Abstract
The effect of physical activity on breast cancer risk may be partly mediated by sex steroid hormones. This review synthesized and appraised the evidence for an effect of physical activity on sex steroid hormones. Systematic searches were performed using MEDLINE (Ovid), EMBASE (Ovid), and SPORTDiscus to identify experimental studies and prospective cohort studies that examined physical activity and estrogens, progestins, and/or androgens, as well as sex hormone binding globulin (SHBG) and glucocorticoids in pre- and postmenopausal women. Meta-analyses were performed to generate effect estimates. Risk of bias was assessed, and the GRADE system was used to appraise quality of the evidence. Twenty-eight randomized controlled trials (RCT), 81 nonrandomized interventions, and six observational studies were included. Estrogens, progesterone, and androgens mostly decreased, and SHBG increased, in response to physical activity. Effect sizes were small, and evidence quality was graded moderate or high for each outcome. Reductions in select sex steroid hormones following exercise supports the biological plausibility of the first part of the physical activity–sex hormone–breast cancer pathway. The confirmed effect of physical activity on decreasing circulating sex steroid hormones supports its causal role in preventing breast cancer.
See related reviews by Lynch et al., p. 11 and Drummond et al., p. 28
Introduction
There is strong evidence that women who accrue higher levels of physical activity may have a reduced risk of developing breast cancer compared with their less active counterparts (1). Despite this, the causal nature of this relationship remains unclear, limiting the certainty with which physical activity can be promoted as a means to reduce breast cancer risk (2). An improved understanding of the mechanistic pathways that may underlie the physical activity–breast cancer relationship will strengthen causal inference.
Sex steroid hormones have been proposed as a key mechanistic pathway underlying the association between higher physical activity and reduced breast cancer risk (3–5). Higher levels of physical activity have, in some instances, been associated with lower levels of circulating sex hormones in both pre- and postmenopausal women (6, 7). In premenopausal women, regular, vigorous-intensity exercise may disrupt menstrual function, and potentially delay the onset of menarche (8–10). Regular physical activity attenuates age-associated weight gain (11), which in turn may reduce levels of circulating estrogens and androgens (12). Breast cancer is widely acknowledged to be a hormone-dependent disease (13). Higher levels of estrogens, progesterone, and androgens, as well as lower levels of sex hormone binding globulin (SHBG) have been implicated in the development of breast cancer (13–15). This could partially explain the link between higher physical activity and reduced breast cancer risk. However, most published reviews relating to the physical activity–sex hormone–breast cancer pathway have been narrative reviews. There is a strong need for systematic review, synthesis of data (including meta-analysis, where possible) and study quality appraisal to conclude that this pathway is a causal contributor to the physical activity–breast cancer association.
The current research utilizes a causal evidence synthesis framework developed by the World Cancer Research Fund (WCRF) International and University of Bristol to review evidence for a physical activity–sex hormone–breast cancer pathway (16). In our protocol article (2), we detailed the initial stage of this process, which used TeMMPo (17), a novel text mining platform, to prioritize sex steroid hormone mediators that may underlie physical activity–breast cancer associations, based on the quantity of evidence for each mediator. In this review, we aim to synthesize and appraise the evidence for an effect of physical activity on each of these sex hormones. The review of the evidence for the latter step assessing the putative pathway of interest is published concurrently (18).
Materials and Methods
The methods for this review have been outlined, in detail, in our protocol article (2), registered on PROSPERO (CRD42020146736) and conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (19). In brief, systematic searches of MEDLINE (Ovid), EMBASE (Ovid), and SPORTDiscus were completed by 30 August 2019. The search terminology is presented in the Supplementary Material (Supplementary Table ST1). Peer-reviewed, experimental studies and prospective cohort studies were included if they examined the effect of physical activity on sex hormones in apparently healthy (i.e., free of a medical diagnosis for a condition that may alter how sex hormones respond to exercise), post-menarche females. Outcomes were estrogens, progesterone, androgens, sex hormone binding globulin (SHBG), and glucocorticoids, which were identified as potential mediators of the physical activity–breast cancer relationship by TeMMPo (2). Following duplicate removal, two reviewers independently screened the titles and abstracts, and then the full texts, and studies that were not relevant were excluded. The Cochrane Collaboration Tool, ROBINS-I, and ROBINS-E were used to assess risk of bias in randomized controlled trials (RCT), nonrandomized interventions, and observational studies, respectively (20–22). To rate the overall quality of evidence and the strength of any findings generated, the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system was used (23). For all outcomes, extracted data were summarized and presented descriptively. Where study design, exposures, outcomes, and analyses were defined consistently in at least three separate RCTs, random-effects meta-analysis was used to generate an effect estimate [standardized mean difference (SMD) 95% confidence interval (CI)]. All statistical analyses were performed using Stata version 16 (Stata Corporation, College Station, Texas).
Results
Search results
Results of the searches are presented in Fig. 1. Of 11,573 results returned across all five database search sets, there were 114 studies (127 publications) that assessed the effect of physical activity or exercise on sex steroid hormones or SHBG. These included 28 RCTs (24–57), 81 nonrandomized interventions (58–145), and six observational studies (6, 7, 146–149). The pre-post studies included 30 that examined the acute effects of exercise (58–110) and 51 that examined the effects of more than a single exercise session on sex steroid hormones (111–145).
Figure 1.
PRISMA flow diagram of literature search, screening, and study selection.
Study characteristics
Study characteristics are presented in Supplementary Methods and Material (Supplementary Tables S2A–S2D). There were nine RCTs that included premenopausal (24–37), 18 RCTs that included postmenopausal (38–56), and one that included perimenopausal (57) women. Sample sizes ranged from 18 to 391 in studies of premenopausal, and from 16 to 382 in studies of postmenopausal women. The intervention consisted of predominantly aerobic exercise in 14 studies (25–27, 31, 32, 34–36, 38, 40, 44, 46, 49, 50), strength training in six (29, 30, 41, 54–56), a combination of aerobic and strength in seven (24, 39, 42, 47, 52, 53, 57), and yoga in one (48), with an intervention duration ranging from 8 weeks to 12 months. An inactive control group served as the comparator in 21 studies (24–26, 29–32, 34–36, 39, 40, 42, 46–49, 53–57). Four studies offered stretch, flexibility, or group information classes during the intervention period (38, 41, 50, 52). Three studies had a different exercise intensity or dose as a comparator (27, 28, 44). Outcomes included circulating SHBG (n = 9; refs. 24, 25, 34, 44, 46, 49, 52, 53), estradiol (n = 15; refs. 26, 27, 34, 35, 40–42, 44, 46, 50, 53, 55–57), estrone (n = 6; refs. 40, 42, 44, 46, 50, 53), estrogen (n = 5; refs. 30–32, 36, 47), free estradiol (n = 6; refs. 34, 40, 44, 46, 50, 53), estrone sulfate (n = 2; refs. 34, 53), bioavailable estradiol (n = 1; ref. 34), 2-OH-E1 (n = 3; refs. 25, 33, 38), 16a-OH-E1 (n = 3; refs. 25, 33, 38), progesterone (n = 5; refs. 32, 34, 48, 56, 57), androstenedione (n = 5; refs. 40, 42, 46, 51, 53), testosterone (n = 14; refs. 24, 27, 29, 34, 40, 41, 46, 51–55, 57), free or bioavailable testosterone (n = 5; refs. 34, 40, 46, 51, 53), dehydroepiandrosterone (DHEA, n = 3; refs. 51, 53, 57), dehydroepiandrosterone sulfate (DHEAS, n = 4; refs. 51, 53, 54, 57), and cortisol (n = 7; refs. 24, 29, 37, 39, 42, 52, 55). In studies of premenopausal women, steroid hormone levels were assessed in the follicular (n = 5; refs. 26, 29–31, 34), luteal (n = 1; ref. 25), or both (n = 3) phases of the menstrual cycle.
Nonrandomized interventions included studies that examined acute hormonal responses to a single exercise session (n = 51; refs. 58–110), or multiple exercise sessions (n = 30; refs. 111–145), with interventions ranging from 5 days to 6 months. Samples included premenopausal women only (acute n = 43, multiple sessions n = 17), postmenopausal women only (acute n = 4, multiple sessions n = 9), or both pre- and postmenopausal women (acute n = 4, multiple sessions n = 4). Sample sizes ranged from n = 5 to n = 75 in acute exercise interventions, and n = 6 to n = 148 in interventions of a longer duration. Exercise interventions included aerobic exercise (acute n = 32, multiple sessions n = 18), anaerobic exercise (acute = 1) strength exercise (acute n = 15, multiple sessions n = 8), and combined aerobic and strength training (acute n = 3, multiple sessions n = 4). Twenty-eight studies had a relevant comparison condition, which included menstrual cycle phase (acute n = 8), participant menopause status (acute n = 3, multiple sessions n = 3), participant fitness or body composition (acute n = 4, multiple sessions n = 1), exercise type or dose (acute n = 6, multiple sessions n = 1), or time of day of exercise (acute n = 3). Outcomes included SHBG (acute n = 2, multiple sessions n = 14), estradiol (acute n = 25, multiple sessions n = 15), estrone (multiple sessions n = 4), estrogen (acute n = 2, multiple sessions n = 3), progesterone (acute = 14, multiple sessions = 12), testosterone (acute = 19, multiple sessions = 13), free testosterone (acute = 5, multiple sessions = 2), androstenedione (acute = 2, multiple sessions = 2), DHEA (acute = 3), DHEAS (acute = 3, multiple sessions = 1), and cortisol (acute = 26, multiple sessions = 4).
Prospective cohort studies included pre- (n = 3; refs. 7, 146, 149) and post- (n = 3; refs. 6, 147, 148) menopausal women and had sample sizes ranging from n = 104 to n = 623 participants. Each used self-reported measures of physical activity, with follow-up duration ranging from one or two menstrual cycles to four years. Outcomes included SHBG (n = 2; refs. 6, 148), estradiol (n = 5; refs. 6, 7, 146–148), estrone (n = 3; refs. 6, 147, 148), free estradiol (n = 2; refs. 6, 148), bioavailable estradiol (n = 2; refs. 6, 148), estrone sulfate (n = 1; ref. 6), progesterone (n = 1; ref. 146), testosterone (n = 4; refs. 6, 147–149), androstenedione (n = 3; refs. 6, 147, 148), DHEA (n = 1; ref. 6), and DHEAS (n = 1; ref. 6).
Risk of bias
Risk of bias results are presented in Supplementary Materials and Methods (Supplementary Tables S3A–S3D). Sources of bias in RCTs included performance bias (all RCTs), due to the inability to blind participants to the fact they are completing exercise, as well as attrition bias, with 12 studies having greater than 10% attrition or noncompliance (25, 27, 32, 34, 35, 39, 42, 48, 54, 57). There was insufficient information regarding selection bias in 19 studies (24, 26, 27, 29–31, 34–36, 39, 41, 42, 47, 49, 52–56) and insufficient information on the measurement, accuracy, reliability, or sensitivity of hormone assays in two studies (24, 39). All nonrandomized interventions had at least a moderate risk of bias owing to the presence of confounding and seven scored serious for confounding as participant body composition was not considered or reported when selecting or describing participants (58, 59, 64, 73, 79, 101, 109, 125). Studies also scored moderate for participant selection (n = 2; refs. 115, 117), intervention classification and the potential for intervention deviation (n = 13; refs. 63, 69, 71, 116, 117, 120, 121, 124, 131, 133, 138, 142, 144), outcome assessment (n = 1), as well as the number of outcomes reported (n = 2; refs. 129, 138). Five observational studies had moderate risk of bias overall, owing to the potential for confounding, self-reported assessment of physical activity, or the number of analyses performed and reported (6, 7, 146, 148, 149). One observational study had serious risk of bias as it did not adjust for important confounders and as the assessment of estradiol lacked adequate sensitivity for approximately half the participants (147).
Effect of physical activity on sex hormones and SHBG
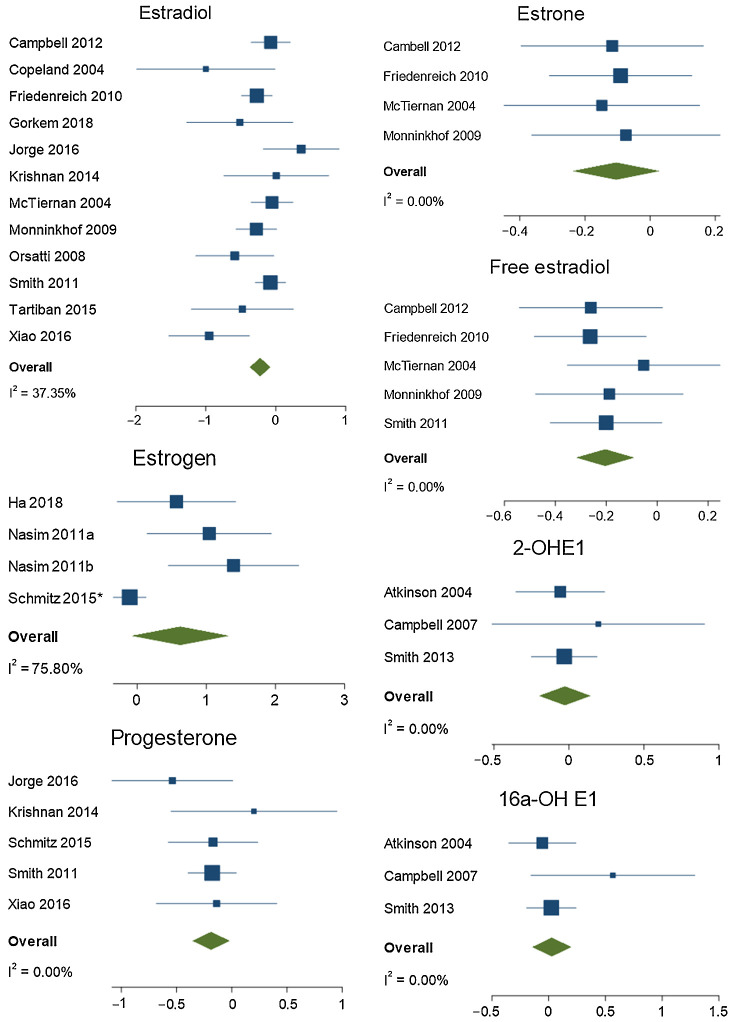
Meta-analysis results are presented in Fig. 2 (estrogens and progesterone) and Fig. 3 (androgens, SHBG, and cortisol) and in Supplementary Methods and Materials (Supplementary Figures 1–4). Results from individual studies that were not included in meta-analyses are presented in Supplementary Methods and Materials (Supplementary Tables S4A–S4D).
Figure 2.
Abbreviated forest plots for estrogens and progesterone. The x-axis represents the SMD between exercise and control groups.
Figure 3.
Abbreviated forest plots for androgens and SHBG. The x-axis represents the SMD between exercise and control groups.
SHBG
A meta-analysis of RCTs (8 studies, n = 1,353) identified a small increase in SHBG following exercise (SMD = 0.13; 95% CI = 0.02, 0.24; I2 = 0%). Only one individual RCT examined exercise dose and SHBG and it did not identify a clear dose–response relationship (44). Nonrandomized interventions that examined the response of SHBG to multiple exercise sessions mostly reported no significant changes from baseline (113, 115, 116, 119, 120, 127, 128, 131, 132, 137, 139, 140, 145). The acute response of SHBG to a single session of exercise was described by two studies. These observed a small increase (∼10%) in SHBG at the conclusion of exercise, which returned to baseline upon recovery (73, 98). Findings from observational studies were consistent with those from the meta-analysis, with 2 of 2 studies identifying higher levels of SHBG in postmenopausal women who reported higher levels of physical activity (6, 148).
Estrogens
Meta-analyses of RCTs identified small decreases in estradiol (12 studies, n = 1,452; SMD = −0.22; 95% CI = −0.37, −0.08; I2 = 37%) and free estradiol (5 studies, n = 1,033; SMD = −0.20; 95% CI = −0.32, −0.09; I2 = 0%) in response to exercise. There was a suggestion of a small, but nonsignificant, decrease in estrone (4 RCTs, n = 878; SMD = −0.10; 95% CI = −0.24, 0.03; I2 = 0%). There was no effect of exercise on estrogen or estrogen metabolites 2-OH-E1 or 16a-OH-E1 identified via meta-analysis. As moderate heterogeneity was evident in the estradiol meta-analysis, subgroup analysis was performed to identify any differences in effect according to exercise type and menopausal status. A decrease in estradiol was evident in studies that prescribed both aerobic (6 studies, n = 1,060; SMD = −0.15; 95% CI = −0.27, −0.03; I2 = 0%) and resistance exercise (3 studies, n = 116; SMD = −0.80; 95% CI = −1.17, −0.43; I2 = 0%), and in studies that enrolled only postmenopausal women (9 studies, n = 1,070; SMD = −0.28; 95% CI = −0.49, −0.06; I2 = 60%). There was no clear evidence of an effect identified in studies that prescribed combined training (2 studies) or yoga (1 study), or studies that included only pre- (2 studies) or peri- (1 study) menopausal women. In individual RCTs, performing a higher quantity of moderate-activity exercise (300 minutes compared with 150 minutes per week) did not result in a greater effect on hormone levels (32, 44), nor did high-intensity interval exercise compared with continuous moderate-to-vigorous aerobic exercise (27, 28).
Of 14 nonrandomized interventions that had more than a single exercise session, seven reported a reduction in resting estradiol levels (117, 121–124, 130, 133, 143). This was more frequently detected in the luteal rather than the follicular phase of the menstrual cycle (121, 124, 130, 133). Two studies that examined differences in hormone responses between pre- and postmenopausal women did not identify an effect of exercise on sex steroid hormones (136, 141). In studies of acute exercise, estradiol appeared to increase following a single session before returning to baseline upon recovery (60–65, 67, 69–72, 75, 79, 81, 83, 84, 89, 96, 97, 107, 110). This relationship was influenced by exercise type, duration and intensity, recent exercise history, and cycle phase. Longer, more intense exercise led to greater increases in estradiol (69, 79, 83). The response was also greater following a week of intense training (75) and greater in the luteal phase of the menstrual cycle (60–63, 68, 81, 89, 94, 96, 97). Although there was no difference between pre- and postmenopausal women in acute responses to prolonged endurance exercise (a 50–100 km run; ref. 71), acute changes in estradiol levels were more evident in pre-menopausal women following strength training (72, 104).
In prospective cohort studies, more physical activity was associated with less estradiol in 1 of 2 studies of premenopausal women and in 1 of 3 studies in postmenopausal women (6, 7, 146–148). Consistent with the meta-analysis results, higher levels of physical activity were associated with less bioavailable estradiol and free estradiol in 2 of 2 studies of postmenopausal women (6, 148). No association between physical activity and estrone levels were identified (6, 147, 148).
Progesterone
In RCTs, progesterone levels decreased in response to exercise (5 studies, n = 548; SMD = −0.19; 95% CI = −0.36, −0.02; I2 = 0%). Only 2 of 12 nonrandomized intervention studies that examined ongoing exercise identified a decrease in progesterone after exercise training (133, 143), with the remaining studies showing no change from baseline. Nonrandomized interventions that examined the acute response of progesterone to a single session of exercise described a brief increase that returned to baseline following recovery (60–62, 67, 79, 81, 83, 94, 96). This was more evident in the luteal phase (81, 83, 94, 96). There was only one relevant observational study, which did not identify an association between weekly physical activity and progesterone levels (146).
Androgens
Meta-analyses of RCTs identified small reductions in testosterone (11 studies, n = 1,434; SMD = −0.11; 95% CI = −0.21, −0.01; I2 = 0%), free testosterone (5 studies, n = 1,187; SMD = −0.12; 95% CI = −0.23, −0.01; I2 = 0%), and DHEA (3 studies, n = 312; SMD = −0.23; 95% CI = −0.46, −0.01; I2 = 0%) levels following exercise. Androstenedione (5 studies, n = 868; SMD = −0.10; 95% CI = −0.23, 0.03; I2 = 0%) and DHEAS (4 studies, n = 309; SMD = −0.28; 95% CI = −0.56, 0.01; I2 = 8%) also declined following exercise, although these effects were not statistically significant.
In nonrandomized interventions, there was no change in resting testosterone levels after ongoing exercise in 10 of 12 studies. In response to acute exercise, testosterone levels increased briefly following exercise then returned to baseline following recovery in some (68, 70, 72, 84, 85, 91, 98, 99, 101, 104), but not all studies (65, 90, 93, 96, 100, 108, 109). The increase was evident in response to both aerobic and strength exercise and during both phases of the menstrual cycle. Both DHEA and DHEAS increased in response to exercise before decreasing in 3 of 5 studies (70, 72, 82).
Results from the prospective cohort studies were inconsistent. One study identified lower levels of testosterone and androstenedione in more physically active postmenopausal women (148). This contrasted with studies that did not identify an association between physical activity and testosterone or androstenedione (6, 147). Physical activity was not linearly associated with a decline in either DHEA or DHEAS in one study (6).
Cortisol
Little evidence of a change in cortisol was evident from a meta-analysis of RCTs (5 studies, n = 328; SMD = 0.08; 95% CI = −0.19, 0.35; I2 = 26%). Following a single session of exercise, some studies documented no change (58, 59, 88, 100), some documented an increase in cortisol during or immediately following exercise (66, 68, 74, 82, 90, 103, 105, 106), and others identified a decrease in cortisol compared with baseline at the conclusion of exercise or following a recovery period (70, 72, 79, 85, 87, 92, 96, 104). The increase in cortisol levels observed in some studies was greater when exercise was performed in the morning rather than the afternoon and was linearly related to exercise intensity (66, 102). The decrease in levels following exercise observed in other studies was more evident in premenopausal than postmenopausal women and was independent of exercise type and intensity (72, 79, 104). No prospective cohort studies that investigated the effect of physical activity on cortisol were identified.
Grade
Results of the GRADE appraisal are presented in Table 1. As the conclusions for each physical activity–sex steroid hormone pathway are based on evidence from several RCTs, the quality of evidence for all physical activity–sex steroid hormone associations was graded as high. The evidence for estradiol, estrogen, androstenedione, DHEAS, and cortisol was graded moderate, owing to publication and imprecision bias.
Table 1.
GRADE evidence table.
| Outcome | Study type, number of studies (participant n) | Effect estimates (SMD, 95% CI) | Quality of evidence |
|---|---|---|---|
| SHBG | RCT, 8 (1,353) | 0.13 (0.02, 0.24) | High |
| Estradiol | RCT, 12 (1,452) | −0.22 (−0.37, −0.08) | Moderatea |
| Estrone | RCT, 4 (878) | −0.10 (−0.24, 0.03) | High |
| Free estradiol | RCT, 5 (1,033) | −0.20 (−0.31, −0.09) | High |
| Estrogen | RCT, 3 (152) | 0.62 (−0.11, 1.35) | Moderateb |
| 2-OH-E1 | RCT, 3 (520) | −0.03 (−0.20, 0.14) | High |
| 16a-OH-E1 | RCT, 3 (520) | 0.03 (−0.18, 0.20) | High |
| Progesterone | RCT, 5 (548) | −0.19 (−0.36, −0.02) | High |
| Testosterone | RCT, 11 (1,434) | −0.11 (−0.21, −0.01) | High |
| Free testosterone | RCT, 5 (1,187) | −0.12 (−0.23, −0.01) | High |
| Androstenedione | RCT, 5 (868) | −0.10 (−0.23, 0.03) | Moderatea |
| DHEA | RCT, 3 (309) | −0.23 (−0.46, −0.01) | High |
| DHEAS | RCT, 4 (242) | −0.28 (−0.56, 0.01) | Moderatea |
| Cortisol | RCT, 5 (328) | 0.08 (−0.19, 0.35) | Moderatea |
aGraded down due to potential publication bias.
bGraded down to imprecision bias.
Discussion
This systematic review and meta-analysis of the effects of physical activity on sex steroid hormones found that physical activity leads to increases in SHBG and decreases in estradiol, free estradiol, progesterone, testosterone, free testosterone, and DHEA. The review also found evidence of possible decreases in estrone, androstenedione, and DHEAS. There was no clear evidence for an effect on estrogen metabolites or cortisol; however, fewer studies examined these outcomes. For all outcomes, the strength of the evidence was graded moderate to high.
This review has several key strengths. It has employed a robust methodology for identifying, synthesizing, and appraising mechanistic evidence to support biological plausibility for the physical activity–sex hormone–breast cancer pathway. In addition, by considering multiple types of studies, including RCTs, nonrandomized interventions, and prospective cohort studies, we have integrated results generated via several different research approaches. This review also has some limitations that must be considered when interpreting the findings. TeMMPo, which was used to prioritize the sex steroid hormones investigated, uses the quantity of evidence for each exposure – mediator and mediator – outcome relationship to score potential mediators. In this sense, it is more suited to identifying what has been most investigated, rather than what may be most important, or novel mediators which warrant attention. To counter this, we supplemented our TeMMPo search with expert input to aid the selection of mediators (2). Our inclusion criteria excluded populations with preexisting menstrual or metabolic disorders, as well as elite athletes. While we consider this restriction a strength of the study, as it limits several factors that may bias findings such as the effects of athlete diet or menstrual dysfunction in athletes, it does potentially limit the scope of the conclusions regarding exercise volume and intensity. As with all reviews, it is possible that some relevant studies were overlooked by the search strategy employed. However, after performing a separate systematic search for each physical activity–sex hormone pathway, as well as examining reference lists of key papers, we are confident the findings presented are an accurate reflection of current evidence.
These findings are consistent with previous reviews that have outlined reductions in sex hormone levels in response to physical activity, as well as broader changes in menstrual function following more intense activity (150–152). Although these prior reviews have employed broader inclusion criteria, allowing for studies with participants that experienced menstrual dysfunction or had metabolic conditions, or examined combined diet and exercise interventions, the consistency supports a robust finding. While acknowledging the consistency of our findings, we note several methodologic advantages of the current review: inclusion of epidemiologic as well as intervention evidence; tight inclusion criteria for participants and exposure to ensure accurate effect estimates; an up to date search for all sex hormone pathways; and, appraisal of the overall evidence quality using GRADE.
The strength of evidence for an effect of physical activity on sex steroid hormones was stronger in postmenopausal women owing to the number and quality of RCTs that included postmenopausal women. However, the low heterogeneity in the meta-analyses, as well as results from subgroup analysis and from several nonrandomized intervention studies (71, 136, 141), suggest that the responses of sex hormones to physical activity are unlikely to differ between pre- and postmenopausal women.
There was no clear evidence as to what type of physical activity or exercise has the strongest effect of sex steroid hormones. However, the primary conclusions of this review are based on meta-analyses of RCTs that employed aerobic, resistance, or combined exercise interventions with carefully prescribed frequency, intensity, time, and type. There were no RCTs that directly assessed the impact of nonprescribed physical activity, which encompasses a broader range of movement, movement intensities, and settings than more deliberate exercise, on sex hormones (153). Observational studies that measured self-reported physical activity did, like the meta-analyses, document lower levels of free and bioavailable estradiol as well as higher levels of SHBG in more active women (6, 148). However, results for estradiol and testosterone in these studies were inconsistent (6, 146–148). These studies may have been limited by the use of self-reported measures of physical activity, which can attenuate physical activity–health outcome associations (154). As such, RCTs or Mendelian randomization studies are needed to better understand the effects of different types of physical activity on sex steroid hormone levels. The use of continuous monitoring/wearables devices may also potentially transform the evidence base for physical activity as an exposure.
Although there were no clear differences in effect between aerobic and resistance exercise, with evidence of an effect on sex hormones identified for both modalities, the evidence should be considered stronger for aerobic exercise. This is due to the larger number of RCTs that investigated the effect of aerobic exercise, as well as the number of participants in these studies and the duration of the interventions employed. In individual RCTs, neither the organization (e.g., high intensity interval training compared with continuous moderate to vigorous exercise) nor the total quantity (e.g., 150 minutes or 300 minutes per week) of aerobic exercise affected the effect on sex hormones in pre- or postmenopausal women (27, 28, 44, 45, 50). In observational studies, the relationship between physical activity and sex hormones was clearer for aerobic than resistance exercise, although this may reflect the activity patterns of the participants more than differences between modalities (155). Also, the association between activity and sex hormones varied little by activity intensity (155).
Physical activity may influence the concentration of sex steroid hormones via several pathways. Low levels of physical activity are associated with increased adiposity and an expansion of peripheral adipose tissue (11), which will facilitate increased aromatization of androgens to estrogens (12, 156). The ability of this review to untangle the direct effect of physical activity on sex hormone levels from the indirect effect via changes in body composition is limited. However, in one RCT included in this review, a decrease in sex hormone levels was present only in the exercisers who lost body fat (50). A mediation analysis of another RCT included in this review suggested that the effect of exercise on sex hormones is at least partially mediated by fat loss (45). In addition, an observational study identified statistically significant effects of physical activity on sex hormones when it did not adjust for BMI, but these effects were no longer significant when BMI was included as a covariate (6). These findings suggest that physical activity–sex hormone associations are, at least somewhat, mediated by body composition; future studies that employ causal mediation techniques may be better able to quantify these effects. This suggests that other weight loss strategies, such as energy restricted diets, could be used to achieve similar effects. However, it is important to note that physical activity is the only intervention strategy that reduces fat mass while preserving lean muscle mass (157).
Physical activity may also influence sex steroid hormones via its effect on SHBG, inflammation, and insulin signaling. In our meta-analysis, physical activity preceded an increase in SHBG, which contributes to the bioavailability of androgens and estrogens (158). Physical inactivity has been associated with increased inflammation, which can promote the upregulation of aromatase and increased production of estrogens by stromal cells of the breast (159, 160). In contrast, increasing physical activity may generate the production and release of anti-inflammatory cytokines while also reducing the production of pro-inflammatory cytokines. Physical inactivity may increase in insulin levels, which in turn may decrease hepatic synthesis of SHBG, increasing the bioavailability of estrogens and androgens (5, 161). Increasing physical activity can increase insulin sensitivity and reduce insulin resistance. These effects can be observed after both an acute bout of physical activity as well as with more regular physical activity (162, 163). Subsequent systematic reviews by our research group will assess the impact of these pathways on breast cancer risk and determine whether physical activity may modulate them (2).
In pre- and postmenopausal women, physical activity and exercise can cause a decrease in levels of select estrogens, progestogens, and androgens, while stimulating a small increase in SHBG. These results support the biological plausibility of the first part of the physical activity–sex hormone–breast cancer pathway.
Authors' Disclosures
C.T.V. Swain reports grants from World Cancer Research Fund during the conduct of the study. L. Boing reports grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) during the conduct of the study. R.L. Milne reports grants from World Cancer Research Fund during the conduct of the study. D.R. English reports grants from World Cancer Research Fund during the conduct of the study. K.A. Brown reports grants from World Cancer Research Fund (funded by Wereld Kanker Onderzoek Fonds) and NIH/NCI R01CA215797 during the conduct of the study. E.H. van Roekel reports grants from Wereld Kanker Onderzoek Fonds (WKOF), as part of the World Cancer Research Fund International grant programme (Grant No. 2016/1620) during the conduct of the study. T.R. Gaunt reports grants from World Cancer Research Fund during the conduct of the study. R.M. Martin reports grants from Cancer Research UK during the conduct of the study. B.M. Lynch reports grants from Victorian Cancer Agency and Wereld Kanker Onderzoek Fonds during the conduct of the study. No disclosures were reported by the other authors.
Acknowledgments
Funding (IIG_2018_1732) was obtained from Wereld Kanker Onderzoek Fonds (WKOF), as part of the World Cancer Research Fund International grant programme. B.M. Lynch was supported by the Victorian Cancer Agency (MCRF18005). K.A. Brown is supported by NIH/NCI R01 CA215797. R.M. Martin was supported by the NIHR Biomedical Research Centre at University Hospitals Bristol and Weston NHS Foundation Trust and the University of Bristol. R.M. Martin was supported by a Cancer Research UK (C18281/A29019) programme grant (the Integrative Cancer Epidemiology Programme).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Note: Supplementary data for this article are available at Cancer Epidemiology, Biomarkers & Prevention Online (http://cebp.aacrjournals.org/).
References
- 1. Chan DSM, Abar L, Cariolou M, Nanu N, Greenwood DC, Bandera EV, et al. World cancer research fund international: Continuous update project—systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control 2019;30:1183–200. [DOI] [PubMed] [Google Scholar]
- 2. Lynch BM, Milne RM, English DR, Brown KA, Drummond AE, Swain CTV, et al. Linking physical activity to breast cancer: a protocol for systematically reviewing three potential mechanistic pathways. Cancer Epidemiol Biomarkers Prev 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer 2008;8:205–11. [DOI] [PubMed] [Google Scholar]
- 4. Koelwyn GJ, Quail DF, Zhang X, White RM, Jones LW. Exercise-dependent regulation of the tumour microenvironment. Nat Rev Cancer 2017;17:620–32. [DOI] [PubMed] [Google Scholar]
- 5. Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. Eur J Cancer 2010;46:2593–604. [DOI] [PubMed] [Google Scholar]
- 6. Bertone-Johnson ER, Tworoger SS, Hankinson SE. Recreational physical activity and steroid hormone levels in postmenopausal women. Am J Epidemiol 2009;170:1095–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jasienska G, Ziomkiewicz A, Thune I, Lipson SF, Ellison PT. Habitual physical activity and estradiol levels in women of reproductive age. Eur J Cancer Prev 2006;15:439–45. [DOI] [PubMed] [Google Scholar]
- 8. Loucks AB. Effects of exercise training on the menstrual cycle: Existence and mechanisms. Med Sci Sports Exerc 1990;22:275–80. [PubMed] [Google Scholar]
- 9. Morris FL, Payne WR, Wark JD. Prospective decrease in progesterone concentrations in female lightweight rowers during the competition season compared with the off season: A controlled study examining weight loss and intensive exercise. Br J Sports Med 1999;33:417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Warren MP, Perlroth NE. The effects of intense exercise on the female reproductive system. J Endocrinol 2001;170:3–11. [DOI] [PubMed] [Google Scholar]
- 11. Jakicic JM, Powell KE, Campbell WW, Dipietro L, Pate RR, Pescatello LS, et al. Physical activity and the prevention of weight gain in adults: a systematic review. Med Sci Sports Exerc 2019;51:1262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Key TJ, Appleby PN, Reeves GK, Roddam AW, Helzlsouer KJ, Alberg AJ, et al. Circulating sex hormones and breast cancer risk factors in postmenopausal women: Reanalysis of 13 studies. Br J Cancer 2011;105:709–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hilton HN, Clarke CL, Graham JD. Estrogen and progesterone signalling in the normal breast and its implications for cancer development. Mol Cell Endocrinol 2018;466:2–14. [DOI] [PubMed] [Google Scholar]
- 14. Hormones E, Group BCC. Endogenous sex hormones and breast cancer in postmenopausal women: Reanalysis of nine prospective studies. J Natl Cancer Inst 2002;94:606–16. [DOI] [PubMed] [Google Scholar]
- 15. Zhang X, Tworoger SS, Eliassen AH, Hankinson SE. Postmenopausal plasma sex hormone levels and breast cancer risk over 20 years of follow-up. Breast Cancer Res Treat 2013;137:883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lewis SJ, Gardner M, Higgins J, Holly JMP, Gaunt TR, Perks CM, et al. Developing the WCRF International/University of Bristol methodology for identifying and carrying out systematic reviews of mechanisms of exposure-cancer associations. Cancer Epidemiol Biomarkers Prev 2017;26:1667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. University of Bristol. Temmpo - text mining for mechanism prioritisation. Available from: https://www.temmpo.org.uk/. 1st June 2019.
- 18. Drummond AE, Swain CTV, Milne RM, English DR, Brown KA, van Roekel EH, et al. Linking physical activity to breast cancer via sex steroid hormones. Part 2: The effect of sex steroid hormones on breast cancer risk. Cancer Epidemiol Biomarkers Prev 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morgan RL, Thayer KA, Santesso N, Holloway AC, Blain R, Eftim SE, et al. A risk of bias instrument for non-randomized studies of exposures: a users' guide to its application in the context of grade. Environ Int 2019;122:168–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. Robins-i: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. Grade: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bell GJ, Syrotuik D, Martin TP, Burnham R, Quinney HA. Effect of concurrent strength and endurance training on skeletal muscle properties and hormone concentrations in humans. Eur J Appl Physiol 2000;81:418–27. [DOI] [PubMed] [Google Scholar]
- 25. Campbell KL, Westerlind KC, Harber VJ, Bell GJ, Mackey JR, Courneya KS. Effects of aerobic exercise training on estrogen metabolism in premenopausal women: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev 2007;16:731–9. [DOI] [PubMed] [Google Scholar]
- 26. Gorkem U, Yamaner F, Demirkan E, Inal HA. Does the spinning exercise affect the ovarian reserve in reproductive-young women? Baltic Journal of Health & Physical Activity 2018;10:175–81. [Google Scholar]
- 27. Kong Z, Fan X, Sun S, Song L, Shi Q, Nie J. Comparison of high-intensity interval training and moderate-to-vigorous continuous training for cardiometabolic health and exercise enjoyment in obese young women: a randomized controlled trial. PLoS One 2016;11:e0158589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kong Z, Sun S, Liu M, Shi Q. Short-term high-intensity interval training on body composition and blood glucose in overweight and obese young women. J Diabetes Res 2016;2016:4073618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marx JO, Ratamess NA, Nindl BC, Gotshalk LA, Volek JS, Hakkinen K, et al. Low-volume circuit versus high-volume periodized resistance training in women. /effet du circuit training d'intensite faible par rapport a la planification de l'entrainement de musculation d'intensite forte chez des femmes. Med Sci Sports Exerc 2001;33:635–43. [DOI] [PubMed] [Google Scholar]
- 30. Moghadasi M, Siavashpour S. The effect of 12 weeks of resistance training on hormones of bone formation in young sedentary women. Eur J Appl Physiol 2013;113:25–32. [DOI] [PubMed] [Google Scholar]
- 31. Nasim H. Effect of moderate walking exercise on body water in sedentary obese and thin women. Ovidius Univ Ann Ser Phys Educ Sport Sci Mov Health 2011;11:101–3. [Google Scholar]
- 32. Schmitz KH, Williams NI, Kontos D, Domchek S, Morales KH, Hwang W-T, et al. Dose-response effects of aerobic exercise on estrogen among women at high risk for breast cancer: a randomized controlled trial. Breast Cancer Res Treat 2015;154:309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith AJ, Phipps WR, Thomas W, Schmitz KH, Kurzer MS. The effects of aerobic exercise on estrogen metabolism in healthy premenopausal women. Cancer Epidemiol Biomarkers Prev 2013;22:756–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith AJ, Phipps WR, Arikawa AY, O'Dougherty M, Kaufman B, Thomas W, et al. Effects of aerobic exercise on premenopausal sex hormone levels: Results of the wiser study, a randomized clinical trial in healthy, sedentary, eumenorrheic women. Cancer Epidemiol Biomarkers Prev 2011;20:1098–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tartibian B, FitzGerald LZ, Azadpour N, Maleki BH. A randomized controlled study examining the effect of exercise on inflammatory cytokine levels in post-menopausal women. Post Reprod Health 2015;21:9–15. [DOI] [PubMed] [Google Scholar]
- 36. Tartibian B, Maleki BH, Abbasi A. The calciotropic hormone response to omega-3 supplementation during longterm weight-bearing exercise training in post menopausal women. J Sports Sci Med 2010;9:245–52. [PMC free article] [PubMed] [Google Scholar]
- 37. Arikawa AY, Thomas W, Patel SR, Kurzer MS. No effect of exercise on urinary 6-sulfatoxymelatonin and catecholamines in young women participating in a 16-week randomized controlled trial. Cancer Epidemiol Biomarkers Prev 2013;22:1634–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Atkinson C, Lampe JW, Tworoger SS, Ulrich CM, Bowen D, Irwin ML, et al. Effects of a moderate intensity exercise intervention on estrogen metabolism in postmenopausal women. Cancer Epidemiol Biomarkers Prev 2004;13:868–74. [PubMed] [Google Scholar]
- 39. Banitalebi E, Faramarzi M, Bagheri L, Kazemi AR. Comparison of performing 12 weeks' resistance training before, after and/or in between aerobic exercise on the hormonal status of aged women: a randomized controlled trial. Horm Mol Biol Clin Investig 2018;35. [DOI] [PubMed] [Google Scholar]
- 40. Campbell KL, Foster-Schubert KE, Alfano CM, Wang C-C, Wang C-Y, Duggan CR, et al. Reduced-calorie dietary weight loss, exercise, and sex hormones in postmenopausal women: Randomized controlled trial. J Clin Oncol 2012;30:2314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Copeland JL, Tremblay MS. Effect of hrt on hormone responses to resistance exercise in post-menopausal women. Maturitas 2004;48:360–71. [DOI] [PubMed] [Google Scholar]
- 42. Figueroa A, Going SB, Milliken LA, Blew RM, Sharp S, Teixeira PJ, et al. Effects of exercise training and hormone replacement therapy on lean and fat mass in postmenopausal women. J Gerontol A Biol Sci Med Sci 2003;58:266–70. [DOI] [PubMed] [Google Scholar]
- 43. Friedenreich CM, Wang Q, Yasui Y, Stanczyk FZ, Duha A, Brenner DR, et al. Long-term effects of moderate versus high durations of aerobic exercise on biomarkers of breast cancer risk: Follow-up to a randomized controlled trial. Cancer Epidemiol Biomarkers Prev 2019;28:1725–34. [DOI] [PubMed] [Google Scholar]
- 44. Friedenreich CM, Neilson HK, Wang Q, Stanczyk FZ, Yasui Y, Duha A, et al. Effects of exercise dose on endogenous estrogens in postmenopausal women: A randomized trial. Endocr Relat Cancer 2015;22:863–76. [DOI] [PubMed] [Google Scholar]
- 45. Friedenreich CM, Neilson HK, Woolcott CG, Wang Q, Yasui Y, Brant RF, et al. Mediators and moderators of the effects of a year-long exercise intervention on endogenous sex hormones in postmenopausal women. Cancer Causes Control 2011;22:1365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Friedenreich CM, Woolcott CG, McTiernan A, Ballard-Barbash R, Brant RF, Stanczyk FZ, et al. Alberta physical activity and breast cancer prevention trial: Sex hormone changes in a year-long exercise intervention among postmenopausal women. J Clin Oncol 2010;28:1458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ha MS, Son WM. Combined exercise is a modality for improving insulin resistance and aging-related hormone biomarkers in elderly korean women. Exp Gerontol 2018;114:13–8. [DOI] [PubMed] [Google Scholar]
- 48. Jorge MP, Santaella DF, Pontes IMO, Shiramizu VKM, Nascimento EB, Cabral A, et al. Hatha yoga practice decreases menopause symptoms and improves quality of life: A randomized controlled trial. Complement Ther Med 2016;26:128–35. [DOI] [PubMed] [Google Scholar]
- 49. Kim J-W, Kim D-Y. Effects of aerobic exercise training on serum sex hormone binding globulin, body fat index, and metabolic syndrome factors in obese postmenopausal women. Metab Syndr Relat Disord 2012;10:452–7. [DOI] [PubMed] [Google Scholar]
- 50. McTiernan A, Tworoger SS, Ulrich CM, Yasui Y, Irwin ML, Rajan KB, et al. Effect of exercise on serum estrogens in postmenopausal women: A 12-month randomized clinical trial. Cancer Res 2004;64:2923–8. [DOI] [PubMed] [Google Scholar]
- 51. McTiernan A, Tworoger SS, Rajan KB, Yasui Y, Sorenson B, Ulrich CM, et al. Effect of exercise on serum androgens in postmenopausal women: A 12-month randomized clinical trial. Cancer Epidemiol Biomarkers Prev 2004;13:1099–105. [PubMed] [Google Scholar]
- 52. Miller GD, Nicklas BJ, Davis CC, Legault C, Messier SP. Basal growth hormone concentration increased following a weight loss focused dietary intervention in older overweight and obese women. J Nutr Health Aging 2012;16:169–74. [DOI] [PubMed] [Google Scholar]
- 53. Monninkhof EM, Velthuis MJ, Peeters PHM, Twisk JWR, Schuit AJ. Effect of exercise on postmenopausal sex hormone levels and role of body fat: A randomized controlled trial. J Clin Oncol 2009;27:4492–9. [DOI] [PubMed] [Google Scholar]
- 54. Nunes PRP, Barcelos LC, Oliveira AA, Furlanetto R, Martins FM, Resende EAMR, et al. Muscular strength adaptations and hormonal responses after two different multiple-set protocols of resistance training in postmenopausal women. J Strength Cond Res 2019;33:1276–85. [DOI] [PubMed] [Google Scholar]
- 55. Orsatti FL, Nahas EAP, Maesta N, Nahas-Neto J, Burini RC. Plasma hormones, muscle mass and strength in resistance-trained postmenopausal women. Maturitas 2008;59:394–404. [DOI] [PubMed] [Google Scholar]
- 56. Xiao C-M, Kang Y, Zhuang Y-C. Effects of elastic resistance band exercise on postural balance, estrogen, bone metabolism index, and muscle strength of perimenopausal period women. J Am Geriatr Soc 2016;64:1368–70. [DOI] [PubMed] [Google Scholar]
- 57. Krishnan S, Gustafson MB, Campbell C, Gaikwad NW, Keim NL. Association between circulating endogenous androgens and insulin sensitivity changes with exercise training in midlife women. Menopause 2014;21:967–74. [DOI] [PubMed] [Google Scholar]
- 58. Altemus M, Rao B, Dhabhar FS, Ding W, Granstein RD. Stress-induced changes in skin barrier function in healthy women. J Invest Dermatol 2001;117:309–17. [DOI] [PubMed] [Google Scholar]
- 59. Altemus M, Roca C, Galliven E, Romanos C, Deuster P. Increased vasopressin and adrenocorticotropin responses to stress in the midluteal phase of the menstrual cycle. J Clin Endocrinol Metab 2001;86:2525–30. [DOI] [PubMed] [Google Scholar]
- 60. Beidleman BA, Rock PB, Muza SR, Fulco CS, Gibson LL, Kamimori GH, et al. Substrate oxidation is altered in women during exercise upon acute altitude exposure. /l'oxydation des substrats energetiques est alteree chez la femme pendant l'exercice realise a haute altitude. Med Sci Sports Exerc 2002;34:430–7. [DOI] [PubMed] [Google Scholar]
- 61. Beidleman BA, Rock PB, Muza SR, Fulco CS, Forte VA, Jr., Cymerman A. Exercise ve and physical performance at altitude are not affected by menstrual cycle phase. J Appl Physiol (1985) 1999;86:1519–26. [DOI] [PubMed] [Google Scholar]
- 62. Bonen A, Haynes FW, Graham TE. Substrate and hormonal responses to exercise in women using oral contraceptives. J Appl Physiol (1985) 1991;70:1917–27. [DOI] [PubMed] [Google Scholar]
- 63. Bonen A, Ling WY, MacIntyre KP, Neil R, McGrail JC, Belcastro AN. Effects of exercise on the serum concentrations of fsh, lh, progesterone, and estradiol. Eur J Appl Physiol Occup Physiol 1979;42:15–23. [DOI] [PubMed] [Google Scholar]
- 64. Bouillon LE, Flynn MG, Lambert CP, Fahlman M, Braun WA, Choi D. Exercise during late-follicular menstrual phase: Influence on immune parameters. J Sports Med Phys Fitness 2006;46:143–51. [PubMed] [Google Scholar]
- 65. Bunt JC, Bahr JM, Bemben DA. Comparison of estradiol and testosterone levels during and immediately following prolonged exercise in moderately active and trained males and females. Endocr Res 1987;13:157–72. [DOI] [PubMed] [Google Scholar]
- 66. Casazza GA, Jacobs KA, Suh S-H, Miller BF, Horning MA, Brooks GA. Menstrual cycle phase and oral contraceptive effects on triglyceride mobilization during exercise. J Appl Physiol (1985) 2004;97:302–9. [DOI] [PubMed] [Google Scholar]
- 67. Chang RT, Lambert GP, Moseley PL, Chapler FK, Gisolfi CV. Effect of estrogen supplementation on exercise thermoregulation in premenopausal women. J Appl Physiol (1985) 1998;85:2082–8. [DOI] [PubMed] [Google Scholar]
- 68. Chearskul S, Srichantaap T. Hormonal and metabolic responses to acute exercise in thai women. J Med Assoc Thai 1994;77:400–9. [PubMed] [Google Scholar]
- 69. Clapp JF, 3rd, Capeless EL, Little KD. The effect of sustained exercise on follicular phase levels of 17 beta-estradiol in recreational athletes. Am J Obstet Gynecol 1993;168:581–4. [DOI] [PubMed] [Google Scholar]
- 70. Consitt LA, Copeland JL, Tremblay MS. Hormone responses to resistance vs. endurance exercise in premenopausal females. Can J Appl Physiol 2001;26:574–87. [DOI] [PubMed] [Google Scholar]
- 71. Copeland JL, Verzosa MLS. Endocrine response to an ultra-marathon in pre- and post-menopausal women. Biol Sport 2014;31:125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Copeland JL, Consitt LA, Tremblay MS. Hormonal responses to endurance and resistance exercise in females aged 19–69 years. J Gerontol A Biol Sci Med Sci 2002;57:B158–65. [DOI] [PubMed] [Google Scholar]
- 73. Cumming DC, Wall SR, Galbraith MA, Belcastro AN. Reproductive hormone responses to resistance exercise. /reponses des hormones sexuelles a l' exercice de resistance. Med Sci Sports Exerc 1987;19:234–8. [PubMed] [Google Scholar]
- 74. Davis SN, Galassetti P, Wasserman DH, Tate D. Effects of gender on neuroendocrine and metabolic counterregulatory responses to exercise in normal man. J Clin Endocrinol Metab 2000;85:224–30. [DOI] [PubMed] [Google Scholar]
- 75. De Cree C, Ball P, Seidlitz B, Van Kranenburg G, Geurten P, Keizer HA. Responses of catecholestrogen metabolism to acute graded exercise in normal menstruating women before and after training. J Clin Endocrinol Metab 1997;82:3342–8. [DOI] [PubMed] [Google Scholar]
- 76. Dohi K, Mastro AM, Miles MP, Bush JA, Grove DS, Leach SK, et al. Lymphocyte proliferation in response to acute heavy resistance exercise in women: Influence of muscle strength and total work. Eur J Appl Physiol 2001;85:367–73. [DOI] [PubMed] [Google Scholar]
- 77. Galassetti P, Mann S, Tate D, Neill RA, Wasserman DH, Davis SN. Effect of morning exercise on counterregulatory responses to subsequent, afternoon exercise. J Appl Physiol 2001;91:91–9. [DOI] [PubMed] [Google Scholar]
- 78. Galliven EA, Singh A, Michelson D, Bina S, Gold PW, Deuster PA. Hormonal and metabolic responses to exercise across time of day and menstrual cycle phase. J Appl Physiol (1985) 1997;83:1822–31. [DOI] [PubMed] [Google Scholar]
- 79. Giraldo E, Garcia JJ, Hinchado MD, Ortega E. Exercise intensity-dependent changes in the inflammatory response in sedentary women: Role of neuroendocrine parameters in the neutrophil phagocytic process and the pro-/anti-inflammatory cytokine balance. NeuroImmunoModulation 2009;16:237–44. [DOI] [PubMed] [Google Scholar]
- 80. Haines M, McKinley-Barnard SK, Andre TL, Gann JJ, Hwang PS, Willoughby DS. Skeletal muscle estrogen receptor activation in response to eccentric exercise up-regulates myogenic-related gene expression independent of differing serum estradiol levels occurring during the human menstrual cycle. J Sports Sci Med 2018;17:31–9. [PMC free article] [PubMed] [Google Scholar]
- 81. Horton TJ, Miller EK, Glueck D, Tench K. No effect of menstrual cycle phase on glucose kinetics and fuel oxidation during moderate-intensity exercise. Am J Physiol Endocrinol Metab 2002;282:E752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Johnson LG, Kraemer RR, Haltom R, Kraemer GR, Gaines HE, Castracane VD. Effects of estrogen replacement therapy on dehydroepiandrosterone, dehydroepiandrosterone sulfate, and cortisol responses to exercise in postmenopausal women. Fertil Steril 1997;68:836–43. [DOI] [PubMed] [Google Scholar]
- 83. Jurkowski JE, Jones NL, Walker C, Younglai EV, Sutton JR. Ovarian hormonal responses to exercise. J Appl Physiol 1978;44:109–14. [DOI] [PubMed] [Google Scholar]
- 84. Keizer HA, Kuipers H, de Haan J, Beckers E, Habets L. Multiple hormonal responses to physical exercise in eumenorrheic trained and untrained women. Int J Sports Med 1987;8:139–50. [DOI] [PubMed] [Google Scholar]
- 85. Kemmler W, Wildt L, Engelke K, Pintag R, Pavel M, Bracher B, et al. Acute hormonal responses of a high impact physical exercise session in early postmenopausal women. Eur J Appl Physiol 2003;90:199–209. [DOI] [PubMed] [Google Scholar]
- 86. Kerksick C, Taylor L, Harvey A, Willoughby D. Gender-related differences in muscle injury, oxidative stress, and apoptosis. Med Sci Sports Exerc 2008;40:1772–80. [DOI] [PubMed] [Google Scholar]
- 87. Kostka T, Patricot MC, Mathian B, Lacour J-R, Bonnefoy M. Anabolic and catabolic hormonal responses to experimental two-set low-volume resistance exercise in sedentary and active elderly people. Aging Clin Exp Res 2003;15:123–30. [DOI] [PubMed] [Google Scholar]
- 88. Kraemer RR, Francois M, Webb ND, Worley JR, Rogers SN, Norman RL, et al. No effect of menstrual cycle phase on glucose and glucoregulatory endocrine responses to prolonged exercise. Eur J Appl Physiol 2013;113:2401–8. [DOI] [PubMed] [Google Scholar]
- 89. Kraemer RR, Heleniak RJ, Tryniecki JL, Kraemer GR, Okazaki NJ, Castracane VD. Follicular and luteal phase hormonal responses to low-volume resistive exercise. /reponses hormonales de la phase lutheale et folliculaire consecutives a un exercise de resistance effectue a un faible volume. Med Sci Sports Exerc 1995;27:809–17. [PubMed] [Google Scholar]
- 90. Kraemer WJ, Fleck SJ, Dziados JE, Harman EA, Marchitelli LJ, Gordon SE, et al. Changes in hormonal concentrations after different heavy-resistance exercise protocols in women. J Appl Physiol (1985) 1993;75:594–604. [DOI] [PubMed] [Google Scholar]
- 91. Lane AR, O'Leary CB, Hackney AC. Menstrual cycle phase effects free testosterone responses to prolonged aerobic exercise. Acta Physiol Hung 2015;102:336–41. [DOI] [PubMed] [Google Scholar]
- 92. Larsen B, Cox AJ, Quinn K, Fisher R, Minahan C. Immune response in women during exercise in the heat: a spotlight on oral contraception. J Sports Sci Med 2018;17:229–36. [PMC free article] [PubMed] [Google Scholar]
- 93. Luk HY, Levitt DE, Boyett JC, Rojas S, Flader SM, McFarlin BK, et al. Resistance exercise-induced hormonal response promotes satellite cell proliferation in untrained men but not in women. Am J Physiol Endocrinol Metab 2019;317:E421–E32. [DOI] [PubMed] [Google Scholar]
- 94. Mastrogiacomo I, Toderini D, Bonanni G, Bordin D. Gonadotropin decrease induced by prolonged exercise at about 55 percent of the vo2max in different phases of the menstrual cycle. Int J Sports Med 1990;11:198–203. [DOI] [PubMed] [Google Scholar]
- 95. Montagnani CF, Arena B, Maffulli N. Estradiol and progesterone during exercise in healthy untrained women. Med Sci Sports Exerc 1992;24:764–8. [PubMed] [Google Scholar]
- 96. Nakamura Y, Aizawa K, Imai T, Kono I, Mesaki N. Hormonal responses to resistance exercise during different menstrual cycle states. Med Sci Sports Exerc 2011;43:967–73. [DOI] [PubMed] [Google Scholar]
- 97. Navalta JW, Sedlock DA, Park K-S, McFarlin BK. Neither gender nor menstrual cycle phase influences exercise-induced lymphocyte apoptosis in untrained subjects. Appl Physiol Nutr Metab 2007;32:481–6. [DOI] [PubMed] [Google Scholar]
- 98. Nindl BC, Kraemer WJ, Gotshalk LA, Marx JO, Volek JS, Bush FA, et al. Testosterone responses after resistance exercise in women: Influence of regional fat distribution. Int J Sport Nutr Exerc Metab 2001;11:451–65. [DOI] [PubMed] [Google Scholar]
- 99. O'Leary CB, Lehman C, Koltun K, Smith-Ryan A, Hackney AC. Response of testosterone to prolonged aerobic exercise during different phases of the menstrual cycle. Eur J Appl Physiol 2013;113:2419–24. [DOI] [PubMed] [Google Scholar]
- 100. Pullinen T, Mero A, Huttunen P, Pakarinen A, Komi PV. Resistance exercise-induced hormonal responses in men, women, and pubescent boys. /reponses hormonales chez les hommes, les femmes et les adolescents de sexe masculin provoquees par un exercice de musculation. Med Sci Sports Exerc 2002;34:806–13. [DOI] [PubMed] [Google Scholar]
- 101. Shangold MM, Gatz ML, Thysen B. Acute effects of exercise on plasma concentrations of prolactin and testosterone in recreational women runners. Fertil Steril 1981;35:699–702. [DOI] [PubMed] [Google Scholar]
- 102. Thuma JR, Gilders R, Verdun M, Loucks AB. Circadian rhythm of cortisol confounds cortisol responses to exercise: implications for future research. J Appl Physiol (1985) 1995;78:1657–64. [DOI] [PubMed] [Google Scholar]
- 103. Traustadottir T, Bosch PR, Cantu T, Matt KS. Hypothalamic-pituitary-adrenal axis response and recovery from high-intensity exercise in women: Effects of aging and fitness. J Clin Endocrinol Metab 2004;89:3248–54. [DOI] [PubMed] [Google Scholar]
- 104. Vajda M, Kovarova J, Okuliarova M, Cvecka J, Schickhofer P, Bohmerova L, et al. Acute hormonal and neuromuscular response to different resistance loading in young pre- and middle-aged postmenopausal women. Gazzetta Medica Ital Arch Sci Mediche 2018;177:443–51. [Google Scholar]
- 105. van der Pompe G, Bernards N, Kavelaars A, Heijnen C. An exploratory study into the effect of exhausting bicycle exercise on endocrine and immune responses in post-menopausal women: Relationships between vigour and plasma cortisol concentrations and lymphocyte proliferation following exercise. Int J Sports Med 2001;22:447–53. [DOI] [PubMed] [Google Scholar]
- 106. van der Pompe G, Bernards N, Meijman TF, Heijnen CJ. The effect of depressive symptomatology on plasma cortisol responses to acute bicycle exercise among post-menopausal women. Psychiatry Res 1999;85:113–7. [DOI] [PubMed] [Google Scholar]
- 107. Vincent S, Berthon P, Zouhal H, Moussa E, Catheline M, Bentue-Ferrer D, et al. Plasma glucose, insulin and catecholamine responses to a wingate test in physically active women and men. Eur J Appl Physiol 2004;91:15–21. [DOI] [PubMed] [Google Scholar]
- 108. Vingren JL, Kraemer WJ, Hatfield DL, Volek JS, Ratamess NA, Anderson JM, et al. Effect of resistance exercise on muscle steroid receptor protein content in strength-trained men and women. Steroids 2009;74:1033–9. [DOI] [PubMed] [Google Scholar]
- 109. Weiss LW, Cureton KJ, Thompson FN. Comparison of serum testosterone and androstenedione responses to weight lifting in men and women. Eur J Appl Physiol Occup Physiol 1983;50:413–9. [DOI] [PubMed] [Google Scholar]
- 110. Wolf MR, Fragala MS, Volek JS, Denegar CR, Anderson JM, Comstock BA, et al. Sex differences in creatine kinase after acute heavy resistance exercise on circulating granulocyte estradiol receptors. Eur J Appl Physiol 2012;112:3335–40. [DOI] [PubMed] [Google Scholar]
- 111. He Z, Rankinen T, Leon AS, Skinner J, Tchernof A, Bouchard C. Plasma steroids are not associated with resting and exercise blood pressure. Int J Sports Med 2018;39:967–71. [DOI] [PubMed] [Google Scholar]
- 112. He Z, Rankinen T, Leon AS, Skinner JS, Tchernof A, Bouchard C. Plasma steroids, body composition, and fat distribution: effects of age, sex, and exercise training. Int J Obes (Lond) 2018;42:1366–77. [DOI] [PubMed] [Google Scholar]
- 113. An P, Rice T, Gagnon J, Borecki IB, Rankinen T, Gu C, et al. Population differences in the pattern of familial aggregation for sex hormone-binding globulin and its response to exercise training: The heritage family study. Am J Hum Biol 2001;13:832–7. [DOI] [PubMed] [Google Scholar]
- 114. An P, Rice T, Gagnon J, Hong Y, Leon AS, Skinner JS, et al. A genetic study of sex hormone–binding globulin measured before and after a 20-week endurance exercise training program: The heritage family study. Metabolism 2000;49:1014–20. [DOI] [PubMed] [Google Scholar]
- 115. Armstrong LE, Maresh CM, Keith NR, Elliott TA, VanHeest JL, Scheett TP, et al. Heat acclimation and physical training adaptations of young women using different contraceptive hormones. Am J Physiol Endocrinol Metab 2005;288:E868–E75. [DOI] [PubMed] [Google Scholar]
- 116. Aubertin-Leheudre M, Lord C, Khalil A, Dionne IJ. Effect of 6 months of exercise and isoflavone supplementation on clinical cardiovascular risk factors in obese postmenopausal women: a randomized, double-blind study. Menopause 2007;14:624–9. [DOI] [PubMed] [Google Scholar]
- 117. Boyden TW, Pamenter RW, Stanforth P, Rotkis T, Wilmore JH. Sex steroids and endurance running in women. Fertil Steril 1983;39:629–32. [DOI] [PubMed] [Google Scholar]
- 118. Bullen BA, Skrinar GS, Beitins IZ, Carr DB, Reppert SM, Dotson CO, et al. Endurance training effects on plasma hormonal responsiveness and sex hormone excretion. J Appl Physiol 1984;56:1453–63. [DOI] [PubMed] [Google Scholar]
- 119. Caballero MJ, Mahedero G, Hernandez R, Alvarez JL, Rodriguez J, Rodriguez I, et al. Effects of physical exercise on some parameters of bone metabolism in postmenopausal women. Endocr Res 1996;22:131–8. [DOI] [PubMed] [Google Scholar]
- 120. Caballero MJ, Maynar M. Effects of physical exercise on sex hormone binding globulin, high density lipoprotein cholesterol, total cholesterol and triglycerides in postmenopausal women. Endocr Res 1992;18:261–79. [DOI] [PubMed] [Google Scholar]
- 121. De Cree C, Malinow MR, van Kranenburg GP, Geurten PG, Longford NT, Keizer HA. Influence of exercise and menstrual cycle phase on plasma homocyst(e)ine levels in young women–a prospective study. Scand J Med Sci Sports 1999;9:272–8. [DOI] [PubMed] [Google Scholar]
- 122. De Cree C, Ball P, Seidlitz B, Van Kranenburg G, Geurten P, Keizer HA. Plasma 2-hydroxycatecholestrogen responses to acute submaximal and maximal exercise in untrained women. J Appl Physiol (1985) 1997;82:364–70. [DOI] [PubMed] [Google Scholar]
- 123. De Cree C, Van Kranenburg G, Geurten P, Fujimori Y, Keizer HA. 4-hydroxycatecholestrogen metabolism responses to exercise and training: possible implications for menstrual cycle irregularities and breast cancer. Fertil Steril 1997;67:505–16. [DOI] [PubMed] [Google Scholar]
- 124. De Cree C, Van Kranenburg G, Geurten P, Fujimura Y, Keizer HA. Exercise-induced changes in enzymatic o-methylation of catecholestrogens by erythrocytes of eumenorrheic women. Med Sci Sports Exerc 1997;29:1580–7. [DOI] [PubMed] [Google Scholar]
- 125. Di Blasio A, Izzicupo P, Di Baldassarre A, Gallina S, Bucci I, Giuliani C, et al. Walking training and cortisol to dhea-s ratio in postmenopause: an intervention study. Women Health 2018;58:387–402. [DOI] [PubMed] [Google Scholar]
- 126. Friedlander AL, Casazza GA, Horning MA, Huie MJ, Piacentini MF, Trimmer JK, et al. Training-induced alterations of carbohydrate metabolism in women: Women respond differently from men. J Appl Physiol 1998;85:1175–86. [DOI] [PubMed] [Google Scholar]
- 127. Hakkinen K, Kraemer WJ, Pakarinen A, Triplett-McBride T, McBride JM, Hakkinen A, et al. Effects of heavy resistance/power training on maximal strength, muscle morphology, and hormonal response patterns in 60–75-year-old men and women. Can J Appl Physiol 2002;27:213–31. [DOI] [PubMed] [Google Scholar]
- 128. Hakkinen K, Pakarinen A, Kraemer WJ, Hakkinen A, Valkeinen H, Alen M. Selective muscle hypertrophy, changes in emg and force, and serum hormones during strength training in older women. J Appl Physiol (1985) 2001;91:569–80. [DOI] [PubMed] [Google Scholar]
- 129. Igwebuike A, Irving BA, Bigelow ML, Short KR, McConnell JP, Nair KS. Lack of dehydroepiandrosterone effect on a combined endurance and resistance exercise program in postmenopausal women. J Clin Endocrinol Metab 2008;93:534–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Keizer HA, Kuipers H, de Haan J, Janssen GM, Beckers E, Habets L, et al. Effect of a 3-month endurance training program on metabolic and multiple hormonal responses to exercise. Int J Sports Med 1987;8Suppl 3(8008349, grk):154–60. [DOI] [PubMed] [Google Scholar]
- 131. Kogure GS, Miranda-Furtado CL, Silva RC, Melo AS, Ferriani RA, De Sa MFS, et al. Resistance exercise impacts lean muscle mass in women with polycystic ovary syndrome. Med Sci Sports Exerc 2016;48:589–98. [DOI] [PubMed] [Google Scholar]
- 132. Miranda-Furtado CL, Ramos FKP, Kogure GS, Santana-Lemos BA, Ferriani RA, Calado RT, et al. A nonrandomized trial of progressive resistance training intervention in women with polycystic ovary syndrome and its implications in telomere content. Reprod Sci 2016;23:644–54. [DOI] [PubMed] [Google Scholar]
- 133. Kossman DA, Williams NI, Domchek SM, Kurzer MS, Stopfer JE, Schmitz KH. Exercise lowers estrogen and progesterone levels in premenopausal women at high risk of breast cancer. J Appl Physiol (1985) 2011;111:1687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kraemer WJ, Staron RS, Hagerman FC, Hikida RS, Fry AC, Gordon SE, et al. The effects of short-term resistance training on endocrine function in men and women. Eur J Appl Physiol Occup Physiol 1998;78:69–76. [DOI] [PubMed] [Google Scholar]
- 135. Nasim H. The effect of short-term weight-bearing exercise on bone mass density in obese and thin young girls. Sport Sci Pract Asp 2010;7:11–6. [Google Scholar]
- 136. Nyberg M, Egelund J, Mandrup CM, Andersen CB, Hansen KMBE, Hergel I-MF, et al. Leg vascular and skeletal muscle mitochondrial adaptations to aerobic high-intensity exercise training are enhanced in the early postmenopausal phase. J Physiol 2017;595:2969–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Petrella JK, Kim J-s, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab 2006;291:E937–46. [DOI] [PubMed] [Google Scholar]
- 138. Prior JC, Vigna Y, Alojada N. Conditioning exercise decreases premenstrual symptoms. A prospective controlled three month trial. Eur J Appl Physiol Occup Physiol 1986;55:349–55. [DOI] [PubMed] [Google Scholar]
- 139. Reis E, Frick U, Schmidtbleicher D. Frequency variations of strength training sessions triggered by the phases of the menstrual cycle. /variations de frequence des sessions d'entrainement de force declenchees par les phases du cycle menstruel. Int J Sports Med 1995;16:545–50. [DOI] [PubMed] [Google Scholar]
- 140. Riesco E, Aubertin-Leheudre M, Maltais ML, Audet M, Dionne IJ. Synergic effect of phytoestrogens and exercise training on cardiovascular risk profile in exercise-responder postmenopausal women: a pilot study. Menopause 2010;17:1035–9. [DOI] [PubMed] [Google Scholar]
- 141. Robles Gil MC, Timon R, Toribio AF, Munoz D, Maynar JI, Caballero MJ, et al. Effects of aerobic exercise on urinary estrogens and progestagens in pre and postmenopausal women. Eur J Appl Physiol 2012;112:357–64. [DOI] [PubMed] [Google Scholar]
- 142. Schmitz KH, Warren M, Rundle AG, Williams NI, Gross MD, Kurzer MS. Exercise effect on oxidative stress is independent of change in estrogen metabolism. Cancer Epidemiol Biomarkers Prev 2008;17:220–3. [DOI] [PubMed] [Google Scholar]
- 143. Timon R, Corvillo M, Brazo J, Robles M, Maynar M. Strength training effects on urinary steroid profile across the menstrual cycle in healthy women. Eur J Appl Physiol 2013;113:1469–75. [DOI] [PubMed] [Google Scholar]
- 144. Walker KZ, Piers LS, Putt RS, Jones JA, O'Dea K. Effects of regular walking on cardiovascular risk factors and body composition in normoglycemic women and women with type 2 diabetes. Diabetes Care 1999;22:555–61. [DOI] [PubMed] [Google Scholar]
- 145. Westerlind KC, Byrnes WC, Freedson PS, Katch FI. Exercise and serum androgens in women. Phys Sportsmed 1987;15:87–90. [DOI] [PubMed] [Google Scholar]
- 146. Ahrens KA, Vladutiu CJ, Mumford SL, Schliep KC, Perkins NJ, Wactawski-Wende J, et al. The effect of physical activity across the menstrual cycle on reproductive function. Ann Epidemiol 2014;24:127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Cauley JA, Gutai JP, Kuller LH, LeDonne D, Powell JG. The epidemiology of serum sex hormones in postmenopausal women. Am J Epidemiol 1989;129:1120–31. [DOI] [PubMed] [Google Scholar]
- 148. Choudhury F, Bernstein L, Hodis HN, Stanczyk FZ, Mack WJ. Physical activity and sex hormone levels in estradiol- and placebo-treated postmenopausal women. Menopause 2011;18:1079–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Sowers MF, Beebe JL, McConnell D, Randolph J, Jannausch M. Testosterone concentrations in women aged 25–50 years: Associations with lifestyle, body composition, and ovarian status. Am J Epidemiol 2001;153:256–64. [DOI] [PubMed] [Google Scholar]
- 150. Hakimi O, Cameron L-C. Effect of exercise on ovulation: a systematic review. Sports Med 2017;47:1555–67. [DOI] [PubMed] [Google Scholar]
- 151. Ennour-Idrissi K, Maunsell E, Diorio C. Effect of physical activity on sex hormones in women: a systematic review and meta-analysis of randomized controlled trials. Breast Cancer Res 2015;17:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. de Roon M, May AM, McTiernan A, Scholten R, Peeters PHM, Friedenreich CM, et al. Effect of exercise and/or reduced calorie dietary interventions on breast cancer-related endogenous sex hormones in healthy postmenopausal women. Breast Cancer Res 2018;20:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep 1985;100:126–31. [PMC free article] [PubMed] [Google Scholar]
- 154. Bassett JK, Swain CTV, Hodge AM, Mahmood S, Csizmadi I, Owen N, et al. Calibration of the Active Australia questionnaire and application to a logistic regression model. J Sci Med Sport 2021;24:474–80. [DOI] [PubMed] [Google Scholar]
- 155.Marquis Hawkins DKT, Alessa HB, Chomistek AK, Barnett JB, Willett WC, Hankinson SE. Objective and self-reported measures of physical activity and sex hormones: Women's lifestyle validation study. J Phys Act Health 2019;16:355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kendall A, Folkerd EJ, Dowsett M. Influences on circulating oestrogens in postmenopausal women: relationship with breast cancer. J Steroid Biochem Mol Biol 2007;103:99–109. [DOI] [PubMed] [Google Scholar]
- 157. Parr EB, Coffey VG, Hawley JA. ‘Sarcobesity’: a metabolic conundrum. Maturitas 2013;74:109–13. [DOI] [PubMed] [Google Scholar]
- 158. Hammond GL. Diverse roles for sex hormone-binding globulin in reproduction. Biol Reprod 2011;85:431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Kolb R, Sutterwala FS, Zhang W. Obesity and cancer: Inflammation bridges the two. Curr Opin Pharmacol 2016;29:77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Zahid H, Simpson ER, Brown KA. Inflammation, dysregulated metabolism and aromatase in obesity and breast cancer. Curr Opin Pharmacol 2016;31:90–6. [DOI] [PubMed] [Google Scholar]
- 161. Kaaks R, Lukanova A, Rinaldi S, Biessy C, Söderberg S, Olsson T, et al. Interrelationships between plasma testosterone, shbg, igf-i, insulin and leptin in prostate cancer cases and controls. Eur J Cancer Prev 2003;12:309–15. [DOI] [PubMed] [Google Scholar]
- 162. Hargreaves M. Skeletal muscle glucose metabolism during exercise: Implications for health and performance. J Sci Med Sport 1998;1:195–202. [DOI] [PubMed] [Google Scholar]
- 163. Conn VS, Koopman RJ, Ruppar TM, Phillips LJ, Mehr DR, Hafdahl AR. Insulin sensitivity following exercise interventions: systematic review and meta-analysis of outcomes among healthy adults. J Prim Care Community Health 2014;5:211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]