-1.71 |
| diazepam*,** | 1.48 | 1.26-1.74 |
| etodolac | 1.79 | 1.32-2.44 |
| ibuprofen | 1.72 | 1.51-1.95 |
| ketoprofen | 2.12 | 1.12-4.02 |
| ketorolac | 1.45 | 1.00-2.08 |
| meloxicam | 1.42 | 1.17-1.73 |
| metaxalone* | 1.51 | 1.23-1.86 |
| naproxen | 1.59 | 1.37-1.84 |
| ondansetron* | 1.28 | 1.06-1.54 |
| Gastrointestinal precipitant | ||
| metoclopramide* | 1.60 | 1.16-2.20 |
| Nutritional precipitant | ||
| niacin | 1.56 | 1.00-2.43 |
| OXYCODONE | ||
| Anti-infective precipitant | ||
| cephalexin | 1.59 | 1.34-1.89 |
| clarithromycin*,** | 1.90 | 1.05-3.44 |
| Cardiovascular precipitant | ||
| telmisartan | 4.21 | 1.88-9.42 |
| Central nervous system precipitant | ||
| acetaminophen | 1.31 | 1.02-1.67 |
| clonazepam*,** | 1.39 | 1.05-1.84 |
| cyclobenzaprine* | 1.46 | 1.28-1.67 |
| ibuprofen | 1.87 | 1.54-2.27 |
| ketorolac | 1.69 | 1.08-2.65 |
| lisdexamfetamine* | 2.18 | 1.43-3.33 |
| naproxen | 1.56 | 1.25-1.94 |
| orphenadrine* | 1.80 | 1.11-2.93 |
| pregabalin | 1.40 | 1.08-1.82 |
| varenicline | 2.09 | 1.15-3.79 |
| Nutritional precipitant | ||
| calcitriol | 2.33 | 1.23-4.43 |
| Renal and genitourinary precipitant | ||
| Metolazone† | 2.39 | 1.31-4.37 |
| TRAMADOL | ||
| Anti-infective precipitant | ||
| cephalexin | 1.58 | 1.29-1.93 |
| quinine* | 2.63 | 1.30-5.33 |
| sulfamethoxazole | 2.02 | 1.63-2.52 |
| trimethoprim | 2.00 | 1.61-2.48 |
| Cardiovascular precipitant | ||
| omega3 polyunsaturated fatty acid | 1.91 | 1.06-3.41 |
| rosuvastatin | 1.52 | 1.05-2.20 |
| Central nervous system precipitant | ||
| acetaminophen | 1.57 | 1.27-1.93 |
| carisoprodol* | 1.47 | 1.03-2.10 |
| diazepam* | 1.59 | 1.17-2.18 |
| ibuprofen | 1.33 | 1.08-1.66 |
| metaxalone* | 1.38 | 1.01-1.89 |
| Naproxen | 1.67 | 1.33-2.11 |
| ondansetron* | 1.30 | 1.00-1.69 |
| orphenadrine* | 2.50 | 1.43-4.36 |
| rizatriptan* | 2.16 | 1.22-3.82 |
| tizanidine* | 1.44 | 1.06-1.94 |
We excluded propoxyphene, a medical product eventually withdrawn from the United States market, from the list of central nervous system precipitants for hydrocodone, tramadol, and oxycodone as this may have represented opioid switching rather than concomitant therapy.
drug interaction with impact on object documented in Micromedex (IBM Watson Health: Cambridge, Massachusetts, United States)
drug interaction with impact on object documented in Facts & Comparisons Clinical Drug Information (Wolters Kluwer: Alphen aan den Rijn, South Holland, Netherlands)
drug interaction with impact on precipitant documented in Micromedex (IBM Watson Health: Cambridge, Massachusetts, United States)
Figure 2. Commonly prescribed opioid + precipitant drug associations with unintentional traumatic injury.
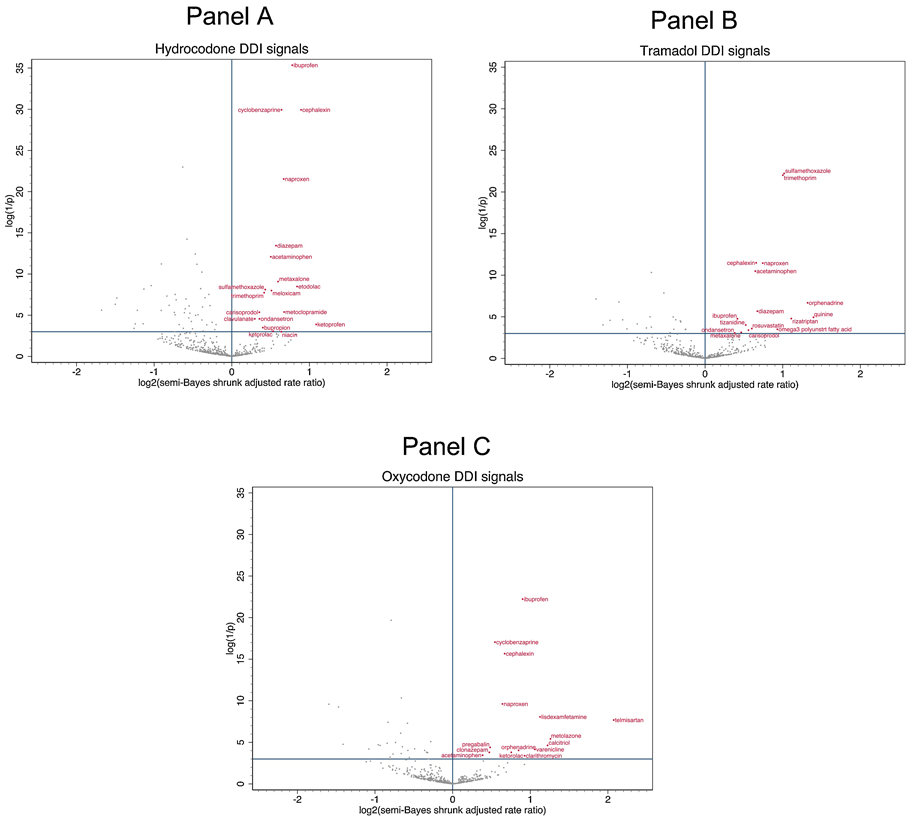
Panel A depicts associations with hydrocodone. Panel B depicts associations with tramadol. Panel C depicts associations with oxycodone. The x-axis represents the log base 2 (semi-Bayes shrunk adjusted rate ratio) for opioid + precipitant vs. opioid. The y-axis represents the log (1 / p-value) for the semi-Bayes shrunk adjusted rate ratio. Data points in the upper right quadrant represent statistically significant elevated rate ratios for the association between opioid + precipitant (vs. opioid) and injury.
4. DISCUSSION
We conducted pharmacoepidemiologic screening to identify potential opioid drug interactions associated with injury. Among 2492 opioid-precipitant pairs, we identified 77 (3.1%) potential drug interaction signals associated with an increased rate of unintentional traumatic injury—53 signals among users of the three most commonly dispensed opioids (i.e., hydrocodone, tramadol, and oxycodone). Among 772 and 177 opioid-precipitant pairs, we identified 0 (0.0%) and 4 (2.3%) potential drug interaction signals associated with increased rates of hip fracture and motor vehicle crash respectively—0 and 4 signals among users of the three most commonly dispensed opioids. Given the semi-automated, high-throughput nature of our investigation, we intend these findings to be interpreted as hypothesis generating and thereby help researchers to target limited available resources to assess etiology.
Despite the clear health burden of opioid drug interactions, few prior studies have rigorously examined their clinical importance.[43] A notable exception includes investigations of opioids with benzodiazepines.[13,44-47] Our study yielded many expected results for this combination. Concomitant use was associated with injury rates potentially increased by: 1.6-fold for alprazolam with morphine; 1.5- to 2.9-fold for diazepam with hydrocodone, tramadol, or codeine; and 1.4-fold for clonazepam with oxycodone. Further, concomitant use of diazepam or alprazolam with oxycodone was associated with 2.6- and 3.0-fold increases in the rate of motor vehicle crash, respectively. Interactions between opioids and benzodiazepines are biologically plausible given additive or synergistic pharmacodynamic (e.g., CNS depression) and/or pharmacokinetic (e.g., altered transport and hepatic metabolism) effects. For example, if diazepam demonstrated in vivo inhibition (currently, only in vitro inhibition has been demonstrated) of hepatic cytochrome P450 (CYPs) 3A4[48]—a pathway involved in the complex metabolism of active hydrocodone to inactive norhydrocodone—this could result in increased hydrocodone concentrations and exaggerated opioid effects, potentially compounded by diazepam’s long half-life and debated anticholinergic properties.[49] Our identification of potential signals for opioids + benzodiazepines, buttressed by mechanistic expectations and prior epidemiologic data, supports the validity of our drug interaction screening approach. Our population based findings are consistent with Food and Drug Administration,[50] Centers for Medicare and Medicaid Services,[51] and American Geriatrics Society[52] recommendations to limit or avoid this combination. The lack of signaling for some expected pairs (e.g., morphine + temazepam, rate ratioinjury = 1.8, 95% CI 0.9–3.3), especially for less commonly used opioids, may be driven by limited statistical precision and suggests that assumptions employed during semi-Bayes shrinkage were appropriately conservative for use in this hypothesis-generating screening context.
We newly identified similar magnitudes of association for opioid + different skeletal muscle relaxants. Concomitant use was associated with injury rates potentially increased by: 2.5-fold for methocarbamol with codeine; 1.8- and 2.5-fold for orphenadrine with oxycodone or tramadol respectively; 1.6- to 2.9-fold for cyclobenzaprine with hydrocodone, morphine, hydromorphone, or codeine; 1.4-fold for tizanidine with tramadol; 1.4- and 1.5-fold for metaxalone with tramadol or hydrocodone respectively; and 1.3- to 2.0-fold for carisoprodol with hydrocodone, tramadol, or codeine. Further, concomitant use of cyclobenzaprine with hydrocodone was associated with a 2.9-fold rate of motor vehicle crash. Potential pharmacokinetic mechanisms (e.g., orphenadrine’s inhibition of CYP2D6 and CYP3A4[53]) and pharmacodynamic effects (e.g., sedation, disorientation, visual disturbances, symptomatic hypotension, psychomotor impairment[54]) of skeletal muscle relaxants may support these associations. Our findings are timely given substantial nationwide increases in chronic use of skeletal muscle relaxants and common co-dispensing with opioids.[55]
We also identified potential signals for opioids + anti-infectives and unintentional traumatic injury. Use of clarithromycin with oxycodone was associated with a 1.9-fold increase in the rate of injury; this finding quantifies the potential importance of a reported pharmacokinetic interaction[56] mediated by potent CYP3A4 inhibition[57] resulting in increased oxycodone plasma concentrations. Use of quinine with tramadol was associated with a 2.6-fold increase in the rate of injury; this seemingly counterintuitive finding highlights the potential importance of patient behavior in response to effects of a reported pharmacokinetic interaction.[56] Quinine may inhibit CYP2D6’s conversion of inactive tramadol (parent compound) to active o-desmethyltramadol.[58] The reduction in active metabolite levels may result in a clinically significant loss of pain control and potentially increased breakthrough opioid consumption;[59] interestingly we did not identify signals for injury with tramadol + other strong CYP2D6 inhibitors (e.g., bupropion, paroxetine, ritonavir). Further, quinine may stimulate insulin release and precipitate hypoglycemia,[60] a major risk factor for traumatic injury.[61] Increased injury rates among users of sulfamethoxazole-trimethoprim with hydrocodone or tramadol may be at least partly related to sulfamethoxazole’s innate hypoglycemic effects.[62] We are unaware of putative mechanisms underlying apparently increased injury rates among concomitant users of opioids with cephalexin or amoxicillin-clavulanate. An alternate explanation for opioid + anti-infective signals may be confounding by indication for the precipitant, as infection alone may alter drug metabolism.
Of other potential drug interaction signals identified, most are biologically plausible. For example, the precipitant’s effects on CYP2D6, serotonin levels, and/or vision[63] may precipitate the apparent 2.2-fold increase in injury risk with oxycodone + lisdexamfetamine. For signals that lack an obvious mechanism (e.g., tramadol + omega-3 polyunsaturated fatty acids) or with less conclusive biologic underpinnings (e.g., hydrocodone + ketoprofen), it is especially unclear whether findings reflect chance, reverse causation, confounding by indication, or important drug interactions that place patients at risk of injury; this is an important concern for precipitants used to treat injuries (e.g., nonsteroidal anti-inflammatory drugs) or their sequelae (e.g., anti-infectives). This represents an especially fertile space for future etiologic investigation.
Our study has strengths. First, we utilized a self-controlled case series design, ideal for drug interaction screening,[64] to minimize confounding. Second, we used a bi-directional implementation of the design to minimize exposure trend bias.[19] Third, we studied clinically meaningful outcomes identified by well-supported algorithms. Finally, we minimized false positive findings by using semi-Bayes shrinkage to account for multiple estimation.
Our study also has limitations. First, prescription dispensings may be imperfect markers for actual drug ingestion. This may be particularly true for opioids. Second, we did not examine higher order drug interactions such as triplets or quadruplets. Such findings may be of future interest given the prevalence of polypharmacy[65] and specific concerns regarding “holy trinity”[66] (i.e., opioid + benzodiazepine + skeletal muscle relaxant) prescribing. Third, the bi-directional self-controlled case series design may be susceptible to reverse causation, especially for suspected drug interactions. If a clinician posited that a precipitant induced an injury in an opioid user (even if concomitant precipitant use had no causal effect on injury), s/he may subsequently discontinue the precipitant; this may result in a spuriously elevated rate ratio for the precipitant. However, we find it unlikely (particularly for non-sedating precipitants) that reverse causation explains the drug interaction signals identified herein. Interactions are often overlooked in clinical practice[67] and therefore clinicians may be unlikely to attribute an injury to an interaction and discontinue a precipitant to reduce future risk. We considered employing a unidirectional design to mitigate reverse causation, but such a methodologic adaptation would be susceptible to potentially substantial exposure trend bias. Fourth, given the hypothesis generating nature of our work, we did not consider injury severity. Fifth, in addition to the potential for bias and confounding, one must consider the role of chance. Finally, our findings may not be generalizable beyond a commercially insured, ambulatory care population.
5. CONCLUSIONS
Evaluating and synthesizing the clinical importance of potential drug interactions is a major unmet information need.[68] Given the evidence gap, a federal framework to end the opioid crisis has called for better research to understand opioid drug interactions.[9] We used longitudinal health insurance data to identify many previously undescribed and/or unappreciated opioid interactions potentially associated with unintentional traumatic injury, typical hip fracture, and/or motor vehicle crash. Our findings provide researchers with an evidence-based list of drug interaction signals, such that limited resources can be directed to confirm (or refute) and elucidate these potential interactions in follow-on etiologic studies.
Supplementary Material
Highlights.
Opioid drug interactions are a high priority target for minimizing patient harms
We identified potential opioid drug interactions associated with unintentional injury
Readers should interpret these drug interaction signals as hypothesis generating
Among identified signals, most were not documented in drug interaction knowledgebases
Findings may help researchers target limited available resources to assess etiology
Acknowledgements
The authors thank Ms. Min Du and Ms. Qing Liu from the University of Pennsylvania for their computer programming support, Ms. Emily K. Acton from the University of Pennsylvania for her expertise on pharmacotherapy, Dr. Mark D. Neuman from the University of Pennsylvania for his expertise on opioid prescribing, and Dr. Meijia Zhou from Johnson & Johnson for sharing Python programming language code to facilitate heat map generation.
Funding Source United States National Institutes of Health
Sources of Funding
The United States National Institutes of Health supported this work (R01AG060975, R01DA048001, R01AG064589, and R01AG025152).
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclosures
Dr. Leonard serves on the Executive Committee of and Dr. Hennessy directs the University of Pennsylvania’s Center for Pharmacoepidemiology Research and Training. The Center receives funding from Pfizer Inc and Sanofi to support trainee education. Dr. Leonard’s spouse is employed by Health Union LLC, a health technology company partly supported by advertising by Abbvie Inc, Adamas Pharmaceuticals, Celgene Corporation, Eli Lilly and Company, Lundbeck A/S, Novartis International AG, and Sunovion Pharmaceuticals Inc. Dr. Bilker has consulted for Genentech Inc. Dr. Dublin has received funding from Jazz Pharmaceuticals to study motor vehicle crashes (unrelated to opioids or other medications); she has pending grant support from GlaxoSmithKline LLC for an unrelated topic. Dr. Oslin has consulted for and receives grant support from Janssen Pharmaceutica. Dr. Hennessy receives salary support from a sponsored research agreement between Pfizer Inc and the University of Pennsylvania. He has consulted for Novo Nordisk, Arbor Pharmaceuticals, Nektar Therapeutics Inc, the Medullary Thyroid Cancer Consortium (Novo Nordisk, AstraZeneca Pharmaceuticals LP, GlaxoSmithKline LLC, Eli Lilly and Company), Merck, Esteve Pharmaceuticals LLC, Purdue Pharma LP, Sanofi US Services Inc, Lexicon Pharmaceuticals Inc, the Transdermal Immediate Release Fentanyl REMS (BioDelivery Sciences International Inc, Insys Therapeutics, Inc, SpecGX LLC [a wholly owned subsidiary of Mallinckrodt Inc], Mylan Inc, Par Pharmaceutical Inc, Sentynl Therapeutics Inc, Teva Pharmaceuticals USA Inc, West Therapeutic Development LLC), Mallinckrodt Pharmaceuticals, SpecGX LLC, and Sage Therapeutics. Other authors report no conflicts.
Statement of Integrity
Dr. Leonard had full access to study data and directed the analyses. He is responsible for the study’s integrity.
Registration None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Centers for Disease Control and Prevention. National Center for Injury Prevention and Control. Welcome to WISQARS. 2020. https://www.cdc.gov/injury/wisqars/index.html. Last updated: 30 March 2020 Last accessed: 26 June 2020.
- [2].Haagsma JA, Graetz N, Bolliger I, Naghavi M, Higashi H, Mullany EC, et al. The global burden of injury: incidence, mortality, disability-adjusted life years and time trends from the Global Burden of Disease study 2013. Inj Prev 2016; 22: 3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Centers for Disease Control and Prevention. National Center for Injury Prevention and Control. WISQARS: 10 leading causes of death, United States. 2018. https://www.cdc.gov/injury/wisqars/LeadingCauses.html. Last updated: 30 March 2020 Last accessed: 07 July 2020. [Google Scholar]
- [4].Hannan EL, Waller CH, Farrell LS, Rosati C. Elderly trauma inpatients in New York state: 1994-1998. J Trauma 2004; 56: 1297–304. [DOI] [PubMed] [Google Scholar]
- [5].McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: A review. Am J Addict 2010; 19: 4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Overholser BR, Foster DR. Opioid pharmacokinetic drug-drug interactions. Am J Manag Care 2011; 17 Suppl 11: S276–87. [PubMed] [Google Scholar]
- [7].Gurwitz JH, Field TS, Harrold LR, Rothschild J, Debellis K, Seger AC, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA 2003; 289: 1107–16. [DOI] [PubMed] [Google Scholar]
- [8].Becker ML, Kallewaard M, Caspers PW, Visser LE, Leufkens HG, Stricker BH. Hospitalisations and emergency department visits due to drug-drug interactions: a literature review. Pharmacoepidemiol Drug Saf 2007; 16: 641–51. [DOI] [PubMed] [Google Scholar]
- [9].U.S. Department of Health and Human Services' Office of Disease Prevention and Health Promotion. National action plan for adverse drug event prevention. 2014. https://health.gov/our-work/health-care-quality/adverse-drug-events/national-ade-action-plan Last updated: 21 October 2019 Last accessed: 07 July 2020. [Google Scholar]
- [10].National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Health Sciences Policy, Committee on Pain Management and Regulatory Strategies to Address Prescription Opioid Abuse. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Phillips JK, Ford MA, Bonnie RJ, editors. Washington (DC): National Academies Press (US); 2017. July 13. [PubMed] [Google Scholar]
- [11].US Department of Health and Human Services. Strategy to combat opioid abuse, misuse, and overdose: A framework based on the five point strategy. 2017. https://www.hhs.gov/opioids/about-the-epidemic/hhs-response/index.html. Last updated: 07 August 2018 Last accessed: 07 July 2020.
- [12].Li G, Chihuri S. Prescription opioids, alcohol and fatal motor vehicle crashes: a population-based case-control study. Inj Epidemiol 2019; 6: 11,019–0187-x. eCollection 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ 2015; 350: h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W. Gabapentin, opioids, and the risk of opioid-related death: A population-based nested case-control study. PLoS Med 2017; 14: e1002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li Y, Delcher C, Wei YJ, Reisfield GM, Brown JD, Tighe PJ, et al. Risk of opioid overdose associated with concomitant use of opioids and skeletal muscle relaxants: a population-based cohort study. Clin Pharmacol Ther 2020. [DOI] [PubMed] [Google Scholar]
- [16].Taylor R Jr, Pergolizzi JV Jr, Puenpatom RA, Summers KH. Economic implications of potential drug-drug interactions in chronic pain patients. Expert Rev Pharmacoecon Outcomes Res 2013; 13: 725–34. [DOI] [PubMed] [Google Scholar]
- [17].Pergolizzi JV Jr, Labhsetwar SA, Puenpatom RA, Joo S, Ben-Joseph R, Summers KH. Exposure to potential CYP450 pharmacokinetic drug-drug interactions among osteoarthritis patients: incremental risk of multiple prescriptions. Pain Pract 2011; 11: 325–36. [DOI] [PubMed] [Google Scholar]
- [18].Pergolizzi JV Jr, Labhsetwar SA, Puenpatom RA, Joo S, Ben-Joseph RH, Summers KH. Prevalence of exposure to potential CYP450 pharmacokinetic drug-drug interactions among patients with chronic low back pain taking opioids. Pain Pract 2011; 11: 230–9. [DOI] [PubMed] [Google Scholar]
- [19].Maclure M, Fireman B, Nelson JC, Hua W, Shoaibi A, Paredes A, et al. When should case-only designs be used for safety monitoring of medical products? Pharmacoepidemiol Drug Saf 2012; 21 Suppl 1: 50–61. [DOI] [PubMed] [Google Scholar]
- [20].Lee KJ, Carlin JB. Fractional polynomial adjustment for time-varying covariates in a self-controlled case series analysis. Stat Med 2014; 33: 105–16. [DOI] [PubMed] [Google Scholar]
- [21].Whitaker HJ, Hocine MN, Farrington CP. The methodology of self-controlled case series studies. Stat Methods Med Res 2009; 18: 7–26. [DOI] [PubMed] [Google Scholar]
- [22].Han X, Chiang CW, Leonard CE, Bilker WB, Brensinger CM, Hennessy S. Biomedical informatics approaches to identifying drug-drug interactions: application to insulin secretagogues. Epidemiology 2017; 28: 459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bykov K, Schneeweiss S, Glynn RJ, Mittleman MA, Gagne JJ. A case-crossover-based screening approach to identifying clinically relevant drug-drug interactions in electronic healthcare data. Clin Pharmacol Ther 2019; 106: 238–44. [DOI] [PubMed] [Google Scholar]
- [24].Leonard CE, Zhou M, Brensinger CM, Bilker WB, Soprano SE, Pham Nguyen TP, et al. Clopidogrel drug interactions and serious bleeding: generating real-world evidence via automated high-throughput pharmacoepidemiologic screening. Clin Pharmacol Ther 2019; 106: 1067–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pottegard A, dePont Christensen R, Wang SV, Gagne JJ, Larsen TB, Hallas J. Pharmacoepidemiological assessment of drug interactions with vitamin K antagonists. Pharmacoepidemiol Drug Saf 2014; 23: 1160–7. [DOI] [PubMed] [Google Scholar]
- [26].Martin-Perez M, Gaist D, de Abajo FJ, Rodriguez LAG. Population impact of drug interactions with warfarin: a real-world data approach. Thromb Haemost 2018; 118: 461–70. [DOI] [PubMed] [Google Scholar]
- [27].Optum Inc. Clinformatics Data Mart. Publication number OPTPRJ5232. 2014. https://www.optum.com/content/dam/optum/resources/productSheets/Clinformatics_for_Data_Mart.pdf. Last accessed: 07 July 2020.
- [28].Statista. Market share of leading health insurance companies in the United States in 2018, by direct premiums written. 2019. https://www.statista.com/statistics/216518/leading-us-health-insurance-groups-in-the-us/. Last accessed: 07 July 2020. [Google Scholar]
- [29].Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med 2006; 25: 1768–97. [DOI] [PubMed] [Google Scholar]
- [30].Juurlink DN, Mamdani M, Kopp A, Laupacis A, Redelmeier DA. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA 2003; 289: 1652–8. [DOI] [PubMed] [Google Scholar]
- [31].Schelleman H, Bilker WB, Brensinger CM, Wan F, Hennessy S. Anti-infectives and the risk of severe hypoglycemia in users of glipizide or glyburide. Clin Pharmacol Ther 2010; 88: 214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Douketis JD, Melo M, Bell CM, Mamdani MM. Does statin therapy decrease the risk for bleeding in patients who are receiving warfarin? Am J Med 2007; 120: 369.e9,369.e14. [DOI] [PubMed] [Google Scholar]
- [33].Centers for Medicare and Medicaid Services. Opioid oral morphine milligram equivalent (MME) conversion factors. 2018. https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Oral-MME-CFs-vFeb-2018.pdf. Last accessed: 07 July 2020. [Google Scholar]
- [34].Fulton J, Wright K, Kelly M, Zebrosky B, Zanis M, Drvol C, et al. Injury risk is altered by previous injury: a systematic review of the literature and presentation of causative neuromuscular factors. Int J Sports Phys Ther 2014; 9: 583–95. [PMC free article] [PubMed] [Google Scholar]
- [35].American College of Surgeons. ACS NTB National Trauma Data Standard: data dictionary. 2016 admissions 2015. https://www.facs.org/quality-programs/trauma/tqp/center-programs/ntdb/ntds/archived-dictionary. Last accessed: 07 July 2020.
- [36].Sears JM, Bowman SM, Rotert M, Hogg-Johnson S. A new method to classify injury severity by diagnosis: validation using workers' compensation and trauma registry data. J Occup Rehabil 2015; 25: 742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Curtis JR, Taylor AJ, Matthews RS, Ray MN, Becker DJ, Gary LC, et al. "Pathologic" fractures: should these be included in epidemiologic studies of osteoporotic fractures? Osteoporos Int 2009; 20: 1969–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2014; 29: 1–23. [DOI] [PubMed] [Google Scholar]
- [39].No authors listed (per PubMed). Recommended framework for presenting injury mortality data. MMWR Recomm Rep 1997; 46: 1–30. [PubMed] [Google Scholar]
- [40].Whitaker HW. Using Stata for the self-controlled case series method. 2005. http://stats-www.open.ac.uk/sccs/stata.htm. Last accessed: 26 June 2020. [Google Scholar]
- [41].Greenland S, Poole C. Empirical-Bayes and semi-Bayes approaches to occupational and environmental hazard surveillance. Arch Environ Health 1994; 49: 9–16. [DOI] [PubMed] [Google Scholar]
- [42].Steenland K, Bray I, Greenland S, Boffetta P. Empirical Bayes adjustments for multiple results in hypothesis-generating or surveillance studies. Cancer Epidemiol Biomarkers Prev 2000; 9: 895–903. [PubMed] [Google Scholar]
- [43].Magro L, Moretti U, Leone R. Epidemiology and characteristics of adverse drug reactions caused by drug-drug interactions. Expert Opin Drug Saf 2012; 11: 83–94. [DOI] [PubMed] [Google Scholar]
- [44].Gressler LE, Martin BC, Hudson TJ, Painter JT. Relationship between concomitant benzodiazepine-opioid use and adverse outcomes among US veterans. Pain 2018; 159: 451–9. [DOI] [PubMed] [Google Scholar]
- [45].Hernandez I, He M, Brooks MM, Zhang Y. Exposure-response association between concurrent opioid and benzodiazepine use and risk of opioid-related overdose in Medicare Part D beneficiaries. JAMA Netw Open 2018; 1: e180919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Dassanayake T, Michie P, Carter G, Jones A. Effects of benzodiazepines, antidepressants and opioids on driving: a systematic review and meta-analysis of epidemiological and experimental evidence. Drug Saf 2011; 34: 125–56. [DOI] [PubMed] [Google Scholar]
- [47].Sun EC, Dixit A, Humphreys K, Darnall BD, Baker LC, Mackey S. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ 2017; 356: j760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res 2018; 46: D1074–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Campbell N, Boustani M. Anticholinergic cognitive burden scale. 2010. https://americandeliriumsociety.org/files/ACB_Handout_Version_03-09-10.pdf. Last accessed: 07 July 2020.
- [50].US Food and Drug Administration. FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. 2016. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-about-serious-risks-and-death-when-combining-opioid-pain-or Last updated 20 September 2017 Last accessed: 07 July 2020.
- [51].Centers for Medicare and Medicaid Services. Reduce risk of opioid overdose deaths by avoiding and reducing co-prescribing benzodiazepines. Report SE19011. 2019. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/SE19011.pdf. Last accessed: 07 July 2020. [Google Scholar]
- [52].The 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. Journal of the American Geriatrics Society 2019; 67: 674–94. [DOI] [PubMed] [Google Scholar]
- [53].Guo Z, Raeissi S, White RB, Stevens JC. Orphenadrine and methimazole inhibit multiple cytochrome P450 enzymes in human liver microsomes. Drug Metab Dispos 1997; 25: 390–3. [PubMed] [Google Scholar]
- [54].Kluwer Wolters. Drug Facts and Comparisons. Centrally acting skeletal muscle relaxants. 2019. https://fco.factsandcomparisons.com/lco/action/login. Last accessed 07 July 2020.
- [55].Soprano SE, Hennessy S, Bilker WB, Leonard CE. Assessment of physician prescribing of muscle relaxants in the United States, 2005-2016. JAMA Netw Open 2020; 3(6): e207664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].IBM Corporation. IBM Micromedex® (electronic version). 2020. https://www.micromedexsolutions.com/home/dispatch/ssl/true. Last accessed: 20 April 2020. [Google Scholar]
- [57].Liukas A, Hagelberg NM, Kuusniemi K, Neuvonen PJ, Olkkola KT. Inhibition of cytochrome P450 3A by clarithromycin uniformly affects the pharmacokinetics and pharmacodynamics of oxycodone in young and elderly volunteers. J Clin Psychopharmacol 2011; 31: 302–8. [DOI] [PubMed] [Google Scholar]
- [58].Simooya OO, Sijumbil G, Lennard MS, Tucker GT. Halofantrine and chloroquine inhibit CYP2D6 activity in healthy Zambians. Br J Clin Pharmacol 1998; 45: 315–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Frost DA, Soric MM, Kaiser R, Neugebauer RE. Efficacy of tramadol for pain management in patients receiving strong cytochrome P450 2D6 inhibitors. Pharmacotherapy 2019; 39: 724–9. [DOI] [PubMed] [Google Scholar]
- [60].Murad MH, Coto-Yglesias F, Wang AT, Sheidaee N, Mullan RJ, Elamin MB, et al. Clinical review: Drug-induced hypoglycemia: a systematic review. J Clin Endocrinol Metab 2009; 94: 741–5. [DOI] [PubMed] [Google Scholar]
- [61].El-Menyar A, Mekkodathil A, Al-Thani H. Traumatic injuries in patients with diabetes mellitus. J Emerg Trauma Shock 2016; 9: 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lee AJ, Maddix DS. Trimethoprim/sulfamethoxazole-induced hypoglycemia in a patient with acute renal failure. Ann Pharmacother 1997; 31: 727–32. [DOI] [PubMed] [Google Scholar]
- [63].Shire US Inc. Vyvanse capsules package insert. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208510lbl.pdf. Last accessed: 07 July 2020.
- [64].Hennessy S, Leonard CE, Gagne JJ, Flory JH, Han X, Brensinger CM, et al. Pharmacoepidemiologic methods for studying the health effects of drug-drug interactions. Clin Pharmacol Ther 2016; 99: 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Quinn KJ, Shah NH. A dataset quantifying polypharmacy in the United States. Sci Data 2017; 4: 170167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Horsfall JT, Sprague JE. The pharmacology and toxicology of the 'holy trinity'. Basic & Clinical Pharmacology & Toxicology 2017; 120: 115–9. [DOI] [PubMed] [Google Scholar]
- [67].Bain KT, Knowlton CH. Role of opioid-involved drug interactions in chronic pain management. J Am Osteopath Assoc 2019; 119: 839–47. [DOI] [PubMed] [Google Scholar]
- [68].Romagnoli KM, Nelson SD, Hines L, Empey P, Boyce RD, Hochheiser H. Information needs for making clinical recommendations about potential drug-drug interactions: a synthesis of literature review and interviews. BMC Med Inform Decis Mak 2017; 17: 21,017-0419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


