Abstract
Single-cell sequencing techniques enable the decades-old T helper subset dogma to be approached in an unsupervised manner, bringing nuances to the definition of known subsets, while simultaneously identifying interesting novel cell states.
Bernardo Buontalenti (1531-1608), a Florentine architect, engineer and artist, is credited to be the inventor of modern gelato, but it is only with Francesco Procopio dei Coltelli (1651-1727), a Sicilian cook, that gelato spread in Europe (https://en.wikipedia.org/wiki/Procopio_Cutò). Migrating to Paris, Francesco exported his expertise on preparing fruit sorbet by mixing juice and ice, and perfectioned gelato preparation that he used to sell in different fruit flavors at the still-existing café Le Procope in the heart of the city. As in gelato, CD4+ effector T cell responses occur in different “flavors” that enable the immune system to act appropriately in different physiological and pathological situations. Seminal work in the 1980s demonstrated that these effector functions are not all performed by one multifunctional T cell, but that the effector T cell pool contains functional heterogeneity, resulting in the definition of T helper 1 (Th1) and Th2 cells on the basis of the cytokines they produced1. The model has since been expanded to include Th17 (producing mainly IL-17), Th22 (IL-22) and Th9 (IL-9), and follicular helper T (Tfh) cells2. The T helper subsets are traditionally characterized by the expression of a few key proteins (the ingredients; Figure 1), including cytokines and chemokine receptors, and transcription factors acting as master regulators of their transcriptional program. T helper cell characterization has for most part been performed on sorted cells, or effector cells generated in vitro from naïve precursors under influence of high concentrations of skewing cytokines. It is still unclear, however, to what extent such specialization occurs in vivo, and whether the often-used markers optimally capture T helper heterogeneity. In this issue of Nature Immunology, Kiner et al. revisit the T helper subset paradigm by using single-cell sequencing approaches to determine what phenotypes colonic effector CD4+ T cells adopt in vivo 3. They show that at the transcriptional level, the majority of colonic effector T cells do not form discrete T helper populations as expected, but instead form a polarized continuum. Still, discrete populations of T cells could be detected, including a cluster of cells highly expressing interferon-signaling genes (ISGs) and a cluster of cells expressing myeloid-associated transcripts.
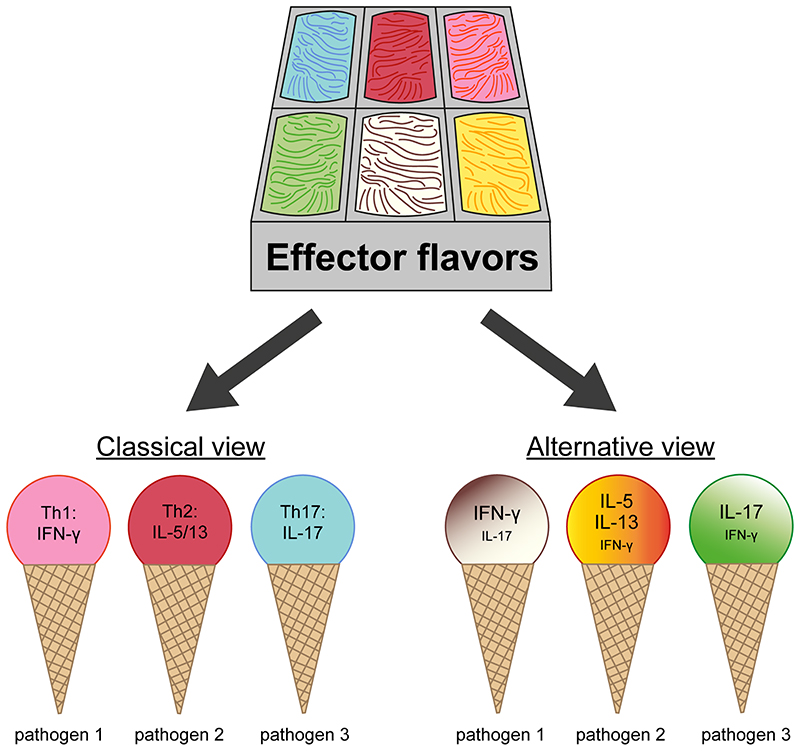
Figure 1. CD4+ T cell effector flavors.
Kiner et al. demonstrate that the in vivo CD4+ effector T cell pool generated in response to various pathogens cannot be easily parsed out into discrete T helper lineages, but instead forms a continuum of polarized phenotypes, and is shaped by the specific pathogen.
Kiner et al. analyzed the transcriptomes of CD4+ T cells isolated from the colon lamina propria of germ-free mice, mice with normal microbiota and mice exposed to pathogens associated with a specific type of T helper response, including Salmonella (Th1), Citrobacter (Th17) and the helminths H. polygyrus and N. brasiliensis (Th2). Both at steady-state and upon infection, they found a clear segregation of naïve, regulatory and effector T cells. However, while they could clearly distinguish T helper subsets by flow cytometry based on the polarized pattern of cytokines expected to be induced in response to infections, they failed to do so by single cell RNA-sequencing (scRNA-seq). Curated gene signatures for Th1, Th2, Th17 and Tfh characterized different areas of the multidimensional scRNA-seq space, thus suggesting some degree of polarization, but did not define discrete T helper clusters, even when combining cells of the different infection models into a single clustering analysis. Instead, the type of infectious agent was a more dominant factor than the expressed cytokines or T helper signatures at driving cellular clustering (Figure 1). Il17a-expressing and Ifng-expressing cells responding to the same infection (Salmonella or Citrobacter) were transcriptionally more similar than effector cells expressing the same cytokine in response to the different pathogens, a finding that was confirmed by bulk RNA-seq using IL-17-reporter mice. Tracing the fate of T cell clones through their T cell receptor sequence revealed that a single clone could, in some cases, give rise to cells expressing Il17, Ifng or both in response to Salmonella infection. This is in line with previous findings showing the response of human memory T cells stimulated in vitro with Mycobacterium tuberculosis or Candida albicans4. The experiments performed by Kiner et al. suggest that the type of pathogen does not solely affect cytokine production, but has a broader impact on the entire transcriptome (Figure 1). This may be linked to a different combination of pathogen associated molecular patterns.
Importantly, the transcriptome is only a snapshot of the cell state at a given moment in time. It has now become clear that the capacity of a cell to express certain genes is controlled at the epigenetic level and thus epigenetic information holds importance for defining cellular plasticity versus stability. Kiner et al. employed the assay for transposase-accessible chromatin using sequencing at the single-cell level (scATAC-seq) to identify heterogeneity in colonic CD4+ effector T cells at the epigenetic level. Open chromatin regions associated with the activity of Tbx21 (encoding T-bet) and Rorc (encoding RORγt), two transcription factors important for Th1 and Th17 cell development, respectively, could clearly separate ATAC-seq profiles from Th1 and Th17 cells generated in vitro, but could not segregate colonic CD4+ effector T cells from Salmonella-infected mice, in line with scRNA-seq. Yet, even though four different infection models were investigated, it begs the question whether in response to other pathogens more polarized and discrete populations can be found. In a house dust mite (HDM) model of allergic airway disease, scRNA-seq could parse effector T cells into several clusters, including Th1 and Th25. In a malaria infection model, scRNA-seq revealed bifurcation of the effector response involving a Tfh and Th1 lineage, although it was noticed that the traditional Th1 markers Ifng, Tbx21 and Cxcr3 were enriched in, but not confined to, the Th1 lineage6. Cxcr6 was instead much better at defining the Th1 cluster6, highlighting that unsupervised approaches can identify more specific markers for detection of distinct T cell subsets. Interestingly, Kiner et al. noticed that the Th2 and Tfh signatures were the most polarized, whereas the least clear demarcation was found for Th1 and Th17 signatures. Their ATAC-seq data showing that open chromatin on the Tbx21 and Rorc loci are not mutually exclusive in Salmonella-specific CD4+ effector T cells is reminiscent of the finding that Mycobacterium tuberculosis-specific CD4+ T cells are preferentially contained in a subset expressing both TBX21 and RORC upon activation7.
Besides antigen-specificity (the what), the where and when are factors that can greatly impact cellular identity. In the HDM study, the authors compared Th2 from the bronchoalveolar lavage with Th2 from mediastinal lymph nodes, and found that these cells exhibited a highly distinct transcriptome including differential expression of cytokines5. As such, the identification of discrete CD4+ populations in one type of tissue, or lack thereof, might not simply apply to other types of tissue. Indeed, all of the pathogens used in the current study infect via the oral route and thus hit the gut which may be intrinsically less prone to a pronounced polarization of the response in order to avoid being exposed to other potential infectious agents as well as pathobionts associated to the microbiota8. Furthermore, immune responses are highly dynamic and thus the timepoint at which cells are isolated determines in part the observed cellular heterogeneity. Kiner et al. wisely looked at multiple days post-infection, but did not find more discrete effector clusters even at later timepoints. Nevertheless, it can be hypothesized that upon secondary infection, more polarized responses occur, as repetitive immunizations induce progressive maturation of CD8+ memory T cell responses9.
The big advantage of single-cell omics is their all-encompassing nature and the ability to detect cellular heterogeneity that is otherwise masked at the bulk level. This makes these approaches particularly efficient at identifying novel cell states or subsets, or at redefining differentiation and functionality of specific lineages, as has recently been reported for human stem-like memory CD8+ T cell precursors10 or murine dendritic and monocyte-derived cells11. Accordingly, Kiner et al. describe several intriguing CD4+ T cell clusters. One discrete cluster of cells expressed an ISG signature, which was also identified in several other models5,12,13 and appears to encompass cells previously assigned to multiple T cell lineages5. Furthermore, the authors identified a cluster of T cells expressing myeloid-associated transcripts including MHC-II. Through some elegantly designed experiments, they were able to rule out that these cells were merely T cell-myeloid cell doublets or T cells that had taken up genetic material from myeloid cells. This opens up a lot of questions for follow-up research. Do these cells represent stable cell states? What are the signals that drive their formation? What is their functional role in the immune response? Are they also involved in pathogenic settings? Are these cells found only in the gut?
Single-cell sequencing approaches have revolutionized the way to detect and define cellular heterogeneity. By using such techniques, Kiner et al. highlight that the in vivo T helper response might not be as clear-cut as perhaps previously thought. These results open up many pressing questions and pave the way for new lines of research.
Acknowledgements
E.L. is funded by grants from the European Research Council (ERC_StG_2014 PERSYST 640511) and by the Associazione Italiana per la Ricerca sul Cancro (AIRC IG 20676 and AIRC 5x1000 UniCanVax 22757). M.R. is funded by grants from the European Research Council (ERC_CoG 615735 Homeogut), AIRC IG 22026 2018 and AIRC 5x1000 UniCanVax 22757.
Footnotes
Conflict of interest disclosure.
The authors declare no competing financial interests.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 2.Sallusto F. Annu Rev Immunol. 2016;34:317–334. doi: 10.1146/annurev-immunol-032414-112056. [DOI] [PubMed] [Google Scholar]
- 3.Kiner E, et al. Nat Immunol. doi: 10.1038/s41590-020-00836-7. [DOI] [Google Scholar]
- 4.Becattini S, et al. Science. 2015;347:400–406. doi: 10.1126/science.1260668. [DOI] [PubMed] [Google Scholar]
- 5.Tibbitt CA, et al. Immunity. 2019;51:169–184.:e5. doi: 10.1016/j.immuni.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Soon MSF, et al. Nat Immunol. 2020;21:1597–1610. doi: 10.1038/s41590-020-0800-8. [DOI] [PubMed] [Google Scholar]
- 7.Acosta-Rodriguez EV, et al. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 8.Faria AMC, Reis BS, Mucida D. J Exp Med. 2017;214:1211–1226. doi: 10.1084/jem.20162014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wirth TC, et al. Immunity. 2010;33:128–140. doi: 10.1016/j.immuni.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galletti G, et al. Nat Immunol. 2020;21:1552–1562. doi: 10.1038/s41590-020-0791-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosteels C, et al. Immunity. 2020;52:1039–1056.:e9. doi: 10.1016/j.immuni.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magen A, et al. Cell Rep. 2019;29:3019–3032.:e6. doi: 10.1016/j.celrep.2019.10.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szabo PA, et al. Nat Commun. 2019;10:4706. doi: 10.1038/s41467-019-12464-3. [DOI] [PMC free article] [PubMed] [Google Scholar]