Abstract
Background and Purpose
UK black African-Caribbean adults have higher risks of stroke than white Europeans and have been shown to have increased carotid intima-media thickness (cIMT). We examined whether corresponding ethnic differences in cIMT were apparent in childhood and, if so, whether these could be explained by ethnic differences in cardiovascular risk markers.
Methods
We conducted a 2-stage survey of 939 children (208 white European, 240 black African-Caribbean, 258 South Asian, 63 other Asian, 170 other ethnicity), who had a cardiovascular risk assessment and measurements of cIMT at mean ages of 9.8 and 10.8 years, respectively.
Results
Black African-Caribbean children had a higher cIMT than white Europeans (mean difference, 0.014 mm; 95% CI, 0.008-0.021 mm; P<0.0001). cIMT levels in South Asian and other Asian children were however similar to those of white Europeans. Among all children, cIMT was positively associated with age, systolic and diastolic blood pressure and inversely with combined skinfold thickness and serum triglyceride. Mean triglyceride was lower among black African-Caribbeans than white Europeans; blood pressure and skinfold thickness did not differ appreciably. However, adjustment for these risk factors had little effect on the cIMT difference between black African-Caribbeans and white Europeans.
Conclusions
UK black African-Caribbean children have higher cIMT levels in childhood; the difference is not explained by conventional cardiovascular risk markers. There may be important opportunities for early cardiovascular prevention, particularly in black African-Caribbean children. (Stroke. 2012;43:00-00.)
Keywords: carotid, intima-media, thickness, childhood, ethnicity
Both in the United Kingdom and in the United States, adults of black African and black Caribbean origin have higher risks of stroke than whites of European origin. 1–3 Carotid intima-media thickness (cIMT) is a strong predictor of stroke and other cardiovascular diseases in healthy adults. 4–6 In population-based studies, black African-Caribbean adults have greater common cIMT than white Europeans, both in the United Kingdom 7 and in the United States. 8–12 Higher blood pressure (BP) levels and an increased prevalence of hypertension among black African-Caribbeans appear to contribute both to increased stroke risk 13,14 and to increased cIMT. 10,11 Among UK South Asians adults, stroke risks are increased, but cIMT levels are similar to or lower than those in white Europeans. 15
The origins of stroke may lie in childhood 16,17 or possibly in infancy or in utero. 17–20 Differences in stroke and cardiovascular disease risk factors between black African-Caribbeans and white Europeans have been reported in childhood and adolescence both in the United Kingdom 21 and in the United States. 22,23 However, information on cIMT levels in childhood in these populations is very limited 24,25 and the extent to which any ethnic differences in cIMT can be explained by differences in BP or other cardiovascular risk factors remains uncertain. We have therefore examined these issues in a study of the cardiovascular health in UK children of black African-Caribbean and white European origin; we also report on similar analyses in children of Asian origin.
Methods
This investigation was carried out within the Child Heart and Health Study in England (CHASE Study), a school-based survey of the cardiovascular health of British primary school children living in London, Leicester, and Birmingham. Full details of the study design have been reported elsewhere. 21 Ethical approval was obtained from the relevant Multicenter Research Ethics Committee. Informed written consent was obtained from parents or guardians at each stage of the investigation. The main study was based in a sample of 200 primary schools providing balanced numbers of children of South Asian origin (including Indians, Pakistanis, and Bangladeshis), black African-Caribbean origin (including black Africans and black Caribbeans), and white European origin. The present investigation was based in the 46 final schools, of which 43 (93%) allowed the CHASE Research Team to return to carry out cIMT measurements in pupils who had previously participated in the main cardiovascular risk factor survey.
Cardiovascular Risk Factor Assessment
A team of 3 trained research nurses and a support fieldworker carried out all measurements between June 2006 and February 2007. Participating children provided a blood sample after an overnight fast and had measurements of height, weight, and waist circumference. Right-sided skinfold thicknesses were measured in 4 sites (biceps, triceps, subscapular, suprailiac); analyses are based on the sum of the 4 measurements. Leg to arm bioimpedance was measured using the Bodystat 1500 bioimpedance monitor (Bodystat Ltd, Isle of Man, UK); fat mass was derived using equations derived specifically for children using dual energy x-ray absorptiometry validation 26 and presented as a fat mass index (fat mass/height 5 ), which was independent of height. Seated BP was measured twice in the right arm after 5 minutes rest using an Omron 907 BP recorder with an appropriately sized cuff; the average of the 2 measures was used. Pubertal status was measured in the girls using Tanner scales. 27 Objective physical activity measurements (described in detail elsewhere 28 ) were made with an Actigraph GT1M activity monitor (ActiGraph, Pensacola, FL), which children wore over the left hip for 7 days. Physical activity data (recorded at 5-second epochs) were downloaded and activity counts per minute were derived. Dietary nutrient intakes were recorded using a structured 24-hour recall method. 29 Participating children provided questionnaire information on parental and grandparental country of birth. The parent or guardian provided information on the ethnicity of both parents and that of the child (coded using a classification similar to the 2001 UK Census) and on their occupation, coded using the National Statistics Socioeconomic Classification. Participant ethnicity was defined using the ethnicity of both parents or the child; in 1% of cases in which parental information was not available, child information on place of birth of parents and grandparents was used to define ethnic origin. 21 All laboratory analyses were carried out blind to participant ethnicity. Analyses of HbA1c, glucose, and blood lipids were carried out in the Department of Clinical Biochemistry, Newcastle Hospitals National Health Service Trust, which received blood samples within 48 hours of collection. Glucose was measured in plasma using the hexokinase method. HbA1c was measured in whole blood by ion exchange high-performance liquid chromatography; HbA1c values were recalculated to adjust for abnormal hemoglobin variants or for increased amounts of normal variant fetal hemoglobin where present. Triglyceride and high-density lipoprotein cholesterol were measured in serum using an Olympus autoanalyzer. Serum, separated and frozen on dry ice after collection, was used for measurement of insulin (Department of Medicine, University of Newcastle, Newcastle, UK) using an enzyme-linked immunosorbent assay method, which does not crossreact with proinsulin and C-reactive protein, which was assayed by ultrasensitive nephelometry (Dade Behring, Milton Keynes, UK). The homeostasis model assessment model equations were used to provide an estimate of insulin resistance. 30
cIMT Measurements
A single research team including 3 vascular technicians carried out all cIMT measurements between June 2007 and April 2008. Both the left and right common carotid arteries were imaged longitudinally 1 cm proximal to the carotid bifurcation following a standardized protocol and using a Zonare ultrasound scanner with a high-resolution probe (Zonare Medical System, Inc, Mountain View, CA). Images were focused on the posterior (far) wall of the artery and the zoom function was used to magnify the area. Ten-second cine loops were recorded in DICOM format and downloaded for offline analysis. Three end-diastolic frames were selected and analyzed for mean cIMT, defined as the interface between lumen-intima and media-adventitia, for both right and left carotid arteries using an automated carotid analyzer (Carotid Analyzer, Iowa City, IA). The images were analyzed by 3 trained readers blinded to participant ethnicity; the mean of the left- and right-sided readings was used in analysis. However, separate analyses based on left- and right-sided measurements yielded essentially similar results.
Statistical Analysis
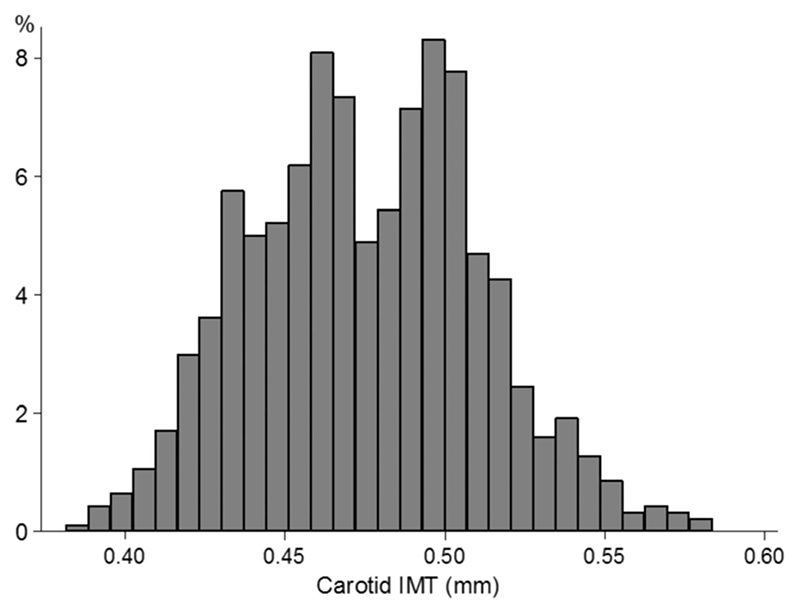
Statistical analyses were carried out using STATA/SE software (Stata/SE 10.1 for Windows; StataCorp LP, College Station, TX). cIMT was approximately normally distributed (Figure). Some markers of adiposity and cardiovascular risk were positively skewed and required log transformation when treated as outcome variables; these included ponderal index, fat mass index, waist circumference, insulin, triglyceride, and C-reactive protein. Sex and ethnic differences in these variables were examined as fixed effects using multilevel linear regression models with school as a random effect to allow for the clustering of children within school. A similar approach was applied to define the absolute difference in cIMT for a 1-SD increase in each cardiovascular risk factor (log-transformed where appropriate) with preliminary examination of data to ensure that assumptions of linearity were robust. All analyses were adjusted for age (except age differences), sex (except sex differences), ethnicity (except ethnic differences), observer (physical measurements only), and month; all were fitted as fixed effects. Tests for interaction were used to examine whether associations between cardiovascular risk factors and cIMT differed by sex or ethnic group.
Figure. Distribution of carotid intima-media thickness.
Results
Among 1409 pupils who had participated in the main CHASE Risk Factor Study in these 43 schools, 939 (67%) participated and had complete cIMT measurements. Participation rates were similar among white Europeans, South Asians, and other Asians (72%, 72%, and 70%, respectively) but slightly lower among black African-Caribbeans and other ethnic groups (61% and 66%, respectively). The mean age of participants at the initial cardiovascular risk survey was 9.8 years and at the cIMT survey was 10.8 years; 47% of participants were boys. The demographic and cardiovascular risk factor characteristics of children in the cardiovascular risk factor survey who did and did not take part in the cIMT survey did not differ appreciably (data not presented).
Sex and Ethnic Differences in cIMT
The distribution of cIMT is summarized in the Figure and cIMT associations with sex and ethnicity in Table 1. There was considerable variation between individuals (SD 0.035 mm, fifth centile 0.420 mm, 95th centile 0.535 mm); overall values were slightly higher in boys than girls. Black African-Caribbeans had a higher cIMT than white Europeans (mean difference, 0.014 mm; 95% CI, 0.008–0.021 mm; P<0.0001), whereas South Asian and other Asian children had cIMT levels similar to those of white Europeans. There were no marked differences in cIMT levels between black African and black Caribbean children or between South Asian children of Indian, Pakistani, or Bangladeshi origin (data not presented).
Table 1. Carotid IMT: Associations With Sex and Ethnicity.
| No. | Mean Carotid IMT, mm (95% CI) | P Value (difference)* | |
|---|---|---|---|
| Boys | 444 | 0.479 (0.475–0.483) | 0.005 |
| Girls | 495 | 0.473 (0.469–0.476) | |
| White European | 208 | 0.472 (0.467–0.477) | |
| Black African-Caribbean | 240 | 0.487 (0.482–0.492) | <0.0001 |
| South Asian | 258 | 0.469 (0.463–0.474) | 0.27 |
| Asian other | 63 | 0.476 (0.467–0.485) | 0.45 |
| Other | 170 | 0.475 (0.470–0.481) | 0.43 |
All means are adjusted for age group, sex (except by sex), ethnicity (except by ethnic group), month, and random effect for school.
IMT indicates intima-media thickness.
P values compare differences between girls and boys and between white European children and children of each specific other ethnic group.
cIMT: Influence of Age, Body Build, and Cardiovascular Risk Factors
Levels of age, body build, and cardiovascular risk factors in the study population (means and SDs) and their associations with cIMT in the whole study population (adjusted for ethnic group) are shown in Table 2. Age, systolic and diastolic BP showed positive associations with cIMT. Physical activity (counts per minute) showed a weak positive association with cIMT at the margins of statistical significance. Sum of skinfolds and triglyceride level were inversely related to cIMT, although other adiposity measures (ponderal index, fat mass index) showed little association. Other blood lipids, insulin and insulin resistance, HbA1c, and C-reactive protein showed no consistent associations with cIMT. The association between triglyceride and cIMT was however attenuated after adjustment for adiposity (P=0.06). The associations observed in the whole study population for age, BP, adiposity markers, and triglyceride were similar in boys and girls and in children from different ethnic groups (all relevant tests for sex*risk factor interaction and ethnicity*risk factor interaction, P>0.1). Childhood dietary intakes (macronutrient and micronutrient) showed no associations with cIMT (data not presented).
Table 2. Associations Between Cardiometabolic Risk Factors and Carotid IMT.
| Anthropometry and Other Markers (N=939) | Mean† | SD† | Difference in IMT, mm, per SD Increase of Marker (95% CI)‡ | P Value (difference) |
|---|---|---|---|---|
| Age, y | 10.8 | 0.4 | 0.0024 (–0.0004 to 0.0052) | 0.10 |
| Height, cm | 139.2 | 7.0 | 0.0011 (–0.0011 to 0.0034) | 0.33 |
| Weight, kg* | 35.3 | 1.3 | 0.0002 (–0.0020 to 0.0025) | 0.83 |
| Ponderal Index, kg/m3 * | 13.1 | 1.2 | –0.0007 (–0.0029 to 0.0015) | 0.54 |
| Sum of skinfolds, mm* | 41.7 | 1.6 | –0.0026 (–0.0048 to –0.0004) | 0.02 |
| Fat mass index, kg/m5 * | 1.9 | 1.5 | –0.0019 (–0.0041 to 0.0003) | 0.09 |
| Systolic BP, mm Hg | 104.4 | 10.6 | 0.0024 (0.0002–0.0046) | 0.03 |
| Diastolic BP, mm Hg | 63.2 | 9.0 | 0.0027 (0.0005–0.0048) | 0.01 |
| Physical activity CPM§ | 468.9 | 105.2 | 0.0030 (0.0000–0.0061) | 0.05 |
| Blood markers (N=831) | ||||
| Total cholesterol, mmol/L* | 4.5 | 1.2 | –0.0013 (–0.0036 to 0.0010) | 0.27 |
| LDL cholesterol, mmol/L* | 2.6 | 1.3 | –0.0005 (–0.0029 to 0.0019) | 0.67 |
| HDL cholesterol, mmol/L | 1.5 | 0.3 | 0.0000 (–0.0024 to 0.0023) | 0.97 |
| Triglyceride, mmol/L* | 0.9 | 1.5 | –0.0031 (–0.0055 to -0.0007) | 0.01 |
| HbA1c, % | 5.3 | 0.3 | 0.0000 (–0.0024 to 0.0023) | 0.97 |
| Glucose, mmol/L | 4.5 | 0.3 | 0.0012 (–0.0011 to 0.0036) | 0.30 |
| Insulin, mU/L* | 7.1 | 1.8 | –0.0015 (–0.0039 to 0.0010) | 0.24 |
| Insulin resistance* | 0.9 | 1.8 | –0.0010 (–0.0034 to 0.0014) | 0.42 |
| C-reactive protein, mg/L* | 0.5 | 3.7 | –0.0015 (–0.0038 to 0.0008) | 0.20 |
IMT indicates intima-media thickness; BP, blood pressure; CPM, counts per minute; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Log-transformed variable.
Geometric means and geometric SD presented for log-transformed variable.
Difference in IMT for SD (or log SD) for log-transformed variables adjusted for sex, age group, ethnicity, month, and random effect for school.
Analyses based on 728 subjects.
Ethnic Differences in Body Build and Cardiovascular Risk Factors: Contribution to Ethnic Differences in cIMT
Patterns of body build and cardiovascular risk factors in different ethnic groups are summarized in Table 3. Black African-Caribbeans were markedly taller and heavier, although adiposity measures (ponderal index, sum of skinfolds, and fat mass index), BP (both systolic and diastolic), and physical activity level were not appreciably different from those in white Europeans. They had lower total and low-density lipoprotein cholesterol and triglyceride, whereas their HbA1c, fasting insulin, and homeostasis model assessment insulin resistance were higher. South Asian children were lighter than white Europeans, had a lower ponderal index, systolic BP, and physical activity levels than white Europeans, whereas their HbA1c, fasting insulin, insulin resistance, triglyceride, and C-reactive protein levels were higher. Children of other Asian origins tended to have lower low-density lipoprotein cholesterol and physical activity levels and higher triglyceride and HbA1c levels. Children of other ethnicity had higher fasting insulin, insulin resistance, and C-reactive protein levels compared with white Europeans.
Table 3. Anthropometry and Blood Markers in Children From Different Ethnic Groups.
| Anthropometry and Other Markers | White European (n=208) Mean (95% CI) | Black African-Caribbean (n=240) | South Asian (n=258) | Asian Other (n = 63) | Other (n=170) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (95% CI) | P Value (difference) | Mean (95% CI) | P Value (difference) | Mean (95% CI) | P Value (difference) | Mean (95% CI) | P Value (difference) | ||
| Age, y | 10.82 (10.73–10.91) | 10.78 (1 0.69–1 0.86) | 0.15 | 10.79 (10.70–10.87) | 0.28 | 10.83 (10.72–10.94) | 0.90 | 10.81 (10.72–10.90) | 0.70 |
| Height, cm | 138.2 (137.2–139.1) | 142.3 (141.4–143.2) | <0.0001 | 137.1 (136.1–138.0) | 0.09 | 138.9 (137.1–140.6) | 0.50 | 139.3 (138.26–140.35) | 0.11 |
| Weight, kg* | 35.0 (33.9–36.2) | 37.7 (36.6–38.9) | <0.001 | 32.9 (31.8–34.0) | 0.01 | 34.9 (33.0–37.0) | 0.94 | 36.0(34.75–37.31) | 0.25 |
| Ponderal Index, kg/m3 * | 13.3 (13.1–13.6) | 13.2 (12.9–13.4) | 0.39 | 12.8 (12.5–13.1) | 0.01 | 13.1 (12.6–13.6) | 0.39 | 13.4(13.04–13.68) | 0.94 |
| Sum of skinfolds, mm* | 42.2 (39.6–45.1) | 39.9 (37.5–42.5) | 0.21 | 42.5 (39.8–45.3) | 0.90 | 43.5 (38.6–49.0) | 0.67 | 41.7 (38.77–44.81) | 0.79 |
| Fat mass index, kg/m5 * | 1.89 (1.78–2.00) | 1.87 (1.77–1.98) | 0.86 | 1.89 (1.78–2.00) | 0.99 | 1.87 (1.69–2.08) | 0.92 | 1.97 (1.85–2.10) | 0.33 |
| Systolic BP, mm Hg | 105.1 (103.6–106.6) | 104.8 (103.3–106.2) | 0.73 | 102.5 (100.9–104.0) | 0.01 | 105.5 (102.8–108.3) | 0.77 | 105.7 (104.03–107.36) | 0.59 |
| Diastolic BP, mm Hg | 63.2 (62.0–64.4) | 63.6 (62.5–64.8) | 0.62 | 62.7 (61.5–63.9) | 0.55 | 64.2 (61.9–66.4) | 0.47 | 62.8(61.48–64.21) | 0.70 |
| Physical activity— CPM† | 483.0 (467.8–498.1) | 492.4 (477.8–507.0) | 0.30 | 436.3 (419.4–453.3) | <0.0001 | 428.4 (404.1–452.7) | <0.0001 | 489.4 (472.3–506.4) | 0.53 |
| Blood markers | (n=195) | (n=197) | (n=229) | (n=53) | (n=157) | ||||
| Total cholesterol, mmol/L* | 4.57 (4.46–4.68) | 4.32 (4.22–4.43) | 0.001 | 4.51 (4.41–4.62) | 0.50 | 4.37 (4.17–4.58) | 0.09 | 4.51 (4.39–4.63) | 0.48 |
| LDL cholesterol, mmol/L* | 2.68 (2.58–2.78) | 2.46 (2.37–2.55) | 0.001 | 2.63 (2.53–2.73) | 0.48 | 2.48 (2.31–2.66) | 0.06 | 2.62(2.51–2.72) | 0.41 |
| HDL cholesterol, mmol/L | 1.58 (1.54–1.63) | 1.55 (1.51–1.60) | 0.31 | 1.52 (1.47–1.56) | 0.04 | 1.51 (1.43–1.60) | 0.15 | 1.55(1.50–1.60) | 0.29 |
| Triglyceride, mmol/L* | 0.82 (0.78–0.87) | 0.75 (0.71–0.80) | 0.02 | 0.96 (0.90–1.02) | <0.0001 | 0.93 (0.84–1.04) | 0.03 | 0.87 (0.82–0.93) | 0.15 |
| HbA1c, % | 5.21 (5.16–5.26) | 5.33 (5.28–5.38) | <0.001 | 5.36 (5.30–5.41) | <0.0001 | 5.31 (5.22–5.40) | 0.07 | 5.27 (5.22–5.33) | 0.09 |
| Glucose, mmol/L | 4.45 (4.40–4.49) | 4.47 (4.42–4.52) | 0.51 | 4.50 (4.45–4.55) | 0.13 | 4.41 (4.32–4.50) | 0.53 | 4.46(4.41–4.52) | 0.61 |
| Insulin, mU/L* | 5.80 (5.29–6.36) | 7.29 (6.64–7.99) | <0.001 | 8.11 (7.37–8.94) | <0.0001 | 6.66 (5.65–7.86) | 0.13 | 7.51 (6.79–8.29) | <0.0001 |
| Insulin resistance* | 0.73 (0.66–0.79) | 0.92 (0.84–1.01) | <0.0001 | 1.01 (0.92–1.11) | <0.0001 | 0.81 (0.68–0.95) | 0.26 | 0.94(0.85–1.04) | <0.0001 |
| C-reactive protein, mg/L* | 0.46 (0.39–0.55) | 0.52 (0.43–0.63) | 0.36 | 0.63 (0.53–0.76) | 0.02 | 0.49 (0.34–0.69) | 0.78 | 0.60(0.49–0.74) | 0.06 |
Means and P values for differences are adjusted for sex, age group, ethnicity, month (bimonthly groups), and random effect for school. BP indicates blood pressure; CPM, counts per minute; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Geometric means shown for log-transformed variables.
Analyses based on 728 subjects.
Ethnic differences in common carotid intima-media thickness were adjusted for ethnic differences in BP, adiposity, blood lipids and insulin resistance, and HbA1c (Table 4). The difference in cIMT between black African-Caribbeans and white Europeans was not materially affected by individual adjustments for BP, adiposity, blood lipids, glucose, and HbA1c; in combination, these factors reduced the differences by less than one fourth. Additional adjustments for physical activity levels (counts per minute), socioeconomic status, C-reactive protein, height, and childhood diet (both macronutrient and micronutrient intakes) made little additional difference (data not presented). Among South Asian and other Asian children (who showed no appreciable difference in cIMT from white Europeans), individual and combined adjustments for these risk factors had no impact on the patterns observed. The sex difference in cIMT was also not abolished by adjustment for these risk factors (data not presented).
Table 4. Ethnic Differences in Carotid IMT Showing the Effect of Adjustments for Cardiometabolic Risk Factors.
| Additional Adjustments (N=833) | Mean Difference in Carotid IMT, mm (95% CI) | ||
|---|---|---|---|
| Black African Caribbean–White European | South Asian–White European | Asian Other–White European | |
| None | 0.0163 (0.0095–0.0231) | –0.0031 (–0.0100 to 0.0039) | 0.0051 (–0.0056 to 0.0157) |
| Systolic and diastolic BP | 0.0164 (0.0096–0.0232) | -0.0028 (-0.0098 to 0.0041) | 0.0049 (-0.0057 to 0.0156) |
| Adiposity (PI, sum of skinfolds, and FMI) | 0.0162 (0.0094–0.0229) | 0.0000 (–0.0071 to 0.0071) | 0.0064 (–0.0042 to 0.0170) |
| Lipids (LDL and HDL cholesterol and triglyceride) | 0.0154 (0.0085–0.0222) | -0.0019 (–0.0089 to 0.0051) | 0.0057 (–0.0050 to 0.0164) |
| Insulin and HbA1c | 0.0170(0.0101–0.0239) | –0.0023 (–0.0095 to 0.0048) | 0.0053 (-0.0053 to 0.0160) |
| BP, adiposity, lipids, insulin, and HbA1c | 0.0157(0.0087–0.0227) | 0.0014 (–0.0059 to 0.0087) | 0.0066 (–0.0041 to 0.0172) |
Analyses adjusted for sex, age group, ethnicity, month, and random effect for school.
IMT indicates intima-media thickness; BP, blood pressure; PI, Ponderal Index; FMI, fat mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Discussion
UK black African-Caribbean adults have higher stroke mortality rates 1 and higher levels of cIMT, 7 a strong predictor of stroke risk. 6 The present study extends these observations by reporting for the first time that cIMT levels are higher in black African-Caribbean children than in white European children at the end of the first decade of life. The study also provides novel large-scale information on the influence of cardiovascular risk factors on cIMT in childhood.
Relation to Earlier Studies
The results of this study are consistent with previous reports in showing appreciable variation between individuals in cIMT as early as 10 years of age. The overall average cIMT level observed in the present study (0.48 mm) is consistent with earlier studies of children focusing on a similar mean age, which reported combined mean cIMTs between 0.42 mm and 0.48 mm. 31–33 The SD of cIMT in the present study (0.035 mm) is likewise similar to previous estimates in children of a similar age range (0.03–0.04 mm). 31,33 The pattern of sex differences, with slightly higher cIMT levels observed in boys than girls, is consistent with the findings of earlier studies both in children 25,33 and in adults. 34 In the present study, BP (both systolic and diastolic) was a major determinant of cIMT; this is consistent with previous reports of the determinants of cIMT in adults 35–37 and in children. 38,39 However, in the present study, low-density lipoprotein cholesterol was unrelated to childhood cIMT, whereas skinfold thickness and triglyceride levels were inversely related. Although earlier studies have suggested that both adult and childhood low-density lipoprotein cholesterol levels are associated with adult cIMT, 40–42 previous evidence for an association between low-density lipoprotein cholesterol and cIMT in childhood is primarily based on small studies comparing children with and without familial hypercholesterolemia, in whom low-density lipoprotein cholesterol differences are marked. 43–47 Our report is however consistent with earlier studies of healthy children in which no associations between total or low-density lipoprotein cholesterol and cIMT were observed. 25,33 The inverse association between triglyceride and childhood cIMT in the present study was markedly attenuated after adjustment for adiposity; the absence of an independent association between triglyceride and childhood cIMT is again consistent with earlier findings. 25 However, the present study is unusual in not observing a positive association between adiposity and childhood cIMT; most previous studies have reported that both marked obesity 48,49 and variation in adiposity in the healthy population 25 are associated with higher childhood cIMT. The reason for this difference is not clear, although the inverse association was only observed for skinfold thickness; there was no appreciable association between other adiposity markers (ponderal index and fat mass index) and cIMT. Although earlier small studies in selected populations suggested that C-reactive protein might be associated with increased cIMT, 31,32 the present, very much larger study shows little evidence of any association between C-reactive protein and cIMT. The higher cIMT levels observed in black African-Caribbeans compared with white Europeans are consistent with adult findings both in the United Kingdom 7 and the United States 4,5,50 and with 1 study in US children. 25 The difference in cIMT in the present study between black African-Caribbean and white European children (0.014 mm) is very similar to that reported in US children 25 and is approximately one third of the absolute difference in middle-aged adults. The failure of conventional cardiovascular risk factors (particularly BP) to explain black African-Caribbean differences in cIMT is consistent with previous reports in adults. 10,11 The absence of appreciable differences in cIMT among South Asians, other Asians, and white Europeans is consistent with the results of an earlier study of UK adults of South Asian and white European origin. 15 The results of other South Asian–white European comparisons of carotid IMT have been inconsistent, with higher levels in rural Indians than Australians 51 but lower levels in South Asian Canadians than Europeans. 52
Strengths and Limitations
The strengths of the present, cross-sectional study include its size (sufficiently large to detect modest differences of 0.4 SD in risk markers between major ethnic groups) and its representation of black African-Caribbean children of both African and Caribbean origin and South Asian children of Indian, Pakistani, and Bangladeshi origin drawn from 3 UK cities accounting for most black African-Caribbeans and South Asians living in the United Kingdom. Classification of ethnicity was based primarily on self-reported parental ethnicity, which agreed closely with ethnicity defined using other approaches, particularly parental and grandparental place of origin. Important potential limitations of the study include its cross-sectional nature, which does not allow us to conclude directly that the higher cIMT level observed in black African-Caribbean children will lead directly to higher adult stroke risk, and the moderate response rates (particularly among black African-Caribbeans). However, response rates were comparable with those of many previous studies; the similar characteristics of responders and nonresponders suggest that limited response rates are unlikely to have accounted for the risk marker patterns observed. Moreover, the patterns and determinants of cIMT observed in the present study are generally consistent with those of earlier population-based studies in children 25 with the exception of the inverse association observed between adiposity (particularly skinfold thickness) and cIMT.
Explanations for cIMT Patterns
The reasons for the higher cIMT levels among black African-Caribbean children remain uncertain. Although BP level was an important determinant of cIMT in the present study, neither this nor other established cardiovascular risk factors could account for the observed differences in cIMT between black African-Caribbeans and white Europeans. BP variability, increased in US black children 53 and which predicts stroke risk independently of BP level, 54 merits further study here. Physical activity, dietary factors, and low birth weight, all of which have been related to cIMT in childhood, or to aortic intima-media thickness in infancy, could be important. 55–57 In the present study, objectively measured physical activity levels were similar in black African-Caribbean and white European children, although lower in South Asians; these differences did not explain ethnic differences in cIMT. Black African-Caribbeans had markedly lower saturated fat intake and higher carbohydrate intake than white Europeans 29 ; these were however unrelated to cIMT level (data not presented). Factors operating before birth could be important. Delayed fetal growth and low birth weight are implicated in the development of cardiovascular risk 17 and related to aortic intima-media thickness in neonates. 56,57 However, in the UK population, South Asians rather than black African-Caribbeans have the lowest mean birth weight and the highest prevalence of low birth weight. 58 Maternal factors, particularly maternal cigarette smoking and low maternal energy intake, are associated with increased offspring cIMT 38,59 and need further exploration. Genetic factors could also be important, although their role remains uncertain. 24 The reason for the higher cIMT levels observed in boys remains unclear, although it does not appear to reflect sex differences in the risk factors measured in the present study (and reported in detail elsewhere 21 ); this finding is consistent with earlier reports. 25
Implications for Stroke Risk and Prevention
Although the results of the present, cross-sectional study should be interpreted with care, the higher levels of cIMT in black African-Caribbean children suggest that it is possible that the higher stroke risks currently occurring in UK adult black African-Caribbeans 1 will persist in the next adult generation. However, if the observed differences in childhood cIMT were to persist into adult life at their present size, their impact on future overall stroke risk (based on the association between cIMT and cardiovascular disease risk reported in a systematic review of adults 6 ) would be modest. A mean cIMT difference of 0.014 mm between black African-Caribbeans and white Europeans could represent an increase in overall stroke rate of 2.3% (based on the absolute intima-media thickness difference) or an increase of 11.7% (based on the SD difference). However, it is also possible that the size of the cIMT difference (and hence its effect on risk) would increase with age to approach (or even exceed) those currently observed in adults. The implications of the results for different stroke subtypes are difficult to assess conclusively. In the United Kingdom, the proportions of ischemic and hemorrhagic stroke are similar among white Europeans and black African-Caribbeans, although 1 ischemic subtype, lacunar stroke, is more prevalent in black African-Caribbeans. 60 Although higher cIMT is associated with increased risk of all stroke subtypes, the impact of raised cIMT may be slightly weaker for lacunar stroke than for other ischemic stroke subtypes. 61 However, until further evidence is available, it would be premature to conclude that the consequences of higher cIMT are less adverse for black African-Caribbeans than white Europeans. The sex difference in childhood cIMT, with boys having higher cIMT levels than girls, could play an important part in emerging sex differences in stroke risk. However, the impact would only be marked if the sex difference were to increase with age; the observed sex difference in cIMT (0.006 mm, less than half the size of the observed ethnic difference) would have only a modest effect on stroke risk. 6
These results may be important for early stroke prevention in the next generation, both for individuals and for specific ethnic groups. The association between BP and cIMT suggests that BP levels in these children are sufficiently high to have adverse effects on the arterial vasculature. In adults, it has been shown that treatment with the BP-lowering calcium antagonist amlodipine reverses the age-related increase in cIMT. 62 Strategies for BP reduction in childhood, particularly based on population-wide non-pharmacological approaches, could help to prevent stroke and other cardiovascular disease in the longer term. Other studies have suggested that favorable changes in diet and physical health behavioral changes could have independent beneficial effects on childhood cIMT, 55 although these exposures showed little association with cIMT in the present study. Further understanding of the reasons for the early emergence of higher levels of cIMT in black African-Caribbeans could facilitate the development of effective strategies for early cardiovascular prevention, particularly in the UK black African-Caribbean population.
Conclusions
Childhood cIMT levels vary between individuals, are associated with BP, and are higher in children of black African-Caribbean origin than in white Europeans and South Asians. Strategies to reduce cIMT levels from childhood onward could help to reduce long-term risks of stroke and other cardiovascular disease, perhaps especially in black African-Caribbeans.
Acknowledgments
We are grateful to the schools, parents, and children who participated in the CHASE Study. We acknowledge the contribution to this study made by Ann Donald, who died during the preparation of the article.
Sources of Funding
This work was supported by project grants from the British Heart Foundation (PG/07/033/22770) and the Wellcome Trust (068362/Z/02/Z).
Footnotes
Disclosures
None.
Contributor Information
Peter H. Whincup, Population Health Research Centre University of Glasgow, Glasgow, UK.
Claire M. Nightingale, Population Health Research Centre University of Glasgow, Glasgow, UK.
Christopher G. Owen, Population Health Research Centre University of Glasgow, Glasgow, UK.
Alicja Rapala, Population Health Research Centre University of Glasgow, Glasgow, UK; Division of Population Health Sciences and Education, St George’s, University of London, London, UK; the Vascular Physiology Unit University of Glasgow, Glasgow, UK.
Devina J. Bhowruth, Population Health Research Centre University of Glasgow, Glasgow, UK; Division of Population Health Sciences and Education, St George’s, University of London, London, UK; the Vascular Physiology Unit University of Glasgow, Glasgow, UK.
Melanie H. Prescott, Population Health Research Centre University of Glasgow, Glasgow, UK; Division of Population Health Sciences and Education, St George’s, University of London, London, UK; the Vascular Physiology Unit University of Glasgow, Glasgow, UK.
Elizabeth A. Ellins, Division of Population Health Sciences and Education, St George’s, University of London, London, UK; the Vascular Physiology Unit University of Glasgow, Glasgow, UK.
Angela S. Donin, Population Health Research Centre University of Glasgow, Glasgow, UK.
Stefano Masi, Division of Population Health Sciences and Education, St George’s, University of London, London, UK; the Vascular Physiology Unit University of Glasgow, Glasgow, UK.
Alicja R. Rudnicka, Population Health Research Centre University of Glasgow, Glasgow, UK.
Naveed Sattar, Institute of Child Health, Guilford Street, London, UK; and the British Heart Foundation Glasgow Cardiovascular Research Centre University of Glasgow, Glasgow, UK.
Derek G. Cook, Population Health Research Centre University of Glasgow, Glasgow, UK.
John E. Deanfield, Division of Population Health Sciences and Education, St George’s, University of London, London, UK; the Vascular Physiology Unit University of Glasgow, Glasgow, UK.
References
- 1.Wild SH, Fischbacher C, Brock A, Griffiths C, Bhopal R. Mortality from all causes and circulatory disease by country of birth in England and Wales 2001–2003. J Public Health (Oxf) 2007;29:191–198. doi: 10.1093/pubmed/fdm010. [DOI] [PubMed] [Google Scholar]
- 2.Pickle LW, Mungiole M, Gillum RF. Geographic variation in stroke mortality in blacks and whites in the United States. Stroke. 1997;28:1639–1647. doi: 10.1161/01.str.28.8.1639. [DOI] [PubMed] [Google Scholar]
- 3.Howard G, Howard VJ, Katholi C, Oli MK, Huston S. Decline in US stroke mortality: an analysis of temporal patterns by sex, race, and geographic region. Stroke. 2001;32:2213–2220. doi: 10.1161/hs1001.096047. [DOI] [PubMed] [Google Scholar]
- 4.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 5.Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–269. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 6.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 7.Markus H, Kapozsta Z, Ditrich R, Wolfe C, Ali N, Powell J, et al. Increased common carotid intima-media thickness in UK African Caribbeans and its relation to chronic inflammation and vascular candidate gene polymorphisms. Stroke. 2001;32:2465–2471. doi: 10.1161/hs1101.098152. [DOI] [PubMed] [Google Scholar]
- 8.Manolio TA, Burke GL, Psaty BM, Newman AB, Haan M, Powe N, et al. Black–white differences in subclinical cardiovascular disease among older adults: the Cardiovascular Health Study. CHS Collaborative Research Group. J Clin Epidemiol. 1995;48:1141–1152. doi: 10.1016/0895-4356(94)00240-q. [DOI] [PubMed] [Google Scholar]
- 9.D’Agostino RB, Jr, Burke G, O’Leary D, Rewers M, Selby J, Savage PJ, et al. Ethnic differences in carotid wall thickness. The Insulin Resistance Atherosclerosis Study. Stroke. 1996;27:1744–1749. doi: 10.1161/01.str.27.10.1744. [DOI] [PubMed] [Google Scholar]
- 10.Schreiner PJ, Heiss G, Tyroler HA, Morrisett JD, Davis CE, Smith R. Race and gender differences in the association of Lp(a) with carotid artery wall thickness. The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb Vasc Biol. 1996;16:471–478. doi: 10.1161/01.atv.16.3.471. [DOI] [PubMed] [Google Scholar]
- 11.Li R, Duncan BB, Metcalf PA, Crouse JR, III, Sharrett AR, Tyroler HA, et al. B-mode-detected carotid artery plaque in a general population. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke. 1994;25:2377–2383. doi: 10.1161/01.str.25.12.2377. [DOI] [PubMed] [Google Scholar]
- 12.Kalra L, Rambaran C, Chowienczyk P, Goss D, Hambleton I, Ritter J, et al. Ethnic differences in arterial responses and inflammatory markers in Afro-Caribbean and Caucasian subjects. Arterioscler Thromb Vasc Biol. 2005;25:2362–2367. doi: 10.1161/01.ATV.0000183887.76087.6a. [DOI] [PubMed] [Google Scholar]
- 13.Chaturvedi N, McKeigue PM, Marmot MG. Resting and ambulatory blood pressure differences in Afro-Caribbeans and Europeans. Hypertension. 1993;22:90–96. doi: 10.1161/01.hyp.22.1.90. [DOI] [PubMed] [Google Scholar]
- 14.Cappuccio FP, Cook DG, Atkinson RW, Strazzullo P. Prevalence, detection, and management of cardiovascular risk factors in different ethnic groups in south London. Heart. 1997;78:555–563. doi: 10.1136/hrt.78.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaturvedi N, Coady E, Mayet J, Wright AR, Shore AC, Byrd S, et al. Indian Asian men have less peripheral arterial disease than European men for equivalent levels of coronary disease. Atherosclerosis. 2006;193:204–212. doi: 10.1016/j.atherosclerosis.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Berenson GS, Srinivasan SR. Cardiovascular risk in young persons: secondary or primordial prevention? Ann Intern Med. 2010;153:202–203. doi: 10.7326/0003-4819-153-3-201008030-00012. [DOI] [PubMed] [Google Scholar]
- 17.Kuh D, Ben-Shlomo Y. A Life Course Approach to Chronic Disease Epidemiology. II ed. Oxford University Press; Oxford, UK: 2004. [Google Scholar]
- 18.Skilton MR. Parity and risk of stroke: fetal origins of adult disease? Neurology. 2010;74:1408–1409. doi: 10.1212/WNL.0b013e3181dd4e20. [DOI] [PubMed] [Google Scholar]
- 19.Barker DJ, Lackland DT. Prenatal influences on stroke mortality in England and Wales. Stroke. 2003;34:1598–1602. doi: 10.1161/01.STR.0000077257.27430.7E. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJ. Early growth, adult income, and risk of stroke. Stroke. 2000;31:869–874. doi: 10.1161/01.str.31.4.869. [DOI] [PubMed] [Google Scholar]
- 21.Whincup PH, Nightingale CM, Owen CG, Rudnicka AR, Gibb I, McKay CM, et al. Early emergence of ethnic differences in type 2 diabetes precursors in the UK: the Child Heart and Health Study in England (CHASE Study) PLoS Med. 2010;7:e1000263. doi: 10.1371/journal.pmed.1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ford ES, Li C, Imperatore G, Cook S. Age, sex, and ethnic variations in serum insulin concentrations among US youth: findings from the National Health and Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29:2605–2611. doi: 10.2337/dc06-1083. [DOI] [PubMed] [Google Scholar]
- 23.Cruickshank JK, Mzayek F, Liu L, Kieltyka L, Sherwin R, Webber LS, et al. Origins of the ‘black/white’ difference in blood pressure: roles of birth weight, postnatal growth, early blood pressure, and adolescent body size: the Bogalusa Heart Study. Circulation. 2005;111:1932–1937. doi: 10.1161/01.CIR.0000161960.78745.33. [DOI] [PubMed] [Google Scholar]
- 24.Urbina EM, Williams RV, Alpert BS, Collins RT, Daniels SR, Hayman L, et al. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension. 2009;54:919–950. doi: 10.1161/HYPERTENSIONAHA.109.192639. [DOI] [PubMed] [Google Scholar]
- 25.Mittelman SD, Gilsanz P, Mo AO, Wood J, Dorey F, Gilsanz V. Adiposity predicts carotid intima-media thickness in healthy children and adolescents. J Pediatr. 2010;156:592–597. doi: 10.1016/j.jpeds.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Clasey J, Bradley K, Bradley J, Long D. A new BIA equation estimating the body composition of young children. Obesity. 2007;15(suppl):A127. doi: 10.1038/oby.2011.158. [DOI] [PubMed] [Google Scholar]
- 27.Tanner JM. Growth at Adolescence. I ed. Blackwell Scientific; Oxford, UK: 1962. [Google Scholar]
- 28.Owen CG, Nightingale CM, Rudnicka AR, Cook DG, Ekelund U, Whincup PH. Ethnic and gender differences in physical activity levels among 9–10-year-old children of white European, South Asian and African-Caribbean origin: the Child Heart Health Study in England (CHASE Study) Int J Epidemiol. 2009;38:1082–1093. doi: 10.1093/ije/dyp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donin AS, Nightingale CM, Owen CG, Rudnicka AR, McNamara MC, Prynne CJ, et al. Nutritional composition of the diets of South Asian, black African-Caribbean and white European children in the United Kingdom: the Child Heart and Health Study in England (CHASE) Br J Nutr. 2010;104:276–285. doi: 10.1017/S000711451000070X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 31.Jarvisalo MJ, Harmoinen A, Hakanen M, Paakkunainen U, Viikari J, Hartiala J, et al. Elevated serum C-reactive protein levels and early arterial changes in healthy children. Arterioscler Thromb Vasc Biol. 2002;22:1323–1328. doi: 10.1161/01.atv.0000024222.06463.21. [DOI] [PubMed] [Google Scholar]
- 32.Iannuzzi A, Licenziati MR, Acampora C, Salvatore V, Auriemma L, Romano ML, et al. Increased carotid intima-media thickness and stiffness in obese children. Diabetes Care. 2004;27:2506–2508. doi: 10.2337/diacare.27.10.2506. [DOI] [PubMed] [Google Scholar]
- 33.Sass C, Herbeth B, Chapet O, Siest G, Visvikis S, Zannad F. Intima-media thickness and diameter of carotid and femoral arteries in children, adolescents and adults from the Stanislas cohort: effect of age, sex, anthropometry and blood pressure. J Hypertens. 1998;16:1593–1602. doi: 10.1097/00004872-199816110-00005. [DOI] [PubMed] [Google Scholar]
- 34.Chambless LE, Folsom AR, Davis V, Sharrett R, Heiss G, Sorlie P, et al. Risk factors for progression of common carotid atherosclerosis: the Atherosclerosis Risk in Communities Study, 1987–1998. Am J Epidemiol. 2002;155:38–47. doi: 10.1093/aje/155.1.38. [DOI] [PubMed] [Google Scholar]
- 35.Ebrahim S, Papacosta O, Whincup P, Wannamethee G, Walker M, Nicolaides AN, et al. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke. 1999;30:841–850. doi: 10.1161/01.str.30.4.841. [DOI] [PubMed] [Google Scholar]
- 36.Baldassarre D, Nyyssonen K, Rauramaa R, de F U, Hamsten A, Smit AJ, et al. Cross-sectional analysis of baseline data to identify the major determinants of carotid intima-media thickness in a European population: the IMPROVE study. Eur Heart J. 2010;31:614–622. doi: 10.1093/eurheartj/ehp496. [DOI] [PubMed] [Google Scholar]
- 37.Vicenzini E, Ricciardi MC, Puccinelli F, Altieri M, Vanacore N, Di Piero V, et al. Common carotid artery intima-media thickness determinants in a population study. J Ultrasound Med. 2007;26:427–432. doi: 10.7863/jum.2007.26.4.427. [DOI] [PubMed] [Google Scholar]
- 38.Gale CR, Jiang B, Robinson SM, Godfrey KM, Law CM, Martyn CN. Maternal diet during pregnancy and carotid intima-media thickness in children. Arterioscler Thromb Vasc Biol. 2006;26:1877–1882. doi: 10.1161/01.ATV.0000228819.13039.b8. [DOI] [PubMed] [Google Scholar]
- 39.Sorof JM, Alexandrov AV, Cardwell G, Portman RJ. Carotid artery intimal-medial thickness and left ventricular hypertrophy in children with elevated blood pressure. Pediatrics. 2003;111:61–66. doi: 10.1542/peds.111.1.61. [DOI] [PubMed] [Google Scholar]
- 40.Salonen R, Salonen JT. Determinants of carotid intima-media thickness: a population-based ultrasonography study in eastern Finnish men. J Intern Med. 1991;229:225–231. doi: 10.1111/j.1365-2796.1991.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 41.Raitakari OT, Juonala M, Kahonen M, Taittonen L, Laitinen T, Maki-Torkko N, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277–2283. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 42.Frontini MG, Srinivasan SR, Xu J, Tang R, Bond MG, Berenson GS. Usefulness of childhood non-high density lipoprotein cholesterol levels versus other lipoprotein measures in predicting adult subclinical atherosclerosis: the Bogalusa Heart Study. Pediatrics. 2008;121:924–929. doi: 10.1542/peds.2007-1472. [DOI] [PubMed] [Google Scholar]
- 43.Aggoun Y, Bonnet D, Sidi D, Girardet JP, Brucker E, Polak M, et al. Arterial mechanical changes in children with familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2000;20:2070–2075. doi: 10.1161/01.atv.20.9.2070. [DOI] [PubMed] [Google Scholar]
- 44.Jarvisalo MJ, Jartti L, Nanto-Salonen K, Irjala K, Ronnemaa T, Hartiala JJ, et al. Increased aortic intima-media thickness: a marker of preclinical atherosclerosis in high-risk children. Circulation. 2001;104:2943–2947. doi: 10.1161/hc4901.100522. [DOI] [PubMed] [Google Scholar]
- 45.Koeijvoets KC, Rodenburg J, Hutten BA, Wiegman A, Kastelein JJ, Sijbrands EJ. Low-density lipoprotein receptor genotype and response to pravastatin in children with familial hypercholesterolemia: substudy of an intima-media thickness trial. Circulation. 2005;112:3168–3173. doi: 10.1161/CIRCULATIONAHA.105.565507. [DOI] [PubMed] [Google Scholar]
- 46.Tonstad S, Joakimsen O, Stensland-Bugge E, Leren TP, Ose L, Russell D, et al. Risk factors related to carotid intima-media thickness and plaque in children with familial hypercholesterolemia and control subjects. Arterioscler Thromb Vasc Biol. 1996;16:984–991. doi: 10.1161/01.atv.16.8.984. [DOI] [PubMed] [Google Scholar]
- 47.Wiegman A, de GE, Hutten BA, Rodenburg J, Gort J, Bakker HD, et al. Arterial intima-media thickness in children heterozygous for familial hypercholesterolaemia. Lancet. 2004;363:369–370. doi: 10.1016/S0140-6736(04)15467-6. [DOI] [PubMed] [Google Scholar]
- 48.Woo KS, Chook P, Yu CW, Sung RY, Qiao M, Leung SS, et al. Overweight in children is associated with arterial endothelial dysfunction and intima-media thickening. Int J Obes Relat Metab Disord. 2004;28:852–857. doi: 10.1038/sj.ijo.0802539. [DOI] [PubMed] [Google Scholar]
- 49.Meyer AA, Kundt G, Steiner M, Schuff-Werner P, Kienast W. Impaired flow-mediated vasodilation, carotid artery intima-media thickening, and elevated endothelial plasma markers in obese children: the impact of cardiovascular risk factors. Pediatrics. 2006;117:1560–1567. doi: 10.1542/peds.2005-2140. [DOI] [PubMed] [Google Scholar]
- 50.Urbina EM, Srinivasan SR, Tang R, Bond MG, Kieltyka L, Berenson GS. Impact of multiple coronary risk factors on the intima-media thickness of different segments of carotid artery in healthy young adults (The Bogalusa Heart Study) Am J Cardiol. 2002;90:953–958. doi: 10.1016/s0002-9149(02)02660-7. [DOI] [PubMed] [Google Scholar]
- 51.Chow CK, McQuillan B, Raju PK, Iyengar S, Raju R, Harmer JA, et al. Greater adverse effects of cholesterol and diabetes on carotid intima-media thickness in South Asian Indians: comparison of risk factor-IMT associations in two population-based surveys. Atherosclerosis. 2008;199:116–122. doi: 10.1016/j.atherosclerosis.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 52.Anand SS, Yusuf S, Vuksan V, Devanesen S, Teo KK, Montague PA, et al. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the Study of Health Assessment and Risk in Ethnic groups (SHARE) Lancet. 2000;356:279–284. doi: 10.1016/s0140-6736(00)02502-2. [DOI] [PubMed] [Google Scholar]
- 53.Chen W, Srinivasan SR, Ruan L, Mei H, Berenson GS. Adult hypertension is associated with blood pressure variability in childhood in blacks and whites: the Bogalusa Heart Study. Am J Hypertens. 2011;24:77–82. doi: 10.1038/ajh.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlof B, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 55.Woo KS, Chook P, Yu CW, Sung RY, Qiao M, Leung SS, et al. Effects of diet and exercise on obesity-related vascular dysfunction in children. Circulation. 2004;109:1981–1986. doi: 10.1161/01.CIR.0000126599.47470.BE. [DOI] [PubMed] [Google Scholar]
- 56.Koklu E, Kurtoglu S, Akcakus M, Yikilmaz A, Coskun A, Gunes T. Intima-media thickness of the abdominal aorta of neonate with different gestational ages. J Clin Ultrasound. 2007;35:491–497. doi: 10.1002/jcu.20335. [DOI] [PubMed] [Google Scholar]
- 57.Skilton MR, Evans N, Griffiths KA, Harmer JA, Celermajer DS. Aortic wall thickness in newborns with intrauterine growth restriction. Lancet. 2005;365:1484–1486. doi: 10.1016/S0140-6736(05)66419-7. [DOI] [PubMed] [Google Scholar]
- 58.Moser K, Stanfield KM, Leon DA. Birthweight and gestational age by ethnic group, England and Wales 2005: introducing new data on births. Health Stat Q. 2008:22–55. [PubMed] [Google Scholar]
- 59.Gunes T, Koklu E, Yikilmaz A, Ozturk MA, Akcakus M, Kurtoglu S, et al. Influence of maternal smoking on neonatal aortic intima-media thickness, serum IGF-I and IGFBP-3 levels. Eur J Pediatr. 2007;166:1039–1044. doi: 10.1007/s00431-006-0376-9. [DOI] [PubMed] [Google Scholar]
- 60.Markus HS, Khan U, Birns J, Evans A, Kalra L, Rudd AG, et al. Differences in stroke subtypes between black and white patients with stroke: the South London Ethnicity and Stroke Study. Circulation. 2007;116:2157–2164. doi: 10.1161/CIRCULATIONAHA.107.699785. [DOI] [PubMed] [Google Scholar]
- 61.Ohira T, Shahar E, Iso H, Chambless LE, Rosamond WD, Sharrett AR, et al. Carotid artery wall thickness and risk of stroke subtypes: the Atherosclerosis Risk In Communities study. Stroke. 2011;42:397–403. doi: 10.1161/STROKEAHA.110.592261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pitt B, Byington RP, Furberg CD, Hunninghake DB, Mancini GB, Miller ME, et al. Effect of amlodipine on the progression of atherosclerosis and the occurrence of clinical events. PREVENT Investigators. Circulation. 2000;102:1503–1510. doi: 10.1161/01.cir.102.13.1503. [DOI] [PubMed] [Google Scholar]