A systematic study applying a variety of biotin-based proximity labeling approaches in several plant systems using various conditions and bait proteins.
Abstract
Proximity labeling is a powerful approach for detecting protein-protein interactions. Most proximity labeling techniques use a promiscuous biotin ligase or a peroxidase fused to a protein of interest, enabling the covalent biotin labeling of proteins and subsequent capture and identification of interacting and neighboring proteins without the need for the protein complex to remain intact. To date, only a few studies have reported on the use of proximity labeling in plants. Here, we present the results of a systematic study applying a variety of biotin-based proximity labeling approaches in several plant systems using various conditions and bait proteins. We show that TurboID is the most promiscuous variant in several plant model systems and establish protocols that combine mass spectrometry-based analysis with harsh extraction and washing conditions. We demonstrate the applicability of TurboID in capturing membrane-associated protein interactomes using Lotus japonicus symbiotically active receptor kinases as a test case. We further benchmark the efficiency of various promiscuous biotin ligases in comparison with one-step affinity purification approaches. We identified both known and novel interactors of the endocytic TPLATE complex. We furthermore present a straightforward strategy to identify both nonbiotinylated and biotinylated peptides in a single experimental setup. Finally, we provide initial evidence that our approach has the potential to suggest structural information of protein complexes.
INTRODUCTION
Protein-protein interaction studies often fail to capture low-affinity interactions, as these are usually not maintained following cell lysis, protein extraction, and protein complex purification. Particularly, this is the case for protein-protein interactions of integral membrane proteins because of the harsh conditions during protein extraction and purification. Biotin-based proximity labeling, on the contrary, uses in vivo covalent biotinylation of proteins that are interactors or near-neighbors of a bait protein of interest (Varnaitė and MacNeill, 2016). Hence, to identify interactions, they do not need to remain intact during purification. Although biotin is an essential cofactor for a small number of omnipresent biotin-dependent enzymes involved mainly in the transfer of CO2 during HCO3−-dependent carboxylation reactions, biotinylation is a relatively rare in vivo protein modification. Moreover, biotinylated proteins can be selectively isolated with high affinity using streptavidin-biotin pairing. Proximity labeling, therefore, permits the identification of both high- and low-affinity in vivo interactions.
Analogous to DamID, in which a prokaryotic Dam methylase is fused to a protein of interest to monitor DNA-protein interactions in eukaryotes (van Steensel and Henikoff, 2000), proximity labeling allows the capture or mapping of protein-protein interactions. More specifically, proximity labeling is based on the activity of native biotin ligases (e.g., Escherichia coli BirA), which catalyze a two-step reaction: first, the generation of reactive biotinyl-AMP (biotinoyl-5′-AMP or bioAMP) from biotin and ATP; and second, the attachment of that bioAMP to a specific Lys of the target protein. Engineered promiscuous biotin ligases (PBLs) have a significantly reduced affinity for the reactive bioAMP intermediate (Choi-Rhee et al., 2004; Kim and Roux, 2016). This intermediate is prematurely released and, due to its high reactivity, will interact with neighboring primary amines (e.g., Lys). Therefore, these variants lead to promiscuous labeling despite their lower affinity for biotin compared with native biotin ligases.
There are several variations of proximity labeling. The first-generation enzymes used were based on the E. coli biotin ligase BirA (Roux et al., 2012). The mutant BirA, designated BirA* (R118G; Kwon and Beckett, 2000), referred to hereafter as BioID, represents a monomeric protein of 35.3 kD and was the first PBL variant used for proximity labeling (Choi-Rhee et al., 2004; Cronan, 2005; Kim and Roux, 2016). A second-generation PBL, called BioID2, was derived from the Aquifex aeolicus biotin ligase (Kim and Roux, 2016). BioID2, which naturally lacks a DNA binding domain that is present in the larger BirA, is approximately one-third smaller than BioID, potentially reducing sterical hindrance of the bait protein (Kim et al., 2016). The third-generation PBLs, called TurboID and mini-Turbo (mTurbo), are derived from the directed evolution of BirA in yeast (Saccharomyces cerevisiae). These two variants showed maximal activity at 30°C, whereas the previous variants showed maximal activity at higher temperatures (Branon et al., 2018). TurboID has the same size as the original BioID tag, albeit with 14 amino acid mutations that greatly increase its labeling efficiency. mTurbo has 12 out of the 14 mutations. The N-terminal DNA binding domain was deleted to reduce its size (28 versus 35 kD), which also slightly impacted its labeling efficiency by reducing it approximately twofold. The first- and second-generation PBLs required ∼18 to 24 h of labeling or sometimes even much longer to produce detectable levels of protein biotinylation, while the TurboID variants required a labeling time in the range of 1 h or less in the various eukaryotic, nonplant systems tested so far (Branon et al., 2018).
Proximity labeling has intrinsic advantages and limitations. In the presence of biotin, the bait-PBL fusion protein labels proximal proteins without the activation by a conditional trigger, thereby tracking all interactions that occurred during a specific time period. The ability for selective capture makes the method generally insensitive to protein solubility or protein complexation, with potential applicability to interactomics studies of membrane proteins and cytoskeletal constituents, providing a major advantage over alternative approaches. Nevertheless, the identity of a candidate interactor does not immediately imply a direct or indirect interaction with the bait but reflects merely proximity (estimated to be ∼10 to 15 nm; Kim et al., 2014). Furthermore, true interactors are missed (false negatives) if they lack accessible primary amines.
So far, PBLs have successfully been used in yeast (Opitz et al., 2017), protozoa (Opitz et al., 2017), amoebae (Batsios et al., 2016), embryonic stem cells (Gu et al., 2017), and xenograft tumors (Dingar et al., 2015) to map a wide range of interactomes in both small-scale (i.e., using a single bait protein) and large-scale network mapping approaches (e.g., the protein interaction landscape of the centrosome-cilium interface or the organization of mRNA-associated granules and bodies [mRNP complexes]; Gupta et al., 2015; Youn et al., 2018).
In plants, the number of reports on the use of PBLs is slowly increasing. So far, four articles describe the application of the first generation of PBLs in plants (Lin et al., 2017; Conlan et al., 2018; Khan et al., 2018; Das et al., 2019). In these first trials, overexpression of BioID was combined with long labeling times, very high biotin levels, and relatively poor labeling efficiencies. These results suggest that first-generation BioID variants do not achieve sufficient activity in plant tissues due to their temperature-activity profiles.
Recently, two studies evaluated several generations of PBLs in plants, including the third-generation TurboID and mTurbo using Nicotiana benthamiana and Arabidopsis (Arabidopsis thaliana) seedlings as model systems, and concluded that TurboID outperforms the other PBLs in its capacity of both cis- and specific trans-biotinylation of both known and novel target proteins under conditions compatible with normal plant growth (Mair et al., 2019; Zhang et al., 2019).
Here, we expand our current knowledge on the use of proximity labeling as an interactomics tool in plants by performing a systematic survey of different approaches in various plant systems. We provide guidelines for the use of proximity labeling in a number of frequently used plant models and highlight the most relevant shortcomings and contingencies. Furthermore, we benchmark different proximity labeling methods at the proteomics level by studying the TPLATE protein complex and its interactors using harsh extraction and washing conditions to maximize the removal of false positives. We also employ a strategy that allows the identification of both nonbiotinylated and biotinylated peptides from a single experiment. Finally, we provide an extensive toolkit to perform proximity labeling in planta and foresee that the methods, tools, and materials described herein will greatly benefit the research community.
RESULTS
PBL-Mediated Biotin Labeling Efficiency Increases upon Biotin Administration in Tomato
To establish proximity labeling in various plant systems, we first tested different PBLs in stable hairy root lines of tomato (Solanum lycopersicum; Figure 1; see Methods). More specifically, we compared the potential applicability of enzyme-catalyzed proximity labeling when using BioID (Roux et al., 2012; Kim et al., 2016), BioID2 (Kim et al., 2016), and TurboID or mTurbo (Branon et al., 2018) as PBL. For this, we fused the engineered PBL to FLAG and enhanced green fluorescent protein (eGFP) tags under the control of the constitutive cauliflower mosaic virus (CaMV) 35S promoter (Supplemental Figures 1 and 2). In all systems tested so far, supplementation of biotin is important for efficient proximity biotin ligation with all the PBLs tested. Plants synthesize biotin endogenously, and thus, in certain systems, the intracellular pool of biotin might be high enough for the PBL. In fact, free biotin accumulates in plant mesophyll cells to a high concentration of approximately 11 μM (Alban et al., 2000), while for example in yeast this concentration is more than 10-fold lower (Pirner and Stolz, 2006). Considering that the Km of BioID for biotin is 0.3 µM, this could, in theory, lead to efficient proximity labeling even in the absence of exogenous biotin supplementation.
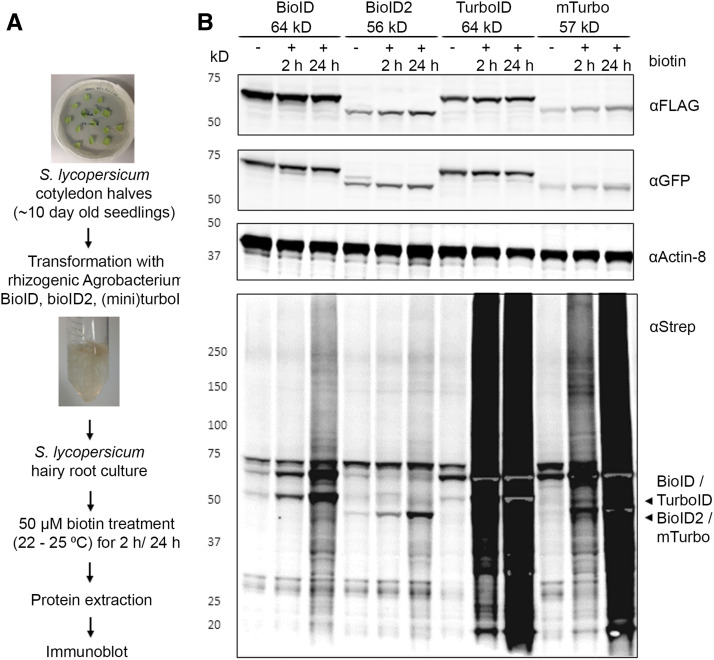
Figure 1.
Characterization of Enzyme-Catalyzed Proximity Labeling in Hairy Root Cultures.
(A) Experimental setup.
(B) Comparison of biotinylation activity in four PBL hairy root cultures from wild-type tomato expressing eGFP-BioID-Flag (∼64 kD), eGFP-BioID2-Flag (∼56 kD), eGFP-Turbo-Flag (∼64 kD), and eGFP-miniTurbo-Flag (∼57 kD). Addition of 50 μM exogenous biotin to 2-week-old hairy root cultures for 2 or 24 h was used for labeling. Arrowheads indicate the expected size of the cis-biotinylation signal. Gray regions in intense black areas represent saturation of the streptavidin-s680 signal and is most prominent in case of self-biotinylation activity. This is a representative experiment repeated twice, and two independent root cultures were analyzed per combination.
We therefore tested biotinylation efficiency in our hairy root system in the presence or absence of biotin using different tagged PBLs as fusion proteins, either codon-optimized for plants or non-codon-optimized (Supplemental Figure 1; Supplemental Table 1; Supplemental File).
As a test case for non-bait-specific biotinylation, PBL-fused eGFP was used. Biotinylation was evident as smears upon streptavidin-HRP-mediated protein immunoblot detection. This smear depicts biotinylation of proteins other than PBLs and will be referred to as “trans-biotinylation.” As a proxy for PBL activity, we used the cis-biotinylation efficiency (i.e., autobiotinylation or self-biotinylation level of PBL fusions) as readout (Figure 1). Manifold faster kinetics for TurboID and mTurbo over BioID and BioID2 could be observed (Figure 1). This is in line with the previously reported lower catalytic activities of the latter PBLs, especially at the growth conditions used (i.e., cultivation of hairy roots was performed at 22 to 25°C; Branon et al., 2018). We note that only residual trans-biotinylation was observed when no exogenous biotin was added to the liquid-grown hairy root cultures. Therefore, the addition of surplus (free) biotin seems also to function as a trigger of proximity labeling in this system. This observation indicates that proximity labeling in plants (to some extent) might also have the capacity to identify the spatiotemporal dynamics of interactome composition.
Proximity Labeling Efficiency Depends on Growth Temperatures, and PBL Can Facilitate Trans-Biotinylation in N. benthamiana
We used transient transformation of N. benthamiana leaf mesophyll cells to test the applicability of proximity labeling in a second model system commonly used for protein expression in planta under various conditions. In this case, biotin was infiltrated directly into leaf tissue 24 h after transformation and harvested 24 h after biotin infiltration (Supplemental Figure 3A). We confirmed that also in this system, the highest cis-biotinylation level was observed for TurboID, and supplementation of biotin was important for the efficient detection of cis-biotinylation (Supplemental Figure 3B). Furthermore, the overall biotinylation output signal in N. benthamiana leaves was higher when biotin concentration was increased from 50 μM to 1 mM (Supplemental Figure 3B).
Evaluation of wild-type BirA showed no trans-biotinylation in the presence of 50 µM exogenous biotin (Supplemental Figure 4A), confirming that the R118G mutation is responsible for promiscuous labeling in plants. Furthermore, a temperature shift from 22 to 28°C increased cis- and trans-biotinylation for both BioID and TurboID, suggesting that temperature control can be used to modulate promiscuous labeling in plants (Supplemental Figures 4A and 4B; see also below).
The effect of temperature on TurboID activity was less apparent compared with that of BioID, consistent with the temperature-activity profiles of the two enzymes (Branon et al., 2018). Interestingly, similar to GFP-TurboID expressed in the hairy root cultures, cis-biotinylation (Figure 1) was saturating already 2 h after biotin addition in N. benthamiana (Supplemental Figure 4D). TurboID and mTurbo were the only PBLs in plants with biotinylation efficiency occurring in the range of a few hours, as other PBLs did not show any visible sign of trans-biotinylation in that time frame (Figure 1).
TurboID Is Useful for the Efficient Capture of Plasma Membrane Interactomes in N. benthamiana
Next, we tested whether we could achieve biotinylation of protein interactors using proximity labeling under the conditions established for N. benthamiana. We observed that the bait proteins used in plants for proximity labeling to date were either membrane-anchored and small proteins (HopF2 [Khan et al., 2018] and AvrPto [Conlan et al., 2018]) or nucleus and/or cytoplasm localized (OsFD2 [Lin et al., 2017], N [Zhang et al., 2019], and FAMA [Mair et al., 2019]).
We therefore tested our conditions using as test cases integral plasma membrane-localized protein complexes with components that reside within a range of a few nanometers. First, we used a known membrane receptor complex from Lotus japonicus comprising two symbiotically active receptor-like kinases (RLKs): the LysM-type RLKs NOD FACTOR RECEPTOR5 (NFR5) and the LRR-RLK SYMBIOTIC RECEPTOR-KINASE (SYMRK). These proteins assemble within the same complex in L. japonicus roots (Ried et al., 2014) as well as in N. benthamiana upon heterologous expression (Antolín-Llovera et al., 2014). By contrast, the brassinosteroid receptor BRASSINOSTEROID INSENSITIVE1 (BRI1) did not coimmunoprecipitate with the symbiotic receptor complex, indicating no or only weak interactions with these RLKs (Antolín-Llovera et al., 2014). However, using bimolecular fluorescence complementation (BiFC), another study reported some interactions between NFR5 and BRI1 as well as with the Arabidopsis innate immune pattern recognition receptor FLAGELLIN SENSING2 (FLS2; Madsen et al., 2011). To further extend the set of control proteins, we additionally included the EF-TU RECEPTOR (EFR), belonging to the LRR family, as well as the LOW TEMPERATURE INDUCED PROTEIN LTI6b that is commonly used as a plasma membrane marker in plant cell biology (Grebe et al., 2003).
In a first experiment, we tested whether cytosolic TurboID would nonspecifically trans-biotinylate the receptors at the plasma membrane. For this, we coexpressed a TurboID-GFP fusion protein with GFP-tagged receptors in N. benthamiana and immunoprecipitated all components using an anti-GFP nanotrap (Supplemental Figure 5A). While all coexpressed proteins could be detected before and after the immunoprecipitation, we only detected cis-biotinylation of TurboID-GFP but not of the receptors (Supplemental Figure 5A). This indicates the absence of nonspecific trans-biotinylation of membrane resident receptors by a soluble TurboID itself. However, it should be clearly stated that prolonged reaction times and increased expression of TurboID will likely result in a certain degree of nonspecificity due to the inherent features of the system.
To test biotinylation between membrane-resident receptors, we coexpressed an NFR5-TurboID (120 kD) fusion protein with either the known NFR5-interacting RLK SYMRK or with BRI1 and FLS2, which may not be stable components of the NFR5/SYMRK receptor complex. As higher degrees of nonspecificity are expected for proteins that reside in close proximity to each other, we tested trans-biotinylation 15 and 30 min after the addition of exogenous biotin (Figure 2; Supplemental Figure 5). As expected, we observed trans-biotinylation of SYMRK-GFP (150 kD) by NFR5-TurboID after 15 min when SYMRK-GFP was immunoprecipitated using anti-GFP nanotrap beads. With the 30-min labeling time, stronger trans-biotinylation of SYMRK5-GFP was detected (Figure 2, top). When applying the same experimental conditions to plants coexpressing BRI1-GFP (157 kD) and NFR5-TurboID, we detected weaker trans-biotinylation after 15 min and still only weak trans-biotinylation after 30 min of BRI1-GFP. These data show that temporal control during labeling experiments is crucial to maintain specificity in the system and that BRI1 may reside in close proximity to the NFR5/SYMRK complex, despite a lack of a stable and physical interaction.
Figure 2.
NFR5-TurboID Shows Strong Biotinylation of Its Known Interactor SymRK-GFP.
Pairwise combination of NFR5-TurboID (120 kD) with either SYMRK-GFP (150 kD) or BRI1-GFP (157 kD) using transient expression in N. benthamiana leaves allowed time-dependent and prevalent biotinylation of SYMRK. Biotin at 50 µM was applied for 15 or 30 min. IB, immunoblot; IP, immunoprecipitation.
Given these results, we sought to test a number of other membrane proteins to elucidate whether the observed levels of nonspecificity are at least partially dependent on the target protein. We coexpressed NFR5-TurboID with the transmembrane proteins FLS2, EFR, and LTI6b. While no trans-biotinylation of EFR and LTI6b was detected, we observed a weak signal for BRI1 as shown above as well as for FLS2, but again considerably lower compared with the levels found for SYMRK, indicating an important impact of the target proteins on the trans-biotinylation patterns (Supplemental Figure 5B). It should be noted that we were not able to detect cis-biotinylated NFR5 after immunoprecipitating SYMRK using GFP nanotraps. This is most likely due to the stringent washing conditions and the possibility that only a fraction of NFR5-TurboID was coimmunoprecipitated together with SYMRK. Taken together, these data are in line with a previously published report (Madsen et al., 2011) and show that predominant trans-biotinylation of proximal membrane-resident proteins is possible, even under constitutive expression in heterologous systems. However, stringent control of experimental conditions such as expression levels and exposure time to biotin is greatly advised.
In summary, these data clearly show that TurboID-mediated proximity labeling can efficiently capture interactors of membrane proteins. Furthermore, it may be advantageous over other methods such as coimmunoprecipitation, as it does not require optimization of the solubilization conditions and provides the possibility to detect transient protein complex constituents.
Application of Proximity Labeling in Arabidopsis Cell Cultures Using the TPLATE Complex as a Case Study
Next, we surveyed the efficiency of trans-biotinylation for a stable multi-subunit plant protein complex. As a test case, we selected the plasma membrane-associated octameric TPLATE complex (TPC; Gadeyne et al., 2014) and used stably transformed Arabidopsis cell suspension cultures as a third plant model system for proximity labeling.
Given the higher biotinylation level observed in N. benthamiana at 28°C (Supplemental Figure 4), we began by evaluating different labeling conditions. To study the temperature effect in this system, we grew cells expressing TPLATE-BioID and GFP-BioID (i.e., proteins fused to the first-generation PBL) at various temperatures in the presence of 50 µM biotin for 24 h. We subsequently isolated the complex under nondenaturing conditions using streptavidin affinity purification (see Methods), performed trypsin on-bead digestion, and analyzed the released nonbiotinylated peptides using liquid chromatography-tandem mass spectrometry (LC-MS/MS).
In order to evaluate the effect of temperature on biotinylation efficiency and on the subsequent identification of the proteins from the isolated complexes, we focused on the other seven TPLATE complex members. We compared their abundances and fold changes with the control setup (35S::GFP-BioID) after streptavidin purification, taking into account label-free protein quantification (LFQ) intensities (Figures 3A and 4; Cox et al., 2014). In addition to the bait, all seven interacting subunits could be detected at all tested temperatures (Figure 4; Supplemental Data Set 1). However, the fold changes observed with respect to the control were not dramatically different between the different temperatures. As we did not observe any major differences with respect to the efficiency of detecting TPC subunits at all tested temperatures, and given the increased efficiency observed in N. benthamiana at 28°C and the likely negative impact of increased temperature on the physiology of the plants, we opted for 28°C as an optimal tradeoff to perform a series of follow-up experiments on the TPC in Arabidopsis cultures.
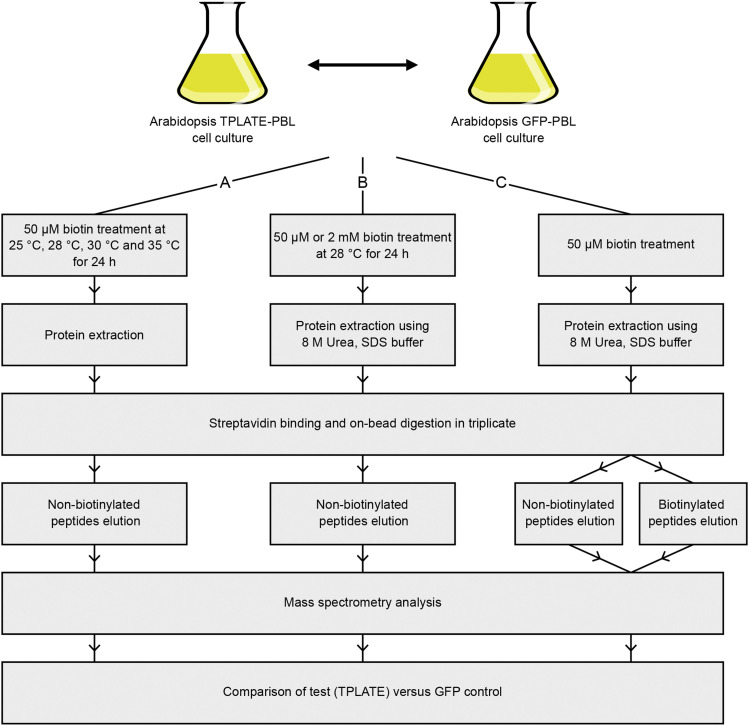
Figure 3.
Schematic Overview of the Subsequent Experimental Procedures Followed.
(A) Initial experimental setup to compare enriched TPC subunits in biotin-treated transformed Arabidopsis cell cultures at different temperatures. Cell cultures (TPLATE/GFP-BioID) were incubated with 50 µM biotin at 25 to 35°C for 24 h before harvesting. Proteins were extracted using a standard protein extraction buffer (see Methods). This protocol was used to obtain the results in Figure 4.
(B) Experimental setup to compare the efficiency of different PBLs with or without long linker sequence. Cell cultures (TPLATE/GFP-linkerBioID, -linkerBioID2, and -linkerTurboID) were incubated with 50 µM or 2 mM biotin at 28°C for 24 h before harvesting. Protein extraction was performed under harsh conditions to exclude false positives (see Methods). This protocol was used to obtain the results in Figures 5 and 6A.
(C) Schematic overview of the optimized and final experimental setup to detect both biotinylated and nonbiotinylated peptides from Arabidopsis cell cultures (TPLATE/GFP-linkerTurboID). Following harsh extraction and on-bead digestion, nonbiotinylated and biotinylated peptides were separately (sequentially) eluted and analyzed. All identified peptides were used for MS analysis. This protocol was used to obtain the results in Figures 6B and 8.
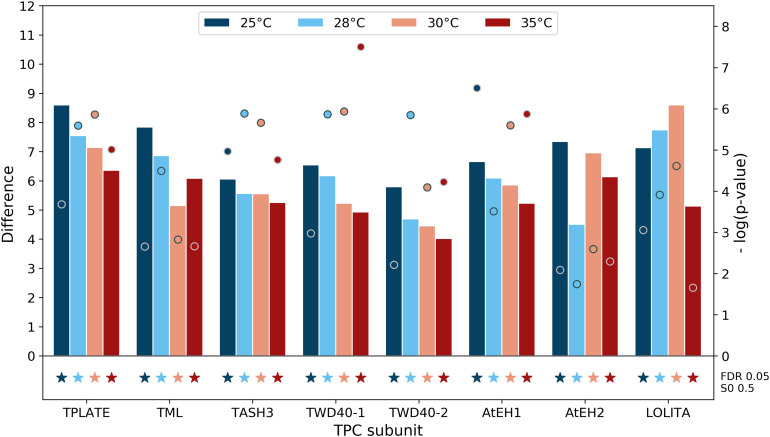
Figure 4.
Detection of TPC Subunits with TPLATE-BioID Is Optimal at 28°C.
Comparison of the enrichment of the TPC subunits in the TPLATE-BioID samples at different temperatures compared with their respective GFP-BioID controls is shown. Difference (bar charts) and −log(p-values) (dots) are derived from t tests in Perseus software, using the average LFQ intensities of three technical replicates of TPLATE-BioID versus three technical replicates of GFP-BioID at similar temperature. All TPC subunits are detected at all four temperatures without major differences and all are significantly enriched with TPLATE-BioID (denoted by stars), as determined by permutation-based FDR, with cutoffs FDR = 0.05 and S0 = 0.5. The full list of significantly enriched identifications with TPLATE-BioID at all tested temperatures can be found in Supplemental Data Set 1.
Various PBLs Affect the Biotinylation of TPC Subunits Differently
The introduction of a flexible linker (Roux et al., 2012) has been successfully used to extend the labeling radius of PBLs (Kim et al., 2016), which is estimated to be ∼10 to 15 nm (Kim et al., 2014). This increased labeling radius may be desirable when the protein of interest is significantly larger than the labeling radius of the PBL alone and/or when the goal is to map the constituency of a larger protein complex or a discrete subcellular region. We thus compared the efficiencies of various PBLs and assessed their biotinylation radius by inserting a 65-amino acid-long flexible linker. Arabidopsis cultures expressing C-terminal fusions of TPLATE with BioID or BioID2 were assessed, with and without a 65-amino acid linker similar to one reported previously (Roux et al., 2012). As controls, we generated GFP fused to BioID or BioID2 without the additional linker (Supplemental Figure 6).
To test the effect of the linker and to further evaluate the activity of different PBLs in Arabidopsis cell culture, transgenic cultures were grown for 24 h, with and without exogenous biotin at 28°C, and expression and biotinylation were assessed via protein immunoblotting (Supplemental Figure 6). For the most part, protein abundance of the BioID and BioID2 constructs was comparable to the respective controls in our cell cultures and was not affected by the addition of biotin; only TPLATE-BioID2 levels were somewhat lower. At the levels of cis- and trans-biotinylation, we observed different patterns for each of the fusion proteins used. Several of the detected bands that increased significantly in the presence of biotin did not correspond to bands in the control or GFP-BioID culture and varied between the different PBLs. We suggest that these likely represent different trans-biotinylated interactors and that the outcome of a BioID-based interaction assay might partially depend on the PBL used. The TPLATE-linkerPBL showed the most complex biotinylation pattern when compared with the other setups expressing BioID and BioID2 fusions (Supplemental Figure 6), suggesting that the addition of a linker may be used to enhance proximity labeling. Consistent with the results described for N. benthamiana, TurboID constructs showed some residual biotinylation without the addition of exogenous biotin, increased biotinylation after 1 h of incubation with biotin, and gave rise to an extensive biotinylation pattern after 24 h of incubation with biotin in both control and bait cultures, suggesting that it is highly promiscuous.
As observed in N. benthamiana (Supplemental Figure 3) using GFP as bait protein, BioID also outperformed BioID2 using TPLATE as bait in this system, although this might (in part) be skewed due to the lower expression levels of the latter. Adding a flexible linker increased cis-biotinylation levels of the bait compared with the constructs without linker (Supplemental Figures 6A and 6C). Overall, our results are consistent with previous observations in nonplant systems suggesting that linkers increase the biotinylation output (Kim et al., 2016).
Following the positive effect of exogenous biotin supplementation (Supplemental Figures 3 and 4), we tested the effect of increasing biotin concentrations on cis-biotinylation efficiency. Cell cultures expressing TPLATE-linkerBioID were grown at 28°C and incubated for 24 h in the presence of increasing concentrations of biotin (50 µM to 4 mM), after which they were analyzed by protein immunoblotting (Supplemental Figure 7A). Supplementing the culture with biotin concentrations in the range of 50 µM to 2 mM increased cis-biotinylation output up to approximately twofold. Increasing biotin concentration >2 mM did not further increase cis-biotinylation efficiency (Supplemental Figure 7B).
We took advantage of the increased biotinylation observed by including a long linker sequence and generated Arabidopsis cultures expressing GFP-linkerTurboID and TPLATE-linkerTurboID. Similar to other reports, when sampling was done 24 h after biotin addition, TurboID efficiency strongly outperformed all other PBLs tested, as evident from the high biotinylation levels observed with and without the addition of exogenous biotin for both the control (GFP) and the TPLATE-expressing cultures (Supplemental Figures 6B and 6D).
To compare the different PBL modules, we processed the isolated proteomes of our cell cultures for LC-MS/MS analysis and focused on the relative levels of the various TPC subunits compared with the control setup. Mass spectrometry (MS) results following streptavidin purification under nondenaturing conditions and on-bead digestion identified all known subunits of the TPC (Figure 4). Given that TPC is a robust multi-subunit complex (Gadeyne et al., 2014) and that we identified only nonbiotinylated peptides with our on-bead digestion protocol, we assumed that the subunits we detect are a combination of direct biotinylation as well as coimmunoprecipitation of the complex as a whole under the nondenaturing conditions. To test this, we adapted our protocol (Figure 3B) and performed protein extraction and stringent washing steps under denaturing conditions using a buffer containing 8 M urea and 2% (w/v) SDS to unfold proteins before streptavidin immunoprecipitation and to remove nonspecific, or indirect, nonbiotinylated protein binders. We also included the TPLATE-linkerBioID setup treated with 2 mM biotin for 24 h to assess if increased biotin concentration improves TPC subunit detection.
In agreement with the higher stringency of the isolation procedure, the smallest TPC subunit, LOLITA, which was robustly detected using affinity purification and mass spectrometry MS (AP-MS; Gadeyne et al., 2014) and, as shown here, without being denatured before binding to streptavidin beads (Figure 4), was no longer detected (Figure 5; Supplemental Data Set 2). LFQ revealed that the remaining seven TPC subunits, including the bait TPLATE, were detectable using BioID, linkerBioID, linkerBioID2, and linkerTurboID, although not all subunits were significantly enriched compared with the GFP PBL control using our statistical threshold criteria (false discovery rate [FDR] of 0.05 and Fudge factor S0 of 0.5). The TASH3 and TWD40-2 subunits, for example, could not be confidently identified with all PBLs. For BioID2, this might be caused by the reduced expression level of the bait in these cultures (Supplemental Figure 6), yet this does not explain why this low level of detection is not observed for the other subunits as well (Figure 5). We also conclude that adding a long linker increased the robustness of prey identification. For example, using TPLATE-linkerBioID, the TASH3 subunit was detected with 15 peptides compared with only 2 peptides when using TPLATE-BioID (Supplemental Table 2). We did not identify TASH3 with TPLATE-BioID2, in contrast to TPLATE-linkerBioID2, where we identified TASH3 with 59 peptides (Supplemental Table 2).
Figure 5.
Different TPLATE-PBLs Affect Biotinylation of TPC Subunits Differently.
Comparison of the enrichment of the TPC subunits with different TPLATE-PBLs versus their respective GFP-PBLs at 28°C is shown. Difference (bar charts) and −log(p-value) (dots) are derived from t tests in Perseus software, using LFQ intensities of three technical replicates of the test compared with three replicates of the respective control. The stars below the graph denote that proteins were found to be significantly different from the control by permutation based-FDR, with cutoffs FDR = 0.05 and S0 = 0.5. The full list of significantly enriched identifications with different TPLATE PBLs at 28°C can be found in Supplemental Data Set 2.
We note that increasing the concentration of biotin from 50 μM to 2 mM adversely affected TPC subunit detection, as only the bait itself could be identified. It is likely that increasing biotin concentrations causes residual free biotin to accumulate in the protein extract, even after protein desalting to deplete free biotin, thereby occupying the streptavidin binding sites on the beads that are saturated at >9 µM biotin. We tested this “saturation hypothesis” using N. benthamiana leaves and protein precipitation to completely remove residual biotin, showing that even at low concentration, residual biotin can saturate the streptavidin beads and incapacitate detection (Supplemental Figure 8). Hence, special care should be taken to avoid an excess of residual free biotin during streptavidin-based capture. A similar conclusion was obtained in other studies combining PBL with MS analysis in planta (Mair et al., 2019; Zhang et al., 2019).
It should be noted that the fold change by which the other TPC subunits were detected with TurboID was comparable or sometimes even lower (e.g., AtEH2/Pan1) compared with the other BioID forms tested (Figure 5). This was due to TPC subunits being identified with higher abundance in the TurboID control samples, resulting in lower relative fold changes. All individual TPC subunits were detected with more than 20 unique peptides using the GFP-linkerTurboID, whereas TWD40-2 was the only TPC subunit detected in the other control GFP-PBLs, which explains its overall low fold change (Supplemental Table 2). Nevertheless, TurboID identified most of the TPC subunits more robustly compared with the other PBLs, as evidenced by the overall higher −log10 P values. So, although in our case TurboID was found to be superior to all others in identifying the other TPC subunits, the lower signal:noise ratio of TurboID, due to its increased activity, might work as a disadvantage to observe differences between bait proteins and control samples. This effect might even be enhanced if the proteins are targeted to specific subcellular locations.
The Structural Composition of Protein Complexes Causes Differences in Detection between Proximity Labeling and AP-MS
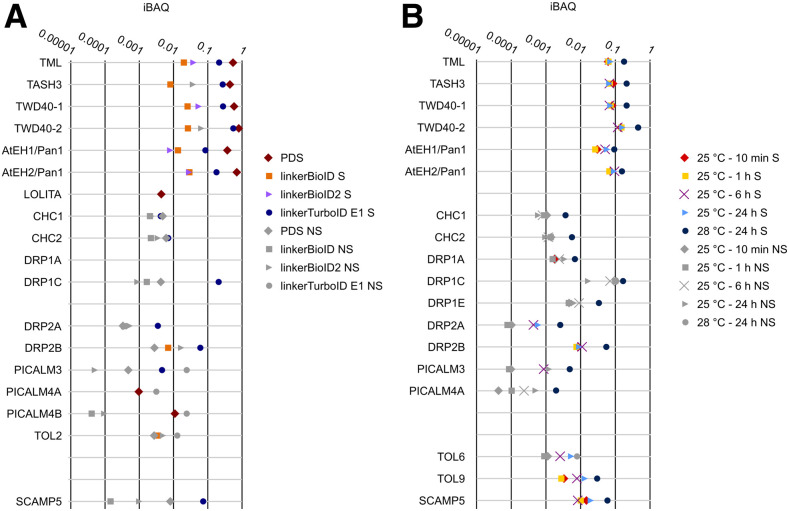
To further evaluate proximity labeling, we measured the relative levels compared with the bait by which the different TPC subunits were detected using our stringent washing protocol with a one-step IgG-based pull-down protocol using the GSrhino tandem affinity purification tag (Van Leene et al., 2019). To do this, we used the MaxQuant intensity-based absolute quantification (iBAQ) value, which is the result of the summed intensity values of the identified peptides divided by the number of theoretical peptides. We calculated these iBAQ values for each TPC subunit, normalized to the value for the bait (TPLATE) to correct for differences in bait fusion expression levels, and compared the values of TPLATE-linkerBioID, TPLATE-linkerBioID2, and TPLATE-linkerTurboID with those from IgG pull-down. When normalized to the bait protein (TPLATE), the other TPC subunits are detected by TurboID at similar levels as compared with IgG pull-down (Figure 6A; Supplemental Data Set 3). The one exception is the subunit LOLITA, which could only be detected by IgG pull-down. The six other TPC subunits could also be significantly detected by BioID and BioID2, although with less efficiency.
Figure 6.
Comparing the Identification of a Subset of Proteins Copurified with TPLATE Using GSrhino Pull-Down (PDS), LinkerBioID, LinkerBioID2, or LinkerTurboID.
(A) Pull-down and proximity biotinylation comparison of a selection of TPLATE interactors. Experiments were performed in triplicate, using TPLATE as bait and using the protocol in Figure 3B. Per set of experiments, MaxQuant iBAQ values, which are the summed intensity values divided by the number of theoretical peptides, were calculated and normalized versus the bait in order to compare the relative abundance of the proteins between the four different approaches. Proteins that were identified significantly (S) in either method are represented with a colored shape. Proteins that were identified below the significance threshold (NS) for a given method are indicated with gray shapes.
(B) Overview of a subset of the identified interactors, color-coded according to their statistical significance in the different experiments (S = significant and NS = not significant) by combining MS data from both elution fractions using the protocol in Figure 3C. Arabidopsis cell cultures expressing TPLATE-linkerTurboID were grown at 25°C and supplemented with exogenous biotin for 10 min, 6 h, or 24 h. Results were compared with the experiment from (A), where the culture was grown at 28°C in the presence of biotin for 24 h.
The complete list of significantly enriched identifications of the experiments shown in (A) and (B), including their normalized average iBAQ values, can be found in Supplemental Data Sets 3 and 4.
The observation that the smallest subunit, LOLITA, could only be identified via AP-MS indicates that this subunit is not biotinylated although it harbors 11 Lys residues, possibly reflecting the structural composition of the TPC. Our results furthermore reveal that, except for LOLITA, all TPC subunits, which are part of a protein complex in the range of 1 MD, can be identified using our stringent wash protocol as a proxy for biotinylation.
TurboID Facilitates Broadening the Interactome of Protein Complexes
We subsequently broadened the analysis toward other interactors and compared all proteins that were significantly enriched in one of the data sets (TPLATE-GSrhino, TPLATE-linkerBioID, TPLATE-linkerBioID2, and TPLATE-linkerTurboID; Supplemental Data Set 3). Whereas the overall number of significant interactors identified with the GSrhino and linkerBioID tags was higher than the number of significant interactors found with linkerTurboID, the latter identified several known players in clathrin-mediated endocytosis (CME) with much stronger statistical significance (Figure 6A). These players included the two Clathrin Heavy Chains (CHCs) and several Dynamin Related Proteins (DRPs). Moreover, TPLATE-linkerTurboID allowed significant enrichment for novel interactors with a clear link to CME, such as the SECRETORY CARRIER MEMBRANE PROTEIN5 (SCAMP5) and an ANTH/ENTH protein, PICALM3 (phosphatidylinositol binding clathrin assembly protein). Integral membrane SCAMP proteins are hypothesized to act in both the exocytic and endocytic pathways between the plasma membrane and the trans-Golgi network (Law et al., 2012). PICALM3 was not identified previously as a TPC interactor, but PICALM4A (AtECA4) and PICALM4B (CAP1) were previously found to be associated with the TPC (Gadeyne et al., 2014), as confirmed here using our IgG pull-down approach (Figure 6A).
Identification of Biotinylated Peptides Enhances the Power of Proximity Labeling and Allows the Mapping of Structural Relationships between Complex Subunits
The interaction between biotin and streptavidin is strong enough to be maintained even under harsh conditions (Supplemental Figure 9). Thus, biotinylated peptides are expected to be retained on the streptavidin beads. Following stringent washing under denaturing conditions, on-bead digestion releases nonbiotinylated proteins, which can subsequently be identified using LC-MS/MS. This approach, however, does not provide direct evidence for biotinylation, and it relies on the assumption that only biotinylated proteins remain bound to the beads after the washing steps. To acquire direct proof of biotinylation, and to further enhance the power of proximity labeling to identify interactors, the release of biotinylated peptides from the streptavidin beads and their subsequent MS-based identification are required.
Thus, we expanded the protocol (Figure 3C) to enable the identification of biotinylated peptides. For this, we included a second elution step (see Methods) to release the biotinylated peptides from the beads using an adapted protocol based on previous work (Schiapparelli et al., 2014). This approach enables the detection of both nonbiotinylated and biotinylated peptides in the same experimental setup.
As a previous report on TurboID describes no major changes in the activity of TurboID between 22 and 30°C and used biotin treatments of only a few hours (Mair et al., 2019), we tested whether we could improve the identification of novel TPC interactors by reducing the time of biotin addition to our cell cultures grown at normal growth temperatures. We performed a series of experiments comparing short (10 min and 1 h), medium (6 h), and long (24 h) biotin treatments at the normal growth temperature (25°C) of our Arabidopsis cell culture. We compared the iBAQ values of all significant hits, using both elutions of each experiment at 25°C and those from our 24-h experiment at 28°C (Figures 3C and 6B; Supplemental Data Set 4). The robustness of detecting interactors clearly increased with longer biotin incubation times. Also, there was a positive effect of working at a slightly elevated temperature (Figure 6B). Combining both elution fractions also increased the robustness of interactor identification. More specifically, including the second elution allowed the identification of additional DRPs and AtECA4, as well as TOL6 and TOL9 (Figure 6B), compared with the results when only the first elution (on-bead digestion) was analyzed (Figure 6A).
Out of the five TOL proteins studied to date, TOL6 and TOL9 localize strongly at the plasma membrane (Moulinier-Anzola et al., 2020). TOL proteins are part of the Endosomal Sorting Complexes Required for Transport pathway and act as gatekeepers for degradative protein sorting (Korbei et al., 2013). We confirmed the association between TPLATE and TOL6, TOL9, and SCAMP5. TOL6-Venus revealed a high degree of colocalization with TPLATE-TagRFP at endocytic foci on the plasma membrane (Figure 7A), which was severely reduced when the image of one channel was flipped horizontally (Figure 7B). Furthermore, quantitative analysis showed TPLATE interacting with TOL9 and SCAMP5 by ratiometric BiFC. The YFP:RFP ratio was significantly higher for all four independent combinations tested compared with a negative control set where we combined TPLATE with the shaggy-like kinase BIN2 (Figures 7C to 7H). The identification and confirmation of these novel interactors shows that proximity labeling can expand our knowledge of the interactomes of multisubunit complexes in plants beyond currently used approaches based on AP-MS.
Figure 7.
TOL6, TOL9, and SCAMP5 Can Be Confirmed as Novel TPC Interactors.
(A) and (B) Representative spinning-disk dual-color images and corresponding quantification of colocalization (%) between TPLATE and TOL6. TPLATE-TagRFP endocytic foci at the plasma membrane were compared with TOL6-Venus foci (A) as well as horizontally flipped TOL6-Venus (TOL6_F) channel images as a control (B). Eight movies from three individual plants, and in total 2607 foci, were analyzed.
(C) to (G) Ratiometric BiFC analysis confirming the interaction of TOL9 ([C] and [D]) and SCAMP5 ([E] and [F]) with TPLATE. BIN2 (G) was used as a control. CC and CN refer to the orientation of the nYFP and cYFP: N-terminal cYFP is annotated as CN and C-terminal cYFP is annotated as CC.
(H) Box plot and Jitter box representation of the quantification of the YFP/RFP fluorescence ratios (n ≥ 15). The black lines represent the median and the red circles represent the mean. Letters above the plots indicate statistical significance using a Welch-corrected ANOVA to account for heteroscedasticity.
Bars = 5 µm ([A] and [B]) or 20 µm ([C] to [G]).
Next to enhancing the robustness of TurboID to identify interactors, the identification of biotinylated peptides also provides direct proof of the proximity of specific domains of the prey proteins with respect to the bait. We therefore tested whether biotinylated peptides could reveal differential proximity between specific domains of TPC subunits using the TPLATE-linkerTurboID as bait (Figure 8; Supplemental Data Set 5). The most biotinylated peptides were identified for TPLATE (44 biotinylated peptides), followed by TWD40-1 (18), AtEH2/Pan1 (16), AtEH1/Pan1 (12), TWD40-2 (9), and TML (3). No biotinylated peptides could be detected for LOLITA, correlating with our previous results. Mapping nonbiotinylated and biotinylated peptides on the different TPC subunits (taking into account their relative abundances) revealed differences in the number of detected peptides as well as differences in the distribution of the biotinylated peptides along the length of the subunits. Whereas the bait, TPLATE, shows a relatively even distribution of biotinylated peptides along the protein sequence, there is a clear tendency of the AtEH1/Pan1, AtEH2/Pan1, and TML subunits toward increased biotinylation at their C-terminal parts (Figure 8).
Figure 8.
Mapping of Biotinylated Versus Nonbiotinylated Peptides Reveals Differential Proximity/Accessibility of Specific TPC Subunit Domains.
Schematic representation of seven TPC subunits and their domains is shown. Identified peptides, color-coded according to their abundance (in gray for nonbiotinylated peptides and from yellow to red for biotinylated peptides), are mapped onto them. The full list of biotinylated and nonbiotinylated peptides identified for TPC subunits in the TPLATE-linkerTurboID culture is shown in Supplemental Data Set 5.
DISCUSSION
We provide a comprehensive comparison of various PBL-based proximity labeling strategies in plants. We show that TurboID is the most promiscuous PBL and that this sometimes leads to a lower signal:noise ratio. We also provide guidelines and approaches for interactome capture in various plant systems as well as a detailed protocol for Arabidopsis cell suspension cultures, specifically focusing on proteins that are intrinsic or peripheral to the plasma membrane (Supplemental Protocol). Furthermore, we show that for each bait/system, conditions might benefit from independent optimization.
We observed that in all three plant systems tested, the exogenous application of biotin enhances proximity labeling output but might not be a strict requirement for the successful application of proximity labeling. This result seems to contradict what has been reported for a related method called INTACT (isolation of nuclei tagged in specific cell types) in plants, which allows for affinity-based isolation of nuclei from individual cell types of tissue. INTACT relies on the endogenous pool of biotin, as no exogenous supplementation is required (Deal and Henikoff, 2011). In INTACT, nuclei are affinity-labeled through transgenic expression of the wild-type variant of BirA, which biotinylates a nuclear envelope protein carrying a biotin ligase recognition peptide from ACC1. This tag acts as a native substrate for the E. coli biotin ligase BirA (Beckett et al., 1999). The use of wild-type BirA along with its preferable substrate could explain the higher affinity for the free biotin pool in INTACT, and the peptide used as fusion is an optimal substrate for the bioAMP intermediate. We assume that various proteins may show variability in functioning as acceptors of bioAMP (e.g., depending on the accessibility of Lys residues).
Proximity labeling utilizing bacterial enzymes poses the question of whether these enzymes could perform adequately in plants (Kim et al., 2016). The activity optimum temperatures for BioID and BioID2 are 37 and 50°C, respectively; thus, BioID2 may be most adequate for use at higher temperature conditions. Both temperatures are however far from the usual growth temperatures of most plant species grown in temperate regions. Both BioID2 and BioID show reduced activity below 37°C (Kim et al., 2016; our results herein). The lower temperature optimum of TurboID (and mTurbo; Branon et al., 2018) would imply that TurboID may function better at normal plant growth temperature. Previous work showed no enhanced activity of TurboID when using temperatures above normal plant growth conditions (Mair et al., 2019). We observed however that TurboID activity increases around twofold from 22 to 28°C and that there is a beneficial effect of slightly increasing the growth temperature of our cell cultures on the identification of specific interactors of TPC. At all tested temperatures, we observed that TurboID (and mTurbo) outperforms other PBLs in terms of speed and promiscuity. Hence, TurboID might be preferred over other PBLs when it concerns the initial study of (transient) complex composition where the generation of as much as possible specific biotinylation output in a short time might be desirable.
However, the strong promiscuity of the control might also work as a disadvantage in revealing specific interactions in cases where the reaction cannot be controlled easily in time or when both the bait and the control would be targeted to a confined intracellular space. Furthermore, controls may express at high levels and show increased diffusion due to their smaller hydrodynamic radius, further skewing results.
We provide evidence that our methods and conditions apply to plasma membrane complexes. We showed that the interaction of the symbiotic RLKs NFR5 and SYMRK can be identified by exploiting proximity labeling, and particularly the PBL TurboID. Furthermore, the use of proper negative controls is imperative. However, even though the brassinosteroid receptor BRI1 was not coimmunoprecipitated with the symbiotic receptors in a previously published data set (Antolín-Llovera et al., 2014), we detected weak biotinylation of this RLK and the immune receptor FLS2. While it could be interpreted as unspecificity within the PBL system, it should also be considered that PBL allows labeling of transient interactions or proximal proteins. As a consequence, continuous unstable interactions accumulate to detectable amounts of proteins and would thus allow their identification. As proximity labeling using TurboID is capable of trans-biotinylation in the range of minutes (15 min under our experimental conditions), the enrichment of unstable interactions would thus be more prominent. Therefore, putative interactions identified by PBL need to be verified using independent experimental systems, but comparisons between the different experimental systems should always reflect the technical limitations of each approach.
By expanding our protocols and PBLs into Arabidopsis cell cultures, we could not only reproduce the composition of the TPC, except for one subunit, but we could also robustly identify and confirm other CME players and novel interactors using the third-generation PBL. We show that MS-based identification of interactors is more robust using prolonged biotin exposure of Arabidopsis cell cultures and that the use of linkers can be advantageous when it comes to identifying protein-protein interactions of multi-subunit complexes. Furthermore, TPLATE-linkerBioID2 shows reduced cis-biotinylation compared with TPLATE-linkerBioID in the presence of exogenous biotin but seems to function in the absence of biotin, suggesting that in plants, BioID2 can function in tissues where exogenous supplementation of biotin may be less effective (e.g., the vasculature). Furthermore, increased biotin application can lead to serious impediments when it comes to the identification of interactors, as this can interfere with biotinylated proteins binding on streptavidin slurries. Caution is warranted to ensure sufficient capture-capacity of biotinylated proteins, since the amount of beads needed for capture should be tested for each experimental model system/setup/protocol.
Complementary to the reports on TurboID in planta published so far (Mair et al., 2019; Zhang et al., 2019), we have established a strategy that uses much harsher conditions, with higher concentrations of SDS and urea for extraction and washing to remove false positives as much as possible (i.e., nonbiotinylated proteins). Finally, we also provide a protocol for the simultaneous identification of biotinylated and nonbiotinylated peptides. This approach allowed us to increase the robustness of interactor identification and provided evidence for the accessibility of different protein domains to proximity labeling. We show that AtEH1/Pan1, AtEH2/Pan1, and TML subunits are preferentially biotinylated at their C-terminal parts, suggesting that their C termini are in closer proximity to the C-terminal end of TPLATE and/or some domains (even complex subunits such as LOLITA) are not accessible for biotinylation. It is tempting to speculate that proximity labeling approaches, combined with harsh extraction/washing conditions, may be able to provide structural information of multi-subunit protein complexes. In light of this, the distribution of biotinylated peptides that we observed for the TPC subunits, as well as their absence, could reflect the proximity of the domains as well as structural constraints with respect to the bait protein and that proximity biotinylation. Next to providing topology information in the case of transmembrane proteins (Kim et al., 2018), proximity labeling might also harness the potential to help deduce structural insight into protein complexes.
Our results are complementary to the work reporting the use of TurboID to identify transient signaling components (Kim et al., 2019) and novel regulators of plant immunity (Zhang et al., 2019) as well as for the efficient capturing of cell- and subcellular compartment-specific interactomes (Mair et al., 2019). Taken together, these four studies provide a new arena for the identification of novel protein-protein interactions in plants.
METHODS
Bacterial Strains
For cloning, Escherichia coli strains DH5α, DH10B, or Top10 were used following standard chemical transformation protocols. Electrocompetent Agrobacterium (Agrobacterium tumefaciens) C58C1 RifR (pMP90), AGL1 RifR, or GV3101 RifR bacterial cells (i.e., a cured nopaline strain commonly used for Nicotiana benthamiana infiltration; Ashby et al., 1988) were used for N. benthamiana infiltration as well as Arabidopsis (Arabidopsis thaliana) cell culture transformation. Electrocompetent rhizogenic Agrobacterium ATCC15834 (Kajala et al., 2014) bacterial cells were used for hairy root transformation.
Cloning of Constructs
For constructs used in hairy roots, constructs encoding the full-length open reading frame of the PBL—BioID (pDEST-pcDNA5-BioID-Flag C-term; a kind gift from the Gingras laboratory (Couzens et al., 2013), BioID2 (MCS-BioID2-HA; Addgene, Plasmid no. 74224 [Kim et al., 2016]), TurboID (V5-TurboID-NES_pCDNA3; Addgene, Plasmid no. 107169 [Branon et al., 2018]), and mTurbo (V5-miniTurbo-NES_pCDNA3; Addgene, Plasmid no. 107170 [Branon et al., 2018])—were PCR amplified using Q5 High-Fidelity DNA Polymerase (New England Biolabs, catalog no. M0491) with oligonucleotide primers containing attB recombination sequences. The forward and reverse primers additionally encoded the GGGGS linker and the Flag tag (DYKDDDDK) followed by a stop codon, respectively. The primer sequences are depicted in Supplemental Table 3. The resultant attB-flanked PCR products were used in a Gateway BP recombination reaction with the pDONR P2r-P3 vector (Life Technologies) according to the manufacturer’s instructions, thereby creating an entry clone. The construct was transformed in DH5α chemical-competent cells and verified by sequencing (i.e., Sanger sequencing). Using a standard multisite (three-fragment) Gateway LR cloning strategy as described by Van Leene et al. (2007), the entry clones together with pEN-L1-F-L2 encoding eGFP (Karimi et al., 2007; https://gateway.psb.ugent.be/search) and pEN-L4-2-R1 encoding the constitutive CaMV 35S promoter (Karimi et al., 2007) were recombined with the multisite Gateway destination vector pKm43GW (Karimi et al., 2007) to generate expression constructs. More specifically, the multisite LR Gateway reaction resulted in translational fusions between the eGFP and the proximity labels, driven by the 35S promoter. In this manner, the following expression constructs were created: Pro35S::eGFP-BioID, Pro35S::eGFP-BioID2, Pro35S::eGFP-TurboID, Pro35S::eGFP-miniTurbo, and Pro35S::eGFP-BioID constructs (in pKm43GW), with a C-terminally triple HA-tagged BioID fused to eGFP.
For constructs used in N. benthamiana, original BioID, BioID2, and TurboID DNA sequences were taken from Roux et al. (2012), Kim et al. (2014), and Branon et al. (2018)) and codon-optimized to Arabidopsis. The Golden Gate-compatible BirA, BioID, BioID2, and TurboID were synthesized and codon-optimized using the codon optimization tool of Integrated DNA Technologies. The open reading frames were synthesized with BsaI overhands and were ligated to the Level1/2 vectors pICSL86900 and pICSL86922, as previously described by Patron et al. (2015). The following expression vectors were used: Pro35S::BirA-Myc, Pro35S::BioID-myc, Pro35S::HF-BioID2-HA, and Pro35S::superfolderGFP-TurboID-FLAG.
The genomic sequence of NFR5 and the coding sequence of BRI1 were synthesized with BsaI overhangs for Golden Gate as Level1 vector (Binder et al., 2014). Pro35S::NFR5-TurboID and Pro35S::BRI1-GFP were created by Golden Gate cloning in Xpre2-S (pCAMBIA) vectors (Binder et al. 2014). Pro35S::FLS2-GFP was kindly provided by the Hemsley lab, University of Dundee. Pro35S::EFR-GFP (Schwessinger et al., 2011) and Pro35S::SymRK-GFP/Pro35S::NFR5-GFP (Madsen et al., 2011; Wong et al., 2019) were kindly provided by Cyril Zipfel (University of Zurich) and Jens Stougaard (Aarhus University).
BiFC constructs were created in the 2in1 BiFC vectors (Grefen and Blatt, 2012). The entry clones were generated by a Gateway BP recombination reaction using coding sequences of SCAMP5 and TOL9 (BioXP/gBlocks, Integrated DNA Technologies). TPLATE was amplified from the pDONR plasmid as described by Gadeyne et al. (2014). All entry clones were sequence verified. The BIN2 entry plasmid was kindly provided by Jenny Russinova (Houbaert et al., 2018). Entry clones were combined in a Gateway LR recombination reaction with an empty BiFC destination vector and selected using LB containing spectinomycin and XgaI. Final BiFC vectors were checked by restriction digestion and sequencing of the recombination borders.
For constructs used in Arabidopsis, BioID and BioID2 DNA sequences were taken from by Roux et al. (2012) and Kim et al. (2014) and codon-optimized for Arabidopsis using the codon optimization tool of Integrated DNA Technologies. The BioID and BioID2 with and without linker (GGGGS)13 with stop codon, flanked by attB2 and attB3 sites (Karimi et al., 2005), were synthesized by Gen9 in the Gm9-2 plasmid. The TurboID sequence (Branon et al., 2018) was codon-optimized to Arabidopsis using the codon optimization tool of Integrated DNA Technologies. TurboID with linker (GGGGS)13 with stop codons, flanked by attB2 and attB3 sites (Karimi et al., 2005), was synthesized by GenScript in the pUC57 plasmid. Entry clones of eGFP (Mylle et al., 2013) and TPLATE (At3g01780; Van Damme et al., 2006) without stop codon were used in a triple Gateway LR reaction, combining pK7m34GW or pH7m34GW (Karimi et al., 2005), pDONRP4-P1R-Pro35, and pDONRP2-P3R-BioID/BioID2/(GGGGS)13BioID/(GGGGS)13BioID2/(GGGGS)13 TurboID to yield pK7m34GW, Pro35S::GFP/TPLATE-BioID, pK7m34GW, Pro35S::GFP, pH7m34GW, Pro35S::TPLATE-BioID2, pK7m34GW, Pro35S::TPLATE-(GGGGS)13BioID/BioID2, pK7m34GW, and Pro35S::GFP/TPLATE-(GGGGS)13 TurboID. Sequences of these constructs can be found in the Supplemental File.
ProTOL6p::TOL6:Ven was obtained by replacing mCherry in ProTOL6::TOL6:mCherry (Korbei et al., 2013) with the Venus tag (Ven), which was PCR amplified with the primer pair NotImcherryu/NotImcherryd from ProPIN2::PIN2:VEN (Leitner et al., 2012).
Plant Transformation
Hairy Roots
Seeds of tomato (Solanum lycopersicum cv Moneymaker) were surface-sterilized in 70% (v/v) ethanol for 10 min and in 3% (v/v) NaOCl for 20 min (rinsing with sterile deionized water was performed in between the two sterilization steps), then rinsed three times 5 min each with sterile deionized water. The seeds were germinated on Murashige and Skoog tissue culture medium containing 4.3 g/L Murashige and Skoog medium (Duchefa, catalog no. M0221.0050), 0.5 g/L MES, 20 g/L Suc, pH 5.8, and 8 g/L agar (Difco, catalog No. 214530) in magenta boxes (∼50 mL). The pH of the medium was adjusted to 5.8 with KOH and autoclaved at 121°C for 20 min. The boxes were covered and placed in the dark at 4°C in a cold room for 2 d. Subsequently, the boxes were transferred to a 24°C growth chamber (16-h-light/8-h-dark photoperiod) for ∼10 d until cotyledons were fully expanded and the true leaves just emerged. Rhizogenic Agrobacterium transformation was essentially performed as described previously by Harvey et al. (2008) with some minor modifications. More specifically, competent rhizogenic Agrobacterium cells were transformed by electroporation (Shen and Forde, 1989) with the desired binary vector, plated on yeast extract beef (YEB) medium plates with the appropriate antibiotics (100 mg/L spectinomycin), and incubated for 3 to 4 d at 28°C. A transformed rhizogenic Agrobacterium culture was inoculated from fresh plates into YEB liquid medium with the appropriate antibiotics added and grown overnight at 28°C with shaking at 200 rpm. The RAB culture was used to transform 20 to 40 tomato cotyledon halves. Using a scalpel, the cotyledons were cut in half from ∼10-d-old tomato seedlings and transferred (adaxial side down) onto Murashige and Skoog liquid medium. The Murashige and Skoog liquid was subsequently removed and the cotyledon halves immediately immersed in a bacterial suspension at an OD at 600 nm of 0.3 in Murashige and Skoog liquid medium for 20 min, blotted on sterile Whatman filter paper, and transferred (adaxial side down) onto Murashige and Skoog agar plates without antibiotics (4.3 g/L Murashige and Skoog medium, 0.5 g/L MES, 30 g/L Suc, pH 5.8, and 8 g/L agar). The cocultivation culture plates were sealed with aeropore tape. After 3 to 4 d of incubation at 22 to 25°C in the dark (Oberpichler et al., 2008), the cotyledons were transferred to Murashige and Skoog agar plates with 200 mg/L cefotaxime (Duchefa, catalog no. c0111.0025) and 50 mg/L kanamycin and returned to 22 to 25°C. Typically, three to five independent roots arise from each cotyledon. The expression of the eGFP marker of antibiotic-resistant roots that emerged was monitored by using fluorescence microscopic imaging (Leica stereomicroscope and imaging DFC7000 T Leica microscope camera). Images were acquired with an ET GFP long pass filter (excitation at 460 to 500 nm and emission from 510 nm and longer). Exposure times ranged from 383 to 586 ms, and a gain between 2.2 and 2.7 was used. For the control picture, an exposure time of 586 ms was used, with a higher gain (7.2) to avoid having no detectable signal (i.e., autofluorescence signal) of the control hairy root visible in the panel. Four to 10 independent roots showing expression of the marker were subcloned for each construct. These roots were subsequently transferred to new selection plates with the same antibiotic concentration for three rounds of subcultivation (∼6 weeks) before antibiotics-free cultivation of the hairy root cultures in liquid Murashige and Skoog medium (in 50-mL Falcon tubes containing 10 to 30 mL of Murashige and Skoog medium at 22 to 25°C and shaking at 300 rpm) and downstream analysis. After three rounds of cultivation, root cultures were maintained and grown in antibiotics-free half-strength Murashige and Skoog medium supplemented with 3% Suc (w/v) at 22 to 25°C.
N. benthamiana
N. benthamiana plants were grown under a normal light and dark regime at 25°C and 70% relative humidity. Three- to 4-week-old N. benthamiana plants were watered from the bottom ∼2 h prior to infiltration. Transformed Agrobacterium strains C58C1 RifR (pMP90), AGL1 RifR, or GV3101 RifR harboring the constructs of interest were used to infiltrate N. benthamiana leaves and used for transient expression of binary constructs by Agrobacterium -mediated transient transformation of lower epidermal leaf cells essentially as described previously by Boruc et al. (2010). Transformed Agrobacterium was grown for ∼20 h in a shaking incubator (200 rpm) at 28°C in 5 mL of Luria-Broth (LB-Miller, 10 g NaCl/l) medium (Carl Roth) or YEB medium, supplemented with appropriate antibiotics (i.e., 100 g/L spectinomycin). After incubation, the bacterial culture was transferred to 15-mL Falcon tubes and centrifuged (10 min, 5000 rpm). The pellets were washed with 5 mL of the infiltration buffer (10 mM MgCl2 and 10 mM MES, pH 5.7), and the final pellet was resuspended in the infiltration buffer supplemented with 100 to 150 μM acetosyringone. The bacterial suspension was diluted with supplemented infiltration buffer to adjust the inoculum concentration to an OD600 value of 0.025 to 1.0. The inoculum was incubated for 2 to 3 h at room temperature before injecting and delivered to N. benthamiana by gentle pressure infiltration of the lower epidermis leaves (fourth and older true leaves were used at approximately four-fifths to full size) with a 1-mL hypodermic syringe without needle (Moschou et al., 2016).
Arabidopsis Cell Suspension
The PSB-D Arabidopsis cell suspension cultures were transformed with the protein of interest (POI): Pro35S::GFP/TPLATE/TML-BioID/BioID2, Pro35S::TPLATE/TML-(GGGGS)13BioID/BioID2, and Pro35S::GFP/TPLATE-(GGGGS)13 TurboID and selected without callus screening, grown, and subcultured as described by Van Leene et al. (2007).
For Arabidopsis plants to express TOL6-Venus, flowering tol2-1/tol2-1/tol5-1/tol5-1/tol6-1/tol6-1/tol9-1/tol9-1 plants, confirmed homozygous by PCR genotyping for the mutant alleles (Korbei et al., 2013), were transformed with Agrobacterium s using the floral dip method (Clough and Bent, 1998). Resulting T2 lines were confirmed for single-transgene insertion sites and propagated for further analysis. The localization of TOL6 was confirmed by characterizing at least three independent transformants (Korbei et al., 2013).
Biotin Treatments
Hairy Roots
For assessing self-biotinylation, 2-week-old 25-mL liquid cultures were added to 5 mL of fresh Murashige and Skoog medium with or without supplemented biotin (i.e., 50 μM final concentration; stock solution dissolved in water) for 2 or 24 h and samples were collected. Two independent root cultures were analyzed per combination, and the experiment was repeated twice with similar results.
N. benthamiana Leaves
Plants were kept under normal growing conditions at 22°C, reinfiltrated with infiltration buffer (no biotin) or, alternatively, infiltration buffer supplemented with biotin (stock solution dissolved in DMSO or water), and samples were collected at the indicated time points. Two infiltrated N. benthamiana leaf segments/leaves were analyzed per combination.
Arabidopsis Cell Cultures
Cell cultures were grown under normal conditions at 25°C and 130 rpm in the dark. At 48 h after subculturing, the required amount of biotin was added and the cell culture was transferred to the desired temperature for the required time at 130 rpm shaking in the dark in an INCLU-line IL56 (VWR) incubator. After the required time, cell cultures were harvested, flash-frozen in liquid nitrogen, and stored at −70° until use.
Protein Extractions
Hairy Roots
The tissue samples were flash-frozen and crushed using a liquid-cooled mortar and pestle. The crushed material was transferred to a 1.5-mL Eppendorf in homogenization buffer (25 mM Tris-HCl, pH 7.6, 15 mM MgCl2, 5 mM EGTA, 150 mM NaCl, 15 mM pNO2PhenylPO4, 15 mM β-glycerolphosphate, 1 mM DTT, 0.1% [v/v] Nonidet P-40, 0.1 mM Na3VO4, 1 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 10 μg/mL leupeptin, 10 μg/mL aprotinin, 10 μg/mL SBTI, 0.1 mM benzamidine, 5 μg/mL antipain, 5 μg/mL pepstatin, 5 μg/mL chymostatin, 1 μM E64, and 5% [v/v] ethylene glycol) with volumes according to the dry weight of the recovered material (1:1, w/v), and protein material was extracted by three repetitive freeze-thaw cycles in liquid nitrogen and the lysate was transferred to a 1.5-mL Eppendorf. The lysates were cleared by centrifugation for 15 min at 16,100g (4°C), and the supernatant was transferred to a new 1.5-mL Eppendorf. This step was repeated two times, and the protein concentration was determined by the DC Protein Assay Kit (Bio-Rad) according to the manufacturer’s instructions.
N. benthamiana Leaves
The tissue samples were crushed using a liquid-cooled mortar and pestle, and the crushed material was transferred to a 1.5-mL Eppendorf in homogenization buffer. Leaves were harvested and directly frozen in liquid nitrogen. Proteins were extracted with buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 10% (v/v) glycerol, 2 mM EDTA, 5 mM DTT, 1 mM phenylmethylsulfonyl fluoride, Protease Inhibitor Cocktail (Roche), and 1% (v/v) IGEPAL CA-630 (Sigma-Aldrich). Extraction buffer was added at 2 mL/g tissue. Extracts were incubated at 4°C for 1 h and then centrifuged at 4°C and 13,000 rpm for 30 min. Supernatants were used directly or filtered through PD-10 columns (GE Healthcare) and incubated with streptavidin (Roche or GE Healthcare) or GFP (Chromotek) beads for 1 h.
For ammonium acetate protein precipitation, supernatants were precipitated using 5× (v/v) prechilled 0.1 M ammonium acetate in methanol at −20°C for 2 h and then centrifuged at 4°C and 13,000 rpm for 15 min. The pellet was washed with prechilled 0.1 M ammonium acetate and dissolved in the same extraction buffer plus 1% (w/v) SDS. Magnetic separation was done using Dynabeads M-280 Streptavidin (Thermo Fisher Scientific) followed by washing five times in buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 10% (v/v) glycerol, 2 mM EDTA, Protease Inhibitor Cocktail (Roche), and 0.5% (v/v) IGEPAL CA-630 (Sigma-Aldrich) and one time in buffer containing 50 mM Tris-HCl (pH 7.5), 1 M NaCl, 10% (v/v) glycerol, 2 mM EDTA, Protease Inhibitor Cocktail (Roche), and 0.5% (v/v) IGEPAL CA-630 (Sigma-Aldrich) at 4°C. To release the proteins, 100 μL of 2× NuPAGE LDS sample buffer (Invitrogen) was added, and samples were heated for 5 min at 95°C.
Arabidopsis Cell Cultures
Total protein extracts were obtained from biotin-treated, harvested, and liquid nitrogen-retched (20 Hz, 1 min), Arabidopsis cell suspension cultures using double the volume (w/2v) of extraction buffer containing 150 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10% (v/v) glycerol, 10 mM EDTA, 1 mM sodium molybdate, 1 mM NaF, and freshly added 10 mM DTT, 1% (w/v) Protease Inhibitor Cocktail (P9599, Sigma-Aldrich [one tablet per 10 mL of elution buffer]), and 1% (v/v) Nonidet P-40. Cell debris was removed by two rounds of centrifugation at 14,000 rpm for 20 min at 4°C, and the supernatant was collected.
SDS-PAGE and Protein Immunoblots
Hairy Roots
Sample loading buffer was added, and equivalent amounts of protein (∼30 μg) were separated by SDS-PAGE (1.0-mm-thick 4 to 12% [w/v] polyacrylamide Criterion Bis-Tris XT gels [Bio-Rad] or equivalent) in MOPS buffer (Bio-Rad) at 150 V. Subsequently, proteins were transferred onto polyvinylidene fluoride (PVDF) membranes with 0.2-μm pore size. Membranes were blocked for 30 min in a 1:1 Tris‐buffered saline (TBS)/Odyssey blocking solution (LI-COR, catalog No. 927-40003) and probed by immunoblotting. Following overnight incubation of primary antibody in TBS‐T (TBS + 0.1% [v/v] Tween 20)/Odyssey blocking buffer and three 10-min washes in TBS‐T, membranes were incubated with secondary antibody for 30 min in TBS‐T/Odyssey blocking buffer followed by three washes in TBS‐T or TBS (last wash step). The antibodies used are listed in Supplemental Table 4. The bands were visualized using an Odyssey infrared imaging system (LI-COR), and the intensity of bands was assessed using the LI-COR Odyssey software for immunoblot image processing.
N. benthamiana
Extracted proteins were loaded onto 12% SDS-PAGE gels and separated for 2 h at 90 to 110 V. SDS-PAGE gels were blotted via wet transfer on PVDF membranes (Carl Roth) overnight at 30 V. Membrane blocking was performed with 3% (w/v) BSA in PBS-T buffer for 1 h at room temperature followed by incubation with mouse anti-GFP (TaKaRa; 1:5000) for 2 h followed by anti-mouse HRP (Sigma-Aldrich; 1:10,000) for 2 h or directly Strep-Tactin-HRP (IBA-Life Sciences; 1:5000) for 2 h. Chemiluminescence was detected with Clarity Western ECL (Bio-Rad).
Input and eluted proteins were loaded onto 12% SDS-PAGE gels and separated for 1 to 2 h at 120 V. SDS-PAGE gels were blotted via wet transfer onto PVDF membranes (Bio-Rad) for 3 h at 300 mA in a cool room. The membrane was blocked with 3% (w/v) BSA in PBS-T buffer for 1 h at room temperature followed by incubation with Streptavidin-HRP (Sigma-Aldrich; 1:25,000) for 2 h. Chemiluminescence was detected with ECL Prime Western Blotting Detection Reagent (GE Healthcare).
Arabidopsis Cell Cultures
The total protein extracts were heated in sample buffer for 10 min at 70°C and loaded in equal amounts (20 μg; protein concentration was measured using a qubit system [Thermo Fisher Scientific]) on a 4 to 20% SDS-PAGE gel. SDS-PAGE-separated proteins were blotted onto PVDF membranes (Thermo Fisher Scientific). Membranes were blocked overnight at room temperature in 5% (w/v) BSA dissolved in 25 mM Tris-HCl, pH 8, 150 mM NaCl, and 0.1% (v/v) Tween 20. The blots were then incubated at room temperature with the Pierce High Sensitivity Streptavidin-HRP (Thermo Fisher Scientific; 1:2000) or Abcam anti-HA-HRP tag antibody (ab1190; 1:5000) in 1% (w/v) BSA made as mentioned above for 2 h. Antigen-antibody complexes were detected using chemiluminescence (Perkin-Elmer).
Image Analysis
A tplate mutant complemented line expressing proLAT52::TPLATE-TagRFP (Wang et al., 2020) was crossed with a quadruple tol (tol2/tol2 tol5/tol5 tol6/tol6 tol9/tol9) mutant line expressing proTOL6::TOL6-Venus. F1 seedlings were imaged using spinning-disk microscopy. Etiolated hypocotyl cells of 4-d-old seedlings expressing TPLATE-TagRFP and TOL6-Venus were imaged with a Nikon Ti microscope equipped with an Ultraview spinning-disk system (Perkin-Elmer) and a 512 × 512 Hamamatsu ImagEM C9100-13 EM CCD camera. Images of hypocotyl epidermal cells were acquired with a 100× oil-immersion objective (Plan Apo; numerical aperture = 1.45). TOl6-Venus was imaged with 514-nm excitation light and an emission window between 525 and 575 nm. TPLATE-TagRFP was imaged with 561-nm excitation light and an emission window between 570 and 625 nm. Dual-color images were acquired sequentially with an exposure time of 500 ms/frame.
Object-based colocalization was performed using the plugin Distance Analysis (DiAna) of ImageJ (Gilles et al., 2017). Prior to analysis with the DiAna plugin, images were processed with ImageJ. Each channel was processed using a Walking Average of 4 and then merged (also rotated if required). Regions of interest within each image were selected based on whether they excluded the border of the cells and still contained a good number of objects. Z-projection images were generated using five frames with average intensity. Then, each channel of Z-projected images was processed with Morphological filters from the MorphoLibJ plugin (Legland et al., 2016), using the parameters white top-hat, disk element, and a two-pixel radius. Objects for each channel were segmented by selecting the 3D Spot segmentation tool. We adapted the calibration by changing the pixel size to 1.00001 for all dimensions. Both the noise and seed threshold values were obtained by averaging the maximum intensity of three regions covering only background signal. The spot was defined using a minimum value of 4 pixels and a maximum value of 36 pixels. The option to exclude objects on XY edges was activated. Default values were used for the other parameters. Results for the number of total objects (Tot) or touching objects (Tou) in image A/B obtained from DiAna were recorded. The colocalization ratio of objects was calculated as follows:
As a control, one of the channels was horizontally flipped, merged with the other channel, and analyzed. Eight cells originating from three seedlings were analyzed.
BiFC
Ratiometric BiFC images were obtained using an Olympus FV1000 inverted confocal microscope equipped with a UPLSAPO 60× water-immersion objective (numerical aperture = 1.2). Images were acquired in line sequential mode, using 515-nm excitation and an emission window between 530 and 548 nm for YFP detection and 559-nm excitation and an emission window between 580 and 615 nm for RFP detection. All images were taken using the same settings. The experiment was independently repeated twice with a similar outcome. BiFC constructs are shown in Supplemental Table 1.
For the quantification of the YFP:RFP ratio, only images with less than 1% saturation in the RFP or YFP channel were analyzed. For each confocal image, parts of the cortical cytoplasm in the RFP channel were traced in ImageJ using the selection brush tool with a width of 15 pixels. Histogram analysis was performed to confirm that less than 1% saturated pixels were present in the region of interest. The average intensity from the obtained region of interest was calculated and divided by the average intensity of the same region in the YFP channel. Ratios were quantified for 15 to 19 individual cells.
Outliers were removed by iterative outlier removal (Leys et al., 2013). Data were analyzed using RStudio (RStudio Team, 2020) with Welch-corrected ANOVA to account for heteroscedasticity. Posthoc pairwise comparison was performed with the package MULTCOMP utilizing the Tukey contrasts (Herberich et al., 2010).
Protein Extraction and Pull-Down for MS Analysis
For Figure 4, Arabidopsis cell cultures expressing different POI were ground in 0.67 volume of extraction buffer containing 150 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10% (v/v) glycerol, 10 mM EDTA, 1 mM sodium molybdate, 1 mM NaF and freshly added 10 mM DTT, 1% (v/v) Protease Inhibitor Cocktail (P9599, Sigma-Aldrich [one tablet per 10 mL of elution buffer]), 1% (v/v) digitonin, and 0.1% (w/v) benzonase. The extract was mixed using ultra-Turrax for 3× 30 s at 16,000 rpm and sonicated for 3× 15 s with a 30-s interval. The extract was incubated on a rotating wheel for 1 h at 4°C. Cell debris was removed by two rounds of centrifugation at 20,000 rpm for 20 min at 4°C, and the supernatant was buffer exchanged using preequilibrated PD-10 columns and eluted in binding buffer (extraction buffer without digitonin and benzonase) at 4°C. Pull-downs were performed in triplicate. For each pull-down, one-third of the soluble protein extract was incubated with a 200-μL slurry of streptavidin-Sepharose high-performance beads (Amersham; preequilibrated with binding buffer) overnight on a rotating wheel at 4°C. The unbound fraction or supernatant was removed after centrifugation at 1500 rpm for 1 min. Beads were transferred to a mobicol column and washed with 2.5 mL of binding buffer followed by washing with 2.5 mL of wash buffer-1 containing 25 mM Tris-HCl (pH 7.5), 150 mM NaCl, and 0.1% (w/v) digitonin. The beads were washed once with wash buffer-2 containing 25 mM Tris-HCl (pH 7.5) and 150 mM NaCl and finally washed once with 50 mM ammonium bicarbonate (pH 8.0). Proteins were digested on beads with Trypsin/LysC (Promega) overnight followed by zip-tip cleanup using C-18 Omix tips (Agilent). Digests containing the unbiotinylated peptides were dried in a SpeedVac and stored at −20°C until LC-MS/MS analysis.
For Figures 5 and 6A, Arabidopsis cell cultures expressing different POI were ground in 0.67 volume of extraction buffer containing 100 mM Tris (pH 7.5), 2% (w/v) SDS, and 8 M urea. The extract was mechanically disrupted using three repetitive freeze-thaw cycles followed by two cycles of sonication at output level 4 with a 40% duty cycle for 50 s with a 35-s interval. The extract was incubated on a rotating wheel for 1 h at room temperature. Cell debris was removed by two rounds of centrifugation at 20,000 rpm for 20 min at room temperature, and the supernatant was buffer exchanged using preequilibrated PD-10 columns and eluted in binding buffer containing 100 mM Tris (pH 7.5), 2% (w/v) SDS, and 7.5 M urea. Pull-downs were performed in triplicate. For each pull-down, one-third of the soluble protein extract was incubated with a 200-μL slurry of streptavidin-Sepharose high-performance beads (Amersham; preequilibrated with binding buffer) overnight on a rotating wheel at room temperature. The unbound fraction or supernatant was removed after centrifugation at 1500 rpm for 1 min. Beads were transferred to a mobicol column and washed with 4 mL of binding buffer for 5 min without agitation, followed by washing with high-salt buffer containing 1 M NaCl, 100 mM Tris-HCl, pH 7.5, and incubated for 30 min. The beads were washed once with ultrapure water, incubated for 5 min, and finally washed with 3.2 mL of 50 mM ammonium bicarbonate (pH 8.0) incubating for 5 min. Proteins were digested on beads with Trypsin/LysC (Promega) overnight followed by zip-tip cleanup using C-18 Omix tips (Agilent). Digests containing the unbiotinylated peptides were dried in a SpeedVac as elution-1 (E1) and stored at −20°C until LC-MS/MS analysis.
After E1, for all linkerTurboID samples (Figure 6B), biotinylated peptides were eluted from the beads by adding 300 μL of the elution buffer containing 0.2% (v/v) trifluoroacetic acid, 0.1% (v/v) formic acid, and 80% (v/v) acetonitrile in water. The eluted peptides were collected by centrifugation at 1500 rpm for 1 min followed by an addition to the beads of 300 μL of the elution buffer, after which the sample was heated at 95°C for 5 min to allow a maximal release of peptides. A short spin at 1500 rpm for 1 min was done to collect the eluted peptides. The two eluates were pooled and dried in a SpeedVac. The dried peptides were dissolved in 1% (v/v) trifluoroacetic acid solution to perform zip-tip cleanup using C-18 Omix tips (Agilent). Digests were dried in a SpeedVac as elution-2 (E2) and stored at −20°C until LC-MS/MS analysis.
TPLATE-GSrhino pull-downs with homemade IgG beads were performed as described by Van Leene et al. (2019).
MS and Data Analysis
Triplicate pull-down experiments were analyzed by LC-MS/MS on Q Exactive (Thermo Fisher Scientific) as previously reported by Nelissen et al. (2015).
For comparison of TPLATE-BioID at 25, 28, 30, and 35°C, raw data of GFP-BioID and TPLATE-BioID triplicates at the different incubation temperatures were searched together with MaxQuant (Tyanova et al., 2016a) using standard parameters (Supplemental Data Set 1). LFQ intensities were used in Perseus software (Tyanova et al., 2016b) to determine the significantly enriched proteins with TPLATE for each sample set, TPLATE versus GFP at 25, 28, 30, and 35°C. Then the MaxQuant protein groups file, with reverse, contaminant, and only identified by site identifications already removed, was loaded in Perseus. Samples were grouped by the respective triplicates and filtered for a minimum of two valid values per triplicate. Missing LFQ values were imputed from normal distribution using standard settings in Perseus, width of 0.3 and down shift of 1.8. Next, t tests were performed and visualized in volcano plots, using permutation-based FDR to determine the significantly different proteins between TPLATE-BioID and GFP-BioID at the different incubation temperatures. As cutoffs, FDR = 0.05 and S0 = 0.5 were applied. S0 is a fudge factor, a small positive constant as proposed by Tusher et al., (2001) in significance analysis of microarrays. Protein lists significantly enriched with TPLATE can be found in Supplemental Data Set 1. For all TPC subunits, the values for difference and −log(p-value) from the Perseus t test were presented in Figure 4, in order to compare the different TPLATE-BioID samples and determine the optimal temperature for BioID.
For comparison of different TPLATE PBLs at 28°C, triplicate TPLATE-BioID, TPLATE-BioID2, TPLATE-linkerBioID, TPLATE-linkerBioID2, TPLATE-linkerTurboID, and respective controls for each PBL: GFP-BioID, GFP-BioID2, and GFP-linkerTurboID raw data was searched together in MaxQuant with standard parameters (Supplemental Data Set 2). Data sets were further processed in the same way as described for the comparison of the different incubation temperatures. GFP-BioID served as a control for TPLATE-BioID and TPLATE-linkerBioID, and GFP-BioID2 served as a control for TPLATE-BioID2 and TPLATE-linkerBioID2. For linkerBioID, in addition to 50 µM biotin incubation, 2 mM biotin incubation also was tested. Pairwise comparisons were made between the different TPLATE PBLs and their respective controls, and cutoffs of FDR = 0.05 and S0 = 0.5 were applied. Protein lists significantly enriched with TPLATE can be found in Supplemental Data Set 2. For all TPC subunits, the values for difference and -log(p-value) from the Perseus t tests are presented in Figure 5, in order to compare the different TPLATE PBLs. TPLATE-GSrhino pull-downs were analyzed as described by Van Leene et al. (2019). Briefly, pull-down triplicates were analyzed by LC-MS/MS on Q Exactive (Thermo Fisher Scientific), raw data were searched with the Mascot search engine (Matrix Science), and average Normalized Spectral Abundance Factors for the identified proteins were compared in a t test versus a large data set of similar experiments consisting of nonrelated baits. Proteins not present in the background list or highly enriched versus the large data set were kept as the significant set. Thresholds used are Normalized Spectral Abundance Factors ratio bait/large data set ≥ 10 and −log(p-value) ≥ 10. In this case, one-peptide identifications were retained; otherwise, the small TPC subunit LOLITA would have fallen out of the data. The significant set can be found in Supplemental Data Set 3.
For comparison of the different PBLs versus GSrhino pull-down samples, a MaxQuant search was performed on all relevant TPLATE raw data together. Since LC-MS/MS analysis is done the same for the GSrhino as for the streptavidin pull-downs, it is also possible to include matching between runs. Next, resulting iBAQ values were used for comparison of the abundance of the identified proteins among the different TPLATE samples. For completeness, one-peptide identifications were allowed, in order to also obtain iBAQ values for the one-peptide identifications. The complete set of significant proteins as determined by previous analysis, for the PBLs and for GSrhino, as described, with their iBAQ values can be found in Supplemental Data Set 3. In Figure 6, a subset of relevant endocytosis-related proteins is presented.
In order to compare TPLATE-linkerTurboID with different incubation times and at different temperatures, triplicate streptavidin pull-downs were performed with TPLATE-linkerTurboID and GFP-linkerTurboID at 25°C with different incubation times with 50 µM biotin. Analysis to determine the significant identifications in each TPLATE-linkerTurboID set versus the respective GFP-linkerTurboID control was done as described before. Significant lists can be found in Supplemental Data Set 4. For a direct comparison of the different incubation times and temperatures with TPLATE-linkerTurboID, a MaxQuant search was performed on all relevant TPLATE raw data together, with matching between runs. Next, resulting iBAQ values were used for comparison of the abundance of the identified proteins among the different sample sets. Again, for completeness, one-peptide identifications were allowed, in order to obtain iBAQ values also for the one-peptide identifications. For this comparison between linkerTurboID samples only, both elutions were taken together for all sample sets, for the determination of the significant sets as well as for the comparison of the iBAQ values. The complete set of significant proteins with their iBAQ values can be found in Supplemental Data Set 4. In Figure 6, a subset of proteins is presented.
For each of the TPC subunits, the identified peptides (Supplemental Data Set 5) in the TPLATE-linkerTurboID replicates were mapped to the protein sequence (Figure 7). Nonbiotinylated and biotinylated peptides of TPC subunits were mapped to the protein sequence by using the Draw Map tool in the MSTools package (http://peterslab.org/MSTools/; Kavan and Man, 2011) and put together using Inkscape v 0.92.4 (www.inkscape.org). Domain annotation of TPC subunits was retrieved using InterPro protein sequence analysis (https://www.ebi.ac.uk/interpro/; Mitchell et al., 2019).
Accession Numbers
The Arabidopsis Information Resource (TAIR) locus identifiers for the genes mentioned in this study are as follows: BRI1 (At4G39400), FLS2 (At5G46330), EFR (At5G20480), LTI6b (At3G05890), TPLATE (At3g01780), TML (At5g57460), TASH3 (At2g07360), TWD40-1 (At3G50590), TWD40-2 (At5G24710), AtEH1/Pan1 (At1G20760), AtEH2/Pan1 (At1G21630), LOLITA (At1g15370), TOL2 (At1G06210), TOL6 (At2G38410), TOL9 (At4G32760), SCAMP5 (At1G32050), CHC1 (At3G11130), CHC2 (At3G08530), DRP1A (At5G42080), DRP2A (At1G10290), DRP2B (At1G59610), PICALM3 (At5G35200), AtECA4/PICALM4A (At2G25430), and CAP1/PICALM4B (At4G32285). We also used Lotus japonicus NFR5 (Q70KR1) and SYMRK (Q8LKX1).
Supplemental Data
Supplemental Figure 1. Overview of available constructs for proximity biotinylation in plants.
Supplemental Figure 2. GFP expression in tomato hairy root cultures produced with rhizogenic Agrobacterium.
Supplemental Figure 3. Characterization of PBL-catalyzed proximity labeling in N. benthamiana.
Supplemental Figure 4. Biotinylation of BioID increases at elevated growth temperature and biotin concentration in Nicotiana benthamiana.
Supplemental Figure 5. Trans-biotinylation within membrane-resident receptor complexes.
Supplemental Figure 6. Different PBL cause different cis- and trans-biotinylation.
Supplemental Figure 7. Cis-biotinylation of TPLATE-linkerBioID increases at higher concentration of exogenous biotin.
Supplemental Figure 8. Exogenous application of biotin can exceed the binding capacity of streptavidin beads.
Supplemental Figure 9. The biotin-streptavidin interaction is retained under harsh conditions.
Supplemental Table 1. List of expression vectors and BiFC constructs used in this study.
Supplemental Table 2. Cell cultures expressing different TPLATE-PBLs identifies TPC subunits with different amount of non-biotinylated peptides.
Supplemental Table 3. List of primers used.
Supplemental Table 4. List of antibodies used.
Supplemental File. List of all PBL sequences used.
Supplemental Protocol. Proximity-dependent biotinylation in Arabidopsis cell cultures: purification of non-biotinylated and biotinylated peptides and MS analysis.
Supplemental Data Set 1. Full list of significantly enriched identifications with TPLATE-BioID versus GFP-BioID at different incubation temperatures for 24 h with 50 µM biotin.
Supplemental Data Set 2. Full list of significantly enriched identifications with TPLATE as bait using BioID, BioID2, linkerBioID, linkerBioID2, linkerTurboID E1, linkerTurboID E2 and linkerTurboID E1+E2, versus the respective GFP PBLs.
Supplemental Data Set 3. Full list of significantly enriched hits with either TPLATE PBLs or GSrhino PD, including average iBAQ values, normalized versus TPLATE.
Supplemental Data Set 4. Full list of significantly enriched hits with TPLATE-linkerTurboID, including average iBAQ values, normalized versus TPLATE, at different incubation times at 25 degrees and 24-h incubation time at 28 degrees, all with 50 µM biotin.
Supplemental Data Set 5. MaxQuant TPC subunits peptides in TPLATE-LTurboID.
DIVE Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Lore Gryffroy (VIB/Ghent University) for providing us with control hairy root cultures. This work was supported by the European Research Council (T-Rex project 682436 to D.V.D. and PROPHECY project 803972 to P.V.D.), the National Science Foundation Flanders (grant G009415N to D.V.D. and G.D.J.), the Deutsche Forschungsgemeinschaft in frame of the Collaborative Research Center 924 (Sonderforschungsbereich 924, grant INST 95/1126-2, Project B4 to T.O. and N.B.A.), the Vetenskapsrådet (VR; grant 21679000 to P.N.M.), the Swedish Research Council Formas (grant 22924-000 to P.N.M.), the Carl Trygger Foundation for Scientific Research (grants 15:336 and 17:317 to P.M.), and from the Foundation of Research and Technology Hellas (IMBB-FORTH; to P.N.M.).
AUTHOR CONTRIBUTIONS
D.A., N.B.A., C.L., P.V.D., K.Y., J.W., B.K., and A.T. designed and/or performed research; L.D.V., B.K., F.I., J.V.L., and D.E. contributed new analytic tools and analyzed data; P.V.D., A.G., G.D.J., T.O., P.N.M., and D.V.D. designed research, analyzed data, and wrote the article; all authors contributed to finalizing the article text.
Footnotes
Articles can be viewed without a subscription.
References
- Alban C., Job D., Douce R.(2000). Biotin metabolism in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51: 17–47. [DOI] [PubMed] [Google Scholar]
- Antolín-Llovera M., Ried M.K., Parniske M.(2014). Cleavage of the SYMBIOSIS RECEPTOR-LIKE KINASE ectodomain promotes complex formation with Nod factor receptor 5. Curr. Biol. 24: 422–427. [DOI] [PubMed] [Google Scholar]
- Ashby A.M., Watson M.D., Loake G.J., Shaw C.H.(1988). Ti plasmid-specified chemotaxis of Agrobacterium tumefaciens C58C1 toward vir-inducing phenolic compounds and soluble factors from monocotyledonous and dicotyledonous plants. J. Bacteriol. 170: 4181–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batsios P., Meyer I., Gräf R.(2016). Proximity-dependent biotin identification (BioID) in Dictyostelium amoebae. Methods Enzymol. 569: 23–42. [DOI] [PubMed] [Google Scholar]
- Beckett D., Kovaleva E., Schatz P.J.(1999). A minimal peptide substrate in biotin holoenzyme synthetase-catalyzed biotinylation. Protein Sci. 8: 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder A., Lambert J., Morbitzer R., Popp C., Ott T., Lahaye T., Parniske M.(2014). A modular plasmid assembly kit for multigene expression, gene silencing and silencing rescue in plants. PLoS One 9: e88218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boruc J., Van den Daele H., Hollunder J., Rombauts S., Mylle E., Hilson P., Inzé D., De Veylder L., Russinova E.(2010). Functional modules in the Arabidopsis core cell cycle binary protein-protein interaction network. Plant Cell 22: 1264–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branon T.C., Bosch J.A., Sanchez A.D., Udeshi N.D., Svinkina T., Carr S.A., Feldman J.L., Perrimon N., Ting A.Y.(2018). Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 36: 880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi-Rhee E., Schulman H., Cronan J.E.(2004). Promiscuous protein biotinylation by Escherichia coli biotin protein ligase. Protein Sci. 13: 3043–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F.(1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Conlan B., Stoll T., Gorman J.J., Saur I., Rathjen J.P.(2018). Development of a rapid in planta BioID system as a probe for plasma membrane-associated immunity proteins. Front. Plant Sci. 9: 1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzens, et al. (2013). Protein interaction network of the mammalian Hippo pathway reveals mechanisms of kinase-phosphatase interactions. science signaling 6: rs15. [DOI] [PubMed] [Google Scholar]
- Cox J., Hein M.Y., Luber C.A., Paron I., Nagaraj N., Mann M.(2014). Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteomics 13: 2513–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J.E.(2005). Targeted and proximity-dependent promiscuous protein biotinylation by a mutant Escherichia coli biotin protein ligase. J. Nutr. Biochem. 16: 416–418. [DOI] [PubMed] [Google Scholar]
- Das P.P., Macharia M.W., Lin Q., Wong S.-M.(2019). In planta proximity-dependent biotin identification (BioID) identifies a TMV replication co-chaperone NbSGT1 in the vicinity of 126 kDa replicase. J. Proteomics 204: 103402. [DOI] [PubMed] [Google Scholar]
- Deal R.B., Henikoff S.(2011). The INTACT method for cell type-specific gene expression and chromatin profiling in Arabidopsis thaliana. Nat. Protoc. 6: 56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingar D., et al. (2015). BioID identifies novel c-MYC interacting partners in cultured cells and xenograft tumors. J. Proteomics 118: 95–111. [DOI] [PubMed] [Google Scholar]
- Gadeyne A., et al. (2014). The TPLATE adaptor complex drives clathrin-mediated endocytosis in plants. Cell 156: 691–704. [DOI] [PubMed] [Google Scholar]
- Gilles L.M., et al. (2017). Loss of pollen-specific phospholipase NOT LIKE DAD triggers gynogenesis in maize. EMBO J. 36: 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebe M., Xu J., Möbius W., Ueda T., Nakano A., Geuze H.J., Rook M.B., Scheres B.(2003). Arabidopsis sterol endocytosis involves actin-mediated trafficking via ARA6-positive early endosomes. Curr. Biol. 13: 1378–1387. [DOI] [PubMed] [Google Scholar]
- Grefen C., Blatt M.R.(2012). A 2in1 cloning system enables ratiometric bimolecular fluorescence complementation (rBiFC). Biotechniques 53: 311–314. [DOI] [PubMed] [Google Scholar]
- Gu B., Lambert J.P., Cockburn K., Gingras A.C., Rossant J.(2017). AIRE is a critical spindle-associated protein in embryonic stem cells. eLife 6: e28131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta G.D., et al. (2015). A dynamic protein interaction landscape of the human centrosome-cilium interface. Cell 163: 1484–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J.J.W., Lincoln J.E., Gilchrist D.G.(2008). Programmed cell death suppression in transformed plant tissue by tomato cDNAs identified from an Agrobacterium rhizogenes-based functional screen. Mol. Genet. Genomics 279: 509–521. [DOI] [PubMed] [Google Scholar]
- Herberich E., Sikorski J., Hothorn T.(2010). A robust procedure for comparing multiple means under heteroscedasticity in unbalanced designs. PLoS One 5: e9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbaert A., et al. (2018). POLAR-guided signalling complex assembly and localization drive asymmetric cell division. Nature 563: 574–578. [DOI] [PubMed] [Google Scholar]
- Kajala K., Coil D.A., Brady S.M.(2014). Draft genome sequence of Rhizobium rhizogenes strain ATCC 15834. Genome Announc. 2: e01108–e01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M., Bleys A., Vanderhaeghen R., Hilson P.(2007). Building blocks for plant gene assembly. Plant Physiol. 145: 1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M., De Meyer B., Hilson P.(2005). Modular cloning in plant cells. Trends Plant Sci. 10: 103–105. [DOI] [PubMed] [Google Scholar]
- Kavan D., Man P.(2011). MSTools: Web based application for visualization and presentation of HXMS data. Int. J. Mass Spectrom. 302: 53–58. [Google Scholar]
- Khan M., Youn J.-Y., Gingras A.-C., Subramaniam R., Desveaux D.(2018). In planta proximity dependent biotin identification (BioID). Sci. Rep. 8: 9212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.I., Birendra K.C., Zhu W., Motamedchaboki K., Doye V., Roux K.J.(2014). Probing nuclear pore complex architecture with proximity-dependent biotinylation. Proc. Natl. Acad. Sci. USA 111: E2453–E2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.I., et al. (2018). BioSITe: A method for direct detection and quantitation of site-specific biotinylation. J. Proteome Res. 17: 759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.I., Jensen S.C., Noble K.A., Kc B., Roux K.H., Motamedchaboki K., Roux K.J.(2016). An improved smaller biotin ligase for BioID proximity labeling. Mol. Biol. Cell 27: 1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.I., Roux K.J.(2016). Filling the void: Proximity-based labeling of proteins in living cells. Trends Cell Biol. 26: 804–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.-W., Park C.H., Hsu C.-C., Zhu J.-Y., Hsiao Y., Branon T., Xu S.-L., Ting A.Y., Wang Z.-Y.(2019). Application of TurboID-mediated proximity labeling for mapping a GSK3 kinase signaling network in Arabidopsis. bioRxiv. [Google Scholar]
- Korbei B., Moulinier-Anzola J., De-Araujo L., Lucyshyn D., Retzer K., Khan M.A., Luschnig C.(2013). Arabidopsis TOL proteins act as gatekeepers for vacuolar sorting of PIN2 plasma membrane protein. Curr. Biol. 23: 2500–2505. [DOI] [PubMed] [Google Scholar]
- Kwon K., Beckett D.(2000). Function of a conserved sequence motif in biotin holoenzyme synthetases. Protein Sci. 9: 1530–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law A.H., Chow C.M., Jiang L.(2012). Secretory carrier membrane proteins. Protoplasma 249: 269–283. [DOI] [PubMed] [Google Scholar]
- Legland D., Arganda-Carreras I., Andrey P.(2016). MorphoLibJ: Integrated library and plugins for mathematical morphology with ImageJ. Bioinformatics 32: 3532–3534. [DOI] [PubMed] [Google Scholar]
- Leitner J., Petrášek J., Tomanov K., Retzer K., Pařezová M., Korbei B., Bachmair A., Zažímalová E., Luschnig C.(2012). Lysine63-linked ubiquitylation of PIN2 auxin carrier protein governs hormonally controlled adaptation of Arabidopsis root growth. Proc. Natl. Acad. Sci. USA 109: 8322–8327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys C., Ley C., Klein O., Bernard P., Licata L.(2013). Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psychol. 49: 764–766. [Google Scholar]
- Lin Q., Zhou Z., Luo W., Fang M., Li M., Li H.(2017). Screening of proximal and interacting proteins in rice protoplasts by proximity-dependent biotinylation. Front. Plant Sci. 8: 749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen E.B., Antolín-Llovera M., Grossmann C., Ye J., Vieweg S., Broghammer A., Krusell L., Radutoiu S., Jensen O.N., Stougaard J., Parniske M.(2011). Autophosphorylation is essential for the in vivo function of the Lotus japonicus Nod factor receptor 1 and receptor-mediated signalling in cooperation with Nod factor receptor 5. Plant J. 65: 404–417. [DOI] [PubMed] [Google Scholar]
- Mair A., Xu S.L., Branon T.C., Ting A.Y., Bergmann D.C.(2019). Proximity labeling of protein complexes and cell-type-specific organellar proteomes in Arabidopsis enabled by TurboID. eLife 8: e47864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A.L., et al. (2019). InterPro in 2019: Improving coverage, classification and access to protein sequence annotations. Nucleic Acids Res. 47: D351–D360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschou P.N., Gutierrez-Beltran E., Bozhkov P.V., Smertenko A.(2016). Separase promotes microtubule polymerization by activating CENP-E-related kinesin Kin7. Dev. Cell 37: 350–361. [DOI] [PubMed] [Google Scholar]
- Moulinier-Anzola J., Schwihla M., De-Araújo L., Artner C., Jörg L., Konstantinova N., Luschnig C., Korbei B.(2020). TOLs function as ubiquitin receptors in the early steps of the ESCRT pathway in higher plants. Mol. Plant 13: 717–731. [DOI] [PubMed] [Google Scholar]
- Mylle E., Codreanu M.-C., Boruc J., Russinova E.(2013). Emission spectra profiling of fluorescent proteins in living plant cells. Plant Methods 9: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen H., et al. (2015). Dynamic changes in ANGUSTIFOLIA3 complex composition reveal a growth regulatory mechanism in the maize leaf. Plant Cell 27: 1605–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberpichler, et al. (2008). Light affects motility and infectivity of Agrobacterium tumefaciens. Environ Microbiol 10: 2020–2029. [DOI] [PubMed] [Google Scholar]
- Opitz N., Schmitt K., Hofer-Pretz V., Neumann B., Krebber H., Braus G.H., Valerius O.(2017). Capturing the Asc1p/Receptor for Activated C Kinase 1 (RACK1) microenvironment at the head region of the 40S ribosome with quantitative BioID in yeast. Mol. Cell. Proteomics 16: 2199–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patron N.J., et al. (2015). Standards for plant synthetic biology: A common syntax for exchange of DNA parts. New Phytol. 208: 13–19. [DOI] [PubMed] [Google Scholar]
- Pirner H.M., Stolz J.(2006). Biotin sensing in Saccharomyces cerevisiae is mediated by a conserved DNA element and requires the activity of biotin-protein ligase. J. Biol. Chem. 281: 12381–12389. [DOI] [PubMed] [Google Scholar]
- Ried M.K., Antolín-Llovera M., Parniske M.(2014). Spontaneous symbiotic reprogramming of plant roots triggered by receptor-like kinases. eLife 3: e03891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux K.J., Kim D.I., Raida M., Burke B.(2012). A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol. 196: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RStudio Team (2020). RStudio: Integrated Development for R. RStudio, PBC: Boston http://www.rstudio.com/
- Schiapparelli L.M., McClatchy D.B., Liu H.H., Sharma P., Yates J.R. III, Cline H.T.(2014). Direct detection of biotinylated proteins by mass spectrometry. J. Proteome Res. 13: 3966–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwessinger B., Roux M., Kadota Y., Ntoukakis V., Sklenar J., Jones A., Zipfel C.(2011). Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genet. 7: e1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusher, et al. (2001). Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. U.S.A. 5116–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyanova S., Temu T., Cox J.(2016a). The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 11: 2301–2319. [DOI] [PubMed] [Google Scholar]
- Tyanova S., Temu T., Sinitcyn P., Carlson A., Hein M.Y., Geiger T., Mann M., Cox J.(2016b). The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 13: 731–740. [DOI] [PubMed] [Google Scholar]
- Van Damme D., Coutuer S., De Rycke R., Bouget F.-Y., Inzé D., Geelen D.(2006). Somatic cytokinesis and pollen maturation in Arabidopsis depend on TPLATE, which has domains similar to coat proteins. Plant Cell 18: 3502–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leene J., et al. (2019). Capturing the phosphorylation and protein interaction landscape of the plant TOR kinase. Nat. Plants 5: 316–327. [DOI] [PubMed] [Google Scholar]
- Van Leene J., et al. (2007). A tandem affinity purification-based technology platform to study the cell cycle interactome in Arabidopsis thaliana. Mol. Cell. Proteomics 6: 1226–1238. [DOI] [PubMed] [Google Scholar]
- Shen, Forde(1989). Efficient transformation of Agrobacterium spp. by high voltage electroporation. nucleic acids research 17: 8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steensel B., Henikoff S.(2000). Identification of in vivo DNA targets of chromatin proteins using tethered dam methyltransferase. Nat. Biotechnol. 18: 424–428. [DOI] [PubMed] [Google Scholar]
- Varnaitė R., MacNeill S.A.(2016). Meet the neighbors: Mapping local protein interactomes by proximity-dependent labeling with BioID. Proteomics 16: 2503–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, et al. (2020). High Temporal Resolution Reveals Simultaneous Plasma Membrane Recruitment of TPLATE Complex Subunits. Plant Physiology 183: 986–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J.E.M.M., et al. (2019). A Lotus japonicus cytoplasmic kinase connects Nod factor perception by the NFR5 LysM receptor to nodulation. Proc. Natl. Acad. Sci. USA 116: 14339–14348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn J.Y., et al. (2018). High-density proximity mapping reveals the subcellular organization of mRNA-associated granules and bodies. Mol. Cell 69: 517–532.e11. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Song G., Lal N.K., Nagalakshmi U., Li Y., Zheng W., Huang P.J., Branon T.C., Ting A.Y., Walley J.W., Dinesh-Kumar S.P.(2019). TurboID-based proximity labeling reveals that UBR7 is a regulator of N NLR immune receptor-mediated immunity. Nat. Commun. 10: 3252. [DOI] [PMC free article] [PubMed] [Google Scholar]