Abstract
Photosynthetic organisms evolved different mechanisms to protect themselves from high irradiances and photodamage. In cyanobacteria, the photoactive Orange Carotenoid-binding Protein (OCP) acts both as a light sensor and quencher of excitation energy. It binds keto-carotenoids and, when photoactivated, interacts with phyco-bilisomes, thermally dissipating the excitation energy absorbed by the latter, and acting as efficient singlet oxygen quencher. Here, we report the heterologous expression of an OCP2 protein from the thermophilic cyanobacterium Fischerella thermalis (FtOCP2) in the model organism for green algae, Chlamydomonas reinhardtii. Robust expression of FtOCP2 was obtained through a synthetic redesigning strategy for optimized expression of the transgene. FtOCP2 expression was achieved both in UV-mediated mutant 4 strain, previously selected for efficient transgene expression, and in a background strain previously engineered for constitutive expression of an endogenous β-carotene ketolase, normally poorly expressed in this species, resulting into astaxanthin and other ketocarotenoids accumulation. Recombinant FtOCP2 was successfully localized into the chloroplast. Upon purification it was possible to demonstrate the formation of holoproteins with different xanthophylls and keto-carotenoids bound, including astaxanthin. Moreover, isolated ketocarotenoid-binding FtOCP2 holoproteins conserved their photoconversion properties. Carotenoids bound to FtOCP2 were thus maintained in solution even in absence of organic solvent. The synthetic biology approach herein reported could thus be considered as a novel tool for improving the solubility of ketocarotenoids produced in green algae, by binding to water-soluble carotenoids binding proteins.
Keywords: Astaxanthin, Ketocarotenoids, Photoprotection, Chlamydomonas, Cyanobacteria, Metabolic engineering
1. Introduction
Photosynthetic organisms need light to drive photosynthetic reactions, producing NADPH and ATP molecules which are essential to satisfy the metabolic demands of the cells. However, in natural environments, these organisms can easily experience rapid changes in light intensity and quality [1]. High light exposure can cause an overexcitation of the photosynthetic apparatus due to the excessive absorption of photons, that cannot be compensated by the capacity of Calvin-Benson cycle to regenerate ADP and NADP+. This condition is extremely harmful for the cell. Indeed, the excess of absorbed light cannot be properly used for charge separation, thus increasing the probability of energy transfer to oxygen molecules and finally leading to the formation of toxic reactive oxygen species (ROS) [1]. Excited and highly reactive intermediates, like ROS, have a high oxidative potential that may damage the photosynthetic apparatus, leading to photodamage or even cell death [1]. To prevent or minimize generation of such oxidizing molecules, photosynthetic organisms have evolved a wide range of photoprotective mechanisms, among which the fastest is the so called non-photochemical quenching (NPQ), which acts through the thermal dissipation of excess energy [1–3].
Cyanobacteria and higher plants show significant differences at the level of their light harvesting systems, despite a conserved structure of the core complex of Photosystems. In cyanobacteria, intramembranous supramolecular assemblies of phycobiliproteins, called phycobilisomes (PBS), act as light harvesting antennae [4], whereas higher plants evolved transmembrane antenna proteins called Light Harvesting Complexes (LHC), which bind carotenoids and chlorophylls [5,6]. LHC complexes or LHC-like proteins can be found in most of the eukaryotic photosynthetic organism, except for glaucophytes, which retained the cyanobacterial PBS, and red algae, which exploit both PBS and LHC proteins [7,8]. Diversity in light harvesting systems caused also a different evolution of NPQ photoprotective mechanisms. Photosynthetic organisms where LHC or LHC-like proteins are present, require some specific subunits to trigger NPQ, as LHCSR (Light Harvesting Complex Stress-related) or their close relative LHCX (Light Harvesting Complex X) and/or PSBS (Photosystem II Subunit S) proteins [9–11]. Either LHCSR or PSBS subunits contain protonatable residues exposed to the lumen, which are responsible for quenching activation upon lumen acidification [12–14]. Differently, in the case of cyanobacteria, NPQ is triggered by an Orange Carotenoid-binding Protein (OCP) [15–20].
Three paralog families of OCP, called OCP1, OCP2, and OCPX have been identified so far: OCPX and OCP1 share a common ancestor, whereas OCP2 and OCP1 are the result of a second divergence [21,22]. Genomic analysis allowed identifying OCP1 as the most widespread OCP protein with members in almost every phylogenetic subclade of cyanobacteria [23]. On the contrary OCP2 is less frequently observed in cyanobacteria genomes and in some cases both OCP1 and OCP2 can be found encoded by the same genome [22]. The third class of OCP proteins, OCPX, is usually present in genomes where OCP1 or OCP2 cannot be found [24]. OCPX is more frequently observed compared to OCP2 especially in filamentous heterocyst-forming cyanobacteria [24]. OCP is a water-soluble protein which binds a single carotenoid molecule, senses blue-light, and interacts with PBS. In particular, OCP has a crucial role in the NPQ mechanism of cyanobacteria because the OCP-PBS interaction allows inducing thermal dissipation of excess energy absorbed by the latter [25,26]. 3-Hydroxyechinenone has been reported as the main carotenoid bound by OCP proteins, even if other types of carotenoids and ketocarotenoids, like echinenone, canthaxanthin and zeaxanthin were also found bound to these soluble protein [25]. Among these ligands ketocarotenoids were specifically reported to be essential for OCP photoactivation and its function in photoprotection [25,27]. Under strong blue-green light, OCPO (orange form) is converted to OCPR (red active form), which can trigger NPQ mechanism [28]. OCP2 and OCPX red active forms can spontaneously revert to OCPO in the dark [22], whereas OCP1 needs a Fluorescence Recovery Protein (FRP) to accelerate OCPR-to-OCPO conversion reaction and for detaching from PBSs [29]. Interestingly, OCP2 and FRP genes were never found in genome where OCP1 was present [22]. OCP shows a modularity in structure; it contains an effector N-terminal domain (NTD), a sensor C-terminal domain (CTD) and a carotenoid. Both NTD and CTD present homologous gene in the majority of cyanobacteria genomes named helix carotenoid proteins (HCPs) in the case of NTD and CTDHs (CTD-like homologs) in the case of CTD [23]. The role of these proteins is still not clear at all but it is proposed that these NTD and CTD homologous can mix generating OCP-like protein with different photoprotective properties [15]. In recent years OCP function and role have acquired an increasing interest, both as a singlet oxygen (1O2) [15] and energy quencher in living cells, but also as an antioxidant molecule for pharmaceutical and cosmetic applications [30,31]. In biological systems, as plants but also animals or humans, excessive light exposure leads to the formation of ROS, that could damage biomolecules, affecting the integrity and stability of cells and tissues. Pathobiochemistry of several diseases of light-exposed tissues is indeed influenced also by these photooxidative processes [32]. For these reasons, research toward the discovery of new bioavailable antioxidant molecules is extremely active and requires the use of highly hydrophilic antioxidants. Water-soluble carotenoid binding proteins, in fact, might be suitable to this purpose, as they are able to confer hydrophilic properties to carotenoids, which otherwise would be relatively hydrophobic and embedded in biological membranes. In addition, it was recently reported the higher antioxidant power of OCP compared to vitamin C, tocopherol and isolated carotenoids [30]. Bourcier de Carbon and co-workers have reported the possibility to produce high amount of holo-OCP protein in Escherichia coli [31] however, to the best of our knowledge, stable production in green microalgae has not been achieved yet. A “green” alternative to bacteria could indeed be represented by microalgae, which are well known as highly efficient CO2-fixing organisms and can be cultivated in a circular economy approach, exploiting nutrients recovered from wastewaters [33,34]. Moreover, the possibility to efficiently manipulate metabolism in microalgae allows redirecting endogenous pathways toward the production of different molecules of interest and high-valuable compounds [35–38]. Recently the model organism for green algae, Chlamydomonas reinhardtii, was engineered to accumulate ketocarotenoids, as canthaxanthin and astaxanthin, suitable for human nutrition or livestock feed [36]. However, carotenoids are hydrophobic molecules requiring an organic solvent or amphiphilic environment to be maintained in solution.
In this work we investigate the expression of OCP2 from Fischerella thermalis (FtOCP2) in a microalgal host. OCP2 was chosen among the different OCP subunits due to its previously reported spontaneous ability to revert to OCPO in the dark even in the absence of FRP [22]. F. thermalis is a thermophile cyanobacterium growing at temperature up to ~60°C presenting in its genome genes for OCP2, HCP2, HCP4 and CTDH subunits [22,39]. FtOCP2 sequence was preferred to OCP2 subunits from other cyanobacteria being F. thermalis a thermophilic microorganism, suggesting a possible higher stability of the proteins encoded by its genome compared to the case of other mesophilic cyanobacteria.
Here, we report the expression and purification of a functional and photoactive FtOCP2 as a carotenoid carrier in C. reinhardtii, in order to improve carotenoids availability in aqueous solution upon extraction.
2. Materials and methods
2.1. Algal strains and culture conditions
C. reinhardtii UVM4 (UV-mediated mutant 4) strain [40] was used for all transformation experiments. Algal cells were cultivated in mixotrophic or photoautotrophic conditions, in Tris-acetate-phosphate (TAP) or High Salts (HS) minimal medium respectively [41,42], as described in the text. Liquid cultures were maintained in shake flasks, whereas agar-solidified medium in plates, at 25 °C and 100–150 μmol photons m−2s−1 of continuous white light, unless otherwise stated. Algal growth and growth tests were conducted with different systems: shaking flasks, stirring flasks or Multi-Cultivator MC-1000 (Photon Systems Instruments, Czech Republic). Temperature was controlled to 25°C while light intensities were varied as indicated in the text.
2.2. Construction of transformation vectors, cloning, transformation and mutant screening
The amino acid sequence of F. thermalis Orange Carotenoid-binding Protein 2 (hereafter FtOCP) (WP_009459388), was synthetically redesigned by codon optimization and intron spreading, to enhance transgene expression, as recently described [43]. The synthesized nucleotide sequence (Thermo Scientific) was cloned into pOpt2_PsaD_mVenus_- Stag_Paro vector [44] to obtain a protein product flanked by the C. reinhardtii photosystem I subunit D (PsaD) chloroplast targeting peptide and a C-terminal mVenus encoding for Yellow Fluorescent Protein (YFP), fused with a Strep-tag® II flag, a short peptide (8 amino acids, WSHPQFEK), which binds with high selectivity to Strep-Tactin®, an engineered streptavidin. The CrBKT (C. reinhardtii β-carotene ketolase) gene (AY860820.1), synthetically redesigned as previously explained [36], was cloned both in pOpt2_PsaD_mVenus_Paro and pOpt2_PsaD_AadA vectors, in the latter fused to AadA (aminoglycoside-3”-adenylyltransferase) gene (pOpt2_PsaD_CrBKT_AadA), conferring resistance to spectinomycin [45].
Stable nuclear transformation was carried out by glass beads agitation, as previously described [46] using 10 μg of linearized plasmid DNA. Selection of transformants was performed on TAP agar plates supplied with antibiotics (12 μg/mL for paromomycin and 200 μg/mL for spectinomycin), for 5–7 days at 200 μmol m−2 s−1 light intensity. Antibiotic resistant colonies were then selected for target construct expression. 50 expressing colonies for both CrBKT_YFP and CrBKT_AadA were pre-screened visually for red coloration before further quantification. FtOCP_YFP antibiotic resistant colonies, instead, were picked to fresh plates and inoculated in 96-well microtiter plates with TAP medium, cultivating at 200 μmol m−2 s−1 light intensity until sufficiently dense. YFP fluorescence was measured in an Infinite PRO 200 plate reader (TECAN, Switzerland) with excitation at 509 nm and emission at 520-560, normalizing it to 720 nm absorbance (cell scattering), to determine the highest expressing strains. Nomenclature of strains generated in this work is reported in Table S1.
2.3. Protein extraction and SDS-PAGE
Total cells or purified proteins were separated by SDS-PAGE as described in [47]. Separated proteins were stained using Coomassie Brilliant Blue solution or were analyzed by immunodetection using anti-GFP (Green Fluorescent Protein) antibody (Sigma-Aldrich) and using an anti-rabbit Immunoglobulin G-Alkaline phosphatase conjugated secondary antibody (Sigma-Aldrich). Protein concentration of isolated proteins was performed measuring absorption at 280 nm by NanoDrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
2.4. Pigment extraction and analysis
Pigments were extracted from 106 intact cells using 80% acetone, buffered with Na2CO3. Pigment extracts were centrifuged at 15,000g and the supernatant loaded in HPLC at the same chlorophyll content (20 μl loaded at 0.01 μg/μl concentration in acetone 80%) determined by absorption spectroscopy as previously reported [49]. In the case of isolated proteins, 25 μl of samples with the same absorption at 500 nm (OD = 0.8) were diluted to 200 μl in acetone buffered with Na2CO3 to obtain a final acetone concentration of 80%. Pigment extracts were then centrifuged at 15,000g before loading into HPLC. HPLC analysis was performed as described in [36] with the difference that here a Jasco LC-4000 Extrema HPLC system (Jasco, Italy) equipped with a C18 column (Synergi 4u Hydro-RP 80A, Phenomenex, USA) and 350–750 nm diode array detector was used. In particular, 15-min gradient of ethyl acetate (0 to 100%) in acetonitrile-water-triethylamine (9:1:0.01, vol/vol/vol) at a flow rate of 1.5 mL/min was applied [48]. Ketocarotenoids peaks were identified by comparing retention times and spectra to commercially available standards (CaroteNature GmbHas, Switzerland) as reported in [36].
2.5. Growth analysis
Cell density of wild type and mutant strains was measured using Countess II FL Automated Cell Counter (Thermo Fisher Scientific, USA), whereas total dry biomass was evaluated by overnight lyophilization of washed cell pellets followed by gravimetric determination.
2.6. Confocal microscopy
The expression and subcellular localization of FtOCP_YFP were examined by confocal microscopy. Images were recorded on a Leica TCS-SP5 inverted confocal microscope (Leica Microsystem, Germany), equipped 63 × oil immersion objectives and 2 Hybrid spectral detectors. mVenus (YFP) and chlorophyll were excited at 514 nm, whereas fluorescence emission was detected at 522–572 nm and 680–720 nm, for YFP and chlorophyll a respectively.
2.7. Protein extraction and purification
FtOCP_YFP protein was purified both from ocp1 and ocp1-bktl lines, exploiting the presence of the Strep-tag® II short peptide at the C-terminal of the recombinant protein. 3 L of saturated culture of each line (9 · 1010 total cells) were pelleted and lysate in Buffer W (100 mM Tris/HCl, 150 mM NaCl, 1 mM EDTA at pH 8) and broken through a Cell Disruptor (Constant Systems Ltd, UK) at 16 kpsi. Crude cell lysate was pelleted at 1000 g to remove cell debris. Soluble recombinant FtOC-P_YFP was purified by affinity chromatography, flowing the obtained supernatant through a Strep-Tactin® immobilized resin, following manufacture’s protocol and eluting with 50 mM Biotin. Eluted solutions were concentrated through membrane spin columns (GE Healthcare, USA) to 1 mL each. Calculation of the fraction of holoprotein compared to the total FtOCP protein content was performed determining the molar ratio between YFP (fused to FtOCP) and carotenoids bound. Molar concentration of YFP and carotenoids were calculated from absorption spectra of isolated OCP samples, pigments extracted from recombinant OCP and isolated YFP, considering the extinction coefficients of carotenoids (114,374 M−1cm−1) and YFP (83,000 M−1cm−1), respectively at 496 nm and 514 nm, and the absorption ratios at 514/496 nm and 496/514 nm respectively for extracted carotenoids and for YFP. Nomenclature of isolated proteins is reported in Table S2.
2.8. Spectroscopic analysis and measurement of recombinant Fischerella thermalis Orange Carotenoid-binding Protein 2 photoconversion kinetics
Absorption spectra and kinetics were collected with Evolution 220 UV–Vis spectrophotometer (Thermo Scientific, USA). Photoconversion of dark-adapted FtOCP_YFP orange-inactive form to the red-active form was tested after 5 min of blue light illumination (λmax = 470 nm) at 900 μmol photons m−2 s−1. The photoconversion and dark recovery kinetics were monitored from the absorption changes at 425–475 nm and 525–575 nm where the orange-inactive form (OCPO) and the red-active form (OCPR) are respectively mainly contributing.
2.9. Experimental replication and statistical treatment
All the experiments herein reported were performed at least three times. Errors are reported as standard deviations.
3. Results
3.1. Heterologous expression of Fischerella thermalis Orange Carotenoid-binding Protein 2
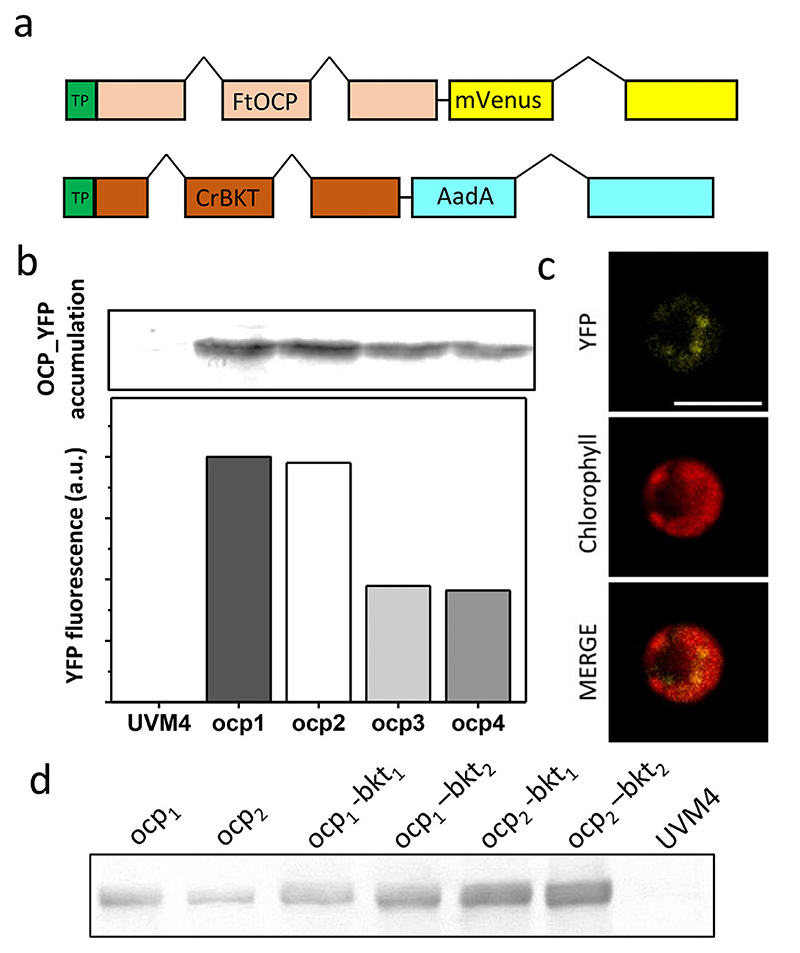
Codon optimization and incorporation of the first intron of C. reinhardtii Ribulose bisphosphate carboxylase small chain 2 is a well-established technique to optimize nuclear transgene expression in this host organism [43]. This strategy was applied to generate an optimized synthetic gene, encoding for the 319 aa OCP2 peptide sequence from F. thermalis (Fig. S1). Optimized gene sequence was cloned into a pOpt2 vector: Photosystem I subunit D (PsaD) transit peptide was used as chloroplast targeting peptide, as previously reported [50], while mVe-nus was fused at the C-terminus of FtOCP, to allow screening of transgene expressing colonies. A Strep-tag® II affinity tag (WSHPQFEK*) was also fused at the C-terminus to allow protein purification. Hsp70A/Rbsc2 hybrid promotor was used to drive gene expression as previously reported [43]. pOpt2_PsaD_FtOCP_YFP vector (Fig. 1a) was used to transform a C. reinhardtii UVM4 strain, a mutant strain previously generated by UV mutagenesis and screened for efficient expression of nuclear transgenes [40]. 96 transformed colonies grown on selective plates were screened for YFP fluorescence and 4 of them, showing the highest YFP fluorescence, were selected to be inoculated. The accumulation of FtOCP_YFP was confirmed in the transgenic cultures, herein called ocp 1–4, by western blot analysis against YFP moiety, showing a 64 kDa protein band which is not present in their background UVM4 strain (Fig. 1d). As reported in Fig. 1b, YFP fluorescence correlates with protein accumulation. The localization of accumulated protein into the chloroplast was confirmed by confocal microscopy, observing the overlap between YFP fluorescence and chlorophyll autofluorescence (Fig. 1c). ocp1 and ocp2 were selected for next steps considering their higher protein accumulation, confirmed both by YFP fluorescence and western blot analysis.
Fig. 1. FtOCP expression in Chlamydomonas reinhardtii: transformation, accumulation and localization.
a) schematic overview of vectors used for transformation of C. reinhardtii with Fischerella thermalis (Ft) OCP (Orange Carotenoid-binding Protein) and C. reinhardtii (Cr) BKT (β-carotene ketolase). Both vectors include Heat shock protein 70/Ribulose bisphosphate carboxylase small chain 2 (Hsp70A/Rbcs2) hybrid promoter. FtOCP vector contains mVenus gene encoding for the YFP (Yellow fluorescence protein) fused to FtOCP, while CrBKT vector contains antibiotic resistance to spectinomycin (AadA, amino-glycoside-3”-adenylyltransferase gene). PsaD (Photosystem I Subunit D) transit peptide (TP) was used to localize both BKT and OCP into the chloroplast. FtOCP and BKT sequences were designed in silico using codon optimization and inserting rbcs2 intron 1. b) YFP fluorescence quantification, expressed as arbitrary unit (a.u.), and western blot analysis showing OCP_YFP accumulation in transformant strains ocp1–4 compared to the background strain UVM4 (UV-mediated mutant 4). c) OCP_YFP cellular localization performed by confocal laser-scanning microscopy. Scale bar (white) represent 5 μm. d) Western blot analysis showing OCP accumulation in transformant ocp1 and ocp2 lines as well as in ocp1–bkt1, ocp1–bkt2, ocp2–bkt1 and ocp2–bkt2 strains.
3.2. Overexpression of Chlamydomonas reinhardtii β-carotene ketolase for ketocarotenoids accumulation
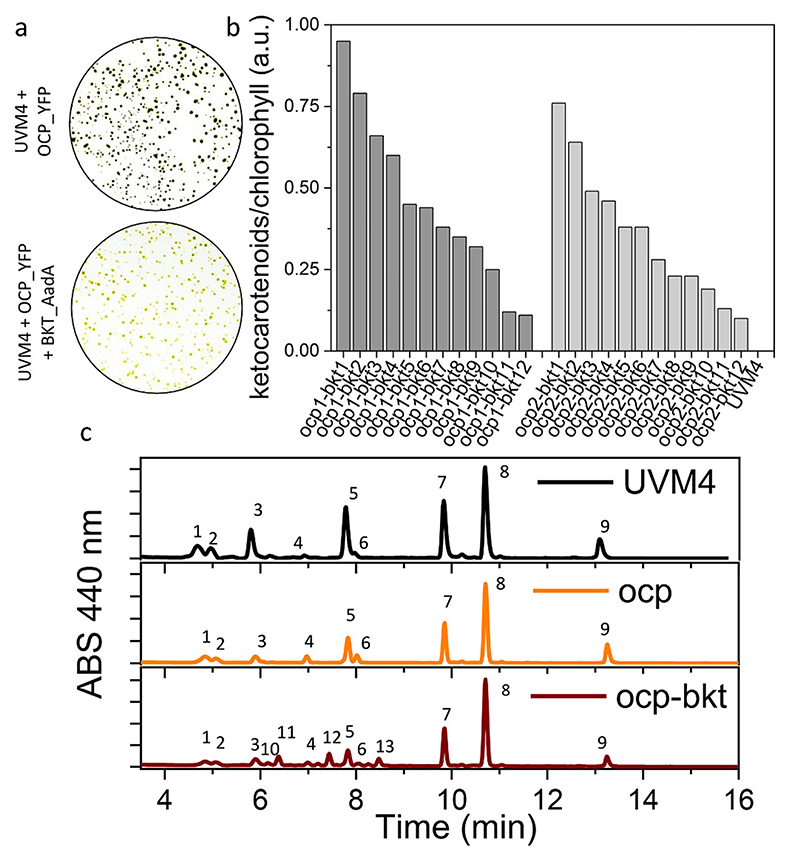
We have recently reported the possibility to selectively accumulate ketocarotenoids in C. reinhardtii by overexpressing an optimized endogenous β-carotene ketolase gene (CrBKT) [36]. In this mutant line, up to 70% of total carotenoids are converted to ketocarotenoids. The same strategy was here used to induce astaxanthin production in FtOCP_YFP transformed lines, considering the ability of OCP to bind ketocarotenoids [15,28]. In particular, the optimized CrBKT, deprived of the last 115 aa and targeted to chloroplast by PsaD transit peptide, was used as previously reported, fused to AadA gene, conferring resistance to spectinomycin [45]. This strategy allows to easily screen for transformed colonies. The gene of interest and resistance marker in fact are expressed as a fused protein, thus the survival of colonies on selective medium ensure the presence of CrBKT. The ability of this fused protein to successfully convert precursors into ketocarotenoids was tested in UVM4 and compared to previously reported CrBKT_YFP. After the transformation with these two expression vectors, an orange phenotype was clearly visible in both cases (Fig. S2). All the colonies transformed with PsaD_BKT_AadA construct showed an altered coloring, even if with different intensity grades, due to the position effect of the genomic insertion of the cassette. Differently, in the case of PsaD_BKT_YFP transformation, only 13% of total colonies showed a red phenotype (Fig. S2). pOpt2_- PsaD_BKT_AadA vector was thus used to transform ocp 1 and ocp 2 lines. Again, as observed for the previous UVM4 transformation on selection plates, most of the surviving colonies were characterized by a clear red/orange phenotype (Fig. 2a). Transformed strains generated using ocp1 or ocp2 background, renamed as ocp 1-bkt 1–12, and ocp2-bkt 1–12, were selected and analyzed for ketocarotenoids accumulation (Fig. 2b). Ketocarotenoids accumulation in selected lines leads to a visible color change in liquid culture (Fig. S3) and the absorption spectra of extracted pigments revealed the presence of red shifted carotenoids, which are not present in UVM4, ocp1 and ocp2. Pigments accumulated by top ketocarotenoids producing line, ocp 1-bkt 1, were further analyzed by high-performance liquid chromatography (HPLC) and compared to the background UVM4 and to the OCP only expressing lines (Fig. 2c; Table S3). HPLC analysis demonstrated similar pigment composition in UVM4 and ocp 1 lines, suggesting that the sole FtOCP expression could not alter the pigment profile in C. reinhardtii (Fig. 2c; Table S3). On the contrary, ocp 1-bkt 1 lines showed the main representative peaks of astaxanthin (peaks 10, 11 and 12) and canthaxanthin (peak 13), as expected due to CrBKT enzyme activity [36] (Fig. 2c; Table S3). The three astaxanthin peaks identified in ocp-bkt lines are respectively related to the astaxanthin isomers as recently reported for the C. reinhardtii strains overexpressing the BKT enzyme [36].
Fig. 2. Ketocarotenoids accumulation in transformed lines.
a) orange color phenotype of transformed lines in UVM4 (UV-mediated mutant 4) background expressing FtOCP (Orange Carotenoid-binding Protein from Fischerella ther-malis) fused to YFP (Yellow Fluorescent Protein) alone or in addition to CrBKT (β-carotene ketolase from Chlamydomonas reinhardtii) fused to spectinomycin resistance (AadA, aminoglycoside-3”-adenylyltransferase gene), indicated respectively as UVM4 + OCP_YFP or UVM4 + OCP_YFP + BKT_AadA. b) Ketocarotenoids accumulation expressed as arbitrary units (a.u.) for transformed lines expression both FtCOP and CrBKT (ocp1_bkt1–12 and ocp2-bkt1–12) compared to the background strain UVM4. c) Representative HPLC of pigments from UVM4 (black) ocp (orange) and ocp-bkt (red) strains. Chromatograms are shown reported the absorbance (ABS) of the different peaks at 440 nm. 1: neoxanthin; 2: loroxanthin; 3: violaxanthin; 4: antheraxanthin; 5: lutein; 6: zeaxanthin; 7: chlorophyll b; 8: chlorophyll a; 9 β-carotene; 10,11, 12: astaxanthin; 13: canthaxanthin. The data reported are representative of three independent experiments.
3.3. Ketocarotenoids accumulation does not interfere with Orange Carotenoid-binding Protein accumulation
Possible effects of ketocarotenoids presence on FtOCP_YFP protein accumulation was evaluated by western blot on transformed lines with different amount of ketocarotenoids (ocp 1-bkt 1, ocp 1-bkt 2, ocp 2-bkt 1 and ocp 2-bkt 2). As shown in Fig. 1d, all tested lines showed similar FtOC-P_YFP protein accumulation, thus excluding negative or positive effects of CrBKT expression of recombinant FtOCP protein.
3.4. Orange Carotenoid-binding Protein and ketocarotenoids accumulation has no effects on growth
Effects of FtOCP_YFP and CrBKT on cell growth were also evaluated in both autotrophy (HS) and mixotrophy (TAP) conditions, using two different light regimes: 100 μmol photons m−2 s−1 for control light and 500 μmol photons m−2 s−1 for high light. Algae were grown in 80 mL airlift photobioreactors systems until reaching the stationary phase. As reported in Fig. S4, ocp 1 and ocp 1-bkt lines show no significant differences in biomass accumulation compared to WT in conditions of autotrophy or mixotrophy at both light intensities herein tested. These results demonstrate that OCP accumulation, neither in absence nor in presence of ketocarotenoids influenced C. reinhardtii growth.
3.5. Orange Carotenoid-binding Protein purification from Chlamydomonas reinhardtii cells
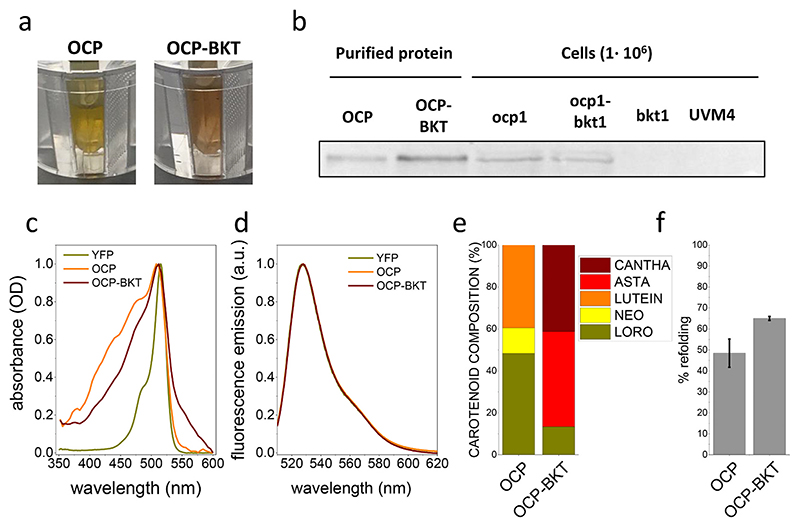
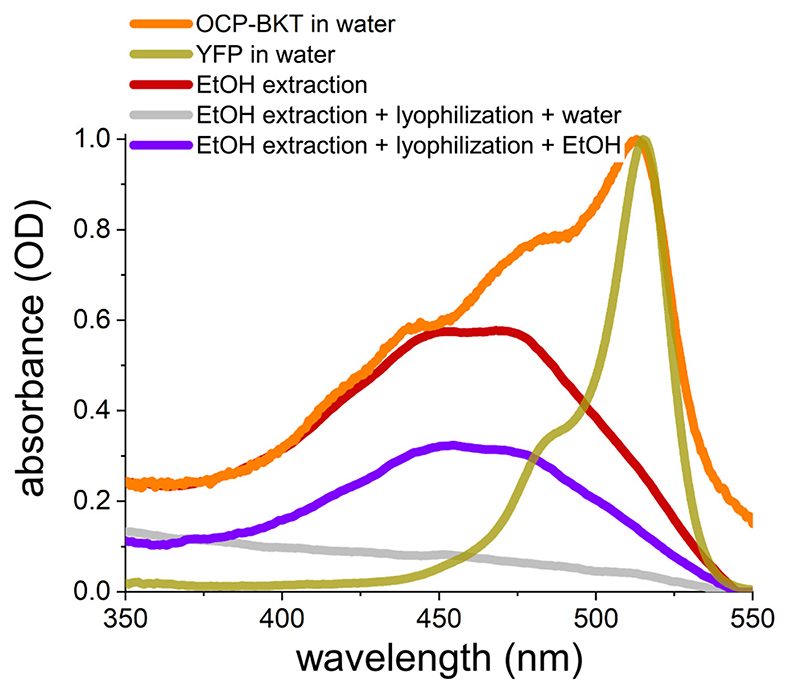
Purification of recombinant FtOCP proteins was then performed from ocp1 and ocp 1-bkt 1 lines grown in a semi-batch system in mixotrophy, at 300 μmol photons m−2 s−1. Cells for both genotypes were harvested and lysed to release soluble proteins. OCP proteins were then purified by affinity chromatography. After elution from Strep-tag® II affinity column, recovered fractions were characterized by a yellow-orange color (Fig. 3a). FtOCP presence was confirmed by western blot analysis in eluted fractions purified from either ocp1 or ocp-bkt 1 (Fig. 3b) and absorption spectra of the eluted samples were collected, showing a main peak at 512-513 nm (Fig. 3c), which is ascribable to the YFP fused to FtOCP, as it can be seen from the comparison with the isolated YFP absorption spectrum in Fig. 3c. The spectra of purified proteins from ocp 1 (OCP) and ocp1-bkt1 (OCP-BKT) show significant differences: OCP presents a blue shifted absorption with peaks at 436 nm and 476 nm while OCP-BKT shows a red-shifted tail in the 550-600 nm region, suggesting different carotenoid binding properties among the two samples. By subtraction of YFP signal it was possible to calculate the absorption spectra of OCP and OCP-BKT deprived of the contribution given by the fluorescence protein (Fig. S5). Fluorescence emission spectra at room temperature were also recorded (Fig. 3d): both OCP andOCP-BKT fluorescence emission spectra perfectly overlap the YFP emission spectra, suggesting a correct folding of fused YFP. As reported in Fig. 3f the fraction of holoproteins compared to total isolated FtOCP protein content was ~50% and ~65% in the case of FtOCP isolated respectively from ocp 1 and ocp 1-bkt 1 lines. The increased fraction of carotenoid binding protein in presence of ketocarotenoid (FtOCP isolated from ocp 1-bkt 1) is consistent with the preferential binding of ketocarotenoids rather than xanthophylls or carotenes to OCP subunits [15,22,27,31]. HPLC was used to evaluate pigments bound to the purified protein from either ocp 1 or ocp 1-bkt 1 lines. FtOCP was shown to bind neoxanthin, loroxanthin and lutein in the ocp 1 line, whereas FtOCP from ocp1_-bkt1 line was found to bind loroxanthin, astaxanthin and canthaxanthin (Fig. 3e). Interestingly, loroxanthin was the xanthophyll molecule with the highest affinity for FtOCP, being the predominant carotenoid found in the holoproteins isolated from ocp 1, while in presence of ketocarotenoids canthaxanthin was the ligand with the highest affinity (Fig. S6). Carotenoids bound by FtOCP are not soluble in water. Data reported in Fig. 4, indeed, show that after pigments extraction using ethanol and subsequent lyophilization, carotenoids bond to FtOCP cannot be solubilized in water but only in organic solvent. FtOCP protein, on the contrary, can be an efficient carrier for xanthophyll or ketocarotenoids in aqueous solution considering its stability in this buffer.
Fig. 3. Purification of OCP proteins.
a) red/orange color of purified OCP (Orange Carotenoid-binding Protein) proteins isolated from Chlamydomonas reinhardtii strains expressing recombinant OCP protein (OCP) or both BKT (β-carotene ketolase) and OCP (OCP-BKT); b) Western blot analysis performed by using specific antibody recognizing YFP (Yellow Fluorescent Protein) fused to OCP proteins in in isolated fractions after affinity chromatography (OCP and OCP-BKT) and in total protein extracts from the background strains UVM4 and from the transformant strains ocp1, ocp1-bkt1 and bkt1 expressing respectively OCP, OCP and BKT or BKT only; c) absorption spectra of isolated proteins reported as optical density (OD); d) fluorescence emission spectra of OCP proteins purified from ocp1 (OCP), ocp1-bkt1 (OCP-BKT) and YFP expressing lines reported as arbitrary units (a.u.); e) composition of carotenoids bound by OCP and OCP-BKT isolated proteins. Data are reported in percentage of total carotenoids. NEO: neoxanthin; CANTHA: canthaxanthin; LORO: loroxanthin; LUTEIN: lutein; ASTA: astaxanthin. f) percentage of refolded FtOCP holoproteins compared to total FtOCP isolated polypeptides. Error bars are reported as standard deviations (n = 3).
Fig. 4. OCP as a soluble carrier of carotenoids in water.
Absorption spectra, reported as optical density (OD) of OCP-BKT (recombinant Orange Carotenoidbinding Protein, OCP, purified from Chlamydomonas reinhardtii strain expressing both OCP and BKT, β-carotene ketolase) in water (orange line), YFP (Yellow Fluorescent Protein) in water (gold line) and OCP-BKT after ethanol (EtOH) extraction (red line). Grey line and violet lines represent spectra of OCP-BKT after ethanol extraction which was then lyophilized and resuspended respectively in water (grey line) or in ethanol (violet line). The data reported are representative of three independent experiments.
3.6. Purified Orange Carotenoid-binding Protein can switch from inactive to active form
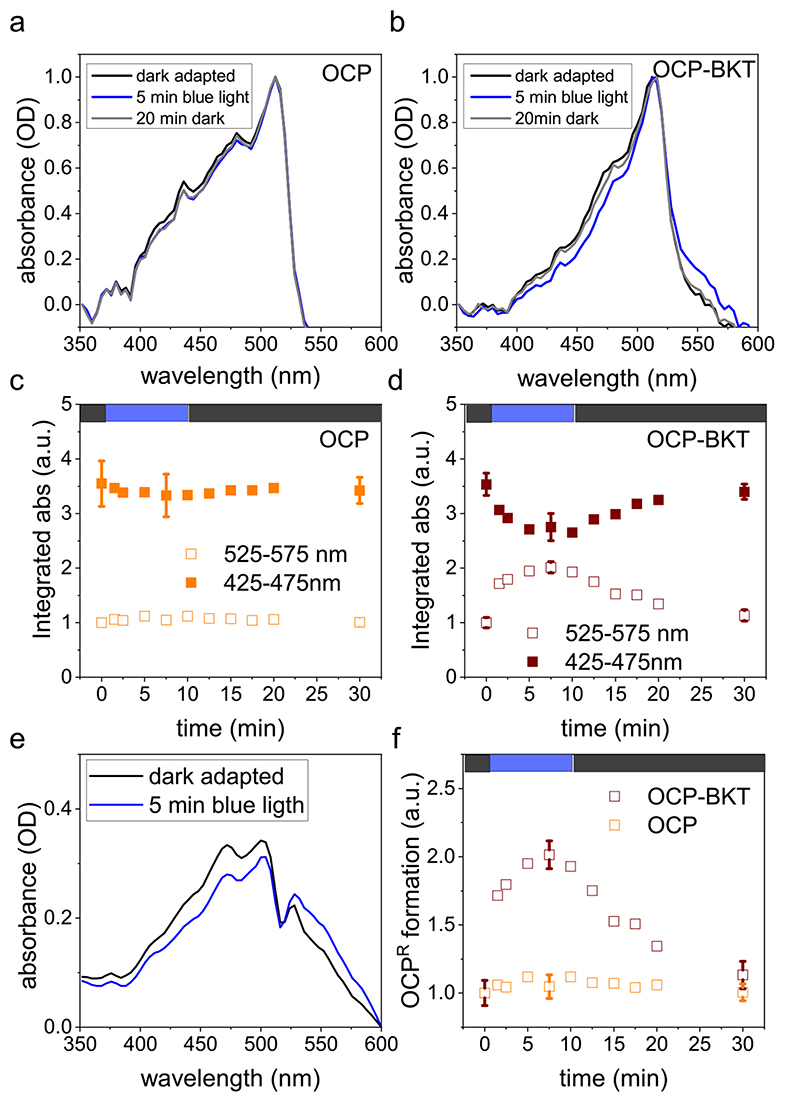
The ability to be photoconverted into the active form was tested for purified FtOCP_YFP obtained from both ocp 1 and ocp 1-bkt 1 lines. The conversion from OCPO, inactive, to the active OCPR has been reported to induce a red shift of the absorption spectrum of the protein [28]. Dark adapted purified proteins were thus illuminated using strong blue light. The conversion rate to the active form was monitored by measuring absorption spectra both at 425–475 nm and 525–575 nm (Fig. 5a, b). Indeed, these regions include respectively the absorption peaks of the inactive and the active form of OCP respectively. As shown in Fig. 5a and c blue light illumination did not induce any significant change in the absorption spectrum of FtOCP_YFP purified from ocp 1, Differently in the case of FtOCP_YFP purified from ocp 1-bkt 1 blue light illumination caused a measurable reduction of absorption values in the 425–475 nm region and, at the same time, an increase in 525–575 region for (Fig. 5b, d, e). When blue light was switched off, an increase in the absorption at 425–475 nm and a simultaneous decrease in 525–575 nm region were recorded, indicating a spontaneously back-conversion from the active to inactive form (Fig. 5b, d, e). of FtOCP_YFP purified from ocp 1, does not bound ketocarotenoids as astaxanthin or canthaxanthin, and it is not able to photoswitch from the inactive to active form, differently from FtOCP_YFP purified from ocp 1-bkt 1 These results confirm the requirement of ketocarotenoids for the photoactivation of OCP proteins (Fig. 5f) [51].
Fig. 5. Blue light excitation of OCP proteins.
a) b) Absorption spectra of recombinant OCP Orange Carotenoid-binding Protein) isolated from transformed Chlamydomonas rein-hardtii strains expressing OCP only (ocp1 a, OCP) or both BKT (β-carotene ketolase) and OCP (ocp1-bkt1 b, OCP-BKT). Absorption spectra are reported as optical density (OD) for dark-adapted protein (black), after 5 min of blue light illumination (blue) and after 20 min of dark recovery (grey). c) d) Absorption spectra integrated in 525–575 nm range (active OCPR) or 425–475 nm range (inactive OCPO) reported as arbitrary units (a.u.). e) Absorption spectra of OCP-BKT after subtraction of YFP (Yellow Fluorescent Protein) signal for dark adapted sample and after 5 min of blue light illumination. f) Comparison of OCPR formation (spectra integrated in 525–575 nm range) for OCP and OCP-BKT. Integrated absorption data reported in c), d) and f) were normalized to 1 in the case of 525–572 nm absorption of dark-adapted samples (time 0). Error bars are reported as standard deviation in c), d) and f) for dark adapted samples, after the blue light treatment and after dark recovery (n = 3).
4. Discussion
Carotenoids are antioxidant molecules which recently raised interest for their possible use as nutraceuticals for human health [52–55]. Ketocarotenoids as astaxanthin or canthaxanthin are, among carotenoid molecules, those with the highest antioxidant properties, with a high potential for their application in different industrial sectors. Microalgae are primary producers of carotenoids, including ketocarotenoids, being thus considered as potential platform for sustainable production of these molecules [52–60]. Differently from other potential heterotrophic sources of carotenoids, as bacteria or yeasts, microalgae have the important benefit of exploiting the photosynthetic process to sustain metabolism, converting light energy into chemical energy and leading to CO2 fixation [61,62]. Carotenoids are essentially hydrophobic and require an amphiphilic carrier to be maintained in aqueous solution for industrial production and application. OCP is a small soluble protein able to bind one carotenoid molecule that could be suitable to this purpose, preventing carotenoids precipitation in aqueous environment (Fig. 4) [31]. OCP proteins in cyanobacteria are responsible for photoprotection, interacting with phycobilisomes and quenching the excess energy to prevent photodamage [63]. Several successful attempts to purify OCP have been performed in cyanobacteria [64] and its recombinant expression in bacteria has also been described [31]. To extend the range of possible platforms to produce carotenoid binding OCP proteins, here we report the overexpression and purification of FtOCP protein, a member of the OCP2 protein family [22], in C. reinhardtii. Using C. reinhardtii as a host for OCP expression permits to induce the formation of OCP holoproteins bound to different carotenoids, which are not available in cyanobacteria. Moreover, the powerful synthetic biology tools recently developed in this model organism allow the application of this strategy [43]. OCP protein was successfully accumulated in Chlamydomonas without any major effect on growth, both in mixotrophy and in autotrophy, even at high light intensity (Fig. S4).
OCP protein has been reported to bind mainly ketocarotenoids in cyanobacteria, with 3’-hydroxyechinenone being the main carotenoid bound [15,23,25,51]. Here we demonstrate that FtOCP can bind, in absence of ketocarotenoids, also loroxanthin, neoxanthin or lutein. In particular, loroxanthin was the xanthophyll for which FtOCP showed the highest affinity (Fig. 3). These results were independent of the relative concentration of loroxanthin among C. reinhardtii carotenoids, which is accumulated to a much lower level compared to other pigments, as lutein or β-carotene [65]. As mentioned above, the use of C. reinhardtii as host for OCP overexpression permit to exploit the synthetic biology tools available for this organism [36,37,43]: the expression of FtOCP in the bkt background allowed accumulating this protein in presence of ketocarotenoids, including astaxanthin (Figs. 2, 3). The results obtained from FtOCP expressed in ocp 1-bkt 1 confirms that FtOCP preferentially binds ketocarotenoids (Fig. 3) and for the first time, to the best of our knowledge, demonstrate the possible direct binding of astaxanthin to a OCP2 subunit (Fig. 3). Indeed, while astaxanthin was reported to bind a novel water-soluble carotenoid binding protein, named AstaP, found in an eukaryotic microalgal strain [66], the affinity of OCP1, OCP2 or OCPX for this ketocarotenoids was not investigated so far. As previously reported, only in the presence of ketocarotenoids, including astaxanthin, recombinant FtOCP behaved as a photoswitchable protein in presence of blue light (Fig. 5). Astaxanthin or canthaxanthin, or both, are thus able to allow OCP conformational change into the OCPR form. Moreover, the ability to solubilize carotenoids in a water-based solution was confirmed also in the case of ketocarotenoid binding FtOCP (Fig. 4). Interestingly, neither accumulation of FtOCP nor ketocarotenoids biosynthesis influenced the biomass production rate of C. reinhardtii (Fig. S4). This result allows considering C. reinhardtii as an optimal host for the expression of OCP, which could act as a carrier of ketocarotenoids in aqueous solution. Metabolic engineering of C. reinhardtii for the production of astaxanthin has been recently reported as a promising step toward efficient and sustainable industrial production of ketocarotenoids for human nutrition, aquaculture or animal feed [36]. Overexpression of OCP in this strain allows further improvement of C. reinhardtii as ketocarotenoid production platform, opening the possibility to keep astaxanthin and canthaxanthin in aqueous solution upon extraction, for specific application requiring the absence of membranes or organic solvents.
However, proper optimization of OCP accumulation and its purification from engineered C. reinhardtii strains are required to make this process feasible at industrial scale. Possibly, other carotenoid binding proteins can be considered as soluble carrier for carotenoids/ketocarotenoids as for instance the C-terminal domain-like carotenoid proteins (CCPs): these subunits are homologs to the C-terminus of the OCP proteins and where recently characterized as a new class of soluble carotenoid binding proteins being able to bind canthaxanthin. Overexpression of CCPs subunits in C. reinhardtii might lead to a further carrier for ketocarotenoids [67]. It is also important to note that the ability to switch from the inactive to active form of FtOCP expressed in bkt background could allow the use of this holoprotein as a blue light-dependent molecular switch, to be used upon further engineering steps for regulating and studying gene expression or other cell activities [68].
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.algal.2021.102255.
Acknowledgements
The research was supported by the ERC Starting Grant SOLENALGAE (679814) to M.B.
No conflicts, informed consent, or human or animal rights are applicable to this study.
Footnotes
CRediT authorship contribution statement
Matteo Pivato: Investigation, Data curation, Validation, Visualization, Writing - original draft; Federico Perozeni: Investigation, Data curation, Validation, Visualization, Writing - original draft; Francesco Licausi: Conceptualization, Writing - review & editing; Stefano Caz-zaniga: Investigation, Data curation, Writing - review & editing; Matteo Ballottari: Conceptualization, Funding acquisition, Supervision, Methodology, Validation, Visualization, Project administration, Writing - original draft, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Niyogi KK. Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol. 1999 Jun;50:333–359. doi: 10.1146/annurev.arplant.50.1.333. [DOI] [PubMed] [Google Scholar]
- [2].Demmig-Adams B, Stewart JJ, Burch TA, et al. Insights from placing photosynthetic light harvesting into context. J Phys Chem Lett. 2014 Aug 21;5(16):2880–2889. doi: 10.1021/jz5010768. [DOI] [PubMed] [Google Scholar]
- [3].Demmig-Adams B, Adams WW, III, Barker DH, et al. Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol Plant. 1996 Oct 01;98(2):253–264. 1996. [Google Scholar]
- [4].Herrero A, Flores E, Flores FG. The Cyanobacteria: Molecular Biology, Genomics, and Evolution. Caister Academic Press; 2008. [Google Scholar]
- [5].Ballottari M, Girardon J, Dall’osto L, et al. Evolution and functional properties of photosystem II light harvesting complexes in eukaryotes. Biochim Biophys Acta. 2012 Jan;1817(1):143–157. doi: 10.1016/j.bbabio.2011.06.005. [DOI] [PubMed] [Google Scholar]
- [6].Croce R, van Amerongen H. Light-harvesting in photosystem I. Photosynth Res. 2013 Oct;116(2-3):153–166. doi: 10.1007/s11120-013-9838-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wolfe GR, Cunningham FX, Durnfordt D, et al. Evidence for a common origin of chloroplasts with light-harvesting complexes of different pigmentation. Nature. 1994 Feb 01;367(6463):566–568. 1994. [Google Scholar]
- [8].Durnford DG, Deane JA, Tan S, et al. A phylogenetic assessment of the eukaryotic light-harvesting antenna proteins, with implications for plastid evolution. J Mol Evol. 1999 Jan;48(1):59–68. doi: 10.1007/pl00006445. [DOI] [PubMed] [Google Scholar]
- [9].Peers G, Truong TB, Ostendorf E, et al. An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature. 2009 Nov 26;462(7272):518–521. doi: 10.1038/nature08587. [DOI] [PubMed] [Google Scholar]
- [10].Park S, Steen CJ, Lyska D, et al. Chlorophyll-carotenoid excitation energy transfer and charge transfer in Nannochloropsis oceanica for the regulation of photosynthesis. Proc Natl Acad Sci U S A. 2019 Feb 26;116(9):3385–3390. doi: 10.1073/pnas.1819011116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li XP, Bjorkman O, Shih C, et al. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature. 2000 Jan 27;403(6768):391–395. doi: 10.1038/35000131. [DOI] [PubMed] [Google Scholar]
- [12].Ballottari M, Truong TB, De Re E, et al. Identification of pH-sensing sites in the light harvesting complex stress-related 3 protein essential for triggering nonphotochemical quenching in Chlamydomonas reinhardtii. J Biol Chem. 2016 Apr 1;291(14):7334–7346. doi: 10.1074/jbc.M115.704601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liguori N, Roy LM, Opacic M, et al. Regulation of light harvesting in the green Chlamydomonas reinhardtii: the C-terminus of LHCSR is the knob of a dimmer switch. J Am Chem Soc. 2013 Dec 11;135(49):18339–18342. doi: 10.1021/ja4107463. [DOI] [PubMed] [Google Scholar]
- [14].Li XP, Gilmore AM, Caffarri S, et al. Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem. 2004 May 28;279(22):22866–22874. doi: 10.1074/jbc.M402461200. [DOI] [PubMed] [Google Scholar]
- [15].Kerfeld CA, Sawaya MR, Brahmandam V, et al. The crystal structure of a cyanobacterial water-soluble carotenoid binding protein. Structure. 2003 Jan;11(1):55–65. doi: 10.1016/s0969-2126(02)00936-x. [DOI] [PubMed] [Google Scholar]
- [16].Wilson A, Ajlani G, Verbavatz JM, et al. A soluble carotenoid protein involved in phycobilisome-related energy dissipation in cyanobacteria. Plant Cell. 2006 Apr;18(4):992–1007. doi: 10.1105/tpc.105.040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Karapetyan NV. Non-photochemical quenching of fluorescence in cyanobacteria. Biochemistry (Mosc) 2007 Oct;72(10):1127–1135. doi: 10.1134/s0006297907100100. [DOI] [PubMed] [Google Scholar]
- [18].Boulay C, Abasova L, Six C, et al. Occurrence and function of the orange carotenoid protein in photoprotective mechanisms in various cyanobacteria. Biochim Biophys Acta. 2008 Oct;1777(10):1344–1354. doi: 10.1016/j.bbabio.2008.07.002. [DOI] [PubMed] [Google Scholar]
- [19].Gwizdala M, Wilson A, Kirilovsky D. In vitro reconstitution of the cyanobacterial photoprotective mechanism mediated by the Orange Carotenoid Protein in Synechocystis PCC 6803. Plant Cell. 2011 Jul;23(7):2631–2643. doi: 10.1105/tpc.111.086884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Berera R, van Stokkum IH, Gwizdala M, et al. The photophysics of the orange carotenoid protein, a light-powered molecular switch. J Phys Chem B. 2012 Mar 1;116(8):2568–2574. doi: 10.1021/jp2108329. [DOI] [PubMed] [Google Scholar]
- [21].Muzzopappa F, Wilson A, Kirilovsky D. Interdomain interactions reveal the molecular evolution of the orange carotenoid protein. Nat Plants. 2019 Oct;5(10):1076–1086. doi: 10.1038/s41477-019-0514-9. [DOI] [PubMed] [Google Scholar]
- [22].Bao H, Melnicki MR, Pawlowski EG, et al. Additional families of orange carotenoid proteins in the photoprotective system of cyanobacteria. Nat Plants. 2017 Jul 10;3:17089. doi: 10.1038/nplants.2017.89. [DOI] [PubMed] [Google Scholar]
- [23].Kerfeld CA, Melnicki MR, Sutter M, et al. Structure, function and evolution of the cyanobacterial orange carotenoid protein and its homologs. New Phytol. 2017 Aug;215(3):937–951. doi: 10.1111/nph.14670. [DOI] [PubMed] [Google Scholar]
- [24].Shih PM, Wu D, Latifi A, et al. Improving the coverage of the cyanobacterial phylum using diversity-driven genome sequencing. Proc Natl Acad Sci U S A. 2013 Jan 15;110(3):1053–1058. doi: 10.1073/pnas.1217107110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kirilovsky D, Kerfeld CA. The orange carotenoid protein in photoprotection of photosystem II in cyanobacteria. Biochim Biophys Acta. 2012 Jan;1817(1):158–166. doi: 10.1016/j.bbabio.2011.04.013. [DOI] [PubMed] [Google Scholar]
- [26].Kirilovsky D, Kerfeld CA. Cyanobacterial photoprotection by the orange carotenoid protein. Nat Plants. 2016 Dec 2;2(12):16180. doi: 10.1038/nplants.2016.180. [DOI] [PubMed] [Google Scholar]
- [27].Leverenz RL, Sutter M, Wilson A, et al. Photosynthesis. A 12 A carotenoid translocation in a photoswitch associated with cyanobacterial photoprotection. Science. 2015 Jun 26;348(6242):1463–1466. doi: 10.1126/science.aaa7234. [DOI] [PubMed] [Google Scholar]
- [28].Wilson A, Punginelli C, Gall A, et al. A photoactive carotenoid protein acting as light intensity sensor. Proc Natl Acad Sci U S A. 2008 Aug 19;105(33):12075–12080. doi: 10.1073/pnas.0804636105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sutter M, Wilson A, Leverenz RL, et al. Crystal structure of the FRP and identification of the active site for modulation of OCP-mediated photoprotection in cyanobacteria. Proc Natl Acad Sci U S A. 2013 Jun 11;110(24):10022–10027. doi: 10.1073/pnas.1303673110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sedoud A, Lopez-Igual R, Ur Rehman A, et al. The cyanobacterial photoactive orange carotenoid protein is an excellent singlet oxygen quencher. Plant Cell. 2014 Apr;26(4):1781–1791. doi: 10.1105/tpc.114.123802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bourcier de Carbon C, Thurotte A, Wilson A, et al. Biosynthesis of soluble carotenoid holoproteins in Escherichia coli. Sci Rep. 2015 Mar 13;5:9085. doi: 10.1038/srep09085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Stahl W, Sies H. Bioactivity and protective effects of natural carotenoids. Biochim Biophys Acta. 2005 May 30;1740(2):101–107. doi: 10.1016/j.bbadis.2004.12.006. [DOI] [PubMed] [Google Scholar]
- [33].Zuliani L, Frison N, Jelic A, et al. Microalgae cultivation on anaerobic digestate of municipal wastewater, sewage sludge and agro-waste. Int J Mol Sci. 2016 Oct 10;17(10) doi: 10.3390/ijms17101692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Koyande AK, Show PL, Guo R, et al. Bio-processing of algal bio-refinery: a review on current advances and future perspectives. Bioengineered. 2019 Dec;10(1):574–592. doi: 10.1080/21655979.2019.1679697. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [35].Lauersen KJ, Wichmann J, Baier T, et al. Phototrophic production of heterologous diterpenoids and a hydroxy-functionalized derivative from Chlamydomonas reinhardtii. Metab Eng. 2018;49:116–127. doi: 10.1016/j.ymben.2018.07.005. [DOI] [PubMed] [Google Scholar]
- [36].Perozeni F, Cazzaniga S, Baier T, et al. Turning a green alga red: engineering astaxanthin biosynthesis by intragenic pseudogene revival in Chlamydomonas reinhardtii. Plant Biotechnol J. 2020 Feb 25; doi: 10.1111/pbi.13364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Crozet P, Navarro FJ, Willmund F, et al. Birth of a photosynthetic chassis: a MoClo toolkit enabling synthetic biology in the microalga Chlamydomonas. ACS Synth Biol. 2018;7(9):2074–2086. doi: 10.1021/acssynbio.8b00251. [DOI] [PubMed] [Google Scholar]
- [38].Poliner E, Farre EM, Benning C. Advanced genetic tools enable synthetic biology in the oleaginous microalgae Nannochloropsis sp. Plant Cell Rep. 2018 Mar; doi: 10.1007/s00299-018-2270-0. [DOI] [PubMed] [Google Scholar]
- [39].Alcorta J, Vergara-Barros P, Antonaru LA, et al. Fischerella thermalis: a model organism to study thermophilic diazotrophy, photosynthesis and multicellularity in cyanobacteria. Extremophiles. 2019 Nov;23(6):635–647. doi: 10.1007/s00792-019-01125-4. [DOI] [PubMed] [Google Scholar]
- [40].Neupert J, Karcher D, Bock R. Generation of Chlamydomonas strains that efficiently express nuclear transgenes. Plant J. 2009 Mar;57(6):1140–1150. doi: 10.1111/j.1365-313X.2008.03746.x. [DOI] [PubMed] [Google Scholar]
- [41].Harris EH. Harris, Introduction to Chlamydomonas and Its Laboratory Use. Academic Press; San Diego: 2008. [Google Scholar]
- [42].Kropat J, Hong-Hermesdorf A, Casero D, et al. A revised mineral nutrient supplement increases biomass and growth rate in Chlamydomonas reinhardtii. Plant J. 2011 Jun;66(5):770–780. doi: 10.1111/j.1365-313X.2011.04537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Baier T, Wichmann J, Kruse O, et al. Intron-containing algal transgenes mediate efficient recombinant gene expression in the green microalga Chlamydomonas reinhardtii. Nucleic Acids Res. 2018 Jul 27;46(13):6909–6919. doi: 10.1093/nar/gky532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wichmann J, Baier T, Wentnagel E, et al. Tailored carbon partitioning for phototrophic production of (E)-alpha-bisabolene from the green microalga Chlamydomonas reinhardtii. Metab Eng. 2018 Jan;45:211–222. doi: 10.1016/j.ymben.2017.12.010. [DOI] [PubMed] [Google Scholar]
- [45].Meslet-Cladiere L, Vallon O. Novel shuttle markers for nuclear transformation of the green alga Chlamydomonas reinhardtii. Eukaryot Cell. 2011 Dec;10(12):1670–1678. doi: 10.1128/EC.05043-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kindle KL. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1228–1232. doi: 10.1073/pnas.87.3.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- [48].Lagarde D, Beuf L, Vermaas W. Increased production of zeaxanthin and other pigments by application of genetic engineering techniques to Synechocystis sp. strain PCC 6803. Appl Environ Microbiol. 2000 Jan;66(1):64–72. doi: 10.1128/aem.66.1.64-72.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ferrante P, Ballottari M, Bonente G, et al. LHCBM1 and LHCBM2/7 polypeptides, components of major LHCII complex, have distinct functional roles in photosynthetic antenna system of Chlamydomonas reinhardtii. J Biol Chem. 2012 May;287(20):16276–16288. doi: 10.1074/jbc.M111.316729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lauersen KJ, Kruse O, Mussgnug JH. Targeted expression of nuclear transgenes in Chlamydomonas reinhardtii with a versatile, modular vector toolkit. Appl Microbiol Biotechnol. 2015 Apr;99(8):3491–3503. doi: 10.1007/s00253-014-6354-7. [DOI] [PubMed] [Google Scholar]
- [51].Wilson A, Punginelli C, Couturier M, et al. Essential role of two tyrosines and two tryptophans on the photoprotection activity of the Orange Carotenoid Protein. Biochim Biophys Acta. 2011 Mar;1807(3):293–301. doi: 10.1016/j.bbabio.2010.12.009. [DOI] [PubMed] [Google Scholar]
- [52].Koyande A, Chew K, Rambabu K, et al. Microalgae: a potential alternative to health supplementation for humans. Food Sci Human Wellness. 2019 Mar;8(1):16–24. 2019. [Google Scholar]
- [53].Zhang J, Sun Z, Sun P, et al. Microalgal carotenoids: beneficial effects and potential in human health. Food Funct. 2014 Mar;5(3):413–425. doi: 10.1039/c3fo60607d. [DOI] [PubMed] [Google Scholar]
- [54].Azqueta A, Collins AR. Carotenoids and DNA damage. Mutat Res. 2012 May;733(1-2):4–13. doi: 10.1016/j.mrfmmm.2012.03.005. [DOI] [PubMed] [Google Scholar]
- [55].Demmig-Adams B, Adams WW. Antioxidants in photosynthesis and human nutrition. Science. 2002 Dec;298(5601):2149–2153. doi: 10.1126/science.1078002. [DOI] [PubMed] [Google Scholar]
- [56].Camacho F, Macedo A, Malcata F. potentialindustrial applications and commercialization of microalgae in the functional food and feed industries: a short review. marine drugs. 2019 Jun;17 doi: 10.3390/md17060312. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sathasivam R, Radhakrishnan R, Hashem A, et al. Microalgae metabolites: a rich source for food and medicine. Saudi J Biol Sci. 2019 May;26(4):709–722. doi: 10.1016/j.sjbs.2017.11.003. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Del Campo JA, Rodríguez H, Moreno J, et al. Accumulation of astaxanthin and lutein in Chlorella zofingiensis (Chlorophyta. Appl Microbiol Biotechnol. 2004 Jun;64(6):848–854. doi: 10.1007/s00253-003-1510-5. [DOI] [PubMed] [Google Scholar]
- [59].Raman R, Mohamad SE. Astaxanthin production by freshwater microalgae Chlorella sorokiniana and marine microalgae Tetraselmis sp. Pak J Biol Sci. 2012 Dec;15(24):1182–1186. doi: 10.3923/pjbs.2012.1182.1186. [DOI] [PubMed] [Google Scholar]
- [60].Li J, Zhu D, Niu J, et al. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol Adv. 2011 Nov-Dec;29(6):568–574. doi: 10.1016/j.biotechadv.2011.04.001. [DOI] [PubMed] [Google Scholar]
- [61].Rosch C, Rossmann M, Weickert S. Microalgae for integrated food and fuel production. Glob Change Biol Bioenergy. 2019 Jan;11(1):326–334. 2019. [Google Scholar]
- [62].Akbari F, Eskandani M, Khosroushahi AY. The potential of transgenic green microalgae; a robust photobioreactor to produce recombinant therapeutic proteins. World J Microbiol Biotechnol. 2014 Nov;30(11):2783–2796. doi: 10.1007/s11274-014-1714-0. [DOI] [PubMed] [Google Scholar]
- [63].Kirilovsky D. hotoprotection in cyanobacteria: the orange carotenoid protein (OCP)-related non-photochemical-quenching mechanism. Photosynth Res. 2007 Jul;93(13):7–16. doi: 10.1007/s11120-007-9168-y. [DOI] [PubMed] [Google Scholar]
- [64].Thurotte A, Lopez-Igual R, Wilson A, et al. Regulation of orange carotenoid protein activity in cyanobacterial photoprotection. Plant Physiol. 2015 Sep;169(1):737–747. doi: 10.1104/pp.15.00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Niyogi KK, Bjorkman O, Grossman AR. The roles of specific xanthophylls in photoprotection. Proc Natl Acad Sci U S A. 1997 Dec;94(25):14162–14167. doi: 10.1073/pnas.94.25.14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kawasaki S, Mizuguchi K, Sato M, et al. A novel astaxanthin-binding photooxidative stress-inducible aqueous carotenoprotein from a eukaryotic microalga isolated from asphalt in midsummer. Plant Cell Physiol. 2013 Jul;54(7):1027–1040. doi: 10.1093/pcp/pct080. [DOI] [PubMed] [Google Scholar]
- [67].Dominguez-Martin MA, Hammel M, Gupta S, et al. Structural analysis of a new carotenoid-binding protein: the C-terminal domain homolog of the OCP. Sci Rep. 2020;10(1):15564. doi: 10.1038/s41598-020-72383-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Dominguez-Martin MA, Kerfeld CA. Engineering the orange carotenoid protein for applications in synthetic biology. Curr Opin Struct Biol. 2019 Aug;57:110–117. doi: 10.1016/j.sbi.2019.01.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.