Study shows that a common insecticide frequently detected in U.S. streams is more toxic than previously thought.
Abstract
Insecticides in streams are increasingly a global concern, yet information on safe concentrations for aquatic ecosystems is sparse. In a 30-day mesocosm experiment exposing native benthic aquatic invertebrates to the common insecticide fipronil and four degradates, fipronil compounds caused altered emergence and trophic cascades. Effect concentrations eliciting a 50% response (EC50) were developed for fipronil and its sulfide, sulfone, and desulfinyl degradates; taxa were insensitive to fipronil amide. Hazard concentrations for 5% of affected species derived from up to 15 mesocosm EC50 values were used to convert fipronil compound concentrations in field samples to the sum of toxic units (∑TUFipronils). Mean ∑TUFipronils exceeded 1 (indicating toxicity) in 16% of streams sampled from five regional studies. The Species at Risk invertebrate metric was negatively associated with ∑TUFipronils in four of five regions sampled. This ecological risk assessment indicates that low concentrations of fipronil compounds degrade stream communities in multiple regions of the United States.
INTRODUCTION
Although the production of synthetic chemicals has increased greatly in recent decades, the effect of these chemicals on nontarget ecosystems is underappreciated (1). There are no data on agricultural insecticides in the surface waters that drain 90% of global croplands, but where data are available, insecticides exceed regulatory thresholds half the time (2). A meta-analysis focusing on agricultural insecticides in surface waters of the United States found that 70% of sampled locations had at least one insecticide that exceeded a regulatory threshold (3). However, these meta-analyses (2, 3) focused only on surface waters influenced by agricultural land uses and are compilations of discrete studies. Pesticides, especially insecticides, also occur at high concentrations in streams draining urban landscapes (4). Comprehensive assessments of pesticides in surface waters draining agricultural and urban landscapes are rare; thus, it is unknown whether pesticides are a large-scale risk to surface water resources and their ecological integrity.
Phenylpyrazoles and neonicotinoids accounted for one-third of the global insecticide market in 2010 (5). In surface waters of the United States, fipronil and its degradates (phenylpyrazoles) are among the most common pesticide compounds detected, often at concentrations that exceed aquatic life benchmarks (6–8). Although neonicotinoids receive attention because of their effects on bees and birds and their ubiquity (9), fipronil is more toxic to fish and birds (10), and other phenylpyrazoles have known herbicidal effects (5). Fipronil is a systemic insecticide used to control insect pests in urban and agricultural settings. In the United States, Japan, and Great Britain, fipronil use has increased substantially since its introduction on the world market in 1993 (5). In the United States, fipronil is used to control ants and termites and is applied to crops including corn (including seed treatment), potatoes, and orchards (11, 12). Agricultural use of fipronil in the United States peaked in 2002 (13), and although there are no national urban-use data available, urban use in California peaked in 2006 and 2015 (https://calpip.cdpr.ca.gov/main.cfm, accessed 2 December 2019). Although fipronil has been found in high concentrations (6.41 μg/liter) in streams of some agricultural areas with high application rates (14), urban streams across the United States typically have more detections and greater concentrations than agricultural streams, with storm events being positively correlated with detection (6, 7, 14–17).
Fipronil enters aquatic ecosystems with runoff or by leaching from soils into streams (7, 14, 18). Fipronil has low volatility (Henry’s law constant of 2.31 × 10−4 Pa m3 mol−1), has low-to-moderate water solubility (3.78 mg/liter at 20°C), has moderate hydrophobicity (log Kow of 3.9 to 4.1), is slightly mobile in soils (log Koc of 2.6 to 3.1) (12, 19), and exhibits low-to-moderate persistence in the environment (20). Fipronil degrades via photolysis, oxidation, pH-dependent hydrolysis, and reduction to form four principal degradates: desulfinylfipronil (desulfinyl), fipronil sulfone (sulfone), fipronil amide (amide), and fipronil sulfide (sulfide). The fipronil degradates tend to be more stable and persistent than the parent compound (21, 22).
The toxicity of fipronil and its degradates to nontarget species, such as aquatic invertebrates, is well documented (14, 15). Fipronil is a neurotoxic compound that interferes with the passage of chloride ions through the γ-aminobutyric acid-regulated chloride channel in insects, leading to hyperexcitation and mortality at sufficient concentrations (20). Fipronil displays selective toxicity, whereby it has a greater receptor-binding affinity for insects than mammals (23). Fipronil degradates vary in their insecticidal activity, with sulfone and sulfide having similar or greater toxicity to freshwater invertebrates than the parent compound, desulfinyl being intermediate in toxicity but less toxic than the parent, and amide being relatively nontoxic (23, 24). Sensitivity of aquatic invertebrates to fipronil and fipronil degradates varies widely within and among taxa groups (15), in some cases by more than an order of magnitude (25). Last, there is evidence that phenylpyrazoles could be more toxic to stream ecosystems than previously thought (3).
Aquatic life benchmarks based on laboratory toxicity tests may underestimate risks to field populations (26–28). Aquatic life benchmarks are typically developed from single-species laboratory toxicity tests that use one or a few aquatic invertebrate species (e.g., Diptera: Chironomidae: Chironomus and Crustacea: Daphnia magna and Hyalella azteca). These test organisms are typically more easily cultured and, in some cases, less sensitive to contaminants than other benthic macroinvertebrate taxa (e.g., Ephemeroptera: mayflies). For example, D. magna is less sensitive to many metals than some insect species, and H. azteca is less sensitive to the pyrethroid insecticide bifenthrin than mayflies (29, 30). Another limitation of existing benchmarks is the endpoints used in their calculation. Acute benchmarks are based on mortality (or, for crustaceans, immobilization), and chronic benchmarks are often based on sublethal endpoints (e.g., growth and reproduction) when such data are available. However, there is a wide range of sublethal effects, such as growth, emergence, paralysis, and developmental delays, that can influence taxa success and community dynamics. As a result, although the benchmarks provide context for biological importance of effects, their ecological relevance as thresholds of toxicity is uncertain.
To better understand the effects of fipronil compounds on benthic aquatic ecosystems (invertebrate and algal), natural benthic communities were brought into a laboratory setting and exposed to a gradient of concentrations of fipronil or one of the four fipronil degradates during a 30-day stream mesocosm experiment. The research goals were to generate species-specific 50% effect concentrations (EC50 values) for each fipronil compound for a broad range of taxa that are representative of stream communities and to identify contaminant-induced effects to community structure and function [i.e., hazard concentration for 5% of affected species (HC5) and indirect effects such as altered emergence and trophic dynamics]. Mesocosm experiment–derived thresholds (compound-specific HC5 values) were then applied to field data collected by the U.S. Geological Survey (USGS) from streams in five regions of the United States (Northeast, Southeast, Midwest, Pacific Northwest, and California Central Coast) as part of the USGS Regional Stream Quality Assessment (https://webapps.usgs.gov/rsqa/#!/). To our knowledge, this is the first ecological risk assessment to comprehensively investigate the effects of fipronil compounds on benthic communities in a controlled mesocosm setting and to then apply those results to a continental-scale field assessment.
METHODS
Mesocosm methods
The 30-day mesocosm experiment was run at the USGS Aquatic Experimental Laboratory (AXL), Fort Collins, CO, from 18 October to 17 November 2017 (1 day for acclimation plus a 30-day experiment). Methods have been previously described (29, 31) and are detailed in the Supplementary Materials. The mesocosm setup consisted of 36 recirculating stream mesocosms contained within four Living Streams (a recirculating water tank). Each Living Stream was equipped with a chiller to maintain water temperature and illuminated on a 16:8 light:dark cycle. The stream mesocosms were stainless steel, suitable for the hydrophobicity of fipronil (log Kow = 4.0) and for use with organic cleaning solvents (fig. S1). Water for the mesocosm experiment was collected from the Cache La Poudre River (headwaters include Rocky Mountain National Park, National Forest, and the Continental Divide) and stored in four polyethylene tanks at the AXL. Previous assessment of sediment and water samples collected from this site returned no pesticide detections (29).
The mesocosm experimental design consisted of 30 treatment streams and 6 control streams. Treatment streams received treated water, each consisting of an unreplicated constant concentration of a fipronil compound: fipronil (Sigma-Aldrich, CAS 120068-37-3), amide (Sigma-Aldrich, CAS 205650-69-7), desulfinyl [U.S. Environmental Protection Agency (EPA) Pesticide Repository, CAS 205650-65-3], sulfone (Sigma-Aldrich, CAS 120068-37-2), and sulfide (Sigma-Aldrich, CAS 120067-83-6); all purities were ≥97.8%. Nominal exposure concentrations ranged from 0.002 to 15.625 μg/liter (fipronil and amide) and from 0.001 to 3.125 μg/liter (desulfinyl, sulfone, and sulfide), chosen on the basis of published response values (7, 15, 16, 18, 21, 23, 25, 32, 33). Concentrated stock solutions were prepared by dissolving a fipronil compound in methanol (Thermo Fisher Scientific, certified American Chemical Society grade) and diluting with deionized water to the desired volume. Because of the different amounts of methanol in a dose, methanol was added to all treatment stream mesocosms and to three of the control mesocosms as needed to ensure the same methanol concentration (0.05 ml/liter) among stream mesocosms. The remaining three control stream mesocosms received river water, no methanol, and were otherwise treated as all other streams.
Temperature, pH, conductivity, and fipronil and fipronil degradates were measured in the stream mesocosms on days 8, 16, and 26. To track degradation of the parent compound fipronil over the duration of the mesocosm experiment, the fipronil (parent) treatment stream mesocosms were sampled three additional days [days 5, 12, and 21 (n = 6)] for temperature, pH, conductivity, fipronil, and the fipronil degradates. Samples for pesticide analysis were collected by filtering 10 ml of stream water through a Whatman 0.7-μm GF/F syringe filter equipped with a large-bore needle into a 20-ml amber glass vial. Samples were immediately frozen and sent to the USGS National Water Quality Laboratory (NWQL), Lakewood, CO, for analysis. Fipronil and the four degradates were measured in water samples by direct aqueous-injection (DAI) liquid chromatography–tandem mass spectrometry (LC-MS/MS; Agilent 6495) using a modification of a previously published method (34). Instrument detection levels (IDLs) were estimated as the lowest calibration standard that met qualitative identification criteria; IDLs were 0.005 μg/liter for fipronil amide and 0.001 μg/liter for the other four fipronil compounds. A complete description of the method used to measure fipronil compounds, including quality control and assurance procedures (e.g., sample recoveries, spikes, third-party checks, and blanks), is provided in the Supplementary Materials.
Enumeration and identification of adult and larval invertebrates, major data collection endpoints, were completed at the end of the 30-day mesocosm experiment. Emergent adult insects were collected daily from nets and frozen in clean 15-ml Falcon centrifuge tubes. At the end of the experiment (day 30), the contents of each stream mesocosm were scrubbed to dislodge any invertebrates and were sieved (250 μm) and preserved in 80% ethanol. Taxonomic identification of larval and adult invertebrates was completed by Timberline Aquatics (Fort Collins, CO) to the lowest taxonomic level possible, typically species. Chlorophyll a was measured in every stream mesocosm on days 9, 19, and 29 in triplicate. All chemical and biological data generated as part of the mesocosm experiment are provided in the companion data release (35).
Field methods
Ecological surveys, with pesticides monitored during a prior index period, were conducted in small (wadeable) streams in five major U.S. regions. Briefly, 77 to 100 sites in each region (444 sites total) were selected on the basis of both agricultural and urban land uses (36–40). One water sample was collected weekly at each site during spring-summer of 1 year (2013–2017) for an index period of 4 to 12 weeks, depending on region and development intensity, except for 11 Northeast sites with little development in the watershed at which only one sample was collected. Because the monitoring period for pesticides varied among regional studies, for purposes of comparison, only the last four samples collected at each site are considered here; the single samples collected at the undeveloped Northeast sites (n = 11) are assumed to be representative of this 4-week sampling period. This approach results in a consistent number of observations of pesticides (except for the 11 sites in the Northeast) and a consistent duration of observations; 4 weeks was considered long enough for chronic exposures to biota and short enough that ecological communities should not have recovered from the exposures.
Where streamflow was sufficient, water samples were collected by an isokinetic, equal-width increment method (41). When streamflow was insufficient for this method, samples were collected either by a depth-integrated sample or by a grab from the centroid of flow. A large-bore syringe and disk filter (0.7 μm) were used to collect 10 ml of filtered sample (42). Water samples were analyzed at the NWQL for 225 pesticides and pesticide degradates, including fipronil and seven degradates (desulfinylfipronil, fipronil sulfide, fipronil sulfone, dechlorofipronil, desulfinylfipronil amide, fipronil amide, and fipronil sulfonate), by DAI LC-MS/MS (34). Typical minimum reporting levels in the field study were 0.004 μg/liter for fipronil, desulfinylfipronil, fipronil sulfide, fipronil sulfone, and dechlorofipronil; 0.009 μg/liter for desulfinylfipronil amide and fipronil amide; and 0.096 μg/liter for fipronil sulfonate.
Invertebrate communities were sampled at the end of each regional study (spring/summer), typically, coincident with the last pesticide sampling event. Sampling was timed to coincide with low-flow conditions following the growing season and high pesticide use and with the time when stream invertebrate communities are mature and predominantly in the larval life stage. Invertebrate community sampling was completed at 437 of the 444 sites using a Surber sampler or D-frame net with 500-μm mesh openings. Sampling methods are described in detail in the Supplementary Materials. All invertebrates were identified and enumerated generally at either genus or species level at the NWQL. All chemical and biological data collected in the field and used in this manuscript can be found in the companion data release (35).
Data analysis
Effect concentrations at which there was a 20 or 50% reduction in larval invertebrates relative to controls (i.e., EC20 and EC50) were calculated for each of the five fipronil compounds used in the mesocosm experiment. The data [x = time-weighted fipronil concentration (see the Supplementary Materials for details), y = larval abundance or other metric] were fit in R (43) extension package “drc” using a three-parameter logistic regression (44). Curves were fit to all (larvae) individual species with adequate abundance and to additional metrics of interest (e.g., taxa richness, total mayfly abundance, and total abundance) to further understand community effects. Model fit was assessed using the Nash-Sutcliff coefficient (45), where a poor model fit can receive infinitely negative values and a perfect fit receives a value of 1.
To explore the effects of fipronil compounds on insect emergence in the experiment, data were evaluated in two ways. First, the cumulative daily emergence (count of all individuals) of insects from each stream mesocosm was normalized to controls by subtracting the mean emergence in the control stream mesocosms from the emergence in each treatment stream mesocosm. These values were plotted against time to understand how emergence in the treatment stream mesocosms deviated from the control stream mesocosms over the 30-day experiment. Second, the percent total emergence for each stream mesocosm, defined as the ratio of the total number of emergers from a given stream mesocosm to the mean number of larvae and adults in the controls, was calculated and fit to a three-parameter logistic regression. All emergent insects collected were from two subfamilies of the family Chironomidae and therefore were combined for analysis.
Changes to community structure, such as taxa loss, can culminate from direct and indirect effects of a toxicant and can result in changes to community function (e.g., a trophic cascade). To test for a trophic cascade, a simple network of cause-effect relationships was evaluated using a path-analytic approach (R package “piecewiseSEM”) (46). For the mesocosm experiment, the presence of fipronil, desulfinyl, sulfide, and sulfone (amide was not tested) in water was hypothesized to reduce the biomass of scrapers, indirectly causing an increase in the biomass of chlorophyll a (47). Compound concentrations were the predictor variables, and scraper and chlorophyll a biomass were response variables. The Fisher’s C statistic was used to assess model fit such that P values of <0.05 indicate a good model fit (46).
To develop a risk-based threshold protective of ecological communities, chronic species sensitivity distributions (SSDs) and hazard concentrations protective of 95% of the affected species (HC5) were derived for each compound. Three SSD datasets were generated: (i) a mesocosm-only dataset, (ii) a dataset inclusive of all the mesocosm data combined with that collected from a query of the EPA ECOTOX database (https://cfpub.epa.gov/ecotox/, accessed 14 March 2019) with a study duration of 4 days or longer, and (iii) a dataset inclusive of all the mesocosm data and the ECOTOX data where the ECOTOX data (acute exposures) were divided by the acute-to-chronic ratio for D. magna (19.39) as a means to account for differences in exposure duration and to approximate chronic EC50 values (12). Our purposes in generating multiple SSD models were (i) to develop HC5 values for comparison with field data (mesocosm-only SSD) and (ii) to evaluate the robustness of mesocosm data relative to data resources more broadly accepted by regulators for inclusion into aquatic life benchmarks and criterion development and thus the utility of mesocosm studies for use in regulatory processes.
SSDs were developed for each dataset using the R package “ssdtools” (48); mean HC5 values and confidence intervals (CIs) were estimated from the SSDs using a bootstrapping (n = 10,000) procedure. Forty-nine taxa responses developed from this study (all taxa identified to genus or species) were combined with 32 taxa responses compiled from six published studies found in the ECOTOX database, combining for a total of 81 taxa responses for use in SSD development. No SSD was developed for amide, as no data were found in the ECOTOX database for amide, and only one EC50 response was derived from the current study. While only 1 EC50 for desulfinyl was found in the ECOTOX database, 12 EC50 values were generated by the current study; therefore, SSDs were developed for desulfinyl.
The fipronil compound-specific HC5 values derived from the mesocosm-only SSD dataset were combined with field data to estimate exposure and potential toxicity to fipronil compounds in the 444 streams from the five U.S. regions assessed. Each detected concentration (nondetections treated as zeros) of a fipronil compound during the final 4-week sampling window was divided by its respective HC5, and the compound ratios for each sample were summed to obtain the total toxic units for fipronil(s) (ΣTUFipronils) for that sample, where ΣTUFipronils > 1 indicates toxicity.
The degree to which the SSD derived from the mesocosm data reflects the broader ecological community sensitivity to fipronil(s) was evaluated by comparing the hazard concentration for 50% of affected species (HC50) to the EC50 value for taxa richness developed from the mesocosm experiment. This comparison allows evaluation of the agreement between the SSD approach, which includes only those taxa responses with dose-response relationships, and the EC50 approach using the metric taxa richness, which includes all unique taxa observed in the mesocosms, including those that did not have a dose-response relationship.
A Species at Risk for pesticides (SPEARpesticides) metric was calculated to investigate the relation between the health of the invertebrate community and ΣTUFipronils at the 437 stream sites where invertebrates were collected. The SPEARpesticides metric converts invertebrate composition into an abundance metric for taxa with physiological and ecological traits that confers susceptibility to pesticides. The SPEARpesticides metric is insensitive to natural covariates (49, 50), although its performance can be affected by severe habitat degradation (51). The field-collected abundance data for each taxonomic unit were harmonized with the taxa key values associated with the ASTERICS software for assessing the ecological quality of rivers (https://gewaesser-bewertung-berechnung.de/index.php/home.html). The data then were imported into the Indicate (http://systemecology.eu/indicate/) software (version 18.05) where, using a European trait database and a database of physiological sensitivity to pesticides, the data at each site were converted into a SPEARpesticides metric. Generalized additive models (GAMs) [“mgcv” package in R (52)] were used to explore associations between the SPEARpesticides metric and ΣTUFipronils [log10(X + 1)–transformed] for each of the five regional studies. Further details on the SPEARpesticides metric and for data analysis can be found in the Supplementary Materials.
RESULTS
Mesocosm results
Water quality metrics were consistent across each stream mesocosm and throughout the duration of the mesocosm experiment. Mean temperature, pH, and conductivity were 13.1°C (± 0.27°C), 7.8 (± 0.12), and 54.1 (± 2.1) μS/cm, respectively (35). Dissolved organic carbon measured in the clean river water was 3.1 mg/liter, and dissolved oxygen was close to saturation in the subset of stream mesocosms deployed with MiniDOT loggers (mean > 8.0 mg/liter), indicating sufficient stream circulation.
Quality control and quality assurance data on fipronil(s) are presented in the companion data release (35). Briefly, laboratory matrix spike and mesocosm sample recoveries were generally within acceptable limits (70 to 130% recovery), IDL standards confirmed the quantitation method, and laboratory and instrument blanks were generally clean, with the few exceptions to these generalizations discussed in the Supplementary Materials.
Measured concentrations of fipronil(s) were generally below target (fig. S2), as expected because of system design (4 to 10 days are necessary to achieve steady state under ideal conditions) (30). Concentrations of desulfinyl and amide were less variable over time than the other fipronil compounds, and, except for low-concentration treatments for sulfone and sulfide, within-treatment variability in concentration was less than differences among treatments. Time-weighted average measured concentration ranges for each treatment group were as follows: fipronil, IDL to 9.07 μg/liter; desulfinyl, IDL to 2.15 μg/liter; amide, IDL to 4.17 μg/liter; sulfide, IDL to 0.57 μg/liter; and sulfone, IDL to 1.13 μg/liter (35). Some stream mesocosms had detections of nontarget fipronil compounds, i.e., a compound not spiked into a particular treatment, but known to be a degradate of the treatment compound. Mesocosms treated with the parent compound fipronil had the greatest number of nontarget degradate detections (desulfinyl, amide, sulfide, and sulfone, when not used as the treatment compound); these likely are the result of compound impurity arising during manufacturing, and (or) degradation processes occurring during storage of stock solutions and (or) during the mesocosm experiment, rather than cross contamination. There were no trends in degradate concentrations observed in the fipronil treatment. Nontarget degradate compounds were detected most frequently in stream mesocosms with the highest treatment concentrations but at concentrations less than the effect concentrations for these nontarget compounds (see effect concentrations in the next section). Thus, because nontarget degradate compounds generally were not detected in the lowest fipronil treatments and because detections were less than effect concentrations in the highest treatments, the effect of these nontarget compounds on analysis was concluded to be minimal.
Benthic macroinvertebrates in the mesocosm experiment were sensitive to fipronil, desulfinyl, sulfone, and sulfide [table S1; raw abundance data available in the companion data release (35)]. Fipronil amide was toxic (lethal) only to the mayfly Rhithrogena sp., which had an EC50 of 2.05 μg/liter [±10.8 (SE)]. Dose-response curves were generated for the 15 unique taxa that exhibited mortality within the range of concentrations tested (table S1) and for aggregated taxonomic groups such as mayflies (fig. S3) and taxa richness (Fig. 1). Ranges of effect concentrations (EC50) for unique taxa were 0.005 to 0.364, 0.002 to 0.252, 0.002 to 0.061, and 0.005 to 0.043 μg/liter for fipronil, desulfinyl, sulfone, and sulfide, respectively, with the most sensitive taxa (e.g., Rhithrogena sp. and Sweltsa sp.; fig. S4) affected at lower concentrations than the more tolerant taxa (e.g., Micropsectra/Tanytarsus and Lepidostoma sp.) (table S1). On the basis of the mean of EC50 values presented for each compound in table S1, sulfone and sulfide were the most potent compounds, whereas invertebrates were generally least sensitive to desulfinyl (amide excluded). Aggregate measures of ecological condition, such as taxa richness, total abundance, total mayflies, and total stoneflies, included taxa and abundances for some taxa so rare in the mesocosms that individual dose-response curves could not be calculated. Thus, these ecological metrics include taxa responses not included in the SSD.
Fig. 1. Response of larval aquatic macroinvertebrates to fipronil and fipronil degradates in a mesocosm experiment.
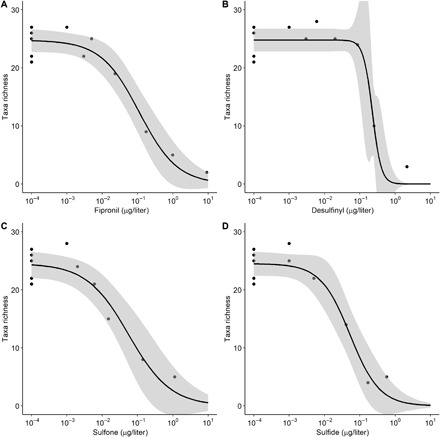
Taxa richness (larvae) as a function of (A) fipronil, (B) desulfinyl, (C) sulfone, and (D) sulfide concentration fitted with a three-parameter logistic function. Each data point represents larvae from an individual stream mesocosm at the end of the 30-day mesocosm experiment. Taxa richness is a count of unique taxa within each stream. Concentration values are time-weighted averages of observed concentrations in each stream mesocosm measured at the end of the 30-day experiment. Fipronil amide (not shown) did not have a relation with taxa richness. Note that the x axes are on the log scale. EC20 and EC50 with SEs are reported in table S1.
Chironomidae emergence rates declined at the highest concentrations of all five fipronil compounds tested; a 50% decline in percent emergence (EC50) was observed at concentrations of 0.03, 0.06, 0.11, 0.78, and 0.97 μg/liter for sulfide, sulfone, fipronil, amide, and desulfinyl, respectively (Fig. 2 and fig. S5). Emergence was delayed in all fipronil, desulfinyl, sulfone, and sulfide treatments for most of the 30-day experiment and was suppressed except in some low-concentration treatments (Fig. 2). In the amide treatments, cumulative emergence was greater than the controls for the 0.286 μg/liter concentration throughout the experiment, the highest concentration (4.164 μg/liter) suppressed emergence throughout the experiment, and the intermediate treatments had emergence similar to that of the controls (Fig. 2).
Fig. 2. Cumulative emergence of adult aquatic insects (Chironomidae) from mesocosm experimental treatments.
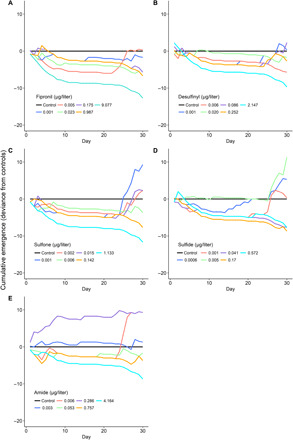
Cumulative emergence is the mean cumulative daily insect emergence from each treatment minus the mean cumulative daily emergence from control stream mesocosms for (A) fipronil, (B) desulfinyl, (C) sulfone, (D) sulfide, and (E) amide. n = 1 except for control (n = 6). Concentration values are time-weighted averages of observed concentrations in each stream mesocosm.
Dose-response curves showed structural changes at the community level in addition to taxa losses. Specifically, mayfly abundance (fig. S3) and taxa richness (Fig. 1) showed a clear dose-response relation to fipronil, desulfinyl, sulfone, and sulfide within the concentration range tested. We therefore explored how these structural changes might result in changes in community function by testing for a trophic cascade. Aquatic invertebrate exposure to fipronil, desulfinyl, sulfide, and sulfone caused direct negative effects on scraper biomass (Fig. 3). Controlling for this negative effect of fipronil(s) on scraper biomass, scrapers also negatively affected chlorophyll a biomass (Fig. 3). The result of these negative path coefficients was net increases in chlorophyll a as fipronil and degradate concentrations increased. These fully mediated path models indicate that an increase in fipronil or fipronil degradate results in a proportionate increase in chlorophyll a (Fig. 3). The direct effect between fipronil or degradate concentrations and chlorophyll a biomass was a priori hypothesized to be zero because fipronil compounds are insecticides, with low direct toxicity to algae (for example, the EPA acute nonvascular plant benchmark is 100 μg/liter for fipronil, desulfinyl, sulfone, and sulfide; https://epa.gov/pesticide-science-and-assessing-pesticide-risks/aquatic-life-benchmarks-and-ecological-risk), and all results (significant models) supported this assumption.
Fig. 3. Structural equation models for indirect effects of fipronil, sulfide, desulfinyl, and sulfone on scraper and chlorophyll a (Chl a) biomass.
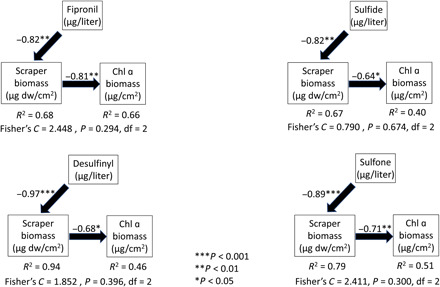
Fipronil(s) significantly reduced the biomass of grazing (scraper taxa as larvae) taxa (direct effect) but had no direct effect on the biomass of chlorophyll a. However, a strong indirect effect of fipronil(s) was that the biomass of chlorophyll a increased in response to less grazing. Arrows indicate standardized path coefficients, and negative signs (−) indicate direction of association. * indicates level of significance.
The three SSDs (mesocosm-only, mesocosm plus ECOTOX data, and mesocosm plus ECOTOX data corrected for differences in exposure duration) yielded nominally different HC5 values (table S3), but results were within an SE of one another. We focus on the mesocosm-only data SSD and the associated HC5 values for the remainder of the study. Refer to the Supplementary Materials for a more complete description of the three SSD evaluations (tables S2 to S5 and figs. S6 and S7). The best-fit data distributions (lowest Akaike Information Criterion score) used in the mesocosm-only SSD plots for the four fipronil compounds (Fig. 4) were the log-gumbel for fipronil and sulfone, weibull for sulfide, and gamma for desulfinyl (table S3). HC5 values derived for each compound are reported in Fig. 4 for mesocosm-only data and in table S3 where HC5 values from all three SSD datasets are reported. The HC50 values for fipronil, sulfide, sulfone, and desulfinyl [22.1 ± 8.78 ng/liter (95% CI, 11.4 to 46.2), 16.9 ± 3.38 ng/liter (95% CI, 11.2 to 24.0), 8. 80 ± 2.66 ng/liter (95% CI, 5.44 to 15.8), and 83.4 ± 32.9 ng/liter (95% CI, 36.4 to 163), respectively] were substantially lower than the EC50 values for taxa richness (total number of unique taxa) for those compounds (table S1; note reported as micrograms per liter in the Supplementary Materials table).
Fig. 4. Species sensitivity distributions for larval macroinvertebrates exposed to fipronil and fipronil degradates in a mesocosm experiment.
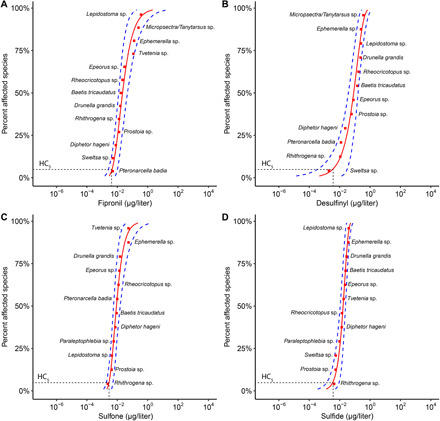
Species sensitivity is depicted as taxa EC50 values when exposed to (A) fipronil, (B) desulfinyl fipronil, (C) fipronil sulfone, (D) fipronil sulfide for 30-days in a mesocosm experiment. Blue dashed lines indicate 95% CIs. Horizontal dashed lines indicate the HC5. HC5 values in nanograms per liter derived for each compound were as follows: fipronil, 4.56 ng/liter (95% CI, 2.59 to 10.2); sulfide, 3.52 ng/liter (1.36 to 9.20); sulfone, 2.86 ng/liter (1.93 to 5.29); and desulfinyl, 3.55 ng/liter (0.35 to 28.4). Note that the x axes are on the log scale.
Field results
Fipronil (parent) was detected at 22% of the 444 field sampling sites from the five regional studies (Table 1). Fipronil, sulfone, and amide were detected with similar frequencies (18 to 22% of samples), sulfide and desulfinyl less often (11 to 13%), with the remaining degradates detected rarely (1% or less) or never (Table 1). Fipronil was most frequently detected in the Southeast (52% of sites) and least frequently in the Northwest (9% of sites), highlighting the variability in phenylpyrazole use and potential stream vulnerability across the country. Degradates generally showed a similar regional pattern, with the highest detection frequencies in the Southeast and lowest in the Northwest or Coastal California. Measured concentrations were highest for fipronil amide, followed by the parent compound fipronil (90th percentile = 10.8 and 6.3 ng/liter, respectively) (Table 1) (35). The highest maximum concentrations (in the last 4 weeks of samples) were measured in the Southeast for fipronil (61.4 ng/liter), desulfinyl (10.6 ng/liter), and sulfide (8.0 ng/liter), and in the Midwest for sulfone (15.7 ng/liter), amide (42.7 ng/liter), desulfinylfipronil amide (14 ng/liter), and fipronil sulfonate (8.1 ng/liter) (35). Fipronil sulfone was the only compound observed at concentrations that exceeded the HC5 (Table 1). Mean ΣTUFipronils ranged widely among regions (Table 1). Nationwide mean ΣTUFipronils was 0.62 (all sites, all regions), while 71 sites (16%) had ΣTUFipronils > 1, indicating likely toxicity to benthic macroinvertebrates. There was a significant relation between SPEARpesticides and ΣTUFipronils in four of the five regions studied (all but the Midwest), with adjusted R2 ranging from 0.07 in Coastal California to 0.34 in the Southeast (Fig. 5).
Table 1. Characteristics (90th percentile concentration, detection frequency, and potential toxicity) of fipronil compounds detected in 444 wadeable streams across the continental United States by region (35).
*Compounds used in the mesocosm experiment. †ΣTUFipronils, median of the sum of toxic units [observed field concentrations for four fipronil compounds/hazard concentration for the fifth percentile of affected species from the SSDs (Fig. 4) for each compound] for fipronil(s) calculated for weekly samples from the last 4 weeks of pesticide samples collected at each site. ‡Number of sites where pesticides were measured. §The 90th percentile was derived from the site maximum observed concentration during the last 4 weeks of pesticide sampling. ║Percentage of samples with detections. ¶The 95% CIs for HC5 values (Fig. 4 and table S3, mesocosm-only) were used to calculate CIs. Dechlorofipronil was analyzed in all regions and never detected. ND, not detected.
| Fipronil* |
Desulfinyl fipronil* |
Fipronil sulfide* |
Fipronil sulfone* |
Fipronil amide* |
Desulfinyl fipronil amide |
Fipronil sulfonate |
Mean ΣTUFipronils† |
% Sites > ΣTUFipronils† = 1 |
Number of sites‡ |
|
| All regions, 90th percentile concentration (ng/liter)§ |
6.3 | 2.2 | 2.0 | 4.2 | 10.8 | ND | ND | |||
| All regions (year)║ |
22% | 11% | 13% | 20% | 18% | 1% | 0.1% | 0.62 (0.27–1.62)¶ | 16% (9–20%)¶ | 444 |
| Midwest (2013)║ |
14% | 9% | 6% | 9% | 5% | 1% | 0.3% | 0.32 (0.14–0.96) | 5% (4–9%) | 100 |
| Southeast (2014)║ |
51% | 30% | 40% | 57% | 56% | 3% | ND | 1.82 (0.80–4.92) | 51% (32–55%) | 77 |
| Northwest (2015)║ |
9% | 3% | 4% | 14% | 2% | ND | ND | 0.13 (0.06–0.28) | 6% (0–9%) | 87 |
| Northeast (2016)║ |
19% | 10% | 11% | 17% | 21% | 0.3% | ND | 0.62 (0.27–1.56) | 14% (8–16%) | 95 |
| Coastal California (2017)║ |
19% | 4% | 8% | 11% | 14% | ND | ND | 0.40 (0.18–0.85) | 12% (6–18%) | 85 |
Fig. 5. Species at Risk pesticides metric (SPEARpesticides) versus the sum of toxic units for fipronil(s) (ΣTUFipronils) at streams sampled in five regional studies (36–40).
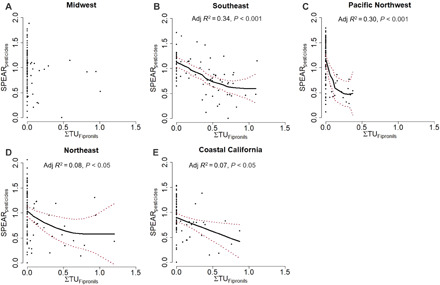
Fipronil toxic units are the measured fipronil(s) concentrations divided by compound-specific HC5 values determined from SSDs (see Fig. 4) derived from the mesocosm experiment. Black lines, generalized additive model (GAM). Red dashed lines, 95% CIs of GAM. ΣTUFipronils are transformed as log10(ΣTUFipronils + 1).
DISCUSSION
The adverse effects of fipronil on nontarget aquatic species are well documented (15, 21, 24, 25, 32, 33), but this is the first study in which communities of sensitive taxa were exposed to fipronil compounds in a controlled laboratory setting and the results were extrapolated at the continental scale. The results of the 30-day mesocosm experiment were able to generate effect concentrations not previously reported in the literature for 15 discrete aquatic insect taxa (table S1), where aquatic insects are notedly underrepresented in toxicity databases (53, 54). Taxon-specific dose-response curves (e.g., EC50) are reflected in community-level changes, such as losses in taxa richness and mayfly abundance, and in functional changes (e.g., trophic cascade and altered emergence). Mesocosm-derived effects were extrapolated to the field, where field-measured fipronil concentrations were correlated with declines in aquatic ecosystem condition in wadeable streams in four of five U.S. regions studied.
The HC5 values protective of 95% of species from the mesocosm experiment indicate that aquatic macroinvertebrate communities overall are substantially more sensitive to fipronil compounds than previously understood. The HC5 values derived (fipronil, 4.56 ng/liter; desulfinyl, 3.55 ng/liter; sulfone, 2.86 ng/liter; and sulfide, 3.52 ng/liter) are a few times (fipronil) to more than three orders of magnitude (desulfinyl) lower than the current EPA chronic invertebrate benchmarks [fipronil, 11 ng/liter; desulfinyl, 10,310 ng/liter; sulfone, 37 ng/liter; and sulfide, 110 ng/liter (8)]. The mesocosm experiment identified numerous taxa that are more sensitive to fipronil(s) than indicated by the EPA chronic invertebrate benchmarks (4 taxa more sensitive to fipronil, 13 to desulfinyl, 11 to sulfone, and 13 to sulfide) (Fig. 4 and table S1). This indicates that the benchmarks are not protective of several species observed in the mesocosms, species that also occur ubiquitously in aquatic ecosystems. The disparities between our results and current benchmarks largely result from a lack of fipronil toxicity test data available for a range of aquatic insect taxa, especially for exposures longer than 4 days and for the fipronil degradates. Most of the insects in the invertebrate communities used in the 30-day mesocosm experiment are more sensitive to fipronil(s) than the common test organism H. azteca (a crustacean), even after correcting EC50s for H. azteca to convert from an acute (typically 96 hours) to a chronic duration of exposure (fig. S7). Better agreement occurred between the mesocosm experiment and studies reported in ECOTOX that used the standard test organism Chironomus dilutus, an insect. It should be no surprise that aquatic insects are particularly sensitive to insecticides. Where no adjustment was made for exposure duration, combined data from the mesocosm experiment and the ECOTOX database showed that numerous taxa were observed to be more sensitive to fipronil compounds than C. dilutus (fig. S6). However, with adjustment for the duration of exposure, C. dilutus was the organism most sensitive to fipronil (parent) and sulfide, although not to sulfone (fig. S7). These results illustrate the importance of including a broad range of types of aquatic organisms, including a diversity of insects, to generate realistic insecticide concentrations that are protective of aquatic life.
The SSD approach may be protective of rare or insensitive taxa for which an EC50 could not be determined, e.g., Cinygmula sp., Isoperla fulva, and Brachycentrus americanus. The EC50 values for taxa richness and mayfly abundance, which reflect changes in community composition, were concordant with the HC50 values from the SSDs for fipronil, sulfone, and sulfide. This agreement supports the idea that the SSD approach for deriving thresholds can be protective of entire communities, including rare or insensitive taxa present in the community. Aquatic life thresholds determined from SSDs that are based on only a small number of taxa or insensitive taxa may be greatly underprotective of aquatic ecosystems. This is the case for desulfinyl (fig. S6B), for which a dearth of data in the ECOTOX database results in the EPA chronic invertebrate benchmark of 10,310 ng/liter, about four orders of magnitude higher than the HC5 of 3.55 ng/liter that results from the diverse set of taxa responses generated in the mesocosm experiment. The lack of toxicity data is particularly problematic for the degradate compounds (fig. S6) and may explain why existing aquatic life benchmarks for sulfone and sulfide are about 15 to 30 times less sensitive, respectively, than mesocosm-based SSD HC5 values. A benefit of the mesocosm approach is the determination of multiple EC50 values in a single experiment sufficient to comprise an entire SSD (e.g., desulfinyl; Fig. 4B and figs. S6B and S7B) with numerous responses for taxa native to the ecosystem to be protected.
The mesocosm experiment demonstrated that fipronil and its degradates can have marked sublethal and indirect adverse effects on community function. Insect emergence in the mesocosm experiment was affected by all five fipronil compounds tested. The contrasting results between the highest and lowest concentrations—suppression and stimulation of emergence of individuals or a change in the timing of emergence—are consistent with results reported previously for mesocosm experiments with the insecticide bifenthrin (29). Emergence of adult insects provides important ecological functions that can be altered by contaminants like fipronil(s) (55, 56). Synchronous emergence is essential not only for insect reproduction and population persistence but also as a supply of mature insects that serve as food to aquatic and terrestrial animals (56). Prevention of emergence can adversely affect the exchange of food between aquatic and riparian ecosystems and propagate the effects of aquatic contaminants into terrestrial ecosystems (55, 56). The decrease in the abundance of scrapers (algae-eating insects) observed in the mesocosm experiment resulted in an increase in chlorophyll a because of reduced consumption of algae (Fig. 3). This trophic cascade, which altered carbon and nitrogen flow within the stream food web, is similar to that observed in a study that evaluated the effects of the pyrethroid bifenthrin on benthic communities (29). Thus, phenylpyrazoles, such as fipronil and its degradates, pyrethroids, and perhaps other classes of insecticides may contribute indirectly to increases in algal biomass and to other effects of carbon and nitrogen perturbations in small streams, which may extend to disruption of carbon and nitrogen cycling between aquatic and terrestrial ecosystems.
The information gained from the mesocosm experiment allowed us to assess the ecological relevance of concentrations of fipronil compounds measured in a large-scale field study in five regions of the United States. Seventeen percent of the 444 small streams sampled had an average (mean value over a 4-week period) concentration of one or more fipronil compounds in excess of an HC5 value derived from the mesocosm experiment. The SSDs from the mesocosm experiment were used to convert measured fipronil compound concentrations to a toxicity-relevant metric, the sum of toxic units (ΣTUFipronils), for which a value of 1 indicates toxicity or cumulative fipronil compound exposures in excess of values known to protect 95% of species. The significant relation between ΣTUFipronils and the SPEARpesticides metric of invertebrate community health in four of the five regions indicates that fipronil may be adversely affecting benthic invertebrate communities in streams from multiple regions of the United States. These results support the supposition of Wolfram et al. (3) that phenylpyrazole insecticide risks to U.S. surface waters are underappreciated because effects to aquatic insects occur below current regulatory thresholds.
Most of the streams where fipronil(s) exceeded toxic levels were in the relatively urbanized Southeast region (https://webapps.usgs.gov/rsqa/#!/region/SESQA). Previous assessments of this region have not only concluded that fipronil was a primary stressor affecting invertebrate community structure in small streams but also cited low dissolved oxygen, elevated nutrients, flow alteration, habitat degradation, and other pesticides and classes of contaminants as important stressors (57). This mix of stressors is consistent with “urban stream syndrome,” the commonly observed degradation of stream ecosystems associated with urban land use (58, 59). The Southeast region has a growing urban land-use signature that is projected to increase as the population of the region grows. Future urban development and effects from pesticides in urban runoff are forecast to increase (4). If urbanization and fipronil use continue to grow, urban uses of this insecticide may increasingly affect stream communities. Although meta-analyses have concluded that agricultural insecticide use threatens stream ecosystems globally (2, 60), we postulate that these assessments have underestimated the total global impact of insecticides by excluding urban uses.
Multiple stressors including pesticides can affect macroinvertebrate communities in developed watersheds (urban, agricultural, and mixed land uses) and may be correlated with land use (58, 59, 61). While this study used the SPEARpesticides metric and fipronil(s) toxicity signature specific to aquatic organisms to minimize the influence of confounding factors, the performance of the SPEARpesticides metric can be influenced by degraded habitat and fipronil(s) can be correlated with other pesticides (4, 17, 51, 57). However, multistressor models developed using field measurements from the first two regional studies (Midwest and Southeast) showed that insecticides were important instream stressors for macroinvertebrate community condition in wadeable streams. Important explanatory variables in these models included pesticides (especially bifenthrin), nutrients, and habitat characteristics in largely agricultural streams of the Midwest and pesticides (especially fipronil), dissolved oxygen, nutrients, and flow alteration in largely urban streams in the Southeast (61, 62). Thus, while the regional studies attempted to address the influence of non-pesticide stressors on the response metrics and tailored the predictive metric to describe the effects due to fipronil(s), the field results in the present investigation support the idea that fipronil(s) should be considered among the most influential stressors of U.S. streams, particularly in the southeastern United States.
The occurrence of pesticide degradates in the environment is poorly documented but may present a greater threat to aquatic organisms than the more commonly measured parents. In the case of fipronil, the field study and mesocosm experiment demonstrate that the degradates are as pervasive in the streams sampled as the parent and are equally or more toxic (Table 1). Fipronil sulfone, indicated by the mesocosm experiment to be one of the most toxic of the pesticide degradates studied and more toxic than the parent compound, was detected in streams at a frequency similar to that of the parent. Potentially toxic events may go unnoticed if only parent pesticides are measured, and the relative lack of toxicity information on pesticide degradates means that their occurrence and consequences, may be overlooked. For example, a comprehensive assessment of pesticides in Swiss streams that included 134 pesticide degradates considered only parent compounds in their ecotoxicological risk assessment because of insufficient information on the toxicity of the degradates (63).
The results of this ecological risk assessment indicate that fipronil compounds adversely affect stream health, and it is reasonable to infer that adverse effects can be observed anywhere fipronil compounds exceed the HC5 levels reported here. The mesocosm experiment results, which are independent of location, indicate that many stream taxa are sensitive to a much lower concentration of fipronil and its degradates than previously documented. We believe that this finding likely can be extended to native taxa from pristine streams in any location. The application of the mesocosm experiment results to a large-scale field study—444 small streams across five major U.S. regions composed of urban, agriculture, and mixed land uses—and the finding that many streams in which fipronil was detected had concentrations predicted to cause toxicity indicates that these results may be extended to other countries in which fipronil is in use. Fipronil use has been reported to be increasing in Japan and Great Britain, as well as in the United States (7). Fipronil is used on virtually every continent, including Australia, South America, and Africa (https://coherentmarketinsights.com/market-insight/fipronil-market-2208). The results of the mesocosm-to-field study presented here indicate that fipronil use may have ecological implications on a global scale.
Supplementary Material
Acknowledgments
We thank E. Jeffery, F. Tremblay, S. Morton, and K. Dowdy for experimental assistance and Timberline Aquatics for invertebrate identification. C. A. Anderson was essential to characterization of fipronil compounds in the mesocosm study. We also thank K. Smalling and J. Steevens for their insightful reviews of this manuscript. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. government. This document contains CAS Registry Numbers, which is a registered trademark of the American Chemical Society. CAS recommends the verification of the CASRNs through CAS Client ServicesSM. Funding: This research was funded by U.S. Geological Survey National Water Quality Assessment Project including a contract to the Colorado Water Center (G19-000810) that, in part, funded J.L.M. Author contributions: J.L.M. and T.S.S. are co–lead authors contributing equally to the writing and statistical analysis presented and in the development and execution of the mesocosm experiment. P.C.V.M. was the Regional Stream Quality Project Leader and contributed to the design of the mesocosm experiment. T.S.S., P.C.V.M., B.J.M., M.W.S., L.H.N., D.M.C., and P.W.M. all contributed to the design and execution of the field study and contributed to data interpretation and writing of the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. All chemical and biological data used in this manuscript can be found in the companion data release (35). Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/43/eabc1299/DC1
REFERENCES AND NOTES
- 1.Bernhardt E. S., Rosi E. J., Gessner M. O., Synthetic chemicals as agents of global change. Front. Ecol. Environ. 15, 84–90 (2017). [Google Scholar]
- 2.Stehle S., Schulz R., Agricultural insecticides threaten surface waters at the global scale. Proc. Natl. Acad. Sci. U.S.A. 112, 5750–5755 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfram J., Stehle S., Bub S., Petschick L. L., Schulz R., Meta-analysis of insecticides in United States surface waters: Status and future implications. Environ. Sci. Technol. 52, 14452–14460 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Van Metre P. C., Waite I. R., Qi S., Mahler B., Terando A., Wieczorek M., Meador M., Bradley P., Journey C., Schmidt T., Carlisle D., Projected urban growth in the southeastern USA puts small streams at risk. PLOS ONE 14, e0222714 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon-Delso N., Amaral-Rogers V., Belzunces L. P., Bonmatin J. M., Chagnon M., Downs C., Furlan L., Gibbons D. W., Giorio C., Girolami V., Goulson D., Kreutzweiser D. P., Krupke C. H., Liess M., Long E., McField M., Mineau P., Mitchell E. A. D., Morrissey C. A., Noome D. A., Pisa L., Settele J., Stark J. D., Tapparo A., Van Dyck H., Van Praagh J., Van der Sluijs J. P., Whitehorn P. R., Wiemers M., Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 22, 5–34 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Metre P. C., Alvarez D. A., Mahler B. J., Nowell L., Sandstrom M., Moran P., Complex mixtures of Pesticides in Midwest U.S. streams indicated by POCIS time-integrating samplers. Environ. Pollut. 220, 431–440 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Budd R., Ensminger M., Wang D., Goh K. S., Monitoring fipronil and degradates in California surface waters, 2008–2013. J. Environ. Qual. 44, 1233–1240 (2015). [DOI] [PubMed] [Google Scholar]
- 8.U.S. Environmental Protection Agency (U.S. Environmental Protection Agency, 2017); https://epa.gov/pesticide-science-and-assessing-pesticide-risks/aquatic-life-benchmarks-and-ecological-risk.
- 9.Morrissey C. A., Mineau P., Devries J. H., Sanchez-Bayo F., Liess M., Cavallaro M. C., Liber K., Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environ. Int. 74, 291–303 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Gibbons D., Morrissey C., Mineau P., A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environ. Sci. Pollut. Res. 22, 103–118 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Environmental Protection Agency, PRD Appendix A: Food/Feed and Non-Food/Non-Feed Uses Considered in Registration Review Work Planning (U.S. Environmental Protection Agency, 2010).
- 12.U.S. Environmental Protection Agency, Problem Formulation for the Environmental Fate and Ecological Risk, Endangered Species and Drinking Water Assessments in Support of the Registration Review of Fipronil (U.S. Environmental Protection Agency Office of Pesticide Programs, 2011).
- 13.U.S. Geological Survey, Estimated Annual Agricultural Pesticide Use, Pesticide Use Maps—Fipronil (U.S. Geological Survey, 2019).
- 14.Mize S. V., Porter S. D., Demcheck D. K., Influence of fipronil compounds and rice-cultivation land-use intensity on macroinvertebrate communities in streams of southwestern Louisiana, USA. Environ. Pollut. 152, 491–503 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Weston D. P., Lydy M. J., Toxicity of the insecticide fipronil and its degradates to benthic macroinvertebrates of urban streams. Environ. Sci. Technol. 48, 1290–1297 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Weston D. P., Chen D., Lydy M. J., Stormwater-related transport of the insecticides bifenthrin, fipronil, imidacloprid, and chlorpyrifos into a tidal wetland, San Francisco Bay, California. Sci. Total Environ. 527, 18–25 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Nowell L. H., Moran P. W., Schmidt T. S., Norman J. E., Nakagaki N., Shoda M. E., Mahler B., Van Metre P. C., Stone W. W., Sandstrom M. W., Hladik M. L., Complex mixtures of dissolved pesticides show potential aquatic toxicity in a synoptic study of Midwestern U.S. streams. Sci. Total Environ. 613–614, 1469–1488 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Gan J., Bondarenko S., Oki L., Haver D., Li J. X., Occurrence of fipronil and its biologically active derivatives in urban residential runoff. Environ. Sci. Technol. 46, 1489–1495 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Clements W. H., Small-scale experiments support causal relationships between metal contamination and macroinvertebrate community composition. Ecol. Appl. 14, 954–967 (2004). [Google Scholar]
- 20.Gunasekara A. S., Truong T., Goh K. S., Spurlock F., Tjeerdema R. S., Environmental fate and toxicology of fipronil. J. Pestic. Sci. 32, 189–199 (2007). [Google Scholar]
- 21.Maul J. D., Brennan A. A., Harwood A. D., Lydy M. J., Effect of sediment-associated pyrethroids, fipronil, and metabolites on Chironomus tentans growth rate, body mass, condition index, immobilization, and survival. Environ. Toxicol. Chem. 27, 2582–2590 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Lin K., Haver D., Oki L., Gan J., Persistence and sorption of fipronil degradates in urban stream sediments. Environ. Toxicol. Chem. 28, 1462–1468 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Hainzl D., Cole L. M., Casida J. E., Mechanisms for selective toxicity of fipronil insecticide and its sulfone metabolite and desulfinyl photoproduct. Chem. Res. Toxicol. 11, 1529–1535 (1998). [DOI] [PubMed] [Google Scholar]
- 24.Schlenk D., Huggett D. B., Allgood J., Bennett E., Rimoldi J., Beeler A. B., Block D., Holder A. W., Hovinga R., Bedient P., Toxicity of fipronil and its degradation products to Procambarus sp.: Field and laboratory studies. Arch. Environ. Contam. Toxicol. 41, 325–332 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Hayasaka D., Korenaga T., Suzuki K., Sánchez-Bayo F., Goka K., Differences in susceptibility of five cladoceran species to two systemic insecticides, imidacloprid and fipronil. Ecotoxicology 21, 421–427 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Chapman P. M., Whole effluent toxicity testing—Usefulness, level of protection, and risk assessment. Environ. Toxicol. Chem. 19, 3–13 (2000). [Google Scholar]
- 27.Conrad A. U., Fleming R. J., Crane M., Laboratory and field response of Chironomus riparius to a pyrethroid insecticide. Water Res. 33, 1603–1610 (1999). [Google Scholar]
- 28.Kimball K. D., Levin S. A., Limitations of laboratory bioassays: The need for ecosystem-level testing. Bioscience 35, 165–171 (1985). [Google Scholar]
- 29.Rogers H. A., Schmidt T. S., Dabney B. L., Hladik M. L., Mahler B. J., Van Metre P. C., Bifenthrin causes trophic cascade and altered insect emergence in mesocosms: Implications for small streams. Environ. Sci. Technol. 50, 11974–11983 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Mebane C. A., Schmidt T. S., Miller J. L., Balistrieri L. S., Bioaccumulation and toxicity of cadmium, copper, nickel, and zinc and their mixtures to aquatic insect communities. Environ. Toxicol. Chem. 39, 812–833 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt T. S., Rogers H. A., Miller J. L., Mebane C. A., Balistrieri L. S., Understanding the captivity effect on invertebrate communities transplanted into an experimental stream laboratory. Environ. Toxicol. Chem. 37, 2820–2834 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Overmyer J. P., Mason B. N., Armbrust K. L., Acute toxicity of imidacloprid and fipronil to a nontarget aquatic insect, Simulium vittatum Zetterstedt cytospecies IS-7. Bull. Environ. Contam. Toxicol. 74, 872–879 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Stark J. D., Vargas R. I., Toxicity and hazard assessment of fipronil to Daphnia pulex. Ecotoxicol. Environ. Saf. 62, 11–16 (2005). [DOI] [PubMed] [Google Scholar]
- 34.M. W. Sandstrom, L. K. Kanagy, C. A. Anderson, C. J. Kanagy, Determination of pesticides and pesticide degradates in filtered water by direct aqueous-injection liquid chromatography-tandem mass spectrometry, in National Field Manual for the Collection of Water-Quality Data: U.S. Geological Survey Techniques of Water-Resources Investigations No. Chapter B11 (U.S. Geological Survey, 2015).
- 35.J. L. Miller, T. S. Schmidt, M. W. Sandstrom, P. C. Van Metre, B. J. Mahler, L. H. Nowell, D. M. Carlisle, P. W. Moran, “Dataset for an ecological risk assessment of Fipronil compounds in U.S. streams” (Data release, U.S. Geological Survey, 2020). [DOI] [PMC free article] [PubMed]
- 36.J. D. Garrett, J. W. Frey, P. C. Van Metre, C. A. Journey, N. Nakagaki, D. T. Button, L. H. Nowell, “Design and methods of the Midwest stream quality assessment (MSQA), 2013” (No. Open-File Report 2017–1073, U.S. Geological Survey, 2017).
- 37.C. A. Journey, P. C. Van Metre, A. H. Bell, J. D. Garrett, D. T. Button, N. Nakagaki, S. L. Qi, P. M. Bradley, “Design and methods of the southeast stream quality assessment (SESQA), 2014” (No. Open-File Report 2015–1095, U.S. Geological Survey, 2015).
- 38.R. W. Sheibley, J. L. Morace, C. A. Journey, P. C. Van Metre, A. H. Bell, N. Nakagaki, D. T. Button, S. L. Qi, “Design and methods of the Pacific Northwest Stream Quality Assessment (PNSQA), 2015” (No. Open-File Report 2017–1103, U.S. Geological Survey, 2017).
- 39.J. F. Coles, K. Riva-Murray, P. C. Van Metre, D. T. Button, A. H. Bell, S. L. Qi, C. A. Journey, R. W. Sheibley, “Design and methods of the US Geological Survey Northeast Stream Quality Assessment (NESQA), 2016” (No. Open-File Report 2018–1183, U.S. Geological Survey, 2019).
- 40.J. T. May, L. H. Nowell, J. F. Coles, D. T. Button, A. H. Bell, S. L. Qi, P. C. Van Metre, “Design and methods of the California Stream Quality Assessment (CSQA), 2017” (No. Open-File Report 2020–1023, U.S. Geological Survey, 2020).
- 41.U.S. Geological Survey, “Chapter A4. Collection of water samples” (Techniques of Water-Resources Investigations 09-A4, U.S. Geological Survey, 2006).
- 42.M. W. Sandstrom, F. D. Wilde, “Syringe-filter procedure for processing samples for analysis of organic compounds by DAI LC-MS/MS” (National Field Manual for the Collection of Water-Quality Data: U.S. Geological Survey Techniques of Water-Resources Investigations No. Chapter 5.2.2.B, U.S. Geological Survey, 2014).
- 43.R Development Core Team (R Foundation for Statistical Computing, 2015).
- 44.Ritz C., Baty F., Streibig J. C., Gerhard D., Dose-response analysis using R. PLOS ONE 10, e0146021 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nash J., Sutcliffe J. V., River flow forecasting through conceptual models, I: A discussion of principles. J. Hydrol. 10, 398–409 (1970). [Google Scholar]
- 46.Lefcheck J. S., piecewiseSEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 7, 573–579 (2016). [Google Scholar]
- 47.J. B. Grace, Structural Equation Modeling and Natural Systems (Cambridge Univ. Press, 2006).
- 48.Thorley J., Schwarz C., ssdtools: An R package to fit species sensitivity distributions. J. Open Source Softw. 3, 1082 (2018). [Google Scholar]
- 49.Beketov M. A., Foit K., Schäfer R. B., Schriever C. A., Sacchi A., Capri E., Biggs J., Wells C., Liess M., SPEAR indicates pesticide effects in streams—Comparative use of species- and family-level biomonitoring data. Environ. Pollut. 157, 1841–1848 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Liess M., Schäfer R. B., Schriever C. A., The footprint of pesticide stress in communities–species traits reveal community effects of toxicants. Sci. Total Environ. 406, 484–490 (2008). [DOI] [PubMed] [Google Scholar]
- 51.Rasmussen J. J., Baattrup-Pedersen A., Larsen S. E., Kronvang B., Local physical habitat quality cloud the effect of predicted pesticide runoff from agricultural land in Danish streams. J. Environ. Monit. 13, 943–950 (2011). [DOI] [PubMed] [Google Scholar]
- 52.S. N. Wood, Generalized additive models: An introduction with R (2nd edition). J. Stat. Softw. 86 (2018).
- 53.Poteat M. D., Buchwalter D. B., Four reasons why traditional metal toxicity testing with aquatic insects is irrelevant. Environ. Sci. Technol. 48, 887–888 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Eisenhauer N., Bonn A., Guerra C. A., Recognizing the quiet extinction of invertebrates. Nat. Commun. 10, 50 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kraus J. M., Schmidt T. S., Walters D. M., Wanty R. B., Zuellig R. E., Wolf R. E., Cross-ecosystem impacts of stream pollution reduce resource and contaminant flux to riparian food webs. Ecol. Appl. 24, 235–243 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Schmidt T. S., Kraus J. M., Walters D. M., Wanty R. B., Emergence flux declines disproportionately to larval density along a stream metals gradient. Environ. Sci. Technol. 47, 8784–8792 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Waite I. R., Munn M. D., Moran P. W., Konrad C. P., Nowell L. H., Meador M. R., Van Metre P. C., Carlisle D. M., Effects of urban multi-stressors on three stream biotic assemblages. Sci. Total Environ. 660, 1472–1485 (2019). [DOI] [PubMed] [Google Scholar]
- 58.Walsh C. J., Roy A. H., Feminella J. W., Cottingham P. D., Groffman P. M., Morgan R. P. II, The urban stream syndrome: Current knowledge and the search for a cure. J. North Am. Benthol. Soc. 24, 706–723 (2005). [Google Scholar]
- 59.Meyer J. L., Paul M. J., Taulbee W. K., Stream ecosystem function in urbanizing landscapes. J. North Am. Benthol. Soc. 24, 602–612 (2005). [Google Scholar]
- 60.Beketov M. A., Kefford B. J., Schäfer R. B., Liess M., Pesticides reduce regional biodiversity of stream invertebrates. Proc. Natl. Acad. Sci. U.S.A. 110, 11039–11043 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmidt T. S., Van Metre P. C., Carlisle D. M., Linking the agricultural landscape of the Midwest to stream health with structural equation modeling. Environ. Sci. Technol. 53, 452–462 (2019). [DOI] [PubMed] [Google Scholar]
- 62.Waite I. R., Van Metre P. C., Multistressor predictive models of invertebrate condition in the Corn Belt, USA. Freshw. Sci. 36, 901–914 (2017). [Google Scholar]
- 63.Moschet C., Wittmer I., Simovic J., Junghans M., Piazzoli A., Singer H., Stamm C., Leu C., Hollender J., How a complete pesticide screening changes the assessment of surface water quality. Environ. Sci. Technol. 48, 5423–5432 (2014). [DOI] [PubMed] [Google Scholar]
- 64.bbe-Moldaenke, BenthoTorch User Manual (bbe-Moldaenke, 2013).
- 65.U.S. Environmental Protection Agency, “National rivers and streams assessment 2008–2009” (Technical Report EPA/841/R-16/008, 2016).
- 66.Gerth W. J., Herlihy A. T., Effect of sampling different habitat types in regional macroinvertebrate bioassessment surveys. J. North Am. Benthol. Soc. 25, 501–512 (2006). [Google Scholar]
- 67.Mohseni O., Stefan H. G., Erickson T. R., A nonlinear regression model for weekly stream temperatures. Water Resour. Res. 34, 2685–2692 (1998). [Google Scholar]
- 68.Benke A. C., Huryn A. D., Smock L. A., Wallace J. B., Length-mass relationships for freshwater macroinvertebrates in North America with particular reference to the southeastern United States. J. North Am. Benthol. Soc. 18, 308–343 (1999). [Google Scholar]
- 69.K. P. Burnham, D. R. Anderson, Model Selection and Multimodal Inference: A Practical Information-Theoretic Approach (Springer-Verlag, ed. 2, 2002). [Google Scholar]
- 70.Knillmann S., Orlinskiy P., Kaske O., Foit K., Liess M., Indication of pesticide effects and recolonization in streams. Sci. Total Environ. 630, 1619–1627 (2018). [DOI] [PubMed] [Google Scholar]
- 71.Agilent Technologies, Agilent MassHunter Workstation Software. Quantitative Analysis Familarization Guide (Agilent Technologies Inc., 2010).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/43/eabc1299/DC1


