Abstract
Postsynaptic nAChRs in the peripheral nervous system are critical for neuromuscular and autonomic neurotransmission. Pre- and peri-synaptic nAChRs in the brain modulate neurotransmission and are responsible for the addictive effects of nicotine. Subtypes of nAChRs in lymphocytes and non-synaptic locations may modulate inflammation and other cellular functions. All AChRs that function as ligand-gated ion channels are formed from five homologous subunits organized to form a central cation channel whose opening is regulated by ACh bound at extracellular subunit interfaces. nAChR subtype subunit composition can range from α7 homomers to α4β2α6β2β3 heteromers. Subtypes differ in affinities for ACh and other agonists like nicotine and in efficiencies with which their channels are opened and desensitized. Subtypes also differ in affinities for antagonists and for positive and negative allosteric modulators. Some agonists are “silent” with respect to channel opening, and AChRs may be able to signal metabotropic pathways by releasing G-proteins independent of channel opening. Electrophysiological studies that can resolve single-channel openings and molecular genetic approaches have allowed characterization of the structures of ligand binding sites, the cation channel, and the linkages between them, as well as the organization of AChR subunits and their contributions to function. Crystallography and cryo-electron-microscopy are providing increasing insights into the structures and functions of AChRs. However, much remains to be learned about both AChR structure and function, the in vivo functional roles of some AChR subtypes, and the development of better pharmacological tools directed at AChRs to treat addiction, pain, inflammation, and other medically important issues.
Introduction
Following the early isolation and characterization of nicotine sensitive acetylcholine-activated ion channels of the neuromuscular junction, similar nicotinic acetylcholine receptors (nAChRs) were found to mediate transmission through autonomic ganglia (for review see (Papke, 2014)). These nAChRs assemble as pentameric complexes that, upon activation by acetylcholine (ACh), form nonspecific cation channels. At neuromuscular junctions and in autonomic ganglia nAChRs are densely packed in specialized structures whose role is to transmit complex patterns of signals rapidly and reliably. Their pharmacology is existential. If they work properly, complex movements and autonomic regulation are possible. Competitive antagonists at controlled doses provide surgical muscle relaxants under controlled conditions (Bowman, 2006). Massive competitive antagonism by cobratoxin (Modahl et al., 2016) or profound desensitization due to nerve gas inhibition of acetylcholinesterase is rapidly lethal (Janik et al., 2019). Selective antagonism of ganglionic nAChRs by blockers like hexamethonium or mecamylamine abolishes all activity of both the sympathetic and parasympathetic branches of the autonomic nervous system, eliminating the dominant tone in all of the various tissues of the body. Without tonic parasympathetic function, heart rate increases, pupils dilate, and gut peristalsis is reduced. Without tonic sympathetic tone, blood vessels relax, and blood pressure and cardiac output are reduced (Katzung et al., 2012).
In the brain, nAChRs are primarily presynaptic or perisynaptic. At these locations they mediate some of the more subtle aspects of cholinergic transmission and are responsible for the addictive properties of nicotine (Dajas-Bailador and Wonnacott, 2004; Gotti et al., 2009; Wonnacott et al., 2000).
Pentameric ligand-gated ion channels
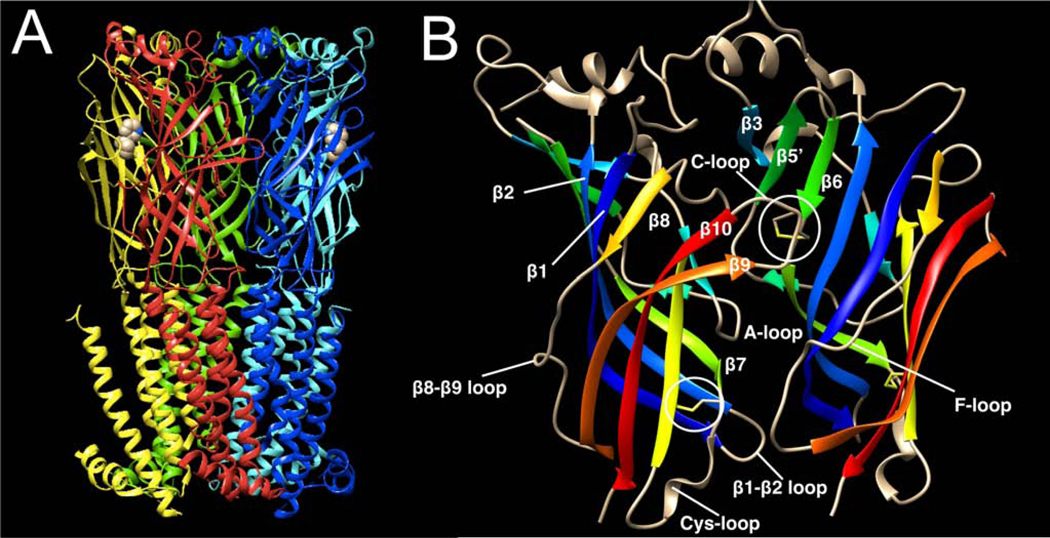
In addition to mediating the rapid ionotropic effects of ACh, related proteins effect inhibitory neurotransmission in the brain by the transmitters GABA and glycine. These proteins, along with one subtype of serotonin receptor, are members of a large superfamily that form pentameric ligand-gated ion channels (Stroud et al., 1990). Each protein subunit (Figure 1) is composed of similar elements: a relatively large extracellular domain (ECD), four transmembrane domains (TM1-TM4) interconnected with linking loops, the third loop comprising an intracellular domain (ICD) between TM3-TM4. Following TM4 there is a short extracellular C-terminal segment. Sequence conservation is greatest in the TM domains and lowest in the ICDs (Stokes et al., 2015), while essential determinants of ligand specificity are in the ECDs. A key feature of these receptors in eukaryotes is a disulfide-linked loop that is positioned at the base of the ECD so it makes an essential contact with the loop connecting the TM2 and TM3 sequences. These elements are vital for coupling ligand binding to channel gating (Bouzat et al., 2008). This structural element has therefore been used to characterize the group as “Cys-loop receptors” (Lester et al., 2004). However, the few homologous proteins that have been found in prokaryotes lack the disulfide link in the domain corresponding to the Cys loop (Tasneem et al., 2005), leading to the proposal that the group be renamed the “Pro-loop receptors” for the universally conserved proline within that domain (Jaiteh et al., 2016).
Figure 1.
Structural models of nAChRs. A) Structure of an α4β2 nAChR homolog binding nicotine at α4/β2 subunit extracellular interfaces based on a recent X-ray crystal structure (Morales-Perez et al., 2016). Note that, although the structure contains the extracellular and transmembrane domains, the more flexibly structured ICD was largely removed in order to obtain the structure. B) An expanded view of an α4/β2 subunit dimer illustrating the key features of primary (left) and complementary (right) surfaces of the orthosteric ligand binding site. Also highlighted is the Cys loop.
Diversity of nicotinic acetylcholine receptors
In total, 16 genes have been identified in mammals that code for full-length nAChR subunits. The nomenclature follows conventions established by the identification of the first four muscle-type nAChR subunits as α1, β1, γ, and δ (Papke, 2014). nAChRs homologous to the mammalian muscle-type nAChRs were biochemically isolated from Torpedo electric ray (Reynolds and Karlin, 1978) and later visualized by electron microscopy (Brisson and Unwin, 1985). The nAChR contained four kinds of homologous subunits. The α subunit was twice as abundant as the other three. Electron micrographs suggested that the nAChR was a pentamer, and so it was correctly deduced that each nAChR had two α1 subunits along with one each of the β1, γ, and δ subunits. The α1β1γδ subunit composition of Torpedo nAChRs is characteristic of embryonic or denervated muscle nAChRs. Receptors of mature neuromuscular junctions were shown to substitute an alternative subunit, ε, for the γ (Gardner, 1990).
The competitive antagonist α-bungarotoxin (α-btx) binds with very high affinity to the α subunits (Haggerty and Froehner, 1981), suggesting location of the ACh binding sites to the α subunits. Studies of ion flux through Torpedo nAChRs reconstituted into lipid vesicles indicated that maximal ion conduction required that both α subunits be available for agonist binding (Sine and Taylor, 1980). Importance of the α subunits for agonist binding was later confirmed by the identification of vicinal cysteines that were unique to the α subunits and when reduced would covalently bind to affinity reagents for the ACh binding site (Kao et al., 1984). As additional nAChR subunit genes were isolated and cloned, the presence of these vicinal cysteines would be the criterion to classify new genes as coding for alpha subunits.
The first clones to be isolated from neuronal tissues included three such alpha subunits (α2, α3, and α4) as well as three subunits lacking the vicinal cysteines, two of which would form functional nAChRs when co-expressed with the muscle genes α1, γ, and ε, leading these subunits to be designated as neuronal beta subunits (β2, β3, and β4), although β3 did not co-assemble with the muscle subunits, and its function remained a mystery for a number of years, as was also the case for the α5 and α6 subunits, which were cloned later (Heinemann et al., 1990).
It was subsequently shown that the α2, α3, or α4 subunits could form functional nAChRs in the Xenopus oocyte heterologous expression system if co-expressed with β2 (Boulter et al., 1987; Deneris et al., 1988; Wada et al., 1988) or β4 subunits (Duvoisin et al., 1989). In situ hybridization showed that the α2, α3, and α4 subunits had distinct, largely non-overlapping patterns of expression in the rat brain, with α4 expressed more widely than the other two subunits, and also that β2 was widely expressed in a pattern that overlapped the expression of all three α subunits (Wada et al., 1989). The overlapping expression pattern of α4 and β2 also corresponded well to autoradiographic localization of sites in the brain that bound radiolabeled nicotine and ACh (Clarke et al., 1985). The purification of the high affinity nAChRs from rat brain confirmed that they contained two types of subunits, with protein sequences predicted for the gene products of α4 and β4 (Whiting and Lindstrom, 1987).
Although, as noted above, some mysteries would take time to resolve regarding the function of certain subunits, these early studies identified basic subtypes of heteromeric neuronal nAChRs, that is, nAChRs that required both alpha and non-alpha subunits for function. Studies of macroscopic and single-channel currents demonstrated that both the alpha and beta subunits were important for determining the pharmacological (Luetje and Patrick, 1991) and kinetic (Papke et al., 1989; Papke and Heinemann, 1991) profiles of the nAChRs. It was also discovered that co-expression of any specific pair of alpha and beta subunits could give rise to more than one channel type, suggesting that nAChRs could form as pentamers like the muscle homologs, but with different stoichiometries (e.g. α/β subunit ratios of 2:3 or 3:2). To test this hypothesis, oocytes were injected with varying ratios of α2 and β2 RNAs to bias the subunit composition. Oocytes injected with equal amounts of α2 and β2 RNA showed both large and small conductance channels, while oocytes injected with α2 RNA and a 9-fold excess of β2 RNA expressed only small conductance channels (Papke et al., 1989).
Studies of site-directed mutants in muscle-type nAChRs (Corringer et al., 2000) indicated that, although the primary surface of the ACh binding sites are on the alpha subunits, functional nAChRs required a complementary surface on the adjacent subunit to complete the binding site. This was confirmed when crystal structures were obtained of ACh-binding proteins isolated from snails (Mukhtasimova et al., 2005). Three subdomains, designated as loops A, B, and C (Figure 1) constitute essential elements on the primary face, the most striking of which in the crystal structures is the C-loop, which forms a sort of lid over a hydrophobic binding pocket for ACh in the apo- (unliganded) form of the nAChR. The alpha subunit vicinal cysteines are at the tip of the C-loop. In the agonist-bound states, the C-loop closes down, allowing the agonist’s cationic core to form π-cation interactions with surrounding aromatic residues (Xiu et al., 2009). These changes reconfigure the structure of subdomains on the complementary surface of the binding site, identified as the D, E, and F, resulting in a concerted conformational change through the entire nAChR, with the Cys loops of the ECDs working with the TM2-TM3 linkers to affect conformation in the pore-forming TM domains. For heteromeric nAChR subtypes, upon agonist binding there is a high initial probability that the nAChR will adopt a conformation in which the TM2 domains form an aqueous channel for ion conduction (Land et al., 1981; Sabey et al., 1999). Channel openings occur in bursts that usually last only a few milliseconds (Papke, 1993), and over time channels are likely to adopt more stable nonconducting (desensitized) conformations (Jones and Westbrook, 1996; Papke, 2010) (Figure 2).
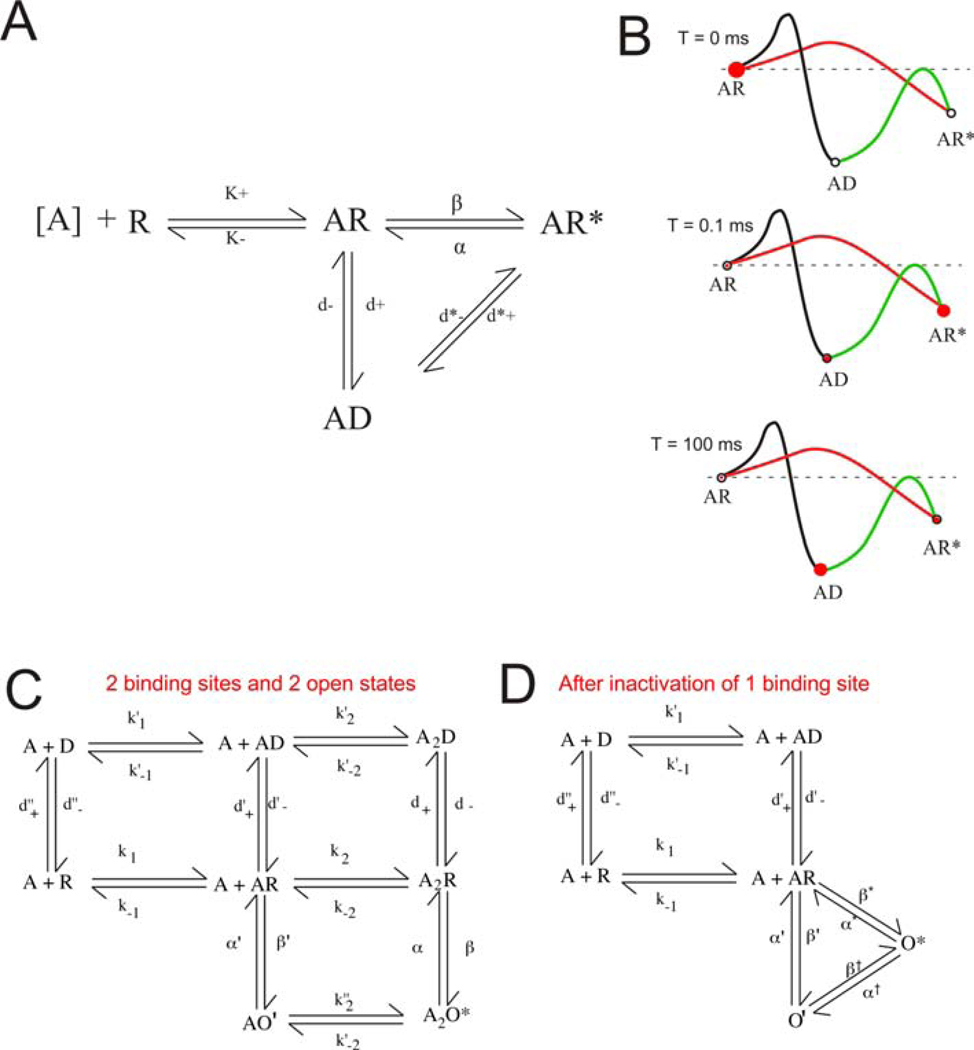
Figure 2.
Activation schemes. A) A minimal model for ligand-gated ion channel activation, involving a single binding site. In the absence of agonist (A), nAChRs are exclusively in the unbound closed state (R). As a function of agonist concentration, nAChRs will occupy the agonist bound state (AR). Note that the forward binding rate (k+) is pseudo first order (M−1s−1); all other rates are first order (s−1). With probability reflected in their relative rates, bound nAChRs will convert to the open state (AR*) or the desensitized state (AD). The model also permits conversion of nAChRs between the AR* and AD states. B) Dynamics of state interconversion as a function of time, the occupancy of specific states represented by the relative size of the red circles. The rate constant for state transitions are inversely proportional to the log of the activation energy barriers connecting the states. Assuming the rapid delivery of a large (saturating), concentration of agonist, at the moment of binding site saturation (t = 0 ms), all nAChRs will be in the AR state. Initially (t = 0.1 ms), nAChR will be most likely to populate the open state. Over time (t = 100 ms), most nAChRs will adopt the low energy desensitized state. C) An expansion of the model in A to account for the presence of two agonist binding sites and the experimental observation that nAChRs show two kinetically distinct open states. D) Collapse of the model in C after a covalent modification of the nAChR that inactivates one of the two ACh binding sites (Williams et al., 2011a).
In a pentameric nAChR, the location of the high affinity ACh binding sites at the interface between non-identical subunits limits the number of such binding sites to two, so that nAChRs contain two ligand-binding dimers and an additional subunit, sometimes considered “structural”. In muscle-type nAChRs, ACh binding sites are between the α1 subunits and either γ, δ, or ε subunits, while the β1 is an obligatory structural subunit. In heteromeric neuronal nAChRs formed by the co-expression of alpha and beta monomers, nAChRs form with an alpha:beta stoichiometry of 2:3 or 3:2 (Nelson et al., 2003). In the laboratory, the relative number of nAChRs taking these two different configurations can be influenced through mass action by biasing the RNA ratios, as was done in the single-channel study previously mentioned (Papke et al., 1989), or controlled more precisely with the use of linked subunit concatamers (Zhou et al., 2003). nAChRs formed with different subunit stoichiometry vary not only in their single-channel properties, but also in their pharmacology (Kuryatov et al., 2008; Lucero et al., 2016; Wang and Lindstrom, 2017; Zwart et al., 2006; Zwart et al., 2008) and ionic permeability (Tapia et al., 2007).
Over time, functional roles were defined for nAChR β3, α5, and α6 subunits, once considered “orphans” (Gerzanich et al., 1996; Gerzanich et al., 1998; Jain et al., 2016; Kuryatov et al., 2000). The α5 subunit, which is part of a gene cluster including α3 and β4 (Boulter et al., 1990), was shown to function as a structural subunit in nAChRs with α3, β2, and β4 (Wang et al., 1996) as well as with α4 and β2 (Kuryatov et al., 2008). The expression of α6 was found to be high in dopaminergic centers of the brain (Charpantier et al., 1998; Goldner et al., 1997), suggesting a potential connection to nicotine addiction. Numerous subsequent studies have confirmed that α6-containing nAChRs play an important role in nicotine-evoked dopamine release in the nucleus accumbens and striatum (Champtiaux et al., 2003; Exley et al., 2008; Gotti et al., 2010; Grady et al., 2007; Jackson et al., 2009; Pons et al., 2008; Wang et al., 2014). Although somewhat difficult to reconstitute in heterologous expression systems (Gerzanich et al., 1996; Kuryatov et al., 2000), in vivo α6 can apparently form nAChRs with other subunits including α4, β2, and β4, often with β3 as a structural subunit (Champtiaux et al., 2003; Cui et al., 2003; Gotti et al., 2010).
Numerous recent studies have highlighted the importance of the specific subunits in the position of the structural subunit, whether in nAChRs containing only one type of α subunit and one type of β subunit or in nAChRs with alternative subunits like α5. This has been best studied with α4β2-containing nAChRs, alone or co-assembled with α5. In addition to differing in single-channel properties, populations of α4(2)β2(3) and α4(3)β2(2) nAChRs are activated by agonists like ACh and nicotine with different potencies; nAChRs with lower EC50 and Imax values are characterized as “high sensitivity” (HS), while “low sensitivity” (LS) α4(3)β2(2) nAChRs express more rapidly, give comparatively larger responses, and respond through a larger range of agonist concentration (Nelson et al., 2003). These differences have been associated with a low-affinity auxiliary agonist binding site at the α4/α4 subunit interface (Wang et al., 2015). These HS and LS forms of α4β2 nAChRs have been shown to differ in other important physiologic properties, including calcium permeability (Tapia et al., 2007), and to have different sensitivity to the experimental agonists, Sazetidine-A (Zwart et al., 2008) and TC-2559 (Moroni et al., 2006), as well as to allosteric modulators (Wang and Lindstrom, 2017) that rely on the difference in subunit interfaces. In vitro, nicotine can serve as a chemical chaperone to increase the absolute level of α4β2 nAChR expression (Moroni et al., 2006) as well as the relative abundance of the HS subtype (Lester et al., 2009), observations that have been proposed to underlie aspects of nicotine dependence and addiction. The α5 subunit will readily co-express with β2–α4 concatamers and function in the position of the structural subunit (Kuryatov et al., 2008). Such α4β2α5 nAChRs have sensitivities to agonists similar to the α4(2)β2(3) subtype (Papke et al., 2013). α3β4α5 nAChRs have lower sensitivities to ACh and nicotine than α4β2 nAChRs and are critical in autonomic ganglia transmission and in the habenula for regulating aversion to high nicotine concentrations and withdrawal from nicotine addiction (Fowler et al., 2011; Gharpure et al., 2019; Lester and Dougherty, 2019; Yuan et al., 2017). α3β4α5 nAChRs are potential targets for drugs for cessation of use of nicotine, alcohol, morphine, and cocaine.
Functions of nicotinic acetylcholine receptors
Studies of nAChRs and other pentameric ligand-gated ion channels usually focus on the eponymous function, binding a ligand (agonist) to promote the opening of an ion channel for the purpose of biological signaling, utilizing the energy of membrane potential and ionic gradients. As noted above, nAChR of the neuromuscular junction and autonomic ganglia function very efficiently in this manner, responding to the synaptic release of ACh with the synchronous activation of large numbers of nAChRs, ultimately leading to the generation of action potentials in the post-synaptic cells. nAChRs in the central nervous system have more diverse and subtle functions, modulating neuronal excitability as well as the release of other neurotransmitters and responses to them due to the presynaptic and perisynaptic expression of these nAChRs (Buhler and Dunwiddie, 2002; Li et al., 1998; Wonnacott, 1997; Wonnacott et al., 2000).
AChRs have been characterized as “allosteric” proteins (Changeux, 1981). The allosteric concept applies to the whole protein and includes homotropic and heterotropic effects (Monod et al., 1965). The most classic example of a homotropic effect in an allosteric protein is the binding of oxygen to either of the heme groups of hemoglobin thereby affecting the affinity of the other. In the case of the nAChR, cooperativity regarding the binding of ACh at the two binding sites would be an example of a homotropic allosteric effect, while increased affinity for ACh at its binding site caused by noncompetitive antagonists is an example of heterotropic allosteric activity. Although these concepts are still applicable, current usage tends to make a distinction between the ACh binding site, referred to as “orthosteric”, and alternative ligand binding sites, which are referred to as “allosteric” if they affect the function of the protein (Williams et al., 2011c). While for the purposes of this review, we will follow the current convention, it should be kept in mind that in the original characterization of nAChRs as “allosteric proteins”, the ACh binding sites were also considered allosteric.
Electrophysiological studies of nAChRs, and single-channel studies in particular, rely on the use of Markov chain models to describe the conformational dynamics of the nAChR (Colquhoun and Hawkes, 1983). Such models assume a relatively small number of discreet conformational states that correspond to what can be observed experimentally as identifiable open and closed states. Virtually all such models are over-simplifications and are really only useful to describe the basic principle of agonist binding leading to channel activation, subsets of interconvertible states such as the openings and closings within single-channel bursts, and the ultimate equilibrium between activation and desensitization. Note that most such models also usually omit the likely existence of the so-called “flip” state, an activated complex preceding channel opening (Lape et al., 2008). One such simplified model is shown in Figure 2A, assuming a single binding site, single open state, and a single desensitized state. Such a model can be used to describe a conformational energy landscape (Papke, 2014) and the probability of state transition over time (Figure 2B). Assuming that all channels are in the resting (bound) closed state at time = 0 ms (Figure 2B, occupancy in a given state represented by the size of the red circle), after a rapid delivery of a saturating concentration of agonist, and since the energy barrier between the resting closed state and the open state is relatively low (i.e. the channel opening rate is relatively high), most channels will enter the open state almost at once. However, after a longer period of time, most channels will be in the more stable desensitized state, although some channels will still return periodically to the open state. This process of low steady-state activity in the presence of predominant desensitization has been referred to as “smoldering” (Campling et al., 2013).
Without explicit knowledge of the precise number of channels contributing to the data, the relatively long closed times between single-channel bursts cannot be ascribed with any confidence to specific closed (unliganded) or desensitized (ligand-bound) states, except arguably at saturating concentrations of agonist, when all nAChRs should be ligand-bound and all long closed states presumably representing desensitized nAChRs (Colquhoun and Ogden, 1988; Sine and Steinbach, 1987). The observations of higher levels of organization in channel behavior, such as groups of clusters of bursts, has been taken as evidence for different levels of desensitization, which necessarily requires expanded models, often based as much on conjecture as on experimental evidence. The model shown in Figure 2C attempts to account for the observation of multiple desensitized states, as well as two different open states, one brief and the other more long lived (Colquhoun and Ogden, 1986; Ogden and Colquhoun, 1985; Sine and Steinbach, 1986). The observation that long duration events were rare at low concentrations of agonist led to the hypothesis that brief events arose from the activation of nAChRs with single agonist molecules bound, while longer duration openings were of nAChRs with both agonist sites bound, although this explanation was not entirely satisfactory since brief events were also occasionally observed with saturating concentrations of agonist (Ogden and Colquhoun, 1985; Papke et al., 1988). It was later shown, with a mutation that permitted the conditional elimination of one agonist binding site, that nAChRs with a single ACh site bound could open to both the brief and long open states under saturating conditions (Williams et al., 2011a), consistent with the model in Figure 2D.
Sequential models are sometimes considered to be at odds with allosteric models (Colquhoun, 2007); however, open channel block, which involves the binding of a noncompetitive antagonist to the first identified putative allosteric site, provides an example of how these different perspectives can be resolved, since the protracted bursts of channel openings, compelled by the binding and unbinding of the blocker, indicate the retention of agonist in its binding site longer than it would be held otherwise (Neher and Steinbach, 1978; Quadri et al., 2019), consistent with the increase in affinity measured long before single-channel currents could be recorded (Changeux and Revah, 1987).
Structural imaging studies of desensitized α4β2 and α3β4 nAChRs reveal that in these desensitized nAChRs, and probably all others, nicotine remains bound to the ACh sites, the activation gate in the middle of the cation channel remains open, and a desensitization gate at the cytoplasmic end of the channel is closed (Gharpure et al., 2019; Walsh et al., 2018). It is likely that type II positive allosteric modulators (PAMs) like Br-PBTC potentiate activation and permit transient reactivation of desensitized nAChRs by promoting opening of the desensitization gate (Norleans et al., 2019).
Diversity of heteromeric nAChRs
As noted above, neuromuscular nAChRs with distinct biophysical properties were observed on mature and embryonic muscle cells, with different subunit composition (Martinou et al., 1991; Mishina et al., 1986). Additionally, nAChRs at mature neuromuscular junctions form in dense structures at specialized endplates (Matthews-Bellinger and Salpeter, 1983), while nAChRs of embryonic or denervated muscle cells are more uniformly distributed over the cell surface (Jaramillo et al., 1988), indicating that the two nAChR subtypes work differently with cytoskeletal proteins (Bloch and Hall, 1982; Huh and Fuhrer, 2002).
Early studies of nAChR subtypes expressed on autonomic neurons showed a great diversity of channel subtypes that varied through development (Moss et al., 1989). While α3 appears to be a common component of most ganglionic neuronal nAChRs, it has been shown to co-assemble with β2 or β4 subunits in varying stoichiometries, sometimes also with α5 (David et al., 2010), each subunit playing a unique role in determining the nAChR’s properties (Luetje and Patrick, 1991; Nai et al., 2003; Papke, 1993; Papke et al., 1991) (Wang et al., 2005).
In the brain there are multiple types of heteromeric nAChRs involving complex combinations and stoichiometries of α4, β2, α5, β4, α2, α6, α3, and β3 subunits (Millar and Gotti, 2009). From the perspective of therapeutics, studies of their pharmacology have been largely focused on nicotine addiction and trying to overcome it. While, as noted above, animal studies implicated α4 and β2 subunits as the primary subunits of high affinity nAChR in the brain, in primates α2 expression may play a larger role in brain nAChR function (Han et al., 2000). From the perspective of nicotine addiction, nAChRs containing α6 and β3, as well as usually α4 and β2 subunits are also important for the rewarding effects of nicotine (Picciotto and Mineur, 2014). Additionally, nAChRs containing α5 subunits have also been shown to influence nicotine use (Frahm et al., 2011; Picciotto and Kenny, 2013) by affecting aversive effects that may limit nicotine use. A specific polymorphism in humans (Bierut et al., 2008; Chen et al., 2009) has been associated with high levels of nicotine use and lung cancer. Some of these effects have been associated with nAChRs containing α5, perhaps in combination with α3 and β4 in the medial habenula (Fowler et al., 2011; Frahm et al., 2011), a brain region with particularly high levels of many nAChR subunits (Connolly et al., 1995; Han et al., 2000; Wada et al., 1989).
Having identified nAChR subtypes associated with addiction, the question is how to target them to aid smoking cessation attempts (Papke and Picciotto, 2012). Nicotine replacement therapies (Rose et al., 1990; Schnoll and Lerman, 2006) do little more than offer a substitute source of nicotine. Modest success has been achieved with high affinity partial agonists for β2-containing nAChRs such as cytisine and varenicline, which selectively desensitize α4β2* nAChRs (the * indicates the possible presence of other subunits, e.g. α6 or β3) (Cahill et al., 2007; Coe et al., 2005; Etter et al., 2008). Such therapies have high rates of relapse, compelling the search for better approaches. Nicotine and varenicline act on α4β2* nAChRs concentrated in reward centers such as the nucleus accumbens and the ventral tegmental area. AT-1001 acts in the brain on α3β4* nAChRs concentrated in the medial habenula and interpecuncular nucleus. AT-1001 also acts thoughout the autonomic nervous system in autonomic ganglia. Knockout of α4 or β2 subunits prevents nicotine self-administration (Marubio et al., 2003; Picciotto et al., 1998). A hypersensitive α4 mutant increases nicotine self-administration (Tapper et al., 2004).
In recent animal studies, attention has focused on AT-1001, a high-affinity desensitizing agonist of α3β4* nAChRs that blocks nicotine self-administration and stress-induced reinstatement of nicotine administration acting in the medial habenula interpeducular pathway. AT-1001 is not re-inforcing, unlike nicotine and varenicline, which are high affinity agonists at α4β2* nAChRs, and where AT-1001 is a lower affinity antagonist (Cippitelli et al., 2015; Gharpure et al., 2019; Lester and Dougherty, 2019; Wu et al., 2014; Yuan et al., 2017; Zaveri et al., 2015). Knocking out α3 subunits prevents autonomic transmission and is lethal (Xu et al., 1999), but antagonism of α3β4 nAChRs with 18-methoxycoronarodine inhibits self-administration of nicotine, morphine, cocaine, and alcohol (Glick and Maisonneuve, 2000; Maisonneuve and Glick, 2003).
A number of other areas have been investigated as potential therapeutic targets related to the heteromeric nAChRs of brain. For example, in Alzheimer’s disease, decreases in the function of both high affinity β2-containing and homomeric α7-type nAChRs have been reported (Levin et al., 2006; Perry et al., 2001), promoting the current use of acetylcholinesterase inhibitors to manage cognitive decline in Alzheimer’s disease. This approach has the advantage of improving the signals mediated by both nicotinic and muscarinic acetylcholine receptors in the brain. An imbalance between nAChR function and other neurotransmitters in the brain has also been associated with depression and related diseases (Mineur and Picciotto, 2010), suggesting the use of the β2 nAChR partial agonists as antidepressants, although the usefulness of these agents has not yet been confirmed in clinical trials in non-smokers (Philip et al., 2009).
Homomeric α7 nAChR
Early autoradiographic studies that identified the high-affinity nAChRs in rat brain, which would come to be associated with the heteromeric nAChR discussed above, also identified a second class of putative nAChR in the brain that bound α-btx (Clarke et al., 1985). These were ultimately associated with the expression of a subunit designated α7 (Couturier et al., 1990; Seguela et al., 1993), which could form functional nAChRs when expressed alone (without the co-expression of a non-alpha subunit). The homomeric nAChRs formed with five α7 subunits were shown to have five potential agonist binding sites (Palma et al., 1996), although it remains unclear whether the binding sites at the α7–α7 interfaces are really identical or functionally equivalent (Gulsevin et al., 2019; Helekar et al., 1994; Rakhilin et al., 1999).
In general, homologs to all the nAChR subunits first cloned from rodents have also been identified in other species, including humans (Anand and Lindstrom, 1992). However, one gene identified as α8 was cloned from chicken, where it is highly expressed in the retina (Gotti et al., 1997), and has not been found to be expressed in mammals.
Unique properties of α7 macroscopic currents
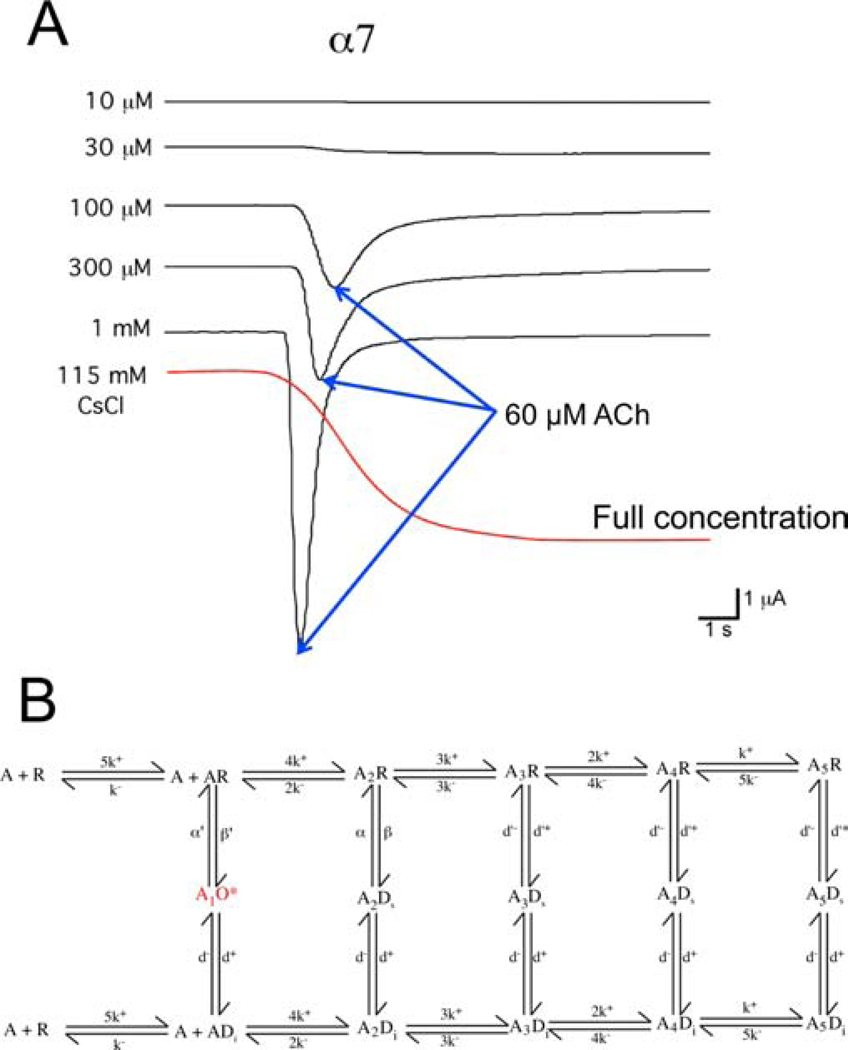
Early studies of α7 expressed in Xenopus oocytes showed that currents in response to ACh applications were very brief, suggesting rapid desensitization. It was also shown that the α7 channels had a relatively high permeability to calcium (Seguela et al., 1993). Both of these features were consistent with the properties of α-btx-sensitive currents of hippocampal neurons (Alkondon and Albuquerque, 1993; Bonfante-Cabarcas et al., 1996). The easiest way to characterize agonist-evoked macroscopic responses, especially in the days before the routine use of computers to store digitized data, was by measurement of peak-current responses. The use of this approach to study the brief currents of α7 nAChRs led to the false conclusion that α7 activation required especially high concentrations (> 200 μM) of ACh to effectively activate the nAChR (Briggs and McKenna, 1998; de Fiebre et al., 1995; Meyer et al., 1998). However, the kinetics of agonist-evoked macroscopic responses are determined by multiple factors including, perhaps most importantly, the kinetics of agonist delivery (Papke, 2010). It is commonly assumed that peak currents occur when the drug concentration is highest; however, through a careful analysis of drug delivery and the timing of α7 nAChR responses, it was discovered that with concentrations of ACh ≥ 60 μM, peak currents occurred prior to the full delivery of the drug (Papke and Thinschmidt, 1998). In fact, channel activation occurred only when the ACh was in the range of 10 – 100 μM, and the peak current always occurred when the ACh concentration was ≈ 60 μM (Figure 3A). The application of ACh concentrations greater than 60 μM had the effect of causing the peak currents to appear earlier, during the leading edge of the drug applications, and with progressively higher concentrations, the time in the effective band of concentration was narrowed, so that the channel activations became more synchronized, resulting in a larger peak current but no more actual nAChR activation when measured as the integrated net charge (Papke and Papke, 2002). Using net charge as a metric, it was shown that the potency of ACh for activating α7 was in fact comparable to that for activating heteromeric nAChR such as those formed by co-expressing monomers of α4 and β2 or α3 and β4 (Papke et al., 2010). What was unique about α7 was the nearly instantaneous effect of high agonist concentrations on desensitizing the nAChR, leading to the hypothesis that activation required only a low level of agonist site occupancy and that higher levels of agonist binding induced the shift to non-conducting conformations (Uteshev et al., 2002). This model for optimal activation of α7 with low fractional occupancy (Figure 3B, model adapted from (Papke et al., 2000)) was supported by studies that showed nAChRs that had covalent inactivation of most of the binding sites could still produce maximal netcharge responses, albeit at higher ACh concentrations (Williams et al., 2011a). Observations related to concentration effects on macroscopic currents from oocyte studies were confirmed with α7 transfected into mammalian cells and studied with rapid agonist applications (Williams et al., 2012). It was also confirmed that single-channel currents of α7 nAChRs with single functional ACh binding sites were indistinguishable from single-channel currents of α7 nAChRs with five intact binding sites (Andersen et al., 2013).
Figure 3.
Concentration-dependent desensitization of α7 nAChR ACh-evoked responses. A) A family of ACh responses of α7 nAChRs expressed in a Xenopus oocyte. Shown in red is an estimation of the rate of solution exchange as measured by the change in junction potential for an open-tipped electrode placed in the recording chamber at the position of an oocyte. By calculating the timing of the peak current relative to the solution exchange profile and the maximum concentration for each application, it was possible to estimate that the ACh concentration at the time of the peak currents was only about 60 μM when concentrations greater that 30 μM were applied (Papke and Thinschmidt, 1998). B) Partial agonist site occupancy model for α7 activation and desensitization (Papke et al., 2000).
Therapeutic targeting of α7 nAChR; selective agonists
The high expression of α7 nAChRs in the hippocampus and cortex, along with its high calcium permeability, made it an attractive therapeutic target for cognitive and neurodegenerative disorders (Kem, 2000). This led to the development of numerous α7-selective agonists, one of the first of which was GTS-21 (DMXBA, 3-(2,4-dimethoxybenzylidene)-anabaseine) (Meyer et al., 1997). The homopentameric structure of α7 suggested that it might be an ancestral type of nAChR. The ACh precursor choline was was found to be an α7-selective agonist (Alkondon et al., 1997; Papke et al., 1996). A structural comparison of a wide range of selective and non-selective nicotinic agonists identified three different structural motifs that could be applied to non-selective agonists to produce an α7-selective agonist or partial agonist (Horenstein et al., 2008). In addition to the choline motif and benzylidene motifs, present in choline and GTS-21, respectively, a third “tropane” motif was identified from a structural dissection of tropisetron (Papke et al., 2005).
Some of the α7-selective drugs identified, GTS-21 included, were partial agonists. Others, such as AR-R17779 (Levin et al., 1999), were potent full agonists. Interestingly, both full and partial agonists were shown to have activity in behavioral assays for learning and memory (Arendash et al., 1995; Levin et al., 1999). It was hypothesized that the calcium permeability of α7 nAChR was important for these in vivo effects, but also a concern that too much activation of a calcium-permeable receptor could lead to cytotoxic effects. While treatment of nerve growth factor-differentiated PC12 cells with low concentrations of GTS-21 could protect cells from dying after nerve growth factor removal, abrupt treatment of PC12 cells with a 100-fold higher concentration of GTS-21 was shown to be toxic. However, if the GTS-21 concentration was elevated slowly, then the high concentrations were not toxic, suggesting qualitative differences in calcium signals based on the mode of delivery (Li et al., 1999). Multiple studies have shown α7 nAChRs capable of modulating intracellular calcium levels (Vijayaraghavan et al., 1992; Yu and Role, 1998; Zhong et al., 2013), though primarily not through nAChR-mediated calcium influx but rather through the control of intracellular calcium release and/or voltage-dependent calcium channels (Dajas-Bailador et al., 2002; King et al., 2018).
Silent agonists and selective desenitizers of α7
Not all compounds that came out of α7 drug development programs were effective in animal models for learning and memory; in fact, one drug, NS6740, stood out for its ability to antagonize the activity of other drugs in such assays (Briggs et al., 2009; Pieschl et al., 2017). NS6740 became a prototype for a class of α7 drugs identified as “silent agonists”. Agonists drive conformational transitions of nAChR through openchannel states then into desensitized states. As noted above, even the most efficacious α7 agonists produce relatively little channel activation and readily drive the nAChRs toward desensitized states. Silent agonists are very weak partial agonists that nonetheless are very effective desensitizers. While the α7 desensitization produced by ACh, choline, or most other agonists is readily reversible, the α7 desensitization produced by NS6740 (Papke et al., 2017) or, to lesser degrees, the desensitization produced by GTS-12 or nicotine (Papke et al., 2009) is very stable and long lived. The desensitization produced by NS6740 appears to be sufficient to block the effects of other α7 drugs (Briggs et al., 2009; Pieschl et al., 2017) and perturb endogenous mechanisms of synaptic plasticity (Papke et al., 2018). The conformational states induced by NS6740 may be associated with alternative forms of α7 signaling (Papke et al., 2015; Thomsen and Mikkelsen, 2012) discussed below.
Alternative binding sites on α7n AChR and nonequivalence of subunit interfaces
Utilizing a bottom-up approach to compare minimally sized non-selective nicotinic agonists, α7-selective agonists, and α7 silent agonists, it was proposed that the binding site for at least some silent agonists is at least partially coincident with the orthosteric agonist (ACh) binding site (Papke et al., 2014a; Quadri et al., 2016). It is unclear, however, whether this simple model can be applied to NS6740 and other large molecules (van Maanen et al., 2015) identified as silent agonists based on their functional properties, or whether large silent agonists might also be working through alternative allosteric binding sites (Gulsevin et al., 2019).
Although α7 subunits assemble as homopentamers, efficient assembly, especially in mammalian cells (Williams et al., 2005), is improved by co-expression with the chaperone protein RIC3 (Halevi et al., 2003) or NACHO (Gu et al., 2016). Two early studies proposed that the α7 subunits in the pentamer are differentially processed, either through proline isomerization (Helekar et al., 1994) or different patterns of disulfide bonding (Rakhilin et al., 1999); however, no subsequent work has supported these studies.
Structural models for α7 ECDs have been proposed (Sixma and Smit, 2003) and progressively refined (Li et al., 2011) (Gulsevin et al, this volume), based originally on crystal structures of acetylcholine binding proteins (AChBPs) isolated from snails (Brejc et al., 2001) and other mollusks (Kaczanowska et al., 2017). These proteins assemble as homopentamers with sequences homologous to the ECDs of nAChRs and therefore have been thought to be particularly useful for modeling the α7 ligand binding domain, especially in the humanized mutant α7–AChBP, where sequence identity to α7 was increased from 26 to 64% (Li et al., 2011). These structures, of course, are not models for functional nAChRs since they lack the transmembrane pore-forming domains and the ICD. While they have not provided evidence for differential processing of subunits that form the pentamer, they have provided evidence for non-equivalent binding of ligands to the five subunit interfaces in the complex for important ligands like GTS-21. Specifically, in three out of the five binding sites in the pentamer, GTS-21 was observed to adopt two alternate orientations in roughly equal proportions. More recently, simulations using molecular dynamics to study a further refined homology model showed that binding of a range of silent agonists differed at the five subunit interfaces, consistent with the concept of allosteric interactions within the complex. One possibility is that ligand binding at one interface could affect subsequent binding of ligands to other interfaces. In addition to identifying differences in the orthosteric binding sites, some ligands were seen to bind preferentially at putative allosteric binding sites (Gulsevin et al., 2019) that were also identified in other computational studies (Delbart et al., 2018; Spurny et al., 2015).
α7 mutants showing altered desenitization
Some of the factors limiting α7 activation were shown to be reduced by site-directed mutants of sites in the ligand binding domain. Most notably, mutations of W55 on the complementary surface of the binding site to valine or alanine slowed the decay of ACh-evoked currents (Gay et al., 2008; Williams et al., 2009). Interestingly, the homologous W57A in β2 subunits yielded α4β2 nAChRs strongly activated by the α7-selective agonist 4OH-GTS-21.
Mutations in the channel domain at either the 6’ or 9’ residues (numbers reflecting the position of the residues within TM2 counting from the intracellular end outward) were also shown to increase the amplitude (Placzek et al., 2005) or reduce the decay rates of α7 currents (Revah et al., 1991), the latter effect proposed to represent decrease in desensitization rates. While nAChRs with the T6’S mutation had fundamentally unchanged pharmacological properties, nAChRs with the greater gain-of-function L9’T mutation profoundly altered the pharmacology, even converting antagonists into activators (Bertrand et al., 1992). The effects of L9’ mutations are not unique to α7 and have been used to produce similar gains of function in heteromeric nAChR (Drenan et al., 2010; Wang et al., 2014). Taken together, the effects of mutations in both the ligand binding domain and the channel suggest global conformation changes involving the ACh binding sites and the cation channel regulate α7 activation and desensitization, and reflect fundamental homologies between homomeric and heteromeric nAChRs.
Allosteric modulation and activation of homomeric α7n AChRs
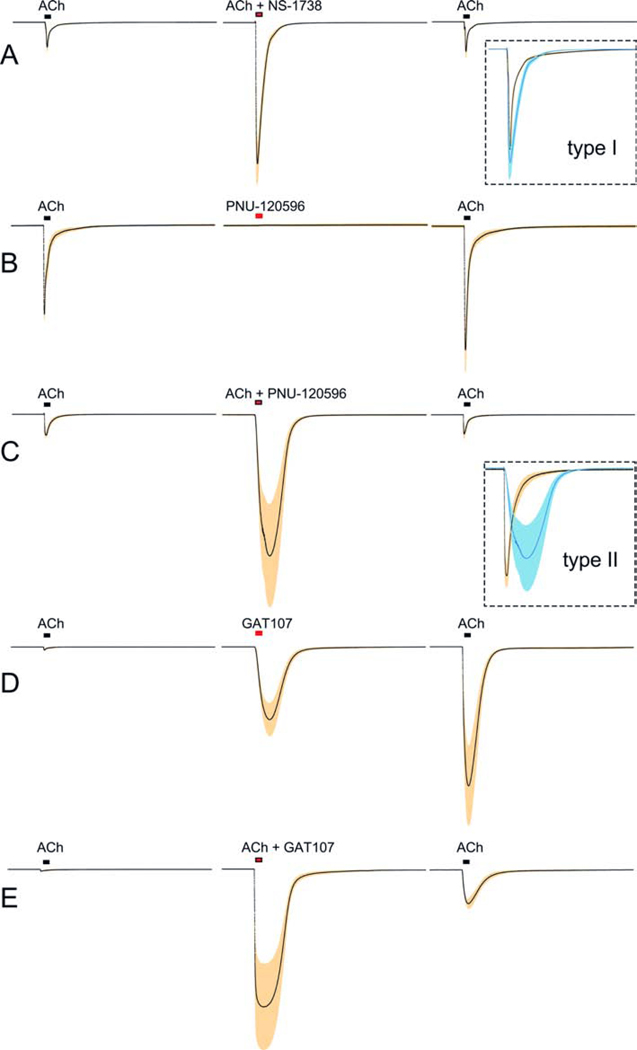
The first drug shown to be a positive allosteric modulator (PAM) of the α7 nAChR was 5-hydroxyindole (5-HI) (Gurley et al., 2000). The relatively modest potentiation (approximately 2-fold increase in peak currents) produced by 5-HI required high concentrations of the drug (≥ 1 mM), and current decay rates were relatively unaffected (Zwart et al., 2002). 5-HI became the prototype for a class of drugs that have been classified as type I α7 PAMs, which primarily increase peak currents without obvious effects on nAChR desensitization (Gronlien et al., 2007; Williams et al., 2011c). NS1738 is a more potent type I PAM (Figure 4A), shown to have cognition-enhancing properties in vivo (Timmermann et al., 2007). A second class of more efficacious PAMs (classified as type II) increase peak currents, greatly decrease current decay rates (Gronlien et al., 2007), and can reactivate nAChRs that were previously desensitized by applications of agonists (Papke et al., 2009). The bath application of PNU-120596, a well-studied and characterized type II PAM, applied on its own does not activate currents in cells expressing α7 (Figure 4B). When co-applied with ACh, PNU-120596 produces large currents of prolonged duration (Figure 4C). Note that in Figure 4B, PNU-120596 was applied after a previous stimulation with ACh; the lack of response indicates that after ACh nAChRs are not maintained in a PAM-sensitive desensitized state. It should be noted that most electrophysiological characterizations of α7-PAMs have been conducted at room temperature, and PAM activity has been shown to be reduced at body temperature (Sitzia et al., 2011). However, endogenous α7 PAMs have been found in serum albumins (Conroy et al., 2003), and these may synergize with synthetic PAMs to reduce the effects of temperature (Williams et al., 2012). Alternatively, the serum albumins may enhance receptor function by extracting compounds, such as long-chain fatty acids, that limit receptor responses in the absence of BSA (Butt et al., 2002).
Figure 4.
Effects of positive allosteric modulators. ACh-evoked responses of α7 receptors expressed in Xenopus oocytes (Gulsevin et al., 2019; Stokes et al., 2019). Each set of traces compares the averaged responses (black line) and the SEM of the averages (shaded areas) for 5 – 8 cells. Individual cell responses were normalized to the initial control responses to 60 μM ACh obtained from the same cells. Each 210 s trace represents 10,000 points acquired at 50 Hz and filtered at 5 Hz. A) Responses to 60 μM ACh co-applied with 10 μM NS-1738, compared to ACh responses before and after the co-application. The inset is a scaled overlay of the ACh control and the potentiated current to illustrate the similarity in kinetics. B) Responses to 10 μM PNU-120596 applied alone, compared to ACh responses before and after the co-application. Note that although there was no response to the PNU-120596, there was a small (p < 0.01) increase in the ACh response after PNU-120596. C) Responses to 60 μM ACh co-applied with 10 μM PNU-120596, compared to ACh responses before and after the co-application. The inset is a scaled overlay of the ACh control and the potentiated current to illustrate the difference in kinetics. D) Responses to 10 μM GAT107 (the active isomer of 4BP-TQS), compared to ACh responses before and after the ago-PAM application. GAT107 application evoked a large response and also produced a large potentiation of the subsequent ACh response. E) Responses to 60 μM ACh co-applied with 10 μM GAT107, compared to ACh responses before and after the co-application.
A large family of allosteric modulators has been developed around the core structure of TQS (4-naphthalene-1-yl-3a,4,5,9b-tetrahydro-3-H-cyclopenta[c]quinoline-8-sulfonic acid amide) (Gill et al., 2012; Gill-Thind et al., 2015). TQS is also a type II PAM, and, as with PNU-120596, nAChRs do not respond when TQS is applied alone, but ACh-evoked responses are increased in amplitude and duration when co-applied with TQS. However, the related compound 4BP-TQS will activate α7 nAChRs when applied alone, identifying it as an allosteric agonist/PAM (ago-PAM) (Gill et al., 2011). Shown in Figure 4D are α7 responses to an application of 10 μM GAT107, the active isomer of 4BP-TQS (Papke et al., 2014b). In addition to the direct allosteric activation produced by GAT107, receptors remained primed for the potentiation of responses evoked by subsequent applications of ACh (Figure 4D) (Papke et al., 2017). GAT107 also very effectively potentiates ACh-evoked responses in co-application (Figure 4E).
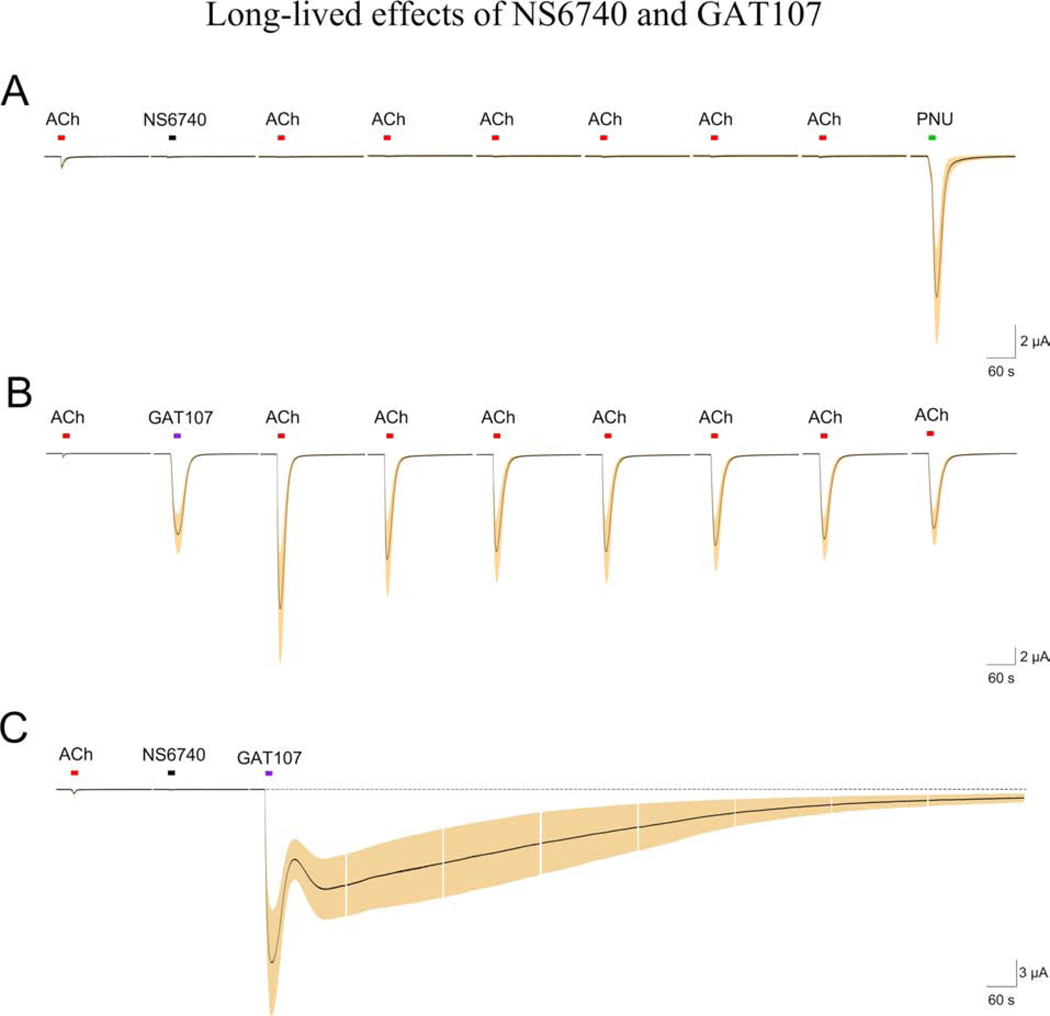
The use of type II PAMs has been very important for confirming the identification of drugs as “silent agonists”, which are able to induce PAM-sensitive desensitization (Papke et al., 2015; Papke et al., 2014a; Quadri et al., 2016). The silent agonist NS6740 is remarkably effective at inducing stable non-conduction states. After application of NS6740, ACh-evoked responses remain desensitized for long periods of time, even after prolonged washout periods, and PNU-120596 applied alone produces large responses from the NS6740-desensitized nAChRs (Papke et al., 2017) (Figure 5). The effects of GAT107 on inducing a stable non-conducting state are also remarkably long-lived but with opposite functional consequences of an NS6740 application, resulting in a unique potential for these agents to interact and produce persistent activation (Figure 5).
Figure 5.
NS6740 and GAT107 produce stable nonconducting states with opposite consequences for subsequent applications of ACh and a unique potential for interaction. A) Single applications of 30 μM NS6740 produce little channel activation but inhibit six subsequent responses to ACh applied at four minute intervals (P < 0.05). Following the applications of ACh, an application 10 μM PNU-120596 produced a response nearly 10-fold larger than the initial ACh controls (p <0.05). B) Currents return to baseline following a single application of 30 μM GAT107; however, receptors remained primed for generation of potentiated ACh-evoked responses over seven subsequent applications of ACh alone (p< 0.05). C) The combined effect of 30 μM NS6740 stable desenitization and the prolonged potentiation produced by 30 μM GAT107 generates persistent currents. The data are taken from Papke et al, 2017 (Papke et al., 2017). Each set of traces illustrates the averaged responses (black line) and the SEM of the averages (shaded areas) for 5 cells. Individual cell responses were normalized to the initial control responses to 60 μM ACh obtained from the same cells. Each 210 s trace represents 10,000 points acquired at 50 Hz and filtered at 5 Hz.
Identifcation of an allosteric modulator site in the transmembrane domains
As noted above, prior to the identification of RIC-3 as a chaperone protein for α7, it was difficult to study α7 expressed in mammalian cell lines. This led to the use of an easily expressible substitute for α7, a chimera between α7 and homopentameric 5-HT3-type serotonin receptors (Craig et al., 2004). nAChRs formed from the chimera that had the ECD of α7 linked to the transmembrane and ICD of the 5HT3 receptor not only expressed well in HEK-293 cells, but also lacked the fast concentration-dependent desensitization characteristic of α7, making the ion currents easier to study, especially in high throughput assay systems. Unlike the α7L9’T mutant, which fails to distinguish between agonists and antagonists, the α7/5-HT3 chimera was a useful reporter for detecting some α7-selective ligands. However, it also gave some misleading results related to the mechanism of α7 activation (Rayes et al., 2009). Although the chimeric nAChRs were reported to have some sensitivity to 5-HI, they were not sensitive to type II PAMs like PNU-120596. This lack of sensitivity to type II PAMs actually allowed the chimera to be a starting point for further studies that located a putative binding site for these PAMs to the α7 transmembrane domains (Bertrand et al., 2008; Young et al., 2008). Ultimately, it was shown that a mutation of the methionine in the 15’ position of the TM2, a residue that is unique to α7, to a leucine, the residue at that site in nearly all other nAChR subunits (all but α9 and α10), eliminated sensitivity to all type II PAMs and ago-PAMs (Papke et al., 2014b; Young et al., 2008). Confirming the importance of the 15’M residue for the activity of α7 PAMs, mutation of the 15’L to M in neuronal β subunits imparted sensitivity to TQS and other select α7 PAMs to heteromeric nAChR (Stokes et al., 2019).
The original observation that allosteric activation by 4BP-TQS was sensitive to the M15’L mutation led to the suggestion that both the allosteric activation and potentiating effects of ago-PAMs were due to binding in the TM2 site (Gill et al., 2011). However, several lines of evidence suggest that direct allosteric activation (DAA) by the active isomer GAT107 requires simultaneous binding to two classes of sites, the TM2 PAM site and additionally an allosteric site in the ECD. Specifically, the kinetics of GAT107 DAA are substantially briefer than the potentiating effects (Papke et al., 2017), suggesting more reversible binding to an allosteric agonism site. The DAA was selectively sensitive to mutations in the ECD that did not substantially affect PAM activity; additionally other TQS analogs that lacked PAM activity were seen to antagonize DAA (Horenstein et al., 2016; Papke et al., 2014b). These observations, along with the identification of a putative allosteric binding site in the α7 vestibule from studies of a humanized α7-AChBP (Delbart et al., 2018; Spurny et al., 2015), suggested that specific ligands might be identified that would bind to a unique extracellular allosteric agonism site and work in concert with a type II PAM binding at the TM2 site to produce channel activation independent of the orthosteric agonist site, providing an alternative mechanism for PAM-dependent silent agonism. Experiments utilized α7C190A, mutants with a disruption of the vicinal disulfide. These nAChRs cannot be activated by ACh alone or in combination with PNU-120596 but are well activated by GAT107. A total of 16 molecules previously identified as silent agonists were used for in silico screening, and 2NDEP (1,1-diethyl-4(naphthalene-2-yl)piperazin-1-ium) showed good predicted binding to the candidate allosteric site. When co-applied with PNU-120596 to α7C190A mutants, 2NDEP produced large responses that were blocked by the allosteric antagonist (silent modulator) 2,3,5,6MP-TQS (Gill-Thind et al., 2015), which also blocked the DAA produced by GAT107 (Horenstein et al., 2016).
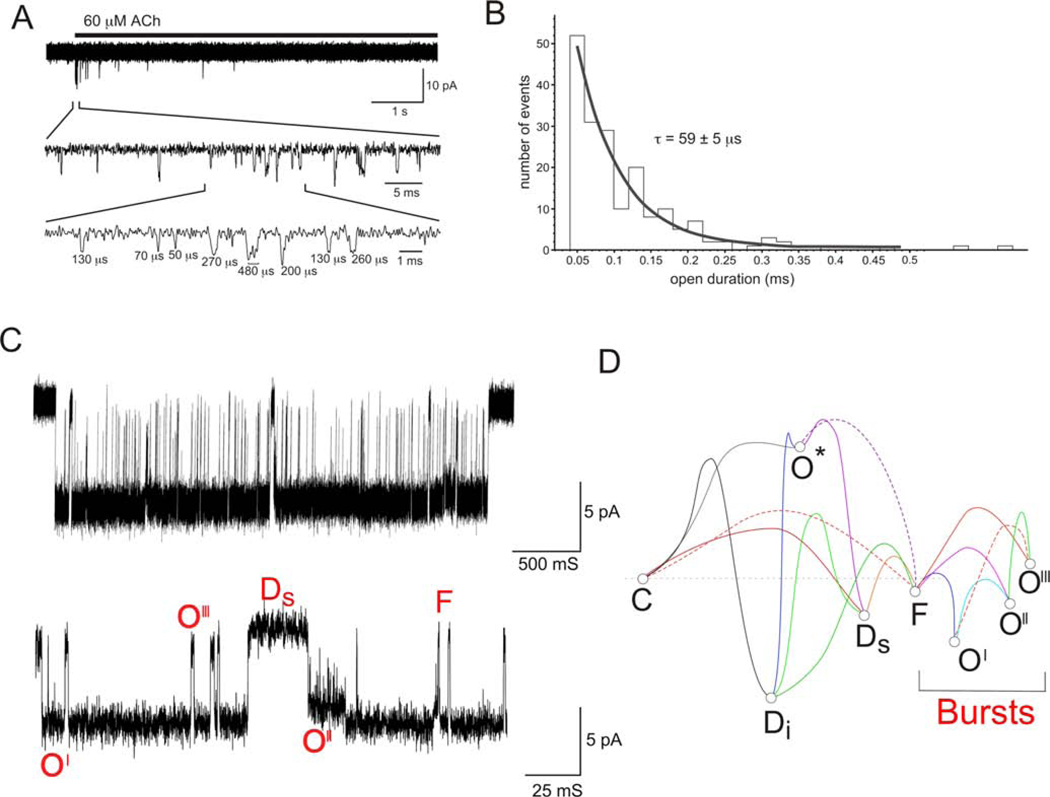
The data therefore suggest at least four distinct classes of sites for potential activation of α7 nAChRs when targeted alone or in combination with PAMs (Figure 5). Of these sites, only the orthosteric agonist site produces significant channel activation in the absence of PAMs, but, as noted earlier, the channel activation through this site is limited by the unique fast desensitization of the nAChR. Activity at the TM2 PAM site overcomes that limitation (Williams et al., 2012; Williams et al., 2011b) but must be combined with simultaneous binding to the orthosteric or allosteric agonist sites. It is likely that each of these sites is present on each subunit or subunit interface, and the details of the conformational dynamics are strongly influenced by the level of ligand occupancy at each site (Williams et al., 2011b). The complexity of the conformational dynamics are most apparent in the single-channel currents recorded from receptors activated by PAMs and ago-PAMs (Andersen et al., 2016; Quadri et al., 2019; Williams et al., 2011b). A representative burst of single α7 nAChR currents in the presence of 10 μM GAT107 is shown in Figure 7. In the absence of a PAM, the average duration of wild-type α7 nAChR single-channel currents is less than 100 μs (Williams et al., 2011b). However, PAM-potentiated currents occur in protracted bursts, typically several seconds in duration. This represents a several hundred thousand-fold increase in the single-channel currents. Another interesting feature of the potentiated currents is the occurrence of subconductance states, the frequency of which will vary depending on the PAM used (Andersen et al., 2016; Quadri et al., 2019; Williams et al., 2011b) and probably on the concentration of the activating ligands, although this has not been studied in detail.
Figure 7.
Single-channel currents of α7 nAChR. A) Representative single-channel currents from an outside-out patch from a cell expressing α7 and Ric-3 and stimulated with the rapid application of ACh. The data is taken from Williams et al, 2011 (Williams et al., 2011b). Single channel current appear as brief isolated events. B) The open time duration distribution was fit with a single time constant of 59 ms. C) Representative burst of single channel activity evoked by 10 μM GAT107, recorded in cell-attached patch-clamp configuration from a cell stably expressing α7 and RIC3 (Quadri et al., 2019). The lower trace displays the center segment of the burst at an expanded time scale in order to resolve the different conductance levels corresponding to the full open state (O’), and two subconductance states (O” and O’’’) (Quadri et al., 2019). There are also multiple intraburst closed states which could correspond to PAM-sensitive desensitized state (Ds) or the active intermediate “Flip” state (F) (Lape et al., 2008). D) A hypothetical energy landscape showing these interconvertable states. Although the absolute assignment of states in the model to conductance levels in the data is only hypothetical, the association of state with the lowest free energy (O’) to the open state with the longest dwell time is reasonable. Likewise, the briefer states, O’’ and O’’’ were assigned to states with higher free energy.
It is important to note the discrepancy between the magnitude of potentiation in macroscopic responses and the single-channel currents. In Figure 4D the net charge of the responses stimulated by 10 μM GAT107 applied alone was 167 (± 20) -fold greater than ACh control responses; this is roughly a thousand-fold less than the effect on the single-channel level. This implies that the effects of the PAMs are large but limited to only a small percentage of the nAChRs at any one time. With such large effects on a small population of channels, it is expected that the variance in PAM-potentiated currents will also be large (Patlak, 1993), consistent with the large standard errors (tan shaded areas) calculated for the averaged current in Figure 4. It should also be noted that the ion conduction pathway in the PAM-potentiated nAChRs is fundamentally different from the channel formed in nAChRs activated by ACh alone. Under normal conditions α7 responses show strong inward rectification, a feature commonly correlated to high calcium permeability (Francis and Papke, 1996; Haghighi and Cooper, 2000). PAM-potentiated currents do not show the inward rectification typical of α7 channels activated by ACh alone (Sitzia et al., 2011), and additionally they have different sensitivity to channel blockers (Peng et al., 2013; Quadri et al., 2019) and reduced calcium permeability (Papke, unpublished).
In recent years there has been a growing appreciation for the importance of α7 expression in non-neuronal tissues, particularly in cells of the immune system (Treinin et al., 2017; Wang et al., 2003). This has created a renewed interest in α7 as a therapeutic target and challenged the perspective that α7 function should only be related to activation of its ion channel and potentially channel-mediated calcium signals. The complex pharmacology and biophysics of α7 nAChRs represent both a challenge and an opportunity for targeted therapeutics, but will require detailed characterization of any candidate compound, especially when considering the possibility for signaling that does not strictly rely on ion channel activation (see below).
α9 α10nAChRs inside and outside the ear
The most well-studied cholinergic efferent synapses in the periphery are those of the neuromuscular junctions and autonomic ganglia, where the principle nAChR subtypes are α1- and α3- containing, respectively. A third descending efferent cholinergic pathway originates in the superior olivary complex of the brainstem and innervates the hair cells of the auditory epithelia (Rasmussen, 1946). This efferent inhibition of hair cells has a unique cholinergic pharmacology. It is blocked by the neuromuscular junction antagonists α-btx and curare, but also by the muscarinic nAChR antagonist atropine and the glycine receptor antagonist strychnine. Curiously, the intracellular response of the outer hair cells to the activation of the cholinergic pathway was observed to be hyperpolarizing (Fuchs and Murrow, 1991). This was apparently due to downstream activation of calcium-dependent potassium channels, suggesting the presence of an nAChR population with high calcium permeability (Elgoyhen and Katz, 2012). While nAChRs formed with α7 subunits had the requisite calcium permeability, they were otherwise a poor match to the pharmacology of the hair cell cholinergic synapse. This motivated a team at the Salk Institute to look further into the rat genomic cDNA library, probing with the α7 sequence at low stringency. This approach led to the cloning of a new nAChR subunit that contained sequence for the extracellular vicinal cysteines and was therefore identified as α9. When expressed alone in Xenopus oocytes, α9 formed nAChRs (albeit somewhat poorly) that showed high calcium permeability and were blocked by α-btx. Other aspects of α9 nAChR pharmacology matched that of the nAChRs in the cochlea, and α9 was confirmed to be expressed at high levels in the inner ear (Elgoyhen et al., 1994). A second related gene, α10 (Boulter et al., 1999), was subsequently found to be co-expressed with α9 in the ear (Elgoyhen et al., 2001). Although α10 did not form functional nAChRs when expressed alone, it improved the expression and function of α9 when the two subunits were co-expressed. Both α9 and α10 are essential for normal cholinergic function in the ear, where the predominant nAChRs are heteropentamers. Studies of the nAChRs expressed in oocytes suggest the usual stoichiometry of two α9 and three α10 subunits (Plazas et al., 2005). Although it was initially proposed that α10 would function strictly by contributing to the complimentary surface of ACh binding sites or as a structural subunit, evidence suggest that it can form the primary surface of ACh binding sites as well (Azam and McIntosh, 2012; Boffi et al., 2017).
Although unique and somewhat unorthodox compared to other neuronal nAChRs, the role played by α9α10 nAChRs in the ear still fits within the usual framework of ligand-gated ion channel function (however, also see below).
AChRs in non-neuronal cells and non-canonical signaling
AChRs expressed are in numerous non-neuronal cell types where they may serve functions that do not require ion channel function at all, but rather affect intracellular signal transduction pathways that are more commonly associated with G-protein-coupled receptors (Bagdas et al., 2017; Egea et al., 2015; King et al., 2017; Rosas-Ballina and Tracey, 2009). Data indicate that the pharmacology for such non-canonical signaling through nAChRs is different from that for optimal channel activation (Horenstein and Papke, 2017; Papke et al., 2015; Thomsen and Mikkelsen, 2012; Zakrzewicz et al., 2017). These differences in pharmacology may indicate that the nAChRs of non-neuronal cells may be physically different from those of neuronal cells due to how the proteins are processed and/or assembled (Richter et al., 2018), or ligands may bind to alternative sites on the nAChRs that modulate (Uteshev, 2014; Wang and Lindstrom, 2017) or activate (Gulsevin et al., 2019) them differently. It is likely that there are ligand-dependent conformational changes affecting the ICDs as well as the channel-forming domains. Just as some ligands (partial agonists), may favor the induction of desensitized states over active states, other ligands, perhaps binding to allosteric sites, may more effectively impact conformational states of the ICDs, where there are specific interactions with signal transduction proteins (Kabbani and Nichols, 2018; Kabbani et al., 2013; King et al., 2018).
The ICDs of nAChRs (Figure 8), especially the non-structured central loop, are the most hypervariable regions of nAChR subunit proteins (Stokes et al., 2015). Although crystal and cryogenic-electron-microscopy structures have recently been published for α4β2 and α3β4 nAChRs that include the transmembrane domains and some helices next to TM3 and TM4 (Gharpure et al., 2019; Morales-Perez et al., 2016), virtually no structural information is available this central loop. The ICDs of nAChR vary greatly in length, with that of α4 being the longest and α5 the shortest (Figure 8). Comparing across species from fish to human, nAChR ICDs vary significantly in sequence conservation. Of the neuronal subunits, the α5 and α7 sequences are among the most well conserved (66.7 and 62.2 % identity, respectively) while α4, α10 and β4 all have only about 36% identity (Stokes et al., 2015).
Figure 8.
ICDs of nAChR subunits illustrating the diversity in length and amino acid character. Intracellular sequences were aligned with Clustal Omega, and colored according to their method (Madeira et al., 2019). Included are the possible helices near TM3 and TM4 with Clustal consensus notations. The putative G-protein binding site (RMKR) of α7 (Kabbani et al., 2013; King et al., 2017) is indicated with a box.
Just as the diversity of ICD sequences suggests unique functional roles for the ICDs of different nAChR subtypes, the evolutionary conservation of sequence for a particular subunit suggests conservation of function. Sites within the ICD are the most likely points for communication between the nAChRs and cellular processes, and that communication most certainly goes in both directions. The phosphorylation states of intracellular sites may regulate functional properties of ion channel signaling, such as desensitization rates; they also affect turnover, assembly, and subcellular localization (for review see (Stokes et al., 2015)). Specific nAChR subtypes each have their own intracellular interactomes involving cytoskeletal proteins and intracellular signaling cascades. For example, electric organ nAChRs were long ago characterized with a rapsyn protein bound to their cytoplasmic domain. While rapsyn has been shown to be involved in stabilizing the semi-crystalline array of nAChRs at neuromuscular junctions (Ramarao and Cohen, 1998), how postsynaptic and presynaptic neuronal nAChRs are anchored remains to be determined.
The discovery of the role of α7 AChR in the vagal-mediated cholinergic anti-inflammatory response (Borovikova et al., 2000; Pavlov et al., 2007; Rosas-Ballina et al., 2009; Rosas-Ballina and Tracey, 2009; van Westerloo et al., 2006) has drawn particular attention to the intracellular interactome of α7 (Paulo et al., 2009). nAChRs in lymphocytes, glia, macrophages, and other non-neural cells are being discovered to have complex roles in suppressing various types of inflammation and associated pain, both peripherally and centrally. Quite where and how some of these nAChRs involved are located and quite how they function is not yet clear. It has been shown that the role of α7 in the cholinergic anti-inflammatory pathway involves signaling through the JAK2/STAT3 pathway, decreasing levels of pro-inflammatory cytokines, such as TNF- α, IL-1β, and IL-6 through inhibition of NF-κB activation and increasing levels of anti-inflammatory cytokines such as IL-10 (Chatterjee et al., 2009; de Jonge et al., 2005; Egea et al., 2015; Marrero and Bencherif, 2009; Zhang et al., 2017). Numerous studies support a role for α7 as a metabotropic as well as an ionotropic nAChR (King et al., 2017; King et al., 2018), and its involvement in the cholinergic anti-inflammatory pathway seems to be independent of ion channel signaling (Kabbani and Nichols, 2018). However, it remains a challenge to sort out exactly how extracellular stimuli are coupling to important sites in the nAChR’s ICD, including a putative G-protein binding epitope (King et al., 2015).
It should be noted that the expression of α9 and α10 nAChRs has also been reported in skin cells (Kurzen et al., 2004), pulmonary (Biallas et al., 2007; Grau et al., 2007) and vascular endothelial cells (Bruggmann et al., 2002), tumor cells (Lee et al., 2010; Mucchietto et al., 2018), and consistently in multiple types of immune cells (Kawashima et al., 2012; Liu et al., 2017; Peng et al., 2004; St-Pierre et al., 2016). Although various functions have been attributed to α9/α10 expression in these non-neuronal tissues, nAChR ion channel currents have not been reported (Peng et al., 2004). As with α7 (see below), the discovery of α9 and/or α10 expression in non-neuronal cells requires that we expand our thinking about nAChR functions and their potential as therapeutic targets, since signaling can clearly occur in the absence of ion channel activation (Kabbani and Nichols, 2018; Lee et al., 2014; Papke et al., 2015; Skok, 2009; Valbuena and Lerma, 2016).
It has been suggested that ion channel-independent activation, whether allosteric or through the induction of specific desensitized states of α7, and perhaps other nAChR subtypes including α9, is associated with release of G-proteins bound to the nAChR cytoplasmic domain. Optimistically, visualizing such silent activation by cryo-EM, especially in the context of a large signaling complex, will someday be possible if the limitations associated with defining structure in the ICD can be overcome.
In conclusion, nAChRs have captured the imaginations of physiologists, pharmacologists, and others for many years. Much has been learned regarding the function of traditional agonists for transient ion channel activation, but much remains to be learned about the complex conformational dynamics of nAChRs regarding non-conducting states and ligands working at allosteric binding sites. Moment to moment we rely on efficient functioning of neuromuscular nAChR; in the words of Charles Sherrington, “To move things is all that mankind can do, for such the sole executant is muscle, whether in whispering a syllable or in felling a forest.” (Linacre lecture, 1924). Likewise, we could shed neither a tear nor a drop of sweat without the action of nAChRs in autonomic ganglia. We still face the challenge of freeing millions of people from the burden of nicotine addiction and the opportunity of developing new therapeutics for cognitive disorders and inflammatory diseases.
Highlights.
Nicotinic acetylcholine receptors (nAChR) function as ligand-gated ion channels.
All nAChR are composed of five homologous or identical subunits.
nAChR are the mediators of all CNS output at neuromuscular and autonomic synapses.
nAChR play diverse and important roles in the central nervous system.
Neuronal nAChR are important therapeutic targets, responsive to ACh and other drugs.
Neuronal nAChR in non-neuronal cells are also therapeutic targets.
nAChR may also function as metabotropic receptors in non-neuronal cells.
Newly discovered allosteric ligands and modulators expand therapeutic possibilities.
Acknowledgements
We thank Dr. Nicole Horenstein for her comments and for the preparation of Figure 1. We also thank Clare Stokes for providing data for Figure 4 and assistance in preparing Figure 8. The data in Figure 6 were provided by Marta Quadri. RLP is supported by NIH RO1 GM057481.
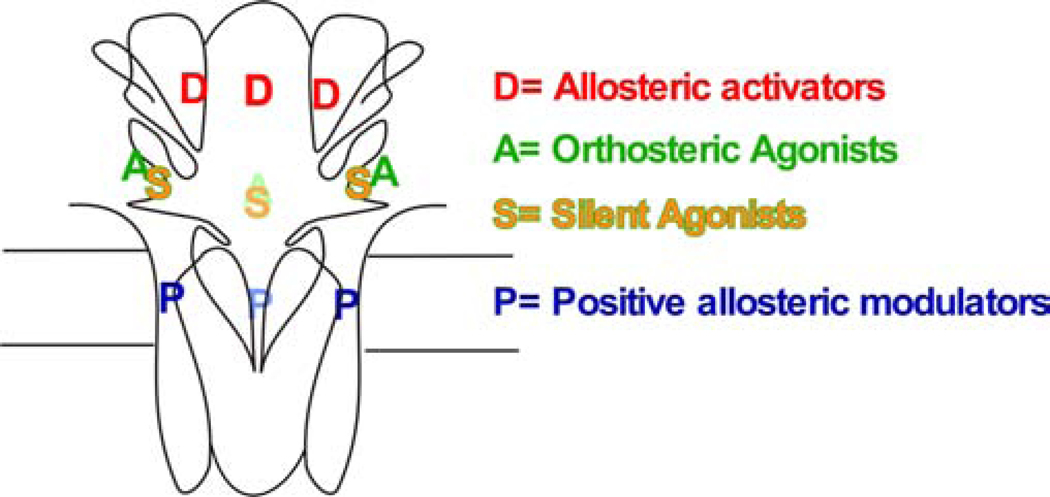
Figure 6.
Orthosteric and allosteric binding sites on an α7 nAChR.
Abbreviations:
- nAChRs
nicotinic acetylcholine receptors
- ACh
acetylcholine
- ECD
extracellular domain
- TM
transmembrane domain
- ICD
intracellular domain
- HS
high sensitivity
- LS
low sensitivity
- GTS-21
3-(2,4-dimethoxybenzylidene)-anabaseine
- AChBP
acetylcholine binding protein
- PAM
positive allosteric modulator
- ago-PAM
allosteric agonist- positive allosteric modulator
- 2NDEP
1,1-diethyl-4(naphthalene-2-yl)piperazin-1-ium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Roger L. Papke, Department of Pharmacology and Therapeutics, University of Florida, P.O. Box 100267, Gainesville, FL 32610-0267, USA
Jon M. Lindstrom, Department of Neuroscience, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, Pennsylvania, USA
References
- Alkondon M, Albuquerque EX, 1993. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J. Pharmacol. Exp. Ther. 265, 1455–1473. [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX, 1997. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci 9, 2734–2742. [DOI] [PubMed] [Google Scholar]
- Anand R, Lindstrom J, 1992. Chromosomal localization of seven neuronal nicotinic acetylcholine receptor subunit genes in humans. Genomics 13, 962–967. [DOI] [PubMed] [Google Scholar]
- Andersen N, Corradi J, Sine SM, Bouzat C, 2013. Stoichiometry for activation of neuronal alpha7 nicotinic receptors. Proc Natl Acad Sci U S A 110, 20819–20824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen ND, Nielsen BE, Corradi J, Tolosa MF, Feuerbach D, Arias HR, Bouzat C, 2016. Exploring the positive allosteric modulation of human alpha7 nicotinic receptors from a single-channel perspective. Neuropharmacology 107, 189–200. [DOI] [PubMed] [Google Scholar]
- Arendash GW, Sengstock GJ, Sanberg PR, Kem WR, 1995. Improved learning and memory in aged rats with chronic administration of the nicotinic receptor agonist GTS-21. Brain Res 674, 252–259. [DOI] [PubMed] [Google Scholar]
- Azam L, McIntosh JM, 2012. Molecular basis for the differential sensitivity of rat and human alpha9alpha10 nAChRs to alpha-conotoxin RgIA. J Neurochem 122, 1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, Gurun MS, Flood P, Papke RL, Damaj MI, 2017. New Insights on Neuronal Nicotinic Acetylcholine Receptors as Targets for Pain and Inflammation: A Focus on alpha7 nAChRs. Curr Neuropharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D, Bertrand S, Cassar S, Gubbins E, Li J, Gopalakrishnan M, 2008. Positive allosteric modulation of the alpha7 nicotinic acetylcholine receptor: ligand interactions with distinct binding sites and evidence for a prominent role of the M2-M3 segment. Mol Pharmacol 74, 1407–1416. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Devillers-Thiéry A, Revah F, Galzi J-L, Hussy N, Mulle C, Bertrand S, Ballivet M, Changeux J-P, 1992. Unconventional pharmacology of a neuronal nicotinic receptor mutated in the channel domain. Proc. Natl. Acad. Sci. USA 89, 1261–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biallas S, Wilker S, Lips KS, Kummer W, Grando SA, Padberg W, Grau V, 2007. Immunohistochemical detection of nicotinic acetylcholine receptor subunits alpha9 and alpha10 in rat lung isografts and allografts. Life Sci 80, 2286–2289. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, Horton WJ, Breslau N, Budde J, Cloninger CR, Dick DM, Foroud T, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Kuperman S, Madden PA, Mayo K, Nurnberger J Jr., Pomerleau O, Porjesz B, Reyes O, Schuckit M, Swan G, Tischfield JA, Edenberg HJ, Rice JP, Goate AM, 2008. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry 165, 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch RJ, Hall ZW, 1982. Cytoskeletal components of the vertebrate neuromuscular junction: vinculin, alpha-actinin and filamin. J. Cell Biol. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffi JC, Marcovich I, Gill-Thind JK, Corradi J, Collins T, Lipovsek MM, Moglie M, Plazas PV, Craig PO, Millar NS, Bouzat C, Elgoyhen AB, 2017. Differential Contribution of Subunit Interfaces to alpha9alpha10 Nicotinic Acetylcholine Receptor Function. Mol Pharmacol 91, 250–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfante-Cabarcas R, Swanson KL, Alkondon M, Albuquerque EX, 1996. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. IV. Regulation by external Ca++ of alpha-bungarotoxin-sensitive receptor function and of rectification induced by internal Mg++. J Pharmacol Exp Ther 277, 432–444. [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ, 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462. [DOI] [PubMed] [Google Scholar]
- Boulter J, Connolly J, Deneris E, Goldman D, Heinemann S, Patrick J, 1987. Functional expression of two neural nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc Natl Acad Sci USA 84, 7763–7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J, Elgoyhen B, Katz E, Rothlin C, Vetter D, 1999. Alpha 10: A novel nicotinic acetylcholine receptor subunit gene. Unpublished GenBank accession number, AF196344. [Google Scholar]
- Boulter J, O’Shea-Greenfield A, Duvoisin RM, Connolly JG, Wada E, Jensen A, Gardner PD, Ballivet M, Deneris ES, McKinnon D, et al. , 1990. Alpha 3, alpha 5, and beta 4: three members of the rat neuronal nicotinic acetylcholine receptor-related gene family form a gene cluster. J Biol Chem 265, 4472–4482. [PubMed] [Google Scholar]
- Bouzat C, Bartos M, Corradi J, Sine SM, 2008. The interface between extracellular and transmembrane domains of homomeric Cys-loop receptors governs openchannel lifetime and rate of desensitization. J Neurosci 28, 7808–7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman WC, 2006. Neuromuscular block. Br J Pharmacol 147 Suppl 1, S277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK, 2001. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 411, 269–276. [DOI] [PubMed] [Google Scholar]
- Briggs CA, Gronlien JH, Curzon P, Timmermann DB, Ween H, Thorin-Hagene K, Kerr P, Anderson DJ, Malysz J, Dyhring T, Olsen GM, Peters D, Bunnelle WH, Gopalakrishnan M, 2009. Role of channel activation in cognitive enhancement mediated by alpha7 nicotinic acetylcholine receptors. Br J Pharmacol 158, 1486–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs CA, McKenna DG, 1998. Activation and inhibition of the human alpha7 nicotinic acetylcholine receptor by agonists. Neuropharmacology 37, 1095–1102. [DOI] [PubMed] [Google Scholar]
- Brisson A, Unwin PNT, 1985. Quaternary structure of the acetylcholine receptor. Nature 315, 474–477. [DOI] [PubMed] [Google Scholar]
- Bruggmann D, Lips KS, Pfeil U, Haberberger RV, Kummer W, 2002. Multiple nicotinic acetylcholine receptor alpha-subunits are expressed in the arterial system of the rat. Histochem Cell Biol 118, 441–447. [DOI] [PubMed] [Google Scholar]
- Buhler AV, Dunwiddie TV, 2002. alpha7 nicotinic acetylcholine receptors on GABAergic interneurons evoke dendritic and somatic inhibition of hippocampal neurons. J Neurophysiol 87, 548–557. [DOI] [PubMed] [Google Scholar]
- Butt CM, Hutton SR, Marks MJ, Collins AC, 2002. Bovine serum albumin enhances nicotinic acetylcholine receptor function in mouse thalamic synaptosomes. J Neurochem 83, 48–56. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T, 2007. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev, CD006103. [DOI] [PubMed] [Google Scholar]
- Campling BG, Kuryatov A, Lindstrom J, 2013. Acute activation, desensitization and smoldering activation of human acetylcholine receptors. PLoS One 8, e79653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, Clementi F, Moretti M, Rossi FM, Le Novere N, McIntosh JM, Gardier AM, Changeux JP, 2003. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci 23, 7820–7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J-P, 1981. The acetylcholine receptor: an “allosteric” membrane protein. Academic Press Inc, New York. [PubMed] [Google Scholar]
- Changeux J-P, Revah F, 1987. The acetylcholine receptor molecule: allosteric sites and the ion channel. Trends Neurosci. 10, 245–250. [Google Scholar]
- Charpantier E, Barneoud P, Moser P, Besnard F, Sgard F, 1998. Nicotinic acetylcholine subunit mRNA expression in dopaminergic neurons of the rat substantia nigra and ventral tegmental area. Neuroreport 9, 3097–3101. [DOI] [PubMed] [Google Scholar]
- Chatterjee PK, Al-Abed Y, Sherry B, Metz CN, 2009. Cholinergic agonists regulate JAK2/STAT3 signaling to suppress endothelial cell activation. Am J Physiol Cell Physiol 297, C1294–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Chen J, Williamson VS, An SS, Hettema JM, Aggen SH, Neale MC, Kendler KS, 2009. Variants in nicotinic acetylcholine receptors alpha5 and alpha3 increase risks to nicotine dependence. Am J Med Genet B Neuropsychiatr Genet 150B, 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Wu J, Gaiolini KA, Mercatelli D, Schoch J, Gorman M, Ramirez A, Ciccocioppo R, Khroyan TV, Yasuda D, Zaveri NT, Pascual C, Xie XS, Toll L, 2015. AT-1001: a high-affinity alpha3beta4 nAChR ligand with novel nicotine-suppressive pharmacology. Br J Pharmacol 172, 1834–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PBS, Schwartz RD, Paul SM, Pert CB, Pert A, 1985. Nicotinic binding in rat brain: autoradiographic comparison of [3H] acetylcholine [3H] nicotine and [125I]-alpha-bungarotoxin. J. Neurosci. 5, 1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD 3rd, O’Neill BT, 2005. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem 48, 3474–3477. [DOI] [PubMed] [Google Scholar]
- Colquhoun D, 2007. Perceptions of a Receptor. Science 315, 1079. [Google Scholar]
- Colquhoun D, Hawkes AG, 1983. The principles of the stochastic interpretation of ion channel mechanisms In: Sakmann B, Neher E, (Eds), Single-Channel Recording. Plenum Press, New York, pp. 135–175. [Google Scholar]
- Colquhoun D, Ogden DC, 1986. States of the nicotinic acetylcholine receptor: enumeration, characteristics and structure In: Maelicke A, (Ed), Nicotinic Acetylcholine Receptor. Structure and Function. Springer-Verlag, Berlin, pp. 197–218. [Google Scholar]
- Colquhoun D, Ogden DC, 1988. Activation of ion channels in the frog end-plate by high concentrations of acetylcholine. J. Physiol. 395, 131–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly JG, Gibb AJ, Colquhoun D, 1995. Heterogeneity of neuronal nicotinic acetycholine receptors in thin slices of rat medial habenula. J. Phys. (Lond.) 4841, 87–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy WG, Liu QS, Nai Q, Margiotta JF, Berg DK, 2003. Potentiation of alpha7-containing nicotinic acetylcholine receptors by select albumins. Mol Pharmacol 63, 419–428. [DOI] [PubMed] [Google Scholar]
- Corringer PJ, Le Novere N, Changeux JP, 2000. Nicotinic receptors at the amino acid level. Annu. Rev. Pharmacol. Toxicol. 40, 431–458. [DOI] [PubMed] [Google Scholar]
- Couturier S, Bertrand D, Matter JM, Hernandez M-C, Bertrand S, Millar N, Valera S, Barkas T, Ballivet M, 1990. A neuronal nicotinic acetylcholine receptor subunit (a7) is developmentally regulated and forms a homo-oligomeric channel blocked by a-btx. Neuron 5, 847–856. [DOI] [PubMed] [Google Scholar]
- Craig PJ, Bose S, Zwart R, Beattie RE, Folly EA, Johnson LR, Bell E, Evans NM, Benedetti G, Pearson KH, McPhie GI, Volsen SG, Millar NS, Sher E, Broad LM, 2004. Stable expression and characterisation of a human alpha 7 nicotinic subunit chimera: a tool for functional high-throughput screening. Eur J Pharmacol 502, 31–40. [DOI] [PubMed] [Google Scholar]
- Cui C, Booker TK, Allen RS, Grady SR, Whiteaker P, Marks MJ, Salminen O, Tritto T, Butt CM, Allen WR, Stitzel JA, McIntosh JM, Boulter J, Collins AC, Heinemann SF, 2003. The beta3 nicotinic receptor subunit: a component of alpha-conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviors. J Neurosci 23, 11045–11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajas-Bailador F, Wonnacott S, 2004. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci 25, 317–324. [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador FA, Mogg AJ, Wonnacott S, 2002. Intracellular Ca2+ signals evoked by stimulation of nicotinic acetylcholine receptors in SH-SY5Y cells: contribution of voltage-operated Ca2+ channels and Ca2+ stores. J Neurochem 81, 606–614. [DOI] [PubMed] [Google Scholar]
- David R, Ciuraszkiewicz A, Simeone X, Orr-Urtreger A, Papke RL, McIntosh JM, Huck S, Scholze P, 2010. Biochemical and functional properties of distinct nicotinic acetylcholine receptors in the superior cervical ganglion of mice with targeted deletions of nAChR subunit genes. Eur J Neurosci 31, 978–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fiebre CM, Meyer EM, Zoltewicz J, Henry JC, Muraskin S, Kem WR, Papke RL, 1995. Characterization of a family of anabaseine-derived compounds reveals that the 3-(4)-dimethylaminocinnamylidine derivative (DMAC) is a selective agonist at neuronal nicotinic a7/[125I]a-bungarotoxin receptor subtypes. Mol Pharm 47, 164–171. [PubMed] [Google Scholar]
- de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE, 2005. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol 6, 844–851. [DOI] [PubMed] [Google Scholar]
- Delbart F, Brams M, Gruss F, Noppen S, Peigneur S, Boland S, Chaltin P, Brandao-Neto J, von Delft F, Touw WG, Joosten RP, Liekens S, Tytgat J, Ulens C, 2018. An allosteric binding site of the alpha7 nicotinic acetylcholine receptor revealed in a humanized acetylcholine-binding protein. J Biol Chem 293, 2534–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneris ES, Connolly J, Boulter J, Wada E, Wada K, Swanson LW, Patrick J, Heinemann S, 1988. Primary structure and expression of b2: a novel subunit of neuronal nicotinic acetylcholine receptors. Neuron 1, 45–54. [DOI] [PubMed] [Google Scholar]
- Drenan RM, Grady SR, Steele AD, McKinney S, Patzlaff NE, McIntosh JM, Marks MJ, Miwa JM, Lester HA, 2010. Cholinergic modulation of locomotion and striatal dopamine release is mediated by alpha6alpha4* nicotinic acetylcholine receptors. J Neurosci 30, 9877–9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvoisin RM, Deneris E, Patrick J, Heinemann S, 1989. The functional diversity of the neuronal acetylcholine receptors is increased by a novel subunit: b4. Neuron 3, 487–496. [DOI] [PubMed] [Google Scholar]
- Egea J, Buendia I, Parada E, Navarro E, Leon R, Lopez MG, 2015. Anti-inflammatory role of microglial alpha7 nAChRs and its role in neuroprotection. Biochem Pharmacol 97, 463–472. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S, 1994. a9: An acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell 79, 705–715. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Katz E, 2012. The efferent medial olivocochlear-hair cell synapse. J Physiol Paris 106, 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J, 2001. alpha10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci U S A 98, 3501–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF, Lukas RJ, Benowitz NL, West R, Dresler CM, 2008. Cytisine for smoking cessation: a research agenda. Drug Alcohol Depend 92, 3–8. [DOI] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ, 2008. Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology 33, 2158–2166. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ, 2011. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature 471, 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm S, Slimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, Auer S, Filkin S, Pons S, Fontaine JF, Tsetlin V, Maskos U, Ibanez-Tallon I, 2011. Aversion to nicotine is regulated by the balanced activity of beta4 and alpha5 nicotinic receptor subunits in the medial habenula. Neuron 70, 522–535. [DOI] [PubMed] [Google Scholar]
- Francis MM, Papke RL, 1996. Muscle-type Nicotinic Acetylcholine Receptor Delta Subunit Determines Sensitivity to Noncompetitive Inhibitors while Gamma Subunit Regulates Divalent Permeability. Neuropharm. 35, 1547–1556. [DOI] [PubMed] [Google Scholar]
- Fuchs PA, Murrow BW, 1991. An unusual cholinergic response in cochlear hair cells Society for Neuroscience. Society for Neuroscience, New Orleans, Louisiana, p. 959. [Google Scholar]
- Gardner PD, 1990. Nucleotide sequence of the epsilon-subunit of the mouse muscle nicotinic acetylcholine receptor. Nucleic Acids Res 18, 6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay EA, Giniatullin R, Skorinkin A, Yakel JL, 2008. Aromatic residues at position 55 of rat alpha7 nicotinic acetylcholine receptors are critical for maintaining rapid desensitization. J Physiol 586, 1105–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzanich V, Kuryatov A, Anand R, Lindstrom J, 1996. “Orphan” alpha6 nicotinic AChR subunit can form a functional heteromeric acetylcholine receptor. Mol. Pharmacol. 51, 320–327. [PubMed] [Google Scholar]
- Gerzanich V, Wang F, Kuryatov A, Lindstrom J, 1998. Alpha5 Subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal alpha 3 nicotinic receptors. J. Pharmacol. Exp. Ther. 286, 311–320. [PubMed] [Google Scholar]
- Gharpure A, Teng J, Zhuang Y, Noviello CM, Walsh RM Jr., Cabuco R, Howard RJ, Zaveri NT, Lindahl E, Hibbs RE, 2019. Agonist Selectivity and Ion Permeation in the alpha3beta4 Ganglionic Nicotinic Receptor. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JK, Dhankher P, Sheppard TD, Sher E, Millar NS, 2012. A series of alpha7 nicotinic acetylcholine receptor allosteric modulators with close chemical similarity but diverse pharmacological properties. Mol Pharmacol 81, 710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JK, Savolainen M, Young GT, Zwart R, Sher E, Millar NS, 2011. Agonist activation of {alpha}7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc Natl Acad Sci U S A 108, 5867–5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill-Thind JK, Dhankher P, D’Oyley JM, Sheppard TD, Millar NS, 2015. Structurally similar allosteric modulators of alpha7 nicotinic acetylcholine receptors exhibit five distinct pharmacological effects. J Biol Chem 290, 3552–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, 2000. Development of novel medications for drug addiction. The legacy of an African shrub. Ann N Y Acad Sci 909, 88–103. [DOI] [PubMed] [Google Scholar]
- Goldner FM, Dineley KT, Patrick JW, 1997. Immunohistochemical localization of the nicotinic acetylcholine receptor subunit alpha6 to dopaminergic neurons in the substantia nigra and ventral tegmental area. Neuroreport 8, 2739–2742. [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M, 2009. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol 78, 703–711. [DOI] [PubMed] [Google Scholar]
- Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, Zanardi A, Rimondini R, Mugnaini M, Clementi F, Chiamulera C, Zoli M, 2010. Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci 30, 5311–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Maggi R, Longhi R, Hanke W, Klinke N, Clementi F, 1997. Alpha7 and alpha8 nicotinic receptor subtypes immunopurified from chick retina have different immunological, pharmacological and functional properties. Eur J Neurosci 9, 1201–1211. [DOI] [PubMed] [Google Scholar]
- Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, Marks MJ, 2007. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol 74, 1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau V, Wilker S, Hartmann P, Lips KS, Grando SA, Padberg W, Fehrenbach H, Kummer W, 2007. Administration of keratinocyte growth factor (KGF) modulates the pulmonary expression of nicotinic acetylcholine receptor subunits alpha7, alpha9 and alpha10. Life Sci 80, 2290–2293. [DOI] [PubMed] [Google Scholar]
- Gronlien JH, Haakerud M, Ween H, Thorin-Hagene K, Briggs CA, Gopalakrishnan M, Malysz J, 2007. Distinct profiles of alpha7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Mol Pharmacol 72, 715–724. [DOI] [PubMed] [Google Scholar]
- Gu S, Matta JA, Lord B, Harrington AW, Sutton SW, Davini WB, Bredt DS, 2016. Brain alpha7 Nicotinic Acetylcholine Receptor Assembly Requires NACHO. Neuron 89, 948–955. [DOI] [PubMed] [Google Scholar]
- Gulsevin A, Papke RL, Stokes C, Garai S, Thakur GA, Quadri M, Horenstein N, 2019. Allosteric agonism of alpha7 nicotinic acetylcholine receptors. Mol Pharmacol 95, 604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley D, Harris EW, Li C, Johnson EC, Lanthorn T, 2000. 5-Hydroxyindole potentiates the nicotinic acetylcholine receptor alpha7 subtype. Soc. Neurosci. Abs. 716.15. [Google Scholar]
- Haggerty JC, Froehner SC, 1981. Restoration of 125I-alpha-bungarotoxin binding activity to the alpha- subunit of Torpedo acetylcholine receptor isolated by gel electrophoresis in sodium dodecyl sulfate. J. Biol. Chem. 256, 8294–8297. [PubMed] [Google Scholar]
- Haghighi AP, Cooper E, 2000. A molecular link between inward rectification and calcium permeability of neuronal nicotinic acetylcholine alpha3beta4 and alpha4beta2 receptors. J Neurosci 20, 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevi S, Yassin L, Eshel M, Sala F, Sala S, Criado M, Treinin M, 2003. Conservation within the RIC-3 gene family. Effectors of mammalian nicotinic acetylcholine receptor expression. J Biol Chem 278, 34411–34417. [DOI] [PubMed] [Google Scholar]
- Han ZY, Le Novere N, Zoli M, Hill JA Jr., Champtiaux N, Changeux JP, 2000. Localization of nAChR subunit mRNAs in the brain of Macaca mulatta. Eur J Neurosci 12, 3664–3674. [DOI] [PubMed] [Google Scholar]
- Heinemann S, Boulter J, Deneris E, Conolly J, Duvoisin R, Papke R, Patrick J, 1990. The brain nicotinic acetylcholine receptor gene family. Prog Brain Res 86, 195–203. [DOI] [PubMed] [Google Scholar]
- Helekar SA, Char D, Neff S, Patrick J, 1994. Prolyl isomerase requirement for the expression of functional homo- oligomeric ligand-gated ion channels. Neuron 12, 179–189. [DOI] [PubMed] [Google Scholar]
- Horenstein NA, Leonik FM, Papke RL, 2008. Multiple pharmacophores for the selective activation of nicotinic alpha7-type acetylcholine receptors. Mol Pharmacol 74, 1496–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horenstein NA, Papke RL, 2017. Anti-inflammatory Silent Agonists. ACS Med Chem Lett 8, 989–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horenstein NA, Papke RL, Kulkarni AR, Chaturbhuj GU, Stokes C, Manther K, Thakur GA, 2016. Critical molecular determinants of alpha7 nicotinic acetylcholine receptor allosteric activation: separation of direct allosteric activation and positive allosteric modulation. J Biol Chem 291, 5049–5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh KH, Fuhrer C, 2002. Clustering of nicotinic acetylcholine receptors: from the neuromuscular junction to interneuronal synapses. Mol Neurobiol 25, 79–112. [DOI] [PubMed] [Google Scholar]
- Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, Damaj MI, 2009. The role of alpha6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. J Pharmacol Exp Ther 331, 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Kuryatov A, Wang J, Kamenecka TM, Lindstrom J, 2016. Unorthodox Acetylcholine Binding Sites Formed by alpha5 and beta3 Accessory Subunits in alpha4beta2* Nicotinic Acetylcholine Receptors. J Biol Chem 291, 23452–23463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiteh M, Taly A, Henin J, 2016. Evolution of Pentameric Ligand-Gated Ion Channels: Pro-Loop Receptors. PLoS One 11, e0151934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janik E, Ceremuga M, Saluk-Bijak J, Bijak M, 2019. Biological Toxins as the Potential Tools for Bioterrorism. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo F, Vicini S, Scheutze SM, 1988. Embryonic acetylcholine receptors guarantee spontaneous contractions in rat developing muscle. Nature 335, 66–68. [DOI] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL, 1996. The impact of receptor desensitization on fast synaptic transmission. Trends Neurosci 19, 96–101. [DOI] [PubMed] [Google Scholar]
- Kabbani N, Nichols RA, 2018. Beyond the Channel: Metabotropic Signaling by Nicotinic Receptors. Trends Pharmacol Sci 39, 354–366. [DOI] [PubMed] [Google Scholar]
- Kabbani N, Nordman JC, Corgiat BA, Veltri DP, Shehu A, Seymour VA, Adams DJ, 2013. Are nicotinic acetylcholine receptors coupled to G proteins? Bioessays 35, 1025–1034. [DOI] [PubMed] [Google Scholar]
- Kaczanowska K, Camacho Hernandez GA, Bendiks L, Kohs L, Cornejo-Bravo JM, Harel M, Finn MG, Taylor P, 2017. Substituted 2-Aminopyrimidines Selective for alpha7-Nicotinic Acetylcholine Receptor Activation and Association with Acetylcholine Binding Proteins. J Am Chem Soc 139, 3676–3684. [DOI] [PubMed] [Google Scholar]
- Kao PN, Dwork AJ, Kaldany RJ, Silver ML, Wideman J, Stein S, Karlin A, 1984. Identification of two alpha-subunit half-cystines specifically labeled by an affinity reagent for the acetylcholine binding site. J. Biol. Chem. 259, 1162–1165. [PubMed] [Google Scholar]
- Katzung BG, Masters SB, Trevor AJ, 2012. Basic and Clinical Pharmacology. McGraw Hill, New York. [Google Scholar]
- Kawashima K, Fujii T, Moriwaki Y, Misawa H, 2012. Critical roles of acetylcholine and the muscarinic and nicotinic acetylcholine receptors in the regulation of immune function. Life Sci 91, 1027–1032. [DOI] [PubMed] [Google Scholar]
- Kem WR, 2000. The brain alpha7 nicotinic receptor may be an important therapeutic target for the treatment of Alzheimer’s disease: studies with DMXBA (GTS-21). Behav Brain Res 113, 169–181. [DOI] [PubMed] [Google Scholar]
- King JR, Gillevet TC, Kabbani N, 2017. A G protein-coupled alpha7 nicotinic receptor regulates signaling and TNF-alpha release in microglia. FEBS Open Bio 7, 1350–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JR, Nordman JC, Bridges SP, Lin MK, Kabbani N, 2015. Identification and Characterization of a G Protein-binding Cluster in alpha7 Nicotinic Acetylcholine Receptors. J Biol Chem 290, 20060–20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JR, Ullah A, Bak E, Jafri MS, Kabbani N, 2018. Ionotropic and Metabotropic Mechanisms of Allosteric Modulation of alpha7 Nicotinic Receptor Intracellular Calcium. Mol Pharmacol 93, 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Olale F, Cooper J, Choi C, Lindstrom J, 2000. Human alpha6 AChR subtypes: subunit composition, assembly, and pharmacological responses. Neuropharmacology 39, 2570–2590. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Onksen J, Lindstrom J, 2008. Roles of accessory subunits in alpha4beta2(*) nicotinic receptors. Mol Pharmacol 74, 132–143. [DOI] [PubMed] [Google Scholar]
- Kurzen H, Berger H, Jager C, Hartschuh W, Naher H, Gratchev A, Goerdt S, Deichmann M, 2004. Phenotypical and molecular profiling of the extraneuronal cholinergic system of the skin. J Invest Dermatol 123, 937–949. [DOI] [PubMed] [Google Scholar]
- Land BR, Salpeter EE, Salpeter MM, 1981. Kinetic paramenters for acetylcholine interaction in intact neuromuscular junction. Proc. Natl. Acad. Sci. USA 78, 7200–7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lape R, Colquhoun D, Sivilotti LG, 2008. On the nature of partial agonism in the nicotinic receptor superfamily. Nature 454, 722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Fakler B, Kaczmarek LK, Isom LL, 2014. More than a pore: ion channel signaling complexes. J Neurosci 34, 15159–15169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Huang CS, Chen CS, Tu SH, Wang YJ, Chang YJ, Tam KW, Wei PL, Cheng TC, Chu JS, Chen LC, Wu CH, Ho YS, 2010. Overexpression and activation of the alpha9-nicotinic receptor during tumorigenesis in human breast epithelial cells. J Natl Cancer Inst 102, 1322–1335. [DOI] [PubMed] [Google Scholar]
- Lester HA, Dibas MI, Dahan DS, Leite JF, Dougherty DA, 2004. Cys-loop receptors: new twists and turns. Trends Neurosci 27, 329–336. [DOI] [PubMed] [Google Scholar]
- Lester HA, Dougherty DA, 2019. Nicotine Bound to Its Receptors: New Structures for a Vexing Pathopharmacological Problem. Neuron 104, 431–432. [DOI] [PubMed] [Google Scholar]
- Lester HA, Xiao C, Srinivasan R, Son CD, Miwa J, Pantoja R, Banghart MR, Dougherty DA, Goate AM, Wang JC, 2009. Nicotine is a selective pharmacological chaperone of acetylcholine receptor number and stoichiometry. Implications for drug discovery. AAPS J 11, 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Bettegowda C, Blosser J, Gordon J, 1999. AR-R17779, and alpha7 nicotinic agonist, improves learning and memory in rats. Behav Pharmacol 10, 675–680. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH, 2006. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 184, 523–539. [DOI] [PubMed] [Google Scholar]
- Li SX, Huang S, Bren N, Noridomi K, Dellisanti CD, Sine SM, Chen L, 2011. Ligand-binding domain of an alpha7-nicotinic receptor chimera and its complex with agonist. Nat Neurosci 14, 1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Rainnie DG, McCarley RW, Greene RW, 1998. Presynaptic Nicotinic Receptors Facilitate Monoaminergic Transmission. J. Neurosci. 18, 1904–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Papke RL, He Y-J, Millard B, Meyer EM, 1999. Characterization of the neuroprotective and toxic effects of a7 nicotinic receptor activation in PC12 cells. Brain Res 81, 218–225. [DOI] [PubMed] [Google Scholar]
- Liu Q, Whiteaker P, Morley BJ, Shi FD, Lukas RJ, 2017. Distinctive Roles for alpha7*- and alpha9*-Nicotinic Acetylcholine Receptors in Inflammatory and Autoimmune Responses in the Murine Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis. Front Cell Neurosci 11, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucero LM, Weltzin MM, Eaton JB, Cooper JF, Lindstrom JM, Lukas RJ, Whiteaker P, 2016. Differential alpha4(+)/(−)beta2 Agonist-binding Site Contributions to alpha4beta2 Nicotinic Acetylcholine Receptor Function within and between Isoforms. J Biol Chem 291, 2444–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetje CW, Patrick J, 1991. Both a- and b-subunits contribute to the agonist sensitivity of neuronal nicotinic acetylcholine receptors. J. Neurosci. 11, 837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, Basutkar P, Tivey ARN, Potter SC, Finn RD, Lopez R, 2019. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res 47, W636-W641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonneuve IM, Glick SD, 2003. Anti-addictive actions of an iboga alkaloid congener: a novel mechanism for a novel treatment. Pharmacol Biochem Behav 75, 607–618. [DOI] [PubMed] [Google Scholar]
- Marrero MB, Bencherif M, 2009. Convergence of alpha 7 nicotinic acetylcholine receptor-activated pathways for anti-apoptosis and anti-inflammation: central role for JAK2 activation of STAT3 and NF-kappaB. Brain Res 1256, 1–7. [DOI] [PubMed] [Google Scholar]
- Martinou JC, Falls DL, Fischbach GD, Merlie JP, 1991. Acetylcholine receptor-inducing activity stimulates expression of the epsilon-subunit gene of the muscle acetylcholine receptor. Proc Natl Acad Sci U S A 88, 7669–7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marubio LM, Gardier AM, Durier S, David D, Klink R, Arroyo-Jimenez MM, McIntosh JM, Rossi F, Champtiaux N, Zoli M, Changeux JP, 2003. Effects of nicotine in the dopaminergic system of mice lacking the alpha4 subunit of neuronal nicotinic acetylcholine receptors. Eur J Neurosci 17, 1329–1337. [DOI] [PubMed] [Google Scholar]
- Matthews-Bellinger JA, Salpeter MM, 1983. Fine structural distribution of acetylcholine receptors at developing mouse neuromuscular junctions. J Neurosci 3, 644–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E, Kuryatov A, Gerzanich V, Lindstrom J, Papke RL, 1998. Analysis of 40H-GTS-21 selectivity and activity at human and rat a7 nicotinic receptors. J Pharmacol Exp Ther 287, 918–925. [PubMed] [Google Scholar]
- Meyer EM, Tay ET, Papke RL, Meyers C, Huang G, de Fiebre CM, 1997. Effects of 3-[2,4-dimethoxybenzylidene]anabaseine (DMXB) on rat nicotinic receptors and memory-related behaviors. Brain Res 768, 49–56. [DOI] [PubMed] [Google Scholar]
- Millar NS, Gotti C, 2009. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology 56, 237–246. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Picciotto MR, 2010. Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis. Trends Pharmacol Sci 31, 580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina M, Takai T, Imoto K, Noda M, Takahashi T, Numa S, Methfessel C, Sakmann B, 1986. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature 321, 406–411. [DOI] [PubMed] [Google Scholar]
- Modahl CM, Mukherjee AK, Mackessy SP, 2016. An analysis of venom ontogeny and prey-specific toxicity in the Monocled Cobra (Naja kaouthia). Toxicon 119, 8–20. [DOI] [PubMed] [Google Scholar]
- Monod J, Wyman J, Changeux JP, 1965. On the Nature of Allosteric Transitions: A Plausible Model. J Mol Biol 12, 88–118. [DOI] [PubMed] [Google Scholar]
- Morales-Perez CL, Noviello CM, Hibbs RE, 2016. X-ray structure of the human alpha4beta2 nicotinic receptor. Nature 538, 411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni M, Zwart R, Sher E, Cassels BK, Bermudez I, 2006. alpha4beta2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Mol Pharmacol 70, 755–768. [DOI] [PubMed] [Google Scholar]
- Moss BL, Schuetze SM, Role LW, 1989. Functional properties and developmental regulation of nicotinic acetylcholine receptors on embryonic chicken sympathetic neurons. Neuron 3, 597–607. [DOI] [PubMed] [Google Scholar]
- Mucchietto V, Fasoli F, Pucci S, Moretti M, Benfante R, Maroli A, Di Lascio S, Bolchi C, Pallavicini M, Dowell C, McIntosh M, Clementi F, Gotti C, 2018. alpha9- and alpha7-containing receptors mediate the pro-proliferative effects of nicotine in the A549 adenocarcinoma cell line. Br J Pharmacol 175, 1957–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhtasimova N, Free C, Sine SM, 2005. Initial coupling of binding to gating mediated by conserved residues in the muscle nicotinic receptor. J Gen Physiol 126, 23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nai Q, McIntosh JM, Margiotta JF, 2003. Relating neuronal nicotinic acetylcholine receptor subtypes defined by subunit composition and channel function. Mol Pharmacol 63, 311–324. [DOI] [PubMed] [Google Scholar]
- Neher E, Steinbach JH, 1978. Local anaesthetics transiently block current through single acetylcholine receptor channels. J. Physiol. 277, 135–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J, 2003. Alternate stoichiometries of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol 63, 332–341. [DOI] [PubMed] [Google Scholar]
- Norleans J, Wang J, Kuryatov A, Leffler A, Doebelin C, Kamenecka TM, Lindstrom J, 2019. Discovery of an intrasubunit nicotinic acetylcholine receptor-binding site for the positive allosteric modulator Br-PBTC. J Biol Chem 294, 12132–12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden DC, Colquhoun D, 1985. Ion channel block by acetylcholine, carbachol and suberyldicholine at the frog neuromuscular junction. Proc. R. Soc. Lond. B 225, 329–355. [DOI] [PubMed] [Google Scholar]
- Palma E, Bertrand S, Binzoni T, Bertrand D, 1996. Neuronal nicotinic alpha 7 receptor expressed in Xenopus oocytes presents five putative binding sites for methyllycaconitine. J Physiol 491, 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, 1993. The kinetic properties of neuronal nicotinic receptors: genetic basis of functional diversity. Prog. in Neurobio. 41, 509–531. [DOI] [PubMed] [Google Scholar]
- Papke RL, 2010. Tricks of Perspective: Insights and limitations to the study of macroscopic currents for the analysis of nAChR activation and desensitization. J. Mol. Neurosci. 40, 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, 2014. Merging old and new perspectives on nicotinic acetylcholine receptors. Biochem Pharmacol 89, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Bagdas D, Kulkarni AR, Gould T, AlSharari S, Thakur GA, Damaj IM, 2015. The analgesic-like properties of the alpha7 nAChR silent agonist NS6740 is associated with nonconducting conformations of the receptor. NeuroPharm. 91, 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Bencherif M, Lippiello P, 1996. An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the a7 subtype. Neurosci. Lett. 213, 201–204. [DOI] [PubMed] [Google Scholar]
- Papke RL, Boulter J, Patrick J, Heinemann S, 1989. Single-channel currents of rat neuronal nicotinic acetylcholine receptors expressed in xenopus laevis oocytes. Neuron 3, 589–596. [DOI] [PubMed] [Google Scholar]
- Papke RL, Chojnacka K, Horenstein NA, 2014a. The minimal pharmacophore for silent agonism of alpha7 nAChR. J. P. E. T. 350, 665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Duvoisin R, Heinemann SF, 1991. The extracellular domain of the neuronal nicotinic subunit b4 determines the pharmacology of receptors formed with a3. Soc. Neurosci. [Google Scholar]
- Papke RL, Heinemann SF, 1991. The role of the b4 subunit in determining the kinetic properties of rat neuronal nicotinic acetylcholine a3-receptor. J. Physiol. (Lond.) 440, 95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Horenstein NA, Kulkarni AR, Stokes C, Corrie LW, Maeng CY, Thakur GA, 2014b. The activity of GAT107, an allosteric activator and positive modulator of alpha7 nicotinic acetylcholine receptors (nAChR), is regulated by aromatic amino acids that span the subunit interface. J Biol Chem 289, 4515–4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Kem WR, Soti F, López-Hernández GY, Horenstein NA, 2009. Activation and desensitization of nicotinic alpha7-type acetylcholine receptors by benzylidene anabaseines and nicotine. J Pharmacol Exp Ther 329, 791–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Meyer E, Nutter T, Uteshev VV, 2000. Alpha7-selective agonists and modes of alpha7 receptor activation. Eur J Pharmacol 393, 179–195. [DOI] [PubMed] [Google Scholar]
- Papke RL, Millhauser G, Lieberman Z, Oswald RE, 1988. Relationships of agonist properties to the single channel kinetics of nicotinic acetylcholine receptors. Biophys. J. 53, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Papke JKP, 2002. Comparative pharmacology of rat and human alpha7 nAChR conducted with net charge analysis. Br J of Pharm 137, 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Peng C, Kumar A, Stokes C, 2018. NS6740, an alpha7 nicotinic acetylcholine receptor silent agonist, disrupts hippocampal synaptic plasticity. Neurosci Lett 677, 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Picciotto MR, 2012. Nicotine Dependence and Depression, What is the Future for Therapeutics? J. Addiction Res. and Ther. 2, 1000e1105. [Google Scholar]
- Papke RL, Schiff HC, Jack BA, Horenstein NA, 2005. Molecular dissection of tropisetron, an alpha7 nicotinic acetylcholine receptor-selective partial agonist. Neurosci. let. 378, 140–144. [DOI] [PubMed] [Google Scholar]
- Papke RL, Stokes C, Damaj MI, Thakur GA, Manther K, Treinin M, Bagdas D, Kulkarni AR, Horenstein NA, 2017. Persistent activation of alpha7 nicotinic ACh receptors associated with stable induction of different desensitized states. Br J Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Stokes C, Muldoon P, Imad Damaj M, 2013. Similar activity of mecamylamine stereoisomers in vitro and in vivo. Eur J Pharmacol 720, 264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Thinschmidt JS, 1998. The correction of alpha7 nicotinic acetylcholine receptor concentration-response relationships in Xenopus oocytes. Neurosci. Let. 256, 163–166. [DOI] [PubMed] [Google Scholar]
- Papke RL, Wecker L, Stitzel JA, 2010. Activation and inhibition of mouse muscle and neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther 333, 501–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak JB, 1993. Measuring kinetics of complex ion channel data using mean-variance histograms. Biophys. J. 65, 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulo J, Brucker W, Hawrot E, 2009. Proteomic Analysis of an alpha7 Nicotinic Acetylcholine Receptor Interactome. J Proteome Res 8, 1849–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Ochani M, Yang LH, Gallowitsch-Puerta M, Ochani K, Lin X, Levi J, Parrish WR, Rosas-Ballina M, Czura CJ, Larosa GJ, Miller EJ, Tracey KJ, Al-Abed Y, 2007. Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med 35, 1139–1144. [DOI] [PubMed] [Google Scholar]
- Peng C, Kimbrell MR, Tian C, Pack TF, Crooks PA, Fifer EK, Papke RL, 2013. Multiple modes of alpha7 nAChR non-competitive antagonism of control agonist-evoked and allosterically enhanced currents. Mol Pharmacol 84, 459–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Ferris RL, Matthews T, Hiel H, Lopez-Albaitero A, Lustig LR, 2004. Characterization of the human nicotinic acetylcholine receptor subunit alpha (alpha) 9 (CHRNA9) and alpha (alpha) 10 (CHRNA10) in lymphocytes. Life Sci 76, 263–280. [DOI] [PubMed] [Google Scholar]
- Perry EK, Martin-Ruiz CM, Court JA, 2001. Nicotinic receptor subtypes in human brain related to aging and dementia. Alcohol 24, 63–68. [DOI] [PubMed] [Google Scholar]
- Philip NS, Carpenter LL, Tyrka AR, Whiteley LB, Price LH, 2009. Varenicline augmentation in depressed smokers: an 8-week, open-label study. J Clin Psychiatry 70, 1026–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto M, Zoli M, Rimondini R, Lena C, Marubio L, Pich E, Fuxe K, Changeux J, 1998. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391, 173–177. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Kenny PJ, 2013. Molecular mechanisms underlying behaviors related to nicotine addiction. Cold Spring Harb Perspect Med 3, a012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Mineur YS, 2014. Molecules and circuits involved in nicotine addiction: The many faces of smoking. Neuropharmacology 76 Pt B, 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieschl RL, Miller R, Jones KM, Post-Munson DJ, Chen P, Newberry K, Benitex Y, Molski T, Morgan D, McDonald IM, Macor JE, Olson RE, Asaka Y, Digavalli S, Easton A, Herrington J, Westphal RS, Lodge NJ, Zaczek R, Bristow LJ, Li YW, 2017. Effects of BMS-902483, an alpha7 nicotinic acetylcholine receptor partial agonist, on cognition and sensory gating in relation to receptor occupancy in rodents. Eur J Pharmacol 807, 1–11. [DOI] [PubMed] [Google Scholar]
- Placzek AN, Grassi F, Meyer EM, Papke RL, 2005. An alpha7 nicotinic acetylcholine receptor gain-of-function mutant that retains pharmacological fidelity. Mol Pharmacol 68, 1863–1876. [DOI] [PubMed] [Google Scholar]
- Plazas PV, Katz E, Gomez-Casati ME, Bouzat C, Elgoyhen AB, 2005. Stoichiometry of the alpha9alpha10 nicotinic cholinergic receptor. J Neurosci 25, 10905–10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, Changeux JP, Maskos U, Fratta W, 2008. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci 28, 12318–12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri M, Garai S, Thakur GA, Stokes C, Gulsevin A, Horenstein NA, Papke RL, 2019. Macroscopic and microscopic activation of alpha7 nicotinic acetylcholine receptors by the structurally unrelated ago-PAMs B-973B and GAT107. Mol Pharmacol 95, 43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri M, Papke RL, Horenstein NA, 2016. Dissection of N,N-diethyl-N’-phenylpiperazines as alpha7 nicotinic receptor silent agonists. Bioorg Med Chem 24, 286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhilin S, Drisdel RC, Sagher D, McGehee DS, Vallejo Y, Green WN, 1999. alpha-bungarotoxin receptors contain alpha7 subunits in two different disulfide-bonded conformations. J. Cell. Biol. 146, 203–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramarao MK, Cohen JB, 1998. Mechanism of nicotinic acetylcholine receptor cluster formation by rapsyn. Proc Natl Acad Sci U S A 95, 4007–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen GL, 1946. The olivary peduncle and other fiber projections of the superior olivary complex. J Comp Neurol 84, 141–219. [DOI] [PubMed] [Google Scholar]
- Rayes D, De Rosa MJ, Sine SM, Bouzat C, 2009. Number and locations of agonist binding sites required to activate homomeric Cys-loop receptors. J Neurosci 29, 6022–6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revah F, Bertrand D, Galzi J-L, Devillers-Thiery A, Mulle C, Hussy N, Bertrand S, Ballivet M, Changeux J-P, 1991. Mutations in the channel domain alter desensitization of a neuronal nicotinic receptor. Nature 353, 846–849. [DOI] [PubMed] [Google Scholar]
- Reynolds JA, Karlin A, 1978. Molecular weight in detergent solution of acetylcholine receptor from Torpedo californica. Biochemistry 17, 2035–2038. [DOI] [PubMed] [Google Scholar]
- Richter K, Sagawe S, Hecker A, Kullmar M, Askevold I, Damm J, Heldmann S, Pohlmann M, Ruhrmann S, Sander M, Schluter KD, Wilker S, Konig IR, Kummer W, Padberg W, Hone AJ, McIntosh JM, Zakrzewicz AT, Koch C, Grau V, 2018. C-Reactive Protein Stimulates Nicotinic Acetylcholine Receptors to Control ATP-Mediated Monocytic Inflammasome Activation. Front Immunol 9, 1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Goldstein RS, Gallowitsch-Puerta M, Yang L, Valdes-Ferrer SI, Patel NB, Chavan S, Al-Abed Y, Yang H, Tracey KJ, 2009. The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med 15, 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina M, Tracey KJ, 2009. Cholinergic control of inflammation. J Intern Med 265, 663–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Levin ED, Behm FM, Adivi C, Schur C, 1990. Transdermal nicotine facilitates smoking cessation. Clin Pharmacol Ther 47, 323–330. [DOI] [PubMed] [Google Scholar]
- Sabey K, Paradiso K, Zhang J, Steinbach JH, 1999. Ligand binding and activation of rat nicotinic alpha4beta2 receptors stably expressed in HEK293 cells. Mol. Pharmacol. 55, 58–66. [PubMed] [Google Scholar]
- Schnoll RA, Lerman C, 2006. Current and emerging pharmacotherapies for treating tobacco dependence. Expert Opin Emerg Drugs 11, 429–444. [DOI] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dinely-Miller K, Dani JA, Patrick JW, 1993. Molecular cloning, functional properties and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci 13(2), 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S, Taylor P, 1980. The relationship between agonist occupation and the permeability response of the cholinergic receptor revealed by bound cobra alpha-toxin. J. Biol. Chem. 255, 10144–10156. [PubMed] [Google Scholar]
- Sine SM, Steinbach JH, 1986. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by low concentrations of agonist. J. Physiol. 373, 129–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine SM, Steinbach JH, 1987. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by high concentrations of agonist. J Physiol 385, 325–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitzia F, Brown JT, Randall AD, Dunlop J, 2011. Voltage- and TemperatureDependent Allosteric Modulation of alpha7 Nicotinic Receptors by PNU120596. Front Pharmacol 2, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sixma TK, Smit AB, 2003. Acetylcholine binding protein (AChBP): A Secreted Glial Protein that Provides a High-Resolution Model for the Extracellular Domain of Pentameric Ligand-Gated Ion Channels. Annu Rev Biophys Biomol Struct 21, 21. [DOI] [PubMed] [Google Scholar]
- Skok MV, 2009. To channel or not to channel? Functioning of nicotinic acetylcholine receptors in leukocytes. J Leukoc Biol 86, 1–3. [DOI] [PubMed] [Google Scholar]
- Spurny R, Debaveye S, Farinha A, Veys K, Vos AM, Gossas T, Atack J, Bertrand S, Bertrand D, Danielson UH, Tresadern G, Ulens C, 2015. Molecular blueprint of allosteric binding sites in a homologue of the agonist-binding domain of the alpha7 nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A 112, E2543–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre S, Jiang W, Roy P, Champigny C, LeBlanc E, Morley BJ, Hao J, Simard AR, 2016. Nicotinic Acetylcholine Receptors Modulate Bone Marrow-Derived Pro-Inflammatory Monocyte Production and Survival. PLoS One 11, e0150230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes C, Garai S, Kulkarni AR, Cantwell LN, Noviello CM, Hibbs RE, Horenstein NA, Abboud KA, Thakur GA, Papke RL, 2019. Heteromeric Neuronal Nicotinic Acetylcholine Receptors with Mutant beta Subunits Acquire Sensitivity to alpha7-Selective Positive Allosteric Modulators. J Pharmacol Exp Ther 370, 252–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes C, Treinin M, Papke RL, 2015. Looking below the surface of nicotinic acetylcholine receptors. Trends Pharmacol Sci 36, 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud RM, McCarthy MP, Shuster M, 1990. Nicotinic acetylcholine receptor superfamily of ligand-gated ion channels. Biochemistry 29, 11009–11023. [DOI] [PubMed] [Google Scholar]
- Tapia L, Kuryatov A, Lindstrom J, 2007. Ca2+ permeability of the (alpha4)3(beta2)2 stoichiometry greatly exceeds that of (alpha4)2(beta2)3 human acetylcholine receptors. Mol Pharmacol 71, 769–776. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA, 2004. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science 306, 1029–1032. [DOI] [PubMed] [Google Scholar]
- Tasneem A, Iyer LM, Jakobsson E, Aravind L, 2005. Identification of the prokaryotic ligand-gated ion channels and their implications for the mechanisms and origins of animal Cys-loop ion channels. Genome Biol 6, R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen MS, Mikkelsen JD, 2012. The alpha7 nicotinic acetylcholine receptor ligands methyllycaconitine, NS6740 and GTS-21 reduce lipopolysaccharide-induced TNF-alpha release from microglia. J Neuroimmunol 251, 65–72. [DOI] [PubMed] [Google Scholar]
- Timmermann DB, Gronlien JH, Kohlhaas KL, Nielsen EO, Dam E, Dyhring T, Ahring PK, Peters D, Holst D, Christensen JK, Malysz J, Briggs CA, Gopalakrishnan M, Olsen GM, 2007. An allosteric modulator of the {alpha}7 nicotinic acetylcholine receptor possessing cognition enhancing properties in vivo. J Pharmacol Exp Ther 323, 294–307. [DOI] [PubMed] [Google Scholar]
- Treinin M, Papke RL, Nizri E, Ben-David Y, Mizrachi T, Brenner T, 2017. Role of the alpha7 Nicotinic Acetylcholine Receptor and RIC-3 in the Cholinergic Anti-inflammatory Pathway. Cent Nerv Syst Agents Med Chem 17, 90–99. [DOI] [PubMed] [Google Scholar]
- Uteshev VV, 2014. The therapeutic promise of positive allosteric modulation of nicotinic receptors. Eur J Pharmacol 727, 181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uteshev VV, Meyer EM, Papke RL, 2002. Activation and inhibition of native neuronal alpha-bungarotoxin-sensitive nicotinic ACh receptors. Brain Res 948, 33–46. [DOI] [PubMed] [Google Scholar]
- Valbuena S, Lerma J, 2016. Non-canonical Signaling, the Hidden Life of Ligand-Gated Ion Channels. Neuron 92, 316–329. [DOI] [PubMed] [Google Scholar]
- van Maanen MA, Papke RL, Koopman FA, Koepke J, Bevaart L, Clark R, Lamppu D, Elbaum D, LaRosa GJ, Tak PP, Vervoordeldonk MJ, 2015. Two novel alpha7 nicotinic acetylcholine receptor ligands: in vitro properties and their efficacy in collagen-induced arthritis in mice. PLoS One 10, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, Ulloa L, Tracey KJ, van der Poll T, 2006. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology 130, 1822–1830. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S, Pugh PC, Zhang Z, Rathouz MM, Berg DK, 1992. Nicotinic receptors that bind a-bungarotoxin on neurons raise intracellular free Ca2+. Neuron 8, 353–362. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW, 1989. Distribution of alpha2, alpha3, alpha4, and beta2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J. Comp. Neurol. 284, 314–335. [DOI] [PubMed] [Google Scholar]
- Wada K, Ballivet M, Boulter J, Connolly J, Wada E, Deneris E, Swanson L, Heinemann S, Patrick J, 1988. Functional expression of a new pharmacological subtype of brain nicotinic acetylcholine receptor. Science 240, 330–334. [DOI] [PubMed] [Google Scholar]
- Walsh RM Jr., Roh SH, Gharpure A, Morales-Perez CL, Teng J, Hibbs RE, 2018. Structural principles of distinct assemblies of the human alpha4beta2 nicotinic receptor. Nature 557, 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Gerzanich V, Wells GB, Anand R, Peng X, Keyser K, Lindstrom J, 1996. Assembly of Human Neuronal Nicotinic Receptor alpha5 Subunits with alpha3, beta2 and beta4 Subunits. J. Biol. Chem. 271, 17656–17665. [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ, 2003. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421, 384–388. [DOI] [PubMed] [Google Scholar]
- Wang J, Kuryatov A, Sriram A, Jin Z, Kamenecka TM, Kenny PJ, Lindstrom J, 2015. An Accessory Agonist Binding Site Promotes Activation of alpha4beta2* Nicotinic Acetylcholine Receptors. J Biol Chem 290, 13907–13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lindstrom J, 2017. Orthosteric and allosteric potentiation of heteromeric neuronal nicotinic acetylcholine receptors. Br J Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Orr-Urtreger A, Chapman J, Ergun Y, Rabinowitz R, Korczyn AD, 2005. Hidden function of neuronal nicotinic acetylcholine receptor beta2 subunits in ganglionic transmission: comparison to alpha5 and beta4 subunits. J Neurol Sci 228, 167–177. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lee JW, Oh G, Grady SR, McIntosh JM, Brunzell DH, Cannon JR, Drenan RM, 2014. Enhanced synthesis and release of dopamine in transgenic mice with gain-of-function alpha6* nAChRs. J Neurochem 129, 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting P, Lindstrom J, 1987. Purification and characterization of a nicotinic acetylcholine receptor from rat brain. Proc. Natl. Acad. Sci. USA 84, 595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Peng C, Kimbrell MR, Papke RL, 2012. The Intrinsically Low Open Probability of alpha7 nAChR Can be Overcome by Positive Allosteric Modulation and Serum Factors Leading to the Generation of Excitotoxic Currents at Physiological Temperatures. Mol Pharmacol 82, 746–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Stokes C, Horenstein NA, Papke RL, 2009. Differential regulation of receptor activation and agonist selectivity by highly conserved tryptophans in the nicotinic acetylcholine receptor binding site. J Pharmacol Exp Ther 330, 40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Stokes C, Horenstein NA, Papke RL, 2011a. The effective opening of nicotinic acetylcholine receptors with single agonist binding sites. J Gen Physiol 137, 369–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Wang J, Papke RL, 2011b. Investigation of the Molecular Mechanism of the Alpha7 nAChR Positive Allosteric Modulator PNU-120596 Provides Evidence for Two Distinct Desensitized States. Mol Pharmacol 80, 1013–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Wang J, Papke RL, 2011c. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: Advantages and limitations. Biochem Pharmacol 82, 915–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ME, Burton B, Urrutia A, Shcherbatko A, Chavez-Noriega LE, Cohen CJ, Aiyar J, 2005. Ric-3 promotes functional expression of the nicotinic acetylcholine receptor alpha7 subunit in mammalian cells. J Biol Chem 280, 1257–1263. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, 1997. Presynaptic nicotinic ACh receptors. TINS 20, 92–98. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Kaiser S, Mogg A, Soliakov L, Jones IW, 2000. Presynaptic nicotinic receptors modulating dopamine release in the rat striatum. Eur. J. Pharmacol. 393, 51–58. [DOI] [PubMed] [Google Scholar]
- Wu J, Perry DC, Bupp JE, Jiang F, Polgar WE, Toll L, Zaveri NT, 2014. [(1)(2)(5)I]AT-1012, a new high affinity radioligand for the alpha3beta4 nicotinic acetylcholine receptors. Neuropharmacology 77, 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu X, Puskar NL, Shanata JA, Lester HA, Dougherty DA, 2009. Nicotine binding to brain receptors requires a strong cation-pi interaction. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Gelber S, Orr-Urtreger A, Armstrong D, Lewis RA, Ou CN, Patrick J, Role L, De Biasi M, Beaudet AL, 1999. Megacystis, mydriasis, and ion channel defect in mice lacking the alpha3 neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A 96, 5746–5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GT, Zwart R, Walker AS, Sher E, Millar NS, 2008. Potentiation of alpha7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc Natl Acad Sci U S A 105, 14686–14691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CR, Role LW, 1998. Functional contribution of the alpha7 subunit to multiple subtypes of nicotinic receptors in embryonic chick sympathetic neurones. J Physiol 509 ( Pt 3), 651–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Malagon AM, Yasuda D, Belluzzi JD, Leslie FM, Zaveri NT, 2017. The alpha3beta4 nAChR partial agonist AT-1001 attenuates stress-induced reinstatement of nicotine seeking in a rat model of relapse and induces minimal withdrawal in dependent rats. Behav Brain Res 333, 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewicz A, Richter K, Agne A, Wilker S, Siebers K, Fink B, Krasteva-Christ G, Althaus M, Padberg W, Hone AJ, McIntosh JM, Grau V, 2017. Canonical and Novel Non-Canonical Cholinergic Agonists Inhibit ATP-Induced Release of Monocytic Interleukin-1beta via Different Combinations of Nicotinic Acetylcholine Receptor Subunits alpha7, alpha9 and alpha10. Front Cell Neurosci 11, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaveri NT, Bertrand S, Yasuda D, Bertrand D, 2015. Functional characterization of AT-1001, an alpha3beta4 nicotinic acetylcholine receptor ligand, at human alpha3beta4 and alpha4beta2 nAChR. Nicotine Tob Res 17, 361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Lu Y, Bian H, Guo L, Zhu H, 2017. Activation of the alpha7 nicotinic receptor promotes lipopolysaccharide-induced conversion of M1 microglia to M2. Am J Transl Res 9, 971–985. [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Talmage DA, Role LW, 2013. Nicotine elicits prolonged calcium signaling along ventral hippocampal axons. PLoS One 8, e82719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Nelson ME, Kuryatov A, Choi C, Cooper J, Lindstrom J, 2003. Human alpha4beta2 acetylcholine receptors formed from linked subunits. J Neurosci 23, 9004–9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart R, Broad LM, Xi Q, Lee M, Moroni M, Bermudez I, Sher E, 2006. 5-I A-85380 and TC-2559 differentially activate heterologously expressed alpha4beta2 nicotinic receptors. Eur J Pharmacol 539, 10–17. [DOI] [PubMed] [Google Scholar]
- Zwart R, Carbone AL, Moroni M, Bermudez I, Mogg AJ, Folly EA, Broad LM, Williams AC, Zhang D, Ding C, Heinz BA, Sher E, 2008. Sazetidine-A is a potent and selective agonist at native and recombinant alpha 4 beta 2 nicotinic acetylcholine receptors. Mol Pharmacol 73, 1838–1843. [DOI] [PubMed] [Google Scholar]
- Zwart R, De Filippi G, Broad LM, McPhie GI, Pearson KH, Baldwinson T, Sher E, 2002. 5-Hydroxyindole potentiates human alpha 7 nicotinic receptor-mediated responses and enhances acetylcholine-induced glutamate release in cerebellar slices. Neuropharm 43, 374–384. [DOI] [PubMed] [Google Scholar]