Exponentially increasing burial rates of microplastics since the 1950s confirm mangrove sediments as long-term sinks.
Abstract
Sequestration of plastics in sediments is considered the ultimate sink of marine plastic pollution that would justify unexpectedly low loads found in surface waters. Here, we demonstrate that mangroves, generally supporting high sediment accretion rates, efficiently sequester plastics in their sediments. To this end, we extracted microplastics from dated sediment cores of the Red Sea and Arabian Gulf mangrove (Avicennia marina) forests along the Saudi Arabian coast. We found that microplastics <0.5 mm dominated in mangrove sediments, helping explain their scarcity, in surface waters. We estimate that 50 ± 30 and 110 ± 80 metric tons of plastic may have been buried since the 1930s in mangrove sediments across the Red Sea and the Arabian Gulf, respectively. We observed an exponential increase in the plastic burial rate (8.5 ± 1.2% year−1) since the 1950s in line with the global plastic production increase, confirming mangrove sediments as long-term sinks for plastics.
INTRODUCTION
The mass production of plastic, starting in the 1950s and growing at 8.4% per year (1), coupled with inefficient disposal and recycling systems, has generated increasingly high loads of waste entering the marine environment (2). The inherent durability of plastic material makes it extremely persistent in seawater (3), where it fragments but does not degrade (4), and its buoyancy facilitates its transport by currents and winds to the most remote locations (5–7). As a result, microplastics, i.e., particles <5 mm in diameter (8), are continuously accumulating across vast areas of the oceans (9–11), with concentrations increasing over time (12). Yet, global assessments indicate that only 1% of plastic entering the marine environment remains floating in surface waters (9, 13), with these plastic stocks particularly depleted in items <1 mm (11). These findings have led some to hypothesize the existence of a range of size-dependent removal processes, such as ingestion by fauna (14–16), sinking to deeper layers of the water column (3, 17, 18), and sequestration in sediments (19).
Burial in sediments is thought to be the major sink of plastic in the marine environment (20). Negatively buoyant plastic material, often ballasted by biofouling (17, 21), sinks and is deposited in sediments, entering long-term sequestration. The signature of plastics in the sediment record provides stratigraphic evidence that, since the mid-20th century, we have entered a new epoch, the Anthropocene (22). Persistent and substantial stocks of microplastics are therefore expected in habitats supporting high sediment accumulation rates, such as vegetated coastal habitats, including seagrasses, salt marshes, and mangroves (23–28). These are termed blue carbon habitats because of their remarkable capacity to sequester and accumulate large stocks of carbon in their sediments at millenary time scales (23). Attenuation of wave action and currents by plant structures promotes sedimentation and reduces resuspension, contributing to the high rates of carbon sequestration in blue carbon habitats (23). Particle retention in blue carbon habitats by these mechanisms may also be effective in supporting the retention of microplastic particles since their shoots, roots, and pneumatophores interact with the top layers of the water column, where microplastics are typically found (18).
The hypothesis that blue carbon sediments may be important sinks for plastics is further supported by recent findings that Red Sea mangrove forests sustain higher loads of plastic than adjacent bare shores, by retaining large plastic items within their mesh of pneumatophores (29). Moreover, the Red Sea has been reported to hold unexpectedly low concentrations of plastics in its surface waters (30). Similarly, low concentrations of plastic in surface waters and high loads in mangrove forests were reported in the Arabian Gulf (29, 31). The low plastic concentration in the surface waters is unexpected given that both the Red Sea and the Arabian Gulf are characterized by an inverse estuarine circulation (for 8 months and all year long, respectively), which should lead to the retention of plastic litter advected in the two seas by surface currents from the Indian Ocean (32–34) and of those entering the Red Sea and the Arabian Gulf from land and ships. Therefore, the low stocks of floating plastics suggest the presence of significant removal processes. Given the extensive coverage of blue carbon habitats, especially mangroves, along the Red Sea and the Arabian Gulf (35, 36), sequestration in blue carbon sediments is likely to be an important removal process for plastics in the region. To test this hypothesis, we collected and extracted plastics from dated sediment cores of mangrove forests of the Red Sea and of the Arabian Gulf and estimated the plastic burial rates in mangrove sediments. Given that plastic production and input to the ocean have increased exponentially since the onset of mass production of plastics in the 1950s (1, 12), we further expect an exponential increase in plastic burial rates in the Red Sea and Arabian Gulf mangrove sediments during the past 70 years.
RESULTS AND DISCUSSION
Small-sized plastics dominate in sediments
Plastic particles were present in all the nine cores sampled in seven mangrove forests of the Red Sea and the Arabian Gulf. We found a total of 126 plastic items, excluding fibers (fig. S1 and data S1), averaging 14 ± 3 plastic particles per core (means ± SE).
Microplastic items smaller than 0.5 mm, which are reported as missing from surface water inventories in the Red Sea and the Arabian Gulf (30, 31), dominated plastic stocks in mangrove sediments (fig. S1A). Specifically, plastic items in the mangrove sediments sampled were significantly smaller than plastic items found in the Red Sea surface waters (fig. S1A; two-sample Kolmogorov-Smirnov test; D = 0.232, P value = 1.5 × 10−4) (30). This tendency is likely due to the higher susceptibility of small plastic items to be entrained by vertical mixing and sink (17, 18, 21). Fragmentation of plastic debris following burial is unlikely since the size distribution of fragments and films in sediments remained uniform with time (fig. S1B). This suggests that plastic sequestration in sediments is size dependent, which helps explain why small microplastics are less frequent than expected in the surface waters of the Red Sea and the Arabian Gulf (30, 31), which is consistent with the depletion of plastic stocks <1 mm in surface waters globally (11). The overall shape, color, and polymer composition of plastics in the sediments sampled did not differ from the plastics reported for the Red Sea and Arabian Gulf surface water (excluding fibers), i.e., majority of blue and white fragments and films made of polyethylene and polypropylene (fig. S1, C to E) (30, 31).
Concentration of plastic in mangrove sediments along a depth profile
We first calculated the concentration of plastic as the number of plastic items per kilogram of dry sediment in the upper 2 and 5 cm of the mangrove sediments sampled in the Arabian Gulf to allow comparisons with results from previous studies conducted in the same region that analyzed plastics in surface sediments. Our estimate of plastic concentration in Arabian Gulf mangrove sediments was higher than the concentration of plastics (excluding fibers) in the top 2 and 5 cm of intertidal beach sediments reported for the same region (Table 1) (31, 37, 38). The difference in plastic item concentration between sediments of vegetated and unvegetated coastal habitats in the Arabian Gulf suggests that mangrove forests might play a key role in the accumulation of plastics in their sediments.
Table 1. Estimates of plastic abundance (excluding fibers) in the upper 2 and 5 cm of sediments in coastal environments (mangroves and beaches) of the Arabian Gulf.
NA, not available.
| Site | Habitat |
Plastic stock in the top 2 cm, n items kg−1, means ± SE (median) |
Plastic stock in the top 5 cm, n items kg−1, means ± SE (median) |
n of samples | Reference |
| Saudi Arabia (Arabian Gulf coast) |
Mangroves | 84 ± 35 (58) | 83 ± 29 (74) | 5 cores | This study |
| Qatar | Beach (intertidal) | 8 ± 7 | NA | 24 | Abayomi et al. (31) |
| Iran (Arabian Gulf coast) | Beach (intertidal) | NA | 9 ± 2 | 15 | Naji et al. (37) |
| Iran (Arabian Gulf coast) | Beach (intertidal) | 15 ± 5 | NA | 12 | Naji et al. (38) |
To account for the size and mass of the plastic items, we then calculated the concentration as milligrams of plastic items per kilogram of sediment. We observed that, generally, the concentration of plastic decreased exponentially, going from the surface to the deeper layers of the sampled mangrove sediments (fig. S2A). However, one sediment core sampled in the Arabian Gulf (core no. 8) presented a very large plastic concentration at approximately 13 cm in depth, much higher than the concentration in the upper sediment layers of that site (fig. S2B and data S1).
Accumulation of plastic litter in mangrove sediments (1930– 2015)
Cores were sliced in 1-cm-thick samples, and each sample was dated (see Materials and Methods), allowing us to obtain the chronology of plastic deposition and sequestration in sediments, with a particular attention to the key milestones of the plastic production history, such as year 1907, the date of invention of plastic polymers; the 1930s, onset of industrial plastic production; and the 1950s, the start of mass plastics production (39). As expected from the chronology of plastic production, plastic particles were not found in sediments dated older than 1907.
The estimated abundance of plastic, excluding fibers, sequestered in sediments accumulated since 1930 (mean depth of 21.0 ± 3.1 cm) was 7840 ± 1630 items m−2 in the Red Sea and Arabian Gulf sampled mangroves. Specifically, we calculated that half of the total abundance, 3920 ± 940 items m−2, was located in the upper 5 cm of surface sediments.
The average stock of plastic buried since 1930 in the mangrove sediments sampled from the Red Sea and the Arabian Gulf is 510 ± 290 × 103 g km−2, with no significant difference between the two basins (Fig. 1; see Materials and Methods). In the Red Sea, we estimated the plastic stock accumulated in sampled mangrove sediments since 1930 (top 21.5 ± 6.2 cm of sediment) as 340 (±200) × 103 g km−2 (Fig. 1). The reported mean concentration of plastic in Red Sea surface waters (top 15 cm) is 1.1 ± 0.3 g km−2 (30). Hence, the plastic stock in 1 km2 of surface water is equivalent to the plastic stock accumulated in 3 m2 (from 1.5 to 10 m2) of mangrove sediments. In the Arabian Gulf, the estimated plastic stock accumulated in sampled mangrove sediments since 1930 (top 20.5 ± 3.3 cm of sediment) is 660 (±510) × 103 g plastic km−2 (Fig. 1). Estimates of floating plastic stocks in Arabian Gulf surface waters are limited to regional surveys. For example, 7.8 ± 5.8 g plastic km−2, excluding fibers, are estimated to be floating in the top 50 cm of Qatar surface waters (Fig. 1; see Materials and Methods) (31). Hence, the plastic stock in 1 km2 of Qatar surface water is equivalent to the stock of plastics in 12 m2 (from 7 to 53 m2) of mangrove sediment. The much higher loads of plastics in mangrove sediments compared to surface waters point to the efficiency of mangrove habitats in sequestering and accumulating plastics in their sediments.
Fig. 1. Plastic stocks in mangrove sediments.
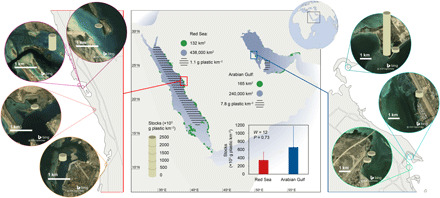
Plastic stocks (in grams of plastic per square kilometer accumulated since 1930) in each of the nine cores sampled in seven mangrove forests of Saudi Arabia (four on the Red Sea coast, framed in red, and three on the Arabian Gulf coast, framed in blue). The sand-colored cylinders on the aerial images indicate the core sampling point in the mangrove forests and the plastic stocks. The bar graph shows the means ± SE of the plastic stocks in the mangrove cores from the Red Sea (in red) and the Arabian Gulf (in blue), which are not significantly different between the two basins (Wilcoxon rank sum test; W = 12 and P = 0.73). The map reports, in green, the area of mangrove forests in the Red Sea (35) and in the Arabian Gulf (36); in blue, the extension of Red Sea waters and Arabian Gulf waters; and in a striped pattern, the areas where the concentration of plastics in surface waters has been assessed for the Saudi Red Sea (30) and Qatari Arabian Gulf (31) waters. Aerial images were printed from Bing Maps (Microsoft product screenshots reprinted with permission from Microsoft Corporation).
We can provide with an estimate of the magnitude of the role of mangrove forests of the Red Sea and the Arabian Gulf in sequestering plastic in their sediments. Extrapolating the calculated plastic stocks to the whole area of mangroves in each basin (132 km2 in the Red Sea and 165 km2 in the Arabian Gulf; Fig. 1) (35, 36), we calculate that a total of 50 ± 30 and 110 ± 80 metric tons of plastic may have been buried since 1930 in the upper approximately 20 cm of sediments in the mangrove forests across the Red Sea and the Arabian Gulf, respectively. This estimate is, despite uncertainties, remarkable when considering that mangroves comprise only 0.03% of the area of the Red Sea and 0.07% of the area of the Arabian Gulf. This suggests that mangroves’ habitats may indeed play an important role as a relevant plastic sink at the basin scale and should encourage efforts to derive more robust estimates. Doing so requires a stratified sampling effort to extend the estimates reported here to other regions in the Red Sea and the Arabian Gulf, representing different mangrove stands and oceanographic settings, and to nearby unvegetated control sites.
Plastic burial rates in mangrove sediments
Plastic burial rates increased exponentially from the beginning of the 20th century in six of seven sampled mangroves (fig. S3). The large burial rate value in one of the Arabian Gulf sites, corresponding to the 1920s (data S1) and derived from the high concentration value in core no. 8 (fig. S2B), leads to the exponential increase being rejected for that site (fig. S3). Such unexpected anomaly may be due to the activity of burrowing animals that may transport recently deposited microplastics deeper into the sediments. Plastic burial rates exponentially increased since 1950 in all sampled mangroves of the Red Sea and the Arabian Gulf (Fig. 2), at a combined rate of 8.5 ± 1.2% year−1 (n = 7; Fig. 2), consistent with the 8.4% annual increase in global plastic production since then (1).
Fig. 2. Plastic burial rates in mangrove sediments since the 1950s.
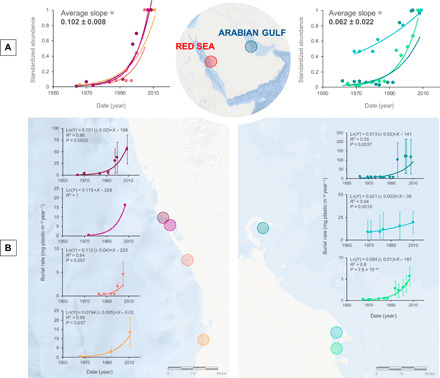
Historical increases in (A) standardized (to the maximum burial rate of each site) plastic burial rates in Red Sea and Arabian Gulf mangrove sites and in each (B) of the four Red Sea and three Arabian Gulf studied mangrove sites, from the 1950s to date (see details in data S1 and in Materials and Methods). Solid lines represent the exponential fit, for which the fitted equation including the SE of the slope, R2, and P value are reported.
The current (2001–2020) burial rate of plastic in the sampled mangroves of the two basins averaged 29 ± 15 and 64 ± 4 mg plastic m−2 year−1. Extrapolating to the whole Red Sea and Gulf mangrove area, we calculate the recent (2001–2020) plastic burial rates of 4 ± 2 and 11 ± 7 metric tons of plastic per year in the mangrove sediments in the Red Sea and the Arabian Gulf, respectively. In a “business-as-usual” scenario, where plastic burial in mangrove sediments continues to increase exponentially at rates of 10.2 ± 0.8% year−1 (Red Sea) and 6.2 ± 2.2% year−1 (Arabian Gulf; Fig. 2), 80 (30 to 160) metric tons and 70 (10 to 220) metric tons of plastic will be buried by year 2050 in the mangrove sediments of the Red Sea and the Arabian Gulf. As previously remarked, these estimates are indicative of the role of mangrove habitats, which should lead to efforts to derive robust basin-scale estimates of plastic burial in the Red Sea and Arabian Gulf mangrove sediments, as well as mangrove sediments elsewhere.
Mangroves are efficient sinks of plastic
In conclusion, our results show that mangrove habitats in the Red Sea and the Arabian Gulf are efficient sinks of marine plastic pollution. As shown by previous studies, small plastic particles undergo rapid changes in their buoyancy (faster than for larger items) when biofouled (17, 21). Especially in blue carbon habitats, they can be readily deposited in seafloor sediments, leading to a size-dependent removal process that is consistent with the depletion of floating microplastic stocks if plastic particles are <0.5 mm (Fig. 3) (11, 35) and supported by our findings that plastics <0.5 mm dominate in mangrove sediments. Once settled and sequestered, plastics can remain undegraded in sediments for decades, as demonstrated by their presence in layers dated from the 1930s, and likely over more extended time scales, given the stability of mangrove sediments reflected in carbon sequestration at the millenary time scale (23), but may be released again if the mangrove cover is disturbed. The plastic loads in mangrove sediments thus provide an archive of historical plastic usage, reflecting the exponential growth in production and subsequent input to the oceans since the emergence of mass plastic production in the 1950s (Fig. 3).
Fig. 3. Conceptual model of plastic burial in mangrove sediments.
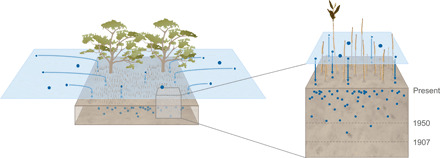
Mangrove forests, through the mesh created by their pneumatophores, enhance the deposition of plastic particles on their sediments. Since sinking of plastics is size dependent (17), mostly small plastic particles reach the bottom, while larger items may remain suspended in surface waters longer. Once deposited, plastics are sequestered for decades undisturbed. Therefore, sediments are an archive of the chronology of plastic consumption, as demonstrated by the absence of plastic particles in sediments dated older than 1907 (year of the invention of the first fully synthetic polymer) and by the exponential increase of plastic particles toward the most recent sediment layer, as a consequence of the mass production that began in the 1950s.
Our results reveal very large burial rates of plastics in mangrove sediments, which are sufficiently high as to help account for the low concentrations of plastic (excluding fibers) found in the surface waters of the Red Sea (30) and of the Arabian Gulf (32) and possibly in other tropical regions where fringing mangroves are abundant. Yet, plastics are also sequestered in sediments beyond mangroves (e.g., seagrass, salt marshes, and non–blue carbon sediments) and structures [e.g., coral reefs (40, 41)] across the region. Hence, the removal of floating plastics from the Red Sea and Arabian Gulf surface waters into sediments and benthic habitats is likely faster than that reflected in sequestration in mangrove sediments alone.
Our results identify mangrove forests as hot spots for plastic sequestration in their sediments. Hence, blue carbon strategies to conserve and restore mangrove habitats are not only effective to mitigate and adapt to climate change (23) but are also critically important to prevent the remobilization of plastic litter accumulated in the sediments. Whether the accumulation of plastic litter in mangrove sediments affects the associated benthic fauna or mangroves themselves is unknown and should be studied to assess the impacts of plastic burial on mangrove ecosystems.
MATERIALS AND METHODS
Study area and design
The Red Sea and the Arabian Gulf are semienclosed basins connected to the Indian Ocean by relatively narrow straits (the Bab-el-Mandeb strait in the Red Sea and the strait of Hormuz in the Arabian Gulf). Both seas are characterized by an inverse estuarine circulation, supported by high evaporation rates, involving an inflow of surface currents and an outflow of deeper denser waters. While this circulation pattern occurs all year long in the Arabian Gulf (34), the Red Sea supports this circulation for 8 months a year, from October to May. From June to September, the main surface circulation in the southern half of the Red Sea reverses and a three-layer current (an inflowing current sandwiched between a surface and deep layer of outflowing currents) flows through the strait (32). Mangrove stands cover 132 and 165 km2 in the Red Sea and the Arabian Gulf, respectively, and, while in the Arabian Gulf, the mangrove coverage is decreasing following the global trend, in the Red Sea, it had an unexpected 12% increase during the last 40 years (35, 36). Given the high temperatures and salinity, the oligotrophic waters, and the lack of permanent riverine inputs in both basins, the mangrove stands of the Arabian regions are often small patches characterized by dwarf trees (42).
Saudi Arabia stretches for approximately 2000 and 560 km on the Red Sea and Arabian Gulf coast and has approximately 50 and 10 km2 of mangrove forests on the two coasts, respectively. We sampled a total of nine cores in four mangrove forests of the Red Sea (four cores collected in 2015) and three forests of the Arabian Gulf (five cores collected in 2016) along the coast of Saudi Arabia (Table 2 and Fig. 1). Sampled mangrove forests are monospecific stands of Avicennia marina.
Table 2. List of cores from the Red Sea and the Arabian Gulf processed for plastic extraction.
We report coordinates (latitude and longitude) of sampled mangrove cores, the name of the site, and MARs (in grams per square centimeter per year) calculated for each core.
| Core ID | Sea | Latitude (N) | Longitude (E) | Site | MAR (g cm−2 year−1) |
| 1 | Red Sea | 22.972008 | 38.848878 | Khor Al-Kharrar | 0.52 ± 0.09 |
| 2 | Red Sea | 22.938872 | 38.878769 | Khor Al-Kharrar | 0.021 ± 0.002 |
| 3 | Red Sea | 22.752567 | 38.997394 | Khor Al-Baqila | 0.29 ± 0.12 |
| 4 | Red Sea | 22.282150 | 39.084325 | Al Taweelah | 0.20 ± 0.06 |
| 5 | Arabian Gulf | 27.28603 | 49.56685 | Abu-Ali | 0.25 ± 0.02 |
| 6 | Arabian Gulf | 27.28283 | 49.56523 | Abu-Ali | 0.45 ± 0.05 |
| 7 | Arabian Gulf | 26.71674 | 50.02111 | Ras Tanura–Safwa 1 | 0.50 ± 0.05 |
| 8 | Arabian Gulf | 26.64246 | 50.01462 | Ras Tanura–Safwa 2 | 0.28 ± 0.09 |
| 9 | Arabian Gulf | 26.63699 | 50.01067 | Ras Tanura–Safwa 2 | 0.44 ± 0.06 |
Sample collection and dating
Sediment cores were collected with polyvinyl chloride (PVC) tubes with a sharp-angled end, carefully hammered into the sediment and retrieved manually or with the help of a winch. White corers (1.7 m long, 6.26 cm in inner diameter) were used to collect sediments in the Red Sea and gray corers (1.4 m long, 7 cm in inner diameter) in the Arabian Gulf. The upper extremity of the core was tapped to allow vacuum and avoid sediment collapse during retrieval from the soil. After collection, cores were stored at 4°C before further processing. Cores were opened using a circular saw, and the length of the sediment retrieved was recorded to later calculate the compression effects against the depth of the core introduced in the soil. Cores were cut in 1-cm slices using a ceramic knife, and each slice (from now on referred also as “sample”) was dried at 60°C. Dry weight was measured, and dry bulk density (g cm−3) was calculated. A compression correction was applied following Serrano et al. (43).
Sediment cores were dated as described by Saderne et al. (44). Specifically, a 5- to 10-g subsample of the top five slices and every second slice from 5- to 19-cm depth of each core were grinded by using a pestle and sieved through a 125-μm mesh and sent to Edith Cowan University (Australia) for 210Pb dating. 210Pb was analyzed via the determination of its granddaughter 210Po. Two hundred to 300 mg of aliquots of sediment from each sample were spiked with a known amount of 209Po, acid was digested using an analytical microwave, and the polonium isotopes were plated onto silver disks. The emissions of the polonium isotopes were measured by alpha spectrometry using passivated implanted planar silicon detectors. The concentrations of excess 210Pb used to obtain the age models were calculated as the difference between total 210Pb and supported 210Pb, continuously produced by decay of 226Ra, the latter measured by low-background liquid scintillation counting (Wallac 1220 Quantulus) or by gamma spectrometry. The constant flux:constant sedimentation model (45, 46) was used to estimate the average soil mass accumulation rates (MARs; in grams of dry weight per square centimeter per year; Table 2) for each core and then divided by the dry bulk density to obtain sediment accretion rates (in centimeters per year). Dates of each sample were then obtained by dividing the depth of the sample (as accumulated mass) by the core MAR.
Plastic extraction
The top 1 cm and other 4 to 12 samples per core were processed for plastic extraction (data S1). The number of samples processed per core depended on the dating results: More and deeper samples were processed for cores with higher sedimentation rates and vice versa since the focus was mainly on samples dated after plastic invention (1907). We processed a total of 88 samples dated from mid-19th century to 2015. We included five samples dated before the invention of plastic in 1907 as a control. Extraction of plastic was implemented using the method described by Coppock et al. (47), being cost efficient and accurate. Particularly, a sediment-microplastic isolation (SMI) unit was manufactured, as per the design proposed by Coppock et al. (47) consisting of a column made by two transparent tubes 14 cm long [we used polymethyl methacrylate (PMMA) instead of PVC] connected by a ball valve (gray, in PVC). We changed the fixed base for a removable one to ease wash of the column after the processing of each sample and to avoid cross-contamination (fig. S4). To test the method, we spiked three clean sediment samples (~50 g) each with 20 green polyethylene beads of four size classes (53 to 63, 212 to 250, 425 to 500, and 850 to 1000 μm) and other three sediment samples each with 20 irregular fragments of biofouled polyethylene of the same size classes but various colors. To extract plastics from the test sediment samples and the samples selected from each core for the analysis, we added each dry sediment sample (<60 g) to the SMI with the ball valve open, together with a magnetic stirring bar and a 700-ml solution of zinc chloride (ZnCl2) (1.5 g cm−3). The top of the unit was closed with aluminum foil, and the solution was stirred on a magnetic stirring plate for 5 min at 500 rpm to allow the complete resuspension of plastic trapped into the sediment. After mixing, the sediment was allowed to decant, and the ball valve was closed. The supernatant, containing particles less dense than 1.5 g cm−3, was vacuum filtered through a 25-μm nylon mesh, and the material retained in the mesh was then inspected under a dissecting microscope (Carl Zeiss, Stemi 2000) in search of plastics. The mesh was thoroughly screened two times at two different magnifications. Putative plastic particles were picked, photographed to be measured with an image analysis software (ImageJ; http://imagej.nih.gov), and rinsed with hydrogen peroxide to eliminate organic fouling and ease the successive characterization at the Fourier transform infrared spectroscopy (FTIR). Particularly, we used Nicolet 10 FTIR for particles ≥250 μm and Nicolet 6700 μFTIR for particles <250 μm. Obtained spectra were matched with the OMNIC spectrum library, and polymer type was assessed. Extracted and confirmed particles were assigned to shape categories: fragments (hard pieces from broken objects), films (bags, wrappings, or pieces of them), foams (expanded cellular plastics), and threads (filaments from fishing lines, ropes, and nets), following the same classification of Martin et al. (33). Fibers (i.e., thin filaments normally originating from textiles) were excluded from the analysis since no appropriate blanks were set during the whole processing. Plastic particles were weighed, and, if weight was <0.01 mg (detection limit of the scale used), then the weight was inferred as the product of volume (obtained from image analyses in ImageJ) and density (obtained from the FTIR characterization of polymers). As a conservative measure to prevent contamination during sampling and core opening procedures, we excluded all plastic particles that could have originated from the PVC corers. Particularly, we excluded all PVC particles (except threads) of white color from the cores collected in the Red Sea and those of gray color from the cores collected in the Arabian Gulf (see data S1). Since the SMI unit is also made of plastic material, it could have been a source of contamination, too. However, no transparent PMMA particles (the material of the tubes in the unit) were encountered in any of the samples, and gray PVC particles (the material of the ball valve in the unit) were excluded from the Red Sea samples, too. Therefore, the number of plastics in the dataset compiled from this study (data S1) should be considered conservative. Since all the white PVC pieces excluded from the Red Sea samples were clearly attributable to the corers (they were bright white, not degraded nor fouled) and since we excluded all gray PVC pieces from the samples of both basins, the results from the two seas are comparable despite the use of corers of two different colors.
Recovery rates for the three test samples spiked with green polyethylene beads were 100% in each sample, showing that the extraction of plastic at the SMI unit is efficient for all sizes tested (50 to 1000 μm). Instead, the recovery was 70 ± 8% in the three samples spiked with irregular biofouled fragments, which were harder to detect at the microscope than green beads, indicating that the limitation of the method is the visual inspection. Pieces of plastic that were not recovered were mainly of the smallest size class (approximately 50 μm), while recovery of larger particles was 91 ± 9%, making the method reliable for items >200 μm.
Data analyses
The size distribution of plastic items recovered (n = 126) was obtained, assigning the sorted plastic pieces, according to their Feret diameter, to four size classes with increasing bin size following a logarithmic scale base 5. Abundance of items in each size class was normalized per width of the size class (in millimeters). Similarly, floating plastics from Red Sea surface waters [data from Martí et al. (30)] were distributed in the same size classes. The cumulative distributions (probability that objects with a size X are smaller than a given size x, F(x) = P[X ≤ x]) in size classes of items retrieved from the Red Sea and Arabian Gulf sediments and from the Red Sea surface waters were compared using a two-sample Kolmogorov-Smirnov test. Moreover, we questioned whether plastic items buried in sediments fragment (and thus become smaller) through time, by testing the null hypothesis that plastic size is independent of the estimated date of accumulation, by fitting a linear model to the data. However, we included in this analysis only the main shapes of regression plastic (fragments, n = 57, and films, n = 35), which we analyzed separately since their different shape may imply a different fragmentation pathway.
To allow comparison with the existing literature covering the Arabian Gulf region, where sediment samples are often collected in the top 2 and 5 cm, we calculated the average abundance of plastic per kilogram of sediment in the top 2 and 5 cm of Arabian Gulf cores. Samples in the top 1 and 2 cm of the cores were all processed, while one to three samples per core were processed between 2 and 5 cm.Since abundance is not informative of the loads of plastic in sediments, which depend on the size of the plastic items, we calculated the concentration of plastic as milligrams of plastic per kilogram of sediment, too.
We then calculated plastic abundance and stocks in mangrove sediments of each basin (Red Sea and Arabian Gulf) as the number and milligrams of plastic per square meter accumulated since year 1930, respectively. Considering that plastic production increased exponentially since the 1950s and that there is always an error associated with the dating, we conservatively selected year 1930 as a baseline to calculate the stocks. Moreover, year 1930 marks the industrial onset of plastic production (39). Specifically, stock values were calculated for each core as the total plastic load in samples from 1930 to present, normalized per surface unit area, considering that the area sampled by each core is 30.78 and 38.48 cm2 for Red Sea and Arabian Gulf cores, respectively (see data S1 for details on calculations). The depth corresponding to year 1930 varied across sites according to sedimentation rates. Particularly, in the processed cores, the year 1930 corresponded to samples ranging from 5.6 to 31 cm in sediment depth (means ± SE; 21.0 ± 3.1 cm; fig. S5). Stock estimates calculated for the Red Sea cores and the Arabian Gulf cores were then compared using a nonparametric test, a Wilcoxon rank sum test, since data were not normally distributed. Normality was tested using a Shapiro-Wilk normality test (W = 0.60449, P value = 0.0001). The average stock of plastic per square meter deposited since year 1930 in the mangrove sediments of each basin was then multiplied by the area covered by mangrove forests in the Red Sea and the Arabian Gulf to obtain the total stock of plastic buried in the mangroves in these two seas. To allow comparison of our results with the existing literature, where most of the samples are collected in the surface sediments, we also calculated the average abundance of plastic (in number of items per square meter) in the upper 5 cm.
We compared the loads of plastics in sampled mangrove sediments with loads in surface waters. Red Sea surface waters (top 15 cm) hold 1.1 ± 0.3 g plastic km−2 (30). For what regards the floating plastic load in the Arabian Gulf, Abayomi et al. (31), reported a mean density of plastic in the Qatar surface waters (top 50 cm) of 4.04 × 105 ± 3.05 × 105 items km−2, ~100 times higher than the one reported by Martí et al. (30) for the Red Sea. However, 93.8% of the total plastic in the Qatar waters was composed of fibers, which were excluded in this study and the study by Martí et al. (30). Hence, the concentration of particles excluding fibers is approximately 25,000 ± 19,000 items km−2. Assuming a similar average weight of single plastic items in the studies by Abayomi et al. (31) and Martí et al. (30), plastic concentration in the surface waters of the Arabian Gulf is 7.8 ± 5.8 g km−2, approximately seven times higher than in the Red Sea.
We calculated the plastic burial rates expressed as milligrams of plastics accumulated per square meter per year, as the product of the MAR of each core (in grams of sediment per square meter per year) and the concentration of plastic (in milligrams of plastic per gram of sediment) in each processed sample. We then plotted a 20-year moving average of burial rates at the y axis against the mid-date of the corresponding 20-year window at the x axis, starting from the oldest processed sample dated >1950 of each core until reaching the most recent processed sample (Fig. 2). Year 1950 was chosen as the baseline because it marks the start of the exponential increase in plastic production. However, we also report the 20-year moving average of plastic burial rates for data before the 1950s for comparison (fig. S3). We used a 20-year window, as the density of plastic particles, constrained by sample size (core diameter), could not be reliably resolved at smaller time intervals. When replicate cores of a site were available (see Table 2), these were considered together when averaging the burial rate, and a single plot was given for each site. We then fitted an exponential curve for each plot to test the hypothesis of exponential increase in plastic burial rates and to obtain the annual rate of increase for each site. The current burial rate (average burial rate of plastic deposited in the most recent 20-year window, from 2001 to 2020) was multiplied by the area covered by mangroves in the two basins to obtain the contemporary rates of total plastic mass buried in each of the Red Sea and Arabian Gulf mangrove sediments.
Statistical analyses were performed in RStudio 1.1.383. Comparisons are considered significantly different when P value is <0.05. All values are reported as means ± SE.
Supplementary Material
Acknowledgments
We thank A. Qasem and P. Priahartato, from Saudi Aramco, for support and advice on sampling design; R. Lindo, R. Magalles, P. Bacquiran, S. Ibrahim, and M. Lopez, at the Marine Studies section of the Center for Environment and Water of King Fahd University of Petroleum and Minerals, for contribution in fieldwork sampling in the Arabian Gulf; and Z. Batang and staff from the Coastal and Marine Resources core laboratory at King Abdullah University of Science and Technology (KAUST) for help with sampling in the Red Sea. We thank I. Schulz, N. Geraldi, K. Rowe, S. Roth, M. Ennasri, and D. Prabowo for helping with processing of the cores. We thank the KAUST Workshop for manufacturing the SMI unit. We thank R. al Nahdi for help during plastic extraction. The International Atomic Energy Agency (IAEA) is grateful for the support provided to its Environment Laboratories by the government of the Principality of Monaco. Funding: This work was supported and funded by KAUST through the baseline funding of C.M.D. and by the Australian Research Council LIEF Project (LE170100219) and the Generalitat de Catalunya (grant 2017 SGR-1588) through the funding provided to P.M. This work is contributing to the ICTA “Unit of Excellence” (MinECo, MDM2015-0552). Author contributions: C.M.D. conceived the work. V.S., M.C., and H.A. collected and processed the sediment cores. L.R., P.K.K., and M.A.Q. contributed to the sampling from the Arabian Gulf. A.A.-O. and P.M. dated the cores with 210Pb. C.M., F.B., and L.V. extracted plastic from sediment samples. C.M. and C.M.D. analyzed data. C.M. and C.M.D. interpreted data. C.M. drafted the manuscript. All authors contributed to the interpretation of the data and to the improvement of the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/44/eaaz5593/DC1
REFERENCES AND NOTES
- 1.Geyer R., Jambeck J. R., Law K. L., Production, use, and fate of all plastics ever made. Sci. Adv. 3, e1700782 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jambeck J. R., Geyer R., Wilcox C., Siegler T. R., Perryman M., Andrady A., Narayan R., Law K. L., Plastic waste inputs from land into the ocean. Science 347, 768–771 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Wang J., Tan Z., Peng J., Qiu Q., Li M., The behaviors of microplastics in the marine environment. Mar. Environ. Res. 113, 7–17 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Andrady A. L., The plastic in microplastics: A review. Mar. Pollut. Bull. 119, 12–22 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Cózar A., Martí E., Duarte C. M., García-de-Lomas J., van Sebille E., Ballatore T. J., Eguíluz V. M., Ignacio González-Gordillo J., Pedrotti M. L., Echevarría F., Troublè R., Irigoien X., The Arctic Ocean as a dead end for floating plastics in the North Atlantic branch of the Thermohaline Circulation. Sci. Adv. 3, e1600582 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lavers J. L., Bond A. L., Exceptional and rapid accumulation of anthropogenic debris on one of the world’s most remote and pristine islands. Proc. Natl. Acad. Sci. U.S.A. 114, 6052–6055 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isobe A., Uchiyama-Matsumoto K., Uchida K., Tokai T., Microplastics in the Southern Ocean. Mar. Pollut. Bull. 114, 623–626 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Barnes D. K. A., Galgani F., Thompson R. C., Barlaz M., Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 364, 1985–1998 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Sebille E., Wilcox C., Lebreton L., Maximenko N., Hardesty B. D., van Franeker J. A., Eriksen M., Siegel D., Galgani F., Law K. L., A global inventory of small floating plastic debris. Environ. Res. Lett. 10, 124006 (2015). [Google Scholar]
- 10.Lebreton L., Slat B., Ferrari F., Sainte-Rose B., Aitken J., Marthouse R., Hajbane S., Cunsolo S., Schwarz A., Levivier A., Noble K., Debeljak P., Maral H., Schoeneich-Argent R., Brambini R., Reisser J., Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic. Sci. Rep. 8, 4666 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cózar A., Echevarría F., González-Gordillo J. I., Irigoien X., Úbeda B., Hernández-León S., Palma Á. T., Navarro S., García-de-Lomas J., Ruiz A., Fernández-de-Puelles M. L., Duarte C. M., Plastic debris in the open ocean. Proc. Natl. Acad. Sci. U.S.A. 111, 10239–10244 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isobe A., Iwasaki S., Uchida K., Tokai T., Abundance of non-conservative microplastics in the upper ocean from 1957 to 2066. Nat. Commun. 10, 417 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Law K. L., Plastics in the marine environment. Ann. Rev. Mar. Sci. 9, 205–229 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Gall S. C., Thompson R. C., The impact of debris on marine life. Mar. Pollut. Bull. 92, 170–179 (2015). [DOI] [PubMed] [Google Scholar]
- 15.S. Kühn, E. L. B. Rebolledo, J. A. van Franeker, in Marine Anthropogenic Litter, M. Bergmann, L. Gutow, M. Klages, Eds. (Springer, 2015), pp. 75–116. [Google Scholar]
- 16.Baalkhuyur F. M., Bin Dohaish E.-J. A., Elhalwagy M. E. A., Alikunhi N. M., AlSuwailem A. M., Røstad A., Coker D. J., Berumen M. L., Duarte C. M., Microplastic in the gastrointestinal tract of fishes along the Saudi Arabian Red Sea coast. Mar. Pollut. Bull. 131, 407–415 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Kooi M., van Nes E. H., Scheffer M., Koelmans A. A., Ups and downs in the ocean: Effects of biofouling on vertical transport of microplastics. Environ. Sci. Technol. 51, 7963–7971 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reisser J., Slat B., Noble K., du Plessis K., Epp M., Proietti M., de Sonneville J., Becker T., Pattiaratchi C., The vertical distribution of buoyant plastics at sea: An observational study in the North Atlantic Gyre. Biogeosciences 12, 1249–1256 (2015). [Google Scholar]
- 19.Van Cauwenberghe L., Vanreusel A., Mees J., Janssen C. R., Microplastic pollution in deep-sea sediments. Environ. Pollut. 182, 495–499 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Van Cauwenberghe L., Devriese L., Galgani F., Robbens J., Janssen C. R., Microplastics in sediments: A review of techniques, occurrence and effects. Mar. Environ. Res. 111, 5–17 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Fazey F. M. C., Ryan P. G., Biofouling on buoyant marine plastics: An experimental study into the effect of size on surface longevity. Environ. Pollut. 210, 354–360 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Waters C. N., Zalasiewicz J., Summerhayes C., Barnosky A. D., Poirier C., Gałuszka A., Cearreta A., Edgeworth M., Ellis E. C., Ellis M., Jeandel C., Leinfelder R., McNeill J. R., deB Richter D., Steffen W., Syvitski J., Vidas D., Wagreich M., Williams M., Zhisheng A., Grinevald J., Odada E., Oreskes N., Wolfe A. P., The Anthropocene is functionally and stratigraphically distinct from the Holocene. Science 351, aad2622 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Duarte C. M., Losada I. J., Hendriks I. E., Mazarrasa I., Marbà N., The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Chang. 3, 961–968 (2013). [Google Scholar]
- 24.Middelburg J. J., Soetaert K., Herman P. M. J., Empirical relationships for use in global diagenetic models. Deep. Res. Part I Oceanogr. Res. Pap. 44, 327–344 (1997). [Google Scholar]
- 25.Mohamed Nor N. H., Obbard J. P., Microplastics in Singapore’s coastal mangrove ecosystems. Mar. Pollut. Bull. 79, 278–283 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Huang Y., Xiao X., Xu C., Perianen Y. D., Hu J., Holmer M., Seagrass beds acting as a trap of microplastics - Emerging hotspot in the coastal region? Environ. Pollut. 257, 113450 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Jones K. L., Hartl M. G. J., Bell M. C., Capper A., Microplastic accumulation in a Zostera marina L. bed at Deerness Sound, Orkney, Scotland. Mar. Pollut. Bull. 152, 110883 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Cozzolino L., Nicastro K. R., Zardi G. I., de Carmen C. B., Species-specific plastic accumulation in the sediment and canopy of coastal vegetated habitats. Sci. Total Environ. 723, 138018 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Martin C., Almahasheer H., Duarte C. M., Mangrove forests as traps for marine litter. Environ. Pollut. 247, 499–508 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Martí E., Martin C., Cózar A., Duarte C. M., Low abundance of plastic fragments in the surface waters of the Red Sea. Front. Mar. Sci. 4, 333 (2017). [Google Scholar]
- 31.Abayomi O. A., Range P., Al-Ghouti M. A., Obbard J. P., Almeer S. H., Ben-Hamadou R., Microplastics in coastal environments of the Arabian Gulf. Mar. Pollut. Bull. 124, 181–188 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Patzert W. C., Wind-induced reversal in Red Sea circulation. Deep. Res. Oceanogr. Abstr. 21, 109–121 (1974). [Google Scholar]
- 33.Martin C., Agustí S., Duarte C. M., Seasonality of marine plastic abundance in central Red Sea pelagic waters. Sci. Total Environ. 688, 536–541 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Kämpf J., Sadrinasab M., The circulation of the Persian Gulf: A numerical study. Ocean Sci. 2, 27–41 (2006). [Google Scholar]
- 35.Almahasheer H., Aljowair A., Duarte C. M., Irigoien X., Decadal stability of Red Sea mangroves. Estuar. Coast. Shelf Sci. 169, 164–172 (2016). [Google Scholar]
- 36.Almahasheer H., Spatial coverage of mangrove communities in the Arabian Gulf. Environ. Monit. Assess. 190, 85 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Naji A., Esmaili Z., Khan F. R., Plastic debris and microplastics along the beaches of the Strait of Hormuz, Persian Gulf. Mar. Pollut. Bull. 114, 1057–1062 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Naji A., Esmaili Z., Mason S. A., Dick Vethaak A., The occurrence of microplastic contamination in littoral sediments of the Persian Gulf, Iran. Environ. Sci. Pollut. Res. 24, 20459–20468 (2017). [DOI] [PubMed] [Google Scholar]
- 39.C. B. Crawford, B. Quinn, Microplastic Pollutants (2016).
- 40.Martin C., Corona E., Mahadik G. A., Duarte C. M., Adhesion to coral surface as a potential sink for marine microplastics. Environ. Pollut. 255, 113281 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Arossa S., Martin C., Rossbach S., Duarte C. M., Microplastic removal by red sea giant clam (Tridacna maxima). Environ. Pollut. , (2019). [DOI] [PubMed] [Google Scholar]
- 42.Almahasheer H., Duarte C. M., Irigoien X., Phenology and growth dynamics of Avicennia marina in the Central Red Sea. Sci. Rep. 6, 37785 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serrano O., Ricart A. M., Lavery P. S., Mateo M. A., Arias-Ortiz A., Masque P., Rozaimi M., Steven A., Duarte C. M., Key biogeochemical factors affecting soil carbon storage in Posidonia meadows. Biogeosciences 13, 4581–4594 (2016). [Google Scholar]
- 44.Saderne V., Cusack M., Almahasheer H., Serrano O., Masqué P., Arias-Ortiz A., Krishnakumar P. K., Rabaoui L., Qurban M. A., Duarte C. M., Accumulation of carbonates contributes to coastal vegetated ecosystems keeping pace with sea level rise in an arid region (Arabian Peninsula). J. Geophys. Res. Biogeo. 123, 1498–1510 (2018). [Google Scholar]
- 45.Krishnaswamy S., Lal D., Martin J. M., Meybeck M., Geochronology of lake sediments. Earth Planet. Sci. Lett. 11, 407–414 (1971). [Google Scholar]
- 46.Arias-Ortiz A., Masqué P., Garcia-Orellana J., Serrano O., Mazarrasa I., Marbá N., Lovelock C. E., Lavery P. S., Duarte C. M., Reviews and syntheses: 210Pb-derived sediment and carbon accumulation rates in vegetated coastal ecosystems - Setting the record straight. Biogeosciences 15, 6791–6818 (2018). [Google Scholar]
- 47.Coppock R. L., Cole M., Lindeque P. K., Queirós A. M., Galloway T. S., A small-scale, portable method for extracting microplastics from marine sediments. Environ. Pollut. 230, 829–837 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/44/eaaz5593/DC1


