Abstract
A common denominator of sexual reproduction in many eukaryotic species is the exposure of an egg to excess sperm to maximize the chances of reproductive success. To avoid potential harmful or deleterious consequences of supernumerary sperm fusion to a single female gamete (polyspermy), many eukaryotes, including plants, have evolved barriers preventing polyspermy. Typically, these checkpoints are implemented at different stages in the reproduction process. The virtual absence of unambiguous reports of naturally occurring egg cell polyspermy in flowering plants is likely reflecting the success of this multiphasic strategy and highlights the difficulty to trace this presumably rare event. We here focus on potential polyspermy avoidance mechanisms in plants and discuss them in light of analogous processes in animals.
Introduction
The mechanisms that bring together male and female gametes are key to reproductive success and plants ability to evolve novel mating strategies has substantially shaped our planet. The evolution of pollen as a sperm transporting system is considered to be one of the most important evolutionary steps taken by land plants [1,2]. Pollen grains have a remarkable morphogenetic capacity: Once they land on a compatible stigmatic surface, they germinate and form tip-growing tubes targeting the ovule. Growth and guidance of the pollen tubes is mediated by several signaling pathways, which have comprehensively been reviewed elsewhere [3• ,4,5•• ,6,7•• ]. The pollen tube typically contains one pair of sperm cells that are linked with a thin cytoplasmic bridge and delivered towards the so-called receptive synergid [8,9]. There are several important implications associated with the number of male gametes delivered. First, flowering plants are characterized by double fertilization, i.e. not only the egg cell but also the adjacent central cell, which is the precursor of the endosperm, gets fertilized by one sperm. The paired delivery of sperm cells enables an almost synchronized fertilization of both female gametes [10], and a coordinated start of egg and central cell into seed development. This is particularly important, as embryo and endosperm development can be initiated in both female gametes independently of one another, which is evidenced e.g. by fertilization experiments using cell-division defective, single sperm containing cdka;1 pollen [11,12].
Second, the delivery of two clonal sperm ensures maximum relatedness of embryo and its compatriot endosperm. Why is this relevant? It has been shown for maize and Arabidopsis, that egg and central cell can become fertilized by genetically different sperm released from two distinct pollen tubes, a process referred to as heterofertilization. In Arabidopsis, heterofertilization was demonstrated combining kokopelli mutant pollen, which contain sperm with reduced fertility thereby causing single fertilization events, with wildtype pollen [13]. With this elegant approach, Maruyama et al. were able to recover seeds with different paternal contribution to embryo and endosperm. The developmental consequences in the resulting Arabidopsis seeds have not yet been investigated. Notably, however, heterofertilization in maize negatively affects resource allocation to the embryo the less related the endosperm is [14]. Co-delivery of two genetically identical sperm in one pollen tube bypasses this kin-conflict.
Reproduction is key to the survival of species and many sexually reproducing eukaryotes respond to this challenge by exposing the egg cell to supernumerary sperm. Notably, the pollen tube of flowering plants typically does not contain more than the required two sperm. This is particularly relevant in the light of polyspermy, which is the fusion of supernumerary sperm with one female gamete.
The conundrum of plant polyspermy
While excess male gametes increase the likelihood of an egg becoming fertilized, an increased sperm pressure enhances the risk of polyspermy, which is lethal in many organisms. Consequently, eukaryotes have evolved mechanisms to prevent fertilization of a single egg by more than one sperm [15]. The respective strategies can be of chemical, physical or electrical nature and are implemented at different levels in the reproductive process [16]. The concept of polyspermy in plants has long been ambiguous: Early observations that were interpreted in the light of polyspermy date back to 1936 when Rhoades identified triploid maize plants, which obviously had inherited two paternal copies [17]. While supernumerary gamete fusion is, indeed, one possible explanation, triploid plants can in theory also result from fusion of an egg with unreduced diploid sperm. This is suggested by crosses between diploid and tetraploid maize, which yield up to 2% fertile seeds [18]. Similarly, the role of polyspermy in the polyploidization of common wheat has been investigated. Crosses between hexaploid plants infrequently generate nonaploid offspring with karyotypes compatible with supernumerary sperm fusion. Nevertheless, it is difficult to conclude polyspermy as a causal agent since unreduced male gamete formation via second meiotic division restitution has the potential to generate polyploids with similar karyotype [19].
Other reports reveal contemporary differences in the convention of using the term polyspermy. Today, polyspermy is commonly used to describe the fusion of more than one sperm with the female gamete [20]. By contrast, in 1970 Vigfússon in his work entitled of ‘polyspermy in sunflower’, refers to polyspermy when describing the release of multiple pollen tubes content in the embryo sac [21].
When investigating the fertilization process in orchids, Hagerup [22] observed polyspermy as infrequent phenomenon. Based on cytological examination of ovules after pollination, he was able to show a single incidence of in vivo gamete attachment and/or plasmogamy involving two sperm cells and an egg cell. It remains unclear, however, whether these sperm nuclei also undergo karyogamy with the egg nucleus and what the fate of this atypical fertilization event is.
In a recent work, Toda et al. [23•• ] made use of isolated egg cells to induce polyspermy by electrical fusion followed by in vitro regeneration of triploid plants. While this artificial setup uses external force to combine the two gametes, it is in support of the hypothesis that polyspermy is not a lethal event in plants [20], which was originally proposed on the basis of the finding that crosses between diploid and tetraploid Arabidopsis plants, yield viable offspring [24,25].
Still, due to the lack of tools to unambiguously identify and trace polyspermy in natural systems, the concept of polyspermy in the egg cell of flowering plants has remained hypothetical. Furthermore, one of the reasons that polyspermy is poorly understood in flowering plants is its rare occurrence, which is likely due to the evolution of polyspermy preventing barriers [20].
Polyspermy blocks and potential polyspermy barriers in flowering plants
A common denominator, which is key to the establishment of a permanent physical block in animal egg cells is the release of Ca2+ from internal stores. Notably, fertilization-dependent changes in calcium homeostasis have also been observed in plants: The literally explosive character of pollen tube discharge deforms egg and central cell and activates characteristic calcium signatures in both female gametes [26•• ,27•• ,28•• ]. These initial calcium peaks might represent the activation of the female gametes by the sperm component [29]. In return the egg cell also activates the sperm cell by secreting EC1 protein, which enables the translocation of sperm specific gamete fusion protein from endomembrane to the cell surface [30]. Interestingly a second peak for cytosolic Ca2+ concentration is observed only in the egg cell (Figure 1a), which is essentially attributed to the gamete fusion event [26•• ,27•• ,31]. One notable difference between the first and second Ca2+ signatures in the egg cell is the pattern of its distribution. During the first peak, it occurs in the entire egg cell, however, the concentration of the second peak starts from the chalazal region of the egg cell and moves towards the whole cell lasting for almost 5 min [27•• ]. Similarly, in vitro fertilization experiments in maize show Ca2+ influx induction after egg-sperm fusion, which transduced like a wavefront from the fusion site to the entire zygote [32,33]. The precise sequence of events has been investigated by several labs independently and has been comprehensively reviewed [34• ,35• ]. In animal oocytes, Ca2+ induces the release of cortical granules, which cause an irreversible biochemical renovation of the extracellular matrix, which transforms from a network of glycoproteins into a stiff shell [36] (Figure 1f, g). Similarly, gamete fusion in the intertidal brown algae Fucus has been shown to be associated with Golgi complex hypertrophy, an accumulation of fibrillar material in large vesicles, and, eventually, the formation of a cell wall, which is absent from unfertilized eggs [37]. Also, maize in vitro fertilization experiments recorded gamete fusion dependent cell wall formation, evidenced by calcofluor white staining [33], suggesting that also flowering plant egg cells mount a physical polyspermy block. Notably, Antoine et al. were able to show that the requirement of gamete fusion for cell wall formation can be bypassed by Ca2+ ionophore induced Ca2+ influx, highlighting the importance of this early egg cell response in maize [33]. Based on these observations, it is conceivable that Ca2+ signaling mediates the renovation of the egg’s extracellular matrix by inducing cell wall formation there-by establishing a physical block to polyspermy.
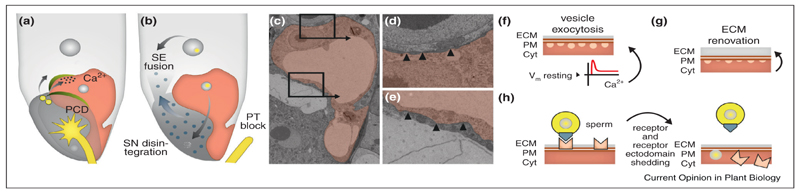
Figure 1. Potential direct and indirect polyspermy avoidance mechanisms.
(a-b) Landmarks in Arabidopsis fertilization process after pollen tube reception. (a) Early fertilization process depicted with male–female gametic cell interaction provoking uneven Ca2+ transient within the egg cell. Actin coronas are depicted in green. Male components are colored yellow. Female cell nuclei are depicted with grey color. (b) The disintegration of second synergid cell is necessary for the establishment of a pollen tube block: Fertilization of the central cell promotes cell fusion between the synergid cell and endosperm causing dilution of pollen tube attractant (blue circles) while fertilization of the egg cell promotes disintegration of the synergid nucleus (grey). (c-e) Transmission electron microscopy visualization of Arabidopsis egg cell revealing ultrastructural cell wall differences between the central cell adjoining side (d) to that of synergid adjoining side (e). Presence of thin furrows in between the thick cell wall structures (arrow head) in the central cell adjoining side of the egg cell. The cell wall structure along the synergid side is uniform. (f-h) Hypothetical mode of polyspermy avoidance mechanisms in flowering plants. (f) The electrical block represented by the changes of resting membrane potential along with Ca2+ changes initiate the exocytosis of materials, which can cause a biochemical renovation of the egg’s ECM (g) and/or shedding of receptor or receptor ectodomain (h). PCD, programmed cell death; SN, synergid nucleus; SE, synergid endosperm; PT, polytubey; ECM, extra cellular matrix; PM, plasma membrane; Cyt, cytoplasm.
In many eukaryotic taxa, the permanent physical block to polyspermy is preceded by a faster membrane block.
In the case of electrical blocks, currents of monovalent ions can result in membrane depolarisation, as observed in echinoderms, or membrane hyperpolarisation, which has been described for decapods [15] (Figure 1f). The changes in the resting potential render the plasma membrane refractory to further sperm entry and bridge the gap until the permanent physical block has been established. In Fucus and Pelvetia, a sodium-dependent membrane potential was shown to critically affect the fusion competence of the egg cell [38], suggesting that also here a fast block to polyspermy is operating.
In addition, research on different eukaryotic taxa has suggested that the membrane block to polyspermy operates through deactivation of the gamete recognition machinery (Figure 1h): It was recently shown that, in mice, the binding of the sperm cell surface protein IZUMO to the egg membrane folate receptor JUNO is required for gamete fusion both in vivo and in vitro [39•• ]. Notably, JUNO disappears from the egg surface 30–45 min after fertilization, which correlates with the time necessary for a mammalian membrane block to become established. Similarly, in the green algae Clamydomonas, the membrane proteins FUS1 and HAP2 are present on plus and minus gametes, respectively. Both proteins are essential for gamete fusion and are rapidly degraded after fertilization, which is likely to render the gametes resistant to further fusion events [40]. An intermediate block of unknown mechanism, that causes additional sperm to detach from the egg soon after sperm fusion, has also been described in fucoid algae [37,38].
While there is some evidence for a polyspermy block mounted in flowering plant egg cells, the situation is less clear regarding the egg cell-adjoining central cell, which in angiosperms is also fertilized to generate the embryonourishing tissue (endosperm). Work by Scott et al. has suggested that an increased sperm pressure can result in central cell polyspermy. The group made use of tetraspore (tes) mutants, which fail to physically separate the four microspores giving rise to coenocytic pollen, which contain a range of sperm numbers and ploidy levels. While pollination with tes pollen did not introduce supernumerary genomic copies to the embryo, the group observed endosperm ploidies that are only compatible with multiple fertilization events [41]. On the other side, Hamamura et al. studied the dynamics of sperm fusion during fertilization making use of live-cell imaging. Their results nicely document that the two functionally equivalent Arabidopsis sperm cells faithfully and almost synchronously fertilize the egg and the central cell, suggesting the presence of a mechanism that prevents the fusion of both sperm with either of the female gamete [10,42].
Potential sperm pressure regulating mechanisms
Formally, all mechanisms that influence sperm number and sperm access to female gametes do have the potential to serve as polyspermy barriers. In internally fertilized animals, the distinct segments of the female reproductive tract impose chemical and physical barriers that strongly reduce the amount of sperm arriving at the ova [43,44]. In addition, many organisms reduce the sperm-receptive area of the egg: in amphibian oocytes, sperm fusion occurs preferentially in the animal hemisphere, and the frog genus Discoglossus even restricts sperm fusion to a single depression at the animal pole [15]. Some animals, like the oviparous salmon, generate a funnel-shaped micropyle, which serves as the sole entry point for the sperm [45]. By contrast, local restrictions to sperm fusion are uncommon in broadcast spawners, which reduce sperm-egg encounters by diluting their gametes in the ocean. Sperm access to the plasma membrane of the oocyte is additionally restricted by the egg’s extracellular matrix (ECM), which, apart from its role in sperm activation, serves to mechanically dilute the sperm population [15]. Interestingly, it has been reported for some plants that the central cell-adjoining egg cell wall differs from the synergid-adjoining egg cell wall in being thicker and slightly indented [46]. Ultrastructural analysis on Arabidopsis egg cells confirm position-dependent cell wall differences in Arabidopsis (Figure 1c–e) with particularly pronounced periodic changes in cell wall thickness at the central cell-adjoining site of the egg cell. Future work will help to elucidate whether the indentations predict the sites of sperm entry and how the cell wall apposition pattern is affected by fertilization. In addition, it is conceivable that the regulation of sperm transport can affect polyspermy. It has been speculated that two actin ‘coronas’ contribute to sperm cell guidance within the female gametophyte. These cytoskeletal structures form after sperm reception, one at the chalazal end of the degenerated synergid and a second at the interface between egg cell and central cell, and disappear soon after fertilization [47,48,49,50]. However, the explosive rupture of the pollen tube appears sufficient to propel the two sperm cells to the fusion site [10]. Nevertheless, mechanisms must exist to selectively and reliably associate the two sperm with egg and central cell and it is conceivable that final micro movements, which have been reported to reposition the two sperm cells prior to plasmogamy [51•• ], are mediated by a gamete-associated actin-based matrix.
Unlike animals, which are equipped with centrosomes and employ microtubules after plasmogamy to direct the female pronucleus towards the male pronucleus [52], flowering plants lack centrosomes and involve their actin cytoskeleton for the completion of fertilization [20]. Recently, Kawashima et al. have investigated the role of F-actin dynamics within female gametes making use of a semi-dominant ACTIN variant, which disrupts F-actin. With this elegant approach the authors could show that an intact F-actin network is required for gamete nuclei migration and successful karyogamy [53•• ,54•• ].
Notably, plants have evolved a pollen tube block, which, in theory, has the potential to further restrict the sperm number arriving at the female gametes. Key to the short-range attraction of pollen tubes are synergids, which generate cysteine-rich peptides necessary and sufficient for pollen tube attraction [55,56,57,58]. Moreover, synergids mediate the discharge of the sperm from the first pollen tube that arrives [59,60,61,62]. This pollen tube reception is accompanied by programmed cell death (PCD) of the first synergid [63] (Figure 1a). Notably, disintegration of the second synergid, and the concomitant termination of pollen tube attraction require double fertilization. This is evidenced by several studies, which have shown that incomplete fertilization or the delivery of gamete fusion defective sperm suppresses disintegration of the second synergid, resulting in the attraction of supernumerary pollen tubes [30,64,65]. In addition, recent work has suggested an involvement of sporophytic tissue, which controls degeneration of the persistent synergid through the Arabinogalactan protein JAGGER [66•• ,67• ]. How is the elimination of the second synergid orchestrated with double fertilization? While sperm fusion with the egg cell appears to mainly trigger disintegration of the synergid nucleus, fertilization of the central cell induces fusion between the second synergid and endosperm [68•• ] (Figure 1b). The discovery of this unprecedented cellular fusion event provides an attractive explanation for the fertilization-associated pollen tube block, as the consumption of the synergid by the voluminous central cell causes rapid dilution of LURE protein which is essential for pollen tube attraction [68•• ]. The mechanisms that translate the fertilization signal into the elimination of the non-receptive synergid involve the Polycomb Repressive Complex 2 and an ethylene response cascade. This is evidenced by mutants in the respective pathways, which fail to induce complete non-receptive synergid disintegration and consequently attract supernumerary pollen tubes despite successful double fertilization [68•• ,69•• ,70]. Whether and to what extend a failure in the establishment of the pollen tube block might affect polyspermy frequencies is currently unclear.
Conclusion
The last two decades have seen a tremendous progress in our understanding of the processes governing plant fertilization, and molecular components involved in pollen tube attraction, pollen tube reception, gamete activation and gamete fusion have been identified. What has remained in the dark, however, is how plants avoid supernumerary gamete fusion to maintain their ploidy. The future development of tools to monitor the presumably rare event of multiple sperm fusion will allow to address the relative importance of individual potential polyspermy barriers and help to identify additional polyspermy relevant checkpoints implemented at the interface between male and female gametes or operating upstream of the discussed processes.
Acknowledgment
We gratefully acknowledge the financial support from the European Research Council (ERC Consolidator Grant “bi-BLOCK” ID. 646644 to R.G.).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Wodehouse RP. Evolution of pollen grains. Bot Rev. 1936;2:67–84. [Google Scholar]
- 2.Kenrick P, Wellman CH, Schneider H, Edgecombe GD. A timeline for terrestrialization: consequences for the carbon cycle in the Palaeozoic. Philos Trans R Soc B: Biol Sci. 2012;367:519–536. doi: 10.1098/rstb.2011.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bircheneder S, Dresselhaus T. Why cellular communication during plant reproduction is particularly mediated by CRP signalling. J Exp Bot. 2016 doi: 10.1093/jxb/erw271. [• In this opinion paper, the authors discuss the diverse role of CRPs in plant reproduction and pathogen fungi defense mechanisms.] [DOI] [PubMed] [Google Scholar]
- 4.Beale KM, Johnson MA. Speed dating, rejection, and finding the perfect mate: advice from flowering plants. Curr Opin Plant Biol. 2013;16:590–597. doi: 10.1016/j.pbi.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Higashiyama T, Takeuchi H. The mechanism and key molecules involved in pollen tube guidance. Annu Rev Plant Biol. 2015;66:393–413. doi: 10.1146/annurev-arplant-043014-115635. [•• This review paper discusses the preovular, synergid cell and male mechanisms governing pollen tube guidance.] [DOI] [PubMed] [Google Scholar]
- 6.Kessler SA, Grossniklaus U. She's the boss: signaling in pollen tube reception. Curr Opin Plant Biol. 2011;14:622–627. doi: 10.1016/j.pbi.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Kanaoka MM, Higashiyama T. Peptide signaling in pollen tube guidance. Curr Opin Plant Biol. 2015;28:127–136. doi: 10.1016/j.pbi.2015.10.006. [•• This review focuses on factors faciliatating and terminating micropylar pollen tube guidance on special emphasis on LURE peptide.] [DOI] [PubMed] [Google Scholar]
- 8.Dumas C, Knox RB, Gaude T. The spatial association of the sperm cells and vegetative nucleus in the pollen grain of Brassica. Protoplasma. 1985;124:168–174. [Google Scholar]
- 9.Kliwer I, Dresselhaus T. Establishment of the male germline and sperm cell movement during pollen germination and tube growth in maize. Plant Signal Behav. 2010;5:885–889. doi: 10.4161/psb.5.7.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamamura Y, Saito C, Awai C, Kurihara D, Miyawaki A, Nakagawa T, Kanaoka Masahiro M, Sasaki N, Nakano A, Berger F, et al. Live-cell imaging reveals the dynamics of two sperm cells during double fertilization in Arabidopsis thaliana. Curr Biol. 2011;21:497–502. doi: 10.1016/j.cub.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Iwakawa H, Shinmyo A, Sekine M. Arabidopsis CDKA;1, a cdc2 homologue, controls proliferation of generative cells in male gametogenesis. Plant J. 2006;45:819–831. doi: 10.1111/j.1365-313X.2005.02643.x. [DOI] [PubMed] [Google Scholar]
- 12.Aw SJ, Hamamura Y, Chen Z, Schnittger A, Berger F. Sperm entry is sufficient to trigger division of the central cell but the paternal genome is required for endosperm development in Arabidopsis. Development. 2010;137:2683–2690. doi: 10.1242/dev.052928. [DOI] [PubMed] [Google Scholar]
- 13.Maruyama D, Hamamura Y, Takeuchi H, Susaki D, Nishimaki M, Kurihara D, Kasahara Ryushiro D, Higashiyama T. Independent control by each female gamete prevents the attraction of multiple pollen tubes. Develop Cell. 25:317–23. doi: 10.1016/j.devcel.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Wu C-C, Diggle PK, Friedman WE. Kin recognition within a seed and the effect of genetic relatedness of an endosperm to its compatriot embryo on maize seed development. Proc Natl Acad Sci. 2013;110:2217–2222. doi: 10.1073/pnas.1220885110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong JL, Wessel GM. Defending the zygote: search for the ancestral animal block to polyspermy. Curr Top Dev Biol. 2006;72:1–151. doi: 10.1016/S0070-2153(05)72001-9. [DOI] [PubMed] [Google Scholar]
- 16.Iwao Y. Egg activation in physiological polyspermy. Reproduction. 2012;144:11–22. doi: 10.1530/REP-12-0104. [DOI] [PubMed] [Google Scholar]
- 17.Rhoades MM. Note on the origin of triploidy in maize. J Genet. 1936;33:355–357. [Google Scholar]
- 18.Pennington PD, Costa LM, Gutierrez-Marcos JF, Greenland AJ, Dickinson HG. When genomes collide: aberrant seed development following maize interploidy crosses. Ann Bot. 2008;101:833–843. doi: 10.1093/aob/mcn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suarez EY, Lopez AG, Naranjo CA. Polyspermy versus unreduced male gametes as the origin of nonaploids (9x) common wheat plants. Caryologia. 1992;45:21–28. [Google Scholar]
- 20.Spielman M, Scott RJ. Polyspermy barriers in plants: from preventing to promoting fertilization. Sexual Plant Reprod. 2008;21:53–65. [Google Scholar]
- 21.VigfÚSson E. On polyspermy in the sunflower. Hereditas. 1970;64:1–52. [Google Scholar]
- 22.Hagerup O. The spontaneous formation of haploid, polyploid, and aneuploid embryos in some orchids. København: I komission hos Munksgaard; 1947. [Google Scholar]
- 23.Toda E, Ohnishi Y, Okamoto T. Development of polyspermic rice zygotes. Plant Physiol. 2016;171:206–214. doi: 10.1104/pp.15.01953. [•• In this study, the authors induced supernumerary sperm fusion in rice egg cells making use of in-vitro electrical fusion. On the basis of tissue culture, they were able to regenerate the resulting zygotes into triploid plants.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henry IM, Dilkes BP, Young K, Watson B, Wu H, Comai L. Aneuploidy and genetic variation in the Arabidopsis thaliana triploid response. Genetics. 2005;170:1979–1988. doi: 10.1534/genetics.104.037788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott RJ, Spielman M, Bailey J, Dickinson HG. Parent-of-origin effects on seed development in Arabidopsis thaliana. Development. 1998;125:3329–3341. doi: 10.1242/dev.125.17.3329. [DOI] [PubMed] [Google Scholar]
- 26.Denninger P, Bleckmann A, Lausser A, Vogler F, Ott T, Ehrhardt DW, Frommer WB, Sprunck S, Dresselhaus T, Grossmann G. Male-female communication triggers calcium signatures during fertilization in Arabidopsis. Nat Commun. 2014:5. doi: 10.1038/ncomms5645. [•• This study together with [27•• ,28•• ] demonstrates the Ca2+ dynamics upon pollen tube reception and gamete fusion.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamamura Y, Nishimaki M, Takeuchi H, Geitmann A, Kurihara D, Higashiyama T. Live imaging of calcium spikes during double fertilization in Arabidopsis. Nat Commun. 2014;5:4722. doi: 10.1038/ncomms5722. [•• This study together with [26•• ,28•• ] demonstrates the Ca2+ dynamics upon pollen tube reception and gamete fusion.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ngo QA, Vogler H, Lituiev DS, Nestorova A, Grossniklaus U. A calcium dialog mediated by the FERONIA signal transduction pathway controls plant sperm delivery. Dev Cell. 2014;29:491–500. doi: 10.1016/j.devcel.2014.04.008. [•• This study together with [26•• ,27•• ] demonstrates the Ca2+ dynamics upon pollen tube reception and gamete fusion.] [DOI] [PubMed] [Google Scholar]
- 29.Han Y-Z, Huang B-Q, Guo F-L, Zee S-Y, Gu H-K. Sperm extract and inositol 1,4,5-triphosphate induce cytosolic calcium rise in the central cell of Torenia fournieri. Sexual Plant Reproduction. 2002;15:187–193. [Google Scholar]
- 30.Sprunck S, Rademacher S, Vogler F, Gheyselinck J, Grossniklaus U, Dresselhaus T. Egg cell-secreted EC1 triggers sperm cell activation during double fertilization. Science. 2012;338:1093–1097. doi: 10.1126/science.1223944. [DOI] [PubMed] [Google Scholar]
- 31.Antoine A-F, Faure J-E, Dumas C, Feijo JA. Differential contribution of cytoplasmic Ca2+ and Ca2+ influx to gamete fusion and egg activation in maize. Nat Cell Biol. 2001;3:1120–1123. doi: 10.1038/ncb1201-1120. [DOI] [PubMed] [Google Scholar]
- 32.Digonnet C, Aldon D, Leduc N, Dumas C, Rougier M. First evidence of a calcium transient in flowering plants at fertilization. Development. 1997;124:2867–2874. doi: 10.1242/dev.124.15.2867. [DOI] [PubMed] [Google Scholar]
- 33.Antoine AF, Faure J-E, Cordeiro S, Dumas C, Rougier M, Feijé JA. A calcium influx is triggered and propagates in the zygote as a wavefront during in vitro fertilization of flowering plants. Proc Natl Acad Sci. 2000;97:10643–10648. doi: 10.1073/pnas.180243697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Gutjahr C, Bleckmann A, Dresselhaus T. Calcium signaling during reproduction and biotrophic fungal interactions in plants. Mol Plant. 2015;8:595–611. doi: 10.1016/j.molp.2015.01.023. [• This review provides a comprehensive overview regarding calcium dynamics in tip-growing structures.] [DOI] [PubMed] [Google Scholar]
- 35.Dresselhaus T, Sprunck S, Wessel GM. Fertilization mechanisms in flowering plants. Curr Biol. 2016;26:R125–R139. doi: 10.1016/j.cub.2015.12.032. [• In this review paper, the authors discuss fertilization mechanisms in flowering plants focusing on the role of calcium during plant reproduction.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papi M, Brunelli R, Sylla L, Parasassi T, Monaci M, Maulucci G, Missori M, Arcovito G, Ursini F, De Spirito M. Mechanical properties of zona pellucida hardening. Eur Biophys J. 2010;39:987–992. doi: 10.1007/s00249-009-0468-3. [DOI] [PubMed] [Google Scholar]
- 37.Brawley SH, Wetherbee R, Quatrano RS. Fine-structural studies of the gametes and embryo of Fucus vesiculosus L. (Phaeophyta). II. The cytoplasm of the egg and young zygote. J Cell Sci. 1976;20:255–271. doi: 10.1242/jcs.20.2.255. [DOI] [PubMed] [Google Scholar]
- 38.Brawley SH. The fast block against polyspermy in fucoid algae is an electrical block. Dev Biol. 1991;144:94–106. doi: 10.1016/0012-1606(91)90482-i. [DOI] [PubMed] [Google Scholar]
- 39.Bianchi E, Doe B, Goulding D, Wright GJ. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature. 2014;508:483–487. doi: 10.1038/nature13203. [•• In this study, the egg specific receptor for IZUMO refered to as JUNO is identified using a protein-protein interaction approach. The study demonstrated the importance of receptor shedding after fertilizaiton to circumvent polyspermy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Misamore MJ, Snell WJ. Membrane fusion triggers rapid degradation of two gamete-specific, fusion-essential proteins in a membrane block to polygamy in Chlamydomonas. Development (Cambridge, England) 2010;137:1473–1480. doi: 10.1242/dev.044743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott RJ, Armstrong SJ, Doughty J, Spielman M. Double fertilization in Arabidopsis thaliana involves a polyspermy block on the egg but not the central cell. Mol Plant. 2008;1:611–619. doi: 10.1093/mp/ssn016. [DOI] [PubMed] [Google Scholar]
- 42.Hamamura Y, Nagahara S, Higashiyama T. Double fertilization on the move. Curr Opin Plant Biol. 2012;15:70–77. doi: 10.1016/j.pbi.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Coy P, Aviles M. What controls polyspermy in mammals, the oviduct or the oocyte? Biol Rev Camb Philos Soc. 2010;85:593–605. doi: 10.1111/j.1469-185X.2009.00117.x. [DOI] [PubMed] [Google Scholar]
- 44.Dale B, DeFelice L. Polyspermy prevention: facts and artifacts? J Assist Reprod Genet. 2011;28:199–207. doi: 10.1007/s10815-010-9513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.M K Blocks to polyspermy in fish: a brief review. Proceedings of the Thirty-second US Japan Symposium on Aquaculture Panel Symposium. Davis and Santa Barbara; California USA: 2003. Aquaculture and pathobiology of crustacean and other species. [Google Scholar]
- 46.Mogensen HL, Suthar HK. Ultrastructure of the egg apparatus of Nicotiana tabacum (Solanaceae) before and after fertilization. Botanical Gazette. 1979;140:168–179. [Google Scholar]
- 47.Huang BQ, Pierson ES, Russell SD, Tiezzi A, Cresti M. Cytoskeletal organisation and modification during pollen tube arrival, gamete delivery and fertilisation in Plumbago zeylanica. Zygote. 1993;1:143–154. doi: 10.1017/s0967199400001404. [DOI] [PubMed] [Google Scholar]
- 48.Huang BQ, Fu Y, Zee SY, Hepler PK. Three-dimensional organization and dynamic changes of the actin cytoskeleton in embryo sacs of Zea mays and Torenia fournieri. Protoplasma. 1999;209:105–119. doi: 10.1007/BF01415706. [DOI] [PubMed] [Google Scholar]
- 49.Fu Y, Yuan M, Huang B-Q, Yang H-Y, Zee S-Y, O'Brien PT. Changes in actin organization in the living egg apparatus of Torenia fournieri during fertilization. Sexual Plant Reprod. 2000;12:315–322. [Google Scholar]
- 50.Huang B-Q, Russell SD. Fertilization in Nicotiana tabacum: cytoskeletal modifications in the embryo sac during synergid degeneration. Planta. 1994;194:200–214. [Google Scholar]
- 51.Huang J, Ju Y, Wang X, Zhang Q. Sodmergen: A one-step rectification of sperm cell targeting ensures the success of double fertilization. J Integr Plant Biol. 2015;57:496–503. doi: 10.1111/jipb.12322. [•• This paper reports on monitors inner embryo sac dynamics with respect to sperm positions prior to gamete fusion.] [DOI] [PubMed] [Google Scholar]
- 52.Reinsch S, Gonczy P. Mechanisms of nuclear positioning. J Cell Sci. 1998;111(Pt 16):2283–2295. doi: 10.1242/jcs.111.16.2283. [DOI] [PubMed] [Google Scholar]
- 53.Kawashima T, Berger F. The central cell nuclear position at the micropylar end is maintained by the balance of F-actin dynamics, but dispensable for karyogamy in Arabidopsis. Plant Reprod. 2015;28:103–110. doi: 10.1007/s00497-015-0259-1. [•• This study shows the importance of F-actin cables for the positioning of the central cell nucleus and karyogamy.] [DOI] [PubMed] [Google Scholar]
- 54.Kawashima T, Maruyama D, Shagirov M, Li J, Hamamura Y, Yelagandula R, Toyama Y, Berger F. Dynamic F-actin movement is essential for fertilization in Arabidopsis thaliana. eLife. 2014;3:e04501. doi: 10.7554/eLife.04501. [•• This study shows the role of Rho-GTPase in regulating F-actin dynamics during the process of sperm nucleus movement towards the female gamete.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Higashiyama T, Yabe S, Sasaki N, Nishimura Y, Miyagishima S, Kuroiwa H, Kuroiwa T. Pollen tube attraction by the synergid cell. Science. 2001;293:1480–1483. doi: 10.1126/science.1062429. [DOI] [PubMed] [Google Scholar]
- 56.Okuda S, Tsutsui H, Shiina K, Sprunck S, Takeuchi H, Yui R, Kasahara RD, Hamamura Y, Mizukami A, Susaki D, et al. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature. 2009;458:357–361. doi: 10.1038/nature07882. [DOI] [PubMed] [Google Scholar]
- 57.Takeuchi H, Higashiyama T. A species-specific cluster of defensin-like genes encodes diffusible pollen tube attractants in Arabidopsis. PLoS Biol. 2012;10:e1001449. doi: 10.1371/journal.pbio.1001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marton ML, Cordts S, Broadhvest J, Dresselhaus T. Micropylar pollen tube guidance by egg apparatus 1 of maize. Science. 2005;307:573–576. doi: 10.1126/science.1104954. [DOI] [PubMed] [Google Scholar]
- 59.Amien S, Kliwer I, Marton ML, Debener T, Geiger D, Becker D, Dresselhaus T. Defensin-like ZmES4 mediates pollen tube burst in maize via opening of the potassium channel KZM1. PLoS Biol. 2010;8:e1000388. doi: 10.1371/journal.pbio.1000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huck N, Moore JM, Federer M, Grossniklaus U. The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development. 2003;130:2149–2159. doi: 10.1242/dev.00458. [DOI] [PubMed] [Google Scholar]
- 61.Rotman N, Rozier F, Boavida L, Dumas C, Berger F, Faure JE. Female control of male gamete delivery during fertilization in Arabidopsis thaliana. Curr Biol. 2003;13:432–436. doi: 10.1016/s0960-9822(03)00093-9. [DOI] [PubMed] [Google Scholar]
- 62.Escobar-Restrepo JM, Huck N, Kessler S, Gagliardini V, Gheyselinck J, Yang WC, Grossniklaus U. The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science. 2007;317:656–660. doi: 10.1126/science.1143562. [DOI] [PubMed] [Google Scholar]
- 63.Sandaklie-Nikolova L, Palanivelu R, King EJ, Copenhaver GP, Drews GN. Synergid cell death in Arabidopsis is triggered following direct interaction with the pollen tube. Plant Physiol. 2007;144:1753–1762. doi: 10.1104/pp.107.098236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beale KM, Leydon AR, Johnson MA. Gamete fusion is required to block multiple pollen tubes from entering an Arabidopsis ovule. Curr Biol. 2012;22:1090–1094. doi: 10.1016/j.cub.2012.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kasahara RD, Maruyama D, Hamamura Y, Sakakibara T, Twell D, Higashiyama T. Fertilization recovery after defective sperm cell release in Arabidopsis. Curr Biol. 2012;22:1084–1089. doi: 10.1016/j.cub.2012.03.069. [DOI] [PubMed] [Google Scholar]
- 66.Pereira Ana M, Nobre Margarida S, Pinto Sara C, Lopes Ana L, Costa Mario L, Masiero S, Coimbra S. Love Is Strong, and You're so Sweet'': JAGGER is essential for persistent synergid degeneration and polytubey block in Arabidopsis thaliana. Mol Plant. 2016;9:601–614. doi: 10.1016/j.molp.2016.01.002. [•• This study (together with 67) reveals a role of the sporophytic ovular tissue for the establishment of a pollen tube block mediated through an Arabinogalactan protein.] [DOI] [PubMed] [Google Scholar]
- 67.Pereira AM, Lopes AL, Coimbra S. JAGGER, an AGP essential for persistent synergid degeneration and polytubey block in Arabidopsis. Plant Signal Behav. 2016;11:e1209616. doi: 10.1080/15592324.2016.1209616. [• This study (together with 66) reveals a role of the sporophytic ovular tissue for the establishment of a pollen tube block mediated through an Arabinogalactan protein.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maruyama D, Volz R, Takeuchi H, Mori T, Igawa T, Kurihara D, Kawashima T, Ueda M, Ito M, Umeda M, et al. Rapid elimination of the persistent synergid through a cell fusion mechanism. Cell. 2015;161:907–918. doi: 10.1016/j.cell.2015.03.018. [•• This study demonstrated the mechanism behind the second synergid degeneration. It provide evidences that after central cell fertilization, the second synergid cell fuses with the endosperm leading to its disintegration. Simulteneously, fertilization of the egg cell activates ethylene signaling pathway which is essential for the disorganization of the second synergid nucleus.] [DOI] [PubMed] [Google Scholar]
- 69.Motomura K, Berger F, Kawashima T, Kinoshita T, Higashiyama T, Maruyama D. Fertilization-independent cell-fusion between the synergid and central cell in the polycomb mutant. Cell Struct Funct. 2016 doi: 10.1247/csf.16010. [•• This paper suggests that the synergid-endosperm fusion reported in [68••] is repressed in a gamete fusion dependent manner by the polycomb complex.] [DOI] [PubMed] [Google Scholar]
- 70.Volz R, Heydlauff J, Ripper D, von Lyncker L, Groß-Hardt R. Ethylene signaling is required for synergid degeneration and the establishment of a pollen tube block. Develop Cell. 2013;25:310–316. doi: 10.1016/j.devcel.2013.04.001. [DOI] [PubMed] [Google Scholar]