Abstract
Background:
Vancomycin is a commonly used antibiotic, which requires therapeutic drug monitoring to ensure optimal treatment. Microsampling assays are attractive tools for pediatric clinical research and therapeutic drug monitoring.
Results:
A LC–MS/MS method for the quantification of vancomycin in human whole blood employing volumetric absorptive microsampling (VAMS®) devices (20 μl) was developed and validated. Vancomycin was stable in human whole blood VAMS under assay conditions. Stability for vancomycin was established for at least 160 days as dried microsamples at -78°C.
Conclusion:
This method is currently being utilized for the quantitation of vancomycin in whole blood VAMS for an ongoing pediatric clinical study and representative clinical data are reported.
Keywords: : human whole blood, LC–MS/MS, pediatrics, therapeutic drug monitoring, vancomycin, volumetric absorptive microsampling
Vancomycin is a glycopeptide antibiotic with excellent activity against Gram-positive bacteria. Due to the rising prevalence of methicillin-resistant Staphylococcus aureus (MRSA) infections, current clinical practice guidelines from the Infectious Diseases Society of America recommend its use in children with suspected serious infections such as bacteremia and meningitis without microbiological confirmation of the infection [1]. In hospitalized children, vancomycin is one of the most commonly administered antibiotics: in a point-prevalence survey involving 2647 patients across 31 pediatric hospitals, vancomycin was the second most commonly prescribed antibiotic in pediatric intensive care units and the third most commonly prescribed antibiotic in neonatal intensive care units [2].
Vancomycin has a narrow therapeutic index and exhibits AUC-dependent efficacy and nephrotoxicity [3]. In adults with MRSA infections, a ratio of the vancomycin AUC over 24 h (AUC24) to the MIC of the bacteria >400 is the therapeutic target associated with improved clinical outcomes [4–6]. In children, a 24-h AUC >800 mg·l/h is associated with an increased risk of acute kidney injury [3]. Nephrotoxicity occurs in up to 25% of vancomycin courses in children [7–9], which is associated with increased mortality and development of chronic kidney disease [10,11]. Considering the tenuous balance between vancomycin efficacy and toxicity, clinicians need reliable approaches to attain target AUC24 to maximize efficacy, minimize toxicity and prevent the development of resistance.
Therapeutic drug monitoring (TDM) is used to minimize drug toxicity and maximize therapeutic efficacy. Vancomycin trough concentrations (Cmin; levels obtained immediately prior to a dose) have traditionally been used as surrogate therapeutic targets for AUC24 [12]. However, guidelines from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America and the Society of Infectious Diseases Pharmacists now recommend AUC/MIC of 400–600 in all patients, including children [13]. Direct estimation of AUC24 is technically difficult, however, requiring multiple blood draws or the use of sophisticated Bayesian dose adaptation approaches. This use of an AUC-directed TDM approach will be challenging in pediatric patients owing to small blood volumes, limited availability of vascular access sites and the desire to avoid painful procedures in children.
Vancomycin concentrations are usually quantified in human plasma or serum and there are several validated LC–MS/MS methods reported for the analysis of vancomycin in these matrices [14–25]. Volumetric absorptive microsampling (VAMS®) is a promising approach [26] for quantitative analysis of drugs, especially in pediatrics where multiple collections of blood volume (1 ml per sample) represents a limitation in conducting clinical studies and routine TDM. VAMS devices have a sampling tip that absorbs the whole blood upon contact, either from blood collected in a tube or from the sampling site (finger or heel stick). They have a fixed volume that helps prevent overfilling when loaded appropriately [27]. VAMS devices enable the collection of fixed volume blood samples (10, 20 or 30 μl) and have been utilized for the quantitative analysis of drugs [28–30]. Other microsampling approaches, such as dried blood spot (DBS), have limitations such as the impact of hematocrit (HCT) on assay performance, variability in spot size and sample heterogeneity [31–35]. The VAMS approach eliminates or minimizes the HCT effect on accurate quantitation while enabling small sample volumes (10–30 μl).
In a recent study comparing the concentrations of vancomycin in DBS and plasma samples, the ratio of plasma to DBS concentrations was highly variable in clinical samples [36]. DBS samples from patients were collected by finger-prick application of one drop of blood to the collection paper, directly from the patient’s finger. While this is an excellent study, DBS is known to be impacted by HCT differences and variable sample volume compared with the fixed volume sample collection with VAMS. Three different correction methods evaluated by Scribel et al. [36] to estimate plasma vancomycin concentrations from DBS concentrations did not work well. Therefore, the prediction of plasma vancomycin concentrations from the DBS measurements were less reliable. A recent bridging study demonstrated the applicability of measuring vancomycin concentrations obtained by capillary microsampling [37]. Paired plasma samples, collected from capillary microsampling and conventional arterial plasma sampling, showed no significant bias. A strong correlation between both sampling methods suggests that capillary microsampling may serve as an alternative to the traditional sampling approach for measuring vancomycin concentrations in plasma.
Recently, Parker et al. [38] reported a vancomycin assay in dried plasma spots and plasma VAMS, and evaluated the effect of drying time on the recovery of vancomycin from plasma microsamples. This study demonstrated that the recovery of vancomycin extracted from dried plasma spots is affected by time, whereas plasma VAMS gave a relatively consistent recovery. Meanwhile, Youhnovski et al. [39] reported that impact assisted extraction (IAE) eliminated the HCT effect on analyte recovery for naproxen and ritonavir. The aim of the current study was to develop and validate a whole blood VAMS assay for the quantification of vancomycin in human whole blood by LC–MS/MS analysis. The quantitation of vancomycin concentrations in children will be helpful to clinicians to prompt dose adjustments that will improve safety and efficacy. The present study also describes the in vitro evaluation of the concentration of vancomycin in whole blood, plasma and whole blood VAMS, which provides further understanding of the blood to plasma partitioning of vancomycin. This is the first study to report a VAMS-LC–MS/MS vancomycin assay in human whole blood, which has been evaluated for the analysis of representative pediatric samples from an ongoing clinical study.
Materials & methods
Materials
Vancomycin hydrochloride was obtained from Toronto Research Chemicals (TRC, Toronto, Canada), atenolol was purchased from Sigma Aldrich (MO, USA) and d12-vancomycin was obtained from AlsaChim (France). All solvent and sample preparation was performed using nanopure water from a Synergy® UV-R system. Formic acid (98%), LC–MS-grade methanol and acetonitrile, and iso-propyl alcohol were obtained from EMD Millipore (MA, USA). The 1600 MiniG, 96-well plates Grenier Bio-One (NC, USA) and cap-mats used for IAE were purchased from SPEX SamplePrep (NJ, USA). VAMS devices (20 μl) were obtained from Neoteryx® (CA, USA). Blank pooled human whole blood was provided by BioIVT (NY, USA) or by healthy volunteers at the Children’s Hospital of Philadelphia (Institutional Review Board [IRB] protocol number 18-015852), which were used for all validation experiments. Eligible volunteers (employees, trainees or students) at the Children's Hospital of Philadelphia were approached for voluntary informed consent, which was obtained prior to any study related procedures being performed. Blood volume from participants may not exceed 50 ml or 3 ml/kg in an 8-week period. Each validation experiment required ≤20 ml of whole blood. If more than 20 ml was needed, multiple participants donated. A schematic of the origin for the blood used in the validation experiments and clinical sample analysis is shown in Figure 1.
Figure 1. . An overview of the origin of human whole blood utilized for assay validation and clinical sample analysis.
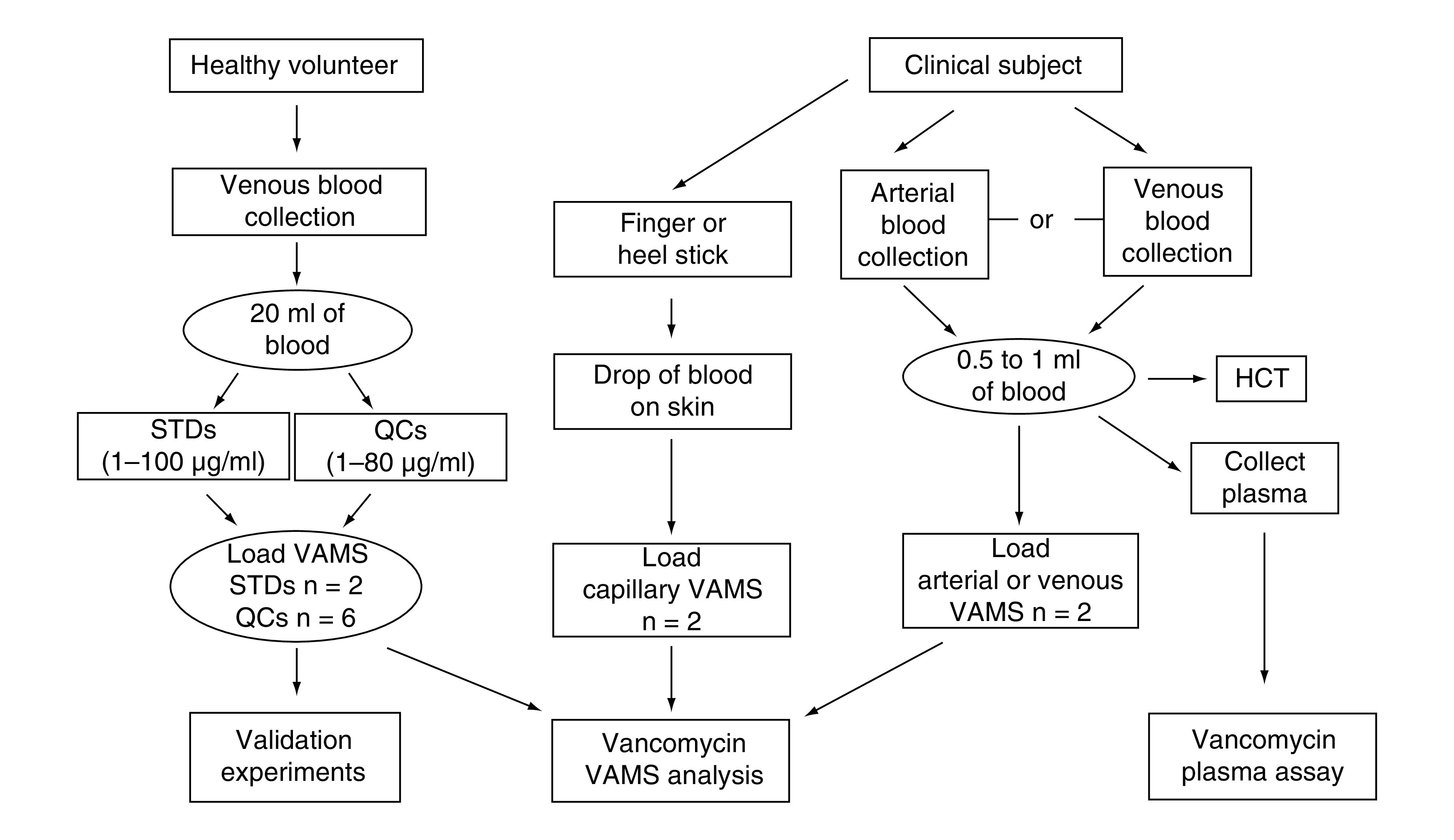
HCT: Hematocrit; QC: Quality control; STD: Standard; VAMS: Volumetric absorptive microsampling.
Stock solutions, calibration standards & quality controls
Two independent primary stock solutions of vancomycin hydrochloride were prepared at a concentration of 10 mg/ml in nanopure water for the preparation of calibration standards (STD) and quality control (QC) working solutions. Both primary stock solutions were prepared in amber glass vials and stored at -20°C. Two independent intermediate working solutions were spiked using fresh human whole blood at a concentration of 1.0 mg/ml and were left to equilibrate for 30 min at room temperature before continuing with further dilutions. The STD working solutions were prepared at concentrations of 1.0, 2.5, 5.0, 10, 25, 50, 75 and 100 μg/ml in human whole blood from the STD intermediate solution. QC working solutions were prepared by serial dilution at the concentrations of 1.0, 2.5, 40 and 80 μg/ml from the QC intermediate working solution. All working solutions were stored for up to 7 days at 4°C [38]. The primary stock solution of atenolol (internal standard) was prepared at 1 mg/ml in LC–MS-grade methanol in an amber glass vial and stored at -20°C when not in use. In order to prepare the final internal standard working solution (ISWS), an intermediate solution was prepared from the primary stock solution at a concentration of 100 ng/ml in methanol. A dilution of the intermediate solution was then prepared at a final concentration of 5 ng/ml in methanol. The primary stock solution for d12-vancomycin (the second internal standard) was prepared at 0.1 mg/ml in water in an amber glass vial and stored at -20°C when not in use. The final working solution concentration for d12-vancomycin was prepared at 100 ng/ml in methanol for sample analysis and both ISWS were stored at 4°C.
Sample preparation
Using the freshly prepared STD and QC working solutions in human whole blood, VAMS devices (20 μl, fixed volume) were loaded according to the instructions from Neoteryx [40,41]. Before the loading of each device, all working solutions were gently mixed using a Thermo Fisher Scientific Vortexer at an approximate speed of six followed by manually inverting each solution at least three to four-times. Previous studies using other antibiotics have shown that the compounds tend to separate quickly in whole blood [28]. All VAMS samples were loaded in sequential order according to the analytical run on the LC–MS/MS instrument submitted for analysis. For example, the first set of STDs were loaded in increasing order of concentration followed by each set of QCs, also in increasing order of concentrations, and then the final calibration curve to end the analytical batch. STDs and QCs loaded onto VAMS devices were placed in a plastic container with desiccants (Whatman®), covered in aluminum foil, and allowed to dry at room temperature for 3 h unassisted (Figure 2). The dried blood tips from the VAMS devices were removed from their plastic holder and placed into their corresponding wells of a 96-well polypropylene sample plate (Wheaton® Company) for extraction. Each loaded sample was reconstituted from its dried state using 50 μl of nanopure water, vortexed for 10 min at 1650 r.p.m. and then incubated at 37°C for 10 min. Next, 400 μl of the ISWS (5 ng/ml of atenolol in methanol) was added to the samples, except double blank samples, which received 400 μl of 100% methanol. Samples were vortexed (15 min at 800 r.p.m.), sonicated (15 min) and centrifuged (30 min at 4000 r.p.m. at 4°C). Each supernatant was transferred (350 μl) to a clean 96 well plate with a multichannel pipette for LC–MS/MS analysis.
Figure 2. . Sample preparation scheme for vancomycin human whole blood VAMS-LC–MS/MS assay.
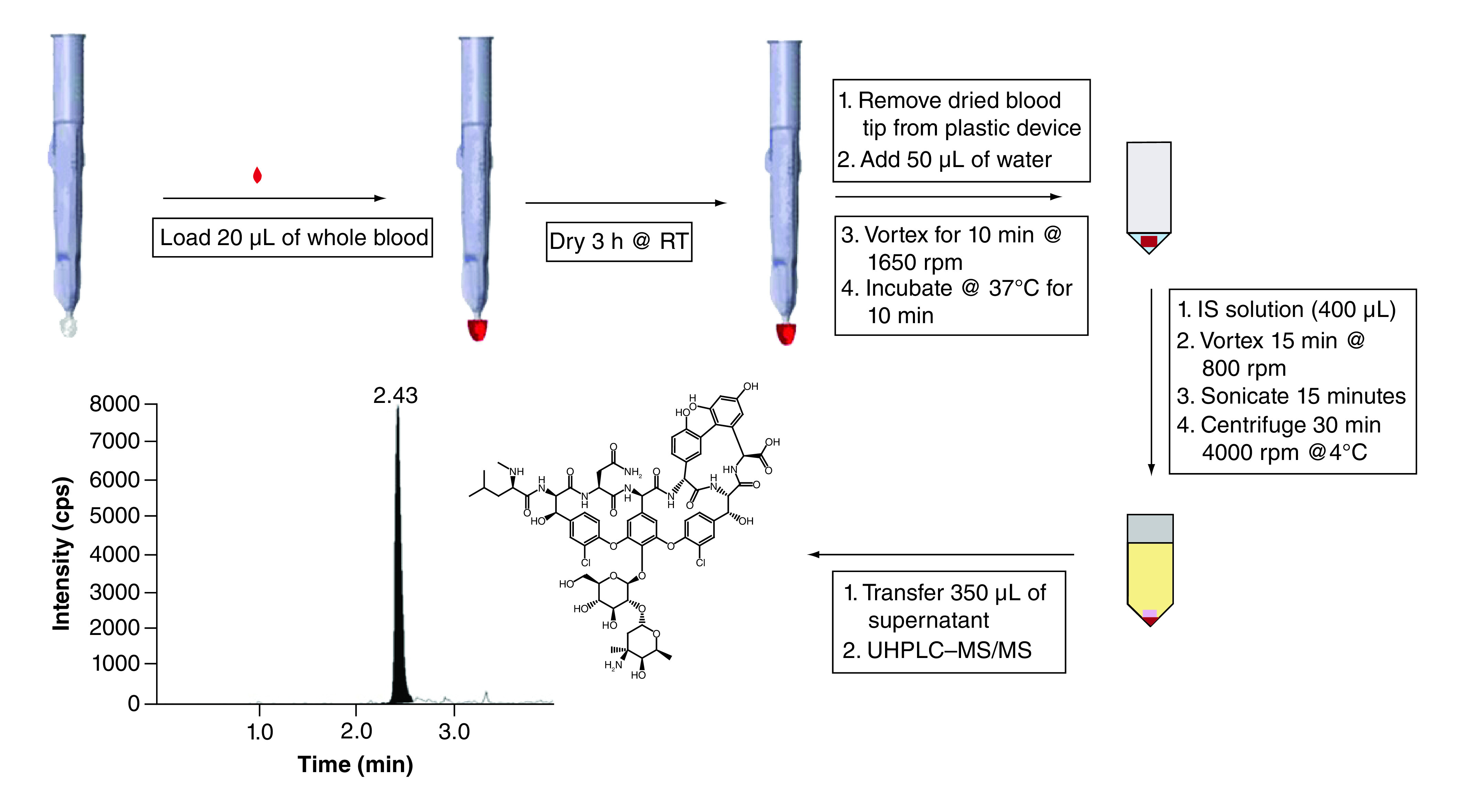
Alternatively, extracting with d12-vancomycin as the ISWS was used with an IAE. Samples were extracted in specific 96-well plates (Grenier Bio-One) and cap-mats both purchased from SPEX SamplePrep. After the addition of the 50 μl of nanopure water and incubation at 37°C for 10 min, a metal ball (from SPEX SamplePrep) was placed into each well. Four hundred microliters of 100 ng/ml d12-vancomycin in methanol was added to each well and the plate was placed in the MiniG for IAE. The samples were shaken vigorously for 15 min at 1200 r.p.m. The plate was removed from the MiniG and centrifuged for 30 min at 4000 r.p.m. at 4°C and 350 μl of each supernatant was transferred to clean wells of a 96-well plate for analysis on LC–MS/MS.
LC–MS/MS analysis
The analysis of vancomycin using atenolol or d12-vancomycin as the IS was performed using an Exion UHLPC coupled to a 6500+ QTrap mass spectrometer (AB Sciex, MA, USA). Vancomycin, d12-vancomycin and atenolol were infused at 1.0 μg/ml in a 1:1 (v/v) 0.1% formic acid in water: 0.1% formic acid in acetonitrile solution to optimize MS conditions. Protonated parent molecular and product ions for vancomycin (m/z 725.4 → 144.0 and 99.8), atenolol (m/z 267.2 → 145.0 and 190.1) and d12-vancomycin (m/z 731.4 → 112.4 and 144.0) were determined through an ESI source in the positive mode. The primary transitions were used for quantitation and the secondary transitions were used for confirmation. Declustering potential, collision energy, entrance potential and exit potential were optimized for vancomycin (57, 18, 9 and 10,), d12-vancomycin (57, 18, 9 and 10) and atenolol (45, 27, 11 and 20). The ion spray voltage (5000 V), curtain gas (35), GS1 (29), GS2 (24), collision gas (10) and source temperature (350°C) were used with nitrogen as the curtain, collision and nebulizer gas. Analyst software (version 1.6.3, AB Sciex) was used for data acquisition and processing.
Chromatographic separation was obtained employing a Kinetex Polar C18 column (100 Å, 2.6 μm, 50 × 2.1 mm) from Phenomenex (CA, USA). Mobile phase A (0.1% formic acid in water) and mobile phase B (0.1% formic acid in acetonitrile) were used at a flow rate of 0.200 ml/min to achieve optimal separation of vancomycin, atenolol and d12-vancomycin. The following gradient was used: 0.0–0.5 min at 2% B; 0.5–1.6 min increased to 40% B; maintained at 40% B until 2.20 min; 2.20–3.20 min increased to 98% B; maintained at 98% B until 3.90 min; 3.90–4.50 min returned to 2% B; and maintained at 2% B until 6.00 min. To reduce carryover, weak and strong washes were used as described previously [30].
Validation
The assay was validated for the quantitative determination of vancomycin in human whole blood VAMS. The 3-day validation was performed based on the US FDA guidance for the bioanalytical validation [42]. The following assessments were performed: linearity, sensitivity, accuracy, precision, selectivity/specificity, matrix effect, recovery, HCT effect, dilution integrity and stability. In addition, in vitro whole blood VAMS, whole blood and plasma comparison studies were performed as described previously [30].
Linearity & sensitivity
The linearity of the assay was evaluated with calibration standards ranging from 1.0 to 100 μg/ml. Linearity was assessed as previously described [7,30]: visual inspection of the calibration plot, the residuals plot and percent relative error (%RE) of back-calculated concentration plot. Standardized residual plot and back-calculated %RE plot were within the ±15 % limits. STD and QC working solutions were prepared from freshly collected human whole blood in lithium heparin tubes each day from healthy volunteers (IRB# 18-015852) who were not receiving vancomycin. Approximately 20 ml of blood was collected for each experiment (Figure 1). The LLOQ for this assay was 1.0 μg/ml. Concentrations of vancomycin in clinical samples collected from patients receiving vancomycin (IRB# 19-016476) are expected to be higher than the LLOQ of 1.0 μg/ml, so a lower range is not needed for analysis [18,43]. The sensitivity of the assay was determined by the signal-to-noise ratio at the LLOQ level.
Accuracy & precision
Four QC concentrations of 1.0, 2.5, 40 and 80 μg/ml of vancomycin in human whole blood (n = 6) were assessed by intra and inter day studies employing the VAMS devices. The intra day accuracies and precisions were calculated using a single analytical validation day run (n = 6 replicates at each QC concentration) for vancomycin and the inter-day accuracies and precisions were calculated within the 3-day validation run (n = 18 replicates at each QC concentration). Accuracy was calculated from the percentage of measured vancomycin concentration compared with the nominal concentration. Precision (% CV) was calculated from the ratio of the standard deviation to the mean of replicate measurements. Accuracies should be within ±15% of the theoretical value (±20% for the LLOQ) and precisions should be ≤15% (≤20% for LLOQ) to be acceptable for all validation studies.
Dilution integrity & carryover
A tenfold dilution integrity was evaluated at 600 μg/ml of vancomycin in human whole blood VAMS (n = 6). Samples were extracted as per the optimized sample preparation method. The extracted sample were diluted using extracts from single blanks (prepared from loaded blank whole blood VAMS devices) and then mixed well for analysis. Carryover was assessed in each analytical batch by placing two double blank samples after the highest calibration standard. After analysis, the peak area of the analyte and IS were compared with that of the LLOQ sample.
Selectivity & specificity
Six individual lots and a pooled lot of human whole blood from healthy volunteers (IRB #18-015852) were collected in lithium heparin tubes for the evaluation of both selectivity and specificity of the assay. The pooled lot was prepared from mixing an aliquot of each of the six individual lots together. Double blanks of each lot were loaded onto VAMS devices (n = 1) along with preparations of the LLOQ in each lot (1.0 μg/ml; n = 3). Each VAMS device was further extracted using the optimized method to ensure interference was absent at the retention times for vancomycin and atenolol (IS), as well as cross-interference between both.
Recovery & matrix effect
Recovery was evaluated from the ratios of the peak areas of extracted sample (vancomycin and atenolol) with double blank samples, which were post spiked in the final extracted matrix with the same concentrations of vancomycin and atenolol at three QC concentrations (n = 6). Matrix effect was evaluated by loading VAMS devices as double blanks using each of the individual lots (n = 6) and pooled lot (n = 1) of human whole blood collected from healthy volunteers. Each sample was post spiked at the low and high QC concentrations into the final extracted matrix. Comparison of the peak areas of the post spiked samples for vancomycin and atenolol to the peak areas of neat samples (n = 6, no matrix) at the same concentrations were assessed.
HCT effect
The HCT effect of vancomycin in human whole blood, VAMS and plasma was evaluated at low (21.7 %), normal (44.0 %) and high (57.8 %) HCT levels. Blood was collected from a healthy volunteer (IRB# 18-015852) with an HCT level of 44.0%. The low and high HCT levels were prepared from an aliquot of this blood sample by adding or removing the appropriate amount of plasma to achieve the correct HCT level for analysis. An aliquot of each prepared HCT level was measured at the Translational Core Lab at the Children’s Hospital of Philadelphia to confirm HCT levels. Whole blood was spiked with 15.0 μg/ml of vancomycin at each HCT level. First, aliquots of human whole blood (20 μl; n = 6) were prepared and analyzed using whole blood vancomycin standards at normal HCT. Next, VAMS (20 μl; n = 6) were loaded with the same human whole blood samples and analyzed following the validated method. Finally, the remaining whole blood spikes were centrifuged, and the plasma was collected, aliquoted (20 μl; n = 6) and analyzed with freshly prepared plasma vancomycin calibration curves. Peak area ratios were compared with determine the recovery of vancomycin and the effect of HCT in whole blood, VAMS and plasma. Nonparametric statistical analysis (Wilcoxon signed-rank test) was performed to compare peak area responses for vancomycin in human whole blood, VAMS and plasma.
Stability
Stability was assessed by loading VAMS (n = 6) with fresh preparations of the low and high QC concentrations in human whole blood and dried for 3 h at room temperature. After the 3 h drying time, each set of QC samples were stored at designated temperatures (room temperature and -78°C) and assessed. Short-term stability for vancomycin was evaluated at room temperature for up to 12 days and long-term stability was evaluated at -78°C for up to 160 days. After the allotted time, samples were taken out of the plastic box and thawed at room temperature before processing. These samples were quantified with freshly prepared standards and QCs in whole blood employing VAMS. Post preparative stability was also evaluated by analyzing samples (in final extracted matrix) left in the autosampler of the instrument for 74 h at 10°C and re-analyzed.
In vitro comparison of VAMS, blood, & plasma concentrations
In vitro blood to plasma distribution of vancomycin was evaluated using human whole blood, VAMS devices loaded with human whole blood, and human plasma at low, medium and high QC levels. Fresh blood was spiked with vancomycin and left at room temperature for 30 min to allow for equilibration mixing every few minutes. Whole blood aliquots (20 μl) were processed according to the validated VAMS method. Using the same whole blood preparations, VAMS (20 μl) were loaded for full quantification (two calibration curves and six sets of QCs at all levels), dried for 3 h, and extracted according to the validated method. An aliquot of the remaining spiked human whole blood samples were centrifuged at 3400 r.p.m. for 15 min at 4°C to collect the plasma. Vancomycin concentrations in plasma samples were measured employing spiked plasma standards. Accuracies for vancomycin at all three concentration levels were compared in each matrix. Blood to plasma and VAMS to plasma ratios were calculated for the estimation of vancomycin concentrations in plasma when analyzed in whole blood or VAMS matrices. Hemolysis was not observed in this study.
Clinical samples
We are conducting a clinical study to compare simultaneous VAMS samples obtained from a finger stick, free flowing blood and plasma obtained from processing the whole blood from the free flowing sample. The principles outlined in the Declaration of Helsinki was followed for this study. The IRB at the Children’s Hospital of Philadelphia approved the protocol (IRB# 19-016476) and informed consent was received from all study participants. This validated method was evaluated for clinical sample analysis of three pediatric subjects. The body weight, intravenous infusion dose of vancomycin, sampling times and the site of sample collection are shown in Table 5. A blood sample was collected from an arterial line or a central venous catheter that was not utilized to administer the vancomycin dose. A part of the sample was loaded to VAMS devices (20 μl, n = 2) according to the optimized procedure (Section 2.3). VAMS samples were allowed to dry at room temperature for a minimum of 3 h, and stored at -78°C until analysis. The remainder of the blood was sent to the Hematology Laboratory for HCT measurements and Chemistry Laboratory at the Children's Hospital of Philadelphia for quantification of vancomycin in plasma. Additionally, a capillary VAMS sample (20 μl, n = 2) was collected by finger stick (within 5 min of arterial or venous sample), according to the manufacturer’s instructions and processed as described above. The measured values from the arterial/venous whole blood VAMS sample, capillary whole blood VAMS sample and plasma were compared.
Table 5. . Vancomycin concentrations measured in whole blood volumetric absorptive microsampling and plasma with estimated plasma concentrations from volumetric absorptive microsampling in pediatric subjects.
| Subject | Dose (mg/kg/dose) | Bodyweight (kg) | Sampling time† (minutes) | Sample collection | HCT (%) | Vancomycin concentration (μg/ml) | ||
|---|---|---|---|---|---|---|---|---|
| Measured whole blood VAMS | Estimated in plasma from VAMS | Measured in plasma | ||||||
| 1 | 15 | 8.8 | 44 | Venous | 24.0 | 21.4 | 29.8 | 29.9 |
| 42 | Capillary | ND | 22.5 | 31.3 | ND | |||
| 2 | 10 | 12 | 199 | Venous | 25.9 | 7.18 | 10.0 | 6.9 |
| 201 | Capillary | ND | 7.66 | 10.7 | ND | |||
| 3 | 20 | 40 | 54 | Arterial | 33.1 | 17.0 | 23.7 | 21.9 |
| 54 | Capillary | ND | 18.9 | 26.3 | ND | |||
After end of infusion.
HCT: Hematocrit; VAMS: Volumetric absorptive microsampling.
Results
Method development
Parker et al. [38], previously evaluated the recovery of vancomycin in human plasma employing 10 μl VAMS devices without an internal standard and showed that the recovery was about 80%. This plasma method was evaluated in initial experiments to extract vancomycin from whole blood VAMS devices where teicoplanin was used as the IS. However, teicoplanin showed large variability in extraction efficiency and resulted in nonlinear vancomycin/teicoplanin peak area ratios across the calibration range. Atenolol proved to be less variable when extracted with vancomycin as an internal standard using a protein precipitation method. D12-vancomycin, a stably labeled internal standard was also evaluated and compared with atenolol for the quantitation of vancomycin. The major product ion of both vancomycin and d12-vancomycin was m/z 144. But, a co-eluting interference peak was detected in the d12-vancomycin channel (731 → 144) that increased with increasing concentration of vancomycin. However, the alternate MRM transition of 731.4 → 112.4 for d12-vancomycin did not have the interfering peak, and provided good chromatography and quantitation for vancomycin with similar results to atenolol as an IS.
Several conditions were evaluated such as solvents, SPE and drying time assessments using the 20 μl VAMS devices. Different extraction solvents were evaluated to obtain the best recovery and clean extracts. A solution of 50% methanol with 0.1% formic acid showed the best recovery of vancomycin; however, samples were still visually unclean requiring filtration. This was likely due to the high percentage of water in the extraction solvent. Several SPE filtration plates were tested for possible sample clean-up options. Both the Strata® X and the Oasis® HLB plates showed good recovery and produced clean extracts. We also evaluated rehydration with nanopure water (50 μl) and 37°C incubation as previously reported [28,30] requiring no SPE or filtration. Results of this experiment showed an increase in recovery of vancomycin, adequate sensitivity at the lower end of the calibration range and clean supernatants eliminating the need for SPE filtration. Method development of vancomycin was also conducted by evaluating several columns for optimization of chromatography separation and sensitivity. The Phenomenex Kinetex Polar C18 100 Å, 2.6 μm (2.1 × 50 mm) column showed good peak shape, and separation of vancomycin, atenolol and d12-vancomycin. Several time points were assessed for drying time of the VAMS devices when sealed in a plastic box containing desiccant (Whatman®) and wrapped in aluminum foil. The time points were evaluated at 2, 3, 24, 48, 96 and 168 h at 1.0 and 100 μg/ml in whole blood using 20 μl VAMS. Results of this experiment showed insignificant differences in peak area between all time points, therefore 3 h was chosen to ensure VAMS devices were completely dried before sample processing or storage. Both extraction approaches using either atenolol as the IS with the original protein precipitation approach or d12-vancomycin as the IS with the IAE showed similar results. However, the IAE improved recovery of vancomycin by about 20%. Either approach can be used for quantitation of vancomycin in whole blood extracted from 20 μl VAMS.
Validation
Linearity & sensitivity
Calibration curves (n = 2 per analysis) ranged from 1.0–100 μg/ml for vancomycin in whole blood collected from a healthy volunteer employing VAMS devices for the 3-day method validation. The calibration curve was linear and reproducible across the concentration range. The mean correlation coefficient (r2) for vancomycin was ≥0.99 for each 3-day validation run employing linear regression (1/x2 weighting). The slope, y-intercept and r2 values (mean ± standard deviation) were 0.176 ± 0.0397, -(1.14 ± 0.350) × 10-2 and 0.997 ± 0.00104, respectively. The accuracies and % CV for calibration standards were 98.1–102%, and 3.90–9.75, respectively. Representative chromatograms of the double blank, single blank and LLOQ in whole blood VAMS are shown in Figure 3. The LLOQ for this assay is 1.0 μg/ml with a signal to noise ratio of >200.
Figure 3. . Representative chromatograms for vancomycin and internal standard.
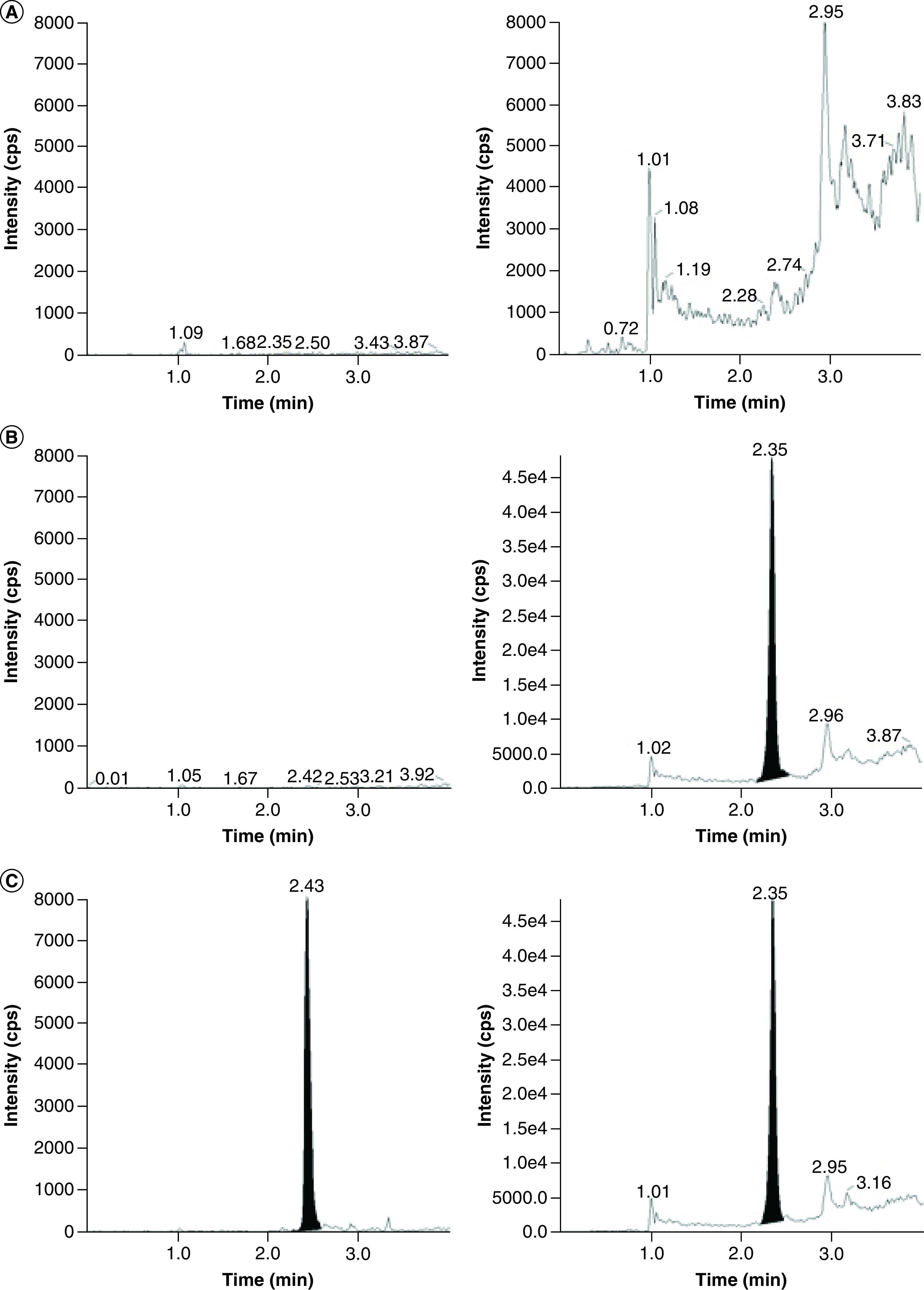
Vancomycin (left), and internal standard atenolol (right) in human whole blood VAMS for (A) double blank, (B) single blank and (C) LLOQ (1.00 μg/ml).
Accuracy & precision
The intra and inter day accuracies and the % CV for vancomycin in whole blood VAMS (n = 6) at QC levels of 1.0, 2.5, 40 and 80 μg/ml were evaluated (Table 1). Intra and inter day accuracies ranged from 90.3–112% with % CV ranging from 2.40–14.0 for all QC levels.
Table 1. . Summary of validation outcomes: intra and inter day accuracies and precisions for vancomycin in human whole blood volumetric absorptive microsampling.
| Conc. | Mean ± SD (μg/ml) | CV (%) | Accuracy (%) | Mean ± SD (μg/ml) | CV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|
| Day 1 (n = 6 replicates) | Day 2 (n = 6 replicates) | |||||
| 1.00 | 1.12 ± 0.129 | 11.5 | 112 | 0.959 ± 0.114 | 11.9 | 95.9 |
| 2.50 | 2.68 ± 0.288 | 9.91 | 107 | 2.48 ± 0.085 | 3.45 | 99.1 |
| 40.0 | 39.8 ± 0.955 | 2.40 | 100 | 38.4 ± 1.73 | 4.50 | 96.1 |
| 80.0 | 84.3 ± 10.0 | 11.9 | 105 | 88.5 ± 9.80 | 11.1 | 111 |
| Day 3 (n = 6 replicates) | Inter day (3 days, n = 18 replicates) | |||||
| 1.00 | 0.903 ± 0.082 | 9.09 | 90.3 | 0.993 ± 0.139 | 14.0 | 99.3 |
| 2.50 | 2.59 ± 0.253 | 9.74 | 104 | 2.59 ± 0.225 | 8.67 | 104 |
| 40.0 | 37.6 ± 1.57 | 4.16 | 94.0 | 38.6 ± 1.65 | 4.28 | 96.5 |
| 80.0 | 76.4 ± 3.76 | 4.92 | 95.4 | 83.0 ± 9.42 | 11.3 | 104 |
Dilution integrity & carryover
Dilution integrity (tenfold) was evaluated by loading VAMS (n = 6) with 600 μg/ml of vancomycin in human whole blood followed by post extraction dilution using single blanks extracts from VAMS loaded with blank human whole blood. The results demonstrated acceptable dilution integrity (85.3 ± 7.07%, n = 6). Signal for the vancomycin peak was <20% of the LLOQ in the single blank VAMS samples after the ULOQ standards throughout the entirety of the validation and no significant carryover was detected.
Selectivity & specificity
Human whole blood (six individual lots and a pooled lot) was obtained and loaded as double blanks (without analyte) onto VAMS devices. After 3 h of drying, all VAMS devices were extracted with methanol according to the validated method. The results of the peak area for vancomycin and atenolol (IS) indicated no interference at the retention times of the analyte and IS. Human whole blood (6 individual lots and a pooled lot) samples spiked with vancomycin at the LLOQ concentration (n = 3) were extracted using the optimized sample processing method then quantified against fresh STDs and QCs loaded onto VAMS devices. The accuracies of vancomycin at the LLOQ concentration for five out of six individual lots were within acceptable range (±20%) between 81.2 and 97.9%.
Recovery & matrix effect
The recovery (mean ± SD) of vancomycin from VAMS was evaluated at three QC concentrations (2.5, 40 and 80 μg/ml; n = 6) and were 14.5 ± 0.784, 17.4 ± 2.39 and 17.9 ± 0.531%, respectively. Recoveries for atenolol ranged from 98.2–101% with % CVs from 5.25–7.92%. Matrix effect in human whole blood (six individual lots and one pooled lot) at the low and high QC levels showed minimal matrix effect with internal standard-corrected (peak area ratio) values with average matrix factors of 82.8 ± 0.07 and 91.3 ± 0.02%, respectively, when extracted from VAMS devices. Non corrected (peak area) values for the matrix effect were 105 ± 0.05 and 109 ± 0.03% for the low and high QC concentrations, respectively. These results showed that vancomycin had consistent recovery across the assay range and had minimal matrix effect.
HCT Effect
The effect of HCT on the quantitation of vancomycin (15 μg/ml) in whole blood, whole blood VAMS and plasma was evaluated in vitro and the results are shown in Table 2. Whole blood and VAMS had similar and consistent peak area ratios across the three HCT levels with a slightly lower recovery from VAMS devices as the HCT levels increased. For whole blood and VAMS, as the HCT levels increased, the peak area ratios decreased. The opposite trend was seen for plasma, whereas the HCT levels increased, the peak area ratios also increased. At high HCT levels, the concentration of vancomycin was higher in the plasma samples (centrifuged from the spiked whole blood samples) compared with the whole blood or VAMS samples. At low versus normal HCT levels and low versus high HCT levels, the peak area response for vancomycin was statistically significant for whole blood, VAMS and plasma samples (p < 0.05). However, at normal versus high HCT levels, the peak area response for vancomycin was not statistically significant in whole blood, VAMS and plasma samples. The lower recovery in whole blood and VAMS devices were expected due to vancomycin’s high protein binding and association with plasma proteins.
Table 2. . The effect of hematocrit on the quantitation of vancomycin in whole blood, whole blood volumetric absorptive microsampling and plasma.
| Matrix | Average peak area ratio ± SD | ||
|---|---|---|---|
| Low HCT (21.7%) | Normal HCT (44.0%) | High HCT (57.8%) | |
| Whole blood | 3.34 ± 0.178 | 2.73 ± 0.200 | 2.79 ± 0.106 |
| VAMS | 3.70 ± 0.268 | 2.32 ± 0.147 | 2.07 ± 0.268 |
| Plasma | 5.72 ± 0.633 | 7.92 ± 1.03 | 9.55 ± 0.7881 |
HCT: Hematocrit.
Stability
Stability assessments of vancomycin (Table 3) were conducted using loaded VAMS devices at the low and high QC concentrations (n = 6). The following conditions were evaluated for stability: 12 days at room temperature, 52 days at -78°C and 160 days at -78°C. Results of the 12-day room temperature assessment showed adequate stability with accuracies of 88.9% (low QC) and 89.1% (high QC). Stability at -78°C for 52 days showed accuracies of 95.3% (low QC) and 88.4% (high QC). Accuracies for the 160 day stability at -78°C showed adequate stability at 91.5% (low QC) and 106% (high QC). Vancomycin was stable in the autosampler at 10°C for at least 74 h post extraction, showing accuracies ranging from 87.0 to 103% with % CVs ranging from 3.67 to 11.6% over all four QC concentrations.
Table 3. . Stability of vancomycin (n = 6) at room temperature and -78°C in human whole blood volumetric absorptive microsampling.
| Concentration (μg/ml) | %Accuracy ± % CV | ||
|---|---|---|---|
| 12 days at RT | 52 days at -78°C | 160 days at -78°C | |
| 2.50 | 88.9 ± 3.36 | 95.3 ± 7.08 | 91.5 ± 6.74 |
| 80.0 | 89.1 ± 11.1 | 88.4 ± 6.18 | 106 ± 8.88 |
In vitro comparison of VAMS, blood & plasma concentrations
Results are summarized in Table 4 for the in vitro comparison of vancomycin in wet whole blood, dried VAMS devices loaded with human whole blood and plasma. The whole blood and whole blood VAMS had similar vancomycin concentrations at the three QC levels. However, vancomycin concentrations in human plasma showed a 30–55% increase compared with the whole blood and VAMS. Blood to plasma and VAMS to plasma ratios for vancomycin were calculated from these results. The ratios for blood to plasma ranged from 0.689 to 0.707 and the VAMS to plasma ratios ranged from 0.644 to 0.767. The mean VAMS to plasma ratio for vancomycin was 0.718, which could be potentially useful in converting whole blood VAMS concentrations to estimate plasma vancomycin concentrations.
Table 4. . Vancomycin concentrations in whole blood, whole blood volumetric absorptive microsampling and plasma (n = 6).
| Vanc. conc. (μg/ml) | Whole blood | VAMS | Plasma | Blood to plasma ratio | VAMS to plasma ratio | |||
|---|---|---|---|---|---|---|---|---|
| Mean concentration ± SD (ng/ml) | % CV | Mean conc. ± SD (ng/ml) | % CV | Mean conc. ± SD (ng/ml) | % CV | |||
| 2.50 | 2.30 ± 0.327 | 13.8 | 2.56 ± 0.278 | 10.8 | 3.34 ± 0.274 | 8.20 | 0.689 | 0.767 |
| 40.0 | 38.4 ± 1.71 | 4.46 | 35.2 ± 1.37 | 3.89 | 54.6 ± 2.69 | 4.93 | 0.703 | 0.644 |
| 80.0 | 82.4 ± 8.03 | 9.75 | 86.7 ± 9.15 | 10.6 | 117 ± 8.17 | 7.01 | 0.707 | 0.744 |
VAMS: Volumetric absorptive microsampling.
Clinical samples
This method was used for vancomycin analysis in VAMS samples from three subjects following an intravenous dose of vancomycin (Table 5) and the representative chromatograms from subject 1 are shown in Figure 4. The results show that the assay is suitable for the analysis of vancomycin using the VAMS devices. Vancomycin concentrations quantified from the capillary whole blood (finger stick) VAMS and the arterial/venous whole blood VAMS samples had similar results. The arterial/venous whole blood VAMS vancomycin concentration was divided by the mean VAMS to plasma partitioning ratio (0.718) to provide an estimate of plasma vancomycin concentration (Table 5). The estimated plasma vancomycin concentrations for the arterial/venous samples was consistent with the measured plasma vancomycin concentration from the Central Lab in two out of three cases.
Figure 4. . Representative chromatograms for vancomycin and d12-vancomycin.
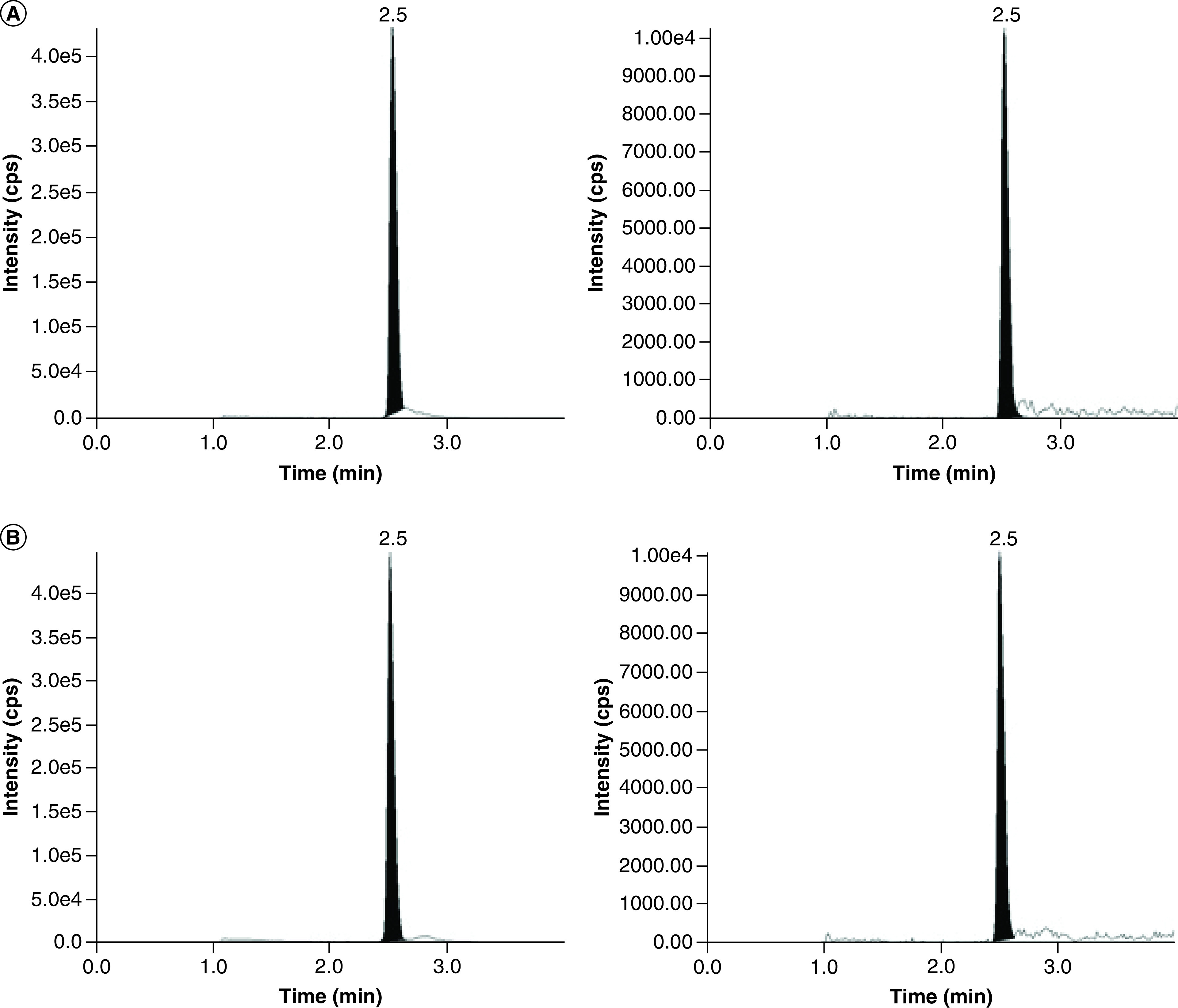
Vancomycin (left), and d12-vancomycin (right) in whole blood volumetric absorptive microsampling from a pediatric patient: (A) venous volumetric absorptive microsampling sample (21.4 μg/ml), and (B) capillary volumetric absorptive microsampling sample (22.5 μg/ml).
Discussion
TDM is crucial to ensure the optimal safety and effectiveness of vancomycin administration. Traditional vancomycin TDM has involved the measurement of trough levels, which has required a collection of a single plasma sample <30 min prior to the next anticipated dose. While it appears straightforward and relatively simple to perform, there are challenges for routine TDM in children. First, clinical care often takes precedence over the collection of blood samples and administration of medications at specific times. In an ideal scenario, vancomycin is consistently administered at regular intervals and the trough is drawn immediately prior to administration of the subsequent dose. However, this rarely occurs in normal clinical practice. Instead, vancomycin troughs are roughly drawn around the time that a true trough would be collected and results are interpreted as an approximation. More importantly, trough concentrations are used as a surrogate for AUC0–24. In adults with normal renal function receiving vancomycin every 12 h, trough levels of 15–20 mg/l approximate an AUC0–24 of 400 mg*h/l. However, in children, standard dosing intervals range from every 6–12 h depending on age and renal maturation. Thus, direct comparison of trough levels across various dosing intervals is not possible, and troughs do not approximate AUC0–24 well in children [44–46]. Because trough measurements reflect a single value taken immediately prior to the next dose, they are influenced by numerous factors including dose, dosing interval and timing of collection, as well as the individual’s pharmacokinetics (clearance and volume of distribution). As with many drugs, there is high variability in vancomycin PK in children and, thus, trough measurements are poor surrogates for AUC, which reflect the full concentration-time profile of a drug within an individual [47–49].
Vancomycin TDM guidelines now call for AUC-directed vancomycin therapy in all patients, and pediatric institutions are working to develop local practices to accommodate this new approach. Due to a paucity of robust population PK models of vancomycin in children to inform a Bayesian modeling approach, the use of a two-point pharmacokinetic estimation strategy may be necessary to estimate AUC0–24 in many pediatric patients. This will involve the collection of two samples for vancomycin concentration measurement during a single dosing interval and calculation of AUC0–24 using log-linear equations. The two-point strategy will be difficult in pediatrics for several reasons: children have small blood volumes and collection of numerous samples is discouraged, especially in neonates and infants; there is a general aversion to the performance of multiple painful procedures in children, such as venipuncture; collection of two timed samples during a brief 5-h window (assuming a 1-h infusion and dosing every 6 h) will be taxing for clinicians and/or phlebotomists, who are often responsible for collection of numerous samples throughout the hospital; and blood sampling from a central venous catheter is discouraged due to infection risks.
VAMS holds promise to improve vancomycin TDM. Through collection of a small volume of blood (20 μl) via finger – or heel-stick, many of the challenges of collecting plasma can be circumvented. Topical lidocaine can be applied in advance of the procedure to minimize pain, and collection of samples at specified times will be more feasible due to the relative ease of capillary sticks compared with venipuncture. The use of microsampling with VAMS holds promise to promote AUC estimation in pediatric patients and improve the safety and effectiveness of vancomycin administration.
Most clinical and pharmacokinetic studies are performed in human plasma, and it is of great interest to understand the ratios of vancomycin in human whole blood to plasma, and VAMS to plasma given the high protein composition of the plasma and the tendency of antibiotics to bind to plasma proteins. Recently the quantification of vancomycin in human whole blood using DBS was reported; however there are no published methods utilizing whole blood VAMS devices for vancomycin quantitation. Consistent with our results, a previous study showed that vancomycin was stable in DBS samples for 7 days at 45°C and 14 days at 22°C [36]. This allows time for VAMS samples to be stored at room temperature until a -78°C freezer is available for storage.
The results of the clinical samples reported in this manuscript are a part of an ongoing clinical study. We have included the results from three subjects to demonstrate the feasibility of sample collection, storage and analysis. A good correlation between the estimated plasma concentrations from the whole blood VAMS assay and measured vancomycin plasma concentrations from the TDM assay was observed in two out of three cases. The present method with limited clinical sample analysis demonstrates the feasibility of conducting a clinical validation [50], but a larger study is needed to formally evaluate the use of VAMS for clinical vancomycin concentration measurement. The ongoing clinical study aims to perform an in-depth comparison of vancomycin concentrations in arterial/venous VAMS, capillary VAMS and plasma samples collected concurrently. The in vitro comparison of VAMS, whole blood and plasma vancomycin concentrations is important as it provides a way to translate whole blood VAMS concentrations to plasma concentrations. Based on the initial results from three subjects (Table 5), prediction of plasma drug concentrations from the VAMS measurements has a potential utility. However, this approach should be used with caution as the in vitro VAMS to plasma ratios were derived from a single HCT value and actual HCT values may vary significantly between clinical samples from different subjects. Other clinical factors (site of sample collection, timing between whole blood and plasma sample collection) may also affect VAMS to plasma ratios. Additional analysis with a larger set of clinical samples is necessary.
Conclusion
A VAMS-LC–MS/MS assay for measuring vancomycin in small volumes (20 μl) of human whole blood was developed with VAMS devices. Method validation demonstrated good accuracy and precision of the assay range of 1–100 μg/ml. The validated method was evaluated for feasibility studies with representative clinical samples from an ongoing clinical study. With fixed volume sampling, VAMS was expected to provide accurate quantitation of vancomycin independent of HCT, compared with DBS. However, we have hypothesized that extreme HCT levels can affect the accurate quantitation of vancomycin in clinical samples based on the in vitro evaluation of HCT effect on the quantitation of vancomycin in whole blood, VAMS and plasma. The ongoing clinical study will provide further insight into the effect of HCT levels on vancomycin measurement in clinical VAMS whole blood microsamples. The established 160 day stability of vancomycin in VAMS at -78°C provides sufficient time to batch clinical samples for analysis. The present method is the first step toward developing an alternative sampling strategy to collect samples from neonates with heel pricks, and children and adults with finger pricks upon a successful clinical validation [50]. Microsampling with VAMS devices has numerous benefits and has the potential to be a valuable tool for pediatric clinical pharmacology studies.
Future perspective
Micosampling provides an alternative approach to traditional plasma sampling in pediatric clinical research and TDM. Enhanced sensitivity of modern instruments provide the capabilities to quantify low concentrations of drugs from small volume of clinical samples. VAMS provides several benefits to patients including the collection of small volumes of blood, reducing the risk for infection and alleviating anxiety with venipuncture blood draws. However, most drugs require establishment of a new therapeutic window utilizing whole blood VAMS to be implemented successfully for TDM. One of the major limitations of the dried whole blood microsampling approach is the inability to measure unbound drug concentrations, as the PK/PD of several drugs are well described by the unbound drug concentrations. Future advances in microsampling to measure plasma and unbound drug concentrations will be of great value for clinical pharmacology studies.
Summary points.
Background
The goal of this study was to develop, validate and evaluate a microsampling assay for vancomycin clinical sample analysis.
Experimental
Volumetric absorptive microsampling (VAMS) devices were extracted and analyzed for vancomycin quantitation (1.0–100 μg/ml) in dried whole blood.
Results
Intra and inter day accuracies were within 90.3–112% and precision (CV) was ≤14% based on a 3-day validation study.
Vancomycin was stable for 12 days at room temperature and 160 days at -78°C as dried microsamples.
Conclusion & future perspective
This assay provides an accurate quantitation of vancomycin in dried whole blood microsamples and has been evaluated for clinical sample analysis.
Additional clinical studies are required to establish the correlation between plasma and dried whole blood microsamples.
Footnotes
Financial & competing interests disclosure
KJ Downes has received research support from Merck & Co. Inc. and Pfizer, Inc. unrelated to the current work, and is supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number K23HD091365 and Children's Hospital of Philadelphia. Collection of clinical samples was performed by nurses from the CHOP Center for Human Phenomic Sciences (CHPS) and supported, in part, by the Penn/CHOP Institutional Clinical and Translational Science Award Research Center through NIH/NCATS (National Center for Advancing Translational Sciences) Grant UL1TR001878. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Liu C, Bayer A, Cosgrove SE. et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52(3), e18–e55 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Grohskopf LA, Huskins WC, Sinkowitz-Cochran RL. et al. Use of antimicrobial agents in United States neonatal and pediatric intensive care patients. Pediatr. Infect. Dis. J. 24(9), 766–773 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Le J, Ny P, Capparelli E. et al. Pharmacodynamic characteristics of nephrotoxicity associated with vancomycin use in children. J. Pediatric Infect. Dis. Soc. 4(4), e109–116 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung Y, Song KH, Cho J. et al. Area under the concentration-time curve to minimum inhibitory concentration ratio as a predictor of vancomycin treatment outcome in methicillin-resistant Staphylococcus aureus bacteraemia. Int. J. Antimicrob. Agents 43(2), 179–183 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Kullar R, Davis SL, Levine DP, Rybak MJ. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin. Infect. Dis. 52(8), 975–981 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Song KH, Kim HB, Kim HS. et al. Impact of area under the concentration-time curve to minimum inhibitory concentration ratio on vancomycin treatment outcomes in methicillin-resistant Staphylococcus aureus bacteraemia. Int. J. Antimicrob. Agents 46(6), 689–695 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Knoderer CA, Nichols KR, Lyon KC, Veverka MM, Wilson AC. Are elevated vancomycin serum trough concentrations achieved within the first 7 days of therapy associated with acute kidney injury in children? J. Pediatric Infect. Dis. Soc. 3(2), 127–131 (2014). [DOI] [PubMed] [Google Scholar]
- 8.McKamy S, Hernandez E, Jahng M, Moriwaki T, Deveikis A, Le J. Incidence and risk factors influencing the development of vancomycin nephrotoxicity in children. J. Pediatr. 158(3), 422–426 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Totapally BR, Machado J, Lee H, Paredes A, Raszynski A. Acute kidney injury during vancomycin therapy in critically ill children. Pharmacotherapy 33(6), 598–602 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Alkandari O, Eddington KA, Hyder A. et al. Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Crit. Care 15(3), R146 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menon S, Kirkendall ES, Nguyen H, Goldstein SL. Acute kidney injury associated with high nephrotoxic medication exposure leads to chronic kidney disease after 6 months. J. Pediatr. 165(3), 522–527 e522 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Martin JH, Norris R, Barras M. et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society Of Infectious Diseases Pharmacists. Clin. Biochem. Rev. 31(1), 21–24 (2010). [PMC free article] [PubMed] [Google Scholar]
- 13.Rybak MJ, Le J, Lodise TP. et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 77(11), 835–864 (2020). [DOI] [PubMed] [Google Scholar]; •• Dosing guidelines for vancomycin in adults and children.
- 14.Andriguetti NB, Lisboa LL, Hahn SR, Pagnussat LR, Antunes MV, Linden R. Simultaneous determination of vancomycin and creatinine in plasma applied to volumetric absorptive microsampling devices using liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 165, 315–324 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Barco S, Castagnola E, Gennai I. et al. Ultra high performance liquid chromatography-tandem mass spectrometry vs. commercial immunoassay for determination of vancomycin plasma concentration in children. Possible implications for everyday clinical practice. J. Chemother. 28(5), 395–402 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Bijleveld Y, de Haan T, Toersche J. et al. A simple quantitative method analysing amikacin, gentamicin, and vancomycin levels in human newborn plasma using ion-pair liquid chromatography/tandem mass spectrometry and its applicability to a clinical study. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 951–952, 110–118 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Chen F, Hu ZY, Laizure SC, Hudson JQ. Simultaneous assay of multiple antibiotics in human plasma by LC–MS/MS: importance of optimizing formic acid concentration. Bioanalysis 9(5), 469–483 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Javorska L, Krcmova LK, Solich P, Kaska M. Simple and rapid quantification of vancomycin in serum, urine and peritoneal/pleural effusion via UHPLC–MS/MS applicable to personalized antibiotic dosing research. J. Pharm. Biomed. Anal. 142, 59–65 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Konig K, Kobold U, Fink G. et al. Quantification of vancomycin in human serum by LC–MS/MS. Clin. Chem. Lab. Med. 51(9), 1761–1769 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Mei S, Wang J, Zhu L. et al. A UPLC–MS/MS method for analysis of vancomycin in human cerebrospinal fluid and comparison with the chemiluminescence immunoassay. Biomed. Chromatogr. 31(8), (2017). [DOI] [PubMed] [Google Scholar]
- 21.Oyaert M, Peersman N, Kieffer D. et al. Novel LC–MS/MS method for plasma vancomycin: comparison with immunoassays and clinical impact. Clin. Chim. Acta 441, 63–70 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Qin F, Wang Y, Wang L. et al. Determination of trantinterol enantiomers in human plasma by high-performance liquid chromatography - tandem mass spectrometry using vancomycin chiral stationary phase and solid phase extraction and stereoselective pharmacokinetic application. Chirality 27(5), 327–331 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Rossmann J, Schubert S, Gurke R, Oertel R, Kirch W. Simultaneous determination of most prescribed antibiotics in multiple urban wastewater by SPE-LC–MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 969, 162–170 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Tsai IL, Sun HY, Chen GY, Lin SW, Kuo CH. Simultaneous quantification of antimicrobial agents for multidrug-resistant bacterial infections in human plasma by ultra-high-pressure liquid chromatography-tandem mass spectrometry. Talanta 116, 593–603 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Zhang T, Watson DG, Azike C. et al. Determination of vancomycin in serum by liquid chromatography-high resolution full scan mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 857(2), 352–356 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Spooner N, Denniff P, Michielsen L. et al. A device for dried blood microsampling in quantitative bioanalysis: overcoming the issues associated blood hematocrit. Bioanalysis 7(6), 653–659 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Protti M, Mandrioli R, Mercolini L. Tutorial: volumetric absorptive microsampling (VAMS). Anal. Chim. Acta 1046, 32–47 (2019). [DOI] [PubMed] [Google Scholar]; •• A review of validation protocols for the quantitative determination of drugs in humans using VAMS.
- 28.Barco S, Castagnola E, Moscatelli A, Rudge J, Tripodi G, Cangemi G. Volumetric adsorptive microsampling-liquid chromatography tandem mass spectrometry assay for the simultaneous quantification of four antibiotics in human blood: method development, validation and comparison with dried blood spot. J. Pharm. Biomed. Anal. 145, 704–710 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Moorthy GS, Vedar C, Zane N, Prodell JL, Zuppa AF. Development and validation of a volumetric absorptive microsampling assay for analysis of voriconazole and voriconazole N-oxide in human whole blood. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1105, 67–75 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Moorthy GS, Vedar C, Zane NR. et al. Development and validation of a volumetric absorptive microsampling- liquid chromatography mass spectrometry method for the analysis of cefepime in human whole blood: application to pediatric pharmacokinetic study. J. Pharm. Biomed. Anal. 179, 113002 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antunes MV, Charao MF, Linden R. Dried blood spots analysis with mass spectrometry: potentials and pitfalls in therapeutic drug monitoring. Clin. Biochem. 49(13–14), 1035–1046 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Evans C, Arnold M, Bryan P. et al. Implementing dried blood spot sampling for clinical pharmacokinetic determinations: considerations from the IQ Consortium Microsampling Working Group. AAPS J. 17(2), 292–300 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guerra Valero YC, Wallis SC, Lipman J, Stove C, Roberts JA, Parker SL. Clinical application of microsampling versus conventional sampling techniques in the quantitative bioanalysis of antibiotics: a systematic review. Bioanalysis 10(6), 407–423 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Kok MGM, Fillet M. Volumetric absorptive microsampling: current advances and applications. J. Pharm. Biomed. Anal. 147, 288–296 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Velghe S, Delahaye L, Stove CP. Is the hematocrit still an issue in quantitative dried blood spot analysis? J. Pharm. Biomed. Anal. 163, 188–196 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Scribel L, Zavascki AP, Matos D. et al. Vancomycin and creatinine determination in dried blood spots: analytical validation and clinical assessment. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1137, 121897 (2020). [DOI] [PubMed] [Google Scholar]; •• Comparison of dried blood spot and plasma sampling for clinical vancomycin analysis.
- 37.Guerra Valero YC, Roberts JA, Lipman J. et al. Analysis of capillary microsamples obtained from a skin-prick to measure vancomycin concentrations as a valid alternative to conventional sampling: a bridging study. J. Pharm. Biomed. Anal. 169, 288–292 (2019). [DOI] [PubMed] [Google Scholar]; •• Comparison of capillary microsampling and conventional plasma sampling for vancomycin analysis.
- 38.Parker SL, Guerra Valero YC, Lipman J, Roberts JA, Wallis SC. Effect of time on recovery of plasma microsamples for the quantitative determination of vancomycin. Bioanalysis 8(21), 2235–2242 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Youhnovski N, Mayrand-Provencher L, Berube ER. et al. Volumetric absorptive microsampling combined with impact-assisted extraction for hematocrit effect free assays. Bioanalysis 9(22), 1761–1769 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Neoteryx. Blood Collection Kit (2019). https://cdn2.hubspot.net/hubfs/1806452/Content/Mitra_Product_Brochure.pdf For Reference
- 41.Neoteryx. How to Take a Sample: The Do's And Don'ts of Using The Mitra Microsampler (2019). https://www.neoteryx.com/how-to-properly-take-a-blood-sample-using-the-mitra-microsampler-vams For Reference
- 42.US FDA. Bioanalytical Methods Validation Guidance for Industry. (2018). https://www.fda.gov/media/70858/download
- 43.Ahsman MJ, Wildschut ED, Tibboel D, Mathot RA. Microanalysis of beta-lactam antibiotics and vancomycin in plasma for pharmacokinetic studies in neonates. Antimicrob. Agents Chemother. 53(1), 75–80 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frymoyer A, Guglielmo BJ, Hersh AL. Desired vancomycin trough serum concentration for treating invasive methicillin-resistant Staphylococcal infections. Pediatr. Infect. Dis. J. 32(10), 1077–1079 (2013). [DOI] [PubMed] [Google Scholar]
- 45.Gordon CL, Thompson C, Carapetis JR, Turnidge J, Kilburn C, Currie BJ. Trough concentrations of vancomycin: adult therapeutic targets are not appropriate for children. Pediatr. Infect. Dis. J. 31(12), 1269–1271 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Le J, Bradley JS, Murray W. et al. Improved vancomycin dosing in children using area under the curve exposure. Pediatr. Infect. Dis. J. 32(4), e155–163 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang D, Chiu NC, Chang L. et al. Vancomycin dosing and target attainment in children. J. Microbiol. Immunol. Infect. 50(4), 494–499 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Kishk OA, Lardieri AB, Heil EL, Morgan JA. Vancomycin AUC/MIC and corresponding troughs in a pediatric population. J. Pediatr. Pharmacol. Ther. 22(1), 41–47 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maloni TM, Belucci TR, Malagutti SR, Furtado GHC. Describing vancomycin serum levels in pediatric intensive care unit (ICU) patients: are expected goals being met. BMC Pediatr. 19(1), 240 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Capiau S, Veenhof H, Koster RA. et al. Official International Association for Therapeutic Drug Monitoring and Clinical Toxicology Guideline: development and validation of dried blood spot-based methods for therapeutic drug monitoring. Ther. Drug Monit. 41(4), 409–430 (2019). [DOI] [PubMed] [Google Scholar]; •• Guidelines for validation of dried blood spot assays for therapeutic drug monitoring.


