Abstract
Glucosylceramidase (GCase) is a lysosomal enzyme that catalyzes the cleavage of β-glucosidic linkage of glucocerebroside (GC) into glucose and ceramide; thereby, plays an essential function in the degradation of complex lipids and the turnover of cellular membranes.
The growing list of 460 mutations in the gene coding for it—glucosylceramidase beta acid 1 (GBA1)—is reported to abolish its catalytic activity and decrease its enzyme stability, associating it with severe health conditions such as Gaucher disease (GD), Parkinson Disease (PD) and Dementia with Lewy bodies (DLB).
Although the three-dimensional structure of wild type glucosylceramidase is elucidated, little is known about its features in human cells. Moreover, alternative sources of GCase that prove to be effective in the treatment of diseases with enzyme treatment therapies, impose the need for a simple and cost-effective procedure to study the enzyme behavior. This work, for the first time, shows a well-established, yet simple, cost- and time-efficient protocol for the study of GCase enzyme in human leukocytes by the artificial substrate p-Nitrophenyl-β-D-glucopyranoside (PNPG). Characterization of the enzyme in human leukocytes for activation parameters (optimal pH, Km, and Vmax) and enzyme inhibition was done. The results indicate that the optimum pH of GCase enzyme with PNPG is 5.0. The Km and Vmax values are 12.6mM and 333 U/mg, respectively. Gluconolactone competitively inhibits GCase, with a Ki value of 0.023 mM and IC50 of 0.047 mM. Glucose inhibition is uncompetitive with a Ki of 1.94 mM and IC50 of 55.3 mM. This is the first report for the inhibitory effect of glucose, δ-gluconolactone on human leukocyte GCase activity.
Keywords: Proteins, Biochemistry, Molecular biology, Hematological system, Clinical research, Glucosylceramidase, Glucosylceramide, PNPG, Human leukocyte, Enzyme kinetics, Inhibition parameters, Gaucher disease, Glucose, Gluconolactone
Proteins; Biochemistry; Molecular Biology; Hematological System; Clinical Research; glucosylceramidase; glucosylceramide; PNPG; human leukocyte; enzyme kinetics; inhibition parameters; Gaucher Disease; glucose; gluconolactone.
1. Introduction
Glucosylceramidase (GCase, EC 3.2.1.45) is a lysosomal enzyme with 497 amino acids and three discontinuous domains [1]. It is an enzyme with glucosidase activity, that catalyzes the cleavage of glucosylceramide (GC) into glucose and ceramide [2]; thereby, it plays a vital function in the degradation of complex lipids and the turnover of cellular membranes [3]. Non-lysosomal GCase (NLGCase), on the other hand, is an isoenzyme of lysosomal GCase that is detectable in trace amounts in leukocytes but has no structural or sequence homology with it [4]. NLGCase has low expression in leukocytes and does not share the same subcellular compartments [5]. The main cellular compartment of GCase is the lysosomal membrane [6]. The activator proteins Saposin A and C, synergistically facilitate the interaction between the lipid substrates and enzymes, resulting in increased GCase enzymatic activity [7, 8]. The substrate (GC) is synthesized from the degradation of glycosphingolipids in the membranes of apoptotic white blood cells and senescent red blood cells [9].
Medically, Gaucher disease (GD, OMIM#230800) is the most protracted health condition related to the defective GCase activity [8]. Recent studies, however, further associate GCase with severe health conditions such as Parkinson's Disease (PD, OMIM#168600) [10], Dementia with Lewy bodies (DLB, OMIM# 127750) [11] and colorectal cancer [12]. GCase is encoded by the housekeeping gene glucosylceramidase beta acid 1 (GBA1, OMIM∗606463) with cytogenetic location 1q21 [13]. More than 460 mutations are reported to disrupt the GBA1 gene [14, 15], thereby abolishing its catalytic activity [16] and decreasing enzyme stability [17]. The improper breakdown of highly hydrophobic GC results in its accumulation in the lysosomes of macrophages, leading to dysfunctions in the spleen, liver, and bone marrow [9, 18]. The abnormal accumulation and storage of these substances damage tissues and organs, causing the clinical manifestations of GD [19], which is the most frequent lysosomal storage disorder [20, 21]. GD is shown to produce a continuum of different clinical conditions, from the fatal perinatal disorder to the asymptomatic one [22]. The efficiency of enzyme replacement therapy (ERT) in GD, has led to extensive studies carried out in alternative sources of GCase [23, 24]. GD has a global occurrence of 1:50,000 [25], but due to the founder effect, it shows a higher prevalence in Ashkenazi Jewish (Eastern European), Spanish, and Portuguese populations [22]. On the other hand, PD is a closely related neurological complication of GD, which is seen with carriers of heterozygous mutations in the GBA1 gene being susceptible to developing it [10]. Recent genetic and pathological reports suggest GBA1 mutations as critical risk factors for DLB as well [26].
Enzymes with glucosidase activity are isolated from organisms of all three domains (Eukarya, Archaea, and Bacteria) [27]. The wide distribution of enzymes with glucosidase activity in nature, resulted in a myriad of applications in food, textile, detergents, pharmaceuticals, and bioethanol conversion industries, rendering them important enzymes both industrially and economically [28]. When these applications are considered together with the wide array of medical conditions related to elevated Glucosylceramidase activity, high demand for the measurement of it emerges. However, the standardized assays employed are expensive and are not readily applicable in many laboratory settings. Modifications to cover the needs of people within diverse social and medical standards include the use of artificial substrates, such as PNPG and 4MUG, which have relative activities similar to the natural substrate [29]. The sufficiently-sensitive fluorogenic 4MUG substrate limits the reproducibility worldwide due to the unavailability of the instrumental setup [30]. As a result, the use of chlorogenic substrate PNPG offers advantages in testing by requiring small amounts of sample, no cell culturing, and widely available testing equipment.
Detection of GD by GCase assay is done in a variety of human tissues, including the liver [31], brain [7], and placenta [32]. Although any human tissue can be used to study GCase activity, in our study, we used human leukocytes as the most readily available ones and did not differentiate between lymphocytes and granulocytes. In vitro, GCase is solubilized from lysosomal membranes using a detergent—TritonX-100 [33, 34, 35]. The non-ionic detergent Triton X-100 removes the endogenous natural lipid activators that are found in lysosomal membranes [36], consequently rendering Glucosylceramidase inactive [33]. Therefore, the reconstitution of GCase activity requires the inclusion of an anionic detergent, such as sodium taurocholate, in the assay medium [37, 38, 39, 40, 41, 42, 43, 44, 45]. The detergent sodium taurocholate is an essential component of GCase assay and can be used with artificial substrates to measure the GCase activity with minimal interference from other enzymes with glucosidase activity [34]. The two isoenzymes (lysosomal and non-lysosomal GCase) respond differently to inhibitors and sodium taurocholate [46]. Sodium taurocholate inhibits completely the non-lysosomal Gcase activity [31], and stimulates lysozomal GCase activity [45]. Therefore, a crude protein extract is suitable without the need for a purified protein. The lysosomal GCase in vivo is active on its natural lipid substrate glucosylceramide. In vitro, artificial substrates such as PNPG (para-Nitrophenyl-β-D-glucopyranoside) [47, 48, 49] and 4MUG (4-methylumbelliferyl-β-D-glucopyranoside) [50, 51, 52] are very convenient ways of studying its activity due to a high yield of products that strongly absorb around 405 nm [53].
In this work, GCase enzyme activity in human leukocytes by using an artificial substrate is studied and for the first time, the inhibitory effects of glucose and δ-gluconolactone are shown. A novel protocol was designed for brief biochemical characterization of activation parameters (optimal pH, Km, and Vmax) and enzyme inhibition.
2. Materials and methods
2.1. Chemicals and samples
p-nitrophenyl-β-D-glucopyranoside (PNPG), pure sodium taurocholate (6 mg/ml), Triton X-100, glucose, and D-gluconolactone (D-glucono-1,5-lactone), were obtained from Sigma-Aldrich (Germany). Additionally, we used pure sodium acetate buffer (50 mM; 0.4355% w/v CH3COO Na/, 0.1089% w/v CH3COOH, pH 5), and lysis buffer (155 mmol/L NH4Cl; 10 mmol/L NaHCO3; 0.1 mmol/L EDTA) obtained from Semikem (Bosnia and Herzegovina). Blood samples were acquired using the guidelines and approval of the University of Burch Research Ethics Committee (30052016). Written informed consent was obtained from two healthy adult male donors. Whole EDTA blood (10 ml) was collected aseptically.
2.2. Isolation of leukocytes
Leukocytes were isolated by centrifugation after specific lysis of erythrocytes, according to the method of Peters et al. [54]. Aseptically collected whole EDTA blood (10 ml) was centrifuged at 1500 g at 4 °C for ten minutes, followed by the removal of plasma. The original blood volume was restored with 0.9% (w/v) NaCl solution and the blood suspension was transferred to a 50 ml conical centrifuge tube, followed by the addition of 40 ml cold hypotonic lysis buffer. The tube was left to stand on the ice and occasionally mixed for approximately ten minutes. The suspension was centrifuged again at 1500 g at 4 °C for five minutes. Leukocytes formed a small, firm pellet on the side of the tube while the erythrocyte ghosts formed a large loose pellet at the bottom of the tube. Both the hemoglobin-containing supernatant and the loose red cell ghosts were removed by aspiration using a Pasteur pipette. Leukocyte pellet was suspended in 5 ml cold hypotonic lysis buffer. The tube was left to stand on the ice. After 10 min, the cell suspension was diluted up to 50 ml with cold 0.9% (w/v) NaCl solution and centrifuged again at 1500 g at 4 °C for five minutes. The supernatant was discarded, and the leukocyte pellet was suspended in 10 ml 0.9% (w/v) NaCl solution followed by centrifugation at 1500 g at 4 °C for ten minutes. The supernatant was removed entirely, and samples were stored at -80 °C for further use. A step by step protocol is uploaded at protocols.io [55].
2.3. Extraction of GCase from leukocyte pellet
GCase extraction protocol is modified from Magalhaes et al. [34]. Leukocyte cell pellets equivalent to 10 ml of whole blood were thawed and suspended in 1.0 ml of 0.1% (v/v) detergent TritonX-100, and subjected to six cycles of freezing-thawing by alternating tubes, at approximately three-minute intervals, using a dry-ice/ethanol bath and water bath (-70 °C/27 °C). The total homogenate was centrifuged at 2100g for 5 min at 10 °C and supernatant was put into a clean tube in an ice bath for assay. Protein concentration was estimated by the Bradford method, using bovine serum albumin as standard [56].
2.4. Leukocyte glucosylceramidase assay
Glucosylceramidase activity was determined spectrophotometrically using 140 μl assay mixtures [57]. The standard reaction mixture consisted of 70 μl of enzyme solution and 70 μl of 5 mM PNPG substrate in 50 mM sodium acetate buffer (pH 5.0), sodium taurocholate was dissolved in the substrate solution [34]. After incubation at 37 °C for 45 min, the reaction was terminated by the addition of 70 μl of 0.5 M sodium carbonate (Na2CO3, pH 10.3). Absorbance was read at 405 nm against the blank solution containing a substrate buffer instead of the enzyme extract. One unit of GCase activity was defined as the amount of enzyme required to release one μmol of p-nitrophenol from PNPG per minute (1 U), at 37 °C and pH 5.0.
In the past, different approaches were employed to study the enzymatic activity of GCase. In vitro approaches by Elliott et al. using the multiplex-tandem mass spectrometry (MS/MS) [58] served as a feasibility study for new-born screening. In vivo activity-based probes using fluorescent reporters allowed the visualization of GCase activity [59], and recently found implementations in the functional annotation of enzymes [60]. There was also interest in the assessment of GCase activity using lyso-GL1 (the deacylated form of Glucosylceramide by acid ceramidase) as a pathophysiologically relevant biomarker [61]. In this work, we report the use of chlorogenic substrate PNPG to spectrophotometrically measure the GCase activity, which offers a simpler [62, 63], inexpensive [64, 65], and time-efficient [66, 67] protocol for the study of GCase enzyme in human leukocytes.
2.5. In vitro inhibition studies and determination of kinetic parameters
All the samples were assayed in the presence of pure sodium taurocholate at pH 5.0. The substrate concentrations for each sample were: 0.71, 1.07, 1.43, 1.79, and 2.50 mmol per liter. Incubations were carried out at 37 °C for 45 min. The Km and Vmax were obtained through the Lineweaver-Burk plot, whereby the best fit lines were calculated by linear regression analysis. Inhibition experiments were performed using PNPG as substrate, and different final concentrations of δ-gluconolactone and glucose as possible inhibitors. A double reciprocal Lineweaver–Burk plot was used to calculate the parameters. The activity of the Glucosylceramidase at four different concentrations of each inhibitor was determined by regression analysis. Results are expressed as % activity, assuming 100% enzyme activity in the absence of an inhibitor. In the presence of possible inhibitors, % activity was calculated by using the following equation: % activity = 100-[(Ao–Ai)/Ao)] x100, where Ao is the initial GCase activity (without inhibitor), and Ai is the GCase activity with the inhibitor. The inhibitor concentration that reduces the enzymatic activity by 50% (I50 values) was determined from the plots.
3. Results and discussion
3.1. GCase extraction and optimization of stability
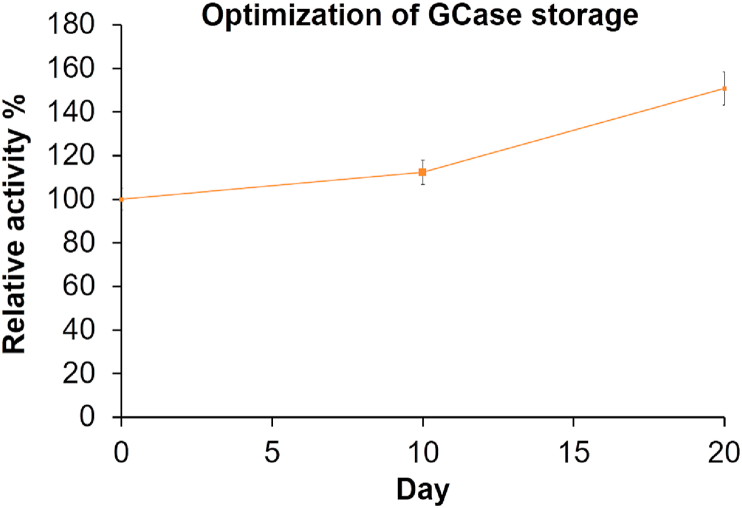
Protein studies require proper preservation of proteins in order to work with the same quality of protein throughout the steps [68]. We performed the optimization study for the stability of GCase in leukocyte homogenate in the course of storage in -20 °C frost-free freezer. It was observed that the activity of GCase, exhibits only minor changes after ten and twenty days of storage, in the presence of pure sodium taurocholate (Figure 1). In our study, the GCase present in the crude protein extract was partially purified. However, the fact that cleavage of PNPG substrate is only possible by GCase, makes our way a convenient one to study the enzyme kinetics of GCase. Furthermore, UV-Vis spectroscopy with an absorbance signal higher than 320nm is widely used to study protein preparations and monitoring enzyme activity [68]. In our case, the product of the catalyzed reaction, p-nitrophenol, was continuously released from PNPG, and this product strongly absorbed light at 405nm [53]. With this theoretical framework, we developed a simple, cost- and time-efficient technique to study the activity of this enzyme with high importance in many diseases and industry.
Figure 1.
Glucosylceramidase (GCase) enzyme stability in human leukocyte homogenate stored at -20 °C. Enzyme activity at day zero was taken as 100%, and the remaining results were normalized according to this value.
3.2. Determination of optimal pH
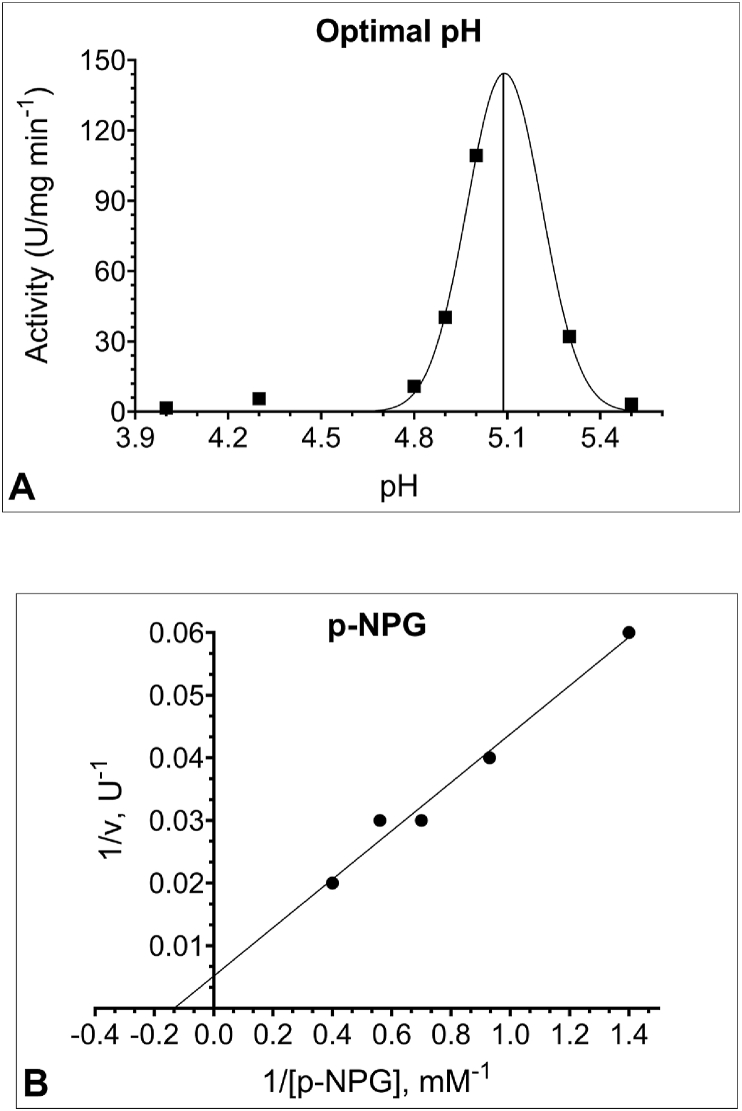
The conformational stability and catalytic activity of enzymes are in direct relationship to their pH [69]. To determine the optimal pH value for the hydrolysis of PNPG by human leukocyte GCase, we examined the effects of variable pH (4.0–5.5) at a constant substrate concentration (5mM). Figure 2A shows the pH activity curve of leukocyte GCase assayed in the presence of sodium taurocholate. Under detergent-containing conditions (Triton X-100, sodium taurocholate, and EDTA) the enzyme activity is solely due to lysosomal GC [70], enabling one to read-out lysosomal GC-specific activity [46]. Leukocyte GCase was optimally active at pH 5.0, after which the enzyme activity started to decline (Figure 2B). The optimal pH of GCase activity from different sources and different substrates is reported to be in 4.7–5.9 intervals [71], which is consistent with its lysosomal function and our finding.
Figure 2.
(A) The optimal pH for human leukocyte Glucosylceramidase (GCase) activity is 5.0. The influence of varying pH values on the activity was determined by using sodium acetate buffer in the pH range 4.0–5.5. (B) Lineweaver-Burk plot for Michaelis constant (Km) determination of GCase at different p-nitrophenyl-β-D-glucopyranoside (PNPG) substrate concentrations (0.71 mM–2.50 mM) and in sodium acetate buffer (50mM, pH 5.0), reveals a Km of 12.6 mM and Vmax of 333 U/mg. All samples were assayed in the presence of 6 mg/ml sodium taurocholate and 70 μl enzyme solution.
3.3. Determination of glucosylceramidase Km and Vmax for PNPG substrate
The Michaelis constant (Km) is a measure of the affinity of the enzyme towards the substrate, with smaller values representing higher affinity. The Michaelis constant (Km) and the maximum rate (Vmax) of leukocyte GCase were obtained through the Lineweaver-Burk plot (Figure 2B) with artificial substrate PNPG in concentrations ranging from 0.71 mM to 2.50 mM. Km and Vmax values were found to be 12.6 mM and 333 U/mg, respectively, in reasonable agreement with previous reports (593 mM) [53].
3.4. In vitro enzyme inhibitor studies
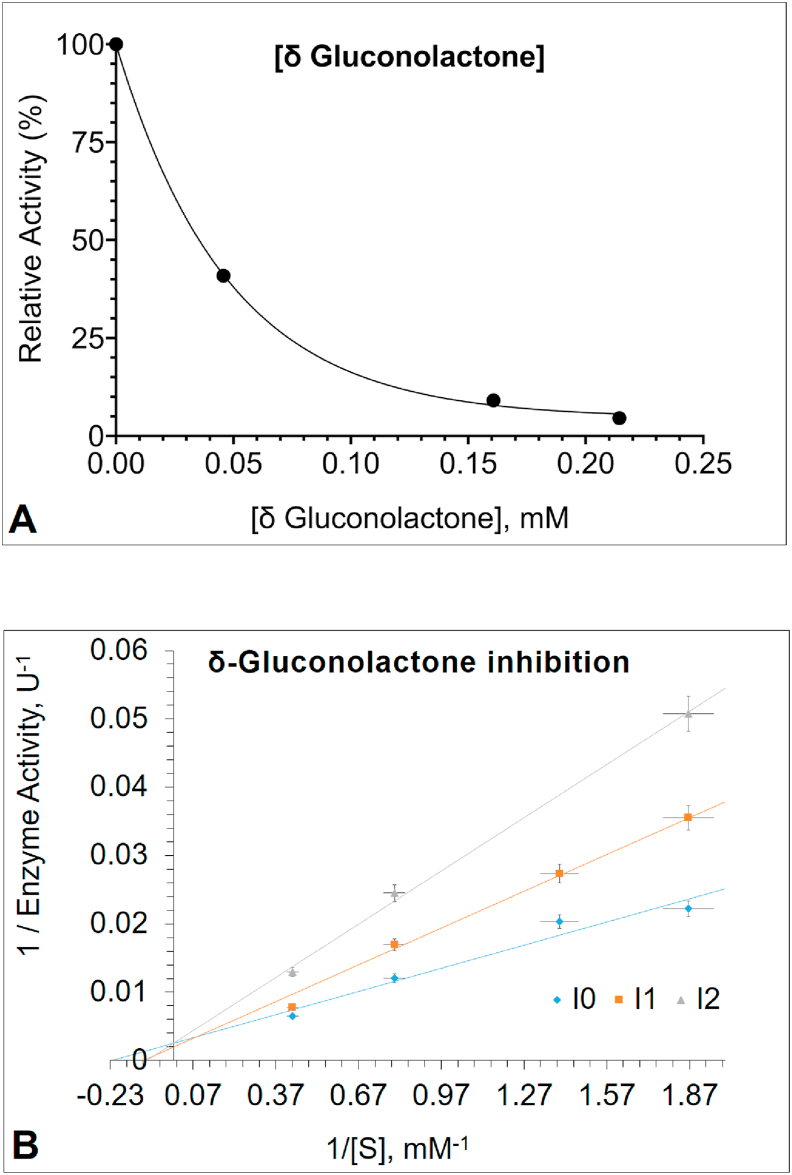
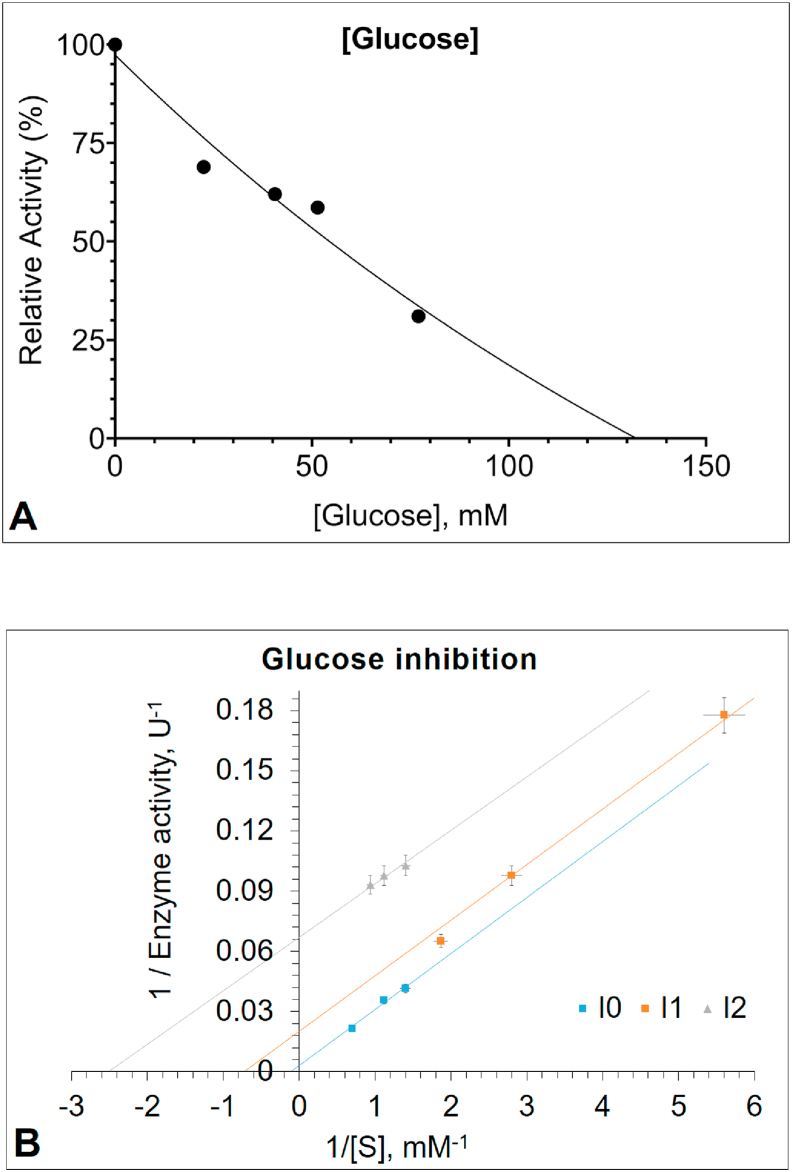
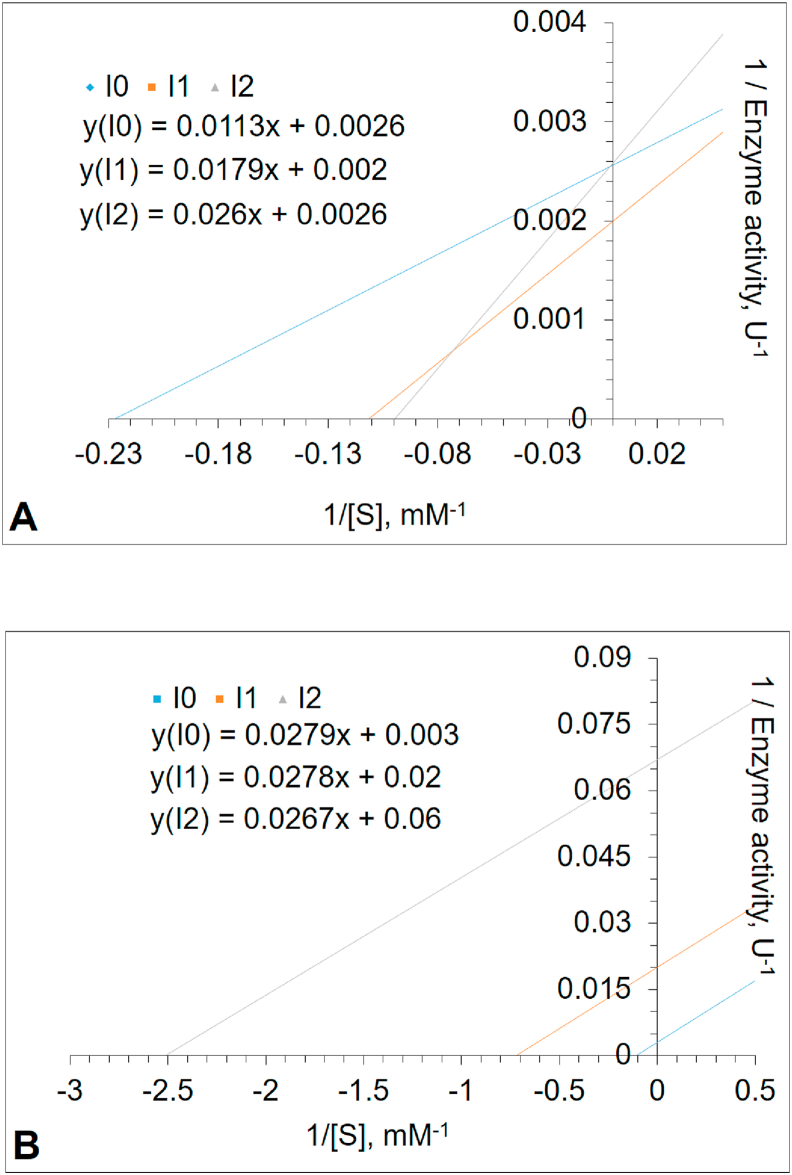
The inhibition study was performed using the PNPG as a substrate. Inhibitors were defined to be gluconolactone and glucose. The results indicated that gluconolactone was the most effective inhibitor acting in a competitive manner, with an IC50 value of 0.047 mM (Figure 3A) and Ki value of 0.023 mM (Figure 3B). The changing apparent Km to higher values in higher gluconolactone concentrations is an indicator of competitive inhibition (Table 1). A report from placental cells has shown similar results [43]. However, as can be seen from Table 1, glucose was shown to be an uncompetitive inhibitor of PNPG hydrolysis, with an IC50 value of 55.3 mM (Figure 4A) and Ki value of 1.94 mM (Figure 4B). The intersection points of Lineweaver–Burk plots in Figures 3B and 4B are shown in Figure 5 in more details. Based on the literature, this is the first study to investigate gluconolactone and glucose inhibition in human leukocyte GCase. However, the inhibition kinetics of the enzymes with glucosidase activity from several plants, fungi and especially microorganisms [24, 27, 28, 72, 73] have extensively been studied using glucose as an inhibitor, since glucose inhibition is undesirable if the enzymatic hydrolysis of cellulose is performed as an industrial process [74, 75].
Figure 3.
(A) The plot of relative enzyme activity (%) of Glucosylceramidase (GCase) in different concentrations of δ-gluconolactone (B) Lineweaver–Burk plot analysis of GCase enzyme, inhibited by the increasing concentration of δ-gluconolactone. GCase activity was measured with p-nitrophenyl-β-D-glucopyranoside (PNPG) as substrate in the absence or presence of δ-gluconolactone (Inhibitor 1 with 0.0128 mM and Inhibitor 2 with 0.0642 mM). The intercept of the plots indicates competitive inhibition by δ-gluconolactone (See Figure 5).
Table 1.
Kinetic parameters of Glucosylceramidase in different concentrations of gluconolactone and glucose.
| I0 |
I1 |
I2 |
Conclusion |
|
|---|---|---|---|---|
| Gluconolactone | ||||
| Vmax and Vmaxapp | 333.3 | 333.3 | 333.3 | Vmax unchanged |
| Km and Kmapp | 3.6 | 5.6 | 8.6 | The apparent Km increased |
| Ki | 0.023 | 0.119 | Competitive Inhibition | |
|
Glucose | ||||
| Vmax and Vmaxapp | 333.3 | 90.9 | 13.5 | Vmax decreased |
| Km and Kmapp | 8.9 | 2.6 | 0.27 | The apparent Km decreased |
| Ki | 2.09 | 1.94 | Uncompetitive Inhibition | |
Figure 4.
(A) The relative activity (%) curve of the Glucosylceramidase (GCase) in the presence of different glucose concentrations (B) Lineweaver–Burk plot analysis of the GCase inhibited by increasing the concentration of glucose. GCase activity was measured with p-nitrophenyl-β-D-glucopyranoside (PNPG) as the substrate, in the absence or presence of glucose concentrations (Inhibitor 1 with 5.07 mM and Inhibitor 2 with 62.8 mM). Glucose inhibits the enzyme in an uncompetitive manner (See Figure 5).
Figure 5.
The intersection points of Lineweaver–Burk plots. The increasing concentration of GCase inhibition by (A) δ-gluconolactone (B) glucose.
Declarations
Author contribution statement
M. Karatas: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
S. Dogan: Conceived and designed the experiments; Wrote the paper.
E. Spahiu: Analyzed and interpreted the data; Wrote the paper.
A. Ašić and L. Bešić: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Y. Turan: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
S.Dogan was supported by the German Research Foundation (DFG-KA 1116/22-1).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Mesut Karatas, Email: mesutkara@yahoo.com.
Emrulla Spahiu, Email: Spahiu.Emrulla@mh-hannover.de.
References
- 1.Smith L., Mullin S., Schapira A.H.V. Insights into the structural biology of Gaucher disease. Exp. Neurol. 2017;298:180–190. doi: 10.1016/j.expneurol.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Beutler E. Gaucher disease: new molecular approaches to diagnosis and treatment. Science. 1992;256:794–799. doi: 10.1126/science.1589760. [DOI] [PubMed] [Google Scholar]
- 3.Magalhaes J., Gegg M.E., Migdalska-Richards A. Autophagic lysosome reformation dysfunction in glucocerebrosidase deficient cells: relevance to Parkinson disease. Hum. Mol. Genet. 2016;25:3432–3445. doi: 10.1093/hmg/ddw185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Interplay between Glucocerebrosidase 1 and Glucocerebrosidase 2; Potential Implications for the Pathogenesis of Gaucher and Parkinson’s Diseases - UCL Discovery. https://discovery.ucl.ac.uk/id/eprint/1571208/
- 5.Matern H., Boermans H., Lottspeich F., Matern S. Molecular cloning and expression of human bile acid beta-glucosidase. J. Biol. Chem. 2001 doi: 10.1074/jbc.M104290200. [DOI] [PubMed] [Google Scholar]
- 6.Yap T.L., Jiang Z., Heinrich F. Structural features of membrane-bound glucocerebrosidase and α-synuclein probed by neutron reflectometry and fluorescence spectroscopy. J. Biol. Chem. 2015;290:744–754. doi: 10.1074/jbc.M114.610584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alroy J., Lyons J.A. Lysosomal storage diseases. J. Inborn. Errors Metab. Screen. 2014;2 2326409813517663. [Google Scholar]
- 8.Grabowski G.A., Gaft S., Horowitz M., Kolodny E.H. Acid β-glucosidase: enzymology and molecular biology of gaucher diseas. Crit. Rev. Biochem. Mol. Biol. 1990;25:385–414. doi: 10.3109/10409239009090616. [DOI] [PubMed] [Google Scholar]
- 9.Glew R.H., Basu A., LaMarco K.L., Prence E.M. Pathology Reviews· 1989. Springer; 1989. Mammalian glucocerebrosidase: implications for Gaucher’s disease; pp. 3–23. [PubMed] [Google Scholar]
- 10.Kim H.-J., Jeon B., Song J. Leukocyte glucocerebrosidase and β-hexosaminidase activity in sporadic and genetic Parkinson disease. Park. Relat. Disord. 2016;23:99–101. doi: 10.1016/j.parkreldis.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Goker-Alpan O., Giasson B.I., Eblan M.J. Glucocerebrosidase mutations are an important risk factor for Lewy body disorders. Neurology. 2006;67:908–910. doi: 10.1212/01.wnl.0000230215.41296.18. [DOI] [PubMed] [Google Scholar]
- 12.Karatas M., Turan Y., Kurtovic-Kozaric A., Dogan Ş. Analysis of gaucher disease responsible genes in colorectal adenocarcinoma. J. Biometrics Biostat. 2016;7 [Google Scholar]
- 13.Cormand B., Montfort M., Chabás A. Genetic fine localization of the β-glucocerebrosidase ( GBA ) and prosaposin ( PSAP ) genes: implications for Gaucher disease. Hum. Genet. 1997;100:75–79. doi: 10.1007/s004390050468. [DOI] [PubMed] [Google Scholar]
- 14.Sheth J., Bhavsar R., Mistri M. Gaucher disease: single gene molecular characterization of one-hundred Indian patients reveals novel variants and the most prevalent mutation. BMC Med. Genet. 2019;20:31. doi: 10.1186/s12881-019-0759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dogan S., Kurtovic-Kozaric A., Karlı G. The Detection of Extremely High and Low Expressed Genes by EGEF Algorithm in Invasive Breast Cancer. J.Biometrics Biost. 2016 [Google Scholar]
- 16.Meivar-Levy I., Horowitz M., Futerman A.H. Analysis of glucocerebrosidase activity using N-(1-[14C]hexanoyl)-D-erythroglucosylsphingosine demonstrates a correlation between levels of residual enzyme activity and the type of Gaucher disease. Biochem. J. 1994;303:377–382. doi: 10.1042/bj3030377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grace M.E., Newman K.M., Scheinker V. Analysis of human acid beta-glucosidase by site-directed mutagenesis and heterologous expression. J. Biol. Chem. 1994;269:2283–2291. [PubMed] [Google Scholar]
- 18.Spahiu E. Thesis, Middle East Technical University; 2015. ATR-FTIR Evaluation of Structural and Functional Changes on Murine Macrophage Cells upon Activation and Suppression by Immuno-Therapeutic Oligodeoxynucleotides. [Google Scholar]
- 19.Beutler E., Demina A., Gelbart T. Glucocerebrosidase mutations in Gaucher disease. Mol. Med. 1994;1:82. [PMC free article] [PubMed] [Google Scholar]
- 20.A Z, A K, T G Gaucher disease. Clinical, laboratory, radiologic, and genetic features of 53 patients. Medicine (Baltim.) 1992;71:337–353. [PubMed] [Google Scholar]
- 21.Dogan S., Mermer E. Comparison of the hemoglobin amount between old and young persons in Bosnia and Herzegovina. J. Biometrics Biostat. 2017;8 [Google Scholar]
- 22.Pastores G.M., Hughes D.A. Gaucher disease. In: Adam M.P., Ardinger H.H., Pagon R.A., editors. GeneReviews®. University of Washington; Seattle, Seattle (WA): 1993. [Google Scholar]
- 23.Naphatsamon U., Ohashi T., Misaki R., Fujiyama K. The production of human β-glucocerebrosidase in nicotiana benthamiana root culture. Int. J. Mol. Sci. 2018;19:1972. doi: 10.3390/ijms19071972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bešić L., Ašić A., Muhović I. Purification and characterization of β-glucosidase from Brassica oleracea. J. Food Process. Preserv. 2017;41 [Google Scholar]
- 25.De Fost M., Aerts J.M., Hollak C.E. Gaucher disease: from fundamental research to effective therapeutic interventions. Neth. J. Med. 2003;61:3–8. [PubMed] [Google Scholar]
- 26.Yang N.-Y., Lee Y.-N., Lee H.-J. Glucocerebrosidase, a new player changing the old rules in Lewy body diseases. Biol. Chem. 2013;394:807–818. doi: 10.1515/hsz-2012-0322. [DOI] [PubMed] [Google Scholar]
- 27.Turan Y., Zheng M. Purification and characterization of an intracellular β-glucosidase from the methylotrophic yeast Pichia pastoris. Biochem. Mosc. 2005;70:1363–1368. doi: 10.1007/s10541-005-0270-5. [DOI] [PubMed] [Google Scholar]
- 28.Ašić A., Bešić L., Muhović I. Purification and characterization of β-glucosidase from agaricus bisporus (white button mushroom) Protein J. 2015;34:453–461. doi: 10.1007/s10930-015-9640-z. [DOI] [PubMed] [Google Scholar]
- 29.Wenger D.A., Clark C., Sattler M., Wharton C. Synthetic substrate ß-glucosidase activity in leukocytes: a reproducible method for the identification of patients and carriers of Gaucher’s disease. Clin. Genet. 1978;13:145–153. doi: 10.1111/j.1399-0004.1978.tb04242.x. [DOI] [PubMed] [Google Scholar]
- 30.Colomer E.G., Gómez M.A.M., Álvarez A.G. Development and application to clinical practice of a validated HPLC method for the analysis of β-glucocerebrosidase in Gaucher disease. J. Pharmaceut. Biomed. Anal. 2014;91:123–130. doi: 10.1016/j.jpba.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 31.Raghavan S.S., Topol J., Kolodny E.H. Leukocyte β-glucosidase in homozygotes and heterozygotes for Gaucher disease. Am. J. Hum. Genet. 1980;32:158. [PMC free article] [PubMed] [Google Scholar]
- 32.Huang C., Freter C. Lipid metabolism, apoptosis and cancer therapy. Int. J. Mol. Sci. 2015;16:924–949. doi: 10.3390/ijms16010924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glew R.H., Basu A., Prence E. Enzymes of Lipid Metabolism II. Springer; 1986. The effects of acidic lipids and heat-stable factor on the physical-chemical and kinetic properties of glucocerebrosidase; pp. 361–370. [Google Scholar]
- 34.Magalhaes J., SáMiranda M.C., Pinto R. Sodium taurocholate effect on β-glucosidase activity: a new approach for identification of Gaucher disease using the synthetic substrate and leucocytes. Clin. Chim. Acta. 1984;141:111–118. doi: 10.1016/0009-8981(84)90003-2. [DOI] [PubMed] [Google Scholar]
- 35.Wattiaux R., De Duve C. Tissue fractionation studies. 7. Release of bound hydrolases by means of Triton X-100. Biochem. J. 1956;63:606. doi: 10.1042/bj0630606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glew R.H., Rosenthal M.D. Oxford University Press; 2006. Clinical Studies in Medical Biochemistry. [Google Scholar]
- 37.Butcher B.A., Gopalan V., Lee R.E. Use of 4-heptylumbelliferyl-β-d-glucoside to identify Gaucher’s disease heterozygotes. Clin. Chim. Acta. 1989;184:235–242. doi: 10.1016/0009-8981(89)90056-9. [DOI] [PubMed] [Google Scholar]
- 38.Daniels L.B., Glew R.H. beta-Glucosidase assays in the diagnosis of Gaucher’s disease. Clin. Chem. 1982;28:569–577. [PubMed] [Google Scholar]
- 39.Ho M.W. Identity of ‘acid’β-glucosidase and glucocerebrosidase in human spleen. Biochem. J. 1973;136:721–729. doi: 10.1042/bj1360721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ho M.W., O’Brien J.S. Gaucher’s disease: deficiency of “cid” β-glucosidase and reconstitution of enzyme activity in vitro. Proc. Natl. Acad. Sci. Unit. States Am. 1971;68:2810–2813. doi: 10.1073/pnas.68.11.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hyun J.C., Misra R.S., Greenblatt D., Radin N.S. Synthetic inhibitors of glucocerebroside β-glucosidase. Arch. Biochem. Biophys. 1975;166:382–389. doi: 10.1016/0003-9861(75)90401-4. [DOI] [PubMed] [Google Scholar]
- 42.Lieberman R.L. A guided tour of the structural biology of Gaucher disease: acid-β-glucosidase and saposin C. Enzym. Res. 2011;2011 doi: 10.4061/2011/973231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michelin K., Wajner A., Bock H. Biochemical properties of β-glucosidase in leukocytes from patients and obligated heterozygotes for Gaucher disease carriers. Clin. Chim. Acta. 2005;362:101–109. doi: 10.1016/j.cccn.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Michelin K., Wajner A., Goulart L da S. Biochemical study on β-glucosidase in individuals with Gaucher’s disease and normal subjects. Clin. Chim. Acta. 2004;343:145–153. doi: 10.1016/j.cccn.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 45.Peters S.P., Coyle P., Glew R.H. Differentiation of β-glucocerebrosidase from β-glucosidase in human tissues using sodium taurocholate. Arch. Biochem. Biophys. 1976;175:569–582. doi: 10.1016/0003-9861(76)90547-6. [DOI] [PubMed] [Google Scholar]
- 46.Berger Z., Perkins S., Ambroise C. Tool compounds robustly increase turnover of an artificial substrate by glucocerebrosidase in human brain lysates. PloS One. 2015;10 doi: 10.1371/journal.pone.0119141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fishman W.H., Springer B., Brunetti R. Application of an improved glucuronidase assay method to the study of human blood β-glucuronidase. J. Biol. Chem. 1948;173:449–456. [PubMed] [Google Scholar]
- 48.Kato K., Yoshida K., Tsukamoto H. Synthesisof p-nitrophenyl β-D-glucopyranosiduronic acid and its utilization as a substrate for the assay of β-glucuronidase activity. Chem. Pharm. Bull. (Tokyo) 1960;8:239–242. [Google Scholar]
- 49.Szasz G. Comparison between p-nitrophenyl glucuronide and phenolphthalein glucuronide as substrates in the assay of β-glucuronidase. Clin. Chem. 1967;13:752–759. [PubMed] [Google Scholar]
- 50.Strasberg P.M., Lowden J.A. The assay of glucocerebrosidase activity using the natural substrate. Clin. Chim. Acta. 1982;118:9–20. doi: 10.1016/0009-8981(82)90222-4. [DOI] [PubMed] [Google Scholar]
- 51.Wenger D.A., Clark C., Sattler M., Wharton C. Synthetic substrate ∖s s-glucosidase activity in leukocytes: a reproducible method for the identification of patients and carriers of Gaucher’s disease. Clin. Genet. 1978;13:145–153. doi: 10.1111/j.1399-0004.1978.tb04242.x. [DOI] [PubMed] [Google Scholar]
- 52.Yu-Bin C., Glew R.H., Diven W.F., Lee R.E. Comparison of various β-glucosidase assays used to diagnose Gaucher’s disease. Clin. Chim. Acta. 1980;105:41–50. doi: 10.1016/0009-8981(80)90090-x. [DOI] [PubMed] [Google Scholar]
- 53.Paal K., Ito M., Withers S.G. Paenibacillus sp. TS12 glucosylceramidase: kinetic studies of a novel sub-family of family 3 glycosidases and identification of the catalytic residues. Biochem. J. 2004;378:141–149. doi: 10.1042/BJ20031028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peters S.P., Lee R.E., Glew R.H. A microassay for Gaucher’s disease. Clin. Chim. Acta. 1975;60:391–396. doi: 10.1016/0009-8981(75)90083-2. [DOI] [PubMed] [Google Scholar]
- 55.Karataş M., Doğan S., Spahiu E., Ašić A., Bešić L., Turan Y. Preparation of leukocytes by differential lysis of erythrocytes. protocols.io. 2020 [Google Scholar]
- 56.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 57.Kara H.E., Sinan S., Turan Y. Purification of beta-glucosidase from olive (Olea europaea L.) fruit tissue with specifically designed hydrophobic interaction chromatography and characterization of the purified enzyme. J. Chromatogr. B. 2011;879:1507–1512. doi: 10.1016/j.jchromb.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 58.Elliott S., Buroker N., Cournoyer J.J. Pilot study of newborn screening for six lysosomal storage diseases using Tandem Mass Spectrometry. Mol. Genet. Metabol. 2016;118:304–309. doi: 10.1016/j.ymgme.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuo C.-L., van Meel E., Kytidou K. Activity-based probes for glycosidases: profiling and other applications. Methods Enzymol. 2018;598:217–235. doi: 10.1016/bs.mie.2017.06.039. [DOI] [PubMed] [Google Scholar]
- 60.Wu L., Armstrong Z., Schröder S.P. An overview of activity-based probes for glycosidases. Curr. Opin. Chem. Biol. 2019;53:25–36. doi: 10.1016/j.cbpa.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 61.Murugesan V., Chuang W.-L., Liu J. Glucosylsphingosine is a key biomarker of gaucher disease. Am. J. Hematol. 2016;91:1082–1089. doi: 10.1002/ajh.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Motabar O., Goldin E., Leister W. A high throughput glucocerebrosidase assay using the natural substrate glucosylceramide. Anal. Bioanal. Chem. 2012;402:731–739. doi: 10.1007/s00216-011-5496-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harlan F.K., Lusk J.S., Mohr B.M. Fluorogenic substrates for visualizing acidic organelle enzyme activities. PloS One. 2016;11 doi: 10.1371/journal.pone.0156312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Geisler J., Thompson T. 2015. Choosing the Best Detection Method: Absorbance vs. Fluorescence.http://www.biocompare.com/Bench-Tips/173963-Choosing-the-Best-Detection-Method-Absorbance-vs-Fluorescence/ [Google Scholar]
- 65.González-Morales D., Valencia A., Díaz-Nuñez A. Development of a low-cost UV-vis spectrophotometer and its application for the detection of mercuric ions assisted by chemosensors. Sensors. 2020;20:906. doi: 10.3390/s20030906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choy F.Y.M., Bouillon L., Laurin C.A. Gaucher disease: accurate identification of asymptomatic French-Canadian carrier using nonlabeled authentic sphingolipid substrate N-palmitoyl dihydroglucocerebroside. Am. J. Med. Genet. 1987;27:895–905. doi: 10.1002/ajmg.1320270416. [DOI] [PubMed] [Google Scholar]
- 67.Barns R.J., Clague A.E. An improved procedure for diagnosis of Gaucher disease using cultured skin fibroblasts and the chromogenic substrate, 2-hexadecanoylamino-4-nitrophenyl-beta-D-glucopyranoside. Clin. Chim. Acta. Int. J. Clin. Chem. 1982;120:57–63. doi: 10.1016/0009-8981(82)90077-8. [DOI] [PubMed] [Google Scholar]
- 68.Raynal B., Lenormand P., Baron B. Quality assessment and optimization of purified protein samples: why and how? Microb. Cell Factories. 2014;13 doi: 10.1186/s12934-014-0180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xia W., Xu X., Qian L. Engineering a highly active thermophilic β-glucosidase to enhance its pH stability and saccharification performance. Biotechnol. Biofuels. 2016;9:147. doi: 10.1186/s13068-016-0560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Körschen H.G., Yildiz Y., Raju D.N. The non-lysosomal β-glucosidase GBA2 is a non-integral membrane-associated protein at the endoplasmic reticulum (ER) and golgi. J. Biol. Chem. 2013;288:3381–3393. doi: 10.1074/jbc.M112.414714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tan Y.L., Genereux J.C., Pankow S. ERdj3 is an endoplasmic reticulum degradation factor for mutant glucocerebrosidase variants linked to gaucher’s disease. Chem. Biol. 2014;21:967–976. doi: 10.1016/j.chembiol.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teugjas H., Väljamäe P. Selecting β-glucosidases to support cellulases in cellulose saccharification. Biotechnol. Biofuels. 2013;6:105. doi: 10.1186/1754-6834-6-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Uchiyama T., Miyazaki K., Yaoi K. Characterization of a novel β-glucosidase from a compost microbial metagenome with strong transglycosylation activity. J. Biol. Chem. 2013;288:18325–18334. doi: 10.1074/jbc.M113.471342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chan C.S., Sin L.L., Chan K.-G. Characterization of a glucose-tolerant β-glucosidase from anoxybacillus sp. DT3-1. Biotechnol. Biofuels. 2016;9:174. doi: 10.1186/s13068-016-0587-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pei J., Pang Q., Zhao L. Thermoanaerobacterium thermosaccharolyticum β-glucosidase: a glucose-tolerant enzyme with high specific activity for cellobiose. Biotechnol. Biofuels. 2012;5:31. doi: 10.1186/1754-6834-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]