Abstract
Background
People presenting with first episode psychosis (FEP) have heterogenous outcomes. More than 40% fail to achieve symptomatic remission. Accurate prediction of individual outcome in FEP could facilitate early intervention to change the clinical trajectory and improve prognosis.
Aims
We aim to systematically review evidence for prediction models developed for predicting poor outcome in FEP.
Methods
A protocol for this study was published on the International Prospective Register of Systematic Reviews (PROSPERO), registration number CRD42019156897. Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidance, we systematically searched six databases from inception to 28th January 2021. We used the CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies (CHARMS) and the Prediction model Risk Of Bias Assessment Tool (PROBAST) to extract and appraise the outcome prediction models. We considered study characteristics, methodology and model performance.
Results
Thirteen studies reporting 31 prediction models across a range of clinical outcomes met criteria for inclusion. Eleven studies employed logistic regression with clinical and sociodemographic predictor variables. Just two studies were found to be at low risk of bias. Methodological limitations identified included a lack of appropriate validation, small sample sizes, poor handling of missing data and inadequate reporting of calibration and discrimination measures. To date, no model has been applied to clinical practice.
Conclusions
Future prediction studies in psychosis should prioritise methodological rigour and external validation in larger samples. The potential for prediction modelling in FEP is yet to be realised.
Introduction
Psychosis is a mental illness characterised by hallucinations, delusions and thought disorder. The median lifetime prevalence of psychosis is around eight per 1000 of the global population.1 Psychotic disorders, including schizophrenia, are in the top 20 leading causes of disability worldwide.2 People with psychosis have heterogeneous outcomes. More than 40% fail to achieve symptomatic remission.3 At present, clinicians struggle to predict long term outcome in individuals with first episode psychosis (FEP).
Prediction modelling has the potential to revolutionise medicine by predicting individual patient outcome.4 Early identification of those with good and poor outcomes would allow for a more personalised approach to care, matching interventions and resources to those most at need. This is the basis of precision medicine. Risk prediction models have been successfully employed clinically in many areas of medicine; for example, the QRISK tool predicts cardiovascular risk in individual patients.5 However, within psychiatry, precision medicine is not yet established within clinical practice. In first episode psychosis, precision medicine could enable rapid stratification and targeted intervention thereby decreasing patient suffering and limiting treatment associated risks such as medication side effects and intrusive monitoring.
Salazar de Pablo et al recently undertook a broad systematic review of individualised prediction models in psychiatry. They found clear evidence that precision psychiatry has developed into an important area of research, with the greatest number of prediction models focussing on outcomes in psychosis. However, the field is hindered by methodological flaws, for example lack of validation. Further, there is a translation gap with only one study considering implementation into clinical practice. Systematic guidance for the development, validation and presentation of prediction models is available.6 Further, the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) statement sets standards for reporting.7 Models that do not adhere to these guidelines result in unreliable predictions, which may cause more harm than good in guiding clinical decisions.8 Salazar de Pablo et al ‘s review was impressive in scope but necessarily limited in detailed analysis of the specific models included.9 Systematic reviews focussing on the predicting the transition to psychosis,10,11 and predicting relapse in psychosis have also been published.12 In our present review, we focus on FEP with the aim to systematically review and critically appraise the prediction models for the prediction of poor outcomes.
Methods
We designed this systematic review in accordance with the CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies (CHARMS).13 A protocol for this study was published on the International Prospective Register of Systematic Reviews (PROSPERO), registration number CRD42019156897.
We developed the eligibility criteria under the Population, Index, Comparator, Outcome, Timing and Setting (PICOTS) guidance (see supplementary materials). A study was eligible for inclusion if it utilised a prospective design, including patients diagnosed with FEP, and developed, updated, or validated prognostic prediction models for any possible outcome, in any setting. We excluded non-English language studies, those where the full text was not available, those involving diagnostic prediction models, and those where the outcome predicted was less than or equal to three months from baseline because we were interested in longer term prediction.
We searched PubMed, PsychINFO, EMBASE, CINAHL Plus, Web of Science Core Collection and Google Scholar from inception up to 28th January 2021. In addition, we manually checked references cited in the systematically searched articles. The search terms were based around three themes – ‘Prediction’, ‘Outcome’ and ‘First Episode Psychosis’ terms. The full search strategy is available in the supplementary materials. Two reviewers (RL and LT) independently screened the titles and abstracts. Full text screening was completed by three independent reviewers (RL, PM and SPL). Disagreements were resolved by consensus.
Data extraction was conducted independently by two reviewers (RL and SPL) following recommendations in the CHARMS checklist.13 From all eligible studies, we collected information on study characteristics, methodology and performance. Study characteristics collected included first author name, year, region, whether multicentre, study type, setting, participant description, outcome, outcome timing, predictor categories and number of models presented. Methodology considered sample size, events per variable (EPV), number of events in validation dataset, number of candidate and retained predictors, methods of variable selection, presence and handling of missing data, modelling strategies, shrinkage, validation strategies (see below), whether models were recalibrated, if clinical utility was assessed and whether the full models were presented. Steyerberg and Harrell outline a hierarchy of validation strategies from apparent (which assesses model performance on the data used to develop it and will be severely optimistic), to internal (via cross validation or bootstrapping), internal-external (for example, validation across centres in the same study) and external validation (to assess if models generalise to related populations in different settings).14 Apparent, internal and internal-external validation use the derivation dataset only, while external validation requires the addition of a validation dataset. Performance for the best performing model per outcome in each article was considered by model validation strategy, including model discrimination (reported as the C-statistic which is equal to the area under the receiver operating characteristic (ROCAUC) curve for binary outcomes), calibration, other global performance measures, and classification metrics. If not reported, where possible, the balanced accuracy (sensitivity + specificity / 2) and the prognostic summary index (positive + negative predictive value - 1) were calculated.
Two reviewers (RL and SPL) independently assessed the risk of bias (ROB) in included studies using the Prediction model Risk Of Bias Assessment Tool (PROBAST), a risk of bias assessment tool designed for systematic reviews of diagnostic or prognostic prediction models.15,16 We considered all models reported in each article and assigned to the article an overall rating. PROBAST uses a structured approach with signalling questions across four domains: ‘participants’, ‘predictors’, ‘outcome’ and ‘statistical analysis’. Signalling questions are answered ‘yes’, ‘probably yes’, ‘no’, ‘probably no’ or ‘no information’. Answering ‘yes’ indicates a low ROB, while ‘no’ indicates high ROB. A domain where all signalling questions are answered as ‘yes’ or ‘probably yes’ indicates low ROB. Answering ‘no’ or ‘probably no’ flags the potential for the presence of bias and reviewers should use their personal judgement to determine whether issues identified have introduced bias. Applicability of included studies to the review question is also considered in PROBAST.
We reported our results according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement (see supplementary materials).17
Results
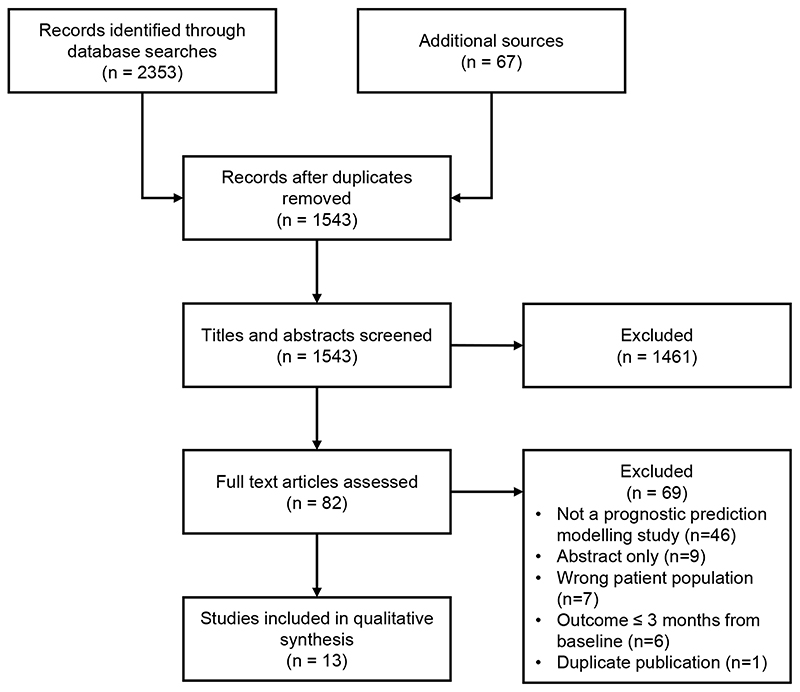
Systematic review of the literature yielded 2353 records from database searches and 67 from additional sources. After removal of duplicates, 1543 records were screened. Of these, 82 full texts were reviewed, which resulted in 13 studies meeting criteria for inclusion in our qualitative synthesis (Figure 1).18,19,28–30,20–27
Figure 1. PRISMA flow diagram.
Study characteristics are summarised in Table 1. The 13 included studies, comprising a total of 19 different patient cohorts, reported 31 different prediction models. Dates of publication ranged from 2006 and 2021. Twelve studies (92%) recruited participants from Europe, with two studies (15%) also recruiting participants from Israel and one study (8%) from Singapore. Over two-thirds (n=9) of studies were multicentre. Ten studies (77%) included participants from cohort studies, three studies (23%) included participants from randomised controlled trials and two studies (15%) included participants from case registries. Two studies (15%) included only out-patients, four (31%) included in-patients and out-patients and the rest did not specify their setting. Cohort sample size ranged from 47 to 1663 patients. The average age of patients ranged from 21 to 28 years, and 49% to 77% of the cohorts were male. Where specified, the average duration of untreated psychosis ranged from 34 to 106 weeks. Ethnicity was reported in 8 studies (62%) with the percentage non-white patients in the cohorts ranging from 4% to greater than 75%. The definition of FEP was primarily non-affective psychosis in the majority of patient cohorts, with the minority also including affective psychosis and two cohorts also including drug-induced psychosis patients. All but one study (92%) considered solely sociodemographic and clinical predictors. A wide range of outcomes were assessed across the 13 included studies including symptom remission in five studies (38%), global functioning in five studies (38%), vocational functioning in three studies (23%), treatment resistance in two studies (15%), rehospitalisation in two studies (15%), and quality of life in one study (8%). All the outcomes were binary. The follow-up period of included studies ranged from 1 to 10 years.
Table 1. Study characteristics.
| Study ID | Country | Multicentre | Recruitment Dates | Type of Study | Setting | Participants included in modelling | Outcome | Predictor Categories | No. of Models | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex (% male) | Age (mean) | Ethnicity | DUP (mean weeks) | FEP Definition | Definition | Timing | ||||||||
| AJNAKINA 2020 | UK | No | Dec 2005 to Oct 2010 | Cohort | In-patients & out-patients | 67.5% | 27.2 (at baseline) | 39.9% white, 60.1% black | 34.3 | Non-affective | Early treatment resistance from illness onset Later treatment resistance |
f/u for 5 years | Socio-demographic, Clinical | 4 |
| BHATTACHARYYA 2021 | UK | No | Sample 1 - 1st Apr 2006 to 31st Mar 2012 Sample 2 - 12th Apr 2002 to 26th Jul 2013 |
Sample 1 - Case Registry Sample 2 - Cohort |
Sample 1 - out-patients Sample 2 - out-patients |
Sample 1 - 63.9% Sample 2 - 60% |
Sample 1 - 24.4 (at onset) Sample 2 - 28.1 (at onset) |
Sample 1 - 31.1% white, 50.6% black Sample 2 - 34.2% white, 54.2% black |
N.R. |
Sample 1 - Non-affective & affective Sample 2 - Non-affective & affective |
Psychiatric rehospitalisation | f/u for 2 years | Socio-demographic, Clinical | 3 |
| CHUA 2019 | Singapore | No | 2001 to 2012 | Cohort | N.R. | 49.2% | 27.5 (at baseline) | 76.7% Chinese | 65.4 | Non-affective | EET status | At 2 years | Socio-demographic, Clinical | 2 |
| DEMJAHA 2017 | UK | Yes | Sep 1997 to Aug 1999 | Cohort | N.R. | 58.4% | 28.9 (at onset) | 48.2% white, 39.8% black | N.R. | Non-affective & affective | Early treatment resistance from illness onset | f/u for 10 years | Socio-demographic, Clinical | 1 |
| DENIJS 2019 | Netherlands & Belgium | Yes | 8th Jan 2004 to 6th Feb 2008 | Cohort | In-patients & out-patients | 76.9% | 27.6 (at baseline) | 85.9% white | N.R. | Non-affective | Andreasen symptom remission (6 months duration) GAF ≥65 | At 3 years & at 6 years | Socio-demographic, Clinical, Genetic, Environmental | 8 |
| DERKS 2010 | Austria, Belgium, Bulgaria, Czech Republic, Germany, France, Israel, Italy, Netherlands, Poland, Rumania, Spain, Sweden & Switzerland | Yes | 23rd Dec 2002 to 14th Jan 2006 | Randomised Controlled Trial | N.R. | 56.5% | 26.0 (at baseline) | N.R. | N.R. | Non-affective | Andreasen symptom remission (6 months duration) | f/u for 1 year | Socio-demographic, Clinical | 1 |
| FLYCKT 2006 | Sweden | Yes | 1st Jan 1996 to 31st Dec 1997 | Cohort | N.R. | 52.9% | 28.8 (at baseline) | N.R. | 62.4 | Non-affective & affective (with mood- incongruent delusions) | Global functioning (independent living, EET status & GAF ≥60) | At mean of 5.4 years | Socio-demographic, Clinical | 1 |
| GONZALEZ-BLANCH 2010 | Spain | No | Feb 2001 to Feb 2005 | Cohort | N.R. | 62% | 26.6 (at baseline) | N.R. | 66.6 | Non-affective | Global functioning (EET status & DAS ≤1) | At 1 year | Socio-demographic, Clinical | 1 |
| KOUTSOULERIS 2016 | Austria, Belgium, Bulgaria, Czech Republic, Germany, France, Israel, Italy, Netherlands, Poland, Rumania, Spain, Sweden & Switzerland | Yes | 23rd Dec 2002 to 14th Jan 2006 | Randomised Controlled Trial | N.R. | 56% | 26.1 (at baseline) | N.R. | N.R. | Non-affective | GAF ≥65 | At 1 year | Socio-demographic, Clinical | 1 |
| LEIGHTON 2019 (1) | UK | Yes | Dev. - 2011 to 2014 Val. - 1st Sep 2006 to 31st Aug 2009 |
Dev. – Cohort Val. - Cohort |
Dev. - Inpatients & out-patients Val. - Inpatients & out-patients |
Dev. - 66% Val. - 68% |
Dev. - 25.2 (at baseline) Val. - 24.6 (at baseline) |
Dev. - 81% white Val. - 96% white |
N.R. | Dev. - Non-affective & affective Val. - Non-affective & affective |
EET Status Andreasen symptom remission (no duration criteria) Andreasen symptom remission (6 months duration) |
At 1 year | Socio-demographic, Clinical | 3 |
| LEIGHTON 2019 (2) | UK & Denmark | Yes | Dev. - Aug 2005 to Apr 2009 Val. UK - 1st Sep 2006 to 31st Aug 2009 & 2011 to 2014 Val Denmark - Jan 1998 to Dec 2000 |
Dev. - Cohort Val. UK - 2 Cohort studies Val. Denmark Randomised Controlled Trial |
Dev. - N.R. Val. UK - In-patients & out-patients Val. Denmark - In-patients & out-patients |
Dev. - 69% Val. UK - 67% Val. Denmark - 59% |
Dev. - 21.3 (at baseline) Val. UK - 24.9 (at baseline) Val. Denmark - 26.6 (at baseline) |
Dev. - 73% white Val. UK - 88% white Val. Denmark - 94% white |
Dev. - 44 Val. UK - 44.4 Val. Denmark - 106 |
Dev. - Non-affective, affective & drug induced Val. UK - Non-affective & affective Val. Denmark - Non-affective |
EET Status GAF ≥65 Andreasen Symptom Remission (6 months duration) Quality of Life | At 1 year | Socio-demographic, Clinical | 4 |
| LEIGHTON 2021 | UK | Yes | Dev. - Aug 2005 to Apr 2009 Val. - Apr 2006 to Feb 2009 |
Dev – Cohort Val - Cohort |
N.R. | Dev. - 68.8% Val. - 61.8% |
Dev - 22.6 (at baseline) Val. - 25.0 (at baseline) |
N.R. | Dev. - 41.3 Val. - 48.9 |
Dev. - Non-affective, affective & drug induced Val. - Non-affective, affective & drug induced |
Andreasen Symptom Remission (6 months duration) | At 1 year | Socio-demographic, Clinical | 1 |
| PUNTIS 2021 | UK | Yes | Dev. - 1st Jan 2011 to 8th Oct 2019 Val. - 31st Jan 2006 to 18th Jun 2019 |
Dev. - Case Registry Val. - Case Registry |
Dev. - out-patients Val. - out-patients |
Dev. - 63% Val. - 63% |
Dev. - 25.6 (at baseline) Val. - 26.7 (at baseline) |
Dev. - 74.8% white Val. - 35.4% white |
N.R. | N.R. | Psychiatric hospitalisation after discharge from early intervention | f/u for 1 year | Socio-demographic, Clinical | 1 |
FEP – first episode psychosis; N.R. – not reported; DUP – duration of untreated psychosis; Dev. – development sample; Val. – validation sample; EET – employment, education or training; f/u – follow-up; GAF – Global Assessment of Functioning; DAS – Disability Assessment Schedule
Study prediction modelling methodologies are outlined in Table 2. Nine (69%) studies pertained solely to model development with the highest level of validation reported being apparent validity in four of the studies, internal validity in three of the studies and internal-external validity (via leave one-site out cross-validation) in two of the studies. The remaining four (31%) studies also included a validation cohort and reported external validity. High dimensionality was common across the study cohorts, with the majority having a very low events per variable (EPV) ratio and up to 258 candidate predictors considered. Some form of variable selection was employed in the majority (62%) of studies. The number of events in the external validation cohort ranged from 23 to 173. All the studies had missing data. Six studies (46%) used complete case analysis, five (38%) used single imputation and the remaining two (15%) applied multiple imputation.
Table 2. Study Methodology.
| Study ID | Sample Size | EPV | No. Events in Validation Dataset | No. Candidate Predictors | No. Retained Predictors | Variable Selection | Missing Data Per Predictor | Handling of Missing Data | Modelling Method | Shrinkage | Validation Method Reported | Re-calibration Performed | Full Model Presented | Clinical Usefulness Assessed |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AJNAKINA 2020 | Recruited – 283; Included in modelling - 190 to 222 | 2 to 4 | No external validation | 13 | 12 to 13 | Full model approach or LASSO | up to 59.9% | Single imputation | Logistic regression via ridge & LASSO | Penalised estimation & then uniform | Internal | Yes | Yes | No |
| BHATTACHARYYA 2021 | Sample 1 - Recruited - 1738; Included in modelling - 1663 Sample 2 - Recruited - 240; Included in modelling - 240 |
4 to 62 | No external validation | 10 to 21 | 10 to 21 | Full model approach | Sample 1 - up to 4.3% Sample 2 - none | Complete case analysis | Logistic regression via MLE | None | Apparent & internal | No | Yes | No |
| CHUA 2019 | Recruited - 1724; Included in modelling - 1177 | 16 | No external validation | 22 | 22 | Full model approach | Yes but N.R. | Complete case analysis | Logistic regression via MLE | None | Apparent | No | No | No |
| DEMJAHA 2017 | Recruited - 557; Included in modelling - 286 |
8 | No external validation | 8 | 6 | LASSO | Yes but N.R. | Complete case analysis | Logistic regression via LASSO | Penalised estimation | Internal | No | Yes | No |
| DENIJS 2019 | Recruited - 1100; Included in modelling - 442 to 523 |
2 | No external validation | 258 | 119 to 152 | Recursive feature elimination | up to 20% | Single imputation | Linear Support Vector Machine | None | Internal & Internal-external | No | No | No |
| DERKS 2010 | Recruited - 498; Included in modelling - 297 |
9 to 18 | No external validation | 10 to 20 | 10 to 20 | Full model approach | Yes but N.R. | Complete case analysis | Logistic regression via MLE | None | Apparent | No | No | No |
| FLYCKT2006 | Recruited 175; Included in modelling - 111 |
2 | No external validation | 32 | 5 | Forward selection | Yes but N.R. | Complete case analysis | Logistic regression via MLE | None | Apparent | No | Yes | No |
| GONZALEZ- BLANCH 2010 | Recruited - 174; Included in modelling – 92 |
4 | No external validation | 23 | 2 | Univariate significance testing (p<0.1) then forward selection | Yes but N.R. | Complete case analysis | Logistic regression via MLE | None | Apparent | No | Yes | No |
| KOUTSOULERIS 2016 | Recruited - 498; Included in modelling - 334 |
<1 | No external validation | 189 | N.R. | Forward selection | up to 20% | Single imputation | Nonlinear Support Vector Machine | None | Internal & Internal-external | No | No | No |
| LEIGHTON 2019 (1) | Dev. - Recruited - 83; Included in modelling - 67 to 75 Val. - Recruited - 79; Included - 64 to 67 |
<1 | 27 to 46 | 56 | 5 to 13 | Elastic net | Dev. - up to 13% Val. - up to 37% |
Single imputation | Logistic regression via elastic net | Penalised estimation | External | No | No | No |
| LEIGHTON 2019 (2) | Dev. - Recruited - 1027; Included in modelling - 673 to 829 Val. UK - Recruited - 162; Included - 47 to 142 Val. Denmark - Recruited - 578; Included - 226 to 553 |
1 to 2 | 23 to 173 | 163 | 17 to 26 | Elastic net | Dev. - up to 20% Val. - Yes but N.R. |
Single imputation | Internal Validation - Logistic regression via elastic net External Validation - Logistic regression via MLE |
Internal-external validation - penalised estimation External validation - none |
Internal-external & external | No | No | No |
| LEIGHTON 2021 | Dev. - Recruited - 1027; Included in modelling – 673 Val. - Recruited - 399; Included - 191 |
25 | 103 | 14 | 14 | Full model approach | Dev.- up to 14.9% Val. - up to 56.5% |
Multiple imputation | Logistic regression via MLE | Uniform | Internal & external | Yes | Yes | Yes |
| PUNTIS 2021 | Dev. - Recruited - N.R.; Included in modelling - 831 Val. - Recruited - N.R.; Included - 1393 |
10 | 162 | 8 | 8 | Full model approach | Dev. - up to 15.4% Val. - up to 5.5% |
Multiple imputation | Logistic regression via MLE | Uniform | Internal & external | Yes | Yes | Yes |
N.R. – not reported; Dev. – development sample; Val. – validation sample; EPV – events per variable; LASSO – least absolute shrinkage and selection operator; MLE – maximum likelihood estimation
The most common modelling methodology was logistic regression fitted by maximum likelihood estimation, then logistic regression with regularisation. Only two studies employed machine learning based methods, both via support vector machines. Just over half of studies (54%) did not use any variable shrinkage and only three studies (23%) recalibrated their models based on validation to improve performance. The full model was presented in seven (54%) studies. Only two studies (15%) assessed clinical utility.
The performance of the best model per study outcome grouped by method of validation to allow for appropriate comparisons is reported in Table 3. For the five studies (38%) reporting only apparent validity, two reported a measure of discrimination and only one considered calibration. For the seven studies (54%) reporting internal validation performance, four reported discrimination with a C-statistic ranging from 0.66 to 0.77 and four reported calibration. For the three studies (23%) reporting internal-external validation only one study considered discrimination with a C-statistic which ranged from 0.703 to 0.736 across each of its four models. None of the studies reporting internal-external validation considered any measure of calibration. All four studies (31%) reporting external validation considered model discrimination with C-statistics ranging from 0.556 to 0.876. However, only two of these studies considered calibration. Table 3 also records any global performance metrics which included the Brier score and McFadden’s pseudo-R2, both of which incorporate aspects of discrimination and calibration. Various classification metrics were reported across the study models, but it is difficult to make any meaningful comparisons between these alone, without considering the models’ corresponding discrimination and calibration metrics which were not universally reported.
Table 3. Performance metrics for best model per outcome in each study.
| Study ID | Outcome | Discrimination C- Statistic | Calibration | Other Global Performance Metrics | Classification Metrics |
|---|---|---|---|---|---|
| Studies Reporting Apparent Validity | |||||
| BHATTACHARYYA 2021 | Psychiatric rehospitalisation | 0.749 | Calibration plot only; No α or β | Brier score - 0.192 | N.R. |
| CHUA 2019 | EET Status at 2 years | 0.759 (95%CI: 0.728, 0.790) | N.R. | N.R. | Classification Accuracy - 0.759; PPV - 0.64; NPV - 0.78; PSI - 0.42 |
| DERKS 2010 | Andreasen symptom remission (6 months duration) with 1 year f/u | N.R. | N.R. | N.R. | Classification Accuracy - 0.63; Balanced Accuracy - 0.665; Sensitivity - 0.73; Specificity - 0.60; PPV - 0.73; NPV - 0.61; PSI - 0.34 |
| FLYCKT 2006 | Global functioning (Independent living, EET status, GAF ≥60) at mean 5.4 years | N.R. | N.R. | N.R. | Classification Accuracy - 0.81; Balanced Accuracy - 0.805; Sensitivity - 0.84; Specificity - 0.77 |
| GONZALEZ-BLANCH 2010 | Global functioning (EET status, DAS ≤1) at 1 year | N.R. | Hosmer–Lemeshow test - p = >0.05 | N.R. | Classification Accuracy - 0.750; Balanced Accuracy - 0.587; Sensitivity - 0.261; Specificity - 0.913; PPV - 0.500; NPV - 0.788; PSI - 0.288 |
| Studies Reporting Internal Validity | |||||
| AJNAKINA 2020 | Early treatment resistance from illness onset with 5 years f/u | 0.77 | α - 0.028; β - 1.264; No calibration plot | N.R. | Balanced Accuracy - 0.5; Sensitivity - 0; Specificity - 1.00; PPV - 0.48, NPV - 0.84; PSI - 0.32 |
| Later treatment resistance with 5 years f/u | 0.77 | α - 0.504; β - 1.838; No calibration plot | N.R. | Balanced Accuracy - 0.81; Sensitivity - 0.62; Specificity - 1.00; PPV - 0.42; NPV - 1.00; PSI - 0.42 | |
| BHATTACHARYYA 2021 | Psychiatric rehospitalisation | 0.66 | Calibration plot only; No α or β | Brier score - 0.232 | N.R. |
| DEMJAHA 2017 | Early Treatment Resistance from Illness Onset with 10 years f/u | N.R. | N.R. | Brier score - 0.146; McFadden pseudo R2 - 0.1 | N.R. |
| DENIJS 2019 | Andreasen Symptom Remission (6 months duration) at 3 years | N.R. | N.R. | N.R. | Balanced Accuracy - 0.644; Sensitivity - 0.76; Specificity - 0.50; PPV - 0.722; NPV - 0.548; PSI - 0.27 |
| GAF ≥65 at 3 years | N.R. | N.R. | N.R. | Balanced Accuracy - 0.676; Sensitivity - 0.749; Specificity - 0.584; PPV - 0.701; NPV - 0.642; PSI - 0.343 | |
| Andreasen symptom remission (6 months duration) at 6 years | N.R. | N.R. | N.R. | Balanced Accuracy - 0.647; Sensitivity - 0.787; Specificity - 0.465; PPV - 0.690; NPV - 0.590; PSI - 0.28 | |
| GAF ≥65 at 6 years | N.R. | N.R. | N.R. | Balanced Accuracy - 0.676; Sensitivity - 0.818; Specificity - 0.477; PPV - 0.718; NPV - 0.616; PSI - 0.334 | |
| KOUTSOULERIS 2016 | GAF ≥65 at 1 year | N.R. | N.R. | N.R. | Balanced Accuracy - 0.738; Sensitivity - 0.667; Specificity - 0.809; PPV - 0.515; NPV - 0.888; PSI - 0.403 |
| LEIGHTON 2021 | Andreasen symptom remission (6 months duration) at 1 year | 0.74 (0.73, 0.75) | β - 0.84 (95%CI: 0.81, 0.86); No calibration plot | N.R. | N.R. |
| PUNTIS 2021 | Psychiatric hospitalisation after discharge from early intervention | 0.76 (0.75, 0.77) | α - 0.01 (95%CI: -0.25, 0.24); β - 0.89 (95%CI: 0.88, 0.89); Calibration plot | Brier score - 0.078 | N.R. |
| Studies Reporting Internal-External Validity | |||||
| DENIJS 2019 | Andreasen symptom remission (6 months duration) at 3 years | N.R. | N.R. | N.R. | Balanced Accuracy - 0.638; Sensitivity - 0.629; Specificity - 0.647; PPV - 0.758; NPV - 0.485; PSI - 0.243 |
| GAF ≥65 at 3 years | N.R. | N.R. | N.R. | Balanced Accuracy - 0.648; Sensitivity - 0.658; Specificity - 0.638; PPV - 0.727; NPV - 0.565; PSI - 0.292 | |
| Andreasen symptom remission (6 months duration) at 6 years | N.R. | N.R. | N.R. | Balanced Accuracy - 0.625; Sensitivity - 0.685; Specificity - 0.565; PPV - 0.743; NPV - 0.493; PSI - 0.236 | |
| GAF ≥65 at 6 years | N.R. | N.R. | N.R. | Balanced Accuracy - 0.640; Sensitivity - 0.718; Specificity - 0.561; PPV - 0.732; NPV - 0.553; PSI - 0.285 | |
| KOUTSOULERIS 2016 | GAF ≥65 at 1 year | N.R. | N.R. | N.R. | Balanced Accuracy - 0.711; Sensitivity - 0.641; Specificity - 0.781; PPV - 0.472; NPV - 0.877; PSI - 0.349 |
| LEIGHTON 2019 (2) | EET Status at 1 year | 0.736 (95%CI: 0.702 - 0.771) | N.R. | N.R. | Classification Accuracy - 0.693 (95%CI: 0.660, 0.725); Balanced Accuracy - 0.694 (95%CI: 0.562, 0.812); Sensitivity - 0.722 (95%CI: 0.573, 0.821); Specificity - 0.666 (95%CI: 0.550, 0.803); PPV - 0.719 (95%CI: 0.673, 0.785); NPV - 0.668 (95%CI: 0.606, 0.736); PSI - 0.387 (95%CI: 0.279, 0.521) |
| GAF ≥65 at 1 year | 0.731 (95%CI: 0.697, 0.765) | N.R. | N.R. | Classification Accuracy - 0.687 (95%CI: 0.657, 0.718); Balanced Accuracy - 0.691 (95%CI: 0.541, 0.825); Sensitivity - 0.722 (95%CI: 0.487, 0.778); Specificity - 0.660 (95%CI: 0.594, 0.871); PPV - 0.650 (95%CI: 0.616, 0.769); NPV - 0.726 (95%CI: 0.655, 0.766); PSI - 0.376 (95%CI: 0.271 - 0.535) | |
| Andreasen symptom remission (6 months duration) at 1 year | 0.703 (95%CI: 0.664, 0.742) | N.R. | N.R. | Classification Accuracy - 0.670 (95%CI: 0.636, 0.703); Balanced Accuracy - 0.668 (95%CI: 0.518, 0.827); Sensitivity - 0.584 (95%CI: 0.491, 0.827); Specificity - 0.751 (95%CI: 0.544, 0.827); PPV - 0.679 (95%CI: 0.601, 0.739); NPV - 0.667 (95%CI: 0.631, 0.734); PSI - 0.346 (95%CI: 0.232, 0.473) | |
| Quality of life at 1 year | 0.704 (95%CI: 0.667, 0.742) | N.R. | N.R. | Classification Accuracy - 0.668 (95%CI: 0.632, 0.704); Balanced Accuracy - 0.667 (95%CI: 0.532, 0.789); Sensitivity - 0.623 (95%CI: 0.512, 0.774); Specificity - 0.711 (95%CI: 0.551, 0.803); PPV - 0.633 (95%CI: 0.575, 0.701); NPV 0.700 (95%CI: 0.659, 0.759); PSI - 0.333 (95%CI: 0.234, 0.460) | |
| Studies Reporting External Validity | |||||
| LEIGHTON 2019 (1) | EET status at 1 year | 0.876 (95%CI: 0.864, 0.887) | N.R. | N.R. | Classification Accuracy - 0.851; Balanced Accuracy - 0.845; Sensitivity - 0.815; Specificity - 0.875; PPV - 0.815; NPV - 0.875; PSI - 0.690 |
| Andreasen symptom remission (no duration criteria) at 1 year | 0.652 (95%CI: 0.635, 0.670) | N.R. | N.R. | Classification Accuracy - 0.612; Balanced Accuracy - 0.623; Sensitivity - 0.578; Specificity - 0.667; PPV - 0.794; NPV - 0.424; PSI - 0.218 | |
| Andreasen symptom remission (6 months duration) at 1 year | 0.630 (95%CI: 0.612, 0.647) | N.R. | N.R. | Classification Accuracy - 0.625; Balanced Accuracy - 0.626; Sensitivity - 0.606; Specificity - 0.645; PPV - 0.645; NPV - 0.606; PSI - 0.251 | |
| LEIGHTON 2019 (2) - Validated in UK | EET Status at 1 year | 0.867 (95%CI: 0.805, 0.930) | N.R. | N.R. | Classification Accuracy - 0.838 (95%CI: 0.775, 0.894); Balanced Accuracy - 0.853 (95%CI: 0.740, 0.935); Sensitivity - 0.898 (95%CI: 0.780, 0.966); Specificity - 0.807 (95%CI: 0.699, 0.904); PPV - 0.766 (95%CI: 0.679, 0.867); NPV - 0.911 (95%CI: 0.840, 0.971); PSI - 0.677 (95%CI: 0.519, 0.838) |
| Andreasen symptom remission (6 months duration) at 1 year | 0.680 (95%CI: 0.587, 0.773) | N.R. | N.R. | Classification Accuracy - 0.695 (95%CI: 0.618, 0.771); Balanced Accuracy - 0.695 (95%CI: 0.535, 0.841); Sensitivity - 0.621 (95%CI: 0.455, 0.773); Specificity - 0.769 (95%CI: 0.615, 0.908); PPV - 0.729 (95%CI: 0.636, 0.854); NPV - 0.667 (95%CI: 0.593, 0.759); PSI - 0.396 (95%CI: 0.229, 0.613) | |
| Quality of life at 1 year | 0.679 (95%CI: 0.522, 0.836) | N.R. | N.R. | Classification Accuracy - 0.702 (95%CI: 0.596, 0.809); Balanced Accuracy - 0.729 (95%CI: 0.407, 0.917); Sensitivity - 0.957 (95%CI: 0.564, 1.000); Specificity - 0.500 (95%CI: 0.250, 0.833); PPV - 0.640 (95%CI: 0.561, 0.800); NPV - 0.900 (95%CI: 0.643, 1.000); PSI - 0.540 (95%CI: 0.204, 0.800) | |
| LEIGHTON 2019 (2) - Validated in Denmark | EET Status at 1 year | 0.660 (95%CI: 0.610, 0.710) | N.R. | N.R. | Classification Accuracy - 0.680 (95%CI: 0.609, 0.725); Balanced Accuracy - 0.655 (95%CI: 0.516, 0.774); Sensitivity - 0.584 (95%CI: 0.457, 0.723); Specificity - 0.726 (95%CI: 0.574, 0.824); PPV - 0.490 (95%CI: 0.421, 0.563); NPV - 0.793 (95%CI: 0.760, 0.831); PSI - 0.283 (95%CI: 0.181, 0.394) |
| GAF ≥65 at 1 year | 0.573 (95%CI: 0.504, 0.643) | N.R. | N.R. | Classification Accuracy - 0.456 (95%CI: 0.328, 0.817); Balanced Accuracy - 0.589 (95%CI: 0.234, 0.926); Sensitivity - 0.781 (95%CI: 0.233, 0.945); Specificity - 0.396 (95%CI: 0.234, 0.906); PPV - 0.179 (95%CI: 0.158, 0.333); NPV - 0.914 (95%CI: 0.876, 0.967); PSI - 0.093 (95%CI: 0.034, 0.300) | |
| Andreasen symptom remission (6 months duration) at 1 year | 0.616 (95%CI: 0.553, 0.679) | N.R. | N.R. | Classification Accuracy - 0.618 (95%CI: 0.524, 0.704); Balanced Accuracy - 0.621 (95%CI: 0.342, 0.864); Sensitivity - 0.612 (95%CI: 0.306, 0.843); Specificity - 0.629 (95%CI: 0.378, 0.885); PPV - 0.476 (95%CI: 0.412, 0.636); NPV - 0.742 (95%CI: 0.687, 0.829); PSI - 0.217 (95%CI: 0.099, 0.465) | |
| Quality of life at 1 year | 0.556 (95%CI: 0.481, 0.631) | N.R. | N.R. | Classification Accuracy - 0.589 (95%CI: 0.540, 0.637); Balanced Accuracy - 0.589 (95%CI: 0.312, 0.845); Sensitivity - 0.876 (95%CI: 0.419, 0.947); Specificity - 0.301 (95%CI: 0.204, 0.743); PPV - 0.559 (95%CI: 0.527, 0.642); NPV - 0.706 (95%CI: 0.555, 0.841); PSI - 0.265 (95%CI: 0.081, 0.483) | |
| LEIGHTON 2021 | Andreasen symptom remission (6 months duration) | 0.73 (95%CI: 0.71, 0.75) | α - 0.12 (95%CI: 0.02, 0.22); β - 0.98 (95%CI: 0.85, 1.11); Calibration plot | N.R. | N.R. |
| PUNTIS 2021 | Psychiatric hospitalisation after discharge from early intervention | 0.70 (95%CI: 0.66, 0.75) | α - -0.01 (95%CI: -0.17, 0.167); β - 1.00 (95%CI: 0.78, 1.22); Calibration plot | Brier score - 0.094 | N.R. |
N.R. – not reported; EET – employment, education or training; GAF – Global Assessment of Functioning; DAS – Disability Assessment Schedule; PPV – positive predictive value; NPV – negative predictive value; PSI – prognostic summary index; f/u – follow-up
We applied the PROBAST tool to the 31 different prediction models across the 13 studies in our systematic review and determined an overall risk of bias rating for each study as summarised in Supplementary Table 1. The majority (85%) of studies had an overall ‘high’ ROB. In each of these studies, the ROB was rated ‘high’ in the analysis domain with one study also having a ‘high’ ROB in the predictors domain. The main reasons for the ‘high’ ROB in the analysis domain were insufficient participant numbers and consequently low EPV, inappropriate methods of variable selection including via univariable analysis, a lack of appropriate validation with only apparent validation, an absence of reported measures of discrimination and calibration, and inappropriate handling of missing data by either complete case analysis or single imputation. Two studies, Leighton et al 202129 and Puntis et al 2021,30 were rated overall ‘low’ ROB. These studies considered symptom remission and psychiatric rehospitalisation outcomes, respectively. Both studies externally validated their prediction model and considered its clinical utility. However, neither study considered the implementation of the prediction model into actual clinical practice. When we assessed the 13 included studies according to PROBAST applicability concerns, all the studies were considered overall ‘low’ concern. This is indicative of the broad scope of our systematic review.
Discussion
Our systematic review identified 13 studies reporting 31 prognostic prediction models for the prediction of a wide range of clinical outcomes. The majority of models were developed via logistic regression. There were several methodological limitations identified including a lack of appropriate validation, issues with handling missing data and a lack of reporting of calibration and discrimination measures. We identified two studies with models at low risk of bias as assessed with PROBAST, both of which externally validated their models.
Principal Findings in Context
Our systematic review found no consistent definition of FEP across the different cohorts used for developing and validating prediction models. A lack of an operational definition for FEP within clinical and research settings has previously been identified as major a barrier to progress.31 The majority of cohorts in our systematic review included only individuals with non-affective psychosis with a minority also including affective psychosis. In contrast, early intervention services typically do not make a distinction between affective and non-affective psychosis in those whom they accept into their service.32 As such, there may be issues with generalisability of prediction models developed in cohorts with solely non-affective psychosis to real-world clinical practice.
A wide range of different outcomes were predicted by the FEP models including symptom remission, global functioning, vocational functioning, treatment resistance, rehospitalisation and quality of life outcomes. This is reflective of the fact that recovery from FEP is not readily distilled down to a single factor like symptom remission. Meaningful recovery is represented by a constellation of multidimensional outcomes unique to each individual.33 We should engage people with lived experience, to ensure that prediction models are welcomed and are predicting outcomes most relevant to the people they are for.
All the prediction models were developed in populations from high-income developed countries and only three studies included participants from countries outside of Europe, an issue not unique to FEP research. Consequently, it is currently unknown how prediction models for FEP would generalise to low-income developing countries. Prediction models may have considerable benefit in developing countries where almost 80% of patients with FEP live but where mental health support is often scarce.34 Prediction models could help prioritise the appropriate utilisation of limited healthcare resources.
Only one study considered predictor variables other than clinical or sociodemographic factors. In this study, the additional predictors did not add significant value.22 In recent years substantial progress has been made in elucidating the pathophysiological mechanisms underpinning the development of psychosis. We now recognise important roles for genetic factors, neurodevelopmental factors, dopamine and glutamate.35 Prediction model performance may be improved by the incorporation of these biological relevant disease markers as predictor variables. However, the cost-benefit of adding more expensive and less accessible disease markers must be carefully considered, especially if models are to be utilised in settings where resources are more limited.
Machine learning can be operationally defined as “models that directly and automatically learn from data”. This is to be contrasted with regression models which “are based on theory and assumptions, and benefit from human intervention and subject knowledge for model specification.”36 Just two studies employed machine learning techniques for their modelling.22,26 The rest of the studies employed logistic regression. We were unable to make any comparison between the discrimination and calibration ability of the two studies employing machine learning and the other studies because these metrics were not provided. However, a recent systematic review found no evidence of superior performance of clinical prediction models using machine learning methods over logistic regression.36 In any case, the distinction between regression models and machine learning has been viewed to be artificial. Instead, algorithms may exist “along a continuum between fully human- guided to fully machine-guided data analysis”.37 An alternative comparison may be between linear and non-linear classifiers. Only one study employed a non-linear classifier,26 but again we were unable to gain meaningful insights into its relative performance because appropriate metrics were not provided.
A principal finding from our systematic review is the presence of methodological limitations across the majority of studies. Steyerberg et al outline four key measures of predictive performance that should be assessed in any prediction modelling study – two measures of calibration (the model intercept (A) and the calibration slope (B)), discrimination via a concordance statistic (C), and clinical usefulness with decision-curve analysis (D).6 Model calibration is the level of agreement between the observed outcomes and the predictions. For example, if a model predicts a 5% risk of cancer, then, according to such a prediction, the observed proportion should be five cancers per 100 people. Discrimination is the ability of a model to distinguish between a patient with the outcome and one without.6 Our review found that only seven studies (54%) reported discrimination and just five (38%) reported any measure of calibration. The remaining studies reported only classification metrics, such as accuracy or balanced accuracy. The problem with solely reporting classification metrics is that they vary both across models and across different probability thresholds for the same model. This renders the comparison between models less meaningful. It is further argued that setting a classification threshold for a probability generating model is premature. Rather, a clinician may choose to set different probability thresholds for the same prediction model depending on the situation at hand in order to optimise the balance between false positives and false negatives. For example, in the case of a model predicting cancer, a clinician may choose a lower probability threshold to offer a non-invasive screening test and a higher probability threshold to suggest an invasive and potentially harmful biopsy. Further, without any measure of model calibration we are unable to assess if the model can make unbiased estimates of outcome.38 The final key step in assessing the performance of a prediction model is to determine its clinical usefulness – that is, can better decisions be made with the model than without? Decision-curve analysis considers the net-benefit (the treatment threshold weighted sum of true- minus false-positive classifications) for a prediction model in comparison the default strategy of treating all or no patients, across an entire range of treatment thresholds.39 Only two studies (15%) included in our review considered whether the model was clinically useful. Without proper validation of prediction models, the reported performances are likely to be overly optimistic. Four studies (31%) report only apparent validity. Just four studies (31%) reported external validation, considered essential before applying a prediction model to clinical practice.14
Altogether, just two studies (15%) had an overall ‘low’ risk of bias according to PROBAST, reflecting these methodological limitations. Neither study considered real-world implementation. To progress with implementation, impact studies are required. These would involve a cluster randomised trial comparing patient outcomes between a group with treatment informed by a clinical prediction model and a control group.40 We are not aware of any such study having been carried out within the field of psychiatry. However, Salazar de Pablo et al suggest that PROBAST thresholds for considering a study to be a ‘low’ risk of bias may be too strict.9 Indeed, in the field of machine learning multiple imputation is frequently computationally infeasible and single imputation may be viewed as sufficient. This is especially true in larger datasets or in the presence of relatively few missing values.41
Strengths and limitations
Our review had a number of strengths. We provide the first systematic overview of prediction modelling studies for use in patients with first episode psychosis. We offer a detailed critique of the study characteristics, their methodologies and model performance metrics. Further, our review adheres to gold standard guidance for extracting data from prediction models and for assessing bias, namely the CHARMS checklist and PROBAST.
There were several limitations. Our initial aim was to perform a meta-analysis of any prediction model which was validated across different settings and populations. However, no meta-analysis was possible because no single prediction model was validated more than once. In addition, as a consequence of poor reporting of discrimination and calibration performance across the studies, it was often difficult to make meaningful comparison between the prediction models. Also, the lack of consensus as to the most important outcome measure in FEP, with six different outcomes considered across only 13 included studies, further hindered efforts at drawing meaningful comparisons between the included studies and their respective prediction models. Likewise, if more studies had considered the same outcome measures, this may have afforded the opportunity to validate existing prediction models rather than necessitating the creation of additional new models. All published prediction modelling studies in FEP reported significant positive findings. It is possible that studies which had negative findings were held back from publication reflecting the possibility of publication bias. We originally intended to evaluate the overall certainty in the body of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework.42 GRADE was originally designed for reviews of intervention studies but has not yet been adapted for use in systematic reviews of prediction models. Consequently, in its current form we did not find GRADE to be a suitable tool for our review and decided not to use it. Future research should consider how to adapt GRADE for use in systematic reviews of prediction models.
Implications for future research
It is clear that there is a growing trend for the development of prediction models in FEP.9 FEP is an illness which responds best to an early intervention paradigm.43 Prediction models have the potential to optimise the allocation of time-critical interventions, like clozapine for treatment resistance.44 However, prior to meaningful implementation into real-world clinical practice several steps are necessary. The field must prioritise external validation and replication of existing prediction models in larger sample sizes to increase the EPV. This is best accomplished by an emphasis on data- sharing and open collaboration. Prediction studies should include FEP cohorts from low-income countries where there is considerable potential for benefit by helping to prioritise limited resources to those most in need. Harmonisation of data collection across the field both in terms of predictors and outcomes measured would facilitate validation efforts. There should be a greater consideration of biologically relevant and cognitive predictors based on our growing understanding of disease mechanisms, which could optimise prediction model performance. Finally, our review highlights considerable methodological pitfalls in much of the current literature. Future prediction modelling studies should focus on methodological rigour with adherence to accepted best practice guidance.6,14,38 Our goal in psychiatry should be to develop an innovative approach to care using prediction models. Application of these approaches into clinical practice would enable rapid and targeted intervention thereby limiting treatment associated risks and reducing patient suffering.
Supplementary Material
Funding
RL is funded by an Institute for Mental Health Priestley Scholarship, University of Birmingham. SPL is funded by a Clinical Academic Fellowship from the Chief Scientist Office, Scotland (CAF/19/04). SJW is funded by the Medical Research Council, UK (MR/K013599).
Footnotes
Declaration of Interest
GVG has received support from H2020-EINFRA, the NIHR Birmingham ECMC, NIHR Birmingham SRMRC, the NIHR Birmingham Biomedical Research Centre, and the MRC HDR UK, an initiative funded by UK Research and Innovation, Department of Health and Social Care (England), the devolved administrations, and leading medical research charities. JC has received grants from Wellcome Trust and Sackler Trust and honorariums from Johnson & Johnson. PKM has received honorariums from Sunovion and Sage and is a Director of Noux Technologies Limited. All other authors declare no competing interests.
Author Contribution
PKM and RL formulated the research question and designed the study. RL, SPL, LT and PKM collected the data. RL, SPL and PKM analysed the data and drafted the manuscript. LT, GVG, SJW,S-JHF, FD and JC critically evaluated and revised the manuscript.
Data Availability
Data is available on request from the corresponding author.
References
- 1.Moreno-Küstner B, Martín C, Pastor L. PLoS ONE. Vol. 13. Public Library of Science; 2018. [cited 2021 May 10]. Prevalence of psychotic disorders and its association with methodological issues. A systematic review and meta-analyses. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IHME. GBD Compare Data Visualization. IHME, University of Washington; Seattle, WA: 2021. Institute for Health Metrics and Evaluation (IHME) [Internet]. Available from: http://vizhub.healthdata.org/gbd-compare. [Google Scholar]
- 3.Lally J, Ajnakina O, Stubbs B, Cullinane M, Murphy KC, Gaughran F, et al. British Journal of Psychiatry. Vol. 211. The Royal College of Psychiatrists; 2017. [cited 2018 Jan 18]. Remission and recovery from first-episode psychosis in adults: Systematic review and meta-analysis of long-term outcome studies [Internet] pp. 350–8. [DOI] [PubMed] [Google Scholar]
- 4.Darcy AM, Louie AK, Roberts LW. Machine Learning and the Profession of Medicine. [cited 2018 Jan 18];JAMA. 2016 Feb 9;315(6):551. doi: 10.1001/jama.2015.18421. [Internet] Available from: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2015.18421. [DOI] [PubMed] [Google Scholar]
- 5.Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. [cited 2018 Jun 29];BMJ. 2017 May 23;357:j2099. doi: 10.1136/bmj.j2099. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. [cited 2018 Jan 18];Eur Heart J. 2014 Aug 1;35(29):1925–31. doi: 10.1093/eurheartj/ehu207. [Internet] Available from: https://academic.oup.com/eurheartj/article-lookup/doi/10.1093/eurheartj/ehu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015 Jan;350:g7594. doi: 10.1136/bmj.g7594. [DOI] [PubMed] [Google Scholar]
- 8.Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E, et al. Prediction models for diagnosis and prognosis of covid-19: Systematic review and critical appraisal. [cited 2021 Jun 16];BMJ. 2020 Apr 7;369:26. doi: 10.1136/bmj.m1328. [Internet] Available from: https://www.bmj.com/content/369/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salazar de Pablo G, Studerus E, Vaquerizo-Serrano J, Irving J, Catalan A, Oliver D, et al. Implementing Precision Psychiatry: A Systematic Review of Individualized Prediction Models for Clinical Practice. [cited 2021 May 10];Schizophr Bull. 2021 Mar 1;47(2):284–97. doi: 10.1093/schbul/sbaa120. [Internet] Available from: https://academic.oup.com/schizophreniabulletin/article/47/2/284/5903901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Studerus E, Ramyead A, Riecher-Rössler A. Psychological Medicine. Vol. 47. Cambridge University Press; 2017. [cited 2021 Oct 20]. Prediction of transition to psychosis in patients with a clinical high risk for psychosis: A systematic review of methodology and reporting [Internet] pp. 1163–78. Available from: https://www.cambridge.org/core/journals/psychological-medicine/article/abs/prediction-of-transition-to-psychosis-in-patients-with-a-clinical-high-risk-for-psychosis-a-systematic-review-of-methodology-and-reporting/1CEA9147A2ED19BE6162ECABD57B129F. [DOI] [PubMed] [Google Scholar]
- 11.Rosen M, Betz LT, Schultze-Lutter F, Chisholm K, Haidl TK, Kambeitz-Ilankovic L, et al. Towards clinical application of prediction models for transition to psychosis: A systematic review and external validation study in the PRONIA sample [Internet] [cited 2021 Oct 20];Neuroscience and Biobehavioral Reviews Neurosci Biobehav Rev. 2021 125:478–92. doi: 10.1016/j.neubiorev.2021.02.032. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan S, Northstone K, Gadd C, Walker J, Margelyte R, Richards A, et al. Models to predict relapse in psychosis: A systematic review. [cited 2021 Oct 20];PLoS One. 2017 Sep 1;12(9):e0183998. doi: 10.1371/journal.pone.0183998. [Internet]. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0183998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moons KGM, de Groot JAH, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, et al. Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modelling Studies: The CHARMS Checklist. [cited 2021 May 6];PLoS Med. 2014 11(10):e1001744. doi: 10.1371/journal.pmed.1001744. [Internet]. Available from: www.plosmedicine.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steyerberg EW, Harrell FE., Jr Prediction models need appropriate internal, internal-external, and external validation. [cited 2019 Mar 19];J Clin Epidemiol. 2016 Jan;69:245–7. doi: 10.1016/j.jclinepi.2015.04.005. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolff RF, Moons KGM, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: A tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med. 2019 Jan 1;170(1):51–8. doi: 10.7326/M18-1376. [DOI] [PubMed] [Google Scholar]
- 16.Moons KGM, Wolff RF, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: A tool to assess risk of bias and applicability of prediction model studies: Explanation and elaboration. Ann Intern Med. 2019 Jan 1;170(1):W1-33. doi: 10.7326/M18-1377. [DOI] [PubMed] [Google Scholar]
- 17.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The BMJ. Vol. 372. BMJ Publishing Group; 2021. [cited 2021 May 6]. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ajnakina O, Agbedjro D, Lally J, Di Forti M, Trotta A, Mondelli V, et al. Predicting onset of early- and late-treatment resistance in first-episode schizophrenia patients using advanced shrinkage statistical methods in a small sample. Psychiatry Res. 2020 Dec 1;294:113527. doi: 10.1016/j.psychres.2020.113527. [DOI] [PubMed] [Google Scholar]
- 19.Bhattacharyya S, Schoeler T, Patel R, di Forti M, Murray RM, McGuire P. Individualized prediction of 2-year risk of relapse as indexed by psychiatric hospitalization following psychosis onset: Model development in two first episode samples. Schizophr Res. 2021 Feb 1;228:483–92. doi: 10.1016/j.schres.2020.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Chua YC, Abdin E, Tang C, Subramaniam M, Verma S. First-episode psychosis and vocational outcomes: A predictive model. Schizophr Res. 2019 Sep 1;211:63–8. doi: 10.1016/j.schres.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Demjaha A, Lappin JM, Stahl D, Patel MX, MacCabe JH, Howes OD, et al. Antipsychotic treatment resistance in first-episode psychosis: Prevalence, subtypes and predictors. [cited 2021 Mar 25];Psychol Med. 2017 Aug 1;47(11):1981–9. doi: 10.1017/S0033291717000435. [Internet] [DOI] [PubMed] [Google Scholar]
- 22.de Nijs J. The outcome of psychosis. Utrecht University; 2019. [Internet]. Available from: https://dspace.library.uu.nl/bitstream/1874/376436/1/22_01_3_jessica_de_nijs_compleet_final.pdf. [Google Scholar]
- 23.Derks EM, Fleischhacker WW, Boter H, Peuskens J, Kahn RS. Antipsychotic drug treatment in first-episode psychosis should patients be switched to a different antipsychotic drug after 2, 4, or 6 weeks of nonresponse? J Clin Psychopharmacol. 2010 Apr;30(2):176–80. doi: 10.1097/JCP.0b013e3181d2193c. [DOI] [PubMed] [Google Scholar]
- 24.Flyckt L, Mattsson M, Edman G, Carlsson R, Cullberg J. Predicting 5-Year Outcome in First-Episode Psychosis: Construction of a Prognostic Rating Scale. [cited 2021 Mar 25];J Clin Psychiatry. 2006 Jun;67(6):916–24. doi: 10.4088/jcp.v67n0608. [Internet] [DOI] [PubMed] [Google Scholar]
- 25.González-Blanch C, Perez-Iglesias R, Pardo-García G, Rodríguez-Snchez JM, Martínez-García O, Vázquez-Barquero JL, et al. Prognostic value of cognitive functioning for global functional recovery in first-episode schizophrenia. [cited 2021 Mar 25];Psychol Med. 2010 Jun;40(6):935–44. doi: 10.1017/S0033291709991267. [Internet] [DOI] [PubMed] [Google Scholar]
- 26.Koutsouleris N, Kahn RS, Chekroud AM, Leucht S, Falkai P, Wobrock T, et al. Multisite prediction of 4-week and 52-week treatment outcomes in patients with first-episode psychosis: a machine learning approach. [cited 2021 Mar 25];The Lancet Psychiatry. 2016 Oct 1;3(10):935–46. doi: 10.1016/S2215-0366(16)30171-7. [Internet] [DOI] [PubMed] [Google Scholar]
- 27.Leighton SP, Krishnadas R, Chung K, Blair A, Brown S, Clark S, et al. Predicting one-year outcome in first episode psychosis using machine learning. Acampora G, editor. [cited 2021 Mar 25];PLoS One. 2019 Mar 7;14(3):e0212846. doi: 10.1371/journal.pone.0212846. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leighton SP, Upthegrove R, Krishnadas R, Benros ME, Broome MR, Gkoutos GV, et al. Development and validation of multivariable prediction models of remission, recovery, and quality of life outcomes in people with first episode psychosis: a machine learning approach. [cited 2021 Jan 29];Lancet Digit Heal. 2019 Oct 1;1(6):e261–70. doi: 10.1016/S2589-7500(19)30121-9. [Internet] [DOI] [PubMed] [Google Scholar]
- 29.Leighton SP, Krishnadas R, Upthegrove R, Marwaha S, Steyerberg EW, Broome MR, et al. Development and validation of a non-remission risk prediction model in First Episode Psychosis: An analysis of two longitudinal studies. Schizophr Bull Open. 2021 doi: 10.1093/schizbullopen/sgab041. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puntis S, Whiting D, Pappa S, Lennox B. Development and external validation of an admission risk prediction model after treatment from early intervention in psychosis services. [cited 2021 Mar 29];Transl Psychiatry. 2021 Jun 1;11(1):35. doi: 10.1038/s41398-020-01172-y. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breitborde NJK, Srihari VH, Woods SW. Review of the operational definition for first-episode psychosis [Internet] [cited 2021 Jun 16];Early Intervention in Psychiatry Early Interv Psychiatry. 2009 3:259–65. doi: 10.1111/j.1751-7893.2009.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Institute for Health and Care Excellence (NICE) Implementing the Early Intervention in Psychosis Access and Waiting Time Standard: Guidance. 2016 [Google Scholar]
- 33.Jääskeläinen E, Juola P, Hirvonen N, McGrath JJ, Saha S, Isohanni M, et al. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr Bull. 2013 Nov;39(6):1296–306. doi: 10.1093/schbul/sbs130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh SP, Javed A. World Psychiatry. Vol. 19. Blackwell Publishing Ltd; 2020. [cited 2021 May 13]. Early intervention in psychosis in low- and middle-income countries: a WPA initiative [Internet] p. 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lieberman JA, First MB. Psychotic Disorders. Ropper AH, editor. [cited 2021 May 13];N Engl J Med. 2018 Jul 19;379(3):270–80. doi: 10.1056/NEJMra1801490. [Internet]. Available from: http://www.nejm.org/doi/10.1056/NEJMra1801490. [DOI] [PubMed] [Google Scholar]
- 36.Christodoulou E, Ma J, Collins GS, Steyerberg EW, Verbakel JY, Van Calster B. Journal of Clinical Epidemiology. Vol. 110. Elsevier; USA: 2019. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models; pp. 12–22. [DOI] [PubMed] [Google Scholar]
- 37.Beam AL, Kohane IS. JAMA - Journal of the American Medical Association. Vol. 319. American Medical Association; 2018. [cited 2021 Oct 20]. Big data and machine learning in health care [Internet] pp. 1317–8. Available from: https://jamanetwork.com/journals/jama/fullarticle/2675024. [DOI] [PubMed] [Google Scholar]
- 38.Harrell FE. Regression Modeling Strategies. Springer International Publishing; Cham: 2015. [cited 2021 May 14]. [Internet]. (Springer Series in Statistics). Available from: http://link.springer.com/10.1007/978-3-319-19425-7. [Google Scholar]
- 39.Vickers AJ, van Calster B, Steyerberg EW. A simple, step-by-step guide to interpreting decision curve analysis. [cited 2021 May 14];Diagnostic Progn Res. 2019 Dec 4;3(1):1–8. doi: 10.1186/s41512-019-0064-7. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moons KGM, Kengne AP, Grobbee DE, Royston P, Vergouwe Y, Altman DG, et al. Heart. Vol. 98. BMJ Publishing Group Ltd and British Cardiovascular Society; 2012. [cited 2021 May 14]. Risk prediction models: II. External validation, model updating, and impact assessment [Internet] pp. 691–8. Available from: http://heart.bmj.com/ [DOI] [PubMed] [Google Scholar]
- 41.Steyerberg EW. Clinical Prediction Models. Springer International Publishing; Cham: 2019. [cited 2021 Oct 20]. [Internet]. (Statistics for Biology and Health). Available from: http://link.springer.com/10.1007/978-3-030-16399-0. [Google Scholar]
- 42.Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies [Internet] [cited 2021 Aug 27];Chinese Journal of Evidence-Based Medicine BMJ. 2009 9:503–8. [Google Scholar]
- 43.Birchwood M, Todd P, Jackson C. Early intervention in psychosis: The critical period hypothesis. Br J Psychiatry. 1998;172(S33):53–9. doi: 10.1192/S0007125000297663. [Internet] [DOI] [PubMed] [Google Scholar]
- 44.Farooq S, Choudry A, Cohen D, Naeem F, Ayub M. Barriers to using clozapine in treatment-resistant schizophrenia: systematic review. [cited 2021 Jan 29];BJPsych Bull. 2019 Feb;43(1):8–16. doi: 10.1192/bjb.2018.67. [Internet]. Available from: https://www.cambridge.org/core. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available on request from the corresponding author.