Abstract
Myopia has become a major cause for concern globally, particularly in East Asian countries. The increasing prevalence of myopia has been associated with a high socioeconomic burden owing to severe ocular complications that may occur with progressive myopia. There is an urgent need to identify effective and safe measures to address the growing number of people with myopia in the general population. Among the numerous strategies implemented to slow the progression of myopia, longer time spent outdoors has come to be recognized as a protective factor against this disorder. Although our understanding of the protective effects of outdoor time has increased in the past decade, considerably more research is needed to understand the mechanisms of action. Here, we summarize the main potential factors associated with the protective effects against myopia of increased outdoor time, namely, exposure to elevated levels and shorter wavelengths of light, and increased dopamine and vitamin D levels. In this review, we aimed to identify safe and effective therapeutic interventions to prevent myopia-related complications and vision loss.
Keywords: Outdoor time, myopia, mechanism, daylight, dopamine, vitamin D
Introduction
The increasing prevalence of myopia over the past few decades has become a significant cause for public health concern globally. In Singapore, the prevalence of myopia among adults older than age 40 years is 38.9%,1 contrary to most Western countries such as the United States (25.1%),2 Barbados (21.9%),3 and Australia (15%).4 Moreover, the percentage is much higher among children in East Asia. Research in Singapore revealed that the prevalence of myopia is 62.0% in children age 7 to 9 years5 and in Guangzhou, China, the prevalence is 49.7%.6 Among university students in central China, the myopia prevalence is 83.2%,7 and it is 96.5% in 19-year-old men in Seoul, Korea.8 The reported prevalence of myopia is 11.9% in Australia,9 20.0% in the United States,10 21.1% in urban India,11 and 16.5% in Nepal.12 Considering the high prevalence of myopia in Singapore, the average cost of eye examinations and visual aid devices13 is 148 USD/child/annum and 709 USD/adult/annum, with additional costs for laser in-situ keratomileusis (LASIK) surgical procedures in adults.14 Similarly, the economic costs associated with myopia are relatively high among adults in the United States, estimated to be USD 4.6 billion/year.15 Additionally, it has been projected that by the end of this decade, 2.5 billion people, accounting for one-third of the world’s population, will be diagnosed with myopia.16
Owing to the increasing global population together with the financial burden caused by myopia, it is crucial to promptly develop safe and effective therapeutic interventions for myopia. A considerable amount of research has been conducted and a number of interventions implemented to prevent the onset or slow the progression of myopia; these include atropine, special multifocal-like soft contact lenses, executive bifocal or progressive addition lenses, and overnight orthokeratology.17–23 Increasing the amount of time spent outdoors has demonstrated protective effects against the onset and progression of myopia.24,25 Therefore, in this paper, we provide a review of the major mechanisms that are potentially involved in the protective effects of increased outdoor time against myopia. We performed a search of the published literature and identified four main mechanisms, those involving high light levels, light chromaticity and spectral composition, dopamine, and vitamin D. We summarize each below.
High light levels
Over the past century, high daylight levels have come to be considered an effective method to prevent myopia.26 Numerous eye specialists have suggested that an adequate amount of daylight at school is necessary for children.27
With rapid technological development, several studies have been performed to identify the evidence and potential mechanisms of the protective effect of high levels of daylight. Animal studies in guinea pigs,28 chicks,29–31 and monkeys32,33 have revealed that high light levels (15,000 to 30,000 lux), both in the form of natural daylight and artificial laboratory light, could retard experimental myopia. In population-based studies, a greater degree of exposure to daylight was observed to be associated with less axial eye growth.34
In a number of epidemiological and animal studies, dopamine (DA) release has been suggested to be triggered by high-intensity outdoor light and rapid changes in luminance. Because DA is an ocular growth inhibitor,35–38 this release may inhibit myopic development.39 This mechanism may explain the protective effect of high light levels. The characteristics of related studies are shown in Table 1.
Table 1.
Characteristics of studies investigating the relationship of high light levels with myopia.
| First author (year) | Location | Sample type (size) |
|---|---|---|
| Jiang L (2014) | China | Albino guinea pigs (n = 303) |
| Cohen Y (2012) | Israel | Chicks (n = 166) |
| Backhouse S (2013) | New Zealand | Chicks (n = 52) |
| Li T (1995) | United States | Chicks (n = 38) |
| Iii ELS (2012) | United States | Infant rhesus monkeys (n = 58) |
| Smith EL 3rd (2013) | United States | Monkeys (n = 64) |
| Read SA (2015) | Australia | Children (n = 101) |
| Rose K (2008) | Australia | Children (n = 752) |
| Rose K (2008) | Australia | Children (n = 4132) |
Light chromaticity and spectral composition
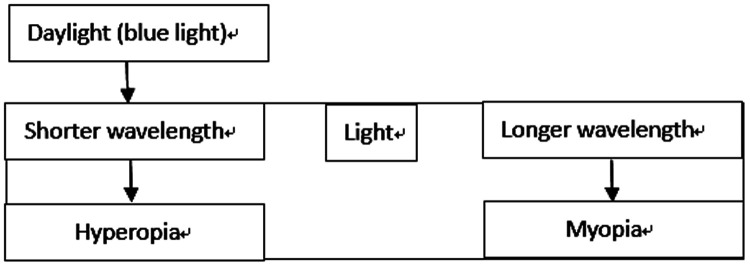
Studies in chicks40 and guinea pigs41–43 (Table 2) have revealed that the spectral composition of light can impact ocular growth. Exposing guinea pigs to short wavelength light led to hyperopic refraction, and exposing chicks to long wavelength light resulted in shorter axial length. In these studies, because the light was not uniformly focused on the retina, there was a remarkable reduction in the contrast of wavelengths that were focused away from the retina.14 Short wavelengths of light (blue light) are focused closer to the lens owing to dispersion produced by the longitudinal chromatic aberration of the human eye; to compensate for this, the eyes develop hyperopic refraction. According to another study,44 myopia is caused by an excess of red light and hyperopia by excess blue light, also suggesting that shorter light wavelengths may be protective against myopia. Because daylight is primarily composed of blue light, myopic inhibition may be attributable to longer time spent outdoors (Figure 1).
Table 2.
Characteristics of studies investigating the spectral composition of light and myopia.
| First author (year) | Location | Sample type (size) |
|---|---|---|
| Rucker FJ (2012) | United States | Chicks (n = 45) |
| Long Q (2009) | China | Guinea pigs (n = 30) |
| Liu R (2011) | China | Guinea pigs (n = 54) |
| Jiang L (2014) | China | Guinea pigs (n = 81) |
Figure 1.
Hypothesis: Daylight composed of shorter-wavelength light impacts ocular growth.
Dopamine (DA)
DA is a neurotransmitter that has an important role in the retina, mediating eye functions such as visual signaling, ocular development, and refractive adjustment.
Over the past few decades, a considerable amount of research in vertebrates has revealed that light and endogenous circadian rhythms may affect the release of DA.45–47 Additionally, scientists believe that retinal DA concentrations indicate the release and synthesis of DA.46,48 Furthermore, pharmacological and genetic studies have provided additional clues about the importance of light or visual input in retinal DA signaling and refractive development.37
Light affects dopamine
Several studies have previously reported that bright light has a protective effect against myopia,49–53 which had been attributed to increased levels of retinal DA and the activation of DA receptors. A 2010 study suggested that blocking D2-like receptor antagonist and spiperone has a protective effect against bright light.52 Image contrast and light intensity, detected by photoreceptors, have been implicated in the release of DA and development of myopia. Therefore, by increasing retinal DA levels, exposure to bright light may limit myopia development, as an environmental intervention.
Typically, two steps are involved in the synthesis of DA from tyrosine, in which tyrosine hydroxylation is considered the highly-regulated and rate-limiting step. It has also been suggested that with concomitant activation of tyrosine hydroxylation, which maintains steady-state reserves of DA upon the enhancement of neuronal activity, light may stimulate the synthesis and release of DA.54,55
The above results indicate that outdoor time may affect the concentration and release of DA.
Dopamine affects myopia
After birth, the growth and refractive development of the eyes are regulated by several messenger molecules, as explained by the biochemical pathways elucidated in several studies using animal models.56–58 DA, released by amacrine and interplexiform cells,59 plays a particularly important role in the modulation of the vertebrate visual system.60
In a number of experiments, DA levels have been raised by either injecting DA directly into the eye or increasing DA synthesis with L-dopamine (L-DOPA).61,62 Furthermore, DA signaling has also been increased with the use of apomorphine, a nonselective DA receptor agonist,63 and 2-amino-6,7-dihydroxy-1,2,3,4-tetrahydronaphthalene hydrobromide.49,64 These results support the hypothesis that increasing DA receptor activity or DA levels has a protective role in myopia.
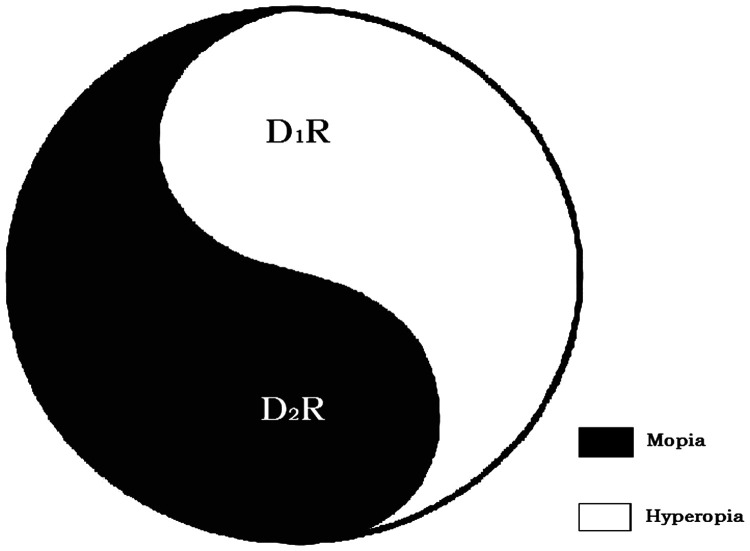
Considering the findings of genetic and pharmacological studies using mouse models of myopia, a working hypothesis about homeostatic control of myopia had recently been proposed, which suggests that D1- and D2-like receptors have contradictory roles in maintaining homeostasis of the emmetropization process; activation of D1-like receptors leads to hyperopia and that of D2-like receptors leads to myopia (Figure 2).65
Figure 2.
Hypothesis: Homeostatic control of myopia via the opposing effects of D1-like and D2-like receptors.
Dopamine-based strategies
DA-based strategies have been used to prevent the occurrence and to delay the progress of myopia in several studies. Reportedly, DA agonists may induce selective inhibition of form-deprivation myopia, without affecting normal visual development in mice.63 Additionally, the influence of L-DOPA on amblyopic vision has been reported in three studies.66–69 However, some researchers disagree with those conclusions;69 there is a need for further studies using larger sample sizes of children with myopia, to clarify the effect of L-DOPA in the prevention of myopia.
The inhibitory effect of DA on myopic eye growth has been evidenced in several works and has recently been considered as a target for treating myopia. However, in the development of myopia, several key questions regarding DA signaling need to be addressed. Further research is necessary to elucidate the exact mechanism of action and potential interactions with retinal pathways of DA.
Vitamin D
Vitamin D is considered a powerful cellular differentiation regulator with strong anticancer and antiproliferative effects.70 Vitamin D may regulate the length and refractive degree of the eye,71 which has a protective effect on myopia. Ciliary muscle enlargement is considered to structurally and functionally affect the eyes by increasing the risk of myopia.72 A recent study demonstrated that the smooth muscle was found to be larger in the eyes of children with myopia.73 Vitamin D, which is beneficial to the functioning of smooth muscle, may subsequently inhibit myopia. Exposure to solar ultraviolet B radiation may trigger vitamin D synthesis,74 which may explain the protective effect of outdoor activities against the disorder.
It has also been suggested that higher levels of vitamin D may inhibit myopia via the following processes: regulating the growth of the sclera, adjusting the smooth ciliary muscle (necessary to achieve a clear retinal image), signaling and regulating the cell cycle,71,75–77 as well as the antiproliferative effect of vitamin D. Studies performed in British children revealed that those who spent more time outdoors appeared to have increased total vitamin D levels, although after controlling for the amount of outdoor time, blood vitamin D levels were not significantly associated with the incidence of myopia. This indicates that vitamin D may be a biomarker for the amount of time spent outdoors. Thus, there is a need for further research to separate the direct effect of vitamin D and its use an alternative to outdoor sunlight exposure.
Other potential mechanisms
According to another hypothesis, the dioptric pattern of the outdoor visual environment has been suggested to inhibit myopia.78
A more uniform pattern of retinal defocus has been considered to be beneficial in protecting against myopia and may influence ocular growth. Additionally, in an outdoor environment, objects are typically further away with fewer dioptric variations across the visual scene, providing a more uniform pattern of retinal defocus. On the contrary, in an indoor visual environment, objects are much closer; the dioptric value is higher at the fixation point and decreases towards the periphery, which leads to higher levels of retinal defocus.
Several studies have proposed potential mechanisms associated with the protective effect of time spent outdoors. In an outdoor visual environment, the pupil constricts under high light intensity, and there is an increase in the accommodative demand for viewing distant objects, resulting in better clarity of the retinal image and an increased depth of focus.35,36,79
Conclusion
Currently, approximately 25% of the global population has myopia.80 If the current increasing trend continues, this percentage will reach 50% (4.76 billion people) by 2050, including nearly one billion people with severe myopia. This situation amounts to a state of emergency. It is therefore crucial to find effective ways to address myopia-related complications.65
Population-based data have demonstrated the protective effect of time spent outdoors, similar to the results of outdoor intervention programs. In this review, we have summarized four possible mechanisms involved in the protective effect against myopia of longer duration of time spent outdoors (Table 3). Further research is needed to explain these mechanisms, and DA-based strategies have yet to be used successfully in clinical settings. To prevent myopia at younger ages, measures must be implemented, such as conducting school classes outdoors, incorporating more outdoor activities into the school curriculum, and providing additional outdoor programs for children on weekends.
Table 3.
Main potential mechanisms involved in the protective effect against myopia of time spent outdoors.
| Possible mechanism | Summary |
|---|---|
| High light levels | Affects axial eye growth and the release of dopamine. |
| Light chromaticity and spectral composition | Shorter wavelengths (daylight) can prevent myopia. |
| Dopamine | Outdoor time affects the concentration and release of dopamine, which inhibit myopic development. |
| Vitamin D | Vitamin D synthesis may be triggered when outdoors, which inhibits myopia. |
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the Major Science and Technology Program of Changzhou Health and Family Planning Commission (ZD201511) and the Science and Technology Support Program of Changzhou (CE20175039).
ORCID iDs
Jun Zhang https://orcid.org/0000-0002-2497-6839
Guohua Deng https://orcid.org/0000-0003-0804-1117
References
- 1.Pan CW, Zheng YF, Anuar ARet al. Prevalence of refractive errors in a multiethnic Asian population: the Singapore epidemiology of eye disease study. Invest Ophthalmol Vis Sci 2013; 54: 2590–2598. [DOI] [PubMed] [Google Scholar]
- 2.Pan CW, Klein BEK, Cotch MFet al. Racial variations in the prevalence of refractive errors in the United States: the multi-ethnic study of atherosclerosis. Am J Ophthalmol 2013; 155: 1129–1138.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu SY, Nemesure B, Leske MC. Refractive errors in a black adult population: the Barbados Eye Study. Invest Ophthalmol Vis Sci 1999; 40: 2179–2184. [PubMed] [Google Scholar]
- 4.Attebo K, Ivers RQ, Mitchell P. Refractive errors in an older population: the Blue Mountains Eye Study. Ophthalmology 1999; 106: 1066–1072. [DOI] [PubMed] [Google Scholar]
- 5.Saw SM, Shankar A, Tan SBet al. A cohort study of incident myopia in Singaporean children. Invest Ophthalmol Vis Sci 2006; 47: 1839. [DOI] [PubMed] [Google Scholar]
- 6.He M, Huang W, Zheng Yet al. Refractive error and visual impairment in school children in rural southern China. Ophthalmology 2007; 114: 374–382.e1. [DOI] [PubMed] [Google Scholar]
- 7.Wei S, Sun Y, Li Set al. Refractive errors in university students in Central China: the Anyang University Students Eye Study. Invest Ophthalmol Vis Sci 2018; 59: 4691–4700. [DOI] [PubMed] [Google Scholar]
- 8.Jung SK, Lee JH, Kakizaki Het al. Prevalence of myopia and its association with body stature and educational level in 19-year-old male conscripts in Seoul, South Korea. Invest Ophthalmol Vis Sci 2012; 53: 5579–5583. [DOI] [PubMed] [Google Scholar]
- 9.Ip J, Huynh S, Robaei Det al. Ethnic differences in refraction and ocular biometry in a population-based sample of 11-15-year-old Australian children. Eye (Lond) 2008; 22: 649–656. [DOI] [PubMed] [Google Scholar]
- 10.Zadnik K. The Glenn A. Fry Award Lecture (1995). Myopia development in childhood. Optom Vis Sci 1997; 74: 603. [PubMed] [Google Scholar]
- 11.Singh NK, James RM, Yadav Aet al. Prevalence of myopia and associated risk factors in schoolchildren in North India. Optom Vis Sci 2019; 96: 200–205. [DOI] [PubMed] [Google Scholar]
- 12.Pokharel GP, Negrel AD, Munoz SRet al. Refractive error study in children: results from Mechi Zone, Nepal. Am J Ophthalmol 2000; 129: 436–444. [DOI] [PubMed] [Google Scholar]
- 13.Lim MC, Gazzard G, Sim ELet al. Direct costs of myopia in Singapore. Eye (Lond) 2009; 23: 1086–1089. [DOI] [PubMed] [Google Scholar]
- 14.Ramamurthy D, Lin CS, Saw SM. A review of environmental risk factors for myopia during early life, childhood and adolescence. Clin Exp Optom 2016; 98: 497–506. [DOI] [PubMed] [Google Scholar]
- 15.Pruett RC. Socioeconomic aspects of laser refractive surgery. Arch Ophthalmol 1995; 113: 1093. [DOI] [PubMed] [Google Scholar]
- 16.Dolgin E. The myopia boom. Nature 2015; 519: 276–278. [DOI] [PubMed] [Google Scholar]
- 17.Cho P, Cheung SW, Edwards M. The longitudinal orthokeratology research in children (LORIC) in Hong Kong: a pilot study on refractive changes and myopic control. Curr Eye Res 2005; 30: 71–80. [DOI] [PubMed] [Google Scholar]
- 18.Walline JJ, Jones LA, Sinnott LT. Corneal reshaping and myopia progression. Br J Ophthalmol 2009; 93: 1181–1185. [DOI] [PubMed] [Google Scholar]
- 19.Chua WH, Balakrishnan V, Chan YHet al. Atropine for the treatment of childhood myopia. Ophthalmology 2012; 119: 347–354. [DOI] [PubMed] [Google Scholar]
- 20.Chia A, Lu QS, Tan D. Five-year clinical trial on atropine for the treatment of myopia 2: myopia control with atropine 0.01% eyedrops. Ophthalmology 2016; 123: 391. [DOI] [PubMed] [Google Scholar]
- 21.Cho P, Cheung SW. Retardation of Myopia in Orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Invest Ophthalmol Vis Sci 2012; 53: 7077–7085. [DOI] [PubMed] [Google Scholar]
- 22.Walline JJ, Greiner KL, Mcvey MEet al. Multifocal contact lens myopia control. Optom Vis Sci 2013; 90: 1207–1214. [DOI] [PubMed] [Google Scholar]
- 23.Huang J, Wen D, Wang Qet al. Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis. Ophthalmology 2016; 123: 697–708. [DOI] [PubMed] [Google Scholar]
- 24.Xiong S, Sankaridurg P, Naduvilath Tet al. Time spent in outdoor activities in relation to myopia prevention and control: a meta‐analysis and systematic review. Acta Ophthalmologica 2017; 95: 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pärssinen O, Lyyra AL. Myopia and myopic progression among schoolchildren: a three-year follow-up study. Invest Ophthalmol Vis Sci 1993; 34: 2794–2802. [PubMed] [Google Scholar]
- 26.Harman NB. A paper on sight-saving classes. Br Med J 1933; 1: 129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashby R. Animal Studies and the Mechanism of Myopia-Protection by Light? Optom Vis Sci 2016; 93: 1052–1054. [DOI] [PubMed] [Google Scholar]
- 28.Jiang L, Long K, Schaeffel Fet al. Effects of dopaminergic agents on progression of naturally occurring myopia in albino guinea pigs (Cavia porcellus). Invest Ophthalmol Vis Sci 2014; 55: 7508–7519. [DOI] [PubMed] [Google Scholar]
- 29.Cohen Y, Peleg E, Belkin Met al. Ambient illuminance, retinal dopamine release and refractive development in chicks. Exp Eye Res 2012; 103: 33–40. [DOI] [PubMed] [Google Scholar]
- 30.Backhouse S, Collins AV, Phillips JR. Influence of periodic vs continuous daily bright light exposure on development of experimental myopia in the chick. Ophthalmic Physiol Opt 2013; 33: 563–572. [DOI] [PubMed] [Google Scholar]
- 31.Li T, Troilo D, Glasser Aet al. Constant light produces severe corneal flattening and hyperopia in chickens. Vision Res 1995; 35: 1203–1209. [DOI] [PubMed] [Google Scholar]
- 32.Smith EL, 3rd, Hung LF, Huang J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci 2012; 53: 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith EL, 3rd, Hung LF, Arumugam Bet al. Negative lens-induced myopia in infant monkeys: effects of high ambient lighting. Invest Ophthalmol Vis Sci 2013; 54: 2959–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Read SA, Collins MJ, Vincent SJ. Light exposure and eye growth in childhood. Invest Ophthalmol Vis Sci 2015; 56: 6779–6787. [DOI] [PubMed] [Google Scholar]
- 35.Rose KA, Morgan IG, Smith Wet al. Myopia, lifestyle, and schooling in students of Chinese ethnicity in Singapore and Sydney. Arch Ophthalmol 2008; 126: 527–530. [DOI] [PubMed] [Google Scholar]
- 36.Rose KA, Morgan IG, Ip Jet al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology 2008; 115: 1279–1285. [DOI] [PubMed] [Google Scholar]
- 37.Feldkaemper M, Schaeffel F. An updated view on the role of dopamine in myopia. Exp Eye Res 2013; 114: 106–119. [DOI] [PubMed] [Google Scholar]
- 38.French AN, Ashby RS, Morgan IGet al. Time outdoors and the prevention of myopia. Exp Eye Res 2013; 114: 58–68. [DOI] [PubMed] [Google Scholar]
- 39.Schwahn HN, Schaeffel F. Flicker parameters are different for suppression of myopia and hyperopia. Vision Res 1997; 37: 2661–2673. [DOI] [PubMed] [Google Scholar]
- 40.Rucker FJ, Wallman J. Chicks use changes in luminance and chromatic contrast as indicators of the sign of defocus. J Vis 2012; 12: 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long Q, Chen D, Chu R. Illumination with monochromatic long-wavelength light promotes myopic shift and ocular elongation in newborn pigmented guinea pigs. Cutan Ocul Toxicol 2009; 28: 176–180. [DOI] [PubMed] [Google Scholar]
- 42.Liu R, Qian YF, He JCet al. Effects of different monochromatic lights on refractive development and eye growth in guinea pigs. Exp Eye Res 2011; 92: 447–453. [DOI] [PubMed] [Google Scholar]
- 43.Jiang L, Zhang S, Schaeffel Fet al. Interactions of chromatic and lens-induced defocus during visual control of eye growth in guinea pigs (Cavia porcellus). Vision Res 2014; 94: 24–32. [DOI] [PubMed] [Google Scholar]
- 44.Foulds WS, Barathi VA, Luu CD. Progressive myopia or hyperopia can be induced in chicks and reversed by manipulation of the chromaticity of ambient light. Invest Ophthalmol Vis Sci 2013; 54: 8004–8012. [DOI] [PubMed] [Google Scholar]
- 45.Adachi A, Nogi T, Ebihara S. Phase-relationship and mutual effects between circadian rhythms of ocular melatonin and dopamine in the pigeon. Brain Res 1998; 792: 361–369. [DOI] [PubMed] [Google Scholar]
- 46.Megaw PL, Boelen MG, Morgan IGet al. Diurnal patterns of dopamine release in chicken retina. Neurochem Int 2006; 48: 17–23. [DOI] [PubMed] [Google Scholar]
- 47.Zawilska JB, Bednarek A, Berezińska Met al. Rhythmic changes in metabolism of dopamine in the chick retina: the importance of light versus biological clock. J Neurochem 2010; 84: 717–724. [DOI] [PubMed] [Google Scholar]
- 48.Iuvone PM, Galli CL, Garrisongund CKet al. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science 1978; 202: 901–902. [DOI] [PubMed] [Google Scholar]
- 49.Ashby R, McCarthy CS, Maleszka Ret al. A muscarinic cholinergic antagonist and a dopamine agonist rapidly increase ZENK mRNA expression in the form-deprived chicken retina. Exp Eye Res 2007; 85: 15–22. [DOI] [PubMed] [Google Scholar]
- 50.Schmid KL, Wildsoet CF. Inhibitory effects of apomorphine and atropine and their combination on myopia in chicks. Optom Vis Sci 2004; 81: 137–147. [DOI] [PubMed] [Google Scholar]
- 51.Norton TT, Siegwart JT., Jr. Light levels, refractive development, and myopia – A speculative review. Exp Eye Res 2013; 114: 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ashby RS, Frank S. The effect of bright light on lens compensation in chicks. Invest Ophthalmol Vis Sci 2010; 51: 5247–5253. [DOI] [PubMed] [Google Scholar]
- 53.Lan W, Feldkaemper M, Schaeffel F. Intermittent Episodes of Bright Light Suppress Myopia in the Chicken More than Continuous Bright Light. PLoS One 2014; 9: e110906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iuvone PM, Galli CL, Garrison-Gund CKet al. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science 1978; 202: 901–902. [DOI] [PubMed] [Google Scholar]
- 55.Kramer SG. Dopamine: a retinal neurotransmitter. I. Retinal uptake, storage, and light-stimulated release of H3-dopamine in vivo. Invest Ophthalmol 1971; 10: 438–452. [PubMed] [Google Scholar]
- 56.Stone RA, Pendrak K, Sugimoto Ret al. Local patterns of image degradation differentially affect refraction and eye shape in chick. Curr Eye Res 2006; 31: 91–105. [DOI] [PubMed] [Google Scholar]
- 57.Wildsoet C. Neural pathways subserving negative lens-induced emmetropization in chicks–insights from selective lesions of the optic nerve and ciliary nerve. Curr Eye Res 2003; 27: 371–385. [DOI] [PubMed] [Google Scholar]
- 58.Diether S, Schaeffel F. Local changes in eye growth induced by imposed local refractive error despite active accommodation. Vision Res 1997; 37: 659. [DOI] [PubMed] [Google Scholar]
- 59.Djamgoz MB, Wagner HJ. Localization and function of dopamine in the adult vertebrate retina. Neurochem Int 1992; 20: 139–191. [DOI] [PubMed] [Google Scholar]
- 60.Witkovsky P. Dopamine and retinal function. Doc Ophthalmol 2004; 108: 17–39. [DOI] [PubMed] [Google Scholar]
- 61.Junfeng M, Shuangzhen L, Wenjuan Qet al. Levodopa inhibits the development of form-deprivation myopia in guinea pigs. Chin Ophthalmic Res 2009; 87: 53. [DOI] [PubMed] [Google Scholar]
- 62.Gao Q, Liu Q, Ma Pet al. Effects of direct intravitreal dopamine injections on the development of lid-suture induced myopia in rabbits. Graefes Arch Clin Exp Ophthalmol 2006; 244: 1329–1335. [DOI] [PubMed] [Google Scholar]
- 63.Yan T, Xiong W, Huang Fet al. Daily injection but not continuous infusion of apomorphine inhibits form-deprivation myopia in mice. Invest Ophthalmol Vis Sci 2015; 56: 2475–2485. [DOI] [PubMed] [Google Scholar]
- 64.Mccarthy CS, Megaw P, Devadas Met al. Dopaminergic agents affect the ability of brief periods of normal vision to prevent form-deprivation myopia. Exp Eye Res 2007; 84: 100–107. [DOI] [PubMed] [Google Scholar]
- 65.Zhou X, Pardue MT, Iuvone PMet al. Dopamine signaling and myopia development: what are the key challenges. Prog Retin Eye Res 2017; 61: 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leguire LE, Rogers GL, Bremer DLet al. Levodopa and childhood amblyopia. J Pediatr Ophthalmol Strabismus 1992; 29: 290. [DOI] [PubMed] [Google Scholar]
- 67.Leguire LE, Rogers GL, Bremer DLet al. Levodopa/carbidopa for childhood amblyopia. Invest Ophthalmol Vis Sci 1993; 34: 3090–3095. [PubMed] [Google Scholar]
- 68.Leguire LE, Walson PD, Rogers GLet al. Longitudinal study of levodopa/carbidopa for childhood amblyopia. J Pediatr Ophthalmol Strabismus 1993; 30: 354. [DOI] [PubMed] [Google Scholar]
- 69.Repka MX, Kraker RT, Dean TWet al. A randomized trial of levodopa as treatment for residual amblyopia in older children. Ophthalmology 2015; 122: 874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin R, White JH. The pleiotropic actions of vitamin D. BioEssays 2010; 26: 21–28. [DOI] [PubMed] [Google Scholar]
- 71.Mutti DO, Marks AR. Blood levels of vitamin D in teens and young adults with myopia. Optom Vis Sci 2011; 88: 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mutti DO, Jones LA, Moeschberger MLet al. AC/A ratio, age, and refractive error in children. Am J Ophthalmol 2000; 130: 690. [DOI] [PubMed] [Google Scholar]
- 73.Bailey MD, Sinnott LT, Mutti DO. Ciliary body thickness and refractive error in children. Invest Ophthalmol Vis Sci 2008; 49: 4353–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 2004; 80: 1678S. [DOI] [PubMed] [Google Scholar]
- 75.Choi JA, Han K, Park YMet al. Low serum 25-hydroxyvitamin D is associated with myopia in Korean adolescents. Invest Ophthalmol Vis Sci 2014; 55: 2041–2047. [DOI] [PubMed] [Google Scholar]
- 76.Annamaneni S, Bindu CH, Reddy KPet al . Association of vitamin D receptor gene start codon (Fok1) polymorphism with high myopia. Oman J Ophthalmol 2011; 4: 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tideman JWL, Polling JR, Voortman Tet al. Low serum vitamin D is associated with axial length and risk of myopia in young children. Eur J Epidemiol 2016; 31: 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Flitcroft DI. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog Retin Eye Res 2012; 31: 622–660. [DOI] [PubMed] [Google Scholar]
- 79.Fujiwara M, Nakanishi R, Tanigawa Ket al. Seasonal variation in myopia progression and axial elongation: an evaluation of Japanese children participating in a myopia control trial. Jpn J Ophthalmol 2012; 56: 401–406. [DOI] [PubMed] [Google Scholar]
- 80.Holden BA, Fricke TR, Wilson DAet al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 2016; 123: 1036–1042. [DOI] [PubMed] [Google Scholar]