Abstract
Background
Numerous iron preparations are available for the treatment of iron deficiency anaemia in pregnancy. We aimed to provide a summary of the effectiveness and safety of iron preparations used in this setting.
Methods
We did a systematic review and network meta-analysis of randomised trials. We searched Medline, Embase, Cochrane Central Register of Controlled Trials, trial registers and grey literature for trials published in any language from Jan 1, 2011 to Feb 28, 2021. We included trials of pregnant women with IDA evaluating iron preparations, irrespective of administration route with ≥ 60 mg of elemental iron, in comparison to another iron or non-iron preparation. Three independent reviewers selected studies, extracted data, and did a risk of bias assessment using the Cochrane tool (version 1·0). The outcomes were haemoglobin (primary) in g/L and serum ferritin in mcg/L (secondary) levels at four weeks from baseline and treatment-related severe and non-severe adverse events. We did random-effects pairwise and network meta-analyses. The effect measure is reported as mean difference (MD) with 95% confidence intervals (CI). Side-effects were reported descriptively for each trial. This study is registered with PROSPERO, number CRD42018100822.
Findings
Among 3037 records screened, 128 full-text articles were further assessed for eligibility. Of the 53 eligible trials (reporting on 9145 women), 30 (15 interventions; 3243 women) contributed data to the network meta-analysis for haemoglobin and 15 (nine interventions; 1396 women) for serum ferritin. The risk of bias varied across the trials contributing to network meta-analysis. Compared with oral ferrous sulfate, intravenous iron sucrose improved both haemoglobin (MD 7·17 g/L, 95%CI 2·62-11·73) and serum ferritin (49·66 mcg/L, 13·63-85·69), and intravenous ferric carboxymaltose (8·52 g/L, 0·51-16·53) improved haemoglobin levels. The evidence for other interventions compared with ferrous sulfate was insufficient. The most common side-effects with oral iron preparations were gastrointestinal effects (nausea, vomiting, and altered bowel movements). Side-effects were less common with parenteral iron preparations, although these included local pain, skin irratation, and, on rare occasions, allergic reactions.
Interpretation
Iron preparations for treatment of iron deficiency anaemia in pregnancy vary in effectiveness, with good evidence of benefit for intravenous iron sucrose and some evidence for intravenous ferric carboxymaltose. Clinicians and policy makers should consider the effectiveness of individual preparations before administration, to ensure effective treatment.
Funding
This work did not receive any funding.
Introduction
Iron deficiency anaemia, the commonest global nutritional deficiency, disproportionately affects women of reproductive age. 1 The burden is particularly severe in pregnancy, affecting half of all pregnant women, due to increased demands, and with many women entering pregnancy with depleted iron stores. A quarter of all mothers are diagnosed with the condition every year even in high-income countries like the UK. 2 Anaemia in pregnancy further predisposes women to maternal mortality 3 and morbidity, including increased haemorrhage, infection, 4 and adverse perinatal outcomes including low birth weight and preterm delivery. 5
Anaemia is characterised by a fall in haemoglobin, resulting from a progressive deficiency of micronutrients including iron. 6,7 Theoretically, treating iron deficiency anaemia should be straightforward: replace the lost iron. Despite the widespread availability of iron preparations, anaemia in pregnancy remains a problem. 4 There are many widely tested as well as new emerging oral and parenteral forms of iron. 8 But there is no comprehensive comparison of the effectiveness of individual iron preparations. Consequently, clinicians tend to prescribe the most readily available oral iron preparation, which may not be the most effective.
Our aim was to synthesise the available data and provide a summary of effectiveness and safety of iron preparations used for the treatment of iron deficiency anaemia in pregnancy.
Methods
Search strategy and selection criteria
Our systematic review with network meta-analysis was guided by a prospectively developed and protocol (Appendix pp 1-11). The study was registered with PROSPERO (CRD42018100822) and reported in accordance with the PRISMA extension for network meta-analysis. 9
We included randomised and quasi-randomised controlled trials (RCTs) published in any language assessing the effectiveness of iron preparation in pregnant women with confirmed iron deficiency anaemia, as defined by trial authors, based on objective testing. Included trials compared one or more iron preparations, with another iron preparation, placebo, no treatment, vitamin (mainly folic acid) and/or mineral supplement (zinc). The iron in the intervention arm was required to contain at least 60 mg of elemental iron, considered the minimum effective dose for treating anaemia. 10,11 We excluded trials comparing two doses of the same iron preparation and those with study groups treated with erythropoietin or blood transfusion, micronutrient or multivitamin supplements, vitamin A, or outdated iron preparations (Appendix p 12). We had originally planned to evaluate the effect of iron preparations in three separate populations: menstruating women, pregnant women and postpartum. In this Article, we present the findings for the pregnant population only, as due to feasibility issues we decided to separate the populations.
Our work builds on two previous Cochrane reviews of iron treatments in pregnant women. 11,12 (Appendix p 12). Thus the literature search was run from 1st January 2011 to 19th July 2018 using a modified search strategy; updated to 28th February 2021 (Appendix p 15). A search, without any language limits, was performed in the major medical literature databases (Appendix p 12). Additionally, we checked the Inside Conferences, Systems for Information in Grey Literature database for grey literature, clinical trial registers for ongoing trials (Appendix pp 12-13) and supplemented this with a random search for relevant trials using Google Scholar. In the first stage, two reviewers (MN, CAP) independently evaluated all retrieved citations, and subsequently the full texts against eligibility criteria. In case of any disagreement, the third reviewer (JD) was consulted.
We collected study-level data using a bespoke data extraction form piloted on five eligible trials. 13–17 We collected information on women’s characteristics, evaluated interventions and routinely collected data about trials (Appendix p 13). The trials were then classified by income group based on the World Bank classification, 18 into low, and lower-middle income counties (LMIC) and upper middle and high-income as high-income countries (HIC). For outcome data reported in various units, we extracted values (and their variances) of haemoglobin and serum ferritin as reported by the authors and converted to g/L and mcg/L respectively; we kept a record of conversions. We also recorded details of blood samples collection (point of care or laboratory tests). Three researchers (MN, CAP and JD) extracted all available data on included trials independently. We did not contact the study authors for any additional information. Publications written in Spanish were translated by CAP, any other non-English publications were translated using Google translate.
The quality of all included trials was assessed using the Cochrane risk of bias tool (version 1·0) classifying trials for each domain, except blinding of outcome assessor, as low, unclear or high risk of bias. 19 We assumed the potential risk of detection bias caused by the lack of blinding of the outcome assessor would be negligible as our main outcome of interest is a laboratory blood test, which is objectively measured.
The assessments of individual domains were then used to obtain a global risk of bias (low, medium or high) for trials contributing to the main network meta-analysis of haemoglobin. We also assessed indirectness of the study groups in accordance with the recommendation of the GRADE working group. 20 The distribution of evidence quality, defined as global risk of bias, is graphically presented for the network analysis of haemoglobin as in Confidence in Network Meta-Analysis approach. 21,22
We determined effectiveness of iron preparations by changes in haemoglobin (the primary outcome) and serum ferritin (secondary outcome). The effect measure for both outcomes is the mean difference (MD) reported with the respective 95% confidence intervals (CIs). We did not undertake quantitative synthesis of side effects, we reported these descriptively for each trial.
The network meta-analysis for haemoglobin comprised of studies comparing individual iron preparations meeting our inclusion criteria. We assumed that all interventions were jointly randomisable and the concomitant interventions (vitamins and/or minerals) did not have a substantial impact on the outcomes. If any included trials comprised study arms of iron preparations with and without concomitant interventions, we combined the data into one arm using recommended methods. 19 The arms containing placebo, no intervention, or vitamins and/or minerals were grouped together and coded as ‘non-iron intervention’.
We anticipated challenges due to variation in treatment duration, the time between the iron intervention administration, and measurement of laboratory outcomes in the included trials. 11,12,23 To address this we consulted an independent panel of experts (obstetric haematologists, midwives and senior obstetricians) from the British Society for Haematology. We held a consultation meeting prior to embarking on the analyses (on 28th November 2018) during which approaches to grouping iron preparations, strategies for analyses and data presentation were discussed. Following this consultation, we decided to record the timing of haemoglobin measurement from baseline in all trials and analyse the change in the blood parameters at the most commonly reported time point. The network map was generated for both efficacy outcomes and examined for its connectivity (presence of closed loops). 22
Data analysis
Firstly, extracted data were inspected in a pairwise meta-analysis where more than two trials for the same comparison were available using a random effects model with the restricted maximum likelihood estimator to account for heterogneity if present. 24,25 We quantified inconsistencies between studies in the pairwise meta-analyses using the I 2 statistic 26 . The network meta-analysis assumed consistency using a frequentist approach with a ‘contrast-based’ model. 22 We assumed constant heterogeneity variance across all comparisons, and estimated the between-study heterogeneity using τ. The within-study correlation because of multi-arm trials was managed using a multivariate random-effects network meta-analysis using the network suite of commands in Stata version 15·1 (StataCorp. Texas, USA). 27 Inconsistency between direct and indirect sources of evidence was examined locally using a node-splitting approach, 22,28,29 and globally using a design-by-treatment interaction model. 30
The ranking of treatments for haemoglobin is presented in a tabulated format ordered according to the mean rank value using the surface under the cumulative ranking (SUCRA) curve. 31 Given the complexity of multiple interventions and comparisons, we used iron ferrous sulfate, the current standard treatment, as the reference arm when presenting and interpreting the data in the analyses for haemoglobin and serum ferritin.
We applied two secondary approaches to grouping of the iron preparations. First, by route of administration (oral, IV, IM) and second by type of iron salt (ferric IM [Fe3+], ferric IV [Fe3+] and ferrous [Fe2+] oral preparations). Lactoferrin, iron amino acid chelate, and arms with ‘no iron preparation’ (such as placebo, vitamins or no intervention) were kept as separate groups throughout. We pre-specified two sensitivity analyses, in the first we explored the impact of interventions administered alongside iron. In the second, we assessed the impact of trial quality by excluding trials classified as ‘at high risk of bias’. Our protocol intended a sensitivity analysis by year of study publication which proved unfeasible (Appendix p 10). Finally, we performed a prespecified subgroup analysis by country income classification.
Role of the funding source
There was no funding source for this study.
Results
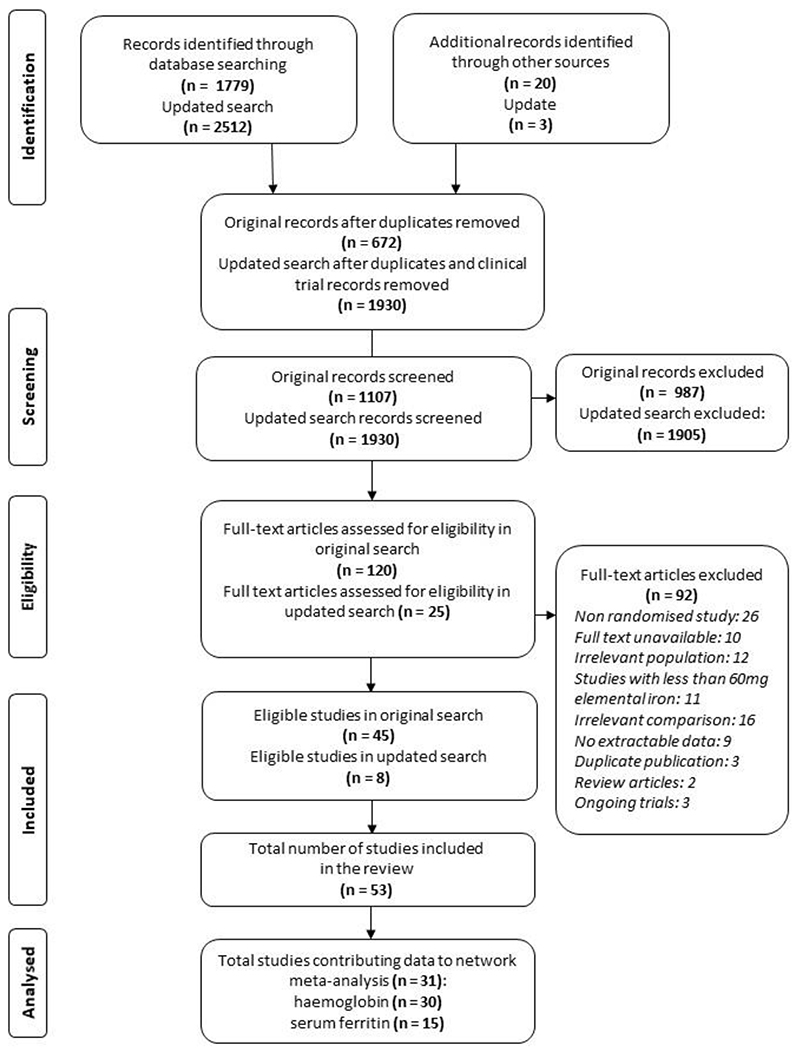
Among 3037 records screened, 128 full-text articles were further assessed for eligibility and 53 trials reporting on 9145 women were included (Figure 1). The main reasons for exclusion were non-RCT design (n=26), irrelevant comparison (dose comparison trials, n=16) and irrelevant study population (non-anaemic pregnant women, n=12) (Figure 1). Not all studies contributed to network meta-analysis due to differences in timing of outcome measurement. 32–62 Additionally, there were issues with data credibility in two studies [unpublished; Mol BW, Bordewijk EM, Rogozinska E et al.] which we chose to exclude from the analyses.
Figure 1. Study selection flow.
The 53 included trials were conducted in 22 different countries between 1969 and 2020, with the majority published after 2000 (n=43). Pregnant women participating in the trials were recruited between the second and third trimester. The baseline haemoglobin level ranged from 60 to 110 g/L with most women having moderate anaemia (67/109 trial arms with haemoglobin ranging from 70 g/L to 99 g/L). The baseline body weight ranged from 45·9 to 61·8 kg in the trials of parenteral (IV and IM) iron. Information on pre-existing health conditions (e.g. haemoglobinopathies) alongside any co-administered treatment (e.g. malaria infection prophylaxis or treatment) can be found in Appendix (pp 16-22). We included trials that evaluated 19 interventions. The total daily dose of elemental iron across the trials of oral preparations ranged from 60mg 53 to 240mg 14 with majority of dosages being between 100-200mg (Appendix pp 23-29).
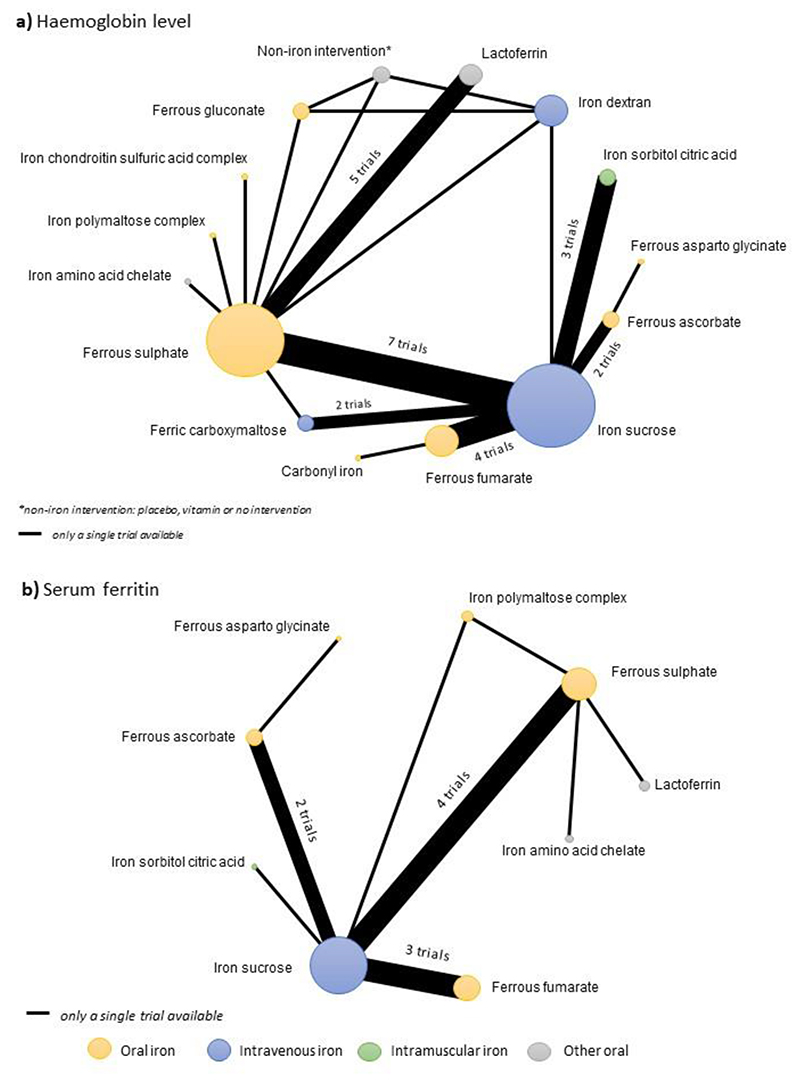
Of all included trials, 30 (62 arms; 3243 women) reported on haemoglobin at four weeks from baseline and were included in the network meta-analysis for haemoglobin. 14,17,34,39,50,51,53,61–82 Characteristics of studies contributing and not contributing data to the network meta-analysis are presented in Table 1. These 30 trials compared 15 different interventions – nine oral iron preparations, three IV preparations, a single IM preparation, lactoferrin and a single ‘non-iron intervention’ (Figure 2A). Six comparisons were evaluated in more than one study and the other comparisons were evaluated in a single trial. IV iron sucrose vs ferrous sulphate were the most frequently compared pair of interventions (seven trials, 695 women), followed by one lactoferrin vs ferrous sulphate (four trials, 457 women), and ferrous fumarate vs IV iron sucrose (four trials, 305 women) (Figure 2A, Appendix p 30).
Table 1. Characteristics of studies contributing and not contributing data to the main network meta-analysis.
| Characteristic | Contributed data to the main NMA | Did not contribute data to the main NMA |
|---|---|---|
| Number of studies | 30 | 23 |
| Total number of women * | 3,243 | 4,854 |
| Publication year (median, min-max) | 2014 (1969, 2020) | 2005 (1978, 2020) |
| Country income group n (%) | ||
| Low and middle-income countries | 22 (73) | 14 (61) |
| Upper-middle and high income countries | 8 (27) | 9 (39) |
| Total number of arms | 62 | 47 ** |
| Anaemia status at baseline *** | ||
| Mild (109-100 g/L) | 11 | 15 |
| Moderate (99-70 g/L) | 44 | 23 |
| Severe (69-40 g/L) | 5 | 3 |
| Not reported | 2 | 6 |
| Number of unique interventions | 15 | 13 |
| Total number of unique comparisons | 19 | 15 |
| Comparisons | ||
| ferrous asparto glycinate vs ferrous ascorbate | 1 | |
| Carbonyl iron vs ferrous fumarate | 1 | |
| iron polymaltose complex vs ferrous fumarate | 1 | |
| Iron chondroitinsulfuric acid complex vs ferrous sulphate | 1 | |
| Iron amino acid chelate vs ferrous sulphate | 1 | |
| ferrous gluconate vs ferrous sulphate | 1 | |
| iron polymaltose complex vs ferrous sulphate | 1 | |
| NaFeEDTA vs ferrous sulphate | 1 | |
| lactoferrin vs ferrous sulphate | 4 | 1 |
| ferrous sulphate vs “no-iron intervention” | 1 | 8 |
| IFB vs “no-iron intervention” | 1 | |
| ferrous gluconate vs “no-iron intervention” | 1 | |
| NaFeEDTA vs “no-iron intervention” | 1 | |
| IV iron dextran vs “no-iron intervention” | 1 | |
| ferrous sulphate vs ferrous sulphate and IV Iron polymaltose complex | 1 | |
| ferrous sulphate vs IV iron sucrose | 7 | 3 |
| ferrous fumarate vs IV iron sucrose | 4 | |
| ferrous ascorbate vs IV iron sucrose | 2 | |
| iron polymaltose complex vs IV iron sucrose | 1 | |
| ferrous sulphate vs IV iron dextran | 1 | |
| ferrous gluconate vs IV iron dextran | 1 | |
| ferrous fumarate vs IV iron dextran | 1 | |
| lactoferrin vs IV iron dextran | 1 | |
| ferrous fumarate vs IV iron polymaltose complex | 1 | |
| ferrous sulphate vs IV ferric carboxymaltose | 1 | |
| IV iron sucrose vs IV ferric carboxymaltose | 2 | |
| IV iron sucrose vs IV iron dextran | 1 | 2 |
| ferrous sulphate vs IM iron dextran | 2 | |
| ferrous sulphate vs IM iron sorbitol citric acid | 1 | |
| IV iron sucrose vs IM iron sorbitol citric acid | 3 | |
NMA, network meta-analysis; IV, intravenous; IM, intramuscular
The number of women analysed in eligible arms
Arms in two originally 3-arm studies with iron and iron and vitamins vs placebo (Sun 2010, Ma 2010) were combined into one
Values correspond to number of arms not studies
Figure 2. Network map for haemoglobin level and serum ferritin measured around four weeks from baseline.
The risk of bias varied across the trials contributing to the network meta-analysis for haemoglobin, with more than two thirds of studies (22 of 30) judged to have a high or medium global risk of bias (appendix pp 32–33). Random sequence generation was correctly implemented in half of the trials (15 [50%] of 30). Allocation concealment frequently could not be assessed due to insufficient information (23 [77%] of 30), although blinding of staff and participants was assessed as low risk of bias in 15 (50%) of the 30 included trials. Incomplete outcome data were deemed at low risk in 21 (70%) of 30 trials and selective reporting of outcomes was assessed as low risk in 22 (73%) trials. The indirectness of the study population in the included trials was assessed as medium risk in three (10%) of 30 trials. An overview of the network for haemoglobin by the global risk of bias of the trials informing the results can be found in the appendix (p 32). Trials not included in the network meta-analysis were more often assessed as being at high risk of bias (appendix pp 32–33).
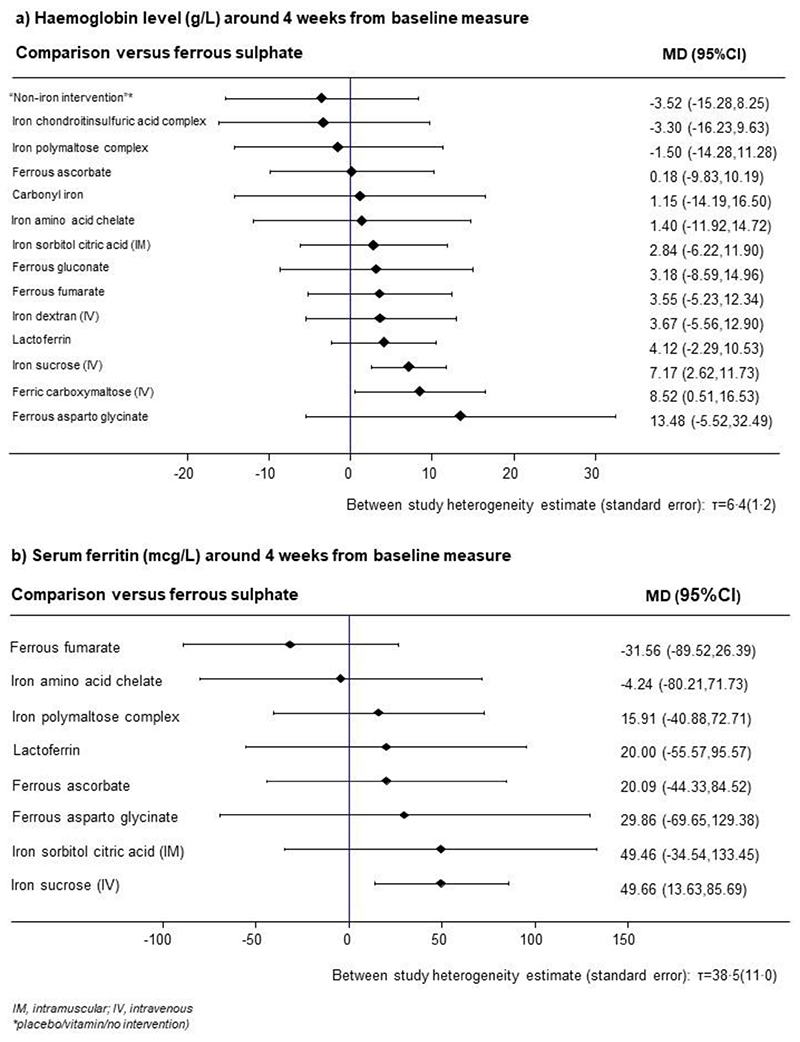
Compared to ferrous sulphate, both IV ferric carboxymaltose (MD 8·52 g/L, 95%CI 0·51-16·53) and IV iron sucrose (MD 7·17 g/L, 95%CI 2·62-11·73) improved haemoglobin levels (Figure 3A). We did not find evidence of an improvement in haemoglobin concentrations between the other interventions and iron ferrous sulfate. There was no evidence to suggest global or local inconsistencies (appendix p 35). The direct and network effects (indirect and direct evidence) were consistent for the majority of comparisons. Interventions with the highest SUCRA values were oral iron ferrous asparto glycinate (85%), intravenous ferric carboxymaltose (81%), and intravenous iron sucrose (78%). Non-iron interventions had the lowest SUCRA value (22%; appendix p 37). The detailed ranking measures, including SUCRA and mean rank, are presented in the appendix (pp 34–38).
Figure 3. The relative effect of evaluated preparations in comparison to ferrous sulphate on haemoglobin levels and serum ferritin around four weeks from baseline.
Additional analyses based on broad grouping of iron preparations (by route of administration and type of iron salt) found intravenous preparations compared best against no intervention (Appendix pp 39–42). In a subgroup analysis by income category, the evidence on different results based on trials from low-middle income countries were similar to those presented in the analysis for haemoglobin (Appendix pp 50–51). Network meta-analysis based on trials from high income countries was not performed due to the small number of studies in this subgroup (appendix p 52). In the sensitivity analyses limited to trials categorised as low and medium risk of bias, the evidence on IV iron sucrose vs ferrous sulphate was robust (MD 8·29g/L, 95%CI 3·47-13·12) while the evidence on IV ferric carboxymaltose vs ferrous sulphate became imprecise (8·35g/L, 95%CI -0·91-17·61, Appendix pp 46–49). Our estimate of between-study heterogeneity remained consistent with that estimated in the network analysis for haemoglobin and sensitivity analyses for this network (appendix pp 43–49).
Fifteen trials (30 arms; 1,396 women) reported on serum ferritin at four weeks from baseline and were included in the network meta-analysis for serum ferritin. 13,17,53,62,64,66–69,73,76–80
The network comprises nine interventions – five oral iron preparations, a single IV and a single IM iron preparation, iron amino acid chelate and lactoferrin. The most frequent comparisons were IV iron sucrose vs ferrous sulphate (four trials, 400 women), IV iron sucrose vs ferrous fumarate (three trials, 216 women) and IV iron sucrose vs ferrous ascorbate (two trials, 400 women) and the other comparisons were evaluated in single trials (Figure 2B).
Compared to ferrous sulphate, IV iron sucrose increased serum ferritin levels (MD 49·66 mcg/L, 95%CI 13·63-85·69) (Figure 3B). There was insufficient evidence of increase of serum ferritin levels between the other interventions vs ferrous sulphate, including IV ferric carboxymaltose vs ferrous sulphate (MD 49·46 mcg/L, 95%CI -34·54-133·45) (Figure 3B). There was no evidence to suggest global (chi-squared = 0·38, p-value = 0·54) or local inconsistencies (Appendix p 36). Interventions with the highest SUCRA were IV iron sucrose (82%) and IV ferric carboxymaltose (74%, appendix 38). The detailed ranking measures, including SUCRA and mean rank, are presented in the appendix (pp 34-38).
Safety reporting in trials of iron interventions in pregnancy were highly variable, with many instances of poor reporting, therefore an analysis by individual preparation proved unfeasible. Overall, gastrointestinal side effects (nausea, vomiting and altered bowel movements) were most common with oral iron preparations. There were no appreciable differences between iron preparations. Allergic reactions, including anaphylaxis, although rare, were more commonly reported with intravenous iron preparations. Other reported side effects to parenteral preparations included injection site pain and inflammation, altered taste and hypotension. A comprehensive summary of all side effects as reported and defined in individual trials can be found in the appendix (pp 53-61).
Discussion
Based on our network meta-analysis of 30 RCTs comparing 15 iron preparations in 3,243 women, IV ferric carboxymaltose and IV iron sucrose were the most effective interventions in improving haemoglobin levels four weeks after starting treatment. The findings on iron ferrous asparto glycinate should be interpreted with caution due to the single small trial with high risk of bias contributing to the evidence. From our network meta-analysis of 15 RCTs comparing nine iron preparations in 1,396 women, IV iron sucrose was the most effective intervention for improving serum ferritin. The evidence from our network meta-analysis for haemoglobin and serum ferritin show the highest certainty for iron sucrose at improving blood values following administration. There were no appreciable differences in rates of side effects between iron preparations.
This is, to our knowledge, the first network meta-analysis to comprehensively assess the effectiveness of many widely available iron treatments for the management of anaemia in pregnancy. We included trials where iron was administered for treating anaemia following a confirmed diagnosis of iron deficiency anaemia based on objective testing,
Our work was guided by a prospectively developed protocol including input from an independent expert clinical panel before analyses were conducted. The panel, comprising senior clinicians and UK policy makers provided advice on the relevance of the iron preparations, the appropriateness of the time points used for the primary and secondary endpoints and on the pre-planned subgroup analyses.
The searches used to identify trials built on two existing Cochrane reviews, 11,12 using several search terms without any limitations, our searches were updated in February 2021, including the most up to date published data. There are several ongoing studies which we were unable to include in the analyses (Appendix pp 62-63).
The included iron interventions were given at variable doses. This reflects real-life clinical practice where no recommended dosing schedules exist, and treatment is largely based on tolerance and response to treatment. Similarly, there was marked variation in the timing of haemoglobin and serum ferritin measurement from commencement of the intervention (e.g. weekly measurements vs just before delivery). We addressed this methodological challenge by using trials evaluating the response to iron interventions four-weeks from commencement. This allowed the largest number of trials to be included, while reducing spurious results from repeated measurements of outcomes. Furthermore, with oral iron treatment and assuming optimal compliance, a rise in haemoglobin level of 10 g/L every two weeks can be expected. 83 Thus, measuring haemoglobin at four weeks from treatment commencement should provide sufficient time to identify some treatment effect.
The pair-wise meta- analysis found statistical heterogeneity, but our explorations did not reveal any obvious sources of between-study differences in treatment effect. Factors such as different dosing regimens, variation in measurement of haemoglobin and iron levels and differences baseline characteristics between women may all play a role. Finally, the evidence contributing to the networks for haemoglobin and serum ferritin were sparse. Most comparisons in the network were single head to head trials, affecting the overall stability. 84
Our work summarises the landscape of clinical trials for the treatment of anaemia caused by iron deficiency in a global pregnant population. Our work allows comparisons across and between individual preparations, giving a more comprehensive overview than the existing pairwise meta-analyses presented in the Cochrane reviews. Our work also incorporates studies published since 2011, 11,12 including newer iron and cofactor preparations. Although iron gluconate and iron isomaltoside are often widely used in clinical practice, these preparations were not included in the trials identified in the systematic review, despite contacting authors for additional non published data.
Existing policy on iron preparations for the treatment of anaemia in pregnancy is highly variable. 83,85 The reasons for this are multifactorial including the numerous causes of iron deficiency that exist globally, differences in antenatal care delivery between regions, and sheer number of small trials testing different preparations of iron where outcomes are measured at different time points 23 . 86 We have addressed some of these challenges in our work, but definitive research, including large scale trials measuring clinically relevant endpoints which have long been called for are needed. 87
The finding from this systematic review show that parenteral iron preparations are more effective at increasing haemoglobin levels compared to oral preparations. This is likely due to improved compliance with parenteral preparations, improved bioavailability and targeted dosing. 1,88 These findings support other existing meta-analyses of iron interventions. 87,89,90 The clinical impact of higher haemoglobin and iron stores such as improvements in clinical outcomes such as maternal and infant wellbeing remain unknown. 91,92 This further emphasizes the need for good quality trials addressing these questions. 92,93
Ferrous sulphate is one of the most widely used oral iron preparations, being cheap and widely available, hence we used this as our reference iron preparations. 85 However, published data suggest that tolerance to ferrous fumerate or alternative dosing schedules such as alternate day may improve adherence. 94 The findings from this systematic review show most oral iron preparations perform similarly, however parenteral preparations fair better. Therefore, policy makers and clinicians to consider which oral iron preparation they are using as first line treatment for anaemic women in pregnancy based on availability, and tolerance for each induvial woman rather than what is most widely used.
Our work suggests insufficient evidence to support lactoferrin, a non-iron based cofactor, as beneficial at improving haemoglobin levels or iron stores in pregnant women. Therefore, further clinical trials, especially in diverse settings, are required before firm conclusions can be made. There are two large ongoing trials of lactoferrin use in pregnancy, which once complete are likely to improve the precision of estimates reported in our work (Appendix p 62-63).
We hope our work improves the available evidence and provide some much-needed clarity on which preparations are the most effective, best tolerated and safest for treating anaemia in pregnancy. Future work, building on this review, could include novel trial methodology testing the top-ranking interventions against each other, increasing the available direct evidence. We hope that these data aid policy makers to reconsider the use of less effective iron preparations when treating anaemia in pregnancy.
Supplementary Material
Research in context.
Evidence before this study
Iron deficiency anaemia is common in pregnancy due to increasing iron demand and is associated with adverse maternal and perinatal outcomes. Numerous iron preparations are available for treatment, but until now these have only been compared in traditional pairwise meta-analyses, the most comprehensive of which are two Cochrane reviews, published in 2011 and 2015. Before this study (in Feb, 2018, we searched Medline, Embase, The Cochrane Library, and the PROSPERO database for completed or ongoing systematic reviews and network meta-analyses of iron treatments for anaemia in pregnancy. We found no published network meta-analyses or available protocols.
Added value of this study
To our knowledge, our network meta-analysis of randomised trials is the first to simultaneously compare all the widely available iron treatments for anaemia in pregnancy against one another. This work updates existing meta-analyses assessing the effectiveness of iron interventions in pregnant women.
Implications of all the available evidence
Treating iron deficiency anaemia in pregnancy remains a priority. Intravenous iron preparations, including iron sucrose and ferric carboxymaltose, are the most effective at improving haemoglobin and iron stores. Our findings suggest that existing policy on the treatment of anaemia in pregnancy could be updated to reflect that some iron preparations are more effective than others for treating anaemia in pregnancy.
Acknowledgements
the following individuals were part of the external clinical experts: Sue Pavord, Joanna Girling, Susan Robinson, members of the British Society for Haematology Obstetric Haematology special interest group. Professor Khalid S. Khan is funded by the Beatriz Galindo (senior modality) grant to the University of Granada by the Spanish Ministry of Education.
Funding
This research did not receive any funding.
Footnotes
Details of Contributions
JD, ER and ST contributed to the study conception and design and planned the statistical analyses. JD, MN and CA collected data, undertook quality assessment. ER and PJG accessed and verified the data. ER, PJG, JZ and CMS analysed the data. JD and ER wrote the first draft of the manuscript and are responsible for the decision to submit the manuscript. RW, PJG, SR, KSK and ST critically revised the manuscript for important intellectual content. All authors commented on the drafts and approved the final draft. JD and ST are the guarantors.
Ethical approval: none required.
Transparency Declaration
JD affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; no important aspects of the study have been omitted; any discrepancies from the study as planned have been explained.
Declarations of Interest
JD was a member of an advisory panel assessing the side effect profiles of intravenous iron preparations for Pharmacosmos in 2018. ER and PJG were supported by the UK Medical Research Council (MC_UU_12023/24).
All other co-authors have no declarations of interest.
Data sharing
The data collected for this systematic review and network meta-analysis can be shared on request, with investigator support and approval thorough a signed data access agreement. This includes aggregate study level data collected, cleaned with a data dictionary and the statistical analysis plan. This will be made available with publication. No additional data are available.
References
- 1.Ganz T. Systemic iron homeostasis. Physiological reviews. 2013;93(4):1721–41. doi: 10.1152/physrev.00008.2013. [DOI] [PubMed] [Google Scholar]
- 2.Barroso F, Allard S, Kahan BC, et al. Prevalence of maternal anaemia and its predictors: a multi-centre study. European journal of obstetrics, gynecology, and reproductive biology. 2011;159(1):99–105. doi: 10.1016/j.ejogrb.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 3.Daru J, Zamora J, Fernández-Félix BM, et al. Risk of maternal mortality in women with severe anaemia during pregnancy and post partum: a multilevel analysis. The Lancet Global Health. 2018;6(5):e548–e54. doi: 10.1016/S2214-109X(18)30078-0. [DOI] [PubMed] [Google Scholar]
- 4.Stevens GA, Finucane MM, De-Regil LM, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: a systematic analysis of population-representative data. The Lancet Global health. 2013;1(1):e16–e25. doi: 10.1016/S2214-109X(13)70001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith C, Teng F, Branch E, Chu S, Joseph KS. Maternal and Perinatal Morbidity and Mortality Associated With Anemia in Pregnancy. Obstet Gynecol. 2019;134(6):1234–44. doi: 10.1097/AOG.0000000000003557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Casal MN, Pasricha SR, Sharma AJ, Pena-Rosas JP. Use and interpretation of hemoglobin concentrations for assessing anemia status in individuals and populations: results from a WHO technical meeting. Ann N Y Acad Sci. 2019 doi: 10.1111/nyas.14090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daru J, Sobhy S, Pavord S. Revisiting the basis for haemoglobin screening in pregnancy. Curr Opin Obstet Gynecol. 2019;31(6):388–92. doi: 10.1097/GCO.0000000000000580. [DOI] [PubMed] [Google Scholar]
- 8.Auerbach M, Gafter-Gvili A, Macdougall IC. Intravenous iron: a framework for changing the management of iron deficiency. The Lancet Haematology. 2020;7(4):e342–e50. doi: 10.1016/S2352-3026(19)30264-9. [DOI] [PubMed] [Google Scholar]
- 9.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 10.Milman N, Bergholt T, Eriksen L, et al. Iron prophylaxis during pregnancy-how much iron is needed? A randomized dose-response study of 20-80 mg ferrous iron daily in pregnant women. Acta obstetricia et gynecologica Scandinavica. 2005;84(3):238–47. doi: 10.1111/j.0001-6349.2005.00610.x. [DOI] [PubMed] [Google Scholar]
- 11.Peña-Rosas JP, De-Regil LM, Garcia-Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database of Systematic Reviews. 2015:7. doi: 10.1002/14651858.CD004736.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reveiz L, Gyte GM, Cuervo LG, Casasbuenas A. Treatments for iron-deficiency anaemia in pregnancy. Cochrane Database of Systematic Reviews. 2011:10. doi: 10.1002/14651858.CD003094.pub3. [DOI] [PubMed] [Google Scholar]
- 13.Al RA, Unlubilgin E, Kandemir O, Yalvac S, Cakir L, Haberal A. Intravenous versus oral iron for treatment of anemia in pregnancy: A randomized trial. Obstetrics and Gynecology. 2005;106(6):1335–40. doi: 10.1097/01.AOG.0000185260.82466.b4. [DOI] [PubMed] [Google Scholar]
- 14.Bayoumeu F, Subiran-Buisset C, Baka NE, Legagneur H, Monnier-Barbarino P, Laxenaire MC. Iron therapy in iron deficiency anemia in pregnancy: Intravenous route versus oral route. American Journal of Obstetrics and Gynecology. 2002;186(3):518–22. doi: 10.1067/mob.2002.121894. [DOI] [PubMed] [Google Scholar]
- 15.Khalafallah A, Chuang A, Kwok C, et al. Treatment of iron deficiency anaemia of late pregnancy with a single intravenous iron polymaltose or ferric carboxymaltose versus oral iron sulphate: A prospective randomized controlled study (tidal) Haematologica. 2014;99 [Google Scholar]
- 16.Froessler B, Cocchiaro C, Saadat-Gilani K, Hodyl N, Dekker G. Intravenous iron sucrose versus oral iron ferrous sulfate for antenatal and postpartum iron deficiency anemia: a randomized trial. The Journal of Maternal-Fetal & Neonatal Medicine 013. 26(7):654–9. doi: 10.3109/14767058.2012.746299. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal RM, Vineet, Panchal Navin A, Patel Nital H, Deshchougule Vrushali V, Jasani Anil. Evaluation of iron sucrose and oral iron in management of iron deficiency anaemia in pregnancy. National Journal of Community Medicine. 2012;3(1):55–60. [Google Scholar]
- 18.The World Bank. [accessed 24th April 2021];The World Bank Group - Country and Lending Groups. 2015 2015 http://data.worldbank.org/about/country-and-lending-groups . [Google Scholar]
- 19.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions Version 5.1.0. The Cochrane Collaboration; 2011. updated March 2011, www.cochrane-handbookorg2012 . [Google Scholar]
- 20.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. Journal of Clinical Epidemiology. 2011;64(12):1303–10. doi: 10.1016/j.jclinepi.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Nikolakopoulou A, Higgins JP, Papakonstantinou T, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Medicine. 2020;17(4):e1003082. doi: 10.1371/journal.pmed.1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PloS one. 2014;9(7):e99682. doi: 10.1371/journal.pone.0099682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malinowski AK, D’Souza R, Khan KS, Shehata N, Malinowski M, Daru J. Reported Outcomes in Perinatal Iron Deficiency Anemia Trials: A Systematic Review. Gynecol Obstet Invest. 2019:1–18. doi: 10.1159/000495566. [DOI] [PubMed] [Google Scholar]
- 24.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemporary clinical trials. 2007;28(2):105–14. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Harville DA. Maximum likelihood approaches to variance component estimation and to related problems. Journal of the American statistical association. 1977;72(358):320–38. [Google Scholar]
- 26.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 27.White IR. Network meta-analysis. Stata Journal. 2015;15(4):951–85. [Google Scholar]
- 28.Dias S, Welton N, Caldwell D, Ades A. Checking consistency in mixed treatment comparison meta-analysis. Statistics in Medicine. 2010;29(7–8):932–44. doi: 10.1002/sim.3767. [DOI] [PubMed] [Google Scholar]
- 29.Caldwell DM, Welton NJ, Ades AE. Mixed treatment comparison analysis provides internally coherent treatment effect estimates based on overviews of reviews and can reveal inconsistency. Journal of Clinical Epidemiology. 2010;63(8):875–82. doi: 10.1016/j.jclinepi.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 30.Efthimiou O, Debray TP, van Valkenhoef G, et al. GetReal in network meta-analysis: a review of the methodology. Research synthesis methods. 2016;7(3):236–63. doi: 10.1002/jrsm.1195. [DOI] [PubMed] [Google Scholar]
- 31.Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PloS One. 2013;8(10):e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al RA, Unlubilgin E, Kandemir O, Yalvac S, Cakir L, Haberal A. Intravenous Versus Oral Iron for Treatment of Anemia in Pregnancy: A randomized trial. Obstet Gynecol. 2005;106:1335–40. doi: 10.1097/01.AOG.0000185260.82466.b4. [DOI] [PubMed] [Google Scholar]
- 33.al-Momen AK, al-Meshari A, al-Nuaim L, et al. Intravenous iron sucrose complex in the treatment of iron deficiency anemia during pregnancy. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 1996;69(2):121–4. doi: 10.1016/0301-2115(95)02538-3. [DOI] [PubMed] [Google Scholar]
- 34.Arzoo S, Yousof S, Rahman J, Chowdhury S. Iron deficiency anemia in pregnancy: Intravenous iron sucrose versus oral iron sulfate. Bangladesh Journal of Obstetrics and Gynecology. 2020;33(1):40–4. [Google Scholar]
- 35.Borg TF, Labiband KM, Darwish GM. A Comparative Study between Lactoferrin versus ferrous sulfate in iron-deficiency during pregnancy. QJM: An International Journal of Medicine. 2020;113(Supplement_1):hcaa056.06 [Google Scholar]
- 36.Dalal M, Goyal R, Nanda S, Dahiya P, Dahiya K, Madan S. Oral versus intravenous iron for treatment of iron deficiency anaemia in pregnancy: a randomized controlled trial. Indian journal of public health research and development. 2018;9(6):1–6. [Google Scholar]
- 37.Darwish AM, Fouly HA, Saied WH, Farah E. Lactoferrin plus health education versus total dose infusion (TDI) of low-molecular weight (LMW) iron dextran for treating iron deficiency anemia (IDA) in pregnancy: a randomized controlled trial. The Journal of Maternal-Fetal & Neonatal Medicine. 2019;32(13):2214–20. doi: 10.1080/14767058.2018.1429396. [DOI] [PubMed] [Google Scholar]
- 38.Darwish AM, Khalifa EE, Rashad E, Farghally E. Total dose iron dextran infusion versus oral iron for treating iron deficiency anemia in pregnant women: a randomized controlled trial. The Journal of Maternal-Fetal & Neonatal Medicine. 2019;32(3):398–403. doi: 10.1080/14767058.2017.1379988. [DOI] [PubMed] [Google Scholar]
- 39.Gawai S, Fonseca M, Kapote D. A randomized controlled trial on lactoferrin versus ferrous sulphate for the treatment of mild to moderate iron deficiency anaemia in pregnancy. Int J Reprod Contracept Obstet Gynecol. 2020;9(2):562–6. [Google Scholar]
- 40.Han XX, Jiang DC, Sun YY, Li Y. Moderate NaFeEDTA and ferrous sulfate supplementation can improve both hematologic status and oxidative stress in anemic pregnant women. Asia Pac J Clin Nutr. 2011;20(4):514–20. [PubMed] [Google Scholar]
- 41.Hayat Q, Ejaz K, Ahmed A. A randomized control trial to assess the efficacy and safety of iron dextran and iron sucrose among ida affected pregnant women. Indo American Journal of Pharmaceutical Sciences. 2019;6(4):7681–6. [Google Scholar]
- 42.Khalafallah A, Dennis A, Bates J, et al. A prospective randomized, controlled trial of intravenous versus oral iron for moderate iron deficiency anaemia of pregnancy. J Intern Med. 2010;268(3):286–95. doi: 10.1111/j.1365-2796.2010.02251.x. [DOI] [PubMed] [Google Scholar]
- 43.Komolafe JO, Kuti O, Ijadunola KT, Ogunniyi SO. A comparative study between intramuscular iron dextran and oral ferrous sulphate in the treatment of iron deficiency anaemia in pregnancy. J Obstet Gynaecol. 2003;23(6):628–31. doi: 10.1080/01443610310001604394. [DOI] [PubMed] [Google Scholar]
- 44.Kumar A, Jain S, Singh NP, Singh T. Oral versus high dose parenteral iron supplementation in pregnancy. International Journal of Gynaecology and. 2005;89(1):7–13. doi: 10.1016/j.ijgo.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 45.Ma AG, Schouten EG, Sun YY, et al. Supplementation of iron alone and combined with vitamins improves haematological status, erythrocyte membrane fluidity and oxidative stress in anaemic pregnant women. Br J Nutr. 2010;104(11):1655–61. doi: 10.1017/S000711451000259X. [DOI] [PubMed] [Google Scholar]
- 46.Mehta MN, Shah JM. Iron deficiency anemia in pregnancy: intravenous versus oral route. Nat J Comm Med. 2014;5(1):10–2. [Google Scholar]
- 47.Menendez C, Todd J, Alonso PL, et al. The effects of iron supplementation attendants, on the prevalence during pregnancy, of anaemia and malaria. Trans R Soc Trop Med Hyg. 1994;88:590–93. doi: 10.1016/0035-9203(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 48.Neogi SB, Devasenapathy N, Singh R, et al. Safety and effectiveness of intravenous iron sucrose versus standard oral iron therapy in pregnant women with moderate-to-severe anaemia in India: a multicentre, open-label, phase 3, randomised, controlled trial. The Lancet Global Health. 2019;7(12):e1706–e16. doi: 10.1016/S2214-109X(19)30427-9. [DOI] [PubMed] [Google Scholar]
- 49.Preziosi P, Prual A, Galan P, Daouda H, Boureima H, Hercberg S. Effectof ironsupplementationon the ironstatusof pregnantwomen:consequencesfor newborn. Am J Clin Nutr. 1997;66:1178–82. doi: 10.1093/ajcn/66.5.1178. [DOI] [PubMed] [Google Scholar]
- 50.Rajwani S, Kshirsagar N, Patil SK. Randomized clinical trial of iv iron sucrose and iv ferric carboxymaltose in the treatment of moderate iron deficiency anaemia in pregnancy. International journal of Research in Pharmaceutical Sciences. 2020;11(3):4937–43. [Google Scholar]
- 51.Sagaonkar S, Sukhija S, Renu T, Sagaonkar PD. Pregnancy induced iron deficiency and the evaluation and comparison of the efficacy and safety of ferrous fumarate and carbonyl iron in its treatment - PERFECT trial. J Obstet Gynecol India. 2009;59(6):552–62. [Google Scholar]
- 52.Samsudin S, Dulasi M, Sany S, Balanathan K, Chong SE, Ali A. Safety and efficacy of intravenous iron sucrose versus low molecular weight iron dextran for treatment of iron deficiency anemia in pregnancy: A randomized controlled trial. International Journal of Women’s Health. 2020;12:1259–70. doi: 10.2147/IJWH.S281826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santiago M, Olivar J, Reyes L. Comparison of the efficacy of iron amino acid chelate and ferrous sulfate in the treatment of iron deficiency anemia among pregnant women seen at out patient department of a tertiary hospital. Journal of Perinatal Medicine. 2019;47:eA163 [Google Scholar]
- 54.Sharma JB, Jain S, Mallika V, et al. A prospective, partially randomized study of pregnancy outcomes and hematologic responses to oral and intramuscular iron treatment in moderately anemic pregnant women. Am J Clin Nutr. 2004;79:116–22. doi: 10.1093/ajcn/79.1.116. [DOI] [PubMed] [Google Scholar]
- 55.Simmons WK, Cook JD, Bingham KC, et al. Evaluation of a gastric delivery system for iron supplementation in pregancy. Am J Clin Nutr. 1993;58:622–6. doi: 10.1093/ajcn/58.5.622. [DOI] [PubMed] [Google Scholar]
- 56.Singh K, Fong YF, Kuperan P. A comDarison between intravenous iron polymdtose complex (Ferrum Hausman@) and oral ferrous fumarate in the treatment of iron deficiency anaemia in pregnancy. Eur J Haematol. 1998;60:119–24. doi: 10.1111/j.1600-0609.1998.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 57.Suharno D, West CE, Muhilal, Karyadi D, Hautvast JGAJ. Supplementation with vitamin A and iron for nutritional anaemia in pregnant women in West Java, Indonesia. Lancet. 1993;342:1325–28. doi: 10.1016/0140-6736(93)92246-p. [DOI] [PubMed] [Google Scholar]
- 58.Sun YY, Ma AG, Jiang DC, et al. A combination of iron and retinol supplementation benefits iron status, IL-2 level and lymphocyte proliferation in anemic pregnant women. Asia Pac J Clin Nutr. 2010;19(4):513–9. [PubMed] [Google Scholar]
- 59.Tanumihardjo SA. Vitamin A and iron status are improved by vitamin A and iron supplementation in pregnant Indonesian women. J Nutr. 2002;132(7):1909–12. doi: 10.1093/jn/132.7.1909. [DOI] [PubMed] [Google Scholar]
- 60.Van Eijk HG, Kroos MJ, Hoogendoorn GA, Wallenburg HCS. Serum ferritin and iron stores during pregnancy. Clinica Chimica Acta. 1978;83:81–91. doi: 10.1016/0009-8981(78)90210-3. [DOI] [PubMed] [Google Scholar]
- 61.Nanthini R, Mamatha KR, Shivmurthy G, Kavitha A comparative prospective study to assess the efficacy and safety of iron sucrose versus iron sorbitol citric acid in pregnant women with iron deficiency anemia in a tertiary care hospital. Natl J Physiol Pharm Pharmacol. 2017;7(5):545–51. [Google Scholar]
- 62.NCT. Intravenous Versus Oral Iron in Late Pregnancy: Results of Treatment (EIVF) 2015. https://clinicaltrialsgov/ct2/show/NCT00746551 .
- 63.Abhilashini GD, Sagili H, Reddi R. Intravenous iron sucrose and oral iron for the treatment of iron deficiency anaemia in pregnancy. J Clin Diagn Res. 2014;8(5):OC04-7. doi: 10.7860/JCDR/2014/6568.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhavi SB, Jaju PB. Intravenous iron sucrose v/s oral ferrous fumarate for treatment of anemia in pregnancy. A randomized controlled trial. BMC Pregnancy Childbirth. 2017;17(1):137. doi: 10.1186/s12884-017-1313-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Breymann C, Milman N, Mezzacasa A, Bernard R, Dudenhausen J investigators F-A. Ferric carboxymaltose vs oral iron in the treatment of pregnant women with iron deficiency anemia: an international, open-label, randomized controlled trial (FER-ASAP) J Perinat Med. 2017;45(4):443–53. doi: 10.1515/jpm-2016-0050. [DOI] [PubMed] [Google Scholar]
- 66.Dalal M, Goyal R, Nanda S, Dahiya P, Dahiya K, Madan S. Oral versus intravenous iron for treatment of iron deficiency anaemia in pregnancy: a randomized controlled trial. Indian Journal of Public Health Research & Development. 2018;9(6):1. [Google Scholar]
- 67.Deeba S, Purandare SV, Sathe AV. Iron deficiency anemia in pregnancy: Intravenous versus oral route. Journal of Obstetrics and Gynecology of India. 2012;62(3):317–21. doi: 10.1007/s13224-012-0222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Digumarthi L, Cheruku V. Comparison of intravenous versus oral iron in iron deficiency anaemia of pregnancy: FC5. 02. BJOG: an international journal of obstetrics and gynaecology. 2008;115 [Google Scholar]
- 69.Gupta A, Manaktala U, Rathore AM. A randomised controlled trial to compare intravenous iron sucrose and oral iron in treatment of iron deficiency anemia in pregnancy. Indian J Hematol Blood Transfus. 2014;30(2):120–5. doi: 10.1007/s12288-012-0224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nappi C, Tommaselli GA, Morra I, Massaro M, Formisano C, Di C. Efficacy and tolerability of oral bovine lactoferrin compared to ferrous sulfate in pregnant women with iron deficiency anemia: A prospective controlled randomized study. Acta Obstetricia et Gynecologica Scandinavica. 2009;88(9):1031–5. doi: 10.1080/00016340903117994. [DOI] [PubMed] [Google Scholar]
- 71.Neeru S, Nair NS, Rai L. Iron sucrose versus oral iron therapy in pregnancy anemia. Indian Journal of Community Medicine. 2012;37(4):214–8. doi: 10.4103/0970-0218.103467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rezk M, Dawood R, Abo-Elnasr M, Al Halaby A, Marawan H. Lactoferrin versus ferrous sulphate for the treatment of iron deficiency anemia during pregnancy: a randomized clinical trial. The Journal of Maternal-Fetal & Neonatal Medicine. 2016;29(9):1387–90. doi: 10.3109/14767058.2015.1049149. [DOI] [PubMed] [Google Scholar]
- 73.Dhanani JV, Ganguly BP, Chauhan LN. Comparison of efficacy and safety of two parenteral iron preparations in pregnant women. J Pharmacol Pharmacother. 2012;3(4):314–9. doi: 10.4103/0976-500X.103688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fochi F, Ciampini M, Ceccarelli G. Efficacy of iron therapy: A comparative evaluation of four iron preparations administered to anaemic pregnant women. Journal of International Medical Research. 1985;13(1):1–11. doi: 10.1177/030006058501300101. [DOI] [PubMed] [Google Scholar]
- 75.Jose A, Mahey R, Sharma JB, et al. Comparison of ferric Carboxymaltose and iron sucrose complex for treatment of iron deficiency anemia in pregnancy- randomised controlled trial. BMC Pregnancy Childbirth. 2019;19(1):54. doi: 10.1186/s12884-019-2200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kamdi SP, Palkar PJ. Efficacy and safety of ferrous asparto glycinate in the management of iron deficiency anaemia in pregnant women. J Obstet Gynaecol. 2015;35(1):4–8. doi: 10.3109/01443615.2014.930098. [DOI] [PubMed] [Google Scholar]
- 77.Kochhar PK, Kaundal A, Ghosh P. Intravenous iron sucrose versus oral iron in treatment of iron deficiency anemia in pregnancy: a randomized clinical trial. J Obstet Gynaecol Res. 2013;39(2):504–10. doi: 10.1111/j.1447-0756.2012.01982.x. [DOI] [PubMed] [Google Scholar]
- 78.Ortiz R, Toblli JE, Romero JD, et al. Efficacy and safety of oral iron(III) polymaltose complex versus ferrous sulfate in pregnant women with iron-deficiency anemia: a multicenter, randomized, controlled study. The Journal of Maternal-Fetal & Neonatal Medicine. 2011;24(11):1347–52. doi: 10.3109/14767058.2011.599080. [DOI] [PubMed] [Google Scholar]
- 79.Paesano R, Berlutti F, Pietropaoli M, Goolsbee W, Pacifici E, Valenti P. Lactoferrin efficacy versus ferrous sulfate in curing iron disorders in pregnant and non-pregnant women. International Journal of Immunopathology and Pharmacology. 2010;23(2):577–87. doi: 10.1177/039463201002300220. [DOI] [PubMed] [Google Scholar]
- 80.Rudra S, Chandna A, Nath J. Comparison of intravenous iron sucrose with oral iron in pregnant women with iron deficiency anaemia. Int J Reprod Contracept Obstet Gynecol. 2016;5(3):747–51. [Google Scholar]
- 81.Singh S, Singh S, Singh PK. A study to compare the efficacy and safety of intravenous iron sucrose and intramuscular iron sorbitol therapy for anemia during pregnancy. Journal of Obstetrics and Gynecology of India. 2013;63(1):18–21. doi: 10.1007/s13224-012-0248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Symonds EM, Radden HS, Cellier KM. Controlled-release iron therapy in pregnancy. The Australian & New Zealand journal of Obstetrics & Gynaecology. 1969;9(1):21–5. doi: 10.1111/j.1479-828x.1969.tb02520.x. [DOI] [PubMed] [Google Scholar]
- 83.Pavord S, Daru J, Prasannan N, Robinson S, Stanworth S, Girling J. UK guidelines on the management of iron deficiency in pregnancy. Br J Haematol. 2020;188(6):819–30. doi: 10.1111/bjh.16221. [DOI] [PubMed] [Google Scholar]
- 84.Lin L, Xing A, Kofler MJ, Murad MH. Borrowing of strength from indirect evidence in 40 network meta-analyses. Journal of Clinical Epidemiology. 2019;106:41–9. doi: 10.1016/j.jclinepi.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.World Health Organization. WHO recommendations on antenatal care for a positive pregnancy experience. World Health Organization; Geneva, Switzerland: 2016. [PubMed] [Google Scholar]
- 86.Moller A-B, Petzold M, Chou D, Say L. Early antenatal care visit: a systematic analysis of regional and global levels and trends of coverage from 1990 to 2013. The Lancet Global Health. 2017;5(10):e977–e83. doi: 10.1016/S2214-109X(17)30325-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haider BA, Olofin I, Wang M, Spiegelman D, Ezzati M, Fawzi WW. Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: Systematic review and meta-analysis. BMJ (Online. 2013;347(7916) doi: 10.1136/bmj.f3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peña-Rosas JP, De-Regil LM, Gomez Malave H, Flores-Urrutia MC, Dowswell T. Intermittent oral iron supplementation during pregnancy. Cochrane Database of Systematic Reviews. 2015:10. doi: 10.1002/14651858.CD009997.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qassim A, Mol BW, Grivell RM, Grzeskowiak LE. Safety and efficacy of intravenous iron polymaltose, iron sucrose and ferric carboxymaltose in pregnancy: A systematic review. Aust N Z J Obstet Gynaecol. 2017 doi: 10.1111/ajo.12695. [DOI] [PubMed] [Google Scholar]
- 90.Daru J, Cooper NA, Khan KS. Systematic review of randomized trials of the effect of iron supplementation on iron stores and oxygen carrying capacity in pregnancy. Acta Obstet Gynecol Scand. 2016;95(3):270–9. doi: 10.1111/aogs.12812. [DOI] [PubMed] [Google Scholar]
- 91.Neogi SB, Devasenapathy N, Singh R, et al. Safety and effectiveness of intravenous iron sucrose versus standard oral iron therapy in pregnant women with moderate-to-severe anaemia in India: a multicentre, open-label, phase 3, randomised, controlled trial. The Lancet Global health. 2019;7(12):e1706–e16. doi: 10.1016/S2214-109X(19)30427-9. [DOI] [PubMed] [Google Scholar]
- 92.Daru J. Iron interventions in pregnancy and better clinical outcomes: the jury is out. The Lancet Global health. 2019;7(12):e1597–e8. doi: 10.1016/S2214-109X(19)30468-1. [DOI] [PubMed] [Google Scholar]
- 93.Randomized controlled trial of the effect of intravenous iron on anaemia in Malawian pregnant women (REVAMP): Third Trimester. 2020. [accessed 24th April 2021]. https://true.mw/revamp-tt/ [DOI] [PMC free article] [PubMed]
- 94.Stoffel NU, Zeder C, Brittenham GM, Moretti D, Zimmermann MB. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica. 2020;105(5):1232–9. doi: 10.3324/haematol.2019.220830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data collected for this systematic review and network meta-analysis can be shared on request, with investigator support and approval thorough a signed data access agreement. This includes aggregate study level data collected, cleaned with a data dictionary and the statistical analysis plan. This will be made available with publication. No additional data are available.