Abstract
Eradication and elimination are increasingly a part of the global health agenda. Once control measures have driven infection to low levels, the ecology of disease may change posing challenges for eradication efforts. These challenges vary from identifying pockets of susceptibles, improving monitoring during and after the endgame, to quantifying the economics of disease eradication versus sustained control, all of which are shaped and influenced by processes of loss of immunity, susceptible build-up, emergence of resistance, population heterogeneities and non-compliance with control measures. Here we discuss how modelling can be used to address these challenges.
Keywords: Elimination, Surveillance, Modelling, Dynamics, Heterogeneity
Introduction
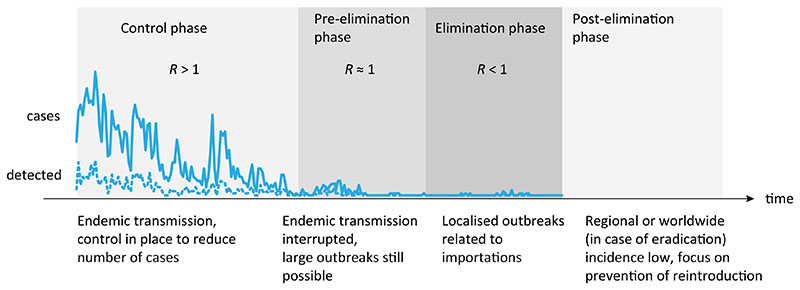
Only two diseases, smallpox and rinderpest, have been eradicated. Yet eradication is increasingly part of the language of the global health community. Calls have been made for the eradication of diseases as diverse as guinea worm and malaria. While each disease poses a unique set of issues, there are a number of recurring challenges that emerge during the endgame, or the phase during which control efforts are intensified and targeted towards achieving elimination locally and eradication globally (see Fig. 1 for a visualization of different control stages towards elimination).
Fig. 1.
Stages towards and after elimination in a given location and milestones on the path to elimination. Adapted from (Townsend et al., 2013b; World Health Organization, 2007). Shading illustrates control intensity (darker grey for heightened efforts).
In order to be successful, eradication effort has to permanently eliminate a pathogen everywhere in the world; pathogen prevalence is globally reduced to zero, thereby removing the risk of re-introduction and re-establishment. Elimination, on the other hand, is a more localized effort that focuses on reduction to zero incidence of a certain pathogen in a given area, with active measures to prevent pathogen re-establishment from other areas after elimination. Since eradication is elimination on global scale, there are many similarities between those two efforts, particularly in dynamical transitions from endemic transmission to elimination and post-elimination period of enhanced vigilance (see Fig. 1). Once infection is driven to very low levels, the ecology of pathogens may change requiring different surveillance and control strategies. Susceptible build-up, waning of immunity, increase in the age of infection, non-compliance of individuals with control measures, pathogen change and emergence of resistance as a result of intensified efforts all become increasingly important during the final stages of eradication programmes. This calls for the development of a research agenda for epidemiological modellers that directly addresses these challenges, from the design of models to target control strategies, to the optimization of surveillance and determining data needs to address, amongst others, the questions we outline below.
In addition to visualizing stages of elimination and corresponding reduction in disease prevalence and change in dynamical regime, Fig. 1 also serves as a timeline of eradication efforts that we use to structure the rest of this manuscript. Before eradication efforts are attempted, is there a way to estimate how likely are they to succeed and how much they are going to cost compared to sustained control? Is there a way to quantify the susceptible landscape that will improve targeting of efforts and monitoring strategies in the pre-elimination and elimination phase?
1. Provide a systematic framework for when we should try to eradicate
Eradication of infectious diseases is a vast public health, political and economic commitment and the intensity of efforts needed to eliminate a disease cannot be sustained indefinitely. The costs and risks are high, as are the potential benefits. During the dynamic transition from endemic transmission to local elimination (Fig. 1) potential shifts in age at first infection, waning of immunity, susceptible build-up, emergence of resistance, etc. can lead to dynamical feedbacks and logistical challenges that can cause unanticipated difficulties for eradication. For emerging infections, modelling pathogen properties demonstrated how timing of infectiousness and appearance of symptoms determines the likely success of isolating infectious individuals and their contacts in controlling an outbreak (Fraser et al., 2004). An analogous framework that can identify what makes a disease “easy” vs. “hard” to eradicate would be a first step in providing a mechanism of prioritizing efforts and strategies.
Such a framework needs to include processes that shape infectious disease dynamics but that operate on very different time scales. For example, intensive efforts exert strong selective pressures on the pathogen and prolonging the elimination phase (Fig. 1) increases the probability of emergence of antimicrobial or insecticide resistance, vaccine escape or antigenic divergence, potentially creating novel problems. While the evolutionary timescales over which drugs fail due to resistance are affected by application strategies or drug regimens, replenishment of susceptible populations and ageing of “naturally immunised”cohorts occur on demographic time-scales determined by turnover, which varies drastically across populations. Models can help identify key-time scales for eradication, how they vary for different pathogens, and how long can intensified elimination efforts be sustained, without detrimental consequences.
Biological feasibility of eradication depends, among other factors, on the pathogen lifecycle, its reservoirs, persistence in the environment, clinical manifestations of disease, sensitivity and specificity of laboratory tests to confirm the disease as well as safe and effective control measures. A related biological factor is the presence of related pathogens that might take advantage of a niche vacated by eradication (Lloyd-Smith, 2013). Although crucial, biological factors are not the only prerequisites - logistic, operational, political and socioeconomic factors are all critical in determining whether or not eradication can be achieved and should be incorporated into models.
2. Develop quantitative models of the economics of control versus eradication
Cost-effectiveness of control methods is increasingly a deciding factor in their implementation (Jit et al., 2008; Baguelin et al., 2012). The reasons for this are fairly intuitive, as it is rational not to attempt something unless the benefits of that action exceed the costs. Yet it can be difficult to accurately estimate costs of control efforts and their benefits when eradication is one of several options. Should we aim for long-term control, tolerating a certain level of infection, or should we push for eradication? When is one option preferable and what kind of models do we need to help distinguish between the two?
Analysis of costs is hard even retrospectively, but estimating these costs in advance is even more challenging. There are several reasons for this. First, costs of expanding control efforts increase; for example, the last foci of infection, or pockets of susceptibility will be those that are hardest to reach, either geographically or socially (e.g. vaccine refusers). The challenge for modelling is to accurately tie the economics of scaling up control programmes with the epidemiology and changing ecology of the disease (Klepac et al., 2011). Second, control efforts are implemented within health systems very differently from eradication efforts. Control programmes are usually integrated in horizontal programmes focused on strengthening primary care and providing ‘health for all’ (Aylward et al., 1998). Elimination and eradication efforts on the other hand often require a targeted ‘vertical approach,’ sometimes at the expense of other public health issues. But elimination efforts can also strengthen primary healthcare by providing basic services and improving surveillance (yaws), training personnel and expanding immunization programmes (smallpox), or establishing a global laboratory network (polio) (Klepac et al., 2013). The impacts on health systems of such secondary or intangible benefits of elimination programmes are particularly hard to measure (Closser et al., 2012), posing a challenge of how to integrate them into models.
Expansion of efforts is very costly and prolonging the endgame leads to donor fatigue risking re-emergence if efforts are scaled-down prematurely. Prolonged low incidence levels during the epidemic tail (as illustrated by the low number of cases in the elimination phase in Fig. 1) can also lead to disengagement of communities with eradication efforts, complacency and ‘individual fatigue’ or even active refusal of vaccination (Saint-Victor and Omer, 2013). In addition, a prolonged epidemic tail may contribute to seeding outbreaks elsewhere (O’Reilly et al., 2011), escalating the costs and jeopardizing chances of success.
For many diseases, the probability of severe complications and the costs of treatment vary with patient age. Population ageing will therefore also affect the costs of control and elimination programmes. The impacts of changing demography might be more costly for sustained, long-term control efforts than for intense, but time-limited eradication programmes which might be a new argument for eradication. Models that consider epidemic, economic and demographic detail on an appropriate time-scale will help answer these questions. Estimates of disease burden that incorporate dynamical effects of control efforts (e.g. Simons et al., 2012) provide a promising avenue for quantifying these costs and benefits.
Finally, in some circumstances, projected costs may suggest that eradication is not feasible. Given that some of the benefits of eradication are difficult or impossible to express in monetary terms (e.g. improving the vaccine supply chain may enable easier and cheaper distribution of future antigens; possible non-linear interactions with other pathogens), financial reasoning and cost-effectiveness may not be the only reasoning to guide elimination efforts (Sabot et al., 2010). Can modelling approaches offer alternatives that take us beyond cost-effectiveness? Developing consistent metrics of health benefits would make it easier to measure total direct and indirect benefits of implemented efforts and improve economic evaluations used to inform public health decisions.
3. Identify the most effective approaches to achieve eradication
The dynamics of infectious diseases close to elimination can be distinctly different from natural dynamics (Fig. 1). The distribution of susceptibility is no longer governed by a combination of replenishment of the susceptible population through births, and immunity through past exposure but, instead, by vaccination coverage, the extent of mass drug administrations (in the case of many campaigns against NTDs (Bockarie et al., 2013)) or behavioural changes (e.g. guinea worm that is on track to be eliminated without the use of drug or vaccine (Biswas et al., 2013; Barry, 2007)). These interventions introduce an element of heterogeneity, which can affect infection dynamics. If those susceptible (e.g., unvaccinated) are preferentially in contact with each other because of geographical and/or social proximity, there is a risk of large outbreaks, which can be hard to predict because introductions into those populations might be rare.
Models need to be designed to take into account these dynamical transitions and predict where and when outbreaks are at most risk of occurring, as well as suggest appropriate prevention and intervention strategies. Using outbreak data from England and Wales, Jansen and colleagues (Jansen et al., 2003) showed that while measles transmission in the UK remained subcritical (locally eliminated with short-lived outbreaks from imported infections) the reproductive number, R, significantly increased in the wake of declining vaccination coverage, suggesting that continued low vaccine uptake could lead to re-establishment of endemic measles. Similar branching process models may have utility for identifying pivotal dynamics during the endgame. Additionally, endgame dynamics should be reconsidered in the light of a heterogeneous landscape of susceptibility and stochastic fade-outs to inform how much control is needed to drive an infection to extinction and, consequently, how to best allocate the resources in eradication efforts.
Approaches during the endgame often differ from sustained, less intense control during the ‘middle game’ (Fig. 1). The smallpox effort switched from mass vaccination campaigns to repetitive active searches conducted by a massive army of health personnel going door-to-door to look for any last remaining cases. Modelling work and statistical inference from polio surveillance data identified the value of switching to a more immunologically effective monovalent vaccine (Grassly et al., 2006) prompting a substantial investment in vaccine development. India was recently certified free from polio, resulting from this switch (WHO, 2014). Using models to assess the limitations of conventional control measures in the face of shifting dynamics, and identifying strategies that increase the probability of eliminating infection, such as the optimal time to intensify efforts or switch strategies, could reduce the length of the epidemic tail (such as in polio).
4. Quantify the landscape of susceptibility
For immunizing (or partially-immunizing) infections, the age, location and social grouping of individuals that remain susceptible as incidence declines to low levels, will be the key determinant of progress towards elimination. The main data-stream available for evaluating this landscape of susceptibility is often surveillance for cases or deaths – susceptibility itself is generally a hidden variable. For completely immunizing infections, susceptible reconstruction can provide some insight into the dynamics of immunity – but inference is weakened specifically where the risk of infection is low (i.e., in an elimination context), since the assumption that everyone can acquire the infection no longer holds.
Expanding the statistical and modelling toolset available for inferring the temporal, geographic, or social patterns of susceptibility is a key challenge in using modelling to support elimination or eradication efforts. This effort will likely include development of models that can synthesize diverse sources of information encompassing serological surveys, coverage estimates, and demographic rates among others. Quantitative descriptions of past dynamics of the population via these variables will inform the likely current proportion of susceptible individuals, their ages, and geographic locations. The history of outbreaks will be an important piece in this puzzle – a large outbreak or campaign could deplete susceptibles such that a long interval without infection (‘honey-moon period’ (McLean and Anderson, 1988)) results; and can be followed by a large, late age outbreak (observed during the recent measles outbreak in Swansea (Wise, 2013)).
Amongst these various data-streams, serological surveys supply perhaps the most direct measure of susceptibility. Nevertheless, there are considerable open challenges in the analyses of serological data. For many infections, vaccine and natural immunity cannot currently be distinguished. Ideally, clinical measures of serology could be improved to allow this, but in the absence of such progress, models that build around other known variables (past outbreak sizes, coverage estimates, etc.) may be able to generate inference to distinguish vaccine coverage from natural immunity. This will allow detection of whether the disease is still circulating in certain populations or spilling over from nearby reservoirs (crucial for diseases such as rinderpest and FMD where premature cessation of vaccination can lead to costly resurgence). More generally, much of the data collected as part of a serological survey is often jettisoned in analyses – in particular, continuous titres are translated into discrete positive/negative variables. Data on the continuum of titre values might be leveraged to infer levels, or recency of exposure via models that incorporate epidemiological data.
As we address increasingly complex pathogens, which may fluctuate in their characteristics over time, space and severity of infections (e.g. malaria, where low immunity is linked to more severe infections (Snow et al., 1997)) such modelling innovations that yield deeper insight into the biology may be key. Furthermore, they may contribute to identifying if and when pathogen escape from chemotherapy or vaccination is occurring. Finally, a challenge is developing models capable of forecasting the likely consequences if an elimination programme were to fail (for example, (Townsend et al., 2013a)).
5. Improve monitoring during and after the endgame
Once a disease is close to elimination locally, the few remaining cases may be concentrated in hard-to-reach groups, for social, logistical or geographic reasons; may be predominantly asymptomatic cases; and may be temporally very irregular. Following elimination, new outbreaks may develop rapidly and be detected through clusters of severe cases, or may develop more slowly and be detected though serological studies. As immunity shifts in the population following elimination, the age-distribution or characteristics of cases may also shift. The question of how to design the right surveillance strategy to assist the drive towards elimination, to confirm that elimination has been achieved and to assist in preventing re-emergence all require a detailed understanding of the characteristics of the disease and its dynamics (see (Woolhouse, 2013)). A classic problem in elimination and re-emergence is that passive surveillance may only capture the ‘tip of the iceberg’-normally the symptomatic cases or those that are laboratory confirmed (Townsend et al., 2013b). Responses based on only these cases may be delayed relative to the outbreak. Either new diagnostics are needed to identify and treat asymptomatic cases (e.g. visceral leishmaniasis (Guerin et al., 2002)), or responses need to take into account the likely pool of infectives around an identified case (the ratio of infections to reported cases). If the dynamics of the disease are such that there are hotspots of infection, this may make a spatial response a very effective tool.
In addition to the challenge of designing the right surveillance method epidemiologically, there are challenges around interpreting existing surveillance data, or designing new surveillance strategies that are logistically feasible. This includes not only monitoring of disease but also of control processes, such as drug distribution, bed net usage (rather than distribution) and systematic uptake or refusal of vaccines (e.g. (Lessler et al., 2011)).
A further complication in designing surveillance is accounting for spatial coupling (e.g. (Metcalf et al., 2013)) and interconnectedness of areas at different stages of elimination. For example, as part of a feasibility study of malaria elimination in Zanzibar, mobile phone movement data suggested that travel from mainland Tanzania was so intense that local controls alone would be unlikely to eliminate malaria due to high rates of importation (Tatem et al., 2009).
6. Identify post-eradication opportunities and threats
Finally, in the event of successful elimination/eradication how long do we need to hold the line? Should one invest in maintaining herd immunity, or implement low-level indefinite control, or stop with the efforts altogether (as with smallpox)? If we were to scale down or stop control efforts, when would it be safe to do so (see (Townsend et al., 2013b))? Should we switch to a different strategy and, if so, when? What are the spatial strategies for maintaining freedom from disease? What defines the width of a protective cordon sanitaire and how should elimination efforts be coordinated across international boundaries?
Models that estimate or account for spatial coupling could guide spatial strategies, to minimize re-introductions from endemic areas (through coordinated efforts) and maintain disease freedom (through for example the design of cordon sanitaires). Finally, models should be able to provide guidance on whether eradication has been achieved, by informing estimates of the proportion of cases detected for a given surveillance programme (Townsend et al., 2013b).
Detection of low-level infection requires ever more sensitive and more accurate detection methods, so surveillance abilities are crucial in post-eradication strategies. Measuring loss of herd immunity or finding asymptomatic carriers might require new surveillance and monitoring techniques and better statistical tools in analysis of the relevant data. All of these need to be considered in a dynamical framework to predict possible shifts in dynamical regimes or potential tipping points for re-emergence of eliminated pathogensor emergence of related ones through competitive release in vacated niches (Lloyd-Smith, 2013).
Conclusions
Eradication initiatives require sustained public health, political, financial and individual efforts. It is a dynamical challenge that requires vast data integration over temporal, geographical and socioeconomic scales. Yet the inherently dynamic nature of infectious diseases can also have unexpected advantages. Even though the malaria eradication programme in the 1950s failed, a number of successful countries have remained malaria free (Smith et al., 2013). Models demonstrated malaria elimination to be a surprisingly stable state (Smith et al., 2013), indicating that elimination could be incrementally achieved without needing the simultaneous and correspondingly expensive effort as required for say polio. The eradication endgame poses a diverse set of challenges that can be addressed in a modelling framework leading to improved surveillance and control.
Acknowledgments
The authors wish to thank Angela McLean and the participants of the Infectious Disease Dynamics workshop at the Newton Institute for Mathematical Sciences in Cambridge. P.K. acknowledges funding from AXA Research Fund. S.F. is supported by a UK Medical Research Council Career Development Award in Biostatistics (MR/K021680/1). C.J.E.M. was funded by the Bill and Melinda Gates Foundation, and the RAPIDD programme of the Science & Technology Directorate, Department of Homeland Security and the Fogarty International Centre, National Institutes of Health. K.H. is supported by the Wellcome Trust and RAPIDD.
References
- Aylward B, Olive JM, Hull HF, De Quadros CA, Melgaard B. In: The Eradication of Infectious Diseases. Dowdle WR, Hopkins D, editors. 1998. Disease eradication initiatives and general health services: ensuring common principles lead to mutual benefits; pp. 61–74. [Google Scholar]
- Baguelin M, Jit M, Miller E, Edmunds WJ. Health and economic impact of the seasonal influenza vaccination programme in England. Vaccine. 2012;30:3459–3462. doi: 10.1016/j.vaccine.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Barry M. The tail end of guinea worm—global eradication without a drug or a vaccine. N Engl J Med. 2007;356:2561–2564. doi: 10.1056/NEJMp078089. [DOI] [PubMed] [Google Scholar]
- Biswas G, Sankara DP, Agua-Agum J, Maiga A. Dracunculiasis (guinea worm disease): eradication without a drug or a vaccine. Philos Trans R Soc B. 2013;368:20120146. doi: 10.1098/rstb.2012.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockarie MJ, Kelly-Hope LA, Rebollo M, Molyneux DH. Preventive chemotherapy as a strategy for elimination of neglected tropical parasitic diseases: endgame challenges. Philos Trans R Soc B. 2013;368:20120144–20120154. doi: 10.1098/rstb.2012.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closser S, Rosenthal A, Parris T, Maes K, Justice J, Cox K, et al. Methods for evaluating the impact of vertical programs on health systems: protocol for a study on the impact of the global polio eradication initiative on strengthening routine immunization and primary health care. BMC Public Health. 2012;12:728. doi: 10.1186/1471-2458-12-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser C, Riley S, Anderson RM, Ferguson NM. Factors that make an infectious disease outbreak controllable. Proc Natl Acad Sci U S A. 2004;101:6146–6151. doi: 10.1073/pnas.0307506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassly NC, Fraser C, Wenger J, Deshpande JM, Sutter RW, Heymann DL, et al. New strategies for the elimination of polio from India. Science. 2006;314:1150–1153. doi: 10.1126/science.1130388. [DOI] [PubMed] [Google Scholar]
- Guerin PJ, Olliaro P, Sundar S, Boelaert M, Croft SL, Desjeux P, et al. Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect Dis. 2002;2:494–501. doi: 10.1016/s1473-3099(02)00347-x. [DOI] [PubMed] [Google Scholar]
- Jansen V, Stollenwerk N, Jensen H, Ramsay M, Edmunds W, Rhodes C. Measles outbreaks in a population with declining vaccine uptake. Science. 2003;301:804–814. doi: 10.1126/science.1086726. [DOI] [PubMed] [Google Scholar]
- Jit M, Choi YH, Edmunds WJ. Economic evaluation of human papillomavirus vaccination in the United Kingdom. Br Med J. 2008;337:a769. doi: 10.1136/bmj.a769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepac P, Laxminarayan R, Grenfell BT. Synthesizing epidemiological and economic optima for control of immunizing infections. Proc Natl Acad Sci U S A. 2011;108:14366–14370. doi: 10.1073/pnas.1101694108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepac P, Metcalf CJE, McLean A, Hampson K. Towards the endgame and beyond: complexities and challenges for the elimination of infectious diseases. Philos Trans R Soc B. 2013;368:20120137. doi: 10.1098/rstb.2012.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessler J, Metcalf CJE, Grais RF, Luquero FJ, Cummings DAT, Grenfell BT. Measuring the performance of vaccination programs using cross-sectional surveys: a likelihood framework and retrospective analysis. PLoS Med. 2011;8:e1001110. doi: 10.1371/journal.pmed.1001110.g003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Smith JO. Vacated niches, competitive release and the community ecology of pathogen eradication. Philos Trans R Soc B. 2013;368:20120150. doi: 10.1098/rstb.2012.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean AR, Anderson RM. Measles in developing countries Part II The predicted impact of mass vaccination. Epidemiol Infect. 1988;100:419–442. doi: 10.1017/s0950268800067170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf CJE, Hampson K, Tatem AJ, Grenfell BT, Bjornstad ON. Persistence in epidemic metapopulations: quantifying the rescue effects for measles, mumps. rubella and whooping cough. PLOS ONE. 2013;8:e74696. doi: 10.1371/journal.pone.0074696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly KM, Chauvin C, Aylward RB, Maher C, Okiror S, Wolff C, et al. A statistical model of the international spread of wild poliovirus in Africa used to predict and prevent outbreaks. PLoS Med. 2011;8:e1001109. doi: 10.1371/journal.pmed.1001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabot O, Cohen JM, Hsiang MS, Kahn JG, Basu S, Tang L, et al. Costs and financial feasibility of malaria elimination. Lancet. 2010;376:1604–1615. doi: 10.1016/S0140-6736(10)61355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Victor DS, Omer SB. Vaccine refusal and the endgame: walking the last mile first. Philos Trans R Soc B. 2013;368:20120148. doi: 10.1098/rstb.2012.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons E, Ferrari M, Fricks J, Wannemuehler K, Anand A, Burton A, et al. Assessment of the 2010 global measles mortality reduction goal: results from a model of surveillance data. Lancet. 2012;379:2173–2178. doi: 10.1016/S0140-6736(12)60522-4. [DOI] [PubMed] [Google Scholar]
- Smith DL, Cohen JM, Chiyaka C, Johnston G, Gething PW, Gosling R, et al. A sticky situation: the unexpected stability of malaria elimination. Philos Trans R Soc B: Biol Sci. 2013;368:20120145. doi: 10.1098/rstb.2012.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow RW, Omumbo JA, Lowe B, Molyneux CS. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet. 1997;349:1650–1654. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]
- Tatem AJ, Qiu Y, Smith DL, Sabot O, Ali AS, Moonen B. The use of mobile phone data for the estimation of the travel patterns and imported Plasmodium falciparum rates among Zanzibar residents. Malar J. 2009;8:287. doi: 10.1186/1475-2875-8-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend SE, Sumantra IP, Pudjiatmoko, Bagus GN, Brum E, Cleaveland S, et al. Designing programs for eliminating canine rabies from islands: Bali, Indonesia as a case study. PLoS Negl Trop Dis. 2013a;7:e2372. doi: 10.1371/journal.pntd.0002372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend SE, Lembo T, Cleaveland S, Meslin FX, Miranda ME, Putra AAG, et al. Surveillance guidelines for disease elimination: a case study of canine rabies. Comp Immunol Microbiol Infect Dis. 2013b;36:249–261. doi: 10.1016/j.cimid.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. India Three Years Polio-free. 2014. [cited 2014 Feb 28]. [Internet]. Available from: http://www.searo.who.int/mediacentre/features/2014/sea-polio/en/
- Wise J. Largest group of children affected by measles outbreak in Wales is 10-18 year olds. BMJ. 2013;346:f2545–f2555. doi: 10.1136/bmj.f2545. [DOI] [PubMed] [Google Scholar]
- Woolhouse MEJ. Theory and Practice of Disease Surveillance. 2013. [Internet], Available from: http://www.newton.ac.uk/programmes/IDD/seminars/2013082110001.html .
- World Health Organization. Malaria Elimination: A Field Manual for Low and Moderate Endemic Countries. 2007.