Abstract
Fragile X-associated tremor/ataxia syndrome (FXTAS) is a progressive neurodegenerative disorder caused by a repeat expansion in the fragile X mental retardation 1 (FMR1) gene. The disorder is characterized by kinetic tremor and cerebellar ataxia, shows age-dependent penetrance, and occurs more frequently in men. This paper summarizes the key emerging issues in FXTAS as presented at the Second International Conference on the FMR1 Premutation: Basic Mechanisms & Clinical Involvement in 2015. The topics discussed include phenotype-genotype relationships, neuro-behavioral function, and updates on FXTAS genetics and imaging.
Keywords: FMR1 premutation, Fragile X-associated tremor/ataxia syndrome (FXTAS), Cognition, Neuroimaging, FMR1 genetics
Introduction
The 2nd International Conference on FMR1 Premutation was a meeting focused on both basic science mechanisms of disease and clinical characteristics seen in carriers of premutation (55–200 CGG repeats) length expansions in the fragile X mental retardation 1 (FMR1) gene (Fig. 1). The premutation was first identified to be associated with symptoms as a distinct entity when fragile X-associated primary ovarian insufficiency (FXPOI) was described for the first time [1, 2], and the importance of this mutation as a specific cause of disease was confirmed when fragile X-associated tremor/ataxia syndrome (FXTAS) was later described [3]. FXTAS, characterized by kinetic tremor, cerebellar gait ataxia, and executive dysfunction, occurs in premutation carriers (PC) typically over the age of 55. Specific neuroimaging abnormalities, such as white matter hyperintensities in the middle cerebellar peduncle, and eosinophilic intranuclear inclusions, both within and outside of the nervous system, are consistently seen in affected individuals. Since the discovery of the disease over 15 years ago, researchers have continued to refine the clinical manifestations and molecular aspects (detailed in another article in this edition of the journal) of FXTAS and to determine whether there are other medical issues distinct from FXTAS in PC. This paper summarizes those abstracts presented at the 2nd International Conference on FMR1 Premutation that are related to the clinical manifestations of FXTAS and PC without FXTAS.
Fig. 1.
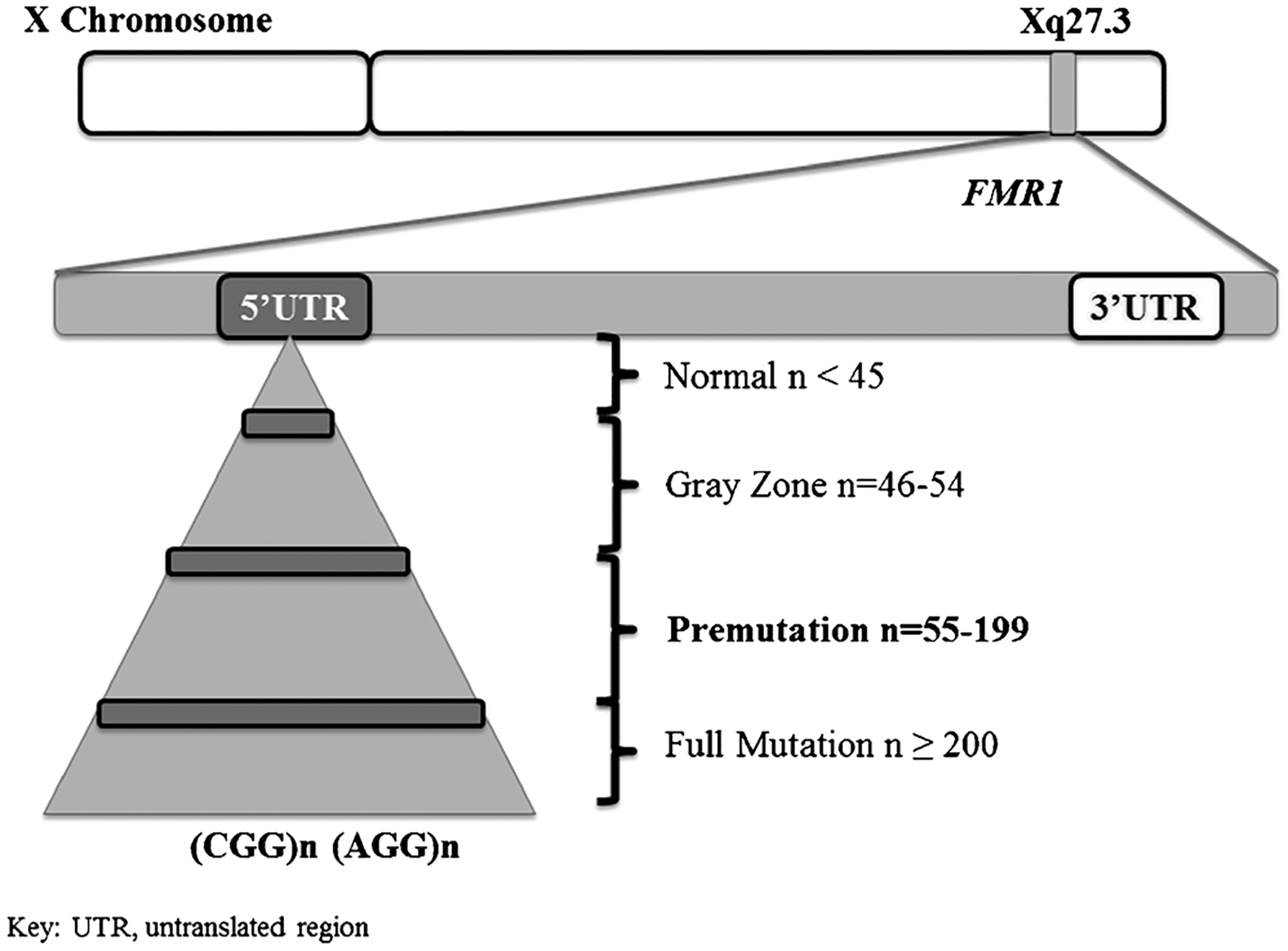
Illustration of the fragile X mental retardation 1 (FMR1) gene
Phenotypic Manifestations
FXTAS
The phenotypic description of the movement disorder and cognitive dysfunction in FXTAS has been expanded since the original discovery of the disease. Hall and colleagues conducted a retrospective chart review of patients seen in the FXTAS clinic at Rush University in Chicago who presented for clinical care between 2009 and 2014 [4]. The data collected included age, sex, FMR1 premutation size, FXTAS diagnosis (possible, probable, or definite), eye movement abnormalities, neuropathic signs, cognitive status, neuropsychiatric signs, FXTAS Rating Scale (FXTAS-RS) score [5], MRI findings, and family history of fragile X disorders. Nineteen patients with complete clinical data (38 % women) were included. FXTAS-RS scores ranged from 30 to 75 (definite FXTAS), 17 to 81 (probable FXTAS), and 7 to 41 (possible FXTAS). Five patients with FXTAS were found to have abnormal eye movements similar to those seen in progressive supranuclear palsy (PSP), with two patients meeting PSP diagnostic criteria [6]. Two additional patients suffered a rapidly progressive decline of their FXTAS signs in the setting of focal spinal cord disease. This case series was the first description of FXTAS patients presenting with a PSP-like phenotype. It also suggested that rapid progression of gait ataxia in FXTAS may warrant investigation for additional spinal cord pathology. Additionally, the FXTAS-RS may not be informative regarding diagnostic certainty.
Quantitative measurement of gait and balance has been a new and important area of research in FXTAS, as affected individuals have a host of gait and balance abnormalities. A novel, inertial sensor-based instrumented Timed Up and Go (i-TUG) was recently used by O’Keefe and colleagues to characterize gait and functional mobility deficits in PC with FXTAS (n = 7) and PC without FXTAS (n = 6) compared to 18 age-matched controls [7]. Those with FXTAS were significantly impaired compared to controls in four of five gait domains, which are thought to reflect independent aspects of the neural control of locomotion [8, 9]. These included gait parameters in the domains of gait speed (p < 0.008), rhythm (p = 0.001), gait phase cycle (p = 0.002), and gait variability (p = 0.002 to <0.001). However, there was no significant difference in the gait domain of asymmetry between controls and PC with and without FXTAS. We also included a movement transition domain to examine possible deficits in turning and sit to and from stand transitions. Those with FXTAS had significantly longer turn (p = 0.003) and turn-to-sit duration times (p < 0.0001). PC without FXTAS did not differ from controls on any i-TUG variables, except that increased time spent in the double-limb support phase of gait approached significance (p = 0.06) and did not differ from PC with FXTAS. Increased double-limb support time is typical in persons with cerebellar ataxia and gait instability [10, 11]. This study suggests that sensitive quantitative measures like the i-TUG may be useful in future outcome and natural history studies, as well as provide possible early markers of gait deficits in FXTAS. This will require verification in future longitudinal studies with much larger subject numbers. Interestingly, this study also found that balance and disability scales correlated highly with many i-TUG parameters, while the FXTAS-RS did not. This suggests that the FXTAS-RS might not be sensitive enough to capture salient features of the gait deficits in FXTAS and therefore may need revision.
O’Keefe and colleagues conducted a second study exploring the association between executive function and balance and gait measures in 32 PC, 17 without FXTAS and 15 with FXTAS. The Symbol Digit Modalities Test (SDMT) was administered to measure information regarding processing speed [12], and the Behavioral Dyscontrol Scale (BDS) was used to measure attention and response inhibition [13]. Lower executive function scores were significantly associated with greater postural sway, delayed postural reflexes, and reduced stability limits, gait speed and cadence (0.04 > p < 0.0001), as well as increased gait variability indices (p < 0.001). Balance and gait are negatively impacted by cognitive deficits in many patient populations [14], predisposing them to falls and increased morbidity [15, 16]. Thus, this preliminary study will lay the foundation for future studies examining the impact of executive function deficits on fall risk and disability levels in FXTAS patients. In addition, greater understanding of the interaction between cognitive deficits and gait and balance impairments is important in the design of therapeutic interventions tailored to both cognitive and motor profiles in FXTAS, as well as in other disorders for which there are combinations of motor and cognitive symptoms.
Lozano and colleagues performed a pilot retrospective controlled study in a convenience sample of 248 controls (130 men, 118 women) and 397 PC with and without FXTAS (176 men, 221 women). All participants were older than 50 years of age (mean 62.3 ± 9.49 for controls and 62.5 ± 9.91 for PC). Genetic testing of CGG repeats and FMR1 mRNA levels was performed. Additional data included body mass index (BMI), cholesterol levels, blood pressure, and HgbA1c levels. The bivariate association between PC status, demographics, and medical conditions was examined using t test and chi-square testing. The PC group had a significantly higher percentage of women (55.7 vs. 47.6 %; p = 0.045), shorter education years (15.5 vs. 16.2 years; p = 0.042), and a higher percentage of non-Hispanic Whites (91.7 vs. 83.9 %; p = 0.022) than the control group. A higher percentage of PC subjects self-reported having a diagnosis of hypertension (49.8 vs. 35.7 %; p = 0.013) and thyroid problems (20.7 vs. 10.4 %; p = 0.017) than control subjects. Blood pressure, blood glucose levels, HgbA1c, and BMI values were not significantly different between the two groups. Higher levels of FMR1 mRNA were associated with higher odds of having hypertension (from the logistic regression model; OR 1.363, 95 % confidence limits 1.03–1.802). Based on these preliminary findings, it appears that PC may have a FMR1 mRNA level-dependent relationship with the risk for hypertension which will require verification in future studies.
Premutation Carriers Without FXTAS
Social cognition and executive functioning may differ among PC women without FXTAS compared to the general population [17–19]. PC have been shown to demonstrate personality features consistent with a genetic liability to autism (i.e., the broad autism phenotype (BAP)) [17]. Losh and colleagues conducted a study to examine the relationship between the results of a battery of executive and social cognitive tasks previously associated with features of the BAP among PC women and autism symptomatology in their children with fragile X syndrome (FXS). PC (n = 51, mean age 44) and non-carrier controls who were parents of typically developing children (n = 54, mean age 41) completed a battery of executive function and social cognitive tasks, as well as measures of the BAP. The Autism Diagnostic Interview-Revised (ADI-R) [20] and Autism Diagnostic Observation Schedule (ADOS-2) [21] were administered to assess autism symptoms in children with FXS. PC performed similarly to controls on IQ, executive functioning, and social cognition overall. However, the subgroup of individuals who displayed features of the BAP showed differences on social cognitive stimuli (e.g., judging complex emotions from faces and biological motion). Parent-child correlations also emerged showing that poorer performance on measures of set shifting and heightened sensitivity to complex emotional stimuli among women with the premutation were associated with increased autism symptoms in their children with FXS (r2 > 0.3, p < 0.05). Results suggest that subtle differences in social cognition are evident in a subgroup of women who carry the PM, and that such differences co-occur with personality and language characteristics of the BAP, showing overlap with profiles observed among parents of individuals with autism. The phenotypes displayed by this subgroup could impact interpersonal functioning and may relate to genetic liability to autism, where subgroups showing autism endophenotypes may also carry mutations for autism risk genes that interact with FMRP. This possibility is supported by associations detected between parent phenotypes and autism symptoms in children with FXS. Finally, the relationship between these personality, language, and social cognitive abilities may help to explain phenotypic heterogeneity in the population of female PC.
Genetics
X-Inactivation
It has been hypothesized that X-inactivation (the transcriptional silencing of one X chromosome in each somatic cell) may be involved in mediating the phenotypic outcome of PC women due to observations that women with FXTAS present with a less severe disease phenotype than men. The ratio of cells containing an active normal FMR1 allele versus an active abnormal FMR1 allele may be skewed in some individuals [22], and variation in this activation ratio (AR; the ratio of cells carrying the normal FMR1 allele on the active X chromosome) appears to contribute to heterogeneity seen in PC women. A few prior studies have examined the association between AR and the presence and severity of FXTAS symptoms [5, 23, 24] as well as balance control [25] in PC women. Robertson and colleagues studied a case series of four PC sisters to determine whether AR, in conjunction with CGG repeat size, could be responsible for phenotypic variability [26]. The sisters were rated using the FXTAS-RS [5], and two sisters underwent balance [27, 28] and gait and functional mobility testing [29]. FMR1 AR measurement was performed using a methylation PCR assay [30]. Sister 1 had the largest CGG expansion, lowest AR, and the most severe clinical presentation. Sister 2 had a lower CGG expansion than sister 1, but a similar AR and milder neurological involvement. The length of CGG expansion in sister 3 was between sisters 1 and 2, but with a slightly higher AR and less neurological symptoms. Sister 4 was completely unaffected by FXTAS, despite having a similar CGG repeat expansion as her sisters, possibly due to her having the largest AR of the four. This case series suggests that there is a role of AR in determining risk for FXTAS and its severity in PC women. The addition of AR results may contribute to diagnostic or prognostic predictions for unaffected women, but larger studies are needed to determine the true clinical utility of this molecular measure.
The phenomenon of skewed X-inactivation is important in women carrying an X-linked disease since skewed X-chromosome inactivation (XCI) in favor of the mutated allele generally protects females from developing the disease [31]. Mila and colleagues studied skewed XCI patterns in 10 women with FXTAS and 21 PC women without FXTAS, and they observed that the distribution of XCI patterns in the FXTAS group and PC without FXTAS group showed severely skewed XCI [32]. In the FXTAS group, all cases preferentially inactivated the non-expanded X chromosome, whereas in the PC without FXTAS group, all preferentially inactivated the expanded X chromosome. Nevertheless, no significant differences were found when comparing XCI global frequencies (X active and X inactive) between PC with and without FXTAS. These results suggest that the skewed XCI of the normal allele may be a risk factor for the development of FXTAS; furthermore, the expression of the normal allele acts as a protective effect.
AGG
The number of AGG interspersions within the FMR1 CGG repeat is associated with gene instability and risk of expansion to a full (>200 CGG) mutation in future generations. It has been postulated that neurological phenotypes are associated with a lack of AGG interspersions regardless of whether the individual is a PC. Hall and colleagues conducted a study to determine whether the number of AGG interspersions within the FMR1 gene in adult patients with cognitive impairment and motor dysfunction with normal FMR1 repeat sizes were different compared to normal controls. Automated FMR1 PCR was performed on existing samples from two longitudinal aging studies at the Rush University Alzheimer Disease (AD) Center, all of whom are organ donors. A detailed clinical evaluation included 17 cognitive tests summarized as a global measure of cognition, a modified Unified Parkinson’s Disease Rating Scale (UPDRS) summarized as a global parkinsonism score, an upper and lower extremity motor strength and motor performance tests summarized as a global motor function score as previously described [33], and clinical diagnosis. Eight hundred and five participants were included (67 % women, 98 % white), mean age of 87.3 ± 6.4 years, mean FMR1 CGG size of 27 ± 4.4, and mean 16.5 ± 3.7 years of education. Autopsy was available on 557 participants with mean age at death of 88.3 ± 6.4 years. Autopsied participants only were given a cognitive diagnosis of normal (n = 202), mild cognitive impairment (n = 83), AD (n = 255), or other dementia (n = 6). There was no statistically significant difference between AGG number and cognitive diagnosis (n = 557), global cognition (n = 805), or parkinsonis n score (n = 805) with regression analysis when analyzing by sex after adjusting for age, race, and ethnicity. However, there was a significant association between AGG number and global motor function (n = 805; p = 0.04), with a complete lack of AGG interspersions being associated with worse global motor function. This effect was only seen in subjects with no AGG interspersions. A larger study to determine if this result remains significant is currently being conducted.
Genetic Modifiers in PC Women
In addition to the 20 % risk of developing fragile-X primary ovarian insufficiency (FXPOI) [34] and the estimated 10–16 % risk of FXTAS [3, 35], PC women have additional medical, neuropsychological, and cognitive features [36]. Several studies have shown an increased risk of up to 47.3 % for developing neuropsychological disorders including major depressive disorder (MDD) in PC women compared to the general population [37–39]. MDD is a complex disorder with multiple genetic and environmental factors that can influence its onset and severity. The polymorphic variants C677T and A1298C of the methylenetetrahydrofolate reductase (MTHFR) gene are being studied in MDD because the T and C alleles reduce enzymatic activity essential for production of the neurotransmitters dopamine, norepinephrine, and serotonin [40, 41]. Santa María and colleagues investigated MTHFR polymorphisms among Chilean PC women, in order to identify genetic factors that could be involved in their neuropsychological features. A total of 48 PC women (18 to 65 years old) referred for cascade screening in the Laboratory at the INTA, University of Chile were genotyped to determine the C677T and A1298C polymorphisms on the MTHFR gene. Allele frequencies and the genotype of cases and 70 controls were compared. The frequencies of both polymorphisms of MTHFR were significantly different between control women and PC women (C677T, p = 0.013; A1298C, p=0.015) and polymorphic allele T frequencies were 33 and 49 %, respectively. Significant differences were also found in the frequency distribution of genotypes (p < 0.001); the genotype CC/CC was not found in PC women and the frequencies of genotype TT/AA were 25 % and 1 % in PC and control women. These results suggest an association between the polymorphic variants of MTHFR in Chilean PC women. A larger study to determine if this result remains significant is necessary to perform. Future directions will be to characterize the neuropsychological phenotype of PC women to understand whether genetic variants of MTHFR are related to risk for depression. The genotype TT/AA, which only has enzyme activity of 25 %, could be treated with L-methylfolate to improve response to treatment in PC women if necessary [42].
Imaging
Diagnostic criteria for FXTAS were proposed in 2003 and require a number of clinical and radiological findings in a PC [43]. Radiological criteria include a major criterion of magnetic resonance imaging (MRI) white matter lesions in the brainstem or in the middle cerebellar peduncle, called the “BMCP” sign. Minor radiological criteria include cerebral white matter lesions and ≥ moderate generalized brain atrophy on MRI. Hyperintensities in the splenium of the corpus callosum (CCS) have recently been proposed as a radiographic diagnostic criterion for FXTAS [44].
FXTAS
Hall and colleagues conducted a study in which MRI from subjects with FXTAS and non-PC disease controls with movement disorders were viewed by a radiologist blinded to gene status and radiographic criteria for FXTAS were scored [43]. Phenotypic data used for diagnosis of FXTAS was collected, including the FXTAS-RS [5]. Twenty-two FXTAS subjects and 23 disease controls (35 % Parkinson’s disease and 25 % ataxia) were included. Cerebral atrophy (p = 0.03), MCP sign (p < 0.01), and brainstem white matter disease (p = 0.02) were more common in the FXTAS compared to the control subjects. Further, the CCS sign was also more common in FXTAS subjects compared to controls (87 vs. 44 %; p = 0.002). It had higher sensitivity (0.87 vs. 0.52), but lower specificity than the MCP sign (0.57 vs. 0.87). However, after controlling for age and sex in a regression model designed to assess the relationship between gene status and all radiologic signs, only the MCP sign was significant as a predictor of FXTAS diagnosis. This study is clinically relevant in that it suggests that although the CCS sign is common in patients with FXTAS, it is seen in other movement disorders, and additional studies evaluating the addition of the CCS sign to the diagnostic criteria for FXTAS need to be conducted.
Granell and Gomez-Anson presented a study of T1 and T2-weighted fluid-attenuated inversion recovery (FLAIR) MRI data from 31 PC (12 with confirmed FXTAS) and 12 controls, matched for age and sex. The images were assessed by two neuroradiologists by visual inspection. There was a gradient of decreased volumes of the cerebellum, brainstem, and brain along the premutation spectrum compared to controls, with FXTAS patients having the greatest loss. Additionally, there were differences in white matter hyperintensities assessed with a visual rating scale [45], with FXTAS having a greater number of lesions than controls (p = 0.052). Atrophy of the corpus callosum and hyperintensities within it were found in 50 % of FXTAS patients, 10 % of PC without FXTAS, and 0 % of controls. Also, the so-called “putaminal” rim—a hyperintense rim along the lateral aspect of the putamen—was present in 41 % of FXTAS subjects, 47 % of PC without FXTAS, and 28 % of controls (FXTAS > control p < 0.001; FXTAS < PC without FXTAS p = 0.024; and PC without FXTAS > controls p < 0.001). The putaminal rim has been considered a radiological hallmark for multiple system atrophy [46], although this sign may be present in healthy subjects, reflecting age-related ferritin deposits [47]. In PCs, and especially in FXTAS, the putaminal rim may reflect advanced white matter degeneration. Overall, this study shows imaging findings that may be present along the premutation spectrum, additionally to the previously reported major and minor radiology criteria for FXTAS.
In FXTAS, both executive function (EF) and processing speed (PS) may be affected. A subset of carriers without FXTAS may have subtle cognitive deficits, and it is unclear whether these are a premutation phenotype or early signs of FXTAS. Grigsby and colleagues conducted a study to determine whether magnetic resonance spectroscopy (MRS) and diffusion tensor imaging (DTI) changes relate to cognition in PC with and without FXTAS. Thirteen PC men, five of whom met criteria for FXTAS, and seven healthy controls were evaluated with MRS and DTI to examine the hippocampus, and the genu and splenium of the corpus callosum, in relation to EF and PS. MRS measures were n-acetyl aspartate (NAA) and choline (Cho). Fractional anisotropy (FA) was obtained from DTI. EF was assessed with the Behavioral Dyscontrol Scale (BDS) [13] and Controlled Oral Word Association Test (COWAT) [48], and PS with the Symbol Digit Modalities Test (SDMT) [12]. In PC with and without FXTAS, significant correlations were found between white matter micro-structural changes and cognitive functioning. Lower BDS and COWAT scores correlated with decreased NAA, while lower BDS, COWAT, and SDMT scores correlated with decreased FA in the genu. This study showed that white matter disease as detected with MRS and DTI correlates with executive impairment and slowed PS in PC with and without FXTAS. Neuroimaging abnormalities in the genu and MCP suggest that disruption of white matter within frontocerebellar networks plays an important role in the cognitive impairment associated with the FMR1 premutation.
Premutation Carriers Without FXTAS
Imaging studies were also reported in PC carriers without FXTAS. Cerebral atrophy was absent in a MRI study reported by Shelton and colleagues who evaluated 20 PC women without FXTAS and 21 age- and IQ-matched control women. However, reduced cortical white matter (r = 0.525, p = 0.018), cortical grey matter (r = 0.613, p = 0.004), and CCS (r = 0.501, p = 0.024), as well as left cerebellar cortex (r = 0.472, p = .036) volumes were correlated with poor paced auditory serial addition test (PASAT) [49] performance in PCs. Increased levels of PC FMR1 mRNA were significantly associated with mean MCP width, but no other brain structural measure. Although volumetric analysis yielded no difference between PC women without FXTAS and controls, the PASAT and FMR1 overexpression of mRNA may prove to be useful in detecting subtle changes in structure (including both the CCS and MCP) prior to any clinical symptoms of FXTAS.
Overall, the neuroanatomical features of FXTAS are being further refined. Reductions in white matter integrity are seminal to the diagnostic criteria for FXTAS, with preliminary evidence suggesting that changes in the volume of the CCS may be a significant predictor of FXTAS. Importantly, neuroimaging changes are likely to develop prior to any overt FXTAS symptoms [50] and may be best detected through thorough investigation of white matter as well as genetic and cognitive testing. Future studies should focus on longitudinal analysis of PC without FXTAS, mapping the trajectory of neural changes associated with FXTAS, as well as adopting elegant statistical approaches to MRI analysis, specifically to assess white matter integrity. This will enable clearer diagnostic criteria that have greater ability to differentiate FXTAS from other movement disorders.
Pathology
Recent studies of mitochondrial dysfunction in fibroblasts from PC with and without FXTAS have suggested that defective iron and zinc metabolism may be one component of disease pathogenesis [51]. Dr. Martínez-Cerdeño tested this hypothesis by studying both iron transportation into the brain and iron metabolism within the brain. A postmortem study was performed using the Perl’s method for detection of iron bound to hemosiderin and immunohistochemistry with antibodies against iron-binding proteins in nine FXTAS and nine control patients. Results showed that iron accumulated in the stroma of the choroid plexus and within the brain capillaries in FXTAS patients. The expression of iron-binding proteins was also modified in FXTAS. Specifically, transferrin, ferroportin, and ceruloplasmin levels were decreased when compared to control cases. In addition, transferrin receptor 1 distribution was shifted from its normal location in the basolateral membrane to a predominantly intracellular location in FXTAS patients [52]. Iron also accumulated within the cells in the basal ganglia in FXTAS. All cell types within the putamen presented with a 5 to 7-fold increase in iron deposits. Iron deposits in microglia and astrocytes occupied the whole cell cytoplasm, while in oligodendrocytes and neurons iron deposits were smaller. Iron binding proteins were also altered in neuronal cells in the putamen. In summary, an alteration in iron content and iron-related proteins was demonstrated in the brain of individuals with FXTAS. These alterations have implications not only for understanding the pathophysiology of FXTAS but also for the development of new clinical treatments that may incorporate selective iron chelation.
Treatment
Only one clinical trial has been conducted in patients with FXTAS [53], in which memantine did not show clinical benefit. There are currently two new ongoing trials. The design of one of these, an allopregnanolone study, was presented by Dr. Hagerman at the meeting. Allopregnanolone (synonyms: 3a, 5a-tetrahydroprogesterone, 3a-hydroxy-5a-pregnan-20-one) is a naturally occurring neurosteroid and positive modulator of GABAA receptors. This compound can stimulate regeneration of neurons by enhancing neurogenesis in the hippocampus, reversing hippocampal-dependent learning and memory problems, enhancing myelination of the central and peripheral nervous systems, and conferring neuroprotection by both reducing the expression of the proapoptotic protein caspase 3 and inhibiting the mitochondrial permeability transition pore, a key process in the intrinsic pathway of apoptosis-induced loss of neurons [54–56].
PC have been shown to have deficits in cerebellar inhibition over primary motor cortex as well as a reduced GABAA- mediated intracortical and afferent inhibition, compared with healthy controls [57]. Premutation neurons in culture have a burst pattern of spikes that can be alleviated by treatment with allopregnanolone [58]. Preliminary data from the mouse model of FXTAS have demonstrated improvements in motor abilities with allopregnanolone treatment (R. Berman, personal communication 2015). An open-label treatment of patients with FXTAS with allopregnanolone over a 12-week period with a once weekly IV infusion has been started. The dose of allopregnanolone is escalated weekly from 2.0 to 4.0 mg, and finally to 6.0 mg, which is the dose that is continued for the rest of the 12-week period. Allopregnanolone is insoluble in water, so it is conjugated with β-cyclodextrin for IV infusion. Baseline and outcome measures include measures of memory, executive function, reaction time, tremor, balance, MRI volumetric changes, and event-related potentials, in addition to mitochondrial function and oxidative stress. Three individuals with FXTAS have begun the protocol and have tolerated all of the dosages of allopregnanolone without sedation or side effects. If improvements are seen in the symptoms of FXTAS, a larger and longer-term study will be conducted.
Summary
In the last 2 years, significant progress has been made in FMR1 premutation-related diseases. Updates in clinical phenotype, genetic underpinnings, imaging, and pathology were presented at the 2nd International Conference on the FMR1 Premutation meeting and provide evidence that there is a robust interest in the research community. A summary of these findings is presented in Fig. 2. Since the discovery of FXTAS, rapid advances have occurred, including the first clinical trial within 15 years of the disease being described. This feat is only possible given the collaborative nature of the fragile X research community, willingness of the patients to participate in studies, and engagement of the healthcare providers who treat these families. Future directions of the group will include the conclusion of two ongoing clinical trials, expansion of imaging and neuropsychological abnormalities, and a better understanding of the brain pathology in these patients.
Fig. 2.
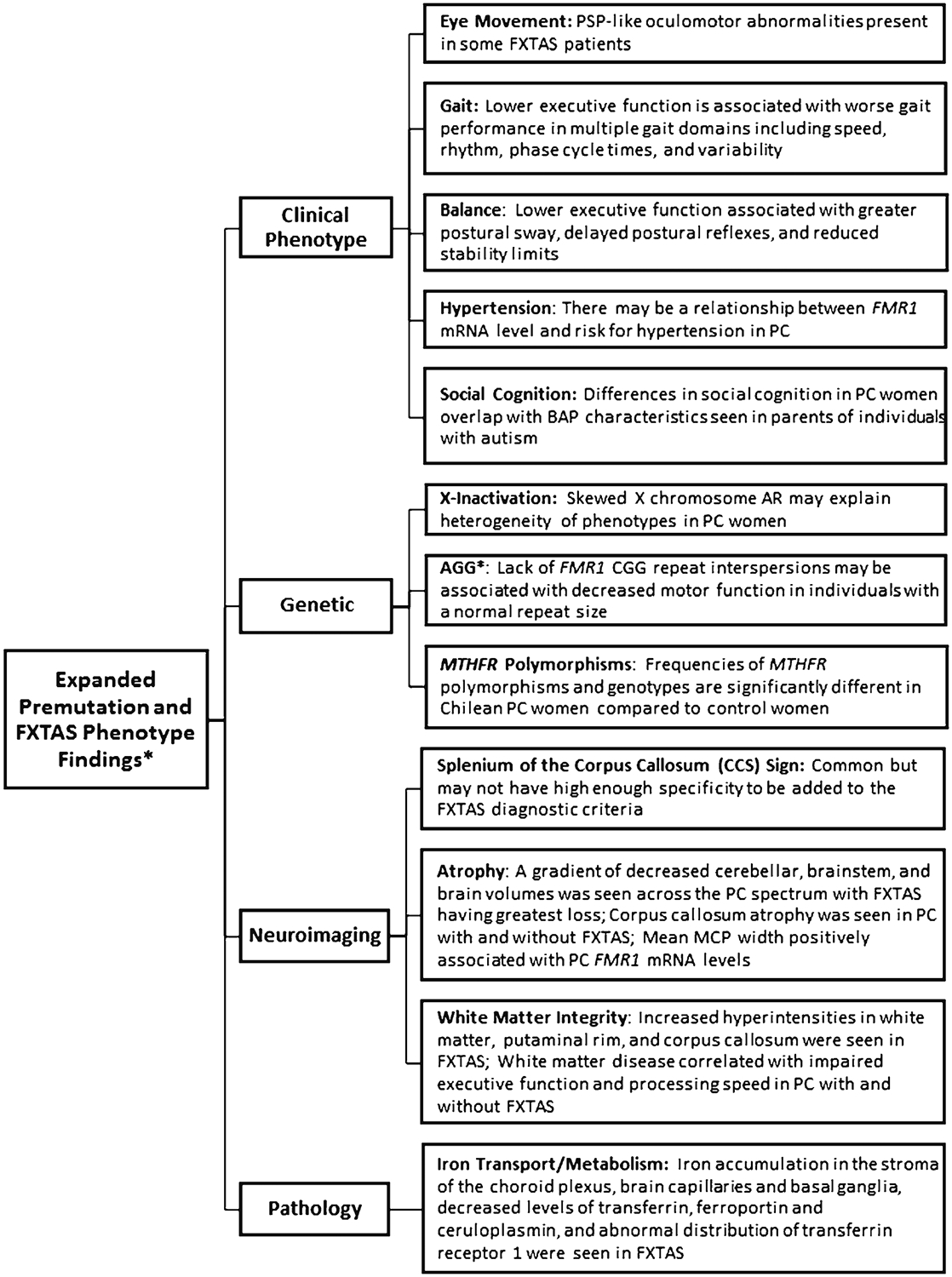
Summary of expanded fragile X-associated tremor/ataxia syndrome (FXTAS) and premutation phenotypes. PSP progressive supranuclear gaze palsy; FMR1 fragile X mental retardation 1 gene; PC premutation carrier; BAP broad autism phenotype; AR activation ratio; MCP middle cerebellar peduncles. Asterisk indicates that this study was conducted in individuals with normal FMR1 repeat size
Acknowledgments
We sincerely thank our premutation carrier and control participants. This work was supported by the following: R01NS082416 (DH); R01MH091131 and R01DC010191 (ML); R01HD032071 (RH); FIS PI 0770 (BGA); NIH (GM082773), Friedman Brain Institute, and Seaver Faculty Scholar (RL); MH094681 (VMC); Strategic Initiative Review Committee of the University of Colorado School of Medicine (JG); Australian Postgraduate Award (ALS); National Fragile X Foundation Fellowship award (ER); and Rush Translational Sciences Consortium Award (JO).
Footnotes
Conflict of Interests Randi Hagerman has received funding from Novartis, Roche, Neuren, and Alcobra for treatment trials in fragile X syndrome (FXS). She has also consulted with Roche/Genentech, Alcobra and Novartis regarding treatment trials in FXS. Elizabeth Berry-Kravis has received funding from Neuren and Alcobra to carry out treatment studies in patients with FXS. She has also received funding from Vtesse to carry out a clinical trial in Niemann-Pick type C. She has also consulted with Neuren, Alcobra, and Neurotrope regarding treatment studies in patients with FXS. Deborah Hall has received research funds from NINDS, Shapiro Foundation, National Parkinson Disease Foundation, Pfizer, and Neurocrine. Dr. Lozano has consulted for Ambry genetics, Courtagen and ClearView Healthcare Partners. The authors declare that they have no conflict of interest.
References
- 1.Murray A, Webb J, MacSwiney F, Shipley E, Morton N, Conway G. Serum concentrations of follicle stimulating hormone may predict premature ovarian failure in FRAXA premutation women. Hum Reprod. 1999;14:1217–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sherman S. Premature ovarian failure in the fragile X syndrome. Am J Med Genet. 2000;97:189–94. [DOI] [PubMed] [Google Scholar]
- 3.Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, et al. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57:127–30. [DOI] [PubMed] [Google Scholar]
- 4.Fraint A, Vittal P, Szewka A. New observations in the fragile X-associated tremor/ataxia syndrome (FXTAS) phenotype. Front Genet. 2014;5:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leehey M, Berry-Kravis E, Goetz C, Zhang L, Hall D, Li L, et al. FMR1 CGG repeat length predicts motor dysfunction in premutation carriers. Neurology. 2008;70:1397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litvan I, Agid Y, Calne D. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. [DOI] [PubMed] [Google Scholar]
- 7.O’Keefe JA, Robertson-Dick EE, Hall DA, Berry-Kravis E. Gait and functional mobility deficits in fragile X-associated tremor/ataxia syndrome. Cerebellum. August 23 2015. doi: 10.1007/s12311-015-0714-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lord S, Galna B, Verghese J, Coleman S, Burn D, Rochester L. Independent domains of gait in older adults and associated motor and nonmotor attributes: validation of a factor analysis approach. J Gerontol A Biol Sci Med Sci. 2013;68:820–7. [DOI] [PubMed] [Google Scholar]
- 9.Hollman JH, McDade EM, Petersen RC. Normative spatiotemporal gait parameters in older adults. Gait Posture. 2011;34:111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serrao M, Pierelli F, Ranavolo A, Draicchio F, Conte C, Don R, et al. Gait pattern in inherited cerebellar ataxias. Cerebellum. 2012;11: 194–211. [DOI] [PubMed] [Google Scholar]
- 11.Ilg W, Golla H, Thier P, Giese MA. Specific influences of cerebellar dysfunctions on gait. Brain. 2007;130:786–98. [DOI] [PubMed] [Google Scholar]
- 12.Smith. The symbol-digit modalities test: a neuropsychologic test of learning and other cerebral disorders In: Helmuth J, editor. Learning disorders Seattle: Special Child Publications; 1968. p. 83. [Google Scholar]
- 13.Luria AR. Human brain and psychological processes. New York: Harper and Row; 1966. [Google Scholar]
- 14.Amboni M, Barone P, Hausdorff JM. Cognitive contributions to gait and falls: evidence and implications. Mov Disord. 2013;28: 1520–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segev-Jacubovski O, Herman T, Yogev-Seligmann G, Mirelman A, Giladi N, Hausdorff JM. The interplay between gait, falls and cognition: can cognitive therapy reduce fall risk? Expert Rev Neurother. 2011;11:1057–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor ME, Delbaere K, Mikolaizak AS, Lord SR, Close JC. Gait parameter risk factors for falls under simple and dual task conditions in cognitively impaired older people. Gait Posture. 2013;37: 126–30. [DOI] [PubMed] [Google Scholar]
- 17.Losh M, Klusek J, Martin GE, Sideris J, Parlier M, Piven J. Defining genetically meaningful language and personality traits in relatives of individuals with fragile X syndrome and relatives of individuals with autism. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:660–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraan CM, Hocking DR, Georgiou-Karistianis N, Metcalfe SA, Archibald AD, Fielding J, et al. Impaired response inhibition is associated with self-reported symptoms of depression, anxiety, and ADHD in female FMR1 premutation carriers. Am J Med Genet B Neuropsychiatr Genet. 2014;165B:41–51. [DOI] [PubMed] [Google Scholar]
- 19.Grigsby J, Cornish K, Hocking D, Kraan C, Olichney JM, Rivera SM, et al. The cognitive neuropsychological phenotype of carriers of the FMR1 premutation. J Neurodev Disord. 2014;6:28,1955-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–85. [DOI] [PubMed] [Google Scholar]
- 21.Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop S. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2). 2nd ed Los Angeles: Western Psychological Services; 2012. [Google Scholar]
- 22.Wang J, Yu R, Shete S. X-chromosome genetic association test accounting for X-inactivation, skewed X-inactivation, and escape from X-inactivation. Genet Epidemiol. 2014;38:483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry-Kravis E, Potanos K, Weinberg D, Zhou L, Goetz CG. Fragile X-associated tremor/ataxia syndrome in sisters related to X-inactivation. Ann Neurol. 2005;57:144–7. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Revenga L, Pagonabarraga J, Gomez-Anson B, Lopez-Mourelo O, Madrigal I, Xuncla M, et al. Motor and mental dysfunction in mother-daughter transmitted FXTAS. Neurology. 2010;75:1370–6. [DOI] [PubMed] [Google Scholar]
- 25.O’Keefe JA, Robertson-Dick E, Dunn EJ, Li Y, Deng Y, Fiutko AN, et al. Characterization and early detection of balance deficits in fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome (FXTAS). Cerebellum. 2015;14:650–62. [DOI] [PubMed] [Google Scholar]
- 26.Hall DA, Robertson-Dick E, O’Keefe JA, Hadd A, Zhou L, Berry-Kravis E. X-inactivation in the clinical phenotype of fragile X premutation carrier sisters. Neurol Genet. 2016. February 3;2(1):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Natus Medical Incorporated. Balance Manager Systems, Clinical Interpretations Guide, Computerized Dynamic Posturography; Natus Medical Incorporated. 2009;D102559–00D:139–145. [Google Scholar]
- 28.Black FO. What can posturography tell us about vestibular function? Ann N Y Acad Sci. 2001;942:446–64. [DOI] [PubMed] [Google Scholar]
- 29.Salarian A, Horak FB, Zampieri C, Carlson-Kuhta P, Nutt JG, Aminian K. iTUG, a sensitive and reliable measure of mobility. IEEE Trans Neural Syst Rehabil Eng. 2010;18:303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L, Hadd AG, Sah S, Houghton JF, Filipovic-Sadic S, Zhang W, et al. High-resolution methylation polymerase chain reaction for fragile X analysis: evidence for novel FMR1 methylation patterns undetected in Southern blot analyses. Genet Med. 2011;13:528–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valencia K, Wutz A. Recent insights into the regulation of X-chromosome inactivation. Adv Genomics Genet. 2015;5:227–38. [Google Scholar]
- 32.Alvarez-Mora M, Rodriguez-Revenga L, Feliu A, Badenas C, Madrigal I, Milà M. Skewed X inactivation in women carrying the FMR1 premutation and its relation with fragile-X-associated tremor/ataxia syndrome, Neurodegener Dis. 2015. [DOI] [PubMed]
- 33.Bennett D, Schneider J, Arvanitakis Z, Wilson R. Overview and findings from the religious orders study. Curr Alzheimers Res. 2012;9:628–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen EG, Sullivan AK, Marcus M, Small C, Dominguez C, Epstein MP, et al. Examination of reproductive aging milestones among women who carry the FMR1 premutation. Hum Reprod. 2007;22:2142–52. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Revenga L, Madrigal I, Pagonabarraga J, Xuncla M, Badenas C, Kulisevsky J, et al. Penetrance of FMR1 premutation associated pathologies in fragile X syndrome families. Eur J Hum Genet. 2009;17:1359–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wheeler AC, Bailey DB Jr, Berry-Kravis E, Greenberg J, Losh M, Mailick M, et al. Associated features in females with an FMR1 premutation. J Neurodev Disord. 2014;6:30,1955-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bailey DB Jr, Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. Am J Med Genet A. 2008;146A:2060–9. [DOI] [PubMed] [Google Scholar]
- 38.Lachiewicz A, Dawson D, Spiridigliozzi G, Cuccaro M, Lachiewicz M, McConkie-Rosell A. Indicators of anxiety and depression in women with the fragile X premutation: assessment of a clinical sample. J Intellect Disabil Res. 2010;54:597–610. [DOI] [PubMed] [Google Scholar]
- 39.Roberts JE, Bailey DB Jr, Mankowski J, Ford A, Sideris J, Weisenfeld LA, et al. Mood and anxiety disorders in females with the FMR1 premutation. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:130–9. [DOI] [PubMed] [Google Scholar]
- 40.Lewis SJ, Lawlor DA, Davey Smith G, Araya R, Timpson N, Day IN, et al. The thermolabile variant of MTHFR is associated with depression in the British Women’s Heart and Health study and a meta-analysis. Mol Psychiatry. 2006;11:352–60. [DOI] [PubMed] [Google Scholar]
- 41.Peerbooms OL, van Os J, Drukker M, Kenis G, Hoogveld L, MTHFR in Psychiatry Group, et al. Meta-analysis of MTHFR gene variants in schizophrenia, bipolar disorder and unipolar depressive disorder: evidence for a common genetic vulnerability? Brain Behav Immun. 2011;25:1530–43. [DOI] [PubMed] [Google Scholar]
- 42.Ginsberg LD, Oubre AY, Daoud YA. L-methylfolate Plus SSRI or SNRI from treatment initiation compared to SSRI or SNRI monotherapy in a major depressive episode. Innov Clin Neurosci. 2011;8:19–28. [PMC free article] [PubMed] [Google Scholar]
- 43.Jacquemont S, Hagerman R, Leehey M, Grigsby J, Zhang L, Brunberg J. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Apartis E, Blancher A, Meissner W. FXTAS: new insights and the need for revised diagnostic criteria. Neurology. 2012;79:1898–907. [DOI] [PubMed] [Google Scholar]
- 45.Scheltens P, Barkhof F, Leys D, Pruvo J, Naura J, Vermersch P, et al. A semiquantitative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci. 1993;114:7–12. [DOI] [PubMed] [Google Scholar]
- 46.Tha KK, Terae S, Tsukahara A, Soma H, Morita R, Yabe I, et al. Hyperintense putaminal rim at 1.5 T: prevalence in normal subjects and distinguishing features from multiple system atrophy. BMC Neurol. 2012;12:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujii S, Matsusue E, Kinoshita T, Sugihara S, Ohama E, Ogawa T. Hyperintense putaminal rim at 3T reflects fewer ferritin deposits in the lateral marginal area of the putamen. AJNR Am J Neuroradiol. 2007;28:777–81. [PMC free article] [PubMed] [Google Scholar]
- 48.Borkowski J, Benton A, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5:135–40. [Google Scholar]
- 49.Wiens A, Fuller K, Crossen J. Paced auditory serial addition test: adult norms and moderator variables. J Clin Exp Neuropsychol. 1997;19:473–83. [DOI] [PubMed] [Google Scholar]
- 50.Brown SS, Stanfield AC. Fragile X premutation carriers: a systematic review of neuroimaging findings. J Neurol Sci. 2015;352(1–2): 19–28. [DOI] [PubMed] [Google Scholar]
- 51.Napoli E, Ross-Inta C, Wong S, Omanska-Klusek A, Barrow C, Iwahashi C, et al. Altered zinc transport disrupts mitochondrial protein processing/import in fragile X-associated tremor/ataxia syndrome. Hum Mol Genet. 2011;20:3079–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ariza J, Steward C, Rueckert F, Widdison M, Coffman R, Afjei A, et al. Dysregulated iron metabolism in the choroid plexus in fragile X-associated tremor/ataxia syndrome. Brain Res. 2015;1598:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seritan AL, Nguyen DV, Mu Y, Tassone F, Bourgeois JA, Schneider A, et al. Memantine for fragile X-associated tremor/ataxia syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2014;75:264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brinton RD. Neurosteroids as regenerative agents in the brain: therapeutic implications. Nat Rev Endocrinol. 2013;9:241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Irwin RW, Brinton RD. Allopregnanolone as regenerative therapeutic for Alzheimer’s disease: translational development and clinical promise. Prog Neurobiol. 2014;113:40–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Irwin RW, Solinsky CM, Brinton RD. Frontiers in therapeutic development of allopregnanolone for Alzheimer’s disease and other neurological disorders. Front Cell Neurosci. 2014;8:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Conde V, Palomar FJ, Lama MJ, Martinez R, Carrillo F, Pintado E, et al. Abnormal GABA-mediated and cerebellar inhibition in women with the fragile X premutation. J Neurophysiol. 2013;109:1315–22. [DOI] [PubMed] [Google Scholar]
- 58.Cao Z, Hulsizer S, Tassone F, Tang HT, Hagerman RJ, Rogawski MA, et al. Clustered burst firing in FMR1 premutation hippocampal neurons: amelioration with allopregnanolone. Hum Mol Genet. 2012;21:2923–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


