Abstract
Mammalian cochlear inner hair cells (IHCs) are specialized sensory receptors able to provide dynamic coding of sound signals. This ability is largely conferred by their ribbon synapses, which tether a large number of vesicles at the IHC’s presynaptic active zones, allowing high rates of sustained synaptic transmission onto the afferent fibres. How the physiological and morphological properties of ribbon synapses change with age is still largely unknown. Here, we have investigated the biophysical and morphological properties of IHC ribbon synapses in the ageing cochlea (9-12 kHz region) of four mouse strains commonly used in hearing research: early-onset progressive hearing loss (C57BL/6J and C57BL/6NTac) and “good hearing” strains (C57BL/6NTacCdh23+ and C3H/HeJ). We found that with age, both modiolar and pillar sides of the IHC exhibited a loss of ribbons, but an increased volume of those that remained. These morphological changes, which only occurred after 6 months of age, were correlated with the level of hearing loss in the different mouse strains, being most severe for C57BL/6NTac and C57BL/6J, less so for C57BL/6NTacCdh23+ and absent in C3H/HeJ strains. Despite the age-related reduction in ribbon number in three out of four strains, the size and kinetics of Ca2+-dependent exocytosis, as well as the replenishment of synaptic vesicles, in IHCs was not affected. The degree of vesicle release at the fewer, but larger, individual remaining ribbon synapses colocalized with the post-synaptic afferent terminals is likely to increase, indicating the presence of a previously unknown degree of functional compensation in the ageing mouse cochlea.
Abbreviations
- ARHL
age-related hearing loss
- OHC
outer hair cells
- IHC
inner hair cells
- PD
postnatal day
- SGN
spiral ganglion neuron
- ΔCm
cell membrane capacitance
- MET
mechanoelectrical transduction
- ABR
auditory brainstem response
Introduction
Age-related hearing loss (ARHL) is the most common form of sensory deficit in the human population (Bowl & Dawson, 2019), causing a progressive, bilateral sensorineural loss associated with decreased hearing sensitivity, decreased ability to understand speech, and impaired sound localization (Gates & Mills, 2005; Gordon-Salant, 2005). Damage to the hair cells has been considered the key contributor to the development of ARHL based on in vivo auditory measurements (Johnsson, 1974; Schuknecht & Gacek, 1993). However, recent evidence has shown that the degeneration of the type I spiral ganglion neurons (SGNs) innervating the inner hair cells (IHCs) is likely to precede hair cell degeneration in the ageing cochlea in mice and humans (Stamataki et al. 2006; Sergeyenko et al. 2013; Kujawa & Liberman, 2015; Wu et al. 2019). Loss of synaptic connections between the IHCs and their type I SGN afferent terminals impacts on the normal encoding of the temporal properties of sound especially in a noisy environment (Costalupes et al. 1984).
In the adult mammalian cochlea, sound-induced displacement of the stereociliary bundles lead to the depolarization of IHCs and the activation of the Cav1.3 Ca2+ channels (Platzer et al., 2000; Brandt et al. 2003; Jeng et al. 2020a), which are clustered at the cell’s presynaptic active zones composed of synaptic ribbons (e.g. Frank et al. 2010; Zampini et al. 2013). Ribbons enable to tether a large number of vesicles, allowing them to facilitate high rates of sustained synaptic transmission onto the auditory afferent fibres (e.g. Glowatzki & Fuchs, 2002; Goutman & Glowatzki, 2007; Keen & Hudspeth, 2006). At mature IHC ribbon synapses, CaV1.3 Ca2+ channels are coupled to glutamate-filled vesicles in either a Ca2+ nanodomain or microdomain (Wong et al. 2014; Johnson et al. 2017; Johnson et al. 2019). Each postsynaptic type I SGN forms only one synapse with an adult IHC (Pujol et al. 1998), with up to ~20 afferent neurons contacting a single IHC in mice (Meyer et al. 2009). These SGNs are segregated around the basolateral membrane of IHCs. In the cat, while high-spontaneous rate (low-threshold) spiral ganglion fibres preferentially contact the pillar side of IHCs (towards the outer hair cells) with small synaptic ribbons, low-spontaneous rate (high-threshold) fibres primarily contact the modiolar side (towards the cochlear nerve) with larger synaptic ribbons (Liberman, 1978; Liberman et al. 1990). The physiological and morphological diversity of SGNs is likely to be important to support the wide dynamic range of sound intensity encoded by the IHCs complement of SGNs (Winter et al. 1990). Currently, very little is known about how ribbon synapses in mouse IHCs, and their associated afferent connections, change with cochlear ageing, and which of those changes directly influences the temporal acquisition of ARHL.
We have identified the initial physiological and morphological changes that occur in ageing IHCs ribbon synapses using mice with differing progressions of hearing loss: early-onset progressive hearing loss (C57BL/6J and C57BL/6NTac) and late-onset hearing loss mice (C3H/HeJ and C57BL/6NTacCdh23+). Like CBA/CaJ, C3H/HeJ mice show a very slow decline in their hearing thresholds with age (Ohlemiller et al. 2016). We found that with age, the ribbon synapses underwent a significant reduction in number and volume, which were correlated with the level of hearing loss in the 9-12 kHz cochlear region of the different mouse strains. C3H mice, which have relatively normal hearing at 18 months of age, had very little or no changes at their IHC ribbon synapses. Despite the synaptic morphological changes in most mouse strains, the size of the Ca2+ current and vesicle exocytosis was not affected with age, highlighting some degree of functional compensation at IHC ribbon synapses in the ageing cochlea.
Materials and Methods
Ethics Statement and animal strains
All animal work was performed at the University of Sheffield (UK) and licensed by the Home Office under the Animals (Scientific Procedures) Act 1986 (PPL_PCC8E5E93) and was approved by the University of Sheffield Ethical Review Committee (180626_Mar).
For in vitro experiments, male mice were killed by cervical dislocation. For in vivo auditory brainstem responses (ABRs) mice were anesthetized using ketamine (100 mg/Kg body weight, Fort Dodge Animal Health, Fort Dodge, USA) and xylazine (10 mg/Kg, Rompun 2%), Bayer HealthCare LLC, NY, USA), which were administered with intraperitoneal injection as previously described (Ingham et al. 2011). At the end of the procedure, mice were either culled by schedule 1 or recovered from anesthesia with intraperitoneal injection of atipamezole (1 mg/Kg). Mice under recovery from anaesthesia were returned to their cage, placed on a thermal mat and monitored over the following 2 to 4 hrs. Once mice were able to move well and respond to external stimuli, they were returned to their holding racks.
Tissue preparation
In vitro recordings were performed from apical-coil IHCs positioned at a frequency range of ~9-12 kHz of the mouse cochlea (Müller et al. 2005; see also Ceriani et al. 2019). Acutely dissected organs of Corti from both male and female mice were obtained from 1 and 18 month old wild-type mice (C57BL/6N, C57BL/6J and C3H/HeJ), and the genome-edited C57BL/6NTacCdh23+ mice (Mianné et al. 2016). The dissection procedure of the aged mouse cochlea was performed as previously described (Jeng et al. 2020b). The excised cochleae were dissected in extracellular solution composed of (in mM): 135 NaCl, 5.8 KCl, 1.3 CaCl2, 0.9 MgCl2, 0.7 NaH2PO4, 5.6 D-glucose, 10 HEPES-NaOH. Sodium pyruvate (2 mM), amino acids and vitamins were added from concentrates (Thermo Fisher Scientific, UK). The pH was adjusted to 7.48 (~308 mmol kg-1). The dissected organ of Corti was then transferred to a microscope chamber, immobilized using a nylon mesh fixed to a stainless steel ring and viewed using an upright microscope (Olympus BX51, Japan; Leica, DMLFS, Germany; Nikon FN-1, Japan). The microscope chamber was continuously perfused with extracellular solution by a peristaltic pump (Cole-Palmer, UK). Hair cells were observed with Nomarski Differential Interference Contrast (DIC) optics (✕63 or ✕60 water immersion objectives) and ✕15 eyepieces.
Single-cell electrophysiology
Real-time changes in membrane capacitance (ΔC m) were recorded at near body temperature (34-37°C) and using 1.3 mM extracellular Ca2+ as previously described (Johnson et al. 2008; 2013; 2017). Recordings were obtained with an Optopatch amplifier (Cairn Research Ltd, UK). Data acquisition was controlled by pClamp software using Digidata 1440A or 1550 boards (Molecular Devices, USA). The patch pipette intracellular solution contained (in mM): 106 Cs-glutamate, 20 CsCl, 3 MgCl2,1 EGTA-CsOH, 5 Na2ATP, 0.3 Na2GTP, 5 HEPES-CsOH, 10 Na2-phosphocreatine, pH 7.3 (294 mmol kg−1). The experimental protocol consisted of a 4 kHz sine wave of 13 mV RMS, which was applied to IHCs from the holding potential of –81 mV, and was interrupted for the duration of the voltage step. The capacitance signal from the Optopatch was filtered at 250 Hz and sampled at 5 kHz. ΔC m was measured by averaging the C m trace over a 200 ms period following the voltage step and subtracting the pre-pulse baseline. Membrane potentials were corrected for the voltage drop across the series resistance and a liquid junction potential of –11 mV. ΔC m experiments were performed during the local perfusion of an extracellular solution containing 30 mM TEA and 15 mM 4-AP (Fluka) to block the BK current (I K,f) (Marcotti et al. 2004) and delayed rectifier K+ currents (I K, previously called I K,neo in the case of pre-hearing IHCs and I K,s in mature IHCs), and linopirdine (80 μM: Tocris) to block I K,n (Marcotti et al. 2003). When the concentration of blockers was ≥1 mM, NaCl was adjusted in the extracellular solution to keep the osmolality constant. The number of vesicles in the RRP was estimated using a conversion factor of 37 aF/vesicle (Lenzi et al. 1999).
Auditory brainstem responses
Auditory brainstem responses (ABRs) were recorded from male and female mice between 1 and 18 months of age from the four strains listed above. Recordings were performed in a soundproof chamber (MAC-3 Acoustic Chamber, IAC Acoustic, UK) as previously described (Ingham et al. 2011). Briefly, stimuli were delivered to the ear by calibrated loudspeakers (MF1-S, Multi Field Speaker, Tucker-Davis Technologies, USA) placed 10 cm from the animal’s pinna. Sound pressure was calibrated with a low-noise microphone probe system (ER10B+, Etymotic, USA). Experiments were performed using a customized software (Ingham et al. 2011) driving an RZ6 auditory processor (Tucker-Davis Technologies). Response thresholds were estimated from the resulting ABR waveform and defined as the lowest sound level where any recognisable feature of the waveform was visible. Responses were measured for clicks and stimulus pure tones of frequencies at 6, 12, 18, 24, 30, 36 and 42 kHz. Stimulus sound pressure levels were typically 0-95 dB SPL, presented in steps of 5 dB SPL. The brainstem response signal was averaged over 256 repetitions. Tone bursts were 5 ms in duration with a 1 ms on/off ramp time, which was presented at a rate of 42.6/sec.
Wave 1 amplitude and latency were measured from ABR recordings obtained by presenting mice with a pure tone (12 kHz, 75 dB SPL). We selected the 12 kHz value because it is close to the frequency range used for the in vitro work. 75dB SPL was the minimal value that allowed the comparison among the different mouse strains (all mice used for this analysis had hearing thresholds below 75 dB at 12 kHz). An initial automatic identification of Wave 1 was carried out using a custom software routine based on the find_peaks function of the scipy.signal Python module (Python 3.7, Python software foundation) (Virtanen et al. 2019). Results were manually reviewed and, if required, adjusted to the correct peak. The Wave 1 amplitude was calculated as the difference between the amplitude of the first peak and the first trough of the ABR waveform; the latency was calculated as the delay of the Wave 1 peak from the beginning of the recording. Since the distance of the speaker from the animal is 10 cm (see above), this leads to a delay in the signal of ~0.3 ms, which was for corrected for.
Immunofluorescence microscopy
Dissected inner ears from the above mouse strains were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4) for 20 minutes at room temperature. Cochleae were dissected, rinsed three times for 10 minutes in PBS, and incubated for 1 hour at room temperature in PBS supplemented with 5% normal goat or horse serum and 0.5% Triton X-100. The samples were then incubated overnight at 37°C with the primary antibody in PBS supplemented with 1% of the specific serum. Primary antibodies were: mouse anti-myosin 7a (1:1000, Developmental Studies Hybridoma Bank, #138-1C), rabbit anti-myosin 7a (1:200, Proteus Biosciences, #25-6790), mouse anti-CtBP2 (C-terminal binding protein 2, 1:200, Biosciences, #612044) and mouse anti-GluR2 (AMPA-type glutamate receptor, 1:200, Millipore, MAB397). All primary antibodies were labelled with species appropriate Alexa Fluor secondary antibodies for 1 hour at 37°C, and then washed 3 times (10 min) in PBS. Samples were then rinsed a final time in PBS (10 min) and mounted in Vectashield. The z-stack images were captured with a Nikon A1 confocal microscope (Nikon CFI Plan Apo 60X Oil objective). The samples used to analyse the pillar-modiolar distribution of the synaptic ribbons were imaged using a Zeiss LSM 880 with AiryScan for super-resolution confocal microscopy. Both confocal microscopes are within the Wolfson Light Microscope Facility at the University of Sheffield. Image stacks were processed with Fiji ImageJ or Imaris analysis software.
The number and volume of synaptic ribbon puncta (and number of GluR2 puncta) was estimated from the z-stack images of the immunolabelled proteins using the Imaris software (Oxford Instruments, UK). Individual puncta were assigned to two separate groups (pillar or modiolar) using k-means clustering (a method of clustering each observation with the nearest mean, MacQueen, 1967) of their position along the pillar to modiolar axis with k=2 clusters. Individual IHCs were manually identified and every protein punctum was automatically assigned to one IHC based on its centroid position.
Statistical analysis
Statistical comparisons of means were made by Student’s two-tailed t-test or, for multiple comparisons, analysis of variance (one-way or 2-way ANOVA followed by a suitable post-test). P < 0.05 was selected as the criterion for statistical significance. Only mean values with a similar variance between groups were compared. Averages are quoted in text and Figures as means ± S.D. For tone burst ABR stimulus data, due to the presence of values outside the threshold limit of our equipment (95 dB), we used non-parametric aligned ranks transformation 2-way ANOVA, followed by Tukey’s post-test or Mann-Whitney U-test with Bonferroni correction for pairwise comparisons. Data are quoted as median, and first and third quartiles.
Results
We performed experiments using four different mouse strains in order to provide a more comprehensive understanding of the pathophysiological differences between those with early-onset (C57BL/6J: 6J, C57BL/6NTac: 6N: Hequembourg & Liberman, 2001; Kane et al. 2012) and “good hearing” late-onset hearing loss (C3H/HeJ: C3H: Trune et al. 1996). The early progression of hearing loss in 6J and 6N mice has been associated with a strain-specific hypomorphic allele in Cadherin 23 (Cdh23ahl: Johnson et al. 1997; Noben-Trauth et al. 2003). Cdh23 encodes cadherin-23 that, together with protocadherin-15, forms the tip links required for gating the mechanoelectrical transducer channels (Kazmierczak et al. 2007; Richardson et al. 2011). When the mutation is corrected with CRISPR/Cas9 in 6N mice, C57BL/6NTacCdh23+ mice (6N-Repaired) showed normal hearing at about 8 months of age (Mianné et al. 2016). Comparing 6N mice with the isogenic 6N-Repaired strain would allow us identifying the possible physiological influence of Cdh23ahl in ageing. In order to identify possible common biological ‘hallmarks’ of cochlear ageing between genetically distinct good-hearing models, 6N-Repaired mice were compared to C3H mice.
Degree of hearing loss in different mouse strains
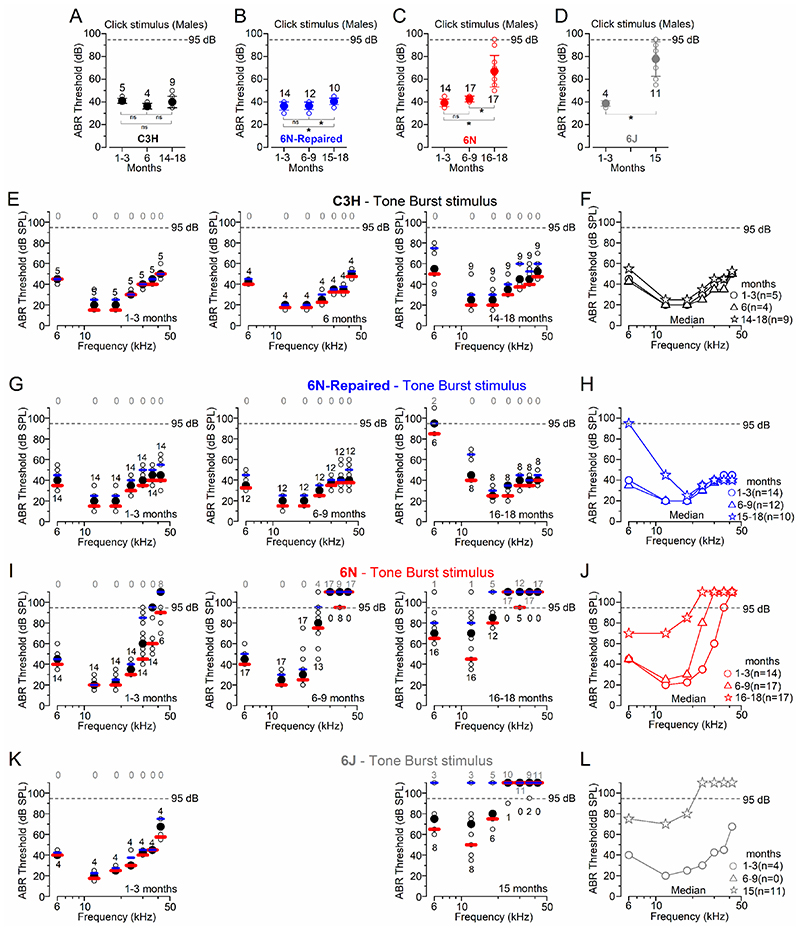
Auditory brainstem responses (ABRs), which measure the summed activity of the auditory nerve fibres (e.g. Kujawa & Liberman, 2009), were used to investigate the hearing sensitivity of the above mouse strains kept under the same environmental conditions (Fig. 1). We found that for click ABR stimuli, C3H male mice showed no significant change in threshold with age (P = 0.2075, one way-ANOVA, Fig. 1A). Male 6N-Repaired, 6N and 6J mice showed significant increases in ABR thresholds with age (6N-Repaired, P = 0.0082, Fig. 1B and 6N: P < 0.0001, Fig. 1C, one way-ANOVA, for post-test see Fig. 1 legend; 6J: P = 0.0003, Fig. 1D, t-test). Strain comparisons showed that ABR click thresholds over the entire age-range investigated (Fig. 1A-C) were comparable between C3H and 6N-Repaired mice (P = 0.8091, Tukey’s post-test, two-way ANOVA), but significantly elevated in the 6N strain (compared to both C3H and 6N-Repaired: P < 0.0001). ABR thresholds in 6J mice were also significantly elevated compared to both C3H and 6N-Repaired strains (P < 0.0001 for both comparisons), but not to 6N mice (P = 0.3546, Tukey’s post-test, two-way ANOVA).
Figure 1. ABR thresholds evoked in ageing C3H, 6N, 6N-Repaired and 6J mice.
A-D, Average ABR thresholds for click stimuli recorded from male C3H (A), 6N-Repaired (B), 6N (C) and 6J (D) mice of increasing age. The number of mice tested for each age/strain is shown above or below the symbols. The dashed lines represent the upper threshold limit of our system (95 dB). Values are mean ± S.D. The age range of the animal tested is shown on the x-axis. One-way ANOVA, Tukey’s post-test: (A) 1-3 vs 6-9 months P = 0.2114; 1-3 vs 15-18 months P = 0.8954; 6-9 vs 15-18 months P = 0.2909; (B) 1-3 vs 6-9 months P = 0.9804; 1-3 vs 15-18 months P = 0.0113; 6-9 vs 15-18 months P = 0.0222; (C) 1-3 vs 6-9 months P = 0.5318; for both 1-3 vs 15-18 and 6-9 vs 15-18 months P < 0.0001. Unpaired t-test (D) P = 0.0003. E-L, ABR thresholds for frequency-specific pure tone stimulation from 6 kHz to 42 kHz recorded from males C3H (E, F), 6N-Repaired (G, H), 6N (I, J) and 6J (K, L) mice at different ages. ABRs data have been grouped in four different age ranges: 1-3 months, 6-9 months and 15-18 months. Because of the several “out of range” (i.e. above the upper threshold limit of our system, 95dB) ABR threshold values at older ages in some strains, tone burst stimuli were plotted as median values (black symbols) and both the first (red lines) and third (blue lines) quartile ranges (panels E, G, I, K). Single data recordings are reported as open circles behind the median and quartiles. Right panels (F, H, J, L) show the direct comparison of the median values only at different ages for the respective mouse strains. The number of mice tested for each age/strain is shown above or below the symbols. The grey numbers above the dashed line represents the number of mice with “out of range” values. Statistical analysis for strain comparisons over the full range of frequencies tested at 14-18 months: P = 0.9607 for C3H vs 6N-Repaired; P < 0.0001 for C3H vs either 6N or 6J; P < 0.0001 for 6N-Repaired vs either 6N or 6J; P = 0.8700 for 6N vs 6J, Tukey’s post-test, aligned ranks transformation 2-way ANOVA. At the 12 kHz region and 14-18 months, statistical analysis for strain comparisons was significantly different between C3H and both 6N (P = 0.0050) and 6J (P = 0.0157); not significantly different for all other interactions: C3H vs 6N-Repaired (P = 0.1177), 6N-Repaired vs 6N (P = 0.0914) and 6J (P = 0.0925); 6N vs 6J (P = 0.6532); aligned ranks transformation 2-way ANOVA with Nonparametric Mann-Whitney U-test with Bonferroni adjustment.
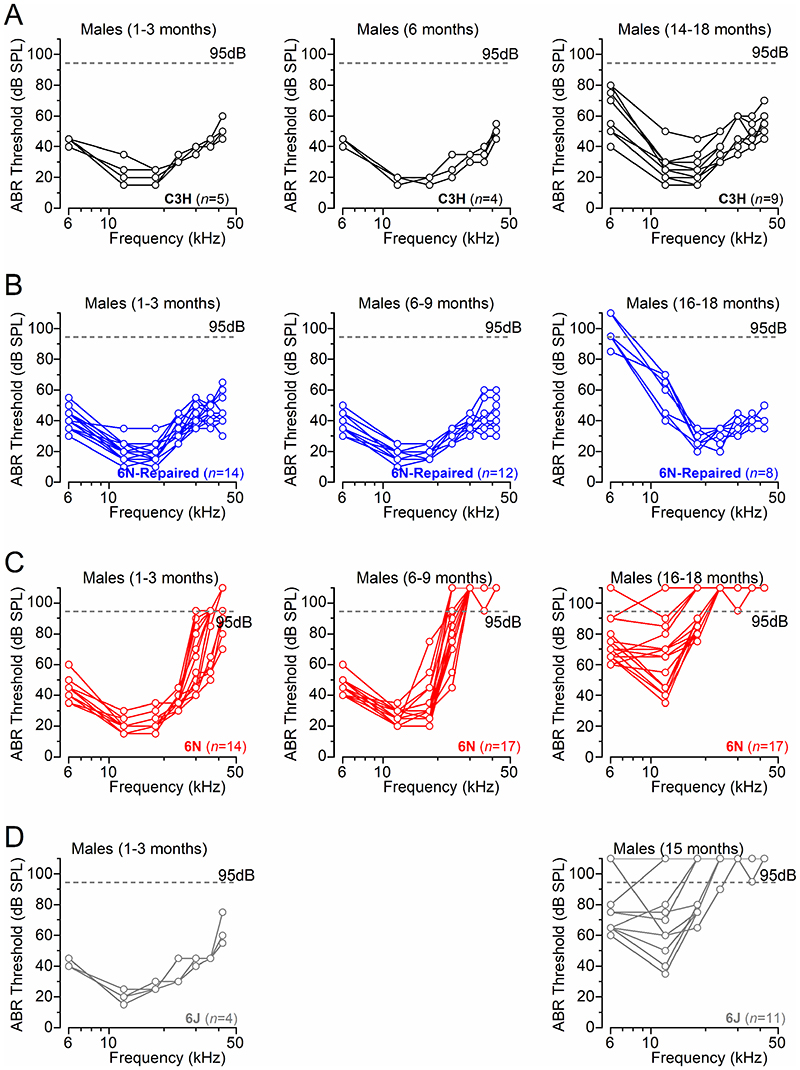
ABR thresholds for pure-tones (6, 12, 18, 24, 36, 42 kHz) were plotted for C3H (Fig. 1E,F, Fig. 2A), 6N-Repaired (Fig. 1G,H, Fig. 2B), 6N (Fig. 1I,J, Fig. 2C) and 6J (Fig. 1K,L, Fig. 2D) male mice. With age and over the 6-42 kHz range, ABR thresholds for pure-tones were significantly increased in 6N-Repaired, 6N and 6J (P < 0.0001, for all comparisons), but not in C3H mice (P = 0.4215, aligned ranks transformation 2-way ANOVA). Strain comparison from aged mice (14-18 months) showed a highly significant difference in ABR thresholds for pure-tones (6-42 kHz range: P < 0.0001, aligned ranks transformation two-way ANOVA). 6N and 6J mice showed significantly elevated thresholds compared to both C3H and 6N-Repaired strains (post-test: see Fig. 1 legend). However, 6N-Repaired mice (Fig. 1G,H) showed comparable ABR thresholds with C3H mice for frequencies above >18 kHz (Fig. 1E,F), but were more similar to the isogenic 6N at lower frequencies (<18 kHz: Fig.1F, G). At the 12 kHz region and 14-18 months, which was in the frequency range used to perform the single cell electrophysiological recordings (see below), 6N-Repaired exhibited an intermediate phenotype since the ABR threshold value was not significantly different from either the good hearing C3H mice nor from the early-onset hearing loss strains (6N and 6J) (statistical results: see Fig. 1 legend).
Figure 2. ABR thresholds evoked from individual ageing C3H, 6N, 6N-Repaired and 6J mice.
A-D, ABR threshold recordings for frequency-specific pure tone stimulation from 6 kHz to 42 kHz recorded from each individual male C3H (A), 6N-Repaired (B), 6N (C) and 6J (D) mice using the same age ranges and data shown in Fig. 1. The dashed lines represent the upper intensity limit of our system, 95dB. Data points above this line indicate recordings that didn’t show any auditory response at 95 dB.
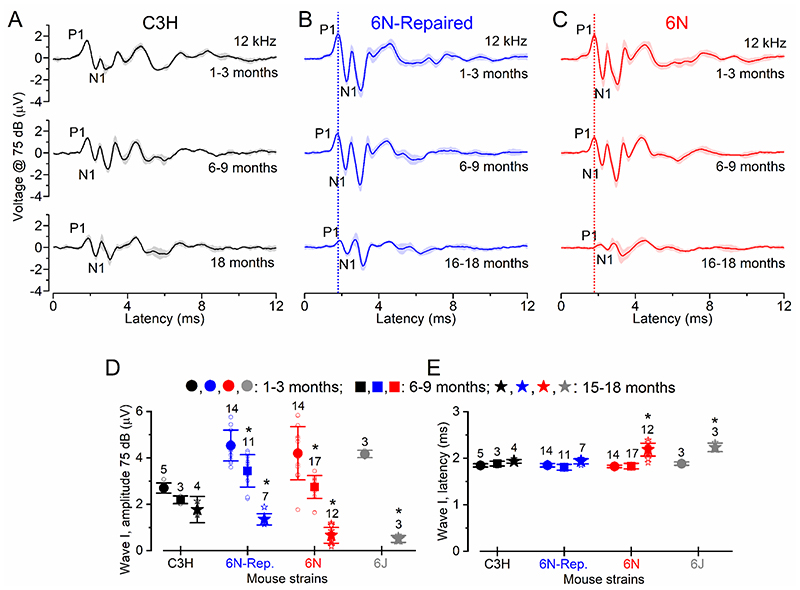
In order to provide some correlation between the ABR data and any possible morphological and/or physiological change identified in the in vitro work (see below), we analysed the amplitude and latency of the ABR wave I, which is generated by the summed response to sound of the afferent nerve fibres innervating the IHCs (Møller & Jannetta, 1982; Schaette & McAlpine, 2011). ABR wave I analysis was investigated at 12 kHz and at a value (75 dB) where signals could be recorded in all mice, even in those with high ABR thresholds (Fig. 3A-E). We found that wave I amplitude was significantly reduced in 6N, 6N-Repaired and 6J male mice between 1-3 months and either 6-9 months or 15-18 months, but not in C3H mice (for statistical analysis see: Fig. 3D). Moreover, wave I latency increased with age only in 6N and 6J mice (Fig. 3E). We also found that in 6N mice, wave I amplitude was significantly reduced (P = 0.0017, Tukey’s post-test, two-way ANOVA, overall strain effect) and latency increased (P < 0.0001) compared to 6N-Repaired. C3H mice had a similar wave I latency to 6N-Repaired (P = 0.5557), but a significantly reduced amplitude (P = 0.0007).
Figure 3. Changes in amplitude and latency of ABR wave in ageing mice.
A-C, Average ABR waveform responses at 12 kHz at 75 dB stimulus intensity in C3H (A), 6N-Repaired (B) and 6N (C) mice as a function of age. Continuous lines represent the average values and the shaded areas the S.D. P1 and N1 indicate the positive and negative peaks of wave I. D, Average amplitude of wave I (from P1 to N1) as a function of age in the four mouse strains. In 6N, 6N-Repaired and 6J mice wave I amplitude was significantly reduced between 1-3 months and both 6-9 and 15-18 months (P < 0.001 for both comparisons: Tukey’s post-test, one-way ANOVA). In C3H mice, wave I amplitude was P > 0.05 for all interactions. E, Average latency of wave I (time between the onset of the stimulus and P1) as a function of age in the four mouse strains. In C3H and 6N-Repaired mice, there was no significant changes in the wave I latency between 1-3 months and either 6-9 or 15-18 months (P > 0.05 for all interactions). For 6N, wave I latency did not significantly change between 1-3 months and 6-9 months of age (P > 0.05), but increased between 6-9 and 15-18 months (P < 0.001) and 1-3 and 15-18 months (P < 0.001). In 6J, it also increased between 1-3 and 15-18 months (P < 0.001). Number of mice tested is shown above the data and single data points are plotted as small open symbols.
The above results have shown a well-defined hearing phenotype among the four mouse strains kept under the same environmental conditions. The level of hearing loss was found to be most severe for C57BL/6NTac and C57BL/6J, less so for C57BL/6NTacCdh23+ and absent in C3H/HeJ, which like CBA mice show a very slow decline in their hearing thresholds with age (Spongr et al. 1997; Sha et al. 2008; Ohlemiller et al. 2010). Although the 6N-Repaired strain has an overall better hearing sensitivity than the isogenic 6N mice (Figs. 1, 2), the correction of the hypomorphic allele Cdh23ahl (Mianné et al. 2016) had only a significant impact on frequencies at or above 12 kHz (Fig. 1). This indicate that progressive low-frequency hearing loss in C57BL/6NTacCdh23+ (6N-Repaired) mice is not related to Cdh23ah, but is instead likely due to a different strain-specific allele(s) present in C57BL/6NTac mice.
Exocytosis at IHC ribbon synapses is stable during ageing
We then investigated whether the different ABR thresholds and wave I among the different mouse strains were due to defects in exocytosis at IHC ribbon synapses with age. Sound-induced stimulation of the IHC stereociliary bundle, generates a receptor potential that drives Ca2+-dependent neurotransmitter release at their ribbon synapses. Synaptic vesicle exocytosis was measured as an increase in cell membrane capacitance (ΔC m) that is interpreted as a sign of neurotransmitter release from presynaptic cells (e.g. Moser & Beutner, 2000; Johnson et al. 2005). Exocytosis at IHC ribbon synapses was investigated using whole-cell patch-clamp recordings from young adults (1 month) and aged (15-19 months) mice from the 9-12 kHz cochlear region.
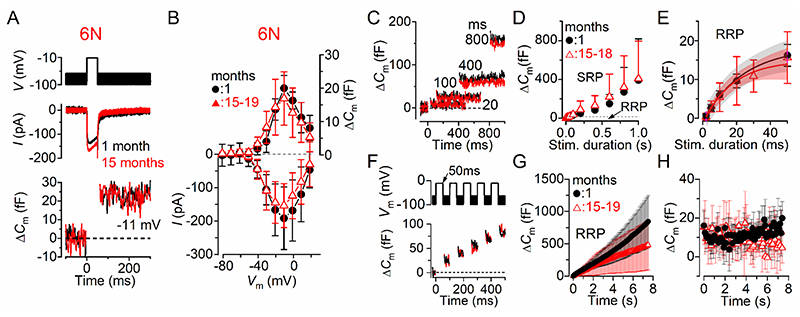
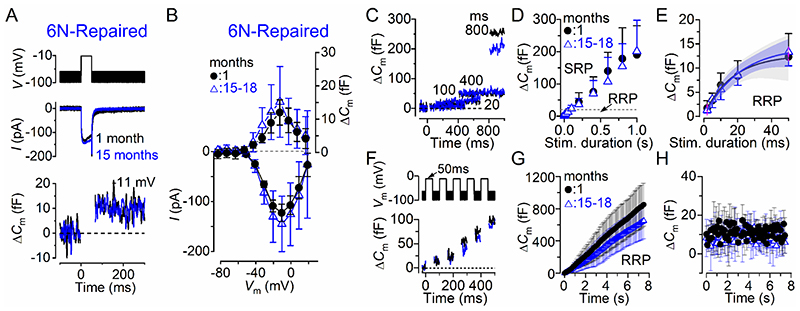
We first investigated exocytosis from IHCs of male and female 6N mice. Fig. 4A,B shows the maximal Ca2+ current (I Ca) and the corresponding ΔC m recorded from IHCs of 1 month and 15-19 months 6N mice using a 50 ms depolarizing voltage step (10 mV nominal increments) from –81 mV. The maximal size of ICa recorded in young-adult IHCs (–191 ± 82 pA, n = 6) was not significantly different compared to that recorded at 15-19 months (–154 ± 66 pA, n = 13, P = 0.2950, t-test). The induced ΔC m was also not significantly different with age (1 month: 20.0 ± 4.5 fF, n = 6; 15-19 months: 16.9 ± 8.0 fF, n = 13; P = 0.3940, t-test). The rate of synaptic vesicle release in IHCs was studied by measuring ΔC m in response to depolarizing voltage steps to –11 mV from –81 mV of varying duration from 2 ms to 1 s (interstep interval was at least 11 s), which allowed us to investigate the emptying of different synaptic vesicle pool populations (Fig. 4C,D). When performing experiments using physiological 1.3 mM extracellular Ca2+ and at body temperature, stimuli up to about 50 ms reveal the number of vesicles docked at the active zones (readily releasable pool: RRP) (Johnson et al. 2010; Johnson et al. 2017). Longer steps induce the release of vesicles from a secondarily releasable pool (SRP) that is located further away from the Ca2+ channels (Moser & Beutner, 2000; 2010; 2017). Voltage steps of up to about 50 ms (RRP) produced an increase in ΔC m that could be approximated with a single exponential (Fig. 4E) under our experimental conditions. The initial release rate of the isolated RRP in 1 month old IHCs (1067 ± 218 fF/s or 28838 ± 5892 vesicles/s, n = 7) was not significantly different from those measured in 15-18 month old cells (894 ± 661 fF/s or 24167 ± 17852 vesicles/s, n = 11, P = 0.5166, t-test). The size of the SRP was also not significantly different between IHCs recorded from young adults and aged mice (Fig. 4D, P = 0.9410, Bonferroni’s post-test, two-way ANOVA).
Figure 4. Size and kinetics of exocytosis in IHCs from ageing 6N mice.
A, ICa and ΔCm from IHCs of 1 month and 15 months old 6N mice. Recordings were obtained in response to 50 ms voltage steps from –81 mV in 10 mV increments. For clarity, only the peak responses at –11 mV are shown. B, Average peak Ca2+ current-voltage (I-Vm, bottom panel) and capacitance-voltage (ΔC m-Vm, top panel) curves from IHCs of 1 month (n = 6) and aged mice (15-19 months: n = 13). C, ΔC m recordings from IHCs at 1 month (black) and 18 months old (red) mice in response to voltage steps of different duration, which are used to recruit the readily releasable pool (RRP: <50 ms steps) and secondary releasable pool (SRP: >100 ms) of vesicles. D, Average ΔCm from 1 month (n = 6) and 15-18 months old (n = 11) IHCs in response to voltage steps to –11 mV between 2 ms and 1 s (holding potential –81 mV) showing the RRP and SRP. E, Size of the RRP (expanded from panel D) approximated with single exponential functions. Exponential fits from single IHC recordings were: 1 month ΔCm = 15.7 ± 3.3 fF, τ = 15.0 ± 4.0 ms, n = 7; 15-18 months: ΔCm = 16.2 ± 6.4 fF, τ = 22.1 ± 8.0 ms, n = 11 (P = 0.8488, P = 0.0466, respectively, t-test). The available RRP consisted of 423 ± 34 vesicles (1 month) and 437 ± 53 vesicles (15-18 months). F, ΔCm elicited using repetitive voltage steps to –11 mV of 50 ms in duration in order to elicit the RRP (inter-step-interval was 50 ms). For clarity, only the first few steps are shown. The voltage protocol used is shown above the traces. G, Average cumulative ΔCm values obtained in response to the 50 ms (75 steps) protocol, respectively, from 1 month (n = 5) and eight 15-18 months old (n = 8) IHCs. H, Individual ΔCm values in 1 month and 15-18 months old IHCs measured following each voltage step from G. In this and Figs. 5, 6, data in panels B,D,E,G and H are reported as mean ± S.D.; panel E also shows the 95% confidence interval for the exponential fit curve.
A rate-limiting step in neurotransmitter release is the vesicle pool depletion/replenishment during prolonged repetitive stimulation. Therefore, we investigated whether vesicle release was limited by the relative pool refilling rates using a train of 50 ms steps to –11 mV (Fig. 4F). The RRP appears to be able to fully replenish following each step in both young adults and aged mice (Fig. 4G,H). The individual ΔC m values were comparable in aged IHCs (9.44 ± 3.46 fF/s, mean ± SD, n = 8) and 1 month old cells (11.27 ± 3.02 fF/s, n = 5, P = 0.3533, t-test) (Fig. 3H).
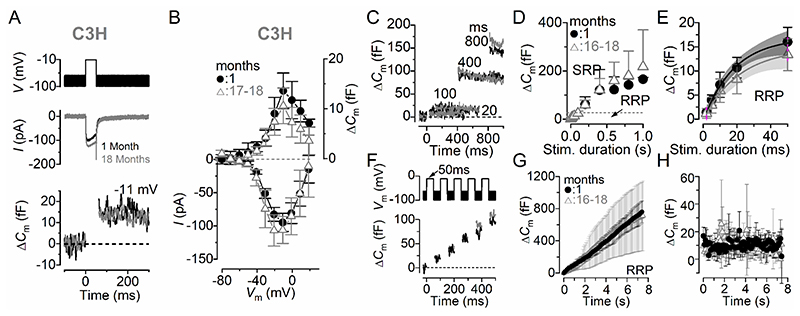
Similar results to those observed in IHCs from 6N strain, were also recorded in 6N-Repaired (Fig. 5) and C3H (Fig. 6) male and female mice. These results indicate that the size, kinetic and replenishment of the synaptic vesicles in IHCs appear unaffected by any pathophysiological change occurring in the ageing cochlea.
Figure 5. Size and kinetics of exocytosis in IHCs from ageing 6N-Repaired mice.
A, ICa and ΔCm from IHCs of 1 month and 15 months old mice. Recordings were obtained as described in Fig. 4. B, Average peak Ca2+ current and ΔC m curves from IHCs of 1 month (n = 7) and aged mice (15-18 months: n = 11). The maximal size of I Ca recorded in young-adult IHCs (–123 ± 17 pA, n = 7) was not significantly different compared to that recorded at 15-18 months (–145 ± 55 pA, n = 11, P = 0.3216, t-test). The induced ΔC m was also not significantly reduced with age (1 month: 12.0 ± 3.8 fF, 15-18 months: 15.2 ± 8.3 fF, P = 0.3555, t-test). C-E, Rate of neurotransmitter release from six 1 month and six 15-18 months old IHCs in response to voltage steps from 2 ms to 1 s (to –11 mV) showing the RRP and SRP (see main text). The size of the SRP was also not significantly different between IHCs recorded from young adults and aged mice (P = 0.9563, Bonferroni’s post-test, two-way ANOVA). The size of the RRP in panel E (expanded from panel D) is approximated with single exponential functions. Exponential fits from single IHC recordings were: 1 month ΔCm = 12.1 ± 5.3 fF, τ = 13.6 ± 6.5 ms, n = 6; 15-18 months: ΔCm = 14.4 ± 3.1 fF, τ = 20.9 ± 7.0 ms, n = 6 (P = 0.3979, P = 0.0910, respectively, t-test). The available RRP consisted of 328 ± 142 vesicles, mean ± SD (1 month) and 388 ± 84 vesicles (15-18 months) (P = 0.3979). The initial release rate of the isolated RRP in 1 month old IHCs (927 ± 182 fF/s or 25057 ± 4912 vesicles/s, n = 6) was not significantly different from those measured in 15-18 month old cells (741 ± 259 fF/s or 20036 ± 6997 vesicles/s, n = 6, P = 0.1807, t-test). F, ΔCm elicited using repetitive voltage steps to –11 mV of 50 ms in duration in order to elicit the RRP (inter-step-interval was 50 ms). G, Average cumulative ΔCm values obtained in response to the 50 ms (75 steps) protocol, respectively, from four 1 month and eight 15-18 months old IHCs. H, Individual ΔC m values in 1 month and eight 15-18 months old IHCs measured following each voltage step from G. The individual ΔCm revealed that RRP refilling rate in aged IHCs was not significantly reduced (8.64 ± 2.56 fF/s, n = 8) compared to 1 month old cells (11.36 ± 3.25 fF/s, n = 4, P = 0.1419, t-test).
Figure 6. Size and kinetics of exocytosis in IHCs from ageing C3H mice.
A, ICa and ΔCm from IHCs of 1 month and 18 months old mice. Recordings were obtained as described in Fig. 4. B, Average peak Ca2+ current and ΔC m curves from IHCs of 1 month (n = 5) and aged mice (16-18 months: n = 5). The maximal size of I Ca recorded in young-adult IHCs (–95 ± 14 pA, n = 5) was not significantly different compared to that recorded at 16-18 months (–106 ± 20 pA, n = 5, P = 0.3282, t-test). The induced ΔC m was also not significantly reduced with age (1 month: 13.6 ± 3.6 fF, 16-18 months: 10.6 ± 3.6 fF, P = 0.2187, t-test). C-E, Rate of neurotransmitter release from five 1 month and six 16-18 months old IHCs in response to voltage steps from 2 ms to 1 s (to –11 mV) showing the RRP and SRP (see main text). The size of the SRP was also not significantly different between IHCs recorded from young adults and aged mice (P = 0.8126, Bonferroni’s post-test, two-way ANOVA). The size of the RRP in panel E (expanded from panel D) is approximated with single exponential functions. Exponential fits from single IHC recordings were: 1 month ΔCm = 18.4 ± 4.8 fF, τ = 23.2 ± 9.3 ms, n = 5; 16-18 months: ΔCm = 15.3 ± 3.1 fF, τ = 24.6 ± 5.2 ms, n = 6 (P = 0.2150, P = 0.7368, respectively, t-test). The available RRP consisted of 498 ± 129 vesicles, (1 month) and 412 ± 83 vesicles (16-18 months) (P = 0.2150). The initial release rate of the isolated RRP in 1 month old IHCs (885 ± 354 fF/s or 23925 ± 9594 vesicles/s, n = 6) was not significantly different from those measured in 15-18 month old cells (660 ± 270 fF/s or 17826 ± 7298 vesicles/s, n = 6, P = 0.2609, t-test). F, ΔCm elicited using repetitive voltage steps to –11 mV of 50 ms in duration in order to elicit the RRP (inter-step-interval was 50 ms). G, Average cumulative ΔCm values obtained in response to the 50 ms (75 steps) protocol, respectively, from four 1 month and eight 16-18 months old IHCs. H, Individual ΔCm values in 1 month and eight 16-18 months old IHCs measured following each voltage step from G. The RRP refilling rate in aged IHCs was not significantly reduced (9.5 ± 4.3 fF/s, n = 5) compared to 1 month old cells (10.2 ± 3.1 fF/s, n = 5, P = 0.2995).
Change in ribbon synapses number and volume in ageing IHCs
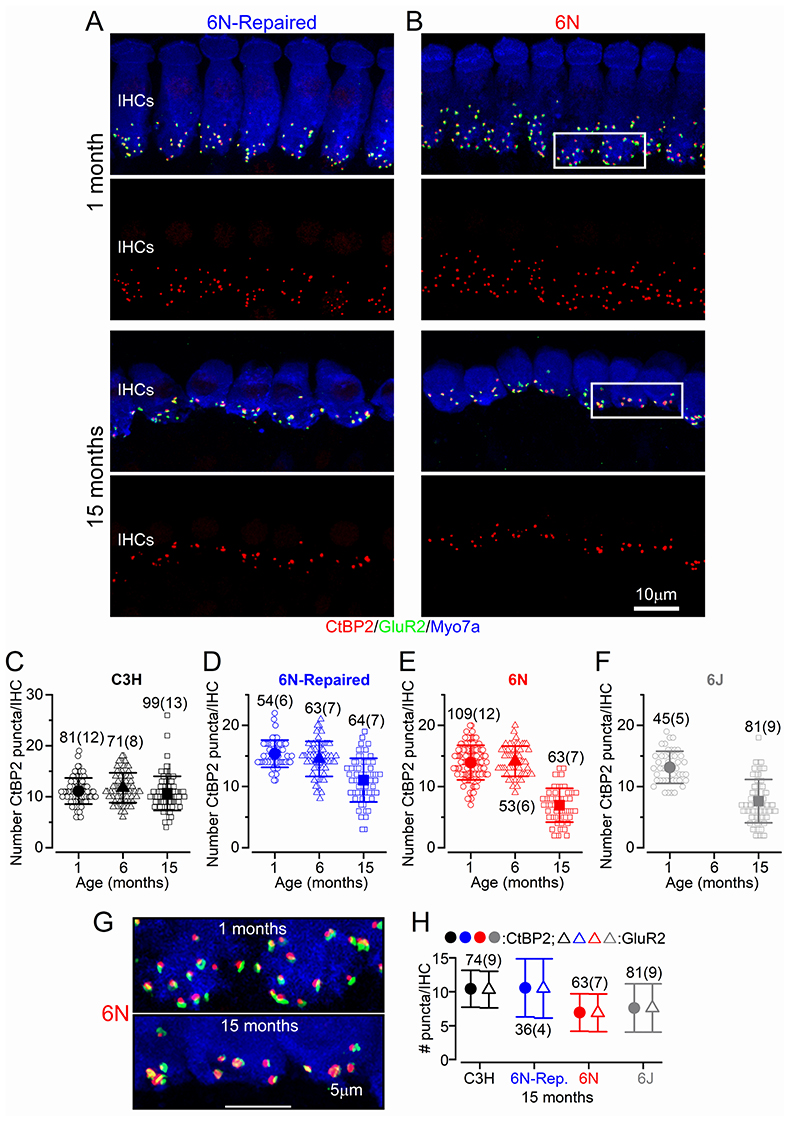
We then evaluated the number of afferent synapses in IHCs from all four mouse strains using antibodies to label the presynaptic ribbon protein RIBEYE (CtBP2) and the postsynaptic AMPA-type glutamate receptor GluR2 (e.g. Liberman et al. 2011; Furman et al. 2013). We found that both CtBP2 and GluR2 puncta were present at the IHC pre- and post-synaptic sites, respectively, in the 9-12 kHz cochlear region in all four aged mouse strains from both sexes (see Fig. 7A,B for 6N and 6N-Repaired mice). While the number of CtBP2 puncta in IHCs of C3H mice did not change significantly with age (P > 0.05 for all interactions, Tukey’s post-test, one-way ANOVA, Fig. 7C), it was significantly reduced in the other 3 strains between 1 and 15 months (6N, 6N-Repaired, 6J: P < 0.001: Fig. 7D-F). No significant changes in CtBP2 puncta were detected between 1 and 6 months old IHCs in both 6N and 6N-Repaired (P > 0.05). Strain comparison showed that the number of ribbons in IHCs from 6N-Repaired mice was significantly different compared to either C3H or 6N mice (P < 0.0001, two-way ANOVA), further supporting the in vivo finding (Figs. 1-3) showing an “intermediate severity” in the phenotype of this strain. We also found that the CtBP2 puncta were always closely colocalized with GluR2 labelling in IHCs from all four strains (Fig. 7G, H).
Figure 7. Ribbon synapse number is reduced in IHCs from most mouse strains.
A,B, Maximum intensity projections of confocal z-stacks of IHCs taken from the apical cochlear region (9-12 kHz) of 6N-Repaired (A) and 6N (B) mice at 1 and 15 months using antibodies against CtBP2 (ribbon synaptic marker: red) and GluR2 (postsynaptic marker: green). Myosin 7a (Myo7a) was used as the IHC marker (blue). Scale bar 10 μm. C-F, Number of CtBP2 puncta as a function of age in IHCs from C3H (C), 6N-Repaired (D), 6N (E) and 6J (F) mice. Data are plotted as mean values (larger closed symbols) and individual CtBP2 counts (smaller open symbols). G, Enlarged view of the two regions highlighted (rectangular white boxes) in panel B, showing the juxtaposed CtBP2 (red) and GluR2 (green) puncta. H, Number of CtBP2 and GluR2 puncta in IHCs from 15 month old mice (C3H, 6N-Repaired, 6N and 6J). Numbers above or below the data in panels C-F and H represent the IHCs (and mice) used for each time point. Average values are mean ± S.D.
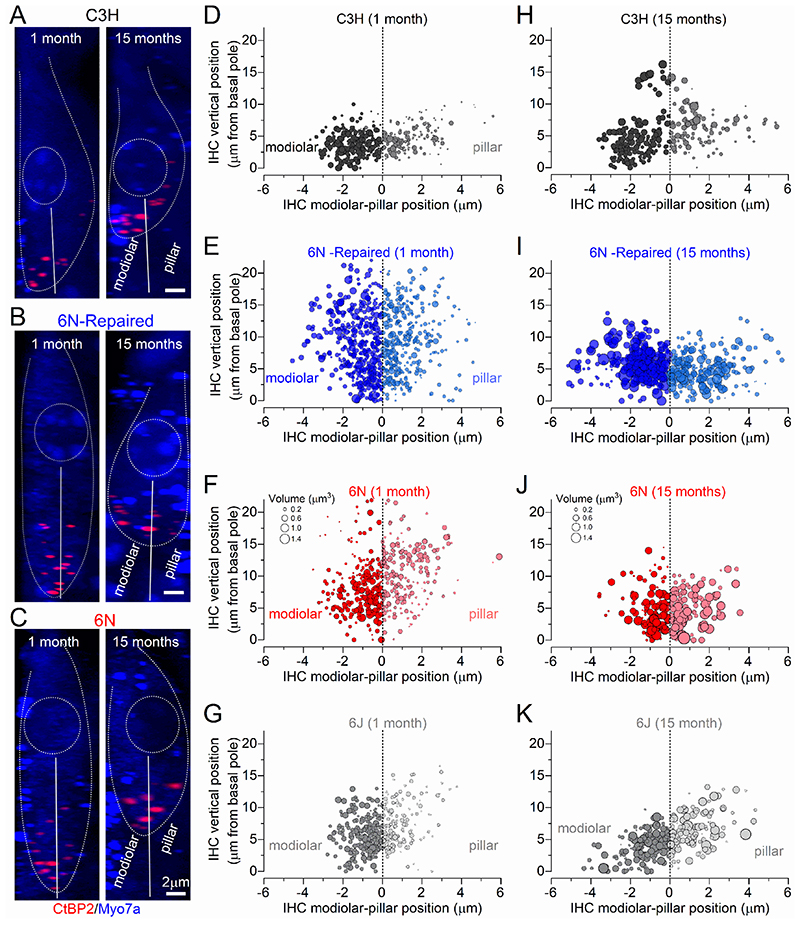
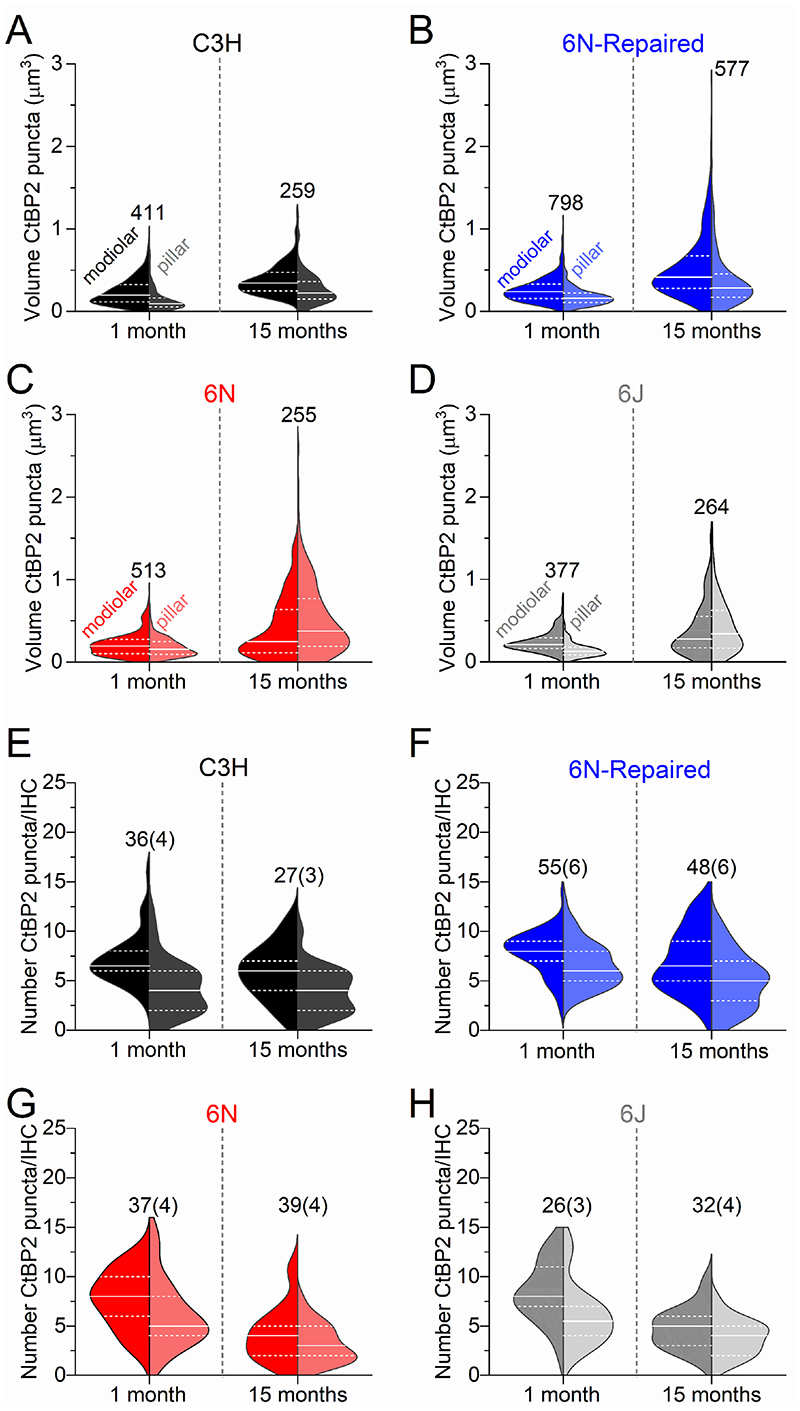
We then investigated possible changes in the distribution and volume of ribbon synapses in young adult and aged IHCs from mice of both sexes (Fig. 8A-K and Fig. 9A-H). Afferent fibres have been shown to be spatially segregated around the IHC basolateral membrane of cats, such that low-spontaneous rate fibres, which tend to have larger presynaptic ribbons, are more likely to contact their modiolar side, and high-spontaneous rate fibres tend to contact the pillar side (Liberman, 1982a; Liberman et al. 1990; Merchan-Perez & Liberman, 1996). The size of the ribbons was estimated by measuring their volume using the CtBP2 immunolabeling and super-resolution microscopy (Liberman et al. 2011; Furman et al. 2013). At 1 month, we found that IHCs from all mouse strains have a significantly larger ribbon volume on the modiolar compared to the pillar side (6N: P = 0.0296, 6J, 6N-Repaired and C3H: P <0.0001, Sidak’s post-test, one-way ANOVA, Figs. 8D-G and Fig. 9A-D). With age, we observed an increased ribbon volume in both the modiolar and pillar side in all strains, although it was much more pronounced in the 6J, 6N, 6N-repaired (Fig. 8I-K and Fig. 9A-C) than in C3H mice (Fig. 8H and Fig. 9A). A previous serial-section ultrastructure study has shown that aged IHCs from 6J mice tend to exhibit synaptic active zones with multiple and larger ribbons compared to young adults (Stamataki et al. 2006). Although it is difficult to distinguish multiple ribbons from larger ribbons with immunostaining and fluorescence microscopy (Figs. 7-9; see also Furman et al. 2013), we did not find any preferential localization of the larger puncta in either the modiolar or pillar side of aged IHCs, in agreement with the electron microscopy study (Stamataki et al. 2006). We then investigated whether the reduction in ribbon number in ageing IHCs from all strains (Fig. 7C-F) had a preferential modiolar-pillar distribution. Using the same ribbon dataset used for volume estimation (Fig. 9A-D), which was a subset of those used in Fig. 7C-F, we found that ribbon number decreased with age on both the modiolar and pillar sides of IHCs from both 6N (P <0.0001, Sidak’s post-test, two-way ANOVA) and 6J (modiolar P < 0.0001; pillar: P = 0.0055). In IHCs from the 6N-repaired strain, ribbon number was significantly changed with age on the pillar (P = 0.0074) but not on the modiolar side (P = 0.4446). In C3H mice there was no significant change of ribbon number in both regions (modiolar: P = 0.1798; pillar: P = 0.9366) (Fig. 9E-H).
Figure 8. Changes in the volume and number of synaptic-ribbons with age.
A-C, Confocal z-stacks of IHCs taken from the apical cochlear region (9-12 kHz) of C3H (A), 6N-Repaired (B) and 6N (C) mice at 1 and 15 months using antibodies against CtBP2. Myosin 7a (Myo7a) was used as the IHC marker (blue). Dotted lines delineate the IHC surface and the nucleus. The continous lines divide the modiolar and pillar side of the IHCs. Scale bar 2 μm. D-K, Bubble plots representing the distribution along the modiolar-to-pillar (horizontal) and cuticular-to-synaptic (vertical) direction of CtBP2 puncta (pooled data from several IHCs: see below). The marker size is proportional to the spot volume (scale volume in panels F,J also applies to all other panels). Data were collected from IHCs at 1 month (D-G) and 15 months (H-K) in C3H, 6N-Repaired, 6N and 6J mice. For number of IHCs and mice tested see Fig. 7.
Figure 9. IHC modiolar-pillar gradient in the volume and number of synaptic-ribbons.
A-D, Violin plots comparing the modiolar-pillar distribution of ribbon volume in IHCs from 1 month (left plots) and 15 months (right plots) in C3H (A), 6N-Repaired (B), 6N (C) and 6J (D) mice (see Fig. 7). Both modiolar and pillar side show an increased CtBP2 volume in all mouse strains (P < 0.0001 for all interactions apart between 1 and 15 months; in the pillar side of C3H mice significance was P = 0.0003, Sidak’s post-test, two-way ANOVA). E-H, Violin plot comparing the modiolar-pillar distribution of ribbon number in IHCs from 1 month (left plots) and 15 months (right plots) in C3H (E), 6N-Repaired (F), 6N (G) and 6J (H) mice. The number of ribbons measured are shown above or next to the violin plots in panels A-D, while the number of IHCs (and mice) used is shown above the violin plots in panels E-H.
Discussion
We have investigated age-related changes in the biophysical and morphological properties of IHC ribbon synapses in the 9-12 kHz cochlear region of four commonly used mouse strains with different progression of hearing loss (C57BL/6J, C57BL/6NTac, C57BL/6NTacCdh23+ and C3H/HeJ). We found that with age, both modiolar and pillar sides of the IHC exhibited a loss of ribbon synapses but an increase in volume of the remaining ribbons. These changes correlate with the degree of hearing loss in the different mouse strains, being most severe for 6N and 6J, less so for 6N-Repaired and absent in C3H strains. Despite the changes in synapse morphology, we provided evidence that the size and kinetics of exocytosis, as well as the replenishment of synaptic vesicles, were not affected with age, indicating that a degree of compensation exists at ribbon synapses that could contribute to maintaining some degree of functionality in aged IHCs.
Age-related morphological changes at IHC ribbon synapses
Type I fibres constitute the majority of spiral ganglion neurons (SGNs) that innervate the mammalian cochlea (∼95%: Ryugo 1992). Each unbranched peripheral axon of these SGNs (Liberman, 1980) receives input from one IHC synapse, which normally containing only one ribbon, although some 20% of synapses appear to have multi ribbon contacts (Merchan-Perez et al. 1996; Michanski et al. 2019). Each IHC can drive the activity of up to ∼20 SGNs depending on their frequency location along the mouse cochlea (Meyer et al. 2009). We found that in 15 month old IHCs, the number of ribbon synapses was lower in 6N and 6J strains, which suffered the worst hearing loss, than in 6N-repaired strain that had only partial hearing loss in the 9-12 kHz region. Although this is consistent with previous immunostaining observations in CBA/CaJ (Sergeyenko et al. 2013) and electron microscopy studies in 6J mice (Stamataki et al. 2006), we showed that it only becomes evident after 6 months of age. Interestingly, IHCs from C3H mice did not appear to lose synaptic ribbons with age but they had significantly fewer at young ages than IHCs from 6J, 6N and 6N-Repaired strains. The smaller number of synapses in C3H mice is likely to be compensated, at least in part, by having about 20% more IHCs in the 9-12 kHz region throughout life compared to the other three strains (Jeng et al. 2020c). Nevertheless, a lower number of ribbons meant that C3H mice showed a significantly smaller wave I ABR amplitude at young ages compared to the other strains. Consistent with the correlation between synapse number and ABR response, the wave I amplitude was significantly reduced with age in 6N-Repaired and even more so in the 6J and 6N mice.
Cochlear ageing has been associated with loss of small afferent terminals in 6J mice (Stamataki et al. 2006), which in the cat have been described as the low spontaneous rate fibres (low-SR: Liberman, 1982a). This has also been seen in the ageing gerbil cochlea (Schmiedt et al. 1996). Since low-SR fibres seem to contact the modiolar side of IHCs in cats and guinea-pigs (Liberman, 1982b; Tsuji & Liberman, 1997), the expectation was that ageing would cause a significantly larger reduction in synaptic counts in this region. However, our data show a reduction in CtBP2 puncta in both the modiolar and pillar regions, with a more prominent decline in the pillar side. It is possible that the spatial distribution of low-SR (modiolar) and high-SR (pillar) afferent fibres around mouse IHCs is less segregated than in the cat. Some support for this hypothesis comes from a recent study showing that the intrinsic voltage-dependence characteristics of the presynaptic Ca2+ channels, which could be a key determinant of the different spontaneous and sound-evoked firing rate among SGNs, is highly variable in both modiolar and pillar sides of mouse cochlear IHCs (Ohn et al. 2016). Moreover, considerable variability in ribbon morphology was also reported within individual IHCs (Michanski et al. 2019). Finally, noise-induced neuropathy, which has been shown to selectively affect the low-SR fibres, caused an increase volume of the synaptic ribbons on both sides of the IHCs, although to a greater extent on the pillar side (Furman et al. 2013). Although the ribbon volume is normally greater on the modiolar side of young adult IHCs (Figs. 8, 9; for CBA/CaJ mice, see: Liberman et al. 2011), we found that with age the remaining ribbons on both IHC sides increased in volume. This finding further supports the idea that the loss of afferent fibres with age in mice is unlikely to be restricted to the modiolar side of IHCs. Alternatively, with age, ribbons may redistribute “equally” around the IHC basal poles after the loss of low-SR fibres as previously suggested for noise-induced cochlear neuropathy (Furman et al. 2013). However, this seems unlikely since in IHCs of C3H mice the ribbons show some degree of volume increase on both the modiolar and pillar sides in aged IHCs, even though they show no synaptic loss.
Functional changes at IHC ribbon synapses with age
Our estimates for the size and kinetics of neurotransmitter release and vesicle replenishment at ribbon synapses are remarkably similar between young (1 month) and old (15-19 months) IHCs from C3H and the other three strains showing a different progression of hearing loss. Considering the large age-related reduction in the number of ribbon synapses in 6J, 6N and 6N-Repaired, the apparently normal vesicle exocytosis indicates that ribbon synapses undergo major morphological re-organization. Compared to young adult IHCs, the bigger or multiple synaptic ribbons opposite to the postsynaptic densities in aged IHCs been shown to hold a significantly larger number of synaptic vesicles, docked at the active zone and also those associated with the more distal pools (Stamataki et al. 2006). Afferent terminals onto IHCs are also significantly larger in aged mice, so despite the lower number of SGN terminals, the overall synaptic area between IHCs and afferent terminals does not change significantly with age (Stamataki et al. 2006). The above morphological changes are also likely to be associated with a redistribution of the Ca2+ channels at the synaptic region (more channels per synapses). This is suggested by the similar kinetics of IHC exocytosis between mice that lose afferent synapses with age (6N and 6N-repaired mice) and those that did not (C3H mice). The significance of such morphological reorganization in aged IHCs is currently unclear but it closely resembles that seen during pre-hearing stages of development (Michanski et al. 2019), possibly as a mechanism that compensates for the loss of SNGs. A similar mechanism in which IHCs apparently recapitulate development has been seen in the aged cochlea, with the efferent system re-forming direct axo-somatic contacts with IHCs (Lauer et al. 2012; Zachary et al. 2015; Jeng et al. 2020c). This process seems to be linked with a malfunction of the mechanoelectrical transducer (MET) channels (Corns et al. 2018).
Mechanisms leading to the progressive loss of ABR thresholds with age
We have demonstrated that the synaptic machinery of IHCs in mice undergoes several changes in the ageing cochlea (12 kHz cochlear region), with smaller numbers of larger synapses correlated with the level of hearing loss in mice. Despite these morphological changes, aged IHCs exhibited normal exocytotic responses, possibly leading to more glutamate-containing vesicles being released by the remaining larger SGN terminals. How these changes impact on the firing activity of the remaining SGNs is difficult to predict. Afferent firing activity depends on a combination of presynapstic (Merchan-Perez et al. 1996; Grant et al. 2010; Ohn et al. 2016), postsynaptic (Liberman et al. 2011) and efferent (Yin et al. 2014) mechanisms, all of which change in the aged cochlea (see above). The observed degree of functional compensation occurring at the IHC ribbon synapses, which could potentially act as a mechanism to preserve at least some hearing via the remaining SGN fibres, is unlikely to counteract the significant loss of SGN connections. Loss of IHC synapses and SGNs would have an impact on the normal encoding of the temporal properties of sound due to stochastic under-sampling (Lopez-Poveda, 2014 ), especially in noisy environments (Costalupes et al. 1984). As such, the proposed loss of low-SR fibres with age (Stamataki et al. 2006; Liberman, 1982a; Schmiedt et al. 1996), which also occurs during noise-exposure (Furman et al. 2013), cannot be the only cause of the wide range of ABR thresholds we observed among the different mouse strains. Other major contributing factors to the different hearing phenotypes among the mouse strains could arise from the differential loss of the electromotile OHCs (e.g. Francis et al. 2003; Sergeyenko et al. 2013), changes in IHC basolateral ion channel biophysics, efferent innervation and in the MET apparatus. Major changes in the number of hair cells in the 9-12 kHz cochlear region are unlikely, since we found comparable results among mouse strains exhibiting very different progressive hearing loss profiles (OHCs: Jeng et al. 2020b; IHCs: Jeng et al. 2020c). On the other hand, the degree of efferent fibres re-reforming axon-somatic connections with aged IHCs, which was demonstrated to occur in C57BL/6J mice (Lauer et al. 2012; Zachary & Fuchs, 2015), was found to be correlated with the degree of hearing loss in the different mouse strains (Jeng et al. 2020b). The contribution of the MET current to the ageing hearing phenotype is supported by the observation that the structural and functional integrity of the stereociliary bundles is progressively altered with age (Bohne et al., 1990; Bullen et al., 2019) and that the hypomorphic Cdh23ahl allele leads to early-onset hearing loss in several inbred mouse strains such as 6J (C57BL/6J) and 6N (C57BL/6NTac) mice (e.g. Johnson et al. 1997; Noben-Trauth et al. 2003). Cdh23 encodes cadherin-23 that, together with protocadherin-15, forms the stereocilia tip links required for gating the mechanoelectrical transducer channels (Kazmierczak et al. 2007). Mice harbouring the Cdh23ahl allele show a reduced MET current in aged hair cells (OHCs: Jeng et al. 2020b; IHCs: Jeng et al. 2020c), but this was not observed in 6N-Repaired mice (Jeng et al. 2020c).
We also found that mice in which the Cdh23ahl allele was repaired with targeted CRISPR/Cas9 gene editing (6N-Repaied: Mianné et al. 2016) exhibited age-related high-frequency hearing thresholds comparable to mouse strains that innately carry the wild-type Cdh23 allele (e.g. C3H). However, both the 6N and 6N-Repaired mice exhibit a comparable progressive low-frequency hearing loss (<18 kHz), which, therefore, is not related to Cdh23, but likely to be due to a different strain-specific allele(s) in C57BL/6NTac mice.
Key Points Summary.
Age-related hearing loss (ARHL) is associated with the loss of IHC ribbon synapses, lower hearing sensitivity and decreased ability to understand speech, especially in a noisy environment.
Little is known about age-related physiological and morphological changes that occur at ribbon synapses.
We show that the differing degrees of ARHL in four selected mouse stains is correlated with the loss of ribbon synapses, being most severe for the strains C57BL/6NTac and C57BL/6J, less so for C57BL/6NTacCdh23-Repaired and lowest in C3H/HeJ.
Despite the loss of ribbon synapses with age, the volume of the remaining ribbons increased and the size and kinetics of Ca2+-dependent exocytosis in IHCs was unaffected, indicating the presence of a previously unknown degree of functional compensation at ribbon synapses.
Although the age-related morphological changes at IHC ribbon synapses contribute to the different progression of ARHL, without the observed functional compensation hearing loss could be greater.
Acknowledgements
The authors thank Lukas Rüttiger and Neil Ingham for advice on the ABR experiments and analysis, and Michelle Bird (University of Sheffield) for her assistance with the mouse husbandry.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
Authors contributions: J-Y.J., F.C., J.O., S.L.J. and W.M. were involved in the acquisition, analysis or interpretation of data for the work. S.D.B., M.C.H., M.R.B. were involved in the initial design and interpretation of data. All authors were involved in revising it critically for important intellectual content. J-Y.J. and WM conceived and designed the study and drafted the paper.
All authors approved the final version of the manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed
Competing interests: The Authors declare no conflict of interest
Funding
This work was supported by the: Wellcome Trust (102892/Z/13/Z) to W.M.; Medical Research Council (MC/UP/1503/2) to M.R.B and (MR/S002510/1) to M.M.
References
- Bohne BA, Gruner MM, Harding GW. Morphological correlates of aging in the chinchilla cochlea. Hear Res. 1990;48:79–91. doi: 10.1016/0378-5955(90)90200-9. [DOI] [PubMed] [Google Scholar]
- Bowl MR, Dawson SJ. Age-related hearing loss. Cold Spring Harb Perspect Med. 2019;9:a033217. doi: 10.1101/cshperspect.a033217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt A, Striessnig J, Moser T. CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells. J Neurosci. 2003;23:10832–10840. doi: 10.1523/JNEUROSCI.23-34-10832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen A, Forge A, Wright A, Richardson GP, Goodyear RJ, Taylor R. Ultrastructural defects in stereocilia and tectorial membrane in aging mouse and human cochleae. J Neurosci Res. 2019 doi: 10.1002/jnr.24556. [DOI] [PubMed] [Google Scholar]
- Ceriani F, Hendry A, Jeng JY, Johnson SL, Stephani F, Olt J, Holley MC, Mammano F, Engel J, Kros CJ, Simmons DD, et al. Coordinated calcium signalling in cochlear sensory and non-sensory cells refines afferent innervation of outer hair cells. EMBO J. 2019;38:e99839. doi: 10.15252/embj.201899839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corns LF, Johnson SL, Roberts T, Ranatunga KM, Hendry A, Ceriani F, Safieddine S, Steel KP, Forge A, Petit C, Furness DN, et al. Mechanotransduction is required for establishing and maintaining mature inner hair cells and regulating efferent innervation. Nature Comm. 2018;9:4015. doi: 10.1038/s41467-018-06307-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costalupes JA, Young, Gibson DJ. Effects of continuous noise backgrounds on rate response of auditory nerve fibers in cat. J Neurophysiol. 1984;51:1326–1344. doi: 10.1152/jn.1984.51.6.1326. [DOI] [PubMed] [Google Scholar]
- Francis HW, Ryugo DK, Gorelikow MJ, Prosen CA, May BJ. The functional age of hearing loss in a mouse model of presbycusis. II. Neuroanatomical correlates. Hear Res. 2003;183:29–36. doi: 10.1016/s0378-5955(03)00212-0. [DOI] [PubMed] [Google Scholar]
- Frank T, Rutherford MA, Strenzke N, Neef A, Pangršič T, Khimich D, Fejtova A, Gundelfinger ED. Liberman MC, Harke B, Bryan KE, et al. Bassoon and the synaptic ribbon organize Ca2+ channels and vesicles to add release sites and promote refilling. Neuron. 2010;68:724–738. doi: 10.1016/j.neuron.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol. 2013;110:577–586. doi: 10.1152/jn.00164.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates GA, Mills JH. Presbycusis. Lancet. 2005;366:1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nat Neurosci. 2002;5:147–154. doi: 10.1038/nn796. [DOI] [PubMed] [Google Scholar]
- Goutman JD, Glowatzki E. Time course and calcium dependence of transmitter release at a single ribbon synapse. Proc Natl Acad Sci USA. 2007;104:16341–16346. doi: 10.1073/pnas.0705756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Salant S. Hearing loss and aging: new research findings and clinical implications. J Rehabil Res Dev. 2005;42:9–24. doi: 10.1682/jrrd.2005.01.0006. [DOI] [PubMed] [Google Scholar]
- Grant L, Yi E, Glowatzki E. Two modes of release shape the postsynaptic response at the inner hair cell ribbon synapse. J Neurosci. 2010;30:4210–4220. doi: 10.1523/JNEUROSCI.4439-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hequembourg S, Liberman MC. Spiral ligament pathology: a major aspect of age-related cochlear degeneration in C57BL/6 mice. J Assoc Res Otolaryngol. 2001;2:118–129. doi: 10.1007/s101620010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane KL, Longo-Guess CM, Gagnon LH, Ding D, Salvi RJ, Johnson KR. Genetic background effects on age-related hearing loss associated with Cdh23 variants in mice. Hear Res. 2012;283:80–88. doi: 10.1016/j.heares.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Müller U, Kachar B. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature. 2007;449:87–91. doi: 10.1038/nature06091. [DOI] [PubMed] [Google Scholar]
- Keen EC, Hudspeth AJ. Transfer characteristics of the hair cell’s afferent synapse. Proc Natl Acad Sci USA. 2006;103:5537–5542. doi: 10.1073/pnas.0601103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear Res. 2015;330:191–199. doi: 10.1016/j.heares.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham NJ, Pearson S, Steel KP. Using the Auditory Brainstem Response (ABR) to Determine Sensitivity of Hearing in Mutant Mice. Curr Protoc Mouse Biol. 2011;1:279–287. doi: 10.1002/9780470942390.mo110059. [DOI] [PubMed] [Google Scholar]
- Jeng JY, Ceriani F, Hendry A, Johnson SL, Yen P, Simmons DD, Kros CJ, Marcotti W. Hair cell maturation is differentially regulated along the tonotopic axis of the mammalian cochlea. J Physiol. 2020a;598:151–170. doi: 10.1113/JP279012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng J-Y, Johnson SL, Carlton AJ, De Tomasi L, Goodyear R, De Faveri F, Furness DN, Wells S, Brown SDM, Holley MC, Richardson GP, et al. Age-related changes in the biophysical and morphological characteristics of mouse cochlear outer hair cells. J Physiol. 2020b doi: 10.1113/JP279795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng J-Y, Carlton AJ, Johnson SL, Brown SDM, Holley MC, Bowl MR, Marcotti W. Biophysical and morphological changes in inner hair cells and their efferent innervation in the ageing mouse cochlea. J Physiol, under final revision. 2020c doi: 10.1113/JP280256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson LG. Sequence of degeneration of Corti’s organ and its first-order neurons. Ann Otol Rhinol Laryngol. 1974;83:294e303. doi: 10.1177/000348947408300303. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/6J mice. Hear Res. 1997;114:83–92. doi: 10.1016/s0378-5955(97)00155-x. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Marcotti W, Kros CJ. Increase in efficiency and reduction in Ca2+ dependence of exocytosis during development of mouse inner hair cells. J Physiol. 2005;563:177–191. doi: 10.1113/jphysiol.2004.074740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Forge A, Knipper M, Münkner S, Marcotti W. Tonotopic variation in the calcium dependence of neurotransmitter release and vesicle pool replenishment at mammalian auditory ribbon synapses. J Neurosci. 2008;28:7670–7678. doi: 10.1523/JNEUROSCI.0785-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Franz C, Kuhn S, Furness DN, Rüttiger L, Münkner S, Rivolta MN, Seward EP, Herschman HR, Engel J, Knipper M, et al. Synaptotagmin IV determines the linear Ca2+ dependence of vesicle fusion at auditory ribbon synapses. Nat Neurosci. 2010;13:45–52. doi: 10.1038/nn.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Kuhn S, Franz C, Ingham N, Furness DN, Knipper M, Steel KP, Adelman JP, Holley MC, Marcotti W. Presynaptic maturation in auditory hair cells requires a critical period of sensory-independent spiking activity. Proc Natl Acad Sci USA. 2013;110:8720–8725. doi: 10.1073/pnas.1219578110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Olt J, Cho S, von Gersdorff H, Marcotti W. The coupling between Ca2+ channels and the exocytotic Ca2+ sensor at hair cell ribbon synapses varies tonotopically along the mature cochlea. J Neurosci. 2017;37:2471–2484. doi: 10.1523/JNEUROSCI.2867-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Safieddine S, Mustapha M, Marcotti W. Hair Cell Afferent Synapses: Function and Dysfunction. Cold Spring Harb Perspect Med. 2019;9:a033175. doi: 10.1101/cshperspect.a033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer AM, Fuchs PA, Ryugo DK, Francis HW. Efferent synapses return to inner hair cells in the aging cochlea. Neurobiol Aging. 2012;33:2892–2902. doi: 10.1016/j.neurobiolaging.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi D, Runyeon JW, Crum J, Ellisman MH, Roberts WM. Synaptic vesicle populations in saccular hair cells reconstructed by electron tomography. J Neurosci. 1999;19:119–132. doi: 10.1523/JNEUROSCI.19-01-00119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC. Auditory-nerve response from cats raised in a low-noise chamber. J Acoust Soc Am. 1978;63:442–455. doi: 10.1121/1.381736. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Morphological differences among radial afferent fibers in the cat cochlea: an electron-microscopic study of serial sections. Hear Res. 1980;3:45–63. doi: 10.1016/0378-5955(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Liberman MC. The cochlear frequency map for the cat: Labeling auditory-nerve fibers of known characteristic frequency. J Acoust Soc Am. 1982a;72:1441–1449. doi: 10.1121/1.388677. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Single-neuron labeling in the cat auditory nerve. Science. 1982b;216:1239–1241. doi: 10.1126/science.7079757. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW, Pierce S. Afferent and efferent innervation of the cat cochlea: quantitative analysis with light and electron microscopy. J Comp Neurol. 1990;301:443–460. doi: 10.1002/cne.903010309. [DOI] [PubMed] [Google Scholar]
- Liberman LD, Wang H, Liberman MC. Opposing gradients of ribbon size and AMPA receptor expression underlie sensitivity differences among cochlear-nerve/hair-cell synapses. J Neurosci. 2011;31:801–808. doi: 10.1523/JNEUROSCI.3389-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Poveda EA. Why do I hear but not understand? Stochastic undersampling as a model of degraded neural encoding of speech. Front Neurosci. 2014;8:348. doi: 10.3389/fnins.2014.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, Johnson SL, Holley MC, Kros CJ. Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells. J Physiol. 2003;548:383–400. doi: 10.1113/jphysiol.2002.034801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, Johnson SL, Kros CJ. Effects of intracellular stores and extracellular Ca(2+) on Ca(2+)-activated K(+) currents in mature mouse inner hair cells. J Physiol. 2004;557:613–33. doi: 10.1113/jphysiol.2003.060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen JB. Some methods for classification and analysis of multivariate observations. Math Statist Prob. 1967;1:281–297. [Google Scholar]
- Merchan-Perez A, Liberman MC. Ultrastructural differences among afferent synapses on cochlear hair cells: correlations with spontaneous discharge rate. J Comp Neurol. 1996;371:208–221. doi: 10.1002/(SICI)1096-9861(19960722)371:2<208::AID-CNE2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Meyer AC, Frank T, Khimich D, Hoch G, Riedel D, Chapochnikov NM, Yarin YM, Harke B, Hell SW, Egner A, Moser T. Tuning of synapse number, structure and function in the cochlea. Nat Neurosci. 2009;12:444–453. doi: 10.1038/nn.2293. [DOI] [PubMed] [Google Scholar]
- Mianné J, Chessum L, Kumar S, Aguilar C, Codner G, Hutchison M, Parker A, Mallon AM, Wells S, Simon MM, Teboul L, et al. Correction of the auditory phenotype in C57BL/6N mice via CRISPR/Cas9-mediated homology directed repair. Genome Med. 2016;8:16. doi: 10.1186/s13073-016-0273-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michanski S, Smaluch K, Steyer AM, Chakrabarti R, Setz C, Oestreicher D, Fischer C, Möbius W, Moser T, Vogl C, Wichmann C. Mapping developmental maturation of inner hair cell ribbon synapses in the apical mouse cochlea. Proc Natl Acad Sci USA. 2019;116:6415–6424. doi: 10.1073/pnas.1812029116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller A, Jannetta P. Evoked potentials from the inferior colliculus in man. Electroencephalogr Clin Neurophysiol. 1982;53:612–620. doi: 10.1016/0013-4694(82)90137-7. [DOI] [PubMed] [Google Scholar]
- Moser T, Beutner D. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proc Natl Acad Sci USA. 2000;97:883–888. doi: 10.1073/pnas.97.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, von Hünerbein K, Hoidis S, Smolders JW. A physiological placefrequency map of the cochlea in the CBA/J mouse. Hear Res. 2005;202:63–73. doi: 10.1016/j.heares.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR. Association of cadherin 23 with polygenic inheritance andgenetic modification of sensorineural hearing loss. Nat Genet. 2003;35:21–23. doi: 10.1038/ng1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Dahl AR, Gagnon PM. Divergent aging characteristics in CBA/J and CBA/CaJ mouse cochleae. J Assoc Res Otolaryngol. 2010;11:605–623. doi: 10.1007/s10162-010-0228-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Jones SM, Johnson KR. Application of Mouse Models to Research in Hearing and Balance. J Assoc Res Otolaryngol. 2016;17:493–523. doi: 10.1007/s10162-016-0589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohn TL, Rutherford MA, Jing Z, Jung S, Duque-Afonso CJ, Hoch G, Picher MM, Scharinger A, Strenzke N, Moser T. Hair cells use active zones with different voltage dependence of Ca2+ influx to decompose sounds into complementary neural codes. Proc Natl Acad Sci USA. 2016;113:E4716–25. doi: 10.1073/pnas.1605737113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Richardson GP, de Monvel JB, Petit C. How the genetics of deafness illuminates auditory physiology. Annu Rev Physiol. 2011;73:311–334. doi: 10.1146/annurev-physiol-012110-142228. [DOI] [PubMed] [Google Scholar]
- Pujol R, Lavigne-Rebillard M, Lenoir M. In: Development of the auditory system. Rubel EW, Popper AN, Fay RR, editors. Springer; New York: 1998. Development of sensory and neural structures in the mammalian cochlea; pp. 146–192. [Google Scholar]
- Ryugo DK. In: The mammalian auditory pathway: Neuroanatomy. Webster DB, et al., editors. Springer; New York: 1992. The auditory nerve: Peripheral innervation, cell body morphology, and central projections; pp. 23–65. [Google Scholar]
- Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci. 2011;31:13452–13457. doi: 10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt RA, Mills JH, Boettcher FA. Age-related loss of activity of auditory-nerve fibers. J Neurophysiol. 1996;76:2799–2803. doi: 10.1152/jn.1996.76.4.2799. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol. 1993;102:1–16. doi: 10.1177/00034894931020S101. [DOI] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci. 2013;33:13686–13694. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha SH, Kanicki A, Dootz G, Talaska AE, Halsey K, Dolan D, Altschuler R, Schacht J. Age-related auditory pathology in the CBA/J mouse. Hear Res. 2008;243:87–94. doi: 10.1016/j.heares.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spongr VP, Flood DG, Frisina RD, Salvi RJ. Quantitative measures of hair cell loss in CBA and C57BL/6 mice throughout their life spans. J Acoust Soc Am. 1997;101:3546–3553. doi: 10.1121/1.418315. [DOI] [PubMed] [Google Scholar]
- Stamataki S, Francis HW, Lehar M, May BJ, Ryugo DK. Synaptic alterations at inner hair cells precede spiral ganglion cell loss in aging C57BL/6J mice. Hear Res. 2006;221:104–118. doi: 10.1016/j.heares.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Trune DR, Kempton JB, Mitchell C. Auditory function in the C3H/HeJ and C3H/HeSnJ mouse strains. Hear Res. 1996;96:41–45. doi: 10.1016/0378-5955(96)00017-2. [DOI] [PubMed] [Google Scholar]
- Tsuji J, Liberman MC. Intracellular labeling of auditory nerve fibers in guinea pig: central and peripheral projections. J Comp Neurol. 1997;381:188–202. [PubMed] [Google Scholar]
- Virtanen P, et al. SciPy 1.0--Fundamental Algorithms for Scientific Computing in Python. arXiv preprint. 2019:arXiv:1907.10121 [Google Scholar]
- Winter IM, Robertson D, Yates GK. Diversity of characteristic frequency rate-intensity functions in guinea pig auditory nerve fibres. Hear Res. 1990;45:191–202. doi: 10.1016/0378-5955(90)90120-e. [DOI] [PubMed] [Google Scholar]
- Wong AB, Rutherford MA, Gabrielaitis M, Pangrsic T, Göttfert F, Frank T, Michanski S, Hell S, Wolf F, Wichmann C, Moser T. Developmental refinement of hair cell synapses tightens the coupling of Ca2+ influx to exocytosis. EMBO J. 2014;33:247–264. doi: 10.1002/embj.201387110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PZ, Liberman LD, Bennett K, de Gruttola V, O’Malley JT, Liberman MC. Primary Neural Degeneration in the Human Cochlea: Evidence for Hidden Hearing Loss in the Aging Ear. Neurosci. 2019;407:8–20. doi: 10.1016/j.neuroscience.2018.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Liberman LD, Maison SF, Liberman MC. Olivocochlear innervation maintains the normal modiolar-pillar and habenular-cuticular gradients in cochlear synaptic morphology. J Assoc Res Otolaryngol. 2014;15:571–583. doi: 10.1007/s10162-014-0462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary SP, Fuchs PA. Re-Emergent Inhibition of Cochlear Inner Hair Cells in a Mouse Model of Hearing Loss. J Neurosci. 2015;35:9701–9706. doi: 10.1523/JNEUROSCI.0879-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampini V, Johnson SL, Franz C, Knipper M, Holley MC, Magistretti J, Masetto S, Marcotti W. Burst activity and ultrafast activation kinetics of CaV1.3 Ca2+ channels support presynaptic activity in adult gerbil hair cell ribbon synapses. J Physiol. 2013;591:3811–3820. doi: 10.1113/jphysiol.2013.251272. [DOI] [PMC free article] [PubMed] [Google Scholar]