Abstract
Children with atopic dermatitis (AD) show an increased risk to develop asthma later in life, a phenomenon referred to as ‘atopic march’, which emphasizes the need for secondary prevention therapies. This study aimed to investigate whether relief of skin inflammation by glucocorticoids and PPARγ-agonists might influence the subsequent development of asthma in a murine model for the atopic march in which mice were repeatedly exposed to house dust mite (HDM) via the skin, followed by exposure to HDM in lungs. To abrogate AD, mice received topical treatment with GR/PPARγ-agonists. Nuclear receptor ligand effects were assessed on primary keratinocytes and dendritic cells, as central players in skin inflammation. Prior HDM-induced skin inflammation aggravates allergic airway inflammation and induces a mixed Th2/Th17 response in the lungs. Cutaneous combined activation of GR/PPARγ reduced skin inflammation to a higher extent compared to single activation. Additive anti-inflammatory effects were more prominent in dendritic cells, as compared to keratinocytes. Alleviation of allergic skin inflammation by activation of GR/PPARγ appeared insufficient to avoid the allergic immune response in the lungs but efficiently reduced asthma severity by counteracting the Th17 response. GR/PPARγ co-activation represents a potent remedy against allergic skin inflammation and worsening of the atopic march.
Keywords: Atopic march, atopic dermatitis, house dust mite, peroxisome proliferator activated receptor γ, glucocorticoid receptor
Introduction
Atopic dermatitis (AD) is a common allergic skin disease among children in wealthy nations (Spergel 2010). Strikingly, 50% of children with AD develop asthma later in life and the risk to develop hay fever is as much as 75% (Thomsen 2015). This phenomenon, whereby allergic manifestations occur in a typical sequence of events is called the ‘atopic march’ (Bantz et al. 2014; Dharmage et al. 2014). In the current study, we developed a model that mimics the atopic march, in which the presence of house dust mite (HDM)-induced AD aggravates the subsequent allergic airway inflammation to a mixed Th2/Th17 response upon HDM reencounter in the lungs. The initiation and perpetuation of allergic inflammation is known to be tightly regulated by an interplay between structural cells (such as bronchial epithelial cells in the lungs or keratinocytes in the skin) and underlying dendritic cells (Deckers et al. 2017a). Attempts to halt the atopic march consist of primary prevention, i.e. before the onset of AD, and secondary prevention, i.e. when children exhibit symptoms of AD (Dharmage et al. 2014). The latter raises the question whether suppression of established skin inflammation is sufficient to prevent the onset or ameliorate the severity of subsequent asthma. Glucocorticoids (GC) are potent anti-inflammatory drugs that serve as the standard treatment for patients with AD (Silverberg et al. 2016). Unfortunately, GC therapy results in detrimental side effects on epidermal barrier integrity such as inhibition of homeostatic keratinocyte proliferation (Schäcke et al. 2002), inhibition of epidermal lipid synthesis (Kao et al. 2003), downregulation of anti-microbial peptides (Aberg et al. 2007) and of epidermal structural proteins such as involucrin, filaggrin and loricrin (Demerjian et al. 2009). Therefore, the quest for new therapies with retained anti-inflammatory properties but with reduced GC-mediated side effects continues. Demerjian et al. showed that the activation of peroxisome proliferator activated receptors (PPARs) and Liver X Receptors (LXRs), two nuclear receptors (NR) regulating cell metabolism and exerting anti-inflammatory effects, reduced GC-induced adverse effects on the epidermis (Demerjian et al. 2009). Additionally, the activation of the glucocorticoid receptor (GR) has been shown to contribute to the anti-inflammatory effects of PPARγ activity (Yamamoto et al. 2014) and the presence of PPARα contributes to the anti-inflammatory activity of GR (Cuzzocrea et al. 2008). Accordingly, the combined activation of GR and PPARα was shown to be effective in suppressing inflammation and reducing GC-mediated side effects in a murine model of AD (Hatano et al. 2011). Previously, we described that GR and PPARα are able to physically interact and provide additive suppressive effects on NFκB-driven reporter genes whereas combined activation of these NR results in an improved GC-induced side-effect profile (Bougarne et al. 2009). Various studies using mouse models report an anti-inflammatory effect of PPARγ-ligands on the onset of or inflammation in established AD (Behshad et al. 2008; Dahten et al. 2007; Jung et al. 2011; Tachibana et al. 2008). Therefore, we asked whether PPARγ might also interact with GR to potentially provide an altered anti-inflammatory profile. We investigated the effect of combined PPARγ/GR activation on cytokine levels in ex vivo cultured primary murine keratinocytes and bone marrow-derived DCs (BMDCs), two major players in the initiation and perpetuation of allergic skin inflammation (Deckers et al. 2017a). We next studied whether GR/PPARγ activation would be beneficial in the treatment of established AD and able to prevent or influence the subsequent development of asthma.
Results
Allergic skin inflammation affects development and severity of subsequent allergic airway inflammation
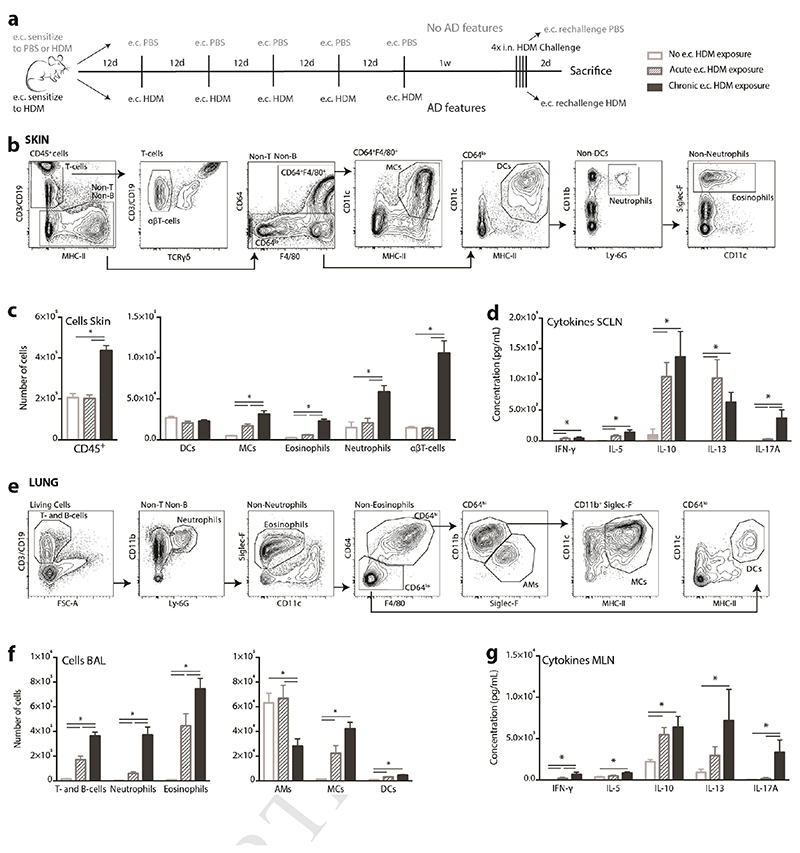
C57Bl/6 mice were subjected to the protocol depicted in Figure 1A. Mice that were chronically exposed to HDM on the skin showed features of AD such as ear thickening, quantified by the weight of dissected ears (Supplementary Figure 1) and they had significantly more infiltrating CD45+ cells in the ear skin compared to mice that were never exposed to HDM on the skin (No e.c. HDM) or only once (Acute e.c. HDM) (Figure 1B-C). Among the CD45+ cells in the skin, eosinophils, monocyte-derived cells (MCs), neutrophils, and αβT-cells were significantly increased in inflamed ears compared to the non-inflamed ears that were only acutely exposed to HDM (Figure 1C). Acute e.c. HDM exposure induced a minor but significant infiltration of eosinophils and MCs, indicating that skin sensitization to HDM, without atopic dermatitis features, may alter the composition of immune cells in the skin (Figure 1C). Ex vivo HDM-restimulated cells from the HDM-sensitized superficial cervical lymph nodes (SCLN), which drain the skin of the ears, mainly produced cytokines associated with a Th2 response (IL-5, IL-13 and IL-10). Remarkably, SCLN cells from chronic e.c. HDM-treated mice produced IL-17A, indicating a cutaneous Th17 response, which was absent in the acute e.c. HDM-treated mice (Figure 1D).
Figure 1.
Chronic epicutaneous HDM exposure results in a mixed Th2/Th17 response in the lungs upon intranasal HDM challenge. C57Bl/6 mice were subjected to the protocol depicted in A). Flow Cytometry analysis of the total cells from (B) the ear skin and (E) bronchoalveolar lavage (BAL) and (C,F) specific subsets. Cytokine secretion by (D) superficial cervical lymph node (SCLN) cells and (G) Mediastinal LN (MLN) cells after 3 days of HDM stimulation ex vivo (ELISA). AD, atopic dermatitis; AMs, alveolar macrophages; DCs, dendritic cells; e.c., epicutaneous; HDM, house dust mite; MCs, monocyte-derived cells. Data represent means + SEM from 3 independent experiments with each 6-8 animals/group and *p < 0,05; analyzed via the Mann-Whitney U-test.
Surprisingly, chronic e.c. HDM exposure aggravated airway inflammation upon lung challenges, reflected by a higher infiltration of inflammatory cells in the broncho-alveolar lavage (BAL) fluid compared to mice that were acutely pre-exposed to HDM via the skin (Figure 1E-F). Chronic e.c. HDM-exposed mice present a mixed neutrophilic/eosinophilic asthma phenotype, whereas the acute e.c. HDM-exposed mice present an eosinophilic asthma phenotype (Figure 1F). Both groups exhibit a Th2 response in the lungs, indicated by the production of Th2-associated cytokines such as IL-5, IL-13 and IL-10 by HDM-restimulated mediastinal LN (MLN) (Figure 1G). Strikingly, also in the lungs, chronic HDM exposure on the skin resulted in a Th17 response, which was absent in mice that were acutely exposed to HDM via the skin (Figure 1G).
PPARγ and GR physically interact and differentially influence gene expression
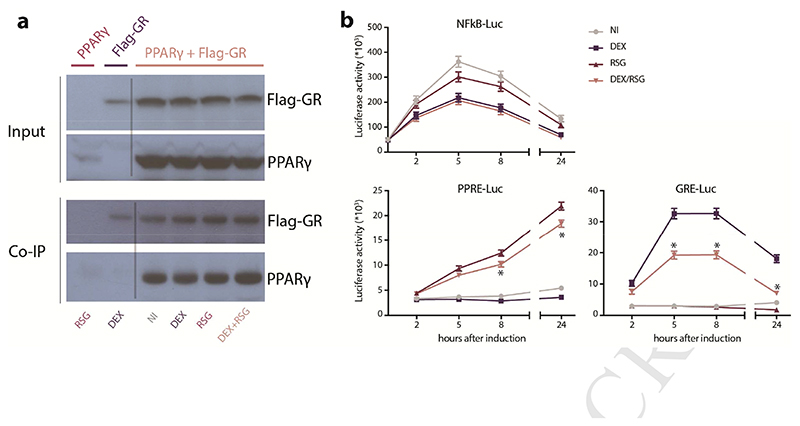
Coimmunoprecipitation analysis of HEK293T cells that were transiently transfected with PPARγ and Flag-tagged GR revealed that these nuclear receptors physically interact (Figure 2A). In line with previous published findings for PPARα (Bougarne et al. 2009), the interaction was ligand independent, yielding similar levels of PPARγ pulled down when cells were stimulated for 1 hour with GR-ligand Dexamethasone (DEX), PPARγ-ligand Rosiglitazone (RSG) or the combination (DEX/RSG) (Figure 2A).
Figure 2. GR and PPARγ physically interact and differentially influence GRE- and PPRE-reporter genes.
A) Input levels and detection of co-immunoprecipitated (Co-IP) PPARγ that was immunoprecipitated with Flag-GR in transfected HEK293T cells, after 1h induction with solvent (not induced, NI), 1μM DEX, 5μM RSG or (DEX/RSG). One out of two independent experiments depicted. B) Luciferase activity upon induction with solvent (NI), 100nM DEX, 1μM RSG or DEX/RSG in stable luciferase-reporter L929sA cells. After 30’, NFκB-Luc expressing L929sA cells were stimulated with 100ng/mL LPS. Data represent means+SE obtained by prediction of Generalized Linear Model, fitted to values from 5 independent experiments, shown on the original scale. Significant differences (*p < 0,05; depicted when combined treatment differs from all other treatments) were calculated by post-hoc t-statistics on log-transformed data.
Although previously described for combined PPARα and GR activation (Bougarne et al. 2009), we found no additive suppressive effect of combined endogenous PPARγ and GR activation on lipopolysaccharide (LPS)-activated NFκB responsive element (NFκBRE)-reporter in stably transfected L929sA cells (Figure 2B). However, the combined treatment with DEX and RSG has a clear antagonizing effect on GRE-driven reporter gene expression and a minor but significant antagonizing effect on PPRE-driven reporter gene expression, both in stably transfected L929sA cells (Figure 2B).
Anti-inflammatory effects of combined PPARγ and GR activation on keratinocytes and BMDCs
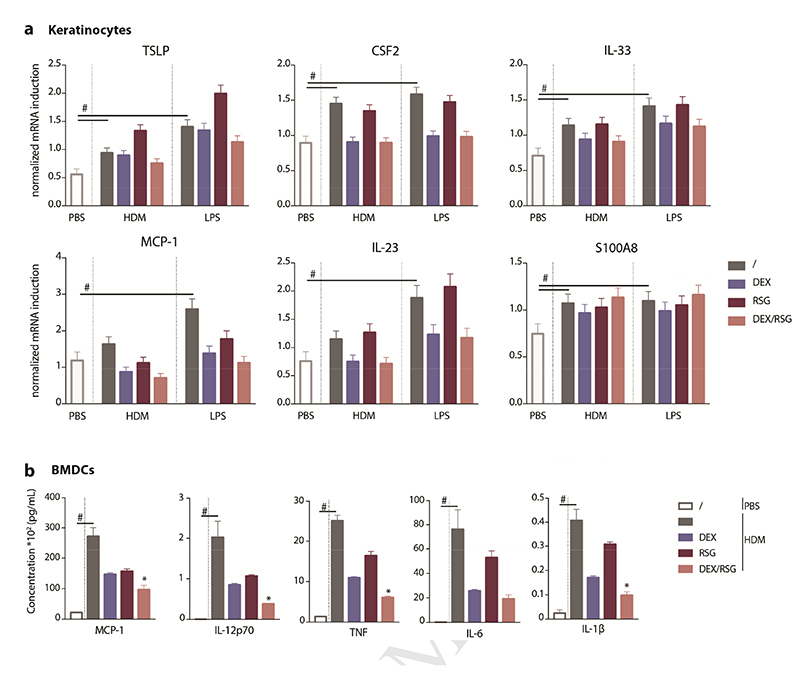
HDM or LPS consistently induced a modest upregulation of innate type 2 cytokines such as TSLP, IL-33 and GM-CSF (encoded by the CSF2 gene) in primary murine keratinocytes (Figure 3A). Similarly, the expression of MCP-1, responsible for attraction of monocytes and monocyte-derived cells (MCs) to the site of inflammation, was increased upon treatment with HDM or LPS as for the expression of IL-23 cytokine and the endogenous TLR4-ligand S100A8, which are known to initiate the Th17 pathway (Yoon et al. 2016). The combination of DEX/RSG had no additive suppressive effect on the expression of these cytokines and mediators (Figure 3A).
Figure 3. Anti-inflammatory capacity of combined PPARγ and GR activation in keratinocytes and BMDCs.
A) Cytokine expression in keratinocytes that were pre-treated for 30’ with solvent (/), 10nM DEX, 5μM RSG or DEX/RSG and subsequently stimulated for 5h with 100μg/mL HDM or 100ng/mL LPS, without replacing the medium. Data represent means+SE obtained by prediction of GLM fitted to values from 4 independent experiments, shown on the original scale. B) Cytokine production by bone marrow derived dendritic cells (BMDCs) that were daily pre-treated for 3 days with solvent (/), 10nM DEX, 5μM RSG or combined DEX/RSG and 1h after the last pre-treatment stimulated for 24h with PBS or 50μg/mL HDM, all without replacing the medium. Data represent means+SEM (n =3-5) and statistical differences (*p<0,05, depicted when combined treatment differs from all other treatments) were calculated by t-tests.
Stimulation of BMDCs with HDM or LPS induced production of MCP-1, IL-12p70, TNF, IL-6 and IL-1β after 24h, although to different extents (Supplementary Figure 2). The combined pre-treatment with DEX/RSG was not able to suppress cytokine production to a higher extent than both compounds alone and this was independent of the type of stimulus (Supplementary Figure 2). However, when BMDC cultures were repeatedly pre-treated from day 6 onwards, the combined activation of PPARγ/GR resulted in an additive anti-inflammatory effect on HDM-induced cytokine production (Figure 3B). In general, treatment with DEX/RSG was unable to suppress cytokine expression in keratinocytes to a higher extent than DEX alone but upon repeated pre-treatments, it was able to provide additive anti-inflammatory effects on HDM-induced cytokine production by BMDCs.
Effects of DEX/RSG on GRE- and PPRE-mediated genes in keratinocytes and BMDCs
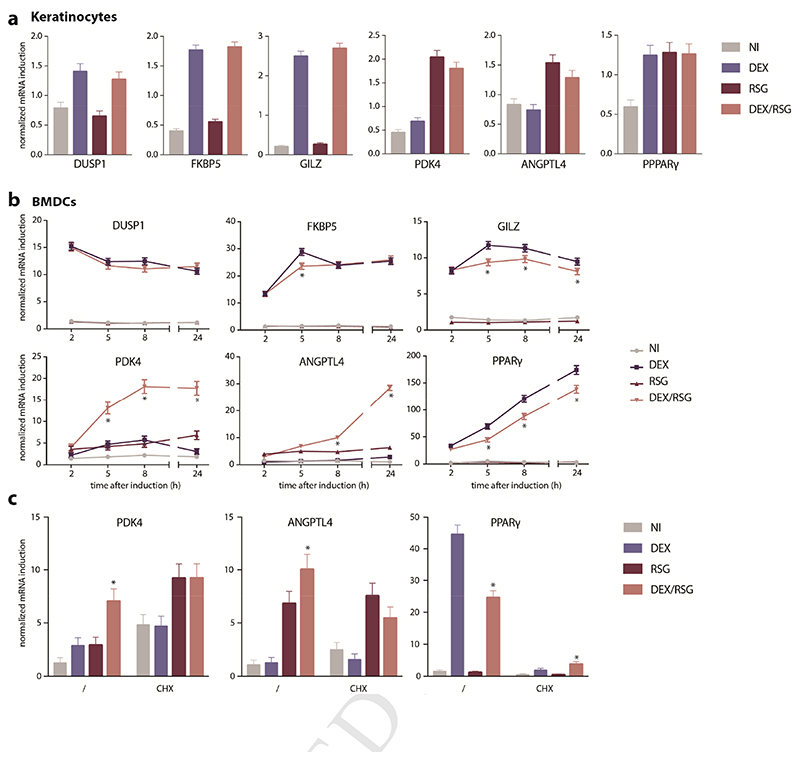
Next, we investigated whether combined activation of PPARγ and GR may have differential effects on GRE- and PPRE-driven genes. In primary keratinocytes that were exposed to compounds and harvested 5 hours later, we did not observe cooperative or additive suppressive effect of the combined treatment with DEX/RSG on any of the GRE- (DUSP1, FKBP5, GILZ, PPARγ) or PPRE-driven (PDK4, ANGPTL4) genes studied (Figure 4A). The effect of DEX/RSG on GRE- and PPRE-driven genes was much more obvious in BMDCs. DEX-activated GRE-driven genes such as GILZ and PPARγ were suppressed when co-treated with RSG from 5 hours upon induction onwards (Figure 4B). There was no clear suppressive effect of additional RSG on DEX-activated GRE-driven gene DUSP1 and FKBP5. Contrary herewith, combined activation of PPARγ/GR resulted in a synergistic effect on PPRE-driven genes such as PDK4 and ANGPTL4 (Figure 4B). There was no significant upregulation of PPARγ protein upon prolonged treatment with DEX or DEX/RSG, indicating that the DEX-mediated induction of PPARγ does not contribute to cooperative effects on PPRE-driven genes (Supplementary Figure 3A-B). Nevertheless, pre-treatment with the mRNA translation blocking agent Cycloheximide (CHX) did abrogate the cooperative effects of DEX/RSG on PPRE-driven mRNA expression of PDK4 and ANGPTL4 (Figure 4C). Of note, CHX treatment elevates base line expression of PDK4 mRNA, most probably because of a lack of mRNA-degrading proteins, since their translation is also blocked by CHX (Figure 4C). The induction of DEX-induced PPARγ expression depended on translation, as shown by the weakened DEX-mediated upregulation of PPARγ in CHX pre-treated BMDCs (Figure 4C). Altogether, these results indicate that combined activation of GR and PPARγ differentially affect GRE- and PPRE-driven genes in BMDCs and that this effect might require de novo synthesis and involvement of other proteins.
Figure 4. Effect of GR/PPARγ on GRE- and PPRE-driven gene expression in keratinocytes versus BMDCs.
A) Gene expression in primary keratinocytes that were either solvent-treated (not induced (NI)) or induced with 100nM DEX, 5μM RSG or DEX/RSG for 5h. B) Kinetics of gene expression in BMDCs that were either NI or induced with 1μpM DEX, 5μM RSG or DEX/RSG. C) Gene expression in BMDCs that were pre-exposed 10μg/mL Cycloheximide (CHX) for 30’ and either NI or induced with 100nM DEX, 5μM RSG or DEX/RSG for 8h. Means+SE were obtained by prediction of GLM, fitted to values from 3-4 biological replicates, shown on the original scale. Statistical differences (*p<0,05, depicted when combined treatment differs from all other conditions) were calculated by post-hoc t-statistics on log-transformed data.
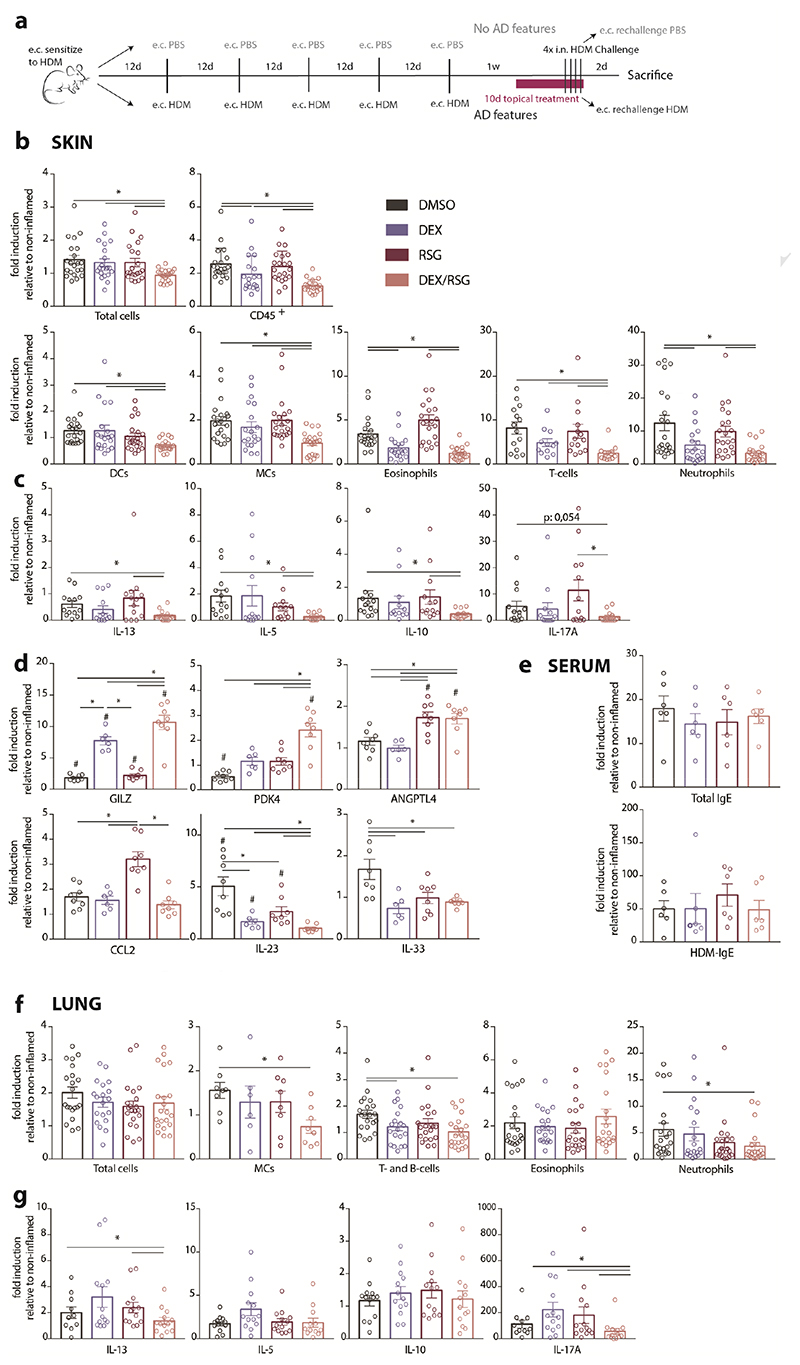
Topically applied DEX/RSG reduces allergic skin inflammation in mice and prevents worsening of subsequent airway inflammation
C57Bl/6J mice were treated according to the protocol depicted in Figure 5A and described in the method section. Regarding cell infiltration, DEX/RSG was able to suppress inflammation to a higher extent than both treatments alone (Figure 5B). Of note, macroscopic examination of ear skin prior to sacrifice did support this data, showing a reduced skin thickening and less scaly skin in the combined treatment group (data not shown). DEX alone had a suppressive effect on the infiltration of neutrophils and eosinophils but only the combined treatment with DEX/RSG was able to significantly suppress infiltration of all measured cell subsets (Figure 5B). Analysis of ex vivo HDM-restimulated SCLN cells from topically treated mice revealed that combined treatment of DEX/RSG significantly suppressed IL-13, IL-5 and IL-10 production. IL-17A cytokine production was not significantly suppressed compared to nontreated group however it was also not increased (Figure 5C). GRE- and GRE/PPRE-target genes (GILZ and PDK4, respectively), but not the PPRE-only target gene ANGPTL4, were differentially expressed in sorted skin cells from the DEX/RSG treated mice (Figure 5D). Interestingly, this topical combined treatment could additively suppress expression of Th17-skewing cytokine IL-23, but not MCs-recruiting chemokine CCL2 and pro-allergic cytokine IL-33 (Figure 5D). However, serum levels of total IgE and HDM-specific IgE were unaltered by topical treatment with compounds (Figure 5E).
Figure 5. Topical applied DEX/RSG suppresses HDM-induced skin inflammation and prevents worsening of subsequent asthma.
C57Bl/6 mice were subjected to the protocol depicted in A) and received daily topical treatment for 10 days with solvent (DMSO), 1,4pg DEX, 100μg RSG or DEX/RSG. B) CD45+ cell infiltrations in ear skin and F) bronchoalveolar lavage (BAL), measured by flow cytometry. C) Cytokine secretion by skin-draining superficial cervical lymph node (SCLN) cells and G) mediastinal LN (MLN) cells, restimulated with HDM for 3 days ex vivo (ELISA). D) mRNA expression of GRE- and PPPRE-target genes and chemokines and cytokines in sorted skin cells (qPCR). E) Serum levels of total and HDM-specific IgE (ELISA). Data represent means+SEM of folds inductions, relative to the mean of the non-inflamed group (1-3 independent experiments, with each 6-8 animals/group). Significant differences between horizontal lines (*p<0,05) were calculated by Mann-Whitney U test.
Regarding inflammation in the lungs, infiltration of total cells and eosinophils in BAL fluid was unaltered by local topical treatment of AD lesions (Figure 5F) and the production of Th2 cytokines IL-5 and IL-10 by HDM-restimulated MLN cells was also not suppressed (Figure 5G). However, compared to the untreated group, infiltration of neutrophils, T- and B-cells as well as IL-13 and IL-17A production was decreased (Figure 5F-G), meaning that the relief of skin inflammation hampered the aggravation of allergic airway inflammation. In conclusion, alleviation of established allergic skin inflammation by DEX/RSG was not sufficient to avoid the progression to eosinophilic airway inflammation but reduced the severity of by counteracting the Th17 pathway.
Discussion
The initiation and perpetuation of the immune reactions to HDM allergens in the lung require a fine-tuned interplay between structural barrier cells and DCs (Hammad et al. 2009; Willart et al. 2012). Also in the skin, keratinocyte-derived factors such as TSLP, IL-25 and IL-33 have been shown to promote epicutaneous sensitization to allergens and overexpression of TSLP in keratinocytes aggravates experimentally induced asthma (Han et al. 2012; Jiang et al. 2012; Leyva-Castillo et al. 2013; Morita et al. 2015; Zhang et al. 2009). These models do not include inflammation in the skin prior to the development of asthma and in the current study, we found that HDM-induced skin inflammation aggravates subsequent development of allergic airway inflammation and influences the immune response. Surprisingly, allergic skin inflammation induced via chronic HDM exposure results in a mixed Th2/Th17 response in the lungs upon HDM airway challenges. This was not the case when mice were acutely sensitized to HDM via the skin, which induces a pure Th2 response, corresponding with our previous findings (Deckers et al. 2017b). Interestingly, epidermal barrier disruption has been shown to induce expression of TSLP in keratinocytes (Oyoshi et al. 2010) and sensitization via a defective skin barrier induces a concomitant Th17 immune response (He et al. 2007; Oyoshi et al. 2009; Yoon et al. 2016). In our model, no severe barrier disruption was induced but the repeated skin occlusion with tape and subsequent scratching behavior might contribute to the HDM-induced Th17 response. Indeed, we previously showed that overnight occlusion with tape induced a mild but significant increase in transepidermal water loss (TEWL), indicative of barrier disruption (Deckers et al. 2017b). However, skin occlusion without repeated HDM exposures (no and acute e.c. HDM exposure groups) did not result in a Th17 response, indicating that repeated subjection to this particular combination is required. Moreover, systemic inflammation markers such as serum levels of IL-6, TNF and IL-1 were not detectable (data not shown), indicating that lung inflammation is probably driven by HDM-primed T-cells that arose in the skin. However, the exact mechanisms and T-cell trafficking should be investigated thoroughly to confirm this.
Children with severe AD are the perfect target for secondary prevention treatment to decrease the severity and the risk of developing other atopic diseases. However, several clinical trials failed to halt the atopic march by treating AD patients with anti-histamine or pimecrolimus (Schneider et al. 2016; Simons 2007). We aimed to test whether the combination of GR and PPARγ agonists could have beneficial effects in the secondary prevention of the atopic march in our newly developed model. An important motivation to use this combination is our previous finding that PPARα and GR physically interact and thereby differentially affect metabolic and anti-inflammatory genes (Bougarne et al. 2009; Ratman et al. 2016). Here, we describe that GR also interacts with the PPARγ family member, which shares homology with PPARα but exerts different tissue distribution and functions. Similarly, we found a clear cooperative or antagonistic effect on PPRE- and GRE-controlled metabolic genes, respectively, upon combined activation of GR and PPARγ but this was only apparent in GM-CSF cultured BMDCs. BMDCs appear a heterogeneous population containing mainly MCs beside a small population of conventional DCs (Helft et al. 2015). Interestingly, we only found a cooperative anti-inflammatory effect of combined treatment with DEX and RSG when we repeatedly treated the BMDCs during the last days of differentiation. Additionally, GRE- and PPRE-driven genes were differentially regulated upon DEX/RSG only in BMDCs and not in keratinocytes. Nevertheless, in skin, the anti-inflammatory GRE-driven gene GILZ is upregulated by a combined treatment of DEX/RSG. Interestingly, expression of the Th17-skewing cytokine IL-23 in the skin, which can be produced both by keratinocytes or DCs/MCs, was additively suppressed by the combined treatment, which could explain the suppression of the type 17 immune response. Since metabolism-controlling genes (PDK4 and ANGPTL4) were also substantially affected, it is tempting to speculate that a combined activation of GR and PPARγ influences the metabolic profile of cells with reported high lipid metabolism (MCs and DCs) and as such influences their responses to immune triggers (Everts and Pearce 2014; Szatmari et al. 2007). Further research is required to untangle via which pathways activated GR/PPARγ mediate their anti-inflammatory effects in DCs. Although from our BMDC experiments, we have an indication that combined GR/PPARγ activation results in suppressive effects on MCs, it is as yet still unknown whether this plays a role in vivo. It was shown before that lung MCs play a role in orchestrating local inflammation during the recall phase of allergic airway inflammation (Plantinga et al. 2013). Due to the lack of tools to specifically target them, the exact function of MCs in allergic skin inflammation has not been described yet. However, we previously showed that these cells are capable of engulfing epicutaneously applied HDM (Deckers et al. 2017b) but they have low migratory capacities (Tamoutounour et al. 2013) indicating they may play a role during local skin inflammation.
Strikingly, although efficiently blocking the severity of subsequent allergic airway inflammation, the DEX/RSG-mediated suppression of inflammation in the skin was not sufficient to completely abrogate the development of Th2-mediated allergic airway inflammation. One possible explanation is that the inflammation in the skin was not completely eradicated when mice were i.n. challenged with HDM. Additionally, unaltered serum levels of HDM-specific IgE suggest that epicutaneous sensitization was not reversible by local treatment with compounds. Even a higher topical dose of DEX (20 μg) was unable to completely abrogate lung inflammation in the atopic march model (data not shown). We used a suboptimal dose of DEX to avoid GC-mediated side effects and to obtain an additive anti-inflammatory effect by addition of PPARγ-agonist. Hence, it would be interesting to search for therapies that effectively halt the atopic march, something that definitely requires more insight in its driving mechanisms.
In conclusion, we developed a murine model that mimics the atopic march in which skin inflammation is associated with a mixed Th2/Th17 phenotype and severe airway inflammation induced by airway challenges to HDM. Combined activation of GR/PPARγ was able to suppress local skin inflammation but this was insufficient to prevent the subsequent development of allergic airway inflammation. However, relief of skin inflammation by GR/PPARγ activation hampered the progression towards neutrophilic/Th17-driven immune responses in the lungs, indicating that this therapy targets specific skin-derived factors involved in the worsening of allergic airway inflammation.
Methods
Animals
C57Bl/6 mice (5 weeks old, females) were obtained from Janvier Labs and were maintained in the animal facility of the Center for Inflammation Research of Ghent under specific pathogen-free conditions. Experiments were approved by the animal ethical committees of Ghent University and Center for Inflammation Research.
Reagents & tools
House dust mite extracts (Dermatophagoides pteronyssinus extracts; Derp1 content: 227,64 μg/vial; Endotoxin content: 1397 EU/vial; Protein content: 5,59 mg/vial, Greer Laboratories) and ultrapure Lipopolysaccharide (LPS) from E.Coli 0111:B4 strain (InvivoGen) were dissolved in phosphate-buffered saline (PBS). Dexamethasone (DEX, Sigma Aldrich) stock solution was made using EtOH for cell culture and DMSO for topical treatment. Rosiglitazone (RSG, Cayman Chemicals) was dissolved in DMSO. Luciferase reagent was prepared as previously described (De Bosscher et al. 1997). Monoclonal antibodies (MoAbs) against GRα (H-300) and PPARγ (H-100) were purchased at Santa Cruz Biotechnology. Reporter gene constructs p(GRE)2-50hu.IL6P-Luc, PDK4-(PPRE)3-Luc or p(IL6-κB)3-50hu.IL6P-Luc reporter gene constructs were generated as previously described (De Bosscher et al. 1997). Flag-GRα was described previously in (Dewint et al. 2008) and PPARγ plasmid was generated in house.
Epicutaneous sensitization and development of AD and allergic airway inflammation
Epicutaneous (e.c.) sensitization was induced in all mice by applying PBS or 30 μg HDM in 20 μL PBS on the dorsal ear skin, which was then covered with a 5mm x 5mm gauze under an acrylic adhesive waterproof tape (Leukoflex). Twelve days later, the ‘no e.c. HDM exposure’ and ‘acute e.c. HDM exposure’ group were e.c. exposed on the same site to PBS under an occlusive tape, whereas the ‘chronic e.c. HDM exposure’ group was e.c. challenged with 30 μg HDM. This cycle was repeated for 5 times with an interval of 12 days in between. One week after the last e.c. exposure, mice were challenged intranasally (i.n.) with 10 μg HDM for 4 days to induce allergic airway inflammation. On the last day of i.n. challenge, mice were rechallenged with PBS or HDM on the ear skin. Two days after the last i.n. challenge and e.c. rechallenge, mice were sacrificed to determine skin and lung inflammation. To test secondary prevention therapy, a daily topical treatment with 1,4 μg DEX, 100 μg RSG or a combination of these (DEX/RSG) was initiated when AD features were established and sustained for 10 days. From the 6th treatment day onwards, mice also received i.n. HDM for 4 days.
Collection of skin, serum, BAL, SCLN and MLN cells
Blood was collected from the iliac vein for serum preparation. Bronchoalveolar lavage (BAL) was obtained by injecting 3 x 1 ml of PBS-EDTA (0,1 mM) in the canulated trachea. Ear skin was collected by cutting at the ear base. SCLN and MLN cells (2*106 cells/ml) were restimulated for 3 days ex vivo with 15 μg/mL HDM extracts in 96 round-bottomed plates. Cytokine production was determined in supernatants by specific ELISA (Ready-SET-Go! Kit, eBioscience).
Skin preparations for flow cytometry
Skin samples were incubated overnight at 4°C with 200 μg/mL Dispase II (from Bacillus polymerase grade 2, Roche) to facilitate manual cutting and isolation of cells. Small skin pieces were further digested with 1,5 mg/mL Collagenase Type 4 (Worthington) and 10 U DNase (Roche) in RPMI medium buffered with Hepes and supplemented with 2% Fetal Calf Serum (FCS). The suspension was every 30’ resuspended and provided with fresh digestion buffer for a total of 90 minutes at 37°C. After digestion, the cell suspension was filtered to remove debris and clots.
HDM-specific ELISA
For the detection of total IgE or HDM-specific IgE in serum, 96-well plates were coated with anti-IgE coating Antibody or 100 μg/mL HDM extracts respectively (overnight at 4°C). After blocking and washing, serum was incubated on the plates for 3h. Biotin-coupled anti-IgE detection antibodies and avidin-HRP reagent were then added for 1h. TMB substrate was added after extensive washing and chemiluminescence was determined by spectrophotometry (absorbance at 450 nm).
Flow Cytometry
To determine the cellular composition of BAL fluids, cells were stained with moAbs directed against MHCII (M5/114.15.2), CD11c (N418), CD19 (1D3) and CD3 (145-2C11), Ly-6C (HK1.4) (eBioscience), Siglec F (E50-2440), Ly-6G (1A8), CD64 (X54-5/7.1), F4/80 (BM8) (BD). For detection and phenotyping of immune cells in cell suspensions of skin, cells were stained with MHCII (M5/114.15.2), CD11c (N418), CD3 (145-2C11), CD19 (1D3) (eBioscience), CD45.2 (104), F4/80 (BM8), CD11b (M1/70), CD64 (X54-5/7.1), Siglec F (E50-2440), Ly-6G (1A8), TCRγδ (GL3) and SAV (BD). Dead cells were always excluded from the analysis by using fixable viability dye eFluor506 (eBioscience). Acquisition of multi-color samples was done on LSR Fortessa flow cytometer and skin cell sorting was performed on Aria III(BD Bioscience). Final analysis and graphical output were performed using FlowJo software (Tree Star Inc.).
Co-immunoprecipitation
HEK293T cells were transiently transfected with Flag-GRα and PPARγ plasmids at a 1:1 ratio, using the CaPO4 procedure. Two days after transfection, cells were treated with compounds for 1 hour and lysed (50 mM Tris-HCl pH7.5, 125 mM NaCl, 1.5 mM MgCl2, 5% Glycerol, 0.2% NP-40, Complete protease inhibitor cocktail (Roche)). Lysates were incubated overnight (at 4°C on a rotor) with 20 μL anti-FLAG M2 affinity gel (Sigma) that was previously blocked during 1 hour using StartingBlock™ (TBS) Blockingbuffer (Thermo Scientific). Next, anti-FLAG beads were washed 3 times in lysisbuffer and then eluted by adding Laemmli buffer and boiling for 5’ at 95°C. Proteins in input samples and Co-IP samples were separated via classical SDS-PAGE and analyzed via a standard Western blotting approach (using MoAbs anti-PPARγ (H-100) and anti-GRα (H-300) Santa Cruz Biotechnology).
Cell culture and treatment with compounds and stimuli
Murine L929sA fibrosarcoma cells and human HEK293T cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), at 37°C and 5% CO2.
To generate BMDCs, bones were isolated from adult C57Bl/6 mice and bone marrow cells were cultured in presence of GM-CSF according to the protocol described in (Lutz et al. 1999). Cells were treated with compounds either on day 8 or from day 6 onwards, for 3 days (as described in Figure Legends) and stimulated with PBS, HDM or LPS on day 8.
To generate primary keratinocytes, back skin was isolated from adult C57Bl/6 mice and epidermal stem cells were cultured under appropriate circumstances to stimulate clonal growth as described earlier (Jensen et al. 2010). Minor changes were made to this protocol; calcium ions were removed from FBS by treatment with a chelating agent and hydrocortisone was removed from the culture medium 5 days before subculturing on collagen I-coated 24-well plates. Two days later, cells were treated with compounds and stimulated as described in Figure Legends.
Reporter assays
Murine L929sA cells were stably transfected by CaPO4 procedure with p(GRE)2-50hu.IL6P-Luc, PDK4-(PPRE)3-Luc or p(IL6-κB)3-50hu.IL6P-Luc reporter gene constructs, generated as described previously (De Bosscher et al. 1997). Luciferase assays were performed as in the protocol from Promega Corp.
RNA isolation and qRT-PCR
RNA was isolated using RNeasy MicroKit (Qiagen) and mRNA was reverse transcribed to cDNA with Primescript RT kit (TaKaRa). cDNA was analyzed by Real-Time PCR with a SYBR Green Master Mix (Roche). Data was analyzed with qBase software (Biogazelle) and normalized to the appropriate reference genes (LDHA/ACTB for keratinocytes, 18S/HPRT for BMDCs and ACTB for sorted skin cells). Primer sequences are available on request.
Statistics
For animal experiments, the difference between 2 groups was calculated using the Mann-Whitney U-test and data from ELISA experiments were analyzed by t-tests (GraphPad Prism). For relative values obtained from qPCR analysis and reporter assays, means and standard errors (SE) were obtained by fitting a log-linear Generalized Linear Model as implemented in Genstat v18 (Poisson distribution, log link). The linear predictor vector for these data can be written as follows: log (μ) = experiment + DEX*RSG. T-statistics were used to assess the significance on the transformed scale. In this case, significant differences were only specified when DEX/RSG treatment was significantly different from all other treatments.
Supplementary Material
Acknowledgements
JD was supported by a personal grant from IWT-Vlaanderen (now called FWO-SB). This work was supported by a grant from FWO-Vlaanderen (G018013N) to KDB and HH.
Footnotes
Conflict Of Interest
The authors state no conflict of interest.
References
- Aberg KM, Radek KA, Choi EH, Kim D-K, Demerjian M, Hupe M, et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J Clin Invest. 2007;117(11):3339–49. doi: 10.1172/JCI31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantz SK, Zhu Z, Zheng T. The Atopic March: Progression from Atopic Dermatitis to Allergic Rhinitis and Asthma. J Clin Cell Immunol. 2014;5(2) doi: 10.4172/2155-9899.1000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behshad R, Cooper KD, Korman NJ. A retrospective case series review of the peroxisome proliferator-activated receptor ligand rosiglitazone in the treatment of atopic dermatitis. Arch Dermatol. 2008;144(1):84–8. doi: 10.1001/archdermatol.2007.22. [DOI] [PubMed] [Google Scholar]
- Bougarne N, Paumelle R, Caron S, Hennuyer N, Mansouri R, Gervois P, et al. PPARalpha blocks glucocorticoid receptor alpha-mediated transactivation but cooperates with the activated glucocorticoid receptor alpha for transrepression on NF-kappaB. Proc Natl Acad Sci USA. 2009;106(18):7397–402. doi: 10.1073/pnas.0806742106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S, Bruscoli S, Mazzon E, Crisafulli C, Donato V, Di Paola R, et al. Peroxisome proliferator-activated receptor-alpha contributes to the anti-inflammatory activity of glucocorticoids. Mol Pharmacol. 2008;73(2):323–37. doi: 10.1124/mol.107.041475. [DOI] [PubMed] [Google Scholar]
- Dahten A, Mergemeier S, Worm M. PPARgamma expression profile and its cytokine driven regulation in atopic dermatitis. Allergy. 2007;62(8):926–33. doi: 10.1111/j.1398-9995.2007.01444.x. [DOI] [PubMed] [Google Scholar]
- De Bosscher K, Schmitz ML, Vanden Berghe W, Plaisance S, Fiers W, Haegeman G. Glucocorticoid-mediated repression of nuclear factor-kappaB-dependent transcription involves direct interference with transactivation. Proc Natl Acad Sci USA. 1997;94(25):13504–9. doi: 10.1073/pnas.94.25.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckers J, De Bosscher K, Lambrecht BN, Hammad H. Interplay between barrier epithelial cells and dendritic cells in allergic sensitization through the lung and the skin. Immunol Rev. 2017a;278(1):131–44. doi: 10.1111/imr.12542. [DOI] [PubMed] [Google Scholar]
- Deckers J, Sichien D, Plantinga M, Van Moorleghem J, Vanheerswynghels M, Hoste E, et al. Journal of Allergy and Clinical Immunology. Elsevier Inc; 2017b. Epicutaneous sensitization to house dust mite allergen requires IRF4-dependent dermal dendritic cells; pp. 1–41. [DOI] [PubMed] [Google Scholar]
- Demerjian M, Choi EH, Man M-Q, Chang S, Elias PM, Feingold KR. Activators of PPARs and LXR decrease the adverse effects of exogenous glucocorticoids on the epidermis. Exp Dermatol. 2009;18(7):643–9. doi: 10.1111/j.1600-0625.2009.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewint P, Gossye V, De Bosscher K, Vanden Berghe W, Van Beneden K, Deforce D, et al. A plant-derived ligand favoring monomeric glucocorticoid receptor conformation with impaired transactivation potential attenuates collagen-induced arthritis. J Immunol. 2008;180(4):2608–15. doi: 10.4049/jimmunol.180.4.2608. [DOI] [PubMed] [Google Scholar]
- Dharmage SC, Lowe AJ, Matheson MC, Burgess JA, Allen KJ, Abramson MJ. Atopic dermatitis and the atopic march revisited. Allergy. 2014;69(1):17–27. doi: 10.1111/all.12268. [DOI] [PubMed] [Google Scholar]
- Everts B, Pearce EJ. Metabolic control of dendritic cell activation and function: recent advances and clinical implications. Front Immunol. 2014;5:203. doi: 10.3389/fimmu.2014.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15(4):410–6. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H, Xu W, Headley MB, Jessup HK, Lee KS, Omori M, et al. Thymic stromal lymphopoietin (TSLP)-mediateddermal inflammation aggravates experimentalasthma. 3. Vol. 5. Nature Publishing Group; 2012. pp. 342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano Y, Elias PM, Crumrine D, Feingold KR, Katagiri K, Fujiwara S. Journal of Investigative Dermatology. 9. Vol. 131. Nature Publishing Group; 2011. Efficacy of Combined PeroxisomeProliferator-Activated Receptor; pp. 1845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He R, Oyoshi MK, Jin H, Geha RS. Epicutaneous antigen exposure induces a Th17 response that drives airway inflammation after inhalation challenge. Proc Natl Acad Sci USA. 2007;104(40):15817–22. doi: 10.1073/pnas.0706942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helft J, Böttcher J, Chakravarty P, Zelenay S, Huotari J, Schraml BU, et al. Immunity. 6. Vol. 42. Elsevier Inc; 2015. GM-CSF Mouse Bone Marrow Cultures Comprise a Heterogeneous Population of CD11c; pp. 1197–211. [DOI] [PubMed] [Google Scholar]
- Jensen KB, Driskell RR, Watt FM. Assaying proliferation and differentiation capacity of stem cells using disaggregated adult mouse epidermis. Nat Protoc. 2010;5(5):898–911. doi: 10.1038/nprot.2010.39. [DOI] [PubMed] [Google Scholar]
- Jiang H, Hener P, Li J, Li M. Skin thymic stromal lymphopoietin promotes airway sensitization to inhalant house dust mites leading to allergic asthma in mice. Allergy. 2012;67(8):1078–82. doi: 10.1111/j.1398-9995.2012.02857.x. [DOI] [PubMed] [Google Scholar]
- Jung K, Tanaka A, Fujita H, Matsuda A, Oida K, Karasawa K, et al. Peroxisome proliferator-activated receptor γ-mediated suppression of dendritic cell function prevents the onset of atopic dermatitis in NC/Tnd mice. J Allergy Clin Immunol. 2011;127(2):420–429.:e1-6. doi: 10.1016/j.jaci.2010.10.043. [DOI] [PubMed] [Google Scholar]
- Kao JS, Fluhr JW, Man M-Q, Fowler AJ, Hachem J-P, Crumrine D, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120(3):456–64. doi: 10.1046/j.1523-1747.2003.12053.x. [DOI] [PubMed] [Google Scholar]
- Leyva-Castillo JM, Hener P, Jiang H, Li M. TSLP produced by keratinocytes promotes allergen sensitization through skin and thereby triggers atopic march in mice. J Invest Dermatol. 2013;133(1):154–63. doi: 10.1038/jid.2012.239. [DOI] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch N, Ogilvie AL, Rössner S, Koch F, Romani N, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223(1):77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- Morita H, Arae K, Unno H, Toyama S, Motomura K, Matsuda A, et al. IL-25 and IL-33 Contribute to Development of Eosinophilic Airway Inflammation in Epicutaneously Antigen-Sensitized Mice. PLoS ONE. 2015;10(7):e0134226. doi: 10.1371/journal.pone.0134226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyoshi MK, Larson RP, Ziegler SF, Geha RS. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol. 2010;126(5):976–84.:984.e1-5. doi: 10.1016/j.jaci.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyoshi MK, Murphy GF, Geha RS. Filaggrin-deficient mice exhibit TH17-dominated skin inflammation and permissiveness to epicutaneous sensitization with protein antigen. J Allergy Clin Immunol. 2009;124(3):485–93.:493.e1. doi: 10.1016/j.jaci.2009.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38(2):322–35. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Ratman D, Mylka V, Bougarne N, Pawlak M, Caron S, Hennuyer N, et al. Chromatin recruitment of activated AMPK drives fasting response genes co-controlled by GR and PPARa. Nucleic Acids Res. 2016;44(22):10539–53. doi: 10.1093/nar/gkw742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacology and Therapeutics. 2002;96(1):23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- Schneider L, Hanifin J, Boguniewicz M, Eichenfield LF, Spergel JM, Dakovic R, et al. Study of the Atopic March: Development of Atopic Comorbidities. Pediatr Dermatol. 2016;33(4):388–98. doi: 10.1111/pde.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg JI, Nelson DB, Yosipovitch G. Addressing treatment challenges in atopic dermatitis with novel topical therapies. J Dermatolog Treat. 2016;27(6):568–76. doi: 10.1080/09546634.2016.1174765. [DOI] [PubMed] [Google Scholar]
- Simons FER. Safety of levocetirizine treatment in young atopic children: An 18-month study. Pediatr Allergy Immunol. 2007;18(6):535–42. doi: 10.1111/j.1399-3038.2007.00558.x. [DOI] [PubMed] [Google Scholar]
- Spergel JM. Epidemiology of atopic dermatitis and atopic march in children. Immunol Allergy Clin North Am. 2010;30(3):269–80. doi: 10.1016/j.iac.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Szatmari I, Töröcsik D, Agostini M, Nagy T, Gurnell M, Barta E, et al. PPARgamma regulates the function of human dendritic cells primarily by altering lipid metabolism. Blood. 2007;110(9):3271–80. doi: 10.1182/blood-2007-06-096222. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Wada K, Katayama K, Kamisaki Y, Maeyama K, Kadowaki T, et al. Activation of peroxisome proliferator-activated receptor gamma suppresses mast cell maturation involved in allergic diseases. Allergy. 2008;63(9):1136–47. doi: 10.1111/j.1398-9995.2008.01677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamoutounour S, Guilliams M, Montanana Sanchis F, Liu H, Terhorst D, Malosse C, et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity. 2013;39(5):925–38. doi: 10.1016/j.immuni.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Thomsen SF. Epidemiology and natural history of atopic diseases. Eur Clin Respir J. 2015;2 doi: 10.3402/ecrj.v2.24642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willart MAM, Deswarte K, Pouliot P, Braun H, Beyaert R, Lambrecht BN, et al. Interleukin-1α controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J Exp Med. 2012;209(8):1505–17. doi: 10.1084/jem.20112691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Kakuta H, Sugimoto Y. International Immunopharmacology. 1. Vol. 22. Elsevier B.V; 2014. Involvement of glucocorticoid receptor activation on anti-inflammatory effect induced by peroxisome proliferator-activated receptor gamma agonist; pp. 204–8. [DOI] [PubMed] [Google Scholar]
- Yoon J, Leyva-Castillo JM, Wang G, Galand C, Oyoshi MK, Kumar L, et al. IL-23 induced in keratinocytes by endogenous TLR4 ligands polarizes dendritic cells to drive IL-22 responses to skin immunization. J Exp Med. 2016;213(10):2147–66. doi: 10.1084/jem.20150376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Hener P, Frossard N, Kato S, Metzger D, Li M, et al. Thymic stromal lymphopoietin overproduced by keratinocytes in mouse skin aggravates experimental asthma. Proc Natl Acad Sci USA. 2009;106(5):1536–41. doi: 10.1073/pnas.0812668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.