Abstract
CRISPR-Cas systems provide versatile tools for programmable genome editing. Here, we developed a caged RNA strategy that allows Cas9 to bind DNA but not cleave until light-induced activation. This approach, referred to as very fast CRISPR, creates doublestrand-breaks (DSBs) at submicron and seconds scales. Synchronized cleavage improved kinetic analysis of DNA repair, revealing that cells respond to Cas9-induced DSBs within minutes and can retain MRE11 after DNA ligation. Phosphorylation of H2AX after DNA damage propagated over 100 kilobases per minute, reaching up to 30 megabases. Using single cell fluorescence imaging, we characterized multiple cycles of 53BP1 repair foci formation and dissolution, with the first cycle taking longer than subsequent cycles and its duration modulated with inhibition of repair. Imaging-guided subcellular Cas9 activation further facilitated genomic manipulation with single allele resolution. Together, very fast CRISPR enables DNA repair studies at high resolution in space, time and genomic coordinates.
One Sentence Summary:
Very fast CRISPR on demand enables DNA repair studies at high resolution in space, time and genomic coordinates.
RNA-guided DNA targeting with CRISPR-Cas9 has revolutionized biomedical research for genome editing and beyond (1). After genomic DNA cleavage by Cas9, DNA damage response (DDR) proteins are recruited to initiate complex repair processes (2). While DDR is known to be influenced by factors such as target sequence (3, 4), cell cycle (5), and chromatin dynamics (6), the precise timing and sequence of cellular events require further investigation. Cas9 has potential as a tool to study the dynamics of DDR, but currently lacks the necessary level of control to initiate precise DNA damage on demand. To unveil the sequence of Cas9-induced DDR events in living cells, an inducible Cas9 system with the spatiotemporal resolution that matches the rapidity and subcellularity of DDR would be powerful.
Numerous inducible Cas9 systems have been developed (7-11). However, these methods often exhibit compromised function in the engineered proteins, coarse temporal control in the hour time scale because Cas9 still has to find the target after induction, and no spatial control or control at millimeters length scale at best.
Here, we report a very fast CRISPR-Cas9 system (vfCRISPR) that allows genome editing on demand at sub-micron space scale and seconds time scale. Through synchronized DSB induction followed by complementary biochemical, sequencing, and imaging-based assays, we characterized the early molecular events that underlie the initiation and progression of DNA repair with high spatiotemporal precision.
The design principle of vfCRISPR is based on the Streptococcus pyogenes Cas9 (Cas9 henceforth) cleavage mechanism. The protospacer adjacent motif (PAM)-proximal 9-10 bp region of guide RNA (gRNA) governs Cas9 binding to its target DNA while additional base pairing at the PAM-distal region (10-20 bp) is required for cleavage (12, 13). Mismatches in the PAM-distal region prevent full unwinding of target DNA (14) and conformational changes of the HNH domain (15) required for cleavage. Based on this mechanistic understanding, we replaced two or three uracils at the PAM-distal region of crRNA with light-sensitive, 6-nitropiperonyloxymethyl modified deoxynucleotide thymine (NPOM-dT) caged nucleotides (16), forming a caged gRNA (cgRNA) once hybridized to wild type tracrRNA (Fig. 1A). The Cas9/cgRNA complex retains the ability to bind its target DNA, but cannot cleave because the steric hindrance imposed by the caging groups prevents full DNA unwinding and nuclease activation. Upon light stimulation at 365 or 405 nm, the caging groups are removed and the pre-bound, now-activated Cas9/cgRNA complex rapidly cleaves target DNA.
Figure 1. Characterization of very fast CRISPR in vitro and in cells.
(A) Schematic of Cas9 activation by modulating base-pairing between the PAM distal region of cgRNA and genomic DNA. (B) Without light, Cas9/cgRNA bound to target DNA without cleavage, causing a clear band shift. Proteinase K degraded Cas9, causing target DNA to shift back to the original position. (C, D) Fast and efficient in vitro cleavage kinetics of Cas9 after light activation. (E) Indels detected by high-throughput sequencing of PCR-amplified genomic DNA extracted from cells without RNP, with RNP but no light, and with RNP 48 h after light activation. (F) DSBs detected by DSB-ddPCR of genomic DNA extracted from cells without RNP, with RNP but no light, and with RNP 30 s after light activation. (G-H) DSB % over time using our method (red) compared to either RNP electroporation (G, target sequence at ACTB) or a chemically inducible system (H, target sequence at MYC). (I) DSBs and Normalized Indels (Materials and Methods) at ACTB over time after Cas9 activation.
An electrophoretic mobility shift assay (EMSA) confirmed that Cas9/cgRNA stably bound to target DNA without light (Fig. 1B). After uncaging with light, the Cas9/cgRNA complex efficiently cleaved DNA within seconds in vitro (Fig. 1C and D, Fig. S1).
Next, we characterized the activity of vfCRISPR in HEK293T cells by targeting four endogenous loci and found light-induced indel efficiency up to 97%, while cells without light exposure had almost no detectable indels (Fig. 1E; S2). Cells exposed to this dosage of light exhibited no apparent phototoxicity (Fig. S3). Importantly, significant DNA cleavage was found within 30 s after light activation (Fig. 1F; S4). Compared to other Cas9 induction methods, vfCRISPR exhibited much faster cleavage kinetics and higher cleavage efficiency (17, 18) (Fig. 1G and H). We attribute the very fast kinetics to skipped nuclear localization or target searching steps, and the higher cleavage efficiency to use of wild type Cas9. Genome-wide analysis of off-target editing using GUIDE-seq (19) also revealed reduced off-target activity compared to wild type gRNA (Fig. S5), consistent with improved specificity from deoxyribonucleotide incorporation into the guide RNA (20). These experiments demonstrated that cgRNA enables very fast and efficient inducible DNA cleavage in mammalian cells.
With a precisely defined time for cleavage, vfCRISPR allowed us to investigate the generation and repair kinetics of Cas9-mediated DSBs. We measured percent of DSBs and indels as a function of time after Cas9 activation at multiple target sites, and adopted mathematical models to describe the kinetics of DSB and indel formation (21) (Fig. 1I; S6; Models I and II, Supplementary theory). Model fitting led us to hypothesize the re-cutting of +1 insertion DNA at ACTB, which we subsequently verified both in vitro and in cells through re-cutting of a monoclonal cell line with a pure +A indel product at ACTB (Fig. S7 and S8).
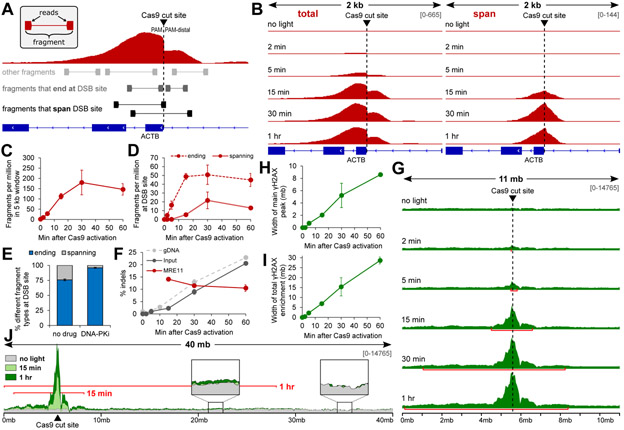
Utilizing highly synchronized DNA cleavage, we performed time-resolved chromatin immunoprecipitation followed by sequencing (trChIP-seq) to track the recruitment of MRE11, which forms the MRN complex with Rad50 and Nbs1, to the ACTB cleavage site (18) (Fig. 2A). We observed rapid MRE11 recruitment that reached half maximal signal between 5 and 15 min (Fig. 2B and C, S9A). This is slower than recruitment of another MRN component, Rad50, after laser microirradiation (22), potentially due to delay in exposure of Cas9-induced DSBs (Fig. S9B). Both ChIP-seq and probe-based ChIP-qPCR detected the emergence of MRE11-bound DNA that spanned the cleavage site at 15 min after Cas9 activation (Fig. 2B and D; S10A). This spanning population, which we attribute to repaired DNA still bound by MRE11, was present across different target sequences and cell types (18) (Fig. S10B to D, S11). Inhibition of DNA-PKcs with KU-0060648 led to a significant reduction in spanning fragments with a concomitant increase in fragments that ends at the cut site (Fig. 2E, S12). Deep amplicon sequencing of spanning DNA revealed consistent 10-15 % indels while total indels rose from 2 % to 20 % within that period of time (Fig. 2F; S13). Together, these results are consistent with transient MRE11 retention on ligated genomic DNA that was processed in a DNA-PKcs-dependent manner.
Figure 2. Time-resolved ChIP-seq reveals recruitment dynamics of MRE11 and γH2AX after synchronized Cas9-induced DSBs.
(A) Schematic of MRE11 ChIP-seq analysis for paired-end reads. (B) Visualization of MRE11 peak features over time after Cas9 activation. The left column (‘total’) piles all fragments. The right column (‘span’) only piles fragments that span the cleavage site. (C) Fragments per million in 5 kb window around the cleavage site over time. (D) Fragments per million that either span or start/end at the cleavage site over time. Fragments that start/end at the DSB site are enriched first, followed with ~15 min delay by fragments that span the DSB site. (E) Proportion of fragments that span the DSB site is depleted with DNA-PKcs inhibition. (F) Indel percent calculated from deep sequencing of PCR amplicons from both ChIP input (dark grey – “Input”) and MRE11 ChIP (red – “MRE11”) DNA that span the cleavage site. Indel kinetics from Figure 1I is included for comparison (light grey, dashed – “gDNA”). (G) γH2AX enrichment over time in a 11 mb window around the cleavage site. Red bars mark the width of the ‘main’ γH2AX peak detected using macs2. (H) Width of the ‘main’ γH2AX peak over time detected using macs2. (I) Width of total enrichment detected using Student’s t-test with Bonferroni correction (p < 0.05), comparing no-light to all afteractivation results. (J) Illustration of enrichment up to ~30 mb along Chr7:1-40,000,000 (p < 0.05). Red bars mark the width of total γH2AX enrichment detected using Student’s t-test.
H2AX is known to be phosphorylated (γH2AX) for 1-2 megabases (mb) around a DSB undergoing active repair (23), but the dynamics of initial γH2AX spreading is unknown. trChIP-seq for γH2AX revealed rapid expansion of a ‘main peak’ around the cleavage site at the speed of ~150 kilobases (kb) per min, reaching 8 mb at 1 h (Fig. 2G and H; S14). Statistical testing (Materials and Methods) revealed another layer of γH2AX enrichment that expanded linearly at 460 kb per min and spanned up to 30 mb at 1 h (Fig. 2I and J; S15). To our knowledge, γH2AX enrichment up to tens of megabases has not been previously reported. While this may be a unique feature of Cas9-induced DSBs, synchronized, high efficiency cleavage with vfCRISPR may have contributed to the detection of lower levels of enrichment.
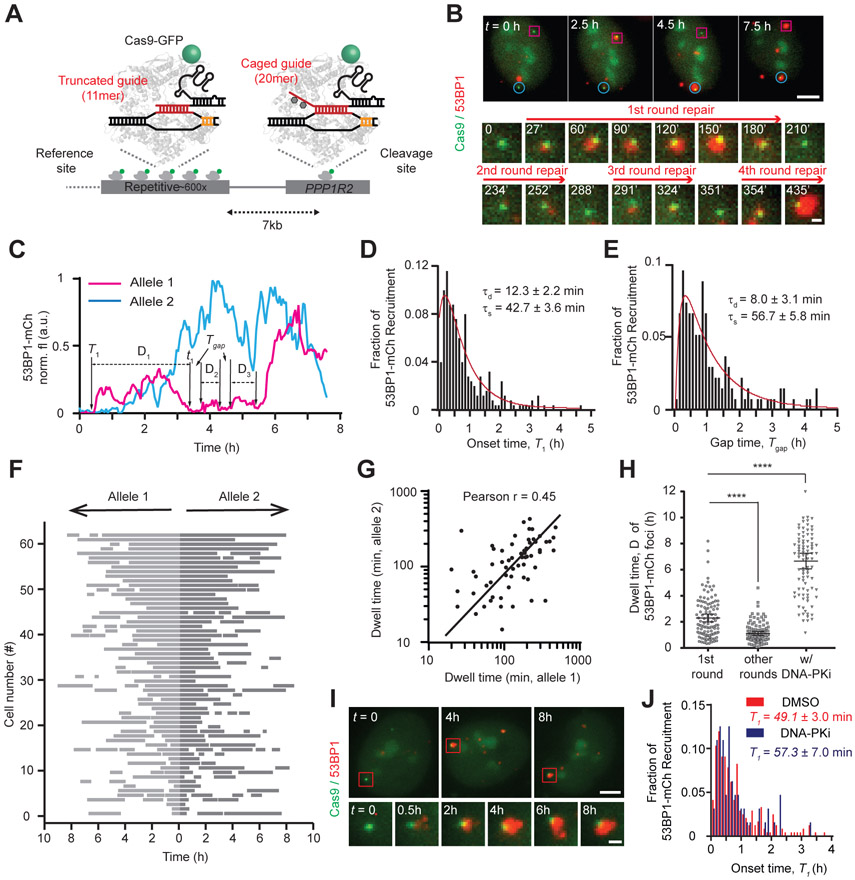
Next, we performed single cell fluorescence imaging to capture the dynamics of repair protein recruitment induced by vfCRISPR. To a monoclonal U-2 OS cell line stably expressing Cas9-EGFP, we co-transfected a truncated gRNA (11-mer in the protospacer) targeting a highly repetitive region in chromosome 3 (Ch3Rep) with a cgRNA targeting the PPP1R2 gene, only 36kb downstream of Ch3Rep (24-26). Upon light activation, a single DSB is generated at PPP1R2, which is fluorescently marked by an array of Cas9-EGFPs decorating Ch3Rep (Fig. 3A). We confirmed recruitment of multiple endogenous repair factors (pATM, MDC1, 53BP1 and γH2AX) to the single break sites (Fig. S16A to D). γH2AX foci size increased over time (Fig. S16D), consistent with γH2AX spreading reported by trChIP-seq.
Figure 3. Live-cell imaging reveals the spatiotemporal dynamics of 53BP1 after synchronized Cas9 DSBs.
(A) Schematic of orthogonal genomic imaging and cleavage using Cas9-EGFP. (B) Snapshots showing 53BP1 recruitment to two different PPP1R2 alleles (magenta square and cyan circle). Zoom-in images show multiple cycles of 53BP1 foci at one PPP1R2 allele (magenta square). (C) Fluorescence intensity traces of 53BP1-mCherry for both alleles monitored in (B). T1, t1 and D1 denote the start, end and dwell times for the first cycle of DNA repair, respectively. Time interval Tg is calculated as Tn+1 – tn, for example, T2 – t1. (D) Histogram of initial 53BP1 recruitment time (T1) at the PPP1R2 locus after light stimulation. (E) Histogram of time interval (Tg) between consecutive 53BP1 cycles for 8 h. (F) Rastergram of 53BP1 foci at 124 paired alleles in 62 cells. Each row displays time courses of 53BP1 foci at a pair of alleles residing in the same nucleus. Grey bars indicate presence and white indicates absence of 53BP1-mCherry at each PPP1R2 allele. Cells are ranked by the mean dwell time of the first 53BP1 recruitment at two alleles (longest to shortest). (G) Scatter plot showing positive correlation in dwell time between two alleles in the same cell nucleus (r = 0.45). (H) Dwell time of 53BP1 foci at PPP1R2 for the first 53BP1 cycle (n = 167 foci, 5 biological replicates), later cycles (n = 109 foci, 5 biological replicates), or after DNA-PKcs inhibition (DNA-PKi; n = 92 foci, 3 biological replicates). Unpaired t-test was performed, ****P < 0.0001. Error bars indicate 95% confidence interval. (I) Snapshots showing longer 53BP1 dwell time in cells with DNA-PKcs inhibition. (J) In DNA-PKcs inhibited cells, 53BP1 recruitment time was unchanged (P > 0.2).
To track real-time repair dynamics through live-cell imaging, we stably coexpressed 53BP1-mCherry (27) with Cas9-EGFP in U-2 OS cells (Fig. 3A to C). The majority of Cas9-EGFP-labeled alleles colocalized with 53BP1-mCherry foci over the course of 8 h after Cas9 activation (Fig. S17A; Supplementary Movie 1), indicating efficient cleavage at PPP1R2. The onset time for 53BP1 recruitment (T1) was heterogeneous between cells and alleles, with most foci appearing within 1 hour (Fig. 3D). Maximum likelihood estimation of a two-step mathematical model for T1 yielded a DSB detection time (τd) of 12 ± 2.2 min and Cas9 target searching time (τs) of 43 ± 3.6 min (Fig. 4C; Supplementary Theory).
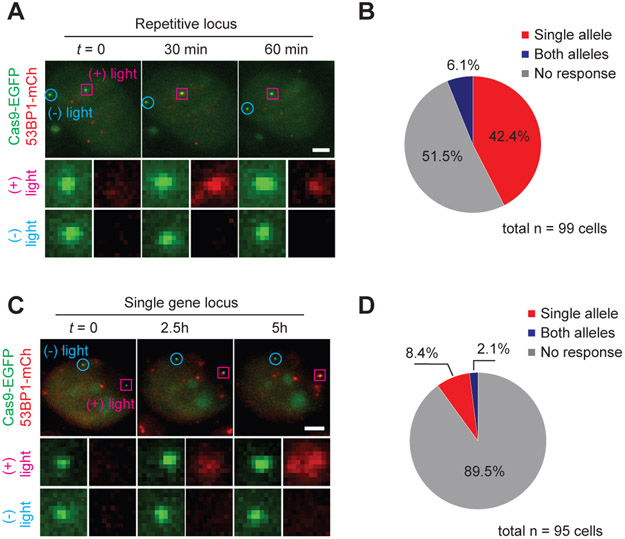
Figure 4. DSB cleavage at single allele resolution using subcellular Cas9 activation.
(A) Single cell snapshots showing targeted cleavage of Ch3Rep at one of the two alleles (pink). (B) Summary of 53BP1-mCh recruitment to Ch3Rep alleles. “Single allele” (red) indicates 53BP1-mCherry recruitment to the targeted allele only, “Both alleles” (blue) indicates recruitment to both the targeted and non-targeted alleles. “No response” (grey) indicates no recruitment to either allele. n = 99, 4 biological replicates. (C) Single cell snapshots showing targeted cleavage of a single PPP1R2 allele (pink). (D) Summary of 53BP1-mCh recruitment to PPP1R2 alleles. n = 95, 4 biological replicates. Single allele specificity is calculated by dividing the percent of mono-allelic activation by the total activation percent (both mono-allelic and bi-allelic activation). For repetitive cutting (B), it is 42.4 / (42.4 + 6.1) x 100 = 87.4%, whereas for single cutting (D), it is 8.4 / (8.4 + 2.1) x 100 = 80%.
Each 53BP1 focus underwent a cycle of enlargement and dissolution, with the majority exhibiting more than one and up to five cycles over 8 h, consistent with a previous report (26) (Fig. S17B). The estimated τs and τd for subsequent rounds of 53BP1 recruitment agree well with τs and τd for the first round, suggesting that each 53BP1 cycle corresponds to at least one repair event (Fig. 3E and Table S6).
Duration of the initial 53BP1 cycle, D1, was on average significantly longer than subsequent cycles (Fig. 3H). D1 varied over a wide range and was positively correlated between two alleles in the same cell but not between different cells (Fig. 3F and G; S18 and S19), suggesting that stochastic differences in chromatin environments between the two alleles is not the main reason for the large variation in D1. Inhibition of ATM using KU-0055933 eliminated 53BP1 foci (Fig. S20), consistent with its role as an upstream regulator of 53BP1 recruitment. Inhibition of DNA-PKcs prolonged 53BP1 foci without affecting 53BP1 recruitment (Fig. 3H to J; S20), leading to fewer 53BP1 cycles and further supporting our interpretation that 53BP1 cycles mark successive rounds of DSB and repair (Fig. S21 to S23).
Finally, we extended vfCRISPR to spatially manipulate single genomic alleles. Both Ch3Rep alleles were bound by an array of Cas9/cgRNAs within the same nucleus. We focused a 405 nm laser beam to one Ch3Rep allele, which locally activated Cas9 to cleave the targeted allele while keeping the other one intact (Fig. S24). Almost half of cells exhibited 53BP1 recruitment to the targeted allele within 1 h, whereas only 6 % of cells showed recruitment to both alleles, showing single allele specificity (Fig. 4A and B; Supplementary Movie 2). We also demonstrated allele-specific manipulation of a nonrepetitive cleavage site at PPP1R2 (Fig. 4C and D; Supplementary Movie 3). We observed only one round of 53BP1-mCherry recruitment, likely because only one round of cleavage is possible due to activation of only a small subset of Cas9/cgRNA in the nucleus (Fig. S25). Laser beam alone was not responsible for 53BP1 recruitment (Fig. S26), and conditions were optimized to maximize single allele specificity, which was 80 % or higher for both experimental schemes (Fig. 4B and D). The capability of vfCRISPR to manipulate single genomic alleles with high specificity motivates applications such as simplifying generation of heterozygous mutants (28) and potentially reducing and/or eliminating off-target genome editing.
To the best of our knowledge, vfCRISPR provides the highest spatial and temporal resolutions to induce site-specific DSBs in living cells. This study sets the blueprint for further systematic studies of the DNA damage response that combines vfCRISPR with time-resolved biochemical, sequencing, and imaging readouts. Use of caged guide RNA with other Cas9-based systems such as nickases, base editors, and prime editors may facilitate study of single strand break, base excision or mismatch, and flap repair, respectively. Combining vfCRISPR with subcellular photoactivation potentially enables precise genome editing with single allele specificity and elimination of off-target activity.
Supplementary Material
Acknowledgements:
We thank Dr. Digvijay Singh for demonstrating initial feasibility on synthetic DNA. We thank Dr. Bong Gu Kang and Prof. Ted Dawson for selection of the monoclonal U-2 OS cell lines. We thank Taylor Cottle for helping with in vitro cleavage assays. We thank Prof. Geraldine Seydoux for access to the Lonza 4D-Nucleofector, Prof. Winston Timp for access to the MiSeq.
Funding: This work was supported by the grants from the National Institutes of Health (R35 GM 122569 to T.H.), the National Science Foundation (PHY 1430124 to T.H., 1817447 to B.W.) and Pew Charitable Trust (00030601 to B.W.). R.S.Z. was supported by the NIH Medical Scientist Training Program Award (T32 GM 007309). Y.N. was supported by Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad. The work was partially supported by NIH GM123130 to Takanari Inoue. T.H. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Competing interests: The authors and Johns Hopkins University have filed a provisional patent application on the method of spatiotemporal control of Cas9 activities via cgRNA.
Data and materials availability: All data associated with this study are present in the paper or Supplementary Materials. All ChIP-seq data is uploaded to Sequence Read Archive under BioProject accession PRJNA609749, with analysis code available on GitHub (https://github.com/rogerzou/chipseq_cgRNA). mCherry-BP1-2 pLPC-Puro was a gift from Titia de Lange.
References and Notes
- 1.Knott GJ, Doudna JA, Science 361, 866–869 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polo SE, Jackson SP, Genes Dev. 25, 409–433 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Overbeek M et al. , Mol. Cell 63, 633–646 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Lemos BR et al. , Proc. Natl. Acad. Sci. U. S. A 115, e2040–2047 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin S, Staahl BT, Alla RK, Doudna JA, Elife 3, e04766 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kallimasioti-Pazi EM et al. , PLoS Biol. 16, e2005595 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dow LE et al. , Nat. Biotechnol 33, 390–394 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zetsche B, Volz SE, Zhang F, Nat. Biotechnol 33, 139–142 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nihongaki Y, Kawano F, Nakajima T, Sato M, Nat. Biotechnol 33, 755–760 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Hemphill J, Borchardt EK, Brown K, Asokan A, Deiters A, J. Am. Chem. Soc 137, 5642–5645 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain PK et al. , Angew. Chem. Int. Ed 55, 12440–12444 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahlman JE et al. , Nat. Biotechnol 33, 1159–1161 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh D, Sternberg SH, Fei J, Doudna JA, Ha T, Nat. Commun 7, 12778 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh D et al. , Nat. Struct. Mol. Biol 25, 347–354 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sternberg SH, Lafrance B, Kaplan M, Doudna JA, Nature 527, 110–113 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lusic H, Young DD, Lively MO, Deiters A, Org. Lett 9, 1903–6 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rose JC et al. , Nat. Methods 14, 891–896 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wienert B et al. , Science 364, 286–289 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai SQ et al. , Nat. Biotechnol 33, 187–197 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin H et al. , Nat. Chem. Biol 14, 311–316 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinkman EK et al. , Mol. Cell 70, 801–813.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aleksandrov R et al. , Mol. Cell 69, 1046–1061 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Clouaire T et al. , Mol. Cell 72, 250–262.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen B et al. , Cell 155, 1479–1491 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma H et al. , Nat. Biotechnol 34, 528–530 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H et al. , Science 365, 1301–1305 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Dimitrova N, Chen YCM, Spector DL, De Lange T, Nature. 456, 524–528 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paquet D et al. , Nature 533, 125–129 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.