Abstract
Neurotransmitters, such as γ-aminobutyric acid, glutamate, acetyl choline, glycine and the monoamines, facilitate the crosstalk within the central nervous system. The designated neurotransmitter transporters (NTTs) both release and take up neurotransmitters to and from the synaptic cleft. NTT dysfunction can lead to severe pathophysiological consequences, e.g. epilepsy, intellectual disability, or Parkinson’s disease. Genetic point mutations in NTTs have recently been associated with the onset of various neurological disorders. Some of these mutations trigger folding defects in the NTT proteins. Correct folding is a prerequisite for the export of NTTs from the endoplasmic reticulum (ER) and the subsequent trafficking to their pertinent site of action, typically at the plasma membrane. Recent studies have uncovered some of the key features in the molecular machinery responsible for transporter protein folding, e.g., the role of heat shock proteins in fine-tuning the ER quality control mechanisms in cells. The therapeutic significance of understanding these events is apparent from the rising number of reports, which directly link different pathological conditions to NTT misfolding. For instance, folding-deficient variants of the human transporters for dopamine or GABA lead to infantile parkinsonism/dystonia and epilepsy, respectively. From a therapeutic point of view, some folding-deficient NTTs are amenable to functional rescue by small molecules, known as chemical and pharmacological chaperones.
Keywords: Neurotransmitter, Transporter, SLC6, Disease variants, Folding, Pharmacochaperoning
1. Introduction
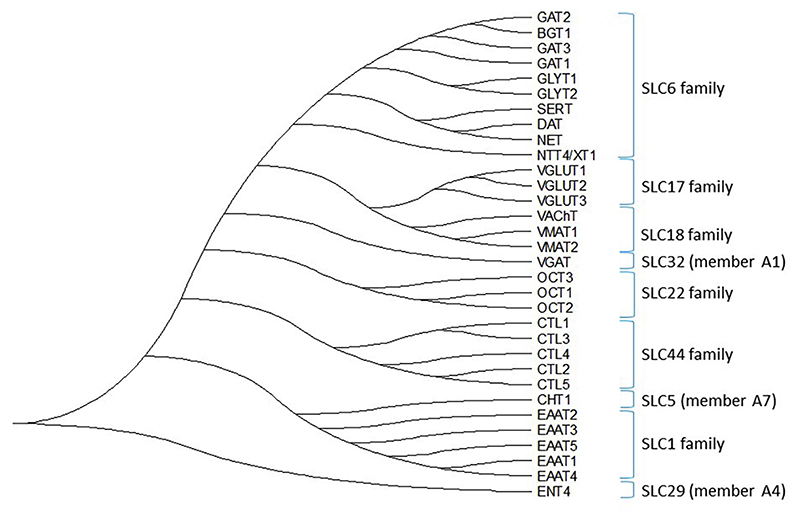
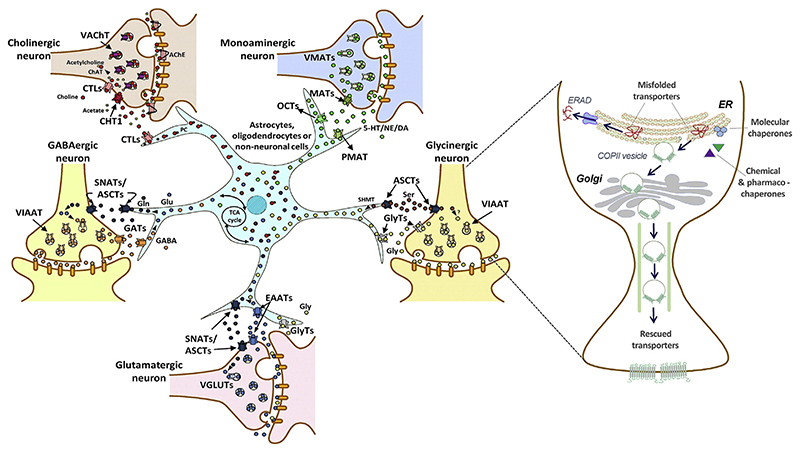
Neurotransmitter transporters (NTTs) belong to the solute carrier (SLC) superfamily of membrane transporters, which is comprised of 65 families (Hu, Tao, Cao, & Chen, 2020). NTTs are responsible for terminating the action of neurotransmitters via rapid reuptake of neurotransmitter molecules from the synaptic cleft, and their subsequent reloading into synaptic vesicles (Nelson, 1998). According to their pertinent sites of action, NTTs can be classified into two major superfamilies (Masson et al., 1999). (i) Plasma membrane transporters, which are further subdivided into the Na+/K+/H+-dependent glutamate transporters (SLC1 gene family), choline-Na+ symporter (SLC5A7 gene), Na+/Cl−-dependent transporters for dopamine, γ-aminobutyric acid (GABA), glycine, norepinephrine, serotonin (SLC6 gene family), Na+-independent organic cation transporters (SLC22 gene family), equilibrative nucleoside transporters (SLC29 gene family) and the choline transporter-like proteins (SLC44 gene family). (ii) The second group encompasses the intracellular H+-dependent vesicular transporters, which are divided into three subclasses: vesicular glutamate transporters (SLC17 gene family), vesicular amine transporters (SLC18 gene family), and the vesicular inhibitory amino acid transporter (SLC32). A phylogenetic tree based on amino acid sequence homology of the protein families belonging to the NTT superfamily is shown in Fig. 1.
Fig. 1.
A phylogenetic tree of neurotransmitter transporter families. The evolutionary history was inferred using the maximum likelihood method and JTT matrix-based model (Jones et al., 1992). The tree with the highest log likelihood is shown. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using a JTT model, and then selecting the topology with superior log likelihood value. This analysis involved 32 amino acid sequences. There were a total of 953 positions in the final dataset. Evolutionary analyses were conducted in MEGA X (Kumar et al., 2018).
By definition, NTTs have a ligand-binding pocket, which affords specificity, selectivity and flexibility to accommodate the cognate substrates and co-substrates and to allow for the conformational transition associated with substrate translocation. Hence, they are posited to be druggable. In fact, several NTTs are prominent pharmacological drug targets. The transporters of the SLC6 family, for instance, are the sites of action of numerous exogenous compounds: e.g. the antiepileptic drug tiagabine blocks the GABA transporter 1 (GAT1, SLC6A1); antide-pressants (tricyclic compounds like imipramine, desipramine and amitryptyline; SSRIs/selective serotonin reuptake inhibitors, SNRIs/serotonin and noreprinephrine reuptake inhibitors), psychostimulants (e.g. cocaine and amphetamines) and anti-addictive drugs (e.g. ibogaine) act on the monoamine transporters for norepinephrine (NET, SLC6A2), dopamine (DAT, SLC6A3), and serotonin (SERT, SLC6A4) (Iversen, 2000; Rask-Andersen, Masuram, Fredriksson, & Schiöth, 2013). The indol alkaloid reserpine and tetrabenazine act on the vesicular monoamine transporters VMATs (e.g. SLC18A2); reserpine was introduced in the treatment of arterial hypertension in the 1950s. It has been abandoned in most countries, because the advent of effective inhibitors of angiotensin II formation and of angiotensin II-receptor anatagonists made reserpine dispensable: reserpine causes severe side effects including depression (due to depletion of serotonin and norepinephrine) and Parkinsonism (due to depletion of dopamine). Tetrabenazine, which is selective for VMAT2/SLC18A2, is still the treatment of choice for chorea/Huntington’s disease (Rask-Andersen et al., 2013).
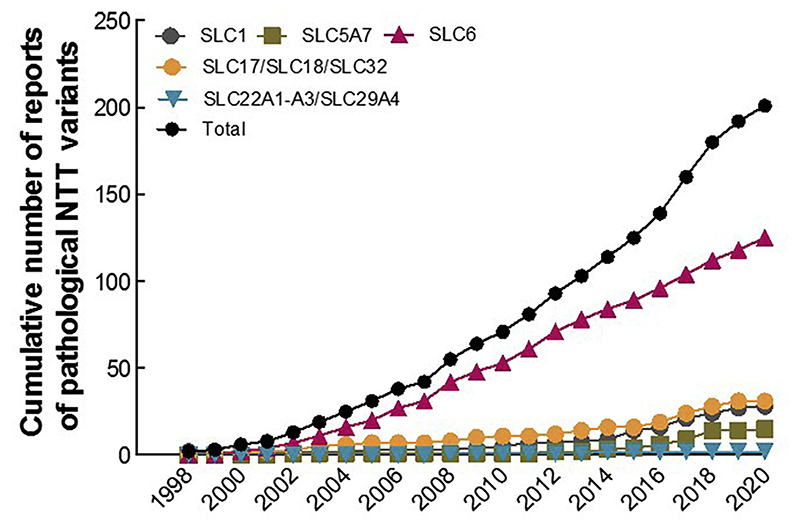
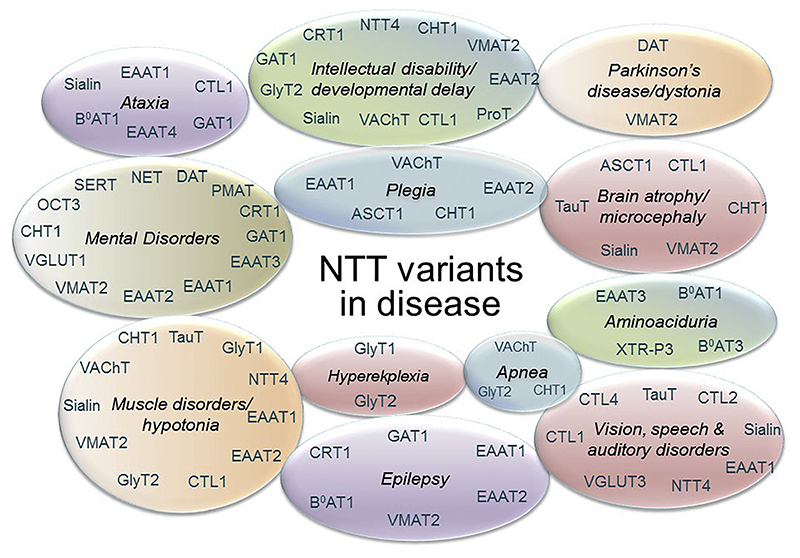
Mutations in NTTs have been linked to a broad range of pathologic conditions in people over the past two decades (Fig. 2). Recent research from our group revealed that point mutations in SLC6 transporters can trigger aberrant protein folding, causing their retention in the endoplasmic reticulum (ER) compartment and hence precluding the delivery of variant transporters to their site(s) of action (e.g. in case of the SLC6 family, at the cell surface). As a consequence, protein misfolding gives rise to severe clinical phenotypes, e.g. folding-deficient variants of the DAT trigger infantile/juvenile parkinsonism-dystonia, of the creatine transporter 1 (CRT1, SLC6A8) cause severe X-linked intellectual disability and of GAT1 cause myoclonic-atonic and other generalized epilepsies. Understanding the molecular mechanisms that lead to protein misfolding and that underlie treatment avenues for folding deficits in NTT disease variants by pharmacological and chemical chaperones have potential for clinical impact.
Fig. 2.
A cumulative number of misfolded disease variants reported among the NTT subfamilies. Numbers of reported pathological NTT variants, belonging to different subfamilies (color-coded), within the given time-span of two decades. The black line represents a total number of disease variants across the NTT protein family.
2. SLC1 family: EAAT and ASCT transporters
2.1. Glutamate transporters
Glutamate is the most abundant neurotransmitter in the mammalian central nervous system (CNS) (Meldrum, 2000). Glutamate clearance from the synaptic cleft is mediated by glutamate transporters, which are expressed both in neurons and astrocytes in the brain (Grewer, Gameiro, & Rauen, 2014). Astrocytes clear a large fraction of glutamate from the synapse; they convert glutamate to glutamine, which is returned into neurons by SLC38 transporters; this glutamate-glutamine shuttle precludes extracellular glutamate mediated excito-toxicity (Bhutia & Ganapathy, 2016). In neurons, glutamine is converted to glutamate, which is refilled into synaptic vesicles. Glutamate transporters include 5 plasmalemmal excitatory amino acid transporters (EAAT1 (SLC1A3), EAAT2 (SLC1A2), EAAT3 (SLC1A1), EAAT4 (SLC1A6), EAAT5 (SLC1A7)) and 2 neutral amino acid transporters (ASCT1/SLC1A4) and (ASCT2/SLC1A5). Glutamate transport is primarily mediated by EAATs, while ASCTs accomplish transport at a low pH (Vadgama & Christensen, 1984). EAATs also have an inherent chloride conductance that may positively influence the concentrative power of these transporters (Vandenberg & Ryan, 2013). ASCT1 and ASCT2 mainly transport neutral amino acids such as alanine, serine, cysteine, and threonine in glial cells and neurons (Utsunomiya-Tate, Endou, & Kanai, 1996; Zerangue & Kavanaugh, 1996). ASCT1 is the major transporter that retrieves D-serine in neurons (Kaplan et al., 2018). ASCT2 has also been shown to retrieve glutamine and asparagine with high affinity, and is proposed to be an active participant in the glutamate-glutamine cycle between glial cells and neurons in the brain (Scalise, Pochini, Console, Losso, & Indiveri, 2018). Owing to the importance of glutamate transporters in glutamate homeostasis, it is plausible that their malfunction plays a role in many neurological diseases (reviewed in Vandenberg & Ryan, 2013; Kanai et al., 2013; O’Donovan et al., 2017). Table 1 lists the disorders arising from point mutations in plasmalemmal glutamate SLC1 transporters, described in further detail below.
Table 1. Mutations in human plasmalemmal SLC1 transporters.
| Gene | Protein name | Missense coding variants | Transport phenotype | Traf ficking to target membrane | Disease phenotype | Mode of inheritance | OMIM# |
|---|---|---|---|---|---|---|---|
| SLC1A1 | EAAT3 | E48*, R445W, I395del | Loss-of-function | Reduced | Dicarboxylic aminoaciduria, autism | AR | 222730 |
| T164A | ? | ? | Obsessive compulsive disorder (OCD) and bipolar disorder | AD | |||
| R280C | ? | ? | Schizotypal personality disorder | SM | 615232 | ||
| SLC1A2 | EAAT2 | N206S | Loss-of-function | Reduced | Sporadic amyotrophic lateral sclerosis | AD | |
| L85P | Loss-of-function | Reduced | Epileptic encephalopathy | AD/CHet | 617105 | ||
| G82R, P289R, L474* | ? | ? | |||||
| A79G | ? | ? | Hereditary spastic paraplegia | AD | |||
| G6S, R31Q | ? | ? | Bipolar disorder and schizophrenia | AD | |||
| SLC1A3 | EAAT1 | P290R | Loss-of-function | Reduced | Episodic ataxia | AD | 612656 |
| C186S | Minimally reduced | Altered | |||||
| M128R, T318A, A329T1, V393I, R454Q, K520R | ? | ? | |||||
| T387P | Loss-of-function | Reduced | Hemiplegia migraines with aura | AD | |||
| A329T2 | ? | ? | Benign essential blepharospasm | AD | |||
| E219D | Gain-of-function | Increased | Tourette syndrome | AD | |||
| SLC1A4 | ASCT1 | E256K, R457W | Loss-of-function | Unaltered | Spastic tetraplegia, thin corpus callosum, and progressive | AR | 616657 |
| Y191*, L315Hfs*42, G381R,W453* | ? | ? | microcephaly; SPATCCM | ||||
AR: Autosomal recessive; AD: Autosomal dominant; CHet: Compound heterozygotes; SM: somatic mosaicism.
2.2. EAAT1 (GLAST; SLC1A3)
EAAT1 knockout mice (EAAT1−/−) do not exhibit spontaneous epileptic seizures, but pentetrazol-induced seizures are enhanced both in their severity and in duration (Watanabe et al., 1999). They also show neurological symptoms commensurate with the prominent expression of EAAT1 in the cerebellum (i.e. reduced motor coordination), the inner ear (hearing loss) and retina (impaired vision). The mice display poor nesting behavior, abnormal sociability and anhedonia (Karlsson et al., 2009; Watase et al., 1998).
Point mutations in EAAT1 (SLC1A3) trigger primary episodic ataxia (EA) in patients harboring the mutations (Jen and Wan, 2018). Episodic ataxia is initiated by paroxysmal cerebellar dysfunction leading to a myriad of symptoms. Episodic ataxia is classified into 8 subtypes (EA1-8) based on the genetic loci affected. Common symptoms in these patients include ataxia with dizziness, migraine, seizures, slurred speech, vertigo, hemiplegia and postural/gait imbalance set off by physical and emotional stress (Choi & Choi, 2016). EAAT1 mutations (Fig. 3a) are categorised as episodic ataxia subtype 6 (EA6). The first EAAT1 mutation associated with EA6, was a heterozygous de novo substitution of proline 290 by arginine (p.(P290R)) (Jen et al., 2005). Functional studies revealed a ~ 90% reduction in radiolabeled glutamate uptake in COS-7 cells expressing the p.(P290R) mutant alone. Co-transfecting the plasmids encoding wild type EAAT1 and p.(P290R) also caused reduced surface trafficking and uptake, indicative of a dominant-negative effect of the mutant on the wild type allele. Despite its low plasma membrane expression, surface-resident p.(P290R) had increased chloride conductance, due to a higher open probability of the chloride channel, contrasting the wild type transporter (Winter, Kovermann, & Fahlke, 2012). This gain-of-function in anionic conductance may explain the more severe symptoms triggered by this variant in people, compared to EAAT1−/− mice or flies (Parinejad et al., 2016). Another ataxia-associated mutation, the p.(C186S) heterozygous variant (De Vries et al., 2009), shows only 18% reduction in glutamate uptake compared to wild type EAAT1, when expressed in COS-7 cells. The mutation appears to disrupt trafficking, rather than the uptake activity of EAAT1 (Hayashi & Yasui, 2015). A patient harboring another EAAT1 variant, p.(T387P), suffered from relatively mild hemiplegic migraine (Kovermann et al., 2017). Biochemical analysis of the mutant revealed reduced trafficking to the plasma membrane. Moreover, an abrogated glutamate transport by p.(T387P), inferred from electrophysiological measurements, impinged on impaired intracellular K+ binding.
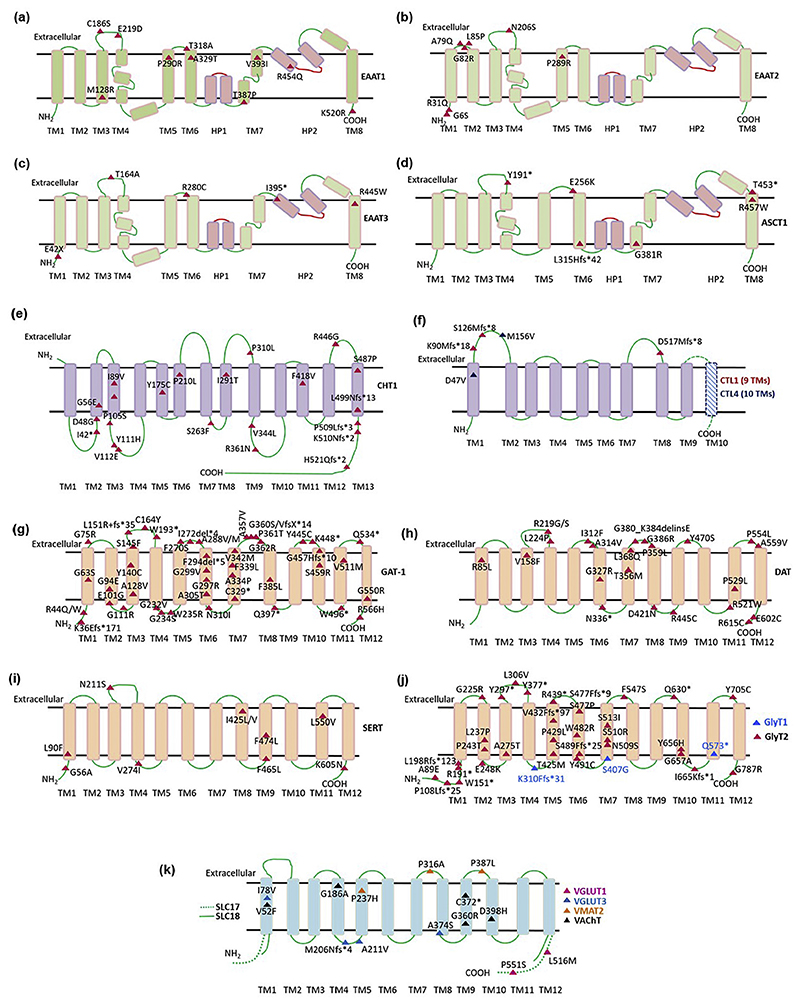
Fig. 3.
Disease-associated variants mapped onto topology diagrams of different NTT subfamilies. The location of point mutations, associated with human disease, are displayed for NTT family members: (a) EAAT1, (b) EAAT2, (c) EAAT3, (d) ASCT1, (e) CHT1, (f) CTL1 (in red) and CTL4 (in blue), (g) GAT1, (h) DAT, (i) SERT, (j) GlyT1 (in blue) andGlyT2(inblack)and (k) vesicular transporters; VGLUT1 (in magenta), VGLUT3 (in blue), VMAT (in orange) and VAChT (in black).
Additional episodic ataxia variants in EAAT1 (Table 1 and Fig. 3a) are yet to be subjected to biochemical and functional characterization (Choi et al., 2017; Choi et al., 2017; D’Adamo et al., 2015; Iwama et al., 2018; Pyle et al., 2015). Most of these variants have been classified as pathogenic, based on the segregation among the affected families and on computational tools. In some cases, EAAT1 mutations are co-inherited with mutations in other genes implicated in the onset of episodic ataxia (Choi, Kim, et al., 2017; D’Adamo et al., 2015). The uncharacterized A329T mutation gives rise to episodic ataxia (Choi, Kim, et al., 2017) and to benign essential blepharospasm (Dong et al., 2020). On the other end of the spectrum, the gain-of-function p.(E219D) EAAT1 variant occurs at higher frequencies in individuals with Tourette syndrome, relative to the general population (Adamczyk et al., 2011). Together, these observations denote that: (i) uncharacterized variants necessitate experimental investigation to unveil their impact on trafficking and the transport cycle, and (ii) in some instances, modifying genes might contribute to the variable penetrance in episodic ataxia.
2.3. EAAT2 (GLT-1; SLC1A2)
EAAT2 is the most abundant glutamate transporter expressed throughout the CNS, accounting for approximately 1% of all brain proteins and 90% of the total glutamate uptake (O’Donovan et al., 2017). EAAT2 knockout mice (EAAT2−/−) are phenotypically normal at birth. The symptoms of hyperactivity and epileptic seizures appear around 3 weeks of age, accompanied by stunted growth relative to wild type littermates. Half of all EAAT2−/− mice die within 4 weeks. These mice exhibit a 95% reduction in glutamate clearance from the synapse, leading to higher extracellular glutamate levels (Tanaka et al., 1997). Heterozygous (EAAT2+/−) mice show milder neurological symptoms, but they have an increased susceptibility to neuronal loss upon traumatic spinal cord injury (Kiryk et al., 2008). Random mutagenesis screening in zebrafish led to the discovery of techno trousers (tnt) locomotor mutants (Granato et al., 1996): the mutant fish larvae show an abnormal escape response with exaggerated body bends in response to touch 2 days after after fertilization. At 4 days post fertilization, they are paralyzed (McKeown et al., 2012). The phenotype is accounted for by a conservative point mutation (A393V) located in TM7b of EAAT2 and can be phenocopied by an EAAT2 inhibitor (McKeown et al., 2012). Collectively, these findings highlight the role of glial EAAT2 in controling neuronal excitability and preventing excitotoxicity by excess synaptic glutamate.
The very first point mutation in human EAAT2 (SLC1A2) was identified in a patient afflicted with sporadic amyotrophic lateral sclerosis (ALS), i.e. the p.(N206S) variant (Aoki et al., 1998) illustrated in Fig. 3b. ALS is a progressive neurodegenerative disease, leading to the loss of voluntary muscle control due to the death of motor neurons in the brain and spinal cord. N206 is highly conserved among EAATs and is a putative site for N-glycosylation. Its substitution by serine impedes glycosylation, trafficking from the ER and Golgi to the plasma membrane, resulting in a loss-of-function phenotype (Trotti et al., 2001).
In addition, EAAT2 point mutations have been identified in several cases of epileptic encephalopathy (EE). Almost all of these mutations (Table 1, Fig. 3b) are inherited in an autosomal dominant fashion. Early onset EEs are epilepsies with heterogeneous genetic causes characterized by several treatment-resistant seizure types, frequent epileptiform activity in the electroenchepalogram (EEG) and global/neuronal developmental delay (McTague et al., 2016). The p.(L85P) variant is recurrent in patients suffering from EE (Epi4K Consortium, 2016; Stergachis et al., 2019). Biochemical analysis revealed that EAAT2-L85P mediates <10% uptake and imposes a dominant negative effect on the wild type transporter. L85P appears to impair either transporter folding, trafficking or assembly of the mixed trimeric transporter complexes as a functional unit (Stergachis et al., 2019). While the EE-associated p.(P289R) variant is yet to be characterized, it is equivalent to and expected to mimic the properties of the p.(P290R) EAAT1 variant, discovered in a patient with episodic ataxia (described above).
Apart from EE, dysregulation of EAAT2 has also been implicated in the development of other neurological and neuropsychiatric disorders, such as hereditary spastic paraplegia, Alzheimer’s disease, depression, autism, schizophrenia and bipolar depression. EAAT2 variants that have not been functionally or biochemically examined are compiled in Table 1 and Fig. 3b (Epi4K Consortium, 2013; Epi4K Consortium, 2016; Fiorentino, Sharp, & McQuillin, 2015; Guella et al., 2017; Meyer et al., 1998; Stergachis et al., 2019; Wagner et al., 2017). In the absence of functional data, their phenotypic repercussions can currently not be surmised.
2.4. EAAT3 (EAAC1, SLC1A1)
EAAT3 is expressed predominantly in neurons of the neocortex, hippocampus and some midbrain regions (Vandenberg & Ryan, 2013). EAAT3 knockout mice (EAAT3−/−) do not exhibit any spontaneous epileptic seizures or neurodegeneration, but they show reduced spontaneous locomotion in an open field test, and are subject to premature ageing. The mice present dicarboxylic aminoaciduria (DA) with increased urinary excretion of aspartate and glutamate (Peghini et al., 1997).
Point mutations in human EAAT3 also lead to DA, an autosomal recessive disorder, manifested by an abnormal excretion of urinary glutamate and aspartate due to the lack of reabsorption of anionic amino acids in the kidney. The first EAAT3 DA mutation, p.(R445W), was identified in an adult patient who, along with his brother, bore kidney stones in early adulthood and had abnormally high urine levels of glutamate and aspartate (Bailey et al., 2011). The second mutation was found in a child harboring a 3-base pair (TCA) deletion at positions c.1184– 1186, leading to the deletion of isoleucine 395 (p.(I395del)). Functional characterization revealed both variants to be trafficking-deficient: the lack of plasma membrane expression was associated with an almost complete abrogation of substrate transport and of transport-associated currents. The patient harboring the p.(R445W) mutation (Fig. 3c) also exhibited an undiagnosed obsessive-compulsive disorder (OCD)-like trait, suggestive of EAAT3 mutations playing a role in the onset of neurological disorders. This is further endorsed by the discovery of uncharacterized EAAT3 variants in patients suffering from severe OCD and bipolar depression (p.(T164A), Wang et al., 2010), schizophrenia/schizotypal personality disorder (p.(R280C), Demily et al., 2018) and autism (p.(E48*), Doan et al., 2019). In fact, EAAT3 was proposed as a novel susceptibility locus in the pathogenesis of schizophrenia and bipolar disorders (Afshari et al., 2015; Myles-Worsley et al., 2013; Rees et al., 2014). Thus, the existing evidence links EAAT3 mutations to a broad spectrum of mental disorders. The actual impact of the uncharacterized EAAT3 mutations on its trafficking and transport cycle ought to be assessed for explaining the phenotypic variation, which may also be influenced by other genes.
2.5. EAAT4 (SLC1A6) and EAAT5 (SLC1A7)
EAAT4 is expressed primarily in Purkinje cells of the cerebellar cortex. It is also sporadically present in the cerebral cortex, vestibular hair cells and calyx endings. EAAT5 is exclusively localized to the retina, with presynaptic expression at the synaptic terminals of cone, rod and bipolar rod cells (Vandenberg & Ryan, 2013). Notably, compared to other transporters, EAAT4 and EAAT5 have a reduced rate of glutamate turnover and a high chloride conductance. Thus, it has been argued that they serve as (inhibitory) glutamate-receptors, rather than as glutamate transporters (Dehnes et al., 1998; Veruki, Mørkve, & Hartveit, 2006). Knocking out EAAT4 in mice (EAAT4−/−) triggers age-dependent progressive motor deficits and ataxia due to Purkinje cell death in the cerebellum (Perkins et al., 2018). Currently, there are no reports on EAAT5 knockout mice or on human disease mutations in either EAAT4 or EAAT5.
2.6. ASCT1 (SLC1A4) and ASCT2 (SLC1A5)
The distribution of ASCT1 and ASCT2 in neurons and glial cells has been well described (Gliddon, Shao, LeMaistre, & Anderson, 2009; Weiss, Derazi, Kilberg, & Anderson, 2001). ASCT1 knockout (ASCT1−/−) or ASCT2 knockout (ASCT2−/−) mice are viable and fertile (Kaplan et al., 2018). ASCT2−/− mice show no behavioral changes. Conversely, ASCT1−/− mice display sensorimotor, behavioral and learning deficits. These phenotypic differences in ASCT1−/− and ASCT2−/− mice also hold true in the corresponding human ASCTs. Point mutations in ASCT1 cause early onset spastic tetraplegia, thin corpus callosum, and progressive microcephaly (SPATCCM), inherited in an autosomal recessive manner (OMIM# 616657). Of the ASCT1 variants (listed in Table 1 and Fig. 3d), Y191* (Abdelrahman, Al-Shamsi, John, Ali, & Al-Gazali, 2019), L315Hfs*42 (Damseh et al., 2015) and W453* (Conroy et al., 2016) code for truncated transporters. While no biochemical characterization data is available, the variants are predicted to have folding and/ or trafficking deficits. The phenotype of other mutants, e.g. the recurrent p.(E256K) (Damseh et al., 2015; Heimer et al., 2015; Srour et al., 2015) and p.(R457W) variants, can be accounted for by their impaired transport cycles. Immunoblots of the surface-labelled protein fractions suggested normal delivery of these mutants to the plasma membrane (Damseh et al., 2015). Trafficking of the p.(R457W) ASCT1 variant to the cell surface is peculiar, since the equivalent mutation in EAAT3 (p. (R445W) Bailey et al., 2011), is in fact ER-retained. The p.(E256K) variant showed markedly reduced levels of uptake of tritiated serine and alanine. Substrate uptake by p.(R457W) was abolished, which rationalizes the more severe clinical symptoms in the patient harboring the latter mutation. Another ASCT1 variant p.(G381R) was predicted to be damaging by in silico modeling, but without further experimental validation (Pironti et al., 2018).
3. SLC5 and SLC44 families: choline transporters
3.1. Choline transporters
Acetylcholine (ACh) exerts its neurotransmitter action in the central and peripheral nervous systems via the cognate nicotinic and muscarinic acetylcholine receptors (Brown, 2019). Upon synaptic release, ACh is degraded to choline and acetate by acetylcholinesterases residing in post-synaptic membranes. While acetate diffuses into the surrounding extracellular space, choline is transported into presynaptic neurons by the designated choline transporters. Choline transporters encompass the high-affinity choline transporter, with a KM of ~2 μM (CHT1, SLC5A7) (Sarter & Parikh, 2005) and the Na+-independent intermediate affinity SLC44 (choline transporter-like proteins, CTLs) transporters (Traiffort, O’Regan, & Ruat, 2013). The latter group is comprised of 5 members (SLC44A1–5), of which CTL3 (SLC44A3) and CTL5 (SLC44A5) are only poorly characterized (Traiffort et al., 2013).
3.2. CHT1 (SLC5A7)
CHT1 is a Na+/Cl−-dependent secondary active transporter (Apparsundaram, Ferguson, George Jr, & Blakely, 2000), with highly regulated assembly and trafficking to the cell membrane (for details see: Ribeiro et al., 2005; Matsuo et al., 2011; Cuddy et al., 2012; Okuda et al., 2012). Choline uptake by CHT1 is crucial: 1) Neurons cannot synthesize choline de novo; i.e., uptake by CHT1 is the main source of choline. 2) Choline provided by CHT1 is converted to ACh by choline acetyltransferase, with CHT1-mediated choline uptake being the ratelimiting step in ACh synthesis (Ferguson & Blakely, 2004; Okuda and Haga, 2003; Ribeiro et al., 2006). 3) CHT1 knockout (CHT1−/−) mice die within 1 h of birth as a result of impaired breathing (Ferguson et al., 2004), 4) Point mutations in CHT1 lead to inheritable neuromuscular junction disorders, transmitted in both, autosomal recessive and dominant manner (Wortmann & Mayr, 2019).
Loss-of-function point mutations in CHT1, inherited in an autosomal dominant manner, lead to the onset of one of the many types of distal hereditary motor neuronopathy (dHMN, for details see Irobi et al., 2006). CHT1 mutations (Table 2 and Fig. 3e) are grouped as type VIIa dHMNs (OMIM#158580), characterized by distal muscular atrophy and unilateral or bilateral vocal cord paralysis (McEntagart et al., 2001). Multiple members of 2 unrelated Welsh kindred, suffering from type VIIa dHMNs, were found to harbor a heterozygous frame shift mutation, leading to the p.(K499Nfs*13) variant (Barwick et al., 2012; Ingram et al., 2016). This truncated variant lacks the cytoplasmic C-terminal domain and the concomitant motifs required for CHT1 trafficking from the ER to the membrane. Unsurprisingly, studies of this mutant in heterologous systems revealed reduced protein abundance/translation and impaired surface trafficking, which led to diminished choline uptake (to ~30% of wild type) upon co-transfection with the wild type CHT1 plasmid. Interestingly, mice with a monoallelic knockout of CHT1 (CHT1+/-) are viable and fertile, with no discernible differences to wild type mice in standard behavioral tests (Bazalakova et al., 2007; Ferguson et al., 2004). These findings are indicative of a dominant negative effect of p.(K499Nfs*13) on the wild type CHT1. Subsequently, 3 additional frameshift variants (p.(P509Lfs*3), p.(H521Qfs*2) and p. (K510Nfs*2)) were identified in patients afflicted with type VIIa dHMNs (Hamanaka et al., 2018; Salter et al., 2018). These variants are expected to be trafficking-deficient due to their truncated C-termini.
Table 2. Mutations in human plasmalemmal choline transporters.
| Gene | Protein name | Missense coding variants | Transport phenotype | Trafficking to target membrane | Disease phenotype | Mode of inheritance | OMIM# |
|---|---|---|---|---|---|---|---|
| SLC5A7 | CHT1 | K499Nfs*13 | Loss-of-function | Reduced | Distal hereditary motor neuronopathy | AD | 158580 |
| P509Lfs*3, K510Nfs*2, H521Qfs*2 | ? | ? | |||||
| D48G, G65E, P105S, R361Q, R446G, S263F | Loss-of-function | No change | Congenital myasthenic syndrome | AR/CHet | 617143 | ||
| S94R, V112E, P210L, | Loss-of-function | Altered | |||||
| I42*, Y111H, Y175C, I291T, P310L, V344L, F418V, S487P | ? | ? | |||||
| SLC44A1 | CTL1 | D517Mfs*19 K90Mfs*18, S126Mfs*8 | Loss-of-function | Unaltered Reduced | Childhood-onset neurodegeneration with ataxia, tremor, optic atrophy, and cognitive decline (CONATOC) | AR | 618868 |
| SLC44A4 | CTL4 | D47V | ? | ? | Age-related macular degeneration and blindness | ? | |
| M156V | Loss-of-function | ? | Postlingual non-syndromic mid-frequency sensorineural hearing loss | AD | 617606 | ||
AR: Autosomal recessive; AD: Autosomal dominant; CHet: Compound heterozygotes.
Patients with homozygous or compound heterozygous mutations in the CHT1 gene suffer from a distinct type of congenital myasthenic syndrome (CMS20, OMIM#617143; Rodríguez Cruz, Palace, & Beeson, 2018). Manifested as susceptibility to fatigue and muscle weakness, these mutations lead to a phenotypic spectrum in patients; while some exhibit classical neonatal onset of CMS accompanied by treatable episodic apneas and a good prognosis, others develop brain atrophy, intellectual disability and developmental delay. Some mutations are lethal and lead to antenatal or infantile lethal arthrogryposis (congenital joint contracture), severe hypotonia and/or akinesia (Banerjee et al., 2018; Bauché et al., 2016; Kashevarova et al., 2014; McMacken et al., 2018; Pardal-Fernández et al., 2018; Wang et al., 2017). Of the 17 variants reported to date (Table 2 and Fig. 3e), the p.(S94R), p.(V112E) and p. (P210L) variants have defective trafficking and accumulate in intracellular compartments (for details, see Section 9). This leads to dramatic reductions in choline uptake (Wang et al., 2017). The remaining characterized mutants (Banerjee et al., 2018; Bauché et al., 2016), all preserved membrane trafficking and had protein levels and stability comparable to the wild type transporter (shown by immunoblots), but had severely reduced choline uptake. This is indicative of intrinsic deficits introduced by mutations in conformational transitions required for substrate translocation. Other, as yet uncharacterized CHT1 mutants (Bauché et al., 2016; McMacken et al., 2018; Pardal-Fernández et al., 2018) are listed in Table 2. Of note, a non-synonymous CHT1 polymorphism coding for a p.(I89V) variant leads to a 40 –50% reduction in maximal choline uptake compared to the wild type CHT1 (Okuda et al., 2002). This particular polymorphism has been implicated in distraction vulnerability (Berry et al., 2014), ADHD (English et al., 2009) and major depressive disorders (Hahn et al., 2008).
3.3. Choline transporter-like proteins (CTL 1–5; SLC44 A1-5)
The cellular distribution of CTL1 (SLC44A1), has been studied in detail (Michel and Bakovic, 2012): there is a plasma membrane fraction, which plays an important role in providing choline for phospholipid synthesis at the cell surface, and a mitochondrial fraction, which provides choline for betaine production in the mitochondria. CTL1 is expressed in neurons as well as in myelinating and mature oligodendrocytes, but it does not overlap with markers of cholinergic neurotransmission (Traiffort, Ruat, O’Regan, & Meunier, 2005). Point mutations in CTL1 lead to an autosomal recessive early onset neurodegeneration with ataxia, tremor, optic atrophy and cognitive decline or CONATOC (OMIM# 618868). Other symptoms of CONATOC include macrocephaly, swallowing difficulties, truncal muscle weakness and hypotonia. So far, 3 CTL1 variants (Table 2 and Fig. 3f) have been identi fied in affected individuals from 3 unrelated consanguineous families (Fagerberg et al., 2020; Reuter et al., 2017). The p.(D517Mfs*19) variant is a truncated protein lacking 2 transmembrane domains and the C-terminus. Despite the truncation, it is trafficked to the cell surface at levels comparable to wild type CTL1, but its choline uptake ability is reduced by 70–80% (Fagerberg et al., 2020). This is indicative of deficits in the catalytic transport activity triggered by the mutation. In contrast, the p.(K90Mfs*18) and p.(S126Mfs*8) variants both resulted in too short a protein fragment to possess any functional capacity. In addition, patient fibroblasts expressing the p.(D517Mfs*19) mutant had altered homeostasis of phosphatidylethanolamine, phosphatidylserine, iron and mitochondrial fatty acids at the cost incurred for maintaining normal levels of phosphatidylcholine. These defects were accompanied by ultrastructural abnormalities, as shown by electron microscopy: an elongated ER, increased mitochondria and small vesicles, but a reduced number of free ribosomes. Interestingly, the pleotropic consequences were reversed by supplementation of choline at high concentrations (Fagerberg et al., 2020), presumably because organic cation transporters, with their low affinity for choline, supported adequate levels of influx.
The cognate substrate of CTL2 (SLC44A2) is not known: circumstantial evidence suggests that CTL2 does transport choline, albeit to a lesser extent than CTL1 (Kommareddi et al., 2010; Nakamura et al., 2010). CTL2 was first identified as an inner ear antigen, targeted by autoantibodies implicated in autoimmune hearing loss (Nair et al., 2004). The expression of CTL2 in glial and sensory cells (=hair cells) of the inner ear is consistent with the hypothesis that CTL2 is required for hair cell survival (Beyer et al., 2011). This conjecture was verified in CTL2 knockout (CTL2−/−) mice (Kommareddi et al., 2015): the mice show agedependent loss of hearing; initially, their perception of high frequencies is impaired, but as they age, the loss of hair cells and neurons in the spiral ganglion progresses, resulting in reduced auditory perception across the entire spectrum of frequencies. Since CTL2 is required for the integrity of hair cells, it is a candidate gene in the screening for Menière’s disease (OMIM# 156000), a disorder associated with episodic (rotational) vertigo, impaired hearing and tinnitus (Nair et al., 2016).
CTL4 (SLC44A4) transports choline and thiamine across plasma membranes (Nabokina et al., 2014; Traiffort et al., 2013). Transcripts are barely detected in the brain, but show pronounced expression in the inner ear, intestine, stomach and kidney (Ma et al., 2017; Traiffort et al., 2013). CTL4, like CTL2, has also been implicated in sensorineural hearing loss. A loss-of-function point mutation p.(M156V) (Fig. 3f and Table 2), inherited in an autosomal dominant manner, was found in multiple members of a Chinese family, who suffered from sensorineural hearing loss, accentuated in the middle frequencies (OMIM# 617606, Ma et al., 2017). Functional characterization of the p.(M156V) variant in heterologous cells revealed significant reduction in choline uptake and subsequent acetyl choline synthesis and secretion. However, cellular trafficking consequences of this mutation were not examined. In the same study, the disruption of CTL4 activity in a zebrafish model led to a significant reduction of hair cells and neuromasts, impairing balance and hearing responses. This is indicative of a vital role for CTL4-mediated choline uptake in the physiology of hair cells in the inner ear. A CTL4 variant p.(D47V) was also screened with other causative genes in patients suffering from age-related macular degeneration and blindness (Cheng et al., 2015). This mutation has not been functionally characterized.
4. SLC6 family: monogenic diseases associated with transporters for γ-aminobutyric acid (GABA), glycine and monoamines
4.1. GABA transporters (GAT1-3, BGT1; SLC6A1,11-13)
GABA is the key inhibitory neurotransmitter in the brain. Similar to glutamate, low levels of extracellular GABA must be maintained to afford a large dynamic range. This is accomplished by plasmalemmal GABA transporters (GATs). The GABA transporter family is comprised of 4 high affinity Na+- and Cl−-coupled symporters: GAT1 (SLC6A1), GAT2 (SLC6A13), GAT3 (SLC6A11) and BGT1 (Betaine transporter, SLC6A12). GAT1 and GAT3 are the most predominant isoforms in the brain; high intensity staining for both isoforms can be seen in the cerebellum (molecular layer), basal ganglia (ventral pallidum, globus pallidus), olfactory bulb (glomerular layer) and the retina (for references see Zhou & Danbolt, 2013; Scimemi, 2014). GAT2 and BGT1, on the other hand, are expressed primarily in the liver and the kidney. GAT1 resides primarily in the axon terminals of GABAergic neurons. GAT3 is predominantly expressed in glial cells.
Phenotypic consequences of non-functional GAT1 were first postulated in patients with a heterozygous microdeletion at 3p25.3 spanning SLC6A1 and SLC6A11 (the genes encoding GAT1 and GAT3, respectively). These patients suffer from developmental delay, intellectually disabilities (ID), ataxia and stereotypic hand movements (Carvill et al., 2015; Dikow et al., 2014; Rauch et al., 2012). In addition, duplication in this locus is implicated in the onset of the Prader-Willi syndrome (Bittel, Kibiryeva, Dasouki, Knoll, & Butler, 2006). Of the >100 GAT1 variants deposited in the ENSEMBL database, 45 point mutations (including 12 frameshift/truncating and 28 missense) are displayed in Fig. 3g and Table 3 (Cai et al., 2019; Carvill et al., 2015; Halvorsen et al., 2015; Islam et al., 2018; Johannesen et al., 2018; Mattison et al., 2018; Palmer et al., 2016; Posar & Visconti, 2019; Rauch et al., 2012; Wang et al., 2020; Zech et al., 2017). The mutations are linked to impaired cognitive development, several epileptic seizure types and mild to moderate ID (mostly language impairment) in the afflicted individuals. Most patients exhibit absence seizures, with the characteristic spike-wave discharges in the electroencephalogram, and behavioral disorders. These symptoms are reminiscent of the phenotypes observed in SLC6A1 −/− mice (Chen et al., 2015; Cope et al., 2009). Myoclonic atonic/astatic epilepsy (Doose Syndrome or MAE, OMIM#:616421) was seen in roughly half the patients, while the others either exhibited different types of generalized or focal epilepsies. Behavioral consequences include aggressiveness, irritability, hyperactivity, ASD and attention deficits (Johannesen et al., 2018). Several de novo missense variants associated with GAT1 have also been implicated in increased susceptibility to autism and schizophrenia (Rees et al., 2020; Satterstrom et al., 2018). Most of the mutations associated with epileptic seizures are de novo, but some are transmitted in an autosomal dominant manner (Carvill et al., 2015; Halvorsen et al., 2015; Johannesen et al., 2018). The dominant negative effect can be rationalized by GAT1 forming constitutive oligomers (see Section 9 for further details, Schmid et al., 2001).
Table 3. Mutations in human plasmalemmal SLC6 neurotransmitter transporters.
| Gene | Protein name | Missense coding variants | Transport phenotype | Trafficking to target membrane | Disease phenotype | Mode of inheritance | OMIM# |
|---|---|---|---|---|---|---|---|
| SLC6A1 | GAT1 | 9/45 variants characterized | Loss-of-function | Myoclonic-atonic epilepsy | AD | 616421 | |
| G94E, W235R, F270S, I272*, Y445C, W496*, G550R | ? | ||||||
| G234S, P361T | Reduced | ||||||
| SLC6A2 | NET | A457P | Loss-of-function | Reduced | Chronic orthostatic intolerance | AD | 604715 |
| SLC6A3 | DAT | A559V, E602G, R615C | Unaltered dopamine uptake, anomalous dopamine efllux | Unaltered | Attention deficit hyperactivity disorder (ADHD), bipolar disorder | AD | |
| R85L, V158F, R219G, R219S, L224P, A314V, G327R, L368Q, G380_K384delinsE, G386R, P395L, R445C, Y470S, R521W, P529L, P554L | Loss-of-function | Reduced | Dopamine transporter defeciency syndrome (Infantile Parkinsonism/dystonia) | AR | 613135 | ||
| V382A | ADHD | AD | |||||
| I312F, D421N | Unaltered | Adult Parkinsonism with ADHD | CHet | ||||
| ΔN336, T356M | Unaltered | Autism spectrum disorder | AD | ||||
| SLC6A4 | SERT | G56A, K605N | Gain-of-function | Unaltered | OCD, depression, Autism spectrum disorder, Asperger’s syndrome, Tourette’s syndrome | AD/AR | 164230 (I425V) |
| I425V, I425L, F465L, L550V | Increased | ||||||
| N211S, V274I, F474L | ? | ? | |||||
| L90F | ? | ? | Anorexia nervosa-restrictive type | AD | |||
| SLC6A5 | GlyT2 | G225R, Y297*, R439*, S477P, S477Ffs*9 | ? | ? | |||
| P108Lfs*25, Y377*, V432Ifs*97, Q630* | Loss-of-function | Reduced | |||||
| W151*, R191*, L198Rfs*123, L237P, P243T, E248K, S489Ffs*39, S513I, F547S, I655Kfs*1, Y656H, G657A | ? | Hyperekplexia | AR | 614618 | |||
| A89E, A275T, L306V, T425M, W482R, Y491C, N509S, G787R | Unaltered | ||||||
| P429L S510R, Y705C | Reduced | AD | |||||
| SLC6A9 | GlyT1 | K310Ffs*31, S407G, Q573* | ? | ? | Glycine encephalopathy | AR | 617301 |
AR: Autosomal recessive; AD: Autosomal dominant; CHet: Compound heterozygotes.
Only a fraction of all GAT1 mutations, reported to date, has been characterized. For instance, the p.(G94E), p.(W235R), p.(F270S), p. (I272del), p.(Y445C), p.(W496*) and p.(G550R) variants showed uptake levels ranging from 0 to 27% of wild type activity (Mattison et al., 2018). Their trafficking is yet to be assessed, i.e. the loss-of-function phenotypes may be due to ER-retention or inactivity at the plasma membrane. Complete functional and trafficking data are available for the p.(G234S) variant identified in a patient with the Lennox-Gastaut syndrome (Cai et al., 2019). Its cell surface expression and GABA uptake were reduced by 30% and 70%, respectively, indicative reduced protein stability, surface trafficking and catalytic transport cycle. The p. (P361T) variant, reported in a patient afflicted with absence and atonic seizures and ASD, is folding-deficient (Wang et al., 2020), it has decreased protein expression, increased ER-retention (determined by immunoblots and confocal microscopy) and > 70% reduction in GABA uptake. Variants such as p.(G362R) (Halvorsen et al., 2015), p.(R44Q) and p.(A288V)(Carvill et al., 2015), are predicted to mimic the synthetic loss-of-function mutations at equivalent residues (Ben-Yona & Kanner, 2013; Rosenberg & Kanner, 2008; Zomot & Kanner, 2003).
The remainder of the known GAT1 variants (Fig. 3g and Table 3) compel functional and biochemical analyses. Mutations that abolish GAT1 activity can compromise synaptic inhibition, as vesicle refilling is contingent on synaptic GABA-uptake. Thus, the depletion of vesicular GABA-stores and the subsequent loss of inhibitory input becomes a possible long-term consequence of GAT1 dysfunction. This prompts one to ruminate over the diversity in phenotypic consequences among the GAT1 variants. It is conceivable that mutations induce variable effects on substrate translocation rates, transport-associated currents, GAT1 delivery to the presynaptic specialization, its internalization from the cell surface and possibly affected interactions with regulatory proteins. This calls for a detailed characterization of pathogenic GAT1 mutants. At this point, no disease relevant mutations have been identified in GAT2, GAT3 and BGT1.
4.2. Norepinephrine transporter (NET; SLC6A2)
The human NET was the first SLC6 transporter, in which a point mutation was reported to trigger protein misfolding with accompanying functional defects (Shannon et al., 2000). The patient (and her identical twin) harbored a loss-of-function p.(A457P) variant, associated with chronic orthostatic intolerance (OMIM# 604715). This condition is manifested by excessive sympathetic activation on physiological cues, inducing the following symptoms: abnormally high plasma norepinephrine concentrations (particularly when standing upright) leading to postural tachycardia, syncope, light-headedness and altered mental activity. The patient was heterozygous for the mutation, indicative of an autosomal dominant mode of inheritance. This was also confirmed biochemically; the p.(A457P) (Table 3) mutant exhibited a severely reduced norepinephrine uptake (<2% of wild type NET) and exerted a dominant-negative effect on wild type NET uptake activity (Hahn, Robertson, & Blakely, 2003). Knock-in mice harboring one allele of p. (A457P) recapitulate the tachycardia (postural hypotension is difficult to reproduce in a 4-legged animal); in addition, they are more anxious, when tested in typical paradigms (elevated plus maze, open field test), but show less signs of behavioral despair in the tail suspension test (Shirey-Rice et al., 2013). Interestingly, in NET+/- mice, substrate uptake is essentially comparable to that seen in wild type animals, despite NET protein levels being reduced by 50%. This alludes to compensatory activation of the transporter by an unknown mechanism. Despite the (near) normal transport activity, NET+/- mice are more anxious, when challenged in the open-field and elevated plus maze tests (Fentress et al., 2013). Similar to p.(A457P) knock-in mice, homozygous NET−/− mice showed less behavioral despair in the forced swim and tail-suspension tests (Xu et al., 2000). In rodents, this response can be elicited by acute administration of antidepressants, which block NET and/or SERT. In fact, this antidepressant-like behavior of NET−/− mice is not further enhanced upon administration of NET-selective antipressants or by an SSRI (selective serotonin-reuptake inhibitor) (Xu et al., 2000). Taken together, the data highlight the complex liaison between transporter activity, maintenance of vesicular stores and synaptic transmission. In NET deficiency, vesicular stores appear to be adequately sustained; the phenotypic consequences are hence more likely to arise from altered rates of norepinehrine clearance from the synaptic cleft.
Apart from p.(A457P) variant, there are several single nucleotide polymorphisms (SNPs) in NET, with altered functional activity and clinical relevance to cardiovascular disorders and the long-QT syndrome (reviewed by Hahn & Blakely, 2002, 2007). One of the SNPs p.(A369P), present at a minor allele frequency of 5%, leads to abolished surface NET expression and concomitant NE clearance presumably due to misfolding (Hahn, Mazei-Robison, & Blakely, 2005). An ADHD-associated variant p.(T283R) (present at a frequency of 0.42%) shows a 30–50% reduction in substrate transport (Hahn, Steele, Couch, Stein, & Krueger, 2009). The trafficking of this variant is yet to be examined.
4.3. Dopamine transporter (DAT; SLC6A3)
DAT knockout (DAT−/−) mice are prone to early death (Giros, Jaber, Jones, Wightman, & Caron, 1996) and require a high-calory diet to reach adulthood. They maintain vesicular dopamine stores by upregulating dopamine synthesis (Efimova, Gainetdinov, Budygin, & Sotnikova, 2016; Gainetdinov, 2008). Their hyperactivity can be accounted for by increased dopamine levels in the synaptic cleft. Approximately a third of all DAT−/− mice sporadically develop a progressive locomotor disorder, characterized by a loss of striatal GABAergic medium-sized spiny neurons. The previously described hyperactive animals developed atactic gait abnormalities, which progress to tremor, weight loss and subsequent death (Cyr et al., 2003). Drosophila melanogaster harboring disrupted dDATs are termed fumin (Japanese for sleepless): they are hyperactive and have a greatly reduced rest or “sleep” time (Kume et al., 2005).
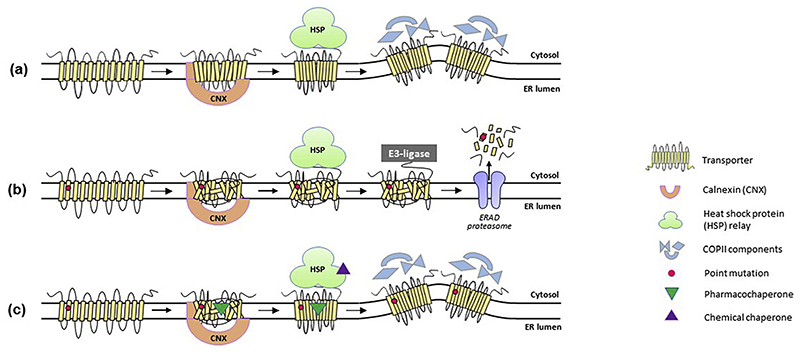
Loss-of-function point mutations in human DAT trigger the syndrome of Parkinsonism and dystonia, with either infantile, juvenile or adult disease onset (Kurian et al., 2009). The infantile and juvenile forms are collectively known as the dopamine transporter deficiency syndrome (DTDS, OMIM#613135). DTDS was first discovered in infants, who manifested abnormal motor development (resting tremor, muscle rigidity, slow dystonic limb movements, abnormal muscle contractions, difficulties in feeding and global developmental delay). So far, at least 14 non-synonymous exonic variants, 3 truncated exonic variants, 7 intronic (including splice site) variants and 1 in frame variant have been identified among DTDS patients (Baga et al., 2020; Heidari, Razmara, Hosseinpour, Tavasoli, & Garshasbi, 2020; Kurian et al., 2009; Kurian et al., 2011; Kuster et al., 2018; Ng et al., 2014; Puffenberger et al., 2012; Yildiz, Pektas, Tokatli, & Haliloglu, 2017). All DTDS mutations (Fig. 3h and Table 3) are inherited in an autosomal recessive manner; patients being either homozygotes or compound heterozygotes. With the exception of p.(R219G/S) and p.(G380_K384delinsE), all exonic mutations have been fully characterized (Asjad et al., 2017; Beerepoot, Lam, & Salahpour, 2016). These variants are folding-deficient, evident from increased complex formation of variants DATs with the cytosolic chaperone HSP70-1A (for details, see Section 9).
The age of onset and the phenotypic spectrum of DTDS appear to be governed by the basal uptake levels of the mutants: e.g., patients harboring the A314V mutation presented DTDS-associated symptoms in adolescence (11 years). This is attributable to A314V having the highest uptake capacity among the known DTDS variants (i.e. 8% of wild type levels) (Ng et al., 2014). This conjecture is further supported by the discovery of a DAT variant identified in a patient with adult onset of Parkinsonism and ADHD (Hansen et al., 2014). The compound heterozygote patient, expressed p.(I312F) and p.(D421N) mutations (Fig. 3h). Individually, p.(I312F) shows ~50%, while p.(D421N) shows only <10% of wild type DAT uptake levels. If both mutants are co-expressed, uptake is reduced to ~30%. The mutants traffic normally to the cell surface and the lack of catalytic activity is caused by perturbed binding of the ligand and Na+ ions to p.(D421N). Both mutants additionally exhibit gain-of-function electrophysiological properties: p.(I312F) shows a large anion conductance and p.(D421N) a large constitutive leak current (Herborg, Andreassen, Berlin, Loland, & Gether, 2018) that may exacerbate reduced DAT function. In addition, p.(D421N) supports anomalous dopamine efflux. Another variant with preserved trafficking, but functional deficits is p.(ΔN336), an in-frame deletion of N336 recently identified in a heterozygous patient suffering from ASD (Campbell et al., 2019). This variant is thought to assume a rate limiting “half-open and inward facing state”, that leads to abrogated dopamine uptake.
A knock-in model in Drosophila melanogaster displayed hyperactivity, impaired social interactions and other behavioral equivalents of ASD. The ADHD-associated SNP, encoding the p.(V382A) variant shows significantly reduced uptake and ligand binding compared to the wild type DAT (Lin and Uhl, 2003). This variant is likely misfolded, trafficking-deficient and impedes wild type DAT delivery to the cell surface, suggestive of a dominant variant effect.
In addition to the loss-of-function mutations, other DAT variants showing defects unrelated to trafficking and catalytic transport have been reported in a clinical setting (Fig. 3h and Table 3). For instance, the p.(A559V) and p.(T356M) variants were screened in patients with bipolar disorder, ASD or ADHD (Bowton et al., 2014; Grünhage et al., 2000; Hamilton et al., 2013; Mazei-Robison, Couch, et al., 2005). These variants exhibit spontaneous anomalous dopamine efflux (Hamilton et al., 2015; Herborg et al., 2018; Horschitz, Hummerich, Lau, Rietschel, & Schloss, 2005; Mazei-Robison, Bowton, et al., 2005). This functional feature was validated in behavioral phenotypes of knock-in animal models (Davis et al., 2018; DiCarlo et al., 2019; Mergy et al., 2014). The functional phenotype of the p.(E602G) variant, identified in a patient with bipolar disorder (Grünhage et al., 2000), is still unknown. There is conflicting evidence in the literature regarding the functional consequences of this variant (Herborg et al., 2018; Horschitz et al., 2005). The variant p.(R615C) (Fig. 3h) was reported in a heterozygous ADHD patient. It supports dopamine uptake to levels comparable to wild type DAT, but underogoes more rapid constitutive and amphetamine-insensitive endocytosis and cell surface recycling. Interestingly, the variant localizes in membrane microdomains different from those inhabited by the wild type DAT (Herborg et al., 2018; Kovtun et al., 2015; Sakrikar et al., 2012). These alterations lead to a gain-of-function effect, reversible by an endocytic brake through cdc42-activated non-receptor tyrosine kinase Ack1 (Wu, Bellve, Fogarty, & Melikian, 2015).
4.4. Serotonin transporter (SERT; SLC6A4)
SERT knockout (SERT−/−) mice are viable and do not have any gross developmental abnormalites (Bengel et al., 1998). They show virtually no serotonin uptake and 60–80% reduction in serotonin levels in various brain regions. These mice are insensitive to the psychostimulant action of 3,4-methylenedioxymethamphetamine (MDMA). SERT−/− mice exhibit anxiety-related behavior, hypolocomotion, predisposition to stress and show less aggression and social interaction (Holmes, Murphy, & Crawley, 2003; Kalueff et al., 2007). The behavioral changes in these mice provide insights into the role of SERT in the progression of affective disorders and other neuropsychiatric diseases in people (Holmes et al., 2003; Kalueff et al., 2007). The only human SERT variant that leads to loss of transport activity is the rare p.(P339L) variant (Glatt et al., 2001), which has dramatically reduced surface expression and serotonin uptake due to misfolding and ER retention (Prasad et al., 2005). Yet, this variant has not been causally linked to any human disease. However, many novel molecular features involved in the folding and trafficking of SERT have been revealed from studies on synthetic misfolded SERT mutants (for details, see Section 9).
Disease relevant point mutations in human SERT are hypermorphic. The mutations were identified by screening patients afflicted with OCD, ASD, eating disorders and other neuropsychiatric disorders (Camarena, González, Hernández, & Caballero, 2012; Delorme et al., 2004; Hernández-Muñoz et al., 2020; Moya et al., 2013; Ozaki et al., 2003; Sutcliffe et al., 2005; Voyiaziakis et al., 2011; Wendland et al., 2008). They are displayed in Fig. 3i and Table 3, and most have been extensively characterized (Kilic et al., 2003; Prasad et al., 2005; Prasad et al., 2009). They show increased rates of serotonin uptake by: 1) increased substrate affinity and/or 2) maximal uptake velocity and/or 3) altered patterns of surface regulation. For instance, the p.(I425V) variant increased the binding and catalytic activity of SERT (reduced KM for 5-HT transport and higher Bmax of radioligand binding), but its trafficking profile remained unchanged relative to wild type (revealed by immunobloting). These properties render p.(I425V) constitutively active in a nitric oxide-independent manner (Kilic et al., 2003). The p.(G56A) variant exhibits elevated basal phosphorylation (Prasad et al., 2009) and assumes a high affinity conformation, which causes an insensitivity to regulation by phosphatases (Quinlan et al., 2019; Quinlan et al., 2020). Experiments on knock-in p.(G56A) mice indicated behavioral responses consistent with autistic symptoms in people, i.e. repetitive behavior, deficits in social interactions and in multisensory processing (Siemann et al., 2017; Veenstra-VanderWeele et al., 2012). Other SERT variants such as p.(F465L) and p.(L550V) preserve their sensitivity to phosphorylation similar to wild type SERT, but display increased trafficking to the cell surface, consequently augmenting catalytic serotonin uptake (Prasad et al., 2009). Uncharacterized SERT variants comprise p. (L90F), p.(N211S), p.(V274I) and p.(F474L).
4.5. Glycine transporter-1 (GlyT1; SLC6A9) and -2 (GlyT2; SLC6A5)
In the brain, GlyT1 is predominantly expressed in astrocytes. GlyT1 knockout mice (GlyT1−/−) are born without any gross abnormalities. Most of these mice die some 6–14 h after birth, largely due to wasting and dehydration caused by their inability to suckle (Eulenburg, Retiounskaia, Papadopoulos, Gomeza, & Betz, 2010; Gomeza et al., 2003; Tsai et al., 2004). Glycine uptake in the frontal brain, brain stem and spinal cord is also greatly diminished in GlyT1−/− mice. In addition, they show severe deficits in motosensory functions, breathing and respiratory rhythmic activity at the pre-Bötzinger complex, arising due to excess glycine receptor activation in the respiratory neurons. Some of the conditional knockout mice survive the critical postnatal period and can have a normal life span. The shocked (sho) gene encodes a mutated GlyT1 gene (G81D in TM2) in zebrafish: the mutation abrogates glycine transport. Homozygous fish embryos do not swim and fail to mount a normal escape response to tactile stimuli (Cui et al., 2005; Mongeon et al., 2008).
Deficits in GlyT1−/− mice parallel the symptoms in people suffering from glycine encephalopathy or non-ketotic hyperglycinemia (NKH), an autosomal recessive disorder with a defective glycine cleavage enzyme complex (Applegarth & Toone, 2006). NKH patients, who were negative for mutations in genes involved in the glycine cleavage system, were subsequently screened for mutations in GlyT1 (Alfadhel et al., 2016; Kurolap et al., 2016): the GlyT1 variants, p.(K310Ffs*31), p. (Q573*) and p.(S407G) (Fig. 3j) were identified in homozygous individuals from 3 unrelated consanguineous families (Table 3). 3 of the 6 affected individuals died within 7 months of age (one death was pre-natal) due to respiratory failure. All patients manifest encephalopathy, shallow breathing, hyperekplexia (exaggerated startle reflex, see below), hypotonia, which paradoxically progresses to muscle hypertonicity resulting in arthrogryposis (joint contracture), and facial dysmorphism (Alfallaj & Alfadhel, 2019). The 2 surviving patients overcame respiratory failure, in a manner similar to some GlyT1−/− mice (see above). Though none of the mutants have been characterized, p. (K310Ffs*31) and p.(Q573*) are predicted to be non-functional transporters, with the former truncated after the 4th transmembrane domain and the latter harboring a premature stop codon in the 11th transmembrane domain.
In contrast to GlyT1, GlyT2 is expressed exclusively in neurons. It is delivered to presynaptic glycinergic terminals, where it retrieves glycine from the synaptic space into the presynaptic neurons. GlyT2 knockout (GlyT2−/−) mice exhibit complex neuromotor deficits that culminate in premature death, after the second post-natal week (Gomeza et al., 2003): on postantal day 10 (P10), GlyT2−/− mice display strong and spontaneous tremor and muscular rigidity. They fail to right themselves when turned on their back. When suspended from their tails, they respond with hind feet clasping. Death occurs due to troubled feeding, desiccation and continued convulsions. These deficits can rationalized by the reduction of glycinergic control in motoneurons.
The complex neuromotor phenotype in GlyT2−/− mice is reminiscent of hyperekplexia (startle disease) in people and other mammals harboring loss-of-function mutations in the key players of glycinergic neurotransmission (Davies et al., 2010; Gill et al., 2011, 2012; Harvey, Topf, Harvey, & Rees, 2008). Hereditary hyperekplexia (human startle disease) is an inherited and genetically heterogenous neurological disorder characterized by an exaggerated startle response to unexpected stimuli, general stiffness following startle events, impaired central pain modulation and other symptoms depending on the gene mutated (Thomas et al., 2013). Thus, startle disease patients were screened for exonic variants in the pre-synaptic neuronal GlyT2 (Carta et al., 2012; Dafsari et al., 2019; Eulenburg et al., 2006; Giménez et al., 2012; Kitzenmaier et al., 2019; Masri et al., 2017; Mineyko et al., 2011; Rees et al., 2006). These studies led to the identification of ~35 disease variants (Fig. 3j and Table 3). In addition to hyperekplexia, most patients with GlyT2 mutations also exhibit hypotonia, recurrent infantile apneas and delayed development (Thomas et al., 2013). The majority of these patients are either homozygotes or compound heterozygotes. Some patients inherit GlyT2 mutations in an autosomal dominant manner (López-Corcuera et al., 2019). The dominant negative effect of the p. (S512R) variants can be rationalized by GlyT2 forming constitutive oligomers (for further details see Section 9). The autosomal dominant p. (Y705C) variant, on the other hand, shows milder functional phenotypes (Giménez et al., 2012). Functional defects, in this variant, arise from the substituted cysteine forming aberrant disulfide bonds with other cysteines in the transporter (as confirmed by cysteine accessibility experiments). Further evidence in support of this proposed mechanism is the rescue of p.(Y705C) to wild type uptake levels by reducing agents (e.g. dithiothreitol) that break such erroneous disulfide bonds (Giménez et al., 2012).
A considerable fraction of GlyT2 disease variants arise from nonsense or frame shift mutations: these truncated transporters are ER-retained (shown by confocal miscroscopy and immunoblotting) and consequently show no glycine uptake (Carta et al., 2012; Eulenburg et al., 2006; Rees et al., 2006). Furthermore, many variants can readily traffic to the plasma membrane, but impair the catalytic function of GlyT2. For instance, the defects in p.(W482R), p.(N509S) and p. (A275T) GlyT2 variants were characterized electrophysiologically. The p.(W482R) variant, showed Na+-dependent transient currents, but no glycine induced currents (Rees et al., 2006). This is indicative of normal surface expression, but perturbed glycine binding in this variant. The p. (N509S) and p.(A275T) variants, on the other hand, showed reduced and voltage-dependent potencies in mediating glycine-induced currents, relative to wild type GlyT2 (Carta et al., 2012; Rees et al., 2006). These variants evidently affect Na+ binding and glycine-Na+ coupling, which are essential for the GlyT2 transport cycle. Molecular modeling studies determined the effects of mutations on the GlyT2 catalytic cycle, revealing that the amino acid transition in the p.(S513I) variant precludes Cl− binding (Carta et al., 2012). Other variants are yet to be characterized.
4.6. Pathological variants in other SLC6 transporters
SLC6A6 encodes TauT, a plasmalemmal transporter which primarily mediates uptake of taurine and, to a lesser extent, GABA (Ramamoorthy et al., 1994; Tomi, Tajima, Tachikawa, & Hosoya, 2008; Uchida et al., 1992). Taurine is a sulfur containing amino acid that is mainly supplied by dietary intake and its entry into various tissues is achieved by TauT (Schuller-Levis & Park, 2003). Taurine is abundantly present in many organs including the brain and retina. It acts as an osmolyte and anti-oxidant in cells; underlying its pleiotropic action in the immune system and neurons. TauT−/−mice exhibit negligible levels of taurine uptake in all tissues, resulting in multisystem failure, lower body mass, impaired fertility, cardiomyopathy, progressive and severe retinal degeneration, liver fibrosis, age-dependent hearing loss and reduced exercise capacity by skeletal muscles (Heller-Stilb et al., 2002; Ito et al., 2008; Warskulat et al., 2007). While the role of TauT deletion in the advent of the 3p-syndrome (OMIM#613792, Patel et al., 1995; Han, Budreau, Chesney, & Sturman, 2000) is unclear, disease relevant loss-of-function mutations in TauT mimic the phenotypic features of TauT−/−mice. Homozygous deletion of the splice site between exon 8 and 9 of TauT was associated with dilated cardiomyopathy in one patient (Shakeel, Irfan, & Khan, 2018). Biallelic mutations in TauT that encode the p.(A78E) and p.(G399V) variants (Table 4) were identified in siblings from 2 unrelated families suffering from rapidly progressive childhood retinal degeneration (Ansar et al., 2020; Preising et al., 2019), accompanied by cardiomyopathy (Ansar et al., 2020). TauT-A78E and G399V were correctly delivered to the cell surface, but their transport activity was impaired (5–15% uptake of wild type TauT). Molecular modeling predicts that glutamate E78 substitution forms an anomalous salt bridge with arginine R284. This salt bridge may stabilize transporter conformations that do not affect folding and trafficking but may perturb taurine and co-substrate binding (Preising et al., 2019). Molecular modeling of the p.(G399V) variant predicted that the valine substitution indirectly slows down conformational transitions needed for the progression along the Taut transport cycle (Ansar et al., 2020). Patients harboring this variant were orally administered high taurine doses, which improved visual performance and remedied the cardiomyopathy symptoms due to taurine supplementation by an unknown transporter.
Table 4. Mutations in other human plasmalemmal SLC6 transporters.
| Gene | Protein name | Missense coding variants | Transport phenotype | Trafficking to target membrane | Disease phenotype | Mode of inheritance | OMIM# |
|---|---|---|---|---|---|---|---|
| SLC6A6 | TauT | A78E, G399V | Loss-of-function | Unaltered | Childhood retinal degeneration with or without cardiomyopathy | AR | |
| SLC6A8 | CRT1 | >80 point mutations reported | Loss-of-function | Reduced in many | Cerebral creatine deficiency syndrome 1 | XLR | 300352 |
| SLC6A17 | NTT4 | G162R, P633R | ? | Unaltered for G162R, altered for P633R | Intellectual disability | AR | 616269 |
| SLC6A18 | B0AT3 | G79S, Y319X, P478L, G496R | ? | Reduced (for G79S,Y319X, G496R), unaltered (for P478L) | Digenic iminoglycinuria (DIG)/hyperglycinuria (HG) when SLC36A2 mutations are co-inherited | AD, AR, DR, CHet | 242600 (DIG) 138500 (HG) |
| SLC6A19 | B0AT1 | >20 mutations | ? | ? | Hartnup Disorder | AR | 234500 |
| IVS7–4G → A | ? | ? | Iminoglycinuria (IG)/hyperglycinuria (HG) when SLC36A2 mutations are co-inherited | AD, AR, DR | 242600 (DIG) 138500 (HG) | ||
| SLC6A20 | XTRP3 | T199M | Reduced | Unaltered | Iminoglycinuria (IG)/hyperglycinuria (HG) when SLC36A2 mutations are co-inherited | AD, AR, DR | 242600 (DIG) 138500 (HG) |
AR: Autosomal recessive; AD: Autosomal dominant; CHet: Compound heterozygotes; XLR: X-linked recessive; DR: Digenic recessive.
SLC6A7 encodes the transporter for L-proline (ProT) in the human brain (Shafqat et al., 1995). Proline is a non-essential amino acid, playing a crucial role in several metabolic pathways (e.g. the synthesis of arginine and glutamate in mitochondria). Many human inherited disorders associated with dysfunction in proline metabolism have been reported, some with neurological consequences (Wyse & Netto, 2011). There are no known disease-associated variants in ProT to date, but a large deletion of the 5q32 region, encompassing the SLC6A7 gene, has been identified in patients with intellectual disabilities and mandibulofacial dysostosis (Vincent et al., 2014).
SLC6A8 encodes the creatine transporter-1 (CRT1, Nash et al., 1994; Dai, Vinnakota, Qian, Kunze, & Sarkar, 1999). Creatine, a precursor of phosphocreatine, acts as an energy buffer to maintain cellular ATP levels (Rae & Bröer, 2015). Over 80 point mutations in the human CRT1 have been identified (Table 4), a large fraction of which code for missense variants (Farr, El-Kasaby, Freissmuth, & Sucic, 2020). Loss-of-function mutations in CRT1 lead to an X-linked creatine transporter deficiency (CTD), clinically manifested as mild to severe intellectual disability, developmental delay, epilepsy and ASD (OMIM#300352, Salomons et al., 2001; Van De Kamp, Mancini, & Salomons, 2014). To date, only a few studies have assessed the molecular features of CTD, by examining their folding and trafficking profiles (Betsalel et al., 2012; Salazar et al., 2020; Uemura et al., 2017; Valayannopoulos et al., 2013). We recently classified 16 loss-of-function missense CRT1 variants as misfolded (see Section 9, El-Kasaby et al., 2019). CRT1 knockout (CrT-/y)micehaveim-paired learning and memory acquisition reflecting the global intellectual deficits in CTD patients (Skelton et al., 2011). SLC6A10 or CRT2, located on chromosome 16, is a pseudogene paralogous to CRT1. SLC6A14 encodes a transporter for neutral, dipolar and basic amino acids (ATB0+), primarily expressed in peripheral tissues (Sloan & Mager, 1999). No disease mutations have been identified in ATB0+ thus far.
The remaining 6 SLC6 transporters (SLC6A15–20) predominantly transport neutral amino acids (Bröer, 2009). Interestingly, of these neutral amino acid transporters, SLC6A17 codes for the vesicular transporter NTT4, which is also abundantly expressed in both dendritic and post- synaptic sites of predominantly glutamatergic and some GABAergic synapses (Hägglund et al., 2013; Iqbal et al., 2015). NTT4 mediates Na+-coupled uptake of alanine, glycine, leucine, proline and glutamate (Parra et al., 2008; Zaia & Reimer, 2009). Disease relevant mutations in NTT4 include 2 missense variants; p.(G162R) and p.(P633R) (Table 4), discovered in unrelated families with an autosomal recessive form of intellectual disability accompanied by progressive tremors, speech impairment and abnormal behavior (OMIM#616269) (Iqbal et al., 2015). In transfected hippocampal neurons, these variants show different phenotypic profiles, evident from confocal microscopy images: 1) the p.(G162R) variant does not exhibit any trafficking deficits, but molecular modeling predicts structural perturbations, which likely impair its transport activity. Through unknown mechanisms, p.(G162R) also appears to suppress dendritic spine formation, alter dendritic morphology and induce global morphological changes in transfected neurons. 2) On the other hand, the trafficking of p.(P633R) to the dendrites is compromised. According to molecular modeling, the substitution of hydrophobic proline, which contacts the membrane lipids, by charged arginine induces anisotropic changes in protein folding. SLC6A15 encodes the sodium-coupled branched-chain amino acid transporter (SBAT1), a protein abundantly expressed in the brain (Bröer et al., 2006; Takanaga, Mackenzie, Peng, & Hediger, 2005). No point mutations in SLC6A15 have been reported, but polymorphisms, which reduce the expression of SBAT1 transcripts, have been linked to an enhanced risk for major depression and stress susceptibility (Kohli et al., 2011). SLC6A16 encodes the poorly characterized NTT5, which is expressed in the brain, among many other tissues (Farmer et al., 2000); no known variants thereof are known as yet.
SLC6A18 (B0AT3), SLC6A19 (B0AT1) and SLC6A20 (XTRP3) encode amino acid transporters, which are expressed in the kidney and intestine, where they facilitate the absorption of glycine, proline, and neutral amino acids, respectively (Bröer, 2006Mutations in these genes, upon co-inheritance with SLC36A2 (proton-coupled amino acid transporter-2, PAT2) mutations, act as modifiers to the complex pathogenesis of digenic iminoglycinuria (OMIM#242600) or hyperglycinuria (OMIM#138500), which results in renal wasting of proline and glycine (Bröer et al., 2008). This study describes 4 B0AT3 point mutations, including p.(G79S), p.(Y319X), p.(L478P) and p.(G496R), a B0AT1 splice site variant and a XTRP3 missense variant p.(T199M). The expression of these variants was abstruse in heterologous systems for functional assays, but cRNA injections in oocytes made it possible to examine their trafficking. The p(L478P) variant was similar to wild type B0AT3, while p.(G79S), p.(Y319X), and p.(G496R) were all trafficking-deficient. It is still unclear whether the missense mutations trigger protein misfolding, or eliminate interactions with collectrin or angiotensin-converting enzyme 2 (ACE2), essential for B0AT3 trafficking to the plasma membrane (Singer et al., 2009). The p.(T199M) XTRP3 variant had an uncharacterized catalytic deficiency in terms of proline uptake with cellular expression resembling the wild type transporter. Besides, >20 B0AT1 mutations (Table 4) inherited in an autosomal recessive manner have been identified in patients suffering from the Hartnup disease (OMIM#234500), a syndrome of renal aminoaciduria, variable manifestations of dermatitis, ataxia and seizures (Bröer, 2009; Kleta et al., 2004; Seow et al., 2004). Like B0AT3, B0AT1 also requires collectrin and ACE2 for proper surface expression. Only a few Hartnup disease-mutations characterized in functional or biochemical assays show ER-retention (due to misfolding) or trafficking deficits (due to altered interactions with collectrin and/or ACE-2) (Camargo et al., 2009).
5. SLC17 family: vesicular transporters GLUTs, sialin and VNUT
The SLC17 family includes 9 structurally related, but functionally divergent proteins. Members SLC17A1, 3 and 4, were originally classified as type 1 phosphate transporters; in fact, they transport organic anions including exogenous (e.g. non-steroidal anti-inflammatory drugs/ NSAIDs) and endogenous substrates (urate) (relevant references in Reimer, 2013; Togawa, Miyaji, Izawa, Omote, & Moriyama, 2012; Togawa et al., 2015). SCL17A2 is structurally different and has since been reclassified as a type 2 phosphate transporter (SLC34A1). SLC17A1–4 transporters are expressed in non-neuronal cells of the kidney, liver and the digestive system. No neurological disorders have been linked to their dysfunction yet, whereas mutations in SLC17A3 lead to hyperuricemia and gout susceptibility in people (OMIM#612671, Jutabha et al., 2010).
5.1. Sialin (AST, ISSD, NSD, SD, SIASD, SLD; SLC17A5)
The ubiquitous and lysosomal membrane-residing sialin (SLC17A5) was classically thought to be responsible for the cytosolic efflux of sialic acid from lysosomes (Reimer, 2013) to support recycling of sialylated proteins and lipids (Schauer, 2008). Of recently, sialin has been highlighted for its under-appreciated role in supporting plasmalemmal uptake of nitrate (Qin et al., 2012) and vesicular uptake of glutamate, aspartate and N-acetylaspartylglutamate (Miyaji, et al., 2008; Mochel et al., 2010: Lodder-Gadaczek et al., 2013). It is therefore not suprising that sialin dysfunction has severe neurological consequences. Patients who recessively inherit point mutations in sialin suffer either from the less severe Salla disease (OMIM# 604369), the more acute intermediate severe Salla disease or the often-fatal infantile sialic acid storage disorder (ISSD, OMIM# 269920) (Aula et al., 2000; Verheijen et al., 1999). These patients commonly present cerebellar atrophy, hypotonia and intellectual disabilities; the symptoms are markedly more severe in ISSD patients that include motor retardation, coarse facial features, hepatospleno- and cardiomegaly, failure to thrive, gross developmental delay and death at age < 5 years (Aula et al., 2000; Varho et al., 2002).
Barring a few exceptions, the basal function of the mutants seems to determine the severity of disease symptoms (Aula, Jalanko, Aula, & Peltonen, 2002; Miyaji et al., 2008; Miyaji et al., 2011; Morin et al., 2004; Wreden, Wlizla, & Reimer, 2005). For instance, ISSD associated sialin variants such as p.(Δ268–272) and p.(H183R) show 1) appropriate targeting to lysosomal and (presumably) vesicular membranes, 2) indistinguishable glutamate and aspartate uptake features relative to wild type sialin and 3) complete loss of sialic uptake capacity. Hence, it is conceivable that the onset of severe phenotypes in ISSD may arise from a type of lysosomal storage disease (Sagné & Gasnier, 2008), wherein the faulty accumulation of sialic acid in lysosomes predominantly leads to fatal neuronal cell death. The Salla disease associated p.(R39C) variant, on the other hand, posesses 1) 10–50% of wild type sialin uptake activity, 2) a slow, yet appreciable trafficking, compared to wild type, to the lysosome at steady state and 3) little to no glutamate and aspartate uptake capacity. It is therefore plausible that neurological symptoms of Salla disease can be explained by lack of vesicular refilling of glutamate and aspartate by this variant. This may, in turn, thwart cognate neuronal signaling. Sialin is hence more than a mere lysosomal transporter; as it may play a key role in neurological function and disorders (Robak et al., 2017) and in phenotypic variability, i.e. from ISSD to Salla disease. These possibilities ought to be explored in future knock-in animal models of sialin variants. The sialin knockout (SLC17A5 −/−) mice display symptoms reminiscent of ISSD in people: gait abnormality, motor retardation, seizures, hypomyelination, leukoencephalopathy and premature death during the third postnatal week (Prolo et al., 2009; Stroobants et al., 2017).
5.2. VGLUT1-3 (1, 2: BNPI, DNPI; 3: DFNA25; SLC17A6-8)
Glutamate is packaged into synaptic vesicles by vesicular glutamate transporters (VGLUT 1–3). VGLUTs transport glutamate (L-enantiomer over D-), but not aspartate or glutamine. The KM of VGLUTs for glutamate is ~2 mM, commensurate with its cytosolic levels in neurons. VGLUT1 (SLC17A7) was originally reported as a brain-specific Na+-dependent inorganic phosphate transporter (BNPI, Ni et al., 1994). It was subsequently shown to be localized in pre-synaptic terminals of neurons, and to mediate vesicular glutamate uptake (Bellocchio, Reimer, Fremeau Jr, & Edwards, 2000; Takamori, Rhee, Rosenmund, & Jahn, 2000). VGLUT2 (SLC17A6) was first referred to as the differentiation-associated Na+-dependent inorganic phosphate transporter (DNPI), because its expression levels were upregulated upon differentiating a rat pancreatic tumour cell to neuronal cells; Aihara et al., 20005). VGLUT2 is found primarily at vesicular membranes of gluta-matergic synapses, but its distribution in the brain is distinct from VGLUT1; in fact, their expression is, for most parts, mutually exclusive (El Mestikawy, Wallén-Mackenzie, Fortin, Descarries, & Trudeau, 2011; Fremeau Jr., Voglmaier, Seal, & Edwards, 2004). The presence of glutamate release in non-glutamatergic neurons led to the identification of VGLUT3 (SLC17A8) (Gras et al., 2002; Schäfer, Varoqui, Defamie, Weihe, & Erickson, 2002).
VGLUT1 knockout (VGLUT1−/−) mice suckle and move normally in their first 2 postnatal weeks. However, after the third week, they fail tothrive(Fremeau Jr. et al., 2004; Wojcik et al., 2004): their body weight is lower than that of wild type and VGLUT1+/- heterozygous littermates. VGLUT1−/− mice die of progressive emaciation between 18 and 21 days post birth if not separated from their littermates (Fremeau Jr., Kam, et al., 2004). This coincides with the time period, where expression of VGLUT2 and of VGLUT1 is down- and upregulated, respectively (FremeauJr.,Kam,etal.,200 ; Wojcik et al., 2004). The surviving VGLUT1−/− mice progressively lose their motor coordination (climbing fibers in the cerelebellum are rich in VGLUT1), they turn blind and have a heightened startle response (Fremeau Jr., Kam, et al., 2004).
VGLUT2 knockout mice (VGLUT2−/−) die at birth (Moechars et al., 2006; Wallén-Mackenzie et al., 2006), which is consistent with the high expression of VGLUT2 in early infancy (see above). Death is due to respiratory arrest: the mice fail to generate a central respiratory rhythm due to the lack of activity in neural circuits at the pre- Bötzinger (PBC) area (Moechars et al., 2006; Wallén-Mackenzie et al., 2006).
The VGLUT3 knockout (VGLUT3−/−) phenotype was originally described in zebrafish (Obholzer et al., 2008): As in zebrafish models, VGLUT3−/− mice are deaf; their auditory nerves are not responsive to acoustic stimuli (Ruel et al., 2008; Seal et al., 2008). VGLUT3 is expressed in hair cells of the cochlea; in the absence of VGLUT3, the terminals of auditory nerve fail to be excited. Interestingly, contrary to asteroid zebrafish larvae, VGLUT3−/− mice have a normal vestibular response (Seal et al., 2008), but they develop epileptic (generalized, synchronous) discharges in the electroencephalogram. These seizure equivalents are, however, not accompanied by motor symptoms, or by behavioral changes (Seal et al., 2008).
Only one study reported human variants of VGLUT1 and their association with mental disorders: In a Taiwanese case-control study, a cohort of 376 schizophrenic patients was matched with 368 control subjects (Shen et al., 2009). 2 missense variants L516M and P551S (Fig. 3k), discovered in the coding region of VGLUT1, both located in exon 12 (Table 5), were detected explicitly in the patient cohort. The functional consequences of these mutations have not yet been investigated. The screen for VGLUT2 variants among the same cohort of patients did not disclose any coding variants in the SLC17A6 gene (Shen et al., 2010).
Table 5. Mutations in human SLC17, SLC18, OCT and PMAT transporters.
| Gene | Protein name | Missense coding variants | Transport phenotype | Trafficking to target membrane | Disease phenotype | Mode of inheritance | OMIM# |
|---|---|---|---|---|---|---|---|
| SLC17A5 | Sialin | >30 mutations | Loss-of-function | Reduced, increased, unaltered or altered | Salla disease, intermediate severe Salla disease or Infantile Sialic Acid Storage Disorder | AR/CHet | 604369 (SD) 269920 (ISSD) |
| SLC17A7 | VGLUT1 | L516M, P551S | ? | ? | Schizophrenia | ? | |
| SLC17A8 | VGLUT3 | A211V | Loss-of-function | Reduced and altered | Non-syndromic sensorineural deafness | AD | 605583 |
| I78V, M206Nfs*4, A374S | ? | ? | |||||
| SLC17A9 | VNUT | R9C, R311N | ? | ? | Disseminated superficial actinic porokeratosis | AD | 616063 |
| SLC18A2 | VMAT2 | P387L, P316A | Loss-of-function | Unaltered protein levels, trafficking studies not undertaken | Brain monoamine vesicular transport disease | AR | 618049 |
| P237H | ? | ? | |||||
| SLC18A3 | VAChT | G360R | Loss-of-function | Protein undetected | Congential myasthenic syndrome | AR | 617239 |
| V52F, G186A, C372*, D398H | ? | ? | |||||
| SLC22A3 | OCT3 | M370I | Loss-of-function | Unaltered | Obsessive Compulsive Disorder | AD | |
| SLC29A4 | PMAT | A138T, D326E | Loss-of-function | Unaltered | Autism spectrum disorder | AD | |
AR: Autosomal recessive; AD: Autosomal dominant; CHet: Compound heterozygotes.
Point mutations in VGLUT3 were reported in patients suffering from autosomal dominant non-syndromic sensorineural deafness (DFNA, loci DFNA25, OMIM#605583), closely resembling presbycusis. The disorder is characterized by a late-onset hearing loss of predominantly high frequency sounds, which progressively intensifies with age (Greene et al., 2001). The VGLUT3 mutation p.(A211V) (Fig. 3k) was identified in several members of 2 supposedly unrelated American families, who probably shared a common ancestor (Ruel et al., 2008). This variant caused a ~ 65% reduction in VGLUT3 protein levels in transfected cells in vitro and in vivo in neurons of the CNS, although its functional impact was modest (Rametetal.,201). The mutation appears to additionally alter the uniform distribution of VGLUT3 across the vesicular pools, explaining the reduced frequencies of mini-excitatory postsynaptic currents in transfected hippocampal autaptic neurons, relative to wild type VGLUT3 controls. While mechanistic insights from VGLUT3−/− mice and zebrafish (see above) can account for the hearing loss in patients afflicted with DFNA25, they do not readily rationalize the delayed onset, the slow progression and the preferential loss of function in hair cells at the base of the cochlea. Knock-in mice harboring 2 alleles for the homologous p.(A224V) recapitulate the slow onset of hearing loss and the predilection for the frequency range of 5–20 kHz; interestingly in these mice the perception of the ultrasonic range is not affected (Joshi et al., 2020). In contrast to the reduced expression in neurons (Rametetal.,201), the levels of p.(A224V) and its distribution in inner hair cells did not differ from the wild type protein. However, the stereocilia bundles were fused in those hair cells required to sense the 5–20 kHz range. This indicates that the primary defect is impaired mechanosensation rather than reduced glutametergic neurotransmission. The candidate link between the mutation and the abnormal assembly of sterocilia is one or several proteins, which – hypothetically – interact with the second intracellular loop (that harbors p.(A211/A224)) of VGLUT3 and is/are required for the assembly of the stereocilia. In 2016, screening for VGLUT3 variants in a South Korean cohort of 87 unrelated patients suffering from DFNA revealed a frameshift mutation (M206Nfs*4, Fig. 3k) in one patient (Table 5). The variant led to a truncated 209 amino acid residue VGLUT3 (Ryu et al., 2016). 2 additional non-synonymous variants were identified, I78V and A374S (Fig. 3k), albeit with minor allele frequencies of 0.1% and 0.2% (from the 1000 Genomes Project Database). The same group discovered a novel splicing variant in a Korean family with 3 generations of DFNA (Ryu et al., 2017). None of the variants have been functionally characterized. It is worth noting that VGLUT3 deficiency in mice results in many behavioral alterations, e.g. increased anxiety (Amilhon et al., 2010), reduced pain threshold (Seal et al., 2009) and increased propensity for cocaine abuse (Sakae et al., 2015). The equivalent changes have not been reported in individuals harboring the mutations.
5.3. VNUT (C20orf59, POROK8; SLC17A9)
SLC17A9 is a synaptic vesicular and lysosomal nucleotide transporter (VNUT), which is expressed in both neuronal and glial cells of the hippo-campus,midbrainandthecerebralcortex(Larssonetal.,2012).VNUTis also expressed in purinergic cells of endocrine glands, such as pancreas, adrenal and thyroid glands, liver and T-cells (Cao et al., 2014; Geisler et al., 2013; Sathe et al., 2011; Sawada et al., 2008; Tokunaga, Tsukimoto, Harada, Moriyama, & Kojima, 2010). VNUT accomplishes the refilling and maintainance of nucleotide (primarily ATP) concentrations at 0.1–1 mM in synaptic vesicles. ATP and other nucleotides are coreleased with neurotransmitters during exocytosis and mediate crucial downstream purinergic responses via purinoreceptors (Miras-Portugal et al., 2019). VNUT knockout mice (VNUT−/−) do not display any gross neurological abnormalities or behavioral alterations (Sakamoto et al., 2014). ATP release is abolished and glutamate release reduced, when hippocampal neurons prepared from these animals are depolarized. The absence of ATP-storage and release in spinal dorsal horn neurons protects against neuropathic pain resulting form peripheral nerve injury (Masuda et al., 2016).
Point mutations of human VNUT were identified in patients suffering from disseminated superficial actinic porokeratosis (DSAP, OMIM#616063; Cui et al., 2014). DSAP is a pre-cancerous skin condition characterized by red brown scaly spots (generally on the limbs) with coronoid lamellae (fine, thready rim on the edge of the spots). 2 VNUT variants, p.(R9C) and p.(R311N), were identified in two unrelated families, and transmissed in an autosomal dominant manner (Table 5). The mutants have not yet been functionally characterized.
6. SLC18 family: vesicular monoamine, acetylcholine and polyamine transporters
The SLC18 family of neurotransmitter transporters is comprised of 4 members: SLC18A1 encodes the vesicular monoamine transporter-1 (VMAT1), SLC18A2 encodes VMAT2, SLC18A3 encodes the vesicular acetylcholine transporter (VAChT) and SLC18B1 encodes the vesicular polyamine transporter (VPAT) (Lawal and Krantz, 2013). SLC18A1/2 and SLC18A3 operate in a relay with the plasmalemmal transporters for monoamines (SLC6A2, SLC6A3, SLC6A4) and choline (SLC5A7), respectively, to recycle neurotransmitters (norepinephrine, dopamine, serotonin) and the neurotransmitter precursor choline to refill synaptic vesicular stores. These transporters thus afford a constant vesicular pool for synaptic transmission events.
6.1. VMAT1 (SLC18A1) and VMAT2 (SLC18A2)
SLC18A1/VMAT1 is the predominant transporter in the peripheral nervous system (sympathetic ganglia including the adrenal medulla and some enteric neurons (Erickson, Schafer, Bonner, Eiden, & Weihe, 1996; Lawal and Krantz, 2013). VMAT2, on the other hand, is expressed exclusively in the CNS, particularly in the monoaminegic neurons of the brain stem. Outside the CNS, VMAT2 is also found in histaminergic and monoaminergic cells of the sympathtic ganglia, adrenall medulla, myenteric and submucosal plexus (Erickson et al., 1996; Lawal and Krantz, 2013). With a few notable exceptions (e.g. fenfluramine) VMAT2 has a higher affinity for serotonin, dopamine, norepinephrine, epinephrine, histamine and for exogenous ligands, such as, reserprine, tetrabenazine, amphetamines and the neurotoxin 1-methyl-4-phenylpyridinium (MPP+) as compared to VMAT1; the difference in affinity varies from modest 2–3 fold (for serotonin, dopamine, (nor)epinephrine, MDMA and reserpine) to pronounced (20–30 fold for histamine and amphetamine), while tetrabenazine does not have any appreciable affinity for VMAT1 (Erickson et al., 1996).
VMAT2 knockout (VMAT2−/−) mice die within days of birth (Fon et al., 1997; Takahashi et al., 1997; Wang et al., 1997). Prior to their death, the mice feed poorly, display hypoactivity and prone to hypothermia. Their brains contain very low levels of monoamines (1–6% of wild type levels), but normal levels of monoamine metabolites indicating an exceedingly high turnover. Mice with one VMAT allele knocked out (VMAT2+/-) or with hypomorphic alleles are phenotypically comparable to wild type mice and survive into adulthood. However, they show 30–50% reduction in monoamine levels in the brain. In addition, their locomotor response to transport inhibition by cocaine (Wang et al., 1997) and to amphetamine-induced reverse transport are augmented (Mooslehner et al., 2001; Takahashi et al., 1997; Wang et al., 1997). This is justified by supersensitivity of (postsynaptic) dopamine receptors (Mooslehner et al., 2001; Wang et al., 1997). VMAT1 knockout (VMAT1−/−) mice display neurocognitive deficits (Multani et al., 2013), suggesting that VMAT1 has physiological roles in the CNS, along with the previously established functions in peripheral neuroendocrine cells.
Although disease-relevant mutations have not been discovered in VMAT1 to date, polymorphisms and rare variants have been implicated in an increased risk for neurological disorders (Bly, 2005; Lohoff et al., 2006; Lohoff et al., 2014). For instance, the p.(T136I) polymorphism is present at higher frequencies in bipolar depression patients, relative to controls (Lohoff et al., 2006). The p.(S98T) variant, on the other hand, was identified in 2 schizophrenic individuals (Lohoff et al., 2014). Both variants significantly increase monoamine uptake in intracellular vesicular compartments of heterologous cells (Lohoff et al., 2014). The molecular causes of this increase in catalytic activity of these as well as other VMAT1 variants are still unknown. Point mutations in VMAT2 (Fig. 3k, Table 5), on the other hand, are known to have neurological consequences. These mutations are inherited in an autosomal recessive manner and lead to a form of infantile Parkinsonism and dystonia, referred to as brain monoamine vesicular transport disease (OMIM#618049). The first identified VMAT2 mutation encodes the p.(P387L) variant found in several members of an extended consanguineous family (Rilstone, Alkhater, & Minassian, 2013). The patients manifested a myriad of symptoms indicative of monoaminergic deficiencies, including dystonia and parkinsonism (reflecting a lack of dopamine), sleep and mood disorders (reflecting a lack of serotonin) and postural hypotension (reflecting a lack of norepinephrine). Immunoblotting did not reveal any differences in the levels and trafficking profiles between the wild type and p.(P387L) proteins. However, serotonin uptake by the mutant was remarkably reduced, alluding to a loss-of-function phenotype. Treatment with pramipexole (D2-receptor agonist) remedied the condition; it corrected the movement deficits and allowed for motor development. Similar responses were seen in additional studies (Jacobsen et al., 2016; Padmakumar et al., 2019; Rath et al., 2017), which identified p.(P237H) and p.(P316A) variants in children from unrelated families exhibiting the symptoms attributable to monoamine deficiency as mentioned above. The variants p.(P237H) and p.(316A), were not functionally characterized. The p.(P387L) variant was also identified in a patient afflicted with severe microphthalmia (an eye developmental disorder with abnormally small eyes), microcephaly, epilepsy, failure to thrive and global developmental delay (Patel et al., 2018). This observation suggests that the development of the human eye involves VMAT2. Upon knock-in of p.(P237H) or p. (P387L) in Caenorhabditis elegans transporter homologue (cat1), the feeding of transgenic worms was impaired (Young et al., 2018): they had reduced grazing and pharyngeal pumping responses, which are regulated by serotonin and dopamine. This points to these 2 VMAT2 variants being loss-of-function mutants.
6.2. VAChT (SLC18A3)
The vesicular acetylcholine transporter VAChT/SLC18A3 is endoded by a single exon, embedded in intron 1 of the choline acetyltransferase (CHAT) gene (Erickson et al., 1994). Accordingly, their expression is linked and both are markers of cholinergic neurons. VAChT is expressed in the striatal magnocellular interneurons, in neurons of the basal forebrain and brain stem, preganglionic sympathetic neurons, pre- and postganglionic parasympathetic neurons and in the motorneurons of the ventral spinal cord (Erickson et al., 1994; Lawal and Krantz, 2013).
VAChT knockout mice (VAChT−/−) die within minutes of birth (De Castro et al., 2009). The newborn pups do not survive due to respiratory failure, arising from the lack of vesicular acetylcholine storage and release at the neuromuscular junction. Targeting the 5′-untranslated region of the SLC18A3 gene afforded the generation of mice with low expressing VAChT (Prado et al., 2006): in homozygous and heterozygous knockdown mutants (VAChTKD), expression is reduced by 65 and 45%, respectively. Homozygous mice are myasthenic, i.e. their muscle strength is reduced and gets aggravated upon physical exertion. In heterozygous VAChTKD, the level of VAChT suffices to maintain normal levels of quantal acetylcholine release at the neuromuscular junction (detected by recording miniature endplate potentials) and muscle strength. However, the reduction in VAChT/synaptic refilling in some brain regions of these mice impairs cholinergic transmission to the extent visible in cognitive tasks, e.g. in paradigms of object recognition and social memory (Prado et al., 2006). This is consistent with cholinergic transmission in the brain being vulnerable and declined in early Alzheimer’s disease (Grothe, Heinsen, & Teipel, 2012).
Deficiencies in VAChT function also have clinical repercussions in people. Patients with impaired VAChT function, arising from biallelic loss, suffer from congential myasthenic syndrome (CMS type 21, OMIM# 617239, Rodríguez Cruz et al., 2018). Earlier clues to this genotype-phenotype correlation were provided by screening of patients with deletions in the loci spanning 10q11.22 and 10q11.23, that encompass VAChT and CHAT (Stankiewicz et al., 2012). These patients present neurological symptoms such as intellectual disabilites, global developmental delay and CMS. Point mutations in VAChT (Fig. 3k and Table 5) linked to CMS were first identified in 2 patients, homozygous for either the p.(G186A) or for the p.(D398H) variant. In both cases, there were symptoms of impaired transmission at the motor endplate, i.e. ptosis, ophthalmoplegia, fatigable weakness and apneic crises (O’Grady et al., 2016). The loss-of-function phenotype of p.(G186A) and p.(D398H) was confirmed using an electrophysiological approach, wherein the patients exhibited a rapid decline in compound muscle action potential upon low-frequency repetitive stimulation. The homozygous p.(G360R) variant was identified in 2 brothers who had severe hypotonia and athrogryposis at birth. One died of respiratory failure 5 days after birth; the other (aged 4.5 years at the time of the study) relied on mechanical ventilation since birth (Aran et al., 2017). Heterologously expressed p.(G360R) was undetectable in immunoblots, although the transfected cells accumulated abundant mRNA levels (Aran et al., 2017). This is consistent with a protein folding defect. Comparably severe symptoms of respiratory distress were seen in a patient, who was hemizygous for the p.(V52F) variant and co-inherited a 10q11.2 deletion in the other allele (Schwartz et al., 2018). Mutations in VAChT can have detrimental effects on fetal development, as they impair movement in utero. In 2 fetuses with akinesia deformation and lethal multiple pterygium syndrome (i.e. growth of skin webs over large joints), the defect was linked to a biallelic mutation which introduces a premature stop codon and hence truncates VAChT at C372 (Hakonen et al., 2019).
6.3. VPAT (SLC18B1)
The vesicular polyamine transporter (VPAT/SLC18B1) was initially annotated as C6ORF192 and subsequently assigned to the SLC18 family, based on sequence homology (Jacobsson et al., 2010). When reconstituted in liposomes, VPAT takes up spermine and spermidine (and serotonin), driven by an antiport of H+ (Hiasa et al., 2014). In the brain, VPUT expression is elevated in the hippocampus, cerebral cortex and the cerebellum. At the cellular level, it is predominantly localized to astrocytes, where it is found in vesicular structures spread throughout the cytoplasm (Hiasa et al., 2014). In mast cells, VPUT carries out the uptake of spermine and spermidine in secretory granules (Takeuchi et al., 2017). In the brain, polyamines are thought to regulate glutamatergic neurotransmission via NMDA-receptor modulation (Moriyama et al., 2020; Ogden and Traynelis, 2011). In VPUT knockout mice (VPUT−/−), the levels of total polyamines in the brain are reduced (Fredriksson et al., 2019), translating to deficits in short- and long-term memories, possibly linked to reduced glutametergic and GABA-ergic signaling (Fredriksson et al., 2019). In addition, a link between SNPs in the human SLC18B1 gene and cognitive performance has been established (Fredriksson et al., 2019). However, no point mutations in VPAT have yet been identified in human disorders.
7. The ’uptake-2′ system: PMATs and OCTs
Extraneuronal biogenic amine uptake transporters, collectively known as the ‘uptake-2’ system, are hypothesized to play a role in the delayed onset of therapeutic action of antidepressant, which target the monoamine transporters (Schildkraut & Mooney, 2004). This system comprises 2 transporter families: organic cation transporters OCT1, 2 and 3 of the SLC22 family (Koepsell et al., 2007)andthe plasma membrane monoamine transporter or PMAT of the SLC29 family (Young, Yao, Baldwin, Cass, & Baldwin, 2013). The members of the uptake-2 system differ from the uptake-1 transporters (i.e. the plasmalemmal monoamine transporters SERT, DAT and NET) in 2 aspects. The fi rst is their extraneuronal distribution, compared to the distribution of the uptake-1 system, which is restricted to pre- synaptic neurons. Secondly, the uptake-2 system mediates Na+-independent and low affinity substrate uptake, albeit with a substantially higher maximal velocity relative to uptake-1 transporters. In fact, it is this high capacity of uptake-2 transporters which is thought to counterpart the uptake efficiency of Na+-dependent, high-affinity and low-capacity properties of uptake-1/monoamine transporters in rapidly terminating neurotransmission (Daws, 2009; Hensler et al., 2013).
OCT1 (SLC22A1),OCT2(SLC22A2) and OCT3 (SLC22A3) belong to the 13-member family of human SLC22 transporters. These are putative 12 transmembrane polyspecific transporters that support uptake, elimination and distribution of a wide range of substrates encompassing cationic drugs, toxins and biogenic amines (Koepsell et al., 2007). The roles of OCT2 and OCT3 in maintaining biogenic amine uptake have been well characterized in the brain (Koepsell, 2013; Yoshikawa & Yanai, 2016). Blocking OCT2 and 3 in mice, either by genetic inactivation or by high affinity antagonists, produces anti-depressant-like effects, which are additive when co-treated with selective serotonin or norepinephrine reuptake inhibitors (Hensler et al., 2013). This is suggestive of potential contribution of OCT2 and OCT3 in a delayed onset of antidepressant action (Schildkraut & Mooney, 2004). The role of OCT1 in the brain is restricted to allowing the passage of endogenous substrates and exogenous drugs across the blood-brain barrier (Koepsell, 2013).
PMAT (SLC29A4) belongs to the 4 member SLC29 family of equilibrative nucleoside transporters (ENTs). ENTs 1–3 are primarily involved in the transport of purine and pyrimidine nucleosides (Young et al., 2013). In contrast, PMAT transports biogenic amines in the brain (Wang, 2016). The pharmacology of PMAT overlaps with that of OCTs indicating that PMAT may contribute to the uptake-2 system (Duan & Wang, 2010).
Murine knockouts of uptake-2 transporters are viable and fertile and show only subtle neurological deficits, indicative of redundancy and compensation, either by each another or by other transporters (Duan & Wang, 2013; Gilman et al., 2018; Jonker et al., 2001; Jonker et al., 2003; Zwart, Verhaagh, Buitelaar, Popp-Snijders, & Barlow, 2001). Most polymorphisms and functional consequences of variations in OCTs have been described in a context to inter-individual variations in response to non-CNS cationic drugs (such as anti-cancer drugs and the antidiabetic drug metformin) and non-CNS diseases (reviewed in Koepsell, 2020). Of note, mutations in OCT3 and PMAT (Table 5) have been identified in patients afflicted with OCD and autism, respectively. The p.(M370I) variant in OCT3 was identified in 3 members of a family suffering from OCD, inherited in an autosomal dominant manner (Lazar et al., 2008). When expressed alone, this variant was delivered to the cell surface at wild type levels, but its norepinephrine uptake was reducedby40%,presumablyduetodecreasedaffinityofthemutanttransporter for norepinephrine. In PMAT, the variants p.(D29G), p.(A138T) and p.(D326E) were identified in autistic patients (Adamsen et al., 2014). These mutants showed cell surface expression comparable to wild type PMAT, dermined by surface biotinylation and immunoblots. Uptake of serotonin, dopamine and MPP+ was modestly reduced in heterologous cells expressing p.(A138T) and p.(D326E). For p.(D29G), the transport defect was restricted to MPP+ (Adamsen et al., 2014). A lot of studies have emphasized the importance of OCT3 and PMAT in response to drugs targeting the monoaaminergic systems (Gasser, 2019; Haenisch & Bönisch, 2009; Hensler et al., 2013; Mayer et al., 2018). It is therefore feasible that genetic variations in uptake-2 play a role in many complex neuropsychiatric disorders and/or individual variability in response to therapeutic interventions.
8. SLC32 family: vesicular GABA transporter (VGAT), vesicular inhibitory amino acid transporter (VIAAT)
The single member of the SLC32 family, SLC32A1 encodes the vesicular GABA transporter (VGAT)/vesicular inhibitory amino acid transporter (VIAAT). Its physiological role is to refill synaptic vesicles with glycine, GABA or β-alanine (Aubrey et al., 2007; Juge et al., 2013; Wojcik et al., 2006). VIAAT exhibits low sequence similarity (of 10–20%) with SLC17 and SLC18 vesicular transporters. This transporter is primarily delivered to the synaptic vesicles of glycinergic and GABA-ergic neurons in the brain and spinal cord (Chaudhry et al., 1998; Gasnier, 2004; McIntire et al., 1997; Sagné et al., 1997). Knockout of VIAAT in mice leads to intrauterine death (Saito et al., 2010; Wojcik et al., 2006); similarly, VIAAT−/− Drosophila melanogaster fail to hatch (Fei et al., 2010). VIAAT−/− murine fetuses adopt a hunched posture due to muscular stiffness (Wojcik et al., 2006). When subjected to preterm delivery by cesarean section on E18.5, they fail to breath (Saito et al., 2010), an observation consistent with abnormal muscle control. Similar to glutamate decaboxylase 67-deficient mice, VIAAT−/− murine fetuses have a cleft palate, which argues for a role of tongue mobility in palate closure. The omphalocele (a prolaps into the umbilical cord of a peritoneal sac containing the gut), seen in VIAAT−/− murine fetuses, phenocopies defects seen in KCC2 (neuron-specific chloride potassium symporter-2, SLC12A5)-deficient mice (Hübner et al., 2001). This can be rationalized as follows: in the absence of KCC2, the chloride gradient in developing neurons is reversed; thus, stimulation of GABAA and glycine receptors is excitatory prior to KCC2 expression during brain development (Rivera et al., 1999; Stein, Hermans-Borgmeyer, Jentsch, & Hübner, 2004). Accordingly, the omphalocele reflects abnormal motor control, rather than structural defects. Point mutations in VIAAT have not yet been reported in human disorders.
9. NTT protein folding: lessons learnt from the SLC6 transporters
Proteins have to adopt a precise 3-dimensional structure to acquire functional activity (Hartl, Bracher, & Hayer-Hartl, 2011). The “thermodynamic hypothesis” described by Christian Anfinsen, almost half a century ago, posits that the natively folded state is reached, when the protein has the lowest Gibbs free energy (Anfinsen, 1973). To attain this energetically favorable conformation, the protein must pass through a variety of intermediate folding states. The folding intermediates are assisted by proteinaceous chaperones, which shield the hydrophobic residues of the protein from the aqueous milieu and thus prevent aggregation with other proteins (Kim et al., 2013). The folding landscape resembles a funnel-shaped energy surface: The unfolded protein is routed along a downhill path towards its native state. However, the energy surface is often rugged, which results in transient accumulation of kinetically trapped conformations (Hartl et al., 2011). Proteinaceous chaperones smoothen the energy landscape, thereby promoting the progression towards the final, natively folded, state (Jahn and Radford, 2005).
The folding trajectory of SLC transporters can be envisioned as follows: like the majority of membrane proteins, SLC transporters co-translationally enter the ER membrane. The translocation process is initiated by the signal-recognition particle (SRP), which recognises the signal sequence of the nascent polypeptide chain as soon as it emerges from the ribosome. The ribosome-nascent-chain-SRP complex is next directed to the ER, where it binds to the SRP receptor. Subsequently, the polypeptide chain is inserted into the protein-conduction channel (the Sec61 translocon complex). Upon entering the channel, the hydrophobic segments are released individually or in pairs into the lipid phase via a lateral gate (Rapoport, 2007). On the lumenal side, the extracellular loop 2 of the nascent transporter is subject to N-linked core glycosylation (Freissmuth, Stockner, & Sucic, 2018). After cleavage of the 2 terminal glucose moieties by α-glucosidase I and α-glucosidase II, the newly synthesized glycoprotein is recognized by the membrane-bound chaperone calnexin (Fig. 4). Calnexin acts as a folding sensor in the ER lumen and its release from the folding intermediate allows for removal of the third glucose moiety by α-glucosidase II (Hebert, Foellmer, & Helenius, 1995). Importantly, the release of calnexin also licenses the transporter to form oligomeric structures (Freissmuth et al., 2018), which is a prerequisite for ER export (Scholze, Freissmuth, & Sitte, 2002). However, if the transporter fails to adopt a proper folded state, it is re-glycosylated by the UDP-glucose: glycoprotein glucosyltransferase (UGT), which allows for a reassociation with calnexin and thus for an additional round of folding. The calnexin cycle continues until the protein either reaches a natively folded state or acquires a terminally misfolded architecture that is no longer recognized by UGT. In the latter case, the protein is eventually subject to proteasome-mediated degradation (Molinari et al., 2005). Concomitantly, the folding process is assisted from the cytosolic side by a relay of heat-shock proteins (HSPs). These molecular chaperones (i.e. an unidentified HSP40 isoform, HSP70-1A and HSP90ß) sequentially engage the C-terminus of the transporter and thus sterically cover the binding site for the coatomer complex II (COPII) machinery component Sec24 (Fig. 4). After reaching the natively folded state, HSP90ß is released, the binding site becomes available and subsequently the transporter is exported from the ER in a COPII-dependent manner (Freissmuth et al., 2018). However, if the transporter is not able to achieve a stable fold, it is ultimately destined for ER-associated degradation (Fig. 4, ERAD) by an unidentified E3-ligase (Chiba, Freissmuth, & Stockner, 2014). Importantly, COPII-mediated ER export is a prerequisite for correct axonal targeting: mutations that disrupt the binding site for Sec24, which acts as a cargo receptor, prevent enrichment of the transporter in the axonal compartment (Montgomery et al., 2014; Reiterer et al., 2008). There are 4 mammalian isoforms of this COPII machinery component, referred to as Sec24 A-D. In case of SLC6 transporters, the relevant isoforms are Sec24D (GAT1, NET, DAT) and Sec24C (SERT, GAT3, GlyT2, BGT1, ATB0,+) (Freissmuth et al., 2018; Kovalchuk et al., 2019; Sucic et al., 2011). Interestingly, the isoform preference between Sec24D and Sec24C, is determined by the residue located at the +2 position, downstream from the ER export motif. While hydrophobic residues (tyrosine or valine) at this position promote the recruitment of Sec24D, hydrophilic residues (lysine, asparagine or glutamine) support the engagement of Sec24 isoform C (Sucic et al., 2013).
Fig. 4.
A magnifying glass view of the folding trajectory and molecular mechanisms involved in the rescue of misfolded NTT transporters. Upon insertion into the ER membrane, SLC6 transporters are engaged by calnexin on the lumenal side and by a relay of HSPs on the cytosolic side. While release of calnexin (CNX) allows for transporter oligomerisation, dissociation of HSPs clears the C-terminal binding site for the COPII component Sec24 and thus allows for ER export via COPII-coated vesicles (a). However, point mutations can impair the folding process of the mutant proteins, causing retention in the ER. Folding-deficient transporters are then marked for ERAD by an unidentified E3-ligase (b). In some instances, such folding defects can be corrected by pharmacological means. Pharmacological chaperones selectively bind to the mutant transporter, which reduces the energy barrier along the folding trajectory and thus promotes native folding. In contrast, HSP inhibitors are thought to relax the stringent ER quality control, which results in improved ER exit (c).
Genetic mutations in SLC proteins can be transmitted either in a dominant or recessive manner. Dominant transmission can be rationalized by a model, where transporters form oligomeric structures prior to their exit from the ER. Accordingly, the mutated transporter oligomerizes with the product of the wild type allele and thus hinders its delivery to the target membrane. Circumstantial evidence in support of this model are autosomal dominant point mutations in glutamate transporters (SLC1 family) and the high affinity choline transporter (CHT1, SLC5A7). CHT1 and most of the SLC1 transporters form homooligomers (Okuda et al., 2012; Vandenberg & Ryan, 2013), which assemble in the ER prior to their export and trafficking to the cell surface as functional units. Several mutations, such as the ALS-linked p.(N206S) EAAT2 variant (Section 2.2), the episodic ataxia causing EAAT1 p. (P290R) variant (Section 2.1), and the dHMN p.(K499Nfs*13) CHT1 variant (Section 3.2) display autosomal dominant features (Barwick et al., 2012; Jen et al., 2005; Trotti et al., 2001). Their co-transfection with wild type counterparts in heterologous cells, drastically reduces surface expression and function, compared to cells expressing wild type transporters alone. Among members of the SLC6 family, the hallmark variant showing autosomal dominant transmission is p.(A457P) in human NET (Section 4.2) that exerts a dominant-negative effect on the wild type transporter (i.e. the product of the healthy allele) (Hahn et al., 2003). The same holds true for the dominant negative GlyT2 variants associated with hyperekplexia, p.(S512R-rodent/S510R-human) and p.(Y705C) (Section 4.5, Giménez et al., 2012; Arribas-González, de Juan-Sanz, Aragón, & López-Corcuera, 2015). These dominant negative effects could be overcome by exposure to the chemical chaperone 4-phenylbutyric acid (4-PBA) or by overexpressing the ER chaperone calnexin, for the p.(S512R) and p.(Y705C) variants, respectively. Such findings are consistent with the notion that SLC transporter oligomerization is a key requirement for their ER export (Anderluh et al., 2014; Farhan et al., 2004; Scholze et al., 2002).
Recessive transmission is readily rationalized by assuming that folding-deficient mutants are stalled in a complex with ER-associated chaperones, which preclude oligomer complex formation. SLC transporter folding is subject to a stringent quality control system, which relies on proteinaceous chaperones. From a teleological perspective, the goal of such a meticulous quality control is precluding the delivery of aggregation-prone proteins to the cell surface (model synapses are illustrated in Fig. 5). This conjecture has been widely explored for SLC6 transporters. Our earlier efforts using synthetic GAT1, SERT and DAT mutants revealed that ER-retention is triggered by increased association of mutants with calnexin and HSPs (El-Kasaby et al., 2010; El-Kasaby, Koban, Sitte, Freissmuth, & Sucic, 2014; Kasture et al., 2016; Korkhov et al., 2008). These observations also apply to disease relevant varinants. For instance, point mutations in DAT lead to infantile/juvenile parkinsonism-dystonia (Kurian et al., 2009; Kurian et al., 2011; Ng et al., 2014). The vast majority of these cause folding defects in DAT, i.e. the mutated DATs accumulate as ER-resident core-glycosylated proteins, complexed with both calnexin and HSPs (Asjad, Kasture, et al., 2017). Missense variants in CRT1, linked to creatine transporter deficiency syndrome, are also known to be misfolded as demonstrated by increased transporter co-localization with calnexin in the ER (El-Kasaby et al., 2019). Consequently, this allows for the premise that alleviating the stringency of ER quality control mechanisms may promote the surface expression of folding-deficient variants. In fact, inhibition of individual components of the HSP relay by pharmacological means restores the cell surface expression of aberrantly folded variants of SERT (El-Kasaby et al., 2014), DAT (Asjad, Kasture, et al., 2017; Kasture et al., 2016) and CRT1 (El-Kasaby et al., 2019).
Fig. 5.
A basic synapse model of key NTT subfamilies and transporter rescue by pharmacochaperoning. A simplified representation of key synapse types, centered around a symbolic glial/ oligondedrocyte/non-neuronal cell: monoaminergic (in blue), glycinergic (in yellow), glutamatergic (in pink), GABAergic (in lime green) and cholinergic (in beige). Neurotransmitters are shown as circles, packaged into vesicles by the designated vesicular transporters, e.g. VMATs sequester biogenic amines dopamine, norepinephrine and serotonin to storage vesicles in monoaminergic neurons, setting them up for the next neurotransmitter release event. To the right, a magnifying glass view over pharmacochaperone action on misfolded NTTs: rescued transporters reach their eponymous site of action at the synapse, where they resume their physiological functions (left, model synapses). Abbreviations: 5-HT – 5-hydroxytryptamine, AChE-Acetylcholinesterase, ChAT-Choline acetyltransferase, DA-dopamine, Glu-glutamate, Gln-glutamine, Gly-glycine, Ser-serine, NE-norepinephrine, PC-phosphatidylcholine, SNATs-sodium-coupled neutral amino acid transporters/SLC38; SHMT-serine hydroxymethyltransferase.
Some point mutations in NTTs lead to correctly folded proteins, which nonetheless fail to reach their target membranes due to trafficking defects. At present, only a few mutations appear to fit into this category: e.g. the p.(C186S) EAAT1 variant shows uptake levels comparable to wild type EAAT1 (De Vries et al., 2009), but its delivery to filopodia in transfected astrocytes is impaired (Hayashi & Yasui, 2015, Section 2.2). In addition, the loss-of-function p.(S94R), p. (V112E) and p.(P210L) variants in CHT1 fail to be sorted to nerve terminals, despite mutant and wild type protein levels being comparable in transfected cells (Wang et al., 2017). Similarly, despite being targeted to neuronal soma and dendrites, the loss-of-function p.(P633R) NTT4 variant does not enter dendritic spines, as does the wild type NTT4 (Iqbal et al., 2015). Disrupted trafficking of transporter mutants can thus lead to either a loss-of-function phenotype or to alterations in their physiological roles (Kasture et al., 2019). Many recessive loss-of-function variants traffic to the cell surface in a manner indistinguishable from wild type proteins. In such instances, mutations most likely impair catalytic transport activity, e.g. altering the binding site/s of substrates and/or co-substrates (Na+/Cl−/K+/H+) or impeding the structural rearrangements necessary for completion of the physiological transport cycle. Elucidating the impaired step(s) is achievable by electrophysiological approaches, which allow for probing distinctive partial reactions of the transport cycle with unrivalled temporal resolution (Campbell et al., 2019; Carta et al., 2012; Herborg et al., 2018; Kovermann et al., 2017; Rees et al., 2006).
10. Pharmacological rescue of folding-deficient NTT variants
Folding-deficient mutants can be functionally rescued by chemical or pharmacological chaperones (Chaudhuri & Paul, 2006). The putative mechanisms of rescue are illustrated in Figs. 4 and 5. Chemical and pharmacological chaperones are small molecules that stabilize the misfolded protein, promote (re)folding and hence facilitate its delivery to the designated cellular locations (Loo and Clarke, 2007). The key difference between chemical and pharmacological chaperones is their substrate specificity: Chemical chaperones, such as glycerol, dimethyl sulfoxide (DMSO) and 4-PBA promote folding of a wide array of proteins (Perlmutter, 2002). In contrast, pharmacochaperones directly bind to and stabilize their cognate target proteins. Most of these small molecules are thought to facilitate the stabilization of the native state, thus enhancing protein expression (Marinko et al., 2019). Nonetheless, this mechanism does not account for the increased surface expression of monoamine transporters by ibogaine and its congeners (Bhat et al., 2020), which appears to occur via stabilization of a folding trajectory intermediate. In addition, the approved drug tafamidis is a pharmacochaperone, which restores the folded state of transthyretin (mutations in transthyretin cause a form of amyloidosis), by preventing protein aggregation and dissolution of aggregates (Coelho et al., 2016). Other prominent examples include migalastat and lumacaftor, which are clinically used in the treatment of Fabry disease (Germain, Hughes, Nicholls, Bichet, et al., 2016) and cystic fibrosis (Wainwright et al., 2015), respectively.
The action of pharmacological chaperones is restricted to specific target proteins (Ringe & Petsko, 2009). Ibogaine is the first pharmacochaperone found to be effective on SERT and several mutants thereof. Ibogaine binds to the inward-facing conformation of SERT and rescues a synthetic SERT-RI607,608AA mutation, located at the ER export motif on the transporter’s C terminus, i.e. the binding site for the COPII component cargo receptor SEC24C (El-Kasaby et al., 2010). The rescue effect was even more potent upon treatment with ibogaine’s metabolite noribogaine (El-Kasaby et al., 2014). Other SERT ligands, including the inhibitor imipramine and substrates serotonin and amphetamine, which bind to the outward-facing and occluded transporter conformations, respectively, produced no rescue effects (El-Kasaby et al., 2010). Interestingly, second site suppressor mutations, which trap SERT in its inward-facing conformational state, can also promote the delivery of folding-deficient mutants to the plasma membrane (Koban et al., 2015). These observations collectively indicate that the folding trajectory of SERT proceeds via the inward-facing conformation (Kasture et al., 2017). Notably, ibogaine, noribogaine and the atypical DAT inhibitor bupropion, can all correct misfolded DTDS variants in the closely related human DAT (Asjad, Kasture, et al., 2017; Beerepoot et al., 2016; Kasture et al., 2016). Ibogaine’s hallucinogenic properties make it an inapt candidate for human therapeutics. However, a recent systematic investigation of partial substrates of monoamine transporters identified PAL1045 as another potent pharmacochaperone, acting on a synthetic folding-deficient SERT mutant PG601,602AA (Bhat et al., 2017). Since the complex ring system of ibogaine is amenable to structural variations and chemical modifications, a consequent fluorinated analog proved valuable in rescuing the equivalent PG584,585AA mutant in DAT, with a higher efficacy than the parent compound ibogaine. This analog also corrected folding defects in 6 DAT mutants associated with DTDS (Bhat et al., 2020).
The pharmacological repertoires of other NTT transporters, such as EAATs and CHT1, have been explored (Ennis & Blakely, 2016; Vandenberg & Ryan, 2013). It is pertinent to elucidate whether the folding trajectories of these transporters also ensue via the inwardfacing state. This conjecture ought to be probed because EAAT3 and CHT1 variants are of clinical interest (see Sections 2.3 and 3.2, respectively). Drugs that inhibit these transporters in a non-competitive manner and show preferential binding to the inward-facing transporter states (e.g., (+)-HIP-B for EAAT3 and ML352 for CHT1) may serve as lead compounds in the search for effective pharmacochaperones of misfolded disease variants in these transporter subfamilies (Callender, Gameiro, Pinto, De Micheli, & Grewer, 2012; Ennis et al., 2015).
Regretably, many SLC transporters do not have a rich pharmacology, confining the opportunities for initial pharmacochaperone screening. In such instances, chemical chaperones can be employed to assist protein folding, as shown for 4-PBA in rescuing misfolded variants of SERT (El-Kasaby et al., 2010), CRT1 (El-Kasaby et al., 2019), and GlyT2 (Arribas-González et al., 2015). Treatment of cells with a HSP70 inhibitor pifithrin-μ also rescued several folding-deficient variants of DAT (Asjad, Kasture, et al., 2017, Kasture et al., 2016). Moreover, both pifithrin-μ and a HSP90 inhibitor 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG) sensitized misfolded SERT mutants to the pharmacochaperoning action of noribogaine (El-Kasaby et al., 2014). HSP inhibitors have additionally been in the spotlight for the development of cytostatic agents (Neckers and Workman, 2012). Their action in alleviating folding deficits can be rationalized as follows: the progression of proteins along the folding trajectory is rigorously monitored by a HSP relay (Section 9). These proteinaceous chaperones must be released prior to the assembly of COPII vesicles and ER export of the protein cargo. HSP inhibition is thought to relax the stringent ER quality control, thus endorsing ER export (Asjad, Nasrollahi-Shirazi, Sucic, Freissmuth, & Nanoff, 2017; Freissmuth et al., 2018). The chemical chaperone 4-PBA prevents protein aggregation by interacting with hydrophobic domains of misfolded proteins. However, 4-PBA has other reputed modes of action, such as modulating HSC70, HSP70 and histone deacetylase levels, the latter leading to transcriptional regulation of genes in the unfolded protein response system (Cousens, Gallwitz, & Alberts, 1979; Rubenstein & Zeitlin, 2000).
Several allosteric modulators, which negatively or positively attune transporter activity, have recently caught attention in extending the already diverse expanse of NTT pharmacology (Hasenhuetl, Bhat, Freissmuth, & Sandtner, 2019; Niello et al., 2020). These drugs stabilize unique conformational states upon binding to their target transporters. As such, they hold significant therapeutic potential in the treatment of both misfolded and catalytically impaired transporter mutants. For instance, Zn2+ rescues the uptake of the ASD associated p.(T356M) DAT variant (see Section 4.3), while reducing spontaneous anomalous dopamine efflux leading to the partial correction of mutant DAT function (Hamilton et al., 2013; Hamilton et al., 2015). Ivacaftor, a renowned CFTR modulator, is in clinical use in the treatment of cystic fibrosis, administered in combination with the corrector molecule lumacaftor (Wainwright et al., 2015).
Pharmacochaperoning of misfolded SLC transporters is not limited to heterologous expression in cell lines. In 2016, Kasture et al. provided a proof-of-principle that folding-deficient mutants of DAT are amenable to rescue in vivo, i.e. utilizing Drosophila melanogaster as a resourceful animal model to demonstrate chemical- and pharmaco-chaperoning (Kasture et al., 2016). Living flies harboring the dDAT-G108Q mutation typically show a sleepless phenotype, reminiscent of a DAT knockout phenotype in flies (Wu, Koh, Yue, Joiner, & Sehgal, 2008). Upon treatment with noribogaine and/or HSP70 inhibitor pifithrin-μ, the mutant transporter reached the axonal compartments and normal sleep time was restored in these flies (Kasture et al., 2016). More importantly, this effect was replicated in flies expressing 2 human DAT variants (V158F and G327R), which give rise to infantile parkinsonism (Asjad, Kasture, et al., 2017) and in the synthetic folding variant DAT-PG584,585AA (Bhat et al., 2020). To the best of our knowledge, pharmacochaperoning has not yet been tested in other animal models.
Pivotal insights into the molecular mechanisms underlying SLC6 transporter folding were granted by in vitro experiments carried out on synthetic mutants, setting the stage for restoring the function of folding-deficient and loss-of-function disease variants in NTTs. Here, pharmacochaperoning may own major therapeutic significance in mending mutation-specific defects. In fact, even seemingly minor improvements in the basal activity of mutant transporters can lead to pronounced alleviation of disease phenotypes, substantiated by observations in DAT and sialin. That is, while only nominal rescue of DAT activity was measured in vitro, complete restoration of dopaminergic function was seen in knock-in flies harboring DTDS mutations (Asjad, Kasture, et al., 2017). Evidently, once the minimal threshold for functional rescue is achieved, increasing the efficacy of pharmacochaperoning in vitro by rational drug design has no further improvement on the behavioral output in vivo (Bhat et al., 2020).
In conclusion, recent findings from our group and others have shed new light onto the prospective uses of pharmacochaperoning in the treatment of many emerging folding diseases among NTT family members. The array of pathologic conditions triggered by anomalous folding in NTTs (Fig. 6) is ever ascending, necessitating the pursuit for novel chaperone compounds. The currently available state-of-the-art molecular dynamics simulations and electrophysiological approaches ought to provide valuable insights that bridge mutation-specific biophysical and structural effects to their biochemical and pharmacological impacts. It is conceivable that such joined endeavours, in consortium with rational drug design, may lead to the development of effective treatment options for many folding diseases in NTTs.
Fig. 6.
Folding diseases in NTT proteins. Folding diseases caused by mutations in NTT genes are displayed in the balloons, with the implicated transporters listed within the circular shapes representing different disorders. Apart from the conditions displayed in the figure, many other symptoms have been linked to specific transporters (e.g. CHT1: akinesia; CTL2: transfusion related acute lung injury; CTL4: sialidosis; EAAT1: vertigo, migraine, postural/gait imbalance; GlyT1: encephalopathy; NET: postural hypotension and long QT syndrome; Sialin: cognitive dysfunction; VMAT2: postural hypotension; VNUT: porokeratosis).
Supplementary Material
Acknowledgments
The authors acknowledge the financial support by the Austrian Science Fund FWF (project P31255-B27) to SS and the Vienna Science and Technology Fund WWTF (LSC17-026) to MF. We are most grateful to Dr. Florian P. Fischer and Roshni Mathur for their assistance with figure preparation.
Abbreviations
- AChT
vesicular acetylcholine transporter
- ASD
autism spectrum disorders
- ASCT
alanine/serine/cysteine transporter
- BGT
betaine/GABA transporter
- CHT
high affinity choline transporter
- CNX
calnexin
- COPII
coatomer complex II
- CRT
creatine transporter
- C-terminus
carboxy terminus
- CTL
choline transporter-like
- d
Drosophila melanogaster
- DAT
dopamine transporter
- DMSO
dimethyl sulfoxide
- EAAT
excitatory amino acid transporter
- EEG
electroencephalography
- ENT
equilibrative nucleoside transporter
- ER
endoplasmic reticulum
- ERAD
ER-associated degradation
- GABA
γ-aminobutyric acid
- GAT
GABA transporter
- GFP
green fluorescent protein
- GlyT
glycine transporter
- h
human
- HEK
human embryonic kidney
- HSP
heat shock protein
- MAE
myoclonic-atonic epilepsy
- MAT
monoamine transporter
- NET
norepinephrine transporter
- NTT
neurotransmitter transporter
- N-terminus
amino terminus
- SERT
serotonin transporter
- SLC
solute carrier
- SRP
signal-recognition particle
- TM
transmembrane segment
- UGT
UDP-glucose: glycoprotein glucosyltransferase
- VGAT
vesicular GABA transporter
- VIAAT
vesicular inhibitory amino acid transporter
- VGLUT
vesicular glutamate transporter
- VMAT
vesicular monoamine transporter
- VNUT
vesicular nucleotide transporter
- WT
wild type
Footnotes
Declaration of competing interest
The authors declare that there are no conflicts of interest.
References
- Abdelrahman HA, Al-Shamsi A, John A, Ali BR, Al-Gazali L. A novel SLC1A4 mutation (p.Y191*) causes spastic tetraplegia, thin corpus callosum, and progressive microcephaly (SPATCCM) with seizure disorder. Child Neurology Open. 2019;6:2329048X19880647. doi: 10.1177/2329048X19880647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamczyk A, Gause CD, Sattler R, Vidensky S, Rothstein JD, Singer H, Wang T. Genetic and functional studies of a missense variant in a glutamate transporter, SLC1A3, in Tourette syndrome. Psychiatric Genetics. 2011;21:90–97. doi: 10.1097/YPG.0b013e328341a307. [DOI] [PubMed] [Google Scholar]
- Adamsen D, Ramaekers V, Ho HT, Britschgi C, Rüfenacht V, Meili D, Thöny B. Autism spectrum disorder associated with low serotonin in CSF and mutations in the SLC29A4 plasma membrane monoamine transporter (PMAT) gene. Molecular Autism. 2014;5:43. doi: 10.1186/2040-2392-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshari P, Myles-Worsley M, Cohen OS, Tiobech J, Faraone SV, Byerley W, Middleton FA. Characterization of a novel mutation in SLC1A1 associated with schizophrenia. Molecular Neuropsychiatry. 2015;1:125–144. doi: 10.1159/000433599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aihara Y, Mashima H, Onda H, Hisano S, Kasuya H, Hori T, Takeda J. Molecular cloning of a novel brain-type Na+-dependent inorganic phosphate cotransporter. Journal of Neurochemistry. 2000;74:2622–2625. doi: 10.1046/j.1471-4159.2000.0742622.x. [DOI] [PubMed] [Google Scholar]
- Alfadhel M, Nashabat M, Qahtani HA, Alfares A, Mutairi FA, Shaalan HA, Ali QA. Mutation in SLC6A9 encoding a glycine transporter causes a novel form of non-ketotic hyperglycinemia in humans. Human Genetics. 2016;135:1263–1268. doi: 10.1007/s00439-016-1719-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfallaj R, Alfadhel M. Glycine transporter 1 encephalopathy from biochemical pathway to clinical disease: Review. Child Neurology Open. 2019;6:1–5. doi: 10.1177/2329048X19831486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amilhon B, Lepicard E, Renoir T, Mongeau R, Popa D, Poirel O, El Mestikawy S. VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety. The Journal of Neuroscience. 2010;30:2198–2210. doi: 10.1523/JNEUROSCI.5196-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anfinsen CB. Principles that govern the folding of protein chains. Science. 1973;181:223–230. doi: 10.1126/science.181.4096.223. [DOI] [PubMed] [Google Scholar]
- Anderluh A, Klotzsch E, Ries J, Reismann AW, Weber S, Fölser M, Schütz GJ. Tracking single serotonin transporter molecules at the endoplasmic reticulum and plasma membrane. Biophysical Journal. 2014;106:L33–L35. doi: 10.1016/j.bpj.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansar M, Ranza E, Shetty M, Paracha SA, Azam M, Kern I, Antonarakis SE. Taurine treatment of retinal degeneration and cardiomyopathy in a consanguineous family with SLC6A6 taurine transporter deficiency. Human Molecular Genetics. 2020;29:618–623. doi: 10.1093/hmg/ddz303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki M, Lin CL, Rothstein JD, Geller BA, Hosler BA, Munsat TL, Brown RH., Jr Mutations in the glutamate transporter EAAT2 gene do not cause abnormal EAAT2 transcripts in amyotrophic lateral sclerosis. Annals of Neurology. 1998;43:645–653. doi: 10.1002/ana.410430514. [DOI] [PubMed] [Google Scholar]
- Apparsundaram S, Ferguson SM, George AL, Jr, Blakely RD. Molecular cloning of a human, hemicholinium-3-sensitive choline transporter. Biochemical and Biophysical Research Communications. 2000;276:862–867. doi: 10.1006/bbrc.2000.3561. [DOI] [PubMed] [Google Scholar]
- Applegarth DA, Toone JR. Glycine encephalopathy (nonketotic hyperglycinemia): Comments and speculations. American Journal of Medical Genetics. 2006;140:186–188. doi: 10.1002/ajmg.a.31030. [DOI] [PubMed] [Google Scholar]
- Aran A, Segel R, Kaneshige K, Gulsuner S, Renbaum P, Oliphant S, Walsh T. Vesicular acetylcholine transporter defect underlies devastating congenital myasthenia syndrome. Neurology. 2017;88:1021–1028. doi: 10.1212/WNL.0000000000003720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arribas-González E, de Juan-Sanz J, Aragón C, López-Corcuera B. Molecular basis of the dominant negative effect of a glycine transporter 2 mutation associated with hyperekplexia. The Journal of Biological Chemistry. 2015;290:2150–2165. doi: 10.1074/jbc.M114.587055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asjad HMM, Kasture A, El-Kasaby A, Sackel M, Hummel T, Freissmuth M, Sucic S. Pharmacochaperoning in a Drosophila model system rescues human dopamine transporter variants associated with infantile/juvenile parkinsonism. The Journal of Biological Chemistry. 2017;292:19250–19265. doi: 10.1074/jbc.M117.797092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asjad HMM, Nasrollahi-Shirazi S, Sucic S, Freissmuth M, Nanoff C. Relax, cool down and scaffold: How to restore surface expression of folding-deficient mutant GPCRs and SLC6 transporters. International Journal of Molecular Sciences. 2017;18(11):2416. doi: 10.3390/ijms18112416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubrey KR, Rossi FM, Ruivo R, Alboni S, Bellenchi GC, Le Goff A, Supplisson S. The transporters GlyT2 and VIAAT cooperate to determine the vesicular glycinergic phenotype. The Journal of Neuroscience. 2007;27:6273–6281. doi: 10.1523/JNEUROSCI.1024-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aula N, Salomäki P, Timonen R, Verheijen F, Mancini G, Månsson J, Peltonen L. The spectrum of SLC17A5-gene mutations resulting in free sialic acidstorage diseases indicates some genotype-phenotype correlation. American Journal of Human Genetics. 2000;67:832–840. doi: 10.1086/303077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aula N, Jalanko A, Aula P, Peltonen L. Unraveling the molecular pathogenesis of free sialic acid storage disorders: Altered targeting of mutant Sialin. Molecular Genetics and Metabolism. 2002;77:99–107. doi: 10.1016/s1096-7192(02)00124-5. [DOI] [PubMed] [Google Scholar]
- Baga M, Spagnoli C, Soliani L, Salerno GG, Rizzi S, Frattini D, Fusco C. Early-onset dopamine transporter deficiency syndrome: Long-term follow-up. The Canadian Journal of Neurological Sciences. 2020:1–6. doi: 10.1017/cjn.2020.144. [DOI] [PubMed] [Google Scholar]
- Bailey CG, Ryan RM, Thoeng AD, Ng C, King K, Vanslambrouck JM, Rasko JE. Loss-of-function mutations in the glutamate transporter SLC1A1 cause human dicarboxylic aminoaciduria. The Journal of Clinical Investigation. 2011;121:446–453. doi: 10.1172/JCI44474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee M, Arutyunov D, Brandwein D, Janetzki-Flatt C, Kolski H, Hume S, Caluseriu O. The novel p.Ser263Phe mutation in the human high-affinity choline transporter 1 (CHT1/SLC5A7) causes a lethal form of fetal akinesia syndrome. Human Mutation. 2018;40:1676–1683. doi: 10.1002/humu.23828. [DOI] [PubMed] [Google Scholar]
- Barwick KE, Wright J, Al-Turki S, McEntagart MM, Nair A, Chioza B, Crosby AH. Defective presynaptic choline transport underlies hereditary motor neuropathy. American Journal of Human Genetics. 2012;91:1103–1107. doi: 10.1016/j.ajhg.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauché S, O’Regan S, Azuma Y, Laffargue F, McMacken G, Sternberg D, Nicole S. Impaired presynaptic high-affinity choline transporter causes a congenital myasthenic syndrome with episodic apnea. American Journal of Human Genetics. 2016;99:753–761. doi: 10.1016/j.ajhg.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazalakova MH, Wright J, Schneble EJ, McDonald MP, Heilman CJ, Levey AI, Blakely RD. Deficits in acetylcholine homeostasis, receptors and behaviors in choline transporter heterozygous mice. Genes, Brain and Behavior. 2007;6:411–424. doi: 10.1111/j.1601-183X.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- Beerepoot P, Lam VM, Salahpour A. Pharmacological chaperones of the dopamine transporter rescue dopamine transporter deficiency syndrome mutations in heterologous cells. The Journal of Biological Chemistry. 2016;291:22053–22062. doi: 10.1074/jbc.M116.749119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio EE, Reimer RJ, Fremeau RT, Jr, Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289:957–960. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- Ben-Yona A, Kanner BI. Functional defects in the external and internal thin gates of the g-aminobutyric acid (GABA) transporter GAT-1 can compensate each other. The Journal of Biological Chemistry. 2013;288:4549–4556. doi: 10.1074/jbc.M112.430215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“ecstasy”) in serotonin transporter-deficient mice. Molecular Pharmacology. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Berry AS, Demeter E, Sabhapathy S, English BA, Blakely RD, Sarter M, Lustig C. Disposed to distraction: Genetic variation in the cholinergic system influences distractibility but not time on-task effects. Journal of Cognitive Neuroscience. 2014;26:1–11. doi: 10.1162/jocn_a_00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsalel OT, Pop A, Rosenberg EH, Fernandez-Ojeda M, Jakobs C, Salomons GS, Creatine Transporter Research, Group Detection of variants in SLC6A8 and functional analysis of unclassified missense variants. Molecular Genetics and Metabolism. 2012;105:596–601. doi: 10.1016/j.ymgme.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Beyer LA, Galano MM, Nair TS, Kommareddi PK, Sha SH, Raphael Y, Carey TE. Age-related changes in expression of CTL2/SLC44A2 and its isoforms in the mouse inner ear. Hearing Research. 2011;282:63–68. doi: 10.1016/j.heares.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat S, Hasenhuetl PS, Kasture A, El-Kasaby A, Baumann MH, Blough BE, Freissmuth M. Conformational state interactions provide clues to the pharmacochaperone potential of serotonin transporter partial substrates. The Journal of Biological Chemistry. 2017;292:16773–16786. doi: 10.1074/jbc.M117.794081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat S, Guthrie GG, Kasture A, El-Kasaby A, Cao J, Bonifazi A, Newman AH. A tropane-based ibogaine analog rescues folding-deficient SERT and DAT. ACS Pharmacology and Translational Science. 2020 doi: 10.1021/acsptsci.0c00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutia YD, Ganapathy V. Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochimica et Biophysica Acta. 2016;1863:2531–2539. doi: 10.1016/j.bbamcr.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittel DC, Kibiryeva N, Dasouki M, Knoll JH, Butler MG. A 9-year-old male with a duplication of chromosome 3p25.3p26.2: Clinical report and gene expression analysis. American Journal of Medical Genetics. 2006;140A:573–579. doi: 10.1002/ajmg.a.31132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bly M. Mutation in the vesicular monoamine gene, SLC18A1, associated with schizophrenia. Schizophrenia Research. 2005;78:337–338. doi: 10.1016/j.schres.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Bowton E, Saunders C, Reddy IA, Campbell NG, Hamilton PJ, Galli A. SLC6A3 coding variant Ala559Val found in two autism probands alters dopamine transporter function and trafficking. Translational Psychiatry. 2014;4:e464. doi: 10.1038/tp.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröer S. The SLC6 orphans are forming a family of amino acid transporters. Neurochemistry International. 2006;48:559–567. doi: 10.1016/j.neuint.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Bröer A, Tietze N, Kowalczuk S, Chubb S, Munzinger M, Bak LK, Bröer S. The orphan transporter v7-3 (slc6a15) is a Na+−dependent neutral amino acid transporter (B0AT2) The Biochemical Journal. 2006;393:421–430. doi: 10.1042/BJ20051273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröer S, Bailey CG, Kowalczuk S, Ng C, Vanslambrouck JM, Rodgers H, Rasko JE. Iminoglycinuria and hyperglycinuria are discrete human phenotypes resulting from complex mutations in proline and glycine transporters. The Journal of Clinical Investigation. 2008;118:3881–3892. doi: 10.1172/JCI36625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröer S. The role of the neutral amino acid transporter B0AT1 (SLC6A19) in Hartnup disorder and protein nutrition. IUBMB Life. 2009;61:591–599. doi: 10.1002/iub.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA. Acetylcholine and cholinergic receptors. Brain and Neuroscience Advances. 2019;3:2398212818820506. doi: 10.1177/2398212818820506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai K, Wang J, Eissman J, Wang J, Nwosu G, Shen W, Kang JQ. A missense mutation in SLC6A1 associated with Lennox-Gastaut syndrome impairs GABA transporter 1 protein trafficking and function. Experimental Neurology. 2019;320:112973. doi: 10.1016/j.expneurol.2019.112973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callender R, Gameiro A, Pinto A, De Micheli C, Grewer C. Mechanism of inhibition of the glutamate transporter EAAC1 by the conformationally constrained glutamate analogue (+)-HIP-B. Biochemistry. 2012;51:5486–5495. doi: 10.1021/bi3006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarena B, González L, Hernández S, Caballero A. SLC6A4 rare variant associated with eating disorders in Mexican patients. Journal of Psychiatric Research. 2012;46:1106–1107. doi: 10.1016/j.jpsychires.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Camargo SM, Singer D, Makrides V, Huggel K, Pos KM, Wagner CA, Verrey F. Tissue-specific amino acid transporter partners ACE2 and collectrin differentially interact with hartnup mutations. Gastroenterology. 2009;136:872–882. doi: 10.1053/j.gastro.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell NG, Shekar A, Aguilar JI, Peng D, Navratna V, Yang D, Galli A. Structural, functional, and behavioural insights of dopamine dysfunction revealed by a deletion in SLC6A3. Proceedings of the National Academy of Sciences of the United States of America. 2019;116:3853–3862. doi: 10.1073/pnas.1816247116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Zhao K, Zhong XZ, Zou Y, Yu H, Huang P, Dong XP. SLC17A9 protein functions as a lysosomal ATP transporter and regulates cell viability. The Journal of Biological Chemistry. 2014;289:23189–23199. doi: 10.1074/jbc.M114.567107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta E, Chung SK, James VM, Robinson A, Gill JL, Remy N, Harvey RJ. Mutations in the GlyT2 gene (SLC6A5) are a second major cause of startle disease. The Journal of Biological Chemistry. 2012;287:28975–28985. doi: 10.1074/jbc.M112.372094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvill GL, Mcmahon JM, Schneider A, Zemel M, Myers CT, Saykally J, Mefford HC. Mutations in the GABA transporter SLC6A1 cause epilepsy with myoclonic-atonic seizures. American Journal of Human Genetics. 2015;96:808–815. doi: 10.1016/j.ajhg.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. The Journal of Neuroscience. 1998;18:9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri TK, Paul S. Protein-misfolding diseases and chaperone-based therapeutic approaches. The FEBS Journal. 2006;273:1331–1349. doi: 10.1111/j.1742-4658.2006.05181.x. [DOI] [PubMed] [Google Scholar]
- Chen L, Yang X, Zhou X, Wang C, Gong X, Chen Y. Hyperactivity and impaired attention in gamma aminobutyric acid transporter subtype 1 gene knockout mice. Acta Neuropsychiatrica. 2015;27:368–374. doi: 10.1017/neu.2015.37. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Yamashiro K, Chen LJ, Ahn J, Huang L, Huang L, Khor CC. New loci and coding variants confer risk for age-related macular degeneration in east Asian. Nature Communications. 2015;6:6063. doi: 10.1038/ncomms7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba P, Freissmuth M, Stockner T. Defining the blanks–pharmacochaperoning of SLC6 transporters and ABC transporters. Pharmacological Research. 2014;83:63–73. doi: 10.1016/j.phrs.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KD, Choi JH. Episodic ataxias: Clinical and genetic features. Journal of Movement Disorders. 2016;9:129–135. doi: 10.14802/jmd.16028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KD, Kim JS, Kim HJ, Jung I, Jeong SH, Lee SH, Choi JH. Genetic variants associated with episodic ataxia in Korea. Scientific Reports. 2017a;7:13855. doi: 10.1038/s41598-017-14254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KD, Jen JC, Choi SY, Shin JH, Kim HS, Kim HJ, Choi JH. Late-onset episodic ataxia associated with SLC1A3 mutation. Journal of Human Genetics. 2017b;62:443–446. doi: 10.1038/jhg.2016.137. [DOI] [PubMed] [Google Scholar]
- Coelho T, Merlini G, Bulawa CE, Fleming JA, Judge DP, Kelly JW, Huertas P. Mechanism of action and clinical application of Tafamidis in hereditary transthyretin amyloidosis. Neurology & Therapy. 2016;5:1–25. doi: 10.1007/s40120-016-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy J, Allen NM, Gorman K, O’Halloran E, Shahwan A, Lynch B, King MD. Novel European SLC1A4 variant: Infantile spasms and population ancestry analysis. Journal of Human Genetics. 2016;61:761–764. doi: 10.1038/jhg.2016.44. [DOI] [PubMed] [Google Scholar]
- Cope DW, Di Giovanni G, Fyson SJ, Orbán G, Errington AC, Lorincz ML, Crunelli V. Enhanced tonic GABAA inhibition in typical absence epilepsy. Nature Medicine. 2009;15:1392–1398. doi: 10.1038/nm.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousens LS, Gallwitz D, Alberts BM. Different accessibilities in chromatin to histone acetylase. The Journal of Biological Chemistry. 1979;254:1716–1723. [PubMed] [Google Scholar]
- Cuddy LK, Gordon AC, Black SA, Jaworski E, Ferguson SS, Rylett RJ. Peroxynitrite donor SIN-1 alters high-affinity choline transporter activity by modifying its intracellular trafficking. The Journal of Neuroscience. 2012;32:5573–5584. doi: 10.1523/JNEUROSCI.5235-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui WW, Low SE, Hirata H, Saint-Amant L, Geisler R, Hume RI, Kuwada JY. The zebrafish shocked gene encodes a glycine transporter and is essential for the function of early neural circuits in the CNS. The Journal of Neuroscience. 2005;25:6610–6620. doi: 10.1523/JNEUROSCI.5009-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Li L, Wang W, Shen J, Yue Z, Zheng X, Zhang X. Exome sequencing identifies SLC17A9 pathogenic gene in two Chinese pedigrees with disseminated superficial actinic porokeratosis. Journal of Medical Genetics. 2014;51:699–704. doi: 10.1136/jmedgenet-2014-102486. [DOI] [PubMed] [Google Scholar]
- Cyr M, Beaulieu JM, Laakso A, Sotnikova TD, Yao WD, Bohn LM, Caron MG. Sustained elevation of extracellular dopamine causes motor dysfunction and selective degeneration of striatal GABAergic neurons. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11035–11040. doi: 10.1073/pnas.1831768100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Adamo MC, Gallenmüller C, Servettini I, Hartl E, Tucker SJ, Arning L, Klopstock T. Novel phenotype associated with a mutation in the KCNA1(Kv1.1) gene. Frontiers in Physiology. 2015;15:525. doi: 10.3389/fphys.2014.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dafsari HS, Kawalia A, Sprute R, Karakaya M, Malenica A, Herkenrath P, Cirak S. Novel mutations in SLC6A5 with benign course in hyperekplexia. Cold Spring Harbour Molecular Case Studies. 2019;5:a004465. doi: 10.1101/mcs.a004465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Vinnakota S, Qian X, Kunze DL, Sarkar HK. Molecular characterization of the human CRT-1 creatine transporter expressed in Xenopus oocytes. Archives of Biochemistry and Biophysics. 1999;361:75–84. doi: 10.1006/abbi.1998.0959. [DOI] [PubMed] [Google Scholar]
- Damseh N, Simonin A, Jalas C, Picoraro JA, Shaag A, Cho MT, Edvardson S. Mutations in SLC1A4, encoding the brain serine transporter, are associated with developmental delay, microcephaly and hypomyelination. Journal of Medical Genetics. 2015;52:541–547. doi: 10.1136/jmedgenet-2015-103104. [DOI] [PubMed] [Google Scholar]
- Davies JS, Chung SK, Thomas RH, Robinson A, Hammond CL, Mullins JG, Rees MI. The glycinergic system in human startle disease: A genetic screening approach. Frontiers in Molecular Neuroscience. 2010;3:8. doi: 10.3389/fnmol.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GL, Stewart A, Stanwood GD, Gowrishankar R, Hahn MK, Blakely RD. Functional coding variation in the presynaptic dopamine transporter associated with neuropsychiatric disorders drives enhanced motivation and contextdependent impulsivity in mice. Behavioural Brain Research. 2018;337:61–69. doi: 10.1016/j.bbr.2017.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC. Unfaithful neurotransmitter transporters: Focus on serotonin uptake and implications for antidepressant efficacy. Pharmacology and Therapeutics. 2009;121:89–99. doi: 10.1016/j.pharmthera.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Castro BM, De Jaeger X, Martins-Silva C, Lima RD, Amaral E, Menezes C, Prado VF. The vesicular acetylcholine transporter is required for neuromuscular development and function. Molecular and Cellular Biology. 2009;29:5238–5250. doi: 10.1128/MCB.00245-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries B, Mamsa H, Stam AH, Wan J, Bakker SLM, Vanmolkot KRJ, van den Maagdenberg AM. Episodic Ataxia associated with EAAT1 mutation C186S affecting glutamate reuptake. Archives of Neurology. 2009;66:97–101. doi: 10.1001/archneurol.2008.535. [DOI] [PubMed] [Google Scholar]
- Dehnes Y, Chaudhry FA, Ullensvang K, Lehre KP, Storm-Mathisen J, Danbolt NC. The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: A glutamate-gated Chloride Channel concentrated near the synapse in parts of the dendritic membrane facing Astroglia. The Journal of Neuroscience. 1998;18:3606–3619. doi: 10.1523/JNEUROSCI.18-10-03606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme R, Betancur C, Wagner M, Krebs MO, Gorwood P, Pearl P, Bourgeron T. Support for the association between the rare functional variant I425V of the serotonin transporter gene and susceptibility to obsessive compulsive disorder. Molecular Psychiatry. 2004;10:1059–1061. doi: 10.1038/sj.mp.4001728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demily C, Hubert L, Franck N, Poisson A, Munnich A, Besmond C. Somatic mosaicism for SLC1A1 mutation supports threshold effect and familial aggregation in schizophrenia spectrum disorders. Schizophrenia Research. 2018;197:583–584. doi: 10.1016/j.schres.2017.11.028. [DOI] [PubMed] [Google Scholar]
- DiCarlo GE, Aguilar JI, Matthies HJ, Harrison FE, Bundschuh KE, West A, Galli A. Autism-linked dopamine transporter mutation alters striatal dopamine neurotransmission and dopamine-dependent behaviours. The Journal of Clinical Investigation. 2019;129:3407–3419. doi: 10.1172/JCI127411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikow N, Maas B, Karch S, Granzow M, Janssen JW, Jauch A, Moog U. 3p25.3 microdeletion of GABA transporters SLC6A1 and SLC6A11 results in intellectual disability, epilepsy and stereotypic behaviour. American Journal of Medical Genetics. 2014;164A:3061–3068. doi: 10.1002/ajmg.a.36761. [DOI] [PubMed] [Google Scholar]
- Doan RN, Lim ET, De Rubeis S, Betancur C, Cutler DJ, Chiocchetti AG, Yu TW. Recessive gene disruptions in autism spectrum disorder. Nature Genetics. 2019;51:1092–1098. doi: 10.1038/s41588-019-0433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Luo Y, Fan S, Yin B, Weng C, Peng B. Screening gene mutations in Chinese patients with benign essential Blepharospasm. Frontiers in Neurology. 2020;10:1387. doi: 10.3389/fneur.2019.01387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Wang J. Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. The Journal of Pharmacology and Experimental Therapeutics. 2010;335:743–753. doi: 10.1124/jpet.110.170142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Wang J. Impaired monoamine and organic cation uptake in choroid plexus in mice with targeted disruption of the plasma membrane monoamine transporter (Slc29a4) gene. The Journal of Biological Chemistry. 2013;288:3535–3544. doi: 10.1074/jbc.M112.436972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimova EV, Gainetdinov RR, Budygin EA, Sotnikova TD. Dopamine transporter mutant animals: A translational perspective. Journal of Neurogenetics. 2016;30:5–15. doi: 10.3109/01677063.2016.1144751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kasaby A, Just H, Malle E, Stolt-Bergner PC, Sitte HH, Freissmuth M, Kudlacek O. Mutations in the carboxyl-terminal SEC24 binding motif of the serotonin transporter impair folding of the transporter. The Journal of Biological Chemistry. 2010;285:39201–39210. doi: 10.1074/jbc.M110.118000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kasaby A, Koban F, Sitte HH, Freissmuth M, Sucic S. A cytosolic relay of heat shock proteins HSP70-1A and HSP90beta monitors the folding trajectory of the serotonin transporter. The Journal of Biological Chemistry. 2014;289:28987–29000. doi: 10.1074/jbc.M114.595090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kasaby A, Kasture A, Koban F, Hotka M, Asjad HMM, Kubista H, Sucic S. Rescue by 4-phenylbutyrate of several misfolded creatine transporter-1 variants linked to the creatine transporter deficiency syndrome. Neuropharmacology. 2019;161:107572. doi: 10.1016/j.neuropharm.2019.03.015. [DOI] [PubMed] [Google Scholar]
- El Mestikawy S, Wallén-Mackenzie A, Fortin GM, Descarries L, Trudeau LE. From glutamate co-release to vesicular synergy: Vesicular glutamate transporters. Nature Reviews Neuroscience. 2011;12:204–216. doi: 10.1038/nrn2969. [DOI] [PubMed] [Google Scholar]
- English BA, Hahn MK, Gizer IR, Mazei-Robison M, Steele A, Kurnik DM, Blakely RD. Choline transporter gene variation is associated with attention-deficit hyperactivity disorder. Journal of Neurodevelopmental Disorders. 2009;1:252–263. doi: 10.1007/s11689-009-9033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis EA, Wright J, Retzlaff CL, McManus OB, Lin Z, Huang X, Wu M, Blakely RD. Identification and characterization of ML352: A novel, noncompetitive inhibitor of the presynaptic choline transporter. ACS Chemical Neuroscience. 2015;6:417–427. doi: 10.1021/cn5001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis EA, Blakely RD. Choline on the move: Perspectives on the molecular physiology and pharmacology of the presynaptic choline transporter. Advances in Pharmacology. 2016;76:175–213. doi: 10.1016/bs.apha.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Epi4K Consortium. De novo mutations in epileptic encephalopathies. Nature. 2013;501:217–221. doi: 10.1038/nature12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epi4K Consortium. De novo mutations in SLC1A2 and CACNA1A are important causes of epileptic encephalopathies. American Journal of Human Genetics. 2016;99:287–298. doi: 10.1016/j.ajhg.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JD, Varoqui H, Schäfer MK, Modi W, Diebler MF, Weihe E, Usdin TB. Functional identification of a vesicular acetylcholine transporter and its expression from a “cholinergic” gene locus. The Journal of Biological Chemistry. 1994;269:21929–21932. [PubMed] [Google Scholar]
- Erickson JD, Schafer MK, Bonner TI, Eiden LE, Weihe E. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:5166–5171. doi: 10.1073/pnas.93.10.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulenburg V, Becker K, Gomeza J, Schmitt B, Becker CM, Betz H. Mutations within the human GLYT2 (SLC6A5) gene associated with hyperekplexia. Biochemical and Biophysical Research Communications. 2006;348:400–405. doi: 10.1016/j.bbrc.2006.07.080. [DOI] [PubMed] [Google Scholar]
- Eulenburg V, Retiounskaia M, Papadopoulos T, Gomeza J, Betz H. Glial glycine transporter 1 function is essential for early postnatal survival but dispensable in adult mice. Glia. 2010;58:1066–1073. doi: 10.1002/glia.20987. [DOI] [PubMed] [Google Scholar]
- Fagerberg CR, Taylor A, Distelmaier F, Schrøder HD, Kibæk M, Wieczorek D, Bakovic M. Choline transporter-like 1 deficiency causes a new type of childhood-onset neurodegeneration. Brain. 2020;143:94–111. doi: 10.1093/brain/awz376. [DOI] [PubMed] [Google Scholar]
- Farhan H, Korkhov VM, Paulitschke V, Dorostkar MM, Scholze P, Kudlacek O, Sitte HH. Two discontinuous segments in the carboxyl terminus are required for membrane targeting of the rat gamma-aminobutyric acid transporter-1 (GAT-1) Journal of Biological Chemistry. 2004;279:28553–28563. doi: 10.1074/jbc.M307325200. [DOI] [PubMed] [Google Scholar]
- Farmer MK, Robbins MJ, Medhurst AD, Campbell DA, Ellington K, Duckworth M, Pangalos MN. Cloning and characterization of human NTT5 and v7-3: Two orphan transporters of the Na+/Cl--dependent neurotransmitter transporter gene family. Genomics. 2000;70:241–252. doi: 10.1006/geno.2000.6387. [DOI] [PubMed] [Google Scholar]
- Farr CV, El-Kasaby A, Freissmuth M, Sucic S. The creatine transporter unfolded: A knotty premise in the cerebral creatine deficiency syndrome. Frontiers in Synaptic Neuroscience. 2020 doi: 10.3389/fnsyn.2020.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei H, Chow DM, Chen A, Romero-Calderón R, Ong WS, Ackerson LC, Krantz DE. Mutation of the Drosophila vesicular GABA transporter disrupts visual figure detection. The Journal of Experimental Biology. 2010;213:1717–1730. doi: 10.1242/jeb.036053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fentress HM, Klar R, Krueger JJ, Sabb T, Redmon SN, Wallace NM, Hahn MK. Norepinephrine transporter heterozygous knockout mice exhibit altered transport and behaviour. Genes, Brain and Behaviour. 2013;12:749–759. doi: 10.1111/gbb.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Blakely RD. The choline transporter resurfaces. New roles for synaptic vesicles? Molecular Interventions. 2004;4:22–37. doi: 10.1124/mi.4.1.22. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Bazalakova M, Savchenko V, Tapia JC, Wright J, Blakely RD. Lethal impairment of cholinergic neurotransmission in hemicholinium-3-sensitive choline transporter knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8762–8767. doi: 10.1073/pnas.0401667101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino A, Sharp SI, McQuillin A. Association of rare variation in the glutamate receptor gene SLC1A2 with susceptibility to bipolar disorder and schizophrenia. European Journal of Human Genetics. 2015;23:1200–1206. doi: 10.1038/ejhg.2014.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fon EA, Pothos EN, Sun BC, Killeen N, Sulzer D, Edwards RH. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron. 1997;19:1271–1283. doi: 10.1016/s0896-6273(00)80418-3. [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Sreedharan S, Nordenankar K, Alsiö J, Lindberg FA, Hutchinson A, Schiöth HB. The polyamine transporter Slc18b1(VPAT) is important for both short and long time memory and for regulation of polyamine content in the brain. PLoS Genetics. 2019;15:e1008455. doi: 10.1371/journal.pgen.1008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freissmuth M, Stockner T, Sucic S. SLC6 transporter folding diseases and pharmacochaperoning. Handbook of Experimental Pharmacology. 2018;245:249–270. doi: 10.1007/164_2017_71. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends in Neurosciences. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Edwards RH. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science. 2004;304:1815–1819. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR. Dopamine transporter mutant mice in experimental neuropharmacology. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2008;377:301–313. doi: 10.1007/s00210-007-0216-0. [DOI] [PubMed] [Google Scholar]
- Gasnier B. The SLC32 transporter, a key protein for the synaptic release of inhibitory amino acids. Pflügers Archiv/European Journal of Physiology. 2004;447:756–759. doi: 10.1007/s00424-003-1091-2. [DOI] [PubMed] [Google Scholar]
- Gasser PJ. Roles for the uptake 2 transporter OCT3 in regulation of dopaminergic neurotransmission and behavior. Neurochemistry International. 2019;123:46–49. doi: 10.1016/j.neuint.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler JC, Corbin KL, Li Q, Feranchak AP, Nunemaker CS, Li C. Vesicular nucleotide transporter-mediated ATP release regulates insulin secretion. Endocrinology. 2013;154:675–684. doi: 10.1210/en.2012-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain DP, Hughes DA, Nicholls K, Bichet DG, Barlow C, Schiffmann R. Treatment of Fabry’s disease with the pharmacologic chaperone migalastat. The New England Journal of Medicine. 2016;375:545–555. doi: 10.1056/NEJMoa1510198. [DOI] [PubMed] [Google Scholar]
- Gill JL, Capper D, Vanbellinghen JF, Chung SK, Higgins RJ, Rees MI, Harvey RJ. Startle disease in Irish wolfhounds associated with a microdeletion in the glycine transporter GlyT2 gene. Neurobiology of Disease. 2011;43:184–189. doi: 10.1016/j.nbd.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JL, James VM, Carta E, Harris D, Topf M, Scholes SF, Harvey RJ. Identification of congenital muscular dystonia 2 associated with an inherited GlyT2 defect in Belgian Blue cattle from the United Kingdom. Animal Genetics. 2012;43:267–270. doi: 10.1111/j.1365-2052.2011.02255.x. [DOI] [PubMed] [Google Scholar]
- Gilman TL, George CM, Vitela M, Herrera-Rosales M, Basiouny MS, Koek W, Daws LC. Constitutive plasma membrane monoamine transporter (PMAT, Slc29a4) deficiency subtly affects anxiety-like and coping behaviours. The European Journal of Neuroscience. 2018;48:1706–1716. doi: 10.1111/ejn.13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giménez C, Pérez-Siles G, Martínez-Villarreal J, Arribas-González E, Jiménez E, Núñez E, López-Corcuera B. A novel dominant hyperekplexia mutation Y705C alters trafficking and biochemical properties of the presynaptic glycine transporter GlyT2. The Journal of Biological Chemistry. 2012;287:28986–29002. doi: 10.1074/jbc.M111.319244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Glatt CE, Deyoung JA, Delgado S, Service SK, Giacomini KM, Edwards RH, Freimer NB. Screening a large reference sample to identify very low frequency sequence variants: Comparisons between two genes. Nature Genetics. 2001;27:435–438. doi: 10.1038/86948. [DOI] [PubMed] [Google Scholar]
- Gliddon CM, Shao Z, LeMaistre JL, Anderson CM. Cellular distribution of the neutral amino acid transporter subtype ASCT2 in mouse brain. Journal of Neurochemistry. 2009;108:372–383. doi: 10.1111/j.1471-4159.2008.05767.x. [DOI] [PubMed] [Google Scholar]
- Gomeza J, Hülsmann S, Ohno K, Eulenburg V, Szöke K, Richter D, Betz H. Inactivation of the glycine transporter 1 gene discloses vital role of glial glycine uptake in glycinergic inhibition. Neuron. 2003a;40:785–796. doi: 10.1016/s0896-6273(03)00672-x. [DOI] [PubMed] [Google Scholar]
- Gomeza J, Ohno K, Hülsmann S, Armsen W, Eulenburg V, Richter DW, Betz H. Deletion of the mouse glycine transporter 2 results in a hyperekplexia phenotype and postnatal lethality. Neuron. 2003b;40:797–806. doi: 10.1016/s0896-6273(03)00673-1. [DOI] [PubMed] [Google Scholar]
- Granato M, van Eeden FJ, Schach U, Trowe T, Brand M, Furutani-Seiki M, Nüsslein-Volhard C. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development. 1996;123:399–413. doi: 10.1242/dev.123.1.399. [DOI] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, El Mestikawy S. A third vesicular glutamate transporter expressed by cholinergic and seroto-ninergic neurons. The Journal of Neuroscience. 2002;22:5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene CC, McMillan PM, Barker SE, Kurnool P, Lomax MI, Burmeister M, Lesperance MM. DFNA25, a novel locus for dominant nonsyndromic hereditary hearing impairment, maps to 12q21-24. American Journal of Human Genetics. 2001;68:254–260. doi: 10.1086/316925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewer C, Gameiro A, Rauen T. SLC1 glutamate transporters. Pflügers Archiv/European Journal of Physiology. 2014;466:3–24. doi: 10.1007/s00424-013-1397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe M, Heinsen H, Teipel SJ. Atrophy of the cholinergic basalforebrain over the adult age range and in early stages of Alzheimer’s disease. Biological Psychiatry. 2012;71:805–813. doi: 10.1016/j.biopsych.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünhage F, Schulze TG, Müller DJ, Lanczik M, Franzek E, Albus M, Nöthen MM. Systematic screening for DNA sequence variation in the coding region of the human dopamine transporter gene (DAT1) Molecular Psychiatry. 2000;5:275–282. doi: 10.1038/sj.mp.4000711. [DOI] [PubMed] [Google Scholar]
- Guella I, McKenzie MB, Evans DM, Buerki SE, Toyota EB, Van Allen MI, Farrer MJ. De novo mutations in YWHAG cause early-onset epilepsy. American Journal of Human Genetics. 2017;101:300–310. doi: 10.1016/j.ajhg.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenisch B, Bönisch H. Interaction of the human plasma membrane monoamine transporter (hPMAT) with antidepressants and antipsychotics. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2009;381:33–39. doi: 10.1007/s00210-009-0479-8. [DOI] [PubMed] [Google Scholar]
- Hägglund MG, Hellsten SV, Bagchi S, Ljungdahl A, Nilsson VC, Winnergren S, Fredriksson R. Characterization of the transporter B0AT3 (Slc6a17) in the rodent central nervous system. BMC Neuroscience. 2013;14:54. doi: 10.1186/1471-2202-14-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MK, Blakely RD. Monoamine transporter gene structure and polymorphisms in relation to psychiatric and other complex disorders. The Pharmacogenomics Journal. 2002;2:217–235. doi: 10.1038/sj.tpj.6500106. [DOI] [PubMed] [Google Scholar]
- Hahn MK, Robertson D, Blakely RD. A mutation in the human norepinephrine transporter gene (SLC6A2) associated with orthostatic intolerance disrupts surface expression of mutant and wild type transporters. The Journal of Neuroscience. 2003;23:4470–4478. doi: 10.1523/JNEUROSCI.23-11-04470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MK, Mazei-Robison MS, Blakely RD. Single nucleotide polymorphisms in the human norepinephrine transporter gene affect expression, trafficking, antidepressant interaction, and protein kinase C regulation. Molecular Pharmacology. 2005;68:457–466. doi: 10.1124/mol.105.011270. [DOI] [PubMed] [Google Scholar]
- Hahn MK, Blakely RD. The functional impact of SLC6 transporter genetic variation. Annual Review of Pharmacology and Toxicology. 2007;47:401–441. doi: 10.1146/annurev.pharmtox.47.120505.105242. [DOI] [PubMed] [Google Scholar]
- Hahn MK, Blackford JU, Haman K, Mazei-Robison M, English BA, Prasad HC, Shelton R. Multivariate permutation analysis associates multiple polymorphisms with subphenotypes of major depression. Genes, Brain and Behavior. 2008;7:487–495. doi: 10.1111/j.1601-183X.2007.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MK, Steele A, Couch RS, Stein MA, Krueger JJ. Novel and functional norepinephrine transporter protein variants identified in attention-deficit hyperactivity disorder. Neuropharmacology. 2009;57:694–701. doi: 10.1016/j.neuropharm.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakonen AH, Polvi A, Saloranta C, Paetau A, Heikkilä P, Almusa H, Aittomäki K. SLC18A3 variants lead to fetal akinesia deformation sequence early in pregnancy. American Journal of Medical Genetics. 2019;179:1362–1365. doi: 10.1002/ajmg.a.61186. [DOI] [PubMed] [Google Scholar]
- Halvorsen M, Petrovski S, Shellhaas R, Tang Y, Crandall L, Devinsky O. Mosaic mutations in early-onset genetic diseases. Genetics in Medicine. 2015;18:746–749. doi: 10.1038/gim.2015.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka K, Takahashi K, Miyatake S, Mitsuhashi S, Hamanoue H, Miyaji Y, Matsumoto N. Confirmation of SLC5A7-related distal hereditary motor neuropathy 7 in a family outside Wales. Clinical Genetics. 2018;94:274–275. doi: 10.1111/cge.13369. [DOI] [PubMed] [Google Scholar]
- Hamilton PJ, Campbell NG, Sharma S, Erreger K, Herborg F, Saunders C, Galli A. De novo mutation in the dopamine transporter gene associates dopamine dysfunction with autism spectrum disorder. Molecular Psychiatry. 2013;18:1315–1323. doi: 10.1038/mp.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton PJ, Shekar A, Belovich AN, Christianson NB, Campbell NG, Sutcliffe JS, Erreger K. Zn(2+) reverses functional deficits in a De novo dopamine transporter variant associated with autism spectrum disorder. Molecular Autism. 2015;6:8. doi: 10.1186/s13229-015-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Budreau AM, Chesney RW, Sturman JA. Does the taurine transporter gene play a role in 3p-syndrome? Advances in Experimental Medicine and Biology. 2000;483:613–619. doi: 10.1007/0-306-46838-7_66. [DOI] [PubMed] [Google Scholar]
- Hansen FH, Skjørringe T, Yasmeen S, Arends NV, Sahai MA, Erreger K, Gether U. Missense dopamine transporter mutations associate with adult Parkinsonism and ADHD. The Journal of Clinical Investigation. 2014;124:3107–3120. doi: 10.1172/JCI73778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Topf M, Harvey K, Rees MI. The genetics of hyperekplexia: More than startle. Trends in Genetics. 2008;24:439–447. doi: 10.1016/j.tig.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Hasenhuetl PS, Bhat S, Freissmuth M, Sandtner W. Functional selectivity and partial efficacy at the monoamine transporters: A unified model of allosteric modulation and amphetamine-induced substrate release. Molecular Pharmacology. 2019;95:303–312. doi: 10.1124/mol.118.114793. [DOI] [PubMed] [Google Scholar]
- Hayashi MK, Yasui M. The transmembrane transporter domain of glutamate transporters is a process tip localizer. Scientific Reports. 2015;5:9032. doi: 10.1038/srep09032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert DN, Foellmer B, Helenius A. Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell. 1995;81:425–433. doi: 10.1016/0092-8674(95)90395-x. [DOI] [PubMed] [Google Scholar]
- Heidari E, Razmara E, Hosseinpour S, Tavasoli AR, Garshasbi M. Homozygous in-frame variant of SCL6A3 causes dopamine transporter deficiency syndrome in a consanguineous family. Annals of Human Genetics. 2020;84:315–323. doi: 10.1111/ahg.12378. [DOI] [PubMed] [Google Scholar]
- Heimer G, Marek-Yagel D, Eyal E, Barel O, Oz Levi D, Hoffmann C, Ben Zeev B. SLC1A4 mutations cause a novel disorder of intellectual disability, progressive microcephaly, spasticity and thin corpus callosum. Clinical Genetics. 2015;88:327–335. doi: 10.1111/cge.12637. [DOI] [PubMed] [Google Scholar]
- Heller-Stilb B, van Roeyen C, Rascher K, Hartwig HG, Huth A, Seeliger MW, Häussinger D. Disruption of the taurine transporter gene (Taut) leads to retinal degeneration in mice. FASEB Journal. 2002;16:231–233. doi: 10.1096/fj.01-0691fje. [DOI] [PubMed] [Google Scholar]
- Hensler JG, Artigas F, Bortolozzi A, Daws LC, De Deurwaerdère P, Milan L, Koek W. Catecholamine/serotonin interactions: Systems thinking for brain function and disease. Advances in Pharmacology. 2013;68:167–197. doi: 10.1016/B978-0-12-411512-5.00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herborg F, Andreassen TF, Berlin F, Loland CJ, Gether U. Neuropsychiatric disease-associated genetic variants of the dopamine transporter display heterogeneous molecular phenotypes. Journal of Biological Chemistry. 2018;293:7250–7262. doi: 10.1074/jbc.RA118.001753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Muñoz S, Camarena-Medellin B, González-Macías L, Aguilar-García A, Flores-Flores G, Luna Dominguez D, Caballero-Romo A. Sequence analysis of five exons of SLC6A4 gene in Mexican patients with anorexia nervosa and bulimia nervosa. Gene. 2020;748:144675. doi: 10.1016/j.gene.2020.144675. [DOI] [PubMed] [Google Scholar]
- Hiasa M, Miyaji T, Haruna Y, Takeuchi T, Harada Y, Moriyama S, Moriyama Y. Identification of a mammalian vesicular polyamine transporter. Scientific Reports. 2014;4:6836. doi: 10.1038/srep06836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Abnormal Behavioral phenotypes of serotonin transporter knockout mice: Parallels with human anxiety and depression. Biological Psychiatry. 2003;54:953–959. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Horschitz S, Hummerich R, Lau T, Rietschel M, Schloss P. A dopamine transporter mutation associated with bipolar affective disorder causes inhibition of transporter cell surface expression. Molecular Psychiatry. 2005;10:1104–1109. doi: 10.1038/sj.mp.4001730. [DOI] [PubMed] [Google Scholar]
- Hu C, Tao L, Cao X, Chen L. The solute carrier transporters and the brain: Physiological and pharmacological implications. Asian Journal of Pharmaceutical Sciences. 2020;15:131–144. doi: 10.1016/j.ajps.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner CA, Stein V, Hermans-Borgmeyer I, Meyer T, Ballanyi K, Jentsch TJ. Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron. 2001;30:515–524. doi: 10.1016/s0896-6273(01)00297-5. [DOI] [PubMed] [Google Scholar]
- Ingram G, Barwick KE, Hartley L, McEntagart M, Crosby AH, Llewelyn G, Morris HR. Distal hereditary motor neuropathy with vocal cord paresis: From difficulty in choral singing to a molecular genetic diagnosis. Practical Neurology. 2016;16:247–251. doi: 10.1136/practneurol-2015-001307. [DOI] [PubMed] [Google Scholar]
- Iqbal Z, Willemsen MH, Papon MA, Musante L, Benevento M, Hu H, van Bokhoven H. Homozygous SLC6A17 mutations cause autosomal-recessive intellectual disability with progressive tremor, speech impairment, and behavioral problems. American Journal of Human Genetics. 2015;96:386–396. doi: 10.1016/j.ajhg.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irobi J, De Jonghe P, Timmerman V. Molecular genetics of distal hereditary motor neuropathies. Human Molecular Genetics. 2006;13:R195–R202. doi: 10.1093/hmg/ddh226. [DOI] [PubMed] [Google Scholar]
- Islam MP, Herman GE, de Los Reyes EC. Language regression in an atypical SLC6A1 mutation. Seminars in Pediatric Neurology. 2018;26:25–27. doi: 10.1016/j.spen.2018.04.001. [DOI] [PubMed] [Google Scholar]
- Ito T, Kimura Y, Uozumi Y, Takai M, Muraoka S, Matsuda T, Azuma J. Taurine depletion caused by knocking out the taurine transporter gene leads to cardiomyopathy with cardiac atrophy. Journal of Molecular and Cellular Cardiology. 2008;44:927–937. doi: 10.1016/j.yjmcc.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Iversen L. Neurotransmitter transporters: Fruitful targets for CNS drug discovery. Molecular Psychiatry. 2000;5:357–362. doi: 10.1038/sj.mp.4000728. [DOI] [PubMed] [Google Scholar]
- Iwama K, Iwata A, Shiina M, Mitsuhashi S, Miyatake S, Takata A, Matsumoto N. A novel mutation in SLC1A3 causes episodic ataxia. Journal of Human Genetics. 2018;63:207–211. doi: 10.1038/s10038-017-0365-z. [DOI] [PubMed] [Google Scholar]
- Jacobsen JC, Wilson C, Cunningham V, Glamuzina E, Prosser DO, Love DR, Lehnert K. Brain dopamine-serotonin vesicular transport disease presenting as a severe infantile hypotonic parkinsonian disorder. Journal of Inherited Metabolic Disease. 2016;39:305–308. doi: 10.1007/s10545-015-9897-6. [DOI] [PubMed] [Google Scholar]
- Jacobsson JA, Stephansson O, Fredriksson R. C6ORF192 forms a unique evolutionary branch among solute carriers (SLC16, SLC17, and SLC18) and is abundantly expressed in several brain regions. Journal of Molecular Neuroscience. 2010;41:230–242. doi: 10.1007/s12031-009-9222-7. [DOI] [PubMed] [Google Scholar]
- Jahn TR, Radford SE. The Yin and Yang of protein folding. The FEBS Journal. 2005;272:5962–5970. doi: 10.1111/j.1742-4658.2005.05021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen JC, Wan J, Palos TP, Howard BD, Baloh RW. Mutation in the glutamate transporter EAAT1 causes episodic ataxia, hemiplegia, and seizures. Neurology. 2005;65:529–534. doi: 10.1212/01.wnl.0000172638.58172.5a. [DOI] [PubMed] [Google Scholar]
- Jen JC, Wan J. Episodic ataxias. Handbook of Clinical Neurology. 2018;155:205–215. doi: 10.1016/B978-0-444-64189-2.00013-5. [DOI] [PubMed] [Google Scholar]
- Johannesen KM, Gardella E, Linnankivi T, Courage C, De Saint Martin A, Lehesjoki AE, Moller RS. Defining the phenotypic spectrum of SLC6A1 mutations. Epilepsia. 2018;59:389–402. doi: 10.1111/epi.13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Computer Applications in the Biosciences. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- Jonker JW, Wagenaar E, Mol CA, Buitelaar M, Koepsell H, Smit JW, Schinkel AH. Reduced hepatic uptake and intestinal excretion of organic cations in mice with a targeted disruption of the organic cation transporter 1 (Oct1 [Slc22a1]) gene. Molecular and Cellular Biology. 2001;21:5471–5477. doi: 10.1128/MCB.21.16.5471-5477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker JW, Wagenaar E, Van Eijl S, Schinkel AH. Deficiency in the organic cation transporters 1 and 2 (Oct1/Oct2 [Slc22a1/Slc22a2]) in mice abolishes renal secretion of organic cations. Molecular and Cellular Biology. 2003;23:7902–7908. doi: 10.1128/MCB.23.21.7902-7908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi Y, Miot A, Guillet M, Sendin G, Bourien J, Wang J, Nouvian R. VGLUT3-p.A211V variant fuses stereocilia bundle and elongates synaptic ribbons in the human deafness DFNA25. Preprint at BioRxiv. 2020 doi: 10.1113/JP282181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juge N, Omote H, Moriyama Y. Vesicular GABA transporter (VGAT) transports β-alanine. Journal of Neurochemistry. 2013;127:482–486. doi: 10.1111/jnc.12393. [DOI] [PubMed] [Google Scholar]
- Jutabha P, Anzai N, Kitamura K, Taniguchi A, Kaneko S, Yan K, Sakurai H. Human sodium phosphate transporter 4 (hNPT4/SLC17A3) as a common renal secretory pathway for drugs and urate. The Journal of Biological Chemistry. 2010;285:35123–35132. doi: 10.1074/jbc.M110.121301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, Fox MA, Gallagher PS, Murphy DL. Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes, Brain and Behaviour. 2007;6:389–400. doi: 10.1111/j.1601-183X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Clémençon B, Simonin A, Leuenberger M, Lochner M, Weisstanner M, Hediger MA. The SLC1 high-affinity glutamate and neutral amino acid transporter family. Molecular Aspects of Medicine. 2013;34:108–120. doi: 10.1016/j.mam.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Zubedat S, Radzishevsky I, Valenta AC, Rechnitz O, Sason H, Wolosker H. ASCT1 (Slc1a4) transporter is a physiologic regulator of brain D-serine and neurodevelopment. Proceedings of the National Academy of Sciences of the United States of America. 2018;115:9628–9633. doi: 10.1073/pnas.1722677115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson RM, Tanaka K, Saksida LM, Bussey TJ, Heilig M, Holmes A. Assessment of glutamate transporter GLAST (EAAT1)-deficient mice for phenotypes relevant to the negative and executive/cognitive symptoms of schizophrenia. Neuropsychopharmacology. 2009;34:1578–1589. doi: 10.1038/npp.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashevarova AA, Nazarenko LP, Skryabin NA, Salyukova OA, Chechetkina NN, Tolmacheva EN, Lebedev IN. Array CGH analysis of a cohort of Russian patients with intellectual disability. Gene. 2014;536:145–150. doi: 10.1016/j.gene.2013.11.029. [DOI] [PubMed] [Google Scholar]
- Kasture A, El-Kasaby A, Szollosi D, Asjad HMM, Grimm A, Stockner T, Sucic S. Functional rescue of a misfolded Drosophila melanogaster dopamine transporter mutant associated with a sleepless phenotype by pharmacological chaperones. The Journal of Biological Chemistry. 2016;291:20876–20890. doi: 10.1074/jbc.M116.737551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasture A, Stockner T, Freissmuth M, Sucic S. An unfolding story: Small molecules remedy misfolded monoamine transporters. The International Journal of Biochemistry & Cell Biology. 2017;92:1–5. doi: 10.1016/j.biocel.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasture AS, Bartel D, Steinkellner T, Sucic S, Hummel T, Freissmuth M. Distinct contribution of axonal and somatodendritic serotonin transporters in Drosophila olfaction. Neuropharmacology. 2019;161:107564. doi: 10.1016/j.neuropharm.2019.03.007. [DOI] [PubMed] [Google Scholar]
- Kilic F, Murphy DL, Rudnick G. A human serotonin transporter mutation causes constitutive activation of transport activity. Molecular Pharmacology. 2003;64:440–446. doi: 10.1124/mol.64.2.440. [DOI] [PubMed] [Google Scholar]
- Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. Molecular chaperone functions in protein folding and proteostasis. Annual Review of Biochemistry. 2013;82:323–355. doi: 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- Kiryk A, Aida T, Tanaka K, Banerjee P, Wilczynski GM, Meyza K, Danysz W. Behavioral characterization of GLT1 (+/-) mice as a model of mild glutamatergic hyperfunction. Neurotoxicity Research. 2008;13:19–30. doi: 10.1007/BF03033364. [DOI] [PubMed] [Google Scholar]
- Kitzenmaier A, Schaefer N, Kasaragod VB, Polster T, Hantschmann R, Schindelin H, Villmann C. The P429L loss of function mutation of the human glycine transporter 2 associated with hyperekplexia. The European Journal of Neuroscience. 2019;50:3906–3920. doi: 10.1111/ejn.14533. [DOI] [PubMed] [Google Scholar]
- Kleta R, Romeo E, Ristic Z, Ohura T, Stuart C, Arcos-Burgos M, Koizumi A. Mutations in SLC6A19, encoding B0AT1, cause Hartnup disorder. Nature Genetics. 2004;36:999–1002. doi: 10.1038/ng1405. [DOI] [PubMed] [Google Scholar]
- Koban F, El-Kasaby A, Hausler C, Stockner T, Simbrunner BM, Sitte HH, Sucic S. A salt bridge linking the first intracellular loop with the C terminus facilitates the folding of the serotonin transporter. The Journal of Biological Chemistry. 2015;290:13263–13278. doi: 10.1074/jbc.M115.641357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: Structure, function, physiological roles, and biopharmaceutical implications. Pharmaceutical Research. 2007;24:1227–1251. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- Koepsell H. The SLC22 family with transporters of organic cations, anions and zwitterions. Molecular Aspects of Medicine. 2013;34:413–435. doi: 10.1016/j.mam.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Koepsell H. Organic cation transporters in health and disease. Pharmacological Reviews. 2020;72:253–319. doi: 10.1124/pr.118.015578. [DOI] [PubMed] [Google Scholar]
- Kohli MA, Lucae S, Saemann PG, Schmidt MV, Demirkan A, Hek K, Binder EB. The neuronal transporter gene SLC6A15 confers risk to major depression. Neuron. 2011;70:252–265. doi: 10.1016/j.neuron.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommareddi PK, Nair TS, Thang LV, Galano MM, Babu E, Ganapathy V, Carey TE. Isoforms, expression, glycosylation, and tissue distribution of CTL2/ SLC44A2. The Protein Journal. 2010;29:417–426. doi: 10.1007/s10930-010-9268-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommareddi P, Nair T, Kakaraparthi BN, Galano MM, Miller D, Laczkovich I, Carey TE. Hair cell loss, spiral ganglion degeneration, and progressive sensorineural hearing loss in mice with targeted deletion of Slc44a2/Ctl2. Journal of the Association for Research in Otolaryngology. 2015;16:695–712. doi: 10.1007/s10162-015-0547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkhov VM, Milan-Lobo L, Zuber B, Farhan H, Schmid JA, Freissmuth M, Sitte HH. Peptide-based interactions with calnexin target misassembled membrane proteins into endoplasmic reticulum-derived multilamellar bodies. Journal of Molecular Biology. 2008;378:337–352. doi: 10.1016/j.jmb.2008.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk V, Samluk Ł, Juraszek B, Jurkiewicz-Trząska D, Sucic S, Freissmuth M, Nałęcz KA. Trafficking of the amino acid transporter B 0,+ (SLC6A14) to the plasma membrane involves an exclusive interaction with SEC24C for its exit from the endoplasmic reticulum. Biochimica at Biophysica Acta Molecular Cell Research. 2019;1866:252–263. doi: 10.1016/j.bbamcr.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovermann P, Hessel M, Kortzak D, Jen JC, Koch J, Fahlke C, Freilinger T. Impaired K+ binding to glial glutamate transporter EAAT1 in migraine. Scientific Reports. 2017;7:13913. doi: 10.1038/s41598-017-14176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtun O, Sakrikar D, Tomlinson ID, Chang JC, Arzeta-Ferrer X, Rosenthal SJ. Single-quantum-dot tracking reveals altered membrane dynamics of an attention-deficit/hyperactivity-disorder-derived dopamine transporter coding variant. ACS Chemical Neuroscience. 2015;6:526–534. doi: 10.1021/cn500202c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K, Kume S, Park SK, Hirsh J, Jackson FR. Dopamine is a regulator of arousal in the fruit Fly. The Journal of Neuroscience. 2005;25:7377–7384. doi: 10.1523/JNEUROSCI.2048-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian MA, Zhen J, Cheng SY, Li Y, Mordekar SR, Jardine P, Maher ER. Homozygous loss-of-function mutations in the gene encoding the dopamine transporter are associated with infantile parkinsonism-dystonia. Journal of Clinical Investigation. 2009;119:1595–1603. doi: 10.1172/JCI39060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian MA, Li Y, Zhen J, Meyer E, Hai N, Christen HJ, Maher ER. Clinical and molecular characterisation of hereditary dopamine transporter deficiency syndrome: An observational cohort and experimental study. The Lancet Neurology. 2011;10:54–62. doi: 10.1016/S1474-4422(10)70269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurolap A, Armbruster A, Hershkovitz T, Hauf K, Mory A, Paperna T, Baris HN. Loss of glycine transporter 1 causes a subtype of glycine encephalopathy with arthrogryposis and mildly elevated cerebrospinal fluid glycine. American Journal of Human Genetics. 2016;99:1172–1180. doi: 10.1016/j.ajhg.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuster A, Arnoux JB, Barth M, Lamireau D, Houcinat N, Goizet C, Individual contributors who contributed to this work Diagnostic approach to neurotransmitter monoamine disorders: Experience from clinical, biochemical, and genetic profiles. Journal of Inherited Metabolic Disease. 2018;41:129–139. doi: 10.1007/s10545-017-0079-6. [DOI] [PubMed] [Google Scholar]
- Larsson M, Sawada K, Morland C, Hiasa M, Ormel L, Moriyama Y, Gundersen V. Functional and anatomical identification of a vesicular transporter mediating neuronal ATP release. Cerebral Cortex. 2012;22:1203–1214. doi: 10.1093/cercor/bhr203. [DOI] [PubMed] [Google Scholar]
- Lawal HO, Krantz DE. SLC18: Vesicular neurotransmitter transporters for monoamines and acetylcholine. Molecular Aspects of Medicine. 2013;34:360–372. doi: 10.1016/j.mam.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar A, Walitza S, Jetter A, Gerlach M, Warnke A, Herpertz-Dahlmann B, Schömig E. Novel mutations of the extraneuronal monoamine transporter gene in children and adolescents with obsessive-compulsive disorder. The International Journal of Neuropsychopharmacology. 2008;11:35–48. doi: 10.1017/S1461145707007742. [DOI] [PubMed] [Google Scholar]
- Lin Z, Uhl GR. Human dopamine transporter gene variation: Effects of protein coding variants V55A and V382A on expression and uptake activities. The Pharmacogenomics Journal. 2003;3:159–168. doi: 10.1038/sj.tpj.6500169. [DOI] [PubMed] [Google Scholar]
- Lodder-Gadaczek J, Gieselmann V, Eckhardt M. Vesicular uptake of N-acetylaspartylglutamate is catalysed by Sialin (SLC17A5) The Biochemical Journal. 2013;454:31–38. doi: 10.1042/BJ20130300. [DOI] [PubMed] [Google Scholar]
- Lohoff FW, Dahl JP, Ferraro TN, Arnold SE, Gallinat J, Sander T, Berrettini WH. Variations in the vesicular monoamine transporter 1 gene (VMAT1/SLC18A1) are associated with bipolar I disorder. Neuropsychopharmacology. 2006;31:2739–2747. doi: 10.1038/sj.npp.1301196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohoff FW, Hodge R, Narasimhan S, Nall A, Ferraro TN, Mickey BJ, Doyle GA. Functional genetic variants in the vesicular monoamine transporter 1 modulate emotion processing. Molecular Psychiatry. 2014;19:129–139. doi: 10.1038/mp.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo TW, Clarke DM. Chemical and pharmacological chaperones as new therapeutic agents. Expert Reviews in Molecular Medicine. 2007;9:1–18. doi: 10.1017/S1462399407000361. [DOI] [PubMed] [Google Scholar]
- López-Corcuera B, Arribas-González E, Aragón C. Hyperekplexia-associated mutations in the neuronal glycine transporter 2. Neurochemistry International. 2019;123:95–100. doi: 10.1016/j.neuint.2018.05.014. [DOI] [PubMed] [Google Scholar]
- Ma Z, Xia W, Liu F, Ma J, Sun S, Zhang J, Ma D. SLC44A4 mutation causes autosomal dominant hereditary postlingual non-syndromic mid-frequency hearing loss. Human Molecular Genetics. 2017;26:383–394. doi: 10.1093/hmg/ddw394. [DOI] [PubMed] [Google Scholar]
- Marinko JT, Huang H, Penn WD, Capra JA, Schlebach JP, Sanders CR. Folding and misfolding of human membrane proteins in health and disease: From single molecules to cellular proteostasis. Chemical Reviews. 2019;119:5537–5606. doi: 10.1021/acs.chemrev.8b00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri A, Chung SK, Rees MI. Hyperekplexia: Report on phenotype and genotype of 16 Jordanian patients. Brain & Development. 2017;39:306–311. doi: 10.1016/j.braindev.2016.10.010. [DOI] [PubMed] [Google Scholar]
- Masson J, Sagné C, Hamon M, El Mestikawy S. Neurotransmitter transporters in the central nervous system. Pharmacological Reviews. 1999;51:439–464. [PubMed] [Google Scholar]
- Masuda T, Ozono Y, Mikuriya S, Kohro Y, Tozaki-Saitoh H, Iwatsuki K, Inoue K. Dorsal horn neurons release extracellular ATP in a VNUT-dependent manner that underlies neuropathic pain. Nature Communications. 2016;7:12529. doi: 10.1038/ncomms12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo A, Bellier JP, Nishimura M, Yasuhara O, Saito N, Kimura H. Nuclear choline acetyltransferase activates transcription of a high-affinity choline transporter. The Journal of Biological Chemistry. 2011;286:5836–5845. doi: 10.1074/jbc.M110.147611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison KA, Butler KM, Inglis GAS, Dayan O, Boussidan H, Bhambhani V, Escayg A. SLC6A1 variants identified in epilepsy patients reduce gammaaminobutyric acid transport. Epilepsia. 2018;59:e135–e141. doi: 10.1111/epi.14531. [DOI] [PubMed] [Google Scholar]
- Mayer FP, Schmid D, Owens WA, Gould GG, Apuschkin M, Kudlacek O, Sitte HH. An unsuspected role for organic cation transporter 3 in the actions of amphetamine. Neuropsychopharmacology. 2018;43:2408–2417. doi: 10.1038/s41386-018-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazei-Robison MS, Couch RS, Shelton RC, Stein MA, Blakely RD. Sequence variation in the human dopamine transporter gene in children with attention deficit hyperactivity disorder. Neuropharmacology. 2005;49:724–736. doi: 10.1016/j.neuropharm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Mazei-Robison MS, Bowton E, Holy M, Schmudermaier M, Freissmuth M, Sitte HH, Blakely RD. Anomalous dopamine release associated with a human dopamine transporter coding variant. The Journal of Neuroscience. 2005;28:7040–7046. doi: 10.1523/JNEUROSCI.0473-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEntagart M, Norton N, Williams H, Teare MD, Dunstan M, Baker P, Rahman N. Localization of the gene for distal hereditary motor neuronopathy VII (dHMN-VII) to chromosome 2q14. American Journal of Human Genetics. 2001;68:1270–1276. doi: 10.1086/320122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature. 1997;389:870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- McKeown KA, Moreno R, Hall VL, Ribera AB, Downes GB. Disruption of Eaat2b, a glutamate transporter, results in abnormal motor behaviors in developing zebrafish. Developmental Biology. 2012;362:162–171. doi: 10.1016/j.ydbio.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMacken G, Whittaker RG, Evangelista T, Abicht A, Dusl M, Lochmüller H. Congenital myasthenic syndrome with episodic apnoea: Clinical, neurophysiological and genetic features in the long-term follow-up of 19 patients. Journal of Neurology. 2018;265:194–203. doi: 10.1007/s00415-017-8689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTague A, Howell KB, Cross JH, Kurian MA, Scheffer IE. The genetic landscape of the epileptic encephalopathies of infancy and childhood. The Lancet Neurology. 2016;15:304–316. doi: 10.1016/S1474-4422(15)00250-1. [DOI] [PubMed] [Google Scholar]
- Meldrum BS. Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. The Journal of Nutrition. 2000;130:1007S–15S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- Mergy MA, Gowrishankar R, Gresch PJ, Gantz SC, Williams J, Davis GL, Blakely RD. The rare DAT coding variant Val559 perturbs DA neuron function, changes behavior, and alters in vivo responses to psychostimulants. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E4779–E4788. doi: 10.1073/pnas.1417294111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Münch C, Völkel H, Booms P, Ludolph CA. The EAAT2 (GLT-1) gene in motor neuron disease: Absence of mutations in amyotrophic lateral sclerosis and a point mutation in patients with hereditary spastic paraplegia. Journal of Neurology, Neurosurgery, and Psychiatry. 1998;65:594–596. doi: 10.1136/jnnp.65.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel V, Bakovic M. The ubiquitous choline transporter SLC44A1. Central Nervous System Agents in Medicinal Chemistry. 2012;12:70–81. doi: 10.2174/187152412800792733. [DOI] [PubMed] [Google Scholar]
- Mineyko A, Whiting S, Graham GE. Hyperekplexia: Treatment of a severe phenotype and review of the literature. The Canadian Journal of Neurological Sciences. 2011;38:411–416. doi: 10.1017/s0317167100011793. [DOI] [PubMed] [Google Scholar]
- Miras-Portugal MT, Menéndez-Méndez A, Gómez-Villafuertes R, Ortega F, Delicado EG, Pérez-Sen R, Gualix J. Physiopathological role of the vesicular nucleotide transporter (VNUT) in the central nervous system: Relevance of the vesicular nucleotide release as a potential therapeutic target. Frontiers in Cellular Neuroscience. 2019;13:224. doi: 10.3389/fncel.2019.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaji T, Echigo N, Hiasa M, Senoh S, Omote H, Moriyama Y. Identification of a vesicular aspartate transporter. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11720–11724. doi: 10.1073/pnas.0804015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaji T, Omote H, Moriyama Y. Functional characterization of vesicular excitatory amino acid transport by human Sialin. Journal of Neurochemistry. 2011;119:1–5. doi: 10.1111/j.1471-4159.2011.07388.x. [DOI] [PubMed] [Google Scholar]
- Mochel F, Engelke UF, Barritault J, Yang B, McNeill NH, Thompson JN, Schiffmann R. Elevated CSF N-acetylaspartylglutamate in patients with free sialic acid storage diseases. Neurology. 2010;74:302–305. doi: 10.1212/WNL.0b013e3181cbcdc4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moechars D, Weston MC, Leo S, Callaerts-Vegh Z, Goris I, Daneels G, Hampson RM. Vesicular glutamate transporter VGLUT2 expression levels control quantal size and neuropathic pain. The Journal of Neuroscience. 2006;26:12055–12066. doi: 10.1523/JNEUROSCI.2556-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari M, Galli C, Vanoni O, Arnold SM, Kaufman RJ. Persistent glycoprotein misfolding activates the glucosidase II/UGT1-driven calnexin cycle to delay aggregation and loss of folding competence. Molecullar Cell. 2005;20:503–512. doi: 10.1016/j.molcel.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Mongeon R, Gleason MR, Masino MA, Fetcho JR, Mandel G, Brehm P, Dallman JE. Synaptic homeostasis in a zebrafish glial glycine transporter mutant. Journal of Neurophysiology. 2008;100:1716–1723. doi: 10.1152/jn.90596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery TR, Steinkellner T, Sucic S, Koban F, Schuchner S, Ogris E, Freissmuth M. Axonal targeting of the serotonin transporter in cultured rat dorsal raphe neurons is specified by SEC24C-dependent export from the endoplasmic reticulum. The Journal of Neuroscience. 2014;34:6344–6351. doi: 10.1523/JNEUROSCI.2991-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooslehner KA, Chan PM, Xu W, Liu L, Smadja C, Humby T, Emson PC. Mice with very low expression of the vesicular monoamine transporter 2 gene survive into adulthood: Potential mouse model for parkinsonism. Molecular and Cellular Biology. 2001;21:5321–5331. doi: 10.1128/MCB.21.16.5321-5331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin P, Sagné C, Gasnier B. Functional characterization of wild type and mutant human sialin. The EMBO Journal. 2004;23:4560–4570. doi: 10.1038/sj.emboj.7600464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama Y, Hatano R, Moriyama S, Uehara S. Vesicular polyamine transporter as a novel player in amine-mediated chemical transmission. Biochimica et Biophysica Acta, Biomembranes. 2020 doi: 10.1016/j.bbamem.2020.183208. [DOI] [PubMed] [Google Scholar]
- Moya PR, Wendland JR, Rubenstein LM, Timpano KR, Heiman GA, Tischfield JA, Murphy DL. Common and rare alleles of the serotonin transporter gene, SLC6A4, associated with Tourette’s disorder. Movement Disorders. 2013;28:1263–1270. doi: 10.1002/mds.25460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multani PK, Hodge R, Estévez MA, Abel T, Kung H, Alter M, Lohoff FW. VMAT1 deletion causes neuronal loss in the hippocampus and neurocognitive deficits in spatial discrimination. Neuroscience. 2013;232:32–44. doi: 10.1016/j.neuroscience.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles-Worsley M, Tiobech J, Browning SR, Korn J, Goodman S, Gentile K, Middleton FA. Deletion at the SLC1A1 glutamate transporter gene cosegregates with schizophrenia and bipolar schizoaffective disorder in a 5-generation family. American Journal of Medical Genetics, Part B Neuropsychiatric Genetics. 2013;162B:87–95. doi: 10.1002/ajmg.b.32125. [DOI] [PubMed] [Google Scholar]
- Nabokina SM, Inoue K, Subramanian VS, Valle JE, Yuasa H, Said HM. Molecular identification and functional characterization of the human colonic thiamine pyrophosphate transporter. The Journal of Biological Chemistry. 2014;289:4405–4416. doi: 10.1074/jbc.M113.528257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair TS, Kozma KE, Hoefling NL, Kommareddi PK, Ueda Y, Gong TW, Carey TE. Identification and characterization of choline transporter-like protein 2, an inner ear glycoprotein of 68 and 72 kDa that is the target of antibody-induced hearing loss. The Journal of Neuroscience. 2004;24:1772–1779. doi: 10.1523/JNEUROSCI.5063-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair TS, Kommareddi PK, Galano MM, Miller DM, Kakaraparthi BN, Telian SA, Carey TE. SLC44A2 single nucleotide polymorphisms, isoforms, and expression: Association with severity of Meniere’s disease? Genomics. 2016;108:201–208. doi: 10.1016/j.ygeno.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Fujiwara R, Ishiguro N, Oyabu M, Nakanishi T, Shirasaka Y, Tamai I. Involvement of choline transporter-like proteins, CTL1 and CTL2, in glucocorticoid-induced acceleration of phosphatidylcholine synthesis via increased choline uptake. Biological and Pharmaceutical Bulletin. 2010;33:691–696. doi: 10.1248/bpb.33.691. [DOI] [PubMed] [Google Scholar]
- Nash SR, Giros B, Kingsmore SF, Rochelle JM, Suter ST, Gregor P, Caron MG. Cloning, pharmacological characterization, and genomic localization of the human creatine transporter. Receptors & Channels. 1994;2:165–174. [PubMed] [Google Scholar]
- Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: Are we there yet? Clinical Cancer Research. 2012;18:64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N. The family of Na+/Cl− neurotransmitter transporters. Journal of Neurochemistry. 1998;71:1785–1803. doi: 10.1046/j.1471-4159.1998.71051785.x. [DOI] [PubMed] [Google Scholar]
- Ng J, Zhen J, Meyer E, Erreger K, Li Y, Kakar N, Kurian MA. Dopamine transporter deficiency syndrome: Phenotypic spectrum from infancy to adulthood. Brain. 2014;137:1107–1119. doi: 10.1093/brain/awu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni B, Rosteck PR, Jr, Nadi NS, Paul SM. Cloning and expression of a cDNA encoding a brain-specific Na(+)-dependent inorganic phosphate cotransporter. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:5607–5611. doi: 10.1073/pnas.91.12.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niello M, Gradisch R, Loland CJ, Stockner T, Sitte HH. Allosteric modulation of neurotransmitter transporters as a therapeutic strategy. Trends in Pharmacological Sciences. 2020;41:446–463. doi: 10.1016/j.tips.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan SM, Sullivan CR, McCullumsmith RE. The role of glutamate transporters in the pathophysiology of neuropsychiatric disorders. NPJ Schizophrenia. 2017;3:32. doi: 10.1038/s41537-017-0037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Grady GL, Verschuuren C, Yuen M, Webster R, Menezes M, Fock JM, Cooper ST. Variants in SLC18A3, vesicular acetylcholine transporter, cause congenital myasthenic syndrome. Neurology. 2016;87:1442–1448. doi: 10.1212/WNL.0000000000003179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obholzer N, Wolfson S, Trapani JG, Mo W, Nechiporuk A, Busch-Nentwich E, Nicolson T. Vesicular glutamate transporter 3 is required for synaptic transmission in zebrafish hair cells. The Journal of Neuroscience. 2008;28:2110–2118. doi: 10.1523/JNEUROSCI.5230-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden KK, Traynelis SF. New advances in NMDA receptor pharmacology. Trends in Pharmacoloical Sciences. 2011;32:726–733. doi: 10.1016/j.tips.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T, Okamura M, Kaitsuka C, Haga T, Gurwitz D. Single nucleotide polymorphism of the human high affinity choline transporter alters transport rate. The Journal of Biological Chemistry. 2002;277:45315–45322. doi: 10.1074/jbc.M207742200. [DOI] [PubMed] [Google Scholar]
- Okuda T, Haga T. High-affinity choline transporter. Neurochemical Research. 2003;28:483–488. doi: 10.1023/a:1022809003997. [DOI] [PubMed] [Google Scholar]
- Okuda T, Osawa C, Yamada H, Hayashi K, Nishikawa S, Ushio T, Haga T. Transmembrane topology and oligomeric structure of the high-affinity choline transporter. The Journal of Biological Chemistry. 2012;287:42826–42834. doi: 10.1074/jbc.M112.405027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki N, Goldman D, Kaye WH, Plotnicov K, Greenberg BD, Lappalainen J, Murphy DL. Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Molecular Psychiatry. 2003;8:933–936. doi: 10.1038/sj.mp.4001365. [DOI] [PubMed] [Google Scholar]
- Padmakumar M, Jaeken J, Ramaekers V, Lagae L, Greene D, Thys C, Freson K. A novel missense variant in SLC18A2 causes recessive brain monoamine vesicular transport disease and absent serotonin in platelets. JIMD Reports. 2019;47:9–16. doi: 10.1002/jmd2.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S, Towne MC, Pearl PL, Pelletier RC, Genetti CA, Shi J, Brownstein CA. SLC6A1 mutation and ketogenic diet in epilepsy with myoclonic-atonic seizures. Pediatric Neurology. 2016;64:77–79. doi: 10.1016/j.pediatrneurol.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardal-Fernández JM, Carrascosa-Romero MC, Álvarez S, Medina-Monzón MC, Caamaño MB, de Cabo C. A new severe mutation in the SLC5A7 gene related to congenital myasthenic syndrome type 20. Neuromuscular Disorders. 2018;28:881–884. doi: 10.1016/j.nmd.2018.06.020. [DOI] [PubMed] [Google Scholar]
- Parinejad N, Peco E, Ferreira T, Stacey SM, van Meyel DJ. Disruption of an EAAT-mediated chloride channel in a Drosophila model of ataxia. The Journal of Neuroscience. 2016;36:7640–7647. doi: 10.1523/JNEUROSCI.0197-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra LA, Baust T, El Mestikawy S, Quiroz M, Hoffman B, Haflett JM, Torres GE. The orphan transporter Rxt1/NTT4 (SLC6A17) functions as a synaptic vesicle amino acid transporter selective for proline, glycine, leucine, and alanine. Molecular Pharmacology. 2008;74:1521–1523. doi: 10.1124/mol.108.050005. [DOI] [PubMed] [Google Scholar]
- Patel A, Rochelle JM, Jones JM, Sumegi J, Uhl GR, Seldin MF, Gregor P. Mapping of the taurine transporter gene to mouse chromosome 6 and to the short arm of human chromosome 3. Genomics. 1995;25:314–317. doi: 10.1016/0888-7543(95)80146-d. [DOI] [PubMed] [Google Scholar]
- Patel N, Khan AO, Alsahli S, Abdel-Salam G, Nowilaty SR, Mansour AM, Alkuraya FS. Genetic investigation of 93 families with microphthalmia or posterior microphthalmos. Clinical Genetics. 2018;93:1210–1222. doi: 10.1111/cge.13239. [DOI] [PubMed] [Google Scholar]
- Peghini P, Janzen J, Stoffel W. Glutamate transporter EAAC-1-deficient mice develop dicarboxylic aminoaciduria and behavioral abnormalities but no neurodegeneration. The EMBO Journal. 1997;16:3822–3832. doi: 10.1093/emboj/16.13.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins EM, Clarkson YL, Suminaite D, Lyndon AR, Tanaka K, Rothstein JD, Jackson M. Loss of cerebellar glutamate transporters EAAT4 and GLAST differentially affects the spontaneous firing pattern and survival of Purkinje cells. Human Molecular Genetics. 2018;27:2614–2627. doi: 10.1093/hmg/ddy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter DH. Chemical chaperones: a pharmacological strategy for disorders of protein folding and trafficking. Pediatric Research. 2002;52:832–836. doi: 10.1203/00006450-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Pironti E, Salpietro V, Cucinotta F, Granata F, Mormina E, Efthymiou S, Di Rosa G. A novel SLC1A4 homozygous mutation causing congenital microcephaly, epileptic encephalopathy and spastic tetraparesis: A video-EEG and tractography – Case study. Journal of Neurogenetics. 2018;32:316–321. doi: 10.1080/01677063.2018.1476510. [DOI] [PubMed] [Google Scholar]
- Posar A, Visconti P. Mild phenotype associated with SLC6A1 gene mutation: A case report with literature review. Journal of Pediatric Neurosciences. 2019;14:100–102. doi: 10.4103/jpn.JPN_2_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado VF, Martins-Silva C, de Castro BM, Lima RF, Barros DM, Amaral E, Prado MA. Mice deficient for the vesicular acetylcholine transporter are myasthenic and have deficits in object and social recognition. Neuron. 2006;51:601–612. doi: 10.1016/j.neuron.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Prasad HC, Zhu CB, McCauley JL, Samuvel DJ, Ramamoorthy S, Shelton RC, Blakely RD. Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11545–11550. doi: 10.1073/pnas.0501432102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad HC, Steiner JA, Sutcliffe JS, Blakely RD. Enhanced activity of human serotonin transporter variants associated with autism. Philosophical Transactions of the Royal Society of London. 2009;364:163–173. doi: 10.1098/rstb.2008.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preising MN, Görg B, Friedburg C, Qvartskhava N, Budde BS, Bonus M, Bolz HJ. Biallelic mutation of human SLC6A6 encoding the taurine transporter TAUT is linked to early retinal degeneration. FASEB Journal. 2019;33:11507–11527. doi: 10.1096/fj.201900914RR. [DOI] [PubMed] [Google Scholar]
- Prolo LM, Vogel H, Reimer RJ. The lysosomal sialic acid transporter sialin is required for normal CNS myelination. The Journal of Neuroscience. 2009;29:15355–15365. doi: 10.1523/JNEUROSCI.3005-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puffenberger EG, Jinks RN, Sougnez C, Cibulskis K, Willert RA, Achilly NP, Strauss KA. Genetic mapping and exome sequencing identify variants associated with five novel diseases. PLoS One. 2012;7:e28936. doi: 10.1371/journal.pone.0028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyle A, Smertenko T, Bargiela D, Griffin H, Duff J, Appleton M, Chinnery PF. Exome sequencing in undiagnosed inherited and sporadic ataxias. Brain. 2015;138(Pt 2):276–283. doi: 10.1093/brain/awu348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Liu X, Sun Q, Fan Z, Xia D, Ding G, Wang S. Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:13434–13439. doi: 10.1073/pnas.1116633109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan MA, Krout D, Katamish RM, Robson MJ, Nettesheim C, Gresch PJ, Blakely RD. Human serotonin transporter coding variation establishes conformational bias with functional consequences. ACS Chemical Neuroscience. 2019;10:3249–3260. doi: 10.1021/acschemneuro.8b00689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan MA, Robson MJ, Ye R, Rose KL, Schey KL, Blakely RD. Ex vivo quantitative proteomic analysis of serotonin transporter Interactome: Network impact of the SERT Ala56 coding variant. Frontiers in Molecular Neuroscience. 2020;13:89. doi: 10.3389/fnmol.2020.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae CD, Bröer S. Creatine as a booster for human brain function. How might it work? Neurochemistry International. 2015;89:249–259. doi: 10.1016/j.neuint.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Rapoport TA. Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature. 2007;450:663–669. doi: 10.1038/nature06384. [DOI] [PubMed] [Google Scholar]
- Rask-Andersen M, Masuram S, Fredriksson R, Schiöth HB. Solute carriers as drug targets: Current use, clinical trials and prospective. Molecular Aspects of Medicine. 2013;34:702–710. doi: 10.1016/j.mam.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Leibach FH, Mahesh VB, Han H, Yang-Feng T, Blakely RD, Ganapathy V. Functional characterization and chromosomal localization of a cloned taurine transporter from human placenta. The Biochemical Journal. 1994;300:893–900. doi: 10.1042/bj3000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramet L, Zimmermann J, Bersot T, Poirel O, De Gois S, Silm K, Mestikawy S. Characterization of a human point mutation of VGLUT3 (p.A211V) in the rodent brain suggests a nonuniform distribution of the transporter in synaptic vesicles. The Journal of Neuroscience. 2017;37:4181–4199. doi: 10.1523/JNEUROSCI.0282-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath M, Korenke GC, Najm J, Hoffmann GF, Hagendorff A, Strom TM, Felbor U. Exome sequencing results in identification and treatment of brain dopamineserotonin vesicular transport disease. Journal of the Neurological Sciences. 2017;379:296–297. doi: 10.1016/j.jns.2017.06.034. [DOI] [PubMed] [Google Scholar]
- Rauch A, Wieczorek D, Graf E, Wieland T, Endele S, Schwarzmayr T, Strom TM. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: An exome sequencing study. Lancet. 2012;380:1674–1682. doi: 10.1016/S0140-6736(12)61480-9. [DOI] [PubMed] [Google Scholar]
- Rees MI, Harvey K, Pearce BR, Chung SK, Duguid IC, Thomas P, Harvey RJ. Mutations in the gene encoding GlyT2 (SLC6A5) define a presynaptic component of human startle disease. Nature Genetics. 2006;38:801–806. doi: 10.1038/ng1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees E, Walters JTR, Chambert KD, O’Dushlaine C, Szatkiewicz J, Richards AL, Kirov G. CNV analysis in a large schizophrenia sample implicates deletions at 16p12.1 and SLC1A1 and duplications at 1p36.33 and CGNL1. Human Molecular Genetics. 2014;23:1669–1676. doi: 10.1093/hmg/ddt540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees E, Han J, Morgan J, Carrera N, Escott-Price V, Pocklington AJ, Owen MJ. De novo mutations identified by exome sequencing implicate rare missense variants in SLC6A1 in schizophrenia. Nature Neuroscience. 2020;23:179–184. doi: 10.1038/s41593-019-0565-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer RJ. SLC17: A functionally diverse family of organic anion transporters. Molecular Aspects of Medicine. 2013;34:350–359. doi: 10.1016/j.mam.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiterer V, Maier S, Sitte HH, Kriz A, Ruegg MA, Hauri HP, Farhan H. Sec24- and ARFGAP1-dependent trafficking of GABA transporter-1 is a prerequisite for correct axonal targeting. The Journal of Neuroscience. 2008;28:12453–12464. doi: 10.1523/JNEUROSCI.3451-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter MS, Tawamie H, Buchert R, Hosny Gebril O, Froukh T, Thiel C, Abou Jamra R. Diagnostic yield and novel candidate genes by exome sequencing in 152 consanguineous families with neurodevelopmental disorders. JAMA Psychiatry. 2017;74:293–299. doi: 10.1001/jamapsychiatry.2016.3798. [DOI] [PubMed] [Google Scholar]
- Ribeiro FM, Black SA, Cregan SP, Prado VF, Prado MA, Rylett RJ, Ferguson SS. Constitutive high-affinity choline transporter endocytosis is determined by a carboxyl-terminal tail dileucine motif. Journal of Neurochemistry. 2005;94:86–96. doi: 10.1111/j.1471-4159.2005.03171.x. [DOI] [PubMed] [Google Scholar]
- Ribeiro FM, Black SA, Prado VF, Rylett RJ, Ferguson SS, Prado MA. The “ins” and “outs” of the high-affinity choline transporter CHT1. Journal of Neurochemistry. 2006;97:1–12. doi: 10.1111/j.1471-4159.2006.03695.x. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Kaila K. The K+/Cl-co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Rilstone JJ, Alkhater RA, Minassian BA. Brain dopamine-serotonin vesicular transport disease and its treatment. New England Journal of Medicine. 2013;368:543–550. doi: 10.1056/NEJMoa1207281. [DOI] [PubMed] [Google Scholar]
- Ringe D, Petsko GA. What are pharmacological chaperones and why are they interesting? Journal of Biology. 2009;8:80. doi: 10.1186/jbiol186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robak LA, Jansen IE, van Rooij J, Uitterlinden AG, Kraaij R, Jankovic J, Shulman JM. Excessive burden of lysosomal storage disorder gene variants in Parkinson’s disease. Brain. 2017;140:3191–3203. doi: 10.1093/brain/awx285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez Cruz PM, Palace J, Beeson D. The neuromuscular junction and wide heterogeneity of congenital myasthenic syndromes. International Journal of Molecular Sciences. 2018;19:1677. doi: 10.3390/ijms19061677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A, Kanner BI. The substrates of the gamma-aminobutyric acid transporter GAT-1 induce structural rearrangements around the interface of transmembrane domains 1 and 6. The Journal of Biological Chemistry. 2008;283:14376–14383. doi: 10.1074/jbc.M801093200. [DOI] [PubMed] [Google Scholar]
- Rubenstein RC, Zeitlin PL. Sodium 4-phenylbutyrate downregulates Hsc70: Implications for intracellular trafficking of ΔF508-CFTR. American Journal of Physiology-Cell Physiology. 2000;278:C259–C267. doi: 10.1152/ajpcell.2000.278.2.C259. [DOI] [PubMed] [Google Scholar]
- Ruel J, Emery S, Nouvian R, Bersot T, Amilhon B, Van Rybroek JM, Puel JL. Impairment of SLC17A8 encoding vesicular glutamate transporter-3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice. American Journal of Human Genetics. 2008;83:278–292. doi: 10.1016/j.ajhg.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu N, Sagong B, Park HJ, Kim MA, Lee KY, Kim UK. Screening of the SLC17A8 gene as a causative factor for autosomal dominant non-syndromic hearing loss in Koreans. BMC Medical Genetics. 2016;17:6. doi: 10.1186/s12881-016-0269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu N, Lee S, Park HJ, Lee B, Kwon TJ, Bok J, Kim UK. Identification of a novel splicing mutation within SLC17A8 in a Korean family with hearing loss by whole-exome sequencing. Gene. 2017;627:233–238. doi: 10.1016/j.gene.2017.06.040. [DOI] [PubMed] [Google Scholar]
- Sagné C, El Mestikawy S, Isambert MF, Hamon M, Henry JP, Giros B, Gasnier B. Cloning of a functional vesicular GABA and glycine transporter by screening of genome databases. FEBS Letters. 1997;417:177–183. doi: 10.1016/s0014-5793(97)01279-9. [DOI] [PubMed] [Google Scholar]
- Sagné C, Gasnier B. Molecular physiology and pathophysiology of lysosomal membrane transporters. Journal of Inherited Metabolic Disease. 2008;31:258–266. doi: 10.1007/s10545-008-0879-9. [DOI] [PubMed] [Google Scholar]
- Saito K, Kakizaki T, Hayashi R, Nishimaru H, Furukawa T, Nakazato Y, Yanagawa Y. The physiological roles of vesicular GABA transporter during embryonic development: A study using knockout mice. Molecular Brain. 2010;3:40. doi: 10.1186/1756-6606-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakae DY, Marti F, Lecca S, Vorspan F, Martín-García E, Morel LJ, El Mestikawy S. The absence of VGLUT3 predisposes to cocaine abuse by increasing dopamine and glutamate signaling in the nucleus accumbens. Molecular Psychiatry. 2015;20:1448–1459. doi: 10.1038/mp.2015.104. [DOI] [PubMed] [Google Scholar]
- Sakamoto S, Miyaji T, Hiasa M, Ichikawa R, Uematsu A, Iwatsuki K, Moriyama Y. Impairment of vesicular ATP release affects glucose metabolism and increases insulin sensitivity. Scientific Reports. 2014;4:6689. doi: 10.1038/srep06689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakrikar D, Mazei-Robison MS, Mergy MA, Richtand NW, Han Q, Hamilton PJ, Blakely RD. Attention deficit/hyperactivity disorder-derived coding variation in the dopamine transporter disrupts microdomain targeting and trafficking regulation. The Journal of Neuroscience. 2012;32:5385–5397. doi: 10.1523/JNEUROSCI.6033-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar MD, Zelt NB, Saldivar R, Kuntz CP, Chen S, Penn WD, Schlebach JP. Classification of the molecular defects associated with pathogenic variants of the SLC6A8 creatine transporter. Biochemistry. 2020;59:1367–1377. doi: 10.1021/acs.biochem.9b00956. [DOI] [PubMed] [Google Scholar]
- Salomons GS, Van Dooren SJ, Verhoeven NM, Cecil KM, Ball WS, Degrauw TJ, Jakobs C. X-linked creatine-transporter gene (SLC6A8) defect: A new creatine-deficiency syndrome. American Journal of Human Genetics. 2001;68:1497–1500. doi: 10.1086/320595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter CG, Beijer D, Hardy H, Barwick KES, Bower M, Mademan I, Crosby AH. Truncating SLC5A7 mutations underlie a spectrum of dominant hereditary motor neuropathies. Neurology Genetics. 2018;4:e222. doi: 10.1212/NXG.0000000000000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Parikh V. Choline transporters, cholinergic transmission and cognition. Nature Reviews Neuroscience. 2005;6:48–56. doi: 10.1038/nrn1588. [DOI] [PubMed] [Google Scholar]
- Sathe MN, Woo K, Kresge C, Bugde A, Luby-Phelps K, Lewis MA, Feranchak AP. Regulation of purinergic signaling in biliary epithelial cells by exocytosis of SLC17A9-dependent ATP-enriched vesicles. The Journal of Biological Chemistry. 2011;286:25363–25376. doi: 10.1074/jbc.M111.232868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterstrom FK, Kosmicki JA, Wang J, Breen MS, De Rubeis S, Joon-Yong An JY, Daly MJ. Novel genes for autism implicate both excitatory and inhibitory cell lineages in risk. Preprint at bioRxiv. 2018 [Google Scholar]
- Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Moriyama Y. Identification of a vesicular nucleotide transporter. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalise M, Pochini L, Console L, Losso MA, Indiveri C. The human SLC1A5 (ASCT2) amino acid transporter: From function to structure and role in cell biology. Frontiers in Cell and Development Biology. 2018;6:96. doi: 10.3389/fcell.2018.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer MK, Varoqui H, Defamie N, Weihe E, Erickson JD. Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. The Journal of Biological Chemistry. 2002;277:50734–50748. doi: 10.1074/jbc.M206738200. [DOI] [PubMed] [Google Scholar]
- Schauer R. Sialic acids as regulators ofmolecular and cellular interactions. Current Opinion in Structural Biology. 2008;19:507–514. doi: 10.1016/j.sbi.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildkraut JJ, Mooney JJ. Toward a rapidly acting antidepressant: The normetanephrine and extraneuronal monoamine transporter (uptake 2) hypothesis. The American Journal of Psychiatry. 2004;161:909–911. doi: 10.1176/appi.ajp.161.5.909. [DOI] [PubMed] [Google Scholar]
- Schmid JA, Scholze P, Kudlacek O, Freissmuth M, Singer EA, Sitte HH. Oligomerization of the human serotonin transporter and of the rat GABA transporter I visualized by fluorescence resonance energy transfer microscopy in living cells. The Journal of Biological Chemistry. 2001;276:3805–3810. doi: 10.1074/jbc.M007357200. [DOI] [PubMed] [Google Scholar]
- Scholze P, Freissmuth M, Sitte HH. Mutations within an intramembrane leucine heptad repeat disrupt oligomer formation of the rat GABA transporter 1. The Journal of Biological Chemistry. 2002;277:43682–43690. doi: 10.1074/jbc.M205602200. [DOI] [PubMed] [Google Scholar]
- Schuller-Levis GB, Park E. Taurine: New implications for an old amino acid. FEMS Microbiology Letters. 2003;226:195–202. doi: 10.1016/S0378-1097(03)00611-6. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Sternberg D, Whalen S, Afenjar A, Isapof A, Chabrol B, Siffroi JP. How chromosomal deletions can unmask recessive mutations? Deletions in 10q11.2 associated with CHAT or SLC18A3 mutations lead to congenital myasthenic syndrome. American Journal of Medical Genetics. 2018;176:151–155. doi: 10.1002/ajmg.a.38515. [DOI] [PubMed] [Google Scholar]
- Scimemi A. Structure, function, and plasticity of GABA transporters. Frontiers in Cellular Neuroscience. 2014;8:161. doi: 10.3389/fncel.2014.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J, Edwards RH. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron. 2008;57:263–275. doi: 10.1016/j.neuron.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462:651–655. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seow HF, Broer S, Broer A, Bailey CG, Potter SJ, Cavanaugh JA, Rasko JE. Hartnup disorder is caused by mutations in the gene encoding the neutral amino acid transporter SLC6A19. Nature Genetics. 2004;36:1003–1007. doi: 10.1038/ng1406. [DOI] [PubMed] [Google Scholar]
- Shafqat S, Velaz-Faircloth M, Henzi VA, Whitney KD, Yang-Feng TL, Seldin MF, Fremeau RT., Jr Human brain-specific L-proline transporter: Molecular cloning, functional expression, and chromosomal localization of the gene in human and mouse genomes. Molecular Pharmacology. 1995;48:219–229. [PubMed] [Google Scholar]
- Shakeel M, Irfan M, Khan IA. Rare genetic mutations in Pakistani patients with dilated cardiomyopathy. Gene. 2018;673:134–139. doi: 10.1016/j.gene.2018.06.019. [DOI] [PubMed] [Google Scholar]
- Shannon JR, Flattem NL, Jordan J, Jacob G, Black BK, Biaggioni I, Robertson D. Orthostatic intolerance and tachycardia associated with norepinephrinetransporter deficiency. The New England Journal of Medicine. 2000;342:541–549. doi: 10.1056/NEJM200002243420803. [DOI] [PubMed] [Google Scholar]
- Shen YC, Liao DL, Chen JY, Wang YC, Lai IC, Liou YJ, Chen CH. Resequencing and association study of vesicular glutamate transporter 1 gene (VGLUT1) with schizophrenia. Schizophrenia Research. 2009;115:254–260. doi: 10.1016/j.schres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Shen YC, Liao DL, Lu CL, Chen JY, Liou YJ, Chen TT, Chen CH. Resequencing of the vesicular glutamate transporter 2 gene (VGLUT2) reveals some rare genetic variants that may increase the genetic burden in schizophrenia. Schizophrenia Research. 2010;121:179–186. doi: 10.1016/j.schres.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Shirey-Rice JK, Klar R, Fentress HM, Redmon SN, Sabb TR, Krueger JJ, Hahn MK. Norepinephrine transporter variant A457P Knock-in mice display key features of human postural orthostatic tachycardia syndrome. Disease Models & Mechanisms. 2013;6:1001–1011. doi: 10.1242/dmm.012203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemann JK, Muller CL, Forsberg CG, Blakely RD, Veenstra-VanderWeele J, Wallace MT. An autism-associated serotonin transporter variant disrupts multisensory processing. Translational Psychiatry. 2017;7:e1067. doi: 10.1038/tp.2017.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer D, Camargo SM, Huggel K, Romeo E, Danilczyk U, Kuba K, Verrey F. Orphan transporter SLC6A18 is renal neutral amino acid transporter B0AT3. The Journal of Biological Chemistry. 2009;284:19953–19960. doi: 10.1074/jbc.M109.011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton MR, Schaefer TL, Graham DL, Degrauw TJ, Clark JF, Williams MT, Vorhees CV. Creatine transporter (CrT; Slc6a8) knockout mice as a model of human CrT deficiency. PLoS One. 2011;6:e16187. doi: 10.1371/journal.pone.0016187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan JL, Mager S. Cloning and functional expression of a human Na+- and Cl−-dependent neutral and cationic amino acid transporterB0,+ . The Journal of Biological Chemistry. 1999;274:23740–23745. doi: 10.1074/jbc.274.34.23740. [DOI] [PubMed] [Google Scholar]
- Srour M, Hamdan FF, Gan-Or Z, Labuda D, Nassif C, Oskoui M, Michaud JLA. Homozygous mutation in SLC1A4 in siblings with severe intellectual disability and microcephaly. Clinical Genetics. 2015;88:e1–e4. doi: 10.1111/cge.12605. [DOI] [PubMed] [Google Scholar]
- Stankiewicz P, Kulkarni S, Dharmadhikari AV, Sampath S, Bhatt SS, Shaikh TH, Shaffer LG. Recurrent deletions and reciprocal duplications of10q11.21q11. 23 including CHAT and SLC18A3 are likely mediated by complex low-copy repeats. Human Mutation. 2012;33:165–179. doi: 10.1002/humu.21614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein V, Hermans-Borgmeyer I, Jentsch TJ, Hübner CA. Expression of the KCl cotransporter KCC2 parallels neuronal maturation and the emergence of low intracellular chloride. The Journal of Comparative Neurology. 2004;468:57–64. doi: 10.1002/cne.10983. [DOI] [PubMed] [Google Scholar]
- Stergachis AB, Pujol-Giménez J, Gyimesi G, Fuster D, Albano G, Troxler M, Rodan LH. Recurrent SLC1A2 variants cause epilepsy via a dominant negative mechanism. Annals of Neurology. 2019;85:921–926. doi: 10.1002/ana.25477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroobants S, Van Acker NG, Verheijen FW, Goris I, Daneels GF, Schot R, D’Hooge R. Progressive leukoencephalopathy impairs neurobehavioral development in Sialin-deficient mice. Experimental Neurology. 2017;291:106–119. doi: 10.1016/j.expneurol.2017.02.009. [DOI] [PubMed] [Google Scholar]
- Sucic S, El-Kasaby A, Kudlacek O, Sarker S, Sitte HH, Marin P, Freissmuth M. The serotonin transporter is an exclusive client of the coat protein complex II (COPII) component SEC24C. The Journal of Biological Chemistry. 2011;286:16482–16490. doi: 10.1074/jbc.M111.230037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucic S, Koban F, El-Kasaby A, Kudlacek O, Stockner T, Sitte HH, Freissmuth M. Switching the clientele: A lysine residing in the C terminus of the serotonin transporter specifies its preference for the coat protein complex II component SEC24C. The Journal of Biological Chemistry. 2013;288:5330–5341. doi: 10.1074/jbc.M112.408237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, Blakely RD. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviours. American Journal of Human Genetics. 2005;77:265–279. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Miner LL, Sora I, Ujike H, Revay RS, Kostic V, Uhl GR. VMAT2 knockout mice: Heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:9938–9943. doi: 10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Takanaga H, Mackenzie B, Peng JB, Hediger MA. Characterization of a branched-chain amino-acid transporter SBAT1 (SLC6A15) that is expressed in human brain. Biochemical and Biophysical Research Communincations. 2005;337:892–900. doi: 10.1016/j.bbrc.2005.09.128. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Harada Y, Moriyama S, Furuta K, Tanaka S, Miyaji T, Hiasa M. Vesicular polyamine transporter mediates vesicular storage and release of polyamine from mast cells. The Journal of Biological Chemistry. 2017;292:3909–3918. doi: 10.1074/jbc.M116.756197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Thomas RH, Chung SK, Wood SE, Cushion TD, Drew CJ, Hammond CL, Rees MI. Genotype-phenotype correlations in hyperekplexia: Apnoeas, learning difficulties and speech delay. Brain. 2013;136:3085–3095. doi: 10.1093/brain/awt207. [DOI] [PubMed] [Google Scholar]
- Togawa N, Miyaji T, Izawa S, Omote H, Moriyama Y. A Na+-phosphate cotransporter homologue (SLC17A4 protein) is an intestinal organic anion exporter. American Journal of Physiology Cell Physiology. 2012;302:C1652–C1660. doi: 10.1152/ajpcell.00015.2012. [DOI] [PubMed] [Google Scholar]
- Togawa N, Juge N, Miyaji T, Hiasa M, Omote H, Moriyama Y. Wide expression of type I Na+-phosphate cotransporter 3 (NPT3/SLC17A2), a membrane potential-driven organic anion transporter. American Journal of Physiology Cell Physiology. 2015;309:C71–C80. doi: 10.1152/ajpcell.00048.2015. [DOI] [PubMed] [Google Scholar]
- Tokunaga A, Tsukimoto M, Harada H, Moriyama Y, Kojima S. Involvement of SLC17A9-dependent vesicular exocytosis in the mechanism of ATP release during T cell activation. The Journal of Biological Chemistry. 2010;285:17406–17416. doi: 10.1074/jbc.M110.112417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomi M, Tajima A, Tachikawa M, Hosoya K. Function of taurine transporter (Slc6a6/TauT) as a GABA transporting protein and its relevance to GABA transport in rat retinal capillary endothelial cells. Biochimica et Biophysica Acta. 2008;1778:2138–2142. doi: 10.1016/j.bbamem.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Traiffort E, Ruat M, O’Regan S, Meunier FM. Molecular characterization of the family of choline transporter-like proteins and their splice variants. Journal of Neurochemistry. 2005;92:1116–1125. doi: 10.1111/j.1471-4159.2004.02962.x. [DOI] [PubMed] [Google Scholar]
- Traiffort E, O’Regan S, Ruat M. The choline transporter-like family SLC44: Properties and roles in human diseases. Molecular Aspects of Medicine. 2013;34:646–654. doi: 10.1016/j.mam.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Trotti D, Aoki M, Pasinelli P, Berger UV, Danbolt NC, Hediger MA. Amyotrophic lateral sclerosis-linked glutamate transporter mutant has impaired glutamate clearance capacity. The Journal of Biological Chemistry. 2001;276:576–582. doi: 10.1074/jbc.M003779200. [DOI] [PubMed] [Google Scholar]
- Tsai G, Ralph-Williams RJ, Martina M, Bergeron R, Berger-Sweeney J, Dunham KS, Coyle JT. Gene knockout of glycine transporter 1: Characterization of the behavioral phenotype. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8485–8490. doi: 10.1073/pnas.0402662101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S, Kwon HM, Yamauchi A, Preston AS, Marumo F, Handler JS. Molecular cloning of the cDNA for an MDCK cell Na+- and Cl−-dependent taurine transporter that is regulated by hypertonicity. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:8230–8234. doi: 10.1073/pnas.89.17.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Ito S, Ohta Y, Tachikawa M, Wada T, Terasaki T, Ohtsuki S. Abnormal N-glycosylation of a novel missense creatine transporter mutant, G561R, associated with cerebral creatine deficiency syndromes alters transporter activity and localization. Biological and Pharmacological Bulletin. 2017;40:49–55. doi: 10.1248/bpb.b16-00582. [DOI] [PubMed] [Google Scholar]
- Utsunomiya-Tate N, Endou H, Kanai Y. Cloning and functional characterization of a system ASC-like Na+-dependent neutral amino acid transporter. The Journal of Biological Chemistry. 1996;271:14883–14890. doi: 10.1074/jbc.271.25.14883. [DOI] [PubMed] [Google Scholar]
- Vadgama JV, Christensen HN. Wide distribution of pH-dependent service of transport system ASC for both anionic and zwitterionic amino acids. The Journal of Biological Chemistry. 1984;259:3648–3652. [PubMed] [Google Scholar]
- Valayannopoulos V, Bakouh N, Mazzuca M, Nonnenmacher L, Hubert L, Makaci FL, de Keyzer Y. Functional and electrophysiological characterization of four non-truncating mutations responsible for creatine transporter (SLC6A8) deficiency syndrome. Journal of Inherited Metabolic Disease. 2013;36:103–112. doi: 10.1007/s10545-012-9495-9. [DOI] [PubMed] [Google Scholar]
- Van De Kamp JM, Mancini GM, Salomons GS. X-linked creatine transporter deficiency: Clinical aspects and pathophysiology. Journal of Inherited Metabolic Disease. 2014;37:715–733. doi: 10.1007/s10545-014-9713-8. [DOI] [PubMed] [Google Scholar]
- Vandenberg RJ, Ryan RM. Mechanisms of glutamate transport. Physiological Reviews. 2013;93:1621–1625. doi: 10.1152/physrev.00007.2013. [DOI] [PubMed] [Google Scholar]
- Varho TT, Alajoki LE, Posti KM, Korhonen TT, Renlund MG, Nyman SR, Aula PP. Phenotypic spectrum of salla disease, a free sialic acid storage disorder. Pediatric Neurology. 2002;26:267–273. doi: 10.1016/s0887-8994(01)00406-4. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, Blakely RD. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5469–5474. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheijen FW, Verbeek E, Aula N, Beerens CE, Havelaar AC, Joosse M, Mancini GM. A new gene, encoding an anion transporter, is mutated in sialic acid storage diseases. Nature Genetics. 1999;23:462–465. doi: 10.1038/70585. [DOI] [PubMed] [Google Scholar]
- Veruki ML, Mørkve SH, Hartveit E. Activation of a presynaptic glutamate transporter regulates synaptic transmission through electrical signaling. Nature Neuroscience. 2006;9:1388–1396. doi: 10.1038/nn1793. [DOI] [PubMed] [Google Scholar]
- Vincent M, Collet C, Verloes A, Lambert L, Herlin C, Blanchet C, Geneviève D. Large deletions encompassing the TCOF1 and CAMK2A genes are responsible for Treacher Collins syndrome with intellectual disability. European Journal of Human Genetics. 2014;22:52–56. doi: 10.1038/ejhg.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyiaziakis E, Evgrafov O, Li D, Yoon HJ, Tabares P, Samuels J, Knowles JA. Association of SLC6A4 variants with obsessive-compulsive disorder in a large multicenter US family study. Molecular Psychiatry. 2011;16:108–120. doi: 10.1038/mp.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Gusic M, Günthner R, Alhaddad B, Kovacs-Nagy R, Makowski C, Wortmann SB. Biallelic mutations in SLC1A2; an additional mode of inheritance for SLC1A2-related epilepsy. Neuropediatrics. 2017;49:59–62. doi: 10.1055/s-0037-1606370. [DOI] [PubMed] [Google Scholar]
- Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, TRAFFIC Study Group,... TRANSPORT Study Group Lumacaftor-Ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. The New England Journal of Medicine. 2015;373:220–231. doi: 10.1056/NEJMoa1409547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallén-Mackenzie A, Gezelius H, Thoby-Brisson M, Nygård A, Enjin A, Fujiyama F, Kullander K. Vesicular glutamate transporter 2 is required for central respiratory rhythm generation but not for locomotor central pattern generation. The Journal of Neuroscience. 2006;26:12294–12307. doi: 10.1523/JNEUROSCI.3855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YM, Gainetdinov RR, Fumagalli F, Xu F, Jones SR, Bock CB, Caron MG. Knockout of the vesicular monoamine transporter 2 gene results in neonatal death and supersensitivity to cocaine and amphetamine. Neuron. 1997;19:1285–1296. doi: 10.1016/s0896-6273(00)80419-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Adamczyk A, Shugart YY, Samuels JF, Grados MA, Greenberg BD, Nestadt G. A screen of SLC1A1 for OCD-related alleles. American Journal of Medical Genetics, Part B Neuropsychiatric Genetics. 2010;153B:675–679. doi: 10.1002/ajmg.b.31001. [DOI] [PubMed] [Google Scholar]
- Wang J. The plasma membrane monoamine transporter (PMAT): Structure, function, and role in organic cation disposition. Clinical Pharmacology and Therapeutics. 2016;100:489–499. doi: 10.1002/cpt.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Salter CG, Refai O, Hardy H, Barwick KES, Akpulat U, Crosby AH. Choline transporter mutations in severe congenital myasthenic syndrome disrupt transporter localization. Brain. 2017;140:2838–2850. doi: 10.1093/brain/awx249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Poliquin S, Mermer F, Eissman J, Delpire E, Wang J, Kang JQ. Endoplasmic reticulum retention and degradation of a mutation in SLC6A1 associated with epilepsy and autism. Molecular Brain. 2020;13:76. doi: 10.1186/s13041-020-00612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warskulat U, Heller-Stilb B, Oermann E, Zilles K, Haas H, Lang F, Häussinger D. Phenotype of the taurine transporter knockout mouse. Methods in Enzymology. 2007;428:439–458. doi: 10.1016/S0076-6879(07)28025-5. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Morimoto K, Hirao T, Suwaki H, Watase K, Tanaka K. Amygdala-kindled and pentylenetetrazole-induced seizures in glutamate transporter GLAST-deficient mice. Brain Research. 1999;845:92–96. doi: 10.1016/s0006-8993(99)01945-9. [DOI] [PubMed] [Google Scholar]
- Watase K, Hashimoto K, Kano M, Yamada K, Watanabe M, Inoue Y, Tanaka K. Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice. The European Journal of Neuroscience. 1998;10:976–988. doi: 10.1046/j.1460-9568.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- Weiss MD, Derazi S, Kilberg MS, Anderson KJ. Ontogeny and localization of the neutral amino acid transporter ASCT1 in rat brain. Brain Research Developmental Brain Research. 2001;130:183–190. doi: 10.1016/s0165-3806(01)00250-4. [DOI] [PubMed] [Google Scholar]
- Wendland JR, DeGuzman TB, McMahon F, Rudnick G, Detera-Wadleigh SD, Murphy DL. SERT Ileu425Val in autism, Asperger syndrome and obsessive-compulsive disorder. Psychiatric Genetics. 2008;18:31–39. doi: 10.1097/YPG.0b013e3282f08a06. [DOI] [PubMed] [Google Scholar]
- Winter N, Kovermann P, Fahlke C. A point mutation associated with episodic Ataxia 6 increases glutamate transporter anion currents. Brain. 2012;135:3416–3425. doi: 10.1093/brain/aws255. [DOI] [PubMed] [Google Scholar]
- Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, Rosenmund C. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7158–7163. doi: 10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik SM, Katsurabayashi S, Guillemin I, Friauf E, Rosenmund C, Brose N, Rhee JS. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron. 2006;50:575–587. doi: 10.1016/j.neuron.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Wortmann SB, Mayr JA. Choline-related-inherited metabolic diseases-A mini review. Journal of Inherited Metabolic Disease. 2019;42:237–242. doi: 10.1002/jimd.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wreden CC, Wlizla M, Reimer RJ. Varied mechanisms underlie the free sialic acid storage disorders. The Journal of Biological Chemistry. 2005;280:1408–1416. doi: 10.1074/jbc.M411295200. [DOI] [PubMed] [Google Scholar]
- Wu MN, Koh K, Yue Z, Joiner WJ, Sehgal A. A genetic screen for sleep and circadian mutants reveals mechanisms underlying regulation of sleep in Drosophila. Sleep. 2008;31(4):465–472. doi: 10.1093/sleep/31.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Bellve KD, Fogarty KE, Melikian HE. Ack1 is a dopamine transporter endocytic brake that rescues a traf ficking-dysregulated ADHD coding variant. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:15480–15485. doi: 10.1073/pnas.1512957112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse AT, Netto CA. Behavioral and neurochemical effects of proline. Metabolic Brain Disease. 2011;26:159–172. doi: 10.1007/s11011-011-9246-x. [DOI] [PubMed] [Google Scholar]
- Xu F, Gainetdinov RR, Wetsel WC, Jones SR, Bohn LM, Miller GW, Caron MG. Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nature Neuroscience. 2000;3:465–471. doi: 10.1038/74839. [DOI] [PubMed] [Google Scholar]
- Yildiz Y, Pektas E, Tokatli A, Haliloglu G. Hereditary dopamine transporter deficiency 452 syndrome: Challenges in diagnosis and treatment. Neuropediatrics. 2017;48:049–052. doi: 10.1055/s-0036-1593372. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Yanai K. Histamine clearance through polyspecific transporters in the brain. Handbook of Experimental Pharmacology. 2016;241:173–187. doi: 10.1007/164_2016_13. [DOI] [PubMed] [Google Scholar]
- Young JD, Yao SY, Baldwin JM, Cass CE, Baldwin SA. The human concentrative and equilibrative nucleoside transporter families, SLC28 and SLC29. Molecular Aspects of Medicine. 2013;34:529–547. doi: 10.1016/j.mam.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Young AT, Ly KN, Wilson C, Lehnert K, Snell RG, Reid SJ, Jacobsen JC. Modelling brain dopamine-serotonin vesicular transport disease in Caenorhabditis elegans. Disease Models & Mechanisms. 2018;11:dmm035709. doi: 10.1242/dmm.035709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaia KA, Reimer RJ. Synaptic vesicle protein NTT4/XT1 (SLC6A17) catalyzes Na+-coupled neutral amino acid transport. The Journal of Biological Chemistry. 2009;284:8439–8448. doi: 10.1074/jbc.M806407200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zech M, Jech R, Wagner M, Mantel T, Boesch S, Nocker M, Winkelmann J. Molecular diversity of combined and complex dystonia: Insights from diagnostic exome sequencing. Neurogenetics. 2017;18:195–205. doi: 10.1007/s10048-017-0521-9. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Kavanaugh MP. ASCT-1 is a neutral amino acid exchanger with chloride channel activity. The Journal of Biological Chemistry. 1996;271:27991–27994. doi: 10.1074/jbc.271.45.27991. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Danbolt NC. GABA and glutamate transporters in brain. Frontiers in Endocrinology. 2013;4:165. doi: 10.3389/fendo.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomot E, Kanner BI. The interaction of the gamma-aminobutyric acid transporter GAT-1 with the neurotransmitter is selectively impaired by sulfhydryl modification of a conformationally sensitive cysteine residue engineered into extracellular loop IV. The Journal of Biological Chemistry. 2003;278:42950–42958. doi: 10.1074/jbc.M209307200. [DOI] [PubMed] [Google Scholar]
- Zwart R, Verhaagh S, Buitelaar M, Popp-Snijders C, Barlow DP. Impaired activity of the extraneuronal monoamine transporter system known as uptake-2 in Orct3/Slc22a3-deficient mice. Molecular and Cellular Biology. 2001;21:4188–4196. doi: 10.1128/MCB.21.13.4188-4196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.