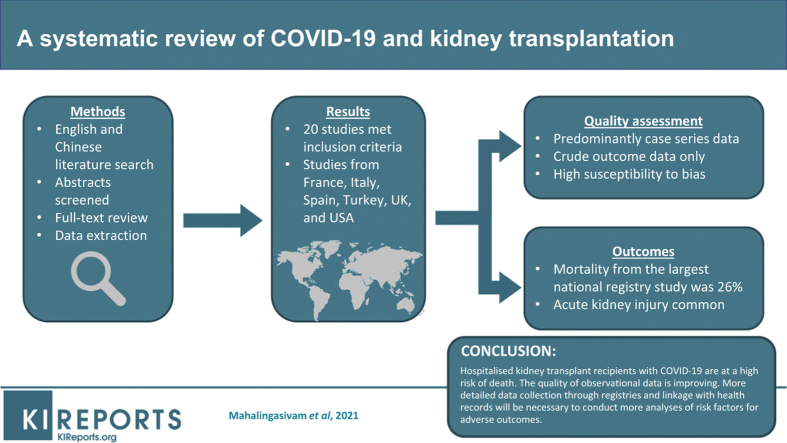
Abstract
Introduction
Kidney transplant recipients are at increased susceptibility to many viral infections leading to justifiable anxiety about the effects of coronavirus disease 2019 (COVID-19).
Methods
We performed literature searches from multiple resources in April and August 2020 for relevant English and Chinese literature. Abstracts were screened, followed by full-text review with data extraction of reports that included at least 20 kidney transplant recipients with confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and completed outcomes.
Results
Twenty studies had sufficient data, which we have summarized. Studies were predominantly descriptive and came from France, Italy, Spain, Turkey, United Kingdom, and United States. Quality assessment demonstrated limitations in selection of comparison groups and controlling for additional factors. Mortality rates from published studies were variable. Based on early data early from Spain, 46% of patients who developed COVID-19 within 60 days of transplantation died. Acute kidney injury was common, and mycophenolate was discontinued in most patients.
Conclusion
Given the rapid global spread of COVID-19, reliable evidence is needed to inform public health policies. Hospitalized kidney transplant recipients with COVID-19 are at a high risk of death in early reports but interpretation of these data requires caution, as studies were susceptible to period effects. Reassuringly, the quality of observational data is improving. Detailed and comprehensive data collection through linked registries will be necessary to conduct accurate analyses of risk factors for adverse outcomes, not least given the risks of stopping imunosuppression. This report highlights the early mortality excess in transplant recipients but medium- and longer-term outcomes remain uncertain and merit careful investigation.
Keywords: COVID-19, kidney transplantation, systematic review
Graphical abstract
On December 31, 2019, the Wuhan Health Commission in China reported an outbreak of atypical pneumonia to the World Health Organization. The causative pathogen was found to be the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) capable of human-to-human transmission through respiratory droplets.1 The associated disease was named COVID-19, and its spectrum of severity ranges from no symptoms to life-threatening organ dysfunction, the scale of which can place extreme burden on health care resources without control of transmission.2 After spread across several continents, COVID-19 was declared a pandemic by the World Health Organization on March 11, 2020.
Severe lung injury and other organ-threatening complications in COVID-19 are understood to be the result of a dysregulated systemic inflammatory response in the days after infection, leading to some immunosuppressive therapies being repurposed both inside and out of clinical trial settings in conventional management.3, 4, 5 Only low-dose dexamethasone has so far demonstrated mortality benefit compared with usual practice.6
Immunosuppressant drugs used to prevent allograft rejection render kidney transplant recipients at increased susceptibility to many viral infections, and such infections are an important cause of morbidity and mortality in this population.7 The immune response to SARS-CoV-2 infection in immunosuppressed kidney transplant recipients, many with other comorbidities, may result in differences in presentation, outcomes, and therapeutic responses compared with the general population.
Given the rapid and global spread of COVID-19, there is a need to gather evidence and disseminate as quickly as possible. Our aim was to conduct a complete systematic review of the early literature to synthesize, analyze, and appraise what has been learned so far to help clinicians and policy makers better understand the risks to kidney transplant recipients, as well as identify gaps for future collaborative research for this global health challenge.
Methods
Search Strategy
Inclusion and exclusion criteria were formulated to ensure comprehensive searching and screening for articles relevant to COVID-19 in chronic kidney disease, specifically including kidney transplantation. Variables of interest were defined based on the PICOS (patient/population, intervention, comparison, outcomes, study designs) strategy.8 Our protocol was prospectively published via PROSPERO (CRD42020182134).
Initial searches were conducted on April 28, 2020, including all relevant English- and Chinese-language research up to that date from December 1, 2019; papers not written in these languages were excluded because of a lack of resources to obtain timely translation. Published and nonpublished literature was searched on MEDLINE (Ovid), EMBASE (Ovid), China National Knowledge Infrastructure, the Wanfang database, the Chinese Biomedical Literature Database, clinicaltrials.gov, the Chinese Clinical Trial Register, the World Health Organization database of COVID-19 research, and the Chinese Medical Journal Network. Search terms are listed in Supplementary Material S1. An updated search was deemed necessary due to the rapidly evolving evidence base and was conducted onAugust 4, 2020, via MEDLINE (Ovid), EMBASE (Ovid), World Health Organization COVID19 database, and bioRxiv and medRxiv preprint servers. The strategy for the update searches is attached in Supplementary Material S2.
Screening, Data Extraction, and Quality Assessment
Duplicates were removed from the studies generated by both searches and the remaining studies were imported to the SysRev Platform (https://sysrev.com). Screening was undertaken at abstract level by separate teams of 2 independent authors for both English and Chinese; a senior author adjudicated where there was nonconcordance. Reference lists were hand-searched for any additional studies that may have been missed. Full-text articles were then further assessed for eligibility, including the exclusion of case reports or studies with populations of interest of fewer than 20 confirmed cases. As all studies were observational, further quality assessment was undertaken using the Newcastle-Ottawa Quality Assessment Scale.9 Where articles did not report all outcomes specifically in kidney transplant populations, we contacted authors to request disaggregated data (Supplementary Material S3).
Data were extracted for each included study, including online publication date, study population, timeframe, total number of cases (including how many patients had completed outcomes, that is, death or recovery to discharge), patient characteristics, clinical presentation, outcomes, baseline immunosuppression adjustment, and COVID-19 therapy. Inpatient mortality for each study was calculated as a proportion of patients with completed outcomes.
Results
Study Identification
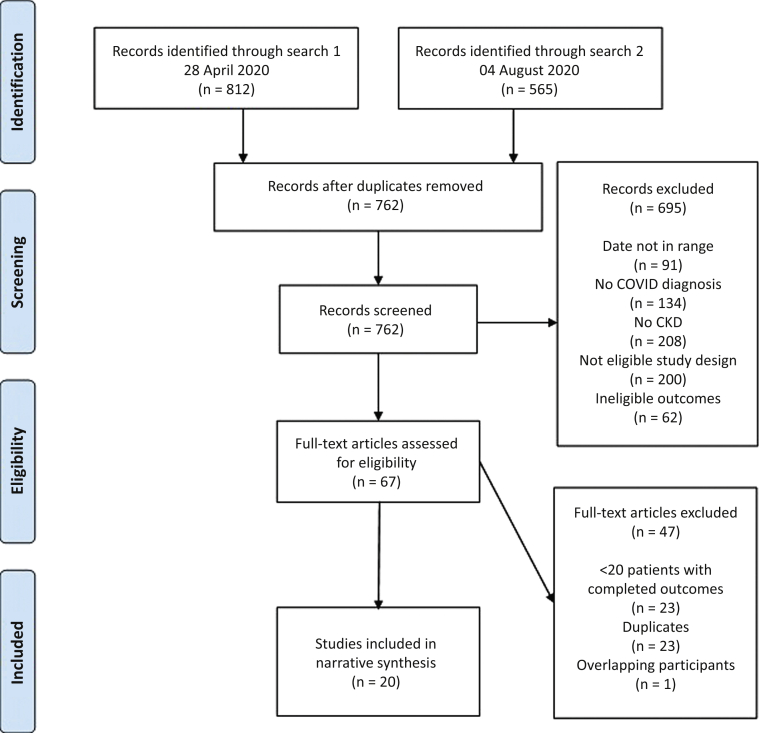
The PRISMA flow diagram is shown in Figure 1. A total of 1377 studies were identified through database searching, after which 762 remained after de-duplication; of these, a further 695 were excluded after abstract screening, leaving 65 that met criteria for full-text assessment for eligibility with no additional articles identified after hand-searching reference lists. A further 47 studies were excluded after detailed assessment. To be more comprehensive, we included 2 additional studies published shortly after our search was completed.10,11 In total, 20 studies underwent data extraction.
Figure 1.
PRISMA flow diagram.
Quality Assessment
There were no randomized controlled trials or case-control studies. Comparative analysis between different exposure groups was limited. Sánchez-Álavarez et al.12 was a report from a national COVID-19 registry across Spain, whereas Pascual et al.13 and Pérez-Sáez et al.14 reported on subgroups from this registry (those within 60 days of transplantation, and those treated with tocilizumab respectively). Bell et al.15 and Ravanan et al.11 reported from national transplant registries in Scotland and England, respectively, whereas Manganaro et al.16 reported on from a regional registry in Italy. Kates et al.10 reported on data entered to a registry by more than 50 transplant centers, almost all from the United States, whereas Cravedi et al.17 was a report of a consortium registry of 12 transplant centers across the United States, Italy, and Spain. Boyarsky et al.18 and Vistoli et al.19 were cross-sectional reports of national surveys from the United States and Italy, respectively. The remaining studies were either single-center or small multicenter case series of either inpatients, or both inpatient and outpatient kidney transplant recipients with COVID-19. Using death as the main outcome of interest, formal quality assessment is shown in Table 1 using the Newcastle-Ottawa Quality Assessment Scale.9
Table 1.
Description of each study design and quality assessment using the Newcastle-Ottawa Quality Assessment Scale, listed by order of online publication date
| Study | Study design | Selection | Comparability | Outcome |
|---|---|---|---|---|
| Manganaro et al.16 | Single-center case series | ☆☆★★ | ☆☆ | ☆☆☆ |
| Boyarksy et al.18 | Cross-sectional national survey | ☆☆☆★ | ☆☆ | ☆☆☆ |
| Pereira et al.21 | Two-center case series | ★☆★★ | ☆☆ | ⋆☆☆ |
| Sánchez-Álvarez et al.12 | National registry cohort | ★★☆★ | ★☆ | ☆★☆ |
| Vistoli et al.19 | Cross-sectional national survey | ★☆☆★ | ☆☆ | ☆☆☆ |
| Rodriguez-Cubillo et al.23 | Single-center case series | ★☆★★ | ☆☆ | ★★★ |
| Pascual. et al.13 | National registry case series | ★☆★★ | ☆☆ | ★☆☆ |
| Chen et al.27 | Single-center case series | ★☆★★ | ☆☆ | ★★★ |
| Mehta et al.28 | Single-center case series | ★☆★★ | ☆☆ | ★★★ |
| Bossini et al.25 | Multicenter case series | ★☆★★ | ☆☆ | ★★★ |
| Cravedi et al.17 | Multicenter case series | ★☆★★ | ☆☆ | ★★☆ |
| Chaudhry et al.26 | Multicenter case series | ★☆★★ | ☆☆ | ★☆☆ |
| Pérez-Sáez et al.14 | National registry case series | ★☆★★ | ☆☆ | ★★★ |
| Demir et al.24 | Multicenter case series | ☆☆★★ | ☆☆ | ★☆☆ |
| Lubetzky et al.22 | Single-center case series | ★☆★★ | ☆☆ | ★★★ |
| Bell et al. (preprint)15 | National registry case series | ★☆★★ | ☆☆ | ★★★ |
| Mohamed et al.20 | Single-center case series | ★☆★★ | ★☆ | ★★★ |
| Kates et al.10 | Multicenter case series | ☆☆★★ | ☆☆ | ★★★ |
| Benotmane et al.29 | Single-center case series | ★☆★★ | ☆☆ | ★★★ |
| Ravanan et al.11 | National registry cohort | ★★★★ | ★☆ | ★★★ |
From left to right, quality items were starred black if they fulfilled predefined criteria: selection was starred on representativeness of patients with the exposure of interest (kidney transplant), selection of the nonexposed group, ascertainment of exposure, and demonstration that outcome of interest (death) was not present at start of the study; comparability was starred on the study controlling for the exposure of interest, and any additional factor; outcome was starred on how the outcome was assessed, whether follow-up was long enough for the outcome to occur, and whether loss to follow-up was adequate enough to be unlikely to introduce bias.
Quality assessment demonstrated consistent weaknesses in selection of control groups (e.g., home dialysis patients on the transplant waiting list) and inadequate control for additional confounding factors. Case series are descriptive and do not make comparisons with a control group, whereas single-center reports may yield biased results when compared with the source population. Kates et al.10 was susceptible to selection bias, as participating centers may not have systematically submitted all cases. Some studies did not report how many patients had been discharged, meaning mortality estimates may have been inaccurate due to misclassification of patients who died after the end of follow-up, but more recent reports had longer and more complete follow-up. Although Boyarsky et al.18 and Vistoli et al.19 had high response rates to their surveys, they may not be completely reliable, as they were not linked to individual patient records.
Study Populations
All studies identified by our search are listed in Supplementary Table S4, along with other studies reporting on ≥5 kidney transplant recipients. Our searches of studies with ≥20 recipients with confirmed COVID-19 and completed outcomes identified studies from only 6 countries (France, Italy, Spain, Turkey, United Kingdom, and United States); at least 5 of the 7 studies with patients from United States included cases from New York City. There have been smaller published studies not included in our review from Belgium, China, Iran, Netherlands, Portugal, and Switzerland.
We note that some studies had overlapping cohorts: patients from Mohamed et al.20 would be included in Ravanan et al.,11 whereas patients in Pereira et al.21 would have been included in Lubetzky et al.22 Some cases from Rodriguez-Cubillo et al.23 are likely to have been included in the registry report by Sánchez-Álvarez et al.,12 whereas Pascual et al.13 and Pérez-Sáez et al.14 were reports of subgroup analyses from this registry. Cravedi et al.17 excluded any patients from studies that had already been published, but these were smaller studies not included in our summary. Kates et al.10 did not report which centers submitted data to its registry but there were more than 50, of which >98% were from the United States; our review includes 6 other studies with data from the United States that may have overlapped. Some centers from the United States and Italy reported in our review are likely to have contributed to the surveys published by Boyarsky et al.18 and Vistoli et al.19
Data Analysis
Data and results from each study are summarized in Table 2. The 20 studies were published online between April 10 and August 11, 2020; one was a preprint with the remainder in journals. The last day of follow-up for each study ranged from March 17 to May 31, 2020. The studies ranged from 24 to 489 kidney transplant recipients in total.
Table 2.
Summary of clinical data and outcomes from all included studies listed by order of online publication date
| Study | Online publication date in 2020 | Study population | Setting | Timeframe in 2020 | Total number of cases | Patient characteristics | Clinical presentation | Outcomes | Baseline IS adjustment | COVID-19 therapy |
|---|---|---|---|---|---|---|---|---|---|---|
| Manganaro et al.16 | 10 April | All inpatients and outpatients with COVID-19 confirmed by swab | 22 Nephrology and Dialysis Units, Piedmont and Aosta Valley, Italy | Up to 27 March | 26 |
|
|
|||
| Boyarsky et al.18 | 13 April | Patients with COVID-19 | 88/111 centers conducting >100 solid organ transplants/year, United States | Up to 24 March to 31 March when survey was conducted | 103 |
|
||||
| Pereira et al.21 | 24 April | All inpatients and outpatients with COVID-19 confirmed by RT-PCR | Two multiple solid organ transplant centers, New York City, United States | 13 March to 3 April | 51 (46 kidney-only, 3 heart-kidney, 1 liver-kidney, 1 pancreas-kidney) |
|
||||
| Sánchez-Álvarez et al.12 | 27 April | Inpatients and outpatients who tested positive for COVID-19 entered to COVID-19 Registry of Spanish Society of Nephrology | Health centers across the Autonomous Communities of Spain | 18 March to 11 April | ∼286 (∼269 hospitalized; ∼122 recovered or died)a |
|
|
|||
| Vistoli et al.19 | 3 June | Reported COVID-19–positive inpatients and outpatients according to survey of kidney transplant centers | 39/41 public kidney transplant centers, Italy | Up to 17 March | 60 (57 hospitalized) |
|
|
|||
| Rodriguez-Cubillo et al.23 | 12 June | Confirmed COVID-19 (RT-PCR) referred to a kidney transplant center | Kidney transplant center, Madrid, Spain | 15 March to 24 April; follow-up to 19 May | 29 (29 recovered to discharge or died) |
|
|
|
|
|
| Pascual et al.13 | 19 June | Inpatients with confirmed COVID-19 (RT-PCR) within 60 d of kidney transplantation, entered to COVID-19 Registry of Spanish Society of Nephrology | 12 transplant centers, Spain | 17 March to 18 April | 24 (of 265 transplants within 60 d) (do not specify how many recovered to discharge) |
|
|
|
|
|
| Chen et al.27 | 23 June | Inpatients with confirmed COVID-19 (RT-PCR) in addition to radiographic evidence | Single center, New York City, United States | 18 March to 10 April | 30 (29 recovered to discharge or died) |
|
|
|
|
|
| Mehta et al.28 | 23 June | Attendees to ED with confirmed COVID-19 (RT-PCR) of 44 who reported symptoms to an outpatient monitoring system | Kidney transplant center, New York City, United States | 15 March to 12 April | 34 (33 recovered to discharge or died) |
|
|
|
|
|
| Bossini et al.25 | 6 July | Symptomatic inpatients and outpatients assessed either in ED or clinic with confirmed COVID-19 (RT-PCR) | Kidney transplant outpatient center and 3 admitting hospitals, Brescia, Italy | 1 March to 16 April | 53 (45 hospitalized; 42 recovered to discharge or died) |
|
|
Inpatients only:
|
Inpatients:
|
|
| Cravedi et al.17 | 10 July | Inpatients with confirmed COVID-19 (RT-PCR) participating in the TANGO consortium (www.tangoxstudy.com) (excluded patients included in prior publications) | 12 transplant centers across the United States (5), Italy (4), and Spain (2) | 2 March to 15 May | 144 (do not specify how many recovered to discharge) |
|
|
|
|
|
| Chaudhry et al.26 | 12 July | All inpatient and outpatient SOT recipients with confirmed COVID-19 (RT-PCR) | 5 hospitals within a quaternary care academic institution, Michigan, United States | 20 March to 18 April | 38 (26 hospitalized)b |
|
|
|
||
| Pérez-Sáez et al.14 | 12 July | Inpatients identified through national COVID-19 registry with confirmed COVID-19 (RT-PCR) who received tocilizumab based on individual hospital protocols for increased disease severity. All patients had at least one of the following: increased IL-6; increase in other inflammatory markers; rapidly progressive ARDS. Additional patients were identified after contacting centers | 29 hospitals, Spain (27 completed request for additional data) | Up to 9 May (follow-up to 15 May) | 80 (of 468 included in the registry) (80 recovered to discharge or died) |
|
|
|
|
|
| Demir et al.24 | 13 July | Inpatients and outpatients with confirmed COVID-19 (RT-PCR) | 5 transplant centers, Istanbul, Turkey | 1 February to 4 May (followed-up for at least 15 d) | 44 (1 excluded as “without typical findings”, 3 lost to follow-up) (39 hospitalized - do not specify how many recovered to discharge) |
|
|
|
|
|
| Lubetzky et al.22 | 17 July | Consecutive inpatients and outpatients with confirmed COVID-19 (RT-PCR) | Transplant center, New York, United States | 13 March to 20 April | 54 (39 hospitalized; 37 recovered to discharge or died) |
|
All patients:
|
Inpatients:
|
|
Inpatient:
|
| Bell et al.15 | 21 July (posted) | Notified confirmed COVID-19 as identified through the Scottish Renal Registry through linkage to Health Protection Scotland | Scotland, UK (100% patient- and unit-level coverage) | Up to 31 May | 24 (of 3286 functioning kidney transplants) |
|
|
|||
| Mohamed et al.20 | 31 July | Consecutive inpatients and outpatients with confirmed COVID-19 (RT-PCR) | Kidney transplant center, London, UK | Up to end of April | 28 (of 1434 functioning transplants) (25 hospitalized – 25 recovered to discharge or died); comparison with 32 patients active on transplant waiting list (of 321) (14 hospitalized) |
|
|
|
|
|
| Kates et al.10 | 7 August | Any inpatient or outpatient SOT recipient with confirmed COVID-19 (RT-PCR) reported through an electronic case report form | >50 transplant centers, >98% United States | 7 March to 15 April; all cases followed-up for 28 d | 318 kidney-only or kidney-pancreas recipients |
|
|
|
|
|
| Benotmane et al.29 | 10 August | Consecutive inpatients with COVID-19 diagnosed by RT-PCR or typical CT chest lesions | Kidney transplant center, Strasbourg, France | 4 March to 7 April; followed-up to 13 May | 40 |
|
|
|
|
|
| Ravanan et al.11 | 11 August | SOT recipients with functioning graft as of 1 February with notified COVID-19 (RT-PCR) as identified through the NHS Blood and Transplant registry with linkage to Public Health England and the NHS Digital Tracing Service | England, UK | 1 February to 20 May | 489 (of 33,972 kidney-only or kidney-pancreas recipients); compared with 188/4241 patients active on the waiting list |
|
ACEI/ARB, angiotensin-converting-enzyme inhibitor/angiotensin II-receptor blocker; AKI, acute kidney injury; AMR, antibody-mediated rejection; ARDS, adult respiratory distress syndrome; ATG, anti-thymocyte globulin; AZM, azithromycin; CAKUT, congenital abnormality of the kidneys and urinary tract; CI, confidence interval; CKD, chronic kidney disease; CNI, calcineurin inhibitor; COPD, chronic obstructive pulmonary disease; COVID-19, Coronavirus Disease 2019; CQ, chloroquine; CRP, C-reactive protein; CT, computed tomography; CXR, chest x-ray; DGF, delayed graft function; DRV/r, darunavir/ritonavir; DVT, deep vein thrombosis; ECMO, extracorporeal membrane oxygenation; ED, emergency department; ESRD, end-stage renal disease; GI, gastrointestinal; GN, glomerulonephritis; HCQ, hydroxychloroquine; HIV, human immunodeficiency virus; ICU, intensive care unit; IHD, ischaemic heart disease; IL-6, interleukin-6; IQR, interquartile range; IS, immunosuppression; LDH, lactate dehydrogenase; LOS, hospital length of stay; LPV/r, lopinavir/ritonavir; MMF, mycophenolate mofetil; MP, methylprednisolone; MPA, mycophenolic acid; mTORi, mammalian target of rapamycin inhibitor; NHS, National Health Service; NIV, non-invasive ventilation; OR, odds ratio; PKD, polycystic kidney disease; RCT, randomized controlled trial; RRT, renal replacement therapy; RT-PCR, reverse transcriptase polymerase chain reaction; SD, standard deviation; SOT, solid organ transplant; TCMR, T-cell mediated rejection; ULN, upper limit of normal; WCC, white cell count.
Note: Mohamed et al.20 overlaps with Ravanan et al.11; Pereira et al.21 overlaps with Lubetzky et al.22; Pascual et al.13, Pérez-Sáez et al.14, ± Rodriguez-Cubillo et al.23 overlap with Sánchez-Álvarez et al.12 Studies may overlap with Kates et al.10
Data reported as percentages of a total 868 patients receiving RRT.
Unclear how many patients were discharged, as authors reported 26 discharged but also reported 7 died and 6 remained ventilated.
Antimetabolite reported withheld in 40 of 40, but only 36 of 40 were on MMF at baseline.
Data available for 27 of 28 patients (missing for 1 patient).
Patient Characteristics
Of the studies with available patient demographic data, average age ranged from 45 years in Demir et al.24 to 66 years in Rodriguez-Cubillo et al.23 The percentage of male patients ranged from 46% in Pascual et al.13 to 79% in Bossini et al.25
Clinical Presentation
Fever was common in studies, ranging from 52% to 95%; cough ranged from 49% to 78%, and dyspnea from 28% to 70%. Gastrointestinal symptoms were also reported, as high as 53% in Chaudhry et al.26 Four studies reported data on acute kidney injury or graft dysfunction at presentation, ranging from 28% in Mohamed et al.20 to 77% in Chen et al.27 No report described asymptomatic infection.
Baseline Immunosuppression Adjustment
A wide range of approaches was taken to adjust immunosuppression both between and within the studies. The dominant practice across other studies was to favor withholding or reducing antiproliferative drugs or mammalian target of rapapmycin inhibitor over reduction in calcineurin inhibitor dose.
COVID-19 Therapy
Thirteen studies reported the use of COVID-19 therapies. Hydroxychloroquine was used in 11 studies, either alone or in combination with another therapy, with the proportion of patients receiving the drug ranging from 38% to 100%. Ten studies either started corticosteroid therapy or increased dosage. High-dose corticosteroid was reported in 6 studies for between 4% and 62% of patients. Remdesivir use was reported in only a few studies, in a small number of patients. Eleven studies reported the use of an anti–interleukin-6 therapy. Pérez-Sáez et al.14 described the experience of tocilizumab use based on inpatient cases entered to the Spanish Society of Nephrology registry.
Outcomes
Inpatient mortality was calculated where possible as a proportion from patients with completed outcomes (i.e., death or recovery to discharge), with the intention of reducing bias by misclassification of patients who remain hospitalized but may die later. In the largest national registry reports, mortality was 43% among mostly inpatients with completed outcomes in Sánchez-Álvarez et al.12 from Spain up until April 11, and 26% in mostly inpatients reported by Ravanan et al.11 from England up until May 20, although the number of patients who may subsequently recover from both studies was not known. In the largest multicenter series, Cravedi et al.17 reported 32% mortality in 144 inpatients, although it did not specify how many recovered to discharge, whereas Kates et al.10 reported 28-day mortality as 18% from its dataset in which 254 of 318 patients were hospitalized. Of other studies from which inpatient mortality could be calculated, mortality was 7 of 39 patients (18%) in Lubetzky et al.22 across New York, 6 of 29 patients (21%) in both Rodriguez-Cubillo et al.23 in Madrid and Chen et al.27 in New York City, 15 of 42 patients (36%) in Bossini et al.25 in Brescia, and 9 of 25 patients (36%) Mohamed et al.20 in London.
Cravedi et al.17 found 7% increased mortality in people aged >60 years compared with those aged ≤60 years. In subgroup reports from the national registry in Spain, Pascual et al.13 reported 46% mortality among patients within 60 days of transplantation, whereas Pérez-Sáez et al.14 reported 33% mortality among patients with severe COVID-19 treated with tocilizumab.
The proportion of patients requiring intensive care was variable, from 9% of inpatients across Spain according to early registry data,12 to 42% of inpatients in the large multicenter series report by Kates et al.10 including 34% requiring mechanical ventilation.10 Acute kidney injury was common, ranging from 41% in Kates et al.10 with 13% requiring extracorporeal renal replacement therapy, to 53% in Mehta et al.,28 although none required renal replacement therapy. There were very few reports of acute rejection, with only 1 case of 318 in Kates et al.10 Benotmane et al.29 reported RNAemia in 26% and seropositivity in 100% of survivors.
Discussion
Our systematic review of the early literature up to August 11, 2020, suggests that kidney transplant recipients hospitalized with COVID-19 experience poor outcomes, especially in the early post-transplant period.
The limitations of the literature so far require appreciation. Over time, published studies evolved from reports from a small number of centers, to larger multicenter studies and national registries. To reduce bias by smaller studies, we reported only those studies with completed outcomes for at least 20 kidney transplant recipients with confirmed COVID-19. Small studies may be more likely to be published by centers who accumulate more complex or unwell patients or by centers who are affected particularly unfavorably, both of which may introduce important bias; they may also exert a period effect, reflecting more overwhelming circumstances in the earlier stages of the pandemic.
Variation in reported mortality also could be due to strong period effects, with differences in thresholds for hospitalization, availability of resources, and management practices. Data from Sánchez-Álvarez et al.12 found mortality of 43% from the Spanish Society of Nephrology COVID-19 registry in which 94% were inpatients. However, this is likely to be exaggerated as this was an early report based on data reported up to April 11, and approximately 147 patients remained alive but not yet recovered so were not included in our mortality calculation; fewer patients were admitted to intensive care unit compared with other reports which also may be an important period effect related to stretched resources.
The other large national registry report was Ravanan et al.,11 who identified all solid organ transplant recipients from the National Health Service Blood and Transplant and linked this to confirmed COVID-19 cases through Public Health England and the National Health Service Digital Tracing Service. Of more than 30,000 prevalent kidney or kidney-pancreas recipients in England, there were 489 cases of COVID-19 of whom 128 died (26%) up to May 20.11 This was compared with deaths in 18 of 188 (10%) patients waitlisted for transplantation but this comparison should be treated with caution, as many of these will have been in-center hemodialysis patients with more access to testing for milder or asymptomatic disease than transplant recipients in the community during the period of study. It was not possible to distinguish inpatients and outpatients from the available data sources, but as data were collected up to May 20, most cases were likely to be inpatients.
Large multicenter series were published by Cravedi et al.17 and Kates et al.10 Cravedi et al.17 reported data for inpatients with COVID-19 from centers in Italy, Spain, and the United States already participating in the TANGO consortium, an international network formed initially to investigate the recurrence of glomerular disease after transplantation. Kates et al.10 reported outcomes from a registry hosted by the University of Washington to which cases were submitted from >50 centers, >98% of which were from United States after invitations through the American Society of Transplantation and American Society of Transplant Surgeons.10 Entered data were not independently verified and its representativeness is uncertain as the extent to which cases from participating centers were systematically submitted is not known, with the authors acknowledging susceptibility to bias.
Studies from the United States reported high proportions of black patients, although no study investigated for associations between ethnicity or other socioeconomic factors with outcomes. From all solid organ transplant recipients in England, 38 of 129 Asian recipients (30%) and 27 of 95 (28%) black recipients died, compared with 79 of 334 white recipients (24%). In total, 2.4% of Asian recipients and 3.6% of black recipients have been diagnosed with COVID-19, compared with 1.0% of white recipients.11
The withdrawal of antiproliferative drugs such as mycophenolate, an inhibitor of T- and B-cell proliferation, was practiced almost universally, in keeping with expert consensus for even mild disease.30,31 Our review highlighted the myriad of different management strategies used in different centers, including antivirals, hydroxychloroquine, corticosteroids, and tocilizumab. Establishing the effectiveness of therapies requires well-designed clinical trials; as high-risk patients, kidney transplant recipients may benefit from both prophylactic and therapeutic trials. The RECOVERY trial demonstrated mortality benefit in treatment with dexamethasone 6 mg daily for patients with COVID-19 requiring oxygen in June 2020, with the World Health Organization consequently recommending the use of systemic corticosteroids in severe and critical cases.6,32 The RECOVERY trial has stopped recruiting patients to its lopinavir/ritonavir arm because of lack of benefit.33 Few patients in studies were reported to have been treated with remdesivir.
Acute kidney injury was seen in several studies, although not all studies reported their definition and alternative terminology such as “renal failure” was also mentioned. Acute kidney injury is not uncommon in COVID-19, and its pathophysiology remains uncertain, but direct parenchymal infection and microangiopathy mediated by complex inflammatory processes have been suggested.34 In transplant kidneys, there may be additional mechanisms, such as acute rejection from underimmunosuppression, or calcineurin inhibitor toxicity through drug-drug interactions (e.g., lopinavir/ritonavir); however, there were few reports of acute rejection in our review, but there may have been less investigation for this because of unwillingness to augment immunosuppression if it were diagnosed; we did not identify any histopathological series; and case reports of biopsies are prone to bias so systematic cross-sectional or longitudinal study designs would need to be considered. Studies in the coming months and years will need to address the longer-term impact of COVID-19 on graft function and permanent graft loss.
Pascual et al.13 reported deaths in almost half the patients in Spain who acquired COVID-19 within 60 days of transplantation (11 of 24 [of 265 transplants in total]) up to April 18. Nearly all were recipients of deceased donor transplants with delayed graft function reported in half, but the authors did not report whether SARS-CoV-2 infection was acquired in health care settings or in the community. Although these findings are alarming, it will also have been affected by period effects from the start of the pandemic and there may now be opportunities to better minimize transmission in acute transplantation through planning and infrastructure. Several deceased- and live-donor programs were suspended with the aim of preventing high-risk patients acquiring SARS-CoV-2 infection perioperatively, limiting the use of lymphocyte-depleting antibodies as induction agents or in treatment of severe rejection, and avoiding use of limited inpatient resources. As services are restored, outcomes should be audited closely, and the risks and benefits should be nevertheless considered on an individualized basis given the apparent increased risk with heightened immunosuppression.
As the pandemic continues, we will need to use more systematic national and international registries with appropriate control groups and linkage to other sources, such as community test results and hospital records, to allow timely, large-scale analyses that can better inform policies and practices. Serological surveys of transplant recipients using validated antibody assays could be valuable in capturing the prevalence of asymptomatic and mild infection that did not result in inpatient admission or community viral RNA testing; such surveys will be important to obtain more accurate mortality and hospitalization estimates, follow-up potential longer-term complications, and identify the factors that are associated with more favorable outcomes.
Limitations
In the absence of available data, we were unable to undertake time-to-event analysis, therefore we report mortality as a proportion of patients with completed outcomes to avoid misclassification of patients who remain hospitalized but may die after surviving the study period. However, this might be biased in the opposite direction if most of those who remained hospitalized were patients who were slowly recovering and more likely to survive.
Our searches included studies in English or Chinese only, with studies meeting inclusion criteria from France, Italy, Spain, Turkey, the United Kingdom, and the United States only. Searches in more languages may have resulted in a broader perspective, including more experiences in middle-income economies. As well as studies from Europe and North America, there were studies published from China and Iran from early in the pandemic that did not fulfill our criteria for inclusion because of smaller numbers of confirmed cases. It would be beneficial to obtain updated reports from centers such as these, as well as others in Africa, Asia, and Latin America.
The Newcastle-Ottawa Quality Assessment Scale was of limited value in objective quality assessment. It is designed to be semi-quantitative, but the crude equivalence of all the constituent assessment items may be misleading.
Conclusion
Our review of the literature in the early phase of the COVID-19 epidemic suggests that hospitalized kidney transplant recipients with COVID-19 are at a high risk of death. The quality of observational data is improving. Detailed and comprehensive data collection through registries and linkage with health records will be necessary to conduct analyses of risk factors for adverse outcomes, not least given the risks of stopping immunosuppression. Indeed, to optimize clinical care, we should ensure that nonhospitalized patients are included and existing registries are supported and commissioned to answer important questions that affect screening and management.
We are reassured that we have developed a reproducible search strategy that can be effectively redeployed at appropriate intervals during the pandemic and beyond to be able to conduct meta-analyses of accumulating data in the future.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We are grateful to the authors of Manganaro et al.,16 Kates et al.,10 and Mohamed et al.20 for providing additional data and clarifications as requested.
Insilica, LLC, owners of Sysrev, gifted the review team a grant worth $2000 total to support screening and data extraction. This research did not receive any other specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary Material S1. Initial search. Tables S1 and S1.1–S1.4 and Appendices S1.5–S1.8.
Supplementary Material S2. Updated searches. Tables S1 and S2.1, Appendices S2.2 and S2.3, and Table S2.4.
Supplementary Material S3. Example data request.
Table S4. List of reports of ≥5 kidney transplant recipients by country and center.
Supplementary Material
Supplementary Material S1. Initial search. Tables S1 and S1.1–S1.4 and Appendices S1.5–S1.8.
Supplementary Material S2. Updated searches. Tables S1 and S2.1, Appendices S2.2 and S2.3, and Table S2.4.
Supplementary Material S3. Example data request.
Table S4. List of reports of ≥5 kidney transplant recipients by country and center.
References
- 1.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller I.F., Becker A.D., Grenfell B.T., Metcalf C.J.E. Disease and healthcare burden of COVID-19 in the United States. Nat Med. 2020;26:1212–1217. doi: 10.1038/s41591-020-0952-y. [DOI] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phua J., Weng L., Ling L. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8:506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alhazzani W., Møller M.H., Arabi Y.M. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19—Preliminary Report [e-pub ahead of print]. N Engl J Med. https://doi.org/10.1056/nejmoa2021436 [DOI] [PMC free article] [PubMed]
- 7.Linares L., Cofán F., Cervera C. Infection-related mortality in a large cohort of renal transplant recipients. Transplant Proc. 2007;39:2225–2227. doi: 10.1016/j.transproceed.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 8.Richardson W.S., Wilson M.C., Nishikawa J., Hayward R.S. The well-built clinical question: a key to evidence-based decisions. ACP J Club. 1995;123:A12–A13. [PubMed] [Google Scholar]
- 9.Deeks J.J., Dinnes J., D’Amico R. Evaluating non-randomised intervention studies. Health Technol Assess (Rockv) 2003;7:1–173. doi: 10.3310/hta7270. iii–x. [DOI] [PubMed] [Google Scholar]
- 10.Kates OS, Haydel BM, Florman SS, et al. COVID-19 in solid organ transplant: a multi-center cohort study [e-pub ahead of print]. Clin Infect Dis. doi: 10.1093/cid/ciaa1097. Accessed September 9, 2020. [DOI]
- 11.Ravanan R., Callaghan C.J., Mumford L. SARS-CoV-2 infection and early mortality of waitlisted and solid organ transplant recipients in England: a national cohort study. Am J Transplant. 2020;20:3008–3018. doi: 10.1111/ajt.16247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sánchez-Álvarez J.E., Fontán M.P., Martín C.J. Status of SARS-CoV-2 infection in patients on renal replacement therapy. Report of the COVID-19 Registry of the Spanish Society of Nephrology (SEN) Nefrologia. 2020;40:272–278. doi: 10.1016/j.nefro.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pascual J., Melilli E., Jiménez-Martín C. COVID-19–related mortality during the first 60 days after kidney transplantation. Eur Urol. 2020;78:641–643. doi: 10.1016/j.eururo.2020.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pérez-Sáez M.J., Blasco M., Redondo-Pachón D. Use of tocilizumab in kidney transplant recipients with COVID-19 [e-pub ahed of print]. Am J Transplant. https://doi.org/10.1111/ajt.16192 [DOI] [PMC free article] [PubMed]
- 15.Bell S., Campbell J., McDonald J. COVID-19 in patients undergoing chronic kidney replacement therapy and kidney transplant recipients in Scotland: findings and experience from the Scottish renal registry. BMC Nephrol. 2020;21:419. doi: 10.1186/s12882-020-02061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manganaro M., Baldovino S., Besso L. First considerations on the SARS-CoV-2 epidemic in the Dialysis Units of Piedmont and Aosta Valley, Northern Italy. J Nephrol. 2020;33:393–395. doi: 10.1007/s40620-020-00732-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cravedi P., Mothi S.S., Azzi Y. COVID-19 and kidney transplantation: results from the TANGO International Transplant Consortium. Am J Transplant. 2020;20:3140–3148. doi: 10.1111/ajt.16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyarsky B.J., Chiang T.P.Y., Werbel W.A. Early impact of COVID-19 on transplant center practices and policies in the United States. Am J Transplant. 2020;20:1809–1818. doi: 10.1111/ajt.15915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vistoli F., Furian L., Maggiore U., Caldara R., Cantaluppi V. COVID-19 and kidney transplantation : an Italian Survey and Consensus. J Nephrol. 2020;33:667–680. doi: 10.1007/s40620-020-00755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohamed I.H., Chowdary P.B., Shetty S. Outcomes of renal transplant recipients with SARS-CoV-2 infection in the eye of the storm [e-pub ahead of print]. Transplantation. https://doi.org/10.1097/tp.0000000000003406 [DOI] [PubMed]
- 21.Pereira M.R., Mohan S., Cohen D.J. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20:1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lubetzky M., Aull M.J., Craig-Schapiro R. Kidney allograft recipients, immunosuppression, and coronavirus disease-2019: a report of consecutive cases from a New York City transplant center. Nephrol Dial Transplant. 2020;35:1250–1261. doi: 10.1093/ndt/gfaa154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez-Cubillo B., de la Higuera M.A.M., Lucena R. Should cyclosporine be useful in renal transplant recipients affected by SARS-CoV-2. Am J Transplant. 2020;20:3173–3181. doi: 10.1111/ajt.16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demir E, Uyar M, Parmaksiz E, et al. COVID-19 in kidney transplant recipients: A multicenter experience in Istanbul [e-pub ahead of print]. Transpl Infect Dis. https://doi.org/10.1111/tid.13371. Accessed August 15, 2020. [DOI] [PMC free article] [PubMed]
- 25.Bossini N., Alberici F., Delbarba E. Kidney transplant patients with SARS-CoV-2 infection: The Brescia Renal COVID task force experience. Am J Transplant. 2020;20:3019–3029. doi: 10.1111/ajt.16176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaudhry Z.S., Williams J.D., Vahia A. Clinical characteristics and outcomes of COVID-19 in solid organ transplant recipients: a cohort study. Am J Transplant. 2020;20:3051–3060. doi: 10.1111/ajt.16188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen T.Y., Farghaly S., Cham S. COVID-19 pneumonia in kidney transplant recipients: focus on immunosuppression management. Transpl Infect Dis. 2020;22:e13378. doi: 10.1111/tid.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta S.A., Leonard J., Labella P. Outpatient management of kidney transplant recipients with suspected COVID-19—Single-center experience during the New York City surge. Transpl Infect Dis. 2020:e13383. doi: 10.1111/tid.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benotmane I., Gautier Vargas G., Wendling M. In-depth virological assessment of kidney transplant recipients with COVID-19. Am J Transplant. 2020;20:3162–3172. doi: 10.1111/ajt.16251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kronbichler A., Gauckler P., Windpessl M. COVID-19: implications for immunosuppression in kidney disease and transplantation. Nat Rev Nephrol. 2020;16:365–367. doi: 10.1038/s41581-020-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maggiore U., Abramowicz D., Crespo M. How should I manage immunosuppression in a kidney transplant patient with COVID-19? An ERA-EDTA DESCARTES expert opinion. Nephrol Dial Transplant. 2020;35:899–904. doi: 10.1093/ndt/gfaa130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization Corticosteroids for COVID-19: living guidance, 2 September 2020. World Health Organization; 2020. License: CC BY-NC-SA 3.0 IGO. https://apps.who.int/iris/handle/10665/334125 Available at:
- 33.Griffin S. Covid-19: Lopinavir-ritonavir does not benefit hospitalized patients, UK trial finds. BMJ. 2020;370:m2650. doi: 10.1136/bmj.m2650. [DOI] [PubMed] [Google Scholar]
- 34.Batlle D., Soler M.J., Sparks M.A. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 2020;31:1380–1383. doi: 10.1681/ASN.2020040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.