Abstract
In utero Bisphenol A (BPA) exposure has been linked to many deficits during brain development, including sexual differentiation, behavior, and motor coordination. Yet, how BPA induces these disorders and whether its effects are long lasting are largely unknown. In this study, using a mouse model, we demonstrated that in utero exposure to an environmentally relevant dose of BPA induced locomotor deficits, anxiety-like behavior, and declarative memory impairments that persisted into old age (18 months). Compared to the control animals, the BPA-exposed mice had a significant decrease in locomotor activity, exploratory tendencies, and long-term memory, and an increase in anxiety. The global brain gene expression profile was altered permanently by BPA treatment and showed regional and sexual differences. The BPA-treated male mice had more changes in the hippocampus, while female mice experienced more changes in the cortex. Overall, we demonstrate that in utero exposure to BPA induces permanent changes in brain gene expression in a region-specific and sex-specific manner, including a significant decrease in locomotor activity, learning ability, long-term memory, and an increase in anxiety. Fetal/early life exposures permanently affect neurobehavioral functions that deteriorate with age; BPA exposure may compound the effects of aging.
Keywords: bisphenol A, neurobehavioral disorder, in utero, memory, locomotor
Bisphenol A (BPA) is a ubiquitous endocrine disrupting compound (EDC) that continues to be utilized in the manufacture of various plastic products, such as baby bottles, water bottles, dental sealants, and food containers. In the United States alone, 93% of the population have detectable BPA in their urine (1). Endocrine-disrupting compound exposure has been linked to reproductive dysfunctions and behavioral abnormalities (2, 3). Of all EDCs, BPA is one of the most pervasive (4, 5), functioning as a xenoestrogen (6). Bisphenol A is easily and rapidly transferred transplacentally from the maternal circulation to the fetus (7–9). Previous studies have shown that in utero exposure to xenoestrogen EDCs disrupt normal development of the female reproductive tract and affects uterine gene regulation (10). In rodent models, in utero BPA exposure also alters mammary gland development (11, 12), reproductive function (13), and advances puberty (14).
Maternal exposure of BPA has been demonstrated to have adverse effects on fetal brain development (15). Vulnerable periods of nervous system development in utero and in early postnatal life, called the “critical windows of development,” are sensitive to hormonal disruption, which may result in permanent damage (16). At low doses, BPA disrupts normal sexual differentiation of the brain and results in altered behavior of offspring, even at doses with no known harmful effects to the reproductive system (17). Prenatal BPA exposure is associated with memory and spatial learning impairment (15, 18, 19) and sex-dependent behavioral deficits (18, 20). In developing Zebrafish, BPA altered motor function and induced muscle damage (21). Although this body of literature indicates the adverse effects of BPA on neurodevelopment, the underlying molecular mechanism is not fully understood (22). Furthermore, in utero BPA exposure has been linked to sex-specific and brain-region-specific changes in social and anxiety-like behavior (23) but has not yet been broadly characterized. The persistent effects of in utero exposure have also not been studied in old subjects. The relationship between and interaction of aging with fetal or early life exposures have not been well characterized.
Here we look at the offspring of BPA-treated dams at 18 months of age and assess their performance in behavioral tests to qualitatively identify behavioral and physical deficits on motor coordination, anxiety, and memory. We then analyze the effect that BPA exposure has on gene expression in different regions of the brain.
Materials and Methods
Animal care and treatment
CD1 mice were obtained from Charles River Laboratories (Wilmington, Massachusetts). Eight-week-old CD-1 female mice were bred to male mice of the same strain and examined every 12 hours until the presence of a vaginal plug was detected. Detection of a vaginal plug was considered as Day 0 of pregnancy. Eight pregnant CD-1 mice were then housed individually and were continuously treated with BPA (Cat# 239658, Sigma-Aldrich, St Louis, Missouri) at 5mg/kg bw/day (serum levels are approximately equivalent to human exposure) or vehicle control via a miniosmotic pump inserted in the peritoneal cavity beginning on Day 9 of gestation for 7 days. We randomly selected 6 offspring per litter, totaling to 48 pups (24 male and 24 female), divided into 24 offspring (12 male and 12 female) in the treatment and the control group, respectively.
The behavioral tests were performed on 18-month-old offspring. After those tests, the mice were euthanized by cervical dislocation under CO2 inhalation anesthesia and the head was removed. The brain was rapidly removed from the skull and chilled in ice-cold normal saline for 5 minutes. The brain was then placed in a glass Petri dish resting on ice / 0.1 M sodium phosphate-buffered saline with a pH of 7.4. Dissection was performed with the brain immersed in saline. The cortex and hippocampus were dissected under a dissection microscope following the standard protocol (24, 25). Forceps placed between the cerebral halves were used to open the cortical halves. The initially encountered corpus callosum was separated from the underlying hippocampus. Large forceps were used to gently pick-up the hippocampus and cortex, and were then transferred to RNA later for RNA isolation and to 4% formalin for immunohistochemistry (IHC) staining. All surgical instruments were cleaned with RNAseZap in between each animal and brain region. The resulting tissues were saved for mRNA analyses. All experiments were conducted with approval by the Yale Institutional Animal Care and Use Committee (IACUC).
Rotarod test
To assess motor learning and coordination, performance on an accelerating rotarod task was measured, according to a previous method (26), with a sample size of 24 control and 24 BPA-treated mice. Briefly, subjects were tested for 3 trials on one day by using Series 8 Rotorod test (MK-610A/RKZ; Muromachi Kikai, Tokyo, Japan). The starting point was increased by 0.5 cm/second every 5 seconds. The latency to fall from a rotating rod was scored automatically with an infrared sensor.
Open field test
The open field test was performed to assess the degree of anxiety and locomotor activity in mice (27). BPA-treated offspring (N = 24) and control (N = 24) mice underwent open field testing at the age of 18 months. The open field test arena is an open square box (50 cm × 50 cm × 40 cm) composed of a clear floor without bedding. The box is virtually demarcated into a central zone and peripheral zones. An experimental mouse was placed in one corner of the box and allowed to explore the arena for 20 minutes. The overall activity of a tested mouse in the box (measured with video track) was recorded by a computer-based video recorder. The time spent, number of entries, and total distance traveled in the central zone were analyzed. The mice with higher anxiety levels tend to spend more time in the periphery and less time in the central area. The overall distance traveled by each mouse was considered an indicator of locomotor activity.
Object recognition test
The object recognition (OR) test was used to evaluate short-term, spatial object recognition memory (28), and the procedure was carried out as described previously (29). Briefly, mice were individually habituated to an open-field box (20 cm x 20 cm x 10 cm) for 3 days. During a training session (Day 1), 2 novel objects were placed into the open field and the animal was allowed to explore for 3 minutes. The objects and the floor of the arena were cleaned with 70% ethanol at the end of each trial to eliminate possible scent/trail markers. Exploratory behavior was considered only when the mice were sniffing or touching the object with the nose. The time spent exploring each object was recorded. During retention tests (Day 3), the animals were placed back into the same box, in which one of the familiar objects used during training was replaced by a novel object, and the animals were allowed to explore freely for 3 minutes. A preference index, the ratio of the amount of time spent exploring any 1 of the 2 similar objects (training session) or the novel one (retention session) over the total time spent exploring both objects, was used to measure recognition memory.
Gene expression analysis
The tissue intended for mRNA analysis was placed in 1 mL TRIzol per 100 mg tissue (Invitrogen, Carlsbad, California). The tissue was homogenized on ice and total RNA was isolated using the methodology described by the manufacturer. Total RNA was divided into 2 pools/group (each pool from 6 mice) and was then labeled and hybridized to an Affymetrix GeneChip 2.0ST (mouse) to profile the whole-genome expression by the Keck Biotechnology Resource Laboratory at Yale School of Medicine. Data was analyzed by using Genome Studio Data analysis software. Data was normalized and genes showing significant positive or negative changes (more than 1.5-fold) versus control were identified. The expression of selected genes was verified by quantitative real time reverse transcription polymerase chain reaction (RT-PCR) using cDNA from individual mice.
Quantitative RT-PCR
Total RNA (500 ng) was reverse transcribed in 20 µl of reaction mixture using the iScript cDNA synthesis kit (Bio-Rad, Hercules, California). The reaction mix was incubated for 5 minutes at 25°C, 30 minutes at 42°C, and 5 minutes at 85°C using an Eppendorf Mastercycler (Eppendorf North America, Framingham, Massachusetts). Quantitative real time RT-PCR reactions were prepared using the iQ SYBR Green Supermix (Bio-Rad, Hercules, California). Each PCR reaction consisted of the following: 5 µl of cDNA template, 0.5 µl of forward primer (10 µM), 0.5 µl of reverse primer (10 µM), 4 µl of nuclease-free H2O, and 10 µl of iQ SYBR Green Supermix. Peptidyl-prolyl cis-trans isomerase A (PPIA) was used as a housekeeping gene. The primers are described in Table 1.
Table 1.
List of primers used in this paper
| Primers | Sequence (5’ » 3’) |
|---|---|
| mTtr-F | ACACTAAGCATGGTCTGTAGC |
| mTtr-R | TGTGATTGAACCCAGGGCTTT |
| mEnpp2-F | GGAACGCAGATGGCATGTTG |
| mEnpp2-R | TTCCAATTTGTTCTTTGGCTCTACC |
| mKL-F | TTCTTAGTCGATCCGGCAGC |
| mKL-R | GCCTCTTCACAGAAGCACAC |
| mPtgs2-F | GTGGTAGCCAGCAAAGCCTA |
| mPtgs2-R | ACCCAATCAGCGTTTCTCGT |
| mRgs4-F | AGAAATGGGCTGAATCGTTGG |
| mRgs4-R | TTCTTATACTCCTCACAGCTGATCC |
| mGfra2-F | GACGAAACCCTCCGCTCTTT |
| mGfra2-R | GCAGCGGCAGTCATACAATG |
| mIghm-F | GAAGCTCGTGGAATCTGGCT |
| mIghm-R | AGACAGGTCAGGTTAGCGG |
| mZic1-F | GTGGTAGCCAGCAAAGCCTA |
| mZic1-R | ACCCAATCAGCGTTTCTCGT |
| mFat2-F | AGCTTCAGGACTTGTTCCCC |
| mFat2-R | CTGGATCTGCTGGCTGAATCTC |
| mPrkcd-F | ACTCTATGGACCAGGAAGCCT |
| mPrkcd-R | TGATGGCAAAGGGAAGGCAA |
| mLrp11-F | TCAGTGGACATGAAGGTGCC |
| mLrp11-R | CCACGGAGTCAGTCACAGTC |
| mRyr3-F | CGACATGGTTCAGAGAGAAATGC |
| mRyr3-R | AGTTCTGCCCATCAGTCCCT |
| mCalb2-F | CGATGCATGAGTTCCTTCGC |
| mCalb2-R | CATTAAGCAAGCTGGTGGGC |
| mHdac4-F | CTGGAGGTTGCATGGACGTA |
| mHdac4-R | AGTAGGGTGTGAATGTGGGG |
| mNsmf-F | TGATGTCCCCATCCGTACCT |
| mNsmf-R | CCCCTGAATACCGCTTGCAT |
| mDbi-F | GGACTCGTGGAACAAGCTGA |
| mDbi-R | GGGAGGAGGAGCAGAGGTTA |
| mPrkcg-F | TACAAGTTACTGAACCAGGAGG |
| mPrkcg-R | GCTCTGCCAGCATTACCTTC |
| mNnat-F | GCTCATCATCGGCTGGTACA |
| mNnat-R | ACCTCACTTCTCGCAATGGG |
| mTmem181a-F | GGAGTACCCTGCGTTCGAG |
| mTmem181a-R | CATGGGTGCCAACCTGTAGT |
| mGit1-F | CTTGGAAACATGTCTGCGCC |
| mGit1-R | GAGACATGCACTTTTGCCGAG |
| mPdk4-F | CCAAGCAGGGAACCGAAGAA |
| mPdk4-R | GCATTGCCAAAGGAGAAGCAG |
| mDctn3-F | TGTGCTGGACAGCGCTTCTATC |
| mDctn3-R | CTTCCAGGAGAGCCTTAGACTC |
| mAtp1a3-F | TGGGCGTGTCCTTCTTCATC |
| mAtp1a3-R | CTTCACCAGGCAGTTCTTGC |
The Bio-Rad iCycler iQ system (Bio-Rad) was used to quantify fluorescence intensity of PCR products during amplification. RT-PCR reactions were performed for 40 cycles at 95°C for 15 seconds and 60°C for 15 seconds, and 72°C for 15 seconds. Relative gene expression was determined by using the 2−ΔΔCT method to normalize with a housekeeping gene, and the results were expressed as fold changes over control mice. Specificity of the amplified products and the absence of primer dimers were confirmed by a melting curve analysis. All PCR products yielded the predicted melting temperature. All experiments were conducted in triplicate. Samples without a cDNA template were used as negative controls.
Immunohistochemistry
Immunohistochemical analysis of Calb2 (1:1000) (12278-1-AP, Life Technologies Corporation DBA, Thermo Fisher Scientific, Waltham, Massachusetts) (30), Lcn2 (1:100) (bs-1373R-TR, Bioss Antibodies) (31), Ttr (1:100) (bs-0152R-TR, Bioss Antibodies) (32), and Enpp2 (1:100) (bs-2437R-TR, Bioss Antibodies, Woburn, Massachusetts) (33) expression was performed. Brain obtained from mice were fixed in formalin, embedded in paraffin, sectioned (thickness, 5 μm), and mounted onto glass slides. Slides were deparaffinized and rehydrated through a series of xylene and ethanol washes. Antigen retrieval was performed by steaming in 0.01 M sodium citrate buffer for 20 minutes, followed by a 45-minute cooling period. Then, the slides underwent three 5-minute washes in phosphate-buffered saline (PBS). Endogenous peroxidases were quenched with 3% hydrogen peroxide for 10 minutes, followed by three 5-minute PBS washes. Slides were circumscribed with a hydrophobic pen, and nonspecific binding was blocked with 5% goat serum in PBST for 1 hour at room temperature. Slides were incubated in the primary antibody overnight at 4°C. Normal goat immunoglobulin G (Santa Cruz Biotechnology, Dallas, Texas) was used as a negative control. ImmPRESS-HRP goat anti-rabbit IgG secondary antibody (Vector Laboratories, San Francisco, California) was applied for 1 hour at room temperature. After washing in PBS, the slides were incubated for 5 minutes in diaminobenzidine (Vector Laboratories). A 16-second exposure to hematoxylin was used as a counterstain. Slides were rehydrated through 5-minute ethanol and 20-minute xylene washes and mounted with Permount. All slides were processed simultaneously. The expression of protein by immunohistochemistry was quantified using the H-scoring method by 2 independent evaluators blinded to the treatment group (34, 35). Intensity of nuclear staining was indicated by a value of 0, 1, 2, or 3 (negative, weak, moderate, or strong, respectively).
Statistical analysis
An unpaired 2-tailed student’s t-test was used for the analysis of qPCR results. The Mann-Whitney U test was used to compare H scores. A P value of < 0.05 was deemed significant. The error bars display the standard error of the mean.
Results
In utero bisphenol A exposure impairs physical movement and memory of adult-aged offspring
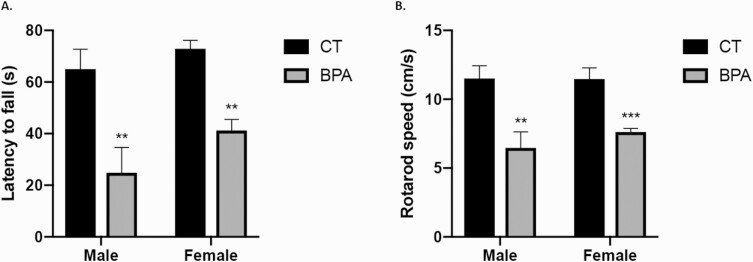
Although the adverse effects of prenatal BPA exposure on neurodevelopment have been previously studied, whether these effects are permanent or transient is unknown. We determined the effects of prenatal BPA exposure on the movement, behavior, and memory of 18-month-old offspring. First, a rotarod performance test was used to assess motor coordination and balance in rodents. The rotarod is mechanically driven to slowly accelerate over time. The time (latency) it takes for the mouse to fall off the rod and the highest rotating speed before the fall were recorded. The mice in the control group stayed on the rotarod for an average of 63.8 seconds, significantly longer than the BPA-exposed group, which averaged 35.2 seconds (P < 0.05) (Fig. 1A). Consistently, the control mice also managed to stay on the rotarod at a higher velocity (average: 11.28 cm/second) than the BPA-exposed mice (average: 7.2 cm/second; P < 0.001) (Fig. 1B). There was no difference in rotarod performance between female and male mice within groups.
Figure 1.
The aged offspring of bisphenol A (BPA)-treated dams present impaired motor coordination and balance. The rotarod test was performed on the 18-month-old offspring of control (CT, n = 24) and BPA-treated (n = 24) dams. A: The time (latency) to fall off the rod. B: The rotation speed (centimeter/second). Bars: mean + standard error of the mean (SEM) of the results; ** P < 0.01, *** P < 0.001 (unpaired 2-tailed Student’s t-test).
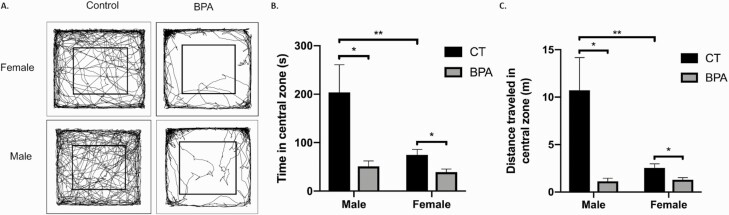
An open field test was performed to evaluate general locomotor activity levels, anxiety, and willingness to explore. The paths of each mouse in the testing zone was tracked (Fig. 2A). The time that a mouse spent in the central zone and cumulative distance traveled in the central zone was measured. The time spent in the central zone (average 203.81 seconds) for the control male mice was significantly higher than that for the BPA-exposed group (average 51.04 seconds; P < 0.05) (Fig. 2B). Similarly, female controls spent more time in the central zone (average: 74.7 seconds) than the female BPA-exposed mice (average 39.0 seconds; P < 0.05) (Fig. 2B). The male control mice traveled a greater distance in the central zone (average 10.72 m) than the male BPA-exposed mice (average 1.14 m; P < 0.05) (Fig. 2C). The female control mice also traveled a greater distance (average 2.54 m) than the female BPA-exposed mice (average 1.29 m; P < 0.05) (Fig. 2C). Intriguingly, the male control group traveled a far greater distance than the female control group (P = 0.006); however, both the male and female BPA-exposed groups traveled similar distances in the central zone. The male control group also stayed in the central zone for longer compared with the female control group (P = 0.01); however, the male and female BPA-exposed groups had no significant difference in the total time spent in central zone. These results demonstrate that the locomotor activity of control male mice is much higher than that of females; however, BPA treatment reduces the locomotor activity of both male and female mice significantly and, to a similar level, eliminates normal sexual dimorphism.
Figure 2.
The offspring of bisphenol A (BPA)-treated dams present with increased anxiety and reduced locomotion. The open field test was performed on the male and female offspring of control (CT, n = 24) and BPA-treated (n = 24) dams. A: Representative tracks. The inner square is the central zone. B: The time (seconds) spent by mice in the central zone, Male CT versus male BPA (P = 0.03), female CT versus female BPA (P = 0.02), male CT versus female CT (P = 0.01). C: The distance (meter) traveled by mice in the central zone. Male CT versus male BPA (P = 0.03), female CT versus female BPA (P = 0.02), male CT versus female CT (P = 0.006). Bars: mean + standard error of the mean (SEM) of the results; * P < 0.05 (unpaired 2-tailed Student’s t-test).
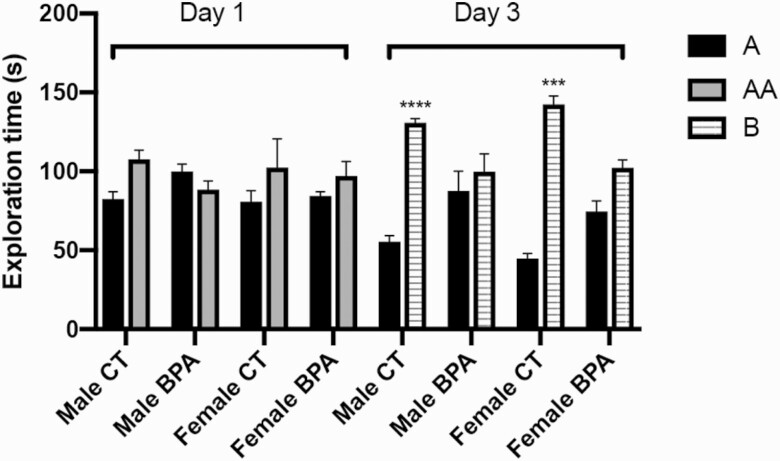
An object recognition test was performed to index long-term memory (Fig. 3). Both the control and BPA-exposed mice on Day 1 demonstrated no significant difference in time expended exploring 2 of the same objects (object A and object AA). The control group mice explored object A for an average of 1:21 minutes, and an identical version of object A, object AA, for 1:46 minutes, while the BPA group mice explored object A for an average of 1:31 minutes and object AA for 1:33 minutes. On Day 3, the control group spent significantly more time exploring the novel object (object B, for an average of 2:13 minutes) than the familiar object (object A, for an average of 0:52 minutes) that had been explored on Day 1 (P < 0.001). The BPA-exposed group spent about the same amount of time exploring the familiar object (object A, for an average of 1:20 minutes) and the novel object (object B, for an average of 1:41 minutes). These results suggest that BPA treatment impairs long-term memory and learning ability.
Figure 3.
Recognition memory is impaired in the offspring of bisphenol A (BPA)-treated dams. The object recognition test was performed on the male and female offspring of control (n = 24) and BPA-treated (n = 24) dams. Mice were allowed to familiarize objects and after 1 day or 3 days, the amount of time taken to explore the familiar object and new object were recorded in seconds. A, first object; AA, identical object to A; B, novel object. Bars: mean + standard error of the mean (SEM) of the results; *** P < 0.001, **** P < 0.0001 (unpaired 2-tailed Student’s t-test).
Bisphenol A alters gene expression differently between males and females
To determine the mechanism of the behavioral changes, a global gene expression microarray was performed for 2 parts of the brain: the cerebral cortex and the hippocampus. The motor cortex and its connected brain regions are responsible for planning and executing volitional movements (36). The medial prefrontal cortex plays a significant role in memory recognition (37) and controlling social behavior in rodents (38). With aging, humans become more reliant on the prefrontal cortex as the central mechanism for motor control (39). Being a cornerstone for both motor function and behavior, the cerebral cortex is the ideal region to analyze and determine a genetic explanation for the BPA-induced changes presented in this study. The hippocampus is crucial to memory-guided exploration (40) and hippocampus-specific deficiencies are tied to an increase of anxiety-like behavior in mice (41). The cortex and hippocampus were chosen given the mice’s old age and the relevance of those 2 regions to motor coordination, behavior, and memory. An ingenuity pathway analysis (IPA) was run to identify key gene expression alterations (>1.5-fold change) and the relation of genes to the function of the brain. Noncoding regions were omitted from this analysis.
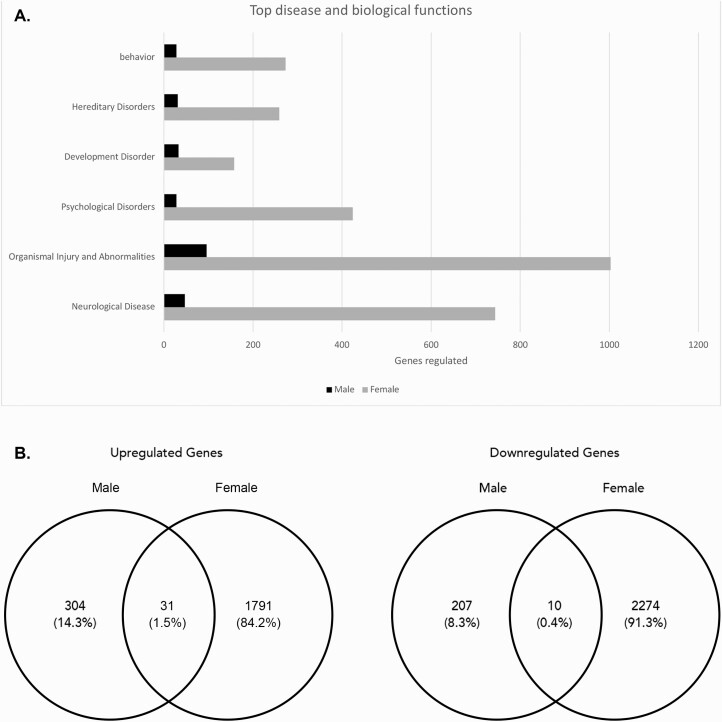
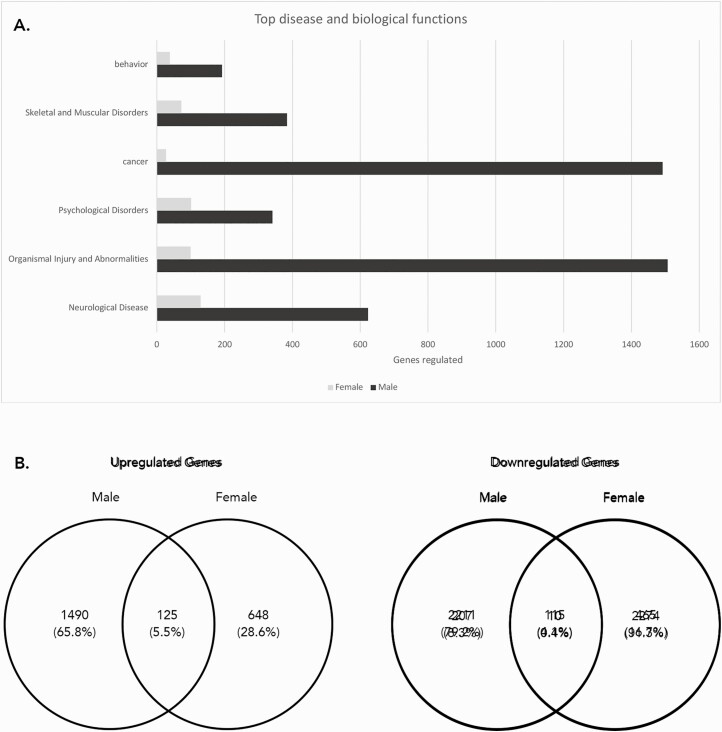
Analysis of the cortex (Fig. 4) showed that most of the altered genes belonged to the biological pathways involved in organismal injury and abnormalities (~1003 genes in females, ~96 genes in males), followed by neurological diseases (~744 genes in females, ~45 genes in males), and psychological disorders (~424 genes in females, ~28 genes in males). Females had a significantly larger change in gene expression within the cortex (4106 genes) compared with males (552 genes), with 1822 of them upregulated and 2284 of them downregulated. Males had 335 genes upregulated and 217 genes downregulated in the cortex. Within the upregulated genes, 31 were upregulated in both males and females. Within the downregulated genes, 10 were downregulated in both males and females.
Figure 4.
Altered gene expression in the cortex of bisphenol A (BPA)-treated offspring. An ingenuity pathway analysis (IPA) analysis was performed for both male and female offspring mice. A: The numbers of genes with altered expression in different biological disease and function groups. B: The Venn diagrams showing the upregulated and downregulated genes in males versus females.
Analysis of the hippocampus (Fig. 5) showed that most of the altered genes were similarly associated with organismal injury and abnormalities (~1507 genes in males, 100 genes in females), followed by cancer (~1492 genes in males, 27 genes in females) and neurological diseases (~623 genes in males, 129 genes in females). Males had a significantly larger change in gene expression within the hippocampus (3941 genes) compared with females (1353 genes), with 1615 of them upregulated and 2326 of them downregulated. Females had 773 genes upregulated and 580 genes downregulated in the hippocampus. Within the upregulated genes, 125 were upregulated in both males and females. Within the downregulated genes, 115 were downregulated in both males and females.
Figure 5.
Altered gene expression in the hippocampus of bisphenol A (BPA)-treated offspring. An ingenuity pathway analysis (IPA) was performed for both male and female offspring mice. A: The numbers of genes with altered expression in different biological disease and function groups. B: The Venn diagrams showing the the upregulated and downregulated genes in males versus females.
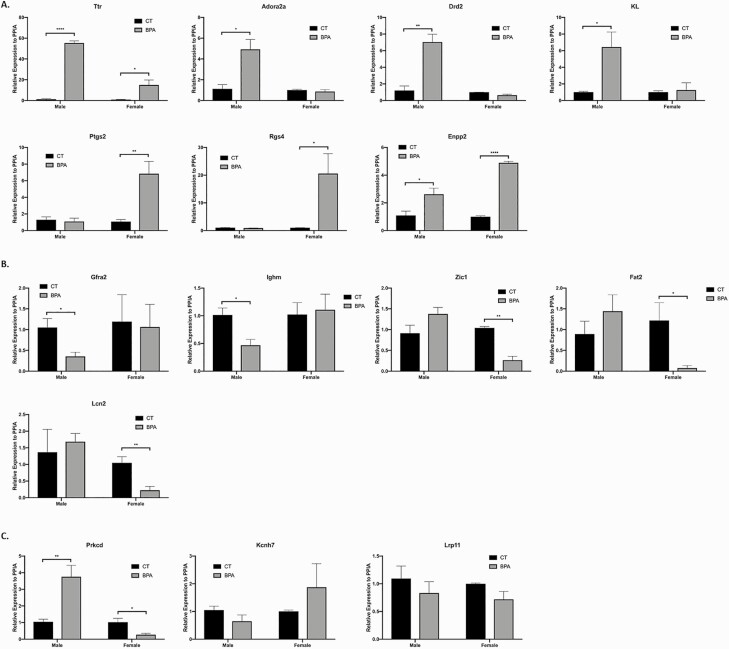
We next validated the top IPA hits that had both a high fold-change and an established association with motor function, behavior, or memory by qRT-PCR and IHC. Overall the qRT-PCR results were consistent with the microarray data. The IHC data also supports the findings exhibited in the PCR results. Figure 6 displays affected genes in the cortex genes validated by qRT-PCR. Expression of Adora2a, Drd2, and KL were all increased in BPA-treated male mice compared with control (P = 0.02, P = 0.006, and P = 0.04, respectively), while Ptgs2 and Rgs4 were increased in the BPA-treated female mice (P = 0.009 and P = 0.05, respectively). Ttr and Enpp2 expression were also increased in both BPA-treated male and female mice (P < 0.0001 and P = 0.02, P = 0.05 and P < 0.0001, respectively). Expression of Gfra2 and Ighm were decreased in the BPA-treated male mice compared with controls (P = 0.04 and P = 0.03, respectively), while Zic1, Fat2, and Lcn2 expression were all downregulated in BPA-treated female mice (P = 0.001, P = 0.03, and P = 0.004, respectively). Prkcd was differentially regulated between males and females, with upregulation in males, while downregulation was observed in females (P = 0.002 and P = 0.04). The expression of Kcnh7 and Lrp11 were not altered by BPA treatment in either sex (P = 0.17 and P = 0.35, P = 0.47 and P = 0.22, respectively).
Figure 6.
Cortex gene expression is altered by in utero exposure to bisphenol A (BPA), as verified by qPCR. A: Representative genes that are upregulated in the in utero BPA-treated male and female mice, including Ttr, Adora2a, Drd2, KL, Ptgs2, Rgs4, and Enpp2 (Ttr: P < 0.0001 and P = 0.02, males and females, respectively; Adora2a: P = 0.02, male; Drd2: P = 0.006, male; KL: P = 0.04, male; Ptgs2: P = 0.009, female; Rgs4: P = 0.05, female; Enpp2: P = 0.05 and P < 0.0001, males and females, respectively). B: Representative genes that are downregulated in the in utero BPA-treated male and female mice, including Gfra2, Ighm, Zic1, Fat2, and Lcn2 (Gfra2: P = 0.04, male; Ighm: P = 0.03, male; Zic1: P = 0.001, female; Fat2: P = 0.03, female; Lcn2: P = 0.004, female). C: Representative genes that are differentially regulated in the in utero BPA-treated male versus female mice, including Prkcd, Kcnh7, and Lrp11 (Prkcd: P = 0.002 and P = 0.04; Kcnh7: P = 0.17 and P = 0.35, Lrp11: P = 0.47 and P = 0.22, males and females, respectively). Bars: mean + standard error of the mean (SEM) of the results; * P < 0.05, ** P < 0.01, **** P < 0.0001 (unpaired 2-tailed Student’s t-test), N = 14. Abbreviation: CT, control.
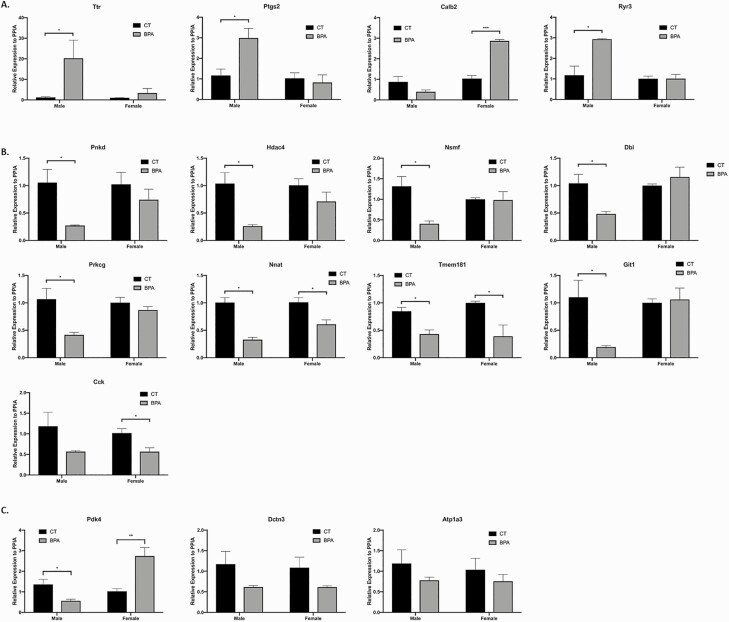
Figure 7 displays affected genes in the hippocampus validated by qRT-PCR. Expression of Ttr, Ptgs2, and Ryr3 were increased in BPA-treated male mice compared with sham (P = 0.05, P = 0.02, and P = 0.02, respectivly), while Calb2 was increased in BPA-treated female mice (P = 0.0002). Expression of Pnkd, Hdac4, Nsmf, Dbi, Prkcg, and Git1 were all decreased in BPA-treated male mice compared with sham (P = 0.03, P = 0.02, P = 0.02, P = 0.05, P = 0.05, and P = 0.04, respectively), while Cck was decreased in BPA-treated female mice (P = 0.03). Nnat and Tmem181 expression were decreased in both treated male and female groups (P = 0.02 and P = 0.002, P = 0.02 and P = 0.04, respectively). Pdk4 was differentially regulated between male and females, with downregulation in males but upregulation in females (P = 0.05 and P = 0.007). The expression of Dctn3 and Atp1a3 were not altered with BPA treatment for either sex (P = 0.22 and P = 0.18, P = 0.40 and P = 0.41, respectively). Ptgs2 expression was upregulated in the hippocampus of males and in the cortex of females. Of note, a significant increase in Ttr expression was observed after BPA treatment in both regions and sexes. In BPA-treated males, there was a ~30-fold increase in Ttr expression in the hippocampus and a ~100-fold increase in the cortex. In BPA-treated females, the increase was ~25-fold in the cortex.
Figure 7.
Hippocampus gene expression is altered by in utero exposure to bisphoenol A (BPA), as verified by qPCR. A: Representative genes that are upregulated in the in utero BPA-treated male and female mice, including Ttr, Ptgs2, Calb2, and Ryr3 (Ttr: P = 0.05, male; Ptgs2: P = 0.02, male; Calb2: P = 0.0002, female; Ryr3: P = 0.02, female). B: Representative genes that are downregulated in the in utero BPA-treated male and female mice, including Pnkd, Hdac4, Nsmf, Dbi, Prkcg, Nnat, Tmem181, Git1, and Cck (Pnkd: P = 0.03, male; Hdac4: P = 0.02, male; Nsmf: P = 0.02, male; Dbi: P = 0.05, male; Prkcg: P = 0.05, male; Nnat: P = 0.02 and P = 0.002, males and females, respectively; Tmem181: P = 0.02 and P = 0.04, male and female, respectively; Git1: P = 0.04, male; Cck: P = 0.03, female). C: Representative genes that are differentially regulated in the in utero BPA-treated male versus female mice, including Pdk4, Dctn3, Atp1a3 (Pdk4: P = 0.05 and P = 0.007, males and females, respectively; Dctn3: P = 0.22 and P = 0.18, males and females, respectively; Atp1a3: P = 0.40 and P = 0.41, males and females, respectively). Bars: mean + standard error of the mean (SEM) of the results. * P < 0.05, ** P < 0.01, *** P < 0.001 (unpaired 2-tailed Student’s t-test), N = 15. Abbreviation: CT, control.
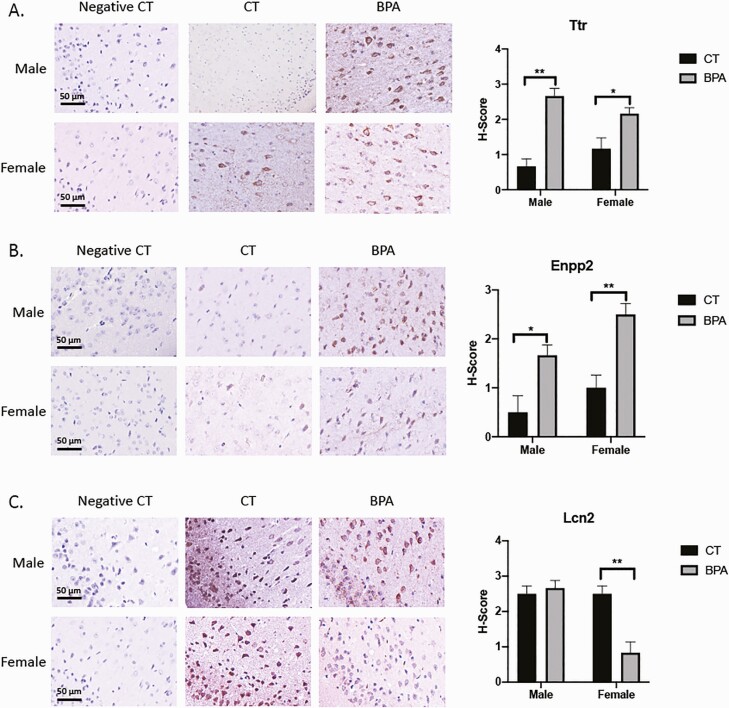
Figure 8 displays affected genes in the cortex validated by immunohistochemistry. Ttr expression was increased in both treated male and female mice (P = 0.002 and P = 0.05, respectively) in the piriform amygdala. Lcn2 expression was decreased in treated female mice (P = 0.009) in the same brain region. The piriform amygdala is a transitional zone at the boundary between the amygdala and the piriform area of the cerebral cortex. Enpp2 expression was increased in both treated male and female mice (P = 0.05 and P = 0.009, respectively) in the retrosplenial cortex. In humans, the retrosplenial cortex is implicated in a wide range of cognitive functions, including episodic memory, navigation, and imagining future events.
Figure 8.
Cortex gene expression is altered by in utero exposure to bisphenol A (BPA), as verified by immunohistochemistry staining. A: Expression of Ttr was increased from controls in both male and female mice in the piriform amygdala area (P = 0.002 and P = 0.05, respectively). B: Expression of Enpp2 was similarly increased in males and females in the retrosplenial cortex (P = 0.05 and P = 0.009, respectively). C: Expression of Lcn2 was decreased in females (P = 0.009) in the piriform amygdala area. Bars: mean + standard error of the mean (SEM) of the results; *, P < 0.05. **, P < 0.01 (Mann-Whitney U test). Abbreviations: CT, vehicle control; negative CT, negative control.
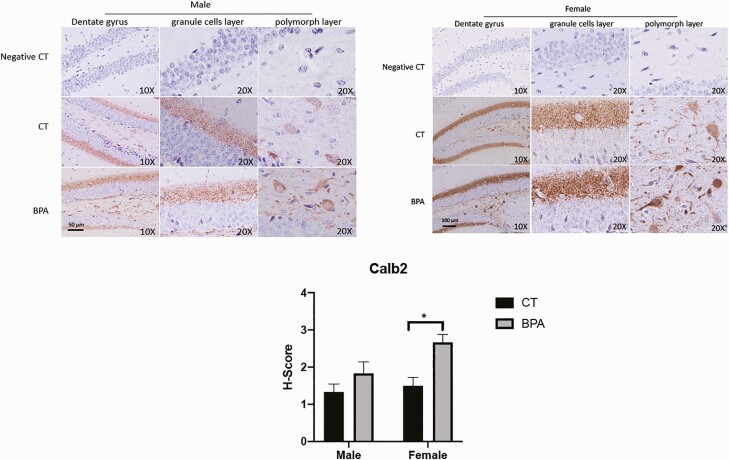
Figure 9 displays altered Calb2 gene expression in the hippocampus, as validated by immunohistochemistry. Calb2 expression in female mice is increased (P = 0.02) in the dentate gyrus’s granule cell layer and polymorph layer. The dentate gyrus is the input region of the hippocampus and plays a critical role in learning, memory, and spatial coding processes.
Figure 9.
Calb2 gene expression is altered in female hippocampus by in utero exposure to bisphenol A (BPA), as verified by immunohistochemistry staining. Calb2 expression is increased (P = 0.02) in the dentate gyrus only in female mice. The regions affected include the dentate gyrus granule cell layer and dentate gyrus polymorph layer. Bars: mean + standard error of the mean (SEM) of the results; * P < 0.05 (Mann-Whitney U test). Abbreviations: CT, vehicle control; negative CT, negative control.
Discussion
The permanent impact of embryonic bisphenol A exposure on offspring brain function
Bisphenol A is an endocrine-disrupting compound that is widely used in the production of plastics and ubiquitously found in common consumer goods (42). Prenatal exposure to BPA, even at levels below those currently considered safe for humans, can result in adverse effects on brain development, sexual differentiation, learning/memory, and social behaviors (43). Using a mouse model, this study provides further evidence that prenatal exposure to BPA impairs learning/memory and social/exploratory behavior. Of note, different from previous work, our study investigated the long-term adverse impact of prenatal BPA exposure on offspring, ie, 18-month-old mice. These results suggest that BPA exposure during embryonic development could cause permanent damage to brain functions. The half-life of BPA is short, and persistent exposure in aged mice is consistent with prior reports that identify epigenetic programming of gene expression after fetal exposure (10, 44).
Aging is associated with a decline in both motor function and cognitive function in both humans and rodents (45–48). The adverse effect of prenatal BPA treatment on motor coordination and cognitive function resembles that of ageing, leading us to postulate that BPA could accelerate the aging process. Indeed, BPA exposure induced senescence in human (49) and mouse cells (50). Bisphenol A has been shown to alter motor behavior, decrease axonal growth of the primary and secondary motoneurons, and induce muscle damage in developing zebrafish (21). However, a mouse model has yet been used in studying the effects of in utero BPA on motor coordination. We found that the control mice outperformed the BPA-treated mice, both male and female, in the rotarod test, a very sensitive and effective index used for assessing motor impairment due to brain injury (51).
The object recognition test examines a rodent’s innate exploratory behavior and working memory, similar to current memory tests used on man, and allows for interspecies comparison (26). On Day 3, the control mice were able to quickly recognize the object from Day 1 and explore the new object, while BPA mice showed no interest in the novel object. These observations are consistent with other recent studies using prenatal or perinatal BPA treatments (19, 22, 52). Object recognition is also a measurement of declarative memory (53), which plays an important compensatory role in memory impairments due to obsessive compulsive disorder (54) and theory of mind and implicit learning deficits in autism spectrum disorder (55, 56). The impairment of declarative memory is often an early symptom of Alzheimer’s disease (57).
The open field test measures anxiety and depression-like behavior. Both the BPA-treated male and female groups spent less time and traveled less distance in the central zone relative to their respective control groups. Interestingly, the male control group exhibited more exploratory behavior than the female control group; however, there was no significant difference between BPA-treated male and female groups regarding the time and distance traveled in the central zone. This leads us to conclude that in utero BPA treatment disrupts males’ exploratory tendencies significantly more than that of females, which resulted in the elimination of differences in exploratory behavior between the sexes, consistent with the findings of a similar open field test for 6- to 9-week-old mice (58). A previous study demonstrated that in utero BPA severely compromised spatial learning abilities and exploratory behavior in male offspring (59). Whereas the same study cited no discernable sexually dimorphic differences, we found a decrease in exploratory and spatial navigation abilities in the BPA-treated female mice, albeit to a lesser extent compared with the males. Our results are consistent with a study conducted on perinatal BPA exposure and the reduction or elimination of sexual differences in behavior (60).
Brain region- and sex-specific effect of bisphenol A on gene expression
Bisphenol A affects brain function in a region-specific manner (43). Previous studies have also documented sex differences due to BPA exposure in the hypothalamus (58, 61, 62) and that the cortex and hippocampus are key brain regions that demonstrate sexual dimorphism in neural development (63). However, BPA effects on sexual dimorphism in these 2 regions have yet been shown. In this study, we demonstrate that in utero BPA exposure affects gene expression in the cortex and hippocampus of male and female mice differentially. There were many more genes regulated by prenatal exposure to BPA in the cortex of females than in males, while significantly more genes regulated in the hippocampus of males than females. There were also genes that were upregulated or downregulated in both males and females, and others that were oppositely regulated between the sexes. These differential changes, however, lead to similar behavioral deficits, as both males and females showed a decrease in motor control, long-term memory, and exploratory behavior. These findings suggest that even though BPA exposure alters the expression of a broad spectrum of genes, it can cause specific neuroendocrine damages common to both sexes. The broad and permanent effect of BPA on brain gene expression suggests that prenatal exposure to BPA is also a risk to human health.
In utero BPA exposure resulted in a significant change in the global gene expression profile in both the cortex and the hippocampus. A large number of genes are related to neurological functions. For example, Pnkd was down-regulated in in the hippocampus of BPA-treated males. Pnkd is important for neurons to maintain cellular redox homeostasis and prevent neurotoxicity. A Pnkd deficiency led to neuronal death in Down’s syndrome and ALS-like transgenic mouse models (64). Anther downregulated gene, Nsmf, is critical for hippocampal long-term potentiation (LTP) at the CA1 synapses and hippocampus-dependent learning (65). The deficiency of Dbi could result in the loss of social interest and motivation (66). Git1 expression was dramatically downregulated after BPA treatment, which could correlate with profound learning and memory defects and reduced synaptic plasticity (67). In the cortex, BPA-treated female mice presented a reduced expression level of Zic1, which plays a critical role in overall cortical development (68). A deficiency in Lcn2 expression in the cortex could impair neurogenesis, neural stem cell cycle progression, and differentiation (69).
A significant number of affected genes are linked to skeletal and muscular disorders in the hippocampus, while none of the genes analyzed for the cortex are related to those conditions. The changes related to skeletal and muscular disorders could underlie the poor performance of BPA-treated mice on the rotarod test. Adult hippocampal neurogenesis is essential for spatial awareness, long term memory, as well as the regulation of mood and anxiety (70). Disruption of normal adult hippocampal neurogenesis is harmful to mental health, specifically in regards to mood, emotion, and cognition (71). Postnatal exposure to a clinically relevant dosage of BPA has been shown to impair hippocampal neurogenesis and result in spatial learning and memory deficits (72).
Conclusion
Our study demonstrates the permanent adverse effects of in utero BPA exposure on offspring brain functions, including motor coordination, memory, learning, and anxiety. Bisphenol A induces gene expression alterations that is sex-specific and different between the hippocampus and cerebral cortex. Although the mouse model of BPA exposure can’t fully recapitulate human exposure to environmental BPA, the results from this mouse model indicates a high and persistent life-long risk of fetal BPA exposure. There are considerable overlaps between BPA effects in aged mice and the effects of aging itself, suggesting that prenatal exposure to BPA may compound the effects of aging on neurobehavioral function.
Acknowledgments
Financial Support: This work was supported by NIH HD076422.
Author Contributions: Z.W, M.H.A, and C.A. performed the experimental procedures. H.S.T conceived, designed, and oversaw the study. Z.W. and H.S.T wrote the paper. All the authors reviewed and/or modified the manuscript.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30(1):75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolstenholme JT, Taylor JA, Shetty SR, Edwards M, Connelly JJ, Rissman EF. Gestational exposure to low dose bisphenol A alters social behavior in juvenile mice. Plos One. 2011;6(9):e25448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biedermann S, Tschudin P, Grob K. Transfer of bisphenol A from thermal printer paper to the skin. Anal Bioanal Chem. 2010;398(1):571–576. [DOI] [PubMed] [Google Scholar]
- 5. Galloway T, Cipelli R, Guralnik J, et al. Daily bisphenol A excretion and associations with sex hormone concentrations: results from the InCHIANTI adult population study. Environ Health Perspect. 2010;118(11):1603–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mueller SO. Xenoestrogens: mechanisms of action and detection methods. Anal Bioanal Chem. 2004;378(3):582–587. [DOI] [PubMed] [Google Scholar]
- 7. Doerge DR, Twaddle NC, Vanlandingham M, Brown RP, Fisher JW. Distribution of bisphenol A into tissues of adult, neonatal, and fetal Sprague-Dawley rats. Toxicol Appl Pharmacol. 2011;255(3):261–270. [DOI] [PubMed] [Google Scholar]
- 8. Nishikawa M, Iwano H, Yanagisawa R, Koike N, Inoue H, Yokota H. Placental transfer of conjugated bisphenol A and subsequent reactivation in the rat fetus. Environ Health Perspect. 2010;118(9):1196–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takahashi O, Oishi S. Disposition of orally administered 2,2-Bis(4-hydroxyphenyl)propane (Bisphenol A) in pregnant rats and the placental transfer to fetuses. Environ Health Perspect. 2000;108(10):931–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bromer JG, Zhou Y, Taylor MB, Doherty L, Taylor HS. Bisphenol-A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response. FASEB J. 2010;24(7):2273–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Markey CM, Luque EH, Munoz De Toro M, Sonnenschein C, Soto AM. In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biol Reprod. 2001;65(4):1215–1223. [DOI] [PubMed] [Google Scholar]
- 12. Muñoz-de-Toro M, Markey CM, Wadia PR, et al. Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice. Endocrinology. 2005;146(9):4138–4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ziv-Gal A, Wang W, Zhou C, Flaws JA. The effects of in utero bisphenol A exposure on reproductive capacity in several generations of mice. Toxicol Appl Pharmacol. 2015;284(3):354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401(6755):763–764. [DOI] [PubMed] [Google Scholar]
- 15. Wang C, Niu R, Zhu Y, et al. Changes in memory and synaptic plasticity induced in male rats after maternal exposure to bisphenol A. Toxicology. 2014;322(1):51–60. [DOI] [PubMed] [Google Scholar]
- 16. Frye CA, Bo E, Calamandrei G, et al. Endocrine disrupters: a review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. J Neuroendocrinol. 2012;24(1):144–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kubo K, Arai O, Omura M, Watanabe R, Ogata R, Aou S. Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci Res. 2003;45(3):345–356. [DOI] [PubMed] [Google Scholar]
- 18. Jašarević E, Williams SA, Vandas GM, et al. Sex and dose-dependent effects of developmental exposure to bisphenol A on anxiety and spatial learning in deer mice (Peromyscus maniculatus bairdii) offspring. Horm Behav. 2013;63(1):180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu X, Zhang J, Wang Y, Ye Y, Luo Q. Perinatal exposure to bisphenol-A impairs learning-memory by concomitant down-regulation of N-methyl-d-aspartate receptors of hippocampus in male offspring mice. Horm Behav. 2010;58(2):326–333. [DOI] [PubMed] [Google Scholar]
- 20. Meng XH, Liu P, Wang H, et al. Gender-specific impairments on cognitive and behavioral development in mice exposed to fenvalerate during puberty. Toxicol Lett. 2011;203(3):245–251. [DOI] [PubMed] [Google Scholar]
- 21. Wang X, Dong Q, Chen Y, et al. Bisphenol A affects axonal growth, musculature and motor behavior in developing zebrafish. Aquat Toxicol. 2013;142-143:104–113. [DOI] [PubMed] [Google Scholar]
- 22. Wang C, Li Z, Han H, et al. Impairment of object recognition memory by maternal bisphenol A exposure is associated with inhibition of Akt and ERK/CREB/BDNF pathway in the male offspring hippocampus. Toxicology. 2016;341-343(1):56–64. [DOI] [PubMed] [Google Scholar]
- 23. Kundakovic M, Gudsnuk K, Franks B, et al. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci. 2013;110(24):9956–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bowyer JF, Thomas M, Patterson TA, George NI, Runnells JA, Levi MS. A visual description of the disection of the cerebral surface vasculature and associated meninges and the choriod plexus from rat brain. J. Vis Exp. 2012;(69):e4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sultan FA. Dissection of different areas from the mouse hippocampus. Bio Protoc. 2013;3(21):e955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holley AJ, Hodges SL, Nolan SO, et al. A single seizure selectively impairs hippocampal-dependent memory and is associated with alterations in PI3K/Akt/mTOR and FMRP signaling. Epilepsia open. 2018;3(4):511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aldad TS, Gan G, Gao XB, Taylor HS. Fetal radiofrequency radiation exposure from 800–1900 mhz-rated cellular telephones affects neurodevelopment and behavior in mice. Sci Rep. 2012;2(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res. 1988;31(1):47–59. [DOI] [PubMed] [Google Scholar]
- 29. Lu C, Wang Y, Xu T, et al. Genistein ameliorates scopolamine-induced amnesia in mice through the regulation of the cholinergic neurotransmission, antioxidant system and the ERK/CREB/BDNF signaling. Front Pharmacol. 2018;9(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. CALB2 (PROTEINTECH CAT# 12278-1-AP, RRID:AB_2228338).
- 31. LCN2 (BIOSS CAT# BS-1373R, RRID:AB_10883642).
- 32. TTR (BIOSS CAT# BS-0152R, RRID:AB_10881832).
- 33. ENPP2 (BIOSS CAT# BS-2437R, RRID:AB_10856152).
- 34. Lessey BA, Castelbaum AJ, Sawin SW, et al. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab. 1994;79(2):643–649. [DOI] [PubMed] [Google Scholar]
- 35. Sharpe-Timms KL, Ricke EA, Piva M, Horowitz GM. Differential expression and localization of de novo synthesized endometriotic haptoglobin in endometrium and endometriotic lesions. Hum Reprod. 2000; 15(10): 2180–2185. [DOI] [PubMed] [Google Scholar]
- 36. Svoboda K, Li N. Neural mechanisms of movement planning: motor cortex and beyond. Curr Opin Neurobiol. 2018;49:33–41. [DOI] [PubMed] [Google Scholar]
- 37. Morici JF, Bekinschtein P, Weisstaub NV. Medial prefrontal cortex role in recognition memory in rodents. Behav Brain Res. 2015;292:241–251. [DOI] [PubMed] [Google Scholar]
- 38. Ko J. Neuroanatomical substrates of rodent social behavior: the medial prefrontal cortex and its projection patterns. Front Neural Circuits. 2017;11(1):2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seidler RD, Bernard JA, Burutolu TB, et al. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34(5):721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Voss JL, Bridge DJ, Cohen NJ, Walker JA. A closer look at the hippocampus and memory. Trends Cogn Sci. 2017;21(8):577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nguyen L, Bohlen J, Stricker J, Chahal I, Zhang H, Pistilli EE. Hippocampus-specific deficiency of IL-15Rα contributes to greater anxiety-like behaviors in mice. Metab Brain Dis. 2017;32(2):297–-302. [DOI] [PubMed] [Google Scholar]
- 42. Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol. 2007;24(2):139–177. [DOI] [PubMed] [Google Scholar]
- 43. Kundakovic M, Champagne FA. Epigenetic perspective on the developmental effects of bisphenol A. Brain Behav Immun. 2011;25(6):1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jorgensen EM, Alderman MH 3rd, Taylor HS. Preferential epigenetic programming of estrogen response after in utero xenoestrogen (bisphenol-A) exposure. FASEB J. 2016;30(9):3194–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rosso AL, Studenski SA, Chen WG, et al. Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci. 2013;68(11):1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sumien N, Sims MN, Taylor HJ, Forster MJ. Profiling psychomotor and cognitive aging in four-way cross mice. Age (Omaha). 2006;28(3):265–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hebert MA, Gerhardt GA. Normal and drug-induced locomotor behavior in aging: comparison to evoked DA release and tissue content in fischer 344 rats. Brain Res. 1998;797(1):42–54. [DOI] [PubMed] [Google Scholar]
- 48. Wooley CM, Xing S, Burgess RW, Cox GA, Seburn KL. Age, experience and genetic background influence treadmill walking in mice. Physiol Behav. 2009;96(2):350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Qin XY, Fukuda T, Yang L, et al. Effects of bisphenol A exposure on the proliferation and senescence of normal human mammary epithelial cells. Cancer Biol Ther. 2012;13(5):296–306. [DOI] [PubMed] [Google Scholar]
- 50. Weinhouse C, Anderson OS, Bergin IL, et al. Dose-dependent incidence of hepatic tumors in adult mice following perinatal exposure to bisphenol A. Environ Health Perspect. 2014;122(5):485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hamm RJ, Pike BR, O’Dell DM, Lyeth BG, Jenkins LW. The Rotarod Test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma. 1994;11(2):187–196. [DOI] [PubMed] [Google Scholar]
- 52. Johnson SA, Javurek AB, Painter MS, et al. Effects of developmental exposure to bisphenol A on spatial navigational learning and memory in rats: a CLARITY-BPA study. Horm Behav. 2016;80(1):139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Manns JR, Stark CEL, Squire LR. The visual paired-comparison task as a measure of declarative memory. Proc Natl Acad Sci. 2000;97(22):12375–-12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rauch SL, Savage CR, Alpert NM, et al. Probing striatal function in obsessive-compulsive disorder: a PET study of implicit sequence learning. J Neuropsychiatry Clin Neurosci. 1997;9(4):568–573. [DOI] [PubMed] [Google Scholar]
- 55. Frith U. Emanuel Miller lecture: confusions and controversies about Asperger syndrome. J Child Psychol Psychiatry. 2004;45(4):672–686. [DOI] [PubMed] [Google Scholar]
- 56. Klinger LG, Dawson G. Prototype formation in autism. Dev Psychopathol. 2001;13(1):111–124. doi: 10.1017/s0954579401001080. [DOI] [PubMed] [Google Scholar]
- 57. Jahn H. Memory loss in Alzheimer’s disease. Dialogues Clin Neurosci. 2013;15(4):445–454. http://www.ncbi.nlm.nih.gov/pubmed/24459411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147(8):3681–3691. [DOI] [PubMed] [Google Scholar]
- 59. Jašarević E, Sieli PT, Twellman EE, et al. Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proc Natl Acad Sci U S A. 2011;108(28):11715–11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gioiosa L, Fissore E, Ghirardelli G, Parmigiani S, Palanza P. Developmental exposure to low-dose estrogenic endocrine disruptors alters sex differences in exploration and emotional responses in mice. Horm Behav. 2007;52(3):307–316. [DOI] [PubMed] [Google Scholar]
- 61. Patisaul H, Fortino A, Polston E. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV☆. Neurotoxicol Teratol. 2006;28(1):111–118. [DOI] [PubMed] [Google Scholar]
- 62. Patisaul HB, Fortino AE, Polston EK. Differential disruption of nuclear volume and neuronal phenotype in the preoptic area by neonatal exposure to genistein and bisphenol-A. Neurotoxicology. 2007;28(1):1–12. [DOI] [PubMed] [Google Scholar]
- 63. Jašarević E, Geary DC, Rosenfeld CS. Sexually selected traits: a fundamental framework for studies on behavioral epigenetics. Ilar J. 2012;53(3-4):253–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Satoh T, Ishige K, Sagara Y. Protective effects on neuronal cells of mouse afforded by ebselen against oxidative stress at multiple steps. Neurosci Lett. 2004;371(1):1–5. [DOI] [PubMed] [Google Scholar]
- 65. Spilker C, Nullmeier S, Grochowska KM, et al. A Jacob/Nsmf gene knockout results in hippocampal dysplasia and impaired BDNF signaling in dendritogenesis. PLoS Genet. 2016;12(3):8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ujjainwala AL, Courtney CD, Rhoads SG, Rhodes JS, Christian CA. Genetic loss of diazepam binding inhibitor in mice impairs social interest. Genes, Brain Behav. 2018;17(5):4–7. [DOI] [PubMed] [Google Scholar]
- 67. Martyn AC, Toth K, Schmalzigaug R, et al. GIT1 regulates synaptic structural plasticity underlying learning. PLoS One. 2018;13(3):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Halfter W, Dong S, Yip YP, Willem M, Mayer U. A critical function of the pial basement membrane in cortical histogenesis. J Neurosci. 2002;22(14):6029–6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ferreira AC, Santos T, Sampaio-Marques B, et al. Lipocalin-2 regulates adult neurogenesis and contextual discriminative behaviours. Mol Psychiatry. 2018;23(4):1031–1039. [DOI] [PubMed] [Google Scholar]
- 70. Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11(5): 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pereira Dias G, Hollywood R, Bevilaqua MC, et al. Consequences of cancer treatments on adult hippocampal neurogenesis: implications for cognitive function and depressive symptoms. Neuro Oncol. 2014;16(4):476–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kim ME, Park HR, Gong EJ, Choi SY, Kim HS, Lee J. Exposure to bisphenol A appears to impair hippocampal neurogenesis and spatial learning and memory. Food Chem Toxicol. 2011;49(12):3383–3389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.