Abstract
The development of cortical maps requires the balanced interaction between genetically determined programs and input/activity-dependent signals generated spontaneously or triggered from the environment. The somatosensory pathway of mice provides an excellent scenario to study cortical map development because of its highly organized cytoarchitecture, known as the barrel field. This precise organization makes evident even small alterations in the cortical map layout. In this review, we will specially focus on the thalamic factors that control barrel field development. We will summarize the role of thalamic input integration and identity, neurotransmission and spontaneous activity in cortical map formation and early cross-modal plasticity.
Keywords: Thalamus, spontaneous activity, somatosensory system, cortical maps, calcium waves, plasticity
Introduction
Sensory information flows from peripheral receptors to cortical structures through a sequence of precisely organized projections. Before reaching primary sensory cortices, topographically organized sensory afferents cluster in modality-specific thalamic nuclei. Subsequently, each thalamic nucleus conveys sensory information to its corresponding cortical region preserving topographical organization (Petersen, 2007; Huberman et al., 2008; Tsukano et al., 2017). This means that besides the message encoded by patterns of action potentials, the specific cortical site where synaptic transmission takes place enriches the neural message with precise positional information, highlighting the relevance of fine-scale connectivity.
Highly elaborated neural networks rely on precise wiring during development. In rodents, the thalamocortical circuits’ assembly starts embryonically and finishes a few days after birth (Jhaveri et al., 1991; Schlaggar & O’Leary, 1994). During this period, the thalamocortical ensemble experiences elongation and branching of projections, synaptogenesis, synaptic deletion, refinement and local synaptic connections. Numerous investigations have provided compelling evidences that intrinsic and extrinsic mechanisms act together to achieve modality-specific and highly organized thalamocortical connections (Hanganu-Opatz, 2010). In particular, the rodent somatosensory system emerged as a helpful model to elucidate connectivity principles due to the unique one-to-one relationship between facial whiskers on the snout and barrel-like modules in layer 4 (L4) of the contralateral primary somatosensory cortex (S1) (Woolsey & Van der Loos, 1970).
Development of the thalamocortical somatosensory system
The topographic map found at every level along the somatosensory system recapitulates the whisker layout on the snout. Each whisker has a specific representation in the ipsilateral principal trigeminal nucleus of the brainstem (PrV), named barrelette (Ma & Woosley, 1984); in the contralateral ventro-posteromedial nucleus of the thalamus (VPM), named barreloid (van der Loos, 1976); and in the contralateral somatosensory cortex (S1), named barrel (Van der Loos & Woolsey, 1973). Every station develops following the same temporal sequence. First, the afferent terminals cluster delineating barrel-like structures. And then, the postsynaptic cells at the brainstem, thalamus and cortex aggregate and refine their dendritic trees in order to overlap the initial presynaptic clusters. Combining both pre- and postsynaptic processes, whisker-related modules arise and the topographical map becomes evident.
In mice, the axons from the PrV project to the thalamus (Erzurumlu et al., 1980), crossing the midline around embryonic day (E)11.5 elongating rostrally towards the contralateral thalamus. These axons reach the VPM around E17.5; they arborize and finally refine forming barreloids during the first postnatal week (Kivrak & Erzurumlu, 2012). Before the trigemino-thalamic axons reach the thalamus, the thalamocortical axons (TCAs) have already started to project to the cortex, independently of the peripheral input (López-Bendito & Molnár, 2003). The extension of the TCAs to the cortex is controlled by the expression of thalamic and subpallial molecular guidance cues, independently of the cortex. Adhesion molecules, guidance cues and guidepost cells, guide TCAs towards the cortex with a highly coordinated topographical pattern (Molnar et al., 1998; Braisted et al., 1999; Molnar et al., 2012; Leyva-Díaz & Lopez Bendito, 2013; Garel & Lopez-Bendito, 2014). Some of these important molecules are Semaphorins/Plexin, Ephrins/Eph, Netrins/DCC and others that are essential for a proper development of the TCAs. Moreover, recent studies have shown that spontaneous calcium activity controls, at least in part, TCAs development by means of transcriptional regulation of critical molecules such as Robo1, Slit1 and DCC (Mire et al., 2012; Castillo-Paterna et al., 2015). These results suggest that spontaneous activity intrinsically modulates the rate of axon extension in the thalamocortical system.
Thalamocortical cells start to extend their axons ventrally from the diencephalon to turn laterally crossing the boundary between the diencephalon and the telencephalon at E12.5. At E13.5, they navigate within the internal capsule (IC) and corridor cells (López-Bendito et al., 2006; Uemura et al., 2007) to arrive to the pallial-subpallial boundary (PSPB) at the entrance of the cortex. Corticothalamic axons (CTAs) leave the cortical plate around the same time and meet the TCAs at the PSPB around E14.5 (Jacobs et al., 2007; Deck et al., 2013; Mandai et al., 2014). Before the neurons from the primary cortical areas are born, the TCAs wait in the subplate region a few days in their specific sensory modality cortex until they enter in their proper cortical layer (López-Bendito & Molnár, 2003). During this period, TCAs transiently connect with subplate neurons and this communication seems to be crucial for the later topographic innervation of the cortical plate (Catalano & Shatz, 1998; Higashi et al., 2002; Hull et al., 2009; Kanold & Luhmann, 2010; Bagnall et al., 2011; Constantinople & Bruno, 2013). The neurites of subplate neurons undergo many processes of remodeling, from a wide pattern reaching the marginal zone to confined localizations in prospective barrel hollows, barrel septa or below L4 (Pinon et al., 2009). As subplate neurons are electrically mature (Luhmann et al., 2003), they could function as an effective bridge between immature thalamic inputs and cortical targets.
Although the extension of the TCAs to the cortex is independent of mechanisms underlying cortical regionalization, intrinsic signals from the cortex are involved in the formation of the final barrel map. Early manipulations of specific genes (Fgf8 and Pax6) cause an abnormal organization of TCAs in the telencephalon, indicating that they use intracortical positional information as well (Fukuchi-Shimogori & Grove, 2003; Shimogori & Grove, 2005; Zembrzycki et al., 2013). Moreover, in animal models that lack transcription factors important for normal arealization of the cortex such as Coup-TF1, Emx1 or Emx2, TCAs are able to reach their proper S1 target area and form the barrel map (Hamasaki et al., 2004; Armentano et al., 2007; Stocker & O’Leary, 2016). Interestingly, TCAs are topographically pre-ordered in the subpallium and this organization seems to be crucial for the development of cortical features. In a relevant study, it was shown that if the topographical positioning of somatosensory TCAs is altered, the barrel map is not formed in S1 (Lokmane et al., 2013). Thus, revealing that sensory map transfer relies on preordering of axons along their trajectory. Therefore, altogether these studies indicate that although TCAs organization in the subpallium is independent of normal cortical arealization, it can severely affect the later formation of the barrel map. Cortical intrinsic information also contributes to the final positioning of TCAs in S1.
The formation of barrels in L4 of S1 comprises two main events. First, immediately after birth, TCAs from VPM are pruned in the cortex giving rise to clusters that delineate the barrel territory. Collaterals that are not confined to a single barrel are eliminated (Rice et al., 1985). Second, L4 spiny stellate neurons surround thalamic axonal clusters to form barrel walls (Killackey, 1973; Woolsey et al., 1975; Simons & Woolsey, 1984; Jensen & Killackey, 1987; Agmon et al., 1995). By postnatal day (P) 4, barrels are evident and each of them receives innervation from a single barreloid (Agmon et al., 1995).
Thalamocortical communication shapes the barrel map
In every relay station throughout the whisker-barrel pathway, afferents imprint their spatial organization to the postsynaptic target (Killackey et al., 1995; Sehara & Kawasaki, 2011). In particular, the thalamocortical projection to L4 has been an experimentally accessible system for understanding the mechanisms involved in the precise formation of circuits in S1. A large body of work indicates that mechanisms dependent on spontaneous and periphery-driven neural activity operate to transmit topological information sequentially from afferents to target neurons.
Spontaneous activity during the early ontogenetic stages regulates, among others, the following physiological processes: neurogenesis, neuronal identity acquisition, cell migration, gene expression, axonal and dendritic projections, growth and differentiation, formation and refinement of topographic maps (Spitzer, 2006; Luhmann, 2017). In sensory systems, correlated spontaneous activity promotes functional maturation of intracortical and thalamocortical circuits (Cang et al., 2005; Hanganu-Opatz, 2010; Ackman & Crair, 2014).
Although the origin and propagation mechanisms of spontaneous correlated activity are still unclear, these activity patterns are transmitted through different structures before sensory onset. In the visual system, retinal waves of newborn rats robustly correlate with spindle burst activity in the contralateral primary visual cortex (V1); however, a large fraction of cortical activity persists when retinal waves are absent suggesting that circuits intrinsic to the brain might be operating to generate early coordinated activity (Hanganu et al., 2006; Colonnese & Khazipov, 2010). In the somatosensory system, while peripheral inputs drive spindle bursts and gamma oscillations in the barrel cortex of newborn rodents, disconnecting peripheral pathways reduces but does not abolish thalamic and cortical activity (Khazipov et al., 2004; Minlebaev et al., 2011; Yang et al., 2013). It is not clear how thalamic and cortical neurons communicate during early stages; an increasing number of publications suggest that subplate neurons are implicated in the generation of cortical oscillatory patterns (Molnár et al., 2003; Kanold & Luhmann, 2010; Hoerder-Suabedissen & Molnar, 2015). Overall, these results suggest an effective vertical communication between developing subcortical and cortical stations even before dedicated sensory processing. In this way, spontaneous electrical activity might function as a messenger carrying topographic information and, therefore, facilitating the formation of sensory maps. The following paragraphs will sum up the current knowledge regarding thalamocortical pre-and postsynaptic elements involved, in an activity-dependent manner, in barrel cortex organization.
Any manipulation to tackle map formation must be done before barrels become apparent in the cortex. In rodents, this sensitive window ends between P3 and P5 (Rice & Van der Loos, 1977; Rice et al., 1985). Indeed, seminal studies demonstrate that short-cutting peripheral input within a few days after birth, by means of infraorbital nerve injury (ION) or damage to the mystacial vibrissae, impairs thalamic afferents clustering and, consequently, L4 cells do not aggregate into barrels (Van der Loos & Woolsey, 1973; Weller & Johnson, 1975; Killackey et al., 1976). Later manipulations did not produce map defects (Woolsey & Wann, 1976) providing strong evidence that an intact periphery, before barrel pattern becomes visible, is an essential condition for a development of a normal map. However, these experiments could not rule out whether the relevant factor for barrel map formation is the presence of the peripheral input or the neural activity that flows through them.
Inspired by results from the visual system (Stryker & Harris, 1986), the idea that neural activity influences barrel formation was tested using pharmacological blockade of cortical NMDA receptors (NMDAR). In these studies, local and systemic inhibition triggered functional and morphological disorders in the barrel map (Fox et al., 1996; Mitrovic et al., 1996). Despite that several lines of evidence supported these results, other reports led to contradictory conclusions (Chiaia et al., 1992; Henderson et al., 1992; Schlaggar et al., 1993). The forthcoming molecular genetics strategies in mice overcome pharmacological data inconsistency demonstrating that targeted deletion of genes related to neural activity induced defects on the whisker representation along the somatosensory pathway (Erzurumlu & Kind, 2001; Wu et al., 2011; Erzurumlu & Gaspar, 2012). Furthermore, this targeted gene deletion allows a comprehensive molecular dissection of the barrel map formation, with the added possibility of spatial and/or temporal control.
Initial approaches using transgenic mice consisted in the ablation of a gene from the whole organism. With this strategy, many null mice were generated for genes involved in presynaptic neurotransmitter release and modulation, and also in postsynaptic activity and signaling. Global knockout of genes related to synaptic function such as adenylyl cyclase 1 (AC1), NMDA receptor subunit 1, metabotropic glutamate receptor 5, phospholipase C-beta1, cAMP-dependent protein kinase type II regulatory subunit, monoamine oxidase A or sodium-dependent 5-HT transporter showed phenotypes with aberrant barrel map organization (Li et al., 1994; Cases et al., 1996; Welker et al., 1996; Iwasato et al., 1997; Abdel-Majid et al., 1998; Hannan et al., 2001; Persico et al., 2001; Salichon et al., 2001; Lu et al., 2003; Rudhard et al., 2003; Gheorghita et al., 2006; Inan et al., 2006; Lu et al., 2006; Watson et al., 2006; Wijetunge et al., 2008; She et al., 2009). Altogether, these studies identified genes required for barrel map formation. However, these findings could not be ascribed to specific effects on the neocortex, as subcortical aberrant maps are more than likely sequentially transmitted to the cortex. A direct assessment of how thalamocortical communication affects barrel formation required site-specific deletions in the thalamus or the cortex.
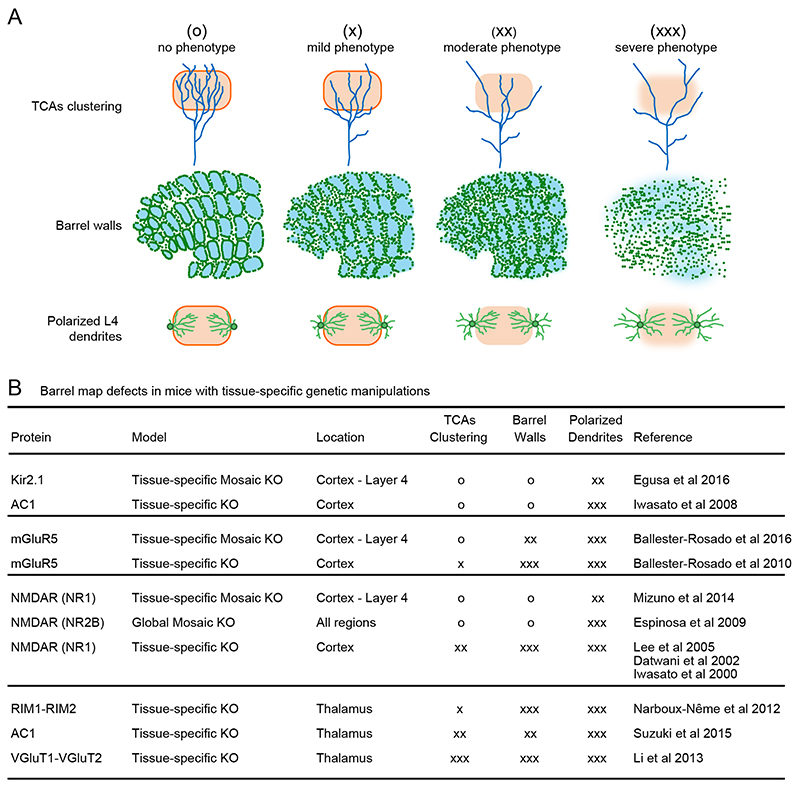
Selective loss-of-function models confine the deletion of a gene to a specific tissue or group of cells (Figure 1). The cortical knockout mice for the NR1 subunit of the NMDAR showed defective thalamocortical neurotransmission (Iwasato et al., 2000; Datwani et al., 2002). In this model, upstream subcortical stations, cortical layers and somatotopy develop normally; but, in the barrel cortex, thalamocortical afferents form rudimentary patches, L4 cells fail to locate around barrel edges and their dendritic field does not orientate towards barrel hollows as in wild type mice. Single axon analyses in these mutant mice revealed that thalamocortical afferents span through larger territories than wild type axons (Lee et al., 2005). A similar aberrant barrel map phenotype is found in another model with defective glutamatergic transmission between thalamocortical innervations and L4 cells. Cortex-specific metabotropic glutamate receptor 5 (mGluR5) knockout mice also exhibit abnormal TCA patterning, as well as L4 cells with cell bodies evenly distributed and symmetric dendrites (Ballester-Rosado et al., 2010). While these studies suggest a pivotal role of postsynaptic glutamatergic receptors in barrel map formation, it is unclear whether postsynaptic defects, such as cell body misplacement or lack of dendritic polarization, reflect a cell-autonomous requirement or they are inherited defects from the presynaptic site due to the abnormal distribution of TCAs.
Figure 1. Increasing severity in barrel map deficits from thalamic to cortical manipulations.
(A) Schematic representation of severity levels in defective phenotypes for three different components of barrel map formation: TCAs clustering (top), alignment to barrel walls of cell bodies from L4 spiny stellate cells (middle), and polarization of their dendritic arbors (bottom). Severity is divided in four categories: no phenotype (circle), mild (one cross), moderate (two crosses), and severe (three crosses). (B) Summary of thalamic and cortical tissue-specific manipulation and their consequences in barrel map formation using the severity code described in A.
A cell-autonomous function of a gene might be assessed in mosaic models in which only a subpopulation of cells carries the deletion. In this way, mutant cells reside in a wild type environment. Loss of the NR1 or NR2B subunit of NMDARs or mGluR5 in a subset of L4 neurons revealed that postsynaptic defects have, partly, a cell-autonomous regulation in S1(Espinosa et al., 2009; Mizuno et al., 2014; Ballester-Rosado et al., 2016). In these mosaic animals, as expected, L4 wild type spiny stellate cells are localized at barrels edges and develop asymmetric dendrites biased towards barrel hollows. Mutant neurons display, on one hand, cell bodies properly aligned to barrel walls in most of the cases (except in mGluR5 mosaic animals; NR1 KO cells seem normal although there is not a direct quantification); and on the other hand, abnormal symmetric dendrites without barrel preference. Same results are found in mice that lack AC1 in the cortex (Iwasato et al., 2008). AC1 participates in the downstream signaling triggered by NMDAR activation. Notably, the same phenotype is observed if L4 neural activity is silenced instead of interfering with glutamatergic transmission, cell body allocation develops properly but the biased arborisation pattern is altered (Egusa et al., 2016). Despite the fact that relevant molecules have been identified, the mechanism of dendritic refinement remains unclear. One possible approach might be chronic calcium imaging experiments over L4 spiny stellate dendrites during the refinement process both in wild type and mutant cells in vivo.
Non-cell autonomous effects indicate that extrinsic factors partly instruct barrel formation. Indeed, perturbations in the presynaptic side, the TCAs, cause even more severe disruptions of barrel map organization. These perturbations end up in nearly abolished or reduced (severity depends on the approach) neurotransmitter release, suggesting a key role of activity-dependent mechanisms. For instance, elimination of vesicular glutamate transporters type 1 and 2 from somatosensory nuclei, nearly prevents neurotransmission. In these mice, thalamic afferents fail to cluster into whisker related patches, L4 spiny stellate characteristic morphology is absent and cortical cytoarchitecture does not emerge (Li et al., 2013). Thus, in addition to recapitulate the cortical phenotype shown by the aforementioned studies in which neurotransmission was disrupted by targeting the postsynaptic glutamate receptors, this model showed dramatic defects on the thalamic afferents too. If neurotransmission is partly affected rather than almost completely blocked, thalamic afferents do cluster, albeit immature, and only the defective cortical phenotype develops strongly. This is the case for the thalamic conditional ablation of Rab3 interacting molecules (RIM) isoforms, components of the synaptic release machinery (Narboux-Neme et al., 2012). Also, the conditional ablation of thalamic AC1 (Suzuki et al., 2015), a molecule that in the presynaptic compartment might regulate glutamate release, shows a stronger cortical phenotype in comparison to thalamic defects –in these mice TCAs clusters are less defined than in the RIM ablation model. These perturbations further support a key role for neurotransmission-mediated mechanisms on barrel map organization.
Thalamic input shapes cortical area identity
The above-mentioned results clearly support a central role of neural activity on barrel map formation. Work in recent years has deepened the knowledge about the function of the thalamus in the development of the somatosensory system, not only in setting the map, but also in influencing the identity of successive relay stations. Sensory pathways are organized in a stereotyped way where the peripheral input contacts a first-order (FO) thalamic nucleus that in turn projects to the corresponding primary sensory cortical area. The output from the primary sensory area projects back to a higher-order (HO) thalamic nucleus that projects to secondary sensory cortical areas. Recent studies suggest that the nature of the input to a territory exerts a strong influence over the postsynaptic molecular and functional differentiation.
Taking advantage of a genetic model of postnatal ablation of the VPM, the FO somatosensory thalamic nucleus, it was shown that the attributes of the L4 thalamorecipient cells in S1 are tightly controlled by the nature of the subcortical input (Pouchelon et al., 2014). In the absence of a VPM input to S1, the posteromedial nucleus (POm), a HO somatosensory nucleus, takes over and acts as the main thalamic driver for L4 cells of S1 (S1L4). Remarkably, in this model S1L4 cells show a genetic differentiation program similar to the one of L4 neurons of secondary areas (S2L4). Moreover, the connectivity and functional properties of S1L4 neurons resemble those of S2L4 neurons. Re-specified L4 neurons respond to both haptic and noxious stimuli, in contrast to the segregation of these sensory modalities in different cortical circuits that occur in normal conditions. Also, barrels are missing in the re-specified S1, and the stimulation of a single whisker leads to a diffuse activation of the immediate–early gene c-fos in S1L4, rather than to the activation of the corresponding barrel. It is interesting to note that in the visual system, the thalamocortical input also drives the differentiation of patterned gene expression that distinguishes primary and HO visual areas (Chou et al., 2013), suggesting that the control of cortical layer properties by the nature of the input may be extensive to other sensory modalities.
The instrumental role of the input in defining target identity is extensive to subcortical stations, such as the thalamus. By means of a transcriptional analysis to compare gene expression of FO and HO thalamic nuclei, it was found that hierarchical order (i.e. first/higher order relationship) rather than the modality of the stimulus is the determinant factor for sharing a similar transcriptional program (Frangeul et al., 2016). HO (LP and POm) and FO (dLGN and VPM) nuclei are segregated in respective groups. The same applies, with less astringency, to the auditory thalamic nuclei. These transcriptional programs are determined by the input from the periphery. Interestingly, in the absence of external inputs, FO thalamic nuclei become HO nuclei or, in other words, HO-type genetic identity is a ground state property from which FO features emerge in the presence of a peripheral input.
The results obtained with the genetic model of postnatal ablation of the VPM (Pouchelon et al., 2014) are consistent with the effect of early ION ablation (Frangeul et al., 2016), as in both paradigms the somatosensory thalamus is rendered to a HO-like identity. This is also convergent to the results obtained in the visual system (Grant et al., 2016). In enucleated animals, corticothalamic L5 neurons aberrantly innervate the (modified) dLGN. Interestingly, blocking the spontaneous activity of the retina with epibatidine leads to similar changes in the pattern of corticothalamic connectivity. This indicates that activity feeds a forward mechanism that controls the identity (or bias the identity) towards a FO in the presence of activity or towards a HO in its absence.
Cross-Modal plasticity affecting the somatosensory cortex: Role of thalamic waves
The plasticity that takes place in the brain represents an adaptive response of neural networks to patterns of stimuli. It is known that patterned neural activity is able to alter the connectivity and the specificity of cortical areas. As aforementioned, a paradigm extensively used to unravel the role of the afferent input and their electrical properties in the development of the cortex, is the deprivation of one sensory modality. The deprivation of a sensory input leads to striking effects on the development of the remaining modalities, and furthermore, an adaptive reorganization of the deprived neurons that integrate a new function. This neuroplastic phenomenon is called “cross-modal plasticity”. The characterization of the functional rearrangement that takes place in the sensory systems after sensory deprivation may help to understand the role of intrinsic and extrinsic mechanisms involved in cortical development.
The classical view of cross-modal plasticity claims that the neural changes observed in the cortical areas of sensory-deprived rodents are due to the increased experience-dependent neuronal activity of the intact sensory system during postnatal life. Studies in rats enucleated at birth, have demonstrated that there is a recruitment of the rostral part of visual cortex from the primary somatosensory cortex, with the consequent higher whisker responsiveness and improved exploring skills (Toldi et al., 1994; Toldi et al., 1996). Moreover, these deprived rats have longer whiskers (Rauschecker et al., 1992) and an increment in the experience-driven activity along the trigeminal pathway (Bronchti et al., 1992; Zheng & Purves, 1995).
While the finding of an experience driven mechanism for cross-modal plasticity was groundbreaking, it was unable to explain the early adaptations found before or soon after the onset of sensory experience. Recent studies, strongly point to the existence of experience-independent mechanisms (Mezzera & Lopez-Bendito, 2015). For example, in mice, enucleations at birth change the expression pattern of genes involved in cortical arealization at P10, before the sensory experience starts (Dye et al., 2012). Moreover, experiments in rats in which enucleations were performed at birth, provoked an increase of the barrel size before active sensory whisking (Fetter-Pruneda et al., 2013; Abbott et al., 2015). Additionally, a recent publication in mice has shown that embryonic enucleation leads to an increase of the barrel size at P4, along with changes in thalamic gene expression (Moreno-Juan et al., 2017). These studies raise the possibility that the barrel cortex expansion is likely due to shifts in the developmental timing of sensory systems, and not due to increased levels of experience-dependent neuronal activity.
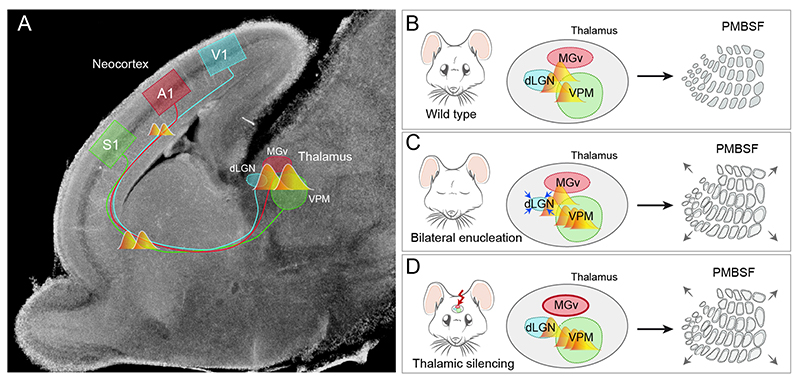
It is clear that early plasticity requires cross-talk among deprived/turned off and spared modalities. A putative scenario for inter-modality communication is the thalamus; the brain structure where peripheral sensory inputs from visual, auditory and somatosensory systems converge before reaching the cortex. Indeed, barrel field expansion in embryonically visually deprived mice has been explained using an experience independent model, whereby spontaneous calcium waves in the thalamus during embryonic stages were shown to be a crucial regulator of cortical reorganization (Moreno-Juan et al., 2017). These waves communicate visual, auditory and somatosensory thalamic nuclei before the arrival of the input from the sensory periphery. Both embryonic binocular enucleation and auditory thalamus silencing cause an increase in the frequency of calcium waves in the somatosensory thalamus that predates an enlargement of the cortical barrel-field (Figure 2). At the molecular level, calcium waves were shown to regulate the nuclear orphan receptor Rorβ in the VPM, which increases the complexity of TCA terminals. Thus, this study demonstrates, on one hand, the existence of a novel mode of communication between distinct sensory-modalities, and on the other hand the mechanism that controls gene expression and triggers barrel size adaptation before the onset of sensory information processing. In sum, intrinsic mechanisms before sensory experience may coexist together with experience-driven activity to promote neuroplastic cross-modal changes after sensory deprivation. The thalamus emerges as a key brain structure to play a role in this early cortical plasticity and therefore, as a potential target for therapeutic interventions.
Figure 2. Thalamic calcium waves communicate sensory modalities and regulate S1 barrels formation.
(A) Embryonic Ca2+ waves propagate among principal thalamic sensory nuclei and reach the neocortex through thalamocortical axons at E16.5 in mice. (B) Bilateral embryonic enucleation at E14.5 triggers more Ca2+ waves in VPM and LGN, eventually leading to an increment in barrel size and posteromedial barrel subfield (PMBSF) area. (C) Silencing Ca2+ waves in the MGv increases Ca2+ waves frequency in the VPM. This change precedes barrel and PMBSF area enlargement.
Concluding remarks
The emergence of the somatosensory cortical map involves the interaction between genes and external input by the environment. What characteristics of the input (trophic or activity-dependent or both) influence the formation of the barrel-field have been recently boosted, especially through the use cell-type-specific manipulation of gene expression and neural activity. Moreover, the role of subcortical regions, such as the thalamus, in controlling the emergence of specific features of the map is gaining emphasis. Thalamic spontaneous synchronous activity not only modulates the formation of the S1 map but also controls its plasticity through the regulation of thalamic gene expression, and ultimately, influencing TCA axonal branching. However, other questions remain opened such as, which are the mechanisms involved in the dendritic refinement of L4 cortical cells? What is the role of the thalamic waves in barrel cortex development? These and other questions should be tackled and responded in the near future.
Acknowledgements
We thank the members of the López-Bendito laboratory for discussions and useful comments on the manuscript. This work was supported by the Spanish MINECO BFU2015-64432-R, the Spanish State Research Agency through the “Severo Ochoa” Programme for Centres of Excellence in R&D (SEV-2013-0317) and the European Commission Grant ERC-2014-CoG-647012. G. L-B is an EMBO YIP Investigator and a FENS-Kavli scholar. We apologize to the authors that could not be cited due to space limitations.
References
- Abbott CW, Kozanian OO, Huffman KJ. The effects of lifelong blindness on murine neuroanatomy and gene expression. Front Aging Neurosci. 2015;7:144. doi: 10.3389/fnagi.2015.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Majid RM, Leong WL, Schalkwyk LC, Smallman DS, Wong ST, Storm DR, Fine A, Dobson MJ, Guernsey DL, Neumann PE. Loss of adenylyl cyclase I activity disrupts patterning of mouse somatosensory cortex. Nat Genet. 1998;19:289–291. doi: 10.1038/980. [DOI] [PubMed] [Google Scholar]
- Ackman JB, Crair MC. Role of emergent neural activity in visual map development. Curr Opin Neurobiol. 2014;24:166–175. doi: 10.1016/j.conb.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agmon A, Yang LT, Jones EG, O’Dowd DK. Topological precision in the thalamic projection to neonatal mouse barrel cortex. J Neurosci. 1995;15:549–561. doi: 10.1523/JNEUROSCI.15-01-00549.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentano M, Chou SJ, Tomassy GS, Leingartner A, O’Leary DD, Studer M. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci. 2007;10:1277–1286. doi: 10.1038/nn1958. [DOI] [PubMed] [Google Scholar]
- Bagnall MW, Hull C, Bushong EA, Ellisman MH, Scanziani M. Multiple clusters of release sites formed by individual thalamic afferents onto cortical interneurons ensure reliable transmission. Neuron. 2011;71:180–194. doi: 10.1016/j.neuron.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester-Rosado CJ, Albright MJ, Wu CS, Liao CC, Zhu J, Xu J, Lee LJ, Lu HC. mGluR5 in cortical excitatory neurons exerts both cell-autonomous and -nonautonomous influences on cortical somatosensory circuit formation. J Neurosci. 2010;30:16896–16909. doi: 10.1523/JNEUROSCI.2462-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester-Rosado CJ, Sun H, Huang JY, Lu HC. mGluR5 Exerts Cell-Autonomous Influences on the Functional and Anatomical Development of Layer IV Cortical Neurons in the Mouse Primary Somatosensory Cortex. J Neurosci. 2016;36:8802–8814. doi: 10.1523/JNEUROSCI.1224-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braisted J, Tuttle R, O’leary D. Thalamocortical axons are influenced by chemorepellent and chemoattractant activities localized to decision points along their path. Dev Biol. 1999;208:430–440. doi: 10.1006/dbio.1999.9216. [DOI] [PubMed] [Google Scholar]
- Bronchti G, Schonenberger N, Welker E, Van der Loos H. Barrelfield expansion after neonatal eye removal in mice. Neuroreport. 1992;3:489–492. doi: 10.1097/00001756-199206000-00008. [DOI] [PubMed] [Google Scholar]
- Cang J, Renteria RC, Kaneko M, Liu X, Copenhagen DR, Stryker MP. Development of precise maps in visual cortex requires patterned spontaneous activity in the retina. Neuron. 2005;48:797–809. doi: 10.1016/j.neuron.2005.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases O, Vitalis T, Seif I, De Maeyer E, Sotelo C, Gaspar P. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Castillo-Paterna M, Moreno-Juan V, Filipchuk A, Rodriguez-Malmierca L, Susin R, Lopez Bendito G. DCC functions as an accelerator of thalamocortical axonal growth downstream of spontaneous thalamic activity. EMBO reports. 2015;16:851–862. doi: 10.15252/embr.201439882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano SM, Shatz CJ. Activity-dependent cortical target selection by thalamic axons. Science. 1998;281:559–562. doi: 10.1126/science.281.5376.559. [DOI] [PubMed] [Google Scholar]
- Chiaia NL, Fish SE, Bauer WR, Bennett-Clarke CA, Rhoades RW. Postnatal blockade of cortical activity by tetrodotoxin does not disrupt the formation of vibrissa-related patterns in the rat’s somatosensory cortex. Brain Res Dev Brain Res. 1992;66:244–250. doi: 10.1016/0165-3806(92)90086-c. [DOI] [PubMed] [Google Scholar]
- Chou SJ, Babot Z, Leingartner A, Studer M, Nakagawa Y, O’Leary DD. Geniculocortical input drives genetic distinctions between primary and higher-order visual areas. Science. 2013;340:1239–1242. doi: 10.1126/science.1232806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnese MT, Khazipov R. “Slow activity transients” in infant rat visual cortex: a spreading synchronous oscillation patterned by retinal waves. J Neurosci. 2010;30:4325–4337. doi: 10.1523/JNEUROSCI.4995-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinople CM, Bruno RM. Deep cortical layers are activated directly by thalamus. Science. 2013;340:1591–1594. doi: 10.1126/science.1236425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datwani A, Iwasato T, Itohara S, Erzurumlu RS. NMDA receptor-dependent pattern transfer from afferents to postsynaptic cells and dendritic differentiation in the barrel cortex. Molecular and cellular neurosciences. 2002;21:477–492. doi: 10.1006/mcne.2002.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deck M, Lokmane L, Chauvet S, Mailhes C, Keita M, Niquille M, Yoshida M, Yoshida Y, Lebrand C, Mann F, Grove EA, et al. Pathfinding of Corticothalamic Axons Relies on a Rendezvous with Thalamic Projections. Neuron. 2013;77:472–484. doi: 10.1016/j.neuron.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye CA, Abbott CW, Huffman KJ. Bilateral enucleation alters gene expression and intraneocortical connections in the mouse. Neural Dev. 2012;7:5. doi: 10.1186/1749-8104-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egusa SF, Inoue YU, Asami J, Terakawa YW, Hoshino M, Inoue T. Classic cadherin expressions balance postnatal neuronal positioning and dendrite dynamics to elaborate the specific cytoarchitecture of the mouse cortical area. Neuroscience research. 2016;105:49–64. doi: 10.1016/j.neures.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Bates CA, Killackey HP. Differential organization of thalamic projection cells in the brain stem trigeminal complex of the rat. Brain Res. 1980;198:427–433. doi: 10.1016/0006-8993(80)90756-8. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Gaspar P. Development and critical period plasticity of the barrel cortex. Eur J Neurosci. 2012;35:1540–1553. doi: 10.1111/j.1460-9568.2012.08075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Kind PC. Neural activity: sculptor of 'barrels' in the neocortex. Trends in Neurosciences. 2001;24:589–595. doi: 10.1016/s0166-2236(00)01958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa JS, Wheeler DG, Tsien RW, Luo L. Uncoupling dendrite growth and patterning: single-cell knockout analysis of NMDA receptor 2B. Neuron. 2009;62:205–217. doi: 10.1016/j.neuron.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetter-Pruneda I, Geovannini-Acuña H, Santiago C, Ibarrarán-Viniegra AS, Martínez-Martínez E, Sandoval-Velasco M, Uribe-Figueroa L, Padilla-Cortés P, Mercado-Célis G, Gutiérrez-Ospina G. Shifts in Developmental Timing, and Not Increased Levels of Experience-Dependent Neuronal Activity, Promote Barrel Expansion in the Primary Somatosensory Cortex of Rats Enucleated at Birth. PloS one. 2013;8:e54940. doi: 10.1371/journal.pone.0054940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K, Schlaggar BL, Glazewski S, O'Leary DD. Glutamate receptor blockade at cortical synapses disrupts development of thalamocortical and columnar organization in somatosensory cortex. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:5584–5589. doi: 10.1073/pnas.93.11.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangeul L, Pouchelon G, Telley L, Lefort S, Luscher C, Jabaudon D. A cross-modal genetic framework for the development and plasticity of sensory pathways. Nature. 2016;538:96–98. doi: 10.1038/nature19770. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Emx2 patterns the neocortex by regulating FGF positional signaling. Nat Neurosci. 2003;6:825–831. doi: 10.1038/nn1093. [DOI] [PubMed] [Google Scholar]
- Garel S, Lopez-Bendito G. Inputs from the thalamocortical system on axon pathfinding mechanisms. Curr Opin Neurobiol. 2014;27:143–150. doi: 10.1016/j.conb.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Gheorghita F, Kraftsik R, Dubois R, Welker E. Structural basis for map formation in the thalamocortical pathway of the barrelless mouse. J Neurosci. 2006;26:10057–10067. doi: 10.1523/JNEUROSCI.1263-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant E, Hoerder-Suabedissen A, Molnar Z. The Regulation of Corticofugal Fiber Targeting by Retinal Inputs. Cereb Cortex. 2016;26:1336–1348. doi: 10.1093/cercor/bhv315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki T, Leingärtner A, Ringstedt T, O'Leary DDM. EMX2 regulates sizes and positioning of the primary sensory and motor areas in neocortex by direct specification of cortical progenitors. Neuron. 2004;43:359–372. doi: 10.1016/j.neuron.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Hanganu IL, Ben-Ari Y, Khazipov R. Retinal waves trigger spindle bursts in the neonatal rat visual cortex. J Neurosci. 2006;26:6728–6736. doi: 10.1523/JNEUROSCI.0752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanganu-Opatz IL. Between molecules and experience: role of early patterns of coordinated activity for the development of cortical maps and sensory abilities. Brain Res Rev. 2010;64:160–176. doi: 10.1016/j.brainresrev.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Hannan AJ, Blakemore C, Katsnelson A, Vitalis T, Huber KM, Bear M, Roder J, Kim D, Shin HS, Kind PC. PLC-beta1, activated via mGluRs, mediates activity-dependent differentiation in cerebral cortex. Nat Neurosci. 2001;4:282–288. doi: 10.1038/85132. [DOI] [PubMed] [Google Scholar]
- Henderson TA, Woolsey TA, Jacquin MF. Infraorbital nerve blockade from birth does not disrupt central trigeminal pattern formation in the rat. Brain Res Dev Brain Res. 1992;66:146–152. doi: 10.1016/0165-3806(92)90152-m. [DOI] [PubMed] [Google Scholar]
- Higashi S, Molnar Z, Kurotani T, Toyama K. Prenatal development of neural excitation in rat thalamocortical projections studied by optical recording. Neuroscience. 2002;115:1231–1246. doi: 10.1016/s0306-4522(02)00418-9. [DOI] [PubMed] [Google Scholar]
- Hoerder-Suabedissen A, Molnar Z. Development, evolution and pathology of neocortical subplate neurons. Nat Rev Neurosci. 2015;16:133–146. doi: 10.1038/nrn3915. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, Isaacson JS, Scanziani M. Postsynaptic mechanisms govern the differential excitation of cortical neurons by thalamic inputs. J Neurosci. 2009;29:9127–9136. doi: 10.1523/JNEUROSCI.5971-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan M, Lu HC, Albright MJ, She WC, Crair MC. Barrel map development relies on protein kinase A regulatory subunit II beta-mediated cAMP signaling. J Neurosci. 2006;26:4338–4349. doi: 10.1523/JNEUROSCI.3745-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasato T, Datwani A, Wolf AM, Nishiyama H, Taguchi Y, Tonegawa S, Knopfel T, Erzurumlu RS, Itohara S. Cortex-restricted disruption of NMDAR1 impairs neuronal patterns in the barrel cortex. Nature. 2000;406:726–731. doi: 10.1038/35021059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasato T, Erzurumlu RS, Huerta PT, Chen DF, Sasaoka T, Ulupinar E, Tonegawa S. NMDA receptor-dependent refinement of somatotopic maps. Neuron. 1997;19:1201–1210. doi: 10.1016/s0896-6273(00)80412-2. [DOI] [PubMed] [Google Scholar]
- Iwasato T, Inan M, Kanki H, Erzurumlu RS, Itohara S, Crair MC. Cortical adenylyl cyclase 1 is required for thalamocortical synapse maturation and aspects of layer IV barrel development. J Neurosci. 2008;28:5931–5943. doi: 10.1523/JNEUROSCI.0815-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EC, Campagnoni C, Kampf K, Reyes SD, Kalra V, Handley V, Xie Y-Y, Hong-Hu Y, Spreur V, Fisher RS, Campagnoni AT. Visualization of corticofugal projections during early cortical development in a τ-GFP-transgenic mouse. European Journal of Neuroscience. 2007;25:17–30. doi: 10.1111/j.1460-9568.2006.05258.x. [DOI] [PubMed] [Google Scholar]
- Jensen KF, Killackey HP. Terminal arbors of axons projecting to the somatosensory cortex of the adult rat. II. The altered morphology of thalamocortical afferents following neonatal infraorbital nerve cut. J Neurosci. 1987;7:3544–3553. doi: 10.1523/JNEUROSCI.07-11-03544.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri S, Erzurumlu RS, Crossin K. Barrel construction in rodent neocortex: role of thalamic afferents versus extracellular matrix molecules. Proc Natl Acad Sci U S A. 1991;88:4489–4493. doi: 10.1073/pnas.88.10.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Annu Rev Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsaki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- Killackey HP. Anatomical evidence for cortical subdivisions based on vertically discrete thalamic projections from the ventral posterior nucleus to cortical barrels in the rat. Brain Res. 1973;51:326–331. doi: 10.1016/0006-8993(73)90383-1. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Belford G, Ryugo R, Ryugo DK. Anomalous organization of thalamocortical projections consequent to vibrissae removal in the newborn rat and mouse. Brain Res. 1976;104:309–315. doi: 10.1016/0006-8993(76)90623-5. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Rhoades RW, Bennett-Clarke CA. The formation of a cortical somatotopic map. Trends in Neurosciences. 1995;18:402–407. doi: 10.1016/0166-2236(95)93937-s. [DOI] [PubMed] [Google Scholar]
- Kivrak BG, Erzurumlu RS. Development of the principal nucleus trigeminal lemniscal projections in the mouse. The Journal of Comparative Neurology. 2012;521:299–311. doi: 10.1002/cne.23183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L-J, Iwasato T, Itohara S, Erzurumlu RS. Exuberant thalamocortical axon arborization in cortex-specific NMDAR1 knockout mice. The Journal of Comparative Neurology. 2005;485:280–292. doi: 10.1002/cne.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyva-Díaz E, Lopez Bendito G. In and out from the cortex: Development of major forebrain connections. Neuroscience. 2013;254:26–44. doi: 10.1016/j.neuroscience.2013.08.070. [DOI] [PubMed] [Google Scholar]
- Li H, Fertuzinhos S, Mohns E, Hnasko TS, Verhage M, Edwards R, Sestan N, Crair MC. Laminar and columnar development of barrel cortex relies on thalamocortical neurotransmission. Neuron. 2013;79:970–986. doi: 10.1016/j.neuron.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Erzurumlu RS, Chen C, Jhaveri S, Tonegawa S. Whisker-related neuronal patterns fail to develop in the trigeminal brainstem nuclei of NMDAR1 knockout mice. Cell. 1994;76:427–437. doi: 10.1016/0092-8674(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Lokmane L, Proville R, Narboux-Nême N, Györy I, Keita M, Mailhes C, Léna C, Gaspar P, Grosschedl R, Garel S. Sensory Map Transfer to the Neocortex Relies on Pretarget Ordering of Thalamic Axons. Current Biology. 2013;23:810–816. doi: 10.1016/j.cub.2013.03.062. [DOI] [PubMed] [Google Scholar]
- López-Bendito G, Cautinat A, Sánchez JA, Bielle F, Flames N, Garratt AN, Talmage DA, Role LW, Charnay P, Marín O, Garel S. Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation. Cell. 2006;125:127–142. doi: 10.1016/j.cell.2006.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bendito G, Molnár Z. Thalamocortical development: how are we going to get there? Nat Rev Neurosci. 2003;4:276–289. doi: 10.1038/nrn1075. [DOI] [PubMed] [Google Scholar]
- Lu HC, Butts DA, Kaeser PS, She WC, Janz R, Crair MC. Role of efficient neurotransmitter release in barrel map development. J Neurosci. 2006;26:2692–2703. doi: 10.1523/JNEUROSCI.3956-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HC, She WC, Plas DT, Neumann PE, Janz R, Crair MC. Adenylyl cyclase I regulates AMPA receptor trafficking during mouse cortical ‘barrel’ map development. Nat Neurosci. 2003;6:939–947. doi: 10.1038/nn1106. [DOI] [PubMed] [Google Scholar]
- Luhmann HJ. Review of imaging network activities in developing rodent cerebral cortex in vivo. Neurophotonics. 2017;4:031202. doi: 10.1117/1.NPh.4.3.031202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann HJ, Hanganu I, Kilb W. Cellular physiology of the neonatal rat cerebral cortex. Brain Res Bull. 2003;60:345–353. doi: 10.1016/s0361-9230(03)00059-5. [DOI] [PubMed] [Google Scholar]
- Ma MP, Woosley TA. Cytoarchitectonic correlates of the vibrissae in the medullary trigeminal complex of the mouse. Brain Research. 1984;306 doi: 10.1016/0006-8993(84)90390-1. [DOI] [PubMed] [Google Scholar]
- Mandai K, Reimert DV, Ginty DD. Linx Mediates Interaxonal Interactions and Formation of the Internal Capsule. Neuron. 2014;83:93–103. doi: 10.1016/j.neuron.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzera C, Lopez-Bendito G. Cross-modal plasticity in sensory deprived animal models: From the thalamocortical development point of view. Journal of chemical neuroanatomy. 2015 doi: 10.1016/j.jchemneu.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Minlebaev M, Colonnese M, Tsintsadze T, Sirota A, Khazipov R. Early gamma oscillations synchronize developing thalamus and cortex. Science. 2011;334:226–229. doi: 10.1126/science.1210574. [DOI] [PubMed] [Google Scholar]
- Mire E, Mezzera C, L-Diaz E, Paternain AV, Squarzoni P, Bluy L, Castillo-Paterna M, Lopez MiaJen, Pi S, Tessier-Lavigne M, Garel SnJGa, et al. Spontaneous activity regulates Robo1 transcription to mediate a switch in thalamocortical axon growth. Nature neuroscience. 2012:1–12. doi: 10.1038/nn.3160. [DOI] [PubMed] [Google Scholar]
- Mitrovic N, Mohajeri H, Schachner M. Effects of NMDA receptor blockade in the developing rat somatosensory cortex on the expression of the glia-derived extracellular matrix glycoprotein tenascin-C. The European journal of neuroscience. 1996;8:1793–1802. doi: 10.1111/j.1460-9568.1996.tb01323.x. [DOI] [PubMed] [Google Scholar]
- Mizuno H, Luo W, Tarusawa E, Saito YM, Sato T, Yoshimura Y, Itohara S, Iwasato T. NMDAR-regulated dynamics of layer 4 neuronal dendrites during thalamocortical reorganization in neonates. Neuron. 2014;82:365–379. doi: 10.1016/j.neuron.2014.02.026. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Adams R, Blakemore C. Mechanisms underlying the early establishment of thalamocortical connections in the rat. J Neurosci. 1998;18:5723–5745. doi: 10.1523/JNEUROSCI.18-15-05723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar Z, Garel S, Lopez-Bendito G, Maness P, Price DJ. Mechanisms controlling the guidance of thalamocortical axons through the embryonic forebrain. Eur J Neurosci. 2012;35:1573–1585. doi: 10.1111/j.1460-9568.2012.08119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár Z, Higashi S, López-Bendito G. Choreography of early thalamocortical development. Cereb Cortex. 2003;13:661–669. doi: 10.1093/cercor/13.6.661. [DOI] [PubMed] [Google Scholar]
- Moreno-Juan V, Filipchuk A, Anton-Bolanos N, Mezzera C, Gezelius H, Andres B, Rodriguez-Malmierca L, Susin R, Schaad O, Iwasato T, Schule R, et al. Prenatal thalamic waves regulate cortical area size prior to sensory processing. Nat Commun. 2017;8:14172. doi: 10.1038/ncomms14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narboux-Neme N, Evrard A, Ferezou I, Erzurumlu RS, Kaeser PS, Laine J, Rossier J, Ropert N, Sudhof TC, Gaspar P. Neurotransmitter Release at the Thalamocortical Synapse Instructs Barrel Formation But Not Axon Patterning in the Somatosensory Cortex. Journal of Neuroscience. 2012;32:6183–6196. doi: 10.1523/JNEUROSCI.0343-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persico AM, Mengual E, Moessner R, Hall FS, Revay RS, Sora I, Arellano J, DeFelipe J, Gimenez-Amaya JM, Conciatori M, Marino R, et al. Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release. J Neurosci. 2001;21:6862–6873. doi: 10.1523/JNEUROSCI.21-17-06862.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CCH. The Functional Organization of the Barrel Cortex. Neuron. 2007;56:339–355. doi: 10.1016/j.neuron.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Pinon MC, Jethwa A, Jacobs E, Campagnoni A, Molnar Z. Dynamic integration of subplate neurons into the cortical barrel field circuitry during postnatal development in the Golli-tau-eGFP (GTE) mouse. J Physiol. 2009;587:1903–1915. doi: 10.1113/jphysiol.2008.167767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouchelon G, Gambino F, Bellone C, Telley L, Vitali I, Luscher C, Holtmaat A, Jabaudon D. Modality-specific thalamocortical inputs instruct the identity of postsynaptic L4 neurons. Nature. 2014;511:471–474. doi: 10.1038/nature13390. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B, Korte M, Egert U. Crossmodal changes in the somatosensory vibrissa/barrel system of visually deprived animals. Proc Natl Acad Sci U S A. 1992;89:5063–5067. doi: 10.1073/pnas.89.11.5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice FL, Gomez C, Barstow C. A comparative analysis of the development of the primary somatosensory cortex: Interspecies similarities during barrel and laminar development. Journal of... 1985 doi: 10.1002/cne.902360405. [DOI] [PubMed] [Google Scholar]
- Rice FL, Van der Loos H. Development of the barrels and barrel field in the somatosensory cortex of the mouse. The Journal of Comparative Neurology. 1977;171:545–560. doi: 10.1002/cne.901710408. [DOI] [PubMed] [Google Scholar]
- Rudhard Y, Kneussel M, Nassar MA, Rast GF, Annala AJ, Chen PE, Tigaret CM, Dean I, Roes J, Gibb AJ, Hunt SP, et al. Absence of Whisker-related pattern formation in mice with NMDA receptors lacking coincidence detection properties and calcium signaling. J Neurosci. 2003;23:2323–2332. doi: 10.1523/JNEUROSCI.23-06-02323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salichon N, Gaspar P, Upton AL, Picaud S, Hanoun N, Hamon M, De Maeyer E, Murphy DL, Mossner R, Lesch KP, Hen R, et al. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-ht transporter knock-out mice. J Neurosci. 2001;21:884–896. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar BL, Fox K, O'Leary DD. Postsynaptic control of plasticity in developing somatosensory cortex. Nature. 1993;364:623–626. doi: 10.1038/364623a0. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, O’Leary DD. Early development of the somatotopic map and barrel patterning in rat somatosensory cortex. J Comp Neurol. 1994;346:80–96. doi: 10.1002/cne.903460106. [DOI] [PubMed] [Google Scholar]
- Sehara K, Kawasaki H. Neuronal circuits with whisker-related patterns. Molecular neurobiology. 2011;43:155–162. doi: 10.1007/s12035-011-8170-8. [DOI] [PubMed] [Google Scholar]
- She WC, Quairiaux C, Albright MJ, Wang YC, Sanchez DE, Chang PS, Welker E, Lu HC. Roles of mGluR5 in synaptic function and plasticity of the mouse thalamocortical pathway. Eur J Neurosci. 2009;29:1379–1396. doi: 10.1111/j.1460-9568.2009.06696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimogori T, Grove EA. Fibroblast growth factor 8 regulates neocortical guidance of area-specific thalamic innervation. J Neurosci. 2005;25:6550–6560. doi: 10.1523/JNEUROSCI.0453-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DJ, Woolsey TA. Morphology of Golgi-Cox-impregnated barrel neurons in rat SmI cortex. J Comp Neurol. 1984;230:119–132. doi: 10.1002/cne.902300111. [DOI] [PubMed] [Google Scholar]
- Spitzer N. Electrical activity in early neuronal development. Nature. 2006;444:707–712. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- Stocker AM, O’Leary DDM. Emx1 Is Required for Neocortical Area Patterning. PLoS ONE. 2016;11:e0149900–0149911. doi: 10.1371/journal.pone.0149900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryker MP, Harris WA. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J Neurosci. 1986;6:2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Lee LJ, Hayashi Y, Muglia L, Itohara S, Erzurumlu RS, Iwasato T. Thalamic adenylyl cyclase 1 is required for barrel formation in the somatosensory cortex. Neuroscience. 2015;290:518–529. doi: 10.1016/j.neuroscience.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toldi J, Farkas T, Volgyi B. Neonatal enucleation induces cross-modal changes in the barrel cortex of rat. A behavioural and electrophysiological study. Neurosci Lett. 1994;167:1–4. doi: 10.1016/0304-3940(94)91014-6. [DOI] [PubMed] [Google Scholar]
- Toldi J, Feher O, Wolff JR. Neuronal plasticity induced by neonatal monocular (and binocular) enucleation. Prog Neurobiol. 1996;48:191–218. doi: 10.1016/0301-0082(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Tsukano H, Horie M, Ohga S, Takahashi K, Kubota Y, Hishida R, Takebayashi H, Shibuki K. Reconsidering Tonotopic Maps in the Auditory Cortex and Lemniscal Auditory Thalamus in Mice. Front Neural Circuits. 2017;11:14. doi: 10.3389/fncir.2017.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura M, Nakao S, Suzuki S, Takeichi M, Hirano S. OL-Protocadherin is essential for growth of striatal axons and thalamocortical projections. Nat Neurosci. 2007;10:1151–1159. doi: 10.1038/nn1960. [DOI] [PubMed] [Google Scholar]
- van der Loos H. Neuronal circuitry and its development. Prog Brain Res. 1976;45:259–278. doi: 10.1016/S0079-6123(08)60994-2. [DOI] [PubMed] [Google Scholar]
- Van der Loos H, Woolsey TA. Somatosensory cortex: structural alterations following early injury to sense organs. Science. 1973;179:395–398. doi: 10.1126/science.179.4071.395. [DOI] [PubMed] [Google Scholar]
- Watson RF, Abdel-Majid RM, Barnett MW, Willis BS, Katsnelson A, Gillingwater TH, McKnight GS, Kind PC, Neumann PE. Involvement of protein kinase A in patterning of the mouse somatosensory cortex. J Neurosci. 2006;26:5393–5401. doi: 10.1523/JNEUROSCI.0750-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker E, Armstrong-James M, Bronchti G, Ourednik W, Gheorghita-Baechler F, Dubois R, Guernsey DL, Van der Loos H, Neumann PE. Altered sensory processing in the somatosensory cortex of the mouse mutant barrelless. Science. 1996;271:1864–1867. doi: 10.1126/science.271.5257.1864. [DOI] [PubMed] [Google Scholar]
- Weller WL, Johnson JI. Barrels in cerebral cortex altered by receptor disruption in newborn, but not in five-day-old mice (Cricetidoe and Muridae) Brain Res. 1975;83:504–508. doi: 10.1016/0006-8993(75)90844-6. [DOI] [PubMed] [Google Scholar]
- Wijetunge LS, Till SM, Gillingwater TH, Ingham CA, Kind PC. mGluR5 regulates glutamate-dependent development of the mouse somatosensory cortex. J Neurosci. 2008;28:13028–13037. doi: 10.1523/JNEUROSCI.2600-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA, Dierker ML, Wann DF. Mouse SmI cortex: qualitative and quantitative classification of golgi-impregnated barrel neurons. Proc Natl Acad Sci U S A. 1975;72:2165–2169. doi: 10.1073/pnas.72.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Wann JR. Areal changes in mouse cortical barrels following vibrissal damage at different postnatal ages. J Comp Neurol. 1976;170:53–66. doi: 10.1002/cne.901700105. [DOI] [PubMed] [Google Scholar]
- Wu C-S, Ballester Rosado CJ, Lu H-C. What can we get from ‘barrels’: the rodent barrel cortex as a model for studying the establishment of neural circuits. European Journal of Neuroscience. 2011;34:1663–1676. doi: 10.1111/j.1460-9568.2011.07892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JW, An S, Sun JJ, Reyes-Puerta V, Kindler J, Berger T, Kilb W, Luhmann HJ. Thalamic network oscillations synchronize ontogenetic columns in the newborn rat barrel cortex. Cereb Cortex. 2013;23:1299–1316. doi: 10.1093/cercor/bhs103. [DOI] [PubMed] [Google Scholar]
- Zembrzycki A, Chou SJ, Ashery-Padan R, Stoykova A, O’Leary DD. Sensory cortex limits cortical maps and drives top-down plasticity in thalamocortical circuits. Nat Neurosci. 2013;16:1060–1067. doi: 10.1038/nn.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Purves D. Effects of increased neural activity on brain growth. Proc Natl Acad Sci USA. 1995;92:1802–1806. doi: 10.1073/pnas.92.6.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]