Abstract
Cannabidiol (CBD) is being investigated as a treatment for several medical disorders but there is uncertainty about its safety. We conducted the first systematic review and meta-analysis of the adverse effects of CBD across all medical indications. Double-blind randomized placebo-controlled clinical trials lasting ≥7 days were included. Twelve trials contributed data from 803 participants to the meta-analysis. Compared with placebo, CBD was associated with an increased likelihood of withdrawal for any reason (OR 2.61, 95% CI: 1.38–4.96) or due to adverse events (OR 2.65, 95% CI: 1.04–6.80), any serious adverse event (OR 2.30, 95% CI: 1.18–4.48), serious adverse events related to abnormal liver function tests (OR 11.19, 95% CI: 2.09–60.02) or pneumonia (OR 5.37, 95% CI: 1.17–24.65), any adverse event (OR 1.55, 95% CI: 1.03–2.33), adverse events due to decreased appetite (OR 3.56, 95% CI: 1.94–6.53), diarrhoea (OR 2.61, 95% CI: 1.46–4.67), somnolence (OR 2.23, 95% CI: 1.07–4.64) and sedation (OR 4.21, 95% CI: 1.18–15.01). Associations with abnormal liver function tests, somnolence, sedation and pneumonia were limited to childhood epilepsy studies, where CBD may have interacted with other medications such as clobazam and/or sodium valproate. After excluding studies in childhood epilepsy, the only adverse outcome associated with CBD treatment was diarrhoea (OR 5.03, 95% CI: 1.44–17.61). In summary, the available data from clinical trials suggest that CBD is well tolerated and has relatively few serious adverse effects, however interactions with other medications should be monitored carefully. Additional safety data from clinical trials outside of childhood epilepsy syndromes and from studies of over-the-counter CBD products are needed to assess whether the conclusions drawn from clinical trials can be applied more broadly.
Subject terms: Outcomes research, Schizophrenia, Pharmacology, Addiction
Introduction
Cannabidiol (CBD) has been approved by both the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) as an add-on treatment for two rare childhood epilepsies: Dravet Syndrome and Lennox-Gastaut Syndrome [1]. Recent clinical trials have also examined CBD as a potential treatment for diabetes [2], fatty liver disease [3] and schizophrenia [4]. CBD is also available in over-the-counter (OTC) preparations which have not been evaluated in clinical trials [5]. To date, most clinical trials of CBD have involved 99% pure CBD at doses of 300–1500 mg/day. OTC preparations typically contain CBD along with other cannabinoids, and provide doses of 5–20 mg/day. However, some OTC preparations reportedly contain much higher or lower quantities of CBD while the market is poorly regulated and many products are mislabeled [5].
The precise molecular mechanism of action of CBD is unclear. Its effects have been related to the inhibition of fatty acid amide hydrolase, inhibition of adenosine reuptake, agonism of transient receptor potential channels, antagonism of the orphan G-protein-coupled receptor GPR55, facilitation of 5-HT1A mediated neurotransmission, and inhibition of inflammatory cytokines via action at receptors such as peroxisome proliferator-activated receptor-gamma [6–9]. It has a low affinity for cannabinoid (CB) receptors, but may act as a negative allosteric modulator at the CB1 receptor [10].
CBD has a reputation for having few adverse effects [11], and this is consistent with data from clinical trials [2–4]. Previous reviews of the safety and tolerability of CBD did not include meta-analyses [12, 13], included data from other cannabinoids [14], or were limited to studies in epilepsy [15]. Given the recent approval of CBD by the FDA and EMA for childhood epilepsy syndromes, and the wide range of other indications for which it is being marketed, a quantitative assessment of its safety and tolerability is timely. The aim of the present study was to address this issue by conducting a systematic review and meta-analysis of the adverse effects of CBD across a range of indications.
Methods
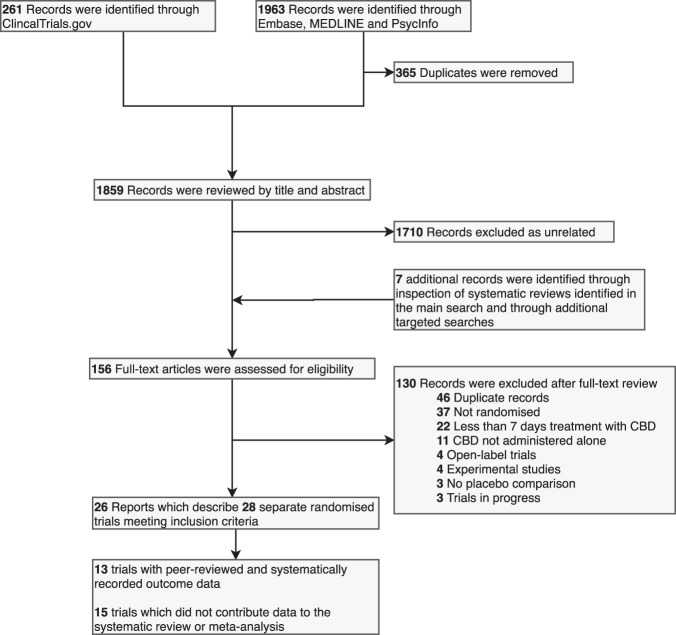
This systematic review and meta-analysis was prospectively registered on PROSPERO (CRD42018111429) and reported according to the PRISMA statement. The PRISMA flowchart is shown in Fig. 1.
Fig. 1. PRISMA Flow Diagram.
Flowchart describing the systematic search strategy, including the identification, screening and inclusion of relevant studies.
Inclusion and exclusion criteria
We included double-blind randomized controlled clinical trials that reported data on withdrawal, serious adverse events or adverse events from placebo-controlled trials of CBD in humans. Studies that were open-label, did not include a placebo comparison, were quasi-randomized, or lasted < 7 days were excluded. There were no restrictions in relation to participant characteristics or disease indication.
Search strategy
We searched Embase, MEDLINE, PsycInfo, and ClinicalTrials.gov. For Embase, MEDLINE, PsycINFO we used the keyword search term ‘(CBD OR cannabidiol) AND trial’. For ClinicalTrials.gov we searched for completed studies with the terms ‘cannabidiol OR CBD’. Each database was searched from its inception until 31/01/2019. We supplemented this search by reviewing the references of papers and systematic reviews identified in the search.
Two reviewers (EC and AG) independently screened the titles and abstracts of all articles identified by the search. The full texts of those selected were then reviewed by two independent authors (EC and AG). Any disagreements were resolved after discussion with a third author (DO).
Data extraction
Two authors (from SS, AG and EC) independently extracted outcome data on withdrawals, serious adverse events and adverse events from each study. Systematically recorded data were extracted from studies that had been published in a peer-reviewed journal or reported on ClinicalTrials.gov; data from posters, abstracts and other informal reports that had not been subject to peer review were excluded. If a study reported both treatment-related adverse events and adverse events we preferentially extracted adverse events, to reduce the risk of unanticipated adverse events being inadvertently disregarded. If two or more reports of the same study contained conflicting data, we preferentially used the data reported on ClinicalTrials.gov. The two sets of extracted data were then compared and any differences were resolved by a third author (EC or DO). Synonymous outcome measures were combined (by EC and DO) and are described in Table S2.
Bias assessment
Each study was assessed for bias using the Cochrane Risk of Bias Tool, which includes the following criteria: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, other bias [16]. Each criterion was rated as being at high, low, or unclear risk of bias by two independent raters (AG and SS). Disagreements were resolved after discussion with a third author (EC or DO).
Statistical analysis
We conducted a pairwise meta-analysis of all outcomes for CBD vs. placebo where more than eight events were recorded across both arms of all studies. As all outcomes were dichotomous, we calculated odds ratios (OR) with 95% confidence intervals using the Mantel–Haenszel method, where an OR > 1 indicates an increased likelihood of the event when treated with CBD compared with placebo and an OR below 1 indicates a reduced likelihood.
To convert mg/kg into a comparable daily dose we multiplied doses by 70 kg. Subgroup analyses of epilepsy studies and non-epilepsy studies were completed. Random-effects models were used to control for heterogeneity and we completed meta-regression analyses of CBD dosage. In such cases, data from the placebo arm were divided by the number of CBD arms contributing data to the meta-regression. Risk of publication bias was tested with the Egger regression intercept method and visual inspection of funnel plots. The significance level was set to p < 0.05 (two tailed). Pairwise analyses were conducted using Review Manager Version 5.3. Meta-regression analyses and publication bias analyses were conducted using Comprehensive Meta-Analysis Version 3.
Results
Identification of studies
The systematic search identified 28 independent randomized double-blind placebo-controlled clinical trials (Fig. 1), comprising data from a total of 1589 participants. Ten studies were excluded from further analysis because they were only reported in abstracts, posters or secondary descriptions from the grey literature. A further five were excluded because they did not report data on withdrawal or adverse events in a systematic way. The remaining 13 trials included a total of 822 participants and reported 266 different adverse outcomes, of which 33 provided sufficient data for meta-analysis (i.e., at least eight events across arms across studies per outcome) (Table 1). One study [3] met all the inclusion criteria but none of the events it reported occurred in outcomes relevant to the meta-analysis. It contributed data to estimates of event rates but did not contribute data to the calculation of OR. This meant that 12 trials using data from 803 participants contributed data to the meta-analysis. The studies that were not included are described in Table S1.
Table 1.
Characteristics of included studies.
| Study | Year | Indication | Length of treatment | Total CBD dose/daya | Type of CBD | Food directions | Number of participants | Age inclusion criteria | Mean age | Male (%) | Clobazam co-prescription (%) | Valproate co-prescription (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Consroe et al. [26] | 1991 | Huntington’s Disease | 6 weeksb | 10 mg/kg | Not specified | Empty stomach with a glass of water | 18 | – | 47.8 | 61.1 | 0 | 0 |
| GWP Fatty Liver Disease NCT01284634 [3] | 2014 | Fatty Liver Disease | 8 weeks | 200/400/800 mg | Plant derived (GWP42003) | Fasted, 30 min before meal | 25 | 18+ | 46.4 | 48 | – | – |
| Jadoon et al. [2] | 2016 | Type II Diabetes | 13 weeks | 200 mg | Plant derived (GWP42003) | Fasted, 30 min before meal | 27 | 18+ | 57.7 | 63 | – | – |
| Naftali et al. [25]c | 2017 | Crohn’s Disease | 8 weeks | 20 mg sublingual | Plant derived | – | 19 | 18–75 | 39 | 57.9 | – | – |
| Devinsky et al. (GWPCARE1) [18] | 2017 | Dravet Syndrome | 11 days titration and 12 weeks maintenance treatment up to 10 days taper period or OLE | 20 mg/kg | GWP42003-P, plant derived | – | 120 | 2–18 | 9.8 | 52 | 65 | 59 |
| Boggs et al. [19] | 2018 | Schizophrenia | 6 weeks | 600 mg | Provided by STI Pharmaceuticals | – | 41 | 18–65 | 47.4 | 69.4 | – | ?d |
| McGuire et al. [4] | 2018 | Schizophrenia | 6 weeks | 1000 mg | Plant derived (GWP42003) | – | 88 | 18–65 | 40.8 | 58 | – | – |
| GWP Clobazam Interaction Study NCT02565108 [20] | 2018 | Clobazam | 10 days titration and 21 days maintenance treatment 10 day taper period or OLE | 20 mg/kg | Plant derived (GWP42003-P) | – | 20 | 18–65 | 36.8 | 50 | 100 | – |
| Devinsky et al. (GWPCARE1 – Dose Ranging) [21] | 2018 | Dravet Syndrome | 3, 7 or 11 days titration 21 days maintenance treatment 10 days taper period or OLE | 5/10/20 mg/kg | Plant derived (GWP42003-P) | – | 34 | 4–10 | 7.6 | 47 | 68 | 65 |
| Devinsky et al. (GWPCARE3) [17] | 2018 | Lennox-Gastaut Syndrome | 7 or 11 days titration 12 weeks maintenance treatment 10 days taper period or OLE | 10/20 mg/kg | Plant derived (GWP42003-P) | – | 225 | 2–55 | 15.6 | 57.3 | 49 | 38 |
| Thiele et al. (GWPCARE4) [22] | 2018 | Lennox-Gastaut Syndrome | 11 days titration 12 weeks maintenance treatment 10 days taper period or OLE | 20 mg/kg | Plant derived (GWP42003-P) | – | 171 | 2–55 | 15.4 | 51.5 | 49 | 40 |
| Taylor et al. [23] | 2018 | Healthy Adults | 1 week | 1.5 g or 3 g per day (half dose on day 7) | Plant derived (GWP42003) | – | 24 | 18–45 | 26 | 33.3 | – | – |
| Hill et al. NCT03102918 [24] | 2019 | Cannabis Use Disorder | 6 weeks | 800 mg | Plant derived (GWP42003) | – | 10 | 18–65 | 30.8 | 40 | 0 | 0 |
OLE open-label extension.
aOral dosing unless stated otherwise.
bCrossover study with a 1 week washout and second 6 weeks treatment period, only pre-crossover data were extracted.
cNaftali 2017 met all inclusion criteria but did not contribute data to meta-analysis.
d25% of participants were prescribed a mood stabilizer which may include valproate.
Of the 13 studies included in the systematic review, 5 involved patients with epilepsy and 2 involved patients with schizophrenia. The remaining six studies involved subjects with problematic cannabis use, Huntington’s disease, type II diabetes, non-alcoholic fatty liver disease, Crohn’s disease and healthy volunteers. The duration of treatment ranged from 1 to 14 weeks, and the dose of oral CBD ranged from 200 to 3000 mg per day. The mean oral dose used was 1132 mg/day and the median dose was 1200 mg/day. One study used sublingual CBD at a dose of 20 mg/day. In epilepsy studies the mean oral dose was 1214 mg/day; in non-epilepsy studies the mean oral dose was 918 mg/day. All trials are believed to have used pure, pharmaceutical-grade CBD, with minimal other cannabinoids.
Meta-analysis
We report on our primary outcome measures below, together with any secondary outcome that achieved statistical significance (p < 0.05) (Fig. 2) and subgroup analyses of epilepsy and non-epilepsy studies (Figs. 3 and 4). Non-significant secondary outcomes are described in Table S4.
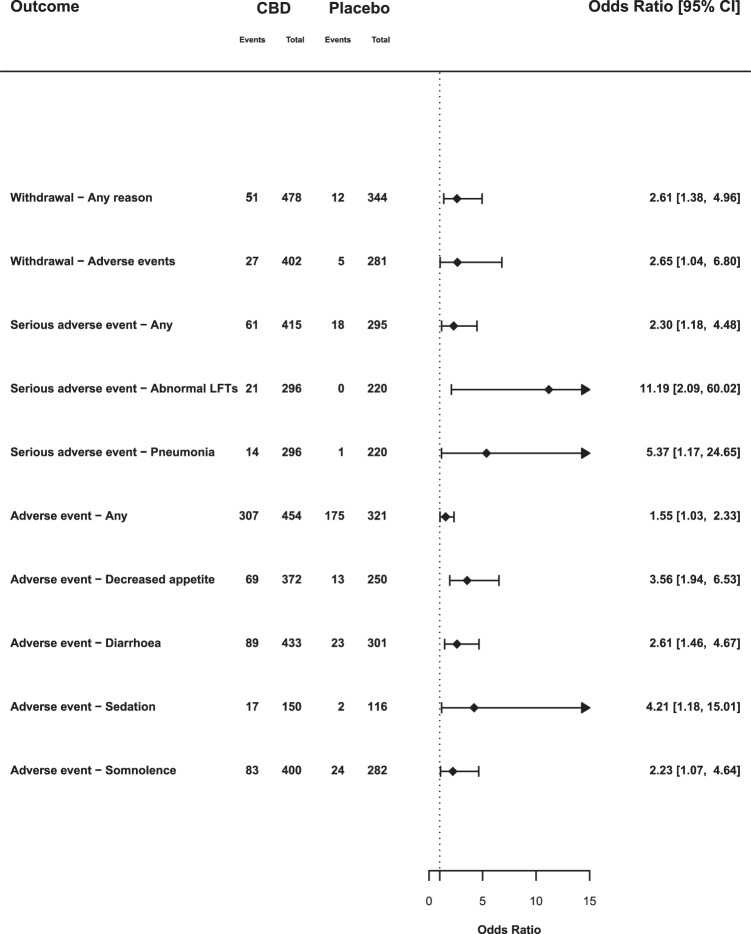
Fig. 2. Summary of meta-analysis results: all studies.
Odds ratios and 95% confience intervals (CIs) comparing CBD with placebo on outcomes which achieved statistical significance (p < 0.05). OR > 1 indicates an increased likelihood of the event when treated with CBD compared with placebo and an OR below 1 indicates a reduced likelihood. Abbreviations: LFTs, liver function tests.
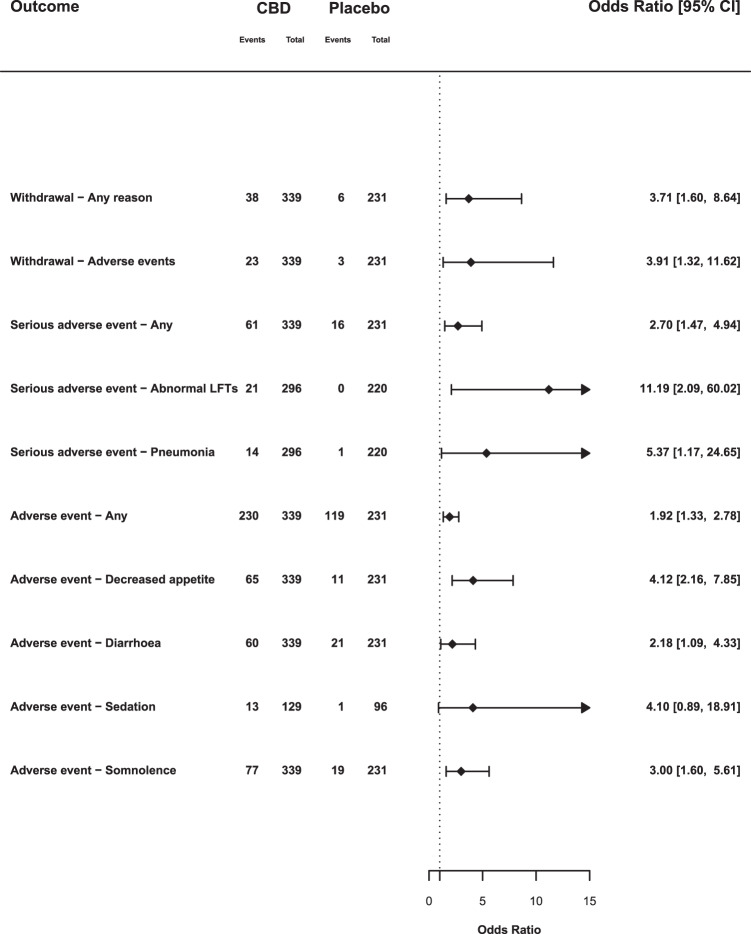
Fig. 3. Summary of subgroup analyses: epilepsy studies.
Odds ratios and 95% confience intervals (CIs) comparing CBD with placebo for selected outcomes from studies in childhood epilepsy syndromes. OR > 1 indicates an increased likelihood of the event when treated with CBD compared with placebo and an OR below 1 indicates a reduced likelihood. Abbreviations: LFTs, liver function tests.
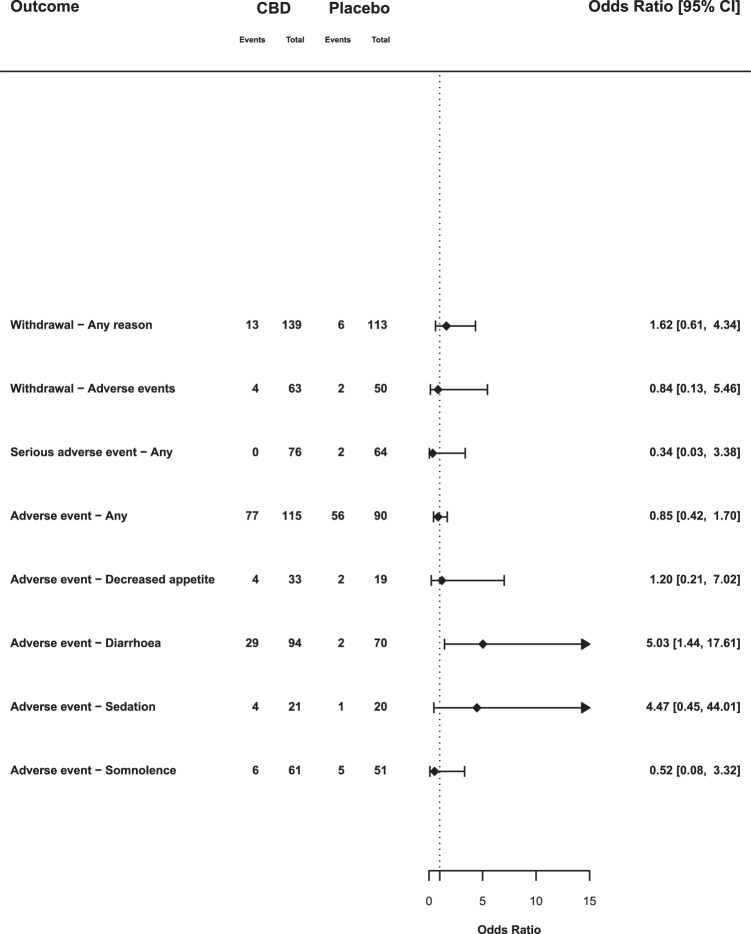
Fig. 4. Summary of subgroup analyses: non-epilepsy studies.
Odds ratios and 95% confience intervals (CIs) comparing CBD with placebo for selected outcomes from non-epilepsy studies. OR > 1 indicates an increased likelihood of the event when treated with CBD compared with placebo and an OR below 1 indicates a reduced likelihood.
Withdrawal
Across all doses, there were more withdrawals due to any reason (OR 2.61, 95% CI: 1.38–4.96) in CBD groups compared with placebo. The likelihood of withdrawal appeared to depend on the dose of CBD. At high doses (1400–3000 mg) 12.9% (35/272) of participants withdrew, compared with 8.8% (14/160) at medium doses (600–1000 mg), and only 4.3% (2/46) at low doses (20–400 mg), a similar rate to placebo (3.5% [12/344]). Meta-regressions indicated that there was a trend for an effect of CBD dose on withdrawal (β = 0.0014; p = 0.077). Withdrawal due to adverse events was also higher in CBD groups compared with placebo (OR 2.65, 95% CI: 1.04–6.80), although there was no significant correlation with CBD dose in the meta-regression (β = 0.0014; p = 0.274).
In studies of patients with rare epilepsy syndromes there was an increased odds of withdrawal due to any reason (OR 3.71, 95% CI: 1.60–8.64), and withdrawal due to adverse events (OR 3.91, 95% CI: 1.32–11.62). However, in non-epilepsy studies withdrawal due to any reason (OR 1.62, 95% CI: 0.61–4.34) or adverse events (OR 0.84, 95% CI: 0.13–5.46) was no more common than with placebo.
Serious adverse events
There were 61 serious adverse events in 415 participants in the CBD arms (14.7%), compared with 18 among 295 participants in the placebo arms (6.1%), an OR of 2.30 (95% CI: 1.18–4.48). The result of the dosage meta-regression was not significant (p = 0.169). In epilepsy studies the OR was 2.70 (95% CI: 1.47–4.94), in non-epilepsy studies it was 0.34 (95% CI: 0.03–3.38). This results from serious adverse events associated with CBD treatment being limited to studies in children with epilepsy.
There was an increased OR for pneumonia (OR 5.37, 95% CI: 1.17–24.65). This reflected 14 cases of pneumonia recorded as a serious adverse event in patients treated with CBD, compared with only 1 in placebo arms. An additional two cases of pneumonia were recorded as (non-serious) adverse events in patients treated with CBD in another study [17]. However, pneumonia was only reported in studies of children with epilepsy.
There was an increased OR for abnormal liver function tests (OR 11.19, 95% CI: 2.09–60.02), with all 21 events occurring in the CBD groups compared with no events in patients treated with placebo. These differences were only evident in studies involving childhood epilepsy, and most of the events (19 of 21) occurred when CBD was given at high doses (20 mg/kg or more). Each of the three trials contributing data to this outcome used a cut-off of >3 times the upper limit of normal for transaminase increases.
Adverse events
The OR for experiencing any adverse event with CBD compared with placebo was 1.55 (95% CI: 1.03–2.33). In total, 67.6% of CBD-treated participants reported an adverse event, compared with 54.5% of those treated with placebo. Here, the results of dosage meta-regression were highly significant (β = 0.0013, p = 0.0023): the likelihood of an adverse event was strongly related to the dose of CBD. The likelihood of adverse events also differed according to the clinical group being treated (χ2 = 4.17; p = 0.04). In the five studies involving children with epilepsy, 67.8% of those given CBD had adverse events, compared with 51.5% on placebo (OR 1.92, 95% CI: 1.33–2.78), whereas in the five non-epilepsy studies the rates were similar: 67.0% and 62.2%, respectively (OR 0.85, 95% CI: 0.42–1.70).
CBD was also associated with a greater OR for decreased appetite (OR 3.56, 95% CI: 1.94–6.53), diarrhoea (OR 2.61, 95% CI: 1.46–4.67), sedation (OR 4.21, 95% CI: 1.18–15.01) and somnolence (OR 2.23, 95% CI: 1.07–4.64). In epilepsy studies, CBD was associated with decreased appetite (OR 4.12, 95% CI: 2.16–7.85), diarrhoea (OR 2.18, 95% CI: 1.09–4.33) and somnolence (OR 3.00, 95% CI: 1.60–5.61). Sedation (OR 4.10, 95% CI: 0.89–18.91) was not statistically different from placebo (p = 0.07). In non-epilepsy studies, the only event that was more frequent with CBD was diarrhoea (OR 5.03, 95% CI: 1.44–17.61).
Other outcomes
All other outcomes were non-significant (p > 0.05; Table S4).
Risk of bias
Ten studies were rated as having a low risk of bias [2–4, 17–23], one was rated as having an unclear risk of bias [24], and two studies were rated as having a high risk, due to selective outcome reporting [25, 26]. Results of the risk of bias assessment are described in Table S3.
Discussion
To our knowledge, this is the first systematic review and meta-analysis of the adverse effects and tolerability of CBD across all indications. We identified a total of 28 trials, of which 13 met inclusion criteria and 12 provided suitable data for meta-analysis from a total of 803 participants. Our main findings were that although CBD was associated with a greater likelihood of adverse events and study withdrawal, this was almost entirely specific to studies in rare forms of epilepsy. These differed from the others we examined, in that they involved relatively high doses of CBD in children, many of whom were also being treated with other anti-epileptic medications.
CBD is a potent inhibitor of the CYP450 enzymes, CYP3A4 and CYP2C19. These hepatic enzymes metabolize clobazam and sodium valproate, which were prescribed in over half of the patients in the three main epilepsy studies included in the meta-analysis [17, 18, 27]. Inhibition of CYP2C19 can increase the levels of clobazam’s metabolite, N-desmethylclobazam, by two- to seven-fold, which has a significant sedative effect [28]. In a recent experimental study, even very high acute doses of CBD (1.5 and 4.5 g orally) had limited effects on alertness, suggesting that CBD alone is not usually sedative [29]. Increased N-desmethylclobazam levels could also account for the increased odds of pneumonia in epileptic patients taking CBD, as this increases the risk of sedation, respiratory depression and aspiration [30]. Again, these events only occurred in patients prescribed high doses of CBD (10–20 mg/kg/day). It is less clear if CBD interacts with clobazam at lower doses, although it has been reported that CBD inhibits CYP2C19 at doses of 5 mg/kg [31], and therefore has the potential to interact with a wide range of other commonly prescribed medications even at low-medium doses. The majority of cases with abnormal liver function tests were in children with epilepsy who were also being treated with sodium valproate. The exact mechanism of this interaction is not fully understood. Co-administration of CBD and valproate does not significantly alter their plasma levels or that of their metabolites [32]. However, the metabolite 7-COOH-CBD, valproate and its metabolite 4-ene-valproic acid may affect hepatic mitochondrial function [33].
An increased incidence of diarrhoea in CBD-treated individuals is consistent with data from a small experimental study, which found that single doses of CBD, 1.5 g and 4.5 g, produced diarrhoea in 10% and 20% of participants, respectively [29]. This may reflect the endocannabinoid system’s role in the regulation of gastrointestinal motility [34]. CBD treatment was also associated with reduced appetite in the epilepsy studies. The endocannabinoid system contributes to the regulation of food intake and moderates the hedonic effect of food consumption [35]. Previous studies have suggested that CBD in cannabis may reduce the appetitive effects of food stimuli [36], but its molecular mechanism of action in this context remains unclear. Rimonabant, a selective inverse agonist at the CB1 receptor, reduces appetite and weight and was approved as a treatment for obesity before being withdrawn because of adverse psychiatric side effects [37]. Dronabinol, a synthetic form of THC, and a partial agonist at CB receptors, has been used as a treatment for anorexia and cachexia in HIV and cancer [38, 39]. In the trials identified by our systematic search, weight was monitored too infrequently to be evaluated.
Strengths of the present study include its pre-registered protocol, comprehensive systematic search strategy, and its timeliness as the first meta-analysis of the safety and tolerability of CBD across all indications. The overall quality of studies included was high and it is unlikely that a significant amount of bias was introduced. Publication bias is also unlikely to have inflated summary ORs, as our target outcomes were not the primary outcome measure, nor the desired outcomes for the studies we examined. Many studies in our systematic review were excluded from the meta-analysis because they did not report adverse events systematically or had not been peer-reviewed. The meta-analysis therefore only contained data from 803 participants, reducing its power to identify uncommon adverse events. We included studies across a range of treatment indications which aids generalizability to different patient groups in clinical practice. However, most (69%) of the participants in our meta-analysis had childhood epilepsy. This is an important consideration, as these studies involved children rather than adults, relatively high doses of CBD, and the patients were often taking other epilepsy medications which can interact with CBD. Although we were able to stratify our analysis to investigate differences between the studies on epilepsy and on other indications, there is a need for further studies that include a broader range of patient groups and indications.
A key consideration in interpreting our findings is that the doses of CBD used in the trials we studied were relatively high (median dose 1200 mg/day; mean dose 1132 mg): only 46 of 822 participants received doses at or below 400 mg/day or 5 mg/kg/day [2, 3, 21, 25]. The doses of CBD provided by health and food supplements are typically much lower (5–20 mg/day [5]), so the incidence of adverse events is likely to be lower. However, this has yet to be demonstrated. This issue requires more research, as the commercial CBD market is poorly regulated. At present, some OTC products are poorly labelled, others do not contain purified CBD, and many contain other cannabinoids or contaminants in addition to CBD [40, 41].
Conclusions
CBD is well tolerated and has few adverse effects. However, its ability to inhibit the hepatic metabolism of other medications (such as clobazam and sodium valproate) has the potential to cause adverse effects. This is demonstrated by the increased rates of adverse events, serious adverse events and withdrawals in trials for childhood epilepsy syndromes but not other indications. Additional safety data from clinical trials outside of childhood epilepsy syndromes and from studies of OTC CBD products would be beneficial.
Funding and disclosure
The authors declare no competing interests.
Supplementary information
Author contributions
All authors contributed to drafting and revising the manuscript and approved its final version. EC was involved in the concept, design and registration of the study, the systematic search, extraction of data and the statistical analysis. DO contributed to the design and registration of the study, systematic search and statistical analysis. AG contributed to the systematic search and extraction of data. SS extracted data. JW, AE, TPF and PM were involved in the design of the study.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-020-0667-2).
References
- 1.Wise J. European drug agency approves cannabis-based medicine for severe forms of epilepsy. BMJ. 2019;366:l5708. doi: 10.1136/bmj.l5708. [DOI] [PubMed] [Google Scholar]
- 2.Jadoon KA, Ratcliffe SH, Barrett DA, Thomas EL, Stott C, Bell JD, et al. Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel group pilot study. Diabetes Care. 2016;39:1777–86. doi: 10.2337/dc16-0650. [DOI] [PubMed] [Google Scholar]
- 3.GW Research Ltd. Study to assess the effect of cannabidiol on liver fat levels in subjects with fatty liver disease. ClinicalTrialsGov; 2014. https://clinicaltrials.gov/ct2/show/NCT01284634. Accessed 15 June 2019.
- 4.McGuire P, Robson P, Cubala WJ, Vasile D, Morrison PD, Barron R, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175:225–31. doi: 10.1176/appi.ajp.2017.17030325. [DOI] [PubMed] [Google Scholar]
- 5.Freeman TP, Hindocha C, Green SF, Bloomfield MAP. Medicinal use of cannabis based products and cannabinoids. BMJ. 2019;365:l1141. doi: 10.1136/bmj.l1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9‐tetrahydrocannabinol, cannabidiol and Δ9‐tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massi P, Valenti M, Vaccani A, Gasperi V, Perletti G, Marras E, et al. 5‐Lipoxygenase and anandamide hydrolase (FAAH) mediate the antitumor activity of cannabidiol, a non‐psychoactive cannabinoid. J Neurochem. 2008;104:1091–100. doi: 10.1111/j.1471-4159.2007.05073.x. [DOI] [PubMed] [Google Scholar]
- 8.McPartland JM, Duncan M, Di Marzo V, Pertwee RG. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol. 2015;172:737–53. doi: 10.1111/bph.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimaraes FS. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos Trans R Soc B Biol Sci. 2012;367:3364–78. doi: 10.1098/rstb.2011.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laprairie RB, Bagher AM, Kelly MEM, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172:4790–805. doi: 10.1111/bph.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rohleder C, Müller JK, Lange B, Leweke FM. Cannabidiol as a potential new type of an antipsychotic. A critical review of the evidence. Front Pharmacol. 2016;7:422. doi: 10.3389/fphar.2016.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iffland K, Grotenhermen F. An update on safety and side effects of cannabidiol: a review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017;2:139–54. doi: 10.1089/can.2016.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huestis MA, Solimini R, Pichini S, Pacifici R, Carlier J, Busardò FP. Cannabidiol adverse effects and toxicity. Curr Neuropharmacol. 2019;17:974–89. doi: 10.2174/1570159X17666190603171901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for medical use a systematic review and meta-analysis. JAMA. 2015;313:2456. doi: 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- 15.Lattanzi S, Brigo F, Trinka E, Zaccara G, Cagnetti C, Del Giovane C, et al. Efficacy and safety of cannabidiol in epilepsy: a systematic review and meta-analysis. Drugs. 2018;78:1791–804. doi: 10.1007/s40265-018-0992-5. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, et al. Effect of cannabidiol on drop seizures in the Lennox–Gastaut syndrome. N Engl J Med. 2018;378:1888–97. doi: 10.1056/NEJMoa1714631. [DOI] [PubMed] [Google Scholar]
- 18.Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N. Engl J Med. 2017;376:2011–20. doi: 10.1056/NEJMoa1611618. [DOI] [PubMed] [Google Scholar]
- 19.Boggs DL, Surti T, Gupta A, Gupta S, Niciu M, Pittman B, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology. 2018;235:1923–32. doi: 10.1007/s00213-018-4885-9. [DOI] [PubMed] [Google Scholar]
- 20.GW Research Ltd. A randomized controlled trial to investigate possible drug-drug interactions between clobazam and cannabidiol. ClinicalTrialsGov; 2018. https://clinicaltrials.gov/ct2/show/NCT02565108. Accessed 15 June 2019.
- 21.Devinsky O, Patel AD, Thiele EA, Wong MH, Appleton R, Harden CL, et al. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90:e1204–e1211. doi: 10.1212/WNL.0000000000005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thiele EA, Marsh ED, French JA, Mazurkiewicz-Beldzinska M, Benbadis SR, Joshi C, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391:1085–96. doi: 10.1016/S0140-6736(18)30136-3. [DOI] [PubMed] [Google Scholar]
- 23.Taylor L, Gidal B, Blakey G, Tayo B, Morrison G. A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs. 2018;32:1053–67. doi: 10.1007/s40263-018-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill KP. Cannabidiol pharmacotherapy for adults with cannabis use disorder (NCT03102918). ClinicalTrialsGov; 2019.
- 25.Naftali T, Mechulam R, Marii A, Gabay G, Stein A, Bronshtain M, et al. Low-dose cannabidiol is safe but not effective in the treatment for Crohn’s Disease, a randomized controlled trial. Dig Dis Sci. 2017;62:1615–20. doi: 10.1007/s10620-017-4540-z. [DOI] [PubMed] [Google Scholar]
- 26.Consroe P, Laguna J, Allender J, Snider S, Stern L, Sandyk R, et al. Controlled clinical trial of cannabidiol in Huntington’s disease. Pharmacol Biochem Behav. 1991;40:701–8. doi: 10.1016/0091-3057(91)90386-G. [DOI] [PubMed] [Google Scholar]
- 27.Thiele EA, Marsh ED, French JA, Mazurkiewicz MB, Benbadis SR, Joshi C, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391:1085–96. doi: 10.1016/S0140-6736(18)30136-3. [DOI] [PubMed] [Google Scholar]
- 28.Groeneveld GJ, Martin JH. Parasitic pharmacology: a plausible mechanism of action for cannabidiol. Br J Clin Pharmacol. 2020;86:189–91. doi: 10.1111/bcp.14028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoedel KA, Szeto I, Setnik B, Sellers EM, Levy-Cooperman N, Mills C, et al. Abuse potential assessment of cannabidiol (CBD) in recreational polydrug users: a randomized, double-blind, controlled trial. Epilepsy Behav. 2018;88:162–71. doi: 10.1016/j.yebeh.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 30.Sun G, Zhang L, Zhang L, Wu Z, Hu D. Benzodiazepines or related drugs and risk of pneumonia: A systematic review and meta-analysis. Int J Geriatr Psychiatry. 2019;34:513–21. doi: 10.1002/gps.5048. [DOI] [PubMed] [Google Scholar]
- 31.FDA Centre for Drug Evaluation and Research. Drug approval package: epidiolex (Cannabidiol). Clin Rev. 2018:30.
- 32.Morrison G, Crockett J, Blakey G, Sommerville K. A Phase 1, open‐label, pharmacokinetic trial to investigate possible drug‐drug interactions between clobazam, stiripentol, or valproate and cannabidiol in healthy subjects. Clin Pharmacol Drug Dev. 2019;8:1009–31. doi: 10.1002/cpdd.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.FDA Centre for Drug Evaluation and Research. Drug approval package: epidiolex (cannabidiol). Summ Rev. 2018:30.
- 34.Pertwee RG. Cannabinoids and the gastrointestinal tract. Gut. 2001;48:859–67. doi: 10.1136/gut.48.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau BK, Cota D, Cristino L, Borgland SL. Endocannabinoid modulation of homeostatic and non-homeostatic feeding circuits. Neuropharmacology. 2017;124:38–51. doi: 10.1016/j.neuropharm.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 36.Morgan CJA, Freeman TP, Schafer GL, Curran HV. Cannabidiol attenuates the appetitive effects of Δ9- tetrahydrocannabinol in humans smoking their chosen cannabis. Neuropsychopharmacology. 2010;35:1879–85. doi: 10.1038/npp.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curioni C, André C. Rimonabant for overweight or obesity. Cochrane Database Syst Rev. 2006;4:CD006162. doi: 10.1002/14651858.CD006162.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badowski ME, Yanful PK. Dronabinol oral solution in the management of anorexia and weight loss in AIDS and cancer. Ther Clin Risk Manag. 2018;14:643–51. doi: 10.2147/TCRM.S126849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andries A, Frystyk J, Flyvbjerg A, Støving RK. Dronabinol in severe, enduring anorexia nervosa: a randomized controlled trial. Int J Eat Disord. 2014;47:18–23. doi: 10.1002/eat.22173. [DOI] [PubMed] [Google Scholar]
- 40.Bonn-Miller MO, Loflin MJE, Thomas BF, Marcu JP, Hyke T, Vandrey R. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318:1708–9. doi: 10.1001/jama.2017.11909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poklis JL, Mulder HA, Peace MR. The unexpected identification of the cannabimimetic, 5F-ADB, and dextromethorphan in commercially available cannabidiol e-liquids. Forensic Sci Int. 2019;294:e25–e27. doi: 10.1016/j.forsciint.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.