Abstract
The hematopoietic system has a very well-studied hierarchy with the long-term (LT) hematopoietic stem cells (HSCs) taking the top position. The pool of quiescent adult LT-HSCs generated during the fetal and early postnatal life acts as a reservoir to supply all the blood cells. Therefore, the maintenance of this stem cell pool is pivotal to maintaining homeostasis in hematopoietic system. It has long been known that external cues, along with the internal genetic factors influence the status of HSCs in the bone marrow (BM). Hypoxia is one such factor that regulates the vascular as well as hematopoietic ontogeny from a very early time point in development. The metabolic outcomes of a hypoxic microenvironment play important roles in functional regulation of HSCs, especially in case of adult BM HSCs. Anaerobic metabolic pathways therefore perform prominent role in meeting energy demands. Increased oxidative pathways on the other hand result in loss of stemness. Recent studies have attributed the functional differences in HSCs across different life stages to their metabolic phenotypes regulated by respective niches. Indicating thus, that various energy production pathways could play distinct role in regulating HSC function at different developmental/physiological states. Here, we review the current status of our understanding over the role that energy production pathways play in regulating HSC stemness.
Keywords: hematopoietic stem cell, metabolism, aging, DNA damage response, ROS, oxidative damage
Introduction
Hematopoietic stem cells are the somatic stem cells which maintain supply of each type of blood cell all through the lifetime (1). Hematopoietic stem cells (HSCs) maintain their pool and produce just the required numbers of mature blood cells through a series of progenitor cells, which become more committed with sequential proliferative events. During development, HSCs emerge from hemogenic endothelium and are highly proliferative, whereas in adults they reside in the bone marrow (BM) cavity and are largely quiescent (2). Dormancy of primitive HSCs ensures maintenance of homeostasis via production of highly proliferative progenitors. The state of dormancy is broken whenever required and is reversible. Physiological requirements of the cells are met by the metabolic pathways employed. Metabolism consists of a series of processes, which help the cells to maintain homeostasis and meet the energy demands for their functions. HSCs show metabolic plasticity that helps in meeting the ever-changing physiological requirements by making logical cell fate decisions. Adult HSCs that are mostly maintained in a state of dormancy, depend greatly on anaerobic glycolysis, which is believed to be aided by the hypoxic niche (3). Their dependence on anaerobic glycolytic pathways is reflected in high levels of glycolytic intermediates and byproducts and relative absence of tricarboxylic acid (TCA) cycle products (4). Confirmation of the role of anaerobic pathways in maintaining stemness came from a variety of genetic mouse models wherein these pathways were modulated (5). Increased O2 consumption rate (OCR) coupled with increased mitochondrial content that leads to elevated levels of oxidative pathways results in loss of quiescence and stemness (6). Interestingly, unlike adult HSCs, fetal HSCs proliferate extensively and transit from one site to another to finally reach the developing BM. Recently, there is interest in examining whether the metabolic principles followed at different developmental stages are indeed distinguishable. Here, we review the recent advances made in understanding the role of hypoxia and related metabolic pathways in hematopoietic events. We discuss how different energy producing metabolic pathways maintain functionally distinct HSCs in pre- and postnatal developmental stages.
Metabolic Regulation of Adult HSCs
HSCs and Their Niche
All types of terminally differentiated, functional blood cells are derived from a single precursor through a series of cell fate decisions made intrinsically, however, in accordance with the extrinsic cues. Identified in early 1960s, this process called hematopoiesis takes place in the marrow of long bones in mammals (7). Although cell-intrinsic properties remained key regulators of their function, it became increasingly clear that extrinsic factors also play crucial role in maintaining the function of HSCs (8). After the description of association of hematopoietic activity near endosteum, osteoblasts were shown to be key regulators of stemness maintenance (9). Following initial studies, the concept emerged that the most primitive HSCs occupied the endosteal niche while the more proliferative counterparts with short-term potential remained near vessels. This concept was strengthened by studies showing that the primitive HSCs reside in the areas with low pO2, as these cells were proposed to occupy locations away from vessels (10). Later, using hypoxia marker pimonidazole and Hoechst perfusion, it was demonstrated that HSCs occupied least perfused locations within the BM (11). Early studies on BM cells cultured in increased O2 concentrations showed concomitant decrease in hematopoietic potential (12). This was found to hold true for human BM progenitors, where 5.8-fold increase in SCID repopulating cells (SRCs) was observed in hypoxic cultures compared to normoxia (13), with hypoxic conditions clearly favoring maintenance of HSC quiescence (14).
With the advent of better imaging techniques and reliable markers for primitive HSCs, it became clear that more primitive HSCs resided next to the sinusoidal areas (15). Elegant studies from Ding et al. showed the importance of perivascular cells in maintenance of primitive HSCs (16). Imaging studies established clearly that it was near the arterioles that the HSCs located themselves around (17). This was contrary to the view that the HSCs preferred less vascularized spaces within the BM. However, two-photon phosphorescence lifetime microscopy based detection of absolute values of pO2 showed that endosteal region is much less hypoxic than the perisinusoidal regions (18). Therefore, the correlation between O2 content in the niche with the stemness of HSCs located in the region is not yet clearly established.
The cells sense the level of oxygen, which plays crucial role in their overall functioning. For stem cells, these pathways become even more important because of their long lifespan wherein oxidative stress becomes a critical factor. Responses to hypoxic conditions are mediated most prominently by hypoxia inducible factors (HIFs) that in heterodimeric form transactivate a great repertoire of genes (19). Three different a-subunits (HIF-1α, 2α, 3α) can bind to one common β-subunit (HIF-β or ARNT) to generate three heterodimers (HIF-1, 2, 3). The prolyl hydroxylase domain proteins (Phds) mediated pathway is the best characterized oxygen sensing pathway in mammalian cells (20). Phds are members of dioxygenase enzyme family that can hydroxylate HIF-1α on conserved prolyl residues within the oxygen-dependent degradation domain (21–23). HIF-1α is the activating subunit of the heterodimeric complex that acts as the key factor mediating crucial transcriptional responses to hypoxia (24). The hydroxylated ODD is recognized by von Hippel-Lindau protein (Vhl) in E3 ubiquitin ligase complex (25). In contrast to HIF-1α stabilization, transcriptional inactivation in Meis1–/– mice led to loss of hematopoietic function (26). Among various BM sub-populations tested, highest level of HIF-1α expression was found in the primitive HSCs, which decreased as differentiation progressed (4). Although tissue specific deletion of HIF-1α in Mx1;HIF-1αfl/fl mice showed no significant hematopoietic defect at steady state, hematopoietic stress condition resulted in loss of HSC quiescence. Stabilization of HIF-1α via the Mx1-Cre mediated deletion of Vhl led to increased proportion of G0 cells within the primitive HSC as well as progenitor population. HIF-1α stabilization using Phd inhibitors DMOG and FG-4497 enhanced the proportion of quiescent HSCs (27). Importantly, HIF-1α stabilization also led to enhanced HSC function as the animals recovered from radiation injury faster and the HSCs showed better long-term repopulation ability. Results also showed that HIF-1α was important for mobilization of HSCs in G-CSF or Plerixafor based regimen and treatment with FG-4497 resulted in better mobilization (28). This was also found true in the case of xenografted human umbilical cord blood (UCB) derived HSCs (29). Cripto/Grp78 pathway was identified as one of the critical mediators of HIF-1α functions (30). Activation of Cripto signaling upregulated glycolytic enzymes and HSCs expressing Cripto receptor Grp78 maintained quiescent state. These cells were more hypoxic as confirmed using hypoxic marker pimonidazole and showed low mitochondrial potential. Upregulation of Cripto and Grp78 expression under hypoxia, and presence of binding site for HIF-1α on Cripto promoter, confirmed involvement of Cripto/Grp78 pathway in hypoxia response of HSCs.
Incidentally, hematopoietic specific loss of Hif-2α did not affect steady state or stress hematopoiesis. Both, Vav-Hif-2α–/– and Mx1-Hif-2α–/– mice showed no significant hematopoietic defect and the HSCs displayed normal serial transplantation capacity (31). In addition, compensation from HIF-1α was ruled out in this case as co-deletion of HIF-1α also could not affect steady state hematopoiesis. Interestingly, the effect of stabilization of HIF-1α in response to Vhl loss was dose dependent. While monoallelic deletion of Vhl led to increased engraftment capacity of HSCs, biallelic deletion led to cell cycle quiescence and impaired transplantation capacity (4).
HIF-1α plays key role in modulating the metabolic phenotype of a cell in response to hypoxia. For example, transcriptional levels of several of the glycolytic enzymes are under the control of HIF-1α (32). In addition, some of its target genes inhibit the enzymes linked to the oxidative pathways. It has been shown to mediate metabolic shift toward exclusive anaerobic glycolysis in embryonic stem cells (ESCs) in a Warburg-like effect (33). In support of this notion, it was demonstrated that the primitive HSCs were low O2 consuming and show high glycolytic flux (26). These cells showed lower mitochondrial mass and upregulation of hypoxia inducible genes under normoxia. These results strongly indicate intrinsic regulation of hypoxic pathways, perhaps independent of O2 levels in the microenvironment. In a comprehensive study, Takubo et al. analyzed the metabolic properties of HSCs and showed that HSCs majorly depend upon anaerobic glycolysis for their energy demands (5). For both aerobic as well as anaerobic energy production pathways glycolysis is the first step that uses glucose as the raw material to generate adenosine triphosphate (ATP) that is the universal energy currency in any cell. Glycolysis results in two ATPs, two NADH and two pyruvate, which under low O2 conditions gets converted to lactate by lactate dehydrogenase (LDHA), producing NAD1 that can be used in glycolysis to produce ATPs. In the presence of abundant O2, pyruvate dehydrogenase (PDH) converts pyruvate produced from glycolysis to acetyl coenzyme A (Ac-CoA). Pyruvate dehydrogenase kinase (PDK) that inactivates PDH by phosphorylating serine residues is the key enzyme that regulates this process. It plays crucial role in metabolic adaptation of a cell in response to hypoxic conditions as it is transcriptionally regulated by HIF-1α (34). Acetyl Co-A enters the citric acid cycle that, in addition to other metabolites, generates three NADH and an FADH2 (35). Electrons from these reductants are then transferred through the electron transport chain (ETC) with chemiosomotically coupled ATP synthesis (36). Mitochondrial ETC has been recognized as one of the major contributors of reactive oxygen species (ROS), which in turn is linked with pathologies of several age-related disorders including that of the hematopoietic system (37).
Detailed metabolic analysis of the LT-HSCs showed enhanced levels of metabolites and enzymes required for anaerobic pathways (5). These include phosphofructokinase-1 (Pfk-1) that is involved in the rate-limiting step of glycolysis, and pyruvate kinase (PK) phosphoenolpyruvate (PEP) that is directly involved in the ATP production step of glycolysis. In support of this, levels of fructose-1,6-bisphosphate (F1,6BP) and pyruvate were elevated in the primitive HSCs. Importantly, the expression of PDK was highest in the LT-HSCs among all the sub-populations. As described earlier, PDK inhibits the influx of glycolytic metabolites toward mitochondrial metabolic pathways. In addition, it was shown that PDK1 attenuates mitochondrial ROS production (34), which is critical as increase in glycolytic flux can be associated with leakage of electrons from respiratory chain resulting in unexpectedly elevated ROS levels. Forced expression of PDK1 mitigated mitochondrial ROS production in Hif1α–/– cells (4,34). In hematopoietic system, deletion of Pdk2 and Pdk4 that showed best correlation with HIF-1α levels in HSCs, resulted in loss of quiescence and affected steady state hematopoiesis significantly (5). Pdk2,4–/– HSCs accumulated mitochondrial ROS and clearly showed diminished repopulation capacity, while multilineage engraftment potential remained unaffected. Overexpression of Pdk2 and Pdk4 also compensated for the loss of HIF-1α. Apart from being produced from pyruvate via PDK action, fatty acid oxidation (FAO) acts as an additional source of Ac-CoA. It was shown that HIF-1α can directly suppress key enzymes involved in FAO such as medium-chain and long-chain acyl-CoA dehydrogenase (22). However, deletion of promyelocytic leukemia (PML)-peroxisome proliferator-activated receptor δ (PPAR-δ) involved in FAO, led to loss of hematopoietic maintenance (38). While Ppard–/– HSCs showed normal homing, their long-term repopulation potential was markedly reduced.
Regulation At The Mitochondrial Level
Lineage commitment of HSCs leads to elevation of mitochondrial pathways. Mediated by increase in mitochondrial content and activity, it results in enhanced OCR (26). The LT-HSCs were distinctly identified by their low mitochondrial content and activity (measured by mitochondrial membrane potential and NADH content). CD34 expression on the HSCs marks the loss of long-term repopulation potential, serial transplantation efficiency and the first step of commitment down the hematopoietic hierarchy to produce required blood cell types (39). Studies indicated that the mitochondrial mass and expression of CD34 might be correlated (40). Lineage commitment of HSCs has been linked with increased ROS production. Indirect evidence comes from the studies that showed ROSlo subpopulation contained the highest self-renewal potential, which was exhausted in ROShi cells (41). Treatment with antioxidant N-acetyl-cysteine (NAC) restored the long-term function of ROShi cells as examined by long-term culture-initiating cell (LTC-IC) assays. In Drosophila, basal levels of ROS were found to be crucial for differentiation into the multipotent hematopoietic progenitors (analogous to mammalian myeloid progenitors). However, elevation in ROS levels led to premature differentiation of the progenitor population into mature blood cell types (42). ROS are produced chiefly by mitochondria as a byproduct of various oxidative metabolic pathways undertaken for energy production. Accumulation of ROS leads to oxidative damage in the cells, specially the long surviving stem cells, causing functional decline. Cell cycle checkpoints play important role in the cellular response to ROS accumulation. Deletion of Ataxia telangiectasia mutated (Atm) gene, one such checkpoint regulator, leads to increased ROS accumulation in the HSCs and start showing bone marrow failure in as early as 24 weeks (43). In these mice as well, treatment with antioxidative agents such as NAC, decreased ROS load and restored the repopulation capacity of the HSCs. Evidently, HSCs depend upon mechanisms that resist oxidative stress for their long-term survival. We recently reported elevated levels of OCR along with enhanced mitochondrial activity and ROS production in proliferative fetal liver (FL) HSCs (44). Gene expression data clearly showed concomitant increase in the expression of genes related to several of the DNA damage response pathways, highlighting the need to balance the oxidative stress in HSCs. Extensive studies have been carried out on the role of another group of transcription factors, the Forkhead Box O (FoxO) subfamily, involved in stress resistance in hematopoietic system. FoxO3 was shown to be a key player in maintaining the expression of Atm in HSCs. Loss of FoxO3 led to increased accumulation of ROS, thereby causing loss of stemness (45). FoxO3a–/– mice showed deteriorated resistance to myelotoxic stress (46). Another report demonstrated that concomitant loss of FoxO1, FoxO3 and FoxO4 in hematopoietic system leads to expansion of myeloid lineage and marked decrease in the lymphoid commitment as well as loss of stem cell population in the BM (47). These mice also showed increased ROS levels in HSCs, reversible by the use of antioxidants restoring long-term repopulation ability. The effects of ROS are mediated by p38 MAPK that limits the lifespan of HSCs (48). Therefore, quite understandably, these FoxO deficient mice as well as the Atm–/– mice showed increased phosphorylation status of p38. In addition to the anti-oxidants, p38 MAPK inhibitors decreased the levels of ROS and restored HSC function to a great extent (41,48). All these findings established that accumulation of ROS, as a result of higher mitochondrial activity, leads to loss of quiescence and activation of differentiation pathways.
Sirtuins (Sirt) are members of a highly conserved family of proteins associated with cellular metabolism including several of the pathways that affect mitochondrial function. Sirt7 is an epigenetic regulator, known for its function to repress the expression of mitochondrial ribosomal proteins and transcription factors. Nrf1 that acts as the master regulator of mitochondrial gene transcription (49) was shown to mediate the binding of Sirt7 to the promoters of several of the mitochondrial ribosomal proteins (mRPs) and translation factors (mTFs) in HEK-293T cells. Deletion of Sirt7 in HSCs resulted in increased mitochondrial mass and ATP levels. Elevated activity of mitochondrial metabolism related enzymes in these cells, eventually led to loss of quiescence (50). Contrary to expectations, Nrf1 inactivation in Sirt7–/– HSCs reduced mitochondrial protein folding stress and improved quiescence showing thus that Sirt7 keeps Nrf1 activity under check. Therefore, Sirt7 was proposed to relieve the HSCs of mitochondrial protein folding stress (PFSmt) through mitochondrial unfolded protein response (UPRmt) also showing that Sirt7 and Nrf1 might be regulating different branches of UPRmt.
As mitochondria act as the leading site of ROS production, a variety of models have been used to examine how overall mitochondrial function and specific mitochondrial metabolic pathways affect hematopoietic system. Several other studies where genetic alterations led to increased mitochondrial function resulted in loss of HSC quiescence. mTOR is one of the most important regulator of mitochondrial function and controls overall oxidative potential of the cells (51). Deletion of tuberous sclerosis complex (TSC), an inhibitor of the mTOR pathway involved in mitochondrial function, led to increased mitochondrial biogenesis, resulting in active cycling of HSCs losing transplantation capability (52). Loss of hematopoietic function was thought to be the result of ROS accumulation that finally culminated in defective functioning of HSCs. As shown in several other studies, ROS clearing had a positive effect on HSC functioning and could show significant recovery of loss of stemness. Inhibition of mTOR pathway along with activation of canonical Wnt pathway ably maintained mouse as well as human LT-HSCs ex vivo in a cytokine-free culture (53). Similar experiments wherein deletion of mitochondrial carrier homologue 2 (MTCH2; in Vav-Mtch2flfl mice) led to increased mitochondrial metabolic pathways, showed HSCs entry into cell cycle (6). Increase in the size and activity of mitochondria in these mice decreased frequency of primitive HSCs. Inactivation of MTCH2 ligand BID in phospho-mutant mice also resulted in a similar effect on mitochondrial mass and activity leading to altered hematopoietic function (54). As BID plays major role in DNA damage response (DDR), these findings indicate that the metabolic-DDR network would be key to the maintenance of HSC quiescence and preserving function following proliferative events during homeostatic physiology.
Is The Metabolic Status of Fetal HSCs Same as in Adults?
During mammalian development, multiple transient hematopoietic sites appear that ultimately lead to establishment of BM as the site of blood formation for most part of the adult life (55,56). In murine embryos, the first wave of hematopoiesis results in appearance of primitive HSCs in the yolk sac (YS) around embryonic day (E) 7.5. This is marked by the appearance of primitive erythrocytes that carry oxygen across rapidly developing embryo (57). This is followed by the second wave of hematopoiesis around E8.5, with the appearance of definitive progenitors in the YS, placenta, umbilical and vitelline arteries and para-aortic-splanchnopleura (P-Sp). Splenic colony forming units (CFU-S) start to appear in the YS and dorsal aorta around E10 (58). This is followed by the final and most important wave of hematopoiesis, which occurs around E10.5. This is marked by initiation of definitive hematopoiesis and formation of hematopoietic clusters in dorsal aorta of the embryo proper (59,60). Endothelial to hematopoietic transition takes place from specific bipotent endothelial cells known as “hemogenic endothelium” (61). Definitive HSCs or dHSCs are formed for the first time in these aortic clusters where Runx1 acts as a key determinant (62). Hypoxia is known to play an important role during embryogenesis. HIFs, the key sensors and effectors of hypoxic response regulate the formation of vasculature and placenta (63). Spatiotemporal correlation between the ontogeny of the vascular and the hematopoietic systems is widely reported (64). In extension, it is logical to hypothesize that HIFs could be important in the development of hematopoietic system as well. Aortic endothelium along with emerging hematopoietic clusters, placenta and FL stained positive for hypoxyprobe pimonidazole (65). The study further showed that vascular endothelium specific deletion of HIF-1α led to defects in hematopoietic emergence. However, it was not clear if hypoxia pathway was crucial for endothelial to hematopoietic transition or expansion of the hematopoietic cluster cells. Interestingly, the effect of HIF-1α deletion was differential in various hematopoietic sites as yolk sac cells did not stain positive for pimonidazole. Moreover, the cells within the aortic clusters were also unequally stained for the hypoxyprobe (65). Role of events downstream of the HIF responses have not been clearly elucidated in the hematopoietic system. Vegf pathway is one of the most crucial downstream cascades that respond to HIF-1α stabilization in a variety of cell types. Expression of Vegf seemed to coincide with the hypoxyprobe staining in E10.5 murine embryo (66). Although a key role for Vegf in vascular development has been clearly elucidated, its function specifically in hematopoietic development has not been delineated yet. E9.5 embryos deficient in Hif-1β expression displayed major vascular defects and almost no hematopoietic clusters in the dorsal aorta (67). Hematopoietic defects along with some vascular abnormalities in these embryos were partially recovered by Vegf treatment. Conditional deletion of Vegf-a in the hematopoietic compartment resulted in impaired engraftment potential of BM HSCs, showing the involvement of an autocrine regulatory loop (68). Expression of Hif-2α in hematopoietic niche, on the other hand was shown to be important for HSC engraftment as even the WT HSCs failed to engraft in Hif-2α–/– BM (31).
Metabolic pathways in response to oxygen levels determine glucose metabolism in a prominent way. Anaerobic pathways for the production of ATPs consume significantly higher amount of glucose than the oxidative pathways. On the other hand, glucose levels also can modulate metabolic properties of a cell. Therefore, it is not surprising that the cases of acute lymphoblastic leukaemia and type 1 diabetes were positively correlated (69). In addition, babies born to mothers suffering from gestation induced diabetes or hypertension were seen to have lower platelet function (70). Also, the neutrophil function and motility of cord blood neutrophils was impaired in these cases (71). All of these observations point to the fact that glucose metabolism induced changes in gross hematopoietic development and function. During zebrafish development, however, it was shown that transient increase in glucose levels resulted in increased hematopoietic function (72). Later it was shown that these effects might have been mediated by PDGFRβ pathway that acts downstream of HIF-1α, which incidentally was stabilized in response to higher glucose levels (73).
FL is the hematopoietic site where dHSCs undergo several rounds of symmetric cell division, which helps build the initial pool of blood cells in the embryo. This increased proliferation poses heightened energy requirements. RNA sequencing data comparing expression profiles of key energy metabolism pathway genes in BM and FL suggested that mitochondrial oxidative phosphorylation pathways were highly enriched in the FL (44). Results showed increased OCR, mitochondrial potential and ROS levels in FL derived HSCs indicating altered metabolic pathways. Deletion of a key component of TCA, Fumarate hydratase (Fh), in hematopoietic tissue-specific manner resulted in decrease in OCR in the FL derived HSCs (74). While no viable Vav-Fh–/– offspring were observed, E14.5 embryos showed severe impairment in FL hematopoiesis. There was no alteration in the numbers of HSCs in FL, but major defects were observed in lineage commitment causing fetal or perinatal lethality. This also might indicate crucial role of ROS signaling pathways in lineage commitment in HSCs. Although no clear studies exist that compare the level of oxygenation in the BM and FL niches wherein HSCs reside, results indicated upregulation of OxPhos related genes in FL-HSC niche (75). As the fetal HSCs differ majorly from their adult counterparts, especially in terms of their proliferative potential, it is pertinent to revisit the metabolic profile of these cells.
Metabolism in Aging
DNA damage signaling and repair pathways that can be directly regulated by oxidative stress in a cell have been linked to the aging process (76), for which stem cells might be playing a central role. Oxidative metabolic pathways are inhibited in adult HSCs and elevation of mitochondrial function leads to loss of quiescence. In this light, loss of quiescence in aged HSCs has been correlated with accumulation of DNA damage due to oxidative stress (77). Results showing increased oxidative pathways in aged HSCs corroborated this hypothesis. While we described that FL HSCs might be more dependent upon mitochondrial pathways than their adult counterparts (44), elegant studies from Ho et al. showed correlation between functional decline in aged HSCs with elevated levels of OxPhos (78). Results clearly showed increased mitochondrial function resulting in enhanced O2 consumption, higher OxPhos and ROS levels. Interestingly, the sub-population of aged HSCs with higher levels of autophagy showed clearing of mitochondria leading to decreased ROS levels. Higher ROS levels in autophagy-low aged HSCs was correlated with increased amount of DNA damage accumulation (78). These results propose higher mitochondrial activity as one of the major causes of functional decline in HSCs in animals with deletion of a variety of factors. These cells exhibited impaired autophagy due to which active mitochondria cannot be eliminated, resulting in loss of quiescence and exhaustion of the primitive stem cell pool and impaired hematopoiesis. Variety of transgenic mouse models resulting in escalated oxidative metabolism showed aging like phenotype of hematopoietic system; namely, decreased repopulation potential, myeloid bias and DNA damage accumulation in HSC population (Table 1). For example, deletion of Sirt7 that led to increased mitochondrial function resulted in loss of hematopoietic function. Interestingly, the expression of Sirt7 is significantly reduced in aged HSCs (50,80), corroborating the link between increased mitochondrial function and aging. ROS accumulation due to increased mitochondrial function, coupled with poor DNA damage response led to functional decline in aged HSCs, also possibly augmenting several of the age specific hematopoietic defects and disorders. It was also observed that aged HSCs show higher mTOR activity, underlining the importance of mTOR regulation for HSC quiescence (81). Increase in mTOR activity following Tsc deletion in Mx1-Tsc–/– mice also led to premature aging of the hematopoietic system. Rapamycin treatment of the old mice restored the self-renewal and repopulation potential of HSCs, increasing their lifespan (81). Similarly, Sirt7 upregulation improved the function of aged HSCs (50). HIF1αΔ/Δ mice also show similar pattern of reduced HSC frequency and number with age exhibiting the role of HIF1α in preserving the HSCs in quiescent stage (4). Activation of anaerobic glycolytic pathways and inhibition of oxidative pathways ensures lower ROS accumulation. Oxidative stress in HSCs has been shown to result in functional decline. In addition, ROS pathway is involved in stem cell differentiation which might lead to lineage bias in aged HSCs. For example, activation of p38MAPK pathway in response to elevated ROS levels upregulate age related markers like p16Ink4a leading to the exhaustion of the stem cell pool (48). Experiments have shown that quiescence of the long-surviving HSCs might be the underlying reason why DNA damage accumulation takes place in aged HSCs. And, the proliferative events might be one of the mechanisms by which HSCs would activate DNA damage response pathways (82).
Table 1. Transgenic mouse models with increased mitochondrial function with concomitant loss of stemness in hematopoietic system.
| Model | Lethality | In vitro | In vivo | Reference |
|---|---|---|---|---|
| Mx-Cre+;FoxO1/3/4l/l | – |
|
|
47 |
| FoxO3a–/– | – |
|
|
46 |
| Atm–/– | By 24 weeks, post BM failure |
|
|
43 |
| Mx1-Cre-;Pdk2,4–/– | – |
|
|
5 |
| Mx-1-Cre;Tscfl/fl | – |
|
|
52 |
| Sirt7–/– | – | Not mentioned |
|
50 |
| Vav1-Cre;Mtch2fl/fl | – |
|
|
6 |
| Mx1-Cre;HIF1afl/fl | – | – |
|
4 |
| Germline Rev1–/– | Lifespan shorter than WT littermates |
|
|
79 |
| Rev1–/– Xpc–/– | ≈3.5 months | – |
|
79 |
Balance Between Oxidative and Non-Oxidative Pathways is the Key
The discussed studies establish clearly that adult HSCs remain metabolically dormant and disturbance of this status can lead to functional loss and spontaneous differentiation. To a large extent, functional decline of HSCs during aging also has been attributed to accumulation of oxidative damage. As described in the earlier sections, functional loss of HSCs due to increased oxidative stress induced by elevated mitochondrial function can be reversed by using anti-oxidants or inhibition of ROS pathway. Recent reports confirmed the importance of balancing oxidative load and proposed it as a key to hematopoietic fitness. Most of the DNA damages are repaired by DDR pathways, specific for different types of damages incurred. While most DNA damages are detected and result in cell cycle arrest or apoptosis, some lesions are bypassed via DNA translesion synthesis that appoints specialized DNA polymerases, wherein Rev1 acts as a core factor. Deletion of Rev1 in hematopoietic system led to loss of engraftment potential of HSCs with functional loss starting as early as in the E14 fetal liver (79). These results clearly emphasize the importance of DDR pathways and predict the effects of DNA damage accumulations. Simultaneous deletion of Xpc, a key nucleotide excise repair gene should exacerbate the generation of DNA lesions. Indeed, the effects of co-deletion of Rev1 and Xpc were rather severe and embryos were born in sub-Mendelian ratios and double knockout littermates showed shortened lifespan of around 3.5 months with major hematopoietic defects. In line with a number of reports that showed restorage of hematopoietic function following anti-oxidant treatment, recent results showed high levels of ascorbate in human as well as mouse HSCs (83). Ascorbate (along with its transporter Slc23α2) was the most enriched metabolite in HSCs and multipotent progenitors (MPPs) as compared with more restricted progenitor populations. Contrary to expectations, depletion of ascorbate led to increase in the number and function of HSCs. However, these effects were mediated by Tet2 function and no effects on ROS accumulation were observed. Interestingly, ascorbate depletion promoted leukemogenesis from Flt3ITD;Tet2Δ/+ cells, resulting in shorter lifespan of the recipients with lacking ascorbate synthesis. The suppressive effects of ascorbate might have been the result of its function as a cofactor for prolyl hydroxylases that degrade HIFs and some other enzymes linked with mitochondrial metabolism. It can be said that a balance between the oxidative and non-oxidative pathways maintains homeostatic function in HSCs, and imbalance will create functional disturbance. For instance, it was observed that overactivation of mitophagy led to hematopoietic defects.
Increased mitophagy resulted from the deletion of AAA1-ATPase Atad3 in adult hematopoietic cells led to poor survival of Atad3αfl/fl;Mx1Cre mice (84). The hematopoietic defects were correlated with decrease in mitochondrial mass and OCR. However, mitochondrial membrane potential was increased in the Mx1;Atad3α–/– HSCs. These changes affected lineage differentiation in major way, however, resulting in increase in the frequency as well as absolute number of stem cell sub-populations due to increased proliferation. In fact, recent results demonstrated that mitochondrial respiration was key to the differentiation process in HSCs (85). Deletion of Rieske iron-sulfur protein (RISP), a complex III subunit led to major fall in mitochondrial respiration and OCR and caused major defects in FL hematopoiesis. While there was no loss of absolute number of HSCs in E15.5 FL tissues, differentiation into MPPs was blocked, resulting in severe loss of differentiated progeny. These results strongly suggested a crucial role of mitochondrial pathways, lineage commitment being integral component of stemness. While anaerobic glycolytic pathways play important role in maintaining quiescence, especially in the case of adult BM HSCs, mitochondrial pathways make sure optimum number of MPPs are produced. Therefore, a fine balance between anaerobic and aerobic arms of respiration should be balanced to achieve homeostasis.
Conclusion
Currently, there is a lot of interest in understanding how a variety of metabolic pathways regulate HSC function. Physiological status of a cell type would affect the metabolic pathways employed. Recent studies confirm that an altered metabolic state in a variety of cell types could influence their function. In the case of adult HSCs, it has been shown that for energy production, they largely depend upon anaerobic pathways. It was also proposed that these metabolic pathways are influenced by the oxygenation status of the niche wherein the HSCs reside. How the cells switch between these pathways in the wake of change in physiological status is not clear. Different developmental stages present differently functioning HSCs. Initial studies have pointed toward altered metabolic status of HSCs across developmental stages that would ultimately define the functional uniqueness of HSCs at that stage. Studies are aimed at modulating key pathways to achieve efficient expansion or directed differentiation into specific hematopoietic populations in order to address specific clinical demands. It has been found that HSC metabolism shows plasticity, which helps them adapt to diverse cell fates. Lineage commitment within the stem cell compartment leads to metabolic fluctuations. However, if the metabolic properties of different HSC sub-populations are regulated by distinct micro-niches that these cells take within the BM, is not very clear. HSCs emerging in the aorta and homed near the arterioles in BM heavily depend upon hypoxic pathways. Whether it depends, at least in part, on the intrinsic regulation is not understood well. Studies are warranted to work out the role of intrinsic versus extrinsic factors that would result in a preferred metabolic pathway. That, hypoxic niche is crucial in maintaining anaerobic respiration in adult HSCs is the most accepted view currently. What are the differences in absolute O2 levels in these sites remains to be seen. Importantly, how functionally distinct HSC subsets locate themselves in accordance to the O2 levels is to be examined. Using modern imaging tools aided by a deeper knowledge on HSC markers, have been able to demonstrate that these subsets might be differently located within the BM. However, these locations are still to be linked with respective O2 levels that might contribute in the regulation of metabolic pathways in HSCs and hence, their function. It is yet to be established if there is any correlation between specific micro-niches taken by specific stem cell sub-population based on their metabolic requirements. Deciphering metabolic networks underlying HSC functional regulation might be the final conquest leading to the HSC becoming amenable to modulation of their function ex vivo. The pluripotent stem cells can provide renewable source of HSCs. However, current methods have proven to be not sufficiently efficient in creating functional HSCs that could be used for transplantations. Better understanding of the metabolic players that could lead to further maturation of the hematopoietic precursors generated via directed differentiation would be a highly sought after step. Overall, this field presents exciting opportunities with immense potential in not only creating better understanding of the developmental and physiological hematopoietic processes but also devising improved methods of HSC expansion and directed differentiation.
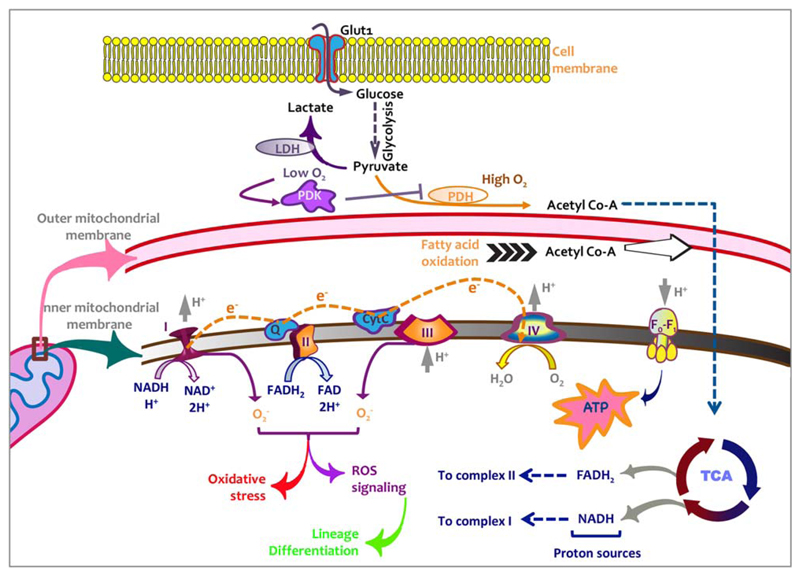
Fig 1.
Energy production pathways depend upon oxygen levels. A variety of metabolic pathways ensure supply of sufficient energy for a cell to perform its function. In case of stem cells thence, these pathways can regulate their self-renewal and differentiation potential. Glucose being the most common raw material for energy production pathways enters the cells through glucose transporters. Glucose is first converted into pyruvate through glycolysis following which metabolic pathways are selected on the basis of availability of O2. Lactate dehydrogenase (LDH) that responds to hypoxia pathways converts pyruvate into lactate in low O2 conditions. Another important metabolic enzyme pyruvate dehydrogenase kinase (PDK) inhibits the activity of pyruvate dehydrogenase (PDH) that generates acetyl-coenzyme A (acetyl co-A) under abundant O2 levels. Under normoxic conditions most of the ATP synthesis depends upon mitochondrial pathways driven by electron transport chain and subsequently ATP synthase activity. These oxidative pathways, however, produce reactive oxygen species (ROS) that leads to oxidative damage in cellular components and affects function negatively. At the same time, ROS signaling events lead to activation of differentiation pathways.
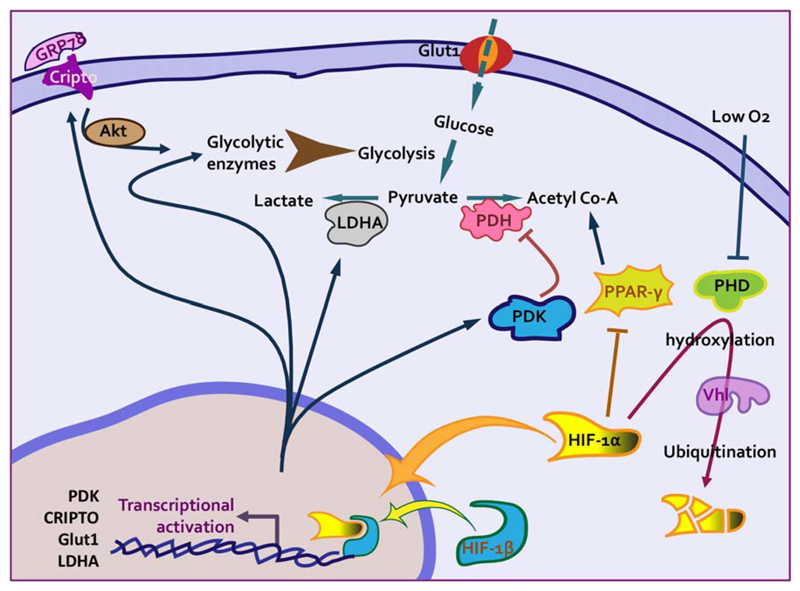
Fig 2.
Hypoxic pathways regulate quiescence of adult hematopoietic stem cells. In adult BM, HSCs have been shown to reside in hypoxic niches. This has been considered as the key factor that determines energy production pathways employed by these cells. O2 levels are sensed by the cells through activity of prolyl hydroxylase domain-containing proteins (PHDs), which degrade HIF1α through VHL ubiquitination pathway. As a consequence of hypoxic niche, HIF-1α gets stabilized causing transcriptional activation of several regulators of anaerobic energy production pathways. HIF target Ldha leads to increased lactate production, which is reflected in elevated extracellular acidification rate in primitive HSCs. In addition, hypoxic response of the cells inhibits several of the pathways key for oxidative mitochondrial pathways such as acetyl co-A production via fatty acid oxidation or by PDH activity. Through multiple mechanisms, including activation of key enzymes, Akt pathway enables glycolytic pathways in HSCs.
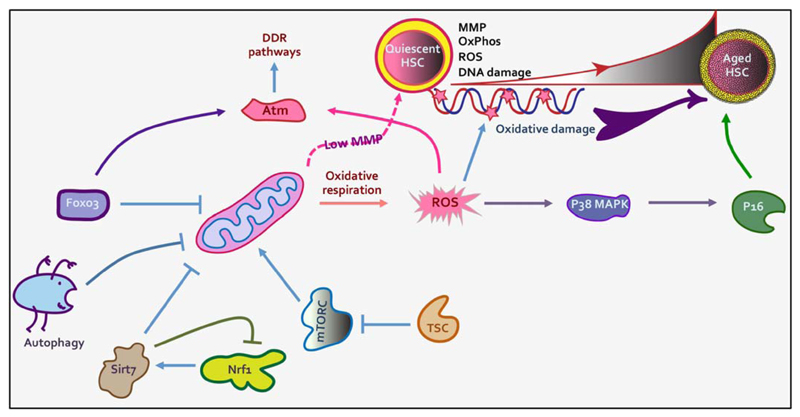
Fig 3.
Mitochondrial activity is associated with decline in stemness. Quiescent BM HSCs show low mitochondrial potential to avoid oxidative stress. Increased oxidative respiratory pathways lead to ROS accumulation leading to decreased stemness and spontaneous differentiation. Accumulation of DNA damage in aged HSCs also has been correlated with increased ROS production. HSCs employ several pathways that ensure glycolytic pathways prevail instead of oxidative means of ATP production. At the same time, DNA damage responses that become insufficient in aged HSCs resulting in accumulation of DNA damage and functional decline. In young HSCs, DDR pathways are activated by Atm kinase through Foxo3, which also keeps mitochondrial activity low. Sirtuins have also been shown to play critical role in keeping mitochondrial activation level under check. While still under investigation, a role of mitophagy in maintaining the content of healthy mitochondria in HSCs is emerging.
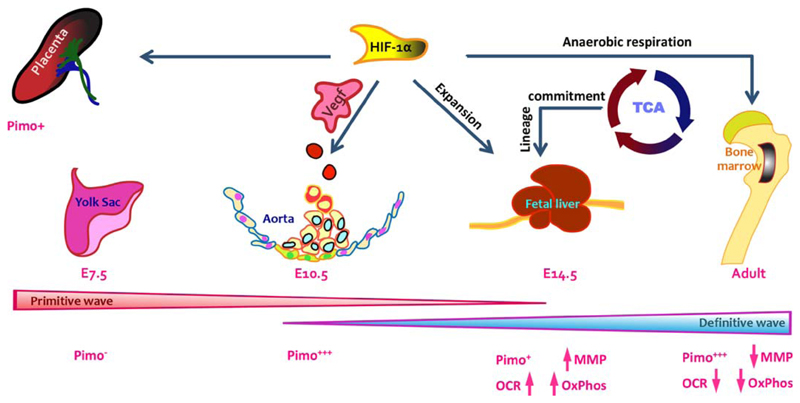
Fig 4.
Across development energy production pathways play important role in regulating hematopoietic function. Across developmental stages, HSCs are known to function in a variety of niches. Several of these niches such as AGM, where definitive HSCs emerge in the embryo, and placenta are hypoxic in nature. In the case of FL HSCs, mitochondrial content and activity has been shown to be elevated. Disruption of ETC blocks the lineage commitment in FL HSCs and they fail to create lineage-committed progenitors. HSCs from most other hematopoietic stages largely depend upon anaerobic energy production pathways. Hematopoietic emergence in AGM within the embryo proper is closely associated with vascular system where hemogenic endothelium gives rise to hematopoietic progenitors. VEGF pathways, one of the most important targets of hypoxia pathway, have been implicated in some of the events leading to hematopoietic emergence. At the same time, importance of anaerobic metabolic pathways in adult BM HSCs is widely accepted.
Fig 5.
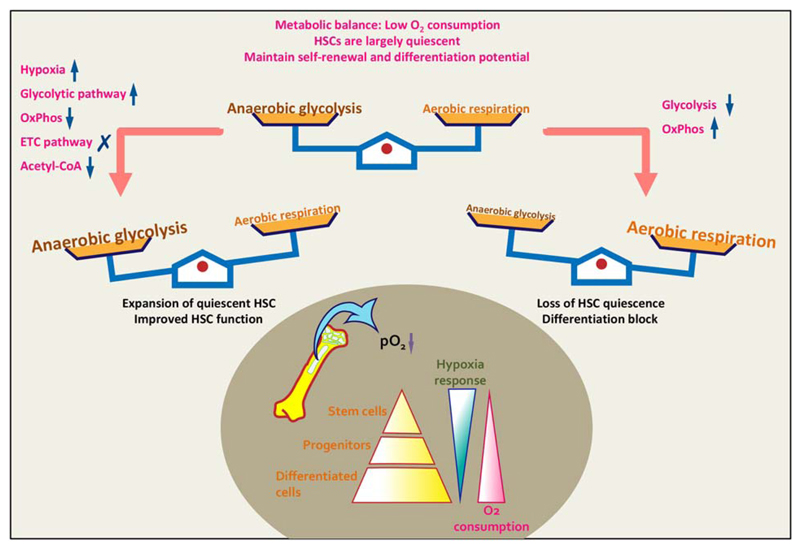
Elevated levels of oxidative pathways result in loss of HSC quiescence. Hematopoietic system is organized in a hierarchical manner with the most primitive HSCs on top and terminally differentiated blood cells at the bottom of the pyramid. The most primitive HSCs depend on anaerobic glycolytic pathways to meet their energy requirements while oxidative pathways become important for more committed progenitors and differentiated cells. However, mitochondrial pathways play important role in creation of the pool of multipotent progenitors from stem cells and further differentiation. A balance between anaerobic and aerobic respiratory pathways is critical for maintaining steady state hematopoietic processes. However, in the case of adult BM HSCs, the balanced state involves production of energy from glycolytic pathways significantly more than mitochondrial oxidative pathways.
Fig 6.
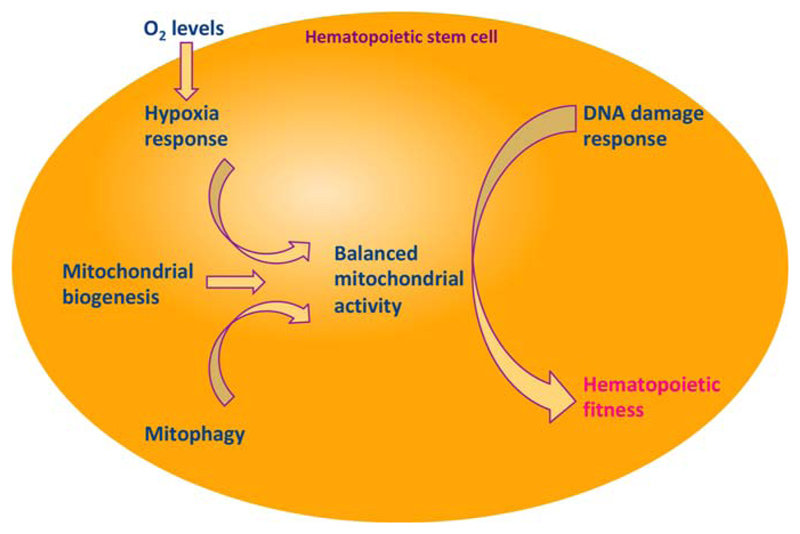
Balance in glycolytic and mitochondrial respiration pathway is the key to hematopoietic fitness. Various studies discussed in this review point toward the importance of anaerobic respiratory pathways in maintenance of quiescence in adult HSCs. In primitive BM HSCs, activation of oxidative pathways resulted in cell cycle entry, also leading to spontaneous differentiation mediated by ROS signaling. Mitochondrial biogenesis and mitophagy provide critical balance in the overall mitochondrial pathways being undertaken by HSCs in the wake of occasional proliferative events. A variety of physiological states might pose requirement for higher rates of oxidative respiration, in which case DNA damage response pathways ably rescue the cells from oxidative stress to the genome. These factors play crucial role in maintaining functional integrity of HSCs.
Acknowledgements
This work was supported by the Wellcome Trust/DBT India Alliance Fellowship (IA/I/15/2/502061) awarded to S.K. and intramural funds from Indian Institute of Science Education and Research Thiruvananthapuram (IISER TVM). I.M.R. is supported by Junior Research Fellowship from University Grants Commission, India. A.B. is supported by IISER TVM. C.M.V. is supported by funds from KU Leuven, FWO and IWT.
Abbreviations
- Ac-CoA
Acetyl Coenzyme A
- Atm
Ataxia telangiectasia mutated
- ATP
Adenosine triphosphate
- BM
Bone Marrow
- CFU-S
Splenic colony forming units
- DDR
DNA Damage response
- dHSCs
Definitive HSCs
- ESCs
Embryonic stem cells
- ETC
Electron Transport Chain
- F1,6BP
Fructose-1,6-bisphosphate
- FAO
Fatty acid oxidation
- FL
Fetal Liver
- FoxO
Forkhead Box O
- HIFs
Hypoxia Inducible Factors
- HSC
Hematopoietic Stem Cells
- LDHA
Lactate dehydrogenase
- LT
Long Term
- LTC-IC
Long-term culture-initiating cell
- MPPs
Multipotent Progenitors
- mRPs
mitochondrial Ribosomal Proteins
- mTFs
mitochondrial Translation Factors
- NAC
N-Acetyl-Cysteine
- OCR
Oxygen consumption rate
- PDH
Pyruvate dehydrogenase
- PDK
Pyruvate dehydrogenase kinase
- PEP
Phospho enol pyruvate
- PFK-1
Phosphofructokinase-1
- PFSmt
mitochondrial protein folding stress
- Phd
Prolyl hydroxylase domain
- PK
Pyruvate Kinase
- PML
Promyelocytic leukemia
- PPAR-δ
Peroxisome proliferator-activated receptor δ
- P-Sp
para-aortic-splanchnopleura
- RISP
Rieske iron-sulfur protein
- ROS
Reactive Oxygen Species
- Sirt
Sirtuins
- SRC
SCID repopulating cells
- TCA
Tri-carboxylic acid
- TSC
Tuberous Sclerosis Complex
- UCB
Umbilical Cord Blood
- UPRmt
mitochondrial unfolded protein response
- VHL
von Hippel-Lindau
- YS
Yolk Sac
References
- [1].Kohli L, Passegue E. Surviving change: the metabolic journey of hematopoietic stem cells. Trends Cell Biol. 2014;24:479–487. doi: 10.1016/j.tcb.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pietras EM, Warr MR, Passegue E. Cell cycle regulation in hematopoietic stem cells. J Cell Biol. 2011;195:709–720. doi: 10.1083/jcb.201102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Eliasson P, Jonsson JI. The hematopoietic stem cell niche: low in oxygen but a nice place to be. J Cell Physiol. 2010;222:17–22. doi: 10.1002/jcp.21908. [DOI] [PubMed] [Google Scholar]
- [4].Takubo K, Goda N, Yamada W, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- [5].Takubo K, Nagamatsu G, Kobayashi CI, et al. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell. 2013;12:49–61. doi: 10.1016/j.stem.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Maryanovich M, Zaltsman Y, Ruggiero A, et al. An MTCH2 pathway repressing mitochondria metabolism regulates haematopoietic stem cell fate. Nat Commun. 2015;6:7901. doi: 10.1038/ncomms8901. [DOI] [PubMed] [Google Scholar]
- [7].Becker AJ, Mc CE, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- [8].Hoggatt J, Kfoury Y, Scadden DT. Hematopoietic stem cell niche in health and disease. Annu Rev Pathol. 2016;11:555–581. doi: 10.1146/annurev-pathol-012615-044414. [DOI] [PubMed] [Google Scholar]
- [9].Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- [10].Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO2 distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models. Biophys J. 2001;81:685–696. doi: 10.1016/S0006-3495(01)75733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cipolleschi MG, Dello Sbarba P, Olivotto M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood. 1993;82:2031–2037. [PubMed] [Google Scholar]
- [13].Danet GH, Pan Y, Luongo JL, Bonnet DA, Simon MC. Expansion of human SCID-repopulating cells under hypoxic conditions. J Clin Invest. 2003;112:126–135. doi: 10.1172/JCI17669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hermitte F, Brunet de la Grange P, Belloc F, Praloran V, Ivanovic Z. Very low O2 concentration (0.1%) favors G0 return of dividing CD341 cells. Stem Cells. 2006;24:65–73. doi: 10.1634/stemcells.2004-0351. [DOI] [PubMed] [Google Scholar]
- [15].Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- [16].Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508:269–273. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Greer SN, Metcalf JL, Wang Y, Ohh M. The updated biology of hypoxia-inducible factor. EMBO J. 2012;31:2448–2460. doi: 10.1038/emboj.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mole DR, Ratcliffe PJ. Cellular oxygen sensing in health and disease. Pediatr Nephrol. 2008;23:681–694. doi: 10.1007/s00467-007-0632-x. [DOI] [PubMed] [Google Scholar]
- [21].Wong BW, Kuchnio A, Bruning U, Carmeliet P. Emerging novel functions of the oxygen-sensing prolyl hydroxylase domain enzymes. Trends Biochem Sci. 2013;38:3–11. doi: 10.1016/j.tibs.2012.10.004. [DOI] [PubMed] [Google Scholar]
- [22].Huang D, Li T, Li X, Zhang L, Sun L, et al. HIF-1-Mediated suppression of acyl-CoA dehydrogenases and fatty acid oxidation is critical for cancer progression. Cell Rep. 8:1930–1942. doi: 10.1016/j.celrep.2014.08.028. [DOI] [PubMed] [Google Scholar]
- [23].Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 2014;9:47–71. doi: 10.1146/annurev-pathol-012513-104720. [DOI] [PubMed] [Google Scholar]
- [25].Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- [26].Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7:380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Forristal CE, Winkler IG, Nowlan B, Barbier V, Walkinshaw G, et al. Pharmacologic stabilization of HIF-1alpha increases hematopoietic stem cell quiescence in vivo and accelerates blood recovery after severe irradiation. Blood. 2013;121:759–769. doi: 10.1182/blood-2012-02-408419. [DOI] [PubMed] [Google Scholar]
- [28].Forristal CE, Nowlan B, Jacobsen RN, Barbier V, Walkinshaw G, et al. HIF-1alpha is required for hematopoietic stem cell mobilization and 4-prolyl hydroxylase inhibitors enhance mobilization by stabilizing HIF-1alpha. Leukemia. 2015;29:1366–1378. doi: 10.1038/leu.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nowlan B, Futrega K, Brunck ME, Walkinshaw G, Flippin LE, et al. HIF-1alpha-stabilizing agent FG-4497 rescues human CD34(1) cell mobilization in response to G-CSF in immunodeficient mice. Exp Hematol. 2017;52:50.e6–55.e6. doi: 10.1016/j.exphem.2017.05.004. [DOI] [PubMed] [Google Scholar]
- [30].Miharada K, Karlsson G, Rehn M, Rorby E, Siva K, et al. Cripto regulates hematopoietic stem cells as a hypoxic-niche-related factor through cell surface receptor GRP78. Cell Stem Cell. 2011;9:330–344. doi: 10.1016/j.stem.2011.07.016. [DOI] [PubMed] [Google Scholar]
- [31].Guitart AV, Subramani C, Armesilla-Diaz A, Smith G, Sepulveda C, et al. Hif-2alpha is not essential for cell-autonomous hematopoietic stem cell maintenance. Blood. 2013;122:1741–1745. doi: 10.1182/blood-2013-02-484923. [DOI] [PubMed] [Google Scholar]
- [32].Gordan JD, Thompson CB, Simon MC. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell. 2007;12:108–113. doi: 10.1016/j.ccr.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhou W, Choi M, Margineantu D, Margaretha L, Hesson J, et al. HIF1alpha induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transition. EMBO J. 2012;31:2103–2116. doi: 10.1038/emboj.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- [35].Hu C, Fan L, Cen P, Chen E, Jiang Z, Li L. Energy metabolism plays a critical role in stem cell maintenance and differentiation. Int J Mol Sci. 2016;17:253. doi: 10.3390/ijms17020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu Rev Biochem. 1985;54:1015–1069. doi: 10.1146/annurev.bi.54.070185.005055. [DOI] [PubMed] [Google Scholar]
- [37].Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- [38].Ito K, Carracedo A, Weiss D, Arai F, Ala U, et al. A PML-PPAR-[delta] pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat Med. 2012;18:1350–1358. doi: 10.1038/nm.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- [40].Mantel C, Messina-Graham S, Broxmeyer HE. Upregulation of nascent mitochondrial biogenesis in mouse hematopoietic stem cells parallels upregulation of CD34 and loss of pluripotency: a potential strategy for reducing oxidative risk in stem cells. Cell Cycle. 2010;9:2008–2017. doi: 10.4161/cc.9.10.11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- [44].Manesia JK, Xu Z, Broekaert D, Boon R, van Vliet A, et al. Highly proliferative primitive fetal liver hematopoietic stem cells are fueled by oxidative metabolic pathways. Stem Cell Res. 2015;15:715–721. doi: 10.1016/j.scr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- [45].Yalcin S, Zhang X, Luciano JP, Mungamuri SK, Marinkovic D, et al. Foxo3 is essential for the regulation of ataxia telangiectasia mutated and oxidative stress-mediated homeostasis of hematopoietic stem cells. J Biol Chem. 2008;283:25692–25705. doi: 10.1074/jbc.M800517200. [DOI] [PubMed] [Google Scholar]
- [46].Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- [47].Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- [48].Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- [49].Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88:611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- [50].Mohrin M, Shin J, Liu Y, Brown K, Luo H, et al. Stem cell aging. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science. 2015;347:1374–1377. doi: 10.1126/science.aaa2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Schieke SM, Phillips D, McCoy JP, Jr, Aponte AM, et al. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J Biol Chem. 2006;281:27643–27652. doi: 10.1074/jbc.M603536200. [DOI] [PubMed] [Google Scholar]
- [52].Chen C, Liu Y, Liu R, Ikenoue T, Guan K-L, et al. TSC–mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Huang J, Nguyen-McCarty M, Hexner EO, Danet-Desnoyers G, Klein PS. Maintenance of hematopoietic stem cells through regulation of Wnt and mTOR pathways. Nat Med. 2012;18:1778–1785. doi: 10.1038/nm.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Maryanovich M, Oberkovitz G, Niv H, Vorobiyov L, Zaltsman Y, et al. The ATM-BID pathway regulates quiescence and survival of haematopoietic stem cells. Nat Cell Biol. 2012;14:535–541. doi: 10.1038/ncb2468. [DOI] [PubMed] [Google Scholar]
- [55].Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cumano A, Godin I. Ontogeny of the hematopoietic system. Annu Rev Immunol. 2007;25:745–785. doi: 10.1146/annurev.immunol.25.022106.141538. [DOI] [PubMed] [Google Scholar]
- [57].Palis J, Segel GB. Developmental biology of erythropoiesis. Blood Rev. 1998;12:106–114. doi: 10.1016/s0268-960x(98)90022-4. [DOI] [PubMed] [Google Scholar]
- [58].Medvinsky AL, Samoylina NL, Muller AM, Dzierzak EA. An early pre-liver intraembryonic source of CFU-S in the developing mouse. Nature. 1993;364:64–67. doi: 10.1038/364064a0. [DOI] [PubMed] [Google Scholar]
- [59].Muller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1:291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- [60].Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- [61].Jaffredo T, Gautier R, Eichmann A, Dieterlen LF. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development. 1998;125:4575–4583. doi: 10.1242/dev.125.22.4575. [DOI] [PubMed] [Google Scholar]
- [62].Yokomizo T, Ogawa M, Osato M, Kanno T, Yoshida H, et al. Requirement of Runx1/AML1/PEBP2alphaB for the generation of haematopoietic cells from endothelial cells. Genes Cells. 2001;6:13–23. doi: 10.1046/j.1365-2443.2001.00393.x. [DOI] [PubMed] [Google Scholar]
- [63].Kotch LE, Iyer NV, Laughner E, Semenza GL. Defective vascularization of HIF-1alpha-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Dev Biol. 1999;209:254–267. doi: 10.1006/dbio.1999.9253. [DOI] [PubMed] [Google Scholar]
- [64].Medvinsky A, Rybtsov S, Taoudi S. Embryonic origin of the adult hematopoietic system: advances and questions. Development. 2011;138:1017–1031. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- [65].Imanirad P, Solaimani Kartalaei P, Crisan M, Vink C, Yamada-Inagawa T, et al. HIF1alpha is a regulator of hematopoietic progenitor and stem cell development in hypoxic sites of the mouse embryo. Stem Cell Res. 2014;12:24–35. doi: 10.1016/j.scr.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ramirez-Bergeron DL, Runge A, Adelman DM, Gohil M, Simon MC. HIF-dependent hematopoietic factors regulate the development of the embryonic vasculature. Dev Cell. 2006;11:81–92. doi: 10.1016/j.devcel.2006.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, et al. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–958. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- [69].Feltbower RG, McKinney PA, Greaves MF, Parslow RC, Bodansky HJ. International parallels in leukaemia and diabetes epidemiology. Arch Dis Child. 2004;89:54–56. [PMC free article] [PubMed] [Google Scholar]
- [70].Strauss T, Maayan-Metzger A, Simchen MJ, Morag I, Shenkmean B, et al. Impaired platelet function in neonates born to mothers with diabetes or hypertension during pregnancy. Klin Padiatr. 2010;222:154–157. doi: 10.1055/s-0030-1249092. [DOI] [PubMed] [Google Scholar]
- [71].Mehta R, Petrova A. Neutrophil function in neonates born to gestational diabetic mothers. J Perinatol. 2005;25:178–181. doi: 10.1038/sj.jp.7211241. [DOI] [PubMed] [Google Scholar]
- [72].Harris JM, Esain V, Frechette GM, Harris LJ, Cox AG, et al. Glucose metabolism impacts the spatiotemporal onset and magnitude of HSC induction in vivo. Blood. 2013;121:2483–2493. doi: 10.1182/blood-2012-12-471201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lim SE, Esain V, Kwan W, Theodore LN, Cortes M, et al. HIF1alpha-induced PDGFRbeta signaling promotes developmental HSC production via IL-6 activation. Exp Hematol. 2017;46:83.e86–95.e86. doi: 10.1016/j.exphem.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Guitart AV, Panagopoulou TI, Villacreces A, Vukovic M, Sepulveda C, et al. Fumarate hydratase is a critical metabolic regulator of hematopoietic stem cell functions. J Exp Med. 2017;214:719–735. doi: 10.1084/jem.20161087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Khan JA, Mendelson A, Kunisaki Y, Birbrair A, Kou Y, et al. Fetal liver hematopoietic stem cell niches associate with portal vessels. Science. 2016;351:176–180. doi: 10.1126/science.aad0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–528. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Nijnik A, Woodbine L, Marchetti C, Dawson S, Lambe T, et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447:686–690. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- [78].Ho TT, Warr MR, Adelman ER, Lansinger OM, Flach J, et al. Autophagy maintains the metabolism and function of young and old stem cells. Nature. 2017;543:205–210. doi: 10.1038/nature21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Martin-Pardillos A, Tsaalbi-Shtylik A, Chen S, Lazare S, van Os RP, et al. Genomic and functional integrity of the hematopoietic system requires tolerance of oxidative DNA lesions. Blood. 2017;130:1523–1534. doi: 10.1182/blood-2017-01-764274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, et al. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5:e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Beerman I, Seita J, Inlay MA, Weissman IL, Rossi DJ. Quiescent hematopoietic stem cells accumulate DNA damage during aging that is repaired upon entry into cell cycle. Cell Stem Cell. 2014;15:37–50. doi: 10.1016/j.stem.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Agathocleous M, Meacham CE, Burgess RJ, Piskounova E, Zhao Z, et al. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature. 2017;549:476–481. doi: 10.1038/nature23876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Jin G, Xu C, Zhang X, Long J, Rezaeian AH, et al. Atad3a suppresses Pink1-dependent mitophagy to maintain homeostasis of hematopoietic progenitor cells. Nat Immunol. 2018;19:29–40. doi: 10.1038/s41590-017-0002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Anso E, Weinberg SE, Diebold LP, Thompson BJ, Malinge S, et al. The mitochondrial respiratory chain is essential for haematopoietic stem cell function. Nat Cell Biol. 2017;19:614–625. doi: 10.1038/ncb3529. [DOI] [PMC free article] [PubMed] [Google Scholar]