The innately heterodimeric K2P filter permits two C-type gating mechanisms, pinching and dilation, to operate in one channel.
Abstract
K2P potassium channels regulate cellular excitability using their selectivity filter (C-type) gate. C-type gating mechanisms, best characterized in homotetrameric potassium channels, remain controversial and are attributed to selectivity filter pinching, dilation, or subtle structural changes. The extent to which such mechanisms control C-type gating of innately heterodimeric K2Ps is unknown. Here, combining K2P2.1 (TREK-1) x-ray crystallography in different potassium concentrations, potassium anomalous scattering, molecular dynamics, and electrophysiology, we uncover unprecedented, asymmetric, potassium-dependent conformational changes that underlie K2P C-type gating. These asymmetric order-disorder transitions, enabled by the K2P heterodimeric architecture, encompass pinching and dilation, disrupt the S1 and S2 ion binding sites, require the uniquely long K2P SF2-M4 loop and conserved “M3 glutamate network,” and are suppressed by the K2P C-type gate activator ML335. These findings demonstrate that two distinct C-type gating mechanisms can operate in one channel and underscore the SF2-M4 loop as a target for K2P channel modulator development.
INTRODUCTION
K2P channels regulate nervous, cardiovascular, and immune system functions (1, 2) through the action of their selectivity filter (C-type) gate (3–6). C-type gating occurs in many potassium channel classes and displays a hallmark sensitivity to external potassium due to its dependency on interactions between the permeant ions and selectivity filter (4, 6, 7–12). Although structural studies of exemplar homotetrameric potassium channels have uncovered various types of selectivity filter rearrangements attributed to C-type gating (11, 13–19), there remains a debate about whether the essence of C-type gating involves pinching (11, 13–16), dilation (12), or more subtle selectivity filter changes (17–19). Furthermore, although structural studies of different K2P family members have revealed changes in the transmembrane helix conformations that affect activity (20–26), no selectivity filter conformational changes that could explain how K2P C-type gating occurs have been observed. This lack of a structural framework has left open questions regarding the extent to which K2P C-type gating mechanisms resemble homotetrameric channels (11, 13–18) and whether the innate heterodimeric K2P selectivity filter architecture confers unique properties to their C-type gates.
Here, combining x-ray crystallography of K2P2.1 (TREK-1) in different potassium concentrations, potassium anomalous scattering, molecular dynamics, and functional studies, we uncover extraordinary, asymmetric, potassium-dependent structural changes that trigger K2P C-type gating. We show that low potassium concentrations evoke conformational changes in selectivity filter strand 1 (SF1), selectivity filter strand 2 (SF2), and the SF2-transmembrane helix 4 loop (SF2-M4 loop) that destroy the S1 and S2 ion binding sites through a mixture of pinching of SF1 and dilation of SF2, leveraging the fundamentally heterodimeric nature of the K2P selectivity filter to exploit two classes of C-type gating mechanisms. Both C-type gate rearrangements are suppressed by binding of the activator ML335 (20) to the K2P modulator pocket in the P1-M4 interface, providing an explanation for how such compounds stabilize the activated state. Shortening the uniquely long SF2-M4 loop to match the canonical length found in the first K2P pore domain (PD1) and in other potassium channels, or disrupting the conserved hydrogen bond network centered on Glu234 from the M3 helix that supports the SF2-M4 loop, the “M3 glutamate network” blunts C-type gate responses to various physical and chemical stimuli. Destabilization of the M3 glutamate network compromises ion selectivity but can be reversed by channel activation, indicating that loss of S1 and S2 ions and associated selectivity filter changes reduce ion selectivity, similar to other channels (27). Together, our data establish that C-type gating occurs through potassium-dependent order-disorder transitions in the selectivity filter and adjacent loops that respond to gating cues relayed through the SF2-M4 loop. These findings underscore the importance of the SF2-M4 loop as a conduit for signals sensed by the cytoplasmic tail and transmitted through the M4 transmembrane helix (3, 4) and highlight the potential for targeting the SF2-M4 loop for the development of new, selective K2P channel modulators.
RESULTS
Potassium-dependent selectivity filter structural changes
Despite the fact that C-type gating is the principal K2P gating mechanism (3–6) and that previously determined K2P structures show major conformational changes that affect function (20–26), all prior K2P structures show identical, canonical selectivity filter conformations and lack changes that could be attributed to C-type gating (fig. S1). Notably, these structures were all determined in the presence of 150 to 200 mM permeant ions, a condition that would be expected to confer considerable C-type gate stabilization based on functional studies (3, 4, 6, 10). In notable contrast, structure determination of a crystallizable K2P2.1 (TREK-1) construct, K2P2.1cryst (20), under a series of seven potassium concentrations, 0, 1, 10, 30, 50, 100, and 200 mM [K+] at resolutions of 3.9, 3.4, 3.5, 3.3, 3.6, 3.9, and 3.7 Å, respectively, revealed obvious potassium-dependent changes in the selectivity filter structure, particularly in SF2 and the SF2-M4 loop (Fig. 1A, figs. S2 and S3, and table S1). These changes manifested at potassium concentrations ≤50 mM and eventually encompassed all of the SF2-M4 loop and the upper portion of the selectivity filter (Gly253-Lys271) (figs. S2 and S3). Additional changes were observed in SF1 residues Gly144-Asn147 at the lowest potassium concentrations (0 and 1 mM) (Fig. 1B and fig. S3, A and B). Structure determination under the same set of potassium concentrations in the presence of the K2P2.1 (TREK-1) activator ML335 (20) at resolutions of 3.4, 2.6, 3.0, 3.2, 3.2, 3.3, and 3.8 Å, respectively, yielded essentially identical structures having canonical selectivity filter conformations at all potassium concentrations (Fig. 1, C and D, and figs. S2 and S3, A and B), a result that agrees with the ability of ML335 to activate the C-type gate directly (20). The observed structural changes were limited to the SF1 and SF2-M4 regions and were uncorrelated with differences in resolution (fig. S2A). Moreover, other parts of the channel remained well defined even when the SF2-M4 loop became disordered (fig. S2, B and C) and had essentially the same conformations as prior K2P2.1 (TREK-1) structures that show the absence of an inner gate (20). Hence, the changes we observe clearly represent a local, specific, potassium-dependent loss of structure.
Fig. 1. K2P2.1 (TREK-1) selectivity filter potassium-dependent conformational changes.
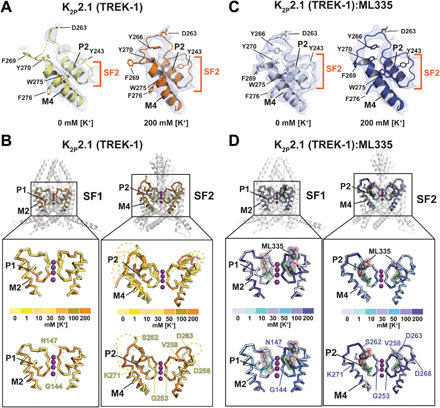
(A) Exemplar 0 and 200 mM [K+] K2P2.1 (TREK-1) SF2 2Fo-Fc electron density (1σ). Select residues and channel elements are indicated. Dashes indicate disordered regions. (B) [K+]-dependent structural changes in K2P2.1 (TREK-1) SF1 (left) and SF2 (right). Top: Superpositions of structures determined in 0 (pale yellow), 1 (yellow), 10 (light orange), 30 (yellow orange), 50 (bright orange), 100 (olive), and 200 (orange) mM [K+]. Bottom: Superposition of 0 and 200 mM [K+] structures. Dashed lines indicate regions absent from the structures. Lower panel labels mark model boundaries. (C) Exemplar 2Fo-Fc electron density (1σ) for the K2P2.1 (TREK-1):ML335 complex SF2 at 0 and 200 mM [K+]. (D) K2P2.1 (TREK-1):ML335 complex structural comparisons of SF1 (left) and SF2 (right). Top: Superposition of structures determined in 0 (blue white), 1 (pale cyan), 10 (aquamarine), 30 (light blue), 50 (marine), 100 (slate), and 200 (deep blue) mM [K+]. Bottom: Superposition of 0 and 200 mM [K+] structures. ML335 is gray and shows its molecular surface. Lower panel labels indicate the equivalent residues from (B). Potassium ions are from the 200 mM [K+] structures and are shown as magenta spheres.
Structural studies of homotetrameric potassium channels have established the intimate connection between the presence of potassium ions in the selectivity filter and the conductive conformation in which the selectivity filter backbone carbonyls coordinate the permeant ions (11, 13–17). Hence, we asked whether the SF1, SF2, and the SF2-M4 loop structural changes in different potassium concentrations were also accompanied by changes to the number of ions in the filter. Comparison of selectivity filter region omit maps (28) showed clear evidence for variation in the number of ions in the filter that paralleled the structural changes in the filter and supporting loops. The 100 and 200 mM [K+] structures showed ions at all four selectivity filter sites, S1 to S4, similar to prior structures determined under similar conditions (20). Whereas in the 0, 1, 10, 30, and 50 mM [K+] structures, the ion densities at sites S1 and S2 were clearly absent, while the S3 and S4 ions persisted to the lowest potassium concentration examined (Fig. 2A and fig. S4A). By contrast, all of the K2P2.1 (TREK-1):ML335 structures showed ions at S1 to S4 regardless of the potassium concentration, underscoring the ability of ML335 to stabilize the filter (Fig. 2B and fig. S4B) and directly activate the C-type gate (20).
Fig. 2. K2P2.1 (TREK-1) selectivity filter ion occupancy as a function of [K+].
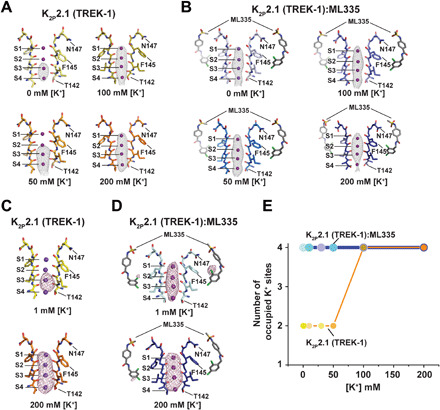
(A and B) Polder omit maps (28) for structures of (A) K2P2.1(TREK-1) and (B) K2P2.1(TREK-1):ML335 determined in 0 mM [K+] [pale yellow (5σ); blue white (4σ)], 50 mM K+ [bright orange (5σ); marine (4σ)], 100 mM [K+] [olive (4σ); slate (4σ)], and 200 mM [K+] [orange (4σ); deep blue (4σ)]. Potassium ions are magenta spheres. Sites S1 to S4 are labeled. ML335 is shown as sticks. (C and D) Potassium anomalous difference maps (29) for (C) K2P2.1(TREK-1) and (D) K2P2.1(TREK-1):ML335 determined in 1 mM [K+] [yellow (4σ); pale cyan (4σ)] and 200 mM [K+] [orange (4σ); deep blue (4σ)]. In (A) and (D), SF1 in the 200 mM [K+] conformation is shown for reference. S1 to S4 sites and select amino acids are labeled. (E) Plot of the number of observed selectivity filter ions as a function of [K+]. Colors correspond to the scheme in Fig. 1 (C and D).
To confirm that the changes in the electron density reflected potassium ion occupancy and were not due to resolution differences, we used long-wavelength x-rays above and below the potassium K-absorption edge (λ = 3.3509 and 3.4730 Å) to measure potassium anomalous scattering (29, 30) from crystals in 1 or 200 mM [K+] in the absence or presence of ML335. Anomalous difference maps showed unequivocally that potassium ions occupy sites S1 to S4 under 200 mM [K+] conditions irrespective of the presence of ML335 (Fig. 2, C and D). By contrast, the density from 1 mM [K+] conditions showed a ML335-dependent difference in the number of potassium ions (Fig. 2, C and D) that agreed with our initial observations (Fig. 2, A and B, and table S2). In the absence of the activator, potassium ions were observed only in the lower portion of the filter, whereas potassium ions are found at all four positions in presence of ML335 (Fig. 2, C and D). Together, these data demonstrate that the loss of structure observed in the upper portion of SF2 as potassium concentrations are lowered is accompanied by a loss of potassium ions at sites S1 and S2 (Fig. 2E). Hence, the well-ordered, fully ion-bound conformations represent the active state of the filter, whereas the low [K+] structures in the absence of ML335 that have various degrees of disorder in SF1, SF2, and the SF2-M4 loop and lack of ions at S1 and S2 reflect low activity conformations of the C-type gate. This assignment agrees with the idea that K2P C-type gate activation involves a rigidification of the filter and surrounding structure (20).
C-type gate and connecting loops are dynamic
To gain further insight into how potassium occupancy and ML335 affect the C-type gate, particularly in the context of a lipid bilayer, we turned to molecular dynamics (MD) simulations of K2P2.1 (TREK-1). Initially, we simulated two conditions: (i) 180 mM [K+] and a +40-mV applied membrane potential (denoted “High [K+]/+40 mV,” 36.5 μs aggregate) and (ii) the same [K+] and potential with bound ML335 (denoted “High [K+]/+40 mV/ML335,” 31.6 μs aggregate). Both conditions showed many permeation events (144 and 253 for High [K+]/+40 mV and High [K+]/+40 mV/ML335, respectively), confirming that the initial structures represent conduction competent states. Nevertheless, the pattern of permeation events with respect to time showed notable differences depending on ML335 (Fig. 3A). Over the course of the simulations, most of the High [K+]/+40 mV/ML335 trajectories (8 of 10) remained in a stable, ion-conducting state. By contrast, most (7 of 12) of the High [K+]/+40 mV trajectories entered long-lived (>1 μs) nonconducting states from which they did not recover and that were characterized by obvious disruptions of the initial selectivity filter conformation. Concordantly, the two conditions had a substantial difference in the current (0.3 pA versus 1.3 pA for High [K+]/+40 mV and High [K+]/+40 mV/ML335, respectively) (Fig. 3B). There were no major changes during the simulations in the M4 helix position or in other parts of the channel outside of the selectivity filter when compared with their starting positions as defined by the crystal structures.
Fig. 3. K2P2.1 (TREK-1) conductance properties and SF conformational dynamics from MD simulations.
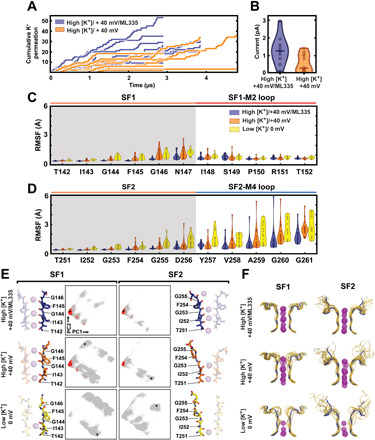
(A) Cumulative K+ ion permeation events over simulation time for all individual trajectories in High [K+]/+40 mV (orange) and High [K+]/+40 mV/ML335 (purple) conditions. (B) Current calculated from the trajectories in (A). Each point shows the average current from one independent trajectory; horizontal bars indicate median. (C and D) Cα RMSF values of the filter and loop regions for (C) pore domain 1 and (D) pore domain 2, for all simulated conditions. Each point represents RMSF calculated from one K2P2.1 (TREK-1) subunit of one trajectory. Conserved selectivity filter signature sequences are shaded gray. (E) PCA analysis of SF1 and SF2 dihedral angles and exemplar filter conformations. Each dot represents the instantaneous conformation of the TIGFG backbone dihedral angles from single selectivity filter. Black stars indicate the location in PC1 vs. PC2 space of the adjacent exemplar. Red dots indicate conformations immediately preceding K+ permeation events. (F) Final SF1 (left) and SF2 and loop (right) ion and backbone conformations from all simulation trajectories. Transparent gold ribbons are the final frame of each trajectory; transparent purple spheres are potassium ions. Solid blue ribbons represent the initial crystal structure conformation.
To determine whether there were differences in C-type gate dynamics across simulation conditions, we examined a number of factors. Because structural waters behind the selectivity filter stabilize both the active and C-type inactivated states of the model homotetrameric channel KcsA (31), we first characterized the role that water molecules have on the K2P2.1 (TREK-1) filter conformation. We found that in conductive states, regardless of the presence of ML335, a characteristic water network behind the filter stabilizes SF1 and SF2 through hydrogen bonds to the backbone amides of Phe145/Gly146 and Phe254/Gly255, respectively (fig. S5, A and B). As the K2P2.1 (TREK-1) filter moves away from the canonical, conductive conformation, these well-organized networks dissolve (fig. S5C). Nevertheless, before dissolution, there were no obvious differences in the water configurations with or without ML335 that would explain the differences in conduction and filter stability. We also note that unlike in KcsA, where water molecules stabilize a discrete nonconducting pinched filter state (11, 14, 31), these K2P2.1 (TREK-1) nonconductive states were heterogeneous, having many different conformations of the filter and surrounding waters.
We next asked whether dynamics in the filter region could explain differences in filter stability. To do so, we calculated root-mean-square fluctuation (RMSF) values for the selectivity filter and the postfilter loops. Because the crystal structures showed that low potassium occupancy in the filter resulted in increased mobility in these regions (Figs. 1, B and D, and figs. S2A and S3), we included a third set of simulations in which K2P2.1 (TREK-1) had only a single ion in the filter under no applied membrane potential (denoted “Low [K+]/0 mV,” 20.6 μs aggregate). This analysis revealed that residues Phe145-Ser149 of SF1, Phe254-Gly261 of SF2, and the SF2-M4 loop comprise the three most dynamic areas near the filter and showed that their mobility was greatly restricted by ML335 (Fig. 3, C and D). Further, under Low [K+]/0 mV conditions, the mobility of these regions exceeded either of the High [K+]/+40 mV conditions (fig. S5D). Together, the simulations indicate that the absence of K+ in the filter versus the presence of ML335 have strong, opposite effects on the dynamics of the selectivity filter and SF2-M4 loop (Fig. 3, C and D).
To determine specific structural features associated with loss of conduction and how these features relate to the broader C-type gating context, we analyzed the backbone dihedral angles of the SF1 and SF2 ion-coordinating “TIGFG” amino acid motifs. We used a simple statistical procedure known as principal components analysis (PCA) to transform the 10 backbone dihedral angles from each TIGFG conformation into a new coordinate system wherein the greatest variance in conformations lies along the first axis (principal component), the second greatest along the second axis, and so on (32). Focus on the first few high-variance components provides a natural way of reducing the dimensionality of the data and reveals collective changes that cannot be gleaned from examining changes in individual dihedral angles. Projecting all simulation snapshots onto the first two principal components (PC1 and PC2) (Fig. 3E) uncovered a distinct grouping of SF1 and SF2 conformations that lack major deviations from the initial structure. All prior K2P selectivity filter structures (fig. S6, A and B) (denoted as the “native state”) and selectivity filters from other potassium channels thought to capture either conducting states (14, 33) or, unexpectedly, C-type inactivated states (14, 17), map to the center of this group (fig. S6C). Additional clustering analysis of all High [K+] selectivity filters in the PC1 to PC3 space separated out many distinct clusters of nonnative conformations in which the backbone dihedral angles deviate substantially from the native state (fig. S6D). Some of these conformations are reached from the canonical SF1 structure via a single discrete backbone “crankshaft” motion between either the S2 and S3 sites (Ile143/Gly144) (fig. S6D, cluster 2) or at the top of the S0 site (Gly146/Asn147) (fig. S6D, cluster 3) that result in a flip of the amide group plane that reorients the backbone carbonyl away from the pore and correspond to conformations suggested to be involved in C-type gating in previous K2P channel simulations (34, 35). The remaining SF1 and SF2 clusters represent larger deviations from the initial state and cannot be described by single amide crankshaft motions. Some of these configurations are reminiscent of the unusual selectivity filter structure of the nonselective channel NaK (fig. S6, C and D) (36), while others represent novel conformations that have not been observed experimentally (fig. S6D). Notably, conformations with multiple crankshaft motions and large dihedral angle changes are more highly populated in the absence of ML335 and are especially abundant under Low [K+] conditions (Fig. 3, E and F).
Of all of the K2P2.1 (TREK-1) conformations observed under high [K+] conditions, only a few are compatible with K+ permeation. Greater than 90% of ion conduction events occurred when all four SF strands occupied the native state conformational cluster. No conduction events were observed when more than two SF strands adopted nonnative states. Under High [K+]/+40 mV/ML335 conditions, SF1 and SF2 were found in the native state cluster 90 and 95% of the time, respectively, while under High [K+]/+40 mV conditions, these values dropped to 64 and 86% (Fig. 3E). Thus, the presence of ML335 reduces the accessible conformational space of the filter, restricting SF1 and SF2 largely to their native, conductive conformations. This conformational restriction causes longer periods of sustained conduction and higher current values relative to the High [K+]/+40 mV condition (Fig. 3, A and B), in line with the fact that ML335 directly activates the K2P2.1 (TREK-1) C-type gate (20).
In all three simulation conditions, most of the nonconductive filter conformations have multiple dihedral angle deviations from the canonical structure (Fig. 3F and fig. S6D) and share a loss of ion binding sites at S1, S2, or both due to rearrangement of the ion coordinating carbonyls (Fig. 3E, bottom and middle right, and fig. S6D). These changes leave only the S3 and S4 sites competent for potassium binding and are in excellent agreement with the crystallographic ion positions observed under low potassium conditions (Figs. 1 to 3F and figs. S2 and S3). Furthermore, examination of the ensemble of final SF1 and SF2 backbone conformations from the simulations under different conditions shows that these structural components display increased conformational disorder and pseudo-fourfold symmetry breaking that is in excellent agreement with the x-ray structures (Fig. 3F). SF1 adopts nonnative conformations, particularly around Asn147, which pinch the conduction pathway, whereas SF2 preferentially dilates out of the pathway (movies S1 and S2). This asymmetry extends beyond the parts of the filter that directly contact the permeant ions. Although the SF1-M2 loop remains largely native-like, despite the changes in SF1, the longer SF2-M4 loop is highly mobile (movie S2). This later observation agrees well with the loss of density for SF2-M4 loop in the low [K+] crystal structures (Fig. 1, A and B). Together, the structures and simulations support the idea that ML335 acts by stabilizing the K2P selectivity filter in a conductive state and indicate that the low [K+] crystal structures represent an inactive C-type gate in which asymmetric disorder in the extracellular portion of the selectivity filter disrupts the S1 and S2 ion binding sites and inhibits ion conduction.
ML335 stabilizes the K2P2.1 (TREK-1) open state
Both the crystallographic and computational data strongly suggest that ML335 stabilizes the conductive state of the C-type gate. To test this idea directly, we recorded K2P2.1 (TREK-1) single channels alone and in the presence of ML335 (Fig. 4, A and B). ML335 activated the channels in the same way regardless of whether it was applied to the bath (Fig. 4A) or through the pipette (Fig. 4B). The effect of bath application was apparent in ~15 min, whereas the pipette application had immediate effects in line with the fact that the K2P modulator pocket faces the extracellular solution. The data clearly show that in both cases, ML335 increases channel open probability but not the single-channel conductance (Fig. 4, C to E). By contrast, the activator BL-1249, which is thought to act by a mechanism different from that of the K2P modulator pocket activators ML335 and ML402 (37, 38), increases both open probability and single-channel conductance (38). The clear effects of ML335 on channel open probability match the expectations from the crystallographic and computational observations that show that ML335 stabilizes the ion-filled conductive state of the selectivity filter C-type gate (Figs. 1 to 3) and support the idea that rigidification of the P1-M4 interface, comprising the K2P modulator pocket, is central to C-type gate activation of K2Ps (20).
Fig. 4. Effects of ML335 on K2P2.1 (TREK-1).
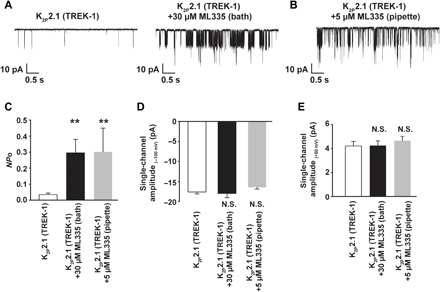
(A) Exemplar K2P2.1 (TREK-1) single-channel recordings at −100 mV before (left) and after (right) application of 30 μM ML335 to the same cell-attached patch. (B) Exemplar K2P2.1 (TREK-1) single-channel recordings at −100 mV in the presence of 5 μM ML335 applied in the pipette solution of a cell-attached patch. (C) Open channel probability at −100 mV from single-channel analysis calculated on recordings of ≥30-s duration. (D and E) Single-channel amplitude at (D) −100 mV and (E) +50 mV. Error bars indicate SEM (n = 5 to 7). “**” indicates P < 0.01 and “N.S.” indicates not statistically different relative to K2P2.1 (TREK-1).
The SF2-M4 loop integrates responses from diverse gating cues
In most potassium channels, including the first K2P pore domain (PD1), a six-residue loop connects the extracellular end of the selectivity filter to the outer transmembrane helix of the pore domain (Fig. 5, A and B, and fig. S7A, and B). K2Ps are unique in that the second pore domain loop (PD2) is longer than this canonical length by six to eight residues in 14 of the 15 K2P subtypes (fig. S7, C and D). Despite these differences, the N-terminal portions of the PD1 and PD2 loops adopt very similar structures up to Pro150 and Ala259, respectively (Fig. 5A). The simulations revealed that loss of SF2-M4 loop stability was accompanied by the disruption of a hydrogen bonding network, the Glu234 network, at the C-terminal end of the PD2 linker involving the M3 Glu234 carboxylate, the SF2-M4 loop Gly260 backbone amide, and the M4 Tyr270 phenolic ─OH (Fig. 5, C and D). Binding of ML335 to the K2P modulator pocket stabilizes the SF2-M4 loop from the opposite side of the Glu234 network (Fig. 5C), increases the strength of the Glu234 hydrogen bonding network in the simulations (fig. S7, E and F), and strongly attenuates potassium-dependent loop dynamics (Figs. 1, B to D, and 3, C and D; and figs. S2 and S3). Conversely, in low [K+] simulations, the Glu234 network is disrupted (figs. S7, E and F), and loop dynamics are enhanced (Fig. 3D). Together, these results suggest that loop dynamics are important for C-type gating, with Glu234 playing a key role by supporting the SF2-M4 loop structure. Notably, the equivalent position of the K2P PD1 outer helix, M1 also has a highly conserved glutamate (fig. S7G) that affects C-type gating through interactions with the short SF1-M2 loop (10, 39) in a manner that is conserved with voltage-gated potassium channels (40, 41). Therefore, given the indications from our structures and simulations that Glu234 network integrity should be important for gating, we set out to test consequences of restricting the SF2-M4 loop mobility and disrupting the Glu234 network.
Fig. 5. K2P SF2-M4 loop is central to C-type gate function.
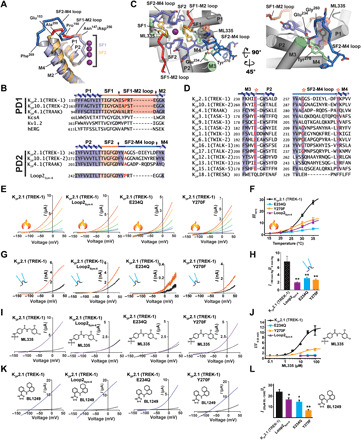
(A) K2P2.1 (TREK-1) P1-SF1-M2 (orange) and P2-SF2-M4 (slate) superposition. SF1-M2 loop (red) and SF2-M4 loop (blue) and portions having a shared conformation (dark blue) are indicated. Residue labels indicate the SF1-M2 and SF2-M4 loop ends and structural divergence point (Pro150/Ala259). (B) Sequence comparison. Arrows denote selectivity filter–outer transmembrane helix linker ends. Red indicates Pro150/Ala259 equivalents. (C) K2P2.1 (TREK-1):ML335 complex SF2-M4 loop details. SF1 (yellow), SF2 (slate), SF1-M2 (red), and SF2-M4 (marine) are indicated. Conserved Glu234 (green), Tyr270 (green), Gly260 (marine) hydrogen bond network is indicted. ML335 is shown as sticks and space filling. (D) Human K2P channel M3 glutamate network conservation (red and asterisks). Figure S7 has sequence codes. (E) Exemplar two electrode voltage clamp (TEVC) recordings at 15°C (blue), 20°C (light green), 25°C (lime green), 30°C (orange), and 35°C (red). (F) Normalized temperature responses (n ≥ 10). (G) Exemplar inside-out pressure response at 0 mmHg (black) and 50 mmHg (orange). (H) Averaged pressure responses (n ≥ 4). (I) Exemplar TEVC recordings for 30 μM ML335 (purple) activation. (J) ML335 dose-response curves (n ≥ 3). EC50 11.3 ± 3.4 and 12.7 ± 4.1 μM, maximum activation 11.9 ± 1.3, and 3.8 ± 0.4 fold for K2P2.1 (TREK1) and K2P2.1 (TREK1) Y270F, respectively. (K) Exemplar TEVC recordings for 20 μM BL-1249 (blue) activation. (L) Normalized responses to 20 μM BL-1249 (n ≥ 7). (F), (H), (J), and (L) show K2P2.1 (TREK1) (black), K2P2.1 (TREK1) Loop2sym6 (purple), K2P2.1 (TREK1) E234Q (light blue), and K2P2.1 (TREK1) Y270F (orange). “*” and “**” indicate P < 0.05 and P < 0.001, respectively.
To create a channel having symmetric length loops between each selectivity filter and its outer transmembrane helix, we transplanted Pro150-Gly155 from PD1 onto PD2, denoted “Loop2Sym-6” (Fig. 5B). Loop2Sym-6 showed blunted responses to temperature (Fig. 5, E and F) and pressure (Fig. 5, G and H). Consistent with the deletion of key ML335-binding SF2-M4 loop residues, Loop2Sym-6 was unresponsive to ML335 (Fig. 5, I and J) but remained partially sensitive to BL-1249 (Fig. 5, K and L), an activator that affects the channel from a site under the selectivity filter (37, 38). Measurement of rectification in inside-out patches, a parameter that is a direct measure of C-type gate activation (5, 20), demonstrated that unlike gain-of-function mutants (20), Loop2Sym-6 does not have a constitutively activated C-type gate that would render it insensitive to gating commands (fig. S8, A and B). Hence, the blunted responses caused by shortening the SF2-M4 loop to the canonical length indicate that the unusual length of the SF2-M4 loop is central to C-type gate control.
Disruption of the Glu234 hydrogen bond network by E234Q and Y270F mutations resulted in channels having severely blunted responses to temperature (Fig. 5, E and F), pressure (Fig. 5, G and H), ML335 (Fig. 5, I and J), and BL-1249 (Fig. 5, K and L). Unlike Loop2Sym-6, both mutations compromised ion selectivity as evidenced by an altered reversal potential (Fig. 5, E, G, I, and K, and fig. S9). This baseline selectivity defect was partially corrected by temperature or pressure activation (Fig. 5, E and G, and fig. S9). Inside-out patch clamp experiments demonstrated that neither mutant resulted in channels having a C-type gate that was activated at rest, although Y270F caused a slight decrease of the rectification coefficient (fig. S8, A and B). Unexpectedly, we also found that E234Q exhibited a time- and voltage-dependent inactivation (fig. S8, C and D), further validating the importance of the Glu234 network for C-type gate control. Together, with prior mutational studies suggesting a role for the SF2-M4 loop in external pH gating (42), these data strongly support the key role that the SF2-M4 loop has in K2P channel gating and underline the importance of SF2-M4 stabilization by the network centered on Glu234.
The M3 glutamate network has a conserved role in C-type gate control
The key elements of the Glu234 network are highly conserved among K2Ps (Fig. 5D). To test its general importance, we disrupted this network in K2P3.1(TASK-1), a K2P from a subfamily distant from K2P2.1 (TREK-1) (2). Structural comparison shows that K2P3.1(TASK-1) Glu182, Leu208, and Tyr220 form a network similar to the K2P2.1 (TREK-1) Glu234-Gly260-Tyr270 network (fig. S10A). Notably, this network is structurally conserved although K2P3.1(TASK-1) has one of the longest SF4-M4 loops (14 residues) (Figs. 5D and fig. S7D) and has a large sidechain, leucine, at the position that contributes the backbone amide (Fig. 5, C and D). Disruption of this network in K2P3.1(TASK-1) had substantial functional consequences. K2P3.1(TASK-1) E182Q failed to produce functional channels (fig. S10C), whereas K2P3.1(TASK-1) Y220F yielded channels that were more readily closed by low pH (fig. S10, D and E). This result phenocopies disruption of interactions on the opposite side of the SF2-M4 loop in P1-M4 interface by the K2P3.1(TASK-1) I88G mutant (3) and indicates that the Y220F mutation destabilized the SF2-M4 loop and C-type gate. Together, our data demonstrate that the Glu234 network and its stabilization of the SF2-M4 loop is a central element of C-type gate control. Because of its conservation and functional importance in diverse K2Ps, we term this network as the M3 glutamate network.
DISCUSSION
Mechanistic implications for K2P channel function
Despite the central role of the selectivity filter C-type gate in K2P channel function (3–6), observation of conformational changes that would provide a framework for understanding the principles of K2P C-type gating has eluded previous structural studies (20–26, 43). Our data establish that control of the K2P C-type gate involves unprecedented, asymmetric, potassium-dependent, order-disorder transitions in the selectivity filter and surrounding loops (Figs. 1 and 6). The selectivity filter conformational changes associated with K2P C-type gating comprise two classes of rearrangements that eliminate the S1 and S2 ion binding sites (Fig. 6 and movies S3 and S4). One pinches the SF1 extracellular side and exposes the Asn147 sidechains to the extracellular solution (Fig. 6A and movie S3), a position that modulates C-type inactivation in homotetrameric potassium channels (44, 45) and that undergoes similar changes in human Ether-à-go-go-Related potassium channel (hERG) simulations (46). Hence, this class of C-type gating mechanism is shared with other potassium channels. The second unwinds SF2 and the SF2-M4 loop, dilates the selectivity filter along the SF2 axis (Fig. 6B and movie S4), depends on the structure of the uniquely long K2P SF2-M4 loop, and is unlike any of the prior structural changes associated with C-type gating (20–26, 43). SF1 pinching and SF2 dilation are not mutually exclusive and are likely to be interdependent given the role of the SF ions in stabilizing the filter. Such asymmetric changes could contribute to the bimodal distribution of closed state dwell times reported for K2P2.1-(TREK-1) (47) and the closely-related K2P10.1 (TREK-2) (48). Further, as K2P heterodimer formation yields channels having two unique SF1-M2 and SF2-M4 loops, this structural diversification together with the two nonmutually exclusive inactivation modes likely provides a mechanism for the emergence of heterodimer properties that differ from either homodimer parent (49–56). The structural rearrangements in the pore and surrounding regions, loss of S1 and S2 ions, and the demonstration that destabilization of the SF2-M4 loop structure compromises ion selectivity are reminiscent of studies of the nonselective bacterial channel NaK, which has only the S3 and S4 sites and can be converted into a potassium-selective channel by forming the S1 and S2 ion binding sites (27). Further, the loss of ion selectivity associated with K2P C-type gating (4, 10, 57) and the strong link between K2P gating and external potassium concentration (3, 4, 6, 10) are in good accord with the structural and functional changes we observe.
Fig. 6. Structural changes associated with C-type gating.
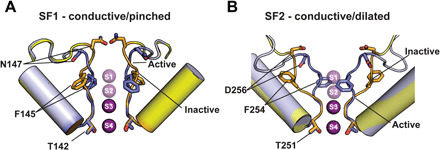
(A) SF1 and (B) SF2 selectivity filter changes between the active (slate) [(A) and (B) conductive] and inactive (yellow orange) [(A) pinched and (B) dilated] conformations based on the 1 mM [K+] and 0 mM [K+]:ML335 structures, respectively. Selectivity filters for 1 mM [K+] and 0 mM [K+]:ML335 show select residues. Potassium ions are magenta spheres.
Although C-type gating is an important mode of channel regulation in many potassium channel classes (38), structural insights into its mechanistic basis are limited to studies of a small number of homotetrameric potassium channel types (11, 13–19) and lack consensus (12), even for the best studied example, KcsA (58–61). Nevertheless, our studies identify a unifying feature shared between K2P C-type gating and homotetrameric potassium channel C-type gating—the importance of the conserved glutamate at the extracellular end of the pore module outer helix (Figs. 5, C and D, and fig. S7G). This site on the K2P PD1 M1 helix affects C-type gating through interactions with the SF1-M2 loop (10, 39) similar to other channels having a canonical six residue loop between the selectivity filter and pore module outer helix (Fig. 5B) (40, 41, 62). The equivalent PD2 glutamate on K2P21 (TREK-1) M3, Glu234, forms a conserved network together with a M4 tyrosine, Tyr270, the M3 glutamate network that supports the uniquely long SF2-M4 loop found throughout the K2P family (fig. S7D). Disruption of the M3 glutamate network blunts responses to diverse stimuli in distantly related K2Ps (Fig. 5 and figs. S9 and S10) and establishes that, together with its role in external pH responses (42), the SF2-M4 loop is a hub that integrates chemical and physical gating cues sensed in other parts of the channel (Fig. 5, E to L) and relayed to the filter via M4 (3, 4). The M3 glutamate network is conserved in every functional K2P except K2P18.1 (TRESK), the only K2P having a short SF2-M4 loop (Fig. 5D and fig. S7D). This conservation, together with the report that a pulmonary hypertension mutation at the conserved M3 glutamate in K2P3.1 (TASK-1), E182K, disrupts function (63) underscores the importance of the M3 glutamate network and SF2-M4 loop in gating throughout the K2P family.
Our studies establish that K2P channel C-type gating entails filter pinching (SF1) and pore dilation (SF2), highlight the dynamic nature of C-type inactivated states (20, 64), and indicate that the innate heterodimeric nature of the K2P filter architecture enables two general C-type gating mechanisms, pinching and dilation (12), which have been viewed as mutually exclusive, to operate in one channel. The substantial differences in the degree of conformational changes between SF1 and SF2 appear to depend on the loop length connecting these elements to the outer transmembrane helix of their respective pore domains. Binding of small molecules, such as ML335, to the K2P modulator pocket enables conduction by stabilizing the SF2-M4 loop and selectivity filter and increasing channel open probability, whereas disruption of the integrity of the SF2-M4 loop blunts transduction of gating cues that originate from the intracellular C-terminal tail (3, 65–70) and pass through M4 to the C-type gate (3, 4). These findings corroborate the ideas that the K2P selectivity filter and its supporting architecture are dynamic under basal conditions (20), that ion permeation requires limiting filter mobility through ligand binding to the K2P modulator pocket or by conformational changes transmitted through the M4 helix (20), that permeant ions organize and stabilize the K2P conductive state (5, 38), and that the inactive state involves an ion-depleted filter (5). Further, our observation that the filter can adopt nonconductive conformations although the M4 transmembrane helix is in the “up” position underscores previous studies indicating that M4 conformation is not the sole determinant of K2P activation (20, 71). The key role for the SF2-M4 loop in transducing gating cues sensed by intracellular channel components to the K2P selectivity filter gate such as temperature and pressure (Fig. 5, E to H), as well as external pH responses (42), demonstrates its pivotal function in K2P gating. These properties, together with the ability of ML335 to increase open probability by stabilizing this loop (Figs. 1 to 5), explain why the P1-M4 interface, which is framed on one side by the SF2-M4 loop, is central to K2P gating (3, 4, 20) and why small molecules bound to this interface activate the channel (20). These findings emphasize the potential for targeting this unique K2P loop for selective small molecule or biologic modulators directed at K2P-dependent processes such as anesthetic responses (72, 73), pain (74–76), arrhythmia (77), ischemia (72, 78), and migraine (52).
MATERIALS AND METHODS
Protein expression and purification
An engineered mouse K2P2.1 (TREK-1), denoted K2P2.1cryst, encompassing residues 21 to 322 and bearing the following mutations: K84R, Q85E, T86K, I88L, A89R, Q90A, A92P, N95S, S96D, T97Q, N119A, S300A, E306A, a C-terminal green fluorescent protein (GFP), and His10 tag was expressed and purified from Pichia pastoris as previously described (20).
Crystallization and refinement
Purified K2P2.1cryst was concentrated to 6 mg ml−1 by centrifugation (Amicon Ultra-15, 50 kDa molecular mass cutoff; Millipore) and crystallized by hanging-drop vapor diffusion at 4°C using a mixture of 0.2 μl of protein and 0.1 μl of precipitant over 100 μl of reservoir containing 20 to 25% polyethylene glycol 400 (PEG400), 200 mM KCl, 1 mM CdCl2, and 100 mM Hepes (pH 8.0). Crystals appeared in 12 hours and grew to full size (200 to 300 μM) in about 1 week.
Crystals were harvested and cryoprotected with buffer D [200 mM KCl, 0.2% octyl glucose neopentyl glycol (OGNG), 15 mM n-heptyl-β-D-thioglucoside (HTG), 0.02% cholesteryl hemisuccinate (CHS), 1 mM CdCl2, and 100 mM Hepes (pH 8.0)] with 5% step increases of PEG400 up to a final concentration of 38%. After cryoprotection, crystals were incubated for 8 hours in buffer E [38% PEG400, 0.2% OGNG, 15 mM HTG, 0.02% CHS, 1 mM CdCl2, and 100 mM Hepes (pH 8.0)] containing 200 mM salt consisting of NaCl and KCl in varied proportions to yield the following K+ concentrations: 0, 1, 10, 30, 50, 100, and 200 mM. In the soaking experiments where the activator was present, ML335 was added to the soaking cocktail to a 1 mM final concentration. The nominal K+ concentration in the 0 mM condition is ~20 nM. Crystals were subsequently harvested and flash-frozen in liquid nitrogen.
Datasets for K2P2.1cryst in the presence of differing potassium concentrations, alone or with ML335, were collected at 100 K using synchrotron radiation at advanced photon source (APS) GM/CAT beamline 23-IDB/D Chicago, Illinois, processed with XDS (79), scaled, and merged with Aimless (80). Final resolution cutoffs were 3.9, 3.5, 3.4, 3.3, 3.6, 3.9, and 3.7 Å for K2P2.1cryst in the presence of 0, 1, 10, 30, 50, 100, and 200 mM potassium, respectively, and were arrived at using the CC1/2 criterion and standard best practices based on map quality (81). Final resolution cutoffs for the K2P2.1cryst:ML335 complex were 3.4, 2.6, 3.0, 3.2, 3.2, 3.3, and 3.8 Å in the presence of 0, 1, 10, 30, 50, 100, and 200 mM potassium, respectively. Structures were solved by molecular replacement using the K2P2.1cryst structure [Protein Data Bank (PDB): 6CQ6] (20) as search model purged of all the ligands. The best resolution structure (1 mM:ML335) had density for head group of the lipid in the phosphatidylinositol 4,5-bisphosphate (PIP2) binding site and was built accordingly. Several cycles of manual rebuilding, using COOT (82), and refinement using REFMAC5 (83) and PHENIX (84) were carried out to improve the electron density map. Twofold local automatic noncrystallographic symmetry restraints were used during refinement.
Two potassium ions were modeled into 2Fo-Fc densities of the Apo K2P2.1cryst 0, 1, 10, and 50 mM structures; whereas, four potassium ions were modeled into 2Fo-Fc densities of the Apo K2P2.1cryst 100 and 200 mM structures. Four potassium ions were modeled for all the K2P2.1cryst:ML335 complexes. To validate the presence of the potassium ions, a polder map (28) was generated for each structure. The polder map of the Apo K2P2.1cryst 50 mM structure showed a density in the filter that extended beyond the S3 site into the S2 site; however, modeling an additional low occupancy K+ ion at this site did not improve the overall statistics. Attempts to refine the occupancy of this third ion using PHENIX (84) yielded an ion having zero occupancy. Hence, the final structure has two ions in the filter, although there may be a low occupancy ion present that is not accountable due to the resolution limit of the data. The final cycle of refinement of each structure was carried out using BUSTER (85).
K+ anomalous data collection
Long-wavelength data were collected at beamline I23, Diamond Light Source (30), UK, at a temperature ~ 50 K at wavelengths of 3.3509 and 3.4730 Å, above and below the potassium K absorption edge, processed and scaled with XDS/XSCALE (79). Anomalous difference Fourier maps to locate the potassium positions were calculated with ANODE (86) using the K2P2.1 (TREK-1) structure (PDB:6CQ6) (20). Peaks present in the maps above but absent in the maps below the absorption edge were assigned as potassium.
Two-electrode voltage-clamp electrophysiology
Two-electrode voltage-clamp recordings were performed on defolliculated stage V to VI Xenopus laevis oocytes 18 to 48 hours after microinjection with 1 to 40 ng of mRNA. Oocytes were impaled with borosilicate recording microelectrodes (0.3- to 3.0-MΩ resistance) backfilled with 3 M KCl. Except where otherwise indicated, recording solution was 96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, and 1.0 mM MgCl2, buffered with 5 mM Hepes at pH 7.4 and was perfused by gravity. For pHo experiment solutions, the standard buffer was replaced with 10 mM tris (pH 9.0 and 8.1), 5 mM Hepes (pH 7.8 and 7.1), or 5 mM MES (pH 6.5 and 5.9).
Currents were evoked from a −80-mV holding potential followed by a 300-ms ramp from −150 to +50 mV. Data were acquired using a GeneClamp 500B amplifier (MDS Analytical Technologies) controlled by pCLAMP software (Molecular Devices) and digitized at 1 kHz using Digidata 1332A digitizer (MDS Analytical Technologies).
For temperature experiments, recording solutions were heated by an SC-20 in-line heater/cooler combined with an LCS-1 liquid cooling system operated by the CL-100 bipolar temperature controller (Warner Instruments). Temperature was monitored using a CL-100–controlled thermistor placed in the bath solution 1 mm upstream of the oocyte. For temperature experiments, perfusate was warmed from 15° to 35°C in 5°C increments, with recordings performed once temperature readings stabilized at the desired values. Temperature response data were fit with the equation where Amin and Amax are the minimum and maximum activation, respectively, T1/2 is the temperature of half maximal activation, and S is the slope factor (4). For pHo experiments, solutions were exchanged consecutively from 9.0 to 5.9 while maintaining the temperature at 22.5°C. pH response data were fit with the equation A = Amin + (Amax − Amin)/(1 + ([H+]o/K1/2)H) where Amin and Amax are the minimum and maximum activation, respectively, K1/2 is the half maximal inhibitory concentration of extracellular protons, and H is the Hill slope.
Dose-response experiments were conducted at room temperature (22°C) and used standard recording solution at pH 7.4 supplemented with 0.2% dimethyl sulfoxide and the indicated concentration of ML335 (20). Dose-response data were fit with the equation A = Amin + (Amax − Amin)/(1 + (EC50/[ML335])H) where Amin and Amax are the minimum and maximum activation, respectively, EC50 is the half maximal effective concentration, and H is the Hill slope. Data analysis and curve fitting were performed using Clampfit and Python according to procedures adapted from (4, 20). X. laevis oocytes were harvested from female X. laevis according to UCSF Institutional Animal Care and Use Commitee (IACUC) Protocol AN178461.
Patch clamp electrophysiology
Human embryonic kidney cells (HEK293) were grown at 37°C under 5% CO2 in a Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 10% l-glutamine, and antibiotics [penicillin (100 IU ml−1) and streptomycin (100 mg ml−1)]. Cells were transfected (in 35-mm-diameter wells) using Lipofectamine 2000 (Invitrogen) and a pIRES-GFP (Invitrogen) plasmid vector into which the gene encoding for mouse K2P2.1 (TREK-1) wild type or mutants has been inserted in the first cassette (4). DNA (1 μg) was used for K2P2.1 (TREK-1) and Loop2-sym-6, whereas 3 μg of DNA was necessary to record reliable currents from E234Q and Y270F. For single-channel experiments, 0.05 to 0.1 μg of DNA was used for K2P2.1 (TREK-1). Transfected cells were identified visually using the GFP expressed in the second cassette of the pIRES-GFP plasmid vector. After a minimum of 6 hours after transfection, cells were plated onto coverslips coated with Matrigel (BD Biosciences). Data acquisition was performed using pCLAMP 10 (Molecular Devices) and an Axopatch 200B amplifier (Molecular Devices).
The inside-out configuration of the patch clamp technique was used to record K+ or Rb+ currents at room temperature (23° ± 2°C) 24 to 48 hours after transfection (5, 20). Pipettes were pulled from borosilicate glass capillaries (TW150F-3, World Precision Instruments) and polished (MF-900 microforge, Narishige) to obtain 1- to 2-MΩ resistances.
Stretch activation of K2P2.1 (TREK-1) and mutants was performed by applying a −50-mmHg pressure to the inside-out patch through a high-speed pressure clamp (HSPC-1, ALA Scientific Instruments) connected to the electrode suction port, after recording the current at 0 mmHg. Pipette solution contained 150 mM NaCl, 5 mM KCl, 1 mM CaCl2, 2 mM MgCl2, and 20 mM Hepes (pH 7.4 with NaOH). Bath solution contained 145 mM KCl, 3 mM MgCl2, 5 mM EGTA, and 20 mM Hepes (pH 7.2 with KOH) and was continuously perfused at 200 ml hour−1 during the experiment. K2P2.1 (TREK-1) currents were elicited by a 1-s ramp from −140 to +50 mV from a −80-mV holding potential.
Single-channel activity was recorded under the cell-attached configuration of the patch clamp technique, using patch pipettes of about 8 MΩ pulled from quartz glass capillaries (QF100-70-7.5, Sutter Instrument, Novato, CA, USA) in a laser-based micropipette puller (P-2000, Sutter Instrument). Both the pipette and bath solutions contained 150 mM KCl, 5 mM EGTA-K, 1 mM EDTA-K, and 10 mM Hepes (pH 7.3 with KOH). Currents were low-pass–filtered at 2 kHz and digitized at a sampling rate of 20 kHz. Threshold detection of channel openings was set at 50%. Channel activity (NPo, where N is the number of channels in the patch and Po is the probability of a channel being open) was determined from ≥30 s of current recordings.
Voltage-dependent activation and inactivation of K2P2.1 (TREK-1) and mutants were recorded from inside-out patches. Pipette solution contained 150 mM KCl, 3.6 mM CaCl2, and 10 mM Hepes (pH 7.4 with KOH). Bath solution contained 150 mM RbCl, 2 mM EGTA, and 10 mM Hepes (pH 7.4 with RbOH) and was continuously perfused at 200 ml hour−1 during the experiment. For voltage-dependent activation, currents were elicited by voltage steps from −100 to +100 mV, from a −80-mV holding potential. For voltage-dependent inactivation, currents were elicited by prepulse voltage steps from −50 to +90 mV from a −80-mV holding potential, each step being followed by a test pulse at +100 mV. All electrophysiology data were analyzed using Clampfit 10.7 (Molecular Devices).
Molecular dynamics
Simulation setup
Initial K2P2.1 (TREK-1) simulations in the absence of ML335 were initiated from PDB:5VK5. Later simulations were based on PDB:6CQ6 (20), which is indistinguishable from PDB:5VK5 except for a minor difference in the C-terminal portion of M4. Simulations in complex with ML335 were constructed from PDB:6W8C. In both cases, models consisted of residues 35 to 321, a disulfide bond was formed between C93 in one subunit with C93 in the other, missing loops were built with RosettaRemodel (87), and N and C termini were capped with methylamide and acetyl groups, respectively. All residues were assigned their standard protonation states at pH 7. Structures were embedded in pure 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC) or POPC + 4% inner leaflet mole fraction 1‑stearoyl-2-arachidonoyl-glycero-3-phosphatidylinositol 4,5‑bisphosphate (18:0-20:4) PIP2 bilayers using CHARMM-GUI (88) and solvated in 180 mM [K+] with neutralizing Cl− (excepting low [K+] simulations, which contained only 4 mM [K+]). Two structural water molecules were added per subunit in the cavity between SF2 and pore helix 2 on the basis of water molecules identified in K2P4.1 (TRAAK) PDB:4I9W (23). For simulations containing PIP2, an additional PIP2 lipid per channel subunit was placed in the lipid binding site adjacent to the M4 helix, as observed in the ML335:1 mM [K+] structure (PDB:6W8C). Extensive preliminary K2P simulations were initiated from a range of filter ion configurations in the S1 to S4 sites including alternating K+ and water with waters at either S1/S3 or S2/S4, all K+, or all K+ with empty sites at S2 or S3. In all cases, the configuration with ions in S1/S2/S4, and an empty S3 was frequently visited during the conduction cycle. Thus, all high [K+] simulations presented here were equilibrated with this configuration. Low [K+] simulations were initiated with a single ion in the selectivity filter placed at the S2, S3, or S4 site. The force fields used for protein, lipids, water, and ML335 were CHARMM36m (89), CHARMM36 (90), TIP3P (91), and CGenFF 3.0.1 (92–94), respectively. Standard CHARMM parameters were used for ions (95).
Simulation details
Production data were collected on two platforms: Anton2 (96) at the Pittsburgh Supercomputing Center and local graphical processing unit (GPU) resources using GROMACS 2018 (97) (see table S3 for a full list). All systems were energy minimized for 8000 steps with 5 kcal/mol per Å2 harmonic restraints on all protein heavy atoms, followed by a multistep equilibration in which protein restraints were gradually reduced over 10 to 12 ns. Next, for systems simulated under a membrane potential, we performed a 10-mV voltage jump every 5 ns until reaching 40 mV using the constant electric field protocol where Eapplied = V/Lz (98). On average Lz = 121 Å in our systems, and the final applied electric field was 0.0076 kcal/mol·Å·e. Note that systems destined for Anton2 were equilibrated with NAMD 2.13 (99). Production run details varied by hardware. Simulations on Anton2 used a 2.5-fs time step, an Martyna-Tobias-Klein barostat (100) with semi-isotropic pressure control at 1 atm, and a Nose-Hoover thermostat (101, 102) with a temperature of 303.15 K. In addition, nonbonded interactions were cut off at 10 Å, long-range electrostatic interactions were calculated using the Gaussian split Ewald method (103), and hydrogens were constrained with SHAKE (104). Meanwhile, GROMACS 2018 runs used either a 2- or 2.5-fs time step, a Parrinello-Rahman barostat (105, 106) with semi-isotropic pressure control at 1 atm, and a Nose-Hoover thermostat set to 310 K. Nonbonded interactions were cut off at 12 Å with force-switching between 10 and 12 Å, long-range electrostatics were calculated with particle mesh Ewald (107), and hydrogens were constrained with the LINCS algorithm (108). For low [K+] simulations, solution ions were excluded from the selectivity filter using a flat bottom restraint on Anton2 or harmonic positional restraints in GROMACS 2018.
Simulation analysis
Ions were tracked within a 22-Å-long cylindrical volume centered on the selectivity filter, and a permeation event was recorded when an ion originating below (above) the midplane of the filter (defined by the plane separating S2 to S3 sites) exited the top (bottom) of the cylinder. The time of the permeation event was recorded as the last time the ion crossed the midplane before exit from the cylinder. PCA was carried out on the backbone dihedral angles of selectivity filter residues (142 to 146 in SF1 and 251 to 255 in SF2) as described in (32), and each strand was treated independently. Formation of hydrogen bonds to carboxylate or carbonyl oxygens was determined on the basis of the H to O distance (>2.5 Å for OH donors, >2.75 Å for NH donors) and C═O H angle >110°. For all analyses, conformations were sampled from the trajectories every 480 to 500 ps. All analysis code was built on top of the MDAnalysis Python package (109).
Statistical tests
For datasets were significance is indicated, a normality test was performed with a Shapiro-Wilk test, followed by an equality of variances test using a Levene’s test. For samples with similar variances, significance was evaluated using either a paired or unpaired Student’s t test. For data that were not normally distributed, a nonparametric Mann-Whitney test or a Wilcoxon signed-rank test for paired analyses was used. For samples with unequal variance, significance was evaluated with a Welch’s t test.
Experiments were nonrandomized and nonblinded, and no prespecified sample size was estimated. Measurements were taken from distinct samples. All data are presented as means ± SEM, and all experiments were repeated from N ≥ 2 different batches to mitigate biological variability. The number of experiments (n) as technical replicates is indicated in the figure legends. Significances are indicated in the figures using the following symbols: “*”, P < 0.05; “**”, P < 0.01; and “N.S.,” not statistically different.
Supplementary Material
Acknowledgments
We thank C. Arrigoni and L. Pope for electrophysiology guidance, C. Colleran for cell culture assistance, B. Roux for guidance on our choice of force fields used in this study, L. Jan for support, and K. Brejc and F. C. Chatelain for comments on the manuscript. Funding: This work was supported by NIH grants R21NS091941 to J.M.R., R01GM089740 to M.G., and R01-MH093603 to D.L.M. and an AHA postdoctoral fellowship to F.A.-A. Anton 2 computer time was provided by the Pittsburgh Supercomputing Center (PSC) through NIH grant R01GM116961. The Anton 2 machine at PSC was made available by D.E. Shaw Research. Simulations were also carried out on the UCSF Wynton Cluster made possible through NIH grant 1S10OD021596. Author contributions: M.L., A.M.N., F.A.-A., S.C., J.M.R., M.G., and D.L.M. conceived the study and designed the experiments. M.L. expressed, purified, and crystallized the proteins, collected diffraction data, and determined the structures. R.D. and A.W. collected anomalous diffraction data. M.L., A.M.N., F.A.-A., and, D.C. performed functional studies. F.A.-A. carried out and analyzed single-channel recordings. A.M.N., S.C., J.M.R., and M.G. designed and executed the simulations. M.L., A.M.N., F.A.-A., D.C., M.G., and D.L.M. analyzed the data. M.G. and D.L.M. provided guidance and support. M.L., A.M.N., F.A.-A., M.G., and D.L.M. wrote the paper. Competing interests: The authors declare that they have no competing interests. Materials and correspondence: Correspondence should be directed to M.G. or D.L.M. Requests for materials should be directed to D.L.M. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Coordinates and structures factors are deposited in the RCSB and will be released immediately upon publication. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/44/eabc9174/DC1
REFERENCES AND NOTES
- 1.Enyedi P., Czirjak G., Molecular background of leak K+ currents: Two-pore domain potassium channels. Physiol. Rev. 90, 559–605 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Feliciangeli S., Chatelain F. C., Bichet D., Lesage F., The family of K2P channels: Salient structural and functional properties. J. Physiol. 593 ( Pt. 12), 2587–2603 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagriantsev S. N., Clark K. A., Minor D. L. Jr., Metabolic and thermal stimuli control K(2P)2.1 (TREK-1) through modular sensory and gating domains. EMBO J. 31, 3297–3308 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagriantsev S. N., Peyronnet R., Clark K. A., Honore E., Minor D. L. Jr., Multiple modalities converge on a common gate to control K2P channel function. EMBO J. 30, 3594–3606 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schewe M., Nematian-Ardestani E., Sun H., Musinszki M., Cordeiro S., Bucci G., de Groot B. L., Tucker S. J., Rapedius M., Baukrowitz T., A non-canonical voltage-sensing mechanism controls gating in K2P K+ channels. Cell 164, 937–949 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piechotta P. L., Rapedius M., Stansfeld P. J., Bollepalli M. K., Erhlich G., Andres-Enguix I., Fritzenschaft H., Decher N., Sansom M. S. P., Tucker S. J., Baukrowitz T., The pore structure and gating mechanism of K2P channels. EMBO J. 30, 3607–3619 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez-Barneo J., Hoshi T., Heinemann S. H., Aldrich R. W., Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels 1, 61–71 (1993). [PubMed] [Google Scholar]
- 8.Baukrowitz T., Yellen G., Modulation of K+ current by frequency and external [K+]: A tale of two inactivation mechanisms. Neuron 15, 951–960 (1995). [DOI] [PubMed] [Google Scholar]
- 9.Pardo L. A., Heinemann S. H., Terlau H., Ludewig U., Lorra C., Pongs O., Stuhmer W., Extracellular K+ specifically modulates a rat brain K+ channel. Proc. Natl. Acad. Sci. U.S.A. 89, 2466–2470 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen A., Ben-Abu Y., Hen S., Zilberberg N., A novel mechanism for human K2P2.1 channel gating. Facilitation of C-type gating by protonation of extracellular histidine residues. J. Biol. Chem. 283, 19448–19455 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Cordero-Morales J. F., Cuello L. G., Zhao Y., Jogini V., Cortes D. M., Roux B., Perozo E., Molecular determinants of gating at the potassium-channel selectivity filter. Nat. Struct. Mol. Biol. 13, 311–318 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Hoshi T., Armstrong C. M., C-type inactivation of voltage-gated K+ channels: Pore constriction or dilation? J. Gen. Physiol. 141, 151–160 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y., MacKinnon R., The occupancy of ions in the K+ selectivity filter: Charge balance and coupling of ion binding to a protein conformational change underlie high conduction rates. J. Mol. Biol. 333, 965–975 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y., Morais-Cabral J. H., Kaufman A., MacKinnon R., Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 Å resolution. Nature 414, 43–48 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Cuello L. G., Cortes D. M., Perozo E., The gating cycle of a K+ channel at atomic resolution. eLife 6, e28032 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuello L. G., Jogini V., Cortes D. M., Perozo E., Structural mechanism of C-type inactivation in K+ channels. Nature 466, 203–208 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pau V., Zhou Y., Ramu Y., Xu Y., Lu Z., Crystal structure of an inactivated mutant mammalian voltage-gated K+ channel. Nat. Struct. Mol. Biol. 24, 857–865 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W., MacKinnon R., Cryo-EM structure of the open human ether-à-go-go-related K+ Channel hERG. Cell 169, 422–430.e10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthies D., Bae C., Toombes G. E. S., Fox T., Bartesaghi A., Subramaniam S., Swartz K. J., Single-particle cryo-EM structure of a voltage-activated potassium channel in lipid nanodiscs. eLife 7, e37558 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lolicato M., Arrigoni C., Mori T., Sekioka Y., Bryant C., Clark K. A., Minor D. L. Jr., K2P2.1 (TREK-1)–activator complexes reveal a cryptic selectivity filter binding site. Nature 547, 364–368 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong Y. Y., Pike A. C. W., Mackenzie A., McClenaghan C., Aryal P., Dong L., Quigley A., Grieben M., Goubin S., Mukhopadhyay S., Ruda G. F., Clausen M. V., Cao L., Brennan P. E., Burgess-Brown N. A., Sansom M. S. P., Tucker S. J., Carpenter E. P., K2P channel gating mechanisms revealed by structures of TREK-2 and a complex with Prozac. Science 347, 1256–1259 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brohawn S. G., del Marmol J., MacKinnon R., Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science 335, 436–441 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brohawn S. G., Campbell E. B., MacKinnon R., Domain-swapped chain connectivity and gated membrane access in a Fab-mediated crystal of the human TRAAK K+ channel. Proc. Natl. Acad. Sci. U.S.A. 110, 2129–2134 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brohawn S. G., Campbell E. B., MacKinnon R., Physical mechanism for gating and mechanosensitivity of the human TRAAK K+ channel. Nature 516, 126–130 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lolicato M., Riegelhaupt P. M., Arrigoni C., Clark K. A., Minor D. L. Jr., Transmembrane helix straightening and buckling underlies activation of mechanosensitive and thermosensitive K(2P) channels. Neuron 84, 1198–1212 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller A. N., Long S. B., Crystal structure of the human two-pore domain potassium channel K2P1. Science 335, 432–436 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Derebe M. G., Sauer D. B., Zeng W., Alam A., Shi N., Jiang Y., Tuning the ion selectivity of tetrameric cation channels by changing the number of ion binding sites. Proc. Natl. Acad. Sci. U.S.A. 108, 598–602 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liebschner D., Afonine P. V., Moriarty N. W., Poon B. K., Sobolev O. V., Terwilliger T. C., Adams P. D., Polder maps: Improving OMIT maps by excluding bulk solvent. Acta Crystallogr. D Struct. Biol. 73, 148–157 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langan P. S., Vandavasi V. G., Weiss K. L., Afonine P. V., el Omari K., Duman R., Wagner A., Coates L., Anomalous x-ray diffraction studies of ion transport in K+ channels. Nat. Commun. 9, 4540 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner A., Duman R., Henderson K., Mykhaylyk V., In-vacuum long-wavelength macromolecular crystallography. Acta Crystallogr. D Struct. Biol. 72, 430–439 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostmeyer J., Chakrapani S., Pan A. C., Perozo E., Roux B., Recovery from slow inactivation in K+ channels is controlled by water molecules. Nature 501, 121–124 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altis A., Nguyen P. H., Hegger R., Stock G., Dihedral angle principal component analysis of molecular dynamics simulations. J. Chem. Phys. 126, 244111 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Long S. B., Tao X., Campbell E. B., MacKinnon R., Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature 450, 376–382 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Brennecke J. T., de Groot B. L., Mechanism of mechanosensitive gating of the TREK-2 potassium channel. Biophys. J. 114, 1336–1343 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrigan M. P., McKiernan K. A., Shanmugasundaram V., Denny R. A., Pande V. S., Markov modeling reveals novel intracellular modulation of the human TREK-2 selectivity filter. Sci. Rep. 7, 632 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi N., Ye S., Alam A., Chen L., Jiang Y., Atomic structure of a Na+- and K+-conducting channel. Nature 440, 570–574 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Pope L., Arrigoni C., Lou H., Bryant C., Gallardo-Godoy A., Renslo A. R., Minor D. L. Jr., Protein and chemical determinants of BL-1249 action and selectivity for K2P channels. ACS Chem. Nerosci. 9, 3153–3165 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schewe M., Sun H., Mert Ü., Mackenzie A., Pike A. C. W., Schulz F., Constantin C., Vowinkel K. S., Conrad L. J., Kiper A. K., Gonzalez W., Musinszki M., Tegtmeier M., Pryde D. C., Belabed H., Nazare M., de Groot B. L., Decher N., Fakler B., Carpenter E. P., Tucker S. J., Baukrowitz T., A pharmacological master key mechanism that unlocks the selectivity filter gate in K+ channels. Science 363, 875–880 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zilberberg N., Ilan N., Goldstein S. A. N., KCNKØ. Neuron 32, 635–648 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Larsson H. P., Elinder F., A conserved glutamate is important for slow inactivation in K+ channels. Neuron 27, 573–583 (2000). [DOI] [PubMed] [Google Scholar]
- 41.Ortega-Sáenz P., Pardal R., Castellano A., López-Barneo J., Collapse of conductance is prevented by a glutamate residue conserved in voltage-dependent K+ channels. J. Gen. Physiol. 116, 181–190 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandoz G., Douguet D., Chatelain F., Lazdunski M., Lesage F., Extracellular acidification exerts opposite actions on TREK1 and TREK2 potassium channels via a single conserved histidine residue. Proc. Natl. Acad. Sci. U.S.A. 106, 14628–14633 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rödström K. E. J., Kiper A. K., Zhang W., Rinné S., Pike A. C. W., Goldstein M., Conrad L. J., Delbeck M., Hahn M. G., Meier H., Platzk M., Quigley A., Speedman D., Shrestha L., Mukhopadhyay S. M. M., Burgess-Brown N. A., Tucker S. J., Müller T., Decher N., Carpenter E. P., A lower X-gate in TASK channels traps inhibitors within the vestibule. Nature 582, 443–447 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Pless S. A., Galpin J. D., Niciforovic A. P., Kurata H. T., Ahern C. A., Hydrogen bonds as molecular timers for slow inactivation in voltage-gated potassium channels. eLife 2, e01289 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lees-Miller J. P., Duan Y., Teng G. Q., Thorstad K., Duff H. J., Novel gain-of-function mechanism in K+ channel-related long-QT syndrome: Altered gating and selectivity in the HERG1 N629D mutant. Circ. Res. 86, 507–513 (2000). [DOI] [PubMed] [Google Scholar]
- 46.Miranda W. E., DeMarco K. R., Guo J., Duff H. J., Vorobyov I., Clancy C. E., Noskov S. Y., Selectivity filter modalities and rapid inactivation of the hERG1 channel. Proc. Natl. Acad. Sci. U.S.A. 117, 2795–2804 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bockenhauer D., Zilberberg N., Goldstein S. A. N., KCNK2: Reversible conversion of a hippocampal potassium leak into a voltage-dependent channel. Nat. Neurosci. 4, 486–491 (2001). [DOI] [PubMed] [Google Scholar]
- 48.Clausen M. V., Jarerattanachat V., Carpenter E. P., Sansom M. S. P., Tucker S. J., Asymmetric mechanosensitivity in a eukaryotic ion channel. Proc. Natl. Acad. Sci. U.S.A. 114, E8343–E8351 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lengyel M., Czirják G., Enyedi P., Formation of functional heterodimers by TREK-1 and TREK-2 two-pore domain potassium channel subunits. J. Biol. Chem. 291, 13649–13661 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levitz J., Royal P., Comoglio Y., Wdziekonski B., Schaub S., Clemens D. M., Isacoff E. Y., Sandoz G., Heterodimerization within the TREK channel subfamily produces a diverse family of highly regulated potassium channels. Proc. Natl. Acad. Sci. U.S.A. 113, 4194–4199 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blin S., Ben Soussia I., Kim E.-J., Brau F., Kang D., Lesage F., Bichet D., Mixing and matching TREK/TRAAK subunits generate heterodimeric K2P channels with unique properties. Proc. Natl. Acad. Sci. U.S.A. 113, 4200–4205 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Royal P., Andres-Bilbe A., Prado P. Á., Verkest C., Wdziekonski B., Schaub S., Baron A., Lesage F., Gasull X., Levitz J., Sandoz G., Migraine-associated TRESK mutations increase neuronal excitability through alternative translation initiation and inhibition of TREK. Neuron 101, 232–245.e6 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Blin S., Chatelain F. C., Feliciangeli S., Kang D., Lesage F., Bichet D., Tandem pore domain halothane-inhibited K+ channel subunits THIK1 and THIK2 assemble and form active channels. J. Biol. Chem. 289, 28202–28212 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Renigunta V., Zou X., Kling S., Schlichthörl G., Daut J., Breaking the silence: Functional expression of the two-pore-domain potassium channel THIK-2. Pflugers Arch. 466, 1735–1745 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Berg A. P., Talley E. M., Manger J. P., Bayliss D. A., Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits. J. Neurosci. 24, 6693–6702 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mathie A., Veale E. L., Cunningham K. P., Holden R. G., Wright P. D., Two-pore domain potassium channels as drug targets: Anesthesia and beyond. Annu. Rev. Pharmacol. Toxicol. 10.1146/annurev-pharmtox-030920-111536 , (2020). [DOI] [PubMed] [Google Scholar]
- 57.Yuill K. H., Stansfeld P. J., Ashmole I., Sutcliffe M. J., Stanfield P. R., The selectivity, voltage-dependence and acid sensitivity of the tandem pore potassium channel TASK-1: Contributions of the pore domains. Pflugers Arch. 455, 333–348 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu S., Focke P. J., Matulef K., Bian X., Moënne-Loccoz P., Valiyaveetil F. I., Lockless S. W., Ion-binding properties of a K+ channel selectivity filter in different conformations. Proc. Natl. Acad. Sci. U.S.A. 112, 15096–15100 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li J., Ostmeyer J., Boulanger E., Rui H., Perozo E., Roux B., Chemical substitutions in the selectivity filter of potassium channels do not rule out constricted-like conformations for C-type inactivation. Proc. Natl. Acad. Sci. U.S.A. 114, 11145–11150 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li J., Ostmeyer J., Cuello L. G., Perozo E., Roux B., Rapid constriction of the selectivity filter underlies C-type inactivation in the KcsA potassium channel. J. Gen. Physiol. 150, 1408–1420 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Devaraneni P. K., Komarov A. G., Costantino C. A., Devereaux J. J., Matulef K., Valiyaveetil F. I., Semisynthetic K+ channels show that the constricted conformation of the selectivity filter is not the C-type inactivated state. Proc. Natl. Acad. Sci. U.S.A. 110, 15698–15703 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van der Cruijsen E. A. W., Nand D., Weingarth M., Prokofyev A., Hornig S., Cukkemane A. A., Bonvin A. M. J. J., Becker S., Hulse R. E., Perozo E., Pongs O., Baldus M., Importance of lipid-pore loop interface for potassium channel structure and function. Proc. Natl. Acad. Sci. U.S.A. 110, 13008–13013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma L., Roman-Campos D., Austin E. D., Eyries M., Sampson K. S., Soubrier F., Germain M., Trégouët D.-A., Borczuk A., Rosenzweig E. B., Girerd B., Montani D., Humbert M., Loyd J. E., Kass R. S., Chung W. K., A novel channelopathy in pulmonary arterial hypertension. N. Engl. J. Med. 369, 351–361 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jekhmane S., Medeiros-Silva J., Li J., Kümmerer F., Müller-Hermes C., Baldus M., Roux B., Weingarth M., Shifts in the selectivity filter dynamics cause modal gating in K+ channels. Nat. Commun. 10, 123 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maingret F., Lauritzen I., Patel A. J., Heurteaux C., Reyes R., Lesage F., Lazdunski M., Honoré E., TREK-1 is a heat-activated background K+ channel. EMBO J. 19, 2483–2491 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patel A. J., Honoré E., Maingret F., Lesage F., Fink M., Duprat F., Lazdunski M., A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 17, 4283–4290 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chemin J., Patel A. J., Duprat F., Lauritzen I., Lazdunski M., Honoré E., A phospholipid sensor controls mechanogating of the K+ channel TREK-1. EMBO J. 24, 44–53 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Honoré E., Maingret F., Lazdunski M., Patel A. J., An intracellular proton sensor commands lipid- and mechano-gating of the K+ channel TREK-1. EMBO J. 21, 2968–2976 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim Y., Bang H., Gnatenco C., Kim D., Synergistic interaction and the role of C-terminus in the activation of TRAAK K+ channels by pressure, free fatty acids and alkali. Pflugers Arch. 442, 64–72 (2001). [DOI] [PubMed] [Google Scholar]
- 70.Kim Y., Gnatenco C., Bang H., Kim D., Localization of TREK-2 K+ channel domains that regulate channel kinetics and sensitivity to pressure, fatty acids and pHi. Pflugers Arch. 442, 952–960 (2001). [DOI] [PubMed] [Google Scholar]
- 71.McClenaghan C., Schewe M., Aryal P., Carpenter E. P., Baukrowitz T., Tucker S. J., Polymodal activation of the TREK-2 K2P channel produces structurally distinct open states. J. Gen. Physiol. 147, 497–505 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heurteaux C., Guy N., Laigle C., Blondeau N., Duprat F., Mazzuca M., Lang-Lazdunski L., Widmann C., Zanzouri M., Romey G., Lazdunski M., TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 23, 2684–2695 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lazarenko R. M., Fortuna M. G., Shi Y., Mulkey D. K., Takakura A. C., Moreira T. S., Guyenet P. G., Bayliss D. A., Anesthetic activation of central respiratory chemoreceptor neurons involves inhibition of a THIK-1-like background K+ current. J. Neurosci. 30, 9324–9334 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alloui A., Zimmermann K., Mamet J., Duprat F., Noël J., Chemin J., Guy N., Blondeau N., Voilley N., Rubat-Coudert C., Borsotto M., Romey G., Heurteaux C., Reeh P., Eschalier A., Lazdunski M., TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 25, 2368–2376 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Devilliers M., Busserolles J., Lolignier S., Deval E., Pereira V., Alloui A., Christin M., Mazet B., Delmas P., Noel J., Lazdunski M., Eschalier A., Activation of TREK-1 by morphine results in analgesia without adverse side effects. Nat. Commun. 4, 2941 (2013). [DOI] [PubMed] [Google Scholar]
- 76.Vivier D., Soussia I. B., Rodrigues N., Lolignier S., Devilliers M., Chatelain F. C., Prival L., Chapuy E., Bourdier G., Bennis K., Lesage F., Eschalier A., Busserolles J., Ducki S., Development of the first two-pore domain potassium channel TWIK-related K+ channel 1-selective agonist possessing in vivo antinociceptive activity. J. Med. Chem. 60, 1076–1088 (2017). [DOI] [PubMed] [Google Scholar]
- 77.Decher N., Ortiz-Bonnin B., Friedrich C., Schewe M., Kiper A. K., Rinné S., Seemann G., Peyronnet R., Zumhagen S., Bustos D., Kockskämper J., Kohl P., Just S., González W., Baukrowitz T., Stallmeyer B., Schulze-Bahr E., Sodium permeable and “hypersensitive” TREK-1 channels cause ventricular tachycardia. EMBO Mol. Med. 9, 403–414 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu X., Liu Y., Chen X., Sun Q., Tang R., Wang W., Yu Z., Xie M., Involvement of TREK-1 activity in astrocyte function and neuroprotection under simulated ischemia conditions. J. Mol. Neurosci. 49, 499–506 (2013). [DOI] [PubMed] [Google Scholar]
- 79.Kabsch W., Xds. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Evans P. R., Murshudov G. N., How good are my data and what is the resolution? Acta Crystallogr. D Biol. Crystallogr. 69, 1204–1214 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karplus P. A., Diederichs K., Assessing and maximizing data quality in macromolecular crystallography. Curr. Opin. Struct. Biol. 34, 60–68 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Emsley P., Cowtan K., Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004). [DOI] [PubMed] [Google Scholar]
- 83.Collaborative Computational Project, Number 4 , The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994). [DOI] [PubMed] [Google Scholar]
- 84.Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L.-W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., Zwart P. H., PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smart O. S., Womack T. O., Flensburg C., Keller P., Paciorek W., Sharff A., Vonrhein C., Bricogne G., Exploiting structure similarity in refinement: Automated NCS and target-structure restraints in BUSTER. Acta Crystallogr. D Biol. Crystallogr. 68, 368–380 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thorn A., Sheldrick G. M., ANODE: Anomalous and heavy-atom density calculation. J. Appl. Cryst. 44, 1285–1287 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang P. S., Ban Y.-E. A., Richter F., Andre I., Vernon R., Schief W. R., Baker D., RosettaRemodel: A generalized framework for flexible backbone protein design. PLOS ONE 6, e24109 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jo S., Kim T., Im W., Automated builder and database of protein/membrane complexes for molecular dynamics simulations. PLOS ONE 2, e880 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang J., Rauscher S., Nawrocki G., Ran T., Feig M., de Groot B. L., Grubmüller H., MacKerell A. D. Jr., CHARMM36m: An improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klauda J. B., Venable R. M., Freites J. A., O’Connor J. W., Tobias D. J., Mondragon-Ramirez C., Vorobyov I., MacKerell A. D. Jr., Pastor R. W., Update of the CHARMM all-atom additive force field for lipids: Validation on six lipid types. J. Phys. Chem. B 114, 7830–7843 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jorgensen W. L., Chandrasekhar J., Madura J. D., Impey R. W., Klein M. L., Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983). [Google Scholar]
- 92.Vanommeslaeghe K., Hatcher E., Acharya C., Kundu S., Zhong S., Shim J., Darian E., Guvench O., Lopes P., Vorobyov I., Mackerell A. D. Jr., CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 31, 671–690 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vanommeslaeghe K., MacKerell A. D. Jr., Automation of the CHARMM General Force Field (CGenFF) I: Bond perception and atom typing. J. Chem. Inf. Model. 52, 3144–3154 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vanommeslaeghe K., Raman E. P., MacKerell A. D. Jr., Automation of the CHARMM General Force Field (CGenFF) II: Assignment of bonded parameters and partial atomic charges. J. Chem. Inf. Model. 52, 3155–3168 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beglov D., Roux B., Finite representation of an infinite bulk system: Solvent boundary potential for computer simulations. J. Chem. Phys. 100, 9050–9063 (1994). [Google Scholar]
- 96.D. E. Shaw, J. P. Grossman, J. A. Bank, B. Batson, J. A. Butts, J. C. Chao, M. M. Deneroff, R. O. Dror, A. Even, C. H. Fenton, A. Forte, J. Gagliardo, G. Gill, B. Greskamp, C. R. Ho, D. J. Ierardi, L. Iserovich, J. S. Kuskin, R. H. Larson, T. Layman, L.-S. Lee, A. K. Lerer, C. Li, D. Killebrew, K. M. Mackenzie, S. Y.-H. Mok, M. A. Moraes, R. Mueller, L. J. Nociolo, J. L. Peticolas, T. Quan, D. Ramot, J. K. Salmon, D. P. Scarpazza, U. B. Schafer, N. Siddique, C. W. Snyder, J. Spengler, P. T. P. Tang, M. Theobald, H. Toma, B. Towles, B. Vitale, S. C. Wang, C. Young, Anton 2: Raising the bar for performance and programmability in a special-purpose molecular dynamics supercomputer, in Proceedings of the International Conference for High Performance Computing, Networking, Storage and Analysis (SC ’14) (IEEE, 2014), pp. 41–53. [Google Scholar]
- 97.Abraham M. J., Murtola T., Schulz R., Páll S., Smith J. C., Hess B., Lindahl E., GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1-2, 19–25 (2015). [Google Scholar]
- 98.Gumbart J., Khalili-Araghi F., Sotomayor M., Roux B., Constant electric field simulations of the membrane potential illustrated with simple systems. Biochim. Biophys. Acta 1818, 294–302 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Phillips J. C., Braun R., Wang W., Gumbart J., Tajkhorshid E., Villa E., Chipot C., Skeel R. D., Kalé L., Schulten K., Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martyna G. J., Tobias D. J., Klein M. L., Constant-pressure molecular-dynamics algorithms. J. Chem. Phys. 101, 4177–4189 (1994). [Google Scholar]
- 101.Nosé S., A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 52, 255–268 (1984). [Google Scholar]
- 102.Hoover W. G., Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 31, 1695–1697 (1985). [DOI] [PubMed] [Google Scholar]
- 103.Shan Y., Klepeis J. L., Eastwood M. P., Dror R. O., Shaw D. E., Gaussian split Ewald: A fast Ewald mesh method for molecular simulation. J. Chem. Phys. 122, 054101 (2005). [DOI] [PubMed] [Google Scholar]
- 104.Ryckaert J.-P., Ciccotti G., Berendsen H. J. C., Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 23, 327–341 (1977). [Google Scholar]
- 105.Parrinello M., Rahman A., Polymorphic transitions in single-crystals: A new molecular-dynamics method. J. Appl. Phys. 52, 7182–7190 (1981). [Google Scholar]
- 106.Nosé S., Klein M. L., Constant pressure molecular-dynamics for molecular systems. Mol. Phys. 50, 1055–1076 (2006). [Google Scholar]
- 107.Darden T., York D., Pedersen L., Particle mesh Ewald: An N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993). [Google Scholar]
- 108.Hess B., Bekker H., Berendsen H. J. C., Fraaije J. G. E. M., LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 18, 1463–1472 (1997). [Google Scholar]
- 109.Michaud-Agrawal N., Denning E. J., Woolf T. B., Beckstein O., MDAnalysis: A toolkit for the analysis of molecular dynamics simulations. J. Comput. Chem. 32, 2319–2327 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guizouarn H., Gabillat N., Motais R., Borgese F., Multiple transport functions of a red blood cell anion exchanger, tAE1: Its role in cell volume regulation. J. Physiol. 535, 497–506 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/44/eabc9174/DC1


