Summary
Understanding how to modulate appetite in humans is key to developing successful weight loss interventions. Here, we show that postprandial glucose dips 2-3h after a meal are a better predictor of postprandial self-reported hunger and subsequent energy intake than peak glucose 0-2h and glucose iAUC 0-2h. We explore the link between postprandial glucose, appetite, and subsequent energy intake in 1070 participants from a UK discovery and US validation cohort, consuming 8,624 standardised meals followed by 71,715 ad libitum meals, using continuous glucose monitors to record postprandial glycemia. For participants eating each of the standardised meals, the average postprandial glucose dip 2-3h relative to baseline level predicts an increase in hunger 2-3h (r=0.16 P=<0.001), shorter time until next meal (r=-0.14 P=<0.001), greater energy intake 3-4h (r=0.19 P=<0.001) and greater energy intake 24h (r=0.27 P<=0.001). Results aredirectionally consistent in the US validation cohort. These data provide a quantitative assessment of the relevance of postprandial glycemia in appetite and energy intake modulation.
Funding
Zoe Global Ltd, Wellcome Trust, NIHR.
Introduction
Obesity represents a major global health challenge, with its prevalence in adults and children increasing worldwide(1). Effective short term interventions have, for the most part, failed to translate into successful long-term healthy behaviour(2). Multiple mechanisms make intentional weight loss difficult and facilitate weight regain ((3),(4). In addition to decreases in resting energy expenditure (5) there is also considerable evidence for increases in postprandial appetite following hypocaloric diets and caloric restriction (e.g. (6),(7),(8)). Attenuating hunger after weight loss has been proposed as a strategy for preventing weight regain(9).
Appetite control is influenced by tonic and episodic signalling systems, formerly known as long-term and short-term control. In the last decade research has shown that processes associated with fat-free mass and resting metabolic rate represent tonic determinants of energy intake (10), confirming consistent outcomes from several studies (eg(11) for review). Equally importantly, research continues to befocussed on episodic processes inthe physiology of satiety with the goal of identifying key post-prandial events that influence the feeling of hunger after eating, and the occurrence and size of subsequent meals. Postprandial satiety is influenced by a series of physiological events following eating, including gastric distension, the release of gastrointestinal peptides and plasma metabolites. Prominent among these metabolites is plasma glucose, modulated by insulin, which has long been regarded as one of the most potentially interesting markers of postprandial satiety.
Animal models have shown that insulin regulates appetite by activating insulin receptors that increase the hypothalamic expression of appetite suppressing (anorexigenic) peptides,and acts on other neurons to inhibit the expression of appetite stimulating (orexigenic) peptides. In parallel, insulin and leptin act to decrease food intake through appetite suppression and changes in energy homeostasis(12). Investigation of glucose-insulin dynamics in some human studies (13,14) suggests that lower glycemic loads and lower glycemic responses can result in lower postprandial appetite and energy intake, particularly in select overweight populations(15).
The notion that blood glucose is involved in appetite expression was originally proposed by Mayer in his ‘glucostatic hypothesis’ (16). However, appetite research in the past decades has focused on seemingly more potent appetite signals such as leptin, GLP-1 and other peptides and their receptors. To date, the role of glucose in appetite expression is not resolved and has not been investigated in free-living conditions. In the current study we reprised Mayer’s idea and investigated the potential role of glucose in appetite control in a large-scale real-world environment. We hypothesize that glucose dynamics after a meal influence self-reported appetite, alertness and subsequent energy intake (calories). We have tested this by taking advantage of digital devices, specifically continuous glucose monitors, wearables, and mobile apps. In the largest such study to date we assessed postprandial glucose and appetite in 1,110 healthy adults from the UK and US without diabetes, following them for 2 weeks at home as they consumed both standardised breakfast and ad libitum meals. By using continuous glucose monitors and a series of standardised meals at breakfast and recording all ad libitum food intake it has been possible to estimate the effect of postprandial glycemia on postprandial appetite and energy intake.
Methods
Study Participants
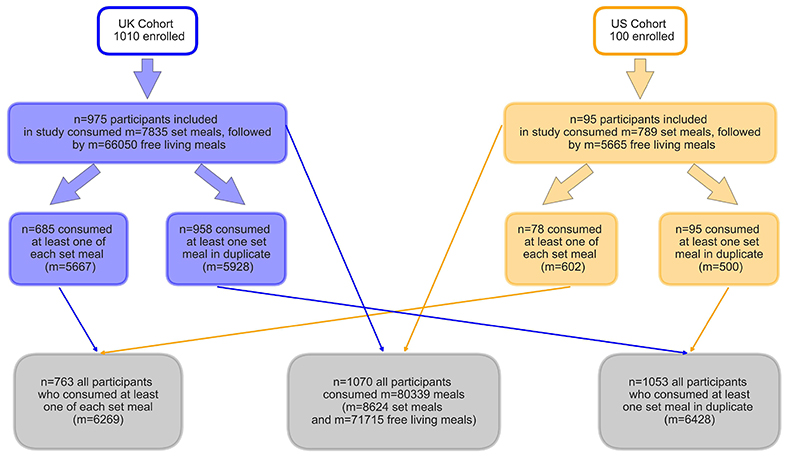
The PREDICT 1 Cohort 1 study(17) (Personalised REsponses to DIetary Composition Trial) was a two-centre study conducted between 2018 and 2019. The first participant was enrolled on 4 August 2018, the last clinical visit was completed on 24 April 2019, with the primary cohort based at King’s College London, UK, and a validation cohort (that underwent the same profiling as in the UK) assessed at Massachusetts General Hospital (MGH) in Boston, Massachusetts, USA. In the UK, participants (target enrolment = 1,000, total enrolled n=1010) were recruited from the TwinsUK cohort, a prospective cohort study and online advertising. In the US, participants (target enrolment = 100, total enrolled = 100) were recruited through online advertising, research participant databases and Rally for Research (https://rally.partners.org/), an online recruiting portal for research trials. Ethical approvals for the studies were obtained in the UK Ethics was granted by the London - Hampstead Research Ethics Committee (REC approval18/LO/0663) Research Ethics Committee and Integrated Research Application System (IRAS 236407) and by the Partners Healthcare Institutional Review Board (IRB 2018P002078). The informed consent and ethical committee approvals covered all analysis reported in the current study in addition to the key primary outcomes described in Berry et al (18). The trial is registered at ClinicalTrials.gov (registration number: NCT03479866) and was run in accordance with the Declaration of Helsinki and Good Clinical Practice. Descriptive characteristics of participants included in this substudy are shown in Supplementary Table 1.
Study Protocol
The full study protocol is described by Berry (17). In brief, the study consisted of two phases: a 1-day clinical baseline visit which included a breakfast and lunch challenge followed by a 13-day at-home study phase. For this study we focused only on the at home phase. During the at-home phase (days 2-14), participants consumed standardised breakfasts (including a repeat of the clinic breakfast and lunch meal, an OGTT and 6 isocaloric meals of which 5 were in duplicate) as described in the protocol (17), varying in macronutrient composition. Analyses of most primary outcomes have been described in (18). Secondary analyses on hunger and postprandial responses after 2-hours are reported here.
Participants were asked to fast for 3 hours following the standardised breakfast meals,and were then free to eat ad libitum. In later phases of the study, some participants were asked to consume meals with different fasting periods; these meals are excluded from the present analysis. Details of the meals included in the analysis are given in Supplementary Table 2. During the assessment period, participants wore continuous glucose monitors (CGM) and accelerometers to assess physical activity and sleep.
Zoe study app and dietary assessment
The Zoe study app prompted participants to report their hunger and alertness levels on visual analogue scales (from 0 to 10) truncated from Flint et al (19)], by displaying the questions “how hungry are you?” and “how alert are you?” above the scales, at 0 minutes (time of logging) and regular intervals thereafter following the logging of a standardised meal.. Participants were supported throughout the study with reminders and communication from study staff through a mobile application (Zoe study app).
Any dietary intake during the study, including test meals, ad libitum meals and accompanying drinks, was recorded in the Zoe study app by participants with the exact time at consumption and ingredient quantities, so that compliance could be monitored by study staff. Only test meals that were completed according to instructions were included in analyses.
QC of the app data: Inclusion criteria for meals
Meals were included in the analysis if they met certain characteristics. Listed below are the exclusions applied to the overall PREDICT 1 Study dataset for the current analysis:
1090 participants completed 9340 standardised meals, of which; 9078 meals were scored as compliant with the study protocol; 8797 meals also had meal valid glucose readings, 8792 meals also occurred during days where the caloric intake was plausible (<6000 kcal), 8683 meals also were fasted until 175 minutes (within 10%, or +/- 100 kcal) and 8624 meals were also part of a daily calorie record that contained at least 5 dishes.
When considering the free-living meals, calorie records were adjusted to remove suspicious and incomplete values. Meal calories were substituted with the food category average calories when missing, or when the reported value was more than 2 standard deviations from the food category average. These adjustments did not affect the significance of the results. When calculating Time until next meal, only meals in excess of 50kcal were included in the analysis to avoid noise from tea, coffee and other non-sugar sweetened beverages.
For the energy intake in the 24 hours after a meal we used a window of time (-4 hours to +20 hours relative to the set meal time) because participants were instructed to eat the set breakfast immediately after waking, and there is some natural variation in waking times. This introduces the risk that the 24hr period following the set breakfast may include the following day’s set breakfast. By calculating the window from -4hr to +20hr, we minimise this issue.
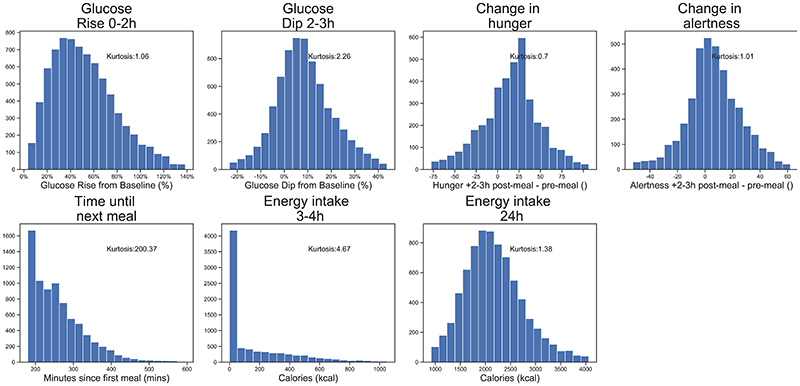
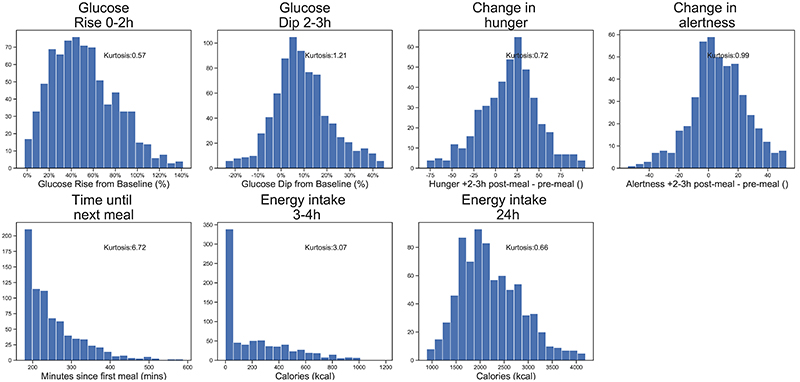
The distributions of all outcome traits are presented in Extended Data Figure 2 (UK cohort) and Extended Data Figure 3 (US cohort).
Data Analysis
From the overall PREDICT 1 dataset (n=1,110) a subset was selected for inclusion in this study (see QC of the app data for inclusion criteria), corresponding to the responses to the standardised breakfast meals taken at home (n=1,070, m=8,624) and the subsequent free-living meals consumed over the course of 24h (m=71,715). We denote the number of individuals who consumed the meal with ‘n’ and to the number of meals consumed with ‘m’.
From the overall set of meals included in the study, two subsets were analysed:
-
a)
Meals from participants who ate each of the types of standardised meals at least once (n=763, m=5667)
-
b)
Meals eaten in duplicate (n=1053, m=6428)
(see Statistical Analysis for power calculations for these two sets of data)
Meal Baseline calculations
The standard approach to the calculation of glycemic responses such as Glucose iAUC0-2h and Glucose Rise0-2h from clinic data is to consider the average glucose level in the 30 minutes before the start of the meal as the “meal baseline”. This baseline can itself therefore be a source of variability in Glucose responses, especially those immediately after waking. Between two repeats of the same meal for the same individual, there was a (r=0.76 p=<0.001) association between the meal baseline values.
We took advantage of the larger dataset provided by the Continuous Glucose monitors to establish a more stable “average baseline” for each individual. This was calculated as the average of the meal baselines for each of the meals included in the study for that individual. By taking a more stable baseline, we were able to improve the repeatability of the glycemic responses that were studied, and observe stronger associations between the Glucose Dip2-3h and the postprandial measures. This choice of baseline does not affect the direction or significance of the main results of this study. A comparison of the main results is presented in Supplementary Table 4
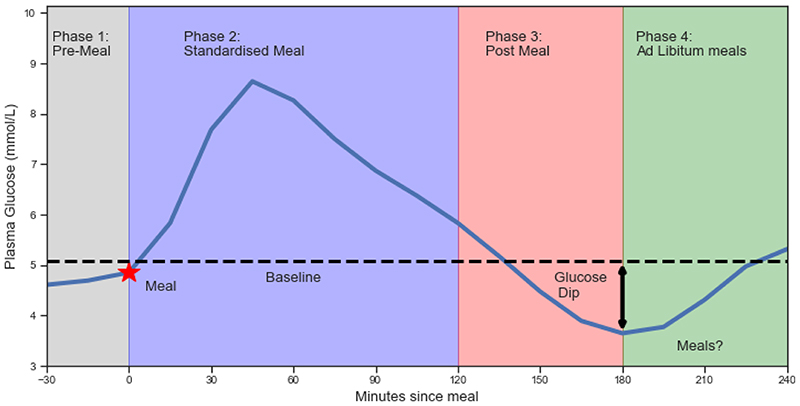
The phases of the study where measures were taken are illustrated in Figure 1 and are as follows:
Average Baseline Level: for each participant, the mean glucose concentration throughout the 30 minutes before each meal eaten in the study, measured across all meals in the study for that participant.
- Phase 1: Pre-Meal: The phase up to 30 minutes before the standardised meal starts. It is characterised by;
- pre-meal Hunger & Alertness: the level of hunger and alertness declared by the participant, in the period +/-5mins around the start of consumption of the meal. Readings are self-reported on a scale of 0-100. Larger values indicate increased perception of hunger and alertness. Hunger and Alertness was asked at 0, 30, 90,120 and 150 minutes after each standardised meal
- Phase 2: Standardised Meal: The phase from 0 to +2 hours is the conventional time period for measuring glucose response. It is characterised by:
- Glucose Rise0-2h: the maximum level above the baseline within the 2h period, as a percentage of the Average Baseline Level;
- GlucoseiAUC0-2h: the incremental area under the glucose curve, measured relative to the Average Baseline Level
- Phase 3: Post-Meal: The phase +2 hours to +3 hours after the meal. Participants who consumed additional food (except water) before the end of phase 3 (+3h hours, +/- 5 mins) were excluded from the analysis.
- Glucose Dip2-3hr: The difference between the lowest glucose reading in hours 2-3, and the Average Baseline Level, as a percentage of Average Baseline Level. The Glucose Dip2-3hr is expressed as a percentage in order to adjust for differences in participants’ baseline levels. Positive Glucose Dip2-3hr values indicate states of mild hypoglycemia, negative Glucose Dip2-3hr values indicate that blood glucose levels remain elevated above baseline level.
- Change In Hunger2-3hr: The difference between a participant’s pre-meal hunger and the average hunger reading in hours 2-3.
- Change In Alertness2-3hr: The difference between a participant’s pre-meal alertness and the average alertness reading in hours 2-3.
- Phase 4: Ad libitum meals: After 3 hours, participants record their food intake using a mobile app.
- Time until next meal: The number of minutes after consumption of the standardised meal that elapsed before the next meal of at least 50 kcal was consumed;
- Energy intake3-4h: The initial consumption is totalled over hours 3-4 including drinks;
Energy intake24h: The total daily consumption covers the full period from 4 hours before the standardised breakfast to 20 hours after/
Figure 1. Average glycemicresponses to standardised breakfasts, illustrating key measures used in study.
(n=1,070, m=8,624).Four phases were studied (see methods) namely: Phase 1: Pre-Meal: (up to 30 minutes before the standardised meal starts. Phase 2: Standardised Meal: (from 0 to +2 hours is the conventional time period for measuring glucose response) Phase 3: Post-Meal: The phase +2 hours to +3 hours after the meal. All participants were asked to fast up until 3 hours post-meal so no energy intake has taken place.Phase 4: Ad libitum meals: when participants were allowed to eat again. See methods for details
The key measures are summarized in Table 1. The Consort Diagram for number of participants and the types of meals are shown in Extended Data Figure 1.
Table 1. Responses to standardised meals (Exploration Cohort).
| Meal | Num | Glucose Rise 0-2h (%) | Glucose Dip 2-3h (%) | Change in hunger | Change in alertness | Time until next meal (mins) | Energy intake 3-4h (kcal) | Energy intake 24h (kcal) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| High Carb | 1,826 | 56% | 24% | 5% | 11% | -12 | 28 | 8 | 20 | 273 | 87 | 156 | 263 | 2189 | 614 |
| High Fat | 381 | 29% | 18% | 8% | 8% | -10 | 24 | 6 | 21 | 264 | 95 | 197 | 286 | 2267 | 696 |
| High Fibre | 886 | 50% | 23% | 6% | 10% | -18 | 26 | 8 | 20 | 277 | 89 | 134 | 243 | 2237 | 647 |
| High Protein | 1,069 | 27% | 17% | 4% | 9% | -16 | 27 | 7 | 20 | 272 | 134 | 163 | 244 | 2231 | 650 |
| OGTT | 1,808 | 77% | 31% | 19% | 17% | 9 | 28 | 3 | 25 | 241 | 85 | 269 | 283 | 2085 | 622 |
| UK Average | 1,865 | 45% | 22% | 6% | 10% | -11 | 26 | 7 | 21 | 272 | 79 | 131 | 220 | 2182 | 632 |
Statistical analyses
In order to test for associations between glucose parameters, hunger and energy intake, standard linear regression models were used. P<.05 was considered as nominally statistically significant, and error bars are shown throughout as 95% confidence intervals. All key measures were approximately normally distributed (γ2: 0.7 to 2.26), except time to next meal and energy intake 3-4h. Distributions are shown in Extended Data Figure 2. Linear regressions were adjusted for age, sex, BMI and weight. Data from questionnaires, clinical visits and laboratory data was entered using comma delimited files, Excel spreadsheets and Microsoft Access. CGM data was imported from text files into the analysis pipeline. Analyses were carried out in R 3.4.2. Core Team, Python 3.7, using Pandas 0.25.1, Scipy 1.3.1, Pingouin 0.3.3.
The key analyses included in this study are multiple linear regressions, with Pearson’s correlations coefficients and 95% CIs used to report effect sizes. Statistical power for the key analyses refer to (i) differences between individuals, for which the sample size is n=763 (see Data Analysis: Set (a), corresponding to the results described in Figure 2), and (ii) differences between m=6428 meals consumed in duplicate; for which the sample size is therefore m=3214 pairs of meals (see Data Analysis: Set(b), corresponding to the results described in Figure 3). The power for the analyses for (i) was carried out adjusting for 5 traits, namely change in hunger, change in alertness, time to next meal, energy intake in the 3 hours after the meal and energy intake in the 24 hours after the meal, and hence an alpha level of 0.01 was chosen. The necessary correlation effect size needed to achieve 80% power with p<0.01 for this sample size is r= 0.124. For the correlations for (ii) to achieve 80% power at the same alpha level the Pearson’s coefficient needs to be r=0.061 or higher.
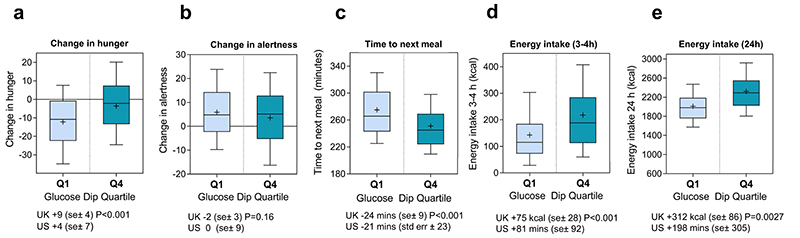
Figure 2. Postprandial measures by top and bottom quartiles of 2-3h Glucose Dip.
(n=763, m=5667 (UK n=685, m=5667, US n=78, m=602). Participants were divided into quartiles of their average Glucose Dip2-3h, following consumption of’ OGTT, High Carb, UK average, High Fat, and High Fibre standardised breakfasts (n=5667). The participants with the largest dips (Q4) are compared to those with the smallest dips (Q1), according to their Change in Hunger2-3h, Change in Alertness2-3h, Time until the next meal, ad libitum Energy Intake3-4h immediately after the end of the fasting period, and ad libitum Energy Intake24h.Boxplots showing median, means (indicated by a + sign) interquartile ranges and 90% confidence intervals are shown. P-values from two sided t-tests are reported. Sample sizes for each panel (a) change in hunger and (b) change in alertness Q1(n=161) Q4(n=162) (c) time in minutes until next meal (d) energy intake between 3 and 4 hours after the meal (e) energy intake in the 24 hours after the meal Q1(n=172) Q4(n=171).Error bars indicate 90% confidence intervals.
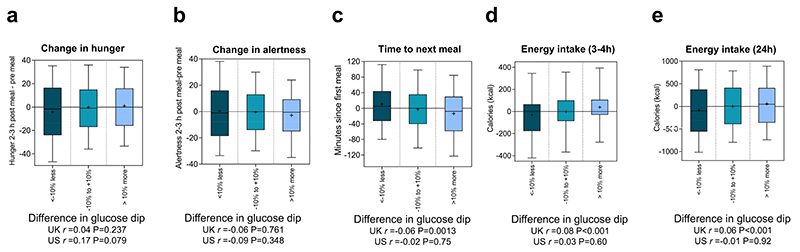
Figure 3. Differences in postprandial measures across repeated meals within individuals.
(n=1053, m=6428 (UK n=958; US n=95, m=500). Standardised breakfasts that were repeated on two occasions, following an overnight fast, separated by 2-5 days. The difference between the first and second repeat of each meal was calculated. We then measured the degree to which the difference in Glucose Dip2-3h was associated with the difference in each of the postprandial measures in the exploration and validation studies. For illustration, the results were grouped according to differences between the Glucose Dip2-3h recorded on the first and second repeat (<-10% less, -10% to +10%, >10% more). Number of pairs of meals foreach box are: for panels (a) and (b) <-10% less m= 177, -10% to +10% m=652, >10% more m=157; for panels (c) (d) and (e) <-10% less m= 501, -10% to +10% m=1960, >10% more m=503. Correlation coefficients (r) were calculated for m= 2964 and m=250 pairs of meals from the UK and US respectively. P-values from two sided tests are shown. Error bars indicate 90% confidence intervals.
Results
The descriptive characteristics of study participants, including their fasting levels of circulating glucose and insulin and their insulin secretion indices from the hospital day meal are presented in Supplementary Table 1. For each type of standardised meal, the mean and standard deviation of responses for the post-prandial measures are presented in Table 1 (data for the validation cohort is shown in Supplementary Table 2(b)). The macronutrient content of the standardised and free-living meals analysed is shown in Supplementary Table 3.
To assess the repeatability of self-reported measures of hunger, alertness, and energy intake, we explored the 6,428 standardised breakfasts that were eaten in duplicate on different days (see Extended Data Figure 1 for meal numbers). Each of the glycemic measures was statistically repeatable, though as expected the strength of correlations varied; Glucose Rise0-2h (r=0.73 P=<0.001; US: r=0.64 P=<0.001), Glucose Dip2-3h (r=0.58 P=<0.001; US: r=0.57 P=<0.001). Intraclass correlations (ICC) for these measures fell within the moderate to good range: Glucose Rise0-2h (ICC(2,1)=0.81; 95% CI [0.78, 0.83]; US: ICC(2,1)=0.78; 95% CI [0.69, 0.85]), Glucose Dip2-3h (ICC(2,1)=0.62; 95% CI [0.59, 0.66]; US: ICC(2,1)=0.68; 95% CI [0.56, 0.78]).
1). Glucose Dips2-3hpredict subsequent hunger and calorie intake
Participants were instructed to eat a range of standardised breakfasts, followed by a 3 hour fast. The post-prandial measures for those participants who consumed each of the 5 core meals (High Carb, High Fat, OGTT, UK Average, High Fibre) (n=763, m=5667; see Supplementary Table 2 for meals included) were averaged for each participant. We examined whether there was an association between glycemic responses - peak glucose rise in hours 0-2 (Glucose Rise0-2h), the glucose incremental area above baseline in hours 0-2 (iAUC0-2h), and the subsequent Glucose Dip2-3h - and postprandial measures of self-reported hunger, alertness, time until next meal, and subsequent energy intake.
Across the postprandial measures, we found a stronger correlation with Glucose Dips 2-3h than with Glucose Rise0-2h, or Glucose iAUC0-2h (Table 2). The Glucose Dip2-3h was statistically significantly associated with a change in Hunger2-3h (r=0.16 P=<0.001; US: r=0.12 P=0.315),,Time until next meal (r=-0.14 P=<0.001; US: r=-0.16 P=0.160), Energy intake3-4h (r=0.19 P=<0.001; US: r=0.24 P=0.032), Energy intake24h (r=0.27 P=<0.001; US: r=0.21 P=0.065). The association with change in Alertness2-3h (r=-0.04 P=0.313; US: r=0.02 P=0.875) was not significant. The Glucose Dip2-3h was as predictive of postprandial responses as the participant’s self-reported hunger.
Table 2. Associations between glucose responses and postprandial measures, between individuals.
| Glucose Rise 0-2h | Glucose iAUC 0-2h | Change in hunger | Glucose Dip 2-3h | |
|---|---|---|---|---|
| Glucose Dip 2-3h | r=0.11 P=0.003 (US: r=0.07 P=0.557) |
r=0.05 P=0.166 (US: r=-0.15 P=0.189) |
r=0.16 P=<0.001 (US: r=0.12 P=0.315) |
|
| Change in hunger | r=0.02 P=0.678 (US: r=0.07 P=0.540) |
r=0.02 P=0.701 (US: r=-0.05 P=0.647) |
r=0.16 P=<0.001 (US: r=0.12 P=0.315) |
|
| Change in alertness | r=0.04 P=0.304 (US: r=-0.07 P=0.571) |
r=0.05 P=0.169 (US: r=0.00 P=0.988) |
r=-0.03 P=0.416 (US: r=0.06 P=0.625) |
r=-0.04 P=0.313 (US: r=0.02 P=0.875) |
| Time until next meal | r=0.13 P=<0.001 (US: r=-0.09 P=0.413) |
r=0.11 P=0.005 (US: r=-0.11 P=0.319) |
r=-0.11 P=0.007 (US: r=-0.02 P=0.876) |
r=-0.14 P=<0.001 (US: r=-0.16 P=0.160) |
| Energy intake 3-4h | r=-0.16 P=<0.001 (US: r=-0.03 P=0.764) |
r=-0.08 P=0.036 (US: r=0.05 P=0.695) |
r=0.21 P=<0.001 (US: r=0.03 P=0.810) |
r=0.19 P=<0.001 (US: r=0.24 P=0.032) |
| Energy intake 24h | r=-0.08 P=0.036 (US: r=-0.02 P=0.835) |
r=-0.05 P=0.170 (US: r=0.05 P=0.677) |
r=0.18 P=<0.001 (US: r=0.10 P=0.375) |
r=0.27 P=<0.001 (US: r=0.21 P=0.065) |
Figure 2 illustrates the difference by dividing the participants into quartiles of Glucose Dip2-3h. The participants with the largest average Glucose Dip2-3h (Q4) also had on average a +9%; (95% CI 5,13) increase in reported Hunger2-3h, a -2% (95% CI 1,5) decrease in Alertness level2-3h, a -24 minutes (95% CI 15,33) shorter time until the next meal, a +75 kcal (95% CI 47,103) higher Energy intake3-4h, and a +312 kcal (95% CI 226,398) higher overall Energy intake24h, compared to the participants with the smallest Glucose Dip2-3h (Q1).
The associations between the Glucose Dips2-3h and the postprandial measures were strongest with the OGTT set meal (which also preceded the largest dips). For each of the set meal subgroups considered independently, the associations between the glucose dip and the postprandial measures were directionally consistent with the overall results and statistically significant, with the exception of (a) Change in Alertness2-3h,,which was not significantly associated at the subgroup level and (b) the High Carb muffin, where the glucose dip was not significantly associated with Energy intake3-4h (r=0.04, p=0.349).
Glucose Dips2-3h had modest and non-significant correlations with individual characteristics, such as age, weight and BMI - except for sex, where males (P=0.005) had slightly larger dips. Glucose Dips2-3h were associated with lower fasting levels of Insulin and C-Peptide, as measured in clinic; Plasma Glucose (r=-0.05 P=0.186; US: r=-0.12 P=0.321), Insulin (r=-0.10 P=0.008; US: r=0.02 P=0.883), C-Peptide (r=-0.14 P=<0.001; US: r=-0.11 P=0.357). Glucose dips2-3h were associated with the Glucose Rise0-2 h, but not with Glucose iAUC0-2 ; Glucose iAUC0-2h (r=0.05 P=0.166; US: r=-0.15 P=0.189), Glucose Rise0-2h (r=0.12 P=0.003; US: r=0.07 P=0.557)
After adjusting for these factors, the postprandial measures (except Change in alertness2-3hr) remained significantly correlated with the Glucose Dips2-3h; Change in hunger2-3hr (r=0.12 P=0.002; US: r=-0.01 P=0.904), Change in alertness2-3hr (r=-0.06 P=0.109; US: r=0.04 P=0.756), Time until next meal (r=-0.15 P=<0.001; US: r=-0.24 P=0.038), Energy intake 3-4h (r=0.18 P=<0.001; US: r=0.23 P=0.055), Energy intake 24h (r=0.25 P=<0.001; US: r=0.12 P=0.316)
2). Glucose Dips2-3h in identical meals consumed by the same person
To understand if the association between Glucose Dips2-3h, hunger, alertness and postprandial energy intake was present even when adjusting for individual characteristics, we used each person as their own control and compared their own responses to the standardised breakfasts that were repeated in duplicate, following an overnight fast, separated by 2-5 days (n=1053, m=6428; see Supplementary Table 2 for meal numbers).
The difference between the first and second repeat of each meal was calculated. We then measured the degree to which the difference in Glucose Dip2-3h was associated with the difference in each of the postprandial measures. Even when holding constant both the food and the individual, Glucose Dips2-3h were modestly but significantly correlated with time until next meal (r=-0.06 P=<0.001), Energy intake3-4h (r=0.08 P=<0.001), and Energy intake24h (r=0.06 P=<0.001). The association with change in Hunger2-3hr (r=0.04 P=0.232) and change in Alertness2-3hr (r=-0.06 P=0.076) was directional, but not significant. None of the correlations in the smaller US validation cohort were significant. Figure 3 illustrates these effects, by comparing the postprandial measures across the largest (above +10%) and smallest (below -10%) differences in Glucose Dip2-3h.
The correlation between difference in glucose dips and difference between the five outcomes shown in Figure 3 for each pair of meals was computed separately for each of four type of standardised meals included in the analysis, namely High Carb, High Protein, OGTT and “UK Average”. The resulting correlation coefficients were then meta-analysed using a fixed effects and a random effects estimate. The results are reported in Supplementary Table 5 and are very similar to those reported in Figure 3.
The fact that associations between Glucose Dips2-3h and postprandial measures still exist between repeated meals for the same individual shows that this relationship is not fully driven by differences between individuals. It is possible that contextual factors (such as exercise, sleep and other meals in the previous day) may be responsible for differences in glucose dips2-3h but we did not explore this avenue further in this study.
Discussion
The notion that blood glucose is involved in appetite was described by Mayer in the ‘glucostatic hypothesis’(16). This concept - that a decline in blood glucose influenced appetite - was subjected to intense study (20)(21,22) but has lost support from front line researchers, partly because of a lack of evidence of any suppression of eating by high glucose availability (23). It has been argued that inadequate delivery of glucose to the brain (‘neuroglucopenia’ or ‘glucoprivation’) activates neurocircuits that drive feeding along with wide-ranging neuroendocrine and autonomic responses(24). A sustained increase in food intake follows, overriding the control exerted by the energy homeostasis system, i.e., irrespective of body fuel stores or plasma levels of leptin or insulin (24,25). However, this mechanism has been dismissed as not relevant to normal feeding and considered as an “emergency response” (e.g.(26)). More recently, an observational (n=31) study (31) of obese and healthy individuals in free-living conditions found that the glucose nadir preceding a meal had statistically significant ability to predict hunger and subsequent energy intake. In the present study, using continuous glucose monitors in an at-home setting in >8,000 controlled meals and> 70,000 ad libitum meals we have been able to show that postprandial glucose dips are associated with appetite expression and energy intake in normal feeding in healthy individuals under what can be regarded as real-world conditions.
We demonstrate in the largest study to date the importance of glucose dynamics in the regulation of hunger and subsequent energy intake in real life scenarios. We report that the key postprandial glycemic measure linked to hunger and subsequent food intake is the Glucose Dip2-3h, not the Glucose Rise0-2h nor the glucose iAUC0-2h. Whilst some of the correlations we report are relatively modest, they reflect the complexity of real-world eating decisions. It is notable that the Glucose Dip2-3h was as good a predictor of subsequent energy intake as the participants’ self-reported Hunger2-3h.
We found these effects to be consistent for the same individual consuming the same meal on two occasions - the meals with larger Glucose Dips2-3h have larger subjectively declared hunger, and greater energy intake afterwards. These results were directionally consistent with those in the US validation cohort, although not all results were significant in this smaller cohort.
We have not fully explored all the possible causes of Glucose Dips in this study; but the findings suggest that both individual characteristics and dietary factors are likely important. Comparing individuals, Glucose Dips2-3h are negatively associated with fasting C-peptide and insulin levels. Between the standardised meals, the largest glucose dips followed the meal with the largest glucose rise (OGTT). Our analysis may partly explain why observational epidemiological ((27)(28)) studies have shown strong correlations between foods with high glycemic loads (such as potatoes and sugar sweetened beverages (29)) and weight gain – as consumption of such foods could lead to glucose dips and subsequent hunger. The link between postprandial blood glucose and satiety suggests novel approaches to predicting and managing hunger using glucose data, especially if it were available in real-time.
The associations demonstrated in our studies are a realistic reflection of how appetite control operates under real world conditions, allowing variation in contextual variables such as exercise, sleep, or meal sequence. These factors may contribute to the observed in-person variability in Glucose Dips. It should be noted that the degree of association (correlation values) between glucose dips and appetite variables is relatively small, and therefore contributes only partly to postprandial satiety. In heavily controlled laboratory studies with uniform conditions and with participants’ physiology and behaviour tightly constrained, much higher correlations can be observed; however, these scientifically ideal conditions are not typical of real-world human activities. The data set reported in this paper is unique - most of the meals were self-determined and consumed according to the individuals’ own schedules. The decision to eat is determined by many social, psychological and physiological factors, and glucose dips are just one part of the picture. Our data confirm that in the realworld blood glucose dips are a physiological mediator of this dietary risk factor, although much of the variance remains to be accounted for.
We note several study limitations. Firstly, given the scale, it was not practical to measure appetite hormones for any of the participants nor measure insulin sensitivity directly with a euglycemic clamp, nor to record insulin dynamics for the at home meals. Secondly, for the ad libitum meals the meal content is self-reported. Great care was taken to ensure the quality and accuracy of these records, as outlined in the methods, and we believe the scale and real-life context of this study more than compensates for this limitation. Thirdly, we relied on the self-reported compliance of the participants with the fasting protocols - 109 meals were excluded from participants that ate before the end of the specified fasting period. (See QC of the app data: Inclusion criteria for meals) - these participants may also have been responding to blood glucose dips. Fourthly, whilst we were able to study energy intake immediately after a meal and total energy intake over 24 hours, we were not able to monitor if participants compensated for short term changes in energy intake over a period of weeks or months. In addition, there was limited ethnic diversity in our study population (97% white). In addition, we have not included some potential confounders in the models, including sleep and physical activity. Finally, we note that some of the outcomes studied, specifically the visual analogue scale for scoring hunger was not perfectly normally distributed. However, we have analysed a very large number of meals (8624 standardised meals and 71,715 ad libitum meals) and linear models are known to be robust to the violation of the assumption of normality when the sample size is large enough for the central limit theorem to be at work (30).
In conclusion, our data show for the first time in a large-scale controlled study of healthy individuals representative of the general population that postprandial glucose dips are common and lead to increased hunger and energy consumption in real world conditions.
Extended Data
Extended Data Figure 1.
Extended Data Figure 2.
Extended Data Figure 3.
Supplementary Material
Acknowledgements
We express our sincere thanks to the participants of the PREDICT 1 study. We thank the staff of Zoe Global, the Department of Twin Research and Massachusetts General Hospital for their tireless work in contributing to the running of the study and data collection. We thank Abbott for their support with using their CGMs. This work was supported by Zoe Global Ltd also received support from grants from the Wellcome Trust (212904/Z/18/Z) and the Medical Research Council (MRC)/British Heart Foundation Ancestry and Biological Informative Markers for Stratification of Hypertension (AIMHY; MR/M016560/1). PWF was supported in part by grants from the European Research Council (CoG-2015_681742_NASCENT), Swedish Research Council, Novo Nordisk Foundation and the Swedish Foundation for Strategic Research (IRC award). AMV was supported by the National Institute for Health Research Nottingham Biomedical Research Centre. TwinsUK is funded by the Wellcome Trust, Medical Research Council, European Union, Chronic Disease Research Foundation (CDRF), Zoe Global Ltd and the National Institute for Health Research (NIHR)-funded BioResource, Clinical Research Facility and Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London.
Footnotes
Role of the sponsor: The sponsor, Zoe Global Ltd, was directly involved in study design, data collection and analysis for this manuscript.
Conflict of interest statement: TD Spector, SE Berry, AM Valdes, PW Franks, A Chan are consultants to Zoe Global Ltd (“Zoe”). J Wolf, G Hadjigeorgiou, H Al Khatib, P Wyatt and I Linenberg are or have been employees of Zoe. JE Blundell is a member of Zoe Global scientific Advisory Board. Other authors have no conflict of interest to declare.
Author contributions: Obtained funding: J.W., G.H., T.D.S.. Study design and developed concept: P.W., A.M.V., Data collection: S.E.B., D.A.D., H.A.K., L.H.N., A.T.C, R.O’D. G.F. Data analysis: P.W., A.M.V., Study coordination: S.E.B., H.A.K., D.A.D., G.H., J.W. I.B. Writing the manuscript P.W. J.B, A.M.V.. All authors reviewed and revised the final manuscript.
Data availability
The data used for analyzing this study are held by the department of Twin Research at King’s College London. The data can be released to bona fide researchers using our normal procedures overseen by the Wellcome Trust and its guidelines as part of our core funding. We receive around 100 requests per year for our datasets and have a meeting three times a month with independent members to assess proposals. Application is via https://twinsuk.ac.uk/resources-for-researchers/access-our-data/. This means that the data needs to be anonymized and conform to GDPR standards. Specifically for this paper, all the variables used in the models can be requested as well as the summary outcome measures for each person
References
- 1.Ng M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young-Hyman D. Introduction to special issue: Self-regulation of appetite—it’s complicated. Obesity. 2017;25:S5–S7. doi: 10.1002/oby.21781. [DOI] [PubMed] [Google Scholar]
- 3.Montesi L, et al. Long-term weight loss maintenance for obesity: a multidisciplinary approach. Diabetes Metab Syndr Obes. 2016;9:37–46. doi: 10.2147/DMSO.S89836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Ghoch M, Calugi S, Dalle Grave R. The Effects of Low-Carbohydrate Diets on Psychosocial Outcomes in Obesity/Overweight: A Systematic Review of Randomized, Controlled Studies. Nutrients. 2016;8 doi: 10.3390/nu8070402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sumithran P, Proietto J. The defence of body weight: a physiological basis for weight regain after weight loss. Clin Sci. 2013;124:231–241. doi: 10.1042/CS20120223. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert JA, et al. Milk supplementation facilitates appetite control in obese women during weight loss: a randomised, single-blind, placebo-controlled trial. Br J Nutr. 2011;105:133–143. doi: 10.1017/S0007114510003119. [DOI] [PubMed] [Google Scholar]
- 7.Hintze LJ, Mahmoodianfard S, Auguste CB, Doucet É. Weight Loss and Appetite Control in Women. Curr Obes Rep. 2017;6:334–351. doi: 10.1007/s13679-017-0273-8. [DOI] [PubMed] [Google Scholar]
- 8.Tremblay A, Lepage C, Panahi S, Couture C, Drapeau V. Adaptations to a diet-based weight-reducing programme in obese women resistant to weight loss. Clin Obes. 2015;5:145–153. doi: 10.1111/cob.12094. [DOI] [PubMed] [Google Scholar]
- 9.Melby CL, Paris HL, Foright RM, Peth J. Attenuating the Biologic Drive for Weight Regain Following Weight Loss: Must What Goes Down Always Go Back Up? Nutrients. 2017;9 doi: 10.3390/nu9050468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam YY, Ravussin E. Indirect calorimetry: an indispensable tool to understand and predict obesity. Eur J Clin Nutr. 2017;71:318–322. doi: 10.1038/ejcn.2016.220. [DOI] [PubMed] [Google Scholar]
- 11.Blundell JE, et al. The drive to eat in homo sapiens: Energy expenditure drives energy intake. Physiol Behav. 2020;219 doi: 10.1016/j.physbeh.2020.112846. 112846. [DOI] [PubMed] [Google Scholar]
- 12.Kleinridders A, Ferris HA, Cai W, Kahn CR. Insulin action in brain regulates systemic metabolism and brain function. Diabetes. 2014;63:2232–2243. doi: 10.2337/db14-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Anton C, et al. Glycemic responses, appetite ratings and gastrointestinal hormone responses of most common breads consumed in Spain. A randomized control trial in healthy humans. Nutrients. 2015;7:4033–4053. doi: 10.3390/nu7064033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonnema AL, Altschwager DK, Thomas W, Slavin JL. The effects of the combination of egg and fiber on appetite, glycemic response and food intake in normal weight adults--a randomized, controlled, crossover trial. Int J Food Sci Nutr. 2016;67:723–731. doi: 10.1080/09637486.2016.1196654. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig DS, et al. High glycemic index foods, overeating, and obesity. Pediatrics. 1999;103:E26. doi: 10.1542/peds.103.3.e26. [DOI] [PubMed] [Google Scholar]
- 16.Mayer J. Glucostatic mechanism of regulation of food intake. N Engl J Med. 1953;249:13–16. doi: 10.1056/NEJM195307022490104. [DOI] [PubMed] [Google Scholar]
- 17.Berry S, et al. Personalised REsponses to DIetary Composition Trial (PREDICT): an intervention study to determine inter-individual differences in postprandial response to foods. 2020 doi: 10.21203/rs.2.20798/v1. [DOI] [Google Scholar]
- 18.Berry SE, et al. Human postprandial responses to food and potential for precision nutrition. Nat Med. 2020;26:964–973. doi: 10.1038/s41591-020-0934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 20.Smith FJ, Campfield LA. Meal initiation occurs after experimental induction of transient declines in blood glucose. Am J Physiol. 1993;265:R1423–9. doi: 10.1152/ajpregu.1993.265.6.R1423. [DOI] [PubMed] [Google Scholar]
- 21.Campfield LA, Smith FJ. Blood glucose dynamics and control of meal initiation: a pattern detection and recognition theory. Physiol Rev. 2003;83:25–58. doi: 10.1152/physrev.00019.2002. [DOI] [PubMed] [Google Scholar]
- 22.Kovacs EMR, et al. Associations between spontaneous meal initiations and blood glucose dynamics in overweight men in negative energy balance. Br J Nutr. 2002;87:39–45. doi: 10.1079/BJN2001473. [DOI] [PubMed] [Google Scholar]
- 23.Woods SC, D’Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab. 2008;93:S37–50. doi: 10.1210/jc.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nat Rev Neurosci. 2014;15:367–378. doi: 10.1038/nrn3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dryden S, Pickavance L, Henderson L, Williams G. Hyperphagia induced by hypoglycemia in rats is independent of leptin and hypothalamic neuropeptide Y (NPY) Peptides. 1998;19:1549–1555. doi: 10.1016/s0196-9781(98)00106-5. [DOI] [PubMed] [Google Scholar]
- 26.Sprague JE, Arbeláez AM. Glucose counterregulatory responses to hypoglycemia. Pediatr Endocrinol Rev. 2011;9:463–73. quiz 474–5. [PMC free article] [PubMed] [Google Scholar]
- 27.TeMorenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ. 2012;346:e7492. doi: 10.1136/bmj.e7492. [DOI] [PubMed] [Google Scholar]
- 28.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bao J, Atkinson F, Petocz P, Willett WC, Brand-Miller JC. Prediction of postprandial glycemia and insulinemia in lean, young, healthy adults: glycemic load compared with carbohydrate content alone. Am J Clin Nutr. 2011;93:984–996. doi: 10.3945/ajcn.110.005033. [DOI] [PubMed] [Google Scholar]
- 30.Pek J, Wong O, Wong ACM. How to Address Non-normality: A Taxonomy of Approaches, Reviewed, and Illustrated. Front Psychol. 2018;9:2104. doi: 10.3389/fpsyg.2018.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used for analyzing this study are held by the department of Twin Research at King’s College London. The data can be released to bona fide researchers using our normal procedures overseen by the Wellcome Trust and its guidelines as part of our core funding. We receive around 100 requests per year for our datasets and have a meeting three times a month with independent members to assess proposals. Application is via https://twinsuk.ac.uk/resources-for-researchers/access-our-data/. This means that the data needs to be anonymized and conform to GDPR standards. Specifically for this paper, all the variables used in the models can be requested as well as the summary outcome measures for each person