Abstract
Purpose
Hypoxia (low oxygen) is a common feature of solid tumours that has been intensely studied for more than six decades. Here we review the importance of hypoxia to radiotherapy with a particular focus on the contribution of hypoxia to immune responses, metastatic potential and FLASH radiotherapy, active areas of research by leading women in the field.
Conclusion
Although hypoxia-driven metastasis and immunosuppression can negatively impact clinical outcome, understanding these processes can also provide tumour-specific vulnerabilities that may be therapeutically exploited. The different oxygen tensions present in tumours and normal tissues may underpin the beneficial FLASH sparing effect seen in normal tissue and represents a perfect example of advances in the field that can leverage tumour hypoxia to improve future radiotherapy treatments.
Keywords: Radiotherapy, Hypoxia, FLASH, Immune system, Metastasis, Cancer, Women in research, Ultra-high dose rate, Tumour Microenvironment
Introduction - The historical importance of hypoxia to radiation responses
Tumours are often found under hypoxic (low oxygen) conditions due to the imbalance between oxygen consumption and supply. The chaotic vasculature and abnormal mitochondrial function are unable to meet the metabolic demands of the growing tumour, despite the induction of angiogenesis (LaGory and Giaccia 2016). As a consequence, pockets of nutrient and oxygen deprivation coupled to high lactate and extracellular acidity form within tumours. Importantly, these microenvironmental features can profoundly impact tumour biology and treatment response (Bertout et al. 2008; Hanahan and Coussens 2012; Yoshimura et al. 2013). Tumour hypoxia is significantly associated with poor patient survival and radiation outcomes (Nordsmark M et al. 1996; Höckel and Vaupel 2001; Overgaard 2011; Sørensen and Horsman 2020; Matuleviciute et al. 2021). Extensive studies by Nordsmark et al., highlighted the prognostic value of oxygenation in cervix and head and neck cancer patients using an oxygen electrode (Nordsmark M. et al. 1996; Nordsmark et al. 2001; Nordsmark and Overgaard 2004; Nordsmark et al. 2005; Nordsmark et al. 2006). Women researchers in the field have also made important contributions to the development of hypoxia gene expression signatures and imaging methods, which have once again highlighted how common hypoxia is in solid tumours e.g. West, Buffa and Lyng (Lyng et al. 2001; Winter et al. 2007; Buffa et al. 2010; Eustace et al. 2013; Harris et al. 2015; Hillestad et al. 2020; Sorensen and Horsman 2020). While work by Denekamp et al., led to clinical trials of accelerated radiotherapy with carbogen and nicotinamide (ARCON), recent studies including those led by Koritzinski and Papandreou suggested metabolic inhibitors such as metformin, papaverine, and atovaquone as radiation sensitisers (Zackrisson et al. 1994; Bernier et al. 2000; Zannella et al. 2013; Ashton et al. 2016; Benej et al. 2018). Targeting hypoxic tumour cells by using prodrugs specifically activated in the reductive environment of hypoxic tumours has also been extensively investigated including with important contributions from McKeown, Robson, McCarthy, Williams, Hammond, and Pedley (McCarthy et al. 2003; McErlane et al. 2005; Dearling et al. 2007; McKeown et al. 2007; Cowen et al. 2008; O’Rourke et al. 2008; O’Connor et al. 2016; Mistry et al. 2017; Mehibel et al. 2021; Skwarska et al. 2021).
Hypoxia leads to cellular stress responses that allow tumour cells to survive and often thrive under what would be considered harsh living conditions for untransformed cells (Giaccia 1996). Severe hypoxia (often termed radiobiological hypoxia when below 10 mmHg) is particularly relevant for tumour radiation responses due to the requirement for oxygen to form free radicals and to induce the downstream DNA breaks and lethality (Hammond et al. 2014; Hall Eric J. and Giaccia 2019). In fact, there is a rapid change in radiosensitivity as oxygen concentrations increase with 0.5% O2 representing a relative halfway in radiosensitivity (Hall Eric J. and Giaccia 2019). Furthermore, the cellular stress response mounted in conditions of radiobiological hypoxia can impact radiation responses and may offer potential therapeutic targets (Wilson WR and Hay 2011; Hasvold et al. 2016). For example, studies from Hammond, Pires, Leszczynska, Olcina and Foskolou have demonstrated that radiobiological hypoxia will lead to replication stress and the induction of a DNA damage response (DDR) (Hammond et al. 2002; Pires I. M. et al. 2010; Olcina et al. 2013; Foskolou et al. 2017). Inhibiting DDR members can sensitise hypoxic cells to radiotherapy (RT) (Fokas et al. 2012; Pires I M et al. 2012; Weber and Ryan 2015; Dillon et al. 2019). Furthermore, the unfolded protein response (UPR) is also induced under these conditions and the study of crosstalk between UPR and DDR signalling could provide a source of future therapeutic targets to improve RT (Wouters and Koritzinsky 2008; Ramachandran et al. 2021).
A key driver of the biological response under hypoxic conditions is hypoxia-inducible factor (HIF), a heterodimeric transcription factor. While the HIFβ subunit is constitutively active, the hydroxylation of HIFα leads to its proteasomal degradation under oxic conditions (Wang GL and Semenza 1993; Maxwell et al. 1999). Under hypoxia, hydroxylation is inhibited and stabilised HIFα forms a heterodimer with HIFβ to transactivate its target genes. HIF activates complex biological responses including altered metabolism, apoptosis, autophagy, metastasis and angiogenesis, which were also demonstrated by female scientists including Simon, Bertout Papandreou, Chan, Rankin, Carroll and Ashcroft (Carroll and Ashcroft 2006; Chan and Giaccia 2007; Bertout et al. 2008; Mijaljica et al. 2010; Krock et al. 2011; Greer et al. 2012; Yang et al. 2012; Rankin and Giaccia 2016).
Previous studies investigating the role of tumour hypoxia on radiation responses have heavily focused on the biological consequences at the level of the tumour cell itself. However, it is becoming increasingly recognised that hypoxia and radiation also have a systemic impact on blood circulation, circulating tumour cells and immune processes (Doedens et al. 2010; Martin et al. 2014; Palazon Asis et al. 2014; Olcina et al. 2019; Matuleviciute et al. 2021).
In this review we highlight the contribution of women leaders in the hypoxia and radiation fields with respect to three key areas that are or have the potential to impact clinical outcome: radiation-induced metastasis, immune responses, and FLASH-RT.
Radiation-induced tumour invasion and metastasis and its crosstalk with hypoxia and redox pathways
Radiation treatment is given as standard-of-care to enhance local tumour control. External radiation is also applied to control local recurrence or to treat bone or brain metastasis, which results in better patient survival. However, local-regional failure or micrometastasis after radiation indicate that there are systemic effects supporting the survival of subsets of tumour cells to become resistant and metastatic.
Historically, tumour bed effects indicate that pre-irradiating normal tissues delays uptake and growth of the primary tumour, suggesting that radiation-induced changes to the microenvironment affect tumour cell growth (Stenstrom et al. 1955). Interestingly, although limiting the primary tumour growth, many studies observed that treating normal tissues or primary tumours with radiation promoted metastatic features of tumour cells (von Essen 1991). Early studies using a mouse xenograft model with subcutaneously injected mammary carcinoma, indicated that radiation (4-10 Gy) induced lung metastasis (Kaplan and Murphy 1949). A subsequent study further suggested that when mouse legs were pre-irradiated (30 Gy) before tumour cell inoculation, lung metastasis was increased while primary tumour growth was delayed (Milas et al. 1987). Although these studies represent radiation treatment to primary tumours versus normal tissues, they suggest systemic radiation responses might result in enhanced dissemination of tumour cells.
In addition to the effects of radiation to tumour cell recruitment to distant organs, tumour self-seeding effects were also observed in mouse breast cancer models by Vilalta and Rafat (discussed in further detail below) (Vilalta et al. 2014; Rafat et al. 2018). The self-seeding effect, referring to circulating tumour cells (CTC) colonising locally, was originally identified by studies undertaken by another female scientist, Park and her colleagues (Park et al. 2012). In this study, tumour-derived interleukin-6 or 8 (IL-6 or IL-8 respectively) were the main factors attracting CTC to the site of origin in a breast cancer model. Vilata and colleagues demonstrated that radiation can induce the self-seeding effect through the secretion of GM-CSF using both in vitro and in vivo models (Vilalta et al. 2014). Following this study, Rafat from the same group, further demonstrated that irradiation of normal tissues also recruited tumour cells from a distant site (Rafat et al. 2018). Although these two studies involved immunocompromised mice, they suggest that secretion factors from irradiated tissues recruit tumour cells from other sites to cause a local recurrence.
There are four mechanisms, which could explain how radiation promotes tumour metastasis: 1) direct radiation effect on tumour cells, 2) radiation effects in distant sites to prime metastatic niches, 3) release of tumour cells into the blood circulation, and 4) increased time for tumour cell release into the bloodstream due to a radiation-induced tumour growth delay (von Essen 1991). In our current understanding, these mechanisms do not seem to separately affect radiation-induced tumour metastasis but rather interplay to select more aggressive and resistant tumour cells. In this part of the review, we will focus on radiation-induced metastasis through expression of tumorigenic factors with a particular focus on those impacted by hypoxia and redox pathways.
It has been speculated that destruction of vasculature is the main cause of tumour cell release into the circulation after radiation treatment. Martin and colleagues detected the presence of CTCs expressing a mesenchymal marker (vimentin) in non-small cell lung cancer patients after radiation (Martin OA et al. 2014). However, before the release of tumour cells, multiple steps are required including hypoxia-mediated angiogenesis and invasion/migration of tumour cells in their primary sites (Sundahl et al. 2018). Radiation effects on hypoxia and angiogenesis were initially demonstrated using a mouse dorsal window chamber model (Moeller et al. 2004). This study found that radiation treatment (5, 10, 15 Gy) enhanced tumour hypoxia and HIF-1 expression through ROS production; and activation of HIF-1 pathways led to enhanced angiogenesis. A study by Williams and colleagues further demonstrated that HIF-1 is a crucial factor for radioresistance using a mouse xenograft model with a HIF-1 deficient hepatoma cell line (Williams et al. 2005). While these studies focused on the role of HIF-1 in radiation resistance, it was further demonstrated that radiation induces tumour hypoxia and angiogenesis, which results in tumour metastasis (Rofstad et al. 2005). This study found that expression of well-known pro-angiogenic factors, IL-8 or urokinase plasminogen activator surface receptor (uPAR) was elevated in primary tumours, which were pre-irradiated 24 hours before tumour inoculation. Their expression, specifically IL-8, was correlated with tumour hypoxia and microvessel density, indicating that hypoxia-induced IL-8 promotes angiogenesis. Inhibition of IL-8 or uPAR decreased tumour metastasis, while it had no effect on primary tumour growth.
These data demonstrated that the hypoxic microenvironment in the pre-irradiated tumour bed governed tumour metastatic potential by inducing IL-8 as an angiogenic factor, or uPAR as an invasive factor. A study by Bouchard and colleagues additionally identified two inflammatory factors, IL-6 and cyclooxygenase-2 (COX-2) as pro-migratory factors in mouse mammary carcinoma grown in pre-irradiated mammary fat pad (Bouchard et al. 2013). Interestingly, while spontaneous metastasis was increased in pre-irradiated mice, tail vein injection did not show significant differences in lung nodule formation between non-irradiated and pre-irradiated mice, confirming that radiation-induced microenvironmental changes contribute to the tumour metastasis. Her subsequent studies further identified that pre-irradiation-mediated lung metastasis was observed specifically in triple negative breast (TNB) cancer models and was dependent on membrane type-1 matrix metalloproteinase (MT1-MMP), which degrades extracellular matrix, by activating MMP2 and MMP9, pro-invasive markers (Bouchard et al. 2016; Bouchard et al. 2017). Bouchard and colleagues also showed that chloroquine treatment inhibits radiation-induced metastasis, and this treatment might be used to enhance disease-free survival of TNB cancer patients. The study by Riekki and her group reported that radiation induced elevation of MMP1 and MMP2 (TIMP1 and TIMP2), two essential matrix metalloproteinases in breast cancer patients who had surgery and radiation (Riekki et al. 2000). Experimental studies by Wild-Bode and colleagues using in vitro and in vivo models also showed that increased MMPs lead to tumour metastasis (Wild-Bode et al. 2001).
Production of reactive oxygen species (ROS) impacts radiation-induced cell killing through indirect DNA damage and altered expression of tumour progression factors including HIF-1 and Transcription growth factor β (TGFβ) (Barcellos-Hoff and Dix 1996; Moeller et al. 2004; Shimura et al. 2018). The work by Barcellos-Hoff and colleagues showed that radiation-induced ROS are responsible for enhanced TGFβ expression (Barcellos-Hoff and Dix 1996). Her later studies also highlighted the role of the tumour microenvironment in metastasis and radiation responses (Illa-Bochaca et al. 2014; Qiang et al. 2016). TGFβ is a major cytokine known for its role in normal tissue fibrosis as a late radiation response, which was supported by studies from female scientists Martin and Rube (Martin M et al. 1997; Rube et al. 2000). Rube and her colleagues performed radiation dose and time dependent studies using non-tumour bearing C57BL/6 mice and showed that TGFβ expression reached the maximum values at 2 and 4 weeks after radiation, which results in injured normal tissue fibrosis. However, TGFβ can also be induced as early as 6 hours after radiation, indicating its effect on early radiation responses. The role of TGFβ in tumour metastasis has been extensively studied in a variety of tumour models including breast and colon cancers (Padua et al. 2008; Calon et al. 2012). Its involvement in endothelial-to-mesenchymal transition (EMT) pathways and metastasis, through regulation of two hypoxia-regulated genes, angiopoietin like-4 (ANGPTL4) and IL11, identified TGFβ as a therapeutic target (Zhang H et al. 2012; Moon et al. 2021). Biswas and her colleagues reported that in MMTV/PyVmT, a transgenic breast cancer mouse model, radiation at 10 Gy induced TGFβ levels in plasma while CTC and lung metastasis were also increased (Biswas et al. 2007). Interestingly, thoracic radiation promoted dissemination of primary tumour cells grown in the mouse mammary fat pad to the mouse lung, suggesting that radiation also amends tissue microenvironment to “recruit” tumour cells. In this study, inhibition of TGFβ signalling, using a conditional TGFβRII knockout animal model or TGFβ antagonising antibody, decreased radiation-induced metastasis and the number of CTC in circulation, confirming that TGFβ is a driving factor of radiation-induced metastasis (Biswas et al. 2007). Subsequent studies have also reported that blocking TGFβ in combination with anti-PD-1, anti-CD137 and irradiation can improve CD8 T cell infiltration into non-irradiated lesions and enhances abscopal responses (tumour eradication outside the irradiation field) (Rodriguez-Ruiz et al. 2019). More recently a combination of radiation with a bifunctional fusion protein simultaneously inhibiting TGFβ and PD-L1 was shown to result in reduced RT-induced fibrosis and spontaneous lung metastasis (Lan et al. 2021), suggesting the beneficial effects of combining targeted and systemic treatments.
While ROS are also known to induce tumour invasion and metastasis after radiation (Kambach et al. 2014; Gu et al. 2015), regulation of redox pathways might also reduce the radiation-mediated metastatic features of cancer. NRF2 is a basic leucine zipper (bZIP) protein, which is well known for its regulation of antioxidant responses (Moon and Giaccia 2015). Although BACH1 was known as a negative regulator of redox pathways via competition with NRF2, a recent study provided significant insights indicating that NRF2 and BACH1 interact to promote tumour metastasis (Lignitto et al. 2019). Although extensive studies by Rosner and Lee have identified the important role of BACH1 in tumour metastasis, the function of BACH1 in radiation responses has not been reported (Sun et al. 2013; Lee et al. 2014; Lee et al. 2019). Studies suggest the importance of NRF2 in tumour biology and radiation resistance (McDonald et al. 2010; Jeong Y et al. 2017). Given that ROS might play a major role in promoting radiation-induced metastasis through upregulation of metastatic factors, it will be important to understand the impact of NRF2 or BACH1 pathways on tumour progression after radiotherapy. A recent study by Moon and colleagues determined that MAFF, an indispensable binding partner of NRF2 and BACH1, is more responsible for hypoxia regulation and tumour metastasis (Moon et al. 2021). Unpublished data by Moon also suggested that MAFF is highly induced by radiation, indicating that its potential role in radiation and radiation-induced metastasis. Therefore, a better understanding of regulation of antioxidant responses could provide therapeutic targets to improve patient outcomes, including in the metastatic setting.
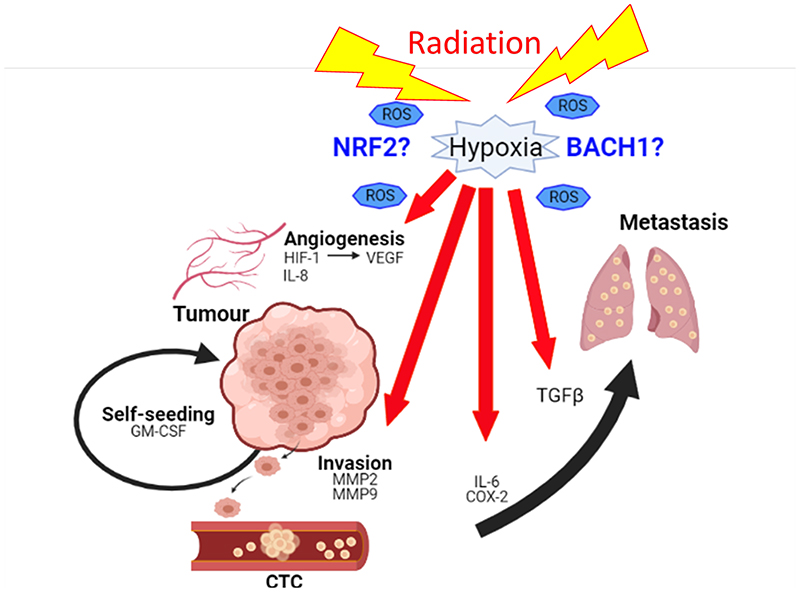
In summary, radiation effects are beneficial to primary tumours by promoting local control. However, multiple studies suggest that local and systemic effects allow tumour cells to invade, release, and to metastasise through hypoxia and ROS-mediated factors, which could lead to loco-regional failure or recurrence of tumour (Figure 1). However, despite numerous preclinical studies, the effect of radiation dose and time on metastasis is still controversial (Sundahl et al. 2018). Although the mechanisms underlying this phenomenon are poorly understood, it seems clear that a more effective treatment strategy is required to modulate tumour hypoxia and redox status of tumours. Also, further investigation regarding the role of radiation-induced systemic changes including immune modulation is required.
Figure 1.
Radiation promotes tumour metastasis through a variety of physiological pathways including angiogenesis (HIF-1, VEGF, IL-8) and invasion (MMP2, MMP9) to release tumour cells into the blood circulation (IL-6, COX-2) as well as to recruit them to a distant (TGFβ) or a primary site (GM-CSF). In these processes, hypoxia and production of reactive oxygen species (ROS) play a key role to regulate responsible molecular factors. Therefore, modulation of redox pathways through NRF2/BACH1 might play a distinct role in radiation-induced metastasis as well as loco-regional recurrence.
Hypoxia and Radiation-Induced Immune Responses
Immune regulation by hypoxia occurs in a number of pathological settings including in response to infections and in the tumour microenvironment (Palazon A. et al. 2014; LaGory and Giaccia 2016). Paradoxically, while in response to infections, movement of immune cells from the oxygen-rich blood to the hypoxic infected tissue contributes to host immune responses, the hypoxic tumour microenvironment is generally considered immunosuppressive (D’Ignazio L. et al. 2016).
While the effects of hypoxia mainly promote immune escape and an overall immunosuppressive environment, radiation can potentiate immune recognition and tumour clearance (Figure 2). Increased antigen release following radiotherapy and immunogenic cell death can enhance immune infiltration and may activate both local and distant site (non-radiated) anti-tumour immunity (Apetoh et al. 2007; Obeid et al. 2007; Ma et al. 2011; Demaria et al. 2015; Vaes et al. 2021; Zhu et al. 2021). Although radiation can also result in suppression of host anti-tumour immunity, its immune-stimulatory effects have sparked renewed interest in radiotherapy, including its potential in combination with immune checkpoint inhibitors (Wirsdorfer et al. 2014; Pilones et al. 2015; Eckert et al. 2019). When considering radiotherapy as a means of boosting anti-tumour immunity it is important to bear in mind that, as mentioned above, most solid tumours are hypoxic and therefore likely under some degree of immunosuppression (Chouaib et al. 2017; Eckert et al. 2019). For example, tumour hypoxia is associated with recruitment of immunosuppressive cell types such as myeloid-derived suppressor cells (MDSCs) and regulatory T-cells (Tregs) as reported by several including Chak-Lui Wong, Jendrossek and colleagues (Yan et al. 2011; Chiu et al. 2017; Westendorf et al. 2017; Jayaprakash et al. 2018). While the exact role of hypoxia in Treg biology remains controversial, HIF-2α but not HIF-1α was recently identified as critical for Treg function with Treg selective knockout of HIF-2α rendering mice resistant to tumour growth and metastasis (in colorectal and melanoma models respectively) (Hsu et al. 2020). HIF-driven secretion of chemokines by tumour cells can also contribute to immunosuppression via MDSC recruitment to the TME Chiu et al. 2016). Furthermore, HIF affects MDSC differentiation into tumour associated macrophages (TAMs) (Corzo et al. 2010; Chiu et al. 2016). HIF-1a also upregulates immune checkpoint factors such as PD-L1 on MDSCs, macrophages, dendritic cells, and tumour cells (Noman et al. 2014). Murphy and Lord recently found that hypoxia inhibits tumour cell expression of IFN-γ-dependent chemokines CXCL9, CXCL10 and MHC Class I. Additionally, T-cells cultured in hypoxia (0.5% O2) following antigenic stimulation, display reduced proliferation and IFN-γ production. Interestingly, the effects on IFN-γ production by T-cells appear HIF-1α-independent and can be rescued by reoxygenation (into 21% O2) (Murthy et al. 2019). Hypoxia can therefore impact immune modulation through both direct effects on immune cells as well as indirectly through the effect that hypoxic tumour cells can have on immune cell recruitment and function.
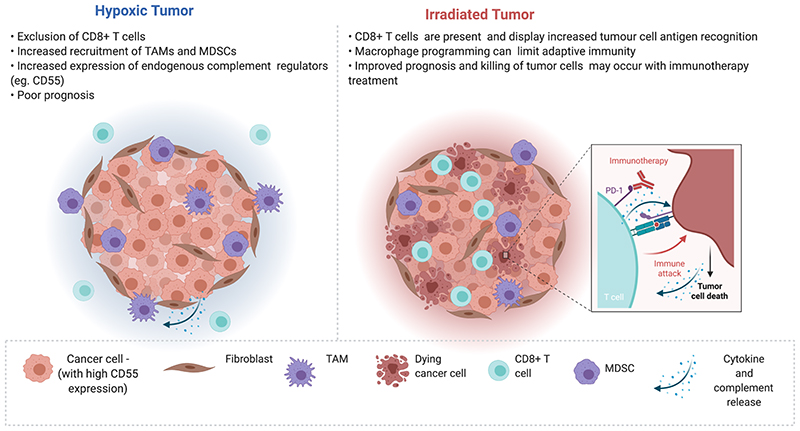
Figure 2.
(Left) Hypoxia within the tumour microenvironment (TME) contributes to immunosuppression and is associated with poor prognosis. Features of the hypoxic tumour contributing to immunosuppression include increased recruitment of myeloid-derived suppressor cells (MDSCs) and tumour associated macrophages (TAMs) with anti-inflammatory, pro-tumorigenic, and pro-angiogenic phenotypes. High expression of endogenous negative complement regulator CD55 in hypoxic tumour cells, may contribute to reduced complement-mediated attack in the TME. (Right) Radiation can potentiate immune recognition and tumour clearance and may therefore potentiate anti-tumour immune responses and immune checkpoint inhibitor treatment (as shown in the inset). However, it is important to bear in mind that radiation (and the hypoxic microenvironment of irradiated tumours) can pose barriers to effective anti-tumour immunity, through for example, macrophage programming or increased PD-L1 expression. Processes associated with regulation of immune responses within the TME are represented in the key at the bottom of the figure. Created with BioRender.com. Adapted from “Cold vs Hot Tumors”, by BioRender.com (2020). Retrieved from https://app.biorender.com/biorender-templates
While hypoxia-mediated immunosuppression can be a challenge for effective RT treatment, it also offers an Achilles’ heel that can be targeted. Indeed, studying hypoxia-induced expression of proteins associated with reduced immune infiltration can serve as a means of identifying therapeutic targets to combine with immune checkpoint blockade and RT. A good example of this is the work carried out by Le, Kuo et al., on galectin 1, a carbohydrate binding protein secreted by hypoxic tumour cells (Le et al. 2005; Kuo and Le 2014). Work from Le and her group has shown that Gal1 expression is inversely correlated with lymphocyte marker CD3 in head and neck (H&N) patients; and that Gal1 and CD3 are predictors of overall survival (Le et al. 2005). Subsequently, Le, Nambiar et al., elegantly showed that tumour Gal 1 reprograms the tumour endothelium to express PD-L1 and galectin 9 thereby preventing T-cell migration. Blocking Gal1 together with anti-PD1 therapy improves tumour response including in combination with radiotherapy in H&N cancer models (Nambiar et al. 2019).
TAMs found in hypoxic regions are associated with an anti-inflammatory, pro-tumorigenic, and pro-angiogenic phenotype that could account for therapy resistance (Henze and Mazzone 2016). The effects of radiation on macrophage biology also support the idea that this cell population is detrimental to radiation response within the TME. The Muschel lab, for example, recently reported on the importance of macrophage programming in the TME following fractionated RT, with FGF2 playing an important role. Interestingly, treatment with FGF2 blocking antibody together with fractionated RT increases tumour growth delay and long-term survival in murine models (Im et al. 2020). Work from the Muschel group had also previously demonstrated that RT of murine colorectal or pancreatic xenografts induces colony-stimulating factor 1 (CSF-1), which is associated with an increase in TAMs with an immunosuppressive phenotype five days post RT. Depleting macrophages (with anti-CSF-1 antibody) and delivering RT resulted in tumour growth delay in a CD8 T cell-dependent manner and was associated with increased antigen priming. These data suggest that adaptive anti-tumour immune responses are limited by TAMs. Interestingly, addition of anti-PD-L1 antibody further enhanced the radiation response of macrophage depleted tumours (Jones et al. 2018). In support of these studies, the G-One lab recently found that TAM depletion (with clodronate) attenuates tumour hypoxia and glycolysis while enhancing T-cell infiltration and PD-L1 expression. This study provides further rationale for combining TAM targeting strategies with anti-PD-L1 antibodies (Jeong H et al. 2019).
Within the TME a survival strategy of the tumour cell involves hijacking those transcription factors that would normally drive inflammation and pathogen clearance (e.g., HIF and NF-κB) in a manner that instead allows tumour immune evasion (Jung et al. 2003; House et al. 2017). While the co-option of these pathways poses a barrier to effective cancer treatment, it also offers insights into particular tumour vulnerabilities. For example, complement-mediated cytotoxicity has been proposed to contribute to tumour cell clearance (Roumenina et al. 2019). However, Olcina and colleagues demonstrated that within the TME, the cytotoxic effects of complement are compromised due to hypoxia and HIF-dependent expression of endogenous complement regulators such as CD55 in colorectal cancer cells (Olcina et al. 2018). HIF-dependent expression of endogenous complement regulators may therefore allow tumours to evade the cytotoxic effects of the complement system while benefiting from the tumour promoting properties of the pathway. Interestingly, increased CD55 expression has been associated with radioresistance and targeting different components of the complement system has been suggested to improve radiation response, albeit with somewhat conflicting results (Elvington et al. 2014; Surace et al. 2015; Leung et al. 2018; Olcina et al. 2020). Early studies from Elvington and her colleagues indicated that, a targeted inhibitor (CR2-Crry) that blocks all complement pathways at the C3 activation step, improved response to fractionated RT (Elvington et al. 2014). However, subsequent reports by Surace, Van den Broek and colleagues, suggested that the positive effects of radiation on anti-tumour immune responses would be diminished if complement inhibition occurred together with primarily high single dose irradiation (20 Gy) (Surace et al. 2015). It is important to note that these two studies used different preclinical murine models, radiation schedules and modes of inhibiting complement, complicating the direct comparison of these two studies. Still, these studies do highlight the need to systematically investigate the contribution of immune-associated processes to radiation responses in single dose and fractionated radiation schedules, side-by-side within the same murine models. Elegant work from Formenti, Demaria and Vanpouille-Box have demonstrated that different radiation regimens can indeed impact the outcome of anti-tumour immune radiation responses, including through regulation of intracellular innate immune processes (Pilones et al. 2015; Vanpouille-Box et al. 2017). Furthermore, when assessing tumour radiation responses in the context of different radiation schedules or novel modalities it will be important to consider the ever-increasing number of non-canonical functions reported for innate immune players (Dunphy et al. 2018; Bai et al. 2019; Olcina et al. 2020).
Modulation of immune responses by hypoxia/HIF dependent mechanism can also occur through crosstalk with NF-κB, the main pro-inflammatory family of transcription factors (Karin, 2006; Rius et al., 2008). Such crosstalk can occur in a number of cell types including macrophages, neutrophils and tumour cells themselves as elegantly reported by the Rocha lab (van Uden et al. 2008; D’Ignazio Laura et al. 2016; D’Ignazio et al. 2020). Importantly, NF-κB is induced in response to hypoxia as well as ionising radiation and is well-known to contribute to intrinsic tumour cell radioresistance, through modulation of pro-survival pathways (Criswell et al. 2003; D’Ignazio Laura et al. 2016). Interestingly, NF-κB activation also occurs on radiosensitive normal tissues where NF-κB-driven pro-survival signalling can confer protection (Wang Y et al. 2004). Finding therapeutic approaches that maximise dynamic modulation of NF-κB signalling to prevent tumour cell radioresistance, while affording productive activation for enhanced anti-tumour and protective normal tissue responses could enhance the therapeutic index of radiotherapy.
Overall, the promise of improved local (and potentially systemic) tumour control by effective immune-modulation and RT combinations might be particularly appealing for hypoxic tumours that currently display immunosuppression, reduced local control and increased rates of distant metastasis.
Is radiation induced transient hypoxia responsible for the FLASH effect?
In recent years, pioneering studies mainly from the Vozenin lab have shown that FLASH irradiation, which is radiation delivered at ultra-high dose rates (>30-40 Gy/s), results in significantly less normal tissue toxicity compared to irradiation at conventional clinical dose rates (few Gy/min) (Favaudon et al. 2014; Loo et al. 2017; Montay-Gruel et al. 2017; Montay-Gruel et al. 2018; Bourhis, Montay-Gruel, et al. 2019; Bourhis, Sozzi, et al. 2019; Fouillade et al. 2019; Montay-Gruel et al. 2019; Simmons et al. 2019; Vozenin, De Fornel, et al. 2019; Wilson JD et al. 2019; Alaghband et al. 2020; Diffenderfer et al. 2020; Levy et al. 2020; Soto et al. 2020; Zhang Q et al. 2020). These studies have primarily been preclinical but a couple of veterinary clinical studies treating canine and feline patients have been reported by Konradsson et al. and Vozenin et al., respectively (Vozenin, De Fornel, et al. 2019; Konradsson et al. 2021), as well as a first patient treated successfully with FLASH-RT (Bourhis, Sozzi, et al. 2019). In addition to limiting toxicities, there have also been reports of FLASH irradiation maintaining the same tumour response as seen following conventional dose rate irradiation, e.g. the excellent work from the Rankin lab investigating the increase in therapeutic index for FLASH in abdominal radiotherapy in mice (Favaudon et al. 2014; Zlobinskaya et al. 2014; Bourhis, Montay-Gruel, et al. 2019; Levy et al. 2020; Montay-Gruel et al. 2021). The FLASH sparing effect has mainly been observed in vivo, though a few studies have shown an effect also in vitro (Buonanno et al. 2019; Fouillade et al. 2019; Montay-Gruel et al. 2019; Adrian et al. 2020; Adrian et al. 2021; Khan et al. 2021). The biological mechanisms responsible for this differential FLASH sparing effect between normal tissue and tumour tissue is not yet known but several hypotheses have been proposed (Wilson JD et al. 2019), e.g. radiochemical depletion of oxygen leading to transient hypoxia (Hendry et al. 1982; Hall E. J. and Brenner 1991; Vozenin, Hendry, et al. 2019), radical-radical interaction (Labarbe et al. 2020; Wardman 2020), and a modified immune response following FLASH relative to conventional dose rate irradiation (Durante et al. 2018; Jin et al. 2020).
In a review of dose rate effects, including in vitro work carried out from the late 1950s, Hall and Brenner (referring to the behaviour of clonogenic cell survival curves) concluded that: “If both the dose and instantaneous dose rate are sufficiently high, the rapid deposition of radiant energy consumes oxygen too quickly for diffusion to maintain an adequate level of oxygenation, and dose-response curves obtained are those characteristic of hypoxia” (Hall E. J. and Brenner 1991). A dose-response characteristic of hypoxia following irradiation at ultra-high dose rate has also previously been shown in vivo by Hendry et al. (Hendry et al. 1982). A recent in vitro study by Adrian et al. showed that the FLASH effect is modified by the oxygen concentration (Adrian et al. 2020), which was also recently shown in vivo; in mice by the Vozenin lab (Montay-Gruel et al. 2019) and by the Beyreuther lab in zebrafish embryos (Pawelke et al. 2021). Following FLASH irradiation, it is hypothesised that the physiological level of oxygen (physoxic) found in normal tissues decreases during the rapid dose delivery, creating an acute period of hypoxia in the irradiated tissue and consequently transient radioresistance. An effect that could be considered less important in tumours, with significant volumes of already hypoxic tissue (Wilson JD et al. 2019), and which was recently indicated by in vivo measurements of oxygen in tumour and normal tissue during ultra-high (300 Gy/s) and conventional dose rate (0.1 Gy/s) irradiation by Cao et al. (Cao et al. 2021). Also, Spitz et al. hypothesized that there are higher levels of redox-active iron (labile iron) in tumour than in normal tissue and that the tissues differ in their oxidative metabolism, with the more rapid removal and decay of the organic hydroperoxides and free radicals derived from peroxidation chain reactions in normal tissue, which could explain the differential effect seen between normal and tumour tissues (Spitz et al. 2019).
The impact of the oxygen consumption following FLASH irradiation has been modelled by several research groups, e.g. the Kirkby lab, and found to fit well to the available biological data (Pratx and Kapp 2019; Petersson et al. 2020; Zhou et al. 2020; Liew et al. 2021; Rothwell et al. 2021). However, studies that model and measure oxygen consumption in water from radiolysis either claim to support the hypothesis (Abolfath et al. 2020; Alanazi et al. 2021) or claim that the oxygen consumption is not a possible explanation for the FLASH effect, as the consumption is small for the amount of dose delivered in FLASH studies and that higher dose rate irradiation consume less oxygen than lower dose rate irradiation (Labarbe et al. 2020; Boscolo et al. 2021; Jansen et al. 2021). To illustrate both sides of this discussion, Figure 3 shows the oxygen consumption as measured in 5 ml cell media in T12.5 flasks, irradiated with 20 Gy at an ultra-high or conventional dose rate, and the clonogenic survival of cells exposed to such beams.
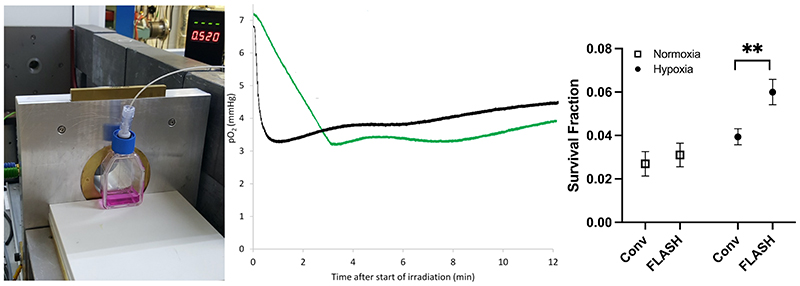
Figure 3.
The setup used (left), the measurement results (middle) of oxygen consumption (measured in media with an Oxylite, Oxford Optronics, Oxford, UK), and clonogenic cell survival (right), following delivery of 20 Gy with ultra-high (2000 Gy/s, black trace) and conventional dose rates (0.1 Gy/s, green trace) to a T12.5 cell flask with a monolayer of H454 murine glioblastoma cells and 5 ml media, from a horizontal 6 MeV electron beam. The beam-on time for FLASH is just 0.01 s. So, for an ideal measurement probe the black trace should drop in this short time but because of averaging of the signal, there is a delay of several seconds. For the same reason, small shoulders are visible for the green trace where the irradiation starts and stops (at around 3 min and 10 s). Similar to data from Cao et al. (Cao et al. 2021), the oxygen consumption is clearly greater for the conventional dose rate, i.e. the p02 value reduction was greater for the green trace (4.0 mmHg, i.e. 0.20 mmHg/Gy) than the black trace (3.5 mmHg, i.e. 0.17-0.18 mmHg/Gy), though the timescale of reduction is very different. Some diffusion of oxygen into the media from the gas in the flask (where the oxygen consumption is far less prominent than in the media) is evident after the end of irradiation. Clonogenic cell survival data show a small non-significant sparing for FLASH irradiation vs. conventional dose rate (CONV) irradiation when cells are prepared in normoxia, which expands and becomes significant (**, p<0.01) when cells are prepared in hypoxia (6 mmHg, following 1-2h in a hypoxia chamber prior to irradiation).
Our figure indicates that measurements of oxygen depletion in water (or media) are likely not adequate to describe the more complex situation that occurs in a cell, through which oxygen diffuses and is consumed differently (Weiss et al. 1974; Wardman 2020; Zhou et al. 2020; Lai et al. 2021). Furthermore, the reactions considered responsible for the depletion of oxygen in water, such as hydrated electrons (eaq −) and H• atoms reacting with O2, are probably unlikely to occur to any significant extent in irradiated cells because of the high concentrations of competing scavengers (Wardman 2020). Consequently, such studies can neither prove or disprove the hypothesis that the FLASH effect is driven by oxygen depletion but can perhaps guide us in the right direction. As expected, oxygen diffusion profiles have been shown to be very different in vivo, where a circulatory system effectively hinders any reduction in oxygen concentration in the tissue irradiated with conventional dose rates, while it cannot hinder the reduction if the irradiation is at an ultra-high dose rate (Cao et al. 2021). From these studies, we can deduce that future in vitro studies on FLASH should predominantly be performed in a controlled oxygen environment (in physoxia or hypoxia), while in vivo studies looking at tumour response should include well-oxygenated as well as hypoxic tumour models. Likely, there is not one simple explanation for the observed effect in vivo but rather a combination of several mechanisms, which result in the very promising FLASH effect.
Conclusion
Though the importance of hypoxia for RT has been studied since the 1950s, it is still being actively researched today and will likely continue to be a relevant research topic in the foreseeable future. In this review, we focused on three highly active fields of radiation research for which the leading researchers are women; metastasis, immune response, and FLASH-RT.
Acknowledgements
This study was funded by the Medical Research Council - MRC (MC_UU_00001/9)(4050620859), (MC_UU_00001/10), (MC_UU_00001/11). The authors would like to thank Jia-Ling Ruan for her work on the clonogenic assay.
Biographies
Biographical Note
Ejung Moon, PhD, is Group Leader and MRC Investigator at the MRC Oxford Institute for Radiation Oncology, University of Oxford, UK.
Kristoffer Petersson, PhD is Group Leader and MRC Investigator at the MRC Oxford Institute for Radiation Oncology, University of Oxford, UK, and at Radiation Physics, Department of Haematology, Oncology and Radiation Physics, Skåne University Hospital, Sweden.
Monica M. Olcina, DPhil, is Group Leader and MRC Investigator at the MRC Oxford Institute for Radiation Oncology, University of Oxford, UK.
Footnotes
Disclosure of Interest: The authors report no conflict of interest.
References
- Abolfath R, Grosshans D, Mohan R. Oxygen depletion in FLASH ultra-high-dose-rate radiotherapy: A molecular dynamics simulation. Med Phys. 2020;47(12):6551–6561. doi: 10.1002/mp.14548. [DOI] [PubMed] [Google Scholar]
- Adrian G, Konradsson E, Beyer S, Wittrup A, Butterworth KT, McMahon SJ, Ghita M, Petersson K, Ceberg C. Cancer Cells Can Exhibit a Sparing FLASH Effect at Low Doses Under Normoxic In Vitro-Conditions. Front Oncol. 2021;11:686142. doi: 10.3389/fonc.2021.686142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian G, Konradsson E, Lempart M, Back S, Ceberg C, Petersson K. The FLASH effect depends on oxygen concentration. Br J Radiol. 2020;93(1106):20190702. doi: 10.1259/bjr.20190702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaghband Y, Cheeks SN, Allen BD, Montay-Gruel P, Doan NL, Petit B, Jorge PG, Giedzinski E, Acharya MM, Vozenin MC, et al. Neuroprotection of Radiosensitive Juvenile Mice by Ultra-High Dose Rate FLASH Irradiation. Cancers (Basel) 2020;12(6) doi: 10.3390/cancers12061671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanazi A, Meesungnoen J, Jay-Gerin JP. A Computer Modeling Study of Water Radiolysis at High Dose Rates. Relevance to FLASH Radiotherapy. Radiat Res. 2021;195(2):149–162. doi: 10.1667/RADE-20-00168.1. [DOI] [PubMed] [Google Scholar]
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- Ashton TM, Fokas E, Kunz-Schughart LA, Folkes LK, Anbalagan S, Huether M, Kelly CJ, Pirovano G, Buffa FM, Hammond EM, et al. The anti-malarial atovaquone increases radiosensitivity by alleviating tumour hypoxia. Nat Commun. 2016;7:12308. doi: 10.1038/ncomms12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Wang W, Li S, Zhan J, Li H, Zhao M, Zhou XA, Li S, Li X, Huo Y, et al. C1QBP Promotes Homologous Recombination by Stabilizing MRE11 and Controlling the Assembly and Activation of MRE11/RAD50/NBS1 Complex. Molecular Cell. 2019 doi: 10.1016/j.molcel.2019.06.023. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol. 1996;10(9):1077–1083. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- Benej M, Hong X, Vibhute S, Scott S, Wu J, Graves E, Le QT, Koong AC, Giaccia AJ, Yu B, et al. Papaverine and its derivatives radiosensitize solid tumors by inhibiting mitochondrial metabolism. Proc Natl Acad Sci U S A. 2018;115(42):10756–10761. doi: 10.1073/pnas.1808945115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier J, Denekamp J, Rojas A, Minatel E, Horiot J, Hamers H, Antognoni P, Dahl O, Richaud P, van Glabbeke M, et al. ARCON: accelerated radiotherapy with carbogen and nicotinamide in head and neck squamous cell carcinomas. The experience of the Co-operative group of radiotherapy of the european organization for research and treatment of cancer (EORTC) Radiother Oncol. 2000;55(2):111–119. doi: 10.1016/s0167-8140(00)00165-1. [DOI] [PubMed] [Google Scholar]
- Bertout Ja, Patel Sa, Simon MC. The impact of O2 availability on human cancer. Nature reviews Cancer. 2008;8:967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Guix M, Rinehart C, Dugger TC, Chytil A, Moses HL, Freeman ML, Arteaga CL. Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest. 2007;117(5):1305–1313. doi: 10.1172/JCI30740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscolo D, Scifoni E, Durante M, Kramer M, Fuss MC. May oxygen depletion explain the FLASH effect? A chemical track structure analysis. Radiother Oncol. 2021;162:68–75. doi: 10.1016/j.radonc.2021.06.031. [DOI] [PubMed] [Google Scholar]
- Bouchard G, Bouvette G, Therriault H, Bujold R, Saucier C, Paquette B. Pre-irradiation of mouse mammary gland stimulates cancer cell migration and development of lung metastases. Br J Cancer. 2013;109(7):1829–1838. doi: 10.1038/bjc.2013.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard G, Therriault H, Geha S, Berube-Lauziere Y, Bujold R, Saucier C, Paquette B. Stimulation of triple negative breast cancer cell migration and metastases formation is prevented by chloroquine in a pre-irradiated mouse model. BMC Cancer. 2016;16:361. doi: 10.1186/s12885-016-2393-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard G, Therriault H, Geha S, Bujold R, Saucier C, Paquette B. Radiation-induced lung metastasis development is MT1-MMP-dependent in a triple-negative breast cancer mouse model. Br J Cancer. 2017;116(4):479–488. doi: 10.1038/bjc.2016.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourhis J, Montay-Gruel P, Goncalves Jorge P, Bailat C, Petit B, Ollivier J, Jeanneret-Sozzi W, Ozsahin M, Bochud F, Moeckli R, et al. Clinical translation of FLASH radiotherapy: Why and how? Radiother Oncol. 2019;139:11–17. doi: 10.1016/j.radonc.2019.04.008. [DOI] [PubMed] [Google Scholar]
- Bourhis J, Sozzi WJ, Jorge PG, Gaide O, Bailat C, Duclos F, Patin D, Ozsahin M, Bochud F, Germond JF, et al. Treatment of a first patient with FLASH-radiotherapy. Radiother Oncol. 2019;139:18–22. doi: 10.1016/j.radonc.2019.06.019. [DOI] [PubMed] [Google Scholar]
- Buffa FM, Harris AL, West CM, Miller CJ. Large meta-analysis of multiple cancers reveals a common, compact and highly prognostic hypoxia metagene. Br J Cancer. 2010;102(2):428–435. doi: 10.1038/sj.bjc.6605450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno M, Grilj V, Brenner DJ. Biological effects in normal cells exposed to FLASH dose rate protons. Radiother Oncol. 2019;139:51–55. doi: 10.1016/j.radonc.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Cespedes MV, Sevillano M, Nadal C, Jung P, Zhang XH, et al. Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22(5):571–584. doi: 10.1016/j.ccr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Zhang R, Esipova TV, Rao Allu S, Ashraf MR, Rahman M, Gunn JR, Bruza P, Gladstone DJ, Williams BB, et al. Quantification of oxygen depletion during FLASH irradiation in vitro and in vivo. International Journal of Radiation Oncology • Biology • Physics. 2021 doi: 10.1016/j.ijrobp.2021.03.056. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll VA, Ashcroft M. Role of hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: implications for targeting the HIF pathway. Cancer Res. 2006;66(12):6264–6270. doi: 10.1158/0008-5472.CAN-05-2519. [DOI] [PubMed] [Google Scholar]
- Chan DA, Giaccia AJ. Hypoxia, gene expression, and metastasis. Cancer and Metastasis Reviews. 2007;26:333–339. doi: 10.1007/s10555-007-9063-1. [DOI] [PubMed] [Google Scholar]
- Chiu DK, Tse AP, Xu IM, Di Cui J, Lai RK, Li LL, Koh HY, Tsang FH, Wei LL, Wong CM, et al. Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat Commun. 2017;8(1):517. doi: 10.1038/s41467-017-00530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu DK, Xu IM, Lai RK, Tse AP, Wei LL, Koh HY, Li LL, Lee D, Lo RC, Wong CM, et al. Hypoxia induces myeloid-derived suppressor cell recruitment to hepatocellular carcinoma through chemokine (C-C motif) ligand 26. Hepatology. 2016;64(3):797–813. doi: 10.1002/hep.28655. [DOI] [PubMed] [Google Scholar]
- Chouaib S, Noman MZ, Kosmatopoulos K, Curran MA. Hypoxic stress: Obstacles and opportunities for innovative immunotherapy of cancer. Oncogene. 2017;36:439–445. doi: 10.1038/onc.2016.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207(11):2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen RL, Garside EJ, Fitzpatrick B, Papadopoulou MV, Williams KJ. Gene therapy approaches to enhance bioreductive drug treatment. Br J Radiol. 2008;81(1):S45–56. doi: 10.1259/bjr/55070206. [DOI] [PubMed] [Google Scholar]
- Criswell T, Leskov K, Miyamoto S, Luo G, Boothman Da. Transcription factors activated in mammalian cells after clinically relevant doses of ionizing radiation. Oncogene. 2003;22:5813–5827. doi: 10.1038/sj.onc.1206680. [DOI] [PubMed] [Google Scholar]
- D’Ignazio L, Bandarra D, Rocha S. NF-kappaB and HIF crosstalk in immune responses. FEBS J. 2016;283(3):413–424. doi: 10.1111/febs.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ignazio L, Bandarra D, Rocha S. NF-κB and HIF crosstalk in immune responses. FEBS Journal. 2016;283:413–424. doi: 10.1111/febs.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ignazio L, Shakir D, Batie M, Muller HA, Rocha S. HIF-1beta Positively Regulates NF-kappaB Activity via Direct Control of TRAF6. Int J Mol Sci. 2020;21(8) doi: 10.3390/ijms21083000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearling JL, Qureshi U, Begent RH, Pedley RB. Combining radioimmunotherapy with antihypoxia therapy 2-deoxy-D-glucose results in reduction of therapeutic efficacy. Clin Cancer Res. 2007;13(6):1903–1910. doi: 10.1158/1078-0432.CCR-06-2094. [DOI] [PubMed] [Google Scholar]
- Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncology. 2015 doi: 10.1001/jamaoncol.2015.2756. [DOI] [PubMed] [Google Scholar]
- Diffenderfer ES, Verginadis II, Kim MM, Shoniyozov K, Velalopoulou A, Goia D, Putt M, Hagan S, Avery S, Teo K, et al. Design, Implementation, and in Vivo Validation of a Novel Proton FLASH Radiation Therapy System. Int J Radiat Oncol Biol Phys. 2020;106(2):440–448. doi: 10.1016/j.ijrobp.2019.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon MT, Bergerhoff KF, Pedersen M, Whittock H, Crespo-Rodriguez E, Patin EC, Pearson A, Smith HG, Paget JTE, Patel RR, et al. ATR inhibition potentiates the radiation-induced inflammatory tumor microenvironment. Clinical Cancer Research. 2019 doi: 10.1158/1078-0432.CCR-18-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, De Nardo DG, Coussens LM, Karin M, Goldrath AW, Johnson RS. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer research. 2010;70:7465–7475. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy G, Flannery SM, Almine JF, Connolly DJ, Paulus C, Jønsson KL, Jakobsen MR, Nevels MM, Bowie AG, Unterholzner L. Non-canonical Activation of the DNA Sensing Adaptor STING by ATM and IFI16 Mediates NF-κB Signaling after Nuclear DNA Damage. Molecular Cell. 2018 doi: 10.1016/j.molcel.2018.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durante M, Brauer-Krisch E, Hill M. Faster and safer? FLASH ultra-high dose rate in radiotherapy. Br J Radiol. 2018;91(1082):20170628. doi: 10.1259/bjr.20170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert F, Zwirner K, Boeke S, Thorwarth D, Zips D, Huber SM. Rationale for Combining Radiotherapy and Immune Checkpoint Inhibition for Patients With Hypoxic Tumors. Front Immunol. 2019;10:407. doi: 10.3389/fimmu.2019.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvington M, Scheiber M, Yang X, Lyons K, Jacqmin D, Wadsworth C, Marshall D, Vanek K, Tomlinson S. Complement-dependent modulation of antitumor immunity following radiation therapy. Cell Reports. 2014;8:818–830. doi: 10.1016/j.celrep.2014.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eustace A, Mani N, Span PN, Irlam JJ, Taylor J, Betts GN, Denley H, Miller CJ, Homer JJ, Rojas AM, et al. A 26-gene hypoxia signature predicts benefit from hypoxia-modifying therapy in laryngeal cancer but not bladder cancer. Clin Cancer Res. 2013;19(17):4879–4888. doi: 10.1158/1078-0432.CCR-13-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaudon V, Caplier L, Monceau V, Pouzoulet F, Sayarath M, Fouillade C, Poupon MF, Brito I, Hupe P, Bourhis J, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med. 2014;6(245):245ra293. doi: 10.1126/scitranslmed.3008973. [DOI] [PubMed] [Google Scholar]
- Fokas E, Prevo R, Pollard JR, Reaper PM, Charlton PA, Cornelissen B, Vallis KA, Hammond EM, Olcina MM, Gillies McKenna W, et al. Targeting ATR in vivo using the novel inhibitor VE-822 results in selective sensitization of pancreatic tumors to radiation. Cell death & disease. 2012;3:e441. doi: 10.1038/cddis.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskolou IP, Jorgensen C, Leszczynska KB, Olcina MM, Tarhonskaya H, Haisma B, D’Angiolella V, Myers WK, Domene C, Flashman E, et al. Ribonucleotide Reductase Requires Subunit Switching in Hypoxia to Maintain DNA Replication. Molecular Cell. 2017;66 doi: 10.1016/j.molcel.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouillade C, Curras-Alonso S, Giuranno L, Quelennec E, Heinrich S, Bonnet-Boissinot S, Beddok A, Leboucher S, Karakurt HU, Bohec M, et al. FLASH irradiation spares lung progenitor cells and limits the incidence of radio-induced senescence. Clin Cancer Res. 2019 doi: 10.1158/1078-0432.CCR-19-1440. [DOI] [PubMed] [Google Scholar]
- Giaccia A. Hypoxic Stress Proteins: Survival of the Fittest. Seminars in radiation oncology. 1996;6:46–58. doi: 10.1053/SRAO0060046. [DOI] [PubMed] [Google Scholar]
- Greer SN, Metcalf JL, Wang Y, Ohh M. The updated biology of hypoxia-inducible factor. The EMBO journal. 2012;31:2448–2460. doi: 10.1038/emboj.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, He Y, Ji J, Yao Y, Shen W, Luo J, Zhu W, Cao H, Geng Y, Xu J, et al. Hypoxia-inducible factor 1alpha (HIF-1alpha) and reactive oxygen species (ROS) mediates radiation-induced invasiveness through the SDF-1alpha/CXCR4 pathway in non-small cell lung carcinoma cells. Oncotarget. 2015;6(13):10893–10907. doi: 10.18632/oncotarget.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall EJ, Brenner DJ. The dose-rate effect revisited: radiobiological considerations of importance in radiotherapy. Int J Radiat Oncol Biol Phys. 1991;21(6):1403–1414. doi: 10.1016/0360-3016(91)90314-t. [DOI] [PubMed] [Google Scholar]
- Hall EJ, Giaccia AJ. Radiobiology for the radiologist. Eighth. Philadelphia: Wolters Kluwer; 2019. [Google Scholar]
- Hammond EM, Asselin MC, Forster D, O’Connor JPB, Senra JM, Williams KJ. The Meaning, Measurement and Modification of Hypoxia in the Laboratory and the Clinic. Clinical Oncology. 2014;26:277–288. doi: 10.1016/j.clon.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Hammond EM, Denko NC, Dorie MJ, Abraham RT, Giaccia AJ. Hypoxia Links ATR and p53 through Replication Arrest. Molecular and Cellular Biology. 2002;22:1834–1843. doi: 10.1128/MCB.22.6.1834-1843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Coussens LM. Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Harris BH, Barberis A, West CM, Buffa FM. Gene Expression Signatures as Biomarkers of Tumour Hypoxia. Clin Oncol (R Coll Radiol) 2015;27(10):547–560. doi: 10.1016/j.clon.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Hasvold G, Lund-Andersen C, Lando M, Patzke S, Hauge S, Suo Z, Lyng H, Syljuasen RG. Hypoxia-induced alterations of G2 checkpoint regulators. Mol Oncol. 2016;10(5):764–773. doi: 10.1016/j.molonc.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry JH, Moore JV, Hodgson BW, Keene JP. The constant low oxygen concentration in all the target cells for mouse tail radionecrosis. Radiat Res. 1982;92(1):172–181. [PubMed] [Google Scholar]
- Henze A-T, Mazzone M. The impact of hypoxia on tumor-associated macrophages. Journal of Clinical Investigation. 2016;126:3672–3679. doi: 10.1172/JCI84427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillestad T, Hompland T, Fjeldbo CS, Skingen VE, Salberg UB, Aarnes EK, Nilsen A, Lund KV, Evensen TS, Kristensen GB, et al. MRI Distinguishes Tumor Hypoxia Levels of Different Prognostic and Biological Significance in Cervical Cancer. Cancer Res. 2020;80(18):3993–4003. doi: 10.1158/0008-5472.CAN-20-0950. [DOI] [PubMed] [Google Scholar]
- Höckel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. Journal of the National Cancer Institute. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- House CD, Jordan E, Hernandez L, Ozaki M, James JM, Kim M, Kruhlak MJ, Batchelor E, Elloumi F, Cam MC, et al. NFkB promotes ovarian tumorigenesis via classical pathways that support proliferative cancer cells and alternative pathways that support ALDHþcancer stem-like cells. Cancer Research. 2017 doi: 10.1158/0008-5472.CAN-17-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu TS, Lin YL, Wang YA, Mo ST, Chi PY, Lai AC, Pan HY, Chang YJ, Lai MZ. HIF-2alpha is indispensable for regulatory T cell function. Nat Commun. 2020;11(1):5005. doi: 10.1038/s41467-020-18731-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illa-Bochaca I, Ouyang H, Tang J, Sebastiano C, Mao JH, Costes SV, Demaria S, Barcellos-Hoff MH. Densely ionizing radiation acts via the microenvironment to promote aggressive Trp53-null mammary carcinomas. Cancer Res. 2014;74(23):7137–7148. doi: 10.1158/0008-5472.CAN-14-1212. [DOI] [PubMed] [Google Scholar]
- Im JH, Buzzelli JN, Jones K, Franchini F, Gordon-Weeks A, Markelc B, Chen J, Kim J, Cao Y, Muschel RJ. FGF2 alters macrophage polarization, tumour immunity and growth and can be targeted during radiotherapy. Nature Communications. 2020;11:4064. doi: 10.1038/s41467-020-17914-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J, Knoll J, Beyreuther E, Pawelke J, Skuza R, Hanley R, Brons S, Pagliari F, Seco J. Does FLASH deplete Oxygen? Experimental Evaluation for Photons, Protons and Carbon Ions. Medical Physics. 2021 doi: 10.1002/mp.14917. [DOI] [PubMed] [Google Scholar]
- Jayaprakash P, Ai M, Liu A, Budhani P, Bartkowiak T, Sheng J, Ager C, Nicholas C, Jaiswal AR, Sun Y, et al. Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy. J Clin Invest. 2018;128(11):5137–5149. doi: 10.1172/JCI96268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H, Kim S, Hong BJ, Lee CJ, Kim YE, Bok S, Oh JM, Gwak SH, Yoo MY, Lee MS, et al. Tumor-Associated Macrophages Enhance Tumor Hypoxia and Aerobic Glycolysis. Cancer Res. 2019;79(4):795–806. doi: 10.1158/0008-5472.CAN-18-2545. [DOI] [PubMed] [Google Scholar]
- Jeong Y, Hoang NT, Lovejoy A, Stehr H, Newman AM, Gentles AJ, Kong W, Truong D, Martin S, Chaudhuri A, et al. Role of KEAP1/NRF2 and TP53 Mutations in Lung Squamous Cell Carcinoma Development and Radiation Resistance. Cancer Discov. 2017;7(1):86–101. doi: 10.1158/2159-8290.CD-16-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JY, Gu A, Wang W, Oleinick NL, Machtay M, Spring Kong FM. Ultra-high dose rate effect on circulating immune cells: A potential mechanism for FLASH effect? Radiother Oncol. 2020;149:55–62. doi: 10.1016/j.radonc.2020.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KI, Tiersma J, Yuzhalin AE, Gordon-Weeks AN, Buzzelli J, Im JH, Muschel RJ. Radiation combined with macrophage depletion promotes adaptive immunity and potentiates checkpoint blockade. EMBO Molecular Medicine. 2018;10 doi: 10.15252/emmm.201809342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17:2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- Kambach DM, Sodi VL, Lelkes PI, Azizkhan-Clifford J, Reginato MJ. ErbB2, FoxM1 and 14-3-3zeta prime breast cancer cells for invasion in response to ionizing radiation. Oncogene. 2014;33(5):589–598. doi: 10.1038/onc.2012.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan HS, Murphy ED. The effect of local roentgen irradiation on the biological behavior of a transplantable mouse carcinoma; increased frequency of pulmonary metastasis. J Natl Cancer Inst. 1949;9(5-6):407–413. [PubMed] [Google Scholar]
- Khan S, Bassenne M, Wang J, Manjappa R, Melemenidis S, Breitkreutz DY, Maxim PG, Xing L, Loo BW, Jr, Pratx G. Multicellular Spheroids as In Vitro Models of Oxygen Depletion During FLASH Irradiation. Int J Radiat Oncol Biol Phys. 2021 doi: 10.1016/j.ijrobp.2021.01.050. [DOI] [PubMed] [Google Scholar]
- Konradsson E, Arendt ML, Bastholm Jensen K, Børresen B, Hansen AE, Bäck S, Kristensen AT, Munck af Rosenschöld P, Ceberg C, Petersson K. Establishment and Initial Experience of Clinical FLASH Radiotherapy in Canine Cancer Patients [Original Research] Frontiers in Oncology. 2021;11(1727) doi: 10.3389/fonc.2021.658004. [DOI] [PMC free article] [PubMed] [Google Scholar]; Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes & cancer. 2011;2:1117–1133. doi: 10.1177/1947601911423654. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo P, Le QT. Galectin-1 links tumor hypoxia and radiotherapy. Glycobiology. 2014;24:921–925. doi: 10.1093/glycob/cwu062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarbe R, Hotoiu L, Barbier J, Favaudon V. A physicochemical model of reaction kinetics supports peroxyl radical recombination as the main determinant of the FLASH effect. Radiother Oncol. 2020;153:303–310. doi: 10.1016/j.radonc.2020.06.001. [DOI] [PubMed] [Google Scholar]
- LaGory EL, Giaccia AJ. The ever-expanding role of HIF in tumour and stromal biology. Nat Cell Biol. 2016;18(4):356–365. doi: 10.1038/ncb3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Jia X, Chi Y. Modeling the effect of oxygen on the chemical stage of water radiolysis using GPU-based microscopic Monte Carlo simulations, with an application in FLASH radiotherapy. Phys Med Biol. 2021;66(2):025004. doi: 10.1088/1361-6560/abc93b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y, Moustafa M, Knoll M, Xu C, Furkel J, Lazorchak A, Yeung TL, Hasheminasab SM, Jenkins MH, Meister S, et al. Simultaneous targeting of TGF-beta/PD-L1 synergizes with radiotherapy by reprogramming the tumor microenvironment to overcome immune evasion. Cancer Cell. 2021 doi: 10.1016/j.ccell.2021.08.008. [DOI] [PubMed] [Google Scholar]
- Le Q-T, Shi G, Cao H, Nelson DW, Wang Y, Chen EY, Zhao S, Kong C, Richardson D, O’Byrne KJ, et al. Galectin-1: A Link Between Tumor Hypoxia and Tumor Immune Privilege. Journal of Clinical Oncology. 2005;23:8932–8941. doi: 10.1200/JCO.2005.02.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Lee J, Farquhar KS, Yun J, Frankenberger CA, Bevilacqua E, Yeung K, Kim EJ, Balazsi G, Rosner MR. Network of mutually repressive metastasis regulators can promote cell heterogeneity and metastatic transitions. Proc Natl Acad Sci U S A. 2014;111(3):E364–373. doi: 10.1073/pnas.1304840111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Yesilkanal AE, Wynne JP, Frankenberger C, Liu J, Yan J, Elbaz M, Rabe DC, Rustandy FD, Tiwari P, et al. Effective breast cancer combination therapy targeting BACH1 and mitochondrial metabolism. Nature. 2019;568(7751):254–258. doi: 10.1038/s41586-019-1005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung TH-Y, Tang HW-M, Siu MK-Y, Chan DW, Chan KK-L, Cheung AN-Y, Ngan HY-S. Human papillomavirus E6 protein enriches the CD55(+) population in cervical cancer cells, promoting radioresistance and cancer aggressiveness. The Journal of Pathology. 2018;244:151–163. doi: 10.1002/path.4991. [DOI] [PubMed] [Google Scholar]
- Levy K, Natarajan S, Wang J, Chow S, Eggold JT, Loo PE, Manjappa R, Melemenidis S, Lartey FM, Schuler E, et al. Abdominal FLASH irradiation reduces radiation-induced gastrointestinal toxicity for the treatment of ovarian cancer in mice. Sci Rep. 2020;10(1):21600. doi: 10.1038/s41598-020-78017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew H, Mein S, Dokic I, Haberer T, Debus J, Abdollahi A, Mairani A. Deciphering Time-Dependent DNA Damage Complexity, Repair, and Oxygen Tension: A Mechanistic Model for FLASH-Dose-Rate Radiation Therapy. Int J Radiat Oncol Biol Phys. 2021 doi: 10.1016/j.ijrobp.2020.12.048. [DOI] [PubMed] [Google Scholar]
- Lignitto L, Le Boeuf SE, Homer H, Jiang S, Askenazi M, Karakousi TR, Pass HI, Bhutkar AJ, Tsirigos A, Ueberheide B, et al. Nrf2 Activation Promotes Lung Cancer Metastasis by Inhibiting the Degradation of Bach1. Cell. 2019;178(2):316–329.:e318. doi: 10.1016/j.cell.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo BW, Schuler E, Lartey FM, Rafat M, King GJ, Trovati S, Koong AC, Maxim PG. (P003) Delivery of Ultra-Rapid Flash Radiation Therapy and Demonstration of Normal Tissue Sparing After Abdominal Irradiation of Mice. International Journal of Radiation Oncology • Biology • Physics. 2017;98(2):E16. [Google Scholar]
- Lyng H, Vorren AO, Sundfor K, Taksdal I, Lien HH, Kaalhus O, Rofstad EK. Assessment of tumor oxygenation in human cervical carcinoma by use of dynamic Gd-DTPA-enhanced MR imaging. J Magn Reson Imaging. 2001;14(6):750–756. doi: 10.1002/jmri.10016. [DOI] [PubMed] [Google Scholar]
- Ma Y, Conforti R, Aymeric L, Locher C, Kepp O, Kroemer G, Zitvogel L. How to improve the immunogenicity of chemotherapy and radiotherapy. Cancer Metastasis Rev. 2011;30(1):71–82. doi: 10.1007/s10555-011-9283-2. [DOI] [PubMed] [Google Scholar]
- Martin M, Vozenin MC, Gault N, Crechet F, Pfarr CM, Lefaix JL. Coactivation of AP-1 activity and TGF-beta1 gene expression in the stress response of normal skin cells to ionizing radiation. Oncogene. 1997;15(8):981–989. doi: 10.1038/sj.onc.1201433. [DOI] [PubMed] [Google Scholar]
- Martin OA, Anderson RL, Russell PA, Cox RA, Ivashkevich A, Swierczak A, Doherty JP, Jacobs DH, Smith J, Siva S, et al. Mobilization of viable tumor cells into the circulation during radiation therapy. Int J Radiat Oncol Biol Phys. 2014;88(2):395–403. doi: 10.1016/j.ijrobp.2013.10.033. [DOI] [PubMed] [Google Scholar]
- Matuleviciute R, Cunha PP, Johnson RS, Foskolou IP. Oxygen regulation of TET enzymes. The FEBS Journalfebs. 2021:15695. doi: 10.1111/febs.15695. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- McCarthy HO, Yakkundi A, McErlane V, Hughes CM, Keilty G, Murray M, Patterson LH, Hirst DG, McKeown SR, Robson T. Bioreductive GDEPT using cytochrome P450 3A4 in combination with AQ4N. Cancer Gene Ther. 2003;10(1):40–48. doi: 10.1038/sj.cgt.7700522. [DOI] [PubMed] [Google Scholar]
- McDonald JT, Kim K, Norris AJ, Vlashi E, Phillips TM, Lagadec C, Della Donna L, Ratikan J, Szelag H, Hlatky L, et al. Ionizing radiation activates the Nrf2 antioxidant response. Cancer Res. 2010;70(21):8886–8895. doi: 10.1158/0008-5472.CAN-10-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McErlane V, Yakkundi A, McCarthy HO, Hughes CM, Patterson LH, Hirst DG, Robson T, McKeown SR. A cytochrome P450 2B6 meditated gene therapy strategy to enhance the effects of radiation or cyclophosphamide when combined with the bioreductive drug AQ4N. J Gene Med. 2005;7(7):851–859. doi: 10.1002/jgm.728. [DOI] [PubMed] [Google Scholar]
- McKeown SR, Cowen RL, Williams KJ. Bioreductive drugs: from concept to clinic. Clin Oncol (R Coll Radiol) 2007;19(6):427–442. doi: 10.1016/j.clon.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Mehibel M, Xu Y, Li CG, Moon EJ, Thakkar KN, Diep AN, Kim RK, Bloomstein JD, Xiao Y, Bacal J, et al. Eliminating hypoxic tumor cells improves response to PARP inhibitors in homologous recombination-deficient cancer models. J Clin Invest. 2021;131(11) doi: 10.1172/JCI146256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijaljica D, Prescott M, Devenish RJ. The intricacy of nuclear membrane dynamics during nucleophagy. Nucleus. 2010 doi: 10.4161/nucl.1.3.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milas L, Hunter N, Peters LJ. The tumor bed effect: dependence of tumor take, growth rate, and metastasis on the time interval between irradiation and tumor cell transplantation. Int J Radiat Oncol Biol Phys. 1987;13(3):379–383. doi: 10.1016/0360-3016(87)90012-5. [DOI] [PubMed] [Google Scholar]
- Mistry IN, Thomas M, Calder EDD, Conway SJ, Hammond EM. Clinical Advances of Hypoxia-Activated Prodrugs in Combination With Radiation Therapy. Int J Radiat Oncol Biol Phys. 2017;98(5):1183–1196. doi: 10.1016/j.ijrobp.2017.03.024. [DOI] [PubMed] [Google Scholar]
- Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5(5):429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Montay-Gruel P, Acharya MM, Goncalves Jorge P, Petit B, Petridis IG, Fuchs P, Leavitt R, Petersson K, Gondre M, Ollivier J, et al. Hypofractionated FLASH-RT as an Effective Treatment against Glioblastoma that Reduces Neurocognitive Side Effects in Mice. Clin Cancer Res. 2021;27(3):775–784. doi: 10.1158/1078-0432.CCR-20-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montay-Gruel P, Acharya MM, Petersson K, Alikhani L, Yakkala C, Allen BD, Ollivier J, Petit B, Jorge PG, Syage AR, et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc Natl Acad Sci U S A. 2019;116(22):10943–10951. doi: 10.1073/pnas.1901777116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montay-Gruel P, Bouchet A, Jaccard M, Patin D, Serduc R, Aim W, Petersson K, Petit B, Bailat C, Bourhis J, et al. X-rays can trigger the FLASH effect: Ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother Oncol. 2018 doi: 10.1016/j.radonc.2018.08.016. [DOI] [PubMed] [Google Scholar]
- Montay-Gruel P, Petersson K, Jaccard M, Boivin G, Germond JF, Petit B, Doenlen R, Favaudon V, Bochud F, Bailat C, et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100Gy/s. Radiother Oncol. 2017;124(3):365–369. doi: 10.1016/j.radonc.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Moon EJ, Giaccia A. Dual roles of NRF2 in tumor prevention and progression: possible implications in cancer treatment. Free Radic Biol Med. 2015;79:292–299. doi: 10.1016/j.freeradbiomed.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon EJ, Mello SS, Li CG, Chi JT, Thakkar K, Kirkland JG, Lagory EL, Lee IJ, Diep AN, Miao Y, et al. The HIF target MAFF promotes tumor invasion and metastasis through IL11 and STAT3 signaling. Nat Commun. 2021;12(1):4308. doi: 10.1038/s41467-021-24631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy A, Gerber SA, Koch CJ, Lord EM. Intratumoral Hypoxia Reduces IFN-gamma-Mediated Immunity and MHC Class I Induction in a Preclinical Tumor Model. Immunohorizons. 2019;3(4):149–160. doi: 10.4049/immunohorizons.1900017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambiar DK, Aguilera T, Cao H, Kwok S, Kong C, Bloomstein J, Wang Z, Rangan VS, Jiang D, von Eyben R, et al. Galectin-1–driven T cell exclusion in the tumor endothelium promotes immunotherapy resistance. Journal of Clinical Investigation. 2019;129:5553–5567. doi: 10.1172/JCI129025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. Journal of Experimental Medicine. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordsmark M, Bentzen SM, Rudat V, Brizel D, Lartigau E, Stadler P, Becker A, Adam M, Molls M, Dunst J, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol. 2005;77(1):18–24. doi: 10.1016/j.radonc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- Nordsmark M, Loncaster J, Aquino-Parsons C, Chou SC, Gebski V, West C, Lindegaard JC, Havsteen H, Davidson SE, Hunter R, et al. The prognostic value of pimonidazole and tumour pO2 in human cervix carcinomas after radiation therapy: a prospective international multi-center study. Radiother Oncol. 2006;80(2):123–131. doi: 10.1016/j.radonc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Nordsmark M, Loncaster J, Chou SC, Havsteen H, Lindegaard JC, Davidson SE, Varia M, West C, Hunter R, Overgaard J, et al. Invasive oxygen measurements and pimonidazole labeling in human cervix carcinoma. Int J Radiat Oncol Biol Phys. 2001;49(2):581–586. doi: 10.1016/s0360-3016(00)01493-0. [DOI] [PubMed] [Google Scholar]
- Nordsmark M, Overgaard J. Tumor hypoxia is independent of hemoglobin and prognostic for loco-regional tumor control after primary radiotherapy in advanced head and neck cancer. Acta Oncol. 2004;43(4):396–403. doi: 10.1080/02841860410026189. [DOI] [PubMed] [Google Scholar]
- Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol. 1996;41(1):31–39. doi: 10.1016/s0167-8140(96)91811-3. [DOI] [PubMed] [Google Scholar]
- Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 1996;41:31–39. doi: 10.1016/s0167-8140(96)91811-3. [DOI] [PubMed] [Google Scholar]
- O’Connor LJ, Cazares-Korner C, Saha J, Evans CN, Stratford MR, Hammond EM, Conway SJ. Design, synthesis and evaluation of molecularly targeted hypoxia-activated prodrugs. Nat Protoc. 2016;11(4):781–794. doi: 10.1038/nprot.2016.034. [DOI] [PubMed] [Google Scholar]
- O’Rourke M, Ward C, Worthington J, McKenna J, Valentine A, Robson T, Hirst DG, McKeown SR. Evaluation of the antiangiogenic potential of AQ4N. Clin Cancer Res. 2008;14(5):1502–1509. doi: 10.1158/1078-0432.CCR-07-1262. [DOI] [PubMed] [Google Scholar]
- Obeid M, Panaretakis T, Joza N, Tufi R, Tesniere A, van Endert P, Zitvogel L, Kroemer G. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14(10):1848–1850. doi: 10.1038/sj.cdd.4402201. [DOI] [PubMed] [Google Scholar]
- Olcina MM, Balanis NG, Kim RK, Aksoy BA, Kodysh J, Thompson MJ, Hammerbacher J, Graeber TG, Giaccia AJ. Mutations in an Innate Immunity Pathway Are Associated with Poor Overall Survival Outcomes and Hypoxic Signaling in Cancer. Cell Rep. 2018;25(13):3721–3732.:e3726. doi: 10.1016/j.celrep.2018.11.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olcina MM, Foskolou IP, Anbalagan S, Senra JM, Pires IM, Jiang Y, Ryan AJ, Hammond EM. Replication stress and chromatin context link ATM activation to a role in DNA replication. Molecular Cell. 2013;52:758–766. doi: 10.1016/j.molcel.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olcina MM, Kim RK, Balanis NG, Li CG, von Eyben R, Graeber TG, Ricklin D, Stucki M, Giaccia AJ. Intracellular C4BPA Levels Regulate NF-κB-Dependent Apoptosis. iScience. 2020;23 doi: 10.1016/j.isci.2020.101594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olcina MM, Kim RK, Melemenidis S, Graves EE, Giaccia AJ. The tumour microenvironment links complement system dysregulation and hypoxic signalling. British Journal of Radiology. 2019;92 doi: 10.1259/bjr.20180069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olcina MM, Melemenidis S, Nambiar DK, Kim RK, Casey KM, von Eyben R, Woodruff TM, Graves EG, Le QT, Stucki M, Giaccia AJ. Targeting C5aR1 Increases the Therapeutic Window of Radiotherapy. bioRxiv. 2020 doi: 10.1101/2020.10.27.358036. [DOI] [Google Scholar]
- Overgaard J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck - A systematic review and meta-analysis. Radiotherapy and Oncology. 2011;100:22–32. doi: 10.1016/j.radonc.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, Massague J. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133(1):66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41(4):518–528. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41:518–528. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JK, Jang SJ, Kang SW, Park S, Hwang SG, Kim WJ, Kang JH, Um HD. Establishment of animal model for the analysis of cancer cell metastasis during radiotherapy. Radiat Oncol. 2012;7:153. doi: 10.1186/1748-717X-7-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelke J, Brand M, Hans S, Hideghety K, Karsch L, Lessmann E, Lock S, Schurer M, Szabo ER, Beyreuther E. Electron dose rate and oxygen depletion protect zebrafish embryos from radiation damage. Radiother Oncol. 2021;158:7–12. doi: 10.1016/j.radonc.2021.02.003. [DOI] [PubMed] [Google Scholar]
- Petersson K, Adrian G, Butterworth K, McMahon SJ. A Quantitative Analysis of the Role of Oxygen Tension in FLASH Radiation Therapy. Int J Radiat Oncol Biol Phys. 2020 doi: 10.1016/j.ijrobp.2020.02.634. [DOI] [PubMed] [Google Scholar]
- Pilones KA, Vanpouille-Box C, Demaria S. Combination of Radiotherapy and Immune Checkpoint Inhibitors. Seminars in Radiation Oncology. 2015;25:28–33. doi: 10.1016/j.semradonc.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Pires IM, Bencokova Z, Milani M, Folkes LK, Li JL, Stratford MR, Harris AL, Hammond EM. Effects of acute versus chronic hypoxia on DNA damage responses and genomic instability. Cancer Res. 2010;70(3):925–935. doi: 10.1158/0008-5472.CAN-09-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires IM, Olcina MM, Anbalagan S, Pollard JR, Reaper PM, Charlton PA, McKenna WG, Hammond EM. Targeting radiation-resistant hypoxic tumour cells through ATR inhibition. British journal of cancer. 2012;107:291–299. doi: 10.1038/bjc.2012.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratx G, Kapp DS. A computational model of radiolytic oxygen depletion during FLASH irradiation and its effect on the oxygen enhancement ratio. Phys Med Biol. 2019;64(18):185005. doi: 10.1088/1361-6560/ab3769. [DOI] [PubMed] [Google Scholar]
- Qiang L, Shah P, Barcellos-Hoff MH, He YY. TGF-beta signaling links E-cadherin loss to suppression of nucleotide excision repair. Oncogene. 2016;35(25):3293–3302. doi: 10.1038/onc.2015.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafat M, Aguilera TA, Vilalta M, Bronsart LL, Soto LA, von Eyben R, Golla MA, Ahrari Y, Melemenidis S, Afghahi A, et al. Macrophages Promote Circulating Tumor Cell-Mediated Local Recurrence following Radiotherapy in Immunosuppressed Patients. Cancer Res. 2018;78(15):4241–4252. doi: 10.1158/0008-5472.CAN-17-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran S, Ma TS, Griffin J, Ng N, Foskolou IP, Hwang MS, Victori P, Cheng WC, Buffa FM, Leszczynska KB, et al. Hypoxia-induced SETX links replication stress with the unfolded protein response. Nat Commun. 2021;12(1):3686. doi: 10.1038/s41467-021-24066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science. 2016;352:175–180. doi: 10.1126/science.aaf4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riekki R, Jukkola A, Sassi ML, Hoyhtya M, Kallioinen M, Risteli J, Oikarinen A. Modulation of skin collagen metabolism by irradiation: collagen synthesis is increased in irradiated human skin. Br J Dermatol. 2000;142(5):874–880. doi: 10.1046/j.1365-2133.2000.03465.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Ruiz ME, Rodriguez I, Mayorga L, Labiano T, Barbes B, Etxeberria I, Ponz-Sarvise M, Azpilikueta A, Bolanos E, Sanmamed MF, et al. TGFbeta Blockade Enhances Radiotherapy Abscopal Efficacy Effects in Combination with Anti-PD1 and Anti-CD137 Immunostimulatory Monoclonal Antibodies. Mol Cancer Ther. 2019;18(3):621–631. doi: 10.1158/1535-7163.MCT-18-0558. [DOI] [PubMed] [Google Scholar]
- Rofstad EK, Mathiesen B, Henriksen K, Kindem K, Galappathi K. The tumor bed effect: increased metastatic dissemination from hypoxia-induced up-regulation of metastasis-promoting gene products. Cancer Res. 2005;65(6):2387–2396. doi: 10.1158/0008-5472.CAN-04-3039. [DOI] [PubMed] [Google Scholar]
- Rothwell BC, Kirkby NF, Merchant MJ, Chadwick AL, Lowe M, Mackay RI, Hendry JH, Kirkby KJ. Determining the parameter space for effective oxygen depletion for FLASH radiation therapy. Phys Med Biol. 2021 doi: 10.1088/1361-6560/abe2ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumenina LT, Daugan MV, Petitprez F, Sautès-Fridman C, Fridman WH. Context-dependent roles of complement in cancer. Nature Reviews Cancer. 2019 doi: 10.1038/s41568-019-0210-0. [DOI] [PubMed] [Google Scholar]
- Rube CE, Uthe D, Schmid KW, Richter KD, Wessel J, Schuck A, Willich N, Rube C. Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int J Radiat Oncol Biol Phys. 2000;47(4):1033–1042. doi: 10.1016/s0360-3016(00)00482-x. [DOI] [PubMed] [Google Scholar]
- Shimura T, Sasatani M, Kawai H, Kamiya K, Kobayashi J, Komatsu K, Kunugita N. Radiation-Induced Myofibroblasts Promote Tumor Growth via Mitochondrial ROS-Activated TGFbeta Signaling. Mol Cancer Res. 2018;16(11):1676–1686. doi: 10.1158/1541-7786.MCR-18-0321. [DOI] [PubMed] [Google Scholar]
- Simmons DA, Lartey FM, Schuler E, Rafat M, King G, Kim A, Ko R, Semaan S, Gonzalez S, Jenkins M, et al. Reduced cognitive deficits after FLASH irradiation of whole mouse brain are associated with less hippocampal dendritic spine loss and neuroinflammation. Radiother Oncol. 2019;139:4–10. doi: 10.1016/j.radonc.2019.06.006. [DOI] [PubMed] [Google Scholar]
- Skwarska A, Calder EDD, Sneddon D, Bolland H, Odyniec ML, Mistry IN, Martin J, Folkes LK, Conway SJ, Hammond EM. Development and pre-clinical testing of a novel hypoxia-activated KDAC inhibitor. Cell Chem Biol. 2021;28(9):1258–1270.:e1213. doi: 10.1016/j.chembiol.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen BS, Horsman MR. Tumor Hypoxia: Impact on Radiation Therapy and Molecular Pathways. Front Oncol. 2020;10:562. doi: 10.3389/fonc.2020.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto LA, Casey KM, Wang J, Blaney A, Manjappa R, Breitkreutz D, Skinner L, Dutt S, Ko RB, Bush K, et al. FLASH Irradiation Results in Reduced Severe Skin Toxicity Compared to Conventional-Dose-Rate Irradiation. Radiat Res. 2020;194(6):618–624. doi: 10.1667/RADE-20-00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz DR, Buettner GR, Petronek MS, St-Aubin JJ, Flynn RT, Waldron TJ, Limoli CL. An integrated physico-chemical approach for explaining the differential impact of FLASH versus conventional dose rate irradiation on cancer and normal tissue responses. Radiother Oncol. 2019;139:23–27. doi: 10.1016/j.radonc.2019.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenstrom KW, Vermund H, Mosser DG, Marvin JF. Effects of roentgen irradiation on the tumor bed. I. The inhibiting action of local pretransplantation roentgen irradiation (1500 r alpha) on the growth of mouse mammary carcinoma. Radiat Res. 1955;2(2):180–191. [PubMed] [Google Scholar]
- Sun M, Song CX, Huang H, Frankenberger CA, Sankarasharma D, Gomes S, Chen P, Chen J, Chada KK, He C, et al. HMGA2/TET1/HOXA9 signaling pathway regulates breast cancer growth and metastasis. Proc Natl Acad Sci U S A. 2013;110(24):9920–9925. doi: 10.1073/pnas.1305172110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundahl N, Duprez F, Ost P, De Neve W, Mareel M. Effects of radiation on the metastatic process. Mol Med. 2018;24(1):16. doi: 10.1186/s10020-018-0015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surace L, Lysenko V, Fontana AO, Cecconi V, Janssen H, Bicvic A, Okoniewski M, Pruschy M, Dummer R, Neefjes J, et al. Complement Is a Central Mediator of Radiotherapy-Induced Tumor-Specific Immunity and Clinical Response. Immunity. 2015;42:767–777. doi: 10.1016/j.immuni.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Sørensen BS, Horsman MR. Tumor Hypoxia. Impact on Radiation Therapy and Molecular Pathways Frontiers in Oncology. 2020;10:562. doi: 10.3389/fonc.2020.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaes RDW, Hendriks LEL, Vooijs M, De Ruysscher D. Biomarkers of Radiotherapy-Induced Immunogenic Cell Death. Cells. 2021;10(4) doi: 10.3390/cells10040930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Uden P, Kenneth NS, Rocha S. Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem J. 2008;412(3):477–484. doi: 10.1042/BJ20080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, Demaria S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nature communications. 2017 doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilalta M, Rafat M, Giaccia AJ, Graves EE. Recruitment of circulating breast cancer cells is stimulated by radiotherapy. Cell Rep. 2014;8(2):402–409. doi: 10.1016/j.celrep.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Essen CF. Radiation enhancement of metastasis: a review. Clin Exp Metastasis. 1991;9(2):77–104. doi: 10.1007/BF01756381. [DOI] [PubMed] [Google Scholar]
- Vozenin MC, De Fornel P, Petersson K, Favaudon V, Jaccard M, Germond JF, Petit B, Burki M, Ferrand G, Patin D, et al. The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients. Clin Cancer Res. 2019;25(1):35–42. doi: 10.1158/1078-0432.CCR-17-3375. [DOI] [PubMed] [Google Scholar]
- Vozenin MC, Hendry JH, Limoli CL. Biological Benefits of Ultra-high Dose Rate FLASH Radiotherapy: Sleeping Beauty Awoken. Clin Oncol (R Coll Radiol) 2019;31(7):407–415. doi: 10.1016/j.clon.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Genetics. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Meng A, Lang H, Brown SA, Konopa JL, Kindy MS, Schmiedt RA, Thompson JS, Zhou D. Activation of nuclear factor kappaB In vivo selectively protects the murine small intestine against ionizing radiation-induced damage. Cancer research. 2004;64:6240–6246. doi: 10.1158/0008-5472.CAN-04-0591. [DOI] [PubMed] [Google Scholar]
- Wardman P. Radiotherapy Using High-Intensity Pulsed Radiation Beams (FLASH): A Radiation-Chemical Perspective. Radiat Res. 2020;194(6):607–617. doi: 10.1667/RADE-19-00016. [DOI] [PubMed] [Google Scholar]
- Weber AM, Ryan AJ. ATM and ATR as therapeutic targets in cancer. Pharmacology and Therapeutics. 2015;149:124–138. doi: 10.1016/j.pharmthera.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Weiss H, Epp ER, Heslin JM, Ling CC, Santomasso A. Oxygen depletion in cells irradiated at ultra-high dose-rates and at conventional dose-rates. Int J Radiat Biol Relat Stud Phys Chem Med. 1974;26(1):17–29. doi: 10.1080/09553007414550901. [DOI] [PubMed] [Google Scholar]
- Westendorf AM, Skibbe K, Adamczyk A, Buer J, Geffers R, Hansen W, Pastille E, Jendrossek V. Hypoxia Enhances Immunosuppression by Inhibiting CD4+ Effector T Cell Function and Promoting Treg Activity. Cell Physiol Biochem. 2017;41(4):1271–1284. doi: 10.1159/000464429. [DOI] [PubMed] [Google Scholar]
- Wild-Bode C, Weller M, Rimner A, Dichgans J, Wick W. Sublethal irradiation promotes migration and invasiveness of glioma cells: implications for radiotherapy of human glioblastoma. Cancer Res. 2001;61(6):2744–2750. [PubMed] [Google Scholar]
- Williams KJ, Telfer BA, Xenaki D, Sheridan MR, Desbaillets I, Peters HJ, Honess D, Harris AL, Dachs GU, van der Kogel A, et al. Enhanced response to radiotherapy in tumours deficient in the function of hypoxia-inducible factor-1. Radiother Oncol. 2005;75(1):89–98. doi: 10.1016/j.radonc.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Wilson JD, Hammond EM, Higgins GS, Petersson K. Ultra-High Dose Rate (FLASH) Radiotherapy: Silver Bullet or Fool’s Gold? Front Oncol. 2019;9:1563. doi: 10.3389/fonc.2019.01563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- Winter SC, Buffa FM, Silva P, Miller C, Valentine HR, Turley H, Shah KA, Cox GJ, Corbridge RJ, Homer JJ, et al. Relation of a hypoxia metagene derived from head and neck cancer to prognosis of multiple cancers. Cancer Res. 2007;67(7):3441–3449. doi: 10.1158/0008-5472.CAN-06-3322. [DOI] [PubMed] [Google Scholar]
- Wirsdorfer F, Cappuccini F, Niazman M, de Leve S, Westendorf AM, Ludemann L, Stuschke M, Jendrossek V. Thorax irradiation triggers a local and systemic accumulation of immunosuppressive CD4+ FoxP3+ regulatory T cells. Radiat Oncol. 2014;9:98. doi: 10.1186/1748-717X-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nature Reviews Cancer. 2008;8:851–864. doi: 10.1038/nrc2501. [DOI] [PubMed] [Google Scholar]
- Yan M, Jene N, Byrne D, Millar EK, O’Toole SA, McNeil CM, Bates GJ, Harris AL, Banham AH, Sutherland RL, et al. Recruitment of regulatory T cells is correlated with hypoxia-induced CXCR4 expression, and is associated with poor prognosis in basal-like breast cancers. Breast Cancer Res. 2011;13(2):R47. doi: 10.1186/bcr2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Staples O, Thomas LW, Briston T, Robson M, Poon E, Simoes ML, El-Emir E, Buffa FM, Ahmed A, et al. Human CHCHD4 mitochondrial proteins regulate cellular oxygen consumption rate and metabolism and provide a critical role in hypoxia signaling and tumor progression. J Clin Invest. 2012;122(2):600–611. doi: 10.1172/JCI58780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Itasaka S, Harada H, Hiraoka M. Microenvironment and radiation therapy. BioMed Research International. 2013;2013 doi: 10.1155/2013/685308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackrisson B, Franzen L, Henriksson R, Littbrand B, Stratford M, Dennis M, Rojas AM, Denekamp J. Acute effects of accelerated radiotherapy in combination with carbogen breathing and nicotinamide (ARCON) Acta Oncol. 1994;33(4):377–381. doi: 10.3109/02841869409098432. [DOI] [PubMed] [Google Scholar]
- Zannella VE, Dal Pra A, Muaddi H, Mc Kee TD, Stapleton S, Sykes J, Glicksman R, Chaib S, Zamiara P, Milosevic M, et al. Reprogramming metabolism with metformin improves tumor oxygenation and radiotherapy response. Clin Cancer Res. 2013;19(24):6741–6750. doi: 10.1158/1078-0432.CCR-13-1787. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wong CC, Wei H, Gilkes DM, Korangath P, Chaturvedi P, Schito L, Chen J, Krishnamachary B, Winnard PT, Jr, et al. HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene. 2012;31(14):1757–1770. doi: 10.1038/onc.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang Q, Cascio E, Li C, Yang Q, Gerweck LE, Huang P, Gottschalk B, Flanz J, Schuemann J. FLASH Investigations Using Protons: Design of Delivery System, Preclinical Setup and Confirmation of FLASH Effect with Protons in Animal Systems. Radiat Res. 2020;194(6):656–664. doi: 10.1667/RADE-20-00068.1. [DOI] [PubMed] [Google Scholar]
- Zhou S, Zheng D, Fan Q, Yan Y, Wang S, Lei Y, Besemer A, Zhou C, Enke C. Minimum dose rate estimation for pulsed FLASH radiotherapy: A dimensional analysis. Med Phys. 2020;47(7):3243–3249. doi: 10.1002/mp.14181. [DOI] [PubMed] [Google Scholar]
- Zhu M, Yang M, Zhang J, Yin Y, Fan X, Zhang Y, Qin S, Zhang H, Yu F. Immunogenic Cell Death Induction by Ionizing Radiation. Front Immunol. 2021;12:705361. doi: 10.3389/fimmu.2021.705361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlobinskaya O, Siebenwirth C, Greubel C, Hable V, Hertenberger R, Humble N, Reinhardt S, Michalski D, Roper B, Multhoff G, et al. The effects of ultra-high dose rate proton irradiation on growth delay in the treatment of human tumor xenografts in nude mice. Radiat Res. 2014;181(2):177–183. doi: 10.1667/RR13464.1. [DOI] [PubMed] [Google Scholar]