Abstract
Radiotherapy should have low toxicity in the entrance channel (normal tissue) and be very effective in cell killing in the target region (tumour). In this regard, ions heavier than protons have both physical and radiobiological advantages over conventional X-rays. Carbon ions represent an excellent combination of physical and biological advantages. There are a dozen carbon-ion clinical centres in Europe and Asia, and more under construction or at the planning stage, including the first in the USA. Clinical results from Japan and Germany are promising, but a heated debate on the cost-effectiveness is ongoing in the clinical community, owing to the larger footprint and greater expense of heavy ion facilities compared with proton therapy centres. We review here the physical basis and the clinical data with carbon ions and the use of different ions, such as helium and oxygen. Research towards smaller and cheaper machines with more effective beam delivery is necessary to make particle therapy affordable. The potential of heavy ions has not been fully exploited in clinics and, rather than there being a single ‘silver bullet’, different particles and their combination can provide a breakthrough in radiotherapy treatments in specific cases.
Radiotherapy with photons started shortly after the discovery of X-rays, but, as early as 1946, Robert R. Wilson proposed using accelerated charged particles—instead. The motivation for doing so was the Bragg peak of charged particles, that is, a plot of energy loss versus distance travelled through matter (the Bragg curve) shows a distinct peak before the particles come to rest (FIG. 1), suggesting that charged particles would be more conformal and produce lower toxicity to normal tissue than photons1. Wilson’s proposal involved protons, but the use of heavier ions in cancer therapy was proposed and pursued at Lawrence Berkeley Laboratory (LBL) by Cornelius A. Tobias2, with the idea that heavier ions have radiobiological properties that can lead to better tumour control than X-rays or protons3.
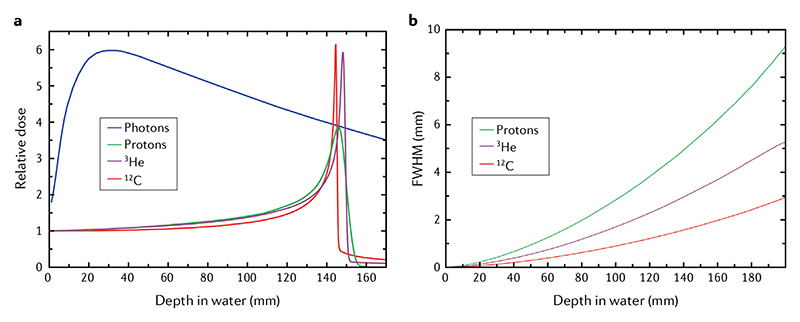
Figure 1. Heavy ion physics.
a| Depth-dose distributions showing the Bragg peak for different ions and the reduced straggling of heavier ions. The X-ray depth–dose curve is calculated for a high-energy 21-MeV linac. Energies of the ions correspond approximately to the same range (15 cm in tissue): 148 MeV1H beam, 170 MeV/n3He beam and 270 MeV/n12C beam. b | Lateral broadening (full width at half maximum (FWHM) of the Gaussian distribution, see Eq. 3) for the same ions. The widening of the beam should be compared with typical entrance spot sizes of proton and ion beams of ~5–10 mm. Image from the GSI image gallery, distributed under Creative Commons CC-BY. Figure is adapted from REF.201, CC BY 4.0.
Patients at LBL were treated in the period 1975-1992 with several ions: He, N, O, C, Ne, Si and Ar (REF.4). Although most of the patients were treated with light He ions, very heavy ions were also used with the goal of overcoming hypoxia, one of the major causes of cancer radioresistance5, which requires charged particles with very high ionization density (linear energy transfer; LET), according to experiments on cells performed at the LBL Bevalac accelerator6. However, the entrance channel LET of very heavy ions is relatively high, resulting in unacceptable patient toxicities. Carbon represents a good compromise, with an LET in the entrance channel of ~12 keV μm-1 and a fairly high LET on the spread-out Bragg peak (SOBP), ranging from 40 to 80 keV μm-1 (REF.7) (FIG. 2). As a result, therapeutic C-ion beams have the advantage of a track structure that is similar to protons in the entrance, but densely ionizing in the tumour, like that of neutrons or α-particles (see FIG. 2 insets and Supplementary Video 1 for the physical and biological tracks). For this very reason,12C ions were chosen for use in cancer therapy first in Japan8 and then in Europe9.
Hypoxia
Reduced oxygen supply in a tissue compared with the normal level (normoxia or physioxia). Tumours are typically hypoxic.
Linear energy transfer
Energy loss of charged particles per unit track length (see Eq. 1).
Spread-out Bragg peak
The monoenergetic beam Bragg peak is too narrow to cover a tumour volume. It must, therefore, be enlarged to provide a uniform biological dose to the target volume.
Track structure
The complete set of ionizations and excitation events caused by a charged particle traversing a medium. Energy is deposited either directly by the traversing ion or by the high-energy electrons emitted by target atom ionization (δ-rays — see Supplementary Video 1).
Conformal radiotherapy
A delivery system that shapes the radiation beams to match the shape of the tumour.
Straggling
Variation in the range of a particle beam caused by the stochastic nature of the energy loss process.
Dose halo
Energy deposited due to scattering in the volume immediately surrounding the target.
Treatment planning
The calculation of the optimal beam directions, energies and intensities to achieve the highest possible dose to the tumour while sparing organs at risk and reducing unnecessary dose to the normal tissue.
Hypofractionation
Reduction of the number of fractions and increase of the dose per fraction compared with the conventional radiotherapy scheme (2 Gy per fraction in 20–30 fractions, one fraction per day).
Gyroradius
Radius of the circular motion of a charged particle in the presence of a uniform magnetic field.
Rigidity
Impact of the magnetic field on the trajectory of a charged particle (Eq. 5).
Passive modulation systems
Systems to produce spread-out Bragg peak from a monoenergetic beam using passive scatterers with different techniques, such as a rotating wheel of different techniques or a scatterer with a collimator and a patient-specific compensator.
Targeted radioimmunotherapy
Cancer therapy that uses a targeting construct (e.g. antibody, peptides or nanoparticles), attached to a radionuclide, to deliver a systemic cytotoxic dose of radiation to malignant tissue.
Reoxygenation
Hypoxic sub-volumes in cancers are radioresistant. During the interval between fractions, the blood can reach the hypoxic niches that survived the previous fraction, making them radiosensitive.
second cancers
malignant neoplasias induced by the treatment to a primary tumour.
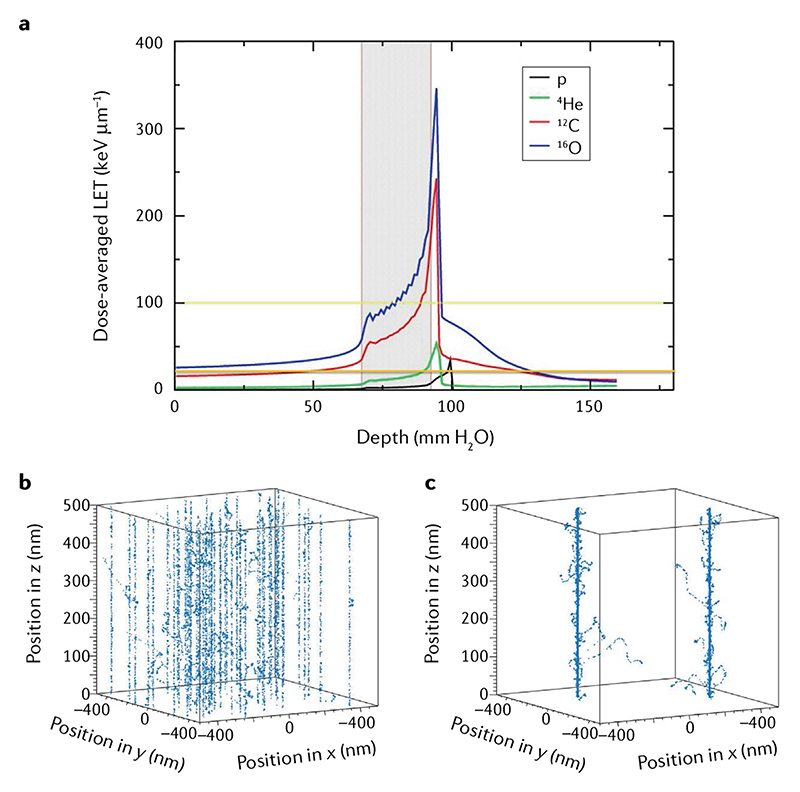
Figure 2. Dose-averaged linear energy transfer versus depth in tissue for a single spread-out Bragg peak of protons, He, C and O providing a uniform physical dose (2 Gy) in the target volume.
a | The grey area represents the tumour region, a 2.5 × 2.5 × 2.5 cm3 volume centred at 8 cm in water. The yellow and orange lines are 100 and 20 keV μm-1 linear energy transfer (LET) levels, respectively. An ideal bullet should have LET < 20 keV μm-1 in the entrance channel and >100 keV μm-1 in the target volume7. b,c | Monte Carlo TRAX code simulation of C-ion tracks in the entrance channel (100 MeV/n; to the left of the grey tumour region in part a) and the Bragg peak (3 MeV/n), providing the same dose in a 1 × 1 × 0.5 μm3 volume. Figure modified from Tommasino et al.167, distributed under Creative Commons CC-BY. Part a is adapted from REF.167, CC BY 4.0.
Although heavy ion therapy is the most advanced radiotherapy technology, patient access is problematic, owing to limited capacity. Radiotherapy technology has been continuously upgraded10, but not all patients benefit from cutting-edge technologies, including particle therapy or X-ray modern techniques, such as stereotactic body radiotherapy (SBRT)11. In the USA, most patients are still treated with conventional 3D conformal radiotherapy, followed by intensity-modulated radiotherapy (IMRT), with only about 1% receiving SBRT or proton therapy12,13. In Europe, about a quarter of cancer patients do not receive the radiotherapy they need14. As for heavy ion therapy, according to the Particle Therapy Co-Operative Group (PTCOG) database15, by the end of 2019, there were eight C-ion centres in Asia and four in Europe that have treated approximately 34,000 patients, with eight more centres under construction or in the planning stage. For comparison, proton therapy is administered at over 100 centres worldwide and has treated over 220,000 patients.
In the US context, the Mayo Clinic in Jacksonville (FL) has advanced plans for building a C-ion therapy facility16,17. Its opening would mark a much awaited return of heavy ion therapy in USA after the end of the LBL pilot trial. Until now, concerns about cost-effectiveness and lack of U.S. Food and Drug Administration certification and approved reimbursement have hampered the introduction of C-ion therapy in the USA18. The financial support to treat patients will need to come from federal grants until enough clinical data are available to approach third-party payers in the USA to consider supporting such therapy. Although high investment costs also affect the well-established proton therapy19, the cost is even higher for carbon, causing resistance even in the proton therapy community20. Yet, some of the clinical results, summarized in this Review, suggest possible improvements in outcomes in some malignancies compared with both X-rays and protons21,22. These results have triggered interest in international comparative trials with C ions and investments in preclinical radiobiological research also in the USA, resulting in recent P20 and R01 NCI grants on planning a C-ion facility and heavy ion radiobiology research. In addition, centres with experience in heavy ion therapy such as the National Institutes for Quantum and Radiological Science and Technology (NIRS-QST) in Chiba (Japan) and the Heidelberg Ion Beam Therapy Center (HIT) at the University of Heidelberg in Germany are now testing the use of different ions (especially He (REF.23) and O (REF.24)) and their combination25– 29 for optimized treatments.
In this Review, we address the question of whether the investment in C-ion therapy is currently justified. Is carbon the ‘silver bullet’ in radiotherapy or should treatments rather use lighter or heavier ions? We first outline the physics and technology of heavy ions, before discussing their radiobiology and surveying relevant clinical results. Finally, we discuss new ions and technologies.
Physics and technology of heavy ions
All charged particles share the same physics of deposition of energy along their path in matter30. The Bragg peak makes particles so appealing compared with X-rays, characterized by a depth-dose curve that decreases exponentially after the initial build-up (FIG. 1). The Bragg peak is the main motivation for charged particle therapy31, but there are some notable physical differences as one moves from hydrogen to heavier nuclei32.
The energy loss per unit mass length is known as stopping power S in physics and LET in radiobiology and radiotherapy. It is described by the Bethe-Bloch equation in the framework of the continuous slowing down approximation theory33:
| (1) |
where e is the electronic charge, NA the Avogadro number, m the mass of the electron, c the speed of light; Zp is the effective charge of the projectile, β its relative velocity and γ the Lorentz factor; ZT is the atomic number of the target material and A T is its mass number. The factor I in the logarithmic term is the mean excitation energy34,35; in human tissues and water, it represents one of the main sources of range uncertainty in particle therapy36,37. The additional correction terms are the shell correction C, Barkas correction L 1, Bloch term L 2, and Mott and density corrections L 3. S is expressed in MeV·cm2 g-1 in physics but, in radiobiology and radiotherapy, LET is expressed in keV μm-1 in water (density 1 g cm-3).
Equation 1 shows that the LET is proportional to the square of the ion charge and, therefore, it can be much higher for heavy ions than for protons, depending on the energy. This is the key to the biological advantages of heavy ions, because the relative biological effectiveness (RBE) increases with LET, owing to the production of more clustered DNA lesions, which are difficult to repair (Supplementary Video 2). Other differences are related to the nuclear interactions and the scattering, as discussed below.
Scattering
Beam dispersion in the longitudinal and lateral directions is an important feature in particle therapy. Longitudinal straggling σR determines the width of the Bragg peak at a range R. For two particles of different mass M at the same range R, it can be shown that30:
| (2) |
Equation 2 shows that longitudinal straggling is lower for heavy ions compared with protons, resulting in a sharper and more precise Bragg peak (FIG. 1).
Lateral scattering for thick targets is dominated by multiple Coulomb scattering and is well described by Molière’s theory of multiple scattering38. At small scattering angles, Molière’s theory approximates the scattering distribution as a Gaussian with standard deviation σθ that can be described as:
| (3) |
where d is the total mass thickness and L rad is the radiation length, which depends on the atomic number Z of the target material. Equation 3 shows that multiple Coulomb scattering increases for thick and heavy targets (because L rad ≈ Z -2), whereas it decreases with particle velocity and, at the same range, with particle mass (FIG. 1). For instance, protons have a lateral scattering that is approximately three times greater than C ions at a depth of 15 cm. However, at greater depths, C-ion projectiles gain substantial contributions to the lateral dose from secondary fragments from nuclear interactions, and the C-ion lateral dose distributions become similar to those of protons39.
Lateral scattering is responsible for the dose halo in treatment planning, and makes dose fall-off sharper for heavy ion treatments (FIG. 3). This property is, of course, attractive for sparing organs at risk (OAR), especially in hypofractionation, when the smooth fall-off can produce substantial doses to the OAR surrounding the tumour.
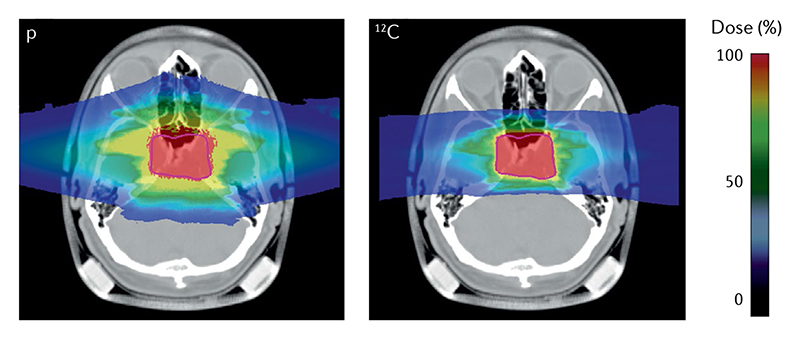
Figure 3. Impact of lateral scattering on treatment planning.
The image shows an optimized plan with two opposite fields for a chordoma patient using protons (left) or 12C ions (right). Colour scale represents the delivered relative biological effectiveness-weighted dose relative to the target dose of 2 Gy, prescribed to the planning target volume (purple line). The dose halo in the proton plan is caused by the larger lateral scattering of the light protons compared with the heavier carbon nuclei. Image from the GSI patient project archive, distributed under Creative Commons CC BY 4.0.
Fragmentation
Nuclear fragmentation is another major difference between protons and heavy ions. Both produce target fragments (that is, fragmentation of target nuclei, which, in the body, are mostly 16O), but protons do not undergo fragmentation after nuclear interactions, whereas heavy ions break into lighter projectile fragments. The nuclear fragmentation cross section σ f at high energies is well described by the geometric Bradt-Peters approximation40:
| (4) |
where r0 is the nucleon radius (~1 fm) and b the overlapping parameter. Equation 4 shows that the cross section increases ~(A p)2/3 and is, therefore, more significant in heavier ions. The projectile fragments have similar velocity and direction to the primary ion but lower charge, and, consequently, have larger range30. They generate a ‘dose tail’ beyond the Bragg peak, which is not observed with protons (FIG. 1). The mean free path for 350 MeV/n carbon ions in water (range 20 cm) is approximately 25 cm (REF.30), meaning that only about 50% of the accelerated 12C ions actually reach a deep tumour, the others undergoing nuclear fragmentation. However, in most practical cases, the tail is within the high-dose region in the patient, because two parallel opposed beams are often used for treatment (FIG. 3). Treatments with single beams are indeed generally avoided because of the problem of the range uncertainty, which is the main physics problem limiting the precision of particle therapy and which also affects proton therapy41.
At the same time, fragmentation can be beneficial for treatment monitoring and reducing range uncertainty. Image guidance is indeed essential for heavy ions, even more so than for X-rays. In fact, the sharp dose gradients in heavy ion therapy become a problem if the target is missed and the Bragg peak occurs in an OAR. In clinical particle therapy, a substantial margin is added to the prescribed range in order to ensure tumour coverage. For instance, in proton therapy, this range margin is on the order of 3.5% of the prescribed range42. Wide margins jeopardize one of the main advantages of the Bragg peak: the steep dose gradients and the potential high accuracy and precision43. It is, therefore, desirable to monitor heavy ion treatments precisely and, thereby, reduce these margins.
Unfortunately, image guidance was originally developed for X-rays and is taking time to be fully exploited in particle therapy, where additional complications can occur, for instance, in using magnetic resonance imaging online guidance. However, the physics of charged particles offers unique opportunities for in vivo range verification30,44. The range verification method that has been tested most extensively in clinical practice is positron emission tomography (PET)45. In C-ion therapy, fragmentation of the primary ion generates positron-emitting isotopes, especially 11C. Positron annihilation with electrons produce two 511-keV γ-rays emitted at 180° to each other that can be visualized by PET. An online PET, that is, a PET system that is positioned around the patient and works during the treatment, was used at GSI for treatment monitoring and range verification46, and offline PET imaging (that is, imaging of the patient with a PET in a different room following the treatment) is currently in use in other C-ion centres47. A similar approach can be used with heavier ions. For example, 16O produces positron-emitting 15O, which can be visualized by PET. In proton therapy, only target fragments can be used for imaging, whereas in heavy ion therapy, the projectile fragments provide a large part of the signal.
However, the activity peak invariably occurs upstream of the Bragg (dose) peak, because the light fragments have shorter range at the same velocity of the primary ion30. The peak shift, along with biological washout and the efficiency of the detectors systems, makes resorting to Monte Carlo simulations in data analysis currently unavoidable. All these corrections currently limit the accuracy of PET-based range verification to about 2-5 mm (REF 44). The problem could be solved by using radioactive ion beams directly for treatment. Radioactive ion beams would improve the count rate by an order of magnitude48, reduce the shift between measured activity and dose, and mitigate the washout blur of the image with short-lived isotopes and in-beam acquisition, eventually leading to sub-millimetre resolutions. However, attempts to use these beams in therapy have been hampered by the low intensity of the secondary beams produced by fragmentation of the primary, stable ion49. With modern, high-intensity accelerators, it is possible to produce radioactive ion beams with an intensity sufficient for therapeutic treatment50, and such beams would pave the way to PET-guided heavy ion treatment. Several studies are ongoing to assess the advantages of radioactive ion beams for therapy51, and a prototype for a compact PET cyclotron producing 11C beams as an injector for a medical synchrotron has been proposed by CERN52.
Accelerators
In the past, orthovoltage X-ray tubes and 60Co γ-rays sources were used for cancer therapy, but, today, all radiotherapy is based on electron or hadron accelerators53. Compact linear accelerators (linacs) are used to accelerate electrons to 6-25 MeV. The electrons then produce high-energy X-rays by bremsstrahlung in a metallic target. Although there are many research projects on the use of linacs for particle therapy54, at present, all particle therapy facilities use circular accelerators, either cyclotrons or synchrotrons30, in which particles are bent by a magnetic field B and accelerated by an electric field. The gyroradius ρ in a particle accelerator is determined by the magnetic rigidity (expressed in Tm):
| (5) |
where p is the momentum of the particle and q is its charge. According to Eq. 5, a synchrotron accelerating 400 MeV/n 12C ions must have a diameter approximately three times larger than a 250-MeV proton synchrotron for equal B field. Most proton therapy centres are based on cyclotrons, but for heavier ions, a cyclotron would be very massive and, currently, all C-ion centres use large synchrotrons. The comparison in FIG. 4a shows the impact of magnetic rigidity on the footprint and complexity of the machine, resulting in increasing cost.
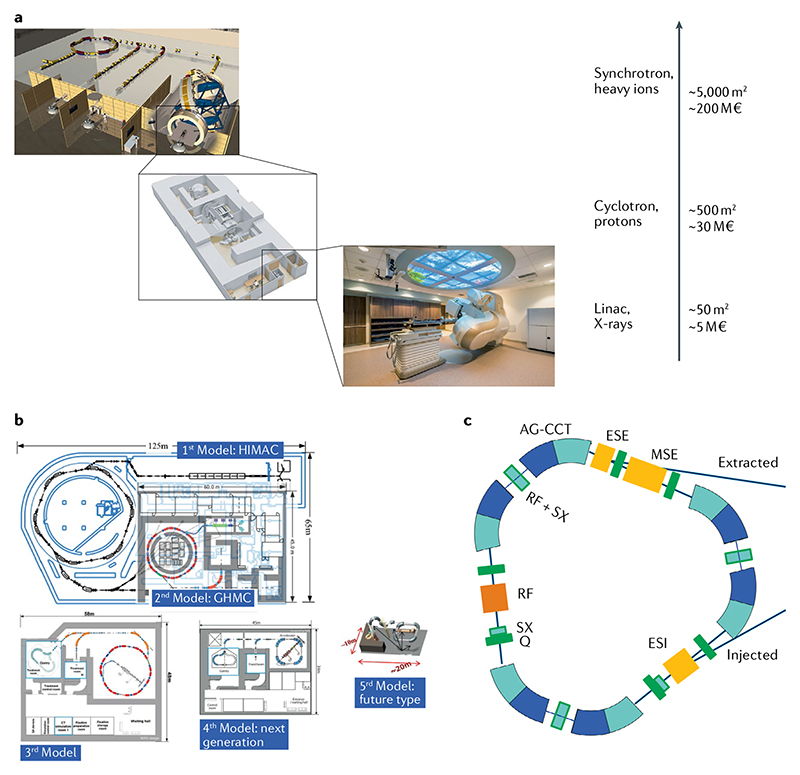
Figure 4. Accelerator technologies in heavy ion therapy.
a | The impact of advancing technology on footprint and costs of radiotherapy facilities. Size and prices can have large variation, but the image gives an indication of the increase in footprint and price. b | Plans at the National Institutes for Quantum and Radiological Science and Technology (NIRS-QST, Chiba, Japan) for reducing the footprint of heavy ion centres. The image shows the large research Heavy-Ion Medical Accelerator (HIMAC) and the subsequent concepts already implemented (such as the Gunma facility) or under development. GHMC, Gunma University Heavy Ion Medical Center. Image courtesy of K. Noda, NIRS-QST. c | An innovative concept of a superconducting synchrotron for heavy ion therapy developed at CERN. The triangular accelerator with a 3.5-T superconducting magnet is capable of accelerating C ions up to 430 MeV/n with 1010 ions per pulse in a footprint approximately one-quarter of the present-day resistive magnet medical synchrotrons in part b. AG-CCT, alternating gradient canted cosine theta; ESE, electrostatic extraction septum; ESI, electrostatic injection septum; MSE, magnetic extraction septum; Q, quadrupole magnet; RF, radiofrequency cavity; SX, sextupole magnet. Image and information courtesy of Elena Benedetto, CERN & TERA Foundation. Part a with permission from © GSI Helmholtzzentrum für Schwerionenforschung GmbH. Part b, image courtesy of Dr Koichi Noda. Part c, image courtesy of Elena Benedetto, TERA Foundation and CERN.
Currently, there are many efforts to reduce the footprint and cost of accelerators. These efforts are mostly directed towards the use of superconducting magnets55 that increase the strength B of the magnetic field (from approximately 1.8 T in resistive magnets up to 4−9 T for superconductive magnets), thus, allowing a reduction of the radius at the same rigidity (Eq. 5). Superconducting cyclotrons are already in use for proton therapy (such as IBA S2C2 and Varian ProBeam), and several projects for heavy ion superconducting cyclotrons56 or synchrotrons57,58 (FIG. 4b) are ongoing. Superconducting magnets in medical accelerators pose several problems. To achieve high fields (B > 3 T), a large quantity of superconducting material is needed, resulting in high cost and challenging cooling. Magnets in synchrotrons need field ramp rates >1 T s-1, a curved shape and, usually, quadrupole integration. The options are Nb–Ti (Nb3Sn)59, cosine theta or canted cosine theta60 and high-temperature superconductors61. Nb–Ti is predominant because of its good industrial development and higher reliability, while Nb3Sn is used only for applications where the very high fields cannot be avoided.
All these options are currently under testing for applications in medical accelerators and gantries. FIGURE 4c shows the concept of a compact medical synchrotron using alternating gradient canted cosine theta superconducting magnets developed at CERN. The circumference is approximately 30 m, whereas the present-day third-generation Japanese models (FIG. 4b) are longer than 60 m.
This R&D is likely to lead to substantially smaller — and cheaper — heavy ion machines in the next 10 years.
Larger uncertainties are associated to the development of new, potentially revolutionary accelerator technologies, such as dielectric wall accelerators62 and laser-driven particle accelerators63– 65. Many research groups have worked on laser-accelerated particles over the past 20 years but, despite many recent efforts for designing beam transport and delivery in therapeutic scenarios66,67, problems remain, including68: increasing the intensity; increasing the maximum particle energy; shielding for secondary radiation (especially the very abundant low-energy ions), which is likely to be bulky and expensive; target stability; improving shot-to-shot reproducibility (at least to the few % level); and addressing quality assurance and patient safety aspects. Full laser-driven ion medical accelerators remain far in the future, but low-energy linear injectors in ring accelerators could be laser-driven. The current plans for miniaturized synchrotrons in Japan (fifth model in FIG. 4b) include the use of a short laser-driven particle accelerator as injector for a high-B-field superconducting synchrotron69.
Beam delivery
New proton therapy centres all deliver the beam using pencil beam scanning (PBS), in which the whole tumour volume is scanned in 3D using a narrow pencil beam30 (Supplementary Video 3). PBS is also used in the majority of C-ion centres, with only a few centres in Japan still using the old passive modulation systems. PBS provides an unsurpassed conformity, but is problematic for moving targets, especially thoracic and abdominal tumours that move with breathing. The problem is caused by the interplay between beam and tumour movements, resulting in poor dose distributions. The problem is tackled with different motion mitigation techniques70– 72, but some simple methods such as gating leave the problem of the residual motion, whereas accurate 3D tumour tracking with the beam requires complex online fast movement and range adaptation. A simpler way to handle range changes and complex motion patterns is 4D treatment planning, in which the plan is optimized from a full 4D computed tomography (CT) scan of the tumour, thus, including the motion. This technique is ideal for particle therapy73, and, in particular, for therapy with heavy ions74, in which the interplay between beam scanning and target motion produces poor target coverage.
An optimal dose distribution always requires irradiation from different angles, and even the Bragg peak means that the number of beams is reduced in particle therapy. In linacs, the beam is bent by a magnet that can rotate around the patient (gantry) to irradiate from different angles or even continuously rotate around the patient (tomotherapy or volumetric modulated arc therapy). Gantries for protons are bigger and separated from the accelerator, with the exception of the Mevion S250 system, in which the cyclotron itself is mounted on a gantry. Gantries for heavy ions are larger and heavier than proton gantries, again to match the increased magnetic rigidity (Eq. 5). The HIT gantry, with resistive magnets, is a 670-tonne structure with diameter of 13 m. It was the first gantry for heavy ions and has been in clinical operation for a decade. As discussed above in the context of synchrotrons, smaller and lighter gantries should use superconducting technology60,61,69. A lighter (300 tonne) superconducting gantry has been installed in the new PBS treatment facility at NIRS75.
Even using modern superconducting magnets, the gantry remains one of the most expensive and bulky items in heavy ion therapy. Certainly an easy way to reduce the cost of a heavy ion facility would be to use only fixed lines, including vertical, horizontal and oblique lines, as is done in several C-ion centres in Europe and Asia. However, this setup limits the angles that can be used, effectively penalizing heavy ion therapy in comparison with IMRT or proton therapy, in which gantries are always provided.
As an alternative to moving the beam, it could be possible to move the patients using a dedicated chair. Irradiation with neutrons and charged particles of patients immobilized on a treatment chair was in use already in the 1950s76, and is still the standard for treating ocular tumours with protons. The method was abandoned for targets outside the skull because the patient must still lie down for the planning CT, and, therefore, the recorded organ positions are different to those when the patient is sitting. However, the situation changed with the introduction of vertical CT, which provides images for planning that are obtained with the patient in the same vertical position as the treatment. The irradiation in upright position of thoracic tumours has the potential additional advantage of reduced respiratory motion77. The Northwestern Medicine Center in Chicago, USA, is currently treating patients in an upright position with protons, following treatment plans from vertical CT. Optimal flexibility in gantry-less systems can be achieved using chairs with six degrees of freedom, that is, using a 360° rotating platform, an XYZ translation and enabling the chair to tilt. A six-degrees-of-freedom chair has been installed at the Shanghai Proton and Heavy Ion Center (SPHIC) C-ion centre in Shanghai, China, for the treatment of head and neck tumours78,79.
Radiobiology
Beyond the physical differences between heavy ions and protons and photons, the real motivation for using heavy ions lies in biology. In fact, as discussed above, heavy ions have higher LET than photons and protons, resulting in more powerful radiobiological effects caused by the different DNA lesion patterns and quality at the micrometre and nanometre scales80 (Supplementary Videos 1 and 2). As shown in FIG. 2, carbon ions have low LET in the entrance channel and high LET in the SOBP. Therefore, their radiobiological properties are similar to protons and X-rays in normal tissue, but similar to neutrons and α-particles in the tumour. Fast neutrons81 and α-particles82 are both used in cancer treatment, but they have caveats compared with heavy ions: for neutrons, the depth–dose distribution is similar to X-rays and the LET is constant along the beam path; short-range α-particles can only be used for internal exposure in the framework of targeted radioimmunotherapy.
The radiobiological features of heavy charged particles have been recently reviewed7,83,84 and are summarized in FIG. 5. The survival curves in FIG. 5 are generally described by the linear–quadratic (LQ) model85:
| (6) |
where S is the surviving fraction to a dose D of radiation. The cell radiosensitivity is determined by the fitting parameters α and β, usually using their ratio. The LQ model is a consequence of Poisson statistics and the quadratic term corresponds to inter-track lethal events, which are more likely for sparsely ionizing radiation than for heavy ions. At high LET, α tends to increase and β tends to decrease86, resulting in survival curves that are almost exponential. The ratio D X/D p of the doses of the reference radiation X (X-rays) and particle radiation p producing the same survival is the RBE for cell killing, which Eq. 6 shows is higher at low doses than at high doses, especially when the α/β ratio is low. Even if the LQ model is only applicable in the low-dose range, typical of conventional fractionation in radiotherapy, it can be extended to high dose per fraction, such as procedures used in SBRT87.
Figure 5. Biological advantages of heavy ions.
D, dose; G1, pre-DNA replication (S) cell cycle phase; G2, post-DNA replication (S) cell cycle phase; LET, linear energy transfer; OER, oxygen enhancement ratio; RBE, relative biological effectiveness; S, survival; VEGF, vascular endothelial growth factor89.
The preclinical studies at LBL6 had already demonstrated that, in addition to the increased RBE for cell killing, heavy ions have reduced oxygen enhancement ratio (OER), reduced dependence from the cell cycle phase and reduced dependence on dose rate or fractionation. More modern observations have shown reduced angiogenesis88,89, as well as synergistic effects in combination with targeted therapy90 and immunotherapy91.
RBE
Certainly the increased RBE for cell killing is the best known advantage of heavy ion therapy. The RBE increases with LET up to a maximum around 100–200 keV μm-1 (REF.86), whereas it decreases again at very high LET (a phenomenon known as the overkilling effect)92. The RBE increases the SOBP peak/plateau ratio in heavy ion therapy compared with proton therapy, because it is higher in the target region (peak; high LET) than in the normal tissue (entrance; low LET). The dose in the target region in heavy ion therapy is usually optimized using a RBE-weighted dose defined by the International Commission on Radiological Units (ICRU) as93
| (7) |
where the physical dose in gray (1 Gy = 1 J kg-1) is corrected by the dimensionless RBE factor, which is a function of the dose, the particle energy E and several other factors (a, b, c…), such as dose rate, oxygen concentration, intrinsic radiosensitivity, among others. Given its dependence on so many parameters, the RBE can only be calculated by a biophysical model such as the micro-dosimetric kinetic model94 or the local effect model95, both based on the LQ model (Eq. 6).
At present, the uncertainty on C-ion RBE models is perceived as a major problem in heavy ion therapy compared with proton therapy3. Although it is generally acknowledged that the proton RBE is also variable96, the variation is considered small enough to allow the use of a simple constant RBE = 1.1 along the whole SOBP97. (However, the situation may change in the future, and, in some centres, RBE models are now used in proton therapy98.) For carbon, the RBE-weighted dose calculated by different models for the same physical C-ion dose can differ substantially99,100, and, for this reason, great care has to be taken in comparing the clinical results from different heavy ion centres, even if the same RBE-weighted dose is reported101,102.
Among the various parameters, notable for radiotherapy is the RBE dependence on the intrinsic radiosensitivity (that is, the α/β ratio, derived from the X-ray dose-response curve; Eq. 6) and on the dose per fraction. The RBE decreases when the intrinsic radiosensitivity of the tissue or the dose per fraction are increased. Accordingly, the maximum RBE advantage is observed for radioresistant tumours (FIG. 6). The fractionation dependence is intertwined with the radiosensitivity, because the RBE decrease at high doses is faster for radioresistant than for radiosensitive tissues, that is, the RBE in hypofractionation decreases103,104 more sensitively for normal tissue (α/β ≈ 2 Gy) than for the tumour (α/β ≈ 10 Gy)105. Therefore, even if the RBE is low at high dose per fraction, hypofractionation is possible and, indeed, is often pursued in clinical treatments with heavy ions106.
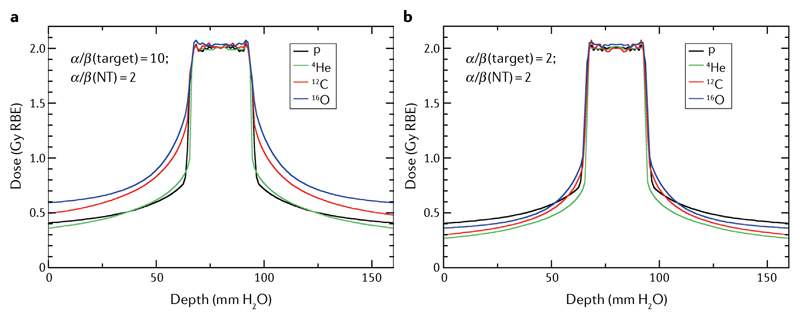
Figure 6. The ‘best’ bullets are those providing the lowest relative biological effectiveness-weighted dose in the normal tissue at the same effect in the target.
Relative biological effectiveness (RBE)-weighted dose optimization for a two-opposite-field treatment of an idealized geometry simulating a typical case of radiosensitive (part a) or radioresistant (part b) tumour. The tumour (2.5 × 2.5 × 2.5 cm3) is centred in the planning target volume. Biological dose optimization was performed using the TRiP98 software and the local effect model (LEM-IV)95. The plan was optimized with different ions to provide the same RBE-weighted dose of 2 Gy in the target volume. Protons are excellent for radiosensitive tumours but the worst for radioresistant tumours. Figure modified from Tommasino et al.167, distributed under Creative Commons CC-BY. Figure is adapted from REF.167, CC BY 4.0.
Hypoxia
Most tumours are hypoxic, which increases their resistance to treatments with either conventional drugs or radiation107. Fractionation in radiotherapy is used to overcome hypoxia, by exploiting interfractional reoxygenation. This advantage is, however, lost in the modern hypofractionated regimes, making hypoxia a major problem for SBRT108,109. The OER — that is, the ratio of the doses producing the same effect in hypoxic (0% pO2) and oxic (20% pO2) conditions — is approximately 3 for X-rays, a value so high that, if translated to the clinic, would make radiotherapy of hypoxic tumours impossible without reoxygenation. In reality, physiological hypoxia is, on average, only 5% in normal tissue and <2% in most tumours110. The OER decreases at high LET111, because, for densely ionizing particles, the damage is induced by direct effects on the DNA molecule and free radicals play a smaller role than they do for X-ray-induced damage. In cellular studies, it is known that the OER drops to 1 at LET ≈ 200 keV μm-1 at physioxic concentrations112. However, this result indicates that the LET for C ions (FIG. 2) is not high enough to overcome hypoxia in the tumour. Thus, interest in heavier ions and multi-ion treatments is justified, as discussed in the next section.
Combined treatments
Radiotherapy is often used in combination with drug treatments, including chemotherapy, targeted therapy and immunotherapy113. Can heavy ions be more effective than X-rays in combined treatments? Some preclinical studies of the combination of carbon ions with targeted therapies suggest a synergistic effect90. The synergism depends on the mechanisms of radiation damage, and, therefore, different drugs can be used for X-rays and heavy ions. However, for immunotherapy, heavy ions have a physical advantage, because they spare much better the patients’ own immune cells, owing to the reduced integral dose compared with other treatments114. This putative advantage is shared with proton therapy91,115, for which reduced lymphopaenia has been observed compared with X-ray treatment for oesophageal cancer116, glioblastoma117 and lung cancer118. Preclinical data in a mouse osteosarcoma model also shows that high C-ion doses have biological advantages compared with X-rays in combination with immune checkpoint inhibitors119,120. Specific trials to address the combination of heavy ions and immunotherapy are highly desirable and may prove a breakthrough in combined treatment protocols using immunotherapy121. Modelling the combination with biophysical models is necessary to achieve an optimal combination122.
Clinical results
The clinical data obtained with carbon ions are reviewed in many recent publications8,16,21,22,83, and, therefore, we only briefly describe below the most promising tumour sites in which differences are expected not only with respect to X-rays but also to proton therapy. We focus on the sites in which carbon ions gave the best results, pending ongoing clinical trials.
Eye tumours
Uveal melanoma is one of the most common diseases treated with proton therapy, but heavier ions have also been used123. Interestingly, the only phase III comparative clinical trial completed for ions heavier than protons is for choroidal and ciliary body melanoma of the eye. The trial compared accelerated He ions to 125I plaque therapy for patients treated in the LBL trial. The results of long-term outcomes of 184 patients treated during the period 1985–1991 were published in 2015 (REF.124) and show significantly improved local control, eye preservation and disease-free survival. Even if this is a limited trial for a rare tumour, it shows that well-designed trials with long outcome can give significant results.
Gastrointestinal cancers
In an independent review of 20 years of C-ion therapy at NIRS-QST in Japan125, the panel noted that the most striking and remarkable outcomes had been observed for gastrointestinal tumours, such as pancreas and local recurrence of rectal cancer.
The results obtained at NIRS-QST for locally advanced pancreatic adenocarcinoma treated with hypofractionated C ions with concurrent gemcitabine126 were confirmed in a multi-institutional Japanese trial127. These results have attracted great interest, because pancreatic cancer is difficult to detect early and is so resistant to treatment that it is becoming a leading cause of cancer death128. Considering that pancreatic cancers are very hypoxic129 and their microenvironment is markedly immunosuppressive130, it has been hypothesized that C ions, with their low OER and increased immunogenic cell death, can be the ‘silver bullet’ for this highly lethal malignancy131. As a result, pancreatic cancer is considered one of the most interesting comparative clinical trials to be pursued with C ions132,133.
Also exceptional are the outcomes of C-ion treatment for another tumour with high lethality, namely, locally recurrent rectal cancer. In a multi-centre Japanese clinical trial, survival rates with concomitant chemotherapy in the high-dose C-ion group was 90% at 2 years and 50% at 5 years134. Similar results were confirmed in a trial with similar doses at the SPHIC135, and good preliminary results were also obtained at the HIT136. These clinical data indicate a clear improvement compared with the best results of chemoradiotherapy using IMRT137, and are similar to the outcome of surgical resection for this malignancy138.
Excellent results have also been reported for hepatocellular carcinoma, especially large tumours close to the porta hepatis139. Both carbon ions and protons present lower toxicity than SBRT or 3D conformal radiotherapy140, but carbon ions are delivered in shorter treatments of about four fractions141 up to a RBE-weighted dose of approximately 60 Gy (REF.142).
Sarcomas
Sarcomas are traditionally considered radioresistant cancers, and, therefore, excellent candidates for heavy ion therapy143. The poor radiation response of sarcomas compared with other cancers, such as carcinomas, does not seem to be linked to genetic radiosensitivity but, rather, to slow growth of the tumours and the presence of hypoxia, especially in large, unresectable malignancies144. Most particle therapy centres treat sarcomas, especially chordomas and chondrosarcomas of the base of the skull145.
The Japanese results with C ions for osteosarcomas of the trunk146 and soft tissue sarcomas147 are excellent, especially for inoperable large tumours that are difficult to treat. First results from the Centro Nazionale di Adroterapia Oncologica (CNAO) in Italy also support these results148. At the HIT, a prospective phase I/II trial with a combined proton and C-ion radiotherapy has been completed, showing favourable toxicity and high response rates149.
The HIT in Heidelberg has made a long-term follow-up of 155 skull base chordoma patients treated in the C-ion pilot project at GSI in Darmstadt over the period 1998–2008 and the results are excellent, with an overall survival of 75% at 10 years150. Excellent results are also reported for skull base chondrosarcoma, but no significant differences between protons and carbon ions were observed in terms of local control or overall survival in 101 patients, with a median follow-up of 40 months from the treatment at the HIT over the period 2009–2014 (REF.151). The hypothesis of the study is that carbon ion radiotherapy can achieve the same local control at lower toxicity than other treatments. The results of the phase III trial are preliminary152 and more time is needed to draw final conclusions, but early results suggest that a favourable physical dose distribution is the main factor to reduce toxicity and achieve local control in chondrosarcoma.
Head and neck tumours
Cancers located in the upper aerodigestive tract are often candidates for particle therapy, because they are surrounded by OAR and patients experience severe toxicities from treatments153. The rationale for using heavy particles rather than protons is both a reduced dose halo (FIG. 3) and the biological advantages (FIG. 5). Physics advantages can be exploited to provide a boost at the end of IMRT treatments154, and the biological advantages can be used for radioresistant histologies155. C-ion therapy is typically indicated for salivary gland tumours such as adenoid cystic carcinoma156 and nasopharyngeal carcinoma157, with randomized clinical trials under way for these pathologies. Comparative trials are important to assess whether heavy ions can do better than protons, which have already demonstrated a significant mitigation of toxicity compared with IMRT in combination with chemotherapy158,159.
Paediatric tumours
Paediatric cancers are typical candidates for proton therapy, considering that sparing normal tissue, often the brain, is particularly important for patients with long life expectancy160. Approximately 10% of paediatric solid tumour patients in the USA were treated with protons in 2012 (REF.13), and this fraction is growing rapidly.
Heavy ions have been traditionally deemed too risky for paediatric patients. In fact, high-LET radiation has high RBE for late effects, including carcinogenesis161. The concern for possible induction of second cancers has, therefore, limited the use of high-LET heavy ions in paediatric patients. However, in a cohort of 83 patients aged <21 years treated at the HIT, no significant differences were observed in normal tissue toxicity after treatment with protons or C ions162. Moreover, the concern for second cancers after high-LET radiation has been contradicted, at least for adult patients, by a retrospective analysis of 1,580 prostate cancer patients treated with C ions at NIRS163. The study found a lower risk of second cancers in patients treated with heavy ions compared with those treated with X-ray therapy. This outcome is also consistent with measurements of secondary radiation, including neutrons, in PBS therapy161, and, therefore, it has been proposed that C ions may be an excellent tool in paediatric patients164.
In addition, helium ions are potentially ideal for children, because they have low RBE, similar to protons (FIG. 2), but sharper dose distributions, thus, providing increased sparing of normal tissue (FIG. 3). An in silico study in different paediatric pathologies has shown advantages of He ions for neuroblastoma, Wilms tumours and ependymoma165.
New ions and technologies
At present, particle therapy is limited to protons and C ions, but several other ions could be used to treat solid cancers. Carbon ions exhibit a good compromise between low LET in the normal tissue and high LET in the target region (FIG. 2). However, for radiosensitive tumours, light ions can have better properties than heavy ions (FIG. 6), and, even for radioresistant tumours, He ions can be as good as carbon in normoxia.
Helium ions
In a pilot project at LBL, over 2,000 patients were treated with 4He ions4. Helium should be selected when one is not faced by a tumour that is particularly radioresistant and/or hypoxic, but one does want an increased precision and reduced dose ‘halo’ (FIG. 3), achieved thanks to the reduced lateral scattering. Acceleration of 4He is complicated by beam contamination with heavier ions with the same q/m ratio from the ion source, which have the same rigidity as helium and are, therefore, accelerated by the synchrotron. For this reason, it has been proposed to use 3He for therapy166, which has similar properties to 4He but with limited contamination problems, and would result in smaller and cheaper He-dedicated accelerators, thanks to the lower rigidity of 3He (Eq. 6). A calculation with two opposite fields using the local effect model (FIG. 6) suggests that, for both a radiosensitive (α/β = 10 Gy) or a radioresistant (α/β = 2 Gy) tumour, helium gives a lower dose to the normal tissue for the same target dose167. These simulations show that helium can be very useful in different scenarios. The HIT will start using helium ions in clinical treatments in 2022, following extensive preclinical studies23,27.
Oxygen ions
Ions heavier than carbon can be interesting for very radioresistant, hypoxic tumours. In simulations of normoxic tumours, ions heavier than carbon do not seem to have advantages (FIG. 6). However, for hypoxic cancers, 16O ions are expected to be more effective than 12C ions168,169. As noted in the introduction, overcoming hypoxia was the main motivation to use very heavy ions in the LBL trial, but very heavy ions such as 40Ar have a high LET, even in the entrance channel, leading to unacceptable toxicities. In the entrance channel, oxygen is still in the low-LET region (FIG. 2), and, therefore, relatively safe. For this reason, the HIT is planning to use oxygen ions for treatment of very hypoxic tumours24,27.
Multi-ion treatment
Even when using ions heavier than carbon, it remains difficult to deliver a high-LET treatment to the whole tumour volume (FIG. 2); only a relatively small fraction of the tumour is exposed to high LET. The optimization on the biological dose can, therefore, jeopardize the putative biological advantages (FIG. 5) of heavy ion therapy7. The problem is now emerging also in the clinics. A retrospective analysis on the C-ion treatment plans at NIRS suggest that the LET is positively associated to local control of pancreas tumours. In particular, patients with higher minimum dose-averaged LET values in the gross tumour volume had lower probability of local failure compared with those with minimum LET values below 40 keV μm-1 (REF.170). Similar results have been observed looking at local recurrence maps in grade 2 chondrosarcoma patients treated at NIRS with C ions171.
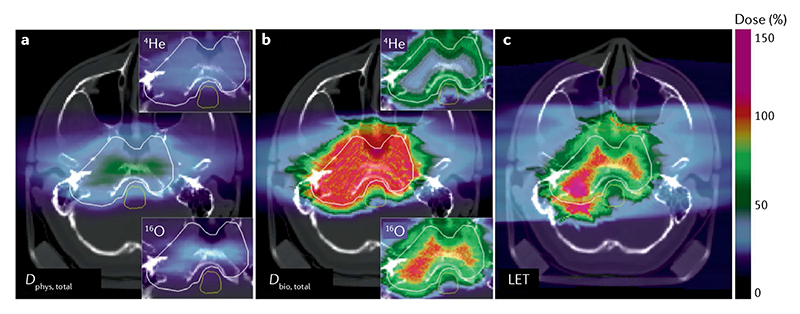
Approaches to extend the high-LET regions with a single ion (LET painting172,173) are hampered by increasing toxicity. However, when multiple ions (light to heavy) are used, it is possible to increase the LET in the target region while maintaining a low toxicity in the normal tissue174. A combination of protons, helium, carbon and oxygen beams, produced at the same synchrotron, can be delivered to achieve a prescribed LET map to the target, in a method known as intensity-modulated composite particle therapy. For instance, in a simulated prostate treatment aiming to deliver 2 Gy to the planning target volume, it is possible to have 80 keV μm-1 in the gross tumour volume, 50 keV μm-1 in the clinical target volume and <30 keV μm-1 in the OAR (rectum)175. This method requires a multi-ion source synchrotron, like those available at the HIT25 or NIRS26,28. In addition, it will be necessary to implement a careful multi-angle dose simulation and verification and fast switching among different ion sources. For applications to hypoxic tumours, multi-ion optimization requires quantitative hypoxia imaging using molecular imaging methods176. If an O2 concentration map is available, multi-ion treatments can optimally increase the LET (decreasing the OER) or the dose in the low-oxygen sub-volumes, resulting in highly effective treatments for hypoxic tumours29 (FIG. 7). Multi-ion therapy, therefore, has a number of technical challenges, but the goal of increasing the LET in the tumour volume is an important step towards the optimization of heavy ion therapy.
Figure 7. Biologically optimized multi-ion plan for a hypoxic skull base chordoma (hypoxia is assumed to be concentred in the central part of the tumour).
a | Total physical dose (D phys, total). Insets correspond to the partial contributions from 16O and 4He fields. Colour scale represents the relative dose compared with the dose of 2 Gy. b | Total biological (relative biological effectiveness–oxygen enhancement ratio (RBE-OER)-weighted) dose (D bio, total). Colour scale represents the relative linear energy transfer (LET) compared with a fixed value of 60 keV μm-1. c | Dose-averaged LET distribution. Colour scale as in part b. Calculation with TRiP98 code from Sokol et al.29, reproduced with permission from IOP. Figure is adapted from REF.29, CC BY 4.0.
New beam delivery methods
We have already noted that the use of very heavy ions, although advantageous for hypoxic tumours, is hampered by normal tissue toxicity. One would, therefore, like a technique that spares normal tissue much more than existing methods do. In this regard, two hot topics in radiotherapy at present are FLASH and spatially fractionated radiotherapy177.
FLASH radiotherapy is a method to deliver the therapeutic dose at very high dose rate (>40 Gy s-1). At ultra-high dose rates, there is a substantial sparing of the normal tissue while maintaining local tumour control178– 180. These extremely high dose rates are difficult to reach with the high-energy X-rays used in radiotherapy, because of the small conversion efficiency of the bremsstrahlung production process. However, the dose rates have been achieved with charged particles, including electrons181 and protons182, and can also be attained at synchrotrons with heavier ions183. It also seems that sparing the normal tissue by FLASH depends not only on the dose rate but on many other parameters, including also total dose, time structure of the beam and tissue oxygenation. The best conditions seem to be achieved around 10 Gy total dose delivered in <100 ms at dose rates >10 Gy s-1 in slightly hypoxic tissues184,185. The topic is alluring for accelerator physicists, who have already produced a number of ideas and prototypes for FLASH-dedicated machines. The laser-driven accelerators (discussed in the section on accelerators) would be ideal for FLASH radiotherapy, given their very high dose per pulse186. However, it should be pointed out that the clinical applications have barely started187, and that the mechanism of the FLASH effect is still unclear. Several models have been proposed based on radiation chemistry mechanisms, such as oxygen depletion188 or free radical recombination189, but none has been supported experimentally190 and the models often also have theoretical flaws191. In the absence of a solid theoretical interpretation, it is unknown whether the FLASH effect will be observed for high-LET radiation. This question is currently under investigation192.
Another delivery technique that substantially spares normal tissue is spatially fractionated radiotherapy, in which the therapeutic beam is divided into beam-lets spaced by a few micrometres (microbeam radiotherapy)193 or millimetres (minibeam radiotherapy)194. This grid configuration greatly improves the repopulation capacity of the normal tissue195, and the target tumour can be covered with interlaced beams196. The method has been used in a rabbit with C ions197 and can be extended to heavier ions198,199, exploiting the spatial fractionation to reduce toxicity.
FLASH and minibeam radiotherapy are approaching clinical utilization, but they have the potential to become useful tools in heavy ion therapy200. These methods widen the therapeutic window that is appealing even for protons, but may allow the use of ions heavier than oxygen against very radioresistant tumours, making possible one of the ideas that sparked their initial development2.
Outlook
The quest for a ‘cure-all’ particle in radiotherapy will never find a simple solution. Cancers have proven to be very different to each other and elusive to the most advanced therapies, and, certainly, there is not a magic particle able to destroy all types of malignancies. Based on the present state of the art, we argue that heavy ion therapy is at a tipping point: it has an enormous potential thanks to favourable physical (FIG. 1) and biological (FIG. 5) properties, but its clinical advantage compared with protons and X-rays — which are both cheaper and smaller technologies (FIG. 4) — remains unproven. Some outstanding results have been obtained in gastrointestinal cancers (especially in the pancreas), sarcomas and nasopharyngeal carcinomas, but randomized clinical trials are ongoing. The plans for new centres in Europe, Asia and the USA are, therefore, welcome, if those centres will be used for clinical research and comparative trials.
At the same time, research must continue to make the therapy cheaper and more effective. In physics, it is particularly important to build smaller and cheaper accelerators and beam delivery systems, exploiting superconducting magnets and gantry-less systems. In biology, it is essential to assess the combination of heavy ions with molecular therapy and immunotherapy, in comparison with X-rays and protons. In clinical research, it is necessary to test hypofractionated regimes, combined protocols and to fully exploit the putative advantages listed in FIG. 5 by increasing the LET in the tumour. In fact, the relatively modest LET values reached in large fractions of the cancer volume treated with C ions (FIG. 2) raises the question of whether the main biological advantages of densely ionizing radiation is being squandered. Higher LET in the target volume can require multi-ion irradiation (FIG. 7), and, therefore, rather than a single silver bullet, in the future, a combination of projectiles may become common to sterilize aggressive, radioresistant malignancies.
Supplementary Material
Key points.
Charged particle therapy is the most advanced radiotherapy technique.
Most of the patients are treated with protons, but heavy ions present additional biological advantages.
Carbon-ion therapy is ongoing in 12 centres worldwide and clinical results are promising, whereas new ions (like 4He and 16O) will be used in the future.
Heavy ion therapy is much more expensive than X-ray therapy and level 1 evidence of superiority is missing.
Radiobiology suggests that heavy ions can be exquisitely effective against hypoxic tumours and improve the effects of immunotherapy.
Rather than a ‘silver bullet’, different particles and their combination can provide optimal results in specific cases.
Acknowledgements
The authors thank Uli Weber, Emanuele Scifoni, Olga Sokol, Daria Boscolo, Burkhard Jakob, Anastasiia Quarz, Koji Noda and Elena Benedetto for their precious assistance in the preparation of the figures. The research activities at GSI and Heidelberg Ion Beam Therapy Center (HIT) are partly supported by the EU Horizon 2020 research and innovation programme under grant agreement no. 101008548 (HITRIplus). Projects on innovative beam delivery at GSI are supported by ERC advanced grant 2020 number 883425 (BARB).
Footnotes
Author contributions
M.D. produced the first draft. J.D. and J.S.L. worked on the biological and clinical sections. All authors edited and modified the manuscript.
Competing interests
M.D. has no conflict of interest. J.S.L. is co-chair of the medical advisory board at Mevion. J.D. received grants from several companies and attended advisory board meetings of Merck KGaA (Darmstadt).
Peer review information
Nature Reviews Physics thanks Hywel Owen, Eleanor Blakely and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
The authors searched PubMed and Scopus using the keywords ‘heavy ions’, ‘carbon ions’, ‘clinical trials’ and ‘comparative’, and selected the period from 2016, considering our previous reviews on the topic21,83. We also searched the ClinicalTrials.gov website with the keywords ‘heavy ions’, ‘carbon ions’, ‘comparative’, ‘randomized’ and ‘phase III’. The website www.ptcog.ch was used for the latest statistics on particle therapy.
References
- 1.Wilson RR. Radiological use of fast protons. Radiology. 1946;47:487–491. doi: 10.1148/47.5.487. [DOI] [PubMed] [Google Scholar]
- 2.Tobias CA. Failla Memorial lecture. The future of heavy-ion science in biology and medicine. Radiat Res. 1985;103:1–33. [PubMed] [Google Scholar]
- 3.Durante M, Loeffler JS. Charged particles in radiation oncology. Nat Rev Clin Oncol. 2010;7:37–43. doi: 10.1038/nrclinonc.2009.183. [DOI] [PubMed] [Google Scholar]
- 4.Castro JR. Results of heavy ion radiotherapy. Radiat Environ Biophys. 1995;34:45–48. doi: 10.1007/BF01210545. [DOI] [PubMed] [Google Scholar]
- 5.Colliez F, Gallez B, Jordan BF. Assessing tumor oxygenation for predicting outcome in radiation oncology: a review of studies correlating tumor hypoxic status and outcome in the preclinical and clinical settings. Front Oncol. 2017;7:10. doi: 10.3389/fonc.2017.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blakely EA, Ngo F, Curtis S, Tobias CA. Heavy-ion radiobiology: cellular studies. Adv Radiat Biol. 1984;11:295–389. [Google Scholar]
- 7.Tinganelli W, Durante M. Carbon ion radiobiology. Cancers. 2020;12:3022. doi: 10.3390/cancers12103022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsujii H, et al. Carbon-Ion Radiotherapy. Springer; 2014. [Google Scholar]
- 9.Kraft G. Tumor therapy with heavy charged particles. Prog Part Nucl Phys. 2000;45:473–544. [Google Scholar]
- 10.Thariat J, Hannoun-Levi J-M, Sun Myint A, Vuong T, Gérard JP. Past, present, and future of radiotherapy for the benefit of patients. Nat Rev Clin Oncol. 2012;10:52–60. doi: 10.1038/nrclinonc.2012.203. [DOI] [PubMed] [Google Scholar]
- 11.Lo SS, et al. Stereotactic body radiation therapy:a novel treatment modality. Nat Rev Clin Oncol. 2010;7:44–54. doi: 10.1038/nrclinonc.2009.188. [DOI] [PubMed] [Google Scholar]
- 12.Pan HY, Jiang J, Shih YCT, Smith BD. Adoption of radiation technology among privately insured nonelderly patients with cancer in the United States, 2008 to 2014: a claims-based analysis. J Am Coll Radiol. 2017;14:1027–1033.:e2. doi: 10.1016/j.jacr.2017.02.040. [DOI] [PubMed] [Google Scholar]
- 13.Waddle MR, et al. Photon and proton radiation therapy utilization in a population of more than 100 million commercially insured patients. Int J Radiat Oncol. 2017;99:1078–1082. doi: 10.1016/j.ijrobp.2017.07.042. [DOI] [PubMed] [Google Scholar]
- 14.Lievens Y, Borras JM, Grau C. Provision and use of radiotherapy in Europe. Mol Oncol. 2020;14:1461–1469. doi: 10.1002/1878-0261.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Particle Therapy Co-Operative Group (PTCOG) Particle therapy facilities in clinical operation. PTCOG. 2021 https://www.ptcog.ch/ [Google Scholar]
- 16.Malouff TD, et al. Carbon ion therapy: a modern review of an emerging technology. Front Oncol. 2020;10:82. doi: 10.3389/fonc.2020.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beltran C, Amos RA, Rong Y. We are ready for clinical implementation of carbon ion radiotherapy in the United States. J Appl Clin Med Phys. 2020;21:6–9. doi: 10.1002/acm2.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pompos A, Durante M, Choy H. Heavy ions in cancer therapy. JAMA Oncol. 2016;2:1539–1540. doi: 10.1001/jamaoncol.2016.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bortfeld TR, Loeffler JS. Three ways to make proton therapy affordable. Nature. 2017;549:451–453. doi: 10.1038/549451a. [DOI] [PubMed] [Google Scholar]
- 20.Jäkel O, Smith AR, Orton CG. The more important heavy charged particle radiotherapy of the future is more likely to be with heavy ions rather than protons. Med Phys. 2013;40:090601. doi: 10.1118/1.4798945. [DOI] [PubMed] [Google Scholar]
- 21.Durante M, Debus J. Heavy charged particles: does improved precision and higher biological effectiveness translate to better outcome in patients? Semin Radiat Oncol. 2018;28:160–167. [Google Scholar]
- 22.Rackwitz T, Debus J. Clinical applications of proton and carbon ion therapy. Semin Oncol. 2019;46:226–232. doi: 10.1053/j.seminoncol.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Tessonnier T, et al. Helium ions at the heidelberg ion beam therapy center: Comparisons between FLUKA Monte Carlo code predictions and dosimetric measurements. Phys Med Biol. 2017;62:6784–6803. doi: 10.1088/1361-6560/aa7b12. [DOI] [PubMed] [Google Scholar]
- 24.Kurz C, Mairani A, Parodi K. First experimentalbased characterization of oxygen ion beam depth dose distributions at the Heidelberg Ion-Beam Therapy Center. Phys Med Biol. 2012;57:5017–5034. doi: 10.1088/0031-9155/57/15/5017. [DOI] [PubMed] [Google Scholar]
- 25.Kopp B, et al. Development and validation of single field multi-ion particle therapy treatments. Int J Radiat Oncol. 2020;106:194–205. doi: 10.1016/j.ijrobp.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Inaniwa T, et al. Application of lung substitute material as ripple filter for multi-ion therapy with helium-, carbon-, oxygen-, and neon-ion beams. Phys Med Biol. 2021;66:055002. doi: 10.1088/1361-6560/abde99. [DOI] [PubMed] [Google Scholar]
- 27.Dokic I, et al. Next generation multi-scale biophysical characterization of high precision cancer particle radiotherapy using clinical proton, helium-, carbon- and oxygen ion beams. Oncotarget. 2016;7:56676–56689. doi: 10.18632/oncotarget.10996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inaniwa T, et al. Experimental validation of stochastic microdosimetric kinetic model for multi-ion therapy treatment planning with helium-, carbon-, oxygen-, and neon-ion beams. Phys Med Biol. 2020;65:045005. doi: 10.1088/1361-6560/ab6eba. [DOI] [PubMed] [Google Scholar]
- 29.Sokol O, Krämer M, Hild S, Durante M, Scifoni E. Kill painting of hypoxic tumors with multiple ion beams. Phys Med Biol. 2019;64:045008. doi: 10.1088/1361-6560/aafe40. [DOI] [PubMed] [Google Scholar]
- 30.Durante M, Paganetti H. Nuclear physics in particle therapy: a review. Rep Prog Phys. 2016;79:096702. doi: 10.1088/0034-4885/79/9/096702. [DOI] [PubMed] [Google Scholar]
- 31.Newhauser WD, Zhang R. The physics of proton therapy. Phys Med Biol. 2015;60:R155. doi: 10.1088/0031-9155/60/8/R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schardt D, Elsässer T, Schulz-Ertner D. Heavy-ion tumor therapy: physical and radiobiological benefits. Rev Mod Phys. 2010;82:383–425. [Google Scholar]
- 33.Bichsel H. Stochastics of energy loss and biological effects of heavy ions in radiation therapy. Adv Quantum Chem. 2013;65:1–38. [Google Scholar]
- 34.Kamakura S, Sakamoto N, Ogawa H, Tsuchida H, Inokuti M. Mean excitation energies for the stopping power of atoms and molecules evaluated from oscillator-strength spectra. J Appl Phys. 2006;100:064905 [Google Scholar]
- 35.Bär E, Andreo P, Lalonde A, Royle G, Bouchard H. Optimized I-values for use with the Bragg additivity rule and their impact on proton stopping power and range uncertainty. Phys Med Biol. 2018;63:165007. doi: 10.1088/1361-6560/aad312. [DOI] [PubMed] [Google Scholar]
- 36.Besemer A, Paganetti H, Bednarz B. The clinical impact of uncertainties in the mean excitation energy of human tissues during proton therapy. Phys Med Biol. 2013;58:887–902. doi: 10.1088/0031-9155/58/4/887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Smet V, Labarbe R, Vander Stappen F, Macq B, Sterpin E. Reassessment of stopping power ratio uncertainties caused by mean excitation energies using a water-based formalism. Med Phys. 2018;45:3361–3370. doi: 10.1002/mp.12949. [DOI] [PubMed] [Google Scholar]
- 38.Embriaco A, Bellinzona EV, Fontana A, Rotondi A. On Molière and Fermi-Eyges scattering theories in hadrontherapy. Phys Med Biol. 2017;62:6290–6303. doi: 10.1088/1361-6560/aa7a94. [DOI] [PubMed] [Google Scholar]
- 39.Ebrahimi Loushab M, Mowlavi AA, Hadizadeh MH, Izadi R, Jia SB. Impact of various beam parameters on lateral scattering in proton and carbon-ion therapy. J Biomed Phys Eng. 2015;5:169–176. [PMC free article] [PubMed] [Google Scholar]
- 40.Zeitlin C, La Tessa C. The role of nuclear fragmentation in particle therapy and space radiation protection. Front Oncol. 2016;6:65. doi: 10.3389/fonc.2016.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lomax AJ. Myths and realities of range uncertainty. Br J Radiol. 2020;93:20190582. doi: 10.1259/bjr.20190582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paganetti H. Range uncertainties in proton therapy and the role of Monte Carlo simulations. Phys Med Biol. 2012;57:R99–R117. doi: 10.1088/0031-9155/57/11/R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Durante M, Flanz J. Charged particle beams to cure cancer: strengths and challenges. Semin Oncol. 2019;46:219–225. doi: 10.1053/j.seminoncol.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Knopf A-C, Lomax A. In vivo proton range verification: a review. Phys Med Biol. 2013;58:R131–R160. doi: 10.1088/0031-9155/58/15/R131. [DOI] [PubMed] [Google Scholar]
- 45.Parodi K. Vision 20/20: positron emission tomography in radiation therapy planning, delivery, and monitoring. Med Phys. 2015;42:7153–7168. doi: 10.1118/1.4935869. [DOI] [PubMed] [Google Scholar]
- 46.Pönisch F, Parodi K, Hasch BG, Enghardt W. The modelling of positron emitter production and PET imaging during carbon ion therapy. Phys Med Biol. 2004;49:5217–5232. doi: 10.1088/0031-9155/49/23/002. [DOI] [PubMed] [Google Scholar]
- 47.Bauer J, et al. Implementation and initial clinical experience of offline PET/CT-based verification of scanned carbon ion treatment. Radiother Oncol. 2013;107:218–226. doi: 10.1016/j.radonc.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 48.Augusto RS, et al. An overview of recent developments in FLUKA PET tools. Phys Med. 2018;54:189–199. doi: 10.1016/j.ejmp.2018.06.636. [DOI] [PubMed] [Google Scholar]
- 49.Durante M, Parodi K. Radioactive beams in particle therapy: past, present, and future. Front Phys. 2020;8:00326. doi: 10.3389/fphy.2020.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Durante M, Golubev A, Park W-Y, Trautmann C. Applied nuclear physics at the new high-energy particle accelerator facilities. Phys Rep. 2019;800:1–37. [Google Scholar]
- 51.Chacon A, et al. Experimental investigation of the characteristics of radioactive beams for heavy ion therapy. Med Phys. 2020;47:3123–3132. doi: 10.1002/mp.14177. [DOI] [PubMed] [Google Scholar]
- 52.Augusto RS, et al. New developments of 11C postaccelerated beams for hadron therapy and imaging. Nucl Instrum Methods Phys Res B. 2016;376:374–378. [Google Scholar]
- 53.Chao AW, Chou W. Reviews of Accelerator Science and Technology. Volume 2: Medical Applications of Accelerators. World Scientific; 2009. [Google Scholar]
- 54.Farr JB, Flanz JB, Gerbershagen A, Moyers MF. New horizons in particle therapy systems. Med Phys. 2018;45:e953–e983. doi: 10.1002/mp.13193. [DOI] [PubMed] [Google Scholar]
- 55.Alonso JR, Antaya TA. Superconductivity in medicine. Rev Accel Sci Technol. 2012;05:227–263. [Google Scholar]
- 56.Jongen Y, et al. Compact superconducting cyclotron C400 for hadron therapy. Nucl InstruMMethods Phys Res A. 2010;624:47–53. [Google Scholar]
- 57.Syresin EM, et al. Superconducting synchrotron project for hadron therapy. Phys ParTNucl Lett. 2012;9:202–212. [Google Scholar]
- 58.Noda K, et al. Recent progress and future plans of heavy-ion cancer radiotherapy with HIMAC. Nucl InstruMMethods Phys Res B. 2017;406:374–378. [Google Scholar]
- 59.Zlobin AV, Schoerling D. In: Nb3Sn Accelerator Magnets. Designs, Technologies and Performance. Schoerling D, Zlobin A, editors. Springer; 2019. pp. 3–22. [Google Scholar]
- 60.Wan W, et al. Alternating-gradient canted cosine theta superconducting magnets for future compact proton gantries. Phys Rev Spec Top Accel Beams. 2015;18:103501 [Google Scholar]
- 61.Baird YTE, Li Q. Optimized magnetic design of superconducting magnets for heavy ion rotating gantries. IEEE Trans Appl Supercond. 2020;30:1–8. [Google Scholar]
- 62.Caporaso GJ, Chen YJ, Sampayan SE. The dielectric wall accelerator. Rev Accel Sci Technol. 2009;02:253–263. [Google Scholar]
- 63.Ma WJ, et al. Laser acceleration of highly energetic carbon ions using a double-layer target composed of slightly underdense plasma and ultrathin foil. Phys Rev Lett. 2019;122:014803. doi: 10.1103/PhysRevLett.122.014803. [DOI] [PubMed] [Google Scholar]
- 64.Higginson A, et al. Near-100 MeV protons via a laser-driven transparency-enhanced hybrid acceleration scheme. Nat Commun. 2018;9:724. doi: 10.1038/s41467-018-03063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahmed H, et al. High energy implementation of coil-target scheme for guided re-acceleration of laser-driven protons. Sci Rep. 2021;11:699. doi: 10.1038/s41598-020-77997-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang KD, et al. Achromatic beamline design for a laser-driven proton therapy accelerator. Phys Rev Accel Beams. 2020;23:111302 [Google Scholar]
- 67.Karsch L, et al. Towards ion beam therapy based on laser plasma accelerators. Acta Oncol. 2017;56:1359–1366. doi: 10.1080/0284186X.2017.1355111. [DOI] [PubMed] [Google Scholar]
- 68.Linz U, Alonso J. Laser-driven ion accelerators for tumor therapy revisited. Phys Rev Accel Beams. 2016;19:124802 [Google Scholar]
- 69.Noda K. Progress of radiotherapy technology with HIMAC. J Phys Conf Ser. 2019;1154:012019 [Google Scholar]
- 70.Kubiak T. Particle therapy of moving targets — the strategies for tumour motion monitoring and moving targets irradiation. Br J Radiol. 2016;89:20150275. doi: 10.1259/bjr.20150275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bert C, Durante M. Motion in radiotherapy: particle therapy. Phys Med Biol. 2011;56:R113–R144. doi: 10.1088/0031-9155/56/16/R01. [DOI] [PubMed] [Google Scholar]
- 72.Riboldi M, Orecchia PR, Baroni PG. Real-time tumour tracking in particle therapy: technological developments and future perspectives. Lancet Oncol. 2012;13:e383–e391. doi: 10.1016/S1470-2045(12)70243-7. [DOI] [PubMed] [Google Scholar]
- 73.Czerska K, et al. Clinical practice vs. state-of-the-art research and future visions: Report on the 4D treatment planning workshop for particle therapy- Edition 2018 and 2019. Phys Med. 2021;82:54–63. doi: 10.1016/j.ejmp.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 74.Graeff C, Lüchtenborg R, Eley JG, Durante M, Bert C. A 4D-optimization concept for scanned ion beam therapy. Radiother Oncol. 2013;109:419–424. doi: 10.1016/j.radonc.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 75.Iwata Y, et al. Design of a superconducting rotating gantry for heavy-ion therapy. Phys Rev Spec Top Accel Beams. 2012;15:044701 [Google Scholar]
- 76.Rahim S, et al. Upright radiation therapy — a historical reflection and opportunities for future applications. Front Oncol. 2020;10:213. doi: 10.3389/fonc.2020.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang J, Chu D, Dong L, Court LE. Advantages of simulating thoracic cancer patients in an upright position. Pract Radiat Oncol. 2014;4:e53–e58. doi: 10.1016/j.prro.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 78.Zhang X, et al. Development of an isocentric rotating chair positioner to treat patients of head and neck cancer at upright seated position with multiple nonplanar fields in a fixed carbon-ion beamline. Med Phys. 2020;47:2450–2460. doi: 10.1002/mp.14115. [DOI] [PubMed] [Google Scholar]
- 79.Sheng Y, et al. Performance of a 6D treatment chair for patient positioning in an upright posture for fixed ion beam lines. Front Oncol. 2020;10:213. doi: 10.3389/fonc.2020.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cornforth MN. Occam’s broom and the dirty DSB: cytogenetic perspectives on cellular response to changes in track structure and ionization density. Int J Radiat Biol. 2020;97:1099–1108. doi: 10.1080/09553002.2019.1704302. [DOI] [PubMed] [Google Scholar]
- 81.Stannard C, et al. Malignant salivary gland tumours: can fast neutron therapy results point the way to carbon ion therapy? Radiother Oncol. 2013;109:262–268. doi: 10.1016/j.radonc.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 82.Parker C, et al. Targeted alpha therapy, an emerging class of cancer agents. JAMA Oncol. 2018;4:1765–1772. doi: 10.1001/jamaoncol.2018.4044. [DOI] [PubMed] [Google Scholar]
- 83.Durante M, Orecchia R, Loeffler JS. Charged-particle therapy in cancer: clinical uses and future perspectives. Nat Rev Clin Oncol. 2017;14:483–495. doi: 10.1038/nrclinonc.2017.30. [DOI] [PubMed] [Google Scholar]
- 84.Blakely EA. The 20th Gray lecture 2019: health and heavy ions. Br J Radiol. 2020;93:20200172. doi: 10.1259/bjr.20200172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fowler JF. 21 years of biologically effective dose. Br J Radiol. 2010;83:554–568. doi: 10.1259/bjr/31372149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Friedrich T, Scholz U, Elsässer T, Durante M, Scholz M. Systematic analysis of RBE and related quantities using a database of cell survival experiments with ion beam irradiation. J Radiat Res. 2013;54:494–514. doi: 10.1093/jrr/rrs114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang JZ, Huang Z, Lo SS, Yuh WTC, Mayr NA. A generalized linear-quadratic model for radiosurgery, stereotactic body radiation therapy, and high-dose rate brachytherapy. Sci Transl Med. 2010;2:39ra48. doi: 10.1126/scitranslmed.3000864. [DOI] [PubMed] [Google Scholar]
- 88.Takahashi Y, et al. Heavy ion irradiation inhibits in vitro angiogenesis even at sublethal dose. Cancer Res. 2003;63:4253–4257. [PubMed] [Google Scholar]
- 89.Liu Y, et al. Effects of carbon-ion beam irradiation on the angiogenic response in lung adenocarcinoma A549 cells. Cell Biol Int. 2014;38:1304–1310. doi: 10.1002/cbin.10327. [DOI] [PubMed] [Google Scholar]
- 90.Konings K, Vandevoorde C, Baselet B, Baatout S, Moreels M. Combination therapy with charged particles and molecular targeting: a promising avenue to overcome radioresistance. Front Oncol. 2020;10:128. doi: 10.3389/fonc.2020.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Durante M, Brenner DJ, Formenti SC. Does heavy ion therapy work through the immune system? Int J Radiat Oncol Biol Phys. 2016;96:934–936. doi: 10.1016/j.ijrobp.2016.08.037. [DOI] [PubMed] [Google Scholar]
- 92.Wulf H, et al. Heavy-ion effects on mammalian cells: inactivation measurements with different cell lines. Radiat Res. 1985;104:122–134. [PubMed] [Google Scholar]
- 93.ICRU Report 93: Prescribing, Recording, and Reporting Light Ion Beam Therapy. J ICRU. 2016;16 [Google Scholar]
- 94.Inaniwa T, et al. Treatment planning for a scanned carbon beam with a modified microdosimetric kinetic model. Phys Med Biol. 2010;55:6721–6737. doi: 10.1088/0031-9155/55/22/008. [DOI] [PubMed] [Google Scholar]
- 95.Grün R, et al. Impact of enhancements in the local effect model (LEM) on the predicted RBE-weighted target dose distribution in carbon ion therapy. Phys Med Biol. 2012;57:7261–7274. doi: 10.1088/0031-9155/57/22/7261. [DOI] [PubMed] [Google Scholar]
- 96.Tommasino F, Durante M. Proton radiobiology. Cancers. 2015;7:353–381. doi: 10.3390/cancers7010353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol. 2014;59:R419–R472. doi: 10.1088/0031-9155/59/22/R419. [DOI] [PubMed] [Google Scholar]
- 98.Mutter RW, et al. Incorporation of biologic response variance modeling into the clinic: limiting risk of brachial plexopathy and other late effects of breast cancer proton beam therapy. Pract Radiat Oncol. 2020;10:e71–e81. doi: 10.1016/j.prro.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang L, Wang W, Hu J, Lu J, Kong L. RBE-weighted dose conversions for patients with recurrent nasopharyngeal carcinoma receiving carbon-ion radiotherapy from the local effect model to the microdosimetric kinetic model. Radiat Oncol. 2020;15:277. doi: 10.1186/s13014-020-01723-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang W, et al. RBE-weighted dose conversions for carbon ionradiotherapy between microdosimetric kinetic model and local effect model for the targets and organs at risk in prostate carcinoma. Radiother Oncol. 2020;144:30–36. doi: 10.1016/j.radonc.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 101.Molinelli S, et al. Dose prescription in carbon ion radiotherapy: How to compare two different RBE-weighted dose calculation systems. Radiother Oncol. 2016;120:307–312. doi: 10.1016/j.radonc.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 102.Fossati P, Matsufuji N, Kamada T, Karger CP. Radiobiological issues in prospective carbon ion therapy trials. Med Phys. 2018;45:e1096–e1110. doi: 10.1002/mp.12506. [DOI] [PubMed] [Google Scholar]
- 103.Friedrich T, Scholz U, Durante M, Scholz M. RBE of ion beams in hypofractionated radiotherapy (SBRT) Phys Med. 2014;30:588–591. doi: 10.1016/j.ejmp.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 104.Yoshida Y, et al. Evaluation of therapeutic gain for fractionated carbon-ion radiotherapy using the tumor growth delay and crypt survival assays. Radiother Oncol. 2015;117:351–357. doi: 10.1016/j.radonc.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 105.Chapman JD. Can the two mechanisms of tumor cell killing by radiation be exploited for therapeutic gain? J Radiat Res. 2014;55:2–9. doi: 10.1093/jrr/rrt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Laine AM, et al. The role of hypofractionated radiation therapy with photons, protons, and heavy ions for treating extracranial lesions. Front Oncol. 2016;5:302. doi: 10.3389/fonc.2015.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Barker HE, Paget JTE, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15:409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Strigari L, Benassi M, Sarnelli A, Polico R, D’Andrea M. A modified hypoxia-based TCP model to investigate the clinical outcome of stereotactic hypofractionated regimes for early stage non-small-cell lung cancer (NSCLC) Med Phys. 2012;39:4502–4514. doi: 10.1118/1.4730292. [DOI] [PubMed] [Google Scholar]
- 109.Toma-Dasu I, Sandström H, Barsoum P, Dasu A. To fractionate or not to fractionate? That is the question for the radiosurgery of hypoxic tumors. J Neurosurg. 2014;121:110–115. doi: 10.3171/2014.8.GKS141461. [DOI] [PubMed] [Google Scholar]
- 110.Mc Keown SR. Defining normoxia, physoxia and hypoxia in tumours — implications for treatment response. Br J Radiol. 2014;87:20130676. doi: 10.1259/bjr.20130676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Furusawa Y, et al. Inactivation of aerobic and hypoxic cells from three different cell lines by accelerated (3) He-, (12)C- and (20)Ne-ion beams. Radiat Res. 2000;154:485–496. doi: 10.1667/0033-7587(2000)154[0485:ioaahc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 112.Tinganelli W, et al. Kill-painting of hypoxic tumours in charged particle therapy. Sci Rep. 2015;5:17016. doi: 10.1038/srep17016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grassberger C, Ellsworth SG, Wilks MQ, Keane FK, Loeffler JS. Assessing the interactions between radiotherapy and antitumour immunity. Nat Rev Clin Oncol. 2019;16:729–745. doi: 10.1038/s41571-019-0238-9. [DOI] [PubMed] [Google Scholar]
- 114.Durante M, et al. X-rays vs. carbon-ion tumor therapy: cytogenetic damage in lymphocytes. Int J Radiat Oncol Biol Phys. 2000;47:793–798. doi: 10.1016/s0360-3016(00)00455-7. [DOI] [PubMed] [Google Scholar]
- 115.Durante M, Formenti S. Harnessing radiation to improve immunotherapy: better with particles? Br J Radiol. 2020;93:20190224. doi: 10.1259/bjr.20190224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Davuluri R, et al. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol. 2017;99:128–135. doi: 10.1016/j.ijrobp.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 117.Mohan R, et al. Proton therapy reduces the likelihood of high-grade radiation-induced lymphopenia in glioblastoma patients: phase II randomized study of protons vs photons. Neuro Oncol. 2021;23:284–294. doi: 10.1093/neuonc/noaa182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim N, et al. Proton beam therapy reduces the risk of severe radiation-induced lymphopenia during chemoradiotherapy for locally advanced non-small cell lung cancer: a comparative analysis of proton versus photon therapy. Radiother Oncol. 2021;156:166–173. doi: 10.1016/j.radonc.2020.12.019. [DOI] [PubMed] [Google Scholar]
- 119.Takahashi Y, et al. Carbon ion irradiation enhances the antitumor efficacy of dual immune checkpoint blockade therapy both for local and distant sites in murine osteosarcoma. Oncotarget. 2019;10:633–646. doi: 10.18632/oncotarget.26551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Helm A, et al. Reduction of lung metastases in a mouse osteosarcoma model treated with carbon ions and immune checkpoint inhibitors. Int J Radiat Oncol. 2021;109:594–602. doi: 10.1016/j.ijrobp.2020.09.041. [DOI] [PubMed] [Google Scholar]
- 121.Marcus D, et al. Charged particle and conventional radiotherapy: current implications as partner for immunotherapy. Cancers. 2021;13:1468. doi: 10.3390/cancers13061468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Friedrich T, Henthorn N, Durante M. Modeling radioimmune response — current status and perspectives. Front Oncol. 2021;11:647272. doi: 10.3389/fonc.2021.647272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang Z, et al. Charged particle radiation therapy for uveal melanoma: a systematic review and meta-analysis. Int J Radiat Oncol. 2013;86:18–26. doi: 10.1016/j.ijrobp.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 124.Mishra KK, et al. Long-term results of the UCSF- LBNL randomized trial: charged particle with helium ion versus iodine-125 plaque therapy for choroidal and ciliary body melanoma. Int J Radiat Oncol Biol Phys. 2015;92:376–383. doi: 10.1016/j.ijrobp.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 125.Kamada T, et al. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol. 2015;16:e93–e100. doi: 10.1016/S1470-2045(14)70412-7. [DOI] [PubMed] [Google Scholar]
- 126.Shinoto M, et al. Carbon ion radiation therapy with concurrent gemcitabine for patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2016;95:498–504. doi: 10.1016/j.ijrobp.2015.12.362. [DOI] [PubMed] [Google Scholar]
- 127.Kawashiro S, et al. Multi-institutional study of carbonion radiotherapy for locally advanced pancreatic cancer: Japan Carbon-ion Radiation Oncology Study Group (J-CROS) study 1403 pancreas. Int J Radiat Oncol. 2018;101:1212–1221. doi: 10.1016/j.ijrobp.2018.04.057. [DOI] [PubMed] [Google Scholar]
- 128.Nevala-Plagemann C, Hidalgo M, Garrido-Laguna I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat Rev Clin Oncol. 2020;17:108–123. doi: 10.1038/s41571-019-0281-6. [DOI] [PubMed] [Google Scholar]
- 129.Yamasaki A, Yanai K, Onishi H. Hypoxia and pancreatic ductal adenocarcinoma. Cancer Lett. 2020;484:9–15. doi: 10.1016/j.canlet.2020.04.018. [DOI] [PubMed] [Google Scholar]
- 130.Ho WJ, Jaffee EM, Zheng L. The tumour microenvironment in pancreatic cancer — clinical challenges and opportunities. Nat Rev Clin Oncol. 2020;17:527–540. doi: 10.1038/s41571-020-0363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huart C, Chen J, Le Calvé B, Michiels C, Wéra AC. Could protons and carbon ions be the silver bullets against pancreatic cancer? Int J Mol Sci. 2020;21:4767. doi: 10.3390/ijms21134767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liermann J, et al. Carbon ion radiotherapy in pancreatic cancer: a review of clinical data. Radiother Oncol. 2020;147:145–150. doi: 10.1016/j.radonc.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 133.Dreher C, Habermehl D, Jäkel O, Combs SE. Effective radiotherapeutic treatment intensification in patients with pancreatic cancer: higher doses alone, higher RBE or both? Radiat Oncol. 2017;12:203. doi: 10.1186/s13014-017-0945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shinoto M, et al. Carbon-ion radiotherapy for locally recurrent rectal cancer: Japan Carbon-ion Radiation Oncology Study Group (J-CROS) study 1404 rectum. Radiother Oncol. 2019;132:236–240. doi: 10.1016/j.radonc.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 135.Cai X, et al. The role of carbon ion radiotherapy for unresectable locally recurrent rectal cancer: a single institutional experience. Radiat Oncol. 2020;15:209. doi: 10.1186/s13014-020-01653-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Habermehl D, et al. Reirradiation using carbon ions in patients with locally recurrent rectal cancer at HIT: first results. Ann Surg Oncol. 2015;22:2068–2074. doi: 10.1245/s10434-014-4219-z. [DOI] [PubMed] [Google Scholar]
- 137.Guren MG, et al. Reirradiation of locally recurrent rectal cancer: a systematic review. Radiother Oncol. 2014;113:151–157. doi: 10.1016/j.radonc.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 138.Venkatesulu BP, Giridhar P, Malouf TD, Trifletti DM, Krishnan S. A systematic review of the role of carbon ion radiation therapy in recurrent rectal cancer. Acta Oncol. 2020;59:1218–1223. doi: 10.1080/0284186X.2020.1769184. [DOI] [PubMed] [Google Scholar]
- 139.Imada H, et al. Comparison of efficacy and toxicity of short-course carbon ion radiotherapy for hepatocellular carcinoma depending on their proximity to the porta hepatis. Radiother Oncol. 2010;96:231–235. doi: 10.1016/j.radonc.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 140.Qi W-X, Fu S, Zhang Q, Guo XM. Charged particle therapy versus photon therapy for patients with hepatocellular carcinoma: a systematic review and meta-analysis. Radiother Oncol. 2015;114:289–295. doi: 10.1016/j.radonc.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 141.Habermehl D, et al. Hypofractionated carbon ion therapy delivered with scanned ion beams for patients with hepatocellular carcinoma-feasibility and clinical response. Radiat Oncol. 2013;8:59. doi: 10.1186/1748-717X-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shibuya K, et al. A feasibility study of high-dose hypofractionated carbon ion radiation therapy using four fractions for localized hepatocellular carcinoma measuring 3 cm or larger. Radiother Oncol. 2019;132:230–235. doi: 10.1016/j.radonc.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 143.Chauvel P. Osteosarcomas and adult soft tissue sarcomas: is there a place for high LET radiation therapy? Ann Oncol. 1992;3:S107–S110. doi: 10.1093/annonc/3.suppl_2.s107. [DOI] [PubMed] [Google Scholar]
- 144.Strander H, Turesson I, Cavallin-ståhl E. A systematic overview of radiation therapy effects in soft tissue sarcomas. Acta Oncol. 2003;42:516–531. doi: 10.1080/02841860310014732. [DOI] [PubMed] [Google Scholar]
- 145.Weber DC, et al. Profile of European proton and carbon ion therapy centers assessed by the EORTC facility questionnaire. Radiother Oncol. 2017;124:185–189. doi: 10.1016/j.radonc.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 146.Matsunobu A, et al. Impact of carbon ion radiotherapy for unresectable osteosarcoma of the trunk. Cancer. 2012;118:4555–4563. doi: 10.1002/cncr.27451. [DOI] [PubMed] [Google Scholar]
- 147.Kamada T, et al. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J Clin Oncol. 2002;20:4466–4471. doi: 10.1200/JCO.2002.10.050. [DOI] [PubMed] [Google Scholar]
- 148.Cuccia F, et al. Outcome and toxicity of carbon ion radiotherapy for axial bone and soft tissue sarcomas. Anticancer Res. 2020;40:2853–2859. doi: 10.21873/anticanres.14260. [DOI] [PubMed] [Google Scholar]
- 149.Seidensaal K, et al. The role of combined ion-beam radiotherapy (CIBRT) with protons and carbon ions in a multimodal treatment strategy of inoperable osteosarcoma. Radiother Oncol. 2021;159:8–16. doi: 10.1016/j.radonc.2021.01.029. [DOI] [PubMed] [Google Scholar]
- 150.Uhl M, et al. Highly effective treatment of skull base chordoma with carbon ion irradiation using a raster scan technique in 155 patients: first long-term results. Cancer. 2014;120:3410–3417. doi: 10.1002/cncr.28877. [DOI] [PubMed] [Google Scholar]
- 151.Mattke M, et al. High control rates of proton- and carbon-ion-beam treatment with intensity-modulated active raster scanning in 101 patients with skull base chondrosarcoma at the Heidelberg Ion Beam Therapy Center. Cancer. 2018;124:2036–2044. doi: 10.1002/cncr.31298. [DOI] [PubMed] [Google Scholar]
- 152.Nikoghosyan AV, et al. Randomised trial of proton vs. carbon ion radiation therapy in patients with low and intermediate grade chondrosarcoma of the skull base, clinical phase III study. BMC Cancer. 2010;10:606. doi: 10.1186/1471-2407-10-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cramer JD, Burtness B, Le QT, Ferris RL. The changing therapeutic landscape of head and neck cancer. Nat Rev Clin Oncol. 2019;16:669–683. doi: 10.1038/s41571-019-0227-z. [DOI] [PubMed] [Google Scholar]
- 154.Akbaba S, et al. Bimodal radiotherapy with active raster-scanning carbon ion radiotherapy and intensity-modulated radiotherapy in high-risk nasopharyngeal carcinoma results in excellent local control. Cancers. 2019;11:379. doi: 10.3390/cancers11030379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shirai K, et al. Prospective observational study of carbon-ion radiotherapy for non-squamous cell carcinoma of the head and neck. Cancer Sci. 2017;108:2039–2044. doi: 10.1111/cas.13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Högerle BA, et al. Primary adenoid cystic carcinoma of the trachea: clinical outcome of 38 patients after interdisciplinary treatment in a single institution. Radiat Oncol. 2019;14:117. doi: 10.1186/s13014-019-1323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kong L, et al. Phase I/II trial evaluating carbon ion radiotherapy for salvaging treatment of locally recurrent nasopharyngeal carcinoma. J Cancer. 2016;7:774–783. doi: 10.7150/jca.14399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Baumann BC, et al. Comparative effectiveness of proton vs photon therapy as part of concurrent chemoradiotherapy for locally advanced cancer. JAMA Oncol. 2020;6:237–246. doi: 10.1001/jamaoncol.2019.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li X, et al. Toxicity profiles and survival outcomes among patients with nonmetastatic nasopharyngeal carcinoma treated with intensity-modulated proton therapy vs intensity-modulated radiation therapy. JAMA Netw Open. 2021;4:e2113205. doi: 10.1001/jamanetworkopen.2021.13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gondi V, Yock TI, Mehta MP. Proton therapy for paediatric CNS tumours — improving treatment-related outcomes. Nat Rev Neurol. 2016;12:334–345. doi: 10.1038/nrneurol.2016.70. [DOI] [PubMed] [Google Scholar]
- 161.Newhauser WD, Durante M. Assessing the risk of second malignancies after modern radiotherapy. Nat Rev Cancer. 2011;11:438–448. doi: 10.1038/nrc3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rieber JG, et al. Treatment tolerance of particle therapy in pediatric patients. Acta Oncol. 2015;54:1049–1055. doi: 10.3109/0284186X.2014.998273. [DOI] [PubMed] [Google Scholar]
- 163.Mohamad O, et al. Risk of subsequent primary cancers after carbon ion radiotherapy, photon radiotherapy, or surgery for localised prostate cancer: a propensity score-weighted, retrospective, cohort study. Lancet Oncol. 2019;20:674–685. doi: 10.1016/S1470-2045(18)30931-8. [DOI] [PubMed] [Google Scholar]
- 164.Ohno T, Okamoto M. Carbon ion radiotherapy as a treatment modality for paediatric cancers. Lancet Child Adolesc Health. 2019;3:371–372. doi: 10.1016/S2352-4642(19)30106-3. [DOI] [PubMed] [Google Scholar]
- 165.Knäusl B, Fuchs H, Dieckmann K, Georg D. Can particle beam therapy be improved using helium ions? - A planning study focusing on pediatric patients. Acta Oncol. 2016;55:751–759. doi: 10.3109/0284186X.2015.1125016. [DOI] [PubMed] [Google Scholar]
- 166.Horst F, et al. Physical characterization of3He ion beams for radiotherapy and comparison with4He. Phys Med Biol. 2021;66:095009. doi: 10.1088/1361-6560/abef88. [DOI] [PubMed] [Google Scholar]
- 167.Tommasino F, Scifoni E, Durante M. New ions for therapy. Int J Part Ther. 2015;2:428–438. doi: 10.14338/IJPT-15-00027.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Scifoni E, et al. Including oxygen enhancement ratio in ion beam treatment planning: model implementation and experimental verification. Phys Med Biol. 2013;58:3871–3895. doi: 10.1088/0031-9155/58/11/3871. [DOI] [PubMed] [Google Scholar]
- 169.Sokol O, et al. Oxygen beams for therapy: advanced biological treatment planning and experimental verification. Phys Med Biol. 2017;62:7798–7813. doi: 10.1088/1361-6560/aa88a0. [DOI] [PubMed] [Google Scholar]
- 170.Hagiwara Y, et al. Influence of dose-averaged linear energy transfer on tumour control after carbon-ion radiation therapy for pancreatic cancer. Clin Transl Radiat Oncol. 2020;21:19–24. doi: 10.1016/j.ctro.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Matsumoto S, et al. Unresectable chondrosarcomas treated with carbon ion radiotherapy: relationship between dose-averaged linear energy transfer and local recurrence. Anticancer Res. 2020;40:6429–6435. doi: 10.21873/anticanres.14664. [DOI] [PubMed] [Google Scholar]
- 172.Bassler N, et al. LET-painting increases tumour control probability in hypoxic tumours. Acta Oncol. 2014;53:25–32. doi: 10.3109/0284186X.2013.832835. [DOI] [PubMed] [Google Scholar]
- 173.Bassler N, Jäkel O, Søndergaard CS, Petersen JB. Dose- and LET-painting with particle therapy. Acta Oncol. 2010;49:1170–1176. doi: 10.3109/0284186X.2010.510640. [DOI] [PubMed] [Google Scholar]
- 174.Ebner DK, Frank SJ, Inaniwa T, Yamada S, Shirai T. The emerging potential of multi-ion radiotherapy. Front Oncol. 2021;11:624786. doi: 10.3389/fonc.2021.624786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Inaniwa T, Kanematsu N, Noda K, Kamada T. Treatment planning of intensity modulated composite particle therapy with dose and linear energy transfer optimization. Phys Med Biol. 2017;62:5180–5197. doi: 10.1088/1361-6560/aa68d7. [DOI] [PubMed] [Google Scholar]
- 176.Horsman MR, et al. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol. 2012;9:674–687. doi: 10.1038/nrclinonc.2012.171. [DOI] [PubMed] [Google Scholar]
- 177.Mazal A, et al. FLASH and minibeams in radiation therapy: the effect of microstructures on time and space and their potential application to protontherapy. Br J Radiol. 2020;93:20190807. doi: 10.1259/bjr.20190807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Favaudon V, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med. 2014;6:245ra93. doi: 10.1126/scitranslmed.3008973. [DOI] [PubMed] [Google Scholar]
- 179.Vozenin MC, et al. The advantage of FLASH radiotherapy confirmed in mini-pig and cat-cancer patients. Clin Cancer Res. 2019;25:35–42. doi: 10.1158/1078-0432.CCR-17-3375. [DOI] [PubMed] [Google Scholar]
- 180.Montay-Gruel P, et al. Hypofractionated FLASH-RT as an effective treatment against glioblastoma that reduces neurocognitive side effects in mice. Clin Cancer Res. 2021;27:775–784. doi: 10.1158/1078-0432.CCR-20-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Di Martino F, et al. FLASH radiotherapy with electrons: issues related to the production, monitoring, and dosimetric characterization of the beam. Front Phys. 2020;8:570697 [Google Scholar]
- 182.Jolly S, Owen H, Schippers M, Welsch C. Technical challenges for FLASH proton therapy. Phys Med. 2020;78:71–82. doi: 10.1016/j.ejmp.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 183.Zakaria AM, et al. Ultra-high dose-rate, pulsed (FLASH) radiotherapy with carbon ions: generation of early, transient, highly oxygenated conditions in the tumor environment. Radiat Res. 2020;194:587–593. doi: 10.1667/RADE-19-00015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vozenin M-C, Montay-Gruel P, Limoli C, Germond J-F. All irradiations that are ultra-high dose rate may not be FLASH: the critical importance of beam parameter characterization and in vivo validation of the FLASH effect. Radiat Res. 2020;194:571–572. doi: 10.1667/RADE-20-00141.1. [DOI] [PubMed] [Google Scholar]
- 185.Rothwell BC, et al. Determining the parameter space for effective oxygen depletion for FLASH radiation therapy. Phys Med Biol. 2021;66:055020. doi: 10.1088/1361-6560/abe2ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chaudhary P, et al. Radiobiology experiments with ultra-high dose rate laser-driven protons: methodology and state-of-the-art. Front Phys. 2021;9:624963 [Google Scholar]
- 187.Bourhis J, et al. Treatment of a first patient with FLASH-radiotherapy. Radiother Oncol. 2019;139:18–22. doi: 10.1016/j.radonc.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 188.Pratx G, Kapp DS. A computational model of radiolytic oxygen depletion during FLASH irradiation and its effect on the oxygen enhancement ratio. Phys Med Biol. 2019;64:185005. doi: 10.1088/1361-6560/ab3769. [DOI] [PubMed] [Google Scholar]
- 189.Labarbe R, Hotoiu L, Barbier J, Favaudon V. A physicochemical model of reaction kinetics supports peroxyl radical recombination as the main determinant of the FLASH effect. Radiother Oncol. 2020;153:303–310. doi: 10.1016/j.radonc.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 190.Jansen J, et al. Does FLASH deplete oxygen? Experimental evaluation for photons, protons, and carbon ions. Med Phys. 2021;48:3982–3990. doi: 10.1002/mp.14917. [DOI] [PubMed] [Google Scholar]
- 191.Boscolo D, Scifoni E, Durante M, Krämer M, Fuss MC. May oxygen depletion explain the FLASH effect? A chemical track structure analysis. Radiother Oncol. 2021;162:68–75. doi: 10.1016/j.radonc.2021.06.031. [DOI] [PubMed] [Google Scholar]
- 192.Weber U, Scifoni E, Durante M. FLASH radiotherapy with carbon ion beams. Med Phys. 2021 doi: 10.1002/mp.15135. [DOI] [PubMed] [Google Scholar]
- 193.Schültke E, et al. Microbeam radiation therapy — grid therapy and beyond: a clinical perspective. Br J Radiol. 2017;90:20170073. doi: 10.1259/bjr.20170073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lamirault C, et al. Short and long-term evaluation of the impact of proton minibeam radiation therapy on motor, emotional and cognitive functions. Sci Rep. 2020;10:13511. doi: 10.1038/s41598-020-70371-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Billena C, Khan AJ. A current review of spatial fractionation: back to the future. Int J Radiat Oncol. 2019;104:177–187. doi: 10.1016/j.ijrobp.2019.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dilmanian FA, et al. Interlaced x-ray microplanar beams: a radiosurgery approach with clinical potential. Proc Natl Acad Sci USA. 2006;103:9709–9714. doi: 10.1073/pnas.0603567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dilmanian FA, et al. Interleaved carbon minibeams: an experimental radiosurgery method with clinical potential. Int J Radiat Oncol Biol Phys. 2012;84:514–519. doi: 10.1016/j.ijrobp.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 198.González W, Prezado Y. Spatial fractionation of the dose in heavy ions therapy: an optimization study. Med Phys. 2018;45:2620–2627. doi: 10.1002/mp.12902. [DOI] [PubMed] [Google Scholar]
- 199.Prezado Y, et al. A potential renewed use of very heavy ions for therapy: neon minibeam radiation therapy. Cancers. 2021;12:1356. doi: 10.3390/cancers13061356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kirkby KJ, et al. Heavy charged particle beam therapy and related new radiotherapy technologies: the clinical potential, physics and technical developments required to deliver benefit for patients with cancer. Br J Radiol. 2020;93:20200247. doi: 10.1259/bjr.20200247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kramer M, et al. Helium ions for radiotherapy? Physical and biological verifications of a novel treatment modality. Med Phys. 2016;43:1995–2004. doi: 10.1118/1.4960373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.