Abstract
The muscular dystrophies are a heterogeneous group of inherited myopathies characterised by the progressive wasting of skeletal muscle tissue. Pericytes have been shown to make muscle in vitro and to contribute to skeletal muscle regeneration in several animal models, although recent data has shown this to be controversial. In fact, some pericyte subpopulations have been shown to contribute to fibrosis and adipose deposition in muscle. In this chapter we explore the identity and the multifaceted role of pericytes in dystrophic muscle, potential therapeutic applications and the current need to overcome the hurdles of characterisation (both to identify pericyte subpopulations and track cell fate), to prevent deleterious differentiation towards myogenic-inhibiting subpopulations, and to improve cell proliferation and engraftment efficacy.
Keywords: pericytes, muscular dystrophy, muscle, stem cells, cell therapy, muscle regeneration, adipogenesis, chondrogenesis, fibrosis, plasticity
1. Skeletal Muscle and Muscular dystrophies
Skeletal muscle is the most abundant tissue in humans and its main roles are to generate movement, support soft tissues, maintain posture, and contribute to energy metabolism and temperature control (Frontera and Ochala, 2015). It is characterised by a well-defined structure of connective tissues and muscle fibres (or myofibres), which are multinucleated, post-mitotic syncytial cells containing contractile units named sarcomeres. During skeletal muscle histogenesis, muscle fibres are generated by the fusion of paired-box transcription factor 3- (Pax3) and Pax7-expressing mesodermal progenitors (Bentzinger et al., 2012; Buckingham, 2006; Comai and Tajbakhsh, 2014). After birth, they grow in size thanks to the fusion of satellite cells (Yablonka-Reuveni, 2011; Yin et al., 2013), a population of muscle stem cells located between the plasma membrane of myofibres (sarcolemma) and the basal lamina, that are responsible for growth, repair, and regeneration of adult skeletal muscle (Mauro, 1961; Relaix and Zammit, 2012). Satellite cells are quiescent in physiological conditions but can be activated after muscle injury or by specific signalling pathways (Dumont et al., 2015; Relaix and Zammit, 2012; Verdijk et al., 2014; Yin et al., 2013). Once activated, they proliferate and the majority of them differentiate along the myogenic programme in order to replace damaged muscle fibres. Alternatively, they undergo self-renewal to replenish the stem cell pool (Rocheteau et al., 2012; Zammit et al., 2004). Satellite cells are characterised by the expression of Pax7, which is SC-specific marker in skeletal muscle. Many also express caveolin-1, integrin-α7, M-cadherin, CD56/NCAM, CD29/integrin-β1 and syndecans 3 and 4, although differences in expression patterns are observed between species, location and activation stage [reviewed in detail in (Boldrin et al., 2010; Tedesco et al., 2010; Tedesco et al., 2017; Yin et al., 2013)]. Satellite cells and their derived myoblast progeny are considered the main muscle stem cells, required for complete myogenic regeneration [reviewed in (Relaix and Zammit, 2012; Zammit et al., 2006)]. In the last two decades, several muscle and non-muscle stem/progenitor cells with variable myogenic potencies have been isolated. For comprehensive reviews on the topic please refer to (Negroni et al., 2016; Tedesco et al., 2010; Tedesco et al., 2017).
Muscular dystrophies are a clinically and genetically heterogeneous group of rare neuromuscular genetic disorders sharing common pathological features (Mercuri and Muntoni, 2013). Despite their heterogeneity in muscle wasting distribution, disease severity, inheritance, age of onset and progression rate, they are characterised by repeated cycles of skeletal muscle degeneration/regeneration, changes in myofibre size and inflammation, which ultimately results in progressive muscle wasting. In the most severe forms, muscle weakness leads to early loss of ambulation and to a premature death by cardiorespiratory failure (Manzur and Muntoni, 2009; Mercuri and Muntoni, 2013). Many muscular dystrophies are caused by mutations in genes coding for proteins that belong to the dystrophin-associated glycoprotein complex (DAGC) (Ervasti and Campbell, 1991). The DAGC is a multiprotein complex located at the muscle fibre membrane (sarcolemma) and provides a strong mechanical link between intracellular cytoskeleton and the extracellular matrix; it plays a pivotal role in stabilising the sarcolemma and in maintaining myofiber integrity during muscle contraction (Emery, 2002; Straub and Campbell, 1997). As a consequence, mutations disrupting the DAGC result in increased sarcolemma fragility and contraction induced-fibre damage, which in turn lead to repeated cycles of myofibre degeneration/regeneration and ultimately to the replacement of the skeletal muscle tissue with fibrotic and adipose tissues (Matsumura and Campbell, 1994; Michalak and Opas, 1997; Straub and Campbell, 1997; Worton, 1995). Other muscular dystrophies can be caused by mutations in ubiquitously expressed proteins that result in muscle pathologies, such as mutations of nuclear envelope components. Recently, nextgeneration sequencing is helping to identify new genes responsible for previously undefined muscular dystrophies (Carss et al., 2013; Hara et al., 2011; Mitsuhashi and Kang, 2012).
The most common are Duchenne (DMD), Becker (BMD) and limb-girdle (LGMD). DMD is caused by mutations in the X-linked gene that codifies for dystrophin, a rod-shaped cytoplasmic protein belonging to the DAGC (Ervasti and Campbell, 1991; Michalak and Opas, 1997; Straub et al., 1992). DMD has an early onset and a severe disease progression. Becker MD (BMD) is the milder allelic variant of DMD, which has a slower progression and later onset. LGMDs represent one of the most heterogeneous groups, which are further subclassified according to the genetic defect responsible for the individual forms and inheritance (Emery, 2002; Mercuri and Muntoni, 2013).
Although muscular dystrophies are often fatal diseases for which no cure currently exists, many therapeutic strategies are being developed and tested in basic, pre-clinical and clinical studies [reviewed in (Benedetti et al., 2013; Bengtsson et al., 2016; Lin and Wang, 2018; Negroni et al., 2016; Pini et al., 2017; Scoto et al., 2018)].
2. Skeletal Muscle Pericytes
2a. Pericyte ontogeny
Pericytes are an heterogeneous population of contractile mural cells that surround and support blood vessels in all vascularised tissues (Hirschi and D'Amore, 1996). They were thought to be exclusively associated with the microvasculature, but evidence supports their presence also on higher order vessels (Campagnolo et al., 2010), except the lymphatic vessels (Norrmen et al., 2011). Pericytes can be defined and distinguished from other perivascular cells, such as smooth muscle cells, by a combination of criteria including anatomical location, morphology and gene/protein expression pattern (Armulik et al., 2011). Notably, pericytes can be distinguished from other endothelial-associated perivascular cells by their location embedded within the vascular basement membrane (Sims, 1986).
Despite pericytes being observed and described for the first time more than a century ago (Eberth, 1871; Rouget, 1873; Zimmermann, 1923), fundamental questions about their origin and functions remain partially unanswered. This is mainly due to the struggle in identifying a common pericyte ancestor. Numerous lineage-tracing experiments have shown that during embryogenesis, pericytes from different tissues originate from diverse sources, so that as a result a single vessel may be composed by pericytes of diverse developmental origins (Majesky, 2007; Majesky et al., 2011). Recently, lineage-tracing studies have shown even more diversity, with subsets of pericytes with hematopoietic (Yamazaki et al., 2017) and macrophage (Prazeres et al., 2018) origin.
Interestingly, pericytes in the aorta appear to have multiple developmental sources (Majesky, 2007; Majesky et al., 2011), adding an additional layer of complexity to the pericyte's ontogeny debate (Birbrair et al., 2017; Dias Moura Prazeres et al., 2017). This hints towards the idea that instead of having a common ancestor, pericytes share a mural precursor with the vascular smooth muscle cells (VSMCs) of the tissue in which they reside (Armulik et al., 2011; Majesky et al., 2011). This might explain the relative heterogeneity of pericytes derived from different tissues. Although undetermined in most organs, there is some evidence of this phenomenon in brain pericyte lineage tracing (Etchevers et al., 2001). In vitro studies using pluripotent stem cells (PSCs) have also alluded to a common mural progenitor (Kumar et al., 2017). While the source of pericytes within many organs has been established, the developmental origin of pericytes in skeletal muscle remains elusive.
2b. Pericyte plasticity
Pericytes have several common functions regardless their tissue of origin, namely blood vessel stabilisation and permeability, vascular development/maturation and regulation of blood flow (Armulik et al., 2011; Enge et al., 2002; Hall et al., 2014; Hellstrom et al., 2001; Leveen et al., 1994; Lindahl et al., 1997; Pallone and Silldorff, 2001; Pallone et al., 1998; Peppiatt et al., 2006; Soriano, 1994). In addition, in the last decade many studies have identified pericytes as tissue-resident progenitors able to form multiple human tissues (Dellavalle et al., 2011; Sacchetti et al., 2016). A recent study by Evans and colleagues challenged this view, showing that Tbx18+ mouse pericytes maintain their mural identity and do not generate other cell types in injured and aging tissues, including brain, heart, fat and skeletal muscle (Guimaraes-Camboa et al., 2017). This suggests that plasticity seen in vitro or after transplantation could be an artefact of ex vivo cell culture. The discrepancy between this data and previous studies suggests that mural cells can behave as progenitors but that this behaviour is dependent on the organ and on the developmental stage. Alternatively, it may be that a small population of pericytes with progenitor capabilities do not express Tbx18, and hence were not labelled in the Tbx18-cre strain. This could be possibly due to the heterogeneous nature of pericytes. The model of endogenous pericytes as tissueresident progenitors might therefore need further investigation, perhaps using additional or alternative pericyte lineage-tracing tools (Cano et al., 2017).
Beside their role in supporting the microvasculature and their putative role as tissue progenitors, pericytes can also display tissue-dependent functions [reviewed in (Holm et al., 2018)]. For example, brain pericytes support the blood-brain barrier integrity (Al Ahmad et al., 2011; Armulik et al., 2010; Daneman et al., 2010; Dohgu et al., 2005; Nakagawa et al., 2007) while in the immune system they contribute to the regulation of lymphocyte activation (Balabanov et al., 1999; Fabry et al., 1993; Tu et al., 2011; Verbeek et al., 1995). In skeletal muscle, pericytes contribute to muscle regeneration, fibrosis, fat deposition and ossification [reviewed in (Birbrair et al., 2015; Murray et al., 2017)]. We shall detail later in this chapter the role of pericytes in these processes in the specific context of muscular dystrophies. Generally, pericytes and their associated blood vessels run parallel to muscle fibres, where cross talk is thought to regulate nutrient uptake and postnatal myogenesis. Early studies suggest that pericyte location in capillary vessels of skeletal muscle is fibre type-specific (Gaudio et al., 1985; Levy et al., 2001) and that specific subset of pericytes within skeletal muscle do have distinct roles (Birbrair et al., 2013b). Interestingly, in contrast to the widely excepted view, there is no clear evidence that pericytes can actively alter blood flow in skeletal muscles [reviewed in (Murray et al., 2017; Sims, 1986)].
2c. Molecular signature and skeletal muscle-specific pericyte subpopulations
As mentioned above, despite their fundamental roles in health and disease and their ubiquitous presence in all body's tissues and organs, pericytes' identification is made difficult by their heterogeneity, which not only concerns origin and distribution but also the pattern and dynamic of molecular markers they do express (Armulik et al., 2011). In general it can be said that i) none of the pericyte markers are specific; ii) not all pericytes do express all the markers at once; iii) pericytes from different tissues express different markers; iv) marker expression is determined by the developmental and activation stage (Armulik et al., 2011). Although efforts are being made to characterise skeletal muscle pericytes, many putative markers overlap with other muscle cells and there is no single all-encompassing pericyte-specific marker in skeletal muscle. For this reason and as for other tissues, skeletal muscle pericytes are often identified as much by their anatomical location as by the expression of a pool of molecular markers/proteins [reviewed in (Tedesco et al., 2017)]. However, it is worth mentioning that in sites of active angiogenesis or disorganised tissue, such as dystrophic muscle, it can be difficult to determine which cells are located within the vascular basement membrane and therefore to define the exact location of cells expressing pericyte markers. In addition, the mechanisms regulating pericyte quiescence, activation and their transition between these two states are still unknown, as most studies have focussed on homing factors or determining final fate.
Some of the most common markers used for pericytes are neural-glia antigen 2 (NG2), platelet derived growth factor receptor β (PDGFRβ), smooth muscle α-actin (α-SMA), desmin, CD13, regulator of G protein signalling 5 (RGS5), CD146 and Nestin (Armulik et al., 2011; Birbrair et al., 2011; Tedesco et al., 2017). None of these markers are unique for pericytes. In the skeletal muscle for example, most proteins are expressed in both satellite cells and a subset of muscle pericytes, including Pax3 (Dellavalle et al., 2007; Sacchetti et al., 2016), which may be due to a shared developmental origin (Esner et al., 2006), and Nestin (Birbrair et al., 2011; Day et al., 2007). A subpopulation of non-myogenic muscle pericytes also share the expression of PDGFRα with fibro/adipogenic progenitors (FAPs), a PDGFRα+/CD34+and stem cell antigen-1 (Sca1)+ muscle interstitial cell population able to differentiate into myofibroblasts and/or adipose cells (Joe et al., 2010; Uezumi et al., 2010).
Fate-tracing experiments in mice have revealed that a subpopulation of muscle pericytes can have myogenic fate. This includes a subpopulation expressing the alkaline phosphatase (AP), which is able to fuse with developing muscle fibres and enter the satellite cell compartment, both during postnatal development and following acute/chronic muscle injury (Dellavalle et al., 2011). Whether they become bona fide, functional satellite cells, however, still needs to be elucidated. In addition, Birbrair et al. used Nestin-GFP/NG2-DsRed double transgenic mice to demonstrate the existence of type-2 (Nestin+/NG2+) and type-1 (Nestin-/NG2+) pericytes. Both populations express the typical pericyte markers PDGFRβ and CD146 and are associated to capillaries. However, type 2 pericytes are able to form myotubes in vitro and in vivo and enter the satellite cell compartment (Birbrair et al., 2013c) while type 1 are PDGFRα+ and contribute to fat accumulation and fibrosis (Birbrair et al., 2013b; Birbrair et al., 2013c). A comparison of the AP+ and Nestin+ populations has not been made.
In humans, a subpopulation of AP+ interstitial muscle cells associated to small vessels has also been observed (Dellavalle et al., 2007). These human interstitial cells (presumed to be of pericyte origin but obtained from un-purified biopsies) were initially characterised as expressing the pericyte markers AP, desmin, PDGFRβ, vimentin, Annexin V and Integrin b1/CD29, whilst being negative for myogenic genes Pax7 and MyoD, CD31, CD34, NCAM/CD56 and CD45. However, these interstitial cells did not express all pericyte markers; expression of M-cadherin/CD146, NG2 and α-SMA was variable among different preparations (Dellavalle et al., 2007). Moreover, variable expression of NCAM/CD56 and myogenic regulatory factors in this population has been observed in a subsequent publication (Meng et al., 2011), further contributing to the evidence that this is a variable population in human muscles. In addition, other studies showed that CD146+ subendothelial cells isolated from the postnatal human skeletal muscle microvasculature have high spontaneous myogenic potential in vitro and they generate myotubes and myofibre in vivo (Sacchetti et al., 2016).
Further studies will be needed to address the relationships existing between these different subpopulations of skeletal muscle pericytes from both murine and human origin.
2d. Pericytes and satellite cells
Within skeletal muscles, satellite cells are located beneath the basement membranes of muscle fibres and are closely connected with capillary endothelial cells. As a result, a close interaction also occurs between pericytes and satellite cells.
This has led to speculation that there is cross talk between these two cell types (Christov et al., 2007; Dellavalle et al., 2011). This relationship is multi-faceted. For example, the juxta-vascular position of satellite cells is thought to enable co-ordinated angio-myogenesis (Christov et al., 2007), whilst Kostallari et al. found that pericytes directly form a niche for satellite cells, regulating their quiescence and contributing to myogenesis, through Angiopotein 1 and insulin-like growth factor 1 (IGF-1), respectively (Kostallari et al., 2015). Whilst the authors proposed that only Nestin+ type-2 pericytes were involved in these processes, future lineage-tracing experiments and selective ablation of type-1 or type-2 subtypes are required to give us a clear answer on this matter.
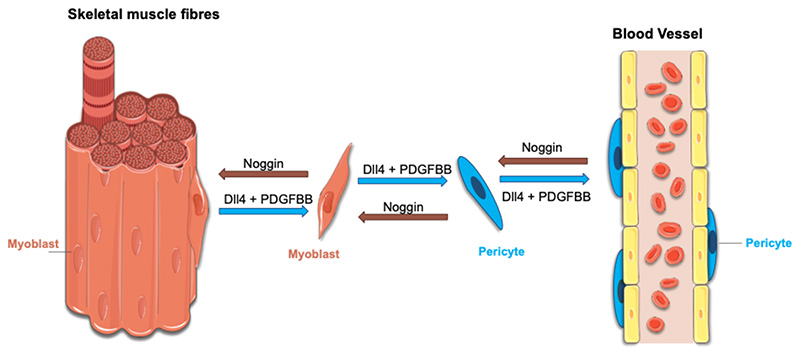
The relationship between pericytes and satellite cells/myoblasts might even be more complex that the one just described above. Several years ago, Cossu and Bianco proposed that during development, cells associated to the growing vessels might be recruited to adopt the local fate of the specific tissue they were invading. In the case of the skeletal muscle, cells associated to the blood vessels that enter the muscle anlagen might be recruited to adopt a myogenic fate and contribute to its histogenesis (Bianco and Cossu, 1999). This concept found a first and partial confirmation in the finding that the embryonic dorsal aorta contains skeletal myogenic cells, named mesoangioblasts, that co-express endothelial and myogenic markers and can contribute to muscle regeneration (De Angelis et al., 1999; Minasi et al., 2002). In vitro co-cultures of embryonic dorsal aortas and murine myotubes demonstrated that Noggin secreted from newly formed muscle fibres recruits NG2+ dorsal aorta progenitors and promotes their conversion to a myogenic fate. Conversely, myogenesis is inhibited by bone morphogenetic factor 2 (BMP2) expressed by the aorta (Ugarte et al., 2012). This data indicates that skeletal muscle and blood vessels compete to recruit mesodermal progenitor cells to a myogenic or to a perivascular fate during foetal muscle development and that the final decision of which cell fate to adopt might be due to the balance existing between Noggin and BMP2 expression. These data also suggest that a fate switch might happen also in the other direction, with skeletal myoblasts being recruited to a pericyte fate. In this direction, Cappellari et al. showed that exposure of both embryonic and foetal skeletal myoblasts to Notch Delta ligand 4 (Dll4), expressed by the developing endothelium (Kume, 2012), and PDGF-BB, which recruits pericytes from the surrounding mesenchyme (Hellstrom et al., 1999), downregulate myogenic genes, upregulate pericyte markers and bring myoblasts to assume a perivascular position when co-cultured with endothelial cells. Moreover, they showed that myoblasts occasionally adopt a perivascular position also in vivo, ruling out that the direct conversion of skeletal myoblasts into pericytes is simply an artefact of ex vivo cell manipulations (Cappellari et al., 2013). Altogether, this data suggests that the endothelium, via Dll4 and PDGF-BB expression, might induce a fate switch in adjacent skeletal myoblasts. Cossu and Cappellari postulated that the reason for this lineage promiscuity between muscle cells and perivascular cells might be explained by developmental timing and the specific need of the skeletal muscle tissue during histogenesis: for the muscle to grow, it recruits not only myoblasts but also unorthodox mesodermal cells that once exposed to muscle-specification molecules undergo myogenesis; once the muscle is grown, hypoxia is triggered with consequent vascular endothelial growth factor release and activation of angiogenesis, with developing blood vessels in the muscles recruiting supporting perivascular cells from the surrounding mesoderm (Figure 1) (Cappellari et al., 2013; Cappellari and Cossu, 2013). Recent work in our laboratory has demonstrated that this mechanism is also conserved in adult murine and human satellite cell-derived myoblasts, and that it can be exploited to enhance migration of myoblasts when transplanted (Gerli et al., 2019).
Figure. 1.
A schematic representation of the hypothetical model explaining lineage promiscuity between muscle pericytes and satellite-cell derived myoblasts during development. The figure has been generated using Servier Medical Art.
3. Pericytes contribution to muscle regeneration
3a. Pericytes as stem/progenitor cells for muscular dystrophies
Stem cell transplantation therapies for muscular dystrophies have long been touted as a method to improve clinical features. Satellite cell-derived myoblasts were initially considered the ideal candidate cell population for the cell therapy of muscular dystrophies (Partridge et al., 1989). However, successive clinical studies have revealed that although some level of dystrophin was produced, no efficacy was achieved in patients with Duchenne Muscular dystrophy (DMD), one of the most severe and common form of muscular dystrophy [reviewed in (Negroni et al., 2016; Partridge, 2000; Tedesco et al., 2010)]. While researchers tried to identify the possible culprit(s) for this result (Fan et al., 1996; Guerette et al., 1997; Huard et al., 1992; Skuk and Tremblay, 2011) and find possible therapeutic solutions (Arpke et al., 2013; Boldrin et al., 2012; Cerletti et al., 2008; Collins et al., 2005; Gilbert et al., 2010; Montarras et al., 2005; Morales et al., 2013; Palmieri et al., 2010; Rocheteau et al., 2012; Sacco et al., 2008; Skuk et al., 2006; Skuk et al., 2007; Skuk et al., 2004; Smythe et al., 2000; Tanaka et al., 2009), the search was started for alternative cell types that could be effective in cell therapy protocols for muscle. Whilst several candidate stem/progenitor populations have been identified as contributing to skeletal muscle regeneration [reviewed in (Loperfido et al., 2015; Negroni et al., 2016; Tedesco et al., 2010; Tedesco et al., 2017)], pericyte-derived cells seem to hold a preferential place.
Data obtained from different laboratories in the past years now support an important role for myogenic pericytes in skeletal muscle regeneration. As mentioned earlier in this chapter, fate-tracing of AP+ murine skeletal muscle pericytes reveals how this subset of pericytes contribute to the formation of new muscle fibres in a limb girdle muscular dystrophy (LGMD) 2D mouse model (Dellavalle et al., 2011). In human skeletal muscle, AP+ pericytes are increased in muscle biopsies of some dystrophic patients compared to healthy controls and neuropathic patients (myopathic 9.4% vs and controls 4.7% vs neuropathic 5.7%) (Diaz-Manera et al., 2012). These results are supported by another study showing an increase in the population of NG2+ pericytes in acute muscular injury (Valero et al., 2012). Conversely, we have reported a significant decrease (of approximately 55%) in the numbers and myogenic capacity of AP+ muscle pericytes both in mice and patients with LGMD2D (Tedesco et al., 2012). This apparently contrasting data could be explained by the different stage of disease progression of the biopsies/samples used in these two studies. We propose that in dystrophic muscles, during the first phase of muscle degeneration/regeneration AP+ skeletal muscle pericytes are transiently amplified to sustain the continuous need for new muscle fibres in cooperation with satellite cells and other muscle stem cells. Over time cycles of muscle degeneration/regeneration lead to an exhaustion of the pool of AP+ pericytes.
Myogenic pericyte transplantation has also been tested in pre-clinical models of muscular dystrophies, where they have the advantageous characteristic of being deliverable through the arterial circulation. These reports show active contribution of pericytes to muscle regeneration in dystrophic animal models, (Berry et al., 2007; Bonfanti et al., 2015; Dellavalle et al., 2007; Diaz-Manera et al., 2010; Domi et al., 2015; Galvez et al., 2006; Iyer et al., 2018; Minasi et al., 2002; Morosetti et al., 2011; Pessina et al., 2012; Quattrocelli et al., 2014; Sampaolesi et al., 2006; Sampaolesi et al., 2003; Sciorati et al., 2006; Tedesco et al., 2011).
As with other myogenic progenitor populations, one of the major hurdles to improve the feasibility of pericytes as a transplantation therapy for muscular dystrophy is the low level of engraftment. Recently, a first-in-human phase I/II clinical trial based upon intra-arterial transplantation of HLA-matched allogeneic pericyte-derived mesoangioblast in 5 DMD boys showed that whilst relatively safe, there was limited dystrophin production (1 of 5 biopsies), probably due to low level of cell engraftment (Cossu et al., 2015). Optimisation of this methodology is therefore required for future therapeutic use.
Lastly, Zazt and colleagues investigated the effect of repeated intra-peritoneal injections of adipose human pericytes on lifespan and motor function of a severe DMD mouse model. They reported that adipose tissue-derived pericytes led to an increased life-span of one month, possibly mediated by immune modulation rather than a regenerative ability (Valadares et al., 2014). In view of the unusual delivery route (intraperitoneal), the lack of histological evidence of engraftment or amelioration of tissue morphology and that none of the functional tests revealed differences between the groups, additional evidence would be required to assess the feasibility and clinical relevance of this strategy.
3b. Limitations to cell therapy and possible solutions
Despite numerous pre-clinical and clinical cell therapy studies on cell transplantation, muscular dystrophies still remain incurable, and there are still several challenges to be addressed before becoming routinely used in a clinical setting, including engraftment efficacy, transplantation route and modulation of the immune response [reviewed in (Maffioletti et al., 2014; Negroni et al., 2016)]. The main challenge is due to the fact that skeletal muscle is the most abundant human tissue, covering 30-38% of total body weight (Janssen et al., 2000). Transplanting stem cells has been shown to result in clinical improvement when specific muscles are affected, such as the recent trial for oculopharyngeal muscular dystrophy using intramuscular injections of autologous myoblasts (Perie et al., 2014). However, replacing large volumes of dystrophic muscle affected in widespread muscular dystrophies (such as DMD) would require the successful engraftment of billions of myogenic progenitors. Indeed, as with other myogenic progenitor populations, there are two major hurdles need to be overcome in order to improve the feasibility of pericytes and pericyte-derived cells as a transplantation therapy for muscular dystrophies: i) the low levels of cell engraftment to the dystrophic muscle and ii) the limited cell expansion potential in vitro.
In this direction, several strategies have been developed with the aim to improve homing and engraftment of pericyte-derived cells. These include making blood vessels more accessible for cell extravasation (Giannotta et al., 2014), treating them with homing factors (Quattrocelli et al., 2014) and modulating the immune response (Maffioletti et al., 2014; Noviello et al., 2014)., Exposure to cytokines and integrins can also improve pericyte-derived mesoangioblast engraftment (Galvez et al., 2006; Palumbo et al., 2004; Tagliafico et al., 2004). However, it is important to assess possible side effects of transplanting engineered cells, as whilst they can promote extravasation and homing, the expression of different surface molecules could modulate the immune response following transplantation, including deleteriously increasing donor cell clearance. The interaction between muscle or pericytes and immune cells via adhesion molecules is well-documented [reviewed in (Maffioletti et al., 2014)(Noviello et al., 2014)]. Of interest, intracellular adhesion molecule 1 (ICAM-1/ CD54) expression is increased in inflamed endothelial cells and muscle fibres (Bartoccioni et al., 1994; Tews and Goebel, 1995). However, leukocyte function-associated antigen 1 (LFA-1) expressed on T cells binds to ICAM-1, resulting in cytotoxic T cell infiltration (Bartoccioni et al., 1994). Also, expression of VCAM-1 and its ligand VLA-4 have been observed in muscle capillaries and infiltrating cells of patients with inflammatory myopathies (Tews and Goebel, 1995). Interestingly, VCAM-1 expression is associated with increased engraftment of CD133+ cells, another class of myogenic vessel-associated cells, which have been transplanted intra-arterially into dystrophic mice (Gavina et al., 2006). In this paper, Gavina and colleagues found that VCAM-1 expression in muscle capillaries increased after exercise and increased engraftment, whilst conversely, blocking VCAM-1 expression significantly reduced engraftment (Gavina et al., 2006). In summary, careful assessment of the transplant population and donor muscle tissue should be performed to maximise engraftment.
Another important factor for optimal muscle cell therapy is the ability of myogenic cells to proliferate in vitro and produce large numbers of transplantable progenitors. This point is of particular importance as in muscular dystrophies the myogenic cells (including pericytes) are exhausted or defective (Blau et al., 1983; Cassano et al., 2011; Kudryashova et al., 2012; Sacco et al., 2010; Tedesco et al., 2012). To overcome the limitation of expansion potential from biopsy-derived cells, induced pluripotent stem cells (iPSCs) can be differentiated towards the myogenic lineage. One such protocol developed by our group is to produce iPS-derived mesoangioblast-like cells, which have an unlimited proliferative potential, which could then efficiently be induced to skeletal myogenesis with a short expression of the myogenesis regulator MyoD (Gerli et al., 2014; Maffioletti et al., 2015; Tedesco et al., 2012).
Another strategy to extend the proliferative potential of pericyte-derived mesoangioblasts is to provide them with an indefinite lifespan via expression of immortalising genes. Our group has recently shown that reversibly immortalising lentiviral vectors expressing the catalytic subunit of human telomerase hTERT and the polycomb gene Bmi-1 is safe and efficacious at extending the proliferative capacity of human DMD pericyte-derived mesoangioblasts, enabling them to have a human artificial chromosome containing the whole dystrophin locus (DYS-HAC) transferred (Benedetti et al., 2018). After DYS-HAC transfer, genetically-corrected DMD pericyte-derived clones were expanded to reach a number of cells potentially sufficient to treat a DMD paediatric patient [in the range of 109cells; (Benedetti et al., 2018; Cossu et al., 2015)].
In the quest for an ideal cell type for muscle cell therapy, our group also explored a different approach by taking advantage of the findings that embryonic and foetal myoblasts could be converted to the pericyte fate following activation of Notch and PDGF pathways via Dll4 and PDGF-BB (Cappellari et al., 2013; Cappellari and Cossu, 2013). As mentioned earlier in this chapter, adult murine and human satellite cell-derived myoblasts exposed to Dll4 and PDGF-BB also acquired perivascular cell features, including transendothelial migration ability, whilst maintain myogenic capacity (Gerli et al., 2019). We propose that this strategy could generate a hybrid pericyte-myoblast cell retaining the two peculiar characteristics of both cell types: the ability to generate muscle with high efficacy (myoblast) alongside transendothelial migration capacity (pericyte-derived cells).
In conclusion, there are several promising strategies in development using pericyte-like cells for cell therapy of muscular dystrophies. Nevertheless, it is crucial that both the transplanted cell and the host environment are considered in order to improve engraftment efficacy. Therefore, it is likely that future clinical experimentation will focus on combined therapies, where stem cell transplantation is merged with another therapeutic intervention, such as administration of anti-fibrotic and pro-angiogenic drugs, which have been shown to improve pathology in mouse models of muscular dystrophy (Cordova et al., 2018; Gargioli et al., 2008).
4. Non-myogenic role of skeletal muscle pericytes
4a. Pericyte contribution to fat accumulation
Intramuscular deposition and accumulation of adipose tissue deposition is a typical hallmark of disease progression and severity in muscular dystrophies, especially in DMD (Lukjanenko et al., 2013; Mankodi et al., 2016; Wren et al., 2008). Pericytes from different tissues, including skeletal muscle, have shown adipogenic potential when cultured in vitro (Crisan et al., 2008a; Farrington-Rock et al., 2004; Minasi et al., 2002). In the skeletal muscle, a subset of quiescent cells expressing the adipogenic progenitor marker PDGFRα are closely associated to the vasculature and located in the interstitial space between muscle fibres. Following muscle injury, these cells, later called fibro/adipogenic progenitors (FAPs), exit quiescence, start proliferating and contribute to ectopic fat accumulation in skeletal muscle (Joe et al., 2010; Rodeheffer, 2010; Uezumi et al., 2010). Other groups have demonstrated that PDGFRα+ type-1 but not PDGFRα- type-2 pericytes have adipogenic potential in vitro (Birbrair et al., 2013a; Gautam et al., 2017). Moreover, cultured type-1 pericytes generated ectopic white fat when delivered intramuscularly in a mouse model of fatty degeneration (Birbrair et al., 2013a). Future lineage-tracing studies might clarify whether type-1 pericytes do indeed contribute to fat accumulation in skeletal muscle in situ. In addition, a recent lineage-tracing study by Strickland and colleagues demonstrated that PDGFRβ+ skeletal muscle pericytes were able to differentiate into perilipin+ adipocytes in a congenital muscular dystrophy model (Yao et al., 2016).
4b. Pericyte contribution to chondrogenesis
In addition to committing to a myogenic fate, pericytes have been shown to undergo chondrogenic and osteogenic differentiation in vitro (Crisan et al., 2008b; Farrington-Rock et al., 2004; James et al., 2012; Levy et al., 2001; Zhang et al., 2011). Whether pericytes contribute to skeletal muscle ossification in vivo, however, remains undetermined. Interestingly, ectopic calcification has been reported in animal models of DMD including the mdx mouse (Geissinger et al., 1990) and dog (Nguyen et al., 2002). Additionally, it has recently been shown that AP+ pericytes are reduced in immune-deficient scgb/Rag2/gc-null mice (a model of LGMD2E), whilst calcification of skeletal muscles is increased (Giovannelli et al., 2018). Unsurprisingly, this has led to the implication that pericytes are involved in the ectopic calcification of blood vessels in skeletal muscle, especially in the context of ongoing angiogenesis [reviewed in (Collett and Canfield, 2005)]. Of note, AP+ mononuclear interstitial cells from adult human skeletal muscle have been shown to express the osteogenic marker osteocalcin when cultured in vitro (Levy et al., 2001), and in fibrodysplasia ossificans progressiva (FOP), progressive ossification of skeletal muscle has been shown to be caused by mesenchymal-like stromal cells expressing smooth muscle markers (Hegyi et al., 2003). Whilst the authors postulate that these cells are pericytes, more recent data suggests it may be due to the Tie2+ FAP population (Lees-Shepard et al., 2018). Again, this points to the requirement for the differentiation between subpopulations of FAPs and pericytes to be properly determined.
4c. Pericytes and fibrosis
Increased fibrosis is a typical feature of aged and dystrophic muscles, which ultimately results in muscle weakness, atrophy and reduction of its regenerative potential (Kragstrup et al., 2011; Mann et al., 2011; Ryall et al., 2008; Thompson, 2009; Walston, 2012). A major contributor to fibrosis is the myofibroblast (Duffield et al., 2013; Humphreys et al., 2010; Lin et al., 2008; Quan et al., 2006; Willis et al., 2006; Wynn, 2008; Zeisberg et al., 2007). Myofibroblasts are responsible for the production and deposition of collagenous extracellular matrix, with consequent reduction of muscle fibre contractility, disruption of the muscle structure and eventually skeletal muscle dysfunction. Several putative myofibroblast progenitor populations have been associated to muscle fibrosis, including FAPs and cells expressing PDGFRα (Uezumi et al., 2010) and ADAM12 (Dulauroy et al., 2012). As some of these markers are also expressed by pericytes, it has been hypothesized that pericytes may be a source of myofibroblasts during skeletal muscle fibrosis. Indeed, Birbrair et al. showed that skeletal muscle PDGFRα+ pericytes are fibrogenic in vitro when cultured in presence of transforming growth factor β (TGFβ), while in vivo they produce collagen, responsible for increasing skeletal muscle fibrosis in old mice (Birbrair et al., 2014; Birbrair et al., 2013c).
In parallel, using a complex triple transgenic mouse that expressed tetracycline under ADAM12 locus, Cre recombinase under control of the tetracycline transactivator and the conditional reporter Rosa26floxSTOP-YFP, Dulauroy et al. showed the existence of a transient subpopulation of ADAM12+ interstitial cells that become active after muscle injury. With this approach they revealed that the large majority of collagen-producing myofibroblasts were generated starting from ADAM12+ cells, which are located in a perivascular position and are positive for PDGFRβ. Moreover, ablation of ADAM12+ cells reduced the number of pro-fibrotic cells and collagen accumulation (Dulauroy et al., 2012). This data corroborates the hypothesis that in skeletal muscle ADAM12 identifies a myofibroblast progenitor with pericyte characteristics.
4d. Pericyte role in the vascular compartment
Muscle ischemia has been observed in biopsies from patients with DMD for many decades (Engel, 1967) and clinical symptoms were once hypothesised to be caused by local infarctions. Whilst there have not been consistent reports in changes to blood flow or microvascular architecture observed in DMD patients [reviewed in (Thomas, 2013)], it has been shown that dystrophin-deficient muscle fibres are more susceptible to muscle ischemia though a neuronal nitric oxide synthase μ (nNOSμ)-specific mechanism. Localised muscle ischemia results in increased exercise-induced fatigue and micro-vessel constriction, elevating clinical symptoms (Kobayashi et al., 2008). This mechanism is not specific to DMD; changes to sarcolemmal nNOS expression have been observed in biopsies from other several muscular dystrophies including limb-girdle and congenital muscular dystrophy (Kobayashi et al., 2008). Whether or not pericytes actively contribute to the vascular pathology observed in muscular dystrophies, it has been shown that improving reduced blood flow improves clinical symptoms and cell therapy in pre-clinical models (Brunelli et al., 2007; Gargioli et al., 2008). Of note, treatment of dystrophic mice with nitric oxide releasing drugs improves the efficacy of mesoangioblast transplantation (Brunelli et al., 2007).
5. Derivation of pericytes from pluripotent stem cells
Pluripotent Stem Cell (PSC)-derived cells are of great importance for studying development and organogenesis, whilst also being promising candidates for cell transplantation studies, due to their unlimited expansion potential. Differentiating PSCs into pericyte-like cells enables the possibility to study developmental relationships between mural cells of different developmental origins. For example, Kumar et al. found using clonal analyses that mesodermal-derived progenitors could make mesenchymal stromal cells, VSMCs and pericytes (Kumar et al., 2017). Additionally, this protocol was used to derive arteriolar and capillary subtypes of pericytes through the modulation of growth factors. Protocols to differentiate vascular cells from different embryonic lineages (neural crest, lateral plate mesoderm and paraxial mesoderm) have also been developed in order to study different forms of vascular development (Cheung et al., 2012; Chin et al., 2018; Cochrane et al., 2018; Orlova et al., 2014). In muscle pathology, PSC-derived pericyte-like cells have shown pathological improvement in pre-clinical models of muscular dystrophy (Tedesco et al., 2012) and ischemia (Dar et al., 2012). Determining whether PSC-derived cells are truly functional pericytes when transplanted in vivo is difficult. However, using lineage determinants to produce a pericyte-like mesenchymal (or neural ectodermal) progenitor, which when transplanted differentiates into a regeneration-supporting cell, could be a more feasible option.
Concluding Remarks
Since their initial description there has been important progress in understanding pericyte biology and function; however, their exact role and involvement in the pathogenic process of muscle degeneration and regeneration is still in need of a definitive model. Nonetheless, the clinical relevance of pericyte is now more important than ever before, both as a target for possible therapeutic intervention (e.g. reduction of fibrosis) or as advanced therapy medicinal products. We foresee that in the upcoming decade the ontogeny and characteristics of this elusive cell type will become clearer, setting the foundation for their organotypic derivation from human pluripotent cells and use in next-generation experimental therapies for muscle diseases.
Acknowledgments
We thank Giulio Cossu for the critical reading of this manuscript. This research was supported by the NIHR Great Ormond Street Hospital Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the National Health Service (NHS), the National Institute for Health Research (NIHR) or the Department of Health. Work in the Tedesco laboratory is funded by the European Research Council (7591108 – HISTOID), Muscular Dystrophy UK, the UK MRC and BBSRC and the AFM-Telethon. F.S.T. is also grateful to previous funding from the European Union's 7th Framework Programme for research, technological development and demonstration under grant agreement no. 602423 (PluriMes), Fundació La Marató de TV3, IMI joint undertaking n° 115582 EBiSC (EU FP7 and EFPIA companies), Duchenne Parent Project Onlus, Takeda New Frontier Science and the NIHR (Academic Clinical Fellowship in Paediatrics ACF-2015-18-001). L.A.M. is supported by the Human Frontiers Science Program.
Bibliography
- Al Ahmad A, Taboada CB, Gassmann M, Ogunshola OO. Astrocytes and pericytes differentially modulate blood-brain barrier characteristics during development and hypoxic insult. J Cereb Blood Flow Metab. 2011;31:693–705. doi: 10.1038/jcbfm.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Arpke RW, Darabi R, Mader TL, Zhang Y, Toyama A, Lonetree CL, Nash N, Lowe DA, Perlingeiro RC, Kyba M. A new immuno-, dystrophin-deficient model, the NSG-mdx(4Cv) mouse, provides evidence for functional improvement following allogeneic satellite cell transplantation. Stem Cells. 2013;31:1611–1620. doi: 10.1002/stem.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanov R, Beaumont T, Dore-Duffy P. Role of central nervous system microvascular pericytes in activation of antigen-primed splenic T-lymphocytes. J Neurosci Res. 1999;55:578–587. doi: 10.1002/(SICI)1097-4547(19990301)55:5<578::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Bartoccioni E, Gallucci S, Scuderi F, Ricci E, Servidei S, Broccolini A, Tonali P. MHC class I, MHC class II and intercellular adhesion molecule-1 (ICAM-1) expression in inflammatory myopathies. Clin Exp Immunol. 1994;95:166–172. doi: 10.1111/j.1365-2249.1994.tb06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti S, Hoshiya H, Tedesco FS. Repair or replace? Exploiting novel gene and cell therapy strategies for muscular dystrophies. FEBS J. 2013;280:4263–4280. doi: 10.1111/febs.12178. [DOI] [PubMed] [Google Scholar]
- Benedetti S, Uno N, Hoshiya H, Ragazzi M, Ferrari G, Kazuki Y, Moyle LA, Tonlorenzi R, Lombardo A, Chaouch S, et al. Reversible immortalisation enables genetic correction of human muscle progenitors and engineering of next-generation human artificial chromosomes for Duchenne muscular dystrophy. EMBO Mol Med. 2018;10:254–275. doi: 10.15252/emmm.201607284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson NE, Seto JT, Hall JK, Chamberlain JS, Odom GL. Progress and prospects of gene therapy clinical trials for the muscular dystrophies. Hum Mol Genet. 2016;25:R9–17. doi: 10.1093/hmg/ddv420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger CF, Wang YX, Rudnicki MA. Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry SE, Liu J, Chaney EJ, Kaufman SJ. Multipotential mesoangioblast stem cell therapy in the mdx/utrn-/-mouse model for Duchenne muscular dystrophy. Regen Med. 2007;2:275–288. doi: 10.2217/17460751.2.3.275. [DOI] [PubMed] [Google Scholar]
- Bianco P, Cossu G. Uno, nessuno e centomila: searching for the identity of mesodermal progenitors. Exp Cell Res. 1999;251:257–263. doi: 10.1006/excr.1999.4592. [DOI] [PubMed] [Google Scholar]
- Birbrair A, Borges IDT, Gilson Sena IF, Almeida GG, da Silva Meirelles L, Goncalves R, Mintz A, Delbono O. How Plastic Are Pericytes? Stem Cells Dev. 2017;26:1013–1019. doi: 10.1089/scd.2017.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Wang ZM, Messi ML, Enikolopov GN, Delbono O. Nestin-GFP transgene reveals neural precursor cells in adult skeletal muscle. PLoS One. 2011;6:e16816. doi: 10.1371/journal.pone.0016816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Files DC, Mannava S, Smith T, Wang ZM, Messi ML, Mintz A, Delbono O. Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem cell research & therapy. 2014;5:122. doi: 10.1186/scrt512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev. 2013a;22:2298–2314. doi: 10.1089/scd.2012.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res. 2013b;10:67–84. doi: 10.1016/j.scr.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. Am J Physiol Cell Physiol. 2013c;305:C1098–1113. doi: 10.1152/ajpcell.00171.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Pericytes at the intersection between tissue regeneration and pathology. Clin Sci (Lond) 2015;128:81–93. doi: 10.1042/CS20140278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau HM, Webster C, Pavlath GK. Defective myoblasts identified in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 1983;80:4856–4860. doi: 10.1073/pnas.80.15.4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrin L, Muntoni F, Morgan JE. Are human and mouse satellite cells really the same? J Histochem Cytochem. 2010;58:941–955. doi: 10.1369/jhc.2010.956201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrin L, Neal A, Zammit PS, Muntoni F, Morgan JE. Donor satellite cell engraftment is significantly augmented when the host niche is preserved and endogenous satellite cells are incapacitated. Stem Cells. 2012;30:1971–1984. doi: 10.1002/stem.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti C, Rossi G, Tedesco FS, Giannotta M, Benedetti S, Tonlorenzi R, Antonini S, Marazzi G, Dejana E, Sassoon D, et al. PW1/Peg3 expression regulates key properties that determine mesoangioblast stem cell competence. Nat Commun. 2015;6:6364. doi: 10.1038/ncomms7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli S, Sciorati C, D’Antona G, Innocenzi A, Covarello D, Galvez BG, Perrotta C, Monopoli A, Sanvito F, Bottinelli R, et al. Nitric oxide release combined with nonsteroidal antiinflammatory activity prevents muscular dystrophy pathology and enhances stem cell therapy. Proc Natl Acad Sci U S A. 2007;104:264–269. doi: 10.1073/pnas.0608277104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr Opin Genet Dev. 2006;16:525–532. doi: 10.1016/j.gde.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Campagnolo P, Cesselli D, Al Haj Zen A, Beltrami AP, Krankel N, Katare R, Angelini G, Emanueli C, Madeddu P. Human adult vena saphena contains perivascular progenitor cells endowed with clonogenic and proangiogenic potential. Circulation. 2010;121:1735–1745. doi: 10.1161/CIRCULATIONAHA.109.899252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano E, Gebala V, Gerhardt H. Pericytes or Mesenchymal Stem Cells: Is That the Question? Cell Stem Cell. 2017;20:296–297. doi: 10.1016/j.stem.2017.02.005. [DOI] [PubMed] [Google Scholar]
- Cappellari O, Benedetti S, Innocenzi A, Tedesco FS, Moreno-Fortuny A, Ugarte G, Lampugnani MG, Messina G, Cossu G. Dll4 and PDGF-BB convert committed skeletal myoblasts to pericytes without erasing their myogenic memory. Dev Cell. 2013;24:586–599. doi: 10.1016/j.devcel.2013.01.022. [DOI] [PubMed] [Google Scholar]
- Cappellari O, Cossu G. Pericytes in development and pathology of skeletal muscle. Circ Res. 2013;113:341–347. doi: 10.1161/CIRCRESAHA.113.300203. [DOI] [PubMed] [Google Scholar]
- Carss KJ, Stevens E, Foley AR, Cirak S, Riemersma M, Torelli S, Hoischen A, Willer T, van Scherpenzeel M, Moore SA, et al. Mutations in GDP-mannose pyrophosphorylase B cause congenital and limb-girdle muscular dystrophies associated with hypoglycosylation of alpha-dystroglycan. Am J Hum Genet. 2013;93:29–41. doi: 10.1016/j.ajhg.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassano M, Dellavalle A, Tedesco FS, Quattrocelli M, Crippa S, Ronzoni F, Salvade A, Berardi E, Torrente Y, Cossu G, et al. Alpha sarcoglycan is required for FGF-dependent myogenic progenitor cell proliferation in vitro and in vivo. Development. 2011;138:4523–4533. doi: 10.1242/dev.070706. [DOI] [PubMed] [Google Scholar]
- Cerletti M, Jurga S, Witczak CA, Hirshman MF, Shadrach JL, Goodyear LJ, Wagers AJ. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C, Bernardo AS, Trotter MW, Pedersen RA, Sinha S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat Biotechnol. 2012;30:165–173. doi: 10.1038/nbt.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin CJ, Li S, Corselli M, Casero D, Zhu Y, He CB, Hardy R, Peault B, Crooks GM. Transcriptionally and Functionally Distinct Mesenchymal Subpopulations Are Generated from Human Pluripotent Stem Cells. Stem Cell Reports. 2018;10:436–446. doi: 10.1016/j.stemcr.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christov C, Chretien F, Abou-Khalil R, Bassez G, Vallet G, Authier FJ, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B, et al. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell. 2007;18:1397–1409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane A, Albers HJ, Passier R, Mummery CL, van den Berg A, Orlova VV, van der Meer AD. Advanced in vitro models of vascular biology: Human induced pluripotent stem cells and organ-on-chip technology. Adv Drug Deliv Rev. 2018 doi: 10.1016/j.addr.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Collett GD, Canfield AE. Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res. 2005;96:930–938. doi: 10.1161/01.RES.0000163634.51301.0d. [DOI] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Comai G, Tajbakhsh S. Molecular and cellular regulation of skeletal myogenesis. Curr Top Dev Biol. 2014;110:1–73. doi: 10.1016/B978-0-12-405943-6.00001-4. [DOI] [PubMed] [Google Scholar]
- Cordova G, Negroni E, Cabello-Verrugio C, Mouly V, Trollet C. Combined Therapies for Duchenne Muscular Dystrophy to Optimize Treatment Efficacy. Front Genet. 2018;9:114. doi: 10.3389/fgene.2018.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossu G, Previtali SC, Napolitano S, Cicalese MP, Tedesco FS, Nicastro F, Noviello M, Roostalu U, Natali Sora MG, Scarlato M, et al. Intra-arterial transplantation of HLA-matched donor mesoangioblasts in Duchenne muscular dystrophy. EMBO Mol Med. 2015;7:1513–1528. doi: 10.15252/emmm.201505636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M, Deasy B, Gavina M, Zheng B, Huard J, Lazzari L, Peault B. Purification and long-term culture of multipotent progenitor cells affiliated with the walls of human blood vessels: myoendothelial cells and pericytes. Methods Cell Biol. 2008a;86:295–309. doi: 10.1016/S0091-679X(08)00013-7. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008b;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar A, Domev H, Ben-Yosef O, Tzukerman M, Zeevi-Levin N, Novak A, Germanguz I, Amit M, Itskovitz-Eldor J. Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb. Circulation. 2012;125:87–99. doi: 10.1161/CIRCULATIONAHA.111.048264. [DOI] [PubMed] [Google Scholar]
- Day K, Shefer G, Richardson JB, Enikolopov G, Yablonka-Reuveni Z. Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells. Dev Biol. 2007;304:246–259. doi: 10.1016/j.ydbio.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis L, Berghella L, Coletta M, Lattanzi L, Zanchi M, Cusella-De Angelis MG, Ponzetto C, Cossu G. Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J Cell Biol. 1999;147:869–878. doi: 10.1083/jcb.147.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, Antonini S, Sambasivan R, Brunelli S, Tajbakhsh S, et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- Dias Moura Prazeres PH, Sena IFG, Borges IDT, de Azevedo PO, Andreotti JP, de Paiva AE, de Almeida VM, de Paula Guerra DA, Pinheiro Dos Santos GS, Mintz A, et al. Pericytes are heterogeneous in their origin within the same tissue. Dev Biol. 2017;427:6–11. doi: 10.1016/j.ydbio.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Manera J, Gallardo E, de Luna N, Navas M, Soria L, Garibaldi M, Rojas-Garcia R, Tonlorenzi R, Cossu G, Illa I. The increase of pericyte population in human neuromuscular disorders supports their role in muscle regeneration in vivo. J Pathol. 2012;228:544–553. doi: 10.1002/path.4083. [DOI] [PubMed] [Google Scholar]
- Diaz-Manera J, Touvier T, Dellavalle A, Tonlorenzi R, Tedesco FS, Messina G, Meregalli M, Navarro C, Perani L, Bonfanti C, et al. Partial dysferlin reconstitution by adult murine mesoangioblasts is sufficient for full functional recovery in a murine model of dysferlinopathy. Cell Death Dis. 2010;1:e61. doi: 10.1038/cddis.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohgu S, Takata F, Yamauchi A, Nakagawa S, Egawa T, Naito M, Tsuruo T, Sawada Y, Niwa M, Kataoka Y. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain Res. 2005;1038:208–215. doi: 10.1016/j.brainres.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Domi T, Porrello E, Velardo D, Capotondo A, Biffi A, Tonlorenzi R, Amadio S, Ambrosi A, Miyagoe-Suzuki Y, Takeda S, et al. Mesoangioblast delivery of miniagrin ameliorates murine model of merosin-deficient congenital muscular dystrophy type 1A. Skelet Muscle. 2015;5:30. doi: 10.1186/s13395-015-0055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host responses in tissue repair and fibrosis. Annual review of pathology. 2013;8:241–276. doi: 10.1146/annurev-pathol-020712-163930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med. 2012;18:1262–1270. doi: 10.1038/nm.2848. [DOI] [PubMed] [Google Scholar]
- Dumont NA, Bentzinger CF, Sincennes MC, Rudnicki MA. Satellite Cells and Skeletal Muscle Regeneration. Compr Physiol. 2015;5:1027–1059. doi: 10.1002/cphy.c140068. [DOI] [PubMed] [Google Scholar]
- Eberth CJ. Handbuch der Lehre von der Gewegen des Menschen und der Tiere. 1871;1 [Google Scholar]
- Emery AE. The muscular dystrophies. Lancet. 2002;359:687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- Enge M, Bjarnegard M, Gerhardt H, Gustafsson E, Kalen M, Asker N, Hammes HP, Shani M, Fassler R, Betsholtz C. Endotheliumspecific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J. 2002;21:4307–4316. doi: 10.1093/emboj/cdf418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel WK. Muscle biopsies in neuromuscular diseases. Pediatr Clin North Am. 1967;14:963–995. doi: 10.1016/s0031-3955(16)32067-3. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Esner M, Meilhac SM, Relaix F, Nicolas JF, Cossu G, Buckingham ME. Smooth muscle of the dorsal aorta shares a common clonal origin with skeletal muscle of the myotome. Development. 2006;133:737–749. doi: 10.1242/dev.02226. [DOI] [PubMed] [Google Scholar]
- Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128:1059–1068. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- Fabry Z, Fitzsimmons KM, Herlein JA, Moninger TO, Dobbs MB, Hart MN. Production of the cytokines interleukin 1 and 6 by murine brain microvessel endothelium and smooth muscle pericytes. J Neuroimmunol. 1993;47:23–34. doi: 10.1016/0165-5728(93)90281-3. [DOI] [PubMed] [Google Scholar]
- Fan Y, Maley M, Beilharz M, Grounds M. Rapid death of injected myoblasts in myoblast transfer therapy. Muscle Nerve. 1996;19:853–860. doi: 10.1002/(SICI)1097-4598(199607)19:7<853::AID-MUS7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–2232. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Ochala J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int. 2015;96:183–195. doi: 10.1007/s00223-014-9915-y. [DOI] [PubMed] [Google Scholar]
- Galvez BG, Sampaolesi M, Brunelli S, Covarello D, Gavina M, Rossi B, Constantin G, Torrente Y, Cossu G. Complete repair of dystrophic skeletal muscle by mesoangioblasts with enhanced migration ability. J Cell Biol. 2006;174:231–243. doi: 10.1083/jcb.200512085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargioli C, Coletta M, De Grandis F, Cannata SM, Cossu G. PlGF-MMP-9-expressing cells restore microcirculation and efficacy of cell therapy in aged dystrophic muscle. Nat Med. 2008;14:973–978. doi: 10.1038/nm.1852. [DOI] [PubMed] [Google Scholar]
- Gaudio E, Pannarale L, Marinozzi G. An S.E.M. corrosion cast study on pericyte localization and role in microcirculation of skeletal muscle. Angiology. 1985;36:458–464. doi: 10.1177/000331978503600708. [DOI] [PubMed] [Google Scholar]
- Gautam J, Nirwane A, Yao Y. Laminin differentially regulates the stemness of type I and type II pericytes. Stem Cell Res Ther. 2017;8:28. doi: 10.1186/s13287-017-0479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavina M, Belicchi M, Rossi B, Ottoboni L, Colombo F, Meregalli M, Battistelli M, Forzenigo L, Biondetti P, Pisati F, et al. VCAM-1 expression on dystrophic muscle vessels has a critical role in the recruitment of human blood-derived CD133+ stem cells after intra-arterial transplantation. Blood. 2006;108:2857–2866. doi: 10.1182/blood-2006-04-018564. [DOI] [PubMed] [Google Scholar]
- Geissinger HD, Rao PV, McDonald-Taylor CK. “mdx” mouse myopathy: histopathological, morphometric and histochemical observations on young mice. J Comp Pathol. 1990;102:249–263. doi: 10.1016/s0021-9975(08)80015-1. [DOI] [PubMed] [Google Scholar]
- Gerli MF, Maffioletti SM, Millet Q, Tedesco FS. Transplantation of induced pluripotent stem cell-derived mesoangioblast-like myogenic progenitors in mouse models of muscle regeneration. J Vis Exp. 2014:e50532. doi: 10.3791/50532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerli MFM, Moyle LA, Benedetti S, Ferrari G, Ucuncu E, Ragazzi M, Constantinou C, Lousa I, Sakai H, Ala P, De Coppi P, et al. Combined notch and PDGF signalling enhances expression of stem cell markers while inducing perivascular cell features in muscle satellite cells. Stem Cell Reports. 2019;12(3):461–473. doi: 10.1016/j.stemcr.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannotta M, Benedetti S, Tedesco FS, Corada M, Trani M, D’Antuono R, Millet Q, Orsenigo F, Galvez BG, Cossu G, et al. Targeting endothelial junctional adhesion molecule-A/ EPAC/ Rap-1 axis as a novel strategy to increase stem cell engraftment in dystrophic muscles. EMBO Mol Med. 2014;6:239–258. doi: 10.1002/emmm.201302520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannelli G, Giacomazzi G, Grosemans H, Sampaolesi M. Morphological and functional analyses of skeletal muscles from an immunodeficient animal model of limb-girdle muscular dystrophy type 2E. Muscle Nerve. 2018 doi: 10.1002/mus.26112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerette B, Skuk D, Celestin F, Huard C, Tardif F, Asselin I, Roy B, Goulet M, Roy R, Entman M, et al. Prevention by anti-LFA-1 of acute myoblast death following transplantation. J Immunol. 1997;159:2522–2531. [PubMed] [Google Scholar]
- Guimaraes-Camboa N, Cattaneo P, Sun Y, Moore-Morris T, Gu Y, Dalton ND, Rockenstein E, Masliah E, Peterson KL, Stallcup WB, et al. Pericytes of Multiple Organs Do Not Behave as Mesenchymal Stem Cells In Vivo. Cell Stem Cell. 2017;20:345–359.:e345. doi: 10.1016/j.stem.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Balci-Hayta B, Yoshida-Moriguchi T, Kanagawa M, Beltran-Valero de Bernabe D, Gundesli H, Willer T, Satz JS, Crawford RW, Burden SJ, et al. A dystroglycan mutation associated with limb-girdle muscular dystrophy. N Engl J Med. 2011;364:939–946. doi: 10.1056/NEJMoa1006939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegyi L, Gannon FH, Glaser DL, Shore EM, Kaplan FS, Shanahan CM. Stromal cells of fibrodysplasia ossificans progressiva lesions express smooth muscle lineage markers and the osteogenic transcription factor Runx2/Cbfa-1: clues to a vascular origin of heterotopic ossification? J Pathol. 2003;201:141–148. doi: 10.1002/path.1413. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–698. [PubMed] [Google Scholar]
- Holm A, Heumann T, Augustin HG. Microvascular Mural Cell Organotypic Heterogeneity and Functional Plasticity. Trends Cell Biol. 2018;28:302–316. doi: 10.1016/j.tcb.2017.12.002. [DOI] [PubMed] [Google Scholar]
- Huard J, Roy R, Bouchard JP, Malouin F, Richards CL, Tremblay JP. Human myoblast transplantation between immunohistocompatible donors and recipients produces immune reactions. Transplant Proc. 1992;24:3049–3051. [PubMed] [Google Scholar]
- Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. The American journal of pathology. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer PS, Mavoungou LO, Ronzoni F, Zemla J, Schmid-Siegert E, Antonini S, Neff LA, Dorchies OM, Jaconi M, Lekka M, et al. Autologous Cell Therapy Approach for Duchenne Muscular Dystrophy using PiggyBac Transposons and Mesoangioblasts. Mol Ther. 2018;26:1093–1108. doi: 10.1016/j.ymthe.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AW, Zara JN, Zhang X, Askarinam A, Goyal R, Chiang M, Yuan W, Chang L, Corselli M, Shen J, et al. Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cells Transl Med. 2012;1:510–519. doi: 10.5966/sctm.2012-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985) 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi YM, Rader EP, Crawford RW, Iyengar NK, Thedens DR, Faulkner JA, Parikh SV, Weiss RM, Chamberlain JS, Moore SA, et al. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456:511–515. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostallari E, Baba-Amer Y, Alonso-Martin S, Ngoh P, Relaix F, Lafuste P, Gherardi RK. Pericytes in the myovascular niche promote post-natal myofiber growth and satellite cell quiescence. Development. 2015;142:1242–1253. doi: 10.1242/dev.115386. [DOI] [PubMed] [Google Scholar]
- Kragstrup TW, Kjaer M, Mackey AL. Structural, biochemical, cellular, and functional changes in skeletal muscle extracellular matrix with aging. Scandinavian journal of medicine & science in sports. 2011;21:749–757. doi: 10.1111/j.1600-0838.2011.01377.x. [DOI] [PubMed] [Google Scholar]
- Kudryashova E, Kramerova I, Spencer MJ. Satellite cell senescence underlies myopathy in a mouse model of limb-girdle muscular dystrophy 2H. J Clin Invest. 2012;122:1764–1776. doi: 10.1172/JCI59581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, D’Souza SS, Moskvin OV, Toh H, Wang B, Zhang J, Swanson S, Guo LW, Thomson JA, Slukvin II. Specification and Diversification of Pericytes and Smooth Muscle Cells from Mesenchymoangioblasts. Cell Rep. 2017;19:1902–1916. doi: 10.1016/j.celrep.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume T. Ligand-dependent Notch signaling in vascular formation. Adv Exp Med Biol. 2012;727:210–222. doi: 10.1007/978-1-4614-0899-4_16. [DOI] [PubMed] [Google Scholar]
- Lees-Shepard JB, Yamamoto M, Biswas AA, Stoessel SJ, Nicholas SE, Cogswell CA, Devarakonda PM, Schneider MJ, Jr, Cummins SM, Legendre NP, et al. Activin-dependent signaling in fibro/adipogenic progenitors causes fibrodysplasia ossificans progressiva. Nat Commun. 2018;9:471. doi: 10.1038/s41467-018-02872-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- Levy MM, Joyner CJ, Virdi AS, Reed A, Triffitt JT, Simpson AH, Kenwright J, Stein H, Francis MJ. Osteoprogenitor cells of mature human skeletal muscle tissue: an in vitro study. Bone. 2001;29:317–322. doi: 10.1016/s8756-3282(01)00585-3. [DOI] [PubMed] [Google Scholar]
- Lin AY, Wang LH. Molecular Therapies for Muscular Dystrophies. Curr Treat Options Neurol. 2018;20:27. doi: 10.1007/s11940-018-0509-2. [DOI] [PubMed] [Google Scholar]
- Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. The American journal of pathology. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Loperfido M, Steele-Stallard HB, Tedesco FS, VandenDriessche T. Pluripotent Stem Cells for Gene Therapy of Degenerative Muscle Diseases. Current gene therapy. 2015;15:364–380. doi: 10.2174/1566523215666150630121207. [DOI] [PubMed] [Google Scholar]
- Lukjanenko L, Brachat S, Pierrel E, Lach-Trifilieff E, Feige JN. Genomic profiling reveals that transient adipogenic activation is a hallmark of mouse models of skeletal muscle regeneration. PLoS One. 2013;8:e71084. doi: 10.1371/journal.pone.0071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffioletti SM, Gerli MF, Ragazzi M, Dastidar S, Benedetti S, Loperfido M, VandenDriessche T, Chuah MK, Tedesco FS. Efficient derivation and inducible differentiation of expandable skeletal myogenic cells from human ES and patient-specific iPS cells. Nat Protoc. 2015;10:941–958. doi: 10.1038/nprot.2015.057. [DOI] [PubMed] [Google Scholar]
- Maffioletti SM, Noviello M, English K, Tedesco FS. Stem cell transplantation for muscular dystrophy: the challenge of immune response. Biomed Res Int. 2014;2014:964010. doi: 10.1155/2014/964010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27:1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- Majesky MW, Dong XR, Regan JN, Hoglund VJ. Vascular smooth muscle progenitor cells: building and repairing blood vessels. Circ Res. 2011;108:365–377. doi: 10.1161/CIRCRESAHA.110.223800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankodi A, Bishop CA, Auh S, Newbould RD, Fischbeck KH, Janiczek RL. Quantifying disease activity in fatty-infiltrated skeletal muscle by IDEAL-CPMG in Duchenne muscular dystrophy. Neuromuscul Disord. 2016;26:650–658. doi: 10.1016/j.nmd.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Munoz-Canoves P. Aberrant repair and fibrosis development in skeletal muscle. Skeletal muscle. 2011;1:21. doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzur AY, Muntoni F. Diagnosis and new treatments in muscular dystrophies. Postgrad Med J. 2009;85:622–630. doi: 10.1136/jnnp.2008.158329. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Campbell KP. Dystrophin-glycoprotein complex: its role in the molecular pathogenesis of muscular dystrophies. Muscle Nerve. 1994;17:215. doi: 10.1002/mus.880170103. [DOI] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. The Journal of biophysical and biochemical cytology. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Adkin CF, Xu SW, Muntoni F, Morgan JE. Contribution of human muscle-derived cells to skeletal muscle regeneration in dystrophic host mice. PLoS One. 2011;6:e17454. doi: 10.1371/journal.pone.0017454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri E, Muntoni F. Muscular dystrophies. Lancet. 2013;381:845–860. doi: 10.1016/S0140-6736(12)61897-2. [DOI] [PubMed] [Google Scholar]
- Michalak M, Opas M. Functions of dystrophin and dystrophin associated proteins. Curr Opin Neurol. 1997;10:436–442. doi: 10.1097/00019052-199710000-00014. [DOI] [PubMed] [Google Scholar]
- Minasi MG, Riminucci M, De Angelis L, Borello U, Berarducci B, Innocenzi A, Caprioli A, Sirabella D, Baiocchi M, De Maria R, et al. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129:2773–2783. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi S, Kang PB. Update on the genetics of limb girdle muscular dystrophy. Semin Pediatr Neurol. 2012;19:211–218. doi: 10.1016/j.spen.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- Morales MG, Gutierrez J, Cabello-Verrugio C, Cabrera D, Lipson KE, Goldschmeding R, Brandan E. Reducing CTGF/CCN2 slows down mdx muscle dystrophy and improves cell therapy. Hum Mol Genet. 2013;22:4938–4951. doi: 10.1093/hmg/ddt352. [DOI] [PubMed] [Google Scholar]
- Morosetti R, Gidaro T, Broccolini A, Gliubizzi C, Sancricca C, Tonali PA, Ricci E, Mirabella M. Mesoangioblasts from facioscapulohumeral muscular dystrophy display in vivo a variable myogenic ability predictable by their in vitro behavior. Cell Transplant. 2011;20:1299–1313. doi: 10.3727/096368910X546571. [DOI] [PubMed] [Google Scholar]
- Murray IR, Baily JE, Chen WCW, Dar A, Gonzalez ZN, Jensen AR, Petrigliano FA, Deb A, Henderson NC. Skeletal and cardiac muscle pericytes: Functions and therapeutic potential. Pharmacol Ther. 2017;171:65–74. doi: 10.1016/j.pharmthera.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Deli MA, Nakao S, Honda M, Hayashi K, Nakaoke R, Kataoka Y, Niwa M. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cell Mol Neurobiol. 2007;27:687–694. doi: 10.1007/s10571-007-9195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negroni E, Bigot A, Butler-Browne GS, Trollet C, Mouly V. Cellular Therapies for Muscular Dystrophies: Frustrations and Clinical Successes. Hum Gene Ther. 2016;27:117–126. doi: 10.1089/hum.2015.139. [DOI] [PubMed] [Google Scholar]
- Nguyen F, Cherel Y, Guigand L, Goubault-Leroux I, Wyers M. Muscle lesions associated with dystrophin deficiency in neonatal golden retriever puppies. J Comp Pathol. 2002;126:100–108. doi: 10.1053/jcpa.2001.0526. [DOI] [PubMed] [Google Scholar]
- Norrmen C, Tammela T, Petrova TV, Alitalo K. Biological basis of therapeutic lymphangiogenesis. Circulation. 2011;123:1335–1351. doi: 10.1161/CIRCULATIONAHA.107.704098. [DOI] [PubMed] [Google Scholar]
- Noviello M, Tedesco FS, Bondanza A, Tonlorenzi R, Rosaria Carbone M, Gerli MFM, Marktel S, Napolitano S, Cicalese MP, Ciceri F, et al. Inflammation converts human mesoangioblasts into targets of alloreactive immune responses: implications for allogeneic cell therapy of DMD. Mol Ther. 2014;22:1342–1352. doi: 10.1038/mt.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova VV, van den Hil FE, Petrus-Reurer S, Drabsch Y, Ten Dijke P, Mummery CL. Generation, expansion and functional analysis of endothelial cells and pericytes derived from human pluripotent stem cells. Nat Protoc. 2014;9:1514–1531. doi: 10.1038/nprot.2014.102. [DOI] [PubMed] [Google Scholar]
- Pallone TL, Silldorff EP. Pericyte regulation of renal medullary blood flow. Exp Nephrol. 2001;9:165–170. doi: 10.1159/000052608. [DOI] [PubMed] [Google Scholar]
- Pallone TL, Silldorff EP, Turner MR. Intrarenal blood flow: microvascular anatomy and the regulation of medullary perfusion. Clin Exp Pharmacol Physiol. 1998;25:383–392. doi: 10.1111/j.1440-1681.1998.tb02220.x. [DOI] [PubMed] [Google Scholar]
- Palmieri B, Tremblay JP, Daniele L. Past, present and future of myoblast transplantation in the treatment of Duchenne muscular dystrophy. Pediatr Transplant. 2010;14:813–819. doi: 10.1111/j.1399-3046.2010.01377.x. [DOI] [PubMed] [Google Scholar]
- Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, Cossu G, Bianchi ME. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol. 2004;164:441–449. doi: 10.1083/jcb.200304135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge T. The current status of myoblast transfer. Neurol Sci. 2000;21:S939–942. doi: 10.1007/s100720070007. [DOI] [PubMed] [Google Scholar]
- Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM. Conversion of mdx myofibres from dystrophin-negative to-positive by injection of normal myoblasts. Nature. 1989;337:176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessina P, Conti V, Tonlorenzi R, Touvier T, Meneveri R, Cossu G, Brunelli S. Necdin enhances muscle reconstitution of dystrophic muscle by vessel-associated progenitors, by promoting cell survival and myogenic differentiation. Cell Death Differ. 2012;19:827–838. doi: 10.1038/cdd.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pini V, Morgan JE, Muntoni F, O’Neill HC. Genome Editing and Muscle Stem Cells as a Therapeutic Tool for Muscular Dystrophies. Curr Stem Cell Rep. 2017;3:137–148. doi: 10.1007/s40778-017-0076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prazeres P, Almeida VM, Lousado L, Andreotti JP, Paiva AE, Santos GSP, Azevedo PO, Souto L, Almeida GG, Filev R, et al. Macrophages Generate Pericytes in the Developing Brain. Cell Mol Neurobiol. 2018;38:777–782. doi: 10.1007/s10571-017-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan TE, Cowper SE, Bucala R. The role of circulating fibrocytes in fibrosis. Curr Rheumatol Rep. 2006;8:145–150. doi: 10.1007/s11926-006-0055-x. [DOI] [PubMed] [Google Scholar]
- Quattrocelli M, Costamagna D, Giacomazzi G, Camps J, Sampaolesi M. Notch signaling regulates myogenic regenerative capacity of murine and human mesoangioblasts. Cell Death Dis. 2014;5:e1448. doi: 10.1038/cddis.2014.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Zammit PS. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development. 2012;139:2845–2856. doi: 10.1242/dev.069088. [DOI] [PubMed] [Google Scholar]
- Rocheteau P, Gayraud-Morel B, Siegl-Cachedenier I, Blasco MA, Tajbakhsh S. A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell. 2012;148:112–125. doi: 10.1016/j.cell.2011.11.049. [DOI] [PubMed] [Google Scholar]
- Rodeheffer MS. Tipping the scale: muscle versus fat. Nat Cell Biol. 2010;12:102–104. doi: 10.1038/ncb0210-102. [DOI] [PubMed] [Google Scholar]
- Rouget C. Mémoire sur le développement, la structure et les proprietés physiologiques des capillaires sanguins et lymphatiques. Arch Phys. 1873;5:603–610. [Google Scholar]
- Ryall JG, Schertzer JD, Lynch GS. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology. 2008;9:213–228. doi: 10.1007/s10522-008-9131-0. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Remoli C, Giannicola G, Kogler G, Liedtke S, Cossu G, Serafini M, Sampaolesi M, Tagliafico E, et al. No Identical “Mesenchymal Stem Cells” at Different Times and Sites: Human Committed Progenitors of Distinct Origin and Differentiation Potential Are Incorporated as Adventitial Cells in Microvessels. Stem Cell Reports. 2016;6:897–913. doi: 10.1016/j.stemcr.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Selfrenewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco A, Mourkioti F, Tran R, Choi J, Llewellyn M, Kraft P, Shkreli M, Delp S, Pomerantz JH, Artandi SE, et al. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell. 2010;143:1059–1071. doi: 10.1016/j.cell.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaolesi M, Blot S, D’Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthelemy I, et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- Sampaolesi M, Torrente Y, Innocenzi A, Tonlorenzi R, D’Antona G, Pellegrino MA, Barresi R, Bresolin N, De Angelis MG, Campbell KP, et al. Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science. 2003;301:487–492. doi: 10.1126/science.1082254. [DOI] [PubMed] [Google Scholar]
- Sciorati C, Galvez BG, Brunelli S, Tagliafico E, Ferrari S, Cossu G, Clementi E. Ex vivo treatment with nitric oxide increases mesoangioblast therapeutic efficacy in muscular dystrophy. J Cell Sci. 2006;119:5114–5123. doi: 10.1242/jcs.03300. [DOI] [PubMed] [Google Scholar]
- Scoto M, Finkel R, Mercuri E, Muntoni F. Genetic therapies for inherited neuromuscular disorders. Lancet Child Adolesc Health. 2018;2:600–609. doi: 10.1016/S2352-4642(18)30140-8. [DOI] [PubMed] [Google Scholar]
- Sims DE. The pericyte--a review. Tissue Cell. 1986;18:153–174. doi: 10.1016/0040-8166(86)90026-1. [DOI] [PubMed] [Google Scholar]
- Skuk D, Goulet M, Roy B, Chapdelaine P, Bouchard JP, Roy R, Dugre FJ, Sylvain M, Lachance JG, Deschenes L, et al. Dystrophin expression in muscles of duchenne muscular dystrophy patients after high-density injections of normal myogenic cells. J Neuropathol Exp Neurol. 2006;65:371–386. doi: 10.1097/01.jnen.0000218443.45782.81. [DOI] [PubMed] [Google Scholar]
- Skuk D, Goulet M, Roy B, Piette V, Cote CH, Chapdelaine P, Hogrel JY, Paradis M, Bouchard JP, Sylvain M, et al. First test of a “high-density injection” protocol for myogenic cell transplantation throughout large volumes of muscles in a Duchenne muscular dystrophy patient: eighteen months follow-up. Neuromuscul Disord. 2007;17:38–46. doi: 10.1016/j.nmd.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Skuk D, Roy B, Goulet M, Chapdelaine P, Bouchard JP, Roy R, Dugre FJ, Lachance JG, Deschenes L, Helene S, et al. Dystrophin expression in myofibers of Duchenne muscular dystrophy patients following intramuscular injections of normal myogenic cells. Mol Ther. 2004;9:475–482. doi: 10.1016/j.ymthe.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Skuk D, Tremblay JP. Intramuscular cell transplantation as a potential treatment of myopathies: clinical and preclinical relevant data. Expert Opin Biol Ther. 2011;11:359–374. doi: 10.1517/14712598.2011.548800. [DOI] [PubMed] [Google Scholar]
- Smythe GM, Fan Y, Grounds MD. Enhanced migration and fusion of donor myoblasts in dystrophic and normal host muscle. Muscle Nerve. 2000;23:560–574. doi: 10.1002/(sici)1097-4598(200004)23:4<560::aid-mus16>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- Straub V, Bittner RE, Leger JJ, Voit T. Direct visualization of the dystrophin network on skeletal muscle fiber membrane. J Cell Biol. 1992;119:1183–1191. doi: 10.1083/jcb.119.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub V, Campbell KP. Muscular dystrophies and the dystrophin-glycoprotein complex. Curr Opin Neurol. 1997;10:168–175. doi: 10.1097/00019052-199704000-00016. [DOI] [PubMed] [Google Scholar]
- Tagliafico E, Brunelli S, Bergamaschi A, De Angelis L, Scardigli R, Galli D, Battini R, Bianco P, Ferrari S, Cossu G, et al. TGFbeta/BMP activate the smooth muscle/bone differentiation programs in mesoangioblasts. J Cell Sci. 2004;117:4377–4388. doi: 10.1242/jcs.01291. [DOI] [PubMed] [Google Scholar]
- Tanaka KK, Hall JK, Troy AA, Cornelison DD, Majka SM, Olwin BB. Syndecan-4-expressing muscle progenitor cells in the SP engraft as satellite cells during muscle regeneration. Cell Stem Cell. 2009;4:217–225. doi: 10.1016/j.stem.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco FS, Dellavalle A, Diaz-Manera J, Messina G, Cossu G. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest. 2010;120:11–19. doi: 10.1172/JCI40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco FS, Gerli MF, Perani L, Benedetti S, Ungaro F, Cassano M, Antonini S, Tagliafico E, Artusi V, Longa E, et al. Transplantation of genetically corrected human iPSC-derived progenitors in mice with limb-girdle muscular dystrophy. Sci Transl Med. 2012;4:140ra189. doi: 10.1126/scitranslmed.3003541. [DOI] [PubMed] [Google Scholar]
- Tedesco FS, Hoshiya H, D’Antona G, Gerli MF, Messina G, Antonini S, Tonlorenzi R, Benedetti S, Berghella L, Torrente Y, et al. Stem cell-mediated transfer of a human artificial chromosome ameliorates muscular dystrophy. Sci Transl Med. 2011;3:96ra78. doi: 10.1126/scitranslmed.3002342. [DOI] [PubMed] [Google Scholar]
- Tedesco FS, Moyle LA, Perdiguero E. Muscle Interstitial Cells: A Brief Field Guide to Non-satellite Cell Populations in Skeletal Muscle. Methods Mol Biol. 2017;1556:129–147. doi: 10.1007/978-1-4939-6771-1_7. [DOI] [PubMed] [Google Scholar]
- Tews DS, Goebel HH. Expression of cell adhesion molecules in inflammatory myopathies. J Neuroimmunol. 1995;59:185–194. doi: 10.1016/0165-5728(95)00045-4. [DOI] [PubMed] [Google Scholar]
- Thomas GD. Functional muscle ischemia in Duchenne and Becker muscular dystrophy. Front Physiol. 2013;4:381. doi: 10.3389/fphys.2013.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LV. Age-related muscle dysfunction. Experimental gerontology. 2009;44:106–111. doi: 10.1016/j.exger.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Z, Li Y, Smith DS, Sheibani N, Huang S, Kern T, Lin F. Retinal pericytes inhibit activated T cell proliferation. Invest Ophthalmol Vis Sci. 2011;52:9005–9010. doi: 10.1167/iovs.11-8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- Ugarte G, Cappellari O, Perani L, Pistocchi A, Cossu G. Noggin recruits mesoderm progenitors from the dorsal aorta to a skeletal myogenic fate. Dev Biol. 2012;365:91–100. doi: 10.1016/j.ydbio.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadares MC, Gomes JP, Castello G, Assoni A, Pellati M, Bueno C, Corselli M, Silva H, Bartolini P, Vainzof M, et al. Human adipose tissue derived pericytes increase life span in Utrn (tm1Ked) Dmd (mdx) /J mice. Stem Cell Rev. 2014;10:830–840. doi: 10.1007/s12015-014-9537-9. [DOI] [PubMed] [Google Scholar]
- Valero MC, Huntsman HD, Liu J, Zou K, Boppart MD. Eccentric exercise facilitates mesenchymal stem cell appearance in skeletal muscle. PLoS One. 2012;7:e29760. doi: 10.1371/journal.pone.0029760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek MM, Westphal JR, Ruiter DJ, de Waal RM. T lymphocyte adhesion to human brain pericytes is mediated via very late antigen-4/vascular cell adhesion molecule-1 interactions. J Immunol. 1995;154:5876–5884. [PubMed] [Google Scholar]
- Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJ. Satellite cells in human skeletal muscle; from birth to old age. Age (Dordr) 2014;36:545–547. doi: 10.1007/s11357-013-9583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walston JD. Sarcopenia in older adults. Current opinion in rheumatology. 2012;24:623–627. doi: 10.1097/BOR.0b013e328358d59b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis BC, duBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc Am Thorac Soc. 2006;3:377–382. doi: 10.1513/pats.200601-004TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worton R. Muscular dystrophies: diseases of the dystrophin-glycoprotein complex. Science. 1995;270:755–756. doi: 10.1126/science.270.5237.755. [DOI] [PubMed] [Google Scholar]
- Wren TA, Bluml S, Tseng-Ong L, Gilsanz V. Three-point technique of fat quantification of muscle tissue as a marker of disease progression in Duchenne muscular dystrophy: preliminary study. AJR Am J Roentgenol. 2008;190:W8–12. doi: 10.2214/AJR.07.2732. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z. The skeletal muscle satellite cell: still young and fascinating at 50. J Histochem Cytochem. 2011;59:1041–1059. doi: 10.1369/0022155411426780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Nalbandian A, Uchida Y, Li W, Arnold TD, Kubota Y, Yamamoto S, Ema M, Mukouyama YS. Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-beta Signaling in Developing Skin Vasculature. Cell Rep. 2017;18:2991–3004. doi: 10.1016/j.celrep.2017.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Norris EH, Mason CE, Strickland S. Laminin regulates PDGFRbeta(+) cell stemness and muscle development. Nat Commun. 2016;7:11415. doi: 10.1038/ncomms11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- Zhang X, Peault B, Chen W, Li W, Corselli M, James AW, Lee M, Siu RK, Shen P, Zheng Z, et al. The Nell-1 growth factor stimulates bone formation by purified human perivascular cells. Tissue Eng Part A. 2011;17:2497–2509. doi: 10.1089/ten.tea.2010.0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann KW. Der feinere Bau der Blutkapillaren. Z Anat Entwicklungsgesch. 1923;68:29–109. [Google Scholar]