Abstract
Chiral molecules with multiple stereocenters are widely present in natural products and pharmaceuticals, whose absolute and relative configurations are both critically important for their physiological activities. In spite of the fact that a series of ingenious strategies have been developed for asymmetric diastereodivergent catalysis, most of these methods are limited to the divergent construction of point chirality. Here we report an enantioselective and diastereodivergent synthesis of trisubstituted allenes by asymmetric additions of oxazolones to activated 1,3-enynes enabled by chiral phosphoric acid (CPA) catalysis, where the divergence of the allenic axial stereogenicity is realized by modifications of CPA catalysts. Density functional theory (DFT) calculations are performed to elucidate the origin of diastereodivergence by the stacking- and stagger-form in the transition state (TS) of allene formation step, as well as to disclose a Münchnone-type activation mode of oxazolones under Brønsted acid catalysis.
Subject terms: Asymmetric catalysis, Organocatalysis, Reaction mechanisms, Stereochemistry, Synthetic chemistry methodology
Despite of the high demand of chiral allenes, their asymmetric synthesis remains a challenge for organic chemists. Here, the authors report a stereodivergent synthesis of trisubstituted allenes via asymmetric additions of oxazolones to activated 1,3-enynes enabled by modification of chiral phosphoric acid catalysts.
Introduction
Chiral allenes are featured in many biologically active natural products, pharmaceuticals, and functional materials1,2. In addition, they also serve as versatile building blocks in organic synthesis due to their diverse reactivities3. Despite of the high demands of chiral allenes, the asymmetric catalytic synthesis of these axially chiral compounds remains a challenge in organic synthesis4–6. In the last two decades, a number of elegant asymmetric catalytic strategies have been developed for chiral allene synthesis, such as nucleophilic additions of 1,3-enynes7–19, dynamic kinetic asymmetric transformations (DyKAT) of racemic allenes20–22, rearrangement of alkynes23–25, coupling of alkynes with diazo compounds26–28 and others29–31. Among these strategies, the direct asymmetric additions of prochiral 1,3-enynes represent as one of the most attractive strategies for synthesis of multiple-substituted chiral allenes, owing to the easy accessibility of these substrates. Since the pioneer work of enantioselective synthesis of boryl, silyl and aryl allenes via chiral Pd and Rh catalyzed asymmetric additions of 1,3-enynes by Hayashi and co-workers7–9, a series of elegant asymmetric reactions have been developed employing this strategy, either through asymmetric transition metal-catalysis7–14 or organocatalysis15–19 (Fig. 1a).
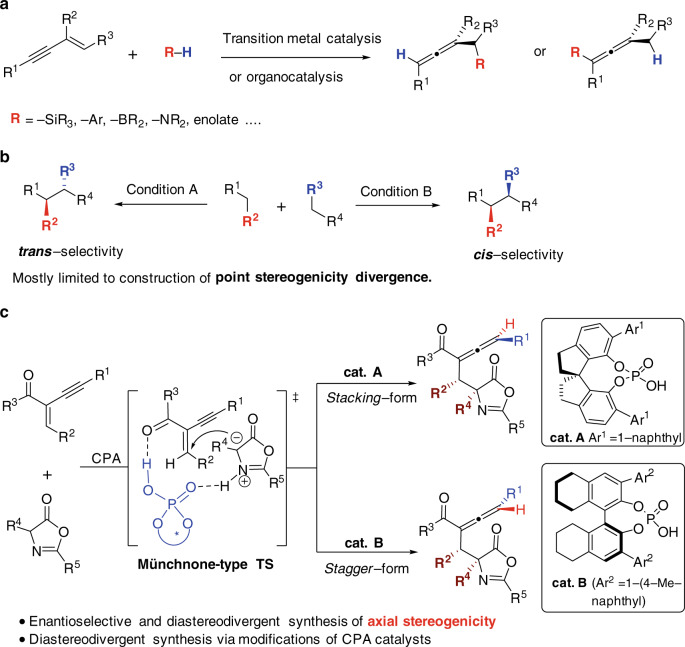
Fig. 1. Asymmetric synthesis of chiral allenes and stereodivergent synthesis.
a Asymmetric synthesis of chiral allenes via enantioselective additions of activated 1,3-enynes. b Asymmetric stereodivergent catalysis was limited to the construction of point stereogenicity divergence. c Asymmetric stereodivergent construction of axial stereogenicity via modifications of chiral phosphoric acid catalysts.
Chiral molecules with multiple stereocenters are widely present in natural products and pharmaceuticals, whose absolute and relative configurations are both critically important for their physiological activities. In the past few decades, numerous highly enantioselective and diastereoselective reactions have been developed. However, modulation the sense of diastereoselectivity in an asymmetric catalytic reaction is still challenging, because the diastereochemical preference is largely governed by the inherent structure and stereoelectronic nature of the substrates32. To address this intrinsic problem, a series of ingenious strategies have been developed for asymmetric diastereodivergent catalysis33–35, such as using distinct catalysts36–38, change of metal cations39, and ligands40,41 of the catalysts, change of reaction conditions42,43, stereodivergent dual catalysis44–50 and stepwise control51–54. Nevertheless, achieving asymmetric diastereodivergent catalysis through modifications of one single type of chiral catalysts remains elusive55,56.
Despite the fact that a large number of asymmetric stereodivergent catalytic methods have been developed, most of these methods have been limited to the construction of point stereogenicity divergence, while stereodivergent synthesis of axial stereogenicity has been rarely explored, except using stepwise control strategy53,54 (Fig. 1b). Herein, we report an enantioselective and diastereodivergent synthesis of trisubstituted allenes via asymmetric conjugate additions of activated 1,3-enynes by oxazolones57–60 enabled by CPA catalysis, in which the diastereodivergent construction of the allenic axial chirality is realized by modifications of CPA catalysts (Fig. 1c). In addition, the origin of the diastereodivergence is well elucidated by DFT calculations, in which a Münchnone-type activation mode of oxazolones under Brønsted acid catalysis is presented.
Results
Reaction optimizations
We commenced our study by selecting α-alkynyl-α,β-enone 1a and 2-para-methoxyphenyl (PMP) substituted oxazolone 2a as model substrates under CPA catalysis (Table 1). Under the promotion of TRIP catalyst (CPA A1, 10 mol%) in toluene (with 3 Å molecular sieves) at room temperature, only two diastereomeric allene products among the four potential ones were detected (Table 1, entry 1), albeit with both poor diastereomeric ratio (dr, 3a:4a 1:2.8) and enantiomeric excess (ee). Subsequently, a series of BINOL and H8-BINOL derived CPA catalysts were screened (entries 2–7). Satisfyingly, the 1-(4-Me-naphthyl) substituted H8-BINOL-derived catalyst B2 provided the allene product in 91% yield with both high diastereoselectivity and enantioselectivity (3a:4a 10:1, 87% ee, entry 7). However, surprisingly, switching the chiral scaffold of 1-naphthyl substituted CPA catalyst form H8-BINOL-type to SPINOL-type (CPA C1) led to the reversal of diastereoselectivity (3a:4a 1:11, entry 8) and formation of product 4a with high ee as well, albeit with moderate yield. To obtain better stereoselectivity control and improve the yield, the effect of the R group at the 2-position of oxazolone was exploited (see Supplementary Table 1), which indicated that 3,5-dimethoxyphenyl group was the optimal one (entry 9 and 10). Finally, a variety of solvents were also screened, and CCl4 was chosen as the optimal solvent (see Supplementary Table 1 for details), in which the chiral allene 3a was obtained in 98% yield, 20:1 dr (3a:4a) with 91% ee in the presence of CPA B2, while diastereomeric chiral allene 4a was generated in 85% yield, 12:1 dr (4a:3a) with 98% ee under the catalysis of CPA C1 (entries 11–12).
Table 1.
Optimizations of reaction conditionsa.
 | ||||||
|---|---|---|---|---|---|---|
| Entry | R | Catalyst | Solvents | Yieldb (%) | drb (3a:4a) | eec (3a/4a, %) |
| 1 | PMP | A1 | toluene | 52 | 1:2.8 | 45/31 |
| 2 | PMP | A2 | toluene | 41 | 1:1 | 41/24 |
| 3 | PMP | A3 | toluene | 51 | 1.4:1 | 35/57 |
| 4 | PMP | A4 | toluene | 50 | 1.3:1 | 72/81 |
| 5 | PMP | A5 | toluene | 68 | 1:2.5 | 24/90 |
| 6 | PMP | B1 | toluene | 57 | 2.7:1 | 91/34 |
| 7 | PMP | B2 | toluene | 91 | 10:1 | 87/– |
| 8 | PMP | C1 | toluene | 51 | 1:11 | –/98 |
| 9 | Ar | B2 | toluene | 99 | 12:1 | 91/– |
| 10 | Ar | C1 | toluene | 80 | 1:9 | –/94 |
| 11 | Ar | B2 | CCl4 | 98 | 20:1 | 91/– |
| 12 | Ar | C1 | CCl4 | 85 | 1:12 | –/98 |
aReactions were performed with 1a (0.15 mmol), 2a (0.1 mmol), cat (0.01 mmol), 3 Å MS (100 mg), solvents (0.5 mL) at ambient temperature for 24 h.
bYields and dr value were determined by crude 1H NMR analysis using 1,2-dimethoxyethane (DME, 0.1 mmol) as internal standard.
cee values were determined by HPLC analysis on a chiral stationary phase. PMP = para-methoxylphenyl, Ar = 3,5-dimethoxyphenyl.
Substrate scope
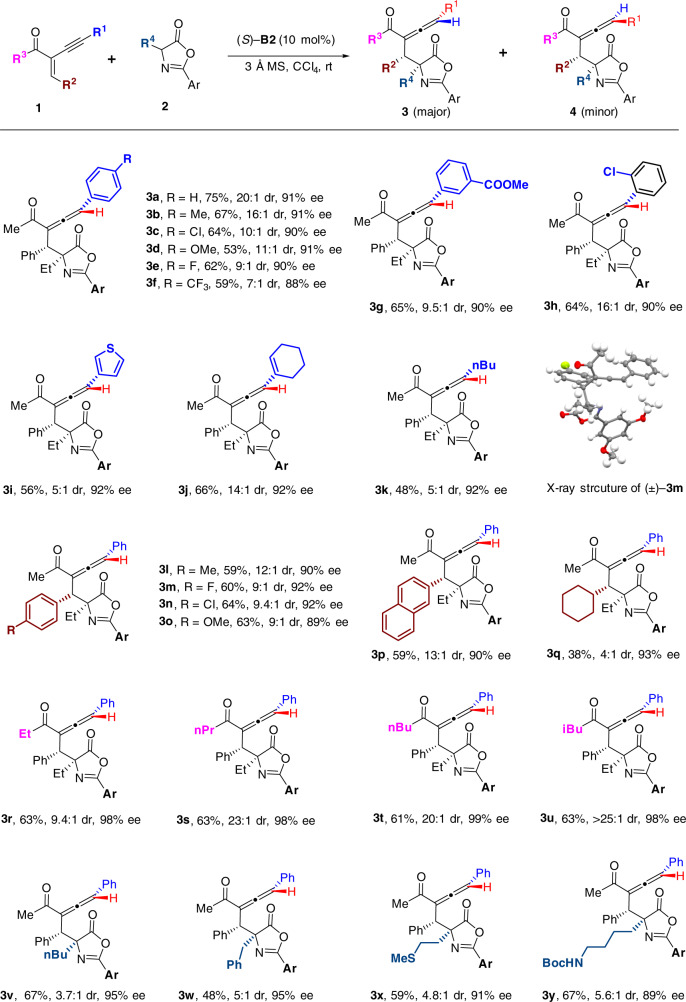
Having established the optimal conditions for stereodivergent synthesis of chiral trisubstituted allenes via modifications of CPA catalysts, the substrate scope under the catalysis of CPA (S)-B2 was firstly investigated (Fig. 2). Various substituted phenylacetylenyl groups were well tolerated under the optimal conditions, regardless of the electronic nature and positions of the substitutions, affording the allene products 3a–3h with high diastereoselectivities (>7:1) and enantioselectivities. In addition, substitutions of the R1 group with heteroaryl, alkenyl and alkyl groups were also amenable, which yielded the products with high enantioselectivities, albeit with moderate to high diastereoselectivity control (3i–3k). Next, a range of R2 groups at the β-positions of the enones were explored, which suggested that various substituted aryl groups (3l–3p) were well tolerated, as well as an alkyl group, albeit with moderate dr value (3q). The relative configurations of the allene products 3 were assigned by analogy to 3m, whose relative structure was confirmed by X-ray crystallography. Subsequently, a range of groups at the ketone site (R3) were also investigated, where the Et-substituted, nPr-substituted, nBu-substituted, and iBu-substituted substrates all generated the chiral allenes with both excellent enantioselectivitie and diastereoselectivitie (>9.4:1 dr, >98% ee, 3r–3u). Finally, a series of substitutions (including some functional group-containing substituents) at the 4-position of oxazolones were also exploited under the optimal conditions, which indicated that the chiral allene products could be produced with high enantioselectivities, albeit with a bit erosive diastereoselectivities (3.7:1–5.6:1, 3v–3y).
Fig. 2. Scope for asymmetric synthesis of trisubstituted allenes 3 catalyzed by CPA catalyst (S)-B2.
Unless otherwise noted, reactions were performed with 1 (0.15 mmol), 2 (0.1 mmol), (S)-B2 catalyst (0.01 mmol) and 3 Å MS (100 mg) in CCl4 (0.5 mL) at room temperature for 24 h. Yields were isolated yields of allenes 3. Dr values were determined by crude 1H NMR analysis. Ee values were determined by HPLC analysis on a chiral stationary phase. Ar = 3,5-dimethoxyphenyl.
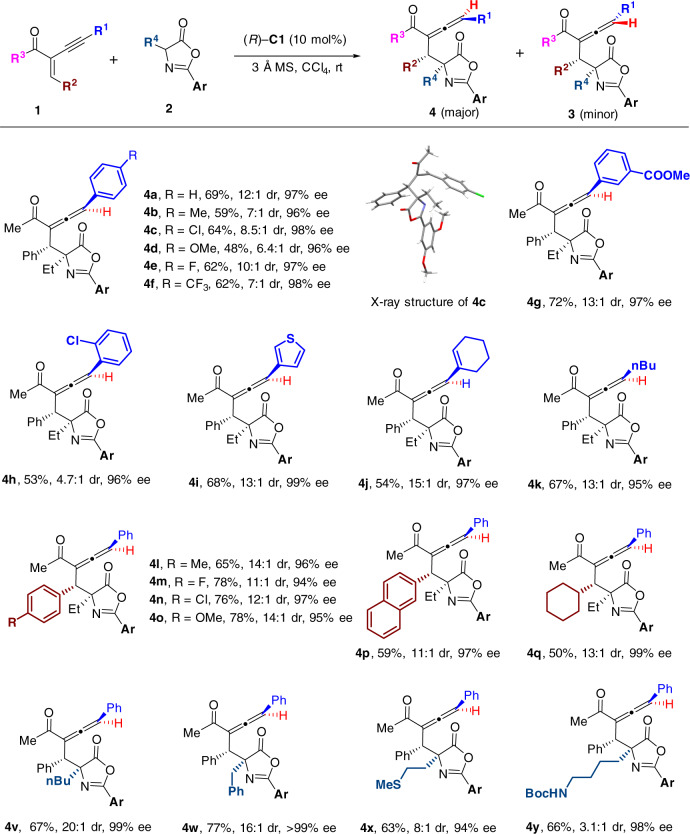
After investigation of the scope under (S)-B2 catalysis, the substrate scope generality with catalyst (R)-C1 was also studied (Fig. 3). All the R1, R2 and R4 substituted substrates explored in Fig. 2 were subjected into investigation under the catalysis of (R)-C1 catalyst, which generated the diastereomeric allenes 4 as the major products with both high dr and ee values for most cases (4a–4y). The absolute structures of the chiral allenes 4 were assigned by analogy to product 4c, whose absolute configuration was unambiguously confirmed by X-ray crystallography. Unfortunately, the variations of the R3 groups of α-alkynyl-α,β-enone 1 under standard conditions were not well tolerated, which led to very low yields and decreased diastereoselectivities (see Supplementary Fig. 1 for details).
Fig. 3. Scope for asymmetric synthesis of trisubstituted allenes 4 catalyzed by CPA catalyst (R)-C1.
Unless otherwise noted, the reaction conditions were the same with those indicated in Fig. 2, except (R)-C1 catalyst (10 mol%) was used instead. Ar = 3,5-dimethoxyphenyl.
Reaction mechanism and origin of diastereodivergence
To investigate the origin of diastereodivergence in construction of the allenic axial chirality, a control experiment was performed. In the presence of CPA catalysts (S)-B2 and (R)-C1, respectively, the asymmetric isomerization of racemic α-alkynyl ketone23 5a proceeded efficiently to give the chiral trisubstituted allene 6a in the opposite enantiomeric bias (Fig. 4a). In addition, some control experiments of the stabilities of the chiral allene products under various conditions were performed (see Supplementary Fig. 2), which indicated that the conversion from a kinetic epimer to a thermodynamic epimer under the reaction conditions is probably not likely. Based on these results and previous reports16–18, a preliminary stepwise mechanism was proposed: (1) oxazolones were activated by CPA catalyst via tautomerism to form active enol intermediates; (2) the Michael addition of α-alkynyl enones by the activated oxazolone intermediates generated another enol intermediates INT A stereoselectively under the guidance of CPA catalysts; (3) the CPA catalyst mediated the proton transfer61,62 of INT A to generate products with the allenic axial stereogenicity (Fig. 4b). For the stereoselectivities of these reactions, we presumed that both (S)-H8-BINOL-B2 and (R)-SPINOL-C1 catalyst generated the same (S,R)-syn-configuration in the Michael addition step, which was followed by the proton transfer step to afford the distinct axial chirality of (S,R,R)-product or (S,R,S)-product, respectively.
Fig. 4. Mechanistic studies.
a Control experiments. b Initially proposed enol-type mode mechanism. c Comparison of the enol-type and Münchnone-type activation modes via calculation. d Energy profiles of Michael-Addition and proton transfer with CPA catalysts via DFT calculations. (The energy shown in kcal/mol, Ar = 3,5-dimethoxyphenyl).
To unveil the different stereochemical control of CPA catalysts in Michael addition and proton transfer steps, density functional theory (DFT) calculations were performed at M06-2X/6-311+ +G(d,p)//M06-2X//6-31G(d) level of theory63–65 (see Supplementary Data 1 for Cartesian coordinates of the optimized structures). Surprisingly, based on a well-accepted enol-type mode (mode A) of oxazolones57,58, initial calculations of the Michael addition step using an achiral phosphoric acid catalyst (dimethyl phosphate, DMP) provided the anti-addition predication of stereochemical outcomes, which were not in agreement with the experimental syn-addition results (Fig. 4c). Further computational evaluations using chiral (S)-B2 and (R)-C1 catalysts remain the wrong (R,R)-anti-addition prediction as well, suggesting that the diastereoselectivity of the Michael addition step did not rely on the chiral scaffolds of CPA catalysts (see Supplementary Fig. 4). Inspired by the basicity of related functional groups of oxazolone, the imine part of oxazolones acting as proton accepter in hydrogen bonding with CPA catalyst is more accessible rather than the carbonyl group (see Supplementary Fig. 4a). Therefore, a Münchnone-type mode59,60,66 (mode B) was proposed and the correct syn-addition outcomes were achieved depended on the achiral DMP catalyst (Fig. 4c). The calculation results suggested that transition state (TS) of enol-type requires 6.1 kcal/mol more activation energy than that of the Münchnone-type by achiral DMP catalyst in the prototropic activation step. For the Michael addition step, Münchnone-type model is also superior to enol-type one. And among a number of our calculated Newman conformations (see Supplementary Table 2), the most favored conformation of syn-addition-TS in Münchnone-type is more stable than the most favored conformation of anti-addition-TS in enol-type by 4.2 kcal/mol, indicating this diastereoselectivity is highly model dependent. The calculations, based on chiral (S)-B2 and (R)-C1 catalysts, provided similar results that phosphoric acid catalysts tend to activate oxazolones in a fashion of Münchnone-type mechanism in these reactions, leading to syn-addition products for the diastereoselectivities (see Supplementary Figs. 3 and 4).
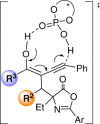
The energy profile of Münchnone-type mechanism was performed in Fig. 4d. Because only the Michael addition and proton transfer steps are responsible for the chirality control in these reactions, the prototropic activation steps forming the key precursor INT2 were not shown here (for the details of these steps see Supplementary Fig. 3). From the related INT2-C/-B, the activated 1,3-enyne 1a undergoes a nucleophilic attack by Münchnone-type intermediate of oxazolone via TS2 accompanying the proton delivery of CPA catalyst. There are four major transition states with different chiral features located, namely syn-isomers (TS2-RS, TS2-SR), anti-isomers (TS2-RR, TS2-SS). Although the CPA catalysts of (S)-B2 and (R)-C1 provide quite similar chiral cavities, they surprisingly display distinct modes of stereoselectivity control in both Michael-addition and proton transfer steps depended on the flexible BINOL backbone and rigid SPINOL backbone, respectively. For (S)-H8-BINOL-B2 catalyst, the final configuration of product Pro-SRR is determined by the proton transfer step with 1.7 kcal/mol free energy difference after the facile and reversible Michael-addition step. Due to sterically repulsive interactions between the phenyl group (R2) at the β-position of 1a and the ethyl group of 2a, the TS2-B-RS is unstable for 1.9 kcal/mol comparing to the favorable TS2-B-SR that is supported by the distortion interaction analysis in Supplementary Fig. 5b. By using the (R)-SPINOL-C1 catalyst, the Michael-addition step mainly determined the reaction rate and led to the more stable INT3-C-SR intermediate readily for proton transfer step forming Pro-SRS. From Supplementary Fig. 5b, the interaction energy of (R)-C1 catalyst and substrate dominates the energy difference of TS2-C-SR and TS2-C-RS probably because of the steric repulsion between 3,3’-substitutent of the C1 catalyst and the ethyl group of 2a, indicating the rigid conformation of SPINOL-C1 catalyst may encounter non-negligible interactions between catalyst and substrate, compared with the relatively flexible conformation of H8-BINOL-B2 catalyst. The stereo-isomerization of enol intermediate INT 3 via low barrier tautomerization and single bond rotation was omitted for clarity. Finally, our calculation predicted the products with 95% ee, 17:1 dr (exp. 91% ee, 20:1 dr) and 98% ee, 15:1 dr (exp. 97% ee, 12:1 dr) under (S)-B2 and (R)-C1 catalysis, respectively, which are in agreement with the experimental results.
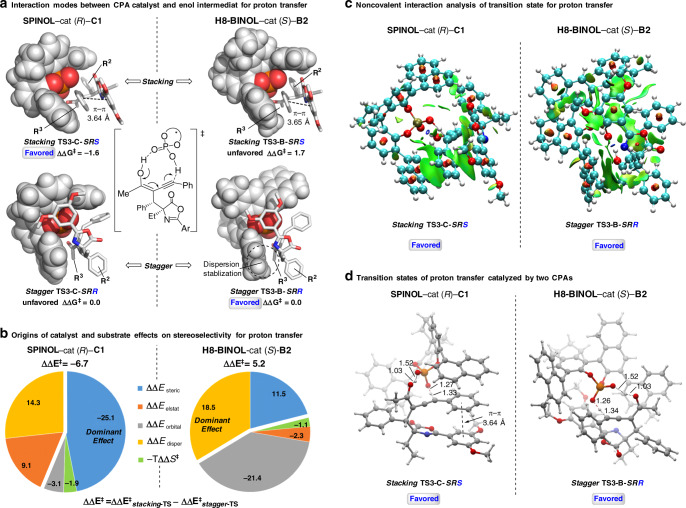
For the origin of chirality control in the proton transfer step for construction of the allenic axial chirality, we found two types of models among our 20 calculated TSs structures (see Supplementary Table 3). In Fig. 5a, stacking-type model tends to form S-axial chirality configuration TSs, where the substitutions of the intermediate in transition state TS3 have strong intramolecular interaction (i.e., π-π interaction supported by the NCIs plots67 of Fig. 5c) and the resonance stabilization between the phenyl group (R1) and the allenic moiety in 1a (see Supplementary Fig. 6 for details). In contrast, stagger-type model prefer R-axial chirality configuration TS with emphasizing the intermolecular interactions of CPA catalyst and substrates (for the distortion-interaction analysis and NCIs plots of TSs see Supplementary Figs. 6 and 7). Due to the relatively rigid conformation of (R)-SPINOL-C1 catalyst, stacking-type model is favored for the TSs of proton transfer step to fit the cavity of catalyst with certain entropy loss, leading to S-axial chirality configuration. That is consistent with less steric repulsion between catalyst and substrate in the stacking-type model, which is the dominant effect by Energy Decomposition Analysis (EDA) calculations68 as shown in Fig. 5b. The (S)-H8-BINOL-B2 catalyst with slightly flexible conformation are more likely to adopt stagger-type mode forming R-axial chirality configuration, which is mainly stabilized by the dispersion effect by EDA calculation. As shown in NCIs plots in Fig. 5c, this dispersion effect between the methyl group of catalyst B2 and substrate in TS3-B-SRR can well rationalize the catalyst B2 featuring a 1-(4-Me-naphthyl) substitution (over just 1-naphthyl in catalyst B1) could improve the diastereoselectivity significantly (2.7:1 to 10:1, see Table 1). For this catalyst, the ΔΔEorbital, a high proportion for energy, stabilized for the unfavorable TS3-B-SRS, which can be rationalized by the dihedral angle ∠C1-C2-C3-H1 of forming hydrogen bond and allene as shown in Supplementary Fig. 8. The dihedral angle in TS3-B-SRS is 85.0°, which is more close to perpendicular than that in TS3-B-SRR with 80.2°. However, this orbital interaction cannot overcome the steric and dispersion effect that favor TS3-B-SRR as a stagger-form in the overall relative energy. The calculated results demonstrate the substrates may dynamically orientate their conformations to interact favorably with various cavities of catalysts under the promotion of inter-/intramolecular interactions.
Fig. 5. Computational studies on origin of diastereoselectivity.
Comparison two types of interaction modes between CPA (R)-C1/(S)-B2 and substrates. a Space-filling model of stacking-TS versus stagger-TS for proton transfer. b Energy Decomposition Analysis (EDA) of transition states for proton transfer at B3LYP-D3/TZ2P level of theory by using ETS-NOCV in ADF. The relative energy (ΔΔE‡) is the sum of ΔΔE0‡ and (-TΔΔS‡), where the entropy term is from Gaussian calculations. For the energy decomposition analysis equation follows: ΔΔE0‡ = ΔΔEsteric + ΔΔEelstat + ΔΔEorbital + ΔΔEdisper. The ΔΔE‡ is calculated from the electronic energy difference between stacking-type TS and stagger-type TS (ΔΔE‡ = ΔEstacking-TS‡ − ΔEstagger-TS‡). Each energy component (ΔΔEsteric, ΔΔEelstat, ΔΔEorbital, and ΔΔEdisper) is calculated in the same fashion. Positive ΔΔE values indicate corresponding interactions promote stagger-type, and R-axial chirality TS is more favored. In contrast, negative ΔΔE values indicate S-axial chirality TS with stacking-type is more stable; Esteric is the positive term as the steric repulsion, and Edisper is the negative term as the dispersion stabilization. c Noncovalent Interactions (NCIs) analysis of transition state for proton transfer (green, dispersion effect; red, steric effect). The gradient isosurfaces (s = 0.3 a.u.) are colored on an BGR scale according to sign(λ2)ρ over the range −0.01 to 0.01 au. d Detailed structural information of proton transfer transition states catalyzed by two CPAs. The energies shown in kcal/mol.
To confirm validity of the stereo-models in the proton transfer step, we performed additional DFT calculations for understanding substituent effects of R2 and R3 group in 1 as shown in Table 2. The calculated diastereoselectivities are in well agreement with the trend of our experimental substituent effects. In B2 catalysis, dispersion effects dominate in the stereo-control. When the cyclohexyl group (Cy) at the R2 of 1 was introduced and TS3-B-SRS-Cy would be stabilized due to the CH-π dispersion effect between cyclohexyl and 4-Me-naphthyl group, which therefore decrease the energy gap for diastereoselectivity. And, n-propyl group (nPr) at R3 in 1 also emphasize the dispersion effect with 4-Me-naphthyl group of B2 catalyst, which mainly stabilize the transition state TS3-B-SRR-nPr and thus increase the diastereoselectivity. In contrast, the relatively rigid conformation and small cavity for C1 catalysis make steric repulsions become important. Introducing the relatively larger cyclohexyl group to substrates would increase energy barriers for both the stacking-forms and stagger-forms of TS3-C, resulting slightly increased energy gap for the diastereoselectivity (see Supplementary Table 5 for details). While switching the R3 group to n-propyl under C1 catalyst led to very low reactivity by our experiment (see Supplementary Fig. 1), and thus further discussions on the diastereoselectivity is trivial.
Table 2.
Calculated free energies of activation for proton transfer transition states for substrates with different R2/R3 substituents.
 |
TS | R2 | R3 | exp ad.r. | exp bΔΔG‡ | calc bΔΔG‡ |
| TS3-B | Ph | Me | 20:1 | 1.8 | 1.7 | |
| Cy | Me | 4:1 | 0.8 | 1.1 | ||
| Ph | nPr | 23:1 | 1.9 | 3.0 | ||
| TS3-C | Ph | Me | 12:1 | 1.5 | 1.6 | |
| Cy | Me | 13:1 | 1.5 | 1.8 |
aIn B2 catalysis, the diastereoselective ratio is 3 (major): 4 (minor), and in C1 catalysis, the diastereoselective ratio is 4 (major): 3 (minor).
bThe value ΔΔG‡ in TS3-B is ΔG‡stacking−TS − ΔG‡stagger−TS, and the value ΔΔG‡ in TS3-C is ΔG‡stagger−TS − ΔG‡stacking-TS. The energy shown in kcal/mol. Ar = 3,5-dimethoxyphenyl.
Derivatizations of the chiral allene products for diversity-oriented synthesis
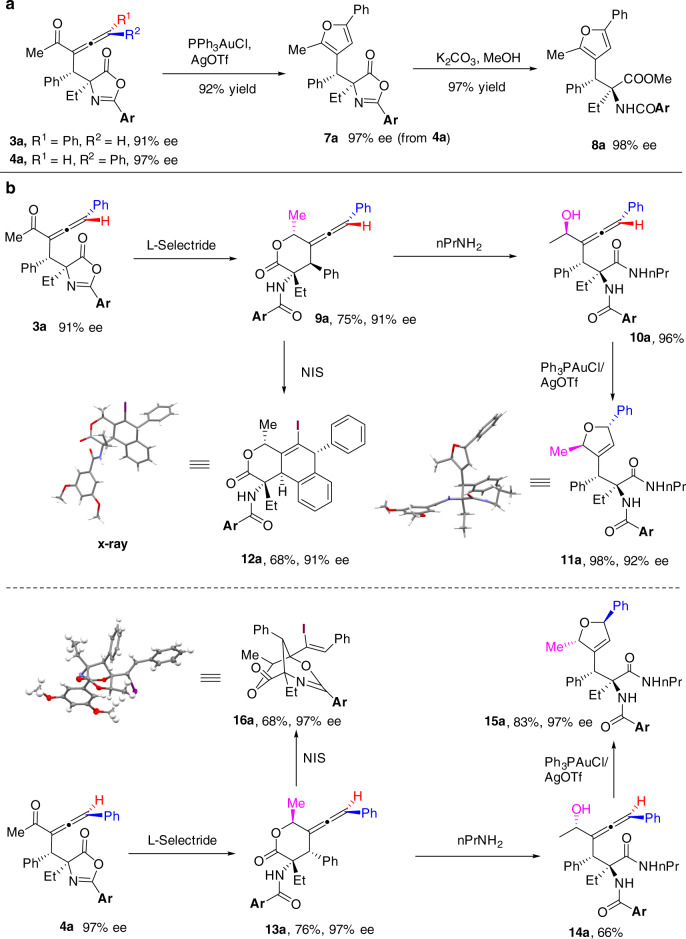
To demonstrate the applicability of these reactions, we devoted our efforts to exploring the derivatizations of the chiral allene products (Fig. 6). Rearrangement of chiral allene 4a into tri-substituted furan 7a was performed under gold(I) catalysis, providing the product in 92% yield. Further alcoholysis of the oxazolone moiety in 7a provided the β,β-di-aryl substituted amino acid derivative 8a, which is a type of important pharmacophore in a series of bioactive small molecules. Analogously, gold(I)-catalyzed rearrangement of 3a afforded the furan derivative 7a with the same bias on a chiral stationary phase, thus confirming the absolute configuration of 3a (Fig. 6a). To achieve diversity-oriented synthesis (DOS)69 from these chiral allene products, diastereoselective reduction of 3a with L-Selectride followed by in-situ intramolecular transesterification provided the lactone 9a (>25:1 dr)70. Aminolysis of lactone 7a with nPrNH2 yielded the allenic alcohol 10a, which was stereospecifically cyclized in the presence of gold(I) catalyst to give the 2,5-dihydrofuran derivatives 11a, whose absolute structure was confirmed by X-ray crystallography. Analogously, treatment of chiral allene 4a with the same three-steps procedure readily provided the diastereomeric chiral 2,5-dihydrofuran 15a (Fig. 6b). Moreover, derivatizations of the chiral allene products were not limited to stereochemical diversities, but could be extended to skeletal diversities. Electrophilic iodocyclization of lactone 9a in the presence of NIS provided the tricyclic product 12a, in which the phenyl group reacted as the nucleophile. On the other hand, treatment of the diastereomeric lactone 13a with the same electrophilic iodination conditions generated the bridged bicyclic product 16a, in which the amide group reacted as the nucleophile; the structures of these cyclization products were all well confirmed by X-Ray crystallography.
Fig. 6. Derivatizations of the chiral allene products.
a Synthesis of chiral furan derivatives. b Derivatizations of the chiral allene products for chemo-diversity-oriented and stereo-diversity-oriented synthesis. Ar = 3,5-dimethoxyphenyl.
Discussion
In summary, we have disclosed an enantioselective and diastereodivergent synthesis of trisubstituted allenes via asymmetric conjugate additions of oxazolones to activated 1,3-enynes under chiral phosphoric acid catalysis, where the axial stereogenicity of the chiral allene products could be well modulated by modifications of the CPA catalysts. The origin of allenic axial diastereodivergence is well elucidated by the stacking-form and stagger-form in transition states from DFT calculations, in which a Münchnone-type model on activation of oxazolones under Brønsted acid catalysis has also been demonstrated with high model dependency of diastereoselectivities. The stereo-specific and chemo-specific transformations of the diastereomeric chiral allenes into more complex stereoisomers and skeletal isomers demonstrate the value of these reactions in organic synthesis, especially in the field of DOS.
Methods
General procedure for asymmetric synthesis of chiral products 3 and 4
To a dried 3 ml vial was added 1 (0.15 mmol), 2 (0.1 mmol), CPA catalyst (0.01 mmol), and activated 3 Å molecular sieves (100 mg). The vial was purged with N2 for 3 times and then followed by adding CCl4 (0.5 mL). After stirring for 24 h at room temperature, the reaction mixture was quenched by adding K2CO3. After filtration, the filtrate was concentrated under vacuum to give a residue, which was purified by flash column chromatography to give the allene products 3 or 4. (Toluene is an alternative choice of solvent used in these reactions, if the usage of CCl4 is restricted). Full experimental details and characterization of new compounds can be found in the Supplementary Information.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This work is dedicated to the 70th anniversary of Shanghai Institute of Organic Chemistry and 100th anniversary of the School of Chemistry and Chemical Engineering, Nanjing University. We gratefully acknowledge NSFC (Grant Nos. 21702138, 21890722, 21702109, 11811530637, 21950410519), the Natural Science Foundation of Tianjin Municipality (18JCYBJC21400, 19JCJQJC62300), Tianjin Research Innovation Project for Postgraduate Students (2019YJSB081), ShanghaiTech University start-up funding, and the Fundamental Research Funds for Central Universities [Nankai University (Nos. 63201043, 63203002)] for financial support. The authors thank the support from Analytical Instrumentation Center (# SPST-AIC10112914), SPST, ShanghaiTech University.
Author contributions
J.W., S.R., and J.X. performed the experiments. S.Z. and Q.P. performed the computational study. N.Y. collected the crystallographic data. Q.P. and X.Y. directed the project and wrote the paper.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and Supplementary Information file, or from the corresponding author upon reasonable request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 1971171, 1971946, 1971947, 1971365, and 1971366. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jiawen Wang, Sujuan Zheng.
Contributor Information
Qian Peng, Email: qpeng@nankai.edu.cn.
Xiaoyu Yang, Email: yangxy1@shanghaitech.edu.cn.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-19294-8.
References
- 1.Rivera-Fuentes P, Diederich F. Allenes in molecular materials. Angew. Chem. Int. Ed. 2012;51:2818–2828. doi: 10.1002/anie.201108001. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann-Röder A, Krause N. Synthesis and properties of allenic natural products and pharmaceuticals. Angew. Chem. Int. Ed. 2004;43:1196–1216. doi: 10.1002/anie.200300628. [DOI] [PubMed] [Google Scholar]
- 3.Yu S, Ma S. Allenes in catalytic asymmetric synthesis and natural product syntheses. Angew. Chem. Int. Ed. 2012;51:3074–3112. doi: 10.1002/anie.201101460. [DOI] [PubMed] [Google Scholar]
- 4.Ogasawara M. Catalytic enantioselective synthesis of axially chiral allenes. Tetrahedron. 2009;20:259–271. doi: 10.1016/j.tetasy.2008.11.039. [DOI] [Google Scholar]
- 5.Ye J, Ma S. Conquering three-carbon axial chirality of allenes. Org. Chem. Front. 2014;1:1210–1224. doi: 10.1039/C4QO00208C. [DOI] [Google Scholar]
- 6.Chu W-D, Zhang Y, Wang J. Recent advances in catalytic asymmetric synthesis of allenes. Catal. Sci. Technol. 2017;7:4570–4579. doi: 10.1039/C7CY01319A. [DOI] [Google Scholar]
- 7.Han JW, Tokunaga N, Hayashi T. Palladium-catalyzed asymmetric hydrosilylation of 4-substituted 1-buten-3-ynes. catalytic asymmetric synthesis of axially chiral allenylsilanes. J. Am. Chem. Soc. 2001;123:12915–12916. doi: 10.1021/ja017138h. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi T, Tokunaga N, Inoue K. Rhodium-catalyzed asymmetric 1,6-addition of aryltitanates to enynones giving axially chiral allenes. Org. Lett. 2004;6:305–307. doi: 10.1021/ol036309f. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura T, Makino H, Nagaosa M, Hayashi T. Rhodium-catalyzed enantioselective 1,6-addition of arylboronic acids to enynamides: asymmetric synthesis of axially chiral allenylsilanes. J. Am. Chem. Soc. 2010;132:12865–12867. doi: 10.1021/ja1066509. [DOI] [PubMed] [Google Scholar]
- 10.Wang M, et al. Synthesis of highly substituted racemic and enantioenriched allenylsilanes via copper-catalyzed hydrosilylation of (Z)-2-alken-4-ynoates with silylboronate. J. Am. Chem. Soc. 2015;137:14830–14833. doi: 10.1021/jacs.5b08279. [DOI] [PubMed] [Google Scholar]
- 11.Yao Q, et al. Efficient synthesis of chiral trisubstituted 1,2-allenyl ketones by catalytic asymmetric conjugate addition of malonic esters to enynes. Angew. Chem. Int. Ed. 2016;55:1859–1863. doi: 10.1002/anie.201509455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y, del Pozo J, Torker S, Hoveyda AH. Enantioselective synthesis of trisubstituted allenyl–B(pin) compounds by phosphine–Cu-catalyzed 1,3-enyne hydroboration. insights regarding stereochemical integrity of Cu–allenyl intermediates. J. Am. Chem. Soc. 2018;140:2643–2655. doi: 10.1021/jacs.7b13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adamson NJ, Jeddi H, Malcolmson SJ. Preparation of chiral allenes through Pd-catalyzed intermolecular hydroamination of conjugated enynes: enantioselective synthesis enabled by catalyst design. J. Am. Chem. Soc. 2019;141:8574–8583. doi: 10.1021/jacs.9b02637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayeh-Romero L, Buchwald SL. Copper hydride catalyzed enantioselective synthesis of axially chiral 1,3-disubstituted allenes. J. Am. Chem. Soc. 2019;141:13788–13794. doi: 10.1021/jacs.9b07582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W, et al. Enantioselective bromolactonization of conjugated (Z)-enynes. J. Am. Chem. Soc. 2010;132:3664–3665. doi: 10.1021/ja100173w. [DOI] [PubMed] [Google Scholar]
- 16.Qian H, Yu X, Zhang J, Sun J. Organocatalytic enantioselective synthesis of 2,3-allenoates by intermolecular addition of nitroalkanes to activated enynes. J. Am. Chem. Soc. 2013;135:18020–18023. doi: 10.1021/ja409080v. [DOI] [PubMed] [Google Scholar]
- 17.Poulsen PH, et al. Organocatalytic formation of chiral trisubstituted allenes and chiral furan derivatives. Angew. Chem. Int. Ed. 2018;57:10661–10665. doi: 10.1002/anie.201806238. [DOI] [PubMed] [Google Scholar]
- 18.Ma Z-G, et al. Asymmetric organocatalytic synthesis of 2,3-allenamides from hydrogen-bond-stabilized enynamides. Org. Lett. 2019;21:2468–2472. doi: 10.1021/acs.orglett.9b00839. [DOI] [PubMed] [Google Scholar]
- 19.Yu X, Ren H, Xiao Y, Zhang J. Efficient assembly of allenes, 1,3-dienes, and 4H-pyrans by catalytic regioselective nucleophilic addition to electron-deficient 1,3-conjugated enynes. Chem. Eur. J. 2008;14:8481–8485. doi: 10.1002/chem.200801004. [DOI] [PubMed] [Google Scholar]
- 20.Deska J, del Pozo Ochoa C, Bäckvall J-E. Chemoenzymatic dynamic kinetic resolution of axially chiral allenes. Chem. Eur. J. 2010;16:4447–4451. doi: 10.1002/chem.201000301. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto T, Sakata K, Tamakuni F, Dutton MJ, Maruoka K. Phase-transfer-catalysed asymmetric synthesis of tetrasubstituted allenes. Nat. Chem. 2013;5:240. doi: 10.1038/nchem.1567. [DOI] [PubMed] [Google Scholar]
- 22.Mbofana CT, Miller SJ. Diastereo- and enantioselective addition of anilide-functionalized allenoates to N-acylimines catalyzed by a pyridylalanine-based peptide. J. Am. Chem. Soc. 2014;136:3285–3292. doi: 10.1021/ja412996f. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Leow D, Huang K-W, Tan C-H. Enantioselective synthesis of chiral allenoates by guanidine-catalyzed isomerization of 3-alkynoates. J. Am. Chem. Soc. 2009;131:7212–7213. doi: 10.1021/ja901528b. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Zhang W, Ma S. A room-temperature catalytic asymmetric synthesis of allenes with ECNU-Phos. J. Am. Chem. Soc. 2013;135:11517–11520. doi: 10.1021/ja406135t. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, et al. Nickel(II)-catalyzed asymmetric propargyl and allyl claisen rearrangements to allenyl- and allyl-substituted β-ketoesters. Angew. Chem. Int. Ed. 2014;53:11579–11582. doi: 10.1002/anie.201404643. [DOI] [PubMed] [Google Scholar]
- 26.Tang Y, et al. Direct synthesis of chiral allenoates from the asymmetric C-H insertion of α-diazoesters into terminal alkynes. Angew. Chem. Int. Ed. 2015;54:9512–9516. doi: 10.1002/anie.201501918. [DOI] [PubMed] [Google Scholar]
- 27.Chu W-D, et al. Enantioselective synthesis of trisubstituted allenes via Cu(I)-catalyzed coupling of diazoalkanes with terminal alkynes. J. Am. Chem. Soc. 2016;138:14558–14561. doi: 10.1021/jacs.6b09674. [DOI] [PubMed] [Google Scholar]
- 28.Tang Y, et al. Asymmetric three-component reaction for the synthesis of tetrasubstituted allenoates via allenoate-copper intermediates. Chem. 2018;4:1658–1672. doi: 10.1016/j.chempr.2018.04.012. [DOI] [Google Scholar]
- 29.Crouch IT, Neff RK, Frantz DE. Pd-catalyzed asymmetric β-hydride elimination en route to chiral allenes. J. Am. Chem. Soc. 2013;135:4970–4973. doi: 10.1021/ja401606e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian D, Wu L, Lin Z, Sun J. Organocatalytic synthesis of chiral tetrasubstituted allenes from racemic propargylic alcohols. Nat. Commun. 2017;8:567. doi: 10.1038/s41467-017-00251-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng W-F, et al. Tetrasubstituted allenes via the palladium-catalysed kinetic resolution of propargylic alcohols using a supporting ligand. Nat. Catal. 2019;2:997–1005. doi: 10.1038/s41929-019-0346-z. [DOI] [Google Scholar]
- 32.Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. Comprehensive Asymmetric Catalysis. Vol. I–III (Springer, 1999).
- 33.Bihani M, Zhao JCG. Advances in asymmetric diastereodivergent catalysis. Adv. Synth. Catal. 2017;359:534–575. doi: 10.1002/adsc.201601188. [DOI] [Google Scholar]
- 34.Lin L, Feng X. Catalytic strategies for diastereodivergent synthesis. Chem. Eur. J. 2017;23:6464–6482. doi: 10.1002/chem.201604617. [DOI] [PubMed] [Google Scholar]
- 35.Krautwald S, Carreira EM. Stereodivergence in asymmetric catalysis. J. Am. Chem. Soc. 2017;139:5627–5639. doi: 10.1021/jacs.6b13340. [DOI] [PubMed] [Google Scholar]
- 36.Li X, et al. Diastereodivergent organocatalytic asymmetric vinylogous Michael reactions. Nat. Commun. 2014;5:4479. doi: 10.1038/ncomms5479. [DOI] [PubMed] [Google Scholar]
- 37.Zhan G, et al. Catalyst-controlled switch in chemo- and diastereoselectivities: annulations of Morita–Baylis–Hillman carbonates from Isatins. Angew. Chem. Int. Ed. 2016;55:2147–2151. doi: 10.1002/anie.201510825. [DOI] [PubMed] [Google Scholar]
- 38.Dai J, et al. Chiral primary amine catalysis for asymmetric mannich reactions of aldehydes with ketimines: stereoselectivity and reactivity. Angew. Chem. Int. Ed. 2017;56:12697–12701. doi: 10.1002/anie.201706304. [DOI] [PubMed] [Google Scholar]
- 39.Lv J, Zhang L, Luo S, Cheng JP. Switchable diastereoselectivity in enantioselective [4+2] cycloadditions with simple olefins by asymmetric binary acid catalysis. Angew. Chem. Int. Ed. 2013;52:9786–9790. doi: 10.1002/anie.201304561. [DOI] [PubMed] [Google Scholar]
- 40.Luparia M, et al. Catalytic asymmetric diastereodivergent deracemization. Angew. Chem. Int. Ed. 2011;50:12631–12635. doi: 10.1002/anie.201106321. [DOI] [PubMed] [Google Scholar]
- 41.Audisio D, Luparia M, Oliveira MT, Klütt D, Maulide N. Diastereodivergent de-epimerization in catalytic asymmetric allylic alkylation. Angew. Chem. Int. Ed. 2012;51:7314–7317. doi: 10.1002/anie.201202853. [DOI] [PubMed] [Google Scholar]
- 42.Tian X, et al. Diastereodivergent asymmetric Sulfa-Michael additions of α-branched enones using a single chiral organic catalyst. J. Am. Chem. Soc. 2011;133:17934–17941. doi: 10.1021/ja207847p. [DOI] [PubMed] [Google Scholar]
- 43.McInturff EL, Yamaguchi E, Krische MJ. Chiral-anion-dependent inversion of diastereo- and enantioselectivity in carbonyl crotylation via ruthenium-catalyzed butadiene hydrohydroxyalkylation. J. Am. Chem. Soc. 2012;134:20628–20631. doi: 10.1021/ja311208a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krautwald S, Sarlah D, Schafroth MA, Carreira EM. Enantio- and diastereodivergent dual catalysis: α-allylation of branched aldehydes. Science. 2013;340:1065. doi: 10.1126/science.1237068. [DOI] [PubMed] [Google Scholar]
- 45.Huo X, He R, Zhang X, Zhang W. An Ir/Zn dual catalysis for enantio- and diastereodivergent α-allylation of α-hydroxyketones. J. Am. Chem. Soc. 2016;138:11093–11096. doi: 10.1021/jacs.6b06156. [DOI] [PubMed] [Google Scholar]
- 46.Cruz FA, Dong VM. Stereodivergent coupling of aldehydes and alkynes via synergistic catalysis using Rh and Jacobsen’s Amine. J. Am. Chem. Soc. 2017;139:1029–1032. doi: 10.1021/jacs.6b10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang X, Beiger JJ, Hartwig JF. Stereodivergent allylic substitutions with aryl acetic acid esters by synergistic iridium and lewis base catalysis. J. Am. Chem. Soc. 2017;139:87–90. doi: 10.1021/jacs.6b11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei L, Zhu Q, Xu S-M, Chang X, Wang C-J. Stereodivergent synthesis of α,α-disubstituted α-amino acids via synergistic Cu/Ir catalysis. J. Am. Chem. Soc. 2018;140:1508–1513. doi: 10.1021/jacs.7b12174. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Q, et al. Stereodivergent coupling of 1,3-dienes with aldimine esters enabled by synergistic Pd and Cu catalysis. J. Am. Chem. Soc. 2019;141:14554–14559. doi: 10.1021/jacs.9b07600. [DOI] [PubMed] [Google Scholar]
- 50.Singha S, Serrano E, Mondal S, Daniliuc CG, Glorius F. Diastereodivergent synthesis of enantioenriched α,β-disubstituted γ-butyrolactones via cooperative N-heterocyclic carbene and Ir catalysis. Nat. Catal. 2020;3:48–54. doi: 10.1038/s41929-019-0387-3. [DOI] [Google Scholar]
- 51.Huang Y, Walji AM, Larsen CH, MacMillan DWC. Enantioselective organo-cascade catalysis. J. Am. Chem. Soc. 2005;127:15051–15053. doi: 10.1021/ja055545d. [DOI] [PubMed] [Google Scholar]
- 52.Huang H, Konda S, Zhao JC-G. Diastereodivergent catalysis using modularly designed organocatalysts: synthesis of both cis- and trans-fused pyrano[2,3-b]pyrans. Angew. Chem. Int. Ed. 2016;55:2213–2216. doi: 10.1002/anie.201510134. [DOI] [PubMed] [Google Scholar]
- 53.Lotter D, Castrogiovanni A, Neuburger M, Sparr C. Catalyst-controlled stereodivergent synthesis of atropisomeric multiaxis systems. ACS Cent. Sci. 2018;4:656–660. doi: 10.1021/acscentsci.8b00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kwon Y, Chinn AJ, Kim B, Miller SJ. Divergent control of point and axial stereogenicity: catalytic enantioselective C−N bond-forming cross-coupling and catalyst-controlled atroposelective cyclodehydration. Angew. Chem. Int. Ed. 2018;57:6251–6255. doi: 10.1002/anie.201802963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan X-X, et al. Highly diastereoselective switchable enantioselective mannich reaction of glycine derivatives with imines. J. Am. Chem. Soc. 2008;130:14362–14363. doi: 10.1021/ja804527r. [DOI] [PubMed] [Google Scholar]
- 56.Uraguchi D, Yoshioka K, Ooi T. Complete diastereodivergence in asymmetric 1,6-addition reactions enabled by minimal modification of a chiral catalyst. Nat. Commun. 2017;8:14793. doi: 10.1038/ncomms14793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang J, Qing J, Gong L-Z. Asymmetric synthesis of 3-amino-δ-lactams and benzo[a]quinolizidines by catalytic cyclization reactions involving azlactones. Chem. Eur. J. 2009;15:7031–7034. doi: 10.1002/chem.200900814. [DOI] [PubMed] [Google Scholar]
- 58.Terada M, Tanaka H, Sorimachi K. Enantioselective direct aldol-type reaction of azlactone via protonation of vinyl ethers by a chiral brønsted acid catalyst. J. Am. Chem. Soc. 2009;131:3430–3431. doi: 10.1021/ja8090643. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Z, et al. Chiral phosphoric acid catalyzed enantioselective 1,3-dipolar cycloaddition reaction of azlactones. Chem. Commun. 2016;52:1377–1380. doi: 10.1039/C5CC08989A. [DOI] [PubMed] [Google Scholar]
- 60.Kanomata K, et al. Mechanism and origin of stereoselectivity in chiral phosphoric acid-catalyzed aldol-type reactions of azlactones with vinyl ethers. Chem. Eur. J. 2020;26:3364–3372. doi: 10.1002/chem.201905296. [DOI] [PubMed] [Google Scholar]
- 61.Xu B, et al. Insertion reaction cooperatively catalyzed by rhodium and chiral spiro phosphoric acids. Angew. Chem. Int. Ed. 2011;50:11483–11486. doi: 10.1002/anie.201105485. [DOI] [PubMed] [Google Scholar]
- 62.Xu B, et al. Highly enantioselective S–H bond insertion cooperatively catalyzed by dirhodium complexes and chiral spiro phosphoric acids. Chem. Sci. 2014;5:1442–1448. doi: 10.1039/c3sc52807c. [DOI] [Google Scholar]
- 63.Lam Y-h, Grayson MN, Holland MC, Simon A, Houk KN. Theory and modeling of asymmetric catalytic reactions. Acc. Chem. Res. 2016;49:750–762. doi: 10.1021/acs.accounts.6b00006. [DOI] [PubMed] [Google Scholar]
- 64.Sunoj RB. Transition state models for understanding the origin of chiral induction in asymmetric catalysis. Acc. Chem. Res. 2016;49:1019–1028. doi: 10.1021/acs.accounts.6b00053. [DOI] [PubMed] [Google Scholar]
- 65.Peng Q, Duarte F, Paton RS. Computing organic stereoselectivity–from concepts to quantitative calculations and predictions. Chem. Soc. Rev. 2016;45:6093–6107. doi: 10.1039/C6CS00573J. [DOI] [PubMed] [Google Scholar]
- 66.Peddibhotla S, Tepe JJ. Stereoselective synthesis of highly substituted Δ-Pyrrolines: exo-selective 1,3-dipolar cycloaddition reactions with azlactones. J. Am. Chem. Soc. 2004;126:12776–12777. doi: 10.1021/ja046149i. [DOI] [PubMed] [Google Scholar]
- 67.Johnson ER, et al. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010;132:6498–6506. doi: 10.1021/ja100936w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qi X, Kohler DG, Hull KL, Liu P. Energy decomposition analyses reveal the origins of catalyst and nucleophile effects on regioselectivity in nucleopalladation of alkenes. J. Am. Chem. Soc. 2019;141:11892–11904. doi: 10.1021/jacs.9b02893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schreiber SL. Target-oriented and diversity-oriented organic synthesis in drug discovery. Science. 2000;287:1964. doi: 10.1126/science.287.5460.1964. [DOI] [PubMed] [Google Scholar]
- 70.Marshall JA, Tang Y. Allene-directed diastereoselection. Additions to chiral allenyl aldehydes and ketones. J. Org. Chem. 1993;58:3233–3234. doi: 10.1021/jo00064a006. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and Supplementary Information file, or from the corresponding author upon reasonable request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 1971171, 1971946, 1971947, 1971365, and 1971366. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.