Abstract
Objectives
The aim of this study was to compare fully quantitative cardiovascular magnetic resonance (CMR) and positron emission tomography (PET) myocardial perfusion and myocardial perfusion reserve (MPR) measurements in patients with coronary artery disease (CAD).
Background
Absolute quantification of myocardial perfusion and MPR with PET have proven diagnostic and prognostic roles in patients with CAD. Quantitative CMR perfusion imaging has been established more recently and has been validated against PET in normal hearts. However, there are no studies comparing fully quantitative CMR against PET perfusion imaging in patients with CAD.
Methods
Forty-one patients with known or suspected CAD prospectively underwent quantitative 13N-ammonia PET and CMR perfusion imaging before coronary angiography.
Results
The CMR-derived MPR (MPRCMR) correlated well with PET-derived measurements (MPRPET) (r = 0.75, p < 0.0001). MPRCMR and MPRPET for the 2 lowest scoring segments in each coronary territory also correlated strongly (r = 0.79, p < 0.0001). Absolute CMR perfusion values correlated significantly, but weakly, with PET values both at rest (r = 0.32; p = 0.002) and during stress (r = 0.37; p < 0.0001). Area under the receiveroperating characteristic curve for MPRPET to detect significant CAD was 0.83 (95% confidence interval: 0.73 to 0.94) and for MPRCMR was 0.83 (95% confidence interval: 0.74 to 0.92). An MPRPET ≤1.44 predicted significant CAD with 82% sensitivity and 87% specificity, and MPRCMR ≤1.45 predicted significant CAD with 82% sensitivity and 81% specificity.
Conclusions
There is good correlation between MPRCMR and MPRPET. For the detection of significant CAD, MPRPET and MPRCMR seem comparable and very accurate. However, absolute perfusion values from PET and CMR are only weakly correlated; therefore, although quantitative CMR is clinically useful, further refinements are still required. (J Am Coll Cardiol 2012;60:1546-55) © 2012 by the American College of Cardiology Foundation
Keywords: coronary disease, ischemia, magnetic resonance imaging, perfusion, positron emission tomography
Noninvasive assessment of myocardial perfusion is clinically important, particularly for the detection and management of patients with coronary artery disease (CAD). Currently, clinical decision making is usually based on visual estimates of relative perfusion. However, this approach relies on the presence of a normally perfused region of myocardium. Quantification of perfusion addresses this limitation, and positron emission tomography (PET) studies have confirmed that it provides incremental value compared with nonquantitative methods (1).
Positron emission tomography can accurately quantify perfusion and myocardial perfusion reserve (MPR) and is currently the noninvasive reference standard. Fully quantitative methods are accurate for the detection of CAD (1) and can result in improved diagnostic accuracy compared with relative perfusion analysis (2). Recent studies have also demonstrated that an abnormal quantitative MPR is an independent predictor of an adverse prognosis (3,4). Furthermore, quantitative data provide unique information about the coronary microcirculation that is not available from nonquantitative methods (5).
Cardiac magnetic resonance (CMR) has, relatively recently, established itself as an accurate and valuable tool for perfusion imaging (6). Currently, visual assessment is standard; however, absolute quantification of perfusion has also been demonstrated to be feasible and accurate (7–10). Quantitative CMR methods have been validated against microspheres in animals (7), coronary sinus flow in patients (9), and PET in healthy volunteers (11–13). However, to date, there is a lack of evidence comparing quantitative CMR with PET in patients with CAD. Patient studies are clinically more relevant, given the inherent differences between patients and healthy volunteers. Therefore, the primary aim of this study was to compare quantitative assessment of myocardial perfusion and MPR with CMR and PET in a cohort of patients with known or suspected CAD.
Methods
Patient population and study design
Patients with a history of stable angina and known or suspected CAD underwent CMR and PET perfusion imaging before planned x-ray coronary angiography. Exclusion criteria: acute coronary syndrome <6 weeks, previous coronary artery bypass graft, previous ST-segment elevation infarction, estimated glomerular filtration rate <30 ml/min, or contraindication to magnetic resonance imaging or adenosine. The local ethics committee approved the study, and the U.K. Administration of Radioactive Substances Advisory Committee licensed radiation exposure. All patients gave written informed consent.
Data acquisition
Patients were instructed to abstain from caffeine and smoking for 24 h before imaging.
CMR imaging
Data were acquired with a 1.5-T scanner (Achieva, Philips, Best, the Netherlands) and a 32-channel coil. Examinations included high-resolution perfusion and functional and scar imaging. Perfusion imaging consisted of 3 short-axis slices acquired every heartbeat covering 16 of the standard myocardial segments (14) (apex excluded). Stress imaging preceded rest by 14 ± 2 min (range 10 to 19 min). Imaging parameters: k-t balanced turbo gradient echo sequence, shortest echo time (range 1.35 to 1.54 ms), shortest repetition time (range 2.64 to 3.12 ms), 50° flip angle; 90° prepulse, 100-ms prepulse delay, and typical acquired resolution 1.7 × 1.9 × 10 mm. For stress imaging, 140 μg/kg/min of adenosine was administered intravenously for 4 min. Imaging commenced 3 min into the infusion and continued for 1 min. A dual bolus (equal volumes of 0.01 mmol/kg followed by 0.1 mmol/kg after a 20–s pause) of contrast agent (gadobutrol/Gadovist, Berlin-Wedding, Schering, Germany) was injected at 4ml/s by a power injector for perfusion imaging (15).
PET imaging
This was performed with a GE Discovery VCT PET-CT scanner (GE Healthcare, Waukesha, Wisconsin) after administration of 13N ammonia, with 47 transaxial slices reconstructed over an axial field of view of 15 cm. Acquired resolution was 4.8 × 4.8 ×4.9 mm. Computed tomography (CT) scout data determined patient position, and a low-dose CT was used for attenuation correction. Acquisition consisted of dynamic scans from 0 to 6 min (12 × 10 s, 6 × 20 s, and 2 × 60 s) and then a single static scan frame for 20 min. For the rest study, a total of 550 MBq of 13N-ammonia was injected intravenously. Stress imaging was performed approximately 50 min later. Adenosine (140 μg/kg/min) was administered intravenously for 6 min, with a second equivalent dose of 13N-ammonia administered 2 min into the infusion. Patients remained on the scanner table throughout the entire study; however, attenuation correction was repeated for the stress study.
X-ray coronary angiography
This was performed according to the standard Judkin’s technique. Multiple projections of the coronary arteries were acquired, including at least 2 orthogonal views to assess stenosis severity.
Visual analysis
Studies were analyzed by 2 independent experts blinded to all other data. The PET scans were classified as positive for CAD in the presence of a stress-induced perfusion defect involving ≥2 myocardial segments and CMR scans in the presence of a stress-induced perfusion defect, which was transmural or involved ≥2 myocardial segments. In the case of disagreement between the observers, the images were reviewed together, and a consensus was reached.
Quantitative analysis
Quantitative analysis was performed by 1 of the experts. Perfusion was quantified in 16 standard American Heart Association segments. Segments were defined separately for the PET and CMR images with the right ventricular insertion points for reference. Segment 17 was excluded. Myocardial border detection was automated and manually corrected where required. Segments unsuitable for analysis due to artifact or poor image quality were excluded. The CMR segments demonstrating late gadolinium enhancement and PET data from patients with >10-mm movement between the CT and PET were considered unreliable and also excluded. Coronary territories were excluded if all relevant segments were unsuitable for analysis.
PET
Original dynamic raw PET scans were used to calculate arterial input function and mean segmental perfusion calculated over the linear portion of the curve from 70 to 210 s, with Quick Cardiac (Hermes Medical Solutions, Stockholm, Sweden) in conjunction with software developed at our institution based on the Patlak method as previously described and validated (16).
CMR
Mean segmental perfusion values were obtained with dedicated ViewForum software (Philips) and the previously validated Fermi deconvolution method (7).
PET AND CMR
Each segment was assigned to the appropriate perfusion territory (14), with segment 15 assigned to the dominant coronary artery (defined by the observer analyzing the angiogram). Myocardial perfusion reserve was defined as stress perfusion divided by rest perfusion and was calculated for each segment and territory. For CAD detection, mean MPR of the 2 lowest scoring segments for each perfusion territory (MPR2) was used for further analysis as described previously (17).
X-RAY ANGIOGRAPHY
All coronary arteries >2 mm in diameter were assessed by an independent cardiologist blinded to all other data. Stenoses <30% and >95% on visual analysis were judged to be nonflow-limiting or flowlimiting, respectively, without further assessment. Quantitative coronary angiography (QCA) was performed for all arteries with a visual stenosis severity of 30% to 95% with a dedicated software program (Medcon UK, Edgware, Middlesex, United Kingdom). Mean diameter stenosis from 2 orthogonal views was recorded, and ≥70% was regarded as significant. Coronary territories subtended by a coronary artery with ≥70% stenosis were classified as stenotic territories, and all others were classified as remote.
Statistical analysis
The IBM SPSS Statistics (version 19.0, SPSS, Chicago, Illinois) and Medcalc software (Medcalc, Mariakerke, Belgium) were used. Data are presented as mean ± SD, except where stated. Shapiro-Wilk analysis defined when nonparametric tests were required. Agreement and correlations between PET and CMR were determined with Bland-Altman plots and Spearman’s test of correlation with a 2-tailed test of significance, respectively. Analyses were performed on a per-observation basis. Because 3 perfusion territories were analyzed/patient, analyses were repeated within each territory to ensure that any strong correlations did not simply reflect high within-subject correlations. Intraobserver and interobserver reproducibility was determined by a coefficient of variation (CV): SD of the differences divided by the mean. Paired and independent t tests were used for comparison of paired and unpaired mean data, respectively. Receiver-operating characteristic (ROC) analysis determined the accuracy of visual analysis, MPR2, and stress perfusion values from PET and CMR for predicting a corresponding coronary artery stenosis of ≥70% on QCA. Optimal cutoffs were determined by the maximum Youden Index. Logistic regression models were used to calculate the area under the receiver-operating characteristic curve (AUC) when information from both CMR and PET were combined, and this was compared with the ROC curves when CMR or PET was used alone. Sensitivity and specificity were compared with McNemar’s test. Significance was determined at <0.05.
Results
Study population
Forty-one patients were recruited. Table 1 shows baseline characteristics. The study protocol was completed in 38 patients (exclusions: 1 atrioventricular block, 1 withdrawal of consent, 1 claustrophobia), and further analysis relates to these patients. Hemodynamic status during imaging is shown in Table 2. Resting heart rate was significantly higher during CMR studies (66 vs. 63 beats/min, respectively). There were no significant differences between rate pressure product, systolic blood pressure, or stress heart rate during CMR and PET studies.
Table 1. Baseline Characteristics of Study Participants.
| Age (yrs) | 63 ± 9 |
| Male | 32 (78%) |
| BMI (kg/m2) | 29 ± 5 |
| LVEF (%) | 63 ± 12 |
| Angina (CCS) | |
| Class 1 | 11 (27%) |
| Class 2 | 22 (54%) |
| Class 3 | 8 (20%) |
| Diabetes | 13 (32%) |
| Hypertension | 30 (73%) |
| Smoking | |
| Current | 5 (12%) |
| Previous | 21 (51%) |
| Family history CAD | 19 (46%) |
| Cerebrovascular disease | 4 (10%) |
| Previous PCI | 13 (32%) |
| Previous myocardial infarct | 5 (12%) |
| Medications | |
| Aspirin | 33 (80%) |
| Clopidogrel | 18 (44%) |
| Statin | 35 (85%) |
| ACE inhibitor | 22 (54%) |
| Angiotensin receptor blocker | 6 (15%) |
| Beta-blocker | 21 (51%) |
| Calcium channel blocker | 13 (32%) |
| Nitrate | 7 (17%) |
| Coronary disease ≥70% | |
| Left anterior descending | 7 (17%) |
| Circumflex | 12 (29%) |
| Right coronary artery | 13 (32%) |
Values are mean ± SD or n (%). Percentages have been rounded to nearest 1% and therefore might not total 100%. ACE = angiotensin-converting enzyme; BMI = body mass index; CAD = coronary artery disease; CCS = Canadian Cardiovascular Society; LVEF = left ventricular ejection fraction; PCI = percutaneous coronary intervention.
Table 2. Summary of Hemodynamic Data for All Participants During Imaging Studies.
| Rest | Stress | |||||
|---|---|---|---|---|---|---|
| PET | CMR | p Value | PET | CMR | p Value | |
| HR | 63 ± 10 | 66 ± 11 | 0.002 | 84 ± 15 | 86 ± 15 | 0.14 |
| SBP | 138 ± 18 | 137 ± 17 | 0.75 | 128 ± 21 | 132 ± 19 | 0.164 |
| RPP | 8,734 ± 336 | 9,122 ± 329 | 0.12 | 10,781 ± 479 | 11,344 ± 445 | 0.08 |
Values are mean ± SD.
CMR = cardiovascular magnetic resonance; HR = heart rate; PET = positron emission tomography; RPP = rate pressure product; SBP = systolic blood pressure.
The interval between PET and CMR scans was 3 ± 6 days; 26 (63%) patients underwent both on the same day. The coronary angiogram was 17 ± 19 days and 17 ± 21 days after the CMR and PET, respectively. Twenty-five patients (61%) had ≥1 stenosis of ≥70% diameter. Nineteen had single-vessel disease, 5 had 2-vessel disease, and 1 had 3-vessel disease. Left ventricular ejection fraction (CMR) was 63 ± 12%.
Myocardial perfusion
All perfusion studies were suitable for visual analysis. Quantitative data were available for 89%, 84%, and 73% of territories with CMR, PET, and both modalities, respectively. Results are summarized in Table 3. In patients with CAD stress perfusion, MPR and MPR2 were significantly lower in stenotic territories compared with remote territories with both PET and CMR. Conversely, rest perfusion was not significantly different.
Table 3. Quantitative Perfusion Values in Patients With and Without Significant CAD.
| CAD | No CAD | |||||
|---|---|---|---|---|---|---|
| Stenotic Territory | Remote Territory | p Value* | All Territories | p Value† | ||
| Stress perfusion (ml/min/g) | ||||||
| PET | 1.24 ± 0.49 | 1.56 ± 0.66 | <0.0001 | 1.72 ± 0.66 | 0.49 | |
| CMR | 1.54 ± 0.34 | 1.94 ± 0.59 | 0.001 | 2.03 ± 0.63 | 0.68 | |
| Rest perfusion (ml/min/g) | ||||||
| PET | 0.77 ± 0.24 | 0.81 ± 0.25 | 0.08 | 0.85 ± 0.26 | 0.71 | |
| CMR | 1.03 ± 0.30 | 1.06 ± 0.33 | 0.28 | 0.98 ± 0.29 | 0.47 | |
| MPR | ||||||
| PET | 1.57 ± 0.31 | 1.87 ± 0.36 | <0.0001 | 2.06 ± 0.44 | 0.20 | |
| CMR | 1.55 ± 0.36 | 1.90 ± 0.48 | 0.001 | 2.20 ± 0.56 | 0.13 | |
| Mean MPR of 2 lowest segments | ||||||
| PET | 1.36 ± 0.32 | 1.74 ± 0.32 | <0.0001 | 1.92 ± 0.39 | 0.14 | |
| CMR | 1.31 ± 0.30 | 1.70 ± 0.42 | <0.0001 | 1.93 ± 0.53 | 0.16 | |
Values are mean ± SD. A stenotic territory is subtended by a coronary artery with ≥70% diameter stenosis, and a remote territory is subtended by coronary arteries with < 70% stenosis.
Significance of the difference between stenotic and remote territory values.
Significance of the difference between the values in patients without coronary artery disease (CAD) and remote territories in patients with CAD.
CMR = cardiovascular magnetic resonance; MPR = myocardial perfusion reserve; PET = positron emission tomography.
The MPR2PET was 1.36 ± 0.32 and 1.74 ± 0.32 in stenotic and remote territories, respectively (p < 0.0001). The MPR2CMR values were 1.31 ± 0.30 and 1.70 ± 0.42 (p < 0.0001). In patients without significant CAD, MPR2PET was 1.92 ± 0.39, and MPR2CMR was 1.93 ± 0.53 (p = 0.14 and p = 0.16, respectively, compared with remote territories in patients with CAD).
Agreement between CMR and PET
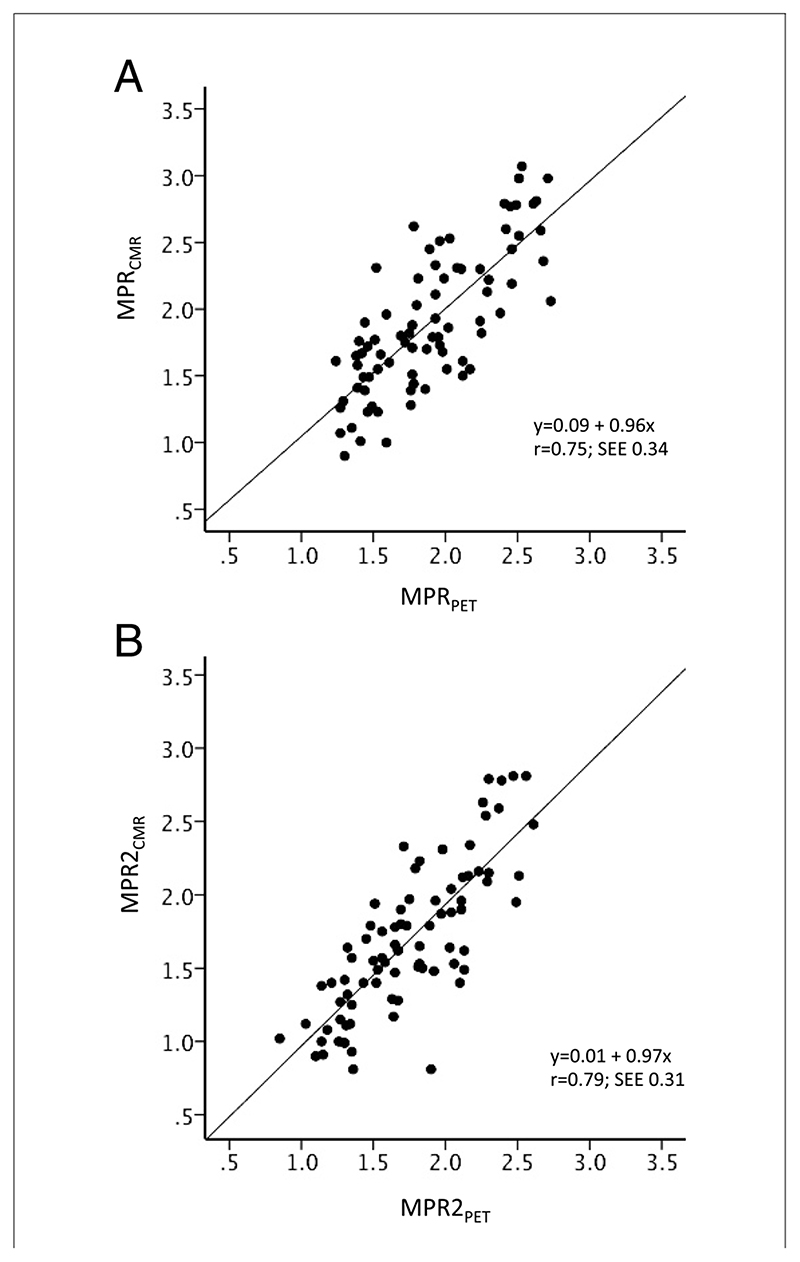
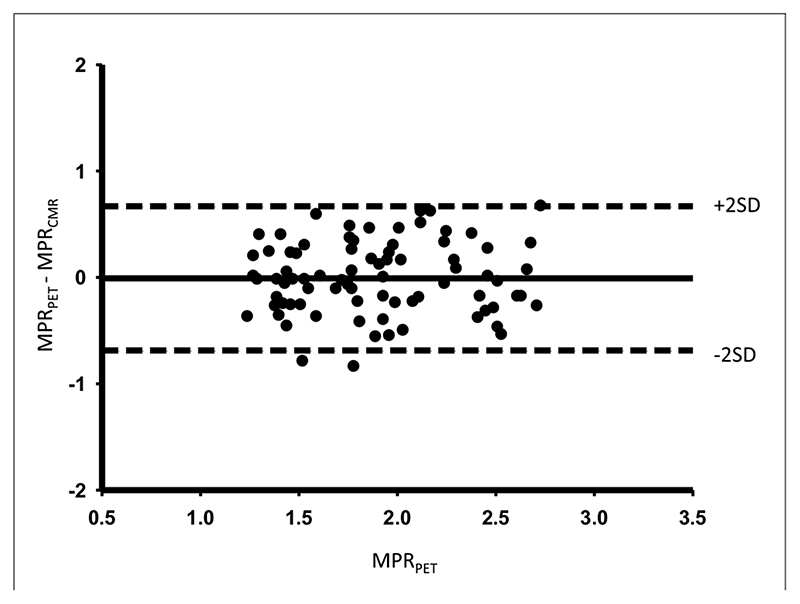
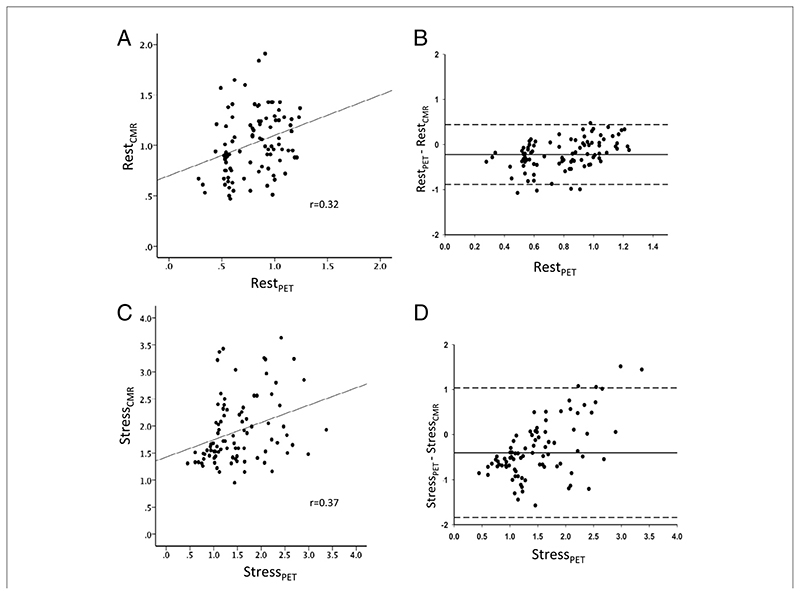
There was good correlation between MPRCMR and MPRPET (r = 0.75; p < 0.0001) (Fig. 1A) and between MPR2CMR and MPR2PET (r = 0.79; p < 0.0001) (Fig. 1B). A Bland-Altman plot demonstrates good agreement between MPRCMR and MPRPET (Fig. 2). Absolute CMR perfusion values correlated significantly, but weakly, with PET values both at rest (r = 0.32; p = 0.002) and during stress (r = 0.37; p < 0.0001) (Fig. 3). Results were similar when analyses were repeated within each territory. The MPRCMR correlated well with MPRPET in the left anterior descending coronary artery (r = 0.79; p < 0.0001), circumflex (r = 0.64; p < 0.0001), and right coronary artery territories (r = 0.77; p < 0.0001). Bland-Altman limits of agreement for MPRCMR and MPRPET also remained similar in the left anterior descending coronary artery (—0.71 to 0.58), circumflex (—0.7 to 0.8), and right coronary artery territories (—0.62 to 0.48).
Figure 1. Scatter Plots Comparing CMR- and PET-Derived MPR.
Scatter plots with fit lines comparing myocardial perfusion reserve (MPR) values from cardiac magnetic resonance (MPRCMR) and positron emission tomography (MPRPET) for the entire myocardial territory (A) and the mean of the lowest 2 segments in each territory (MPR2) (B).
Figure 2. Agreement Between CMR and PET MPR.
Bland-Altman plot showing the agreement between CMR- and PET-derived absolute MPR measurements. Abbreviations as in Figure 1.
Figure 3. I Correlation and Agreement Between CMR- and PET-Derived Absolute Perfusion Values.
Scatter plots illustrating the correlation between absolute measures of myocardial perfusion at rest (A) and during peak stress (C), along with the corresponding Bland-Altman plots (B and D, respectively) with limits of agreement lines (2 SDs). CMR = cardiac magnetic resonance; PET = positron emission tomography.
Reproducibility
Intraobserver CV for PET stress perfusion, rest perfusion, and MPR were 9%, 12%, and 15%, respectively. Corresponding CV for inter-observer reproducibility were 17%, 13%, and 18%, respectively. The CMR intraobserver CV were 9%, 14%, and 20%, respectively, and interobserver CV were 16%, 18%, and 22%, respectively.
Diagnosis of CAD. VISUAL ANALYSIS
Sensitivity and specificity against QCA were: PET 92% (95% confidence interval [CI]: 72% to 99%) and 69% (95% CI: 41% to 88%); CMR 86% (95% CI: 64% to 96%) and 76% (95% CI: 50% to 92%). The CMR and PET sensitivity and specificity were not significantly different (p = 0.65 and 0.71, respectively). The AUC were: PET 0.81 (95% CI: 0.66 to 0.96); CMR 0.81 (95% CI: 0.65 to 0.96). If only those patients with quantitative data suitable for analysis were considered, sensitivity and specificity changed to 94% (95% CI: 71% to 99%) and 67% (95% CI: 39% to 87%), and 85% (95% CI: 61% to 96%) and 79% (95% CI: 49% to 94%) for PET and CMR, respectively.
Quantitative Analysis
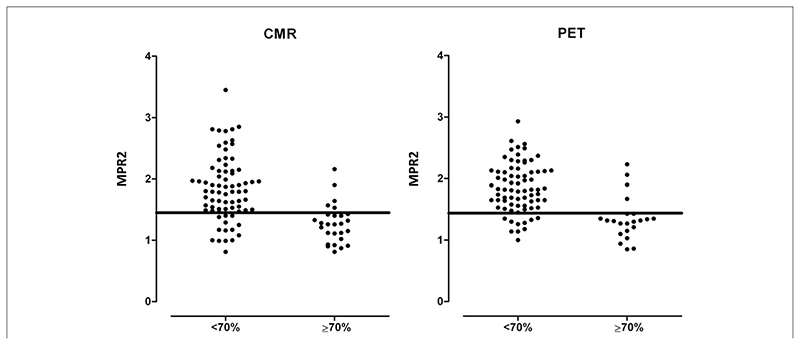
The AUC for MPR2PET to detect significant CAD was 0.83 (95% CI: 0.73 to 0.94) and for MPR2CMR was 0.83 (95% CI: 0.74 to 0.92) (p = 0.96). Sensitivity and specificity against QCA were: MPR2PET ≤1.44: 82% and 87%; and MPRCMR ≤1.45: 82% and 81% (Fig. 4). The AUC was not significantly different from AUC for visual analysis (p = 0.73 and p = 0.79 for PET and CMR, respectively). An example case is shown in Figure 5.
Figure 4. Diagnosis of Significant CAD on Angiography With Quantitative CMR- and PET-Derived MPR.
Mean myocardial perfusion reserve (MPR) of the lowest 2 segments (MPR2) in remote (<70%) and stenotic (≥70%) territories. The best cutoff values for the detection of coronary artery disease (CAD) (MPRCMR 1.45 and MPRPET 1.44) are shown. CMR = cardiac magnetic resonance; PET = positron emission tomography.
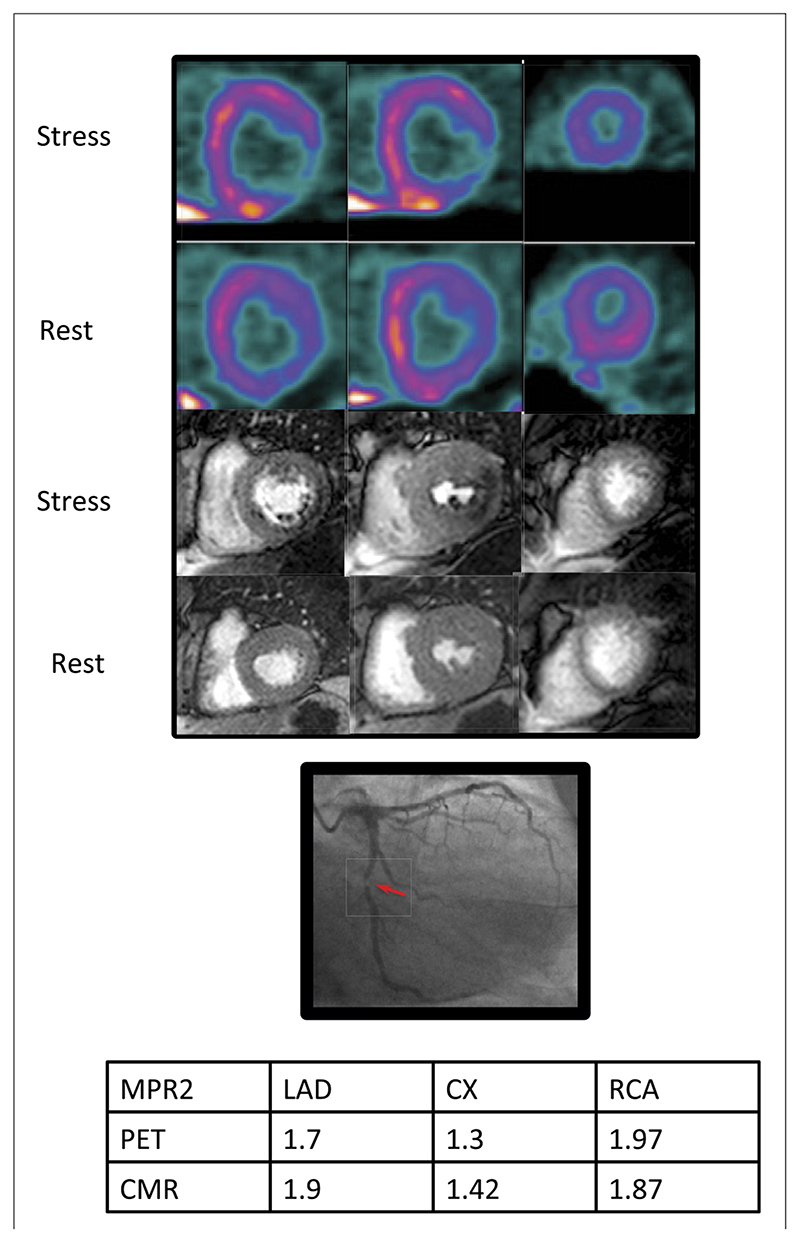
Figure 5. Case Example.
Positron emission tomography (PET) (top), cardiac magnetic resonance (CMR) (middle), and the x-ray angiogram of the left coronary artery of a 54-year-old patient with diabetes and exertional angina. Basal, mid, and apical slices have been taken from the PET study, which approximately correspond to the CMR slices. There is a stress-induced perfusion defect in the infero-lateral region from base to apex visible on both PET and CMR images. There is a corresponding severe (>95%) stenosis of the proximal circumflex artery. There was no other significant angiographic disease. Myocardial perfusion reserve of the lowest 2 segments (MPR2) for each territory are shown in the table. The MPR2 for the circumflex artery (CX) is below the cutoff of 1.44 and 1.45 for both PET and CMR, respectively. LAD = left anterior descending artery; RCA = right coronary artery.
The AUC was 0.85 (95% CI: 0.75 to 0.95) for MPR2PET and MPR2CMR combined. There was no significant difference between AUC when MPR2PET and MPR2CMR were both included in the model and when MPR2PET (p = 0.406) or MPR2CMR (p = 0.4749) alone were used.
The MPR2PET and MPR2CMR were inversely related to the severity of CAD by QCA. For QCA subgroups <30%, 30% to 49%, 50% to 69%, 70% to 95%, and >95% MPR2PET and MPR2CMR were 1.93 ± 0.39 and 1.89 ± 0.46, 1.73 ± 0.33 and 1.86 ± 0.67, 1.58 ± 0.4 and 1.54 ± 0.47, 1.49 ± 0.33 and 1.28 ± 0.42, and 1.18 ± 0.20 and 1.26 ± 0.22, respectively.
The AUC for absolute perfusion values during stress to detect significant CAD were: PET 0.69 (95% CI: 0.56 to 0.81) and CMR 0.72 (95% CI: 0.61 to 0.83). This was significantly lower than MPR2 AUC with PET (p = 0.049) but not CMR (p = 0.12). Optimal absolute stress perfusion cutoffs were: PET ≤1.48 ml/min/g (sensitivity 80%, specificity 53%); and CMR ≤1.50 ml/min/g (sensitivity 63%, specificity 76%).
Discussion
We have demonstrated that, in patients with known or suspected CAD, there is good correlation between MPRPET and MPRCMR. The MPR2CMR also seems to have a similar sensitivity and specificity to MPR2PET for the detection of significant coronary artery stenoses. However, the correlation between the absolute perfusion values from PET and CMR is relatively weak.
To our knowledge, there are no previously published studies comparing quantitative CMR myocardial perfusion with PET in patients. Previous studies have been limited to semi quantitative analysis methods (18,19) or have involved healthy volunteers (11–13). Semi-quantitative analysis has been shown to be useful (20) and to correlate well with MPRPET in patents with CAD (18,19). However, unlike fully quantitative analysis, these methods only provide an index of perfusion reserve and not a true MPR and might substantially underestimate perfusion, particularly at higher perfusion values (19).
Studies using fully quantitative CMR methods are limited to small numbers of healthy volunteers. The results of these studies are inconsistent and difficult to compare, because each study employed different PET and CMR methods. Pärkkä et al. (12) studied 18 healthy volunteers with 15O-water PET and CMR. They used compartmental modeling and found that MPR and stress perfusion values were significantly correlated (r = 0.48 and r = 0.7, respectively). However, rest perfusion values were not significantly correlated. Fritz-Hansen et al. (11) studied 10 healthy volunteers with 13N-ammonia PET with the same CMR quantification method. They demonstrated, similar to our findings, a significant correlation between global MPRPET and MPRCMR (r = 0.7). They found an even stronger correlation for the change in myocardial perfusion between rest and stress (r = 0.96). Both of these studies found a tendency for CMR to underestimate MPR compared with PET. In a smaller study of 5 volunteers, Pack et al. (13) found that 3-T CMR and 13N-ammonia PET perfusion and MPR values are well correlated but that MPR might be in better agreement.
Both PET and CMR perfusion and MPR values in our study are physiologically plausible and in the same range as in some previous studies (11,21–25) but lower than those reported by others (26). Patients are known to have lower stress and rest perfusion than normal volunteers even in remote territories (22,23). The mean difference between PET and CMR perfusion values is small; however, the limits of agreement are broad, particularly at higher stress perfusion values. The findings that MPR, a ratio of stress and rest perfusion values, correlates well but that the absolute perfusion values correlate relatively poorly suggests that the errors in quantification had a similar influence on both rest and stress perfusion values and were subsequently cancelled by the calculation of MPR. These errors might be a result of either methodological or physiological factors.
Methodological considerations
Differences in voxel size and acquisition methods (3-dimensional segments were acquired with PET and 10-mm slices through the 16 segments with CMR) and the use of different postprocessing methods mean that, despite the calculation of mean values in standard segments and territories, there is likely to have been a small degree of misregistration between the modalities. Furthermore, although partial volume effects are corrected for with compartmental PET analysis (16), these were not taken into account with CMR.
Positron emission tomography and CMR have both been validated against gold-standard techniques; however, both still have limitations. The PET studies have used different tracers (e.g., 13N-ammonia vs. 15O water), acquisition protocols, and analysis methods (e.g., Patlak, compartmental). In the present study, the method might underestimate absolute perfusion (27)—an effect that would also be cancelled by the calculation of MPR.
Quantitative CMR perfusion studies have used different magnetic field strengths, contrast agents, perfusion sequences, and post-processing algorithms. One of the main challenges faced by CMR quantification is that, at higher concentrations of contrast agent, there is a loss of linearity between contrast agent concentration and signal intensity. It is possible that these signal saturation effects influenced our CMR results. However, we used a dual bolus method to preserve signal linearity in the left ventricular blood pool and allow calculation of the arterial input function. In addition, a previous study has demonstrated that myocardial signal intensity continues to increase at much higher doses of contrast agent (up to at least 0.15 mmol/kg) than those used here (28), although this study was in normal hearts imaged with a hybrid echo planar pulse sequence. Furthermore, a 14-min interval between stress and rest imaging is insufficient for complete dissipation of the contrast agent. We used a published method (29) of baseline correction to account for this; however, this might also have introduced some error into rest perfusion measurements. We did not find, in contrast to the previous volunteer studies, any evidence of systematic errors for low or high values, indicating that CMR is valid for a wide range of perfusion values.
It is also possible that systematic errors could relate to the fact that PET (30,31) and particularly CMR (32) have only moderate inter-study reproducibility. Regional measurements of perfusion in particular tend to be less reproducible than global measurements. Because the reproducibility of data analysis was relatively high, this might also be partly explained by physiological variation of perfusion.
Physiological considerations
There are also likely to have been real changes in perfusion, even though the interval between the PET and CMR scans was short. Little is known, for example, as to whether diurnal variation or changes in hydration affect myocardial perfusion. It is also possible that such differences are more pronounced in patients than in volunteers—for example, as a result of the use of medications that affect perfusion.
Clinical implications
The novel finding that MPRCMR correlates strongly with MPRPET is important, given the proven utility of MPRPET. The MPRPET correlates inversely with the degree of coronary artery stenosis at angiography (33). Furthermore, recent studies have demonstrated that MPRPET is an independent predictor of outcome and predicts major adverse cardiac events and cardiac death (3) in patients with suspected impaired perfusion and reduced survival in patients with left ventricular impairment (4).
In this study, we identified MPR2CMR and MPR2PET cutoff values that detected CAD with a high sensitivity and specificity. The MPR was superior to absolute stress perfusion values for this purpose. The MPR2PET AUC was significantly higher than absolute stress perfusion values AUC, and there was also a trend toward significance with MPR2CMR. This was reflected by optimal cutoff values for absolute stress perfusion values that would be clinically less useful due to modest sensitivity (CMR) or specificity (PET). This is in contrast to some limited recent PET data suggesting that a single absolute stress perfusion cutoff value might be superior to MPR for the detection of CAD (21). In addition, there does not seem to be any incremental value in combining MPR data from both PET and CMR for the diagnosis of CAD.
Quantification of images in general results in more precise, reproducible, and user-independent results. Although in this study there was no incremental benefit over visual analysis, noninvasive quantification of perfusion might be particularly useful in cases where visual assessment is difficult, such as multivessel disease, severe left ventricular impairment, and after coronary artery bypass grafting. Moreover, it might eventually allow the definition of thresholds of perfusion associated with myocardial ischemia (34) and viability, both of which are known to be important for selecting patients for revascularization procedures. This study reinforces the evidence that quantitative CMR perfusion data can be useful for the assessment of patients with CAD, and at present, we can accurately differentiate normal from abnormal myocardial territories. However, further method refinement is required before the benefits can be fully realized.
Study limitations
The sample size in this study is modest. However, this is the first study in patients and larger than previous volunteer studies. Furthermore, this limitation applies more to the secondary objective of exploring diagnostic accuracy than to the primary aim of comparing quantitative perfusion. Large sample sizes will be required to demonstrate any significant differences in diagnostic accuracy. We compared functional tests against an anatomic reference standard (QCA), despite the well-documented limitations of this approach. It might be better to use fractional flow reserve as a reference standard in future studies. However, x-ray coronary angiography is widely used as the reference standard in both studies and in clinical practice, and again, this does not affect the comparisons made between PET and CMR, which was the main goal of our study.
The patients included in this study had overall good left ventricular function, and it is not clear how well these methods translate into patients with left ventricular systolic dysfunction (35). Finally, the relatively high prevalence of CAD and the exclusion of segments in which image quality precluded quantitative assessment means that these results are likely to represent best possible results with currently available quantitative techniques.
Conclusions
There is a strong correlation between MPR derived from quantitative CMR and PET data, which is important given the proven value of MPRPET for detection of CAD, prognostication, and assessment of the microcirculation. The MPRPET and MPRCMR seem to predict significant CAD equally well and accurately. However, in patients, the absolute perfusion values from PET and CMR are only weakly correlated, suggesting that further refinement of quantitative techniques is required.
Acknowledgment
The authors thank Siobhan Crighton of King’s College London for her assistance with the statistical analysis.
This work was supported by a European Union Grant (Grant number 224495 to Drs. Morton and Nagel); the British Heart Foundation (Research Excellence Award RE/08/003 and FS/10/029/28253 to Drs. Schuster, Perera, and Nagel); the Biomedical Research Centre (Grant number BRC-CTF 196 to Drs. Schuster, Perera, and Nagel); the Wellcome Trust and EPSRC (Grant number WT 088641/Z/09/Z to Drs. Chiribiri and Nagel); and the Academy of Finland Centre of Excellence in Molecular Imaging in Cardiovascular and Metabolic Research, Helsinki, Finland (Dr. Knuuti.). Dr. Nagel has received grant support from Philips Healthcare and Bayer Schering Pharma. Dr. Hedstrom has received grant support from Covidien Dr. Knuuti has received grant support from Gilead Inc.; and is a consultant to Lantheus Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Abbreviations and Acronyms
- AUC
area under the receiver-operating characteristic curve
- CAD
coronary artery disease
- CI
confidence interval
- CMR
cardiovascular magnetic resonance
- CT
computed tomography
- CV
coefficient of variation
- MPR
myocardial perfusion reserve
- PCI
percutaneous coronary intervention
- PET
positron emission tomography
- QCA
quantitative coronary angiography
- ROC
receiver-operating characteristic
References
- 1.Knuuti J, Kajander S, Mäki M, Ukkonen H. Quantification of myocardial blood flow will reform the detection of CAD. J Nucl Cardiol. 2009;16:497–506. doi: 10.1007/s12350-009-9101-1. [DOI] [PubMed] [Google Scholar]
- 2.Kajander SA, Joutsiniemi E, Saraste M, et al. Clinical value of absolute quantification of myocardial perfusion with (15)O-water in coronary artery disease. Circ Cardiovasc Imaging. 2011;4:678–84. doi: 10.1161/CIRCIMAGING.110.960732. [DOI] [PubMed] [Google Scholar]
- 3.Herzog BA, Husmann L, Valenta I, et al. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol. 2009;54:150–6. doi: 10.1016/j.jacc.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 4.Tio RA, Dabeshlim A, Siebelink H-MJ, et al. Comparison between the prognostic value of left ventricular function and myocardial perfusion reserve in patients with ischemic heart disease. J Nucl Med. 2009;50:214–9. doi: 10.2967/jnumed.108.054395. [DOI] [PubMed] [Google Scholar]
- 5.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–40. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 6.Nandalur KR, Dwamena BA, Choudhri AF, Nandalur MR, Carlos RC. Diagnostic performance of stress cardiac magnetic resonance imaging in the detection of coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2007;50:1343–53. doi: 10.1016/j.jacc.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 7.Christian TF, Rettmann DW, Aletras AH, et al. Absolute myocardial perfusion in canines measured by using dual-bolus first-pass MR imaging. Radiology. 2004;232:677–84. doi: 10.1148/radiol.2323030573. [DOI] [PubMed] [Google Scholar]
- 8.Patel AR, Antkowiak PF, Nandalur KR, et al. Assessment of advanced coronary artery disease: advantages of quantitative cardiac magnetic resonance perfusion analysis. J Am Coll Cardiol. 2010;56:561–9. doi: 10.1016/j.jacc.2010.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ichihara T, Ishida M, Kitagawa K, et al. Quantitative analysis of first-pass contrast-enhanced myocardial perfusion MRI using a Patlak plot method and blood saturation correction. Magn Reson Med. 2009;62:373–83. doi: 10.1002/mrm.22018. [DOI] [PubMed] [Google Scholar]
- 10.Jerosch-Herold M, Wilke N, Stillman AE. Magnetic resonance quantification of the myocardial perfusion reserve with a Fermi function model for constrained deconvolution. Med Phys. 1998;25:73–84. doi: 10.1118/1.598163. [DOI] [PubMed] [Google Scholar]
- 11.Fritz-Hansen T, Hove JD, Kofoed KF, Kelbaek H, Larsson HB. Quantification of MRI measured myocardial perfusion reserve in healthy humans: a comparison with positron emission tomography. J Magn Reson Imaging. 2008;27:818–24. doi: 10.1002/jmri.21306. [DOI] [PubMed] [Google Scholar]
- 12.Pârkkâ JP, Niemi P, Saraste A, et al. Comparison ofMRI and positron emission tomography for measuring myocardial perfusion reserve in healthy humans. Magn Reson Med. 2006;55:772–9. doi: 10.1002/mrm.20833. [DOI] [PubMed] [Google Scholar]
- 13.Pack NA, DiBella EVR, Rust TC, et al. Estimating myocardial perfusion from dynamic contrast-enhanced CMR with a modelindependent deconvolution method. J Cardiovasc Magn Reson. 2008;10:52. doi: 10.1186/1532-429X-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 15.Ishida M, Schuster A, Morton G, et al. Development of a universal dual-bolus injection scheme for the quantitative assessment of myocardial perfusion cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2011;13:28. doi: 10.1186/1532-429X-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi Y, Huang SC, Hawkins RA, et al. A simplified method for quantification of myocardial blood flow using nitrogen-13-ammonia and dynamic PET. J Nucl Med. 1993;34:488–97. [PubMed] [Google Scholar]
- 17.Lockie T, Ishida M, Perera D, et al. High-resolution magnetic resonance myocardial perfusion imaging at 3.0-Tesla to detect hemo-dynamically significant coronary stenoses as determined by fractional flow reserve. J Am Coll Cardiol. 2010;57:70–5. doi: 10.1016/j.jacc.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 18.Schwitter J, Nanz D, Kneifel S, et al. Assessment of myocardial perfusion in coronary artery disease by magnetic resonance: a comparison with positron emission tomography and coronary angiography. Circulation. 2001;103:2230–5. doi: 10.1161/01.cir.103.18.2230. [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim T, Nekolla SG, Schreiber K, et al. Assessment of coronary flow reserve: comparison between contrast-enhanced magnetic resonance imaging and positron emission tomography. J Am Coll Cardiol. 2002;39:864–70. doi: 10.1016/s0735-1097(01)01829-0. [DOI] [PubMed] [Google Scholar]
- 20.Nagel E, Klein C, Paetsch I, et al. Magnetic resonance perfusion measurements for the noninvasive detection of coronary artery disease. Circulation. 2003;108:432–7. doi: 10.1161/01.CIR.0000080915.35024.A9. [DOI] [PubMed] [Google Scholar]
- 21.Hajjiri MM, Leavitt MB, Zheng H, Spooner AE, Fischman AJ, Gewirtz H. Comparison of positron emission tomography measurement of adenosine-stimulated absolute myocardial blood flow versus relative myocardial tracer content for physiological assessment of coronary artery stenosis severity and location. J Am Coll Cardiol Img. 2009;2:751–8. doi: 10.1016/j.jcmg.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Iida H, Kanno I, Takahashi A, et al. Measurement of absolute myocardial blood flow with H215O and dynamic positron-emission tomography. Strategy for quantification in relation to the partialvolume effect. Circulation. 1988;78:104–15. doi: 10.1161/01.cir.78.1.104. [DOI] [PubMed] [Google Scholar]
- 23.Karamitsos T, Leccisotti L, Arnold J, et al. Relationship between regional myocardial oxygenation and perfusion in patients with coronary artery disease: insights from cardiovascular magnetic resonance and positron emission tomography. Circ Cardiovasc Imaging. 2010;3:32–40. doi: 10.1161/CIRCIMAGING.109.860148. [DOI] [PubMed] [Google Scholar]
- 24.Czernin J, Müller P, Chan S, et al. Influence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation. 1993;88:62–9. doi: 10.1161/01.cir.88.1.62. [DOI] [PubMed] [Google Scholar]
- 25.Selvanayagam J, Cheng A, Jerosch-Herold M, et al. Effect of distal embolization on myocardial perfusion reserve after percutaneous coronary intervention: a quantitative magnetic resonance perfusion study. Circulation. 2007;116:1458–64. doi: 10.1161/CIRCULATIONAHA.106.671909. [DOI] [PubMed] [Google Scholar]
- 26.Nesterov SV, Han C, Mâki M, et al. Myocardial perfusion quantitation with 15O-labelled water PET: high reproducibility of the new cardiac analysis software (Carimas) Eur J Nucl Med Mol Imaging. 2009;36:1594–602. doi: 10.1007/s00259-009-1143-8. [DOI] [PubMed] [Google Scholar]
- 27.Mullani NA. Is the Patlak graphical analysis method applicable to measurement of myocardial blood flow with nitrogen-13-ammonia? J Nucl Med. 1993;34:1831–4. [PubMed] [Google Scholar]
- 28.Giang TH, Nanz D, Coulden R, et al. Detection of coronary artery disease by magnetic resonance myocardial perfusion imaging with various contrast medium doses: first European multi-centre experience. Eur Heart J. 2004;25:1657–65. doi: 10.1016/j.ehj.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 29.Jerosch-Herold M, Seethamraju RT, Swingen CM, Wilke NM, Stillman AE. Analysis of myocardial perfusion MRI. J Magn Reson Imaging. 2004;19:758–70. doi: 10.1002/jmri.20065. [DOI] [PubMed] [Google Scholar]
- 30.Kaufmann PA, Gnecchi-Ruscone T, Yap JT, Rimoldi O, Camici PG. Assessment of the reproducibility of baseline and hyperemic myocardial blood flow measurements with 15O-labeled water and PET. J Nucl Med. 1999;40:1848–56. [PubMed] [Google Scholar]
- 31.Nagamachi S, Czernin J, Kim AS, et al. Reproducibility of measurements of regional resting and hyperemic myocardial blood flow assessed with PET. J Nucl Med. 1996;37:1626–31. [PubMed] [Google Scholar]
- 32.Jerosch-Herold M, Vazquez G, Wang L, Jacobs DR, Folsom AR. Variability of myocardial blood flow measurements by magnetic resonance imaging in the Multi-Ethnic Study of Atherosclerosis. Invest Radiol. 2008;43:155–61. doi: 10.1097/RLI.0b013e31815abebd. [DOI] [PubMed] [Google Scholar]
- 33.Uren NG, Melin JA, De Bruyne B, Wijns W, Baudhuin T, Camici PG. Relation between myocardial blood flow and the severity of coronary-artery stenosis. N Engl J Med. 1994;330:1782–8. doi: 10.1056/NEJM199406233302503. [DOI] [PubMed] [Google Scholar]
- 34.Vogel R, Indermuhle A, Seiler C. Determination of the absolute perfusion threshold preventing myocardial ischaemia in humans. Heart. 2007;93:115–6. doi: 10.1136/hrt.2005.087031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuster A, Morton G, Chiribiri A, Perera D, Vanoverschelde J, Nagel E. Imaging in the management of ischemic cardiomyopathy: special focus on magnetic resonance. J Am Coll Cardiol. 2012;59:359–70. doi: 10.1016/j.jacc.2011.08.076. [DOI] [PubMed] [Google Scholar]