Abstract
Antibody (Ab) responses to SARS-CoV-2 can be detected in most infected individuals 10-15 days following the onset of COVID-19 symptoms. However, due to the recent emergence of this virus in the human population it is not yet known how long these Ab responses will be maintained or whether they will provide protection from re-infection. Using sequential serum samples collected up to 94 days post onset of symptoms (POS) from 65 RT-qPCR confirmed SARS-CoV-2-infected individuals, we show seroconversion in >95% of cases and neutralizing antibody (nAb) responses when sampled beyond 8 days POS. We demonstrate that the magnitude of the nAb response is dependent upon the disease severity, but this does not affect the kinetics of the nAb response. We further reveal that the nAb response after SARS-CoV-2 infection is typical of an acute viral infection with declining nAb titres observed following an initial peak. Whilst some individuals with high peak ID50 (>10,000) maintained nAb titres >1,000 at >60 days POS, some with lower peak ID50 had nAb titres approaching baseline within the follow up period. A similar decline in nAb titres was also observed in a cohort of seropositive healthcare workers from Guy’s and St Thomas’ Hospitals. This study has important implications when considering widespread serological testing, Ab protection against re-infection with SARS-CoV-2 and the durability of vaccine-induced protection.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a betacoronavirus responsible for coronavirus disease-19 (COVID-19). Spike (S) is the virally encoded surface glycoprotein facilitating angiotensin converting enzyme-2 (ACE-2) receptor binding on target cells through its receptor binding domain (RBD). In a rapidly evolving field, researchers have already shown that, in most cases, individuals with a confirmed PCR diagnosis of SARS-CoV-2 infection develop IgM, IgA and IgG against the virally encoded surface spike protein (S) and nucleocapsid protein (N) within 1-2 weeks post onset of symptoms (POS) and remain elevated following initial viral clearance.1–7 S is the target for nAbs, and a number of highly potent monoclonal antibodies (mAbs) have been isolated that predominantly target the receptor binding domain.8–11 A wide range of SARS-CoV-2 neutralizing antibody (nAb) titres have been reported following infection and these vary depending on the length of time from infection and the severity of disease.4,3,5,12,13 Further knowledge on the magnitude, timing and longevity of nAb responses following SARS-CoV-2 infection is vital for understanding the role nAbs might play in disease clearance and protection from reinfection (also called renewed or second wave infections) or disease. Further, as a huge emphasis has been placed on Ab reactivity assays to determine seroprevalence against SARS-CoV-2 in the community and estimating infection rates, it is important to understand immune responses following infection to define parameters in which Ab tests can provide meaningful data in the absence of PCR testing in population studies.
Ab responses to other human coronaviruses have been reported to wane over time.14–17 In particular, Ab responses targeting endemic human alpha- and betacoronaviruses can last for as little as 12 weeks,18 whereas Abs to SARS-CoV and MERS can be detected in some individuals 12-34 months after infection.15,19 Cross-sectional studies in SARS-CoV-2 infected individuals have so far reported lower mean nAb titres for serum samples collected at later time points POS (23-52 days).20,4,7 However, there is currently a paucity of information on the kinetics and longevity of the nAb response using multiple sequential samples from individuals in the convalescent phase beyond 30-40 days POS.3,5,21 This study uses sequential samples from 65 individuals with PCR confirmed SARS-CoV-2 infection and 31 seropositive healthcare workers (HCW) up to 94 days POS to understand the kinetics of nAb development and the magnitude and durability of the nAb response.
Here, we measured the Ab binding response to S, the receptor binding domain (RBD) of S and N, as well as the neutralization potency against SARS-CoV-2 using a surrogate HIV-1 based pseudotype viral entry inhibition assay and a wild type virus neutralization assay. We show that IgM and IgA binding responses decline after 20-30 days POS. We demonstrate that the magnitude of the nAb response is dependent upon the disease severity but this does not impact on the time to ID50 peak (serum dilution that inhibits 50% infection). In some individuals that develop modest nAb titres following infection (100-300 range), titres become undetectable (ID50 <50) or are approaching baseline after ~50 days highlighting the transient nature of the Ab response towards SARS-CoV-2 in some individuals. In contrast, those with high peak ID50 for neutralization maintain nAb titres in the 1,000-3,500 range at the final timepoint tested (>60 days POS). This study has important implications when considering protection against re-infection with SARS-CoV-2 and the durability of vaccine protection.
Results
Cohort description
The antibody response in 65 RT-qPCR confirmed SARS-CoV-2-infected individuals was studied over sequential time points. The cohort consisted of 59 individuals admitted to, and 6 healthcare workers (HCW) at, Guy’s and St Thomas’ NHS Foundation Trust (GSTFT). The cohort were 77.2% male with average age of 55.2 years (range 23-95 years) (Table 1). Ethnicity information was not collected on this cohort. A severity score was assigned to patients based on the maximal level of respiratory support they required during their period of hospitalisation. The score, ranging from 0-5 (see methods), was devised to mitigate underestimating disease severity in patients not for escalation above level one (ward-based) care. This cohort included the full breadth of COVID-19 severity, from asymptomatic infection to those requiring extra corporeal membrane oxygenation (ECMO) for severe respiratory failure. Comorbidities included diabetes mellitus, hypertension, and obesity, with a full summary in Table S1. Sequential serum samples were collected from individuals at time-points between 1- and 94-days post onset of symptoms (POS) and were based upon availability of discarded samples taken as part of routine clinical care, or as part of a HCW study.
Table 1. Cohort description.
Gender, severity, age, and outcome.
| Male | 51 (78.5%) |
| Female | 14 (21.5%) |
| Age | |
| Mean | 55.2 years (23-95) |
| Severity | |
| 0 | 14 |
| 1 | 10 |
| 2 | 7 |
| 3 | 2 |
| 4 | 25 |
| 5 | 7 |
| Outcome | |
| HCW | 6 |
| Died | 12 |
| Discharged | 41 |
| Still in hospital | 5 |
| Transferred to local | 3 |
Antibody binding responses to SARS-CoV-2
The IgG, IgM and IgA response against S, RBD and N were measured by ELISA (enzyme-linked immunosorbent assay) over multiple time points (Figure 1 and S1).6 >300 pre-COVID-19 healthy control samples and >100 sera from PCR confirmed SARS-CoV-2 infected individuals were previously used to validate the ELISA setup.6 Initially, the optical density at 1:50 serum dilution was measured for 300 samples from the 65 individuals (Figure 1 and S1). Only 2/65 individuals (3.1%) did not generate a detectable Ab response against any of the antigens in the follow up period (Table S2). However, sera were only available up until 2- and 8-days POS for these two individuals and as the mean time to seroconversion against at least 1 antigen was 12.6 days POS, it is likely these individuals may have seroconverted at a later time point after they were discharged from hospital. IgG responses against S, RBD and N antigens were observed in 92.3%, 89.2% and 93.8% of individuals respectively (Table S2). The frequency of individuals generating an IgM response was similar to IgG, with 92.3%, 92.3% and 95.4% seropositive against S, RBD and N respectively. The frequency of individuals with an IgA response to RBD and N was lower, with only 72.3% and 84.6% seropositive respectively (Table S2) whereas the IgA to S frequency was similar to the IgM and IgG.
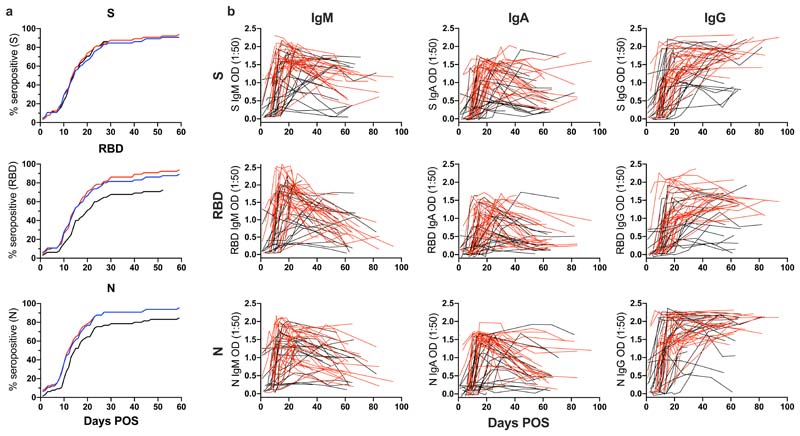
Figure 1. Kinetics of antibody development against SARS-CoV-2 antigens over time.
A) A cumulative frequency analysis describing the point of seroconversion for each person in the cohort. Graph shows the percentage of individuals in the cohort that become IgM, IgA or IgG positive to S, RBD and N each day post onset of symptoms. A serum sample is considered positive when the OD is 4-fold above background. B) OD values at 1:50 serum dilution for IgM, IgA and IgG against S, RBD and N overtime. Each line represents one individual. Severity 0-3 are shown in black and severity 4/5 are shown in red. Development of the ELISA assay is described in Pickering et al 6.
A cumulative frequency analysis of positive IgG, IgA and IgM responses against S, RBD and N across the cohort did not indicate a more rapid elicitation of IgM and IgA responses against a particular antigen (Figure 1A and S2A) and may reflect the sporadic nature in which sequential serum samples were collected. Therefore, a subset of donors from whom sera was collected over sequential time points early in infection (<14 days POS) were analysed further and different patterns of seroconversion were observed (Figure S2B). 51.6% (16/31) of individuals showed synchronous seroconversion to IgG, IgM and IgA whilst some individuals showed singular seroconversion to IgG (9.7%), IgM (9.7%) and IgA (9.7%). 58.1% (18/31) of individuals showed synchronous seroconversion to S, RBD and N, whereas singular seroconversion to N or S were both seen in 16.1% of individuals.
Longitudinal analysis across sequential samples highlighted the rapid decline in the IgM and IgA response to all three antigens following the peak OD between 20- and 30-days POS for IgM and IgA respectively (Figure 1b and S1a) as might be expected following an acute viral infection.14,22–24 For some individuals sampled at time points >60 days POS, the IgM and IgA responses were approaching baseline (Figure 2b and S1a). In contrast, the IgG OD (as measured at 1:50 dilution) remained high in the majority of individuals, even up to 94 days POS (Figure 1b and S1a). However, differences were apparent when patients were stratified by disease severity (Figure 2b) and when half maximal binding (EC50) was measured (see below, Figure 4b-d).
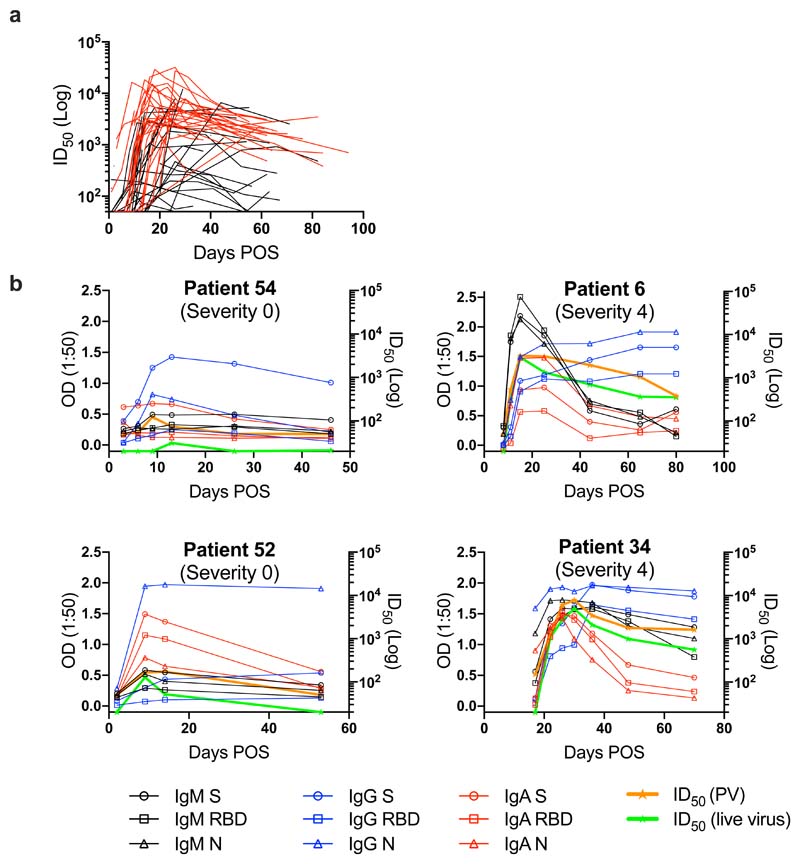
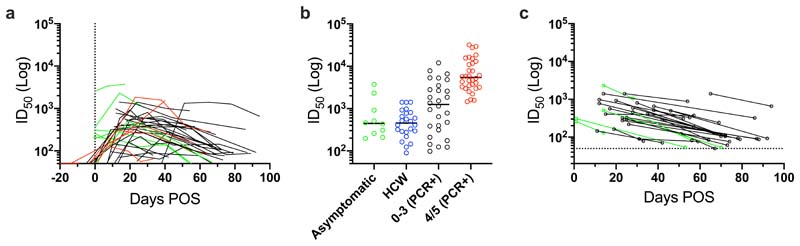
Figure 2. Kinetics of neutralizing antibody responses in SARS-CoV-2 infection.
a) nAb ID50 change related to days POS. ID50 measured using HIV-1 based virus particles (PV), pseudotyped with SARS-CoV-2 S. Each line represents one individual. Severity 0-3 are shown in black and severity 4/5 are shown in red. b) Example kinetics of Ab responses (IgM, IgA, IgG binding to S, RBD and N, and ID50 against PV and wild type virus) for four individuals during acute infection and the convalescent phase. Graphs show comparison between severity 0 (left) and severity 4 (right) rated disease. The cut-off for the pseudovirus and wild-type virus neutralization assays are 1:50 and 1:20 respectively. Error bars for OD values represent the range of the value for experiments performed in duplicate (not shown when smaller than symbol size).
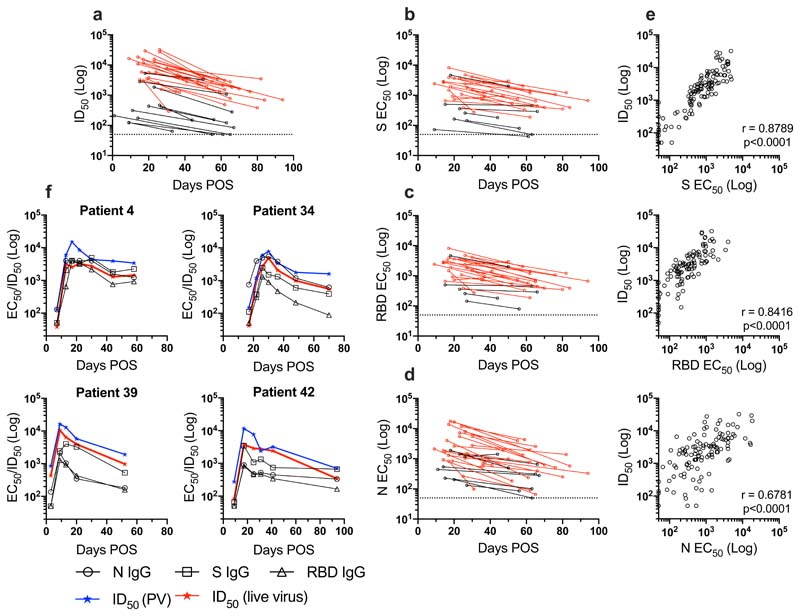
Figure 4. Longevity of the nAb response.
a) ID50 at peak neutralization (measured using HIV-1 based virus particles, pseudotyped with SARS-CoV-2 S) is plotted against the donor matched ID50 at the last time point sera was collected. Only individuals where the peak ID50 occurs before the last time point, and where the last time point is >30 days POS are included in this analysis. The dotted line represents the cut-off for the pseudovirus neutralization assay. b-c) EC50 values for IgG binding to S, RBD and N were calculated at time points with peak ID50 and the final time point sera was collected. EC50 at peak neutralization is plotted with the donor matched EC50 at the last time point sera was collected. Individuals with a disease severity 0-3 are shown in black and those with 4/5 are shown in red. The dotted line represents the cut-off for EC50 measurement. e) Correlation of ID50 with IgG EC50 against S (r 2=0.8293), RBD (r 2=0.7128) and N (r 2=0.4856) (Spearman correlation, r. A linear regression was used to calculate the goodness of fit, r 2). f) Change in IgG EC50 measured against S, RBD and N and ID50 using pseudovirus and wild type virus over time for 4 example patients (all severity 4). The lowest dilution used for the pseudovirus and wild-type virus neutralization assays are 1:50 and 1:20 respectively.
Neutralizing antibody responses to SARS-CoV-2
We next measured SARS-CoV-2 neutralization potency using a surrogate viral inhibition assay that utilises HIV-1 (human immunodeficiency virus type-1) based virus particles, pseudotyped with SARS-CoV-2 S25,26 and a HeLa cell-line stably expressing the ACE2 receptor. Increased neutralization potency was observed with increasing days POS (Figure 2a) with each individual reaching a peak neutralization titre (ranging from 98 to 32,000) after an average of 23.1 days POS (range 1-66 days) (Figure S1b). Only two individuals (3.1%) did not develop a nAb response (ID50 <50) which was consistent with their lack of binding Abs at the time points tested (<8 days POS). Pre-COVID-19 healthy control samples also did not show any neutralization at a 1:20 serum dilution (Table S3). At peak neutralization, 7.7% had low (50-200), 10.8% medium (201-500), 18.5% high (501-2,000) and 60.0% potent (2,001+) neutralizing titres. For serum samples collected after 65 days POS, the percentage of donors with potent nAbs (ID50 >2,000) had reduced to 16.7% (Table S3). Neutralization ID50 values correlated well with IgG, IgM and IgA binding OD values to all three antigens, S, RBD and N (Figure S3a), and the best fit (r 2) was observed between ID50 and the OD for S IgA and S IgM. The average time to detectable neutralization was 14.3 days POS (range 3-59 days). At earlier time points POS, some individuals displayed neutralizing activity before an IgG response to S and RBD was detectable by ELISA (Figure S2c). This highlights the capacity of S- and RBD-specific IgM and IgA to facilitate neutralization in acute infection in the absence of measurable IgG.27
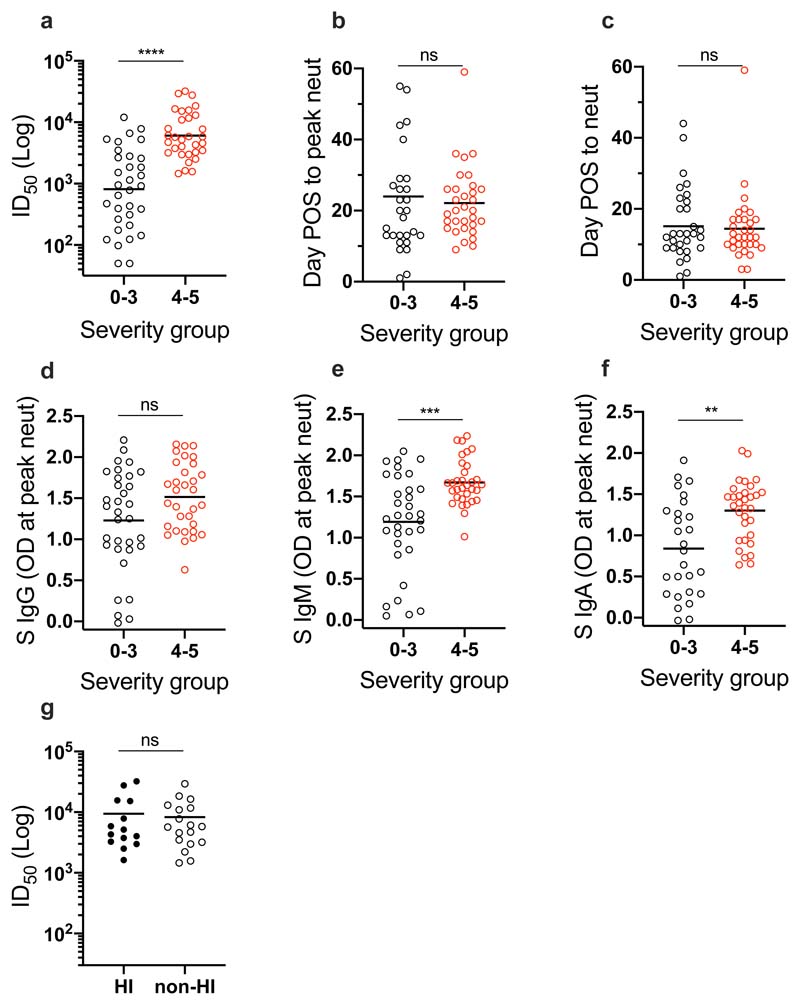
To determine how disease severity impacts Ab titres, we compared the peak ID50 values between individuals with 0-3 disease severity with those in the 4/5 group (Figure 3). Although the magnitude of the nAb response at peak neutralization was significantly higher in the severity 4/5 group (Figure 3a), the mean time taken to measure detectable nAb titres (Figure 3c) and the mean time to reach peak neutralization (Figure 3b) did not differ between the two groups suggesting disease severity enhances the magnitude of the Ab response but does not alter the kinetics. Comparison of the IgG, IgM and IgA OD values against S at peak neutralization showed significantly higher IgA and IgM ODs in the severity 4/5 group but no significant difference was observed for IgG to S (Figure 3d-f). This observation may further highlight a potential role for IgA and IgM in neutralization.27 Within the severity 4/5 group, a proportion of patients were treated with immunomodulation for a persistent hyperinflammatory state characterized by fevers, markedly elevated C-reactive protein (CRP) and ferritin, and multi-organ dysfunction. Despite an initial working hypothesis that antibody responses may differ either as a cause or consequence of this phenotype, no difference in ID50 was observed between these individuals and the remainder of the severity 4/5 cases (Figure 3g).
Figure 3. Impact of disease severity on Ab responses in SARS-CoV-2 infection.
Comparison for individuals with 0-3 or 4/5 disease severity for a) peak ID50 of neutralization (p<0.0001), b) the time POS to reach peak ID50 (p=0.674), and c) the time POS to detect neutralizing activity (p=0.9156). ID50 measured using HIV-1 based virus particles, pseudotyped with SARS-CoV-2 S. Comparison in OD values for individuals with 0-3 or 4/5 disease severity for d) IgG (p=0.0635), e) IgM (p=0.0003) and f) IgA (p=0.0018) against S measured at peak ID50. G) Comparison of the peak ID50 value for individuals who were treated for hyperinflammation or not, and had 4/5 disease severity (p>0.999). Statistical significance was measured using a Mann-Whitney test. ns = not significant.
Longevity of the Ab response
Following the peak in neutralization, a waning in ID50 was detected in individuals sampled at >40 days POS. Comparison of the ID50 at peak neutralization and ID50 at the final time point collected showed a decrease in almost all cases (Figure 4a). For some individuals with severity score 0, where the peak in neutralization was in the ID50 range 100-300, neutralization titres became undetectable (ID50 <50) in the pseudotype neutralization assay at subsequent time points (Figure 4a and 2b). For example, donors 52 and 54 both generated a low nAb response (peak ID50 of 174 and 434 respectively) but no neutralization could be detected (at 1:50 dilution) against the pseudotyped virus 39 and 34 days after the peak in ID50 respectively (Figure 2b). To determine whether similar neutralization trends were observed with infectious wild type virus, we selected a subset of individuals, representative of the range of nAb responses observed using the pseudotyped virus, for further comparison. As shown by others, neutralization titres against authentic virus correlated very well (r 2 = 0.9532, p<0.0001) with those measured using the SARS-CoV-2 pseduovirus8,28,29 (Figure S3b) and the same trends in neutralization decline were observed for these selected donors (Figure 2b and 4f). Neutralization could also not be detected at 1:20 dilution in donors 52 and 54 at the final timepoints (Figure 2b).
To gain a more quantitative assessment of the longevity of the IgG binding titres specific for S, RBD and N, EC50 values were measured by ELISA at the peak of neutralization and compared to the EC50 at the final time point collected. A stronger correlation was observed between ID50 and EC50 values compared to the OD values (Figure 4e and S3). Similar to neutralization potency, a decrease in EC50 was observed within the follow up period for S, RBD and N (Figure 4b-d). For those whose nAb titre decreased towards baseline, the EC50 for IgG to S and RBD also decreased in a similar manner. Finally, to determine whether the reduction in IgG titres might plateau, EC50 values for all time points for four representative individuals were measured who had multiple samples collected in the convalescent phase (Figure 4f). A steady decline in neutralization was accompanied by a decline in IgG EC50 to all antigens within the time window studied. Further assessment of Ab binding and neutralizing titres in samples collected >94 days POS will be essential to fully determine the longevity of the nAb response.
Ab responses in a Healthcare worker cohort
To gain further understanding of Ab responses in SARS-CoV-2 infection we next analysed sequential serum samples from 31 seropositive (as determined by an IgG response to both N and S)6 healthcare workers (HCW) from GSTFT. Ab responses in these individuals are likely to be more akin to those who were never hospitalised. Sera were collected every 1-2 weeks from March - June 2020 and any symptoms relating to COVID-19 recorded. Acute infection, as determined by detectable SARS-CoV-2 RNA on RT-qPCR, was not measured routinely. 80.6% (25/31) of seropositive individuals recorded COVID-19 compatible symptoms (including fever, cough and anosmia) since 1st February 2020, and 19.4% (6/31) reported none.
IgG and IgM binding to S, RBD and N by ELISA and pseudovirus neutralization titres were measured over time using sequential samples (Figure 5a and S4a). Similar to the patient cohort, ID50 values correlated with the OD values for IgG and IgM against S and RBD (Figure S4b). However, in contrast, the IgM and IgG responses to N in HCW correlated poorly (r2 = 0.030 and 0.381 respectively) (Figure S4b). Comparison of the peak ID50 between asymptomatic individuals, and symptomatic HCWs showed a very similar mean peak ID50. In contrast, both groups had lower mean ID50 values compared to hospitalized individuals in the 0-3 and 4/5 severity groups (Figure 5b). Importantly, some asymptomatic individuals generated neutralization titres >1,000. Similar to the cohort with confirmed SARS-CoV-2 infection, a decline in ID50 was observed following peak neutralization. For many individuals with a peak ID50 in the 100-500 range, neutralization was approaching baseline after 50 days POS (Figure 5c). As the mean peak ID50 was lower in the HCW cohort, the decline in nAb titres towards baseline was seen more frequently compared to the patient cohort.
Figure 5. Ab responses in a healthcare worker cohort.
a) ID50 values plotted against the time post onset of symptoms (POS) at which sera was collected. Each line represents one individual. ID50 measured using HIV-1 based virus particles, pseudotyped with SARS-CoV-2 S. Asymptomatic individuals shown in green, symptomatic individuals shown in black and PCR+ HCW shown in red (for comparsion). The dotted line represents day 0 post onset of symptoms. b) Comparison of the peak ID50 between asymptomatic individuals (n=10, includes 7 HCW and 3 hospital patients), healthcare workers (n=24 symptomatic HCW with no PCR test), and PCR+ individuals with either severity 0-3 (n=28) or 4/5 (n=32). The 2 PCR+ individuals sampled at early time points (<8 days POS) and did not seroconvert were not included in this analysis. c) ID50 at peak neutralization is plotted with the donor matched ID50 at the last time point sera was collected. The dotted line represents the cut-off for the SARS-CoV-2 pseudotyped virus particles neutralization assay. Asymptomatic donors are shown in green and symptomatic donors shown in black.
Discussion
The sequential serum samples collected up to 94 days post SARS-CoV-2 infection allowed evaluation of the kinetics and longevity of the nAb response in much greater detail than has hitherto been possible. As exemplified by Figures 2b and 4f, the kinetics of the Ab response in SARS-CoV-2 infection is typical of an acute viral infection.14,22–24 The peak in nAbs arises due to a rapid production of short-lived plasmablasts30 that secrete high titres of Abs; this is subsequently followed by a decline in virus-specific Abs as these cells die. We observed a wide range of peak nAb titres (98-32,000), similar to other cross-sectional cohorts4,21,31 and disease severity was associated with higher nAb titres. It is not clear yet why nAb responses correlate with disease severity.32 A higher viral load may lead to more severe disease and generate a stronger Ab response through increased levels of viral antigen. Alternatively, Abs could have a causative role in disease severity, although there is currently no evidence for antibody dependent enhancement in COVID-19.33
Comparison of the peak ID50 value for each individual and ID50 at their final timepoint collected, showed a decline in neutralizing titres regardless of disease severity. The decline in nAbs was mirrored in the reduction in IgG binding titres (EC50) to S and RBD and also IgM and IgA binding to S and RBD (OD values) for the PCR+ cohort (Figure 4b). For some individuals with a peak ID50 in the 100-300 range, neutralizing titres were at, or below, the level of detection (ID50 <50) after only ~50 days from the measured peak of neutralization (although IgG binding to N, S and RBD were still detected). This trend was also seen in the HCW cohort, and reveals that in some individuals, SARS-CoV-2 infection generates only a transient nAb response that rapidly wanes. For the majority of individuals with peak ID50 titres >4,000, despite a decline in nAb titres ranging from 2- to 23-fold over an 18-65 day period, nAb titres remain in the 1,000-3,500 range at the final time point. Whilst the lowest serum dilution used in the pseudovirus neutralization assay is relatively high (1:50), donors that lacked detectable neutralization also showed no neutralizing activity against wild-type virus at 1:20.
The magnitude of the following decline in nAb titres reported here is similar to that observed in several newly reported pre-prints.12,13,20,31,34–39 In these studies, similar to our observations, those who generated a high nAb titre still had high nAb titres regardless of the initial decline,12,13,31,35,39 and for those with lower disease severity, two studies reported 7/3413 and 5/8031 individuals with a decline to undetectable nAbs (ID50 <20 or ID50 <50, respectively) at the last time point studied. In contrast, several studies report a sustained Ab response in the first 3 months following SARS-CoV-2 infection but these studies report changes in binding antibodies only.34,37,38 Although binding titres to S and RBD correlate with nAb titres, this difference in Ab measurement may account for the differences in kinetics described. Further follow up in these longitudinal cohorts is required to determine whether the nAb decline will continue on a downward trajectory or whether the nAb titres will plateau to a steady state, facilitated through production of nAbs by long-lived plasma cells. Importantly, class-switched IgG memory B cells against S and RBD have been detected in blood of COVID-19 patients showing memory responses are generated during infection that have the potential to be activated to rapidly produce nAbs upon re-exposure to SARS-CoV-2 to prevent infection and/or disease.8,29,40,41 Indeed, highly potent neutralizing monoclonal antibodies (mAbs) with protective capacity have been isolated from memory B cells of both symptomatic and asymptomatic individuals.8,9,42
The longevity of Ab responses to other human coronaviruses have been previously studied.14–17 In contrast to SARS-CoV-2 infection, SARS-CoV infection typically caused more severe disease and asymptomatic, low severity cases were less common.43 The Ab response following SARS-CoV infection in a cohort of hospitalized patients peaked around day 30 (average titre 1:590)16 and a general waning of the binding IgG and nAb followed during the 3-year follow up. Low nAb titres of 1:10 were detected in 17/18 individuals after 540 days.16 In a second study, low nAb titres (mean titre, 1:28) could still be detected up to 36 months post infection in 89% of individuals.19 The lower nAb responses in the 0-3 disease severity cases in our two cohorts may reflect more the immune response to endemic seasonal coronaviruses (i.e. those associated with the common cold) which have also been reported to be more transient and where re-infections do occur.2,18 For example, individuals experimentally infected with endemic alphacoronavirus 229E, generated high Ab titres after 2 weeks but these rapidly declined in the following 11 weeks and by 1 year, the mean Ab titres had reduced further.14 Subsequent virus challenge lead to reinfection (as determined by virus shedding) yet individuals showed no cold symptoms.14
The nAb titre required for protection from re-infection and/or disease in humans is not yet understood. Neutralizing monoclonal antibodies (mAbs) isolated from SARS-CoV-2 infected individuals can protect from disease in animal challenge models in a dose dependant manner highlighting nAbs as a correlate of protection.9–11 SARS-CoV-2 infected rhesus macaques, which developed nAbs titres of ~100 (range 83-197), did not show any clinical signs of illness when challenged 35 days after the first infection.44 However, virus was still detected in nasal swabs, albeit 5-logs lower than in primary infection, suggesting immunologic control rather than sterilizing immunity. In contrast, a second study showed no detectable virus following re-challenge with nAb titres in the 8-20 range.45 Many current COVID-19 vaccine efforts focus on eliciting a robust nAb response to provide protection from infection. Our observation that nAb titres decline to low levels following low severity disease suggest that vaccines should aim to elicit titres similar to those generated by severe disease and boosting may be required to maintain nAb titres. The first results from phase I clinical trials showed peak median nAb titres of 654 and 3,906 following 2-doses of an mRNA vaccine encoding SARS-CoV-2 S (mRNA-1273) by Moderna46 and 2-doses of a recombinant nanoparticle Spike vaccine by Novavax47, respectively. Vaccine challenge studies in macaques can give some limited insights into nAb titres required for protection from re-infection.48–51 For example, a DNA vaccine encoding SARS-CoV-2 S generated nAb titres between 100-200 which were accompanied by a lowering of the viral load by 3-logs. nAb titres in vaccinated animals were shown to strongly correlate with viral load.50 Further, vaccine mRNA-1273 generated geometric mean titres of 3,481 which was shown to prevent viral replication in the upper and lower respiratory tract of macaques.52 The role of T-cell responses generated through either infection53 or vaccination play in controlling disease cannot be discounted in these studies and defining further the correlates and longevity of vaccine-induced protection is needed. Taken together, despite the lower nAb titres measured at the latest timepoints in some individuals, it is possible that nAb titres will still be sufficient to provide protection from COVID-19 for a period of time. However, sequential PCR testing and serology studies in individuals known to have been infected will be critical for understanding the ability of nAbs to protect from renewed infection in humans.
In summary, using sequential samples from SARS-CoV-2 infected individuals collected up to 94 days POS, we demonstrate a typical Ab response following an acute viral infection where a peak response was detected 3-4 weeks post infection which then wanes. For those who develop a low nAb response (ID50 100-300), titres can return to base line over a relatively short period, whereas those who develop a robust response maintain titres >1,000 despite the initial decline. Further studies using samples collected from these individuals at extended time points is required to determine the longevity of the nAb response as well as the nAb threshold for protection from re-infection and/or disease.
Methods
Ethics
Surplus serum from patient biochemistry samples taken as part of routine care were retrieved at point of discard, aliquoted, stored and linked with a limited clinical dataset by the direct care team, before anonymization. Work was undertaken in accordance with the UK Policy Framework for Health and Social Care Research and approved by the Risk and Assurance Committee at Guy’s and St Thomas’ NHS Foundation Trust (GSTFT). Serum was collected from consenting healthcare workers with expedited approval from GSTFT Research & Development office, Occupational Health department and Medical director.
The Health Research Authority (HRA) UK has provided informed consent exemption for research limited to acellular material (e.g. plasma, serum, DNA) extracted from tissue previously collected in the course of normal care. This exclusion is only provided if the patients are not identifiable to the research team carrying out the research. In the study the patient samples were collected and anonymised by the direct care team prior to providing them to the research team and therefore is exempted from requiring informed consent. Informed consent was provided by in the healthcare worker arm of the study.
Patient and sample origin
269 individual venous serum samples collected at St Thomas’ Hospital, London from 59 patients diagnosed as SARS-CoV-2 positive via real-time RT-PCR, were obtained for serological analysis. Samples ranged from 1 to 94 days after onset of self-reported symptoms or, in asymptomatic cases, days after positive PCR result. Patient information is given in Table S1.
Healthcare worker (HCW) cohort
Sequential serum samples were collected every 1-2 weeks from healthcare workers at GSTFT between 13th March and 10th June 2020. Seropositivity to SARS-CoV-2 was determined using sera collected in April and early May 2020 using ELISA. Individuals were considered seropositive if sera (diluted 1:50) gave an OD for IgG against both N and S that was 4-fold above the negative control sera.6 Self-reported COVID-19 related symptoms were recorded by participants and days post onset of symptoms in seropositive individuals was determined using this information. For asymptomatic, seropositive individuals, days POS was defined as the first timepoint SARS-CoV-2 Abs were detected. Six participants had confirmed PCR+ infection and were included with the PCR+ hospitalized patients in the initial analysis. An additional 31 HCW were found to be seropositive.
COVID-19 severity classification
Patients diagnosed with COVID-19 were classified as follows:
-
0 -
asymptomatic OR no requirement for supplemental oxygen.
-
1 -
requirement for supplemental oxygen (FiO2 <0.4) for at least 12 hrs.
-
2 -
requirement for supplemental oxygen (FiO2 ≥0.4) for at least 12 hrs.
-
3 -
requirement for non-invasive ventilation (NIV)/ continuous positive airways pressure (CPAP) OR proning OR supplemental oxygen (FiO2 >0.6) for at least 12 hrs AND not a candidate for escalation above level one (ward-based) care.
-
4 -
requirement for intubation and mechanical ventilation OR supplemental oxygen (FiO2 >0.8) AND peripheral oxygen saturations <90% (with no history of type 2 respiratory failure (T2RF)) OR <85% (with known T2RF) for at least 12 hrs.
-
5 -
requirement for extracorporeal membrane oxygenation (ECMO).
Protein expression
N protein was obtained from Leo James and Jakub Luptak at LMB, Cambridge. The N protein used is a truncated construct of the SARS-CoV-2 N protein comprising residues 48-365 (both ordered domains with the native linker) with an N terminal uncleavable hexahistidine tag. N was expressed in E. coli using autoinducing media for 7h at 37°C and purified using immobilised metal affinity chromatography (IMAC), size exclusion and heparin chromatography.
S protein consists of a pre-fusion S ectodomain residues 1-1138 with proline substitutions at amino acid positions 986 and 987, a GGGG substitution at the furin cleavage site (amino acids 682-685) and an N terminal T4 trimerisation domain followed by a Strep-tag II.8 The plasmid was obtained from Philip Brouwer, Marit van Gils and Rogier Sanders at The University of Amsterdam. The protein was expressed in 1 L HEK-293F cells (Invitrogen) grown in suspension at a density of 1.5 million cells/mL. The culture was transfected with 325 μg of DNA using PEI-Max (1 mg/mL, Polysciences) at a 1:3 ratio. Supernatant was harvested after 7 days and purified using StrepTactinXT Superflow high capacity 50% suspension according to the manufacturer’s protocol by gravity flow (IBA Life Sciences).
The RBD plasmid was obtained from Florian Krammer at Mount Sinai University.1 Here the natural N-terminal signal peptide of S is fused to the RBD sequence (319 to 541) and joined to a C-terminal hexahistidine tag. This protein was expressed in 500 mL HEK-293F cells (Invitrogen) at a density of 1.5 million cells/mL. The culture was transfected with 1000 μg of DNA using PEI-Max (1 mg/mL, Polysciences) at a 1:3 ratio. Supernatant was harvested after 7 days and purified using Ni-NTA (Nickel-Nitrilotriacetic acid) agarose beads.
ELISA protocol
ELISA was carried out as previously described.6 All sera/plasma were heat-inactivated at 56°C for 30 mins before use in the in-house ELISA. High-binding ELISA plates (Corning, 3690) were coated with antigen (N, S or RBD) at 3 μg/mL (25 μL per well) in PBS, either overnight at 4°C or 2 hr at 37°C. Wells were washed with PBS-T (PBS with 0.05% Tween-20) and then blocked with 100 μL 5% milk in PBS-T for 1 hr at room temperature. Wells were emptied and sera diluted at 1:50 in milk was added and incubated for 2 hr at room temperature. Control reagents included CR3009 (2 μg/mL), CR3022 (0.2 μg/mL), negative control plasma (1:25 dilution), positive control plasma (1:50) and blank wells. Wells were washed with PBS-T. Secondary antibody was added and incubated for 1 hr at room temperature. IgM was detected using Goat-anti-human-IgM-HRP (horseradish peroxidase) (1:1,000) (Sigma: A6907), IgG was detected using Goat-anti-human-Fc-AP (alkaline phosphatase) (1:1,000) (Jackson: 109-055-043-JIR) and IgA was detected Goat-anti-human-IgA-HRP (1:1,000) (Sigma: A0295). Wells were washed with PBS-T and either AP substrate (Sigma) was added and read at 405 nm (AP) or 1-step TMB (3,3’,5,5’-Tetramethylbenzidine) substrate (Thermo Scientific) was added and quenched with 0.5 M H2S04 before reading at 450 nm (HRP). ELISA measurements were performed in duplicate and the mean of the two values was used. Measurements were carried out in duplicate.
EC50 values were measured using a titration of serum starting at 1:50 and using a 5-fold dilution series. Half-maximal binding (EC50) was calculated using GraphPad Prism. Measurements were carried out in duplicate.
SARS-CoV-2 pseudotyped virus preparation
Pseudotyped HIV virus incorporating the SARS-Cov-2 spike protein was produced in a 10 cm dish seeded the day prior with 3.5x106 HEK293T/17 cells in 10 ml of complete Dulbecco’s Modified Eagle’s Medium (DMEM-C, 10% FBS and 1% Pen/Strep) containing 10% (vol/vol) foetal bovine serum (FBS), 100 IU/ml penicillin and 100 μg/ml streptomycin. Cells were transfected using 35 μg of PEI-Max (1 mg/mL, Polysciences) with: 1500 ng of HIV-luciferase plasmid, 1000 ng of HIV 8.91 gag/pol plasmid and 900 ng of SARS-2 spike protein plasmid.25,26 The media was changed 18 hours post-transfection and supernatant was harvested 48 hours post-transfection. Pseudotype virus was filtered through a 0.45μm filter and stored at -80°C until required.
Viral entry inhibition assay with SARS-CoV-2 pseudotyped virus
Serial dilutions of serum samples (heat inactivated at 56°C for 30mins) were prepared with DMEM media (10% FBS and 1% Pen/Strep) and incubated with pseudotype virus for 1-hour at 37°C in 96-well plates. Next, Hela cells stably expressing the ACE2 receptor (provided by Dr James Voss, The Scripps Research Institute) were added (12,500 cells/50uL per well) and the plates were left for 72 hours. Infection level was assessed in lysed cells with the Bright-Glo luciferase kit (Promega), using a Victor™ X3 multilabel reader (Perkin Elmer). Measurements were performed in duplicate and duplicates used to calculate the ID50.
Virus strain and propagation
Vero E6 (Cercopithecus aethiops derived epithelial kidney cells, provided by Prof Wendy Barclay, Imperial College London) cells were grown in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) supplemented with GlutaMAX, 10% fetal bovine serum (FBS), 20 μg/mL gentamicin, and incubated at 37°C with 5% CO2. SARS-CoV-2 Strain England 2 (England 02/2020/407073) was obtained from Public Health England. The virus was propagated by infecting 60-70% confluent Vero E6 cells in T75 flasks, at an MOI of 0.005 in 3 ml of DMEM supplemented with GlutaMAX and 10% FBS. Cells were incubated for 1 hr at 37°C before adding 15 ml of the same medium. Supernatant was harvested 72h post-infection following visible cytopathic effect (CPE), and filtered through a 0.22 μm filter to eliminate debris, aliquoted and stored at -80C. The infectious virus titre was determined by plaque assay also in Vero E6 cells.
Live virus neutralization assay
Vero E6 cells were seeded at a concentration of 20,000 cells/100uL per well in 96-well plates and allowed to adhere overnight. Serial dilutions of serum samples (heat inactivated at 56°C for 30mins) were prepared with DMEM media (2% FBS and 1% PS) and incubated with authentic SARS Cov2 for 1 hour at 37°C. The media was removed from the pre-plated Vero E6 cells and the serum-virus mixtures were added to the Vero E6 cells and incubated at 37°C for 24 h. These virus/serum mixture was aspirated and each well was fixed with 150μL of 4% formalin at room temperature for 30 min and then topped up to 300μL using PBS. The cells were washed once with PBS and permeabilised with 0.1% Triton-X in PBS at room temperature for 15 min. The cells were washed twice with PBS and blocked using 3% milk in PBS at room temperature for 15 min. The blocking solution was removed and a N-specific mAb (murinized-CR3009) was added at 2μg/mL (diluted using 1% milk in PBS) at room temperature for 45 min. The cells were washed twice with PBS and horse-anti-mouse-IgG-conjugated to HRP was added (1:2000 in 1% milk in PBS, Cell Signaling Technology, S7076) at room temperature for 45 min. The cells were washed twice with PBS, developed using TMB substrate for 30 min and quenched using 2M H2SO4 prior to reading at 450 nm. Measurements were performed in duplicate and duplicates used to calculate the ID50.
Statistical analysis
Analyses were performed using R (version 4.0.0) and GraphPad Prism (version 8.4.2). On charts showing OD/ID50 and days post-infection, the overall trend in the data was indicated by lines generated using Loess regressions (span 1.5) with ribbons depicting the 95% confidence intervals.
Supplementary Material
Acknowledgements
King’s Together Rapid COVID-19 Call awards to MHM, KJD, SJDN and RMN.
MRC Discovery Award MC/PC/15068 to SJDN, KJD and MHM.
AWS and CG were supported by the MRC-KCL Doctoral Training Partnership in Biomedical Sciences (MR/N013700/1).
GB and JMJ-G was supported by the Wellcome Trust (106223/Z/14/Z to MHM).
SA was supported by an MRC-KCL Doctoral Training Partnership in Biomedical Sciences industrial Collaborative Award in Science & Engineering (iCASE) in partnership with Orchard Therapeutics (MR/R015643/1).
NK was supported by the Medical Research Council (MR/S023747/1 to MHM).
MSH is supported by the National Institute for Health Research Clinician Scientist Award (CS-2016-16-011). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care.
SP, HDW and SJDN were supported by a Wellcome Trust Senior Fellowship (WT098049AIA). Fondation Dormeur, Vaduz for funding equipment (to KJD).
Development of SARS-CoV-2 reagents (RBD) was partially supported by the NIAID Centers of Excellence for Influenza Research and Surveillance (CEIRS) contract HHSN272201400008C.
Thank you to Florian Krammer for provision of the RBD expression plasmid, Philip Brouwer, Marit Van Gils and Rogier Sanders (University of Amsterdam) for the S protein construct, Leo James, Jakub Luptak and Leo Kiss (LMB) for the provision of purified N protein, and James Voss for providing the Hela-ACE2 cells. We thank Laura Mccoy (UCL) for the provision of the CR3009 mAb. We thank Isabella Huettner for assistance with figures. We are extremely grateful to all patients and staff at St Thomas’ Hospital who participated in this study.
We thank the COVID-19 core research team members including Olawale Tijani, Kate Brooks, Michael Flanagan, Robert Kaye, Raenelle Williams, Cristina Blanco-Gil, Helen Kerslake, Annelle Walters, Rizwana Dakari, Jennifer Squires, Anna Stanton, Sherill Tripoli, Andrew Amon, Isabelle Chow and Olanike Okolo.
Footnotes
Author contributions:
KJD, BM, SJDN, MHM and JDE designed the study.
JS, CG, SA, KJAS, OH, AO’B, NK, IH, KJD performed ELISAs.
JS, CG, SP performed neutralization assays.
HW, CK, RMN, MJL, JMJ-G prepared pseudovirus or wild-type virus.
JS, CG, BM, SA, KJD, MHM, SJDN analysed and interpreted data.
SP, RPG, GB, HDW, AWS curated hospital serum samples.
BM, KB, AP, MKIT, LOC, GO’H, EM, SD, GN, RB assisted in collection of samples from hospitalized patients.
BM, LS, KB, AM, AG, LM, BS, JH, AI-B, GA, AP, RB assisted in collection of samples from healthcare workers.
GO’H, EM, SD, GN assisted in project administration.
NT provided new reagents.
KJD, MHM, SJDN, BM, JDE, JS, CG, SP, RPG, MSH drafted the work or substantially revised it.
Competing interests:
The authors declare no competing interests.
Data availability statement
The data sets generated during the current study are available from the corresponding author on reasonable request.
References
- 1.Amanat F, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020 doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorse GJ, Donovan MM, Patel GB. Antibodies to coronaviruses are higher in older compared with younger adults and binding antibodies are more sensitive than neutralizing antibodies in identifying coronavirus-associated illnesses. J Med Virol. 2020;92:512–517. doi: 10.1002/jmv.25715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long QX, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 4.Luchsinger LL, et al. Serological Analysis of New York City COVID19 Convalescent Plasma Donors. medRxiv. 2020 doi: 10.1101/2020.06.08.20124792. [DOI] [Google Scholar]
- 5.Okba NMA, et al. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients. Emerg Infect Dis. 2020;26:1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pickering PGB, Galao Rui Pedro, Merrick Blair, Signell Adrian W, Wilson Harry D, Ik Kia Mark Tan, Seow Jeffrey, Graham Carl, Acors Sam, Kouphou Neophytos, Steel Kathryn JA, et al. Comparative assessment of multiple COVID-19 serological technologies supports continued evaluation of point-of-care lateral flow assays in hospital and community healthcare settings. 2020 doi: 10.1101/2020.06.02.20120345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prevost J, et al. Cross-sectional evaluation of humoral responses against SARS-CoV-2 Spike. bioRxiv. 2020 doi: 10.1101/2020.06.08.140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brouwer PJM, et al. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science. 2020 doi: 10.1126/science.abc5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers TF, et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020 doi: 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao Y, et al. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell. 2020 doi: 10.1016/j.cell.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi R, et al. A human neutralizing antibody targets the receptor binding site of SARS-CoV-2. Nature. 2020 doi: 10.1038/s41586-020-2381-y. [DOI] [PubMed] [Google Scholar]
- 12.Long QX, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 13.Crawford KHD, D AS, Eguia Rachel, Wolf Caitlin R, Wilcox Naomi, Logue Jennifer K, Shuey Kiel, Casto Amanda M, Fiala Brooke, Wrenn Samuel, Pettie Deleah, et al. Dynamics of neutralizing antibody titers in the months after SARS-CoV-2 infection. medRxiv. 2020 doi: 10.1101/2020.08.06.20169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callow KA, Parry HF, Sergeant M, Tyrrell DA. The time course of the immune response to experimental coronavirus infection of man. Epidemiol Infect. 1990;105:435–446. doi: 10.1017/s0950268800048019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kellam P, Barclay W. The dynamics of humoral immune responses following SARS-CoV-2 infection and the potential for reinfection. J Gen Virol. 2020 doi: 10.1099/jgv.0.001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mo H, et al. Longitudinal profile of antibodies against SARS-coronavirus in SARS patients and their clinical significance. Respirology. 2006;11:49–53. doi: 10.1111/j.1440-1843.2006.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore JP, Klasse PJ. SARS-CoV-2 vaccines: ‘Warp Speed’ needs mind melds not warped minds. J Virol. 2020 doi: 10.1128/JVI.01083-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edridge AWD, K JM, Hoste Alexis CR, Bakker Margreet, Klein Michelle, Jebbink Maarten F, Matser Amy, Kinsella Cormac, Rueda Paloma, Prins Maria, Sastre Patricia, et al. Coronavirus protective immunity is short-lasting. medRxiv. 2020 doi: 10.1101/2020.05.11.20086439. [DOI] [PubMed] [Google Scholar]
- 19.Cao WC, Liu W, Zhang PH, Zhang F, Richardus JH. Disappearance of antibodies to SARS-associated coronavirus after recovery. N Engl J Med. 2007;357:1162–1163. doi: 10.1056/NEJMc070348. [DOI] [PubMed] [Google Scholar]
- 20.Lee WT, RC G, Dupuis Alan P, II, Kulas Karen E, Payne Anne F, Wong Susan J, Arinsburg Suzanne, Nguyen Freddy T, Mendu Damodara Rao, Firpo-Betancourt Adolfo, Jhang Jeffrey, et al. Neutralizing Antibody Responses in COVID-19 Convalescent Sera. medRxiv. 2020 doi: 10.1101/2020.07.10.20150557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu FAW, Liu Mei, Wang Qimin, Chen Jun, Xia Shuai, Ling Yun, Zhang Yuling, Xun Jingna, Lu Lu, Jiang Shibo, Lu Hongzhou, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv. 2020 doi: 10.1101/2020.03.30.20047365. [DOI] [Google Scholar]
- 22.Wang M, et al. Antibody dynamics of 2009 influenza A (H1N1) virus in infected patients and vaccinated people in China. PLoS One. 2011;6:e16809. doi: 10.1371/journal.pone.0016809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 24.Choe PG, et al. MERS-CoV Antibody Responses 1 Year after Symptom Onset, South Korea, 2015. Emerg Infect Dis. 2017;23:1079–1084. doi: 10.3201/eid2307.170310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grehan K, Ferrara F, Temperton N. An optimised method for the production of MERS-CoV spike expressing viral pseudotypes. MethodsX. 2015;2:379–384. doi: 10.1016/j.mex.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson C, G N, Paton Robert, Lourenço José, Penman Bridget, Lee Lian Ni, Odon Valerie, Mongkolsapaya Juthathip, Chinnakannan Senthil, Dejnirattisai Wanwisa, Edmans Matthew, et al. Neutralising antibodies to SARS coronavirus 2 in Scottish blood donors - a pilot study of the value of serology to determine population exposure. medRxiv. 2020 doi: 10.1101/2020.04.13.20060467. [DOI] [Google Scholar]
- 27.Sterlin Delphine, M A, Miyara Makoto, Mohr Audrey, Anna Francois, Claer Laetitia, Quentric Paul, Fadlallah Jehane, Ghillani Pacale, Gunn Cary, Hockett Rick, et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. medRxiv. 2020 doi: 10.1101/2020.06.10.20126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt F, et al. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. J Exp Med. 2020;217 doi: 10.1084/jem.20201181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ju B, et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020 doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- 30.Laing AG, et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med. 2020 doi: 10.1038/s41591-020-1038-6. [DOI] [PubMed] [Google Scholar]
- 31.Muecksch F, et al. Longitudinal analysis of clinical serology assay performance and neutralising antibody levels in COVID19 convalescents. medRxiv. 2020 doi: 10.1101/2020.08.05.20169128. [DOI] [Google Scholar]
- 32.Lee N, et al. Anti-SARS-CoV IgG response in relation to disease severity of severe acute respiratory syndrome. J Clin Virol. 2006;35:179–184. doi: 10.1016/j.jcv.2005.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwasaki A, Yang Y. The potential danger of suboptimal antibody responses in COVID-19. Nat Rev Immunol. 2020;20:339–341. doi: 10.1038/s41577-020-0321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wajnberg AFA, Firpo Adolfo, Altman Deena, Bailey Mark, Mansour Mayce, McMahon Meagan, Meade Philip, Mendu Damodara Rao, Muellers Kimberly, Stadlbauer Daniel, Stone Kimberly, et al. SARS-CoV-2 infection induces robust, neutralizing antibody responses that are stable for at least three months. medRxiv. 2020 doi: 10.1101/2020.07.14.20151126. [DOI] [Google Scholar]
- 35.Iyer AS, et al. Dynamics and significance of the antibody response to SARS-CoV-2 infection. medRxiv. 2020 doi: 10.1101/2020.07.18.20155374. [DOI] [Google Scholar]
- 36.Beaudoin-Bussières GAL, Anand Sai Priya, Prévost Jérémie, Gasser Romain, Goyette Guillaume, Medjahed Halima, Perreault Josée, Tremblay Tony, Lewin Antoine, Gokool Laurie, Morrisseau Chantal, et al. Decline of humoral responses against SARS-CoV-2 Spike in convalescent individuals. medRxiv. 2020 doi: 10.1101/2020.07.09.194639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isho BKTA, Zuo Michelle, Jamal Alainna J, Rathod Bhavisha, Wang Jenny H, Li Zhijie, Chao Gary, Rojas Olga L, Bang Yeo Myong, Pu Annie, Christie-Holmes Natasha, et al. Evidence for sustained mucosal and systemic antibody responses to SARS-CoV-2 antigens in COVID-19 patients. medRxiv. 2020 doi: 10.1101/2020.08.01.20166553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J, Liang Boyun, Chen Cunrong, Wang Hua, Fang Yaohui, Shen Shu, Yang Xiaoli, Wang Baoju, Chen Liangkai, Chen Qi, Wu Yang, et al. SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19. medRxiv. 2020 doi: 10.1101/2020.07.21.20159178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang K, et al. Longitudinal dynamics of the neutralizing antibody response to SARS-CoV-2 infection. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seydoux E, et al. Analysis of a SARS-CoV-2-Infected Individual Reveals Development of Potent Neutralizing Antibodies with Limited Somatic Mutation. Immunity. 2020 doi: 10.1016/j.immuni.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodda LB, N J, Shehata Laila, Pruner Kurt B, Morawski Peter M, Thouvenel Christopher, Takehara Kennidy K, Eggenberger Julie, Hemann Emily A, Waterman Hayley R, Fahning Mitchell L, et al. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. medRxiv. 2020 doi: 10.1101/2020.08.11.20171843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robbiani DF, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020 doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petersen eKM, Go U, Hamer DH, Petrosillo N, Castelli F, Storgaard M, Al Khalili S, Simonsen L. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infection. 2020 doi: 10.1016/S1473-3099(20)30484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chandrashekar A, et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science. 2020 doi: 10.1126/science.abc4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng W, et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science. 2020 doi: 10.1126/science.abc5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackson LA, et al. An mRNA Vaccine against SARS-CoV-2 - Preliminary Report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keech CGA, Reed Patricia, Neal Susan, Plested Joyce S, Zhu Mingzhu, Cloney-Clark Shane, Zhou Haixia, Patel Nita, Frieman Matthew B, Haupt Robert E, Logue James, et al. First-in-Human Trial of a SARS CoV 2 Recombinant Spike Protein Nanoparticle Vaccine. medRxiv. 2020 doi: 10.1101/2020.08.05.20168435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith TRF, et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat Commun. 2020;11:2601. doi: 10.1038/s41467-020-16505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Doremalen N, et al. ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. bioRxiv. 2020 doi: 10.1101/2020.05.13.093195. [DOI] [PubMed] [Google Scholar]
- 50.Yu J, et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020 doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao Q, et al. Rapid development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020 doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corbett KS, et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N Engl J Med. 2020 doi: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karolinska COVID-19 Study Group. Sekine T, P-P A, Rivera-Ballesteros Olga, Strålin Kristoffer, Gorin Jean-Baptiste, Olsson Annika, Llewellyn-Lacey Sian, Kamal Habiba, Bogdanovic Gordana, Muschiol Sandra, Wullimann David J, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. medRxiv. 2020 doi: 10.1101/2020.06.29.174888. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated during the current study are available from the corresponding author on reasonable request.