AKI and CKD are interconnected syndromes with common outcomes, including progressive CKD, cardiovascular events, poor quality of life, and death.1 However, outpatient care after AKI is often fragmented, with limited evidence to inform care recommendations. These observations prompt the question, “Can changes in care after an episode of AKI reduce these risks?”
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) convened a workshop on January 30 and 31, 2019, that included a multidisciplinary group of clinicians, scientists, and other stakeholders. Participants were tasked with identifying potential gaps in care after AKI, populations at risk, outcomes of interest, potential interventions, and possible trial designs. In light of NIDDK initiatives to include patient voices in all aspects of clinical research, ranging from suggesting content areas to participation in Steering Committees, Safety Monitoring Boards, and the planning, conduct, and reporting of clinical trials,2 a panel of patients who experienced AKI was invited to participate in the workshop.
Speaker Session Synopses
What Clinical Outcomes following Discharge after an AKI Hospitalization May Be Modifiable?
Several studies have reported a lack of nephrology follow-up after AKI and the potential benefit of such care.3 Given the paucity of therapeutic agents to effect kidney recovery, it is important to define specific elements of care that may improve outcomes. With mechanistic and clinical studies providing strong links between AKI and CKD progression and potentially cardiovascular disease, early discussions centered on identifying intermediate outcomes along these axes.
Preclinical studies indicate that AKI can worsen traditional risk factors for CKD and cardiovascular disease, including salt sensitivity of BP and urine protein excretion. Emerging clinical data also demonstrate robust associations between AKI, hypertension, and proteinuria.4,5 Given that cardio- and kidney-protective medications are often discontinued after AKI and that kidney function and proteinuria are infrequently assessed, the lack of timely recognition and management of these risk factors represents a potentially important gap in care.
Recurrent AKI, which affects up to 25% of AKI survivors, is another intermediate outcome that can threaten recovery and contribute to faster declines in kidney function.6 Both traditional factors (e.g., CKD and diabetes) and AKI-related factors (e.g., severity) pose risks for AKI recurrence. One potential area for decreasing risk is minimizing nephrotoxic exposures. Quality improvement programs have successfully reduced nephrotoxin exposure in hospitalized patients but have not been extended to outpatient settings. Outpatient providers have a critical role and may not be aware of the associated hazards of AKI nor potential approaches to mitigate risk for further kidney injury. Discharge summaries (when available) may not document that AKI has occurred as AKI may not be prioritized among competing issues. Further, providers may not be aware of the increased risk of nephrotoxins following AKI, such as nonsteroidal anti-inflammatory agents, or that the risk may be heightened when taken concomitantly with renin-angiotensin-aldosterone system (RAAS) inhibitors and diuretics. AKI survivors are also at higher risk for adverse drug events due to poor recognition of changing kidney function, highlighting the need for improved medication reconciliation.7 Lastly, patients themselves may be unaware of their AKI or its consequences. Collectively, these findings suggest a need for approaches to improve awareness and education regarding the minimization of nephrotoxin exposure after AKI among both providers and patients.
Other factors potentially affecting recovery or the risk for recurrent AKI include the degree of BP control and RAAS inhibition. Preclinical studies indicate that AKI results in autoregulatory derangements that may increase susceptibility to future kidney injury. Given the known effects of RAAS inhibition on vascular tone, it is unclear whether such medications may unfavorably alter the risk-benefit ratio for kidney health in this population. Several recent observational studies attempting to quantify the risks and benefits of continued RAAS inhibition have been published, with conflicting findings.8–10 Some studies have suggested an increased risk for hospitalization for kidney events, whereas others have shown no increase in risk for recurrent AKI. However, studies have also demonstrated that the potential risks of these medications may be offset by favorable effects on survival and long-term kidney function, if applied cautiously. The optimal approach and timing to reinitiate/initiate RAAS inhibition after AKI are unknown.
Similarly, impaired autoregulation may increase susceptibility to future AKI in patients with lower BP. To our knowledge, no study has examined the effect of intensive BP control after AKI. Although not directly comparable, post hoc analyses from the Systolic Blood Pressure Intervention Trial study in patients with underlying CKD suggest that, although intensive BP control may increase the risk of AKI events, this effect may be predominately hemodynamic. The increased risk of AKI events did not eliminate the benefits regarding all-cause and cardiovascular mortality.11
What Is Most Important to Patients Who Have Experienced AKI?
Survivors of AKI often experience poor quality of life and worsening frailty.12,13 The latter has been observed in both patients with AKI receiving dialysis and patients with AKI not requiring dialysis. Poor quality of life and frailty may reflect characteristics of populations with comorbidities placing them at high risk for AKI and poor outcomes. Therefore, critical attention has been focused on patient-centered outcomes in these populations. In a highlight of the workshop, three patients who experienced severe AKI described their experiences and commented on patient priorities. Two patients experienced dialysis-requiring AKI and eventually recovered, whereas the third patient experienced postoperative AKI that did not require dialysis.
All patients perceived severe decrements in quality of life. Chief among these were physical symptom burden (fatigue, weakness, anorexia, pruritis, and edema) and the effect of external restrictions (time traveling to appointments and dietary constraints). These burdens, coupled with uncertainty regarding recovery of kidney function, exerted a psychologic toll that contributed to anxiety, depression, and social isolation that were often unaddressed by clinicians. These features have been described by other AKI survivors.14 Patients also experienced fragmented care, often because of limited communication among health care providers. Patient participants noted a lack of meaningful advocates to foster engagement, education, and preparation for living with the short-term and long-term consequences of AKI. Overall, patients recommended a more holistic approach to assessing measurable outcomes in clinical studies that prioritizes improving quality of life and education through targeting the physical and psychologic burdens experienced during and after AKI. Each patient had a distinct experience, implying that a “one-size-fits-all” approach is unlikely to be successful.
Patient Populations and Settings of Interest
As AKI is a heterogenous set of disorders, the risk for subsequent adverse outcomes also varies. Risk stratification tools can predict loss of kidney function at hospital discharge, and they demonstrate good discrimination, calibration, and predictive value.15 Individuals with moderate to severe AKI, AKI superimposed upon CKD, and incomplete recovery are at highest risk of future complications.
The use of biomarkers to enhance risk stratification in AKI clinical trials has primarily focused on enriching study populations to test interventions for preventing AKI in specific settings (e.g., perioperative or following radiographic contrast). Only a few studies have demonstrated a moderate association between novel biomarkers and long-term outcomes after AKI, such as cardiovascular disease and mortality.16 However, one recent multicenter study demonstrated that albuminuria can serve as a valuable risk stratification tool for CKD progression after AKI.17
Potential Interventions and Design Considerations
Numerous pharmacotherapeutic pathways have been tested for the prevention and treatment of AKI, with limited results, but few have targeted the transition from AKI to CKD. Potential therapeutic targets include microvascular damage and tissue fibrosis. Phase 2 trials in CKD have yielded mixed results,18 suggesting that data are currently insufficient to warrant larger-scale testing following AKI. Further, pathways involved in the transition from AKI to CKD may have similarities across causes of AKI and may also have distinctive features across these causes, such as when comparing drug toxicity with vascular insufficiency.
Addressing potential gaps in care after AKI will positively affect clinical and patient-centered outcomes. For example, bundled process of care interventions have shown promise in preventing AKI in specific clinical situations,19 but they have not been applied at or following hospital discharge following AKI. Potential components of an intervention include improving medication reconciliation, nephrotoxin avoidance, systematic monitoring for recovery and recurrent AKI, strategic initiation/reinitiation of cardio- and kidney-protective medications, management of extracellular volume overload, and facilitating timely and appropriate nephrology referral.
The need for interventions directed at alleviating symptom burden, mood, and psychosocial barriers; promoting physical and psychologic rehabilitation; improving engagement and self-management; and providing patient education and better coordination of care was recognized. Patient navigators to guide patients through clinical pathways during the postrecovery period, interact with health care teams, and improve coordination and patient advocacy were identified as a potentially useful tool.
Challenges to the execution of AKI trials include patient engagement, as many patients are already taxed by the burden of illness and competing health care needs. Potential solutions to promote patient engagement include the use of technologies to reduce the burden of trial participation, including point-of-care home monitoring, telemedicine, and digital mobile applications that leverage behavioral tools to foster self-monitoring, medication adherence, and patient-provider communication, and the use of electronic health records to assess outcomes. Nephrologists or primary care providers might also be relied on to implement potential interventions, particularly in a pragmatic study.
Breakout Group Reports
Interventional studies should be enriched with patients at high risk for poor outcomes, excluding those with transient AKI or whose etiologies have effective treatments (e.g., acute GN or urinary tract obstruction). Given the competing risks of death, investigators should consider the challenges of enrolling and retaining patients with severe comorbid disease, the possibly limited benefits of interventions in the severely ill, exclusion of patients with limited life expectancy, and other barriers to participation. An intervention would ideally start at or shortly after hospital discharge depending on the outcome. Several study designs were considered, and strengths and limitations were discussed. Depending on the intervention and end points, study designs including 1:1 randomization to multicenter, cluster-randomized studies could be used. Pragmatic studies were also discussed, including potential cluster-randomized designs to test process of care interventions. Multiple implementation challenges would need to be overcome, including buy-in from stakeholders, ensuring standardized data collection, and maximizing follow-up.
Clinical outcomes of interest include intermediate events such as recurrent AKI, all-cause hospitalizations/emergency room visits, and adverse drug events, which could be combined into a composite of event-free days. Additional intermediate clinical outcomes of interest include major adverse kidney events (a composite of death, dialysis, and persistent kidney dysfunction; e.g., at 90 days), albuminuria/proteinuria, and BP. Workshop participants thought, partly due to the inclusion of patient voices, that trials should also prioritize patient-centered outcomes, including health-related quality of life, physical and psychologic symptom burden, and functional status. The duration of the intervention(s) and primary outcome assessment would likely vary on the basis of the outcome evaluated. In general, a minimum of 90–180 days after AKI was felt to be necessary. Additional secondary follow-up beyond the initial outcome assessment period to link intermediate to longer-term clinical outcomes (e.g., CKD and heart failure) would be informative.
Each breakout group proposed a potential clinical trial to address the potential gaps in care. Although the trial designs varied, each group proposed to address both patient-centered and clinical outcomes. One group proposed a study focused on improving quality of life and well-being among patients with moderate to severe AKI using rehabilitation and educational interventions, as well as improving transitions of care using patient navigators. The proposed primary end point was quality of life at 6 months after discharge, with secondary end points including rehospitalizations with or without recurrent AKI. A second group proposed a clinical trial to test whether a postdischarge AKI care bundle could reduce the rates of rehospitalization within 90–180 days. Additional end points included recurrent AKI, adverse drug events, eGFR decline and recovery, proteinuria, volume status, BP, and death, as well as patient-centered outcomes such as quality of life and anxiety/depression. The last breakout group proposed that a cluster-randomized trial using a post-AKI care bundle could improve 90-day clinical and patient-centered outcomes in patients with moderate to severe AKI, nonrecovered AKI, or AKI superimposed on CKD. The primary outcome proposed was event-free days/time to event at day 90. Events of interest included recurrent AKI, all-cause rehospitalizations/emergency room visits, and adverse drug events. Secondary end points of interest included major adverse kidney events (death, dialysis, or a 50% increase in baseline serum creatinine concentration), health-related quality of life, and Beck Depression Inventory score. This group also proposed additional follow-up extending to 1–3 years.
Testing interventions to improve clinical and patient-centered outcomes after hospitalized AKI is warranted (Figure 1). Given the challenges with transition from the hospital to the outpatient setting expressed by survivors of AKI, workshop participants felt that studies should focus on interventions that begin at or shortly after discharge and target intermediate clinical outcomes that may yield both short-term and long-term benefits. Important concerns reported by patients included symptoms and quality of life after AKI. Patient perspectives are critical in devising feasible interventions that can target meaningful outcomes. Patients, clinicians, and policy makers should be incorporated into planning clinical trial designs.
Figure 1.
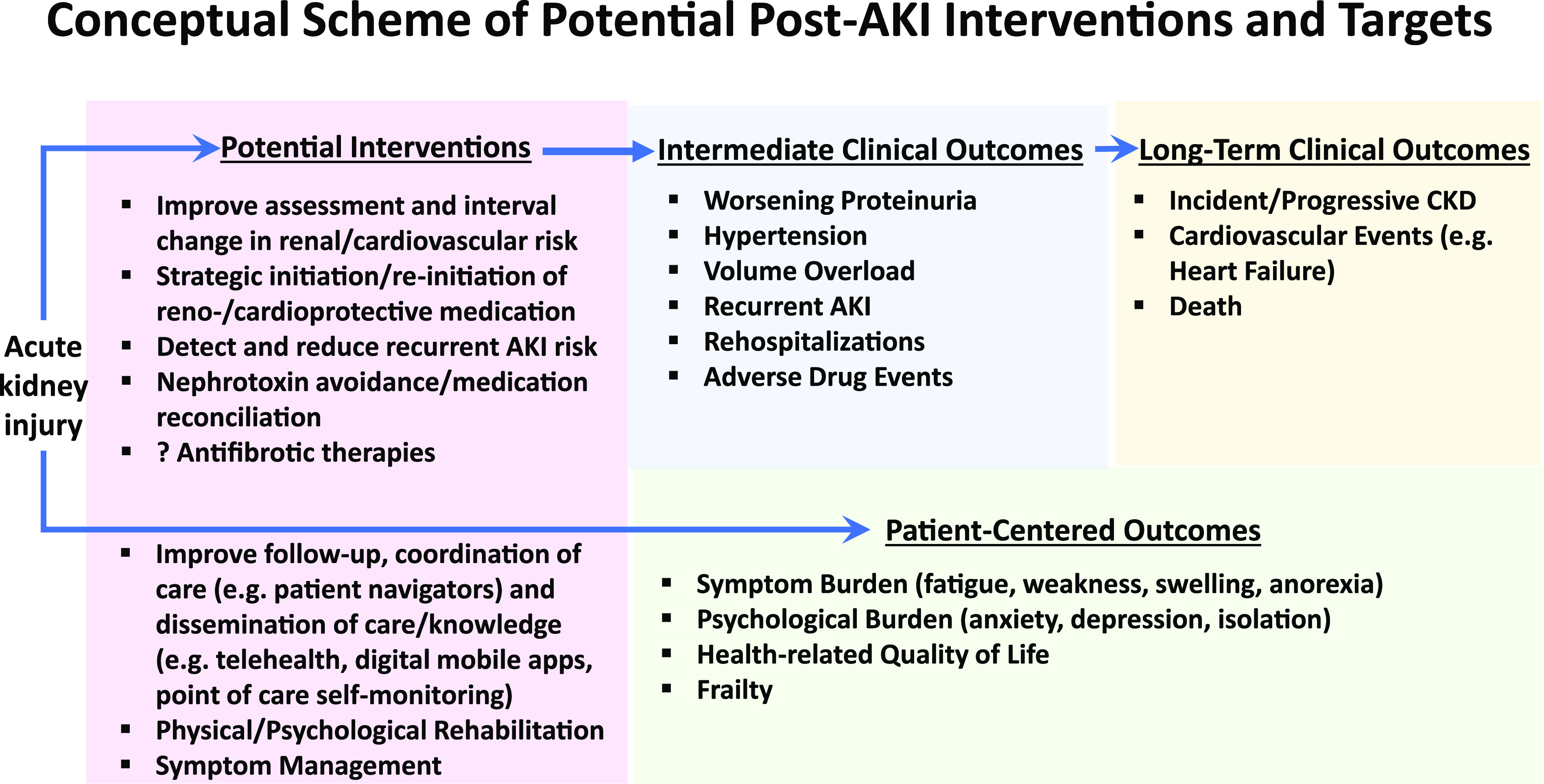
Multi-faceted interventions that target both intermediate clinical and patient-centered outcomes may improve long-term outcomes after AKI. The schematic figure identifies potential intermediate clinical outcomes (blue) that may mediate the association between AKI and poor long-term clinical outcomes (yellow). Important patient-centered outcomes were identifed by the invited patient panel (green). Some potential Interventions discussed that might improve these outcomes are listed (pink).
Disclosures
L. Dember receives compensation from the National Kidney Foundation as a Deputy Editor of the American Journal of Kidney Diseases. T. Greene reports consultancy agreements: AstraZeneca, Boehringer-Ingleheim, CSL, DURECT Corporation, Janssen Pharmaceuticals, and Pfizer Inc.; and research funding: AstraZeneca, Boehringer-Ingleheim, CSL, DURECT Corporation, Janssen Pharmaceuticals, and Pfizer Inc. M. James is the principal investigator of an investigator-initiated research grant from Amgen, Canada, which is not related to this work. C. Parikh serves on the advisory board of Genfit Biopharmaceutical Company. All remaining authors have nothing to disclose.
Funding
E. Siew is supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant U01DK082192 and U.S. Department of Veterans Affairs grant IIR 13-073. K. Liu is supported by National Institutes of Health (NIH) grant R01DK122797. C. Parikh is supported by NIH grant R01-HL-085757, O’Brien Kidney Center grant P30-DK-079310, and is a member of the NIH-sponsored Assessment, Serial Evaluation, and Subsequent Sequelae of Acute Kidney Injury (ASSESS-AKI) Consortium (grant U01-DK-082185).
Acknowledgments
The opinions expressed in this paper do not necessarily reflect those of NIDDK, the National Institutes of Health (NIH), the Department of Health and Human Services, and the government of the United States.
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendations. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or JASN. Responsibility for the information and views expressed herein lies entirely with the author(s).
Dr. Laura M. Dember reports receiving grants from NIDDK; consulting fees from Merck; and personal fees from GlaxoSmithKline, outside the submitted work. Dr. Timothy D. Girard reports grants from National Institutes of Health (NIH), outside the submitted work. Dr. Adrian F. Hernandez reports grants and personal fees from AstraZeneca; personal fees from Amgen; personal fees from Bayer; grants and personal fees from Merck; grants and personal fees from Boehringer Ingelheim; and grants and personal fees from Novartis, outside the submitted work. Dr. T. Alp Ikizler reports personal fees from Abbott Renal Care, Fresenius Kabi, and the International Society of Nephrology, outside the submitted work. Dr. Matthew T. James reports research support from the Canadian Institutes of Health Research New Investigator Award, outside of the submitted work. Dr. Neesh Pannu reports receiving research support from the Canadian Institutes of Health Research and Alberta Health Services, outside the submitted work. Dr. Kathleen D. Liu reports personal fees from American Thoracic Society, AstraZeneca, Baxter, Biomerieux, Durect, Potrero Med, Quark, and Theravance; and other from Amgen, the National Policy Forum on Critical Care and ARF, and UpToDate, outside the submitted work. Dr. Paul M. Palevsky reports personal fees from Baxter and grants from Dascena and BioPorto, outside the submitted work. Dr. Chirag R. Parikh reports other from Akebia Therapeutics and Renalytix AI, outside of the submitted work. Dr. Edward D. Siew reports consulting fees for Akebia, Inc. in 2019; honorarium as an invited speaker at the Da Vita Annual Physician Leadership Conference in 2019; royalties as an author for UpToDate; and compensation for serving on the editorial board for the Clinical Journal of the American Society of Nephrology, outside the submitted work. Dr. Samuel A. Silver reports speaking fees from Baxter Canada, outside the submitted work.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Chawla LS, Eggers PW, Star RA, Kimmel PL: Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371: 58–66, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimmel PL, Jefferson N, Norton JM, Star RA: How community engagement is enhancing NIDDK research. Clin J Am Soc Nephrol 14: 768–770, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harel Z, Wald R, Bargman JM, Mamdani M, Etchells E, Garg AX, et al. : Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int 83: 901–908, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Hsu CY, Hsu RK, Yang J, Ordonez JD, Zheng S, Go AS: Elevated BP after AKI. J Am Soc Nephrol 27: 914–923, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu CY, Hsu RK, Liu KD, Yang J, Anderson A, Chen J, et al. ; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators and the Assessment, Serial Evaluation, and Subsequent Sequelae of Acute Kidney Injury (ASSESS-AKI) Study : Impact of AKI on urinary protein excretion: Analysis of two prospective cohorts. J Am Soc Nephrol 30: 1271–1281, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siew ED, Parr SK, Abdel-Kader K, Eden SK, Peterson JF, Bansal N, et al. : Predictors of recurrent AKI. J Am Soc Nephrol 27: 1190–1200, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hung AM, Siew ED, Wilson OD, Perkins AM, Greevy RA Jr., Horner J, et al. : Risk of hypoglycemia following hospital discharge in patients with diabetes and acute kidney injury. Diabetes Care 41: 503–512, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brar S, Ye F, James MT, Hemmelgarn B, Klarenbach S, Pannu N; Interdisciplinary Chronic Disease Collaboration : Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with outcomes after acute kidney injury. JAMA Intern Med 178: 1681–1690, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu CY, Liu KD, Yang J, Glidden DV, Tan TC, Pravoverov L, et al. : Renin-angiotensin system blockade after acute kidney injury (AKI) and risk of recurrent AKI. Clin J Am Soc Nephrol 15: 26–34, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou YH, Huang TM, Pan SY, Chang CH, Lai CF, Wu VC, et al. : Renin-angiotensin system inhibitor is associated with lower risk of ensuing chronic kidney disease after functional recovery from acute kidney injury. Sci Rep 7: 46518, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung AK, Rahman M, Reboussin DM, Craven TE, Greene T, Kimmel PL, et al. ; SPRINT Research Group : Effects of intensive BP control in CKD. J Am Soc Nephrol 28: 2812–2823, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansen KL, Smith MW, Unruh ML, Siroka AM, O’Connor TZ, Palevsky PM; VA/NIH Acute Renal Failure Trial Network : Predictors of health utility among 60-day survivors of acute kidney injury in the Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network Study. Clin J Am Soc Nephrol 5: 1366–1372, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdel-Kader K, Girard TD, Brummel NE, Saunders CT, Blume JD, Clark AJ, et al. : Acute kidney injury and subsequent frailty status in survivors of critical illness: A secondary analysis. Crit Care Med 46: e380–e388, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wise M: On the Toss of a Coin: “A Memoir of a Near-Death Illness... and My Fight for Survival”, LAicester, United Kingdom, Troubador Publishing Ltd, 2017 [Google Scholar]
- 15.James MT, Pannu N, Hemmelgarn BR, Austin PC, Tan Z, McArthur E, et al. : Derivation and external validation of prediction models for advanced chronic kidney disease following acute kidney injury. JAMA 318: 1787–1797, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parikh CR, Puthumana J, Shlipak MG, Koyner JL, Thiessen-Philbrook H, McArthur E, et al. : Relationship of kidney injury biomarkers with long-term cardiovascular outcomes after cardiac surgery. J Am Soc Nephrol 28: 3699–3707, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu CY, Chinchilli VM, Coca S, Devarajan P, Ghahramani N, Go AS, et al. ; ASSESS-AKI Investigators : Post-acute kidney injury proteinuria and subsequent kidney disease progression: The Assessment, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury (ASSESS-AKI) study. JAMA Intern Med 180: 402–410, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuttle KR, Brosius FC 3rd, Adler SG, Kretzler M, Mehta RL, Tumlin JA, et al. : JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: Results from a phase 2 randomized controlled clinical trial. Nephrol Dial Transplant 33: 1950–1959, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, et al. : Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: The PrevAKI randomized controlled trial [published correction appears in Intensive Care Med 43: 1749, 2017]. Intensive Care Med 43: 1551–1561, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]


