Abstract
A considerable share of bacterial species maintains segmented genomes. Plant symbiotic α-proteobacterial rhizobia contain up to six repABC-type replicons in addition to the primary chromosome. These low or unit-copy replicons, classified as secondary chromosomes, chromids, or megaplasmids, are exclusively found in α-proteobacteria. Replication and faithful partitioning of these replicons to the daughter cells is mediated by the repABC region. The importance of α-rhizobial symbiotic nitrogen fixation for sustainable agriculture and Agrobacterium-mediated plant transformation as a tool in plant sciences has increasingly moved biological engineering of these organisms into focus. Plasmids are ideal DNA-carrying vectors for these engineering efforts. On the basis of repABC regions collected from α-rhizobial secondary replicons, and origins of replication derived from traditional cloning vectors, we devised the versatile family of pABC shuttle vectors propagating in Sinorhizobium meliloti, related members of the Rhizobiales, and Escherichia coli. A modular plasmid library providing the elemental parts for pABC vector assembly was founded. The standardized design of these vectors involves five basic modules: (1) repABC cassette, (2) plasmid-derived origin of replication, (3) RK2/RP4 mobilization site (optional), (4) antibiotic resistance gene, and (5) multiple cloning site flanked by transcription terminators. In S. meliloti, pABC vectors showed high propagation stability and unit-copy number. We demonstrated stable coexistence of three pABC vectors in addition to the two indigenous megaplasmids in S. meliloti, suggesting combinability of multiple compatible pABC plasmids. We further devised an in vivo cloning strategy involving Cre/lox-mediated translocation of large DNA fragments to an autonomously replicating repABC-based vector, followed by conjugation-mediated transfer either to compatible rhizobia or E. coli.
Keywords: plasmid cloning vehicle, Cre/loxP, repABC, in vivo cloning, Sinorhizobium, Rhizobiales
Plasmid vectors are valuable tools in genetic engineering and synthetic biology. Most well established cloning vectors have specifically been selected and designed to be applied in Escherichia coli. Plasmids with a broader host range that can be transferred between different microorganisms and function in diverse genomic backgrounds represent a natural source of orthogonal DNA elements autonomously replicating in bacteria. While medium to high copy plasmids are usually randomly distributed during cell division, low copy plasmids employ a segregation mechanism to assort plasmid copies to each daughter cell.1 Plasmids can be considered as ideal DNA-carrying vectors with respect to the ease of plasmid engineering, modularization, and standardization.2 Yet, variations in copy number and propagation stability, as well as limited cloning capacity and combinability are unfavorable properties of plasmid vectors. Unreliable plasmid propagation has to be overcome by selection pressure that may negatively affect cell growth and physiology. The main bacterial chromosome has been used as vector for assembly and stable propagation of megasize DNA,3−5 and also engineered or synthetic chromosomes potentially offer high propagation stability and cloning capacity.6−8
Several members of the Rhizobiales belonging to the class of α-proteobacteria are best known for their ability to establish a nitrogen-fixing symbiosis with legumes9,10 or as plant pathogens of the genus Agrobacterium causing crown gall tumors on many vascular plants.11,12 Advances in synthetic biology have increasingly moved utilization of genetic resources and processes underlying the microbe-plant interactions of these α-proteobacteria into focus. This includes the high value of symbiotic nitrogen fixation for sustainable agriculture,13 but also their biotechnological potential with respect to polysaccharide14,15 and vitamine16,17 production and Agrobacterium-mediated plant transformation.18 However, the toolbox of available plasmid vectors is underdeveloped in this group of bacteria.
In α-proteobacteria, genome segmentation is a common phenomenon. Especially the fast-growing rhizobia harbor multipartite genomes which are usually composed of a main chromosome and additional low or unit-copy replicons classified as secondary chromosomes, chromids, or megaplasmids,19,20 some of which even exceed 2 Mb in size. For instance, Mesorhizobium loti MAFF303099 possesses a chromosome and two megaplasmids (pMla, pMlb),21 whereas Sinorhizobium meliloti Rm1021 harbors a chromosome, a chromid (pSymB), and a megaplasmid (pSymA).22Rhizobium etli CFN42 even contains six megaplasmids (p42a-f) beside its main chromosome,23 and Agrobacterium tumefaciens C58 maintains two chromosomes, a circular and a linear one, and two plasmids (pTi, pAt).24,25 The unrivaled variety of α-rhizobial genome architecture offers a rich resource of megaplasmids that are faithfully inherited to the daughter cells. These are ideal candidates for plasmid vector construction.
DNA replication origins of these extra-chromosomal replicons usually belong to the repC superfamiliy,20,26,27 named after the DNA replication initiator RepC, which appears to be unique to α-proteobacteria. In repABC family plasmids, repC including the cognate oriV forms an operon with repA and repB.27 RepA and RepB belong to the ParAB family of partitioning proteins27 mediating segregation of newly replicated oriVs. Segregation is initiated by specific binding of RepB to its cognate cis-acting centromere-like repS sites.27 Highly reliable inheritance, even of dispensable repABC-type replicons such as most of the R. etli megaplasmids,28−30 can be attributed to proper function of the segregation machinery.31,27,32
Replicon copy number is supposed to be mainly transcriptionally controlled by RepAB-mediated autorepression of the repABC operon20,26 and post-transcriptionally by a small counter-transcribed RNA, which is usually encoded by a locus between repB and repC (ctRNA).26,33,34 Strict regulation of repABC expression is the mechanistic basis of incompatibility groups allowing stable coexistence of several repABC replicons in the cell.20,27,34−37 High variability of incompatibility factors, such as repS sites, the ctRNA and occasionally the C-terminal domain of RepC, may explain the compatibility of heterologous megaplasmids in related species.38
Here, we explored the naturally occurring low or unit-copy number extrachromosomal elements of members of the Rhizobiaceae and Phylobacteriaceae to design the versatile family of modular standardized pABC plasmids for this important group of bacteria. In S. meliloti, pABCs were shown to mimic megaplasmid-like characteristics, such as high propagation stability, unit-copy number and combinability of compatible repABC-type plasmids, rendering them highly suitable for complex ectopic expression systems. We further devised an in vivo cloning strategy based on Cre/lox-mediated site-specific recombination and an IPTG-inducible engineered oriVrepABC, allowing for translocation of DNA fragments to an autonomously replicating repABC-based vector and conjugation-mediated transfer either to compatible rhizobia or to E. coli.
Results and Discussion
Autonomous Plasmid Replication Mediated by Heterologous repABC Regions in S. meliloti
Initially, we tested the ability of various repABC regions to mediate plasmid maintenance in S. meliloti, which is a well-characterized model of nitrogen-fixing plant-symbiotic α-rhizobia. To this end, repABC regions derived from megaplasmids of S. meliloti (pSymA), Rhizobium etli (p42b, p42d, p42e, p42f), Mesorhizobium loti (pMla, pMlb), Sinorhizobium medicae (pSMED01, pSMED02), Sinorhizobium fredii (pNGR234b), and Agrobacterium tumefaciens (pTi) were inserted into the suicide vector pK18mob2 (Table 1), which is replicative in E. coli, but nonreplicative in S. meliloti.39 Plasmids carrying the repABC cassettes from R. etli, M. loti and A. tumefaciens were successfully established in the S. meliloti Rm1021 wild type strain. In contrast, plasmids carrying the repABC regions derived from pSymA, pSMED01, pSMED02, and pNGR234b were not maintained in this strain, probably due to incompatibility between the indigenous megaplasmids and these heterologous replicons derived from closely related α-rhizobia. In line with this observation, the construct harboring the repABCpSymA cassette propagated in the pSymA-cured S. meliloti Rm1021 derivative SmA818, but not in the wild type. The identified set of eight heterologous repABC regions autonomously replicating in the S. meliloti Rm1021 wild type strain provided the foundation for construction of the family of pABC vectors.
Table 1. Bacterial Strains and Plasmids Used in This Study.
| strains/plasmids | description | refs |
|---|---|---|
| S. meliloti | ||
| Rm1021 | wild type strain | (22) |
| ΔhsdR | Rm1021 strain with hsdR deletion | (45) |
| SmCreΔhsdR | Rm1021 cre expression strain with hsdR deletion, tauX:: cre-tetRA (Tcr) | (45) |
| SmA818 | strain 2011 cured of megaplasmid pSymA | (79) |
| JDSm106 | Rm1021 bearing tetR-YFP-mChr-parBYp (pJD120) under control of PtauAB | this study |
| MWSm8 | Rm1021 bearing pMW1 genomically integrated (Kmr) | this study |
| MWSm37 | ΔhsdR bearing pMW28 for constitutive egfp expression (Kmr) | this study |
| SmCre_exo-IN | SmCreΔhsdR bearing pIso and Pmin2-loxR-aacC1 integrated up- and downstream of the exo gene cluster, respectively (Kmr, Gmr) | this study |
| SmCre_exo-OUT | SmCreΔhsdR carrying pIso-exo isolated from pSymB (IPTG dependent) (Kmr, Specr) | this study |
| A. tumefaciens | ||
| C58 | wild type strain | (24, 25) |
| C58 exoB | exhibiting a CFGE-mediated inactivation of exoB (Gmr) | (45) |
| exoB/pIso-exo | C58 exoB carrying pIso-exo (IPTG dependent) (Gmr, Kmr, Specr) | this study |
| R. etli | ||
| CFN42 | wild type strain | (80) |
| S. medicae | ||
| WSM419 | wild type strain | (81) |
| CC169 | wild type strain | (82) |
| S. fredii | ||
| NGR234 | wild type strain | (83) |
| M. loti | ||
| MAFF303099 | wild type strain | (21) |
| R. leguminosarum | ||
| Norway | wild type strain | (84) |
| M. extorquens | ||
| AM1 Δcel | strain AM1 deficient in cellulose production | (85) |
| C. crescentus | ||
| CB15 N | wild type strain | (86) |
| E. coli | ||
| DH5α | used for cloning procedures and plasmid extraction | (87) |
| S17–1 | used for conjugation with S. meliloti | (88) |
| XL1-Blue | used as recipient of pRK2013 (Tcr) | Stratagene |
| XL1Blue-pRK2013 | used for triparental matings (Tcr, Kmr) | this study |
| AB3219 | used as Clm resistant recipient of pISO-exo (Clmr) | (89) |
| Plasmids | ||
| pK/G18mob2 | suicide vector carrying replication origin pMB1 | (39) |
| pK18mobsacB | suicide vector carrying sacB for sucrose selection | (39) |
| pSRK-Gm/Km | broad-host-range expression vector | (90) |
| pXG-10 | carrying replication origin pSC101* (derivative of pSC101 exhibiting reduced copy number in E. coli) | (91) |
| pACYC184 | carrying replication origin p15A | (92) |
| pPHU231 | broad-host-range expression vector | (93) |
| pRK2013 | helper plasmid for mobilization of nonself-transmissible plasmids | (94) |
| pLAU44 | pUC18 derivate carrying tet operator (tetO) cassettes | (57) |
| pART | artificial mini-replicon based on a pSymA derived repA2B2C2 cassette | (58) |
| pK18mob2-repABCpSymA | pK18mob2 carrying a pSymA derived repA2B2C2 cassette | this work |
| p42b_p | pK18mob2 carrying repABCp42b based oriV (R. etli) | this work |
| p42d_p | pK18mob2 carrying repABCp42d based oriV (R. etli) | this work |
| p42e_s | pK18mob2 carrying a truncated repABC region derived from p42e (R. etli) | this work |
| p42f1_p | pK18mob2 carrying repABCp42f1 based oriV (R. etli) | this work |
| p42f2_p | pK18mob2 carrying repABCp42f2 based oriV (R. etli) | this work |
| pMla_s | pK18mob2 carrying a truncated repABC region derived from pMla (M. loti) | this work |
| pMlb_p | pK18mob2 carrying repABCpMlb based oriV (M. loti) | this work |
| pSMED01_p | pK18mob2 carrying repABCpSMED01 based oriV (S. medicae) | this work |
| pSMED02_p | pK18mob2 carrying repABCpSMED02 based oriV (S. medicae) | this work |
| pNGR234b_p | pK18mob2 carrying repABCpNGR234b based oriV (S. fredii) | this work |
| pTi_p | pK18mob2 carrying repABCpTi based oriV (A. tumefaciens). | this work |
| pMW1 | integrative pK18mob2 derivative conferring Kmr | this work |
| pMW28 | integrative pK18mob2 derivative carrying Pmin2-egfp | this work |
| pJD120 | pK18mobsacB derivative, integration of tetR-YFP-mChr-parBYp under control of PtauAB | this work |
| pJD211 | pK18mob2 derivative carrying Pmin2-loxR-aacC1 | this work |
| pPHU231-egfp | pPHU231 derivative carrying a constitutive egfp cassette | this work |
| pABCa-egfp | pABCa carrying a constitutive egfp cassette | this work |
| pABCa-egfp-expR | pABCa carrying constitutively expressed egfp-expR | this work |
| pABCa-tetO | pABCa carrying a tetO array | this work |
| pABCb-egfp | pABCb carrying a constitutive egfp cassette | this work |
| pABCb-egfp-expR | pABCb carrying constitutively expressed egfp-expR | this work |
| pABCc-egfp-expR | pABCc carrying constitutively expressed egfp-expR | this work |
| pABCc-parSYp | pABCc carrying parSYp | this work |
| pABCc-mob-egfp | pABCc-mob carrying a constitutive egfp cassette | this work |
| pABC1-egfp | pABC1 carrying a constitutive egfp cassette | this work |
| pABC1-egfp-expR | pABC1 carrying constitutively expressed egfp-expR | this work |
| pABC1-egfp_f | pABC1 carrying a forwardly integrated SD-egfp fragment | this work |
| pABC1-egfp_r | pABC1 carrying a reversely integrated SD-egfp fragment | this work |
| pABC1-T2-egfp_f | pABC1-T2 carrying a forwardly integrated SD-egfp fragment | this work |
| pABC1-T2-egfp_r | pABC1-T2 carrying a reversely integrated SD-egfp fragment | this work |
| pABC1-T3-egfp_f | pABC1-T3 carrying a forwardly integrated SD-egfp fragment | this work |
| pABC1-T3-egfp_r | pABC1-T3 carrying a reversely integrated SD-egfp fragment | this work |
| pABC2-egfp | pABC2 carrying a constitutive egfp cassette | this work |
| pABC2-egfp-expR | pABC2 carrying constitutively expressed egfp-expR | this work |
| pABC3-egfp | pABC3 carrying a constitutive egfp cassette | this work |
| pABC4-egfp | pABC4 carrying a constitutive egfp cassette | this work |
| pABC4-egfp-expR | pABC4 carrying constitutively expressed egfp-expR | this work |
| pABCb-lacI | pABCb carrying lacI for constitutive LacI expression | this work |
| pMlb_lacO | pK18mob2 carrying repABCpMlb bearing a lacO cassette at the predicted transcription start site | this work |
| pIso | ABC cloning vector integrating upstream of the exo gene cluster on pSymB | this work |
| pIso-exo | isolated ABC cloning vector carrying the exo gene cluster (replicative upon IPTG supplementation) | this work |
| pABC Library Plasmids Carrying Module Parts | ||
| pLoriVSm | pK18mob2 derivative, unloaded library vector for oriVSm parts | this work |
| pLsynTer-MCS | pK18mob2 derivative, unloaded library vector for synTer-MCS parts | this work |
| pLoriT | pK18mob2 derivative, unloaded library vector for oriT parts | this work |
| pLAR | pK18mob2 derivative, unloaded library vector for AR parts | this work |
| pLoriV-42b | pLoriVSm derivative providing the oriVp42b region (R. etli) | this work |
| pLoriV-42d | pLoriVSm derivative providing the oriVp42d region (R. etli) | this work |
| pLoriV-42e | pLoriVSm derivative providing the oriVp42e region (R. etli) | this work |
| pLoriV-42f1 | pLoriVSm derivative providing the repA1B1C1 based oriVp42f region (R. etli) | this work |
| pLoriV-42f2 | pLoriVSm derivative providing the repA2B2C2 based oriVp42f region (R. etli) | this work |
| pLoriV-Mla | pLoriVSm derivative providing the oriVpMla region (M. loti) | this work |
| pLoriV-Mlb | pLoriVSm derivative providing the oriVpMlb region (M. loti) | this work |
| pLoriV-Ti | pLoriVSm derivative providing the oriVpTi region (A. tumefaciens) | this work |
| pLsynTer-1 | pLsynTer-MCS derivative providing synTer1-MCS | this work |
| pLsynTer-2 | pLsynTer-MCS derivative providing synTer2-MCS | this work |
| pLsynTer-3 | pLsynTer-MCS derivative providing synTer3-MCS | this work |
| pLsynTer-1-lox | pLsynTer-MCS derivative providing synTer1-MCS-loxL | this work |
| pLsynTer-2-lox | pLsynTer-MCS derivative providing synTer2-MCS-loxL | this work |
| pLsynTer-3-lox | pLsynTer-MCS derivative providing synTer3-MCS-loxL | this work |
| pLoriT-1 | pLoriT derivative providing a mob site | this work |
| pLoriT-1-lox | pLoriT derivative providing mob-loxR | this work |
| pLoriT-1-loxT | pLoriT derivative providing mob-loxR-rrnBT2 | this work |
| pLAR-Km | pLAR derivative providing a kanamycin resistance cassette | this work |
| pLAR-Spec | pLAR derivative providing a spectinomycin resistance cassette | this work |
| pLAR-Tc | pLAR derivative providing a tetracyclin resistance cassette | this work |
| pLAR-Hyg | pLAR derivative providing a hygromycin resistance cassette | this work |
| pLAR-Tmp | pLAR derivative providing a trimethoprim resistance cassette | this work |
| pLAR-Gm | pLAR derivative providing a gentamicin resistance cassette | this work |
pABC Vector Design
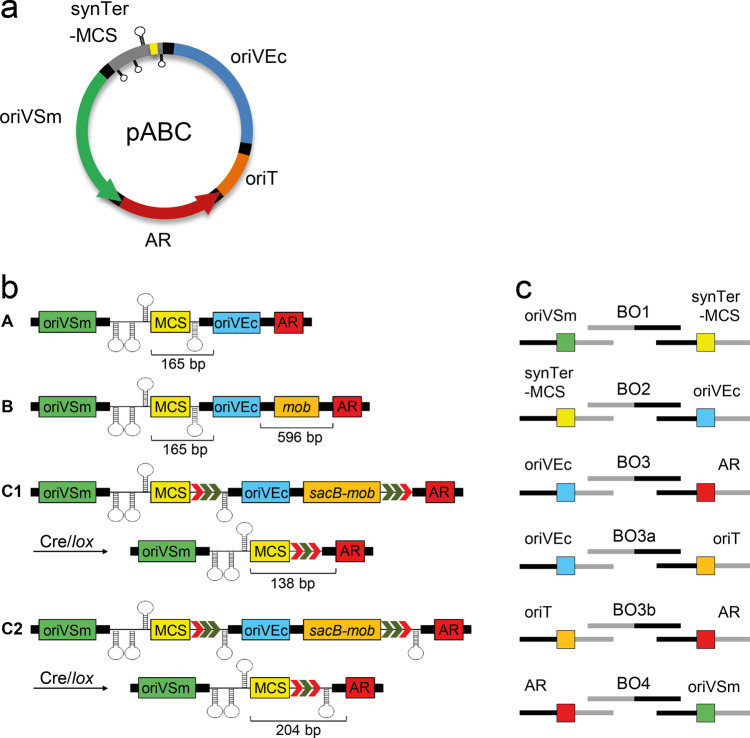
Simple design concepts and modularity facilitating assembly and exchange of functional modules have become standard in vector development for applications in synthetic biology.40−43Figure 1a depicts the blueprint of the pABC vector family composed of five basic modules: oriVSm (repABC cassette for propagation in S. meliloti), oriVEc (plasmid-derived origin of replication for propagation in E. coli), oriT (RK2/RP4 mobilization site), AR (antibiotic resistance gene cassette), and synTer-MCS (multiple cloning site flanked by transcription terminators).
Figure 1.
pABC vector concept and assembly strategy. (a) A pABC is composed of up to five module library parts. Modules oriVSm and oriVEc mediate propagation in S. meliloti and E. coli, respectively. To ensure proper transcriptional control of the repABC operon, the multiple cloning site (yellow box) of the synTer-MCS module, serving as insertion site for the gene load, is flanked by transcriptional terminators (loop structures). A common RK2/RP4 mobilization site (module oriT) enables conjugal transfer of pABCs from E. coli to S. meliloti. For reliable selection in both organisms standardized antibiotic resistance cassettes were developed (module AR). Each module is flanked by linker sequences for standardized LCR-based assembly (black boxes). In the pABC design, order and orientation of modules is intended to minimize mutual influences of adjacent regions. (b) Detailed scheme of pABC design types A to C. The size of design-derived homologous regions potentially occurring in multi-pABC systems is denoted. Setups C1 and C2 differ in the number of transcription terminator sequences (loop structures) protecting the MCS after Cre-mediated deletion of modules oriVEc and oriT. (c) Module-specific linker sequences (gray and black lines) facilitate LCR-based assembly of module parts with standardized bridging oligonucleotides BO1 to BO4. Integration of the oriT module requires BO3a and BO3b.
One of our motivations for developing this vector family is the possibility of coexistence of multiple pABC replicons derived from compatible megaplasmids in one cell. Natural occurrence of α-rhizobial species with up to six compatible repABC-type replicons indicate the high potential of combinability.23 Previously, for a high-throughput plasmid integration study,44 we determined 220 bp sharing at least 80% sequence similarity with the target region as the minimal size promoting homologous recombination at a significant frequency in S. meliloti Rm1021. To limit the possibility of homologous recombination between corresponding members of the pABC vector family, parts were designed on the premise of avoiding high and extended sequence identity (Table S1). Multiple parts per module allow for assembly of combinable pABC vectors. The only exception is the oriT module that comprises the ∼100 bp common mob site within a 464 bp fragment.39 Because of extended sequence identity, it is therefore not recommendable to combine pABC plasmids with this module in one cell.
Three basic pABC types were devised (Figure 1b). pABCs of type A, lacking the oriT module, have to be introduced to S. meliloti by electroporation. pABCs of type B contain an oriT module (pABC-mob) enabling conjugal transfer. Hence, unintended co-integration by homologous recombination between type B pABC vectors through the oriT module appears possible. This is circumvented by design type C which offers the possibility of excision of the oriVEc and oriT modules by Cre/lox-mediated site-specific recombination in S. meliloti.45,46
LCR (ligase cycling reaction)47 is our procedure of choice for restriction enzyme-independent assembly of the building blocks to pABC vectors. LCR was shown to efficiently assemble up to ten 2 kb DNA fragments.47 Library plasmids serving as a template for PCR amplification of the 23 parts generated in our study were constructed (Table 1). Each part is flanked by a module-specific linker, which enable LCR-based assembly promoted by the standardized bridging oligonucleotides BO1-4 (Figure 1c, Table S2). Following in vitro assembly, E. coli DH5α was directly transformed with the purified LCR reaction mix, and correct assembly of pABC vectors was verified by DNA sequencing. Usually more than 75% of tested clones exhibited correct assembly, indicating a suitably efficient assembly system.
Library parts for each module (Table 1 and Table S3) and pABC vectors (Table 2 and Table S4) generated in this study are described in the SEVA-SIB database (http://seva.cnb.csic.es).40 To translate individual pABCs into the SEVA-SIB collection, a unique identifier was assigned to each insert in accordance with an adapted SEVA nomenclature (Tables S3 and S4).
Table 2. pABCs Generated in This Studya.
| modules |
|||||||
|---|---|---|---|---|---|---|---|
| plasmid | oriVSm (oriV origin) | synTer-MCS (relevant fragment) | oriVEc (oriV origin) | oriT (relevant fragment) | AR (resistance) | size (kb) | design type |
| No Linker | |||||||
| pABCa | pMlb | synTer1 | p15A | Gm | 6.7 | A | |
| pABCb | p42b | synTer2 | pMB1 | Sp | 6.5 | A | |
| pABCc | p42d | synTer3 | pSC101* | Km | 11.5 | A | |
| pABCc-mob | p42d | synTer3 | pSC101* | mob | Km | 12.0 | B |
| Module Library Based Construction | |||||||
| pABC1 | p42b | synTer1 | pMB1 | Sp | 6.7 | A | |
| pABC1-loxA | p42b | synTer1-loxL | pMB1 | mob-loxR | Sp | 9.5 | C1 |
| pABC1-loxB | p42b | synTer1-loxL | pMB1 | mob-loxR-rrnBT2 | Sp | 9.6 | C2 |
| pABC1-sT2 | p42b | synTer2 | pMB1 | Sp | 6.7 | A | |
| pABC1-sT3 | p42b | synTer3 | pMB1 | Sp | 6.7 | A | |
| pABC2 | pMla | synTer2 | p15A | Gm | 7.2 | A | |
| pABC3 | pTi | synTer3 | pSC101* | Hyg | 8.6 | A | |
| pABC4 | p42e | synTer1 | pMB1 | Sp | 7.0 | A | |
| pABC5 | p42f1 | synTer2 | pMB1 | Sp | 7.2 | A | |
| pABC6 | p42f2 | synTer2 | pMB1 | Sp | 7.3 | A | |
pABCa, pABCb, and pABCc are based on building blocks lacking the module-specific linker sequences and a transcription terminator downstream of the MCS.
Parts Characterization
oriVSm Module
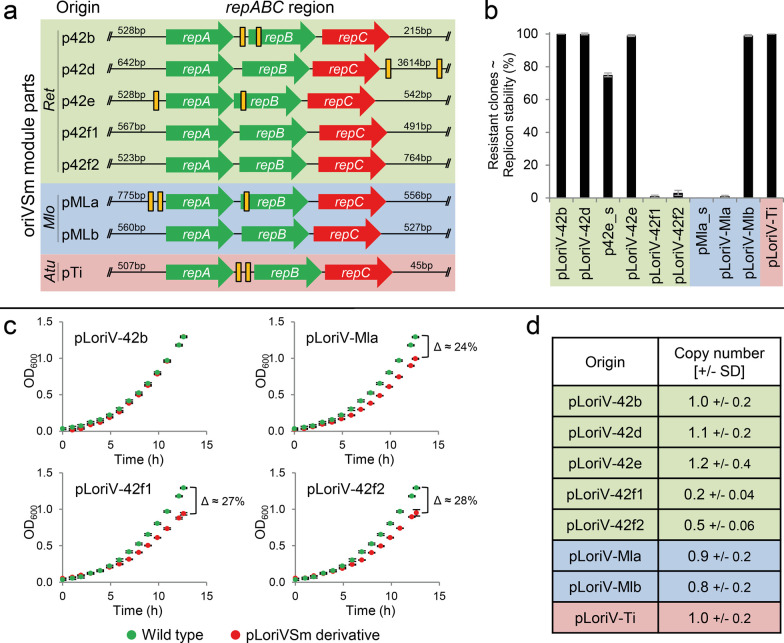
The common element of the pABC vector family is the repABC region which naturally acts as replication origin of megaplasmids of various α-proteobacteria.20,26repABC cassettes of different megaplasmids were integrated into pK18mob2 derivative pLoriVSm resulting in library module vectors which provide the repABC cassettes of R. etli p42b,d,e,f (pLoriV-42b, -42d, -42e, -42f1, -42f2), M. loti pMla,b (pLoriV-Mla, -Mlb), and A. tumefaciens pTi (pLoriV-Ti). Figure 2a depicts the genetic structure of the eight repABC regions, which constitute the basis of the oriVSm module parts used in this study. To test for autonomous propagation and stability in the absence of selective pressure, S. meliloti Rm1021 was transformed with the pLoriVSm library plasmids. Restriction enzyme-digested pLoriVSm plasmids purified from the S. meliloti transformants showed the expected electrophoresis banding patterns (Figure S1), verifying autonomous propagation of these replicons in S. meliloti.
Figure 2.
Characterization of oriVSm module parts. (a) repABC cassettes analyzed in this study. Regions are derived from R. etli (Ret), M. loti (Mlo), and A. tumefaciens (Atu) plasmids. Since functional elements, such as partitioning sites (repS, yellow boxes) or the promoter of the antisense RNA incα potentially are located outside of the repABC operon, repABC cassettes were expanded several hundred base pairs up- and downstream of the coding regions. Although a suicide vector carrying the repABC operon of p42d plus 500 bp downstream of the repC stop codon was able to propagate in R. etli CFNX101,77 the downstream region was generously expanded in order to include a further predicted repS site.78 (b) Stability assay of pLoriVSm derivatives. S. meliloti Rm1021 carrying pLoriV-42b, -42d, 42e, -Mla, -Mlb, or -Ti, or the shorter constructs p42e_s or pMla_s was grown for 72 h in nonselective TY medium and diluted every 12 h to an OD600 of ∼0.05. Single colonies were examined for antibiotic resistance (n = 100 for pLoriVSm derivatives; n = 50 for p42e_s and pMla_s). Error bars indicate the standard deviation calculated from three technical replicates. (c) Growth of S. meliloti Rm1021 carrying selected pLoriVSm plasmids compared to MWSm8 (wild type, Kmr). Cultures were adjusted to an OD600 of 0.1 and grown for 12 h in selective TY medium. Error bars indicate the standard deviation calculated from four technical replicates. (d) qPCR-based copy number determination of pLoriVSm derivatives (-42b, -42d, -42e, -42f1, -42f2, -Mla, -Mlb, and -Ti) in exponentially growing S. meliloti. Measurements are based on eight technical replicates from two independent clones (see Table S6). SD: standard deviation.
pLoriV-42b, -42d, -42e, -Mlb, and -Ti exhibited highly stable propagation despite cultures being kept in exponential growth for 72 h in nonselective rich medium (Figure 2b). The repABCp42e region used for construction of library plasmid pLoriV-42e is characterized by a 542 bp 3′UTR. A shorter variant with a 26 bp 3′UTR (p42e_s) was less stable, indicating that genetic elements required for faithful replication or segregation of these replicons can be located further downstream of the repABC operon.30 Plasmids harboring oriVp42f1, oriVp42f2, and oriVpMla derived cassettes were virtually lost in the same period of time. Expanding the 3′UTR of repABCpMla from 45 bp (pMla_s) to 556 bp (pLoriV-Mla) did not improve plasmid maintenance. Slight incompatibility between repABCpMla and the oriV regions of the indigenous megaplasmids may account for this viable but unstable coexistence. Cross-interactions between components of indigenous and heterologous RepABC systems may impair regulatory control of the repABC operons or function of the gene products in replication initiation or plasmid segregation. This may affect cell cycle progression and diminish cell growth. Indeed, pLoriV-42f1, -42f2, and -Mla negatively affected S. meliloti cell growth, whereas stably propagating plasmids bearing oriV cassettes from p42b, p42e, pMlb, and pTi had no significant influence on growth (Figures 2c and S2). This is supposed to be a critical prerequisite for a multi-pABC setup under nonselective conditions since a negative effect on cell growth would promote plasmid loss.
We aimed at preserving the unit-copy number or low copy number of megaplasmids in the free-living state as an important attribute of pABC vectors. The copy number of pLoriVSm plasmids in S. meliloti was determined by qPCR and data were normalized to pK18mob2-repABCpSymA, which in a calibration experiment was shown to exhibit the same copy number as the chromosome (Table S5). The stably propagating pLoriV library plasmids roughly matched a unit-copy number, whereas instable plasmids showed values below 1 (Figure 2d and Table S6). Since a qPCR-based copy number determination was performed on crude cell extracts, a significant number of pLoriV plasmid-negative cells could account for the low values determined for the unstable plasmids pLoriV-42f1 and -42f2 (Figure 2d).
Considering stability and copy number, the oriVSm module vectors pLoriV-42b, -42d, -42e, -Mlb, and -Ti exhibited the desired megaplasmid-like characteristics and thus appear to be highly suitable for construction of the pABC vector system.
synTer-MCS Module
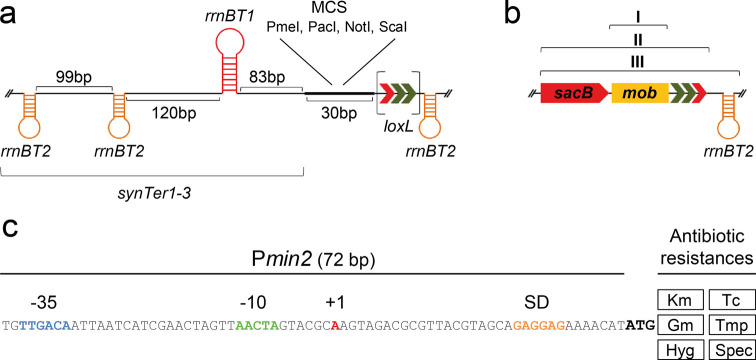
This module provides a multiple cloning site offering recognition sites for the rare-cutting restriction enzymes PmeI, PacI, and NotI. Transcriptional read-through into the repABC operon was found to interfere with its strict native expression control. The MCS module was therefore additionally equipped with flanking transcription terminators to protect the oriVSm module and the MCS position from transcriptional read-through emanating from sequences inserted into the MCS or adjacent modules. To this end, synthetic termination regions (synTer1–3) flanking the MCS were designed based on the E. coli rrnBT1 and rrnBT2 terminators (Figure 3a) and cloned into pK18mob2 to generate the pLsynTer library plasmids (Table 1). Effective transcription termination was verified by interrupting expression of an egfp reporter gene from a strong constitutive promoter by inserting the synTer1–3 cassettes downstream of the promoter (Figure S3).
Figure 3.
Setup of pABC library modules. (a) The synTer-MCS module plasmids provide three ∼550 bp synTer regions composed of Rho-independent E. coli terminators rrnBT1 (90 bp) and rrnBT2 (60 bp) separated by unique synthetic sequences. Terminator sites (loop structures) are intended to protect the oriVSm module and the MCS, which provides unique recognition sites for rare cutters. pLsynTerX-lox plasmids are further equipped with a left arm mutated loxL site (arrows in brackets). (b) The oriT module comprises a 464 bp DNA fragment containing the RK2-based mob site (I). For Cre/lox applications the region is extended by sacB-loxR (II) and a further rrnBT2 transcription terminator (III). (c) Module AR contains the short Pmin2 fragment conferring resistance to kanamycin (Km), tetracycline (Tc), gentamicin (Gm), trimethoprim (Tmp), hygromycin (Hyg), or spectinomycin (Spec) in E. coli and S. meliloti.
oriVEc and oriT Modules
The oriVEc module enables E. coli-based cloning of pABCs. However, construction of standardized library plasmids carrying a further E. coli replication origin (besides the native oriVpMB1) failed. For this reason, oriVEc parts harboring oriVpMB1, oriVp15A, and oriVpSC101* were PCR-amplified from nonlibrary plasmids by use of extended primers carrying the module specific linker sequences. The oriT module provides an RP4-derived mob site which mediates conjugal transfer from E. coli to a wide range of Gram-negative bacteria48 including S. meliloti. This module is available on the pLoriT library plasmids (Table 1).
The low variability of oriVEc and oriT module parts is associated with a reduced combinability of pABC vectors since homologous regions provoke unintended replicon fusions. For this reason we equipped the synTer-MCS and oriT modules with Cre recognition (lox) sites, resulting in the pLsynTer-x-lox and pLoriT-x-lox library plasmids, respectively (Figure 3a–b, Table 1). These lox sites allow Cre-mediated deletion of these modules from the pABC vectors in the α-rhizobial host strain SmCreΔhsdR as demonstrated in Figure S4.
AR Module
This module comprises selection markers conferring resistance to the antibiotics gentamicin, hygromycin, kanamycin, tetracycline, trimethoprim, and spectinomycin in E. coli and S. meliloti (Figure S5). For the AR module, we chose a standardized design with the 72 bp Pmin2 promoter driving expression of the resistance genes (Figure 3c). This promoter was selected from PaacC1 and Ptrp derivatives (Figure S6). AR module parts are available on the pLAR library plasmids (Table 1).
Characterization of pABC Shuttle Vectors
We constructed 14 pABCs representing design types A to C utilizing all oriVSm module parts described above (Table 2 and Table S7). pABCa-c are based on constructs lacking the standard linker sequences flanking the module library parts. They basically match the structure of pABC1 to six that are composed of library parts assembled via module-specific linker sequences and include an additional transcription terminator downstream of the MCS (Figure S7a). Dependent on the pABC design, transformation of S. meliloti Rm1021 was carried out via electroporation or E. coli S17-1 mediated conjugal transfer. Following electroporation, transformation rates of S. meliloti Rm1021 with pABCs were massively higher than with the common replicative vectors pSRKGm and pPHU231.45 Compared to pSRKGm, pABCa, pABCb, and pABCc achieved ∼7200-, 88-, and 22-fold higher transformation efficiencies, respectively, making cotransformation of S. meliloti with up to three pABCs possible (Figure S7b). Successful introduction of pABCs into S. meliloti and autonomous propagation was verified by plasmid purification from transconjugants and restriction enzyme digest-derived fragment patterns (Figure S8).
Inheritance of pABC vectors was studied by flow cytometry-based single cell analysis applying S. meliloti strains carrying pABC-egfp derivatives (Table S8). In contrast to the preliminary selection marker-based assay of maintenance of the pLoriVSm library plasmids (Figure 2b), which tends to preselect fast growing cells, the flow cytometry-based assay allows for unbiased determination of the ratio of pABC-positive and -negative cells and is highly sensitive (Figure S9). Cultures were incubated in nonselective rich medium and diluted twice a day to allow for continuous growth in the range of OD600 of 0.05 to 1.0.
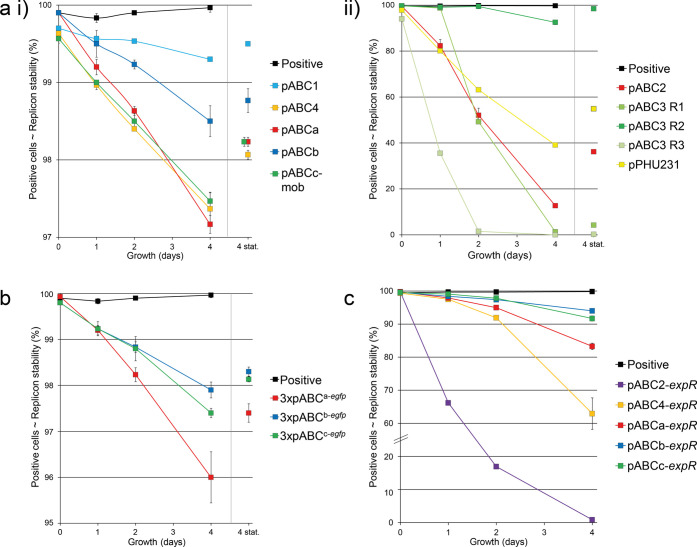
Consistent with the properties of the corresponding pLoriVSm plasmids, derivatives of pABCa (oriVpMlb), pABCb (oriVp42b), pABCc-mob (oriVp42d), pABC1 (oriVp42b), and pABC4 (oriVp42e) were remarkably stably propagated over the time course of 96 h (Figure 4a,i). After 24 h even ∼99% of measured cells were eGFP-positive and therefore supposed to maintain the respective pABC replicon. After 96 h of incubation still 97% of cells were positive, indicating a highly reliable propagation of the pABC derivatives in S. meliloti Rm1021. In contrast, egfp expressing derivatives of pABC2 (oriVpMla) and the low-copy plasmid pPHU231 showed a pronounced plasmid loss of ∼20% after 24 h (Figure 4a,ii). After 96 h, the proportion of positive cells even decreased to 13% and 39%, respectively. Notably, flow cytometry revealed dramatic variations in propagation stability between biological replicates of clones bearing the oriVpTi-based pABC3-egfp (99%, 49%, and 2% positive cells after 48 h) (Figure 4a,ii). Mutation of the cognate repABC cassette was excluded by resequencing. However, we cannot exclude mutations in other pABC modules or the S. meliloti background genome. Previously, regulatory mechanisms affecting replication and thereby the copy number of pTi in A. tumefaciens have been reported.49 The heterogeneity in propagation stability observed in our study and variations in copy number of pTi characterize oriVpTi as unsuitable part for construction of stably propagating single copy pABC vectors in S. meliloti. The pABC maintenance assay identified oriVpMlb, oriVp42b, oriVp42d, and oriVp42e as highly suitable origins of replication for generation of S. meliloti pABC vectors.
Figure 4.
Inheritance stability of pABCs in S. meliloti Rm1021. S. meliloti carrying pABC-egfp (-expR) derivatives were grown in nonselective TY medium at 30 °C for 96 h. During the time course, cultures were diluted every 12 h to an OD600 of ∼0.05. An eGFP signal of 10.000 cells was measured by flow cytometry at time point 0 and after 1, 2, and 4 days. Additionally, after the initial 24 h, subcultures were incubated for 48 h until the stationary phase was reached, and reinoculated at day 3 (4 stat., separated by a line). Varying ordinate labeling should be noted. Positive: S. meliloti MWSm37 (genomically integrated egfp). Error bars indicate standard deviation calculated from three biological replicates. (a) Maintenance of single pABC-egfp derivatives. (i) Stable pABC-egfp constructs which did not deceed 97% propagation stability. (ii) Replicon stability of instable pABC-egfp derivatives and low copy plasmid pPHU231-egfp. Because of the heterogeneous behavior of pABC3-egfp, measurements from individual clones are shown (R1–3). (b) Replicon stability of pABCs in a triple pABC system. 3xpABCa-egfp: pABCa-egfp, pABCb, pABCc-mob; 3xpABCb-egfp: pABCa, pABCb-egfp, pABCc-mob; 3xpABCc-egfp: pABCa, pABCb, pABCc-mob-egfp. (c) S. meliloti carrying pABC-egfp-expR derivatives.
To rule out mutual influences of the repABC cassettes in multi-pABC setups we further studied replicon stability in S. meliloti strains simultaneously carrying two or three pABCs. pABCa–pABCc retained highly stable propagation (>96%) throughout the time course and achieved stability values which were only marginally lower as compared to that of the strains carrying only one of these pABCs (Figure 4b). pABC3 and pABC4 were combined to study the influence of an instably propagating pABC on maintenance of a stably inherited pABC. In combination, pABC3 and pABC4 showed the same properties as each did individually; namely, biological replicates show great variation in the maintenance of pABC3, while pABC4 was stably inherited (Figure S10). In accordance with the previous growth studies of strains harboring stably propagating pLoriVSm plasmids (Figure 2c and S2), the corresponding pABCs did not affect S. meliloti cell growth even when present in combination (Figure S11).
We further tested maintenance of stably propagating pABCs under more challenging conditions by loading pABC vectors with the expR gene, whose presence restrains growth of S. meliloti.50 In the reference strain Rm1021, expR is disrupted by an insertion element.51 Complementation of this mutation results in a strong increase in galactoglucan production since the LuxR-type regulator ExpR is a key activator of biosynthesis of these exopolysaccharides.51−55expR under the control of PlacZ was integrated into pABCs based on repABC cassettes derived from pMlb (pABCa-egfp-expR), p42b (pABCb-egfp-expR), p42d (pABCc-egfp-expR), p42e (pABC4-egfp-expR), and pMla (pABC2-egfp-expR). Constitutive expression of expR significantly decreased cell growth and resulted in a mucoid colony phenotype, indicating a proper function of the expR cassette in the pABCs (Figure S12a). We compared maintenance of expR-positive and expR-negative pABCs reliably and unreliably propagating in S. meliloti. After 96 h, the expR-positive derivative of the unreliably propagating pABC2 (pABC2-egfp-expR) was virtually lost (Figure 4c), whereas the derivative lacking expR (pABC2-egfp) was still present in 17% of the cells (Figure 4a,ii). Maintenance of pABCs characterized as very reliably inherited to the daughter cells was less affected by the presence of expR. After 96 h, 63%, 83%, and 92 to 94% of cells contained pABC4, pABCa, and pABCb,c, respectively. Nevertheless, derivatives of pABCa to pABCc and pABC4 were reliably maintained with 98 to 99% positive cells after 24 h, despite carrying a functional copy of expR throughout the entire time course (Figure S12b). Hence, vectors bearing oriV cassettes derived from p42b, p42d, p42e, and pMlb exhibit remarkable inheritance even when imposing a fitness burden on the cell.
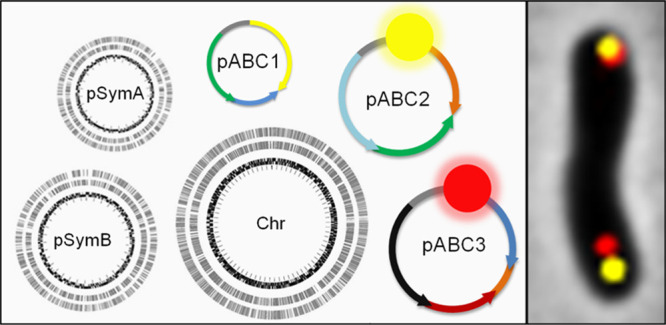
To validate the unit copy number of stably propagating pABCs and to rule out potential morphological aberrations of pABC-bearing cells, fluorescently labeled pABC derivatives were applied for microscopy analysis. To this end, Yersinia pestis and fluorescent repressor operator system (FROS) derived parS/mChr-ParB56 and tetO/TetR-YFP57 pairs were applied as a taurine-inducible dual color label system. Heterologously expressed DNA-binding proteins mChr-ParB and TetR-YFP bind to parS and tetO sequences, respectively, allowing for specific labeling of individual pABCs. Localization patterns of parS/mChr-ParB and tetO/TetR-YFP clusters implied single copy replication of pABCa to pABCc, pABC1 and pABC4, since the number of fluorescent foci never exceeded two per cell (Figure S13a). This observation is in accordance with the copy number of pLoriVSm constructs determined by qPCR (Figure 2d). Cell populations of the S. meliloti strains with the unreliably inherited pABC2 (oriVpMla), pABC5 (oriVp42f1) and pABC6 (oriVp42f2) vectors contained a substantial proportion of cells lacking fluorescent foci, indicating loss of the respective vector (Figure S13b). Aberrant cell morphologies, such as branching and elongation, were not observed. Previously, we observed that the repA2B2C2 region derived from pSymA is sufficient to confer the spatiotemporal pattern of this megaplasmid to a small plasmid.58 Although, spatiotemporal dynamics and integration of pABC into the cell cycle must be examined in more detail in future studies, microscopic analyses suggest a megaplasmid-like coordination with subpolar localization in small cells at the beginning of the cell cycle and in elongated predivisional cells (Figure 5a).
Figure 5.
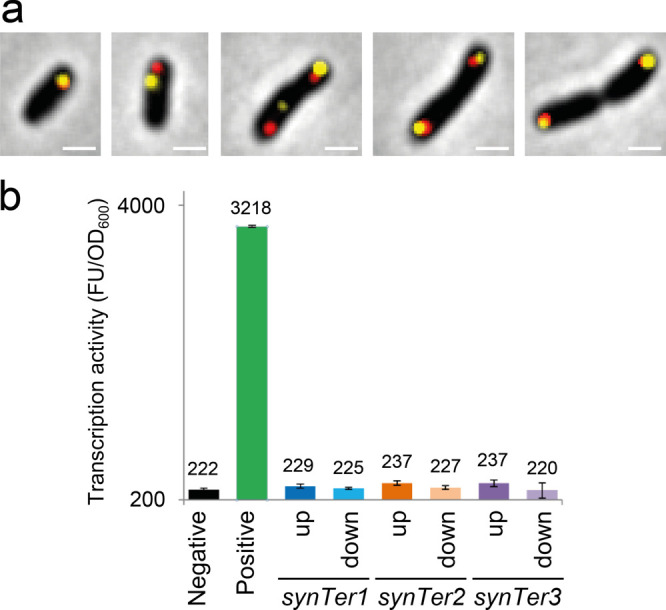
Examination of pABCs in S. meliloti. (a) Fluorescence microscopy of S. meliloti JDSm106 carrying pABCa-tetO, pABCb, and pABCc-parSYp. Expression of reporters tetR-YFP (yellow) and mChr-parBYp (red) was induced with 15 mM taurine for 4 h. Snapshots show cells at different stages of the cell cycle. Scale bar: 1 μm. (b) eGFP fluorescence of S. meliloti Rm1021 carrying pABC1-egfp_f/r (synTer1), pABC1-ST2-egfp_f/r (synTer2), and pABC1-ST3-egfp_f/r (synTer3) was taken as a measure for the transcription activity in the MCS of library-derived pABCs. Promoter-less egfp_f/r cassettes were either forwardly or reversely (corresponding to up/down orientation) integrated into the MCS. Negative: S. meliloti/pABC1; positive: S. meliloti/pABC1-egfp. Error bars indicate standard deviation calculated from three technical replicates. FU: fluorescence units.
A well-defined insulated cloning site is a main requirement for the applicability of the pABC vectors. Insulation was tested by measuring fluorescence derived from promoter-less reporter gene insertions into the cloning site (Figure 5b and S14). Thus, pABCa, pABCb, pABCc, pABC1, and pABC4 are supposed to be the most reliable candidates for applications in S. meliloti.
repABC Mediated in Vivo Cloning in S. meliloti (ABC Cloning)
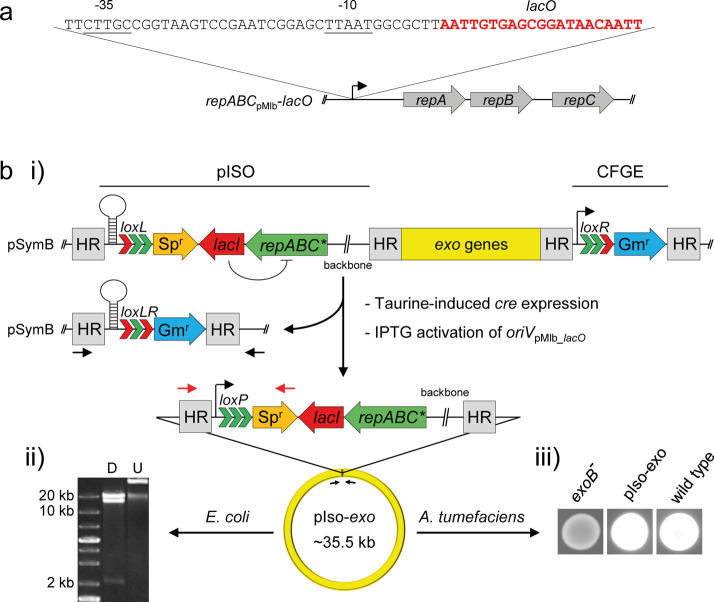
Several methods have been developed which enable direct cloning of large gene clusters, including transformation-associated recombination (TAR) in Saccharomyces cerevisiae,59−62 RecET-mediated linear-plus-linear homologous recombination (LLHR) in E. coli,63 and φBT1 integrase-mediated site-specific recombination in Streptomyces species.64 The Cre/loxP plus BAC strategy65 and oriT-directed cloning66 are well established in vivo approaches which facilitate cloning of large DNA fragments derived from α-rhizobia. The former strategy is based on Cre/loxP-mediated circularization of the DNA fragment of interest bearing a BAC oriV, which is purified from the crude cell culture and introduced to E. coli by electroporation.65 The latter strategy involves a Flp-mediated excision of the FRT site bordered DNA fragments including an oriT site and an E. coli plasmid oriV, instantly followed by mobilization of the excised DNA to E. coli.66 These direct cloning methods have proven to be very useful. However, they do not allow reliable maintenance of excised DNA in propagating donor cells, but require either DNA purification from treated cells and subsequent transformation of the crude mix into a suitable host or excision and transfer in a single step and therefore entail reduced efficiency and increased screening efforts. Properties of α-rhizobial megaplasmids such as single copy number, faithful inheritance to daughter cells, and large size make repABC-type oriVs ideal tools for in vivo cloning strategies. We devised an in vivo cloning strategy involving Cre/lox mediated excision of the target region, followed by stable extrachromosomal establishment of the excised region on an autonomously replicating repABC-type replicon, and demonstrated its feasibility by translocation of the ∼24 kb exo gene cluster from pSymB to a repABC-type replicon (Figure 6).
Figure 6.
repABC mediated in vivo cloning in S. meliloti (ABC cloning). (a) The promoter region of repABCpMLb was predicted according to the consensus motifs of S. meliloti σ70 promoters (−35 and −10 elements are underlined). A single lacO box (red letters) was integrated at the predicted transcription start site (+1). (b) ABC cloning of the exo gene cluster in S. meliloti SmCre. Three consecutive arrow heads indicate lox sites with native (green) or mutated arms (red). (i) A gentamicin resistance cassette carrying a right arm-mutated loxR site (Pmin2-loxR-aacC1) was integrated downstream of the gene cluster via cloning-free genome editing45 (CFGE). Subsequently, pISO bearing repABCpMlb-lacO (repABC*) was integrated via a ∼550 bp homologous region (HR) upstream of the gene cluster, resulting in S. meliloti SmCre_exo-IN. Taurine induction of Cre expression and simultaneous IPTG activation of oriVpMLb_lacO gave rise to SmCre_exo-OUT which carries the relocated ∼35.5 kb region (pIso-exo) comprising the entire exo gene cluster. Both the deletion site on pSymB and the fusion site on pIso-exo were PCR-amplified with primers 94 + Rev (black arrows) and 680 + 128 (red arrows), respectively, and sequencing of PCR products confirmed proper Cre-mediated recombination. (ii) pISO-exo was transferred to E. coli via triparental mating, resulting in E. coli/pIso-exo. Purified plasmid DNA was digested with NheI, resulting in fragments of 2.03 kb, 13.8 kb, and 19.6 kb fragments (D). Sequencing of the fusion site comprising loxP and aadA1 with primers 680 and 128 (red arrows) further confirmed successful cloning. U: Undigested plasmid DNA. (iii) Calcofluor fluorescence assay. pISO-exo was transferred to the exopolysaccharide-deficient A. tumefaciens C58 exoB mutant (exoB-) via triparental mating, giving rise to A. tumefaciens exoB/pIso-exo complemented for exopolysaccharide production (pIso-exo), and thus showing UV-induced fluorescence on Calcofluor-containing medium. Wild type: A. tumefaciens C58.
This strategy requires genomic integration of a repABC-type oriV region adjacent to the region of interest. To enable stable integration, we constructed a LacI-silenced and IPTG-inducible repABCpMlb operon by insertion of a lac operator sequence into the repABC promoter region (Figure 6a). Constitutive lacI expression strongly affected the capacity of pMlb_lacO bearing the engineered repABCpMlb-lacO to transform S. meliloti, most probably due to repression of the repABC operon (Figure S15a). Whereas establishment of pMlb_lacO in S. meliloti carrying the medium copy plasmid pSRKGm as LacI donor was completely abolished, S. meliloti harboring the single copy plasmid pABCb-lacI exhibited still sufficiently reduced transformation rates. Thus, lacI expression from a single copy locus achieved sufficient inactivation of repABCpMlb-lacO.
Figure 6b,i depicts the genomic situation of S. meliloti SmCre_exo-IN bearing pIso and Pmin2-loxR-aacC1 integrated up- and downstream of the exo gene cluster, respectively. Whereas the downstream fragment constitutes a functional gentamicin resistance cassette which is separated from the exo gene cluster by the Cre recognition site loxR, pIso provides a loxL site which is preceded by a terminator sequence preventing transcription of the promoter-less spectinomycin resistance gene aadA1. pIso further offers a constitutively expressed lacI gene encoding the transcriptional repressor which mutes the adjacent repABCpMlb_lacO operon in the absence of IPTG. This repression of oriV activity enables integration of pIso into the genome. Accordingly, the efficiency of S. meliloti transformation with pIso was ∼4000-fold decreased when mating mixtures were incubated on TY agar lacking IPTG and ∼20% of transconjugants carried the construct integrated into the genome (Figure S15a). Induction of Cre expression and simultaneous activation of oriVpMlb_lacO by IPTG resulted in excision and maintenance of the floxed DNA fragment, respectively, giving rise to strain S. meliloti SmCre_exo-OUT carrying pIso-exo as an autonomous mini-replicon. Successful Cre-mediated recombination, resulting in an active loxP site on pIso-exo and loxLR remaining on pSymB, was verified via sequencing (Figure S15b). Since the recombination event caused an antibiotic resistance switch, the screening effort for identifying positive clones was minimized. ABC cloning provided high efficiency rates of ∼75% after Cre/lox-mediated isolation, which can be attributed to highly efficient Cre recombination in SmCre derivatives45 in general, and suitable function of the engineered repABC-type oriV in particular. Since multiple lox sites recombine in an unpredictable manner, a single copy number of activated repABCpMlb-lacO plasmids is an important prerequisite for manageable Cre reactions. Promoter modification appeared to affect the propagation stability of pMlb_lacO and pIso-exo, while retaining copy number control, as suggested by the qPCR-based copy number determination (Figure S15c).
Transfer of pIso-exo to E. coli was achieved via triparental mating. oriVpMB1 on the pIso backbone enabled replication in E. coli due to the high copy number efficient purification of the large DNA molecule from this host (Figure 6b,ii). Moreover, conjugation-mediated transfer to A. tumefaciens C58 exoB resulted in complementation of the exopolysaccharide-deficient phenotype (Figure 6b,iii), demonstrating propagation of the engineered repABC cassette also in this host (Figure S15d).
Applicability of repABC-Based Plasmids in the Rhizobiales
To further explore the potential applicability of heterologous repABC-based vectors beyond S. meliloti, a pilot survey was performed in Agrobacterium tumefaciens C58, Mesorhizobium loti MAFF303099, Sinorhizobium medicae CC169, Sinorhizobium fredii NGR234, Rhizobium leguminosarum Norway, Methylobacterium extorquens AM1 Δcel, and Caulobacter crescentus CB15N (Table S9). In the diverse tested members of the Rhizobiales, four to seven of the eight probed repABC regions mediated plasmid replication. Even in the distantly related C. crescentus, one of the eight repABC regions (oriVpMlb) mediated plasmid replication, although growth on medium selecting for presence of this repABC-based plasmid was impaired. This may indicate unreliable replication or segregation of this plasmid, or interference with cellular functions in C. crescentus. The initial survey of the pilot set of repABC modules in α-proteobacteria suggests that this vector family is probably applicable in the Rhizobiales beyond the Rhizobiaceae, particularly considering the possibility of further expanding the portfolio of repABC modules in the future.
Conclusions
The currently available ectopic expression systems applicable in S. meliloti and related bacteria are based on a small number of low-copy and multicopy vectors. Because of incompatibilities, options for combining different vectors in one cell are very limited. Moreover, different copy number ranges and cell-to-cell variation complicate balanced gene expression even from a set of compatible plasmids. While chromosomal integration of expression constructs solves copy number problems, stable integration of multiple constructs is still cumbersome and integration sites may influence gene expression. In this study, we established the pABC vector family which circumvents these issues and highly facilitates the setup of combinatorial expression systems.
pABC vectors provide a well-defined integration site in single copy and we demonstrated stable coexistence of up to three compatible pABCs. Occurrence of α-rhizobial species harboring up to six RepABC-family plasmids23 suggests that even more than three pABC plasmids may be combined in a suitable host. The number of available resistance cassettes and restraints in the applicability of multiple antibiotics selection pressure potentially hampers combinatorial use of pABC vectors. However, oriVrepABC-based multireplicon systems benefit from robust inheritance under nonselective conditions. A future route to further stabilization of pABC plasmids, independent of antibiotic resistance cassettes, could be the integration of toxin–antitoxin (TA)-based maintenance systems, which frequently occur in S. meliloti.67 Integration of additional modules is facilitated by the modular pABC design, which also facilitates construction of pABCs with multifarious part combinations. pABC plasmids were designed as shuttle vectors capable of replication in E. coli and S. meliloti. This is an advantageous feature when it comes to E. coli-based cloning procedures and plasmid DNA purification. Nevertheless, some applications in S. meliloti may favor pABC plasmids lacking oriVEc and oriT. We demonstrated that these modules can be efficiently removed on demand by induction of Cre/lox-mediated site specific recombination in S. meliloti.
Furthermore, we took a stab at the broad potential of pABC applications by devising the in vivo ABC cloning procedure, which provides high flexibility due to isolation and immediate maintenance of the fragment of interest in a repABC-based vector. This opens up multifarious follow-up processes including intracellular relocation of the isolated fragment by Cre/lox mediated recombination or conjugal transfer to a compatible host organism. In a future perspective, the available set of nonpromiscuous lox spacer mutants45 paves the way to serial engineering steps, such as in vivo assembly of indigenous or heterologous DNA fragments on pABC plasmids. Thus, the family of pABC vectors has the potential to significantly advance metabolic and genome engineering in S. meliloti and related species and to provide a platform for in vivo DNA assembly.
Methods
Bacterial Strains and Growth Conditions
Bacterial strains used in this study are derivatives of Escherichia coli K12, Sinorhizobium meliloti Rm1021, Agrobacterium tumefaciens C58, Rhizobium etli CFN42, Sinorhizobium fredii NGR234, Mesorhizobium loti MAFF303099, Rhizobium leguminosarum Norway, Methylobacterium extorquens AM1 Δcel, Sinorhizobium medicae WSM419 and CC169, and Caulobacter crescentus CB15N. E. coli strains were cultivated at 37 °C, whereas A. tumefaciens, M. loti, R. etli, S. fredii, and S. medicae were grown at 30 °C in Luria–Bertani (LB) medium.68S. meliloti strains were cultivated at 30 °C in tryptone yeast extract (TY) medium.69 C. crescentus was grown at 28 °C on peptone-yeast extract (PYE) medium.70 If necessary, the following antibiotics were used: gentamicin (8 μg/mL for E. coli, 30 μg/mL for S. meliloti, and 50 μg/mL for A. tumefaciens), kanamycin (25 μg/mL for C. crescentus, 30 μg/mL for S. fredii, and R. leguminosarum, 50 μg/mL for E. coli, M. loti, and M. extorquens, 70 μg/mL for A. tumefaciens, 200 μg/mL for S. meliloti), streptomycin (600 μg/mL for S. meliloti and R. leguminosarum), spectinomycin (100 μg/mL for E. coli, 200 μg/mL for S. meliloti, and 500 μg/mL for A. tumefaciens), hygromycin (100 μg/mL for both E. coli and S. meliloti), nalidixic acid (10 μg/mL for A. tumefaciens, S. fredii, M. loti, and S. medicae), trimethoprim (1000 μg/mL for E. coli, 700 μg/mL for S. meliloti), chloramphenicol (20 μg/mL for E. coli) or tetracycline (10 μg/mL for E. coli and 3 μg/mL for S. meliloti). Cre/lox applications were performed as described before,46 excluding tetracycline supplementation during induction of cre expression. Calcofluor fluorescence assays of A. tumefaciens C58 exoB carrying pIso-exo were performed on LB agar supplemented with gentamycin and spectinomycin, 200 μg/mL calcofluor, and 500 μM IPTG. Cultures of an optical density at 600 nm (OD600) of 0.2 were spotted on agar medium and grown overnight at 30 °C. Calcofluor fluorescence was excited by UV transillumination.
DNA Manipulation and Plasmid Extraction
Standard techniques were employed for common cloning procedures.68 Primers and PCR products were 5′-phosphorylated applying T4 Polynucleotide Kinase (Thermo Scientific, Germany). Dephosphorylation of 5′-ends of DNA was achieved by use of FastAP Thermosensitive Alkaline Phosphatase (Fermentas). Blunting of DNA ends was performed by use of DNA Polymerase I (Large Klenow fragment) (New England Biolabs (U.K.)). Plasmid DNA was generally purified using the “E.Z.N.A. Plasmid Mini Kit” (Omega Bio-Tek). For gel extractions of DNA the “illustra GFX PCR DNA and Gel Band Purification Kit” (GE Healthcare Life Sciences) was employed.
Plasmid and Strain Construction
Strains and constructs generated in this study are listed in Table 1 and Table S10. Assembled pABCs are listed in Table 2. A detailed description of plasmids is given in Tables S7 and S11. Oligonucleotides and synthetic DNA fragments were provided by Sigma-Aldrich (USA) and Integrated DNA Technologies (USA), respectively (Table S2). DNA fragments were PCR amplified using Q5 High-Fidelity DNA Polymerase (New England Biolabs) or Taq DNA Polymerase (New England Biolabs). DNA sequence verification was performed by Eurofins Genomics (Germany). For transformation of S. meliloti, the respective strains were either conjugated or electroporated as described before.45 Preparation of electrocompetent cells was done as described by Ferri et al. (2010).
Construction of pABC mini-replicons: Amplification of module parts from library plasmids was performed with Q5 polymerase applying module specific primer sets oriVSm_for/rev (pLoriVSm derivatives), synTer-MCS_for/rev (pLsynTer derivatives), oriT_for/rev (pLoriT derivatives), and AR_for/rev (pLAR derivatives). oriVEc module parts were amplified from pK18mob2 (pMB1), pACYC184 (p15A), and pX-G10 (pSC101*) with primer sets pMB1_for/rev, p15A_for/rev, and pSC101*_for/rev, respectively. Subsequently, matching DNA fragments (blunt-end and 5′ phosphorylated) were fused via ligase cycling reaction (LCR) according to de Kok et al. (2014). For LCR 0.3 U Taq ligase (New England Biolabs), 3 nM DNA parts, 30 nM bridging oligos, and 8% (v/v) DMSO were applied. The following conditions were used: 2 min at 94 °C and then 50 cycles of 10 s at 94 °C, 30 s at 60 °C, and 60 s at 65 °C, followed by incubation at 4 °C. Reaction mixtures were purified using the E.Z.N.A. Cycle-Pure Kit (Omega Bio-Tek). A detailed pABC construction manual is provided in the Supporting Information.
For live-cell localization studies of pABC vectors, a dual label system based on the fluorescent reporter gene fusions tetR-eYFP (derived from the fluorescent repressor operator system, FROS57) and mChr-parBYp (parB derived from Yersinia pestis) was developed. Homologous regions comprising the upstream region of tauA (pSymB position 1050453–1050952) and PtauAB-tauA (pSymB position 1050781–1051458) were integrated into pK18mobsacB, thereby flanking tetR-YFP-mChr-parBYp. S. meliloti Rm1021 was transformed with the resulting plasmid pJD120. Loss of the plasmid backbone was forced by sucrose selection resulting in strain JDSm106 carrying the tetR-YFP-mChr-parBYp reporter construct stably integrated into the chromosome downstream of the tauA promoter. Localization studies of pABCs carrying either a pLAU44-derived tetO array57 (position 5117-9290) or a pMT1-derived, 86 bp parSYp sequence71 were performed in this dual label S. meliloti reporter strain JDSm106.
Control strains for cell growth and plasmid inheritance experiments were generated as follows. A homologous region comprising exsA (pSymB position 1166189-1166773) was cloned into pK18mob2, resulting in pMW1. Integration of a Pmin2-egfp cassette upstream of the exsA region in pMW1 gave rise to pMW28. pMW1 was integrated into pSymB in S. meliloti Rm1021 by homologous recombination, resulting in the kanamycin resistant strain MWSm8. Integration of pMW28 into the genome of S. meliloti ΔhsdR by homologous recombination gave rise to strain MWSm37 exhibiting constitutive egfp expression.
An S. meliloti strain suitable for ABC cloning of the exo gene cluster was constructed in a two-step cloning strategy applying cloning-free genome editing (CFGE).45 Pmin2-loxR-aacC1 (PCR amplified from pJD211 with primers Uni/Rev) was blunt-end ligated with a ∼550 bp fragment covering the exoP-thiD intergenic region (pSymB position 1190665-1191220; PCR amplified with primers 86/87). Purified ligation products were then introduced to SmCreΔhsdR by electroporation. Finally, a transformant exhibiting Pmin2-loxR-aacC1 correctly integrated into the genome downstream of the exo gene cluster was transformed with pIso, giving rise to strain SmCre_exo-IN.
DNA Transfer
Preparation of electrocompetent cells and transformation of S. meliloti, A. tumefaciens, R. etli, S. fredii, M. loti, R. leguminosarum, and S. medicae cells was performed as described by Ferri et al., 2010. Electroporation of M. extorquens and C. crescentus was carried out as described previously.72,73 To transfer pISO-exo from S. meliloti into different species, triparental matings between S. meliloti SmCre_exo-OUT and E. coli XL1blue-pRK2013, with A. tumefaciens C58 exoB or E. coli AB3219 were performed.
For triparental matings the cell density of fresh overnight cultures was adjusted to an OD600 of 2.0 and equal volumes of donor, recipient, and helper strain were mixed. A 50 μL aliquot of cell suspension was dropped on LB agar supplemented with 500 μM IPTG and incubated overnight. Cells were recovered in 1 mL sterile 0.9% (w/v) NaCl solution. For the determination of conjugation efficiency, OD600 of the cell suspensions was adjusted to 2.0 and appropriate dilutions were plated on selective LB agar containing 500 μM IPTG. The efficiency of conjugation was determined by plating the conjugation cell suspension on media selective for transconjugants as well as on media selective for all recipient cells. Conjugation frequency was expressed as the number of colony forming units (CFU) transconjugants per CFU recipient cells.
Plasmid Stability Assays
To assess inheritance stability of pLoriVSm derivatives, S. meliloti cultures were grown over 72 h at 30 °C in TY medium supplemented with streptomycin. Cell suspensions were diluted every 12 h to an OD600 of ∼0.05. Finally, dilution series were plated on nonselective TY agar and emerging colonies were streaked on both selective (Km) and nonselective TY agar. Antibiotic resistance was correlated with the presence of the assessed plasmid.
For determination of propagation stability of pABC-egfp derivatives, S. meliloti cultures were incubated over 96 h at 30 °C in TY medium. Probes were taken 0, 24, 48, and 96 h postinoculation and immediately diluted to an OD600 of 0.1 in 0.9% (w/v) NaCl. eGFP fluorescence was detected using a BD Fortessa flow cytometer. For FACS analysis 10.000 gated events were acquired giving rise to the ratio between eGFP-negative and positive cells. Sufficient sensitivity was verified (Figure S9).
qPCR-Based Copy Number Determination
S. meliloti cultures were grown in selective TY medium until OD600∼1.0 was reached and adjusted to ∼1.39 × 105 cells/μL (≙ 1 ng DNA/μL). Cell suspensions that had been incubated at 95 °C for 15 min served as template. The DNA concentration of cell lysates containing pLoriVSm derivatives was verified via Qubit fluorometric quantitation (HS Assay Kit, Thermo Fisher Scientific). qPCR was carried out in a qTOWER Thermal Cycler (Analytik Jena, Germany) using the TaykonNo Rox SYBRMasterMix dTTP Blue Kit. Reactions were performed according to the manufacturer’s instructions in a 5 μL reaction volume. Replicon copy number was calculated according to Lee et al. (2006).74
Microscopy
Phase contrast and fluorescence pictures of S. meliloti were taken using an Eclipse Ti-E inverse research microscope (Nikon) and the live imaging modus of the NIS elements software (Nikon). For fluorescence microscopy with a 100x CFI Apo TIRF Oil objective (numerical aperture of 1.49) a green DPSS solid state laser (561 nm, 50 mW; Sapphire) and a multiline Argonlaser (65 mW; Melles Griot) were applied with AHF HC filter sets F36-504 TxRed (excitation bp 562/40 nm, beam splitter 593 nm, emission bp 624/40 nm) and F36-513 YFP (excitation bp 500/24 nm, beam splitter 520 nm, emission bp 542/27 nm). Background fluorescence was reduced by applying the highly inclined laminated optical sheet (HILO) technique.75 Cell cultures were grown in TY medium supplemented with suitable antibiotics to an OD600 of 0.1 and expression of the fluorescent labeling system was induced with 15 mM taurine for 4 h. Living cells were placed onto 1% (w/v) TY agarose pads.
Bioinformatics Tools
To generate neutral orthogonal DNA sequences for module linker fragments, the web-based tool R2oDNA Designer76 was applied. To estimate the recombination potential of distinct pABCs we investigated the local sequence homology of all module parts (including linker sequences). We applied a window-based local alignment approach to focus on the local sequence homology. For a pair of parts we therefore extracted all 200 bp sequence fragments of one part and performed a local alignment on the full sequence of the second part. The best alignment similarity of all 200 bp fragments with the second vector was taken as the similarity value of both vectors.
Acknowledgments
We thank Gabriele Malengo for supporting the FACS analyses, Daniela Kiekebusch and Maritha Lippmann for technical assistance in Caulobacter handling, and Macarena Marín for providing R. leguminosarum strain Norway. Financial support from the German Research Foundation (Collaborative Research Centre TRR 174 “Spatiotemporal dynamics of bacterial cells” and INST 160/536-1 FUGG), the LOEWE program of the State of Hesse (Germany) (SYNMIKRO), the Max Planck Society, and the European Research Council (ERC 637675 “SYBORG”) is acknowledged.
Glossary
Abbreviations
- Clm
chloramphenicol
- Gm
gentamicin
- h
hour
- Hyg
hygromicin
- Km
kanamycin
- oriV
replication origin
- SD
Shine–Dalgarno sequence
- Sp
spectinomycin
- Str
streptomycin
- TA
toxin–antitoxin
- Tc
tetracycline
- Tmp
trimethoprim
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acssynbio.6b00320.
Table S1: Examination of sequence identity of pABC module parts. Table S2: Primers and synthetic DNA fragments used in this study. Table S3: Designation of pABC library parts according to the SEVA-SIB nomenclature. Table S4: Translation of pABC plasmids into the SEVA-SIB collection. Table S5: qPCR indicates a unit copy number of plasmids carrying a repABCpSymA cassette. Table S6: Copy number determination of oriVSm library plasmids. Table S7: Detailed description of pABCs generated in this study. Table S8: Flow cytometry measurements of S. meliloti Rm1021 carrying pABC-egfp derivatives. Table S9: Replication capacity of repABC-based replication origins in various α-proteobacteria. Table S10: Strains and plasmids used in Supporting Information. Table S11: Detailed description of constructs generated in this study. Figure S1. Electrophoresis banding patterns of oriVSm library plasmids. Figure S2. Growth of S. meliloti carrying stably propagating pLoriVSm library plasmids. Figure S3. Activity of E. coli transcription terminators derived from rrnBT1 and rrnBT2 in S. meliloti. Figure S4. Cre-mediated deletion of modules oriVEc and oriT in S. meliloti strain SmCreΔhsdR. Figure S5. Examination of standardized antibiotic resistance cassettes. Figure S6. Examination of promoter activity of Ptrp derivatives in S. meliloti. Figure S7. pABC setup and electroporation efficiency. Figure S8. Electrophoresis banding pattern of digested pABCs purified from S. meliloti. Figure S9. Verification of Flow Cytometry measurements. Figure S10. Propagation stability of pABC3-egfp and pABC4-egfp. Figure S11. Growth of S. meliloti Rm1021 harboring stably propagating pABCs. Figure S12. Characterization of pABC-egfp-expR derivatives. Figure S13. Fluorescence microscopy analysis of fluorescently labeled pABCs. Figure S14. Analysis of transcriptional insulation of the multiple cloning site of pABCa–c. Figure S15. Examination of ABC cloning procedures (PDF)
Author Contributions
# J.D. and M.W. contributed equally. A.B., J.D., and M.W. designed the research. J.D., M.W., and C.H. performed the experimental work. M.C., T.J.E., and M.T. contributed to the assays of repABC-based plasmids in diverse bacteria. P.S. computationally analyzed parts for sequence similarities. J.D. and M.W. analyzed all data. J.D. and A.B. wrote the paper.
The authors declare no competing financial interest.
Supplementary Material
References
- Gerdes K.; Howard M.; Szardenings F. (2010) Pushing and pulling in prokaryotic DNA segregation. Cell 141, 927–942. [DOI] [PubMed] [Google Scholar]
- Martinez-Garcia E.; Benedetti I.; Hueso A.; Lorenzo V. de (2015) Mining environmental plasmids for synthetic biology parts and devices. Microbiol. Spectrum 3, PLAS-0033-2014 10.1128/microbiolspec.PLAS-0033-2014. [DOI] [PubMed] [Google Scholar]
- Itaya M. (1999) Effective cloning of unmarked DNA fragments in the Bacillus subtilis 168 genome. Biosci., Biotechnol., Biochem. 63, 602–604. 10.1271/bbb.63.602. [DOI] [PubMed] [Google Scholar]
- Itaya M.; Tsuge K.; Koizumi M.; Fujita K. (2005) Combining two genomes in one cell: stable cloning of the Synechocystis PCC6803 genome in the Bacillus subtilis 168 genome. Proc. Natl. Acad. Sci. U. S. A. 102, 15971–15976. 10.1073/pnas.0503868102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S.; Akioka M.; Tsuge K.; Itaya M. (2005) DNA shuttling between plasmid vectors and a genome vector: systematic conversion and preservation of DNA libraries using the Bacillus subtilis genome (BGM) vector. J. Mol. Biol. 349, 1036–1044. 10.1016/j.jmb.2005.04.041. [DOI] [PubMed] [Google Scholar]
- Liang X.; Baek C.-H.; Katzen F. (2013) Escherichia coli with two linear chromosomes. ACS Synth. Biol. 2, 734–740. 10.1021/sb400079u. [DOI] [PubMed] [Google Scholar]
- Messerschmidt S. J.; Kemter F. S.; Schindler D.; Waldminghaus T. (2015) Synthetic secondary chromosomes in Escherichia coli based on the replication origin of chromosome II in Vibrio cholerae. Biotechnol. J. 10, 302–314. 10.1002/biot.201400031. [DOI] [PubMed] [Google Scholar]
- Milbredt S.; Farmani N.; Sobetzko P.; Waldminghaus T. (2016) DNA Replication in engineered Escherichia coli genomes with extra replication origins. ACS Synth. Biol. 17, 2016. 10.1021/acssynbio.6b00064. [DOI] [PubMed] [Google Scholar]
- MacLean A. M.; Finan T. M.; Sadowsky M. J. (2007) Genomes of the symbiotic nitrogen-fixing bacteria of legumes. Plant Physiol. 144, 615–622. 10.1104/pp.107.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturi V.; Keel C. (2016) Signaling in the rhizosphere. Trends Plant Sci. 21, 187–198. 10.1016/j.tplants.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Zhu J.; Oger P. M.; Schrammeijer B.; Hooykaas P. J. J.; Farrand S. K.; Winans S. C. (2000) The bases of crown gall tumorigenesis. J. Bacteriol. 182, 3885–3895. 10.1128/JB.182.14.3885-3895.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. F.; Townsend C. O. (1907) A plant-tumor of bacterial origin. Science 25, 671–673. 10.1126/science.25.643.671. [DOI] [PubMed] [Google Scholar]
- Mus F.; Crook M. B.; Garcia K.; Garcia Costas A.; Geddes B. A.; Kouri E. D.; Paramasivan P.; Ryu M.-H.; Oldroyd G. E. D.; Poole P. S.; Udvardi M. K.; Voigt C. A.; Ané J.-M.; Peters J. W. (2016) Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Appl. Environ. Microbiol. 82, 3698–3710. 10.1128/AEM.01055-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellane T. C. L.; Lemos M. V. F.; Lemos E. G. de M. (2014) Evaluation of the biotechnological potential of Rhizobium tropici strains for exopolysaccharide production. Carbohydr. Polym. 111, 191–197. 10.1016/j.carbpol.2014.04.066. [DOI] [PubMed] [Google Scholar]
- Wu D.; Li A.; Ma F.; Yang J.; Xie Y. (2016) Genetic control and regulatory mechanisms of succinoglycan and Curdlan biosynthesis in genus Agrobacterium. Appl. Microbiol. Biotechnol. 100, 6183–6192. 10.1007/s00253-016-7650-1. [DOI] [PubMed] [Google Scholar]
- Tazoe M.; Ichikawa K.; Hoshino T. (1999) Production of vitamin B6 in Rhizobium. Biosci., Biotechnol., Biochem. 63, 1378–1382. 10.1271/bbb.63.1378. [DOI] [PubMed] [Google Scholar]
- Dong H.; Li S.; Fang H.; Xia M.; Zheng P.; Zhang D.; Sun J. (2016) A newly isolated and identified vitamin B12 producing strain: Sinorhizobium meliloti 320. Bioprocess Biosyst. Eng. 39, 1527–1537. 10.1007/s00449-016-1628-3. [DOI] [PubMed] [Google Scholar]
- Gelvin S. B. (2003) Agrobacterium-mediated plant transformation: the biology behind the ″gene-jockeying″ tool. Microbiol. Mol. Biol. Rev. 67, 16–37. 10.1128/MMBR.67.1.16-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. W.; Lower R. P.; Kim N. K.; Young J. P. (2010) Introducing the bacterial ’chromid’: not a chromosome, not a plasmid. Trends Microbiol. 18, 141–148. 10.1016/j.tim.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Mazur A.; Koper P. (2012) Rhizobial plasmids — replication, structure and biological role. Cent. Eur. J. Biol. 7, 571–586. 10.2478/s11535-012-0058-8. [DOI] [Google Scholar]
- Kaneko T.; Nakamura Y.; Sato S.; Asamizu E.; Kato T.; Sasamoto S.; Watanabe A.; Idesawa K.; Ishikawa A.; Kawashima K.; Kimura T.; Kishida Y.; Kiyokawa C.; Kohara M.; Matsumoto M.; Matsuno A.; Mochizuki Y.; Nakayama S.; Nakazaki N.; Shimpo S.; Sugimoto M.; Takeuchi C.; Yamada M.; Tabata S. (2000) Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res. 7, 331–338. 10.1093/dnares/7.6.331. [DOI] [PubMed] [Google Scholar]
- Galibert F.; Finan T. A.; Long S. R.; Pühler A.; Abola P.; Ampe F.; Barloy-Hubler F.; Barnett M. J.; Becker A.; Boistard P.; Bothe G.; Boutry M.; Bowser L.; Buhrmester J.; Cadieu E.; Capela D.; Chain P.; Cowie A.; Davis R. W.; Dreano S.; Federspiel N. A.; Fisher R. F.; Gloux S.; Godrie T.; Goffeau A.; Golding B.; Gouzy J.; Gurjal M.; Hernandez-Lucas I.; Hong A.; Huizar L.; Hyman R. W.; Jones T.; Kahn D.; Kahn M. L.; Kalman S.; Keating D. H.; Kiss E.; Komp C.; Lelaure V.; Masuy D.; Palm C.; Peck M. C.; Pohl T. M.; Portetelle D.; Purnelle B.; Ramsperger B. U.; Surzycki R.; Thébault P.; Vandenbol M.; Vorhölter F.-J.; Weidner S.; Wells D. H.; Wong K.; Yeh K.-C.; Batut J. (2001) The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293, 668–672. 10.1126/science.1060966. [DOI] [PubMed] [Google Scholar]
- González V.; Santamaría R. I.; Bustos P.; Hernández-González I.; Medrano-Soto A.; Moreno-Hagelsieb G.; Janga S. C.; Ramírez M. A.; Jiménez-Jacinto V.; Collado-Vides J.; Dávila G. (2006) The partitioned Rhizobium etli genome: genetic and metabolic redundancy in seven interacting replicons. Proc. Natl. Acad. Sci. U. S. A. 103, 3834–3839. 10.1073/pnas.0508502103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. W.; Setubal J. C.; Kaul R.; Monks D. E.; Kitajima J. P.; Okura V. K.; Zhou Y.; Chen L.; Wood G. E.; Almeida N. F. Jr; Woo L.; Chen Y.; Paulsen I. T.; Eisen J. A.; Karp P. D.; Bovee D.; SR; Chapman P.; Clendenning J.; Deatherage G.; Gillet W.; Grant C.; Kutyavin T.; Levy R.; Li M. J.; McClelland E.; Palmieri A.; Raymond C.; Rouse G.; Saenphimmachak C.; Wu Z.; Romero P.; Gordon D.; Zhang S.; Yoo H.; Tao Y.; Biddle P.; Jung M.; Krespan W.; Perry M.; Gordon-Kamm B.; Liao L.; Kim S.; Hendrick C.; Zhao Z. Y.; Dolan M.; Chumley F.; Tingey S. V.; Tomb J. F.; Gordon M. P.; Olson M. V.; Nester E. W. (2001) The genome of the natural genetic engineer Agrobacterium tumefaciens C58. Science 294, 2317–2323. 10.1126/science.1066804. [DOI] [PubMed] [Google Scholar]
- Goodner B.; Hinkle G.; Gattung S.; Miller N.; Blanchard M.; Qurollo B.; Goldman B. S.; Cao Y.; Askenazi M.; Halling C.; Mullin L.; Houmiel K.; Gordon J.; Vaudin M.; Iartchouk O.; Epp A.; Liu F.; Wollam C.; Allinger M.; Doughty D.; Scott C.; Lappas C.; Markelz B.; Flanagan C.; Crowell C.; Gurson J.; Lomo C.; Sear C.; Strub G.; Cielo C.; Slater S. (2001) Genome sequence of the plant pathogen and biotechnology agent Agrobacterium tumefaciens C58. Science 294, 2323–2328. 10.1126/science.1066803. [DOI] [PubMed] [Google Scholar]
- Pinto U. M.; Pappas K. M.; Winans S. C. (2012) The ABCs of plasmid replication and segregation. Nat. Rev. Microbiol. 10, 755–765. 10.1038/nrmicro2882. [DOI] [PubMed] [Google Scholar]
- Cevallos M. A.; Cervantes-Rivera R.; Gutiérrez-Ríos R. M. (2008) The repABC plasmid family. Plasmid 60, 19–37. 10.1016/j.plasmid.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Brom S.; García de los Santos A.; Stepkowsky T.; Flores M.; Dávila G.; Romero D.; Palacios R. (1992) Different plasmids of Rhizobium leguminosarum bv. phaseoli are required for optimal symbiotic performance. J. Bacteriol. 174, 5183–5189. 10.1128/jb.174.16.5183-5189.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brom S.; García-de los Santos A.; Cervantes L.; Palacios R.; Romero D. (2000) Rhizobium etli symbiotic plasmid transfer, nodulation competitivity and cellular growth require interaction among different replicons. Plasmid 44, 34–43. 10.1006/plas.2000.1469. [DOI] [PubMed] [Google Scholar]
- Ramírez-Romero M. A.; Soberón N.; Pérez-Oseguera A.; Téllez-Sosa J.; Cevallos M. A. (2000) Structural elements required for replication and incompatibility of the Rhizobium etli symbiotic plasmid. J. Bacteriol. 182 (11), 3117–3124. 10.1128/JB.182.11.3117-3124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLellan S. R.; Zaheer R.; Sartor A. L.; MacLean A. M.; Finan T. M. (2006) Identification of a megaplasmid centromere reveals genetic structural diversity within the repABC family of basic replicons. Mol. Microbiol. 59 (5), 1559–1575. 10.1111/j.1365-2958.2006.05040.x. [DOI] [PubMed] [Google Scholar]
- Landeta C.; Dávalos A.; Cevallos M. Á.; Geiger O.; Brom S.; Romero D. (2011) Plasmids with a chromosome-like role in rhizobia. J. Bacteriol. 193, 1317–1326. 10.1128/JB.01184-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Rivera R.; Romero-López C.; Berzal-Herranz A.; Cevallos M. A. (2010) Analysis of the mechanism of action of the antisense RNA that controls the replication of the repABC plasmid p42d. J. Bacteriol. 192, 3268–3278. 10.1128/JB.00118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Urbalejo A.; Pérez-Oseguera Á.; Carreón-Rodríguez O. E.; Cevallos M. A. (2015) Mutations in an antisense RNA, involved in the replication control of a repABC plasmid, that disrupt plasmid incompatibility and mediate plasmid speciation. Plasmid 78, 48–58. 10.1016/j.plasmid.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Pérez-Oseguera A.; Cevallos M. A. (2013) RepA and RepB exert plasmid incompatibility repressing the transcription of the repABC operon. Plasmid 70, 362–376. 10.1016/j.plasmid.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Yip C. B.; Ding H.; Hynes M. F. (2015) Counter-transcribed RNAs of Rhizobium leguminosarum repABC plasmids exert incompatibility effects only when highly expressed. Plasmid 78, 37–47. 10.1016/j.plasmid.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Koper P.; Żebracki K.; Marczak M.; Skorupska A.; Mazur A. (2016) RepB proteins of the multipartite Rhizobium leguminosarum bv. trifolii genome discriminate between centromere-like parS sequences for plasmid segregational stability. Mol. Microbiol. 2, 102–446. 10.1111/mmi.13472. [DOI] [PubMed] [Google Scholar]
- Ding H.; Hynes M. F. (2009) Plasmid transfer systems in the rhizobia. Can. J. Microbiol. 55, 917–927. 10.1139/W09-056. [DOI] [PubMed] [Google Scholar]
- Schäfer A.; Tauch A.; Jäger W.; Kalinowski J.; Thierbach G.; Pühler A. (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145, 69–73. 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
- Silva-Rocha R.; Martínez-García E.; Calles B.; Chavarría M.; Arce-Rodríguez A.; de las Heras A.; Páez-Espino A. D.; Durante-Rodríguez G.; Kim J.; Nikel P. I.; Platero R.; de Lorenzo V. (2013) The Standard European Vector Architecture (SEVA): a coherent platform for the analysis and deployment of complex prokaryotic phenotypes. Nucleic Acids Res. 41, D666–675. 10.1093/nar/gks1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E.; Engler C.; Gruetzner R.; Werner S.; Marillonnet S. (2011) A modular cloning system for standardized assembly of multigene constructs. PLoS One 6 (2), e16765. 10.1371/journal.pone.0016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty R. P.; Endy D.; Knight T. F. Jr. (2008) Engineering BioBrick vectors from BioBrick parts. J. Biol. Eng. 2, 5. 10.1186/1754-1611-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucca S.; Pasotti L.; Politi N.; Cusella De Angelis M. G.; Magni P. (2013) A standard vector for the chromosomal integration and characterization of BioBrick parts in Escherichia coli. J. Biol. Eng. 7 (1), 12. 10.1186/1754-1611-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A.; Barnett M. J.; Capela D.; Dondrup M.; Kamp P.-B.; Krol E.; Linke B.; Rüberg S.; Runte K.; Schroeder B. K.; Weidner S.; Yurgel S. N.; Batut J.; Long S. R.; Pühler A.; Goesmann A. (2009) A portal for rhizobial genomes: RhizoGATE integrates a Sinorhizobium meliloti genome annotation update with postgenome data. J. Biotechnol. 140, 45–50. 10.1016/j.jbiotec.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döhlemann J.; Brennecke M.; Becker A. (2016) Cloning-free genome engineering in Sinorhizobium meliloti advances applications of Cre/loxP site-specific recombination. J. Biotechnol. 233, 160–170. 10.1016/j.jbiotec.2016.06.033. [DOI] [PubMed] [Google Scholar]
- Harrison C. L.; Crook M. B.; Peco G.; Long S. R.; Griffitts J. S. (2011) Employing site-specific recombination for conditional genetic analysis in Sinorhizobium meliloti. Appl. Environ. Microbiol. 77, 3916–3922. 10.1128/AEM.00544-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kok S.; Stanton L. H.; Slaby T.; Durot M.; Holmes V. F.; Patel K. G.; Platt D.; Shapland E. B.; Serber Z.; Dean J.; Newman J. D.; Chandran S. S. (2014) Rapid and reliable DNA assembly via ligase cycling reaction. ACS Synth. Biol. 3, 97–106. 10.1021/sb4001992. [DOI] [PubMed] [Google Scholar]
- Datta N.; Hedges R. W.; Shaw E. J.; Sykes R. B.; Richmond M. H. (1971) Properties of an R factor from Pseudomonas aeruginosa. J. Bacteriol. 108, 1244–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P. L.; Farrand S. K. (2000) The replicator of the nopaline-type Ti plasmid pTiC58 is a member of the repABC family and is influenced by the TraR-dependent quorum-sensing regulatory system. J. Bacteriol. 182, 179–188. 10.1128/JB.182.1.179-188.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoenpanich P.; Soto M. J.; Becker A.; McIntosh M. (2015) Quorum sensing restrains growth and is rapidly inactivated during domestication of Sinorhizobium meliloti. Environ. Microbiol. Rep. 7, 373–382. 10.1111/1758-2229.12262. [DOI] [PubMed] [Google Scholar]
- Pellock B. J.; Teplitski M.; Boinay R. P.; Bauer W. D.; Walker G. C. (2002) A LuxR homolog controls production of symbiotically active extracellular polysaccharide II by Sinorhizobium meliloti. J. Bacteriol. 184, 5067–5076. 10.1128/JB.184.18.5067-5076.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M.; Chen H.; Eberhard A.; Gronquist M. R.; Robinson J. B.; Rolfe B. G.; Bauer W. D. (2005) sinI- and expR-dependent quorum sensing in Sinorhizobium meliloti. J. Bacteriol. 187, 7931–7944. 10.1128/JB.187.23.7931-7944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang H. H.; Becker A.; González J. E. (2004) The LuxR homolog ExpR, in combination with the Sin quorum sensing system, plays a central role in Sinorhizobium meliloti gene expression. J. Bacteriol. 186, 5460–5472. 10.1128/JB.186.16.5460-5472.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn S. A.; Gurich N.; Feeney M. A.; González J. E. (2007) The ExpR/Sin quorum-sensing system controls succinoglycan production in Sinorhizobium meliloti. J. Bacteriol. 189, 7077–7088. 10.1128/JB.00906-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marketon M. M.; Glenn S. A.; Eberhard A.; Gonzalez J. E. (2003) Quorum sensing controls exopolysaccharide production in Sinorhizobium meliloti. J. Bacteriol. 185, 325–331. 10.1128/JB.185.1.325-331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. A.; Shapiro L. (2011) An SMC ATPase mutant disrupts chromosome segregation in Caulobacter. Mol. Microbiol. 82, 1359–1374. 10.1111/j.1365-2958.2011.07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau I. F.; Filipe S. R.; Søballe B.; Økstad O.-A.; Barre F.-X.; Sherratt D. J. (2003) Spatial and temporal organization of replicating Escherichia coli chromosomes. Mol. Microbiol. 49, 731–743. 10.1046/j.1365-2958.2003.03640.x. [DOI] [PubMed] [Google Scholar]
- Frage B.; Döhlemann J.; Robledo M.; Lucena D.; Sobetzko P.; Graumann P. L.; Becker A. (2016) Spatiotemporal choreography of chromosome and megaplasmids in the Sinorhizobium meliloti cell cycle. Mol. Microbiol. 100, 808–823. 10.1111/mmi.13351. [DOI] [PubMed] [Google Scholar]
- Cocchia M.; Kouprina N.; Kim S.-J.; Larionov V.; Schlessinger D.; Nagaraja R. (2000) Recovery and potential utility of YACs as circular YACs/BACs. Nucleic Acids Res. 28, e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem S.-H.; Noskov V. N.; Park J.-E.; Kim S. I.; Larionov V.; Kouprina N. (2003) Optimum conditions for selective isolation of genes from complex genomes by transformation-associated recombination cloning. Nucleic Acids Res. 31, e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z.; Kim J. H.; Brady S. F. (2010) Fluostatins produced by the heterologous expression of a TAR reassembled environmental DNA derived type II PKS gene cluster. J. Am. Chem. Soc. 132, 11902–11903. 10.1021/ja104550p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z.; Luo Y.; Zhao H. (2011) Rapid characterization and engineering of natural product biosynthetic pathways via DNA assembler. Mol. BioSyst. 7, 1056–1059. 10.1039/c0mb00338g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J.; Bian X.; Hu S.; Wang H.; Huang F.; Seibert P. M.; Plaza A.; Xia L.; Müller R.; Stewart A. F.; Zhang Y. (2012) Full-length RecE enhances linear-linear homologous recombination and facilitates direct cloning for bioprospecting. Nat. Biotechnol. 30, 440–446. 10.1038/nbt.2183. [DOI] [PubMed] [Google Scholar]
- Du D.; Wang L.; Tian Y.; Liu H.; Tan H.; Niu G. (2015) Genome engineering and direct cloning of antibiotic gene clusters via phage ϕBT1 integrase-mediated site-specific recombination in Streptomyces. Sci. Rep. 5, 8740. 10.1038/srep08740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S.; Liu Z.; Zhang X.; Zhang G.; Xie Y.; Ding X.; Mo X.; Stewart A. F.; Fu J.; Zhang Y.; Xia L. (2016) ″Cre/loxP plus BAC″: a strategy for direct cloning of large DNA fragment and its applications in Photorhabdus luminescens and Agrobacterium tumefaciens. Sci. Rep. 6, 29087. 10.1038/srep29087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chain P. S.; Hernandez-Lucas I.; Golding B.; Finan T. M. (2000) oriT-directed cloning of defined large regions from bacterial genomes: Identification of the Sinorhizobium meliloti pExo megaplasmid replicator region. J. Bacteriol. 182, 5486–5494. 10.1128/JB.182.19.5486-5494.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milunovic B.; diCenzo G. C.; Morton R. A.; Finan T. M. (2014) Cell growth inhibition upon deletion of four toxin-antitoxin loci from the megaplasmids of Sinorhizobium meliloti. J. Bacteriol. 196, 811–824. 10.1128/JB.01104-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., and Sambrook J. (2012) Molecular cloning: A laboratory manual, 4th ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [Google Scholar]
- Beringer J. E. (1974) R factor transfer in Rhizobium leguminosarum. Microbiology 84, 188–198. 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Poindexter J. S. (1964) Biological properties and classification of the Caulobacter group. Bacteriol. Rev. 28, 231–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindler L. E.; Plano G. V.; Burland V.; Mayhew G. F.; Blattner F. R. (1998) Complete DNA sequence and detailed analysis of the Yersinia pestis KIM5 plasmid encoding murine toxin and capsular antigen. Infect. Immun. 66, 5731–5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama H.; Anthony C.; Lidstrom M. E. (1998) Construction of insertion and deletion mxa mutants of Methylobacterium extorquens AM1 by electroporation. FEMS Microbiol. Lett. 166, 1–7. 10.1111/j.1574-6968.1998.tb13175.x. [DOI] [PubMed] [Google Scholar]
- Ely B. (1991) Genetics of Caulobacter crescentus. Methods Enzymol. 204, 372–384. 10.1016/0076-6879(91)04019-K. [DOI] [PubMed] [Google Scholar]
- Lee C.; Kim J.; Shin S. G.; Hwang S. (2006) Absolute and relative QPCR quantification of plasmid copy number in Escherichia coli. J. Biotechnol. 123, 273–280. 10.1016/j.jbiotec.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Tokunaga M.; Imamoto N.; Sakata-Sogawa K. (2008) Highly inclined thin illumination enables clear single-molecule imaging in cells. Nat. Methods 5, 159–161. 10.1038/nmeth1171. [DOI] [PubMed] [Google Scholar]
- Casini A.; Christodoulou G.; Freemont P. S.; Baldwin G. S.; Ellis T.; MacDonald J. T. (2014) R2oDNA designer: computational design of biologically neutral synthetic DNA sequences. ACS Synth. Biol. 3, 525–528. 10.1021/sb4001323. [DOI] [PubMed] [Google Scholar]
- Cervantes-Rivera R.; Pedraza-López F.; Pérez-Segura G.; Cevallos M. A. (2011) The replication origin of a repABC plasmid. BMC Microbiol. 11, 158. 10.1186/1471-2180-11-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignell C.; Thomas C. M. (2001) The bacterial ParA-ParB partitioning proteins. J. Biotechnol. 91, 1–34. 10.1016/S0168-1656(01)00293-0. [DOI] [PubMed] [Google Scholar]
- Oresnik I. J.; Liu S. L.; Yost C. K.; Hynes M. F. (2000) Megaplasmid pRme2011a of Sinorhizobium meliloti is not required for viability. J. Bacteriol. 182, 3582–3586. 10.1128/JB.182.12.3582-3586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinto C.; de la Vega H.; Flores M.; Fernández L.; Ballado T.; Soberón G.; Palacios R. (1982) Reiteration of nitrogen fixation gene sequences in Rhizobium phaseoli. Nature 299, 724–726. 10.1038/299724a0. [DOI] [Google Scholar]
- Reeve W.; Chain P.; O’Hara G.; Ardley J.; Nandesena K.; Bräu L.; Tiwari R.; Malfatti S.; Kiss H.; Lapidus A.; Copeland A.; Nolan M.; Land M.; Hauser L.; Chang Y.-J.; Ivanova N.; Mavromatis K.; Markowitz V.; Kyrpides N.; Gollagher M.; Yates R.; Dilworth M.; Howieson J. (2010) Complete genome sequence of the Medicago microsymbiont Ensifer (Sinorhizobium) medicae strain WSM419. Stand. Genomic Sci. 2, 77–86. 10.4056/sigs.43526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casse F.; Boucher C.; Julliot J. S.; Michel M.; Dénarié J. (1978) Identification and characterization of large plasmids in Rhizobium meliloti using agarose gel electrophoresis. J. Gen. Microbiol. 113, 229–242. 10.1099/00221287-113-2-229. [DOI] [Google Scholar]
- Perret X.; Fellay R.; Bjourson A. J.; Cooper J. E.; Brenner S.; Broughton W. J. (1994) Subtraction hybridisation and shot-gun sequencing: a new approach to identify symbiotic loci. Nucleic Acids Res. 22, 1335–1341. 10.1093/nar/22.8.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossmann J. A.; Markmann K.; Brachmann A.; Rose L. E.; Parniske M. (2012) Polymorphic infection and organogenesis patterns induced by a Rhizobium leguminosarum isolate from Lotus root nodules are determined by the host genotype. New Phytol. 196 (2), 561–73. 10.1111/j.1469-8137.2012.04281.x. [DOI] [PubMed] [Google Scholar]
- Delaney N. F.; Kaczmarek M. E.; Ward L. M.; Swanson P. K.; Lee M. C.; Marx C. J. (2013) Development of an optimized medium, strain and high-throughput culturing methods for Methylobacterium extorquens. PLoS One 8 (4), e62957. 10.1371/journal.pone.0062957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evinger M.; Agabian N. (1977) Envelope-associated nucleoid from Caulobacter crescentus stalked and swarmer cells. J. Bacteriol. 132, 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. (1983) Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166, 557–580. 10.1016/S0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Simon R.; Priefer U.; Pühler A. (1983) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology 1, 784–791. 10.1038/nbt1183-784. [DOI] [Google Scholar]
- Zähringer F.; Lacanna E.; Jenal U.; Schirmer T.; Boehm A. (2013) Structure and signaling mechanism of a zinc-sensory diguanylate cyclase. Structure 21, 1149–1157. 10.1016/j.str.2013.04.026. [DOI] [PubMed] [Google Scholar]
- Khan S. R.; Gaines J.; Roop R. M. 2nd; Farrand S. K. (2008) Broad-host-range expression vectors with tightly regulated promoters and their use to examine the influence of TraR and TraM expression on Ti plasmid quorum sensing. Appl. Environ. Microbiol. 74, 5053–5062. 10.1128/AEM.01098-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J. H.; Vogel J. (2007) Translational control and target recognition by Escherichia coli small RNAs in vivo. Nucleic Acids Res. 35, 1018–1037. 10.1093/nar/gkl1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C. Y.; Cohen S. N. (1978) Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134, 1141–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner P.; Willison J. C.; Vignais P. M.; Bickle T. A. (1991) Expression of regulatory nif genes in Rhodobacter capsulatus. J. Bacteriol. 173, 2993–2999. 10.1128/jb.173.9.2993-2999.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H.; Helinski D. R. (1979) Replication of an origin-containing derivative of RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. U. S. A. 76, 1648–1652. 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.