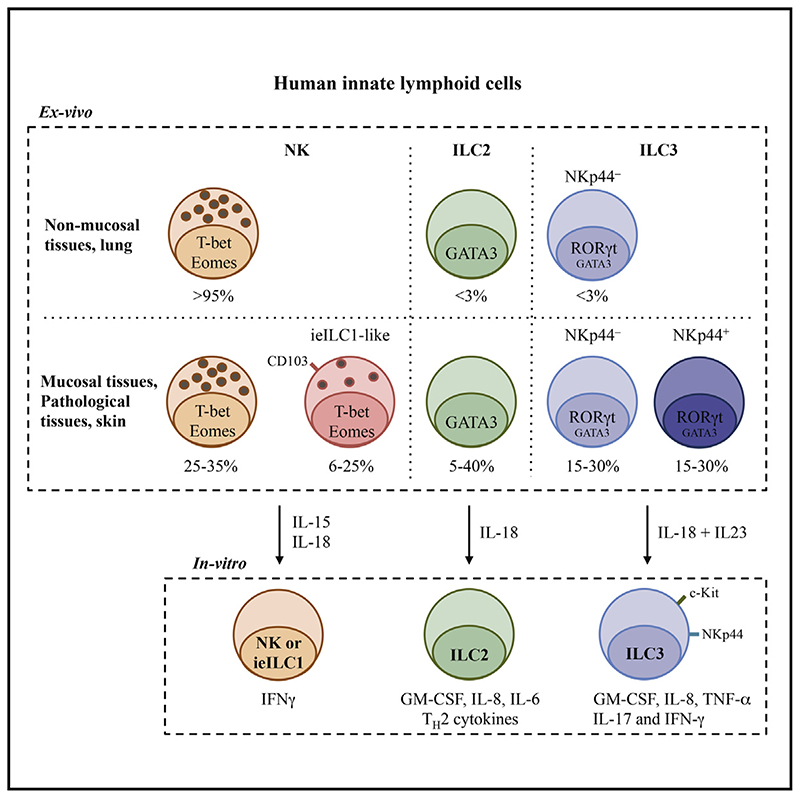
Summary
Animal models have highlighted the importance of innate lymphoid cells (ILCs) in multiple immune responses. However, technical limitations have hampered adequate characterization of ILCs in humans. Here, we used mass cytometry including a broad range of surface markers and transcription factors to accurately identify and profile ILCs across healthy and inflamed tissue types. High dimensional analysis allowed for clear phenotypic delineation of ILC2 and ILC3 subsets. We were not able to detect ILC1 cells in any of the tissues assessed, however, we identified intra-epithelial (ie)ILC1-like cells that represent a broader category of NK cells in mucosal and non-mucosal pathological tissues. In addition, we have revealed the expression of phenotypic molecules that have not been previously described for ILCs. Our analysis shows that human ILCs are highly heterogeneous cell types between individuals and tissues. It also provides a global, comprehensive, and detailed description of ILC heterogeneity in humans across patients and tissues.
Graphic abstract.
Introduction
Innate lymphoid cells (ILCs) represent a heterogeneous population of innate immune cells in mice and humans that include classically defined natural killer (NK) cells as well as more recently described helper-type ILCs (ILC1, ILC2, and ILC3) (Artis and Spits, 2015; Cella et al., 2009; Satoh-Takayama et al., 2008). These cells can be involved in infection, chronic inflammation, and in metabolic disease (Artis and Spits, 2015; Diefenbach et al., 2014; McKenzie et al., 2014). In contrast to T- and B-lymphocytes, ILCs lack somatic rearrangement of antigen receptors (e.g., TCR, BCR), and can be activated by cytokines and/or through natural cytotoxicity receptors (e.g., NKp44) (Glatzer et al., 2013). In contrast to NK cells, helper-type ILCs do not possess efficient cytotoxic capacity (Hazenberg and Spits, 2014). In mice and humans, helper-type ILCs can be subdivided into three main groups analogous to the main subsets of T helper lymphocytes. ILC1 cells can produce T helper-1 (Th1) cell signature cytokines (i.e., IFN-γ) and express the transcription factor T-bet (Bernink et al., 2013), whereas ILC2 cells are capable of secreting Th2 cell-type cytokinesand display a GATA3+ phenotype. ILC3 cells have been shown to be associated with interleukin-17 (IL-17) and IL-22 production, as well as RORγt transcription factor expression, characteristic of Th17 helper T cells (Hazenberg and Spits, 2014). Moreover, similar to ILC2 cells, ILC3 cells express CD127 (IL-7R), which is crucial for their development and survival (Vonarbourg and Diefenbach, 2012). Murine models have demonstrated that ILC subset diversity, assessed using single analyses including mass cytometry, can be heavily influenced by pathogens exposure (Gury-BenAri et al., 2016).
ILC1 cells have been controversially discussed in the literature due to their similarity with NK cells (Serafini et al., 2015). In mice and humans, two non-NK cell ILC1 cell subsets have been described. One subset expresses CD127 but no cytotoxic granules and plays a role in intestinal pathologies (Bernink et al., 2013; McKenzie et al., 2014). The other subset has been described as intraepithelial ILC1 cells (ieILC1) that do not express CD127, but are capable of producing cytotoxic granules (Fuchs et al., 2013). Recently, a transcriptomic in-depth analysis of murine ILC subsets has demonstrated that gene-expression patterns of ILC1 cells overlap with NK cells. This raises the question of whether NK cells and ILC1 cells can truly be classified as distinct cell types (Robinette et al., 2015). In humans, single-cell mRNA analysis has shown that ILC1 cells express transcripts that encode variable regions of the TCR, as well as various other specific T cell markers (Björklund et al., 2016). Consistent with this, another recent paper has shown that the majority of ILC1 cells also express CD5, which is expressed by T cells but not ILCs (Vely et al., 2016).
Murine models have helped to effectively classify ILCs, especially since similarities have been observed between ILCs identified in mice and humans. However, differences in murine and human immunology pose a challenge. The characterization of human ILCs is mainly based on ex vivo analysis and on in vitro-derived cell lines (Bernink et al., 2013) and although human specimens have been used to analyze helper-type ILCs in different tissues (Hazenberg and Spits, 2014), studies analyzing all known ILC subsets for individual tissues in parallel are lacking. In addition, Hazenberg and Spits have demonstrated that a clear definition of ILC2 and ILC3 populations in humans by multi-color flow cytometry requires at least eight fluorescence channels (Hazenberg and Spits, 2014). Consequently, technical limitations of flow cytometry devices such as spectral overlap hamper a further in-depth characterization of these cells. Moreover, reported discrepancies in defining ILC subsets between studies have complicated an accurate comparison of these cells.
Here, we use mass cytometry (cytometry by time of flight, CyTOF), to analyze NK, ILC1, ieILC1, ILC2, and ILC3 cells in parallel across nine different healthy tissues and three pathological tissues. To these ends, we simultaneously assessed a wide range of surface markers, cytotoxic granule components, transcription factors, and activation and proliferation markers on a single cell basis. Our data provide a clear global picture of ILC diversity and heterogeneity in humans.
Results
Mass Cytometry Allows the Identification of Human ILC Subsets
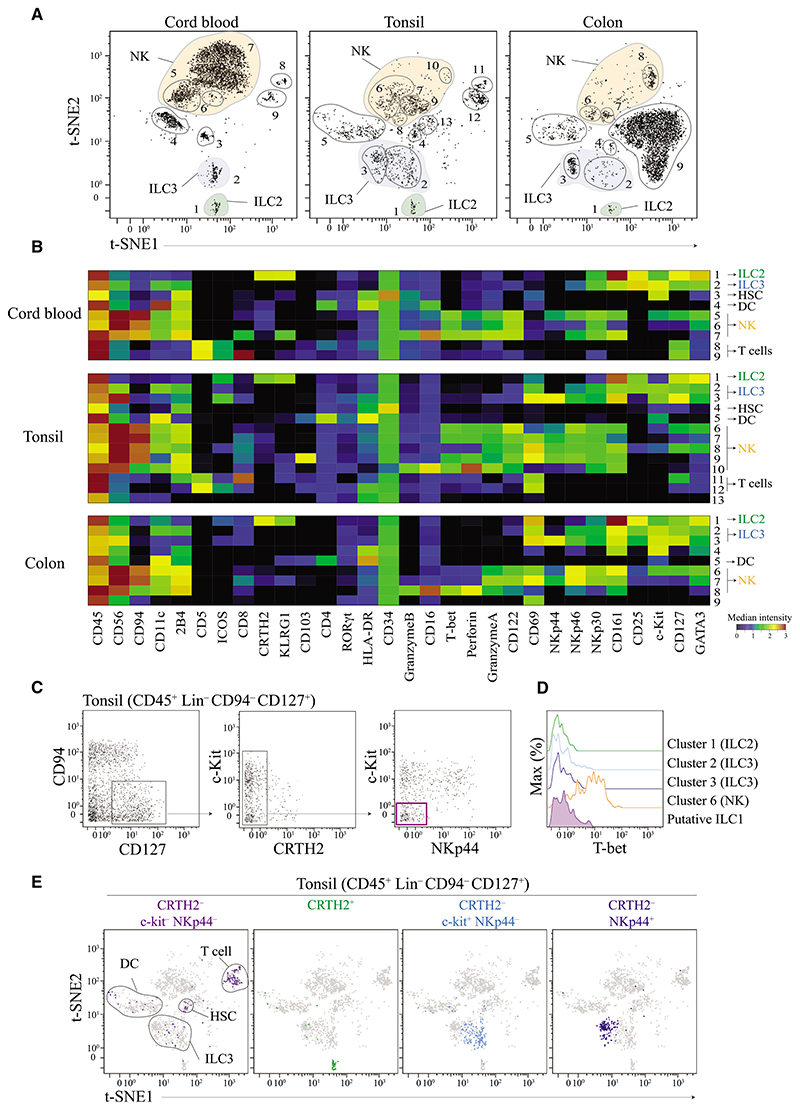
To resolve inconsistencies in ILC subset definitions, we developed a mass cytometry panel consisting of 38 heavy metal-labeled antibodies to identify and characterize ILC populations in humans. This panel included a broad range of phenotypic markers that allow segregation of previously described ILC subsets. Additional markers allowed for simultaneous quantification of lineage defining transcription factors as well as other phenotypic and functional cellular proteins. To demonstrate this approach, we used cord blood, tonsils, and colon specimen because these tissues are known to contain all ILC subsets (NK, ieILC1, ILC1, ILC2, and ILC3 cells). Prior to CyTOF acquisition, T cells (CD3+) and B cells (CD20+) were depleted. Using standard biaxial gating, we further excluded monocytes (CD14+), plasmacytoid dendritic cells (pDC) (CD123+), mast and/or basophils (FcεR1α+), as well as contaminating B cells (CD19+) from live immune cells (Cisplatin– DNA+ CD45+) (Figure S1A). To assess ILC profiles, we also performed t-Distribution Stochastic Neighbor Embedding (t-SNE) analysis. t-SNE projects high dimensional data into low dimensional space by making a pairwise comparison of cellular phenotypes to optimally plot similar cells close to each other (see Supplemental Experimental Procedure). For our analysis, we used t-SNE to reduce 29 parameters (dimensions) into two dimensions (t-SNE1 and t-SNE2). Based on this projection, we identified up to 13 distinct cell clusters in each tissue (Figure 1A). For each cluster, the median intensity of each single marker molecules assessed was quantified and summarized by using a heat-map illustration (Figure 1B). We identified a group of clusters corresponding to contaminating CD4+ T cells (CD5+ CD4+), CD8+ T cells (CD5+ CD8+), hematopoietic stem cells (HSC) (CD34+), and dendritic cells (DC) (HLA-DR+ CD11c+), as well as unidentified cell populations (clusters 13 in tonsils and 9 in colon tissue, which did not express any known ILC marker. This analysis also segregated ILC2 cells (green cluster, CD127+ CRTH2+ KLRG1+ CD25+ GATA3high) and ILC3 cells (blue clusters, CD127+ CRTH2– NKp44+ or –), as well as various NK cell subsets (orange clusters, CD56+ NKp46+ T-bet+ CD16+ Granzyme+ Perforin+). Notably, in tonsils, we observed a cluster that shared all characteristics with previously described mucosal associated ieILC1 cells, which was consistent across different donors (cluster 9 CD56+ T-bet+ CD103+ NKp44+ CD94+, Figures S1B and S1C) (Fuchs et al., 2013).
Figure 1. t-SNE Analysis Objectively Delineates ILCs Populations—with the Exception of ILC1 Cells.
(A) Visualized t-SNE map of cells isolated from cord blood, tonsil, and colon tissue. t-SNE was performed after depleting B and T cells and gating on live CD45+ Lin− (FcεR1α− CD14− CD19− CD123−, see Figure S1A for gating strategy). Clusters (cells populations) were identified by manual gating. Data shown are representatives of one donor per tissue from 3 to 10 independent experiments.
(B) Based on the clusters identified in (A), median intensities for each of the marker were calculated and plotted as a heatmap to identify the respective ILC populations. Data shown are from donor represented in (A).
(C) Identification of putative ILC1 cells using standard bi-axial gating (CD45+ Lin− CD94− CD127− CRTH2− c-Kit− NKp44−) from tonsil. Data shown are from donor represented in (A).
(D) Histogram of T-bet expression by putative ILC1 cells in tonsils. Cell clusters corresponding to ILC2, ILC3, and NK cells were used as controls. Data shown are from donor represented in (A).
(E) Visualization of all different ILC subsets identified by standard bi-axial gating by t-SNE. Data shown are from donor represented in (A).
Please also see Figures S1 and S2.
Helper-Type ILC1 Cells Are Undetectable in Human Tissues
None of the clusters determined by t-SNE analysis was in line with previous ILC1 cell definitions. To reconcile our findings with previous observations, we used a standard bi-axial gating strategy (CD45+ Lin− CD94− CD127+ CRTH2− c-Kit− NKp44−) (Bernink et al., 2013) to delineate putative ILC1-like cells (Figure 1C). However, none of these cells expressed T-bet, a defining signature transcription factor that should be expressed by all cells in the ILC1 lineage (Bernink et al., 2013) (Figure 1D). When we plotted these putative ILC1 cells using the t-SNE, no distinct cluster representing ILC1 cells was observed. Instead, these cells overlapped with T cells, dendritic cells, and ILC3 cell clusters (Figure 1E). In contrast, we observed that manual gating on ILC2 cells, NKp44− ILC3 or NKp44+ ILC3 cells resulted in the identification of independent individual clusters on the two dimensional t-SNE projection map (Figure 1E). Similar findings were observed when analyzing cord blood, colon tissue, or fresh tissues (Figures S2A–S2C). In all cases, we detected the expression of CD5 (contaminating CD4+ or CD8+ T cells), CD11c (contaminating DC) or CD34 (contaminating HSC) on putative ILC1 cells. It is plausible that contamination with T cells, DCs, ILC3 cells, HSCs, and NK cells might have resulted in an inaccurate definition of ILC1 cells in previous studies. This hypothesis was further supported by the observation of an NK cell population that expressed low amount of CD127 and shared other phenotypic characteristics of ILC1 cells (CD127+ T-bet+ CRTH2− c-Kit− NKp44−) (Figure S2D). However, these cells expressed key markers that characterize NK cells but should not be found on helper type ILC1 cells, such as CD94, CD56, NKp46, and perforin (Bernink et al., 2013).
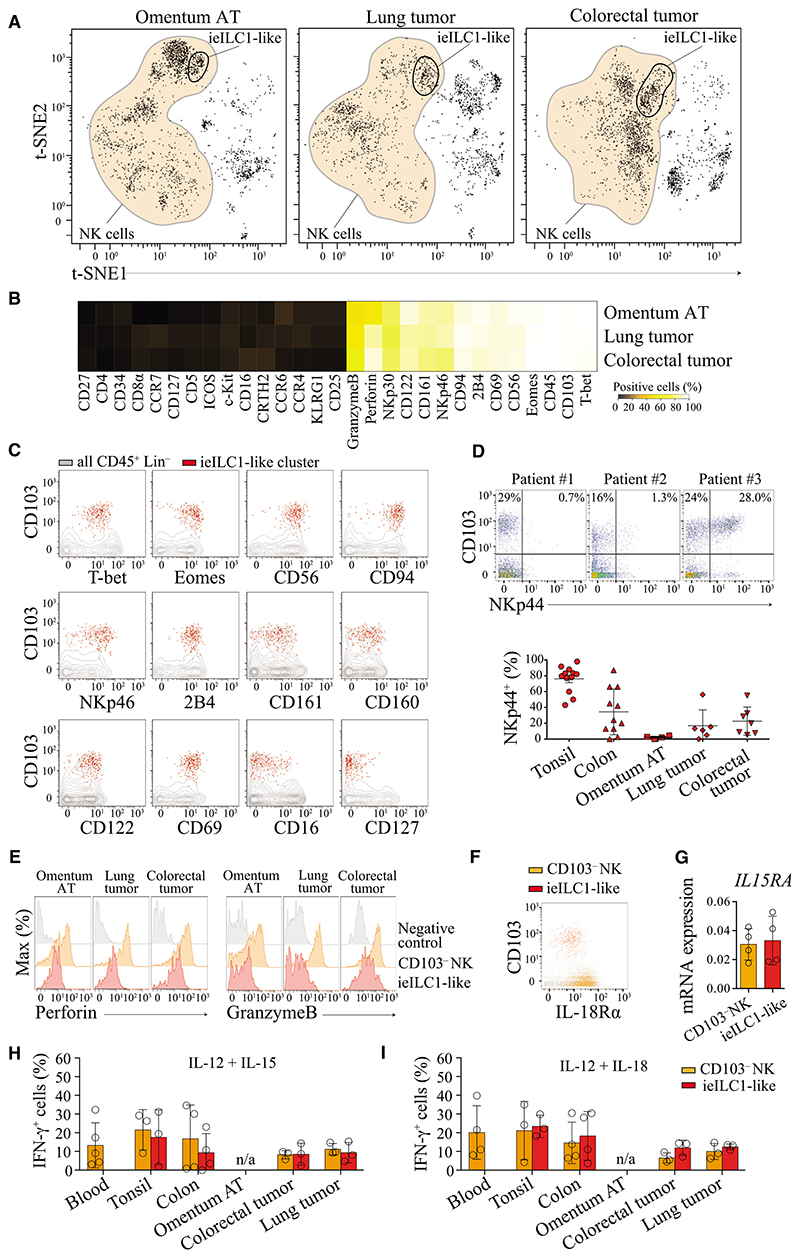
An ieILC1-like Population Can Be Found that Is Not Restricted to Mucosal Tissues and Displays Similarity to NK Cells
Intraepithelial (ie)ILC1 cells have previously been described as cells present in mucosal tissues with an intraepithelial location, producing IFN-γ and cytotoxic granules and having an unique integrin-expression profile and implicated in Crohn’s disease (Fuchs et al., 2013). We also found a cell population displaying phenotypic characteristics of ieILC1 cells in non-mucosal pathological tissues (hereby referred to as ieILC1-like cells). Using t-SNE, we identified a single cluster of these cells in pathological omentum adipose tissues (omentum AT) from obese patients (Ouchi et al., 2011), lung tumors, and colorectal tumors (Figure 2A). Compared to NK cells from the same tissue, these ieILC1-like cells expressed CD103, the transcription factors T-bet and EOMES, as well as surface NK cell markers CD56, NKp46, CD94, 2B4, CD161, CD160, CD122, CD69, and CD49a, but did not express CD16 or CD127 (Figures 2B, 2C, and S3A). On the basis of this analysis, we found that CD103 was consistently expressed on this population, implying that CD103 can be used to identify these cells by standard biaxial gating (Figure S3B). NKp44 is a marker that has been used to characterize ieILC1 cells (Bernink et al., 2015; Fuchs et al., 2013). However, we found that ieILC1-like cells heterogeneously expressed this marker across different individuals and tissues (Figure 2D). Unlike in tonsils where more than 75% of these cells expressed NKp44, frequencies of cells expressing this marker were lower and more variable in colon tissue, colorectal tumors, and lung tumors. Moreover, in omentum AT, NKp44 expression was almost absent on ieILC1-like cells (Figure 2D, lower panel). Using t-SNE, we observed that the ieILC1-like cells did not entirely cluster together and were strongly associated with various NK cell populations (Figure 2A), thus suggesting a close similarity between these two populations.
Figure 2. ieILC1-like Cells Are Present in Non-Mucosal Inflamed Tissues.
(A) Visualized t-SNE map of cells from omentum adipose tissue (AT), lung tumor, and colorectal tumor. t-SNE was performed after depleting B and T cells and gating on live CD45+ Lin− (FcεR1α− CD14− CD19− CD123−). Cluster corresponding to NK cells is represented in orange. Data representative of one donor by tissue from 3 to 5 independent experiments.
(B) Frequencies of cells positive for each marker molecule assessed. Based on the gates shown in (A), the frequencies of positive cells for each marker were calculated and represented as a heatmap.
(C) Dot plots showing markers expressed by ieILC1-like cells. According to the clusters identified by t-SNE (A) ieILC1-like cells (red) were displayed on a biaxial dot plot. CD45+ Lin− (gray) cells were used as controls. Representative data from one donor (lung tumor) is shown from 3 to 5 independent experiments.
(D) Top shows dot plots representing NKp44 expression by ieILC1 cells from three colorectal tumor samples (three donors). Data shown is gated on NK and ieILC1-like cells (see gating Figure S3B). Bottom shows expression of NKp44 by ieILC1 cells from tonsil, colon, omentum AT, lung, and colorectal tumor tissue (for gating see Figure S3B). Data shown are the mean values ± SD from at least 3 independent experiments.
(E) Ex vivo expression of perforin and granzymeB by ieILC1 cells (red) and NK cells (orange) from omentum AT, lung, and colorectal tumor tissue. Helper type ILCs (gray) were used as controls (for gating see Figure S4A). Data shown are one representative from three different donors.
(F) Dot plot representing expression of IL18Rα on ieILC1 (red) and CD103− NK cells (orange). Representative data from one individual from 3 independent experiments.
(G) IL15RA mRNA expression of ieILC1 and CD103− NK cells (see sorting gating strategy Figure S3C), means ± SD, n = 4.
(H and I) Frequencies of IFN-γ producing ieILC1-like or CD103– NK cells from PBMC, tonsil, colon, lung tumor, and colorectal tumor tissue following stimulation with IL-12 and IL-15 (H) or IL-12 and IL-18 (I). Data shown are the mean values ± SD from 3 independent experiments. n/a (data not available) (see Figure S3D for staining).
Please also see Figure S3.
To explore this hypothesis, we investigated functional characteristics of these cells. Ex vivo ieILC1-like cells produced gran-zyme B and perforin but showed lower and more variable amounts of both cytotoxic granules as compared to CD103− NK cells of the same individual (Figure 2E). We found that both ieILC1-like and CD103− NK cells, expressed IL-18R (Figure 2F) and mRNA coding for IL-15Rα (IL15RA) (Figures 2G and S3C). Consistent with previous ieILC1 studies (Fuchs et al., 2013), we also found that ieILC1-like cells derived from various tissues produced IFN-γ in response to IL-15 or IL-18 stimulation at comparable quantities as detected for CD103− NK cells derived from the same tissues (Figures 2H and 2I, S3D).
Human ILC2 and ILC3 cells Cannot Exclusively Be Defined by Transcription Factors GATA3 and RORγt
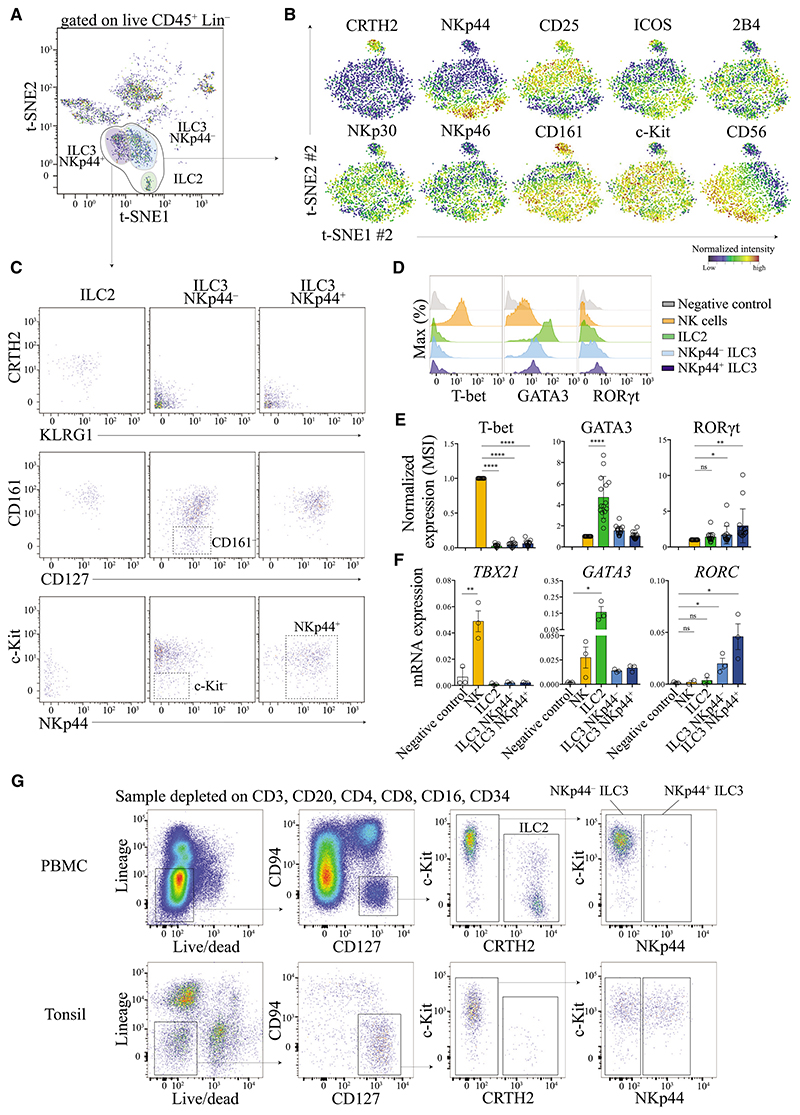
To investigate the heterogeneity of helper type ILC subsets, we performed a second round of t-SNE analysis (t-SNE #2) to zoom-in exclusively on ILC2 and ILC3 cells (Figures 3A and 3B). This gave rise to the identification of a homogeneous and distinct cluster of cells that matched the previous definitions of ILC2 cells (CD127+ CD161+ CRTH2+ KLRG1+) (Mjosberg et al., 2011) (Figure 3C). A higher degree of heterogeneity could be observed within the ILC3 cellular compartment (CD127+ CRTH2−) (Figures 3B and S4A). For instance, we observed a diverse expression of markers such as CD56, ICOS, 2B4, and natural cytotoxicity receptors (NCRs) such as NKp30, NKp46, and NKp44 across this plot (Figures 3B, S4A). Consistent with previous reports, we noted that cells could clearly be segregated based on their expression of NKp44 and this also correlated with other markers such as CD25 and ICOS (Figure 3A). It is clear, however, that these two cell subsets of ILC3 cells (NKp44 positive versus negative) were highly diverse in their expression of other NCRs that we tested (NKp30 and NKp46). This is in contrast to murine cells, where NCR expression in general has been used to segregate two major subsets of ILC3 cells. These findings suggest that human ILC3 cell subsets cannot be identified based on NCR expression alone. Nonetheless, on the basis of this analysis and the differences observed between tissues (e.g., absence of NKp44+ ILC3 cells in blood, or cord blood, Figure 1), we think that it is useful to segregate the two major subsets of ILC3 cells based on NKp44 expression. We also observed, although to a lesser degree, some diversity in terms of CD161 expression (Figure 3C). In particular, we found that NKp44− ILC3 cells displayed a broad distribution of CD161 and c-Kit expression, which we think precludes the utility of these markers as defining features of this ILC3 population (Figure 3C).
Figure 3. t-SNE Analysis Objectively Delineates Helper-Type ILC Populations.
(A) Visualized t-SNE map of cells from tonsil tissue. t-SNE was performed after depleting B and T cells and gating on live CD45+ Lin− (FcεR1α− CD14− CD19− CD123−). Manual gating identified three clusters corresponding to ILC2 (green), NKp44− ILC3 (light blue), and NKp44+ ILC3 (dark blue) cells. Data shown is from three donors.
(B) A second round of t-SNE analysis (t-SNE #2) was applied to zoom-in exclusively on ILC2 and ILC3 cells cluster (black circle, A). Normalized expression intensities of each marker were calculated and overlayed on the t-SNE plot (see Figure S4A for others markers).
(C) Representation of marker expression by standard bi-axial gating according to the gates shown in (A).
(D) Expression of T-bet, GATA3, and RORγt by each ILC population. HSC (CD34+) were used as negative control. Representative data from one donor (tonsil) is shown.
(E) Normalized mean signal intensities (MSI) of T-bet, GATA3, or RORγt of each ILC population. Data from >5 independent experiments, n = 17 tonsil tissue, paired t test, ns, non significant, *p ≤ 0.05, **p ≤ 0.01, ****p ≤ 0.0001.
(F) mRNA expression of TBX21, GATA3, and RORC of each ILC population. Data from one experiment, n = 3 tonsil tissue, unpaired t test, ns, non significant, *p ≤ 0.05, **p ≤ 0.01.
(G) Flow cytometry based gating strategy for the identification of each helper-type ILC population from PBMCs and tonsil. Lineage markers: CD14, CD5, CD19, FcεR1α, CD123, CD11c.
To assure that the t-SNE analysis was reliable in the delineation of these helper type ILC subsets, we also investigated transcription factor expression in these cells. In comparison to NK and ILC3 cells, we found that ILC2 cells expressed significantly higher amount of transcription factor GATA3 (Figures 3D and 3E and S3A). This was further confirmed by measuring of the mRNA amount of sorted cells and we found that both, NK and ILC3 cells expressed transcripts for GATA3 (Figure 3F). This observation is consistent with previous reports in mice (Zhong et al., 2015) and demonstrates that GATA3 is highly expressed by ILC2 cells, but cannot exclusively be used to distinguish ILC2 cells from other ILC types. We detected the expression of RORγt in both ILC3 subsets by using either antibody staining or quantitative gene-expression analysis of pre-sorted cells. Notably, NKp44+ ILC3 cells expressed higher amounts of RORγt protein and RORC mRNA transcripts than NKp44− ILC3 cells (Figures 3D–3F). We further found that T-bet and TBX21 mRNA expression were restricted to NK cells and was not detected for ILC2 and ILC3 cells.
In summary, based on the high-dimensional perspective provided by these data and the t-SNE analysis, we found that helper-type ILCs cannot be defined solely by the expression of different transcription factors. First, due to the weak RORγt antibody stainings (especially for NKp44− ILC3 cells, see Discussion), it is not possible to accurately discern ILC3 cells by using this marker in humans. Similarly, because GATA3 is expressed by other ILC subsets, the usage of this marker alone would not be an accurate approach to discriminate ILC2 cells from other ILC subsets. Thus, among helper-type ILCs (CD45+ Lin− CD11c− CD5− CD34− CD127+ CD94−), ILC2 cells can be defined as CD161+ CRTH2+, and ILC3 cells can be defined as CRTH2− which can further be divided into two major subpopulations based on the expression of NKp44 (Figure S4B). It is noteworthy, that the use of exclusion markers is important to remove contaminating cells, especially CD5 and CD11c. Based on these observations, we developed a panel and a gating strategy, which can be used by flow cytometry to identify helper-type ILC subsets in different tissues (Figure 3G, Experimental Procedures).
Human ILC2 and ILC3 Cells Are Under-Represented in Non-mucosal and Lung Tissues
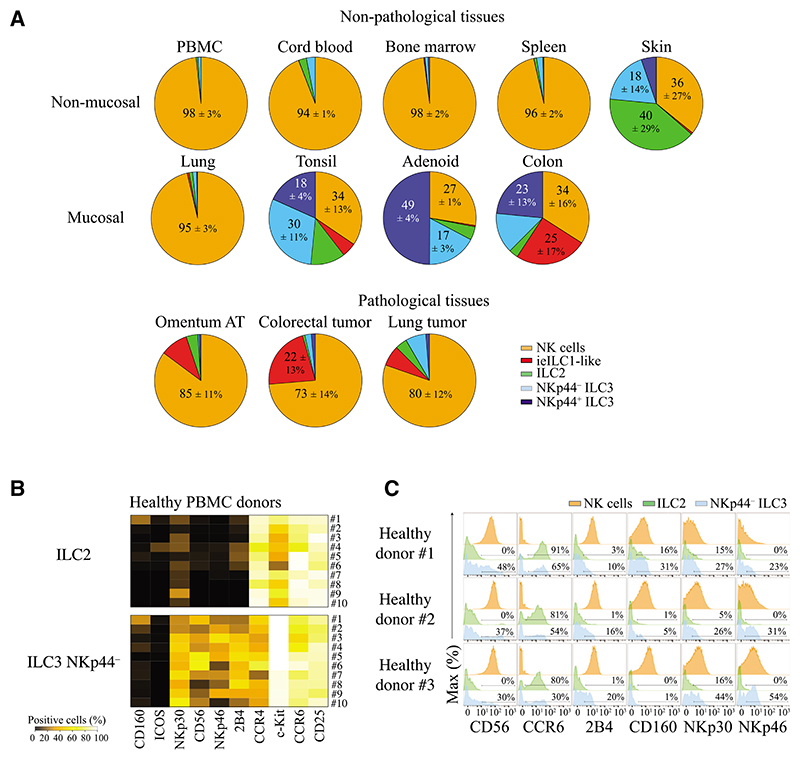
Next, we analyzed the frequencies and phenotypic profiles of each of the ILC subsets across nine different non-pathological and three different pathological tissues by mass cytometry. Therefore, we used the gating strategies described above to simultaneously analyze all ILC subsets (Figure S4B). Due to the large sample size, we were unable to collect data for all different tissues simultaneously in a single batch of experiment, which is needed for an accurate t-SNE analysis. We consequently used manual gating to quantify the frequencies of cells expressing marker molecules fitting the definitions of each ILC subset described for each of the cellular proteins measured across tissues and donors.
In non-pathological tissues, we observed a clear distinction between mucosal and non-mucosal tissues (except for lung) with regard to the frequencies of different ILC subsets. In non-mucosal tissues (PBMCs, cord blood, bone marrow, spleen) and lung, ILC2, and ILC3 cells represented only a minority of the total ILC numbers (0.1% to 5%). In these tissues, NKp44+ ILC3 cells represented less than 0.5% of all ILCs. It is notable though, that the frequencies of ILC2 and NKp44− ILC3 cells were higher in cord blood as compared to adult blood (2.7% versus 0.63%, respectively, for ILC2 and 3.09% versus 1.05% for NKp44− ILC3 cells) (Figure 4A and Table S1). In lung, ILC2 and ILC3 cells represented less than 3% of total innate lymphoid cells (mostly composed of NK cells). In mucosal tissues (tonsil, adenoid, colon) and skin, helper-type ILC frequencies were higher than those of NK cells. As described above, among non-pathological tissues we found ieILC1-like cells only in tonsils (5% ± 4.1%) and colon tissue (25.1% ± 17%). NKp44+ ILC3 cells were highly represented (>14% of total ILCs) in these barrier tissues (tonsil, adenoid, colon, skin). In human skin, ILC2 cells represented the main population of ILCs, similar to what has been observed in murine dermis (Roediger et al., 2013). In pathological tissues, we observed ILC2 and ILC3 cell recruitment in all tissues studied (colorectal tumors, lung tumors, and omemtum AT). However, more than 95% of ILCs infiltrating these inflamed tissues were composed of NK and ieILC1-like cells (Figure 4A). As reflected by the standard deviation, we observed a remarkable heterogeneity in ILC frequencies between individuals that were higher in mucosal and skin tissues as compared to other tissues (Table S1). Despite this variation, the general ILC composition of each tissue was relatively constant (e.g., in all individuals helper-type ILCs outnumbered NK cells in colon, tonsil, adenoid, and skin).
Figure 4. Frequencies of ILC Populations in Human Tissues.
(A) Pie charts displaying the frequencies of NK (orange), ieILC1-like (red), ILC2 (green), NKp44− ILC3 (light blue), and NKp44+ ILC3 (dark blue) cells in human tissues. Data shown are the mean values from at least 9 independent experiments, Non-pathological tissues included PBMC (n = 15), cord blood (n = 3), bone marrow (n = 4), spleen (n = 6), lung (n = 6), skin (n = 4), tonsil (n = 9), adenoid (n = 4), and colon (n = 9) tissue. Pathological tissues included omentum AT (n = 4), lung tumor (n = 6), and colorectal tumor (n = 7) (Table S1).
(B) Heatmap displaying the frequencies of positive cells for markers expressed by ILC2 and NKp44− ILC3 cells. Data shown are PBMCs (n = 10) from 4 independent experiments.
(C) Histograms displaying the percentages of ILC2 and NKp44− ILC3 cells expressing markers. Data shown are PBMCs from 3 different healthy donors from 4 independent experiments.
Please also see Figure S4.
In addition to the overall ILC and NK cell subset composition, we observed phenotypic variations across different donors. Due to limitations in sample size and patient data from tissues, we highlight donor-to-donor variations by using ten PBMC samples from healthy donors without infection, chronic disease, or medical treatments (see Experimental Procedures). We observed a high degree of variation in the expression of c-Kit, CD56, CCR4, CCR6, 2B4, NKp30, and NKp46. Expression of some of the markers assessed, such as CD160 (not previously described for ILC), were observed only in few patients (Figures 4B and 4C). As compared to murine studies, genetic variation and environmental exposure can greatly influence the immune cell composition of humans. The variability in expression of these markers in healthy donors might influence their response to stimuli and therefore should be considered in future human ILC studies.
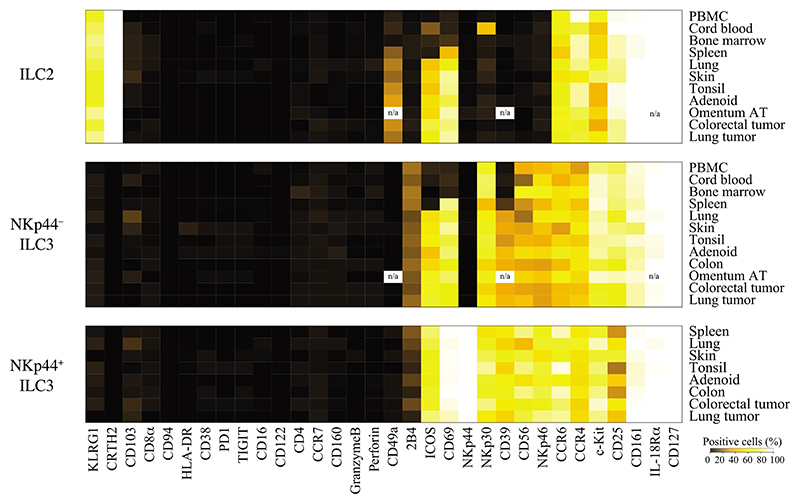
Variable Tissue-Specific Phenotypic Profiles of Helper-Type ILCs
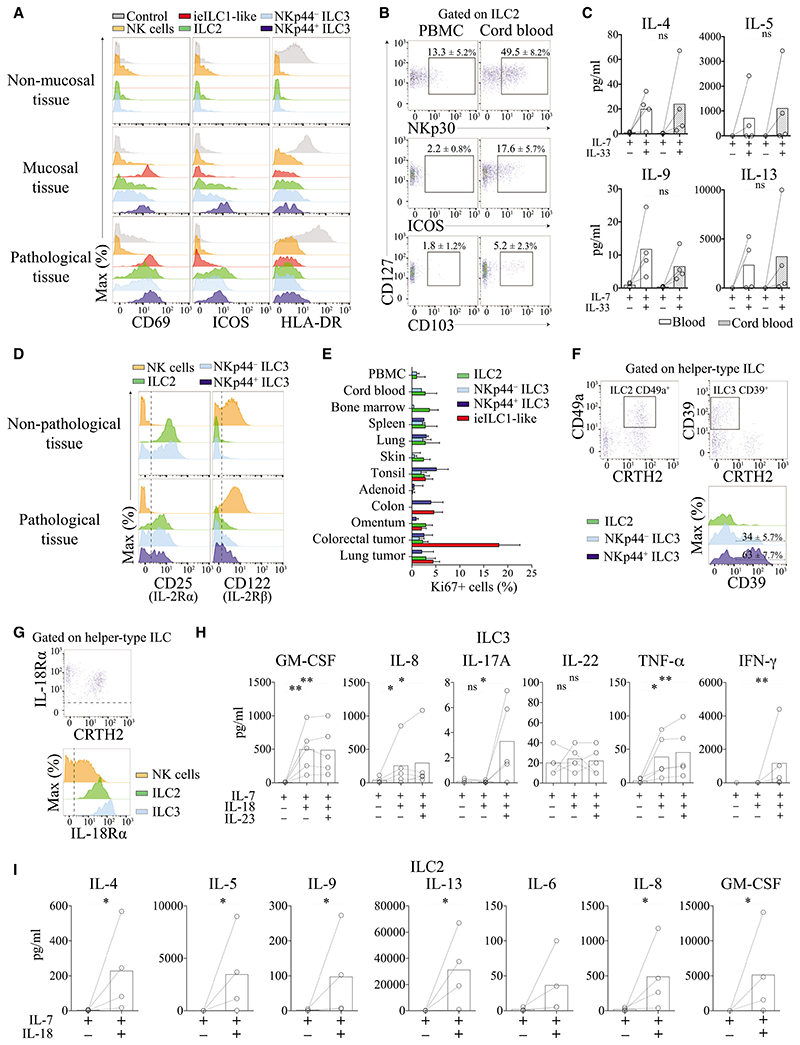
As observed for helper-type ILC frequencies (Figure 4A), we also observed a clear distinction between non-pathological, mucosal, and pathological tissues in terms of phenotypic profiles of ILCs (Figure 5). Under non-pathological condition, the majority of ILC2 cells expressed KLRG1, CD25, CCR6, and CCR4 and exhibited variable c-Kit expression. We found that CD69 expression on ILC2 cells was restricted to skin, mucosal tissues, and a majority of cells found in the spleen (Figures 5 and 6A). ICOS expression is required for survival and efficient cytokine production by ILC2 cells (Maazi et al., 2015). Notably, ICOS expression was not ubiquitous and mainly limited to ILC2 cells derived from mucosal tissues (Figures 5 and 6A). In contrast to adult PBMCs, cord blood ILC2 cells expressed ICOS at a relatively high frequency (17.6% ± 5.7%). Some cord-blood-derived ILC2 cells also expressed NKp30 (49.5% ± 8.2%) and CD103 (5.2% ± 2.3%) (Figure 6B). On the basis of these findings, we investigated whether these phenotypic differences between adult PBMCs and cord blood ILC2 cells were indicative of functional differences. We found, however, that stimulation of sorted ILC2 cells with IL-33, a cytokine known to stimulate Th2 cell responses, elicited comparable quantities of IL-4, IL-5, IL-9, and IL-13 production by these cells (Figure 6C).
Figure 5. Heterogeneity of Helper-Type ILC between Tissues and Donors.
Heatmap displaying the frequencies of ILC2 and ILC3 cells positive for 32 different marker molecules. Data shown are from at least 13 independent experiments, n/a (data not available).
Figure 6. Heterogeneity of Helper-Type ILCs.
(A) Expression of CD69, ICOS, and HLA-DR by ILC populations in non-mucosal (blood), mucosal (tonsil), and pathological tissue (lung tumor). Data shown is one representative from 3 different donors.
(B) Comparison of NKp30, ICOS, and CD103 expression by ILC2 cells derived from adult PBMCs and cord blood. Data shown are the mean values ± SD from at least 6 independent experiments (n = 14 for PBMCs, and n = 3 for cord blood). n/a (data not available).
(C) Expression of IL-4, IL-5, IL-9, and IL-13 by sorted ILC2 cells from blood (white) or cord blood (gray) after culture with or without IL-33. Data shown are the mean values ± SD from 2 independent experiments. n = 4, Mann-Whitney test, ns: non-significant, *p ≤ 0.05, **p ≤ 0.01.
(D) Expression of CD25 and CD122 by ILC population in non-pathological (PBMCs) and pathological tissue (lung tumor). Data shown is one representative from two different donors from at least 6 independent experiments.
(E) Frequencies of proliferating ILCs (Ki67+) in all tissues analyzed. Data shown are the mean values ± SD from at least 9 independent experiments (see Figure S5A for staining).
(F) Expression of CD49a and CD39 by ILC2 and ILC3 cell populations in tonsils. Data shown are the mean values ± SD from at least 6 independent experiments.
(G) Expression of IL-18Rα by NK (orange), ILC2 (green), and ILC3 (blue) cells. Representative dot plot from one donor (PBMC) from at least 6 independent experiments.
(H) Expression of GM-CSF, IL-8, IL-17A, TNFα, and IL-13 by sorted ILC3 cells from PBMCs after culture with or without IL-18 and IL-23. Data shown are the mean values ± SD from 2 independent experiments, n = 5, Mann-Whitney test, ns: non significant, *p ≤ 0.05, **p ≤ 0.01.
(I) Expression of IL-4, IL-5, IL-9, IL-13, GM-CSF, IL-8, and IL-6 by sorted ILC2 cells from PBMCs after culture with or without IL-18 and IL-23. Data shown are the mean values ± SD from 2 independent experiments, n = 4, Mann-Whitney test, ns: non significant, *p ≤ 0.05, **p ≤ 0.01 (see Figure S5D for sorting strategy).
Please also see Figure S5.
Similar to ILC2, NKp44− ILC3 cells exhibited heterogeneous phenotypes across different tissues. ICOS and CD69 were only expressed on NKp44− ILC3 cells from skin and mucosal tissues (Figures 5 and 6A). The majority of these cells expressed CD25, NKp30, CCR6, and CCR4 and more than 40% expressed CD56, 2B4, and NKp46 across all tissues (Figure 5). We also observed differences in phenotypes of NKp44− ILC3 cells as compared to NKp44+ ILC3 cells. The average frequency of CD25 expression was lower on NKp44+ ILC3 cells as compared to NKp44− ILC3 cells in all tissues except for skin where both cell types displayed high frequencies of CD25 expression. In contrast, CD56, NKp46, and ICOS were highly expressed on NKp44+ ILC3 cells as compared to their NKp44− counterparts. In some patients, we also noticed expression of CD103 on various ILC2 and ILC3 cell subsets. Less than 5% of all of these ILC subsets were observed to be CD103+ (see Figure 6B for ILC2 cell staining).
Within pathological tissues, we found that helper-type ILC displayed a phenotype similar to that observed in mucosal tissues. We observed an upregulation of CD69 and ICOS expression (Figures 5 and 6A), while expression of others markers remained similar to those observed in mucosal tissues (Figure 5). We were not able to detect significant differences in major histocompatibility complex (MHC) class II (HLA-DR) molecule expression between ILC subsets within the same tissues tested (Figure 6A). Nevertheless, since in some experiments we detected a slightly higher expression of MHCII expression in mucosal and pathological tissues as compared to non-mucosal tissue (e.g., Figure 6A). As previously described, ILCs express CD25, the non-signaling IL-2Rα subunit that increases the affinity of IL-2R (CD122 and γc) for IL-2. Under pathological conditions, we found that helper-type ILCs slightly upregulated CD122 expression in tissues. However, this expression remained very low as compared to NK cells (Figure 6D). One of the main roles of IL-2 signaling is the maintenance and proliferation of immune cells. In line with this, the frequency of proliferating helper-type ILCs (Ki67+) was relatively low in non-pathological, mucosal, or pathological tissues (< 5%) (Figure 6E). For instance, in colorectal tumors, we detected a high frequency of proliferating (Ki67+) ieILC1-like and/or NK cells but not helper-type ILCs (Figures 6E and S5A).
We were also able to identify a number of previously unreported markers expressed by ILCs. For instance, we found that ILC3 cells can express 2B4 (CD244), a receptor having an activating or inhibitory function (Figures 5 and 3B). Moreover, we found that ILC2 cells can express integrin CD49a. However, unlike ieILC1-like cells, which also express CD49a, CD49a+ ILC2 cells did not necessarily express integrin CD103 (data not shown). We further found that ILC3 cells can also express CD39, an ectoenzyme involved in suppression of inflammation (Figure 6F). Here, CD39 was mostly restricted to NKp44+ ILC3 cells from mucosal and inflamed tissues (Figure 5). We did not detect expression of any other receptors involved in immune suppression, such as PD-1, TIGIT, or CD38 by any of the ILC subsets across all tissues tested (Figure 5).
Human ILC2 and ILC3 Cells Respond to IL-18 Stimulation by Secreting Th1, Th2, or Th17 Cytokines
We observed the expression of IL-18Rα by ILC2 and ILC3 cells (Figure 6G), which has not been reported previously. By using quantitative-PCR, we confirmed that ILC2 and ILC3 cells express mRNA coding for both chains of the IL-18 receptor (Figure S5B). Interaction between IL-18R and IL-18 is implicated in promoting production of Th1 cell-related cytokines. We found that ILC2 cells produced IL-4, IL-5, IL-9, IL-13, GM-CSF, IL-6, and IL-8 in response to IL-18 stimulation (Figure 6I). This cytokine profile produced by ILC2 cells was similar to that observed in response to IL-33 (Figure S5C) and demonstrates that IL-18 has a similar effect as IL-33 on ILC2 cell activation. ILC3 cells produced GM-CSF, TNF-α, and IL-8 in response to stimulation with IL-18, as well as in combination with IL-23 (Figure 6H) but only low amounts of IL-17. We did not detect IL-22 production by ILC3 cells in response to IL-18 stimulation. We also observed IFN-γ secretion by ILC3 cells in the presence of IL-18 and IL-23. After in vitro culture with IL-18 and IL-23, these IFN-γ producing ILC3 cells maintained their ILC3 cell phenotypes (i.e., expression of c-Kit and upregulation of NKp44; Figure S5D). This confirms that ILC3 cells can be plastic in terms of cytokine production in vitro as described previously (Cella et al., 2009), but do not acquire an ILC1 cellular phenotype.
Discussion
Our study aimed to clarify the composition and phenotypic profiles of ILCs in humans. In contrast to NK ILC2 and ILC3 cells, we did not detect any ILC1 population as previously described (Bernink et al., 2015; Bernink et al., 2013). Although we observed populations with shared phenotypic characteristics of previously defined ILC1 cells (CD127+ CRTH2− c-Kit− NKp44−), other markers expressed by these cells were not compatible with the current definitions of ILC1 cells. Our analysis, including the t-SNE analysis, showed that these cells derived from contaminating T cells, DCs, HSC, ILC3, and/or NK cells. Recently, as part of a single-cell transcriptomic analysis of ILC cells, it was reported that tonsil ILC1 cells express transcripts coding for rearranged TCR, as well as various other T cell-specific genes (Björklund et al., 2016). This together with another recent report using CD5 staining (Vely et al., 2016) supports our findings that T cells can heavily contaminate ILC1 cells. In vitro observations about ILC1 cell plasticity, differentiation into ILC3 cells, and the ability to produce both, Th1- and Th17 cell-related cytokines (Bernink et al., 2015) can also be explained by contaminating NK and ILC3 cells in ILC1 gating as we describe here. Furthermore, the first publication that describes ILC1 cells showed a bi-modal expression of T-bet by these cells (Bernink et al., 2013). This confirms that ILC1 cells are composed of at least two cell populations. Because a large number of markers was required for this detailed and accurate characterization of putative ILC1 cells, we think that it might be possible that previous identifications of human ILC1 cells comprised cellular contaminations, which could not be excluded due to technical limitations.
Using t-SNE analysis, we identified a unique subset of NK cells (ieILC1-like cells) that matches previous descriptions of ieILC1 cells (Fuchs et al., 2013), except for variable NKp44 expression between tissues and individuals. Remarkably, this population was also observed in non-mucosal pathological tissues. A similar population was previously described in uterine tissue from pregnant women and was termed decidual NK cells (Kopcow et al., 2005). As reported, we found that this population also expressed NKp44 and CD103 and had a low expression of cytotoxic granule components. In vitro experiments showed that decidual stromal medium (containing TGF-ß) can convert blood peripheral NK cells into decidual NK cells sharing a similar phenotype. The profiles of these ieILC1-like cells also appeared to match those of a previously reported subset of intrahepatic NK cells and consistent with this, we found that this population expressed integrin CD49a. All of these observations strongly suggest that ieILC1-like cells do not represent an independent lineage of innate lymphoid immune cells specific to mucosal tissues, but a NK cell subset that acquired this specific phenotype under the influence of mucosal or inflamed tissue environments.
Consistent with previous studies, our t-SNE analysis confirmed that helper-type ILCs can be divided into three main populations: ILC2, NKp44– ILC3, and NKp44+ ILC3 cells. The use of t-SNE was also useful for delineating ILC3 cells. By this analysis, we observe that all ILC3 cells coalesce into one amorphous population of cells. We observed diversity within these cells that results from the variable expression of numerous markers, such as CD56, ICOS, and the NCRs. As described for mice, this diversity might be related to how ILCs can integrate and differentiate in response to signals from the microenvironment (Gury-BenAri et al., 2016). Nonetheless, we found that NKp44 expression functions to divide this population, and this distinction is especially meaningful when using it to divide subsets of ILC3 cells and to compare the compositions of different tissue types. We also noted that RORγt antibody staining was more difficult to detect in the NKp44− subset of ILC3 cells. Because this antibody staining was weak, we also sorted these cells and showed that RORC expression could be detected. However, because the gene expression was not assessed at a single cell level, this does not rule out the possibility that some of the ILC3 cells (especially the NKp44− subset) do not express RORγt. Nonetheless, we argue that the t-SNE analysis, which takes into account the composite of all markers (Newell and Cheng, 2016), should be an accurate mean to delineate this subset of cells. In contrast to published murine studies, we did not observe a coordinate segregation of ILC3 cells based on NKp46 or CCR6 expression in humans. Furthermore, we found that c-Kit and CD161 were not sufficiently reliable for the identification of NKp44− ILC3 cells. We did not observe an ILC3 cell subset expressing T-bet as it has been reported in mice (Klose et al., 2013). However, we found that all human ILC subsets (NK and ILC3 cells included) expressed at least a low amount of GATA3. This is consistent with the role of GATA3 in promoting IL-22 expression in ILC3 cells as it has been observed in mice (Serafini et al., 2014; Zhong et al., 2015). On the basis of these observations, we propose a panel and gating strategy to identify human helper-type ILCs using flow cytometry, which we further confirmed by qPCR and functional studies of sorted cells. Importantly, the expression of various markers on ILCs can be modulated in response to cytokine exposure (e.g., CD127, CD94, CRTH2) (Cella et al., 2009). Therefore, we think that this gating strategy should be adjusted depending on the tissue and/or inflammatory conditions being studied.
Human tissues can be divided into two categories based on their overall ILC subsets composition. Under non-pathological conditions, non-mucosal and lung tissues displayed low frequencies of ILC2 and ILC3 cells (<5% of ILC), this questioning the importance of these cells in tissue homeostasis and immune surveillance in comparison to NK cells (>95%). NKp44+ ILC3 cells were nearly absent in these tissues (<0.5%). However, this observation does not undermine the role of these cells in infections and pathologies such as lung inflammation, where it is clear that rare cells can have profound effects (Huang et al., 2015). Our study demonstrates that ILC2 cells found in cord blood are functionally similar to ILC2 cells derived from adult blood when stimulated with IL-33. However, we cannot exclude the possibility that alternative stimulation conditions would be able to trigger functional differences between these cells. These cells displayed an increased frequency and distinct phenotypic profiles when compared with their counterparts derived from adult blood (expressing more ICOS, CD103 and NKp30—a receptor also involved in virus or parasite recognition), which suggests a possible role for ILC2 cells in normal neonatal immunity. Under inflammatory conditions, more than 95% of ILCs recruited were composed of NK or eILC1-like cells in all tissues analyzed. Despite a low frequency of helper-type ILCs, these cells represent a phenotype that is similar to mucosal ILCs with a remarkably high expression of CD69 and ICOS.
In contrast to non-mucosal and lung tissues, oral and gastro-intestinal mucosal and skin tissues contained high frequencies of helper-type ILCs, which is consistent with the role of these cells in human barrier surface immunity and which has already been partially confirmed in mice (Artis and Spits, 2015; Song et al., 2015). This distinction between mucosal and non-mucosal tissues also corresponds with an upregulation of CD69 and ICOS. Recently, it has been demonstrated that ICOS–ICOS-L interaction between human ILC2 cells is required for efficient function and survival of these cells (Maazi et al., 2015). Expression of ICOS on ILC3 cells suggests a role for ICOS-L-mediated activation. However, this further raises the question about a potential ICOS–ICOS-L interaction between ILC2 and ILC3 cells and the cytokines that might be produced by these cells (TH2 or TH17 cell-associated cytokines). Our analyses also identified a number of previously unreported markers expressed by helper-type ILCs in oral and gastrointestinal mucosal, as well as skin tissues. We found that ILC2 cells expressed CD49a, an integrin providing tissue retention and survival signals. We report that 2B4 was expressed on ILC3 cells. This might be relevant since, depending on the tissue location and inflammatory condition, 2B4–CD48 interactions can inhibit cytokine production by human NK cells (Morandi et al., 2005). We also found that CD39 was expressed by ILC3 cells, and particularly by NKp44+ ILC3 cells, which can play a role in immunosuppression through its ability to degrade extracellular ATP and the modulation of purinergic signaling. These observations about helper type ILC phenotypes could be considered as additional targets for therapeutics approaches.
Across all tissues studied, we were unable to clearly detect MHC II expression by ILCs. As compared to mice, this observation can be explained by different molecular mechanisms regulating MHCII expression in humans (Robinette and Colonna, 2016). We can hypothesize that MHC II expression on ILCs is restricted to some specific inflammatory conditions. As reported, ILC2 and ILC3 cells proliferate in response to IL-2 stimulation (Cella et al., 2009; Moro et al., 2010). However, we observed that helper-type ILCs do not abundantly express CD122 (IL-2Rß). A weak expression of CD122 on ILC3 can be explained by the fact that IL-2 signaling constrains IL-17 production (Laurence et al., 2007). However, helper-type ILCs highly express CD25, the non-signaling subunit that increases the affinity of IL-2R. As observed for DCs, CD25 on helper-type ILCs could trans-present IL-2 to other immune cells as a way to provide survival signal (Boyman and Sprent, 2012). In this study, we show that both ILC2 and ILC3 cell subsets expressed a functional IL-18R in all tissues analyzed. This receptor binds IL-18, a cytokine associated with Th1 cell cytokine production (Dinarello et al., 2013). We observed that IL-18 could elicit Th1 cell cytokine production by ILC3 cells. Despite this, we found that these cells maintained their ILC3 phenotype, excluding plasticity toward an ILC1 cellular phenotype. Importantly, we did not detect T-bet expression on ILC3 cells ex vivo. These observations confirm a functional plasticity of ILC3 cells in vitro, which needs to be further confirmed in vivo. We also found that both, ILC2 and ILC3 cells produce GM-CSF and IL-8 in response to IL-18, two cytokines promoting the recruitment and activation of myeloid cells, such as monocytes and neutrophils. Our data show that ILC2 cells can also produce Th2 cell-associated cytokines in response to this cytokine stimulation, at similar amounts as by IL-33 stimulation. In contrast, IL-18 in combination with IL-23 induced the secretion of IL-17 but not IL-22 by ILC3 cells. This might be relevant because human intestinal epithelial cells are capable of producing high amounts of IL-18 in Crohn’s disease (Pizarro et al., 1999). Furthermore, macrophages that are present in inflamed tissues produce large amounts of IL-18 (Dinarello et al., 2013). Taken together, these observations reveal the potential for novel mechanisms of ILC2 and ILC3 cell activation under inflammatory conditions.
In summary, this study provides a global, comprehensive, and detailed description of human heterogeneity in ILCs across patients, tissues, in non-pathological conditions, and within various pathological environments. Despite a homology with mice, our study highlights the uniqueness of human ILCs in terms of their composition, phenotypes, and heterogeneity. These differences between mice and humans should be considered as results from mouse ILC studies that are translated into humans.
Experimental Procedures
Human Samples and Cells Isolation
The use of human tissues was approved by the appropriate IRBs, A*STAR, the Singapore Immunology Network and the Health Sciences Authority of Singapore or the KwaZulu-Natal Research Institute for TB- HIV (K-RITH). Briefly, tissues were mechanically dissociated in small pieces and incubated at 37°C for 15 to 40 min in media with Collagenase IV and DNase. Dissociatedtissues were filtered and washed. Except to indication, all samples were cryopreserved and stored in liquid nitrogen. see Supplemental Experimental Procedure and Table S3 for more details about samples details and preparation.
CyTOF Staining, Data Analysis, and t-SNE
Samples were depleted of T cells (αCD3) and B cells (αCD20) using anti-Mouse IgG microbeads (Miltenyi). Prior to surface staining, cells were stained with Cisplatin (viability marker). Cells were then stained witha first antibody cocktail containing: αCD122, αCCR4, αCCR6, αNKp30, αNKp44, αNKp46, αCRTH2-FITC, αc-Kit-APC during for 15 min (see Table S2 for clone list and metal). After washing, cells were stained with the remaining antibodies for 15 min. After two washing steps, cells were fixed in fixation FoxP3 buffer (eBioscience) for 30 min. After washing in perm buffer, cells were stained with αEomes-PE or RORγt-PE during 30 min in perm buffer. Cells were washed and stained with αPE, αT-bet, αPerforin, αGranzymeA, αGranzymeB, αKi67 for 30 min in perm buffer. After two washing steps, cells were stained for barcoding, fixed in PBS 2% PFA overnight, and stained for DNA. see Supplemental Experimental Procedure for more details.
Antibody Conjugation
Purified antibodies lacking carrier proteins were purchased from the companies listed in Table S2. Antibody conjugation was performed according to the protocol provided by Fluidigm (see Figure S1 for each staining).
Flow Cytometry Staining and Cell Sorting
For NK or ieILC1 staining, samples were stained with αCD14, αCD3, αCD19, αCD56, αCD103, Live–dead. Cells were sorted according to gating strategy shown in Figure S4B.
For ILC subsets, samples were depleted of contaminating cells using αCD3, αCD20, αCD8, αCD4, αCD34, and αCD16 using anti-Mouse IgG microbeads (Miltenyi). Cells were then stained first with antibodies cocktail containing αCRTH2-FITC, αCD127-biotinylate, αNKp44-APC for 15 min. After washing, cells were stained with αCD94-PercP-Cy5.5, αc-KIT-BV421, Streptavidine PE-Cy7, and lineage markers: αCD14-PE, αCD5-PE, αCD11c-PE, αCD19-PE, αFcER1a-PE, αCD123-PE, Live-dead for 15 mn.
Cells were sorted according to gating strategy shown in Figure 3D. see Supplemental Experimental Procedures for more details and clones listed.
qPCR
From sorted cells mRNA was extracted using RNeasy Micro Kit (QIAGEN). cDNA was generated using high-capacity cDNA reverse transcription kit (Life Technologies). qPCR was performed using TaqMan® Universal Master Mix II (Applied Biosystems) and TaqMan primer for ACTB, RORc, TBX21, GATA3, IL15RA, IL18R1, IL18RAP, and IL1RAPL1. Results were normalized to the housekeeping gene ACTB. see Supplemental Experimental Procedures for more details.
Cell Culture and LuminexAssays
For NK or ieILC1-like stimulation, fresh samples were dissociated as described. Cell suspensions were incubated overnight in the presence of IL-12, IL-15, and IL-18 (50 ng/mL). BrefeldinA was finally added for 4 hr. Cells were stained for CyTOF or FACS analysis as described previously.
Sorted ILC2 or ILC3 cells were cultured for 3–4 days, with IL-7, IL-33, IL-18, and IL-23 (50 ng/mL) as indicated. Supernatant was analyzed for cytokines using Luminex drop array. see Supplemental Experimental Procedures for more details.
Supplementary Material
Highlights.
Comprehensive profiling of human ILCs across tissues
Detailed description of previously defined ILC subsets except helper-type ILC1
ieILC1-like cells are present in several tissues and functionally similar to NK cells
Identification of markers expressed on ILCs, including functional IL-18R
Acknowledgments
The authors thank all members of E.N. and F.G. laboratory, Etienne Becht, Harsimran Singh, the SIgN community, and the SIgN flow cytometry and luminex facilities. This study was funded by A-STAR/SIgN core funding, A-STAR/SIgN immunomonitoring platform funding, a Translational & Clinical Research grand from the National Medical Research Council, Singapore (NMRC/TCR/007-NCC/2013) and the National Cancer Center Singapore CG/007/2013-SD2 (NCCSPG-YR2015-JUL-13). P.K. is supported by NIHR Biomedical Research Centre, Oxford, an NIHR Senior Fellowship and WT109965MA.
Footnotes
Author contributions
Y.S. designed and did research, analyzed data, and wrote the paper. M.F. provided technical help with CyTOF, performed experiments, and reviewed the paper. C.Y.L. helped to process samples. S.-L.K., N.M., S.L., A.K., J.R.F., C.-L.T., M.H.K., K.D., T.K.H.L., A.C.Y.F., C.W.H., J.K.Y.C., M.C.L., S.N., H. K.K.T., R.A., E.-H.T., A.T., and D.S.W.T. provided samples. S.B., M.S., and S.-A.E.S.T. provided omentum adipose. F.G., H.N.K., P.K., A.L., and I. B.T. provided samples and discussed data. E.W.N. initiated and led the project, developed scripts for CyTOF analysis, and helped write the paper.
References
- Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand N, Buskens CJ, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14:221–229. doi: 10.1038/ni.2534. [DOI] [PubMed] [Google Scholar]
- Bernink JH, Krabbendam L, Germar K, de Jong E, Gronke K, Kofoed-Nielsen M, Munneke JM, Hazenberg MD, Villaudy J, Buskens CJ, et al. Interleukin-12 and -23 Control Plasticity of CD127(+) Group 1 and Group 3 Innate Lymphoid Cells in the Intestinal Lamina Propria. Immunity. 2015;43:146–160. doi: 10.1016/j.immuni.2015.06.019. [DOI] [PubMed] [Google Scholar]
- Björklund AK, Forkel M, Picelli S, Konya V, Theorell J, Friberg D, Sandberg R, Mjösberg J. The heterogeneity of human CD127(+) innate lymphoid cells revealed by single-cell RNA sequencing. Nat Immunol. 2016;17:451–460. doi: 10.1038/ni.3368. [DOI] [PubMed] [Google Scholar]
- Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach A, Colonna M, Koyasu S. Development, differentiation, and diversity of innate lymphoid cells. Immunity. 2014;41:354–365. doi: 10.1016/j.immuni.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin-18 and IL-18 binding protein. Front Immunol. 2013;4:289. doi: 10.3389/fimmu.2013.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, Cella M, Colonna M. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity. 2013;38:769–781. doi: 10.1016/j.immuni.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatzer T, Killig M, Meisig J, Ommert I, Luetke-Eversloh M, Babic M, Paclik D, Blüthgen N, Seidl R, Seifarth C, et al. RORγt+ innate lymphoid cells acquire a proinflammatory program upon engagement of the activating receptor NKp44. Immunity. 2013;38:1223–1235. doi: 10.1016/j.immuni.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Gury-BenAri M, Thaiss CA, Serafini N, Winter DR, Giladi A, Lara-Astiaso D, Levy M, Salame TM, Weiner A, David E, et al. The Spectrum and Regulatory Landscape of Intestinal Innate Lymphoid Cells Are Shaped by the Microbiome. Cell. 2016;166:1231–1246.:e13. doi: 10.1016/j.cell.2016.07.043. [DOI] [PubMed] [Google Scholar]
- Hazenberg MD, Spits H. Human innate lymphoid cells. Blood. 2014;124:700–709. doi: 10.1182/blood-2013-11-427781. [DOI] [PubMed] [Google Scholar]
- Huang Y, Guo L, Qiu J, Chen X, Hu-Li J, Siebenlist U, Williamson PR, Urban JF, Jr, Paul WE. IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat Immunol. 2015;16:161–169. doi: 10.1038/ni.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose CS, Kiss EA, Schwierzeck V, Ebert K, Hoyler T, d’Hargues Y, Goppert N, Croxford AL, Waisman A, Tanriver Y, Diefenbach A. AT-bet gradient controls the fate and function of CCR6-RORγt+ innate lymphoid cells. Nature. 2013;494:261–265. doi: 10.1038/nature11813. [DOI] [PubMed] [Google Scholar]
- Kopcow HD, Allan DS, Chen X, Rybalov B, Andzelm MM, Ge B, Strominger JL. Human decidual NK cells form immature activating synapsesand are not cytotoxic. Proc Natl Acad Sci USA. 2005;102:15563–15568. doi: 10.1073/pnas.0507835102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Maazi H, Patel N, Sankaranarayanan I, Suzuki Y, Rigas D, Soroosh P, Freeman GJ, Sharpe AH, Akbari O. ICOS:ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity. 2015;42:538–551. doi: 10.1016/j.immuni.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie AN, Spits H, Eberl G. Innate lymphoid cells in inflammation and immunity. Immunity. 2014;41:366–374. doi: 10.1016/j.immuni.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- Morandi B, Costa R, Falco M, Parolini S, De Maria A, Ratto G, Mingari MC, Melioli G, Moretta A, Ferlazzo G. Distinctive lack of CD48 expression in subsets of human dendritic cells tunes NK cell activation. J Immunol. 2005;175:3690–3697. doi: 10.4049/jimmunol.175.6.3690. [DOI] [PubMed] [Google Scholar]
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- Newell EW, Cheng Y. Mass cytometry: blessed with the curse of dimensionality. Nat Immunol. 2016;17:890–895. doi: 10.1038/ni.3485. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro TT, Michie MH, Bentz M, Woraratanadharm J, Smith MF, Jr, Foley E, Moskaluk CA, Bickston SJ, Cominelli F. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn’s disease: expression and localization in intestinal mucosal cells. J Immunol. 1999;162:6829–6835. [PubMed] [Google Scholar]
- Robinette ML, Colonna M. Innate lymphoid cells and the MHC. HLA. 2016;87:5–11. doi: 10.1111/tan.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immunological Genome Consortium Immunological Genome Consortium. Robinette ML, Fuchs A, Cortez VS, Lee JS, Wang Y, Durum SK, Gilfillan S, Colonna M. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat Immunol. 2015;16:306–317. doi: 10.1038/ni.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, Mitchell AJ, Tay SS, Jain R, Forbes-Blom E, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14:564–573. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Serafini N, Klein Wolterink RG, Satoh-Takayama N, Xu W, Vosshenrich CA, Hendriks RW, Di Santo JP. Gata3 drives development of RORγt+ group 3 innate lymphoid cells. J Exp Med. 2014;211:199–208. doi: 10.1084/jem.20131038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini N, Vosshenrich CA, Di Santo JP. Transcriptional regulation of innate lymphoid cell fate. Nat Rev Immunol. 2015;15:415–428. doi: 10.1038/nri3855. [DOI] [PubMed] [Google Scholar]
- Song C, Lee JS, Gilfillan S, Robinette ML, Newberry RD, Stappenbeck TS, Mack M, Cella M, Colonna M. Unique and redundant functions of NKp46+ ILC3s in models of intestinal inflammation. J Exp Med. 2015;212:1869–1882. doi: 10.1084/jem.20151403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vely F, Barlogis V, Vallentin B, Neven B, Piperoglou C, Perchet T, Petit M, Yessaad N, Touzot F, Bruneau J, et al. Evidence of innate lymphoid cell redundancy in humans. Nat Immunol. 2016;17:1291–1299. doi: 10.1038/ni.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonarbourg C, Diefenbach A. Multifaceted roles of interleukin-7 signaling for the development and function of innate lymphoid cells. Semin Immunol. 2012;24:165–174. doi: 10.1016/j.smim.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Zhong C, Cui K, Wilhelm C, Hu G, Mao K, Belkaid Y, Zhao K, Zhu J. Group 3 innate lymphoid cells continuously require the transcription factor GATA-3 after commitment. Nat Immunol. 2015;17:169–178. doi: 10.1038/ni.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.