Abstract
Thymus albicans is an endemic species of the Iberian Peninsula with a vulnerable conservation status. In an attempt to contribute to the valorization of this species, the present study brings new insights on the antifungal and anti-inflammatory mechanism of action of T. albicans essential oil. The antifungal activity of the oil and its major compounds was assessed for the first time against standard and clinically isolated strains of yeasts and filamentous fungi. The effect on the two major virulence factors of Candida albicans (germ tube formation and biofilm disruption) was considered in more detail. At 0.08 μL/mL, the oil inhibited C. albicans germ tube formation by more than 40% and decreased biofilm biomass at MIC values, thus pointing out its antivirulent potential. The anti-inflammatory activity of the essential oil was investigated on LPS-stimulated mouse macrophages (RAW 264.7) by evaluating the levels of several pro-inflammatory mediators, namely nitric oxide (NO), inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). T. albicans oil reduced the production of nitrites, a NO derived sub-product, at non-cytotoxic concentrations of 0.32 and 0.64 μL/mL, by 27 and 41%, respectively. In addition, the iNOS protein levels of essential oil pre-treated cells were reduced by 14%. Overall, the high essential oil yield of T. albicans as well as its bioactive effects at concentrations without cytotoxicity, encourage further studies on the potential pharmacological applications of this species. Furthermore, these results raise awareness for the need to preserve endangered species that may hold relevant medicinal value.
Subject terms: Drug discovery, Plant sciences
Introduction
Thyme species are among the most important medicinal and aromatic crops in non-tropical environments1. The medicinal and non-medicinal uses of these plants are mainly related to their volatile extracts. Several Thymus spp. extracts are widely used for the treatment of gastrointestinal, respiratory and skin disorders, and their use is regulated by some entities and agencies, such as the European Medicines Agency2, European Scientific Cooperative on Phytotherapy3, German Commission E and World Health Organization4,5.
Food and flavour industries take advantage of the aromatic and antiseptic properties of thyme essential oils applying them in the preservation of food products and in the production of cosmetics and toiletries6. Moreover, due to its aromatic characteristics, thyme is a daily used culinary herb all over the world.
Thymus albicans Hoffm. and Link, popularly known in Portugal as tomilho-alvadio [syn. Origanum albicans (Hoffmans. and Link) Kuntze; Thymus mastichina var. micranthus Boiss.; Thymus tomentosus var. virescens Coss.; Thymus virescens (Coss.) Pau]7 is an endemic species to the coastal area of the south-west Iberian Peninsula currently listed as vulnerable (meaning at high risk of extinction) by the IUCN Red List of Threatened Species8. It can only be found in the region of Algarve in Portugal, and in the provinces of Sevilla and Cádiz in Spain, being presently distributed in disperse and severely fragmented populations with a decreasing trend9. The main threats are related to the pressure exerted by mass tourism, agriculture and aquaculture practices.
Thymus albicans belongs to Sect. Mastichina, together with Thymus mastichina (L.) L. subsp. mastichina and Thymus mastichina subsp. donyanae R. Morales 10. The aerial flowering parts of the plant are traditionally used for the same purposes of T. mastichina subsp. mastichina, mainly for the treatment of oral and pharyngeal inflammation and dermatitis11. Additionally, there is ethnopharmacological evidence of its use as a stomach tonic in Spain12.
Thymus mastichina is one of the most industrially relevant thyme species, currently employed in the production of cleaning products, perfumes, fragrances, soaps and other cosmetics13,14. It is widely cultivated in Portugal and Spain, and has an ISO standard (4728:2003) specifying the quality parameters of the essential oil for industrial and commercial uses15. Several pharmacological activities have been reported for T. mastichina essential oil, including antimicrobial activity16–19, anti-inflammatory potential20,21, antioxidant activity22–24 and cytotoxicity against cancer cell lines25–27. Due to the similarities between the chemical profile of T. mastichina and T. albicans essential oils, it seems reasonable to assume that both species might possess similar bioactive properties. This assumption is in part corroborated by previous studies showing the antibacterial activity of T. albicans essential oil against Gram-positive bacteria28, its antioxidant activity29,30, its capacity to scavenge nitric oxide and to inhibit the activity of lipoxygenase in vitro, both commonly used as markers of anti-inflammatory potential21. As far as we know, those are the only studies on the bioactive potential of T. albicans essential oil. More studies are needed to further confirm the reported effects and to explore new bioactivities.
In this regard, the present study aimed to investigate the chemical profile, and the antifungal and anti-inflammatory activities of Thymus albicans essential oil from Portugal and its major compounds, concomitantly highlighting the mechanisms of action underlying these effects. These bioactivities were selected taking into account their current relevance in the clinic. In fact, invasive fungal infections are an increasingly important global health burden accounting for 1.66 million deaths every year31. While these mainly affect immunocompromised individuals, superficial fungal infections are reported to affect between 20 and 25% of the world’s population, including immunocompetent individuals32. Treatment failure and high relapse rates are mainly correlated with the extensive use of a limited number of non-selective antifungal drugs (e.g. fluconazole, amphotericin B and terbinafine) which leads to off-target toxicity and to the emergence of resistance among the main etiologic agents33. Chronic inflammation is another factor that contributes to the successful colonization of the host tissues by pathogenic fungi and may impede disease eradication34. Taken together, these facts justify the urgency for therapeutic alternatives, preferentially combining selective antifungal and anti-inflammatory activities.
Results and discussion
Essential oil composition
Prior to this study, several essential oils from T. albicans plants collected in different regions of Algarve (Portugal) were chemically characterized, revealing chemical variability regarding the four major compounds 1,8-cineole (29–43%), linalool (5–25%), α-terpineol (4.5–9.8%) and borneol (2.2–8.0%). The most predominant chemical profile was characterized by high contents of 1,8-cineole and linalool. Therefore, for the present study we selected the sample that better represented the chemical profile of the essential oils from Portuguese T. albicans. This sample was obtained from populations with the same chemical profile growing in Foz de Almargem coastal lagoon in Algarve. The chemical characterization of the obtained essential oil is shown in Table 1.
Table 1.
Composition of the essential oil of Thymus albicans.
| RIa | RIp | Compoundsa | % |
|---|---|---|---|
| 922 | 1030 | α-Thujene | 0.2 |
| 930 | 1030 | α-Pinene | 1.7 |
| 943 | 1073 | Camphene | 2.3 |
| 964 | 1128 | Sabinene | 1.1 |
| 970 | 1118 | β-Pinene | 1.9 |
| 980 | 1161 | Myrcene | 0.7 |
| 1006 | 1185 | α-Terpinene | 0.4 |
| 1013 | 1272 | p-Cymene | 0.1 |
| 1020 | 1206 | Limonene | 0.6 |
| 1020 | 1212 | 1,8-Cineole | 40.5 |
| 1035 | 1250 | trans-β-Ocimene | 0.2 |
| 1047 | 1250 | γ-Terpinene | 0.3 |
| 1050 | 1459 | trans-Sabinene hydrate | 0.1 |
| 1081 | 1543 | Linalool | 25.0 |
| 1115 | 1516 | Camphor | 3.4 |
| 1129 | 1668 | trans-Verbenol | 0.1 |
| 1142 | 1667 | δ-Terpineol | 3.0 |
| 1142 | 1693 | Borneol | 6.4 |
| 1158 | 1595 | Terpinen-4-ol | 1.4 |
| 1166 | 1692 | α-Terpineol | 4.5 |
| 1178 | 1780 | Myrtenol | 0.1 |
| 1210 | 1764 | Citronellol | 0.2 |
| 1233 | 1842 | Geraniol | 0.4 |
| 1266 | 1574 | Bornyl acetate | 0.3 |
| 1359 | 1755 | Geranyl acetate | 0.1 |
| 1382 | 1585 | β-Elemene | 0.3 |
| 1411 | 1590 | trans-β-Caryophyllene | 0.5 |
| 1498 | 1751 | γ-Cadinene | 0.1 |
| 1526 | 2070 | Elemol | 0.1 |
| 1557 | 1968 | Caryophyllene oxide | 0.4 |
| 1569 | 2072 | Viridiflorol | 0.2 |
| 1579 | 2025 | Ledol | 0.1 |
| 1615 | 2153 | T-Cadinol | t |
| 1628 | 2218 | α-Cadinol | t |
| 1628 | 2208 | α-Eudesmol | t |
| Monoterpene hydrocarbons | 9.5 | ||
| Oxygen containing monoterpenes | 85.5 | ||
| Sesquiterpene hydrocarbons | 0.9 | ||
| Oxygen containing sesquiterpenes | 0.9 | ||
| Total | 96.8 |
RIa, retention indices on the SPB-1 column relative to C8 to C24 n-alkanes. RIp, retention indices on the SupelcoWax-10 column.
aCompounds listed in order of their elution on the SPB-1 column; t, traces (≤ 0.05%).
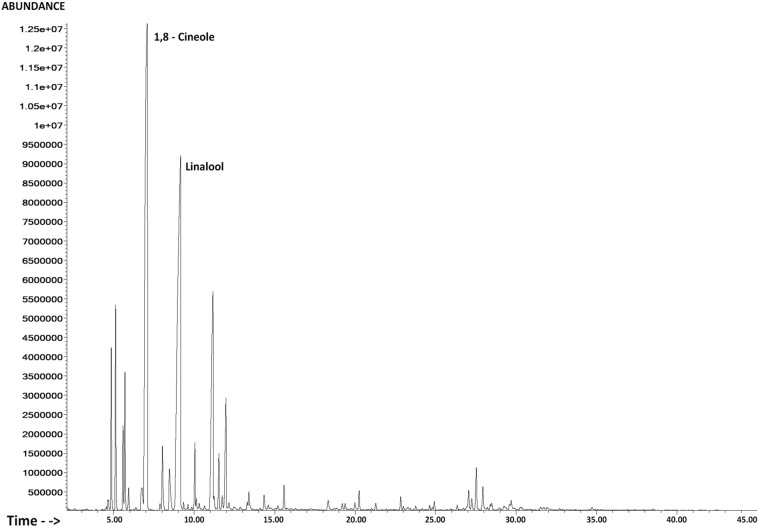
Oxygen containing monoterpenes represented the major fraction of the oil (85.5%). The major compounds identified were 1,8-cineole (40.5%) and linalool (25.0%), followed by borneol (6.4%) and α-terpineol (4.5%). The Total Ion Current (TIC) chromatogram, processed using HP Enhanced ChemStation software, is shown in Fig. 1. Our results are in accordance with the works of Morales Valverde10 and Salgueiro, et al.35 that pointed out 1,8-cineole as the major compound of T. albicans essential oil from Portugal followed by linalool. Miguel, et al.36 studied the oils obtained from leaves and from flowers, distinguishing the chemotypes 1,8-cineole and 1,8-cineole/linalool.
Figure 1.
Total ion current chromatogram of the essential oil of Thymus albicans.
Thymus albicans essential oil, in the present study, was obtained from the flowering aerial parts with a yield of 2.4% (v/w). Our results are in agreement with the study of Aazza, et al.21 reporting a yield of 2.2%. This yield is quite relevant since it is higher than that obtained with industrially relevant species, such as T. vulgaris, T. zygis and T. mastichina, thus highlighting the industrial potential of T. albicans.
Fungal growth (MIC and MLC)
As far as it is known, this is the first report on the antifungal effect of T. albicans essential oil. The oil showed some degree of inhibition against all Candida, Cryptococcus neoformans, dermatophytes and Aspergillus strains tested (Table 2), thus showing a broad-spectrum antifungal effect. Moreover, for the majority of yeast and all dermatophyte strains, MIC values were similar to MLC ones, pointing out a fungicidal effect of the oil. The highest antifungal activity was observed against Trichophyton rubrum CECT 2794 and Epidermophyton floccosum FF9 (MIC and MLC of 0.64 μL/mL).
Table 2.
Antifungal activity (MIC and MLC) of Thymus albicans essential oil and its major compounds (1,8-cineole, linalool, borneol and α-terpineol) against collection type and clinical strains of Candida, Cryptococcus neoformans, dermatophytes and Aspergillus.
| Strains | Thymus albicans | 1,8-Cineole | Linalool | Borneol | α-Terpineol | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MICa | MLCa | MICa | MLCa | MICa | MLCa | MICa | MLCa | MICa | MLCa | |
| Candida albicans ATCC 10231 | 1.25 | 2.5 | 10 | 10 | 5 | 5 | 2.5 | > 20 | 1.25 | 2.5 |
| Candida tropicalis ATCC 13803 | 2.5 | 2.5 | 20 | 20 | 5 | 5 | 2.5 | > 20 | 1.25 | 2.5 |
| Candida krusei H9 | 2.5 | 2.5 | 10 | 10 | 10 | 10 | 5 | > 20 | 1.25 | 2.5 |
| Candida guilliermondii MAT23 | 1.25 | 1.25 | 10 | 10 | 5 | 10 | 2.5 | 2.5 | 1.25 | 1.25–2.5 |
| Candida parapsilosis ATCC 90,018 | 2.5 | 2.5 | 10 | 10 | 10 | 10 | 5 | > 20 | 0.64–1.25 | 2.5 |
| Cryptococcus neoformans CECT 1078 | 1.25 | 1.25 | 5–10 | 10 | 5 | 5 | 1.25 | 1.25 | 0.64–1.25 | 1.25 |
| Epidermophyton floccosum FF9 | 0.64 | 0.64 | 5 | 5 | 1.25–2.5 | 2.5 | 2.5 | 2.5 | 1.25 | 1.25 |
| Microsporum canis FF1 | 1.25 | 1.25 | 5 | 5 | 2.5 | 2.5 | 2.5 | 2.5 | 1.25 | 1.25 |
| Microsporum gypseum CECT 2908 | 1.25 | 1.25 | 5–10 | 10 | 1.25–2.5 | 2.5 | 2.5 | 2.5 | 1.25 | 1.25 |
| Trichophyton mentagrophytes FF7 | 1.25 | 1.25 | 5 | 5 | 1.25 | 2.5 | 2.5 | 5 | 1.25 | 2.5 |
| Trichophyton mentagrophytes var. interdigitale CECT 2958 | 1.25 | 1.25 | 10 | 10 | 2.5 | 2.5–5 | 2.5 | 5 | 0.64 | 1.25 |
| Trichophyton rubrum CECT 2794 | 0.64 | 0.64 | 2.5–5 | 5 | 1.25 | 1.25–2.5 | 2.5 | 2.5 | 1.25 | 1.25–2.5 |
| Trichophyton verrucosum CECT 2992 | 1.25 | 1.25 | 10 | 10–20 | 1.25–2.5 | 1.25–2.5 | 2.5 | 2.5 | 0.64 | 1.25 |
| Aspergillus niger ATCC 16404 | 2.5 | 10–20 | 10 | > 20 | 5 | ≥ 20 | 5 | > 20 | 0.64 | 10–20 |
| Aspergillus fumigatus ATCC 46645 | 2.5 | 5 | 10 | 10–20 | 2.5 | 20 | 2.5 | > 20 | 0.32–0.64 | 2.5 |
| Aspergillus flavus F44 | 5 | 5–10 | 20 | 20 | 10 | ≥ 20 | 5 | > 20 | 0.64–1.25 | 2.5 |
aMIC and MLC determined by macrodilution method and expressed as μL/mL.
Other essential oils with high amounts of 1,8-cineole, such as the ones isolated from Salvia fruticosa, Thymus capitellatus and Thymus mastichina, were previously shown to exert antifungal activity against several fungal species16,37,38.
Regarding the antifungal activity of T. albicans major compounds, 1,8-cineole, linalool, borneol and α-terpineol, only the later showed a slightly stronger effect than the essential oil. Among the strains tested, C. parapsilosis ATCC 90018, Cryptococcus neoformans CECT 1078 and Aspergillus strains were the most susceptible to α-terpineol with MIC values ranging from 0.32 to 1.25 μL/mL. Also, Hammer, et al.39 demonstrated that α-terpineol was more active than 1,8-cineole against some of the fungi species tested, namely C. albicans, C. parapsilosis, E. floccosum, M. canis, T. mentagrophytes var. interdigitale, T. rubrum, A. niger, A. fumigatus and A. flavus. Although 1,8-cineole had a weak activity, several studies reported synergistic effects of this compound with other monoterpenes40. Moreover, this compound has also shown synergistic effects with chlorhexidine, a commonly used antiseptic41,42.
Candida albicans virulence factors
Among Candida species, C. albicans is the main etiological agent of candidiasis and one of the main causes of hospital-acquired infections43. The pathogenicity of C. albicans is associated with two major virulence factors: germ tube and biofilm formation. The germ tube (filamentation) confers increased resistance to phagocytosis and allows the penetration into deeper layers of the mucosa leading to invasive infections44. Biofilms, formed on both implanted medical devices and host tissues, potentiate C. albicans dissemination and increase its resistance to conventional drugs45. They act as reservoirs of multi-resistant yeast cells, which upon filamentation trigger resistant systemic infections. Despite the major role of these virulence factors in the pathogenesis of invasive Candida infections, to date, no-specific germ tube and biofilm antifungal drugs are available.
Candida albicans germ tube formation
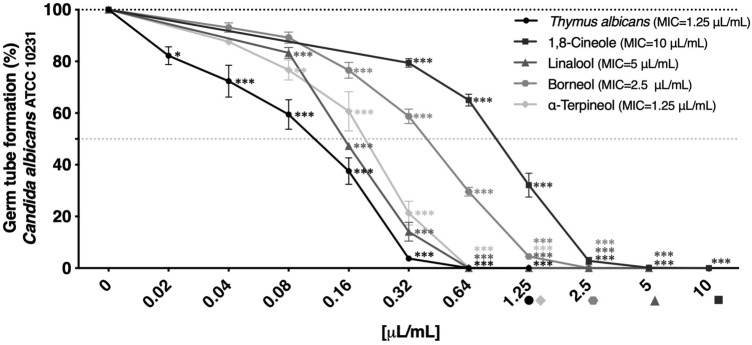
In our study, T. albicans essential oil was able to strongly inhibit the germ tube formation of C. albicans at concentrations much lower than the respective MIC. At 0.08 μL/mL the oil inhibited germ tube formation by 40.5% and at 0.32 μL/mL a 96.3% inhibition was attained (Fig. 2). These results are quite interesting since fluconazole, a conventional antifungal drug widely used in the clinic, despite inhibiting fungal growth at very low concentrations, is ineffective in decreasing germ tube formation. Indeed, even at concentrations up to 200 µg/mL the percentage of inhibition did not exceed 10% (Figure S1). Conversely, T. albicans essential oil was able to strongly reduce C. albicans germ tube formation at concentrations as low as 0.08 µL/mL.
Figure 2.
Germ tube formation in Candida albicans ATCC 10231 treated with sub-inhibitory concentrations—ranging from MIC to MIC/64—of Thymus albicans essential oil and its main compounds (1,8-cineole, linalool, borneol and α-terpineol). The values are presented as percentage of control (oil-free samples with 1% DMSO (v/v)) ± SEM of at least three independent experiments performed in duplicate. Statistical differences were calculated by one-way ANOVA followed by Dunnett’s post hoc test (*p < 0.05, **p < 0.01, ***p < 0.001).
Regarding the major compounds tested, only linalool could completely inhibit germ tube formation at sub-MIC concentrations (0.64 μL/mL). Hsu et al.46 and Zore et al.47 also reported the ability of linalool to decrease C. albicans germ tube formation at sub-inhibitory concentrations. The results obtained for 1,8-cineole are consistent with those reported by Pina‐Vaz et al.16. Moreover, in the same study, phenolic thyme essential oils from T. vulgaris and T. zygis were less effective than T. albicans in inhibiting germ tube formation, although both oils showed better antifungal activity (lower MIC values) against C. albicans.
Disruption of preformed biofilms in Candida albicans
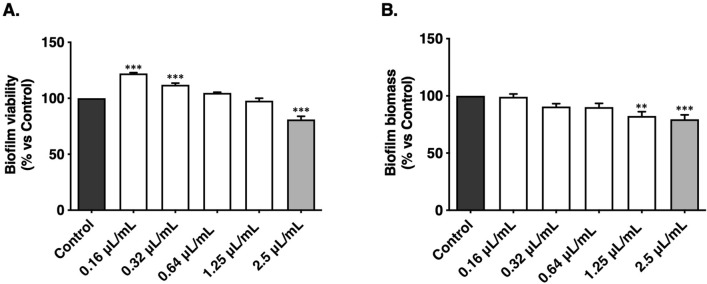
Besides germ tube formation, the pathogenicity of C. albicans is also associated with biofilm formation. In the present study, T. albicans essential oil was assessed for its capacity to decrease biofilm mass and viability (Fig. 3). At MIC values, the oil decreased biofilm mass, while fluconazole had no effect.
Figure 3.
Influence of Thymus albicans essential oil on Candida albicans ATCC 10231 biofilm viability (A) and effect on biofilm biomass after 24 h (B). Each value represents the mean ± SEM of at least three independent experiments performed in duplicate. Statistical differences were calculated by one-way ANOVA followed by Dunnett’s post hoc test (**p < 0.01, ***p < 0.001). The grey bar represents the minimum lethal concentration (MLC) of Thymus albicans essential oil against Candida albicans ATCC 10231.
The main compound of T. albicans essential oil, 1,8-cineole, was shown to inhibit C. albicans biofilms at higher concentrations than the oil41. Also, other major compounds, namely linalool, borneol and α-terpineol were previously reported to disrupt C. albicans biofilm formation46,48,49.
Germ tube formation and biofilm integrity in C. albicans are important virulence factors that allow the transition from a superficial mycosis to a systemic infection. Overall, our results show that T. albicans essential oil presents antivirulent potential against C. albicans since it was able to inhibit both of these features. The possibility of targeting virulence factors at non-growth inhibitory concentrations is a promising therapeutic strategy, particularly because it may reduce the toxicity of antifungal therapy simultaneously reducing not only the selective pressure for the emergence of resistance among pathogenic fungi but also the impact on the host’s natural microbiota44.
Anti-inflammatory activity
LPS-stimulated mouse macrophages (RAW 264.7) were used as an in vitro model of acute inflammation. Briefly, LPS, a Toll-like receptor 4 agonist and a component of the outer membrane of Gram-negative bacteria, acts upstream of the NF-κB signaling pathway inducing the phosphorylation and degradation of its inhibitory protein κB (IκB). Once released from inhibition, NF-κB translocates into the nucleus activating the transcription of genes coding for the inducible form of nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), two pro-inflammatory enzymes, among others. iNOS catalyzes the synthesis of NO through oxidation of L-arginine and COX-2 the conversion of arachidonic acid to prostaglandin E2 (PGE2)50.
In our study, the levels of NO stable metabolites (nitrites) before and after T. albicans oil treatment were measured by the colorimetric Griess reaction and used as a parameter to determine the anti-inflammatory potential of the essential oil.
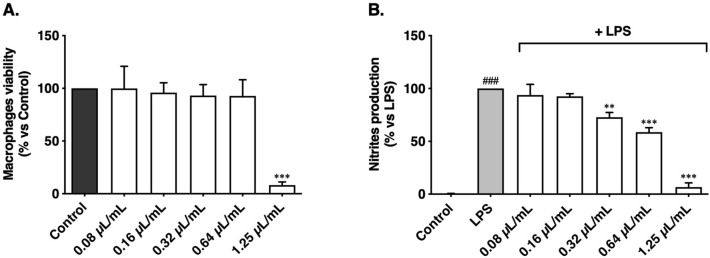
Our results demonstrate that T. albicans oil was able to inhibit nitrites production by LPS-stimulated macrophages in a dose-dependent manner. Importantly, a significant reduction by 27.16 and 41.32%, was observed at the non-cytotoxic concentrations of 0.32 and 0.64 μL/mL, respectively, in comparison with the LPS stimuli (Fig. 4). In an analogous experiment, Zuzarte et al.51 evaluated the effect of 1,8-cineole on the same parameter, demonstrating that it also inhibited the production of nitrites, however at higher concentrations (2.25 μL/mL). This indicates that other compounds may contribute to the anti-inflammatory activity of T. albicans oil.
Figure 4.
Effect of Thymus albicans essential oil on macrophages viability (A) and nitrites production (B). Cell viability is expressed as a percentage of MTT reduction in comparison to control cells (100% viability). Nitrite production is expressed as a percentage of nitrite production in comparison to cells stimulated with LPS alone (100% nitrite production). Each value represents the mean ± SEM from three experiments, performed in duplicate. Statistical differences between groups were calculated by one-way ANOVA followed by Dunnett’s post hoc test (###p < 0.001, compared to control (B); **p < 0.01, ***p < 0.001 compared to control (A) and to LPS (B)).
Mechanism of action: scavenging capacity or gene expression regulation?
To clarify whether the lower levels of nitrites observed after T. albicans essential oil treatment were due to its direct scavenging potential, a reaction between a NO donor (SNAP) and different concentrations of the oil was performed. Since T. albicans essential oil failed to scavenge NO (Fig. 5), we could exclude this hypothesis to justify the reduction of nitrite levels in LPS-stimulated macrophages. In the study of Aazza, et al.21, T. albicans essential oil was shown to scavenge NO in vitro, however at much higher concentrations than those tested in our study.
Figure 5.
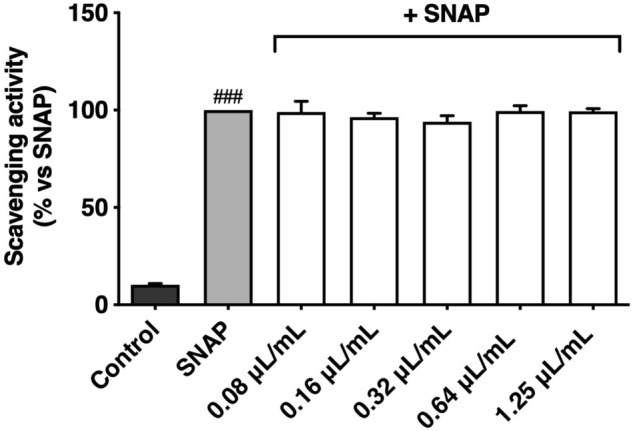
NO scavenging potential of Thymus albicans essential oil. Different concentrations of essential oil (0.08–1.25 μL/mL) were incubated with SNAP (300 μM) in culture medium for 3 h. Results are expressed as a percentage of NO release triggered by SNAP. Each value represents the mean ± SEM of three independent assays, performed in duplicate. Statistical differences were calculated by one-way ANOVA followed by Dunnett’s post hoc test (###p < 0.001, compared to control).
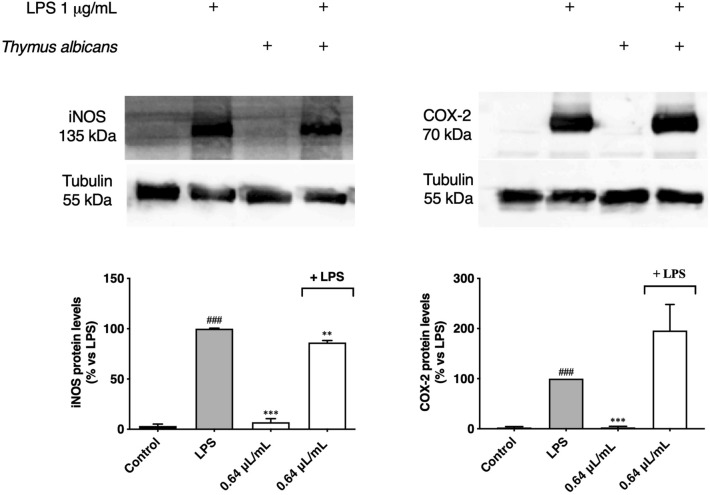
Another mechanism by which T. albicans essential oil could reduce the nitrite levels is the modulation of genes coding for key enzymes along the inflammatory pathway, namely iNOS and COX-2. Our results show that the expression of both enzymes by LPS-stimulated macrophages was significantly higher (p < 0.001) in comparison to the LPS-free control (Fig. 6), demonstrating the ability of LPS to induce the expression of these enzymes in our inflammatory-like model. Cells treated only with essential oils revealed neglectable levels of iNOS and COX-2 expression, ruling out possible pro-inflammatory effects of the oil.
Figure 6.
Effect of Thymus albicans essential oil on the iNOS and COX-2 protein levels in LPS stimulated RAW 264.7 macrophages. Cells (1.2 × 106 cells/well) were kept 24 h in medium (control) or pre-treated for 1 h with 0.64 μL/mL of T. albicans essential oil and then stimulated with LPS (1 μg/mL). Total cell lysates were analyzed by western blot. Results are expressed as protein levels compared to LPS. Statistical differences were calculated by two-tailed unpaired student’s t-test (###p < 0.001, compared to control; **p < 0.01, ***p < 0.001, compared to LPS).
At 0.64 μL/mL, T. albicans essential oil inhibited by 13.64% iNOS expression in comparison to the LPS stimulus (p < 0.01). This slight decrease of iNOS protein levels in LPS-stimulated macrophages pre-treated with T. albicans essential oil does not reflect the significant NO reduction evaluated through Griess reaction. It seems that T. albicans essential oil may exert its anti-inflammatory activity also by modulating iNOS activity or by inhibiting other signaling pathways. Further studies should be performed to deeply explore this hypothesis. Regarding COX-2 levels, no significant result was observed, indicating a selective effect of the oil towards iNOS expression (Fig. 6). Uncropped blots are provided in Supplementary Information (Fig. S2).
The anti-inflammatory activity of T. albicans main compounds is broadly reported in both pre-clinical and clinical studies. Accordingly, in a clinical study conducted by Juergens et al.52, 1,8-cineole revealed anti-inflammatory activity in patients with severe asthma. Using human cell lines, Greiner et al.53 demonstrated the ability of 1,8-cineole to attenuate the activity of NF-κB, thus decreasing its nuclear translocation and consequently reducing the expression of its target genes coding for pro-inflammatory mediators. Nogueira et al.54 showed that it modulates the production of pro-inflammatory cytokines by LPS-stimulated macrophages by interfering with NF-κB, p38 or ERK/MAPK pathways. Peana et al.55 demonstrated the capacity of linalool to reduce NO production in LPS-stimulated macrophages, although it failed to inhibit iNOS expression. The authors suggested that linalool could possibly act as a scavenger of NO or as an inhibitor of iNOS activity.
Huo et al.56 also showed the anti-inflammatory activity of linalool using in vitro and in vivo models. Linalool inhibited NF-kB and MAPK activation in LPS-stimulated macrophages and protected mice against LPS-induced acute lung injury by reducing the production of TNF-α and IL-6. Borneol was shown to decrease the expression of cytokines (IL-6 and IL-8) in human gingival fibroblasts57. These studies confirm the contribution of 1,8-cineole, linalool, borneol and α-terpineol to the anti-inflammatory activity of T. albicans essential oil.
Other thyme essential oils rich in 1,8-cineole were previously reported to have anti-inflammatory potential, namely T. mastichina, which was shown to inhibit lipoxygenase activity21,29, T. hyemalis, which reduced the secretion of pro-inflammatory cytokines (TNF-α, IL1β, and IL-6) in an inflammatory model of human macrophages stimulated with Cu2+-oxidized LDLs58, and T. camphoratus (chemotype 1,8-cineole/borneol) that inhibited both iNOS and COX-2 in the same model of LPS-stimulated macrophages used in this study59.
Conclusion
Thymus albicans essential oil showed a broad spectrum antifungal activity. The oil was able to inhibit germ tube formation and biofilm integrity in Candida albicans, which are two key virulent factors of this highly prevalent etiologic agent of topical and invasive candidiasis in humans. In addition, at concentrations devoid of toxicity, Thymus albicans essential oil was able to reduce the production of the pro-inflammatory mediator NO. This anti-inflammatory effect was shown to be partly related to the reduction of iNOS levels.
The combination of antifungal and anti-inflammatory effects together with the high essential oil yield (2.4%) and absence of cytotoxicity at bioactive concentrations demonstrate the therapeutic potential of Thymus albicans, an endangered Iberian species. Moreover, the results obtained in the present study corroborate the traditional use of Thymus species as antimicrobial and anti-inflammatory agents.
Also, T. albicans revealed higher essential oil yield than other widely marketed thyme species like Thymus vulgaris L., Thymus zygis L. and T. mastichina. It is important to underline that although T. albicans and T. mastichina are both species of the Mastichina section with high content of 1,8-cineole, only T. mastichina oil has industrial relevance, proven by the established ISO 4728:2003 standard15. Indeed, the bioactivities herein reported confirm the potential of T. albicans essential oil paving the way for further studies on the pharmaceutical applications of this species, which might contribute to improve its conservation status.
Material and methods
Plant material
Flowering parts of Thymus albicans Hoffmanns. and Link were collected at the same time in the area of Foz de Almargem coastal lagoon, Algarve, Portugal. All plants presented a similar stage of development. Voucher specimens were included in the Herbarium of the University of Coimbra (COI), with the accession number Moura 4796. Dr. Jorge Paiva, a taxonomist at the University of Coimbra, confirmed species authenticity and plant names were checked with https://www.theplantlist.org.
Essential oil isolation and analysis
The essential oils from the aerial parts of T. albicans plants were obtained by hydrodistillation for 3 h, using a Clevenger-type apparatus60. Chemical analyses were carried out by both gas chromatography (GC-FID) and gas chromatography-mass spectroscopy (GC–MS) using fused silica capillary columns with two stationary phases (SPB-1 and SupelcoWax 10) as previously reported61.
The compounds were identified based on their GC retention indices on both columns and through their mass spectra. Retention indices, calculated by linear interpolation relative to the retention times of C8–C24 n-alkanes (Eq. 1)62, were compared with those of authentic products included in the Faculty of Pharmacy of the University of Coimbra laboratory database and/or literature data63,64. Acquired mass spectra were compared with reference spectra from the laboratory database, Wiley/NIST library and literature data63,65. Relative amounts of individual components were calculated based on GC raw data areas without a response factor correction for flame ionization detection.
| 1 |
Iφ, experimental retention indices of the analyte “a” in the stationary phase “φ”; z, number of carbons of the n-alkane with the closest previous elution to “a”; T(z), retention time of the n-alkane with the closest previous elution to “a”; T(z+1), retention time of the n-alkane with the closest after elution to “a”; T(a), Retention time of the analyte “a”.
Pure and reference compounds
The following synthetic compounds were purchased: 1,8-cineole (extra pure, Merck, Darmstadt, Germany), linalool (pure, Fluka, Steinheim, Germany), borneol (pure, Fluka, Steinheim, Germany) and α-terpineol (pure, Extrasynthese, Genay, France).
Fungal growth (MIC and MLC)
The antifungal activity of T. albicans essential oil and its major compounds (1,8-cineole, linalool, borneol and α-terpineol) was evaluated against two Candida strains isolated from recurrent cases of vulvovaginal and oral candidiasis (C. krusei H9, C. guilliermondii MAT23) and three type strains (C. albicans ATCC 10231, C. tropicalis ATCC 13803, C. parapsilosis ATCC 90018); a Cryptococcus neoformans type strain (C. neoformans CECT 1078); three clinical dermatophyte strains isolated from nails and skin (Epidermophyton floccosum FF9, Microsporum canis FF1, Trichophyton mentagrophytes FF7) and four type strains (M. gypseum CECT 2908, T. mentagrophytes var. interdigitale CECT 2958, T. rubrum CECT 2794, T. verrucosum CECT 2992); one Aspergillus clinical strain isolated from bronchial secretions (A. flavus F44) and two type strains (A. niger ATCC 16404, A. fumigatus ATCC 46645). The fungal isolates were stored on Sabouraud broth with 20% of glycerol at − 80 °C and previously cultured on Sabouraud Dextrose Agar (SDA) or Potato Dextrose Agar (PDA) to ensure optimal growth characteristics and purity for the antifungal susceptibility testing.
Minimal Inhibitory Concentrations (MICs) and Minimal Lethal Concentrations (MLCs) were determined using the macrodilution method, according to Clinical and Laboratory Standards Institute guidelines for yeasts and filamentous fungi as described by Zuzarte et al.51.
Candida albicans virulence factors
Germ tube inhibition
Cell suspensions (1.0 ± 0.2 × 106 CFU/mL) from overnight SDA cultures were prepared in NYP medium [N-acetylglucosamine (Sigma-Aldrich, St. Louis, MO, USA; 10–3 mol/L), yeast nitrogen base (Difco, Detroit, MI, USA; 3.35 g/L), proline (Fluka; 1023 mol/L) and NaCl (4.5 g/L), pH 6.7 ± 0.1]. Serial two-fold essential oil dilutions were previously prepared in DMSO [maximum concentration of 1% (v/v)], and added to the inoculum suspensions to obtain a range of test concentrations from MIC value to MIC/64 (1.25–0.02 μL/mL). Oil-free controls with and without DMSO were included. After 3 h of incubation in a static chamber at 37 °C, homogenized cell suspensions of each treatment were mounted onto a Neubauer chamber; 100 cells per replica were counted and visually scored, excluding gemulating cells. The percentage of filamentation was defined as the number of cells in which the size of the germ tube was equal or bigger than the blastopore’s diameter.
Disruption of preformed biofilms
The effect of the T. albicans essential oil on the disruption of C. albicans ATCC 10231 preformed biofilm was investigated following the method described by Alves-Silva et al.66. Briefly, a cell suspension of C. albicans was prepared in Yeast Peptone Dextrose (YPD) broth (1% yeast extract, 2% peptone, and 2% dextrose) and incubated for 24 h at 37 °C. A final suspension of 1 × 106 CFU/mL was added to 96-well microtiter plates and incubated for 24 h at 37 °C, to form the biofilms. After three washing steps with PBS, the essential oils (0.16–2.5 µL/mL) prepared in RPMI were added and incubated for 24 h at 37 °C. Negative and positive controls were included using sterile and inoculated RPMI media, respectively.
Anti-inflammatory activity
Cell line and culture conditions
The mouse macrophage cell line, RAW 264.7 (ATCC-TIB-71) was cultured in Dulbecco’s modified eagle medium (DMEM) (Invitrogen, California, USA) supplemented with 10% (v/v) of non-inactivated fetal bovine serum (Invitrogen), 3.02 g/L sodium bicarbonate (Sigma), 100 μg/mL streptomycin (Sigma) and 100 U/mL penicillin (Sigma). The cells were maintained at 37 °C in humidified atmosphere with 5% CO2 and 95% air. Adhesion, growth and morphological patterns were monitored by optical microscopy. Cells were regularly sub-cultured every two–three days and kept in culture for a maximum of three months.
Cell viability
The effect of T. albicans essential oil on macrophages viability (0.3 × 106 cells/well of 48-well plates) was evaluated by MTT assay, as described by Zuzarte et al.59.
Nitric oxide production
The effect of the essential oil on NO production was evaluated by quantifying the concentration of its stable metabolites (nitrites) in macrophages culture supernatants, using the Griess colorimetric reagent, following the protocol described by Zuzarte et al.59.
Essential oil’s mechanism of action in LPS-stimulated macrophages
Nitric oxide scavenging potential
The NO-scavenging potential of T. albicans essential oil was evaluated using S-nitroso-N-acetyl-DL-penicillamine (SNAP) reagent as a NO donor. The essential oil dilutions in culture medium were incubated with 300 μM SNAP for 3 h in the dark at room temperature. An oil free control was included. Nitrite quantification was performed as described previously for nitric oxide production.
Expression of inducible nitric oxide synthase and cyclooxygenase-2
Western blot analyses were performed to evaluate the influence of the essential oil on the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) on LPS-stimulated macrophages. Total cell lysates, protein concentration determination and western blot analysis were performed according to Zuzarte et al.59. Membranes were revealed using a Typhoon FLA 9000 fluorescence reader (GE Healthcare, Chicago, IL, USA) and protein bands were measured by densitometric analysis using TotalLab TL120 (TotalLab, Newcastle, UK).
Statistical analyses
The results are expressed as mean ± SEM of three independent experiments performed in duplicate. Differences between groups were calculated using one-way ANOVA followed by Dunnett’s post hoc test or t-test, using GraphPad Prism v7.0a for Mac OS X.
Supplementary information
Acknowledgements
We would like to thank Dr. Otília Vieira (CEDOC - Chronic Diseases Research Center) for kindly providing the mouse macrophage cell line (RAW 264.7).
Author contributions
M.R., M.Z., M.G. and J.S. performed experiments and analyzed data. C.C. performed GC and GC–MS analysis. M.R. made the literature review and wrote the first draft of the manuscript. M.Z. and M.C. reviewed the manuscript. L.S. supervised the work and reviewed the manuscript.
Data availability
The data that support the findings of this study are available upon request from the corresponding author, MZ.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-75244-w.
References
- 1.Douglas M, Heyes J, Smallfield B. Herbs, spices and essential oils: post-harvest operations in developing countries. UNIDO and FAO. 2005;61:1–8. [Google Scholar]
- 2.EMA. (ed Committee on Herbal Medicinal Products (HMPC) European Medicines Agency) (Herba, Final Document of 12 November 2012 Doc. Ref. EMA/HMPC/342332/2013, 2007).
- 3.ESCOP. Thymi herba (thyme). ESCOP Monographs Second edition (2003).
- 4.Blumenthal M, Busse WR, Goldberg A, Gruenwald J, Hall T, Riggins CW, Rister RS. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Austin, Newton: American Botanical Council/Integrative Medicine Communications; 1998. [Google Scholar]
- 5.WHO . WHO monographs on selected medicinal plants. Vol. 2. Geneva: World Health Organization; 1999. [Google Scholar]
- 6.Stahl-Biskup E, Venskutonis R. Thyme. In: Peter KV, editor. Handbook of Herbs and Spices. Amsterdam: Elsevier; 2012. pp. 499–525. [Google Scholar]
- 7.WFO. Thymus albicans Hoffmanns and Link. https://www.worldfloraonline.org/taxon/wfo-0000323640 (2020).
- 8.Buira, A., Carapeto, A., & Monteiro-Henriques, P. G. Thymus albicans. The IUCN Red List of Threatened Species 2017e.T103496011A103496020 (2017).
- 9.Girón V, et al. Geographical distribution of diploid and tetraploid cytotypes of Thymus sect. Mastichina (Lamiaceae) in the Iberian Peninsula, genome size and evolutionary implications. Folia Geobotanica. 2012;47:441–460. doi: 10.1007/s12224-012-9126-0. [DOI] [Google Scholar]
- 10.Morales Valverde, R. Taxonomía de los géneros Thymus (excluida de la sección serpyllum) y Thymbra en la Península Ibérica. (CSIC-Real Jardín Botánico (RJB), 1986).
- 11.Silva A, et al. Plantas Aromáticas e Medicinais do Parque Natural da Serra da Estrela. Seia: CISE; 2011. [Google Scholar]
- 12.Blanca, G. et al. Libro rojo de la flora silvestre amenazada de Andalucía. I. Especies en peligro de extinción. (Junta de Andalucía, 1999).
- 13.Méndez-Tovar I, Sponza S, Asensio-S-Manzanera M, Novak J. Contribution of the main polyphenols of Thymus mastichina subsp. mastichina to its antioxidant properties. Ind. Crops Prod. 2015;66:291–298. doi: 10.1016/j.indcrop.2014.11.029. [DOI] [Google Scholar]
- 14.ECHA. Thyme, Thymus mastichina, ext.https://echa.europa.eu/registration-dossier/-/registered-dossier/27004/3/1/4 (2020).
- 15.ISO. Oil of Spanish wild marjoram (Thymus mastichina L.) (No. 4728), https://www.iso.org/standard/30436.html (2013).
- 16.Pina-Vaz C, et al. Antifungal activity of Thymus oils and their major compounds. J. Eur. Acad. Dermatol. Venereol. 2004;18:73–78. doi: 10.1111/j.1468-3083.2004.00886.x. [DOI] [PubMed] [Google Scholar]
- 17.Fraternale D, Giamperi L, Ricci D. Chemical composition and antifungal activity of essential oil obtained from in vitro plants of Thymus mastichina L. J. Essent. Oil Res. 2003;15:278–281. doi: 10.1080/10412905.2003.9712142. [DOI] [Google Scholar]
- 18.Faleiro M, et al. Antimicrobial activity of essential oils isolated from Portuguese endemic species of Thymus. Lett. Appl. Microbiol. 2003;36:35–40. doi: 10.1046/j.1472-765X.2003.01259.x. [DOI] [PubMed] [Google Scholar]
- 19.Ballester-Costa C, Sendra E, Fernández-López J, Pérez-Álvarez JA, Viuda-Martos M. Chemical composition and in vitro antibacterial properties of essential oils of four Thymus species from organic growth. Ind. Crops Prod. 2013;50:304–311. doi: 10.1016/j.indcrop.2013.07.052. [DOI] [Google Scholar]
- 20.Cutillas A-B, Carrasco A, Martinez-Gutierrez R, Tomas V, Tudela J. Thymus mastichina L. essential oils from Murcia (Spain): composition and antioxidant, antienzymatic and antimicrobial bioactivities. PLoS ONE. 2018;13:e0190790. doi: 10.1371/journal.pone.0190790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aazza S, et al. Antioxidant, anti-inflammatory and anti-hyperglycaemic activities of essential oils from Thymbra capitata, Thymus albicans, Thymus caespititius, Thymus carnosus, Thymus lotocephalus and Thymus mastichina from Portugal. Nat. Prod. Commun. 2016;11:1029–1038. [PubMed] [Google Scholar]
- 22.Miguel G, et al. Composition and antioxidant activities of the essential oils of Thymus caespititius, Thymus camphoratus and Thymus mastichina. Food Chem. 2004;86:183–188. doi: 10.1016/j.foodchem.2003.08.031. [DOI] [Google Scholar]
- 23.Delgado T, et al. Antioxidant activity of twenty wild Spanish Thymus mastichina L. populations and its relation with their chemical composition. LWT Food Sci. Technol. 2014;57:412–418. doi: 10.1016/j.lwt.2013.12.041. [DOI] [Google Scholar]
- 24.Bentes J, et al. Antioxidant activities of the essential oils and extracts of Portuguese Thymbra capitata and Thymus mastichina. Italian J. Food Sci. 2009;21:183–195. [Google Scholar]
- 25.Miguel MG, et al. Antioxidant and antiproliferative activities of the essential oils from Thymbra capitata and Thymus species grown in Portugal. Evid. Complement. Altern. Med. 2015;2015:851721. doi: 10.1155/2015/851721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arantes SM, et al. Toxicological and pharmacological properties of essential oils of Calamintha nepeta, Origanum virens and Thymus mastichina of Alentejo (Portugal) Food Chem. Toxicol. 2019;133:110747. doi: 10.1016/j.fct.2019.110747. [DOI] [PubMed] [Google Scholar]
- 27.Gordo J, et al. Thymus mastichina: chemical constituents and their anti-cancer activity. Nat. Prod. Commun. 2012;7:1491–1494. [PubMed] [Google Scholar]
- 28.Faleiro, L., Miguel, G., Guerrero, C. & Brito, J. In II WOCMAP Congress Medicinal and Aromatic Plants, Part 2: Pharmacognosy, Pharmacology, Phytomedicine, Toxicology 501. 45–48.
- 29.Miguel MG, Costa LA, Figueiredo AC, Barroso JG, Pedro LG. Assessment of the antioxidant ability of Thymus albicans, T. mastichina, T. camphoratus and T. carnosus essential oils by TBARS and micellar model systems. Nat. Prod. Commun. 2007;2:399–406. [Google Scholar]
- 30.Miguel MG, et al. Effect of the volatile constituents isolated from Thymus albicans, Th. mastichina, Th. carnosus and Thymbra capitata in sunflower oil. Food/Nahrung. 2003;47:397–402. doi: 10.1002/food.200390089. [DOI] [PubMed] [Google Scholar]
- 31.GAFFI. Burden of common life-threatening fungal infections. https://www.gaffi.org/why/fungal-disease-frequency/ (2017).
- 32.Brown GD, et al. Hidden killers: human fungal infections. Sci. Transl. Med. 2012;4:165rv113. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 33.Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet. Infect. Dis. 2017;17:e383–e392. doi: 10.1016/S1473-3099(17)30316-X. [DOI] [PubMed] [Google Scholar]
- 34.Romani L. Immunity to fungal infections. Nat. Rev. Immunol. 2004;11:275–288. doi: 10.1038/nri2939. [DOI] [PubMed] [Google Scholar]
- 35.Salgueiro LR, et al. Composition and variability of the essential oils of Thymus species from section Mastichina from Portugal. Biochem. Syst. Ecol. 1997;25:659–672. doi: 10.1016/S0305-1978(97)00046-X. [DOI] [Google Scholar]
- 36.Miguel MG, Duarte F, Venâncio F, Tavares R. Composition of the essential oils from Portuguese Thymus albicans collected at different regions of Ria Formosa (Algarve) J. Essent. Oil Res. 2004;16:308–311. doi: 10.1080/10412905.2004.9698729. [DOI] [Google Scholar]
- 37.Adam K, Sivropoulou A, Kokkini S, Lanaras T, Arsenakis M. Antifungal activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia, and Salvia fruticosa essential oils against human pathogenic fungi. J. Agric. Food Chem. 1998;46:1739–1745. doi: 10.1021/jf9708296. [DOI] [Google Scholar]
- 38.Salgueiro LR, et al. Antifungal activity of the essential oil of Thymus capitellatus against Candida, Aspergillus and dermatophyte strains. Flavour Fragr. J. 2006;21:749–753. doi: 10.1002/ffj.1610. [DOI] [Google Scholar]
- 39.Hammer K, Carson C, Riley T. Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J. Appl. Microbiol. 2003;95:853–860. doi: 10.1046/j.1365-2672.2003.02059.x. [DOI] [PubMed] [Google Scholar]
- 40.Van Zyl RL, Seatlholo ST, Van Vuuren SF, Viljoen A. Pharmacological interactions of essential oil constituents on the viability of microorganisms. Nat. Prod. Commun. 2010;5:1381–1386. [PubMed] [Google Scholar]
- 41.Hendry E, Worthington T, Conway BR, Lambert P. Antimicrobial efficacy of eucalyptus oil and 1,8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. J. Antimicrob. Chemother. 2009;64:1219–1225. doi: 10.1093/jac/dkp362. [DOI] [PubMed] [Google Scholar]
- 42.Şimşek M, Duman R. Investigation of effect of 1,8-cineole on antimicrobial activity of chlorhexidine gluconate. Pharmacogn. Res. 2017;9:234–237. doi: 10.4103/0974-8490.210329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lohse MB, Gulati M, Johnson AD, Nobile CJ. Development and regulation of single-and multi-species Candida albicans biofilms. Nat. Rev. Microbiol. 2018;16:19–31. doi: 10.1038/nrmicro.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vila T, et al. Targeting Candida albicans filamentation for antifungal drug development. Virulence. 2017;8:150–158. doi: 10.1080/21505594.2016.1197444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fanning S, Mitchell AP. Fungal biofilms. PLoS Pathog. 2012;8:e1002585. doi: 10.1371/journal.ppat.1002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu C-C, Lai W-L, Chuang K-C, Lee M-H, Tsai Y-C. The inhibitory activity of linalool against the filamentous growth and biofilm formation in Candida albicans. Med. Mycol. 2013;51:473–482. doi: 10.3109/13693786.2012.743051. [DOI] [PubMed] [Google Scholar]
- 47.Zore GB, Thakre AD, Jadhav S, Karuppayil SM. Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomedicine. 2011;18:1181–1190. doi: 10.1016/j.phymed.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 48.Ramage G, et al. Antifungal, cytotoxic, and immunomodulatory properties of tea tree oil and its derivative components: potential role in management of oral candidosis in cancer patients. Front. Microbiol. 2012;3:220. doi: 10.3389/fmicb.2012.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manoharan RK, Lee J-H, Lee J. Antibiofilm and antihyphal activities of cedar leaf essential oil, camphor, and fenchone derivatives against Candida albicans. Front. Microbiol. 2017;8:1476. doi: 10.3389/fmicb.2017.01476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75:639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 51.Zuzarte M, et al. Antifungal and anti-inflammatory potential of Lavandula stoechas and Thymus herba-barona essential oils. Ind. Crops Prod. 2013;44:97–103. doi: 10.1016/j.indcrop.2012.11.002. [DOI] [Google Scholar]
- 52.Juergens UR, et al. Anti-inflammatory activity of 1,8-cineol (eucalyptol) in bronchial asthma: a double-blind placebo-controlled trial. Respir. Medi. 2003;97:250–256. doi: 10.1053/rmed.2003.1432. [DOI] [PubMed] [Google Scholar]
- 53.Greiner JF-W, et al. 1,8-Cineol inhibits nuclear translocation of NF-κB p65 and NF-κB-dependent transcriptional activity. Biochim. et Biophys. Acta Mol. Cell Res. 2013;1833:2866–2878. doi: 10.1016/j.bbamcr.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Nogueira M, Aquino S, Junior CR, Spolidório DMP. Terpinen-4-ol and alpha-terpineol (tea tree oil components) inhibit the production of IL-1β, IL-6 and IL-10 on human macrophages. Inflamm. Res. 2014;63:769–778. doi: 10.1007/s00011-014-0749-x. [DOI] [PubMed] [Google Scholar]
- 55.Peana AT, Marzocco S, Popolo A, Pinto A. (−)-Linalool inhibits in vitro NO formation: probable involvement in the antinociceptive activity of this monoterpene compound. Life Sci. 2006;78:719–723. doi: 10.1016/j.lfs.2005.05.065. [DOI] [PubMed] [Google Scholar]
- 56.Huo M, et al. Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model. J. Surg. Res. 2013;180:e47–e54. doi: 10.1016/j.jss.2012.10.050. [DOI] [PubMed] [Google Scholar]
- 57.Ehrnhöfer-Ressler MM, et al. Identification of 1,8-cineole, borneol, camphor, and thujone as anti-inflammatory compounds in a Salvia officinalis L. infusion using human gingival fibroblasts. J. Agric. Food Chem. 2013;61:3451–3459. doi: 10.1021/jf305472t. [DOI] [PubMed] [Google Scholar]
- 58.Ocaña A, Reglero G. Effects of thyme extract oils (from Thymus vulgaris, Thymus zygis, and Thymus hyemalis) on cytokine production and gene expression of oxLDL-stimulated THP-1-macrophages. J. Obes. 2012;2012:104706. doi: 10.1155/2012/104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zuzarte M, et al. New insights on the anti-inflammatory potential and safety profile of Thymus carnosus and Thymus camphoratus essential oils and their main compounds. J. Ethnopharmacol. 2018;225:10–17. doi: 10.1016/j.jep.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 60.Council of Europe. In European Pharmacopoeia. Vol. 1 (2010).
- 61.Cavaleiro C, Salgueiro L, Miguel MG, Da Cunha AP. Analysis by gas chromatography–mass spectrometry of the volatile components of Teucrium lusitanicum and Teucrium algarbiensis. J. Chromatogr. A. 2004;1033:187–190. doi: 10.1016/j.chroma.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 62.Van den Dool H, Kratz PD. A generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J. Chromatogr. 1963;11:463–471. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- 63.Adams RP. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. 4. Carol Stream: Allured; 2007. [Google Scholar]
- 64.Stein SE. Retention indices by NIST Mass Spec Data Center. In: Linstrom PJ, Mallard WG, editors. NIST Chemistry WebBook, NIST Standard Reference Database Number 69. Gaithersburg: National Institute of Standards and Technology; 2017. [Google Scholar]
- 65.McLafferty FW. Wiley Registry of Mass Spectral Data/NIST08. New York: Wiley; 2009. [Google Scholar]
- 66.Alves-Silva JM, et al. New claims for wild carrot (Daucus carota subsp. carota) essential oil. Evid. Complement. Altern. Med. 2016;2016:9045196. doi: 10.1155/2016/9045196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author, MZ.