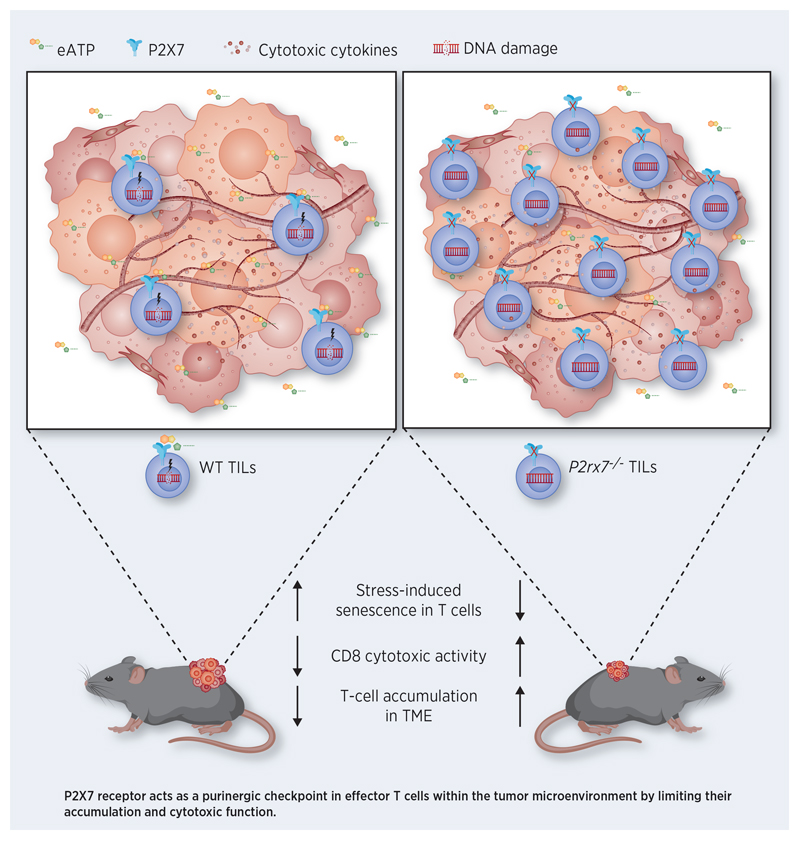
Abstract
Extracellular adenosine triphosphate (eATP) is a signaling molecule which variably affects all cells of the immune system either directly or after hydrolysis to adenosine. Although eATP is virtually absent in the interstitium of normal tissues, it can be present in the hundreds of micromolar range in tumors, a concentration compatible with activation of the ATP-gated ionotropic P2X7 receptor. Here we show that P2X7 activity in tumor-infiltrating T cells (TILs) induces cellular senescence and limits tumor suppression. P2X7 stimulation affected cell cycling of effector T cells and resulted in generation of mitochondrial reactive oxygen species (ROS) and p38 MAPK-dependent upregulation of cyclin-dependent kinase inhibitor 1A (Cdkn1a, encoding for p21Waf1/Cip1). Lack of P2X7 promoted a transcriptional signature that correlated with enhanced cytotoxic T cell response in human solid tumors. In mice, transfer of tumor specific T cells with deletion of P2rx7 significantly reduced tumor growth and extended survival. Collectively, these findings uncover a purinergic checkpoint that can be targeted to improve the efficacy of cancer immunotherapy strategies.
Introduction
More than 30 years have passed since the first tumor infiltrating lymphocytes (TILs) were conditioned for treating patients with metastatic melanoma (1). Today, checkpoint inhibitors and chimeric antigen receptor (CAR) T cells have succeeded in promoting effective anti-tumor cytotoxicity with unprecedented durable responses in a variety of cancers, thereby establishing new immunotherapeutic paradigms for oncologic patients (2,3). Unfortunately, a substantial fraction of patients does not respond to current immunotherapy treatments. Thus, it has become important to identify factors that limit efficient T cell responsiveness in the tumor microenvironment (TME), and to develop strategies that could potentially increase the patient response rates.
The TME can impair the effector functions of TILs by various mechanisms, including nutrients depletion (4,5) and release of immunosuppressive molecules, such as indoleamine 2,3-dioxygenase (IDO) (6), by cancer cells; recruitment of myeloid suppressor cells that release immunomodulators, such as arginase and nitrous oxide synthase (7); hypoxia (8) and release of intracellular potassium ions by tumor-associated necrosis, both of which suppress T cell effector function (9). A characteristic feature of the tumor interstitium is the elevated concentration of extracellular ATP (eATP) (10), a pleiotropic signaling molecule, which can act as a danger-associated molecular pattern (DAMP). In fact, eATP contributes to adjuvant’s efficacy in vaccination (11) and promotes immunogenic cell death of cancer cells by attracting antigen presenting cells and activating pro-inflammatory cascades (12); however, it can also limit pro-inflammatory T cell effector function (13) or generate immunosuppressive adenosine through the activity of plasma membrane ectonucleotidases (14). Therefore, the final effect of eATP in the tumor microenvironment would depend on the nature of the immune cell infiltrate, the composition of receptors for extracellular nucleotides and the activity of ATP-degrading ectonucleotidases.
Plasma membrane receptors for extracellular nucleotides, termed P2 receptors, are divided into two families, P2X and P2Y (15). P2X1 to P2X7 receptors are ATP gated non-selective cation channels, whereas P2Y receptors are guanine nucleotide–binding protein–coupled receptors (GPCRs), which bind also to ADP, UDP, UTP, or UDP-glucose. The P2X7 receptor is widely expressed with highest levels in the nervous and immune systems; it is characterized by dual gating that depends on the saturation level of the ligand binding sites. Activation of P2X7 by low agonist concentrations results in slow desensitizing currents, whereas saturating or repetitive stimulations generate high-amplitude currents that lead to dilation of a pore permeable to nanometer-size dyes and eventually cell death (16,17). Albeit a detailed analysis of αβTCR repertoire was not performed in naïve T cells from P2rx7-/- mice, P2rx7 expression did not apparently influence αβ T cell development in the thymus (18). We found that P2rx7 is robustly upregulated in T effector memory (TEM) cells compared to the naïve counterpart and its activity in TILs promotes cell cycle arrest, cellular senescence and impairs the tumoricidal response. Our results unravel P2X7 as a possible target to foster the adaptive T cell response against cancer cells in immunotherapeutic approaches.
Materials and Methods
Mice and in vivo experiments
All animal experiments were performed in accordance with the Swiss Federal Veterinary Office guidelines and approved by the Ethical Committee of the Cantonal Veterinary with authorization number TI 37/2016. C57BL/6J, P2rx7-/- (B6.129P2-P2rx7tm1Gab/J), Cd3ε-/-, OT-II Rag1-/- [B6.Cg-Tg(TcraTcrb)425Cbn/J], OT-I Rag1-/- [B6.Cg-Tg(TcraTcrb)1100Mjb/DcrJ], CD45.1 (B6.SJL-Ptprca Pepcb/BoyJ) mice were bred in specific pathogen-free (spf) facility at the Institute for Research in Biomedicine, Switzerland. OT-II Rag1-/- P2rx7-/- and OT-I Rag1-/- P2rx7-/- were generated by crossing OT-II Rag1-/- or OT-I Rag1-/- with P2rx7-/- mice. Genotyping was accomplished by the polymerase chain reaction (PCR) method according to the manufacturer’s protocol. Mice were housed, five per cage, in ventilated cages under standardized conditions (20 ± 2°C, 55 ± 8% relative humidity, 12 h light/dark cycle). Food and water were available ad libitum, and mice were examined daily. To analyze anti-tumor response, CD4+ or CD8+ T naïve were sorted from C57BL/6J and P2rx7-/- mice as described below. 2.5x105 T naïve cells were injected into Cd3ε-/- mice. Melanoma B16F10 cells were harvested at exponential growth. After five days from transfer of T cells, melanoma cells were resuspended in PBS at a concentration of 5x106 cells/mL and a volume of 0.1 mL (5x105 tumor cells) was injected subcutaneously into the back of adoptively transferred Cd3ε-/- mice. To analyse the T cell response against a tumor specific antigen, C57BL/6 or CD45.1 mice were injected subcutaneously with 5x105 B16-OVA or MC38-OVA cells. After 5 or 7 days, 1x106 Rag1-/- or Rag1-/-/P2rx7-/- congenically marked CD4+ OT-II or CD8+ OT-I, that had been previously activated in vitro with anti-CD3 and anti-CD28 antibodies for 72h and IL-2 in the last 24h prior to injection (19), were transferred into mice randomized from littermate cages and tumor growth was assessed. Tumor growth was scored with a caliper by measuring the greatest tumor diameter and its perpendicular to determine an average and then the area was calculated as: (average/2)2π. Tumor-bearing animals were sacrificed after 20 days or earlier when showing any sign of discomfort. For analysis of survival, mice were sacrificed when they reached human endpoint, defined by: tumor volume [estimated with the formula (lengh x width2)/2] bigger than 1,5 cm3 or severe signs of discomfort.
Cell isolation from mice organs
For in vitro experiments and adoptive transfer, CD4+ or CD8+ T naïve, TEM or congenically marked OT-II or OT-I cells were sorted at FACSAria (BD Biosciences) from pooled cell suspensions of spleen, inguinal, axillary, brachial, cervical and mesenteric lymph nodes collected from C57BL/6J and P2rx7-/- mice. T naïve cells were sorted as CD4+ or CD8+, CD62L+, CD44- and CD25- cells, TEM cells were sorted as CD4+ or CD8+, CD62L-, CD44+ and CD25- cells. OT-I and OT-II cells were sorted as CD8+ and CD4+, respectively, CD25- and CD45.1+ or CD45.1- cells. Magnetic Cell Sorting (MACS, Miltenyi Biotec) with anti-CD4 and anti-APC mAbs were used to enrich cell subsets from complex cell mixtures.
Tumor cell lines
B16F10, B16F10-OVA (B16-OVA), MC38-OVA cells were cultured in RPMI-1640 supplemented with 10% heat-inactivate fetal bovine serum, 100 U/mL penicillin/streptomycin and 100 U/mL kanamycin. Cells were tested for the absence of mycoplasma and maintained in 5% CO2 at 37°C. Frozen aliquots were thawed for each in vivo experiment and passaged in vitro for the minimum time required. Tumor cells at 70-80% confluency were harvested by diluting them 1:5 in 0.25% trypsin. B16F10 and B16-OVA cells were obtained from Dr. Matteo Bellone, HSR Scientific Institute, Milan, Italy. MC38-OVA cells were obtained from Dr. Maria Rescigno, Humanitas University, Rozzano (MI), Italy.
Generation of stable B16F10-pmeLUC transfectants
B16F10 melanoma cells were transfected with 1μg/ml of pmeLUC-pcDNA3 plasmid by Lipofectamine LTX (ThermoFisher), according to the manufacturer’s instructions. For stable transfection, cells were kept in continuous presence of G418 (0.4mg/ml) (Calbiochem) for two weeks. B16F10-pmeLUC clones were obtained by limiting dilution and B16F10pmeLUC positive clones were selected by luminescence assay in the presence of the Luciferase substrate D-luciferin (Promega). Stably transfected clones were maintained in culture in the presence of G418 (0.2mg/ml) (20).
Luciferase and in vivo imaging
Luciferase luminescence was acquired with a total body luminometer (IVIS Lumina, Caliper-PerkinElmer). Mice were anesthetized with 2.5% isofluorane, intraperitoneally (i.p.) injected with 150 mg/kg D-luciferin (PerkinElmer), and luminescence quantified after 15 minutes. Regions of interest (ROI) from displayed images were identified at tumour sites and quantified as Total Flux (photons/sec) using the Living Image software.
In vitro calibration of B16F10-pmeLUC cells
B16F10-pmeLUC cells, 1x105 per well, plated in 24 wells plate, were incubated in RPMI 1640 medium and challenged with increasing concentration of ATP in the presence of D/luciferine, 60μg/ml. Luminescence was acquired with the IVIS luminometer for 1 min. Total luminescence emission was acquired from each well and quantified as Total Flux (photons/sec) using the Living Image software. Luminescence was expressed as Total Flux (photons/sec) as a function of the added ATP concentration.
Cell isolation from tumor tissue
Tumors were cut in small pieces and resuspended in RPMI-1640 with 1.5 mg/mL type I collagenase (Sigma), 100 μg/mL DNase I (Roche) and 5% FBS, digested for 45 min at 37°C under gentle agitation. The digestion product was then passed through a 70 μm cell strainer to obtain a single cell suspension. Lymphocytes were then enriched by Percoll density gradient following manufacturer’s protocol.
Time monitoring of DAPI uptake
Purified T naïve or TEM cells were resuspended at 1x106 cells/mL and loaded with DAPI (1μg/ml). After 30 seconds of measurement for DAPI basal level, cells were stimulated with BzATP and DAPI uptake was monitored over time (250 seconds) at LSRFortessa and the kinetics analyzed using FlowJo software.
Gene expression profiling of ex vivo purified CD4+ cells
Gene expression profiling of ex vivo purified CD4+ cells was performed on MG-430 PM Array Strip (Affymetrix, Santa Clara, CA, USA). Briefly, total RNA was extracted using TriPure (Roche) from ex vivo purified CD4+ naïve and TEM cells from WT (n=2 and n=3 independent samples for naïve and TEM, respectively) and P2rx7-/- (n=2 and n=3 independent samples for naïve and TEM, respectively) mice. RNA quality and purity were assessed on the Agilent Bioanalyzer 2100 (Agilent Technologies, Waldbronn, Germany); RNA concentration was determined using the NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies Inc.). In vitro transcription, hybridization and biotin labeling were performed according to Affymetrix GeneChip 3’IVT protocol and processed using GeneAtlas™ Platform (Affymetrix, Santa Clara, CA, USA). Scanning and data exporting was performed using standard Affimetrix protocols. All microarray data analyses were performed in R (version 3.3.2) using Bioconductor libraries (BioC 3.1) and R statistical packages. Probe level signals were converted to expression values using robust multi-array average procedure RMA (21) of Bioconductor affy package and a custom definition file for mouse HT array plates based on Entrez genes from BrainArray (version 22.0.0; http://brainarray.mbni.med.umich.edu/Brainarray/Database/CustomCDF/22.0.0/entrezg.asp). Before downstream analysis, expression values have been batch-corrected using the ComBat function of the sva package. Raw data are available at Gene Expression Omnibus under accession number GSE118146. To identify genes associated with P2rx7 deletion in ex vivo purified CD4+ TEM cells, we compared the expression levels of WT and P2rx7-/- TEM cells using the Significance Analysis of Microarray (SAM) (22) algorithm coded in the samr R package. In SAM, we estimated the percentage of false positive predictions (i.e. False Discovery Rate, FDR) with 100 permutations and selected those probe sets with FDR ≤ 5% and absolute fold change larger than a selected threshold (e.g. ≥ 1.5) in the comparison of TEM cells from P2rx7-/- and WT mice (Tables S1 and S3). Principal component analysis (PCA) has been performed using the function prcomp of R stats package. Before unsupervised PCA, to reduce the effect of noise from non-varying genes, we removed those probe sets with a coefficient of variation smaller than the 95th percentile of the coefficients of variation in the entire dataset. The filter retained 904 genes that are more variable across samples in any of the 4 subsets (naïve and TEM cells from P2rx7-/- and WT mice). Supervised clustering was performed using the function hclust of R stats package with Pearson correlation as distance metric and average agglomeration method. Gene expression heatmap has been generated using the function heatmap.2 of R gplots package after row-wise standardization of the expression values. The volcano plot, showing the most significantly differentially expressed genes in the comparison of ex vivo purified CD4+ TEM cells from P2rx7-/- and WT mice, was generated using the ggplot function of the ggplot2 R package. P-values were derived from SAM q-values using the function samr.pvalues.from.perms of the samr R package (Table S3). Functional over-representation was performed using Gene Set Enrichment Analysis (GSEA; http://software.broadinstitute.org/gsea/index.jsp) and the curated gene sets of the Molecular Signatures Database (MSigDB) derived from the KEGG pathway database (http://software.broadinstitute.org/gsea/msigdb/genesets.jsp?collection=CP:KEGG). GSEA was applied on log2 expression data of P2rx7-/- and WT TEM cells. Prior to GSEA analysis, we converted mouse Entrez IDs into the corresponding human homologous genes using the HUGO Gene Nomenclature Committee (HGNC) database (https://www.genenames.org/cgi-bin/hcop). Gene sets were considered significantly enriched at FDR≤0.25 when using Signal2Noise as metric and 1,000 permutations of gene sets.
Gene expression data that support the findings of our study have been deposited in NCBI Gene Expression Omnibus (GEO) and are accessible through the link: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE118146 by using the following secure token: oxmpkqkotfidbuz.
Statistical analyses
Statistical analysis was performed with the Prism software (GraphPad). Comparisons of two groups were calculated using nonparametric Mann Whitney test or Student’s unpaired t test. Comparisons for more than two groups were calculated using Kruskall-Wallis test followed by Dunn’s multiple comparison test. Results are presented as mean values ± SEM or SD. Values of p<0.05 were considered significant.
Results
Enhanced anti-tumor response by P2rx7-/- CD8 T cells in lymphopenic mice
P2rx7 is the most expressed P2rx gene in both CD4+ T naïve and TEM cells (Fig. S1A). Nevertheless, real-time quantitative reverse transcription PCR (qRT-PCR) and western blot (WB) revealed significantly increased levels of P2rx7 transcript and P2X7 protein, respectively, in TEM versus naïve cells (Fig. S1, B and C). The analysis of pore opening in flow cytometry in CD4+ T cells upon stimulation with the selective agonist 3’-O-(4-benzoyl) benzoyl ATP (BzATP) at 150 μM revealed that only a small fraction of naïve cells was sensitive to P2X7-mediated DAPI uptake, as compared to the vast majority of TEM cells (Fig. S1D), indicating enhanced sensitivity of CD4 cells to eATP after stimulation by cognate antigen and differentiation to effector/memory cells. An analogous upregulation of P2rx7 transcripts was observed in CD8+ TEM cells (Fig. S1E).
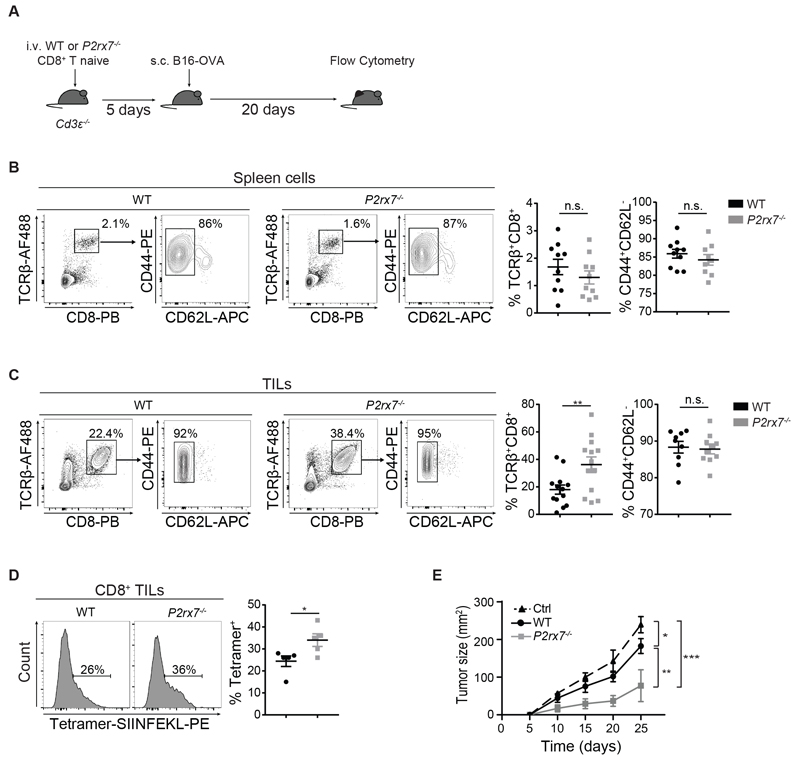
In orthotopic human melanoma xenografts, eATP is present in the hundreds micromolar range (10,23). The analysis of B16F10 melanoma composed by cells stably expressing the eATP reporter pmeLUC (23) at day 7 after transplant into wild-type (WT) mice, revealed concentrations of eATP in the TME from tens to hundreds of micromolar (Fig. S1F). We addressed whether P2X7 stimulation in the TME affected control of tumor growth by CD8+ TILs. The transfer of P2rx7-/- CD8+ naïve T cells into Cd3ε-/- mice that were subsequently engrafted with OVA expressing B16F10 (B16-OVA) melanoma cells (Fig. 1A) resulted in enhanced accumulation of P2rx7-/- TEM cells in the tumor but not in the spleen with respect to WT cells (Fig. 1, B and C). Tetramer staining revealed increased percentages of P2rx7-/- versus WT TILs specific for the H-2Kb restricted OVA peptide 257-264 (Fig. 1D). Moreover, tumor growth was significantly delayed by P2rx7-/- with respect to WT cells (Fig. 1E).
Fig. 1. Enhanced accumulation and control of tumor growth by P2rx7-/- CD8+ TILs.
(A-E) Cd3ε-/- mice were adoptively transferred with 2.5 x 105 WT or P2rx7-/- naive CD8+ T cells, engrafted with B16-OVA cells and sacrificed as indicated. (A) Experimental design. (B-C) Representative flow cytometry plots and statistical analysis (mean ± SEM) for CD8+ TCRβ+ cells within (B) splenocytes (n=10) and (C) TILs (n=13), with distribution for CD44 and CD62L expression among TCRß+CD8+ cells. Percentages of positive cells among total live (Zombie Green-) cells (see methods for tumors) in each gate are shown. Data from three independent experiments. (D) Flow cytometry histograms and statistical analysis (mean ± SEM) for tetramer positive TILs gated as CD8+ TCRβ+ cells (n=5). Data from one experiment representative of three. Percentages of positive cells are shown. (E) Kinetics of tumor growth. Data from one experiment representative of two (n=5). Two-tailed Mann-Whitney U test for the comparison of two groups or two-way ANOVA for comparison of tumor size was used. n.s. not significant, * p<0.05, ** p<0.01, *** p<0,001.
Enhanced control of tumor growth and survival of mice by lack of P2X7 activity in tumor specific CD8 cells
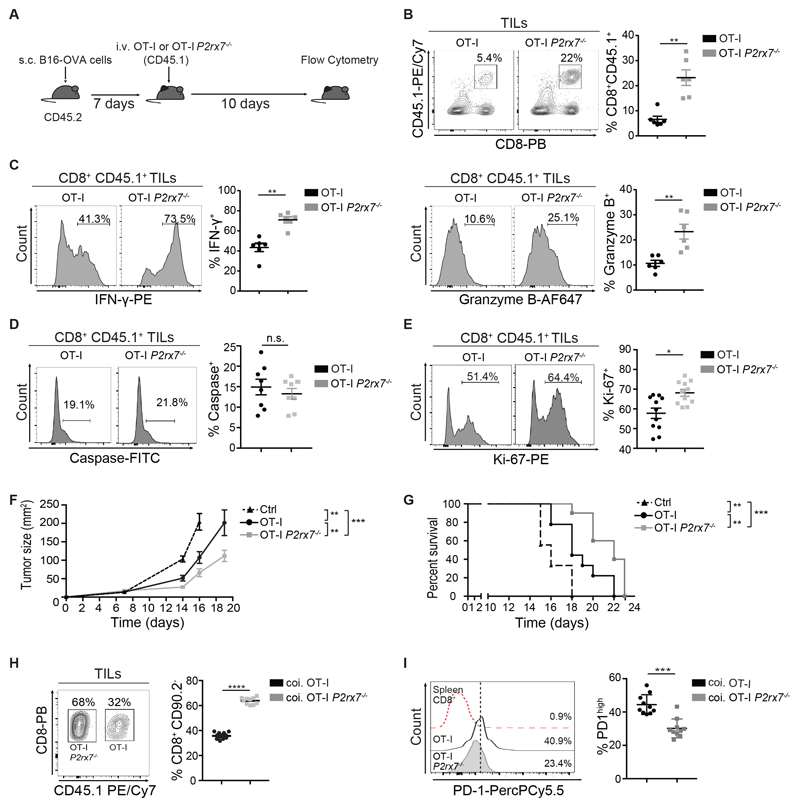
Adoptive transfer of in vitro-primed congenic Rag1-/-/P2rx7+/+ (OT-I) or Rag1-/-/P2rx7-/- (OT-I P2rx7-/-) OT-I TCR transgenic CD8+ cells specific for chicken ovalbumin peptide 257-264 presented by H2Kb, into WT mice bearing B16-OVA tumors (Fig. 2A), resulted in increased absolute number (Fig. S2A) and percentage of tumor-infiltrating OT-I P2rx7-/- cells (Fig. 2B) that showed enhanced secretion of IFN-γ and Granzyme B with respect to OT-I cells (Fig. 2C). The increase in the percentage of P2rx7-/- cells did not impact on the frequency of endogenous CD8+ TILs (Fig. S2C).
Fig. 2. Enhanced control of B16-OVA tumor growth and mice survival by P2rx7-/- OT-I TILs.
(A-G) CD45.2+ mice were engrafted with B16-OVA cells and injected with 1 x 106 CD45.1+CD8+ Rag1-/-/P2rx7+/+ (OT-I) or Rag1-/-/P2rx7-/- (OT-I P2rx7-/-) cells, initially primed in vitro with anti-CD3/CD28 antibodies. (A) Experimental design. (B-C) Representative flow cytometry plots and statistical analysis (mean ± SEM) for CD45.1+CD8+ TILs, gated on CD3+ cells (B), IFN-γ (left) and Granzyme B (right) secretion in CD45.1+CD8+ TILs (C) (n=6). Data from one experiment representative of two. (D-E) Flow cytometry histograms and statistical analysis (mean ± SEM) for cells positive for activated caspase (n=8) (D) and Ki-67 (n=11) (E) within CD45.1+CD8+ TILs. Percentages of positive cells in the indicated gates are shown. Data from two independent experiments. (F) Kinetics of tumor growth and (G) Kaplan-Meier survival plot. One experiment representative of two (n=9 for each group). (H-I) Tumor bearing CD45.2+CD90.2+ mice were co-injected with 1 x 106 CD45.1+CD90.1+ Rag1- /-/P2rx7+/+ (OT-I) and 1 x 106 CD45.2+CD90.1+ Rag1-/-/P2rxT/ (OT-I P2rx7-/-) cells. Representative flow cytometry plot and statistical analysis (mean ± SEM) for CD90.2-CD45.1+CD8+ (OT-I) or CD90.2-CD45.1-CD8+ (OT-I P2rx7-/-) TILs (H) and PD-1hi cells within CD90.2- CD45.1+CD8+ (OT-I) or CD90.2-CD45.2+CD8+ (OT-I P2rx7-/-) TILs, and CD8+TCRβ+ cells from spleen (Spleen) (I). Percentages of positive cells in each gate are shown. Data from 2 independent experiments (n=10). Two-tailed Mann-Whitney U test for the comparison of two groups or two-way ANOVA for comparison of tumor size was used. Mantel- Cox test for analysis of survival. n.s. not significant, * p<0.05, ** p<0.01, *** p<0,001, **** p<0,0001.
We checked phosphatidylserine exposure and IFN-γ secretion before injection; no significant differences were detected between P2rx7+/+ and P2rx7-/- cells (Fig. S2, D and E). Moreover, since P2X7 activity can result in diminished recovery of particular T cell subsets during the ex vivo isolation procedure (24), we purified TILs in the presence of the selective P2X7 antagonist A-438079. We did not detect any difference in the percentages of OT-I or OT-I P2rx7-/- cells within CD8+ TILs irrespective of drug’s addition (Fig. S2F). In spite of the function of P2X7 in promoting caspase-mediated cell death (13), we found no difference in caspase activation between P2rx7+/+ and P2rx7-/- TILs (Fig. 2D). However, P2rx7-/- cells were characterized by an increased proliferative potential than WT cells, as shown by staining with Ki-67 antibodies (Fig. 2E). Tumor infiltration by OT-I P2rx7-/- cells resulted in significant inhibition of tumor growth as compared to mice transferred with OT-I cells (Fig. 2F). Importantly, the transfer of OT-I P2rx7-/- cells promoted extended survival of mice (Fig. 2G), suggesting that P2X7 limits the expansion and tumoricidal activity of TILs. Consistent with enhanced capacity of OT-I P2rx7-/- cells to accumulate in the TME, co-transfer of OT-I and OT-I P2rx7-/- cells at 1:1 ratio (Fig. S2G) into WT mice bearing B16-OVA tumors resulted in the significant dominance of OT-I P2rx7-/- TILs (Fig. 2H). Notably, OT-I P2rx7-/- cells were characterized by reduced expression of PD-1, which distinguishes dysfunctional CD8 cells (Fig. 2I), whereas other checkpoint proteins, including Tim3 and CTLA4, were not differentially expressed by co-infiltrating P2rx7+/+ and P2rx7-/- cells (Fig. S2D). These data suggest that lack of P2X7 endows TILs with improved resistance to dysfunctionality induced by the TME.
To substantiate these results within a different TME, we engrafted WT mice with OVA expressing MC-38 (MC38-OVA) colon adenocarcinoma cells and adoptively transferred OT-I or OT-I P2rx7-/- CD8+ cells (Fig. S3A). As observed in B16-OVA tumors, P2rx7-/- TILs were significantly increased with respect to P2rx7+/+ cells (Fig. S2B and S3B) and expressed lower levels of PD-1 in the plasma membrane (Fig. S3C). Moreover, mice transferred with P2rx7-/- cells showed significantly reduced tumor growth and enhanced survival (Fig. S3, D and E), suggesting P2X7 deficient OT-I cells displayed enhanced anti-tumor functionality also in the MC-38 colon adenocarcinoma conditioned microenvironment.
Enhanced expansion of P2rx7-/- CD4 TEM cells in the TME
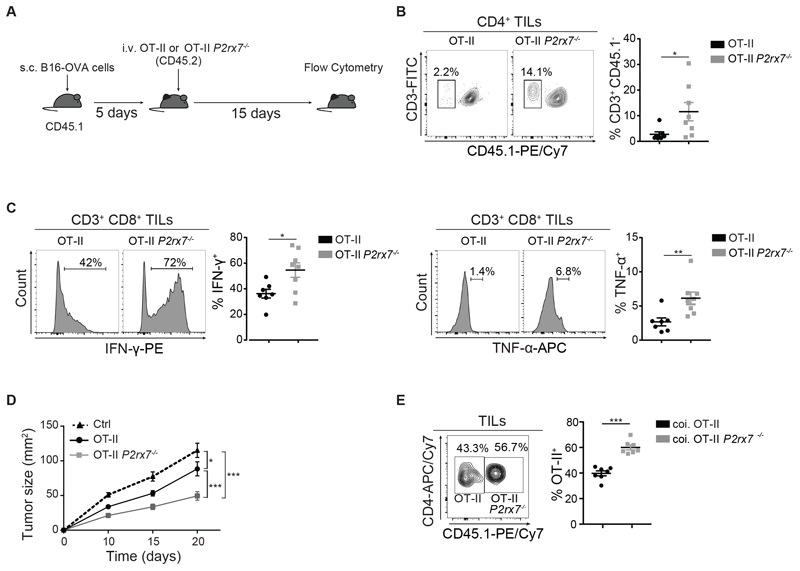
To address whether P2X7 activity could limit CD4+ T cells abundance in the TME, we adoptively transferred CD4+ naïve cells from WT or P2rx7-/- mice into Cd3ε-/- mice that were subsequently engrafted with B16F10 melanoma cells (Fig. S4A). Flow cytometry at 20 d after tumor transplant revealed a significant increase in P2rx7-/- with respect to WT TILs with TEM phenotype, but not in the spleen (Fig. S4, B and C). We did not detect differences in tumor growth between Cd3ε-/- mice either non-transferred or transferred with P2rx7-/- or WT CD4+ naïve cells, consistent with lack of CD8+ T cell mediated cytotoxicity in Cd3ε-/- mice (Fig. S4D). To mimic the effector response of CD4 cells to a tumor specific antigen in an immunocompetent organism, we adoptively transferred either Rag1-/-/P2rx7+/+ (OT-II) or Rag1-/-/P2rx7-/- (OT-II P2rx7-/-) congenic CD4+ OT-II TCR transgenic T cells specific for I-Ab restricted OVA peptide 323-339 into WT mice bearing B16-OVA tumors (Fig. 3A). As observed in mice adoptively transferred with OT-I P2rx7-/- cells, the increased proportion of OT-II P2rx7-/- cells did not influence the frequency of endogenous CD4+ TILs (Fig. S5A). As for in vitro primed CD8+ OT-I naïve cells, we checked phosphatidylserine exposure, and IFN-γ as well as IL-17 secretion before injection; no significant differences were detected between WT and P2rx7-/- cells (Fig. S5, B and C). In mouse naïve CD4 T cells, ADP-ribosylation of P2X7 by the ectoenzyme ADP-ribosyltransferase 2.2 (ARTC2.2) is responsible for nicotinamide adenine dinucleotide (NAD)-induced T cell death (NICD) (24). The analysis of OT-II T cells after in vitro activation and before injection into tumor bearing mice showed lack of ARTC2.2 expression, ruling out a possible function of ADP-rybosylation in impairing the expansion of WT with respect to P2rx7-/- cells (Fig. S5C). After 15 d, both OT-II and OT-II P2rx7-/- cells were barely detectable in the spleen (Fig. S5D), however OT-II P2rx7-/- cells were significantly increased in tumors as compared to OT-II cells (Fig. 3B). The percentage of Foxp3+ immunosuppressive T regulatory cells (Tregs) infiltrating the tumor tissue was not influenced by the expression of P2X7 in transferred Rag1-/- OT-II cells (Fig. S5E). The analysis of endogenous CD8+ TILs showed the significant increase of IFN-γ and TNF-α secreting cells in mice transferred with OT-II P2rx7-/- cells (Fig. 3C), suggesting lack of P2X7 fostered helper function to cytotoxic T cells that could contrast tumor growth more efficiently (25). Accordingly, tumor growth was significantly delayed in mice transferred with P2X7 deficient cells (Fig. 3D). Analogously to the results obtained with CD8 cells, co-transfer of OT-II and OT-II P2rx7-/- cells at 1:1 ratio (Fig. S5G) into WT mice bearing B16-OVA tumors resulted in significant dominance of P2rx7-/- over P2rx7+/+ cells (Fig. 3E), indicating that P2X7 deficient CD4 TEM cells are endowed with enhanced tumor infiltrating potential with respect to P2X7 proficient cells.
Fig. 3. P2X7 activity limits CD4+ TILs expansion in B16 melanoma.
(A-D) CD45.1+ mice were engrafted with B16-OVA cells and injected with in vitro primed 1 x 106 CD45.2+CD4+ Rag1-/-/P2rx7+/+ (OT-II) or Rag1-/-/P2rx7-/- (OT-II P2rx7-/-) cells. (A) Experimental design. (B) Representative flow cytometry plots and statistical analysis (mean ± SEM) for CD3+CD45.1- cells gated on CD4+ TILs. (C) Flow cytometry histograms and statistical analysis (mean ± SEM) for IFN-γ (left) and TNF-α (right) secretion in CD3+CD8+ TILs. Percentages of positive cells in the indicated gates are shown. (D) Kinetics of tumor growth. Data from two independent experiments (n=8). (E) Tumor bearing CD45.2+ mice were co-injected with 1 x 106 CD45.2+CD4+Vα2Vβ5.1/5.2+ Rag1 -/-/P2rx7+/+ (OT-II) and 1 x 106 CD45.1+CD4+Vα2Vβ5.1/5.2+ Rag1-/-/P2rx7-/- (OT-II P2rx7-/-) cells. Representative flow cytometry plots of CD45.1 and CD4 staining on gated Vα2Vβ5.1/5.2+ cells and statistical analysis of OT-II and OT-II P2rx7-/- TILs (mean ± SEM). Data from one experiment representative of two (n=7). Two- tailed Mann-Whitney U test for the comparison of two groups or two-way ANOVA for comparison of tumor size was used. * p<0.05, ** p<0.01, *** p<0,001.
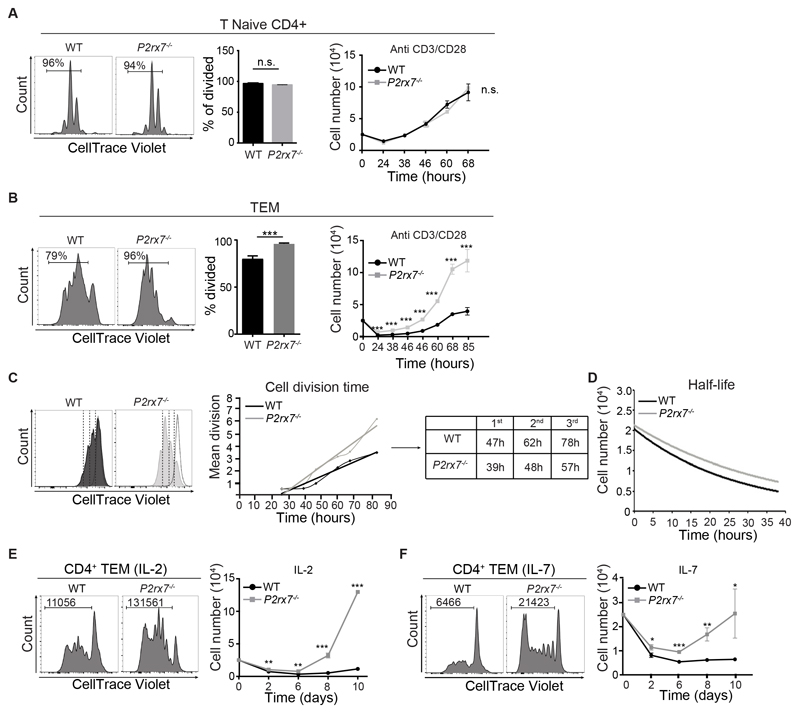
Regulated cell cycling in TEM cells by P2X7 activity
In CD4 naïve T cells, P2X receptors activation concomitantly to TCR stimulation contributes to productive T cell activation. In fact, P2X inhibition by the pharmacological antagonist periodate-oxidized ATP (oATP) in cells stimulated with anti-CD3/CD28 antibodies promotes T cell anergy (26). P2rx7-/- CD4 naïve cells did not show any difference in cell proliferation as compared to WT cells (Fig. 4A), suggesting that P2X1 and/or P2X4 could compensate for the lack of P2X7 activity, as observed in human T cells (27). In contrast, stimulation of P2rx7-/- TEM cells revealed a peculiar enhancement of cell proliferation with respect to the WT counterpart (Fig. 4B). To better address the contribution of P2X7 in regulating cell survival versus cycling, we applied a mathematical model to quantify division times and death rates within a time-course experiment in CD3/CD28 stimulated TEM cells (28). Graphical data extrapolation showed that P2rx7-/- TEM cells progressed earlier in the first cell division and required less time to enter subsequent cell divisions (Fig. 4C). At the same time, P2rx7-/- TEM cells were characterized by a reduced rate of cell death overtime as indicated by the slower exponential decay of the relative constant (Fig. 4D). These results indicate that P2rx7-/- TEM cells “perform” better than WT cells following TCR stimulation. To see whether an analogous difference could be detected following cytokine driven stimulation (without TCR engagement), we stimulated purified TEM cells with IL-2 or IL-7. In contrast to WT cells, which poorly proliferated, P2rx7-/- TEM cells effectively expanded with both IL-2 and IL-7 (Fig. 4, E and F). Altogether, these data suggest that P2X7 activity limits TEM cell proliferation.
Fig. 4. P2X7 activity inhibits proliferation of mouse TEM but not naïve cells.
(A-B) Mouse WT and P2rx7-/- CD4+ T naïve or TEM cells were stimulated with anti-CD3/CD28 antibodies. Representative CellTrace Violet dilution in WT and P2rx7-/- T naïve (A) and TEM (B) cells after 72 h stimulation and statistical analysis of divided cells. Numbers in histogram plots indicate percentages of divided cells. The graph on the right shows cell recoveries at different time points after stimulation. (C) Linear regression analysis of mean division number against harvest time.
The table on the right shows the time taken for WT and P2rx7-/- TEM cells to enter into each subsequent division. (D) Overtime analyses of TEM cells half-life. (E-F) Representative CellTrace Violet dilution in flow cytometry of WT and P2rx7-/- TEM cells after 10 days stimulation in IL-2 (E) or IL-7 (F). The absolute number of dividing cells is reported in the left corner of the histogram plot. The graph on the right shows cell recoveries at different time points after stimulation. Data are from three independent experiments with five pooled mice per sample. Statistical analysis shows means ± SD. Two-tailed Mann-Whitney U test for the comparison of two groups or Kruskall-Wallis test for the comparison of more than two groups. n.s. not significant, * p<0.05, ** p<0.01 *** p<0,001.
Transcriptional regulation of cell cycling by P2X7 in TEM but not naïve CD4 cell
To explore the transcriptional impact of P2rx7 deletion in CD4+ T naïve and TEM cells, we performed genome-wide expression profiling to compare ex vivo purified cells from WT and P2rx7-/- mice. Unsupervised principal component analysis (PCA) of gene expression levels showed that naïve cells grouped independently from the genotype, while P2rx7-/- TEM cells were clearly separated from the WT counterpart, suggesting that P2rx7 deletion substantially influences gene transcription in TEM but not naïve cells (Fig. S6A). Differential expression analysis resulted a transcriptional signature of 158 upregulated and 255 downregulated genes in P2rx7-/- TEM with respect to WT cells (FDR≤5% and absolute fold change≥1.5; Table S1) that discloses how P2rx7 deficiency induces in TEM cells a transcriptional pattern intermediate between naïve and WT TEM cells (Fig. S6B). Functional over-representation analysis revealed that gene sets associated to DNA replication were enriched in P2rx7-/- TEM cells (Fig. S6C), whereas TCR/cytokine signaling, apoptosis and cell cycle arrest signatures were enriched in WT TEM cells (Fig. S6D). These results suggest that lack of P2X7 might confer greater proliferation potential to TEM cells in the eATP-rich TME. The efficacy of cytotoxic T cell response in human solid tumors has been associated to the magnitude of infiltrating CD8+ cells expressing the integrin alpha E chain (CD103) together with the ATP-hydrolysing plasma membrane ectonucleoside triphosphate diphosphohydrolase-1 (CD39), suggesting that limiting P2X7 signaling in TILs could enhance the tumoricidal activity of these cells (29–31). Notably, genes over-expressed in P2rx7-/- TEM cells (Table S2) were significantly enriched in purified CD103highCD39+ TILs (Fig. S6E) as well as in non-small-cell lung cancers (NSCLCs) and skin cutaneous melanoma (SKCM) transcriptomes from patients showing significantly improved survival (Fig. S6, F and G). These data suggest that diminished P2X7 activity could promote a transcriptional program, which more effectively controls tumor progression in humans.
Induction of T cell senescence by P2X7 activation
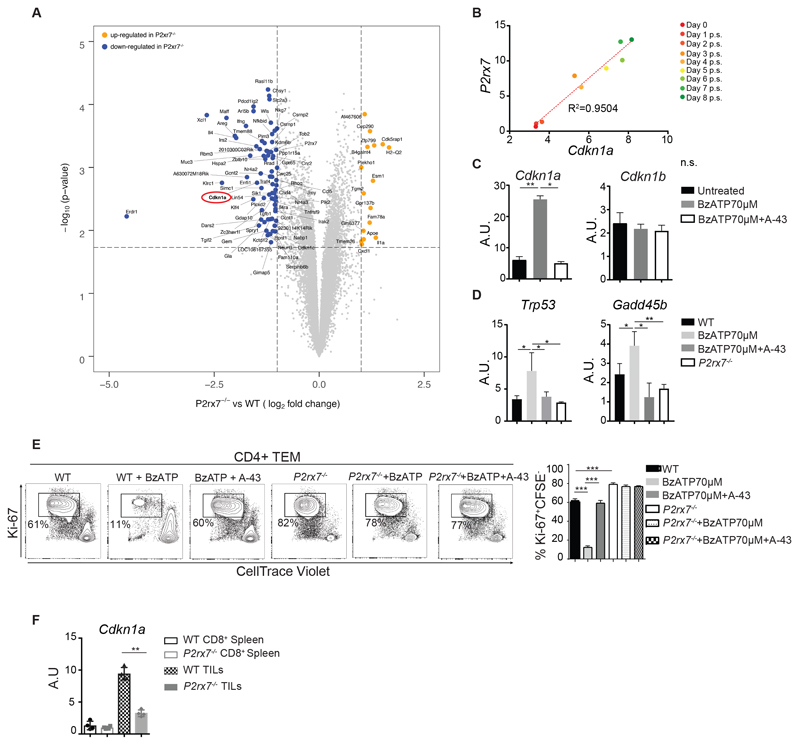
The genome-wide transcriptional analysis evidenced Cdkn1a, encoding for p21Waf1/Cip1, as one of the most down-regulated transcript in P2rx7-/- TEM cells (Fig. 5A, and Table S3). P2rx7 expression at different times after in vitro stimulation of naïve CD4+ T cells directly correlated with Cdkn1a, suggesting signaling by P2X7 positively regulated Cdkn1a transcription (Fig. 5B). Accordingly, qRT-PCR on purified TEM cells stimulated with BzATP alone or together with A-438079 confirmed that signaling by P2X7 induced Cdkn1a; this induction was selective and did not affect Cdkn1b transcription (Fig. 5C). Coherently with gene array data and the function of P2X7 in regulating cell cycling activity, the transcription of Trp53 and Gadd45b genes was also induced by BzATP (Fig. 5D). Moreover, addition of BzATP to in vitro stimulated WT CD4+ TEM cells with anti-CD3/CD28 antibodies resulted in reduced Ki-67 staining and CellTrace Violet dilution, indicating inhibition of cell cycling activity. This effect was abrogated by A-438079 (Fig. 5E). BzATP showed a dose-dependent effect on cell proliferation that was observed also in CD8+ WT TEM cells albeit with higher doses of BzATP (Fig. S7, A and B) and Cdkn1a was upregulated in vitro by BzATP (Fig. S7C). Notably, Cdkn1a transcript levels were significantly increased in WT as compared to P2rx7-/- CD8+ TILs (Fig. 5F).
Fig. 5. P2X7 stimulation in TEM cells induces cell cycle arrest.
(A) Volcano plot showing the most significantly differentially expressed genes in the comparison of ex vivo purified CD4+ TEM cells from P2rx7-/- (n = 3 independent samples) and WT (n = 3 independent samples) mice. Blue and yellow dots indicate down- and up-regulated genes in ex vivo P2rx7-/- CD4+ TEM cells (absolute fold change ≥2 and FDR ≤5%; table S3). P- values on the y axis were derived from Significance Analysis of Microarray (see Methods). Unadjusted P values are shown. (B) Correlation between Cdkn1a and P2rx7 transcript levels in mouse CD4+ T cells at different times post stimulation (p.s.) with anti-CD3 and anti-CD28 antibodies. (C-D) WT and P2rx7-/- CD4+ TEM cells were stimulated in vitro with BzATP for 24 h in the presence of the P2X7 inhibitor A-438079 (A-43) 50 μM where indicated. Data are from three independent experiments with five pooled mice per sample. (C) qRT-PCR of Cdkn1a (left) and Cdkn1b (right) transcripts. (D) qRT-PCR of Trp53 (left) and Gadd45b (right) transcripts. (E) WT and P2rx7-/- CD4+ TEM cells were stimulated with anti- CD3/CD28 antibodies for 72 h in presence of BzATP and A-43 50 μM where indicated. Representative flow cytometry plots for CellTrace Violet dilution and Ki-67 staining. Percentages of cycling Ki-67+ cells in the displayed gate and relative statistics are shown. Data are from three independent experiments with five pooled mice per sample. (F) qRT-PCR of Cdkn1a in CD8+ splenocytes and TILs from Cd3ε-/- mice adoptively transferred with WT or P2rx7-/- CD8+ T naive cells and subsequently engrafted with B16-OVA cells (n=4). Data from one experiment representative of two. A.U., Arbitrary Unit. Statistical analysis shows means ± SD. Two-tailed Mann-Whitney U test for the comparison of two groups or Kruskall-Wallis test for the comparison of more than two groups. n.s. not significant, * p<0.05, ** p<0.01 *** p<0,001.
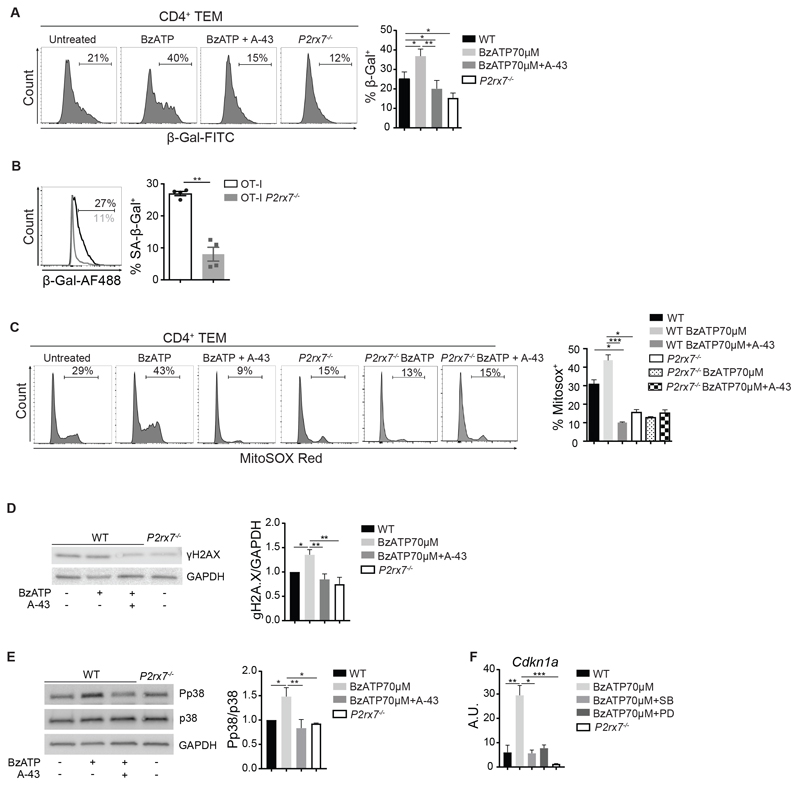
Cdkn1a is a well-characterized inhibitor of cell cycle progression and its activation by stress-induced p53 contributes to the onset of cellular senescence (32). To address whether P2X7 activity promoted TEM cells senescence, we stimulated ex vivo purified WT and P2rx7-/- TEM cells with anti-CD3/28 antibodies for 72h and analysed senescence-associated (SA) β-galactosidase (SA-β-gal). We observed reduced basal levels in P2rx7-/- cells and addition of BzATP to the culture of WT cells resulted in increase in SA-β-gal, suggesting P2X7 stimulation in TEM cells by eATP induces cellular senescence (Fig. 6A). Consistent with occurrence of the same phenomenon by P2X7 stimulation in effector T cells infiltrating the TME, SA-β-gal+ cells were significantly increased among OT-I with respect to OT-I P2rx7-/- TILs (Fig. 6B). P2X7 activity in T cells was associated with enhanced generation of mitochondrial ROS (33). MitoSOX staining of CD4 TEM cells showed an increase in mitochondrial ROS generation following BzATP stimulation and the frequency of P2rx7-/- cells stained with MitoSOX Red was significantly diminished with respect to WT cell, suggesting that lack of P2X7 resulted in reduced production of superoxide by mitochondria (Fig. 6C). ROS promote the formation of DNA damage foci that contain H2A.X histone phosphorylated at Ser139 (γH2A.X) (34). Consistent with enhanced ROS production, WT TEM cells showed increased γH2A.X histone by P2X7 stimulation in Western blot that was inhibited by A-438079 (Fig. 6D). Cellular senescence in T cells can be actively maintained by p38 MAPK signaling (35). Stimulation of P2X7 induced p38 MAPK Thr180/Tyr182 phosphorylation (Fig. 6E), while pharmacological inhibition of p38 MAPK resulted in restoring basal levels of Cdkn1a transcripts (Fig. 6F). These results suggest that in the TME, P2X7 activation in TILs promotes cell cycle arrest and p38 MAPK mediated cellular senescence (Fig. S8).
Fig. 6. P2X7 stimulation in TEM cells leads to premature senescence.
(A) Representative flow cytometry histograms and statistical analysis for SA β-galactosidase staining on ex vivo CD4+ TEM cells stimulated with anti-CD3/CD28 antibodies for 72 h in the presence of the indicated treatments. Percentages of positive cells are shown. Data are from three independent experiments with five pooled mice per sample. (B) Representative flow cytometry histograms and statistical analysis for SA β-galactosidase staining of Rag1-/-/P2rx7+/+ (OT-I) and Rag1-/- /P2rx7-/- (OT-I P2rx7-/-) TILs from B16-OVA tumor bearing WT mice. Cells were gated as CD8+CD45.1+. Percentages of positive cells are shown (n=4). Data from one experiment representative of two. (C-E) Ex vivo CD4+ TEM cells were stimulated with anti-CD3/CD28 antibodies for 72 h in the presence of the indicated treatments. Where indicated, cells were pretreated with A-438079 (A-43) 50 μM for 30 min. Data are from three independent experiments with five pooled mice per sample. (C) Representative flow cytometry histograms and statistical analysis of WT and P2rx7-/- CD4+TEM cells labelled with MitoSOX Red. Numbers in graphs indicate the percentage of positive cells. (D) Western blot on protein extracts from WT and P2rx7-/- CD4+ TEM cells. Membranes were probed for histone H2AX phosphorylated at serine 139 (γ-H2Ax) and GAPDH. Histograms show the statistical analysis of densitometric values of γ-H2Ax normalized on GADPH expression. (E) Western blot for p38 MAPK phosphorylation at Thr180/Tyr182 (Pp38), total p38 protein and GAPDH. Histograms show the statistical analysis of densitometric values of Pp38 normalized on total p38. (F) qRT-PCR of Cdkn1a transcript. WT and P2rx7-/- CD4+ TEM cells were stimulated in vitro with BzATP for 24 h in the presence of the p38 MAPK inhibitors SB-239063 (SB) 5 μM or PD-169316 (PD) 5 μM where indicated. Data are from three independent experiments with five pooled mice per sample. A.U., Arbitrary Unit. Statistical analysis shows means ± SD. Two-tailed Mann-Whitney U test for the comparison of two groups or Kruskall-Wallis test for the comparison of more than two groups. n.s. not significant, * p<0.05, ** p<0.01 *** p<0,001.
Regulated cell cycling in human TEM cells by P2X7 activity
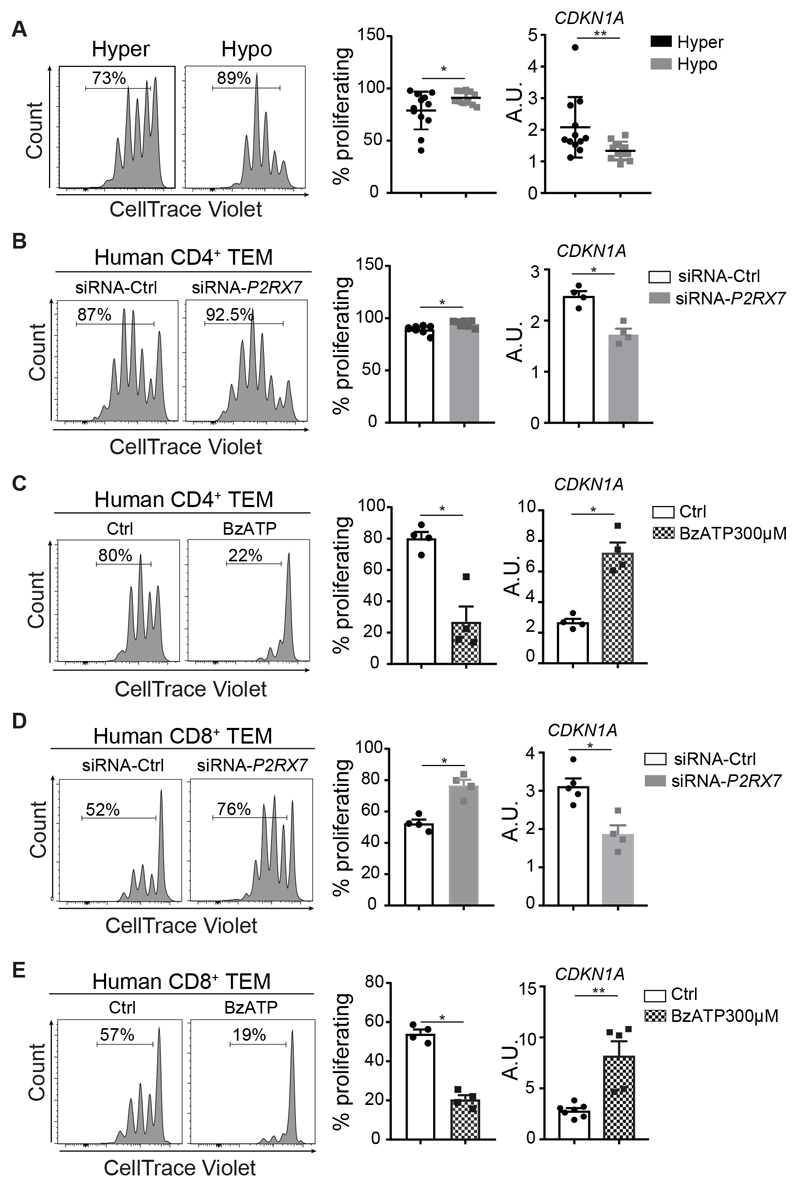
In humans, P2RX7 gene polymorphisms generate functionally different P2X7 isoforms. To address possible differences in the regulation of cell cycling by P2X7 activity in human TEM cells, we genotyped a large survey of the general population of the northern area of Milan (n= 2.606, see Supplementary Materials and Methods) (36,37) for two variants in the P2RX7 locus: the rs11065464, g.36458C>A intron variant, associated with loss-of-function P2X7 pore forming (38) and the rs1718119 Ala348Thr missense variant, determining increase in receptor activity (39). Analogously to murine P2rx7-/- cells, human TEM cells bearing the hypomorphic P2X7 variant rs11065464 showed a significant increase in the frequency of proliferating cells after TCR stimulation compared to cells purified from subjects carrying the hyperactive rs1718119 SNP variant (Fig. 7A). The impaired progression in the cell cycle of cells bearing hyperactive P2X7 correlated with increased levels of CDKN1A transcripts (Fig. 7A). Knockdown of P2RX7 in human CD4 or CD8 TEM cells (Fig. S9A) resulted in the increase of cycling cells after TCR stimulation with concomitant reduction of CDKN1A expression (Fig. 7, B and D). The function of P2X7 in conditioning human TEM cells proliferation was confirmed by addition of BzATP during TCR stimulation that blocked cell cycle progression and upregulated CDKN1A (Fig. 7, C and E, and Fig. S9B). These results indicate that P2X7 activity limits the expansion potential of human TEM cells.
Fig. 7. P2X7 activity controls CDKN1A transcription and proliferation in human TEM cells.
(A) TEM cells from human subjects expressing the P2RX7 hypermorphic (hyper) (n=12) or hypomorphic (hypo) (n=11) gene variant were stimulated with anti-CD3/CD28 antibodies for 72 h. Left: representative histograms of CellTrace Violet dilution and statistical analysis of proliferating cells. Numbers in the histogram plots represent the percentage of proliferating cells. Right: qRT-PCR for CDKN1A transcript on ex vivo TEM cells from the same subjects. (B,D) Left: representative CellTrace Violet dilution in human CD4+ (B) or CD8+ (D) TEM cells transfected with siRNA-Ctrl or siRNA-P2RX7 and stimulated with anti-CD3/CD28 antibodies for 96 h. Numbers in the plots represent the percentage of proliferating cells. Right: qRT-PCR for CDKN1A transcript on the same cells. (C,E) Left: representative CellTrace Violet dilution in CD4+ (C) and CD8+ (E) TEM cells stimulated with anti-CD3/CD28 antibodies for 96 h either without (Ctrl) or with BzATP 300 μM and statistical analysis of proliferating cells. Numbers in the plots represent the percentage of proliferating cells. Right: qRT-PCR for CDKN1A transcript on same cells. The two experimental groups in panels B-E were generated from 4 to 7 independent donors and mean ± SEM are shown. A.U., Arbitrary Unit. Two-tailed Mann-Whitney U test for the comparison of two groups or Kruskall-Wallis test for the comparison of more than two groups. In panel A, samples are normally distributed and Student’s unpaired t test was used. * p<0.05, ** p<0.01.
Discussion
T cells are potent effectors in controlling tumor growth; in fact, the extent of tumor infiltration by T cells has been generally considered a good prognostic marker in a number of tumor types (40). However, the peculiar nature of the TME results in a T cell response that is not proficient in controlling tumor growth. In melanoma patients, early effector T cells progress to a highly proliferating dysfunctional state (41). The composition of distinct dysfunctional CD8+ subsets within TILs differentially influences the control of tumor growth and sensitivity to checkpoint blockade (42). Antigen recognition within the TME is hypothesized to be important in driving the expansion of dysfunctional cells and in fact, elegant experiments in different tumors have shown that cytotoxicity is confined to non-tumor specific bystander cells that infiltrate the TME (43,44). In some cases, bystander cells dominated the pool of infiltrating CD8 cells, suggesting that the tumoricidal response would benefit from improving the quality of tumor-specific T cells (43). T cells transduced with chimeric antigen receptors (CARs) can mediate specific destruction of hematological malignancies and yield durable therapeutic responses (45). However, in solid tumors, the induction of a dysfunctional state and loss of T cell effector function by chronic antigen stimulation limit the efficacy of this immunotherapeutic approach (46–48).
The three nuclear receptor (NR) transcription factors 4A (NR4A), together with nuclear factor of activated T cells (NFAT), were shown to play an important role in controlling the cell-intrinsic program of CD8+ TILs hyporesponsiveness. The NFAT-NR4A axis controls the expression of multiple inhibitory receptors; CAR T cells with deletion of the three Nr4a genes promote regression of solid tumors and prolonged survival, thereby suggesting NR4A inhibition could constitute a promising strategy in cancer immunotherapy (49). Interestingly, Nr4a2 and Nr4a3 were downregulated in P2rx7-/- TEM cells, suggesting a possible contribution of P2X7 activity to this negative regulatory pathway in TILs. The P2X7 receptor plays pleiotropic roles in shaping T cell function. In Tfh cells it triggers caspase activation and pyroptosis, a mechanism that limits the expansion of pathogenic CD4 cells in systemic lupus erythematosus (13). An analogous signaling is likely responsible of cell death induced by bacteria derived ATP in Tfh cells in the Peyer’s patches of the small intestine to ensure controlled generation of T cell dependent secretory IgA and host-microbiota mutualism (50). Among downregulated genes between WT and P2rx7-/- TEM cells, we identified erythroid differentiation regulator 1 (Erdr1), which encodes for a secreted protein that induce Fas-dependent T cell apoptosis, suggesting P2X7 activity can promote T cell death also by this signaling pathway (51). Importantly, acute TCR stimulation of Tfh cells robustly downregulates P2rx7 expression, thus protecting antigen responding T cell from cell death (50). Similar results have been obtained in tissue resident memory T cells, suggesting that selective downregulation of P2rx7 in T cells that productively respond to cognate antigen would ensure the amplification of pathogen-destructing cells during infections (52). In contrast, P2X7 activity is required for the establishment and maintenance of long-lived central and tissue-resident memory CD8 T cells in mice, probably reflecting the function of P2X7 as ion channel in promoting mitochondrial function and metabolic fitness (53). Herein, we have shown that P2X7 stimulation in tumor-specific T cells within the TME results in stress-induced premature senescence (SIPS) that limits the expansion of tumoricidal cells. Differently from replicative senescence, SIPS is induced after exposure to outside factors that act as cellular stressor (54). For example, Treg cells can force both CD4+ and CD8+ effector T cells into SIPS by metabolic competition that causes DNA damage (55). Likewise, P2X7 activity in T cells evokes a similar signaling signature characterized by MAPK-P38 activation, DNA damage induction, increased p21 expression and β-gal activity with concomitant inhibition of T cell proliferation. In vitro generated data by pharmacological antagonism of P2X7 were mirrored by P2rx7-/- TILs in vivo. We hypothesize targeting of P2X7 in effector TILs might provide a rejuvenating signal able to perpetuate the tumoricidal response.
It is important to consider that stimulation of P2X7 in cells of the innate immune system and/or cancer cells by eATP can contribute to T cell priming and control of tumor growth (12). It was recently shown that enhancement of P2X7 mediated activation of NLRP3 inflammasome in myeloid cells by an anti-CD39 antibody that inhibits ectonucleotidase activity, promoted the anti-tumor response by CD8+ TILs (56). Accordingly, tumor bearing P2X7 null mice showed lack of inflammatory infiltration and accelerated tumor progression (57). Nevertheless, an opposite outcome (i.e. enhanced control of tumor growth) with enhancement of pro-inflammatory TILs was observed by treating WT mice after tumor engrafting with a P2X7 selective antagonist (23). These data show how the eATP/P2X7 axis can dramatically influence tumor progression depending on the timing and cell types it activates. Our observations are important to discriminate the possible overall effect of P2X7 inhibition in the TME versus T cell selective P2X7 inhibition that could improve the tumoricidal potential of CAR T cells or TILs expanded in vitro in immunotherapeutic approaches.
Supplementary Material
Statement of significance.
Findings suggest that the purinergic checkpoint P2X7 may be targeted to enhance T cell-mediated cancer immunotherapy and improve T effector cell accumulation in the tumor microenvironment.
Acknowledgments
We thank Sara Maffei (Institute for Research in Biomedicine, Bellinzona) for help with mice experiments, the NIH Tetramer Core Facility for providing tetramers. The work was supported by grant KFS–4110-02-2017-R of the Swiss Cancer Research, 310030_159491 and IZCNZ0-174704 of the Swiss National Science Foundation, Fondazione Gelu, Fondazione per la Ricerca sulla Trasfusione e sui Trapianti (to F. Grassi), AIRC Special Program Molecular Clinical Oncology ‘5 per mille’ grant 10016 and Italian Ministry of Education, University and Research and the National Research Council grant Italian Epigenomics Flagship Project (Epigen) (to S. Bicciato).
The work of the PLIC study was supported by grants 2015-0524 and 2015-0564 (to A.L. Catapano) and 2016-0852 (to G.D. Norata) of Fondazione Cariplo, Italy; H2020 REPROGRAM PHC-03-2015/667837-2 (to A.L. Catapano); GR-2011-02346974 of Ministry of Health, Italy (to G.D. Norata).
Footnotes
Conflict of interest disclosure statement:
The authors declare potential conflict of interest.
References
- 1.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676–80. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 2.Paucek RD, Baltimore D, Li G. The Cellular Immunotherapy Revolution: Arming the Immune System for Precision Therapy. Trends Immunol. 2019;40:292–309. doi: 10.1016/j.it.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Topalian SL, Wolchok JD, Chan TA, Mellman I, Palucka K, Banchereau J, et al. Immunotherapy: The path to win the war on cancer? Cell. 2015;161:185–6. doi: 10.1016/j.cell.2015.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–41. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho PC, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R, et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell. 2015;162:1217–28. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munn DH, Mellor AL. IDO and tolerance to tumors. Trends Mol Med. 2004;10:15–8. doi: 10.1016/j.molmed.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–79. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 8.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol. 2005;5:712–21. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 9.Eil R, Vodnala SK, Clever D, Klebanoff CA, Sukumar M, Pan JH, et al. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature. 2016;537:539–43. doi: 10.1038/nature19364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One. 2008;3:e2599. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vono M, Taccone M, Caccin P, Gallotta M, Donvito G, Falzoni S, et al. The adjuvant MF59 induces ATP release from muscle that potentiates response to vaccination. Proc Natl Acad Sci U S A. 2013;110:21095–100. doi: 10.1073/pnas.1319784110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kepp O, Loos F, Liu P, Kroemer G. Extracellular nucleosides and nucleotides as immunomodulators. Immunol Rev. 2017;280:83–92. doi: 10.1111/imr.12571. [DOI] [PubMed] [Google Scholar]
- 13.Faliti CE, Gualtierotti R, Rottoli E, Gerosa M, Perruzza L, Romagnani A, et al. P2X7 receptor restrains pathogenic Tfh cell generation in systemic lupus erythematosus. J Exp Med. 2019 doi: 10.1084/jem.20171976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatfield SM, Sitkovsky M. A2A adenosine receptor antagonists to weaken the hypoxia-HIF-1alpha driven immunosuppression and improve immunotherapies of cancer. Curr Opin Pharmacol. 2016;29:90–6. doi: 10.1016/j.coph.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burnstock G. Purinergic signalling--an overview. Novartis Found Symp. 2006;276:26–48. discussion -57, 275-81. [PubMed] [Google Scholar]
- 16.Browne LE, Compan V, Bragg L, North RA. P2X7 receptor channels allow direct permeation of nanometer-sized dyes. J Neurosci. 2013;33:3557–66. doi: 10.1523/JNEUROSCI.2235-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khadra A, Tomic M, Yan Z, Zemkova H, Sherman A, Stojilkovic SS. Dual gating mechanism and function of P2X7 receptor channels. Biophys J. 2013;104:2612–21. doi: 10.1016/j.bpj.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frascoli M, Marcandalli J, Schenk U, Grassi F. Purinergic P2X7 receptor drives T cell lineage choice and shapes peripheral gammadelta cells. J Immunol. 2012;189:174–80. doi: 10.4049/jimmunol.1101582. [DOI] [PubMed] [Google Scholar]
- 19.Klein Geltink RI, O’Sullivan D, Corrado M, Bremser A, Buck MD, Buescher JM, et al. Mitochondrial Priming by CD28. Cell. 2017;171:385–97.:e11. doi: 10.1016/j.cell.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Marchi E, Orioli E, Pegoraro A, Adinolfi E, Di Virgilio F. Detection of Extracellular ATP in the Tumor Microenvironment, Using the pmeLUC Biosensor. Methods Mol Biol. 2020;2041:183–95. doi: 10.1007/978-1-4939-9717-6_13. [DOI] [PubMed] [Google Scholar]
- 21.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–64. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 22.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–21. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Marchi E, Orioli E, Pegoraro A, Sangaletti S, Portararo P, Curti A, et al. The P2X7 receptor modulates immune cells infiltration, ectonucleotidases expression and extracellular ATP levels in the tumor microenvironment. Oncogene. 2019 doi: 10.1038/s41388-019-0684-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adriouch S, Hubert S, Pechberty S, Koch-Nolte F, Haag F, Seman M. NAD+ released during inflammation participates in T cell homeostasis by inducing ART2-mediated death of naive T cells in vivo. J Immunol. 2007;179:186–94. doi: 10.4049/jimmunol.179.1.186. [DOI] [PubMed] [Google Scholar]
- 25.Borst J, Ahrends T, Babala N, Melief CJM, Kastenmuller W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018 doi: 10.1038/s41577-018-0044-0. [DOI] [PubMed] [Google Scholar]
- 26.Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, et al. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008;1:ra6. doi: 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- 27.Woehrle T, Yip L, Elkhal A, Sumi Y, Chen Y, Yao Y, et al. Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood. 2010;116:3475–84. doi: 10.1182/blood-2010-04-277707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawkins ED, Hommel M, Turner ML, Battye FL, Markham JF, Hodgkin PD. Measuring lymphocyte proliferation, survival and differentiation using CFSE time-series data. Nat Protoc. 2007;2:2057–67. doi: 10.1038/nprot.2007.297. [DOI] [PubMed] [Google Scholar]
- 29.Duhen T, Duhen R, Montler R, Moses J, Moudgil T, de Miranda NF, et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun. 2018;9:2724. doi: 10.1038/s41467-018-05072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganesan AP, Clarke J, Wood O, Garrido-Martin EM, Chee SJ, Mellows T, et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat Immunol. 2017;18:940–50. doi: 10.1038/ni.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savas P, Virassamy B, Ye C, Salim A, Mintoff CP, Caramia F, et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat Med. 2018;24:986–93. doi: 10.1038/s41591-018-0078-7. [DOI] [PubMed] [Google Scholar]
- 32.Krenning L, Feringa FM, Shaltiel IA, van den Berg J, Medema RH. Transient activation of p53 in G2 phase is sufficient to induce senescence. Mol Cell. 2014;55:59–72. doi: 10.1016/j.molcel.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Foster JG, Carter E, Kilty I, MacKenzie AB, Ward SG. Mitochondrial superoxide generation enhances P2X7R-mediated loss of cell surface CD62L on naive human CD4+ T lymphocytes. J Immunol. 2013;190:1551–9. doi: 10.4049/jimmunol.1201510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–68. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 35.Akbar AN, Henson SM, Lanna A. Senescence of T Lymphocytes: Implications for Enhancing Human Immunity. Trends Immunol. 2016;37:866–76. doi: 10.1016/j.it.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Baragetti A, Norata GD, Sarcina C, Rastelli F, Grigore L, Garlaschelli K, et al. High density lipoprotein cholesterol levels are an independent predictor of the progression of chronic kidney disease. J Intern Med. 2013;274:252–62. doi: 10.1111/joim.12081. [DOI] [PubMed] [Google Scholar]
- 37.Norata GD, Garlaschelli K, Ongari M, Raselli S, Grigore L, Catapano AL. Effects of fractalkine receptor variants on common carotid artery intima-media thickness. Stroke. 2006;37:1558–61. doi: 10.1161/01.STR.0000221803.16897.22. [DOI] [PubMed] [Google Scholar]
- 38.Todd JN, Poon W, Lyssenko V, Groop L, Nichols B, Wilmot M, et al. Variation in glucose homeostasis traits associated with P2RX7 polymorphisms in mice and humans. J Clin Endocrinol Metab. 2015;100:E688-96. doi: 10.1210/jc.2014-4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stokes L, Fuller SJ, Sluyter R, Skarratt KK, Gu BJ, Wiley JS. Two haplotypes of the P2X(7) receptor containing the Ala-348 to Thr polymorphism exhibit a gain-of-function effect and enhanced interleukin-1beta secretion. FASEB J. 2010;24:2916–27. doi: 10.1096/fj.09-150862. [DOI] [PubMed] [Google Scholar]
- 40.Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013;39:61–73. doi: 10.1016/j.immuni.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H, van der Leun AM, Yofe I, Lubling Y, Gelbard-Solodkin D, van Akkooi ACJ, et al. Dysfunctional CD8 T Cells Form a Proliferative, Dynamically Regulated Compartment within Human Melanoma. Cell. 2019;176:775–89.:e18. doi: 10.1016/j.cell.2018.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller BC, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, et al. Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol. 2019;20:326–36. doi: 10.1038/s41590-019-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheper W, Kelderman S, Fanchi LF, Linnemann C, Bendle G, de Rooij MAJ, et al. Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat Med. 2019;25:89–94. doi: 10.1038/s41591-018-0266-5. [DOI] [PubMed] [Google Scholar]
- 44.Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW, et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557:575–9. doi: 10.1038/s41586-018-0130-2. [DOI] [PubMed] [Google Scholar]
- 45.June CH, Sadelain M. Chimeric Antigen Receptor Therapy. N Engl J Med. 2018;379:64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmed N, Brawley VS, Hegde M, Robertson C, Ghazi A, Gerken C, et al. Human Epidermal Growth Factor Receptor 2 (HER2) -Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J Clin Oncol. 2015;33:1688–96. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moon EK, Wang LC, Dolfi DV, Wilson CB, Ranganathan R, Sun J, et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin Cancer Res. 2014;20:4262–73. doi: 10.1158/1078-0432.CCR-13-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017:9. doi: 10.1126/scitranslmed.aaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen J, Lopez-Moyado IF, Seo H, Lio CJ, Hempleman LJ, Sekiya T, et al. NR4A transcription factors limit CAR T cell function in solid tumours. Nature. 2019;567:530–4. doi: 10.1038/s41586-019-0985-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Proietti M, Cornacchione V, Rezzonico Jost T, Romagnani A, Faliti CE, Perruzza L, et al. ATP-gated ionotropic P2X7 receptor controls follicular T helper cell numbers in Peyer’s patches to promote host-microbiota mutualism. Immunity. 2014;41:789–801. doi: 10.1016/j.immuni.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 51.Soto R, Petersen C, Novis CL, Kubinak JL, Bell R, Stephens WZ, et al. Microbiota promotes systemic T-cell survival through suppression of an apoptotic factor. Proc Natl Acad Sci U S A. 2017;114:5497–502. doi: 10.1073/pnas.1619336114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stark R, Wesselink TH, Behr FM, Kragten NAM, Arens R, Koch-Nolte F, et al. T RM maintenance is regulated by tissue damage via P2RX7. Sci Immunol. 2018:3. doi: 10.1126/sciimmunol.aau1022. [DOI] [PubMed] [Google Scholar]
- 53.Borges da Silva H, Beura LK, Wang H, Hanse EA, Gore R, Scott MC, et al. The purinergic receptor P2RX7 directs metabolic fitness of long-lived memory CD8(+) T cells. Nature. 2018;559:264–8. doi: 10.1038/s41586-018-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ott C, Jung T, Grune T, Höhn A. SIPS as a model to study age-related changes in proteolysis and aggregate formation. Mech Ageing Dev. 2018;170:72–81. doi: 10.1016/j.mad.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 55.Liu X, Mo W, Ye J, Li L, Zhang Y, Hsueh EC, et al. Regulatory T cells trigger effector T cell DNA damage and senescence caused by metabolic competition. Nat Commun. 2018;9:249. doi: 10.1038/s41467-017-02689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li XY, Moesta AK, Xiao C, Nakamura K, Casey M, Zhang H, et al. Targeting CD39 in Cancer Reveals an Extracellular ATP- and Inflammasome-Driven Tumor Immunity. Cancer Discov. 2019;9:1754–73. doi: 10.1158/2159-8290.CD-19-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adinolfi E, Capece M, Franceschini A, Falzoni S, Giuliani AL, Rotondo A, et al. Accelerated tumor progression in mice lacking the ATP receptor P2X7. Cancer Res. 2015;75:635–44. doi: 10.1158/0008-5472.CAN-14-1259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.