Abstract
Regulating social emotional actions is essential for coping with life stressors and is associated with control by the anterior prefrontal cortex (aPFC) over the amygdala. However, it remains unclear to what extent prefrontal emotion regulation capacities contribute to resilience against developing post-traumatic stress disorder (PTSD) symptoms. Here, 185 police recruits who experienced their core trauma in the line of duty participated ina prospective longitudinal study. Pre-and post-trauma, they performed a well-established functionalMRI approach-avoidance task, mapping impulsive and controlled emotional actions. Higher baseline aPFC, dorsal and medial frontal pole activity was related to lower PTSD-symptoms after trauma exposure.aPFC activity predicted symptom development over and above self-reported and behavioral measures. Trauma-exposure, but not trauma-symptoms, predicted amygdala activation at follow-up. These findings suggest that prefrontal emotion regulation activity predicts increased resilience against developing post-traumatic stress symptoms, and may provide fruitful starting points for prediction and intervention studies.
Introduction
The ability to control emotional responses is crucial for adaptive behaviour, especially when one is confronted with adverse or stressful situations. Post-traumatic stress disorder (PTSD) is characterized by reduced prefrontal activation and aberrant emotion regulationabilities1–3. However, it is currently unclear to what extent prefrontal emotion regulation capabilities form a predisposing factor, protecting against the development of post-traumatic stress symptoms after trauma, or whether individual differences in emotion regulationare rather acquired, forming a consequence of trauma exposure. Identifying predictive resilience factors, is essential not only for selection purposes, but also to guide development of preventive interventions. Resilience can be operationalized as stable or only moderately deteriorated mental health despite stressor exposure4. According to this resilience framework, prospective longitudinal studies in PTSD are crucial to identify resilience factors4, but such studies are challenging because they require participants to be assessed before the traumatizing event occurs5.
About 80% of the general population encounters at least one traumatic event during their lifetime, of which about 10% develops PTSD6, indicating that there are vast individual differences in resilience against development of the disorder. PTSD has been conceptualized as an emotion regulation disorder7, linked with impaired down-regulation of emotional, physiological and neural responding towards threatening and trauma-related stimuli8,9. Neurobiological correlates of PTSD include hypoactivity of the prefrontal cortex, including the inferior frontal gyrus, and ventromedial (vmPFC) and anterior (aPFC) parts, and hyperactivity within salience processing areas, including the amygdala and dorsal anterior cingulate cortex9–13. Additionally, decreased resting-state connectivity between the vmPFC, amygdala and hippocampus in PTSD suggest diminished top-down prefrontal control over the (amygdala-mediated) fear response14,15. This mechanism is thought to underlie difficulties in down-regulating emotional responses towards trauma-related stimuli and reduced fear inhibition16,17. The aPFC is particularly relevant for control over emotional approach-avoidance action tendencies18–21. However, the aPFC has not been a key focus of neurobiological models of PTSD so far. This may be due to the fact that traditional - often animal-based - models mostly focus on hyperarousal and memory processing, which is associated with a network including the amygdala, dACC and vmPFC9,16. So far, these models have been largely applied to emotional memory, reappraisal and symptom provocation in individuals with PTSD - but not on the actual control over emotional actions such as avoidance22. This is important, because active avoidance and aggressive behavior, for instance when confronted with trauma reminders, is an important hallmark of PTSD (DSM5; American Psychiatric Association, 2013). There is increasing interest in the aPFC - or more precisely the lateral portion of the frontal pole or Brodmann area 1024 -, which has a particular cytoarchitectonic organization, optimized for the integration of multiple inputs25 and structural connections to the amygdala26,27. Several meta-analyses show its involvement in emotion regulation28–30 and multiple neuroimaging studies report decreased activation and connectivity of this region in PTSD patients31,32. Recent analyses of cortical thickness in PTSD also point specifically towards abnormalities in the aPFC33–35.
However, little is known about whether these regions may play a role in resilience against PTSD, as only a limited number of studies investigated the neural correlates of PTSD development prospectively. Admon and colleagues36,37 showed in a military sample tested pre-deployment that increased amygdala activation during gambling and passive picture viewing tasks predicts PTSD symptom increase after a stressful deployment period. Evidence for acquired changes in amygdala reactivity comes from a study by van Wingen and colleagues38, that indicates that amygdala responses to threatening stimuli increase after military deployment and is related to trauma exposure instead of PTSD symptoms. The latter finding is in accordance with meta-analytic evidence demonstrating higher amygdala activation in both PTSD patients and trauma-exposed individuals, compared to non-trauma-exposed healthy individuals10. Also, structural and functional vmPFC abnormalities and changes in its connectivity with the hippocampus have been suggested to be acquired rather than predisposing5. However, these studies investigated threat/reward anticipation37 or passive processing of emotional stimuli36,38 and did not test active emotion regulation by the prefrontal cortex, despite its key role in PTSD2,3. Therefore, the aim of the present prospective longitudinal study is to test whether relatively high frontal activation and relatively low amygdala activation during emotional action control constitute resilience factors against the development of post-traumatic stress symptoms. To be able to disentangle symptom-predictive factors from exposure-related acquired factors we selected a relatively resilient group of early-career police officers before they were enrolled in what is known to be a stressful period in their training (Fig 1A). During this training they are exposed to many potentially traumatic events and previous work showed that over 34% of Dutch police officers developed post-traumatic stress symptoms or sub-syndromal PTSD within one year after a traumatic event39. We used a well-established emotional action control task known to elicit increased anterior prefrontal cortex (aPFC) activation and reductions in speed and accuracy during emotion control (Fig 2A). As documented in our protocol article40, our prespecified primary hypothesis was that police-officers who show higher activation in the prefrontal cortex during emotion control, develop less PTSD symptoms after trauma exposure. Further, based on the aforementioned research we explored potential acquired neural deficits as well as the previously reported role of the amygdala in PTSD development5 and trauma exposure38, although this was not a prespecified analysis.
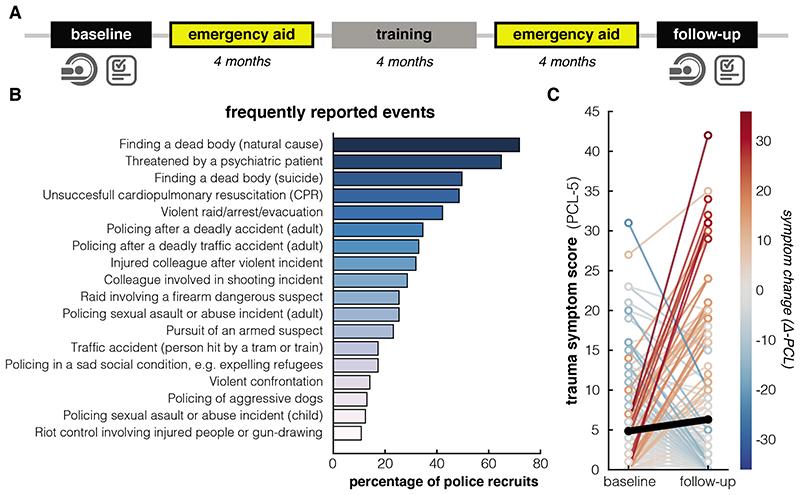
Figure 1. Study timeline, reported trauma exposure and symptoms.
A. Timeline of the study. Between baseline and follow-up, police officers gained experience in emergency aid (two periods) as part of their training, encountering potentially trauma-related events. B. Potentially traumatic events from the Police Life Events checklist (PLES)68 reported by the police officers (N = 185) between baseline and follow-up measurement. All events reported by >10% of the participants are listed. C. PTSD symptoms on the PCL-checklist (PCL-5)66 for the baseline and follow-up measurement (Δ-PCL score M = 1.46, SD = 8.48, t(184) = 2.34, p = .02, Cohen’s d = 0.17, 95% CI = [0.23 - 2.69]). Colors of the lines map the individual differences in symptom change (N = 185). The black line depicts the average change.
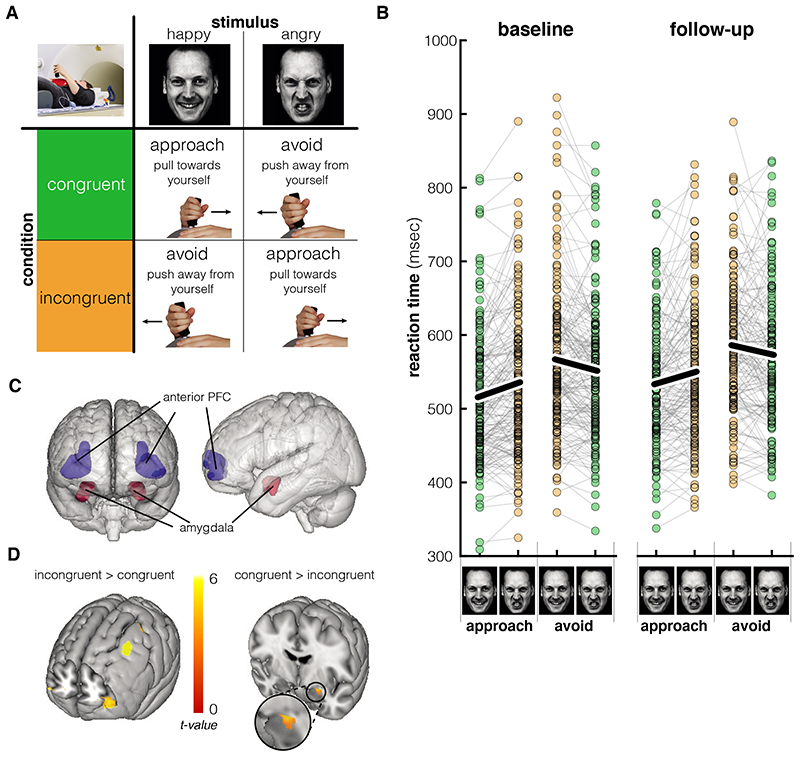
Figure 2. Experimental paradigm and main task effects.
A. Response mapping for the different conditions during the approach-avoidance task. In congruent blocks, participants approach happy faces and avoid angry faces by pulling or pushing a joystick, respectively. In incongruent blocks, participants avoid happy faces and approach angry faces. Adapted from Volman et al.19; images are AM10HAS and AM10ANS from the Karolinska Directed Emotional Faces database71. B. While participants (N = 160) overall responded slower on avoid trials, the main effect of interest is that the response time slows when the action (approach/avoid) is incongruent with the emotion portrayed in the face (happy/angry) (F(1,159) = 41.48, p < .001, ). This effect did not change significantly between baseline and follow-up (p = .64). Black lines show average reaction times. C. Regions of interest (bilateral) for the small-volume corrections.24,79 D. Collapsed over both measurement sessions (baseline and follow-up, N = 160), the bilateral anterior prefrontal cortex (aPFC) showed stronger activation during incongruent compared to congruent trials (SVC pFWE = .002), while the left amygdala showed decreased activation during incongruent compared to congruent trials (SVC pFWE = .001), thresholded at p < .001 for the aPFC and p < .005 for the amygdala for visualization. Brain images are created with Mango software (Research Imaging Institute, UTHSCSA)
Results
Trauma exposure and symptom development
Participants experienced a greater number of traumatic events between baseline and follow-up than before the baseline measurement (Δ-PLES vs. PLES baseline; t(184) = 20.29, p < .001, Cohen’s d = 1.49, 95% CI = [4.47 - 5.43])). See Fig 1B for an overview of potentially traumatic events between baseline and follow-up. PTSD symptom severity increased after exposure to traumatic events on average (Δ-PCL score M = 1.46, SD = 8.48, t(184) = 2.34, p = .02, Cohen’s d = 0.17, 95% CI = [0.23 - 2.69]) – See Fig 1C. As can be seen in Fig 1C, there appeared to be different trajectories of symptom change, which was confirmed by an automatic 2-step clustering analysis, revealing 3 clusters: symptom increase (N = 65, Δ-PCL M = 8.82 (SD 8.87), t(64) = 8.02, p < .001, Cohen’s d = 0.99, 95% CI = [6.62 - 11.01]), symptom decrease (N = 38, Δ-PCL M = -7.39 (SD 4.68), t(37) = -9.736, p < .001, Cohen’s d = -1.58, 95% CI = [-8.93 - -5.86]) and no symptom change (N = 82, Δ-PCL M = -.27 (SD 2.80), t(81) = -.868, p = .39, Cohen’s d = -0.10, 95% CI = [-0.88 - 0.35]). The number of traumatic events experienced between baseline and follow-up (Δ-PLES, median 6, interquartile range 5) correlated positively with trauma symptom increase (r = .20, p = .006, 95% CI = [0.06 - 0.33]).
Emotional control (approach-avoidance) task validation
Behavioural task effects
Participants responded slower and made more errors during trials that required a response incongruent to the automatic tendency (i.e. approaching angry faces and avoiding happy faces) compared to congruent trials at the baseline measurement (Valence x Movement interaction for Reaction Time (RT): F(1,184) = 38.43, p < .001, Fig 2B; Error rate (ER): F(1,184) = 30.31, p < .001, ). This indicates that participants had greater difficulty to perform the required actions during incongruent trials, in line with previous findings41–43. Apart from this interaction effect of interest, we found the following main effects: Reaction times were on average slower for avoidance trials (main effect movement: F(1,184) = 165.08, p < .001, ). Error rates were on average higher for happy expressions (main effect Valence: F (1,184) = 25.01, p < .001, ) and trials requiring an avoidance response (main effect Movement: F(1,184) = 15.06, p < .001, ).
Participants were overall (i.e. unspecific to any task condition) slower to respond during the follow-up measurement (main effect of Time [baseline vs. follow-up]: F (1,159) = 13.81, p < .001, ). However, the change in the congruency effects at follow-up was not statistically significant (Time x Valence x Movement interaction RT: p = .64; ER: p = .26; Valence x Movement collapsed across baseline and follow-up: RT F (1,159) = 41.48, p < .001, , ER: F(1,159) = 41.70, p < .001, ). Moreover, neither behavioural congruency effects or the change in congruency effects over time were significantly associated with PTSD symptom increase (Δ-PCL x Valence x Movement interaction RT: p = .59, ER: p = .67; Time x ΔPCL x Valence x Movement interaction RT: p = .22, ER: p = .67). Also, there was no credible evidence for a relationship between symptom increases and (changes in) valence and movement effects in reaction times or error rates (all p > .07). With respect to trauma load, we found no evidence for an effect of trauma exposure (Δ-PLES) on behavioural congruency effects (all p > .19). However, Trauma load (Δ-PLES) affected the main effect of valence on errors over time (Time x ΔPLES x Valence, F(1,155) = 5.79, p = .017, ). Individuals who encountered more traumatic events showed a decrease in error rate in trials containing a happy facial expression specifically (Time x ΔPLES for happy expressions F (1,155) = 4.09, p = .045, ).
Neural task effects
At baseline, participants showed increased bilateral aPFC activation during the incongruent condition [approach angry and avoid happy] compared to the congruent condition [approach happy and avoid angry] (Fig2C; left aPFC xyz: -32, 54, 6, SVC pFWE < .001, right aPFC xyz: 30, 52, 8, SVC pFWE = .012; effects are also bilaterally significant at the whole-brain level, see Supl. Table 1). This reflects increased recruitment of this frontal region during the control over automatic emotional response tendencies, in line with previous results18–21. Across the baseline and follow-up measurements, there was no credible evidence that activation in the left anterior prefrontal cortex changed over time (i.e. there was no congruency x time [baseline vs. follow-up] interaction in the bilateral aPFC; congruency effect in left aPFC collapsed across both measurements: xyz: -32, 58, 6, SVC pFWE = .002). As far as the amygdala, there was no statistically significant congruency effect at baseline (SVC p FWE > .05), but there was an increase in right amygdala activation for congruent>incongruent trials at the follow-up measurement (indicated by a time by congruency interaction: xyz: 34, 0, -22; SVC p FWE < .001; also significant at the whole-brain level, see Suppl. Table 1; congruent>incongruent right amygdala activation at follow-up: xyz: 22, -2, -14; SVC p FWE < .001). All effects remained significant after inclusion of Δ-PCL, PCL baseline score, PLES baseline and Δ-PLES as covariates.
Predictive effects of neural activation during emotional action control
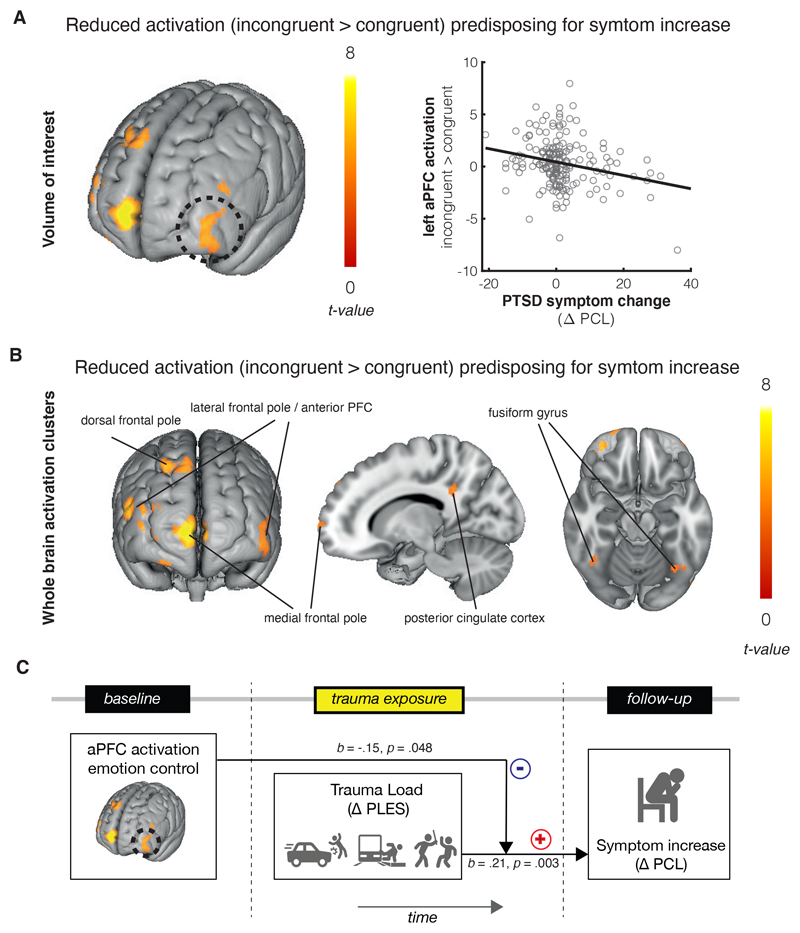
We first assessed the predisposing relationship between neural activation during emotional action control at baseline and PTSD symptom development. At the whole-brain level, decreased activation in the bilateral lateral frontal pole (overlapping with the left aPFC region of interest), dorsal frontal pole and medial frontal pole/paracingulate gyrus was associated with an increase in PTSD symptoms at follow-up (Δ-PCL). Other regions in which reduced activation predicted PTSD symptoms include the posterior cingulate cortex and visual processing regions including the fusiform gyrus and calcarine cortex (see table S1). Focusing on the a-priori regions of interest, less left aPFC activation during emotionally incongruent vs. congruent actions at baseline was associated with stronger increases in PTSD symptoms between baseline and follow-up (Δ-PCL, xyz: -40, 58, -6, SVC pFWE = .003, Fig3 A; this effect was also significant at whole-brain cluster-level, see table S1). This indicates that increased aPFC activation during emotional control measured pre-trauma predicted a smaller increase in PTSD symptoms after trauma exposure. This predictive effect was not significantly different for angry and happy faces (Steiger’s Z = -1.86, p = .06, see supplementary Fig 2A). Post-hoc correlations between extracted activation from the aPFC cluster and symptom increase for each subcluster (increase/decrease/stable), revealed that this negative relationship between prefrontal activation and symptoms was significant in the subgroup showing actual symptom increase (r = -.37, p = .002, 95% CI = [-.61 - -.11]; see supplementary Fig 3), but not in the subgroups showing symptom stability (r = -.07, p = .53, 95% CI = [-.34 - .20]) or decrease (r = .13, p = .446, 95% CI = [-.15 - .41]). The relationship between PTSD symptom increase and left aPFC activation remained significant after correcting for baseline symptom severity (baseline PCL) and trauma exposure history (baseline PLES and Δ-PLES). Moreover, post-hoc tests showed that these effects remained significant after correcting for time between assessments (see supplement). Amygdala activation (incongruent > congruent contrast) did not moderate the relationship between aPFC activation and PTSD symptom increase (see supplement). Notably, follow-up analyses showed that aPFC activation during emotion control moderates the previously mentioned positive relationship between trauma load (Δ-PLES) and symptom increase (Δ-PCL). Increased trauma exposure was only associated with increased symptoms in those individuals who had relatively low prefrontal activation during emotion control at baseline (interaction between aPFC activation and Δ-PLES: F(1,181) = 3.93, p = .049, effect of Δ-PLES on Δ-PCL at 16th percentile (low) of aPFC activation: p = .006, β = .35, 95% CI = [.15-.54]; 50th percentile (middle): p = .023, β = .21, 95% CI = [.08-.36]; 84th percentile (high): p = .35, β = .08, 95% CI = [-.09-.26]). This moderating role was only found for the left aPFC region, and no statistically significant interaction was found for the other 3 frontal clusters that emerged from the whole brain analysis (all p > .20).
Figure 3. Predisposing effects for PTSD symptom development.
A. Left anterior prefrontal activation during emotion control preceding trauma exposure was associated with less symptom development after trauma exposure (pFWE = .003). The scatterplot illustrates the relationship between PTSD symptom increase as scored on the PTSD Checklist for DSM 5 (PCL 5) and mean beta-values for the left aPFC activation cluster, extracted from the individual incongruent>congruent contrast maps (N = 185). B. Whole brain clusters at baseline that showed a negative relationship with symptom increase at follow-up (N = 185). See also table S1. C. Anterior PFC activation moderated the positive relationship between trauma exposure and symptom development (N = 185, F(1,181) = 3.93, p = .049, ). There was a positive relationship between trauma exposure and symptom development when aPFC activation was low (p = .006, β = .35, 95% CI = [.15-.54]), but no significant relationship between trauma exposure and symptom development when aPFC activation was high (p = .35, β = .08, 95% CI = [-.09 - .26]). Brain images are created with Mango software (Research Imaging Institute, UTHSCSA)
As an additional follow-up analysis, we investigated whether the relationship between left aPFC activation and symptom increase (Δ-PCL) could be attributed to a clinically significant increase in one of the four PTSD symptom clusters specifically, using binary logistic regression analyses. Participants were considered to show a clinically significant increase on a symptom cluster when at least one symptom changed from clinically non-significant (PCL symptom severity score < 2) to clinically significant (severity ≥ 2). Decreased left aPFC activation was significantly associated with an increase in avoidance symptoms (Cluster C; W = 6.82, p = .009, OR = 0.48, 95% CI = [0.28 – 0.83]), but not with an increase in the other symptom clusters (Cluster-B intrusions, W = 2.79, p = .095, OR = 0.70, 95% CI = [0.46 – 1.063]; Cluster-D alterations in cognitions and mood, W = 3.44, p = .064, OR = 0.67, 95% CI = [0.44 – 1.02]; Cluster-E Hyperarousal, W = .77, p = .38, OR = 0.85, 95% CI = [0.58 – 1.23]; Bonferroni-corrected α-level = .0125).
To further investigate the predictive value of the aPFC activation in concert with other measures, such as the self-reported traumatic events and the behavioral measures from the approach-avoidance task, we ran a hierarchical regression analysis with symptom change as the dependent variable, building on the relationships described above. The base model contained self-reported traumatic events (both before baseline and between baseline and follow-up) and symptoms at baseline as predictors. This model was significant (F (3,181) = 8.95, R2 = .13, p < .001) and featured significant contributions of trauma load increase and baseline symptoms. Addition of the congruency effect on reaction time and error rates did not significantly increase the explained variance of the model (F(2,179) = 0.19, R2 -change = .002, p = .83). However, addition of the activation for the congruency contrast, extracted from the aPFC cluster explained significantly more variance (F(1,178) = 11.627, R2-change = .053, p = .001). To summarize, the hierarchical regression analysis showed that the symptom change can be best predicted by looking at subjective reports of traumatic events and symptoms in concert with our objective neural marker of frontal activation.
Amygdala activation during emotion control at baseline was not significantly associated with trauma symptom increase (Δ-PCL) or baseline trauma symptoms (PCL-baseline) (no clusters p FWE<.05). The amount of experienced traumatic events before baseline (PLES-baseline) was associated with relatively more left amygdala activation during emotion control at baseline (xyz: -28, -4, -22, SVC pFWE = .003). Activation dependent on valence (angry vs. happy) or movement (avoid vs. approach), regardless of congruency, did not significantly predict symptoms (no clusters p FWE<.05).
Acquired changes in neural activation during emotion regulation related to traumatic events and symptoms
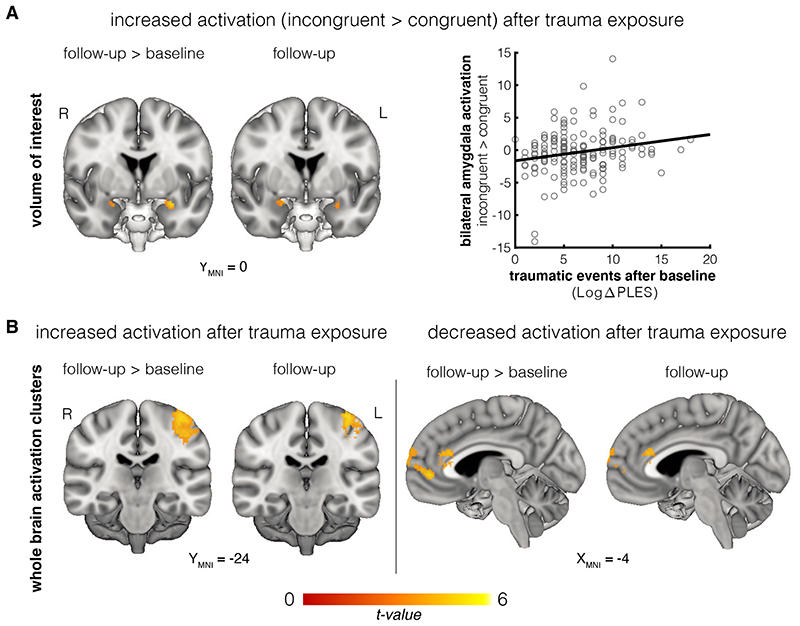
Next, we assessed which changes in neural activity were associated with (i) trauma exposure and (ii) PTSD symptom development, thus likely reflecting acquired neural effects. As far as effects of police-related experiences, the previously described positive association between potentially traumatic events before baseline and left amygdala activation during emotion regulation was reduced at follow-up (Change over time in association between PLES-baseline and congruency effect: left amygdala xyz -28, -4, -22, SVC pFWE = .002; association between PLES-baseline and congruency at follow-up: no clusters p FWE<.05). Instead, increased bilateral amygdala activation during emotion control at the follow-up assessment was positively associated with experienced traumatic events between baseline and follow-up measurement (Fig 3B; Change over time in association between Δ-PLES and congruency effect: left amygdala xyz -28, -2, -16, SVC pFWE < .001; right amygdala xyz 22, 2, -20, SVC pFWE = .017; association between Δ-PLES and congruency at follow-up: left amygdala xyz -24, -2, -18, SVC pFWE = .016; right amygdala xyz 20, 2, -18, SVC pFWE = .005). See Fig 4A and supplementary Fig 4 for a visualization of the amygdala effects. This effect was not significantly different for angry and happy faces (Steiger’s Z = 0.30, p = 0.77, see supplement Fig 2B). Moreover, post-hoc tests showed that these effects remained significant after correcting for time between assessments (see supplement). The whole brain analysis further revealed that exposure to traumatic events was associated with increased activation during emotion control (incongruent>congruent) of the left pre-and post-central gyrus (i.e. primary motor and sensory cortices), and reduced activation in the anterior cingulate cortex and medial frontal pole/paracingulate cortex (see table S2).These results have been corrected for baseline PCL, Δ-PCL, and baseline PLES.
Figure 4. Effects of trauma exposure on activation for incongruent > congruent responses.
A. The number of experienced different traumatic events was positively associated with increased bilateral amygdala activation during emotion control after trauma exposure (N = 160, left amygdala: SVC pFWE < .001; right amygdala: SVC pFWE = .017). The scatterplot illustrates the relationship between potentially traumatic events as scored on the Police Life Events Questionnaire (PLES) and mean beta-values for the bilateral amygdala activation cluster, extracted from the individual incongruent>congruent contrast maps. The image is thresholded at p < .005 for visualization. B. At the whole brain level, trauma exposure was associated with increased activation in the left pre-and post-central gyrus, and decreased activation in the medial frontal pole and anterior cingulate gyrus (N = 160). Images are thresholded at p < .001. Brain images are created with Mango software (Research Imaging Institute, UTHSCSA)
As far as acquired changes in relation to symptom development, increases in traumatic symptoms were not significantly related to left aPFC activation at follow-up (Δ-PCL x congruency x time: xyz -40, 58, -4, SVC pFWE = .009; Δ-PCL x congruency at follow-up pFWE > .999), in contrast to the relationship found at baseline. This indicates that reduced left anterior PFC activation during emotion control predicts future trauma symptom development prospectively, but not retrospectively in this sample. The same holds true for most of the other frontal clusters reported in the analysis of predictive factors, including the medial frontal pole, dorsal frontal pole and posterior cingulate (See table S2 for all whole brain results). These results have been corrected for baseline PCL, baseline PLES and Δ-PLES. Activation dependent on valence (angry vs. happy) or movement (avoid vs. approach), regardless of congruency, did not change as a function of symptoms or trauma load.
To investigate the role of aPFC-amygdala connectivity in PTSD resilience, we conducted a generalized psycho-physiological interaction (gPPI) analysis44 with the bilateral amygdala volume of interest as seed region and the different task conditions as regressors. No statistically significant congruency effects on aPFC-amygdala connectivity were found, nor any significant relationship between PTSD symptomatology and aPFC-amygdala connectivity, both for the task performed at baseline and at follow-up.
To summarize, relatively lower PTSD symptom increase could be predicted by relatively higher left anterior PFC activation during emotion control preceding a period of trauma exposure. Trauma exposure itself was related to more amygdala activation during emotion control following the period of trauma exposure.
Discussion
This prospective longitudinal study shows that strong prefrontal emotion control is associated with increased resilience against developing PTSD, providing a promising resilience marker for PTSD-symptoms. By testing a healthy sample of police recruits with high risk for trauma exposure on an emotion control task before and after trauma exposure, we could disentangle predictive and acquired effects of post-traumatic stress symptoms, without potential disease-related confounding factors such as medication. Those recruits who activated the aPFC more strongly when controlling emotional action tendencies, showed relatively less increase in PTSD symptoms after trauma exposure. Similar relationships were found with other prefrontal regions, including more medial and dorsal portions of the frontal pole. As far as acquired effects, the experience of traumatic events -independent of traumatic symptoms- was associated with increased amygdala activation, as well as decreased activation in the para/anterior cingulate cortex, during emotion control at follow-up. Thus, predictive and acquired effects are linked to distinct parts of the emotion-control network, with frontal control being a possible resilience marker for symptom development, and increased amygdala activation being a consequence of trauma exposure.
Emotion dysregulation is a hallmark of PTSD and inadequate development of emotion regulatory systems have been hypothesized as a vulnerability factor for the disorder1,7. In line with this, previous cross-sectional neuroimaging studies showed the involvement of reduced prefrontal control in the disorder2,10. We extend these findings by showing that reduced prefrontal control may already exist prior to exposure to the traumatic event(s) that trigger development of PTSD symptoms. More specifically, reduced prefrontal activation during the regulation of approach-avoidance behaviour was predictive for a clinically significant increase in avoidance symptoms, adding to our understanding of one of the core features of PTSD. The previous prospective PTSD studies did not report prefrontal activation as a predisposing factor in PTSD development36,37. This may be related to the fact that these studies involved different tasks, including passive picture viewing36 and gambling tasks37. These tasks capture the processes of emotional reactivity and risk-and reward sensitivity, respectively, but not into active emotion control as in the current study. Changes in PTSD symptoms were not related to individual differences in the recruitment of the anterior prefrontal cortex during emotion control at the follow-up measurement. Trauma exposure, but not traumatic stress symptom increase, was related with decreased anterior cingulate and medial frontal activation and increased amygdala and motor cortex activation during emotion control at follow-up. Increased amygdala activation in combination with reduced medial prefrontal activation is widely considered to play a key role in PTSD13,16,17 and increased amygdala and dACC activation specifically has been shown to be a predisposing factor5. Possibly, our results reflect the early effects of trauma exposure on the emotion regulation system, mainly reflecting hypervigilance45. This could eventually lead to PTSD symptomatology when it does not return to pre-trauma levels on the long term, due to other vulnerability factors or additional traumatic events5. This mechanism would be in line with a longitudinal study in deployed soldiers also showed a positive association between trauma exposure and amygdala activity, independently of symptom development, which normalized when a longer time (1.5 years) had passed since the trauma exposure45. Furthermore, childhood trauma is a well-known risk factor for PTSD development in adulthood46, and has been consistently associated with amygdala hyperactivity47,48. Taken together, our finding of early trauma-induced amygdala hyperactivity could provide a potential mechanism of cumulative effects of trauma exposure on PTSD symptom severity5,49.
In the current study, we focused on a specific sub-region of the PFC, the anterior PFC. Across studies, activation in this region is consistently associated with control over emotionally incongruent actions in the approach-avoidance task as well as in complex emotion regulation tasks30. Moreover, individual differences in task-related activation of this region has been linked to various types of psychopathology, such as psychopathy and borderline personality disorder50,51, and we have recently shown, in an overlapping sample, that higher activation in this area predicts milder acute cortisol responses after a stress induction procedure52. It has recently been argued activation of the aPFC in the context of emotion reflects monitoring of alternative emotion regulation strategies or actions30. It is unlikely, however, that the aPFC is the only prefrontal sub-region predicting PTSD vulnerability. In line with this, the whole brain analysis showed that reduced activation in various other frontal regions, including medial and dorsal portions of the frontal pole, before trauma exposure was also associated with increased PTSD symptoms at follow-up (See Table S1). Only activation in anterior PFC, however, moderated the relationship between trauma exposure and symptom increase, suggesting it may play an important role in coping with emotional and stressful events.
The approach-avoidance task measures emotional action control, which requires control over the interaction between the emotional valence of a stimulus and action tendencies43,53. As such, this task may be closer actual to coping with real-life emotional events than tasks that involve resolving conflict at the stimulus level, such as in an emotional Stroop task54. Our findings are linked to emotional action control and could therefore extend existing neurobiological models of PTSD that stress the importance of the dACC and vmPFC as key neural regions, but are mainly build on evidence from tasks that probe different aspects of emotion processing and regulation1,11,13. This is exemplified by the fact that frontal activation predicted a clinically significant increase in avoidance symptoms specifically, which is a core symptom and maintaining factor of the disorder23,55.
Apart from providing a marker for resilience, our findings may have (preventive) treatment implications. Improving emotion regulation capacities is a key feature of PTSD treatments, such as cognitive behavioural therapy (CBT)56,57. Our results suggest that improving emotion regulation capacities prior to exposure to traumatic events may help to prevent PTSD symptoms from developing in the first place. Such a training could be targeted at at-risk populations, such as first responders or soldiers, or shortly after trauma exposure in the general population. Neurofeedback may be a promising candidate for this training, as a recent study showed the potential of real time fMRI neurofeedback to help PTSD patients to upregulate prefrontal activation and downregulate amygdala activation58. Interestingly, a recent study indicates that successful psychotherapy of PTSD patients is associated with increased activation of the left lateral aPFC activity and aPFC – vmPFC connectivity at follow up. subsequent transcranial magnetic stimulation revealed that this connectivity reflected downstream modulation of the vmPFC by the aPFC59. Together with previous work showing connectivity between these regions during fear downregulation60, this provides a potential mechanism of how the aPFC may be connected to the network of regions traditionally associated with PTSD symptomatology.
Our findings should be interpreted in light of several strengths and limitations. A strength of this study is that it investigates emotional control abilities as a resilience factor for PTSD development prospectively. However, the police officers have passed stringent selection and may therefore not be representative of the general population, which limits the generalizability. Moreover, the sample as a whole is relatively resilient and only a few cases of full-blown PTSD occurred. Nevertheless, both the sample itself and the experienced traumatic events are more heterogeneous thanthose samples and events observed in other prospective studies, which investigated deployed soldiers. In accordance with previous fMRI studies, we used an approach-avoidance task with a relatively mild social challenge optimized to detect individual differences in neural effects while keeping performance relatively stable across participants61. This may explain why we did not find a relationship between task performance and PTSD symptomatology. Behavioural studies often employ a more provocative task version in which the faces increase and decrease in size with approach-and avoidance movements, respectively. This may elicit stronger individual differences in task performance, but is less suitable for fMRI because of the perceptual confounds of the zooming of the faces.
Our results suggest that stronger neural emotion regulation capabilities, reflected by aPFC activation, predict increased resilience against developing post-traumatic stress symptoms. In contrast, acquired effects after trauma are manifested in increased amygdala activity during emotion control. These findings suggest frontal emotion-control is a promising marker for stress-resilience and can provide fruitful starting points for prediction and preventive intervention studies.
Methods
The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Independent Review Board Nijmegen, the Netherlands. All participants provided written consent before study participation.
Participants
All participants were recruits from the Dutch Police Academy. 713 police recruits were assessed for eligibility, of whom 57 were excluded, 314 declined to participate, and 342 (48.0%) provided informed consent. Exclusion criteria were any current psychiatric or neurological disorder; history of, or current, neurological or endocrinological treatment; current use of psychotropic medication; and current drug-or alcohol abuse. The sample size was chosen to facilitate stable estimates of correlations with a small to medium effect size62. Three-hundered-forty participants completed the baseline assessment, of whom 271 (79.7%) completed follow-up, 21 (6.2%) completed follow-up symptom assessments only, and 48 (14.1%) were lost to follow-up. Study attrition was not related to demographics, baseline clinical characteristics or trauma history. As we aimed to predict development of trauma-related symptoms, we included participants who experienced their core traumatic event between baseline and follow-up (N = 222), as assessed with a clinical interview (Clinician-Administered PTSD scale, CAPS63) and who did not have PTSD symptoms above the clinical cut-off at baseline (PCL-5 total score > 3364 which led to the exclusion of one additional participant. Participants without trauma exposure data (PLES, see below; 6), no fMRI approach-avoidance task data acquired at baseline (13), with anatomical abnormalities or poor MRI quality (1), excessive movement during MRI acquisition (i.e. maximal framewise displacement > 3 mm; 16), were excluded. Consequently, the final analyses included 185 (140 males) participants between 18 and 45 years (median: 23, interquartile range: 6). The final sample showed no significant differences compared to other participants who were considered for participation (N = 527), not in terms of age (included 24.15 (SD 5.32) vs. excluded: 24.69 (SD 6.25), t(706) = 1.06, p = .289), nor in terms of gender (included: 26% females vs. excluded 30% females, χ 2 = 0.73, p = .45). See consort flow diagram in supplement. For the analyses that included MRI data at the follow-up measurement, a subsample of 160 (86%) participants was available (122 males, age median 23, interquartile range: 5). Participants were financially compensated with EUR 50.
Procedure
All reported experiments were conducted as part of a large prospective study assessing the role of automatic defensive responses in the development of trauma-related psychopathology in police recruits (Netherlands Trial Registry NTR6355). See Koch et al.40 for details on the general procedure and for details on the emotion control task see52,65. Baseline assessments took place when the police officers were still in the safe environment of the police academy. Follow-up assessments took place after the first periods of emergency aid experiences with potentially trauma-related events (Fig 1A), on average after 16 months (M = 483 days, SD = 57 days, range 349-679 days). Upon arrival in the lab, participants provided written informed consent, completed several questionnaires and performed several behavioural and fMRI tasks (not reported here). In the MRI scanner, participants underwent a 7-min structural T1-weighted scan, and completed the approach-avoidance task (12-min), which was preceded by a 3-min approach-avoidance task training session. The procedure was similar for the baseline and follow-up session.
Questionnaires
As mentioned in the protocol article of this study40, the primary outcome measure was change in PTSD symptom severity assessed by the PTSD checklist for DSM-5 (PCL-5)64,66. The PCL-5 was used to assess PTSD symptom severity, by self-rating of all DSM-5 PTSD symptoms in the last month, ranging from 0 (absent) to 4 (extreme/incapacitating). The PCL had a good to excellent reliability score at baseline (Crombach’s α = .85) and follow-up (Crombach’s α = .93). To assess specifically the increase in PTSD symptoms, we calculated a PTSD change score (Δ-PCL) by subtracting the baseline PCL-5 score from the follow-up score. As part of a follow-up analysis, we used automatic clustering of Δ-PCL scores using the two-step cluster method67, to derive different symptom groups. Participants filled out the Police Life Events Scale (PLES) to measure police work-related trauma incidence before and during the emergency aids68. This is a list of 40 possible traumatic police work-related events that are violent (for example shootings or escalating riot situations) or tragic (for example finding a corpse or being confronted with severely mutilated victims). The baseline PLES inquired about all events that participants had previously encountered during their life, but the follow-up PLES inquired specifically about all events during the period of approximately 16 months between baseline and follow-up. The PLES score reflects the number of different encountered traumatic events. The change is trauma exposure is captured by the follow-up PLES, which is therefore referred to as ‘Δ-PLES’ for the remainder of this report for consistency.
Experimental task
The approach-avoidance task is a well-established task41,53, requiring participants to respond as fast as possible to pictures of facial expressions by moving a joystick (Fig 2A). The automatic tendency is to approach happy and avoid angry faces (affect-congruent actions). Execution of the opposite - affect-incongruent - actions requires control over these automatic action tendencies, reflected in longer reaction times and stronger aPFC recruitment18,26. At the start of congruent blocks, participants were instructed to pull the joystick in response to happy faces and to push in response to angry faces. The instruction was reversed for incongruent blocks. 4 congruent and 4 incongruent blocks (12 trials each) were presented alternatingly, with an inter-block interval of 21-24 s and an inter-trial interval of 2-4 s. The first block type was counterbalanced across participants. The facial expressions (36 models, 18 male, 2 emotions each), originated from multiple databases69–72. Presentation software version 16 (www.neurobs.com) was used for stimuli presentation and the acquisition of joystick positions. See Tyborowska et al.73 for further details.
Materials and apparatus
All MRI data were acquired with a Siemens 3T Magnetom Prisma scanner (Siemens Medical Solution) with a 32-channel head coil. Structural images were obtained using a combined MPRAGE and GeneRalized Autocalibrating Partially Parallel Acquisitions (GRAPPA) sequence (192 slices, TR: 2300, TE: 3.03, FOV: 256 mm, flip angle 8°, voxel size = 1.0 x 1.0 x 1.0 mm). Functional scans were acquired with an ascending dual-echo echoplanar imaging (EPI) sequence with optimal sensitivity for both prefrontal areas and ventral areas including the amygdala (37 transversal slices, TR: 1740 ms; TE: 11 and 25 ms, FOV 212 mm flip angle 90°, voxel size = 3.3 x 3.3 x 3.0 mm)74.
Preprocessing of behavioural data
Behavioural task effects were analysed using Matlab 2012 (Mathworks) and SPSS Statistics 23 (IBM). The joystick displacement measures for each trial were reconstructed into a reliable measure of joystick movement onset73. Trials that contained a movement in the wrong direction or no movement at all were considered as errors. Trial blocks with an error rate (ER) at or above chance level were excluded from ER and reaction time (RT) analyses. In total, 13 out of 185 participants had one or two blocks excluded, resulting in the exclusion of 16 out of 1480 blocks (1%) for the total dataset. RT was defined as the time between the onset of the presentation of the facial expression and the onset of the movement. For the RT analyses, error trials, trials with an extreme RT (<100 or >1500 ms), and trials with an RT >3 SDs from the individual mean were excluded. Reaction times were log-transformed on the individual levels to correct for a skewed distribution. Mean RTs and ERs were calculated for each level of the experimental factors (valence and movement), resulting in 4 variables (approach-happy, approach-angry, avoid-happy, avoid-angry) per participant.
Behavioural analysis
Two separate repeated-measure ANOVAs were run to analyse mean RTs and ERs, with within-subject factors movement (approach/avoid) and valence (happy/angry). Δ-PCL (follow-up-baseline), log-transformed PCL baseline score, log-transformed PLES baseline score and log-transformed Δ-PLES score were included as covariates. Log-transformations were applied to correct for a skewed distribution. The first model included all data acquired at the baseline measurement. The second model included all baseline and follow-up data and was run to assess time effects (follow-up vs. baseline). This model included time as an extra within-subjects factor. The α-level was set at 0.05.
fMRI preprocessing
The Matlab toolbox SPM12 (Statistical Parametric Mapping, www.fil.ion.ucl.uk/spm) was used to pre-process and analyse the imaging data. To allow for T1 equilibration, the first 6 volumes of each subject’s dataset were discarded. The two echoes were combined to form a single time series, using echo-time weighted combination. subsequently, principal component analysis (PCA) was used to filter out motion-related slice-specific noise components75. The image time series were spatially realigned using a least-squares approach with the six rigid-body transformation parameters, and slice-time corrected. After spatial co-registration to the mean of the functional images, the T1-weighted image was segmented into grey matter, white matter and cerebrospinal fluid (CSF) compartments.
After normalization to standard Montreal Neurological Institute (MNI) space, the functional images were spatially smoothed using an isotropic 8 mm full-width at half-maximum Gaussian kernel. Finally, an automated removal of motion artefacts (AROMA) procedure was conducted to filter out motion-related artefacts76.
fMRI subject-level analysis
The fMRI time series were analysed using an event-related approach within the framework of the general linear model, following Volman et al. and Tyborowska et al.18,73. The following effects were considered separately: approach-happy, approach-angry, avoid-happy and avoid-angry. The time of stimulus presentation (onset) and the time between stimulus presentation and response (duration), were convolved with the canonical hemodynamic response function. Misses and on-screen information (instructions preceding each block and feedback messages) were modelled as separate regressors. Potentially confounding effects of residual head movement were modelled using original, squared, cubic, first-order, and second-order derivatives of the movement parameters77. Three further regressors described the time course of signal intensities of white matter, CSF, and the portion of the MR image outside the skull78. The fMRI time series were high-pass filtered (cut-off 128s). Temporal autocorrelation was corrected by a first-order autoregressive model (AR1).
fMRI group-level analyses
To test the predictive effects of neural emotional control for symptom development, contrast images of the 4 task conditions at baseline were entered into a random-effects multiple regression analysis, separately for each condition (4 regressors). We first contrasted affect-incongruent trials (approach-angry and avoid-happy) with affect-congruent trials (approach-happy and avoid-angry) to test for the previously reported congruency effects. subsequently, Δ-PCL values, log-transformed baseline PCL values, log-transformed PLES baseline values and log-transformed Δ-PLES values were added as condition-specific covariates, yielding 16 extra regressors. We assessed the interaction of the congruency effects with the trauma symptom (Δ-PCL) and trauma events (Δ-PLES) measures, by contrasting these condition-specific covariates (e.g. [Δ-PLES*approach-angry and Δ-PLES*avoid-happy] vs. [Δ-PLES*approach-happy and Δ-PLES*avoid-angry]).
To test the acquired effects PTSD symptomatology on approach-avoidance task activation, contrast images of the 4 task conditions for both assessment times (baseline and follow-up) were entered into a random-effects multiple regression analysis, separately for each condition and each assessment (8 regressors). We first contrasted the congruency effect at follow-up with the congruency effect at baseline to assess overall differences in task-related activation. Δ-PCL values, log-transformed baseline PCL values, PLES baseline values and Δ-PLES values were added as condition-specific covariates for baseline and follow-up separately, yielding 32 extra regressors. subsequently, we assessed the interaction between time (follow-up vs. baseline), congruency effects, trauma symptom (Δ-PCL) and trauma events (Δ-PLES) measures. Significant time interaction effects were follow-up by post-hoc models assessing the effect at baseline and follow-up separately. Main effects of valence ([happy-approach and happy-avoid] vs. [angry-approach and angry-avoid]), of movement ([happy-approach and angry-approach] vs. [happy-avoid and angry-avoid], and vice versa), and their interactions with the predictors of interest (symptoms (Δ-PCL) and trauma events (Δ-PLES)) were also assessed.
Effects were assessed at the whole-brain level and within a priori defined regions of interests: the bilateral anterior PFC (aPFC), which has been shown to be sensitive to approach-avoidance task congruency effects18,19 and the bilateral amygdala. Following previous work52,65,73, we used the lateral frontal pole region, or lateral Brodmann area 10, as a volume of interest (VOI) for the aPFC (Centres of gravity in MNI space [-26, 54, 0] and [26, 54, 0], See Fig 2C). This region is anatomically defined in a frontal cortex parcellation atlas24 based on the structural connectivity profile of the anterior and ventrolateral prefrontal cortex (available at: http://www.rbmars.dds.nl/CBPatlases.htm). We used an anatomically defined VOI for the bilateral amygdala, based on the Automated Anatomical Labelling Atlas79. The reported activation clusters were corrected for multiple comparisons using a family-wise error (FWE) correction. Statistical inferences were made at the cluster-level for the whole-brain analyses (initial threshold p < .001; FWE: p < .05) and at the peak-level for the volumes of interest (small volume correction SVC, FWE: p < .05). Anatomical inference was drawn by superimposing the thresholded SPM T-maps on the canonical SPM single subject T1 image.
In follow-up analyses, we investigated whether left aPFC activation at baseline predicted a clinically significant increase in specific PTSD symptom clusters. A clinically significant increase was defined as the development of at least one symptom (severity ≥ 2 on PCL-checklist66) between baseline and follow-up assessments, resulting in a binary score for each participant on each cluster (i.e. clinically significant increase - yes/no). The percentage of participants showing a clinically significant symptom increase were as follows: Cluster-B intrusions: 15.1%; Cluster-C avoidance: 8.1%; Cluster-D alterations in cognitions and mood: 14.6%; Cluster-E Hyperarousal: 18.9%). Binary logistic regression analyses were conducted on development of symptoms at each cluster, with extracted mean beta-values from the incongruent>congruent contrast of the cluster predicting PTSD symptoms as independent variable. Results were bonferroni-corrected for multiple comparisons (4 models). Additional follow-up analyses included a hierarchical regression analysis to assess the added value of incorporating behavioral (congruency effects reaction times and error rates) and neural (congruency effect in left anterior PFC; extracted beta-values) in predicting PTSD symptom increase (Δ-PCL) (see results section and supplement). Finally, we conducted a moderation analysis of baseline prefrontal activation (extracted beta values from congruency contrast) on the relationship between trauma load (Δ-PLES) and symptom increase (Δ-PCL).
Supplementary Material
Acknowledgements
We thank all participants for their willingness to participate in this study. The authors thank Annika Smit and other personnel of the Dutch Police Academy for their valuable help with recruiting participants and facilitating this study. We gratefully acknowledge contributions of Ingrid Kersten, Tiele Döpp, Naomi de Valk, Leonore Bovy and Lisanne Nuijen in participant recruitment and data acquisition, Naomi Garaux in assistance with data analysis and Vanessa van Ast for her help with setting up the study. This study was funded by the Netherlands Organization for Scientific Research (NWO VICI-grant 453-12-0010) and a starting grant from the European Research Council (StG2012 313749), both awarded to Karin Roelofs. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Author Contributions
R.K., M.M.H., W.Z., S.B.J.K, F.K. and K.R. contributed to the study design. R.K., W.Z., and M.M.H. conducted the experiment. R.K. performed data analysis. R.K., S.B.J.K. and K.R. drafted the manuscript. R.K., M.M.H., W.Z., S.B.J.K, F.K. and K.R. edited the manuscript and approved the final version of the manuscript for submission.
Competing Interests
The authors declare no competing interests
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
The computer code that supports the findings of this study is available from the corresponding author upon reasonable request.
References
- 1.Liberzon I, Abelson JL. Context Processing and the Neurobiology of Post-Traumatic Stress Disorder. Neuron. 2016;92:14–30. doi: 10.1016/j.neuron.2016.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabinak CA, et al. Focal and aberrant prefrontal engagement during emotion regulation in veterans with posttraumatic stress disorder. Depress Anxiety. 2014;31:851–861. doi: 10.1002/da.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzgerald JM, Digangi JA, Phan KL. Functional Neuroanatomy of Emotion and Its Regulation in PTSD. Harv Rev Psychiatry. 2018;26:116–128. doi: 10.1097/HRP.0000000000000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalisch R, et al. The resilience framework as a strategy to combat stress-related disorders. Nat Hum Behav. 2017;1:784–790. doi: 10.1038/s41562-017-0200-8. [DOI] [PubMed] [Google Scholar]
- 5.Admon R, Milad MR, Hendler T. A causal model of post-traumatic stress disorder: Disentangling predisposed from acquired neural abnormalities. Trends Cogn Sci. 2013;17:337–347. doi: 10.1016/j.tics.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 6.de Vries G-J, Olff M. The Lifetime Prevalence of Traumatic Events and Posttraumatic Stress Disorder in the Netherlands. J Trauma Stress. 2009;22:259–267. doi: 10.1002/jts.20429. [DOI] [PubMed] [Google Scholar]
- 7.Lanius RA, Frewen PA, Vermetten E, Yehuda R. Fear conditioning and early life vulnerabilities: two distinct pathways of emotional dysregulation and brain dysfunction in PTSD. Eur J Psychotraumatol. 2010;1:5467. doi: 10.3402/ejpt.v1i0.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehlers A, et al. Heart rate responses to standardized trauma-related pictures in acute posttraumatic stress disorder. Int J Psychophysiol. 2010;78:27–34. doi: 10.1016/j.ijpsycho.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord. 2012;2:1–13. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttraumatic stress disorder and beyond: A meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2012;36:2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Michopoulos V, Norrholm SD, Jovanovic T. Diagnostic Biomarkers for Posttraumatic Stress Disorder: Promising Horizons from Translational Neuroscience Research. Biol Psychiatry. 2015;78:344–353. doi: 10.1016/j.biopsych.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitman RK, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch SBJ, et al. Intranasal Oxytocin Normalizes Amygdala Functional Connectivity in Posttraumatic Stress Disorder. Neuropsychopharmacology. 2016;41:2041–2051. doi: 10.1038/npp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sripada RK, et al. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci. 2012;37:241–249. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rauch SL, Shin LM, Phelps Ea. Neurocircuitry Models of Posttraumatic Stress Disorder and Extinction: Human Neuroimaging Research-Past, Present, and Future. Biological Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry. 2010;167:648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volman I, Toni I, Verhagen L, Roelofs K. Endogenous testosterone modulates prefrontal-amygdala connectivity during social emotional behavior. Cereb Cortex. 2011;21:2282–90. doi: 10.1093/cercor/bhr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volman I, et al. Reduced serotonin transporter availability decreases prefrontal control of the amygdala. J Neurosci. 2013;33:8974–9. doi: 10.1523/JNEUROSCI.5518-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radke S, et al. Testosterone biases the amygdala toward social threat approach. Sci Adv. 2015;1:e1400074–e1400074. doi: 10.1126/sciadv.1400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertsch K, et al. Neural correlates of emotional action control in angerprone women with borderline personality disorder. J Psychiatry Neurosci. 2018;43:161–170. doi: 10.1503/jpn.170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LeDoux JE, Moscarello J, Sears R, Campese V. The birth, death and resurrection of avoidance: A reconceptualization of a troubled paradigm. Mol Psychiatry. 2017;22:24–36. doi: 10.1038/mp.2016.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. American Psychiatric Publishing; 2013. [Google Scholar]
- 24.Neubert FX, Mars RB, Thomas AG, Sallet J, Rushworth MFS. Comparison of Human Ventral Frontal Cortex Areas for Cognitive Control and Language with Areas in Monkey Frontal Cortex. Neuron. 2014;81:700–713. doi: 10.1016/j.neuron.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- 26.Bramson B, et al. Human lateral frontal pole contributes to control over emotional approach–avoidance actions. J Neurosci. 2020;40:2925–2934. doi: 10.1523/JNEUROSCI.2048-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folloni D, et al. Dichotomous organization of amygdala/temporal-prefrontal bundles in both humans and monkeys. Elife. 2019;8:1–23. doi: 10.7554/eLife.47175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalisch R. The functional neuroanatomy of reappraisal: Time matters. Neurosci Biobehav Rev. 2009;33:1215–1226. doi: 10.1016/j.neubiorev.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Morawetz C, Bode S, Derntl B, Heekeren HR. The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: A meta-analysis of fMRI studies. Neurosci Biobehav Rev. 2017;72:111–128. doi: 10.1016/j.neubiorev.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Koch SBJ, Mars RB, Toni I, Roelofs K. Emotional control, reappraised. Neurosci Biobehav Rev. 2018;95:528–534. doi: 10.1016/j.neubiorev.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Lanius RA, et al. Functional connectivity of dissociative responses in posttraumatic stress disorder: A functional magnetic resonance imaging investigation. Biol Psychiatry. 2005;57:873–884. doi: 10.1016/j.biopsych.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Morey RA, Petty CM, Cooper DA, LaBar KS, McCarthy G. Neural systems for executive and emotional processing are modulated by symptoms of posttraumatic stress disorder in Iraq War veterans. Psychiatry Res - Neuroimaging. 2008;162:59–72. doi: 10.1016/j.pscychresns.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun D, et al. Brain structural covariance network topology in remitted posttraumatic stress disorder. Front Psychiatry. 2018;9:1–10. doi: 10.3389/fpsyt.2018.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadeh N, et al. Neurobiological indicators of disinhibition in posttraumatic stress disorder. Hum Brain Mapp. 2015;36:3076–3086. doi: 10.1002/hbm.22829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sadeh N, et al. SKA2 methylation is associated with decreased prefrontal cortical thickness and greater PTSD severity among trauma-exposed veterans. Mol Psychiatry. 2016;21:357–363. doi: 10.1038/mp.2015.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Admon R, et al. Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proc Natl Acad Sci U S A. 2009;106:14120–14125. doi: 10.1073/pnas.0903183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Admon R, et al. Imbalanced neural responsivity to risk and reward indicates stress vulnerability in humans. Cereb Cortex. 2013;23:28–35. doi: 10.1093/cercor/bhr369. [DOI] [PubMed] [Google Scholar]
- 38.Van Wingen GA, Geuze E, Vermetten E, Fernández G. Perceived threat predicts the neural sequelae of combat stress. Mol Psychiatry. 2011;16:664–671. doi: 10.1038/mp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carlier IVE, Lamberts RD, Gersons BP. Risk Factors for Posttraumatic Stress Symptomatology in Police Officers: A Prospective Analysis. J Nerv Ment Dis. 1997;185:498–506. doi: 10.1097/00005053-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Koch SBJ, et al. The role of automatic defensive responses in the development of posttraumatic stress symptoms in police recruits: protocol of a prospective study. Eur J Psychotraumatol. 2017;8 doi: 10.1080/20008198.2017.1412226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen M, Bargh JA. Consequences of Automatic Evaluation: Immediate Behavioral Predispositions to Approach or Avoid the Stimulus. Personal Soc Psychol Bull. 1999;25:215–224. [Google Scholar]
- 42.Rotteveel M, Phaf RH. Automatic affective evaluation does not automatically predispose for arm flexion and extension. Emotion. 2004;4:156–172. doi: 10.1037/1528-3542.4.2.156. [DOI] [PubMed] [Google Scholar]
- 43.Bramson B, Jensen O, Toni I, Roelofs K. Cortical Oscillatory Mechanisms Supporting the Control of Human Social–Emotional Actions. J Neurosci. 2018;38:5739–5749. doi: 10.1523/JNEUROSCI.3382-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. Neuroimage. 2012;61:1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Wingen GA, Geuze E, Vermetten E, Fernández G. The neural consequences of combat stress: Long-term follow-up. Mol Psychiatry. 2012;17:116–118. doi: 10.1038/mp.2011.110. [DOI] [PubMed] [Google Scholar]
- 46.Yehuda R, Halligan SL, Grossman R. Childhood trauma and risk for PTSD: Relationship to intergenerational effects of trauma, parental PTSD, and cortisol excretion. Dev Psychopathol. 2001;13:733–753. doi: 10.1017/s0954579401003170. [DOI] [PubMed] [Google Scholar]
- 47.Tottenham N, et al. Elevated amygdala response to faces following early deprivation. Dev Sci. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dannlowski U, et al. Limbic scars: Long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 49.Yehuda R, et al. Post-traumatic stress disorder. Nat Rev Dis Prim. 2015;1:15057. doi: 10.1038/nrdp.2015.57. [DOI] [PubMed] [Google Scholar]
- 50.Volman I, et al. Testosterone Modulates Altered Prefrontal Control of Emotional Actions in Psychopathic Offenders. eNeuro. 2016;3:1–12. doi: 10.1523/ENEURO.0107-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bertsch K, et al. Out of control? Acting out anger is associated with deficient prefrontal emotional action control in male patients with borderline personality disorder. Neuropharmacology. 2019;156 doi: 10.1016/j.neuropharm.2018.12.010. 107463. [DOI] [PubMed] [Google Scholar]
- 52.Kaldewaij R, et al. Frontal Control Over Automatic Emotional Action Tendencies Predicts Acute Stress Responsivity. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:975–983. doi: 10.1016/j.bpsc.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 53.Roelofs K, Minelli A, Mars RB, van Peer J, Toni I. On the neural control of social emotional behavior. Soc Cogn Affect Neurosci. 2009;4:50–58. doi: 10.1093/scan/nsn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams JMG, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychol Bull. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- 55.Davies CD, Niles AN, Pittig A, Arch JJ, Craske MG. Physiological and behavioral indices of emotion dysregulation as predictors of outcome from cognitive behavioral therapy and acceptance and commitment therapy for anxiety. J Behav Ther Exp Psychiatry. 2015;46:35–43. doi: 10.1016/j.jbtep.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 56.Schnyder U, et al. Psychotherapies for PTSD: What do they have in common? Eur J Psychotraumatol. 2015;6 doi: 10.3402/ejpt.v6.28186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bryant RA, et al. Augmenting cognitive behaviour therapy for post-traumatic stress disorder with emotion tolerance training: A randomized controlled trial. Psychol Med. 2013;43:2153–2160. doi: 10.1017/S0033291713000068. [DOI] [PubMed] [Google Scholar]
- 58.Nicholson AA, et al. The neurobiology of emotion regulation in posttraumatic stress disorder: Amygdala downregulation via real-time fMRI neurofeedback. Hum Brain Mapp. 2017;38:541–560. doi: 10.1002/hbm.23402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fonzo GA, et al. Selective effects of psychotherapy on frontopolar cortical function in PTSD. Am J Psychiatry. 2017;174:1175–1184. doi: 10.1176/appi.ajp.2017.16091073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klumpers F, et al. Prefrontal mechanisms of fear reduction after threat offset. Biol Psychiatry. 2010;68:1031–1038. doi: 10.1016/j.biopsych.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 61.Price CJ, Friston KJ. Scanning Patients With Tasks They Can Perform. Hum Brain Mapp. 1999;8:102–108. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<102::AID-HBM6>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schönbrodt FD, Perugini M. At what sample size do correlations stabilize? J Res Pers. 2013;47:609–612. [Google Scholar]
- 63.Boeschoten MA, et al. Clinician administered PTSD scale for DSM-5--Dutch version. Diemen, Netherlands Arq Psychotrauma Expert Groep. 2014 [Google Scholar]
- 64.Weathers FW, et al. The PTSD Checklist for DSM-5 (PCL-5) 2013.
- 65.Kaldewaij R, et al. High Endogenous Testosterone Levels Are Associated With Diminished Neural Emotional Control in Aggressive Police Recruits. Psychol Sci. 2019;30:1161–1173. doi: 10.1177/0956797619851753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boeschoten MA, Bakker A, Jongedijk RA, Olff M. PTSD checklist for DSM-5 – Dutch version. 2014.
- 67.Peter-Hagene LC, Ullman SE. Sexual Assault Characteristics Effects on PTSD and Psychosocial Mediators: A Cluster Analysis Approach to Sexual Assault Types. Psychol Trauma. 2015;7:162–170. doi: 10.1037/a0037304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carlier IVE, Gersons BPR. Development of a scale for traumatic incidents in police work. Psychiatr Fenn. 1992;23:59–70. [Google Scholar]
- 69.Ekman P, Friesen WV. Pictures of facial affect. Consult Psychol Press; 1976. [Google Scholar]
- 70.Matsumoto D, Ekman P. Japanese and caucasian facial expressions of emotion (JACFEE) and neutral faces. San Fr Dep Psychiatry, Univ Calif; 1988. [Google Scholar]
- 71.Lundqvist D, Flykt A, Ohman A. The Karolinska directed emotional faces (KDEF) CD ROM from Department of Clinical Neuroscience, Psychology section, Karolinska Institutet; 1998. [Google Scholar]
- 72.Martinez AM, Benavente R. The AR face database. CVC Tech Rep. 1998;24 [Google Scholar]
- 73.Tyborowska A, Volman I, Smeekens S, Toni I, Roelofs K. Testosterone during Puberty Shifts Emotional Control from Pulvinar to Anterior Prefrontal Cortex. J Neurosci. 2016;36:6156–64. doi: 10.1523/JNEUROSCI.3874-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Poser BA, Versluis MJ, Hoogduin JM, Norris DG. BOLD contrast sensitivity enhancement and artifact reduction with multiecho EPI: Parallel-acquired inhomogeneity-desensitized fMRI. Magn Reson Med. 2006;55:1227–1235. doi: 10.1002/mrm.20900. [DOI] [PubMed] [Google Scholar]
- 75.Nieuwhof F, et al. Impaired dual tasking in Parkinson’s disease is associated with reduced focusing of cortico-striatal activity. Brain. 2017;140:1384–1398. doi: 10.1093/brain/awx042. [DOI] [PubMed] [Google Scholar]
- 76.Pruim RHR, et al. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–277. doi: 10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- 77.Lund TE, Nørgaard MD, Rostrup E, Rowe JB, Paulson OB. Motion or activity: Their role in intra- and inter-subject variation in fMRI. Neuroimage. 2005;26:960–964. doi: 10.1016/j.neuroimage.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 78.Verhagen L, Grol MJ, Dijkerman HC, Toni I. Studying visually-guided reach-to-grasp movements in an MR-environment. Neuroimage. 2006;31:S45. [Google Scholar]
- 79.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The computer code that supports the findings of this study is available from the corresponding author upon reasonable request.