Abstract
In this pilot, single-blind, randomized controlled trial, we investigated the effects of intensified oral hygiene care (IOHC) on reducing stroke-associated pneumonia (SAP) incidence. Patients admitted within 24 hours of stroke onset were recruited and randomized to receive IOHC or routine oral hygiene care. The occurrence of SAP was checked and oral swabs were obtained during the 7-day follow-up. The SAP incidence was lower, though not significantly, in the IOHC group than in the control group. IOHC successfully decreased SAP incidence among patients who were male, had higher National Institutes of Health Stroke Scale and Debris Index scores, and lower Glasgow Coma Scale and Gugging Swallowing Screen scores. Furthermore, IOHC significantly decreased the prevalence of oral suspected SAP pathogens. These results suggest that IOHC can decrease the incidence of SAP in the most vulnerable patient groups and lower the prevalence of suspected oral SAP pathogens.
Keywords: pneumonia, stroke, oral hygiene, deglutition disorders, randomized controlled trial
What do we already know about this topic?
Cohort studies have documented that dysphagia screening and intensified oral hygiene care (IOHC) may successfully decrease the risk of pneumonia among hospitalized stroke patients.
How does your research contribute to the field?
Our pilot, single-blind, randomized controlled trial showed that IOHC could successfully decrease the incidence of newly defined stroke-associated pneumonia when stroke patients were male or had higher National Institutes of Health Stroke Scale and Debris Index scores, and lower Glasgow Coma Scale and Gugging Swallowing Screen scores.
What are your research’s implications toward theory, practice, or policy?
Increased attention to oral care is required among patients who are male or have a greater stroke severity, worse consciousness, worse swallowing function, and poorer oral hygiene.
Stroke is a common and serious health issue worldwide, and it is associated with high mortality and disability rates.1 Pneumonia is a major complication after stroke, with a frequency of up to 56.6% in neurological intensive care units,2 and is a major cause of mortality, poorer outcomes, and increased length of hospitalization among stroke survivors.3,4 As a result of increasing recognition in recent years of pneumonia as an important complication following stroke, a consensus was established in 2015 for the term “stroke-associated pneumonia” (SAP), being defined as “the spectrum of lower respiratory tract infections within the first 7 days after stroke onset.”5 Therefore, reducing the SAP rate may greatly alleviate the global burden related to stroke.
The primary mechanism by which SAP occurs is the aspiration of oral content contaminated by microbial pathogens implicated in pneumonia, in conjunction with stroke-induced immunosuppression.2 Previous cross-sectional studies have suggested that oral hygiene, dysphagia, and severity of stroke are important independent risk factors for pneumonia after stroke.6 Findings from cohort studies have documented that dysphagia screening and intensified oral hygiene care (IOHC), especially chlorhexidine mouth rinses, may successfully decrease the risk of pneumonia among hospitalized stroke patients compared to historical controls.7,8 However, for the newly defined SAP, there is little evidence about the effectiveness of oral hygiene practices, especially basing on clinical randomized controlled trials. Moreover, there remains a paucity of studies investigating the impact factors influencing the effectiveness of oral hygiene practices on pneumonia after stroke.
Conceptual Framework
We hypothesize that IOHC could decrease the incidence of newly defined SAP and the detection rates of suspected oral SAP pathogens. Moreover, the effectiveness of IOHC on SAP may be influenced by the characteristics of the patients or the disease, such as patients’ age or gender and severity of stroke or dysphagia.
Purpose
Therefore, the objective of our study was to perform a pilot, single-blind, randomized controlled trial to investigate whether IOHC could decrease the incidence of SAP, as well as lead to a reduction in the detection rates of suspected oral SAP pathogens; and if factors including age, sex, stroke type, initial oral hygiene, the severity of stroke, coma, and dysphagia would influence the effects of IOHC on the incidence of SAP.
Methods
Study Design
This study was a pilot prospective, single-blind, 2-arm, parallel randomized controlled trial with an allocation ratio of 1:1. The Ethics Committee of the Beijing Stomatological Hospital evaluated and approved the study protocol (Reference number: CMUSH-IRB-KJ-PJ-2019-03F). The study was registered in the Chinese Clinical Trial Registry prior to commencement (Registration number: ChiCTR-IPR-17013403).
Participants
Consecutive patients admitted to one neurological intensive care unit in a hospital in China from June 2017 to September 2018 were screened. Inclusion criteria were as follows: (1) a clinical diagnosis of acute stroke; (2) admission within 24 hours after stroke onset; and (3) age 18 years or older. Participants were excluded if they were (1) diagnosed with pneumonia or showed clinical signs of infection on admission; (2) required mechanical ventilation; (3) were prescribed antibiotics or immunosuppressive agents within the preceding 2 months; (4) were unable to receive oral care within 12 hours of admission; (5) had an allergy to chlorhexidine; or (6) were pregnant. All participants were screened for eligibility by the nursing staff and provided with an information sheet outlining the details of the study. Written informed consent was provided by all participants prior to recruitment into the study. Direct relatives provided written informed consent if participants were deemed to lack the capacity to do so.
Interventions
After recruitment into the study, participants were immediately randomized to receive either IOHC or routine oral hygiene care, for a duration of 7 days. In the routine oral hygiene care group, cognitively intact participants with adequate manual dexterity and unimpaired mouth opening capacity were asked to perform oral care by themselves, with or without the help of a nursing assistant. Those participants lacking the ability to perform oral care, including all participants in the intensive care unit, received oral swabbing with saline (2-minute duration, twice daily). In the IOHC group, in addition to oral self-care (or instead of routine saline swabbing), all teeth and oral soft tissues (including the gingiva, vestibule, buccal mucosa, floor of the mouth, tongue dorsum, and pharynx oralis), were swabbed with 0.12% chlorhexidine digluconate mouth wash (5-minute duration, 3 times daily). All interventions were performed by nurses who had been trained by a dental professional prior to the commencement of the study.
Outcomes and Data Collection
The follow-up period was from recruitment to 7 days after the stroke onset. The follow-up period was terminated earlier if participants developed SAP, received ventilation, died, or were discharged from the hospital. Oral examination and oral swab collection were performed by one senior dentist.
SAP Screening
The primary outcome was the rate of SAP, defined by the Pneumonia in Stroke Consensus Group criteria as pneumonia within the first 7 days after stroke onset in non-ventilated patients.5 The definition of probable SAP was used in this study, because not all participants with suspected SAP received a repeat chest X-ray for confirmation. One examiner was responsible for the daily screening of participants for SAP. A diagnosis of SAP required at least one of the following: (1) fever (>38°C) with no other recognized cause; (2) leukopenia (<4000 WBC/mm3) or leukocytosis (>12 000 WBC/mm3); or (3) for adults ≥70 years old, altered mental status with no other recognized cause. It also required at least 2 of the following: (1) new onset of purulent sputum or a change in the characteristics of sputum over a 24-hour period, increased respiratory secretions, or increased suctioning requirements; (2) new onset or worsening of cough, dyspnea, or tachypnea (respiratory rate > 25/min); (3) rales, crackles, or bronchial breath sounds; or (4) worsening gas exchange.
Systematic Dysphagia Screening
The same occupational therapist measured swallowing function in all participants at baseline with the Gugging Swallowing Screen (GUSS) method.9 The GUSS uses a scoring system ranging from 0 to 20 points and delineates the severity of dysphagia and aspiration risk, with a higher score indicating better swallowing function. If there was any doubt regarding the outcome of the GUSS, the participant was further subjected to video fluoroscopic evaluation of swallowing. The diet of all participants was guided via the GUSS score, which recommended either a normal diet, soft diet, thickened fluid, or tube feeding. The screening for SAP and dysphagia was performed by one senior neurologist who is skilled in swallowing function rehabilitation. Before the study began, 10 stable stroke patients were screened by the neurologist twice using GUSS method with an interval of 24 hours, and the consistency was 100%.
Oral Examination
The number of teeth and oral hygiene were examined at baseline. Oral hygiene was assessed with the Oral Hygiene Index.10 The whole mouth was divided into 6 quadrants, and the Debris Index and Calculus Index were recorded for the most severe tooth in each quadrant. The mean value for each index was calculated for the whole mouth. Before the study began, 10 stable patients were screened by the dentist twice using Oral Hygiene Index with an interval of 24 hours, and the value of intraclass correlation coefficient were 0.87 and 0.98 for Debris Index and Calculus Index respectively.
Collection of Oral Swabs and the Detection of Suspected SAP Pathogens
Oral swabs were obtained from each participant at baseline, prior to the administration of oral care interventions, and again at the end of the follow-up period. A sterile swab was passed over the entire dorsal surface of the tongue. The tip of each swab was soaked and mixed into 3 mL of brain heart infusion broth. Each swab was subsequently streaked onto blood agar (Thermo Fisher Scientific, Waltham, MA, USA). All plates were incubated at 37°C for 24 hours and then examined visually. Identification of suspected SAP pathogen colonies was performed using an automated mass spectrometry microbial identification system (Vitek MS system, bioMerieux, Craponne, France).
Clinical Data and Demographic Characteristics
Additional clinical data were obtained from medical records, including stroke subtype, National Institutes of Health Stroke Scale (NIHSS) score, Glasgow Coma Scale (GCS) score, medical history, age, sex, etc. Tolerance to the administered oral care was also monitored by the nurses.
Randomization and Blinding
Before the start of recruitment, the coordinator prepared a set of subject identification numbers (SIDs), which identified the treatment assignments for participants based on the odevity of a random number table. Following the screening and enrollment of each patient, study nurses called the coordinator to obtain each sequential SID, and subsequently assigned the intervention. Intervention assignment was blinded to all assessors, including the neurologist who screened for SAP and dysphagia, the dentist who performed the oral examinations and collected oral swabs, one examiner who detected suspected SAP pathogens, and one medical assistant who recorded clinical data and demographic characteristics. Furthermore, the nurses and assessors were forbidden to talk about the intervention assignment.
Data Analyses
Continuous variables were expressed as either mean (standard deviation), or median (interquartile range) in cases of significant non-normality, and qualitative variables were expressed as a count value (percentage). Participants who received mechanical ventilation, died, or were discharged from the hospital within 48 hours were excluded from the analysis without the risk of bias, and data from participants who withdrew their consent during the study were not used in the analysis. Categorical variables were compared between groups using the chi-square test, and continuous variables were compared between groups using the Student’s t-test or Mann–Whitney U test when required. Binary logistic regression analysis was performed, and the odds ratio (OR) and 95% confidence interval (CI) for SAP in the IOHC group versus the control group for all participants, and in each subgroup, were calculated. The scores of NIHSS, GCS, GUSS, and DI were divided into 2 parts by the median value or by common grading to enable subgroup analysis. Therefore, the study groups were subdivided into participants aged <60 years and participants aged ≥60 years; males and females; participants with ischemic stroke and those with hemorrhagic stroke; participants with NIHSS < 10 and those with NIHSS ≥ 10; participants with GCS < 12 and those with GCS ≥ 12; participants with GUSS < 15 and those with GUSS ≥ 15; and participants with DI < 12 and those with DI ≥ 12. All tests were performed at a significance level of α = 0.05, and P values were 2-sided. The statistical calculations were performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA).
Results
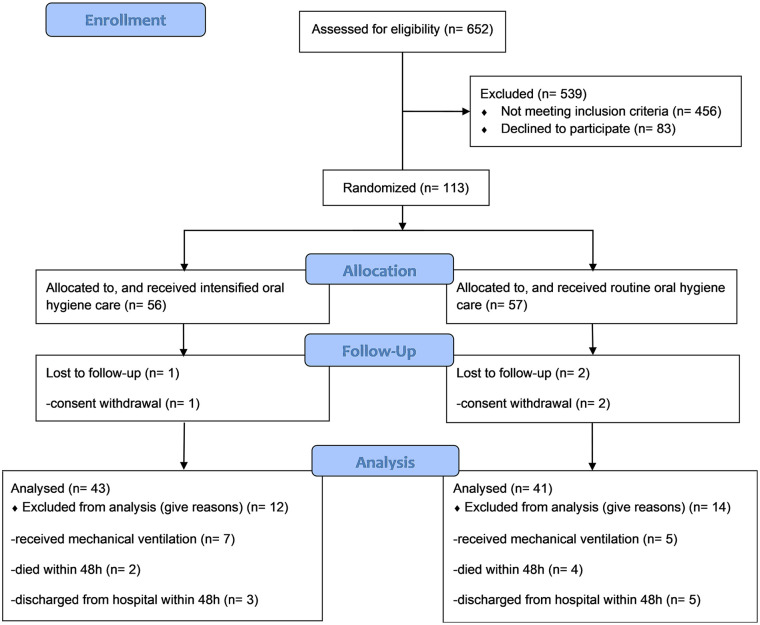
A total of 652 consecutive patients were assessed for eligibility, and 113 patients were randomized, with 84 being included in the analysis (Figure 1). Baseline demographic and clinical characteristics were well balanced between the 2 groups (Table 1). SAP was diagnosed in 6 (14%) and 13 (31.7%) of participants in the intervention and control groups, respectively (OR = 0.349, 95% CI [0.118-1.033], P = .052). No adverse events related to the intraoral intervention were observed during the study period.
Figure 1.
Recruitment and assignment of participants.
Table 1.
Baseline Characteristics and the Incidence of SAP in the IOHC and Control Groups.
| Intervention n = 43 | Control n = 41 | P value | |
|---|---|---|---|
| Age, mean (SD) | 57.1 (13.4) | 60.3 (13.7) | .279 |
| Sex (male), n (%) | 24 (55.8) | 27 (65.9) | .346 |
| History of chronic respiratory disease, n (%) | 3 (7.0) | 2 (4.9) | .165 |
| Smoking, n (%) | 7 (16.3) | 13 (31.7) | .097 |
| Stroke type, n (%) | .763 | ||
| Ischemic | 25 (58.1) | 25 (61.0) | |
| Intracerebral hemorrhage | 8 (18.6) | 9 (22.0) | |
| Subarachnoid hemorrhage | 10 (23.3) | 7 (17.1) | |
| Stroke more than once, n (%) | 7 (16.3) | 11 (26.8) | .239 |
| NIHSS score, median (25%, 75%) | 9 (1, 18) | 10 (1.5, 17) | .907 |
| GCS score, median (25%, 75%) | 14 (9, 15) | 13 (10.5, 15) | .740 |
| GUSS level, n (%) | .773 | ||
| 0-9 | 18 (41.9) | 18 (43.9) | |
| 10-14 | 2 (4.7) | 2 (4.9) | |
| 15-19 | 6 (14.0) | 6 (14.6) | |
| 20 | 17 (39.5) | 15 (36.6) | |
| Remaining teeth number, median (25%, 75%) | 27 (26, 30) | 27 (20.5, 30) | .341 |
| DI score, mean (SD) | 1.8 (0.5) | 1.9 (0.5) | .131 |
| CI score, mean (SD) | 2.2 (0.7) | 2.1 (0.9) | .626 |
| SAP, n (%) | 6 (14.0) | 13 (31.7) | .052 |
CI = Calculus Index; DI = Debris Index; GCS = Glasgow Coma Scale; GUSS = Gugging Swallowing Screen; IOHC = intensified oral hygiene care; NIHSS = National Institutes of Health Stroke Scale (NIHSS); SD = standard deviation; SAP = stroke-associated pneumonia.
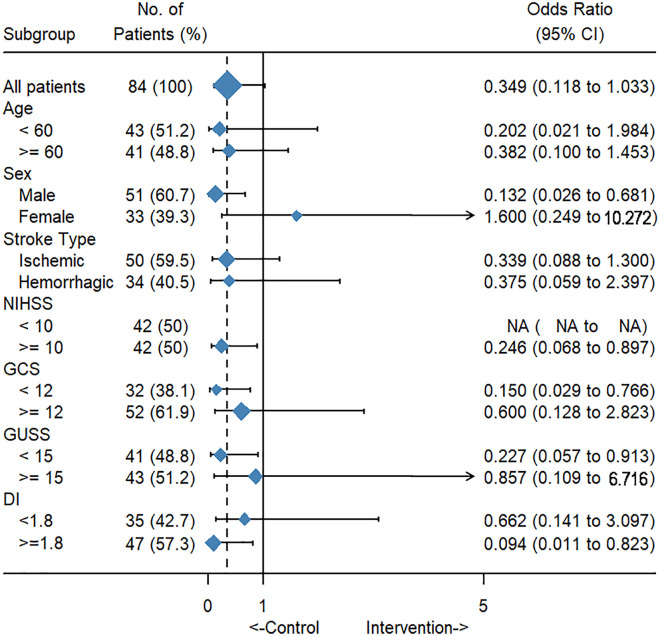
The results of subgroup analysis showed that the intervention significantly reduced the rate of SAP in participants who were male (OR = 0.132, 95% CI [0.026-0.68], P = .008), had a higher NIHSS score (OR = 0.246, 95% CI [0.068-0.897], P = .03), had a lower GCS score (OR = 0.15, 95% CI [0.029-0.766], P = .017), had a lower GUSS score (OR = 0.227, 95% CI [0.057-0.913], P = .031), and had a higher DI score (OR = 0.094, 95% CI [0.011-0.823], P = .013) (Figure 2).
Figure 2.
Effects of IOHC on the incidence of SAP, with associated 95% confidence intervals for all patients and subgroups.
DI = Debris Index; GCS = Glasgow Coma Scale; GUSS = Gugging Swallowing Screen; IOHC = intensified oral hygiene care; NIHSS = National Institutes of Health Stroke Scale; SAP = stroke-associated pneumonia.
Suspected SAP pathogens were documented at baseline in 1 and 3 participants in the intervention and control groups, respectively. No significant differences in the detection rate of SAP pathogens were observed at baseline between the intervention and control groups (P = .283). At the end of the follow-up period, 31.0% of all participants had detectable oral SAP pathogens, with the pathogen prevalence being significantly lower in the intervention group (20.9%) compared to the control group (41.5%; P = .042). The following suspected SAP pathogens were detected in oral swab samples during the follow-up period: Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Candida albicans, and Pseudomonas aeruginosa (Table 2).
Table 2.
Oral SAP Pathogens in the IOHC and Control Groups at the End of the Follow-Up Period.
| Oral SAP pathogens | Intervention n = 43 | Control n = 41 |
|---|---|---|
| Staphylococcus aureus, n (%) | 5 (11.6) | 5 (12.2) |
| Klebsiella pneumoniae, n (%) | 3 (7.0) | 6 (14.6) |
| Acinetobacter baumannii, n (%) | 0 (0.0) | 4 (9.8) |
| Candida albicans, n (%) | 1 (2.3) | 1 (2.4) |
| Pseudomonas aeruginosa, n (%) | 0 (0.0) | 1 (2.4) |
| No SAP pathogen, n (%) | 34 (79.1)* | 24 (58.5) |
IOHC = intensified oral hygiene care; SAP = stroke-associated pneumonia.
P < .05 (comparison of the SAP pathogen prevalence between the 2 groups).
Discussion
Results of this study showed that IOHC could successfully decrease the incidence of SAP when stroke patients were male, or had higher NIHSS, lower GCS, lower GUSS, or higher DI scores. Furthermore, this study confirmed that IOHC could decrease the prevalence of suspected SAP pathogens in the oral cavity.
This study adhered to the recently established 2015 consensus for the diagnosis of SAP, which is defined as “the spectrum of lower respiratory tract infections within the first seven days after stroke onset.” It is clear that there is a close association between lower respiratory tract infection and stroke.5 The first week after the onset of stroke is the time when pneumonia is most likely to occur, this being due to a combination of factors including stroke-related dysphagia, impaired consciousness, and suppressed immune response. For this reason, in this study, participants were recruited within 24 hours after stroke onset and conducted a 7-day follow-up. By the end of this randomized clinical trial, SAP was diagnosed in 31.7% and 14% of participants in the control and intervention groups, respectively. Although there was an obvious trend toward the reduction in SAP rate with the implementation of IOHC, this did not reach statistical significance, perhaps due to the small sample size. The results of 2 prior studies using historical controls have suggested that either IOHC plus systematic dysphagia screening or IOHC alone can reduce the incidence of pneumonia among individuals after stroke.7,8
The currently recognized pathophysiological mechanism for SAP is the aspiration of oral content combined with stroke-induced immunosuppression.2 Therefore, the swallowing function of the participants were carefully evaluated with the GUSS method and strictly followed its recommendations for dietary modifications. In this current study, participants were subdivided according to age, sex, stroke type, severity of stroke, level of consciousness, swallowing function, and oral hygiene. Not unexpectedly, the study showed that stroke patients who had a greater stroke severity, worse consciousness, worse swallowing function, and poorer oral hygiene were more likely to require IOHC. Interestingly, this study also suggested that male stroke patients were more likely to benefit from IOHC. This may have been due to the poorer oral hygiene and higher rate of smoking among male patients, as smoking may reduce respiratory resistance to infection and worsen oral hygiene. The results of this study suggest that increased attention to oral care is required among patients who are male or have a greater stroke severity, worse consciousness, worse swallowing function, and poorer oral hygiene.
To further investigate the effects of IOHC on SAP, we detected the occurrence of suspected oral SAP pathogens at baseline, prior to the intervention period, and on follow-up. S. aureus, K. pneumoniae, A. baumannii, C. albicans, and P. aeruginosa were the species detected, and these are consistent with the most commonly implicated etiological agents for pneumonia.11 While pathogen prevalence was low at baseline, SAP pathogens were isolated on follow-up in 41.5% and 20.9% of participants in the control and intervention groups, respectively. These results are supported by a previous study that documented large increases in oral aerobic Gram-negative bacteria during the first 2 weeks after acute stroke.12 The present study demonstrated that IOHC was associated with a significantly lower prevalence of oral SAP pathogens, compared to routine oral hygiene care, which confirms the effectiveness of IOHC against the microbiological causes of SAP. A study by Ab Malik et al also suggested that though IOHC with an electric tooth brush and chlorhexidine oral gel did not show superior effects on dental plaque reduction when compared with the conventional oral hygiene care over 6 months, it did have more effects on reducing the prevalence of oral opportunistic pathogens, including oral yeast and S. aureus.13,14
This study was a pilot, single-blind, randomized controlled trial with a limited sample size. Although we observed a trend for the effectiveness of IOHC on SAP, especially among the most vulnerable patient groups, these preliminary results are insufficient to draw firm conclusions. Also, the methods for the detection of suspected oral SAP pathogens were relatively rough. Further randomized controlled studies with larger sample sizes are required to confirm the effects of IOHC on SAP and inform the development of specific oral care protocols for patients after acute stroke.
The results of this study suggest that IOHC can successfully decrease the incidence of SAP among acute stroke patients who are male, or who have a greater stroke severity, worse consciousness, worse swallowing function, or poorer oral hygiene, providing reference for clinical work. Furthermore, our study indicated that IOHC can decrease the prevalence of suspected SAP pathogens in the oral cavity. Future clinical trials with larger sample sizes are required to confirm the effectiveness of IOHC, especially among the most vulnerable patient groups.
Acknowledgments
The authors would like to acknowledge the contribution of Yu Lei and Dan Su to the provision of oral care.
Footnotes
Author Contributions: YW and SC conceived and designed this study. DY, JZ, XW, SC, and YW collected and analyzed the data. YD wrote the manuscript. All authors revised and approved the final manuscript for submission.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Beijing Science and Technology Committee (grant number Z151100004015041) and the Beijing Stomatological Hospital Subject Construction Fund (grant number 16-09-20).
ORCID iD: Yue Wang  https://orcid.org/0000-0001-7537-5278
https://orcid.org/0000-0001-7537-5278
References
- 1. Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hannawi Y, Hannawi B, Rao CPV, Suarez JI, Bershad EM. Stroke-associated pneumonia: major advances and obstacles. Cerebrovasc Diss. 2013;35(5):430-443. [DOI] [PubMed] [Google Scholar]
- 3. Kishore AK, Vail A, Chamorro A, et al. How is pneumonia diagnosed in clinical stroke research? A systematic review and meta-analysis. Stroke. 2015;46(5):1202-1209. [DOI] [PubMed] [Google Scholar]
- 4. Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. 2011;11:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith CJ, Kishore AK, Vail A, et al. Diagnosis of stroke-associated pneumonia: recommendations from the pneumonia in stroke consensus group. Stroke. 2015;46(8):2335-2340. [DOI] [PubMed] [Google Scholar]
- 6. Wastfelt M, Cao Y, Strom JO. Predictors of post-stroke fever and infections: a systematic review and meta-analysis. BMC Neurol. 2018;18(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sorensen RT, Rasmussen RS, Overgaard K, Lerche A, Johansen AM, Lindhardt T. Dysphagia screening and intensified oral hygiene reduce pneumonia after stroke. J Neurosci Nurs. 2013;45(3):139-146. [DOI] [PubMed] [Google Scholar]
- 8. Wagner C, Marchina S, Deveau JA, Frayne C, Sulmonte K, Kumar S. Risk of stroke-associated pneumonia and oral hygiene. Cerebrovasc Dis. 2016;41(1-2):35-39. [DOI] [PubMed] [Google Scholar]
- 9. Trapl M, Enderle P, Nowotny M, et al. Dysphagia bedside screening for acute-stroke patients: the Gugging Swallowing Screen. Stroke. 2007;38(11):2948-2952. [DOI] [PubMed] [Google Scholar]
- 10. Silness J, Loe H. Periodontal disease in pregnancy. ii. correlation between oral hygiene and periodontal condtion. Acta odontol Scand. 1964;22:121-135. [DOI] [PubMed] [Google Scholar]
- 11. Kishore AK, Vail A, Jeans AR, et al. Microbiological etiologies of pneumonia complicating stroke: a systematic review. Stroke. 2018;49(7):1602-1609. [DOI] [PubMed] [Google Scholar]
- 12. Boaden E, Lyons M, Singhrao SK, et al. Oral flora in acute stroke patients: a prospective exploratory observational study. Gerodontology. 2017;34(3):343-356. [DOI] [PubMed] [Google Scholar]
- 13. Ab Malik N, Abdul Razak F, Mohamad Yatim S, et al. Oral health interventions using chlorhexidine-effects on the prevalence of oral opportunistic pathogens in stroke survivors: a randomized clinical trial. J Evid Based Dent Pract. 2018;18(2): 99-109. [DOI] [PubMed] [Google Scholar]
- 14. Ab Malik N, Mohamad Yatim S, Abdul Razak F, et al. A multi-centre randomised clinical trial of oral hygiene interventions following stroke-A 6-month trial. J Oral Rehabil. 2018;45(2):132-139. [DOI] [PubMed] [Google Scholar]