Abstract
In December 2019, an outbreak of pneumonia caused by a novel Coronavirus (COVID-19) spread rapidly worldwide. Although the clinical manifestations of COVID-19 are dominated by respiratory symptoms, the cardiovascular system is extensively affected at multiple levels. Due to the unprecedented consequences of the COVID-19 pandemic, the ARTERY society decided to launch the Covid-19 effects on ARTErial StIffness and vascular AgeiNg (CARTESIAN) study — the first international multicentre study into the effects of COVID-19 on non-invasive biomarkers of vascular ageing. The main study objective is to evaluate the presence of Early Vascular Ageing (EVA) 6 and 12 months after COVID-19 infection. Secondary objectives are to study the effect of COVID-19 disease severity on EVA, to investigate the role of psychosocial factors in COVID-19 induced EVA, and to investigate the potential modifying effect of comorbidities and chronic treatments. In the CARTESIAN study, a broad array of cardiovascular measurements, including carotid-femoral pulse wave velocity, central blood pressure, carotid ultrasound, brachial flow-mediated dilatation, will be performed. To date, 43 centres from 21 countries have agreed to participate, with an expected study population of >2500 individuals. To our knowledge, CARTESIAN will be the first study to provide insight into the relationship between COVID-19, its severity, and early vascular ageing in a large cohort, potentially enabling future care and diagnostics to be more focused on the most vulnerable.
Keywords: COVID-19, coronavirus, inflammation, vascular ageing, arterial stiffness
1. Introduction
Vascular ageing, an age-related deterioration in vascular structure and function, is an integrated marker of overall Cardiovascular (CV) risk burden on the vasculature over time. While age-dependent arterial damage typically appears in the fifth decade of life, there is strong variability between individuals with some displaying Early Vascular Ageing (EVA). Vascular ageing, driven by chronological ageing and accelerated by risk factors such as hypertension and diabetes, occurs both in the micro- and macro-vasculature: the cross-talk between the two perpetuates a vicious cycle further aggravating diabetes and hypertension, ultimately leading to CV events [1]. Life-time exposure to risk factors promotes the development and accumulation of subclinical vascular changes that directs an individual towards a trajectory of EVA [2]. This has led to the notion that vascular age may better relate to CV outcomes than chronological age [3].
In December 2019, an outbreak of pneumonia caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), occurred in Wuhan (Hubei province, China), and spread rapidly throughout China and worldwide, representing an unprecedented challenge for humanity. Although the clinical manifestations of the disease caused by SARS-CoV-2 (coronavirus disease 2019, COVID-19) are dominated by respiratory symptoms, the CV system is also extensively affected [4,5]. For this reason, the Association for Research into Arterial Structure and Physiology (ARTERY) Society, launched an initiative to study the effects of COVID-19 infection on non-invasive biomarkers of early vascular ageing. This activity is supported by the European Cooperation in Science and Technology (COST) Action CA18216 VascAgeNet [6].
2. Scientific Background
Severe acute respiratory syndrome coronavirus 2 infection affects the CV system at multiple levels. First, SARS-CoV-2 is able to directly infect endothelial cells, inducing marked endothelial damage and inflammation [7]. In autopsies from COVID-19 patients, cardiac infection was demonstrated [8]; endothelial injury and endothelialitis (with infiltration of inflammatory cells into vessel walls) was consistently present in multiple vascular beds [9]. In addition, an increase of a Kawasaki-like inflammatory syndrome, the most common primary vasculitis in children (known to be associated with aortic stiffness [10]), has also been observed [11]. These vascular changes may result from a complex process involving either disturbed cell metabolism as a result of cell infection or immune impairment mediated by cellular immunity and cytokine actions. SARS-CoV-2 is able to infect endothelial cells through Angiotensin-converting Enzyme 2 (ACE2), and downregulates ACE2 expression such that the enzyme is unable to exert protective effects, thus resulting in organ injury [12]. Impaired ACE2 activity enhances vascular inflammation and plaque formation, and increases vascular stiffness in experimental animals [13,14]. In people with diabetes and hypertension low ACE2 activity is associated with endothelial dysfunction and arterial stiffening [15], thus suggesting that accelerated vascular ageing might occur by this pathway after SARS-CoV-2 infection.
Second, COVID-19-associated inflammation may induce immune-mediated vascular damage, as already shown in a wide range of primarily inflammatory diseases [16]. Dysregulated immune response and cytokine-release syndrome, due to overactivation of innate immunity characterizing severe COVID-19, may result in indirect, immune-mediated damage to the systemic vasculature and to increased risk of CV events [17]. For example, survivors of hospitalised pneumonia have an increased risk of CV disease during up to 10-years follow-up and it is likely that cases infected via respiratory virus outbreaks will experience similar adverse outcomes [18], which could be prevented by timely vaccination [19]. COVID-19-associated severe systemic inflammation may induce immune-mediated damage to the vasculature, thus increasing long-term risk of CV events. A large body of evidence shows that accelerated vascular ageing (with arterial stiffening and increased risk of CV events) occurs in chronic inflammatory diseases [16,20,21]. Preliminary studies suggest that arterial stiffening may also occur after sepsis [16,22].
Third, COVID-19 alters metabolic and cardiac markers. Survivors of SARS-CoV infection had a markedly increased prevalence of metabolic complications, including impaired glucose metabolism and dyslipidaemia, 12 years after infection [23], possibly related to marked release of inflammatory mediators, especially interleukin (IL)-1β and tumor necrosis factor α(TNF-α), which promote systemic insulin resistance and β-cell damage [24]. Alternatively, these consequences could be related to treatments administered during the acute phase of infection, such as corticosteroids, antiviral drugs, or immunomodulator drugs [23]. The metabolic consequences of COVID-19 are still unknown, but may be similar and further accelerate vascular ageing and increase long-term CV risk. In addition, one study has reported diastolic dysfunction in 16% of patients with COVID-19 [25], consistent with previous findings in SARS-CoV patients [26]; this is compatible with our current hypotheses that SARS-CoV-2 seems to particularly affect small vessels and capillaries and that cardiac diastolic dysfunction may relate to abnormalities in the micro-circulation. Alternatively, the observed diastolic dysfunction could be attributable to myocarditis [27,28]. A significant proportion of patients have shown increased cardiac troponin-I levels, associated with worse prognosis [29,30].
Fourth, psychosocial factors may play a role in COVID-19-associated vascular ageing. These factors are increasingly recognised as a relevant risk factor to take into account in CV disease prevention [31]. A significant proportion of patients having survived COVID-19 show Post-traumatic Stress Disorder (PTSD), anxiety, depression, and insomnia [32]; this phenomenon might be amplified if the patient needed intensive care [33]. Indeed, survivors of intensive care hospitalisation have impaired quality of life [34], and roughly half of them have symptoms of depression, anxiety and PTSD, associated with increased mortality during follow-up [35]. A direct correlation between PTSD symptoms and arterial stiffness has been demonstrated in Polish survivors from deportation to Siberia in their childhood [36], suggesting that PTSD may contribute to the increased CV risk also in COVID-19 survivors. Social inequalities also play a crucial role in accelerating vascular ageing [37], and foster health disparities in COVID-19 severity and mortality [38].
Fifth, chronic or acute treatments and comorbidities may play a role in COVID-19-associated vascular ageing. Previous chronic treatments (e.g., renin–angiotensin system blockers) or treatments administered in the acute phase (such as corticosteroids, immunomodulators, antiviral drugs, and invasive respiration) may have direct consequences on vascular ageing in COVID-19 patients, being either protective or deleterious. For example, corticosteroid use is associated with better survival outcome in COVID-19 patients [39], but its chronic use has been associated with increased risk of CV events in the general population [40]. Some authors suggest that chronic use of drugs protecting the endothelium might attenuate COVID-19 severity [7,41]. Studies exploring the effect of blockers of the renin-angiotensin system on COVID-19 outcome found either a beneficial [42] or neutral impact [43,44]. Moreover, these drugs may be protective on long-term CV consequences of SARS-CoV-2 infection. CV comorbidities, including ischemic heart disease, hypertension, obesity and diabetes are the most important contributors to the severity of COVID-19 [45–47]. Impaired vascular function in these patients may play a role in worsening COVID-19 prognosis. Diabetic patients in particular, due to a compromised innate immune response, exhibit increased susceptibility and enhanced disease severity following SARS-CoV-2 infection [48].
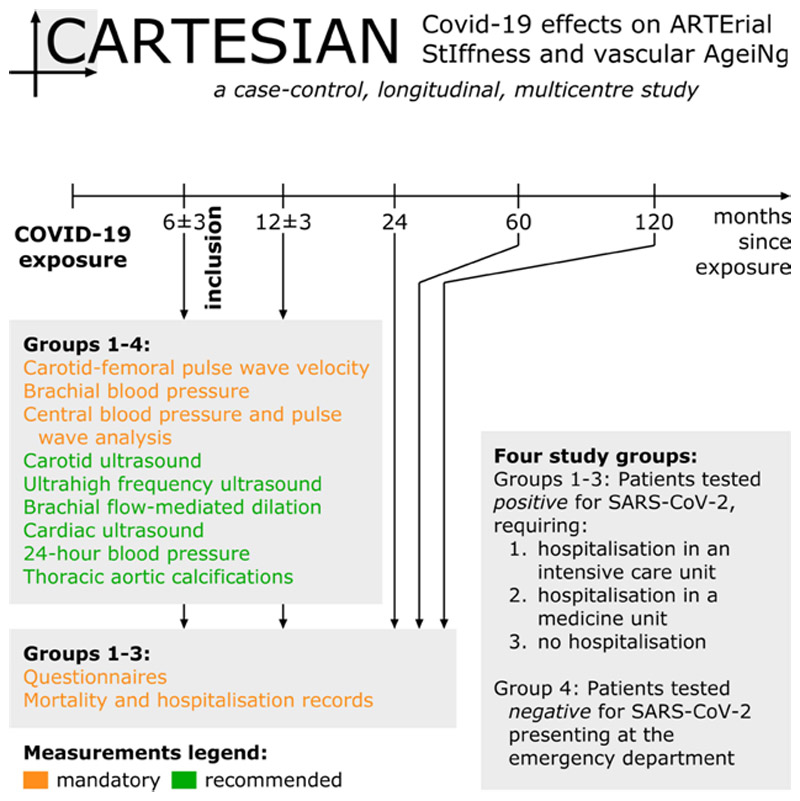
Taken together, accurate CV phenotyping associating arterial and cardiac measurements of stiffness, and structure and function tests (endothelial function), assessing both the micro- and the macro-vasculature and their cross-talk, is needed to ascertain whether COVID-19 infection is associated with EVA and to establish which mechanisms are involved in COVID-19 related accelerated vascular ageing. It is therefore that we are presenting the Covid-19 effects on ARTErial StIffness and vascular AgeiNg (CARTESIAN) study (Figure 1).
Figure 1. CARTESIAN study overview.
3. Hypotheses and Objectives
We hypothesise that accurate CV phenotyping by structural and functional tests, assessing both the micro- and the macro-vasculature and their cross-talk, will demonstrate the presence of accelerated vascular ageing in COVID-19 survivors. We further hypothesise that COVID-19-related vascular ageing will correlate with COVID-19 severity and with pre-existing cardiometabolic disease. We will also explore the role of pre-existing cardiometabolic disease; including metabolic alterations, residual chronic inflammation and psychosocial factors and the possible influence of chronic and/or acute treatments in COVID-19-associated accelerated vascular ageing.
The main objective of the CARTESIAN study is to evaluate the presence of EVA after COVID-19 infection. The primary endpoint will be carotid-femoral Pulse Wave Velocity (PWV), an established biomarker of EVA. PWV will be evaluated at 6 ± 3 and 12 ± 3 months after infection. PWV-derived vascular age and difference between chronological and vascular age will also be calculated according to comparison to reference values [49] as well as according to a more recent approach, validated for cardiovascular event prediction [50].
Secondary objectives are:
-
(1)
To investigate the presence of altered arterial properties in the carotid, radial and digital arteries by standard and ultrahigh-frequency ultrasound; endothelial dysfunction in the brachial artery by flow mediated dilation; micro-macrovasculature crosstalk alterations by wave separation/intensity analysis of aortic pressure and flow curves; 24 h-central blood pressure (in equipped centers);
-
(2)
To investigate the presence of: cardiac dysfunction by cardiac ultrasound (in equipped centers);
-
(3)
To investigate temporal evolution of vascular ageing biomarkers between 6 and 12 months in the four groups;
-
(4)
To investigate the role of psychosocial factors (including PTSD) in COVID-19-related EVA; and
-
(5)
To investigate the association with EVA of previous chronic treatments or treatments administered in the acute phase, as well as of pre-existing cardiometabolic disease.
As a pre-planned study extension, in COVID-positive patients, mortality causes and hospitalization data from electronic medical records will be collected 2, 5, and 10 years after the beginning of the study. Questionnaires to evaluate health status and psychometric tests will be also sent by email/mail.
The resulting findings have the potential to improve CV risk assessment and patient management in the long term.
4. Materials and Methods
The CARTESIAN study is a prospective, multicentre, cohort study.
4.1. Study Population
The study will include three groups of individuals with recent (6 ± 3 months) documented exposure to SARS-CoV-2 and one control group (Figure 1):
-
(1)
Patients with confirmed infection by SARS-CoV-2, requiring hospitalisation in an intensive care unit.
-
(2)
Patients with confirmed infection by SARS-CoV-2, requiring hospitalisation in a medicine unit.
-
(3)
Patients with confirmed infection by SARS-CoV-2, not requiring hospitalisation.
-
(4)
Individuals tested for SARS-CoV-2 infection, but who tested negative.
Written informed consent will be obtained from all included individuals. COVID-19 status will be assessed through Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR). A positive serology for COVID-19 will be also considered. Participants enrolled in groups 1 and 2 will be recruited during their planned follow-up visits. Group 3 will be recruited from individuals who tested positive for COVID-19 but did not need hospitalization. This may include individuals who were asymptomatic or minimally symptomatic but were picked up through routine testing. The comparison between these three groups will provide insight into the role of disease severity in COVID-19-associated vascular damage, and to assess whether milder forms of COVID-19 are associated with evidence of vascular damage. Group 4, constituted by individuals who underwent testing for SARS-CoV-2 infection (RT-PCR testing or serology) but tested negative, represents the negative control group for this study. A negative control group is crucial in this study in order to confirm the study hypothesis, i.e., the presence of accelerated vascular ageing in COVID-19 survivors. Exclusion criteria are inability to provide written consent, pregnancy or breastfeeding, diseases carrying out a life expectancy of <1 year according to clinical judgment, or any other circumstance that would preclude full participation in the study.
Comparison with data from historical cohorts has been also planned, to consolidate acquired results against an unexposed group and to permit exploration of the effects of social measures to control spread of COVID-19 on EVA.
4.2. Schedule and Study Duration
Patients will attend for their first visit 6 ± 3 months after COVID-19 exposure, and a second one 12 ± 3 months thereafter. At both visits, CV measurements will be taken for all four groups (Figure 1). Questionnaires will be completed and outcomes and hospitalisations recorded at these visits, as well as 24, 48, and 120 months after recruitment. The duration of participation for each participant is thus 6 months for the main study and 10 years for the pre-planned study extension.
4.3. Measurements
To ensure a broad inclusion of centres, only a core set of measurements (carotid-femoral PWV, brachial and central blood pressure) is considered mandatory, whereas the majority of the measurements described below are optional but recommended (Figure 1). An overview of all measurements and parameters is given in Table 1.
Table 1. Overview of measured parameters.
| Measurement | Location | Derived parameters | Mandatory? |
|---|---|---|---|
| Applanation tonometry | Carotid, femoral | PWV; carotid PP– * | Yes |
| Peripheral blood pressure | Brachial | SBP/DBP/MAP | Yes |
| Central blood pressure | Radial (recording site) | SBP/DBP; AP; AIx, AIx@75; RM; P f; P b | Yes |
| Ultrasound | Carotid | IMT; A s; A d; DC; PWVcar | No |
| Ultrasound | Brachial | FMD: ∆d/d 0; ∆Q/Q 0 | No |
| Ultrasound | Cardiac | LVMI; E/e’; LAVI; TR; SV; CO; CI; proximal aortic A s/A d/DC | |
| Ultrahigh frequency ultrasound | Carotid, radial, digital | IMT; A s/A d/DC | No |
| 24-h blood pressure | Brachial, central | Brachial SBP/DBP/MAP; central SBP/DBP; AP; AIx, AIx@75; RM; P f; P b | No |
| Computed tomography | Thorax | Aortic calcification score; CAC | No |
| Questionnaires | – | SF-12; ISI; HADS; DSM-5; EPICES; CBS | No |
Carotid PP measurement is optional and to be used together with carotid ultrasound measurements to compute local carotid distensibility. PP, pulse pressure; SBP/DBP, systolic/diastolic blood pressure; MAP, mean arterial pressure; AP, augmentation pressure; AIx, augmentation index; AIx@75, AIx normalised for a heart rate of 75 beats per minute; RM, reflection magnitude; P f and P b, absolute amplitude of the forward and backward wave; IMT, intima-media thickness; A s/A d, systolic and diastolic cross-sectional area; DC, distensibility coefficient; PWVcar, local carotid PWV; FMD, flow-mediated dilation; ∆d/d 0, relative flow-mediated diameter increase; ∆Q/Q 0, relative flow increase; CAC, coronary artery calcium; LVMI, left ventricular mass index; E/e’, ratio between early mitral inflow velocity and mitral annular early diastolic velocity; LAVI, left atrial volume index; TR, tricuspid regurgitation; SV, stroke volume; CO, cardiac output; CI, cardiac index; SF-12, 12-item short form survey score; ISI, insomnia severity index; HADS, hospital anxiety and depression scale; DSM-5, diagnostic and statistical manual of mental disorders scale; EPICES, evaluation de la précarité et des inégalités de santé dans les centres d’examens de santé/evaluation of deprivation and inequalities in health examination centres; CBS, caregiver burden scale.
4.3.1. Carotid-femoral pulse wave velocity
Large artery stiffness will be assessed by carotid-femoral PWV [51]. Waveforms will be recorded at the femoral and carotid site, using validated devices, such as SphygmoCor (AtCor Medical, Sydney, NSW, Australia), Complior (Alam Medical, Vincennes, France), PulsePen (PulsePen, DiaTecne, Milan, Italy), or Vicorder (Skidmore Medical, Bristol, UK). Briefly, a probe or cuff is placed on the selected artery while 10–15 subsequent heartbeats are recorded. PWV is calculated from the pulse Transit Time (TT) using the 80% of direct distance method, as
| (1) |
with d the surface distance between the two recording sites [52].
4.3.2. Brachial blood pressure
Brachial blood pressure will be measured using a validated device according to the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) protocol [53]. Three measurements should be performed in the supine position at the non-dominant arm at intervals of 2 min after 10 min of rest. Blood pressure will also be measured in the supine posture directly before starting the PWV measurement.
4.3.3. Central blood pressure and micro-macrocirculation cross-talk
Peripheral pressure waveforms will be recorded from the radial artery at the wrist, using applanation tonometry with a high-fidelity tonometer. After 20 sequential high-quality waveforms have been acquired, a validated, Federal Drug Administration (FDA)-approved generalised transfer function will be used to generate the corresponding central aortic pressures and pressure waveforms (SphygmoCor). Waveforms will be calibrated with brachial mean and diastolic blood pressure. Blood pressure will be measured with a validated, automated, oscillometric, sphygmomanometer and mean blood pressure calculated as diastolic blood pressure + 0.4 * pulse pressure.
Augmentation Pressure (AP) — the difference between the second and first systolic peak will be determined. Augmentation Index (AIx) will be calculated as the ratio of AP to Pulse Pressure (PP), expressed as a percentage. In addition, an index normalised for a heart rate of 75 beats per minute (AIx@75) will be calculated.
Micro-macrocirculation cross-talk will be assessed by wave separation analysis [54]. The absolute amplitude of the antegrade and retrograde pressure waves will be quantified using the ARCSolver method [55] from measured pressure waveforms and modelled flow waveforms. Reflection magnitude, as well as absolute amplitude of the forward and backward wave, will be calculated.
4.3.4. Carotid ultrasound
Carotid ultrasound scans will be obtained by high-resolution B-mode ultrasound by a ≥7.5 MHz linear array transducer by a trained operator. The systolic and diastolic left and right carotid diameters will be automatically measured on the distal wall 1–2 cm proximal to the bifurcation by a real-time computerised contour tracking system “Cardiovascular Suite” (Quipu srl, Pisa, Italy). Cross-sectional Distensibility Coefficient (DC) will be estimated through the variations in arterial cross-sectional area and pulse pressure. DC will be calculated as
| (2) |
where A d is the diastolic lumen area, ∆A is the stroke change in lumen area (A s − A d with A s the systolic lumen area), and ∆P is the local pulse pressure. Lumen area and stroke change in lumen area will be calculated from diameter and distension values, assuming the cross section of the artery to be circular. Local carotid pulse pressure will be acquired by applanation tonometry (SphygmoCor). The carotid tonometer trace will be calibrated with brachial diastolic and mean blood pressure (calculated as diastolic blood pressure + 0.4 * pulse pressure). Finally, DC will be converted into local, carotid PWV (PWVcar) by using [56]:
| (3) |
where ρ is the blood mass density, assumed to be 1050 kg/m3. Common carotid Intima-media Thickness (IMT) will be automatically and simultaneously measured as the mean of the relative values of the last 10 s on the same image sequences. IMT of the far wall of the common carotid artery will be measured over a region 1 cm proximal to the carotid bulb [57– 59].
4.3.5. Carotid, radial, and digital ultrahigh frequency ultrasound
Carotid, radial, and digital remodelling (diameter and thickness) and distensibility will be assessed by ultrahigh frequency ultrasound by Vevo® MD (FUJIFILM VisualSonics Inc., Toronto, ON, Canada) in equipped centres. This high-frequency ultrasound machine is equipped with three probes operating at 22, 48, 70 MHz, with a spatial resolution of 100, 50, 30 μm, respectively. Edge detection and contour tracking techniques will be applied on high-resolution ultrasound images (Cardiovascular Suite, Quipu srl), in order to obtain arterial diameter, thickness and distensibility for each arterial site (as described above) [59].
4.3.3. Brachial artery flow-mediated dilation
Conduit artery endothelial function by brachial artery flow-mediated dilation and will include assessment of reactive hyperaemic response, a proxy for microvascular function, in equipped centres. This response will be assessed as increase of the artery diameter in response to increased blood flow [60]. The brachial artery will be imaged in longitudinal section, about 5 cm proximal to placement of a blood pressure cuff, just below the antecubital fossa. The probe (linear, >7.5 MHz) will be held in a stereotactic clamp to avoid movement artefacts. A Doppler flow signal will be recorded from the centre of the vessel with the range gate set at 1.5 mm. The baseline image and Doppler signal will be recorded for 1 min, following which a blood pressure cuff will be inflated to supra-systolic pressure for 5 min (300 ± 30 mmHg). The cuff will then be rapidly deflated and the artery imaged and Doppler signal recorded for 5 min post-cuff deflation. Brachial artery flow-mediated dilation is calculated as the maximum change in diameter from baseline, expressed as a percentage. On cuff deflation the resultant reactive hyperaemia is calculated as the flow change from baseline, expressed as a percentage change in blood flow using an automatic edge detection system (Cardiovascular Suite, Quipu srl).
4.3.7. Echocardiography
Participants will undergo standard echocardiograms at baseline and during follow-up, following standard recommendations [61–63] and according to each centre’s pre-specified protocol by expert sonographers. Left Ventricular (LV) Mass Index (LVMI) will calculated by Devereux’s formula and indexed to body surface area. The geometric classification will be based on the combination of LVMI and relative wall thickness. Transmitral peak E wave (pulse Doppler) and early diastolic Tissue Doppler velocity of mitral annulus (e’) will be used to assess LV diastolic function together with other parameters [E/e’, Left Atrial (LA) volume index, and peak Tricuspid Regurgitation (TR)]. Doppler echocardiography combined with two-dimensional imaging will be used to estimate Stroke Volume (SV), Cardiac Output (CO), and the cardiac index. Proximal aorta imaging (including M-mode images obtained from the parasternal long-axis view) will be used to assess the end-systolic aortic dimension and end-diastolic aortic dimension 2–3 cm above the aortic valve using the leading edge-to-leading edge technique. Using these measurements, aortic indices such aortic distensibility, strain and stiffness index will be estimated. In addition to standard echocardiography and when available in the participating centres, Global peak systolic Longitudinal Strain (GLS) will be assessed using 2D speckle tracking echocardiography.
4.3.8. 24-h brachial and central blood pressure
24-h brachial and central blood pressure will be measured using the Mobil-o-graph Pulse Wave Analysis (PWA) device (IEM GmbH, Stolberg, Germany). An appropriately sized cuff used for oscillometric reading is inflated at a pre-defined steady-state pressure level to obtain waveform signals which are analysed with proprietary methodologies. The central blood pressure measurement follows the brachial blood pressure assessment; brachial pressure waveforms are therefore auto-calibrated to brachial blood pressure values. The protocol for ambulatory blood pressure measurements will follow published recommendations [64].
4.3.9. Thoracic aortic calcifications
Assessment of presence or absence of thoracic aortic calcifications will be performed in any thoracic Computed Tomography (CT) scan available in the recruited population. The extent of aortic calcification will be assessed by a semi-qualitative method and will be defined as none, mild (1 sector [ascending, arch, descending]), moderate (>1 sector), or severe (>1 sector, concentric) [65]. Similarly, assessment of presence or absence of Coronary Artery Calcium (CAC) will be performed and the extent of CAC will be also assessed by a semi-qualitative method. CAC will be characterised as none, mild, moderate, or severe based on visual assessment that has been shown to be comparable to the Agatston score [66]. This information will be included in the baseline characteristics of the participants in the study. Further, quantitative assessment of arterial calcifications (coronary or/and thoracic) might be pursued as a post hoc analysis based on availability of baseline and follow-up scans.
4.3.10. Questionnaires
The following psychometric tests will be included:
Quality of life (12-item short form survey, SF-12)
Insomnia Severity Index (ISI)
Hospital Anxiety and Depression Scale (HADS)
PTSD questionnaires (PTSD checklist for DSM-5, PCL-5)
Socio-economic status (evaluation de la précarité et des inégalités de santé dans les centres d’examens de santé/evaluation of deprivation and inequalities in health examination centres, EPICES)
Chronic burden scale (CBS)
4.3.11. Mortality and hospitalisation records
All participants will be linked to relevant health surveillance, electronic heath record and mortality data in participating countries at visit 1. Follow up of these data is planned for visit 2, and at 2, 5 and 10 years after recruitment (Figure 1). Questionnaires to evaluate health status and psychometric tests will be also sent by email/mail.
4.4. Statistical Analysis
First, we will compare the trajectories of vascular ageing variables between COVID-19 patients and controls (group 4), and between COVID-19 patients with various degrees of severity, accounting for a set of fixed and time-varying confounders. This will be performed using mixed models suited for repeated assessments. Adjustment for age, sex, and mean arterial pressure will be performed. Further adjustment for classical CV risk factors (obesity, diabetes, renal function) will be performed if deemed necessary.
Second (mid-term/long-term), we will compare the risk of CV disease between the four groups. For this purpose, Cox proportional hazards regression will be performed, accounting for differences in trajectory of vascular ageing between infected and noninfected patients will be used.
Third, we will compare among infected patients: (a) the treatment/management practices (acute treatments for COVID-19, chronic renin-angiotensin system (RAS) blocker treatment); (b) the prevalence of pre-existing cardiovascular comorbidities; (c) the psychosocial characteristics, including PTSD; and (d) their potential impact on trajectory of vascular ageing and clinical events. To minimise indication bias in objective (d), propensity score analysis will be carried out.
Since COVID-19 is a completely new condition, sample size calculation cannot be based on preliminary data. However, we estimated that the minimal detectable difference in PWV between the control group and any of the other COVID-19 positive groups in a Dunnett’s test will be 0.35 m/s for a total population of 1500 participants, 0.3 m/s for 2000 participants, and 0.28 m/s for 2500 participants, given a standard deviation of 1.2 m/s, α = 0.05, a global statistical power (1-β) of 0.80, and 10% of subjects lost to follow-up.
5. Ethical Considerations
The CARTESIAN study is a research study involving human participants with minimal risks and burden. Accordingly, it cannot be carried out on a person without his/her freely given and informed consent, obtained expressly after the person has been given the information. An adequate reflection period will be given to the individual between the time when he or she is informed and when he or she signs the consent form. A copy of the information sheet and consent form, signed and dated by the research participant and by a study investigator, will be given to the individual prior to their participation in the study. This research concerns only people physically able to give her/his written consent. Given the real-life nature of this study, no prohibition from participating in another clinical study or exclusion period after the study is foreseen.
6. Data Collection and Management
Data will be held centrally in the European coordinating centre (Institut national de la santé et de la recherche médicale - INSERM U970, Paris France). An electronic Case Report Form (eCRF) is set up (RedCAP) via a web browser. The eCRF used for this research is implemented in accordance with European (General Data Protection Regulation) regulations.
INSERM will be responsible for data hosting and management. A data transfer agreement will be signed between INSERM and study promoter in order to authorise INSERM to secured data storage and management. INSERM will send to the study promoter a cleaned database version, via secured electronic transfer. Ownership of any data transferred to the eCRF and centralized database will be retained by the site that contributed it. All analysis of pooled data will be undertaken with the explicit agreement of each contributing site. Research data will be shared with public health authorities as needed.
7. Conclusion
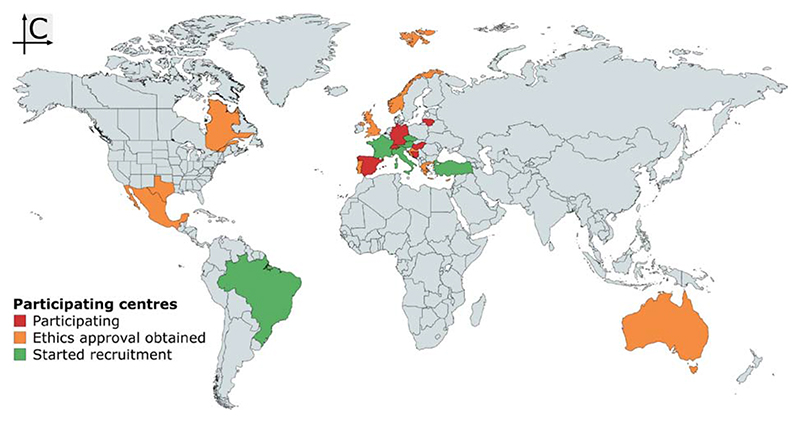
There is strong reason to believe that COVID-19 has long-term consequences for arterial structure and function, and thus for CV prognosis in survivors. Therefore, in this paper, we present the protocol of a large multi-centre prospective study investigating the effects of COVID-19 on EVA. Although this protocol details many measurements, we took great care to ensure that only the core ones are mandatory, ensuring inclusiveness for as many centres as possible. At the time of writing (10 November 2020), 43 centres from 21 countries had expressed interest in participating (Figure 2) with a total expected number of >2500 included patients. A list of centres that have obtained Ethics Committee approval/started recruitment is provided in Supplemental Table S1 in Supplemental Digital Content 1.
Figure 2. Map of participating centres as of 10 November 2020. Map created with mapchart.net.
In summary, data from the CARTESIAN study should provide insight in the relationship between COVID-19, its severity, and early vascular ageing in a large cohort. To our knowledge, this will be the first study to provide such insight, potentially enabling future care and diagnostics to be more focused on those most affected.
Supplementary Material
Funding
The CARTESIAN study is supported by a grant from the ARTERY Society, covering administrative expenses for the initiation of the study (e.g., fees for submission to the ethical committee) for participating centres. If the number of centres willing to participate exceeds the allocated budget, priority will be given to centres with the greatest recruitment capacity. Any centre fulfilling the requirements can participate in the study at its own expenses. COST Action CA18216 VascAgeNet is supported by COST (European Cooperation in Science and Technology, https://www.cost.eu). BS is supported by the European Union’s Horizon 2020 research and innovation program (grant 793805).
Footnotes
Authors’ Contribution
RMB and BP contributed in study conceptualization and writing (original draft, review & editing) the manuscript. BH, PL, CCM, MLM, CR, DTP, TW and TWH contributed in review & editing. PB supervised the project.
Conflicts of Interest
The authors declare they have no conflicts of interest.
References
- [1].Climie RE, van Sloten TT, Bruno RM, Taddei S, Empana JP, Stehouwer CDA, et al. Macrovasculature and microvasculature at the crossroads between type 2 diabetes mellitus and hypertension. Hypertension. 2019;73:1138–49. doi: 10.1161/HYPERTENSIONAHA.118.11769. [DOI] [PubMed] [Google Scholar]
- [2].Laurent S, Boutouyrie P, Cunha PG, Lacolley P, Nilsson PM. Concept of extremes in vascular aging. Hypertension. 2019;74:218–28. doi: 10.1161/HYPERTENSIONAHA.119.12655. [DOI] [PubMed] [Google Scholar]
- [3].Hamczyk MR, Nevado RM, Barettino A, Fuster V, Andres V. Biological versus chronological aging: JACC focus seminar. J Am Coll Cardiol. 2020;75:919–30. doi: 10.1016/j.jacc.2019.11.062. [DOI] [PubMed] [Google Scholar]
- [4].Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–60. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–32. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- [6].Climie RE, Mayer CC, Bruno RM, Hametner B. Addressing the unmet needs of measuring vascular ageing in clinical practice–European COoperation in Science and Technology Action VascAgeNet. Artery Res. 2020;26:71–5. [Google Scholar]
- [7].Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–18. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lindner D, Fitzek A, Brauninger H, Aleshcheva G, Edler C, Meissner K, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5:1281–5. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ding Y, Wang H, Shen H, Li Z, Geng J, Han H, et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200:282–9. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gordon JB, Kahn AM, Burns JC. When children with Kawasaki disease grow up: myocardial and vascular complications in adulthood. J Am Coll Cardiol. 2009;54:1911–20. doi: 10.1016/j.jacc.2009.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–8. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Monteil V, Kwon H, Prado P, Hagelkruys A, Wimmer RA, Stahl M, et al. Inhibition of SARS-CoV-2 Infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905.:e7-13.e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Thomas MC, Pickering RJ, Tsorotes D, Koitka A, Sheehy K, Bernardi S, et al. Genetic Ace2 deficiency accentuates vascular inflammation and atherosclerosis in the ApoE knockout mouse. Circ Res. 2010;107:888–97. doi: 10.1161/CIRCRESAHA.110.219279. [DOI] [PubMed] [Google Scholar]
- [14].Patel VB, Zhong JC, Fan D, Basu R, Morton JS, Parajuli N, et al. Angiotensin-converting enzyme 2 is a critical determinant of angiotensin II-induced loss of vascular smooth muscle cells and adverse vascular remodeling. Hypertension. 2014;64:157–64. doi: 10.1161/HYPERTENSIONAHA.114.03388. [DOI] [PubMed] [Google Scholar]
- [15].Srivastava P, Badhwar S, Chandran DS, Jaryal AK, Jyotsna VP, Deepak KK. Imbalance between Angiotensin II - Angiotensin (1-7) system is associated with vascular endothelial dysfunction and inflammation in type 2 diabetes with newly diagnosed hypertension. Diabetes MetabSyndr. 2019;13:2061–8. doi: 10.1016/j.dsx.2019.04.042. [DOI] [PubMed] [Google Scholar]
- [16].Zanoli L, Briet M, Empana JP, Cunha PG, Mäki-Petäjä KM, Protegerou AD, et al. Vascular consequences of inflammation: a position statement from the ESH Working Group on Vascular Structure and Function and the ARTERY Society. J Hypertens. 2020;38:1682–98. doi: 10.1097/HJH.0000000000002508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].He L, Ding Y, Zhang Q, Che X, He Y, Shen H, et al. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol. 2006;210:288–97. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Corrales-Medina VF, Alvarez KN, Weissfeld LA, Angus DC, Chirinos JA, Chang CC, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313:264–74. doi: 10.1001/jama.2014.18229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vlachopoulos CV, Terentes-Printzios DG, Aznaouridis KA, Pietri PG, Stefanadis CI. Association between pneumococcal vaccination and cardiovascular outcomes: a systematic review and meta-analysis of cohort studies. Eur J PrevCardiol. 2015;22:1185–99. doi: 10.1177/2047487314549512. [DOI] [PubMed] [Google Scholar]
- [20].Zanoli L, Boutouyrie P, Fatuzzo P, Granata A, Lentini P, Oztürk K, et al. Inflammation and aortic stiffness: an individual participant data meta-analysis in patients with inflammatory bowel disease. J Am Heart Assoc. 2017;6:e007003. doi: 10.1161/JAHA.117.007003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Roman MJ, Devereux RB, Schwartz JE, Lockshin MD, Paget SA, Davis A, et al. Arterial stiffness in chronic inflammatory diseases. Hypertension. 2005;46:194–9. doi: 10.1161/01.HYP.0000168055.89955.db. [DOI] [PubMed] [Google Scholar]
- [22].Nagayama D, Imamura H, Endo K, Saiki A, Sato Y, Yamaguchi T, et al. Marker of sepsis severity is associated with the variation in cardio-ankle vascular index (CAVI) during sepsis treatment. Vasc Health Risk Manag. 2019;15:509–16. doi: 10.2147/VHRM.S228506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wu Q, Zhou L, Sun X, Yan Z, Hu C, Wu J, et al. Altered lipid metabolism in recovered SARS patients twelve years after infection. Sci Rep. 2017;7:9110. doi: 10.1038/s41598-017-09536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Odegaard JI, Chawla A. Connecting type 1 and type 2 diabetes through innate immunity. Cold Spring Harb Perspect Med. 2012;2:a007724. doi: 10.1101/cshperspect.a007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Szekely Y, Lichter Y, Taieb P, Banai A, Hochstadt A, Merdler I, et al. Spectrum of cardiac manifestations in COVID-19 - a systematic echocardiographic study. Circulation. 2020;142:342–53. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li SSl, Cheng Cw, Fu Cl, Chan Yh, Lee Mp, Chan JWm, et al. Left ventricular performance in patients with severe acute respiratory syndrome: a 30-day echocardiographic follow-up study. Circulation. 2003;108:1798–803. doi: 10.1161/01.CIR.0000094737.21775.32. [DOI] [PubMed] [Google Scholar]
- [27].Grimaud M, Starck J, Levy M, Marais C, Chareyre J, Khraiche D, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020;10:69. doi: 10.1186/s13613-020-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zeng JH, Liu YX, Yuan J, Wang FX, Wu WB, Li JX, et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020;48:773–7. doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–18. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63:390–1. doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2016;37:2315–81. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gennaro Mazza M, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain BehavImmun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tingey JL, Bentley JA, Hosey MM. COVID-19: understanding and mitigating trauma in ICU survivors. Psychol Trauma. 2020;12:S100–S4. doi: 10.1037/tra0000884. [DOI] [PubMed] [Google Scholar]
- [34].Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38:1276–83. doi: 10.1097/CCM.0b013e3181d8cc1d. [DOI] [PubMed] [Google Scholar]
- [35].Hatch R, Young D, Barber V, Griffiths J, Harrison DA, Watkinson P. Anxiety, Depression and post traumatic stress disorder after critical illness: a UK-wide prospective cohort study. Crit Care. 2018;22:310. doi: 10.1186/s13054-018-2223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Walczewska J, Rutkowski K, Wizner B, Cwynar M, Grodzicki T. Stiffness of large arteries and cardiovascular risk in patients with post-traumatic stress disorder. Eur Heart J. 2011;32:730–6. doi: 10.1093/eurheartj/ehq354. [DOI] [PubMed] [Google Scholar]
- [37].Climie RE, Boutouyrie P, Perier MC, Guibout C, van Sloten TT, Thomas F, et al. Individual and neighborhood deprivation and carotid stiffness. Hypertension. 2019;73:1185–94. doi: 10.1161/HYPERTENSIONAHA.118.12186. [DOI] [PubMed] [Google Scholar]
- [38].Dorn AV, Cooney RE, Sabin ML. COVID-19 exacerbating inequalities in the US. Lancet. 2020;395:1243–4. doi: 10.1016/S0140-6736(20)30893-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Recovery Collaborative Group, Dexamethasone in hospitalized patients with Covid-19 — preliminary report. N Engl J Med. 2020:NEJMoa2021436 [Google Scholar]
- [40].Fardet L, Petersen I, Nazareth I. Risk of cardiovascular events in people prescribed glucocorticoids with iatrogenic Cushing’s syndrome: cohort study. BMJ. 2012;345:e4928. doi: 10.1136/bmj.e4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yang G, Tan Z, Zhou L, Yang M, Peng L, Liu J, et al. Effects of ARBs and ACEIs on virus infection, inflammatory status and clinical outcomes in COVID-19 patients with hypertension: a single center retrospective study. Hypertension. 2020;76:51–8. doi: 10.1161/HYPERTENSIONAHA.120.15143. [DOI] [PubMed] [Google Scholar]
- [42].Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, et al. Association of inpatient use of angiotensin–converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–81. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin–angiotensin–aldosterone system blockers and the risk of covid-19. N Engl J Med. 2020;382:2431–40. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhang J, Wang M, Ding W, Wan J. The interaction of RAAS inhibitors with COVID-19: current progress, perspective and future. Life Sci. 2020;257:118142. doi: 10.1016/j.lfs.2020.118142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020;382:e102. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [46].Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Docherty AB, Harrison EM, Green CA, Hardwick HE, Holden KA, Dondelinger F, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang A, Zhao W, Xu Z, Gu J. Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID-19) is urgently needed. Diabetes Res Clin Pract. 2020;162:108118. doi: 10.1016/j.diabres.2020.108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Reference Values for Arterial Stiffness Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31:2338–50. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bruno RM, Nilsson PM, Engström G, Wadström BN, Empana JP, Boutouyrie P, et al. Early and supernormal vascular aging: clinical characteristics and association with incident cardiovascular events. Hypertension. 2020;76:1616–24. doi: 10.1161/HYPERTENSIONAHA.120.14971. [DOI] [PubMed] [Google Scholar]
- [51].Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30:445–8. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- [52].Huybrechts SAM, Devos DG, Vermeersch SJ, Mahieu D, Achten E, de Backer TLM, et al. Carotid to femoral pulse wave velocity: a comparison of real travelled aortic path lengths determined by MRI and superficial measurements. J Hypertens. 2011;29:1577–82. doi: 10.1097/HJH.0b013e3283487841. [DOI] [PubMed] [Google Scholar]
- [53].Stergiou GS, Alpert B, Mieke S, Asmar R, Atkins N, Eckert S, et al. A universal standard for the validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. J Hypertens. 2018;36:472–8. doi: 10.1097/HJH.0000000000001634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Westerhof N, Sipkema P, van den Bos GC, Elzinga G. Forward and backward waves in the arterial system. Cardiovasc Res. 1972;6:648–56. doi: 10.1093/cvr/6.6.648. [DOI] [PubMed] [Google Scholar]
- [55].Weber T, Wassertheurer S, Rammer M, Haiden A, Hametner B, Eber B. Wave reflections, assessed with a novel method for pulse wave separation, are associated with end-organ damage and clinical outcomes. Hypertension. 2012;60:534–41. doi: 10.1161/HYPERTENSIONAHA.112.194571. [DOI] [PubMed] [Google Scholar]
- [56].Bramwell JC, Hill AV. The velocity of the pulse wave in man. Proc R Soc Lond B. 1922;93:298–306. [Google Scholar]
- [57].Engelen L, Ferreira I, Stehouwer CD, Boutouyrie P, Laurent S. Reference Values for Arterial Measurements Collaboration. Reference intervals for common carotid intima-media thickness measured with echotracking: relation with risk factors. Eur Heart J. 2013;34:2368–80. doi: 10.1093/eurheartj/ehs380. [DOI] [PubMed] [Google Scholar]
- [58].Engelen L, Bossuyt J, Ferreira I, van Bortel LM, Reesink KD, Segers P, et al. Reference values for local arterial stiffness. Part A: carotid artery. J Hypertens. 2015;33:1981–96. doi: 10.1097/HJH.0000000000000654. [DOI] [PubMed] [Google Scholar]
- [59].Bruno RM, Cartoni G, Stea F, Armenia S, Bianchini E, Buralli S, et al. Carotid and aortic stiffness in essential hypertension and their relation with target organ damage: the CATOD study. J Hypertens. 2017;35:310–18. doi: 10.1097/HJH.0000000000001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Thijssen DHJ, Bruno RM, van Mil ACCM, Holder SM, Faita F, Greyling A, et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J. 2019;40:2534–47. doi: 10.1093/eurheartj/ehz350. [DOI] [PubMed] [Google Scholar]
- [61].Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1.e14–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- [62].Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- [63].Goldstein SA, Evangelista A, Abbara S, Arai A, Asch FM, Badano LP, et al. Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the European Association of Cardiovascular Imaging: endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2015;28:119–82. doi: 10.1016/j.echo.2014.11.015. [DOI] [PubMed] [Google Scholar]
- [64].Parati G, Stergiou G, O’Brien E, Asmar R, Beilin L, Bilo G, et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32:1359–66. doi: 10.1097/HJH.0000000000000221. [DOI] [PubMed] [Google Scholar]
- [65].Pedrosa JF, Barreto SM, Bittencourt MS, Ribeiro ALP. Anatomical references to evaluate thoracic aorta calcium by computed tomography. Curr Atheroscler Rep. 2019;21:51. doi: 10.1007/s11883-019-0811-9. [DOI] [PubMed] [Google Scholar]
- [66].Chiles C, Duan F, Gladish GW, Ravenel JG, Baginski SG, Snyder BS, et al. Association of coronary artery calcification and mortality in the national lung screening trial: a comparison of three scoring methods. Radiology. 2015;276:82–90. doi: 10.1148/radiol.15142062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.