Abstract
Background
To optimize vaccine implementation visits for young children, it could be efficient to administer the first RTS,S/AS01 malaria vaccine dose during the Expanded Programme on Immunization (EPI) visit at 6 months of age together with Vitamin A supplementation and the third RTS,S/AS01 dose on the same day as yellow fever (YF), measles and rubella vaccines at 9 months of age. We evaluated the safety and immunogenicity of RTS,S/AS01 when co-administered with YF and combined measles-rubella (MR) vaccines.
Methods
In this phase 3b, open-label, controlled study (NCT02699099), 709 Ghanaian children were randomized (1:1:1) to receive RTS,S/AS01 at 6, 7.5 and 9 months of age, and YF and MR vaccines at 9 or 10.5 months of age (RTS,S coad and RTS,S alone groups, respectively). The third group received YF and MR vaccines at 9 months of age and will receive RTS,S/AS01 at 10.5, 11.5 and 12.5 months of age (Control group). All children received Vitamin A at 6 months of age. Non-inferiority of immune responses to the vaccine antigens was evaluated 1 month following co-administration versus RTS,S/AS01 or EPI vaccines (YF and MR vaccines) alone using pre-defined non-inferiority criteria. Safety was assessed until Study month 4.5.
Results
Non-inferiority of antibody responses to the anti-circumsporozoite and anti-hepatitis B virus surface antigens when RTS,S/AS01 was co-administered with YF and MR vaccines versus RTS,S/AS01 alone was demonstrated. Non-inferiority of antibody responses to the measles, rubella, and YF antigens when RTS,S/AS01 was co-administered with YF and MR vaccines versus YF and MR vaccines alone was demonstrated. The safety profile of all vaccines was clinically acceptable in all groups.
Conclusions
RTS,S/AS01 can be co-administered with Vitamin A at 6 months and with YF and MR vaccines at 9 months of age during EPI visits, without immune response impairment to any vaccine antigen or negative safety effect.
Keywords: Non-inferiority, RTS, S/AS01, Co-administration, Yellow fever, Measles, Rubella
1. Introduction
An estimated 219 million malaria cases and 435 000 deaths from malaria occurred worldwide in 2017 [1]. Africa accounted for more than 90% of malaria cases and deaths, and children younger than five years were the most vulnerable age group [1,2]. Current malaria control measures rely on chemoprophylaxis and vector control interventions [1,3]. Although these methods have contributed to the decline in the number of malaria cases, complementary tools, such as vaccines, are needed, especially since anti-malarial drug and insecticide resistance continue to increase [1].
In this context, the pre-erythrocytic Plasmodium falciparum malaria vaccine (RTS,S/AS01, GSK vaccine) has been developed [4]. RTS,S/AS01 has received a positive scientific opinion from the European Medicines Agency under an Article 58 regulatory procedure in July 2015 [5]. The WHO recommended pilot implementation of RTS,S/AS01 as a four-dose regimen in African settings with moderate-to-high malaria transmission [6]. The first dose should be administered after the age of 5 months and should be followed by the second and third doses with a minimum interval of 4 weeks and the third dose completed by 9 months of age. The fourth dose should be given around the second birthday.
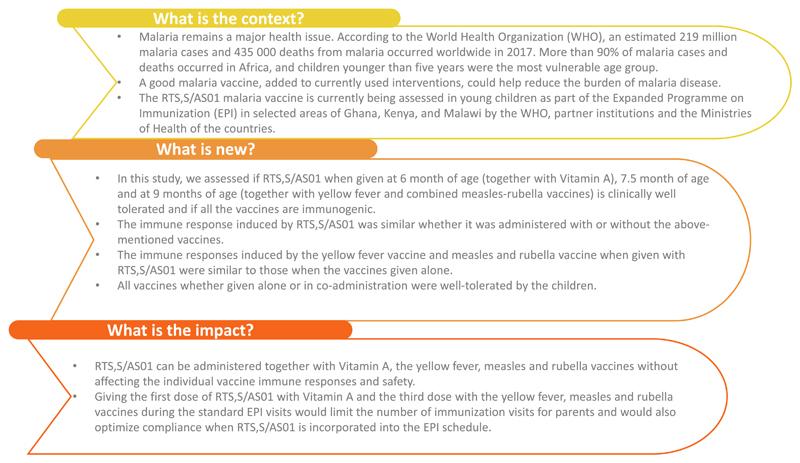
The EPI delivery system is an effective means of achieving rapid high vaccination coverage in Africa. Co-administration of multiple vaccines ensures that children are protected when they need it most, reduces the number of vaccination visits required, and allows an easier incorporation of new vaccines into existing schedules. However, the risks of immune interference or enhancing effects of the co-administered vaccines always need to be evaluated before their incorporation into an existing immunization program [7–11]. In sub-Saharan Africa, measles, rubella and yellow fever (YF) vaccines are given at 9 months of age [12]. A previous phase 4 study conducted in The Gambia has shown that the coadministration of a combined measles-rubella (MR) vaccine and a YF vaccine at 9 months of age was associated with a reduction in the anti-rubella and anti-YF antibody levels, which were further reduced by the concomitant administration of the IPV vaccine, but that seroconversion rates were maintained to both antigens [13]. In regions with Vitamin A deficiency, the WHO recommends Vitamin A supplementation twice yearly for children 6–59 months of age [14]. Co-administration with Vitamin A and with MR and YF vaccines would therefore facilitate the implementation of RTS,S/AS01. In a previous study, the co-administration of RTS,S/AS01 with YF and measles vaccines at 9 months of age did not show immunological interferences for the YF and measles immune responses [11]. However, the study design did not allow for the evaluation of the impact of co-administration on the anti-CS antibody response and was conducted in infants 6 weeks of age at the first dose with co-administration of RTS,S/AS01 with rubella vaccine not assessed. The present study evaluated the immunogenicity, safety and reactogenicity of RTS,S/AS01 in co-administration with a YF and a MR vaccine in Ghanaian children. A summary contextualizing the results, the potential clinical research relevance, and the impact of our study is described in the Plain Language Summary (Fig. 1).
Fig. 1. Plain language summary.
2. Methods
2.1. Study design and participants
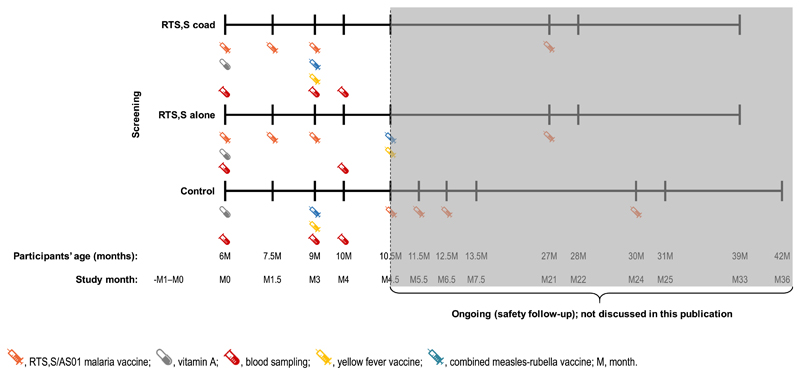
This phase 3b, open-label, controlled study conducted in two centers in Ghana (Kintampo Health Research Center and Kwame Nkrumah University of Science & Technology/Agogo Presbyterian Hospital) started in May 2017. Here, we present results obtained up to March 2018 (Study month 4.5; Fig. 2) following completion of all immunogenicity analyses. The safety results obtained up to the end of the study will be presented elsewhere.
Fig. 2.
Study design.  RTS.S/ASOl malaria vaccine;
RTS.S/ASOl malaria vaccine;  vitamin A;
vitamin A;  blood sampling;
blood sampling;  yellow fever vaccine;
yellow fever vaccine;  combined measles-rubella vaccine; M, month. The data presented here are the results of an interim analysis where all the immunogenicity endpoints were analyzed. The study is currently ongoing and the highlighted area of the study design after the marking are not presented in this manuscript.
combined measles-rubella vaccine; M, month. The data presented here are the results of an interim analysis where all the immunogenicity endpoints were analyzed. The study is currently ongoing and the highlighted area of the study design after the marking are not presented in this manuscript.
Participants were randomized 1:1:1 in three groups: the RTS,S coad, the RTS,S alone and the Control group (Fig. 2). All children received Vitamin A at 6 months of age. Children in the RTS,S coad and RTS,S alone groups received three RTS,S/AS01 doses at 6, 7.5 and 9 months of age, and the YF vaccine (Stamaril, Sanofi-Pasteur) and a combined MR vaccine (MR-VAC, Serum Institute of India) at 9 or 10.5 months of age, respectively. Children in the Control group received the YF and combined MR vaccines at 9 months of age, according to the Ghanaian Expanded Programme on Immunization (EPI) [12], and will receive three RTS,S/AS01 doses at 10.5, 11.5 and 12.5 months of age during the continued follow-up of the trial. All children will receive a booster dose of RTS,S/AS01 approximately 20 months after the third dose of RTS,S/AS01. Participant allocation to a study group was performed using a centralized randomization system. The randomization algorithm used a minimization procedure accounting for center. The randomization of supplies within blocks was performed with SAS (Cary, NC, USA).
The study population consisted of healthy male or female children aged 6 months at the time of first vaccination and/or Vitamin A supplementation, who had documented evidence of previous vaccination with three consecutive doses of diphtheria, tetanus, whole-cell pertussis and hepatitis B vaccine (following vaccination with the pentavalent vaccine which included Hib), and oral polio vaccine, and whose parents/legally acceptable representatives provided written informed consent and were able to comply with the study procedures, as assessed by the investigators. Children with a history of previous vaccination against YF, measles or rubella, or previous administration of Vitamin A, were excluded from study participation. A full list of inclusion and exclusion criteria is provided in Supplementary text S1.
The study was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice, all applicable privacy requirements and the guiding principles of the Declaration of Helsinki. The study protocol and amendment, the informed consent forms and other information that required pre-approval were reviewed and approved by national and investigational center ethics committees and the Ghana Food and Drug Authority. Written/thumb printed and witnessed informed consent was obtained from the parents/legally acceptable representatives of each participant prior to the performance of any study-specific procedure. The study is registered at http://www.clinical-trials.gov (NCT02699099). The protocol is available at http://www.gsk-clinicalstudyregister.com (ID200596).
2.2. Study objectives
The primary objective was to demonstrate non-inferiority of the 1 month post-dose 3 antibody response to the circumsporozoite (CS) antigen when the third dose of RTS,S/AS01 was coadministered with the YF and MR vaccines compared to RTS,S/AS01 administered alone.
Secondary objectives included the demonstration of the noninferiority of the immune responses induced by the YF and MR vaccines when co-administered with the third dose of RTS,S/AS01 compared to their administration without RTS,S/AS01. The noninferiority of the hepatitis B virus surface antigen (HBs) immune response was also calculated when the third dose of RTS,S/AS01 was co-administered with the YF and MR vaccines compared to RTS,S/AS01 administered alone. The antibody responses to CS, HBs, measles, rubella and YF antigens were evaluated following vaccination in all groups, and the safety and tolerability of RTS,S/AS01 and co-administered vaccines were assessed.
2.3. Study vaccines
The RTS,S/AS01 malaria vaccine contains the RTS,S hybrid antigen, a portion of the Plasmodium falciparum CS protein fused to HBs, co-expressed with unfused HBs in yeast. The composition of the RTS,S/AS01 vaccine was previously described in detail [4].
The MR vaccine was a live attenuated measles virus and rubella virus vaccine containing 1000CCID50 of Edmonston-Zagreb measles virus and 1000CCID50 of Wistar RA 27/3 rubella virus. The live attenuated YF vaccine contained the 17D strain of the YF virus.
Each dose of RTS,S/AS01 (0.5 ml) was administered as an intramuscular injection in the left deltoid region of the arm. Each dose of MR vaccine (0.5 ml) was administered subcutaneously into the left anterolateral thigh. The YF vaccine (0.5 ml) was administered intramuscularly into the right anterolateral thigh. A single 100 000 international units (IU) dose of Vitamin A (30 mg retinol equivalent) was administered orally.
2.4. Immunogenicity assessment
Blood samples were collected at pre-vaccination (all groups), before and 1 month after the third dose of RTS,S/AS01 (RTS,S coad and RTS,S alone groups) and before and 1 month post-vaccination with the MR and YF vaccines (RTS,S coad and Control groups) (Fig. 2).
Anti-CS antibodies were quantified by enzyme-linked immunosorbent assay (ELISA) at the Center for Vaccinology (Ghent, Belgium) with a cut-off of 1.9 ELISA units (EU)/mL. Antirubella and anti-measles antibodies were quantified by ELISA at GSK laboratory (Clinical Laboratory Sciences in Rixensart and Wavre, Belgium; NEOMED-LABS Inc, Canada) with cut-offs of 4 international units (IU)/mL and 150 mIU/mL, respectively. Anti-YF antibodies were quantified by plaque neutralization assay at Focus Diagnostics, Inc (Cypress, United States) with a cut-off of 10 (endpoint dilution 50 [ED50]). Anti-HBs antibodies were quantified by chemiluminescence enzyme immunoassay at GSK laboratory with a cut-off of 6.2 mIU/mL.
Seroprotection rates for anti-HBs antibodies were defined as percentages of children with antibody concentrations ≥ 10 mIU/mL. Seroconversion rates for anti-measles antibodies were defined as percentages of children with a pre-vaccination anti-measles antibody concentration < 150 mIU/mL and a post-vaccination anti-measles antibody concentration ≥ 150 mIU/mL. Seroconversion rates for anti-rubella antibodies were defined as percentages of children with a pre-vaccination anti-rubella antibody concentration ≥ 4 IU/mL and a post-vaccination anti-rubella antibody concentration < 4 IU/mL.
2.5. Safety and reactogenicity assessment
Solicited local adverse events (AEs) (injection site pain, redness and swelling), solicited general AEs (drowsiness, fever, irritability/fussiness, loss of appetite and presence of measles/rubella-like rash) were collected over a 7-day follow-up period (day of vaccination and 6 subsequent days) after administration of study vaccines at 6 and 7.5 months of age and over a 14-day follow-up period (day of vaccination and 13 subsequent days) after the vaccination at 9 months of age. A 14-day safety follow-up period was applied at 9 months of age because the peak of fever usually occurs between 5 and 12 days following MR vaccination [15]. The list of products administered, route and site of administration per study group at each visit is provided in Supplementary Table S1.
Unsolicited AEs were collected over a period of up to 30 days (day of vaccination and 29 subsequent days) after administration of study vaccines at 6 and 7.5 months of age and over a 42-day period (day of vaccination and 41 subsequent days) after the vaccination at 9 months of age. The occurrence of serious AEs (SAEs) and potential immune-mediated diseases (pIMDs) was recorded during the entire study period (Study month 33 for the RTS;S coad and RTS,S alone groups and Study month 36 for the Control group), but are presented up to Study month 4.5 here. Other AEs of specific interest were also collected: meningitis occurring during the entire study period, all seizures occurring within 30 days after administration of study vaccines at 6 and 7.5 months of age and within 42 days after the vaccination at 9 months of age, and generalized convulsive seizures occurring over a 7-day follow-up period after administration of study vaccines at 6 and 7.5 months of age and over a 14-day follow-up period after the vaccination at 9 months of age.
All AEs were graded on scale of 1 (mild) to 3 (severe). AEs with a grade 3 intensity were defined as injection site redness and swelling with a diameter > 20 mm, fever with temperature > 39.0 °C, and preventing normal daily activity for all other AEs. Solicited AEs were recorded on diary cards by trained study personnel who visited the children. Unsolicited AEs were recorded through passive surveillance at inpatient and outpatient facilities and during study visits. All solicited local reactions were considered causally related to vaccination. The causality of all other AEs was assessed by the investigator.
The study was overseen by an Independent Data Monitoring Committee operating under a charter and assisted by two Local Safety Monitors.
2.6. Statistical analyses
Assuming a drop-out rate of 12%, 233 children were planned to be enrolled in each group to have 205 evaluable children per group. For the primary endpoint, the study had ≥90% power to rule out a two-fold decrease in anti-CS antibody geometric mean concentrations (GMCs) in the RTS,S coad group versus the RTS,S alone group with a two-sided 5% alpha level and an assumed anti-CS log standard deviation ≤0.9. For the secondary endpoints, the study had >90% power to demonstrate non-inferiority in terms of seroconversion for anti-rubella [16] and anti-measles antibodies [17], and >99% power to demonstrate non-inferiority in terms of seropositivity for anti-YF antibodies [17] in the RTS,S coad group compared with the Control group, assuming that seroconversion and seropositivity rates of 90% and 95% would be reached, based on previous studies.
Safety was assessed in the exposed set, which included all children who received at least one study treatment (study vaccine or Vitamin A). Immunogenicity results are presented for the per-protocol set for immunogenicity, which included all evaluable children meeting all eligibility criteria, complying with the protocol, and with no elimination criteria during the study.
Non-inferiority of the anti-CS and anti-HBs immune responses in the RTS,S coad group compared to the RTS,S alone group was defined as an upper limit (UL) of the two-sided 95% confidence interval (CI) around the anti-CS and anti-HBs antibody GMC ratio (RTS,S alone group/RTS,S coad group) < 2 at 1 month after the third RTS,S/AS01 dose. Non-inferiority of the anti-YF, anti-measles and anti-rubella immune responses in the RTS,S coad group compared with the Control group was defined as an UL of the 95% CIs around the differences (Control group minus RTS,S coad group) in seroconversion rates for anti-measles and anti-rubella antibodies and in seropositivity rates for anti-YF antibodies < 10% at 1 month post-YF and MR vaccination.
Seroprotection and seropositivity rates were calculated with their associated two-sided 95% CIs using the Clopper-Pearson method [18]. Differences in seroconversion rates for anti-measles and anti-rubella antibodies and in seropositivity rates for anti-YF antibodies at 1 month post-vaccination between groups (Control group minus Coad group) were calculated with their associated CIs using the Miettinen and Nurminen method [19]. Antibody GMCs/geometric mean titers (GMTs) were calculated by taking the anti-log of the mean of the logarithmical transformations. Antibody concentrations/titers below the assay cut-off were given an arbitrary value of half the cut-off for GMC/GMT calculations. The 95% CIs of the means of log-transformed concentrations/titers were obtained assuming that log-transformed concentrations/titers were normally distributed with unknown variance. The 95% CIs of GMCs/GMTs were then obtained by exponential transformations of the 95% CIs for the means of the log-transformed concentrations/titers. The between groups GMC ratios (RTS,S alone group/RTS,S coad group) at 1 month after the third dose of RTS,S/AS01 were obtained using an analysis of variance (ANOVA) model that included the study group as fixed effect. The GMC ratios and their two-sided 95% CIs were derived as exponential transformations (base 10) of the corresponding group contrasts in the model. The number and percentages of doses followed by solicited AEs and the number and percentages of children with unsolicited AEs were tabulated with exact 95% CIs. Statistical analyses were performed using the Statistical Analysis System (SAS) statistical software package version 9.2. (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Study population
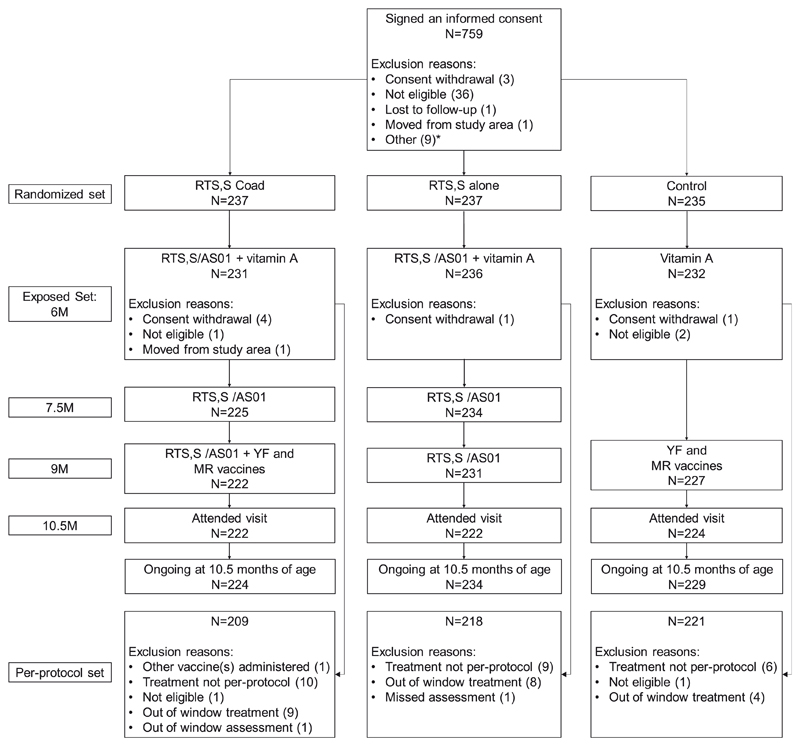
Out of 759 children for whom an informed consent form was signed, 709 were randomized to the three study groups (Fig. 3). Of the randomized children, 699 were vaccinated (231 in the RTS,S coad group, 236 in the RTS,S alone group and 232 in the Control group) and 687 were still continuing the study at the data lock point for this analysis. A total of 648 children were included in the per-protocol set for immunogenicity (209 in the RTS,S coad group, 218 in the RTS,S alone group and 221 in the Control group) (Fig. 3). The demographic characteristics of the children included in the three study groups were similar (Table 1).
Fig. 3.
Flow of participants. The RTS,S coad group received the RTS,S/AS01 vaccines at 6, 7.5 and 9 months of age and the YF and MR vaccines at 9 months of age; the RTS,S alone group received the RTS,S/AS01 vaccines at 6, 7.5 and 9 months of age; and the Control group received the YF and MR vaccines at 9 months of age. N, number of children, M, month; RTS,S/AS01, pre-erythrocytic Plasmodium falciparum malaria vaccine; YF, yellow fever; MR, combined measles-rubella. *An informed consent form was signed for 9 children when the enrolment target was met and these children were not included in the study.
Table 1. Summary of demographic characteristics at Vitamin A administration and/or RTS,S/AS01 vaccination - exposed set.
| RTS,S coad | RTS,S alone | Control | |
|---|---|---|---|
| N = 231 | N = 236 | N = 232 | |
| Age (months), mean ± SD | 6.3 ± 0.3 | 6.3 ± 0.3 | 6.3 ± 0.3 |
| Male gender, n (%) | 115 (49.8) | 117 (49.6) | 119 (51.3) |
| Mean length for age Z-score ± SD | −0.5 ± 1.0 | −0.5 ± 1.1 | −0.6 ± 1.1 |
| Mean weight for age Z-score ± SD | −0.8 ± 1.1 | −0.7 ± 1.1 | −0.8 ± 1.1 |
RTS,S coad, children who received the RTS,S/AS01 vaccines at 6, 7.5 and 9 months of age and the YF and MR vaccines at 9 months of age; RTS,S alone, children who received the RTS,S/AS01 vaccines at 6, 7.5 and 9 months of age; Control, children who received the YF and MR vaccines at 9 months of age; N, number of children; SD, standard deviation; n (%), number (percentage) of children in a given category; Z-score, SD score calculated as: (observed value minus the median value of the reference population) / standard deviation value of the reference population; YF, yellow fever; MR, combined measles-rubella. At Visit 2, RTS,S/AS01 was given with Vitamin A in the RTS,S coad and the RTS,S alone groups and the Control group received only Vitamin A.
3.2. Immunogenicity
3.2.1. Immune response to RTS,S/AS01
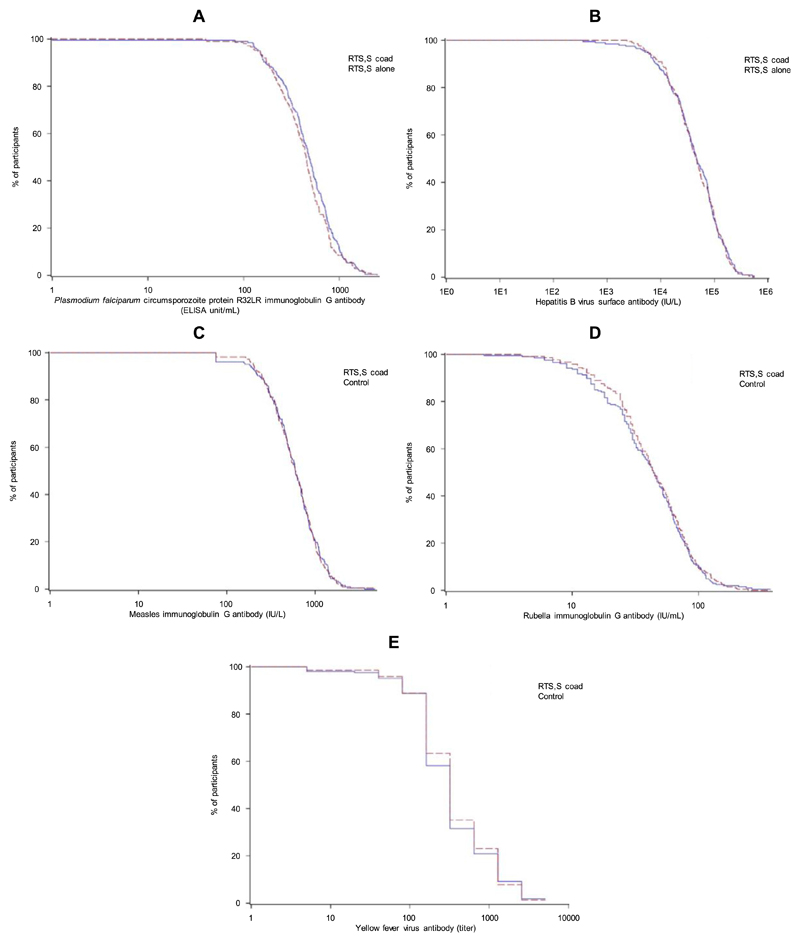
At pre-vaccination, anti-CS antibody seropositivity rates were low in the RTS,S coad and the RTS,S alone groups, ranging from 0.5% to 0.9%. One month after the third dose of RTS,S/AS01, seropositivity rates for anti-CS antibodies were 99.5% and 100%, and anti-CS antibody GMCs were 452.9 and 422.5 EU/mL in the RTS,S coad and the RTS,S alone groups, respectively (Table 3), with similar reverse cumulative curves (RCCs) of the distribution of anti-CS antibody concentrations (Fig. 4A). The primary objective was met as the anti-CS antibody response was shown to be noninferior in the RTS,S coad group compared to the RTS,S alone group. The UL of the two-sided 95% CI around the anti-CS GMC ratio (RTS, S alone group/RTS,S coad group) was 1.07, which was below the pre-defined criterion for non-inferiority of 2 (Table 2).
Table 3. Immune responses before vaccination and at 1 month after the third dose of RTS,S/AS01 vaccine (RTS,S coad and RTS,S alone groups) and prior to and at 1 month after the MR and YF vaccination (RTS,S coad and Control groups) - per-protocol set.
| Antibody | Parameter | Timepoint | RTS,S coad | RTS,S alone | Control | |||
|---|---|---|---|---|---|---|---|---|
| N | Value (95% CI) | N | Value (95% CI) | N | Value (95% CI) | |||
| Anti-CS | Seropositivity rate (concentration ≥ 1.9 | Pre | 209 | 0.5 (0.0–2.6) | 218 | 0.9 (0.1–3.3) | – | – |
| EU/mL) | Post | 207 | 99.5 (97.3–100.0) | 213 | 100.0(98.3–100.0) | – | – | |
| GMC (EU/mL) | Pre | 209 | 0.96 (0.94–0.97) | 219 | 0.96 (0.94–0.98) | – | – | |
| Post | 207 | 452.87 (406.99–503.92) | 219 | 422.49 (385.42–463.12) | – | – | ||
| Anti-HBs | Seropositivity rate (concentration ≥ 6.2 | Pre | 206 | 97.1 (93.8–98.9) | 213 | 97.2 (94.0–99.0) | – | – |
| mIU/mL) | Post | 204 | 100.0 (98.2–100.0) | 210 | 100.0 (98.3–100.0) | – | – | |
| GMC (mIU/mL) | Pre | 206 | 393.29 (315.23–490.66) | 213 | 420.79 (335.10–528.39) | – | – | |
| Post | 204 | 42096.33 (35558.62–49836.05) | 210 | 43261.19 (37275.19–50208.47) | − | − | ||
| Seroprotection rate (concentration ≥ 10 | Pre | 206 | 95.6 (91.9–98.0) | 213 | 96.2 (92.7–98.4) | – | – | |
| mIU/mL) | Post | 204 | 100.0 (98.2–100.0) | 210 | 100.0 (98.3–100.0) | – | – | |
| Anti- | measles | Seropositivity rate | Pre | 206 | 0.0 | (0.0–1.8) | ||
| − | − | (concentration ≥ 150 mIU/mL) | 220 | 0.0 (0.0–1.7) | ||||
| Post | 207 | 96.1 (92.5–98.3) | – | – | 217 | 98.2 (95.3–99.5) | ||
| GMC (mIU/mL) | Pre | 206 | <150* | – | – | 220 | <150* | |
| Post | 207 | 564.32 (511.88–622.13) | − | − | 217 | 572.09 (523.83–624.79) | ||
| Anti-rubella | Seropositivity rate (concentration ≥ 4 IU/mL) | Pre | 206 | 2.4 (0.8–5.6) | − | − | 220 | 1.8 (0.5–4.6) |
| Post | 207 | 99.5 (97.3–100.0) | – | – | 217 | 100.0 (98.3–100.0) | ||
| GMC (IU/mL) | Pre | 206 | 2.08 (1.99–2.18) | – | – | 220 | 2.09 (1.98–2.20) | |
| Post | 207 | 39.38 (35.20–44.06) | − | − | 217 | 42.45 (38.4246.92) | ||
| Anti-YF | Seropositivity rate (Titre ≥ 10 ED50) | Post | 206 | 98.1 (95.1–99.5) | – | – | 216 | 98.6 (96.0–99.7) |
| GMT (ED50) | Post | 206 | 318.93 (269.65–377.21) | 216 | 346.73 (296.62–405.30) | |||
The RTS,S coad group received the RTS,S/AS01 vaccines at 6, 7.5 and 9 months of age and the YF and MR vaccines at 9 months of age; the RTS,S alone group received the RTS,S/AS01 vaccines at 6, 7.5 and 9 months of age; and the Control group received the YF and MR vaccines at 9 months of age.
N, number of children with available results; CI, confidence interval; Anti-CS: antibodies to Plasmodium falciparum circumsporozoite (CS) protein; Anti-HBs: antibodies to hepatitis B surface antigen; Pre, pre-vaccination; Post, one month after the third dose of RTS,S/AS01 vaccine alone (RTS,S alone group), the third dose of RTS,S/AS01 vaccine given with YF and MR vaccines (RTS,S coad group) and the YF and MR vaccines (Control group); GMC, geometric mean concentration; HBs, hepatitis B surface antigens; YF, yellow fever; GMT, geometric mean titer; MR, combined measles-rubella. * Indicates that all children were seronegative and had a value below the cut-off of the assay.
Fig. 4.
Reverse cumulative distributions of anti-CS (A), anti-HBs (B), anti-measles (C), anti-rubella (D), anti-YF (E) antibodies at 1 month after the third dose of RTS,S/AS01 vaccine (RTS,S coad and RTS,S alone groups) and at 1 month after the MR and YF vaccination (RTS,S coad and Control groups) - per-protocol set. The RTS,S coad group received the RTS,S/AS01 vaccines at 6, 7.5 and 9 months of age and the YF and MR vaccines at 9 months of age; the RTS,S alone group received the RTS,S/AS01 vaccines at 6, 7.5 and 9 months of age; and the Control group received the YF and MR vaccines at 9 months of age. CS, circumsporozoite; HBs, hepatitis B surface antigens; YF, yellow fever; MR, combined measles-rubella. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Non-inferiority assessment of the anti-CS and anti-HBs antibody responses at 1 month after the third dose of RTS,S/AS01 vaccine (RTS,S coad and RTS,S alone groups) and of the anti-measles, anti-rubella and anti-YF antibody responses at 1 month after the MR and YF vaccination (RTS,S coad and Control groups) - per-protocol set.
| RTS,S alone | RTS,S coad | GMC ratio (RTS,S alone/RTS,S coad) | |||
|---|---|---|---|---|---|
| Antibody | N | GMC (95% CI) | N | GMC (95% CI) | Value (95% CI) |
| Anti-CS (EU/mL) | 213 | 422.49 (382.89–466.18) | 207 | 452.87 (409.85–500.41) | 0.93 (0.81–1.07) |
| Anti-HBs (mIU/mL) | 210 | 43261.19 (36964.93–50629.89) | 204 | 42096.33 (35887.11–49379.88) | 1.03 (0.82–1.29) |
| RTS,S coad | Control | Absolute difference in percentage (Control minus RTS,S coad) | |||
| Antibody | N | % (95% CI) | N | % (95% CI) | % (95% CI) |
| Anti-measles (mIU/mL) | 204 | 96.08 (92.4–98.3) | 216 | 98.15 (95.3–99.5) | 2.07 (-1.27–5.92) |
| Anti-rubella (IU/mL) | 199 | 99.50 (97.2–100.0) | 212 | 100.00 (98.3–100.0) | 0.50 (-1.29–2.80) |
| Anti-YF (ED50) | 206 | 98.06 (95.1–99.5) | 216 | 98.61 (96.0–99.7) | 0.55 (-2.31–3.67) |
The RTS,S coad group received the RTS,S/AS01 vaccines at 6, 7.5 and 9 months of age and the YF and MR vaccines at 9 months of age; the RTS,S alone group received the RTS,S/AS01 vaccines at 6, 7.5 and 9 months of age; and the Control group received the YF and MR vaccines at 9 months of age.
N, number of children with seronegative results at pre-vaccination and available post-vaccination results (for anti-measles and anti-rubella antibodies) or number of children with post-vaccination results available (anti-CS, anti-HBs and anti-YF antibodies); GMC, geometric mean concentration; CI, confidence interval; CS, circumsporozoite; HBs, hepatitis B surface antigens; %, percentage of seropositive (anti-YF), or seroconverted (anti-measles and anti-rubella children at 1 month post-vaccination; YF, yellow fever; MR, combined measles-rubella.
Bold: primary objective of the study.
At pre-vaccination, anti-HBs antibody seroprotection rates were high in the RTS,S coad (95.6%) and the RTS,S alone groups (96.2%). Anti-HBs antibody GMCs further increased from 393.3 to 42096.3 mIU/mL in the RTS,S coad group and from 420.8 to 43261.2 mIU/mL in the RTS,S alone group between pre-vaccination and 1 month after the third dose of RTS,S/AS01, resulting in 100% anti-HBs antibody seroprotection rates in both groups (Table 3) and overlapping RCCs of the distribution of anti-HBs antibody concentrations (Fig. 4B). Anti-HBs antibody response was shown to be noninferior in the RTS,S coad group compared to the RTS,S alone group. The UL of the two-sided 95% CI around the anti-HBs GMC ratio (RTS,S alone group/RTS,S coad group) was 1.29, which was below the pre-defined criterion for non-inferiority of 2 (Table 2).
3.2.2. Immune response to co-administered vaccines
At pre-vaccination, seropositivity rates for anti-measles and anti-rubella antibodies were low in the RTS,S coad and the Control groups, ranging from 0.0% to 2.4%. One month post-vaccination, seropositivity rates were 96.1% and 98.2% against measles, 99.5% and 100% against rubella, and 98.1% and 98.6% against YF in the RTS,S coad and the Control groups, respectively (Table 3). RCCs of the distribution of 1 month post-vaccination anti-measles, antirubella and anti-YF antibody concentrations were very similar between the RTS,S coad and the Control groups (Fig. 4C, D and E). One month post-vaccination, anti-measles, anti-rubella, and anti-YF antibody responses were shown to be non-inferior in the RTS,S coad group compared to the Control group. The ULs of the 95% CIs around the differences (Control group minus RTS,S coad group) in seroconversion rates of anti-measles and anti-rubella antibodies, and seropositivity rates of anti-YF antibodies were 5.92%, 2.80% and 3.67% respectively, which were below the predefined criteria for non-inferiority of 10% (Table 2).
3.3. Safety
At the data lock point, children in the RTS,S coad and RTS,S alone groups had received 3 doses of RTS,S/AS01, while those in the Control group had not received any RTS,S/AS01 dose. Overall, following administration of the three doses of RTS,S/AS01, pain was the most frequently reported solicited local AE at the RTS,S/AS01 injection site in children who received the third dose of RTS,S/AS01 either co-administered with MR and YF vaccines (RTS,S coad group; following 5.3% of doses) or alone (RTS,S alone group; following 5.4% of doses) (Table 4). Grade 3 solicited local AEs were uncommon (0–0.1% of doses overall) in both groups. The most frequently reported solicited general symptom was low-grade fever (temperature ≥ 37.5 °C) in all groups (following 19.7% and 18.2% of doses in the RTS,S coad and the RTS,S alone groups, respectively) (Table 4). Grade 3 fever (temperature > 39.0 °C) was reported following ≤ 1.3% of doses in both groups.
Table 4. Overall incidence of solicited local and general adverse events reported following 3 doses of the RTS,S/AS01 vaccine in the RTS,S coad and RTS,S alone groups (overall per dose) - exposed set.
| Symptoms | RTS,S coad | RTS,S alone | ||||
|---|---|---|---|---|---|---|
| N | n | % (95% CI) | N | n | % (95% CI) | |
| Solicited local adverse events at the RTS,S/AS01 injection site | ||||||
| Pain | 675 | 36 | 5.3 (3.8–7.3) | 698 | 38 | 5.4 (3.9–7.4) |
| Redness | 675 | 11 | 1.6 (0.8–2.9) | 698 | 16 | 2.3 (1.3–3.7) |
| Swelling | 675 | 15 | 2.2 (1.2–3.6) | 698 | 15 | 2.1 (1.2–3.5) |
| Solicited general adverse events | ||||||
| Drowsiness | 676 | 17 | 2.5 (1.5–4.0) | 698 | 15 | 2.1 (1.2–3.5) |
| Irritability/Fussiness | 676 | 36 | 5.3 (3.8–7.3) | 698 | 43 | 6.2 (4.5–8.2) |
| Loss of appetite | 676 | 19 | 2.8 (1.7–4.4) | 698 | 24 | 3.4 (2.2–5.1) |
| Measles/Rubella-like rash | 676 | 0 | 0.0 (0.0–0.5) | 698 | 0 | 0.0 (0.0–0.5) |
| Fever* | ||||||
| All | 676 | 133 | 19.7 (16.7–22.9) | 698 | 127 | 18.2 (15.4–21.3) |
| Grade 3 | 676 | 9 | 1.3 (0.6–2.5) | 698 | 6 | 0.9 (0.3–1.9) |
The RTS,S coad group received the RTS,S/AS01 vaccines at 6, 7.5 and 9 months of age and the YF and MR vaccines at 9 months of age; the RTS,S alone group received the RTS,S/AS01 vaccines at 6, 7.5 and 9 months of age.
N, number of documented doses on all visits combined; n, number of doses followed by the symptom after all visits; %, percentage of doses followed by the symptom after all visits; CI, confidence interval.
Fever was defined as temperature ≥ 37.5 °C for oral, axillary or tympanic route. Grade 3 Fever was defined as temperature > 39.0 °C.
At 9 months of age, the RTS,S coad group received their third RTS,S/AS01 dose together with YF and MR vaccines, the RTS,S alone group received their third dose of RTS,S/AS01 alone, and the Control group received the YF and MR vaccines. Pain at the RTS,S/AS01 injection site was reported at similar incidences in the RTS, S coad and the RTS,S alone groups (3.6% and 4.4% of children) (Table 5). Pain at the YF and MR injection sites was reported for 3.2% and 3.7% of children in the RTS,S coad group, respectively, and for 0.4% of children (for each injection site) in the Control group. Redness and swelling were infrequent irrespective of the injection site in all groups (≤1.4% of children). No grade 3 solicited local AEs were reported. At 9 months of age, the most frequently reported solicited general AE in all groups was fever, reported at similar incidences in the RTS,S coad and the RTS,S alone groups (24.4% and 21.8% of children) but at a lower incidence in the Control group (7.6% of children). Grade 3 fever was reported by ≤ 1.4% of children in all groups (Table 5).
Table 5. Incidence of solicited adverse events reported during the 14-day follow-up period post-vaccination at 9 months of age - exposed set.
| Symptom and vaccine site | RTS,S coad | RTS,S alone | Control | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | n | % (95% CI) | N | n | % (95% CI) | N | n | % (95% CI) | |
| Solicited local adverse events | |||||||||
| Pain | |||||||||
| RTS,S/AS01 dose 3 site | 220 | 8 | 3.6 (1.6–7.0) | 228 | 10 | 4.4 (2.1–7.9) | – | – | – |
| Combined measles-rubella vaccine site | 218 | 8 | 3.7 (1.6–7.1) | – | – | – | 225 | 1 | 0.4 (0.0–2.5) |
| Yellow fever vaccine site | 218 | 7 | 3.2 (1.3–6.5) | – | – | – | 225 | 1 | 0.4 (0.0–2.5) |
| Redness | |||||||||
| RTS,S/AS01 dose 3 site | 220 | 0 | 0.0 (0.0–1.7) | 228 | 1 | 0.4 (0.0–2.4) | – | – | – |
| Combined measles-rubella vaccine site | 218 | 0 | 0.0 (0.0–1.7) | – | – | – | 225 | 2 | 0.9 (0.1–3.2) |
| Yellow fever vaccine site | 218 | 0 | 0.0 (0.0–1.7) | – | – | – | 225 | 2 | 0.9 (0.1–3.2) |
| Swelling | |||||||||
| RTS,S/AS01 dose 3 site | 220 | 3 | 1.4 (0.3–3.9) | 228 | 3 | 1.3 (0.3–3.8) | – | – | – |
| Combined measles-rubella vaccine site | 218 | 0 | 0.0 (0.0–1.7) | – | – | – | 225 | 1 | 0.4 (0.0–2.5) |
| Yellow fever vaccine site | 218 | 0 | 0.0 (0.0–1.7) | – | – | – | 225 | 1 | 0.4 (0.0–2.5) |
| Solicited general adverse events | |||||||||
| Drowsiness | 221 | 7 | 3.2 (1.3–6.4) | 229 | 6 | 2.6 (1.0–5.6) | 225 | 0 | 0.0 (0.0–1.6) |
| Irritability/Fussiness | 221 | 11 | 5.0 (2.5–8.7) | 229 | 8 | 3.5 (1.5–6.8) | 225 | 1 | 0.4 (0.0–2.5) |
| Loss of appetite | 221 | 8 | 3.6 (1.6–7.0) | 229 | 6 | 2.6 (1.0–5.6) | 225 | 2 | 0.9 (0.1–3.2) |
| Measles/Rubella-like rash | 221 | 0 | 0.0 (0.0–1.7) | 0 | 0.0 (0.0–1.6) | 225 | 0 | 0.0 (0.0–1.6) | |
| Fever* | |||||||||
| All | 221 | 54 | 24.4(18.9–30.6) | 229 | 50 | 21.8 (16.7–27.8) | 225 | 17 | 7.6 (4.5–11.8) |
| Grade 3 | 221 | 3 | 1.4 (0.3–3.9) | 229 | 2 | 0.9 (0.1–3.1) | 225 | 2 | 0.9 (0.1–3.2) |
The RTS,S coad group received the RTS,S/AS01 vaccines at 6, 7.5 and 9 months of age and the YF and MR vaccines at 9 months of age; the RTS,S alone group received the RTS,S/AS01 vaccines at 6, 7.5 and 9 months of age; and the Control group received the YF and MR vaccines at 9 months of age.
N, Number of children with the corresponding documented dose; n, number of children in whom the symptom was reported following the corresponding dose; %, percentage of children in whom the symptom was reported following the corresponding dose; CI, confidence interval; RTS,S/AS01, pre-erythrocytic Plasmodium falciparum malaria vaccine; YF, yellow fever; MR, combined measles-rubella.
Fever was defined as temperature ≥ 37.5 °C for oral, axillary or tympanic route, or ≥ 38.0 °C for rectal route. The preferred route for recording temperature was axillary. Grade 3 Fever was defined as temperature > 39.0 °C.
Overall, unsolicited AEs were similar in nature and intensity in all groups. Comparable incidences were reported in the three groups (82.7%, 78.8% and 75.0% of children in the RTS,S coad, RTS,S alone and Control groups, respectively). Upper respiratory tract infections (48.1%, 45.3% and 43.5% of children in the RTS,S coad, RTS,S alone and Control groups, respectively) and gastroenteritis (35.1%, 37.7% and 31.9% of children in the RTS,S coad, RTS, S alone and Control groups, respectively) were the most frequently reported unsolicited AEs (Table 6 and Supplementary Table S2). Unsolicited AEs considered by the investigator as causally related to study vaccines were reported for two children in the RTS,S coad group (injection site inflammation considered related to RTS,S/AS01 vaccination and increased intracranial pressure considered related to Vitamin A administration) and for one child in the RTS, S alone group (injection site abscess). No causally related unsolicited AEs were reported in the Control group. Grade 3 unsolicited AEs were reported in 3.9%, 1.7% and 3.0% of children in the RTS,S coad, RTS,S alone and Control groups, respectively; none were considered related to the study vaccines by the investigators.
Table 6. Percentage of children reporting the most common (occurrence > 5% in at least one group) unsolicited adverse events - exposed set.
| RTS,S coad N = 231 | RTS,S alone N = 236 | Control N = 232 | ||||
|---|---|---|---|---|---|---|
| Unsolicited adverse events in all children | n | % (95%CI) | n | % (95% CI) | n | % (95% CI) |
| All | 191 | 82.7 (77.2–87.3) | 186 | 78.8 (73.0–83.8) | 174 | 75.0 (68.9–80.4) |
| Grade 3 | 9 | 3.9 (1.8–7.3) | 4 | 1.7 (0.5–4.3) | 7 | 3.0 (1.2–6.1) |
| Unsolicited adverse events reported by > 5% of children | ||||||
| Enteritis | 27 | 11.7 (7.8–16.5) | 27 | 11.4 (7.7–16.2) | 20 | 8.6 (5.3–13.0) |
| Conjunctivitis | 19 | 8.2 (5.0–12.5) | 17 | 7.2 (4.3–11.3) | 14 | 6.0 (3.3–9.9) |
| Dermatitis infected | 7 | 3.0 (1.2–6.1) | 18 | 7.6 (4.6–11.8) | 13 | 5.6 (3.0–9.4) |
| Gastroenteritis | 81 | 35.1 (28.9–41.6) | 89 | 37.7 (31.5–44.2) | 74 | 31.9 (25.9–38.3) |
| Malaria | 37 | 16.0 (11.5–21.4) | 37 | 15.7 (11.3–21.0) | 54 | 23.3 (18.0–29.3) |
| Otitis media | 15 | 6.5 (3.7–10.5) | 20 | 8.5 (5.3–12.8) | 10 | 4.3 (2.1–7.8) |
| Pneumonia | 32 | 13.9 (9.7–19.0) | 25 | 10.6 (7.0–15.2) | 19 | 8.2 (5.0–12.5) |
| Respiratory tract infection | 43 | 18.6 (13.8–24.2) | 52 | 22.0 (16.9–27.9) | 42 | 18.1 (13.4–23.7) |
| Rhinitis | 9 | 3.9 (1.8–7.3) | 12 | 5.1 (2.7–8.7) | 7 | 3.0 (1.2–6.1) |
| Upper respiratory tract infection | 111 | 48.1 (41.5–54.7) | 107 | 45.3 (38.9–51.9) | 101 | 43.5 (37.1–50.2) |
| Dermatitis | 12 | 5.2 (2.7–8.9) | 16 | 6.8 (3.9–10.8) | 8 | 3.4 (1.5–6.7) |
The RTS,S coad group received the RTS,S/AS01 vaccines at 6, 7.5 and 9 months of age and the YF and MR vaccines at 9 months of age; the RTS,S alone group received the RTS,S/AS01 vaccines at 6, 7.5 and 9 months of age; and the Control group received the YF and MR vaccines at 9 months of age.
N, number of children with at least one documented dose; n, number of children in whom the symptom was reported at least once; %, percentage of children in whom the symptom was reported at least once; CI, confidence interval; YF, yellow fever; MR, combined measles-rubella.
The follow-up period for unsolicited AEs was 30 days post-vaccination for the doses given at 6 and 7.5 months of age, and 42 days post-vaccination for the doses given at 9 months of age.
Over the entire study period, there was no imbalance in the reporting of SAEs between groups (6.1%, 5.1% and 9.1% of children in the RTS,S coad, RTS,S alone and Control groups, respectively). Two fatal SAEs were reported: one child in the RTS,S coad group died from septic shock due to pneumococcal sepsis and the other death in the Control group, which occurred prior to vaccination, was concluded to be of unknown cause despite investigations and efforts to obtain a verbal autopsy. None of the SAEs were assessed by the investigators as related to vaccination or Vitamin A administration. No pIMDs, meningitis or seizures were reported during the study period.
4. Discussion
We evaluated whether RTS,S/AS01 could be integrated into the EPI schedule to potentially limit the number of visits for parents and maximize the compliance. Therefore, we assessed the safetyand immunogenicity of RTS,S/AS01 when co-administered with Vitamin A supplementation at 6 months of age, given alone at 7.5 months of age, and co-administered with YF and MR vaccines at 9 months of age in Ghanaian children. We evaluated whether the co-administration of RTS,S/AS01 and vaccines included in the EPI schedule interfered with immune responses to any antigen and whether a clinically acceptable safety profile was maintained.
We demonstrated the non-inferiority of the anti-CS, anti-HBs, anti-measles, anti-rubella and anti-YF immune responses induced by the concomitant administration of the third dose of RTS,S/AS01 with a MR vaccine and a YF vaccine compared to their separate administration. Non-inferiority of the anti-measles and anti-YF immune responses when the third dose of RTS,S/AS01 was coadministered with the YF vaccine and another measles vaccine (Rouvax, Aventis Pasteur), compared with the EPI vaccines given alone, has been shown in a previous study conducted in infants 6-10 weeks of age who received RTS,S/AS01 according to a 0, 1 and 7 months vaccination schedule [11]. However, in this previous study, the non-inferiority of the anti-CS and anti-HBs immune responses was not assessed and RTS,S/AS01 was not coadministered with a rubella-containing vaccine. Our study demonstrates thus for the first time that the co-administration of RTS,S/AS01 with these EPI vaccines does not negatively impact the immune responses induced by RTS,S/AS01. Since the combined MR vaccine is currently the vaccine of choice for EPI, it is also reassuring that its co-administration with RTS,S/AS01 does not negatively affect the humoral responses to the rubella antigen.
The similar anti-CS and anti-HBs antibody GMCs and reverse cumulative distribution curves observed in children who received the third dose of RTS,S/AS01 co-administered with MR and YF vaccines versus RTS,S/AS01 alone are in line with the results of the previous study conducted in younger children who received the third RTS,S/AS01 dose (0, 1, 7-month schedule) co-administered with the YF vaccine and another measles vaccine [11]. The results of the previous study suggested that the co-administered vaccines did not interfere with the immune responses induced by RTS,S/AS01, even though a formal non-inferiority assessment was not performed. Likewise, the similar distribution of anti-measles and anti-YF antibody concentrations in our study and in the previous study in infants further demonstrated that measles and YF vaccines can be concomitantly administered with RTS,S/AS01 without impairment of the immune response to either vaccine antigen [11]. Moreover, the similar anti-rubella antibody concentrations and distribution in children who received the MR and YF vaccines with or without RTS,S/AS01 showed for the first time that there was no interference with the immune response to the rubella antigen upon co-administration of the MR vaccine with RTS,S/AS01. Since the anti-CS and anti-HBs antibody responses measured in our study were in line with previous observations, our results also suggest that the first RTS,S/AS01 dose can be co-administered with the Vitamin A supplementation at 6 months of age [20–22].
In our study, the RTS,S/AS01 vaccine was given within the age range recommended by the WHO for its pilot implementation [6], but the interval between the three primary doses was longer (6 weeks) than in the schedule evaluated in a previous phase 3 efficacy study in children aged 5-17 months (4 weeks) [20–22]. Since the anti-CS antibody responses were in the range of what was previously observed, our results suggest that there is some potential flexibility in the RTS,S/AS01 vaccination schedule. As previously observed [7], the RTS,S/AS01 vaccine also had a positive effect on the anti-HBs antibody GMCs due to the adjuvanted HepB vaccine component. Each dose of RTS,S/AS01 boosted the anti-HBs immune response.
Co-administration of EPI vaccines in infants is common practice even if it results in some increased reactogenicity [23]. In our study, the safety and reactogenicity profiles of all vaccines were clinically acceptable in the three groups. No safety concerns related to vaccination were identified during the entire study and no imbalance was observed between groups in terms of reported unsolicited AEs and SAEs. Pain at any injection site and fever tended to be reported more frequently when all three vaccines were co-administered as compared to administration of YF and MR vaccines alone, but incidences were within the same ranges as those observed after administration of RTS,S/AS01 alone and the reported pain and fever were predominantly mild or moderate. Similar results were also obtained in the previous study conducted in younger infants, where the occurrence of fever was more frequent upon co-administration of RTS,S/AS01 with YF and measles vaccines than after administration of YF and measles vaccines alone [11]. The similar incidence of post-vaccination fever when RTS,S/AS01 was given alone or co-administered with YF and MR vaccines and the absence of febrile convulsions in our study are important findings because an increased incidence of febrile convulsions was previously observed post-RTS,S/AS01 vaccination [24]. There were a limited number of malaria cases, which were observed with comparable proportions in both RTS,S/AS01 vaccinated groups and with a higher proportion in the Control group. The absence of pIMDs and meningitis and the fact that the two coincidental fatalities were not related to vaccination were also reassuring.
The main strengths of this study were that it was powered to demonstrate the non-inferiority of the immune responses to all antigens induced by the co-administered vaccines compared to their separate administration and the results were conclusive. The randomized design allowed a comparable demographic profile of children in the three groups. However, this study was limited by its open-label design due to the between-group differences in immunization schedules. The open-label design might have induced some level of bias in the safety assessments but not in the immunogenicity results since the laboratories where the assays were performed were blinded to the treatment.
5. Conclusion
The results of this study demonstrated that RTS,S/AS01 can be concomitantly administered with Vitamin A at 6 months of age, given alone at 7.5 months of age and co-administered with YF and MR vaccines at 9 months of age without impairment of the immune response to either vaccine antigen or a negative safety effect. The safety profile of RTS,S/AS01 when co-administered with the YF and MR vaccines was clinically acceptable and comparable to that of RTS,S/AS01 given alone. Co-administration of the first dose of RTS,S/AS01 with Vitamin A and of the third dose with YF and MR vaccines during the standard EPI visits would limit the number of immunization visits for parents and may contribute to maximize the adherence to the vaccination schedule.
Supplementary Material
Acknowledgements
The authors would like to thank all study participants, their families, community leaders and traditional Chiefs. We are grateful for the support of the Management of Ghana Health Service and all collaborating institutions in Kintampo North Municipality, Kintampo South District, Nkoranza North District and Techiman North District. We thank Ashura Bakari, Maame Anima Attobrah Sarfo, Maame Fremah Peprah, Felix Owusu Bonsu, Alimatu Salam, Joyce Bening and Lawrence Osei-Tutu from Komfo Anokye Teaching Hospital (Kumasi, Ghana), Kingsley Kayan for laboratory support, Jacqueline Gyapomaa Asibey and Zakariah B. Buwah. Authors would like to acknowledge the staff of the Agogo Presbyterian Hospital for their support and collaboration in this study. We thank PATH’s Malaria Vaccine Initiative for financial support. We thank our GSK colleagues: Amanda Leach, Muriel Debois, Yolanda Guerra-Mendoza, Erik Jongert and Nathalie Baudson for their contribution to the study as well as Johan Vekemans (GSK employee at the time of the study; current affiliation: Initiative for Vaccine Research, WHO). The authors also thank the clinical operations, laboratory sciences, data management and scientific writing teams of GSK who worked on this study for their contribution to the study. The authors thank the Modis platform for editorial assistance and manuscript coordination, on behalf of GSK; Claire Verbelen provided medical writing support and Fabienne Danhier coordinated the manuscript development and provided editorial support.
Abbreviations
- AE
adverse event
- CI
confidence interval
- CS
circumsporozoite
- ED50
endpoint dilution 50
- ELISA
enzyme-linked immunosorbent assay
- EPI
Expanded Programme on Immunization
- EU
ELISA units
- GMC
geometric mean concentration
- GMT
geometric mean titer
- HBs
hepatitis B virus surface antigen
- IU
international unit
- MR
combined measles-rubella
- pIMD
potential immune-mediated disease
- RCC
reverse cumulative curve
- RTS,S/AS01
pre-erythrocytic Plasmodium falciparum malaria vaccine
- SAE
serious adverse event
- UL
upper limit
- WHO
World Health Organization
- YF
yellow fever
Footnotes
Author contributions
All authors either participated in the design, implementation or analysis, and interpretation of the study, as well as the development of this manuscript. All authors had full access to the data and granted their final approval of the paper before submission.
Data sharing statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: LS, ML and OOA are employees of the GSK group of companies. The current affiliation of CA is Kemri-Wellcome Trust Research Programme and KWTRP played no role in the study, holds no responsibility relative to the data presented and the views expressed here do not represent KWTRP views. EG was an employee of GSK at the time of the study. LS, CA, PV, OOA have shares or stock options in the GSK group of companies. KPA, DA, SA, EG, HOB, SOA, TA, TR, DS, OB, DD, EA, LNB, FP, PBYB, JAA, SK, PAD, SBEH, EAW, AAD, YN, CA, FP declare no competing interests.
Financial disclosure
This study and related publication were sponsored by GlaxoSmithKline Biologicals S.A. GlaxoSmithKline Biologicals SA was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also took responsibility for all costs associated with the development and publishing of the present manuscript.
Trademark statement
Stamaril is a trademark owned by or licensed to Sanofi-Pasteur. MR-VAC is a trademark owned by or licensed to Serum Institute of India. Rouvax is a trademark owned by or licensed to Aventis Pasteur.
Contributor Information
Kwaku Poku Asante, Email: kwakupoku.asante@kintampo-hrc.org.
Seyram Kaali, Email: kaali.seyram@kintampo-hrc.org.
Marc Lievens, Email: marc.lievens@gsk.com.
Prince Agyapong Darko, Email: agyapong.darko@kintampo-hrc.org.
Owusu Boahen, Email: owusu.boahen@kintampo-hrc.org.
Clara Agutu, Email: CAgutu@kemri-wellcome.org.
Samuel Benard Ekow Harrison, Email: samuel.harrison@kintampo-hrc.org.
Japhet Adomako Anim, Email: japhet.anim@kintampo-hrc.org.
Elisha Adeniji, Email: elisha.adeniji@kintampo-hrc.org.
David Dosoo, Email: david.dosoo@kintampo-hrc.org.
Elvis Ato Wilson, Email: elvis.wilson@kintampo-hrc.org.
Pascale Vandoolaeghe, Email: pascale.vandoolaeghe@gsk.com.
Lode Schuerman, Email: Lode.Schuerman@gsk.com.
Seth Owusu-Agyei, Email: sowusuagyei@uhas.edu.gh.
Tsiri Agbenyega, Email: tsiri@ghana.com.
References
- [1].World Health Organization. [accessed 13 August 2019];World malaria report. 2018 http://apps.who.int/iris/bitstream/handle/10665/275867/9789241565653-eng.pdf?ua=1.
- [2].World Health Organization. [accessed 30 May 2018];Malaria in children under five. 2018 http://www.who.int/malaria/areas/high_risk_groups/children/en/
- [3].World Health Organization. [accessed 13 August 2019];Larval source management: a supplementary measure for malaria vector control. An operational manual. 2013 http://apps.who.int/iris/bitstream/handle/10665/85379/9789241505604_eng.pdf;jsessionid=5D313FF934519E9FE9578359D9F9916A?sequence=1.
- [4].Cohen J, Nussenzweig V, Nussenzweig R, Vekemans J, Leach A. From the circumsporozoite protein to the RTS, S/AS candidate vaccine. Hum Vaccin. 2010;6:90–6. doi: 10.4161/hv.6.1.9677. [DOI] [PubMed] [Google Scholar]
- [5].European Medicines Agency. [accessed 13 August 2019];Committee for medicinal products for human use. Assessment report - Mosquirix. 2015 https://www.ema.europa.eu/en/documents/medicine-outside-eu/mosquirix-public-assessment-report_en.pdf.
- [6].World Health Organization. Malaria vaccine: WHO position paper-January 2016. Wkly Epidemiol Rec. 2016;91:33–51. [PubMed] [Google Scholar]
- [7].Valea I, Adjei S, Usuf E, Traore O, Ansong D, Tinto H, et al. Immune response to the hepatitis B antigen in the RTS, S/AS01 malaria vaccine, and coadministration with pneumococcal conjugate and rotavirus vaccines in African children: A randomized controlled trial. Hum Vaccin Immunother. 2018;14:1489–500. doi: 10.1080/21645515.2018.1442996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Abdulla S, Oberholzer R, Juma O, Kubhoja S, Machera F, Membi C, et al. Safety and immunogenicity of RTS, S/AS02D malaria vaccine in infants. N Engl J Med. 2008;359:2533–44. doi: 10.1056/NEJMoa0807773. [DOI] [PubMed] [Google Scholar]
- [9].Ota MO, Odutola AA, Owiafe PK, Donkor S, Owolabi OA, Brittain NJ, et al. Immunogenicity of the tuberculosis vaccine MVA85A is reduced by coadministration with EPI vaccines in a randomized controlled trial in Gambian infants. Sci Transl Med. 2011;3:88ra56. doi: 10.1126/scitranslmed.3002461. [DOI] [PubMed] [Google Scholar]
- [10].Roy Chowdhury P, Meier C, Laraway H, Tang Y, Hodgson A, Sow SO, et al. Immunogenicity of yellow fever vaccine coadministered with MenAfriVac in healthy infants in Ghana and Mali. Clin Infect Dis. 2015;61(Suppl 5):S586–93. doi: 10.1093/cid/civ603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Agnandji ST, Asante KP, Lyimo J, Vekemans J, Soulanoudjingar SS, Owusu R, et al. Evaluation of the safety and immunogenicity of the RTS, S/AS01E malaria candidate vaccine when integrated in the expanded program ofimmunization. J Infect Dis. 2010;202:1076–87. doi: 10.1086/656190. [DOI] [PubMed] [Google Scholar]
- [12].Ministry of Health/Ghana Health Service. [accessed 13 August 2019];Immunization programme comprehensive multi-year plan (2010-2014) 2011 http://www.nationalplanningcycles.org/sites/default/files/country_docs/Ghana/revised_cmyp_2010_-_2014.pdf.
- [13].Clarke E, Saidu Y, Adetifa JU, Adigweme I, Hydara MB, Bashorun AO, et al. Safety and immunogenicity of inactivated poliovirus vaccine when given with measles-rubella combined vaccine and yellow fever vaccine and when given via different administration routes: a phase 4, randomised, non-inferiority trial in The Gambia. Lancet Glob Health. 2016;4:e534–47. doi: 10.1016/s2214-109x(16)30075-4. [DOI] [PubMed] [Google Scholar]
- [14].World Health Organization. [accessed 13 August 2019];Guideline: Vitamin A supplementation in infants and children 6-59 months of age. 2011 https://apps.who.int/iris/bitstream/handle/10665/44664/9789241501767_eng.pdf;jsessionid=0FBD1354F93D1AE4F41A5128E08E175C?sequence=1.
- [15].Shinefield H, Black S, Digilio L, Reisinger K, Blatter M, Gress JO, et al. Evaluation of a quadrivalent measles, mumps, rubella and varicella vaccine in healthy children. Pediatr Infect Dis J. 2005;24:665–9. doi: 10.1097/01.inf.0000172902.25009.a1. [DOI] [PubMed] [Google Scholar]
- [16].World Health Organization. Rubella vaccines: WHO position paper. Wkly Epidemiol Rec. 2011;86:301–16. [PubMed] [Google Scholar]
- [17].Asante KP, Abdulla S, Agnandji S, Lyimo J, Vekemans J, Soulanoudjingar S, et al. Safety and efficacy of the RTS, S/AS01E candidate malaria vaccine given with expanded-programme-on-immunisation vaccines: 19 month follow-up of a randomised, open-label, phase 2 trial. Lancet Infect Dis. 2011;11:741–9. doi: 10.1016/s1473-3099(11)70100-1. [DOI] [PubMed] [Google Scholar]
- [18].Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–13. doi: 10.1093/biomet/26.4.404. [DOI] [Google Scholar]
- [19].Nurminen M, Miettinen O, Gart JJ, Nam J-m. Confidence intervals for the ratio of the parameters of two independent binomials. Biometrics. 1990;46:269–72. [Google Scholar]
- [20].RTS, S clinical trials partnership. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365:1863–75. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- [21].RTS, S clinical trials partnership. Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med. 2014;11:e1001685. doi: 10.1371/journal.pmed.1001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].RTS, S clinical trials partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45. doi: 10.1016/s0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dolan S, Wallace A, Burnett E, Ehlman D, Sui W, Garon J, et al. In: SAGE Yellow Book, editor. Summary of evidence on the administration of multiple injectable vaccines in infants during a single visit: safety, immunogenicity, and vaccine administration practices; Prepared for the April 2015 SAGE Meeting; Geneva, Switzerland. 2015. pp. 222–64. [Google Scholar]
- [24].Guerra Mendoza Y, Garric E, Leach A, Lievens M, Ofori-Anyinam O, Pirçon J-Y, et al. Safety profile of the RTS, S/AS01 malaria vaccine in infants and children: additional data from a phase III randomized controlled trial in sub-Saharan Africa. Hum Vaccines Immunotherapeutics. 2019:1–13. doi: 10.1080/21645515.2019.1586040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.